Abstract
Objectives
To evaluate the antiplaque and antibacterial efficacy of commercially available mouthwashes containing aloe vera (AV), hydrogen peroxide (HP), and cetylpyridinium chloride (CPC) in a 4-day plaque regrowth study.
Methods
Plaque score and salivary samples were assessed (Day-0 and Day-4) in 96 participants in a randomised, double-blind prospective parallel-arm 4-day plaque regrowth study. Participants were divided into five groups who refrained from engaging in regular oral hygiene measures during the study period and used commercially available mouthwashes containing AV, HP, and CPC as test products with distilled water (DW) and chlorhexidine (CHX) mouthwash as negative and positive controls, respectively. Salivary bacterial count was expressed as colony-forming units (CFU) (culture method).
Results
There was a significant difference both in plaque score (p < 0.001) and in CFU (p < 0.001) among the study mouthwashes at Day-4. The plaque score and CFU of AV were significantly higher and lower than those of CHX and DW, respectively. The plaque score of HP was significantly higher than that of AV (p = 0.016) and CPC (p < 0.001). No significant difference was observed between AV and CPC (p = 0.70). Moreover, the CFU of HP was significantly higher than that of CPC (p = 0.04). There was no statistically significant difference between the CFU of mouthwashes containing AV and HP (p = 0.912) or AV and CPC (p = 0.280). No significant difference was seen in the inhibition of plaque and salivary bacterial count between AV, HP, and CPC.
Conclusion
The antiplaque and antibacterial efficacy of commercially available AV mouthwash was similar to that of CPC and significantly better than that of HP mouthwash and can be a natural alternative to chemically formulated mouthwashes.
Keywords: Antiplaque, Biofilm, Cetylpyridinium chloride, Clinical trial, Hydrogen peroxide
الملخص
أهداف البحث
تقييم الفعالية المضادة للبكتيريا والترسبات لغسول الفم المتاح تجاريًا المحتوي على الصبار وبيروكسيد الهيدروجين وكلوريد سيتيل بيريدينيوم من خلال دراسة إعادة نمو الترسبات لمدة 4 أيام.
طرق البحث
تم تقييم درجة الترسبات والعينات اللعابية (اليوم 0 واليوم 4) لعدد 96 مشاركا في تجربة عشوائية منضبطة متوازية الذراع لمدة 4 أيام. تم تقسيم المشاركين إلى خمس مجموعات امتنعت عن تدابير نظافة الفم المعتادة خلال فترة الدراسة واستخدموا غسول الفم المتاح تجاريا والذي يحتوي على الصبار أو بيروكسيد الهيدروجين أو كلوريد سيتيل بيريدينيوم كمنتجات اختبار مع الماء المقطر كعنصر تحكم سلبي وغسول الفم الكلورهيكسيدين كعنصر تحكم إيجابي. تم التعبير عن العد البكتيري اللعابي كوحدات تشكيل مستعمرة (طريقة الاستزراع).
النتائج
كان هناك فرق كبير في درجة الترسبات و العد البكتيري اللعابي بين غسولات الفم في اليوم الرابع من الدراسة. كانت درجة الترسبات و العد البكتيري اللعابي لغسول الفم المحتوي على الصبار أقل بكثير من الماء المقطر وأعلى بكثير من غسول الفم الكلورهيكسيدين. كانت درجة الترسبات لغسول الفم المحتوي على بيروكسيد الهيدروجين أعلى بكثير من غسول الفم المحتوي على الصبار وغسول الفم المحتوي على كلوريد سيتيل بيريدينيوم . لم يلاحظ أي فرق بين غسول الفم المحتوي على الصبار و كلوريد سيتيل بيريدينيوم . أيضًا ، العد البكتيري اللعابي لبيروكسيد الهيدروجين كان أعلى من كلوريد سيتيل بيريدينيوم. لم يكن هناك فرق إحصائي بين العد البكتيري اللعابي لغسول الفم المحتوي على الصبار وبيروكسيد الهيدروجين أو الصبار وكلوريد سيتيل بيريدينيوم لم يلاحظ أي فرق للحد من الترسبات و العد البكتيري اللعابي بين غسول الفم المتاح تجاريا المحتوي على الصبار وبيروكسيد الهيدروجين وكلوريد سيتيل بيريدينيوم.
الاستنتاجات
كانت الفعالية المضادة للبلاك والمضادة للبكتيريا لغسول الفم المتاح تجاريا المحتوي على الصبار مماثلة لـغسول الفم المحتوي على كلوريد سيتيل بيريدينيوم وأفضل بكثير من غسول الفم المحتوي على بيروكسيد الهيدروجين ومن الممكن أن تكون بديلا طبيعيا لغسول الفم المصنوع كيميائيا.
الكلمات المفتاحية: تجربة سريرية, بيروكسيد الهيدروجين, كلوريد سيتيل بيريدينيوم, مضاد الترسبات, غشاء حيوي
Introduction
Microbial dental plaque is a tenaciously adhering biofilm that grows intra-orally on hard and soft tissues.1 Bacterial colonies can accumulate on tooth surfaces and lead to periodontal disease.2 Gram-positive aerobic bacteria are early colonisers of dental plaque, followed by gram-negative anaerobic and fusiform bacteria.3,4 The primary goal of periodontal disease prevention is plaque reduction.5 Although tooth brushing is the most reliable source for mechanical plaque control, factors such as lack of manual dexterity, skill, or motivation hamper its effectiveness.6 Hence, chemical antiplaque agents in various formulations have been introduced to improve oral health. Rinsing with mouthwashes is easier to perform than tooth brushing, and it aids in controlling supragingival plaque and gingivitis along with mechanical plaque control.7
Although chemical plaque control measures help maintain oral health, they come with a fair share of side effects.8, 9, 10 Therefore, there is a constant quest for mouthwashes with comparable efficacy and fewer side effects. In recent times, much emphasis has been placed on natural products that can aid oral hygiene with minimal or no side effects. Aloe vera (AV) is a naturally occurring plant and has been reported to have several medicinal properties.11, 12, 13, 14 Aloe vera has been widely used medicinally, yet there is little and inconclusive evidence of its antiplaque and antibacterial efficacy.15 Hydrogen peroxide (HP) is another compound that acts on microorganisms by releasing nascent oxygen and has several therapeutic uses, including antiplaque, anti-inflammatory, and antimicrobial activity.16
Although these formulations have been studied for their antiplaque and antibacterial activities, most studies have examined their use in conjunction with regular mechanical plaque control measures. There is thus limited evidence on the efficacy of these commercially available mouthwashes in inhibiting de-novo plaque regrowth using a non-brushing model, which warrants exploration. Therefore, the objective of this study was to evaluate the antiplaque and antibacterial efficacy of commercially available mouthwashes containing aloe vera, hydrogen peroxide, and cetyl pyridinium chloride in a 4-day plaque regrowth study.
Materials and Methods
Study population and design
This was a randomised, double-blind, placebo-controlled, prospective parallel-arm clinical and microbiological trial (Figure 1). All participants were informed about the study and provided their written consent before participating in the study. All participants included in the study were undergraduate dental students at our institute who met the selection criteria: falling within the age group of 18 to 25 years, having an erupted dentition with at least 20 evaluable teeth (minimum five teeth per quadrant), and being willing to participate and follow the schedule. Those excluded had a history of smoking, tobacco consumption, scaling in the past three months; had participated in similar investigations in the past four weeks; or had evidence of gingival inflammation, periodontitis, untreated dental caries, and removable or fixed prosthesis and orthodontic appliances. Those with systemic disease/conditions (diabetes mellitus, xerostomia, oral desquamative lesions, or those who were immunocompromised or physically or mentally challenged) that might influence the conduct of the study, who had used antibiotics within three months of the baseline, had known allergies to any mouthwash, pharmaceutical products, or components in the test products and who had used any mouthwash in the past four weeks were also excluded. Pregnant women and lactating mothers were not included.
Figure 1.
CONSORT flow Chart.
Sample size determination
A-priori analysis conducted using a software program17 at the 5% significance level with a power value of 0.8 and effect size of 0.4 estimated the total sample size to be 80. Since there were five groups, each group was allocated with twenty participants considering a ten percent probable loss to follow-up.
Study procedures
The clinical trial comprised the screening stage and data collection at baseline (Day-0) and Day-4. During the screening visit, the participants' detailed medical history was assessed, and oral examination was conducted by a single examiner (RS) who determined the participants’ eligibility for the study and enrolled them into the trial according to the inclusion criteria. The study was preceded by thorough prophylaxis by the same experienced examiner one week prior to the allocation, and the participants were asked to continue with their regular oral hygiene measures until further instructions.
Randomisation, concealment, and allocation
A lottery method was used to randomly allocate mouthwashes and code numbers to identify participants throughout the study. Two independent designated faculty members assigned a unique code (bearing mouthwash type and participant number) at the institute. The faculty member then carefully sealed each code in separate identical envelopes and dispensed 80 ml of study mouthwashes according to the code in identical amber colour bottles, which were indistinguishable in terms of colour, packaging, and labelling. Participants were assigned to either Group I (DW): distilled water (Negative control); Group II (CHX): 0.2% chlorhexidine gluconate containing mouthwash (Clohex®, Dr Reddy Laboratories Ltd, India (Positive control)); Group III (AV): 20% aloe vera containing mouthwash (The Natural Dentist®, Revive Personal Products Company, NJ, USA); Group IV (HP): 1.5% hydrogen peroxide containing mouthwash (Colgate® Peroxyl® Mouth Sore Rinse, Colgate Palmolive Company, NY, USA); or Group V (CPC): 0.075% cetylpyridinium chloride containing mouthwash (Colgate Total® advanced Pro-shield ®, Colgate Palmolive Company, NY, USA). Only the two previously designated faculty members at the institute, who were not the study investigators, were aware of the codes used for the actual identification of participants and mouthwashes.
On Day-0, each participant opened a sealed envelope to receive the code. Plaque scores were recorded following a plaque disclosing agent (PLAKSEE MD, ICPA, Ankleshwar, India), and salivary samples were collected in the morning on Day-0 and Day-4. The disclosed plaque was recorded by a single examiner (NP) from four surfaces (mesiobuccal, buccal, distobuccal, lingual/palatal) of all fully erupted permanent teeth, excluding the third molars using a plaque index.18 For the collection of unstimulated saliva, the same examiner (NP) asked the participants to sit comfortably upright and slightly lean forward and tip their heads down to pool saliva in their mouth, thus allowing the saliva to drip from the lower lip into a graduated sterile container (Drooling method)19 to collect two ml of saliva.
After data at baseline (Day-0) were collected, each participant was provided with a bottle containing the study mouthwash labelled with their code and was asked to refrain from tooth brushing, flossing, or any other mechanical oral hygiene maintenance measures during the study period. During that period, participants were instructed to rinse 10 ml of the allocated mouthwash for 30s twice daily (in the morning and evening after eating) and not to rinse with water. Moreover, participants were instructed to avoid rinsing their mouths with water or eating food during the first hour after rinsing with the allocated mouthwash. On Day-4, participants reported for data collection and returned their allocated bottles.
A clinical coordinator (RM) who was not involved in the randomisation or allocation sent a telephone message to each participant every day during the study period to remind them to rinse and to immediately report the occurrence of any side effects or adverse events. They were interviewed and examined to record their reported side effects or adverse events.
Microbiological assessment (salivary bacterial count)
The collected salivary samples were code-labelled and immediately transported to the Department of Microbiology for further processing by the microbiologist (AKP) within 4 h. The Miles and Misra Method20 was used to determine the number of CFU of bacteria in the salivary samples. Serial dilution of the inoculums was conducted using phosphate buffer saline (1X to 9X). All culture plates (Nutrient agar, MacConkey agar and blood agar (Hi–Media Laboratories Pvt. Limited, Mumbai, India)) were inoculated with each dilution. Using spread plate techniques, 20 μl inoculum drops were absorbed in the culture plates and were allowed to dry for 15–20 min. All culture plates were evenly divided into eight sectors and appropriately labelled to facilitate their traceability. In each sector, 1 × 20 μl of the dilution was dropped onto the surface of the agar to allow drop spread. Care was taken not to touch or contaminate the surface of agar in the culture plates and they were incubated at 37 °C for 18 to 24 h. Colonies were counted with a magnifying colony counter. Each sector was observed for growth of microorganisms. A luxuriant growth was observed at higher concentrations throughout the drop area, and tiny colonies were frequently united. Colonies were identified and counted in the sector with the largest concentration of full-size discrete colonies. To increase the certainty of the results, the average reading of three plates was considered. The number of colony-forming units (CFU) per ml from the original aliquot per sample was calculated by multiplying the colony count for a dilution with 50 times the dilution factor.
Statistical analysis
The collected data were analysed using a statistical software package (IBM SPSS Statistics 20, Chicago, Illinois, USA). The distribution of variables was checked as per the Shapiro–Wilk test for normality. An intra-examiner reliability and reproducibility assessment of the single expert examiner (NP) who recorded the plaque score was carried out using repeated measurements (30 min interval) and yielded a satisfactory score (κ > 0.8). The Kruskal–Wallis test was used to compare the mean differences in age among the five groups. A Chi Square Test assessed the difference in the distribution of gender. A one-way analysis of variance (ANOVA) was used to compare the scores between the groups. Once it was established that significant differences existed between the mouthwashes, a post-hoc Tukey's test was carried out for multiple comparisons to determine which group differed from the others. Additionally, the Student's paired t-test was used to compare observations within the study group before and after the intervention. The significance level was set at p ≤ 0.05.
Results
Study population
Of the 350 screened participants, 100 participants were enrolled in our study. After the start of the study, four participants could not continue due to personal reasons. The remaining 96 participants (45 males, 51 females; age range, 18–23 years; mean 20.58 ± 1.18 years) completed the rinsing regimens in a satisfactory manner without any severe side effects. Table 1 presents the demographic characteristics of the participants. There were no statistically significant differences in the age of participants or the gender distribution among the five treatment groups.
Table 1.
Demographic characteristics of participants.
| DW (n = 19) |
CHX (n = 20) |
AV (n = 19) |
HP (n = 19) |
CPC (n = 19) |
P value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| Age (years) | 20.68 | 1.25 | 20.80 | 1.51 | 20.32 | 1.11 | 20.63 | 1.01 | 20.47 | 0.96 | 0.597∗ |
| Male/Female | 12/7 | 12/8 | 6/13 | 12/7 | 9/10 | 0.222# | |||||
DW = Distilled water; CHX= Chlorhexidine; AV = Aloe vera; HP= Hydrogen peroxide; CPC= Cetylpyridinium chloride; ∗ Kruskal–Wallis test; # Chi Squared Test; n = No. of participants; SD = Standard Deviation; Significant at p < 0.05.
Plaque score
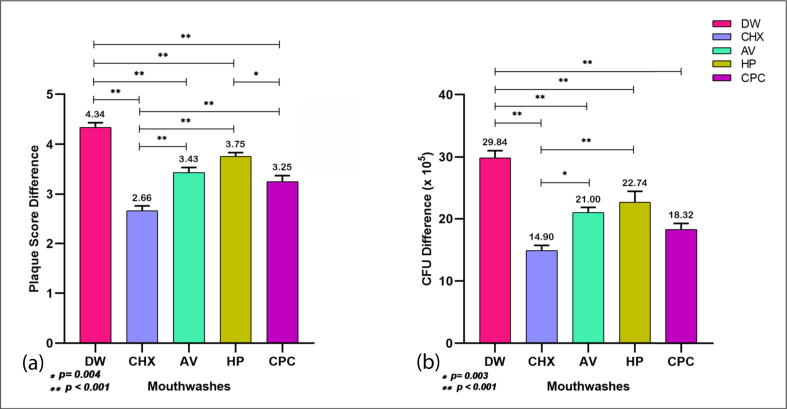
There was no statistically significant difference in mean plaque score (p = 0.794) among the five groups at baseline, but the difference was statistically significant (p < 0.001) at Day-4, with the mean plaque score being highest in the negative control group (distilled water) and lowest in the positive control (CHX) group (Table 2). Among the test mouthwashes, the plaque score with HP (4.13 ± 0.25) was highest and was followed by AV (3.75 ± 0.45) and CPC (3.60 ± 0.42). A post-hoc analysis (Table 3) of Day-4 revealed that the plaque score of AV was significantly lower than that of HP (p = 0.016) and comparable to that of CPC (p = 0.70). In addition, the plaque score of HP was significantly higher than that of CPC (p < 0.001). There was a statistically significant increase (p < 0.001) in the plaque scores of all mouthwashes on Day-4 in comparison to baseline (Table 4), and the increase was observed to be lowest with CHX (Figure 2), indicating the highest inhibition of plaque growth from baseline. Multiple comparisons revealed that there was no statistically significant difference in the inhibition of plaque from baseline by the AV mouthwash when compared with HP (p = 0.147) and CPC (p = 0.68) (Figure 2).
Table 2.
Comparison of plaque score and salivary bacterial count (CFU x 105) at baseline and at Day-4.
| Parameter | Time point | Group | Mean | SD | 95% CI |
p value∗ | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Plaque Score | At Day-0 F = 0.42; df = 4 |
DW | 0.31 | 0.23 | 0.20 | 0.42 | 0.794 |
| CHX | 0.33 | 0.17 | 0.25 | 0.41 | |||
| AV | 0.32 | 0.07 | 0.28 | 0.35 | |||
| HP | 0.37 | 0.19 | 0.28 | 0.47 | |||
| CPC | 0.35 | 0.18 | 0.26 | 0.43 | |||
| At Day-4 F = 55.59; df = 4 | DW | 4.64 | 0.24 | 4.53 | 4.76 | <0.001 | |
| CHX | 2.99 | 0.40 | 2.80 | 3.17 | |||
| AV | 3.75 | 0.45 | 3.53 | 3.97 | |||
| HP | 4.13 | 0.25 | 4.01 | 4.25 | |||
| CPC | 3.60 | 0.42 | 3.39 | 3.80 | |||
|
Salivary Bacterial Count CFU (X 105) |
At Day-0 F = 0.31; df = 4 |
DW | 24.00 | 2.60 | 22.75 | 25.25 | 0.873 |
| CHX | 23.75 | 2.22 | 22.71 | 24.79 | |||
| AV | 24.58 | 2.52 | 23.36 | 25.80 | |||
| HP | 24.16 | 2.43 | 22.99 | 25.33 | |||
| CPC | 24.21 | 2.30 | 23.10 | 25.32 | |||
| At Day-4 F = 27.52; df = 4 | DW | 53.84 | 3.67 | 52.07 | 55.61 | <0.001 | |
| CHX | 38.65 | 2.80 | 37.34 | 39.96 | |||
| AV | 45.58 | 2.81 | 44.22 | 46.94 | |||
| HP | 46.89 | 8.27 | 42.91 | 50.88 | |||
| CPC | 42.53 | 3.91 | 40.64 | 44.41 | |||
DW = Distilled water; CHX= Chlorhexidine; AV = Aloe vera; HP= Hydrogen peroxide; CPC= Cetylpyridinium chloride; CFU = Colony Forming Unit ∗ One way ANOVA; SD = Standard Deviation; CI = Confidence Interval; Significant at p < 0.05.
Table 3.
Post–hoc multiple comparisons of plaque score and salivary bacterial count (CFU X 105) at Day-4.
| Parameter | GP | DW |
CHX |
AV |
HP |
||||
|---|---|---|---|---|---|---|---|---|---|
| Mean Diff | p value∗ | Mean Diff | p value∗ | Mean Diff | p value∗ | Mean Diff | p value∗ | ||
| Plaque Score | CHX | −1.66 | < 0.001 | ||||||
| AV | −0.89 | < 0.001 | 0.76 | < 0.001 | |||||
| HP | −0.52 | < 0.001 | 1.14 | < 0.001 | 0.38 | 0.016 | |||
| CPC | −1.05 | < 0.001 | 0.61 | < 0.001 | −0.15 | 0.70 | −0.53 | <0.001 | |
|
Salivary Bacterial Count CFU (X 105) |
CHX | −15.19 | < 0.001 | ||||||
| AV | −8.26 | < 0.001 | 6.93 | < 0.001 | |||||
| HP | −6.95 | < 0.001 | 8.25 | < 0.001 | 1.32 | 0.912 | |||
| CPC | −11.32 | < 0.001 | 3.88 | < 0.001 | −3.05 | 0.280 | −4.37 | 0.04 | |
DW = Distilled water; CHX= Chlorhexidine; AV = Aloe vera; HP= Hydrogen peroxide; CPC= Cetylpyridinium chloride; CFU = Colony Forming Unit; ∗Tukey's Test; SD = Standard Deviation; ∗ Significant at p < 0.05.
Table 4.
Comparison of plaque score and salivary bacterial count (CFU x 105) for each group between Day-0 and Day-4.
| Parameter | Group | Mean Difference | SD | 95% CI Diff |
t | ∗p value | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Plaque Score | DW | 4.34 | 0.39 | 4.15 | 4.53 | 48.51 | <0.001 |
| CHX | 2.66 | 0.43 | 2.45 | 2.86 | 27.7 | <0.001 | |
| AV | 3.43 | 0.43 | 3.22 | 3.64 | 34.54 | <0.001 | |
| HP | 3.75 | 0.34 | 3.59 | 3.92 | 48.24 | <0.001 | |
| CPC | 3.25 | 0.52 | 3 | 3.5 | 27.05 | <0.001 | |
| Salivary Bacterial Count CFU (X 105) | DW | 29.84 | 5.11 | 27.38 | 32.31 | 25.44 | <0.001 |
| CHX | 14.90 | 3.75 | 13.14 | 16.66 | 17.75 | <0.001 | |
| AV | 21.00 | 3.67 | 19.23 | 22.77 | 24.96 | <0.001 | |
| HP | 22.74 | 7.62 | 19.06 | 26.41 | 13.00 | <0.001 | |
| CPC | 18.32 | 4.26 | 16.26 | 20.37 | 18.76 | <0.001 | |
DW = Distilled water; CHX= Chlorhexidine; AV = Aloe vera; HP= Hydrogen peroxide; CPC= Cetylpyridinium chloride; CFU= Colony forming unit; ∗ Student's paired t-test; SD = Standard Deviation; CI = Confidence Interval; Significant at p < 0.05.
Figure 2.
Post–hoc multiple comparisons of (Day-4 – Day-0) difference in (a) Plaque score and (b) CFU (X 105) among the mouthwashes, DW = Distilled water; CHX= Chlorhexidine; AV = Aloe vera; HP= Hydrogen peroxide and CPC= Cetylpyridinium chloride (∗Tukey's test; p < 0.05).
Salivary bacterial count
The salivary bacterial count was expressed as the number of colony-forming units (CFU x 105). There was no statistically significant difference (p = 0.873) among the five groups at baseline, but the difference was statistically significant (p < 0.001) at Day-4, with the salivary bacterial count highest in the negative control group (distilled water) and lowest in the positive control (CHX) group (Table 2). Among the test mouthwashes, the salivary bacterial count with HP (46.89 ± 8.27) was highest, followed by AV (45.58 ± 2.81) and CPC (42.53 ± 3.91). A post-hoc analysis (Table 3) of Day-4 revealed that the salivary bacterial count with HP was significantly higher than that with CPC (p = 0.04) and there was no statistically significant difference in the salivary bacterial count between AV and HP (p = 0.912) or AV and CPC (p = 0.28). There was a statistically significant increase (p < 0.001) in the salivary bacterial count with all mouthwashes on Day-4 in comparison to the baseline (Table 4), and the increase was observed to be lowest with CHX (Figure 2), indicative of the highest inhibition of bacterial growth from the baseline. Multiple comparisons revealed that there was no statistically significant difference in the inhibition of salivary bacterial growth from the baseline by AV mouthwash when compared with HP (p = 0.0.83) and CPC (p = 0.488) (Figure 2). Moreover, no significant differences were observed between HP and CPC (p = 0.065).
Discussion
This randomised, double-blind, parallel group study was undertaken to assess the antiplaque and antibacterial efficacy of three commercially available mouthwashes containing aloe vera, hydrogen peroxide, and cetylpyridinium chloride on a 4-day plaque regrowth model. The mouthwashes containing AV and CPC exhibited similar and better antiplaque and antimicrobial efficacy compared to the mouthwash containing HP. CHX, which was included as a positive control, demonstrated the highest antiplaque and antimicrobial activity. Similarly, as expected, distilled water, taken as a negative control, showed the least antiplaque and antimicrobial activity.
The 4-day plaque regrowth experimental model employed in the study has been accepted in the literature to study the antiplaque efficacy of several oral hygiene products.21, 22, 23 The efficacy of this model is based on the fact that there is a measurable accumulation of undisturbed growth of microbial plaque in the absence of oral hygiene practices (non-brushing model).24
In our study, mouthwash containing chlorhexidine was used as a positive control as in several previous studies for comparison with other mouthwash formulations or products.25, 26, 27, 28 CHX is considered the gold standard owing to its superior antiplaque and antimicrobial efficacy.29,30 It is helpful to include the most efficacious and widely used products in comparative studies for greater validity. Our study demonstrated that chlorhexidine was the most effective antiplaque and antimicrobial agent, in accordance with the results of previous studies.31,32 In their study, Roberts et al.33 showed that a single rinse with CHX could reduce oral flora from 50% to 90% for several hours. Our study also demonstrated the effective antiplaque and antibacterial effect of CPC, which is a quaternary ammonium compound with a similar cationic surface-active agent to that of CHX.34,35 CPC is also a formulation that is widely used in lieu of CHX as an antiplaque and antimicrobial agent.
The present study reported the antiplaque and antimicrobial efficacy of HP mouthwash to be the lowest compared to other test mouthwashes. Macperson et al.36 reported a one-third reduction in plaque accumulation with one percent hydrogen peroxide used under pressure for 15s compared to plain water rinsing. Our findings showed a similar decrease in plaque accumulation with the use of HP mouthwash. Hydrogen peroxide is more effective in anaerobic infections37,38; therefore, our study revealed the minimal effect of HP in inhibiting day-four plaque and salivary bacterial load, as the microflora was mostly aerobic.39,40 However, studies by Donna et al.,41 Grundeman et al.,42 Maruniak et al.,43 and Jhingta et al.44 showed that the use of HP in addition to CHX is very beneficial in lowering plaque and preventing stain. In a recent systematic review, Muniz et al.16 reported little differences in the antiplaque efficacy of HP and negative controls.
Mouthwashes containing CHX and CPC have been reported to have several disadvantages owing to which the search for herbal alternatives has been a prevalent research interest for quite some time now. Several herbal or plant extracts have been tested as antiplaque and antimicrobial agents, such as Azadirachta indica,45 Terminalia chebula,46 Piper betle,47 and Ocimum sanctum.48 These herbal or plant extracts possess several medicinal properties such as antibacterial, antiplaque, and ulcer-healing properties.49 Among these formulations, aloe vera has shown antiplaque and antimicrobial effects. While most studies have used non-standardised aloe vera formulations, a commercially available mouthwash containing aloe vera was tested in our study. The results of the present study indicated that AV mouthwash was able to inhibit plaque growth; this is in concurrence with the study by Haffajee et al., 50 and it was found that the herbal mouthwash (though less effective than CHX) was efficient in inhibiting oral biofilm, mostly the Actinomyces species, T. forsythia, E. nodatum, and P. intermedia. Karim et al.51 used 100% aloe vera and found no difference between CHX and aloe vera mouthwashes in terms of their antiplaque and anti-inflammatory efficacy. In a similar study, Gupta et al.52 found no statistically significant differences in the antiplaque efficacy of mouthwashes containing aloe vera and CHX in a 4-day plaque regrowth model, stating that aloe vera was as efficient as chlorhexidine with no side effects.
The findings of our study are not in agreement with those of the studies conducted by Karim et al.51 and Gupta et al.,52 who found that aloe vera was as efficacious an antiplaque agent as CHX. In contrast, our study shows that AV is less effective than CHX. This could be because we used a 20% extract as compared to the 100% aloe vera extract as used in their study. Further, while the substantivity of CHX is adequately documented and is one of the critical elements in its antiplaque efficacy, the substantivity of aloe vera is still unclear.
Our study showed a significant inhibition of salivary microflora count by the mouthwash containing aloe vera compared to the negative control. The antimicrobial effect of aloe vera was reported by Lee et al.49 in an in-vitro study in which they demonstrated that AV was effective in inhibiting a wide range of oral microorganisms including S. mutans, S. sanguis, A. viscosus, and C. albicans. A similar study by Kaim et al.53 reported that an antiseptic aloe vera mouthwash significantly reduces salivary aerobic, micro-aerophilic, and anaerobic bacteria for up to 2 h. Although the antimicrobial efficacy of AV in our study was observed to be significantly lower than CHX, it was comparable to both HP and CPC in terms of CFU count on Day-4 and inhibition of salivary bacteria (Day-4 – Day-0). The antimicrobial effects of aloe vera may be attributed to anthraquinones and aloin derivatives, which facilitate cellular invasion and have potent antibacterial, antifungal, and antiviral activity. Although CPC and AV did not significantly differ in antiplaque and antimicrobial efficacy in the present study, the long-term use of CPC mouthwash may also lead to staining teeth, tongue, and restoration.54,55 No such side effects have been reported for aloe vera.51 The prevalence of side effects for mouthwashes decreases their acceptability for long-term use.56
In our study, the clinical and microbial efficacies of all of the test mouthwashes were clearly demonstrated, and the microbiological findings supported the clinical data. However, the study had several limitations. Although the widely accepted 4-day non-brushing plaque regrowth model adopted in our study has several advantages, it also has a very short follow-up period, limiting its clinical applicability in clinical settings in which a mouthwash is used as an adjunct to mechanical plaque control in general. Few studies employing the same model have also assessed gingival inflammation, which was not conducted in this study since the participants underwent professional oral prophylaxis a week before commencement.
Further, although dental students have been used as participants in trials of oral hygiene products in several studies similar to ours, such a practice limits the external validity of the study results. Further, the participants in this clinical trial may have experienced Hawthorne57 and novelty effects,58 which could contribute to behavioural changes due to their awareness that they were part of a clinical trial regardless of the mouthwash they receive.59 In contrast, non-compliance with the proper usage of mouthwash could also occur. No severe side effects or adverse events were reported by any of the study participants, which could be due to the short follow-up duration. However, additional enquiries on subjective findings such as taste perception and opinion would have been beneficial.
Only the salivary bacterial CFU count was assessed, and bacterial isolation and identification was not conducted separately, which would have provided information about the antibacterial activity on specific bacteria. Future studies should conduct a detailed exploration of several types of periodontal microflora, both in terms of their number (quantitative) and type (qualitative), in a larger sample population with varying concentrations of aloe vera mouthwash to provide greater clarity about aloe vera's properties and its possible applications in oral hygiene maintenance and periodontal therapy.
Conclusions
In consideration of the limitations of this study, it can be concluded that the antiplaque and antibacterial efficacy of mouthwash containing aloe vera was similar to that of mouthwash containing cetylpyridinium chloride and significantly better than mouthwash containing hydrogen peroxide. Therefore, a mouthwash containing aloe vera may be a natural alternative to chemically formulated mouthwashes.
Source of funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors have no conflict of interest to declare.
Ethical approval
This study was approved by the institutional ethical committee of IMS and SUM Hospital, Siksha ‘O’ Anushandhan (Deemed to be University) (IMS/CRL/IEC/193/1; Dated 29.10.2013) and was conducted in accordance with the Declaration of Helsinki of 1975, as revised in 2013.
Authors contributions
NP, RM, AS, RN, RS, and AKP contributed to the conceptualisation and design of this study and the intellectual content, literature survey, data collection, manuscript drafting, editing, and review. AS contributed to data analysis, statistical analysis, interpretation, and led the writing process. All authors have critically reviewed and approved the final draft and are responsible for the content and similarity index of the manuscript.
Acknowledgment
The authors wish to thank all the participants in the study for their cooperation.
Footnotes
Peer review under responsibility of Taibah University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jtumed.2021.10.006.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Fine D.H. Mouthrinses as adjuncts for plaque and gingivitis management. A status report for the American Journal of Dentistry. Am J Dent. 1988;1(6):259–263. [PubMed] [Google Scholar]
- 2.Caton J.G., Armitage G., Berglundh T., Chapple I.L.C., Jepsen S., Kornman K.S., et al. A new classification scheme for periodontal and peri-implant diseases and conditions - introduction and key changes from the 1999 classification. J Periodontol. 2018;89:S1–S8. doi: 10.1002/JPER.18-0157. [DOI] [PubMed] [Google Scholar]
- 3.Marsh P.D. Dental plaque as a microbial biofilm. Caries Res. 2004;38(3):204–211. doi: 10.1159/000077756. [DOI] [PubMed] [Google Scholar]
- 4.Seneviratne C.J., Zhang C.F., Samaranayake L.P. Dental plaque biofilm in oral health and disease. Chin J Dent Res. 2011;14(2):87–94. [PubMed] [Google Scholar]
- 5.Santos A. Evidence-based control of plaque and gingivitis. J Clin Periodontol. 2003;30(Suppl 5):13–16. doi: 10.1034/j.1600-051x.30.s5.5.x. [DOI] [PubMed] [Google Scholar]
- 6.Bollero P., Franco R., Cecchetti F., Miranda M., Barlattani A., Jr., Dolci A., et al. Oral health and implant therapy in Parkinson's patients: review. Oral Implantol (Rome) 2017;10(2):105–111. doi: 10.11138/orl/2017.10.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prasad K.A., John S., Deepika V., Dwijendra K.S., Reddy B.R., Chincholi S. Anti-plaque efficacy of herbal and 0.2% chlorhexidine gluconate mouthwash: a comparative study. J Int Oral Health. 2015;7(8):98–102. [PMC free article] [PubMed] [Google Scholar]
- 8.Flotra L., Gjermo P., Rolla G., Waerhaug J. Side effects of chlorhexidine mouth washes. Scand J Dent Res. 1971;79(2):119–125. doi: 10.1111/j.1600-0722.1971.tb02001.x. [DOI] [PubMed] [Google Scholar]
- 9.Parwani S.R., Parwani R.N., Chitnis P.J., Dadlani H.P., Prasad S.V. Comparative evaluation of anti-plaque efficacy of herbal and 0.2% chlorhexidine gluconate mouthwash in a 4-day plaque regrowth study. J Indian Soc Periodontol. 2013;17(1):72–77. doi: 10.4103/0972-124X.107478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Satpathy A., Ravindra S., Porwal A., Das A.C., Kumar M., Mukhopadhyay I. Effect of alcohol consumption status and alcohol concentration on oral pain induced by alcohol-containing mouthwash. J Oral Sci. 2013;55(2):99–105. doi: 10.2334/josnusd.55.99. [DOI] [PubMed] [Google Scholar]
- 11.Ciancio S.G. Antiseptics and antibiotics as chemotherapeutic agents for periodontitis management. Comp Cont Educ Dent. 2000;21(1):59–62. 4, 6 passim; quiz 78. [PubMed] [Google Scholar]
- 12.Vangipuram S., Jha A., Bhashyam M. Comparative efficacy of aloe vera mouthwash and chlorhexidine on periodontal health: a randomized controlled trial. J Clin Exp Dent. 2016;8(4):e442–e447. doi: 10.4317/jced.53033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chhina S., Singh A., Menon I., Singh R., Sharma A., Aggarwal V. A randomized clinical study for comparative evaluation of Aloe Vera and 0.2% chlorhexidine gluconate mouthwash efficacy on de-novo plaque formation. J Int Soc Prev Community Dent. 2016;6(3):251–255. doi: 10.4103/2231-0762.183109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shamim R., Satpathy A., Nayak R., Mohanty R., Panda S. Efficacy of fresh Aloe vera extract in postoperative healing following periodontal surgery in patients with chronic periodontitis: a randomized clinical trial. J Dent Allied Sci. 2016;5(2):70–73. [Google Scholar]
- 15.Al-Maweri S.A., Nassani M.Z., Alaizari N., Kalakonda B., Al-Shamiri H.M., Alhajj M.N., et al. Efficacy of aloe vera mouthwash versus chlorhexidine on plaque and gingivitis: a systematic review. Int J Dent Hyg. 2019;18(1):44–51. doi: 10.1111/idh.12393. [DOI] [PubMed] [Google Scholar]
- 16.Muniz F.W.M.G., Cavagni J., Langa G.P.J., Stewart B., Malheiros Z., Rösing C.K., et al. A systematic review of the effect of oral rinsing with H2O2 on clinical and microbiological parameters related to plaque, gingivitis, and microbes. Int J Dent. 2020;2020:1–18. doi: 10.1155/2020/8841722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faul F., Erdfelder E., Lang A.G., Buchner A.G. ∗Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 18.Turesky S., Gilmore N.D., Glickman I. Reduced plaque formation by the chloromethyl analogue of victamine C. J Periodontol. 1970;41(1):41–43. doi: 10.1902/jop.1970.41.41.41. [DOI] [PubMed] [Google Scholar]
- 19.Granger D.A., Johnson S.B., Szanton S.L., Out D., Schumann L.L. Incorporating salivary biomarkers into nursing research: an overview and review of best practices. Biol Res Nurs. 2012;14(4):347–356. doi: 10.1177/1099800412443892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miles A.A., Misra S.S., Irwin J.O. The estimation of the bactericidal power of the blood. J Hyg. 1938;38(6):732–749. doi: 10.1017/s002217240001158x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Badger S.J., Butler T., Kim C.K., Johnston K.H. Experimental Eikenella corrodens endocarditis in rabbits. Infect Immun. 1979;23(3):751–757. doi: 10.1128/iai.23.3.751-757.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jenkins S., Addy M., Newcombe R. Toothpastes containing 0.3% and 0.5% triclosan. I. Effects on 4-day plaque regrowth. Am J Dent. 1989;2(Spec No):211–214. [PubMed] [Google Scholar]
- 23.Hatti S., Ravindra S., Satpathy A., Kulkarni R.D., Parande M.V. Biofilm inhibition and antimicrobial activity of a dentifrice containing salivary substitutes. Int J Dent Hyg. 2007;5(4):218–224. doi: 10.1111/j.1601-5037.2007.00249.x. [DOI] [PubMed] [Google Scholar]
- 24.Pires J.R., Rossa Junior C., Pizzolitto A.C. In vitro antimicrobial efficiency of a mouthwash containing triclosan/gantrez and sodium bicarbonate. Braz Oral Res. 2007;21(4):342–347. doi: 10.1590/s1806-83242007000400011. [DOI] [PubMed] [Google Scholar]
- 25.Lamarque G.C.C., Mendez D.A.C., Gutierrez E., Dionisio E.J., Machado M., Oliveira T.M., et al. Could chlorhexidine be an adequate positive control for antimicrobial photodynamic therapy in- in vitro studies? Photodiagnosis Photodyn Ther. 2019;25:58–62. doi: 10.1016/j.pdpdt.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Newman M.G., Flemmig T.F., Nachnani S., Rodrigues A., Calsina G., Lee Y.S., et al. Irrigation with 0.06% chlorhexidine in naturally occurring gingivitis. II. 6 months microbiological observations. J Periodontol. 1990;61(7):427–433. doi: 10.1902/jop.1990.61.7.427. [DOI] [PubMed] [Google Scholar]
- 27.Nadkerny P.V., Ravishankar P.L., Pramod V., Agarwal L.A., Bhandari S. A comparative evaluation of the efficacy of probiotic and chlorhexidine mouthrinses on clinical inflammatory parameters of gingivitis: a randomized controlled clinical study. J Indian Soc Periodontol. 2015;19(6):633–639. doi: 10.4103/0972-124X.168491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alnouri D.M.A., Kouchaji C., Nattouf A.H., AlSayed Hasan M.M.A. Effect of aloe vera mouthwash on dental plaque and gingivitis indices in children: a randomized controlled clinical trial. Pediatr Dent J. 2020;30(1):1–8. [Google Scholar]
- 29.Jones C.G. Chlorhexidine: is it still the gold standard? Periodontol 2000. 1997;15:55–62. doi: 10.1111/j.1600-0757.1997.tb00105.x. [DOI] [PubMed] [Google Scholar]
- 30.Pattnaik S., Anand N., Chandrasekaran S.C., Chandrashekar L., Mahalakshmi K., Satpathy A. Clinical and antimicrobial efficacy of a controlled-release device containing chlorhexidine in the treatment of chronic periodontitis. Eur J Clin Microbiol Infect Dis. 2015;34(10):2103–2110. doi: 10.1007/s10096-015-2459-x. [DOI] [PubMed] [Google Scholar]
- 31.Addy M., Wright R. Comparison of the in vivo and in vitro antibacterial properties of providone iodine and chlorhexidine gluconate mouthrinses. J Clin Periodontol. 1978;5(3):198–205. doi: 10.1111/j.1600-051x.1978.tb02280.x. [DOI] [PubMed] [Google Scholar]
- 32.Segreto V.A., Collins E.M., Beiswanger B.B., de La Rosa M., Isaacs R.L., Lang N.P., et al. A comparison of mouthrinses containing two concentrations of chlorhexidine. J Periodontal Res. 1986;21:23–32. [Google Scholar]
- 33.Roberts W.R., Addy M. Comparison of the in vivo and in vitro antibacterial properties of antiseptic mouthrinses containing chlorhexidine, alexidine, cetyl pyridinium chloride and hexetidine. Relevance to mode of action. J Clin Periodontol. 1981;8(4):295–310. doi: 10.1111/j.1600-051x.1981.tb02040.x. [DOI] [PubMed] [Google Scholar]
- 34.Quirynen M., Soers C., Desnyder M., Dekeyser C., Pauwels M., van Steenberghe D. A 0.05% cetyl pyridinium chloride/0.05% chlorhexidine mouth rinse during maintenance phase after initial periodontal therapy. J Clin Periodontol. 2005;32(4):390–400. doi: 10.1111/j.1600-051X.2005.00685.x. [DOI] [PubMed] [Google Scholar]
- 35.Shim J.Y., Yim S.B., Chung J.H., Hong K.S. Antiplaque and antigingivitis effects of a mouthrinse containing cetylpyridinium chloride, triclosan and dipotassium glycyrrhizinate. J Periodontal Implant Sci. 2012;42(2):33–38. doi: 10.5051/jpis.2012.42.2.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Macpherson L.M., Stephen K.W., Joiner A., Schafer F., Huntington E. Comparison of a conventional and modified tooth stain index. J Clin Periodontol. 2000;27(11):854–859. doi: 10.1034/j.1600-051x.2000.027011854.x. [DOI] [PubMed] [Google Scholar]
- 37.Gusberti F.A., Sampathkumar P., Siegrist B.E., Lang N.P. Microbiological and clinical effects of chlorhexidine digluconate and hydrogen peroxide mouthrinses on developing plaque and gingivitis. J Clin Periodontol. 1988;15(1):60–67. doi: 10.1111/j.1600-051x.1988.tb01556.x. [DOI] [PubMed] [Google Scholar]
- 38.Wennstrom J., Lindhe J. Effect of hydrogen peroxide on developing plaque and gingivitis in man. J Clin Periodontol. 1979;6(2):115–130. doi: 10.1111/j.1600-051x.1979.tb02190.x. [DOI] [PubMed] [Google Scholar]
- 39.Faran Ali S.M., Tanwir F. Oral microbial habitat a dynamic entity. J Oral Biol Craniofac Res. 2012;2(3):181–187. doi: 10.1016/j.jobcr.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ross P.W. Quantitative studies on the salivary flora. J Clin Pathol. 1971;24(8):717–720. doi: 10.1136/jcp.24.8.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dona B.L., Grundemann L.J., Steinfort J., Timmerman M.F., van der Weijden G.A. The inhibitory effect of combining chlorhexidine and hydrogen peroxide on 3-day plaque accumulation. J Clin Periodontol. 1998;25(11 Pt 1):879–883. doi: 10.1111/j.1600-051x.1998.tb02385.x. [DOI] [PubMed] [Google Scholar]
- 42.Grundemann L.J., Timmerman M.F., Ijzerman Y., van der Weijden G.A., van der Weijden G.A. Stain, plaque and gingivitis reduction by combining chlorhexidine and peroxyborate. J Clin Periodontol. 2000;27(1):9–15. doi: 10.1034/j.1600-051x.2000.027001009.x. [DOI] [PubMed] [Google Scholar]
- 43.Maruniak J., Clark W.B., Walker C.B., Magnusson I., Marks R.G., Taylor M., et al. The effect of 3 mouthrinses on plaque and gingivitis development. J Clin Periodontol. 1992;19(1):19–23. doi: 10.1111/j.1600-051x.1992.tb01143.x. [DOI] [PubMed] [Google Scholar]
- 44.Jhingta P., Bhardwaj A., Sharma D., Kumar N., Bhardwaj V.K., Vaid S. Effect of hydrogen peroxide mouthwash as an adjunct to chlorhexidine on stains and plaque. J Indian Soc Periodontol. 2013;17(4):449–453. doi: 10.4103/0972-124X.118315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schumacher M., Cerella C., Reuter S., Dicato M., Diederich M. Anti-inflammatory, pro-apoptotic, and anti-proliferative effects of a methanolic neem (Azadirachta indica) leaf extract are mediated via modulation of the nuclear factor-kappaB pathway. Genes Nutr. 2011;6(2):149–160. doi: 10.1007/s12263-010-0194-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang M.H., Ali Z., Khan I.A., Khan S.I. Anti-inflammatory activity of constituents isolated from Terminalia chebula. Nat Prod Commun. 2014;9(7):965–968. [PubMed] [Google Scholar]
- 47.Alam B., Akter F., Parvin N., Sharmin Pia R., Akter S., Chowdhury J., et al. Antioxidant, analgesic and anti-inflammatory activities of the methanolic extract of Piper betle leaves. Avicenna J Phytomed. 2013;3(2):112–125. [PMC free article] [PubMed] [Google Scholar]
- 48.Penmetsa G., Pitta S. Efficacy of Ocimum sanctum, Aloe vera and chlorhexidine mouthwash on gingivitis: a randomized controlled comparative clinical study. AYU (An Int Quarterly J Res Ayurveda) 2019;40(1) doi: 10.4103/ayu.AYU_212_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee S.S., Zhang W., Li Y. The antimicrobial potential of 14 natural herbal dentifrices: results of an in vitro diffusion method study. J Am Dent Assoc. 2004;135(8):1133–1141. doi: 10.14219/jada.archive.2004.0372. [DOI] [PubMed] [Google Scholar]
- 50.Haffajee A.D., Yaskell T., Socransky S.S. Antimicrobial effectiveness of an herbal mouthrinse compared with an essential oil and a chlorhexidine mouthrinse. J Am Dent Assoc. 2008;139(5):606–611. doi: 10.14219/jada.archive.2008.0222. [DOI] [PubMed] [Google Scholar]
- 51.Karim B., Bhaskar D.J., Agali C., Gupta D., Gupta R.K., Jain A., et al. Effect of Aloe vera mouthwash on periodontal health: triple blind randomized control trial. Oral Health Dent Manag. 2014;13(1):14–19. [PubMed] [Google Scholar]
- 52.Gupta R.K., Gupta D., Bhaskar D.J., Yadav A., Obaid K., Mishra S. Preliminary antiplaque efficacy of aloe vera mouthwash on 4 day plaque regrowth model: randomized control trial. Ethiop J Health Sci. 2014;24(2):139–144. doi: 10.4314/ejhs.v24i2.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaim J.M., Gultz J., Do L., Scherer W. An in vitro investigation of the antimicrobial activity of an herbal mouthrinse. J Clin Dent. 1998;9(2):46–48. [PubMed] [Google Scholar]
- 54.Herrera D., Escudero N., Perez L., Otheo M., Canete-Sanchez E., Perez T., et al. Clinical and microbiological effects of the use of a cetylpyridinium chloride dentifrice and mouth rinse in orthodontic patients: a 3-month randomized clinical trial. Eur J Orthod. 2018;40(5):465–474. doi: 10.1093/ejo/cjx096. [DOI] [PubMed] [Google Scholar]
- 55.Rahman B., Alkawas S., Al Zubaidi E.A., Adel O.I., Hawas N. Comparative antiplaque and antigingivitis effectiveness of tea tree oil mouthwash and a cetylpyridinium chloride mouthwash: a randomized controlled crossover study. Contemp Clin Dent. 2014;5(4):466–470. doi: 10.4103/0976-237X.142813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tartaglia G.M., Tadakamadla S.K., Connelly S.T., Sforza C., Martin C. Adverse events associated with home use of mouthrinses: a systematic review. Ther Adv Drug Saf. 2019;10 doi: 10.1177/2042098619854881. 2042098619854881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McCarney R., Warner J., Iliffe S., van Haselen R., Griffin M., Fisher P. The Hawthorne Effect: a randomised, controlled trial. BMC Med Res Methodol. 2007;7:30. doi: 10.1186/1471-2288-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gomes C.E., Cavalcante D.G., Filho J.E., da Costa F.N., da Silva Pereira S.L. Clinical effect of a mouthwash containing Anacardium occidentale Linn. on plaque and gingivitis control: a randomized controlled trial. Indian J Dent Res. 2016;27(4):364–369. doi: 10.4103/0970-9290.191883. [DOI] [PubMed] [Google Scholar]
- 59.Choonhakarn C., Busaracome P., Sripanidkulchai B., Sarakarn P. The efficacy of aloe vera gel in the treatment of oral lichen planus: a randomized controlled trial. Br J Dermatol. 2008;158(3):573–577. doi: 10.1111/j.1365-2133.2007.08370.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.