Abstract
Background
Ferroptosis, as a new form of cell death, is different from other cell deaths such as autophagy or senescence. Ferroptosis involves in the pathophysiological progress of several diseases, including cancers, cardiovascular diseases, nervous system diseases, and kidney damage. Since oxidative stress and iron deposition are the broad pathological features of neurological diseases, the role of ferroptosis in neurological diseases has been widely explored.
Scope of review
Ferroptosis is mainly characterized by changes in iron homeostasis, iron-dependent lipid peroxidation, and glutamate toxicity accumulation, of which can be specifically reversed by ferroptosis inducers or inhibitors. The ferroptosis is mainly regulated by the metabolism of iron, lipids and amino acids through System Xc−, voltage-dependent anion channels, p53, p62-Keap1-Nrf2, mevalonate and other pathways. This review also focus on the regulatory pathways of ferroptosis and its research progress in neurological diseases.
Major conclusions
The current researches of ferroptosis in neurological diseases mostly focus on the key pathways of ferroptosis. At the same time, ferroptosis was found playing a bidirectional regulation role in neurological diseases. Therefore, the specific regulatory mechanisms of ferroptosis in neurological diseases still need to be further explored to provide new perspectives for the application of ferroptosis in the treatment of neurological diseases.
Keywords: Ferroptosis, Oxidative stress, Iron homeostasis, Neurological diseases, Cell death
1. Introduction
As one of the most important essential trace elements in the human body, iron is widely involved in a variety of metabolic processes. Ferroptosis was first proposed by Dixon et al. [1] as a unique iron-dependent form of cell death triggered by the erastin, which could not be blocked by inhibitors of apoptosis, autophagy, and other cell deaths [2]. However, it could be inhibited by specific inhibitors and lipophilic antioxidants, such as ferritin and iron chelator [3]. Ferroptosis is mainly caused by the imbalance between the production and degradation of lipid reactive oxygen species (ROS). In the process of iron-containing mitochondrial oxyphosphorylation, the oxidative stress caused by exceeded ROS will directly or indirectly damage macromolecular substances [4]. In morphology, ferroptosis is mainly manifested as cell membrane breakage and blistering, mitochondrial volume reduction, increased membrane density, decreased or even disappeared mitochondria cristae, other than the morphological characteristics of apoptosis, such as cell shrinkage, chromatin condensation, skeleton disintegration and formation of apoptosis body [5]. Ferroptosis is not sensitive to the inhibitors of apoptosis, pyrolysis, and autophagy, but is sensitive against the iron chelators and antioxidants. As the central link of ferroptosis, the accumulation of iron and ROS could reduce the uptake of cystine, consume glutathione (GSH) and release arachidonic acid to inhibit the activity of cystine/glutamate antiporter system (System Xc−) and glutathione peroxidase 4 (GPX4) [3]. Besides, a variety of abnormal expression of genes could be observed, especially those related to iron metabolism, such as transferrin receptor (TFRC), divalent metal transporter 1 (divalent metal transporter 1, DMT1), ferritin heavy chain 1 (FTH1), nuclear receptor coactivator 4 (NCOA4), etc. [6]. This manuscript intends to systematically review the regulation and related pathways of ferroptosis, and mainly focus on its progress in neurological diseases, with the perspective of providing new insight of ferroptosis in neurological diseases.
2. Main characteristics of ferroptosis
2.1. Changes in iron homeostasis
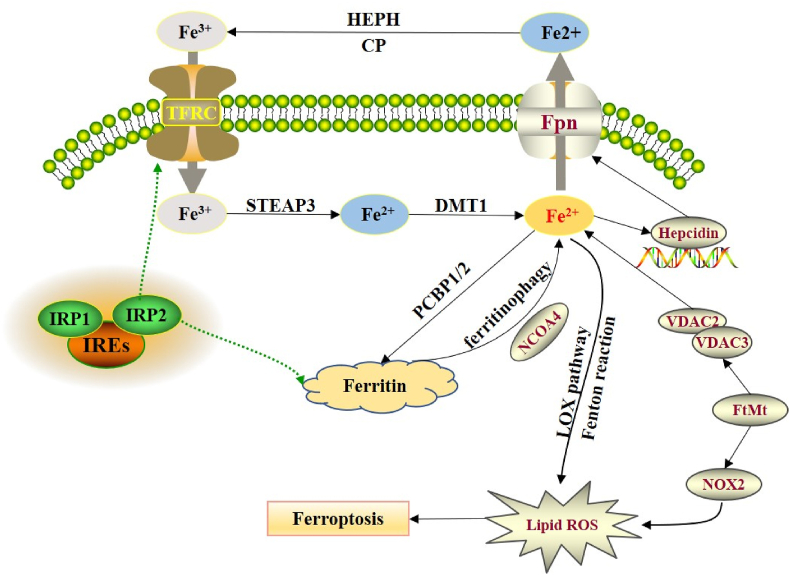
The iron homeostasis has been proven as a key factor of ferroptosis [7,8]. Excess Fe2+ will lead to the overproduction of ROS through the fenton reaction [9]. The iron bind to the transferrin in the form of Fe3+ to transfer into the cell through TFRC [10], which will be reduced to Fe2+ by the STEAP3 in endosomes (six-transmembrane epithelial antigen of prostate 3) [11]. The reduced Fe2+ will react with ferritins rich in light or heavy chain [12,13], which will be transferred out of the cell by ferroportin (Fpn) [14]. In the duodenum, the basolateral ferrous oxidase (hephaestin, HEPH) or ceruloplasmin (CP) can act synergistically with transferrin to transfer Fe2+ out of the cell and transform the Fe2+ to Fe3+ to form the circulation [15]. Besides, The ApoTf and TFRC will be recycled back to the cell membrane to re-recognize the free iron and transferrin complexes [16,17].
At the transcription level, the expression level of hepcidin is closely related to the iron content, erythropoietin and cell environment. The binding of hepcidin to Fpn after translation will cause the degradation of Fpn to inhibit the output of iron [18]. In addition to transcriptional regulation, iron homeostasis can also be regulated at the translation level. Iron regulatory protein 2 (IRP2), an RNA binding protein, controls the translation of a group of mRNAs involved in iron homeostasis. In the untranslated regions (UTRs) of genes encoding a variety of iron regulatory molecules (including DMT1 and TFR1), the IRP1 and IRP2 bind to the iron response elements (IREs). In an iron-deficient state, the combination of IRP2 and IREs can maximize intracellular iron levels. When the iron content increases, the extracellular iron regulatory pathway (IRE/IRP system) will be activated to weaken the iron overload state [[19], [20], [21]]. Cooperman et al. [22]found that IRP2 expression could reduce iron absorption by reducing TFR expression, and increase free iron storage by inducing ferritin expression (Figure 1).
Figure 1.
Regulation of iron homeostasis in ferroptosis. HEPH and CP can transform the Fe2+ to Fe3+, which will be transferred into the cell through TFRC. The cellular Fe3+ will be reduced to Fe2+ by the STEAP3, which was then transported to the cytoplasm through DMT1. Excess Fe2+ generates ROS through the fenton reaction in cell, which could also be transferred out to the cell through Fpn. The binding of hepcidin to Fpn after translation will cause the degradation of Fpn to inhibit the output of iron. The combination of IRP1/2 and IREs could reduce TFRC expression to induce ferritin expression. PCBP1/2 are responsible for the transportation of iron to ferritin, NCOA4 could mediate autophagy of ferritin. FtMt could regulate the content of Fe2+ and ROS through VDAC2/3 and NOX2.
ZRT/IRT-like proteins (ZIP) 8/14 is essential for prion protein to reduce non-transferrin-bound iron and mediate the uptake of non-transferrin-bound iron [23,24]. In acidic endosomes, the STEAP family reductase can convert Fe3+ (released by transferrin) to Fe2+, which will be transported to the cytoplasm through DMT1. On the contrary, extracellular iron can be converted into Fe2+ through STEAP family reductase and enter the cell directly through ZIP14 transporter, which leads to the instability of the iron pool [25]. Poly-(rC)-binding protein (PCBP) and nuclear receptor co-activator 4 (NCOA4) are also responsible for the release of ferritin-bound iron. PCBP1 and PCBP2, as cytoplasmic iron partners, are responsible for the transportation of iron to ferritin for metabolism, storage, and transportation [26,27]. NCOA4 could mediate autophagy of ferritin, which is responsible for the delivery of ferritin to lysosomes [28,29].
Iron could be stored in mitochondria by ferritin, and the recombinant ferritin mitochondrial (FtMt) can significantly regulate the metabolism of cellular iron [30]. The ferroptosis model of drosophila cells induced by erastin was established to reveal that the protection of FtMt in ferroptosis is mainly rely on the inhibition of voltage-dependent anion channels 2 and 3 (VDAC2/3) and NOX2. Activation of NOX2, the main NADPH oxidase isomer produced by ROS, could significantly promote pathological oxidative stress in the central nervous system [31]. Ferritinophagy refers to the autophagy degradation process of ferritin and is closely related to ferroptosis [32,33]. Salinomycin can accumulate and sequester iron in the lysosome to trigger ferritinophagy, leading to excessive accumulation of iron and ferroptosis [34]. Inhibitors of lysosomal such as Baf A1 and Pep A-Me can inhibit the ferroptosis by attenuating ferritinophagy [35,36]. NCOA4 also participates in ferroptosis by regulating ferritinophagy [37]. Knockdown of NCOA4 can inhibit ferritinophagy, which in turn inhibits ferroptosis induced by erastin [38].
2.2. Lipid peroxidation
TFR1-mediated iron absorption is offset by iron excretion by transferrin. Excess iron is stored at ferritin to prevent cytotoxicity, and only a small part of iron, as a free unstable pool, can stimulate the formation of ROS. The harmful effects of iron overload begin to appear when the complex bound with iron saturated. Iron may directly catalyze the formation of free radicals in the cytoplasm or generate ROS through fenton reaction to participate in ferroptosis [7].
Superoxide radical (O2−) is the initial active species produced in the redox reaction. The radical SOD can neutralize the O2−, then the product hydrogen peroxide can be metabolized by catalase into a non-toxic product, or combined with glutathione. Similarly, the ubiquitous thioredoxin system also plays an important role in maintaining the redox state of cells. Thioredoxin is a small protein with two adjacent cysteine residues that can undergo reversible oxidation to form disulfide bonds. The thioredoxin system can protect cells from oxidative stress with enough NADPH. However, when O2− is not neutralized, it can form more active species, such as nitric oxide (NO) and chlorine (Cl−), and cause further oxidative damage [39].
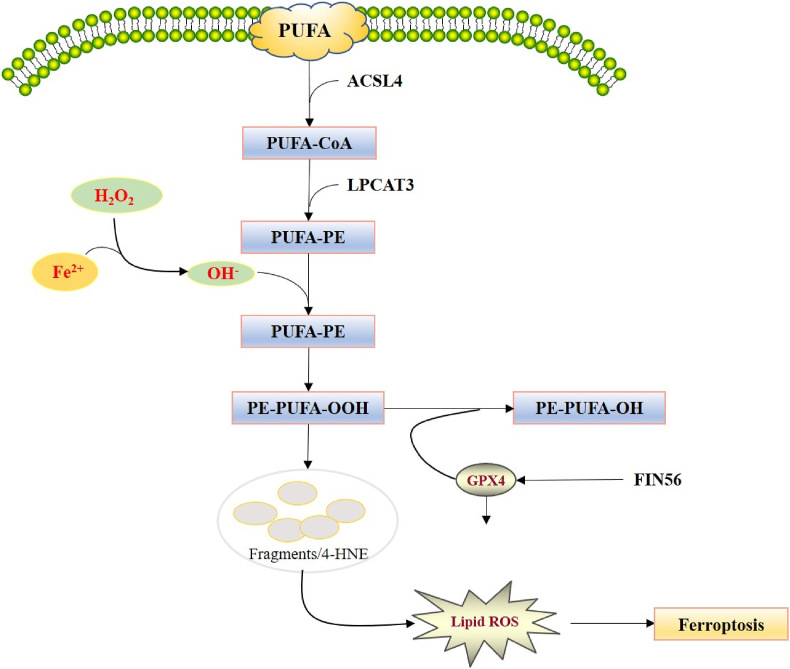
However, in the presence of Fe2+, hydrogen peroxide can generate hydroxyl radicals (OH−) through the fenton reaction, thereby promoting the oxidation of polyunsaturated fatty acid (PUFAs) on the cell membrane, resulting in the accumulation of deadly lipid peroxides and other hydroperoxides. PUFAs, as substrates of lipoxygenase, can be oxidized into lipid hydrogen peroxide, which in turn generates multiple aldehydes including 4-hydroxynonenal (4-HNE). PUFAs can be repaired by GPX4 through two GSH molecules act as electron donors to reduce lipid hydrogen peroxide to protect the membrane function (Figure 2). Oxidation of PUFA and free radical-mediated damage will eventually fragment PUFA to various products, leading to the destruction of cell membrane [[40], [41], [42]].
Figure 2.
Regulation of lipid peroxidation in ferroptosis. In the presence of Fe2+, hydrogen peroxide can generate hydroxyl radicals (OH−) through the fenton reaction, thereby promoting the oxidation of PUFAs on the cell membrane. ACSL4 incorporates PUFAs into the membrane as PUFA-CoA, and LPCAT3 inserts the acyl group into the lysophospholipid as PUFA-PE, of which will lead to the accumulation of PE-PUFA-OOH and in turn generate multiple aldehydes including 4-HNE. PE-PUFA-OOH can be repaired by GPX4 to reduce lipid hydrogen peroxide to protect the membrane function, and FIN56 can reverse the process through inhibiting GPX4.
Nevertheless, the precise mechanism through which lipid peroxidation leads to ferroptosis remains unclear. The accumulation of PL-OOH is proven as an important feature of ferroptosis, which mainly depends on the content of highly oxidized PUFAs that are esterified to phospholipids. Therefore, the molecular pathways responsible for the free diffusion of fatty acid transposase, fatty acid transporter or AA/AdA as well as activation and esterification into phospholipids will affect the sensitivity of cell membranes to phospholipid peroxidation [43,44].
Eran et al. [45] found that acyl-CoAsynthetase long-chain family member 4 (ACSL4) and lysophosphatidylcholine Acyltransferase 3 (LPCAT3) were the key mediator of fatty acid metabolism and lipid remodeling in ferroptosis. ACSL4 incorporates PUFAs into the membrane, and LPCAT3 inserts the acyl group into the lysophospholipid to promotes ferroptosis. The fragmentation of PUFA and membrane lipid damage may be sufficient to irreversibly permeate the plasma membrane. In addition, actived lipid intermediates produced by oxidation of PUFA could promote cell death by covalently modifying and inactivating basic intracellular proteins.
2.3. Accumulation of glutamate toxicity
Under oxidative conditions, the uptake of cystine through the System Xc− will be combined with the release of glutamate [46,47]. High concentrations of glutamate may cause excitotoxicity both in vitro and in vivo, which plays key role in several neurodegenerative diseases, including amyotrophic lateral sclerosis, Alzheimer's disease and Huntington's disease. The interaction of several different activating factors leads to the “glutamatergic” excitatory input on neurons, resulting in ferroptosis [48]. Therefore, intervention of glutamate metabolism relating to ferroptosis has the potential in improving the progression of neurological diseases [49,50].
3. Molecular mechanisms of ferroptosis
3.1. System Xc-/cysteine/GSH
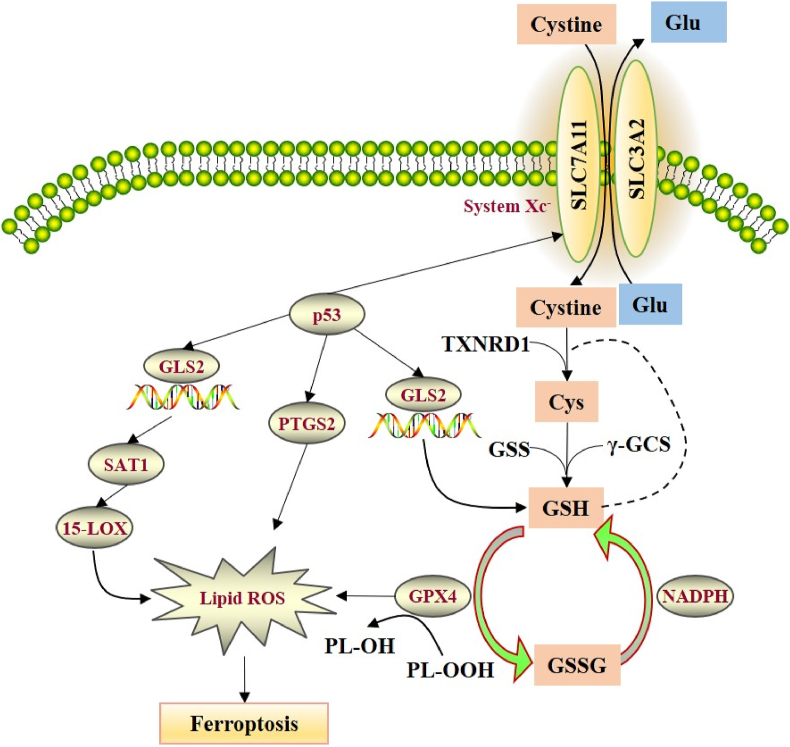
The System Xc− is a heterodimeric amino acid transporter composed of two subunits, recombinant solute carrier family 7 member 11 (SLC7A11) and recombinant solute carrier family 3 member 2 (SLC3A2). Under oxidative conditions, System Xc-extracellular uptake of cystine to release glutamate, which is considered to be the most upstream event in the ferroptosis. Once cystine enters the cell, it is reduced to cysteine by GSH or thioredoxin reductase (TXNRD1). Cysteine is mainly applied for the synthesis of GSH, which is gradually produced by cysteine synthase γ-GCS) and glutathione synthase (GSS) [51]. The GSH molecules of GPX4 are considered as electron donors to reduce phospholipid hydrogen peroxide (PL-OOH) to the corresponding alcohol. NADPH can also reduce GSSG (oxidized GSH) to GSH by glutathione reductase. GSH biosynthesis is critical to the functional activity of GPX4. GSH depletion induces GPX4 inactivation, which will increase intracellular lipid peroxidation and lead to ferroptosis (Figure 3) [52,53].
Figure 3.
The regulation mechanisms of System Xc- and p53 pathway in ferroptosis. The System Xc-is a heterodimeric amino acid transporter composed of two subunits (SLC7A11and SLC3A2). Under oxidative conditions, System Xc-extracellular uptake of cystine to release glutamate. The cellular cystine will be reduced to cysteine by GSH or TXNRD1. Cysteine is gradually produced by γ-GCS and GSS. The GSH molecules of GPX4 act as electron donor to reduce phospholipid hydrogen peroxide (PL-OOH) to the corresponding alcohol. NADPH can also reduce GSSG (oxidized GSH) to GSH by glutathione reductase. Up-regulating the p53 gene can reduce the mRNA and protein expression levels of SLC7A11. Several p53 target genes have been found with the ability of promoting cell ferroptosis, such as glutaminase 2 (GLS2), prostaglandin endoperoxide synthase 2 (PTGS2), spermidine/spermine N1-acetyltransferase 1 (SAT1) and so on. GLS2 can catalyze the hydrolysis of glutamine to glutamate and reduce the antioxidant capacity by reducing GSH levels to increase the sensitivity against ferroptosis. Activated p53 can directly bind to the GLS2 promoter region to promote the transcription of GLS2. The increase of PTGS2 expression is dependent on p53. SAT1 can promote the expression of arachidonic acid 15-LOX, resulting in lipid ROS.
3.2. VDACs
VDACs contains 18 exons and 17 introns, including multiple subtypes such as VDAC1, VDAC2, and VDAC3. VDACs are a kind of porins widely present in eukaryotes, mainly located in the outer mitochondrial membrane, and participate in the transmission of substances and energy inside and outside the mitochondria and the process of programmed cell death mediated by mitochondria [54]. Yagoda et al. [55] found that VDAC2 and VDAC3 were the targets of ferroptosis inducer erastin on the mitochondria. Knockout of VDAC2/3 could lead to the decrease of the sensitivity of tumor cells against erastin. The interaction of erastin with VDACs can cause mitochondrial dysfunction and increase the production of ROS, leading to the ferroptosis.
3.3. p53 pathway
p53 is an important anti-cancer gene, which slows down or monitors cell division under normal conditions. Wild-type p53 can repair gene defects and induce apoptosis to exert the tumor suppressor effect. Deletion of p53 gene involves in the occurrence of several tumors. The mutant p53 loses its regulatory effect on cell growth, apoptosis and DNA repair due to changes in its spatial conformation, and the p53 gene will be transformed from a tumor suppressor gene to an oncogene. Jiang et al. [56,57] first linked p53 to ferroptosis. They found that up-regulating the p53 gene in tumor cells can significantly reduce the mRNA and protein expression levels of SLC7A11, confirming SLC7A11 as the target of p53. They further constructed acetylation-deficient p533KR mutant cells, which lack the classic functions of inducing cell cycle arrest and apoptosis, but retain the ability of p53 to inhibit SLC7A11 transcription. When the ferroptosis inducer erastin was used to treat p533KR mutant and p53-deficient cells, it was found that the p533KR mutant cell death rate was greater than 90%, while the p53-deficient cell death rate was less than 10%. When SLC7A11 was overexpressed in p533KR mutant cells, it could significantly inhibit erastin-induced cell death. All above indicated that p53 can inhibit the expression of SCL7A11 through transcription to inhibit the uptake of cystine of System Xc−, resulting in the decrease of the synthesis of GSH and the activity of GSH-dependent GPX4, as well as the reduction of cellular antioxidant capacity and accumulation of lipid peroxides.
In addition to SLC7A11, several p53 target genes have been found with the ability of promoting cell ferroptosis, such asGLS2, PTGS2, SAT1and so on. GLS2 can catalyze the hydrolysis of glutamine to glutamate and reduce the antioxidant capacity by reducing GSH levels to increase the sensitivity against ferroptosis. Activated p53 can directly bind to the GLS2 promoter region to promote the transcription of GLS2 [58]. PTGS2 is a key enzyme in the biosynthesis of prostaglandins, which increases the sensitivity of cells against ferroptosis by regulating membrane phospholipids. Yang et al. [59] discovered that the expression of PTGS2 was up-regulated during ferroptosis, and the increase of PTGS2 expression was dependent on p53. SAT1, a direct target gene of p53, can promote the expression of arachidonic acid 15-LOX, which is an iron-dependent polyunsaturated fatty acid oxidase that can catalyze lipid peroxidation. The transcriptional expression of SAT1 induced by p53 will lead to the increase of the production of lipid peroxides, thereby enhancing the sensitivity against ferroptosis (Figure 3) [60]. The activation of the p53-p21 pathway could also inhibit ferroptosis by increasing the expression of GSH and inhibiting the accumulation of lipid ROS [61].
Conversely, other studies have shown that p53 can reduce the sensitivity of cells to ferroptosis. p53 cannot persist in the resting state and will be ubiquitinated and degraded by the ubiquitin ligase MDM2. Pretreatment of tumor cells with nutlin3, a small molecule inhibitor of MDM2, can delay the process of cell ferroptosis by stable p53 at the resting state [61]. In addition, p53 can also delay the occurrence of cell ferroptosis by regulating the transcription level of p21 [62].
3.4. p62-Keap1-Nrf2
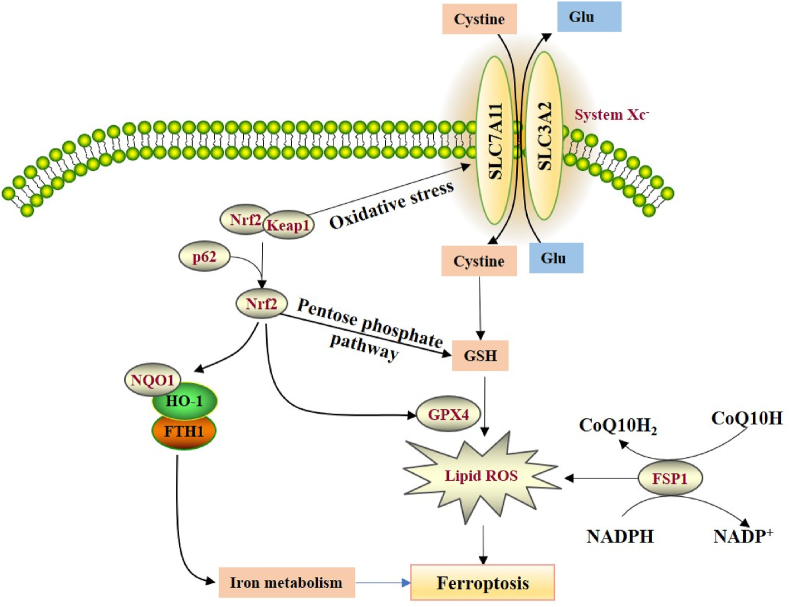
Nuclear factor-erythroid 2-related factor 2 (Nrf2), a key regulator of the antioxidant response, will be ubiquitination degraded in the proteasome to maintain the inactive state by binding to Keap1. Under oxidative stress conditions, Nrf2 will be released from the conjugate with Keap1 and translocate to the nucleus. The Nrf2 heterodimer binds to the small Maf nucleoprotein and the antioxidant response element to activate cytoprotective genes to resist toxic damage. When exposed to erastin, p62 protein competitively binds to Keap1, and Nrf2 will be released to enhance the subsequent increase of Nrf2 into the nucleus, thereby activating quinone oxidoreductase 1 (NQO1), hemo oxygenase-1 (HO-1) and ferritin heavy chain 1 (FTH1) to participate in iron metabolism and lipid peroxidation [63,64]. Knocking out p62, NQO1, HO-1, and FTH1 could promote the occurrence of ferroptosis in liver cancer [63], which demonstrated that the p62-Keap1-Nrf2 antioxidant signaling pathway is involved in the regulation of ferroptosis in liver cancer. Interestingly, other studies have shown that the Nrf2/keap1 pathway can promote the expression of System Xc− under oxidative stress, and SLC7A11 serves as the transcription target of Nrf2, which indicated that the SLC7A11 gene may be involved in the protection of Nrf2-mediated ferroptosis (Figure 4) [65]. SLC7A11 knockout mice was also established to prove that the expression of SLC7A11 can be regulated through ROS-Nrf2-ARE pathway [66].
Figure 4.
The regulation mechanisms of p62-Keap1-Nrf2 and FSP1 in ferroptosis. During ferroptosis, p62 protein competitively binds to Keap1, and Nrf2 will be released to enhance the subsequent increase of Nrf2 into the nucleus, thereby activating NQO1, HO-1 and FTH1 to participate in iron metabolism and lipid peroxidation. The Nrf2/keap1 pathway can promote the expression of System Xc-under oxidative stress, and SLC7A11 serves as the transcription target of Nrf2. Nrf2 can also inhibit ferroptosis through GPX4 and participate in the pentose phosphate pathway to produce NADPH. FSP1 can inhibit lipid oxidation and ferroptosis through its CoQ oxidoreductase activity.
Nrf2 acts as a key switch for cells to adapt and survive under oxidative stress. In folic acid-induced acute kidney injury mice, Nrf2 exerted its renal protective effect by inhibiting ferroptosis through regulating the downstream gene GPX4. The up-regulation of GPX4 in Nrf2+/+ animals could cope with oxidative stress [67]. Nrf2 also participate in the pentose phosphate pathway to produce NADPH, which in turn affect the function of GSH and ferroptosis [68].
3.5. Mevalonate pathway
GPX4 has a selenocysteine activation site (Sec). Selenium can regulate ferroptosis by regulating the abundance and activity of GPX4. Inhibitors of the mevalonate pathway, such as statins, have been proven to interfere with the biosynthesis of Sec and GPX4 to inhibit ferroptosis, which indicated that the mevalonate pathway is involved in the regulation of ferroptosis [69]. FIN56 is a new type of ferroptosis-inducing factor, which could mediate ferroptosis through the mevalonate pathway. FIN56 reduces the enrichment of GPX4 and activates the downstream target protein squalene synthase (SQS) to induce ferroptosis. SQS is a squalene molecule connected by two famesyl pyrophosphate (FPP) molecules, while FPPs can reverse the cytotoxicity induced by FIN56 [69,70].
3.6. The transsulfuration pathway
In addition to the extracellular uptake of cysteine, methionine can also act as a sulfur donor in mammalian cells to synthesize nascent cysteine from the homocysteine and cystathionine through the transsulfuration pathway. While the uptake mechanism is inhibited, the transsulfuration pathway is critical to cell survival. The transsulfuration pathway has been proven involving in ferroptosis. The loss of cysteinyl-tRNA synthetase (CARS) activates the transsulfuration pathway to induce the accumulation of cystathionine and upregulation of genes related to serine biosynthesis and transsulfurization, thereby inhibiting ferroptosis caused by cystine deprivation [71]. Telorack M et al. [72] further confirmed that the thioredoxin reductase system is involved in the antioxidant effect of ferroptosis.
3.7. FSP1
FSP1, formerly known as apoptosis-inducing factor mitochondrial-associated protein 2 (AIF-M2), is a pro-apoptotic gene. Bersuker et al. [73] performed genome-wide screening through CRISPR/Cas9 and found that when the FSP1 protein was knocked out, the ferroptosis induced by the GPX4 inhibitor RSL3 was greatly increased, proving that FSP1 can resist the occurrence of cell ferroptosis. In addition, by measuring FSP1 gene expression and drug resistance in more than 800 tumor cell lines, the authors found that the higher the FSP1 expression, the stronger the ferroptosis resistance existed. Doll et al. [74] found that GPX4 and FSP1 proteins overexpressed in ferroptosis-resistant cells, supplementing FSP1 after GPX4 deficiency can significantly inhibit the ferroptosis, which confirmed that FSP1 is a brand new ferroptosis inhibitor. FSP1, a NADH-dependent CoQ oxidoreductase, can convert CoQ into lipophilic antioxidants RTA and idebenone. Further mechanism study revealed that FSP1 inhibited lipid oxidation and ferroptosis through its CoQ oxidoreductase activity (Figure 4). Therefore, FSP1 is a glutathione-independent ferroptosis inhibitor, which participates in the inhibition of cell ferroptosis in a parallel manner with the GPX4 protein.
4. Ferroptosis in neurological diseases
4.1. Acute central nervous system injury
4.1.1. Ischemic stroke
Stroke includes hemorrhagic stroke and ischemic stroke, both have been found in association with ferroptosis. Tuo et al. [75] found that ferroptosis was involved in ischemia-reperfusion injury. The 3-month-old tau knockout mice could resist ischemia-reperfusion injury since no iron deposition in the brain. The protective effects will be lost at the older mice due to the increased iron deposition. The ferroptosis inhibitors liproxstatin-1 and Fer-1 could recover the protective effects at the tau knockout mice, which indicated that tau-mediated iron transfer can inhibit the role of ferroptosis in acute cerebral infarction. Iron deposition occurs in the basal ganglia, thalamus, periventricular, and subcortical white matter areas during the brain injury caused by severe ischemia [76]. In mouse models of ischemic stroke, the GSH content and GPX4 activity decreased in the neuronal cell, and lipid peroxide production increased [77], indicating that ferroptosis is the main form of neuronal death after ischemic stroke.
Subsequently, the regulatory mechanism related to ferroptosis in ischemic stroke was further revealed. Lu et al. [78] found that lncRNA PVT1 regulates ferroptosis through miR-214-mediated TFR1 and TP53 expression in acute ischemic stroke. Chen et al. [79]found that MCAO modeling reduces ferritin expression, and overexpression of ferritin can regulate p53/SLC7A11-mediated ferroptosis to improve the learning and memory function of MCAO model, and reduce tau hyperphosphorylation, neuronal death and oxidative stress. Besides, inhibiting ferroptosis has been proven as a promising therapeutic approach against ischemic stroke. The ferroptosis inhibitors liproxstatin-1 and Fer-1 could prevent ischemia–reperfusion injury of mice [75]. Compounds disrupting the NCOA4-FTH1 interaction blocked ferroptosis to ameliorates the ischemic-refusion injury [80]. Compound 2-(1-(4-(4-methylpiperazin-1-yl)phenyl)ethyl)-10H-phenothiazine (51) could prevent ferroptosis of the erastin-induced HT1080 cell and the MCAO ischemic stroke model [81].
4.1.2. Spontaneous intracerebral hemorrhage
Spontaneous intracerebral hemorrhage (ICH) has high mortality and high morbidity, the accumulated blood compresses the surrounding brain tissue, leading to the tissue damage and neuronal death [[82], [83]]. Evidence from preclinical and clinical studies indicated that released toxins may exacerbate ICH [84]. Hemoglobin (Hb), the most abundant protein, will be released from lysed red blood cells after ICH, which could be taken into microglia and metabolized to ferrous/ferric iron, thereby inducing lethal ROS [85]. The iron released by Hb is transported from microglia, but instead of stimulating the production of ROS in the cell, it forms highly toxic hydroxyl free radicals to attack DNA, protein and lipid membrane to destroy cell functions and neuronal death [86,87].
Many forms of cell death have been identified for ICH, including apoptosis [[88], [89], [90]], necrosis [91,92], and autophagy [93,94]. Hb, heme and iron play an important role in the production of ROS and lipid ROS after ICH [95,96]. IREB2, CS, RPL8, and ATP5G3 are also considered to be essential ferroptosis genes in several cancers [97], of which IREB2 is a master regulator that encodes iron metabolism. Neurons lacking IREB2 are highly resistant to the toxicity of Hb [98]. Knockout of IREB2 can increase perihematoma ferritin expression and cell survival after ICH [99]. The mRNA expression levels of IREB2 and ATP5G3 are also up-regulated after ICH in vivo [100]. Fer-1, a ferroptosis inhibitor, can inhibit the death of organotypic hippocampal slice cultures (OHSC) caused by ferrous iron and Hb, reduce the lipid ROS, improve the reduction of GPX activity in OHSC, and inhibit the expression of cyclooxygenase 2 (COX-2) [101]. It is worth noting that COX-2 is encoded by PTGS2, which is induced in ferroptosis cells [59]. While COX-2 is highly expressed in neurons after ICH, the inhibition of COX-2 could reduce the secondary brain damage caused by ICH [102,103]. At the same time, the inhibitory effect of Fer-1 on ferroptosis has a long-term neuroprotective effect in the acute phase of ICH [104]. Another study also showed that the expression of GPX4 in brain tissue decreased significantly after 24 hours of ICH. Up-regulating of GPX4 or ferroptosis inhibitors can effectively protect the brain damage caused by hemorrhage [105]. Epicatechin, a flavanol that could permeate the brain, could reduce early brain damage in ICH, reduce lesion volume, and improve nervous system defects. In addition to providing neuroprotection effect through the Nrf2 signaling pathway, it also reduced the expression of HO-1, activator protein-1 (AP-1), matrix metalloprotein 9 (MMP-9) and iron deposition through non-Nrf2-dependent pathways, as well as increasing the expression of lipocalin-2 (LCN2). LCN2 can capture iron particles, reduce cell death, and act as an antioxidant by regulating iron homeostasis in ICH to intervene ferroptosis [100,106,107]. Intracellular cysteine can promote the production of GSH, and the cell-permeable cysteine analogue N-acetyl-cysteine (NAC) can protect brain tissue from the cell deaths related to ICH [108]. Baicalin, as well as Fer-1, inhibited ferroptosis to alleviate motor deficits and brain injury of the ICH mice [101,109]. Isorhynchophylline could inhibit the ferroptosis and oxidative stress levels induced by ferric ammonium citrate (FAC), and improve the mNSS score of ICH model [110]. In summary, inhibiting ferroptosis can protect hemorrhagic encephalopathy, which has the potential to be developed into clinical therapy for ICH.
4.1.3. Subarachnoid hemorrhage
Subarachnoid hemorrhage (SAH) is a severely life-threatening subtype of stroke [111]. Angiographic vasospasm and delayed cerebral ischemia were once considered to be the main factors of poor prognosis after SAH, but anti-vasospasm drugs did not significantly improve the prognosis in clinical trials [112]. Therefore, it is particularly important to explore novel mechanisms for the occurrence and development of SAH. At present, a variety of cell deaths are involved in SAH, such as apoptosis, autophagy, and necrosis. Recent studies have reported that in the in vitro SAH model treated with oxyhemoglobin, the iron content the expression of TFR1 and Fpn increased significantly. In addition, in SAH models, the GSH content and GPX4 activity decreased, while lipid peroxides increased in oxyhemoglobin-treated cells. Fer-1 can up-regulate Fpn, reduce iron content, and improve lipid peroxidation. At the same time, Fer-1 had no effect on cell apoptosis, suggesting that ferroptosis is closely related to SAH, while ferroptosis inhibitor Fer-1 reduced ferroptosis and inhibited lipid peroxidation to exhibit the neuroprotective effect against SAH [113]. Liproxstatin-1 was found with the protection effect on the neural function and neuronal cell death in vivo [114]. Besides, the p53 inhibitor could block cortical SAH-induced ferroptosis, which provides new insight of ferroptosis in SAH [115].
4.1.4. Traumatic brain injury
Ferroptosis is also associated with traumatic brain injury (TBI). In the rat cortex-impact model of TBI, elevated levels of oxidized PE lipids can be detected 1 h after injury, which is consistent with the loss of GPX4 function [116]. In the mouse cortex-impact model of TBI, the reduction of iron-positive cells can be observed at and near the injury site [117]. In addition, treatment with Fer-1 found that cell death and related behavioral changes were reduced. It has been revealed that the way of cell death in brain injury is related to the type of stress it endures [91]. Cerebral ventricular injection of Fer-1 could reduce the neuronal degeneration induced by ferroptosis to improve the motor and cognitive function [117]. Melatonin was also found as the ferroptosis inhibitor, which inhibit neuronal Fth-mediated ferroptosis of TBI [118]. Although, the mechanism of ferroptosis in TBI need to be further explored, anti-ferroptosis provides a potential therapeutic target for TBI.
4.1.5. Spinal cord injury
Spinal cord injury (SCI) is a serious clinical traumatic disease, iron overload, ROS production, lipid peroxidation, and glutamate accumulation have been discovered during SCI, and both of those are the features of ferroptosis [[119], [120], [121]].
Chen et al. found that conditional ablation of GPX4 can induce degeneration and paralysis of motor neurons in model mice, while vitamin E can delay the above process, suggesting that the ferroptosis can aggravate the progress of SCI [122]. Yao et al. found that deferoxamine can significantly improve the morphology of mitochondria in SCI rats, increase the expression of GPX4, xCT and glutathione, and increase the expression levels of ferroptosis-related genes acyl-CoA synthase family member 2 (ACSF2) and IREB2 to increase the survival rate of neurons and inhibit glial hyperplasia [123]. The ferroptosis inhibitor SRS16-86 also played a similar role in SCI [124]. Zhang et al. found that at the cellular level (primary neurons and SH-SY5Y cells), deferoxamine can also protect primary cortical neurons from ferroptosis induced by erastin [125].
4.2. Ferroptosis in neurodegenerative diseases
4.2.1. Alzheimer's disease
Alzheimer's disease (AD) is currently considered to be a complex neurodegenerative disease. The most typical histopathological features of AD are the deposition of extracellular amyloid protein (Aβ) and the formation of senile plaques (SPs), and intracellular neurofibrillary tangles (NETs) formed by hyperphosphorylated tau protein. Disorders of iron homeostasis and reduced endogenous antioxidant systems (including GPX) are closely related to the pathology of AD. Brain iron levels are positively correlated with AD progression and cognitive decline [126,127]. MRI scan of patients with AD showed that areas of the brain affected by AD, such as the severely damaged hippocampus, have elevated iron content [128]. Patients with mild cognitive impairment showed higher cortical iron with high Aβ plaque load, which increased the risk of AD [[129], [130], [131]]. Studies have proven that the imbalance of brain iron homeostasis is closely related to Aβ plaques and NETs. Aβ precursor protein (APP) is a type 1 transmembrane glycoprotein. APP m RNA encodes IREs in the 5′-untranslated region (5′-UTR mRNA), which is closely related to the iron content [132]. The increased concentration of iron could up-regulate the translation of APP by virtue of the mRNA IRE in the 5′-UT to increase the amount of APP protein and Aβ [[133], [134], [135]]. In addition, iron also directly binds to His6, His13, His14 and amino acid residues in β to enhance the neurotoxicity of Aβ [136,137]. Under physiological conditions, tau acts as the microtubule-associated protein to support the microtubule structure of neurons to protect the axon transport, protein transport and cognitive functions [138,139]. However, hyperphosphorylation of tau protein aggregates into NFTs, which is the main pathological feature of AD. It is generally believed that brain iron homeostasis is closely related to the formation of NFTs and the progression of tau-mediated neurodegeneration. Iron can not only regulate the phosphorylation of tau protein, but also induce the aggregation of hyperphosphorylated tau protein [140,141]. Indeed, iron deposition and NFTs colocalize in specific areas of the brain associated with the progression of neurodegeneration [142]. Under normal physiological conditions, tau protein can transport iron to the cell surface by transporting APP [143], and the hyperphosphorylation and aggregation of tau protein disrupt the surface transport of APP, resulting in the accumulation of toxic neuronal iron and aggravation of NFTs to form the vicious circle [144]. Due to the high demand for dynamic energy metabolism and the low antioxidant defense, the brain is more susceptible to oxidative damage than other tissues [145]. The progressive loss of neurons is the direct cause of the clinical symptoms associated with AD, and oxidative stress is an important pathological mechanism of AD [146,147]. Iron is an essential cofactor for metabolic reactions, but iron can also generate ROS in the same microenvironment and cause oxidative stress. Iron-induced oxidative stress directly leads to damaging DNA, lipid and protein damage, resulting in cell death [135]. The lipid peroxidation and abnormal iron dynamics and accumulation in AD are the two basic conditions of ferroptosis [148].
GPX4, a unique anti-peroxidase, inhibits lipid peroxidation by directly reducing membrane lipid hydroperoxide to lipid alcohol [149], which acts as the central regulator of ferroptosis [150]. Studies have shown that lipid peroxidation and reduction in glutathione levels are the features of ferroptosis, as well as AD. The expression of GSH in the hippocampus and frontal cortex has the potential to become predictive biomarkers of AD and MCI [151]. Ablation of GPX4 can induce ferroptosis of spinal cord motor neurons, leading to the rapid occurrence and development of paralysis and death of adult mice. Further studies have shown that in the process of hippocampal neurodegeneration, ferroptosis triggers neuronal death in the hippocampus through the ablation of forebrain neuron GPX4, which is directly related to cognitive dysfunction. In vivo experiments have found that GPX4-deficient mice can show obvious cognitive impairment and hippocampal neuronal degeneration. The administration of vitamin E or ferroptosis inhibitor lipoxstatin-1 can significantly improve the degree of neurodegeneration. The characteristics of ferroptosis (abnormal iron regulation, lipid peroxidation, glutathione metabolism disorder, inflammation) are considered to be important preclinical signs of AD and cognitive impairment [152,153], and targeted ferroptosis therapy may lead to further excitotoxicity and energy deficiency [154]. In addition, alpha-lipoic acid (LA) can prevent tau-induced iron overload, lipid peroxidation and inflammation, which are all related to ferroptosis [155]. Iron not only aggravates the polymerization of toxic Aβ and hyperphosphorylated tau, but also directly leads to neuronal oxidative damage [156]. Considering the particularity and importance of iron in ferroptosis and the pathological mechanism of AD, ferroptosis may provide new insights into the molecular pathophysiology of the disease [157,158].
4.2.2. Parkinson's disease
Parkinson's disease (PD) is the second most common neurodegenerative disease of the nervous system. The main pathological feature of PD is the degeneration of dopaminergic neurons in the substantia nigra compact area (SNpc) with rich iron [159], which is the important participant in tyrosine hydroxylase-dependent dopamine synthesis and other dopamine metabolism processes [160,161]. The GSH depletion, lipid peroxidation, and elevated levels of ROS are the common features of PD and ferroptosis [[162], [163], [164]]. In the main event of PD [165], the iron chelator DFP can reduce oxidative stress and increase dopamine activity to improve the existing motor nerve symptoms and reduce the deterioration of motor function, resulting in the neuroprotective effect in the early stage of PD [160]. The MPP+-induced SH-SY5Y (often used Parkinson's disease model) cell line is not programmed cell death, but has some similarities with ferroptosis, involving in lipid peroxidation and suppressed by DIM and fer-1. Iron chelating agents not only inhibit ferroptosis, but also protect dopamine neurons from cell death [159]. In addition, GSH in the MPTP mouse model was reduced [166], and GSH depletion enhanced the MPP+ toxicity of substantia nigra dopaminergic neurons [167]. These studies indicate that ferroptosis is related to the degeneration of dopamine neurons in PD. Therefore, inhibiting the ferroptosis of dopamine neurons may become one of the strategies for the treatment of PD.
Ferroptosis is also involved in the PC12 cell line, which could be developed as PD model. The increased production of lipid ROS, the decreased ratio of GSH/GSSG, and the decreased expression of GPX4 were found at t-BHP-induced PC12 cells, which could be reversed by Fer-1, DFO and FAC, indicating that t-BHP-induced cell death was iron-dependent [168]. In the synucleinopathy model of PD, the incorporation of excessive α-synuclein oligomers into the membrane induced abnormal calcium signaling to further induce lipid peroxidation. Inhibition of lipid peroxidation could eliminate the above effects and prevent oligomer-induced neuronal toxicity, further confirming the role of ferroptosis in PD [169]. Ferroptosis was also observed in 6-hydroxydopamine (6-OHDA)-induced PD models (zebrafish and SH-SY5Y cells). The activation of p62-Keap1-Nrf2 pathway can prevent 6-OHDA-induced ferroptosis [170]. Tian et al. found that the expression of FTH1 in PD rats induced by 6-OHDA was significantly down-regulated to regulate ferritinophagy, microtubule-associated protein light chain 3 and NCOA4. While ferritinophagy inhibitors could inhibit the degradation of ferritin and ferroptosis induced by 6-OHDA [171]. When FAC was used to simulate the iron overload during PD progression, it was found that low concentrations of FAC could induce ferroptosis, and as the concentration increased, the main cell death mode tended to apoptosis, and ferroptosis inhibitors could rescue the above process rely on regulating the p53 signaling pathway, while the apoptosis inhibitor does not have the above functions [172].
4.2.3. Huntington's disease
The key trigger and pathological feature of huntington's disease (HD) is mutant Htt (m Htt). The N-terminus of m Htt is truncated and can be cleaved to generate monomers or small oligomeric fragments with abnormal conformations (such as β-sheet structures). These toxic fragments in the cytoplasm may damage the system that processes abnormal proteins in neurons, such as proteasomes, chaperones, and neuronal autophagy. Toxic macromolecules entering the nucleus of neurons can also interfere with the transcription of antioxidant genes [173]. Therefore, both cytoplasmic and nuclear localized toxic fragments can cause mitochondrial abnormalities, such as the decrease of ATP and the increase of ROS [174]. Although the pathological mechanism of HD is complicated, oxidative stress is the initial factor of its pathogenesis [175]. In transgenic HD mouse models and patients, no nucleus or cytoplasmic vesicles, apoptotic bodies, or DNA fragmentation could be observed, and indicating that new type of cell death is involved [176]. High levels of lipid peroxidation are the main feature of HD [177]. In the R6/2 HD mouse model, the increased lipid peroxidation co-existed with m Htt inclusion bodies in striatal neurons [178]. Similarly, the increased lipid peroxidation can be detected in the cortical oral brain slices of the m N90Q73 HD mouse model [179], as well as the cerebrospinal fluid of HD [180]. The inhibition of lipid peroxidation by Fer-1 can significantly improve the neuropathology of the R6/2 HD mouse model. Low glutathione level is another characteristic of HD [181]. The plasma GSH content and GPX4 protein activity of HD patients are significantly lower than that at healthy people [141]. Kumar et al. consistently revealed that the reduction of GSH and GSH-S transferase was detected in the striatum, cortex and hippocampus of HD mice induced by 3-nitropropionic acid (3-NP) [182], which could be reversed by supplementation of cysteine and cysteamine [183]. Excessive accumulation of iron is the main cause of neuronal hypoxic stress and another important triggering factor in HD [184]. Excessive iron deposition in the occipital cortex, globus pallidus, and putamen could be observed through MRI [185]. Quantitative susceptibility maps showed that the iron content increased in the putamen, core and globus pallidus [186]. Correspondingly, the content of ferritin iron in the striatum is also significantly increased [187], as well as iron-related proteins. In the R6/2 HD mouse model, the increase of ferritin in the striatum and cortex of HD, the decrease of IRP and TFR, and the increase of Fpn can be detected [188,189], as well as the changed expression of Fe–S enzymes in the HD striatum [190]. Iron supplementation further reduced the size of the striatum and worsened neurodegeneration in HD mice [191]. In contrast, intraventricular injection of iron chelator (DFO) improved the pathology and motor phenotype of the striatum in R6/2 HD mice [178]. The administration of ferroptosis inhibitor Fer-1 and iron chelator can significantly reduce the death of nerve cells in the isolated brain slices of HD rats [179]. Above, the correlation between ferroptosis and HD could be conducted.
4.2.4. Amyotrophic lateral sclerosis
Abnormal accumulation of irons could be found in spinal nerve cells of Amyotrophic lateral sclerosis (ALS) patients [192]. In addition, it was also found that the production of lipid peroxidation increased in ALS patients, which led to the excessive consumption of GSH and aggravated degeneration of motor neurons [193]. A large-scale phase III clinical trial (Mitotarget/TRO19622) study found that the levels of ferroptosis-related markers (4-HNE and FT, etc.) in ALS were related to the decline in ALSFRS-r scores. Ferroptosis led to disintegration of the axon skeleton and DNA fragmentation, and secondary increase of Nfl and 8-oxo-dG [194]. hTBK1-c.978T > A mutation induced ferroptosis and inhibited the proliferation of NSC-34 cells through KEAP1/NRF2/p62 signaling, while the effect of TBK1 mutation was significantly reversed by Fer-1 or p62 siRNA [195], indicating the inevitable connection between ALS and ferroptosis.
4.2.5. Friedreich ataxia
Friedreich ataxia (FRDA) is caused by decreased expressionn and function loss of frataxin in spinal cord neurons. Frataxin is mainly located in mitochondria and participates in the synthesis of iron-sulfur clusters and hemoglobin. As an important iron-binding protein in mitochondria, frataxin participates in iron storage and transportation. Due to the dynamic mutation of the frataxin gene, the iron will be abnormal deposited with increased oxidative stress and ROS, and ultimately lead to the neuronal degeneration and necrosis [196]. DFO could induce the increase of iron content and ROS level in FRDA cell model [197,198], suggesting that ferroptosis may have a certain correlation with FRDA. FRDA cells are more sensitive to the ferroptosis inducer erastin. FAC and BSO can cause the decrease of glutathione-dependent peroxidase activity and increase of lipid peroxidase, while ferroptosis inhibitors can reverse the above process and cell death due to the knockdown of frataxin [199]. Knockdown of frataxin can enhance the cell ferroptosis by accelerating the accumulation of free iron and lipid peroxidation, while overexpression of frataxin can protect cells from ferroptosis [200]. In addition, Nrf2 is a key factor regulating ferroptosis in FRDA [201].
4.3. Ferroptosis in brain cancers
Ferroptosis is also involved in the occurrence of brain cancers. As the key factor in the metabolism of glutamate, cystine and glutathione, the glutamate exchanger xCT (SLC7a11) is closely related to the progression of brain cancers. Inhibition of xCT will destroy the neurodegeneration and microenvironmental toxicity of glioma [202,203]. The activating transcription factor-4 (ATF4) promotes the malignancy of primary brain cancers (grade III and IV gliomas) by shaping the vascular structure through transcription targeting System Xc−. The proliferation activity induced by ATF4 can be attenuated by System Xc− inhibitor, ferroptosis inducer (erastin) and GPX4 inhibitor (RSL3). siRNA-mediated knockout of ATF4 can also attenuate the malignant characteristics of gliomas. Therefore, not only ferroptosis is involved in the formation of brain cancers, but ATF4 has also become an effective target that affects tumor growth and vasculature function [204].
The Nrf2-Keap1 pathway, another key target of ferroptosis, was also found in the fourth ventricle of patients with glioma. Activation of Nrf2-Keap1 signal up-regulates System Xc− and enhances glutamate secretion to affect the tumor microenvironment, resist cell ferroptosis, and promote cell proliferation. Database analysis showed that Nrf2 was at relatively low levels in different brain regions. In primary brain cancers, the expression of Nrf2 was positively correlated with the malignancy and prognosis of the cancer. Compared with Nrf2 overexpressing cells, knockdown of Keap1 would increase the proliferation rate [205]. ACSL4 has also been found in gliomas. Overexpression of ACSL4 can reduce the expression of GPX4 and increase the levels of ferroptosis biomarkers (5-HETE, 12-HETE and 15-HETE), resulting in the increased LDH release and decreased cell viability [206].
Wang et al. found that Pseudolaric acid B triggers ferroptosis in glioma cells by activating NCOA4 to further inhibit the activity of the cystine/glutamate transporter on the cell membrane [207]. ATF4 reduced the sensitivity of glioblastoma to ferroptosis inducers by promoting the expression of cystine/glutamate transporter-related proteins. Silencing the expression of cystine/glutamate transporter related proteins in glioblastoma enhanced the sensitivity of glioma cells against temozolomide. The specific inducer of ferroptosis, erastin, inhibited the activity of the cystine/glutamate transporter, resulting in insufficient synthesis of reduced glutathione, thereby sensitizing the effect of temozolomide against glioma cells [204]. The necrosis of glioblastoma is also regulated by ferroptosis triggered by neutrophils. Neutrophils transferred granules containing myeloperoxidase to cancer cells and induced the iron-dependent accumulation of lipid peroxidation to form a positive feedback loop connecting ferroptosis and necrosis [208].
Dihydroartemisinin (DHA) can cause endoplasmic reticulum stress in glioma cells, up-regulating protein kinase R-like ER kinase (PERK) to activate ATF4 and induce HSPA5 expression, then the expression and activity of GPX4 were increased to neutralize the lipid peroxidation induced by DHA to protecti the ferroptosis of glial cells [209]. Amentoflavone (AF) can trigger ferroptosis through triggering autophagy-dependent ferroptosis in glioma [210]. Ibuprofen can down-regulate the expression of Nrf2, GPX4 and SLC7A11 in glioblastoma cells, and induce ferroptosis [211]. Gastrodin inhibits H2O2-induced ferroptosis through its antioxidative effect in glioma cells [212]. Disulfiram (DSF) induces ferroptosis of glioblastoma and triggers lysosomal membrane permeabilization (LMP) in a ROS-dependent manner to enhance its radiosensitivity [213].
4.4. Ferroptosis in other neurological diseases
Periventricular leukomalacia (PVL) is a kind of white matter brain damage, which is the basis of most neurological diseases encountered by many premature infants. Hypoxia during the perinatal period causes ischemia in the periventricular area of the child, resulting in the damage of neuron and oligodendrocytes (OLs), and the disorder of myelination. These features will cause the softening of the white matter around the ventricle, leading to bilateral spastic hemiplegia, quadriplegia, and intellectual disability [214]. At the cellular level, it is caused by the loss of oligodendrocytes. Analysis of patient samples showed that there are abundant lipid peroxidation products in the cerebrospinal fluid of children with white matter damage, such as 8-isoproterenol and malondialdehyde. The consumption of GSH in rat alcohol cultures can cause cell death. Combined Fer-1 or its analogues with vitamin E can reverse the process caused by GSH depletion, which provided the basis of the relationship between ferroptosis and PVL [205].
5. Discussion and conclusion
With the deepening of research, ferroptosis has been found to play a key regulatory role in various diseases, which provides new ideas and treatment potential for related diseases. In addition, ferroptosis, as an independent cell death, has been found in a variety of diseases to promote the progress of the disease together with other types of cell death methods, which provides the possibility for the development of drug combination therapy in the future, as well as the novel treatment of such diseases. However, the current research on ferroptosis is still in the initial stage, and many obstructions remain to be resolved. For example, the underlying mechanism of the bidirectional regulation of p53 in ferroptosis is still unclear; the relationship between ferroptosis, apoptosis and autophagy need to be further explored. Iron has been proven as the key role of ferroptosis, which could catalyze lipid peroxidation through fenton reaction to mediate ferroptosis. However, other studies have also showed that other metal element, such as copper, can participate in the redox reaction and participate in ferroptosis. Therefore, are there other metal ions involved in regulating cell ferroptosis? Is iron necessary for ferroptosis, or can it be replaced by other elements? Currently known upstream genes involved in regulating iron metabolism, including FPN, TFR1 and DMT1, can affect the occurrence of ferroptosis, but what is the specific molecular mechanism downstream of iron metabolism? Ferroptosis is involved in the regulation of inflammatory response, but the specific mechanism of ferroptosis promoting inflammation is currently unclear. Besides, the specifical biomarker for ferroptosis is urgent needed.
In summary, as a new type of cell death, the in-depth research of ferroptosis will bring new directions for the treatment of neurological diseases. The regulatory mechanism of ferroptosis in neurological diseases and how to effectively regulate ferroptosis are urgently needed to be explored to provide the theoretical basis for the prevention and treatment of neurological diseases.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
Not applicable.
Funding
The work is supported by the National Natural Science Foundation of China (No. 8210131157), Science and Technology Development Foundation of Nanjing Medical University (No. NMUB2020296 and NMUB2020297), Wuxi Municipal Health Commission (No. Q202101, ZH202110 and Q202167), Wuxi Taihu Talent Project (No. WXTTP2020008 and WXTTP2021) and a grant from Jiangsu Research Hospital Association for Precision Medication (JY202105).
Author contributions
Zhou. Z.H. conceived the study, Jiang. Y., Zhu. H.H., Ji. Y.Y., Zhou. Q., Ou. M.M., Du. Z. Q. performed literature searching and summary; Jiang. Y. and Zhu. H.H. wrote the manuscript; and Li. D. and Zhou. Z.H. edited the manuscript.
Acknowledgments
Not applicable.
Conflict of interest
The authors declare no conflict of interest.
Contributor Information
Haohao Zhu, Email: zhuhaohao233@163.com.
Zhenhe Zhou, Email: zhouzh@njmu.edu.cn.
Abbreviations
- 3-NP
3-nitropropionic acid
- 4-HNE
4-hydroxynonenal
- Aβ
amyloid protein
- ACSL4
acyl-CoAsynthetase long-chain family member 4
- AD
alzheimer's disease
- AIF-M2
apoptosis-inducing factor mitochondrial-associated protein 2
- ALS
amyotrophic lateral sclerosis
- ApoTf
apotransferrin
- APP
Aβ precursor protein
- CARS
cysteinyl-tRNA synthetase
- CP
ceruloplasmin
- DFO
deferoxamine
- DMT1
divalent metal transporter 1
- Fer-1
ferrostatin-1
- FPP
famesyl pyrophosphate
- FRDA
friedreich ataxia
- FTH1
ferritin heavy chain 1
- FTL
ferritin light chain
- FtMt
ferritin mitochondrial
- Fpn
ferroportin
- GCL
glutamate cysteine ligase
- γ-GCS
cysteine synthase
- GLS2
glutaminase 2
- GPX4
glutathione peroxidase 4
- GSH
glutathione
- GSS
glutathione synthase
- HD
huntington's disease
- HEPH
hephaestin
- HO-1
hemo oxygenase-1
- IREs
iron response elements
- IRP2
iron regulatory protein 2
- LA
lipoic acid
- LOX
lipoxygenase
- LPCAT3
lysophosphatidylcholine acyltransferase 3
- MCI
mild cognitive impairment
- NCOA4
nuclear receptor coactivator 4
- NETs
neurofibrillary tangles
- Nrf2
nuclear factor-erythroid 2-related factor 2
- NQO1
quinone oxidoreductase 1
- PCBP
poly-(rC)-binding protein
- PD
parkinson's disease
- PTGS2
prostaglandin endoperoxide synthase 2
- PUFA
polyunsaturated fatty acid
- ROS
reactive oxygen species
- SAT1
spermidine/spermine N1-acetyltransferase 1
- SLC7A11
recombinant solute carrier family 7 member 11
- SLC3A2
recombinant solute carrier family 3 member 2
- SNpc
substantia nigra compact area
- SPs
senile plaques
- SQS
squalene synthase
- STEAP3
six-transmembrane epithelial antigen of prostate 3
- System Xc-
cystine/glutamate antiporter system
- TBK1
TANK binding kinase 1
- TFRC
transferrin receptor
- TfR1
transferrin receptor 1
- TfR2
transferrin receptor 2
- TXNRD1
thioredoxin reductase 1
- UTRs
untranslated regions
- VDAC
voltage-dependent anion channels
References
- 1.Dixon S.J., Lemberg K.M., Lamprecht M.R., Skouta R., Zaitsev E.M., Gleason C.E., et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hao S., Liang B., Huang Q., Dong S., Wu Z., He W., et al. Metabolic networks in ferroptosis. Oncology Letters. 2018;15:5405–5411. doi: 10.3892/ol.2018.8066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doll S., Conrad M. Iron and ferroptosis: a still ill-defined liaison. IUBMB Life. 2017;69:423–434. doi: 10.1002/iub.1616. [DOI] [PubMed] [Google Scholar]
- 4.Yu H.T., Guo P.Y., Xie X.Z., Wang Y., Chen G. Ferroptosis, a new form of cell death, and its relationships with tumourous diseases. Journal of Cellular and Molecular Medicine. 2017;21:648–657. doi: 10.1111/jcmm.13008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mou Y.H., Wang J., Wu J.C., He D., Zhang C.F., Duan C.J., et al. Ferroptosis, a new form of cell death: opportunities and challenges in cancer. Journal of Hematology & Oncology. 2019;12 doi: 10.1186/s13045-019-0720-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang S.H., Luo J., Zhang Z.H., Dong D.D., Shen Y., Fang Y.W., et al. Iron and magnetic: new research direction of the ferroptosis-based cancer therapy. American Journal of Cancer Research. 2018;8:1933–1946. [PMC free article] [PubMed] [Google Scholar]
- 7.Brown C.W., Amante J.J., Mercurio A.M. Cell clustering mediated by the adhesion protein PVRL4 is necessary for alpha 6 beta 4 integrin-promoted ferroptosis resistance in matrix-detached cells. Journal of Biological Chemistry. 2018;293:12741–12748. doi: 10.1074/jbc.RA118.003017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grzelak A., Wojewodzka M., Meczynska-Wielgosz S., Zuberek M., Wojciechowska D., Kruszewski M. Crucial role of chelatable iron in silver nanoparticles induced DNA damage and cytotoxicity. Redox Biology. 2018;15:435–440. doi: 10.1016/j.redox.2018.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yan H.X., Pan Y.S., Liao X.B., Zhu Y., Huang R., Pan C.L. Significantly enhanced Fenton-based oxidation processes with CuS-Cu9S8 as co-catalyst by accelerating the Fe3+/Fe2+cycles. Applied Surface Science. 2021;559 [Google Scholar]
- 10.Fillebeen C., Charlebois E., Wagner J., Katsarou A., Mui J., Vali H., et al. Transferrin receptor 1 controls systemic iron homeostasis by fine-tuning hepcidin expression to hepatocellular iron load. Blood. 2019;133:344–355. doi: 10.1182/blood-2018-05-850404. [DOI] [PubMed] [Google Scholar]
- 11.Ohgami R.S., Campagna D.R., Greer E.L., Antiochos B., McDonald A., Chen J., et al. Identification of a ferrireductase required for efficient transferrin-dependent iron uptake in erythroid cells. Nature Genetics. 2005;37:1264–1269. doi: 10.1038/ng1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zarjou A., Black L.M., McCullough K.R., Hull T.D., Esman S.K., Boddu R., et al. Ferritin light chain confers protection against sepsis-induced inflammation and organ injury. Frontiers in Immunology. 2019;10 doi: 10.3389/fimmu.2019.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mukherjee C., Kling T., Russo B., Miebach K., Kess E., Schifferer M., et al. Oligodendrocytes provide antioxidant defense function for neurons by secreting ferritin heavy chain. Cell Metabolism. 2020;32:259–+. doi: 10.1016/j.cmet.2020.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geng N., Shi B.J., Li S.L., Zhong Z.Y., Li Y.C., Xua W.L., et al. Knockdown of ferroportin accelerates erastin-induced ferroptosis in neuroblastoma cells. European Review for Medical and Pharmacological Sciences. 2018;22:3826–3836. doi: 10.26355/eurrev_201806_15267. [DOI] [PubMed] [Google Scholar]
- 15.Xu E., Chen M., Zheng J., Maimaitiming Z., Zhong T., Chen H. Deletion of hephaestin and ceruloplasmin induces a serious systemic iron deficiency and disrupts iron homeostasis. Biochemical and Biophysical Research Communications. 2018;503:1905–1910. doi: 10.1016/j.bbrc.2018.07.134. [DOI] [PubMed] [Google Scholar]
- 16.Zheng J.S., Jiang R.W., Chen M., Maimaitiming Z., Wang J.Z., Anderson G.J., et al. Multi-copper ferroxidase-deficient mice have increased brain iron concentrations and learning and memory deficits. Journal of Nutrition. 2018;148:643–649. doi: 10.1093/jn/nxy012. [DOI] [PubMed] [Google Scholar]
- 17.Chen Z., Jiang R.W., Chen M.X., Zheng J.S., Chen M., Braidy N., et al. Multi-copper ferroxidase deficiency leads to iron accumulation and oxidative damage in astrocytes and oligodendrocytes. Scientific Reports. 2019;9 doi: 10.1038/s41598-019-46019-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abreu R., Quinn F., Giri P.K. Role of the hepcidin-ferroportin axis in pathogen-mediated intracellular iron sequestration in human phagocytic cells. Blood Advances. 2018;2:1089–1100. doi: 10.1182/bloodadvances.2017015255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanchez M., Galy B., Muckenthaler M.U., Hentze M.W. Iron-regulatory proteins limit hypoxia-inducible factor-2alpha expression in iron deficiency. Nature Structural & Molecular Biology. 2007;14:420–426. doi: 10.1038/nsmb1222. [DOI] [PubMed] [Google Scholar]
- 20.Hentze M.W., Muckenthaler M.U., Galy B., Camaschella C. Two to tango: regulation of Mammalian iron metabolism. Cell. 2010;142:24–38. doi: 10.1016/j.cell.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 21.Rouault T.A. The role of iron regulatory proteins in mammalian iron homeostasis and disease. Nature Chemical Biology. 2006;2:406–414. doi: 10.1038/nchembio807. [DOI] [PubMed] [Google Scholar]
- 22.Cooperman S.S., Meyron-Holtz E.G., Olivierre-Wilson H., Ghosh M.C., McConnell J.P., Rouault T.A. Microcytic anemia, erythropoietic protoporphyria, and neurodegeneration in mice with targeted deletion of iron-regulatory protein 2. Blood. 2005;106:1084–1091. doi: 10.1182/blood-2004-12-4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao N., Zhang A.S., Worthen C., Knutson M.D., Enns C.A. An iron-regulated and glycosylation-dependent proteasomal degradation pathway for the plasma membrane metal transporter ZIP14. Proceedings of the National Academy of Sciences of the U S A. 2014;111:9175–9180. doi: 10.1073/pnas.1405355111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang C.Y., Jenkitkasemwong S., Duarte S., Sparkman B.K., Shawki A., Mackenzie B., et al. ZIP8 is an iron and zinc transporter whose cell-surface expression is up-regulated by cellular iron loading. Journal of Biological Chemistry. 2012;287:34032–34043. doi: 10.1074/jbc.M112.367284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tripathi A.K., Haldar S., Qian J., Beserra A., Suda S., Singh A., et al. Prion protein functions as a ferrireductase partner for ZIP14 and DMT1. Free Radical Biology and Medicine. 2015;84:322–330. doi: 10.1016/j.freeradbiomed.2015.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nandal A., Ruiz J.C., Subramanian P., Ghimire-Rijal S., Sinnamon R.A., Stemmler T.L., et al. Activation of the HIF prolyl hydroxylase by the iron chaperones PCBP1 and PCBP2. Cell Metabolism. 2011;14:647–657. doi: 10.1016/j.cmet.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frey A.G., Nandal A., Park J.H., Smith P.M., Yabe T., Ryu M.S., et al. Iron chaperones PCBP1 and PCBP2 mediate the metallation of the dinuclear iron enzyme deoxyhypusine hydroxylase. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:8031–8036. doi: 10.1073/pnas.1402732111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mancias J.D., Wang X.X., Gygi S.P., Harper J.W., Kimmelman A.C. Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy. Nature. 2014;509:105. doi: 10.1038/nature13148. -+ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nai A., Lidonnici M.R., Federico G., Pettinato M., Olivari V., Carrillo F., et al. NCOA4-mediated ferritinophagy in macrophages is crucial to sustain erythropoiesis in mice. Haematologica. 2021;106:795–805. doi: 10.3324/haematol.2019.241232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Y.Q., Chang S.Y., Wu Q., Gou Y.J., Jia L.P., Cui Y.M., et al. The protective role of mitochondrial ferritin on erastin-induced ferroptosis. Frontiers in Aging Neuroscience. 2016;8 doi: 10.3389/fnagi.2016.00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lou Z., Wang A.P., Duan X.M., Hu G.H., Song G.L., Zuo M.L., et al. Upregulation of NOX2 and NOX4 mediated by TGF-beta signaling pathway exacerbates cerebral ischemia/reperfusion oxidative stress injury. Cellular Physiology and Biochemistry. 2018;46:2103–2113. doi: 10.1159/000489450. [DOI] [PubMed] [Google Scholar]
- 32.Li N., Wang W., Zhou H., Wu Q.Q., Duan M.X., Liu C., et al. Ferritinophagy-mediated ferroptosis is involved in sepsis-induced cardiac injury. Free Radical Biology and Medicine. 2020;160:303–318. doi: 10.1016/j.freeradbiomed.2020.08.009. [DOI] [PubMed] [Google Scholar]
- 33.Masaldan S., Clatworthy S.A.S., Gamell C., Meggyesy P.M., Rigopoulos A.T., Haupt S., et al. Iron accumulation in senescent cells is coupled with impaired ferritinophagy and inhibition of ferroptosis. Redox Biology. 2018;14:100–115. doi: 10.1016/j.redox.2017.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao Y., Zhao W., Lim Y.C., Liu T. Salinomycin-loaded gold nanoparticles for treating cancer stem cells by ferroptosis-induced cell death. Molecular Pharmaceutics. 2019;16:2532–2539. doi: 10.1021/acs.molpharmaceut.9b00132. [DOI] [PubMed] [Google Scholar]
- 35.Xiao J.T., Zhang S.S., Tu B.J., Jiang X.J., Cheng S.Q., Tang Q.H., et al. Arsenite induces ferroptosis in the neuronal cells via activation of ferritinophagy. Food and Chemical Toxicology. 2021;151 doi: 10.1016/j.fct.2021.112114. [DOI] [PubMed] [Google Scholar]
- 36.Sun Y., Zheng Y.F., Wang C.X., Liu Y.Z. Glutathione depletion induces ferroptosis, autophagy, and premature cell senescence in retinal pigment epithelial cells. Cell Death & Disease. 2018;9 doi: 10.1038/s41419-018-0794-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gao M.H., Monian P., Pan Q.H., Zhang W., Xiang J., Jiang X.J. Ferroptosis is an autophagic cell death process. Cell Research. 2016;26:1021–1032. doi: 10.1038/cr.2016.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gryzik M., Asperti M., Denardo A., Arosio P., Poli M. NCOA4-mediated ferritinophagy promotes ferroptosis induced by erastin, but not by RSL3 in HeLa cells. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 2021;1868:118913. doi: 10.1016/j.bbamcr.2020.118913. [DOI] [PubMed] [Google Scholar]
- 39.Ahammed G.J., Wu M.J., Wang Y.Q., Yan Y.R., Mao Q., Ren J.J., et al. Melatonin alleviates iron stress by improving iron homeostasis, antioxidant defense and secondary metabolism in cucumber. Scientia Horticulturae. 2020;265 [Google Scholar]
- 40.Kang Y.Y., Tiziani S., Park G., Kaul M., Paternostro G. Cellular protection using Flt3 and PI3K alpha inhibitors demonstrates multiple mechanisms of oxidative glutamate toxicity. Nature Communications. 2014;5 doi: 10.1038/ncomms4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mayr L., Grabherr F., Schwrzler J., Reitmeier I., Sommer F., Gehmacher T., et al. Dietary lipids fuel GPX4-restricted enteritis resembling Crohn's disease. Nature Communications. 2020;11 doi: 10.1038/s41467-020-15646-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Forcina G.C., Dixon S.J. GPX4 at the crossroads of lipid homeostasis and ferroptosis. Proteomics. 2019;19 doi: 10.1002/pmic.201800311. [DOI] [PubMed] [Google Scholar]
- 43.Miotto G., Rossetto M., Di Paolo M.L., Orian L., Venerando R., Roveri A., et al. Insight into the mechanism of ferroptosis inhibition by ferrostatin-1. Redox Biology. 2020;28 doi: 10.1016/j.redox.2019.101328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maiorino M., Conrad M., Ursini F. GPx4, lipid peroxidation, and cell death: discoveries, rediscoveries, and open issues. Antioxidants and Redox Signaling. 2018;29:61–74. doi: 10.1089/ars.2017.7115. [DOI] [PubMed] [Google Scholar]
- 45.Agmon E., Solon J., Bassereau P., Stockwell B.R. Modeling the effects of lipid peroxidation during ferroptosis on membrane properties. Scientific Reports. 2018;8 doi: 10.1038/s41598-018-23408-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sato H., Tamba M., Okuno S., Sato K., Keino-Masu K., Masu M., et al. Distribution of cystine/glutamate exchange transporter, system x(c)-, in the mouse brain. Journal of Neuroscience. 2002;22:8028–8033. doi: 10.1523/JNEUROSCI.22-18-08028.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lewerenz J., Klein M., Methner A. Cooperative action of glutamate transporters and cystine/glutamate antiporter system Xc- protects from oxidative glutamate toxicity. Journal of Neurochemistry. 2006;98:916–925. doi: 10.1111/j.1471-4159.2006.03921.x. [DOI] [PubMed] [Google Scholar]
- 48.Ishii T., Warabi E., Mann G.E. Circadian control of BDNF-mediated Nrf2 activation in astrocytes protects dopaminergic neurons from ferroptosis. Free Radical Biology and Medicine. 2019;133:169–178. doi: 10.1016/j.freeradbiomed.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 49.Lewerenz J., Maher P. Chronic glutamate toxicity in neurodegenerative diseases-what is the evidence? Frontiers in Neuroscience. 2015;9 doi: 10.3389/fnins.2015.00469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tabassum R., Jeong N.Y., Jung J.Y. Protective effect of hydrogen sulfide on oxidative stress-induced neurodegenerative diseases. Neural Regeneration Research. 2020;15:232–241. doi: 10.4103/1673-5374.265543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ingold I., Berndt C., Schmitt S., Doll S., Poschmann G., Buday K., et al. Selenium utilization by GPX4 is required to prevent hydroperoxide-induced ferroptosis. Cell. 2018;172:409. doi: 10.1016/j.cell.2017.11.048. -+ [DOI] [PubMed] [Google Scholar]
- 52.Hou L.Y., Huang R.X., Sun F.Q., Zhang L., Wang Q.S. NADPH oxidase regulates paraquat and maneb-induced dopaminergic neurodegeneration through ferroptosis. Toxicology. 2019;417:64–73. doi: 10.1016/j.tox.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 53.Zhang Y.L., Swanda R.V., Nie L.T., Liu X.G., Wang C., Lee H., et al. mTORC1 couples cyst(e)ine availability with GPX4 protein synthesis and ferroptosis regulation. Nature Communications. 2021;12 doi: 10.1038/s41467-021-21841-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hemono M., Ubrig E., Azeredo K., Salinas-Giege T., Drouard L., Duchene A.M. Arabidopsis voltage-dependent anion channels (VDACs): overlapping and specific functions in mitochondria. Cells. 2020;9 doi: 10.3390/cells9041023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yagoda N., von Rechenberg M., Zaganjor E., Bauer A.J., Yang W.S., Fridman D.J., et al. RAS-RAF-MEK-dependent oxidative cell death involving voltage-dependent anion channels. Nature. 2007;447:864–868. doi: 10.1038/nature05859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jiang L., Hickman J.H., Wang S.J., Gu W. Dynamic roles of p53-mediated metabolic activities in ROS-induced stress responses. Cell Cycle. 2015;14:2881–2885. doi: 10.1080/15384101.2015.1068479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jiang L., Kon N., Li T.Y., Wang S.J., Su T., Hibshoosh H., et al. Ferroptosis as a p53-mediated activity during tumour suppression. Nature. 2015;520:57. doi: 10.1038/nature14344. -+ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gao M.H., Monian P., Quadri N., Ramasamy R., Jiang X.J. Glutaminolysis and transferrin regulate ferroptosis. Molecular Cell. 2015;59:298–308. doi: 10.1016/j.molcel.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang W.S., SriRamaratnam R., Welsch M.E., Shimada K., Skouta R., Viswanathan V.S., et al. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156:317–331. doi: 10.1016/j.cell.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ou Y., Wang S.J., Li D.W., Chu B., Gu W. Activation of SAT1 engages polyamine metabolism with p53-mediated ferroptotic responses. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:E6806–E6812. doi: 10.1073/pnas.1607152113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tarangelo A., Magtanong L., Bieging-Rolett K.T., Li Y., Ye J.B., Attardi L.D., et al. p53 suppresses metabolic stress-induced ferroptosis in cancer cells. Cell Reports. 2018;22:569–575. doi: 10.1016/j.celrep.2017.12.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tarangelo A., Dixon S. The p53-p21 pathway inhibits ferroptosis during metabolic stress. Oncotarget. 2018;9:24572–24573. doi: 10.18632/oncotarget.25362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sun X.F., Ou Z.H., Chen R.C., Niu X.H., Chen D., Kang R., et al. Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology. 2016;63:173–184. doi: 10.1002/hep.28251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Roh J.L., Kim E.H., Jang H., Shin D. Nrf2 inhibition reverses the resistance of cisplatin-resistant head and neck cancer cells to artesunate-induced ferroptosis. Redox Biology. 2017;11:254–262. doi: 10.1016/j.redox.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Itoh K., Tong K.I., Yamamoto M. Molecular mechanism activating Nrf2-Keap1 pathway in regulation of adaptive response to electrophiles. Free Radical Biology and Medicine. 2004;36:1208–1213. doi: 10.1016/j.freeradbiomed.2004.02.075. [DOI] [PubMed] [Google Scholar]
- 66.Wang H., An P., Xie E.J., Wu Q., Fang X.X., Gao H., et al. Characterization of ferroptosis in murine models of hemochromatosis. Hepatology. 2017;66:449–465. doi: 10.1002/hep.29117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Angeli J.P.F., Schneider M., Proneth B., Tyurina Y.Y., Tyurin V.A., Hammond V.J., et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nature Cell Biology. 2014;16 doi: 10.1038/ncb3064. 1180-U1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shimada K., Hayano M., Pagano N.C., Stockwell B.R. Cell-line selectivity improves the predictive power of pharmacogenomic analyses and helps identify NADPH as biomarker for ferroptosis sensitivity. Cell Chemical Biology. 2016;23:225–235. doi: 10.1016/j.chembiol.2015.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shimada K., Skouta R., Kaplan A., Yang W.S., Hayano M., Dixon S.J., et al. Global survey of cell death mechanisms reveals metabolic regulation of ferroptosis. Nature Chemical Biology. 2016;12:497. doi: 10.1038/nchembio.2079. -+ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kagan V.E., Mao G.W., Qu F., Angeli J.P.F., Doll S., St Croix C., et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nature Chemical Biology. 2017;13:81–90. doi: 10.1038/nchembio.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hayano M., Yang W.S., Corn C.K., Pagano N.C., Stockwell B.R. Loss of cysteinyl-tRNA synthetase (CARS) induces the transsulfuration pathway and inhibits ferroptosis induced by cystine deprivation. Cell Death & Differentiation. 2016;23:270–278. doi: 10.1038/cdd.2015.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Telorack M., Meyer M., Ingold I., Conrad M., Bloch W., Werner S. A glutathione-Nrf2-thioredoxin cross-talk ensures keratinocyte survival and efficient wound repair. PLoS Genetics. 2016;12 doi: 10.1371/journal.pgen.1005800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bersuker K., Hendricks J.M., Li Z.P., Magtanong L., Ford B., Tang P.H., et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature. 2019;575:688–+. doi: 10.1038/s41586-019-1705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Doll S., Freitas F.P., Shah R., Aldrovandi M., da Silva M.C., Ingold I., et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature. 2019;575:693–+. doi: 10.1038/s41586-019-1707-0. [DOI] [PubMed] [Google Scholar]
- 75.Tuo Q.Z., Lei P., Jackman K.A., Li X.L., Xiong H., Li X.L., et al. Tau-mediated iron export prevents ferroptotic damage after ischemic stroke. Molecular Psychiatry. 2017;22:1520–1530. doi: 10.1038/mp.2017.171. [DOI] [PubMed] [Google Scholar]
- 76.Valdes Hernandez M.D., Case T., Chappell F.M., Glatz A., Makin S., Doubal F., et al. Association between striatal brain iron deposition, microbleeds and cognition 1 Year after a minor ischaemic stroke. International Journal of Molecular Sciences. 2019;20 doi: 10.3390/ijms20061293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen J.M., Yang L., Geng L.X., He J.N., Chen L., Sun Q., et al. Inhibition of acyl-CoA synthetase long-chain family member 4 facilitates neurological recovery after stroke by regulation ferroptosis. Frontiers in Cellular Neuroscience. 2021;15 doi: 10.3389/fncel.2021.632354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lu J.J., Xu F., Lu H. LncRNA PVT1 regulates ferroptosis through miR-214-mediated TFR1 and p53. Life Sciences. 2020;260 doi: 10.1016/j.lfs.2020.118305. [DOI] [PubMed] [Google Scholar]
- 79.Chen W., Jiang L.F., Hu Y.Q., Tang N., Liang N., Li X.F., et al. Ferritin reduction is essential for cerebral ischemia-induced hippocampal neuronal death through p53/SLC7A11-mediated ferroptosis. Brain Research. 2021;1752 doi: 10.1016/j.brainres.2020.147216. [DOI] [PubMed] [Google Scholar]
- 80.Fang Y., Chen X., Tan Q., Zhou H., Xu J., Gu Q. Inhibiting ferroptosis through disrupting the NCOA4-FTH1 interaction: a new mechanism of action. ACS Central Science. 2021;7:980–989. doi: 10.1021/acscentsci.0c01592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yang W., Liu X., Song C., Ji S., Yang J., Liu Y., et al. Structure-activity relationship studies of phenothiazine derivatives as a new class of ferroptosis inhibitors together with the therapeutic effect in an ischemic stroke model. European Journal of Medicinal Chemistry. 2021;209:112842. doi: 10.1016/j.ejmech.2020.112842. [DOI] [PubMed] [Google Scholar]
- 82.Dasari R., Bonsack F., Sukumari-Ramesh S. Brain injury and repair after intracerebral hemorrhage: the role of microglia and brain-infiltrating macrophages. Neurochemistry International. 2021;142 doi: 10.1016/j.neuint.2020.104923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wasserman J.K., Schlichter L.C. Neuron death and inflammation in a rat model of intracerebral hemorrhage: effects of delayed minocycline treatment. Brain Research. 2007;1136:208–218. doi: 10.1016/j.brainres.2006.12.035. [DOI] [PubMed] [Google Scholar]
- 84.Wei J.L., Novakovic N., Chenevert T.L., Xi G.H., Keep R.F., Pandey A.S., et al. Perihematomal brain tissue iron concentration measurement by MRI in patients with intracerebral hemorrhage. CNS Neuroscience and Therapeutics. 2020;26:896–901. doi: 10.1111/cns.13395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Huang F.P., Xi G., Keep R.F., Hua Y., Nemoianu A., Hoff J.T. Brain edema after experimental intracerebral hemorrhage: role of hemoglobin degradation products. Journal of Neurosurgery. 2002;96:287–293. doi: 10.3171/jns.2002.96.2.0287. [DOI] [PubMed] [Google Scholar]
- 86.Gaasch J.A., Lockman P.R., Geldenhuys W.J., Allen D.D., Van der Schyf C.J. Brain iron toxicity: differential responses of astrocytes, neurons, and endothelial cells. Neurochemical Research. 2007;32:1196–1208. doi: 10.1007/s11064-007-9290-4. [DOI] [PubMed] [Google Scholar]
- 87.He Q., Song N., Jia F.J., Xu H.M., Yu X.J., Xie J.X., et al. Role of alpha-synuclein aggregation and the nuclear factor E2-related factor 2/heme oxygenase-1 pathway in iron-induced neurotoxicity. The International Journal of Biochemistry & Cell Biology. 2013;45:1019–1030. doi: 10.1016/j.biocel.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 88.Wang Z., Zhou F., Dou Y., Tian X.D., Liu C.L., Li H.Y., et al. Melatonin alleviates intracerebral hemorrhage-induced secondary brain injury in rats via suppressing apoptosis, inflammation, oxidative stress, DNA damage, and mitochondria injury. Translational Stroke Research. 2018;9:74–91. doi: 10.1007/s12975-017-0559-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhou Y., Wang S.H., Li Y.X., Yu S.S., Zhao Y. SIRT1/PGC-1 alpha signaling promotes mitochondrial functional recovery and reduces apoptosis after intracerebral hemorrhage in rats. Frontiers in Molecular Neuroscience. 2018;10 doi: 10.3389/fnmol.2017.00443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yang G.Q., Huang J.C., Yuan J.J., Zhang Q., Gong C.X., Chen Q., et al. Prdx1 reduces intracerebral hemorrhage-induced brain injury via targeting inflammation- and apoptosis-related mRNA stability. Frontiers in Neuroscience. 2020;14 doi: 10.3389/fnins.2020.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li Q., Weiland A., Chen X.M., Lan X., Han X.N., Durham F., et al. Ultrastructural characteristics of neuronal death and white matter injury in mouse brain tissues after intracerebral hemorrhage: coexistence of ferroptosis, autophagy, and necrosis. Frontiers in Neurology. 2018;9 doi: 10.3389/fneur.2018.00581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shen H.T., Liu C.L., Zhang D.P., Yao X., Zhang K., Li H.Y., et al. Role for RIP1 in mediating necroptosis in experimental intracerebral hemorrhage model both in vivo and in vitro. Cell Death & Disease. 2017;8 doi: 10.1038/cddis.2017.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Duan X.C., Wang W., Feng D.X., Yin J., Zuo G., Chen D.D., et al. Roles of autophagy and endoplasmic reticulum stress in intracerebral hemorrhage-induced secondary brain injury inrats. CNS Neuroscience and Therapeutics. 2017;23:554–566. doi: 10.1111/cns.12703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shi H., Wang J., Wang J., Huang Z.M., Yang Z. IL-17A induces autophagy and promotes microglial neuroinflammation through ATG5 and ATG7 in intracerebral hemorrhage. Journal of Neuroimmunology. 2018;323:143–151. doi: 10.1016/j.jneuroim.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 95.Katsu M., Niizuma K., Yoshioka H., Okami N., Sakata H., Chan P.H. Hemoglobin-induced oxidative stress contributes to matrix metalloproteinase activation and blood-brain barrier dysfunction in vivo. Journal of Cerebral Blood Flow and Metabolism. 2010;30:1939–1950. doi: 10.1038/jcbfm.2010.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zheng J.W., Shi L.G., Liang F., Xu W.L., Li T., Gao L.S., et al. Sirt3 ameliorates oxidative stress and mitochondrial dysfunction after intracerebral hemorrhage in diabetic rats. Frontiers in Neuroscience. 2018;12 doi: 10.3389/fnins.2018.00414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tang D.L., Kepp O., Kroemer G. Ferroptosis becomes immunogenic: implications for anticancer treatments. OncoImmunology. 2021;10 doi: 10.1080/2162402X.2020.1862949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Regan R.F., Chen M., Li Z., Zhang X.F., Benvenisti-Zarom L., Chen-Roetling J. Neurons lacking iron regulatory protein-2 are highly resistant to the toxicity of hemoglobin. Neurobiology of Disease. 2008;31:242–249. doi: 10.1016/j.nbd.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chen M., Awe O.O., Chen-Roetling J., Regan R.F. Iron regulatory protein-2 knockout increases perihematomal ferritin expression and cell viability after intracerebral hemorrhage. Brain Research. 2010;1337:95–103. doi: 10.1016/j.brainres.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chang C.F., Cho S., Wang J. (-)-Epicatechin protects hemorrhagic brain via synergistic Nrf2 pathways. Annals of Clinical and Translational Neurology. 2014;1:258–271. doi: 10.1002/acn3.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li Q., Han X.N., Lan X., Gao Y.F., Wan J.R., Durham F., et al. Inhibition of neuronal ferroptosis protects hemorrhagic brain. JCI Insight. 2017;2 doi: 10.1172/jci.insight.90777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wu T., Wu H., Wang J., Wang J.A. Expression and cellular localization of cyclooxygenases and prostaglandin E synthases in the hemorrhagic brain. Journal of Neuroinflammation. 2011;8 doi: 10.1186/1742-2094-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chu K., Jeong S.W., Jung K.H., Han S.Y., Lee S.T., Kim M., et al. Celecoxib induces functional recovery after intracerebral hemorrhage with reduction of brain edema and perihematomal cell death. Journal of Cerebral Blood Flow and Metabolism. 2004;24:926–933. doi: 10.1097/01.WCB.0000130866.25040.7D. [DOI] [PubMed] [Google Scholar]
- 104.Chen B., Chen Z.H., Liu M.J., Gao X.R., Cheng Y.J., Wei Y.X., et al. Inhibition of neuronal ferroptosis in the acute phase of intracerebral hemorrhage shows long-term cerebroprotective effects. Brain Research Bulletin. 2019;153:122–132. doi: 10.1016/j.brainresbull.2019.08.013. [DOI] [PubMed] [Google Scholar]
- 105.Zhang Z.W., Wu Y., Yuan S., Zhang P., Zhang J.Y., Li H.Y., et al. Glutathione peroxidase 4 participates in secondary brain injury through mediating ferroptosis in a rat model of intracerebral hemorrhage. Brain Research. 2018;1701:112–125. doi: 10.1016/j.brainres.2018.09.012. [DOI] [PubMed] [Google Scholar]
- 106.Dong M., Xi G.H., Keep R.F., Hua Y. Role of iron in brain lipocalin 2 upregulation after intracerebral hemorrhage in rats. Brain Research. 2013;1505:86–92. doi: 10.1016/j.brainres.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Speer R.E., Karuppagounder S.S., Basso M., Sleiman S.F., Kumar A., Brand D., et al. Hypoxia-inducible factor prolyl hydroxylases as targets for neuroprotection by "antioxidant" metal chelators: from ferroptosis to stroke. Free Radical Biology and Medicine. 2013;62:26–36. doi: 10.1016/j.freeradbiomed.2013.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Karuppagounder S.S., Alin L., Chen Y., Brand D., Bourassa M.W., Dietrich K., et al. N-acetylcysteine targets 5 lipoxygenase-derived, toxic lipids and can synergize with prostaglandin E2 to inhibit ferroptosis and improve outcomes following hemorrhagic stroke in mice. Annals of Neurology. 2018;84:854–872. doi: 10.1002/ana.25356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Duan L., Zhang Y., Yang Y., Su S., Zhou L., Lo P.C., et al. Baicalin inhibits ferroptosis in intracerebral hemorrhage. Frontiers in Pharmacology. 2021;12:629379. doi: 10.3389/fphar.2021.629379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhao H., Li X., Yang L., Zhang L., Jiang X., Gao W., et al. Isorhynchophylline relieves ferroptosis-induced nerve damage after intracerebral hemorrhage via miR-122-5p/TP53/SLC7A11 pathway. Neurochemical Research. 2021;46:1981–1994. doi: 10.1007/s11064-021-03320-2. [DOI] [PubMed] [Google Scholar]
- 111.Savarraj J., Parsha K., Hergenroeder G., Ahn S., Chang T.R., Kim D.H., et al. Early brain injury associated with systemic inflammation after subarachnoid hemorrhage. Neurocritical Care. 2018;28:203–211. doi: 10.1007/s12028-017-0471-y. [DOI] [PubMed] [Google Scholar]
- 112.Liu W., Li R., Yin J., Guo S., Chen Y., Fan H., et al. Mesenchymal stem cells alleviate the early brain injury of subarachnoid hemorrhage partly by suppression of Notch1-dependent neuroinflammation: involvement of Botch. Journal of Neuroinflammation. 2019;16:8. doi: 10.1186/s12974-019-1396-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li Y., Liu Y., Wu P., Tian Y., Liu B., Wang J., et al. Inhibition of ferroptosis alleviates early brain injury after subarachnoid hemorrhage in vitro and in vivo via reduction of lipid peroxidation. Cellular and Molecular Neurobiology. 2021;41:263–278. doi: 10.1007/s10571-020-00850-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cao Y., Li Y., He C., Yan F., Li J.R., Xu H.Z., et al. Selective ferroptosis inhibitor liproxstatin-1 attenuates neurological deficits and neuroinflammation after subarachnoid hemorrhage. Neuroscience Bulletin. 2021;37:535–549. doi: 10.1007/s12264-020-00620-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kuang H., Wang T., Liu L., Tang C., Li T., Liu M., et al. Treatment of early brain injury after subarachnoid hemorrhage in the rat model by inhibiting p53-induced ferroptosis. Neuroscience Letters. 2021;762:136134. doi: 10.1016/j.neulet.2021.136134. [DOI] [PubMed] [Google Scholar]
- 116.Wenzel S.E., Tyurina Y.Y., Zhao J., St Croix C.M., Dar H.H., Mao G., et al. PEBP1 wardens ferroptosis by enabling lipoxygenase generation of lipid death signals. Cell. 2017;171:628–641. doi: 10.1016/j.cell.2017.09.044. e626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Xie B.S., Wang Y.Q., Lin Y., Mao Q., Feng J.F., Gao G.Y., et al. Inhibition of ferroptosis attenuates tissue damage and improves long-term outcomes after traumatic brain injury in mice. CNS Neuroscience and Therapeutics. 2019;25:465–475. doi: 10.1111/cns.13069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rui T., Wang H., Li Q., Cheng Y., Gao Y., Fang X., et al. Deletion of ferritin H in neurons counteracts the protective effect of melatonin against traumatic brain injury-induced ferroptosis. Journal of Pineal Research. 2021;70:e12704. doi: 10.1111/jpi.12704. [DOI] [PubMed] [Google Scholar]
- 119.Meng F.X., Hou J.M., Sun T.S. Effect of oxidative stress induced by intracranial iron overload on central pain after spinal cord injury. Journal of Orthopaedic Surgery and Research. 2017;12 doi: 10.1186/s13018-017-0526-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Li X., Zhan J.H., Hou Y., Hou Y.H., Chen S.D., Luo D., et al. Coenzyme Q10 regulation of apoptosis and oxidative stress in H2O2 induced BMSC death by modulating the Nrf-2/NQO-1 signaling pathway and its application in a model of spinal cord injury. Oxidative Medicine and Cellular Longevity. 2019;2019 doi: 10.1155/2019/6493081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang H.S., Yu G., Wang Z.T., Yi S.P., Su R.B., Gong Z.H. Changes in VGLUT1 and VGLUT2 expression in rat dorsal root ganglia and spinal cord following spared nerve injury. Neurochemistry International. 2016;99:9–15. doi: 10.1016/j.neuint.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 122.Chen L., Hambright W.S., Na R., Ran Q. Ablation of the ferroptosis inhibitor glutathione peroxidase 4 in neurons results in rapid motor neuron degeneration and paralysis. Journal of Biological Chemistry. 2015;290:28097–28106. doi: 10.1074/jbc.M115.680090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yao X., Zhang Y., Hao J., Duan H.Q., Zhao C.X., Sun C., et al. Deferoxamine promotes recovery of traumatic spinal cord injury by inhibiting ferroptosis. Neural Regeneration Research. 2019;14:532–541. doi: 10.4103/1673-5374.245480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang Y., Sun C., Zhao C., Hao J., Zhang Y., Fan B., et al. Ferroptosis inhibitor SRS 16-86 attenuates ferroptosis and promotes functional recovery in contusion spinal cord injury. Brain Research. 2019;1706:48–57. doi: 10.1016/j.brainres.2018.10.023. [DOI] [PubMed] [Google Scholar]
- 125.Zhang Y., Fan B.Y., Pang Y.L., Shen W.Y., Wang X., Zhao C.X., et al. Neuroprotective effect of deferoxamine on erastininduced ferroptosis in primary cortical neurons. Neural Regeneration Research. 2020;15:1539–1545. doi: 10.4103/1673-5374.274344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ayton S., Fazlollahi A., Bourgeat P., Raniga P., Ng A., Lim Y.Y., et al. Cerebral quantitative susceptibility mapping predicts amyloid-beta-related cognitive decline. Brain. 2017;140:2112–2119. doi: 10.1093/brain/awx137. [DOI] [PubMed] [Google Scholar]
- 127.Derry P.J., Kent T.A. Correlating quantitative susceptibility mapping with cognitive decline in Alzheimer's disease. Brain. 2017;140:2069–2072. doi: 10.1093/brain/awx167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lou B.H., Jiang Y.W., Li C.M., Wu P.Y., Li S.H., Qin B., et al. Quantitative analysis of synthetic magnetic resonance imaging in alzheimer's disease. Frontiers in Aging Neuroscience. 2021;13 doi: 10.3389/fnagi.2021.638731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yan N., Zhang J.J. Iron metabolism, ferroptosis, and the links with alzheimer's disease. Frontiers in Neuroscience. 2020;13 doi: 10.3389/fnins.2019.01443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.van Bergen J.M.G., Li X., Hua J., Schreiner S.J., Steininger S.C., Quevenco F.C., et al. Colocalization of cerebral iron with amyloid beta in mild cognitive impairment. Scientific Reports. 2016;6 doi: 10.1038/srep35514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lupton M.K., Benyamin B., Proitsi P., Nyholt D.R., Ferreira M.A., Montgomery G.W., et al. No genetic overlap between circulating iron levels and alzheimer's disease. Journal of Alzheimer's Disease. 2017;59:85–99. doi: 10.3233/JAD-170027. [DOI] [PubMed] [Google Scholar]
- 132.Rogers J.T., Bush A.I., Cho H.H., Smith D.H., Thomson A.M., Friedlich A.L., et al. Iron and the translation of the amyloid precursor protein (APP) and ferritin mRNAs: riboregulation against neural oxidative damage in Alzheimer's disease. Biochemical Society Transactions. 2008;36:1282–1287. doi: 10.1042/BST0361282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Becerril-Ortega J., Bordji K., Freret T., Rush T., Buisson A. Iron overload accelerates neuronal amyloid-beta production and cognitive impairment in transgenic mice model of Alzheimer's disease. Neurobiology of Aging. 2014;35:2288–2301. doi: 10.1016/j.neurobiolaging.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 134.Peters D.G., Connor J.R., Meadowcroft M.D. The relationship between iron dyshomeostasis and amyloidogenesis in Alzheimer's disease: two sides of the same coin. Neurobiology of Disease. 2015;81:49–65. doi: 10.1016/j.nbd.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Telling N.D., Everett J., Collingwood J.F., Dobson J., van der Laan G., Gallagher J.J., et al. Iron Biochemistry is correlated with amyloid plaque morphology in an established mouse model of alzheimer's disease. Cell Chemical Biology. 2017;24:1205. doi: 10.1016/j.chembiol.2017.07.014. -+ [DOI] [PubMed] [Google Scholar]
- 136.Weiland A., Wang Y., Wu W., Lan X., Han X., Li Q., et al. Ferroptosis and its role in diverse brain diseases. Molecular Neurobiology. 2019;56:4880–4893. doi: 10.1007/s12035-018-1403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nikseresht S., Bush A.I., Ayton S. Treating Alzheimer's disease by targeting iron. British Journal of Pharmacology. 2019;176:3622–3635. doi: 10.1111/bph.14567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang Y.P., Mandelkow E. Tau in physiology and pathology. Nature Reviews Neuroscience. 2016;17:5–21. doi: 10.1038/nrn.2015.1. [DOI] [PubMed] [Google Scholar]
- 139.Joppe K., Roser A.E., Maass F., Lingor P. The contribution of iron to protein aggregation disorders in the central nervous system. Frontiers in Neuroscience. 2019;13 doi: 10.3389/fnins.2019.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kim A.C., Lim S., Kim Y.K. Metal ion effects on A and tau aggregation. International Journal of Molecular Sciences. 2018;19 doi: 10.3390/ijms19010128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rao S.S., Adlard P.A. Untangling tau and iron: exploring the interaction between iron and tau in neurodegeneration. Frontiers in Molecular Neuroscience. 2018;11 doi: 10.3389/fnmol.2018.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Spotorno N., Acosta-Cabronero J., Stomrud E., Lampinen B., Strandberg O.T., van Westen D., et al. Relationship between cortical iron and tau aggregation in Alzheimer's disease. Brain. 2020;143:1341–1349. doi: 10.1093/brain/awaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lei P., Ayton S., Finkelstein D.I., Spoerri L., Ciccotosto G.D., Wright D.K., et al. Tau deficiency induces parkinsonism with dementia by impairing APP-mediated iron export. Nature Medicine. 2012;18:291–295. doi: 10.1038/nm.2613. [DOI] [PubMed] [Google Scholar]
- 144.Tsatsanis A., Wong B.C.X., Gunn A.P., Ayton S., Bush A.I., Devos D., et al. Amyloidogenic processing of Alzheimer's disease beta-amyloid precursor protein induces cellular iron retention. Molecular Psychiatry. 2020;25:1958–1966. doi: 10.1038/s41380-020-0762-0. [DOI] [PubMed] [Google Scholar]
- 145.Endres D., van Elst L.T., Meyer S.A., Feige B., Nickel K., Bubl A., et al. Glutathione metabolism in the prefrontal brain of adults with high-functioning autism spectrum disorder: an MRS study. Molecular Autism. 2017;8 doi: 10.1186/s13229-017-0122-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Thapa A., Carroll N.J. Dietary modulation of oxidative stress in alzheimer's disease. International Journal of Molecular Sciences. 2017;18 doi: 10.3390/ijms18071583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cobley J.N., Fiorello M.L., Bailey D.M. 13 reasons why the brain is susceptible to oxidative stress. Redox Biology. 2018;15:490–503. doi: 10.1016/j.redox.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Masaldan S., Bush A.I., Devos D., Rolland A.S., Moreau C. Striking while the iron is hot: iron metabolism and ferroptosis in neurodegeneration. Free Radical Biology and Medicine. 2019;133:221–233. doi: 10.1016/j.freeradbiomed.2018.09.033. [DOI] [PubMed] [Google Scholar]
- 149.Cozza G., Rossetto M., Bosello-Travain V., Maiorino M., Roveri A., Toppo S., et al. Glutathione peroxidase 4-catalyzed reduction of lipid hydroperoxides in membranes: the polar head of membrane phospholipids binds the enzyme and addresses the fatty acid hydroperoxide group toward the redox center. Free Radical Biology and Medicine. 2017;112:1–11. doi: 10.1016/j.freeradbiomed.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 150.Seibt T.M., Proneth B., Conrad M. Role of GPX4 in ferroptosis and its pharmacological implication. Free Radical Biology and Medicine. 2019;133:144–152. doi: 10.1016/j.freeradbiomed.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 151.Ayton S., Wang Y.M., Diouf I., Schneider J.A., Brockman J., Morris M.C., et al. Brain iron is associated with accelerated cognitive decline in people with Alzheimer pathology. Molecular Psychiatry. 2020;25:2932–2941. doi: 10.1038/s41380-019-0375-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chen L.J., Hambright W.S., Na R., Ran Q.T. Ablation of the ferroptosis inhibitor glutathione peroxidase 4 in neurons results in rapid motor neuron degeneration and paralysis. Journal of Biological Chemistry. 2015;290:28097–28106. doi: 10.1074/jbc.M115.680090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Huang L., McClatchy D.B., Maher P., Liang Z.B., Diedrich J.K., Soriano-Castell D., et al. Intracellular amyloid toxicity induces oxytosis/ferroptosis regulated cell death. Cell Death & Disease. 2020;11 doi: 10.1038/s41419-020-03020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ashraf A., Jeandriens J., Parkes H.G., So P.W. Iron dyshomeostasis, lipid peroxidation and perturbed expression of cystine/glutamate antiporter in Alzheimer?s disease: evidence of ferroptosis. Redox Biology. 2020;32 doi: 10.1016/j.redox.2020.101494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhang Y.H., Wang D.W., Xu S.F., Zhang S., Fan Y.G., Yang Y.Y., et al. alpha-Lipoic acid improves abnormal behavior by mitigation of oxidative stress, inflammation, ferroptosis, and tauopathy in P301S Tau transgenic mice. Redox Biology. 2018;14:535–548. doi: 10.1016/j.redox.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Fernandez-Bertolez N., Costa C., Bessa M.J., Park M., Carriere M., Dussert F., et al. Assessment of oxidative damage induced by iron oxide nanoparticles on different nervous system cells. Mutation Research: Genetic Toxicology. 2019;845 doi: 10.1016/j.mrgentox.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 157.Morris G., Berk M., Carvalho A.F., Maes M., Walker A.J., Puri B.K. Why should neuroscientists worry about iron? The emerging role of ferroptosis in the pathophysiology of neuroprogressive diseases. Behavioural Brain Research. 2018;341:154–175. doi: 10.1016/j.bbr.2017.12.036. [DOI] [PubMed] [Google Scholar]
- 158.Leong Y.Q., Ng K.Y., Chye S.M., Ling A.P.K., Koh R.Y. Mechanisms of action of amyloid-beta and its precursor protein in neuronal cell death. Metabolic Brain Disease. 2020;35:11–30. doi: 10.1007/s11011-019-00516-y. [DOI] [PubMed] [Google Scholar]
- 159.Ayton S., Lei P. Nigral iron elevation is an invariable feature of Parkinson's disease and is a sufficient cause of neurodegeneration. BioMed Research International. 2014;2014 doi: 10.1155/2014/581256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Do Van B., Gouel F., Jonneaux A., Timmerman K., Gele P., Petrault M., et al. Ferroptosis, a newly characterized form of cell death in Parkinson's disease that is regulated by PKC. Neurobiology of Disease. 2016;94:169–178. doi: 10.1016/j.nbd.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 161.Belarbi K., Cuvelier E., Destee A., Gressier B., Chartier-Harlin M.C. NADPH oxidases in Parkinson's disease: a systematic review. Molecular Neurodegeneration. 2017;12 doi: 10.1186/s13024-017-0225-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sian-Hulsmann J., Riederer P. The role of alpha-synuclein as ferrireductase in neurodegeneration associated with Parkinson's disease. Journal of Neural Transmission. 2020;127:749–754. doi: 10.1007/s00702-020-02192-0. [DOI] [PubMed] [Google Scholar]
- 163.Enogieru A.B., Omoruyi S.I., Hiss D.C., Ekpo O.E. Potential antiparkinsonian agents derived from South African medicinal plants. Journal of Herbal Medicine. 2018;13:1–7. [Google Scholar]
- 164.Kim T.Y., Leem E., Lee J.M., Kim S.R. Control of reactive oxygen species for the prevention of Parkinson's disease: the possible application of flavonoids. Antioxidants-Basel. 2020;9 doi: 10.3390/antiox9070583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Abeyawardhane D.L., Fernandez R.D., Murgas C.J., Heitger D.R., Forney A.K., Crozier M.K., et al. Iron redox chemistry promotes antiparallel oligomerization of alpha-synuclein. Journal of the American Chemical Society. 2018;140:5028–5032. doi: 10.1021/jacs.8b02013. [DOI] [PubMed] [Google Scholar]
- 166.Feng G.S., Zhang Z.J., Bao Q.Q., Zhang Z.J., Zhou L.B., Jiang J., et al. Protective effect of chinonin in MPTP-induced C57BL/6 mouse model of Parkinson's disease. Biological & Pharmaceutical Bulletin. 2014;37:1301–1307. doi: 10.1248/bpb.b14-00128. [DOI] [PubMed] [Google Scholar]
- 167.Takahashi S., Hisatsune A., Kurauchi Y., Seki T., Katsuki H. Polysulfide protects midbrain dopaminergic neurons from MPP+-induced degeneration via enhancement of glutathione biosynthesis. Journal of Pharmacological Sciences. 2018;137:47–54. doi: 10.1016/j.jphs.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 168.Wu C., Zhao W., Yu J., Li S., Lin L., Chen X. Induction of ferroptosis and mitochondrial dysfunction by oxidative stress in PC12 cells. Scientific Reports. 2018;8:574. doi: 10.1038/s41598-017-18935-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Angelova P.R., Choi M.L., Berezhnov A.V., Horrocks M.H., Hughes C.D., De S., et al. Alpha synuclein aggregation drives ferroptosis: an interplay of iron, calcium and lipid peroxidation. Cell Death & Differentiation. 2020;27:2781–2796. doi: 10.1038/s41418-020-0542-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sun Y., He L., Wang T., Hua W., Qin H., Wang J., et al. Activation of p62-keap1-Nrf2 pathway protects 6-hydroxydopamine-induced ferroptosis in dopaminergic cells. Molecular Neurobiology. 2020;57:4628–4641. doi: 10.1007/s12035-020-02049-3. [DOI] [PubMed] [Google Scholar]
- 171.Tian Y., Lu J., Hao X.Q., Li H., Zhang G.Y., Liu X.L., et al. FTH1 inhibits ferroptosis through ferritinophagy in the 6-OHDA model of Parkinson's disease. Neurotherapeutics. 2020;17:1796–1812. doi: 10.1007/s13311-020-00929-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zhang P., Chen L., Zhao Q.Q., Du X.X., Bi M.X., Li Y., et al. Ferroptosis was more initial in cell death caused by iron overload and its underlying mechanism in Parkinson's disease. Free Radical Biology and Medicine. 2020;152:227–234. doi: 10.1016/j.freeradbiomed.2020.03.015. [DOI] [PubMed] [Google Scholar]
- 173.Bardile C.F., Garcia-Miralles M., Caron N.S., Rayan N.A., Langley S.R., Harmston N., et al. Intrinsic mutant HTT-mediated defects in oligodendroglia cause myelination deficits and behavioral abnormalities in Huntington disease. Proceedings of the National Academy of Sciences of the United States of America. 2019;116:9622–9627. doi: 10.1073/pnas.1818042116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ayala-Pena S. Role of oxidative DNA damage in mitochondrial dysfunction and Huntington's disease pathogenesis. Free Radical Biology and Medicine. 2013;62:102–110. doi: 10.1016/j.freeradbiomed.2013.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Chen C.M., Wu Y.R., Cheng M.L., Liu J.L., Lee Y.M., Lee P.W., et al. Increased oxidative damage and mitochondrial abnormalities in the peripheral blood of Huntington's disease patients. Biochemical and Biophysical Research Communications. 2007;359:335–340. doi: 10.1016/j.bbrc.2007.05.093. [DOI] [PubMed] [Google Scholar]
- 176.Crotti A., Benner C., Kerman B.E., Gosselin D., Lagier-Tourenne C., Zuccato C., et al. Mutant Huntingtin promotes autonomous microglia activation via myeloid lineage-determining factors. Nature Neuroscience. 2014;17:513–U558. doi: 10.1038/nn.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Hatami A., Zhu C.N., Relano-Gines A., Elias C., Galstyan A., Jun M., et al. Deuterium-reinforced linoleic acid lowers lipid peroxidation and mitigates cognitive impairment in the Q140 knock in mouse model of Huntington's disease. FEBS Journal. 2018;285:3002–3012. doi: 10.1111/febs.14590. [DOI] [PubMed] [Google Scholar]
- 178.Agrawal S., Fox J., Thyagarajan B., Fox J.H. Brain mitochondrial iron accumulates in Huntington's disease, mediates mitochondrial dysfunction, and can be removed pharmacologically. Free Radical Biology and Medicine. 2018;120:317–329. doi: 10.1016/j.freeradbiomed.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Skouta R., Dixon S.J., Wang J.L., Dunn D.E., Orman M., Shimada K., et al. Ferrostatins inhibit oxidative lipid damage and cell death in diverse disease models. Journal of the American Chemical Society. 2014;136:4551–4556. doi: 10.1021/ja411006a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Reddy P.H., Shirendeb U.P. Mutant huntingtin, abnormal mitochondrial dynamics, defective axonal transport of mitochondria, and selective synaptic degeneration in Huntington's disease. BBA Molecular Basis of Disease. 2012;1822:101–110. doi: 10.1016/j.bbadis.2011.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Mason R.P., Casu M., Butler N., Breda C., Campesan S., Clapp J., et al. Glutathione peroxidase activity is neuroprotective in models of Huntington's disease. Nature Genetics. 2013;45 doi: 10.1038/ng.2732. 1249-U1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kumar P., Kalonia H., Kumar A. Nitric oxide mechanism in the protective effect of antidepressants against 3-nitropropionic acid-induced cognitive deficit, glutathione and mitochondrial alterations in animal model of Huntington's disease. Behavioural Pharmacology. 2010;21:217–230. doi: 10.1097/fbp.0b013e32833a5bf4. [DOI] [PubMed] [Google Scholar]
- 183.Mao Z.K., Choo Y.S., Lesort M. Cystamine and cysteamine prevent 3-NP-induced mitochondrial depolarization of Huntington's disease knock-in striatal cells. European Journal of Neuroscience. 2006;23:1701–1710. doi: 10.1111/j.1460-9568.2006.04686.x. [DOI] [PubMed] [Google Scholar]
- 184.Agrawal S., Fox J., Thyagarajan B., Fox J.H. Brain mitochondrial iron accumulates in Huntington's disease, mediates mitochondrial dysfunction, and can be removed pharmacologically. Free Radical Biology and Medicine. 2018;120:317–329. doi: 10.1016/j.freeradbiomed.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Rosas H.D., Chen Y.I., Doros G., Salat D.H., Chen N.K., Kwong K.K., et al. Alterations in brain transition metals in huntington disease an evolving and intricate story. Archives of neurology. 2012;69:887–893. doi: 10.1001/archneurol.2011.2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.van Bergen J.M.G., Hua J., Unschuld P.G., Lim I.A.L., Jones C.K., Margolis R.L., et al. Quantitative susceptibility mapping suggests altered brain iron in premanifest huntington disease. American Journal of Neuroradiology. 2016;37:789–796. doi: 10.3174/ajnr.A4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Bartzokis G., Lu P.H., Tishler T.A., Fong S.M., Oluwadara B., Finn J.P., et al. Myelin breakdown and iron changes in Huntington's disease: pathogenesis and treatment implications. Neurochemical Research. 2007;32:1655–1664. doi: 10.1007/s11064-007-9352-7. [DOI] [PubMed] [Google Scholar]
- 188.Simmons D.A., Casale M., Alcon B., Pham N., Narayan N., Lynch G. Ferritin accumulation in dystrophic microglia is an early event in the development of Huntington's disease. Glia. 2007;55:1074–1084. doi: 10.1002/glia.20526. [DOI] [PubMed] [Google Scholar]
- 189.Chen J.F., Marks E., Lai B., Zhang Z.J., Duce J.A., Lam L.Q., et al. Iron accumulates in huntington's disease neurons: protection by deferoxamine. PLoS One. 2013;8 doi: 10.1371/journal.pone.0077023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Charvin D., Vanhoutte P., Pages C., Borelli E., Caboche J. Unraveling a role for dopamine in Huntington's disease: the dual role of reactive oxygen species and D2 receptor stimulation (vol 102, pg 12218, 2005) Proceedings of the National Academy of Sciences of the United States of America. 2005;102:16530. doi: 10.1073/pnas.0502698102. 16530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Berggren K.L., Chen J.F., Fox J., Miller J., Dodds L., Dugas B., et al. Neonatal iron supplementation potentiates oxidative stress, energetic dysfunction and neurodegeneration in the R6/2 mouse model of Huntington's disease. Redox Biology. 2015;4:363–374. doi: 10.1016/j.redox.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Deschauer M., Gaul C., Behrmann C., Prokisch H., Zierz S., Haack T.B. C19orf12 mutations in neurodegeneration with brain iron accumulation mimicking juvenile amyotrophic lateral sclerosis. Journal of Neurology. 2012;259:2434–2439. doi: 10.1007/s00415-012-6521-7. [DOI] [PubMed] [Google Scholar]
- 193.Bonnefont-Rousselot D., Lacomblez L., Jaudon M.C., Lepage S., Salachas F., Bensimon G., et al. Blood oxidative stress in amyotrophic lateral sclerosis. Journal of Neurological Sciences. 2000;178:57–62. doi: 10.1016/s0022-510x(00)00365-8. [DOI] [PubMed] [Google Scholar]
- 194.Devos D., Moreau C., Kyheng M., Garcon G., Rolland A.S.O., Blasco H., et al. A ferroptosis-based panel of prognostic biomarkers for Amyotrophic Lateral Sclerosis. Scientific Reports. 2019;9 doi: 10.1038/s41598-019-39739-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Zhang Y.J., Fan D.S., Liu X.Y., Liu X.L., He J., Zhang N., et al. hTBK1-c.978T > A mutation promotes the ferroptosis in NSC-34 cells via mediation of KEAP1/NRF2/p62 signaling. American Journal of Tourism Research. 2020;12:7386. -+ [PMC free article] [PubMed] [Google Scholar]
- 196.Tamarit J., Obis E., Ros J. Oxidative stress and altered lipid metabolism in Friedreich ataxia. Free Radical Biology and Medicine. 2016;100:138–146. doi: 10.1016/j.freeradbiomed.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 197.Wong A., Yang J., Cavadini P., Gellera C., Lonnerdal B., Taroni F., et al. The Friedreich's ataxia mutation confers cellular sensitivity to oxidant stress which is rescued by chelators of iron and calcium and inhibitors of apoptosis. Human Molecular Genetics. 1999;8:425–430. doi: 10.1093/hmg/8.3.425. [DOI] [PubMed] [Google Scholar]
- 198.Li K.Y., Besse E.K., Ha D., Kovtunovych G., Rouault T.A. Iron-dependent regulation of frataxin expression: implications for treatment of Friedreich ataxia. Human Molecular Genetics. 2008;17:2265–2273. doi: 10.1093/hmg/ddn127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Cotticelli M.G., Xia S.J., Lin D., Lee T., Terrab L., Wipf P., et al. Ferroptosis as a novel therapeutic target for friedreich's ataxia. Journal of Pharmacology and Experimental Therapeutics. 2019;369:47–54. doi: 10.1124/jpet.118.252759. [DOI] [PubMed] [Google Scholar]
- 200.Du J., Zhou Y., Li Y.C., Xia J., Chen Y.J., Chen S.F., et al. Identification of Frataxin as a regulator of ferroptosis. Redox Biology. 2020;32 doi: 10.1016/j.redox.2020.101483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.La Rosa P., Petrillo S., Turchi R., Berardinelli F., Schirinzi T., Vasco G., et al. The Nrf2 induction prevents ferroptosis in Friedreich's Ataxia. Redox Biology. 2021;38 doi: 10.1016/j.redox.2020.101791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Savaskan N.E., Heckel A., Hahnen E., Engelhorn T., Doerfler A., Ganslandt O., et al. Small interfering RNA-mediated xCT silencing in gliomas inhibits neurodegeneration and alleviates brain edema. Nature Medicine. 2008;14:629–632. doi: 10.1038/nm1772. [DOI] [PubMed] [Google Scholar]
- 203.Robert S.M., Buckingham S.C., Campbell S.L., Robel S., Holt K.T., Ogunrinu-Babarinde T., et al. SLC7A11 expression is associated with seizures and predicts poor survival in patients with malignant glioma. Science Translational Medicine. 2015;7 doi: 10.1126/scitranslmed.aaa8103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Chen D., Fan Z., Rauh M., Buchfelder M., Eyupoglu I.Y., Savaskan N. ATF4 promotes angiogenesis and neuronal cell death and confers ferroptosis in a xCT-dependent manner. Oncogene. 2017;36:5593–5608. doi: 10.1038/onc.2017.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Fan Z., Wirth A.K., Chen D., Wruck C.J., Rauh M., Buchfelder M., et al. Nrf2-Keap1 pathway promotes cell proliferation and diminishes ferroptosis. Oncogenesis. 2017;6 doi: 10.1038/oncsis.2017.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Cheng J., Fan Y.Q., Liu B.H., Zhou H., Wang J.M., Chen Q.X. ACSL4 suppresses glioma cells proliferation via activating ferroptosis. Oncology Reports. 2020;43:147–158. doi: 10.3892/or.2019.7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Wang Z.Q., Ding Y., Wang X.Z., Lu S., Wang C.C., He C., et al. Pseudolaric acid B triggers ferroptosis in glioma cells via activation of Nox4 and inhibition of xCT. Cancer Letters. 2018;428:21–33. doi: 10.1016/j.canlet.2018.04.021. [DOI] [PubMed] [Google Scholar]
- 208.Yee P.P., Wei Y.J., Kim S.Y., Lu T., Chih S.Y., Lawson C., et al. Neutrophil-induced ferroptosis promotes tumor necrosis in glioblastoma progression. Nature Communications. 2020;11 doi: 10.1038/s41467-020-19193-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Chen Y.B., Mi Y.J., Zhang X.F., Ma Q., Song Y.C., Zhang L.W., et al. Dihydroartemisinin-induced unfolded protein response feedback attenuates ferroptosis via PERK/ATF4/HSPA5 pathway in glioma cells. Journal of Experimental & Clinical Cancer Research. 2019;38 doi: 10.1186/s13046-019-1413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Chen Y., Li N., Wang H.J., Wang N.N., Peng H., Wang J., et al. Amentoflavone suppresses cell proliferation and induces cell death through triggering autophagy-dependent ferroptosis in human glioma. Life Sciences. 2020;247 doi: 10.1016/j.lfs.2020.117425. [DOI] [PubMed] [Google Scholar]
- 211.Gao X.C., Guo N., Xu H., Pan T., Lei H., Yan A.L., et al. Ibuprofen induces ferroptosis of glioblastoma cells via downregulation of nuclear factor erythroid 2-related factor 2 signaling pathway. Anti-Cancer Drugs. 2020;31:27–34. doi: 10.1097/CAD.0000000000000825. [DOI] [PubMed] [Google Scholar]
- 212.Jiang T., Chu J., Chen H.J.T., Cheng H., Su J.J., Wang X.C., et al. Gastrodin inhibits H2O2-induced ferroptosis through its antioxidative effect in rat glioma cell line C6. Biological & Pharmaceutical Bulletin. 2020;43:480–487. doi: 10.1248/bpb.b19-00824. [DOI] [PubMed] [Google Scholar]
- 213.Qiu C., Zhang X., Huang B., Wang S., Zhou W.J., Li C., et al. Disulfiram, a ferroptosis inducer, triggers lysosomal membrane permeabilization by up-regulating ROS in glioblastoma. OncoTargets and Therapy. 2020;13:10631–10640. doi: 10.2147/OTT.S272312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Kim T.K., Park D., Ban Y.H., Cha Y., An E.S., Choi J., et al. Improvement by human oligodendrocyte progenitor cells of neurobehavioral disorders in an experimental model of neonatal periventricular leukomalacia. Cell Transplantation. 2018;27:1168–1177. doi: 10.1177/0963689718781330. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.