Abstract
Abstract
Objective
To study the association between behavioural factors and incidence rates of SARS-CoV-2 infection.
Design
Case–control web-based questionnaire study.
Setting
Questionnaire data were collected in the Capital Region of Denmark in December 2020 when limited restrictions were in place, while the number of daily SARS-CoV-2 cases increased rapidly.
Participants
8913 cases of laboratory-confirmed SARS-CoV-2 infection were compared with two groups of controls: (1) 34 063 individuals with a negative SARS-CoV-2 test from the same date (negative controls, NCs) and 2) 25 989 individuals who had never been tested for a SARS-CoV-2 infection (untested controls, UC). Controls were matched on sex, age, test date and municipality.
Exposure
Activities during the 14 days prior to being tested positive for SARS-CoV-2 or during the same period for matched controls and precautions taken during the entire pandemic.
Main outcomes and measures
SARS-CoV-2 infection incidence rate ratios (IRR).
Results
Response rate was 41.4% (n=93 121). Using public transportation, grocery shopping (IRR: NC: 0.52; UC: 0.63) and outdoor sports activities (NC: 0.75; UC: 0.96) were not associated with increased rate of SARS-CoV-2 infection. Most precautions, for example, using hand sanitizer (NC: 0.79; UC: 0.98), physical distancing (NC: 0.79; UC: 0.82) and avoiding handshakes (NC: 0.74; UC: 0.77), were associated with a lower rate of infection. Activities associated with many close contacts, especially indoors, increased rate of infection. Except for working from home, all types of occupation were linked to increased rate of infection.
Conclusions
In a community setting with moderate restrictions, activities such as using public transportation and grocery shopping with the relevant precautions were not associated with an increased rate of SARS-CoV-2 infection. Exposures and activities where safety measures are difficult to maintain might be important risk factors for infection. These findings may help public health authorities tailor their strategies for limiting the spread of SARS-CoV-2.
Keywords: COVID-19, Epidemiology, INFECTIOUS DISEASES
Strengths and limitations of this study.
This study had a large sample size of 68 965 Danish citizens, which provided precision and power to our findings.
Using a web-based questionnaire survey enables a large-scale collection of self-reported data, but data can be affected by recall and selection bias.
The confirmed SARS-CoV-2 positive cases were compared with two different groups of controls, matched on age, sex, municipality and test date.
Our results could be very dependent on the stage of the pandemic at the time and therefore cannot necessarily be extrapolated to other stages of the pandemic.
All of the analyses performed were exploratory, and no adjustment for multiple testing was made.
Background
The rapid global spread of SARS-CoV-2, the novel virus causing COVID-19,1,3 has created an unprecedented public health emergency worldwide. Non-pharmaceutical interventions have played an important role in the COVID-19 response and are likely to continue as the key interventions for the predictable future despite the promising advances in vaccination programmes. Optimally, these interventions should be based on evidence about transmission patterns of SARS-CoV-2. This evidence could help governments and public health authorities direct restrictions against sectors of the society where transmission of SARS-CoV-2 is high and scale down restrictions on low-transmission activities.
Viral transmission is influenced by contact patterns, environmental and socioeconomic factors.4 It can occur everywhere; however, some settings are more likely to increase the risk of transmission due to a mixture of behavioural and environmental factors.5 Increased risk for SARS-CoV-2 transmission is reported in settings where there is close proximity contact, contact over an extended period of time or multiple contacts in a confined, poorly ventilated space.5 Evidence suggest that the risk of SARS-CoV-2 transmission is the highest in household settings, and that living in a multiple occupancy or overcrowded household elevates the risk of becoming infected.6 Working in healthcare has been associated with increased risk of SARS-CoV-2 infection.7 8 Also, specific community settings have been associated with an increased risk of infection. The starting point for large SARS-CoV-2 outbreaks has been identified and linked to hospitality venues such as restaurants, night clubs and bars.9,11 Occupational settings including factories, warehouses and educational institutions have also been reported as sites for SARS-CoV-2 outbreaks.12 However, data about the role of community-related factors in facilitating transmission are still limited, despite being critical for designing evidence-based control measures for SARS-CoV-2 transmission.6
We investigated the association between behavioural factors and SARS-CoV-2 infection rate by means of a web-survey-based case–control study conducted in Denmark during a period of rapidly increasing infection rate.
Methods
Study design and case definition
We conducted a web-survey-based retrospective case–control study in the Capital Region of Denmark. We invited 25 000 cases of laboratory-confirmed SARS-CoV-2 infection, tested from 2 November to 13 December 2020. A confirmed case of SARS-CoV-2 infection was defined by a positive result on a reverse transcription PCR (RT-PCR) assay of a specimen collected from the nasopharynx or oropharynx.13 SARS-CoV-2 positive cases were matched first with a control group of 100 000 cases who had a negative test performed the same day (denoted negative controls, NCs) and, second, with a randomly selected group of 99 689 people from the background population who had never been tested for SARS-CoV-2 infection (denoted untested controls, UCs). Controls were matched on sex, age and municipality, and the UCs were provided a ‘test date’ corresponding to the matched case. The overall response was moderate and to allow most cases to be matched with at least one control we rounded the matching age by 5 years and the test date by 5 days. The participants did not receive compensation.
Setting
During the study period of November and December 2020, the official restrictions instituted by the Danish authorities entailed a curfew for restaurants, cafés and bars to close at 22:00. Face masks were mandatory indoors in all public places, including in public transport and retail stores as well as in restaurants and bars when not sitting down. Public gatherings were allowed at a maximum of 10 people. Nightclubs were closed. Spectators at sporting events, concerts and religious services were restricted to a maximum of 500 people all facing the same direction. People were asked to practice 1 m physical distancing, work from home and limit the use of public transport. Meanwhile, the number of SARS-CoV-2 infected cases in Denmark increased from 1270 daily cases on 2 November to 3163 cases on 13 December 2020. The population of Denmark consisted of 5 837 213 individuals by the end of 2020.13
Data collection and sources
In December 2020 and January 2021, 224 689 participants over the age of 18 were asked to complete an online questionnaire. Participants were invited to participate in the web-survey via the secure digital platform, ‘e-Boks’. The e-Boks is a secure national digital post box used by 92.1% of the Danish adult population by the second quarter of 2020.14 A list of SARS-CoV-2 positive individuals was obtained from the Danish Microbiology (MiBa) database, which holds data for all SARS-CoV-2 PCR tests in Denmark, provided by the Danish Health Data Authority.15 Invitations were sent to participants via e-Boks between 13 December 2020 and 2 January 2021 together with written project information and a link to a questionnaire on demographic data, known exposure to COVID-19, behavioural factors and symptomatology related to COVID-19. A reminder was sent to the non-respondents via e-Boks 24–28 days after the invitations were distributed. The last questionnaire was received digitally by 25 January 2021. The study was also presented on Danish national television and shared on several social media platforms prior to the distribution. The questionnaire was constructed and managed using REDCap electronic data capture tools hosted by the Capital Region of Denmark,16 17 and was tested on approximately 60 people without a healthcare background before distributing, to ensure that the questions were correctly understood. The survey data were linked to each participant’s individual background data obtained from the Danish nationwide registries in the research environment of Statistics Denmark. The linkage was possible by using the participant’s individual Central Person Register number, which is assigned to all Danish residents on birth or immigration.18 The Danish Civil Registration System was used to obtain information on age, sex, residency, ethnicity and household size.18 Information regarding household income and educational level was collected from the Danish Registers on Personal Income and Transfer Payments and the Danish Education Registers, respectively.19 20
Variables
The relevant time interval for transmission factors was defined as the period 2 weeks before the date of their RT-PCR test. The online questionnaire assessed exposure to known SARS-CoV-2 infected cases, exposure attributes during contact with the case or cases during the defined time interval, travel history, self-reported health, occupation and demographic variables. Participants were questioned about their behaviour during the 2 weeks prior to their RT-PCR test date or, if applicable (UCs), a 2-week period before a specific date matched to the corresponding case.
Participants were asked about possible community exposure activities (eg, gatherings with >30 people indoors/outdoors, eg, in a home/public event/work-related; grocery shopping; dining at a restaurant or café; going to a bar, gym, public swimming pool; or using public transportation) on a 5-point Likert-type scale ranging from ‘never/less than once a month’ to ‘more than once per day’. Community activity responses were dichotomised as never versus one or more times during the 2 weeks before the date of their RT-PCR test. Precautions to avoid viral transmission taken since 1 March 2020 were assessed and participants were asked to quantify degree of adherence to recommendations such as wearing a face mask, frequent use of hand sanitizers, physical distancing, avoiding handshakes, hugs and social gatherings, with response options ranging from ‘never’ to ‘almost always’. The date of onset and type of symptoms experienced by the individual, if any, were also recorded. The translated survey questions are listed in eText 1 and variables obtained from Statistics Denmark are listed in online supplemental eTable 1. Some of the answers are not covered in this publication.
Statistical methods
Categorical variables were summarised by counts and percentages, while continuous variables were summarised by medians and quartiles. The nested case–control data were obtained as described in section ‘Study design and case definition’ and analysed using Cox regression with time-dependent exposure and baseline incidence rate function stratified for age (in 5-year intervals), test date (in 5-day intervals), sex and municipality via risk set matching.21 22 The associations between each exposure separately and the infection rates were reported as incidence rate ratios (IRRs) adjusted for age, sex and municipality. The models assume that the IRRs were constant in the study period (proportional hazards assumptions). All of the analyses performed were exploratory, and no adjustment for multiple testing was made. IRRs were reported with their 95%CIs. The analyses were repeated in subgroups defined by sex, age, educational level, household size, ethnicity and occupation. The level of statistical significance was set at 5%. All data management and statistical analyses were performed using R statistical software, V.4.0.3.23
The patient and public involvement statement
Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research.
Results
Participant characteristics
A total of 93 121 individuals (41.1%) responded to the survey, of which 11 854 (47.4 %) were cases, 45 405 (45.4 %) were NCs and 35 862 (35.9 %) were UCs.
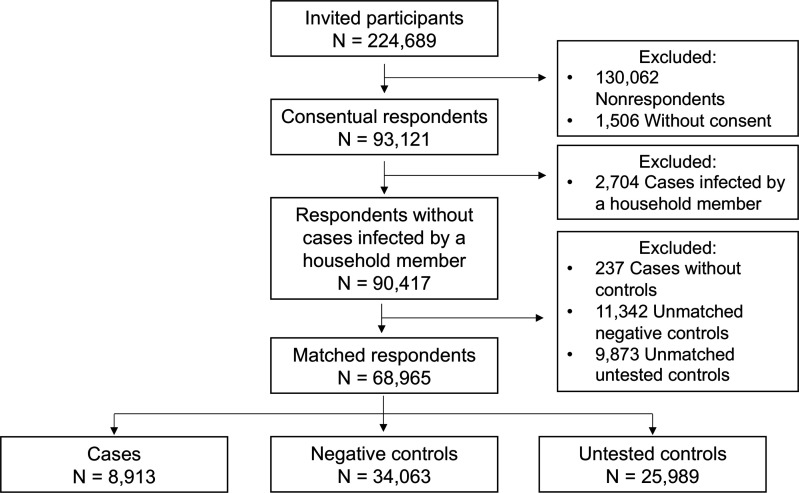
Among cases, 2704 (22.8%) reported having a member of their household as the presumed source of infection. Likely, these cases had not contracted SARS-CoV-2 in the community setting. Consequently, we decided to exclude these cases when investigating behavioural risk factors for SARS-CoV-2 community-related transmission. After matching, participants consisted of 8942 cases, 34 165 NCs and 26 006 UCs (figure 1).
Figure 1. Flowchart describing study participants.
Participants (n=68 965, median age 44 years) were predominantly women (58.9%), of Danish ethnicity (90.8%), self-employed (35.1%) and lived in a household of 1–2 individuals (59.1%) (table 1). The baseline characteristics of respondents and non-respondents are described in online supplemental eTable 2.
Table 1. Demographics and SARS-CoV-2 transmission characteristics by survey group.
| Characteristics | Cases (n=8913) | Negative controls (n=34 063) | Untested controls (n=25 989) | Total (n=68 965) |
| Age, median (IQR) | 43 (29, 55) | 44 (30, 54) | 45 (31, 55) | 44 (30, 55) |
| Sex, n (%) | ||||
| Female | 5065 (56.8) | 19 745 (58.0) | 15 816 (60.9) | 40 626 (58.9) |
| Male | 3848 (43.2) | 14 318 (42.0) | 10 173 (39.1) | 28 339 (41.1) |
| Ethnicity, n (%) | ||||
| Danish | 7640 (85.7) | 30 334 (89.1) | 24 668 (94.9) | 62 642 (90.8) |
| Other | 1273 (14.3) | 3729 (10.9) | 1321 (5.1) | 6323 (9.2) |
| Household size, n (%) | ||||
| 1–2 persons | 5234 (59.7) | 19 438 (57.8) | 16 246 (63.0) | 40 918 (60.0) |
| 3–4 persons | 2931 (33.4) | 12 156 (36.1) | 8290 (32.2) | 23 377 (34.3) |
| ≥5 persons | 606 (6.9) | 2046 (6.1) | 1241 (4.8) | 3893 (5.7) |
| Missing | 142 | 423 | 212 | 777 |
| Primary employment, n (%) | ||||
| Student | 746 (9.4) | 2734 (8.7) | 3617 (14.8) | 7097 (11.1) |
| Public employee | 1111 (14.0) | 4059 (12.9) | 2570 (10.5) | 7740 (12.1) |
| Private employee | 2545 (32.1) | 10 210 (32.4) | 4848 (19.9) | 17 603 (27.6) |
| Self-employed | 2739 (34.5) | 11 352 (36.0) | 10 127 (41.5) | 24 218 (37.9) |
| Non-employed | 529 (6.7) | 2036 (6.5) | 1988 (8.2) | 4553 (7.1) |
| Other | 264 (3.3) | 1134 (3.6) | 1237 (5.1) | 2635 (4.1) |
| Missing | 979 | 2538 | 1602 | 5119 |
| Type of occupation, n (%) | ||||
| Non-employed | 847 (10.7) | 3132 (10.0) | 3999 (16.5) | 7978 (12.6) |
| At home | 1470 (18.6) | 7673 (24.5) | 6858 (28.3) | 16 001 (25.2) |
| Own office | 601 (7.6) | 2477 (7.9) | 1916 (7.9) | 4994 (7.9) |
| Shared office | 1358 (17.2) | 5029 (16.0) | 4134 (17.1) | 10 521 (16.6) |
| Retail, library and factory work | 558 (7.1) | 1814 (5.8) | 1946 (8.0) | 4318 (6.8) |
| Social care and education | 1489 (18.9) | 5012 (16.0) | 1714 (7.1) | 8215 (12.9) |
| Healthcare sector | 746 (9.4) | 3098 (9.9) | 708 (2.9) | 4552 (7.2) |
| Transport (eg, bus/taxi driver) | 63 (0.8) | 240 (0.8) | 192 (0.8) | 495 (0.8) |
| Outdoors (eg, gardener) | 178 (2.3) | 714 (2.3) | 792 (3.3) | 1684 (2.7) |
| Other | 587 (7.4) | 2193 (7.0) | 1953 (8.1) | 4733 (7.5) |
| Missing | 1016 | 2681 | 1777 | 5474 |
| Contact with SARS-CoV-2 positive, n (%) | ||||
| None | 4025 (48.7) | 22 320 (68.0) | 24 166 (94.9) | 50 511 (75.9) |
| Other contact | 1715 (20.8) | 5683 (17.3) | 815 (3.2) | 8213 (12.3) |
| Close contact (<2 m, >30 min) | 2522 (30.5) | 4829 (14.7) | 477 (1.9) | 7828 (11.8) |
| Missing | 651 | 1231 | 531 | 2413 |
| Test reason, n (%) | ||||
| Symptoms | 3323 (40.0) | 5471 (16.7) | ||
| SARS-CoV-2 contact tracing app | 57 (0.7) | 1814 (5.5) | ||
| Prior to medical treatment | 85 (1.0) | 1982 (6.0) | ||
| Contact with SARS-CoV-2 positive | 3678 (44.3) | 9288 (28.3) | ||
| Work-related test reason | 260 (3.1) | 2889 (8.8) | ||
| Visit high risk person | 71 (0.9) | 2026 (6.2) | ||
| Travel plans | 25 (0.3) | 668 (2.0) | ||
| Prior to social event | 113 (1.4) | 2086 (6.4) | ||
| Suspected infection | 197 (2.4) | 1223 (3.7) | ||
| Other | 489 (5.9) | 5382 (16.4) | ||
| Missing | 615 | 1234 | ||
| Presumed source of infection, n (%) | ||||
| Unknown | 2876 (34.8) | |||
| Household | 0 (0.0) | |||
| Family/close friend | 2229 (27.0) | |||
| Colleague/acquaintance | 3152 (38.2) | |||
| Missing | 656 | |||
For both cases and NCs, close contact with a SARS-CoV-2-positive individual was the most common reason for being tested (cases: 41.3%, NC: 27.3%), while having COVID-19-associated symptoms was the second-most common reason (cases: 37.3%, NC: 16.1%). A total of 32.3% of cases reported suspected source of infection as unknown (table 1).
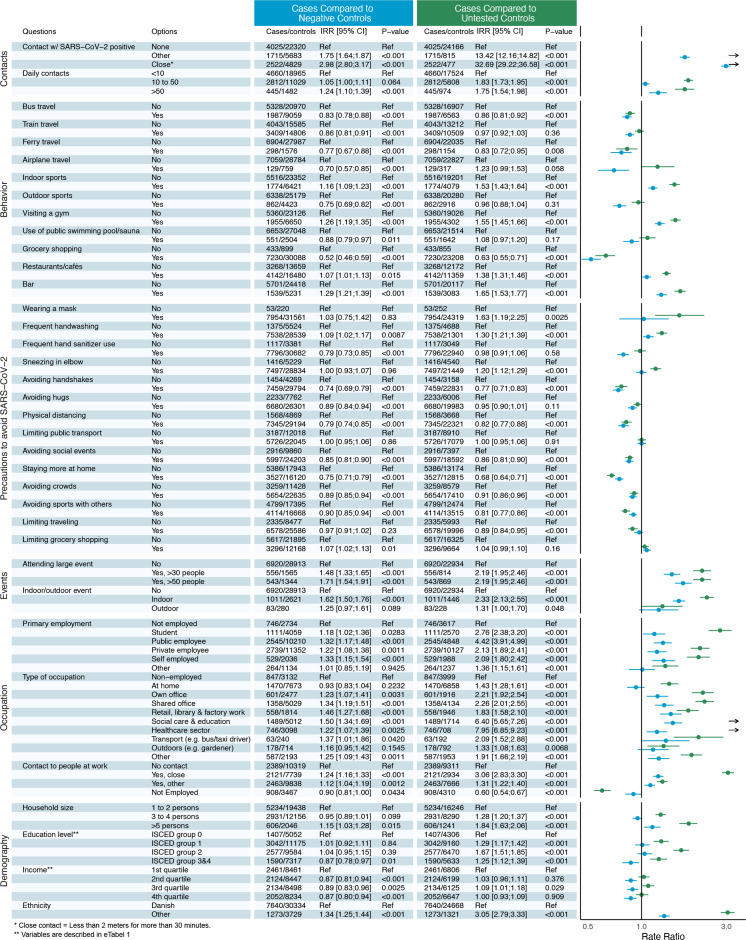
Figure 2 presents the IRRs for the different exposures when comparing cases with either NCs or UCs.
Figure 2. The infection incidence rate ratios (IRRs) for the different exposures when comparing cases with either negative controls or untested controls. Blue: cases compared with negative controls. Green: cases compared with untested controls.
Social contacts
Close contact (less than 2 m for more than 30 min) with a SARS-CoV-2-positive person was associated with the highest relative rate of testing positive for SARS-CoV-2 (IRR: NC: 2.98; UC: 32.69). Having had some other form of contact with a SARS-CoV-2-positive person was also associated with an elevated rate of infection (IRR: NC: 1.75; UC: 13.42). Similarly, we found a high number of daily contacts to be associated with increased SARS-CoV-2 infection rates, particularly when having more than 50 daily contacts (IRR: NC: 1.24; UC: 1.75). Attending large, indoor social events with more than 30 or 50 attendees increased the infections rates. Attending large outdoor events increased the rate as well, however, to a lesser degree.
Behavioral risk factors
Visiting a gym (IRR: NC: 1.26; UC: 1.55) or participating in indoor sports activities (IRR: NC: 1.16; UC: 1.53) were associated with an increased rate of testing positive for SARS-CoV-2. No statistically significantly elevated infection rate was found for visiting indoor public swimming pools. Outdoor exercising was associated with a lower SARS-CoV-2 infection rate (IRR: NC: 0.75; UC: 0.96 (p=0.31)). Bar visits were associated with a higher rate of testing positive for SARS-CoV-2 than visiting a restaurant or café. Neither grocery shopping (IRR: NC: 0.52; UC: 0.63), use of public transport (bus travel IRR: NC: 0.83; UC: 0.86), train travel: IRR: NC: 0.86; UC: 0.97), nor travelling by ferry (IRR: NC: 0.77; UC: 0.83) or aeroplane (IRR: NC: 0.70; UC: 1.23 (p=0.058)) were associated with increased infection rate.
Precautions taken in order to avoid SARS-CoV-2 infection
Using hand sanitizer (IRR: NC: 0.79; UC: 0.98 (p=0.58), practising physical distancing (IRR: NC: 0.79; UC: 0.82) and staying more at home (IRR: NC: 0.75; UC: 0.68) were all associated with a reduced rate of infection. Avoiding handshakes, hugs, large crowds and social events were associated with lower rate of infection. Frequent handwashing appeared to be a risk factor for SARS-CoV-2 infection (IRR: NC: 1.09; UC: 1.30). However, no significantly increased rates were found in subgroup analysis when excluding participants in high-risk occupations (IRR: NC: 0.98 (p=0.52); UC: 1.05 (p=0.21)) (healthcare, social care and education) (online supplemental eTable 3).
Occupational risk factors
Working in healthcare (IRR: NC: 1.22; UC: 7.95), social care and educational institutions (IRR: NC: 1.50; UC: 6.40) was associated with increased rate of SARS-CoV-2 infection. All types of employment were significantly associated with a higher rate of infection compared with being unemployed. Having close contact or some other form of contact with clients, patients or customers during work was an additional risk factor.
Demographic risk factors
Living in larger households>5 persons (IRR: NC: 1.15; UC: 1.84) and having an ethnic background other than Danish (IRR: NC: 1.34; UC: 3.05) were associated with an elevated rate of SARS-CoV-2 infection. Household income and level of education were not significantly related to infection rate.
Subgroup analyses
When only including the 2704 cases who had reported a member of their household as the assumed source of infection, IRRs varied greatly from the estimates found in the main analysis and were often counterintuitive (online supplemental eTable 2). These findings support the decision to exclude the presumed household-infected cases when investigating risk factors for community transmission. Additional subgroup analyses are shown in online supplemental eTables 4–22.
Moreover, the main analysis was repeated adjusted for wearing a mask, frequent hand washing, as well as both mask wearing and hand washing (online supplemental eTables 18–20). The analysis adjusted for handwashing shows IRR’s almost identical to the main analysis in figure 2, where the analysis adjusted for wearing a mask shows multiple of the precautions to be associated with an increased rate of infection (online supplemental eTables 18–20).
When comparing the symptomatic cases with the symptomatic NCs, having close contact to a SARS-CoV-2-positive person (IRR: 8.78), going to the gym (IRR: 1.43), doing indoor sports (IRR: 1.38), visiting restaurants/cafés (IRR: 1.35), visiting a bar (IRR: 1.66) and attending a large event (IRR>30 attendees: 1.91 and IRR>50 attendees: 2.13) were all associated with an increased rate of infection (online supplemental eTable 21).
The main analysis was repeated for cases with an unknown source of infection compared with NC and UC (online supplemental eTable 22). In this analysis, doing indoor sports (IRR: NC: 1.26; UC: 1.68), visiting a gym (IRR: NC: 1.52; UC: 1.87), visiting restaurants/cafés (IRR: NC: 1.33; UC: 1.77), visiting a bar (IRR: NC: 1.67; UC: 2.20) and attending a large event (IRR>30 attendees - NC: 1.78; UC: 2.67. IRR>50 attendees - NC: 2.37; UC: 3.22) were all associated with an increased rate of infection. In this analysis, many precautions had IRRs above 1 but the findings were not statistically significant (online supplemental eTable 22).
Discussion
This large retrospective case–control study aimed to investigate the behavioural factors associated with the risk of SARS-CoV-2 infection. During the study period of November and December 2020, restrictions instituted by the Danish authorities were limited and the infection rate rapidly increasing. Our results indicate that going grocery shopping, using public transport and swimming pools as well as participating in outdoor sports are not associated with an increased incidence rate of SARS-CoV-2 infection. Importantly, our study was conducted in a community setting with moderate restrictions. The non-pharmaceutical interventions instituted by the Danish authorities in the study period included reserved seating in regional trains, mandatory masking when using public transportation and while standing indoors in all public places, and a physical distance≥1 m was encouraged in all social settings. These non-pharmaceutical interventions may be required in order to sustain a reduced viral transmission in public transportation and other community settings in the future.
An essential part of the Danish authorities’ initiatives to mitigate viral transmission has been to recommend a series of behavioural changes through TV ads and posters in public places. Encouragingly, our study showed that frequent use of hand sanitizer, physical distancing, as well as avoiding handshakes, hugs, social events and large gatherings were associated with a lower rate of SARS-CoV-2 infection. This is in line with recent reports showing that the implementation of non-pharmaceutical interventions is enough to achieve control of a SARS-CoV-2 outbreak.24 25 Non-pharmaceutical interventions reduce presymptomatic transmission, which previous studies have found to constitute a high proportion of the total transmission.26 27 Bans on large public gatherings and making hand sanitizer available in public places are relatively inexpensive measures that should help reduce SARS-CoV-2 infections.
In our population, frequent handwashing appeared to be a risk factor for SARS-CoV-2 infection. However, this increased rate was not found in subgroup analysis when excluding participants in high-risk occupations (working in healthcare, social care, retirement homes and educational institutions) (online supplemental eTable 3). Consequently, the initial finding may be explained by the frequent handwashing of high-risk occupations. The use of masks did not appear to be preventative. During the time period when this study was conducted, masks were mandatory indoors in all public places, for example, in bars/restaurants and in public transportation. The rate estimates presented here may be confounded by several factors and the lack of a protective effect for mask wearing may be more linked to the general behaviour of the individuals more than to the effect of the masks themselves. When looking at the supplementary analysis adjusted for wearing a mask, multiple of the precautions are associated with an increased rate of infection. This suggests that wearing a mask, or the general behaviour of the individuals who wear a mask, could be a substantial confounder for the protective properties of many of the safety measures in this study (online supplemental eTable 18).
Attending meetings with many people, visiting bars and restaurants and having many activities in public places would require frequent mask wearing. Whereas, staying at home and reducing social activities to a minimum would practically eliminate the need to wear a mask.
Having more than 50 daily contacts was associated with higher rates of SARS-CoV-2 infection. Another important factor was attending large indoor events with more than 30 attendees. In several cases, these large gatherings have been the source of ‘superspreading events’ in which many people are infected within a short period of time.28 Increasing evidence indicates that superspreading events play a dominant role in SARS-CoV-2 transmission,28 29 and that superspreading events are critical for maintaining the epidemic.29 Restrictions on large gatherings seem warranted. The increase in infection rate was less if the events were held outdoors. This supports existing evidence that the rate of SARS-CoV-2 transmission is lower outdoors.30
Every type of occupation carried out outside one’s own home was associated with increased rate of infection, with outdoor occupations having the lowest increase in rate, numerically. Physical proximity to other people, especially close contacts, during work increased rate of infection. These findings support the recommendation for working from home and limiting physical contact at work, when possible.
Going to the gym and doing indoor sports were associated with increased rate of infection, in line with previous reports of SARS-CoV-2 outbreaks from indoor sporting facilities.31,33 Considering that SARS-CoV-2 is transmitted by respiratory droplets, group exercise in a closed indoor space could provide an environment highly prone to SARS-CoV-2 transmission.31 The increased rate of infection in bars and to a lesser extent in restaurants corresponds to previous reports about these locations being the centre for superspreading events.10 11 34 Reports of exposures in restaurants have been linked to air circulation.11 Eating and drinking may obstruct efficient mask use, whereas masks can be effectively worn while shopping and during numerous other indoor activities.
In Denmark, great attention has been devoted to subpopulations throughout the pandemic, because big differences in infection rates were observed between groups. Some of the more important groupings were by ethnicity, age and occupation. We believe that presenting the risk in subgroups of populations of specific interest would be useful to guide policy-makers, as effects of interventions could be very different across these subpopulations.
Considering the risk of selection bias, the SARS-CoV-2 positive cases were compared with both a group of tested individuals (NC) and a group of individuals who were never tested (UC).
The association between some behavioural factors and SARS-CoV-2 transmission was markedly different when cases were compared with NCs versus UCs. Although the IRRs mostly pointed in the same direction, the IRR was often markedly higher when using UCs as the control group. The reason for this is unclear but could be due to unaccounted for bias/confounding. UCs may be more careful and cautious, for example, have fewer daily contacts, than the NCs. Also, differences in access to SARS-CoV-2 testing could explain some of the difference seen. The fact that the results when comparing the symptomatic cases with the symptomatic NCs were almost identical to the main findings in figure 2 suggests that the behavioural factors associated with an increased rate of infection are the same regardless of the reason for being tested.
The findings in this report are subject to limitations. We used a case–control design from which results should be interpreted as hypothesis-generating only. Only a moderate number of invitees answered the survey, possibly limiting generalisability. Participants were aware of their SARS-CoV-2 test results, which could have influenced their responses to questions about community exposures and close contacts leading to recall bias. Similarly, UCs may have more of a problem with recall bias than those who were tested. We recruited and asked questions on a digital platform leading to exclusion of participants not using this platform (mostly elderly people). Very few of our participants had been hospitalised indicating that our data are skewed towards those less affected by SARS-CoV-2. Likewise, we could not include people who ultimately died from the disease. Factors identified in this study might therefore be less applicable to the elderly or those with severe disease. Finally, our results could be very dependent on the stage of the pandemic at the time and therefore cannot necessarily be extrapolated to other stages of the pandemic.
Among the strengths of our study is a large sample size, that most participants answered all the questions in the comprehensive questionnaire and the choice to use two control groups matched by age, sex, municipality and test date.
Conclusions
Using a case–control design, we identified activities including grocery shopping and using public transport that do not seem to increase the rate of getting infected with SARS-CoV-2 in a community with moderate restrictions. Exposures and activities associated with many and close contacts, especially indoors, increased the rate of infection. Importantly, continued assessment of various types of activities and exposures in other settings and in the next phases of the pandemic are needed as communities reopen. The results presented here should help public health authorities and individuals tailor their strategies for limiting the spread of SARS-CoV-2.
supplementary material
Footnotes
Funding: The study has received funding from IMK Almene Fond. IMK Almene Fond had no role in the design and conduct of the study, collection, management, analysis, interpretation of the data, preparation, review, approval of the manuscript or decision to submit the manuscript for publication. Grant number N/A.
Prepublication history and additional supplemental material for this paper are available online. To view these files, please visit the journal online (http://dx.doi.org/10.1136/bmjopen-2021-056393).
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Ethics approval: This study involves human participants and was approved by registry-based studies and surveys with the purpose of scientific research do not require ethical committee approval in Denmark. Approval to use data sources was granted by the data responsibility institute in the Capital Region of Denmark (approval number P-2019-191), and the Danish Patient Safety Authority approved the use of patient data for the survey (project ID 31-1521-266). Informed consent was obtained from all participants. Participants gave informed consent to participate in the study before taking part.
Data availability free text: Data are available on reasonable request by emailing CT-P, email address: christian.torp-pedersen@regionh.dk.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Correction notice: The article has been corrected since it was published online. The first author's name has been updated from Mille Dybdal Cajar to Mille Dybdal Bager.
Data availability statement
Data are available upon reasonable request.
References
- 1.Sanche S, Lin YT, Xu C, et al. High contagiousness and rapid spread of severe acute respiratory syndrome coronavirus 2. Emerg Infect Dis. 2020;26:1470–7. doi: 10.3201/eid2607.200282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wölfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–9. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 3.Zhou P, Yang X-L, Wang X-G, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–3. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cevik M, Marcus J, Buckee C, et al. SARS-CoV-2 transmission dynamics should inform policy. SSRN Journal. 2020 doi: 10.2139/ssrn.3692807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meyerowitz EA, Richterman A, Gandhi RT, et al. Transmission of SARS-CoV-2: a review of viral, host, and environmental factors. Ann Intern Med. 2021;174:69–79. doi: 10.7326/M20-5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee EC, Wada NI, Grabowski MK, et al. The engines of SARS-CoV-2 spread. Science. 2020;370:406–7. doi: 10.1126/science.abd8755. [DOI] [PubMed] [Google Scholar]
- 7.Leclerc QJ, Fuller NM, Knight LE, et al. What settings have been linked to SARS-CoV-2 transmission clusters? Wellcome Open Res. 2020;5:83. doi: 10.12688/wellcomeopenres.15889.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nguyen LH, Drew DA, Graham MS, et al. Risk of COVID-19 among front-line health-care workers and the general community: a prospective cohort study. The Lancet Public Health. 2020;5:e475–83. doi: 10.1016/S2468-2667(20)30164-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fisher KA, Tenforde MW, Feldstein LR, et al. Community and close contact exposures associated with COVID-19 among symptomatic adults ≥18 years in 11 outpatient health care facilities — United States, July 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1258–64. doi: 10.15585/mmwr.mm6936a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Furuse Y, Sando E, Tsuchiya N, et al. Clusters of coronavirus disease in communities, Japan, January–April 2020. Emerg Infect Dis. 2020;26:2176–9. doi: 10.3201/eid2609.202272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu J, Gu J, Li K, et al. COVID-19 outbreak associated with air conditioning in restaurant, Guangzhou, China, 2020. Emerg Infect Dis. 2020;26:1628–31. doi: 10.3201/eid2607.200764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ismail SA, Saliba V, Lopez Bernal J, et al. SARS-CoV-2 infection and transmission in educational settings: a prospective, cross-sectional analysis of infection clusters and outbreaks in England. Lancet Infect Dis. 2021;21:344–53. doi: 10.1016/S1473-3099(20)30882-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Denmark S. Population figures. 2021. https://www.dst.dk/en/Statistik/emner/borgere/befolkning/befolkningstal
- 14.Agency TDD. Statistics on digital post. 2020. https://digst.dk/it-loesninger/digital-post/om-loesningen/tal-og-statistik-om-digital-post/
- 15.Voldstedlund M, Haarh M, Mølbak K, et al. The Danish microbiology database (MiBa) 2010 to 2013. Euro Surveill. 2014;19 doi: 10.2807/1560-7917.ES2014.19.1.20667. [DOI] [PubMed] [Google Scholar]
- 16.Harris PA, Taylor R, Minor BL, et al. The REDCap Consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pedersen CB. The Danish civil registration system. Scand J Public Health. 2011;39:22–5. doi: 10.1177/1403494810387965. [DOI] [PubMed] [Google Scholar]
- 19.Baadsgaard M, Quitzau J. Danish registers on personal income and transfer payments. Scand J Public Health. 2011;39:103–5. doi: 10.1177/1403494811405098. [DOI] [PubMed] [Google Scholar]
- 20.Jensen VM, Rasmussen AW. Danish education registers. Scand J Public Health. 2011;39:91–4. doi: 10.1177/1403494810394715. [DOI] [PubMed] [Google Scholar]
- 21.Essebag V, Platt RW, Abrahamowicz M, et al. Comparison of nested case-control and survival analysis methodologies for analysis of time-dependent exposure. BMC Med Res Methodol. 2005:5. doi: 10.1186/1471-2288-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klein JP, van Houwelingen HC, Ibrahim JG, et al., editors. Handbook of Survival Analysis. 1st. Chapman and Hall/CRC; 2013. https://doi.org/10.1201/b16248 edn. Available. [DOI] [Google Scholar]
- 23.Team RC . R: a language and environment for statistical computing. R foundation for statistical computing. Vienna, Austria: 2018. https://www.R-project.org/ [Google Scholar]
- 24.Al-Tawfiq JA, Rodriguez-Morales AJ. Super-spreading events and contribution to transmission of MERS, SARS, and SARS-CoV-2 (COVID-19) J Hosp Infect. 2020;105:111–2. doi: 10.1016/j.jhin.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sneppen K, Nielsen BF, Taylor RJ, et al. Overdispersion in COVID-19 increases the effectiveness of limiting nonrepetitive contacts for transmission control. Proc Natl Acad Sci U S A. 2021;118 doi: 10.1073/pnas.2016623118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bulfone TC, Malekinejad M, Rutherford GW, et al. Outdoor transmission of SARS-CoV-2 and other respiratory viruses: a systematic review. J Infect Dis. 2021;223:550–61. doi: 10.1093/infdis/jiaa742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pan A, Liu L, Wang C, et al. Association of public health interventions with the epidemiology of the COVID-19 outbreak in Wuhan, China. JAMA. 2020;323:1915–23. doi: 10.1001/jama.2020.6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flaxman S, Mishra S, Gandy A, et al. Estimating the effects of non-pharmaceutical interventions on COVID-19 in Europe. Nature. 2020;584:257–61. doi: 10.1038/s41586-020-2405-7. [DOI] [PubMed] [Google Scholar]
- 29.Hu S, Wang W, Wang Y, et al. Infectivity, susceptibility, and risk factors associated with SARS-CoV-2 transmission under intensive contact tracing in Hunan, China. medRxiv. 2020 doi: 10.1101/2020.07.23.20160317. 03 Nov 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferretti L, Wymant C, Kendall M, et al. Quantifying SARS-CoV-2 transmission suggests epidemic control with digital contact tracing. Science. 2020;368 doi: 10.1126/science.abb6936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bae S, Kim H, Jung T-Y, et al. Epidemiological characteristics of COVID-19 outbreak at fitness centers in Cheonan, Korea. J Korean Med Sci. 2020;35:e288. doi: 10.3346/jkms.2020.35.e288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Groves LM, Usagawa L, Elm J, et al. Community transmission of SARS-CoV-2 at three fitness facilities — Hawaii, June–July 2020. MMWR Morb Mortal Wkly Rep. 2021;70:316–20. doi: 10.15585/mmwr.mm7009e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jang S, Han SH, Rhee J-Y. Cluster of coronavirus disease associated with fitness dance classes, South Korea. Emerg Infect Dis. 2020;26:1917–20. doi: 10.3201/eid2608.200633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Althouse BM, Wenger EA, Miller JC, et al. Superspreading events in the transmission dynamics of SARS-CoV-2: opportunities for interventions and control. PLoS Biol. 2020;18:e3000897. doi: 10.1371/journal.pbio.3000897. [DOI] [PMC free article] [PubMed] [Google Scholar]