Abstract
Objective
The establishment of reference intervals (RIs) for complement 3 (C3) and complement 4 (C4) is rare, especially by indirect methods. Therefore, this study aims to establish regional RIs for C3 and C4 by an indirect method, using relevant statistical methods.
Methods
Total of 12,313 data points for C3 and 12,125 data points for C4 were obtained from the First Hospital of Jilin University's database in China and standardised using the Tukey and Box–Cox statistical methods. The coefficients of the skewness-median-coefficient of variation curves (LMS) were used to determine the critical value for age, and a subsequent z test used to compare the differences. A non-parametric method was used to establish the RIs.
Results
The C3 and C4 concentrations showed no significant differences by sex, and a weak correlation with age. No significant difference was found after calculating the z value for the age points on the LMS curves. The RIs for C3 and C4 were 0.83–1.58 g/L and 0.15–0.40 g/L, respectively. The RIs all passed verification.
Conclusion
Suitable RIs for C3 and C4 were established for the local population, and will benefit clinical diagnosis. The feasibility and practicability of the indirect method were demonstrated.
Keywords: China, Data mining, Indirect method, Reference intervals, Serum complement 3, Serum complement 4
الملخص
أهداف البحث
الفترات المرجعية للمتممة 3 والمتممة 4 نادرة، خاصة بالطرق غير المباشرة. تهدف هذه الدراسة إلى إنشاء فترات مرجعية إقليمية للمتممة 3 والمتممة 4 باستخدام الطريقة غير المباشرة بالأساليب الإحصائية المناسبة.
طرق البحث
تم تضمين 12313 نقطة بيانات للمتممة 3 و 12125 نقطة بيانات للمتممة 4 من قاعدة بيانات المستشفى الأول بجامعة جيلين في الصين، وتم توحيدها بواسطة الأساليب الإحصائية. تم استخدام معاملات التجانف - الوسيط – معامل التفاوت لتحديد القيمة الحرجة للعمر، مع اختبار "زي" اللاحق لمقارنة الاختلافات. تم استخدام الطريقة غير المتثابتة لإنشاء الفترات المرجعية.
النتائج
لم تظهر التراكيز للمتممة 3 والمتممة 4 فروقا ذات دلالة احصائية مع الجنس وأظهرت ارتباطا ضعيفا مع العمر. كما لم يتم العثور على فروق ذات دلالة إحصائية بعد حساب قيمة "زي" للنقاط العمرية على المنحنيات المستخدمة. كانت الفترات المرجعية للمتممة 3 هو 0,83-1,58 جم/لتر وللمتممة 4 هو 0.15-0.40 جم/لتر. اجتازت الفترات المرجعية جميع أدوات التحقق.
الاستنتاجات
تم إنشاء الفترات المرجعية المناسبة للمتممة 3 وللمتممة 4 للسكان المحليين وستفيد في التشخيص السريري. تم إثبات جدوى وعملية الطريقة غير المباشرة.
الكلمات المفتاحية: طريقة غير مباشرة, فترات مرجعية, متممة المصل 3, متممة المصل 4, التنقيب عن المعطيات, الصين.
Introduction
The complement system plays a crucial role in the innate immune system, and promotes inflammatory and immune responses.1 Many compounds are produced when members of the complement system perform their immunological functions in destroying pathogenic microorganisms. The complement system was recently shown to predict in-hospital mortality in the case of SARS-CoV-2 infected patients.2 Research on SARS-CoV, which is closely related to SARS-CoV-2, has demonstrated that the activation of complement 3 (C3) exacerbates disease in SARS-CoV-associated ARDS3,4; additionally, the complement system is more than just a first line of defence: its considerable degree of sophistication during the course of evolution enables it to interact efficiently with other effector systems of innate immunity as well as those of adaptive immunity.5 This raises its importance, to a certain extent. Moreover, C3 and complement 4 (C4) are closely related to metabolic diseases, such as insulin resistance and other cardiometabolic diseases. Because C3 and C4 play such a vital role in various infectious and inflammatory diseases, and gradually occupy a place in other emerging diagnostic fields, such as myocardial and metabolic diseases,6 the need to establish suitable reference intervals (RIs) for them should be emphasised in clinical practice.
RIs are critical for clinicians to interpret laboratory results and make subsequent diagnostic and intervention decisions.7 However, the RIs recommended by manufacturers are not always applicable to local populations, since biomedical analyte concentrations are affected by region, experimental instrument, or other factors. Hence, from 1987 to 1991, the International Federation of Clinical Chemistry (IFCC) published a series of 6 papers, in which it was recommended that each laboratory should follow defined procedures to produce its own RIs.8, 9, 10, 11 According to the principle of statistics, the analyte level of a healthy population in a region can represent the normal level for the whole region, to a certain extent. The establishment of RIs is a process of using the analyte data of healthy subjects to calculate the analyte concentration range that is suitable for healthy people through statistical methods. This normal range can be used clinically as a critical state to evaluate the health status of patients; the critical state is affected by age, gender, region, and other factors. According to the IFCC's and the Clinical and Laboratory Standards Institute's (CLSI) recommendations, the so-called ‘direct method’ is preferred for establishing RIs, in which it is necessary to select healthy individuals' data for analysis.12,13 Nevertheless, one of the difficulties in this method is that many measures are required to select healthy subjects. In addition, when establishing RIs for paediatric and geriatric groups, or those involving other uncommon sample types (e.g. cerebrospinal fluid),14 collecting enough samples is undeniably twice as tricky. Moreover, there are labour and time constraints. These limitations, coupled with the development of medical information systems,15 have led to the emergence of a new indirect method that uses modern medical databases. Researchers prefer the indirect method from clinical practice to the direct method in clinical decision-making. Generally, researchers have noted the following advantages of the indirect method over the direct method: (1) less time-consuming and costly, (2) guaranteed sample size for each interval when partitioning, (3) reflection of routine laboratory operating conditions, and (4) ethical advantages, as, for a reference interval study, participants are not subjected solely to venesection.16
The establishment of C3 and C4 RIs plays a significant role in the diagnosis of clinical immune diseases and the assessment of immune status and inflammatory response. Suitable RIs for a specific region can greatly improve diagnosis efficiency and avoid the diagnosis error caused by differences in population characteristics in different regions. Most previous studies have selected healthy individuals as the reference population to establish RIs, based on questionnaire investigation or voluntary registration. However, few RIs have been established using data from hospital databases of health examination records. In addition, the concentrations of immunoglobulins and complement components may vary in diverse geographical regions, due to sex, age, and race. However, these differences are generally not significant, while knowledge of these subtle varieties is sometimes critical for clinical explanation.17 Because the indirect method and the RIs of C3 and C4 are assessed separately, the purpose of this study is to use data from the Laboratory Information System (LIS) to establish age- and sex-specific RIs for C3 and C4 by the indirect method, to promote diagnostic efficiency in various diseases. A comparison with Chinese national standards and a verification of the RIs were also performed for this method to be helpful in interpreting laboratory data.
Material and Methods
Study group
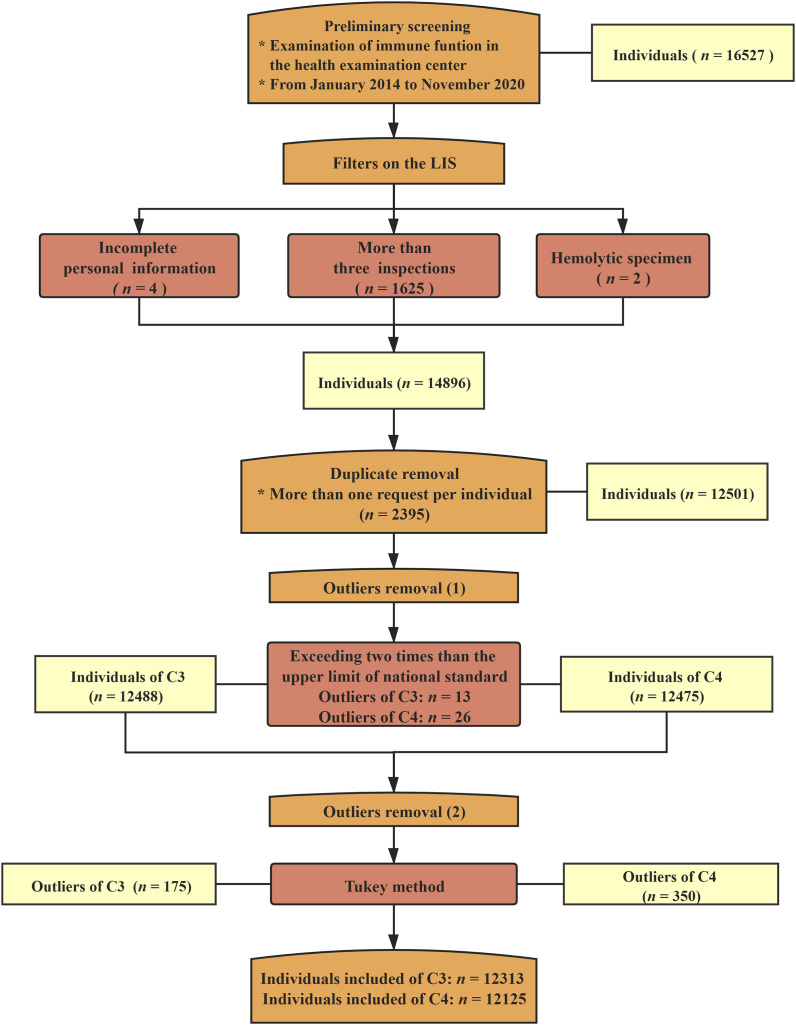
C3 and C4 records were collected from the LIS in the First Hospital of Jilin University, China. All the individuals who had undergone immune testing in the health examination centre were enrolled in the study from January 2014 to November 2020. To improve the accuracy of the RIs in this study, the following exclusion criteria were implemented: a recent history of acute or chronic infection; digestive, kidney, metabolic, rheumatic, and thyroid diseases; any systemic disease; atherosclerosis and vascular disease; heart disease; malignant tumours; burns and muscle damage; weight and height exceeding 10% of the average for the same sex; hypertension (systolic blood pressure ≥140 mm Hg and diastolic blood pressure ≥90 mm Hg). The specific protocol for data removal is shown in Figure 1. Among the procedures, 12,313 C3 and 12,125 C4 data points were included for further research.
Figure 1.
Protocol for the elimination of individuals. National standards: C3, 0.7–1.4 g/L; C4, 0.1–0.4 g/L.
Analytical measurement and quality control
C3 and C4 were quantitatively measured using the immunonephelometric method on Siemens BN Ⅱ automatic protein analyser (Equipment Number: YZB/GEM 1473-2009/223034), according to the manufacturer's package inserts and standard laboratory operating procedures. N Antiserum to Human C3c and C4 were detected by means of test kits from Siemens Healthcare Diagnostics Products GmbH. Reagents were stored at + 2 °C to +8 °C for direct examination, while the precipitate did not contain any particles or residual fibres after the centrifugation of the serum samples. The assay was calibrated by Siemens multiple protein calibrators (2014 No. 3402493) and quality control material (Bole liquid immunology and protein control material: No. 20142405918). Both the calibration and the assay reagents were the same across the whole study period. The typical within-day and between-days CV%s for C3 were CV% ≤ 6.25% and CV% ≤ 8.33%, respectively, while for C4, they were CV% ≤ 6.25% and CV% ≤ 8.33%, respectively, during the quality control measurements. Moreover, internal quality control was performed to test N/T protein controls for SL/L, M, and H, after establishing reference curves, using antiserum for the first time and running samples for a round. In addition, quality assurance was performed according to laboratory standard procedures.
Evaluation of factors of RIs
Harris and Boyd's method (z test) was used to determine sex differences for C3 and C4. Spearman's rank correlation was performed to determine whether the concentrations of analytes were age-independent. In addition, in recent studies, the coefficient of skewness-median-coefficient of variation (LMS) method was used to divide subgroups and establish RIs.19 In this method, three parameters, namely, the power index of the Box–Cox transformation λ (L), median μ (M), and coefficient of variation σ (S), were used to calculate the percentiles, followed by the adjustments of the valid degrees of freedom to obtain smooth curves.20 The centile 100α of y at t was given by C100α(t) = M(t)[1 + L(t)S(t)Zα]1/L(t), where Zα was the normal equivalent deviate of size α.21 Through the curves, the critical segmentation point for age could be observed. Subsequently, z and the critical z (z∗) values were calculated to review the difference between the two age subgroups against the C28-A3 guideline issued by the CLSI.13 A subgroup was considered significant if z > z∗. The formulas were as follows:
| (A1) |
| (A2) |
¯X1 and ¯X2 are the mean values for the first and second groups, respectively; S1 and S2 are the standard deviations (SD) for the first and second groups, respectively; n1 and n2 are the numbers of observations in the first and second groups, respectively.
Establishment and verification of RIs
The lower limits (LRL) and the upper limits (URL) of the RIs (2.5th and 97.5th percentiles), and the 90% confidence intervals of the above two limits were calculated using a nonparametric method and a bootstrap program through random resampling of the same dataset for 1000 times.13,22 According to the IFCC's recommendation for evaluating the accuracy of the established RIs, 100 individuals were randomly selected from the health examination centre in the First Hospital of Jilin University. The proportion of individuals outside the RIs was calculated. The RIs were verified if the rate was less than 5%. A comparison and verification exercise was performed according to the Chinese national standards for C3 and C4 issued in 2018.
Statistical methods
All the analyses were performed using Microsoft Excel for Windows, SPSS version 22.0 (SPSS), and Minitab version 19.0. GraphPad Prism 8.0.2 software (GraphPad Software) and LMS chartmaker Light 2.54 (Medical Research Council) were used as auxiliary software. The process of establishing RIs involved mainly the normal transformation, outlier elimination, correlation analysis of factors, and the establishment and accuracy verification of the RIs against Chinese national standards. The specific steps were as follows.
Data pre-processing
The skewness-Kurtosis test was used to evaluate the normal distribution of the data.18 If the data showed a non-Gaussian distribution, a Box–Cox transformation was performed to transform the data to an approximately normal distribution.
Outlier removal
The Tukey method was used to remove outliers.12 Briefly, Q1 (1st quartile) and Q3 (3rd quartile) were calculated, which involved the 25th (P25) and 75th percentiles (P75), respectively, followed by calculation of interquartile ranges (IQR: Q3 minus Q1). Thereafter, the lower and upper limits were calculated according to the formulas, P25–1.5 × IQR and P75 + 1.5 × IQR, respectively. Outliers were identified whenever the data lay outside the range of the above two limits.
Results
Distribution and elimination of enrolled data
Data on 16,527 individuals were collected from the LIS. Of these, 4,214 C3 and 4,402 C4 data points were excluded, due to either incomplete information or duplicate data, or for exceeding twice the national standard or that of the Tukey method. Finally, 12,313 data points for C3 (6,028 males and 6,285 females aged 6–100 years) and 12,125 data points for C4 (5,965 males and 6,160 females aged 6–102 years) were included after the exclusions. The distributions of C3 and C4 were skewed, according to the skewness-kurtosis test. Therefore, the skewed data were transformed to an approximate Gaussian distribution using the Box–Cox method.
Analysis of variable factors and establishment of RIs
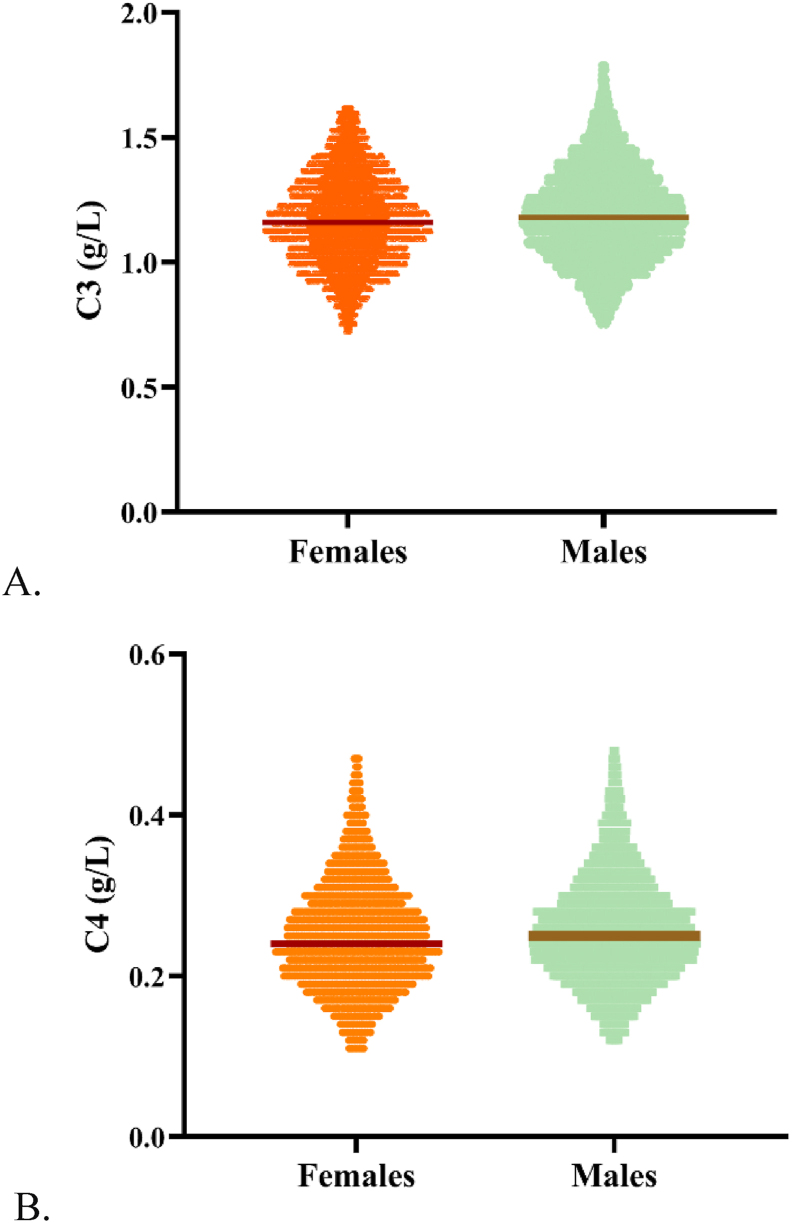
The sex difference was not statistically significant for C3 (z = 3.14, z∗ = 21.48) and C4 (z = 5.28, z∗ = 21.32), while the concentration distribution for sex for C3 and C4 is shown in Figure 2. Weak positive correlations were found between age and analyte concentration for both C3 and C4 (C3: r = 0.02, P < 0.05; C4: r = 0.1, P < 0.05). However, although the differences between the parameters and age were statistically significant from the correlation coefficient, the correlation between the analytes and age was extremely weak and almost negligible, which was also verified in the subsequent statistical analysis.
Figure 2.
Concentration trends for C3 and C4 in males and females: A for C3 and B for C4.
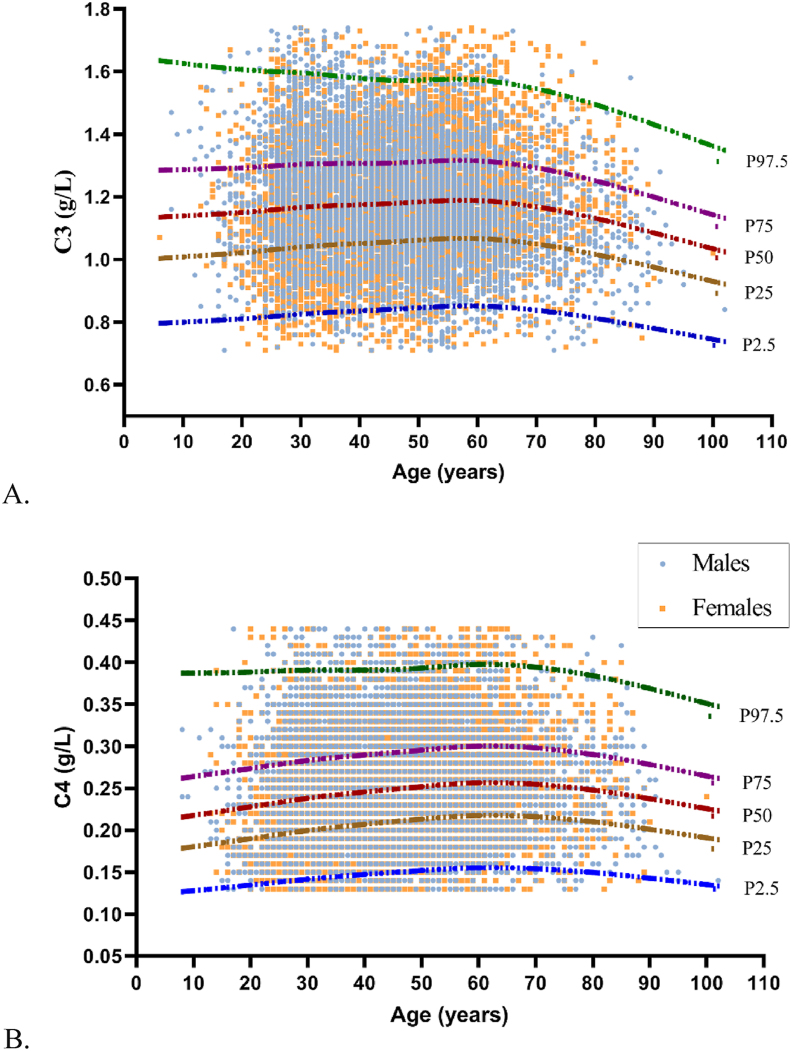
The continuous percentile curves for C3 and C4 are shown in Figure 3. The following results were interpreted by the medium lines (50th percentiles) of the charts since they are the most stable and representative of physiology. The C3 concentrations remained stable throughout the age range, but began to decrease slightly from 60 years old. The concentration of C4 increased slightly at 60 years old, with a subtle decreasing trend thereafter. There was no statistical significance at the cut-off point of 60 years old for either C3 or C4 (C3: 60 years old, z = 1.37, z∗ = 21.48; C4: 60 years old, z = 4.72, z∗ = 21.32). According to the above calculation, no analytes were partitioned by sex or age. The 2.5th percentile, 97.5th percentile, and 90% confidence intervals are presented in Table 1.
Figure 3.
The combination of continuous percentile curves and scatter plots of C3 and C4. A for C3 and B for C4. P stands for percentile. P2.5, P25, P50, P75, and P97.5 denote, respectively, the 2.5th, the 25th, the median, the 75th, and the 97.5th values of the group.
Table 1.
Establishment and verification of RIs for C3 and C4.
| Parameter | Medium | P2.5 | 90% CI |
P97.5 | 90% CI |
Rate (%) |
|||
|---|---|---|---|---|---|---|---|---|---|
| LL | UL | LL | UL | National standard | Present RIs | ||||
| C3 (g/L) | 1.17 | 0.83 | 0.83 | 0.84 | 1.58 | 1.58 | 1.59 | 89 | 97 |
| C4 (g/L) | 0.25 | 0.15 | 0.15 | 0.15 | 0.40 | 0.39 | 0.40 | 100 | 97 |
Abbreviations: LL, lower limits; UL, upper limits; Rate%, percentage of individuals who passed the validation exercise. P2.5 and P97.5 are the 2.5th and the 97.5th percentiles, respectively.
Comparison and verification of RIs
The RIs for the Chinese national standards (C3, 0.7–1.4 g/L; C4, 0.1–0.4 g/L) were determined without age or sex partitioning, as in this study. The national standards for C3 were wider than those in this study, while the present RIs for C4 were similar to the national standards. The differences between this study and other studies are shown in Table 2.
Table 2.
Comparison with previous studies.
| Countries | Equipment | Age (y) | C3 (g/L) |
C4 (g/L) |
||
|---|---|---|---|---|---|---|
| Males | Females | Males | Females | |||
| Present studya | Deling BN-II specific protein analyser | 0.83–1.58 | 0.15–0.40 | |||
| Francea | Optilite® turbidimetric analyser | 0.89–1.87 | 0.15–0.45 | |||
| Russiaa | Beckman AU5800 | 0.78–1.34 | 0.15–0.45 | |||
| SE Africa | UniCel DxC (BC) | 0.82–1.57 | 0.80–1.48 | 0.14–0.41 | 0.14–0.40 | |
| Japan | UniCel DxC (BC) | 0.74–1.35 | 0.71–1.26 | 0.12–0.31 | 0.11–0.30 | |
| Indiaa | Beckman Coulter Immage 800 | 0.85–1.82 | 0.16–0.55 | |||
| Chinese multicentre studya | Beckman AU5800 | <45 y | 0.76–1.27 | 0.15–0.45 | ||
| ≥45 y | 0.81–1.34 | 0.17–0.51 | ||||
Abbreviations: y, years old
One range indicates no sex difference in this group, while the RIs for males and females are the same.
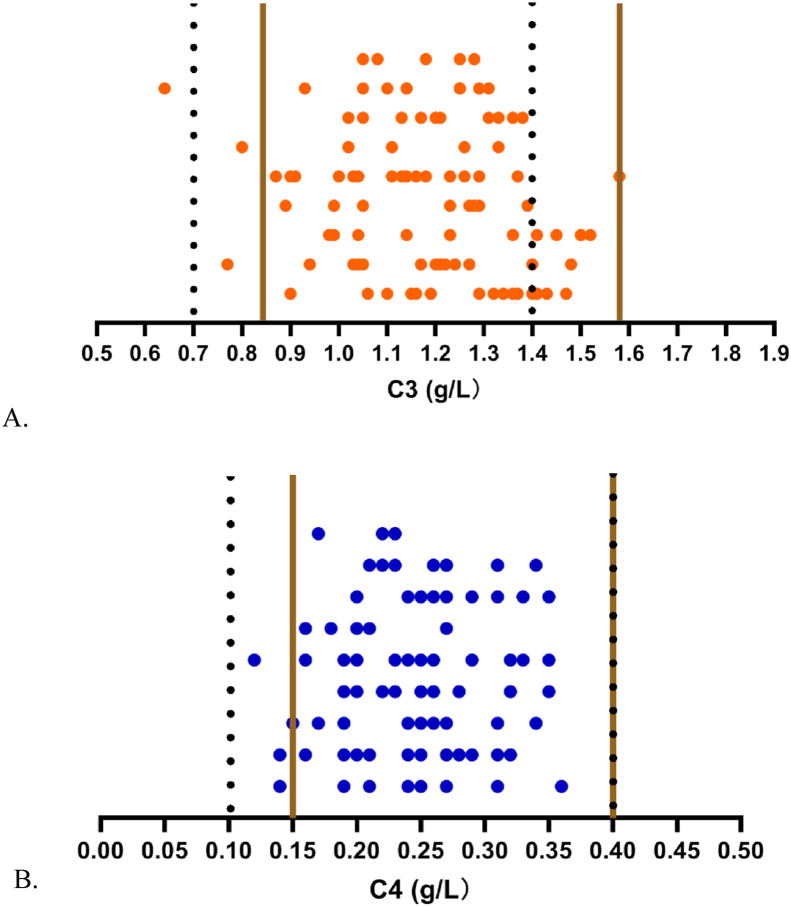
The results of the applicable verification of RIs are shown in Table 1. The RIs in this study were considered valid, although more than 5% of the values fell outside the Chinese national standards, as shown in more detail in Figure 4.
Figure 4.
Validation of RIs for C3 and C4 by the indirect method. The solid straight lines indicate the RIs by the indirect method, with the lower limit on the left and the upper limit on the right; the dotted straight lines indicate the manufacturer's RIs, with the lower limit on the left and the upper limit industry standard on the right. A for C3 and B for C4.
Discussion
To the best of our knowledge, this was one of the few RI studies on C3 and C4 that used data from the LIS and the indirect method. No age or sex partitions were required for C3 and C4; thus, the RIs were calculated from pooled data. The validation of the accuracy of the RIs by randomly selecting 100 subjects represented an added value over previous reports on C3 and C4 RIs.
In this study, the individuals were selected from the LIS's health examination centre to avoid patients with major diseases. These people had an extremely low probability of illness, and were more reliable than past hospital patients. After all, avoiding extreme values due to disease was an essential prerequisite for establishing the RIs. Noteworthily, the C3 and C4 samples for further analysis were different; indeed, this phenomenon was also found in previous studies.22,23 In this study, the reason was that the individuals whose data exceeded twice the URL of the Chinese national standards were excluded to bring the skewness and kurtosis of the overall data distribution within a reasonable range. This procedure could also be considered a method to avoid extreme values. The distribution of the C3 data was better than that of the C4 data, which led to more excluded data for C4.
The concentrations of C3 and C4 were influenced by sample type, instruments, and race. A subtle difference in the RIs for C3 and C4 was found between this study, which used serum samples, and a previous study that used plasma samples (C3: 0.85–1.80 g/L; C4: 0.13–0.39 g/L).24 This phenomenon may be due to the difference in the composition of plasma and serum.
Both the LRL and URL for C3 in this study were higher than those of Russian and Asian countries.25, 26, 27 Noteworthily, the opposite result was obtained in France's study on the Optilite® turbidimetric analyser28 and India's study on the Beckman Coulter Immage 800 autoanalyser.22 In this study, the RIs for C4 were similar to those of the Chinese national standards, European countries, and Southeast Asia. Both the LRL and URL for C4 in this study were lower than those in India's study but higher than those in Japan's study. The differences may be due to the statistical methods and instruments used and the variety of immune functions affected by genes and race. The parametric method was used to calculate the RIs, while the latent abnormal-value exclusion method was used to eliminate the potential outliers for the second time in some of the above studies, which narrowed down the RIs. Several studies have shown that the RIs established by the parametric method are narrower than those established by the nonparametric method.22,29 Furthermore, different compositions and performances were found in the instruments, which led to various test results. The difference in the RIs for C3, compared with other studies, was more significant than that for C4; this phenomenon has occurred in other studies. The reason may be the more complex mechanism of action for C3 in the body's immune system and the more possibilities of being affected by various factors.
Some literature, including official documents, indicates that the RIs for C3 and C4 are not partitioned by sex and age.22,26,28 Nevertheless, some studies have pointed out that different trends are shown among males and females or in a certain age group.23,27 The physiological trend for C3 and C4, which showed no sex difference in this study, was consistent with all the compared studies except those for Southeast Asia and Japan. First, this may be due to a discrepancy in physical conditions and the ratio of males to females. In addition, various statistical methods can also lead to this phenomenon. Furthermore, the RIs for C3 and C4 in this study were not partitioned by age, as with those in the Chinese national standards and previous studies, but different from those in the multicentre study in China.23 The multicentre study suggested that RIs should be partitioned for subjects who are 45 years old. Although this was true for 60-year-olds in the current study, the z test result did not show a significant difference at this age. This may be because the sample was selected in various regions. In addition, even if the concentrations of some analytes in females fluctuated at middle age due to menopause or oestrogen treatment, this fluctuation might not be significant enough to show a noticeable age difference in analyte concentrations in this region.
The RIs for C3 and C4 in this study all passed the verification test; thus, the accuracy of the RIs established by the indirect method in this study was confirmed. Surprisingly, the applicability of the C3 national standard for the local population was slightly low, which may be because the concentrations of C3 were influenced by various physical conditions between the local and national populations. This phenomenon may also verify the practical clinical significance of this study for establishing regional RIs.
The strengths of this study were the indirect sampling technique and special statistical methods for the establishment of the RIs. Although the direct sampling technique is deemed an a priori approach, it is particularly helpful in drawing on resources from modern laboratory databases when the establishment of RIs faces practical constraints or difficulties.16 In addition, the LMS method and z test were used to determine the cut-off point for age. In recent years, some scholars have proposed applying the LMS method to the establishment of RIs and age partition.19,30 More intuitive judgment could be provided to partition the age point by the LMS method, while the smooth curves could reflect the age change of analytes more minutely.
The study was not sufficiently stringent in screening the physical conditions of healthy individuals; thus, some non-healthy individuals might have been included. In addition, the age cut-off point was observed by the visual method of LMS curves, and therefore the result might inevitably have been affected by subjective judgment. Furthermore, there was no unified standard for the indirect method published officially to establish RIs; thus, different statistical methods and sample sizes could lead to different results.
Conclusions
In conclusion, this study established the local RIs for C3 and C4, which exhibited no significant differences in sex and age with the indirect method. In addition, verification was performed and obtained a satisfactory result, thus confirming the accuracy of the established RIs. Certainly, the RIs in this study may reflect the fluctuation in C3 and C4 in the local population, and improve the efficiency of clinical diagnosis, whether in immune system diseases or diseases of other areas. It is uncommon to establish the RIs of the complement system using the indirect method, with data mining in the local area or the world. Therefore, this study has provided a new pattern for the establishment of RIs for immune indicators.
Source of funding
This work was supported by grants from the First Hospital Translational Funding for Scientific & Technological Achievements (no. CGZHYD202012-005 to Dr. Jiancheng Xu).
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical approval
This study (number: 2019-249) was a retrospective study that was approved by the ethics committee of the First Hospital of Jilin University on May 18, 2019, and was performed in conformity with the Declaration of Helsinki.
Authors contributions
JTC conceived and designed the study, conducted research, provided research materials, wrote the initial and final drafts of the article, and provided logistical support. ZYS and DYX collected, organised, and analysed the data. QZ and JCX guided the writing process for the thesis. All authors have critically reviewed and approved the final draft and are responsible for the content and similarity index of the manuscript.
Acknowledgment
Acknowledgement is extended to all the participants and their families.
Footnotes
Peer review under responsibility of Taibah University.
References
- 1.Copenhaver M.M., Yu C.-Y., Zhou D., Hoffman R.P. Relationships of complement components C3 and C4 and their genetics to cardiometabolic risk in healthy, non-Hispanic white adolescents. Pediatr Res. 2020;87:88–94. doi: 10.1038/s41390-019-0534-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sinkovits G., Mező B., Réti M., Müller V., Iványi Z., Gál J., Gopcsa L., Reményi P., Szathmáry B., Lakatos B., Szlávik J., Bobek I., Prohászka Z.Z., Förhécz Z., Csuka D., Hurler L., Kajdácsi E., Cervenak L., Kiszel P., Masszi T., Vályi-Nagy I., Prohászka Z. Complement overactivation and consumption predicts in-hospital mortality in SARS-CoV-2 infection. Front Immunol. 2021;12:663187. doi: 10.3389/fimmu.2021.663187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Risitano A.M., Mastellos D.C., Huber-Lang M., Yancopoulou D., Garlanda C., Ciceri F., Lambris J.D. Complement as a target in COVID-19? Nat Rev Immunol. 2020;20:343–344. doi: 10.1038/s41577-020-0320-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gralinski L.E., Sheahan T.P., Morrison T.E., Menachery V.D., Jensen K., Leist S.R., Whitmore A., Heise M.T., Baric R.S. Complement activation contributes to severe acute respiratory syndrome coronavirus pathogenesis. mBio. 2018;9 doi: 10.1128/mBio.01753-18. e01753-18,/mbio/9/5/mBio.01753-18.atom . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song W.-C., Rosa Sarrias M., Lambris J.D. Complement and innate immunity. Immunopharmacology. 2000;49:187–198. doi: 10.1016/S0162-3109(00)80303-3. [DOI] [PubMed] [Google Scholar]
- 6.P.-J. Haas, J. van Str, Their role in bacterial infection and inflammation, (n.d.) vol. 15. [DOI] [PubMed]
- 7.Cheng D., Li X., Zhao S., Hao Y. Establishment of thromboelastography reference intervals by indirect method and relevant factor analyses. J Clin Lab Anal. 2020;34 doi: 10.1002/jcla.23224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Solberg H.E. Approved recommendation (1986) on the theory of reference values. Part 1. The concept of reference values. Clin Chim Acta. 1987;165:111–118. doi: 10.1016/0009-8981(87)90224-5. [DOI] [PubMed] [Google Scholar]
- 9.Solberg H.E. Approved recommendation (1987) on the theory of reference values. Part 5. Statistical treatment of collected reference values. Determination of reference limits. Clin Chim Acta. 1987;170:S13–S32. doi: 10.1016/0009-8981(87)90151-3. [DOI] [Google Scholar]
- 10.Solberg H.E., Stamm D. IFCC recommendation — theory of reference values. Part 4. Control of analytical variation in the production, transfer and application of reference values. Clin Chim Acta. 1991;202:S5–S11. doi: 10.1016/0009-8981(91)90266-F. [DOI] [PubMed] [Google Scholar]
- 11.Ozarda Y. Reference intervals: current status, recent developments and future considerations. Biochem Med. 2016:5–16. doi: 10.11613/BM.2016.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Q., Guo L., Lu Z., Gu J. Reference intervals established using indirect method for serum ferritin assayed on Abbott Architect i 2000 SR analyzer in Chinese adults. J Clin Lab Anal. 2020;34 doi: 10.1002/jcla.23083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Defining, Establishing, and Verifying Reference Intervals in the Clinical Laboratory;, CLSI; 2008. P. C28-A3. Approved guidline-Third Edition (n.d.).
- 14.Katayev A., Balciza C., Seccombe D.W. Establishing reference intervals for clinical laboratory test results: is there a better way? Am J Clin Pathol. 2010;133:180–186. doi: 10.1309/AJCPN5BMTSF1CDYP. [DOI] [PubMed] [Google Scholar]
- 15.Delgado J.A., Bauça J.M., Pastor M.I., Barceló A. Use of data mining in the establishment of age-adjusted reference intervals for parathyroid hormone. Clin Chim Acta. 2020;508:217–220. doi: 10.1016/j.cca.2020.05.030. [DOI] [PubMed] [Google Scholar]
- 16.Indirect Reference Intervals Harnessing the power of stored laboratory data. Cell Biol Rev CBR. 2019;40:99–111. doi: 10.33176/AACB-19-00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kardar G., Oraei M., Shahsavani M., Namdar Z., Kazemisefat G., Ashtiani M.H., Shams S., Pourpak Z., Moin M. Reference intervals for serum immunoglobulins IgG, IgA, IgM and complements C3 and C4 in. Iran Healthy Child. 2012;41:5. [PMC free article] [PubMed] [Google Scholar]
- 18.Inal T.C., Serteser M., Coşkun A., Özpinar A., Ünsal I. Indirect reference intervals estimated from hospitalized population for thyrotropin and free thyroxine. Croat Med J. 2010;51:124–130. doi: 10.3325/cmj.2010.51.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang Q., Lew H.Y., Peh R.H.H., Metz M.P., Loh T.P. An automated and objective method for age partitioning of reference intervals based on continuous centile curves. Pathology. 2016;48:581–585. doi: 10.1016/j.pathol.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 20.Cole T.J., Green P.J. Smoothing reference centile curves: the lms method and penalized likelihood. Stat Med. 1992;11:1305–1319. doi: 10.1002/sim.4780111005. [DOI] [PubMed] [Google Scholar]
- 21.Zhu X., Wang K., Zhou Q., Guo W., Jia Y., Xu J. Age- and sex-specific pediatric reference intervals of serum electrolytes in Jilin province of China using the A priori approach. Am J Clin Pathol. 2020;154:708–720. doi: 10.1093/ajcp/aqaa093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shah S.A.V., Ichihara K., Dherai A.J., Ashavaid T.F. Reference intervals for 33 biochemical analytes in healthy Indian population: C-RIDL IFCC initiative. Clin Chem Lab Med CCLM. 2018;56:2093–2103. doi: 10.1515/cclm-2018-0152. [DOI] [PubMed] [Google Scholar]
- 23.Qin X., Tang G., Qiu L., Li P.C., Xia L., Chen M., Tao Z., Li S., Liu M., Wang L., Gao S., Yu S., Cheng X., Han J., Hou L., Kawano R., Ichihara K. A multicenter reference intervals study for specific proteins in China. Medicine. 2015;94 doi: 10.1097/MD.0000000000002211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nespola B., Comitogianni H., Jahn I., Goetz J. Evaluation of the Optilite® analyser for determination of total complement activity and C3 and C4 fractions. Ann Biol Clin. 2019;77:447–452. doi: 10.1684/abc.2019.1453. [DOI] [PubMed] [Google Scholar]
- 25.Jones G.R.D., Haeckel R., Loh T.P., Sikaris K., Streichert T., Katayev A., Barth J.H., Ozarda Y. Indirect methods for reference interval determination – review and recommendations. Clin Chem Lab Med CCLM. 2018;57:20–29. doi: 10.1515/cclm-2018-0073. [DOI] [PubMed] [Google Scholar]
- 26.Evgina S., Ichihara K., Ruzhanskaya A., Skibo I., Vybornova N., Vasiliev A., Kimura S., Butlitski D., Volkova E., Vilenskaya E., Emanuel V. Establishing reference intervals for major biochemical analytes for the Russian population: a research conducted as a part of the IFCC global study on reference values. Clin Biochem. 2020;81:47–58. doi: 10.1016/j.clinbiochem.2020.04.001. [DOI] [PubMed] [Google Scholar]
- 27.Ichihara K., Ceriotti F., Tam T.H., Sueyoshi S., Poon P.M.K., Thong M.L., Higashiuesato Y., Wang X., Kataoka H., Matsubara A., Shiesh S.-C., Muliaty D., Kim J.-H., Watanabe M., Lam C.W.K., Siekmann L., Lopez J.B., Panteghini M. The Asian project for collaborative derivation of reference intervals: (1) strategy and major results of standardized analytes. Clin Chem Lab Med. 2013:51. doi: 10.1515/cclm-2012-0421. [DOI] [PubMed] [Google Scholar]
- 28.Majerus V., Dubucquoi S., Labalette M., Lefèvre G., Launay D., Rogeau S., Deleplancque A.-S., Moitrot E., Maanaoui M., Joudinaud R., Ledoult E., Bertier N., Lopez B. Classical pathway activity C3c, C4 and C1-inhibitor protein reference intervals determination in EDTA plasma. Biochem Med (Online) 2019;29:559–569. doi: 10.11613/BM.2019.030707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Omuse G., Ichihara K., Maina D., Hoffman M., Kagotho E., Kanyua A., Mwangi J., Wambua C., Amayo A., Ojwang P., Premji Z., Erasmus R. Determination of reference intervals for common chemistry and immunoassay tests for Kenyan adults based on an internationally harmonized protocol and up-to-date statistical methods. PLoS One. 2020;15 doi: 10.1371/journal.pone.0235234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loh T.P., Antoniou G., Baghurst P., Metz M.P. Development of paediatric biochemistry centile charts as a complement to laboratory reference intervals. Pathology. 2014;46:336–343. doi: 10.1097/PAT.0000000000000118. [DOI] [PubMed] [Google Scholar]