Abstract
Piper betle L. (synonym: Piper betel Blanco), or betel vine, an economically and medicinally important cash crop, belongs to the family Piperaceae, often known as the green gold. The plant can be found all over the world and is cultivatedprimarily in South East Asian countries for its beautiful glossy heart‐shaped leaves, which are chewed or consumed as betelquidand widely used in Chinese and Indian folk medicine, as carminative, stimulant,astringent, against parasitic worms, conjunctivitis, rheumatism, wound, etc., andis also used for religious purposes. Hydroxychavicol is the most important bioactive compound among the wide range of phytoconstituents found in essential oil and extracts. The pharmacological attributes of P. betle are antiproliferation, anticancer, neuropharmacological, analgesic, antioxidant, antiulcerogenic, hepatoprotective, antifertility, antibacterial, antifungal and many more. Immense attention has been paid to nanoformulations and their applications. The application of P. betle did not show cytotoxicity in preclinical experiments, suggesting that it could serve as a promising therapeutic candidate for different diseases. The present review comprehensively summarizes the botanical description, geographical distribution, economic value and cultivation, ethnobotanical uses, preclinical pharmacological properties with insights of toxicological, clinical efficacy, and safety of P. betle. The findings suggest that P. betle represents an orally active and safe natural agent that exhibits great therapeutic potential for managing various human medical conditions. However, further research is needed to elucidate its underlying molecular mechanisms of action, clinical aspects, structure–activity relationships, bioavailability and synergistic interactions with other drugs.
Keywords: Betelvine (Piper betle L.), ethnobotany, hydroxychavicol, nanoparticles, pharmacology, phytochemicals
1. INTRODUCTION
Piper betle L. (synonym: Piper betel Blanco) (Piperaceae) is a widely known perennial creeping plant belonging to the genus Piperaceae and originates from central and eastern Peninsular Malaysia and is distributed to East Africa and tropical countries of Asia. 1 It is a commercial cash crop cultivated mainly in India, Bangladesh, Sri Lanka Thailand, Taiwan, Malaysia and few other Southeast Asian countries. 2 , 3 The betelvine is called the ‘green gold of India’ because almost 20 million people depend on this plant to derive their source of income from the production, transportation, handling, processing and preparation of betel leaves. 4 , 5 The betel vine is usually an asexually propagated plant that has various cultivars and bears both male and female plants. About a hundred varieties of betel plants are found across the world, among them 40 varieties are found only in India and of which 30 are recorded from West Bengal and Bangladesh. 6 The most common varieties of betel are Magadhi, Salem, Mysore, Bangla, Kauri, Venmony, Meetha, Kapoori, Sanchi, Banarasi, Desavari, Kasi, Ghanagete and Bagerhati, which are mainly based upon their colour, aroma, taste and size. 1 P. betle is known by various names in different countries around used globe, though ‘Paan’ is the most used in India, Pakistan, Nepal and Bangladesh. 7 The betel leaf and areca nuts play a central role in Hindu culture as they are used in a variety of social, cultural and religious ceremonies. 1 Betel quid is a common practice in many countries because it acts as a natural tonic and mouth refresher to prevent oral malodour. The International Agency for Research on Cancer surveyed and estimated that there are 200–600 million users present globally (Refs 251 , 271 ; IARC).
The use of P. betle is found in many traditional medicinal systems, such as the Indian Ayurvedic medicinal system, traditional Chinese medicine, and also in the folklore medicinal system of the West Indies and Latin America. In the Ayurvedic medicine system, P. betle plants are used as preparation varieties for the treatment of many diseases, known as Lokantha Rasa, Puspadhava Rasa, Laghu‐sutaseknara Rasa, Lanha, Brhat sarwajwarahara and Brhat visamaj warantaka Rasa. The juice prepared from the betle leaf is generally used as an adjuvant in many herbal combinations with different other medicinal plants for better results in Ayurveda. 8 Traditionally, the plant is used to cure many ailments such as cold, bronchial asthma, cough, stomachalgia and rheumatism, and it is used for the treatment of other diseases such as boils, bad breath, constipation, conjunctivitis, gum swelling, abscesses, injuries and cuts, which are communicable or noncommunicable. 9 The use of this plant is also found in other purposes, such as in fish poisoning, fish bait, insecticides, ornaments, oils, perfumes and hallucinogens. 10
Pharmacological properties of medicinal plants are primarily attributed to a variety of bioactive phytochemicals with biomedical and pharmaceutical significance. 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 Plants are known to house a number of different classes of phytoconstituents 19 , 20 , 21 such as alkaloids, glycosides, tannins, phenolic compounds, flavonoids, terpenes and oligosaccharides. 22 , 23 , 24 Such phytochemicals have also been reported against an array of human ailments. 20 , 21 , 25 The strong pungent aroma comes from the leaves of betel because the essential oil contains a good quantity of terpenes and phenols. 26 , 27 , 28 The essential oil from betel leaf is to some extent a greasy, slippery and viscous liquid at room temperature. A wide diversity of bioactive compounds is present in the leaves of betel, this difference is based on the environment, soil types, the location of growing and types of landraces. 7 The wide range of phytochemicals present in the betel plants was identified as chavicol, chavibetol, hydroxychavicol, eugenol, estragole, methyl eugenol, hydroxycatechol, α‐pinene, caryophyllene, β‐pinene, 1,8‐cineol and others. 29 In a recent report, combining herbs (P. betle leaves) and herbo‐minerals (Swarna bhasma) in a dosage‐dependent manner was used to treat Corona patients by enhancing their prophylactic and therapeutic effects. 30
Various extraction methods are used for the extraction of volatile oils from the leaves of betel, including hydrodistillation, steam distillation, solvent extraction and supercritical fluid extraction that were characterized by GC‐MS, NMR. 1 Huge numbers of studies have revealed the efficacy of the bioactive compounds present in essential oil as antioxidants to prevent cancer, inflammation, neurodegenerative disorders, and also as antimutagenic, antifertility, antilipidaemic, antiglycaemic, cardioprotective, etc. 31 , 32 The essential oil of betel leaves can also combat bacterial, protozoan and fungal infections and insect attacks.
This review comprehensively summarizes the botanical description, economic status, pharmacological properties, nanoformulations and their applications taking into account the safety and toxicity. In addition, the underlying molecular basis of the action of plant extracts or phytochemicals are also discussed. Considering the immense potential of this underexploited medicinal plant, the present review comprehensively describes the present state of the art research on this plant with an interdisciplinary approach that includes the pharmacology, nanotechnology, preclinical and clinical studies and also potential toxicological considerations of using P. betle preparations. However, more studies are needed to enumerate the structure–activity relationships behind the pharmacological activity of plant constituents. Detailed clinicalstudies are also needed, and the pharmacokinetic properties and druggability ofsuch preparations need to be elucidated.
2. TAXONOMY
Taxonomical classification
Kingdom: Plantae
Division: Magnoliophyta
Class: Magnoliopsida
Order: Piperales
Family: Piperaceae
Genus: Piper
Species: Piper betle L. 33
3. BOTANICAL DESCRIPTION
The plant (Figure 1) is a dioecious root climber, and the shoots reach any height from 3 to 10 m according to available facilities for climbing. The plant bears lateral branches along its entire length that grow a couple of feet from the ground. The stems are swollen and articulate, with dichotomous branching and rooting at the nodes. The stems are stout, almost terete, slightly flattened; when young, they are light green and marked by short, raised, whitish streaks and with pinkish stripes along the node. The internodes generally attain a length of about 12 cm. and a diameter of 1.2 cm. Leaves are characterized as a simple blade, alternate, spiral and ex‐stipulate; petioles are 2–5 mm long, pubescent and channelled. Leaf blades are glabrous, coriaceous, fleshy, greenish to yellowish, shining, broadly ovate, width 7–8.5 cm, length 9–11 cm; base cordate; apex acuminate; margin is entire, narrowly recurved; venation reticulate, 7–9 veins in two or three pairs coming from the midrib, one pair elevating from base. The inflorescence is an axillary spike up to 5.5 cm long. The male inflorescence forms a cylindrical pendulous catkin of 10 cm in length and 2 cm in diameter. Female spikes are also cylindrical, pendulous; length 2.5–4 cm and diameter 0.5 cm. Individual flowers are very minute and unisexual, reduced, consisting of a couple of stamens and stigmas inserted into the axil of each bract. The bracts are orbicular, peltate, arranged in a thickly crowded spiral series. The mature inflorescence is strongly aromatic. Fruiting spikes are 3–5 cm in length, orange and drupping, entrenched on the rachis of the mature inflorescence. 34 , 35
FIGURE 1.
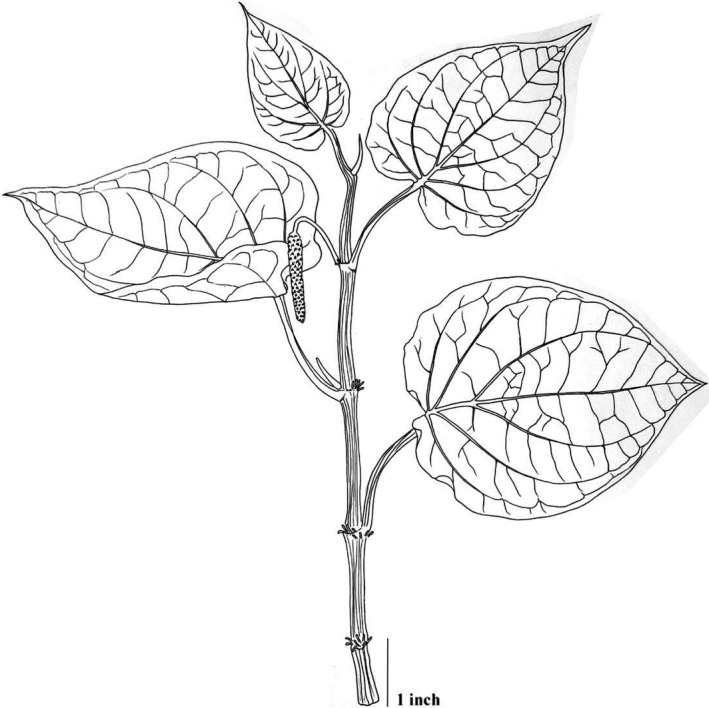
Piper betle L.: Habit sketch
4. VERNACULAR NAME
Vernacular names in Indian languages
Sanskrit: Tambool, Mukhbhushan, Nagavalli, Varnalata, Nagavallari
Hindi, Bengali, Urdu: Paan
Telugu: Nagballi, Tamalapaku
Tamil: Vetrilai
Gujarati: Nagarbael
Marathi: Vidyache pan
Malayalam: Vettilakkoti, Vettila
Kannada: Veeleya, Veeleyada yele, Vilya, Villayadel
Konkani: Phodi paan
Other Asian languages
English: Betle, Betle pepper, Betle‐vine
Vietnamese: Tråu
Khmer: Maluu
Mon: Plu
Thai: Plue, Pelu
Persian: Burg‐e‐Tanbol
Chamorro: Papulu
Javanese: Suruh, Sirih, Bodeh
Arabic: Tanbol
Sakai: Jerak
Semang: Seresa, Be, Cabe
Sinhalese: Bulath
Jakun: Kerekap, Kenayek
Malay: Daun sirih, Sirih hudang, Sirih Carang, Sirih melayu
5. DISTRIBUTION AND CULTIVATION
The betel vine is believed to have originated in Malaysia. 38 The plant is widely grown in forests that are generally damp and also in hot and moist climatic conditions of India and many other countries in South and South East Asia, viz. China and Vietnam. Piper betle is believed to have first emerged in tropical Asia and then spread to East Africa and Madagascar. Betel is widely grown in India, Sri Lanka, Bangladesh, Indonesia, Nepal, Pakistan, Vietnam, Thailand, Laos, Kampuchea, Philippine Islands, Burma, Malaysia, Taiwan, Malay Peninsula and many countries in Southeast Asia and is known to have a long history, mentioning the presence of the betel plant over 2000 years. 39 Figure 2 presents the worldwide distribution of the species. In India, the plant is found in Bengal, Bihar, Orissa, Andhra Pradesh, Karnataka, Uttar Pradesh and Tamilnadu. 40 , 41 , 42 About a hundred betel plant varieties are found all over the world,among them, 40 are grown in India, along with 30 are grown in Bangladesh and West Bengal. 43 Various types of Piper betle are found across the world, for instance, Magadhi, Kauri, Meetha, Salem, Venmony, Bangla, Banarasi, Kapoori, Kasi, Sanchi, Mysore, Desavari, Ghanagete and Bagerhati according to their size, colour and aroma. 44
FIGURE 2.
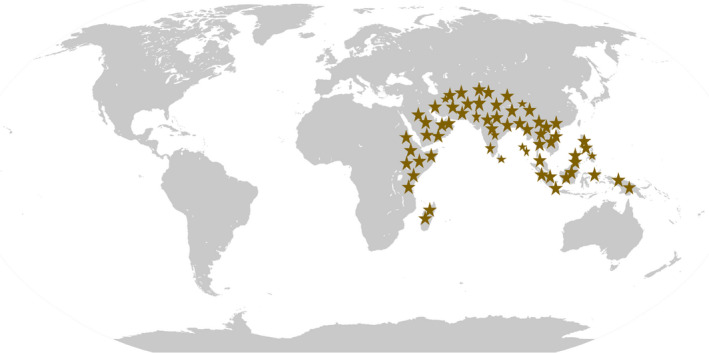
Geographical distribution of Piper betle L. throughout the world
Piper betle is generally propagated asexually by cutting stems rather than germinating seeds. 45 It needs a compatible tree or long support for its creeping habit. Betel vine cultivation is a very typical type of farming. For betel cultivation, the best choices are highlands and especially fertile sandy or sandy clay or sandy loam soil with a well drainage system and a pH range of 5.6–8.2, thus, saline and alkali soils where water logging are a problem is not suitable; about 2250–4750 mm rainfall, relative humidity 40–80% and temperature range 15–40°C are considered suitable. In Bangladesh, farmers prepare a special garden called ‘barouj’ which is fenced with bamboo sticks and coconut leaves and on top of the fench is covered with paddy leaves to grow betel. The farming land is well dug into furrows of approximately 10–15 m long, 75 cm wide and 75 cm deep. The furrows are thoroughly manured with cow dung, rotten farmyard manure, oil cakes, leaves and wood ash. After proper dressing, the cuttings are planted at the beginning of the monsoon, in the months of May to June. Then, the plants are parallelly arranged in rows with a distance of two feet between each plant and are bound with a string around upright sticks of split bamboo or short plants for support. Proper shade and frequent irrigation are necessary in areas where rainfall is lower about 1500–1700 mm; regular watering is required in summer and watering every 3–4 days is sufficient in winter, and a proper drainage system is mandatory at the time of rainy season for the successful cultivation of this crop. After 1 year of planting, the leaves of the plant turn out to be ready for plucking, and the production of betel leaf from the barouj lasts for more than a few years from the time of planting. 41 , 43 , 46
6. ECONOMIC STATUS
In the Indian climate, the female plants of Piper betle rarely produce flowers or fruit. Betel vines are cultivated and harvested mainly for their heart‐shaped green leaves. 41 This crop has a vast economic potentiality which can be effectively recognized by the piece of evidence that more or less 15–20 million of people in India have the habit of using betel leaves regularly 47 not only that, there are more than 2 billion people from many other countries who are recognized as regular users of betel leaves from all over the world. 48 Most importantly, the economic status of betel leaves is dependent on the physical character of the end products in the worldwide market. The betel leaf and products produced in different forms such as powder, capsules, liquid and various types of value‐added products are available on a broad spectrum in the market as beverages, in oral care, pharmaceutical products and cosmetics. 41 The annual turnover national income is Rs 7000–10,000 million, and from this, the state, West Bengal, gains an income of 800–1000 million rupees per year. The leaves were exported to various countries around the world where the plant is not grown naturally or the local supply could not meet the requirements. Betel leaves are generally exported to Hong Kong, Pakistan, Italy, Bahrain, Canada, Great Britain, Kuwait, Saudi Arab, Nepal and several other countries in Europe. 47 , 49
7. TRADITIONAL AND ETHNO‐MEDICINAL USE
Traditional medicine has played a crucial role in the health care of the rural and urban people. 246 , 247 , 248 Ethno‐medico‐botanicals have been used across almost all the cultures worldwide against an array of human medical conditions. 50 , 51 , 52 , 53 The use of betel leaf alone and with a combination of other plants or medicines for better therapeutic effects is mentioned in the Ayurvedic literature, which was almost 1400 BC ago. 54 Atharved, the ancient Vedic literature, mentioned the usefulness of the betel plant against numerous diseases at about 3000–2500 BC before. 55 Saptasira, the Vedic name of the leaves of betel, is mentioned in the Kamasutra of Vatsyayan as having aphrodisiac properties. 44 In the ayurvedic and Unani system of medicine, the betel plant is used as an anthelmintic, appetite stimulant, vermifuge, astringent, diarrhoea, aphrodisiac, breath freshener, carminative, cardiac tonic, dentifrice, in the prevention of diuretic emmenagogues, induction and increase of menstrual flow, laxative, strengthen gums, nerve tonic and also in the treatment of urinary disorders. Betel leaves are mostly chewed by about 200 million people on a regular basis throughout the south Asia and western part of the Pacific basin in a special shape of packets known as ‘Betel quid’, which is prepared from Piper betle leaves brushed with burnt lime and contain few pieces of areca nut, flavours, often cardamom or cloves, are added with or without tobacco according to choice. 56 Chaveerach et al. stated that the betel leaf is a most important material in Thai ceremonies. Elderly people chew betel leaves to prepare quid. In weddings, the family members of the bridegroom place money along with the betle leaves in a bowl, which together is known as khun maak. The ethnic group Kui, from the southern division of North East Thailand, uses betel leaf (locally, raam phi taan) in the ‘Spirit dancing’ ceremony to chase away evil spirits or fend off bad luck from the patients from the family or the village. They use betel leaves as stimulant, exhilarant, antiseptic and antioxidant, to treat kidney inflammation and thirst resulting from diabetes, strength to stomach, as expectorant for asthma, coughs and bronchitis, and antiflatulent element. 34 Decoction of P. betle leaves used to prevent body odour and treat diarrhoea, sore throat, skin allergies and fluor albus, leaves are cooked and added to vegetable soup. 57 In Southeast Asia, Betel chewing with its associated discoloration of the teeth is the ascriptions of the teeth blackening practice related to sexual maturation and becoming a full member of society in Masticans. 58 In the Laleng community, people use betel leaf to chew and at the sociocultural festival. They oil the leaf with mustard oil and place it on the naval area to relieve liver pain. 59 The Rabha community of Mataikhar forest, Assam, the Torajanese, the Bugis community and Lakshadweep people also use betel leaf for chewing and in religious festivals. 60 , 61 , 62 , 63 People in Parsa district, Nepal, chew betel leaf or mix leaf juice with hot water, honey or milk mild stimulant, cure worm, remedy for bad breath and provides mouth refreshment, improve digestion, strengthen teeth and gums, palate cleaner, treatment of nervous pains and exhaustion, ease of urination, analgesic, reduce cough and cold. 64 The ethno‐medicinal uses of P. betle in the area and community are listed in Table 1.
TABLE 1.
Ethnomedicinal uses of P. betle
| Local name | Community/tribe and region | Part used and preparation | Medicinal property/used against | Reference |
|---|---|---|---|---|
| Paan | Dibru‐Saikhowa Biosphere Reserve of Northeast India | leaf infusion | abdominal pain | 267 |
| Daing | Kadazandusun communities around Crocker range | leaf tea taken orally, paste applied topically | cough, scabies, boils, nosebleed | 239 |
| – | Thailand | leaves | stimulant, exhilarant, antiseptic and antioxidant, kidney inflammation and thirst resulting from diabetes, strength to stomach, expectorant effect for coughs, asthma and bronchitis, antiflatulent material | 34 |
| paan | Assam | crushed leaf juice | pediculosis | 269 |
| Tamalapaku | Andhra Pradesh, India | leaves | asthma | 271 |
| Paan | Garo tribal community, Netrakona district, Bangladesh | paste of leaf and petiole singly or in combination | against bronchitis, indigestion, and as an antidote to poison against bronchitis, indigestion, and as antidote to poison | 268 |
| Vettrilai | Villupuram district of Tamil Nadu, India | fresh leaves chewed or immersed with sesame oil, then warmed with flame | for digestive, stimulative, carminative, aphrodisiac, applied for headaches and lactogogue | 270 |
| Eman | Bulu and inland Kaulong of Papua New Guinea | pulped leaves used topically | to cure swollen limbs | 262 |
| Vertrilai | Kalrayan Hills, Eastern Ghats, Tamil Nadu | leaves | digestive problem | 255 |
| paan | tribal and native people of Madhupur forest area, Bangladesh | decoction of leaves, leaf juice | nerve pain. for joint pain, cough, and oedema | 250 |
| Paan | Rabha community of Mataikhar reserve forest, Kamrup district, Assam, India | leaf | castor oil is smeared on leaves, warmed and applied to affected areas for arthritis, cold, cough and headache | 61 |
| Base, sirih, | Bali, Indonesia | decoction of leave | body odour, and for treating diarrhoea, sore throat, skin allergies, fluor albus | 57 |
| patiwa | Chungtia village, Nagaland, India | leaf paste used topically or chewed with lime, areca nut and tobacco | cure cuts and wounds, to treat dental caries | 253 |
| pan | Parsa district, Nepal | leaf chewing, leaf juice mixed with hot water, honey or milk | mild stimulant, cure worm, remedy for bad breath and provides mouth refreshment, improve digestion, strengthen teeth and gums, palate cleaner, treatment of nervous pains and nervous exhaustion, ease urination, analgesic, reduce cough and cold | 64 |
| Ikmo | Sambal‐Bolinao of Pangasinan, Philippines | leaves heat with oil and salt | rub on the body the body of jaundice patient | 249 |
| betle | Tobelo Dalam tribe in Aketajawe Lolobata National Park Area | leaves boil with water and taken orally | postpartum pain | |
| Sirih/ betle | Southern slope of Mount Merapi, Yogyakarta, Indonesia | leaf | relative cough | 257 |
8. PHYTOCHEMICAL PROFILE
Piper betle is one of the extensively investigated plants for its various phytochemical constituents present in it, and the study revealed that the plant contains a wide range of phytochemicals that are biologically active. Compound concentrations depend on the different varieties of the plant, season, climate and may geographical location and also might be influenced by various factors such as soil, humidity, agronomic practices, rainfall, season and type of plant. 65 The main phytochemical constituents of the essential oil of the betel leaf are mainly phenols and terpenes. 66 The phenol content varies by gender, total phenols are three times higher in male plants, and the thiocyanate content is two times higher compared to female plans. Leaf quality is basically dependent on the phenol content; more phenol content comes with better leaf quality. 67 The typical pungent aroma of the betel leaves is the result of the phenols present in them. Preliminary photochemical studies of aqueous and methanol extracts of betel leaves revealed the presence of alkaloids, flavonoids, tannins, sterols, phenols, glycosides, saponins and terpenoids. 68 Syahidah et al. also identified alkaloids, phenols, flavonoids, saponins, steroids, tannins, terpenoids and glycosides from qualitative analysis of the methanolic extract of the betel leaves. 175 Leaves also contain bitter compounds (0.7–2.6%). 2 Terpenoids and their acetates, including cadinene, 1,8‐cineole, chavicol, chavibetol, safrole, camphene, limonene, caryophyllene, pinene, carvacrol, allylpyrocatechol and eugenol, are present in P. betle as the main phenols. 2 , 69 A recent work with the leaves was found to contain starch, diastases, sugars (0.8 to 1.8%) and an essential oil in an amount of 4.2%. 70 The presence of tannins and steroidal components was revealed by phytochemical investigation on leaves. 71 The main components of betel leaf oil are safrole (48.7%), chavibetol acetate (12.5%) allylpyrocate choldiacetate (34.0%), along with ρ–cymene, 4‐terpinol, eugenol, β‐caryophyllene. 72 There are two sesquiterpenes, cadinene and caryophyllene and safrole (52.7%), eugenyl acetate (5.8%), allylpyrocatecholdiacetate (15.4%) and eugenol (6.4%) are also reported as the main elements of the essential oil of the P. betle leaf from Sri Lanka. 67 The leaves were also found to produce an alkaloid, namely arakene, which possesses properties similar to those of cocaine. The chemical compositions of essential oil differ in different parts: leaf, stem, stalk and root contain safrole, while fruits contain β ‐phellandrene. Younger leaves of betel contain more amount of essential oil. 73 Phytochemical analysis of two varieties of betel leaves, Kamarvetrilai and Kumbakonamvetrilai, confirmed cardiac glycosides, acids and steroids along with tannins, saponins and flavonoids. 74 In another experiment, four cultivars of P. betle—Banarasi, Calcutta, Kammar and Kumbakonam—showed positive results in tannin, flavonoid and terpenoid tests,plobatannins found in the Banarasi cultivar, Banarasi and Kammar gave positive results for saponins,cardiac glycosides found in the Banarasi and Kumbakonam cultivars. 75 Pipercerebrosides A and B are two new sphingolipids isolated and identified by NMR (Nuclear magnetic resonance) spectroscopy of betel leaf extract. 76 GC‐MS (Gas chromatography–mass spectrometry) studies identified all compounds that can be divided as monoterpene (α‐thujene, α‐pinene, camphene, sabinene, myrcene, β‐phellandrene, α‐terpinene, (e)‐β‐ocimene, 1,8‐cineole/eucalyptol, γ‐terpinene, terpinolene, linalool, terpinen‐4‐ol, α‐terpineol), sesquiterpenes (δ‐elemene, α‐copaene, β‐copaene, β‐elemene, e‐β‐caryophyllene, γ‐elemene, β‐selinene, aromadendrene, α‐humulene, germacrene d, α‐selinene, γ‐muurolene, bicyclogermacrene, α‐muurolene, cis‐β guaiene, δ‐cadinene, palustrol, spathulenol, caryophyllene oxide, globulol, viridiflorol, cubenol, α‐cadinol), and phenylpropane (estragole/methyl chavicol, chavicol, anethole/isoestragole, safrole, chavicol, acetate, eugenol, methyl eugenol, eugenol acetate). 77 Betel vine also contains dotriacontanoic acid, stearic acid, piperlonguminine, hentriacontane, n‐triacontanol, pentatriacontane, triotnacontane, isoeugenol, allylpyrocatecholdiacetate, α‐pinene, β‐sitosteryl palmitate, 1, 8‐cineol, ursolic acid, β‐sitosterol, β‐pinene, sitosterol, ursolic acid 3β‐acetate and stigmasterol. Betel roots possess ursonic acid, piperlonguminine, stearic acid, β‐sitosteryl palmitate, β‐sitosterol, 3β‐acetyl ursolic acid, 4‐allyl resorcinol, aristololactam A II and stigmast‐4‐en‐3, 6‐dione. The betel stems were found to have stigmast‐4‐en‐3, piperine, piperlonguminine, piperdardine, dehydropipernonaline, guineensine, 6‐dione, aristololactam A‐II, pellitorine, 4‐allyl resorcinol, syringaresinol‐O‐β‐D‐glucopyranoside, N‐isobutyl‐2E,4E‐dodecadienamide, pinoresinol, piperolein‐B, cepharadione A, dotriacontanoic acid, β‐daucosterol, tritriacontane, β‐sitosterol, α‐ethyl glucoside (2E,4E)‐N‐isobutyl‐7‐(3’,4'‐methylenedioxyphenyl)‐2,4‐heptadienamide, 23‐hydroxyursan‐12‐en‐28‐oic acid, (2S)‐4'‐hydroxy‐ 2,3‐dihydrofl avonone‐7‐O‐β‐D‐glucoside and β‐sitosterol‐3‐O‐β‐D‐glucoside‐6'‐O‐palmitate. 78 , 79 , 80 , 81 Gas chromatography mass spectrometry (GC–MS) analysis of fresh and cured leaves of the essential oil of P. betle var Bangla fresh and cured leaves revealed a total of thirty‐three phytochemicals and a total of thirty volatile components, respectively, with high abundance of estragole, eugenol, linalool, anethole, α‐copaene, chavicol and caryophyllene. 1 Very recently, Islam et al. studied volatile oils from five varieties of betel such as Bangla, Misti, Khasi, Sanchi and Bari and found a total of 101 volatile oil compounds, which are much higher in number than previous reports with 50 compounds identified for the first time. 82 Table 2 represents the phytochemical constituents of P. betle. Figure 3 represents the chemical structures of some phytochemicals reported from the species.
TABLE 2.
Phytochemical constituents of P. betle
| Plant part/Extract/Essential oil | Techniques | Chemical compounds | References |
|---|---|---|---|
| Aqueous extract of leaves | GC/MS | 2,3‐bis(hydroxy)propyl ester, 2‐monopalmitin, α‐hydroxy, alpha‐hydroxyphenyl, benzeneacetic acid, benzeneacetic acid, hexadecanamide, hexadecanoic acid, hexadecanoic acid, hydroxychavicol, myristic acid, octadecanoic acid, octadecanoic acid | 258 |
| Essential oil from leaves | GC/MS | 4‐allyl‐1,2‐diacetoxybenzene, acetyleugenol, bicyclo(4.1.0)hept‐3‐en‐ camphene, chavicol, cis‐ocimene, cyclohexene,4‐methyl‐decanal, eugenol, germacrene B, germacrene D, globulol, ledene, linalyl acetate, l‐limonene, methyl‐eugenol, phenyl acetylaldehyde, t‐caryophyllene, t‐ocimene, undecanal, α –humulene, α‐pinene, β‐elemene, β‐myrcene, γ‐cadinene, γ‐ionene, γ‐muurolene | 261 |
| Leaf extract | DART‐MS | chavicol, allylpyrocatechol, chavibetol, phenyl alanine, chavicol acetate, allylpyrocatechol acetate, chavibetol acetate, allylpyrocatechol, diacetate | 244 |
| Acetone extract and different fractions of leaf | UV/VIS/NIR, NMR, HR‐ESI‐MS, GC/MS |
Sphingolipids ‐ pipercerebroside A pipercerebroside B |
76 |
| Volatile oil from leaves | GC/MS | β‐ caryophyllene, α‐farnesene, α‐humulene, germacrene b, germacrene d | 260 |
| Hexane extract of leaves | GC/MS | 2,3‐dihydro‐3,5‐dihydroxy‐6‐methyl‐4h‐pyran‐4‐one, phellandrene, α‐terpinene, p‐cymene, sabinene, ɣ‐terpinene, o‐guaiacol, linalool, tujene, terpine‐1‐ol, terpine‐4‐ol, α‐terpineol, safrole, eugenol, isoeugenol, α‐copaene, β –bourbonene, methyleugenol, β –caryophyllene, β –cubebene, ɣ‐cadinene, α‐humulene, β‐selinene, α‐selinene, caryophyllene oxide, camphene, germacrene b, longifolene, phytol | 245 |
| Ethanol extract of leaves | GC/MS | heptafluorobutyrate, ethyl diazoacetate, 4‐(2‐propenyl)phenol, 3‐fluoro‐2‐propynenitrite, eugenol, tris(trifluoromethyl)phosphine | |
| Aqueous and ethanol extracts of leaves | GC/MS |
amino acid: alanine, valine, isoleucine, proline fatty acids: palmitic acid, linoleic acid, linolenic acid, oleic acid, stearic acid, palmitic acid derivatives sterols: cholesterol, cholesterol derivatives, stigmasterol, β ‐sitosterol |
259 |
| Ethanol extract of leaves | GC/MS | 1‐phenylpropene‐3,3‐diol diacetate, eugenol, 4‐chromanol, 4‐allyl‐1,2‐diacetoxybenzene, hydroxychavicol (1‐allyl‐3, 4‐dihydroxybenzene) | 256 |
| Chloroform extract of leaves | 1D NMR, 2D NMR, ESI‐MS, FT‐IR and HR‐ESI‐MS | s 1‐n‐dodecanyloxy resorcinol (H1) and desmethylenesqualenyl deoxy‐cepharadione‐A | 243 |
| Ultrasound‐assisted extract of leaves | GC/MS | hydroxychavicol, eugenol, isoeugenol, and 4‐allyl‐1,2‐diacetoxybenzene | 240, 241 |
| Leaf aqueous extract of varieties bangla, bagerhati, manikdanga, meetha, kalibangla, chhaanchi, ghanagete and haldi | GC/MS |
amino acids: l‐glutamic acid (dehydrated), l‐pyroglutamic acid, l‐tryptophan, organic acids: citric acid, 3,4‐dihydroxyphenylacetic acid, fumaric acid, gluconic acid, gluconic acid lactone, glyceric acid, glycolic acid, 3‐hydroxy‐3‐methylglutaric acid, 4‐hydroxyphenylacetic acid, isocitric acid, l‐(+) lactic acid, maleic acid, malic acid, malonic acid, nicotinic acid, oxalic acid, 3‐phenyllactic acid, ribonic acid‐gamma‐lactone, succinic acid, sugars: methyl‐β‐d‐galactopyranoside, isopropyl‐β‐d‐1‐thiogalactopyranoside, phenyl‐β‐glucopyranoside, sucrose, raffinose, d‐(+)trehalose, sugar alcohols: arabitol, galactinol, glycerol, d‐mannitol, d‐sorbitol fatty acids: lauric acid, myristic acid, palmitic acid, stearic acid, phenols: o‐acetylsalicylic acid, p‐anisic acid, benzene‐1,2,4‐triol, caffeic acid, chlorogenic acid, chrysin, cinnamic acid, coniferyl alcohol 3,4‐dihydroxybenzoic acid, ferulic acid, gentisic acid, hydroquinone, 2‐hydroxyacetophenone, 4‐hydroxybenzoic acid, hydroxychavicol, 3‐hydroxycinnamic acid, 4‐hydroxycinnamic acid, 4‐hydroxy‐3‐methoxybenzoic acid, 2‐(4‐hydroxyphenyl)ethanol, 4‐(2‐hydroxyethyl)phenol (tyrosol), 3‐(4‐hydroxyphenyl)propionic acid(synonym: hydro‐p‐coumaric acid), piceatannol, shikimic acid, quinic acid, terpenoid, loganin other organic compounds: adenosine, (‐)‐epinephrine, indole‐3‐acetamide, porphine |
77 |
| Leaves volatile compound from five varieties (bangla, khasia, misti, sanchi, bari) | SDE‐ GC/MS | (E)‐2‐hexenyl acetate, (E)‐cadina‐1,4‐diene, (E)‐cadinol, (E)‐calamenene, (E)‐ocimene, (E)‐Verbenol, (Z)22‐pentenyl acetate, (Z)‐3‐hexenyl‐1‐acetate, (Z)‐a‐ bergamotene, (Z)‐a‐bisabilene, (Z)‐aabinene hydrate, 4‐d‐carene, 1‐hexanol, 1‐H‐indol, 1‐nor‐bourbonanonee c, 2,3‐butanediyl diacetate, 2‐ethylfuran, 2‐hexen‐1‐ol, 2‐hexenal, 2‐penten‐1‐ol, 2‐pentylfuran, 2‐phenylethyl acetate, 3‐hexen‐1‐ol, 3‐hexenal, 4‐allylphenyl acetate, 4‐vinyl guaiacol, 9‐epi‐b‐caryophyllene, α‐amorphene, α ‐curcumene, α ‐guaiene, α ‐humulene, α ‐muurolene, α ‐muurolol, α ‐nerolidola c, α ‐pinene, aromadendrene, α ‐terpineol, α ‐thujene, β‐(z)‐bergamotene, β ‐bisabolol, β ‐caryophyllene, β ‐cyclocitral, β ‐elemene, benzaldehyde, benzyl acetate, β ‐pinene, β ‐selinene, β ‐spathulenol, cadalene, cadin‐4‐en‐10‐ol, cadina‐1(6),4‐diene, c‐amorphene, camphene, caryophyllene oxide, ɣ‐elemene, chavicol, ɣ ‐muurolene, ɣ ‐terpinene, cubebol, δ‐cadinene, decanal, dehydrocineole, dimethylallyl acetate, epicubenol, eremophilene, estragole, eugenol, eugenyl acetate, farnesyl acetate, farnesyl acetone, furfuraldehyde, guaiac acetate, hexanal, humulene epoxide ii, isogermacrene d, isophytol, limonene, linalool, linalool oxide acetate, methyl heptenone, methyl salicylate, methyleugenol, n‐butyl benzene, n‐decyl acetate, n‐dodecanal, n‐hexyl acetate, nonanal, octadecanol acetate, oxophorone, p‐cymen‐8‐ol, p‐cymene, phenyl acetaldehyde, phenylethyl alcohol, phytone, pogostol, salvial‐4(14)‐en‐1‐one, sesquisabinene, terpinen‐4‐ol, tetradecanal, undecan‐2‐one, valencene | 82 |
| P. betle var. haldia and maghai | 1D NMR, 2D NMR, ESI‐MS, FT‐IR and HR‐ESI‐MS | 1‐n‐decanoyl hydroxybenzoic acid/1‐n‐decanoyl phenol and 3‐butylphenol | 242 |
FIGURE 3.
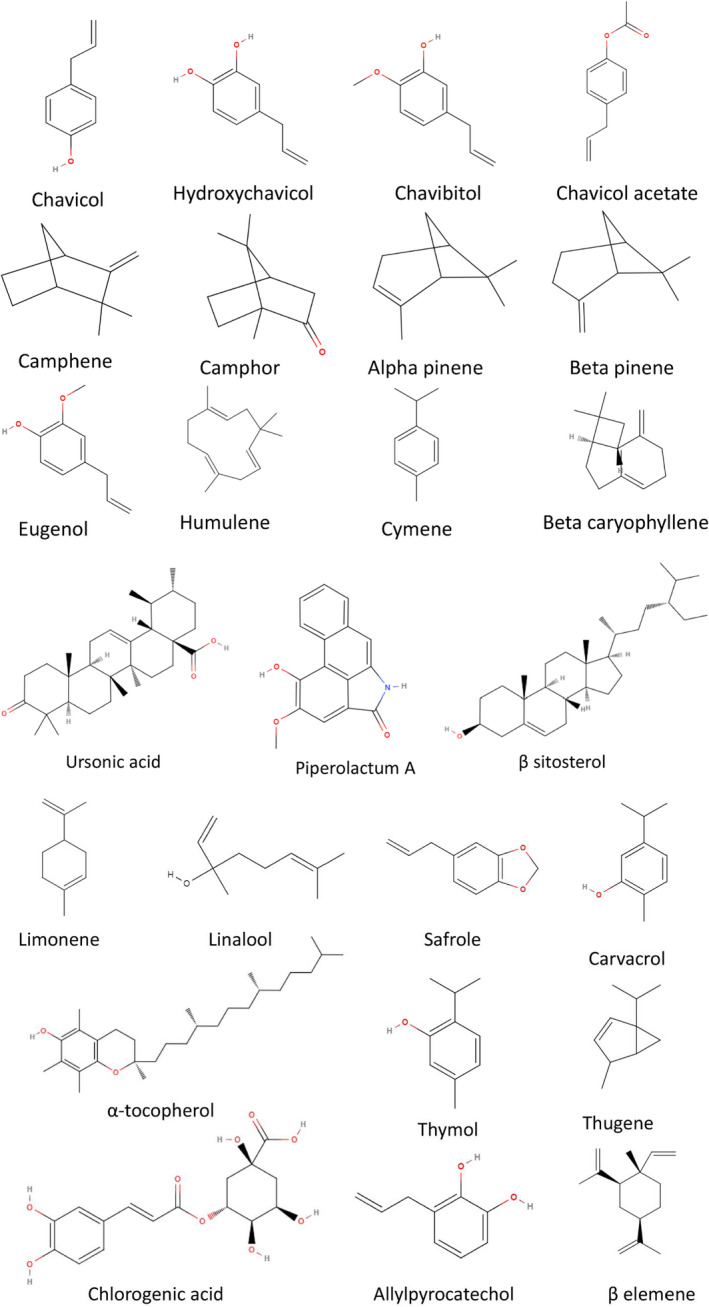
Chemical structures of some phytochemicals reported from the species
9. PHARMACOLOGICAL ACTIVITIES
The following section summarizes the various pharmacological attributes of P. betle (Table 3).
TABLE 3.
Pharmacological activities of P. betle
| Activity | Part used (compound) | Design | Model | Effects | Reference |
|---|---|---|---|---|---|
| Anticancer/Anti tumour/Anti proliferative activity | aqueous extract of leaves | tumour inhibition assay | benzo(a)pyrene‐induced tumours in hamster buccal pouches | both short‐term and long‐term studies, expressed complete or partial suppression of tumour | 232 |
| aqueous extract of the leaves | tumour inhibition assay | dimethylbenz[a]anthracene (DMBA)‐induced mammary carcinogenesis in Holtzman rats | higher doses of the extract inhibited the emergence of tumours | 91 | |
| alcoholic extract (eugenol, hydroxychavicol, β‐carotene and α‐tocopherol) | anticarcinogenicity studies | benzo[a]pyrene‐induced foestomach neoplasia in male Swiss mice | decreased number of papillomas per animal (by β‐carotene and α –tocopherol) | 92 | |
| leaf extract (hydroxychavicol) | tumour suppression assay | 4‐(N‐nitrosomethylamino)‐1‐(3‐pyridyl)‐1‐butanone induced mutagenesis and tumorigenesis in mice | reduced the tumorigenic effects by 25% | 93 | |
| leaf extract (beta‐carotene, alpha‐tocopherol, eugenol and hydroxychavicol) | tumour inhibition assay by topical administration and intraperitoneal injection | 7,12‐dimethylbenz(a)anthracene (DMBA) induced skin tumours in mice | inhibition of tumour formation by 83–94%; eugenol showed minimal protection | 72 | |
| leaf extract (β‐carotene and α‐tocopherol) or combined with turmeric | tumour inhibition assay | methyl (acetoxymethyl) nitrosamine‐induced hamster oral carcinogenesis | inhibition of tumour incidence, reduction of tumour burden, extension of the tumour latency period, and regression of established, frank tumours | 94 | |
| ethanol extract of leaves | morphological studies, MMTV‐RT assay | mammary tumour virus‐induced and 7–12‐dimethylbenz(a)anthracene‐induced rodent mammary tumours | reduced tumour incidence by 75%, tumour burden by >90% | 95 | |
| methanol extract | inhibitory assay of Epstein‐Barr virus (EBV) activation | Raji cells induced by 12‐O‐hexadecanoylphorbol‐13‐acetate | antitumour activity in terms of cancer chemoprevention | 96 | |
| leaf aqueous extract | in vitroneutral red cytotoxicity assay | KB and HeLa cell line | cytotoxicity on the KB cell line 29.5 ± 0.3 µg/ml, no effect towards HeLa cell line | 97 | |
| leaf ethanol extract | in vitroMTS assay | breast cancer cell line T47D | inhibit cell proliferation with IC50 55.2 µg/ml | 101 | |
| water, methanol, ethyl acetate and hexane extracts of leaves | in vitroMTT assay | breast cancer cell line, MCF‐7 | the ethyl acetate and hexane extracts showed dose‐dependent inhibitory effects with IC50 values of 65.00 and 163.30 μg/ml | 99 | |
| methanol extract of leaves (hydroxychavicol) | in vivotumour growth and bioluminescent imaging, MTT assay | prostate cancer PC‐3 cells implanted in male BALB/c nude mice | oral feeding effective in tumour growth inhibition | 100 | |
| aqueous extract of root | in vitroMTS assay | T47D human ductal breast epithelial tumour cell line | reduce 2.8% cell proliferation, induce apoptosis 9.45% | 98 | |
| ethanol extract of leaves | inhibiting proliferation cells and by SubG1 flow cytometry | cervical cancer cells HeLa | growth inhibition with IC50 value 7.13 µg/ml, apoptotic activity with IC50 value 12.5 µg/ml (95.87%) | 102 | |
| leaf extract (hydroxychavicol) | in vitroMTT assay, in vivo histopathologic and immunohistochemical analysis | androgen‐independent human prostate cancer cells, PC‐3, DU145, C4‐2, and 22Rv1; BALB/c nude mice y injected with PC‐3‐luc cells | sensitivity was 22Rv1> C4‐2> PC‐3> DU14; inhibits growth and proliferation via ROS generation and caspase dependant pathway in P‐3 cells | 9 | |
| leaf acetone extract | in vitroMTT assay | lung cancer cell line (A549) | cell toxicity‐ 88.7%, cell death 11.4% | 103 | |
| petroleum ether, ethyl acetate, aqueous, and ethanol extract of leaves | in vivotumour growth study | B16F10 melanoma in C57BL/6 Mice | ethyl acetate extract showed the highest dose dependant reduction in tumour size | 104 | |
| crude ethanol extract | cytotoxicity and suppression of cell migration determination, SRB wound healing assays, evaluation of transdermal patches | human breast cancer MCF‐7 cells | cytotoxicity with an IC50 of 114.3 µg/ml, suppressed cell migration at a dose of 25 µg/ml, developed a transdermal patch containing 0.03% of the extract | 105 | |
| leaf extract (hydroxychavicol) | colony formation assay, Annexin‐V/PI assay, cell cycle and cell death analysis, comet assay, scratch assay, Transwell migration and invasion assays | MIA PaCa‐2, PANC‐1, L929, INT407, NIH‐3T3, Vero and HEK293 cells | inhibits proliferation and epithelial‐mesenchymal transition, migration and invasion of cells, induces DNA damage, mitotic catastrophe and apoptosis | 106 | |
| Analgesic, anti‐inflammatory, antinocepective activity | hot and cold water extract of leaves | tail flick, hot plate, and formalin tests | cross bred albino mice | hot plate and formalin tests were most effective mediated via opioid mechanisms | 110 |
| ethanol extract of leaves | Freund's adjuvant‐induced model of arthritis | rat | anti‐inflammatory and anti‐arthritic effect by down regulation nitric oxide | 111 | |
| water, ethanol, ethyl acetate and hexane extract of leaves | hyaluronidase (HYA), xanthine oxidase (XOD), and lipoxygenase (LOX) inhibition assay | in vitroassay | all extracts showed significant inhibition activity | 112 | |
| ethanol extract of leaves | carrageenan‐induced hind paw oedema model, hot plate, writhing, and formalin tests | Swiss albino mice and Wistar rats | inhibit paw oedema, also reduced writhing and number of lickings in dose dependant manner | 113 | |
| ethanol extract of leaves | acetic acid induced writhing test | Swiss Albino mice | reduced writhing response via modulation of the arachidonic acid pathway | 114 | |
| aqueous extract of leaves | eddy hot plate and heat conduction method | Mice and rats | significant analgesic activity, dose‐dependent increase in latency period | 115 | |
| methanol extract of leaves (9 varieties) | ‐ | LPS induced RAW 264.7 cell line | five varieties showed anti‐inflammatory activity | 116 | |
| Betle leaf essential oil | detection of MMP‐2 and MMP9 using Gelatin Zymography | In vitroassay | 85% anti‐inflammatory activity | 117 | |
| Antidepressant | ethanol extract of leaves | forced swim test and tail suspension test | Swiss albino mice | reduction in the duration of immobility compared to imipramine | 121 |
| hydroalcoholic extract | forced swim test and tail suspension test | Swiss albino mice | reduced the immobility time | 122 | |
| volatile oil | forced swim method | albino mice | reduced immobility than standard fluoxetine | 252 | |
| Anti axiety | hydroalcoholic extract | light/dark exploration test and elevated plus maze test | Swiss albino mice | gradual increase in the dose of extract showed improvement of anxiety | 122 |
| Anti stress | ethanol extract of leaves | behavioural study, luciferase reporter gene assay, melatonin estimation, gene expression study | dexamethasone (DEX) induced stress in zebrafish larvae | improved behavioural and gene expression level similar to the positive control melatonin | 124 |
| Anticholinesterase activity | methanol extract (hydroxychavicol and chlorogenic acid) | bio‐autographic method | ‐ | AChE and BChE inhibition (IC50) are 21.23 ± 0.33 μg/ml and 45.55 ± 1.89 μg/ml, respectively | 126 |
| aqueous and ethanol extract, hydroxychavicol | 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide reduction and lactate dehydrogenase leakage | human neuroblastoma cells (SH‐SY5Y) | activity against both AChE and BChE, cytotoxic to human neuroblastoma cells at concentrations higher than 500 μg/ml | 125 | |
| Alzheimer's disease | aqueous extract of leaves | Morris water maze test and Passive avoidance test | aluminium chloride (AlCl3) induced Alzheimer's disease in Wistar rats | reduced mean escape latency period, improved retention of spatial memory | 127 |
| Nootropic effect | hydroalcoholic extract | visual observation | Swiss male albino mice | increase in discrimination index | 128 |
| aqueous extract of leaves | Y‐maze Test | Scopolamine induced amnesia in albino rats | reversal effect against amnesia with a momentous decrease in retention latency, and a major decrease in inflexion ratio | 129 | |
| Antioxidant activity | inflorescence extract | H2O2, superoxide, hydroxyl radical scavenging assay | in vitroassay | free radical scavenging with 50% inhibitory concentration | 213 |
| aqueous extract of leaves of Kauri variety, Ghanagete, Bagerhati (chevibetol, allylpyrocatechol) | DPPH, superoxide radical scavenging activity in a riboflavin/light/NBT system, hydroxyl radical scavenging activity and inhibition of lipid peroxidation induced by FeSO4 in egg yolk | in vitroassay | antioxidant capacity in order of Kauri>Ghanagete>Bagerhati | 132 | |
| cold ethanol extract, hot water extract of leaves and essential oil | DPPH free radical scavenging assay | in vitroassay | free radical scavenging effects decreased in the order Cold ethanol extract >essential oil >hot water extract | 133 | |
| ethyl acetate, methanol, water, petroleum ether extract of leaves | DPPH assay, TBARS assay, hydroxyl radical scavenging assay | in vitroassay | significant antioxidant activity by all extracts | 32 | |
| aqueous extract of leaves | DPPH radical scavenging assay | T47D human ductal breast epithelial tumour cell line | 83% antioxidant activity | 98 | |
| ethanol extract of leaves | superoxide dismutase activity assay | HeLa cell line | scavenged more than 50% free radical | 102 | |
| methanol, ethanol, acetone, ethyl acetate, and distilled water extract of leaves (Banarasi, safeda, Calcutta, Cuttack, Desibagla, Maharashtra and Sofia varieties) | DPPH, ABTS radical scavenging activity FRAP, and photochemiluminescence assay | in vitroassay | FRAP and ABTS assay of the Banarasi and safeda varieties and the photochemiluminescence assay for the Calcutta variety showed the highest antioxidant activity | 134 | |
| crude ethanol extract of leaves | DPPH radical scavenging assay | human breast cancer MCF‐7 cells | antioxidant activity with (IC50) of 30.0 ± 0.1 µg/ml | 105 | |
| ethanol, ethyl acetate, hexane +petroleum ether and aqueous extract of leaves | DPPH scavenging assay, reducing power activity, hydrogen peroxide scavenging assay | in vitroassay | all extracts showed good antioxidant properties in all assays in concentration dependant manner | 135 | |
| ethanol extract of leaves | oxygen radical absorbance capacity (ORAC) Assay | in vitroassay | potential free radical scavenging activity | 136 | |
| leaf methanol extract | nitric oxide, hydroxyl radical and reducing power assay, ferric ion RPA method | in vitroassay | fewer antioxidant activity compared to eugenol | 137 | |
| Hepatoprotective activity | aqueous extract or leaf | biochemical estimation | ethanol‐induced hepatotoxic and oxidative damage in Wistar rat | decreased aspartate aminotransferase (AST), alanine aminotransferase (ALT), thiobarbituric acid reactive substances (TBARS), and lipid hydroperoxides; improved non‐enzymatic antioxidants and free radical detoxifying enzymes | 138 |
| aqueous extract of leaf | biochemical and histopathological study | Wistar rats liver fibrosis induced with carbon tetrachloride (CCl4) and corn oil | inhibited AST and ALT activities; attenuated total glutathione S‐transferase activity (GST); enhanced superoxide dismutase (SOD) and catalase (CAT) activities; attenuated liver fibrosis, decreased expression of α‐smooth muscle actin (α‐SMA), induced expression of active matrix metalloproteinase‐2 (MMP2), and inhibited TIMP2 level | 140 | |
| leaf extract | ‐ | oxidative stress‐induced D‐galactosamine intoxication in Wistar rat | improved antioxidants –lipid hydroperoxidase, SOD, GSH peroxidise, vitamin C, vitamin E, GSH; decreased TBARS, hydroperoxidase and liver marker enzymes AST, ALT, alkaline phosphate (ALP), gamma glutamyltranspeptidase (GGTP) | 139 | |
| ethanol extract of leaves | acute toxicity, serum hepatic enzyme level and antioxidant enzyme level study | liver damage in Wistar rats induced with CCl4 | reduced serum glutamate oxaloacetate transaminase (SGOT), serum glutamate pyruvate transaminase (SGPT), (ALP), acid phosphatase, lipid peroxidation; improved catalase, SOD, glutathione (GSH) in liver | 141 | |
| ethanol extract of leaves | Biochemical and histopathological assay | cadmium chloride‐induced liver dysfunction in Wister rat | altered elevated level of serum AST, serum ALT, ALP, lactate dehydrogenase (LDH), gamma‐glutamyl transpeptidase (GGTP), bilirubin; oxidative stress markers TBARS, lipid hydroperoxide (LOOH), protein carbonyl, conjugated dienes, reduced SOD, CAT, GST vitamin C and vitamin E in the liver | 142 | |
| ethanol extract of betle leaves | biochemical and histochemical studies | methotrexate‐induced hepatotoxicity in Sprague‐Dawley rats | reduced ALT, AST, ALP level; reduced central vein dilatation, leukocyte infiltration, normalized hepatocellular architecture, reduced LPO, increased depleted GSH level and SOD, CAT, and GPx | 143 | |
| Anti ulcer activity | ethanolic extract of leaves | assay of MDA, oxidatively damaged protein, SOD, CAT, hexosamine, mucus, and free radical scavenging activity | indomethacin‐induced gastric lesion in Sprague‐Dawley rats | Significant protection against gastric lesions, increased SOD and CAT activity, increased mucus, hexosamine and total thiol group content, reduced oxidative damaged protein and peroxidized lipid level, increased free radical scavenging action | 144 |
| ethanol extract of leaves | histochemical investigation | NSAID‐induced ulcer in Charles Foster rats | increased antioxidative factors, mucus, and total gastric tissue sulfhydryl group | 145 | |
| leaf ethanol extract (isolated allylpyrocatechol) | histological and biochemical investigation | indomethacin‐induced stomach ulceration in Sprague‐Dawley rats | reduced the ulcer index by 93.4%, accelerated ulcer healing, improved the mucin content of gastric tissues, showed normal malondialdehyde (MDA) and protein level, increased the SOD and CAT | 146, 147 | |
| hydroalcoholic extract of leaves | acute toxicity test and ulcer index study | HCl‐ethanol, acute stress, and pylorus ligation models in Wistar rats and Swiss albino mice | decreased ulcer index, increased gastric pH, and decreased gastric fluid volume | ||
| hot and cold aqueous extract of leaves | effects on mucus content of the gastric mucosa, total and free acidity, volume and pH of the gastric juice study | Ethanol‐induced crossbreed albino rats | increased the mucus content adhering to the wall of the gastric mucosa and inhibited the volume of gastric acid | 31 | |
| Antihyperglycaemic activity | leaf suspension | plasma levels of glucose and glycosylated haemoglobin, activities of liver hexokinase and gluconeogenic enzymes assay | streptozotocin diabetic albino Wistar rats | reduction in blood glucose and glycosylated haemoglobin, decreased activities of liver glucose‐6‐phosphatase and fructose‐1,6‐bisphosphatase, increased liver hexokinase in a dose dependant manner | 149 |
| methanol extract of leaf (Bangla variety) | biochemical study, spectroscopic study | in vitroBSA‐glucose model | inhibit glucose‐induced glycation, thiol group modification and carbonyl formation | 150 | |
| ethanol extract of leaves | aldose reductase assay | in vitroassay | inhibition of human recombinant aldose reductase (HRAR) contradiction | 136 | |
| Antihyperlipidaemic activity | aqueous extract of leaves | ‐ | brain of ethanol administered Wistar rats | co‐administration resulted in reduction of lipid levels (free fatty acids, cholesterol, and phospholipids) and lipid peroxidation markers | 151 |
| methanol extract of leaves | fat diet induced hyperlipidemia in Wistar rat | depletion in total cholesterol (TC), triglycerides (TG), low‐density lipoprotein (LDL) and very low‐density lipoprotein‐cholesterol (VLDL) activity levels in serum | 152 | ||
| Anti‐ atherogenic activity | ethanol extract of leaves | ‐ | Triton WR‐1339‐induced hypercholesterolemia in Wistar rat | ameliorated hypercholesterolemia induced high level of TC, TG, LDL and VLDL and low level of enzymatic and non‐enzymatic antioxidants | 153 |
| ethanol extract of leaves (eugenol) | Biochemical and histopathological study | Atherogenic diet fed Wister rat | lower levels of TC, TG, LDL and VLDL cholesterol in serum and liver tissue; low aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, lactate dehydrogenase, and lipid‐metabolizing enzymes in serum; low enzymatic antioxidant; higher malondialdehyde in haemolysate and hepatic tissue | 154 | |
| Cardioprotective activity | hydroalcoholic extract of leaves | surgery for haemodynamic, measurement of left ventricular function measurement, biochemical study | rat with isoproterenol (ISP)‐induced myocardial infarction | modulated haemodynamic systolic, diastolic, mean arterial pressure (SAP, DAP, and MAP) and ventricular function parameters‐ contractility (+LVdP/dt), and relaxation (‐LVdP/dt), heart rate (HR), restored SOD, CAT, GSH, and GPx, reduced leakage of the CK‐MB isoenzyme and LDH, decreased lipid peroxidation in the heart | 155 |
| ethyl acetate extract of leaves (eugenol) | intracellular ROS levels assay, cellular antioxidant enzyme profile, detection of apoptosis with annexin V‐PI | rat heart cell line H9c2 incubated with H2O2 | cytoprotective effect against H2O2 induced oxidative stress, decreased intracellular ROS and apoptosis | 156 | |
| Antifertility | Stalk alcoholic extract | ‐ | adult male and female rats and rabbits | number of pups reduced, anti‐oestrogenic property recorded, mild progestational activity in immature oestrogen‐primed rabbits with some follicle depressant type in their regressive phase | 157 |
| ethyl alcohol extract of leaf stalk | sperm motility and count, fertility, biochemical study | male Swiss albino mice | reduced fertility to 0%, suppressed sperm mobility and cauda epididymal sperm count, reduced fructose content in the seminal vesicle, increased cholesterol in testes | 158 | |
| ethanol extract of petiole | oestrus cycle, fertility, litters per rat and oestradiol concentration, haematology and serum biochemistry study | female albino Wistar rats (Rattus norvegicus) | reduction in reproductive organ weights, oestrogen level, fertility, litter number, serum glucose concentration, acid phosphatase, SGOT and SGPT activity, increased cholesterol and ascorbic acid activity, non‐utilization of cholesterol and mobilization of ascorbic acid, irregular oestrus cycle, no change in haematological parameters | 159 | |
| aqueous and methanol extract of leaves | Fertility study, effect on oestrous cycle | vaginal smear of female albino Wistar rat | irregular and prolonged oestrous cycle which result in infertility | 68 | |
| root extract (Piperolactam A) | molecular docking, ligand binding affinity, molecular dynamics study | in silicostudy | potential contraceptive activity with high binding affinity to the oestrogen and progesterone receptor, the binding site has more hydrogen binding with receptor | 160 | |
| petroleum ether, ethanol, and water extract of whole plant | antifertility, reproductive outcome, anti‐implantation, abortifacient, hormonal study | adult female Wistar rat | significant antifertility, anti‐implantation and abortifacient activity, reduced level of follicle stimulating hormone (FSH), luteinizing hormone (LH), progesterone, anti‐oestrogenic activity, irregular oestrous cycle | 161 , 162 | |
| Antiplatelet | n butanol extract and fractions of roots (isolated ursonic acid, 3B‐acetyl ursolic acid and B‐sitosterol) | in vitrostudy | arachidonic acid, platelet activation factor (PAF), and adenosine diphosphate (ADP)‐induced human platelet aggregation (PA) | ursonic acid, 3B‐acetyl ursolic acid, and B‐sitosterol have decreasing potency for arachidonic acid‐induced PA inhibition; ursonic acid and B‐sitosterol inhibited PAF‐induced PA, B‐sitosterol inhibited ADP‐ induced PA | 212 |
| aqueous extract of inflorescence | in vitroassay | arachidonic acid (AA) induced and collagen‐induced rabbit platelet aggregation | inhibited platelet aggregation with IC50 207 and 335 µg/ml, inhibited AA‐, collagen, and thrombin‐induced thromboxane B2 (TXB2) production by >90% | 213 | |
| Anti‐halitosis | methanol extract and fractions of leaves and isolated compound allylpyrocatechol (APC) | MIC, Biofilm, methyl mercaptan and hydrogen sulphide volatile sulphur compound (VSC) assay | in vitrosaliva chip model | the reduction in the VSC production by oral anaerobic bacteria due to the antimicrobial activity of APC also prevented periodontal infection | 214 |
| Antiallergic activity | ethanol extract of leaves | histamine and granulocyte macrophage colony‐stimulating factor (GM‐CSF); eotaxin and IL‐8 production study | in vitroassay | decreased histamine and GM‐CSF production and inhibited eotaxin and IL‐8 secretion | 215 |
| Anti‐asthmatic effect | ethanol extract | calculation of proconvulsive time | histamine aerosol induced asthma in guinea pig | significant anti‐asthmatic effect at doses of 100 mg/kg and 200 mg/kg body weight, prolonged the latent period of convulsions | 216 |
| Anti dermatophytic activity | ethanol extract of leaves | broth dilution, disc diffusion assay | against selected zoonotic dermatophytic fungi, viz. Microsporum canis, Microsporum gypseum, Trichophyton mentagrophyte and Candida albicans | very effective antifungal activity with IC50 values ranging from 110.44 to 119.00 µg/ml | 217 |
| Anti‐hematolytic activity | water, methanol, ethyl acetate, and petroleum ether extracts of leaves | ‐ | in vitroH2O2 treated human erythrocytes model | reduced haemolysis without any toxicity | 32 |
| Thyroid function | leaf aqueous extract | triiodothyronine T3 and thyroxine T4concentratiodetermination | Swiss albino male mice | higher doses decreased T3 and increased T4 concentrations, the lowest dose increased T3 and decreased T4 | 218 |
| Immunomodulatory activity | methanol extract of leaves | lymphocyte proliferation assay, delayed type hypersensitivity reaction, determination of antibody titre | in vitroassay and in vivo assay in Swiss albino mice immunized with sheep red blood cells | immunosuppressive effect on cellular and humoral response by dose‐dependent suppression of peripheral blood lymphocyte proliferation, decreased antibody titre, increased inflammation suppression | 71 |
| crude methanol and n‐hexane fraction of plant | assessment of humoral immune response, cellular immune response, flow cytometry | in vivoassay in Balb/c mice infected with the human lymphatic filarial parasite Brugia malayi | enhancement of both humoral and cell‐mediated immune responses, increased population of T cells and B cells, produced type 1 and type 2 cytokine responses | 204 | |
| Radioprotective activity | ethanol extract of leaves | lipid peroxidation, DNA strand break, 2‐deoxyribose, superoxide scavenging, and lymphoproliferation assay | in vitro ɣ‐irradiated rat liver mitochondria and pBR322 plasmid DNA as two model | prevented ɣ‐ray induced lipid peroxidation (thiobarbituric acid reactive substrates, lipid hydroperoxide and conjugated diene), DNA strand breaks, improved hydroxyl and superoxide radical scavenging property along with its lymphoproliferative activity in a concentration‐dependent manner | 55 |
| Activity against acne | cream dose of ethanol extract | disc diffusion method, minimum inhibitory concentration | bacteria Staphylococcus aureus and Propionibacterium acnes | antibacterial activity with a MIC value of 4.5% and 4.0% | 219 |
| noisome gel containing leaf essential oil | Franz diffusion cell | Propionibacterium acnes | inhibition of bacteria | 221 | |
| ethanol extract cream | disc diffusion method | Propionibacterium acnes | 15% cream‐containing extract showed highest inhibition | 220 |
9.1. Antitumour/anticancer/antiproliferative activity
One of the promising therapeutic strategy to inhibit cancer cell proliferation is to facilitate apoptosis. In cancer research, finding apoptosis‐inducing agents derived from plant sources has become popular due to the fact that existing anti‐apoptotic drugs, many of which are derived from chemical substances, often fail to combat cancer development and progression. 83 In addition to the destruction of rapidly proliferating cancer cells, many anticancer compounds also kill normal cells in the body. Cancer can be treated with chemotherapy and/or radiotherapy, but both can cause numerous adverse health effects, and in many instances, cancer cells develop resistance to anticancer medications. However, of late few compounds obtained from natural sources, which cannot even be synthesized in the most advanced chemical synthesis laboratories, have shown great promise in the cancer treatment. 84 , 85 , 86 , 87 , 88 , 89
The first report of antitumour activity of P. betle came from Rao. He studied the activity of the aqueous extract prepared from leaves in benzo(a)pyrene‐induced tumours in buccal pouches of hamsters. The result revealed that the betel leaf extract was very effective in inhibiting preneoplastic and neoplastic changes; partial and complete tumour suppression was also observed in both short‐term (10 days) and long‐term (6 months) treatment. 90 Again, the effect of the aqueous extract of leaves on dimethyl benz (a) anthracene (DMBA)‐ induced carcinogenesis in the mammary grand of Holtzman rats was evaluated. When leaf extract was administered orally at higher doses, it showed the inhibitory result on tumour emergence. 91 Bhide et al. investigated the result of the alcoholic extract of betel leaves and its few constituents (hydroxychavicol, α‐tocopherol, eugenol and β‐carotene) against benzo[a]pyrene‐induced neoplasia in the forestomach of Swiss male mice. The leaf extract of betel and the constituents present in it were able to decrease the number of papilloma, and the highest protection was shown by α‐tocopherol and β‐carotene. 92 A study of the effect of leaf extracts on the carcinogenic and mutagenic actions of nitrosamines, 4‐(N‐nitrosomethylamino)‐1‐(3‐pyridyl)‐1‐butanone (NNK), which is one of the most potent chemicals specific to tobacco, was carried out in mice. The result showed that leaf extract and hydroxychavicol were able to reduce the tumour‐forming efficacy of NNK by approximately 25%, and inhibited the decrease in vitamin A levels by the induction activity of NNK in plasma and liver. 93 Azuie et al. studied the tumour inhibition activity of the betel leaf and its constituents in 7,12‐dimethylbenz(a)anthracene (DMBA) induced skin tumours in mice and found inhibition of tumour formation by 83–84%. 72 They also investigated oral carcinogenesis induced by methyl (acetoxymethyl) nitrosamine in hamster, extract treatment resulted in inhibition of tumour incidence, reduction of tumour burden, extension of tumour latency period, and regression of established and honest tumours, suggesting that betel can be used to develop a potential chemopreventive agent for human oral cancer. 94 In another experiment, Bhide et al. showed that the incidence of virus‐induced and 7–12‐dimethylbenz (a) anthracene‐induced rodent mammary gland tumours can be reduced by 75% and tumour burden by >90% by the administration of the ethanol extract of the leaves. 95 The methanol extract prepared from the leaves was able to exhibit antitumour activity in terms of cancer chemoprevention in Raji cells induced by 12‐O‐hexadecanoylphorbol‐13‐acetate. 96 The aqueous extract of leaves and the ethanol extract of leaves in KB cell line (human epithelial carcinoma cells) 97 and the ethanol extract in the breast cancer T47D cell line 98 exhibited cytotoxic and antiproliferative activity with IC50 values of 29.5 ± 0.3 and 55.2 µg/ml, respectively. Abrahim et al. evaluated the anticancer activity of extracts of water, methanol, ethyl acetate and hexane from leaves in the MCF‐7 breast cancer cell line. Ethyl acetate and hexane extracts showed a dose‐dependent inhibitory effect with IC50 values of 65.00 and 163.30 μg/ml, respectively. 99 The anticancer benefits of betel leaves and bioguided fractionation were evaluated for prostate cancer management and found that hydroxychavicol is the most potent component to inhibit tumour formation in the PC 3 cell line. 100 In another experiment, Widowati et al. found that the aqueous extract of P. betle root can effectively reduce cell proliferation by 2.8% and induce apoptosis by 9.45% in the T47D cell line (human ductal breast epithelial tumour) 101 ,ethanolic extract of leaves can inhibit the growth of HeLa cervical cancer cells with an IC50 value of 7.13 µg/ml and exhibit apoptotic activity with an IC50 value of 12.5 µg/ml (95.87%). 102 The in vitro anticancer efficacy of hydroxychavicol‐containing leaf extract showed sensitivity to androgen‐independent human prostate cancer cells (22Rv1> C4‐2> PC‐3> DU14) and the activity of P. betle in BALB/c nude mice y injected with PC‐3‐luc cells by inhibiting growth and proliferation through ROS (Reactive oxygen species) generation and caspase‐dependent pathway. 9 An experiment with the MTT assay, 88.7% cell toxicity and 11.4% cell death were observed in the lung cancer cell line (A549) applying acetone extract of betel leaves. 103 Shah et al. studied the tumour inhibition assay of B16F10 melanoma in mice (C57BL/6) with leaves ethyl acetate, petroleum ether, aqueous and ethanol extracts. The result revealed that the ethyl acetate extract showed the highest dose‐dependent reduction in tumour size. 104 Recently, Boontha et al. used a crude ethanolic extract of betel leaf to assess anticancer activity in human breast cancer cells (MCF‐7) and found that the extract showed cytotoxicity with an IC50 value of 114.3 µg/ml, suppressed cell migration at a dose of 25 µg/ml and developed a transdermal patch containing 0.03% extract. 105 Another in vitro experiment with leaf extract containing hydroxychavicol in pancreatic cancer cell lines, viz. MIA PaCa‐2, PANC‐1, L929, INT407, NIH‐3T3, Vero and HEK293 cells exhibited inhibition of cell proliferation and epithelial‐to‐mesenchymal transition in cell lines, invasion and migration of cells through generalized gene repression, induced DNA damage, and also resulted in mitotic catastrophe and apoptosis through the JNK pathway and the caspase‐mediated pathway. 106
9.2. Analgesic/anti‐inflammatory/antinociceptive activity
The term ‘inflammation’ refers to the complex pharmacological process of the tissues in response to harmful stimuli viz. damaged cells, pathogens or irritants, which is characterized by swelling, warmth, redness and pain. 107 There has been a growing interest in developing safe and effective drugs for pain and inflammation from both academia and the pharmaceutical industry. 108 By different types of inflammatory model tests, researchers found that food supplements could be considered as safe natural analgesics which act as adjuvant for various clinical pain and inflammation by modulation of TRPM8/TRPA1 channels and endogenous opioids signalling pathways. 109 The antinociceptive activity of P. betle was investigated using hot and cold‐water extracts of various concentrations in tail flick test, hot plate test and formalin test models of cross‐bred albino mice. The cold extract showed higher antinociceptive activity than the hot extract via the opioid‐mediated pathway. 110 The anti‐inflammatory efficacy of the betel leaf ethanol extract was studied in arthritic rats with a complete Freund adjuvant‐induced model. Ethanol extract was found to reveal anti‐inflammatory and anti‐arthritic activity by down‐regulating nitric oxide generation in a dose‐dependent manner compared to positive control dexamethasone. 111 Pin et al. investigated the anti‐inflammatory activity of P. betle leaves using various solvents (ethanol, ethyl acetate, water and hexane) by in vitro inhibition assay of hyaluronidase (HYA), xanthine oxidase (XOD) and lipoxygenase (LOX). The extracts did not show a good inhibitory effect in the HYA assay, but showed a greater inhibition of more than 70% in the XOD and LOX assay. The order of increasing inhibitory activity of the extracts was aqueous < ethyl acetate < ethanol < hexane. 112 In another experiment, betel leaves methanol extract was used to study anti‐inflammatory activity with the carrageenan‐induced hind paw oedema model and analgesic activity was studied using hot plate, formalin test and writhing test. Administration of the extract significantly (p < 0.05) reduced carrageenan‐induced paw oedema and reduced the number of acetic acid‐induced writhing and formalin‐induced licks in a dose‐dependent manner. 113 De et al. also observed a reduced writhing response through modulation of the arachidonic acid pathway in the acetic acid‐induced writhing test on Swiss Albino mice using ethanolic extract of leaves. 114 The analgesic effect of the betel leaf was evaluated using the heat conduction process and the hot plate method of the eddy in mice and rat models. Dose‐dependent analgesic effect was observed by increasing the latency period. 115 The in vitro anti‐inflammatory effects of several varieties of P. betle leaf methanolic extracts were evaluated in the cell line (RAW 264.7) induced by E. coli lipopolysaccharide (LPS). Five varieties among the nine varieties showed significant anti‐inflammatory activity. 116 Another experiment was carried out in which leaf essential oil was used to evaluate the anti‐inflammatory activity of P. betle using the detection of MMP‐2 (metalloproteinase‐2) and MMP‐9 (metalloproteinase‐9) using the gelatin zymography method in vitro. An effective anti‐inflammatory activity with 85% inhibition was observed. 117
9.3. Neuropharmacological property
Numerous neurological and psychiatric disorders such as Alzheimer's disease and Parkinson's disease as well as epilepsy, migraine and essential tremors have caused severe human morbidity and mortality. 118 , 119 , 120 Depression, anxiety disorders and cognitive impairment are the most common comorbid diagnoses in neurological diseases. Treatment options include medications, cognitive‐behavioural therapy, somatic interventions or electroconvulsive therapy. Although oral antidepressants have some advantages, they also present few limitations like side effects, interaction with other medications, incompatibility and inefficiency. To find a better and safer alternative treatment of neurological conditions, natural compounds of plant origin such as terpenes, alkaloids, flavonoids, lipids and phenolic acids are being studies extensively. 87
9.3.1. Antidepressant activity
The antidepressant activity of the ethanol extract of betel leaves was evaluated in Swiss albino mice using the forced swim test and the tail suspension test. Oral administration of leaf extract showed notable antidepressant activity by reducing the duration of immobility compared to imipramine‐treated control mice. 121 Gulhane et al. in their experiment also found that the hydroalcoholic extract from betel leaves is capable of controlling depression by reducing immobility time in the tail suspension test and forced swim test, when imipramine was used as standard drug. 122 The volatile oil obtained from the P. betle fruit also showed a significant antidepressant effect in albino mice using the forced swim method compared to the standard antidepressant drug fluoxetine. 252
9.3.2. Anti‐anxiety activity
Anxiety is characterized as being an unpleasant emotional state for which the cause cannot be identified or perceived as uncontrollable, which impairs efficiency and induces insomnia, as well as resulting in a wide range of medically unexplained symptoms. 123 The hydroalcoholic extract of P. betle leaves was used to assess antianxiety activity in Swiss albino mice. A gradual dose‐dependent improvement was observed using the light/dark exploration test and an increase in plus compared to the control group receiving diazepam as standard in the antianxiety model. 122
9.3.3. Antistress activity
The effect of P. betle was evaluated to understand its potential role in stress‐mediated sleep disruption mediated by early exposure to life. For this study, betel leaf ethanol extract was administered under post‐fertilization stress induced by dexamethasone (DEX) in zebrafish larvae. The results showed improved levels of melatonin‐related behavioural gene expression (MT1, MT2, aanat1 and aanat2) and stress‐related gene expression (NF‐kB) similar to positive control melatonin. 124
9.3.4. Anticholinesterase activity and against Alzheimer's disease
The neurotransmitter acetylcholine is cleaved by acetylcholinesterase (AChE) and butyrylcholinesterase (BchE); therefore, inhibition of AchE and BchE is important to enhance brain activity. Alzheimer’s disease, which is a neurodegenerative disorder that causes dementia, impaired memory and impaired cognitive function in elderly people, can be managed by the application of cholinesterase inhibitor drugs. The in vitro anticholinesterase activity of P. betle was investigated in human neuroblastoma cells (SH‐SY5Y) by studying viability by reducing the leakage of 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide and lactate dehydrogenase. Both the aqueous extract and the ethanol extract exhibited strong inhibitory activity on AchE and BchE. 125 Dalai et al. also evaluated the inhibitory efficacy of standardized betel leaf methanol extract, containing hydroxychavicol and chlorogenic acid against AchE and BchE. Hydroxychavicol was found to have a more potent cholinergic effect than chlorogenic acid, but a combination of both (1:1) showed the highest inhibitory activity with an IC50 of 21.23 ± 0.33 μg/ml and 45.55 ± 1.89 μg/ml against AchE and BchE, respectively. 126
The effects of P. betle leaf extract on memory and learning ability were evaluated in Wistar rats with aluminium chloride‐induced (AlCl3) Alzheimer’s disease. Two tests, the passive avoidance test and the Morris water maze test, showed that the administration of the aqueous extract of leaves reduced the mean escape latency period and improved spatial memory retention in the same way as in rivastigmine‐treated mice. 127 These investigations suggest that P. betle could be an excellent anticholinergic agent with potential in the therapeutic management of Alzheimer's disease.
9.4. Nootropic effect
The hydroalcoholic extract of P. betle leaves was shown to have nootropic effect by the experiment in which the extract was administered to Swiss albino mice and the result showed an increase in discrimination index in the object recognition test. 128 In another experiment, the nootropic effect of P. betle in scopolamine‐induced amnesia in albino rats was evaluated using the Y‐maze test and it was found that the aqueous extract of leaves can reverse the effect against amnesia with a significant decrease in retention latency, a major decrease in the inflection ratio. 129
9.5. Antioxidant activity
Formation of reactive oxygen species (ROS) is one of the major markers in any disease pathology. An antioxidant acts as a protective barrier against ROS, which causes chronic and degenerative diseases. 130 The main health problems such as cancer, cardiovascular diseases, rheumatoid arthritis, Alzheimer’s disease and other neurodegenerative disorders may be caused by the formation of free radicals. Antioxidants are very beneficial because they scavenge these free radicals and help prevent these kinds of disorders because they reduce the oxidative injury of cell proteins, carbohydrates and lipids. 131 The extract of the inflorescence of P. betle was found to scavenge free radical (H2O2, superoxide, hydroxyl radical) with a 50% inhibitory concentration using an in vitro assay. 2,2‐diphenyl‐1‐picrylhydrazyl (DPPH), superoxide and hydroxyl radical scavenging activity was calculated in a riboflavin/light/NBT (9 nitro blue tetrazolium) system and inhibition of lipid peroxidation induced by FeSO4 using the aqueous extract of leaves of three varieties of betel in egg yolk (Kauri, Ghanagete, Bagerhati). The antioxidant capacity was observed in the order of Bagerhati < Ghanagete < Kauri. 132 Arambewela et al., in the DPPH free radical scavenging assay, found that the free radical scavenging effects decreased in the order of cold ethanolic extract > essential oil > hot water extract of leaves. 133 Significant antioxidant efficacy of betel leaves methanol, water, petroleum ether and ethyl acetate extracts observed using the in vitro DPPH assay and the TBARS (Thiobarbituric acid reactive substance assay), hydroxyl radical scavenging assay. 32 The leaf aqueous extract in DPPH, free radical scavenging assay showed 83% antioxidant activity in the human ductal breast epithelial tumour (T47D) cell line 98 and the ethanolic extract of leaves scavenged more than 50% free radical in the HeLa cell line using the superoxide dismutase activity assay. 102 Jaiswal et al. performed an antioxidant assay using methanol, ethanol, acetone, ethyl acetate and distilled water extract from betel leaves (Banarasi, safeda, Calcutta, Cuttack, Desibagla, Maharashtra and Sofia varieties). The highest antioxidant activity was observed in the FRAP assay (Ferric reducing antioxidant power) and the ABTS (2,2′‐Azinobis‐(3‐Ethylbenzthiazolin‐6‐Sulfonic Acid) assay of Banarasi safeda and photochemiluminescence assay for the Calcutta variety. 134 The DPPH radical scavenging assay in human breast cancer MCF‐7 cells using crude ethanolic extract of leaves showed antioxidant activity with (IC50) of 30.0 ± 0.1 µg/ml. 105 used different solvent extracts of leaves (ethanol, ethyl acetate, hexane +petroleum ether and aqueous extract of leaves) and found potential antioxidant properties of all extracts in all assays (DPPH, reducing power activity and hydrogen peroxide scavenging assay) in a concentration‐dependent manner 135 Ethanolic extract from leaves using the in vitro ORAC (oxygen radical absorbance capacity) assay 136 and methanol extract of leaves using in vitro nitric oxide, hydroxyl radical and reducing power assay, ferric ion RPA (Robotic Process Automation) method 137 showed potential antioxidant activity through free radical scavenging.
9.6. Hepatoprotective property
The hepatoprotective activity of P. betle was investigated using a model of ethanol‐intoxicated hepatotoxic injury in the Wistar rat. Oral administration of betel leaf ethanolic extract at a dose of 300 mg/kg bw was found to show the highest activity, namely decreased AST (aspartate aminotransferase), TBARS (thiobarbituric acid reactive substances), ALT (alanine aminotransferase) and lipid hydroperoxides; improved non‐enzymatic antioxidants such as reduced GSH (glutathione), vitamin E and vitamin C, and free radical detoxifying enzymes such as CAT (Catalase), SOD (Superoxide dismutase) and GSH peroxidase in kidney and liver of rats. 138 Treatment with betel leaf extract improved D‐galactosamine intoxication induced by oxidative stress in Wistar rats. The extract at a dose of 200 mg/kg bw improved antioxidant levels such as lipid hydroperoxidase (LOOH), SOD, GSH, GSH peroxidise, vitamin E and vitamin C,decreased TBARS, hydroperoxidase and liver marker enzymes such as ALT, AST, alkaline phosphate (ALP) and gamma glutamyl transpeptidase (GGTP). 139 To evaluate the hepatoprotective activity of P. betle, Young et al. induced liver fibrosis with carbon tetrachloride (CCl4) and corn oil in Wistar rats. The leaf aqueous extract was found to attenuate liver fibrosis by inhibiting AST and ALT activities, attenuating total glutathione S‐transferase activity (GST) and decreasing the expression of a‐smooth muscle actin (α‐SMA). The extract also enhanced the expression of active matrix metalloproteinase‐2 (MMP2) induced by SOD and CAT activities and inhibited the level of TIMP2 (Tissue inhibitor of metalloproteinases 2). 140 Manigauha et al. also studied the effect of ethanolic extract from betel leaves against CCl4 induced liver damage in Wistar rats. Administration of leaf extract significantly reduced serum glutamate oxaloacetate transaminase (SGOT), serum glutamate pyruvate transaminase (SGPT), (ALP), acid phosphatase and lipid peroxidation, it also improved CAT, SOD, GSH in the liver of rats. 141 Altered levels of elevated serum AST, ALT, ALP, lactate dehydrogenase (LDH), GGTP, bilirubin,TBARS, LOOH, protein carbonyl, conjugated dienes, reduced SOD, CAT, GST, vitamin C and vitamin E were observed when liver dysfunction induced with cadmium chloride in Wister rats treated with ethanol extract of betel leaves. 142 Ethanol extract prepared from betel leaves was also found to mitigate methotrexate‐induced hepatotoxicity in rats (Sprague‐Dawley) by reducing ALT, AST and ALP levels. Histological studies showed that the extract reduced central vein dilation, leukocyte infiltration and normalization of the hepatocellular architecture,the extract also reduced LPO levels and increased the depleted GSH level and SOD, CAT and GPx (glutathione peroxidase) by methotrexate. 143
9.7. Antiulcerogenic property
The prolonged use of non‐steroidal anti‐inflammatory drugs (NSAID) is one of the main causes of peptic ulcer, and there are other factors such as alcohol abuse and acute/chronic stress. Majumder et al. evaluated the antiulcerogenic efficacy of P. betle against gastric injury induced by indomethacin in Sprague‐Dawley rats. Oral administration of ethanol leaf extract at 200 mg / kg bw of dose for ten days showed noteworthy protection against gastric lesions, increase in SOD and CAT activity, amplification of mucus quantity, increase in hexosamine and total thiol group quantity, but reduced the amount of damaged oxidative protein and peroxidized lipid level, increased free radical proving the antiulcerogenic potential of betel by antioxidant mechanism. 144 The authors in another experiment also found that the ethanolic extract of betel leaves can protect NSAID‐induced ulcer in Charles Foster rats by increasing the antioxidative factors, mucus and the total gastric tissue sulfhydryl group. 145 The ethanol extract of betel leaves and the isolated compound allylpyrocatechol were found to have excellent healing properties against indomethacin‐induced stomach ulceration in rats (Sprague‐Dawley). The extract reduced the ulcer index by 93.4%, accelerated ulcer healing, improved the mucin content of gastric tissues, showed normal levels of malondialdehyde (MDA) and protein, and also increased levels of SOD and CAT levels. 146 , 147 The antiulcer activity of P. betle was also investigated in HCl‐ethanol, acute stress and pylorus ligation models in Wistar rats and Swiss albino mice using the hydroalcoholic extract of leaves. The results showed a decrease in ulcer index, gastric fluid volume, and an increase in gastric pH. An experiment was carried out using HAE (hot aqueous extract) and CEE (cold ethanolic extract) of betel leaves to assess gastroprotective efficacy in gastric ulcer induced by ethanol in crossbreed albino rats. When HAE and CEE were administered orally, they showed remarkable protection against gastric damage induced by absolute ethyl alcohol in a dose‐dependent manner by increasing the adhesion of the mucus content to the wall of the gastric mucosa and inhibiting the volume of gastric acid. 31 All these experiments proved the traditional claim that P. betle could be an excellent gastroprotective and antiulcerogenic agent in therapeutics.
9.8. Antihyperglycaemic activity
The antihyperglycaemic efficacy of P. betle was investigated in streptozotocin‐diabetic albino Wistar rats. Leaf suspension was administered orally at a dose of 75 and 150 mg/kg body weight, for 30 days and the glucose level in plasma and glycosylated haemoglobin, liver hexokinase and gluconeogenic enzyme activities were tested. The result showed a decrease in blood glucose level from 205.00 mg/dl to 151.30 mg/dl, reduced glycosylated haemoglobin level, decreased liver fructose‐1,6‐bisphosphatase and glucose‐6‐phosphatase activity and also increased liver hexokinase in a dose‐dependent mode. 149 The effect of betel leaf methanolic extract on in vitro protein glycation was investigated using a BSA (Bovine Seram Albumin)‐glucose model. The methanol extract inhibited glucose‐induced protein glycation in different stages and also inhibited the dose‐dependent modification of the thiol group and carbonyl formation. The early stage or the formation of amadori products was identified by haemoglobin‐δ‐gluconolactone, middle stage or the formation of the oxidative cleavage product was identified by BSA‐methylglyoxal, and the last stage or the production of advanced glycation ends (AGE) was identified by BSA‐glucose. 150 Fatmawati and Shimizu, by in vitro aldose reductase assay, identified that the ethanolic extract of betel leaves can inhibit human recombinant aldose reductase (HRAR), which is the key compound in the polyol signalling pathway in converting glucose to sorbitol,therefore, long‐term diabetic complications develop contradictory with the value of IC50 18.8 μg/ml. 136
9.9. Antihyperlipidaemic activity
The Wistar rat brain when treated with ethanol showed that lipid peroxidation, lipids and turbulence in antioxidant protection are increased. Different doses of administration of the aqueous extract of the P. betle leaf showed improvement in toxicity symptoms. Co‐administration of the aqueous extract at the 300 mg/kg dose rate showed the highest activity and appreciably abridged the levels of lipids such as phospholipids, free fatty acids and cholesterol, and also reduced markers for lipid peroxidation such as TBARS and hydroperoxides, and increased antioxidants, such as SOD, reduced GSH, vitamin E, vitamin C, CAT and GSH peroxidase. 151 The hypolipidaemic activity of betel leaf was evaluated in hyperlipidaemic rats fed a high‐fat diet. The leaf methanolic extract showed depletion in TC (total cholesterol), TG (triglycerides), LDL (low‐density lipoprotein) and VLDL (very low‐density lipoprotein‐cholesterol) activities in serum. 152
9.10. Anti‐atherogenic activity
Atherosclerosis is a major health problem, which subsequently leads to cardiovascular disease caused by hypercholesterolemia. Venkadeswaran et al. studied the anti‐atherogenic potential of the P. betle plant and the active constituents present in it in the Triton WR‐1339‐induced hypercholesterolaemic Wistar rat. The betel leaf ethanol extract at a dose of 500 mg/kg w and its constituent eugenol at a dose of 5 mg/kg wt for 7 days of administration ameliorated hypercholesterolemia‐induced high levels of TC, TG, LDL and VLDL and low levels of enzymatic and non‐enzymatic antioxidants as standard lipid lowering drug, lovastatin. 153 In another experiment, the authors fed Wistar rats an atherogenic diet and biochemical and histopathological experiments exhibited that the ethanolic leaf extract and the active constituent eugenol are capable of lowering the amount of TG, TC, LDL‐cholesterol and VLDL‐cholesterol in serum and liver tissue. The extract and eugenol also reduced AST, alkaline phosphatase, ALT, enzymes for lipid metabolization and lactate dehydrogenase in serum, decreased the antioxidant enzyme, and induces malondialdehyde in liver tissue and hemolysate. 154
9.11. Cardioprotective activity
The effectiveness of P. betle in cardioprotection was evaluated in rats with isoproterenol (ISP)‐induced myocardial infarction. Oral administration of betel leaf hydroalcoholic extract significantly modulated the haemodynamic of systolic pressure, diastolic pressure, mean arterial pressure (DAP, MAP and SAP) and parameters for ventricular function such as contractility (+LVdP/dt) and relaxation (LVdP/dt), heart rate (HR); the extract restored the level of catalase (CAT), glutathione peroxidase (GPx), GSH and SOD, decreased leakage of creatine phosphokinase‐MB (CK‐MB) isoenzyme of and LDH, reduced lipid peroxidation in the heart showing a protection effect against ISP‐induced myocardial infarction. 155 Piper betle was found to prevent oxidative cardiac cell injury through an in vitro study using the rat heart cell line H9c2 incubated with H2O2. The leaf extracted using ethyl acetate and the isolated bioactive component eugenol protected against oxidative stress induced by H2O2, decreased intracellular ROS and apoptosis and improved the cellular defence system at a dose of 10 μg/ml. 156
9.12. Antifertility activity
The first report on the antifertility potential of P. betle was probably from Tewari et al., who found that the alcoholic extract of the betel stalk can reduce the number of pups; anti‐oestrogenic property recorded in adult male, female rats and rabbits. Gentle progestational action was also found in oestrogen‐primed immature rabbits with few types of follicle depressant in their regressive phase. 157 To evaluate the antifertility efficacy of P. betle, alcoholic extract of leaf stalks is administered orally to male Swiss albino mice at a dose of 500 mg initially for 30 days and after that a dose of 1000 mg/kg body weight for another 30 days per animal per day. After 60 days of treatment, fertility was reduced to 0%. The extract suppressed sperm mobility and cauda epididymal sperm count, reduced fructose content in the seminal vesicles and weights of reproductive organs, and also increased cholesterol in the testes. The altered parameters were found to recover after discontinuation of the extract, suggesting P. betle as a contraceptive agent without altering hormonal balance. 158 The antifertility efficacy of betel petiole extract was studied in female albino Wistar rats. Petiole ethyl alcohol extract at a dose of 100 mg/day/rat for 30 days showed a reduction in fertility, reproductive organ weights, oestrogen level, litter number, serum glucose concentration, acid phosphatase, SGOT and SGPT activity,increased cholesterol and ascorbic acid activity. The extract revealed that cholesterol was not used and there was no mobilization of ascorbic acid, irregular oestrus cycle and no change in haematological parameters. 159 The application of methanol and the aqueous extract of betel leaf extract on the female Wistar rat revealed an irregular and prolonged oestrous cycle, which results in infertility. 68 In silico study of the antifertility effect of P. betle root extract containing piperolactam A exhibited potential contraceptive activity with high binding affinity to the oestrogen and progesterone receptor (8.9 and 9.0 Kcal/mol, respectively), the binding site showed more hydrogen binding to the receptor than Rohitukine and OrgC. 160 Shah and Jhade studied the antifertility effect of the betel plant on adult female Wistar rats using the water, petroleum ether and ethanol extract of the whole plant. The results showed significant antifertility potential with anti‐implantation and abortifacient activity, reduced level of follicle stimulating hormone (FSH), luteinizing hormone (LH), progesterone, anti‐oestrogenic activity and irregular oestrous cycle. 161 , 162
10. ANTIMICROBIAL ACTIVITIES
The following section presents the antibacterial and antifungal properties of the plant (Table 4).
TABLE 4.
Antimicrobial activities of P. betle
| Effect | Extract (isolates) | Active against | Result | Reference |
|---|---|---|---|---|
| Antibacterial activity | stalk ethyl acetate ethanol, hexane, benzene extract | Vibrio cholerae Ogawa, Staphylococcus aureus, Diplococcus pneumoniae, and Klebsiella aerogenes | ethyl acetate, ethanol extract showed significant activity, hexane, and benzene extract showed moderate activity | 165 |
| Methanolic and aqueous extract of leaves | Pseudomonas aeruginosa, P. testosteroni, P. pseudoalcaligenes, Staphylococcus aureus, S. epidermidis, S. subflava, Proteus mirabilis, Pr. vulgaris, Pr. morganii, B. cereus, B. subtilis, B. megaterium, Citrobacter freundii, Micrococcus flavus, Alcaligenes fecalis, Enterobacter aerogenes, Salmonella typhimurium, K. pneumoniae, Escherichia. coli, Streptococcus fecalis, St. cremoris, St. agalactiae | methanol extract is more potent than aqueous extract | 166 | |
| essential oil | S. aureus | potential antibacterial activity | 167 | |
| ethanol extract of leaves | S. aureus, Pseudomonas aeruginosa, and K. pneumoniae, Pr. vulgaris | potent antibacterial activity with MIC range of 25 µg to 40 µg | 170 | |
| essential oil from leaves of vellaikodi variety | S. aureus, St. mutans, Lactobacillus acidophilus | potential antibacterial activity | 169 | |
| ethanol and aqueous extract of leaves | B. subtilis, S. aureus, Micrococcus luteus, E. coli, P. aeruginosa | ethanol extract is more potent than aqueous extract. The water extract did not show activity against E. coli and P. aeruginosa | 171 | |
| water, methanol, ethyl acetate and petroleum ether extracts of leaves | St. pyogenes, S. aureus, Pr. vulgaris and E. coli | all extracts showed antibacterial activity against all tested bacteria | 32 | |
| cold aqueous, ethanol, methanol, and ethyl acetate extracts of leaves (Desawari, Desi, Bangladeshi and Jaleswar varieties) | P. aeruginosa, S. aureus and E. coli | all varieties in all solvents are effective against all bacteria; Bangladeshi and Jaleswar, the varieties in Ethanol, Ethyl Acetate, and Methanol solvents were most effective | 172 | |
| essential oil from leaves of two varieties Bangladeshi and Deshwari | S. aureus, St. epidermidis, K. pneumoniae | potential antibacterial activity | 168 | |
| crude aqueous extract diluted in ethanol | E. coli, K. pneumoniae, Proteus sp., P. aeruginosa, Vibrio cholerae, S. aureus, St. faecalis | most bacteria were found to be susceptible with the highest bactericidal activity towards E. coli, P. aeruginosa and S. aureus | 174 | |
| methanol extract of leaves (eugenol, hydroxychavicol) | Bacillussp., Enterococcus faecalis, S. aureus, St. agalactiae, Aeromonas hydrophila, E. coli, K. pneumoniae, P. aeruginosa, V. alginolyticus | promising concentration dependant antibacterial activity | 175 | |
| ethanol extract from leaves | S. aureus from conjunctivitis patient | potential antibacterial activity | 254 | |
| essential oil from fresh and cured leaves | Mycobacterium smegmatis, S. aureus and P. aeruginosa | cured leaf essential oil exhibited higher antimicrobial activity towards M. smegmatis | 1 | |
| ethanol extract of leaves | S. simulans, S. chromogenes, S. mitis, St. dysgalactiae, St. agalactiae, St. uberis, St. sanguinis | antibacterial effect at MIC 12.5 mg/ml | 173 | |
| crude water extract of young and mature leaves | St. agalactiae and E. coli | both extracts showed antibacterial activity; 30% extract of young leaf showed the highest activity against S. agalactiae | 176 | |
| acetone and ethanol extract of leaves (Barguna and Moheshkhali) varieties | B. cereus, S. aureus and E. coli | Barguna showed MIC of 2.12 to 4.25 mg/ml and Moheshkhali showed MIC 2.12–8.5 mg/ml | 177 | |
| Antifungal activity | methanolic extract | Candida tropicalis | potential antifungal activity | 166 |
| essential oil, methanol and aqueous extract | Candida albicansand Malassezia pachydermatis | potential antifungal activity | 167 | |
| leaf aqueous extract, chloroform fraction (hydroxychavicol) | C. albicans, C. glabrata, C. krusei, C. parapsilosis, C. tropicalis, C. neoformans, Aspergillus flavus, A. fumigatus, A. niger, A. parasiticus, E. floccosum, M. canis, M. gypsium, T. mentagrophytes, T. rubrum | concentration‐dependent antifungal activity against all fungi and inhibited biofilm of C. albicans | 186 | |
| Leaf extract by hydrodistillation | C. albicans and Saccharomyces cerevisiae | potential antifungal activity | 169 | |
| essential oil from leaves | C. albicans, C. rugosa, Saccharomyces cerevisiae, A. flavus | potential antifungal activity | 168 | |
| plant extract and methanol fraction | A. flavus, C. albicans, Microsporum canis, Trichophyton mentagrophytes and T. rubrum | antifungal activity against all fungi with maximum activity against A. flavus | 187 | |
| leaves crude extract and chloroform fraction (hydroxychavicol) | Colletotrichum gloeosporioides, Rhizoctonia solani, Fusarium oxysporum f. sp. cubense, Sphaceloma ampelinum, C. capsici | concentration‐dependent fungicidal and fungistatic activity | 188 | |
| leaf essential oil | C. albicans | moderate antifungal activity with MIC 0.4% | 183 | |
| ethanol extract of leaves | C. albicans | good anticandidal activity with MIC values 125 μg/ml | 184 | |
| ethanol extract of leaves | A. flavus | complete inhibition of fungal mycelia | 185 | |
| leaf extract | C. albicans | good antifungal activity | 182 |
10.1. Antibacterial activity
The global epidemic of infectious diseases caused by microbes has a high mortality rate, resulting in a high global health burden. Antimicrobial resistance and the lack of novel vaccines make infectious diseases one of the greatest threats to human health globally. Various factors are contributing to the rise in antibiotic resistance among human invasive organisms. 163 , 164 The antimicrobial efficiency of the P. betle leaf stalk was studied against the human pathogenic bacteria Staphylococcus aureus, Vibrio cholerae Ogawa, Klebsiella aerogenes and Diplococcus pneumoniae. Among the extracts, the ethyl acetate and ethanol extracts exhibited remarkable activity, the hexane and benzene extracts exhibited moderate activity towards the majority of the bacteria. 165 Nair and Chanda tested the antibacterial effect of betel leaf against several gram +ve and gram‐ve bacteria Pseudomonas aeruginosa, P. testosteroni, P. pseudoalcaligenes, Staphylococcus aureus, S. epidermidis, S. subflava, Proteus mirabilis, P. vulgaris, P. morganii, B. cereus, B. subtilis, B. megaterium, Citrobacter freundii, Micrococcus flavus, Alcaligenes faecalis, Enterobacter aerogenes, Salmonella typhimurium, Klebsiella pneumoniae, E. coli, Streptococcus faecalis, St. cremoris and St. agalactiae and found that methanol extract is more potent than aqueous extract in comparison with the standard drug Piperacillin and gentamicin. 166 Essential oil of betel leaves of the Vellaikodi, Bangladeshi and Deshwari varieties showed potential antibacterial activity against S. aureus, St. mutans, Lactobacillus acidophilus, St. epidermidis, K. pneumoniae e. 167 , 168 , 169 Antibacterial activity was found with a MIC range of 25–40 µg against S. aureus, Pseudomonas aeruginosa and K. pneumoniae, P. vulgaris using leaf ethanol extract. 170 Kaveti et al. evaluated the antibacterial efficacy of leaf ethanol and aqueous betel extracts against S. aureus, Micrococcus luteus, B. subtilis, P. aeruginosa and E. coli and found that ethanol leaf extract is more potent in efficacy than aqueous extract, while water extract showed no efficacy against E. coli and P. aeruginosa. 171 The methanol, water, petroleum ether and ethyl acetate extracts of leaves found to restrict the growth of St. pyogenes, S. aureus, P. vulgaris, E. coli, P. aeruginosa, Bacillus sp., Enterococcus faecalis, St. agalactiae, Aeromonas hydrophila, K. pneumoniae, Vibrio cholerae, V. alginolyticus, S. simulans, S. chromogenes, S. mitis, St. dysgalactiae, St. agalactiae, St. uberis, St. sanguinis, K. pneumoniae e, Proteus sp., S. aureus and St. faecalis in different experiments. 32 , 172 , 173 , 174 , 175 . Lubis and Marlisa collected S. aureus from patients with swab of the conjunctivitis patients and observed that the ethanol extract of the leaves effectively inhibited bacteria. 254 Essential oils from fresh and cured leaves were used to investigate the bactericidal activity of P. betle. The results showed that the cured leaf essential oil exhibited higher antimicrobial activity towards M. smegmatis. 1 Surjowardojo et al. tested the crude water extract of young and mature leaves in St. agalactiae and E. coli and found that both extracts showed antibacterial activity, while 30% of the young leaf extract showed the highest activity against S. agalactiae. 176 A recent experiment compared antibacterial efficacy between acetone extract and ethanol extract from leaves (Barguna and Moheshkhali) against the varieties of S. aureus, E. coli and B. cereus. The Barguna showed a MIC (minimum inhibition concentration) value of about 2.12 to 4.25 mg/ml, and the Moheshkhali variety showed a MIC of 2.12 to 8.5 mg/ml. 177 The ethanol extract of betel leaves also showed antibacterial efficacy against foodborne bacteria such as E. coli, Shigella dysenteriae, Staphylococcus aureus and Vibrio cholera with MIC values in the range of 0.625–0.75% (w/v). 178 The inhibition activity of food and waterborne pathogens from betel leaf was evaluated for multidrug resistant Staphylococcus aureus, Salmonella typhi, P. aeruginosa, B. cereus, E. coli and B. subtilis. Different solvent extracts, viz. methanol, ethanol and water showed significant antibacterial potency against all bacteria tested. 179 The antibacterial experiment of P. betle showed that betel leaf extract in n‐hexane and ethyl acetate promisingly inhibits fish pathogens such as Aeromonas hydrophila, Vibrio alginolyticus and Edwardsiella tarda, demonstrating the application of betel extract in fish preservation. 180 , 181
10.2. Antifungal activity
Various preclinical studies proved the antifungal potential of P. betle against a number of fungi by different solvent extracts. Essential oils and ethanolic extract from leaves showed potential antifungal activity against Candida albicans. 182 , 183 , 184 Complete inhibition of Aspergillus flavus fungal mycelia 185 by ethanol extract and inhibition of Candida tropicalis 166 were also observed in two different experiments. Essential oil, methanolic and aqueous leaf extracts of betel against Candida albicans and Malassezia pachydermatis 167 and leaf extract by hydrodistillation against Saccharomyces cerevisiae and Candida albicans 169 exhibited significant antifungal activity. Ali et al., tested leaf aqueous extract and chloroform fraction (isolated compound hydroxychavicol) against C. albicans, C. glabrata, C. krusei, C. parapsilosis, C. tropicalis, C. neoformans, A. flavus, A. fumigatus, A. niger, A. parasiticus, M. canis, M. gypsium, T. mentagrophytes, T. rubrum and E. floccosum. The result showed concentration‐dependent antifungal activity against all fungi, and inhibition of the C. albicans biofilm was also observed. 186 The potential antifungal efficacy of the essential oil and the methanol extract of betel leaves was found in C. rugosa, C. albicans, A. flavus, Saccharomyces cerevisiae, Microsporum canis, Trichophyton mentagrophytes and T. rubrum. 168 , 187 In another experiment, crude leaf extract and chloroform fraction containing hydroxychavicol applied on Colletotrichum gloeosporioides, Rhizoctonia solani, Fusarium oxysporum f. sp. cubense, Sphaceloma ampelinum and C. capsici and the result showed concentration‐dependent fungicidal and fungistatic activity. 188 The ethanol extract of P. betle, when tested against foodborne fungi Aspergillus niger, A. oryzae and Penicillium s sp. in agar diffusion assay, exhibited complete fungal inhibition at a concentration of >1.50% (v/v). 189 The addition of betel essential oil at a safe concentration to apple juice and tomato paste was also found to improve antioxidant capacity and inhibit microbial growth, such as Aspergillus flavus and Penicillium expansum, enhancing shelf life under refrigerator conditions. 190 , 191
10.3. Antiparasitic activities
The following section presents the antiparasitic (anthelmintic, anti‐protozoan and antifilarial) activities of P. betle (Table 5).
TABLE 5.
Antiparasitic activities of P. betle
| Anthelmintic property | leaf extract | Pheretima posthuma | required less time for paralysis and death compared albendazole | 193 |
| stem extract | Pheretima posthuma | caused death | 192 | |
| leaf extract | Eisenia fetida | less time for paralysis and death | 194 | |
| essential oil from leaves | Ascaridia galli | significant anthelmintic activity | 195 | |
| Anti‐protozoan activity | chloroform leaf extract | Giardia intestinalis | anti‐giardial activity with MIC 250 (μg/ml) and IC50 value 51.57 (μg/ml) | 196 |
| ethanol extract of leaves | Leishmania donovani | inhibited promastigotes and amastigotes by apoptosis and morphological changes, mitochondrial membrane potential loss, DNA fragmentation, and cell‐cycle arrest at G0/G1 phase | 197 | |
| methanol extract of leaf extract (Bangla Mahoba variety) | Leishmania donovani | inhibited promastigotes and amastigotes, accelerated apoptosis, generated ROS targeting mitochondria | 200 | |
| leaves ethanol extract | Leishmania donovani | inhibited promastigotes at a concentration of 8.42 ± 2.03 mg/ml and 50.2 ± 13.75 mg/ml after 24 h and 48 h, respectively | 199 | |
| root extract | Leishmania donovani | Inhibited axenic and intracellular amastigotes | 198 | |
| methanol extract of leaves | Plasmodium berghei | significant (p < 0.05) schizonticidal activity at a dose of 50–400 mg/kg in ICR mice | 201 | |
| leaf extract | Toxoplasma gondii | 25 µg/ml inhibited parasite invasion into host human foreskin fibroblast cells, reduced parasite burden in the brains of BALB/c mice | 202 | |
| leaf extract | Neospora caninum | inhibit parasite growth in human foreskin fibroblast cells, increased survival of C57BL/6 mice | 203 | |
| Antifilarial activity | crude methanol extract, n‐hexane, and chloroform fractions | Brugia malayi | suppressed microfilaraemia, potential macrofilaricidal efficacy, sterilized female worms, increased antifilarial IgG antibody | 204 |
10.3.1. Anthelmintic property
Helminths produce substances, which have substantial toxicity towards humans, that are found in foods acquired from livestock, causing a serious hazard to human health, including lymphatic filariasis or elephantiasis, onchocerciasis, and schistosomiasis. The anthelmintic activity of P. betle was studied using aqueous and ethanol extracts of the stem against the adult Indian earthworm Pheretima posthuma. The results showed that the time required to cause paralysis and death is less in ethanol extract and aqueous extract than in the standard drug albendazole. 192 Akter et al. also observed the same activity when using leaf methanol extract instead of stem extract. 193 The anthelmintic efficacy of the crude aqueous leaf extract of P. betle was also evaluated in adult earthworm Eisenia fetida. The result expressed anthelmintic activity in terms of less time for paralysis and death of the earthworm. 194 The anti‐helmintic activity of the essential oil of P. betle from leaves was also found to inhibit the burden of Ascaridia galli in poultry birds. 195
10.3.2. Anti‐protozoan activity
Giardiasis is the most common protozoan parasitic infection of the human intestine. Anti‐giardial activity was observed using chloroform extract of P. betle leaves against trophozoites of Giardia intestinalis with MIC 250 (μg/ml) and IC50 value 51.57 (μg/ml). 196 Leishmaniasis is also a protozoan parasitic infection caused by Leishmania that results in a broad spectrum of clinical representation with significant morbidity and also mortality throughout the world. Ethanolic betel leaf extract showed antileishmanial potency towards both promastigotes with IC50 value of 9.8 and against amastigotes with IC50 value 5.45 μg/ml of Leishmania donovani, mediated by apoptosis and morphological changes, loss of mitochondrial membrane potential, DNA fragmentation and cell cycle arrest in the sub‐G0/G1 phase. 197 Antileishmanial activity was also reported in which the use of leaf ethanol extract and root extract inhibited promastigotes and amastigotes of Leishmania donovani. 198 , 199 Inhibition of promastigotes and amastigotes, acceleration in apoptosis, and ROS generation targeting Leishmania mitochondria was also observed using methanol extract of betel leaves of Bangla Mahoba variety. 200 Plasmodium berghei, the causal agent for human malaria, is parasitic protozoa of mosquito. The betel leaf extract showed notable schizonticidal activity (p < 0.05) and an antiplasmodial effect at a dose of 50–400 mg/kg in ICR mice. 201 Leesombun et al. described that the betel leaf extract is capable of inhibiting the invasion of the Toxoplasma gondii parasite into human foreskin fibroblast cells at a dose of 25 µg/ml dose and reduced parasite burden in the brains of BALB/c mice. 202 The authors also found that betel leaf extract can inhibit the growth of Neospora caninum parasites in human foreskin fibroblast cells and increase the survival of C57BL/6 mice. 203
10.3.3. Antifilarial activity
In vivoantifilarial activity of P. betle was evaluated using crude methanolic extract, chloroform, and n‐hexane extracts were administered at different doses to Balb/c mice. All extracts showed antigen‐specific immune response, increased antifilarial IgG antibody and also suppressed microfilaraemia, showed potential macrofilaricidal efficacy, and induced sterilization of female worms. 204
10.4. Insecticidal activities
The insecticidal activity of P. betle was evaluated using an aged grain assay against bean weevil (Sitophilus zeamais), lesser grain borer (Rhyzopertha dominica) and cowpea weevil cowpea weevil (Callosobruchus maculatus). The 30% volatile oil dust formulation exhibited toxicity against adult insects, prevented the survival of adult C. maculatus, and up to 52% protected corn against S. zeamais and R. dominica; also inhibited living and emerging progeny. 205 Nair and Kavrekar found that the methanol extract of betel leaves can exhibit good insecticidal activity against insects such as Bruchus pisorum, Tribolium castaneum and Sitophilus oryzae. 206 The following section presents the insecticidal activities of P. betle (Table 6).
TABLE 6.
Insecticidal, larvicidal and adulticidal activities of P. betle
| Insecticidal activity | essential oil | Sitophilus zeamais motschulsky, Rhizopertha dominica, Callosobruchus maculatus | inhibition of living and emerging progenies | 205 |
| methanol extract of leaves | Bruchus pisorum, Tribolium castaneum, Sitophilus oryzae | good insecticidal activity | 206 | |
| Larvicidal activity | essential oil and methanol extract of leaves | Aedes aegypti | for essential oil at 2 h and 24 h LD50 value of 86 and 48 ppm; for methanol extract at 2 h and 24 h LD50 value of 153 and 125 ppm, respectively | 167 |
| methanol extracts of leaves | Aedes aegypti | LC50 values 313.58 and 122.99 ppm, respectively after 24 and 48 h | 207 | |
| essential oil | Aedes aegypti | 24 h exposed, LC50 = 13, l ppm – For the 48‐h exposed, LC50: 1l,2 ppm | 208 | |
| essential oil | Aedes aegypti | for larvicide activity, the LC50 values at 1 h, 24 h and 48 h are 183, 92.7 and 59.8 ppm | 210 | |
| essential oil from betle leaf | Chrysomya bezziana | 4% essential oil killed all first instar larvae in 2 h while killing second instar larvae in 4 h | 209 | |
| essential oil from betle leaf | Chrysomya megacephala | 3 and 4% essential oil killed 100% larvae in 3.5 h | 211 | |
| methanol extract of leaves | Drosophila melanogaster | dose‐dependent larvicidal activity with a reducing effect on the nucleic acid and protein content | 206 | |
| Mosquito adulticidal activity | essential oil | Aedes aegypti | concentration of 2.5 μl/ml, caused 100% mortality in adult mosquitoes within 15–30 min | 210 |
10.5. Larvicidal property
The mosquito larvicidal activity of P. betle against Aedes aegypti was evaluated using methanol extract and essential oil of the leaves. For essential oil, the LD50 values were found to be 86 and 48 ppm at 2 and 24 h; for the methanol extract, the LD50 values at 2 and 24 h are 153 and 125 ppm, respectively. 167 Tennyson et al. observed larvicidal activity with LC50 values 313.58 and 122.99 ppm, respectively, after 24 and 48 h using leaves methanol extract. 207 The essential oil of the betel leaves can also inhibit the larval growth of Aedes aegypti. When the third instar larvae were exposed for 24 h, the LC50 value found l3, l ppm, and for the 48‐h exposure, the LC50 value is 1l, 2 ppm. 208 Essential oil from obtained betel leaf was also treated on Chrysomya bezziana larvae, and the result showed that 4% essential oil killed all first instar larvae in 2 h while killing the second instar larvae in 4 h. 209 Larvicidal activity using P. betle essential oil was also observed with LC50 183 ppm 92.7 ppm and 59.8 ppm and LC90 637 ppm 525 ppm and 434.7 ppm, after 1, 24 and 48 h after treatment, respectively. 210 Another experiment showed that 3 and 4% essential oils are also capable of killing 100% Chrysomya megacephala larvae in 3.5 h. 211 Drosophila melanogaster larvae were also found to be killed with the administration of methanol extract of the leaves in a dose‐dependent manner by reducing the effect on the nucleic acid and protein content. 206
11. MISCELLANEOUS ACTIVITIES
11.1. Antiplatelet activity
Three compounds (B‐sitosterol, ursonic acid and 3B‐acetyl ursolic acid) from betel root extract were isolated and identified to evaluate the antiplatelet activity of P. betle arachidonic acid (AA), platelet activation factor (PAF) and Adenosine diphosphate (ADP) induced human platelet aggregation (PA). An in vitro study showed that all three compounds have potency in inhibiting PA. The order of inhibition of AA‐induced PA inhibition is B‐sitosterol < 3B‐acetyl ursolic acid < ursonic acid. Only B‐sitosterol and ursonic acid have inhibitory activity towards PAF and inducing activity to PA, whereas B‐sitosterol only showed inhibitory activity against ADP‐induced PA. 212 The antiplatelet activity of the aqueous extract of the inflorescence of P. betle was investigated in collagen‐induced and AA‐induced rabbit PA. In vitro treatment of the extract inhibited platelet aggregation induced by collagen and AA‐ with IC50 values of 207 and 335 µg/ml, respectively, inhibited the production of AA, collagen and thrombin‐induced thromboxane B2 (TXB2), induced by >90%, indicating that the extract of P. betle contains compounds that can inhibit platelet aggregation by ROS elimination or inhibition of TXB2 production (Table 3). 213
11.2. Anti‐halitosis activity
Halitosis is the degradation of proteins and amino acids present in saliva, gingival cervical fluid or food retained in the teeth that causes bad breath or oral malodour due to microbial activity. The methanol extract and fractions of leaves (isolated compound allylpyrocatechol—APC) showed antibacterial activity against oral bacteria and reduced the production of volatile sulphur compound (VSC) by oral anaerobic bacteria using an in vitro saliva chip model. APC also potentially reduced methyl mercaptan and hydrogen sulphide and prevented periodontal infection. 214
11.3. Antiallergic activity
To know about the antiallergic activity of the ethanol extract of P. betle leaf on the synthesis of GM‐CSF (granulocyte macrophage colony‐stimulating factor) and histamine by BMMC (murine bone marrow mast cells) and also the activity on the human lung epithelial cell line, BEAS‐2B mediated the secretion of eotaxin and IL‐8 was evaluated in vitro. Treatment with extract markedly reduced histamine and IgE‐mediated hypersensitive reaction‐mediated GM‐CSF production; it also inhibited eotaxin and IL‐8 secretion produced by an allergic reaction induced by TNF‐α and IL‐4. This experiment suggests that P. betle controls allergic diseases by inhibiting the production of allergic mediators and can be used as a therapeutic antiallergic agent. 215
11.4. Anti‐asthmatic activity
The anti‐asthmatic activity of P. betle against 0.2% histamine‐induced bronchospasm in guinea pigs was evaluated using ethanol extract. Treatment of the extract, with a dose of 100 and 200 mg/kg bw, exhibited a prominent anti‐asthmatic effect with a prolonged latent period of convulsions compared to the standard antihistaminic drug, chlorpheniramine. 216
11.5. Dermatological activities
To evaluate the dermatological activity of P. betle, the crude ethanolic extract of the leaves and the formulated cream were tested for certain zoonotic dermatophytic fungi, that is Trichophyton mentagrophyte, Microsporum gypseum, Microsporum canis and Candida albicans. The broth dilution and disc diffusion assay revealed significant antifungal activity with a range of IC50 values 110.44–119.00 µg/ml. The result suggests that P. betle has notable therapeutic importance for the treatment of dermatophytosis comparable to the ketoconazole drug, proving the traditional claim. 217
11.6. Antihemolytic activity
The antihemolytic efficacy of the betel plant was investigated in an in vitro H2O2‐treated human erythrocyte model. Different solvent extracts, such as water, ethyl acetate, petroleum ether and methanol extracts from leaves, were used in the study, and the result showed reduced haemolysis without any toxicity as compared to ascorbic acid, taken as a positive control. Further lipid peroxidation was tested in terms of malonaldehyde production, showing reduced peroxidation in H2O2‐induced RBC (Red blood cell) cells by the effect of leaf extracts. 32
11.7. Role in thyroid function
Panda and Kar in an experiment found that P. betle leaf extract showed a dual role on thyroid function in rats. The leaf aqueous extract was administered to Swiss albino male mice and changes in the concentrations of thyroid hormone, LPO (lipid peroxidation), SOD and CAT activity were investigated. Higher doses increased LPO concentration and decreased SOD and CAT activities. Higher doses decreased triiodothyronine (T3) and increased thyroxine (T4) concentrations, while the lowest dose increased T3 and decreased T4 concentrations. 218
11.8. Immunomodulatory effect
Many in vivo and in vivo experiments were performed to prove P. betle as a novel immunomodulatory plant. The methanol extract of betel leaves in an in vitro study showed that the proliferation of peripheral blood lymphocytes was significantly induced by the suppression of phytohaemagglutinin in a dose‐dependent manner. Furthermore, the activity of P. betle was studied in mice that were immunized with sheep red blood cells using the extract at different dose levels to observe the cellular and humoral immune responses. The extract showed dose‐dependent suppression of T‐cell, B‐cell and immune response mediated by antibody, decrease in antibody titre, increase in inflammation suppression; delayed T‐cell‐mediated hypersensitivity reaction. The methanol extract prepared from betel leaves at a dose of 500 mg/kg showed immunosuppression that was in correlation with cyclophosphamide, an immunosuppressive drug (2 mg/kg) suggesting P. betle as a potent therapeutic agent for the treatment of various autoimmune disorders and immune disorders. 71 The crude n‐hexane and methanol extracts of P. betle showed immunomodulatory effectiveness in Balb/c mice infected with Brugia malayi, a parasite of human lymphatic filaria. In vivo experiment showed enhancement in both humoral immune responses by increasing plaque‐forming cells and hemagglutination titre,enhanced cell‐mediated immune responses such as lymphoproliferation, delayed type of immune responses for hypersensitivity, macrophage activation; increased population of B cells (CD19 +) and T cells (CD4 +, CD8 +) and produced type‐1 and type‐2 cytokine responses. 204
11.9. Radioprotective activity
Mitochondria from rat liver and plasmid DNA (pBR322), the two models were used to evaluate the radioactive property of the P. betle plant. Treatment of ethanol extract of leaves on in vitro irradiated mitochondria of rat liver and plasmid DNA (pBR322) prevented ray‐induced lipid peroxidation (thiobarbituric acid—TBA, reactive substrates of TBA, conjugated diene and LOOH) and DNA strand breaks; the extract also improved HO and SOD radical scavenging activity together with the lymphoproliferative property in a concentration‐dependent method. 55
11.10. Anti‐acne activity
Acne, an inflammatory skin disease, caused by Propionibacterium acnes and Staphylococcus aureus due to blocking of polysebase. To evaluate the efficacy of P. betle against acne, a cream dose of betel leaf ethanol extract was prepared and applied to P. acnes and S. aureus using a disc diffusion process and the MIC was calculated. The result showed antibacterial efficacy with MIC values of 4.5% and 4.0%. 219 Meinisasti et al. also showed that the cream formulation prepared from ethanol extract is effective against P. acnes. 220 In another experiment, noisome gel containing essential oil from betel leaves was prepared which also inhibited P. acnes in Franz diffusion cell. 221
12. TOXICITY PROFILE
An acute and chronic preclinical toxicity study was performed using the alcoholic extract of P. betle leaf stalk in different doses in mice and rats. Hematological, biochemical and chemical evaluations indicated that the alcoholic extract is devoid of any toxicity at the dose level of 100, 200 and 300 mg/kg bodyweight for 60 days and also interestingly 3200 mg/kg bw did not show any toxicity. 222 An acute toxicity study in guinea pigs was studied by administering betel leaf extract that did not show death within 24 h of a dose of 100 and 200 mg/kg but at a dose, more than 300 mg/kg was found to exhibit 50% mortality, suggesting that doses of 100–200 mg/kg are safe. 223 Venkateswarlu and Devanna reported that the leaf aqueous extract of P. betle up to the dose of 1000 mg/kg (po) body weight is safe when administered to Albino rats. 115 Doses of up to 2000 mg/ kg were also found to be without toxicity in mice administered with hydroalcoholic betel leaf extract. No occurrence of death, no abnormal general symptoms, no effect of necropsy and histopathological lesions observed for 14 days after the methanol extract of betel leaf administered to ICR mice at a dose of up to 5000 mg/kg. 201 De et al. also found that the ethanol extract of betel leaves is safe up to 2000 mg/kg bw without any toxicity or morbidity during the 14‐day observation period in Sprague‐Dawley rats. 143 All of these studies suggest that P. betle is safe at higher doses and can be used as a therapeutic agent to treat various maladies.
13. NANOFORMULATIONS
Nanotechnology aims to synthesize materials with unique properties such as at least in one‐dimension, small size, surface charge, high surface energy, porosity and a large surface area/volume ratio, proving advantageous for catalysis and interacting with other molecules. Scientists have developed green chemistry methods with the synthesis of nanomaterials using different biological sources which are more sustainable, cleaner and eco‐friendly, non‐toxic, energy‐efficient, that eliminates the need for high energy, pressure, temperature, and needs no stabilizing, reducing and capping agents from outside. 50 , 51 , 224 . The petiole extract of P. betle leaf was used to synthesize stable silver nanoparticles with or without CTAB (cetyltrimethylammonium bromide) and SDS (sodium dodecyl sulphate). The polyphenolic groups contained in the leaf extract 225 are the main agents responsible for the reduction of n Ag+ions into metallic Ag0 and also for stabilizing and capping. The morphology and crystalline phase were characterized by selected area electron diffraction (SAED) and transmission electron microscopy (TEM). 226 , 227 . Green synthesis of silver nanoparticles from betel leaf extract was also confirmed and characterized by energy‐dispersive X‐ray analysis (EDX), X‐ray diffraction (XRD), scanning electron micrograph (SEM) and Fourier transform infrared (FTIR) studies. 228 The ethanolic leaf extract of P. betle was also used for the successful synthesis of AuNPs (gold nanoparticles), which were characterized by TEM, Fourier transform infrared (FT‐IR), EDX and XRD. These nanoparticles were tested non‐toxic to MCF‐7 and HeLa (cancer) cell lines. 229 Gadolinium‐doped titanium dioxide nanoparticles (GdT NPs) were also synthesized from the leaf of P. betle using the hydrothermal method. GdT NP showed high antibacterial activity against S. aureus, E. coli and C. albicans at 25µg/ml and also showed promising antioxidant activity in the DPPH radical scavenging method. 230 The extract of the leaf of P. betle was also used in the synthesis of titanium dioxide nanoparticles (TiO2NP). TiO2 nanoparticles were characterized by TEM, XRD and FTIR, and antioxidant activity was evaluated using the DPPH assay, which showed promising antioxidant activity with the lowest IC50 value. 231 The leaf of P. betle helps stabilize and capping in the phytofabrication of zinc oxide nanoparticles (PZnO). These PZnO exhibited antibacterial activity towards pathogens related to dental infections such as Lactobacillus acidophilus and St. mutans in the well diffusion test at low concentrations of 3.25 μg/ml and also demonstrated a high antioxidant efficacy of approximately 70% at a concentration of 200 μg/ml concentration in the DPPH assay. 232 An experiment showed that Piper betle leaf extract‐mediated silver protein (core‐shell) nanoparticles (Ag NP) showed less toxicity against Daphnia magna than chemically synthesized AgNPs. 233 Copper oxide nanoparticles (CuONPs) synthesized using P. betle leaf extract efficiently inhibited the growth of phytopathogens such as Xanthomonas axonopodis and Ralstonia solanacearum and also exhibited a cytotoxic effect on rat splenocytes by decreasing cell viability to 94% at 300 μg/ml. 234 Silver nanoparticles coated with polyaniline (AgNP) synthesis from P. betle leaf extracts were evaluated for antimicrobial potency. The result exhibited 32.78 ± 0.64 mm inhibition zone for S. aureus, a maximum 29.55 ± 0.45 mm inhibition zone against S. typhi, 21.95 ± 0.45 mm for P. aeruginosa and 27.12 ± 0.38 mm for E. coli compared to the standard drug norfloxacin. 235 Silver nanobioconjugates synthesized from the leaf extract of betel and its chief compound eugenol showed potent anticancer activity in lung adenocarcinoma (A549 cell line) with low viability and nuclear fragmentation in the MTT ‐ (3‐[4,5‐dimethylthiazol‐2‐yl]‐2,5 diphenyl tetrazolium bromide) assay and techniques for staining with orange acridine or ethidium bromide, also showed no toxicity against non‐cancerous human peripheral blood lymphocytes. 236 Another experiment showed that silver nanoparticles synthesized using P. betle showed antifungal potency against Fusarium solani and Alternaria brassicae in a dose‐dependent method. 237 , 238 The Betel leaf extract was used to synthesize a silver‐gold nanocomposite (Ag‐Au NCPs) through the reduction of silver nitrate and gold chloride by the biological reduction method and was confirmed by XTD, SEM, FTIR and EDX. This bimetallic composite nanoformulation significantly inhibited B. subtilis and K. Planticola with higher antibacterial activity against B. subtilis. 228 Green synthesized CaO calcium oxide nanoparticles from betel leaf extract, which are also able to inhibit E. coli, P. aeruginosa, S. aureus and St. mutans with the highest activity against E. coli and St. mutans. CaO nanoparticles also exhibited anticancer efficacy against the A549 cell line using an MTT assay with an IC50 value of 92.08 mg/ml.
14. CONCLUSIONS AND FUTURE PROSPECTS
Piper betle is a world‐known herbal cash crop of tremendous, social, economic and therapeutic importance and known as ‘green gold’. The mention of betel leaf is found in various ancient medicinal literatures, and the plant is still used in traditional and folklore medicinal systems. Traditional knowledge and preclinical studies revealed the use of P. betle which has potential multitherapeutic efficacy in various diseases such as cancer, inflammation, neurodegenerative disorders, asthma, dental and oral infections, allergy, thyroid, diabetes and skin diseases. Essential oils and extracts showed great results in antifertility, cardioprotection, hepatoprotection and antiplatelet. Various researchers have reported remarkable inhibition efficacy against insects, larvae, and improvement in microbial infections, parasitic infections. The plant contains a treasure of bioactive phytochemicals belonging to different classes such as phenol, tannin, terpenoid, alkaloids and flavonoids, which are responsible for healing of various diseases. The beautiful and pungent aroma of the plant is due to its phenolic and terpenoid compounds, which made betel an eminent flavouring agent. In addition to that, betel has a notably nutritional value and is considered GRAS (generally recognized as safe) for consumption.
Piper betle is found in many varieties and cultivars, and there is a problem in synonym and proper authentication; therefore, proper taxonomic identification of landraces is the most important criterion in research. Genetic and molecular markers must be used to differentiate the different varieties of betel. Different landraces contain different amounts and combinations of chemical constituents; therefore, efforts must be made to identify phytochemicals using modern extraction and detection techniques. Standardization and validation of chemical constituents for quantitative and qualitative evaluations must also be taken care of. As the quality and quantity of the chemical constituents vary with soil and environmental factors, therefore, the optimization of the highest‐yielding soil quality and factor must be studied for large‐scale commercial cultivation purposes. Although there are many reports available on preclinical treatment using P. betle in various diseases, the mechanism of the reaction is not mentioned in most reports. A computer‐aided drug discovery program can be used since it clarifies the molecular mechanisms, correlates the pharmacological responses with experimental data, and has a crucial role in boosting medical and pharmaceutical innovation. The docking analysis performed in this research provided valuable insights into the bindings of bioactive isolates towards many protein targets, including those involved in antidepressant, anti‐inflammatory and thrombolytic cascades. 237 , 238 Broad‐spectrum clinical studies are also a major lacuna, which is incomplete in the pharmacological study. Therefore, more attention must be paid to the mechanism of action in different disease management in clinical studies that may open up an innovative avenue in therapeutics. Unexplored landraces must be further studied, and new varieties with a high number of bioactive compounds can be developed by the use of biotechnological methods. Furthermore, proper attention must be paid to the management of pests and diseases of betel plants and long‐term storage of leaves and extracts for commercial use.
The present review encompasses the traditional uses, preclinical and clinical aspects of P. betle with notes on its toxicological attributes and safety considerations. However, further studies are needed to explore its efficacy via proper elucidation of its underlying molecular mechanisms of action against various disease pathology, structure–activity relationships, bioavailability and synergism. In addition, well‐designed clinical studies involving statistically significant number of human patients are needed to assess the clinical significance of the plant preparations and the derived compounds. Lastly, its high abundance, low‐cost production, exportation and potential therapeutic aspects made the betel plant very distinguished throughout the world and opened myriad possibilities for future studies.
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest.
AUTHOR CONTRIBUTIONS
Protha Biswas:Conceptualization (supporting); methodology (supporting);writing ‐ original draft (supporting). Uttpal Anand: Conceptualization (lead); investigation (supporting); methodology (supporting);writing ‐ original draft (supporting). Suchismita Chatterjee Saha: Formal analysis (supporting); investigation (supporting); methodology (supporting);writing ‐ original draft (supporting). Nishi Kant: Methodology (equal). Tulika Mishra: Methodology (equal); writing, review, editing (equal). Harison Masih: Methodology (equal); writing, review, editing (equal). Ananya Bar: Methodology (equal); writing, review, editing (equal). Devendra Kumar Pandey: Data curation (supporting); formal analysis (supporting); writing, review, editing (supporting). Niraj Kumar Jha: Formal analysis (supporting); writing, review, editing (supporting). Madhumita Majumder: Formal analysis (supporting); writing, review, editing (supporting). Neela Das: Formal analysis (supporting); writing, review, editing (supporting). Vijaykumar Shivaji Gadekar: Methodology (equal); writing, review, editing (equal). Mahipal S. Shekhawat: Formal analysis (supporting); writing, review, editing (supporting). Manoj Kumar: Formal analysis (supporting); writing, review, editing (supporting). Radha: Data curation (supporting); writing, review, editing (supporting). Jarosław Proćków: Data curation (supporting); formal analysis (supporting); funding acquisition (lead); investigation (supporting); project administration (lead); resources (lead); supervision (lead); validation (supporting); visualization (supporting); writing, review, editing (supporting). José M. Pérez de la Lastra: Conceptualization (supporting); funding acquisition (lead); project administration (lead); resources (supporting); supervision (supporting); writing, review, editing (supporting). Abhijit Dey: Conceptualization (lead); formal analysis (lead); project administration (lead); resources (lead); supervision (lead); validation (supporting); visualization (supporting); writing, review, editing (supporting).
Biswas P, Anand U, Saha SC, et al. Betelvine (Piper betle L.): A comprehensive insight into its ethnopharmacology, phytochemistry, and pharmacological, biomedical and therapeutic attributes. J Cell Mol Med. 2022;26:3083–3119. doi: 10.1111/jcmm.17323
Funding information
This research was funded by projects APOGEO (Cooperation Program INTERREG‐MAC 2014–2020, with European Funds for Regional Development‐FEDER, ‘Agencia Canaria de Investigación, Innovación y Sociedad de la Información (ACIISI) del Gobierno de Canarias’ (project ProID2020010134), and CajaCanarias (project 2019SP43).
Protha Biswas and Uttpal Anand contributed equally to this study and are the first authors.
Contributor Information
Jarosław Proćków, Email: jaroslaw.prockow@upwr.edu.pl.
José M. Pérez de la Lastra, Email: jm.perezdelalastra@csic.es.
Abhijit Dey, Email: abhijit.dbs@presiuniv.ac.in, Email: abhijitbio25@yahoo.com.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
REFERENCES
- 1. Madhumita M, Guha P, Nag A. Extraction of betel leaves (Piper betle L.) essential oil and its bio‐actives identification: process optimization, GC‐MS analysis and anti‐microbial activity. Ind Crops Prod. 2019;138:111578. [Google Scholar]
- 2. Bajpai V, Sharma D, Kumar B, Madhusudanan KP. Profiling of Piper betle Linn. cultivars by direct analysis in real time mass spectrometric technique. Biomed Chromatogr. 2010;24(12):1283‐1286. [DOI] [PubMed] [Google Scholar]
- 3. Sudjaroen Y. Evaluation of ethnobotanical vegetables and herbs in Samut Songkram province. Procedia Eng. 2012;32:160‐165. [Google Scholar]
- 4. Das S, Parida R, Sandeep IS, Nayak S, Mohanty S. Biotechnological intervention in betelvine (Piper betle L.): a review on recent advances and future prospects. Asian Pacific J Trop Med. 2016;9(10):938‐946. [DOI] [PubMed] [Google Scholar]
- 5. Jane NS, Deshmukh AP, Joshi MS. Review of study of different diseases on betelvine plant and control measure. Int J Appl Innov Eng Manag. 2014;3(3):560‐563. [Google Scholar]
- 6. Khan AA, Bhatnagar SP, Sinha BN, Lal UR. Pharmacognostic specifications of eight cultivars of Piper betle from eastern region of India. Pharmacog J. 2013;5(4):176‐183. [Google Scholar]
- 7. Guha P, Nandi S. Essential oil of betel leaf (Piper betle L.): a novel addition to the world food sector. In Malik, S ed. Essential oil research. Springer; 2019:149‐196. [Google Scholar]
- 8. Taukoorah U, Lall N, Mahomoodally F. Piper betle L. (betel quid) shows bacteriostatic, additive, and synergistic antimicrobial action when combined with conventional antibiotics. South Afr J Botany. 2016;105:133‐140. [Google Scholar]
- 9. Gundala SR, Yang C, Mukkavilli R, et al. Hydroxychavicol, a betel leaf component, inhibits prostate cancer through ROS‐driven DNA damage and apoptosis. Toxicol Appl Pharmacol. 2014;280(1):86‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ghosh R, Darin K, Nath P, Deb P. An overview of various Piper species for their biological activities. Int J Pharm Res Rev. 2014;3(1):67‐75. [Google Scholar]
- 11. Anand U, Jacobo‐Herrera N, Altemimi A, Lakhssassi N. A comprehensive review on medicinal plants as antimicrobial therapeutics: potential avenues of biocompatible drug discovery. Metabolites. 2019;9(11):258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Anand U, Nandy S, Mundhra A, Das N, Pandey DK, Dey A. A review on antimicrobial botanicals, phytochemicals and natural resistance modifying agents from Apocynaceae family: possible therapeutic approaches against multidrug resistance in pathogenic microorganisms. Drug Resist Updates. 2020;51:100695. [DOI] [PubMed] [Google Scholar]
- 13. Das T, Anand U, Pandey SK, et al. Therapeutic strategies to overcome taxane resistance in cancer. Drug Resist Updat. 2021;55:100754. [DOI] [PubMed] [Google Scholar]
- 14. Datta S, Ramamurthy PC, Anand U, et al. Wonder or evil?: multifaceted health hazards and health benefits of Cannabis sativa and its phytochemicals. Saudi J Biol Sci. 2021;28(12):7290‐7313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mitra S, Anand U, Sanyal R, et al. Neoechinulins: molecular, cellular, and functional attributes as promising therapeutics against cancer and other human diseases. Biomed Pharmacother. 2022;145:112378. [DOI] [PubMed] [Google Scholar]
- 16. Mohammed MJ, Anand U, Altemimi AB, Tripathi V, Guo Y, Pratap‐Singh A. Phenolic composition, antioxidant capacity and antibacterial activity of white wormwood (Artemisia herba‐alba). Plants. 2021;10(1):164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Paul S, Chakraborty S, Anand U, et al. Withania somnifera (L.) Dunal (Ashwagandha): a comprehensive review on ethnopharmacology, pharmacotherapeutics, biomedicinal and toxicological aspects. Biomed Pharmacother. 2021;143:112175. [DOI] [PubMed] [Google Scholar]
- 18. Tandon B, Anand U, Alex BK, et al. Statistical optimization of in vitro callus induction of wild and cultivated varieties of Mucuna pruriens L. (DC.) using response surface methodology and assessment of L‐Dopa biosynthesis. Ind Crops Prod. 2021;169:113626. [Google Scholar]
- 19. Ağagündüz D, Çelik MN, Dazıroğlu MEÇ, Capasso R. Emergent drug and nutrition interactions in COVID‐19: a comprehensive narrative review. Nutrients. 2021;13(5):1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Anand U, Cabreros C, Mal J, et al. Novel coronavirus disease 2019 (COVID‐19) pandemic: from transmission to control with an interdisciplinary vision. Environ Res. 2021;197:111126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Anand U, Jakhmola S, Indari O, et al. Potential therapeutic targets and vaccine development for COVID‐19 management: a review on the recent update. Front Immunol. 2021a;12:2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bari MS, Khandokar L, Haque E, et al. Ethnomedicinal uses, phytochemistry, and biological activities of plants of the genus Gynura . J Ethnopharmacol. 2021;271:113834. [DOI] [PubMed] [Google Scholar]
- 23. Fernández J, Silván B, Entrialgo‐Cadierno R, et al. Antiproliferative and palliative activity of flavonoids in colorectal cancer. Biomed Pharmacother. 2021;143:112241. [DOI] [PubMed] [Google Scholar]
- 24. Martínez V, Iriondo De‐Hond A, Borrelli F, Capasso R, Del Castillo MD, Abalo R. Cannabidiol and other non‐psychoactive cannabinoids for prevention and treatment of gastrointestinal disorders: useful nutraceuticals? Int J Mol Sci. 2020;21(9):3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tallei TE, Niode NJ, Idroes R, et al. A comprehensive review of the potential use of green tea polyphenols in the management of COVID‐19. Evid Based Complement Alternat Med, 2021;2021:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bhagath B, Guha P. Development of novel Sooji Halwa with unique properties of essential oil of betel leaf. Int J Agric Food Sci Technol. 2014;5:87‐93. [Google Scholar]
- 27. Bhoite VS, Kamble DK, Patil YN. Effect of different levels of Piper betel leaves on physico‐chemical attributes of ice‐cream. Int J Chem Stud. 2019;7(5):168‐171. [Google Scholar]
- 28. Roy A, Guha P. Development of a novel cup cake with unique properties of essential oil of betel leaf (Piper betle L.) for sustainable entrepreneurship. J Food Sci Technol. 2015;52(8):4885‐4894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Arambewela LSR, Kumarathunge KGA, Dias K. Studies on Piper betel of Sri Lanka. J Natl Sci Foundation Sri Lanka. 2013;33(2):133‐139. [Google Scholar]
- 30. Soni H, Sharma S, Malik JK. Synergistic prophylaxis on COVID‐19 by nature golden heart (Piper betle) & Swarna Bhasma. Asian J Res Dermatol Sci. 2020;3:21‐27. [Google Scholar]
- 31. Arawwawala L, Arambewela LSR, Ratnasooriya WD. Gastroprotective effect of Piper betle Linn. leaves grown in Sri Lanka. J Ayurveda Integr Med. 2014;5(1):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chakraborty D, Shah B. Antimicrobial, antioxidative and antihemolytic activity of Piper betel leaf extracts. Int J Pharm Pharm Sci. 2011;3(3):192‐199. [Google Scholar]
- 33. Cronquist A. An Integrated System of Classification of Flowering Plants. Columbia University Press; 1981. [Google Scholar]
- 34. Chaveerach A, Mokkamul P, Sudmoon R, Tanee T. Ethnobotany of the genus Piper (Piperaceae) in Thailand. Ethnobotany Res Appl. 2006;4:223‐231. [Google Scholar]
- 35. Chibber HM. (1913). The morphology and histology of Piper betle, Linn.(the Betel‐vine). Bot J Linn Soc 41(283), 357‐383. [Google Scholar]
- 36. Dwivedi V, Tripathi S. Review study on potential activity of Piper betle . J Pharmacogn Phytochem. 2014;3(4):93‐98. [Google Scholar]
- 37. Vikash C, Shalini T, Verma NK, Singh DP, Chaudhary SK, Asha R. Piper betel phytochemistry, traditional use & pharmacological activity a review. Int J Pharm Res Dev. 2012;4(04):216‐223. [Google Scholar]
- 38. Chattapdayay SP, Maity S. Diseases of Betelvine and Species. ICAR; 1967. [Google Scholar]
- 39. Wendy Voon WY, Ghali NA, Rukayadi Y, Meor Hussin AS. Application of betel leaves (Piper betle L.) extract for preservation of homemade chili bo. Int Food Res J. 2014;21(6):2399‐2403. [Google Scholar]
- 40. Arambewela L, Kumaratunga KGA, Dias K. Studies on Piper betle of Sri Lanka. J Natl Sci Foundation Sri Lanka. 2005a;33(2):133. [Google Scholar]
- 41. Guha P. Betel leaf: the neglected green gold of India. J Human Ecol. 2006;19(2):87‐93. [Google Scholar]
- 42. Jayaweera DMA. Medicinal Plants Used in Ceylon. National Science Council of Sri Lanka. 1982;5:201. [Google Scholar]
- 43. Guha P, Jain RK. Status report on production, processing and marketing of betel leaf (Piper betle L.). Agricultural and Food Engineering Department, IIT, Kharagpur, India, 15‐22.
- 44. Satyavati GV, Raina MK, Sharma M. Medicinal Plants of India. Indian Council of Medical Research; 1987. [Google Scholar]
- 45. Verma A, Kumar N, Ranade SA. Genetic diversity amongst landraces of a dioecious vegetatively propagated plant, betelvine (Piper betle L.). J Biosci. 2004;29(3):319‐328. [DOI] [PubMed] [Google Scholar]
- 46. Kumar N. (02) Betelvine (Piper Betle L.) cultivation: a unique case of plant establishment under anthropogenically regulated microclimatic conditions. Indian J Hist Sci. 1999;34(1):19‐32. [Google Scholar]
- 47. Jana B. (1996) Improved technology for betel leaf cultivation. A paper presented in the “Seminar‐cum‐Workshop on Betel leaf Marketing”, held at State cashew nut farm, Directorate of Agricultural Marketing, Digha, Midnapur (WB), India.
- 48. Jeng J‐H, Chen S‐Y, Liao C‐H, et al. Modulation of platelet aggregation by areca nut and betel leaf ingredients: roles of reactive oxygen species and cyclooxygenase. Free Radic Biol Med. 2002;32(9):860‐871. [DOI] [PubMed] [Google Scholar]
- 49. Singh KK, Balasubrahmanyam VR, Kochhar VK. Effect of different packing methods, temperature conditions, treatment with chemicals on the senescence and storage behaviour of betel (Piper betle L.) leaves. J Plantation Crops. 1990;18(1):23‐28. [Google Scholar]
- 50. Anand U, Carpena M, Kowalska‐Góralska M, et al. Safer plant‐based nanoparticles for combating antibiotic resistance in bacteria: a comprehensive review on its potential applications, recent advances, and future perspective. Sci Total Environ. 2022;821:153472. [DOI] [PubMed] [Google Scholar]
- 51. Anand U, Tudu CK, Nandy S, et al. Ethnodermatological use of medicinal plants in India: from ayurvedic formulations to clinical perspectives – a review. J Ethnopharmacol. 2022a;284:114744. [DOI] [PubMed] [Google Scholar]
- 52. Shahbaz A, Abbasi BA, Iqbal J, et al. Chemical composition of Gastrocotyle hispida (Forssk.) bunge and Heliotropium crispum Desf. and evaluation of their multiple in vitro biological potentials. Saudi J Biol Sci. 2021;28(11):6086‐6096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Shoshan‐Barmatz V, Anand U, Nahon‐Crystal E, Di Carlo M, Shteinfer‐Kuzmine A. Adverse effects of metformin from diabetes to COVID‐19, cancer, neurodegenerative diseases, and aging: is VDAC1 a common target? Front Physiol. 2021;12:1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Madhumita M, Guha P, Nag A. Processing and potential health benefits of betel leaf (Piper betle L.). In Sen, S & Chakraborty, R eds. Herbal Medicine in India. Springer; 2020:237‐246. [Google Scholar]
- 55. Bhattacharya S, Subramanian M, Roychowdhury S, et al. Radioprotective property of the ethanolic extract of Piper betel leaf. J Radiat Res. 2005;46(2):165‐171. [DOI] [PubMed] [Google Scholar]
- 56. Norton SA. Betel: consumption and consequences. J Am Acad Dermatol. 1998;38(1):81‐88. [DOI] [PubMed] [Google Scholar]
- 57. Sujarwo W, Keim AP, Savo V, Guarrera PM, Caneva G. Ethnobotanical study of Loloh: traditional herbal drinks from Bali (Indonesia). J Ethnopharmacol. 2015;169:34‐48. [DOI] [PubMed] [Google Scholar]
- 58. Zumbroich TJ. The ethnobotany of teeth blackening in Southeast Asia. Ethnobotany Res Appl. 2009;7:381‐398. [Google Scholar]
- 59. Partha P. Ethnobotany of the Laleng (Patra) community in Bangladesh. J Pharmacog Phytochem. 2014;2(6):173‐184. [Google Scholar]
- 60. Aziz IR, Raharjeng ARP, Nasution J. Ethnobotany of traditional wedding: a comparison of plants used by Bugis, Palembang, Sundanese and Karo ethnic in Indonesia. J Phys Conf Ser. 2019;1175:1‐7. [Google Scholar]
- 61. Das C, Teron R. Ethnobotanical notes of the Rabha community in Mataikhar reserve forest of Kamrup district, Assam, India. Res J Recent Sci. 2014;2277:2502. [Google Scholar]
- 62. John KJ, Nair RA, Suma A, Unnikrishnan M, Arunachalam V. Agro‐biodiversity and ethnobotany of Lakshadweep Islands of India. Genet Resour Crop Evol. 2018;65(8):2083‐2094. [Google Scholar]
- 63. Widjaja EA. Ethnobotany of the funeral ceremony of the Torajanese. Econ Bot. 1988;42(2):250‐254. [Google Scholar]
- 64. Singh S. Ethnobotanical study of some climbers of Parsa district forest of Nepal. J Med Plants. 2016;4:6‐10. [Google Scholar]
- 65. Garg SC, Jain R. Volatile constituents of the essential oil of Piper betle L. (Cultiver Sagar Bangla). Indian J Chem B Organ Chem. 1996;35(8):874‐875. [Google Scholar]
- 66. Atal CK, Dhar KL, Singh J. The chemistry of Indian Piper species. Lloydia. 1975;38(3):256‐264. [PubMed] [Google Scholar]
- 67. Mohottalage S, Tabacchi R, Guerin PM. Components from Sri Lankan Piper betle L. leaf oil and their analogues showing toxicity against the housefly, Musca domestica . Flavour Fragr J. 2007;22(2):130‐138. [Google Scholar]
- 68. Biswal S. Phytochemical analysis and a study on the antiestrogenic antifertility effect of leaves of Piper betel in female albino rat. Anc Sci Life. 2014;34(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Revankar GD, Sen DP. Antioxidant effect of betel leaf and its extracts on storing of fish oil. J Oil Technol Assoc India. 1978;10(4):156‐157. [Google Scholar]
- 70. Chopra RN. Chopra's indigenous drugs of India. U.N. Dhur and sons; 1958. [Google Scholar]
- 71. Kanjwani DG, Marathe TP, Chiplunkar SV, Sathaye SS. Evaluation of immunomodulatory activity of methanolic extract of Piper betel . Scand J Immunol. 2008;67(6):589‐593. [DOI] [PubMed] [Google Scholar]
- 72. Azuine MA, Amonkar AJ, Bhide SV. Chemopreventive efficacy of betel leaf extract and its constituents on 7, 12‐dimethylbenz (a) anthracene induced carcinogenesis and their effect on drug detoxification system in mouse skin. Indian J Exp Biol. 1991;29(4):346‐351. [PubMed] [Google Scholar]
- 73. Rastogi RP, Mehrotra BN. Compendium of Indian Medicinal Plants. Central Drug Research Institute; (1990); 1:388‐389. [Google Scholar]
- 74. Sweatha R, Vinitha V, Thilagavathy D, Yuvasri L, Subhashini S, Sakthivel R. A study on phytochemical analysis and antibacterial activity of Piper betel varieties (Kamar And Kumbakonam Vetrilai). Eureka. 2019;2582:1571. [Google Scholar]
- 75. Prakash UNK, Smila KH, Priyanka JD, Srinithya B, Sripriya N. Studies on phytochemistry and bioefficancy of cultivars of Piper betle Linn. Int J Res Pharm Sci. 2014;5(2):94‐98. [Google Scholar]
- 76. Chen D‐Z, Xiong H‐B, Tian K, Guo J‐M, Huang X‐Z, Jiang Z‐Y. Two new sphingolipids from the leaves of Piper betle L. Molecules. 2013;18(9):11241‐11249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Karak S, Acharya J, Begum S, Mazumdar I, Kundu R, De B. Essential oil of Piper betle L. leaves: chemical composition, anti‐acetylcholinesterase, anti‐β‐glucuronidase and cytotoxic properties. J Appl Res Med Arom Plants. 2018;10:85‐92. [Google Scholar]
- 78. Ghosh K, Bhattacharya T. Chemical constituents of Piper betle Linn. (Piperaceae) roots. Molecules. 2005;10(7):798‐802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Huang X, Yin Y, Huang W, et al. Alkaloids and lignans from stems of Piper betle . Zhongguo Zhong Yao Za Zhi. 2010;35(17):2285. [PubMed] [Google Scholar]
- 80. Parmar VS, Jain SC, Gupta S, et al. Polyphenols and alkaloids from Piper species. Phytochemistry. 1998;49(4):1069‐1078. [Google Scholar]
- 81. Sankar CR, Sridevi D, Babu MK. Studies on essential oil and oil constituents of betel vine cultivars. Andhra Agric J. 1996;43(1):24‐26. [Google Scholar]
- 82. Islam MA, Ryu KY, Khan N, et al. Determination of the volatile compounds in five varieties of Piper betle L. from Bangladesh using simultaneous distillation extraction and gas chromatography/mass spectrometry (SDE‐GC/MS). Anal Lett. 2020;53(15):2413‐2430. [Google Scholar]
- 83. Rahman MM, Reza AA, Khan MA, et al. Unfolding the apoptotic mechanism of antioxidant enriched‐leaves of Tabebuia pallida (lindl.) miers in EAC cells and mouse model. J Ethnopharmacol. 2021;278:114297. [DOI] [PubMed] [Google Scholar]
- 84. Ahmed S, Khan H, Aschner M, Mirzae H, Küpeli Akkol E, Capasso R. Anticancer potential of furanocoumarins: mechanistic and therapeutic aspects. Int J Mol Sci. 2020;21(16):5622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Küpeli Akkol E, Genç Y, Karpuz B, Sobarzo‐Sánchez E, Capasso R. Coumarins and coumarin‐related compounds in pharmacotherapy of cancer. Cancers. 2020;12(7):1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Akkol EK, Tatlı II, Karatoprak GŞ, et al. Is emodin with anticancer effects completely innocent? Two sides of the coin. Cancers. 2021a;13(11):2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Akkol EK, Çankaya IT, Karatoprak GŞ, Carpar E, Sobarzo‐Sánchez E, Capasso R. Natural compounds as medical strategies in the prevention and treatment of psychiatric disorders seen in neurological diseases. Front Pharmacol. 2021b;12:1‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Bandopadhyay S, Anand U, Gadekar VS, et al. Dioscin: a review on pharmacological properties and therapeutic values. BioFactors. 2021;48:1‐34. [DOI] [PubMed] [Google Scholar]
- 89. Mitra S, Anand U, Jha NK, et al. Anticancer applications and pharmacological properties of piperidine and piperine: a comprehensive review on molecular mechanisms and therapeutic perspectives. Front Pharmacol. 2021;12:772418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Rao AR. Modifying influences of betel quid ingredients on B (a) P‐induced carcinogenesis in the buccal pouch of hamster. Int J Cancer. 1984;33(5):581‐586. [DOI] [PubMed] [Google Scholar]
- 91. Rao KP, Sreeramulu SH. Ethnobotany of selected medicinal plants of Srikakulam district, Andhra Pradesh. Anc Sci Life. 1985;4(4):238. [PMC free article] [PubMed] [Google Scholar]
- 92. Bhide SV, Padma PR, Amonkar AJ. Antimutagenic and anticarcinogenic effects of betel leaf extract against the tobacco‐specific nitrosamine 4‐(N‐nitrosomethylamino)‐1‐(3‐pyridyl)‐1‐butanone (NNK). IARC Sci Publ. 1991;(105):520‐524. [PubMed] [Google Scholar]
- 93. Bhide SV, Zariwala MBA, Amonkar AJ, Azuine MA. Chemopreventive efficacy of a betel leaf extract against benzo [a] pyrene‐induced forestomach tumors in mice. J Ethnopharmacol. 1991;34(2–3):207‐213. [DOI] [PubMed] [Google Scholar]
- 94. Azuine MA, Bhide SV. Protective single/combined treatment with betel leaf and turmeric against methyl (acetoxymethyl) nitrosamine‐induced hamster oral carcinogenesis. Int J Cancer. 1992;51(3):412‐415. [DOI] [PubMed] [Google Scholar]
- 95. Bhide SV, Azuine MA, Lahiri M, Telang NT. Chemoprevention of mammary tumor virus‐induced and chemical carcinogen‐induced rodent mammary tumors by natural plant products. Breast Cancer Res Treat. 1994;30(3):233‐242. [DOI] [PubMed] [Google Scholar]
- 96. Murakami A, Jiwajinda S, Koshimizu K, Ohigashi H. Screening for in vitro anti‐tumor promoting activities of edible plants from Thailand. Cancer Lett. 1995;95(1–2):139‐146. [DOI] [PubMed] [Google Scholar]
- 97. Fathilah AR, Sujata R, Norhanom AW, Adenan MI. Antiproliferative activity of aqueous extract of Piper betle L. and Psidium guajava L. on KB and HeLa cell lines. J Med Plants Res. 2010;4(11):987‐990. [Google Scholar]
- 98. Widowati W, Mozef T, Risdian C, Yellianty Y. Anticancer and free radical scavenging potency of Catharanthus roseus, Dendrophthoe petandra, Piper betle and Curcuma mangga extracts in breast cancer cell lines. Oxid Antioxid Med Sci. 2013a;2(2):137‐142. [Google Scholar]
- 99. Abrahim NN, Kanthimathi MS, Abdul‐Aziz A. Piper betle shows antioxidant activities, inhibits MCF‐7 cell proliferation and increases activities of catalase and superoxide dismutase. BMC Complement Altern Med. 2012;12(1):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Paranjpe R, Gundala SR, Lakshminarayana N, et al. Piper betel leaf extract: anticancer benefits and bio‐guided fractionation to identify active principles for prostate cancer management. Carcinogenesis. 2013;34(7):1558‐1566. [DOI] [PubMed] [Google Scholar]
- 101. Widowati W, Mozef T, Risdian C, Ratnawati H, Tjahjani S, Sandra F. The comparison of antioxidative and proliferation inhibitor properties of Piper betle L., Catharanthus roseus [L] G. Don, Dendrophtoe petandra L., Curcuma mangga Val. extracts on T47D cancer cell line. Int Res J Biochem Bioinform. 2011;1(2):22‐28. [Google Scholar]
- 102. Widowati W, Wijaya L, Wargasetia TL, Bachtiar I, Yellianty Y, Laksmitawati DR. Antioxidant, anticancer, and apoptosis‐inducing effects of Piper extracts in HeLa cells. J Exp Integr Med. 2013b;3(3):225‐230. [Google Scholar]
- 103. Padmapriya N, Poonguzhali T. Anticancer activity from the leaf acetone extract of Piper betle using human lung cancer cell line (A549). Indian J Appl Res. 2015;5(4):48‐50. [Google Scholar]
- 104. Shah GA, Shah TA, Telang S. Inhibition of cell division by Piper betle against B16F10 melanoma in an in‐vivo experimental model. Int J Adv Res Sci Eng. 2018;7(4):1495‐1502. [Google Scholar]
- 105. Boontha S, Taowkaen J, Phakwan T, et al. Evaluation of antioxidant and anticancer effects of Piper betle L (Piperaceae) leaf extract on MCF‐7 cells, and preparation of transdermal patches of the extract. Trop J Pharm Res. 2019;18(6):1265‐1272. [Google Scholar]
- 106. Majumdar AG, Subramanian M. Hydroxychavicol from Piper betle induces apoptosis, cell cycle arrest, and inhibits epithelial‐mesenchymal transition in pancreatic cancer cells. Biochem Pharmacol. 2019;166:274‐291. [DOI] [PubMed] [Google Scholar]
- 107. Sreeja PS, Arunachalam K, de Oliveira Martins DT, et al. Sphenodesme involucrata var. paniculata (CB Clarke) Munir.: chemical characterization, anti‐nociceptive and anti‐inflammatory activities of methanol extract of leaves. J Ethnopharmacol. 2018;225:71‐80. [DOI] [PubMed] [Google Scholar]
- 108. Chy MNU, Adnan M, Chowdhury MR, et al. Central and peripheral pain intervention by Ophiorrhiza rugosa leaves: potential underlying mechanisms and insight into the role of pain modulators. J Ethnopharmacol. 2021;276:114182. [DOI] [PubMed] [Google Scholar]
- 109. Freitas MA, Vasconcelos A, Gonçalves EC, et al. Involvement of opioid system and TRPM8/TRPA1 channels in the antinociceptive effect of Spirulina platensis . Biomolecules. 2021;11(4):592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Arambewela LSR, Arawwawala L, Ratnasooriya WD. Antinociceptive activities of aqueous and ethanol extracts of Piper betle. leaves in rats. Pharm Biol. 2005b;43(9):766‐772. [Google Scholar]
- 111. Ganguly S, Mula S, Chattopadhyay S, Chatterjee M. An ethanol extract of Piper betle Linn. mediates its anti‐inflammatory activity via down‐regulation of nitric oxide. J Pharm Pharmacol. 2007;59(5):711‐718. [DOI] [PubMed] [Google Scholar]
- 112. Pin KY, Chuah AL, Rashih AA, et al. Antioxidant and anti‐inflammatory activities of extracts of betel leaves (Piper betle) from solvents with different polarities. J Trop Forest Sci. 2010;22:448‐455. [Google Scholar]
- 113. Alam B, Akter F, Parvin N, et al. Antioxidant, analgesic and anti‐inflammatory activities of the methanolic extract of Piper betle leaves. Avicenna J Phytomed. 2013;3(2):112. [PMC free article] [PubMed] [Google Scholar]
- 114. De S, Maroo N, Saha P, Hazra S, Chatterjee M. Ethanolic extract of Piper betle Linn. leaves reduces nociception via modulation of arachidonic acid pathway. Indian J Pharmacol. 2013;45(5):479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Venkateswarlu K, Devanna N. Pharmacological evaluations (Analgesic Activity) of ‘Piper betel’. Int J Pharmamedix India. 2014;2(2):688‐693. [Google Scholar]
- 116. Rintu D, Shinjini M, Kaustab M, Pramathadhip P, Umesh PS, Banerjee ER. Anti‐oxidant and anti‐inflammatory activities of different varieties of Piper leaf extracts (Piper betle L.). Nutr Food Sci. 2015;5(5):1‐15. [Google Scholar]
- 117. Paridhi B, Ashita U, Swati P, Dilip N. An invitro study of determination of anti‐bacterial, antioxidant, anti‐inflammatory potential of Piper betel essential oil. Natl J Integr Res Med. 2015;6(2):37‐44. [Google Scholar]
- 118. Banerjee S, Anand U, Ghosh S, et al. Bacosides from Bacopa monnieri extract: an overview of the effects on neurological disorders. Phytother Res. 2021;35(10):5668‐5679. [DOI] [PubMed] [Google Scholar]
- 119. Halder S, Anand U, Nandy S, et al. Herbal drugs and natural bioactive products as potential therapeutics: a review on pro‐cognitives and brain boosters perspectives. Saudi Pharm J. 2021;29(8):879‐907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Ogunmokun G, Dewanjee S, Chakraborty P, et al. The potential role of cytokines and growth factors in the pathogenesis of Alzheimer’s disease. Cells. 2021;10(10):2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Vinayak M, Ruckmani A, Chandrashekar K, et al. Antidepressant activity of ethanolic extract Piper betle leaves in mice. Curr Res Neurosci. 2012;2(1):11‐16. [Google Scholar]
- 122. Gulhane H, Misra AK, Reddy P, Pandey D, Gulhane R, Varma SK. Effects of Piper betle leaves (paan) extract as anti‐depressant and anti‐anxiety in experimental animals. Mintage J Pharm Med Sci. 2015;12‐15. [Google Scholar]
- 123. Goni O, Khan MF, Rahman MM, et al. Pharmacological insights on the antidepressant, anxiolytic and aphrodisiac potentials of Aglaonema hookerianum Schott. J Ethnopharmacol. 2021;268:113664. [DOI] [PubMed] [Google Scholar]
- 124. Kumari Y, Choo BKM, Shaikh MF, Othman I. Melatonin receptor agonist Piper betle L. ameliorates dexamethasone‐induced early life stress in adult zebrafish. Exp Therap Med. 2019;18(2):1407‐1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Ferreres F, Oliveira AP, Gil‐Izquierdo A, Valentão P, Andrade PB. Piper betle leaves: profiling phenolic compounds by HPLC/DAD–ESI/MSn and anti‐cholinesterase activity. Phytochem Anal. 2014;25(5):453‐460. [DOI] [PubMed] [Google Scholar]
- 126. Dalai MK, Bhadra S, Bandyopadhyay A, Mukherjee PK. Evaluation of anti‐cholinesterase activity of the standardized extract of Piper betel L. leaf. Oriental Pharm Exp Med. 2014;14(1):31‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Upadhyaya S, Gangachannaiah S, Chandrashekar PL. Effect of Piper betel leaf extract on learning and memory in Aluminium chloride induced Alzheimer’s disease in Wistar rats. Biomed Pharmacol J. 2019;12(3):1425‐1431. [Google Scholar]
- 128. Vyawahare NS, Bodhankar SL. Neuropharmacological profile of Piper betel leaves extract in mice. Pharmacologyonline. 2007;2:146‐162. [Google Scholar]
- 129. Saka VP, Babu SP, Himaja V. Effect of aqueous Piper betle leaf extract against scopolamine induced amnesia on albino rats. J Chem Pharm Sci. 2017;10(1):116‐120. [Google Scholar]
- 130. Sinan KI, Akpulat U, Aldahish AA, et al. LC‐MS/HRMS analysis, anti‐cancer, anti‐enzymatic and anti‐oxidant effects of Boerhavia diffusa extracts: a potential raw material for functional applications. Antioxidants. 2021;10(12):2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Halliwell B. Drug antioxidant effects. A basis for drug selection? Drugs. 1991;42(4):569‐605. doi: 10.2165/00003495-199142040-00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Dasgupta N, De B. Antioxidant activity of Piper betle L. leaf extract in vitro. Food Chem. 2004;88(2):219‐224. [Google Scholar]
- 133. Arambewela L, Arawwawala M, Rajapaksa D. Piper betle: a potential natural antioxidant. Int J Food Sci Technol. 2006;41:10‐14. [Google Scholar]
- 134. Jaiswal SG, Patel M, Saxena DK, Naik SN. Antioxidant properties of Piper betel (L) leaf extracts from six different geographical domain of India. J Bioresour Eng Technol. 2014;1:18‐26. [Google Scholar]
- 135. Manideep KV, Anusha P, Babu MR, Gupta BS, Swathi M, Rekha VPB. Evaluation of antifibrin, antioxidant and antimicrobial activities of betel leaves (Piper betel L.). Eur J Med Plants. 2019;27(1):1‐9. [Google Scholar]
- 136. Fatmawati S, Shimizu K. (2019) Anti‐oxidant and aldose reductase inhibitory activity of Piper betle extracts: biological activity of Piper betle extracts. Proc Pakistan Acad Sci B Life Environ Sci, 56(3):75‐82. [Google Scholar]
- 137. Aara A, Chappidi V, Ramadas MN. Antioxidant activity of eugenol in Piper betel leaf extract. J Family Med Prim Care. 2020;9(1):327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Saravanan R, Prakasam A, Ramesh B, Pugalendi KV. Influence of Piper betle on hepatic marker enzymes and tissue antioxidant status in ethanol‐treated Wistar rats. J Med Food. 2002;5(4):197‐204. [DOI] [PubMed] [Google Scholar]
- 139. Pushpavalli G, Veeramani C, Pugalendi KV. Influence of Piper betle on hepatic marker enzymes and tissue antioxidant status in D‐galactosamine‐induced hepatotoxic rats. J Basic Clin Physiol Pharmacol. 2008;19(2):131‐150. [DOI] [PubMed] [Google Scholar]
- 140. Young S‐C, Wang C‐J, Lin J‐J, Peng P‐L, Hsu J‐L, Chou F‐P. Protection effect of Piper betel leaf extract against carbon tetrachloride‐induced liver fibrosis in rats. Arch Toxicol. 2007;81(1):45‐55. [DOI] [PubMed] [Google Scholar]
- 141. Manigauha A, Patel S, Ali H, Chandy A, Maheshwari MU. Study the effect of phytochemical constituents of Piper betle leaves extracts on liver disorders by in vivo model. J Pharm Res. 2009;2(3):353‐356. [Google Scholar]
- 142. Prabu SM, Muthumani M, Shagirtha K. Protective effect of Piper betle leaf extract against cadmium‐induced oxidative stress and hepatic dysfunction in rats. Saudi J Biol Sci. 2012;19(2):229‐239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. De S, Sen T, Chatterjee M. Reduction of oxidative stress by an ethanolic extract of leaves of Piper betle (Paan) Linn. decreased methotrexate‐induced toxicity. Mol Cell Biochem. 2015;409(1):191‐197. [DOI] [PubMed] [Google Scholar]
- 144. Majumdar B, Chaudhuri SR, Ray A, Bandyopadhyay SK. Potent antiulcerogenic activity of ethanol extract of leaf of Piper betle Linn by antioxidative mechanism. Indian J Clin Biochem. 2002;17(1):49‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Majumdar B, Chaudhuri SGR, Ray A, Bandyopadhyay SK. Effect of ethanol extract of Piper betle Linn leaf on healing of NSAID–induced experimental ulcer – a novel role of free radical scavenging action. Indian J Exp Biol. 2003;41:311‐315. [PubMed] [Google Scholar]
- 146. Bhattacharya S, Banerjee D, Bauri AK, Chattopadhyay S, Bandyopadhyay SK. Healing property of the Piper betel phenol, allylpyrocatechol against indomethacin‐induced stomach ulceration and mechanism of action. World J Gastroenterol. 2007;13(27):3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Bhattacharya S, Chaudhuri SR, Chattopadhyay S, Bandyopadhyay SK. Healing properties of some Indian medicinal plants against indomethacin‐induced gastric ulceration of rats. J Clin Biochem Nutr. 2007;41(2):106‐114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Vyawahare NS, Kagathara VG, Katedeshmukh RG, Sharma PK, Mohod SM. Evaluation of antiulcer activity of Piper betel leaves extract in rats. Res J Pharmacol Pharmacodynamics. 2010;2(4):278‐282. [Google Scholar]
- 149. Santhakumari P, Prakasam A, Pugalendi KV. Antihyperglycemic activity of Piper betle leaf on streptozotocin‐induced diabetic rats. J Med Food. 2006;9(1):108‐112. [DOI] [PubMed] [Google Scholar]
- 150. Bhattacherjee A, Chakraborti AS. Inhibitory effect of Piper betle Linn. leaf extract on protein glycation‐quantification and characterization of the antiglycation components. Indian J Biochem Biophys. 2013;50:529‐536. [PubMed] [Google Scholar]
- 151. Saravanan R, Rajendra Prasad N, Pugalendi KV. Effect of Piper betle leaf extract on alcoholic toxicity in the rat brain. J Med Food. 2003;6(3):261‐265. [DOI] [PubMed] [Google Scholar]
- 152. Thirumalai T, Tamilselvan N, David E. Hypolipidemic activity of Piper betel in high fat diet induced hyperlipidemic rat. J Acute Dis. 2014;3(2):131‐135. [Google Scholar]
- 153. Venkadeswaran K, Muralidharan AR, Annadurai T, et al. Antihypercholesterolemic and antioxidative potential of an extract of the plant, Piper betle, and its active constituent, eugenol, in triton WR‐1339‐induced hypercholesterolemia in experimental rats. Evid Based Complement Alternat Med. 2014;2014:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Venkadeswaran K, Thomas PA, Geraldine P. An experimental evaluation of the anti‐atherogenic potential of the plant, Piper betle, and its active constitutent, eugenol, in rats fed an atherogenic diet. Biomed Pharmacother. 2016;80:276‐288. [DOI] [PubMed] [Google Scholar]
- 155. Arya DS, Arora S, Malik S, Nepal S, Kumari S, Ojha S. Effect of Piper betle on cardiac function, marker enzymes, and oxidative stress in isoproterenol‐induced cardiotoxicity in rats. Toxicol Mech Methods. 2010;20(9):564‐571. [DOI] [PubMed] [Google Scholar]
- 156. Savsani H, Srivastava A, Gupta S, Patel K. Strengthening antioxidant defense & cardio protection by Piper betle: an in‐vitro study. Heliyon. 2020;6(1):e03041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Tewari PV, Chaturvedi C, Dixit SN. Antifertility effect of betal leaf stalk (Tambul patrabrint): a preliminary experimental study. J Res Indian Med. 1970;4(2):143‐151. [PubMed] [Google Scholar]
- 158. Sarkar M, Gangopadhyay P, Basak B, et al. The reversible antifertility effect of Piper betle Linn. on Swiss albino male mice. Contraception. 2000;62(5):271‐274. [DOI] [PubMed] [Google Scholar]
- 159. Sharma JD, Sharma L, Yadav P. Antifertility efficacy of Piper betle Linn. (Petiole) on female albino rats. Asian J Exp Sci. 2007;21(1):145‐150. [Google Scholar]
- 160. Amin SA, Bhattacharya P, Basak S, Gayen S, Nandy A, Saha A. Pharmacoinformatics study of Piperolactam A from Piper betle root as new lead for non steroidal anti fertility drug development. Comput Biol Chem. 2017;67:213‐224. [DOI] [PubMed] [Google Scholar]
- 161. Shah SK, Jhade DN.Antifertility activity of ethanolic and aqueous extracts of Piper betle petiole on female Wistar rats. Int J Green Pharm. 2017;10(04):1‐7. [Google Scholar]
- 162. Shah SK, Jhade DN. Evaluation of antifertility potential of Piper betle (Petiole) on female wistar rats “rising approaches of herbal contraception’’. Biochem Biophys Rep. 2018;15:97‐102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Hossain S, Urbi Z, Karuniawati H, et al. Andrographis paniculata (burm. F.) wall. Ex nees: an updated review of phytochemistry, antimicrobial pharmacology, and clinical safety and efficacy. Life. 2021;11(4):348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Khare T, Anand U, Dey A, et al. Exploring phytochemicals for combating antibiotic resistance in microbial pathogens. Front Pharmacol. 2021;12:1‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Shitut S, Pandit V, Mehta BK. The antimicrobial efficiency of Piper betle Linn leaf (stalk) against human pathogenic bacteria and phytopathogenic fungi. Cent Eur J Public Health. 1999;7(3):137‐139. [PubMed] [Google Scholar]
- 166. Nair R, Chanda S. Antimicrobial activity of Terminalia catappa, Manilkara zapota and Piper betel leaf extract. Indian J Pharm Sci. 2008;70(3):390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Row LCM, Ho JC. The antimicrobial activity, mosquito larvicidal activity, antioxidant property and tyrosinase inhibition of Piper betle . J Chin Chem Soc. 2009;56(3):653‐658. [Google Scholar]
- 168. Saxena M, Khare NK, Saxena P, Syamsundar KV, Srivastava SK. Antimicrobial activity and chemical composition of leaf oil in two varieties of Piper betle from northern plains of India. J Sci Ind Res. 2014;73:95‐99. [Google Scholar]
- 169. Sugumaran M, Gandhi MS, Sankarnarayanan K, Yokesh M, Poornima M, Rajasekhar SR. Chemical composition and antimicrobial activity of vellaikodi variety of Piper betle Linn leaf oil against dental pathogens. Int J PharmTech Res. 2011;3(4):2135‐2139. [Google Scholar]
- 170. Datta A, Ghoshdastidar S, Singh M. Antimicrobial property of Piper betel leaf against clinical isolates of bacteria. Int J Pharm Sci Res. 2011;2(3):104‐109. [Google Scholar]
- 171. Kaveti B, Tan L, Sarnnia KTS, Baig M. Antibacterial activity of Piper betle leaves. Int J Pharm Teach Pract. 2011;2(3):129‐132. [Google Scholar]
- 172. Agarwal T, Singh R, Shukla AD, Waris I, Gujrati A. Comparative analysis of antibacterial activity of four Piper betle varieties. Adv Appl Sc Res. 2012;3:698‐705. [Google Scholar]
- 173. Harjanti DW, Ciptaningtyas R, Wahyono F. (2019). Phytochemical properties and antibacterial activity of Ageratum conyzoides, Piper betle, Muntinga calabura and Curcuma domestica against mastitis bacteria isolates. In IOP Conference Series: Earth and Environmental Science,47, 012049. IOP Publishing. doi: 10.1088/1755-1315/247/1/012049 [DOI]
- 174. Subashkumar R, Sureshkumar M, Babu S, Thayumanavan T. Antibacterial effect of crude aqueous extract of Piper betle L. against pathogenic bacteria. Int J Res Pharm Biomedical Sci. 2013;4:42‐46. [Google Scholar]
- 175. Syahidah A, Saad CR, Hassan MD, Rukayadi Y, Norazian MH, Kamarudin MS. Phytochemical analysis, identification and quantification of antibacterial active compounds in betel leaves, Piper betle methanolic extract. Pakistan J Biol Sci. 2017;20(2):70‐81. [DOI] [PubMed] [Google Scholar]
- 176. Surjowardojo P, Setyowati E, Ambarwati I. Antibacterial effects of green betel (Piper betle Linn.) leaf against Streptococcus agalactiae and Escherichia coli . AGRIVITA J Agric Sci. 2019;41(3):569‐574. [Google Scholar]
- 177. Avijit B, Zerin T, Rajia S. Comparative phytochemical and antibacterial properties of Piper betle leave extracts from Barguna and Moheshkhali, Bangladesh. Iran J Med Microbiol. 2020;14(2):125‐132. [Google Scholar]
- 178. Hoque MM, Rattila S, Shishir MA, Bari ML, Inatsu Y, Kawamoto S. Antibacterial activity of ethanol extract of betel leaf (Piper betle L.) against some food borne pathogens. Bangla J Microbiol. 2011;28(2):58‐63. [Google Scholar]
- 179. Rahman MS, Wadud MA, Islam T, Hussain MS, Bristy EMS, Tuhin AM. Evaluation of antibacterial activity of Piper betel leaves and Nigella sativa seeds against multidrug resistant food and water borne pathogenic bacteria: an in vitro study model. Microbiol Res J Int. 2017;22:1‐11. [Google Scholar]
- 180. Bond MM, Senggagau B. Application of piper betel leaf (Piper betle linn) extract to control fish pathogenic bacteria in‐vitro. In IOP Conference Series: Earth and Environmental Science (Vol. 383, No. 1, p. 012030). IOP Publishing. [Google Scholar]
- 181. Kalaimani N, Muraleedharan V, Joseph KG, Nair TU. Antioxidant effect of betel leaf extract on dry‐cured fish. Fishery Technol. 1984;21:37‐40. [Google Scholar]
- 182. Kumpanich J, Eiampongpaiboon T, Kanchanavasita W, Chitmongkolsuk S, Puripattanavong J. Effect of Piper betle extract on anti‐candidal activity, gelation time, and surface hardness of a short‐term soft lining material. Dent Mater J. 2020;39(6):1016‐1021. [DOI] [PubMed] [Google Scholar]
- 183. Makkar N, Prasanna SB, Singla H. Comparative evaluation of antifungal activity of Piper betel leaf oil, Origanum vulgare essential oil and fluconazole suspension on Candida albicans − an in vitro study. J Indian Assoc Public Health Dent. 2017;15(1):89. [Google Scholar]
- 184. Sivareddy B, Reginald BA, Sireesha D, Samatha M, Reddy KH, Subrahamanyam G. Antifungal activity of solvent extracts of Piper betle and Ocimum sanctum Linn on Candida albicans: an in vitro comparative study. J Oral Maxillofac Pathol. 2019;23(3):333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Boonpawa R, Sriyoha F, Kamnon G. Antifungal activity of clove and betel extract against Aspergillus flavus during dried chili storage. Asia Pacific Environ Occup Health J. 2019;5(2):17‐22. [Google Scholar]
- 186. Ali I, Khan FG, Suri KA, et al. In vitro antifungal activity of hydroxychavicol isolated from Piper betle L. Ann Clin Microbiol Antimicrob. 2010;9(1):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Nazmul MHM, Rashid MA, Jamal H. Antifungal activity of Piper betel plants in Malaysia. Drug Discov. 2013;6(17):16‐17. [Google Scholar]
- 188. Singburaudom N. Hydroxychavicol from Piper betel leave is an antifungal activity against plant pathogenic fungi. J Biopestic. 2015;8(2):82. [Google Scholar]
- 189. Wanchaitanawong P, Chaungwanit P, Poovarodom N, Nitisinprasert S. In vitro antifungal activity of Thai herb and spice extracts against food spoilage fungi. Agric Nat Resour. 2005;39(3):400‐405. [Google Scholar]
- 190. Basak S, Guha P. Betel leaf (Piper betle L.) essential oil microemulsion: Characterization and antifungal activity on growth, and apparent lag time of Aspergillus flavus in tomato paste. LWT. 2017a;75:616‐623. [Google Scholar]
- 191. Basak S, Guha P. Use of predictive model to describe sporicidal and cell viability efficacy of betel leaf (Piper betle L.) essential oil on Aspergillus flavus and Penicillium expansum and its antifungal activity in raw apple juice. LWT. 2017b;80:510‐516. [Google Scholar]
- 192. Adate PS, Parmesawaran S, Chauhan Y. In vitro anthelmintic activity of stem extracts of Piper betle Linn against Pheritima posthuma. Pharmacogn J. 2012;4(29):61‐65. [Google Scholar]
- 193. Akter KN, Karmakar P, Das A, Anonna SN, Shoma SA, Sattar MM. Evaluation of antibacterial and anthelmintic activities with total phenolic contents of Piper betel leaves. Avicenna J Phytomed. 2014;4(5):320. [PMC free article] [PubMed] [Google Scholar]
- 194. Sandhya S, Sachin F, Mahesh S. Anthelmintic activity of Piper betle Linn, (Paan/Nagavalli) aqueous extract. Res J Pharm Biol Chem Sci. 2012;3(4):467‐470. [Google Scholar]
- 195. Raza A, Muhammad F, Bashir S, Aslam B, Anwar MI, Naseer MU. In‐vitro and in‐vivo anthelmintic potential of different medicinal plants against Ascaridia galli infection in poultry birds. Worlds Poult Sci J. 2016;72(1):115‐124. [Google Scholar]
- 196. Sawangjaroen N, Subhadhirasakul S, Phongpaichit S, Siripanth C, Jamjaroen K, Sawangjaroen K. The in vitro anti‐giardial activity of extracts from plants that are used for self‐medication by AIDS patients in southern Thailand. Parasitol Res. 2005;95(1):17‐21. [DOI] [PubMed] [Google Scholar]
- 197. Sarkar A, Sen R, Saha P, Ganguly S, Mandal G, Chatterjee M. An ethanolic extract of leaves of Piper betle (Paan) Linn mediates its antileishmanial activity via apoptosis. Parasitol Res. 2008;102(6):1249. [DOI] [PubMed] [Google Scholar]
- 198. Bhattacharya P, Mondal S, Basak S, Das P, Saha A, Bera T. In Vitro susceptibilities of wild and drug resistant Leishmania donovani amastigotes to piperolactam A loaded hydroxypropyl‐β‐cyclodextrin nanoparticles. Acta Trop. 2016;158:97‐106. [DOI] [PubMed] [Google Scholar]
- 199. Singh SK, Bimal S, Narayan S, et al. Leishmania donovani: assessment of leishmanicidal effects of herbal extracts obtained from plants in the visceral leishmaniasis endemic area of Bihar, India. Exp Parasitol. 2011;127(2):552‐558. [DOI] [PubMed] [Google Scholar]
- 200. Misra P, Kumar A, Khare P, Gupta S, Kumar N, Dube A. Pro‐apoptotic effect of the landrace Bangla Mahoba of Piper betle on Leishmania donovani may be due to the high content of eugenol. J Med Microbiol. 2009;58(8):1058‐1066. [DOI] [PubMed] [Google Scholar]
- 201. Al‐Adhroey AH, Nor ZM, Al‐Mekhlafi HM, Amran AA, Mahmud R. Antimalarial activity of methanolic leaf extract of Piper betle L. Molecules. 2011;16(1):107‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Leesombun A, Boonmasawai S, Shimoda N, Nishikawa YJPO. Effects of extracts from Thai Piperaceae plants against infection with Toxoplasma gondii . PloS one. 2016;11(5):e0156116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Leesombun A, Boonmasawai S, Nishikawa Y. Effects of Thai piperaceae plant extracts on Neospora caninum infection. Parasitol Int. 2017;66(3):219‐226. [DOI] [PubMed] [Google Scholar]
- 204. Singh M, Shakya S, Soni VK, Dangi A, Kumar N, Bhattacharya S‐M. The n‐hexane and chloroform fractions of Piper betle L. trigger different arms of immune responses in BALB/c mice and exhibit antifilarial activity against human lymphatic filarid Brugia malayi . Int Immunopharmacol. 2009;9(6):716‐728. [DOI] [PubMed] [Google Scholar]
- 205. Gragasin MCB, Wy AM, Roderos BP, Acda MA, Solsoloy AD. Insecticidal activities of essential oil from Piper betle Linn. against storage insect pests. Form Philipp Agric. 2006;89(3):212‐216. [Google Scholar]
- 206. Nair SS, Kavrekar V. In vitro screening of larvicidal and insecticidal activity of methanolic extracts of Artocarpus heterophyllus, Artocarpus altilis and Piper betle . Int J Environ Agric Biotechnol. 2017;2(1):238672. [Google Scholar]
- 207. Tennyson S, Arivoli S, Raveen R, Bobby M, Dhinamala K. Larvicidal activity of Areca catechu, Nicotiana tabacum and Piper betle leaf extracts against the dengue vector Aedes aegypti (L.)(Diptera: Culicidae). Int J Res Biol Sci. 2012;2(4):157‐160. [Google Scholar]
- 208. Wahyuni D. Larvicidal activity of essential oils of Piper betle from the Indonesian plants against Aedes aegypti L. J Appl Environ Biol Sci. 2012;2(6):249‐254. [Google Scholar]
- 209. Wardhana AH, Kumarasinghe SPW, Arawwawala L, Arambewela LSR. Larvicidal efficacy of essential oil of betel leaf (Piper betle) on the larvae of the old World screwworm fly, Chrysomya bezziana in vitro. Indian J Dermatol. 2007;52(1):43. [Google Scholar]
- 210. Martianasari R, Hamid PH. Larvicidal, adulticidal, and oviposition‐deterrent activity of Piper betle L. essential oil to Aedes aegypti. Vet World. 2019;12(3):367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Kumarasinghe SPW, Karunaweera ND, Ihalamulla RL, Arambewela LSR, Dissanayake RD. Larvicidal effects of mineral turpentine, low aromatic white spirits, aqueous extracts of Cassia alata, and aqueous extracts, ethanolic extracts and essential oil of betel leaf (Piper betle) on Chrysomya megacephala . Int J Dermatol. 2002;41(12):877‐880. [DOI] [PubMed] [Google Scholar]
- 212. Saeed SA, Farnaz S, Simjee RU, Malik A. Triterpenes and B‐sitosterol from Piper betle: isolation, antiplatelet and anti‐inflammatory effects. Biochem Soc Trans. 1993;21(4):462S. [DOI] [PubMed] [Google Scholar]
- 213. Lei D, Chan CP, Wang YJ, et al. Antioxidative and antiplatelet effects of aqueous inflorescence Piper betle extract. J Agric Food Chem. 2003;51(7):2083‐2088. doi: 10.1021/jf0210223 [DOI] [PubMed] [Google Scholar]
- 214. Ramji N, Ramji N, Iyer R, Chandrasekaran S. Phenolic antibacterials from Piper betle in the prevention of halitosis. J Ethnopharmacol. 2002;83(1–2):149‐152. [DOI] [PubMed] [Google Scholar]
- 215. Wirotesangthong M, Inagaki N, Tanaka H, Thanakijcharoenpath W, Nagai H. Inhibitory effects of Piper betle on production of allergic mediators by bone marrow‐derived mast cells and lung epithelial cells. Int Immunopharmacol. 2008;8(3):453‐457. [DOI] [PubMed] [Google Scholar]
- 216. Misra KH, Kodanda Ramu B, Bandyopadhyay M. Evaluation of antiasthmatic effect of ethanol extract of Piper betle Linn. against histamine induced bronchospasm in guinea pigs. Int J Basic Appl Chem Sci. 2014;4(1):67‐73. [Google Scholar]
- 217. Trakranrungsie N, Chatchawanchonteera A, Khunkitti W. Ethnoveterinary study for antidermatophytic activity of Piper betle, Alpinia galanga and Allium ascalonicum extracts in vitro. Res Vet Sci. 2008;84(1):80‐84. [DOI] [PubMed] [Google Scholar]
- 218. Panda S, Kar A. Dual role of betel leaf extract on thyroid function in male mice. Pharmacol Res. 1998;38(6):493‐496. [DOI] [PubMed] [Google Scholar]
- 219. Budiman A, Rusnawan DW, Yuliana A. Antibacterial activity of Piper betle L. extract in cream dosage forms against Staphylococcus aureus and Propionibacterium acne . J Pharm Sci Res. 2018;10(3):493‐496. [Google Scholar]
- 220. Meinisasti R, Muslim Z, Sunita R. The effectiveness test of betel leaf ethanol extract cream (Piper betle Linn) toward Propionibacterium acnes bacterial growth. Biosci Med. 2020;4(2):10‐17. [Google Scholar]
- 221. Jufri M, Humairah E, Purwaningsih EH. Stability of anti‐acne niosome gels containing betel leaf (Piper betle L.) essential oil. Int J Appl Pharm. 2017;130‐134. [Google Scholar]
- 222. Sengupta A, Adhikary P, Basak BK, et al. Pre‐clinical toxicity evaluation of leaf‐stalk extractive of Piper betle Linn. in rodents. Indian J Exp Biol. 2000;38:338‐342. [PubMed] [Google Scholar]
- 223. Hajare R, Darvhekar VM, Shewale A, Patil V. Evaluation of antihistaminic activity of Piper betel leaf in guinea pig. Afr J Pharm Pharmacol. 2011;5(2):113‐117. [Google Scholar]
- 224. Iqbal J, Abbasi BA, Ahmad R, et al. Phytogenic synthesis of nickel oxide nanoparticles (NiO) using fresh leaves extract of Rhamnus triquetra (wall.) and investigation of its multiple in vitro biological potentials. Biomedicines. 2020;8(5):117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Nouri L, Nafchi AM, Karim AA. Mechanical and sensory evaluation of noodles incorporated with betel leaf extract. Int J Food Eng. 2015;11(2):221‐227. [Google Scholar]
- 226. Khan Z, Bashir O, Hussain JI, Kumar S, Ahmad RJC, Biointerfaces SB. Effects of ionic surfactants on the morphology of silver nanoparticles using Paan (Piper betel) leaf petiole extract. Colloids Surf B Biointerfaces. 2012;98:85–90. [DOI] [PubMed] [Google Scholar]
- 227. Mallikarjuna K, Dillip GR, Narasimha G. Phytofabrication and characterization of silver nanoparticles from Piper betle broth. Res J Nanosci Nanotechnol. 2012;2(1):17‐23. [Google Scholar]
- 228. Lagashetty A, Ganiger SK. Synthesis, characterization and antibacterial study of Ag–Au Bi‐metallic nanocomposite by bioreduction using Piper betle leaf extract. Heliyon. 2019;5(12):e02794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Punuri JB, Sharma P, Sibyala S, Tamuli R, Bora U. Piper betle‐mediated green synthesis of biocompatible gold nanoparticles. Int Nano Lett. 2012;2(1):1‐9. [Google Scholar]
- 230. Hunagund SM, Desai VR, Barretto DA, et al. Photocatalysis effect of a novel green synthesis gadolinium doped titanium dioxide nanoparticles on their biological activities. J Photochem Photobiol A. 2017;346:159‐167. [Google Scholar]
- 231. Hunagund SM, Desai VR, Barretto DA, Kadadevarmath JS, Vootla S, Sidarai AH. Free radical scavenging activities of thermally stable green synthesized titanium dioxide nanoparticles. Adv Sci Eng Med. 2017;9(6):453‐460. [Google Scholar]
- 232. Rao SP, Byrappa K, Keerthiraj N, Chatterjee J, Mustak MS. Phyto‐fabrication of ZnO nanoparticles using Piper betel aqueous extract and evaluation of its applicability in dentistry. Pharm Nanotechnol. 2018;6(3):201‐208. [DOI] [PubMed] [Google Scholar]
- 233. Rani PU, Rajasekharreddy P. Green synthesis of silver‐protein (core–shell) nanoparticles using Piper betle L. leaf extract and its ecotoxicological studies on Daphnia magna . Colloids Surf, A. 2011;389(1–3):188‐194. [Google Scholar]
- 234. Praburaman L, Jang J‐S, Muthusamy G, et al. Piper betle‐mediated synthesis, characterization, antibacterial and rat splenocyte cytotoxic effects of copper oxide nanoparticles. Artif Cells Nanomed Biotechnol. 2016;44(6):1400‐1405. [DOI] [PubMed] [Google Scholar]
- 235. Rashida MMO, Islam MS, Haque MA, Rahman MA, Hossain MT, Hamid MA. Antibacterial activity of polyaniline coated silver nanoparticles synthesized from Piper betle leaves extract. Iranian J Pharm Res. 2016;15(2):591. [PMC free article] [PubMed] [Google Scholar]
- 236. Preethi R, Padma PR. Green synthesis of silver nanobioconjugates from Piper betle leaves and its anticancer activity on A549 cells. Asian J Pharm Clin Res. 2016;9(1):252‐257. [Google Scholar]
- 237. Khan MF, Kader FB, Arman M, et al. Pharmacological insights and prediction of lead bioactive isolates of Dita bark through experimental and computer‐aided mechanism. Biomed Pharmacother. 2020;131:110774. [DOI] [PubMed] [Google Scholar]
- 238. Khan S, Singh S, Gaikwad S, Nawani N, Junnarkar M, Pawar SV. Optimization of process parameters for the synthesis of silver nanoparticles from Piper betle leaf aqueous extract, and evaluation of their antiphytofungal activity. Environ Sci Pollut Res. 2020;27(22):27221‐27233. doi: 10.1007/s11356-019-05239-2 [DOI] [PubMed] [Google Scholar]
- 239. Ahmad FB, Ismail G. Medicinal plants used by Kadazandusun communities around Crocker Range. ASEAN Review of Biodiversity and Environmental Conservation (ARBEC). 2003;1:1‐10. [Google Scholar]
- 240. Ali A, Lim XY, Chong CH, Mah SH, Chua BL. Optimization of ultrasound‐assisted extraction of natural antioxidants from Piper betle using response surface methodology. LWT. 2018a;89:681‐688. [Google Scholar]
- 241. Ali A, Lim XY, Chong CH, Mah SH, Chua BL. Ultrasound‐assisted extraction of natural antioxidants from betel leaves (Piper betle): extraction kinetics and modeling. Sep Sci Technol. 2018b;53(14):2192‐2205. [Google Scholar]
- 242. Atiya A, Salim MA, Sinha BN, Ranjan Lal U. Two new anticancer phenolic derivatives from leaves of Piper betle Linn. Nat Prod Res. 2020;35:1‐9. [DOI] [PubMed] [Google Scholar]
- 243. Atiya A, Sinha BN, Ranjan Lal U. New chemical constituents from the Piper betle Linn. (Piperaceae). Nat Prod Res. 2018;32(9):1080‐1087. [DOI] [PubMed] [Google Scholar]
- 244. Bajpai V, Pandey R, Negi MPS, Bindu KH, Kumar N, Kumar B. Characteristic differences in metabolite profile in male and female plants of dioecious Piper betle L. J Biosci. 2012;37(1):1061‐1066. [DOI] [PubMed] [Google Scholar]
- 245. Bhat KS, Thomas J, Kempraj V, Danesh A, Sridhara BY. Betel vine leaf extract inhibits mildew fungus of nyctanthes arbor‐tristis growth under in vitro conditions. Int J Plant Pathol. 2015;6:1‐7. doi: 10.3923/ijpp.2015 [DOI] [Google Scholar]
- 246. Dutta T, Nandy S, Dey A. Urban ethnobotany of Kolkata, India: a case study of sustainability, conservation and pluricultural use of medicinal plants in traditional herbal shops. Environ Dev Sustain. 2022;24(1):1207‐1240. [Google Scholar]
- 247. Dey A, De JN. Ethnobotanical survey of Purulia district, West Bengal, India for medicinal plants used against gastrointestinal disorders. J Ethnopharmacol. 2012;143(1):68‐80. [DOI] [PubMed] [Google Scholar]
- 248. Dey A, Gorai P, Mukherjee A, Dhan R, Modak BK. Ethnobiological treatments of neurological conditions in the Chota Nagpur Plateau, India. J Ethnopharmacol. 2017;198:33‐44. [DOI] [PubMed] [Google Scholar]
- 249. Fajardo WT, Cancino LT, Dudang EB, et al. (2017) Ethnobotanical study of traditional medicinal plants used by indigenous Sambal‐Bolinao of Pangasinan, Philippines. PSU J Nat Allied Sci. 1(1), 52‐63. [Google Scholar]
- 250. Islam MK, Saha S, Mahmud I, et al. An ethnobotanical study of medicinal plants used by tribal and native people of Madhupur forest area, Bangladesh. J Ethnopharmacol. 2014;151(2):921‐930. [DOI] [PubMed] [Google Scholar]
- 251. Jeng JH, Chang MC, Hahn LJ. Role of areca nut in betel quid‐associated chemical carcinogenesis: current awareness and future perspectives. Oral Oncol. 2001;37(6):477‐492. [DOI] [PubMed] [Google Scholar]
- 252. Khan M. Comparative physicochemical evaluation of fruits and antidepressant potential of volatile oils of fruits of local Piper species. Orient J Chem. 2015;31(1):541‐545. [Google Scholar]
- 253. Kichu M, Malewska T, Akter K, et al. An ethnobotanical study of medicinal plants of Chungtia village, Nagaland, India. J Ethnopharmacol. 2015;166:5‐17. [DOI] [PubMed] [Google Scholar]
- 254. Lubis RR, Marlisa DDW. Antibacterial activity of betle leaf (Piper betle l.) extract on inhibiting Staphylococcus aureus in conjunctivitis patient. Am J Clin Exp Immunol. 2020;9(1):1. [PMC free article] [PubMed] [Google Scholar]
- 255. Manikandan S, Lakshmanan GMA. Ethnobotanical survey of medicinal plants in kalrayan hills, eastern Ghats, Tamil Nadu. Int Lett Nat Sci. 2014;12(2):111‐121. [Google Scholar]
- 256. Muruganandam L, Krishna A, Reddy J, Nirmala GS. Optimization studies on extraction of phytocomponents from betel leaves. Resour Effic Technol. 2017;3(4):385‐393. [Google Scholar]
- 257. Nahdi MS, Kurniawan AP. The diversity and ethnobotanical study of medicinal plants in the southern slope of Mount Merapi, Yogyakarta, Indonesia. Biodiversitas. 2019;20(8):2279‐2287. [Google Scholar]
- 258. Nalina T, Rahim ZHA. The crude aqueous extract of Piper betle L. and its antibacterial effect towards Streptococcus mutans . Am J Biotechnol Biochem. 2007;3(1):10‐15. [Google Scholar]
- 259. Oliveira AP, Ferreira JG, Riboira S, Andrade PB, Valentão P. Bioactive natural products from Piper betle L. leaves and their α‐glucosidase inhibitory potential. Rec Nat Prod. 2016;10(6):771. [Google Scholar]
- 260. Parthasarathy U, Asish GR, Saji KV, Johnson GK, Leela NK, Mathew PA. Diversity study of leaf volatile oil constituent of Piper species based on GC/MS and spatial distribution. J Spices Aromat Crops. 2014;23(1):10‐16. [Google Scholar]
- 261. Prakash B, Shukla R, Singh P, Kumar A, Mishra PK, Dubey NK. Efficacy of chemically characterized Piper betle L. essential oil against fungal and aflatoxin contamination of some edible commodities and its antioxidant activity. Int J Food Microbiol. 2010;142(1–2):114‐119. [DOI] [PubMed] [Google Scholar]
- 262. Prescott TAK, Kiapranis R, Maciver SK. Comparative ethnobotany and in‐the‐field antibacterial testing of medicinal plants used by the Bulu and inland Kaulong of Papua New Guinea. J Ethnopharmacol. 2012;139(2):497‐503. [DOI] [PubMed] [Google Scholar]
- 263. Purkayastha J, Nath SC, Islam M. Ethnobotany of medicinal plants from Dibru‐Saikhowa biosphere reserve of Northeast India. Fitoterapia. 2005;76(1):121‐127. [DOI] [PubMed] [Google Scholar]
- 264. Rahmatullah M, Mukti IJ, Haque A, et al. An ethnobotanical survey and pharmacological evaluation of medicinal plants used by the Garo tribal community living in Netrakona district, Bangladesh. Adv Nat Appl Sci. 2009;3(3):402‐418. [Google Scholar]
- 265. Saikia AP, Ryakala VK, Sharma P, Goswami P, Bora U. Ethnobotany of medicinal plants used by Assamese people for various skin ailments and cosmetics. J Ethnopharmacol. 2006;106(2):149‐157. [DOI] [PubMed] [Google Scholar]
- 266. Sankaranarayanan S, Bama P, Ramach J, et al. Ethnobotanical study of medicinal plants used by traditional users in Villupuram district of Tamil Nadu, India. J Med Plants Res. 2010;4(12):1089‐1101. [Google Scholar]
- 267. Savithramma N, Sulochana C, Rao KN. Ethnobotanical survey of plants used to treat asthma in Andhra Pradesh, India. J Ethnopharmacol. 2007;113(1):54‐61. [DOI] [PubMed] [Google Scholar]
- 268. Valle DL, Puzon JJM, Cabrera EC, Rivera WL. Thin layer chromatography‐bioautography and gas chromatography‐mass spectrometry of antimicrobial leaf extracts from Philippine Piper betle L. against multidrug‐resistant bacteria. Evid Based Complement Altern Med 2016;1‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. World Health O, Humans IWGotEoCRt, & International Agency for Research on C (2004). Betel‐quid and areca‐nut chewing and some areca‐nut‐derived nitrosamines (Vol. 85). IARC. [PMC free article] [PubMed] [Google Scholar]
- 270. Yakub A, Leksono AS, Batoro J. Ethnobotany of medicinal and edible plants of Tobelo Dalam Tribe in Aketajawe Lolobata National Park Area. Indonesian J Environ Sustain Dev. 2019;10(1):45–50. [Google Scholar]
- 271. Yoonus J, Resmi R, Beena B. Greener nanoscience: Piper betel leaf extract mediated synthesis of CaO nanoparticles and evaluation of its antibacterial and anticancer activity. Mater Today Proceed. 2021;41:535‐540. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.


