Abstract
Background
Although the clinical course of the COVID-19 in adults has been extensively described, the impact of the co-detection of SARS-CoV-2 and rhinovirus on severity outcomes is not understood.
Objectives
This study aimed to compare the risk of hospitalization of outpatients with COVID-19 with and without the co-detection of rhinovirus in southern Brazil. Secondarily, such risk was also compared between all individuals with COVID-19 and those with single rhinovirus infection.
Study design
Outpatients (>18 years) with acute signs of cough, fever, or sore throat were prospectively enrolled at two emergency departments from May to September 2020. Sample collection was performed to detect SARS-CoV-2 and other 20 respiratory pathogens. Participants were followed for 28 days through telephone interviews.
Results
1,047 participants were screened and 1,044 were included. Of these, 4.9% were lost during follow-up, and 993/1,044 (95.1%) were included in severity-related analysis. Rhinovirus was the most prevalent pathogen (25.0%, 248/993), followed by SARS-CoV-2 (22.6%, 224/993), with coinfection of these two viruses occurring in 91/993 (9.2%) participants. The risk of COVID-19-related hospitalizations were not different between individuals with and without co-detection of rhinovirus (9.9% vs. 7.6%, respectively, P = 0.655). Conversely, subjects with COVID-19 had a higher hospitalization risk than single rhinovirus infection (8.3 vs 0.4%, respectively, P < 0.001).
Conclusions
The co-detection of SARS-CoV-2 and rhinovirus did not change the risk of hospitalizations in adults. Furthermore, COVID-19 was more severe than single rhinovirus infection.
Keywords: COVID-19, SARS-CoV-2, Rhinovirus, Coinfection
1. Introduction
The COVID-19 pandemic has been the most significant health crisis of the 21st century. Despite all global efforts made to contain the spread of the Severe Acute Respiratory Syndrome Coronavirus type 2 (SARS-CoV-2) since 2020, more than 6 million people have died up to date [1]. Older age and obesity are the most important risk factors for respiratory failure and death [2], [3], [4], [5], [6], [7].
The preventive measures to contain the pandemic, which included the widespread use of face masks, hand hygiene, travel restrictions, school closures, and physical distancing, had an important impact on the epidemiology of many infectious diseases [8]. A substantial global decline in the detection of common respiratory viruses, such as influenza and respiratory syncytial virus, was reported, especially during the most rigid restrictions [9], [10], [11], [12]. However, the rhinovirus detection was shortly reduced or even not reduced in some settings, making this virus the main co-circulating with SARS-CoV-2 during the COVID-19 pandemic [13], [14], [15], [16]. Despite the accumulating epidemiological evidence mentioned above, the impact of respiratory viral co-detections, including rhinovirus, in the severity of COVID-19 is not completely understood.
The rapid development of highly effective vaccines against SARS-CoV-2 through many different platforms had an important impact in the course of the COVID-19 pandemic, reducing the risks of both severity-related outcomes and transmission [17], [18], [19], [20]. However, many factors such as the inequality regarding vaccine distribution, vaccine hesitancy and the emergence of variants of concern may perpetuate SARS-CoV-2 as a global threat [21], [22], [23].
All factors mentioned above reinforce the need to evaluate the interactions of SARS-CoV-2 and other respiratory viral infections. The main aim of this study is to evaluate the impact of rhinovirus co-detection on the risk of hospitalization in adults enrolled as outpatients with COVID-19. As a secondary aim, the risk of hospitalization in individuals with single rhinovirus detection and all those with COVID-19 was also compared.
2. Materials and methods
2.1. Participants’ selection
A prospective cohort study was done at emergency rooms (ERs) of Hospital Moinhos de Vento and Hospital Restinga e Extremo Sul in Porto Alegre, Southern Brazil. Hospital Moinhos de Vento, which is a tertiary and a private hospital, has 372 general wards and 113 ICU beds. Hospital da Restinga Extremo Sul is a tertiary hospital with 485 beds and is a reference for Restinga district, providing care exclusively through the Brazilian public health system. From May to September 2020, adults (≥18 years old) presenting at least one sign or symptom suggestive of COVID-19 (cough, fever, or sore throat) within 14 days were screened. The failure in sample collection was the exclusion criteria. Clinical and demographic data, comorbidities, and signs or symptoms suggestive of COVID-19 infection were collected at baseline. After the inclusion at ER, the participants were followed for 28 days through phone calls (on the 7th, 14th, and 28th day) and hospitalization outcomes (hospital admission, use of supplemental oxygen, admission at intensive care unit (ICU), use of invasive mechanical ventilation, and death) were assessed, as described elsewhere [4]. The review of medical records was performed at these hospitals when no information was obtained through the phone calls. The losses were defined if no contact was possible within the 28-day follow-up or no admission at both institutions.
2.2. Pathogen detection
At enrollment, oropharyngeal and bilateral nasopharyngeal swab collection for SARS-CoV-2 detection was performed for all participants and analyzed through qualitative reverse transcription polymerase chain reaction (RT-PCR) assay, as described elsewhere [24] . Participants with detection of SARS-CoV-2, regardless of the occurrence of other respiratory pathogens co-detections, were classified as having COVID-19.
A real-time PCR respiratory panel was done from a second bilateral nasopharyngeal swab, collected at enrollment, and stored at −80 °C. It was assessed the presence of Bordetella pertussis; Chlamydophila pneumoniae; Mycoplasma pneumoniae; adenovirus; bocavirus; coronavirus types HKU1, 229E, NL63, and OC43; influenza A virus types H1 and H3; influenza B virus; human enterovirus; human metapneumovirus; parainfluenza virus types 1, 2, and 3; RSV types A and B; and rhinovirus. Acid nucleic extraction was done using the MagMax™ Viral/Pathogenic Nucleic Acid Isolation (Applied Biosystems) in the KingFisher Duo Prime System platform (ThermoFisher, USA). NanoDrop™ Lite Spectrophotometer (ThermoFisher, Wilmington, Delaware, USA) was used to sample quantification, and then, the samples were diluted between 0.5 and 2.0 ng/µL. RT-PCR assay was done using the Path™ 1-Step RT-qPCR Master Mix CG (A15299, Applied Biosystems) and TaqMan® Microbial Assays-single tube assay (Applied Biosystems, Pleasanton, California, USA). The probe access codes for the target pathogens are listed in Supplementary Table 1. As the reaction control, we used the TaqMan®Respiratory Tract Microbiota Amplification Control (A39178, Thermofisher).
All the samples were analyzed in the Molecular Biology Laboratory at Hospital Moinhos de Vento.
2.3. Statistical analysis
Data normality assumptions were verified for continuous variables, and values were presented as median and interquartile ranges (IQR). Categorical variables were described as percentages, and Pearson's Chi-square test or Fisher's Exact test were used to evaluate the association of clinical and demographic data, as well as severity-related outcomes, between individuals with SARS-CoV-2 single detection and those with both SARS-CoV-2 and rhinovirus co-detection. The same comparisons were also performed between those with single rhinovirus detection and all individuals with COVID-19, regardless of respiratory pathogens co-detection. The cycle threshold (ct) values of ORF1ab, S and N SARS-CoV-2–specific targets were analyzed by the two-tailed Mann–Whitney–Wilcoxon test between the groups (SARS-CoV-2 single detection and the co-detection with rhinovirus). The power estimation was performed using the observed values of the hospitalization outcome according to the groups cited above. The “ES.w2” function was used to calculate the effect size, and then “pwr.chisq.test” function with (both from the “pwr” package [25] was applied. Multivariable logistic regression analyses were performed to evaluate the risk of hospitalization, using the backward stepwise method with P value cutoff < 0.20, considering the results from univariate analyses presented in Table 2, in addition to other relevant predictors, as obesity [4]. All data preprocessing and analyses were performed in R 4.1.1 statistical software.
Table 2.
Demographic and clinical characteristics of the subjects positive for SARS-CoV-2, and rhinovirus and SARS-CoV-2 coinfection. (IQR) interquartile range. (yr) years. (ICU) intensive care unit.
| Characteristics | SARS-CoV-2 single detection (n = 224) | Rhinovirus and SARS-CoV-2 co-detection (n = 91) | P value |
|---|---|---|---|
| Age, median (IQR) | 38.2 (30.4–46.5) | 34.4 (28.7–43.0) | 0.030* |
| Female sex, n (%) | 138 (61.6) | 53 (58.2) | 0.669† |
| Racial or ethnic group | |||
| Caucasian, n (%) | 165/220 (75.0) | 67 (73.6) | 0.912† |
| Non-caucasian, n (%) | 55 (25.0) | 24 (26.4) | |
| Duration of symptoms at inclusion | |||
| Days, median (IQR) | 3.0 (2.0–5.0) | 3.0 (2.0–4.0) | 0.992* |
| Hospital at inclusion | |||
| Private hospital, n (%) | 108 (48.2) | 38 (41.8) | 0.359† |
| Public hospital, n (%) | 116 (51.8) | 53 (58.2) | |
| Underlying medical conditions | |||
| Obesity, n (%) | 64/214 (29.9) | 37/90 (41.1) | 0.078† |
| Hypertension, n (%) | 37 (16.5) | 6 (6.6) | 0.032† |
| Asthma, n (%) | 13 (5.8) | 5 (5.5) | 1.000† |
| Diabetes mellitus, type 1 or 2, n (%) | 10 (4.5) | 1 (1.1) | 0.187‡ |
| Chronic obstructive pulmonary disease, n (%) | 4 (1.8) | 0 (0.0) | 0.328‡ |
| Malignancy, n (%) | 3/223 (1.3) | 2 (2.2) | 0.629‡ |
| Previous transplantation, n (%) | 1/223 (0.4) | 0 (0.0) | 1.000‡ |
| Symptoms | |||
| Respiratory symptoms**, n (%) | 216 (96.4) | 88 (96.7) | 1.000† |
| Gastrointestinal symptoms***, n (%) | 116/223 (52.0) | 49 (53.8) | 0.865† |
| Fever, n (%) | 146/217 (67.3) | 54/90 (60.0) | 0.277† |
| Headache, n (%) | 202/223 (90.6) | 82 (90.1) | 1.000† |
| Malaise, n (%) | 170 (75.9) | 70 (76.9) | 0.961† |
| Myalgia, n (%) | 170/222 (75.9) | 70 (76.9) | 1.000† |
| Chills, n (%) | 134 (59.8) | 57 (62.6) | 0.736† |
| Dysgeusia, n (%) | 115/223 (51.6) | 47 (51.6) | 1.000† |
| Anosmia, n (%) | 110/220 (50.0) | 46 (50.5) | 1.000† |
| Appetite loss, n (%) | 111/223 (49.8) | 47/90 (52.2) | 0.789† |
| Conjunctivitis, n (%) | 80 (35.7) | 32 (35.2) | 1.000† |
| Skin rash, n (%) | 6 (2.7) | 4 (4.4) | 0.665† |
| Duration of symptoms at hospitalization | |||
| Days, median (IQR) | 8.0 (6.0–9.0) | 8.0 (7.0–9.0) | 0.603* |
| Hospitalization | |||
| Hospital admission, n (%) | 17 (7.6) | 9 (9.9) | 0.655† |
| Use of supplemental oxygen, n (%) | 15 (6.7) | 7 (7.7) | 0.944† |
| Admission at ICU, n (%) | 5 (2.2) | 2 (2.2) | 1.000‡ |
| Use of invasive mechanical ventilation, n (%) | 2 (0.9) | 1 (1.1) | 1.000‡ |
| Deaths, n (%) | 3 (1.3) | 0 (0.0) | 0.559‡ |
Mann-Whitney-Wilcoxon test; † Pearson's Chi-squared test; ‡ Fisher's exact test.
Respiratory symptoms comprise: cough, coryza, sore throat, stuffy nose, dyspnea or sputum production;.
Gastrointestinal symptoms comprise: nausea, diarrhea or vomiting.
2.4. Ethical approval
The study was performed following the Decree 466/12 of the National Health Council [26] and Good Clinical Practice Guidelines, after approval by the Hospital Moinhos de Vento Institutional Review Board (IRB n° 4.637.933). All participants included in this study provided written informed consent.
3. Results
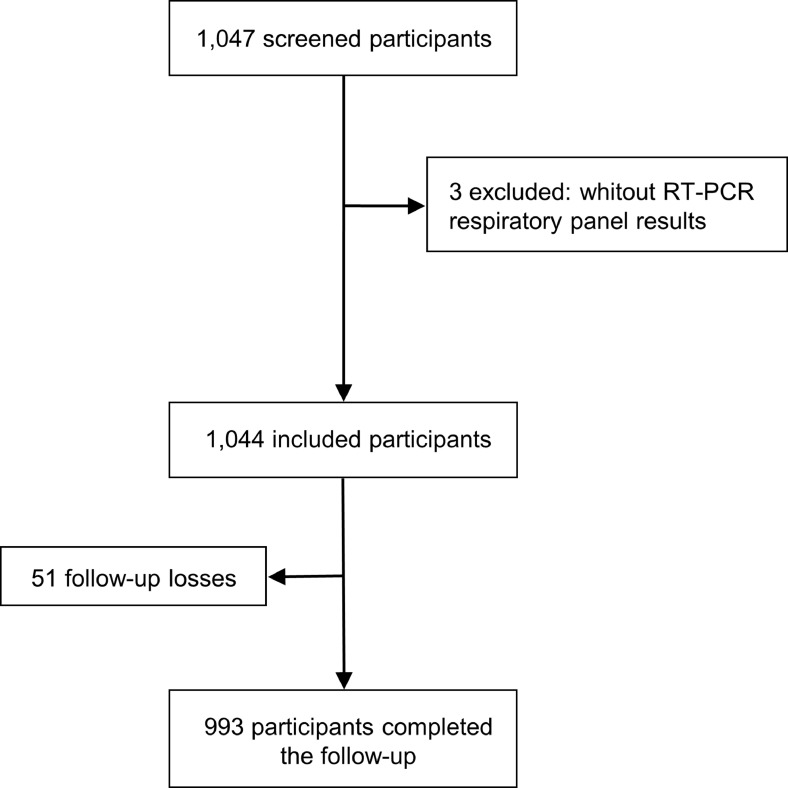
In this study, 1047 outpatient participants were screened, and three were excluded (for failure in sample collection). As shown in Fig. 1 , 1044 adults were included. Of these, 34 (3.3%) subjects required hospitalization. The 28-days follow-up was successfully obtained for 993 (95.1%) participants.
Fig. 1.
Study flowchart.
Among the 993 participants that completed the follow-up, 25.0% (248) were diagnosed positive for rhinovirus, followed by SARS-CoV-2 (22.6%, 224) and Mycoplasma pneumoniae (0.4%, 4) as single detections, as shown in Table 1 . The coinfection of rhinovirus and SARS-CoV-2 occurred in 91 (9.2%) participants, and one had also Mycoplasma pneumoniae co-detection associated. Other multiple detections, such as SARS-CoV-2 and other pathogens (coronavirus types HKU1 or NL63; enterovirus; adenovirus; metapneumovirus; or Mycoplasma pneumoniae), and rhinovirus and Mycoplasma pneumoniae, were observed in 16 (1.6%) and 10 (1.0%) participants, respectively. No pathogen detection occurred in 400 (40.3%) individuals.
Table 1.
Pathogen detection of included participants.
| Pathogens | Total(n = 1044) | Completed follow-up(n = 993) |
|---|---|---|
| Single detection | ||
| Rhinovirus | 262 (25.1) | 248 (25.0) |
| SARS-CoV-2 | 235 (22.5) | 224 (22.6) |
| Mycoplasma pneumoniae | 4 (0.4) | 4 (0.4) |
| Multiple detections | ||
| Rhinovirus and SARS-CoV-2 | 93 (8.9) | 91 (9.2) |
| SARS-CoV-2 and others | 18 (1.7) | 16 (1.6) |
| Rhinovirus and Mycoplasma pneumoniae | 10 (1.0) | 10 (1.0) |
| No pathogen detection | 422 (40.4) | 400 (40.3) |
No detection of Bordetella pertussis; Chlamydophila pneumoniae; bocavirus; coronavirus types 229E, and OC43; influenza A virus types H1 and H3; influenza B virus; parainfluenza virus types 1, 2, and 3; RSV types A and B was found.
The median age of the included participants was 36.4 years (IQR, 28.8–44.9, range 18.0–89.1), 62.7% (655/1044) were female, and the median days of symptoms onset to inclusion was 3.0 (IQR, 2.0–5.0, range 0.0–14.0), as shown in Supplementary Table 2.
The co-detection of rhinovirus did not influence the risk of hospital admission in individuals with COVID-19 (9.9% vs. 7.6%, P = 0.655), as shown in Table 2 . However, there was a higher association with hospitalization, use of supplemental oxygen and admission at intensive care unit (ICU) in participants with COVID-19, regardless of co-detection status, than in those with rhinovirus single detection, as depicted in Supplementary Table 3. Adults with rhinovirus co-detection were younger than those with COVID-19 only (P = 0.030), and the prevalence of hypertension was positively associated with subjects with COVID-19 single detection (16.5% vs. 6.6%, P = 0.032). In multivariable modeling, participants who required hospitalization were significantly older and obese (OR = 1.00, 95%CI 1.00–1.01, P = 0.001; OR = 1.13, 95%CI 1.07–1.20, P < 0.001, respectively). Whereas the presence of hypertension and virus co-detections had no relevance for hospitalization in our cohort (OR = 0.98, 95%CI 0.89–1.08, P = 0.694; OR = 1.02, 95%CI 0.96–1.09, P = 0.567, respectively).
The median, IQR and the comparison between ct values of the probes considering the SARS-CoV-2 single detection and the co-detection with rhinovirus are, respectively: ORF1ab (20.7, IQR 17.7–24.9 and 20.7, IQR 17.2–26.2; P = 0.886), N (20.4, IQR 16.2–24.5 and 19.9, IQR 16.4–24.8; P = 0.949) and S (20.8, IQR 17.6–24.8 and 20.1, IQR 16.8–23.2; P = 0.424).
4. Discussion
This is the first prospective study assessing the impact of rhinovirus co-detection on hospitalizations of COVID-19 in adults. Our results strongly suggest that rhinovirus detection does not affect the risk of hospitalization in individuals with COVID-19. Moreover, the risk of hospitalization in adults with COVID-19 was much higher than in those with single rhinovirus detection.
The epidemiology of many communicable diseases was markedly changed during the COVID-19 pandemic. Detections of highly prevalent agents of respiratory infections such as influenza and respiratory syncytial viruses decreased worldwide during 2020 [9,10]. In the same way, other viruses such as adenovirus, parainfluenza, and metapneumovirus also were less detected compared to previous seasons [27,28]. Conversely, rhinovirus was reported as the main co-circulating virus during the pandemic. A decrease of its detection during the most restrictive periods of social isolation but an earlier relapse as soon as restrictions were relaxed were reported in many countries, mainly through surveillance studies [15,16,29]. Our findings reinforce with prospectively collected data all these epidemiological changes during the first year of pandemic. In addition, age and obesity were the most critical risk factors associated with hospital admission [4].
Throughout the COVID-19 pandemic, much was discussed about the comparison regarding the severity of the infection due to SARS-CoV-2 and other respiratory viruses, as well as the role of respiratory coinfections. However, as the spread of most prevalent respiratory viruses was reduced, as mentioned above, many of these comparisons were mostly based on historical or retrospective data [30,31]. Our findings provide a direct comparison between the single infections and the co-detections of the two main co-circulating viruses in 2020. Furthermore, respiratory symptoms were more associated with rhinovirus single detection, whereas general symptoms were observed in individuals with SARS-CoV-2 infection. Although rhinovirus might be only a bystander in some situations, it has been consistently related to lower respiratory tract disease in older adults and asthma exacerbation [32], [33], [34]. However, the risk of hospitalization due to rhinovirus was much lower than those secondary to COVID-19 in our study.
The role of coinfection of SARS-CoV-2 with other respiratory viruses is not fully understood. Some experimental data suggested that the simultaneous infection by rhinovirus might protect individuals from severe COVID-19 due to Interferon stimulation [35]. Nonetheless, our results did not support any changes in the severity of COVID-19 when rhinovirus was co-detected. Before the COVID-19 pandemic, most of the available evidence suggested that detection of multiple respiratory viruses was not associated with changes in severity and our findings are in line with previous evidence [36,37]. However, the severity of multiple infections, including SARS-CoV-2, might be different, although our findings do not suggest this association in SARS-CoV-2 and rhinovirus co-detection.
Regarding the SARS-CoV-2 variants, the B.1.1.28, B.1.1.33, B.1.91, B.1.1 and B.1.1.143 lineages were reported to predominate at the time of the study [38,39], with special attention to the emergence of the B.1.1.28 lineage (which originated the Gamma lineage – P.1) that was described in November 2020. Although our enrollment was completed in September, it is unlikely that the Gamma lineage was represented in our samples.
This study has some limitations. First, as the incidence of most respiratory viruses sharply decreased during the pandemic, only the impact of SARS-CoV-2 and rhinovirus co-detection was possible to assess. Second, as participants were mostly middle-aged outpatients enrolled at the beginning of the clinical course with mild disease, evaluation of severity-related outcomes other than hospitalization was not possible. Third, technically it is not possible to differentiate between actual rhinovirus infection and its detection as a bystander. Fourth, the use of azithromycin at inclusion might mask the prevalence of atypical bacteria. Fifth, this study was done before the emergence of variants of concern. On the other hand, no previous study directly compared prospectively the impact of rhinovirus co-detection on the risk of hospitalization due to COVID-19 in adults, evaluating co-detections using a broad viral/bacterial panel, a large sample size, and 28 days of follow-up.
In summary, our results strongly suggest that rhinovirus co-detection has no prognostic role in COVID-19. Moreover, rhinovirus single detection was associated with milder disease than COVID-19. As COVID-19 is expected to become endemic, further studies comparing the severity of SARS-CoV-2 to other viral agents and further combinations of co-detections will be essential to understand the epidemiology of respiratory infections, including SARS-CoV-2.
Funding
This work was supported by the Brazilian Ministry of Health, through the Institutional Development Program of the Brazilian National Health System (PROADI-SUS) in collaboration with Hospital Moinhos de Vento.
CRediT authorship contribution statement
Marcelo Comerlato Scotta: Conceptualization, Investigation, Formal analysis, Data curation, Visualization, Writing – original draft, Project administration. Luciane Beatriz Kern: Conceptualization, Resources, Validation, Investigation, Writing – original draft. Márcia Polese-Bonatto: Conceptualization, Methodology, Resources, Validation, Investigation, Data curation, Writing – original draft, Supervision. Thais Raupp Azevedo: Resources, Validation, Investigation, Writing – original draft. Fernanda Hammes Varela: Conceptualization, Methodology, Investigation, Visualization, Writing – original draft. Gabriela Oliveira Zavaglia: Resources, Investigation, Writing – original draft. Ingrid Rodrigues Fernandes: Methodology, Resources, Investigation, Writing – original draft. Caroline Nespolo de David: Methodology, Resources, Investigation, Writing – original draft, Project administration. Tiago Fazolo: Resources, Investigation, Writing – review & editing. Marcela Santos Corrêa da Costa: Resources, Investigation, Writing – review & editing. Felipe Cotrim de Carvalho: Resources, Investigation, Writing – review & editing. Ivaine Tais Sauthier Sartor: Conceptualization, Software, Investigation, Formal analysis, Data curation, Visualization, Writing – original draft. Alexandre Prehn Zavascki: Conceptualization, Investigation, Data curation, Visualization, Writing – review & editing, Supervision. Renato T. Stein: Conceptualization, Writing – review & editing, Supervision.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgments
We thank Adriane Isabel Rohden, Amanda Paz Santos, Camila Dietrich, Caroline Cabral Robinson, Catia Moreira Guterres, Charles Francisco Ferreira, Débora Vacaro Fogazzi, Fernanda Lutz Tolves, Elvira Alicia Aparicio Cordero, Fernando Rovedder Boita, Jaina da Costa Pereira, Maristênia Machado Araújo, Shirlei Villanova Ribeiro, and Thainá Dias Luft. We thank the Scientific Committee of the Research Support Nucleus (NAP) of Moinhos de Vento Hospital for technical-scientific consultancy. We thank the assistance team, laboratory team, and local staff from Hospital Moinhos de Vento and Hospital Restinga e Extremo Sul.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jcv.2022.105197.
Appendix. Supplementary materials
References
- 1.2022. WHO Coronavirus (COVID-19) Dashboard.https://covid19.who.int (n.d.) accessed May 20. [Google Scholar]
- 2.Bastos G.A.N., de Azambuja A.Z., Polanczyk C.A., Gräf D.D., Zorzo I.W., Maccari J.G., Haygert L.S., Nasi L.A., Gazzana M.B., Bessel M., Pitrez P.M., de Oliveira R.P., Scotta M.C. Clinical characteristics and predictors of mechanical ventilation in patients with COVID-19 hospitalized in Southern Brazil. Rev. Bras. Ter. Intensiva. 2020;32 doi: 10.5935/0103-507X.20200082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fazolo T., Lima K., Fontoura J.C., de Souza P.O., Hilario G., Zorzetto R., Júnior L.R., Pscheidt V.M., de Castilhos Ferreira Neto J., Haubert A.F., Gambin I., Oliveira A.C., Mello R.S., de Bastos Balbe e Gutierres M., Gassen R.B., Coimbra L.D., Borin A., Marques R.E., Sartor I.T.S., Zavaglia G.O., Fernandes I.R., Nakaya H.I., Varela F.H., Polese-Bonatto M., Borges T.J., Callegari-Jacques S.M., da Costa M.S.C., de Araujo Schwartz J., Scotta M.C., Stein R.T., Bonorino C. Pediatric COVID-19 patients in South Brazil show abundant viral mRNA and strong specific anti-viral responses. Nat. Commun. 2021;12:6844. doi: 10.1038/s41467-021-27120-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.I.T.S. Sartor, C.N. de David, G.H. Telo, G.O. Zavaglia, I.R. Fernandes, L.B. Kern, M. Polese-Bonatto, T.R. Azevedo, A.P. Santos, W.A.F. de Almeida, V.B.G. Porto, F.H. Varela, M.C. Scotta, R.G. Rosa, R.T. Stein, Covid. study Group, Association between obesity and hospitalization in mild COVID-19 young adult outpatients in Brazil: a prospective cohort study, (2021) 2021.08.04.21261538. https://doi.org/10.1101/2021.08.04.21261538.
- 5.Mehta N.S., Mytton O.T., Mullins E.W.S., Fowler T.A., Falconer C.L., Murphy O.B., Langenberg C., Jayatunga W.J.P., Eddy D.H., Nguyen-Van-Tam J.S. SARS-CoV-2 (COVID-19): what do we know about children? A systematic review. Clin. Infect. Dis. 2020;71:2469–2479. doi: 10.1093/cid/ciaa556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tartof S.Y., Qian L., Hong V., Wei R., Nadjafi R.F., Fischer H., Li Z., Shaw S.F., Caparosa S.L., Nau C.L., Saxena T., Rieg G.K., Ackerson B.K., Sharp A.L., Skarbinski J., Naik T.K., Murali S.B. Obesity and mortality among patients diagnosed with COVID-19: results from an integrated health care organization. Ann. Intern. Med. 2020;173:773–781. doi: 10.7326/M20-3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang J., Ma Z., Lei Y. A meta-analysis of the association between obesity and COVID-19. Epidemiol. Infect. 2020;149:e11. doi: 10.1017/S0950268820003027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friedrich F., de C. e Garcia L., Petry L.M., Pieta M.P., Carvalho G.E., Zocche G., Ongaratto R., Lumertz M.S., Brum M., Stein R.T., Scotta M.C., Jones M.H., Pinto L.A. Impact of nonpharmacological COVID-19 interventions in hospitalizations for childhood pneumonia in Brazil. Pediatr. Pulmonol. 2021;56:2818–2824. doi: 10.1002/ppul.25570. [DOI] [PubMed] [Google Scholar]
- 9.Friedrich F., Ongaratto R., Scotta M.C., Veras T.N., Stein R.T., Lumertz M.S., Jones M.H., Comaru T., Pinto L.A. Early impact of social distancing in response to coronavirus disease 2019 on hospitalizations for acute bronchiolitis in infants in Brazil. Clin. Infect. Dis. 2021;72:2071–2075. doi: 10.1093/cid/ciaa1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Varela F.H., Scotta M.C., Polese-Bonatto M., Sartor I.T.S., Ferreira C.F., Fernandes I.R., Zavaglia G.O., de Almeida W.A.F., Arakaki-Sanchez D., Pinto L.A., Nader Bastos G.A., Nasi L.A., Falavigna M., Pitrez P.M., Stein R.T. Absence of detection of RSV and influenza during the COVID-19 pandemic in a Brazilian cohort: likely role of lower transmission in the community. J. Glob. Health. 2021;11:05007. doi: 10.7189/jogh.11.05007. n.d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Brusselen D., De Troeyer K., Ter Haar E., Vander Auwera A., Poschet K., Van Nuijs S., Bael A., Stobbelaar K., Verhulst S., Van Herendael B., Willems P., Vermeulen M., De Man J., Bossuyt N., Vanden Driessche K. Bronchiolitis in COVID-19 times: a nearly absent disease? Eur. J. Pediatr. 2021;180:1969–1973. doi: 10.1007/s00431-021-03968-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olsen S.J. Decreased influenza activity during the COVID-19 Pandemic — United States, Australia, Chile, and South Africa, 2020. MMWR Morb. Mortal Wkly. Rep. 2020;69 doi: 10.15585/mmwr.mm6937a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marriott D., Beresford R., Mirdad F., Stark D., Glanville A., Chapman S., Harkness J., Dore G.J., Andresen D., Matthews G.V. Concomitant marked decline in prevalence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and other respiratory viruses among symptomatic patients following public health interventions in Australia: data from St Vincent's Hospital and associated screening clinics, Sydney, NSW. Clin. Infect. Dis. 2021;72:e649–e651. doi: 10.1093/cid/ciaa1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park S., Michelow I.C., Choe Y.J. Shifting patterns of respiratory virus activity following social distancing measures for coronavirus disease 2019 in South Korea. J. Infect. Dis. 2021 doi: 10.1093/infdis/jiab231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodgers L., Sheppard M., Smith A., Dietz S., Jayanthi P., Yuan Y., Bull L., Wotiz S., Schwarze T., Azondekon R., Hartnett K., Adjemian J., Kirking H.L., Kite-Powell A. Changes in seasonal respiratory illnesses in the United States during the coronavirus disease 2019 (COVID-19) pandemic. Clin. Infect. Dis. 2021;73:S110–S117. doi: 10.1093/cid/ciab311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Redlberger-Fritz M., Kundi M., Aberle S.W., Puchhammer-Stöckl E. Significant impact of nationwide SARS-CoV-2 lockdown measures on the circulation of other respiratory virus infections in Austria. J. Clin. Virol. 2021;137 doi: 10.1016/j.jcv.2021.104795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singanayagam A., Hakki S., Dunning J., Madon K.J., Crone M.A., Koycheva A., Derqui-Fernandez N., Barnett J.L., Whitfield M.G., Varro R., Charlett A., Kundu R., Fenn J., Cutajar J., Quinn V., Conibear E., Barclay W., Freemont P.S., Taylor G.P., Ahmad S., Zambon M., Ferguson N.M., Lalvani A., Badhan A., Dustan S., Tejpal C., Ketkar A.V., Narean J.S., Hammett S., McDermott E., Pillay T., Houston H., Luca C., Samuel J., Bremang S., Evetts S., Poh J., Anderson C., Jackson D., Miah S., Ellis J., Lackenby A. Community transmission and viral load kinetics of the SARS-CoV-2 delta (B.1.617.2) variant in vaccinated and unvaccinated individuals in the UK: a prospective, longitudinal, cohort study. Lancet Infect. Dis. 2022;22:183–195. doi: 10.1016/S1473-3099(21)00648-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tenforde M.W., Self W.H., Adams K., Gaglani M., Ginde A.A., McNeal T., Ghamande S., Douin D.J., Talbot H.K., Casey J.D., Mohr N.M., Zepeski A., Shapiro N.I., Gibbs K.W., Files D.C., Hager D.N., Shehu A., Prekker M.E., Erickson H.L., Exline M.C., Gong M.N., Mohamed A., Henning D.J., Steingrub J.S., Peltan I.D., Brown S.M., Martin E.T., Monto A.S., Khan A., Hough C.L., Busse L.W., ten Lohuis C.C., Duggal A., Wilson J.G., Gordon A.J., Qadir N., Chang S.Y., Mallow C., Rivas C., Babcock H.M., Kwon J.H., Halasa N., Chappell J.D., Lauring A.S., Grijalva C.G., Rice T.W., Jones I.D., Stubblefield W.B., Baughman A., Womack K.N., Rhoads J.P., Lindsell C.J., Hart K.W., Zhu Y., Olson S.M., Kobayashi M., Verani J.R., Patel M.M. Influenza and other viruses in the acutely ill (IVY) network, association between mRNA vaccination and COVID-19 hospitalization and disease severity. JAMA. 2021;326:2043–2054. doi: 10.1001/jama.2021.19499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jara A., Undurraga E.A., González C., Paredes F., Fontecilla T., Jara G., Pizarro A., Acevedo J., Leo K., Leon F., Sans C., Leighton P., Suárez P., García-Escorza H., Araos R. Effectiveness of an Inactivated SARS-CoV-2 vaccine in Chile. N. Engl. J. Med. 2021;385:875–884. doi: 10.1056/NEJMoa2107715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.NEJM; 2022. Effect of Vaccination on Household Transmission of SARS-CoV-2 in England.https://www.nejm.org/doi/full/10.1056/nejmc2107717 n.d. accessed May 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dror A.A., Eisenbach N., Taiber S., Morozov N.G., Mizrachi M., Zigron A., Srouji S., Sela E. Vaccine hesitancy: the next challenge in the fight against COVID-19. Eur. J. Epidemiol. 2020;35:775–779. doi: 10.1007/s10654-020-00671-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tatar M., Shoorekchali J.M., Faraji M.R., Wilson F.A. International COVID-19 vaccine inequality amid the pandemic: perpetuating a global crisis? J. Glob. Health. 2021;11:03086. doi: 10.7189/jogh.11.03086. n.d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tatsi E.-.B., Filippatos F., Michos A. SARS-CoV-2 variants and effectiveness of vaccines: a review of current evidence. Epidemiol. Infect. 2021;149:e237. doi: 10.1017/S0950268821002430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Polese-Bonatto M., Sartor I.T.S., Varela F.H., Gianinni G.L.T., Azevedo T.R., Kern L.B., Fernandes I.R., Zavaglia G.O., de David C.N., de Almeida W.A.F., Porto V.B.G., Scotta M.C., Stein R.T., Covid. study Group Children have similar RT-PCR cycle threshold for SARS-CoV-2 in comparison with adults. MedRxiv. 2021 doi: 10.1101/2021.04.20.21255059. 2021.04.20.21255059. [DOI] [Google Scholar]
- 25.Csicsman J., Sep C. 2022. Effect Size Calculation in Power Estimation For the Chi-square Test of Preliminary Data in Different Studies.https://www.academia.edu/27853396/Effect_Size_Calculation_in_Power_Estimation_for_the_Chi_square_Test_of_Preliminary_Data_in_Different_Studies n.d. accessed May 20. [Google Scholar]
- 26.2021. Ministerio Da Saude.https://bvsms.saude.gov.br/bvs/saudelegis/cns/2013/res0466_12_12_2012.html n.d. accessed July 13. [Google Scholar]
- 27.Kitanovski S., Horemheb-Rubio G., Adams O., Gärtner B., Lengauer T., Hoffmann D., Kaiser R. Respiratory virus network, rhinovirus prevalence as indicator for efficacy of measures against SARS-CoV-2. BMC Public Health. 2021;21:1178. doi: 10.1186/s12889-021-11178-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poole S., Brendish N.J., Clark T.W. SARS-CoV-2 has displaced other seasonal respiratory viruses: results from a prospective cohort study. J. Infect. 2020;81:966–972. doi: 10.1016/j.jinf.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuitunen I., Artama M., Haapanen M., Renko M. Rhinovirus spread in children during the COVID-19 pandemic despite social restrictions-A nationwide register study in Finland. J. Med. Virol. 2021;93:6063–6067. doi: 10.1002/jmv.27180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang X., Du R.-.H., Wang R., Cao T.-.Z., Guan L.-.L., Yang C.-.Q., Zhu Q., Hu M., Li X.-.Y., Li Y., Liang L.-.R., Tong Z.-.H., Sun B., Peng P., Shi H.-.Z. Comparison of hospitalized patients with ARDS caused by COVID-19 and H1N1. Chest. 2020;158:195–205. doi: 10.1016/j.chest.2020.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deng L.-.S., Yuan J., Ding L., Chen Y.-.L., Zhao C.-.H., Chen G.-.Q., Li X.-.H., Li X.-.H., Luo W.-.T., Lan J.-.F., Tan G.-.Y., Tang S.-.H., Xia J.-.Y., Liu X. Comparison of patients hospitalized with COVID-19, H7N9 and H1N1. Infect. Dis. Poverty. 2020;9:163. doi: 10.1186/s40249-020-00781-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi T., Arnott A., Semogas I., Falsey A.R., Openshaw P., Wedzicha J.A., Campbell H., Nair H. The etiological role of common respiratory viruses in acute respiratory infections in older adults: a systematic review and meta-analysis. J. Infect. Dis. 2019:jiy662. doi: 10.1093/infdis/jiy662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee N., Smith S., Zelyas N., Klarenbach S., Zapernick L., Bekking C., So H., Yip L., Tipples G., Taylor G., Mubareka S. Burden of noninfluenza respiratory viral infections in adults admitted to hospital: analysis of a multiyear Canadian surveillance cohort from 2 centres. CMAJ. 2021;193:E439–E446. doi: 10.1503/cmaj.201748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pierangeli A., Scagnolari C., Selvaggi C., Verzaro S., Spina M.T., Bresciani E., Antonelli G., Bertazzoni G. Rhinovirus frequently detected in elderly adults attending an emergency department. J. Med. Virol. 2011;83:2043–2047. doi: 10.1002/jmv.22205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dee K., Goldfarb D.M., Haney J., Amat J.A.R., Herder V., Stewart M., Szemiel A.M., Baguelin M., Murcia P.R. Human rhinovirus infection blocks severe acute respiratory syndrome coronavirus 2 replication within the respiratory epithelium: implications for COVID-19 epidemiology. J. Infect. Dis. 2021;224:31–38. doi: 10.1093/infdis/jiab147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scotta M.C., Chakr V.C.B.G., de Moura A., Becker R.G., de Souza A.P.D., Jones M.H., Pinto L.A., Sarria E.E., Pitrez P.M., Stein R.T., Mattiello R. Respiratory viral coinfection and disease severity in children: a systematic review and meta-analysis. J. Clin. Virol. 2016;80:45–56. doi: 10.1016/j.jcv.2016.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Asner S.A., Science M.E., Tran D., Smieja M., Merglen A., Mertz D. Clinical disease severity of respiratory viral co-infection versus single viral infection: a systematic review and meta-analysis. PLoS ONE. 2014;9:e99392. doi: 10.1371/journal.pone.0099392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.da S. Francisco Jr R., Benites L.F., Lamarca A.P., de Almeida L.G.P., Hansen A.W., Gularte J.S., Demoliner M., Gerber A.L., de C Guimarães A.P., Antunes A.K.E., Heldt F.H., Mallmann L., Hermann B., Ziulkoski A.L., Goes V., Schallenberger K., Fillipi M., Pereira F., Weber M.N., de Almeida P.R., Fleck J.D., Vasconcelos A.T.R., Spilki F.R. Pervasive transmission of E484K and emergence of VUI-NP13L with evidence of SARS-CoV-2 co-infection events by two different lineages in Rio Grande do Sul, Brazil. Virus Res. 2021;296 doi: 10.1016/j.virusres.2021.198345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sartor I.T.S., Varela F.H., Meireles M.R., Kern L.B., Azevedo T.R., Giannini G.L.T., da Silva M.S., Demoliner M., Gularte J.S., de Almeida P.R., Fleck J.D., Zavaglia G.O., Fernandes I.R., de David C.N., Santos A.P., de Almeida W.A.F., Porto V.B.G., Scotta M.C., Vieira G.F., Spilki F.R., Stein R.T., Polese-Bonatto M. Y380Q novel mutation in receptor-binding domain of SARS-CoV-2 spike protein together with C379W interfere in the neutralizing antibodies interaction. Diagn. Microbiol. Infect. Dis. 2022;102 doi: 10.1016/j.diagmicrobio.2022.115636. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.