Abstract
The use of opioids in pain management is hampered by the emergence of analgesic tolerance, which leads to increased dosing and side effects, both of which have contributed to the opioid epidemic. One promising potential approach to limit opioid analgesic tolerance is activating the CNS endocannabinoid system, via activation of CB1 receptors (CB1Rs) in the descending pain inhibitory pathway. In this review, we first discuss preclinical and clinical evidence revealing the potential of pharmacological activation of CB1Rs in modulating opioid tolerance, including activation by phytocannabinoids, synthetic CB1R agonists, endocannabinoid degradation enzyme inhibitors, and recently discovered CB1R positive allosteric modulators. On the other hand, as nonpharmacological pain relief is advocated by the US-NIH to combat the opioid epidemic, we also discuss contributions of peripheral neuromodulation, involving the electrostimulation of peripheral nerves, in addressing chronic pain and opioid tolerance. The involvement of supraspinal endocannabinoid systems in peripheral neuromodulation-induced analgesia is also discussed.
Keywords: Opioid epidemic, opioid tolerance, endocannabinoids, CB1 receptor, monoacylglycerol lipase, fatty acid amide hydrolase, peripheral neuromodulation
1. Opioid epidemic
The opioid epidemic is an issue of great concern in Northern America and many European countries (OECD, 2019) due to a continuously increasing death rate from misuse or overuse of opioids. In 2018, opioids contributed to more than two-third of overdose deaths in the US (US-CDC, 2020), exerting an enormous economic burden to the healthcare system (Hagemeier, 2018). Opioids are commonly prescribed for relieving cancer pain, postoperative pain or severe neuropathic pain. However, patients often continued to consume opioids even though opioids are categorized as a second or third-line treatment for chronic neuropathic pain (Urits et al., 2020). This is possibly due to persistent painful conditions or postsurgical opioid over-prescription (Neuman et al., 2019). Besides prescription opioids, improper disposal of leftover opioids and the availability of over-the-counter opioids have also substantially contributed to opioid misuse (Sobczak and Gorynski, 2020). Repeated opioid use leads to analgesic tolerance, dependence, and abuse (Skolnick, 2018). Increasing dosing of opioids for better pain control also increases the risk of constipation and respiratory depression, due to the excessive activation of opioid receptors in gastrointestinal and respiratory systems. The latter leads to opioid overdose death. In fact, the consumption of opioids for relieving non-cancer pain, as compared with other analgesics, positively correlates with an increased risk of all-cause mortality (Ray et al., 2016). Thus, unresolved chronic pain and potentially mismanaged opioid administration are significant contributors to the ongoing opioid crisis (Skolnick, 2018). Although there are several non-opioid analgesic options, opioids retain their central role in the approach of many practitioners to pain management, despite inconclusive or negative findings in benefit to risk analyses.
2. Opioids, endorphins, and descending pain inhibition
2.1. Opioid-induced analgesia
There are four members in the opioid receptor family, μ- (MOR), κ- (KOR) and δ- (DOR) opioid receptors as well as NOP receptors (NOR) (Alexander et al., 2019). The analgesic effect of clinically used opioids is mainly mediated by MORs, which are abundantly expressed in neuronal tissues involved in the descending pain inhibitory pathway, including the periaqueductal gray (PAG), rostral ventromedial medulla (RVM) and spinal cord, as well as in sensory nerve endings (Stein, 2016). MOR activation leads to neuronal inhibition by activating G protein-coupled-inward rectifying K+ (GIRK) channels and/or inhibiting Ca2+ channels, both mechanisms can contribute to opioid-induced analgesia. GIRK channel activation induces neuronal membrane hyperpolarization and thus directly inhibits neuronal excitability, which probably contributes to the analgesic effect of opioids at peripheral and spinal levels (Nockemann et al., 2013). Ca2+ channel inhibition at nerve terminals either leads to reduced glutamate release at the spinal dorsal horn or produces disinhibition in the PAG by reducing GABA release onto the glutamatergic neurons in the PAG, which project to the RVM, and subsequently suppress nociceptive transmission at the spinal cord (Bagley and Ingram, 2020).
2.2. Opioid tolerance and the descending pain inhibitory pathway
Repeated application of MOR agonists typically elicits MOR desensitization (Williams et al., 2013) and internalization (Koch and Hollt, 2008) via β-arrestin-2- and/or PKA-dependent pathways, both of which may contribute to analgesic tolerance of opioids. The descending pain inhibitory pathway, where MORs are densely expressed, plays an important role in the generation of analgesic tolerance, especially the PAG. Direct microinjection of morphine into the PAG induced a stronger analgesic effect and quickly elicited analgesia tolerance, compared to injection at the dorsal raphe nucleus (Campion et al., 2016). Similar susceptibility of the PAG to morphine analgesic tolerance was observed in another comparative study between PAG and RVM (Morgan et al., 2005). These data demonstrated that opioid-sensitive neurons in the PAG play a prominent role in the development of opioid analgesic tolerance. Indeed, electrophysiological studies in PAG slices of morphine tolerant mice, found reduced responsiveness of the PAG neurons towards an MOR agonist, suggesting down-regulation of MOR number and/or dysfunctional MOR activity (Bagley et al., 2005).
2.3. Endogenous opioids in PAG stimulation-induced analgesia and stress-induced analgesia
Electrical stimulation of the PAG was able to produce analgesia, which is mediated by endogenous opioids in rodents (Cannon et al., 1982) and humans (Young and Chambi, 1987). This PAG stimulation-produced analgesia (PAG-SPA) was naloxone-sensitive (Akil et al., 1976), mediated by a mechanism similar to morphine analgesia, and exhibited analgesic tolerance after repeated administrations (Mayer and Hayes, 1975). PAG-SPA is known to exhibit crosstalk with the neuronal circuit involved in the phenomenon of stress-induced analgesia (SIA) (Terman et al., 1985), an evolutionarily conserved, self-protecting mechanism in mammals engaged during life-threatening situations. During stress, endogenous opioids, e.g., β-endorphin (Rubinstein et al., 1996), are released to induce analgesia via activating the descending pain inhibitory pathway.
In addition to opioid-dependent PAG-SPA, a naloxone-insensitive PAG-SPA was also reported (Yaksh et al., 1976), but its mechanism(s) remained unclear for decades. Similarly, opioid-independent SIA was also reported (Terman et al., 1986), which, unlike opioid-dependent SIA, did not develop tolerance after repeated induction (Suplita et al., 2008). The opioid-independent form of SIA was, very much later, revealed to be mediated, at least in part, via the endocannabinoid (eCB) system (Hohmann et al., 2005; Lee et al., 2016; Lee et al., 2020). Thus, it is not surprising that the (endo)cannabinoid system has been regarded as a viable alternative pharmacological target for analgesia, although the efficacy of its clinical application remains inconclusive. In some countries, cannabinoids have been suggested as second- or third-line treatments for chronic pain (Urits et al., 2020).
3. Cannabinoid-induced analgesia
3.1. Cannabinoid pharmacology
Cannabinoids include phytocannabinoids, synthetic cannabinoids and eCBs, as reviewed by Schurman et al. (2020). Phytocannabinoids are isolated from marijuana and hemp (Cannabis sativa), where Δ9-tetrahydrocannabinol (THC) is the major psychomimetic constituent. Synthetic cannabinoids, such as CP-55,940, WIN-55,212, levonantradol, etc, have been synthesized for research or drug development purposes and have higher affinity and often higher efficacy at cannabinoid receptors, as compared to THC. eCBs are endogenous ligands of cannabinoid receptors. They are notable for being synthesized on demand, unlike classical neurotransmitters that are pre-synthesized, stored in vesicles of nerve terminals, and rapidly released following increases in nerve terminal calcium. Anandamide (AEA) and 2-arachydononylglycerol (2-AG) are two main eCBs, and both are well documented for their roles in analgesia. 2-AG, in particular, appears to be synthesized in postsynaptic neurons and then travels retrogradely to activate presynaptic cannabinoid CB1 receptors (CB1Rs) to inhibit neurotransmitter release. The synthesis and degradation of these two eCBs are enzymatically regulated, which will be discussed in the later section.
Cannabinoids can produce several biological activities via CB1Rs and CB2Rs, such as analgesia, anti-emesis, hyperthermia, appetite stimulation, anti-spasticity, anti-epilepsy, anti-inflammation and anti-tumorigenesis (Alexander and Molina-Holgado, 2019). Among these, the analgesic effect mediated by CB1Rs is noteworthy, although CB2Rs also have a role in pain regulation (Iyer et al., 2020). CB1Rs are expressed in neuronal tissues along the descending pain inhibitory pathway, including the PAG (Bouchet and Ingram, 2020; Lau and Vaughan, 2014), RVM and spinal cord (Kelly and Chapman, 2001), as well as in some peripheral nerves endings (Kelly et al., 2003). At the cellular level, CB1R activation, via Gi/o protein activation, leads to inhibition of adenylyl cyclase, activation of K+ channels, and inhibition of Ca2+ channels, which can contribute to the analgesic effects of cannabinoids (Alexander and Molina-Holgado, 2019).
Interestingly, opioids and cannabinoids share the same cellular action mechanisms and a similar regional distribution in the descending pain inhibitory pathway (Wilson-Poe et al., 2012), while both exert their analgesic effects through their respective receptors (Lau and Vaughan, 2014). Cannabinoids can also activate the descending pain inhibitory pathway, which encompasses the midbrain PAG and its downstream RVM, and ultimately inhibit pain transmission at the spinal cord (Bouchet and Ingram, 2020). Through its distinct pharmacological receptors and profiling, the cannabinoid system is regarded as a main non-opioid analgesic mechanism to complement opioid analgesia in the descending pain inhibitory pathway, especially in the PAG (Lau and Vaughan, 2014). Thus, cannabinoids are well-positioned to be a non-opioid alternative in pain management.
3.2. Cannabinoids and pain relief
Through their evaluation in multiple preclinical pain models, both phytocannabinoids and synthetic cannabinoids have been reported to suppress acute and chronic pain, mainly, via CB1Rs (Narouze, 2020). However, like opioids, the tolerance development is also a concern when using cannabinoids as an alternative pain-relieving agent. Like MORs, prolonged activation of CB1Rs may result in β-arrestin-dependent desensitization (Daigle et al., 2008; Nguyen et al., 2012), which may contribute to analgesic tolerance of cannabinoids. The analgesic effect of repeated low doses of THC, although less susceptible to tolerance development (McKinney et al., 2008), was found to be associated with cognitive dysfunction in rodents (Sarne et al., 2011). Clinically, substantial human studies unfortunately also indicated a limited efficacy of using CB1R agonists in relieving chronic non-cancer pain (Stockings et al., 2018). Often, the therapeutic benefits of CB1R agonists are limited by the risk or emergence of adverse psychoactive effects.
3.3. Endocannabinoids and pain relief
Besides exogenous agonists, pharmacological activation of CB1Rs can be achieved via increasing eCB levels through inhibiting their respective degradation enzymes, i.e. fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL) for AEA and 2-AG, respectively (Donvito et al., 2018). At CB1Rs, AEA is a low efficacy agonist, whereas 2-AG is a high efficacy agonist, although AEA has a higher affinity than 2-AG (Narouze, 2020). An electrophysiological study has shown that inhibiting either FAAH or MAGL enhanced GABAergic transmission via CB1Rs in rat PAG slices (Lau et al., 2014), which can lead to PAG disinhibition, subsequently activating the descending pain inhibitory pathway and inducing analgesia (Guindon et al., 2013; Lau and Vaughan, 2014). Several in vivo preclinical studies also confirmed the analgesic effect of eCB degradation inhibitors in various animal models of inflammatory and neuropathic pain, as reviewed previously (Donvito et al., 2018; Hossain et al., 2020). It is noteworthy that majority of these preclinical studies demonstrated that eCB degradation inhibitors possess antiallodynic and/or anti-hyperalgesia effect in inflammatory and neuropathic pain models (Donvito et al., 2018; Hossain et al., 2020), insufficient finding are available in the literature to demonstrate whether both classes of eCB degradation inhibitors (FAAH and MAGL inhibitors), can enhance pain threshold in naïve animals, similar to opioids.
Nonetheless, similar to exogenous CB1R agonists, repeated CB1R-activation by eCBs, especially by 2-AG, can also cause analgesic tolerance. This was used to explain the underlying cause of analgesic tolerance observed in repeated administrations of MAGL inhibitors, such as JZL184 and KML29 (Crowe et al., 2017; Schlosburg et al., 2010), due to the eCB overload phenomenon (Lichtman et al., 2010). Repeated administrations of a low dose of JZL184, which was expected to modestly increase level of 2-AG and activate CB1Rs in a manner similar to low doses of THC, seems to induce analgesia without tolerance in mice (Kinsey et al., 2013). On the other hand, although repeated administration of exogenous AEA caused analgesic tolerance (Welch, 1997), repeated administration of its degradation enzyme (FAAH) inhibitor generally does not elicit analgesic tolerance (Kiso et al., 2020; Slivicki et al., 2018a; Slivicki et al., 2019), but see Okine et al. (2012).
To date, the successful application of FAAH and MAGL inhibitors in pain management is at the preclinical stage only. An earlier clinical study with an FAAH inhibitor, PF-04457845, did not show significant analgesia in phase II trial in patients with osteoarthritic pain (Huggins et al., 2012). The phase I clinical trial of another FAAH inhibitor, BIA 10–2474, was terminated early due to flaws in research design and a likely off target effect (Kaur et al., 2016). A clinical study of an MAGL inhibitor, ABX-1431, for experimental pain and peripheral neuropathy is completed, but the results have not been revealed (Deng and Li, 2020). Further clinical studies may be planned to investigate the efficacy of FAAH and/or MAGL inhibitors in humans.
Recently, Hohmann’s group identified a novel pharmacological approach to enhance eCB-CB1R-mediated analgesia without tolerance by using a positive allosteric modulator (PAM) of CB1Rs. This PAM retained its analgesic efficacy after chronic administration without tolerance in animal models of inflammatory pain and chemotherapy-induced neuropathic pain (Slivicki et al., 2018b). This pharmacological manipulation has the potential to enhance eCB-CB1R transmission at low or normal levels of eCBs and thus minimize the occurrence of CB1R desensitization due to eCB overload. However, further studies will be needed to determine its role in managing chronic pathological pain in humans.
4. Opioid-sparing effects by cannabinoids in opioid-tolerant subjects
4.1. Synergistic analgesia between opioids and cannabinoids
Concurrent activation of MORs and CB1Rs was found to synergistically induce analgesia in preclinical studies, where co-administration of THC with opioids, including morphine and codeine, was found to increase the potency of opioids by 1.2–24.8 folds, as compared with the group co-administered with the vehicle of THC (Cichewicz, 2004). Thus, co-administration of opioids with cannabinoids is considered to be a potential strategy to reduce the amount of opioid needed to produce analgesia, i.e., provide an opioid-sparing effect, in chronic opioid users. If co-administration of a cannabinoid reduces the amount of opioid needed for satisfactory analgesia, this may be a way to decrease the development of opioid analgesic tolerance. In this section, we will discuss the preclinical and clinical evidence of the opioid-sparing effect of cannabinoids.
In rodents, systemic administration of a CB1R agonist together with morphine can induce synergistic analgesia in neuropathic (Kazantzis et al., 2016) and inflammatory (Chen et al., 2019) pain models. Interestingly, several studies also showed synergistic analgesia when morphine was co-administered with an ineffective dose of cannabinoids (Alsalem et al., 2019), or when both morphine and cannabinoids were at sub-effective doses (Smith et al., 2007). The PAG is an important site of action for this synergistic analgesia. Repeated intra-PAG (i.pag.) microinjections of a CB1R agonist enhanced the analgesic efficacy of subsequent i.pag. morphine injection without producing analgesic tolerance (Wilson et al., 2008). Thus, the synergistic analgesic effect of cannabinoids with low doses of opioids may be utilized to reduce opioid tolerance. Interestingly, a continuation study by the same group showed that the analgesic synergism between CB1R and MOR agonists in rat PAG is bidirectional (Wilson-Poe et al., 2013). Nevertheless, an asymmetrical analgesic synergism was reported when CB1R and MOR agonists were systemically administered (Vigano et al., 2005). In morphine (i.p.)-tolerant rats, i.p. injection of a CB1R agonist can induce analgesia, but not vice versa. The synergistic analgesia produced by opioids and cannabinoids was also reported in nonhuman primates (Nilges et al., 2019), a preclinical model known to closely mimic human responses.
However, the outcomes of clinical studies examining cannabinoid/opioid interactions are not as consistently promising as expected from preclinical studies (Nielsen et al., 2017). In healthy volunteers, cannabis enhances the analgesic effects of sub-threshold oxycodone (Cooper et al., 2018). However, coadministration of dronabinol with oxycodone not only did not enhance oxycodone-induced analgesic effect, but it also increased abuse- and impairment-related subjective responses in healthy volunteers (Babalonis et al., 2019). In patients suffering from chronic non-cancer pain, indeed some studies showed a positive correlation of enhanced opioid analgesia with cannabis co-administration (Degenhardt et al., 2015) and some patients consider using cannabinoids as a substitution for opioids (Takakuwa and Sulak, 2020). However, in cancer pain patients with optimized opioid therapy, add-on administration with a cannabinoid did not show superior pain relief as compared with placebo (Lichtman et al., 2018). Meta-analysis studies also indicated that no significant opioid reduction was achieved by medical cannabis or CB1R agonists in patients with postoperative pain (Abdallah et al., 2020), non-cancer chronic pain (Okusanya et al., 2020) and cancer-related chronic pain (Boland et al., 2020). Furthermore, some clinical studies indicated that cannabinoid treatment is associated with higher pain intensity (Liu et al., 2019), increased opioid consumption (Bhashyam et al., 2018), and enhanced opioid abuse potential (Babalonis et al., 2019) in patients with acute or postoperative pain. Furthermore, a history of cannabis use can increase the tendency of inpatient opioid use (Dalal et al., 2020).
4.2. Synergistic analgesia between opioids and eCBs
As for the potential synergistic effect of eCBs on opioid analgesia, either through inhibiting eCB degradation or CB1R PAMs, so far, only preclinical studies are available. When administered alone, an FAAH inhibitor or MAGL inhibitor, which was known to increase AEA or 2-AG levels, significantly inhibited chemotherapy-induced (Guindon et al., 2013) and chronic constriction injury (CCI)-induced (Kinsey et al., 2009) neuropathic pain in mice, via CB1Rs as analgesia was prevented by pretreatment with a CB1R antagonist. Table 1 compiles the available reports on the opioid-sparing and analgesic tolerance-preventive effects of eCB degradation enzyme inhibitors. Co-administration of a selective MAGL inhibitor with a sub-effective dose of morphine was synergistic in eliciting analgesia in formalin-induced inflammatory pain in rats (Clapper et al., 2018) or in CCI-induced neuropathic pain in mice (Wilkerson et al., 2016). Interestingly, repeated co-administrations of these agents did not show analgesic tolerance (Wilkerson et al., 2016). A similar opioid-sparing effect was observed in rodents co-treated with morphine and a selective FAAH inhibitor (Hasanein and Ghafari-Vahed, 2016; Slivicki et al., 2018a) or a dual FAAH/MAGL inhibitor (Wilkerson et al., 2017). Repeated co-administrations of an FAAH inhibitor (Fotio et al., 2020; Hasanein and Ghafari-Vahed, 2016) or a dual inhibitor (Wilkerson et al., 2017) with morphine also prevented or attenuated morphine analgesic tolerance.
Table 1.
Synergistic effects on opioid analgesia induced by endocannabinoids.
| Pain Model | Animal | eCB enhancer | Interaction with morphine | Reference | ||
|---|---|---|---|---|---|---|
| AEA | 2-AG | |||||
| FAAH inhibitor | MAGL inhibitor | Acute morphinea (Dose) | Repeated morphineb (Dose, days) | |||
| Inflammatory pain (formalin test) | SD rat | ABD-1970 | Synergistic analgesia (2.49 mg kg−1, s.c.) | Clapper et al., 2018 | ||
| Neuropathic pain (CCI) | C57BL/6J mouse | MJN110 | Synergistic analgesia (0.82 mg kg−1, i.p.) | Reduced tolerance (0.82 mg kg−1, 2× day−1, 6 days) | Wilkerson et al., 2016 | |
| Acute pain (tail-flick) | Wistar rat | URB597 | Reduce tolerance (10 mg kg−1, 2× day−1, 7 days) | Hasanein and Ghafari-Vahed, 2016 | ||
| Neuropathic pain (chemotherapy) | C57BL/6J mouse | URB597 URB937 | Reduction of morphine ED50 | Slivicki et al., 2018a | ||
| Neuropathic pain (CCI) | C57BL/6J mouse | SA-57 (dual inhibitor) | Reduction of morphine ED50 | Without tolerance (1.12 mg kg−1, 2× day−1, 5 days) | Wilkerson et al., 2017 | |
| Acute pain (tail-immersion) | CD1 mouse | URB597 | No interaction (15 mg kg−1, s.c.) | Reduced tolerance (15–30 mg kg−1, 2× day−1, 7 days) | Fotio et al., 2020 | |
| URB937 | No interaction (15 mg kg−1, s.c.) | No interaction (15–30 mg kg−1, 2× day−1, 7 days) | ||||
| Neuropathic pain (chemotherapy) | C57BL/6J mouse | GAT211 (CB1R PAM) | Reduction of morphine ED50 | Without tolerance (10 mg kg−1, 1× day−1, 20 days) | Slivicki et al., 2020 | |
The acute dose of morphine when co-administrated with the eCB enhancer.
The repeated doses of morphine and treatment duration when co-administrated with the eCB enhancer. URB597 is a BBB-permeable and URB937 is a BBB-impermeable FAAH inhibitors. SA-57 is an FAAH and MAGL dual inhibitor. 2-AG: 2-arachidonoylglycerol; AEA: anandamide; CB1R PAM: CB1 receptor positive allosteric modulator; CCI, chronic constriction injury; eCB, endocannabinoid; FAAH, fatty acid amide hydrolase; i.p., intraperitoneal injection; MAGL, monoacylglycerol lipase; s.c., subcutaneous injection; SD rat, Sprague Dawley rat.
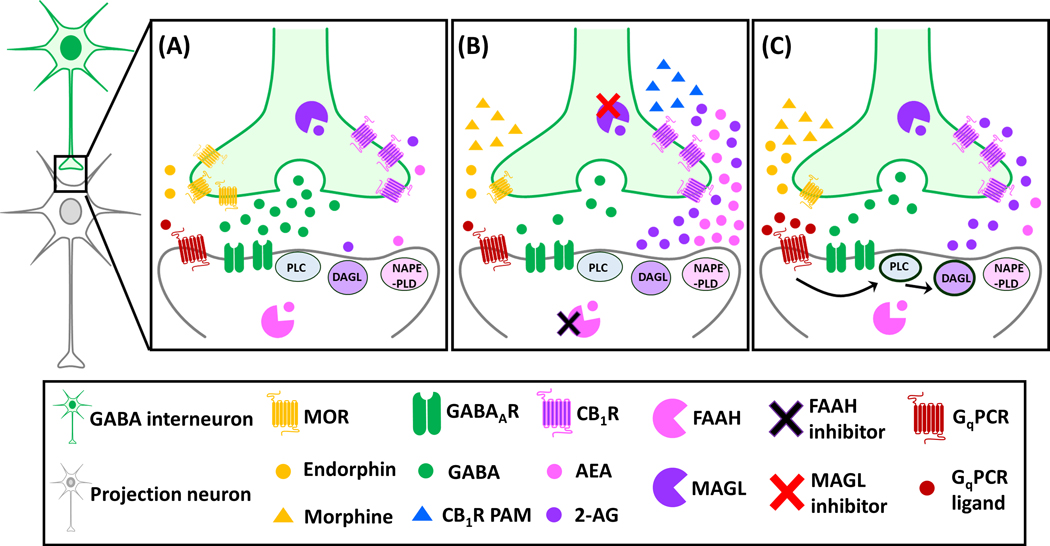
Fig. 1 (A & B) depicted a working model of the possible signaling pathway in the PAG explaining how eCB degradation enzymes restore analgesia in opioid-tolerant conditions. Briefly, downregulation of MORs in GABA interneurons of the PAG following repeated exposure to exogenous opioids, causes analgesic tolerance as it impairs opioid-induced disinhibition signaling onto the projection neuron that is required to activate the descending inhibitory pathway (Bagley and Ingram, 2020; Bouchet and Ingram, 2020). Pharmacological inhibition of MAGL and/or FAAH, via their respective inhibitors, causes an accumulation of eCBs that can activate the CB1Rs on GABA interneurons, thus restoring the disinhibition signaling in the PAG (Fig. 1A). On the other hand, a recent study demonstrated that a CB1R PAM co-administered with morphine augmented the analgesic effect of morphine and prevented the development of morphine tolerance (Slivicki et al., 2020). This may be attributed to an enhancement of the effect of eCBs on presynaptic CB1Rs on GABAergic terminals of PAG neurons, leading to the restoration of PAG disinhibition, which was impaired during opioid-tolerant conditions (Fig. 1B). Taken together, similar to preclinical studies of CB1R agonists, eCB degrading enzyme inhibitors and a CB1R PAM showed convincing analgesia and attenuated opioid tolerance, but further clinical studies will need to be conducted to provide translational validity of their pharmacotherapeutic potentials.
Fig. 1. A working model of possible interactions between opioids and endocannabinoids in the periaqueductal gray (PAG) in control (A) and opioid-tolerant conditions during pharmacological (B) and peripheral neuromodulation (C) interventions.
(A) Under control condition, the glutamatergic projection neuron (grey neuron) in the PAG receives GABA (green circles) inhibitory transmission via GABAA receptors (green receptors). The inhibition of GABA release from the presynaptic GABA interneuron (green neuron) (disinhibition) can be achieved via presynaptic activation of either μ-opioid receptors (MORs, yellow receptors) by endorphins (yellow circles) or CB1 receptors (CB1Rs, pinkish-purple receptors) by endocannabinoids (eCBs), i.e., 2-arachidonoylglycerol (2-AG, purple circles) or anandamide (AEA, pink circles). The levels of 2-AG and AEA are regulated by their respective degradation enzymes, monoacylglycerol lipase (MAGL, purple circular sectors in the presynaptic GABAergic terminal) or fatty acid amide hydrolase (FAAH, pink circular sectors in the postsynaptic neuron). (B) During opioid-tolerant conditions, the expression of MORs on GABA interneurons is downregulated due to repeated treatments with exogenous opioids, e.g., morphine (yellow triangles), leading to a reduced inhibition of GABAergic transmission by opioids, i.e., reduced disinhibition. This reduction of disinhibition on the postsynaptic glutamatergic projection neuron can possibly be restored by pharmacological inactivation of eCB degradation enzymes with an MAGL inhibitor (red cross) and/or an FAAH inhibitor (black cross), or by a CB1R positive allosteric modulator (PAM). (C) On the other hand, the reduction of disinhibition on the postsynaptic projection neuron during opioid-tolerant conditions can also be achieved via peripheral neuromodulation through an opioid-independent and cannabinoid-dependent mechanism. That is, electrostimulation at peripheral nerves, e.g., the median nerve, can lead to activation of the Gq protein-coupled receptors (GqPCRs) by increasing the release of GqPCR ligands (e.g., orexins or glutamate) in the PAG. Via the phospholipase C (PLC)-diacylglycerol lipase (DAGL) pathway, 2-AG can be synthesized and produce retrograde inhibition of GABA release via presynaptic CB1Rs. The images of neurons, ligands and receptors are adapted from Illustration Toolkit Neuroscience by Motifolio.
It is noteworthy that the abovementioned preclinical studies on the analgesic effect of cannabinoids and eCBs use acute neurogenic pain (thermal or mechanical), inflammatory pain or chronic neuropathic pain (surgical-, chemotherapy-, or diabetic induced) models. Although they are often assumed to be translationally relevant for clinical pain, they may not adequately mimic the multidimensionality of clinical pain conditions, e.g., the motor, neurological and psychological complications (Finn et al., 2021) associated with chronic pain in humans. Further studies are required to collectively examine the potential effects of cannabinoids and eCBs on the full multidimensionality of clinical pain conditions in humans.
5. Peripheral neuromodulation and chronic pain relief
5.1. Peripheral neuromodulation
The search for pharmacological replacements for opioid analgesics is regarded as an important component to resolve the opioid crisis (Yaksh et al., 2018). However, the financial and time costs of novel drug development are considerable barriers to a rapid solution. The US National Institute of Health has recognized that nonpharmacological treatments of pain may be an alternative approach to address chronic pain as well as the opioid crisis (Abbasi, 2018). Peripheral neuromodulation may be one of the feasible options.
The main principle of peripheral neuromodulation involves the application of electrical stimulation on the peripheral nerves to achieve therapeutic effects, including analgesia (Slavin et al., 2015). In an early implementation, peripheral modulation was achieved via peripheral nerve stimulation (PNS), where large electrodes were implanted next to the target nerves via open surgery (Slavin et al., 2015). Gradually, several less invasive procedures of peripheral neuromodulation were introduced, including transcutaneous electrical nerve stimulation (TENS), percutaneous electrical nerve stimulation (PENS), and ultrasound-guided percutaneous PNS (pPNS). TENS involves delivering transcutaneous electrical stimulation through intact skin to activate the underlying nerve fibers (Teoli and An, 2021). When TENS is applied to an acupoint is referred as transcutaneous acupoint electrical stimulation (TEAS) (Liang et al., 2019). PENS is conducted via inserting acupuncture-like needles into the dermatome(s) of the target peripheral nerve (Ghoname et al., 1999). The later ultrasound-guided pPNS was developed by percutaneously inserting stimulating electrodes to the target nerve guided under ultrasound, utilizing similar principles as conventional PNS while avoiding an invasive open surgery (Gabriel and Ilfeld, 2021).
5.2. Analgesic mechanisms of peripheral neuromodulation
5.2.1. Opioid-dependent
Since the first report of peripheral nerve stimulation for pain management in 1967 (Wall and Sweet, 1967), the Gate Theory of Pain or Gate Control Theory is attributed as the underlying mechanism (Melzack and Wall, 1965). This theory posits that nociceptive transmission via Aδ and C fibers can be interfered with by non-painful stimuli on Aβ fibers, with the spinal dorsal horn as the “gate” to suppress pain transmission to the brain. However, the gate theory cannot explain the systemic analgesia or the pain relief at the location distal to the stimulating point of peripheral neuromodulation (Gozani, 2019). Thus, mechanisms other than the gate theory may be involved.
Endogenous opioids are one of the candidates that contribute to peripheral neuromodulation-induced analgesia. PNS can increase β-endorphin levels in human cerebrospinal fluid (CSF) (Clement-Jones et al., 1980; Salar et al., 1981). Direct PAG electrostimulation can also induce β-endorphin elevation in the CSF (Amano et al., 1980), which contributes to SPA in humans (Hosobuchi et al., 1979). Thus, it is likely that PNS can activate the opioid system in the brain, releasing β-endorphin that subsequently triggers the PAG-RVM-spinal descending inhibitory pathway. Indeed, Sluka’s group reported that peripheral neuromodulation-induced analgesia can be reversed by blocking opioid receptors at PAG (DeSantana et al., 2009), RVM (Kalra et al., 2001) and spinal (Sluka et al., 1999) levels in rats with inflammatory pain. Preclinical reports on naloxone-sensitive peripheral neuromodulation are also available in the literature (Chen et al., 1996; Jorum and Shyu, 1988).
5.2.2. Opioid-independent
The opioid-dependent analgesic effects induced by peripheral neuromodulation or SPA, similar to morphine-induced analgesia, also develop tolerance after repeated stimulations, and displayed cross-tolerance to morphine in humans (Leonard et al., 2011; Young and Chambi, 1987) and rats (Mayer and Hayes, 1975). Nonetheless, numerous longitudinal studies have found that peripheral neuromodulation can surprisingly induce long-term analgesic effects, ranging from months to decades (Cohen et al., 2019; Johnson and Goebel, 2016; Kupers et al., 2011). Thus, tolerance-prone opioid signaling is unlikely to be the underlying mechanism. Indeed, the SPA induced by PAG stimulation in rats was only partially reversed by naloxone (Akil et al., 1976; Morozova and Zvartau, 1986) at the dose that completely reversed morphine-induced analgesia, suggesting an involvement of a non-opioid mechanism in the PAG, which is supported by a later clinical study (Young and Chambi, 1987).
Similarly, the evidence supporting that a naloxone-insensitive mechanism can contribute to peripheral neuromodulation-induced analgesia has been reported in humans since 1981. In patients with primary dysmenorrhea, Walker and Katz (1981a) found that repeated electrostimulation at the radial, median and/or saphenous nerves exerted a prolonged systemic analgesic effect that was naloxone-insensitive and did not show cross-tolerance with morphine. Their next publication in the same year emphasized that the same mode of PNS did not develop analgesic tolerance after repeated treatments (Walker and Katz, 1981b). The authors claimed that this was the first clinical evidence that described a “non-opioid pathway can produce lasting pain relief in patients with severe clinical symptoms” (Walker and Katz, 1981a).
The studies conducted in nonhuman primates by Willis’s group also demonstrated the involvement of a non-opioid mechanism in the analgesia induced by peripheral neuromodulation. For instance, electrostimulation at the tibial, sciatic or median nerve significantly suppressed neuronal activity in the spinothalamic tract (STT) activated by painful sural nerve stimulation, suggesting a central participation in peripheral neuromodulation (Chung et al., 1984b). Naloxone slightly but significantly reversed the suppression induced by tibial nerve stimulation on the elicited STT neuronal activity (Chung et al., 1984a). Furthermore, TENS at the hindlimb nerves also inhibited STT neuronal activity and induced analgesia (Foreman et al., 1975) in a manner not reversed by naloxone (Lee et al., 1985). These data suggested an opioid-independent mechanism contributes to peripheral neuromodulation-induced analgesia.
5.2.3. Involvement of eCBs
Among reported analgesic mechanisms other than opioids, the eCB system in the PAG is the most likely candidate involved in peripheral neuromodulation-induced analgesia. The first report indicating that the PAG eCB system can be activated by peripheral neuromodulation was demonstrated by Longhurst’s group in a study investigating the cardiovascular suppressive effect induced by peripheral neuromodulation in rats. They found that electrical stimulation at PC5 (Jianshi) and PC6 (Neiguan) acupoints, i.e. electroacupuncture at PC5-PC6, reduced GABA levels in the PAG in a manner reversed by a CB1R antagonist (Fu and Longhurst, 2009). PC5 and PC6 acupoints are known to overlie the median nerve (Joo Oh et al., 2012). Chiou’s group subsequently substantiated that median nerve stimulation (MNS) can trigger a CB1R-mediated inhibition of GABA release, i.e. disinhibition, in the PAG (Chen et al., 2018). That is, MNS at the PC6 acupoint (MNS-PC6) significantly suppressed the hot-plate nociceptive response in normal mice and mechanical allodynia in CCI-mice, via a CB1R-mediated disinhibition in the PAG through an endogenous orexin-initiated eCB signaling (Chen et al., 2018). This will be discussed in section 6. The same study also indicated that MNS-PC6-induced analgesia is naloxone-insensitive, in agreement with earlier studies in humans (Walker and Katz, 1981a; Walker and Katz, 1981b) and nonhuman primates (Lee et al., 1985) that found an opioid-independent mechanism underlying this form of peripheral neuromodulation-induced analgesia.
Besides MNS, stimulation of other peripheral nerves by the electroacupuncture procedure can also induce analgesia via the CB1R-mediated disinhibition mechanism in the PAG. A study reported that electroacupuncture at acupoints GB30 (Huantiao) and GB34 (Yanglingquan), a procedure similar to percutaneous sciatic nerve stimulation (Shao et al., 2015), significantly suppressed inflammatory and neuropathic pain responses in mice via CB1R-mediated inhibition of GABA neurons in the PAG (Zhu et al., 2019). In addition to the PAG, eCB-CB1R transmission in the periphery and spinal cord are also involved in peripheral neuromodulation-induced analgesia. TENS of the hindpaw of mice was found to induce analgesia, accompanied by increased anandamide and CB1R expression in paw, spinal, and PAG tissues (de Oliveira et al., 2020).
5.3. Opioid-sparing effect of peripheral neuromodulation providing pain relief in opioid-tolerant subjects
In clinical practice, the treatment duration of prescription opioids in an opioid-naïve postoperative patient strongly correlates with the likelihood to develop opioid dependence, which may lead to unintentional overdose or misuse of opioids. In addition to pharmacological intervention with cannabinoids, peripheral neuromodulation has been demonstrated to have opioid-sparing effects in patients with postoperative pain (Gabriel and Ilfeld, 2021). Table 2 summarizes the available literature on the opioid-sparing effect of peripheral neuromodulation. In patients with varied chronic pain conditions, direct PNS of the forearm nerves (ulnar, median or radial nerves) by implanted electrodes has been reported to remarkably reduce opioid consumption in 23 out of 24 (Strege et al., 1994) and 8 out of 9 (Deer et al., 2010) patients. Recently, clinical reports indicated that percutaneous PNS at the femoral and sciatic regions provided adjunct analgesic effect in patients receiving total knee arthroplasty surgery and led to an earlier cessation of opioid consumption (Ilfeld et al., 2019). Several placebo-controlled clinical studies also demonstrated that the consumption of opioids to relieve postoperative pain was significantly reduced by TENS treatment at the dermatome of the skin incision sites in patients receiving major spinal surgery (Unterrainer et al., 2010) or major gynecological procedures (Hamza et al., 1999; Wang et al., 1997). Interestingly, transcutaneous acupoint stimulation (TEAS) treatments also significantly reduced post-operative opioid consumption with the stimulating acupoints including the LI4 (Hegu) acupoint (Lan et al., 2012; Wang et al., 1997) targeting the radial nerve (Umemoto et al., 2019), the ST36 (Zusanli) acupoint (Chen et al., 1998; Lan et al., 2012) targeting the sciatic nerve (Jung et al., 2018), and the PC6 acupoint (Lan et al., 2012) targeting the median nerve (Chen et al., 1998) (Table 2). Besides acute postoperative pain, the requirement for oral opioids in patients with lower back pain was reported to be relieved by regional PENS or pPNS of the lower back (Kapural et al., 2018). Interestingly, a reduction in opioid consumption by TENS was accompanied by decreased cortisol levels 24 h after surgery (Szmit et al., 2021), suggesting that the TENS procedure reduces stress.
Table 2.
Opioid-sparing effect of peripheral neuromodulation in chronic pain or postoperative pain relief in clinical setting.
| Pain types | Subjecta | Target nerve (Acupoint) | Stimulation Mode | Opioid | Opioid Reduction (PostOP time)b | Reference |
|---|---|---|---|---|---|---|
| Chronic peripheral nerve pain | American (24 vs 0) | Ulnar/ Median/ Radial | PNS | Narcotics (Meperidine) | Opioid cessation in 23/24 patients. | Strege et al. (1994) |
| Chronic pain (Carpal tunnel syndrome) | American (9 vs 0) | Median | PNS | Oral narcotics | Opioid reduction in 8/9 patients. | Deer et al. (2010) |
| Postoperative (total knee arthroplasty) | American (7 vs 0) | Femoral/ sciatic | pPNS | Oxycodone | Opioid cessation: 45–60 to 6 days. | Ilfeld et al. (2019) |
| Postoperative (major spinal surgery) | Austrian (14 vs 11) | Dermatome of incision site | TENS | Piritramide | 62.1%. | Unterrainer et al. (2010) |
| Postoperative (major gynaecological procedures) | American♀ (25 vs 25) | Dermatome of incision site | TENS | Morphine | 50% (24 hr) 53% (overall). | Hamza et al. (1999) |
| Postoperative (lower abdominal surgery) | American♀ (25 vs 25) | Radial (LI4) | TEAS | Hydromorphone | 34% (24 hr) 46% (overall). | Wang et al. (1997) |
| Postoperative (total abdominal hysterectomy/ myomectomy surgery) | American♀ (25 vs 25) | Sciatic (ST36) | TEAS /TENS | Hydromorphone | 39% (24 hr) 38% (overall). | Chen et al. (1998) |
| Postoperative (total hip arthroplasty) | Chinese; (30 vs 30) | Median (PC6) /Radial (LI4) /Sciatic (GB31-ST36) | TEAS | Fentanyl | 37% (24 hr) 31% (48hr). | Lan et al. (2012) |
| Postoperative (inguinal hernia repair) | Polish (24 vs 23) | Radial (LI4)/ Dermatome of incision site | TEAS /TENS | Morphine | 51.6% (24 hr). | Szmit et al. (2021) |
The number of subjects in the treatment and placebo groups, respectively. Both genders were recruited, unless specified.
The opioid reduction percentage 24 hours post-operation or overall. PNS: direct peripheral nerve stimulation; PostOP: post-operation; pPNS: ultrasound-guided percutaneous peripheral nerve stimulation; TEAS: transcutaneous acupoint electrical stimulation at the GB31 (Fengshi), LI4 (Hegu), PC6 (Neiguan) or ST36 (Zusanli) acupoint. TENS: transcutaneous electrical nerve stimulation.
To the best of our knowledge, until now, only one preclinical study has investigated the potential beneficial effect of peripheral neuromodulation in opioid-tolerant animals. Taking advantage of the naloxone-insensitive analgesic effect induced by MNS-PC6 (Chen et al., 2018), Chiou’s group revealed that analgesic tolerance did not develop after repeated MNS-PC6 treatments in mice with neuropathic pain. They also found that MNS-PC6 was able to provide significant analgesia in neuropathic mice that had developed tolerance to escalating doses of morphine (Lee et al., 2021). MNS-PC6-induced analgesia is mediated by an eCB (2-AG)-mediated disinhibition of the PAG, a sequence after activation of orexin 1 receptors (OX1Rs), a type of Gq protein-coupled receptors (GqPCRs) (Fig. 1C). This study indirectly supports the notion that the eCBs, which are synthesized on demand and released under optimal spatial (in the synaptic cleft) and temporal (upon OX1R activation) conditions can induce analgesia without causing eCB overload and tolerance, an important distinction to the effect of pharmacological elevation of eCBs (Lichtman et al., 2010), thus avoiding CB1R desensitization. Although the mechanistic findings from the preclinical model are somewhat in line with prior clinical reports of peripheral neuromodulation-induced analgesia, it should be noted that these studies may not fully reflect other neurological and psychological aspects of chronic pain and/or opioid use disorders, thus further studies should be conducted.
6. Involvement of the orexin-eCB signaling in the PAG in analgesia induced by peripheral neuromodulation and stress
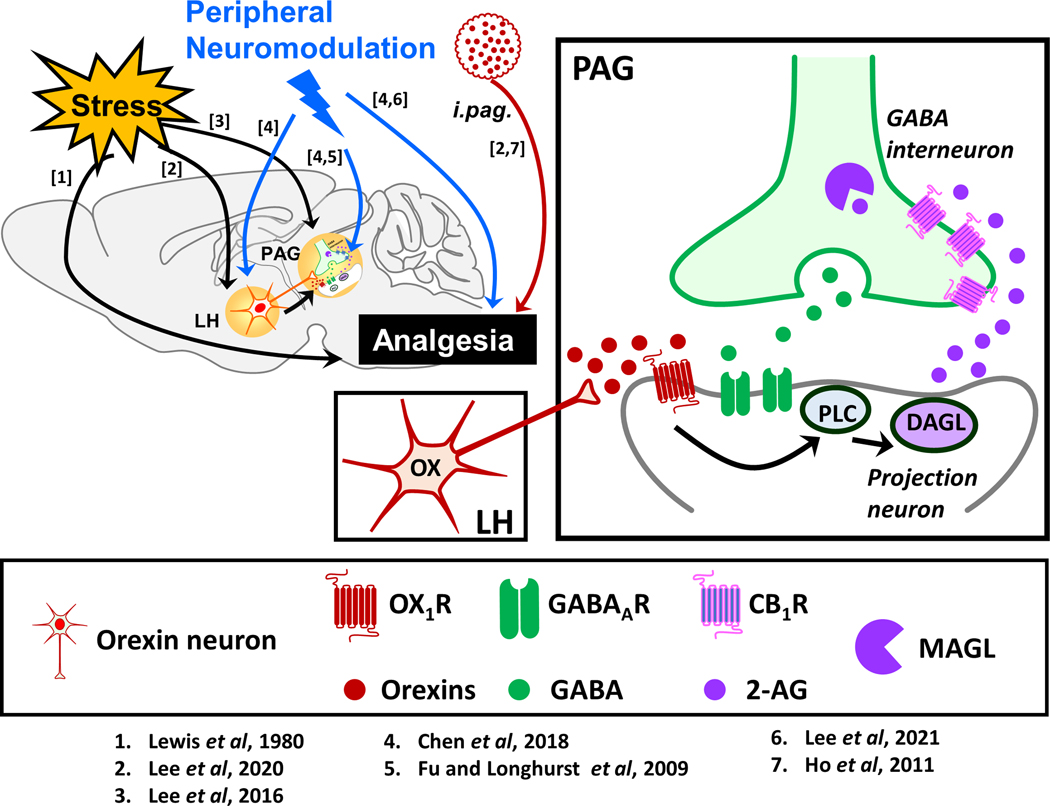
The orexin system consists of two hypothalamic neuropeptides, orexin-A and orexin-B, and two receptors, OX1 and OX2. Orexins are well-known to be involved in arousal, hormonal, metabolic and cardiovascular functions (Li and de Lecea, 2020), and also in pain regulation (Chiou et al., 2010). Using electrophysiological and behavioral approaches, Chiou’s group has reported that the analgesic effect induced by MNS-PC6 is opioid-independent (Chen et al., 2018), as observed in the opioid-independent form of SIA (Lee et al., 2016; Lee et al., 2020). Both modes of analgesia share the same disinhibition mechanism mediated by an endogenous orexin-initiated eCB cascade, as first revealed in the PAG (Ho et al., 2011). This mechanism can lead to analgesia via activating the descending pain inhibitory pathway. As depicted in Fig. 2, when orexin neurons in the lateral hypothalamus are activated by acute stress (Lee et al., 2016) or MNS-PC6 (Chen et al., 2018), orexins are released in the PAG to activate postsynaptic OX1 receptors (OX1Rs), a GqPCR, resulting in the synthesis of 2-AG via a phospholipase C (PLC)–diacylglycerol lipase (DAGL) enzymatic pathway. 2-AG then produces retrograde inhibition of GABA release by activating CB1Rs on GABAergic terminals, leading to disinhibition of the PAG excitatory neurons that project to the RVM that in turn send inhibitory inputs to the spinal cord, culminating in the activation of the descending pain inhibitory pathway that is constituted by the PAG–RVM–spinal cord circuit and ultimately leading to analgesia (Ho et al., 2011). It is noteworthy that activation of other GqPCRs in the PAG, e.g. mGlu5 receptors was shown to cause GABA disinhibition via a similar downstream signaling pathway (Drew et al., 2008) and mediate SIA in mice (Lee et al., 2020). Further studies will need to be carried out to discern their possible involvement in peripheral neuromodulation-induced analgesia.
Fig. 2. A proposed working model demonstrating the involvement of orexin-initiated endocannabinoid signaling in stress- and peripheral neuromodulation-induced analgesia.
The periaqueductal gray (PAG) is known to be a pivotal brain region mediating the supraspinal analgesic effect of orexins (red circles) as intra-PAG (i.pag.) microinjection of orexin was shown to produce analgesia in rodents via activation of OX1 receptors (OX1Rs, red receptor) in the projection neuron (grey neuron). Through the phospholipase C (PLC)–diacylglycerol lipase (DAGL) pathway, activation of OX1Rs leads to the production of 2-arachidonoylglycerol (2-AG, purple circles), an endocannabinoid (eCB) that travel retrogradely to activate presynaptic CB1 receptors (CB1Rs, pinkish-purple receptor) on the terminal of the GABA neuron (green neuron), resulting in decreased GABA (green circles) release, and thus disinhibition of the projection neuron, leading to analgesia. This orexin-initiated eCB signaling in the PAG was reported to mediate opioid-independent stress- and peripheral neuromodulation-induced analgesia, via activating orexin neurons (red neuron) in the lateral hypothalamus (LH). The numbers in square brackets refer to the numbers assigned to the supporting studies depicted below the schemas. The images of neurons, ligands and receptors are adapted from Illustration Toolkit Neuroscience by Motifolio.
7. Conclusions and future perspectives
Opioid analgesics are the “gold standard” in pain management and remain irreplaceable, although their chronic clinical use is limited by several unwanted side effects, especially the analgesic tolerance that leads to a dose escalation and ultimately increases the risk of respiratory depression. Therapeutic interventions to delay the emergence of opioid tolerance or maintain the analgesic efficacy after repeated opioid dosing may be achieved by activating the cannabinoid system in the descending pain inhibitory pathway. Mechanistic studies in laboratory animals support this notion, including the finding that exogenous cannabinoids (Chen et al., 2019) or inhibitors of eCB degradation (Wilkerson et al., 2017; Wilkerson et al., 2016) have an opioid-sparing effect by preventing opioid analgesic tolerance. However, inconclusive findings were reported in the clinical setting (Le Foll, 2021), and often the analgesic benefits of cannabinoids were masked by their neurocognitive side effects (Yanes et al., 2019). As direct CB1R activation is subject to tolerance as well, the cannabis-use history and the amount of cannabinoid intake may need to be established to ensure a higher success rate in clinical trials. Recent advancement in cannabinoid pharmacology is the introduction of CB1R PAMs, which have been reported to be devoid of analgesic tolerance and cannabimimetic side effects, such as hypothermia and catalepsy in rodents (Ignatowska-Jankowska et al., 2015; Slivicki et al., 2018b). Thus, CB1R PAMs seem to be a promising therapeutic agent for opioid-independent chronic pain control, provided that the efficacy is translated in clinical studies. Furthermore, their safety pharmacology and toxicology profiles should be established to ensure an adequate therapeutic window when used for chronic pain management.
On the other hand, peripheral neuromodulation via activating the eCB system in the descending pain pathway may be a potential alternative nonpharmacological option for chronic pain management. Since its clinical introduction in the 1960s, peripheral nerve stimulating devices have evolved, due to the advancement of biomedical technology, from surgically implanted electrodes to minimally invasive miniature implants (Banks and Winfree, 2019), and even more recently simplified to non-invasive wearable devices (Kong and Gozani, 2018). Thus, peripheral neuromodulation has become easily accessible. Extensive mechanistic studies (Chen et al., 2018; Lee et al., 2021) in animals have supported the involvement of endocannabinoids in the analgesic mechanism of peripheral neuromodulation by MNS. It can be speculated that this mode of opioid-independent analgesic management may be easily achieved by wearable devices in patients with opioid tolerance, providing satisfactory clinical outcomes, and thus may be of great benefit in palliative care or reducing opioid use and lessening the opioid crisis. Although clinical case studies and animal studies both showed similar efficacy in pain suppression, larger-scale randomized control trials for peripheral neuromodulation in opioid-tolerant patients should be conducted to discern the efficacy in this unique patient population.
Clinically, prescription opioids and chronic pain are both reported to be associated with depression and anxiety (Rosoff et al., 2021). The opioid and endocannabinoid systems in periaqueductal gray are known to, at least in part, be involved in these neuropsychiatric disorders. Furthermore, other domains of opioid use disorders, such as dependence, withdrawal, rewards, etc. have been demonstrated to be subject to cannabinoid-opioid interactions (Mohammadkhani and Borgland, 2020; Norris et al., 2019). Nonetheless, the neuropsychiatric interactions between opioid and cannabinoid systems would be another interesting and crucial topic of review.
Acknowledgements
This study was supported by the grants from the Ministry of Science and Technology, Taiwan (MOST 104-2745-B-002-004, MOST 106-2321-B-002-019; MOST 107-2321-B-002-010; MOST 108-2321-B-002-005; MOST 108-2320-B-002-029-MY3 and MOST 109-2320-B-002-042-MY3 to LCC; 107-2811-B-002 -008 to MTL), National Health Research Institutes, Taiwan (NHRI-EX109-10733NI to LCC), the Ministry of Education, Taiwan (107M4022-3 to LCC), National Institute of Health, USA (DA041229 and DA047858 to KM), Fundamental Research Grant Scheme, Ministry of Higher Education, Malaysia (FRGS/1/2021/WAB13/UCSI/02/1 to MTL) and the UCSI University Research Excellence and Innovation Grant, Malaysia (REIG-FPS-2020/065 to MTL).
Abbreviations:
- 2-AG
2-arachidonoylglycerol
- BBB
blood brain bar
- CB1R
CB1 receptor
- CB2R
CB2 receptor
- CCI
chronic constriction injury
- CSF
cerebrospinal fluid
- DAGL
diacylglycerol lipase
- DOR
δ-opioid receptor
- eCB
endocannabinoid
- i.p.
intraperitoneal injection
- FAAH
fatty acid amide hydrolase
- GIRK
G protein-coupled-inward rectifying K+ channels
- GqPCRs
Gq protein-coupled receptors
- KOR
κ- opioid receptor
- LH
lateral hypothalamus
- MAGL
monoacylglycerol lipase
- MOR
μ-opioid receptor
- MNS
median nerve stimulation
- MNS-PC6
median nerve stimulation at PC6 acupoint
- NOR
NOP receptor
- OX1R
OX1 receptor
- PAG
periaqueductal gray
- PAM
positive allosteric modulator
- PLC
phospholipase C
- PNS
direct peripheral nerve stimulation
- PostOP
post-operation
- pPNS
ultrasound-guided percutaneous peripheral nerve stimulation
- RVM
rostral ventromedial medulla
- s.c.
subcutaneous injection
- SIA
stress-induced analgesia
- SPA
stimulation-produced analgesia
- STT
spinothalamic tract
- TEAS
transcutaneous acupoint electrical stimulation at acupoint
- TENS
transcutaneous electrical nerve stimulation
- THC
Δ9-tetrahydrocannabinol
Footnotes
Data Availability Statement
Data sharing is not applicable to this article because no new data were created or analysed in this study.
Conflict of Interest Statement
The authors declare no conflict of interest.
References
- Abbasi J (2018). Robert Kerns, PhD: Researching Nondrug Approaches to Pain Management. JAMA 319: 1535–1537. [DOI] [PubMed] [Google Scholar]
- Abdallah FW, Hussain N, Weaver T, Brull R (2020). Analgesic efficacy of cannabinoids for acute pain management after surgery: a systematic review and meta-analysis. Reg Anesth Pain Med 45: 509–519. [DOI] [PubMed] [Google Scholar]
- Akil H, Mayer DJ, Liebeskind JC (1976). Antagonism of stimulation-produced analgesia by naloxone, a narcotic antagonist. Science 191: 961–962. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Christopoulos A, Davenport AP, Kelly E, Mathie A, Peters JA, et al. (2019). THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: G protein-coupled receptors. Br J Pharmacol 176 Suppl 1: S21–S141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Molina-Holgado F (2019). Cannabinoids and their actions: An update. Br J Pharmacol 176: 1359–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsalem M, Altarifi A, Haddad M, Aldossary SA, Kalbouneh H, Aldaoud N, et al. (2019). Antinociceptive and Abuse Potential Effects of Cannabinoid/Opioid Combinations in a Chronic Pain Model in Rats. Brain Sci 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano K, Kitamura K, Kawamura H, Tanikawa T, Kawabatake H, Notani M, et al. (1980). Alterations of immunoreactive beta-endorphin in the third ventricular fluid in response to electrical stimulation of the human periaqueductal gray matter. Appl Neurophysiol 43: 150–158. [DOI] [PubMed] [Google Scholar]
- Babalonis S, Lofwall MR, Sloan PA, Nuzzo PA, Fanucchi LC, Walsh SL (2019). Cannabinoid modulation of opioid analgesia and subjective drug effects in healthy humans. Psychopharmacology (Berl) 236: 3341–3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagley EE, Chieng BC, Christie MJ, Connor M (2005). Opioid tolerance in periaqueductal gray neurons isolated from mice chronically treated with morphine. Br J Pharmacol 146: 68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagley EE, Ingram SL (2020). Endogenous opioid peptides in the descending pain modulatory circuit. Neuropharmacology 173: 108131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks GP, Winfree CJ (2019). Evolving Techniques and Indications in Peripheral Nerve Stimulation for Pain. Neurosurg Clin N Am 30: 265–273. [DOI] [PubMed] [Google Scholar]
- Bhashyam AR, Heng M, Harris MB, Vrahas MS, Weaver MJ (2018). Self-Reported Marijuana Use Is Associated with Increased Use of Prescription Opioids Following Traumatic Musculoskeletal Injury. J Bone Joint Surg Am 100: 2095–2102. [DOI] [PubMed] [Google Scholar]
- Boland EG, Bennett MI, Allgar V, Boland JW (2020). Cannabinoids for adult cancer-related pain: systematic review and meta-analysis. BMJ Support Palliat Care 10: 14–24. [DOI] [PubMed] [Google Scholar]
- Bouchet CA, Ingram SL (2020). Cannabinoids in the descending pain modulatory circuit: Role in inflammation. Pharmacol Ther 209: 107495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campion KN, Saville KA, Morgan MM (2016). Relative contribution of the dorsal raphe nucleus and ventrolateral periaqueductal gray to morphine antinociception and tolerance in the rat. Eur J Neurosci 44: 2667–2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon JT, Prieto GJ, Lee A, Liebeskind JC (1982). Evidence for opioid and non-opioid forms of stimulation-produced analgesia in the rat. Brain Res 243: 315–321. [DOI] [PubMed] [Google Scholar]
- Chen L, Tang J, White PF, Sloninsky A, Wender RH, Naruse R, et al. (1998). The effect of location of transcutaneous electrical nerve stimulation on postoperative opioid analgesic requirement: acupoint versus nonacupoint stimulation. Anesth Analg 87: 1129–1134. [PubMed] [Google Scholar]
- Chen X, Cowan A, Inan S, Geller EB, Meissler JJ, Rawls SM, et al. (2019). Opioid-sparing effects of cannabinoids on morphine analgesia: participation of CB1 and CB2 receptors. Br J Pharmacol 176: 3378–3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XH, Geller EB, Adler MW (1996). Electrical stimulation at traditional acupuncture sites in periphery produces brain opioid-receptor-mediated antinociception in rats. J Pharmacol Exp Ther 277: 654–660. [PubMed] [Google Scholar]
- Chen YH, Lee HJ, Lee MT, Wu YT, Lee YH, Hwang LL, et al. (2018). Median nerve stimulation induces analgesia via orexin-initiated endocannabinoid disinhibition in the periaqueductal gray. Proc Natl Acad Sci U S A 115: E10720-E10729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung JM, Fang ZR, Hori Y, Lee KH, Willis WD (1984a). Prolonged inhibition of primate spinothalamic tract cells by peripheral nerve stimulation. Pain 19: 259–275. [DOI] [PubMed] [Google Scholar]
- Chung JM, Lee KH, Hori Y, Endo K, Willis WD (1984b). Factors influencing peripheral nerve stimulation produced inhibition of primate spinothalamic tract cells. Pain 19: 277–293. [DOI] [PubMed] [Google Scholar]
- Cichewicz DL (2004). Synergistic interactions between cannabinoid and opioid analgesics. Life Sci 74: 1317–1324. [DOI] [PubMed] [Google Scholar]
- Clapper JR, Henry CL, Niphakis MJ, Knize AM, Coppola AR, Simon GM, et al. (2018). Monoacylglycerol Lipase Inhibition in Human and Rodent Systems Supports Clinical Evaluation of Endocannabinoid Modulators. J Pharmacol Exp Ther 367: 494–508. [DOI] [PubMed] [Google Scholar]
- Clement-Jones V, McLoughlin L, Tomlin S, Besser GM, Rees LH, Wen HL (1980). Increased beta-endorphin but not met-enkephalin levels in human cerebrospinal fluid after acupuncture for recurrent pain. Lancet 2: 946–949. [DOI] [PubMed] [Google Scholar]
- Cohen SP, Gilmore CA, Rauck RL, Lester DD, Trainer RJ, Phan T, et al. (2019). Percutaneous Peripheral Nerve Stimulation for the Treatment of Chronic Pain Following Amputation. Mil Med 184: e267–e274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ZD, Bedi G, Ramesh D, Balter R, Comer SD, Haney M (2018). Impact of co-administration of oxycodone and smoked cannabis on analgesia and abuse liability. Neuropsychopharmacology 43: 2046–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe MS, Wilson CD, Leishman E, Prather PL, Bradshaw HB, Banks ML, et al. (2017). The monoacylglycerol lipase inhibitor KML29 with gabapentin synergistically produces analgesia in mice. Br J Pharmacol 174: 4523–4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daigle TL, Kearn CS, Mackie K (2008). Rapid CB1 cannabinoid receptor desensitization defines the time course of ERK1/2 MAP kinase signaling. Neuropharmacology 54: 36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalal RS, Palchaudhuri S, Snider CK, Lewis JD, Mehta SJ, Lichtenstein GR (2020). Preadmission Cannabis Use Is Positively Correlated With Inpatient Opioid Dose Exposure in Hospitalized Patients With Inflammatory Bowel Diseases. Inflamm Bowel Dis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira HU, Dos Santos RS, Malta IHS, Pinho JP, Almeida AFS, Sorgi CA, et al. (2020). Investigation of the Involvement of the Endocannabinoid System in TENS-Induced Antinociception. J Pain 21: 820–835. [DOI] [PubMed] [Google Scholar]
- Deer TR, Levy RM, Rosenfeld EL (2010). Prospective clinical study of a new implantable peripheral nerve stimulation device to treat chronic pain. Clin J Pain 26: 359–372. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Lintzeris N, Campbell G, Bruno R, Cohen M, Farrell M, et al. (2015). Experience of adjunctive cannabis use for chronic non-cancer pain: findings from the Pain and Opioids IN Treatment (POINT) study. Drug Alcohol Depend 147: 144–150. [DOI] [PubMed] [Google Scholar]
- Deng H, Li W (2020). Monoacylglycerol lipase inhibitors: modulators for lipid metabolism in cancer malignancy, neurological and metabolic disorders. Acta Pharm Sin B 10: 582–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSantana JM, Da Silva LF, De Resende MA, Sluka KA (2009). Transcutaneous electrical nerve stimulation at both high and low frequencies activates ventrolateral periaqueductal grey to decrease mechanical hyperalgesia in arthritic rats. Neuroscience 163: 1233–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donvito G, Nass SR, Wilkerson JL, Curry ZA, Schurman LD, Kinsey SG, et al. (2018). The Endogenous Cannabinoid System: A Budding Source of Targets for Treating Inflammatory and Neuropathic Pain. Neuropsychopharmacology 43: 52–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew GM, Mitchell VA, Vaughan CW (2008). Glutamate spillover modulates GABAergic synaptic transmission in the rat midbrain periaqueductal grey via metabotropic glutamate receptors and endocannabinoid signaling. J Neurosci 28: 808–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn DP, Haroutounian S, Hohmann AG, Krane E, Soliman N, Rice ASC (2021). Cannabinoids, the endocannabinoid system, and pain: a review of preclinical studies. Pain 162: S5–S25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foreman RD, Applebaum AE, Beall JE, Trevino DL, Willis WD (1975). Responses of primate spinothalamic tract neurons to electrical stimulation of hindlimb peripheral nerves. J Neurophysiol 38: 132–145. [DOI] [PubMed] [Google Scholar]
- Fotio Y, Palese F, Guaman Tipan P, Ahmed F, Piomelli D (2020). Inhibition of fatty acid amide hydrolase in the CNS prevents and reverses morphine tolerance in male and female mice. Br J Pharmacol 177: 3024–3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu LW, Longhurst JC (2009). Electroacupuncture modulates vlPAG release of GABA through presynaptic cannabinoid CB1 receptors. J Appl Physiol (1985) 106: 1800–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel RA, Ilfeld BM (2021). Acute postoperative pain management with percutaneous peripheral nerve stimulation: the SPRINT neuromodulation system. Expert Rev Med Devices: 1–6. [DOI] [PubMed] [Google Scholar]
- Ghoname EA, White PF, Ahmed HE, Hamza MA, Craig WF, Noe CE (1999). Percutaneous electrical nerve stimulation: an alternative to TENS in the management of sciatica. Pain 83: 193–199. [DOI] [PubMed] [Google Scholar]
- Gozani SN (2019). Remote Analgesic Effects Of Conventional Transcutaneous Electrical Nerve Stimulation: A Scientific And Clinical Review With A Focus On Chronic Pain. J Pain Res 12: 3185–3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guindon J, Lai Y, Takacs SM, Bradshaw HB, Hohmann AG (2013). Alterations in endocannabinoid tone following chemotherapy-induced peripheral neuropathy: effects of endocannabinoid deactivation inhibitors targeting fatty-acid amide hydrolase and monoacylglycerol lipase in comparison to reference analgesics following cisplatin treatment. Pharmacol Res 67: 94–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagemeier NE (2018). Introduction to the opioid epidemic: the economic burden on the healthcare system and impact on quality of life. Am J Manag Care 24: S200–S206. [PubMed] [Google Scholar]
- Hamza MA, White PF, Ahmed HE, Ghoname EA (1999). Effect of the frequency of transcutaneous electrical nerve stimulation on the postoperative opioid analgesic requirement and recovery profile. Anesthesiology 91: 1232–1238. [DOI] [PubMed] [Google Scholar]
- Hasanein P, Ghafari-Vahed M (2016). Fatty acid amide hydrolase inhibitor URB597 prevented tolerance and cognitive deficits induced by chronic morphine administration in rats. Behav Pharmacol 27: 37–43. [DOI] [PubMed] [Google Scholar]
- Ho YC, Lee HJ, Tung LW, Liao YY, Fu SY, Teng SF, et al. (2011). Activation of orexin 1 receptors in the periaqueductal gray of male rats leads to antinociception via retrograde endocannabinoid (2-arachidonoylglycerol)-induced disinhibition. J Neurosci 31: 14600–14610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann AG, Suplita RL, Bolton NM, Neely MH, Fegley D, Mangieri R, et al. (2005). An endocannabinoid mechanism for stress-induced analgesia. Nature 435: 1108–1112. [DOI] [PubMed] [Google Scholar]
- Hosobuchi Y, Rossier J, Bloom FE, Guillemin R (1979). Stimulation of human periaqueductal gray for pain relief increases immunoreactive beta-endorphin in ventricular fluid. Science 203: 279–281. [DOI] [PubMed] [Google Scholar]
- Hossain MZ, Ando H, Unno S, Kitagawa J (2020). Targeting Peripherally Restricted Cannabinoid Receptor 1, Cannabinoid Receptor 2, and Endocannabinoid-Degrading Enzymes for the Treatment of Neuropathic Pain Including Neuropathic Orofacial Pain. Int J Mol Sci 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huggins JP, Smart TS, Langman S, Taylor L, Young T (2012). An efficient randomised, placebo-controlled clinical trial with the irreversible fatty acid amide hydrolase-1 inhibitor PF-04457845, which modulates endocannabinoids but fails to induce effective analgesia in patients with pain due to osteoarthritis of the knee. Pain 153: 1837–1846. [DOI] [PubMed] [Google Scholar]
- Ignatowska-Jankowska BM, Baillie GL, Kinsey S, Crowe M, Ghosh S, Owens RA, et al. (2015). A Cannabinoid CB1 Receptor-Positive Allosteric Modulator Reduces Neuropathic Pain in the Mouse with No Psychoactive Effects. Neuropsychopharmacology 40: 2948–2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilfeld BM, Ball ST, Gabriel RA, Sztain JF, Monahan AM, Abramson WB, et al. (2019). A Feasibility Study of Percutaneous Peripheral Nerve Stimulation for the Treatment of Postoperative Pain Following Total Knee Arthroplasty. Neuromodulation 22: 653–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer V, Slivicki RA, Thomaz AC, Crystal JD, Mackie K, Hohmann AG (2020). The cannabinoid CB2 receptor agonist LY2828360 synergizes with morphine to suppress neuropathic nociception and attenuates morphine reward and physical dependence. Eur J Pharmacol 886: 173544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S, Goebel A (2016). Long-Term Treatment of Chronic Neuropathic Pain Using External Noninvasive External Peripheral Nerve Stimulation in Five Patients. Neuromodulation 19: 893–896. [DOI] [PubMed] [Google Scholar]
- Joo Oh H, Ko YK, Cho SS, Yoon SP (2012). A cadaveric study of needle insertion at PC6 in eight wrists of four subjects and an understanding of the anatomy. Acupunct Med 30: 44–46. [DOI] [PubMed] [Google Scholar]
- Jorum E, Shyu BC (1988). Analgesia by low-frequency nerve stimulation mediated by low-threshold afferents in rats. Pain 32: 357–366. [DOI] [PubMed] [Google Scholar]
- Jung SJ, Kook MG, Kim S, Kang KS, Soh KS (2018). Homing of the Stem Cells from the Acupoint ST-36 to the Site of a Spinal Cord Injury: A Preliminary Study. J Acupunct Meridian Stud 11: 133–136. [DOI] [PubMed] [Google Scholar]
- Kalra A, Urban MO, Sluka KA (2001). Blockade of opioid receptors in rostral ventral medulla prevents antihyperalgesia produced by transcutaneous electrical nerve stimulation (TENS). J Pharmacol Exp Ther 298: 257–263. [PubMed] [Google Scholar]
- Kapural L, Gilmore CA, Chae J, Rauck RL, Cohen SP, Saulino MF, et al. (2018). Percutaneous Peripheral Nerve Stimulation for the Treatment of Chronic Low Back Pain: Two Clinical Case Reports of Sustained Pain Relief. Pain Pract 18: 94–103. [DOI] [PubMed] [Google Scholar]
- Kaur R, Sidhu P, Singh S (2016). What failed BIA 10–2474 Phase I clinical trial? Global speculations and recommendations for future Phase I trials. J Pharmacol Pharmacother 7: 120–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazantzis NP, Casey SL, Seow PW, Mitchell VA, Vaughan CW (2016). Opioid and cannabinoid synergy in a mouse neuropathic pain model. Br J Pharmacol 173: 2521–2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly S, Chapman V (2001). Selective cannabinoid CB1 receptor activation inhibits spinal nociceptive transmission in vivo. J Neurophysiol 86: 3061–3064. [DOI] [PubMed] [Google Scholar]
- Kelly S, Jhaveri MD, Sagar DR, Kendall DA, Chapman V (2003). Activation of peripheral cannabinoid CB1 receptors inhibits mechanically evoked responses of spinal neurons in noninflamed rats and rats with hindpaw inflammation. Eur J Neurosci 18: 2239–2243. [DOI] [PubMed] [Google Scholar]
- Kinsey SG, Long JZ, O’Neal ST, Abdullah RA, Poklis JL, Boger DL, et al. (2009). Blockade of endocannabinoid-degrading enzymes attenuates neuropathic pain. J Pharmacol Exp Ther 330: 902–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey SG, Wise LE, Ramesh D, Abdullah R, Selley DE, Cravatt BF, et al. (2013). Repeated low-dose administration of the monoacylglycerol lipase inhibitor JZL184 retains cannabinoid receptor type 1-mediated antinociceptive and gastroprotective effects. J Pharmacol Exp Ther 345: 492–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiso T, Watabiki T, Sekizawa T (2020). ASP8477, a fatty acid amide hydrolase inhibitor, exerts analgesic effects in rat models of neuropathic and dysfunctional pain. Eur J Pharmacol 881: 173194. [DOI] [PubMed] [Google Scholar]
- Koch T, Hollt V (2008). Role of receptor internalization in opioid tolerance and dependence. Pharmacol Ther 117: 199–206. [DOI] [PubMed] [Google Scholar]
- Kong X, Gozani SN (2018). Effectiveness of fixed-site high-frequency transcutaneous electrical nerve stimulation in chronic pain: a large-scale, observational study. J Pain Res 11: 703–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupers R, Laere KV, Calenbergh FV, Gybels J, Dupont P, Baeck A, et al. (2011). Multimodal therapeutic assessment of peripheral nerve stimulation in neuropathic pain: five case reports with a 20-year follow-up. Eur J Pain 15: 161 e161–169. [DOI] [PubMed] [Google Scholar]
- Lan F, Ma YH, Xue JX, Wang TL, Ma DQ (2012). Transcutaneous electrical nerve stimulation on acupoints reduces fentanyl requirement for postoperative pain relief after total hip arthroplasty in elderly patients. Minerva Anestesiol 78: 887–895. [PubMed] [Google Scholar]
- Lau BK, Drew GM, Mitchell VA, Vaughan CW (2014). Endocannabinoid modulation by FAAH and monoacylglycerol lipase within the analgesic circuitry of the periaqueductal grey. Br J Pharmacol 171: 5225–5236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau BK, Vaughan CW (2014). Descending modulation of pain: the GABA disinhibition hypothesis of analgesia. Curr Opin Neurobiol 29: 159–164. [DOI] [PubMed] [Google Scholar]
- Le Foll B (2021). Opioid-sparing effects of cannabinoids: Myth or reality? Prog Neuropsychopharmacol Biol Psychiatry 106: 110065. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Chang LY, Ho YC, Teng SF, Hwang LL, Mackie K, et al. (2016). Stress induces analgesia via orexin 1 receptor-initiated endocannabinoid/CB1 signaling in the mouse periaqueductal gray. Neuropharmacology 105: 577–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KH, Chung JM, Willis WD Jr. (1985). Inhibition of primate spinothalamic tract cells by TENS. J Neurosurg 62: 276–287. [DOI] [PubMed] [Google Scholar]
- Lee MT, Chen YH, Mackie K, Chiou LC (2021). Median Nerve Stimulation as a Nonpharmacological Approach to Bypass Analgesic Tolerance to Morphine: A Proof-of-Concept Study in Mice. J Pain 22: 300–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MT, Chiu YT, Chiu YC, Hor CC, Lee HJ, Guerrini R, et al. (2020). Neuropeptide S-initiated sequential cascade mediated by OX1, NK1, mGlu5 and CB1 receptors: a pivotal role in stress-induced analgesia. J Biomed Sci 27: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard G, Cloutier C, Marchand S (2011). Reduced analgesic effect of acupuncture-like TENS but not conventional TENS in opioid-treated patients. J Pain 12: 213–221. [DOI] [PubMed] [Google Scholar]
- Li SB, de Lecea L (2020). The hypocretin (orexin) system: from a neural circuitry perspective. Neuropharmacology 167: 107993. [DOI] [PubMed] [Google Scholar]
- Liang Y, Bao G, Gong L, Zhou J, Kong X, Ran R, et al. (2019). Evaluating the analgesic effect and advantage of transcutaneous electrical acupoint stimulation combined with opioid drugs for moderate to severe cancer-related pain: a study protocol for a randomized controlled trial. Trials 20: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman AH, Blankman JL, Cravatt BF (2010). Endocannabinoid overload. Mol Pharmacol 78: 993–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman AH, Lux EA, McQuade R, Rossetti S, Sanchez R, Sun W, et al. (2018). Results of a Double-Blind, Randomized, Placebo-Controlled Study of Nabiximols Oromucosal Spray as an Adjunctive Therapy in Advanced Cancer Patients with Chronic Uncontrolled Pain. J Pain Symptom Manage 55: 179–188 e171. [DOI] [PubMed] [Google Scholar]
- Liu CW, Bhatia A, Buzon-Tan A, Walker S, Ilangomaran D, Kara J, et al. (2019). Weeding Out the Problem: The Impact of Preoperative Cannabinoid Use on Pain in the Perioperative Period. Anesth Analg 129: 874–881. [DOI] [PubMed] [Google Scholar]
- Mayer DJ, Hayes RL (1975). Stimulation-produced analgesia: development of tolerance and cross-tolerance to morphine. Science 188: 941–943. [DOI] [PubMed] [Google Scholar]
- McKinney DL, Cassidy MP, Collier LM, Martin BR, Wiley JL, Selley DE, et al. (2008). Dose-related differences in the regional pattern of cannabinoid receptor adaptation and in vivo tolerance development to delta9-tetrahydrocannabinol. J Pharmacol Exp Ther 324: 664–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzack R, Wall PD (1965). Pain mechanisms: a new theory. Science 150: 971–979. [DOI] [PubMed] [Google Scholar]
- Mohammadkhani A, Borgland SL (2020). Cellular and behavioral basis of cannabinioid and opioid interactions: Implications for opioid dependence and withdrawal. J Neurosci Res. [DOI] [PubMed] [Google Scholar]
- Morgan MM, Clayton CC, Boyer-Quick JS (2005). Differential susceptibility of the PAG and RVM to tolerance to the antinociceptive effect of morphine in the rat. Pain 113: 91–98. [DOI] [PubMed] [Google Scholar]
- Morozova AS, Zvartau EE (1986). Stimulation-produced analgesia under repeated morphine treatment in rats. Pharmacol Biochem Behav 25: 533–536. [DOI] [PubMed] [Google Scholar]
- Narouze S (2020). Antinociception mechanisms of action of cannabinoid-based medicine: an overview for anesthesiologists and pain physicians. Reg Anesth Pain Med. [DOI] [PubMed] [Google Scholar]
- Neuman MD, Bateman BT, Wunsch H (2019). Inappropriate opioid prescription after surgery. Lancet 393: 1547–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen PT, Schmid CL, Raehal KM, Selley DE, Bohn LM, Sim-Selley LJ (2012). beta-arrestin2 regulates cannabinoid CB1 receptor signaling and adaptation in a central nervous system region-dependent manner. Biol Psychiatry 71: 714–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen S, Sabioni P, Trigo JM, Ware MA, Betz-Stablein BD, Murnion B, et al. (2017). Opioid-Sparing Effect of Cannabinoids: A Systematic Review and Meta-Analysis. Neuropsychopharmacology 42: 1752–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilges MR, Bondy ZB, Grace JA, Winsauer PJ (2019). Opioid-enhancing antinociceptive effects of delta-9-tetrahydrocannabinol and amitriptyline in rhesus macaques. Exp Clin Psychopharmacol. Nockemann D, Rouault M, Labuz D, Hublitz P, McKnelly K, Reis FC, et al. (2013). The K(+) channel GIRK2 is both necessary and sufficient for peripheral opioid-mediated analgesia. EMBO Mol Med 5: 1263–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris C, Szkudlarek HJ, Pereira B, Rushlow W, Laviolette SR (2019). The Bivalent Rewarding and Aversive properties of Delta(9)-tetrahydrocannabinol are Mediated Through Dissociable Opioid Receptor Substrates and Neuronal Modulation Mechanisms in Distinct Striatal Sub-Regions. Sci Rep 9: 9760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OECD (2019). Addressing Problematic Opioid Use in OECD Countries. edn. [Google Scholar]
- Okine BN, Norris LM, Woodhams S, Burston J, Patel A, Alexander SP, et al. (2012). Lack of effect of chronic pre-treatment with the FAAH inhibitor URB597 on inflammatory pain behaviour: evidence for plastic changes in the endocannabinoid system. Br J Pharmacol 167: 627–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okusanya BO, Asaolu IO, Ehiri JE, Kimaru LJ, Okechukwu A, Rosales C (2020). Medical cannabis for the reduction of opioid dosage in the treatment of non-cancer chronic pain: a systematic review. Syst Rev 9: 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray WA, Chung CP, Murray KT, Hall K, Stein CM (2016). Prescription of Long-Acting Opioids and Mortality in Patients With Chronic Noncancer Pain. JAMA 315: 2415–2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosoff DB, Smith GD, Lohoff FW (2021). Prescription Opioid Use and Risk for Major Depressive Disorder and Anxiety and Stress-Related Disorders: A Multivariable Mendelian Randomization Analysis. JAMA Psychiatry 78: 151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein M, Mogil JS, Japon M, Chan EC, Allen RG, Low MJ (1996). Absence of opioid stress-induced analgesia in mice lacking beta-endorphin by site-directed mutagenesis. Proc Natl Acad Sci U S A 93: 3995–4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salar G, Job I, Mingrino S, Bosio A, Trabucchi M (1981). Effect of transcutaneous electrotherapy on CSF beta-endorphin content in patients without pain problems. Pain 10: 169–172. [DOI] [PubMed] [Google Scholar]
- Sarne Y, Asaf F, Fishbein M, Gafni M, Keren O (2011). The dual neuroprotective-neurotoxic profile of cannabinoid drugs. Br J Pharmacol 163: 1391–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosburg JE, Blankman JL, Long JZ, Nomura DK, Pan B, Kinsey SG, et al. (2010). Chronic monoacylglycerol lipase blockade causes functional antagonism of the endocannabinoid system. Nat Neurosci 13: 1113–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurman LD, Lu D, Kendall DA, Howlett AC, Lichtman AH (2020). Molecular Mechanism and Cannabinoid Pharmacology. Handb Exp Pharmacol 258: 323–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao XM, Shen Z, Sun J, Fang F, Fang JF, Wu YY, et al. (2015). Strong Manual Acupuncture Stimulation of “Huantiao” (GB 30) Reduces Pain-Induced Anxiety and p-ERK in the Anterior Cingulate Cortex in a Rat Model of Neuropathic Pain. Evid Based Complement Alternat Med 2015: 235491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skolnick P (2018). The Opioid Epidemic: Crisis and Solutions. Annu Rev Pharmacol Toxicol 58: 143–159. [DOI] [PubMed] [Google Scholar]
- Slavin KV, Carayannopoulos AG, Plazier M, Vanneste S, De Ridder D (2015). Peripheral Nerve Stimulation. In: Knotkova H, Rasche D (ed)^(eds). Textbook of Neuromodulation: Principles, Methods and Clinical Applications, edn. New York, NY: Springer New York. p^pp 19–33. [Google Scholar]
- Slivicki RA, Iyer V, Mali SS, Garai S, Thakur GA, Crystal JD, et al. (2020). Positive Allosteric Modulation of CB1 Cannabinoid Receptor Signaling Enhances Morphine Antinociception and Attenuates Morphine Tolerance Without Enhancing Morphine- Induced Dependence or Reward. Front Mol Neurosci 13: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slivicki RA, Saberi SA, Iyer V, Vemuri VK, Makriyannis A, Hohmann AG (2018a). Brain-Permeant and -Impermeant Inhibitors of Fatty Acid Amide Hydrolase Synergize with the Opioid Analgesic Morphine to Suppress Chemotherapy-Induced Neuropathic Nociception Without Enhancing Effects of Morphine on Gastrointestinal Transit. J Pharmacol Exp Ther 367: 551–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slivicki RA, Xu Z, Kulkarni PM, Pertwee RG, Mackie K, Thakur GA, et al. (2018b). Positive Allosteric Modulation of Cannabinoid Receptor Type 1 Suppresses Pathological Pain Without Producing Tolerance or Dependence. Biol Psychiatry 84: 722–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slivicki RA, Xu Z, Mali SS, Hohmann AG (2019). Brain permeant and impermeant inhibitors of fattyacid amide hydrolase suppress the development and maintenance of paclitaxel-induced neuropathic pain without producing tolerance or physical dependence in vivo and synergize with paclitaxel to reduce tumor cell line viability in vitro. Pharmacol Res 142: 267–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluka KA, Deacon M, Stibal A, Strissel S, Terpstra A (1999). Spinal blockade of opioid receptors prevents the analgesia produced by TENS in arthritic rats. J Pharmacol Exp Ther 289: 840–846. [PubMed] [Google Scholar]
- Smith PA, Selley DE, Sim-Selley LJ, Welch SP (2007). Low dose combination of morphine and delta9-tetrahydrocannabinol circumvents antinociceptive tolerance and apparent desensitization of receptors. Eur J Pharmacol 571: 129–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobczak L, Gorynski K (2020). Pharmacological Aspects of Over-the-Counter Opioid Drugs Misuse. Molecules 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein C (2016). Opioid Receptors. Annu Rev Med 67: 433–451. [DOI] [PubMed] [Google Scholar]
- Stockings E, Campbell G, Hall WD, Nielsen S, Zagic D, Rahman R, et al. (2018). Cannabis and cannabinoids for the treatment of people with chronic noncancer pain conditions: a systematic review and meta-analysis of controlled and observational studies. Pain 159: 1932–1954. [DOI] [PubMed] [Google Scholar]
- Strege DW, Cooney WP, Wood MB, Johnson SJ, Metcalf BJ (1994). Chronic peripheral nerve pain treated with direct electrical nerve stimulation. J Hand Surg Am 19: 931–939. [DOI] [PubMed] [Google Scholar]
- Suplita RL 2nd, Eisenstein SA, Neely MH, Moise AM, Hohmann AG (2008). Cross-sensitization and cross-tolerance between exogenous cannabinoid antinociception and endocannabinoid-mediated stress-induced analgesia. Neuropharmacology 54: 161–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szmit M, Agrawal S, Gozdzik W, Kubler A, Agrawal A, Pruchnicki P, et al. (2021). Transcutaneous Electrical Acupoint Stimulation Reduces Postoperative Analgesic Requirement in Patients Undergoing Inguinal Hernia Repair: A Randomized, Placebo-Controlled Study. J Clin Med 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakuwa KM, Sulak D (2020). A Survey on the Effect That Medical Cannabis Has on Prescription Opioid Medication Usage for the Treatment of Chronic Pain at Three Medical Cannabis Practice Sites. Cureus 12: e11848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teoli D, An J (2021). Transcutaneous Electrical Nerve Stimulation. In: (ed)^(eds). StatPearls, edn. Treasure Island (FL). p^pp. [PubMed] [Google Scholar]
- Terman GW, Lewis JW, Liebeskind JC (1986). Two opioid forms of stress analgesia: studies of tolerance and cross-tolerance. Brain Res 368: 101–106. [DOI] [PubMed] [Google Scholar]
- Terman GW, Penner ER, Liebeskind JC (1985). Stimulation-produced and stress-induced analgesia: cross-tolerance between opioid forms. Brain Res 360: 374–378. [DOI] [PubMed] [Google Scholar]
- Umemoto K, Naito M, Tano K, Terayama H, Koike T, Ohmichi M, et al. (2019). Acupuncture Point “Hegu” (LI4) Is Close to the Vascular Branch from the Superficial Branch of the Radial Nerve. Evid Based Complement Alternat Med 2019: 6879076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unterrainer AF, Friedrich C, Krenn MH, Piotrowski WP, Golaszewski SM, Hitzl W (2010). Postoperative and preincisional electrical nerve stimulation TENS reduce postoperative opioid requirement after major spinal surgery. J Neurosurg Anesthesiol 22: 1–5. [DOI] [PubMed] [Google Scholar]
- Urits I, Li N, Berardino K, Artounian KA, Bandi P, Jung JW, et al. (2020). The use of antineuropathic medications for the treatment of chronic pain. Best Pract Res Clin Anaesthesiol 34: 493–506. [DOI] [PubMed] [Google Scholar]
- US-CDC (2020). Drug Overdose Deaths. [Google Scholar]
- Vigano D, Rubino T, Vaccani A, Bianchessi S, Marmorato P, Castiglioni C, et al. (2005). Molecular mechanisms involved in the asymmetric interaction between cannabinoid and opioid systems. Psychopharmacology (Berl) 182: 527–536. [DOI] [PubMed] [Google Scholar]
- Walker JB, Katz RL (1981a). Non-opioid pathways suppress pain in humans. Pain 11: 347–354. [DOI] [PubMed] [Google Scholar]
- Walker JB, Katz RL (1981b). Peripheral nerve stimulation in the management of dysmenorrhea. Pain 11: 355–361. [DOI] [PubMed] [Google Scholar]
- Wall PD, Sweet WH (1967). Temporary abolition of pain in man. Science 155: 108–109. [DOI] [PubMed] [Google Scholar]
- Wang B, Tang J, White PF, Naruse R, Sloninsky A, Kariger R, et al. (1997). Effect of the intensity of transcutaneous acupoint electrical stimulation on the postoperative analgesic requirement. Anesth Analg 85: 406–413. [DOI] [PubMed] [Google Scholar]
- Welch SP (1997). Characterization of anandamide-induced tolerance: comparison to delta 9-THCinduced interactions with dynorphinergic systems. Drug Alcohol Depend 45: 39–45. [DOI] [PubMed] [Google Scholar]
- Wilkerson JL, Ghosh S, Mustafa M, Abdullah RA, Niphakis MJ, Cabrera R, et al. (2017). The endocannabinoid hydrolysis inhibitor SA-57: Intrinsic antinociceptive effects, augmented morphine-induced antinociception, and attenuated heroin seeking behavior in mice. Neuropharmacology 114: 156–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkerson JL, Niphakis MJ, Grim TW, Mustafa MA, Abdullah RA, Poklis JL, et al. (2016). The Selective Monoacylglycerol Lipase Inhibitor MJN110 Produces Opioid-Sparing Effects in a Mouse Neuropathic Pain Model. J Pharmacol Exp Ther 357: 145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JT, Ingram SL, Henderson G, Chavkin C, von Zastrow M, Schulz S, et al. (2013). Regulation of mu-opioid receptors: desensitization, phosphorylation, internalization, and tolerance. Pharmacol Rev 65: 223–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson-Poe AR, Morgan MM, Aicher SA, Hegarty DM (2012). Distribution of CB1 cannabinoid receptors and their relationship with mu-opioid receptors in the rat periaqueductal gray. Neuroscience 213: 191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson-Poe AR, Pocius E, Herschbach M, Morgan MM (2013). The periaqueductal gray contributes to bidirectional enhancement of antinociception between morphine and cannabinoids. Pharmacol Biochem Behav 103: 444–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson AR, Maher L, Morgan MM (2008). Repeated cannabinoid injections into the rat periaqueductal gray enhance subsequent morphine antinociception. Neuropharmacology 55: 1219–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaksh TL, Hunt MA, Dos Santos GG (2018). Development of New Analgesics: An Answer to Opioid Epidemic. Trends Pharmacol Sci 39: 1000–1002. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Yeung JC, Rudy TA (1976). An inability to antagonize with naloxone the elevated nociceptive thresholds resulting from electrical stimulation of the mesencephalic central gray. Life Sci 18: 1193–1198. [DOI] [PubMed] [Google Scholar]
- Yanes JA, McKinnell ZE, Reid MA, Busler JN, Michel JS, Pangelinan MM, et al. (2019). Effects of cannabinoid administration for pain: A meta-analysis and meta-regression. Exp Clin Psychopharmacol 27: 370–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RF, Chambi VI (1987). Pain relief by electrical stimulation of the periaqueductal and periventricular gray matter. Evidence for a non-opioid mechanism. J Neurosurg 66: 364–371. [DOI] [PubMed] [Google Scholar]
- Zhu H, Xiang HC, Li HP, Lin LX, Hu XF, Zhang H, et al. (2019). Inhibition of GABAergic Neurons and Excitation of Glutamatergic Neurons in the Ventrolateral Periaqueductal Gray Participate in Electroacupuncture Analgesia Mediated by Cannabinoid Receptor. Front Neurosci 13: 484. [DOI] [PMC free article] [PubMed] [Google Scholar]