25-28 June 2022
Athens, Greece
The Abstracts
ORAL ABSTRACTS
S1a: Pharmacogenomics in clinical practice
11
IMPACT OF THE GENOTYPE AND PHENOTYPE OF CYP3A AND P-GP ON THE APIXABAN AND RIVAROXABAN BLOOD CONCENTRATIONS IN REAL-WORLD SETTING
Lenoir C1, Terrier J2, Gloor Y1, Daali Y1, Gosselin P2, Niederer A1, Doffey Lazeyras F1, Desmeules J1, Samer C1, Reny J2, Rollason V1
1 Division of Clinical Pharmacology and Toxicology, Department of Anaesthesiology, Pharmacology, Intensive Care and Emergency Medicine, Geneva University Hospitals, Geneva, Switzerland
2 Division of General Medicine, Department of Medicine, Geneva University Hospitals, Geneva, Switerzland
Introduction: Apixaban and rivaroxaban, two oral direct factor Xa inhibitors (DOACs), have become an alternative to vitamin K antagonists because of their ease of use. However, safety and efficacy concerns have emerged with the availability of real-world data, particularly regarding their concomitant use with other drugs which may cause bleeding or with CYP3A and/or P-gp modulators. While age, weight and renal function are known factors for dose adjustment, CYP3A/P-gp genotypic and phenotypic activities are not routinely considered although they are involved in their metabolism and transport.
Objectives: We aimed to determine whether CYP3A/P-gp genotypes and phenotypic activities could have a significant impact on apixaban and rivaroxaban blood exposure.
Methods: This real-life observational study included 164 and 136 hospitalized patients treated with apixaban or rivaroxaban, respectively. CYP3A and P-gp phenotypic activities were assessed with the Geneva cocktail administered orally on the day of the study. Capillary blood samples were collected at t0 and 2h, 3h and 6h after cocktail administration to assess OH-midazolam/midazolam (CYP3A) metabolic ratios (MR) and fexofenadine (P-gp) AUC, and to assess apixaban and rivaroxaban blood concentrations. CYP3A and P-gp genetic polymorphisms were also measured.
Results: Substantial interindividual variability was observed in dose-normalized blood concentrations and AUC of apixaban (117.4±55.9 ng/mL) and rivaroxaban (once a day: 21.3±12.4 ng/mL and twice a day: 28.3±8.9 ng/mL), as well as CYP3A (0.45±0.42 for apixaban cohort and 0.44±0.44 for rivaroxaban cohort) and P-gp (222.8±219.4 for apixaban cohort and 200.1±150 for rivaroxaban cohort) phenotypic activities. A linear mixed model for apixaban and rivaroxaban AUC predicted 40% and 18% of the variability, respectively. The covariables were the weight, age, gender, GFR (Cockcroft), ALAT level, dose, log10(MRmidazolam) and log10(AUCfexofenadine). Log10(AUCfexofenadine) reflecting P-gp activity (169.76 (79.4 to 260.12); p=0.0003) and GFR (Cockcroft) (-2.10 (-3.74 to -0.45); p=0.0127) were variables that significantly affected apixaban AUC. AUCfexofenadine per log10 (232.51 (105.69 to 359.33); p=0.0004) also had a significant impact on rivaroxaban AUC. CYP3A phenotype and SNPs tested had no significant impact on the pharmacokinetics of both molecules of both molecules.
Conclusion: Our study demonstrated the significant impact of P-gp activity on apixaban and rivaroxaban blood concentrations, which could have a clinically relevant impact on drug response. P-gp activity could therefore be considered a relevant factor for DOACs’ dose adjustment in the future.
196
Added value of DPYD whole exon sequencing to explain severe fluoropyrimidine-induced toxicity
Zhai Q1, van der Lee M1, Lunenburg C2, Henricks L3, Böhringer S1,4, de Man F5,6, Offer S7, Baars A8, Creemers G9, Dezentjé V6,10, Droogendijk H11, Hamberg P12, Imholz A13, Jansen R14, Jeurissen F15, Koopman M16, Mandigers C17, Nieboer P18, van de Poel M19, Portielje J2,20, van Schaik R21, Gelderblom H2, Mathijssen R6, Schellens J22, Cats A23, Guchelaar H1, Swen J1
1 Department of Clinical Pharmacy and Toxicology, Leiden University Medical Center, Leiden, The Netherlands
2 Department of Medical Oncology, Leiden University Medical Center, Leiden, The Netherlands
3 Department of Clinical Chemistry and Laboratory Medicine, Leiden, The Netherlands
4 Department of Biomedical Data Sciences, Leiden University Medical Center, Leiden, The Netherlands
5 Department of Medical Oncology, Erasmus MC Cancer Institute, Erasmus University Medical Center, Rotterdam, The Netherlands
6 Department of Internal Medicine, Reinier de Graaf Hospital, Delft, The Netherlands
7 Department of Molecular Pharmacology and Experimental Therapeutics, Mayo Clinic, Rochester, MN, USA
8 Department of Internal Medicine, Hospital Gelderse Vallei, Ede, The Netherlands
9 Department of Medical Oncology, Catharina Hospital, Eindhoven, The Netherlands
10 Division of Medical Oncology, The Netherlands Cancer Institute, Amsterdam, The Netherlands
11 11Department of Internal Medicine, Bravis Hospital, Roosendaal, The Netherlands
12 Department of Internal Medicine, Franciscus Gasthuis en Vlietland, Rotterdam, The Netherlands
13 Department of Internal Medicine, Deventer Hospital, Deventer, The Netherlands
14 Department of Internal Medicine, Maastricht University Medical Center, Maastricht, The Netherlands
15 Department of Internal Medicine, Haaglanden Medical Center, The Hague, The Netherlands
16 Department of Medical Oncology, University Medical Center Utrecht, Utrecht, The Netherlands
17 Department of Internal Medicine, Canisius-Wilhelmina Hospital, Nijmegen, The Netherlands
18 Department of Internal Medicine, Wilhelmina Hospital, Assen, The Netherlands
19 Department of Internal Medicine, Laurentius Hospital, Roermond, The Netherlands
20 Department of Medical Oncology, Haga Hospital, The Hague, The Netherlands
21 Department of Clinical Chemistry, Erasmus University Medical Center, Rotterdam, The Netherlands
22 Department of Pharmaceutical Sciences, Utrecht University, Utrecht, The Netherlands
23 Department of Gastroenterology and Hepatology, Division of Medical Oncology, The Netherlands Cancer Institute, Amsterdam, The Netherlands
Introduction: Fluoropyrimidines are commonly used in the treatment of cancer. Up to 30% of patients treated with fluoropyrimidines experience severe toxicity (≥3 grade), primarily caused by a deficiency in dihydropyrimidine dehydrogenase (DPD). Prospective genotyping for four genetic variants (c.1905+1G>A, c.1679T>G, c.1236G>A, and c.2846A>T) in the gene encoding for DPD (DPYD) followed by individual dose reductions have been proven to reduce fluoropyrimidines-related severe toxicity. However, substantial fluoropyrimidines-induced toxicity remains, which might be attributed to rare deleterious variants in DPYD.
Objectives: In this retrospective analysis of a large prospective study (clinicaltrial.gov identifier NCT02324452), we tested the hypothesis that rare variants in DPYD contribute to the occurrence of severe fluoropyrimidine-induced toxicity.
Methods: Exon sequencing (including 20bp flanking) of DPYD was performed for 1,103 patients treated with fluoropyrimidines who participated in the Alpe DPD study(1). Carriers of one of the four clinical DPYD variant alleles (n=85) who received dose reductions were excluded from the analyses. The potential impact of all non-synonymous DPYD variants was assessed with two in silico tools, DPYD-Varifier and MMsplice, and an in vitro expression system in HEK293T/c17 cells(2). Variants were considered deleterious if predicted so by at least one tool. For toxicity analysis, a matched-pair analysis was performed in which, for each deleterious variant carrier, three matched DPYD wild-type patients were identified based on three clinical criteria, including treatment regimens, tumor type, and disease stage.
Results: In the 1,018 patients included in the primary analysis, 24 non-synonymous genetic variants in DPYD were found. Of these, a total of 7 variants was defined as deleterious. Five variants (c.1670C>T, c.1913T>C, c.1925T>C, c.506delC, c.731A>C), were identified as deleterious by at least the in vitro assay or the DPYD-Varifier. Another two variants (c.1740+1G>T and c.763-2A>G) were predicted deleterious by MMsplice. In total, ten patients carried one of these seven predicted deleterious variants, of whom three experienced severe toxicity such as gastrointestinal toxicity. These ten patients showed a 2.14-fold (95%CI: 0.408-11.255, p= 0.388) increased risk of severe toxicity compared to matched wild-type controls.
Conclusion: Rare deleterious variants detected by exon sequencing in the DPYD gene might lead to an increased risk of severe fluoropyrimidine-induced toxicity. Whole exon sequencing detected an additional 1% deleterious allele variants in the DPYD gene. The impact of these allele variants in daily clinical practice is unsure yet, as the number of patients in this study was too small to draw definitive conclusions.
(1) Henricks, L.M. et al. DPYD genotype-guided dose individualisation of fluoropyrimidine therapy in patients with cancer: a prospective safety analysis. Lancet Oncol 19, 1459-67 (2018).
(2) Offer, S.M., Fossum, C.C., Wegner, N.J., Stuflesser, A.J., Butterfield, G.L. & Diasio, R.B. Comparative functional analysis of DPYD variants of potential clinical relevance to dihydropyrimidine dehydrogenase activity. Cancer Res 74, 2545-54 (2014).
208
Direct oral anticoagulant-related bleeding in atrial fibrillation patients alters DNA methylation of NOS3 and KDR genes
Ragia G1,2, Thomopoulos T3, Atzemian N1,2, Tsaliki V4, Chalikias G4, Kolios G1,2, Tziakas D4, Trikas A3, Manolopoulos V1,2,5
1 Laboratory of Pharmacology, Medical School, Democritus University of Thrace, Alexandroupolis, Greece
2 Individualised Medicine & Pharmacological Research Solutions Center (IMPReS), Alexandroupolis, Greece
3 Department of Cardiology, "Elpis" General Hospital of Athens, Athens, Greece
4 Cardiology Department, Medical School, Democritus University of Thrace, Alexandroupolis, Greece
5 Clinical Pharmacology Unit, Academic General Hospital of Alexandroupolis, Alexandroupolis, Greece
Introduction: Direct Oral Anticoagulants (DOACs) are now standard therapy for patients with atrial fibrillation (AF). Despite their effectiveness, several patients experience bleeding events. No studies have addressed how epigenetic modifications may affect DOAC treatment. To fill this gap, we are currently conducting a prospective clinical trial in newly AF diagnosed patients eligible for DOAC treatment.
Objectives: The objective of our study is to follow in time changes of DNA methylation pattern and microRNA expression in naïve AF patients starting DOAC therapy. Herein, we present preliminary results of DNA methylation analyses of two genes associated with endothelium healing, endothelial nitric oxide synthase (NOS3) and VEGF-A receptor 2 (KDR).
Methods: This is an ongoing study. So far, we have enrolled 44 AF patients treated with dabigatran, rivaroxaban or apixaban and 18 non-AF controls. Genomic DNA has been isolated at baseline (t0, controls and patients prior to DOAC initiation), and, for patients, at t0 plus at 7 (t1) and 28 (t2) days of DOAC treatment. Whole blood genomic DNA was isolated by use of MagCore® system and was bisulfite converted prior to methylation analyses. Promoter DNA methylation of NOS3 (CRE and PRDI/II elements) and KDR genes was analyzed with qMSP-PCR.
Results: In our study, no major bleeding or thrombotic events were recorded. A total of 15 minor bleeding events occurred. In regression analysis adjusted for DOAC dose, gender, age, co-morbidities and bleeding-inducing drug interactions, hypertension increased risk for bleeding (OR 28.587, 95% C.I. 1.954-418.117, p=0.014). In both patients and controls, NOS3 CRE and PRDI/II elements were heavily methylated, while KDR promoter was lightly methylated. Percentage of DNA methylation of NOS3 and KDR at baseline did not differ between AF patients and non-AF controls. In patient cohort, DOAC therapy did not alter NOS3 or KDR methylation at different timepoints. When patients were categorized into experiencing bleeding events (cases) or not (controls), in cases NOS3 CRE was demethylated from t0 to t2 (-16.36% in cases vs. 1.32% in controls, p=0.017) and from t1 to t2 (-21.44% in cases vs. 4.67% in controls, p=0.045). No differences were found for NOS3 PRDI/II and KDR methylation. In addition, we observed decreased KDR methylation (by 0.71%, p=0.003) in cases compared to non-bleeding patients at 7 days of therapy.
Conclusion: This is the first time that the effect of DOACs on DNA methylation is studied. DOAC-related bleeding leads to NOS3 CRE demethylation increasing thus NOS3 expression. This finding highlights NOS3 implication in endothelium healing and is in line with the critical role of NO in endothelium homeostasis. Hypomethylation of KDR in bleeding patients at 7 days of therapy, a critical timepoint for the occurrence of DOAC bleedings, supports KDR activation by VEGF-A towards regulation of angiogenesis and vascular permeability.
Funding: Financial support for project IMPReS (MIS 5047189) was provided by the Program “Competitiveness, Entrepreneurship and Innovation” (NSRF 2014–2020) co-financed by Greece and the European Union (European Regional Development Fund).
S1b: Immunopharmacology
233
Impact of Obesity on Dexamethasone Pharmacokinetic in COVID-19 hospitalized patients: an observational exploratory study
Abouir K1,2, Gosselin P3, Guerrier S2,4, Daali Y1,2,5, Desmeules J1,2,5, Grosgurin O3, Reny J3, Samer C1,5, Calmy A6, Lorenzini K1
1 Division of Clinical Pharmacology and Toxicology, Geneva University Hospitals, Geneva, Switzerland
2 Institute of Pharmaceutical Sciences of Western Switzerland, University of Geneva, Geneva, Switzerland
3 Department and Division of Primary Care Medicine, University Hospital of Geneva, Geneva, Switzerland
4 Geneva School of Economics and Management, University of Geneva, Geneva, Switzerland
5 Swiss Centre for Applied Human Toxicology (SCAHT), Geneva, Switzerland
6 Division of Infectious Diseases, Geneva University Hospitals, Geneva, Switzerland
Introduction: In the context of the last pandemic, the scientific community leveraged the already marketed drugs’ safety profile to manage patients with SARS-CoV2 infection, or COVID-19. Dexamethasone (DEX) has been shown to improve the survival of moderately or severely ill patients. [1] Therefore systemic corticosteroids are recommended by the World Health Organization (WHO) and the National Institutes of Health (NIH) for the treatment of patients with severe or critical COVID-19.[2,3] Studies showed a high frequency of obesity among patients admitted to intensive care for SARS‐CoV‐2 infection, underlining that it is indeed a risk factor for developing severe forms of COVID-19.
Objectives: Little information is available in the literature regarding DEX dose adjustment based on BMI or body weight. Accordingly, we conducted an exploratory study to assess the impact of obesity on the pharmacokinetics (PK) of DEX in COVID-19 hospitalized patients.
Methods: Two groups of patients were enrolled: one group with a BMI between 18.5 and 25 kg/m² (normal-weight) and the second ≥ 30 kg/m² (obese). The 30 patients of the study were all hospitalized at the Geneva University Hospitals (Switzerland) with a diagnosis of moderate to severe COVID-19 requiring oxygen and received the standard of care therapy of daily 6 mg oral DEX. Capillary blood samples were collected before DEX administration and after to assess DEX PK profile.
Results: The mean DEX AUC0-inf and Cmax were lower in the obese compared to the normal weight group (572 ± 258 vs. 926 ± 552 ng. h/ml, and 138 ± 68 vs. 203 ± 126 ng/ml, respectively). We observed a decrease in DEX AUC0-inf of 4% per additional BMI unit and defined a significant relationship between weight and DEX AUC0-inf (P-value 0.004, 95% CI: 2% - 7%). We also observed a statistically significant impact of gender. In women, DEX AUC0-inf increased by 214% as compared to men (P-value <0.001, 95% CI: 154% - 298%). Similarly, the mean Cmax increased by 205% in women (P-value <0.001, 95% CI: 141%-297%). On the other hand, exploratory treatment outcomes, such as the length of hospitalization, did not show any significant difference between obese and normal-weight groups.
Conclusion: We demonstrated a statistically significant difference in mean DEX AUC0-inf and Cmax between the normal and obese groups. We conclude that different dosing would be needed to reach DEX similar exposure in obese and normal-weight COVID-19 hospitalized patients.
1. University of Oxford. Low-cost dexamethasone reduces death by up to one-third in hospitalized patients with severe respiratory complications of COVID-19. 2021; Available from: https://www.recoverytrial.net/news/low-cost-dexamethasone-reduces-death-by-up-to-one-third-in-hospitalised-patients-with-severe-respiratory-complications-of-covid-19.
2. World Health Organization. Corticosteroids for Covid-19 - Living Guidance. 2020 01.03.2022]; Available from: https://www.who.int/publications/i/item/WHO-2019-nCoV-Corticosteroids-2020.1.
3. National institutes of health. Covid-19 treatment guidelines- corticosteroids 2021; Available from: https://www.covid19treatmentguidelines.nih.gov/therapies/immunomodulators/corticosteroids/.
238
Intestinal permeability in transplant patients: are systemic short-chain fatty acids an early biomarker?
Brossier C1, Jardou M1, Pinault E1, Sauvage F1, Lawson R1
1 Univ. Limoges, Inserm U1248, IPPRITT, Limoges, France
Introduction: Intestinal permeability is characterized by a loss of the gut epithelial wall integrity allowing different sizes of compounds (food antigens, commensal or pathogenic bacteria, and their metabolites) to enter the systemic circulation. In transplant patients, this intestinal permeability could be accentuated by immunosuppressive therapy with deleterious consequences such as chronic systemic inflammation. For instance, the digestive accumulation of mycophenolic acid (MPA), one of the most widely used immunosuppressants, is known to alter gut epithelial cells homeostasis and tight-junction proteins expression. Furthermore, chronic inflammation can explain the elevated risk of post-transplant cardio-metabolic complications and graft loss. The severity of intestinal permeability is known to be correlated with the molecular weights of permeable materials. Lipopolysaccharides (LPS) have high molecular weight and they are validated to diagnose severe and late intestinal permeability. Therefore, there is an urgent need to early identify patients at risk of intestinal permeability. This can be achieved by investigating the permeation of small-size molecules such as short-chain fatty acids (SCFAs), a collection of bacterial metabolites exclusively produced by the gut microbiota.
Objective: This study aims at investigating SCFAs as an early biomarker of intestinal permeability in a cohort of transplant patients.
Methods: Plasma samples from biological collections (Limoges University Hospital) of a heart-transplant patients cohort (n=18) at one-year post-transplantation were investigated in comparison to healthy volunteers (n=10). LPS maximum concentration (ELISA method) in healthy volunteers has been used to set an arbitrary threshold in order to create two groups of patients in the transplant cohort (high LPS and normal LPS). These two groups have been compared for systemic SCFAs quantitation (cumulation of acetate, propionate, and butyrate, LC-MS/MS method), and for exposition to MPA (AUC/dose).
Results: In healthy volunteers, plasma LPS ranges from 10 to 186 pg/mL. Thus, a threshold of 200 pg/mL was chosen to set high LPS and normal LPS groups in transplant patients. Regarding this threshold, LPS quantitation for the normal LPS group (60 ± 6 pg/mL, n=9) was similar to healthy volunteers (66 ± 18 pg/mL) but was six times higher for the high LPS group (394 ± 46 pg/mL, n=9). Despite this significant difference for LPS, systemic SCFAs concentrations were not different between both groups (high LPS, 299 ± 41 μM vs normal LPS, 252 ± 31 μM, p>0.05, unpaired t-test) but two-fold higher than healthy volunteers (volunteers 116 ± 16 μM, p<0.05, one-way ANOVA, Dunnett multiple comparison tests). The exposition to MPA was similar between the two groups probably due to the efficiency of therapeutics drugs monitoring (high LPS 0.024 ± 0.004 mg.h/L/mg vs normal LPS 0.029 ± 0.006 mg.h/L/mg).
Conclusion: These results suggest that SCFAs could be used as a biomarker to early diagnose intestinal permeability. Additional studies are needed for the monitoring of systemic SCFAs that could be used to decipher the early stage of intestinal permeability development in the post-transplant period.
S1c: COVID-19 - Update on therapies
70
Immunogenicity 5-months after homologous or heterologous booster vaccination in Health Care Workers primed with Ad26.COV2.S
Sablerolles R1,9, Rietdijk W1, Goorhuis A2, Postma D3, Visser L4, Koopmans M5, Dalm V6, Kootstra N7, Huckriede A8, Lafeber M9, van Baarle D8, GeurtsvanKessel C5, de Vries R5, van der Kuy H1
1 Department of Hospital Pharmacy, Erasmus Medical Center, Rotterdam, Netherlands
2 Center of Tropical Medicine and Travel Medicine, Department of Infectious Diseases, Amsterdam University Medical Centers, Amsterdam, Netherlands
3 Department of Internal Medicine and Infectious Diseases, University Medical Center Groningen, Groningen, Netherlands
4 Department of Infectious Diseases, Leiden University Medical Center, Leiden, Netherlands
5 Department of Viroscience, Erasmus Medical Center, Rotterdam, Netherlands
6 Department of Internal Medicine, Division of Allergy & Clinical Immunology and Department of Immunology, Erasmus Medical Center, Rotterdam, Netherlands
7 Department of Experimental Immunology, Amsterdam University Medical Centers, Amsterdam, Netherlands
8 Department of Medical Microbiology and Infection Prevention, University Medical Center Groningen, Groningen, Netherlands
9 Department of Internal Medicine, Erasmus Medical Center, Rotterdam, Netherlands
Introduction: The Janssen vaccine (Ad26.COV2.S), approved as a single-shot regimen, is effective against severe coronavirus disease-2019 (COVID-19). However, this vaccine induces lower severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike (S)–specific antibody levels than messenger RNA (mRNA)–based vaccines. There is evidence that heterologous boosting (with BNT162b2 or mRNA-1273) of Ad26.COV2.S primed individuals resulted in higher (S)-specific antibody levels than homologous boosting (with Ad26.COV2.S). Because of the emergence of SARS-CoV-2 variants, questioning arises on the long-term protection of homologous and heterologous vaccination strategies after Ad26.COV2.S primed individuals.
Methods: The SWITCH trial is a single-blind, multicenter, randomized, controlled trial among ±450 healthcare workers (HCW) and investigated the immunogenicity and reactogenicity of a homologous (Ad26.COV2.S) or heterologous (BNT162b2 or /mRNA-1273) vaccination strategy among Health Care Workers (HCW) who were primed with Ad26.COV2.S. We focused on development of short (1-month)- and long-term immune responses (up to 5 months post booster).
Objectives: The primary end-point is the level of S-specific binding antibodies, and the secondary end points are the levels of neutralizing antibodies and S-specific T-cell responses.
Results: Homologous or heterologous booster vaccination resulted in higher levels of S-specific binding antibodies, neutralizing antibodies, and T-cell responses than a single Ad26.COV2.S vaccination. Responses were significantly higher with heterologous regimens compared to homologous booster; mRNA-1273 was most immunogenic. Antibody and T-cells measured in whole blood waned at 5 months post booster vaccination; however, levels were still significantly higher after BNT162b2 or mRNA-1273 booster vaccination, compared to Ad26.COV2.S booster. When assessing cross-reactivity of neutralizing antibodies with the emerging Delta and Omicron variants, participants that were vaccinated and boosted with Ad26.COV2.S, had relatively low levels of neutralizing antibodies to Delta and could not cross-neutralize Omicron. Participants boosted with an mRNA vaccine had high titers to Delta, and cross-neutralized Omicron.
Conclusion: We showed that mRNA booster vaccination after Ad26.COV2.S priming induces strong humoral and cellular immune responses, which are detectable up to 5 months after booster vaccination. However, although waning was observed and cross-neutralization of emerging variants is less likely, the fact that immune responses were detected in almost all study participants early and later after booster vaccinations indicates that immunological memory was properly performed. Our results do not directly lead to a recommendation for a second boost within 5 months after the first boost, provided there are no alarming variants of concern.
76
Geographic variation in top-10 prescribed medication and potentially inappropriate prescription in Portugal: an ecological study of 2.2 million older adults
Rocha V1, Plácido A2, Rodrigues D2, Tavares A2, Figueiras A3, Roque F2, Herdeiro M1
1 Institute of Biomedicine (iBiMED) and Department of Medical Sciences (DCM), University of Aveiro, Aveiro, Portugal
2 Research Unit for Inland Development, Polytechnic of Guarda (IPG-UDI), Guarda, Portugal
3 Department of Preventive Medicine and Public Health, Faculty of Medicine, University of Santiago de Compostela, Santiago de Compostela, Spain
Introduction: The use of multiple medications by older adults is considered a Public Health concern since it is associated to a higher risk of adverse drug reactions and potentially inappropriate medication (PIM).
Objectives: This study aimed to describe the top-10 prescribed active substances in older adults considering geographical distribution and PIM prescription.
Methods: A retrospective ecological study was conducted using data on the prescribed active substances during 2020 to people with 65 years or older. Information on active substances and defined daily doses (DDD) by age group, sex and region were retrieved from a Portuguese health administrative database. The average number of prescribed packages and DDD per 1000 inhabitants per day of top-10 active substances were calculated. Each active sustance was considered PIM if listed on the European Union(7)-PIM list.
Results: A total of 2228090 older adults (58% females) were included. The active substances with higher prescription rates (mean DDD/1000 inhabitants/day) in all ARS were furosemide and atorvastatin in both males and females, compared to the other active substances of the top-10. Geographic differences in prescription were observed (higher prescription in ARS North and Centre and lower in ARS Algarve). In females, 2/10 most prescribed active substances were PIM (benzodiazepines and opioids) with geographic disparities across regions.
Conclusions: Most prescribed active substances to older adults belong to the cardiovascular system. The prescription of benzodiazepines and opioids in females, classified as PIM, alert for the need of public health policies to reduce inappropriate prescribing. Geographic differences in the top-10 most prescribed active substances and in PIM highlighted the importance of medication optimisation across regions.
S2a: OMICS and Targeted Biomarkers
54
Quantitative Proteomics of Hepatic Drug-Metabolizing Enzymes and Transporters in Patients with Colorectal Cancer Liver Metastasis
Vasilogianni A1, Al-Majdoub Z1, Achour B1, Peters S2, Barber J1, Rostami-Hodjegan A1
1The University of Manchester, Manchester, UK, 2Merck KGaA, Dramstadt, Germany
Introduction: Colorectal cancer with liver metastasis (CRLM) is a leading cause of death. Liver is the main site of drug metabolism, and thus the presence of tumour can affect pharmacokinetics (PK) of drugs used in cancer patients. Therefore, studying the impact of cancer on parameters that affect drug PK (e.g. abundance of drug metabolising enzymes and transporters) may lead to better PK predictions in cancer patients.
Objectives: This study aims to provide for the first time population-specific abundance data of drug-metabolizing enzymes (DMEs) and transporters for CRLM patients (CRLM) that are necessary for PBPK modelling.
Methods: Microsomes were prepared from liver tissue taken from 15 healthy individuals and 18 cancer patients (2 primary, 16 metastatic). Patient samples included tumors and matching histologically normal tissue. LC-MS/MS targeted proteomics was used for the quantification of 22 DMEs and 25 transporters.
Results: The levels of cytochrome P450 (CYPs 2B6, 2D6, 2E1, 3A4, 3A5) and uridine 5′-diphospho-glucuronosyltransferases (UGTs 1A1, 1A6, 1A9, 2B15, 2B4, 2B7) were lower in histologically normal tissue from patients relative to healthy controls (up to 6.6-fold) and decreased further in tumours (up to 21-fold for CYPs and 58-fold for UGTs). BSEP, and MRPs were also suppressed in histologically normal (up to 3.1-fold) and tumorous tissue (up to 6.3-fold) relative to healthy individuals. Expression of OCT3, OAT2, OAT7 and OATPs followed similar trends (up to 2.9-fold lower in histologically normal tissue and up to 16-fold lower in tumours). Expression of NTCP and OCT1 was also significantly suppressed (up to 9-fold). Interestingly, the monocarboxylate transporter MCT1 was more abundant (3.3-fold) in tumours, the only protein of interest to show this pattern. Inter-individual variability was substantially higher in the cancer set. Proteomics-informed physiologically-based pharmacokinetic (PBPK) models of 50 drugs with different attributes and hepatic extraction ratios (Simcyp Simulator) showed substantially lower drug clearance with cancer-specific parameters compared to default parameters.
Conclusion: Overall, this study provides values for decreased expression of DMEs and transporters in liver cancer, which enables using population-specific abundance data for these patients when conducting PBPK modelling.
231
Pharmacological characterization of a novel lipid-rich breast cancer patient-derived xenograft
Papapostolou I1, Sereti E2, Koutsougianni F1, Bouloutsou E1, Konteles V3, Lambrianidou A1, Poultsidi A4, Ioannou M5, Tsezou A3, Dimas K1
1 Department of Pharmacology University Of Thessaly, Larisa, Greece
2 Department of Translational Medicine, Division of Urological Cancers, Lund University, Sweden
3 Laboratory of Cytogenetics and Molecular Genetics University of Thessaly, Larisa, Greece
4 Department of Surgery, University of Thessaly, Larisa, Greece
5 Department of Pathology, University of Thessaly, Larisa, Greece
Introduction: Lipid-rich breast cancer (LRBC) is a rare subtype of breast cancer, highly metastatic and with a poor prognosis. It is reported to generally be negative for ER/PR receptors but highly positive for HER2 expression with triple-negative (TN) cancers to be even rarer accounting for 1-2% of all malignant breast cancers. Noteworthy, there are no models available for studies on this rare type of cancer so far.
Objective: To develop and pharmacologically characterize a PDX from a patient with LRBC.
Methods: The model was developed in immunocompromised mice after direct engraftment of tumor fragments surgically excised from the patient. Firstly, one priority was to determine the growth curves and take rate of this PDX in three gradually immunocompromised mice – Rag1 (B6.129S7-Rag1tm1Mom/J), NOD/SCID (NOD/ShiLtSz-Prkdcscid) and NSG (NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ) strains. After that, the PDX was further evaluated pharmacologically following the patient’s schedule. Specifically, docetaxel, cyclophosphamide, doxorubicin, and its liposomal form, Caelyx, were administrated. Histological, karyotypic and NGS analyses were performed for this type of cancer both in patients’ and mice’s tumors.
Results: We observed that tumors grow slower in the NOD/SCID mice in comparison to NSG mice, suggesting that the presence of NKs may be a crucial parameter for fighting this tumor. Of note, the same procedure into Rag1 mice that develop fully functional NK cells did not result to the development of tumors which supports the hypothesis for a crucial role of NK in the immunity against this type of tumor. Although, through direct fragment implantation in Rag1 mice it indeed resulted to tumor development, suggesting a noticeable distinguishment in the ability of NK to allow tumor growth when it is in fragments instead of single cells. Pharmacological characterization in NSG and NOD/SCID mice revealed that the xenograft responded well to cyclophosphamide and docetaxel, as was expected, but doxorubicin was found to be highly toxic. As an alternative Caelyx® (stealth liposomal doxorubicin) was for the first time tested on this type of breast cancer and found to be highly efficient with lower toxicity. Karyotyping revealed polyploidy, while NGS analysis the presence of a pathogenic mutation in the MSH2 gene (c.482T> A, p. Val161Asp) in both the patient and the xenograft. Data suggest that this mutation may be a driver mutation.
Conclusion: This is the first report on the development of a PDX for a triple-negative LRBC, a model that we anticipate will be an extremely valuable tool for developing novel treatments and understanding the biology of this rare type of breast cancer.
S2c: Mixed Oral Presentations (1)
96
Multiple sclerosis drugs and dental and gingival disorders: an observational retrospective study and disproportionality analysis in the world pharmacovigilance database
Bouazzaoui L1, Fedrizzi S2, Chretien B2, Nguyen S2, Chatellier A5, Nicot R3, Dolladille C2, Defer G4, Peyro-Saint-Paul L1
1 Department of Clinical Research and Innovation, CHU de Caen Normandie, Caen, France
2 Department of Pharmacology, CHU de Caen Normandie, Caen, France
3 Department of Oral and Maxillofacial Surgery, University of Lille, CHU Lille, INSERM U 1008: Controlled Drug Delivery Systems and Biomaterials, Lille, France
4 Department of Neurology, MS Expert Centre, CHU de Caen Normandie, Caen, France
5 Department of Maxillofacial and Plastic Surgery, CHU de Caen Normandie, Caen, France
Introduction: Multiple sclerosis (MS) is a chronic, inflammatory, demyelinating, neurodegenerative disease of the central nervous system. MS patients tend to have oral pathologies; the impact of MS drugs is poorly investigated.
Objectives: The aim of this study was to assess the putative association of each MS drug with dental and gingival disorders (DGDs).
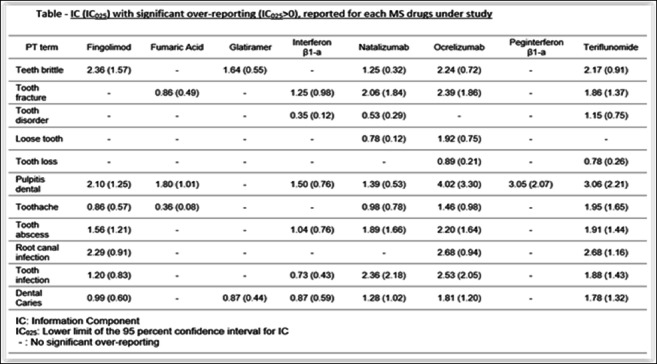
Methods: Using VigiBase®, the WHO Pharmacovigilance database, disproportionality of DGDs reporting was assessed among adverse drug reactions related to any MS drug, performed on February 03, 2022, through a case-non-case design. MS drugs included injectable drugs (interferon β-1a Avonex®, interferon β-1a Rebif®, interferon β-1b Betaferon®, interferon β-1b Extavia®, peginterferon β-1a, glatiramer acetate), intravenous therapies (natalizumab, ocrelizumab), and oral agents (fingolimod, teriflunomide, and dimethyl fumarate). Cases were identified with the HLGT « Dental and Gingival conditions » of the Medical Dictionary for Regulatory Activities, version 24.1. A signal of disproportionate adverse event reporting was defined by the lower 95% confidence limit of the Information Component (IC) greater than 0.
Results: Out of 29,512,078 de-duplicated cases reported up to 03 February 2022 in Vigibase®, 811,863 involved at least one MS drug, among which 4,498 were related to DGD (0.55%). The female-male sex ratio was 4.3 and the median [interquartile range] age: 48 [40-56] years. We found a signal of disproportionate reporting for MS drugs in general (IC [IC025 –IC975]: 0.20 [0.15-0.23]) with the highest association for « pulpitis dental » (IC025>2 for ocrelizumab, teriflunomide and peginterferon β-1a). The 4 most reported PTs among all MS drugs were «Toothache» (n=760, 16.9%), «Tooth disorder» (n=584, 13.0%), «Tooth infection» (n=569, 12.7%) and «Tooth fracture» (n=515, 11.4%). Results are shown in Table.
Conclusion: DGD could have an endodontic origin (from caries to pulp necrosis) and-or periodontal origin (alveolar bone destruction and-or connective tissue attachment from periodontitis to tooth loss). MS condition is associated to dry mouth that can induce dental demineralization. Manual dexterity can be decreased in MS, and loss of muscular coordination results in increased difficulty maintaining adequate oral hygiene. Pathogenesis of MS could involve a demyelinating process causing caries to be less painful and leading to most severe damage (as pulp disorders). Drugs could have a direct impact, both positive (by decreasing MS relapses) and negative (enhancing the risk of oral infection through their immunomodulating properties). This observational study must be interpreted as an exploratory analysis, and these results should be refined by future epidemiological studies. MS patients require specific protocols regarding prevention and care of DGD.
Tabel/Image
97
Safety, tolerability and pharmacokinetic profile of Macozinone (PBTZ169) formulated as native crystal powder: multiple ascending-doses, phase-Ib trial in healthy volunteers
Chtioui H1, Prod’Hom S1, Desfontaine V1, André P1, Moulfi F2, Bardinet C1, Abo-Loha C1, Tessieras J1, Rothuizen L1, Diezi L1, Guidi M1, Ivanyuk A1, Decosterd L1, Cole S2, Buclin T1
1 Service of Clinical Pharmacology, CHUV - Lausanne University Hospital, and University of Lausanne, Lausanne, Switzerland
2 Innovative Medicines for Tuberculosis (iM4TB), EPFL Innovation Parc, Lausanne, Switzerland
Introduction: Tuberculosis, particularly multidrug-resistant, represents a neglected medical need. Macozinone, a benzothiazinone derivative blocking arabinose synthesis and cell wall construction in mycobacteria, is a promising antitubercular agent.
Objectives: In this Phase Ib clinical trial, healthy volunteers received macozinone orally as native crystal powder for 14 days at increasing daily doses up to 600 mg/d, to confirm its safety and tolerability, assess its metabolic and pharmacokinetic (PK) profile and explore its drug interaction potential using a microdosed cocktail for cytochrome phenotyping.
Method: This single centre, prospective, randomized, parallel-group, sequential-design, ascending multiple doses, placebo controlled, double-blind trial included 5 consecutive panels of 8 healthy males aged 18-48. They received multiple doses of either macozinone or matching placebo for 14 days. The treatment was diluted in OraSweet® syrup for panels A (150 mg bid), B (300 mg bid) and C (600 mg qd), and in water for panels E (300 mg qd) and F (300 mg bid). All subjects underwent 2 detailed investigations on D1 and D14.
Results: Overall safety and tolerability of oral macozinone was excellent in all panels. It is rapidly absorbed, with concentrations peaking dose-dependently for each formulation. Cmax was however 4-fold higher with water suspension than syrup (p<0.001). The log-concentration profile follows a biphasic course, with a median distribution half-life of 2 h followed by an elimination half-life of 16 h (range 6.5-30 h). Accumulation occurs accordingly with a D14/D1 concentration ratio of 1.7 (0.9-4.7). The AUC increases dose-dependently for each formulation, with a relative bioavailability of 32% for syrup compared to water. The H2-metabolite circulates at levels 6-fold higher than macozinone. Other metabolites are lower but undergo significant urinary excretion conversely to PBTZ169 and the H2-metabolite, whose renal elimination is negligible. Phenotyping tests in panels A,B,C did not reveal any discernible interaction potential on the tested metabolic routes.
Conclusion: this trial provides important insights into the complex PK profile of macozinone and its metabolites. The rather large PK variability is not surprising for a drug absorbed with limited absolute bioavailability and eliminated by metabolic routes. The sensitivity to the syrup excipient illustrates the importance of thorough galenical optimization before Phase II investigations.
133
Peptidomimetic structure of dipeptidyl peptidase-4 inhibitors and the risk of sulfonylurea-induced severe hypoglycemia: a population-based cohort study
Dimakos J1, Cui Y2, Platt R2,3,4, Renoux C2,3,5, Filion K1,2,3, Douros A1,2,3,6
1 Department of Medicine, McGill University, Montreal, Canada
2 Centre for Clinical Epidemiology, Lady Davis Institute, Montreal, Canada
3 Department of Epidemiology, Biostatistics and Occupational Health, McGill University, Montreal, Canada
4 Department of Pediatrics, McGill University, Montreal, Canada
5 Department of Neurology and Neurosurgery, McGill University, Montreal, Canada
6 Institute of Clinical Pharmacology and Toxicology, Charité-Universitätsmedizin Berlin, Berlin, Germany
Introduction: Dipeptidyl peptidase-4 (DPP-4) inhibitors have a very low intrinsic risk of hypoglycemia due to their glucose-dependent mode of action. However, DPP-4 inhibitors can augment sulfonylurea pharmacodynamics through the activation of incretin signaling, which then leads to insulin secretion in pancreatic β-cells. Therefore, concomitant use of sulfonylureas and DPP-4 inhibitors is known to increase the risk of sulfonylurea-induced hypoglycemia. Currently, it is unclear whether the risk of this potentially fatal adverse effect varies with the pharmacologic properties of DPP-4 inhibitors (peptidomimetic versus non-peptidomimetic).
Objectives: To assess the risk of severe hypoglycemia associated with concomitant use of sulfonylureas with peptidomimetic DPP-4 inhibitors (i.e., saxagliptin, vildagliptin) as compared to concomitant use of sulfonylureas with non-peptidomimetic DPP-4 inhibitors (i.e., sitagliptin, linagliptin, alogliptin) among patients with type 2 diabetes.
Methods: We conducted a retrospective cohort study using the United Kingdom’s Clinical Practice Research Datalink, a primary care database with electronic medical records of >40 million individuals, which was linked to hospitalization and vital statistics data. Participants were patients initiating treatment with sulfonylureas between January 17, 2007, and June 30, 2020. Using a time-varying exposure definition, we compared current concomitant use of sulfonylureas with peptidomimetic DPP-4 inhibitors to current concomitant use of sulfonylureas with non-peptidomimetic DPP-4 inhibitors. Severe hypoglycemia was defined as hospitalization with or death due to hypoglycemia. We used Cox proportional hazards models to estimate hazard ratios with 95% confidence intervals of severe hypoglycemia, adjusted for several confounders measured at baseline. Secondary analyses stratified by age and sex, and assessed a potential duration-response relation between concomitant use of sulfonylureas and peptidomimetic DPP-4 inhibitors and the risk of severe hypoglycemia.
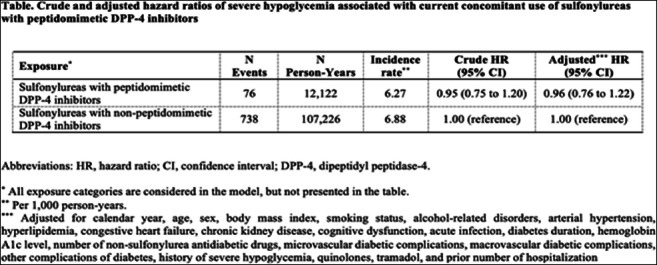
Results: Our cohort included 196,138 patients initiating sulfonylureas. There were 8,576 events of severe hypoglycemia during a median follow-up of 6.0 years, generating a crude incidence rate of 7.17 (95% confidence interval, 7.02 to 7.32) per 1000 person-years. The Table shows that when compared to concomitant use of sulfonylureas with non-peptidomimetic DPP-4 inhibitors, concomitant use of sulfonylureas with peptidomimetic DPP-4 inhibitors was not associated with an increased risk of severe hypoglycemia (adjusted hazard ratio, 0.96; 95% confidence interval, 0.76 to 1.22). An effect modification by sex was observed. Among female patients, concomitant use of sulfonylureas and peptidomimetic DPP-4 inhibitors was associated with a non-significant trend towards an increased risk of severe hypoglycemia (adjusted hazard ratio, 1.32; 95% confidence interval, 0.97 to 1.81). Among male patients, an association with a decreased risk was observed (adjusted hazard ratio, 0.69; 95% confidence interval, 0.48 to 0.99; p for heterogeneity <0.01). Age or duration of concomitant use did not modify the association.
Conclusion: Our large population-based cohort study showed no increased risk of severe hypoglycemia associated with concomitant use of sulfonylureas and peptidomimetic DPP-4 inhibitors compared to concomitant use of sulfonylureas and non-peptidomimetic DPP-4 inhibitors. Further research is needed to verify the observed effect modification by sex.
Tabel/Image
187
Intoxication Risk in Children of Family Members Prescribed Opioids: A Nationwide, Case-control Study in Denmark
Finkelstein Y1, Szépligeti S2, Horváth-Puhó E2, Pedersen L2, Freedman S3, Sørensen H2, Cohen E1
1 The Hospital For Sick Children, Toronto, Canada
2 Aarhus University Hospital, Aarhus, Denmark
3 Alberta Children Hospital, Calgary, Canada
Introduction: Worldwide, more than 350,000 deaths are attributable to opioids each year. The direct downstream risk and harm to children from opioids prescribed to family members is unknown.
Objectives: To determine the risk of serious opioid intoxication events (SOE) in children of family members prescribed opioids.
Methods: To determine the odds of a SOE (i.e., death, hospitalization, or emergency department care) in children associated with redemption of an opioid prescription by a family member, we conducted a nationwide, nested case-control study among all Danish residents <20 years old, over a 23-year period (April 1, 1995 – December 31, 2017). Cases were individuals who experienced a SOE. For each case, we matched 10 population-matched controls with no SOE. In the primary analysis, we compared the odds of a SOE in children whose family member redeemed an opioid prescription in the preceding year with that of children of unexposed family members using multivariable logistic regression, adjusting for sociodemographic (age, sex, marital status) and mental health covariates. An additional analysis compared the unexposed group with children of family members prescribed non-opioid analgesics (non-steroidal anti-inflammatory drugs [NSAIDs]). In a secondary analysis, we excluded children with unexposed family members, and directly compared SOE risk in children of family members exposed to opioids vs. NSAIDs in the year preceding the SOE date. lastly, we replicated the above analyses restricting the time interval between a redeemed prescription and SOE to one month and three months, and to subgroups stratified by age, sex, and patient disposition.
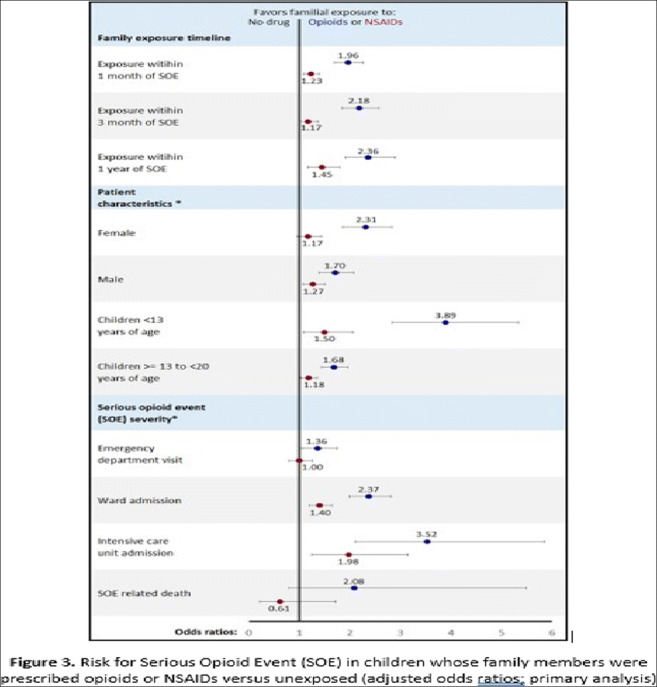
Results: The incidence of SOE among Danish children almost doubled during the study period (from 2.03/100,000 in 1996 to 3.75/100,000 in 2017). 1,752 (54.5% male) experienced an SOE (“cases”) and were matched to 17,401 controls. Among cases of SOE, 1,091 (62.3%) were hospitalized, 143 (8.2%) to intensive care units, and 46 (2.6%) died.
Compared to controls, case parents were less likely employed, married or have higher education, and more likely to have a substance abuse or documented mental illness.
Primary analysis - A redeemed opioid prescription by a family member within the year prior to index date was associated with twice the odds of a child’s SOE (adjusted odds ratio [aOR] 1.96, 95% confidence interval [95%CI] 1.70 to 2.26) compared with a child with unexposed family members. Secondary analysis - Children of family members prescribed opioids versus NSAIDs had increased odds of SOE (aOR 1.61, 95%CI 1.39 to 1.88). Increased SOE risk for both analyses persisted for both sexes, age brackets, shorter time intervals between drug redemption and SOE, and for all dispositions (discharge home, ward or ICU admission).
Conclusions: Children of family members prescribed opioids are at a markedly increased risk of opioid intoxication requiring hospital care, admission or death. Socioeconomic determinants affect the risk. Physicians, pharmacists and parents should take measures to mitigate the risk of opioid-related harm to children, such as prescribing smaller quantities, emphasizing the importance of secure medication storage, and the prompt disposal of unused opioids.
Tabel/Image
219
Safety and applicability of a phenotyping cocktail of seven cytochrome P450 -selective substrate drugs for clinical trials
Aurinsalo L1,2,3, Lapatto-Reiniluoto O1,4, Neuvonen M1,2, Kiiski J1,2, Niemi M1,2,3, Tornio A5,6, Backman J1,2,3
1 Department of Clinical Pharmacology, University of Helsinki, Helsinki, Finland
2 Individualized Drug Therapy Research Program, Faculty of Medicine, University of Helsinki, Helsinki, Finland
3 Department of Clinical Pharmacology, HUS Diagnostic Center, Helsinki University Hospital, Helsinki, Finland
4 HUS Pharmacy, Helsinki University Hospital, Helsinki, Finland
5 Integrative Physiology and Pharmacology, Institute of Biomedicine, University of Turku, Turku, Finland
6 Unit of Clinical Pharmacology, Turku University Hospital, Turku, Finland
Introduction: The information obtained from a single clinical drug-drug interaction trial is often limited to the effects of the investigated drug on one cytochrome P450 (CYP) enzyme. Efficient phenotyping of drug metabolism, for example to investigate the influence of disease states or genetic backgrounds, can be facilitated by measuring the activities of multiple CYP enzymes simultaneously. To gain more information from a single clinical trial, cocktails of substrate drugs of several CYP enzymes are increasingly used. However, in this setting the risk of adverse effects and substrate-substrate interactions is increased and therefore these cocktails rarely include a comprehensive collection of CYP enzymes. For example, CYP2C8 is often neglected from these cocktails despite its importance in drug metabolism.
Objectives: The aim was to add the CYP2C8 substrate repaglinide to a previously validated phenotyping cocktail, the Geneva cocktail, to build-up a cocktail of the seven most important human drug-metabolizing CYP enzymes for clinical trials. Additional targets were assessment of the effects of clopidogrel and gemfibrozil on this cocktail of seven CYP enzymes and evaluation of their CYP2C8 inhibition selectivity in vivo.
Methods: In a 5-phase randomized controlled trial of 16 healthy volunteers a 0.05 mg dose of repaglinide was administered alone and together with the Geneva cocktail, containing oral probe substrates for six CYP enzymes (50 mg caffeine/CYP1A2, 20 mg bupropion/CYP2B6, 10 mg flurbiprofen/CYP2C9, 10 mg omeprazole/CYP2C19, 10 mg dextromethorphan/CYP2D6 and 1 mg midazolam/CYP3A4), and the effects of clopidogrel and gemfibrozil on the cocktail including repaglinide were assessed. Venous blood samples were collected before the administration of the pretreatment drugs and 5 minutes before and 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12 and 23 hours after the administration of the study drugs. Blood glucose values and adverse effects were monitored up to 12 hours. Standardized meals were served 1, 3, 7 and 9 hours after the administration of the study drugs. Concentrations of the probe substrates and their metabolites were quantitated with LC-MS/MS techniques.
Results: Neither symptoms of hypoglycaemia nor any other drug-related adverse effects were reported during the study. Blood glucose levels were ≥3.5 mmol/l in all participants during all five study phases. The minimum ± S.D. blood glucose concentrations following study drug intake were 4.8±0.5 mmol/l with the Geneva cocktail, 4.6±0.6 mmol/l with repaglinide alone, 4.8±0.5 mmol/l with repaglinide and the Geneva cocktail, 4.5±0.5 mmol/l with repaglinide, the Geneva cocktail and clopidogrel as pretreatment, and 4.5±0.5 mmol/l with repaglinide, the Geneva cocktail and gemfibrozil as pretreatment.
Conclusions: Addition of a 0.05 mg dose of repaglinide to the Geneva cocktail was well tolerated and did not cause any significant decline in blood glucose levels, even with the strong CYP2C8 inhibitors gemfibrozil and clopidogrel. Considering its safety profile, this cocktail of seven CYP probe drugs is suitable for clinical drug-drug interaction and phenotyping trials. Pharmacokinetic data will be presented at the 15th Congress of the European Association for Clinical Pharmacology and Therapeutics.
260
The use of screening data for the carrier of multi-resistant microorganisms to determine strategies for antibiotic prophylaxis and treatment
Kasimova A1,2, Vilium I1, Kolbin A1
1 Pavlov First Saint Petersburg State Medical University, Saint-Petersburg, Russia
2 Russian Scientific Research Institute of Traumatology and Orthopedicsnamed after R.R. Vreden, Saint-Petersburg, Russia
Objectives: The aim of the study was to assess the impact of the introduction of screening on the carriage of multi-resistant microorganisms in surgical patients before hospitalisation on the optimisation of the use of antibiotics. The new schemes of antibiotic prophylaxis and empirical antibiotic therapy take into account the results of screening for the carriage of multi-resistant microorganisms: MRSA, MRSCons, VIM, IMP and NDM, KPC, OXA-48, ESBL, AmpC.
Methods: The data on the antibiotics for periods before (control) and after (study) the introduction of screening, the duration of hospital stay, the duration of stay in the intensive care unit for similar periods were analyzed. The assessment of the structure and volume of antibiotic consumption was carried out on the basis of the ACT/DDD methodology.
Results: Data from about 11,000 out of 15,550 patient histories were studied, 6,820 (62%) of them were prescribed antimicrobial drugs. The profiles of interventions are gynaecological, oncological, cardiovascular, orthopedic, urological, endocrinological. When the carrier of multi-resistant microorganisms was detected, in addition to standard prophylaxis, antibacterial drugs such as clindamycin, fosfomycin were prescribed to patients. When prescribing empirical therapy, antibiotics of the reserve group were not used in the absence of data for the carriage of multi-resistant microorganisms. ACT/DDD analysis showed a decrease in antibiotic consumption after the introduction of screening by 27%. A change in the length of stay in the hospital and in the intensive care unit was observed for the profile of cardiovascular and orthopedic surgery. Economic analysis has determined a cost reduction of drug provision.
Conclusions: The introduction of screening for the carrier of resistant microorganisms and the use of its results in determining antibiotic prophylaxis and prescribing empirical therapy leads to a reduction in the consumption of antibiotics and, as a consequence, a reduction in the cost of this group
S3a: Advanced Therapies
71
Pharmacokinetics and safety of DWJ211, injectable deoxycholic acid, after subcutaneous administration in healthy Korean subjects
Chung W1, Hwang S1,2, Cho S3, Na J1, Kim B1, Lee S1, Oh J1, Jang I1
1 Department of Clinical Pharmacology and Therapeutics, Seoul National University College of Medicine and Hospital, Seoul, Republic of Korea
2 Integrated Major in Innovative Medical Science, Seoul National University Graduate School, Seoul, Republic of Korea
3 Daewoong Pharm. Co., Ltd., Seoul, Republic of Korea
Introduction: Deoxycholic acid (DCA) injection is used for the improvement of convexity or fullness associated with submental fat. DWJ211 is an injectable form of synthetic DCA that is under clinical development.
Objectives: This study aimed to evaluate the safety, tolerability, and pharmacokinetics (PK) of DWJ211 after subcutaneous (SC) administration in healthy subjects.
Methods: A randomized, open-label, 2-period, parallel group study was conducted in healthy Korean subjects. Subjects randomly received one of the three SC dose (50 mg, 100 mg, and 200 mg) of DWJ211 into submental and abdominal fat, in the first and second period, respectively. PK blood samples were collected up to 24 hours before and after administration of DWJ211, and PK parameters were analyzed by non-compartmental analysis. Safety and tolerability were evaluated by adverse events (AEs), 12-lead electrocardiograms, vital signs, physical examinations, and laboratory clinical tests.
Results: A total of 18 subjects were randomized and 16 subjects completed the study as planned. DWJ211 was rapidly absorbed, reaching the maximum concentration (Cmax) after 0.08 to 4 hours of drug administration. The plasma concentration of DCA returned to the baseline levels after 24 hours of post-dose, and the area under the concentration-time curve (AUC) increased proportionally. After the abdominal fat injection, the dose-normalized Cmax and AUC were 0.8-fold lower and 1.1-fold higher than after the submental fat injection, respectively. All AEs were mild and there were no serious AEs.
Conclusion: The DWJ211 injection showed linear PK profile after SC administration, and the relative bioavailability similar between after the abdominal fat injection and after the submental fat injection. The DWJ211 were generally well tolerated in healthy subjects.
109
Multidisciplinary collaboration to address the urgent medical need of patients with severe COVID-19 ARDS: COVID-AT Study
Caballero-Bermejo A1, Ramírez-García A1, Darnaude-Ximénez I1, Payares-Herrera C1, Ruiz-Antorán B1, Malo de Molina R2, Lipperheide I3, Martinez-Muñoz M4, Avendaño-Solá C1
1 Clinical Pharmacology Department. Hospital Universitario Puerta De Hierro-Majadahonda, Madrid, Spain
2 Respiratory Medicine Department. Hospital Universitario Puerta de Hierro-Majadahonda, Madrid, Spain
3 Intensive Care Unit. Hospital Universitario Puerta de Hierro-Majadahonda, Madrid, Spain
4 Hematology Department. Hospital Universitario Puerta de Hierro-Majadahonda, Madrid, Spain
Introduction: The emergence of COVID19 lead to an overwhelming scenario with no vaccines or specific pharmacological agents to treat moderate/ severe disease forms. Mesenchymal stromal cells (MSC), with attributed immunomodulatory/ tissue-regenerative properties and limited prior evidence in the treatment of ARDS, emerged as a potential option for patients with ARDS.
Objectives: To assess the efficacy/safety of allogeneic bone marrow-derived MSC in patients with moderate/severe COVID19 acute respiratory distress syndrome (ARDS).
Methods: Our multidisciplinary team (composed by Clinical Pharmacology, Intensive Care, Respiratory Medicine and Hematology specialists) conducted a randomized, academic, double-blind, controlled study with 1-year follow-up. Patients were randomized (1:1) to an IV dose of 1x106 MSC/kg or equivalent matching placebo, plus standard of care. Primary endpoint: change from baseline to day 7 in the PaO2/FiO2 ratio. Key secondary endpoints: clinical improvement in the WHO 7-point ordinal scale, oxygen requirements, 1-year mortality, cumulative incidence of infusion and treatment-related adverse events.
Results: From 01/10/2020-04/12/2020, 20 patients were randomized and treated. The change in the PaO2/FiO2 ratio at day 7 from baseline was 83.3 in the MSC group vs 57.6 in the control (p=0.318). In addition, time to reduction of supplemental oxygen requirements to WHO ≤4 was significantly shorter for the experimental arm (15.0 vs 22.6 days; p=0.013). Improvement of at least one category of the WHO 7-point ordinal scale at day 7 was greater in the experimental group (5 patients, 50%) than in the control (0 patients, 0%; p=0.033). No differences were detected in other secondary endpoints, including 1–year mortality. No infusion or treatment-related serious adverse events occurred in the study.
Conclusion: In the midst of the 2nd wave of the COVID pandemic in Spain, a multidisciplinary team joined their efforts to give response to an urgent medical need by conducting a well-designed trial. Although statistically significant differences were not demonstrated for the primary endpoint, some clinical benefits were observed and the benign safety of MSC was confirmed. Overall, multidisciplinary collaboration is a valuable asset in times of crisis.
134
Association of CYP2C19 Polymorphism with Treatment Response Among Multiple Myeloma Patients on Bortezomib Based Induction Treatment
Gupta P1, Goel L1, Kumar L1, Singh A1
1 All India Institute of Medical Sciences, New Delhi, India
Introduction: Multiple myeloma (MM) accounts for 1 - 1.8% of all cancers and is the second most common blood cancer. Bortezomib, 26S proteasome inhibitor, is a frontline antimyeloma drug which is associated with significant improvement in progression-free survival (PFS). However, the major drawback is that 10 - 30% patients either do not respond or are resistant to it. Bortezomib is metabolized through oxidative deboronation by cytochrome P450 (CYP) enzymes, primarily CYP3A4 (69.8%) and CYP2C19 (33.5%). CYP3A4 is polymorphic (CYP3A4*1B) in 1.2% Indians. On the other hand, CYP2C19 polymorphism has been reported to be relatively higher in the Indian population.
Objective: To study the association of CYP2C19 polymorphism with response to treatment in multiple myeloma.
Methods: Treatment naive MM patients were screened from August 2016 till May 2021. Patients ≥18 years age, either sex, and eligible for bortezomib induction therapy were recruited after obtaining written informed consent. Those receiving concurrent CYP3A4/ CYP2C19 modulators were excluded. The demographic details, disease staging, and related investigations were recorded. The treatment response was assessed at the end of four induction cycles as per International Myeloma Working Group Uniform Response Criteria. The CYP2C19 polymorphism was assessed using polymerase chain reaction- restriction fragment length polymorphism (PCR-RFLP) technique for *2, *3 and *17 allele. Based on genotyping, patients were stratified as extensive (EMs), intermediate (IMs), poor (PMs) and ultrarapid (UMs) metabolizers.
Results: A total of 352 patients were screened and 220 were enrolled. The mean (SD) age of the enrolled patients was 55.6 (9.5) years, and 65.9% patients were male. Of the 220 patients, 15.9%, 30.9% and 53.2% were in ISS stage I, II and III respectively while 13.6%, 20.9% and 65.5% were in R-ISS stage I, II and III respectively. For myeloma subtype: 65.1%, 13.2% and 21.7% patients had IgG, IgA and light chain myeloma type respectively. The mean (SD) haemoglobin was 9.3 (2.5) g/dL while the albumin was 3.6 (0.9) g/dL. The estimated glomerular filtration rate (eGFR), calculated using Cockcroft-Gault formula, was 63.6 (40.5) mL/min. The mean (SD) serum calcium was 9.5 (1.9) mg/dL. The 195 responders and 25 non-responders were comparable for baseline characteristics, except ISS stage. There was no non-responder in ISS-II stage (p= 0.001). The CYP2C19 was polymorphic in 38.6%, 2.3% and 23.7% participants for *2, *3 and *17 allele respectively. The association was computed between treatment response and metabolizer status (p=0.06). Further, a subgroup analysis was performed for the four phenotypes of metabolizers separately. The difference between the EM versus IM, PM and UM group was statistically significant (p=0.005) as all 54 participants in EM group were treatment responders.
Conclusion: This study reports, for the first time, that extensively metabolizing (EM) patients responded well to the treatment. The observation has the potential to individualize bortezomib based treatment for multiple myeloma patients.
S3b: Clinical Pharmacology of cardiovascular drugs
5
SGLT2 inhibitors in diabetes, a systematic review and meta-analysis of cardiovascular outcome trials balancing their benefits against their risks
Marilly E1, Cottin J1, Cabrera N2, Cornu C3, Boussageon R4, Moulin P5, Lega J6, Gueyffier F2, Cucherat M2, Grenet G1
1 Service Hospitalo-Universitaire De Pharmacotoxicologie, Hospices Civils De Lyon, Lyon, France
2 Laboratoire De Biométrie Et Biologie Evolutive Umr 5558, Cnrs, Université Lyon 1, Université De Lyon, Lyon, France
3 Cic 1407 Inserm, Hospices Civils De Lyon, Lyon, France
4 Département De Médecine Général, Université De Lyon, Lyon, France
5 Fédération D’endocrinologie, Maladies Métaboliques, Diabète Et Nutrition, Inserm Umr 1060Carmen Hospices Civils De Lyon, Université Lyon 1, Lyon, France
6 Service De Médecine Interne Et Vasculaire, HôpitalLyon Sud, Hospices Civils De Lyon, Lyon, France
Introduction: Cardiovascular outcome trials (CVOTs) demonstrated benefits of SGLT2 inhibitors (SGLT2i). However, serious adverse drug reactions (ADRs) have been reported. Previous meta-analyses assessed their efficacy and their safety separately. The extent to which these ADRs might negate the CV benefits of SGLT2i remains unclear.
Objectives: We estimated their benefit-risk balance (BRB) in patients with type 2 diabetes (T2D).
Methods: We conducted a systematic review and meta-analysis of randomized CVOTs assessing SGLT2i on cardiovascular (CV) events in patients with T2D. Our primary outcomes were overall mortality, BRB integrating the primary efficacy outcomes– major adverse CV events (MACEs), hospitalization for heart failure (HHF)– and the primary safety outcomes –amputation, diabetic ketoacidosis (DKA) and genital infections (GIs)–. For each outcome, we estimated the Incidence Rate Ratio (IRR) and computed the number of events expected spontaneously and under SGLT2i, then provided an integrated BRB of SGLT2i. We conducted a sensitivity analysis including an early-ended CVOT. A prespecified protocol was registered.
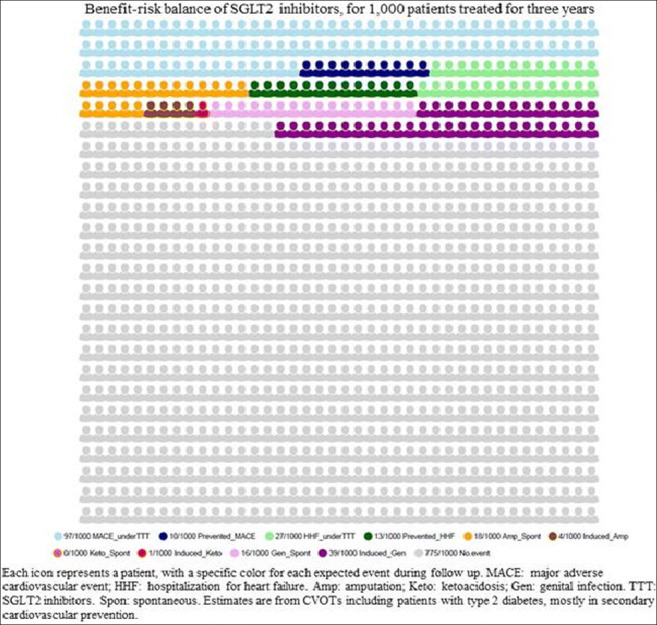
Results: A total of 46,969 patients, mostly at high CV risk, from five trials (mean follow-up: 3 years) were included. The reporting of GIs appeared heterogeneous across the CVOTs: from “Genital mycotic infection” to “Genital infections that led to discontinuation of the trial regimen or were considered to be serious adverse events”. The use of SGLT2i decreased the risk of all-cause death (IRR: 0.86 [95%CI 0.78 to 0.95]), MACEs (IRR: 0.91 [95%CI 0.86 to 0.96]),and HHF (IRR: 0.69 [95%CI 0.62 to 0.76]), and conversely increased the risk of DKA (IRR: 2.59 [95% CI 1.57 to 4.27]),and GIs (IRR: 3.50 [95%CI 3.09 to 3.95]), but not of amputation (IRR: 1.23 [95%CI 1.00 to 1.51]). For 1,000 patients treated for 3.0 years, SGLT2i are expected, on average, to decrease the number of deaths from 73 to 63; 10 MACEs and 13 HHF are expected to be prevented, versus 1 DKA and 39 GIs to be induced. Eighteen amputations are expected spontaneously in this population. If SLGT2i do induce amputations, four other patients might be harmed this way. 775 patients are expected to experience neither of the following outcomes: MACE, HHF, amputation, DKA and GI. The balance between MACE and amputation and MACE and DKA remained in favor of SGLT2i use (net benefit: -0.006 and -0.009, respectively), as the balance between HHF and amputation and HHF and DKA (net benefit: -0.009; and -0.012, respectively). However, the number of reported GIs exceeded the number of reported MACEs and reported HHF (+0.029 and +0.026, respectively) (see figure). In the sensitivity analysis, the balance between MACE and amputation, DKA and GI remained closed to the primary analysis, as for the balance between HHF and amputation, DKA and GI.
Conclusion: We provided an original synthesis of the BRB of SGLT2i, using powerful trials and data at low risk of bias. The BRB of SGLT2i remains in favor of their use in T2D patients at high CV risk, but remains unclear in primary CV prevention (excluding indication as heart failure and chronic kidney disease).
Tabel/Image
170
Effects of acute triiodothyronine treatment in patients with anterior myocardial infarction undergoing primary angioplasty. Evidence from a pilot randomized trial
Mourouzis I1, Trikas A2, Pissimisis E3, Grigoriou K2, Stougiannos P2, Dimopoulos A3, Linardakis S3, Alexopoulos N4, Evdoridis C2, Gavrielatos G2, Patsourakos N3, Papakonstantinou N3, Theodosis-Georgilas A3, Pantos C1
1 Medical School, National And Kapodistrian University Of Athens, Athens, Greece
2 Department of Cardiology, ELPIS General Hospital of Athens, Athens, Greece
3 Department of Cardiology, Tzaneio General Hospital of Piraeus, Piraeus, Greece
4 Department of Radiology, IASO Hospital of Athens, and Cardiovascular Imaging Unit, Department of Radiology, Athens Euroclinic, Athens, Greece
Introduction: Despite current treatments, acute myocardial infarction is associated with high incidence of heart failure and mortality. Thyroid hormone has reparative effects on ischemic myocardium due to its differential action on healthy and injured heart. Triiodothyronine (T3) administration was shown to improve postischemic cardiac function while limited apoptosis in experimentally induced ischemia.
Objectives: Τhe aim of the present study was to investigate potential effects of acute liothyronine (LT3) treatment in the clinical setting of anterior myocardial infarction. (Clinical Trials Registration: EudraCT 2016-000631-40)
Methods: This study is a pilot, randomized, double blind, placebo-controlled trial (ThyRepair study). 52 patients were randomized and thirty-seven patients (n=16 placebo and n=21 LT3) were included in the final analysis. LT3 treatment started after stenting as an intravenous (i.v.) bolus injection of 0.8μg/kg of LT3 followed by a constant infusion of 0.113μg/kg/h i.v. for 48 hours. All patients had cardiac magnetic resonance (CMR) at hospital discharge and 6 months follow-up. The primary end point was CMR left ventricular (LV) ejection fraction (LVEF) and secondary endpoints were LV volumes, infarct volume (IV) and safety.
Results: CMR LVEF% at 6 months was 53.6±9.5 for LT3 treated group and 48.6±11 for placebo, p=0.15. Acute LT3 treatment resulted in significant lower LV end-diastolic volume index (92.2±16.8 ml/m2 vs 107.5±22.2, p=0.022) and LV systolic volume index (47.5±13.9 ml/m2 vs 61.3±21.7, p=0.024) at hospital discharge. LV volumes between hospital discharge and 6 months were not different in either group. There was no significant difference in CMR infarct volume at hospital discharge between the groups. CMR infarct volume was significantly lower in LT3 treated group at 6months follow-up (18.7±9.5 vs 25.9±11.7, in placebo, p=0.05). Serious, life-threatening events related to LT3 treatment were not observed. A trend for an increased incidence of atrial fibrillation was found in LT3 group during the first 48 hours (19% for T3 group vs 5% for placebo, p=0.13).
Conclusion: This pilot RCT study suggests potential favourable effects (reduction in cardiac dilatation and infarct volume) of LT3 administration early after myocardial infarction, which should be tested in a larger scale study.
235
Characterising disease and prescribing patterns in patients with heart failure and multimorbidity: a single-centre descriptive cohort study
Bahar J1, Saied S1, Heron O1, Mashida K1, Akpan A1,2, Sankaranarayanan R1,2, Walker L1,2
1 Liverpool University Hospitals NHS Foundation Trust, Liverpool, United Kingdom
2 Liverpool Centre for Cardiovascular Science, University of Liverpool, Liverpool, United Kingdom
Introduction: Heart failure (HF) co-exists with multi-morbidity like renal impairment, diabetes, chronic respiratory diseases, frailty and anaemia. The management of HF patients with multimorbidity is complex, involving numerous therapeutics, which have potential for drug-drug and drug-disease interactions.
Objectives:
1. To characterise prescribing patterns in HF patients with multimorbidity
2. To identify inappropriate polypharmacy and therefore targets for de-prescribing
Methods: This was a retrospective cohort study involving patients under the care of HF multimorbidity, multidisciplinary team at Aintree University Hospital from January 2020-February 2021. Data was extracted from 234 adult HF patients with multimorbidity. We also recorded age, sex, number of medications and presence/absence of inappropriate dual antiplatelet therapy(DAPT) and proton-pump inhibitor(PPI) use were recorded. Inappropriate medication use was determined according to NICE prescribing guidance. Age-adjusted Charlson Comorbidity Index (CCI), Rockwood Clinical Frailty score (CFS<6=mild/no frailty,≥6+moderate/severe frailty) and anticholinergic burden (ACB) score were calculated. CFS of 7-9 was used to determine patients considered to be approaching end of life(12-24 months).
Results: Mean age was 71.5±13.9 and 44% patients were female. CCI was 6.9±3.3, Rockwood Frailty Score 5.5±3.2, polypharmacy burden high at 10.2±3.9 and ACB 1.45±0.9. ACB was higher in patients with CFS≥6 vs. those with CFS<6 (1.5±1.1 vs. 1.1±0.9;p=0.02). Proportion of HF patients on treatment for depression was 19.7%, chronic pain 35%, and chronic constipation 19.7%. Regular oral iron was prescribed in 15% of those appropriate for intravenous iron replacement. 17.9% of the cohort were estimated to be approaching end of life. Regarding potential inappropriate prescribing; 9% were on either DAPT/anticoagulant plus anti-platelet therapy beyond 12 months of acute coronary event. 20.1% patients were inappropriately prescribed regular PPI without clear indication.
Conclusion: Frail HF patients have a higher ACB and this observational study identifies clear targets for de-prescribing intervention in HF patients, like inappropriate PPI and DAPT/anticoagulant plus anti-platelet therapy, affecting 1:5 and 1:10 patients in the clinic respectively. Clear de-prescribing guidelines for these medications should be developed to support shared decision making between patients and clinicians to reduce the drug burden in this complex cohort.
S3c: New ways of learning Clinical pharmacology
56
An inter-professional student-run medication review programme: The clinical outcomes of a clinical controlled trial in a geriatric outpatient clinic
Sultan R1,2, Reumerman M1, Richir M1,2, Daelmans H3, Grijmans E4, Muller M5, van Agtmael M1,2, Tichelaar J1,2
1 AmsterdamUMC, department of internal medicine, section pharmacotherapy, Amsterdam, Nederland
2 RECIPE - Amsterdam, Amsterdam, Nederland
3 Amsterdam UMC, Vrije Universiteit Amsterdam, Faculty of Medicine, Skills training department, Amsterdam, Nederland
4 Hogeschool Inholland, Amsterdam, Nederland
5 Department of Internal Medicine section Geriatric Medicine, Amsterdam, Nederland
, juni 27, 2022, 09:00 - 10:30
Introduction: As the population ages, more people will have comorbid disorders and polypharmacy. Medication should be reviewed regularly in order to avoid adverse drug reactions and medication-related hospital visits, but this is often not done. An inter-professional student-led medication review program (ISP) team reviewing medication in geriatric patients has shown promising educational and clinical results.
Objectives: As part of our student-run clinic project, we investigated whether an ISP-team added to standard care at a geriatric outpatient clinic leads to better prescribing 1 and 3 months after the patient visit.
Methods: In this clinical controlled trial, patients visiting a memory outpatient clinic were allocated to standard care (control group) or standard care plus the ISP-team (intervention group). The medications of all patients were reviewed by a review panel (‘gold standard’), clinic physicians, and in the intervention arm also by an ISP-team consisting of a group of students from the medicine and pharmacy faculties and students from the higher education school of nursing for advanced nursing practice. For both groups, the number of STOPP/START-based medication changes mentioned in general practitioner (GP) correspondence and the implementation of these changes about 1 and 3 months after the outpatient visit were investigated. The ISP-team also performed a follow-up telephone call to the general practice office 6 weeks after the outpatient visit to inform the status of the medication advice and to nudge when advice were overlooked.
Results: The data of 216 patients were analysed (control group n=116, intervention group n=100). At baseline, the review panel identified 251 STOPP/START items (100%) in the control group (mean 2.2) and 206 items (100%) in the intervention group (mean 2.1). Of these items, the physician identified 17 (7%) in the control group and 14 (7%) in the intervention group; the ISP-team identified 128 STOPP/START items (62%) items in the intervention group. In total, 61 items (24%) in the control group and 89 items (43%) in the intervention group (p=<0.001) were mentioned as recommended medication changes in the GP correspondence. About 1 month later, medication changes based on 22 STOPP/START items (9%) in the control group and 39 (19%) in the intervention group had been implemented (p=0.001). Six weeks later, the ISP-team performed 116 follow-up telephone calls (control group = 51, intervention group 65). In both groups over 40% of the GP’s had not read the medication advice. When nudged to take on the medication advice most (>70%) planned to take action. In 20% of the cases the GP did not agree with the medication advices given, mostly because they had information not present at the review. In other cases (35%) the GP already planned to perform the medication changes in the next (planned/unplanned) patient visit. Three months after the outpatient visit, medication changes based on 40 STOPP/START items (16%) in the control group and 79 (38%) in the intervention group had been implemented (p=0.001).
Conclusion: The ISP-team is an effective intervention for optimizing pharmacotherapy and medication safety in a geriatric outpatient clinic on top of standard care.
57
An inter-professional student-run medication review programme. Reducing adverse drug reactions in a memory outpatient clinic: A controlled clinical trial
Reumerman M1, Richir M1,2, Sultan R1,2, Daelmans H3, Grijmans E4, Springer H4, Muller M5, van Agtmael M1,2, Tichelaar J1,2
1 AmsterdamUMC, department of internal medicine, section pharmacotherapy, Amsterdam, Nederland
2 RECIPE - Amsterdam, Amsterdam, Nederland
3 VUmc School of Medical Sciences, Amsterdam, Nederland
4 Hogeschool Inholland, Amsterdam, Nederland
5 Department of Internal Medicine, section Geriatric medicine, Amsterdam, Nederland
Introduction: The incidence of adverse drug reactions (ADRs) among community dwelling elderly patients is high, however most ADRs go undetected and untreated. Delayed detection or treatment of ADRs can seriously reduce patient quality of live, cause serious harm and lead to unplanned hospital admissions. Previous studies regarding student run clinics have suggested that students can play a crucial role in optimizing medication, however it is still unknown if they can play a role in reducing ADRs at an outpatient clinic.
Objectives: We describe and evaluate whether the addition of an inter-professional student-led medication review team (ISP-team) on top of standard care is associated with a reduction in ADR’s three months after the outpatient visit compared to standard care.
Methods: In this controlled clinical trial, patients visiting a memory outpatient clinic were allocated to standard care (control group) or standard care plus the ISP-team (intervention group) by a medical secretary. This ISP-team consists of students in medicine, pharmacy, master advanced nursing practice, and master physician assistant. The ISP-team performed a medication and ADR interview and provided additional medication interventions to reduce the number of ADRs. Three months after the outpatient visit, a clinical pharmacologist who was blinded for allocation, performed a follow-up telephone interview to determine which ADRs were still present.
Results: In total 142 patients were included in the analysis (intervention group n=76, control group n=66). During the outpatient clinic visit, significantly more (p<0.001) ADRs were detected in the intervention group (n=48) compared to the control group (n=10). In both groups, 60-63% of all detected ADRs received an intervention. Three months after the outpatient visit, significantly fewer ADRs (p=0.006) were detected in the patients of the intervention group. Most noticeable is the significant (p=0.022) improvement of dizziness and risk of falls and improvement (p=0.043) of ADRs related to antihypertensive drugs compared to the control group.
Conclusion: An ISP team in addition to standard care increases the detection and management of ADRs in elderly patients attending an outpatient memory clinic, resulting in fewer mild and moderately severe ADRs.
127
Novel teaching resources for the European Open Platform for Prescribing Education (EurOP2E) – a nominal group technique study
Bakkum M1,2, Loobeek B1,2, Richir M1,2, Papaioannidou P3,4, Likic R3,5, Sanz E3,6, Christiaens T3,7, Costa J3,8, Dima L3,9, de Ponti F10, Kramers C11, van Smeden J12, van Agtmael M1,2,3, Tichelaar J1,2,3
1 Amsterdam UMC, Vumc, Amsterdam, Netherlands
2 Research and Expertise Centre in Pharmacotherapy Education (RECIPE), Amsterdam, Netherlands
3 European Association for Clinical Pharmacology and Therapeutics (EACPT) Education Working Group
4 Aristotle University of Thessaloniki, Faculty of Health Sciences, Thessaloniki, Greece
5 University of Zagreb School of Medicine and Clinical Hospital Centre Zagreb, Zagreb, Croatia
6 Universidad de La Laguna, school of Health Sciences, La Laguna, Spain
7 Heymans Institute of Pharmacology Ghent University, Ghent, Belgium
8 Laboratory of Clinical Pharmacology and Therapeutics, Faculty of Medicine, Lisbon, Portugal
9 Transilvania University of Brașov, Faculty of Medicine, Brașov, Romania
10 Department of Medical and Surgical Sciences, Pharmacology Unit, Alma Mater Studiorum, University of Bologna, Bologna, Italy
11 Department of Internal Medicine and Pharmacology-Toxicology, Radboud University Medical Center, Nijmegen, Netherlands
12 Division of education, Centre for Human Drug Research, Leiden, Netherlands
Introduction: The European Open Platform for Prescribing Education (EurOP2E) aims to improve and harmonize European clinical pharmacology and therapeutics education by facilitating international collaboration and sharing open educational resources. The COVID-19 pandemic has forced teachers to switch to online teaching, highlighting the need for high-quality online teaching materials.
Objectives: The goal of this study was to establish the resources needed to sustain prescribing education during the pandemic and thereafter.
Methods: A nominal group technique study was conducted with prescribing teachers from several European countries and combined with thematic analysis.
Results: In four meetings, 20 teachers from 15 countries ranked 35 teaching materials. Ten themes were identified: prescribing scenarios; interactivity & gamification; re-usable materials; online case discussions; practical aspects of prescribing; teaching the teacher; knowledge multimedia; topical issues; personalized & evidence-based prescribing; and essential formularies.
Conclusion: By making teaching materials related to the learning outcomes of CPT, format of teaching and resource and faculty development openly available, EurOP2E will help to make high-quality prescribing education available to all. The role of the platform will range from facilitating collaboration to educating the teachers and/or providing ready-to-use teaching materials.
Tabel/Image
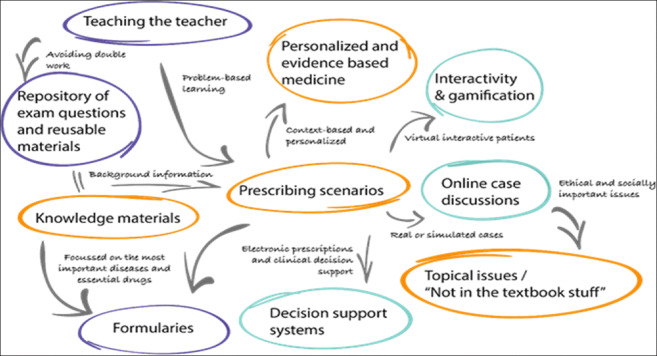
S4b: Mixed Oral Presentations (2)
3
ABCG2, SCN1A and CYP3A5 genes polymorphism and drug-resistant epilepsy in children: A case-control study
Gholami O1, Mousavi S1, Hasanpour K1, Nazarzadeh M2, Rad A1
1 Sabzevar University Of Medical Sciences, Sabzevar, Iran
2 Deep Medicine, Oxford Martin School, University of Oxford, Oxford, United Kingdom
Abstract
Purpose: Drug Resistance Epilepsy is still a major challenge in pharmacotherapy of epilepsy. Pharmacogenetic pathways are one of the critical factors that could predict the drug response and assist clinicians for personalized medications. Genetic alterations in drug target and transporter proteins, in part, could explain the development of drug resistance epilepsy. We sought to assess the association of CYP3A5 (rs776746), SCN1A (rs2298771) and ABCG2 (rs2231137) polymorphisms with pharmacoresistance among Iranian epileptic children.
Methods: Genotyping of CYP3A5 (rs776746), SCN1A (rs2298771) and ABCG2 (rs2231137) polymorphisms using the high-resolution melting (HRM) method were performed in 46 children with drug resistance epilepsy and 47 healthy control subjects. The logistic regression model was used to estimate the odds ratio (OR) for each polymorphism.
Results: There was a significant increase in the chance of pharmacoresistance in patients with candidate polymorphism in ABCG2 (adjusted odds ratio [OR] 2.41, confidence interval [CI] 0.99 to 5.87, P=0.05). No significant association between CYP3A5 (OR 0.92, CI 0.33 to 2.60, P= 0.88), SCN1A (OR 0.65, 95% CI 0.34 to 1.23, P= 0.19) and drug resistance was found.
Conclusion: In absence of genome-wide association studies, we found evidence for the relationship between the ABCG2 gene polymorphism (genotype C versus T) and drug resistance in a sample of children. This finding may have important implications for understanding the role of genetic factors in drug resistance epilepsy.
8
Eliciting drug safety signals from patient records: a language-agnostic approach
Kaas-Hansen B1,2,3, Placido D2, Rodríguez C2, Thorsen-Meyer H4, Gentile S5, Nielsen A2, Brunak S2, Jürgens G2, Andersen S2
1 Clinical Pharmacology Unit, Zealand University Hospital, Roskilde, Denmark
2 NNF Center for Protein Research, University of Copenhagen, Copenhagen, Denmark
3 Section of Biostatistics, Department of Public Health, University of Copenhagen, Copenhagen, Denmark
4 Department of Intensive Care Medicine, Copenhagen University Hospital, Copenhagen, Denmark
5 Region Zealand, Sorø, Denmark
Introduction: Text mining in pharmacovigilance often hinges on hand-curated reference sets, codifying free-text terms so they can be used as structured data. The consequent limited availability of tools and resources (including corpora) for non-English textual data (e.g. MedDRA is the official adverse drug reaction [ADR] vocabulary of the Danish Medicines Agency) hinders the use of non-English textual data for pharmacovigilance. Thus, the potential of unsupervised and automatic information extraction from, for vast screening of, clinical free text deserve exploration, to complement vocabulary-based approaches.
Objective: To create a drug safety signalling pipeline associating latent information in clinical free text with exposure profiles to highlight potential ADRs to single drugs and drug pairs.
Methods: We used inpatient visits of a 500,000-patient sample from two Danish regions, between 18 May 2008 and 30 June 2016. Tokens from clinical notes recorded within 48 hours of admission were operationalised with a fastText embedding. For each of the 10,720 single-drug and drug-pair exposures from doorstep (i.e. at time of admission) medication profiles, we trained a feed-forward neural network predicting the risk of exposure using embedding vectors as inputs. We assessed signal relevance by manually reviewing top signals for UKU items (in 4 domains).
Results: 2,905,251 inpatient visits comprised 13,740,564 doorstep drug prescriptions; the median number of prescriptions was 5 (IQR: 3-9) and in 1,184,340 (41%) admissions patients used ≥5 drugs concurrently. 10,788,259 clinical notes were included, with 179,441,739 tokens retained after pruning. Of 345 single-drug signals reviewed, 28 (8.1%) represented possibly undescribed relationships; 186 (54%) signals were clinically meaningful. 16 (14%) of the 115 drug-pair signals were possible interactions and 2 (1.7%) were known. See figure for details.
Conclusion: We built a language-agnostic pipeline for mining associations between free-text information and medication exposure without manual curation, by predicting not the likely outcome of a range of exposures, but the likely exposures for outcomes of interest. Our approach may help overcome limitations of text mining methods relying on curated data in English and makes our method appealing in settings that must make sense of non-English free text for pharmacovigilance.
Tabel/Image
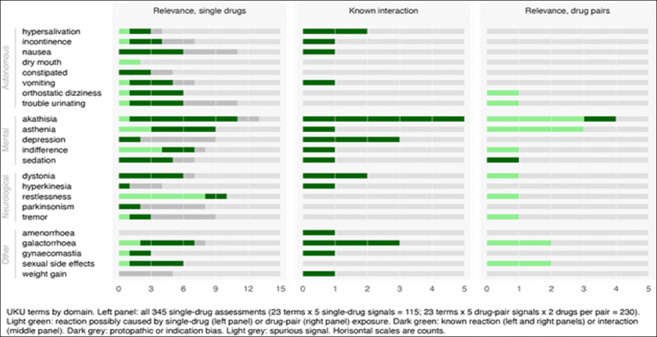
108
Clinical characterization and prognosis outcomes of carbon monoxide poisoning in Spain. The AMICO study
Caballero-Bermejo A1, Dueñas-Ruiz A2,3, Rodríguez-Aguilar L4, Ramírez-García A1, Darnaude-Ximénez I1, Sancho-López A1, Salgado-García E5, Homar-Amengual C6, Avendaño-Solá C1, Dueñas-Laita A2,3, Ruiz-Antorán B1, Puiguriguer-Ferrando J6
1 Clinical Pharmacology Department. Hospital Universitario Puerta De Hierro-Majadahonda, Madrid, Spain
2 Clinical Toxicology Unit. Hospital Universitario Río Hortega, Valladolid, Spain
3 Toxicology Section. Universidad de Valladolid, Valladolid, Spain
4 Faculty of Medicine. Universidad Autónoma de Madrid, Madrid, Spain
5 Clinical Toxicology Unit. Emergency Department. Hospital Clínic Barcelona, Barcelona, Spain
6 Clinical Toxicology Unit. Emergency Department. Hospital Universitario Son Espases, Palma, Spain
Introduction: Carbon monoxide (CO) poisoning debuts with heterogenous symptoms and is clearly underdiagnosed in Europe. The most serious complication is delayed neurologic sequelae (DNS), which occurs in up to 30% of patients.
Objectives: To describe clinical characteristics of CO poisoning and identify prognosis factors of developing DNS after an initial episode of CO poisoning.
Methods: A retrospective review of all cases of pure CO poisoning (those poisoned by fire smoke were excluded) presenting to the Emergency Department of 2 tertiary hospitals in Spain during the last 10 years. We analyzed demographics and clinical characteristics at the time of the episode. In patients with follow-up data available, we evaluated the appearance of DNS and its relationship with different variables in the initial exposure to CO.
Results; 136 cases were identified. Median age was 36.3 years (IQR, 20.6-50.2). 52.9% were female. Seven (5.1%) cases were related to suicide attempts and 126 cases (92.6%) were accidental. The leading causes of poisoning were malfunctioning braziers (38, 27.9%), poor combustion of the boiler (19, 14.9%) and deficient combustion stoves (19, 14.9%). Median hospital stay was 6 hours (IQR, 4-9). Nine (6.6%) patients required hospitalization, 7 patients (5.1%) were admitted to the ICU and none died.
Main symptoms were headache (76, 55.9%), dizziness (74, 54.4%) and loss of consciousness (52, 38.2%). Initial mean (SD) Glasgow score was 13.7 (3.2). Median first COHb levels were 13.6% (IQR, 9.7-18.8). Of the 21 patients with available follow up, 8 patients (38.1%) developed DNS, 7 of these (87.5%) presented MRI alteration. 63% of the patients who were admitted at the hospital developed DNS vs 33.3% of the patients who did not require hospital admission.
Only the Glasgow scale score was identified as a predictive factor of DNS (10.6 (5.3) in patients with DNS vs 14.5 (1.4); p=0.019). No statistically significant differences were found in any other demographics, clinical or analytical variables analyzed (COHb: DNS 21.1% vs 19.5% no DNS, p=0.725).
Conclusions: The Glasgow score appears to be a predictive factor of development of DNS. Due to the high incidence of DNS (38% in our cohort), we consider essential the development of multidisciplinary clinical protocols for the follow-up of these patients.
132
Concomitant use of sulfonylureas and warfarin and the risk of severe hypoglycemia: a population-based cohort study
Dimakos J1, Cui Y2, Platt R2,3,4, Renoux C2,3,5, Filion K1,2,3, Douros A1,2,3,6
1 Department of Medicine, McGill University, Montreal, Canada
2 Centre for Clinical Epidemiology, Lady Davis Institute, Montreal, Canada
3 Department of Epidemiology, Biostatistics and Occupational Health, McGill University, Montreal, Canada
4 Department of Pediatrics, McGill University, Montreal, Canada
5 Department of Neurology and Neurosurgery, McGill University, Montreal, Canada
6 Institute of Clinical Pharmacology and Toxicology, Charité-Universitätsmedizin Berlin, Berlin, Germany
Introduction: The vitamin K antagonist warfarin has been shown to interact with sulfonylureas via different pharmacokinetic mechanisms including competitive cytochrome P450 mediated metabolism and plasma protein displacement. This interaction could lead to elevated systemic levels of sulfonylureas and potentially further increase the risk of sulfonylurea-induced hypoglycemia.
Objective: To assess whether the concomitant use of sulfonylureas and warfarin is associated with an increased risk of severe hypoglycemia as compared to the use of sulfonylureas alone among patients with type 2 diabetes.
Methods: We used the Clinical Practice Research Datalink, a large electronic healthcare database from the United Kingdom containing medical records of over 40 million patients, that was linked to the Hospital Episodes Statistics and the Office for National Statistics databases. We assembled a study cohort including patients who initiated treatment with sulfonylureas between April 1, 1998, and June 30, 2020. Severe hypoglycemia was defined as hospitalization with or death due to hypoglycemia. Using a time-varying exposure definition, we compared current concomitant use of sulfonylureas and warfarin with current use of sulfonylureas alone. Cox proportional hazards models estimated hazard ratios with 95% confidence intervals of severe hypoglycemia. The analyses were adjusted for numerous confounders including demographic characteristics, anthropometric information, comorbidities, markers of diabetes severity, comedications, and proxies of overall health, all measured at cohort entry. Supplementary analyses using ‘active comparators’ (i.e., concomitant use of sulfonylureas and antiplatelet agents; concomitant use of sulfonylureas and direct oral anticoagulants) as reference group were performed to account for the potential impact of residual confounding. Sensitivity analyses accounted for selection bias and information bias.
Results: Our cohort included 325,549 patients initiating sulfonylureas. During a mean (standard deviation) follow up of 8.4 (5.7) years, there were 23,039 events of severe hypoglycemia, generating a crude incidence rate of 8.42 (95% confidence interval, 8.31 to 8.53) per 1000 person-years. Compared to use of sulfonylureas alone, concomitant use of sulfonylureas and warfarin was associated with an increased risk of severe hypoglycemia (adjusted hazard ratio, 1.25; 95% confidence interval, 1.16 to 1.35). Use of active comparators led to an attenuation of the risk (versus concomitant use of sulfonylureas and antiplatelet agents: adjusted hazard ratio, 1.20; 95% confidence interval, 1.11 to 1.30) or even a disappearance of the risk (versus concomitant use of sulfonylureas and direct oral anticoagulants: adjusted hazard ratio, 1.01; 95% confidence interval, 0.67 to 1.52) (Figure). Sensitivity analyses yielded findings that were consistent with those of the primary analysis.
Conclusion: Our large population-based study showed that compared to use of sulfonylureas alone, concomitant use of sulfonylureas and warfarin was associated with a modest increase in the risk of severe hypoglycemia. However, the increase in the risk is likely a result of residual confounding and does not seem to reflect a true effect. Our findings should provide reassurance to treating physicians and patients regarding the safety of the concomitant use of these drugs.
Tabel/Image
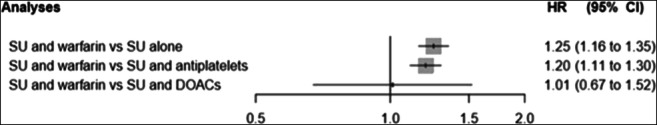
254
Impact of OPRM1 and ABCB1 gene polymorphisms on the perioperative remifentanil requirements and pain perception in patients undergoing elective thyroidectomy
Ntenti C1, Pourzitaki C2, Tsaousi G3, Soultati I3, Kotsovolis G4, Papavramidis T5, Goulas A1
1 1st Laboratory of Pharmacology, School of Medicine, Faculty of Health Sciences, Aristotle University of Thessaloniki, Thessaloniki, Greece
2 Laboratory of Clinical Pharmacology, School of Medicine, Faculty of Health Sciences, Aristotle University of Thessaloniki, Thessaloniki, Greece
3 Department of Anesthesiology and Intensive Care Unit, AHEPA Univ. Hospital, School of Medicine, Faculty of Health Sciences, Aristotle University of Thessaloniki, Thessaloniki, Greece
4 InterBalkan Medical Center, Thessaloniki, Greece
5 1st Department of Surgery, AHEPA University Hospital, School of Medicine, Faculty of Health Sciences, Aristotle University of Thessaloniki, Thessaloniki, Greece
Introduction: The response to opioid drugs varies among patients, indicating that part of the variation could be genetic. Remifentanil is a synthetic opioid with rapid onset and short duration of action, independent of the duration of infusion, usually administered intraoperatively. The preferred method of infusion is by continuous, commonly target-controlled infusion (TCI), in which the infusion rate is calculated based on pharmacokinetic (PK) and pharmacodynamic (PD) modeling and patient anthropometric characteristics.
Objective: This is a prospective observational cohort study aiming to examine the association of OPRM1 and ABCB1 gene polymorphism with the intraoperative needs of remifentanil in patients undergoing elective total thyroidectomy.
Methods: Ninety consecutive patients, 18 years or older, scheduled to undergo elective thyroidectomy for malignancy, benign disease, or hormonal disease not responsive to medical management, were included in the study. Exclusion criteria were age < 18 years, ASA PS ≥3, analgesic use up to one week prior to surgery, emergency thyroidectomy, severe thyroid hormone levels disturbance, pregnancy, and diagnosis of a personality disorder. Intraoperatively analgesia was achieved by TCI remifentanil (1.5 to 3 μg/mL), adjusted to manage hemodynamic changes exceeding 20% of the baseline values. Postoperatively, patients were assessed for pain by Visual Analogue Scale (VAS) at 15, 30, and 60 minutes and at 2 and 6 hours after surgery completion. DNA was extracted from patients’ peripheral blood using a commercial DNA extraction kit (Quick-DNA Miniprep Kit, Zymo Research, Irvine, CA, USA) while the polymorphisms were genotyped with PCR-RFLP methods.
Results: Study of the association of both polymorphisms on post-operative pain perception, operationalized through VAS ratings at different time points, provided evidence for a tentative time-dependent effect of ABCB1 C3435T,with carriers of the TT genotype experiencing significantly less pain compared to C allele carriers at time t = 30 min following completion of the operation; this effect was particularly evident among patients requiring additional analgesic treatment (VAS30min = 1.57 ± 1.272 vs. 3.39 ± 2.011, respectively; p = 0.024). Otherwise, a trend was noted with respect to OPRM1 A118G and intra-operative remifentanil consumption, while a marginally significant association has emerged between the strongest systolic pressure (SP) reduction, at the time of surgical cut, and OPRM1 A118G, with GG genotypes displaying consuming less remifentanil and greater SP reduction (p = 0.079 and 0.043, respectively).
Conclusions: The significantly stronger effect of OPRM1 118GG on intra-operative systolic pressure reduction suggests that its carriers appear to be more sensitive to the effect of remifentanil. The effect of ABCB1 C3435T on post-operative pain perception, may be related to a recently suggested involvement of P-glycoprotein in the efflux of endogenous opioids from the brain.
257
Unraveling Potential Pharmacological Target Genes from Transcriptomic Profiles in the Development of Persistent Atrial Fibrillation: A Bioinformatics Approach
Atzemian N1,2, Dovrolis N1,2, Ragia G1,2, Kolios G1,2, Manolopoulos V1,2,3
1Laboratory of Pharmacology, Medical School, Democritus University of Thrace, Alexandroupolis, Greece, 2 Individualised Medicine & Pharmacological Research Solutions Center (IMPReS), Alexandroupolis, Greece, 3Clinical Pharmacology Unit, Academic General Hospital of Alexandroupolis, Alexandroupolis, Greece
Introduction: Atrial fibrillation (AF) is the most common cardiac arrhythmia worldwide and it is characterized by uncoordinated contraction, which can potentially lead to thrombus formation, ischemic strokes, even death. Expression differences in genes involved in fibrosis or inflammation can promote cardiac remodeling leading to AF development and maintenance, although the underlying molecular mechanisms are not yet well understood. Due to this inadequate knowledge of AF mechanisms, management strategies are limited to preventing its complications such as stroke, and not the actual disease.
Objectives: Our aim was to identify novel genes playing a crucial role in AF development, which can provide clues as to the mechanism of disease pathogenesis and may also be used as pharmacological targets to reverse the pathophysiological mechanism of disease.
Methods: We analyzed data from 4 publicly available gene expression datasets from cardiac biopsies taken from Gene Expression Omnibus database and European Nucleotide Archive (GSE79768, GSE41177, PRJNA667522, GSE135445). A total of 46 and 31 atrial samples from patients with persistent AF and sinus rhythm respectively were included. Differential gene expression analysis was performed (>1.5-fold difference) using the GEO2R platform for each microarray data collection and RaNA-Seq for RNA-Seq data collection. Comparison between differentially expressed genes (DEGs) was performed using molbiotools.com to identify the common DEGs among the datasets.
Results: We identified the molecular signature of AF which includes 32 overexpressed and 12 underexpressed genes that were common among datasets. The most notably overexpressed gene in all 4 datasets is SERBP1 (SERPINE1 MRNA Binding Protein 1), a protein that is physiologically underexpressed in heart and involved in neuromuscular diseases. Additionally, ASAH1 (N-acylsphingosine amidohydrolase 1) is overexpressed in our datasets and its variant has been found involved in AF development while a ASAH1-related disorder is characterized by muscle weakness and degeneration. Also, CDH2 (Cadherin 2) was found overexpressed in our analysis and physiologically its expression plays a role in the establishment of left-right asymmetry, whereas its variants induce arrhythmias. Lastly, CASQ2 (Calsequestrin 2) was found overexpressed, and its dysregulation can conduct arrhythmias. The most underexpressed gene in all 4 datasets is IER3 (Immediate Early Response 3), which physiologically has an anti-apoptotic role in the heart, and it is the first time that is found dysregulated in AF. Three more genes that are identified underexpressed in AF are ADAMTSL4 (ADAMTS Like 4), BMP7 (Bone Morphogenetic Protein 7), which are involved in cardiac fibrosis, and ANKRD23 (Ankyrin Repeat Domain 23) which may be involved in angiogenesis.
Conclusion: With our bioinformatics approach, the molecular signature of Persistent AF was elucidated. This signature unravels novel pharmacological targets that can play a pivotal role in reversing the pathophysiological mechanism of AF and may potentially improve our therapeutic arsenal.
Funding: The study is funded by the program "Competitiveness, Entrepreneurship, Innovation" of REGIONAL EXCELLENCE, with a Grant for the project "Creation of Center of Excellence in Pharmacological Research and Precision Medicine" (MIS: 5047189).
270
Therapeutic Drug Monitoring of anticancer monoclonal antibodies by mass spectrometry approaches
Kotteas E2, Kamperi N1, Trontzas I2, Orfanou I1, Grapsa D2, Karampelas T1, Paloumpis N1, Syrigos K2, Tamvakopoulos C1
1 Biomedical Research Foundation, Academy of Athens, PharmacologyOf Athens, Athens, Greece
2 Oncology Unit, 3rd Department of Medicine, Athens Medical School, Sotiria General Hospital, Athens, Greece
Introduction: Therapeutic Drug Monitoring (TDM) defined as the measurement of drug levels in a biological fluid, has gained interest in Oncology over the last years. It enables the evaluation of drug efficacy or toxicity allowing, thus, to optimize the drug dosage scheme and overall clinical outcome of the patient. Cancer patients can benefit significantly from TDM as there are a number of anticancer agents that either have a narrow therapeutic index, which could lead to severe or even life-threatening toxicities, or have significant pharmacokinetic variability potentially resulting in suboptimal systemic drug exposure and reduced therapeutic efficacy. Cancer treatment remains primarily guided by tumor genotyping, while failing to take into consideration the additional key role of interpatient variability with respect to pharmacokinetics and tissue distribution of anticancer agents. TDM though remains an underused tool due to the lack of advanced analytical instrumentation and expertise in the clinic and lack of sensitive and specific assays.
Objectives: Our aim is to contribute in individualization of cancer therapy through TDM of immune checkpoint inhibitors (ICIs) or other monoclonal antibodies for targeted therapies, such as tyrosine kinase inhibitors (TKIs), and platinum-based drugs.
Our laboratory participates in EATRIS-GR, the Network of Greek Translational Research Infrastructures (RIs) based on the collective effort of Greek universities and research institutes, contributing to early-phase trials, bioequivalence studies, drug metabolism and pharmacokinetics studies. In the context of participation in the EATRIS program, TDM measurements in collaboration with the Oncology Clinic represent a unique opportunity to highlight the translational value of RIs.
Methods: Enzyme linked immunosorbent assay (ELISA) methods were initially used for the quantification of monoclonal antibodies (mAbs) such as pembrolizumab (Pbz) and trastuzumab (TZM) in human plasma or serum. Interim results were obtained from a cohort of lung cancer patients that had received 2 or 3 cycles of immunotherapy (monotherapy). A State-of-the-Art bioanalytical methodology based on high pressure liquid chromatography-tandem mass spectrometry (LC-MS/MS) was then developed for mAb quantification by monitoring of selected peptides derived from the mAbs of interest following tryptic digestion. The approach was used for Pbz and TZM, in biological fluids (human plasma or serum). The sample preparation technique is challenging, as it necessitates efficient extraction of the monoclonal antibody drugs from the biological fluid before digestion to surrogate peptides.
Results: Preliminary experiments were conducted using a series of protein precipitation and tryptic digestion protocols of the Pbz and TZB. By the described approach Pbz was detectable with a limit concentration of 0.5 μg/mL in human plasma showing excellent potential to further transfer the method for the evaluation of clinical samples. A similar strategy was followed for TZB with somewhat higher limits of quantification in serum (1 μg/mL).
Conclusion: We anticipate that adaptable bioanalytical mass spectrometry-based methods for anticancer agents monitoring can be developed, allowing us to proceed with simultaneous measurements of anticancer therapeutics (e.g., immunotherapeutic and cytotoxic), thus further enhancing our understanding of drug pharmacokinetics and patient response in selected clinical cohorts.
S5c: Changing Benefit-Risk Balance of Medicines (challenging the mantra)
36
French academic and industrial experience on financial compensation of participants in clinical trials
BOUAZZAOUI L1, MORDEL P1, CHAILLOT F1, LECRUX B1, GAILLARD C1, GOURIO C2,3, MORELLO R1,3, Peyro-Saint-Paul L1
1 DRI CHU de Caen Normandie, Caen, France
2 Pharmacie CHU de Caen Normandie, Caen, France
3 CPP NO III, Caen, France
Introduction: Financial compensation to research participants depends on the sponsor and the trial. European legislation indicates “that no inappropriate pressure, including of a financial nature, should be exerted on the subjects in order to obtain that they participate in a clinical trial (CT)”. Compensation is calculated according to the constraints; risks should not be considered. In France, it is subject to the opinion of one of the 39 ethics committees (ECs), up to 4500€ per year and per participant.
Objectives: The aim of our study was to compare if the awarding of financial compensation to research participants was consistent with the opinion of an EC depending on whether the sponsor was academic or industrial.
Methods: We reviewed all studies authorized from 2017 to 2021 sponsored by CHU de Caen allocated to studies by industry according to the phase. Blinded to compensation amount, an adjudication committee (AC) composed by two EC members examined constraints then established if compensation was indicated (yes/no/no opinion). Cohen’s Kappa Statistic was used to measure agreement between sponsors and AC. The Kappa statistic (K) was performed considering “no opinion” as a positive agreement. We interviewed AC about its methodology for assessing the need for compensation.
Results: Among the 50 studies, there were 4% phase 1, 48% phase 2, 28% phase 3 and 20% phase 4. Consistency between actual compensations or not and AC opinion are reported in table.
AC reported the lack of criteria to assist in decision making ("no opinion”: 17/50).
Conclusion: Concerning compensation in CTs, our academic practices seem more ethical than those of industry. Neither both sponsors compensate participants excessively. Participants are sometimes not compensated when they should be, particularly by industry. In 2018, a Malawi guideline for a fair participant compensation was published. National or even European more precise recommendations could be useful for sponsors and ECs. AC proposed a list of criteria that could help to constitute a tool depending on the participants’ constraints.
Tabel/Image
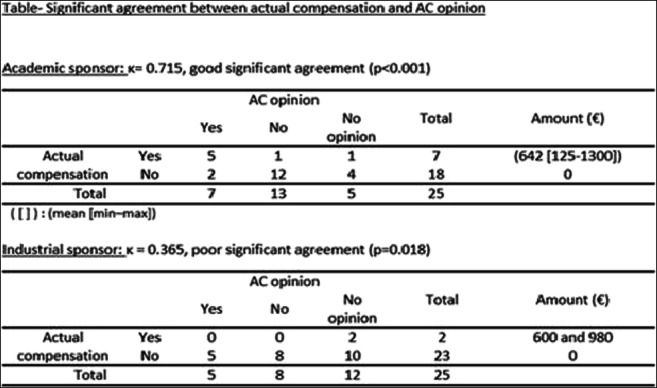
130
Antineoplastic drug approvals by European Medicines Agency (EMA) and Food and Drug Administration (FDA) between 2018-2021. Evaluation of potential differences
Alcubilla P1, Arellano L1, Aparicio L1, Burunat L1, Sánchez C1, Sáez-Peñataro J1
1 Clinical Pharmacology Department. Hospital Clinic, Barcelona, Spain
Introduction: New cancer treatments are continuously being approved by regulatory agencies in a wide variety of indications. In this regard, it seems relevant to evaluate if these new treatments are synchronically approved in Europe and United States for the same indications, and which is the quality of the evidence provided for efficacy at the time of approval.
Objectives: Perform a descriptive comparison between anticancer drug approvals by EMA and FDA during period 2018 - 2021, to detect potential differences among agencies in terms of timing of approvals, indications approved and utilization of accelerated/special approval paths. We also revised the available efficacy evidence for each treatment at the time of approval.
Methods: Revision of public scientific reports, summaries of product characteristics and labels on antineoplastic agent approvals by EMA and FDA from January 2018 to December 2021. Data on the exact indication, special approval paths used, and quality of evidence from pivotal clinical trials was extracted for analysis. Cut-off date for data extraction was 24 of February 2022.
Results: From January 2018 to December 2021, between both agencies 62 new therapies for 99 indications were approved. 43 new therapies were approved by both agencies. 19 drugs were approved by FDA and not by EMA. Of those, 13 are being revised by EMA, 2 were withdrawn by holder during revision, and 4 are not submitted. Among drugs approved by both (N=43,) median time for approval between FDA and EMA was 10 months (IQR 5.5 – 17 months) Among those approved by both, 27 were for same indication(s), 12 presented slight indication differences and 4 presented significant indication differences or were approved for different indications. 82% of therapies approved by FDA were in a special assessment program (Fast-track, accelerated approval, breakthrough therapy, priority review, assessment aid or Project Orbis) but only 30% of those approved by the EMA (accelerated assessment, PRIME, conditional marketing authorization, or approval under exceptional circumstances). Primary efficacy endpoint for approval was Overall Survival in 10%, Progression-free-survival in 37% and overall response rate in 53%. Pivotal trials that led to approvals were single arm in 53% of approvals and randomized trials in 47%
Conclusion: It seems that new anticancer therapies are first available in the US and then in Europe, with delays ranging between few months and more than a year. Unlike the EMA, we noticed a widespread utilization of special assessment programs by the FDA that could partially explain the timing differences, although it is acknowledged that holders tend to submit first the applications to the FDA. When drug was approved by both agencies, significant differences in approved indications were uncommon. Overall, proof of evidence at the time of approval was mainly based on surrogate endpoints, with a significant proportion of uncontrolled single-arm studies.
202
What has happened to medicines after a Conditional Marketing Authorisation (CMA) was granted by European Medicines Agency (EMA) from 2006-2020
Arellano L1, Alcubilla P1, De Dios V1, Olesti E1, Sáez J1, Varea S1
1 Clinical Pharmacology Department, Hospital Clinic of Barcelona, Barcelona, España
Introduction: CMA granted by EMA allows for early approval based on less complete clinical data than normally required, because the benefits of earlier patient access outweigh the potential risks of limited data and it also likely fulfils an unmet medical need. CMA are valid for one year; it can be renewed annually or convert to full authorisation if the holder fulfils specific obligations within defined timelines. It seems relevant to evaluate what has happened to medicines after authorisation by this fast-track approval.
Objectives: Perform a descriptive study of medicines granted a CMA by EMA since the inception of the program in 2006, to describe the characteristics of the initial authorisation, the requirements and procedural steps taken post-authorisation.
Methods: Revision of the EMA website of publicly available initial marketing-authorisation documents. Analysis of the changes made in approval documents since their initial release upon CMA authorization, covering the period 2006 to 2020. Data on the type of drug, clinical areas, pivotal trial, and changes made on type of authorisation through the years was extracted for analysis. Cut-off date for data extraction was 28 of February 2022.
Results: From 2006 to December 2020, 59 new medicines were granted a CMA. Of those, the most frequent were classified as antineoplastics/immunomodulators (35, 59.3%) and anti-infectives or antivirals for systemic use (12, 20.3%). The most frequent areas with a CMA drug were haemato-oncology (57.6%) and infections (18.6%). Most of the CMA medicines were authorised based on one (40, 67.8%) pivotal clinical trial. Of these, 28 (82.4%) were for haemato-oncology area drugs. ORR was the primary endpoint leading to CMA in 31% of medicines. Post authorisation requirements for most of medicines were based on the submission of data of ongoing trials or the commitment to conduct confirmatory trials. The changes of initial authorisations were conversion to full marketing authorisation (16, 38.9%), renewal of CMA (31, 52.2%), revoked by the European Commission (1, 1.7%), withdrawn by the holder (3, 5.1%) and no data available in one. The median time to conversion to full marketing authorisation was 92.8 months (7.7 years) (range: 14.7-333.7).
Conclusion: It seems that anticancer therapies are the most frequent medicines to be granted a CMA, requiring initially only one pivotal clinical trial. Most of the CMA medicines are either renewing annually the authorisation or in lesser degree converting to full marketing authorisation. For those drugs finally granted a full authorization, median time to fulfillment of regulatory obligations was long (more than 5 years).
S6a: TDM of mAbs
99
Is there an association between valproic acid serum levels and remission in epileptic children?
Charfi R1, Affes R1, Ferchichi K1,2, Ben Sassi M1,2, Gaies E1,2, El Jebari H1,2, Daghfous R1,2, Trabelsi S1,2
1 National Centre Chalbi Belkahia of Pharmacovigilance, Department of clinical pharmacology, LR16SP02, Tunis, Tunisia
2 University of Tunis El Manar - Faculty of Medicine of Tunis, Tunis, Tunisia
Introduction: Valproic acid (VA) is a broad spectrum first generation antiepileptic drug widely prescribed in epileptic children because of its. Because of its pharmacokinetic variability and the influence of intrinsic and extrinsic factors such as therapeutic compliance, therapeutic drug monitoring of VA is essential in epileptic children.
Objective: we aimed to compare VA trough plasma levels (TL) in epileptic children, in remission and in those with ongoing seizures.
Methods: We conducted a retrospective study (january 2009 – january 2022). We included epileptic children (2-18 years), who were addressed at least two times to the department of clinical pharmacology for a VA TL measurement. Blood samples were collected, at the steady state, just before the next administration of VA. We used chimiluminescence method to measure VA TL. VA therapeutic range is 50-100 μg/mL.
According to the International League Against Epilepsy, patients in remission should have as lasting three times the longest pre-treatment seizure-free interval and more than one year [3]. This rule of three has been recently validated on a statistical basis [4]. The population was divided in two groups:
Group 1: epileptic children in remission;
Group 2: epileptic children with ongoing seizures.
In this study, we took in consideration only the last VA TL.
Results: In this study, we included 147 children with generalized epilepsy (147 blood samples). Sex ratio boys/girls was 1.37. Children’s median age was 8.1 years (2.4 to 17.8 years). Median VA normalized daily dose was 26.09 mg/kg/day ( 5.06-56.25 mg/kg/day ) and median VA TL was 64.2 μg/mL (20.9-149.8 μg/mL). There was no correlation between VA normalized dose and VA TL.
Group 1 was composed of 81 epileptic children in remission and group 2 of 66 ones with ongoing seizures (table 1).
Conclusions: In this study, we found no correlation between normalized doses of VA and VA TL because of the interindividual variability of VA pharmacokinetics.
Besides, there was a difference in VA TL in the two groups with a significantly lower VA TL in epileptic children in remission with a ceiling of 112.5 μg/mL and a significant difference in drug association in the two groups probably due to an interindividual variability of resistance profile to VA.
These results are in favor of the rule of the therapeutic drug monitoring of VA to help drug adjustment.
Tabel/Image
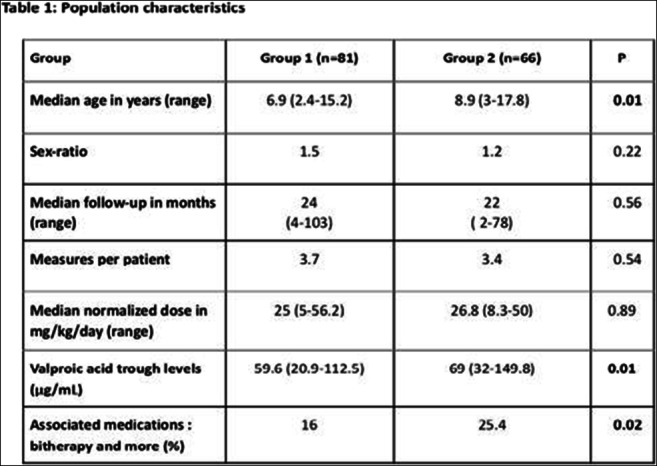
194
Development and validation of an LC-MS method for drug exposure measurement of 57 oral antitumor drugs in human plasma
Kehl N1, Dürr P1, Schlichtig K1, Maas R1, Fromm M1, Gessner A1, Taudte V1
1 Friedrich-Alexander University Erlangen-Nuremberg, Erlangen,, Germany
Introduction: During the last two decades, oral antitumor drugs (OADs) have significantly improved the therapy for multiple tumor entities. Most of these drugs are prescribed in a one dose fits all approach (i.e. identical dosing for each patient), irrespective of individual factors such as body surface or kidney function. As a result, highly variable plasma concentrations are observed between patients, which in turn may lead to a reduced therapeutic effect, or an increased risk of side effects. Drug exposure monitoring constitutes a useful tool to detect increased or reduced patient plasma levels of OADs and consequently can help to improve outcomes for patients. Currently existing methods for exposure monitoring cover only a comparatively small number of OADs (with a maximum of 17) compared to the relatively large and increasing number of these drugs on the market. Consequently, monitoring of a wide variety of OADs would require different analytical methods and possibly the time-consuming change of analytical equipment. To address this shortcoming of currently published methods for exposure monitoring of OADs we developed an analytical method, which covers 57 OADs in a single run. This method can help to improve the applicability of drug exposure monitoring of oral antitumor drugs in clinical routine.
Objectives: The aim of this project was to develop and validate a liquid chromatography high resolution mass spectrometry method for the simultaneous quantification of 57 oral antitumor drugs in human plasma.
Methods: A simple and robust method for the quantification of 57 OADs was developed and validated according to the FDA guideline. Sample preparation includes protein precipitation and dilution followed by the analysis via ultra-high performance liquid chromatography coupled to an Orbitrap mass spectrometer operated in full scan mode. The method was applied to 71 real world samples from 39 patients currently under therapy with an OAD at the Comprehensive Cancer Center Erlangen-EMN.
Results: The validation of the method was successful according to FDA recommendations for all 57 OADs. Quantification of OADs in the 71 plasma samples confirmed high interindividual variability in OAD plasma concentrations, which is in accordance to reports in the literature. The method proved its applicability to clinical practice and demonstrates the strong need for further investigations focusing on the correlation between plasma concentrations and clinical outcome in patients during therapy with OADs.
Conclusion: We developed and validated a liquid chromatography-mass spectrometer method which allows for simultaneous quantification of 57 OADs in human plasma, without the need for changing analytical hardware. This method sets a foundation for future clinical studies, to investigate the benefits of drug exposure measurement of a wide variety of OADs in routine clinical practice.
226
A RETROSPECTIVE STUDY OF ANTI-XA ACTIVITY IN PATIENTS WITH RENAL INSUFFICIENCY RECEIVING RENAL-BASED REDUCED OR NOT-REDUCED THERAPEUTIC DOSES OF DALTEPARIN
Mitrov-winkelmolen L1,2, Pires K1, le h1, Bouwhuis J1, Kuijper M1, Bosch T1
1 Maasstad Ziekenhuis, Rotterdam, The Netherlands
2 Ikazia Ziekenhuis, Rotterdam, The Netherlands
Introduction: Renally impaired (RI) patients have an increased potential to accumulate dalteparin and consequently an increased risk of (major) bleeding events. To reduce this risk, guidelines advice dalteparin dose reduction with anti-Xa monitoring. However, clinical experience has shown that dalteparin has a minimal tendency to accumulate in RI patients.
Objectives: The objective of this study was to compare the anti-Xa activity between a 75% and 100% weight-based therapeutic dose. Furthermore, we aimed to investigate the association between anti-Xa activity and the occurrence of bleeding.
Method: This study was a multicentre, retrospective observational study including non-intensive care unit (non-ICU) and ICU patients (≥18 years) with an estimated glomerular filtration rate (eGFR) <60 mL/min who received therapeutic doses of dalteparin (≥ 7500 IU daily). Anti-Xa levels were eligible for inclusion when they were sampled at peak concentrations during steady state. Primary outcome was the median anti-Xa level. The Wilcoxon test was used for analysis of the primary outcome, comparing 75% with 100% weight-based therapeutic dose of 200 units/kg/day dalteparin. The data was stratified for different patient groups, dose frequencies and dose reduction. The secondary outcome was the occurrence of bleeding.
Results: The median anti-Xa levels of non-ICU and ICU patients who received 75% or 100% weight-based therapeutic dose were mainly around or below the lower limit of the therapeutic ranges, as shown in table 1. In group A median anti-Xa levels of 75% dose were significantly lower than in 100% dose. In the other groups no significance was found between the median anti-Xa levels of 75% and 100% dose.
Preliminary data: Seven bleeding events occurred in total in non-ICU patients with an eGFR<30ml/min (group A and C combined). Three events (9,7%, all minor) occurred in patients receiving 75% of the weight-based therapeutic dose. Four events (8,9%, one major, three minor) occurred in patients receiving 100% of the weight-based therapeutic dose. No bleeding events occurred in non-ICU patients with an eGFR of 30-60 ml/min (group B and D combined).
Conclusion: Dose reduction of dalteparine in renal-impaired non-ICU and ICU patients predominantly results in lower anti-Xa activity than the recommended ranges in the guidelines. So far, our findings shows that the occurrence of bleeding is similar when dalteparine is dosed 75% and 100%. We therefore recommend that initial dose adjustment is not necessary in RI patients. Nonetheless, preventing high anti-Xa levels requires monitoring for these levels in renally impaired patients.
Tabel/Image
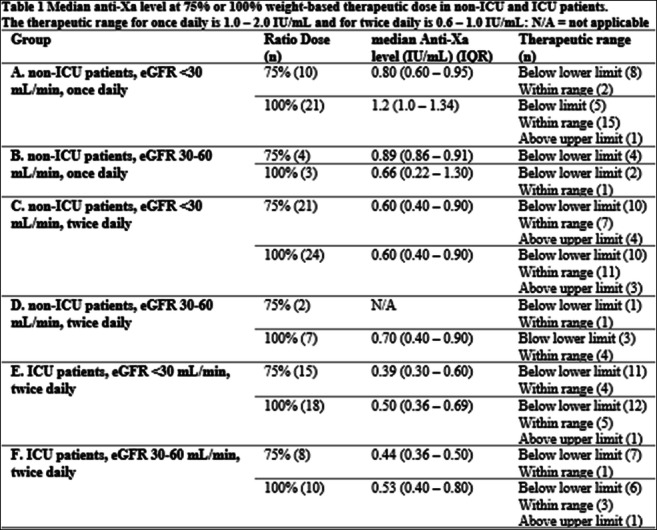
S6b: Public-private collaboration in drug repurposing: challenges and opportunities
14
Repurposing the Japanese heartburn drug camostat to potential antiviral treatment in COVID-19 – a human trial.
Breining P1, Gunst J2, Saedder E1, Sogaard O2, Breining P1
1 Dept. of Clinical Pharmacology, Aarhus University Hospital and Dept. of Biomedicine, Aarhus University, Aarhus, Denmark
2 Department of Infectious Diseases, Aarhus University, Aarhus, Denmark
The coronavirus responsible for COVID-19, SARS-CoV-2, utilizes a viral membrane spike protein for host cell entry. For the virus to engage in host membrane fusion, SARS-CoV-2 utilizes the human transmembrane surface protease, TMPRSS2, which cleaves and activates the spike protein. Camostat, an orally available serine protease inhibitor, is a potent inhibitor of TMPRSS2 and has been hypothesized as a potential antiviral drug against COVID-19. We therefore initiated a clinical trial for testing efficacy and safety of the TMPRSS2 inhibitor in patients with COVID-19 in a randomized international multicenter trial. Participants were randomly assigned in a 2:1 ratio to receive placebo or camostat 200 mg three times daily for 5 days. In total 137 patients were assigned to receive camostat and 68 to placebo. Median time to clinical improvement was 5 days (interquartile range [IQR], 3 to 7) in the camostat group and 5 days (IQR, 2 to 10) in the placebo group (P = 0.31). The hazard ratio for 30-day mortality in the camostat compared with the placebo group was 0.82 (95% confidence interval [CI], 0.24 to 2.79; P = 0.75). The frequency of adverse events was similar in the two groups. Median change in viral load from baseline to day 5 in the camostat group was -0.22 log10 copies/mL (p <0.05) and -0.82 log10 in the placebo group (P <0.05).
At this intervention timepoint in the disease development and with this dose, camostat mesilate treatment was not associated with increased adverse events during hospitalization for Covid-19 but the treatment did not significantly affect time to clinical improvement, progression to ICU admission or mortality.
Gunst et al., EClinicalMedicine (Lancet), 2021, Breining et al., BCPT 2021, Hoffmann et al. EBiomedicine (Lancet), 2021.
23
Defibrotide in the prevention and treatment of acute respiratory distress syndrome in patients with COVID-19. Preliminary safety results update.
Rodríguez-Fortúnez P1, Martínez-Mellado A2, Jara-Rubio R2, Blanquer-Blanquer M2, Castro-Rebollo P3, Carrillo-Alcaraz A4, Rodríguez-Jiménez C1, Albendin H2, Solano E2, Pareja A2, García-Clavel J5, López-Bernús A6, Onteniente M7, Moraleda-Jiménez J5
1 Clinical Trials Unit. University Hospital of the Canary Islands. Spanish Clinical Research Network (SCReN), Tenerife, Spain
2 University Clinical Hospital Virgen de la Arrixaca. IMIB., Murcia, Spain
3 Clínic Hospital, Barcelona, Spain
4 University Hospital Morales Meseguer, Murcia, Spain
5 Murcia University. University Clinical Hospital Virgen de la Arrixaca. IMIB., Murcia, Spain
6 Clinical Hospital of Salamanca, Salamanca, Spain
7 University Hospital Reina Sofía, Córdoba, Spain
Introduction: Currently, the search for treatments that demonstrate safety and efficacy in the treatment of SARS-CoV-2 infection is an unmet need. Defibrotide (DF), given its pleiotropic properties, including endothelial protection, anti-inflammatory and immunomodulatory activity, as well as anticoagulant and fibrinolytic activity, could become a therapeutic option for COVID-19 patients, particularly those with severe manifestations. Defibrotide has already been approved for veno-occlusive disease of the liver (VOD), which would facilitate its immediate access to patients.
Objectives: To evaluate the safety and efficacy of intravenous infusion of DF in the prevention and treatment of acute respiratory distress and cytokine release syndrome in patients with SARS-CoV-2 infection.
Methods: Prospective, multicenter, randomised, parallel, double-blind, placebo-controlled, prospective phase IIb clinical trial (CT). DF was infused as a 24-hour continuous intravenous infusion at a total dose of 25mg/kg/day for 15 days. Preliminary results from 150 patients are presented. This CT has been approved by the Spanish Medicines Agency and the Ethics Committee of the Hospital Clínico Universitario Virgen de la Arrixaca. EudraCT No: 2020-001409-21. Clinicaltrials.gov: NCT04348383
Results: 150 patients with PCR-documented SARS-CoV-2 infection and respiratory insufficiency that required hospitalization, with risk factors (IL-6 levels >3 normal levels) were recruited between April 2020 and January 2022. The mean age of the series was 61 years (range: 34-89 years), 79% were male. Out of 150 patients, 108 (72%) were grade 4-5, and 42 (28%) were grade 6 of the scale of 7 grades of the WHO.
We collected 821 adverse events (AE), 57 (6.9%) were considered serious adverse events (SAEs) and the 39 patients who experienced them (41 SAEs), died.
The reported SAEs were 57 (54 patients; mean age 63,48 years, 87,7% male) and are summarized as follows: elevated fibrin D-dimer (3 SAEs), elevated transaminase (1 SAE), necrotising pneumonia (1 SAE), mechanical ventilation (8 SAEs), interruption of treatment (1 SAE), chest drainage (1 SAE), thrombosis (8 SAEs), haemorrhage (1 SAE), distributive shock (2 SAEs), cardiorespiratory arrest (4 SAEs), atrial fibrillation (1 SAE), pulseless electrical activity (1 SAE), arrhythmia (1 SAE), tachycardia (1 SAE), decreased level of consciousness (1 SAE), tonic seizure (1 SAE), coma (1 SAE), perforation (1 SAE), multi-organ dysfunction syndrome (8 SAEs), respiratory failure (6 SAEs) and hypoxia (5 SAEs).
Only one SAEs was considered related to the investigational product (haemorrhage) and was considered as an expected adverse reaction (listed in the product data sheet). These data were consistent with the known favorable safety profile of DF in VOD.
Conclusion: To date, there is no evidence of relevant safety risks associated with the use of Defibrotide in continuous intravenous infusion in patients with COVID-19.
Acknowledgments: We are indebted to Maria Muñoz from the Clinical Trials Unit. Foundation for Health Training and Research of the Region of Murcia. Spain.
Note: Given the blind nature of the study, aggregated data are presented.
Tabel/Image
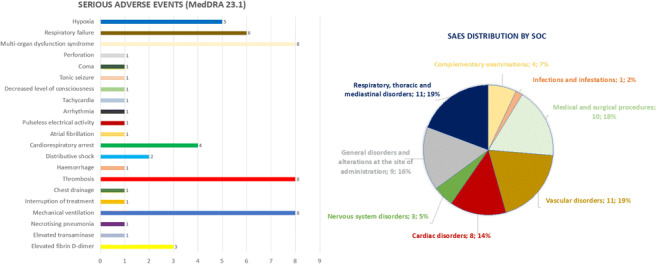
210
Pharmacokinetics and Pharmacodynamics for Optimal Drug Repurposing of Imatinib from Cancer to COVID-19
Bartelink I1, Baalbaki N2, Aman J3, Bet P1, Duijvelaar E3, Twisk J4, Longo C2, Mahmoud K1, Maitland-van der Zee A2, Bogaard H3, Swart E1
1 Department of Pharmacy and Clinical Pharmacology, Amsterdam UMC, location VUmc, Amsterdam, The Netherlands
2 Department of Respiratory Medicine, Amsterdam UMC, location AMC, Amsterdam, The Netherlands
3 Department of Pulmonary Medicine, Amsterdam UMC, location VUmc, Amsterdam, The Netherlands
4 Department of Epidemiology and Data Science, Amsterdam UMC, location VUmc, Amsterdam, The Netherlands
Introduction: In the randomized double-blind placebo-controlled CounterCovid study, imatinib reduced mortality in COVID-19 patients. High levels of alpha-1 acid glycoprotein (AAG) were associated with increased total imatinib concentrations in COVID-19 patients, which seems to explain the marked difference compared to the pharmacokinetics in cancer patients.
Aims: We aimed to study the difference in imatinib exposure in COVID-19 compared to cancer patients, to identify possible relationships between pharmacokinetic (PK) profiles of oral imatinib in COVID-19 patients and pharmacodynamic (PD) outcomes and to identify clinically relevant covariates for PK and PD in COVID-19 patient. We hypothesize that patient covariates may differ between cancer and COVID-19, which influence the exposure response relationship.
Methods: COVID-19 patients enrolled in the CounterCovid study used 400 mg oral imatinib once daily for 9 days following a loading dose of 800 mg. 648 Total plasma concentrations were obtained from 168 patients, and used to compare PK-data of a historical dataset of 475 total PK-samples of 20 patients with gastrointestinal stromal tumor (GIST) and 85 chronic myeloid leukemia (CML). PK was analyzed using a previously published AAG-binding model (Ref: Bartelink IH et al). For further PKPD analyses, profiles were expressed as total trough concentration at steady state (Css) and AUC average (AUCave). PD responses in Covid-19 patients were the ratio between partial oxygen pressure and fraction of inspired oxygen(P/F), WHO ordinal scale for clinical improvement(WHO-score) and oxygen supplementation liberation(O2lib). Linear regression, linear mixed effects models and time-to-event analysis were performed, possible confounders identified and adjusted for.
Results: AUCave was 2.21-(CI95% 2.07-2.37), and Css 1.53-fold (1.44-1.63) lower, for CML/GIST patients compared with COVID-19 patients. AAG and albumin explained 80% of variability in exposure between all patients. In CounterCovid patients, Css (not AUCave) associated significantly with P/F (β=-199,42; p-value=0.013) and O2lib (HR 0.75; p-value= 0.021), adjusted for sex, age, neutrophil-lymphocyte ratio, dexamethasone usage, AAG and baseline P/F-and WHO-score. Css not AUCave associated significantly with WHO-score.
Concusion: Higher total exposure following oral imatinib in COVID-19 patients was observed compared to CML/GIST patients, which associated with differences in plasma proteins among diseases. Higher total exposure (AUC or trough concentration) did not associate with improved clinical outcomes. Css showed an inverse association with PD-outcomes, when adjusted for patient and disease related confounders. This association may be biased by disease-course, processes related to vascular leakage in COVID-19, variability in metabolic rate and/or protein binding. Therefore, additional PKPD analyses into unbound imatinib and its main metabolite at Css may better explain exposure-response associations. This study shows that disease specific PK, PD and covariate analyses should be performed when defining the optimal exposure for drug repurposing of imatinib in COVID-19 patients.
Reference
Bartelink IH, Bet PM, Widmer N, Guidi M, Duijvelaar E, Grob B, Honeywell R, Evelo A, Tielbeek IPE, Snape SD, Hamer H, Decosterd LA, Jan Bogaard H, Aman J, Swart EL. Elevated acute phase proteins affect pharmacokinetics in COVID-19 trials: Lessons from the CounterCOVID - imatinib study. CPT Pharmacometrics Syst Pharmacol. 2021 Dec;10(12):1497-1511
S6c: Cultural versus genetic diversity
24
Applications of the REMEDI[e]S tool: implicit and explicit criteria for optimizing drug prescribing in older adults
Laroche M2, Grau M3, Roux B1
1 Pharmacovigilance Center of Limoges, LIMOGES, France
2 UR 24134, LIMOGES, France
3 Centre Bien Vieillir CHU Limoges, LIMOGES, France
Introduction: Reducing potentially inappropriate prescriptions (PIP) in older adults is a public health challenge. Many tools with explicit or implicit criteria exist, while some researchers highlight a best success of a combined approach to reduce harms associated with PIP. So, the REMEDI[e]S tool was developed to list implicit and explicit criteria of PIP in French older adults aged 75 years and over or 65 years and over with multimorbidity [Roux B et al., Eur J Clin Pharmacol 2021].
Objectives: To describe REMEDI[e]S tool and its applications.
Methods: A Delphi survey with 15 experts (geriatrics, general practice, pharmacy, clinical pharmacology) established: a) a seven-step algorithm (implicit criteria) encompassing the three main domains of PIP (overuse, underuse and misuse), and b) 104 explicit criteria divided into 6 tables (inappropriate drug duplications, omissions of medications and/or medication associations, medications with an unfavourable benefit/risk ratio and/or a questionable efficacy, medications with an unsuitable dose or duration, drug-disease and drug-drug interactions). Rationale and therapeutic alternatives are exposed.
Results: The design of REMEDI[e]S tool has been conceived to meet 3 objectives. Firstly, in daily clinical practice, healthcare professionals can review their patients’ prescriptions in order to decide a switch through a safer therapeutic alterative or to deprescribe. Secondly, the tool can be used for training of undergraduate and postgraduate physicians or pharmacists. The seven-step prescribing algorithm referring to different sets of explicit criteria allows the acquisition of a systematic reasoning. Thirdly, explicit criteria can be used to investigate the quality of drug use and associated outcomes in pharmacoepidemiologic studies.
Conclusion: REMEDI[e]S enables PIP detection both at the individual level in clinical practice and at the population level in large-scale studies in order to optimize care in older adults. An integrated computerized decision support tools in electronic health records is being developed to assist clinicians in their daily clinical practice and training.
38
Placebo response in Raynaud’s Phenomenon clinical trials: the prominent role of regression towards the mean
Roustit M1,2, Jullien A2, Jambon-Barbara C1, Goudon H1, Blaise S1,2, Cracowski J1,2, Khouri C1,2
1 Universite Grenoble Alpes, Grenoble, France
2 Grenoble Alpes University Hospital, Grenoble, France
Introduction: Substantial placebo response has been observed in trials assessing treatments in Raynaud’s Phenomenon (RP), which makes any treatment effect difficult to detect. However, whether this response is due to a real placebo effect or to other nonspecific effects, such as regression towards the mean (RTM), has not been explored.
Objectives: Our objectives were to explore and quantify placebo response in RP, and to evaluate the magnitude of RTM contribution.
Methods: We combined trial-level and individual-level data from a series of n-of-1 trials and a network meta-analysis, respectively. Main outcomes were the daily frequency and the mean duration of RP attacks, as well as the Raynaud’s Condition Score (RCS). We estimated the placebo response by the mean difference between the placebo period (or arm) and the baseline. RTM was estimated by the relationship between placebo response and baseline, and with Galton squeeze plots. Finally, we simulated the effect of the threshold used for inclusion in clinical trials on RTM.
Results: We observed a large and significant placebo response from both individual and trial data for RCS [-1.20 (-1.63, -0.77) and -0.65 (-0.89, -0.41)] and the daily frequency of RP [-0.61 (-0.85, -0.37) and -0.75 (-0.95, -0.54)]. Outcome at baseline was significantly associated with placebo response, suggesting the presence of RTM. The latter was confirmed on individual data, through Galton squeeze plots (Figure).
Conclusion: Placebo response is large in RP trials, and likely due to regression towards the mean rather than ‘true’ placebo effect. This should be carefully considered when designing future trials.
Figure legend. Galton squeeze plots representing z-score for each patient (thin lines) and by quartile (bold lines), at baseline and on placebo. The p-value of the quartile*time interaction was <0.001 for all three outcomes.
Tabel/Image
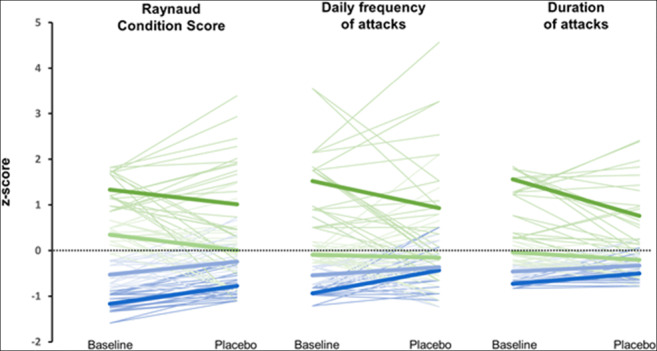
282
EFFECTS OF CYP3A4 GENETIC POLYMORPHISMS ON THE EFFICACY AND SAFETY PROFILES OF DIAZEPAM IN PATIENTS WITH ALCOHOL WITHDRAWAL SYNDROME
Skryabin V1,2, Zastrozhin M1,2
1 Moscow Research And Practical Centre For Narcology, Moscow, Russian Federation
2 Russian Medical Academy for Continuous Professional Education, Moscow, Russian Federation
Background: Diazepam therapy is often ineffective and some patients suffer from dose-dependent adverse drug reactions, reducing the efficacy of the therapy of alcohol withdrawal syndrome. The presence of some polymorphic markers of CYP3A4 decreases the amount of isoenzyme to be expressed or reduces its activity resulting in the changes biotransformation and elimination rates of the medication. Currently there is no data on correlation between the CYP3A4 genetic polymorphisms and efficacy and safety of diazepam among the Russian patients.
Objective: To investigate the effects of CYP3A4 genetic polymorphisms on the efficacy and safety of diazepam in patients with alcohol withdrawal syndrome in order to develop the algorithms of optimization of diazepam therapy for reducing the risk of dose-dependent undesirable side effects and pharmacoresistance.
Methods: The study involved 95 male patients (average age: 36.42±9.72 years) with alcohol withdrawal syndrome who were hospitalized in Moscow Research and Practical Centre of Addictions of the Moscow Department of Healthcare. Inclusion criteria were 5-day diazepam therapy in intramuscular (IM) injections and age of 18 to 75. Exclusion criteria were presence of any antipsychotics in the treatment regimen, creatinine clearance values <50 mL/min, creatinine concentration in plasma ≥1.5 mg/dL (133 mcmol/L); body weight less than 60 kg or greater than 100 kg; and presence of any contraindications for diazepam use. Venous blood samples collected in vacuum tubes VACUETTE® (Greiner Bio-One, Austria) on the sixth day of the diazepam therapy were used for genotyping. The real-time polymerase chain reaction was performed using DNA amplifiers “Dtlite” by DNA Technology (Moscow, Russia) and CFX96 Touch Real Time System with CFX Manager software of Bio-Rad Laboratories Inc. (USA) and sets “SNP-screen” by “Syntol” (Russia). A series of psychometric scales were used in the research. Genotyping of C>T intron 6 of CYP3A4*22 (rs35599367) was performed using the real-time polymerase chain reaction.
Results: According to results of U-test Mann-Whitney, statistically significant differences between the efficacy and safety of diazepam were obtained on the 1st and 6th days of therapy in patients with CC and CT+TT genotypes (Differences in mean CIWA-Ar scores: -9.0 [-12.0; -7.0] for CC genotype carriers vs -13.0 [-14.25; -12.75] (p < 0.001) for CT and TT genotype carriers; differences in mean Udvald for Kliniske Undersogelser Side Effect Rating Scale scores: 8.0 [6.0; 9.0] (p < 0.001) for CC genotype carriers vs 10.0 [9.5; 12.0] for CT and TT genotype carriers. The results of our study should be taken into consideration when prescribing diazepam to patients with alcohol withdrawal syndrome since it will allow increasing the efficacy of the therapy and decreasing the risk of undesirable side effects.
Conclusion: This study demonstrated the higher efficacy and lower safety of diazepam in patients with alcohol withdrawal syndrome carrying the CT and TT genotypes of CYP3A4*22 intron 6 C>T polymorphism (rs35599367). This should be considered when prescribing this medication to such patients to reduce the risk of undesirable side effects and pharmacoresistance.
S7a: Mixed Oral Presentations (3)
22
RISK OF SKIN ULCER AND USE OF COX-2 INHIBITORS: A NATIONAL POPULATION-BASED NESTED CASE CONTROL STUDY
Perez J1, Roustit M1, Bezin J2, Blaise S1, Cracowski J1, Khouri C1
1 Grenoble Alpes University Hospital, Grenoble, France
2 Bordeaux University Hospital, Bordeaux, France
Introduction: Cardiovascular adverse effects of COX-2 inhibitors are well described. Nevertheless, no study explored the cutaneous and vascular adverse effects of COX-2 inhibitors, in particular the risk of skin ulcer.
Objective: To identify and quantify the risk of skin ulcer (diabetic, pressure, venous or mixed) associated with COX-2 inhibitors in real world setting.
Design: A population-based nested case control study was conducted from a representative sample of the French nationwide claims database for the 2012-2016 period.
Setting: This database includes a 1/97th random sample of the population covered by the French healthcare insurance system.
Participants: Cases were defined as patients who had consumed at least two boxes of wound dressing during one month between 2012 and 2016. The index date was the date of first period of reimbursement of wound dressing for skin ulcer. Up to four potential controls were selected for each case among subjects free from skin ulcers.
Exposure: Exposure to COX-2 inhibitors was estimated using dispensing data. Cumulative exposure was expressed by Defined Daily Dose and anteriority of exposure by the Coverage End Date.
Main Outcomes and Measures: Conditional logistic models were used to estimate adjusted odds ratios (aORs) and their 95% CIs. Cumulative exposure was considered using quartile as a categorical variable (0 to 14 days, 14 to 30 days, 30 to 90 days, ≥ 90 days and non-exposed). The anteriority of exposure was defined using a categorical variable (exposure during the period of 8 months before the index date: « recent users », the exposition overlap the index date: « current users » and exposure more than 8 months before the index date: « past users »).
Results: We identified 2237 cases of diabetic foot ulcers and 8948 controls, 4225 cases of pressure ulcers and 16900 controls and 32246 cases of venous or mixed ulcers and 128902 controls, fulfilling the inclusion criteria. An increased risk of all types of ulcers was found for a cumulative exposure to COX-2 inhibitors exceeding 90 days with aOR= 1.35 (95% CI 1.03-1.77) for diabetic foot ulcer, aOR= 2.04 (95% CI 1.63-2.56) for pressure ulcers, aOR=1.89 (95% CI 1.71-2.11) for venous or mixed ulcers. Current exposure was associated with a higher risk of skin ulcer with aOR=1.63 (95% CI 1.06-2.49) for diabetic foot ulcer, aOR= 2.20 (95% CI 1.52-3.19) for pressure ulcers, aOR: 3.0 (95% CI 2.57-3.52) for vascular ulcers.
Conclusions and Relevance: Our study suggests a risk of diabetic foot, venous or mixed and pressure ulcer associated with the use of COX-2 inhibitors. Given the observational nature of this study, we cannot exclude unmeasured and residual confounding.
30
Pharmacokinetics and pharmacodynamics of intravenous and oral fexuprazan, a potassium-competitive acid blocker, after single administration in healthy Korean and Chinese
Park J1, Jeong S1, Kim B1, Cho J1, Lee S1, Lee A2, Jang I1
1 Department of Clinical Pharmacology and Therapeutics, Seoul National University College of Medicine and Hospital, Seoul, Republic of Korea
2 Daewoong Pharmaceutical Co., Seoul, Republic of Korea
Introduction: Gastroesophageal reflux disease(GERD) is rising in Asian countries and proton pump inhibitors (PPIs) is mainly used to decrease gastric acid secretion so far. However, PPI has several limitations like slow onset, weak acid suppression and drug-drug interactions. Fexuprazan is a novel potassium-competitive acid blocker that is under clinical development.
Objectives: This study aimed to compare the pharmacokinetics (PK), pharmacodynamics (PD), and safety profile of intravenous (IV) and oral formulation of fexuprazan 40 mg in healthy subjects.
Methods: A randomized, open-label, single-dose, two-way, two-period, crossover study was conducted in six Korean and two Chinese subjects. Subjects received a fexuprazan 40 mg via 3-minute IV infusion or oral route with 7 days of washout in between each dose. Serial blood samples were collected for PK analysis and 24-hour ambulatory intragastric pH monitoring was performed for PD analysis. Tolerability was evaluated throughout the study.
Results: All subjects completed the study as planned. The mean oral bioavailability of fexuprazan 40 mg was 0.46. After treatment of IV fexuprazan, the overall PD effect was greater and the onset of action was faster than after treatment of oral fexuprazan. The mean percentage of time with pH≥4 (%Time≥4) were 71.6% and 47.5% after intravenous and oral dose, respectively (p = 0.0037). The %Time≥4 before lunch (up to 4 hours post-dose) were 80.32% and 58.92% after intravenous and oral dose, respectively (p < 0.0001). Korean and Chinese subjects showed similar results on both PK and PD parameters. Both of IV and oral formulations were well tolerated in the subjects and there were no subject with intolerable hypergastrinemia (serum gastrin > 200 pg/mL).
Conclusion: The absolute bioavailability of fexuprazan 40 mg intravenous formulation was about 2-fold higher than the oral formulation, and the intravenous formulation showed better PD effect than the oral formulation.
37
Effect of tacrolimus formulation (prolonged-release vs. immediate-release) on the extent of the pharmacokinetic interaction with St. John's wort
Teegelbekkers A1, Gümüs K1, Förster K1, Burhenne J1, Meid A1, Weiß J1, Haefeli W1, Czock D1
1 Heidelberg University Hospital, Heidelberg, Germany
Introduction: Tacrolimus, an often-used immunosuppressant, is metabolized by cytochrome P450 3A (CYP3A) and is susceptible to interaction with the CYP3A4 and P-glycoprotein inducer St. John’s wort (SJW). CYP3A enzymes are predominantly expressed in the small intestine and liver. Prolonged-release tacrolimus formulations are absorbed in more distal intestinal sections and can potentially bypass first-pass metabolism, especially Envarsus®, a tacrolimus formulation that is absorbed largely in the colon and is considerably less susceptible to CYP3A inhibition by voriconazole.
Objectives: To analyse the effect of SJW on tacrolimus pharmacokinetics using immediate-release (Prograf®; IR-Tac) and prolonged-release (Envarsus®; PR-Tac) formulations and to explore whether individual factors (CYP3A4 activity estimated with a midazolam microdose, CYP3A5 genetic polymorphism) correlate with the pharmacokinetic changes.
Methods: In a randomized, cross-over, phase I clinical trial with 4 study periods, 18 healthy volunteers (including 7 CYP3A5 expressors) received a single oral tacrolimus dose (IR-Tac or PR-Tac, 5 mg each) alone or during SJW. The administration of SJW (300 mg TID) started 10 days before tacrolimus in the SJW periods and was continued for 3 more days. Concentrations were quantified using UPLC-MS/MS methods. Pharmacokinetics were analysed by non-compartmental methods.
Results: SJW decreased IR-Tac exposure (AUC) 0.73-fold (90% CI 0.60–0.88) and maximum concentration (Cmax) 0.61-fold (0.52-0.73). With PR-Tac, the decrease in AUC was 0.67-fold (0.55–0.81) and Cmax 0.69-fold (0.58-0.82), with no statistical difference between the two formulations (p = 0.60). Higher baseline CYP3A4 activity and presence of a functional CYP3A5*1 allele both appeared to attenuate the extent of the interaction.
Conclusions: In contrast to CYP3A inhibition, CYP3A induction by SJW showed a comparable extent of interaction with both tacrolimus formulations. Individuals with larger metabolic capacity and CYP3A5 expressors appeared to be less susceptible to or even protected from CYP3A induction by SJW, which might be explained by presystemic SJW metabolism or by limited inducibility in individuals with already high metabolic capacity.
53
Genetic variability in phospholipase genes affect nephrosclerosis risk and associated cardiovascular outcomes
Gonzalez L1, Robles N2, Mota-Zamorano S1, Arévalo-Lorido J3, Valsivielso J4, Lopez-Gomez J5, Gervasini G1
1 Dpt of Medical and Surgical Therapeutics, Institute of Molecular Pathology Biomarkers, University of Extremadura, Badajoz, Spain
2 Service of Nephrology, Badajoz University Hospital, Badajoz, Spain
3 Service of Internal Medicine, Badajoz University Hospital, Badajoz, Spain
4 Vascular and Renal Translational Research Group, UDETMA, ISCIII REDinREN, IRBLleida, Lleida, Spain
5 Service of Clinical Analyses, Badajoz University Hospital, Badajoz, Spain
Introduction: Nephrosclerosis patients have a high cardiovascular (CV) risk that is very often of more concern than the renal disease itself.
Objetives. We aimed to determine whether variants in phospholipase-related genes, associated with atherosclerosis and CV outcomes in the general population, could constitute biomarkers of nephrosclerosis and/or its associated CV risk.
Methods: We screened 1209 nephrosclerosis patients and controls for 86 tag-SNPs that were identified in the SCARB1, PLA2G4A and PLA2G7 gene loci. Regression models were utilized to evaluate their effect on several clinical parameters.
Results: Most notably, rs10846744 and rs838880 in SCARB1 showed significant odds ratios (OR) of 0.66 (0.51-0.87), p=0.003 and 1.48 (1.11-1.96), p=0.007 for nephrosclerosis risk. PLA2G4A and PLA2G7 harboured several SNPs associated with atherosclerosis measurements in the patients, namely common carotid intima media thickness (ccIMT), presence of plaques, number of plaques detected and 2-year ccIMT progression (significant p-values ranging from 0.0004 to 0.047). Eight SNPs in PLA2G4A were independent risk factors for CV events in nephrosclerosis patients. Their addition to a ROC model containing classic risk factors significantly improved its predictive power from AUC=69.1%(61.4-76.9) to AUC=79.1%(73.1-85.1%), p=0.047. Finally, PLA2G4A rs932476AA and rs6683619AA genotypes were associated with lower CV event-free survival after controlling for confounding variables [49.59 (47.97-51.21) vs. 51.81 (49.93-51.78) months, p=0.041 and 46.46 (41.00-51.92) vs. 51.17 (50.25-52.08) months, p=0.022, respectively].
Conclusion: Variability in phospholipase-related genes play a relevant role in nephrosclerosis and associated atherosclerosis measurements and CV events.
64
The identification of adverse drug reactions in medical hospital admissions: A one month prospective analysis
Osanlou R1,2, Walker L1,2, Pirmohamed M1,2
1 Department of Pharmacology and Therapeutics, University of Liverpool, United Kingdom
2 Liverpool University Hospital Foundation NHS Trust, United Kingdom
Introduction: Adverse drug reactions (ADRs) are an important cause of morbidity and mortality, with significant health implications and associated economic burden. Identification of adverse reactions is critical for appropriate and timely management to reduce their severity and improve outcomes.
Objectives: To assess one full month of medical hospital admissions determining the number of adverse drug reactions and if they were identified by the medical team.
Methods: Data was prospectively collected for one month at Liverpool University Hospital Foundation NHS Trust, England. Patient notes, observations, prescriptions and investigations were reviewed to determine if an ADR occurred by two clinical pharmacologists and a third when agreement was not met. ADR definition as per Edwards and Aronson criteria. The Liverpool causality assessment tool (LCAT) was used to assess the likelihood of potential missed ADRs. Evidence of identification of an ADR by the admitting team included explicit documentation, reduction or withdrawal of suspected causative medicine, or treatment with a reversal agent.
Results: From 1,187 admissions we identified 235 adverse drug reactions in 218 (18.4%) patients. Out of the 235 ADRs, 177 (75.3%) were identified by the medical team. Of the 58 not identified by the medical team, 42 were possible ADRs, 10 probable and 6 definite.
Conclusion: Up to a quarter of adverse drug reactions are not identified by the admitting medical team but were identified by a clinical pharmacologist. Most of the missed adverse events were rated as possible ADRs using LCAT criteria, with only 16 (6.8%) rated probable or definite ADRs that were missed.
Further efforts are required to improve pharmacovigilance in medical admissions. The study suggests clinical pharmacologists could play a role in increasing the identification of adverse drug reactions in medical hospital admissions.
80
Comprehensive pharmacogenomics study of simvastatin pharmacokinetics: major role of SLCO1B1 and CYP3A4
Mykkänen A1,2, Taskinen S1,2, Neuvonen M1,2, Paile-Hyvärinen M1,2, Tarkiainen K1,2, Lilius T1,2, Tapaninen T1,2, Backman J1,2, Tornio A1,2, Niemi M1,2
1 Department of Clinical Pharmacology, University of Helsinki and Helsinki University Hospital, Helsinki, Finland
2 Individualized Drug Therapy Research Program, University of Helsinki, Helsinki, Finland
Introduction: Simvastatin is a 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitor widely used in the treatment of hypercholesterolemia. In its pharmacokinetics, marked interindividual variability exists, and genetic variation affects simvastatin exposure and adverse-effect risk.
Objectives: The aim of this study was to comprehensively characterize the effect of genetic variation on simvastatin pharmacokinetics by use of genome-wide methods.
Methods: We investigated the single-dose pharmacokinetics of simvastatin in a prospective clinical trial of 170 and a retrospective cohort of 59 healthy Finnish volunteers. The participants were genotyped using a genome-wide microarray.
Results: In a genome-wide association study with the prospective data, the SLCO1B1 c.521T>C (p.Val174Ala, rs4149056) single nucleotide variation (SNV) showed the strongest, genome-wide significant association with the area under the plasma simvastatin acid concentration-time curve (AUC) (P=6.0×10-10). Meta-analysis with the retrospective cohort strengthened the association (P=1.6×10-17). In a candidate gene analysis, SLCO1B1 c.521T>C (P=1.9×10-13) and the CYP3A4 c.664T>C (p.Ser222Pro, rs55785340, P=0.023) were associated with increased simvastatin acid AUC. Moreover, the SLCO1B1 c.463C>A (p.Pro155Thr, rs11045819, P=7.2×10-6) and c.1929A>C (p.Leu643Phe, rs34671512, P=5.3×10-4) missense variants associated with decreased simvastatin acid AUC. Based on these results, we classified the volunteers into genotype-predicted OATP1B1 and CYP3A4 phenotype groups. Compared to the normal OATP1B1 function group, simvastatin acid AUC was 273% higher in the poor (90% confidence interval, 137%, 488%; P=3.1×10-6), 40% higher in the decreased (8%, 83%; P= 0.036), and 67% lower in the highly increased group (46%, 80%; P=2.4×10-4). Heterozygous carriers of either CYP3A4 c.664T>C (CYP3A4*2) or CYP3A4*22 (rs35599367) were classified as intermediate CYP3A4 metabolizers. They had 87% (39%, 152%, P=6.4×10-4) larger simvastatin acid AUC than normal metabolizers.
Conclusions: Genetic variability in SLCO1B1 and CYP3A4 plays an important role in simvastatin pharmacokinetics, and may affect the efficacy and safety of simvastatin therapy.
S7b: Psychopharmacology
126
A randomized controlled trial to evaluate the effect of alpha lipoic acid in patients with treatment resistant schizophrenia
Mishra A1, Reeta K1, Sarangi S1, Sood M1
1 All India Institute Of Medical Sciences, New Delhi, India
Introduction: Treatment resistant schizophrenia (TRS) has been defined as persistence of symptoms despite at least two antipsychotics trial of adequate dose and duration and has been prevalent in approximately 30% of patients with schizophrenia. Though the exact pathophysiology is unknown; NMDA receptor hypofunction, dopamine receptor sensitivity, increase in oxidative stress markers has been found in patients with TRS. Clozapine is the only drug approved for TRS and helps in relief in approximately 60-70% patients. Alpha lipoic acid, an antioxidant has been shown to decrease oxidative stress, cause downregulation of dopamine receptors, reverse NMDA receptor hypofunction and increase neurotrophic factors.
Objectives: This study evaluates the effect of alpha lipoic acid (ALA) on psychopathological scores (positive, negative, cognitive), neurotrophic factors and oxidative stress.
Methods: A randomized double-blind placebo-controlled parallel design trial was conducted in 20 patients with treatment-resistant schizophrenia (TRS). After initial screening, participants were randomized into test (add-on ALA) and control (add-on placebo) groups. After recruitment, clinical evaluations with scale for assessment of positive symptoms and negative symptoms (SAPS & SANS), schizophrenia cognitive rating scale (SCoRS), UKU side effect rating scale were done. Serum levels of BDNF, MDA, and GSH were estimated. Patients were followed up for 8 weeks, and clinical and biochemical evaluations were repeated. Adherence to medication was evaluated at follow up.
Results: A significantly greater improvement was found in SANS score in the test group when compared to control (4.671; 95% CI: 1.36 to 7.98; p = 0.008), whereas there was no significant improvement in SAPS score (Mann Whitney U = 41.5; p = 0.780). A significant increase in BDNF levels was observed in the control group when compared to ALA (U = 20.0; p = 0.041). No significant differences were found between the test and control groups in serum MDA (0.114; 95% CI -0.104 to 0.332; p = 0.282), serum GSH (-0.748; 95% CI -3.79 to 2.29; p = 0.611) and medication adherence rating scale (MARS) scores (0.156; 95% CI -1.763; p=0.866). However, serum MDA levels decreased significantly in add-on ALA group when compared to baseline values. There was a significant difference in number of responders in test group when compared to control group (p = 0.033).
Conclusion: ALA supplementation improved psychopathology and decreased oxidative stress in patients with TRS. This study thus shows the potential of adjunctive ALA in TRS.
129
Use of maximal urine concentrating capacity markers to evaluate lithium-induced nephrogenic diabetes insipidus
Zittema D1, van der Aa M2, Doornebal J3, Klumpers U4, Bisseling E5, Kupka R4, Nijenhuis T6, Kerckhoffs A2
1 Amsterdam UMC, Amsterdam, The Netherlands
2 Jeroen Bosch Ziekenhuis, Den Bosch, The Netherlands
3 Isala, Zwolle, The Netherlands
4 GGZ inGeest, Amsterdam, The Netherlands
5 Canisius Wilhelmina Hospital, Nijmegen, The Netherlands
6 Radboudumc, Radboud, The Netherlands
Introduction: Lithium is the cornerstone in pharmacological treatment of patients with bipolar disorder. A common early renal side effect of lithium treatment is nephrogenic diabetes insipidus (NDI), which refers to a decreased maximal urine concentrating capacity causing polyuria (urine production >3L per day) and polydipsia. Long term lithium therapy can also lead to chronic kidney disease (CKD) with a decrease in glomerular filtration rate (GFR) and in rare cases end stage kidney disease. To evaluate NDI, a water deprivation test or DDAVP test is performed to determine maximal urine concentrating capacity, but this is time consuming and therefore not feasible in clinical practice. Consequently, NDI is probably underdiagnosed in this population with risk of significant interference of patient daily routine and occupational activities and possible irreversible kidney damage. Moreover, feasible diagnostic tools for NDI could lead to better understanding of the correlation between NDI and CKD.
Objectives: To evaluate feasible markers to determine maximal urine concentrating capacity to evaluate lithium-induced NDI in clinical practice.
Methods: 98 patients with a mood disorder treated with lithium at the outpatient psychiatry clinics of the Canisius Wilhelmina Hospital (CWZ), Nijmegen and GGZ inGeest Mental Health Center, Amsterdam, the Netherlands, underwent a desmopressin (DDAVP) test at to determine maximal urine concentrating capacity after inclusion in 2012/2013. Urine and blood samples were stored frozen for subsequent measurement of plasma copeptin, a precursor of vasopressin, and urine biomarkers for tubular injury AQP-2, α1M, NGAL, KIM-1 and NAG to study the association with maximal urine concentrating capacity. Next to these biomarkers, urine urea and plasma urea was measured to calculate the urine-to-plasma urea ratio (UPU) to study the association with maximal urine concentrating capacity as well. Urine samples for biomarker measurement were collected as spot samples and corrected for urine creatinine. Multivariable lineair regression models were used to test associations between different variables. Non lineair variables were log (LN) transformed to attain normal distribution.
Results: Demographics of the 98 DDAVP test participants are depicted in the table. 50 patients (51%) had an impaired maximal urine concentrating capacity (urine osmolality 600-800 mOsmol/kg) and 17 patients (17%) had NDI (urine osmolality <600 mOsmol/kg). Baseline eGFR, UPU, copeptin and NGAL were significantly associated with maximal urine concentrating capacity (St. β=0.44, p<0.001, St. β=0.30, p=0.003; St. β=-0.36, p<0.001; St. β=-0.25, p=0.01, respectively). UPU, copeptin and NGAL remained significantly associated after adjustment for age, sex and eGFR (St. β= 0.22, p=0.02; St. β= -0.22, p<0.03; St. β= -0.26, p<0.01). Other urine biomarkers were not associated with maximal urine concentrating capacity.
Conclusion: UPU, copeptin and NGAL were associated with maximal urine concentrating capacity, independent of eGFR, and therefore show promising value to screen for lithium-induced NDI. If an association between maximal urine concentrating capacity and kidney function decline were to be demonstrated, these markers could be used to identify patients at risk for kidney function decline.
Tabel/Image
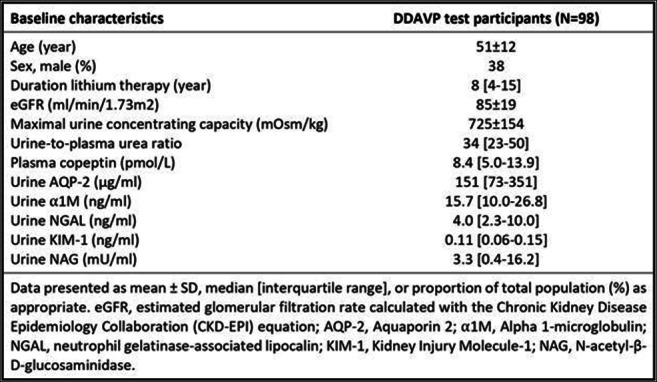
S7c: Physiology-based pharmacokinetic modeling to guide personalized drug dosing
63
Pharmacokinetics and pharmacodynamics of HIP1601, a novel dual delayed-release esomeprazole, compared to a conventional delayed-release esomeprazole in healthy male subjects
Kim H1, Yang E1, Park Y1, Jeong S1, Lee S1, Cho J1, Jang I1
1 Department of Clinical Pharmacology and Therapeutics, Seoul National University College of Medicine and Hospital, Seoul, Republic of Korea
Introduction: Esomeprazole has short plasma half-life which can cause insufficient gastric acid suppression during the nighttime in some patients. To overcome the shortcoming of the esomeprazole, a novel dual delayed-release formulation of esomeprazole (HIP1601) was developed.
Objectives: This study aimed to compare the pharmacokinetics (PKs) and pharmacodynamics (PDs) of HIP1601 and conventional delayed-release esomeprazole formulation (Nexium®).
Methods: Two (part A and B) randomized, open-label, multiple doses, two-way crossover studies were conducted. Subjects received HIP1601 or Nexium® 40 mg in part A and 20 mg in part B, respectively, once daily for 7 days in each period with 7-day washout in between the periods. Serial plasma concentration samples were collected for 24 hours after the first dose. Intragastric pH was continuously monitored for 24 hours on day -1 (baseline), 1 and 7 in each period.
Results: A total of 44 and 38 subjects completed the study in Part A and B, respectively. The dual-release pattern was observed after treatment of HIP1601, and it exhibited more sustained plasma concentration-time profiles compared to Nexium®. After the 1st and 7th dose of HIP1601 and Nexium®, the geometric mean ratios and its 90% confidence intervals for the area under the plasma concentration-time curve and for percent decrease from baseline in integrated gastric acidity during 24 hours were within the bioequivalence range of 0.80 – 1.25. In part A, the percent of nocturnal acid breakthrough (NAB) after the 7th dose were 15% and 22% for HIP1601 and Nexium®, respectively and those values were 34% and 42% in part B.
Conclusion: The overall plasma exposure and PD profile of HIP1601 were comparable to Nexium®, but the rate of NAB was significantly lower after treatment of HIP1601 than the Nexium®.
110
Evolution of 25(OH)D plasma levels in adults with hypovitaminosis D after a 4-month treatment with calcifediol: An Interim Analysis
Guerra López P1, Vega-Gil N2, Sánchez Santiago B2, Zorrilla Martínez I3,4, Jiménez-Mercado M3, García-Bea A5, Landeta Manzano A5, Campo Hoyos C5, Frías Iniesta J1,6
1 Clinical Trials Unit, Pharmacology Department, Universidad Autónoma de Madrid, Madrid, Spain
2 Valdecilla Clinical Trials Unit, Hospital Universitario Marqués de Valdecilla- IDIVAL, Santander, Spain
3 Clinical Trials Unit, IIS BIOARABA, OSI Araba, Vitoria, Spain
4 Universidad del País Vasco (UPV-EHU), Spain
5 Faes Farma, Medical Affairs, Leioa, Spain
6 Clinical Pharmacology Service, Hospital Universitario La Paz, Madrid, Spain
Introduction: Vitamin D deficiency is a common condition worldwide, with increasing evidence of its influence in a variety of extra-skeletal diseases. Calcifediol, direct precursor of calcitriol, is the only vitamin D metabolite used to determine the vitamin D status of a subject and a treatment of choice for hypovitaminosis D.
Objectives: The aim of this phase I multicenter clinical trial was to assess two different treatment schedules (monthly or biweekly) according to subjects’ baseline 25(OH)D level in terms of the percentage of adult population with vitamin D deficiency (25(OH)D <20 ng/mL) who achieved plasmatic levels within the optimal range (20-60 ng/mL) after a 4-month treatment with calcifediol 0.266 mg soft capsules.
Methods: Adult volunteers (aged 18-55) with hypovitaminosis D were supplemented with calcifediol 0.266 mg capsules for 4 months: severe deficiency (25(OH)D <10 ng/mL) was supplemented biweekly, while mild-moderate deficiency (25(OH)D 10-19.99 ng/mL) was supplemented monthly. Once within the optimal range, subjects were randomly allocated to monthly treatment (placebo or calcifediol 0.266 mg), for 5 additional months.
Results: 79 subjects (65% female, mean age of 31.2 years) were included in the present interim analysis; 8.9% with severe vitamin D deficiency and 91.1% with mild-moderate vitamin D deficiency. Two subjects were excluded according to the study protocol.
Regarding the primary endpoint, 79% of subjects achieved optimal 25(OH)D levels (>20 ng/mL) at month 4, (100% in biweekly and 78% in monthly treatment groups, respectively). When the threshold was increased to >30 ng/mL approximately 40% of subjects reached target levels (100% of the volunteers in the biweekly treatment group).
In the severe deficient group, baseline 25(OH)D levels of 8.35 ng/mL increased by 17.05 ng/mL and 33.05 ng/mL, at month 1 and month 4, respectively. In the mild-moderate deficient group, baseline 25(OH)D levels (14.42 ng/mL) increased by 5.78 ng/mL at month 1 and by 12.18 ng/mL at month 4. In the overall population, the baseline 25(OH)D average level was 14.02 ng/mL and increased by 6.51 ng/mL (month 1) and by 13.54 ng/mL (month 4), reaching a final average level of 27.56 ng/mL at month 4.
Several bone metabolism parameters, including calcium, PTH, albumin, phosphate, etc. were analysed in all subjects, levels increased slightly at month 1, showing no significant changes throughout the study.
Treatment with calcifediol was safe and well tolerated, no patient reached risk toxic 25(OH)D levels, nor serious adverse events were reported.
Conclusion: Calcifediol 0.266 mg soft gelatine capsules can be administered biweekly or monthly, in adults with vitamin D severe or mild to moderate deficiency, according to the increase of serum 25(OH)D levels needed to achieve the optimal range for vitamin D normalization. Calcifediol has shown to be an effective and safe treatment, without toxicity related to high 25(OH)D levels or safety concerns associated to significant variations in bone metabolism parameters.
POSTER ABSTRACTS
Advanced therapies
31
EFFECT OF FOOD ON PHARMACOKINETICS AND SAFETY OF DA-8010, A NOVEL SELECTIVE MUSCARINIC RECEPTOR ANTAGONIST, IN HEALTHY SUBJECTS
Khwarg J1, Yang E1, Chung W1, Park S1, Jang I1, Cho J1, Ryu C2, Lee D2, Lee M2, Kim H3, Lee S1
1 Department of Clinical Pharmacology and Therapeutics, Seoul National University College of Medicine and Hospital, Seoul, Republic of Korea
2 Dong-A ST Research Institute, Yongin, Republic of Korea
3 Dong-A ST Co Ltd, Seoul, Republic of Korea
Introduction: DA-8010 is a novel selective muscarinic receptor antagonist under development for the treatment of overactive bladder. Compared to other muscarinic receptor antagonists, DA-8010 is expected to reduce the side effects (including dry mouth), owing to its high selectivity for muscarinic receptors in urinary bladder.
Objectives: The objective of this study was to evaluate the effect of food on the pharmacokinetics (PK) and safety of DA-8010 in healthy subjects.
Methods: A randomized, open-label, single-dose, 4-sequence, 4-treatment, 4-period crossover study was conducted. According to their randomly assigned sequences, subjects received a single oral dose of 2.5 mg or 5 mg DA-8010 in fasted or fed (high-fat meal) state in each period with a 7-day washout. Blood samples for PK analysis were collected up to 48 h post-dose. PK parameters including maximum plasma concentration (Cmax) and area under the time-concentration curve from zero to the last measurable point (AUClast) were calculated using a non-compartmental analysis, and were compared between fed and fasted states. Safety data were also collected and evaluated.
Results: 29 subjects were randomized, and 27 subjects completed the study. Under fed condition, systemic exposure of DA-8010 increased. Mean Cmax and AUClast of DA-8010 showed 2.3- and 1.5-fold increase for the 2.5mg dose, respectively, and 2.0- and 1.3-fold increase for the 5 mg dose, respectively, when administered under fed condition compared to fasted condition. The times to reach Cmax were comparable regardless of dose or food intake, showing median values of 4.5-5.0 hours. No clinically significant changes were also observed in safety parameters regardless of food intake.
Conclusion: These results suggest a modest increase of the extent of DA-8010 absorption by food intake, but the extent of food effect of DA-8010 was difficult to be considered clinically significant. DA-8010 was safe and well tolerated, regardless of food intake.
Antimicrobials
43
SERBIAN HEALTH PROFESSIONS STUDENTS’ KNOWLEDGE, ATTITUDES AND BEHAVIOUR TOWARDS ANTIBIOTIC USE: IS THERE ROOM FOR IMPROVEMENT?
Horvat O1, Tomas A1, Paut Kusturica M1, Bukumirić D2, Rašković A1, Jovančević B1, Kovačević Z3
1 Faculty of Medicine, Department of Pharmacology Toxicology and Clinical Pharmacology, University of Novi Sad, Novi Sad, Serbia
2 Department of Planning, Analyzing and Statistics, Primary Health Care Center, Pančevo, Serbia
3 Faculty of Agriculture, Department of Veterinary Medicine, University of Novi Sad, Novi Sad, Serbia
Introduction: The complex problem of antimicrobial resistance (AMR) requires actions taken with the One Health approach, involving both human and veterinarian medicine. It can spread from animals to humans through the food chain or direct contact. Health professions students, as the future antibiotic providers, can greatly impact antibiotic-related issues in the future.
Objectives: The study was conducted to evaluate knowledge, attitudes and behaviour of prospective antibiotic prescribers in relation to prudent use of antibiotics.
Methods: This cross-sectional questionnaire-based study was performed on 400 students of health professions allowed to prescribe antibiotics (Medicine (M), Dentistry (D) and Veterinary medicine (V)) of the University of Novi Sad, Serbia.
Results: M and D students showed a significantly higher knowledge score compared to V students (p = 0.001). Multivariate regression identified the following predictors of adequate antibiotic knowledge: being female student (B=0,571; p=0,020)), higher grade average (B=1,204; p=0,001), students of M (B=0,802; p=0,006) and D (B=0,769; p=0,026), and students who used antibiotics during the last infection until the bottle was finished (B=0,974; p=0,001) or for the period advised by the doctor (B=1.964; p=0.001). Out of the total sample, self-medication was reported among 42.8% of students. The identified predictors of of self-medication were: more frequent (B=0,587; p=0.001) and irregular (B=0,719; p=0,007) antibiotic use, using antibiotics until symptoms resolved (B=2,142; P=0.001) or until the bottle was finished (B=1,010; p=0.001) during the last infection.
Conclusion: It seems prudent to to re-evaluate the educational curricula regarding antibiotic use and AMR in Serbia, specifically teaching of clinical pharmacology. This work was supported by the Ministry of Education, Science and Technological Development, the Republic of Serbia, project No
III 41012; and the Provincial Secretariat for Higher Education and Scientific Reasearch project No 142-451-2574//2021-01/01].
79
eHealthResp: educational intervention to improve antibiotic use in primary care – a protocol for a pilot study
Herdeiro M1, Magalhães Silva T1, Rocha V1, Figueiras A2,3,4, Roque F5,6, Herdeiro M1
1 Institute of Biomedicine (iBiMED) and Department of Medical Sciences (DCM), University of Aveiro, Aveiro, Portugal
2 Department of Preventive Medicine and Public Health, Faculty of Medicine, University of Santiago de Compostela, Santiago de Compostela, Spain
3 Institute of Health Research of Santiago de Compostela (IDIS), Santiago de Compostela, Spain
4 Consortium for Biomedical Research in Epidemiology and Public Health (CIBER Epidemiology and Public Health—CIBERESP), Madrid, Spain
5 Research Unit for Inland Development, Polytechnic of Guarda (IPG-UDI), Guarda, Portugal
6 Health Sciences Research Centre, University of Beira Interior (CICS-UBI), Covilhã, Portugal
Introduction: Antibiotic resistances are among the most threatening public health issues worldwide, being highly associated with inadequate antibiotic use. To tackle this challenge, it is crucial to educate health professionals to appropriately prescribe and dispense antibiotics. Thus, out team developed eHealthResp, an educational intervention composed by two online courses and a clinical decision support system in the form of a mobile app directed to primary care physicians and community pharmacists, aiming to improve antibiotic prescribing and dispensing in respiratory tract infections.
Objectives: The main goal of this pilot study is to validate the eHealthResp online courses and the clinical decision support system (mobile app), involving a small group of health professionals.
Methods: Aproximately 15 physicians and 15 pharmacists will be recruited to participate in the study. Participants will have complete autonomy to explore and evaluate the eHealthResp mobile app and online courses, composed by six modules on respiratory tract infections for physicians (i) acute otitis media, ii) acute rhinosinusitis, iii) acute pharyngotonsilitis, iv) acute tracheobronchitis, v) community-acquired pneumonia, and vi) COVID-19), and three modules for pharmacists (i) common cold and flu, ii) acute rhinosinusitis, acute pharyngotonsilitis, and acute tracheobronchitis, and iii) acting protocol). Each online course is also composed by four clinical cases and the most recommended pharmacological therapy. Additionally, for the the global validation of the online course and the mobile app, participants will be invited to complete a questionnaire including three sections of questions. The first part, consisting of five brief questions, will allow the collection of sociodemographic data. The second part contains four groups of closed questions, and the third part consists of four open-answer questions, both aiming to evaluate the online course and mobile app elements.
Results: After the assessment made by the physicians and pharmacists who agreed to participate in the pilot study, the data obtained will be duly analyzed and integrated by the research team. The appropriate changes will be incorporated into the e-Health platforms to improve the quality of both the online courses and the eHealthResp mobile app.
Conclusions: The findings of this pilot study will provide important information for the next stage of the project, ensuring the feasibility of the educational interventions in a group of primary care physicians and community pharmacists from the Centre region of Portugal, using a randomized controlled trial designed by clusters.
182
Impact of COVID-19 pandemic on antimicrobial consumption in Croatia
Rubinić I1, Palčevski D1, Payerl-Pal M2, Vlahović-Palčevski V1,3
1 Clinical Hospital Centre Rijeka, Rijeka, Croatia
2 ISKRA, Ministry of Health, Zagreb, Croatia
3 Faculty of Health Studies, Rijeka, Croatia
Introduction: COVID-19 had a tremendous effect on every aspect of healthcare system during the past two years. It was uncertain how it will influence antimicrobial consumption (AMC) and consequently antimicrobial resistance (AMR). A lot of unnecessary antimicrobials were prescribed because of the fear of bacterial coinfections that were later proven to be sporadic. Contrastingly, preventive measures are responsible for a reduction in the incidence of respiratory infections that would have been treated with antimicrobials.
Objectives: To assess the impact of COVID-19 pandemic on AMC in the community and hospital sector in 2020 in Croatia in comparison to previous 4 years. It is necessary to assess AMC to adapt AMS programmes accordingly.
Methods: Data reported to European Surveillance of Antimicrobial Consumption Network and the official body of the Croatian Ministry of Health responsible for surveillance of antimicrobial consumption and resistance (ISKRA) were used. Antimicrobials were classified using Anatomical Therapeutic Chemical (ATC) classification and the consumption was expressed in DDDs per 1000 inhabitants per day for both community and hospital sector, and additionally in DDDs per 100 bed days for the hospital sector. Linear regression was conducted to assess 5-year trend. Statistical significance was reported if the p-value was ≤ 0.05.
Results: In the community sector, the 5-year trend for the consumption of antibacterials for systemic use (ATC group J01) did not change significantly, even though a decrease by 17% is reported in 2020 compared to 2019. Broad-spectrum AMC shows a statistically significant increase between 2016 and 2020 compared to narrow-spectrum AMC. Several antimicrobials show statistically significant change during the observed period.
In the hospital sector, the consumption of systemic antibacterials did not significantly change in the 5-year period when expressed in DDDs per 1000 inhabitants per day. Azithromycin consumption increased significantly. Contrastingly, when AMC is expressed in DDDs per 100 bed days, several ATC level 3 groups and several individual antibacterials show statistically significant changes.
Conclusion: COVID-19 pandemic caused an overall decrease in the consumption of antibacterials for systemic use but also the pattern. Hospital sector consumption expressed in DDDs per 1000 inhabitants per
243
ANALYSIS OF ANTIBIOTIC CONSUMPTION IN THE NATIONAL CENTER OF CHILDREN'S REHABILITATION FOR 2019-2020 IN THE CONDITIONS OF THE COVID-19 PANDEMIC
Bulekbayeva S1, Makalkina L1,2, Shakenov M1, Ikhambaeva A2, Seikenova Z1, Aldiyarova N2
1 National center for children’s rehabilitation, University medical center, Nur-Sultan, Kazakhstan
2 Astana Medical University, Nur-Sultan, Kazakhstan
3 Professional Association Of Clinical pharmacologist and pharmacists, Nur-Sultan, Kazakhstan
Introduction: The COVID-19 pandemic has posed a major challenge for all humanity and health systems in all countries. Excessive and unjustified use of antibiotics is one of the most important factors in the development of antibiotic resistance.
Objectives: to identify the dynamics of antibiotic consumption in a children's rehabilitation center during the COVID-19 pandemic for 2019-2020 and to identify factors affecting the incidence of infectious diseases and the consumption of antibiotics.
Methods: the calculation of antibiotic consumption was carried out according to the ATC / DDD methodology of the WHO Collaborating Center for Drug Statistics Methodology using the WHO database [4] to assign the ATC index and DDD values of drugs
Results: The total consumption of antibacterial agents for systemic use (group J01) showed a decrease in DBD in 2020 compared to 2019 by 40% and amounted to 39.29 DBD in 2019 and 28.04 DBD in 2020. When assessing dynamic consumption antibiotics on arrival, we have identified a downward trend in antibiotic consumption after the introduction of strict quarantine measures at the Center. That, combined with data for 2020 on a decrease in the number of patients who were prescribed antibiotics and a decrease in the number of antibiotics per patient, showed the high effectiveness of restrictive measures at the Center related to the COVID-19 pandemic.
Conclusions: the results of the study of antibiotic consumption for 2019-2020. according to the ATC / DDD methodology, it revealed an overall decrease in consumption in 2020 compared to 2019 by 40%, which is a positive result of the strict quarantine measures and an increase in the justification for prescribing antibiotics at the Center during the COVID-19 pandemic.
247
Longitudinal study on utilization of antibacterials in European Countries
Papaioannidou P1, Stergiopoulou T1
1 1st Department of Pharmacology, School of Medicine, Faculty of Health Sciences, Aristotle University of Thessaloniki, Thessaloniki, Greece
Introduction: Antimicrobial resistance is a global concern and is related to antimicrobial use. Aiming at reducing antimicrobial resistance and improving antimicrobial therapy, an effort has been made during the last decade, with the purpose to reduce antibacterial consumption in European Countries.
Objectives: The aim of this work was to study antibacterial utilization in various European countries, to calculate differences in antibacterial use between the years 2010 and 2019, and to evaluate the achievement in reducing antibacterial consumption.
Methods: Data on antibacterial consumption in 22 European countries were collected from the Organisation for Economic Cooperation and Development (OECD) data bases. Antibacterial consumption was expressed in defined daily dosages (DDDs) per 1,000 inhabitants per day, and calculations referred to years 2010 and 2019.
Results: In 2010, Greece, Luxemburg, Italy and Slovak Republic were the countries with the largest antibacterial consumption, ranging from 36 to 24 DDDs per 1,000 inhabitants per day. In 2019, Greece France and Spain were the countries with antibacterial consumption higher than 24 DDDs per 1,000 inhabitants per day. Among 22 European countries, 14 countries managed to lower antibacterial use between the years 2010 and 2019. Finland, Luxemburg and Slovak Republic were on the top of the list of countries that achieved to reduce antibacterial consumption by 27,6%, 26% and 21% respectively (corresponding to amounts of 5.6, 7.55 and 5.2 DDDs per 1,000 inhabitants per day, respectively).
Conclusion: During the study decade (2010-2019), 14 out of 22 European countries reduced antibacterial consumption. However, in 8 countries, an increase in antibacterial consumption was observed.
253
EVALUATING THE EFFICIENCY OF SPENDING ON ANTIMICROBIAL THERAPY IN THE PULMONOLOGY DEPARTMENT OF A MULTIDISCIPLINARY HOSPITAL IN A PANDEMIC SETTING
Utepova D1, Assan A2, Kerimbayeva Z1, Magzumova R1, Sychev D3, Tsoy L4, Aldiyarova N5
1 Astana Medical University, Nur-Sultan, Kazakhstan
2 Khoja Akhmet Yassawi International Kazakh-Turkish University, Turkestan, Kazakhstan
3 Russian Medical Academy of Continuous Professional Education, Moscow, Russian Federation
4 Regional Clinic Hospital, Shymkent, Kazakhstan
5 Professional Association Of Clinical pharmacologist and pharmacists, Nur-Sultan, Kazakhstan
Introduction: Antimicrobial resistance is recognized as one of the top 10 threats to public health. Due to recent circumstances with the 2019 Covid pandemic worldwide, the urgency of monitoring antibiotic consumption and rational use of medications has increased. According to WHO recommendations, countries should aim to increase the proportion of Access group antibiotics consumption to 60% and higher in AWaRe classification system (Access, Watch and Reserve). The ABC/VEN analysis (80%, 15%, 5% of spending) is the simplest and most relevant method for evaluating the effectiveness of antibiotic therapy expenditures.
Objectives: Evaluating the cost-effectiveness of antibiotic therapy in the Department of Pulmonology.
Methods: ABC/VEN analysis was performed with data on antibiotic costs in the pulmonology department (30 beds) of a multidisciplinary regional hospital (844 beds in total) with 1 full-time clinical pharmacologist for no clinical pharmacy or pharmacology service. To analyze antibiotic consumption patterns according to the AWaRe 2021 classification, we used data on the number of antibiotics procured.
Results: The results of the antibiotics spending analysis from 2019-2021 showed that all antibiotics from the most costly group A (80% of total spending) are in the Watch group (J01DH Carbapenems – Ertapenem, Doripenem, Meropenem; J01MA Fluoroquinolones – Levofloxacin; J01DD Third-generation-cephalosporins – Ceftazidime, Ceftriaxone and J01DE Fourth-generation-cephalosporins: Cefepime).
Meanwhile, there has been an increase in the share of spending on the most consumed group of antibiotics, J01DH Carbapenems, from 42.9% in 2019 to 62.8% by 2021. On the contrary, there is downward trend in spending on the third-generation-cephalosporins which was 35.6% in 2019 and only 6.7% by 2021. Assessment of antibiotic prescription patterns in the pulmonology department based on classification AWaRe 2021 and WHO Model List of Essential Medicines (EML) 2021 (22nd edition) revealed a negative trend in the use of the most costly group (A) of antibiotics with a low level of evidence of efficiency or safety in pulmonology: Doripenem, Ertapenem, Levofloxacin, Cefepime. However, there is a positive result in the work of the clinical pharmacology service - the drugs mentioned above were moved into group B (medium-cost) by 2021, except for Cefepim, which was not purchased at all.
Conclusion: Despite the positive trend in antibiotic consumption patterns (transfer of antibiotics with efficiency proof from gr A to gr B), current antibiotic therapy in the pulmonology department needs comprehensive optimization of approach to rational antibiotic use, strengthening pharmaceutical care by implementing a clinical pharmacy service that will conduct regular systematic evaluation and contribute to the pharmacoeconomic expediency of antibiotic therapy. Such measures lead to an improvement of the quality of medical care for the population and reduce the cost of this nosology, which proves that there is a need for a comprehensive detailed analysis.
265
The use of the "benefit-risk" concept to determine the dosage regimen of drugs on the example of anti-tuberculosis drugs
Kasimova A1,2, Vilium I1, Kolbin A1
1 Pavlov First Saint Petersburg State Medical University, Saint-Petersburg, Russia
2 Russian Scientific Research Institute of Traumatology and Orthopedicsnamed after R.R. Vreden, Saint-Petersburg, Russia
Objectives: The aim of the work was to develop algorithms for dosing oral anti-tuberculosis drugs, if necessary, dividing tablets and determining the dose interval closest to the calculated patient weight in accordance with the patient's risk factors regarding the tolerability of therapy.
Methods: Data on recommended dosage regimens for different weight categories of patients were analysed. The greatest difficulties arise in the treatment of patients with extremely low or high body weight, as the risk of overdose of anti-tuberculosis drugs increases, exceeding the dosage according to the instructions or, conversely, the use of ineffective doses. In this regard, using data on the risk factors of anti-tuberculosis therapy, these instructions for use and epidemiological data of real clinical practice, an approach was developed to determine the dose in the direction of a smaller or larger dose interval.
Results: Recommendations on dosing, instructions for the use of basic anti-tuberculosis drugs were studied. The data of the sections "Contraindications", "With caution", "Side effect", "Special instructions" and "Interactions" were taken into account. In accordance with the clinical conditions indicated in these sections, risk factors were determined for each drug, dictating the use of the minimum value of the dose interval and the division of the finished form of the oral drug, as well as conditions allowing the use of the drug in maximum dosages. Algorithms have been developed for more than 10 anti-tuberculosis drugs for each weight value from 40 to 100 kg and rules for dividing tablets.CONCLUSIONS: The introduction of screening for the carrier of resistant microorganisms and the use of its results in determining antibiotic prophylaxis and prescribing empirical therapy leads to a reduction in the consumption of antibiotics and, as a consequence, a reduction in the cost of this group of medicines.
Conclusions: The use of the "benefit-risk" concept to determine the dosage regimen of anti-tuberculosis drugs allowed us to unify the approach for choosing the dosing interval, as well as to develop rules for dividing fixed tablet forms if it is impossible to use them in the full dose of the release form.
321
Identification of Heat Shock Protein-90 as a New Potential Drug Target for the Treatment of SLE, by the Lincs1000 Software.
Nikolakis D1, Banos A2, Filia A2, Myrianthopoulos V3, Garantziotis P4, Verginis P2, Mikros E3, Boumpas D2
1 Amsterdam UMC, University of Amsterdam, Department of Experimental Immunology, Amsterdam institute for Infection & Immunity, Amsterdam institute of Gastroenterology Endocrinology and Metabolism, Amsterdam, Netherlands
2 Center of Clinical, Experimental Surgery & Translational Research, Biomedical Research Foundation, Academy of Athens, Athens, Greece
3 Department of Pharmaceutical Chemistry, School of Pharmacy,National and Kapodistrian University of Athens, Athens, Greece
4 Department of Clinical Immunology and Rheumatology, Medical University Hannover, Hannover, Germany
Introduction: Systemic lupus erythematosus (SLE) is a systemic autoimmune disease with complex pathogenesis including genetic and environmental factors. Production of interferon α and autoantibodies, as well as, activation of neutrophils and monocytes are key pathogenetic events in SLE. Current therapies used are the disease-modifying antirheumatic drugs (DMARDs), corticosteroids, the non-steroid anti-inflammatory drugs (NSAIDs), and biologics such as, monoclonal antibodies. Even though biological agents are very efficient in reducing the progression of the disease, nevertheless are associated with several other drawbacks, including high cost, inadequate clinical response over time, need of parenteral administration, as well as increased risk of several infections, due to the progressively diminished immune response. There is a clear need for orally administered, well-tolerated and inexpensive drugs to treat SLE.
Objectives: Based on RNA sequencing data of lupus patients, we tried to identify novel compounds and potential therapeutic targets for the treatment of SLE, by the aid of the Lincs1000 drug repurposing bioinformatic tool.
Methods: We performed RNA sequencing in peripheral blood monocytes from 15 active SLE patients and age and sex matched healthy controls. We further analyzed differentially expressed genes by the use of the bioinformatic software Lincs 1000. This program enables the user to identify patented small molecules from the Lincs database, that can mimic or reverse a specific gene expression profile. As an input signature, we used significantly differentially expressed genes (DEGs) (Fold Change≥ 1.5, p≤0.01), from RNA seq data from peripheral blood monocytes derived from 2 different groups of SLE patients. The first group included 7 SLE patients (6 females and 1male) with disease activity index(SLEDAI) higher than 8 (active disease), as well as, characterized by high IFN-α serum levels. The second group included 8 SLE patients (6 females and 2 males) with SLEDAI higher than 10 (active disease).
Results: We identified two compounds -geldanamycin and NVP-AUY922- that could reverse the gene expression profile of monocytes derived from lupus patients while having the same protein target, the heat shock protein 90 (HSP90). HSP90 has been linked with SLE pathogenesis, since it is associated with the delivery of TLR7/9 receptors from the endoplasmic reticulum to early endosomes for ligand (nucleic-acid) recognition and therefore to IFN-α production in plasmacytoid dendritic cells from SLE patients or lupus‐prone MRL/lpr mice.
Conclusions: Application of Lincs1000 can provide an unbiased approach into drug discovery in SLE providing biologically meaningful drug targets such as Heat Shock Protein-90 (HSP-90).
Bioinformatics and systems pharmacology
78
Non-targeted metabolomics and machine learning for the identification of plasma metabolites associated with organic anion transporting polypeptide 1B1 function
Hämäläinen K1,2, Neuvonen M1,2, Hirvensalo P1,2, Lehtonen M3, Auriola S3, Tornio A1,2, Backman J1,2,4, Niemi M1,2,4
1 Department of Clinical Pharmacology, University of Helsinki, Helsinki, Finland
2 Individualized Drug Therapy Research Program, Helsinki, Finland
3 School of Pharmacy, University of Eastern Finland, Kuopio, Finland
4 Department of Clinical Pharmacology, HUS Diagnostic Center, Helsinki, Finland
ABSTRACT TITLE: Non-targeted metabolomics and machine learning for the identification of plasma metabolites associated with organic anion transporting polypeptide 1B1 function
Introduction: Glycochenodeoxycholate 3-O-glucuronide (GCDCA-3G) and glycodeoxycholate 3-O-glucuronide (GCDCA-3G) were recently identified as promising endogenous biomarkers for the hepatic influx transporter organic anion transporting polypeptide 1B1 (OATP1B1). OATP1B1 is an influx transporter expressed on the sinusoidal membrane of human hepatocytes and also functions as an uptake regulator of multiple drugs, such as statins.
Objectives: The aim of this study was to identify plasma biomarkers associated with OATP1B1 activity using a hypothesis free metabolomics approach.
Methods: We carried out a liquid chromatography-mass spectrometry based non-targeted metabolomics analysis in the fasting plasma samples from 356 healthy volunteers. Based on decreased (*5 and *15) and increased (*14 and *20) function SLCO1B1 alleles, we grouped the participants into poor, decreased, normal, increased, and highly increased OATP1B1 function classes. Random forest (RF) regressor, gradient boosted decision tree (GBDT) regressor, and linear regression analysis were employed to identify metabolite features associated with the OATP1B1 functional classes.
Results: A total of 9152 metabolite features were found in the plasma samples, of which 21 showed a significant association with the OATP1B1 functional classes in the linear regression analysis with a Bonferroni-corrected P-value threshold of 10-5. The strongest associations were observed with two features representing GDCA-3G (P=1.2×10-20 and P=1.7×10-19, metabolite identification level L1). A feature identified as GCDCA-3G (L1) followed thereafter (P=2.7×10-16). Both the RF and GBDT regressors robustly identified OATP1B1 functional classes using the whole metabolome dataset. Moreover, they found a metabolite feature identified as GCDCA-3G to be the most strongly associated one, as quantified by Gini impurity decrease of 0.41 for RF and 0.17 for GBDT.
Conclusion: These data indicate that plasma GDCA-3G and GCDCA-3G are robust biomarkers for OATP1B1 activity. Other metabolite features found in the non-targeted plasma metabolomics analysis provide little additional value for measuring OATP1B1 activity.
214
Elucidating the biological and pharmacological background of two Heart Failure subtypes, Dilated and Ischemic Cardiomyopathy; a multi-omics bioinformatics approach
Portokallidou K1,2, Dovrolis N1,2, Ragia G1,2, Kolios G1,2, Manolopoulos V1,2,3
1 Laboratory of Pharmacology, Medical School, Democritus University of Thrace, Alexandroupolis, Greece
2 Individualised Medicine & Pharmacological Research Solutions Center (IMPReS), Alexandroupolis, Greece
3 Clinical Pharmacology Unit, Academic General Hospital of Alexandroupolis, Alexandroupolis, Greece
Introduction: Dilated Cardiomyopathy (DCM) and Ischemic Cardiomyopathy (ICM) are the most common causes of heart failure (HF), a condition with an estimated prevalence of 64.3 million people worldwide.
Objectives: The aim of the present study is to elucidate the molecular pathways and genetic signature of DCM and ICM, coined as DiSig and IsSig, respectively, via multi-omics bioinformatics analyses.
Methods: We have included a total of 10 transcriptomic (microarray and RNA-Seq) and proteomic (mass spectrometry) datasets collected from the Gene Expression Omnibus (GEO) database and the Proteomics Identifications Database (PRIDE) Archive, respectively. Datasets consist of DCM and ICM patients, whereas non-failed left ventricle samples served as a control group. Differentially Expressed Genes (DEGs) were identified using the GEO2R and RaNASeq tools. Venn diagrams were used to identify common genes within transcriptomics and proteomics datasets. Additionally, Gene Ontology (GO), Reactome and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses were performed to explore the biological functions and signaling pathways. Protein–protein interaction (PPI) networks were assembled with STRING-db to identify hub genes related to the pathogenesis of DCM and ICM, followed by a comparison between the two subtypes of HF.
Results: For DCM, a total of 106 DEGs (78 upregulated and 28 downregulated genes) were identified as the intersection of three microarray datasets, three RNASeq datasets and one mass spectrometry dataset. Of these DEGs, 10 were common in all lists.
For ICM, we have identified 187 DEGs (147 upregulated and 40 downregulated genes) as the intersection of three microarray datasets, four RNASeq datasets and one mass spectrometry dataset. Of these, 15 DEGs, all upregulated, were common in all lists.
When looking deeper in the 10 DEGs of DiSig and the 15 DEGs of IsSig, we found that they share 8 common genes. These genes can be categorized as cardioprotective proteins (HSPA2, SOD3) and genes having a major role in cardiac remodeling processes (AEBP1, CA3, THBS4, UCHL1). Hemoglobin types HBA2 and HBB are overexpressed in both DCM and ICM patients. IsSig has six additional extracellular matrix proteins associated with its incidence (BGN, CBGN, COL14A1, LUM, LTBP2, MFAP4) and upregulated expression of apolipoprotein A1 (APOA1), while DiSig has two downregulated genes MYH6 and SERPINA3.
We have further compared the top 100 statistically significant GO pathways of the two HF subtypes and we have found that 27 are common between DCM and ICM. These pathways mainly include extracellular matrix organization, cellular response to stress and transforming growth factor beta, and transmembrane transport of ions, particularly calcium.
Conclusion: The findings from this holistic, multi-omics bioinformatics network analysis provide evidence for the gene signatures of DCM and ICM (DiSig and IsSig). Our approach holds promise to increase current knowledge and understanding of the mechanisms underlying DCM and ICM. DiSig and IsSig, ultimately, can be used to identify novel therapeutic targets for these two subtypes of HF.
Funding: Financial support for project IMPReS (MIS 5047189) was provided by the Program “Competitiveness, Entrepreneurship and Innovation” (NSRF 2014–2020) co-financed by Greece and the European Union (European Regional Development Fund).
298
Nifuroxazide has better efficacy than probiotic treatment in adult patients with acute diarrheal syndrome
Husic-Selimovic A, Zahiragic N, Sivac N, Glamoclija U, Sukalo A, Mehic M, Limo S, Huduti D
1 European Association of Clinical Pharmacology and Therapeutics, Sarajevo, BiH
Introduction: The aim of this observational clinical study was to compare the efficacy of nifuroxazide and probiotic preparation containing lactic acid bacteria in the treatment of acute diarrheal syndrome.
Objectives: The study included 72 patients (38 in nifuroxazide and 34 in the probiotic group) who suffered from acute infective diarrhoea or traveller’s diarrhoea for ≤72 hours and had ≥3 unformed stools per day, with no administration of antibiotics during previous 10 days.
Methods: A subjective evaluation of efficacy, tolerance of the medication and health improvement (number of stools per 24 hours, stool consistency and vomiting) were monitored at the baseline, third and seventh day of the treatment. Already at the third day of treatment, patients in nifuroxazide group had lower number of stools [3 (2-4) vs. 4 (3-5), p<0.001] and were vomiting less (89.5% not vomiting vs. 70.6% not vomiting, rho=0.235, p=0.047).
Results: At the seventh day of treatment, nifuroxazide group was superior regarding the stool consistency (rho=0.554, p<0.001), subjective evaluation of efficacy (rho=0.321, p=0.006) and subjective perception of health status improvement (rho=0.521, p<0.001).
Conclusion: Nifuroxazide should be recommended as the first choice empirical treatment in adult patients with the acute diarrheal syndrome.
Cancer Treatment
51
PHARMACOKINETICS AND PHARMACODYNAMICS OF IV AND ORAL FORMULATIONS OF CG-745 AFTER A SINGLE ADMINISTRATION IN HEALTHY KOREAN SUBJECTS
Kim B1, Bae S1, Park S1, Jang I1, Oh J1, Cho S2, Kim Y2, Lee S1
1 Department of Clinical Pharmacology and Therapeutics, Seoul National University College of Medicine and Hospital, Seoul, Republic of Korea
2 Division of Clinical Development, CrystalGenomics, Inc., Korea Bio Park, Seongnam-si, Republic of Korea
Introduction: The deacetylation of histones and non-histone proteins by HDACs (histone deacetylases) play an important role in transcription regulation of eukaryotic cells. HDAC inhibitors are novel class of small-molecule drugs for the treatment of diseases including T-cell lymphoma, multiple myeloma, epilepsy, and bipolar disorders. CG-750 is an oral formulation of CG-745, an HDAC inhibitor.
Objectives: This study aimed to compare the pharmacokinetics (PK), pharmacodynamics (PD) and tolerability between IV (CG-745) and oral (CG-750) formulations of CG-745 in healthy volunteers.
Methods: A randomized double-blind, placebo-controlled, two-treatment, two-period crossover study was conducted in 3 cohorts. Subjects were randomized (6:2) to receive either CG-745 IV (cohort 1 and 3: 125 mg; cohort 2: 250 mg) or placebo, followed by CG-750 (cohort 1: 125 mg; cohort 2: 375 mg; cohort 3: 750 mg) or placebo after a 14-day washout period. Blood samples for PK and PD assessment were collected up to 72 hours post-dose, and non-compartmental analysis was performed. Histone H3 acetylation at sites K9, K9/K14 and K27 were assessed for area under the % acetylation induction vs time curve (AUEC). Tolerability profiles were assessed throughout the study.
Results: A total of 25 subjects were randomized and 23 completed the study. The mean bioavailability of CG-750 was 10.6% (range: 4.18 – 21.33%), and displayed linear PK in the dose range of 125 – 750 mg. AUEC of all acetylation sites in cohort 3 were comparable between CG-745 IV and CG-750 (p = 0.3125, 0.8438, 0.3125 for K9, K9/14, K27, respectively), with CG-750 showing comparable or higher acetylation in all three comparisons. All AEs were transient and of mild or moderate intensity.
Conclusion: The mean bioavailability of CG-750 was 10.6 % and displayed linear PK. CG-750 oral formulation and CG-745 IV were generally well tolerated after a single oral and IV administration.
83
Role of patient reported outcomes in clinical trials in metastatic colorectal cancer: clinical decision aid or just nice to have?
Veluwenkamp-Worawutputtapong P1, Tichelaar F2, De Groot J1, Postma M2, Touw D2,3, Maring J1
1 Isala Hospital, Zwolle, The Netherlands
2 University of Groningen, Groningen, The Netherlands
3 University Medical Center Groningen, Groningen, The Netherlands
Introduction: Over the past few years, the use of Patient Reported Outcomes (PROs) has gained increasing interest, especially in cancer treatment. PROs provide reports from patients on their own health, quality of life, or functional status associated with the health care they have received. Since the emerging role of PROs in clinical trials has not been studied extensively so far, we decided to perform a review on the use of PROs in clinical trials evaluating systemic treatment options in patients with metastatic colorectal cancer (mCRC).
Objectives
• To evaluate the use of PROs in clinical trials in mCRC cancer started and/or published between 2010 and 2021
• To evaluate the relationship between PROs and clinical outcomes
• To evaluate the role of PROs in the assessment of the value of new treatment modalities
Methods: To obtain an overview of all mCRC related phase III trials carried out between 2010 and 2021, a search on clinicaltrials.gov was performed. Publications related to the selected studies were acquired using Embase and MedLine. A quantitative analysis on the use of PROs was performed on data derived from the clinicaltrials.gov database. Data from recruiting, ongoing and completed trials were included.
A qualitive analysis regarding the value of PROs in the final assessment of new treatment modalities was performed on published data from a subset of completed clinical trials. For each paper relevant study characteristics were collected, including design, size, objectives, endpoints, type of PRO-measure (PROM), PRO outcomes, clinical outcomes and role of PROs in the discussion section of the published paper.
Results: Seventy-one clinical trials and 28 peer reviewed publications met the predefined inclusion criteria. The QLQ-C30 was the most often selected PROM with 29 times. The EQ-5D and the QLQ-CR29 were used in 15 and 6 clinical trials, respectively. In the remaining trials a large range of alternative PROMs was used. We observed an increase in the number of clinical trials including PROs after 2015 and also a trend towards standardization in PROM use (see figure). In the 28 selected articles, PROs were included as secondary (93%) or tertiary endpoints (7%). Twenty studies were carried out as a randomized trial. Interestingly, in 7 studies reporting better clinical outcomes for a new treatment, this was in only 3 studies (43%) supported by superior PROs. In 12 studies with equal clinical outcomes, PROs were supportive, neutral or unfavourable in 3 (25%), 7 (58%) and 2 (17%) trials, respectively.
Strikingly, 9 out of 28 papers (32%) did not include PROs in the final assessment and position of the new treatment compared to the reference treatment. Even more striking is the fact that in 6 papers (21%) PROs were not mentioned at all in the discussion section of the article.
Conclusion: PROs are increasingly becoming a regular part of clinical studies. Standard PROM tools seem to be used more often during recent years. The importance attributed to PROs in determining the value of new treatments is however highly variable and unstructured.
Tabel/Image
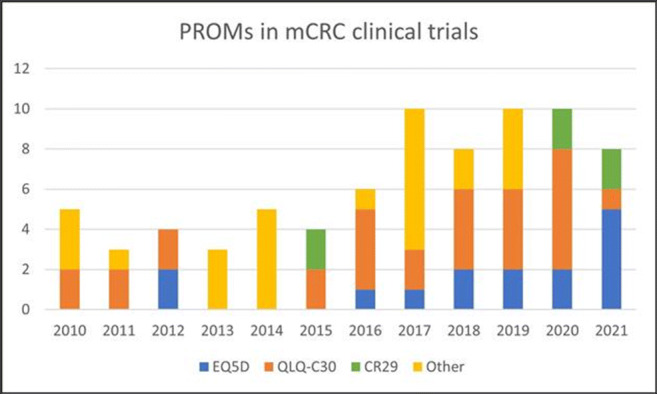
118
Early outcomes of the establishment of an orthotopic patient-derived xenografts (PDX) model for pancreatic ductal adenocarcinoma
Magouliotis D1, Lafazanis K2, Koutsougianni F2, Sakellaridis N2, Ioannou M3, Zacharouli K3, Zacharoulis D4, Dimas K2
1 University Of Thessaly, Larissa, Greece
2 Department of Pharmacology, University of Thessaly, Larissa, Greece
3 Department of Pathology, University of Thessaly, Larissa, Greece
4 Department of Surgery, University of Thessaly, Larissa, Greece
Introduction: Pancreatic cancer (PC) is one of the leading causes of cancer-related death. Due to the limitations of the existing preclinical cancer models, the research for new treatments and biomarkers for PC remains slow.
Objectives: The purpose of the present study was to establish a patient-derived orthotopic xenograft model (PDOX) for pancreatic ductal adenocarcinoma (PDAC), thus providing a tumor microenvironment resembling that of the human pancreas to identify novel potential biomarkers and treatment regimens.
Methods: PDAC tissue samples were received from 35 patients, following informed consent, and three mouse strains were implemented.
Results: Successful PDOX engraftment was performed in NOD/SCID and NOD/SCID gamma (NSG) mice. Nonetheless, we found a higher rate of successful engraftment and tumor growth in NSG compared to NOD/SCID mice, possibly owning to the different levels of immunosuppression and more specifically of the natural killer cells presence.
Conclusion: Our suggested PDOX model represents a preclinical cancer research model with a high affinity to the patient’s tumor microenvironment, thus enabling the acceleration of PDAC research.
259
Squalene synthase inhibitors as anti-cancer/metastasis agents: a preliminary in vitro investigation
Katavati T1, Matralis A2, Aidinis V2, Kourounakis A1
1 National And Kapodistrian University Of Athens, Athens, Greece
2 Biomedical Sciences Research Center "Alexander Fleming", Athens, Greece
Introduction: Cholesterol is an essential biomolecule involved in various aspects of human health and disease. Elevated cholesterol levels have been linked to a variety of diseases, including atherosclerosis, neurodegenerative disorders, cancer and metastasis through various molecular mechanisms. Although great progress has been made in understanding the underling events of cancer and metastasis, new approaches for potential treatments are still ongoing. The multifactorial pathophysiology of cancer remains a significant challenge in the field of oncology research as is the discovery of novel therapies that will selectively target molecular mechanisms favoring carcinogenesis. The de novo biosynthesis of cholesterol is strictly regulated under normal conditions. However, in cancer cells, where transcription factors, oncogenes, and various signaling pathways are stimulated, this biosynthetic pathway is disrupted in order to serve high energy and biosynthetic needs. Since these abnormalities are associated with the development of cancer, enzymes involved in the biosynthesis of cholesterol, such as squalene synthase, have attracted interest as potential targets for anti-cancer therapy. 1-4
Objectives: Given the lack of adequate therapies and experimental data on the understanding of cholesterol homeostasis in cancer cells, we proceeded to study the contribution of squalene synthase in cancer cell lines of different metastatic potential, by molecules that inhibit this enzyme.
Methods: Three high affinity inhibitors were selected and tested pharmacologically in vitro at different concentrations for their toxicity in both normal primary cells (fibroblasts) and various cancer cell lines. Further, the mechanism by which these molecules inhibit the progression of cancer was investigated by examining how they are involved and affect important cell processes such as proliferation, invasion/migration, attachment, cell death stages, cell cycle phases and apoptosis. The study included use of the following assays: MTT, wound scratch assay (quantification of cellular migration on two-dimensional (2-D) surfaces over time upon different treatments), Propidium Iodide staining protocol, apoptosis detection assay (with FITC Annexin V and propidium iodide), adhesion assay and JC-10 mitochondrial membrane potential assay.
Results and Conclusion: The three inhibitors investigated are not toxic in normal primary cells but significantly and selectively toxic to cancer cells at low concentrations. One of the inhibitors seems to contribute to mitochondrial apoptosis and partially inhibits plate-adhesion of cancer cells. Another inhibitor affected cell cycle phases (reduced cells found in S phase and increased those in G2). In summary, SQS seems to be associated with cancer by a variety of mechanisms which have not been fully investigated as yet. Based on our findings, SQS inhibitors could be a new direction of research with promising perspectives as a new therapeutic strategy.
1. Codini M et al. Cholesterol and Sphingolipid Enriched Lipid Rafts as Therapeutic Targets in Cancer. Int J Mol Sci. 2021 22(2):726.
2. Kourounakis, A. et al. New applications of squalene synthase inhibitors: Membrane cholesterol as a therapeutic target. Archiv Der Pharmazie 2020, 353.
3. Xu H et al. Cholesterol metabolism: New functions and therapeutic approaches in cancer. Biochim Biophys Acta Rev Cancer. 2020 1874(1):188394.
279
Assessment of the in Vitro Effect of the Cannabinoids Cannabidiol and Cannabigerol on Gastric and Oesophageal Cancer Cell Lines
Saleh A1,2, Dobbins B2, Saha A2, Phillips R1, Javid F1
1 University Of Huddersfield, Huddersfield, United Kingdom
2 Calderdale and Huddersfield NHS Foundation Trust, Huddersfield, United Kingdom
Introduction: Globally there are one million new gastric cancer, and 570 thousand new oesophageal cancer diagnoses per annum. These cancers cause approximately 1.3 million deaths worldwide annually. In addition to being associated with high mortality rates these conditions are linked with high rates of morbidity and poor quality of life. For both conditions, surgery in combination with chemotherapy is the only curative option, however the treatment is highly invasive and only a relatively small proportion of patients are either fit enough for or suitable for surgery. It would therefore be beneficial to determine if novel agents could play a role in the treatment of these cancers and improve outcomes for patients. There is evidence that the non-psychoactive cannabinoids cannabidiol (CBD) and cannabigerol (CBG) have anti-cancer properties however the evidence in gastric and oesophageal cancer is currently limited. Recent changes to legislation in the United Kingdom have paved the way for research involving cannabinoids and given rise to the potential to use them as therapeutic agents in clinical practice.
Objectives: To determine the in vitro effects of the cannabinoids CBD and CBG on a panel of gastric and oesophageal cancer cell lines.
Methods: Three gastric (AGS, HS746T and SK-GT-2) and three oesophageal (OE33, KYSE 410 and FLO1) cancer cell lines were maintained and subcultured as per American Type Culture Collection (ATCC) guidelines. When cells reached 70% confluence, they were seeded in 96 well plates and treated with CBD or CBG concentrations ranging from 0.2μm to 100μm. After 96 hours contact time, cell viability was assessed using a 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT) assay. Experiments were repeated independently four times. Cell viability at each concentration was recorded and half maximal inhibitory concentration (IC50) was calculated.
Results: The IC50 concentrations are displayed in the table in micromolar. The standard errors of the mean (SEM) are included in parentheses.
Conclusion: Exposure to CBD and CBG leads to an in vitro cytotoxic effect on both gastric and oesophageal cancer cells lines. The results thus far are promising and provide a solid foundation for further investigation into the effects of cannabinoids on these cancer cells in addition to the mechanisms that underpin them.
Tabel/Image
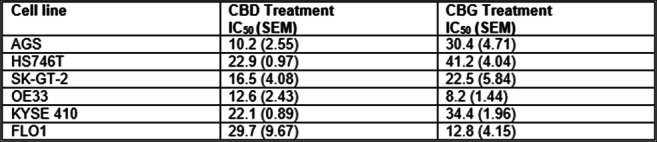
Cardiovascular treatment
45
ENFLUANCE OF PLANT ORIGIN SOLUBLE EPOXIDE HYDROLASE INHIBITORS ON HEMODYNAMIC PARAMETERS IN HYPERTENSIVE RATS
Papiashvili N3, Papiashvili N1, Bakuridze K1, Ghongadze M2, Bakuridze A1, Gongadze N2
1 Department of Pharmaceutical Technology, Tbilisi State Medical University, Tbilisi, Georgia
2 Department of Medical Pharmacology, Tbilisi State Medical University, Tbilisi, Georgia
3 Department of Pharmacology, LEPL. State Regulation Agency for Medical Activities, Tbilisi, Georgia
Introduction: Epoxyeicosatrienoic acids (EETs) revealing vasodilatory action (VA) are converted by soluble epoxide hydrolase (sEH) to less active dihydroxyeicosatrienoic acids (DHETs). Studies provide evidence that sEH inhibitors (sEHI) may exert beneficial effects in hypertensive states alleviating vascular homeostasis.
Objective: The goal of this study was to estimate the influence of plant origin sEHI on hemodynamic indices and baroreflex sensitivity (BRS) in sham-operated (SO) and hypertensive rats (HR).
Methods: Experiments were performed in male unanesthetized Wistar-Kyoto rats. Animals were divided into the two groups: I – SO and HR (two-kidney, one clip) with preliminary implanted catheters into carotid artery and jugular vein. Blood pressure (BP), heart period (HP) and BRS were assessed before and after 2 weeks of i.p. injection of sEHI-3mg/kg. Based on our preliminary studies it revealed high VA on isolated mesenteric artery in rats. For determination of plasma content of EETs blood samples were drawn from carotid artery.
Results: In HR BP was 160±10 mmHg correlated with mean values of HP-140±6ms and BRS-0.58±0.04 ms mmHg-1 vs. to SO rats indices of BP-135±8mmHg, Hp-156±4ms and BRS-0.84±0.06ms mmHg-1, respectively. sEHI in HR markedly decreased BP (134±8mmHg, P<0.05) and increased HP (150±4ms, P<0.05) and BRS (0.7±0.08ms mmHg-1, P<0.05) in comparison with the same values in SO rats with slight reduction in BP (128±5 mmHg) small increase in HP (160±6ms) and BRS (0.88±0.05 ms mmHg-1). The plasma content of EETs in HR (15.8±0.6 ng/ml) treated (T) with sEHI significantly exceeded this value in untreated (U) HR (11.2±0.4 ng/ml, P<0.05) without marked differences between U (18.5±0.4 ng/ml) and T (19.6±0.2 ng/ml) in SO rats.
Conclusion: Our results suggest, that by enhancing EETs VA, the plant origin sEHI may provide beneficial effect on hemodynamic parameters and BRS in hypertensive state being promising compound for improvement of different vascular disorders.
91
Pharmacological evidence to the usefulness of Coumarin in metabolic syndrome
Mehmood M1, Hussain M1, Malik A2, Gilani A3
1 Department of Pharmacology, Faculty of Pharmaceutical Sciences Government College University, Faisalabad, Pakistan
2 Faculty of Pharmacy, University of Sargodha, Sargodha, Pakistan
3 University of Haripur, Hari Pur, Pakistan
Background: Prevalence of metabolic syndrome in population is growing rapidly throughout the world along with developing countries. Now it becomes worldwide major public health problem. It is primarily causes increasing risk of development of diabetes and cardiovascular disorders, which are contributing up to 5 folds causing high mortality. Coumarins are a group of secondary metabolites, belong to the benzopyrone family. These are found in many edible plants such as tonka bean extracts, woodruff, cassia, cinnamon, green tea, peppermint, celery, bil berries, lavender, honey, carrots.
Objectives: Literature revealed diverse beneficial effects of coumarins in chemically-induced diabetes and its complications; So far, contributing mechanisms of action of coumarin has not been identified yet. This study was carried out in order to attest the usefulness of Coumarin in cardiometabolic disorders.
Method: In the in-vitro assay, rat isolated aortae were used in a tissue organ bath assembly coupled with an isometric transducer and a PowerLab data acquisition system. In vivo assays were carried in fructose-fed animal model representing characteristics of cardiometabolic disorders.
Results: In the isolated rat aorta preparation, Coumarin caused a concentration-dependent (0.3-100 μM) relaxation of low K+ (25 mM)-induced contractions whereas partial relaxation was observed against high K+ (80 mM) contractions. Pretreatment of the tissue with Glibenclamide (10 μM) had a negligible effect on the inhibitory effect of Coumarin on low K+ (25 mM)-induced contractions, while 4-aminopyridine (1 mM) completely obstructed this effect. Tetraethyl ammonium (TEA; 10 mM) shifted the inhibitory effect of Coumarin towards a higher dose but with less efficacy than 4-aminopyridine. In fructose-fed animal model, Coumarin administration showed improvement in obesity, hyperlipidemia, hypertension and endothelial dysfunction characteristic features of cardiometabolic disorders. These results indicate that Coumarin possesses potential against cardiometabolic disorders and also showed a vasodilatory effect mediated possibly through the dominant activation of voltage-dependent K+ channels followed by non-specific K+ channels with a weak effect on Ca++ influx.
Conclusion: This study provides pharmacological basis for the possible future development of Coumarin as a drug candidate for the management of cardiometabolic disorders including hypertension.
101
INFLUENCE OF PLANT ORIGIN SOLUBLE EPOXIDE HYDROLASE INHIBITORS ON HEMODYNAMIC PARAMETERS IN HYPERTENSIVE RATS
Papiashvili N1, Papiashvili N3, Bakuridze K1, Ghongadze M2, Bakuridze A1, Gongadze N2
1 Department of Pharmaceutical Technology, Tbilisi State Medical University, Tbilisi, Georgia
2 Department of Medical Pharmacology, Tbilisi State Medical University, Tbilisi, Georgia
3 Department of Pharmacology, LEPL. State Regulation Agency for Medical Activities, Tbilisi, Georgia
Introduction: Epoxyeicosatrienoic acids (EETs) revealing vasodilatory action (VA) are converted by soluble epoxide hydrolase (sEH) to less active dihydroxyeicosatrienoic acids (DHETs). Studies provide evidence that sEH inhibitors (sEHI) may exert beneficial effects in hypertensive states alleviating vascular homeostasis.
Objective: The goal of this study was to estimate the influence of plant origin sEHI on hemodynamic indices and baroreflex sensitivity (BRS) in sham-operated (SO) and hypertensive rats (HR).
Methods: Experiments were performed in male unanesthetized Wistar-Kyoto rats. Animals were divided into the two groups: I – SO and HR (two-kidney, one clip) with preliminary implanted catheters into carotid artery and jugular vein. Blood pressure (BP), heart period (HP) and BRS were assessed before and after 2 weeks of i.p. injection of sEHI-3mg/kg. Based on our preliminary studies it revealed high VA on isolated mesenteric artery in rats. For determination of plasma content of EETs blood samples were drawn from carotid artery.
Results: In HR BP was 160±10 mmHg correlated with mean values of HP-140±6ms and BRS-0.58±0.04 ms mmHg-1 vs. to SO rats indices of BP-135±8mmHg, Hp-156±4ms and BRS-0.84±0.06ms mmHg-1, respectively. sEHI in HR markedly decreased BP (134±8mmHg, P<0.05) and increased HP (150±4ms, P<0.05) and BRS (0.7±0.08ms mmHg-1, P<0.05) in comparison with the same values in SO rats with slight reduction in BP (128±5 mmHg) small increase in HP (160±6ms) and BRS (0.88±0.05 ms mmHg-1). The plasma content of EETs in HR (15.8±0.6 ng/ml) treated (T) with sEHI significantly exceeded this value in untreated (U) HR (11.2±0.4 ng/ml, P<0.05) without marked differences between U (18.5±0.4 ng/ml) and T (19.6±0.2 ng/ml) in SO rats.
Conclusion: Our results suggest, that by enhancing EETs VA, the plant origin sEHI may provide beneficial effect on hemodynamic parameters and BRS in hypertensive state being promising compound for improvement of different vascular disorders.
113
Influence of the Covid-19-pandemic on hypertension treatment in rural areas of Romania
Lechsner P1, Balan M1, Dho-Nagy E1, Ban E1
1 UMFST George Emil Palade Targu Mures, Targu Mures, Romania
Introduction: Hypertension is a silent disease which is affecting almost 50% of the adult population in Romania, according to the most recent national survey conducted in 2017. This cardiovascular illness requires chronic, life-long medical therapy and regular checkups by the physician. If the therapeutic treatment fails or the disease is left untreated, the severity of hypertension will increase, hospitalization may become necessary and other complications might occur.
Objectives: Objective of the study was to evaluate how the SARS-CoV-2 pandemic and the lockdown periods influenced the pharmacological therapy of hypertensive, adult patients in the rural parts of Romania.
Methods: We have performed a prospective, cross-sectional pharmaco-epidemiologic questionnaire-based survey in a rural area of Romania among a population which we are monitoring from the point of view of hypertension since 2002.
Results: Our questionnaire with 410 participants revealed a prevalence of hypertension of 53%, determined based on the patients’ knowledge of the diagnosis, showing a slight decrease in comparison with previous data obtained in 2002 and 2018. Summarizing the changes that occurred among the 220 hypertensive patients during the pandemic and the lockdown period in spring of 2020. While 100 patients suffered from an increase in severity of hypertension, change of the pharmaceutical treatment was only initiated in 70 patients. From those who suffered from a Covid-infection, in exactly half was reported, by the medical doctor, that the presence of hypertension was considered a risk factor in the progression of the disease.
Conclusion: Based on our results we observed a slight decrease in hypertension prevalence compared to previous data could be due to the fact that in this pandemic period, presentation for regular check-ups at the family physician was neglected, so the “accidental” diagnosis of the still silent hypertension, as it is often common, was not possible. Secondly, in over 45% of hypertensive patients, the disease progressed to more severe stages and complications occurred with a high incidence as well. It is unclear why not all patients with worsened hypertension were started on a changed drug treatment; this might be in relation to the reduced or missing visits to the family physician. Overall, the pandemic and the enforced restrictions appear to have had a negative influence on the pharmacological therapy ensured control of hypertension in the observed rural population.
Tabel/Image
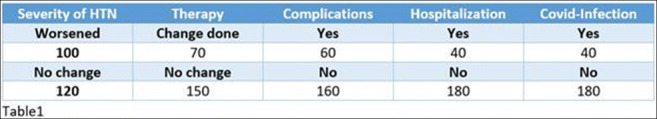
124
Prognostic value of platelet to lymphocyte ratio in patients undergoing myocardial revascularization with non-ST elevation acute coronary syndrome
Ygiyeva D2, Batenova G1
1 Pirogov Russian National Research Medical University, Moscow, Russia
2 Semey Medical University, Semey, KAZAKHSTAN
Introduction: ACS in patients is one of the main reasons for visiting the emergency department. Acute coronary syndrome (ACS), characterized by unstable atherosclerotic lesions, is the leading cause of death.
Currently, cardiac markers - troponins and creatine phosphokinase MB-fraction (CPK-MB) - act as the "gold standard" for the diagnosis of ACS. It is known that inflammatory processes are of great importance in the pathogenesis of ACS, which arouses interest in studying the role of inflammatory markers in the development of coronary thrombosis, both for diagnostic and prognostic purposes. In recent years, research findings have emerged indicating that the platelet/lymphocyte ratio is able to predict the severity of coronary artery injury and left ventricular dysfunction.
Objective: To assess the predictive role of TLR in relation to the severity of ACS without ST elevation in patients undergoing myocardial revascularization with single or multiple coronary vascular injury and LV dysfunction.
Research materials and methods: Inclusion criteria: patients with severe stenosis (> 50%) of one or more epicardial coronary arteries requiring cardiac intervention were included in the main study group. The comparison group included patients with minor lesions (normal coronary arteries, minimal plaques, stenoses <50%).
Exclusion Criteria: Patients with ST elevation >20 sec. in two consecutive ECG leads; with atrial fibrillation or supraventricular tachyarrhythmias, with ventricular tachycardia, idioventricular rhythm, atrioventricular blockade of 2 or 3 degrees, asystole, sepsis, oncological, systemic diseases, who received chemotherapy.
Results of the study: This study was conducted on the basis of the BSMP of the city of Semey. The study included 106 patients who were admitted to the emergency department with non-ST elevation ACS, with a history of revascularization and underwent coronary angiography from September 2021 to February 2022.
The main clinical manifestations of ACS were angina pectoris pain, sudden shortness of breath, asthma attacks, sudden loss of consciousness, acute heart failure or hypotension within 3 hours after the onset of symptoms. All patients underwent ECG analysis, biochemical tests and complete blood count (CBC), chest x-ray, echocardiography, troponin I and TLR.
For the period from September 2021 to February 2022, 2,880 therapeutic patients were admitted to the emergency department. Among which 1497 (52%) were cardiac patients. The proportion of patients with NSTE-ACS among cardiac patients is 10%.
Conclusions:
1) The proportion of patients with NSTE-ACS among patients admitted to the emergency department is 10%.
2) ETR has a strong correlation with the degree of coronary artery stenosis (0.814) and the left ventricular ejection fraction (-0.703). The higher the TLR value, the greater the damage to the coronary artery and the lower the EF.
3) TLR is a simple, fast and low-cost parameter that can predict the severity of IB and left ventricular systolic dysfunction in patients with non-ST elevation ACS.
228
Relaxation of venous bypass graft induced by procyanidin B2
Novakovic A1, Novakovic A1, Jankovic G1, Stojanovic I2,3, Milojevic P2,3, Nenezic D2,3, Kanjuh V4, Yang Q5,6, He G6
1 Department of Pharmacology, Faculty of Pharmacy, University of Belgrade, Belgrade, Serbia
2 Faculty of Medicine, University of Belgrade, Belgrade, Serbia
3 Institute for Cardiovascular Diseases “Dedinje”, Belgrade, Serbia
4 Academy of Sciences and Arts, Belgrade, Serbia
5 Department of Surgery, The Chinese University of Hong Kong, Hong Kong
6 TEDA International Cardiovascular Hospital, Medical College, Nankai University, Tianjin, China
Introduction: Interest in the therapeutic potentials of medicinal plants as an inexhaustible source of polyphenols is still high. In plant polyphenols, the most active fractions have been found in procyanidins which are shown to have different benefits on cardiovascular health. Vasodilation seems to be one of their cardioprotective mechanisms.
Objectives: Aim of our study was to investigate vasorelaxant effect of procyanidin B2 on human saphenous vein (HSV), and its underlying mechanisms which are still unclear.
Methods: Discarded segments of HSV were collected from patients undergoing coronary artery bypass grafting and studied in organ baths.
Results: Procyanidin B2 relaxed phenylephrine-induced contraction of HSV rings in concentration-dependent manner. The relaxation was strongly affected by the inhibitors of NO/cGMP pathway, L-NAME, hydroxocobalamin or ODQ. Indomethacin, a cyclooxygenase inhibitor, significantly affected only the relaxation produced by the highest concentrations of procyanidin B2. Combination of apamin and TRAM-34, selective blockers of small- and intermediate-conductance Ca2+-activated K+ (KCa) channels (SKCa and IKCa), in the presence of L-NAME and indomethacin, did not additionally decreased procyanidin B2-induced relaxation. Furthermore, relaxation induced by procyanidin B2 was partially attenuated by 4-aminopyridine, a predominant blocker of voltage-gated K+ (KV) channels, significantly inhibited by glibenclamide, a selective ATP-sensitive K+ channels (KATP) inhibitor, and almost abolished by iberiotoxin, a highly selective blocker of large-conductance KCa (BKCa).
Conclusions: Our results revealed that procyanidin B2 acts as a potent vasodilator on the isolated human venous graft. Mechanism of this endothelium- and concentration-dependent relaxation of HSV induced by procyanidin B2 probably involves stimulation of NO production, as well K+ channels opening, especially BKCa, and partially KATP and KV.
250
Effect of therapy with direct oral anticoagulants on PITX2 gene methylation in atrial fibrillation patients
Maslarinou A1,2, Ragia G1,2, Thomopoulos T3, Dovrolis N1,2, Kolios G1,2, Trikas A3, Manolopoulos V1,2,4
1 Laboratory of Pharmacology, Medical School, Democritus University of Thrace, Alexandroupolis, Greece
2 Individualised Medicine & Pharmacological Research Solutions Center (IMPReS), Alexandroupolis, Greece
3 Department of Cardiology, "Elpis" General Hospital of Athens, Athens, Greece
4 Clinical Pharmacology Unit, Academic General Hospital of Alexandroupolis, Alexandroupolis, Greece
Introduction: Atrial fibrillation (AF) is the most common cardiac arrhythmia and the most frequent cause of death in elderly people. Direct Oral Anticoagulants (DOACs) are now standard therapy for AF patients. PITX2 gene encodes for a transcription factor involved in the expression regulation of several ion channel subunits and in the development of left right cardiac asymmetry during embryonic life. Several studies report that PITX2 gene variations and epigenetic modifications (including PITX2 promoter methylation) are associated with AF incidence.
Objectives: The aim of the present study was to compare PITX2 gene promoter methylation pattern between DOAC treated AF patients and non-AF controls. Additionally, we have assessed the impact of DOAC therapy on PITX2 methylation.
Methods: Peripheral blood samples were drawn from 41 newly diagnosed AF patients eligible for DOAC treatment and 18 non-AF individuals (control group). For AF patients, samples were collected at three different points of their anticoagulation therapy: at the time of AF diagnosis prior to DOAC dosing (t0), a week after DOAC initiation (t1) and four weeks after DOAC initiation (t2). Genomic DNA extracted from all samples was bisulfite converted and PITX2 methylation was analyzed by use of qMSP-PCR. Methylation percentage of PITX2 promoter region was calculated and compared between patients and controls. Within the patient group, the methylation percentage was further compared among different therapy timepoints. The online tool NetworkAnalyst was used to create a gene-transcription factor network enabling further bioinformatics approaches.
Results: In patient group, a total of 14 minor bleeding events occurred, whereas no major bleeding or thrombotic events were recorded. Twenty-nine patients were treated with high DOAC doses. In both patients and controls, PITX2 promoter was lightly methylated and did not differ between two groups. In patients, DOAC therapy did not alter PITX2 methylation at different timepoints. When patients were categorized into experiencing bleeding events (cases) or not (controls), no differences were found for PITX2 methylation. Between high and low DOAC dose groups, multivariable linear regression analysis with % reduction of PITX2 methylation at t1 from baseline showed that high DOAC dose and increasing age reduce PITX2 methylation change (β= -1.968, 95% C.I. -3.567, -0.369, p=0,017 and β= -0.110, 95% C.I. -0.196, -0.024, p=0,013, respectively). Through gene-transcription factor network analysis, 8 transcription factors are potentially mediating PITX2 interaction with the DOAC targets thrombin and factor X.
Conclusion: PITX2 methylation did not differ between patients and non-AF controls. The effect of high DOAC dose on reduced PITX2 methylation change merits further investigation. Bioinformatics analysis supports the interaction of PITX2 and DOAC targets through a network of transcription factors that up- or downregulate coagulation cascade.
Funding: Financial support for project IMPReS (MIS 5047189) was provided by the Program “Competitiveness, Entrepreneurship and Innovation” (NSRF 2014–2020) co-financed by Greece and the European Union (European Regional Development Fund).
302
Correlation between risk reduction of major bleeding, thrombosis, and death in non-valvular atrial fibrillation and venous thromboembolism: metaregression of antithrombotic
Kilo R1,2, LEGA J1,3,4, PROVENCHER S5, BERTOLETTI L4,6
1 Univ Lyon 1, UMR CNRS 5558, Laboratoire de Biométrie et Biologie Evolutive, Equipe Evaluation Et Modélisation Des Effets Thérapeutiques, Université Claude Bernard Lyon 1, Lyon, France
2 Pôle de Santé Publique, Hospices Civils De Lyon, Lyon, France
3 Service de Médecine Interne et Vasculaire, Hôpital Lyon Sud, Hospices Civils De Lyon, Lyon, France
4 Groupe d’Etude Multidisciplinaires des Maladies Thrombotiques (GEMMAT), Hospices Civils de Lyon,, Lyon, France
5 Pulmonary Hypertension Research Group (http://phrg.ca), Institut universitaire de cardiologie et de pneumologie de Québec Research Center, Department of medicine, Université Laval, Quebec City, Canada
6 Service de Médecine Vasculaire et Thérapeutique, CHU de Saint-Étienne; INSERM, UMR1059, Equipe Dysfonction Vasculaire et Hémostase, Université Jean-Monnet; INSERM, CIC-1408, CHU Saint-Etienne, F-42055, Saint-Etienne, France
Background: The failure in preventing thrombotic recurrence in non-valvular atrial fibrillation (NVAF) and acute venous thromboembolism (VTE) and major bleeding (MB) related to anticoagulants may conduct to fatal outcomes. However, the relation between these outcomes is complex and poorly evaluated. This study aimed to estimate the correlation between risk reduction of MB, thrombotic events (TE), and all-cause mortality (ACM) in randomized trials assessing antithrombotics.
Methods: Randomized control trials comparing antithrombotics with placebo or standard treatment in acute VTE and NVAF were extracted from 1990 to December 2020. A weighted linear regression model was performed to quantify the trial-level coefficient of determination (R²trial) between the relative risk (RR) of MB or TE and ACM.
Results: Sixty-five eligible studies totaling 174,173 patients were included. In the 37 NVAF studies, treatment-related effects on ACM, TE and MB did not correlate, with R²trial for ACM-TE and ACM-MB of 0.00 (95%CI 0.00-0.45, p=0.74) and 0.04 (95%CI 0.00-0.35, p=0.34), respectively. However, sensitivity analysis showed a relation between ACM and MB in double-blind NVAF studies (R²trial=0.42; 95%CI 0.00-0.74, p=0.01). Conversely, the R²trial for ACM-TE and ACM-MB were 0.25 (95%CI 0.06-0.67, p<0.0001) and 0.19 (95%CI 0.00-0.58, p=0.02), respectively, in the 28 acute VTE studies.
Conclusion: The magnitude of reduction of MB and TE correlates with ACM in VTE trials. This association may be restricted to double-blind studies in NVAF. This trial-level analysis suggests that the association between treatment-related effects on TE recurrence, MB, and ACM differs according to the medical condition, patients characteristics, and design of the study.
304
MEASURING ANTIHYPERTENSIVE DRUG LEVELS TO DETERMINE NON-ADHERENCE IN HYPERTENSIVE PATIENTS WITH AND WITHOUT KIDNEY TRANSPLANTATION
Peeters L1, Hesselink D1, de Winter B1, Lafeber M1, Severs D1, van den Hoogen M1, Sonneveld M1, Ramakers C1, Bahmany S1, van Gelder T1, Koch B1, Versmissen J1
1 Erasmus Medical Center, Rotterdam, Netherlands
Introduction: Non-adherence to antihypertensive drugs (AHDs) is one of the main causes of resistant hypertension which is defined as uncontrolled blood pressure despite the prescription of at least 3 AHDs.
Objectives: Our primary objective was to determine the prevalence of non-adherence to AHDs in patients visiting the nephrology and vascular outpatient clinics. Inclusion rates at both departments allowed comparison between non-adherence of kidney transplantation (KT) patients with patients without KT as secondary objective.
Methods: This was a prospective observational study called RHYME-AD (Resistant Hypertension: MEasure Antihypertensive Drugs, MEC-2020-0096). Patients were eligible if they used at least 2 AHDs that could be measured with a validated UPLC-MS/MS method and had an office blood pressure >140 and/or 90 mmHg. After informed consent was signed, rest material was collected from a regular venipuncture measurement. The total absence of drug in blood (<lower limit of detection) was defined as non-adherent. We made comparisons between patients with and without KT and with and without resistant hypertension, defined as an office blood pressure >140 and/or 90 mmHg despite the use of 3 or more AHDs.
Results: Material was available from 143 patients of which 93 had a KT and 66 patients fulfilled the definition of resistant hypertension. The overall adherence rate was 78.3%. From the non-adherent patients 87.8% was partially non-adherent and 12.2% non-adherent for all prescribed AHDs. When looking at the two separate groups, with and without KT, more patients in the non-KT cohort fulfilled the definition of resistant hypertension as compared to the KT-cohort (62.0% vs 37.6%, p=0.005). There was a significant difference between having resistant hypertension and being non-adherent (non-adherence RH vs non-RH, 31.8% vs 13.0%, p=0.023) and having KT and being non-adherent (non-adherence KT vs non-KT, 14.0% vs 36.0%, p=0.005). In addition, patients with KT and no resistant hypertension were the most adherent to AHDs (91.2%) (Table 1).
Conclusions: Hypertensive patients with a KT are less likely to be non-adherent to their AHDs as compared to hypertensive patients without KT. However, fulfilling the definition of resistant hypertension, independent of KT, seems to increase the risk of being non-adherent to AHDs.
Tabel/Image
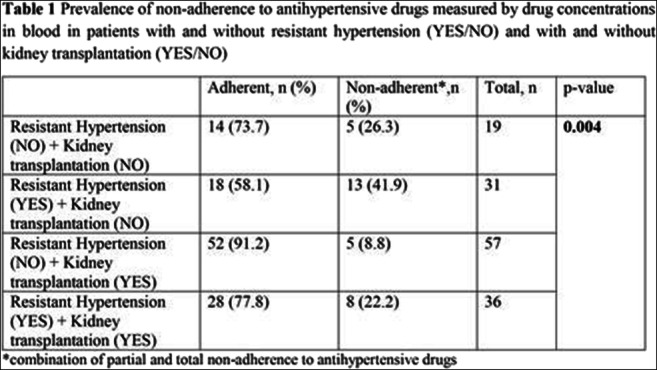
305
MEASURING DRUG LEVELS USING A DRIED BLOOD SPOT COMBINED WITH PERSONALIZED FEEDBACK TO IMPROVE RESISTANT HYPERTENSION: A RANDOMIZED CONTROLLED TRIAL
Peeters L1, Kappers M2, Hesselink D1, van Gelder T1, Koch B1, Versmissen J1
1Erasmus Medical Center, Rotterdam, Netherlands, 2Amphia Hospital, Breda, Netherlands
Introduction: Identification of non-adherence to antihypertensive drugs is crucial to improve resistant hypertension (RH). For this therapeutic drug monitoring is the most reliable method.
Objectives: The primary objective of this study is to determine whether drug levels measured with a dried blood spot (DBS) method combined with personalized feedback leads to a decrease in prevalence of RH after 12 months due to an increase in adherence.
Methods: This is a multi-center single-blinded randomized controlled trial (RHYME-RCT, NL6736). Eligible patients based on previous blood pressure readings underwent DBS sampling and a 24-hour ambulatory blood pressure measurement (ABPM) simultaneously. Patients with a daytime systolic blood pressure (SBP) >135 and/or diastolic blood pressure (DBP) >85 mmHg were randomized to standard treatment (control) or intervention. The intervention was performed by the treating physician and included information on drug levels and a personalized feedback conversation based on a feedback tool. The follow-up period was one year and included visits at 3, 6 and 12 (T=12) months inclusion. At each visit an 24h-ABPM and DBS were performed.
Results: A total of 141 patients were measured at baseline, of which 100 patients had blood pressures >135/85 mmHg and were randomized to the intervention (n=49) or control arm (n=51). The adherence rate in the excluded population (n=41) was 78.0%. The randomized patients had a mean age of 59 ± 11 years and were predominantly male (70.0%). The prevalence of RH decreased from 100% in both arms to 73.0% at T=12 in the intervention arm (p=0.002, n=37) and 60.0% at T=12 in the control arm (p<0.001, n=35). Adherence rates improved from 67.3% to 92.1% in the intervention arm (p=0.008) and 68.6% to 71.4% in the control arm (p=0.268). No statistically significant differences were found after 12 months between the two groups in the degree of RH (p=0.243) or adherence (p=0.056).
Conclusions: Measuring blood pressure and drug levels led to a decrease in the prevalence of RH and improved non-adherence. However, this improvement could not be linked to the actual intervention. The fact that one third was excluded at baseline because of normotension suggests that participation in a study already leads to improvement of adherence and thus blood pressure control.
310
Dietary remodeling of intestinal microbiota to reduce the number of cardiovascular events in patients after acute myocardial infarction
Sergazy S1, Zhashkeyev A2, Gulyayev A1, Aljofan M1, Zhumadilov Z1
1 Nazarbayev University, NUR-SULTAN, KAZAKHSTAN
2 Karaganda Medical University, Karaganda, Kazakhstan
Introduction: Intestinal microbiome is one of important regulators of the pathogenesis of atherogenesis and other related diseases. Changes in the level of trimethylamine N-oxide (TMAO) and oxidative stress in blood plasma are can be used as biomarkers to determine the relationship between cardiovascular diseases and the state of the intestinal microbiome (“gut-heart axis”), as well as the degree of risk of cardiovascular events. It is proposed that correcting the state of the gut microbiome with plant polyphenols, as determined by the dynamics of TMAO and indicators of oxidative stress in blood plasma will reduce the risk of cardiovascular events in patients with coronary heart disease.
Objective: To investigate the relationship between cardiovascular events in patients with acute myocardial infarction during dietary remodeling of the intestinal microbiota using grape polyphenol concentrate.
Methods: We initiated a randomized, placebo-controlled clinical trial in 150 patients to evaluate the effect of daily (for 3 months of the year, starting one week after acute myocardial infarction with coronary artery stenting) oral administration of 15 Cabernet Sauvignon grape polyphenol concentrate (a dietary supplement with a concentration of polyphenols 2 mg/ml). We measured the change in TMAO concentration, the level of oxidative stress, as well as the number of cardiovascular events during the year in patients after acute myocardial infarction with ST segment elevation (STEMI). Secondary endpoint: was to measure the degree of progression of coronary atherosclerotic plaques according to the SYNTAX scale.
Results: Up until now (6 months of the study), the administration of grape polyphenol concentrate in patients after acute myocardial infarction, resulted in a significant reduction of oxidative stress level and approximately 2-fold increase in the antioxidant capacity of blood plasma, compared to placebo (placebo - red grape juice). On average the initial level of TMAO concentration decreased by 38.0 ± 1.27% in patients who had a three-month intake of grape polyphenol concentrate, which might be due to the correction of the state of the intestinal microbiome. Also, there were no major CV events reported in patients in the polyphenol concentrate group for 6 months.
Conclusion: The observed reduction of oxidative stress and TMAO formation during the dietary intervention with grape polyphenol concentrate can be regarded a significant improvement and positive biomarkers of the relationship between the state of the intestinal microbiome and the state of the cardiovascular system. The established changes in biomarkers are evidence of the correction of the intestinal microbiome and may be predictors of a decrease in the risk of cardiovascular events. Evidence for the prevention of major CV events in patients after myocardial infarction with grape polyphenol concentrate is expected to come at the end of the clinical trial.
Chronic diseases
16
Oleic Acid Chronopharmacology on Obese Patients Adipose Tissue Biological Response
RODRIGUEZ F1, MARTIN F1, GARCIA S2, HO A1, RODRIGUEZ C1, LOPEZ C1, VALDES S2, MORENO F2, FERNANDEZ J3, GARCIA E1
1 Ibima- Hospital Universitario Virgen de la Victoria, Malaga, Spain
2 Ibima-Hospital Regional de Malaga, Malaga, Spain
3 Facultad de Telecomunicaciones-Universidad de Vigo, Vigo, Spain
TITLE: Oleic Acid Chronopharmacology on Obese Patients Adipose Tissue biological response
Introduction: Chronopharmacology examines the influence administration time of a substance in the biological response and the effect of timing on the biological rhythms. Chronobiologycal effects of diet composition are not clarified yet, and the importance of the time of intake has become important nowadays. We have reported that oleic acid (OA) the major component of Mediterranean diet, restores core circadian clock genes rhythmicity in gastrointestinal explants from patients with morbid obesity applied at 9 am. Obesity shows a chronodisruption associated and the molecular circadian clock plays a role in metabolic homeostasis. Knowing the interconnections between circadian clocks, diet and concretely lipid metabolism, are all a potential applications for the future of medicine.
Objetives: Study of OA effect at 9am and 9 pm on core circadian clock genes and lipid metabolism on adipose tissue (AT) of obese patients.
Patients and Methods: AT explants from obese patients were incubated with/without OA at 9am/9 pm and samples were takeout every 6 hours during 24h. We have studied the circadian rhythmicity of BMAL1, CLOCK, PER1-3, REVERBα, CRY1-2 and FAS, SREBP1, LPL and CPT1 enzymes by RT-PCR approach.
Results: As it is shown on Table1, AT explants showed an altered circadian rhythm in all genes studied and OA led to the emergence rhythmicity in most of them. On CLOCK and BMAL1 OA delayed the acrophase at 9am. On Per1-3, OA enhanced amplitude at 9am and delayed acrophase at 9pm, and the same effect has been found on REVERBα. OA on CRY1-2 led to emergence rhythmicity only at 9am. Respect to lipid enzymes, the 4 genes studied show circadian rhythmicity less SREBP. OA effect on FAS show an enhanced amplitude at 9am and a delayed on acrophase at 9pm with similar effect by OA on SREBP and LPL, enhanced amplitude and delayed acrophase both at 9am. However the OA effect on CPT1 was significant at 9pm, enhanced amplitude and delayed acrophase.
Conclusion: OA restored the rhythmicity of most genes studied but tends to increase the amplitude at 9am and to delay acrophase at 9pm. These behavioral differences must be further studied in order to have a key to improve our time to eat some nutrients in order to restore circadian rhythms correctly.
Key words: Obesity, Chronobiology, Metabolism, Lipid enzymes, clock genes
Tabel/Image
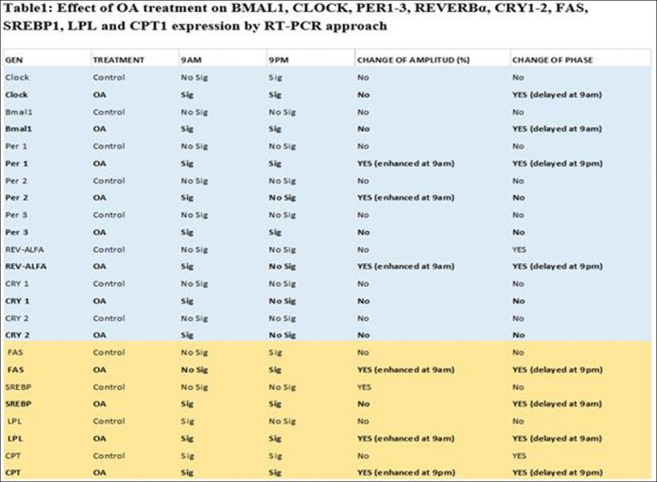
81
H1 ANTIHISTAMINES EFFECT ON INFLAMMATORY MARKERS IN GRASS POLLEN ALLERGIC RHINITIS
Bocsan I1, Muntean I1, Miron N2, Sabin O1, Deleanu D1, Buzoianu A1
1 Iuliu Hatieganu University of Medicine And Pharmacy, Cluj Napoca, Romania
2 Department of Clinical Immunology, Sahlgrenska University Hospital, Gothenburg, Sweden
Adhesion molecules like ICAM-1 and VCAM-1 play important roles in pathophysiology of allergic rhinitis (AR). Treatment with H1 antihistamines improves the symptoms of AR and in vitro studies showed that they might reduce the levels of adhesion molecules. The aim of the study was to evaluate serum levels of ICAM-1 and VCAM-1 in patients with AR to grass pollen and the effect of 2nd generation H1 antihistamines on them. Material and methods: Fifty patients with AR to grass pollen were clinically and biologically evaluated before and after 1 month treatment with Levocetirizine and Desloratadine. ICAM-1 and VCAM-1 serum levels were evaluated during pollen season before and after treatment, through the ELISA method. Results: ICAM-1, VCAM-1, eosinophils, and total IgE were elevated in patients with AR, compared to healthy subjects. Both Levocetirizine and Desloratadine improved significantly specific symptoms of AR and increased patients’ quality of life during pollen season and they reduced VCAM-1, ICAM-1, and total IgE but not significantly. The reduction in ICAM-1 and VCAM-1 was noticed in less than half of the patients. Patients with increased baseline values tend to remain with increased ones after one-month treatment. The reduction of adhesion molecules were more significant in patients with moderate severe forms of AR compared to mild AR. Conclusions: ICAM-1 and sVCAM-1 levels are higher in patients with grass pollen-induced AR than healthy controls during pollen exposure. Baseline values of CAMs tend to remain higher during pollen exposure and they were not changed significantly despite AH1 treatment.
90
Therapeutic care of headache according to family medicine practitioners
Sanaï S2, Charfi R1,2, Khammassi N2, Daghfous R1,2, Trabelsi S1,2
1 National Centre Chalbi Belkahia of Pharmacovigilance, Department of clinical pharmacology, LR16SP02, Tunis, Tunisia
2 University of Tunis El Manar, Faculty of Medicine of Tunis, Tunis, Tunisia
Background: Headache represents 1 to 2% of emergency room consultation. Although they may be perceived as trivial, they can also signify a serious medical condition. So even a mild headache can be very disabling and requires appropriate treatment.
The aims of our present of our research are to assess the therapeutic management of acute and chronic headache by general practitioners and family medicine residents, and to estimate the rate of chronic headache due to drug abuse as well as compliance and self-medication.
Methods: We conducted a descriptive cross-disciplinary study through a Google-forms online self-administered questionnaire intended for general practitioners and family medicine residents, throughout a 2-month time period, from october 1st to december 1st, 2021.
Results: A total of 50 physicians responded to our questionnaire, 84% of whom were family medicine residents. The majority worked in a university hospital center 74%. The median of patients who consulted for a headache per month was 10. The estimated frequency of headaches in current practice is more than 5% for 38% of physicians. The majority of doctors surveyed (88%) prescribed a first-line treatment (paracetamol in 98% of cases).
Regarding adverse events, gastric damage is reported in 42% of cases and dependence or overconsumption in 10% of cases. In case of adverse reaction appearance, in 62%, physians stopped treatment and only 12% referred their patients for pharmacovigilance.
For treatment adherence, 52% said that patients with chronic headache very frequently to frequently have good adherence. The majority of physicians (98%) said that patients self-medicate very frequently to frequently. Among questioned physicians, 64% stated that they did not experience chronic daily headaches due to drug abuse. Almost the majority of doctors who answered our questionnaire feel the need to improve their knowledge (98%) in terms of acute and chronic headaches (postgraduate teaching sessions and workshops).
Conclusion: General practitioners play an essential role in the treatment and screening of headache which can cause significant personal suffering, a deterioration of the quality of life and an important financial health cost.
137
Agreement between Study Protocols and Journal Publications of Pulmonary Hypertension-Specific Therapy Clinical Trials Subgroup Analyses: A Systematic Review
Rodríguez Ramallo H1, Báez Gutiérrez N2, Otero Candelera R1, Abdel-kader Martín L1
1 Hospital Universitario Virgen del Rocío, Sevilla, Spain
2 Hospital Universitario Reina Sofía, Córdoba, Spain
Introduction: Subgroup analysis (SA) may generate research hypotheses and influence clinical practice. As trial protocols provide a complete insight into the analysis methods utilized in randomized controlled trials (RCTs), they should be consulted when assessing critical aspects of the credibility of SA.
Objectives: To evaluate the agreement in SA planned in the trial protocol of pulmonary hypertension-specific therapy RCTs (PHST-RCTs) and those finally reported in the journal publication.
Methods: A systematic review of Medline, including PHST-RCT published between January 2000 and December 2020, was carried out to identify SA reports.
For RCTs reporting subgroup analyzes, we actively searched for published study protocols on www.clinicaltrials.gov and the publisher web page.
We assessed the agreement between the journal publication and the trial protocol on the number of subgroup factors, SA, and outcomes, as well as the pre-specification of such analyses.
Results: A total of 30 PHST-RCTs reported SA, of which only eight (26,7%) made the protocol available. Significant differences were found for all eight of the RCTs between the trial protocol and the published manuscript:
Subgroup analyses: Six RCTs reported fewer numbers of SA than prespecified in the protocol. The two RCTs remaining reported subgroup analyses that were not prespecified in the protocol; in both cases, these analyses were characterized as prespecified in the published manuscript.
Subgroup Factors: The number of subgroup factors reported differed between the protocol and the published manuscript in seven cases: five RCTs reported fewer factors than those specified in the protocol, the remaining two added several subgroup factors not previously defined.
Selective reports of subgroup analyses by outcome: There were differences in the number of subgroup analyses reported for the primary outcome in seven RCTs. In addition, in four protocols, authors specified that subgroup analysis would be carried out for primary and secondary endpoints; however, the published manuscript only reported the subgroup analyses for the primary endpoint on three of these RCTs.
Conclusion: Most PHST-RCTs did not publish the study protocol. For those studies whose protocol was available, SA reported in the manuscript lacked description and significantly differed from those planned in the protocol.
138
Credibility of subgroup Claims in Pulmonary Hypertension-Specific-Therapy Clinical Trials: A Systematic Review
Rodríguez Ramallo H1, Rodríguez Ramallo H2, Otero Candelera R2, Abde-kader Martín L2
1 Hospital Universitario Reina Sofía, Córdoba, Spain
2 Hospital Universitario Virgen del Rocío, Seville, Spain
Introduction: Pulmonary hypertension treatment decisions are driven by randomized controlled trials (RCTs) results. Subgroup analyses (SA) are often performed, and their wrongful interpretation may result in denial of a beneficial treatment or even the administration of potentially harmful treatment.
Objectives: This study aims to assess the quality of SA and the credibility of subgroup effect claims in pulmonary-hypertension specific therapy (PHST)-RCTs.
Methods: A systematic review of Medline, including PHST-RCTs published between January 2000-December 2020, was carried out to identify SA reports.
The following variables were collected: number of SA, pre-specification of SA, and use of interaction test.
In order to evaluate the credibility of subgroup claims for RCT primary outcomes, the ten criteria of Sun et al. 2012 were applied.
The review protocol was registered with the PROSPERO register: CRD42021242265.
Results: A total of 30 studies reported SA. The median SA reported was 7 (range 2-36). In 14 RCT, the SA were prespecified, and in 11, an interaction test was used.
11 RCT reported 13 claims of subgroup difference for the primary outcome. Authors included subgroup variables for the primary outcome measured at baseline for 13 claims (100%), used subgroup variable as stratification factor at randomization for 3 (23.1%), clearly prespecified their hypothesis for 3 (23.1%), the subgroup effect was one of a small number of hypothesized effects tested for one (7.7%), carried out a test of interaction that provides statistically significant for 4 (30.8%), documented replication of a subgroup effect with previously related studies for 8 (68.5%), identified the consistency of a subgroup effect across related outcome for none, and provided a biological rationale for 6 (46.2%). 12 out of 13 claims met four or fewer of the ten criteria.
The inter-reviewer agreement for assessing the credibility of the subgroup claims was 0.88 (95% CI: 0.77-0.98).
Conclusion: SA in PHST-RCTs are of poor quality. Most claims of subgroup effect did not meet critical criteria. Thus, although SA in published PHST-RCTs may generate research hypotheses, scepticism is recommended in their interpretation due to methodological limitations.
157
Predictors of bisoprolol effects in acute coronary syndrome patients
Zagorodnikova K1, Shumkov V, Murzina A, Boldueva S
1 North-western State Medical University N.a.i.i.mechnikov
Objectives: We aimed at evaluation of the individual factors predictive of bisoprolol effects in real clinical practice.
Methods: We included patients who were prescribed bisoprolol after having suffered acute coronary syndrome (n 102). Blood samples were taken for genotyping. CYP2D6*3, *4; CYP3A5*3 carriers were identified using real-time pcr (“DNA-technology”, Russia). All patients had 24-hours ECG monitoring on day 10 after the start of treatment. Standard biochemistry and concomitant medications were registered.
Results: The patients age was 63±10 years; 62 men, 40 women. CYP3A5*3 allele prevalence was 0.93; CYP2D6*4 – 0.15; CYP2D6*3 was not found. Genotype distribution was in Hardy-Weinberg equilibrium (p>0.05). Despite that CYP2D6*4 carriage was negatively associated with lower heart rate at exertion (Spearman r-0.21; р<0.05) regression analysis revealed that this was only due to older age of CYP2D6*4 carriers, and the latter factor was the only predictor for this variable (β = -0.6; SE = 0.07; p <0.001). CYP3A5*1 carriers required higher bisoprolol dose (0.07 [0.06; 0.11] mg/kg) to achieve a similar mean heart rate as CYP3A5*1 non-carriers (0.06[0.03;0.06] mg/kg), Mann-Whitney p<0.05). The only CYP3A5*1*1 carrier had maximal dose of 10 mg/day. Since renal function was another factor associated with bisoprolol dose variability we created another regression model, which included renal function (Cockroft-Gault equation; functional class of heart failure, gender, age, number of CYP3A5 substrated). CYP3A5 genotype was the only predictor for the bisoprolol dose (F=8.5; p 0.005; R2=0.096).
Conclusion: We found that CYP3A5 genotype was associated with bisoprolol individual dose requirement in a setting of acute coronary syndrome patients. This conclusion is hypothesis-generating and should be tested in a more controlled manner on a larger population.
183
Rhabdomylosis in severe acute asthma
Fathallah N1, Ouni B1, Tej A2, Kebaili R2, Ben Salem C1, Slim R1
1 Department of Pharmacology, Laboratoire de Biophysique Métabolique et Toxicologie Professionnelle et Environnementale Appliquée LR12ES02. Université de Sousse, Faculté de Medecine, Sousse
2 Department of Pediatry, Farhat Hached University Hospital, Sousse
Introduction: Asthma is the most common chronic illness in childhood. It represents a significant burden to health care and educational systems. Severe acute asthma is a complication encountered in asthma’s illness. Rhabdmoylosis associated with severe acute asthma is an uncommon complication not well known by clinicians.
Method: We present a case of rhabdomylosis occurring after severe acute asthma in an 11 year-old infant and we discuss the main origins of this event.
Case report: An 11-year-old boy with history of asthma controlled with short-acting β2-agonist upon request, had developed coughing and dyspnea after dust’s exposure. Respiratory function had worsened and he was transferred to the pediatric department. Physical examination showed an apyretic patient, tachycardia (120bpm) polypnea, dyspnea, cyanosis and use of accessory muscles of respiration. The diagnosis of severe acute asthma was maid and the infant was incubated and ventilated. He received salbutamol 0.25mg/h, solumedrol 80 mg intravenous then 40 mg intravenous every 6 hours. Respiratory signs improved gradually. However, at the biological test, an increased CPK at 1626 UI/ml was noticed. Otherwise,his renal and liver function was normal. K+ was at 5.74 mmol/L, Na+ was at 137mmol/L. Blood gas measurements showed respiratory acidosis. Pharmacovigilance department was advised. Rhabdomylosis secondary to high doses of corticosteroids was suspected. A decrease in doses of solumedrol was recommended. The monitoring of CPK values revealed a decrease in CPK values (1478 then 800).
Discussion: This infant had developed a rhabdomylosis in the course of severe acute asthma. The association of rhaddomyolysis and asthma has been described at the first time by Chud et al, however; the problem consists to determine the cause of rhabdomylosis occurring in such cases. In the literature, some hypothesis could be found to explain such event. Indeed, theophylline intoxication, hypokalemia secondary to excessive use of β2-agonists or infection with legionella pneumophila and Mycolpasma pneumonia are likely induce rhabdomylosis. Yet, the most common cause seems to be hypoxia, acidosis and intensive muscular work of respiratory muscles. In our case, rhabdomyolysis is most likely to be induced by high doses of corticosteroids since decreasing of CPK values when clinicians decreased doses of corticoids given to this infant. Moreover, his potassium blood rate was within normal range. High doses of corticosteroids may induce myopathy which is related to rhabdomyolysis.
Conclusion: Close monitoring of CPK in severe acute asthma remains preferable due to the risk of occurrence of rhabdomyolysis.
190
THE USE OF ADALIMUMAB IN CHILDREN WITH JUVENILE IDIOPATHIC ARTHRITIS ASSOCIATED WITH UVEITIS. CLINICAL OBSERVATION
Tanuarbek U1, Temirgalieva E2, Mukhitova D3
1 Kazakh National Medical University named after S.D.Asfendiyarov, Almaty, Republic of Kazakhstan
2 Kazakh National Medical University named after S.D.Asfendiyarov, Almaty, Republic of Kazakhstan
3 Kazakh National Medical University named after S.D.Asfendiyarov, Almaty, Republic of Kazakhstan
Introduction: One of the urgent problems of modern pediatrics and pediatric rheumatology is juvenile idiopathic arthritis (JIA), a multifactorial disease with a complex immunoaggressive pathogenesis characterized by a steadily progressive course and development of joint destruction, as well as a wide range of extra—articular manifestations. To date, tumor necrosis factor (TNF) undoubtedly plays a key role in the pathogenesis of rheumatoid arthritis and its importance as a target for anti-rheumatic therapy. In the Republic of Kazakhstan, human monoclonal antibodies to tumor necrosis factor (TNF) - Adalimumab, Infliximab, Golimumab — are allowed for use for antirheumatic therapy. The drug Adalimumab is included in the clinical protocol of the Ministry of Health of the Republic of Kazakhstan for the diagnosis and treatment of juvenile idiopathic arthritis dated 03.05.2019. We conducted a clinical observation of the successful use of Adalimumab in a child with juvenile idiopathic arthritis associated with uveitis.
Objectives: The format of clinical observation for the successful use of Adalimumab in a patient with juvenile idiopathic arthritis (JIA) associated with uveitis is presented.
Methods: The design of the study is analytical retrospective. Clinical observation of 1 patient diagnosed with Juvenile idiopathic arthritis associated with uveitis on the basis of one of the multidisciplinary clinics. Patient S., born in 2005, diagnosed with Juvenile idiopathic arthritis (JIA), pauciarticular variant, with eye damage of the type of chronic uveitis, moderate activity. The patient's condition before switching to Adalimumab: JIA was first diagnosed in November 2013, uveitis joined in 2016. From the moment of diagnosis until 2017, therapy was carried out with drugs of systemic glucocorticosteroid drugs and antimetabolites, without effect. Taking into account the long-term immunosuppressive therapy, with the absence of positive dynamics, deterioration of visual acuity, in April 2017, the patient was prescribed genetically engineered biological therapy with Adalimumab at the rate of 24 mg / sq.m 40mg. Duration of clinical observation: April 2017 - December 2021). The effectiveness of therapy is assessed according to clinical data: the presence of pain syndrome, the volume of movement in the joints, laboratory data: leukocyte counts, erythrocyte sedimentation rate (ESR), Antistreptolysin-O (ASLO).
Results: During the period of clinical observation of the use of Adalimumab in the 40 mg mode 1 time in 2 weeks subcutaneously: the severity of the pain syndrome decreased, the volume of movements in the joints increased, it became easier to walk, daily activity expanded. The patient began to lead an active social life, engage in physical education. There is a significant improvement in his psycho-emotional state. According to ophthalmological data, the deterioration of visual acuity did not progress, according to laboratory data: leukocyte counts decreased by 17.6%, ESR decreased by 83.33%, the ASLO index decreased by 51%. Not a single undesirable phenomenon has been registered.
Conclusion: Thus, this drug Adalimumab demonstrates high efficacy, positive effect on physical activity and quality of life in a patient with juvenile idiopathic arthritis associated with uveitis.
232
Niclosamide attenuates inflammatory mediators-associated profibrotic responses in subepithelial lung myofibroblasts
Spathakis M1,2, Tarapatzi G1,2, Filidou E1,2, Kandilogiannakis L1,2, Karatzas E3, Steiropoulos P4, Mikroulis D5, Spyrou G6, Manolopoulos V1,2, Kolios G1,2, Arvanitidis K1,2
1 Laboratory of Pharmacology, Faculty of Medicine, Democritus University of Thrace, Alexandroupolis, Greece
2 Individualised Medicine & Pharmacological Research Solutions Center (IMPReS), Alexandroupolis, Greece
3 Institute for Fundamental Biomedical Research, BSRC “Alexander Fleming”, Athens, Greece
4 Department of Pneumonology, Medical School, Democritus University of Thrace, Alexandroupolis, Greece
5 Department of Cardiothoracic Surgery, Medical School, Democritus University of Thrace, Alexandroupolis, Greece
6 Bioinformatics Department, The Cyprus Institute of Neurology and Genetics, Nicosia, Cyprous
Introduction: Niclosamide is a compound primarily used for the treatment of helminth infestations. Recently, data derived from a bioinformatics analysis have proposed this drug as an anti-inflammatory and anti-fibrotic agent in fibrotic diseases such as Idiopathic Pulmonary Fibrosis.
Objective: The aim of our study was to investigate the effect of Niclosamide on the fibrotic response in Subepithelial Lung Myofibroblasts (SELMs) after stimulation with pro-inflammatory and pro-fibrotic cytokines.
Methods: Human SELMs were isolated from tissue biopsies of patients undergoing surgery for lung cancer and then set to culture. SELMs were stimulated for 6 hours with TNF-α (50ng/ml), IL-1α (5ng/ml), added alone or in combination, and TGF-β1 (5ng/ml) in the presence or not of Niclosamide (30nM and 100nM). Total RNA was isolated and the expression of Collagen type I, Collagen type III and Fibronectin was studied by qRT-PCR. Migration of SELMs was studied using a Wound Healing Assay.
Results: Niclosamide had no effect on baseline mRNA expression levels of Collagen type I, III or Fibronectin of SELMs. Stimulation with TGF-β1, IL-1α and/or TNF-α upregulated the expression of Collagen Type I, type III and Fibronectin. Treatment with Niclosamide at 100nM ameliorated the effect of almost every cytokine stimulation on the expression levels of Collagen type I (IL-1α: 0.52-fold, ±0.0, TNF-α: 0.74-fold, ±0.03 p<0,0001 and IL-1α+TNF-α: 0.76-fold, ±0.07, p<0.05, TGF-β1:1.31-fold, ±0.16, p<0.05), type III (IL-1α: 0.75-fold, ±0.02, TNF-α: 1.07-fold, ±0.19, IL-1α+TNF-α: 0.81-fold, ±0.11, p<0.05 and TGF-β1:0.77-fold, ±0.31, p<0.01) and Fibronectin (IL-1α: 1.02-fold, ±0.08, TNF-α: 0.77-fold, ±0.03 and IL-1α+TNF-α: 1.11-fold, ±0.06, p<0.05), while Niclosamide treatment at 30nM exhibited a weaker effect. SELMs migration was increased in the presence of TGF-β1 (118.3%, ±8.8, p<0.05), while addition of the higher dose of Niclosamide debilitated the migratory ability of both stimulated (68.02%, ±2.96, p<0.0001) and unstimulated SELMs (69.75%, ±4.24, p<0.0001).
Conclusion: In this study we showcase an anti-fibrotic effect of Niclosamide on SELMs stimulated with profibrotic and pro-inflammatory cytokines. These data suggest a possible therapeutic effect of Niclosamide in pulmonary fibrosis.
Funding: This study was partially supported by «Strategic expansion of the Greek Biobanking Infrastructure» (BBMRI‐GR) in the framework of the Action "Enhancing Research and Innovation Infrastructure - Second Cycle" (NSRF 2014-2020) and by the project IMPReS (MIS 5047189) financially supported by the Program “Competitiveness, Entrepreneurship and Innovation” (NSRF 2014–2020) co-financed by Greece and the European Union (European Regional Development Fund).
249
Alterations in glutamatergic function of Fmr1 Knock out rats, an animal model of autism spectrum disorders
Ntoulas G1, Brakatselos C1, Asprogerakas M1, Nakas G1, Kokras N2,3, Polissidis A1,4, Dalla C2, Antoniou K1
1 University of Ioannina, Faculty of Medicine, Department of Pharmacology, Ioannina, Greece
2 Department of Pharmacology, Medical School,National and Kapodistrian University of Athens, Athens, Greece
3 First Department of Psychiatry, Eginition Hospital, Medical School, National and Kapodistrian University of Athens, Athens, Greece
4 Center of Clinical, Experimental Surgery and Translational Research, Biomedical Research Foundation of the Academy of Athens, Athens, Greece
Introduction: Fragile X Syndrome (FXS) is a neurodevelopmental disorder with autism-like symptomatology that is caused by the loss of Fragile X Mental Retardation protein (FMRP). The majority of animal studies for approaching this genetic disorder have employed mice models while a few studies have focused on Fmr1 knockout rats (Fmr1KO), an alternative preclinical approach of FXS.
Objectives: In this study we evaluated the biophenotype of Fmr1KO rats using behavioral tasks related to motor responses and cognitive functions along with neurobiological markers which associate to glutamatergic activity.
Methods: Male 12-16 weeks-old rats, LE-Fmr1em2Mcwi referred to as Fmr1KO and WT littermates, were used in this study. Spontaneous and habituated motor activity was evaluated using an open field apparatus. In addition, behavioral motor habituation was assessed as an index of non-associative learning and its retention in both groups. Glutamatergic function was evaluated in terms of neurotransmitter activity and receptor protein expression, in distinct rat brain regions, namely the Prefrontal Cortex, Dorsal and Ventral Hippocampus.
Results: Our findings show a specific bio-phenotype of Fmr1KO rats as compared to their WT counterparts. Fmr1KO rats displayed elevated spontaneous and habituated motor activity over the course of the experiment and disrupted behavioural habituation. Protein expression levels of NMDA and AMPA receptor subunits were altered in a region-specific manner. Glutamate and GABA levels were estimated through HPLC-ED and found to be genotype dependent.
Conclusion: Based on our findings a stimulated motor profile was observed in Fmr1KO rats along with glutamatergic region-specific alterations. Present results essentially contribute to issues related to preclinical approaches of FXS and autism spectrum disorders.
This research has been co‐financed by the European Regional Development Fund of the European Union and Greek national funds through the Operational Program Competitiveness, Entrepreneurship and Innovation, under the call RESEARCH – CREATE – INNOVATE project code:T2EDK-02075
255
Anti-VEGF intravitreal injections in age-related macular degeneration of wet type, 1 Year follow up at the General Hospital of Kilkis
Vousnakis D1, Karailidou P1, Kazantzidis L1, Kalaitzidou A1, Tataridis G1, Demirtsoglou D1, Papadopoulos A1
1 Ophthalmology department, Kilkis, Greece
Dimitrios Vousnakis, Panagiota Karailidou, Lazaros Kazantzidis, Alina Kalaitzidou, Despina Demirtsoglou, Georgios Tataridis, Adamantios Papadopoulos
Ophthalmology Clinic, General Hospital of Kilkis
Introduction: Age related macular degeneration (AMD) is a chronic disease which has two forms: wet and dry type. The wet type is treated with anti-VEGF intravitreal injections.
Objectives: To evaluate the effectiveness of anti-VEGF injections therapy of wet AMD during a follow-up period of 1 year.
Methods: Information was drawn from the clinic's records from 22 patients with AMD who underwent Anti-VEGF treatment with the PRN protocol (pro re nata, "as needed"). The factors examined were age (<80, 80 years and above), gender (male, female), bilateral involvement of the eyes, the pharmaceutical substance used (ranibizumab, aflibercept), the number of injections and the change in vision after 1 year of treatment. For the processing of the data, the necessary statistical tests were performed: one-sample Kolmogorov-Smirnov test, independent-samples t test, paired-samples t test.
Results: The statistical analysis showed, that after a year of treatment, patients with an average of 4 injections (mean=4, with several patients reaching 5 and 6 injections) showed an improvement in their vision by about 1/10 (Mean difference=0.762, p=0.032). For every other factor examined, no statistically significant correlation was found (p>0.05). It is also noted that it was not possible to compare the effectiveness of the two drugs as only 3 patients were treated with alfibercept.
Conclusion: Patient vision improved on average about 1/10 after 1 year of treatment. However, for the rest factors examined, we consider that the number of individual subgroups statistically analyzed was small and for that reason it is necessary to conduct future analyses on a larger sample of patients in order to draw safer conclusions.
256
Anti-VEGF intravitreal injections in diabetic macular edema, 1 Year follow up at the General Hospital of Kilkis
Vousnakis D1, Karailidou P1, Kazantzidis L1, Kalaitzidou A1, Tataridis G1, Demirtsoglou D1, Papadopoulos A1
1 Ophthalmology department, Kilkis, Greece
Introduction: Diabetic macular edema (DME) is a long term complication of Diabetes which is treated with anti-VEGF intravitreal injections.
Objectives: To evaluate the effectiveness of anti-VEGF injections therapy of DME over a follow-up period of 1 year.
Methods: From the clinic's records, information of 21 patients was drawn and examined. They were all treated according to the PRN protocol (pro re nata, "as needed"), where in the case of bilateral eye involvement, one eye was randomly selected. The factors examined were: age (65, 66-75, >76), sex (women, men), chronicity of diabetes mellitus (3 groups: <5 years, 5-9 years, >9 years), type of treatment (tablets, insulin), bilateral eye involvement, the pharmaceutical substance used (ranibizumab, aflibercept), the number of injections and the change in visual acuity after 1 year of treatment. To extract the results, the necessary statistical tests were performed: one-sample Kolmogorov-Smirnov test, independent-samples t test, paired-samples t test, one-way anova.
Results: Statistical analysis of the data showed that the visual acuity of the patients improved by about 1/10 after 1 year of treatment (Mean difference=1,387, p=0,001). For every other factor examined, no statistically significant correlation was found (p>0.05). At the same time, it is noted that patients with ranibizumab needed about 4 injections (Mean=4.25), while those with alfibercept need about 6 injections (Mean=5.73), while there was no difference in the effectiveness of the two drugs (p>0.05).
Conclusions: After 1 year of treatment, patients' vision improved by an average of 1/10. However, for the rest factors examined, we consider that the number of individual subgroups statistically analyzed was small and for that reason it is necessary to conduct analyses on a larger sample of patients in order to draw safer conclusions.
263
Nutritional derangement of one-carbon metabolism pathway might induce kidney and cardiac disease that are aggravated in case of diabetes
Liapi C1, Strilakou A1, Al- Humadi A2
1 Medical School, National And Kapodistrian University Of Athens, Athens, GREECE
2 Conway Institute, University College Dublin, Dublin, IRELAND
Introduction: One-carbon metabolism encompasses a broad range of biosynthetic reactions driven by folate and methionine cycles generating methyl groups for the synthesis of DNA, amino acids and phospholipids. Choline is a vitamin-like soluble essential nutrient involved in many biologic functions such as lipid transport, energy homeostasis and nerve impulse transmission while is considered a crucial methyl donor in the maintenance of one-carbon metabolism. Choline deficiency setting is an established experimental model of non-alcoholic steatohepatitis (NAFLD) associated with glucose intolerance, increased oxidative stress and mitochondrial dysfunction. Diabetes mellitus is a chronic disease with an increasing prevalence worldwide due to sedentary lifestyle, obesity and aging. Among the most serious complications of diabetes are the cardiac and kidney disease. Diabetic cardiomyopathy is characterized by cardiac stiffness and diastolic dysfunction with preserved ejection fraction while diabetic nephropathy by albuminuria, glomerular and tubular lesions, loss of glomerular filtration rate and mesangial expansion.
Objectives: The aim of the present study was to investigate the effects of dietary choline deprivation on the heart and kidney tissue architecture and functional integrity and also evaluate the role of diabetes mellitus in this setting.
Methods: Male Wistar Albino rats were divided randomly into four groups: control (C) and diabetic (DM) rats fed with standard diet, choline-deprived (CD) and diabetic choline-deprived (DM+CD) rats fed with choline deficient diet. Diabetes was experimentally induced by a single intraperitoneal injection of streptozotocin at a dose of 50 mg/kg body weight. After five weeks of dietary intervention heart function was evaluated by echocardiography study, histopathology analyses and immunohistochemical assessment of vascular endothelial growth factor (VEGF-A) on the cardiac and renal tissue were performed. Moreover, the renal immunohistochemical expression of kidney injury molecule (KIM-1) as a sensitive biomarker of acute and chronic kidney injury was evaluated.
Results: Choline deficiency was characterized by diastolic dysfunction with focal inflammation and cardiac interstitial fibrosis that resembles the features of diabetic cardiomyopathy. In the case of the concurrent presence of choline deficiency and diabetes mellitus, the functional impairment was preserved but the echocardiographic dimensions of the cardiac chambers were characterized by significant decreased left ventricle posterior wall thickness and dilation of the left atrium as compared to either the diabetic or choline-deprived rats alone (p=0.041 vs DM, p=0.009 vs CDD and p=0.015 vs C). VEGF-A cardiac immunohistochemical expression showed a significant increase in all groups compared to control, while the renal immunohistochemical expression was more intense in the DM group and was significantly suppressed in the DM +CD group. KIM-1 immunohistochemical expression showed a significant increase in the DM+CD group compared to all other groups (p<0.001versus the control and DM groups and p<0.01 versus the DM group).
Conclusion: Choline deprivation seems to aggravate the collagen deposition in the diabetic myocardium triggering however at the same time the transition of a restrictive type of cardiomyopathy to a potential dilated type. Furthermore, under choline deficiency conditions diabetic nephropathy is deteriorated which in turn increases the cardiovascular morbidity and mortality.
272
Novel multipotent antihyperlipidemic - antioxidant derivatives as potential agents for neuroinflammation
Tzara A1, Kleftaras A1, Kourounakis A1
1 National And Kapodistrian University Of Athens, Athens, Greece
Introduction: The multifactorial nature of many complex diseases, such as metabolic syndrome, cancer and neurodegeneration, is shifting scientific research interest towards a multitarget approach; compounds, bearing a combination of two pharmacophores, each directed towards a different pharmacological target, seem to be a more suitable strategy to tackle such pathological conditions.
Squalene synthase (SQS) inhibitors, apart from acting as antihyperlipidemic agents, have been further directed towards various other potential therapeutic areas such as Alzheimer’s or cancer. Imbalance in cell cholesterol levels may lead to alterations in neuronal cell membranes, influencing neuronal survival and therefore the course of Alzheimer’s2. At the same time, there has been a scientific return towards cholinergic modulation in Alzheimer’s, as part of a multitherapy approach3.
Objectives: This project involves the investigation of a series of multipotent molecules 1-5, combining moieties with antioxidant, anti-inflammatory and squalene synthase (SQS) inhibitory properties as an extension of a previous study.1
Methods: The newly designed compounds were synthesized in good yields, characterized via 1H and 13C NMR spectroscopy and pharmacologically evaluated, both in vitro and in vivo, for their antioxidant, anti-inflammatory, anti-hyperlipidemic and potential anti-neurodegenerative activity. Antioxidant activity was evaluated in vitro via inhibition of microsomal-membrane lipid-peroxidation and free-radical scavenging, as well as in vivo by determining total antioxidant capacity. Anti-inflammatory properties were studied in vitro, via inhibition of the enzyme lipoxygenase, and in vivo in an paw-edema protocol. Antihyperlipidemic activity was evaluated in vivo while inhibition of the enzyme acetylcholinesterase was determined in vitro.
Results: All new derivatives successfully maintained or even exceeded the antioxidant activity of their parent molecules, in the corresponding assays, with the most active compound 4 bearing an IC50 of 0.6 μM for lipid peroxidation. They also demonstrated satisfactory activity as lipoxygenase inhibitors, bearing IC50 values between 140-190 μM, significantly increased compared to all parent molecules. The new compounds also reduced in vivo carrageenan-induced mouse paw edema by 35-45%. All compounds reduced lipidemic parameters in vivo, with compound 1 being the most active antihyperlipidemic agent, reducing lipidemic parameters (total cholesterol and triglycerides) by approx. 50%. Finally, the in vitro acetylcholinesterase inhibition, as a potential “anti-neurodegenerative” indication, showed that several of the newly designed molecules inhibited the enzyme’s activity by 31-42% at a concentration of 300μΜ.
Conclusion: The improved antihyperlipidemic, antioxidant and anti-inflammatory activity of the new under investigation derivatives, provide an interesting basis for their potential application not only in cardiovascular disorders but further in interlinked neuroinflammatory conditions. Further investigation of these multipotent derivatives may render them a promising therapeutic approach towards such interrelated conditions.
1 Matralis, AN; Kourounakis, AP. ACS Med. Chem. Lett. 2019, 10(1), 98-104
2 Kourounakis AP; Bavavea E. Arch. Pharm. (Weinheim) 2020, 353(9):e2000085
3 Douchamps, V; Mathis, C. Behav. Pharmacol. 2017, 28, 112-123
286
Perspective for the development of new medicines based on purified Naftalan oil for the treatment of dermatological diseases
Zaychenko G1, Horbach A1
1 Department of Pharmacology, Bogomolets National Medical University, Kyiv, Ukraine
Introduction: Psoriasis is a complex, chronic inflammatory disease of the skin, manifested in little red and scaly plaques on any part of the body. Topical drugs are used to treat dermatological conditions, particularly psoriasis, effectively. Dermal drugs should include the following pharmacological activities: affect psoriatic inflammation, keratinocyte hyperproliferation, and angioproliferation.
Objectives: The study's primary is the scientific substantiation of the search for new drugs that have a complex effect on the main pathogenesis links of psoriasis and have a favourable safety profile. For 60 years, ointments with petroleum products have been used for treating psoriasis. The previous generation, which contained crude Naftalan oil, had several disadvantages (specific oil odour, dark brown colour, contaminated clothing, and bedding). Prolonged use of crude Naftalan oil led to dry skin, toxic liver damage, and was carcinogenic
Methods: Pharmacological analysis of medicinal properties of promising topical drugs based on purified Naftalan oil for the treatment of dermatological diseases.
Results: The new generation of new topical dosage forms proposed contain purified Naftalan oil, more saturated with gapans and steranes, standardized according to the European Pharmacopoeia, free of harmful organoleptic properties, and with improved pharmacological properties, and safer, reduced overall and dermal toxicity. Urea and drotaverine have been added to enhance the pharmacological action of new drugs with purified Naftalan oil. According to the projected data, such drugs should have pharmacological action: wound healing, keratolytic, antimicrobial, analgesic, and photoprotective.
Conclusion: The development of new drugs in mild dosage forms based on purified Naftalan oil will effectively treat chronic dermatological diseases, such as psoriasis. There are many medicines for the topical treatment of dermatological conditions. The use of new drugs may, in the long run, reduce doses of corticosteroids and expand the range of medications used to treat dermatological diseases such as psoriasis.
287
Perspective for the development of new medicines based on purified Naftalan oil for the treatment of dermatological diseases
Zaychenko G1, Horbach A1
1 Department of Pharmacology, Bogomolets National Medical University, Kyiv, Ukraine
Introduction: Psoriasis is a complex, chronic inflammatory disease of the skin, manifested in little red and scaly plaques on any part of the body. Topical drugs are used to treat dermatological conditions, particularly psoriasis, effectively. Dermal drugs should include the following pharmacological activities: affect psoriatic inflammation, keratinocyte hyperproliferation, and angioproliferation.
Objectives: The study's primary is the scientific substantiation of the search for new drugs that have a complex effect on the main pathogenesis links of psoriasis and have a favourable safety profile. For 60 years, ointments with petroleum products have been used for treating psoriasis. The previous generation, which contained crude Naftalan oil, had several disadvantages (specific oil odour, dark brown colour, contaminated clothing, and bedding). Prolonged use of crude Naftalan oil led to dry skin, toxic liver damage, and was carcinogenic
Methods: Pharmacological analysis of medicinal properties of promising topical drugs based on purified Naftalan oil for the treatment of dermatological diseases.
Results: The new generation of new topical dosage forms proposed contain purified Naftalan oil, more saturated with gapans and steranes, standardized according to the European Pharmacopoeia, free of harmful organoleptic properties, and with improved pharmacological properties, and safer, reduced overall and dermal toxicity. Urea and drotaverine have been added to enhance the pharmacological action of new drugs with purified Naftalan oil. According to the projected data, such drugs should have pharmacological action: wound healing, keratolytic, antimicrobial, analgesic, and photoprotective.
Conclusion: The development of new drugs in mild dosage forms based on purified Naftalan oil will effectively treat chronic dermatological diseases, such as psoriasis. There are many medicines for the topical treatment of dermatological conditions. The use of new drugs may, in the long run, reduce doses of corticosteroids and expand the range of medications used to treat dermatological diseases such as psoriasis.
299
The RASGRP1.rs8032939 in Kazakhstani patients with Seropositive Rheumatoid arthritis
Nurgaziyev M1, Issilbayeva A1,2, Ainabekova B2, Zhetkenev S1, Meiramova A1,2, Ahmetova Z1,2, Kushugulova A1
1 Laboratory of Human Microbiome and longevity, Center for life sciences, National Laboratory Astana, Nazarbayev University, Nur-Sultan, Kazakhstan
2 NJSC Medical University Astana, Department of internal Medicine with the course of gastroenterology, endocrinology and pulmonology, Nur-Sultan, Kazakhstan
Introduction: Rheumatoid arthritis (RA) is an autoimmune disease characterized by a genetic predisposition. Seropositive (RF+) form of this disease is the most common one. We studied the relationship between single nucleotide polymorphisms (SNP) RASGRP1.rs8032939 with RA in Kazakhstanis.
Objectives: Our study aims to investigate whether there are genetic links with RF status in Kazakhstani patients with RA.
Methods: We enrolled 70 RA patients all female and 113 healthy control subjects. The blood was collected to the test tubes with EDTA. The Genomic DNA was extracted using Promega Wizard genomic DNA Purification Kit according to manufacturer`s protocol. All DNA samples were stored at -20°C temperature. We genotyped all samples for RASGRP1.rs8032939 by Real-time polymerase chain reaction (RT-PCR) using TaqMan technology. Comparison of genotypes distribution between RA patients and healthy controls was carried out by the Chi-square (χ2) test, an odds ratio (ORs) and 95% confidence intervals (95% CIs) were used. Correlation of the associated SNP with Rheumatoid factor (RF) status among RA cases was performed with χ2 test.
Results: We hadn`t revealed any significant predominance of RASGRP1.rs8032939 in RA patients group compared to healthy subjects. Stratifying the data by RF presence, a significant association was revealed between the C/T genotype of RASGRP1 and seropositive (RF+) RA patients (OR= 4.67 [95CI 1.42-15.31], p= 0.00776) in overdominant mode of inheritance and in codominant mode of inheritance (OR= 2.33 [95CI 0.54-10.14], p= 0.00512).
Conclusion: Our study had revealed strong association between RASGRP1.rs8032939 and RF+ RA form. We need further studies on larger cohorts to confirm and be able to extrapolate our results.
315
Effects of type 2 diabetes treatment on TNFα plasmatic concentrations in the neuropathy risk
Balderas Vazquez C1, Valenzuela Limón O2, Bernal Morales B1, García Montalvo E2, Rodríguez Landa J1, Balderas Vazquez C1
1 Instituto de Neuroetología, Universidad Veracruzana, Xalapa, México
2 Facultad de Ciencias Químicas, Universidad Veracruzana, Orizaba, México
Introduction: The type 2 diabetes is a complex disorder frequently found amongst populations that involves the pancreas and insulin system. Manage patients include dietary adequacy and pharmacology treatment metformin. The tumor necrosis factor alpha (TNFα) is a pleiotropic pro-inflammatory cytokine involved in the mechanism of neuroinflammation. Several studies have researched the relationship between drugs approved for management of type 2 diabetes and its anti-inflammatory properties, but in Mexico especially Veracruz these studies are scarce. Aim: Assess the effect of pharmacology treatment on TNFα plasmatic concentrations and its association with protective role on peripheral neuropathy in diabetic patients of the central zone in Veracruz. Materials and methods: A cross sectional study was conducted according to a prior approval by an ethical committee. The study included participants with diagnostic of type 2 diabetes. Each participant signed the informed consent letter. Demographic and clinical aspects were explored by questionnaires. Neuropathy symptoms were assessed by Neuropathic Pain Questionnaire. Blood samples were obtained by venipuncture to assess glucose concentration and %HbA1c (spectrophotometric technique), and TNFα (Enzyme Linked Immuno-Sorbent Assay, ELISA). Non-parametric tests and logistic regression were performed for statistical analyses. Results: A total of 81 residents in central zone in Veracruz were included in the study. The average age was 48 years (22- 70). Of the participants, 80% were women. Pharmacological therapy includes insulin, glibenclamide and metformin. The geometric mean of TNFα was 7.7 ± 5.2 pg/mL (2 – 32.6), and it was significantly higher in patients with higher treatment time (p<0.05). The TNFα concentrations tended to be low in patients with metformin therapy and were the lowest in the metformin-insulin combination (p=0.058). A protective effect to neuropathy symptoms was recorded in the metformin treatment (Odds ratio= 0.031, IC 95%= 0.09 - 1.06). Conclusion: The results suggest that pharmacological treatment, especially which includes metformin, has potential to prevent an inflammatory stage down-regulating pro-inflammatory cytokines. Further studies should be performed to assess pro-inflammatory cytokines changes with pharmacotherapy to elucidate which drugs are most beneficial for prevention of neuropathy symptoms.
Computerized decision support
27
2PRESCRIBE AS INTERNATIONAL AND DIGITAL CASE-BASED PHARMACOTHERAPY EDUCATION AND RESEARCH PLATFORM
van Doorn A1, Salvo F2, Rosellini P2, Bijl G4, Elseviers M3, Coorevits P3, Vander Stichele R3
1 University Medical Center Groningen, Dep. of Clinical Pharmacy and Pharmacology, Groningen, The Netherlands
2 University of Bordeaux, Fac. of Medical Sciences, Dep. of Public Health/Med. Pharmacology, Bordeaux, France
3 Ghent University, Fac. of Medicine and Health Sciences, Dep. of Public Health and Primary Care, Ghent, Belgium
4 4, Groningen, The Netherlands
Introduction: The international online and case-based pharmacotherapy (FT) education and research platform "2Prescribe" (https://www.pscribe.eu/), based on the WHO-6-Step patient treatment model for rational prescribing is used in the Netherlands, Belgium, France and Portugal. It’s already offered in three languages (Dutch, English and French). The platform is now provided with more research tools to efficiently map the rational prescribing process (RPP) of more than 42,000 students. To this end, we have developed a data tracking tool (DTT) collecting data to analyse the RPP of groups and individuals. As an additional part of the DTT, we are also developing and testing the web-service tool “Pharmagrader” to (semi-)automatically assess prescriptions using Artificial Intelligence Algorithms (AI/A).
Objectives: We present RPP data of 1st year medical students that have been collected and mapped by the DTT and Pharmagrader.
Methods: To demonstrate the potential of the DTT in collecting and mapping the RPP data (graphics and tables) in combination with the collected data of Pharmagrader immediately after ending an assignment, we took a 2Prescribe/hypertension case (X). The DTT online data will be visually presented with graphics. In the same time parts of completed prescriptions (open & structured formats) from a group with randomly selected 1st year medical students (n=58) were parsed with Pharmagrader. Data from Pharmagrader will serve as input to feed AI/A’s in the future.
Results: A graphical representation of the DTT outcomes with data of the drug choices (number of times chosen by the students) is shown in figure 1. A typical example of the Pharmagrader outcomes will be shown on the congress.
Conclusion: This approach of directly mapping students' RPP through this DTT shows that the value of such a research tool in combination with the Pharmagrader web-service is very encouraging. It might be a first step to make adaptive feedback and personalized FT education feasible on the one hand, and on the other hand to enable teachers to assess students' prescriptions more efficiently and reliably (semi-)automatically.
Tabel/Image
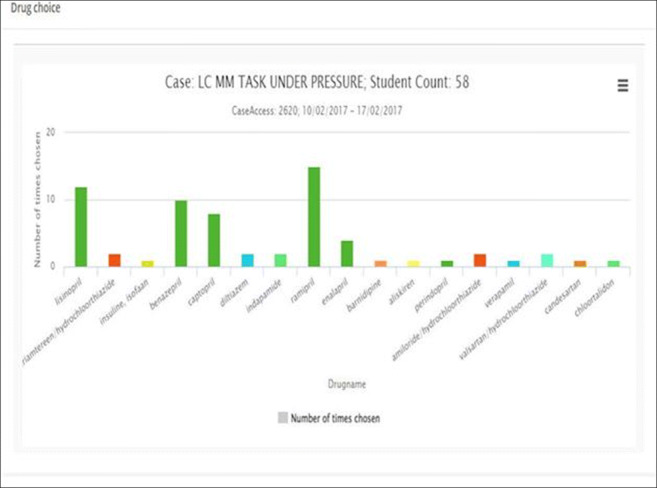
52
The ways to the quality and safety of the multicomponent therapy in phthisiology
Lugovkina T 1
1 "Ural Research Institute Of Phthisiopulmonology"(Ekaterinburg) – Branch Of FGBU "Scientific Medical Research Center of phthisiopulmonology and infection diseases " under Russian Healthcare ministry (Moscow), Ekaterinburg, Russia
Introduction: The doctors are forced to prescribe multicomponent drugs therapy for a long treatment period, when tuberculosis is due to the resistant strains of M. tuberculosis. The use of multicomponent drugs therapy is often followed by the high risks of adverse events. It demands the search of triggers and signals of adverse events for prospective minimization of risks by monitoring the drug prescribing quality.
Objectives: Methodology for constructing of the multimodal medical electronic platform to prospective search of triggers and signals, prevent the risks of adverse events, decision support and monitoring of drug prescribing.
Methods: The conceptual scheme of Clinical Practice Information Space for phthisiology, the digital reflection of the diagnosis components of Clinical Situations, the environment of the diagnosis components performed on the basis of the systemic principles: hierarchy, uniformity, sufficient diversity, complementarity, symmetry of reflection, irreversibility of time. The tools of ontologies and Intelligent Systems based on expert knowledge were used to provide electronic search of triggers and signals of adverse events and decision support for improving drug therapy.
Results: A conceptual scheme of the Information Field of the diagnosed Clinical Situations components was constructed. A dictionary of general terms was compiled and the corresponding concepts were formulated. The conceptual schemes were tested in the prototype of multimodule medical electronic platform. The search of triggers and signals in data bases was realized by ontology tools. The transformation of the components of the diagnosed Clinical Situations into the digital codes was realized by the program algorithm.
Conclusion: The tools of ontologies and Intelligent Systems based on expert knowledge and used according to the described methodology of systematization of clinical information space have a great potential to improve the quality and safety of drug treatment in the subject fields of Clinical Practice.
198
Clarity, clinical applicability and relevance of absolute contraindications in official ‘Summaries of Product Characteristics’ (SmPCs)
Andrikyan W1, Then M1, Dürr P1,2, Jung-Poppe L1, Schuster A3, Weisbach L3, Farker K3, Fromm M1, Maas R1
1 Friedrich-Alexander-Universität Erlangen-Nürnberg, Institute of Experimental and Clinical Pharmacology and Toxicology, Fahrstraße 17, 91054 Erlangen, Germany
2 Erlangen University Hospital, Pharmacy Department, Palmsanlage 3, 91054 Erlangen, Germany
3 Jena University Hospital, Hospital Pharmacy, University Center for Pharmacotherapy and Pharmacoeconomics, Erlanger Allee 101, 07747 Jena, Germany
Introduction: Absolute contraindications in ‘Summaries of Product Characteristics’ (SmPCs) define situations in which a drug must be avoided due to potential patient harm. Expert-based analyses of medical records indicate, however, that contraindicated prescriptions are more common than one would expect. Rare clinical situations that justify deviation from the official recommended use aside, alternative explanations have to be considered, such as clinically unclear definitions or wording which may leave too much room for interpretation.
Objectives: The aim of this work was to assess the clarity, clinical applicability and relevance of common absolute contraindications used in SmPCs.
Methods: In preparation of an overarching use case of the German Medical Informatics Initiative ‘POLAR_MI - POLypharmacy, Drug interActions, Risks’ (supported by the Federal Ministry of Education and Research, 01ZZ1910O, 01ZZ1910C), an anonymous online survey was conducted among physicians and pharmacists from a broad spectrum of clinical backgrounds. For this purpose, 24 examples of absolute contraindications of German SmPCs were selected after an expert-based consensus evaluation of commonly missed contraindications in different university hospitals. Participants were given up to two patient cases for each contraindication and were asked to assess two main questions:
1. Is in this particular case the definition of the contraindication fulfilled from your point of view? (17 cases)
2. Is the contraindication in this particular case clinically relevant? (24 cases)
Results: Questionnaires were obtained from 27 physicians and 27 pharmacists, most of them with clinical or academic background. For none of the questions, all experts agreed on the same answer. In 12 of 17 (70.6%) cases, a majority of the experts agreed that the definition of the contraindication was fulfilled. In 5 of 17 (29.4%) cases, no simple majority was given for an answer option by the experts. For instance, experts gave a broad spectrum of different answers on the definition of an ‘active liver disease’, which is a contraindication for simvastatin according to its SmPC. With respect to the clinical relevance, in 16 of 24 (66.7%) patient cases a majority of the experts agreed on the clinical relevance, in 1 of 24 (4.2%) the majority disagreed on the clinical relevance. For 7 of 24 (29.2%) cases, a simple majority was not given. The contraindication of the daily administration of diclofenac for a patient with chronic pain and heart failure (NYHA II) was rated as the most clinically relevant, whereas the clinical relevance of a single dose for the same patient was assessed to be less clinically relevant not justifying an alert for all patients and classification as medication error.
Conclusion: This online survey indicates that SmPCs are too vague regarding the definition of contraindications. Furthermore, the clinical relevance of a contraindication highly depends on the clinical situation (e.g., long-term vs. single application), which may be insufficiently accounted for in some current SmPCs.
Tabel/Image
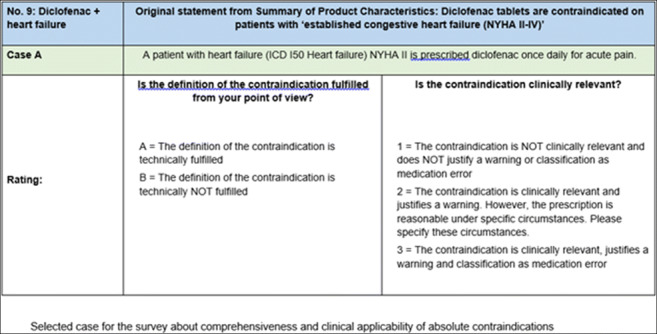
222
Electronic DDI system effect on optimal treatment in an outpatients setting: a literature review
Pancere J1,2,3, Lasys T1,4, Stankeviciute S1,2,3, Karinauske E1,5
1 Gydytoju klinikiniu farmakologu asociacija, Kaunas, Lithuania
2 Lithuanian University Of Health Sciences Kaunas Clinics, Kaunas, Lithuania
3 Lithuanian University Of Health Sciences, Kaunas, Lithuania
4 Utrecht University, Utrecht, Netherlands
5Lithuanian University Of Health Sciences Hospital of Kaunas, Kaunas, Lithuania
Background: In Lithuania, the Ministry of Health launched an initiative to reduce drug-drug interactions in the outpatient prescriptions (DDIs) via an electronic DDI alert system in 2019. The primary goal of such a system is to optimize the treatment by warning physicians about possible DDI during the prescribing process. However, previous research suggests that such systems should be used with caution since some DDIs are inevitable in clinical practice, and alerts targeting them could lead to alert fatigue, reducing compliance even for serious DDIs that could be avoided.
Objectives: To determine the effect electronic DDI systems had on outpatient clinical practice. We searched for studies evaluating adverse effects before and after the electronic DDI system was introduced as a primary goal. A secondary goal was to examine the effects of using electronic DDI system overrides as a surrogate to the effectiveness of a system.
Methods: A systematic literature review was conducted in MEDLINE and EMBASE up to 3 February 2022 to identify studies investigating the effect of the implementation of electronic drug-drug interaction alert systems in routine primary care practices. We excluded studies assessing specific DDIs, studies conducted in the inpatient setting and studies based on questionnaires and surveys. From the eligible studies, we extracted information on how many prescriptions were examined, how many patients were involved and how the effectiveness of such systems were measured.
Results: Our search on PubMed identified 732 articles, of which 21 were included for full-text review. After full-text review 12 articles were included in the analysis. In the selected studies, 65 – 95,7% of DDI alerts were overridden. The most common reasons for overriding drug-drug interactions were “will monitor as recommended”, “patient has already tolerated combination”, “no reasonable alternative”. Only in two articles the efficacy of DDIs alerts was evaluated by assessing adverse drug interactions related to DDIs. Few articles evaluated the appropriateness of alert overrides. However, different study designs and methodology complicate the study results’ comparability that also significantly depend on DDIs selected in specific systems.
Conclusions: A significant part of DDI alerts in identified studies were overridden, suggesting a need for better tailored electronic DDI alert systems.
COVID-19 therapies and vaccines
10
COVID-19 and eicosanoids
Hoxha M1, Zappacosta B1
1 Catholic University Our Lady Of Good Counsel, Department of Chemical -Toxicological and Pharmacological Evaluation of Drugs, Tirana, Albania
Introduction: COVID-19 or severe acute respiratory syndrome coronavirus 2 (SARS-COV-2) is an infectious viral disease caused by SARS-CoV-2 virus.
Objectives: Eicosanoids (prostaglandins and leukotrienes) are released once the organism is infected by the virus. The aim of this study is to evaluate the role of eicosanoids and their therapeutic targeting in COVID-19.
Methods: Pubmed and Scopus databases were the main databases used to identify all the studies conformed to the eligibility criteria.
Results: Macrophages, T/B cells, leukocytes are some of the cells that when affected by SARS-CoV-2 release arachidonic acid (AA), which acts as antiviral compound by inactivating the virus. Humans are more vulnerable to SARS-CoV-2 when they have a deficiency in AA levels. Cytosolic phospholipase A2 (cPLA2), through which AA is released from membrane glycerophospholipids is very important for the replication of coronavirus; cPLA2 inhibition in cell culture influences an early step of coronavirus replication. Once a patient is affected by SARS-CoV-2, eicosanoids are released. PGE2 and leukotriene B4/D4/E4 (LTB4/LD4/LTE4) are proinflammatory mediator that play a significant role in COVID-19 pathophysiology. PGE2 levels were found to be higher in males, and increased with age, and in obese patients. The inhibition of human microsomal prostaglandin E synthase-1 (mPGES-1) can be a novel therapeutic strategy for preventing either severe disease, or death by lowering PGE2 levels, and not affecting other prostaglandins level. Moreover, mPGES-1 selective inhibitor (sonlicromanol) prevented ROS-driven cell death. LXA4 other than inhibiting interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) synthesis, suppresses also PGE2 and LTs’ synthesis and enhances the production of M1 phenotype and M2 macrophages.
Conclusion: mPGES-1 inhibition is suggested to be a better pharmacological approach in COVID-19 in confront to NSAIDs, which can lead to gastric and cardiovascular problems. Since corticosteroids or statins can alter the bioactive lipids, the oral or intravenous administration of AA can facilitate the recovery, increase the resistance of COVID-19 patients, or even prevent it. Moreover, the nanoencapsulation of AA, or of its metabolite can bring to novel safer treatment. The therapeutic role of 5-LOX inhibitors in COVID -19 should be also further tested.
13
Camostat mesylate against SARS-CoV-2 and COVID-19—Rationale, dosing and safety
Breining P1, Saedder E1, Nielsen L1, Breining P1
1 Aarhus University Hospital and Aarhus University, Aarhus, Denmark
The coronavirus responsible for COVID-19, SARS-CoV-2, utilizes a viral membrane spike protein for host cell entry. For the virus to engage in host-membrane-fusion, SARS-CoV-2 interacts with the human transmembrane surface protease, TMPRSS2, and to a lesser degree TMPRSS11D/E/F, and -13, to cleave and activate the spike protein. Camostat mesylate, an orally available well-known serine protease inhibitor, is a potent inhibitor of TMPRSS2 (and 11D/E/F and -13) and has been hypothesized as a potential antiviral drug against COVID-19. In vitro human cell and animal studies have shown that camostat and the metabolite GBPA inhibits virus-cell membrane fusion and hence viral replication. In mice, camostat mesylate treatment during acute infection with influenza virus, another TMPRSS2 dependent virus, leads to decreased viral load. In the clinic, a reduced viral load may lead to improved patient outcome. Because camostat mesylate is administered as an oral drug, it may be used in outpatient as well as inpatients at all disease stages of SARS-CoV-2 infection if it is shown to be an effective antiviral agent. Clinical trials have been initiated to test whether this well-known drug could be repurposed and utilized to combat the current pandemic.
Breining et al. BCPT, 2021, Hoffmann et al., EBiomedicine, 2021.
26
Social impact of the clinical trials on COVID-19-related vaccines and other drugs
Casino G1, Prados-Bo A2, Bosch-Llonch F3
1 Iberoamerican Cochrane Centre - IIB Sant Pau, Barcelona, Spain
2 Pompeu Fabra University, Barcelona, Spain
3 Dr Antoni Esteve Foundation, Barcelona, Spain
Introduction: The COVID-19 pandemic has focused social attention on biomedicine and drugs. The social attention on papers about drugs for COVID-19 can be measured with altmetrics. This bibliometric alternative estimates the social impact of papers, based on their mentions in social media, traditional media, and other digital platforms.
Objectives: To analyze the social impact of clinical trials on COVID-19-related vaccines and other drugs. To analyze which drugs and academic journals related to these trials have reached the highest social impact.
Methods: We searched the Clarivate Web of Science (WoS) database for academic articles on drugs and covid-19 (TS=drug* and TS=covid-19) and then applied the clinical trial filter. Then, we estimated the social impact of these articles by their Altmetric Attention Score (AAS) using the Altmetric bookmarklet. For the 50 most cited articles in WoS, we also registered all the variables included in the AAS algorithm and other details provided by Altmetric. WoS and Altmetric searches were performed on December 11, 2021.
Results: We identified 627 articles about clinical trials on COVID-19 drugs. The article with the highest AAS was a clinical trial on the Russian vaccine Sputnik V (Gam-COVID-Vac), which is the 10th with the highest AAS of the 20 million articles monitored by Altmetric and with the highest AAS of those in The Lancet. Seven of the 50 most cited articles on clinical trials of COVID-19 drugs are in the Altmetric top 100 of all time. Clinical trials on COVID-19 vaccines accounted for 58.2% of the AAS of the 50 most cited articles, antivirals for 32.1%, immunomodulators (corticosteroids) for 4.5%, monoclonal antibodies for 3.0% and combined therapies for 2.1%. The five drugs with highest AAS were —from higher to lower scoring— tozinameran (Pfizer/BioNtech), ChAdOx1 (AstraZeneca/Oxford) and Gam-COVID-Vac (Sputnik V) vaccines, remdesivir and hydroxychloroquine. Articles published in NEJM (25) and The Lancet (10) account for 82.4% of the AAS of the 50 most cited articles.
Conclusion: Some of the articles on clinical trials of COVID-19 drugs are among the 100 with the highest social impact of the 20 million articles of all time monitored by Altmetric. Vaccine clinical trials had higher social impact than trials on other types of drugs for COVID-19. NEJM and The Lancet have garnered most of the social attention on clinical trials of vaccines and drugs for COVID-19.
28
Impact of vaccine hesitancy on onset, severity and type of self-reported adverse events
Khouri C1, Larabi A1, Verger P2,3, Gauna F3, Cacowski J1, Ward J2,4
1 Grenoble Alpes University Hospital, France
2 VITROME (Aix Marseille Université, IRD, AP-HM, SSA), Marseille,, France
3 Observatoire régional de la santé PACA (ORS Paca), Aix-Marseille Université, Marseille, France
4 CERMES3 (INSERM, CNRS, EHESS, Université de Paris), Villejuif, France
Little is known about the impact of mandatory vaccination on the onset, severity and characteristics of adverse events, in people who are reluctant to be vaccinated. We used a cross-sectional online survey conducted in 2021 among a representative sample of the French population aged 18 years and older (n=2015) to explore the relationship between vaccine hesitancy and self-reported adverse events. We found that the proportion of reported adverse events ranged from 18%, of which 5% were considered severe, to 65%, of which 41% were considered severe, for not reluctant to very reluctant responders respectively. Although the adverse events profile remains similar between groups. Our results suggest that vaccine hesitancy could be a major driver of patient reported vaccine related adverse events and their perceived impact.
40
COVID-19 Vaccines Adverse Effects: Potential Underlying Molecular Mechanisms
Lamprinou M1, Stamoula E1, Sachinidis A2, Vavilis T3, Papazisis G1
1 Laboratory of Clinical Pharmacology, School of Medicine, Aristotle University of Thessaloniki, Thessaloniki, Greece
2 4th Department of Internal Medicine, Hippokration General Hospital of Thessaloniki, School of Medicine, Aristotle University of Thessaloniki, Thessaloniki, Greece
3 Laboratory of Medical Biology and Genetics, School of Medicine, Aristotle University of Thessaloniki, Thessaloniki, Greece
Introduction: COVID-19 is an infectious disease caused by SARS-CoV-2, an ssRNA virus. The disease, since its first outbreak in Wuhan, China, in December 2019, has led to a global pandemic. Fortunately, several vaccines, which are based on different vector technologies, have been developed against the virus. Of note, among these vaccines, seven have been fully approved by WHO. However, despite the benefits of COVID-19 vaccination, some rare adverse effects have been reported and have been associated with the use of the vaccines developed against SARS-CoV-2, especially those based on mRNA and non-replicating viral vector technology. Rare adverse effects reported include allergic and anaphylactic reactions, thrombosis and thrombocytopenia, myocarditis/pericarditis, autoimmunity flares, neurological disorders, and others.
Objectives: Discuss the potential molecular mechanisms leading to these rare adverse effects of interest and also attempt an association with the various vaccine components and platforms.
Methods: A review of the literature, using databases such as PubMed, based on keywords such as COVID-19 Vaccines, Adverse Effects, Side Effects and Mechanisms.
Results: Identification of specific vaccine components (e.g. PEG) as causal factors of some of the rare adverse effects. Furthermore, characterization of the most dominant/common molecular mechanisms, like the molecular mimicry and the stimulation of TLRs, leading to post-vaccination adverse reactions.
Conclusions: Indubitably, there is room for COVID-19 vaccines improvement. A better understanding of the underlying mechanisms, according to which the vaccines cause side effects, in conjunction with the identification of the vaccine components and/or platforms that are responsible for these reactions, could probably enable the improvement of future vaccines against COVID-19 and/or even other pathological conditions.
65
Tolerability of different SARS-CoV-2-Vaccine-Combinations in hospital staff (HelCo-Vac Study)
Dedroogh S1,2, Graf K5, Schmiedl S3,4,5, Thürmann P3,4, Koß R1, Theis C1, Tebbenjohanns J1, Thal S6, Dedroogh M1
1 Helios Klinikum Hildesheim, Germany
2 Georg-August-University, Göttingen, Germany
3 Philipp Klee-Institute for Clinical Pharmacology, Helios University Hospital Wuppertal, Germany
4 Department of Clinical Pharmacology, Witten/Herdecke University, Germany
5 Center for Clinical Trials, Witten/Herdecke University, Germany
6 Department of Anesthesia I, Witten/Herdecke University, Germany
Introduction: Due to the novel mechanism of action, the tolerability of new COVIS-19-vaccines was of special concern.
Objectives: The reactogenicity of COVID-19 vaccines in different combinations was evaluated by the collective of clinic employees at Helios Klinikum Hildesheim.
Methods: 1206 subjects were included in the prospective observational study, of which 1123 were available for evaluation after completion of the basic immunization:
BNT162b2 (BNT) with a vaccination interval of 3, respectively 6 weeks (BNT-3: n=248, BNT-6: n=262), two groups with ChAdOx1 (AZ) prime vaccination followed by AZ (homologous AZ: n=270) or BNT (heterologous AZ-BNT: n=343) for second vaccination after 12 weeks.
The vaccine-mediated reactogenicity was recorded as local and systemic adverse drug reaction (ADR) within one week after first and second vaccination using standardized questionnaires.
The statistical analysis of categorical data was performed using frequencies, percentages and corresponding tests (Chi2- or Fisher's exact test).
Results: AZ caused local and/or systemic ADR in more than 90% of subjects after initial administration, while more than 1/4 of the subjects showed no ADR after first BNT vaccination (p<0.001). All clinically relevant systemic ADR were more common under AZ initial vaccination (p<0.001 each). AZ was significantly less reactive after second vaccination, after all, 44% had no reactions after homologous AZ vaccination.
A BNT-boost after 3 weeks was significantly more reactogenic (p=0.007) compared to 6 weeks. The tolerability of a heterologous vaccination with a BNT-boost at 12 weeks was as well tolerated as a homologous BNT-vaccination with a 6-week interval (p=0.461) and less reactogenic than in the BNT-3-group (p=0.031).
Conclusion: The four SARS-CoV-2-Vaccine combinations analyzed differed clinically and statistically significantly regarding ADR. Serious vaccination reactions requiring hospitalisation did not occur.
This investigation was partially funded by Helios Kliniken GmbH, Grant-ID:2021_037.
Tabel/Image
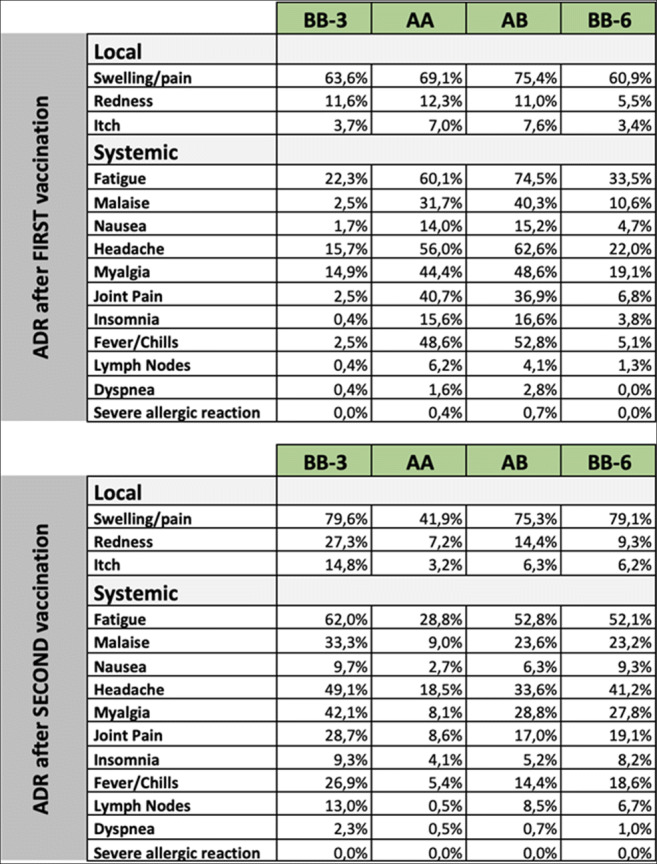
93
Practical guideline for Managing Potential Drug Interactions of Nirmatrelvir/Ritonavir (Paxlovid) in COVID-19 Patients
Goldstein L1,8, Zhurat S2,9, De Haan T2,9, Guy-Alfandary S3, Gueta I4,9, Shihmanter R5,10, Golik A2,7, Eyal S6, Berkovitch M2,9, Berlin M2,9
1 Clinical Pharmacology Unit, Haemek Medical Center, Affiliated to the Bruce Rapapport School of Medicine, Technion, Israel, Afula, Israel
2 Clinical Pharmacology and Toxicology Unit, Shamir Medical Center, Zerrifin, Israel
3 Pharmacy and Pharmacology Department, Health Division, Maccabi Healthcare Services, Tel Aviv, Israel
4 The Institute of Clinical Pharmacology and Toxicology, Internal Medicine A, Sheba Medical Center, Tel Hashomer, Israel
5 Clinical Pharmacology Unit, Kaplan Medical Center, Rehovot, Israel
6 Institute for Drug Research, School of Pharmacy, Jerusalem, Israel
7 Adelson School of Medicine, Ariel University, Ariel, Israel
8 Bruce Rapapport School of Medicine, Technion, Haifa, Israel
9 Sackler Faculty of Medicine, Tel-Aviv University, Tel Aviv, Israel
10 School of Medicine, Hebrew University of Jerusalem, Jerusalem, Israel
Introduction: Paxlovid was approved by the Israeli Ministry of Health (MOH) in December 2021, for treating patients with mild-to-moderate COVID-19, as described in its FDA Emergency Use Authorization. The emergency authorization, the multiple drug interactions expected due to the ritonavir component together with Israel being in the midst of the fifth wave of the COVID-19 pandemic led us to believe that effort should be put into maximizing the number of patients eligible for this life saving treatment.
Objective: Compile a guidance document for clinicians suggesting medicines which could be temporarily withheld, medicines which could be continued despite the potential interaction, and medicines which should be contraindicated. The guidance document should be practical and simple, with clear guidance on dose reduction, temporary stopping and resuming interacting drug and how the patient should be followed, all taking into account that the patient is not hospitalized and in isolation.
Methods: The FDA and EMA authorization documents were reviewed, and potential interactions were identified. Prescribing instructions of each interacting medication was reviewed together with relevant literature. Information on clinical effect and safety of continuing treatment despite short term interaction (5 days) or of abrupt withdrawal of interacting drug together with pharmacokinetic data such as half-life, alternative metabolizing pathways, were gathered.
Results: A practical guideline was compiled and placed online on the MOH website and periodically updated. Recommendations on interacting drugs were divided into four categories:
1. Contraindicated: a. Due to induction of CYP 3A4 (reducing Paxlovid efficacy). Induction was considered to be of 7days minimum duration. b. Due to inability to temporarily stop drug due to safety or futility
2. Dose reduction
3. Temporary withdrawal of drug (considering safety and efficacy on specific patient) Guidance on time before first PAXLOVID dose, and time after last dose before resuming treatment and if needed, alternative drug replacement, and follow up measures)
4. Coadministration of drug and PAXLOVID with guidance on followup measures, and potential effects on patients.
Conclusions: Practical and simple guidance for physicians in an emergency situation is essential and feasible, and should be undertaken by clinical pharmacologists
155
DESCRIPTIVE OBSERVATIONAL STUDY OF HOSPITALIZED PATIENTS DIAGNOSED WITH COVID-19 TO EVALUATE THE PHARMACOLOGICAL TREATMENT USED TO TREAT THIS DISEASE. COVID-19Registry(NCT04347278)
Lavin-Alconero L1,2, Bautista-Blazquez A2, Mazon-Maraña I1,2, Gonzalez-Samperio C1,2, Valencia-Lopez D2, Cuellar-Gomez D2, Alonso-Gomez B2, Vega-Gil N2, Sanchez-Santiago M1,2, Garcia-Saiz M1,2
1 IDIVAL, Santander, España
2 HOSPITAL UNIVERSITARIO MARQUES DE VALDECILLA, Santander, Cantabria
Objectives:
- To describe the frequency of use regarding treatments employed during the first wave of the pandemic in patients hospitalized for COVID-19 in Cantabria (Northern Spain).
- To assess the clinical situation of hospitalized patients with COVID-19 in terms of mortality.
- To Identify and quantify the adverse effects of the drugs used.
Methods: Prospective observational study, with IDI-REM-2020 code and EUPAS34551 registry. 249 patients hospitalized for COVID-19 between March and April 2020 in Northern Spain (Cantabria) were included. The data was analyzed using the SPSS program to obtain results on the frequency of treatments used, adverse reactions and their clinical implications.
Results: We analyzed the treatments received in 249 patients hospitalized in 4 centers in Cantabria. The majority treatment was hydroxychloroquine in 98% of cases, followed by lopinavir/ritonavir in 82.7%, tocilizumab and iv corticoids in 20.1% and 24.5% respectively.
Regarding clinical implications, the treatments administered with tocilizumbab and/or iv corticosteroids presented a trend to significantly reduce mortality p=0.059, OR=0.474 in the case of IV corticosteroids.
The main adverse reactions (AR) detected were diarrhea (20.9%) and vomiting/nausea (10.8%) with treatment having to be adjusted in 31.7%. Other AR identified were liver function alterations (3.2%) and skin hypersensitivity (2.4%).
Conclusions: Taking into account that this is a descriptive observational study, the results shown only offer information based by the use of standardized treatment guidelines in all hospitalized patients. The ARs identified are within those expected according to the Summary of Product Characteristics of the drugs collected and allow us to reinforce the information published in the first wave of the pandemic.
This reinforces the need for clinical trials to obtain evidence of the efficacy of treatments administered even in emergency situations.
161
Triiodothyronine prevents tissue hypoxia in experimental sepsis: potential implications for COVID-19 therapy
Mourouzis I1, Lourbopoulos A1,2,3, Pantelidaki A1, Trikas A1, Tseti I1, Pantos C1
1 Department of Pharmacology, National and Kapodistrian University of Athens, 75 Mikras Asias Ave.,11527 Goudi, Athens, Greece
2 Institute for Stroke and Dementia Research (ISD), University of Munich Medical Center, Munich, Germany
3 Neurointensive Care Unit, Schoen Klinik Bad Aibling, Kolbermoorerstrasse 72 Bad Aibling, Bayern, Germany
Introduction: Tissue hypoxia occurs frequently in sepsis even after apparent restoration of stable systemic hemodynamics (macro- to micro-circulation uncoupling).
Objectives: Τhe present study explored the potential of triiodothyronine (T3) to prevent tissue hypoxia which occurs early in experimental sepsis.
Methods: Sepsis was induced in adult male mice by ligation distal to the ileocecal valve and perforation by a single puncture (CLP). Animals were treated with a single dose of either vehicle (n=8, placebo group) or T3 (n=8, 0.3μg/g, T3 group) intraperitoneally. Naive animals were used as control (n=9, naive group). Animals were sacrificed 18hours after CLP procedure. Cardiac and liver hypoxia at cellular level was detected using Hypoxyprobe™ Plus kit (pimonidazole hydrochloride, PMZ) PMZ staining was used to detect tissue pO2 <10 mmHg. Cardiac performance was assessed by echocardiography.
Results: Cardiac output (ml/min) was 14.7 (SEM, 1.0), 12.1(0.7) and 14(1.0) and heart rate (beats /min) 444(23), 439(16) and 427(9) for naïve, placebo and T3 respectively (p=ns). CLP resulted in increased lactate levels and cardiac and liver hypoxia at cellular level (PMZ staining) in placebo group. Early T3 treatment prevented tissue hypoxia and significantly reduced circulating lactate. In addition, T3 treatment significantly reduced the extent of myocardial tissue hypoxia from 4 ± 0.5% in untreated animals to 1.5% ± 0.5 in treated ones, at 18hrs post sepsis initiation. In accordance, T3 treatment also reduced liver tissue hypoxia.
Conclusions: The present study demonstrated that T3 treatment can prevent tissue hypoxia in cardiac and liver samples which occurs early in experimental sepsis before cardiac output is impaired. T3 treatment was also shown to significantly lower circulating lactate levels probably due to the prevention of tissue hypoxia. Preliminary data from a small clinical study driven by our laboratory showed that acute administration of T3 in septic patients with COVID19 infection significantly reduced Erythrocyte Sedimentation Rate within 48h of infusion. Interestingly, this effect was associated with a trend towards lower troponin levels in treated patients. This novel T3 action could be relevant to the observed favorable effect on sepsis-induced microcirculatory failure and tissue hypoxia and merits further investigation (Clin Hemorheol Microcirc 2021;79(3):485-488).
174
Cardiovascular therapy and in-hospital death in patients with severe COVID-19: a Swiss cohort study
Follonier C1, Tessitore E1, Handgraaf S1, Carballo D1, Achard M1, Péchère-Bertschi A1, Mach F1, Herrmann F1, Girardin F2
1 University Hospital of Lausanne (CHUV), Geneva, Switzerland
2 University Hospital of Lausanne (CHUV), Lausanne, Switzerland
Introduction: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has a significant impact on cardiovascular risk factors and cardiac comorbidities in patients with COVID-19: the prescription sequences and dosage of cardiovascular medications remain controversial.
Methods: Logistic regression models adjusted for potential confounders were used to assess the associations of use (i.e., pre-hospital use, in-hospital use) and modifications in exposure (i.e., discontinuation on admission, initiation during hospitalization) to of eight common cardiovascular therapies with the risk of in-hospital events using data from a retrospective cohort study including all hospitalized adult patients with confirmed COVID-19 at the Geneva University Hospitals from February 26, 2020, and discharged by June 5, 2020.
Results: Of 838 inpatients with COVID-19, 468 (55.8%) were exposed to cardiovascular therapies before hospitalization, 779 (92.5%) had at least one of their cardiovascular drug prescriptions modified, 453 (54.1%) were male, and 152 (18.1%) died before discharge. The mean age was 66.5 ± 17.6 years. Overall, no cardiovascular therapy used before the hospitalization was associated with the risk of in-hospital death after accounting for potential confounders. During the hospitalization, the use of diuretics (adjusted odds ratio (aOR) 2.59 [1.68-3.98], p<0.001) was associated with an increase, and the use of agents acting on the renin-angiotensin system (aOR 0.39 [0.23-0.64], p<0.001) and lipid-lowering agents (aOR 0.41 [0.24-0.68], p=0.001) were associated with a reduction in the odds of in-hospital death, respectively. Exposure modifications associated with decreased survival were the discontinuation of an agent acting on the renin-angiotensin system (aOR 4.42 [2.08-9.37], p<0.001), a β-blocking agent (aOR 5.44 [1.16-25.46], p=0.031), a lipid-modifying agent (aOR 3.26 [1.42-7.50], p=0.005) or an anticoagulant (aOR 5.85 [1.25-27.27], p=0.025), and the initiation of a diuretic (aOR 5.19 [2.98-9.03], p<0.001) and an antiarrhythmic (aOR 6.62 [2.07-21.15], p<0.001). Exposure modification associated with impoved survival was the initiation of an agent acting on the renin-angiotensin system (aOR 0.17 [0.03-0.82], p=0.028).
Conclusion: In hospitalized patients with COVID-19, there was no detrimental association of the pre-hospital use of any cardiovascular medication category. These therapies therefore appear to be able to be continued as is recommended. Agents acting on the renin-angiotensin system and lipid-modifying agents tend to benefit in some patients with COVID-19. Regular cardiovascular medications could be continued in hospitalized patients with COVID-19.
Tabel/Image
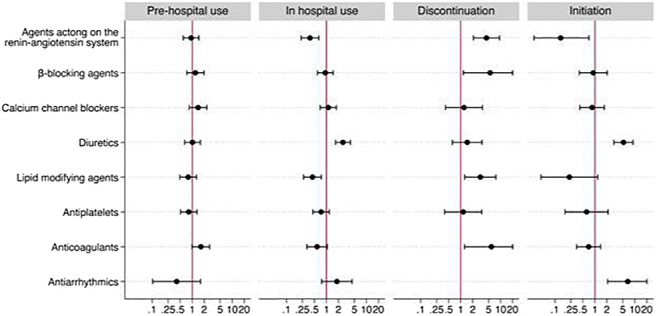
177
SSRI Antidepressant Use and Risk of Death due to Coronavirus Disease 2019 in Europe
Ascencao R1, Mainoli B2, Tobias A3, Costa J2
1 Instituto de Medicina Preventiva e Saúde Pública, Faculdade De Medicina, Universidade De Lisboa, Lisboa, Portugal
2 Laboratório de Farmacologia Clínica e Terapêutica, Faculdade de Medicina, Universidade de Lisboa, Lisboa, Portugal
3 Institute of Environmental Assessment and Water Research, Spanish Council for Scientific Research, Barcelona, Spain
Introduction: Selective serotonin reuptake inhibitors (SSRI) are routinely used for treating mood and depressive disorders. The repurposing of SSRI as a pharmacological option to treat coronavirus disease 2019 (COVID-19) has recently gained attention due to its potential role in the immune response following severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.
Objectives: To explore a possible association between SSRI antidepressant use and risk of death due to COVID-19 in Europe, as assessed by a population-level analysis.
Methods: The limited data available was only fit to be analyzed using unadjusted linear regression modelling. Exploratory analysis included single-adjustment for gross domestic product (GDP) per capita, given that income inequality and national wealth are strong determinants for population health and mortality.
Results: Data for COVID attributed mortality and SSRI use was available for 12 out of the 27 European countries only. We found a non-significant positive association between SSRI use and COVID-19 mortality (β=3.77; p=0.624 unadjusted), and a non-significant negative association between SSRI use and all-cause mortality (β=-6,44; p=0.063 unadjusted). GDP-adjusted analysis showed similar results.
Conclusion: This study failed to suggest an association between SSRI use across European countries and a lower COVID-19 related mortality, although results are biased because of the scarcity of data. Despite the important findings from recent randomized controlled trials on the efficacy of fluvoxamine in outpatients with COVID-19, several questions remain unanswered. Efforts should be made to enhance the quality of publicly available data on SSRI use in Europe in other to assist the development of well-built ecological studies.
181
Acute urticaria secondary to covid 19 vaccines: about 14 cases
Fathallah N1, Aounallah A2, Slim R1, Mokni S2, Atig A3, Ouni B1, Ben Salem C1
1 Department of Pharmacology, Laboratoire de Biophysique Métabolique et Toxicologie Professionnelle et Environnementale Appliquée LR12ES02. Université de Sousse, Faculté de Medecine, Sousse
2 Department of Dermatology, Farhat Hached University Hospital of Sousse, Sousse
3 Department of internal Medicine, Sousse
Introduction: Several adverse effects were associated with the covid19 vaccination, ranging from mild to severe reactions. Immediate hypersensitivity reactions such as urticaria secondary to the vaccine are rarely reported.
Objectives: We studied the clinical, epidemiological characteristics in a group of patients who presented with acute urticaria secondary to COVID19 vaccination.
Methods: We included all cases of urticaria secondary to covid19 vaccines notified to the pharmacovigilance department of Sousse.
Results: We included 14 patients with a female predominance (8 women and 6 men) and a sex ratio M/F of 0.75. The average age was 41.64 years with extremes between 17 and 57 years. All patients were vaccinated with the first dose of the vaccine and the type of vaccine received was respectively an RNA vaccine in 7 cases (respectively Pfizer (5 cases) and moderna (2 cases)), adenovirus (astrazeneca type in 4 cases) and Coronavac type inactivated virus in a single case. The reaction described was localized or generalized acute urticaria, associated in two cases with facial edema, without associated respiratory signs. The mean time to onset was 4 hours with extremes between 30 minutes and 24 hours, a compatible time in all cases. The evolution was favorable in all cases. The management was to switch for the second dose to another family of vaccine in 10 cases, and the resumption of the booster with the same initial vaccine in 4 cases. The evolution after booster was favorable in all cases. No recurrence of urticaria was objectified.
Conclusion: Several studies have reported secondary skin reactions to the COVID-19 vaccine. In the study by Stingeni et al., six patients developed mucocutaneous allergic reactions after the first dose of the Pfizer-BioNTech vaccine and resolved spontaneously. Urticarial rash are described with all vaccines. They occur, in the majority of cases, within 12 to 24 hours after the injection. In the classification of Gell and Coombs, acute urticaria is classified as immediate type IgE-mediated hypersensitivity reaction, the risk of serious recurrence after subsequent re-exposure remains possible. In hypersensitivity reactions to the vaccine, and apart from the presence of signs of severity, re-administration of the vaccine is possible.
184
Covid-19 and EMA Authorized Medicines
Tesseromatis C1, Tounta D
1 National and Kapodistrian University of Athens, School of Medicine, Athens, Greece
2 National and Kapodistrian University of Athens, Athens, Greece
Viruses like SARS-CoV-2 plagued people throughout history.They cause severe acute respiratory syndrome (SARS). COVID-19 affects different people in different ways. Most infected people have mild to moderate symptoms and may recover without treatment. Many other suffer from severe acute acute respiratory syndrome with common symptoms, like fever, cough, fatigue, loss of taste (ageusia) or of smell (anosmia). Against COVID-19 disease EMA authorized for use in the European Union the following medicines: Kineret (anakinra), Paxlovid (PF-07321332 / ritonavir), Regkirona (regdanvimab), RoActemra (tocilizumab), Ronapreve (casirivimab / imdevimab), Veklury (remdesivir), Xevudy (sotrovimab). Authorisation was granted from 03/07/2020 to 28/01/2022. Moreover disease that insists may be treated with monoclonal antibodies [Ronapreve (casirivimab/imdevimab) and Regkirona (regdanvimab)], convalescent plasma, or SARS-CoV-2 immunoglobulins.
186
Tunisian experience on therapeutic drug monitoring of hydroxychloroquine in patients with COVID-19
Daly W1,2, Ben Sassi M1,2, Gaies E1,2, El Jebar H1,2, Charfi R1,2, Jlassi S1,2, Jebabli N1,2, Salouage I1,2, Daghfous R1,2, Trabelsi S1,2
1 Service de Pharmacologie Clinique-National Centre Chalbi Belkahia of Pharmacovigilance (CNPV), Tunis, Tunisia
2 Laboratoire de Recherche de Pharmacologie Clinique et expérimentale LR16SP02-Tunis-Tunisia, Tunis, Tunisia
Background: COVID-19 is an emerging disease caused by SARS-CoV-2, which appeared in China in December 2019 and has induced a pandemic in March 2020. Currently, there is no specific anti-viral treatment for Covid -19.
During the first wave, hydroxychloroquine (HCQ) was used by several teams around the world, including, Tunisia, as part of an ethical project called MEURI.
HCQ is an alkaloid belonging to quinoline group used in COVID-19 for its anti-inflammatory effect.
After approximately 12 months of use, we evaluated, at the Clinical Pharmacology department of CNPV, the clinical and epidemiological characteristics of patients.
Objective: Evaluate the plasma concentration of HCQ in patients with COVID-19.
Methodology: This is a retrospective study, established in the Clinical Pharmacology department of CNPV over a 12-month period from April 8, 2020, to Marsh 19, 2021. The quantification of HCQ was established by a high-performance liquid chromatographic method developed in our department. The therapeutic interval of HCQ is between 100 and 1000 ng/ml.
Results: This study has included 29 patients, corresponding to 38 samples. Eighteen of these patients were male, with a sex ratio M/F of 1.6. Median age was 58 years and median weight 80 kg.
Median daily dose of HCQ was 400 mg/day with a maximal dose of 900mg/day and a minimal dose of 250 mg/day. The median weight-based dosing was 5 mg/kg/day.
Median plasma concentration was 187 ng/ml.
Among the 38 samples; 13 presented an infra-therapeutic concentration (34%), 1 presented a supra-therapeutic concentration and the remaining were in the therapeutic-interval.
Over the 13 samples that presented an infra-therapeutic concentration, six samples had zero concentration.
Conclusion: HCQ is usually a well tolerated drug, but because of its narrow therapeutic interval, inefficiency risk and its severe cardiac side effects, a therapeutic drug monitoring is recommended according to the French society of pharmacology and therapeutics.
223
Utilization of Azithromycin during the COVID-19 pandemic in Thessaloniki, Greece
Papaioannidou P1, Michailidou M1, Tsaluchidu S1
1 1st Department of Pharmacology, School of Medicine, Faculty of Health Sciences, Aristotle University of Thessaloniki, Thessaloniki, Greece
Introduction: In the beginning of COVID 19 pandemic, the National Organization of Public Health in Greece (EODY) recommended the administration of azithromycin to patients with symptoms of COVID-19, partially due to its chemoprophylactic / chemotherapeutic effects and partially due to its immunomodulating properties. According to a new guideline of EODY in November 2020, the use of azithromycin was not recommended any more.
Objectives: The aim of this pilot study was to explore the use of azithromycin in the community of Thessaloniki, Greece, due to COVID-19, and to check its correspondence to the waves of the COVID 19 pandemic in Greece.
Methods: The sample was collected by using data from the new Electronic Health Records that have been applied in community pharmacy stores in Thessaloniki. The collected data corresponded to two time periods: March 2019 to January 2020 and March 2020 to January 2021. Specifically, we recorded and compared the monthly consumption of azithromycin for the above time intervals. The consumption of azithromycin was expressed in units of Daily Defined Doses (DDDs).
Results: There was an important increase in azithromycin utilization in July 2020 by 200%, in November 2020 by 150% and in December 2020 by 63%, compared to azithromycin utilization in the corresponding months of 2019. The majority of prescriptions of azithromycin in July, November and December 2020 concerned patients with COVID-19 infection.
Surprisingly, azithromycin utilization for most of the remaining months of 2020 and for January 2021 was lower compared to the same months of 2019 and to January 2020.
Conclusion: The remarkable increase in azithromycin utilization in July, November and December of 2020, is consistent with the increased COVID 19 cases in July 2020 and with the outbreak of the second wave of the COVID-19 pandemic in Greece that was observed in November and December 2020. It reflects the increased cases of COVID-19 patients and the choice of azithromycin as possible therapeutic choice against coronavirus in the community.
The overall decrease in azithromycin use during the remaining months, in which COVID 19 cases were under control, could be attributed to the fact that the implemented measures for the confinement of the pandemic (like the mandatory use of mask, staying at home and keeping distances) seem to have reduced the prevalence of most common respiratory infections in the community.
237
Novel pharmacological targets during COVID-19 infection: a bioinformatics approach
Anatolou D1,2, Dovrolis N1,2, Ragia G1,2, Kolios G1,2, Manolopoulos V1,2,3
1 Laboratory of Pharmacology, Medical School, Democritus University Of Thrace, Alexandroupolis, Greece
2 Individualised Medicine & Pharmacological Research Solutions Center (IMPReS), Alexandroupolis, Greece
3 Clinical Pharmacology Unit, Academic General Hospital of Alexandroupolis, Alexandroupolis, Greece
Introduction: COVID-19 is a respiratory infectious disease induced by the novel coronavirus SARS-CoV-2, causing a worldwide health crisis. The disease exhibits a diversity of symptoms, varying from asymptomatic or common cold-like conditions to severe acute respiratory syndrome and sometimes death. For this reason, it is crucial to characterize the immunological basis of the disease and provide valuable information for therapeutic approaches.
Objectives: This study aims to elucidate potential pharmacological targets using open-access transcriptomic data from two different tissues.
Methods: Expression data from twelve publicly available datasets were obtained from Gene Expression Omnibus (GEO). Five of them contained RNA-seq data from lung autopsies of 61 patients and 30 healthy individuals, while the rest contained RNA-seq data from whole blood samples of 371 patients and 102 healthy participants. Each dataset was analyzed individually and combined using the platform RaNA-seq, comparing the patient group with the healthy control group, and the differentially expressed genes (DEGs) were extracted. The statistically significant genes were selected from each dataset and the intersections between them and between the two tissues were found via Multiple List Comparator of molbiotools. The protein-protein interactions and the functional networks were created via the platform Metascape. The Gene Ontology, Reactome and Kegg databases were used for the emergence of the related pathways.
Results: Among the different datasets and tissues, several DEGs were identified. Specifically, the intersection of the lung datasets suggests 82 upregulated and 204 downregulated genes, while the combined analysis of the whole blood datasets revealed >500 upregulated and only 20 downregulated genes. Regarding the related processes, the extracellular matrix pathway was associated with both the upregulated and downregulated genes revealed from the lung samples. Respectively, mediators of immune responses were highlighted in data from whole blood samples, exposing the dysregulated condition of the immune system. Comparing the transcriptomic profiles from the two different tissues, we identified several DEGs and perturbated pathways that were found common in both lung and whole blood samples and may help unravel the mechanisms behind the disease. There were 15 dysregulated genes that were found in both tissues. Some of these genes are involved in processes, such as cell proliferation (TOP2A, ANLN, MKI67), activation of immune cells (ACSL1, CD177) or extracellular matrix organization (MMP8, TNFAIP6), and may have roles in the pathogenesis of the disease.
Conclusion: The results of this study suggest that a bioinformatics pipeline can improve our understanding of the pathophysiology of COVID-19. The novel pharmacological and therapeutic targets or the potential biomarkers that arise from the bioinformatics analyses could be furtherly explored.
Funding: Financial support for project IMPReS (MIS 5047189) was provided by the Program “Competitiveness, Entrepreneurship and Innovation” (NSRF 2014–2020) co-financed by Greece and the European Union (European Regional Development Fund).
269
Longitudinal study of anti-SARS-CoV-2-Spike IgG antibodies after the second dose of COVID 19 vaccine
Papaioannidou P1, Skoumpa K2, Tsalidou M1, Samaras C2, Varsou E2, Goutsiou R2, Bostanitis C1, Gara S2
1 1st Department of Pharmacology, School of Medicine, Faculty of Health Sciences, Aristotle University of Thessaloniki, Thessaloniki, Greece
2 Microbiological Deparment, General Hospital of Katerini, Katerini, Greece
Introduction:The successful immunization of health care providers is of great importance worldwide. Although the contribution of anti-SARS-CoV-2-Spike IgG antibodies in the real immunity against SARS-CoV-2 remains unclear, their serum title represents a simple and easily measurable index of immunity against SARS-CoV-2, after COVID 19 vaccination.
Objectives: The aim of this work was the longitudinal study of changes in anti-SARS-CoV-2-Spike IgG antibodies in health care professionals after the second dose of vaccination with the BNT162b2 COVID 19 vaccine.
Methods: A total of 180 health care professionals were included in the study. The participants were vaccinated with the BNT162b2 COVID 19 vaccine. Blood sampling was drawn in two time points: 1) two to four weeks following the second dose of the vaccine, and 2) six months after the first blood sample was drawn. Measurement of serum IgG antibodies against the spike domain of SARS-CoV-2 was performed using the SARS-CoV-2 IgG II Quant assay, a chemiluminescent microparticle immunoassay (CMIA) provided by Abbot Diagnostics. The SPSS 25.0 statistical package was used and p<0.05 was considered statistically significant.
Results: 61 out of 180 (34%) health care professionals were male and 119 out of 180 (66%) were female; there mean age was 50.9±8.0 years. The first measurement of IgG antibodies gave the following results: All participants (180/180, 100%) had sufficient serum IgG (normal range 50-25.000 AU/ml). The mean IgG title was 11,885±62 AU/ml, with the majority of titles (77.8 %) ranging from 5000 to 20000 AU/ml. Only 1 out of180 participants (0.5 %) had low IgG levels (<1000 AU/ml) but none below the detection limit (<50 UA/ml). The second measurement of IgG antibodies showed a dramatic decrease in the IgG titles: the mean IgG title was 564±775 AU/ml, showing a decrease of 95%, and reduced to just 5% of the initial title. Nevertheless, almost all participants (176/180, 97.8%) had sufficient serum IgG titles; only 4 participants (1%) had IgG titles below the detection limit (<50 UA/ml).
Conclusion: It seems that BNT162b2 COVID 19 vaccine offers high protection against SARS-CoV-2, since the two-dose immunization was successful to all participants and the initial IgG titles were very high. Nevertheless, six months after the first measurement, the initially high mean IgG title was reduced to just 5% of the initial title of serum anti-SARS-CoV-2-Spike IgG antibodies. Although the exact meaning of this decrease cannot be evaluated, the results of this study support the importance of the third dose of COVID 19 vaccine.
273
Off-label covid-19 targeted therapies-related adverse reactions in a tertiary hospital in Porto during the Portuguese pandemic first wave: retrospective description
Cordeiro G1, Souto M1, Lopes D1, Pinto R1,2,3, Reina-Couto M1,2,4
1 Clinical Pharmacology Unit, São João Hospital and University Centre, Porto, Portugal
2 Biomedicine Department, Pharmacology and Therapeutics Unit, Faculty of Medicine, University of Porto, Porto, Portugal
3 Cardiology Department, São João Hospital and University Centre, Porto, Portugal
4 Intensive Care Department, São João Hospital and University Centre, Porto, Portugal
Introduction: During the first wave of the COVID 19 pandemic, pharmacologic treatments were lacking to show proven efficacy and safety. To overcome this problem, compassionate and off label use programs were instituted(1-3). A COVID 19 treatment protocol and a prescription monitoring program were developed at our institution with the aim of guiding prescription, evaluate the clinical course and monitor adverse drug reactions (ADRs). The hospital clinical pharmacology unit (CPU), the first of its kind in Portugal, had active participation in the construction of the treatment protocol and was responsible for the COVID 19 therapies related ADRs data collection.
Objectives: This work aims to deliver a detailed description of those off label COVID 19 targeted therapies related ADRs in our institution during the Portuguese pandemic first wave.
Methods: A retrospective observational study, based in medical records of adult patients with COVID 19 hospitalized until April 30th of 2020. Description of all off label therapies applied were included, as well other descriptive demographic and clinical variables of interest. Additionally, all ADRs were detailed.
Multivariate logistic regression analysis was performed to identify which risk factors could have affected the likelihood of any ADRs. Significant differences were considered with a p result <0,05.
Results: Five hundred patients were admitted with SARS CoV 2 infection during the study period, with 263 (52.6%) being treated with off label therapies. Patients in this subgroup presented a mean age of 69,9 years, 145 (55,1%) were male, 166 (63.1%) had arterial hypertension, 92 (35.0%) had type 2 diabetes and 62 (23.6%) were obese. All patients were treated with hydroxychloroquine, and five were treated concomitantly with other off label drugs: one with tocilizumab, one with remdesivir, one with anticoagulant drugs, and two with lopinavir/ritonavir. The big majority, 213 (81%), were treated before the institutional protocol was approved, on April 9th of 2020, and presented no ADRs (84.4%). Abnormal liver blood tests were the most frequent ADRs reported, with 9 (3.42%), 8 (3.04%) and 10 (3.8%) presenting cytocholestasis, cytolysis and cholestasis respectively. The logistic regression analyses showed that the likelihood of ADRs significantly increased in the presence of hypertension (P=0.040) and dementia syndrome (P=0.017) comorbidities, and especially critically ill patients needing mechanical ventilation (P<0.001).
Conclusions: These results showed that critically ill patients and those with hypertension and dementia treated with off label COVID 19 targeted therapies could be at higher risk of developing ADRs. Due to the exploratory nature and the sample size of this study, no definite conclusion can be made.
275
Effect and Tolerability of a Nutritional Supplement in Volunteers Receiving the Influenza or the COVID-19 Vaccine: Randomized, Double-Blind, Placebo-Controlled Study
Mateus Rodriguez J1, Bifano M1, Mendez Placencia C1, Olivé Torralba A1, Fontseca Martin E1, Roy Millan P1
1 Hospital Mare de Déu de la Mercè, Barcelona, Spain
Introduction: Nutritional supplements aimed at enhancing immune defense mechanisms for an effective antiviral immune response have been a growing focus of interest, particularly in the face of immunosenescence and to compensate for specific micronutrient deficiencies among elderly subjects. In Covid-19 infection and in the fall/winter season with influenza virus, additional tools for immunity enhancement provided by vaccines are sought. ABBC1 is a nutritional supplement a combination of beta-1,3 / 1,6-glucan with inactivated Saccharomyces cerevisae rich in selenium and zinc that allows a dosage compatible with the usual medication in patients vulnerable to this type of infections, often polymedicated, allowing to add an additional therapeutic tool in the fight against the pandemic.
Objectives: The aim of the study was to determine whether nutritional supplementation could improve the immune response to these vaccines and the micronutrient status of the participants. The high tolerance, safety, and immediate availability of ABB C1® in all types of subjects receiving the influenza or COVID-19 vaccines including geriatric and immunocompromised populations.
Methods: A single-center, randomized, double-blind, placebo-controlled study was conducted in 72 volunteers who received a synergistic combination of yeast-based ingredients with a unique β-1,3/1,6-glucan complex and a consortium of heat-treated probiotic Saccharomyces cerevisiae rich in selenium and zinc (ABB C1®) or placebo on the next day after getting vaccinated against influenza (Chiromas®) (n = 34) or the COVID-19 (Comirnaty®) (n = 38). The duration of treatment was 30 and 35 days for the influenza and COVID-19 vaccine groups, respectively.
Results: Mean levels of CD4+T cells increased from 910.7 at baseline to 1000.2 cells/μL after the second dose of the COVID-19 vaccine in the ABB C1® group, whereas there was a decrease from 1055.1 to 929.8 cells/μL in the placebo group. Changes of CD3+T and CD8+T lymphocytes showed a similar trend. In the COVID-19 cohort, the increases in both IgG and IgM were higher in the ABB C1® supplement than in the placebo group. Serum levels of selenium and zinc showed a higher increase in subjects treated with the active product than in those receiving placebo. No serious adverse events related to ABB C1® or tolerance issues were reported. The study findings validate the capacity of the ABB C1® product to stimulate trained immunity.
Conclusions: The administration of a nutritional supplement (ABB C1®) based on a combination of β-glucan and probiotic S. cerevisiae yeasts enriched with selenium and zinc in volunteers in association with influenza and COVID-19 mRNA vaccines appeared to be able to stimulate trained immunity as compared with placebo, which indicates that ABB C1® provides a reliable source of absorbable micronutrients relevant to enhance the immune function.
Tabel/Image
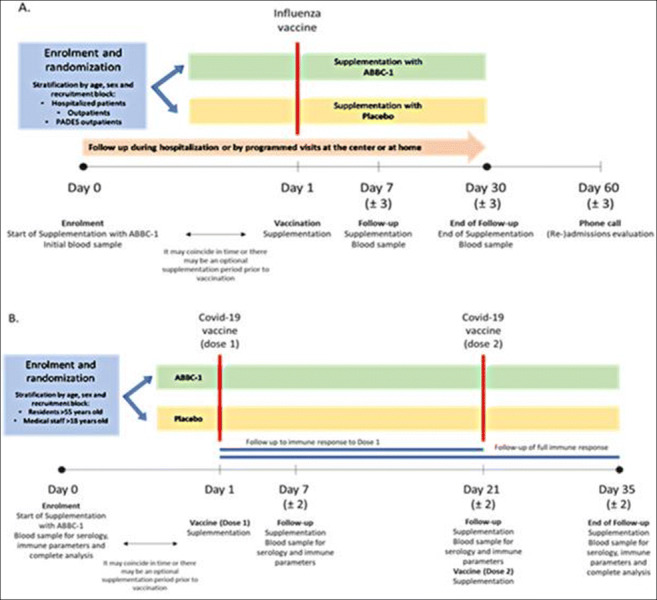
277
Utilization of injectable anticoagulants in a tertiary Hospital of Thessaloniki during the second wave of COVID 19 pandemic in Greece
Papaioannidou P1, Michailidou M1
1 1st Department of Pharmacology, School of Medicine, Faculty of Health Sciences, Aristotle University of Thessaloniki, Thessaloniki, Greece
Introduction: In spite of measures against the COVID 19 pandemic, the outbreak of the second wave of the pandemic in Greece during the months of November and December of 2020, resulted in remarkable increase of hospitalization of COVID 19 patients. Among other therapeutic strategies at that time, the use of injectable anticoagulants was strongly recommended for hospitalized patients, due to its beneficial effect against increased thrombotic risk.
Objectives: The aim of this study was to investigate the use of injectable anticoagulants during the second wave of COVID 19 pandemic in a tertiary Hospital of Thessaloniki, Greece, and to explore any possible differences with the previous use of injectable anticoagulants before the COVID-19 pandemic.
Methods: The data were collected from the Pharmacy of a tertiary Hospital in Thessaloniki, by examining prescriptions of injectable anticoagulants for hospitalized patients during the time periods of November to December 2020 and November 2019 to December 2019. Specifically, we recorded and compared the monthly consumption of enoxaparin, nadroparin, tinzaparin, heparin, bemiparin and fondaparinux between the above time intervals.
Results: The consumption of injectable anticoagulants in 2020 was much higher than the relative consumption in 2019 (about double).
In November 2019, out of 15,986 DDDs of injectable anticoagulants, the percentage of relative consumption of fondaparinux, enoxaparin, nadroparin, heparin, tinzaparin, and bemiparin was 7.3%, 33.8%, 0.1%, 16.2%, 32.2% and 10.2%, respectively. In November 2020, out of 32,025 DDDs of injectable anticoagulants, the percentage of relative consumption of fondaparinux, enoxaparin, nadroparin, heparin, tinzaparin, and bemiparin was 2.2%, 72.2%, 0.0%, 7.4%, 14.4% and 3.8% respectively. In December 2019, out of 15,880 DDDs of injectable anticoagulants, the percentage of relative consumption of fondaparinux, enoxaparin, nadroparin, heparin, tinzaparin, and bemiparin was 5.9%, 36.0%, 0.45%, 15.6%, 30.6% and 11.4% respectively. In December 2020, out of 33.406 DDDs of injectable anticoagulants, the percentage of relative consumption of fondaparinux, enoxaparin, nadroparin, heparin, tinzaparin, and bemiparin was 3.3%, 71.3%, 0.0%, 7,2%, 15,2% and 3.0% respectively.
Compared to enoxaparin utilization in November and December of 2019, a huge increase in enoxaparin utilization was observed in November and December of 2020 (328% and 317% respectively). The majority of enoxaparin prescriptions in November and December 2020 concerned patients with COVID-19 infection.
Conclusion: Injectable anticoagulant therapy, and especially enoxaparin, was administered in COVID-19 patients, as a therapeutic choice to reduce the high risk of thrombosis in hospitalized patients during the outbreak of the second wave of COVID-19 pandemic in Greece in November and December 2020.
283
Capillary Leak Syndrome and COVID-19 Vaccines: An Analysis of the European Spontaneous Reporting System EudraVigilance
Di Napoli R1,2, Balzano N1,2, Fraenza F1,2, Ruggiero R1,2, Capuano A1,2, Rossi F1, Rafaniello C1,2
1 Section of Pharmacology “L. Donatelli”, Department of Experimental Medicine, University of Campania “Luigi Vanvitelli”, Naples, Europe
2 Campania Regional Centre for Pharmacovigilance and Pharmacoepidemiology, University of Campania "Luigi Vanvitelli", Department of Experimental Medicine, Napoli, Italy, Naples, Europe
Introduction: Recently, capillary leak syndrome (CLS) emerged as new suspected adverse event after immunization (AEFI) associated to COVID-19 vaccination. The CLS is rare condition characterized by increased capillary permeability, resulting in hypoalbuminemia, hypotension, and edema. This condition is rare, but serious and potentially fatal.
Objectives: Our pharmacovigilance study aims to evaluate the onset of CLS as AEFI with COVID-19 mRNA vaccines (Spikevax and Comirnaty) compared to viral vector vaccines (Janssen and Vaxzevria).
Methods: We carried out descriptive and disproportionality analyses of all Individual Case Safety Reports (ICSRs) reporting a vaccine COVID-19 as suspected drug and the CLS as AEFI, which were collected in the pharmacovigilance database EudraVigilance from January 1st, 2021, to January 14th, 2022. For the disproportionality analysis we applied the Reporting Odds Ratio (ROR) 95% CI.
Results: During our study period, CLS was described as AEFI in 84 out of 1,357,962 ICRs reporting a vaccine COVID-19 as suspected drug and collected in the EV database.
Overall, the ICSR reported by CLS were mainly related to the viral vector COVID-19, Vaxzevria®, (N=36) and Janssen®, (N=9), while the ICSR reported to vaccines COVID-19 mRNA were 39 (Comirnaty®, N=33; Spikevax®, N=6). Majority of ICSRs were reported by healthcare professionals (N=60; 71.4%). The non-healthcare professional represented the primary source in the 41.7% of Vaxzevria®-related ICSRs.
Majority of the patients were adult (N=49; 58.3%). The female gender accounted in more than 65% of ICSRs referred both to mRNA and viral vector vaccines. In particular, women were more represented in ICSRs referred to Spikevax® (83.3%) and to Vaxzevria® (72.2%).
The CLS outcome was more frequently favorable in mRNA ICSRs (N=13; 33,3%) than the viral vector ones (N=6; 13.3%). On the other hand, among the ICSRs reporting CLS with unfavorable outcome (N = 25; 29.8%) we found also 9 ICSRs describing fatal CLS (Comirnaty® N = 1; Vaxzevria® N = 4; Janssen® N = 4).
In 27 ICSRs it was reported at least a concomitant drug. The most frequently reported ones were psychoanaleptics (N=9), drugs for obstructive airway diseases (N=8), agents acting on the renin-angiotensin system and analgesics (N=6, both).
From our disproportionality analysis emerged a lower CLS reporting probability after COVID-19 vaccination with mRNA vaccines compared to viral vector-based ones.
Conclusions: According to our results, few ICSRs describing CLS have been collected in EV in front of billion administered doses. This could underline the rarity of this AEFI or the limit of underreporting of spontaneous reporting and therefore also our study. Since the significant clinical relevance of CLS, this AEFI requires a careful monitoring.
Healthcare professionals as well as patients should be aware of the signs and symptoms of CLS. Patients with a history of CLS require particular attention because of a possible risk of flare-up of disease. Since a precise mechanism is still not identified, further studies are necessary to confirm the causal relationship between CLS and COVID-19 vaccination.
288
Safety of mRNA-platform based COVID-19 vaccines in pediatric population: an Analysis of the European pharmacovigilance database Eudravigilance
Ruggiero R1,2, Stelitano B1,2, Mascolo A1,2, Gaio M1,2, Balzano N1, Sportiello L1,2, Capuano A1,2, Rossi F2
1 Campania Regional Centre for Pharmacovigilance and Pharmacoepidemiology, Naples, Europe
2 Department of Experimental Medicine – Section of Pharmacology “L. Donatelli”, Naples, Europe
Introduction: Recently mRNA-based COVID-19 vaccines have been approved also for use in pediatric population. Vaccines safety require particular attention in this population, considering its vulnerability. Analysis of pharmacovigilance database allows to extrapolate important information in order to identify possible safety signals or new/unknown adverse events following immunization (AEFIs).
Objective: Our pharmacovigilance study aims to describe and evaluate the onset of AEFIs with COVID-19 mRNA vaccines (Spikevax and Comirnaty) in the pediatric population.
Methods: We retrieved all the Individual Case Safety Reports (ICSRs) collected in the European pharmacovigilance database, Eudravigilance from the 1st Jan 2021 to 4th March 2022 and reporting AEFIs related to mRNA COVID-19 vaccines (Spikevax and Comirnaty) occurred in pediatric population (aged 0-17 years).
Results: Overall, we retrieved in Eudravigilance 25.019 ICSR related to Comirnaty and 1.862 ICSRs referred to Spikevax reporting AEFI occurred after COVID-19 vaccination in pediatric patients. The majority of Comirnaty-related ICSRs were reported by non-healthcare professionals (59.6%), while there was no difference considering the reporter type in Spikevax ICSRs. The majority of ICSRs reported well-known general disorders like headache, pyrexia, fatigue and nausea, which represented the most frequently reported AEFIs for both mRNA vaccines. Overall, no substantial gender difference emerged in our dataset, even if we found a slight prevalence for female gender for both mRNA vaccine (Spikevax, 52.6 %; Comirnaty, 53.9%). Considering specific AEFIs of interest such as reproductive system disorders, these were more frequently referred to adolescent female than male patients. These AEFIs included menstrual disorders (Comirnaty, N=609; Spikevax, N=23), amenorrhoea (Comirnaty, N=408; Spikevax, N=16) or intermenstrual bleeding (Comirnaty, N=169; Spikevax, N=10), polymenorrhoea (Comirnaty, N=172; Spikevax, N=10). The few cases describing AEFIs related to reproductive system disorders in males were mainly related to vaccination with Comirnaty. In particular, the reported AEFIs included testicular pain (Comirnaty, N=14; Spikevax, N=1), erectile dysfunction (Comirnaty, N=5), testicular torsion (Comirnaty, N=4) or swelling (Comirnaty, N=4), scrotal pain (Comirnaty, N=3) or oedema (Comirnaty, N=1). Majority of AEFIs had a favorable outcome in more than 50% of cases for both mRNA vaccines, including a complete resolution of the events in more than 30% of cases or an ongoing resolution at the moment of reporting in 20% of cases. Finally, from our disproportionality analysis emerged a statistically significant ROR for menstrual disorders (ROR 1.72, 95%CI 1.43-2.10; p <0.05), vaccination failure (ROR 8.11, 95%CI 5.05- 13.97; p <0.05) and seizure (ROR 1.54, 95%CI 1.03-2.41; p= 0.037) when compared Comirnaty versus Spikevax.
Conclusion: According to our results, the majority of reported AEFIs occurred in pediatric population are mild and with a positive outcome, supporting the role of ongoing COVID-19 vaccination campaign in this population as a critical public health tool for disease prevention and control of pandemic. However, from our analysis emerged that Comirnaty was associated with an increased reporting probability of menstrual disorders, vaccination failure and seizure when compared to Spikevax in our population of interest. Further investigations are needed to establish the causal relationship.
289
Safety of mRNA-platform based COVID-19 vaccines in pediatric population: an Analysis of the European pharmacovigilance database Eudravigilance
Ruggiero R1,2, Stelitano B1,2, Mascolo A1,2, Gaio M1,2, Balzano N1, Sportiello L1,2, Capuano A1,2, Rossi F2
1 Campania Regional Centre for Pharmacovigilance and Pharmacoepidemiology, Naples, Europe
2 Department of Experimental Medicine – Section of Pharmacology “L. Donatelli”, Naples, Europe
Introduction: Recently mRNA-based COVID-19 vaccines have been approved also for use in pediatric population. Vaccines safety require particular attention in this population, considering its vulnerability. Analysis of pharmacovigilance database allows to extrapolate important information in order to identify possible safety signals or new/unknown adverse events following immunization (AEFIs).
Objective: Our pharmacovigilance study aims to describe and evaluate the onset of AEFIs with COVID-19 mRNA vaccines (Spikevax and Comirnaty) in the pediatric population.
Methods: We retrieved all the Individual Case Safety Reports (ICSRs) collected in the European pharmacovigilance database, Eudravigilance from the 1st Jan 2021 to 4th March 2022 and reporting AEFIs related to mRNA COVID-19 vaccines (Spikevax and Comirnaty) occurred in pediatric population (aged 0-17 years).
Results: Overall, we retrieved in Eudravigilance 25.019 ICSR related to Comirnaty and 1.862 ICSRs referred to Spikevax reporting AEFI occurred after COVID-19 vaccination in pediatric patients. The majority of Comirnaty-related ICSRs were reported by non-healthcare professionals (59.6%), while there was no difference considering the reporter type in Spikevax ICSRs. The majority of ICSRs reported well-known general disorders like headache, pyrexia, fatigue and nausea, which represented the most frequently reported AEFIs for both mRNA vaccines. Overall, no substantial gender difference emerged in our dataset, even if we found a slight prevalence for female gender for both mRNA vaccine (Spikevax, 52.6 %; Comirnaty, 53.9%). Considering specific AEFIs of interest such as reproductive system disorders, these were more frequently referred to adolescent female than male patients. These AEFIs included menstrual disorders (Comirnaty, N=609; Spikevax, N=23), amenorrhoea (Comirnaty, N=408; Spikevax, N=16) or intermenstrual bleeding (Comirnaty, N=169; Spikevax, N=10), polymenorrhoea (Comirnaty, N=172; Spikevax, N=10). The few cases describing AEFIs related to reproductive system disorders in males were mainly related to vaccination with Comirnaty. In particular, the reported AEFIs included testicular pain (Comirnaty, N=14; Spikevax, N=1), erectile dysfunction (Comirnaty, N=5), testicular torsion (Comirnaty, N=4) or swelling (Comirnaty, N=4), scrotal pain (Comirnaty, N=3) or oedema (Comirnaty, N=1). Majority of AEFIs had a favorable outcome in more than 50% of cases for both mRNA vaccines, including a complete resolution of the events in more than 30% of cases or an ongoing resolution at the moment of reporting in 20% of cases. Finally, from our disproportionality analysis emerged a statistically significant ROR for menstrual disorders (ROR 1.72, 95%CI 1.43-2.10; p <0.05), vaccination failure (ROR 8.11, 95%CI 5.05- 13.97; p <0.05) and seizure (ROR 1.54, 95%CI 1.03-2.41; p= 0.037) when compared Comirnaty versus Spikevax.
Conclusion: According to our results, the majority of reported AEFIs occurred in pediatric population are mild and with a positive outcome, supporting the role of ongoing COVID-19 vaccination campaign in this population as a critical public health tool for disease prevention and control of pandemic. However, from our analysis emerged that Comirnaty was associated with an increased reporting probability of menstrual disorders, vaccination failure and seizure when compared to Spikevax in our population of interest. Further investigations are needed to establish the causal relationship.
314
Comparative tolerability profile of mRNA vaccines during mass vaccination of healthcare professionals at a tertiary hospital. An active pharmacovigilance study
Sáez-peñataro J1, Torres F1,2, Bartra J3,4,5, Bascuas J1, Vilella A6, Tortajada M7, Quesada S7, González E6, López-Suñé E8, Castells A9, Serrano S2, Camacho C, Trilla A6, Calvo G1
1 Medicines Division. Department of Clinical Pharmacology. Hospital Clínic, Barcelona, Barcelona, Spain
2 IDIBAPS Biostatistics Core Facility. Medicines Division. Department of Clinical Pharmacology. Hospital Clínic, Barcelona, Barcelona, Spain
3 Department of Pneumology, Allergy Section, Institut Clínic Respiratori (ICR), Hospital Clínic de Barcelona, Barcelona, Spain
4 Institut d'Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Universitat de Barcelona, Barcelona, Spain
5 Spanish Network for Allergy - RETIC de Asma, Reacciones adversas y Alérgicas (ARADYAL), Madrid, Spain
6 Department of Preventive Medicine and Epidemiology. Hospital Clínic, Barcelona, Barcelona, Spain
7 Department of Occupational Health Care. Hospital Clínic, Barcelona, Barcelona, Spain
8 Medicines Division. Department of Hospital Pharmacy. Hospital Clínic, Barcelona, Barcelona, Spain
9 Chair of the Pharmacovigilance Technical Committee (PhVTC). Medical Director of Hospital Clínic of Barcelona. Hospital Clínic, Barcelona, Barcelona, Spain
10 Medicines Division. Hopsital Clínic, Barcelona, Barcelona, Spain
Background: Further understanding on the safety profile of vaccines in a “real-world” still need to be elucidated, such as the comparative tolerability and reactogenicity of mRNA vaccines (BNT162b2 and MRNA-1273) beyond the controlled context of clinical trials.
An active pharmacovigilance study was designed to capture a complete short-term safety profile of two mRNA vaccines BNT162b2 and mRNA-1273, targeting incidence rates of adverse reactions within a pre-defined denominator of vaccinated healthcare professionals.
Methods: A prospective active surveillance study was implemented during the vaccination campaign at Hospital Clínic by a multidisciplinary team, involving the Pharmacovigilance Technical Committee, the Department of Preventive Medicine and Epidemiology and the Department of Occupational Health Care of the Hospital. Target population of the program included vaccinated professionals from Hospital Clínic and affiliated institutions, who were vaccinated with BNT162b2 and mRNA-1273. The program was based on the delivery of a structured questionnaire by telephonic interview after each vaccine dose. A total of 64% of vaccinated professionals completed the questionnaire (n=5088).
Results: A total of 85% subjects suffered at least 1 AR reaction with the vaccine. The proportion of professionals developing any AR was 2878 (81.2%) after vaccination with BNT162b2 and 1463 (92.9%) after vaccination with mRNA-1273. Severe ARs (VAS scoring ≥ 7) were reported in 1184 (33.7%) professionals after vaccination with BNT162b2 and 886 (56.4%) after mRNA-1273. In the multivariate analysis, mRNA-1273 showed a greater reactogenicity than BNT162b2 (OR=3.04 (95% CI 2.48 - 3.73; p-value: <0.0001)).
Conclusions: Our study shows that mRNA-1273 has greater reactogenicity than BNT162b2. Overall, both mRNA vaccines had a reasonable tolerability profile, compared in a real-world scenario. This can be understood as a reassuring message for the medical and scientific community.
317
Impact of demographics on the inmunogenicity after the third dose of the mRNA-1273 vaccine against the SARS-CoV-2 in cancer patients
Jiménez Ortega A1, Benitez Fuentes J2, de Luna Aguilar A2, Flores Navarro P2, Bartolomé Arcilla J2, Baos Muñoz E3, Delgado-Iribarren García-Campero A3, Gil Useros S2, Pérez Segura P2, Sánchez-Ramón S4, Mohamed Mohamed K4, Guevara-Hoyer K4, García Bravo L4
1 Clinical Pharmacology Department, IdISSC, Hospital Clinico San Carlos, Madrid, Madrid, 28040, Spain, Madrid, Spain
2 Medical Oncology Department, IdISSC, Hospital Clinico San Carlos, Madrid, Spain
3 Clinical Microbiology Department, IML and IdISSC, Hospital Clínico San Carlos, Madrid, Spain
4 Inmunology Department, IdISSC, Hospital Clinico San Carlos, Madrid, Spain
Introduction: Data regarding cancer patients is scarce, as oncology patients were not systematically included in phase III clinical trials. It is important to produce clinical data to ascertain the level of protection provided by SARS-CoV-2 vaccines among cancer patients.
Objectives: To describe the immunogenicity achieved by covid-naïve oncology patients after the second and third dose and to describe the impact of prognostic baseline covariates and factors on the antibody levels after the third dose.
Methods: This observational prospective study took place in the Hospital Clinico San Carlos (Madrid) between April 19, 2021, and December 31, 2021. We included patients 18-years or older with solid malignancies who were under active anticancer therapy and received the three-dose schedule of the mRNA9 1273 vaccine. Patient electronic medical records were reviewed to gather data regarding demographics and antibody levels. Patients with documented previous infection by SARS-Cov-2, positive baseline antibody levels or incomplete data were excluded from the study. We performed a Kruskal Wallis test to determine if there were differences in antibody levels after the third dose between different Eastern Cooperative Oncology Group (ECOG) scores, from 0 to 2. We performed a linear regression to ascertain the effect of different ECOG scores on immunogenicity after the third dose adjusted by sex, age and antibody levels after the second dose.
Results: A total of 93 patients were included. Median levels after the second and third dose were 5528,2 AU/ml (range 82513 AU/mL) and 22376 U/mL (range 79980 AU/mL) respectively. Distributions of antibody levels were not similar for all groups (ECOG 0, ECOG 1 and ECOG 2), as assessed by means of an inspection of a boxplot. Antibody levels were significantly different between the different levels of ECOG, χ2(2) = 45,823 p = <0,01. We performed pairwise comparisons using the Dunn’s procedure with a Bonferroni correction. This analysis revealed statistically significant differences between the groups ECOG 0 (mean rank=81,10) and 2 (14,44), p < 0,001; and the ECOG 0 and 1 (42,24) (p < 0,001) No difference was found between ECOG 2 and 1. The multiple regression model statistically significantly predicted the square root of antibodies after the third dose. F (5, 87) 12,48, p < .001, adjusted R-squared = 0,384. Sex and age weren’t significantly related to the dependent variable. There was a statistically significant and direct relationship between antibody levels after the second dose and after the third dose (p=0,017). We found an inverse relationship between higher ECOG scores and antibody levels after the third dose which resulted statistically significant (p < .00
Conclusion: oncology patients with higher ECOG scores appear to achieve lower antibody levels after vaccination against SARS-CoV-2. Immunogenicity after the previous dose is also a statistically significant predictor. Further studies are needed to ascertain if those findings are clinically significant and result in higher infection rates across these subgroups.
Drug Regulation
20
Interchangeability of biosimilars from a regulatory standpoint: Croatian experience (2018 - 2020)
Arapovic Dzakula S1, Juricic Nahal D1, Lovrek Romcevic M1, Tomic S1
1 Croatian Agency For Medicinal Products And Medical Devices (HALMED), Zagreb, Croatia
Introduction: Biosimilarity concept is widely accepted by the scientific community and the regulators. Biosimilar medicinal products are highly similar to their reference biologic medicines in terms of structure, efficacy, safety and immunogenicity. In the EU, biosimilars are approved by the European Commission following thorough assessment and a favourable opinion by the European Medicines Agency (EMA). However, the issue of interchangeability is dealt with at national level. In June 2018 Croatian national competent authority (HALMED) issued updated recommendation to encourage interchangeability of biosimilars.
Objectives: To review utilisation of 3 biologic medicines (infliximab, etanercept and adalimumab) and their biosimilars during the review period (2018-2020).
To review ADR reporting for reference biologic medicines and their biosimilars during the review period.
To follow up on the preliminary analysis done for 2018 which was presented at the EACPT 2019.
Methods: VigiLyze database was searched for ICSRs for 3 biologic medicines and their biosimilars from 1 January 2018 to 31 December 2020 for Croatia. Details of ICSRs between reference biologic medicines and their biosimilars were compared.
Utilisation data from 2018 to 2020 were extracted from the national utilisation database.
Results: Utilisation of reference biologic medicines and their respective biosimilars increased during the 3-year review period. The highest increase in utilisation was recorded for adalimumab. The total number of ADRs for infliximab decreased during the review period, remained almost unchanged for etanercept and increased significantly for adalimumab. An increase in ADR reporting for adalimumab was observed for reference adalimumab as well as for the available biosimilars. The total number of ADRs per year for each biologic active substance was low. Several new biosimilars became available in 2019 and particularly 2020.
Conclusion: Following the encouragement of interchangeability of biosimilars in Croatia in 2018, further data were collected and analysed for 3 consecutive years (2018 – 2020). Initial enhanced public focus was observed. Utilisation data confirmed expected market penetration of biosimilars. No universal trend in ADR reporting was observed. Encouraging interchangeability of biosimilars on a national level is associated with a wider availability of biologic medicines.
50
Sepsis in pregnancy and the puerperium: a comparison of 4 international guidelines
Boureka E1, Tsakiridis I1, Lallas K2, Dagklis T1, Papazisis G3,4
1 Third Department of Obstetrics and Gynaecology, School of Medicine, Aristotle University of Thessaloniki, Greece
2 Department of Medical Oncology, Papageorgiou General Hospital, School of Medicine, Aristotle University, Thessaloniki, Greece
3 Department of Clinical Pharmacology, School of Medicine, Aristotle University, Thessaloniki, Greece
4 Clinical Trials Unit, Special Unit for Biomedical Research and Education, School of Medicine, Aristotle University of Thessaloniki, Greece
Introduction: Despite the new diagnostic techniques and therapeutical strategies, sepsis remains an important cause of maternal mortality, which combined with antibiotic resistance makes early diagnosis and management of sepsis even more urgent.
Objectives: To review the optimal management of sepsis in pregnancy and the puerperium with the comparison of guidelines.
Methods: Four international guidelines on the diagnosis, management and prevention of sepsis in pregnancy and the puerperium were reviewed and compared. Specifically, a descriptive review of the guidelines of Royal College of Obstetricians and Gynaecologists (RCOG), Society for Maternal-Fetal Medicine (SMFM), Society of Obstetric Medicine of Australia and New Zealand (SOMANZ) and World Health Organization (WHO) was conducted.
Results: RCOG, SMFM and SOMANZ provide information on the diagnosis and management of sepsis, whereas WHO suggests preventive methods. More specifically, regarding diagnosis, RCOG, SMFM and SOMANZ strongly recommend culture obtainment, measurement of serum lactate and imaging, when required. As for the management, necessity of primary antibiotic administration, during the first hour, is underlined, with differentiation on the antibiotics, depending on the site of infection. In addition, delivery of the fetus should be attempted for obstetric indications only. Finally, WHO recommends preventive antibiotic administration on certain cases, such as preterm premature rupture of membranes.
Conclusion: Despite the differences from this comparison, all four guidelines agree on the necessity of early diagnosis and proper therapeutic management on controlling sepsis in pregnancy and the puerperium. Meanwhile, preventive strategies aim on reducing the increasing mortality of the disease.
69
High-dose MTX intoxication treated with dose-capped glucarpidase
Engels F1, van der Sluis I1, Dijkman M2, Koppen A2
1 Princess Maxima Center For Pediatric Oncology, Utrecht, The Netherlands
2 Dutch National Poisons Information Center, Utrecht, The Netherlands
Introduction: High dose methotrexate (HDMTX) is an essential part of pediatric oncology treatment. HDMTX-associated acute kidney injury due to delayed MTX clearance is a serious toxicity and linked to an excess in MTX induced toxicities. Glucarpidase is a recombinant enzyme that rapidly hydrolyzes MTX into two non-toxic metabolites, DAMPA and glutamic acid. The recommended dose is 50 IE/kg, however no formal dose-finding studies were performed as part of the authorization application. In our institution patients are treated with a capped dose of 1000 IE, resulting in doses <50 IE/kg. The enzyme activity of glucarpidase together with several case reports highly suggest that lower doses of glucarpidase might be equally effective in lowering MTX levels.
Objectives: Here we assessed the effect of dose-capped dosing of glucarpidase on MTX levels and kidney function.
Methods: Twelve patients (the majority of which were leukemia patients, HDMTX 5 g/m2 in 24 hours) with toxic MTX levels following HDMTX were treated with glucarpidase 1000 IE (median 25 IE/kg, range 13-53 IE/kg). Creatinine levels together with MTX levels (immunoassay) prior and post (≥ 48 hours) glucarpidase administration were retrospectively assessed.
Results: All patients experienced HDMTX associated acute kidney injury (median increase in creatinine levels at 48 hours after HDMTX compared to baseline of 311%, range 144-623%) and showed toxic MTX levels (median 15 μmol/L range 8,3-140 μmol/L) before glucarpidase administration. Glucarpidase was administered 41-54 hours (median 50 hours) after HDMTX initiation. MTX levels decreased to levels < 0,25 μmol/L by 216 hours (range 209-253 hours) after HDMTX start (i.e. 169 hours after glucarpidase administration, range 156-212 hours). Creatinine levels were ≤ 1,5 times baseline value within 223 hours (range 164-356 hours).
Conclusion: For glucarpidase there are no data that allow for an assessment of the relationship between exposure and efficacy of MTX conversion. As such the minimal effective dose is unknown. Based upon in vitro enzyme activity (1 unit of glucarpidase activity catalyses the hydrolysis of 1 μmol of MTX in 1 ml in 1 minute) the recommended dose seems to be more than sufficient even taking into account redistribution of MTX. Our patients were all treated with 1000 IE and time to MTX < 0,25 μmol/L was comparable between patients. MTX levels were measured by immunoassay which does not allow for distinction between DAMPA and MTX. Alternative approaches to monitor MTX levels after glucarpidase administration, such as HPLC, are required in order to better evaluate the effectiveness of glucarpidase at lower doses than recommended. Glucarpidase is an expensive drug. By limiting the dose to 1000 IE we required 20 vials less than otherwise required (savings 460.000 euros).
82
Real life experience of medicinal cannabis derivatives use in children and adults in Uruguay between 2018 and 2021
Speranza N1, De Santis A1, Galarraga F1, Wood I1
1 Departamento de Farmacología y Terapéutica, Hospital de Clínicas Dr. Manuel Quintela, Facultad de Medicina, Universidad de la República, Montevideo, Uruguay
Introduction: Medical cannabis use has been legally contemplated in Uruguay since 2013. Consumption, possession and commercialization is regulated for recreational and medicinal use. Until the introduction of industrialized cannabis to the national market, access was by compassionate use or by artisanal producers. Since 2017, five legalized presentations with different percentages of cannabidiol have been incorporated. There are few national published studies on medical cannabis use.
Objective: To describe medical cannabis use profile in 3 cohorts of treated patients from Uruguay: two adult’s cohorts and one child and adolescent’s cohort of patients, between 2018 and 2021.
Methods: We summarize three descriptive, observational studies based on telephone interviews to patients under treatment with medical cannabis. Cohorts data were obtained from a private clinic in 2018 and artisanal cannabis producers in 2019 (adult cohorts) and a pediatric hospital and private clinic (pediatric cohort) in 2021. All participants or their guardians had to give consent to be included. Obtained data was analyzed using descriptive statistics. All of them were carried out with students from the Faculty of Medicine (Universidad de la República) within the framework of Scientific Methodology II subject.
Results: Sixty six, 32 and 26 patients were included in 2018, 2019 and 2021, respectively. The most frequent symptom described in adults was pain, with arthrosis as the main cause. Refractory epilepsy was the most frequent pediatric indication. Medical cannabis used were cannabidiol or artisanal medical cannabis. Most frequent adverse event was dry mouth in adults and drowsiness in children. Almost all patients had high expectations before use and had a good perception of improvement (numerical pain scale in adults or number of epileptic seizures in children) (Table 1)
Conclusions: Medical cannabis main uses were pain in adults and refractory epilepsy in children and adolescents. The only approved indication for medical cannabis in Uruguay is refractory epilepsy in children older than 2 years. There were few adverse events reported in each cohort, mostly already known events. It is important to continue analyzing its use, mainly in pain conditions including long-term effectiveness and safety monitoring and also incorporate drug prescription attitudes and behavior studies, in order to contribute to the evidence base for medical cannabis.
Tabel/Image
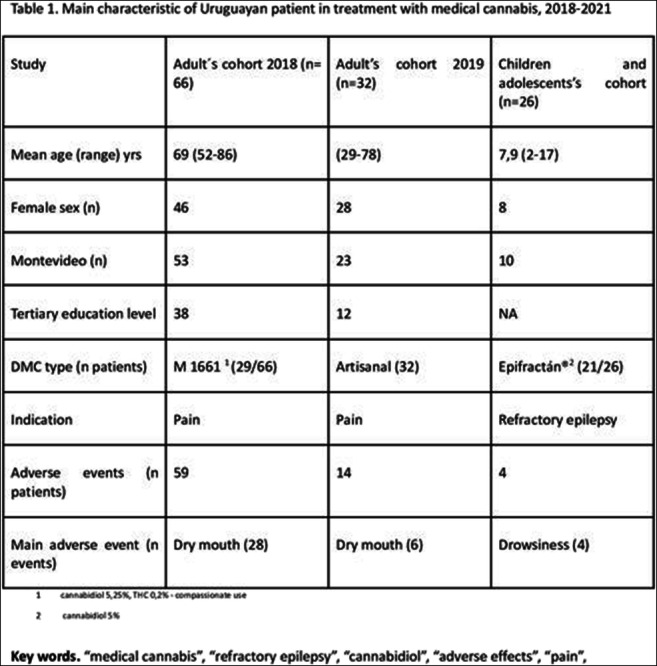
84
Real-world evidence of pain treatment with medical cannabis in two populations in Uruguay during 2018-2019
De Santis A1, Speranza N, Galarraga F
1 Departamento de Farmacología y Terapéutica, Facultad de Medicina, Universidad de la República, montevideo, Uruguay
Introduction: Medical cannabis use has been legally contemplated in Uruguay since 2013. Consumption, possession and commercialization is regulated for recreational and medicinal use. Until the introduction of industrialized cannabis to the national market, access was by compassionate use or by artisanal producers. Since 2017, five legalized presentations with different percentages of cannabidiol have been incorporated, only for refractory epilepsy indication.
Objective: To describe medical cannabis use for pain treatment in two adult cohorts in Uruguay between 2018 and 2019.
Methods: We summarize two descriptive, observational, studies based on telephone interviews to adult patients under treatment with medical cannabis. Cohort's data were obtained from a private clinic in 2018 and from artisanal cannabis producers in 2019. Obtained data was analyzed using descriptive statistics.
Results: Sixty six and 32 patients were included in 2018 and 2019, respectively. In both studies the most frequent use of medical cannabis was pain, with osteoarthritis as main cause. In 2018’s cohort, 45 patients use medical cannabis for pain treatment, followed by Parkinson's disease (n=7). Other causes of pain were spine pathologies (n=7). Pre-treatment mean in the numerical pain scale was 8.4±1.03. The post-treatment mean was 5.4 ± 0.7, regardless of the type of medical cannabis used, and the pain etiology. M1661, Epifractan® and artisanal cannabis had significant changes in the intensity of pain (3 or more points in the scale of pain). In 2019’s cohort, 26 of 32 patients had as main symptom the pain. The most frequent etiology of pain was osteoarthritis (n=12), followed by fibromyalgia (n=7). Prior to the treatment, no user considered their pain to be of mild intensity in the numerical pain scale. Post treatment, only 9 patients considered their pain in this range. Twenty one patients, prior to treatment, considered their pain to be of severe intensity, while in the second instance, only 9 classified it as such, regardless of the type of cannabis derivative and the etiology of pain. Thirteen patients were under treatment only with medical cannabis. In both studies dry mouth was the most frequent adverse event.
Conclusions: The main uses of medicinal cannabis in these cohorts of adults in Uruguay were pain, mainly in women with osteoarthritis. Most of the patients improved their symptoms. The most frequent adverse event was dry mouth. An in-depth evaluation of this use and discussion of its possible inclusion as a label indication, is needed.
Tabel/Image
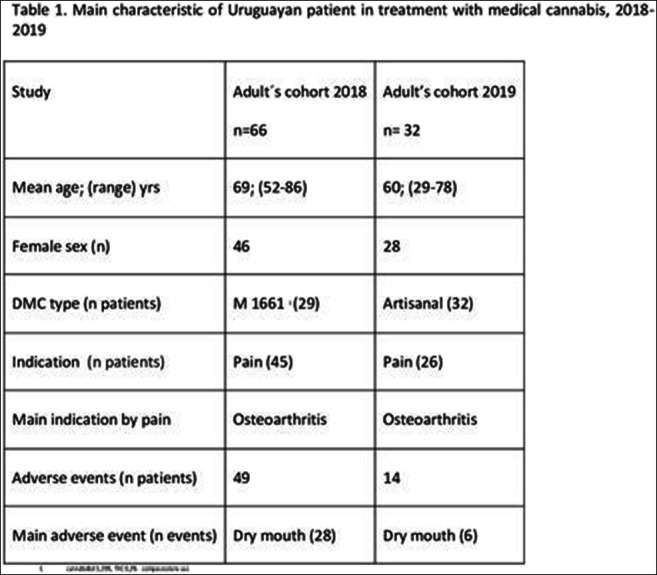
95
Extent of use of QT interval prolonging medication in Psychiatry In- Patient in a tertiary care hospital
Sarkar C1, Nongpiur A1, Wahlang J1, Das B2
1 Neigrihms,shillong,meghalaya, India, SHILLONG, India
2 AIIMS, RISHIKESH, Rishikesh, India
Introduction: Psychiatric patients constitute a population at notable risk of drug induced QT-prolongation. Quite a number of antipsychotic and antidepressant drugs are known to cause significant QT-prolongation.
Objectives: The aims were to explore extent of use of QTc-interval prolonging agents in Psychiatry In- Patient in a tertiary care hospital in India.
Methods: The study was carried out in the psychiatry In- Patient at NEIGRIHMS, Shillong, India. For each patient, the entire medication list was analyzed for the possibility of interactions, with particular attention on the high-risk QT prolonging ones. Arizona Center for Education and Research on Therapeutics (AZCERT) QT drug lists were used to classify TdP risks of psychotropic and other medications.
Results: 246 patients attending the psychiatry department during the 3 months study period were scrutinized. 149 patients (61%) were males whereas 97 (39%) were females in our study. Of the 246 patients, 207 patients (84%) were identified as receiving interacting medications with the ability to induce torsades de pointe (TdP). 349 (51.8%) interacting medications with torsadogenic risk were encountered out of total 674 medication prescribed to 246 patients. The most frequently interacting medications were from antidepressant (190), antipsychotic (132), antidementia (14), proton pump inhibitor (7) therapeutic categories. As per AZCERT classification (CredibleMeds TdP risk-stratification lists), 110 (31.5%), 46 (13.2%) and 193 (55.3%) of the interacting medications were associated with known, possible, and conditional risk of TdP, respectively.
Conclusions: Concurrent prescriptions of QT-prolonging drugs is frequent in psychiatry setting. Appropriate precautions should be instituted to provide caregivers with clear guidelines on how to use these drugs in a responsible and safe way.
103
Trends of Potentially Inappropriate Medication prescription in older adults: a population-based study in Portugal
A. Rodrigues D1, I. Plácido A1, Tavares A1, Azevedo D1, Mateos-Campos R2, Figueiras A3,4,5, Herdeiro M6, Roque F1,7
1 Research Unit for Inland Development, Polytechnic of Guarda (UDI-IPG)), Guarda, Portugal
2 Area of Preventive Medicine and Public Health, Department of Biomedical and Diagnostic Sciences, University of Salamanca, Salamanca, Spain
3 Department of Preventive Medicine and Public Health, University of Santiago de Compostela, Santiago de Compostela, Spain
4 Consortium for Biomedical Research in Epidemiology and Public Health (CIBER Epidemiology and Public Health-CIBERESP), Madrid, Spain
5 Health Research Institute of Santiago de Compostela (IDIS), Santiago de Compostela, Spain
6 Department of Medical Sciences, Institute of Biomedicine (iBiMED), University of Aveiro, Aveiro, Portugal
7 Health Sciences Research Centre, University of Beira Interior (CICS-UBI), Covilhã, Portugal
Introduction: Age-related comorbidities prone older adults to multiple treatments, increasing the complexity of therapeutic management and potentiating the occurrence of medication-related problems. The use of Potentially Inappropriate Medication (PIM) in older people is associated with an increased risk of hospitalization and poor clinical outcomes. Portugal has a high percentage of older adults (23.4%) and one of the highest old-age dependency ratios in Europe (34.7%).
Objectives: To evaluate trends of PIM prescription to Portuguese older adults according to the EU(7)-PIM list criteria, to analyze the change rate of PIM prescribing over time and, to assess the geographical variability between different regions of mainland Portugal.
Methods: A retrospective ecological study in primary health care between January 2019 and September 2021 for PIM prescribing data published in a national public database for all persons aged 65 and older in mainland Portugal, according to the Portuguese EU(7)-PIM list and from three perspectives: (a) PIM defined daily dose (DDD) frequency (%), (b) DDD per 1000 inhabitants per day (DID) value, and (c) PIM DID change rate (%). PIM were excluded if they are: (a) dose/duration/drug-regimen-dependent, (b) without marketing holder in Portugal, (c) not currently marketed in Portugal, and/or (d) without DDD information.
Results: A total of 140 PIM were included in this study, within 138 active substances and 2 drug classes. The study population comprised 2.3 million older people (≥ 65 years old), belonging to the 5 Regional Health Administrations of mainland Portugal. A total of 1,232 million DDD of PIM were prescribed. Overall, the PIM DDD frequency is 9.20%, with high values in Alentejo and Centro (10.58% and 10.22%, respectively). Alprazolam, fluoxetine, and rivaroxaban were the PIM with the highest DDD frequency values. Prescription of alprazolam and fluoxetine decreased over time (3.80% and 14.86%, respectively) and were most prescribed in older women, while for rivaroxaban an increase was observed (18.54%), mostly in older men. The biggest increases were registered in Lisbon and Tagus Valley while the biggest decreases occurred in the north.
Conclusions: Since Portugal has one of the highest rates of population over 65 years old in Europe, this study provides relevant knowledge to design new strategies to identify PIM-related factors in primary care for improving polypharmacy management in older adults.
107
Opportunities for de-prescribing in a tertiary UK hospital
Cole C1, Pirmohamed M1, Tsakiroglou M1
1 University of Liverpool, Liverpool, United Kingdom
Introduction: Aspirin reduces the risk of cardiovascular disease (CVD) but increases the risk of major bleeding. Guidelines (ESC and NICE) recommend low-dose aspirin in patients with high risk of CVD and concomitant use of a gastro-protective agent if required. However, aspirin could be considered for de-prescribing in patients with low risk of CVD and/or increased risk of gastro-intestinal (GI) bleed.
Objective: We audited if physicians at a large acute hospital in the UK discontinued aspirin based on patient’s risk of CVD and GI bleed.
Method: The records of patients with a diagnosis of oesophagitis, gastritis, duodenitis, GI ulcer or bleed admitted at the Royal Liverpool Hospital from 1st October to 31st December 2019 were reviewed to identify those taking aspirin and its appropriateness. We used STOPP/START Medication Tool, QRisk3 score and current guidelines to assess whether aspirin de-prescribing should have been considered.
Results: We reviewed 122 records and identified 51 (42%) patients on aspirin.
Primary prevention: Aspirin was prescribed in 39% of patients (n=20) with the majority (n=18, 90%) being high risk (QRisk3≥10%). Concomitant proton pump inhibitor (PPI) was prescribed for 65% (n=13). Of three (15%) patients with a history of GI ulcer, one was not on PPI and one was low risk for CVD.
Secondary prevention: Aspirin was prescribed in 31 (61%) patients with established CVD, 2 of whom were high risk of GI bleeding with one having aspirin discontinued and the other remaining on it without PPI.
In total, although aspirin was discontinued in only four patients, we identified eight to 24 (16%-47%) patients who could have been considered for aspirin de-prescribing (Table 1).
Conclusion: Our findings indicate that de-prescribing of aspirin, even when used for primary prevention, is infrequent despite increased GI bleeding risk. A dedicated service to review medications and de-prescribing education are needed.
Tabel/Image
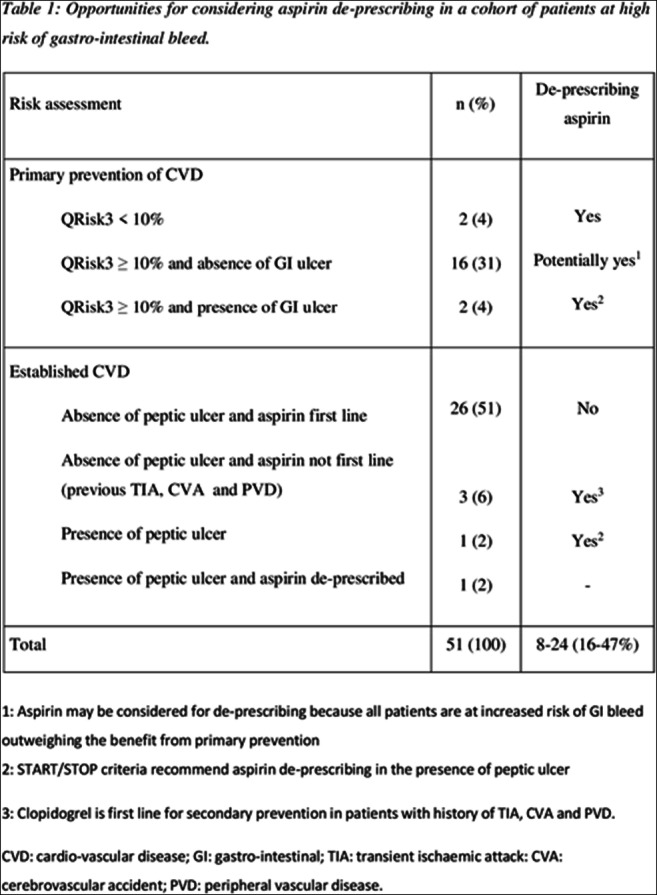
114
The impact of the COVID-19 pandemic on natural remedy use for hypertension
Balan M1, Lechsner P1, Dho-Nagy E1, Ban E1
1 UMFST George Emil Palade Targu Mures, Targu Mures, Romania
Introduction: Hypertension reached the rank of an epidemic, being considered the “silent killer”. Less than half of the adults suffering from hypertension are actually diagnosed and treated. The poor control of the disease is mainly due to the absence of the patients’ medical education. Patients are unaware of the possible complications of hypertension and the severity of its progression when not being treated. The rural population in Romania has a known interest in the use of natural remedies and popular healing methods.
Objectives: This study aims to evaluate whether the SARS-COV-2 pandemic and the periods of lockdown had an influence on the general approach of the patients suffering from hypertension towards the use of natural remedies.
Methods: We have performed a prospective, cross-sectional pharmaco-epidemiologic questionnaire-based survey in a rural area of Romania with collection of 1230 questionnaires.
Results: The demographic characteristics: age, gender distribution and the prevalence of hypertension in the population included in the current study are following the national trends. The study population included 1230 patients, with 1020 of these patients under chronic pharmacological treatment. 19.51% of the patients who participated in the study believe in natural remedies as an adjuvant therapy, besides the medical pharmacological prescriptions, but a total of 39.02% of the ones under chronic treatment also stated that they would be willing to try the natural remedies From the ones willing to change their therapy, 214 patients (53.65%) actually did change it, using exclusively natural remedies after the start of the pandemic. We noticed that the great majority (81.81%), of the patients who changed their therapy, did so in the far beginning of the first lockdown, although they also stated that they could reach medical care throughout the entire lockdown period.
Conclusion: During the pandemic and the lockdown periods, the use of natural remedies among adult residents of rural areas has increased. This rise in prevalence was especially observed in patients who were under chronic medical therapy. Further, we observed a negative correlation between choosing to self-medicate with natural remedies instead of medical drug therapy and the opinion of patients regarding accessibility of medical services. Unfortunately, the risk of suffering complications and worsening of the disease due to lack of medical therapy is increasing with the use of natural remedies as self-medication.
119
Off-label use of rituximab in patients with systemic lupus erythematosus with extrarenal disease activity
Sans Pola C1,2,3, Agustí A1,2,3, Bosch J4,5, Alerany C6, Cortés J7, Danés I1,2,3
1 Department of Clinical Pharmacology, Vall D’hebron Hospital Universitari, Barcelona, Spain
2 Department of Pharmacology, Therapeutics and Toxicology, Universitat Autònoma de Barcelona, Bellaterra, Spain
3 Immunomediated Diseases and Innovative Therapies Research Group, Vall d’Hebron Institut de Recerca (VHIR), Vall d’Hebron Hospital Universitari, Barcelona, Spain
4 Department of Internal Medicine, Vall d’Hebron Hospital Universitari, Barcelona, Spain
5 Department of Internal Medicine, Universitat Autònoma de Barcelona, Barcelona, Spain
6 Pharmacy Service, Vall d’Hebron Hospital Universitari, Barcelona, Spain
7 Department of Rheumatology, Vall d’Hebron Hospital Universitari, Barcelona, Spain
Introduction: Off-label use of rituximab (RTX) is sometimes requested for patients with resistant systemic lupus erythematosus (SLE) as a treatment option. However, data on its efficacy and safety on these patients is still controversial.
Objectives: To evaluate the off-label use of RTX in adult patients with extrarenal SLE disease activity treated at Vall d’Hebron University Hospital from 2013 to 2020, and to assess the outcomes and tolerability. Pacients with lupus nephritis were included only when the indication for RTX was not the renal activity. Treated patients were followed-up until December 2021.
Methods: Data were retrieved retrospectively from electronic medical records. Remission was classified according to the current guidelines and SLEDAI-based definitions. Disease flares during follow-up were defined as an increase in disease activity and immunosuppressive drugs.
Results: A total of 49 requests were received for 39 patients during the study period, and 43 RTX cycles were approved and administered to 33 (84.6%) patients. Median age was 45 years (IQR 36-55) and 97% were female. Most patients had received previous immunosuppressive therapies, with a median of 5 (IQR 3-6) different medications, and had refractory or relapsing disease. The median time from diagnosis to the first RTX cycle was 75 months (IQR 31-144). The symptoms that motivated RTX use were thrombocytopenia (30%), arthritis (30%), neurological manifestations (24.2%), cutaneous lupus (15.2%), neutropenia (3%), hemolytic anemia (3%) and optic neuritis (3%). Median number of RTX cycles for each patient was 1 (IQR 1-2). After the majority of RTX cicles a partial remission was achieved (69.8%); however, 25.5% of cicles did not achieve a response. A complete remission was achieved in 4.7%. All patients needed further immunosuppressive therapies during follow-up to maintain remission or to treat new flares. The median number of post-RTX immunosuppressants was 3 (IQR 2-5). Nine (27.6%) patients presented adverse reactions: infusion-related reactions (6), anaphylactic shock (1), serum sickness-like reaction (1) and hypogammaglobulinemia (1). During the study period, 19 (57.6%) patients presented infectious complications, mostly respiratory (9) and urinary tract (8) infections.
Conclusion: Some response was observed after most RTX cycles in patients with extrarenal SLE manifestations. Infusion-related reactions were the most common adverse events.
120
Translating pharmacological developments into clinical practice: case study of Ronapreve for COVID-19
Page C1, Smith D1, Rajasundaram S2, Gill D1, Kumar V1
1 2St George's University Hospitals NHS Foundation Trust, London, UK
2 Imperial College Healthcare NHS Trust, London, UK
Introduction: The management of SARS-CoV-2 has evolved rapidly since its emergence in 2019 and incorporating new therapies into acute medical practice poses challenges. In June 2021, the RECOVERY trial reported that casirivimab/imdevimab (Ronapreve) reduced the relative risk of mortality in seronegative, hospitalised COVID-19 patients by 20%¹. Ronapreve was licensed for the treatment of acute COVID-19 infection in August² with the expectation that all provider organisations prescribe Ronapreve to eligible patients³. We conducted a 3- cycle iterative service evaluation with three main aims; 1) To ensure that Ronapreve was accessible, 2) to ascertain compliance with national guidelines and 3) to identify barriers to Ronapreve’s administration.
Methods: Data was collected for all three PDSA cycles during 1st October – 20th November 2021 and qualitative insights were obtained from discussions with stakeholders. The standard was defined using national guidance³.
Results: Initially the standard was not met as the majority of eligible patients (55%) did not receive antibody testing (Figure 1), and very few patients were offered Ronapreve. Limited clinician awareness of eligibility criteria and antibody testing were the main barriers identified. Interventions within the cycles resulted in the number of appropriate antibody test requests increasing from 45% to 100% (Figure 1), leading to more Ronapreve doses being successfully administered (figure 2). Clinician understanding and engagement also improved.
Discussion: Service evaluation showed that initially, administration of Ronapreve was inadequate. Although data was collected retrospectively by a single clinician and the sample size is small, we improved the provision of Ronapreve to almost all eligible patients. We did this by updating guidance, targeted education, liaising with stakeholders, systemic changes to ordering phlebotomy tests and increasing pharmacy support. Implementing other pharmacological developments into practice come with similar challenges to those identified in this project and our interventions could be used with equal success by other trusts.
Tabel/Image
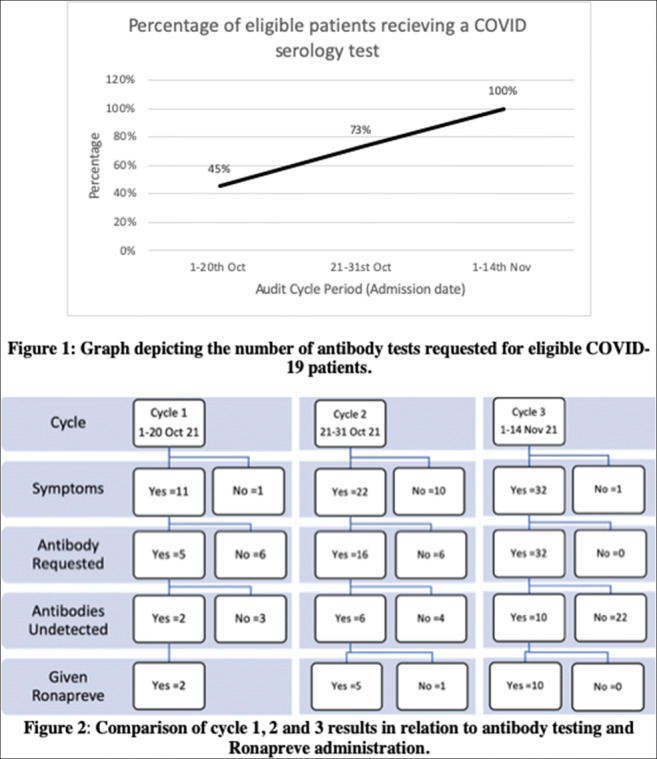
121
STUDY THE AWARENESS OF MEDICINE CONSUMER ABOUT THE PROBLEM OF MEDICINAL POLLUTION OF THE ENVIRONMENT,NECESSITY TO DEVELOP, INTRODUCE PREVENTIVE MEASURES
Makhmudova O, Khaziakhmetova V, Rakhimova Z
Irrational use of medicines and baseless polypragmasy increase the amount of pharmaceutical waste and risk of pollution. According to WHO experts, public education, explanation the severity of the problem, propaganda of responsible use and disposal of medicines are the effective measures to minimize environmental pollution [1].
From 2019 to 2021 we have conducted a questionnaire survey on Google, where we have assessed the attitude of population to the problem of rational use of medications, proper disposal of medications and the desire of medicine consumers to give unused / expired medications in special collection points. In the first survey 287 respondents took part, in the second - 159. Surveys have shown that most of drug users take medications for medicinal purposes (93,1%). The aim of medications disposal for 88% of respondents is the expired drug shelf life.
According to the 2019 results, 80.1% of the respondents discarded expired medications in the household garbage, and 2020 - 93,7%. The idea of separate collection of drugs and creation of special points of return for expired and unused drugs was supported by 62.4% in 2019 and 86.1% of respondents in 2020.
162
An insight into the storage, use and disposal of drugs among Serbian students through in-home drug inventory
Tomas A1, Paut Kusturica M, Prodanović D, Rašković A, Horvat O, Stilinović N, Tomić Z, Sabo A
1 University Of Novi Sad, Faculty Of Medicine, Department Of Pharmacology, Toxicology And Clinical Pharmacology, Novi Sad, Serbia
Introduction: The review of home pharmacies provides insights into the many habits of the population in relation to medicines, their storage, use and disposal. The results of the study of home pharmacies on the general population cannot be generalized to students due to sociodemographic and other specific differences.
Objectives: To determine the volume and structure of drugs, the rate of self-medication, as well as the storage and disposal of drugs among students in Novi Sad.
Methods: This cross-sectional study included 70 student accommodations in Novi Sad in the period from November 1st, 2018 until December 20th, 2018. Two trained interviewers performed the survey by visiting each student accommodation. The study consisted of making a direct insight into inventory of medicines in student dormitories and a semi-structured interview about drug use practices and perceptions.
Results: Medicines were found in all of the students’ rooms. During the insight into in-home drug inventory, a total of 337 packages of medicines were identified and 71.43% were found in one designated place, a home-pharmacy. The most common medicines in home pharmacies were drugs that affect nervous, muscle-bone system and anti-infectives. Most common drugs stored in student rooms were ibuprofen, paracetamol and diclofenac, accounting for 30.86% of the total number of drugs.
Over 70% of medicines were purchased for self-medication, a much higher proportion than observed in general population in the city of Novi Sad. Antibiotics accounted for 5.93% of total drugs found, and from 20 packages of antibiotics, 6 were purchased self-initiatively (30%). About half of found antibiotics were not currently in use. About 10% of students store drugs with expired shelf life, 75% of medicines are kept properly, but a negligible part of them is properly disposed. Even though the majority (74.29%) considered that throwing medicines into the garbage and toilet is bad for the environment, most (41.43%) answered that this type of disposal is the easiest and the most convenient method of drug disposal. Majority of drugs in solid/semisolid pharmaceutical form are disposed together with the household garbage (67.14%), and the same was shown for liquid forms (62.86%). Less than 3% of the respondents stated returning drugs to pharmacy to be properly disposed.
Conclusion: Self-medication is very common among students. The majority of medicines from student households are kept in the proper manner, but are not disposed properly.
Funding: This work was supported by the Ministry of science, education and technological development project No. 451-03-68/2022-14/200114
165
Covid-19 and Infodemic: Impact on the Consumption of Hydroxychloroquine, Ivermectin, Acetaminophen and Ibuprofen in Uruguay
Garcia J1, Toledo M1, Penengo M1, Perin F1
1 Universidad Claeh, Punta del Este, Uruguay
Misinformation and infodemic have led to the consumption of drugs with potential impact on the prevention and treatment of COVID-19 in Uruguay (UY). The first wave took place between March and June of 2020. At that time, as US President, D. Trump, and French Health Minister, O. Veran, tweeted about drugs (with little basis), the first papers about the potential benefit of Ivermectin were published, resulting in large range media repercussion.
In UY, accessibility to drugs is ample. Pharmacies do not require medical prescriptions, their sales being an indicator of self-medication. Medical prescriptions are mainly dispensed by the national health system ISNIS) with mandatory prescription.
Objective: To establish the evolution in the consumption of hydroxychloroquine (HCQ), ivermectin (IVM), acetaminophen (AC) and ibuprofen (IB) in UY between 1/18 and 12/20 (January 2018 and December 2021)
Methodology: Observational, descriptive and retrospective study based on commercialization data collected by the consulting firm IMS in community pharmacies and the national health system.
Consumption is reported in defined daily dose (DDD) per 1,000 inhabitants per day (DHD).
Results: Consumption of IVM increased in relation to pre-pandemic values and is persisting still (N DHD 4/18: 0,0216; 4/19: 0,0275; 4/20:0,0612; 4/21: 0,2316; 12/21: 0.1104). HCQ consumption increased strongly over 3-4/20 (N DHD: 2/20: 0,04; 3/20: 1,47; 4/20: 0,17; 6/20: 0,02). The consumption of IB decreased and that of AC grew, a still persisting trend.
Discussion: The increase in the consumption of HCQ, AC and IVM and the decrease in IB consumption coincided with the first COVID-19 wave and the tweets of influential leaders. In times of uncertainty and anxiety, non rational factors influence prescription and self-medication. Information with no reliable scientific basis influenced the consumption of drugs with no proven evidence.
Conclusions: During the first COVID-19 wave the demand for HCQ, AC and IVM increased and that of IB decreased. Information is crucial for decision-making in health. Active efforts must be made to mitigate infodemic and promote health literacy to reduce irrational use or consumption of drugs.
Tabel/Image
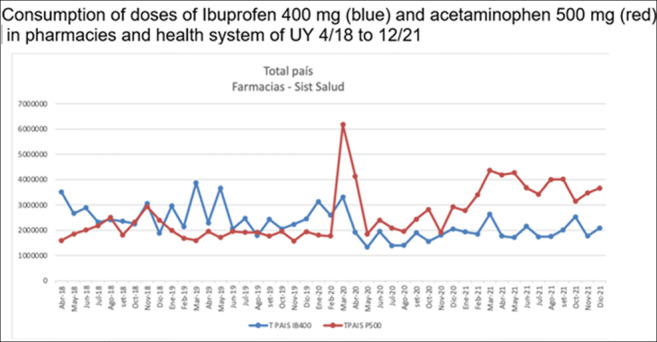
189
Subdermal Contraceptive Implants and Reasons for Removal in Public Healthcare Centers in Maldonado, Uruguay
Del Bene Simonetti C1, Campisi V1, García J1, Penengo M1, Toledo M1
1 Universidad CLAEH, Punta Del Este, Uruguay
Introduction: Subdermal contraceptive implants have become a popular method: reversible, hormonal and long-lasting. Jadelle® is the one available in our public healthcare centers. These implants present adverse drug reactions (ADR), an important reason for early removal. Changes in menstrual bleeding are the most frequent cause.
Objective: To record the most frequent ADR and reasons for their early removal in the population studied.
Materials and Methods: An observational, cross-sectional, retrospective and quantitative clinical research was conducted. The information was collected in public health Gynecology clinics between 2015 and 2021 in Maldonado, Uruguay. Patients were women of childbearing age with public health coverage, users of subdermal implants.
Results: The sample was made up of 655 patients in the 12-50 age range, with an average of 25,2± 8,5 years of age. The minimum duration of the implant was 17 days, given the patient's contraindication, and the maximum duration was 72 months, exceeding the validity of the method. Another 20 cases exceeded this period. The average duration was 43.7±20.1 months. 433 out of the total requested removal due to expiry date, ADR or contraindications. The reason for removal is known in 209 cases and unknown in 224. Out of the 224 cases, the time between implant insertion and removal was less than 60 months, while in 149 cases was equal to or over 60 months. Out of the 209 cases, 38.7% of removal matches expiry date and 45.9% was due to ADR. Among them, in decreasing order: metrorraghia 22.0%, weight gain 8.6%, headaches 8.6%, pain in the insertion area 3.3%, mastalgia 0.9%; the remaining 2.3% reported the following ADR: cramps in the arm of insertion, loss of libido, fibromyalgia, breast cyst and deep vein thrombosis. Patients aged 19-25 years requested early removal more frequently due to the three main ADR.
Conclusion: Out of the 224 cases with no recorded cause for removal, 75 of them were removed early, inferring ADR as one of the probable causes. Taking into account the recorded cases of early removal due to ADR (45.9%) plus the cases with no recorded causes, early removal percentage increases. This analysis suggests that the implant’s ADR play a major role and impact on tolerance and adherence to the method. All the above mentioned should be taken into account so to provide better information to the users. Furthermore, strategies should be devised in order to correct unwanted effects.
200
Characteristics, Influential Factors, and Adverse Reactions of Long-Term Use of Sleep Medications in the Elderly: Cross-Sectional Survey of Patients
Lavon O1,2, Mottes Y2
1 Clinical Pharmacology and Toxicology Unit, Carmel Medical Center, Haifa, Israel
2 Rappaport Faculty of Medicine, Tecnion-Israel Institute of Technology, Haifa, Israel
Introduction: Use of sleep medications is widespread, especially in the elderly. These medications include mainly benzodiazepines and z-drugs, and may be used for a long duration of time, even in increased doses (more than once a day). This treatment can potentially induce significant adverse reactions. There are limited data on patients' personal experience, attitude and knowledge regarding this treatment.
Objectives: To evaluate the personal characteristics of prolonged use of sleep medications among elderly patients, the reasons and factors influencing this use, from the perspective of the patient himself, and adverse reactions to the treatment.
Methods: A hospital-based, cross-sectional study of patients aged 65 years and over with regular use of sleep medications. Patients were personally interviewed using a designated structured questionnaire. The results of the survey were statistically analyzed.
Results: 88 patients completed the full survey. The average age was 81 years. Most patients were treated with brotizolam regularly. Almost all patients reported not receiving any explanation from the prescribing physician about treatment safety, recommended duration of treatment, or treatment alternatives for their sleep disorder. About two-thirds (64.8%) of patients expressed a desire to discontinue treatment, but most patients (87.5%) expressed some anxiety regarding this action. Most patients reported that they were unaware of any adverse effects related to the sleep medications. Nevertheless, nearly one-third (31.8%) of patients reported at least one fall during treatment, and 20.5% reported memory disorders of varying degrees. X2 test analysis did not reveal any statistically significant effect of the patients' characteristics, such as age, sex or type of sleep drug on the results distributions.
Conclusions: According to the study, adults over the age of 65 years treated with sleep medications are not provided by their prescribing physicians with relevant or sufficient information regarding their treatment. Appropriate intervention is recommended, including improving information provision and patient support, as well as, better training and supporting the treating physician.
278
Gabapentinoid Prescribing in the Management of Diabetic Neuropathic Pain in the Irish Primary Care Setting.
Morris C1, Gallagher H1
1 School of Medicine, Conway Institute, University College Dublin, Dublin 4, IRELAND
Introduction: Licensed uses of gabapentinoids include epilepsy and neuropathic pain, such as that associated with diabetes mellitus. Prescribing of gabapentinoids is increasing internationally amid growing concerns over their misuse. In Ireland, increased pregabalin-positive poisoning deaths correlate with increased pregabalin dispensing.
Objectives: Since diabetes mellitus is also increasing in prevalence worldwide, we here aimed to examine general practitioners’ (GPs’) knowledge, attitudes and behaviours related to gabapentinoid prescribing and misuse prevention, with a focus on their use in diabetic neuropathic pain (DNP).
Method: A 16-question survey was developed using an online survey tool. The survey link was emailed to 348 GP practices, yielding 42 responses (response rate:12%). A further 40 responses were obtained from circulating the survey link to GP groups. Nine returned surveys were excluded from analysis as they were incomplete. Descriptive statistics and non-parametric statistical analysis were performed using IBM SPSS Statistics 26. Kruskal-Wallis, Spearman’s Rho and Kendall’s Tau tests were applied.
Results: One-fifth of respondents reported prescribing gabapentin/pregabalin first-line for DNP. A moderate correlation was found between GPs’ opinions on the efficacy of pregabalin and that of gabapentin in treating DNP (correlation coefficient=0.638, p<0.001); with 41.1% and 45.2% agreeing that pregabalin and gabapentin, respectively, are effective DNP treatments. Respondents reported low levels of prescribing gabapentinoids for unlicensed indications. The majority (73.9%) agreed that pregabalin should be reclassified as a controlled drug. Less experienced GPs were more likely to prescribe an initial gabapentinoid trial (p= 0.02). While 48.7% of GPs with <10 years’ experience strongly agreed that they always prescribe an initial trial, this dropped to 11.8% for GPs with >20 years’ experience (p=0.023). Urban GPs are more confident than rural GPs in managing signs of drug diversion (p=0.017; Table 1).
Conclusions: Although most GPs do not report using gabapentinoids as first-line treatment for DNP, over 40% consider them effective in this condition. Less experienced GPs are more cautious in their gabapentinoid prescribing for DNP. GPs are aware of risks of gabapentinoid misuse, as evidenced by the majority favouring reclassification of pregabalin. Supports for GPs in safe gabapentinoid prescribing could be improved, particularly in rural settings.
Tabel/Image
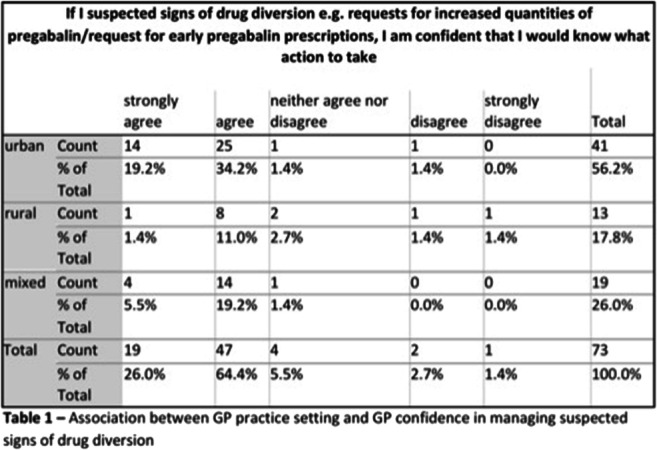
285
Features associated with premature termination of clinical trials: an analysis of the Spain national database
Filippi-Arriaga F1, Boy R1, Díaz Del Gobbo A1, Rodríguez Gallego A1,2
1 Clinical Pharmacology Department, Hospital Universitari Vall d'Hebron, Barcelona, Spain
2 Ethics Committee Support Unit-Vall d’Hebron Institut de Recerca (VHIR), Barcelona, Spain
Introduction: Premature termination of a clinical trial directly affects patients, researchers and other agents, like regulatory agencies, healthcare professionals and sanitary administrations. In previous research, we found that the main reasons for premature termination of clinical trials were patient recruitment issues, efficacy or futility problems, and commercial or strategic decisions of the sponsor. But the information about the features of the clinical trials that are associated with its premature termination is scarce.
Objective: Determine the features associated with the risk of premature termination of the clinical trials registered in the Spanish Registry of Clinical Studies (REec).
Methods: We performed a retrospective observational study of all the clinical trials registered in the REec, from inception until November 2021. To determine the association of premature termination according to the clinical trials features, we calculated the relative risks with 95% confidence intervals, using dichotomous variables that included the presence versus the absence of the feature analyzed (Table 1).
Results: We identified a total of 7402 clinical trials registered in REec. Of these 46% (3419) were terminated. Among terminated trials 79% (2701) were terminated as planned and 21% (718) were prematurely terminated. Multicenter clinical trials are associated with a 32% increased risk of premature termination, phase II study with a 21%, rare diseases with a 36% and female gender with a 62%. The use of placebo was associated with a doubled risk of premature termination. According to the objective, the efficacy and pharmacodynamics were associated with a 98% and 28% increase in the risk of premature termination respectively. The extreme ages (pediatric and elderly population) were associated with an increased risk of premature termination: in the pediatric population the risk was more intense as age decreased (92% with newborns, 47% with preschoolers, 29% with children) and it was 27% in people over 64 years of age. Other features associated with an increased risk of premature termination were: pregnant women (71%), participants in emergency situations (58%), cáncer studies (37%) and digestive pathologies studies (65%).
Conclusion: We found that 21% of clinical trials registered in the REec were prematurely terminated. The main features associated with a higher risk of premature termination were: use of placebo, the objective of efficacy, extreme ages of life, the inclusion of participants in emergency situations, the inclusion of pregnant women and the study of cancer and digestive pathologies. Within these, the use of placebo doubled the risk of premature termination. The knowledge of the association of these features with a higher risk of premature termination of clinical trials can be useful to plan and execute them.
Tabel/Image
296
In silico and experimental toxicity studies of new ethylcarbamates derivatives with ectoparasiticide activity
Prado Ochoa M 1
1 Universidad Nacional Autónoma De México
Ethyl-4-bromophenyl-carbamate (LQM 919) and Ethyl-4-chlorophenyl-carbamate (LQM 996) are compounds with ectoparasiticidal activity proposed for the control of ticks in cattle. The use of these compounds represents a risk to human health and the environment. Therefore, it is mandatory to determine its toxicity. In this study we conducted an in silico study to predict the acute and subchronic toxicity of these compounds and compared it with the results of toxicity studies in rats.
Quantitative Structure Relationship (QSAR) models were made for the prediction of each toxicity endpoint: LD50 (lethal dose 50%) and NOAEL (nonobserved adverse effect level) using the QSAR Toolbox software according to workflow of the hazard assessment process. A conformational study of the molecules was conducted, the values of the molecular descriptors were calculated with the MOPAC software and the linearity were verified. The model equation for each endpoint, the statistics of the prediction model and the The predicted target values for LD50 and NOAEL are shown in table 1.
Female and male Wistar rats were used to determine the acute oral and subchronic toxicities of the ethyl-carbamates according to the OECD guidelines for testing of chemicals. The oral LD50 of each carbamate was 300 to 2000 mg/kg. NOAELs were 12.5 mg/kg/day for both the female and male rats. Subchronic exposure (90 days) of > 25 mg/kg/days of these carbamates produced alterations in water consumption, hematocrit, percentages of reticulocytes, plasma proteins, and some biochemical parameters (aspartate aminotransferase, gamma-glutamyl transpeptidase, cholinesterase and creatinine).
In conclusion, QSAR studies are good predictors of the toxicity of new compounds. According to this study, the toxicity of the new ethylcarbamates is low, however, further studies are required to determine their risk of use.
This study was supported by the PAPIIT/UNAM Project IN211222
Tabel/Image
312
Trends in utilization of antidepressants in twenty European Countries
Papaioannidou P1, Tsaluchidu S1, Stergiopoulou T1
1 1st Department of Pharmacology, School of Medicine, Faculty of Health Sciences, Aristotle University of Thessaloniki, Thessaloniki, Greece
Introduction: The use of antidepressants seems to be increasing in most countries worldwide, probably due to the increasing burden of stressful life (1). Apart from their therapeutic application, antidepressants are sometimes used as lifestyle drugs. Monitoring antidepressant usage is crucial to prevent unnecessary consumption and avoid adverse effects and additional costs (2).
Objectives: The aim of this work was to study trends in antidepressants utilization in various European countries, and to note changes in their usage between the years 2013 and 2019, before the outbreak of COVID 19 pandemic.
Methods: Data on antidepressants consumption in 20 European countries were collected from the Organization for Economic Cooperation and Development (OECD) data bases. Antidepressants consumption was expressed in Defined Daily Doses (DDDs) per 1,000 inhabitants per day, and calculations referred to years 2013 and 2019. Changes in antidepressants use during this six-year period in each European country were assessed. The statistical package SPSS (Chicago, IL, USA) was used for calculations.
Results: There was a huge variation in antidepressants usage among the 20 countries of our study. The mean consumption of antidepressants was 52.67 DDDs per 1,000 inhabitants per day in 2013 (range 10.2-113.7 DDDs) and 62.51 DDDs per 1,000 inhabitants per day in 2019 (range 17.6-146.0 DDDs), with a mean increase of 9.84 DDDs per 1,000 inhabitants per day (18.68%) in just six years.
The countries with the highest consumption of antidepressants in 2013 were Iceland (113.7 DDDs), Portugal (87.5 DDDs), Sweden (84.3 DDDs), Belgium (72.1 DDDs), Finland (69.4 DDDs) and Spain (65.2 DDDs). The countries with the highest consumption of antidepressants in 2019 were Iceland (146.0 DDDs), Portugal (123.7 DDDs), Sweden (102.7 DDDs), Spain (83.6 DDDs) and Belgium (81.9 DDDs).
The countries with the lowest consumption of antidepressants in 2013 were Latvia (10.2 DDDs), Estonia (21.4 DDDs), Lithuania (24.7 DDDs), and Hungary (27,5 DDDs). The countries with the lowest consumption of antidepressants in 2019 were Latvia (17.6 DDDs), Hungary (29.5 DDDs), Estonia (34,8 DDDs) and Lithuania (35,4 DDDs).
The use of antidepressants was increased in all European countries in the study period. There was only one exception: Finland, being one the countries with the highest consumption of antidepressants, reduced their use by 13%. In the countries with the lowest consumption of antidepressants (Latvia, Estonia and Lithuania), the increase in antidepressants usage was higher than 40%. A similarly high increase (41.37%) was also observed in Portugal, which was second in antidepressant use in both years studied (2013 and 2019). The Countries with the lowest increase (less than 5%) were Austria, Norway and Luxemburg, which displayed an average consumption of antidepressants in the study period.
Conclusion: There was a huge variation in antidepressants use among the 20 European countries of our study. A trend for increase in antidepressants use was observed in almost all countries during the six-year study period.
References
1. Papaioannidou P, et al. Pharmacoepidemiol Drug Safety 2016, 25 S3: 665
2. Papaioannidou P, & Ntaralas A. Pharmacoepidemiol Drug Safety 2014, 23 S1: 115
313
Valproate using trends among women of childbearing age in Tunisia between 2015 and 2021
Ferchichi K1,2, CHARFI R1,2, Ben Sassi M1,2, Gaies E1,2, Ben Hammamia S1,2, El Jebari H2, Jebabli N2, Salouage I1,2, Daghfous R1,2, Trabelsi S1,2
1 University Of Tunis El Manar Faculty Of Medecine Of Tunis, Tunis, Tunisia
2 Centre Chalbi Belkahia of Pharmacovigilance, Department of clinical pharmacology, Research Laboratory of Clinical and Experimental Pharmacology (LR16SP02), Tunis, Tunisia
Introduction: Valproate use among women of childbearing age has been related to fetal congenital malformations and neurodevelopmental disorders. Since 2013-2014, the European Medicines Agency (EMA) has issued guidance to boost valproate-related warnings in order to reduce valproate use among women of childbearing age. In May 2018, the French Agency for the Safety of Health Products (ANSM) approved new restrictive measures including a contraindication of valproate in most cases of pregnancy, a pregnancy prevention program, educational material distribution, and changes in the product’s information. Recent studies in Europe or other continents report mainly signs of an enduring decline in valproate use among females of childbearing age. However, in Tunisia, the lack of regulations restricting the use of valproate among young women was the trigger behind that work aimed to evaluate the change in the number of female valproate users in Tunisia from 2015 to 2021 referring to the therapeutic valproate monitoring data.
Method: We conducted a retrospective study based on the department of clinical pharmacology database for six years (January 2015-December 2021). We included female patients aged between ten and 40 years.
Results: We included 410 female patients aged between ten and 40 years. The median age was 15,53 years. Ninety-six point three percent of the patients were using valproate for epilepsy and 3.6% for bipolar disorder. The absolute number of female patients under 40 using valproate decreased by 54% between 2015 and 2020. By 2021 there was an increase of about 44% of the patients using valproate.
Conclusion: The EMA and the ANSM referral approach of restricting the use of valproate in females of childbearing age was followed by a significant decline in the number of female valproate users in Tunisia. But the valproate prescription is mainly influenced by the other antiepileptic drugs availability which explains the increase of the female using valproate between 2020 and 2021.
Tabel/Image
316
Trends in utilization of lipid-modifying drugs in European Countries
Papaioannidou P1, Stergiopoulou T1
1 1st Department of Pharmacology, School of Medicine, Faculty of Health Sciences, Aristotle University Of Thessaloniki, Thessaloniki, Greece
Introduction: Lipid-modifying drugs (LMDs) can effectively improve lipid abnormalities and prevent cardiovascular events. Although their use is not therapeutic but rather preventive against cardiovascular complications, LMDs are among the most prescribed drugs worldwide (1,2).
Objectives: The aim of this work was to study trends in LMDs utilization in various European countries, and to note changes in their usage between the years 2013 and 2019.
Methods: Data on LMD usage in 20 European countries were collected from the Organization for Economic Cooperation and Development (OECD) data bases. LMD use was expressed in Defined Daily Doses (DDDs) per 1,000 inhabitants per day, and calculations referred to years 2013 and 2019. Changes in LMD use during this six-year period in each European country were assessed. The statistical package SPSS (Chicago, IL, USA) was used for calculations.
Results: There was a great variation in LMD usage among the 20 countries of our study. The mean consumption of LMDs was 88.7 DDDs per 1,000 inhabitants per day in 2013 (range 15.3-152.9 DDDs) and 108.9 DDDs per 1,000 inhabitants per day in 2019 (range 58.9-147.7 DDDs), with a mean increase of 20.2 DDDs per 1,000 inhabitants per day (23%) in six years.
The countries with the highest use of LMDs in 2013 were Slovak Republic, Belgium, Norway, Luxemburg and Netherlands with 152.9, 130.1, 121.0, 116.4, 111.8 DDDs per 1,000 inhabitants per day, respectively. The countries with the lowest use of LMDs in 2013 were Lithuania, Estonia, Latvia, Austria and Germany with 15.3, 43.7, 51.8, 69.4 and 73.0 DDDs per 1,000 inhabitants per day, respectively.
The countries with the highest use of LMDs in 2019 were Norway, Belgium, Slovenia, Czech Republic and Netherlands with 147.7, 147.6, 146.3, 145.5 and 136.1 DDDs per 1,000 inhabitants per day, respectively. The countries with the lowest use of LMDs in 2019 were Lithuania, Estonia, Latvia, Iceland and Italy with 58.9, 69.1, 92.1, 94.1 and 97.1 DDDs per 1,000 inhabitants per day, respectively. In the countries with the lowest use of LMDs in 2013, a high increase in the consumption of LMDs was observed in 2019: in Lithuania 284.9% increase, in Latvia 77.8% increase, in Estonia 58.1% increase, in Sweden 56.3% increase, and in Austria 46.5% increase.
Conclusion: The use of LMDs was increased in almost all European countries in the study period. Exceptions were Slovak Republic and Luxemburg, in which a reduction of lipid-modifying drugs was observed. Since a high consumption of lipid-modifying agents was reported in these countries in 2013, the decrease in their use justifies a more reasonable prescription approach.
References
1. Papaioannidou P, & Ntaralas A. Pharmacoepidemiol Drug Safety 2015, 24 S1: 144
2. Papaioannidou P, & Ntaralas A. Clinical Therapeutics 2015, 37 8S: e148
318
The role of DOACs in anticoagulant treatment in a tertiary Hospital of Thessaloniki, Greece
Michailidou M1, Papaioannidou P1
1 1st Department of Pharmacology, School of Medicine, Faculty of Health Sciences, Aristotle University of Thessaloniki, Thessaloniki, Greece
Introduction: Low molecular weight heparins are used extensively in anticoagulant therapy, due to their safer profile, in comparison to other anticoagulants. Direct Oral AntiCogulants (DOACs) have been initiated in anticoagulant therapy as a safer treatment choice than coumarin derivatives.
Objectives: The aim of this study was to investigate the use of oral and injectable anticoagulants, and especially the place of DOACs in anticoagulant treatment, in a tertiary Hospital of Thessaloniki, Greece.
Methods: The data were collected by investigating prescriptions from the Hospital Pharmacy of a tertiary Hospital in Thessaloniki, Greece. Prescriptions of oral and injectable anticoagulants for hospitalized patients were collected during the period from June to September 2021. The consumption of the following oral and injectable anticoagulants was recorded in DDDs: acenocumarol, rivaroxaban, apixaban, dabigatran, heparin, enoxaparin, tinzaparin, bemiparin and fondaparinux.
Results: The total amount of anticoagulants used was 53,041 DDDs, of which 97,9% were injectable anticoagulants whereas 2,1% were oral anticoagulants. DOACs represented the 1,8% of the anticoagulants used. The consumption of injectable anticoagulants for the hospitalized patients was 51,936 DDDs, of which 63.5% was enoxaparin, 18.5% was tinzaparin, 6.3% was heparin, 6.1% was bemiparin, and 5.6% was fondaparinux. The consumption of acenocumarol was 176 DDDs and the consumption of DOACs was 929 DDDs, with the percentage of rivaroxaban, apixaban, and dabigatran being 46%, 45% and 9% respectively.
Indications with the highest prevalence for patients on enoxaparin was COVID 19, heart failure, stroke, angina pectoris, malignancy. Indications with the highest prevalence for patients on tinzaparin was COVID 19, malignancy, stroke. Indications with the highest prevalence for patients on bemiparin was malignancy, COVID 19, aortic valve disease, stroke. Heart failure, stroke and atrial fibrillation were the indications with highest prevalence in patients on DOACs. Acenocumarol was used mainly for heart failure, stroke and aortic valve stenosis.
Conclusion: Injectable anticoagulants, and mainly low molecular weight heparins were the treatment of choice in hospitalized patients. Oral anticoagulants represented only a very small proportion (2,1%) of the anticoagulants used. DOACs have replaced coumarin derivatives, representing the 86% of oral anticoagulants in clinical use. Nevertheless, the percentage of DOACs was very low (1.8%) in the total consumption of anticoagulants, with rivaroxaban and apixaban being the most commonly used DOACs. Injectable anticoagulants, especially enoxaparin, are preferred by the clinicians as a safer choice for managing high risk thrombosis in hospitalized patients. DOACs, Direct Oral AntiCogulants, anticoagulants, NOACs
319
Trends in utilization of peptic ulcer and gastro-oesophageal reflux drugs in European Countries
Papaioannidou P1, Stergiopoulou T1
1 1st Department of Pharmacology, School of Medicine, Faculty of Health Sciences, Aristotle University of Thessaloniki, Thessaloniki, Greece
Introduction: Peptic ulcer and gastro-oesophageal reflux disease drugs (PU-GOR-Ds), such as proton pump inhibitors and histamine H2-receptor antagonists, are among the most used drugs worldwide, and they are accessible either by prescription or over the counter.
Objectives: The aim of this work was to study trends in utilization of PU-GOR-Ds in various European countries, and to investigate changes in their usage between the years 2013 and 2019.
Methods: Data on PU-GOR-Ds usage in 20 European countries were collected from the Organization for Economic Cooperation and Development (OECD) data bases. PU-GOR-Ds usage was expressed in Defined Daily Doses (DDDs) per 1,000 inhabitants per day, and calculations referred to years 2013 and 2019. Changes in PU-GOR-Ds use during this six-year period in each European country were assessed. The statistical package SPSS (Chicago, IL, USA) was used for calculations.
Results: There was a great variation in PU-GOR-Ds usage among the 20 countries of our study. The mean consumption of PU-GOR-Ds was 65,4 DDDs per 1,000 inhabitants per day in 2013 (range 30,9 - 121,8 DDDs) and 76,0 DDDs per 1,000 inhabitants per day in 2019 (range 32,1 - 128,1 DDDs), with a mean increase of 10.6 DDDs per 1,000 inhabitants per day (15.3%) in six years.
The countries with the highest use of PU-GOR-Ds in 2013 were Spain, Netherlands, Belgium and Portugal, with 121.8, 104.0, 91.3 and 90.3 DDDs per 1,000 inhabitants per day, respectively. The countries with the lowest use of PU-GOR-Ds in 2013 were Latvia, Lithuania, Estonia and Slovak Republic, with 30.9, 33.7, 33.8 and 40.6 DDDs per 1,000 inhabitants per day, respectively.
The countries with the highest use of PU-GOR-Ds in 2019 were Netherlands, Spain, Iceland, Portugal and Belgium, with 128.1, 127.2, 109.0, 102.8 and 100.0 DDDs per 1,000 inhabitants per day, respectively. The lowest consumption in 2019 was reported in Austria, Lithuania, Latvia, Slovak Republic and Estonia, with 32.1, 46.3, 46.8, 53.4 and 55 DDDs per 1,000 inhabitants per day, respectively.
There was a trend for increase in the usage of PU-GOR-Ds in almost all European countries, except Austria, Italy, and Germany. In Austria, a huge reduction (53.3%) in the use of PU-GOR-Ds was reported, reflecting probably changes in methodology and maybe in reimbursement of this category of drugs during the study period.
Conclusion: A trend for increase in the usage of PU-GOR-Ds was observed in almost all European countries. The great variation that was observed in the use of PU-GOR-Ds among the countries may be attributed probably to differences in methodology of reporting and in reimbursement of this category of drugs in various countries.
320
Pharmaceutical Strategy for Europe: Emergency Use and Intellectual Property amid the COVID-19 pandemic
Ussai S1, Lauria B2, Pistis M1
1 University Of Cagliari, Clinical Pharmacology And Toxicology, Cagliari, Italy
2 Gulotta Foundation, Florence, Italy
Pharmaceutical Strategy for Europe: Emergency Use and Intellectual Property amid the COVID-19 pandemic
Introduction: In November 2020, the European Commission adopted the Pharmaceutical Strategy for Europe, a regulatory framework aiming at responding to the citizen’s therapeutical needs as well as to address weakness of the current pharmaceutical market. The initiative is based on four pillars: i. access to essential and affordable medicines; ii. Support to an innovative and sustainable pharmaceutical industry; iii. preparedness and response mechanisms amid crisis, including pandemics; iv. advocacy interventions. The COVID-19 emergency has posed at greatest challenge the global public health, with direct implications across all the four EU’s Pharmaceutical Strategy pillars.
Objectives: The abstract aims to describe the link between the conditional market approvals for COVID-19 vaccines, and related intellectual property issues, and the newly established EU’s Pharmaceutical Strategy.
Methods: In response to the pandemic, unprecedented efforts to rapidly review the safety, efficacy, and quality of COVID-19 vaccines led the EU to widely adopting Emergency Use Listing (EUL) decisions. At the same time, the introduction of innovative technologies poses questions on their affordable access, in light of the WTO Agreement on Trade-Related Aspects of Intellectual Property Rights (TRIPS, also known as Doha Declaration). This abstract has descriptively reviewed the (EUL position papers as well as the TRIPS Agreement and identified links and future implications with the EU’s Pharmaceutical Strategy.
Results: EUL decisions are rapid review procedures for granting a conditional marketing authorization for medicines based on rolling clinical data, where the benefit of immediate availability of the medicine outweighs the risk inherent in the fact that additional data are still required. These actions are therefore fully aligned with all the four pillars foreseen by the EU’s Pharmaceutical Strategy.
With regard to the sustainable intellectual property, the Doha Declaration confirms that countries are free to determine the grounds for granting compulsory licenses, and to determine what constitutes a national emergency. Recently, the TRIPS Agreement has been amended to provide for an additional type of compulsory licensing, recognizing that countries unable to manufacture pharmaceuticals should be able to obtain cheaper copies produced under compulsory licenses elsewhere if necessary. This ultimately led to the proposal of a TRIPS Waiver on COVID-19 vaccines, submitted by India and South Africa in the World Trade Organization.
Overall, the policy is in line with the sustainable access to innovative medicines expressed by the EU’s Pharmaceutical Strategy.
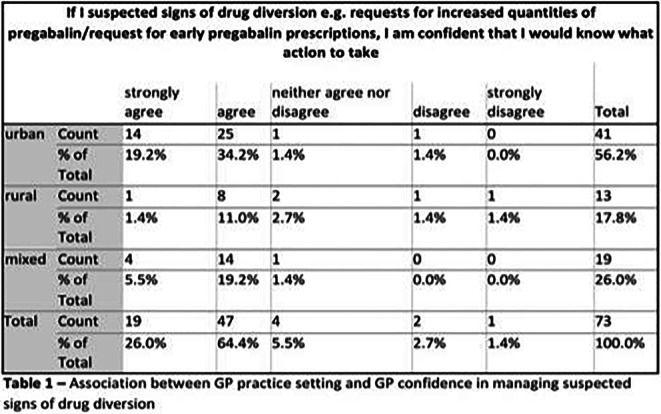
Conclusion: This establishment and adoption of the EUL as well as the TRIPS Agreements are harmonized according to the EU’s Pharmaceutical Strategy.
There are, however, open discussions. Among others, critics about TRIPS waiver are based on the idea that this policy can affect the incentive for industry to take research and development risks. Similarly, the EUL to be effective needs to have in place post-marketing risk management and surveillance steps which are delegated to national authorities and therefore may be differently addressed across the continent.
Drug-drug interactions
68
In Vitro Inhibitory Effects and Mechanisms of Tyrosine Kinase Inhibitors on Warfarin Metabolism
Jin S1,2, Paludetto M1, Kurkela M1, Kahma H1, Neuvonen M1, Cai W2, Backman J1
1 Department of Clinical Pharmacology and Individualized Drug Therapy Research Program, University of Helsinki, Helsinki, Finland
2 School of Pharmacy, Fudan University, Shanghai, China
Introduction: Tyrosine kinase inhibitors (TKIs) and the anticoagulant drug warfarin are commonly co-prescribed in patients with cancer-associated venous thromboembolism. Many TKIs have been suspected of causing hazardous drug-drug interactions (DDIs) with warfarin on the basis of case studies, but the biochemical mechanisms causing these interactions have not been demonstrated.
Objectives: As inhibition of cytochrome P450 (CYP) enzymes is one of the central DDI mechanisms, we investigated 11 TKIs for their inhibitory effects on the major enzymes involved in the metabolism of warfarin enantiomers.
Methods: An in vitro cocktail assay with three CYP enzymes was applied in this study. Diclofenac 4'-hydroxylation, midazolam 1'-hydroxylation and tacrine 1-hydroxylation were used as marker reactions for CYP2C9, CYP3A and CYP1A2 activity, respectively.
Results: After a 30-min preincubation with NADPH to screen for possible time-dependent inhibition, trametinib, alvolcidib and cediranib exhibited an increased inhibition of CYP3A, tozasertib an increased inhibition of both CYP3A and CYP2C9, and linsitinib and masitinib an increased inhibition of both CYP3A and CYP1A2, compared to experiments with no preincubation. In detailed experiments, tozasertib and linsitinib were identified as time-dependent inhibitors of CYP3A, with inhibitor concentration that supports half-maximal rate of inactivation (KI) and maximal inactivation rate (kinact) of 400 μM and 0.026 min-1, and 231μM and 0.056 min-1, respectively. Linsitinib was the only inhibitor causing a time-dependent inhibition of CYP1A2 with KI and kinact of 9.06 μM and 0.017 min-1. However, trametinib, alvolcidib and cediranib were negative for significant time-dependent inhibition of CYP3A. Moreover, the competitive inhibitory effects of linsitinib, tozasertib and trametinib towards CYP2C9 were strong, with a Ki of 1.43, 2.85 and 0.56 μM, respectively. Masitinib and vatalanib exhibited competitive inhibition of both CYP3A (Ki=1.33 μM, Ki=0.26 μM) and CYP2C9 (Ki=1.98 μM, Ki=0.30 μM). Vatalanib also noncompetitively inhibited CYP1A2 with a Ki of 1.96 μM.
Conclusion: The results suggest that several of the TKIs studied may cause DDIs by inhibition of CYP1A2, CYP2C9 or CYP3A4. More attention should be paid on to avoid unnecessary clinical DDI risks when such TKIs are co-administrated with warfarin.
Tabel/Image
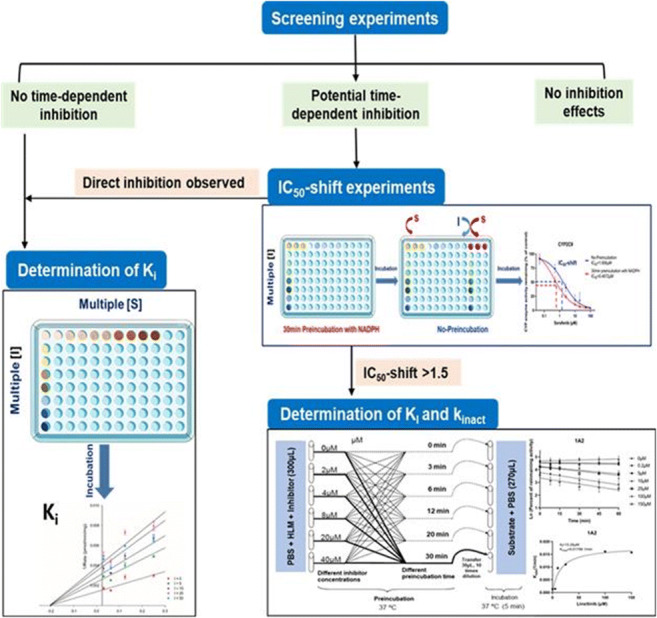
Keywords
tyrosine kinase inhibitors, warfarin, drug-drug interaction
143
Prevalence and predictors of potentially severe drug-drug interactions in patients with myasthenia gravis
Aleksic D1, Milosavljevic M2, Stefanovic S3, Bukonjic A3, Milosavljevic J4, Jankovic S2, Bozovic I5, Peric S6, Lavrnic D6
1 University of Kragujevac, Faculty of Medical Sciences, Department of Neurology, Clinic of Neurology, University Clinical Centre Kragujevac, Kragujevac, Serbia
2 University of Kragujevac, Faculty of Medical Sciences, Department of Pharmacology and Toxicology, Kragujevac, Serbia
3 University of Kragujevac, Faculty of Medical Sciences, Department of Pharmacy, Kragujevac, Serbia
4 University of Kragujevac, Faculty of Medical Sciences, Department of Anatomy, Kragujevac, Serbia
5 Neurology Clinic, Clinical Center of Serbia, Belgrade, Serbia, Belgrade, Serbia
6 Neurology Clinic, Clinical Center of Serbia, Belgrade, Serbia, University of Belgrade, School of Medicine, Belgrade, Serbia
Introduction: Patients suffering from myasthenia gravis (MG) are likely to be exposed to potentially severe drug interactions (ps-DDIs), especially during hospital treatment and if they have multiple comorbidities that require polypharmacy. However, unlike other chronic diseases, specific reports on the relevance of ps-DDIs in patients with MG are still lacking.
Objectives: To assess the prevalence and predictors of ps-DDIs among hospitalized patients with MG.
Methods: A retrospective cross-sectional study was carried out at the Neurology Clinic of the Clinical Center of Serbia, Belgrade. We enrolled subjects with the first hospitalization due to MG and those hospitalized due to exacerbation of MG. Data were collected by reviewing medical records and discharge summaries over a 10-year period. ps-DDIs were identified using the Micromedex online checker by combining the total number of contraindicated and major pDDIs into a single outcome of interest. The proportion of patients who were exposed to each type of ps-DDIs, as well as the number of ps-DDIs per patient was also determined. Multivariable linear regression was used to reveal potential predictors of number of ps-DDIs.
Results: A total of 697 patients with a median age of 55 years (IQR 35-69) were enrolled, most of whom were women (55.6%). The vast majority of them (over 80%) had generalized form of MG, and slightly more than one third were classified as type IIb, the most commonly observed stage of the disease. At least one ps-DDI was observed in 355 participants (51%), median 0 (IQR 0-2). Among them, 62 (17.5%) had contraindicated pDDIs. Only two contraindicated pDDIs were identified: atropine-potassium chloride (61/8.75%) and cyclosporin-lercanidipine (1/0.1%). The most common major pDDIs included potassium chloride-spironolactone (60/8.6%) and enalapril-azathioprine (59/8.5%). Diabetes mellitus, hypertension, indicated thymectomy, total number of drugs-used and use of antiplatelets were identified as the relevant predictors for number of ps-DDIs (R2=0.473, F = 56.840, p<0.001), while history of cancer was inversely correlated with such an outcome.
Conclusion: The prevalence of ps-DDIs in hospitalized MG patients is high and may be influenced by certain comorbidities and therapeutic factors. Clinicians should always be aware of the possibility of serious drug interactions during the treatment of patients with MG.
159
Initiation of glucose-lowering diabetes drugs decreases the blood thinning effect of warfarin
Dunvald A1, Pottegård A1, Stage T1
1 University Of Southern Denmark, Odense, Denmark
Introduction: Type 2 diabetes is a major public health concern and is associated with an increased risk of late complications including cardiovascular disease and ischemic stroke. The risk is modifiable and can be reduced through lifestyle changes and medical treatment. Previous studies indicate that glucose-lowering drugs reduce the efficacy of the blood thinning drug warfarin, supposedly through induction of warfarin drug metabolizing cytochrome P450 enzymes CYP2C9 and CYP3A4.
Objectives: This study aims to assess if initiation of glucose-lowering drugs lead to reduced blood thinning effect of warfarin and whether change in glucose correlates to the impact of this putative drug-drug interaction.
Methods: We conducted a self-controlled register-based cohort study using data from The Copenhagen Primary Care Laboratory Database (CopLAB) and the Danish National Prescription Registry in the period of 2000-2015. Individuals with current use of warfarin, two or more measures of International Normalized Ratio (INR) and a filled prescription for a glucose-lowering drug were included in the study. INR measurements obtained before and after initiation of the glucose-lowering drug were compared using a paired t-test. Subgroup analyses were performed for the individual diabetes drug classes. To assess the correlation of glucose-lowering effect to extent of drug-drug interaction, individuals were grouped based on pre-treatment HbA1c (average blood glucose) and change of HbA1c following initiation of glucose-lowering drug. Further replication is currently in preparation in a Scottish Cohort.
Results: A total of 677 individuals initiated a glucose-lowering drug while receiving warfarin treatment. Mean INR levels decreased from 2.49 to 2.27 within 1 to 3 weeks of initiating a glucose-lowering drug (mean decrease of -0.22, 95% CI -0.30; -0.13). A total of 39% of individuals experienced subtherapeutic INR levels (<2.0) within 1 to 3 weeks after initiating a glucose-lowering drug compared to 28% during a similar interval prior to diabetes treatment. Similar changes in INR are observed across different classes of glucose-lowering drugs. Individuals with normal blood glucose (HbA1c <48 mmol/mol) before initiation of a glucose-lowering drug did not experience a decrease in INR (mean: -0.11, 95% CI -0.34; 0.11) while individuals with severe hyperglycemia (HbA1c >75 mmol/mol) experienced decreased INR (mean: -0.27, 95% CI -0.45; -0.09). Furthermore, change in HbA1c following initiation of a glucose-lowering drug correlated to change in INR; individuals with an increase of HbA1c following initiation did not experience decreased INR (mean: -0.11, 95% CI -0.34; 0.11) while individuals with large decrease in HbA1c following initiation (>10 mmol/mol) experienced reduced INR (mean: -0.31, 95% CI -0.52; -0.09).
Conclusion: The blood thinning effect of warfarin decreases after initiation of a glucose-lowering diabetes drug. The observed effect supports a drug-disease-drug interaction; the decrease in blood-thinning effect correlates to the degree of hyperglycemia prior to initiation of glucose-lowering drugs and to the change in glucose level following initiation of a glucose-lowering drug.
192
Hydroxychloroquine is metabolized by CYP2D6, CYP3A4 and CYP2C8, and inhibits CYP2D6, while its metabolites also inhibit CYP3A4 in vitro
Paludetto M1, Kurkela M1, Kahma H1, Backman J1,2, Niemi M1,2, Filppula A1,3
1 Department of Clinical Pharmacology and Individualized Drug Therapy Research Program, Faculty of Medicine, University of Helsinki, Helsinki, Finland
2 HUS Diagnostic Center, Helsinki University Hospital, Helsinki, Finland
3 Pharmaceutical Sciences Laboratory, Faculty of Science and Engineering, Åbo Akademi University, Turku, Finland
Introduction: Hydroxychloroquine is used for the treatment of malaria, rheumatoid arthritis and lupus erythematosus. In 2020, hydroxychloroquine was also repurposed for the treatment of COVID-19. Although current evidence does not encourage the use of hydroxychloroquine to treat COVID-19, its therapeutic and prophylactic use against COVID-19 is still investigated in clinical trials. Despite being in clinical use for more than 60 years, its clinical pharmacology is not well understood. Hydroxychloroquine is metabolized into three active metabolites, but the key metabolizing enzymes have not been unambiguously identified. Moreover, little is known about the inhibitory effects of hydroxychloroquine on cytochrome P450 (CYP) enzymes.
Objectives: This study aimed to investigate the CYP metabolic and inhibitory profile of hydroxychloroquine and its three metabolites in vitro.
Methods: Hydroxychloroquine metabolism was studied in human liver microsomes (HLM) and recombinant CYP enzymes using substrate depletion and CYP-selective inhibitors. The inhibitory effects of hydroxychloroquine and its metabolites on nine CYP enzymes were also determined in HLM, using automated probe substrate cocktail assays.
Results: Based on screening experiments, CYP3A4, CYP2D6 and CYP2C8 were the key enzymes involved in hydroxychloroquine metabolism in vitro. Although the intrinsic clearance (CLint) value of hydroxychloroquine depletion by recombinant CYP2D6 (0.87 μl/min/pmol) was more than 10-fold higher than that by CYP3A4 (0.075 μl/min/pmol), scaling of the recombinant data to HLM level resulted in similar CLint values for CYP2D6 and CYP3A4 (11 and 14 μl/min/mg) because of the much greater abundancy of CYP3A4 than that of CYP2D6. The scaled HLM CLint of CYP2C8 was 5.7 μl/min/mg. Data in HLM with CYP-selective inhibitors also suggested relatively equal roles for CYP2D6 and CYP3A4 in hydroxychloroquine metabolism, and a smaller contribution for CYP2C8. In CYP inhibition experiments, hydroxychloroquine and its three metabolites were direct CYP2D6 inhibitors (50% inhibitory concentration IC50 18-135 μM), while all metabolites were CYP3A time-dependent inhibitors (IC50 12-117 μM, IC50 shift 2.2-3.4-fold). CYP2D6 inhibition explains the reported clinical drug-drug interaction between hydroxychloroquine and the CYP2D6 substrate metoprolol. The present data, together with the inhibitors’ estimated intracellular hepatocyte concentrations, were successfully used in a static model to predict the fold increase in metoprolol AUC (predicted: 2.3-2.8-fold, observed: 1.65-fold).
Conclusion: The present study unambiguously demonstrates that hydroxychloroquine is metabolized mainly by CYP2D6, CYP3A4 and CYP2C8 in vitro. Moreover, hydroxychloroquine and its three metabolites are CYP2D6 reversible inhibitors, and hydroxychloroquine metabolites are CYP3A time-dependent inhibitors. The current data can be used in static and physiologically-based pharmacokinetic models to predict hydroxychloroquine drug-drug interaction potential, as shown with the successful prediction of hydroxychloroquine - metoprolol drug-drug interaction.
Tabel/Image
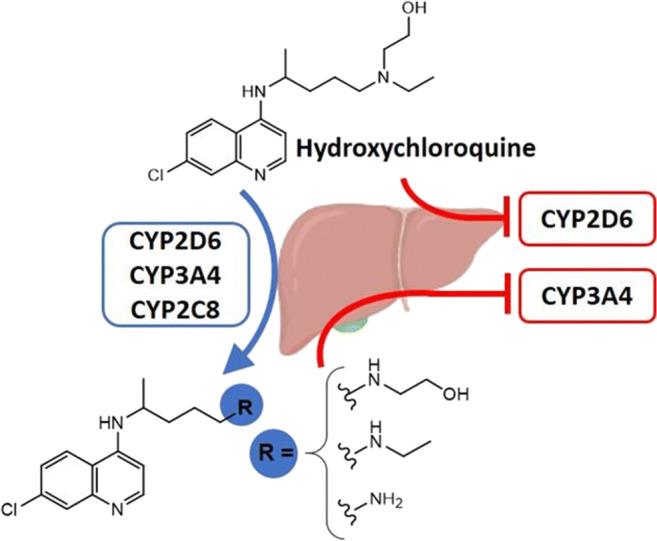
207
Promising pleiotropism of statins and its clinical proof of concept
Mohan P1, Prabhakaran K2, Roy N4, Sinha S3
1 Armed Forces Medical Services India, Shillong, India
2 Resident Dept of Pharmacology AFMC Pune, Pune, India
3 Professor Dept of Pharmacology AFMC Pune, Pune, India
4 Professor Dept of Surgery AFMC Pune, Pune, India
Introduction: With increasing incidence of dyslipidaemia, there has been an increase in statin usage. They have been found to possess analgesic properties in animal studies; and we could demonstrate that this property is additive with the effect of other analgesics, in one of our experimental work. We further hypothesized that patients taking statins for dyslipidaemia (which is a large and increasing cohort) may require lower doses of analgesics (as and when required) compared to similar patients not on statins. To test this, we designed this prospective cohort study wherein we compared the analgesic usage in post operative period, following minor surgical procedures.
Objectives:
• To study the analgesic prescription and utilisation during 2 to 7 days post minor surgical procedures.
• To compare the analgesic usage during 2 to 7 days post-surgery with respect to history of statin usage.
Methods: Adult patients having undergone day based minor surgical procedures (dermoid cyst removal, lipoma excision, etc), in post-operative period (day 2 to day 7), were included. The patients were prescribed Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) for post-operative analgesia on an ‘as and when required’ (SOS) basis. The entire cohort was divided into two groups (Group 1: on statins for past 6 months; Group 2: with no history of statin use). Data regarding analgesic consumption was collected as daily defined doses (DDD) and total amount of analgesics consumed during the study period. Number of patients consuming 20% (or more) less analgesics vis a vis the prescribed dose, was the outcome of interest in both the groups. Also calculated was the average amount of analgesics consumed in both the groups.
Results: Total 245 participants (56 in group 1 and 189 in group 2) were included. Rosuvastatin and atorvastatin were the two statins being prescribed. > 90% of statin prescriptions were as per their respective DDDs. Paracetamol (229 patients) and paracetamol + ibuprofen combination (16 patients) were the NSAIDs prescribed in this study and were prescribed in lesser DDDs in all the patients. Greater percentage of patients in Group 1 i.e. statin users (63.17%) had the outcome of interest compared to those in Group 2 (36.83%) (p<0.00001). Since > 90% patients were prescribed tab paracetamol, the mean consumption of paracetamol during the study period between the two groups was compared. Mean paracetamol consumption in Group 1 (3.47 gm+/- 2.15) was significantly lesser as compared to Group 2 (4.46 gm +/- 2.29) (p=.0053)
Conclusion: Analgesic properties of statins have been experimentally established and so has been its additive interaction with known analgesics. In our study, patients on long term statins consumed significantly less analgesics as compared to the comparator group. The present study provides early evidence that patients on long term statins may be requiring less amounts of analgesics. However, before prescribing lesser dose of NSAIDs to patients on statins, the findings warrant confirmation by Randomised Control Trials.
212
Potential drug-drug-interactions in the Swedish ICU: Should we worry about ICU polypharmacy?
Myllymäki L1, Bottiger Y
1 Linköping University Hospital, Sweden, LINKÖPING, Sverige
Introduction: Drug-drug interactions (DDI) are generally a significant cause of morbidity and mortality, as well as increased costs and length of hospital stay. In Sweden today, electronic health records with integrated DDI warnings have been implemented in virtually all hospitals, with the exception of the intensive care units, where the medications charts are either still on paper or, if electronic, still not connected to DDI warning systems. However, in the ICU, it may well be that the clinical relevance of interaction warnings differ from ordinary care, due to the type of medications used, as well as the close monitoring of the patients.
Objectives: This study aimed to determine the frequency of potential DDIs and clinically relevant DDIs during the hospitalization of patients in three different Swedish ICUs at the same university hospital.
Methods: This observational pilot study was conducted at a mixed ICU, a cardiothoracic ICU and a neurosurgical ICU over the course of a total of 5 months during the covid-19 pandemic year 2021. The investigator visited the ward once weekly and checked all prescribed medications on that day for each patient against the DDI database SFINX/Janusmed Interactions. The result was communicated to the physician in charge.
Results: The sample size included 172 patients. A total of 53 patients (31%) were found to have at least one potential DDI (pDDI). The most common pDDIs in all three ICUs were drugs with risk of QT prolongation and drugs with increased risk of serotonergic toxicity. 29-41% of the pDDIs in the different ICUs were drugs with risk of QTprolongation, the most frequent drugs being amiodarone, antibiotics (erythromycin, moxifloxacin and ciprofloxacin) and ondansetrone. 7-24% of the pDDIs in the different ICUs were drugs with increased risk of serotonergic toxicity, the most frequent drugs being selective serotonin reuptake inhibitors (SSRI), fentanyl, remifentanil, pethidine and metoclopramide. Neurosurgical intensive care patients were exposed to higher frequency of pDDI with serotonergic toxicity compared with the other intensive care unit-patients.
Observed pDDIs led to dose-adjustment in 6 cases and exchange of drugs in 4 cases. No adverse drug reactions (ADRs) were observed.
Conclusion: Potential DDIs are common in ICU patients, but far from all are clinically relevant. We need to learn more about the clinical relevance of the pDDIs in this patient setting, as a basis for customized either manual or computerized decision support algorithms to decrease the risk of unfavorable outcomes due to DDIs.
251
Metronidazole does not significantly elevate plasma concentrations of the CYP2C9 substrate fluvastatin in healthy volunteers
Kaartinen T1,2, Tornio A3,4, Niemi M1,2, Backman J1,2
1 Department of Clinical Pharmacology, Faculty of Medicine, University of Helsinki and HUS Helsinki University Hospital, Helsinki, Finland
2 Individualized Drug Therapy Research Program, Faculty of Medicine, University of Helsinki, Helsinki, Finland
3Integrative Physiology and Pharmacology, Institute of Biomedicine, University of Turku, Turku, Finland, 4Unit of Clinical Pharmacology, Turku University Hospital, Turku, Finland
Introduction: Metronidazole, an antimicrobial agent mainly used to treat anaerobic bacterial and protozoan infections, elevates stereoselectively the plasma concentrations of the more pharmacologically active S-enantiomer of warfarin. Although the precise underlying mechanism of this interaction remains unclear, previous studies suggest that metronidazole is an inhibitor of CYP2C9 or down-regulates CYP2C9 in hepatocytes. However, the evidence regarding metronidazole’s potential to inhibit CYP2C9 is sparse.
Objectives: The objective of this study was to evaluate the effect of a 3-day clinically used dosing of metronidazole on the pharmacokinetics of fluvastatin, whose 3S,5R-enantiomer is a relatively sensitive substrate of CYP2C9.
Methods: We conducted a randomized placebo-controlled crossover pharmacokinetic study, where 8 healthy volunteers were administered a single oral dose of 40 mg fluvastatin 1 hour after the last dose of 3-day pretreatment with 400 mg oral metronidazole or placebo three times daily. Plasma concentrations of 3R,5S- and 3S,5R-fluvastatin enantiomers, metronidazole and its metabolites were measured up to 12 hours in both study phases by liquid chromatography-tandem mass spectrometric methods. Pharmacokinetic parameters for each analyte were determined using noncompartmental analysis. Each subject was genotyped for the CYP2C9*2 and *3 alleles and the SCLO1B1*1B, *5, *14, and *15 alleles.
Results: Metronidazole caused no statistically significant changes in the main pharmacokinetic variables of either of the fluvastatin enantiomers. In the metronidazole phase, the AUC from 0 to 12 hours of both 3S, 5R- and 3R, 5S-fluvastatin was 1.10-fold (90% confidence intervals 0.86-1.39-fold and 0.92-1.31-fold, respectively), compared to that in the placebo phase. The Cmax values of 3S, 5R- and 3R, 5S-fluvastatin were 0.90-fold (90% CIs 0.47-1.70-fold and 0.50-1.60-fold, respectively) compared to those in the placebo phase. There was a trend for delayed absorption and secondary peaks in the metronidazole phase. One of the study subjects had the CYP2C9*3/*3 genotype and had very high fluvastatin enantiomer concentrations in both phases. The exclusion of this CYP2C9 poor metabolizer from the statistical analysis did not alter the conclusions.
Conclusion: 400 mg of metronidazole three times daily does not cause a statistically significant elevation of plasma concentration of oral fluvastatin indicating that there is no clinically significant interaction between metronidazole and fluvastatin. Moreover, this study suggests that metronidazole is not a moderate or strong CYP2C9 inhibitor.
271
Pharmacokinetic interaction between valproic acid and carbapenem antibiotics: a systematic review
Mazón Maraña I1, Valencia López D2, Llorente Cantalapiedra A2, Cadenas Manceñido Á2, Bautista Blázquez A2, Alonso Gómez B2, Cuellar Gómez D2, Gaztelumendi Martín G2, González Samperio C1, Lavin Alconero L1, Vega Gil N2, Pena Pardo M2, Cos Cossio M2, Sánchez Santiago M2, García Saiz M2
1 Instituto De Investigación Sanitaria Valdecilla (idival), Santander, Spain
2 Hospital Universitario Marqués de Valdecilla, Santander, Spain
Introduction: Plasma concentrations (PC) of valproic acid (VPA) are known to decrease during the concomitant administration of carbapenem antibiotics (CPA). There are occasional references in the literature about the clinical severity of the interaction. However, despite the widespread use of these drugs, the mechanism and magnitude are not well clarified and the clinical management recommendations are insufficient.
Objectives: The objective is to review the reports about the VPA-CPA interaction in the literature and to make a descriptive analysis that would permit to set up a recommendation for its clinical management.
Methods: A systematic search was performed in Pubmed, EMBASE and Cochrane databases to identify bibliography published on the interaction between VPA and CPA until january 2022. 57 articles were found. After a validation we finally selected 30. Subsequently, descriptive statistics were performed with SPSS taking into account: country, year of publication, age, CPA used, previous VPA PC, time elapsed, therapeutic decisions and recovery time.
Results: 51 cases and 10 studies were included from 1997 to 2021. Regarding the studies, the most frequent design was retrospective observational (60%) and the total number of patients was n=705. Patients’ mean age was 38.7±25.7 years. The most associated CPAs were Meropenem (52.9%) and Ertapenem (15.7%). Dosages of VPA and CPA showed a wide variability. All cases presented a rapid decrease in PC of VPA after the start of CPA. The average decrease percentage was 63.3%±25.8 and it was similar when separated by type of CPA, except in Doripenem, which seemed to be higher (92.1%±2.4) although sample size was not sufficient to confirm the difference. The mean time to reach minimum PC was 4.7±3.3 days and the mean recovery after discontinuation of CPA was 13.8±14.7 days. The most frequent clinical decision was to increase the dose of VPA (50%), followed by suspension/change of CPA (35%) or simply waiting (10%), only 5% opted to change the antiepileptic.
Conclusion: The interaction between VPA and CPA is potentially serious, especially due to the frequent use of both drugs, the rapid decrease in PC of VPA with the subsequent slow increase, and the known epileptogenic capacity of some CPAs. The interaction is so strong that the use of CPA has been evaluated in VPA poisoning. Unfortunately, the quality of the reports found does not usually clarify concomitant medications that could have intercurrent importance. In any case, all the studies reviewed agree in recommending vigilance and avoiding the concomitant use of these drugs. It seems clear that strategies based on increasing the dose of VPA are ineffective. It should always be recommended to replace antibiotic therapy when possible, but if this is not the case, considering the risk of seizures, the change of antiepileptic drug before starting antibiotic therapy should be standardized, for example to levetiracetam.
Tabel/Image
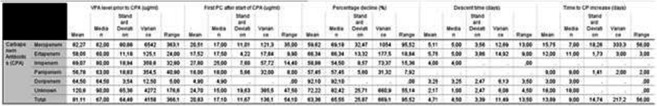
Education
21
How would final years’ medical students perform if their skill-based prescription assessment was real life?
Kalfsvel L1, Hoek K1, Bethlehem C1, van der Kuy H1, van den Broek W2, Versmissen J3, van Rosse F1
1 Hospital pharmacy, Erasmus Medical Center, Rotterdam, the Netherlands
2 Institute of Medical Education Research Rotterdam, Erasmus Medical Center, Rotterdam, the Netherlands
3 Department of Internal Medicine, Erasmus Medical Center, Rotterdam, the Netherlands
Introduction: Prescribing errors occur frequently, especially among junior doctors. Furthermore, the majority of final-year medical students feet their medical curriculum does not adequately prepare them for their future prescribing responsibilities.
Objectives: The aim of our study was to investigate the prescribing errors made by final years’ medical students. Information on the type, amount and severity of these errors can help to improve education on and assessment of clinical pharmacotherapy (CPT).
Methods: This was a retrospective cohort study amongst final years’ medical students at Erasmus Medical Centre, The Netherlands. Errors made in the final prescribing assessment were analysed. This test consists of four cases for which students need to write a prescription. For one of those cases, students need to fill out an additional WHO-six step model. Each test consists of at least an opioid case, a pediatric case and a case in which the dose needs to be adjusted to the kidney function. Errors were categorized by type of error, possible consequence of the error and the possibility of reaching the patient in a real life scenario.
Results: A total of 381 students wrote 1502 analysable prescriptions. 40% of the prescriptions contained at least one error. The majority of errors were of the inadequate information type (54%). The mean grade of prescriptions for children was lower than for other question categories (e.g. opioids) (P = <0.001). 50% of all errors were classified as ‘would have reached the patient but would not have had the potential to cause patient harm’. In total 253 (29%) errors would not have been intercepted by an electronic prescribing system or a pharmacist. 10 (4%) of these would probably have caused harm in the patient.
Conclusions: There is a high rate of errors in prescriptions written by final years’ medical students. Most errors were of the inadequate information type indicating that students had difficulties determining the content and amount of information needed to make treatment successful. Prescriptions for children contained most errors. Curricula could be improved by offering more case-based CPT education focusing on the practical issues of prescribing, especially for pediatric cases, and offering more practice time for prescribing during clerkships.
58
Dr. Vigilance, a teacher’s guide and training videos commissioned by EurOP2E for educating clinical pharmacovigilance in undergraduate students.
Reumerman M1,2, Richir M1,2, van Agtmael M1,2, Tichelaar J1,2, on behalf of the EurOP2E consortium
1AmsterdamUMC, department of internal medicine, section pharmacotherapy, Amsterdam, Nederland, 2RECIPE - Amsterdam, Amsterdam, Nederland
Introduction: Previous studies have shown the lack of clinical pharmacovigilance competences in (under)graduate healthcare students/professionals. This lack of competences is concerning since they negatively affect doctors, pharmacists and prescribing nurses adverse drug reaction (ADR) detection and reporting rates and have shown to negatively affect ADR-treatment. Despite the necessary pharmacovigilance learning outcomes set out by the education working group of the European Association for Clinical Pharmacology and Therapeutics (EACPT) only few educational training methods targeting day-to-day clinical activities as divided by the WHO pharmacovigilance core curriculum (understanding the importance of pharmacovigilance and preventing, recognizing, managing and reporting ADRs) exist. Because of this lack of high quality and clinical pharmacovigilance educational methods we were commissioned by the European Open Platform for Prescribing Education (EurOP2E) to develop suitable pharmacovigilance teaching methods.
Objectives: To develop free of costs, ready to use, evidence based, open licensed educational teacher’s guide and accompanying training video’s for educating clinical pharmacovigilance in medical curricula.
Methods: The Dr. Vigilance working group performed a review of available educational materials targeting clinical pharmacovigilance in undergraduate healthcare students. Included materials were reviewed according to the key training aspects of the WHO pharmacovigilance core curriculum, enrichment of learning-context and if the training method was evidenced based. According to these aspects an easy to use flowchart was created in which teachers can choose their most suitable clinical pharmacovigilance education for their need and be referred to the accompanying manual or video. In addition, the working group performed an online survey in healthcare students to explore what type of clinical educational pharmacovigilance videos the students would prefer.
Results: The current Dr. Vigilance teacher’s guide on EurOP2E has already incorporated 20 different available educational materials targeting clinical pharmacovigilance in undergraduate healthcare students and is expanding rapidly. The Dr. Vigilance survey has shown many opportunities for clinical pharmacovigilance educational videos which have been grouped into two types: “Dr. Vigilance – explains” and Dr. Vigilance – in the clinic” videos. “Dr. Vigilance – explains” is a series of short videos explaining one specific ADR (e.g. angioedema with ACE-inhibitors and euglycemic diabetic ketoacidosis in SGLT-2 inhibitors). All videos are made in the same format and explain the incidence of the ADR, differential diagnosis (not necessarily an ADR), time-relationship, pharmacological mechanism involved and treatment options. “Dr. Vigilance – in the clinic” shows teachers and students how to get students involved in real-life clinical pharmacovigilance experiences in a general practice office or hospital setting. These videos are all based on published studies such as the junior-adverse drug event managers or ADR interviews at an outpatient clinic.
Conclusion: The Dr. Vigilance module in EurOP2E, guides teachers to choose the most suitable free, evidence based, open licensed, clinical pharmacovigilance materials available and helps them to incorporate this training into their own curriculum. The Dr. Vigilance videos are ready to use, student inspired and a great addition to stimulate real-life, high context, pharmacovigilance education in Europe.
111
Understanding therapeutic reasoning: insights from cognitive psychology
Hartjes M1,2, Richir M1,2, van Agtmael M1,2, Tichelaar J1,2
1 AmsterdamUMC, Vrije Universiteit Amsterdam, Department of Internal Medicine, section Pharmacotherapy, Amsterdam, The Netherlands
2 RECIPE (Research and Expertise Center in Pharmacology Education), Amsterdam, The Netherlands
Introduction: Prescribing medicines is one of the most important treatment options for patients which happens on a daily basis. In particular young doctors are responsible for most hospital prescriptions. Prescribing is a complex task which depends on several factors and prescribing errors are common. However, it is still not clear how prescribers choose their therapy. In order to improve the prescribing skills and reduce prescribing errors, it is necessary to understand the therapeutic reasoning process. Based on these insights, it is possible to improve the Clinical Pharmacology & Therapeutics (CPT) education.
Objectives: The aim of this review is to get insight into the therapeutic reasoning process in order to improve CPT education.
Methods: A search on the literature about decision making has been conducted.
Results: Based on the literature about cognitive psychology, diagnostic and therapeutic reasoning, it can be assumed that when a patient is diagnosed, a primary, automatic response arises based on pattern recognition via therapy scripts. This process is also called type 1 thinking. This primary response can be either correct or incorrect. If this is incorrect, an alternative has to be formed through analytical, also called type 2, thinking. The reflective mind, of which metacognition is an important part, is part of type 2 thinking and must recognize when this response is incorrect and needs further analysis. Then better alternatives can be considered through the algorithmic mind, which is also part of type 2 thinking. Based on this reasoning process, the best drug can be chosen. After choosing the right therapy, new therapy scripts develop through metacognition. Experienced doctors have more and richer therapy scripts and therefore their primary response is more often correct.
Conclusion: A model of the therapeutic reasoning process has been made in order to improve the prescribing process. However, there are some uncertainties, for example whether it is possible to apply theories of cognitive psychology and diagnostic reasoning to therapeutic reasoning. Further research on this topic needs to be done.
Tabel/Image
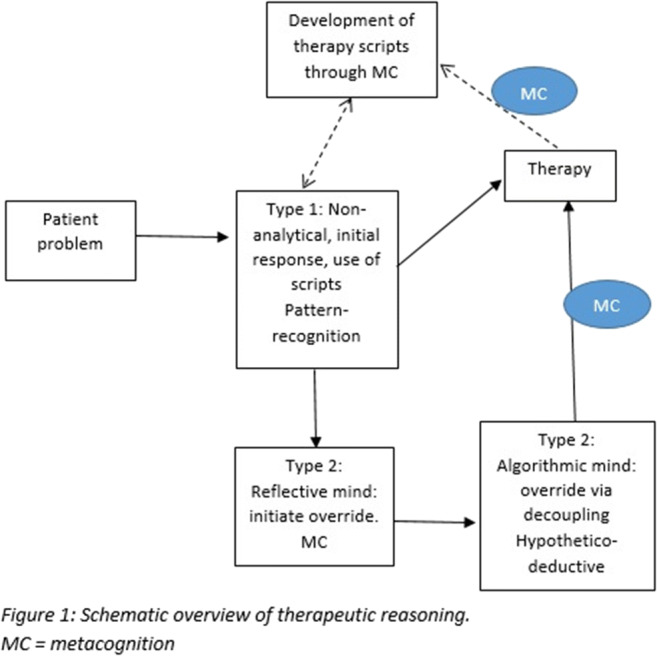
147
European List of Essential Medicines for Medical Education: a modified Delphi study
Donker E1,2, Spitaleri Timpone P8, Brinkman D1,2, Richir M1,2, Papaioannidou P3, Likic R4, Sanz E5, Christiaens T6, Costa J7, De Ponti F8, Böttiger Y9, Gatti M8, Pandit R11, Kramers C10, Van Agtmael M1,2, Tichelaar J1,2
1 Department of Internal Medicine, section Pharmacotherapy, Amsterdam Umc, Location Vumc, Amsterdam, The Netherlands
2 Research and Expertise Centre in Pharmacotherapy Education (RECIPE), Amsterdam, The Netherlands
3 1st Department of Pharmacology, School of Medicine, Faculty of Health Sciences, Aristotle University of Thessaloniki, Thessaloniki, Greece
4 Unit of Clinical Pharmacology, Department of Internal Medicine, University Hospital Centre Zagreb and University of Zagreb School of Medicine, Croatia
5 School of Health Science, Universidad de La Laguna, Tenerife, Spain And Hospital Universitario de Canarias, Tenerife, Spain
6 Department of Clinical Pharmacology, Ghent University, Ghent, Belgium
7 Laboratory of Clinical Pharmacology and Therapeutics, Faculty of Medicine, University of Lisbon, Lisbon, Portugal
8 Pharmacology Unit, Department of Medical and Surgical Sciences, Alma Mater Studiorum, University of Bologna, Bologna, Italy
9 Department of Medical and Health Sciences, Linköping University, Linköping, Sweden
10 Department of Pharmacology-Toxicology, Radboud University Medical Center, Nijmegen, The Netherlands
11 Department of Translational Neuroscience, Brain Center Rudolf Magnus, University Medical Center, Utrecht, The Netherlands
Introduction: Having sufficient prescribing knowledge and skills is essential to prescribe safely and effectively in clinical practice. However, due to the expanding drug arsenal, and increasing amount of patients with polypharmacy, prescribing medicines has become an increasingly complex task. To prepare junior doctors for this difficult task, (inter-)national projects have been developed to improve the undergraduate teaching in clinical pharmacology and therapeutics (CP&T). However, a European list of medicines that junior doctors should be able to independently prescribe safely and effectively without direct supervision is lacking. Such a list can be used for the European Prescribing Exam (EuroPE+) and could also form the basis for country specific lists with the aim to harmonize the teaching in CP&T in Europe.
Objectives: To reach consensus on a list of medicines that are widely prescribed and available in Europe and which junior doctors working in Europe should be able to prescribe safely and effectively without direct supervision.
Methods: This is a modified Delphi study to research consensus among CPT teachers, medical specialists, pharmacists and junior doctors working in Europe. In the first part, an extensive list of available medicines (≥80% of the European countries) was compiled. In the second part, two Delphi rounds were carried out. In each round the participants had to indicate whether a medicine should be included in the final list (5-point Likert scale). Medicines on which ≥80% of all respondents agreed or strongly agreed were included in the final list.
Results: In total, 187 (41%) participants with a diverse background and from 24 European countries completed the study. Of the 416 medicines on the initial list, a total of 98 medicines are included in the final list, see Figure 1.
Conclusion: This is the first Delphi consensus study among European experts working in varies fields to form a list of medicines that junior doctors should be able to prescribe safely and effectively without direct supervision. The European List of Essential Medicines for Medical Education contains 98 medicines. The list will be an extra step towards harmonization of CP&T education in Europe.
Tabel/Image
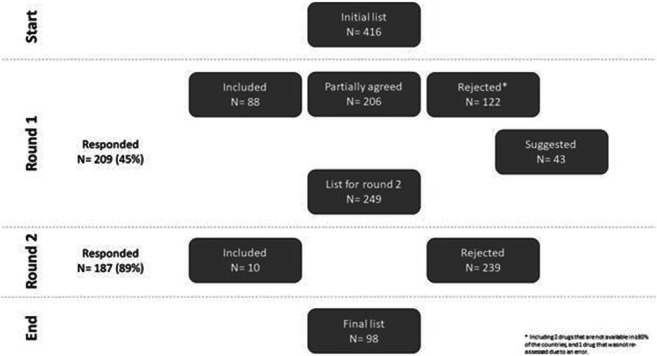
153
Situational tasks as a tool for admitting doctors to independent practice. Russian experience
Kasimova A1,2, Kolbin A1
1 Pavlov First Saint Petersburg State Medical University, Saint-Petersburg, Russia
2 Russian Scientific Research Institute of Traumatology and Orthopedicsnamed after R.R. Vreden, Saint-Petersburg, Russia
Introduction: Medical education in Russia is characterized by a complex multi-stage structure. To become a doctor, applicant need to get a specialist degree. To obtain a clinical specialty, you need to study for 6 years. Graduates of medical universities are required to undergo the primary accreditation procedure, which includes checking the theoretical knowledge and practical skills acquired by students in the learning process. After completing the training, the specialist has a choice. He can immediately try to enroll in a two-year residency or start working as a primary level specialist, thus increasing his chances of entering the residency. In addition to work experience, when entering the residency, the results of primary accreditation, research experience, volunteer activity and other achievements in education are taken into account.
The residency in clinical pharmacology lasts 2 years. During this time, the resident deepens his knowledge of fundamental and clinical pharmacology and develops practical skills. After completing the residency, the graduate will receive primary specialized accreditation - an exam that tests theoretical knowledge and the ability to act in clinical situations.
Objectives: To share the experience of creating situational tasks for primary specialized accreditation in clinical pharmacology.
Methods: The development of situational tasks began 2 years before the introduction of new rules for the certification of graduates. The development was carried out by a team of specialists under the auspices of the Russian Association of Clinical Pharmacologists. Uniform rules were prepared for all developers. The situational task should include the following sections: description of the clinical situation, all necessary examination results, established diagnosis. Questions should be aimed at prescribing treatment, checking the knowledge of dosages, the main adverse reactions and the ability to prescribe treatment to pregnant and lactating women. Also, in the course of solving tasks, skills such as pharmacovigilance, pharmacoeconomical research and knowledge of legislation in the field of circulation of medicines are tested.
Results: More than 1,500 situational tasks have been developed, including all the mandatory conditions. The final exams with their use began in 2020. The experience of two years of using situational tasks for final exams demonstrates their versatility. Graduates are more motivated to solve multi-cases than to conduct a traditional oral exam
Conclusion: Situational tasks should be a mandatory element in the final examinations of specialists. Since it is with their help that you can check the readiness of a specialist to make decisions in routine clinical practice. The development of situational tasks should be handled by professional associations, and the tasks should be based on clinical recommendations
234
Teaching nocebo effects and adverse nondrug reactions to medical students
De La Rosa Loppacher G, Núñez M, Hladun O, Montané E, Pérez-Mañá C, Papaseit E, Farré M
1 Hospital Universitari Germans Trias I Pujol, Universitat Autónoma de Barcelona, Barcelona, Spain
Introduction: Adverse reactions are defined as a noxious and unintended response to a medicine. In clinical trials and daily activities, a percentage of notified adverse drug reactions are just symptoms that can appear in subjects not taking drugs and/or without pathology or comorbidities. These symptoms are sometime called adverse nondrug reactions. Most of these symptoms are very frequently reported as adverse reaction in clinical trials.
Teaching medical students about placebo induced adverse effects (nocebo effect) could be difficult and needs good examples. We used a list of adverse nondug reactions to facilitate the understanding of the nocebo effects and to explain medical students about its frequency in clinical trials and medical health care, and to present the difficulties to evaluate causality in research and medical care.
Objectives: The aim of the present study was to evaluate nocebo effect and its frequency when using a list of most common adverse nondrug reactions in a group of 5th and 6th year medical students taking no medication.
Methods: The study was done during a seminar about placebo/nocebo effects in a Clinical Pharmacology course in the 5th – 6th year of Medicine (Universitat Autònoma de Barcelona, School of Medicine, Hospital Universitari Germans Trias i Pujol, Badalona, Spain). Participant answered a Spanish version of the “Questionnaire for side effects of drugs” (Reidenberg and Löwenthal, 1968; Meyer et al.,1996). It includes a list of 23 symptoms (YES/NO response) that occurred in the last 72 hours, and medicines taken in the same period. A general comment on the results were provided at the end of the seminar to explain the relevance of nondrug symptoms in clinical research, and medical care, and the placebo/nocebo effects. The actual results were compared to previous publications.
Results: A total of 260 medical students answered the questionnaire, 149 did not reported to take any medicine or had an illness. Mean age was 23,5 years old, and 61,7% where women. The most frequent symptoms reported were fatigue (81%), inability to concentrate (48%), excessive sleepiness (41%), irritability (34%), pain in muscles (32%), bad dreams (30%) and headache (28%). Headache, and constipation were significantly more frequent in women than in men. Symptoms ranged from 0 to 12, with a mean number of 5 per subject, and only 5 participants (3,4%) did not report any symptoms.
Conclusions: Our study showed a relative high prevalence of some adverse nondrug reactions in subjects not taking medications. The results were like those found in previous articles in American and German medical students that were published more than thirty years ago. Adverse nondrug reactions are common and should be considered in the interpretation of adverse drug reaction. This activity can facilitate understanding the difficulties to evaluate causality of adverse drug reactions in medical students.
266
ROMANIAN MEDICAL STUDENTS’ PERCEPTION OF ONLINE LEARNING OF PHARMACOLOGY DURING THE COVID-19 PANDEMIC: A SURVEY STUDY
Ioniță I1, Neculau A1, Rogozea L1, Moga M1, Dima L1
1Transilvania University of Brașov, Brașov, Romania
Introduction: COVID-19 pandemic has had a great impact on medical education, disrupting teaching and bringing many changes worldwide in medical education methods. One of these changes is the transition from traditional, face-to-face education to online education
Objectives: The aim of this study was to identify the perception of medical students from all universities in Romania of online education of Pharmacology, in order to see which are the strengths and weaknesses of online education in Romania.
Methods: We conducted a survey by distributing an online questionnaire to Romanian medical students in 13 universities. We included students in their 3rd to 6th year of study and 2020 and 2021 medical faculties’ graduates. The questionnaire consisted of 44 questions and included items concerning demographic details, questions about advantages and disadvantages of online learning of pharmacology, the resources used and the general level of acceptance of online education. We also included items where the respondents had to rate a series of affirmations on online education of pharmacology or compare it to face-to-face education using the 5-point Likert scale.
Results: 178 students answered the questionnaire, most of them in their 3rd and 4th year of study (57.3%). The main advantages of online learning were the ability to stay at home (82%), comfortable surroundings (60.1%) and permanent access to online materials (57.9%). The most rated disadvantages of online learning of pharmacology were the lack of proper student-teacher communication (67.4%), lack of interactions with patients (67.4%), social isolation (66.3%), being easily distracted at home (63.5%) and lack of self-discipline (55.6%). While the most frequently used resource was videoconference (84.8%), the most useful resources were considered to be case-based discussions (74.7%) and quizzes (71.3%), which are used in a significantly lower proportion than videoconference. According to the respondents’ answers, most of them do not consider online education a stimulating environment for learning pharmacology (53.7%) and many do not consider that online learning of pharmacology prepares them as face-to-face education (46.1%). Most of them consider that it did not help them acquire prescribing competencies (55.1%) and they fear they will not be able to acquire the right prescribing skills through online education (62.4%). Overall, only 5.1% of the students would prefer studying pharmacology through online education only, the rest of them considering combined face-to-face education with online education and traditional face-to-face education more beneficial (55.1% and 39.9%). Out of the 178 respondents, only 10.1% are familiar with online websites of pharmacology and only 29.2% utilize mobile applications of pharmacology.
Conclusions: While e-learning methods of pharmacology are modern and make education possible during difficult times when otherwise there would be no education, there is still a lot of work to do for a proper implementation of e-learning and for addressing the students’ needs – more dynamic, more interactive and more stimulating.
308
Drug Information Unit, Medical Faculty of Novi Sad – 15 Years’ Experience
Vukmirovic S1, Tomic Z1, Stilinovic N1, Raskovic A1, Milijasevic B1, Horvat O1, Mijatovic-Jovin V1, Djanic M1, Tomas A1, Martic N1, Bukumiric Z2
1 Department of Pharmacology, Toxicology and Clinical Pharmacology, Medical Faculty, University of Novi Sad, Novi Sad, Serbia
2 Institute for Medical Statistics and Informatics, Medical faculty, University of Belgrade, Belgrade, Serbia
Introduction: There are several ways to obtain necessary information on drugs in Serbia. Medical and pharmaceutical professionals usually use information provided by National Agency of Drugs and Medical Devices or international databases. General practitioners and pharmacists are main source of information for general population. Drug Information Unit at the Medical Faculty, University of Novi Sad, is a regional drug info centre which has been providing information on drugs for 15 years to both professionals and general population in Vojvodina (approximately 1,900,000 inhabitants).
Objecitves: The aim of the study was to analyse request on drug information sent to Drug information unit at Medical faculty, University of Novi Sad.
Methods: Data on requests on drug information sent to drug info unit at Medical faculty, University of Novi Sad during the period from 2007 to 2021 were collected and analysed. We analysed type of the clients sending request (general population, health care professionals) and the type of information requested (e.g. drug interactions, side effects, use of drugs in pregnancy and lactation etc.)
Results: During the observed period of 15 years there were 3444 requests on drug information. The vast majority of request were generated by phone (96,7%). About 15,7% of all requests were coming from the general population (usually questions on interactions, side effects, use of drugs in pregnancy and lactation, dosing and administration); 79% from health care professionals (19% from GPs, 57,7% from specialists and 2,3% from nurses) and 5,3% pharmaceutical professionals. The most frequent request by health care professionals were related to treatment of choice in bacterial infections (17,6%), drug interactions (16,3%) and drug use in pregnancy and lactation (15%). According to frequency these requests were followed by questions on pharmacokinetics, most frequently related to drug pharmacokinetics in kidney/liver failure. Patients/general population were most frequently interested in interactions (26,7%), use of drugs in pregnancy and lactation (24,4%) and side effects (17,8%). Pharmacists were most frequently interest in drug use after expiration date (26,7%).
Conclusion: It can be concluded that the Drug Information Unit is a useful source of information for both professionals and the general population offering various information on different topics related to drugs. In health care professionals there is a growing demand for information on use of antibacterial drugs, especially in case of drug resistant bacteria, and pharmacokinetics of drugs in patients with failure of excretory organs.
Geriatric treatment
76
Geographic variation in top-10 prescribed medication and potentially inappropriate prescription in Portugal: an ecological study of 2.2 million older adults
Rocha V1, Plácido A2, Rodrigues D2, Tavares A2, Figueiras A3, Roque F2, Herdeiro M1
1 Institute of Biomedicine (iBiMED) and Department of Medical Sciences (DCM), University of Aveiro, Aveiro, Portugal
2 Research Unit for Inland Development, Polytechnic of Guarda (IPG-UDI), Guarda, Portugal
3 Department of Preventive Medicine and Public Health, Faculty of Medicine, University of Santiago de Compostela, Santiago de Compostela, Spain
Introduction: The use of multiple medications by older adults is considered a Public Health concern since it is associated to a higher risk of adverse drug reactions and potentially inappropriate medication (PIM).
Objectives: This study aimed to describe the top-10 prescribed active substances in older adults considering geographical distribution and PIM prescription.
Methods: A retrospective ecological study was conducted using data on the prescribed active substances during 2020 to people with 65 years or older. Information on active substances and defined daily doses (DDD) by age group, sex and region were retrieved from a Portuguese health administrative database. The average number of prescribed packages and DDD per 1000 inhabitants per day of top-10 active substances were calculated. Each active sustance was considered PIM if listed on the European Union(7)-PIM list.
Results: A total of 2228090 older adults (58% females) were included. The active substances with higher prescription rates (mean DDD/1000 inhabitants/day) in all ARS were furosemide and atorvastatin in both males and females, compared to the other active substances of the top-10. Geographic differences in prescription were observed (higher prescription in ARS North and Centre and lower in ARS Algarve). In females, 2/10 most prescribed active substances were PIM (benzodiazepines and opioids) with geographic disparities across regions.
Conclusions: Most prescribed active substances to older adults belong to the cardiovascular system. The prescription of benzodiazepines and opioids in females, classified as PIM, alert for the need of public health policies to reduce inappropriate prescribing. Geographic differences in the top-10 most prescribed active substances and in PIM highlighted the importance of medication optimisation across regions.
156
Risk of upper gastrointestinal tract bleeding in patients up to 65 years and older, depending on gender and pharmacotherapy
Yurina Y1, Bondarenko N, Karandov A, Sarmanaev S
1 Clinical Hospital № 85 Federal Medical Biological Agency, Academy of postgraduate education Federal Scientific Glinical, Moscow, Russian Federation
Introduction: Gender identity, including in geriatrics (patients >65 years), have special significance, both for the analysis of the pharmacoepidemiology of diseases, and for the study of the effect of pharmacotherapy on the risk of gastrointestinal tract bleeding (GITB), depending on gender and comorbidity (Graham D.J. et al., 2019, Barnes G.D., 2018).
Objectives: Сomparative assessment of the risk upper GITB in patients younger than 65 years and older; study of the influence of gender and pharmacotherapy features on the risk of bleeding.
Methods: Retrospective analysis of 288 medical records of inpatients who underwent gastroscopy to diagnose the source of bleeding (2018). Group I -134 patients were identified under 65 years, 90 men, and group II – 154 patients over 65 years including 89 men. The most common causes of upper GITB (78%) were stomach and duodenal ulcers, and variceal esophageal veins (22%). Comorbid background in group I and II was represented by cancer (18% and 55%, accordingly), cardiovascular diseases (25 % and 40%), liver cirrhosis (20% and 5%). Features of pharmacotherapy: taking warfarin (1% and 8%), oral anticoagulants (1% and 3%), acetylsalicylic acid (0%% and 25%), other non-steroidal anti-inflammatory drugs (5% and 15%), drug interaction (1% and 5%). Quantitative risk assessment was carried out using key indicators (absolute risk reduction (ARR), odds ratio (OR), number need to treat (NNT)).
Results: Risk of upper GITB had no significant differences between the groups despite the high comorbid background in patients older than 65 years (ARR – 6,9%, CI =95%, p=0,11, OR=1,32, NNT=14), but it was significantly higher in men in both groups compared to women (I - ARR – 34.3%, CI =95%, p<0,0001, OR=0,24, NNT=3; II-ARR=15,6%, p=0,009, OR=0,53; NNT=6) and in patients over 65 years of age who received pharmacotherapy due to comorbid background (ARR – 37,1%, CI =95%, p<0,0001, OR=0,19, NNT=3).
Conclusion: An objective assessment of risk of upper GITB showed that it was significantly higher in patients older than 65 years with a comorbid background receiving pharmacotherapy, as well as in men in both groups compared to women.
246
Deprescribing in palliative care; is there a role for clinical pharmacology to support decision making?
Wightman F4, Rowland T1, Hanchanale S3, Dickman A3, Walker L2
1 Department of Clincial Pharmacology and Therapeutics, Liverpool University Foundation Hospitals Trust, Liverpool, UK
2 Institute of Systems, Molecular and Integrative Biology, University of Liverpool, Liverpool, UK
3 Department of Palliative Care, Liverpool University Hospitals Foundation Trust, Liverpool, UK
4 University of Bristol Medical School, Bristol, UK
Introduction: Reducing medication burden at the end of life has a number of potential benefits, including reduced rates of referral to acute care, reduced risk of falls and improved adherence with essential medication. Many patients remain on medications that are of questionable benefit in frail elderly patients, despite being identified as being in the last months of life.
Objectives: We aimed to quantify the burden of polypharmacy in patients that are referred to the palliative care team of a large teaching hospital in the United Kingdom, and to assess whether medications of questionable benefit were reviewed. We looked at whether advice was given regarding deprescribing and whether patients were still prescribed medications of questionable benefit at the point of death or discharge from hospital. We assessed the impact of education sessions and the implementation of deprescribing guidance.
Methods: Electronic notes and prescription charts for inpatients referred to palliative care were assessed for documentation of palliative review and evidence of any decision to deprescribe medicines during February 2019. Electronic prescription charts were reviewed for medicines of questionable benefit, determined using the STOPPFrail criteria. Analysis was repeated for palliative referrals in June 2021 after implementation of a proforma to guide deprescribing of medicines in palliative care.
Results: Analysis of deprescribing decisions was completed for 133 patients referred to the palliative care team in the first audit cycle and 50 patients in the follow-up cycle. Advice on medication deprescribing given by the palliative care team increased from 16% to 22% of patients referred following the intervention. Overall 6-month mortality was 85%. Despite recognition of limited life expectancy by the referring team, and the implementation of deprescribing guidance, 41% of all patients referred in February 2019 and 48% in June 2021 were still prescribed potentially inappropriate medicines at point of death or discharge from hospital.
Conclusion: Our findings suggest a need for greater clinical support to facilitate pragmatic use of medicines at end of life. Our initial interventions had limited impact. We plan to introduce clinical pharmacology inpatient reviews, in collaboration with the palliative care team, to provide expert advice for deprescribing medications of questionable benefit at the end of life.
Health economy
29
Analysis of Clinical Trials Extraordinary Costs in Oncology: Is There an Economic Impact for the Health System?
Astasio O1, García-Arenillas M1,2, Portolés-Pérez P1,2, Vargas-Castrillón P
1 Clinical Pharmacology Dept, Hospital Clínico San Carlos, IdISSC, Madrid, Spain
2 Pharmacology and Toxicology Dept, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain
Introduction: Cancer is one of the main causes of morbidity and mortality worldwide, as well as a main area for clinical research. In Spain, 2015, it was the second more frequent disease causing years of life lost, and direct costs for hospital treatment of cancer were estimated at 2,8 M€ (million €) (almost 10% of Spanish healthcare spending and 0.66% of GDP that year).
Objetives: Revise and describe the direct costs of drugs and complementary tests associated with conducting clinical trials (CT) in oncology in a university hospital (Hospital Clínico San Carlos, Madrid, Spain).
Methods: Economic evaluation study of the consumption of resources in drugs and complementary tests, in oncology clinical trials (bladder, prostate and digestive), on a retrospective observational study (2014-2020). Medical history were reviewed and total cost of drugs was assessed within the clinical trial, and according to the usual clinical practice (SEOM and ESMO treatment guidelines). Drug prices were obtained from the Nomenclátor (laboratory prices). Complementary tests prices were obtained from published official public prices in the regions of Andalucía and Extremadura.
Results: 27 CTs were identified (10 bladder, 9 prostate, 8 digestive), including 187 patients (only 181 were evaluated and 25 were still in treatment at the end of the study), corresponding to a total treatment period of 66976 days (median treatment: 175 days/patient). We evaluated 36 different drugs (21 experimental drugs, 7 have not yet been approved). Cancer drugs total cost (experimental or not) was 8,31 M€, of which 7,26 had been provided by the CT account. The estimated cost of treating those kind and number of patients according to usual clinical practice would have been 3,77 M€, avoid cost of treatment was 2,76M€. The cost of the complementary tests carried out on these patients during the CTs was between 320.016€ and 521.470 € (depending on the price source consulted) and the corresponding cost for usual clinical practice was estimated at least between 264.839€ and 434.680€. Estimated treatment cost/patient was 66.896€ (prostate), 50.527€ (bladder), 23.943€ (digestive) and complementary test/patient was between 1.808€ and 2.946€. Nine experimental drugs accrued 5,35 M€ in financial benefit from early access to subsequently approved therapies.
Conclusion: Regardless of their effectiveness, which is not the aim of this analysis, oncological CTs facilitate access to experimental treatments, and constitute a funding resource for health care.
Tabel/Image
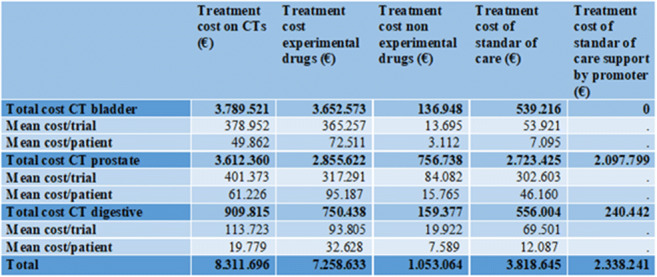
141
The incremental cost-effectiveness ratio of the innovative pelvic floor muscle training in women with stress urinary incontinence treated by duloxetine
Svihra J1, Hagovska M2, Breza jr. J3, Dubravicky J4, Vargovcak M5
1 Jessenius Faculty of Medicine, Martin, Comenius University Bratislava, Martin, Slovak Republic
2 Faculty of Medicine, PJ Safarik University, Kosice, Slovak Republic
3 Faculty of Medicine, Comenius University, Bratislava, Slovak Republic
4 University Hospital Bratislava, Bratislava, Slovak Republic
5 Railway Hospital, Kosice, Slovak republic
Introduction: The stress urinary incontinence (SUI) is defined as the complaint of any involuntary loss of urine on effort or physical exertion or on sneezing or coughing. The SUI significantly affects a women’s quality of life. The Quality Adjusted Life Years (QALYs) measure impact of disease on quality of life during defined period of life. The incremental cost-effectiveness ratio (ICER) provides the incremental cost of QALYs gained from one treatment compared to another.
Objectives: The aim of this study was to measure the impact of the innovative pelvic floor muscle training (iPFMT) on the Quality Adjusted Life Years (QALYs) in women with stress urinary incontinence (SUI) treated by duloxetine.
Methods: This analysis is a part of the clinical trial realized between February 2019 and 2020. It was a randomized intervention, parallel, multicentre study at urological outpatient clinics for 12 weeks. Women were assigned in a 1:1 ratio to the experimental and control groups, an estimated 63 women were required for each group. The control group received oral duloxetine treatment (40 mg BID), the experimental group received oral duloxetine treatment (40 mg BID) and iPFMT with lumbopelvic stabilization. The iPFMT was performed 5 times a week for 20–30 minutes a day, in cooperation with a physiotherapist. The SUI was analysed during a baseline and a final period according to the International Consultation on Incontinence Questionnaire - Urinary Incontinence - Short Form (ICIQ-UI SF) with the range from 0 (without SUI) to 21 (the most severe SUI). The calculation of the weighting factor (WF) was done by a linear transformation of the ICIQ-UI SF (WF = 1 – ICIQ-UI-SF score/21). The QALYs gained were calculated by multiplying the study period (SP) by a weighting factor (QALYs = SP * WF). The ICER was calculated according to the ratio of cost differences vs QALYs differences.
Results: The study included 158 women, of whom 129 women (81.6%) were analysed in the control group (n = 64) and experimental group (n = 65), mean age of 55.2±13.0 years (range 29-80 years). The stress urinary incontinence evaluated with the ICIQ-UI-SF was decreased in control vs experimental group by 37.0±22.3% vs 45.0±23.3% (p<0.05). The calculated mean baseline QALYs gained per year in control vs experimental group was 0.27±0.08 vs 0.28±0.07 and final QALYs 0.53±0.20 vs 0.60±0.18 (p<0.05). The cost of the experimental vs. control treatment reached of 381.48 vs. 94.08 EUR. The experimental treatment had positive financial benefit because an ICER was 4105.71 EUR per one QALY gained.
Conclusion: IPFMT treatment in a population of women with stress urinary incontinence treated with duloxetine is a cost-effective treatment and increases the quality of life of patients.
160
Competitiveness of the pharmaceutical industry of Kazakhstan
Almurzayeva A1, Zhakipbekov K, Ashirov M
1 Kazakh National Medical University named after S.D.Asfendiyarov, Almaty, Kazakhstan
Introduction: At the present stage of development of market relations, the effectiveness of the activities of pharmaceutical enterprises in Kazakhstan as subjects of the pharmaceutical market largely depends on the level of their competitiveness, as well as the government's course for modernization, innovative development of the economy and the existing significant need for effective medicines (drugs), which make the country attractive and a promising market for large international companies. Under these conditions, the main task of any organization is to search for and develop effective mechanisms for organizing and managing activities, assessing strengths, developing and multiplying them, identifying weaknesses and developing measures to eliminate them or level the negative impact, search and attract customers.
Objectives: To determine evidence-based approaches to increase the level of competitiveness.
Methods: Comparative, descriptive, systemic, retrospective methods.
Results: In total, 7455 names of drugs are registered in the country, of which domestic production (DP) - 12% (922 names), foreign manufacturers - 88% (7106 names). To date, 96 enterprises are employed in the domestic pharmaceutical industry, 33 of which produce drugs (of which 23 production sites have GMP), 41 are medical devices and 22 enterprises are medical equipment. In 2020, DP increased production by 34.1%, or 81.5 billion tenge (2019 - 57.6 billion tenge). Investments in the industry increased by 5.2% and amounted to 4.1 billion tenge (2019 - 4.09 billion tenge). According to IQVIA, by 2024, the overall Kazakh pharmaceutical market is projected to grow by 10%. The conducted SWOT-ANALYSIS indicates that: the strengths of DP are state support for development as an organization of procurement procedures through a single distributor system and the formation of a stable dozen of leaders in domestic drug production, which provide 87.4% of the total state order. However, the low export potential of DP and the lack of well-established interaction between science and production represent the weaknesses of the domestic pharmaceutical market.
Conclusion: To increase the competitiveness of domestic DPs, it is proposed to strengthen the implementation of GMP, update drug production technologies and commission new capacities, introduce state orders for domestic drugs and the support system in general, create favorable conditions for investment activities, create a single information space, improve the pricing system for drugs, etc.
230
A model of clinical management of new expensive medicines and their impact on the budget in a large University Hospital
Danés I1,2,3, Diogène E1,2,3, Ballarín E1,3, Alerany C4, Vancells G4, Gorgas M4, Vila M5, Cortés S5, Abadías M6, Agustí A1,2,3
1 Clinical Pharmacology Service, Vall d’Hebron University Hospital, Vall d’Hebron Barcelona Hospital Campus, Barcelona, Spain
2 Department of Pharmacology, Therapeutics and Toxicology, Universitat Autònoma de Barcelona, Bellaterra, Spain
3 Immunomediated Diseases and Innovative Therapies Group, Vall d’Hebron Research Institute, Barcelona, Spain
4 Pharmacy Service, Vall d’Hebron University Hospital, Vall d’Hebron Barcelona Hospital Campus, Barcelona, Spain
5 Financial Resources Direction, Vall d’Hebron University Hospital, Vall d’Hebron Barcelona Hospital Campus, Barcelona, Spain
6 Medical Direction, Vall d’Hebron University Hospital, Vall d’Hebron Barcelona Hospital Campus, Barcelona, Spain
Introduction: Many expensive drugs recently marketed are used to treat patients with pathologies managed by highly specialized physicians. They have become a challenge to maintain excellence in patient care and health care system sustainability. Late 2015, a model for the clinical management of such drugs was set up at Vall d’Hebron University Hospital, the largest in Catalonia (Spain). It is based on multidisciplinary meetings (managers, clinicians, clinical pharmacologists and pharmacists) with selected clinical services to decide measures to minimize drug expenditure while preserving the quality of care.
Objectives: To describe the model for the clinical management of new expensive medicines at the hospital and the principal results of the activity.
Methods: A descriptive analysis of this activity during 2016-2020 was carried out by reviewing the minutes of the meetings. The meeting agenda (adapted to each specific service) includes: a) information on newly approved drugs (including financing conditions set by the public health system) and on drugs in advanced stages of authorization or investigation; b) the review of new guidelines; c) follow-up of drug expenditure and number of treated patients; d) analysis of the registry of treated patients (compulsory for the reimbursement of these drugs), and e) follow-up of indicators, if available. The specific agreements are included in the meeting minutes.
Results: A total of 142 meetings with 12 different clinical services took place during this period (median 27/year). A total of 154 different agreements were reached during the whole period. The most frequent were related to the need to improve the registry information (23.4%), to promote actions to enhance the use of biosimilars or generic drugs (22.1%) or other specific actions to reduce drug expenditure (15.6%). Changes in global annual expenditure compared to the previous year varied between -4.4% and +7.4% depending on the year analyzed. Important differences in the evolution of drug expenditure between services were observed; decreases have been mainly observed when agreements/indicators on the use of biosimilars were applied. The use of biosimilars saved €2,000,387 during 2020.
Conclusion: A model for the clinical management of new expensive drugs has been implemented and consolidated, and includes agreements where both economic and clinical criteria are taken into account. It is difficult to quantify its global effect and what would have been the evolution without this activity. Nevertheless, it is useful to provide a stable multidisciplinary forum for the discussion of the impact of new drugs, their regulation and financing status and to monitor their use.
Intensive care treatment
274
Dealing with Extreme Scarcity of Intensive Care Resources in Covid-19 Pandemic: National Guidance on Triage in Slovakia
Glasa J1,2,3, Glasova H1,3, Krčméryová T2, Soboňová K3,4
1 Department of Clinical Pharmacology, Faculty of Medicine, Slovak Medical University in Bratislava, Bratislava, Slovakia
2 Institute of Health Care Ethics, Faculty of Nursing and Professional Health Studies, Slovak Medical University in Bratislava, Bratislava, Slovakia
3 Slovak Society of Clinical Pharmacology, Slovak Medical Association, Bratislava, Slovakia
4 Clinic of Clinical Pharmacology of the Faculty of Medicine of the Slovak Medical University in Bratislava and of the Teaching Hospital in Nové Zámky, Nové Zámky, Slovakia
Introduction: The Covid-19 pandemic put the overall health care, and especially intensive care resources under unprecedented strains worldwide. Temporary scarcity or depletion of these resources led to the gut-wrenching necessity of triage, i.e., choosing among the eligible patients for the provision of the potentially life-saving intensive care. Usually, the triage process is implemented in the management of local disasters that overwhelm available rescue and emergency care capacities. During the Covid-19 pandemic, however, it had to be implemented on a larger, regional, or even national scale. As the triage process decisions are among the hardest encountered in clinical practice, those must be done impartially, and in the fullest possible respect of the relevant medical, ethical, and legal standards.
In Slovakia (SR), the developing critical situation of scarcity of intensive care resources during the 2nd wave of the pandemic, led the Ministry of Health to tasking an ad hoc expert group by producing of a national guidance document, aimed to provide for standard, nation-wide triage criteria, and decision-making procedures.
Objectives: To analyse guiding medical, ethical, and legal principles, and their implementation in developing the SR national guidance document on triage process regarding allocation of scarce intensive care resources to medically indicated patients during the Covid-19 pandemic, and to draw some practice-oriented conclusions regarding the similar national and international health care challenges.
Methods: A practice-oriented analysis and subsequent synthesis of lessons learned in the development of the above-mentioned national triage guidance, based upon the available insiders’ factual and conceptual information, seen in comparison with the pertinent international contexts.
Results: The national guidance document on triage process for allocation of intensive care resources in the critical situation of their serious scarcity or depletion due to catastrophic course of the Covide-19 pandemic in SR, developed by the ad hoc expert working group called up by the SR Ministry of Health, was based on the combination of a thorough study and use of similar national or international guidance documents and relevant professional literature available at its drafting time, and the hands-on experience of top country experts in the fields of intensive care and emergency services, health care ethics, and law. The criteria of medical utility were aimed to be balanced with the respective deontological concerns. The use of age criterion was avoided. The modified frailty criteria were used instead. The triage procedure “algorithm” and scoring tables were pilot tested in real-life practice by the members of the working group and their collaborators. The document was positively accepted by the concerned health professionals and by the media. Its actual use was limited to five weeks period during the 3rd pandemic wave.
Conclusion: The SR guidance on the triage process in the critical situation of scarcity of the necessary intensive care resources during the Covid-19 pandemic constitutes an example of an originally developed and practically used guiding document with possible use as a conceptual resource for similar materials developed ex necessitate in the future.
293
Design considerations in clinical trials with adaptive stopping, arm-dropping and randomisation
Granholm A1, Kaas-Hansen B1,2, Lange T2, Schjørring O3,4, Andersen L5,6,7, Perner A1, Jensen A2, Møller M1
1 Department of Intensive Care, Copenhagen University Hospital – Rigshospitalet, Copenhagen, Denmark
2 Section of Biostatistics, Department of Public Health, University of Copenhagen, Copenhagen, Denmark
3 Department of Anaesthesia and Intensive Care, Aalborg University Hospital, Aalborg, Denmark
4 Department of Clinical Medicine, Aalborg University, Aalborg, Denmark
5 Research Center for Emergency Medicine, Department of Clinical Medicine, Aarhus University and Aarhus University Hospital, Aarhus, Denmark
6 Department of Anaesthesiology and Intensive Care, Aarhus University Hospital, Aarhus, Denmark
7 Prehospital Emergency Medical Services, Central Denmark Region, Aarhus, Denmark
Introduction: Conventional randomised clinical trials are inflexible and risk running longer than needed or turning out inconclusive, especially if sample sizes rely on inflated expectations, as is common. Adaptive clinical trials may remedy this by increasing trial efficiency and the chance of allocating participants to more promising interventions.
Objectives: To provide guidance on key decisions pertaining to planning adaptive clinical trials with adaptive stopping, adaptive arm dropping and/or response-adaptive randomisation (RAR).
Methods: We built and used adaptr (R package available on CRAN; see https://inceptdk.github.io/adaptr) and ran 10,000 simulations for each of 3 scenarios (no, large and unimportant effects), each with a 4-arm, binary-outcome trial and maximum 10,000 participants.
Results: We identified 7 key methodological decisions of composite nature. #1: Decide on appropriate interventions and, if relevant, specify the common control. #2: Choose an apt outcome and the statistical model to guide and underpin adaptive analyses; consider follow-up duration and expected data completeness. #3: Decide on timing and frequency of adaptive analyses; consider using a burn-in period without adaptations to prevent undue influence of random fluctuations. #4: Define trial-stopping and arm-dropping rules: when to consider an arm superior, inferior, practically equivalent, or futile to the control or (for all but futility) all other arms. #5: Set up the initial allocation scheme (e.g. equal allocation in the absence of a control arm) and randomisation scheme (e.g. fixed, RAR in all arms, or RAR in non-control arms); decide whether to tailor randomisation for the control arm (e.g. matching allocation probability with the best-performing arm). #6: Choose appropriate performance metrics, e.g. total sample size required to stop the trial, probability of conclusiveness (power), and ideal design percentage (IDP). The choice involves a trade-off considering logistics, economic constraints, and weighing benefits of enrolled (internal) against those of future (external) patients. #7: Devise realistic scenarios with reasonable outcome values (e.g. event rates for binary outcomes) in each arm and report pertinent results prior to trial initiation. Use a null scenario to estimate the risk of type-1 errors.
Our simulations indicated that higher control-arm allocation and RAR in non-control arms be preferable in trials with a common control; some level of restriction on RAR may strike a good balance between maximising IDP and minimising sample sizes and event counts; and different designs can perform similarly, i.e., multiple designs may be reasonable insofar as obviously inferior ones be disregarded.
Conclusion: Trials using adaptive stopping, arm-dropping and randomisation strategies require thorough planning and methodological knowhow. In this overview we have outlined 7 key decisions pertaining to their design and crafted freely available software to facilitate this process, enabling fellow trialists to hit the ground running when embarking on this endeavour. In theory, one could consider myriad trial designs but focusing on select meaningful designs and iteratively submitting them to simulation-based comparison likely suffices to select a prudent and performant design.
Misuse of medicines / substances
44
Pharmacological Pathways Modulated by Some Heavy Metals’ Exposure in Rat Detrusor Muscle
Senbel A1, Taha S, Daabees T, Aly R
1 College of Pharmacy, Arab Academy For Science Technology And Maritime Transport, Alexandria, Egypt
2 Faculty of Pharmacy, Alexandria University, Alexandria, Egypt
3 Faculty of Medicine, Alexandria University, Alexandria, Egypt
Introduction: Metals have played a crucial role in the development of humankind. This study is one of a few comparing the effect of lead, cadmium, and iron overload on the in-vitro neurogenic activity of rat detrusor muscle which plays an important role in bladder voiding.
Methods: Effect of lead acetate, cadmium sulfate or ferrous sulfate in a subacute toxicity study (21-day i.p. treatment in male rats), was evaluated on electrical field stimulation (EFS)-induced contractility of isolated detrusor muscle. Pharmacological modulators of main neurogenic pathways have been used in search for the potential mechanism of toxic action. Serum urea and creatinine levels were measured as well as metal concentrations in blood and urinary bladder.
Results: EFS (1-16 Hz) induced a frequency-dependent contraction of the rat detrusor muscles. At 16 Hz EFS, both low and high doses (3, 30 mg/kg) of lead acetate inhibited contraction with a maximum of -44.44±2.57% and -52.77±4.21% respectively. Only the high dose (1 mg/kg) of cadmium sulfate caused a significant inhibition of EFS-induced contraction at same frequency by -47.77±4.91% as well as the high dose (30 mg/kg) of ferrous acetate by -42.5±5.72%.
In the presence of tetraethylammonium (K+ channel blocker, 10-3 M), the inhibitory effect of lead acetate was significantly potentiated, reaching -51.52±2.78% compared to -36.93±3.81% in its absence. In presence of NG-nitro-L-arginine methyl ester, NOS inhibitor, 10-4 M) or the sGC inhibitor methylene blue (10-4 M), the inhibitory effect of lead acetate was also significantly potentiated. Atropine reversed the inhibitory effect of lead acetate with no effect on cadmium action. Trifluoperazine (calmodulin inhibitor, 10-5 M) significantly hampered the inhibitory effect of cadmium sulfate.
High dose of lead acetate increased urea level (54.0±3.59 compared to 27.43±3.06 mg/dl as control). The accumulation of lead in bladder reached 2.03±0.31 μg/g after treatment compared to 0.09±0.02 μg/g as control. Cadmium and iron accumulation levels also increased dramatically with associated significant histopathological changes.
Conclusion: Lead, cadmium, and iron overload subacutely induce hypoactivity in the detrusor muscle. Lead inhibitory effect may be mediated by muscarinic receptors but not K + /NO/cGMP, while cadmium inhibitory effect may be mediated by inhibiting the Ca 2+ /calmodulin pathway.
86
Self-medication for headaches in medical and pharmacy students
Khammassi N2, Charfi R1,2, Sanaï S2, Khammassi G3, Mouna M4, Aissi M4, Daghfous R1,2, Trabelsi S1,2
1 National Centre Chalbi Belkahia of Pharmacovigilance, Department of clinical pharmacology, LR16SP02, Tunis, Tunisia
2 University of Tunis El Manar, Faculty of Medicine of Tunis, Tunis, Tunisia
3 University of Monastir. Faculty of Pharmacy, Monastir, Tunisia
4 University of Monastir. Faculty of Medicine of Monastir, Monastir, Tunisia
Introduction: Headaches are one of the most common affection with the highest impact on the quality of life. According to the WHO, nearly half of the adult population has had at least one headache in the past year. This can lead the patient to self-medication, which has become an emerging phenomenon and increasingly threatening public health.
Methods: We conducted a cross-sectional study on students with chronic headaches from december 1st, 2021 to february 1st, 2022. We used a questionnaire about self-medication. Student from medical and pharmacy faculties answered anonymously on Google forms to this questionnaire.
Objectives: To assess the prevalence and characteristics of self-medication for headaches in students and to evaluate the perception of self-medication in this group
Results: We included 201 students. Sex ratio (male/female) was 0.16. Among the students, 79.8% were under 25 years old and 94% were in the 2nd cycle. They came in 53%from pharmacy and in 47% from medical studies. Self-medication was reported in 94% of students. The frequency of self-medication was 39% at least once a month and 21% at least once a week. Among the motivating factors for self-medication, 47% of students responded by the benign character of headache, 36% responded by knowing the appropriate treatment. Drugs used were taken from home in 96% of cases. Paracetamol was used in 92,5% of the students, followed by non-steroidal anti-inflammatory drugs in 11% of the cases. Other treatments were included in students responses such as benzodiazepines and triptans. The two main sources of treatments were the family medicine box (45.5%) and the retail pharmacy (43.5%). Before self-medication, 43% of students simply used the treatment they knew, while 33.8% read the package leaflet. Among the students, 82.5% had not reported adverse effects during self-medication. In cases of persistent or unusual headaches, 61.5% of the students consulted a doctor. Medication overuse headache was reported in 3 students (1.5%). Among the responders, 53% thought that self-medication helped them cope, 26.8% considered it was dangerous but for them it remains the best solution for their headaches. Sex, age, type of study were tested for their potential association with self-medication. No significant differences were found in our study by sex (p=0.26), age (p=0.67), type of study (p=0.64).
Conclusion: Self-medication was very common among medical and pharmacy students. Paracetamol and NSAIDs were the most commonly used treatments for headaches. Association of self-medication with sex was the closest to the significance.
104
Serum ethanol concentrations in patients admitted to a large Emergency Department during 2015-2020
Bratberg A1, Nygaard I1, Løhne K2, Gustavsen I1, Opdal M1,3
1 Department of Pharmacology, Oslo University Hospital, Ullevål, Oslo, Norway
2 Department of Medical Biochemistry, Oslo University Hospital, Ullevål, Oslo, Norway
3 Institute of Clinical medicine, University of Oslo, Oslo, Norway
Introduction/Objectives: Ethanol is a common drug of abuse and intake is associated with impairment. Serum ethanol concentration (SEC) is a marker of acute ethanol impairment. The aims of this study were to examine the frequency of patients tested for SEC at admission in the largest Emergency Department in Norway, and the measured levels of SEC grouped by age and gender.
Materials and Methods: Serum ethanol from venous blood was analyzed by enzymatic method using Cobas 8000 c502. The calibrator range was 0.10-4.98 g/L with cut off < 0.2 g/L. Results from ethanol analyses of patient samples requested by the Emergency Department were retrieved from the laboratory information system, Swisslab, retrospectively for 2015–2020. The data were analyzed using SPSS and Microsoft Excel 2010 and are presented below. Mann-Whitney test (p=0.05) was used to compare ethanol concentrations for age groups.
Results: A total of 174 378 patients were admitted to the Emergency Department during 2015-2020 (approx. 29 000 per year). 12 685 (7.3 %) of the patients had a blood sample taken for analysis of SEC and 4476 (35.3 %) of these were positive. To avoid intra-individual variation we included only the first positive test per patient, n=3607. Of these, 73 % were men, 44 (14-96) years and 27 % were women, 45 (14-98) years. The median SEC for both men and women was 1.9 g/L. The figure shows the distribution of SEC by age groups, showing that SEC was significantly higher in the age group 40-59 years compared to the age groups 18-39 years and 60 years and above.
Conclusion: 7.3 % of admitted patients to the Emergency Unit were tested for SEC, and about 1/3 of these were positive. The median SEC in unique patients of both genders were 1.9 g/L, a level where most patients will be impaired. More than 70 % of unique patients with positive SEC were men. The highest SEC were detected in patients between 40-59 years.
Tabel/Image
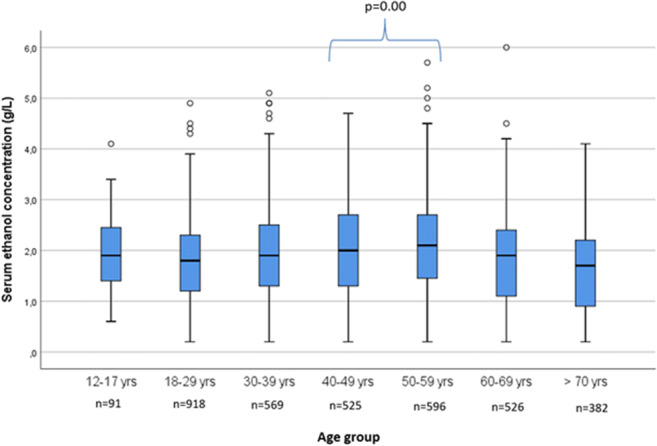
188
Predictors of Severe Outcome Following Opioid Intoxication in Children
Finkelstein Y1,2, Cohen N1,2, Mathew M1,2, Davis A1,2, Brent J3, Wax P4, Schuh S1,2, Freedman S5, Froberg B6, Schwarz E7, Canning J8, Tortora L8, Hoyte C9, Koons A10, Burns M11, McFalls J12, Wiegand T13, Hendrickson R14, Judge B15, Quang L16, Hogdman M17, Chenoweth J18, Algren D19, Carey J20, Caravati M21, Akpunonu P22, Geib A23, Seifert S24, Kazzi Z25, Othong R26, Greene S27, Holstege C28, Tweet M29, Vearrier D30, Pizon A31, Campleman S32, Li S32, Aldy K12
1 The Hospital For Sick Children, Toronto, Canada
2 University of Toronto, Toronto, Canada
3 University of Colorado, Aurora, USA
4 University of Texas, Dallas, USA
5 University of Calgary, Calgary, Canada
6 Indiana University, Indianapolis, USA
7 Washington University, St. Louis, USA
8 Banner University Medical Centre, Phoenix, USA
9 Rocky Mountain Poison and Drug Centre, Denver, USA
10 USF Morsani College of Medicine, Allentown, USA
11 Boston Children's Hospital, Boston, USA
12 University of Texas, Dallas, USA
13 University of Rochester, Rochester, USA
14 Oregon University, Portland, USA
15 Michigan States University, Grand Rapids, USA
16 University of Arkansas, Little Rock, USA
17 Upstate Medical University, Syracuse, USA
18 University of California, Sacramento, USA
19 University of Missouri, Kansas City, USA
20 University of Massachusetts, Worcester, USA
21 University of Utah, Salt Lake City, USA
22 University of Kentucky, Lexington, USA
23 Carolinas Medical Centre, Charlotte, USA
24 University of New Mexico, Albuquerque, USA
25 Emory University, Atlanta, USA
26 Navamindradhiraj University, Bangkok, USA
27 University of Houston, Houston, USA
28 University of Virginia, Charlottesville, USA
29 Toxicon Consortium, Chicago, USA
30 University of Mississippi, Jackson, USA
31 University of Pittsburgh, Pittsburgh, USA
32 American College of Medical Toxicology, Phoenix, USA
Introduction: While the opioid crisis has claimed the lives of nearly 500,000 in the United States over the past two decades, and pediatric cases of opioid intoxications are increasing, only sparse data exist regarding risk factors for severe outcome in children following an opioid intoxication.
Objectives: We explore predictors of severe outcome (i.e., intensive care unit [ICU] admission or in-hospital death) in children who presented to the Emergency Department with an opioid intoxication.
Methods: In this prospective cohort study we collected data on all children (0–18 years) who presented with an opioid intoxication to the 50 medical centers in the US and two international centers affiliated with the Toxicology Investigators Consortium (ToxIC) of the American College of Medical Toxicology, from August 2017 through June 2020, and who received a bedside consultation by a medical toxicologist. We collected relevant demographic, clinical, management, disposition, and outcome data, and we conducted a multivariable logistic regression analysis to explore predictors of severe outcome. The primary outcome was a composite severe outcome endpoint, defined as ICU admission or in-hospital death. Covariates included sociodemographic, exposure and clinical characteristics.
Results: Of the 165 (87 females, 52.7%) children with an opioid intoxication, 89 (53.9%) were admitted to ICU or died during hospitalization, and 76 did not meet these criteria. Seventy-four (44.8%) children were exposed to opioids prescribed to family members. Fentanyl exposure (adjusted OR [aOR] = 3.6, 95% CI: 1.0 to 11.6; P=0.03) and age ≥10 years (aOR= 2.5, 95% CI: 1.2 to 4.8; P=0.01) were independent predictors of severe outcome.
Conclusions: Children with an opioid toxicity that have been exposed to fentanyl and those aged ≥10 years had 3.6 and 2.5 higher odds of ICU admission or death, respectively, than those without these characteristics. Prevention efforts should target these risk factors to mitigate poor outcomes in children with an opioid intoxication.
Tabel/Image
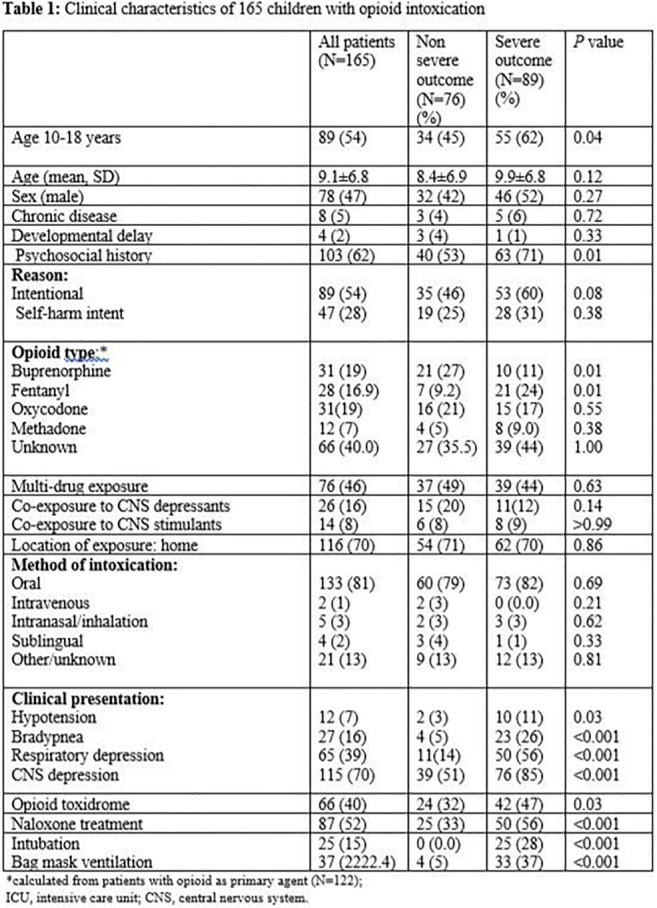
290
Therapeutic protocol in COVID-19: useful or misleading about antibiotics?
Sabin O1, Cizmaș I1, Pop R1, Bocșan I1, Buzoianu A1
1 Department of Pharmacology, Toxicology and Clinical Pharmacology, Faculty of Medicine, Iuliu Hatieganu Medicine And Pharmacy University Cluj-napoca, Romania, Cluj-Napoca, România
During the COVID-19 pandemic, several drugs were used to control SARS-CoV-2 infection in the absence of a specific treatment, for different periods. Sometimes they were prescribed without enough evidence and this strategy later proved to be useless or even dangerous for the patients. In Romania, a therapeutic protocol was published in the first days of the pandemic, with periodic changes according to the new evidence that appeared. This study started from the hypothesis that there is a reluctance to make changes to a protocol already used and once treatment was introduced, it remains in the prescribing habit of doctors. The first therapeutic protocol for COVID-19 in Romania included the formulation “antibiotics can be used in case of...", and it was changed on 27.11.2020 with the clear statement that "no antibiotics shall be used unless a bacterial infection was detected". The present study investigated how the doctors changed their prescription strategy for antibiotics in COVID-19 based on the new version of the protocol. Methods: The observational study evaluated the use of antibiotics and antisecretory treatments in patients hospitalized with COVID-19 between October 2020 and April 2021. We have chosen these two classes because they are associated with an increased risk of Clostridium difficile infection. Three hundred consecutive patients with COVID-19, hospitalized in an Internal medicine department of a tertiary hospital in Romania were included. The hospital has no antibiotic stewardship program or a clinical pharmacologist.
Results: 257 patients (pts) (85.3%) received at least one antibiotic during hospital admission, although only 89 patients (29.7%) had evidence of bacterial infections (positive sputum, urine or blood cultures). Also, 23.3% of COVID-19 patients received antibiotic treatment before admission (up to 1 month), and most of them were treated with Azithromycin (32 pts; 45,7%). Almost all admitted patients (99%) had received antisecretory drugs (proton pump inhibitors or H2 antihistamines). Twenty-six pts (8,7%) had clinical manifestation of colitis and a positive stool test for C. difficile toxin (a prevalence of 56,5 cases/ 10.000 patient-days). No statistically significant difference was observed in the prescribing of antibiotics before and after the aforementioned change in the protocol. Conclusions: Although it was not recommended by the protocol except in patients with proven bacterial infections, antibiotic treatment for patients admitted for COVID-19 was almost the rule rather than the exception. The change in the treatment protocol for COVID-19 has not altered the prescribing habits of antibiotics, continuing their prescription without justification. Antisecretory drugs were also excessively used, without clinical justification. The introduction of an antibiotic stewardship program in Romania is an emergency.
Nephropharmacology
34
Time trends in number and acceptance of pharmacist interventions during nephrology ward rounds: 5-year data
Van Den Oever F2, Vasbinder E1, Bingöl M, Schrama Y1, van Gurp E, van den Bemt P1, van Gelder T1
1 Franciscus Gasthuis And Vlietland, Rotterdam, the Netherlands
2 Leiden University Medical Center, Leiden, the Netherlands
Introduction: Drug related problems (DRPs) are common in patients with chronic kidney disease. They contribute substantially to drug-associated morbidity and mortality, leading to prolonged hospitalization and increased healthcare costs. Participation of clinical pharmacists in ward rounds reduces DRPs in hospitalized patients, but this requires acceptance of pharmacist advices by the prescriber. Over the years the number of interventions may decrease while acceptance rates may increase, due to the learning curve of both the pharmacist and the medical team, and the time it takes to acquire a role in the medical team, but this has not been explored before.
Objectives: We designed this study to explore the time trend in both number of interventions and acceptance rate of pharmacist interventions.
Methods: In this retrospective, single-center study data were extracted from medical records. The study period ranged from 2015 to 2019. Primary outcome measure was the number of interventions, secondary outcome measure was the acceptance rate of pharmacist interventions. The Cochran-Armitage test for trend was used to analyse both outcome measures.
Results: Patients had a mean age of 69 years, used 13 different drugs, and 58% of them were male. The prevalence of CKD stage 3 or higher was 81%. Approximately 46% of patients were on renal replacement therapy, including renal transplantation. The most frequent reasons for hospitalisation were infections (40%), acute kidney injury (38%), fluid retention (15%), diagnosis or analysis of renal disease (15%), and start of dialysis treatment (12%).
A significant linear increasing trend in the number of interventions per patient was observed (see table 1). The mean acceptance rate of pharmacist interventions over the five year study period was 67.3% and did not change over time.
Table 1. Acceptance rate and number of interventions per patient
Conclusions: In contrast to our hypothesis, the number of interventions per patient increased over time. This may be explained by the increased complexity of the patient population over time. The mean acceptance rate of pharmacist interventions was 67.3%, which is similar to earlier Dutch data from two ICUs. The acceptance rate did not change over time.
Tabel/Image
92
Impact of CYP3A5 6986A>G on the correlation trough concentration / area under the curve of tacrolimus in renal transplantation
Guettari M1, Charfi R1, Sfar I2,3, Daldoul M1,2, Daghfous R1,2, Trabelsi S1,2
1 National Centre Chalbi Belkahia of Pharmacovigilance, Department of clinical pharmacology, LR16SP02, Tunisia
2 University of Tunis El Manar, Faculty of Medicine of Tunis, Tunis, Tunisia
3 Charles Nicole Hospital, Department of immunology, LR03SP01, Tunis, Tunisia
Introduction: Tacrolimus (Tac) is largely used in renal transplantation to prevent allograft rejection. Therapeutic drug monitoring of Tac is necessary because of its large inter-individual variability. This variability may be explained partly by the polymorphism in the gene coding for CYP3A5 mediating its hepatic metabolism. Among these genes, single nucleotide polymorphism (SNP) in CYP3A5, 6986A>G, leads to the loss of part of or all the enzyme expression leading to two phenotypes: CYP3A5 Expressors A/A, A/G and CYP3A5 non expressors G/G affecting Tac trough blood concentration (C0). As C0 is widely used as a surrogate of the area under the curve (AUC) because of a good correlation between the two variables, this study aims to investigate the impact of CYP3A5 SNP on this correlation.
Objective: To assess the impact of CYP3A5 6986A>G on C0-AUC correlation of Tac.
Methods: We conducted a retrospective study (2011-2015) including renal transplant recipients who were genotyped for CYP3A5 6986A>G and divided into two groups, G1=CYP3A5 Expressors and G2= CYP3A5 Non expressors. Tac blood concentrations’ measurement was achieved by Architect® using a chimiluminescence immunoassay method. Bayesian estimation was employed to estimate the AUC. The CYP3A5 genotypes were characterized by using polymerase chain reaction/restriction fragment length polymorphism. Therapeutic ranges are 5-10 ng/mL for C0 and 100-190 h.μg/L for AUC. Statistical analysis was performed using the software Graph Pad.
Results: We included 39 patients. Median age was 31.5 (18 to 58 years). Sex ratio M/W was 3.33. There was nine patients in G1 and 30 in G2. C0 and AUC were in the therapeutic in 43 and 54%, respectively. They were subtherapeutic in 31 and 28%, respectively. And supratherapeutic in 26 and 18%, respectively. Correlations (r²) between C0 and AUC in each group were calculated (table 1). Tac exposition (AUC) was well reflected by C0 in G1 in 8 cases and in group 2 in 23 cases. It was underestimated by C0 in 3 cases in G2 and overestimated in one case in G1 and 4 cases in G2 (p=0.002).
Conclusion: C0 displayed good correlations to AUC in both groups with a higher r² in expressors group A/G.
Tabel/Image
Patient empowerment
140
Features and Functionalities of Smartphone Apps related to Psoriasis: A Systematic Search and Content Evaluation.
Fernández González M1, Rodríguez Ramallo H2, Rodríguez Ramallo H1
1 Hospital Universitario Virgen del Rocío, Sevilla, Spain
2 Hospital Universitario Reina Sofía, Córdoba, Spain
Introduction: Psoriasis-related apps are frequently consulted by patients and healthcare professionals, however, knowledge on the number and quality of apps is lacking.
Objectives: To identify and evaluate apps aimed at patients and healthcare professionals regarding psoriasis.
Methods: An observational, cross-sectional descriptive study was conducted. A systematic search was carried out in September 2021 in 'App Store' and 'Play Store'. Key terms employed were "psoriasis" and "psoriatic". Apps in English and Spanish language were included. Apps unavailable in Spain and apps that included inappropriate content were excluded.
Variables collected: Platform (iOS, Android), name, category, cost, latest update, target user, purpose, and participation of health professionals in their development.
To assess quality, the mobile application rating scale (MARS) was employed. This evaluation includes 5 domains: Engagement, Functionality, Aesthetics, Information, and Subjective quality. Each domain was rated from 1 (inadequate) to 5 (Excellence). Mann-Whitney U test was performed to analyse the difference in means.
Results: Overall, 15 apps were included ( 8 iOs, 2 Android, 5 both platforms); The most frequent category was Medicine 80% (12). All apps were free. 10 apps were updated last year. 8 apps were aimed at healthcare professionals and 7 at patients and caregivers. The most frequent purposes were general information (87%), monitoring (67%), and providing advice (40%). Healthcare professionals participated in the development of 9 apps (60%).
The median score in MARS was 3.35 (Range: 1.5 - 4.2) considered as an acceptable score (>3). Differences in scores in dominions were similar among all apps. The median subjective dominion score evaluation was the lowest among dominions: 2.3 (range 1.8 - 3.8). In this evaluation, reviewers would not recommend most apps (66.7%) to patients or healthcare professionals.
iOs apps and Healthcare professionals' participation was associated with higher mean scores (+0,29 and +0,5 respectively), however, these differences were not statistically significant (P>0.05).
Conclusion: Knowledge of the quality of psoriasis-related apps is critical, as they aim to serve as daily tools for professionals and patients.
The quality of psoriasis-related apps was acceptable; however, in most cases, the subjective evaluation revealed that reviewers would not have recommended its use to patients or healthcare professionals.
239
Clinical Pharmacology and Patients’ Empowerment in Slovakia
Glasova H1,3,5, Glasa J1,2,3,5, Soboňová K3,5, Tomek D1,4, Lévyová M4
1 Department of Clinical Pharmacology, Faculty of Medicine, Slovak Medical University In Bratislava, Bratislava, Slovakia
2 Institute of Health Care Ethics, Faculty of Nursing and Advanced Health Studies, Slovak Medical University in Bratislava, Bratislava, Slovakia
3 Slovak Society of Clinical Pharmacology, Slovak Medical Association, Bratislava, Slovakia
4 Association for the Protection of Patients’ Rights in Slovakia, Bratislava, Slovakia
5 Clinic of Clinical Pharmacology, Faculty of Medicine, Slovak Medical University in Bratislava, and of the Teaching Hospital in Nové Zámky, Nové Zámky, Slovakia
Introduction: The empowerment of patients is considered to be one of the cornerstones of modern days rational pharmacotherapy. In Slovakia (SR), the continued, concerted professional and lay activities to this effect started about two decades ago, with strong involvement of the discipline of clinical pharmacology (CP).
Objectives: To report on developments and unique experiences gained in fostering patients’ empowerment in SR, a country having undergone considerable economic, political, and health care transitions ever since the 1990ies.
Methods: Developing a practice-oriented analysis and subsequent synthesis of lessons learned, based upon the insiders’ factual and conceptual information and knowledge of the country’s developments and their driving, and slowing down forces and factors, as seen in the pertinent international contexts.
Results: The developments leading to ever more pronounced and practical empowerment of patients in Slovakia were much enhanced, and even politically supported, after the launching of unprecedented multifaceted transitions following the Velvet Revolution in 1989. Setting up independent patients’ organizations, their coming together forming the alliances and, later on, establishing common associations (such as Association for the Protection of Patients’ Rights in SR (AOPP; see www.aopp.sk)) aimed at fostering patients’ rights (that felt under some pressures from the deep transitional changes occurring within the health care system), were paralleled by growing academic and political interest (e. g., national Patients’ Rights Charter approved by the SR Government in 2001), as well as by SR patients’ representatives taking part in various international initiatives and organizations (e. g., EPF, ELPA, EUPATI, etc.). Starting from quickly developing informal contacts, consultations, increasingly professional discussions, and help in education (incl. issues of better access to modern pharmacotherapy modalities), SR’s CP(-ists) became involved in mutual activities fostering patients’ and patients’ representatives’ education, such as regular annotated courses “Patient and Medicaments” at the Slovak Medical University in Bratislava, invitations to annual CP conferences and other meetings. In 2015, EUPATI.sk was established. In 2017, a national, multistakeholder project “Medicaments with Reason” started, led by AOPP, with the Slovak Society of CP being its scientific guarantor. Successful long-term collaboration of AOPP with SR CP(-ists) and other stakeholders, including payers (health insurance companies in SR), Slovak Chamber of Pharmacists, politicians, academia, and some of the leading SR media, brought about more effective patients’ representatives’ involvement in legislation activities, high-level health policies negotiations, diagnostic and therapeutic standards and guidelines development, and a more adequate media coverage. These aspects, including collaborations with CP(-ists), were further strengthened during the Covid-19 pandemic. A patients’ representative became a regular member of the newly established national SR Clinical Trials Ethics Committee (established under EU CTs Reg. No. 536/2014).
Conclusion: Empowerment of patients ascertained by fostering long-term collaborations and synergies among decisive stakeholders involved, including important professional contributions of CP(-ists), as shown by decades-long experience in Slovakia, can contribute in a substantial manner to continuous, patient-friendly optimization of the national medicinal drugs policies and development of national pharmacotherapy standards and guidelines.
Pediatric treatment
72
Parents’ perceptions of determinants of their decision to enroll or not their infant in a clinical trial: a qualitative analysis
CORNU C1,2, CARLE Q3, POITE M3, ERPELDINGER S3, MEUNIER-BEILLARD N4, BINQUET C4, GINHOUX T1, SAIDI M1, LAMOTTE-FELIN A4, BERARD A2,5, PARIS A6, LAMORT-BOUCHE M3,7, KIEFFER F8, WALLON M9
1 INSERM, CIC1407, Hospices Civils de Lyon, LYON, FRANCE
2 UMR 5558, Université Claude Bernard Lyon 1, LYON, FRANCE
3 Collège universitaire de médecine générale (CUMG), Université Lyon 1, LYON, FRANCE
4 CHU Dijon Bourgogne, INSERM, Université de Bourgogne, CIC 1432, Module Épidémiologie Clinique, DIJON, FRANCE
5 Faculté de pharmacie, Université de Montréal, and CHU Ste-Justine Research Center, MONTREAL, CANADA
6 CIC 1406, Inserm, GRENOBLE, FRANCE
7 Research on Healthcare Performance RESHAPE, INSERM U1290, Université Claude Bernard Lyon 1, LYON, FRANCE
8 Service de néonatologie, Hôpital Armand Trousseau, PARIS, FRANCE
9 Service de Parasitologie et de Mycologie Médicale, Hospices Civils de Lyon, LYON, FRANCE
Introduction: Clinical trials are the cornerstone of drug evaluation but are difficult to perform in children; obtaining written informed consent from both parents can be challenging.
Objectives: This qualitative study aimed at identifying determinants for parents’ enrollment or not of their child in a clinical trial a randomized controlled trial evaluating the occurrence of a new chorioretinitis after a 3-month vs 12 month-treatment in newborns with moderate toxoplasmosis congenital (TOSCANE trial).
Methods: We used a Grounded Theory qualitative approach, based on semi-structured interviews with parents who were solicited some years earlier for their consent to enroll their child in the TOSCANE trial, in which 301 children aged 3 to 6 month were included between 2010 and 2017. An interview guide based on bibliographic references, expert consultations and work meetings with TOSCANE investigators were used for video interviews, conducted until saturation was reached. These were audio recorded, transcribed anonymously into a text format, and double coded (open, axial, and selective coding).
Results: Between April 2020 and April 2021, 18 interviews were conducted (9 parents who consented to the enrolment of their child, and 9 who did not). Saturation was reached after 16 interviews. Important determinants for the parents’ decision were: a) The investigator perceived as very human and competent; b) The parents’ altruism, willing to help other parents; c) Parents having a health related professional activity; and d) A strong preference for one of the treatment groups. Most of these determinants could result in a decision of enrolling the child or not. Familial context was also important in the decision process. In addition to decision triggers previously described in the literature, this study identified parents feeling guilty for toxoplasmosis transmission- as a factor mostly associated with non-consenting, and specific to the context of maternal transmission of diseases.
Conclusion: Several determinants could lead to the decision to consent to enrolment of a child. The parents' decision depends on a set of determinants related to their family history, their personality, and their perception of the disease and of research, without any of them being in the forefront. This suggests that a patient-centered approach could be applied to the context of participation in research. This would require an appropriate training of investigators.
175
Effectiveness and Safety of Tranexamic Acid in Pediatric Trauma: A Systematic Review and Meta-Analysis
Kornelsen E2, Kuppermann N3, Nishijima D3, Ren L4, Rumantir M1, Gill P1,2, Finkelstein Y1,2
1 The Hospital For Sick Children, Toronto, Canada
2 University of Toronto, Toronto, Canada
3UC Davis School of Medicine, Sacramento, USA, 4Stanford University, Palo Alto, USA
Introduction: Worldwide, trauma is a leading cause of childhood death. To-date, there are no medications proven to improve survival in pediatric trauma. Tranexamic acid (TXA) is an antifibrinolytic that has been shown beneficial in adult trauma. Based on data from clinical trials in adults, TXA has been endorsed by professional societies and incorporated into treatment protocols of severely injured children by many hospitals.
Objective: Our primary goal was to determine the effectiveness of tranexamic acid (TXA) in improving survival in pediatric trauma. we also explored its safety in this context.
Methods: MEDLINE (OVID), Embase (OVID), Cochrane Central Register databases, CINAHL (EBSCO), Web of Science (Clarivate Analytics), and grey literature sources were searched for publications reporting survival and safety outcomes in children receiving TXA in acute trauma, with no language restrictions, published until February 11, 2021. Two independent researchers assessed studies for eligibility, bias, and quality. Data on the study setting, injury type, participants, design, interventions, TXA dosing and outcomes were extracted. The primary outcome was survival in children who received TXA following trauma. Forest plots of effect estimates were constructed for each study. Heterogeneity was assessed and data were pooled by meta-analysis using a random-effects model.
Results: Fourteen articles met inclusion criteria - six single-institution and eight multicentre retrospective cohort studies. Overall, TXA use was not associated with increased survival in pediatric trauma (adjusted odds ratio [aOR]:0.61, 95% CI: 0.30–1.22) after adjustment for patient-level variables, such as injury severity (Figure 1). Increased survival was documented in the subset of children experiencing trauma in combat settings (aOR: 0.31, 95% CI: 0.14–0.68). There were no differences in the odds of thromboembolic events (OR 1.15, 95%CI: 0.46–2.87) in children who received TXA versus not.
Conclusions: The utility of TXA in children with trauma is unclear. Guidelines supporting TXA use in pediatric trauma may not be based on the available evidence of its use in this context. Rigorous trials measuring survival and other meaningful outcomes and exploring optimal TXA dosing are urgently needed.
Tabel/Image
Pharmacogenetics / omics
4
Effect of CYP2C9, CYP2C8 gene polymorphisms on the efficacy and safety of analgesia with ketoprofen in patients after cardiac surgery
Shatskiy D1, Morozova T1, Shikh N1, Shikh E1, Andrushchyshina T1, Lukina M1, Kachanova A2, Denisenko N2, Sychev D2
1 I.M. Sechenov First Moscow State Medical University (Sechenov University), Moscow, Russian Federation
2 Russian Medical Academy of Continuous Professional Education, Moscow, Russian Federation
Introduction: Conducting cardiac interventions has to do with post-sternotomy pain syndrome. A lot of postoperative complications, including infections and thrombosis, may be caused by pain syndrome. Ketoprofen is widely used non-steroidal anti-inflammatory drug for postoperative analgesia in cardiac surgery. Cytochrome P-450 is the enzyme associated with metabolizing of most of NSAIDs. Gene polymorphisms may have impact on metabolism of NSAIDs by CYP2C9 and CYP2C8 enzymes.
Objectives: Evaluation of the influence of CYP2C9, CYP2C8 gene polymorphisms on the efficacy and safety of postoperative analgesia with ketoprofen in patients with coronary artery disease after cardiac surgery.
Methods: This study included 90 patients. Numeric Rating Scale (NRS) was used for measure of pain intensity. Gastrointestinal Symptom Rating Scale (GSRS) was used for evaluation of severity of dyspepsia. Acute kidney injury was determined by Kidney Disease Improving Global Outcomes criteria. Polymerase chain reaction was performed for determination of CYP2C9*3 (1075А>С) rs1057910, CYP2C8 (T>C) rs10509681 gene polymorphisms.
Results: There were not deviations from the Hardy–Weinberg equilibrium for CYP2C9*3 rs1057910, CYP2C8 rs10509681. Significantly lower pain intensity was shown in patients with the CYP2C9*3 AC: 7,06 ± 2,11 and 5,46 ± 1,39 points (p=0,003), 6,94 ± 2 and 5.38 ± 1,4 points (p=0,04), 5,84 ± 2,17 and 4,69 ± 1,03 points (p=0,04), 4,41 ± 1,77 and 3,3 ± 1.84 points (p=0,02). There were no significant differences in pain intensity by the NRS in patients with different genotypes for CYP2C8 rs10509681. GSRS score was significantly higher in patients with CT genotype, than with TT genotype for CYP2C8 rs10509681: 22,6 ± 7,64 and 18,97 ± 4,25 (p=0,038). There were no significant differences in acute kidney injury frequency in patients with CYP2C9*3 rs1057910, CYP2C8 rs10509681 gene polymorphisms.
Conclusion: CYP2C9*3 rs1057910 gene polymorphism affect efficacy of postoperative analgesia with ketoprofen in patients with coronary artery disease after cardiac surgery. Further pharmacokinetics trials are needed for details.
Tabel/Image
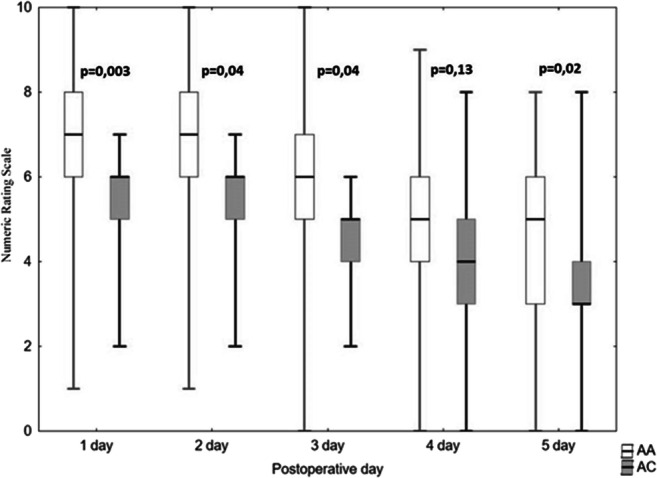
25
Toxicity and Therapy Outcome Associations in High-grade Serous Ovarian Cancer
Deng F1,2, Laasik M3, Salminen L3, Lapatto L4, Huhtinen K4,5, Li Y4, Hautaniemi S4, Hynninen J3, Niemi M1,2,6, Lehtonen R4,7
1 Department of Clinical Pharmacology, Faculty of Medicine, University of Helsinki, Helsinki, Finland
2 Individualized Drug Therapy Research Program, Faculty of Medicine, University of Helsinki, Helsinki, Finland
3 Department of Obstetrics and Gynecology, Turku University Hospital and University of Turku, Turku, Finland
4 Research Program in Systems Oncology, Research Programs Unit, Faculty of Medicine, University of Helsinki, Helsinki, Finland
5 Institute of Biomedicine and FICAN West Cancer Centre, Turku University Hospital and University of Turku, Turku, Finland
6 Department of Clinical Pharmacology, HUS Diagnostic Center, Helsinki University Hospital, Helsinki, Finland
7 Research Program in Tumor Genomics, Research Programs Unit, Biomedicum Helsinki, University of Helsinki, Finland, Helsinki, Finland
Background: High-grade serous ovarian cancer (HGSOC) is the most prevalent subtype of epithelial ovarian cancers. It is usually diagnosed in advanced stage and has a poor 5-year survival rate of 43%. In the advanced stage, HGSOC is treated with debulking surgery followed by chemotherapy. Adverse effects are the major limiting factor in combinatorial chemotherapies in cancer patients.
Objective: The purpose of the study was to investigate the genetic association between selected candidate polymorphisms, and chemotherapy-induced toxicity and therapeutic outcome in a Finnish HGSOC patient cohort.
Methods: The cohort consisted of 101 patients with histologically confirmed HGSOC, who completed their primary carboplatin-paclitaxel therapy. We designed a multiplex custom single nucleotide polymorphism genotyping assay, genotyped 19 candidate variants, and applied case-control association statistics and the log-rank Mantel-Cox test.
Results: We observed three significant, multiple-testing corrected genetic associations: LIG3 rs1052536 T allele associated with an increased risk of grade 1-3 neuropathy (p = 0.03) and SLCO1B3 rs1052536 A allele with a reduced overall risk of any grade 3-5 toxicities (p = 0.047). GSTP1 rs1695 G allele associated with complete response in the first-line chemotherapy when compared to partially responding and progressive cases (p = 0.02). In survival analysis, ABCB1 rs2032582 TT genotype was associated with shorter overall survival (uncorrected p = 0.02), and OPRM1 rs544093 GG and GT genotypes with shorter platinum-free interval (uncorrected p = 0.03) and progression-free survival (uncorrected p = 0.01).
Conclusion: Results suggest that LIG3 and SLCO1B3 variants contribute to the risk of adverse effects, and the GSTP1 variant may affect the first-line treatment response. Moreover, ABCB1 and OPRM1 variants may have an impact on prognosis. Further studies with larger numbers of patients are warranted to verify the clinical relevance of these findings.
39
Improvement of postoperative nausea and vomiting risk prediction scores through identification of genetic susceptibility markers
Gloor Y1, Czarnetzki C2,3, Curtin F1,4, Gil-Wey B2, Tramèr M2, Desmeules J1
1 Division of Clinical Pharmacology and Toxicology, Department of Anesthesiology, Pharmacology, Intensive care and Emergency medicine, Geneva University Hospitals (HUG), Geneva, Switzerland
2 Division of Anesthesiology, Department of Anesthesiology, Pharmacology, Intensive care and Emergency medicine, Geneva University Hospitals (HUG), Geneva, Switzerland
3 Division of Anesthesiology, Department of Anesthesiology, Intensive care and Emergency medicine, Ospedale Regionale di Lugano, Lugano, Switzerland
4 Personalized Health Programs, Swiss Federal Institute of Technology Zurich (EPFZ), Zurich, Switzerland
Introduction: Postoperative nausea and vomiting (PONV) are frequently occurring adverse effects following surgical procedures, affecting around 30% of the patients despite predictive risk scores and a pallet of antiemetic treatments. PONV affects the well-being of surgical patients and increases the burden of post-operative care.
Objectives: The aim of the current study is to characterize selected genetic risk factors of PONV to improve the identification of at risk patients.
Methods: We genotyped 601 patients followed during the first 24 hours after surgery for apparition of PONV symptoms in the absence of any antiemetic prophylaxis. Patients with a very high risk of PONV or those undergoing procedures requiring strict antiemetic controls were excluded from the cohort. We examined 28 individual single nucleotide polymorphisms (SNPs) located around 13 different genes. In addition, we predicted the activity of 6 liver cytochromes (CYP) based on their described genetic polymorphisms. We assessed the impact of each of those SNP and CYP enzymatic activities on the occurrence of PONV using a multivariate logistic regression analysis considering known risk factors as co-variables (age, gender, smoking status, type of surgery, history of PONV, use of volatile anesthetics and perioperative opioid dosage).
Results: The overall PONV incidence in the cohort was 44%. We found an association between occurrence of PONV and five polymorphisms, four located around the Type 3B serotonin receptor gene (HTR3B) and the last one in the promoter region of the neurokinin receptor TACR1. Modelling of the data showed that integrating the genetic information of HTR3B SNP rs3782025 in the risk score enhanced our prediction potential.
Conclusion: Our genetic study confirms the importance of specific genetic predisposition in the occurrence of PONV and suggest that integration of rs3782025 genotype in preoperative risk assessment may help targeting prophylaxis towards patients at risk of PONV.
55
Polymorphisms in glucose homeostasis candidate genes affect cardiovascular outcomes of patients with diabetic nephropathy
Mota-Zamorano S1,2, González L1, González-Rodríguez L1,2, Robles N2,3, Valdivielso J2,4, Arévalo-Lorido J5, López-Gómez J6, Gervasini G1,7
1 Department Of Medical And Surgical Therapeutics, Medical School, Universidad De Extremadura, Badajoz, Spain
2 RICORS2040 Renal Research Network, Spain
3 Service of Nephrology, Badajoz University Hospital, Badajoz, Spain
4 Vascular and Renal Translational Research Group, UDETMA, IRBLleida, Lleida, Spain
5 Service of Internal Medicine, Badajoz University Hospital, Badajoz, Spain
6 Service of Clinical Analyses, Badajoz University Hospital, Badajoz, Spain
7 Institute of Biomarkers of Molecular and Metabolic Pathologies, Universidad de Extremadura, Badajoz, Spain
Introduction: Patients with diabetic nephropathy (DN), the greatest contributor to end-stage kidney disease, not only show a disproportionately higher risk of cardiovascular disease (CVD) than the general population, but this risk is also greatly elevated in comparison with diabetics who have normal renal function.
Objetives: Our aim was to determine whether common, functional SNPs in genes involved in glucose homeostasis are associated with the risk of DN and/or CV traits and events in these patients.
Methods: We screened 824 subjects, 318 patients with DN (stage 3 or higher) and 506 controls with normal renal function for 23 SNPs in six genes related to glucose homeostasis. These were four genes coding for glucose transporters (SLC2A1, SLC2A2, SLC5A1 and SLC5A2) and two genes (KCNJ11 and ABCC8) key for insulin secretion. Genetic analyses were carried out by allelic discrimination using a customized panel (TaqMan® OpenArray Genotyping). Participants were followed for a median of 46.46 (4.11) months. Regression models including confounding variables were utilized to identify genetic associations with the risk of DN and renal and CV parameters and outcomes in the DN patients.
Results: Common carotid intima media thickness median values were higher in patients carriers of ABCC8 rs3758953 and rs2188966 than in non-carriers [0.78(0.25) vs. 0.72(0.22) mm, p<0.05 and 0.79(0.26) vs. 0.72(0.22) mm, p<0.05], respectively. Furthermore, KCNJ11 rs5219 and ABCC8 rs1799859 were linked to presence of plaque in these patients [OR=0.54(0.29-1.03), p<0.05 and 1.89(1.03-3.46), p<0.05, respectively]. After adjusting for traditional risk factors, SLC2A2 (GLUT1) rs8192675 and SLC5A2 (SGLT2) rs9924771, were associated with better [OR=0.48(0.29-0.81),p<0.01] and worse [OR=1.86(1.09-3.15),p<0.05] CV event-free survival. With regard to renal function, two SLC2A1 SNPs, rs841848 and rs710218, and the rs3813008 variant in SLC5A2 were associated with eGFR values. Median (interquartile range) values of carriers vs. non-carriers were 30.41 (22.57) vs. 28.25 (20.10), p<0.05; 28.95 (21.11) vs. 29.52 (21.66), p<0.05 and 32.03 (18.06) vs. 28.14 (23.06) ml/min/1.73 m2, p<0.05, respectively. Furthermore, ABCC8 rs3758947 was significantly associated with higher albumin-to-creatinine ratios [193.5 (1139.91) vs. 160 (652.90) mg/g creatinine]. No SNPs were found to affect DN risk.
Conclusion: Polymorphisms in genes coding for proteins and transporters key for glucose homeostasis may affect CV-related outcomes in patients with DN. Specifically, polymorphisms in genes coding for the two subunits that form the KATP channel regulating insulin secretion (ABCC8 and KCNJ11) were significantly associated with atherosclerosis in these subjects. In addition, variability in GLUT2, also involved in insulin secretion, and in the glucose transporter SGLT2 resulted in altered CV event-free survival, which is particularly relevant in a pathology such as DN, which confers an exceptionally high CV risk
59
Individualized genotype-guided treatment with siponimod
Ramírez-García A1, Caballero-Bermejo A1, Darnaude Ximénez I1, Sancho-López A1, Payares-Herrera C1, Ruiz-Antorán B1, Avendaño Solá C1
1 Clinical Pharmacology Department. Hospital Universitario Puerta De Hierro Majadahonda, Madrid, Spain
Introduction: Siponimod was granted marketing authorization for treatment of secondary progressive multiple sclerosis provided that prior CYP2C9 genotyping was performed to adjust titration schedule and maintenance dose. After commercial availability in April 2021, we implemented a clinical pharmacology consultation following reduced activity *2 and *3 alleles genotyping.
Objectives: To present our preliminary results of genotype-guided siponimod treatment recommendations
Methods: Retrospective analysis of all requests in our first year experience
Results: From April 2021 to January 2022 we conducted 34 pre-emptive pharmacogenetic tests. 9/34 patients (26.5%) presented a CYP2C9 variant (8 patients with *1/*3, intermediate metabolizer with activity score 1 and 1 patient with *2/*3, poor metabolizer with activity score 0.5) that required dose-adjustment. Our results differ slightly from the frequencies expected in Caucasian population, obtaining *1*1,*1/*2,*2/*2,*1/*3,*2/*3 and *3/*3 in 16/34 (47 %), 9/34 (26.5%), 0%, 8/34 (23.5%), 1/34 (3%) and 0% of the samples, respectively. No treatment was prevented because of genotyping that would contraindicate it. 12 patients initiated treatment up to this date, with an average follow-up of 5.13 months from start. Lymphopenia reaction was observed in 100%, 9/12 reaching grade 3-4 (2 eventually requiring suspension and 2 dose adjustment). The relative reduction of baseline lymphocyte count was 71.52% far superior to what was found in RCT (20-30%). Lymphopenia was apparently unrelated to genotype, 11/12 were normal metabolizers and received the standard 2 mg and 1 had an intermediate phenotype and received the 1 mg posology accordingly) or basal lymphopenia (only 4/12).
Conclusion: More than one quarter of the target population benefited from CYP2C9 genotyping for dose-adjustment. However, this genotype-guided dose-adjustment apparently did not prevent the appearance of a generalized intense lymphopenia that motivated further down-titration or discontinuation in one third of treated patients. This fact triggered the planning of a prospective registry to investigate individual factors other than the identified allelic variants that could suppose a risk factor for lymphopenia in the treatment with siponimod.
67
Clinical utility and feasibility of pharmacogenetic testing in psychiatry
Ramírez-García A1, Caballero-Bermejo A1, Mejia-Abril G2, Zubiaur P2, Darnaude Ximénez I1, Payares-Herrera C1, Navares-Gomez M2, Sancho-López A1, Ruiz-Antorán B1, Abad-Santos F2, Avendaño-Solá C1
1 Clinical Pharmacology Department. Hospital Universitario Puerta De Hierro Majadahonda (Madrid, Spain)
2 Clinical Pharmacology Department. Hospital Universitario La Princesa (Madrid, Spain)
Introduction: There is a strong relation between genetic variants of CYP2D6 and CYP2C19, their enzymatic activity and plasma concentrations of antidepressants and antipsychotics. Although clinical utility and feasibility of pharmacogenetic testing in guiding psychiatric therapy is controversial, its implementation was demanded by clinical psychiatrists.
Objectives: To present the experience of a Clinical Pharmacology (CP) Department at a tertiary hospital implementing therapy advice based on CYP2D6 and CYP2C19 genotyping and the current evidence-based dosing guidelines for using pharmacogenetics for antidepressants/antipsychotics (CPIC/DPWG)
Methods: Retrospective analysis of all requests in our 3-year experience
Results: From 2019 to 2021, genotyping was requested for only 13 patients. The average age was 16 (range 8-34). Most patients had multiple concomitant psychiatric conditions (n=7, including autism, psychosis, manic disease, schizophrenia, retardation disorders, obsessive-compulsive disorder and behavioural disorder). Most frequent condition was autism (N=6). All patients had ≥2 psychotherapeutic agents.
Main trigger for request was the combination of inefficacy and appearance of adverse effects (n=6), followed by isolated appearance of adverse effects (n=5) or inefficacy (n=1). There was one request prior to therapy initiation with the goal of long-term treatment planning.
Most (10) had a CYP2D6 normal metabolizer phenotype and 3 intermediate. For CYP2C19, findings were normal (7), intermediate (3), poor (1) and ultrarapid (2). Only 5/13 patients (38.5%) had a gene alteration for which the actual psychiatric treatment could have been affected; 2 of them also received an interaction recommendation. Other 2 patients received treatment recommendations based on interactions but with no pharmacogenetic alteration. In total, 7/13 patients (53.9%) benefited from CP advice regarding their psychiatric treatment.
Conclusion: Psycotherapy-related genotyping is currently infrequent and reactive rather than pre-emptive, more frequently requested by child and adolescent psychiatrists in patients with multiple psychiatric comorbidities and difficult control even with polymedication. More than half of the patients benefited from CP advice regarding their psychiatric treatment but only one third of suboptimal treatment or adverse events could be explained by a genetic alteration.
98
CYP2C8 pharmacogenetic risk profiles affecting enzymatic H2O2 production and uncoupling of substrate metabolism ex vivo
Yamoune S1,2, Stingl J1
1 Institute of Clinical Pharmacology, University hospital of RWTH, Aachen, Germany
2 Federal institute for drugs and medical devices, research division, BfArM, Bonn, Germany
Background: Pharmacogenetic polymorphisms in drug metabolizing enzymes as cytochrome P450 monooxygenases affect individual drug exposure. Depending on the location of respective polymorphisms within the protein, either folding, substrate binding or catalytic capacity can be altered. CYP2C8 activity is determined by a genetic polymorphism leading to decreased metabolism of drugs and endogenous CYP2C8 substrates as arachidonic acid. Alterations within the catalytic cycle of CYP450 enzymes can lead to decomposition of reactive intermediates and release of dioxide- and peroxide-anions, which in turn cause oxidative stress.
Objectives: The aim of this study was to characterize CYP2C8, CYP2C8*2 and CYP2C8*3 functionality und uncoupling susceptibility of human recombinant enzyme.
Methods: Enzyme functionality was determined by quantification of Amodiaquine (AQ) N-desethylation (NDAQ) over time by isolated recombinant CYP2C8, CYP2C8*2, CYP2C8*3. Using HPLC-MS/MS, AQ and NDAQ levels were determined and the respective peak area was quantified and put to ratio. Determination of H2O2 levels was performed using the amplex red/ horse radish peroxidase assay, allowing the determination of H2O2 molecules based on amplex red oxidation to resorufin with a stoichiometry of 1:1.
Results: Quantification of NDAQ formation by isolated recombinant CYP2C8, CYP2C8*2 and CYP2C8*3 revealed only 20% of metabolite formation by CYP2C8*2 and 80% of metabolite formation by CYP2C8*3 compared to the levels reached by CYP2C8 wildtype enzyme. Addition of H2O2 approximately quadrupled the metabolite formation in CYP2C8*2 and CYP2C8*3 reactions. Determination of H2O2 concentrations showed increased peroxide production in CYP2C8*3, compared to the other two enzyme variants. Addition of AQ recovered the increased peroxide levels, whereas addition of arachidonic acid did not result in the same effect.
Conclusion: CYP2C8*2 and CYP2C8*3 enzyme variants show increased substrate specific uncoupling of the CYP450 reaction pathway. Increased turnover rates in presence of μM H2O2 levels indicate substrate oxidation via H2O2 shunt pathway, distinctively characterized in CYP450 enzyme catalysis.
112
Regulation of cytochrome P450 enzyme activity and metabolism by estrogen receptor mediated steroid hormone effects
Müller J1, Hoffmann M1, Folliot A1, Yamoune S1,2, Stingl J1
1 Uniklinik RWTH Aachen, Aachen, Germany
2 Federal Institute for Drugs and Medical Devices, Bonn, Germany
Introduction: Drug metabolism is influenced by estrogen in several ways. Beside the induction through nuclear receptors, which is taking place in high estrogen or other steroid hormone concentrations, estrogens also modulate CYP activity by action on estrogen receptors α and β (ERα/β). They are expressed throughout the body, and especially in hormone receptor positive breast cancer have been shown to be involved in regulation of CYP1B1 and 2B6. The reduced activity variant CYP2B6*6 is linked to increased breast cancer risk, while increased CYP1B1 activity is linked to cancer development and progression.
Objectives: The objective of this study was to elucidate the role of ERα versus β in the modulation of CYP1B1 and 2B6 metabolic activity.
Methods: To study the regulation of CYP enzymes via estrogen we used the breast cancer cell lines T47D (ERα positive) and MDA-MB-231 (ERα negative, triple negative). We treated the cells with estradiol (E2) and measured differences in transcription of ERα, ERβ, CYP1B1 and 2B6 by quantitative real-time PCR. To discriminate the role of ERα and β in the regulation of CYP1B1 and 2B6, and the interplay between the receptors, we overexpressed both in ERα positive and negative cell lines.
Results: CYP1B1 was transcriptionally upregulated by E2 in ERα positive and negative cell lines. However, CYP2B6 was only detected in ERα positive cells and treatment with E2 led to a reduction of CYP2B6 mRNA levels around 0.5-fold, with a concomitant ERβ upregulation of about 2.5-fold. Conversely, prior estrogen-starvation of ERα-positive cells, led to an increase of CYP2B6 mRNA levels of roughly 2-fold. We then studied the impact of the ERα and β ratio on CYP regulation. Transient overexpression of ERα in T47D cells led to the upregulation of ERβ, while ERβ overexpression reduced ERα mRNA levels. Interestingly, there was no differential effect on CYP1B1 and 2B6 mRNA levels with either ERα or ERβ overexpression.
Conclusion: In breast cancer cells, regulation of CYP enzymes by E2 is mediated by ERα and β. We demonstrate that the withdrawal of estrogens has a direct influence on the outcome of E2 signaling by the example of CYP2B6 induction. E2 treatment and transient ERα overexpression induced ERβ, while ERβ overexpression reduced ERα, demonstrating an interplay between both receptors. This suggests that an altered ratio of ERα and β may lead to different outcomes in E2-mediated signaling.
139
Importance of Phenotype-Guide-Dosing of Voriconazole in pediatric hematologic patients: a case report
Rodríguez Ramallo H1, Rodriguez Ramallo H2, Álvarez del Vayo Benito C2, Sánchez Martín A3, Pérez Hurtado J2, Merino Bohorquez V4
1 Hospital Universitario Reina Sofía, Córdoba, Spain
2 Hospital Universitario Virgen del Rocío, Sevilla, Spain
3 Hospital Universitario Virgen de las Nieves, Granada, Spain
4 Hospital Virgen Macarena, Sevilla, Spain
Introduction: Voriconazole is a broad-spectrum antifungal agent used for the treatment of various fungal infections. Therapeutic drug monitoring (TDM) is highly recommended due to this drug interpatient variability. CYP2C19 polymorphisms mainly cause highly variable and non-linear pharmacokinetics of voriconazole.
Objectives: We aim to illustrate the difficulties of voriconazole treatment in rapid metabolizers.
Methods; We present the case of an 8-years-old caucasian girl (19 kg) diagnosed with acute myelocytic leukemia. She was treated according to NOPHO-AML protocol.
During the induction phase, she developed bilateral pulmonary aspergillosis that was initially treated with caspofungin 50 mg/m2 and voriconazole 9 mg/kg/12h.
Throughout the following six months, therapeutic drug monitoring resulted in subtherapeutic voriconazole concentrations prompting multiple dose adjustments.
The patient’s initial voriconazole dose of 171 mg twice daily produced a trough level of 0.31 mg/L, leading to a dose increase to 200 mg twice daily. On this dose, the voriconazole trough level was 0,37 mg/L. Due to the subtherapeutic voriconazole serum concentration reached, the genotyping of CYP2C19 (* 2, * 3, and * 17) was requested.
Results: The patient had the CYP2C19 * 1/* 17 genotype corresponding to an ultrarapid metabolizer (UM) activity phenotype, which explained the difficulty of reaching therapeutic levels of voriconazole. According to the Clinical Pharmacogenetics Implementation
Consortium guidelines, the probability of reaching therapeutic concentrations is small, and it is recommended to choose a drug not metabolized by CYP2C19.
For this patient, it was decided to optimize the treatment with increased doses guided by TDM. A subsequent increase to 300 mg twice a day led to undetectable levels, inciting a dose increase to 375 mg/12 h ( 20 mg/kg/12 h), reaching the target concentration (4,8 mg/L) finally. However, this patient maintained high-dose therapy for months, resulting in significant hepatotoxicity, eventually leading to the discontinuation of voriconazole.
Conclusion: The prescription of voriconazole in pediatrics clinical practice should be personalized according to the CYP2C19 phenotype, followed by TDM to guide dose adjustment.
CYP2C19 UM requires high doses of voriconazole to achieve therapeutic concentrations with an associated high risk of adverse events. For these patients, the balance risk/benefit should be evaluated.
164
V89L(rs523349) and A49T(rs9282858) variations on SRD5A2 gene on dutasteride efficacy and safety in bladder cancer patients: A pilot study
Incir C1, Gumustekin M1, Korhan P2, Ozer M3, Sarıkaya A3, Bozkurt O3, Atabey N2, Esen A3, Tuncok Y1
1 Dokuz Eylul University, School of Medicine, Department of Medical Pharmacology, Izmir, Turkey
2 Dokuz Eylul University, Izmir Biomedicine and Genom Center, Izmir, Turkey
3 Dokuz Eylul University, School of Medicine, Department of Urology, Izmir, Turkey
Introduction: Human steroid 5α-reductase 2 (SRD5A2) coded by SRD5A2 gene is an enzyme that catalyzes the reduction of testosterone to dihydrotestosterone. Dutasteride, an SRD5A2 inhibitor, is a widely used antiandrogen for the treatment of benign prostate hyperplasia. Multiple variations have been identified in the SRD5Ar gene. Some of these variations may affect the efficacy and safety of SRD5A2 inhibitors. Dutasteride has also been investigated for intermediate and high-risk non-muscle-invasive urothelial bladder cancer treatment with the combination of BCG (Bacillus Calmette-Guerin).
Objectives: The study aims to evaluate the potential impact of V89L (rs523349) and A49T (rs9282858) variations on the SRD5A2 gene on dutasteride efficacy and safety in bladder cancer patients that have been enrolled in Phase 2 clinical trial entitled "Efficacy and safety of a 5-alpha reductase inhibitor, dutasteride, added to Bacillus Calmette Guerin (BCG) immunotherapy in the prevention of recurrence and progression of intermediate and high risk non-muscle invasive bladder cancer: A single-arm, Phase 2 clinical trial"
Methods: Twenty-one patients on BCG and dutasteride in the Phase 2 clinical trial were included in the study. Genomic DNA was obtained from whole blood samples, and evaluation of V89L (rs523349) (G>C) and A49T (rs9282858) (C>T) variations on the SRD5A2 gene was performed by using TaqMan SNP Genotyping Assay. The severity of the adverse events was graded by the United States National Cancer Institute- Common Terminology Criteria for Adverse Events 5.0. The causality assessment of adverse drug reactions was performed using Liverpool Causality Assessment Tool, Naranjo Algorithm, and World Health Organization-Uppsala Drug Monitoring Centre Causality Assessment System. The response to dutasteride was evaluated as the presence of bladder cancer recurrence. The Chi-Square test was used for testing the relationship between categorical variables. P values of <0.05 were considered significant.
Results: All patients were homozygous GG for V89L variation on the SRD5A2 gene. Regarding the A49T variation, only one patient was homozygous CC, 8 patients were homozygous TT and 12 patients were heterozygous TC. One of the 8 patients (%12) was homozygous TT and 3 of 12 patients (%25) were heterozygous TC had bladder cancer recurrence. There was no statistically significant difference between bladder cancer recurrence and A49T variation (p=0.803). None of the adverse events were associated with dutasteride treatment whereas some of the adverse events, mostly urinary tract infections, were associated with the BCG. Other adverse events were upper respiratory tract infections, COVID-19, abdominal pain, vomiting, and loss of appetite. Serious adverse events were coronary artery disease, dyspnea, hypotension, and urethral stricture. None of the serious adverse events were associated with dutasteride or BCG treatment.
Conclusion: Neither V89L nor A49T variation on the SRD5A2 gene was found to be associated with the efficacy and safety of dutasteride in medium and high-risk bladder cancer patients. Further studies of these variations with larger sample sizes and/or healthy control groups may lead to a better understanding of the impact of these variations on the efficacy and safety of dutasteride.
168
Following a case: The role of the systematic study of the pharmacogenetics of tamoxifen, an open discussion
Darnaude-Ximénez I1, Ramírez-García A1, Caballero-Bermejo A1, Payares-Herrera C1, Sancho-López A1, Ruiz-Antorán B1, Mejía-Abril G2, Zubiaur P2, Navares-Gomez M2, Abad-Santos F2, Avendaño-Solá C1
1 Clinical Pharmacology Department Hospital Universitario Puerta De Hierro, Majadahonda, Spain
2 Clinical Pharmacology Department Hospital Universitario La Princesa, Madrid, Spain
Introduction: Individual responses to hormonal treatments used in breast cancer present a great variability due, in part, to genetic polymorphisms. For tamoxifen, CYP2D6 can predict the degree of conversion into endoxifen, the desired metabolite due to its higher activity responsible for the therapeutic effect. Routine CYP2D6 testing is currently not recommended, however, an altered CYP2D6 function could affect the outcome in patients treated with tamoxifen, in particular when other factors coexist.
Objectives: To describe a case in which the CYP2D6 pharmacogenetic study led to a change of antineoplastic therapy and to discuss the adequacy of implementing a systematic study of said polymorphism prior to starting tamoxifen treatment.
Methods: We present a case of a patient for whom we assessed the risk of pharmacological interactions with tamoxifen. Due to the need to use of CYP2D6 inhibitors long-term, a Pharmacogenetic test was performed prior to starting tamoxifen therapy
Results: The case concerned a 44-year-old woman with bipolar disorder and obsessive-compulsive disorder (treated with lithium and sertraline) diagnosed of breast cancer, candidate to receive treatment with tamoxifen. In addition to assessing potential pharmacological interactions, we also performed a pharmacogenetic study of CYP2D6, finding genotype 1*/4* (intermediate metabolizer), which is associated with reduced efficacy, even without other coexisting risk factors. A recommendation to use a different oncologic therapy was issued.
Conclusions: Current clinical guidelines do not recommend systematic CYP2D6 testing as a predictor of tamoxifen response, outside of a clinical trial setting. In our case, a patient with other risk factors for reduced tamoxifen effect (unavoidable use of CYP2D6 inhibitors) benefited from the pharmacogenetic test: results identified a variant that could further reduce efficacy and increase the risk of relapse, leading to a treatment change that might have also changed the patient’s outcome. Therefore, while universal testing may not be necessary, it may play a role in those patients at risk of reduced efficacy due to other coexisting factors. In that particular setting, pharmacogenetic testing may contribute to identify the best treatment for an individual patient.
215
Preemptive genotyping of DPYD in routine clinical practice: experience and results at Hospital Universitario de La Princesa
Mejía Abril G1, Navares Gómez M1, Campodónico D1, Zubiaur Precioso P1,2, Soria Chacartegui P1, Abad Santos F1,2
1 Clinical Pharmacology Department, La Princesa University Hospital, Instituto Teófilo Hernando, Instituto de Investigación Sanitaria La Princesa (IP), Universidad Autónoma de Madrid (UAM), Madrid, Spain
2 Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas (CIBERehd), Madrid, Spain
Introduction: Fluoropyrimidines are used for the management of common malignancies particularly colon and breast cancer, either as a single agent or in combination with other cytotoxic agents. The dihydropyrimidine dehydrogenase (DPD) enzyme is the principal enzyme involved in fluoropyrimidines metabolism. On 30 April 2020, the European Medicines Agency (EMA) recommended that patients should be genotyped for DPYD*2A (c.1905+1G>A), *13 (c.1679T>G), rs67376798 (c.2846A>T) and HapB3 (c.1236G>A), to identify intermediate (IM) and poor metabolizers (PM), who are at increased risk of severe toxicity during treatment with fluoropyrimidines. It is now accepted that treatment with these drugs is contraindicated in PMs. In addition, in IMs a reduced initial dose should be considered.
Objectives: To describe the results of DPYD genotyping in patients treated with fluoropyrimidines in a tertiary hospital in Spain.
Methods: Retrospective analysis of all requests in our 9-year experience.
Results: The genotyping of 278 patients was requested between 2013 and 2022. Prior to the EMA recommendation, 13 determinations were performed (1.86 requests/year), mainly motivated by fluoropyrimidine-associated toxicity. Since 2020, previous to treatment, an average of 10 requests per month (122 per year) have been received. In total, 15 patients (5.40%) carried a decreased or no-function allele corresponding to a DPD intermediate metabolizer phenotype: 5 patients (1.80%) carried the *2A allele, 8 (2.88%) carried the *HapB3 allele and 2 (0.72%) carried the rs67376798 allele. Only one patient (0.36%) carried two decreased function alleles (rs67376798/*HapB3).
Conclusion: The implementation of pharmacogenetics is a safe practice that enables the optimization of treatment in cancer patients. Preemptive genotyping of DPYD is required in the fluoropyrimidines label; since the recommendation by the competent authorities, the number of requests has increased exponentially, preventing the possible occurrence of serious adverse reactions. To avoid toxicity in one patient, it is necessary to genotype 18 patients, which is very cost-effective.
225
Risk of acute hemolytic anemia in COVID-19 patients treated with hydroxychloroquine in the context of G6PD Deficiency
Gómez A1, Villapalos-García G1, Zubiaur P1, Campodónico D1, Casajús A1, Navares-Gómez M1, Soria-Chacartegui P1, Mejía-Abril G1, Parra R1, Abad-Santos F1
1 Hospital Universitario La Princesa, Madrid, Madrid, Spain
Introduction: Hydroxychloroquine is a well-known drug, classically used for the prevention and treatment of malarian non complicated disease and immune-related conditions as rheumatoid arthritis, widely prescribed for the off-label management of COVID-19 patients in 2020. Glucose-6-phosphate dehydrogenase (G6PD) deficiency is associated with development of acute hemolytic anemia (AHA) induced by a number of drugs, as indicated in the FDA label for hydroxychloroquine. The Clinical Pharmacogenetics Implementation Consortium (CPIC) published a guideline on rasburicase-induced AHA in G6PD deficient patients; for hydroxychloroquine, the level of evidence for this association remains unclear.
Objectives: We aim to investigate the influence of G6PD deficiency on the incidence of AHA in COVID-19 patients who were prescribed hydroxychloroquine between March and April 2020 at Hospital Universitario de La Princesa.
Methods: This study was designed as a retrospective observational case-control study. Cases were considered individuals carrying G6PD Asahi/Hechi (rs1050828-T), A- (rs1050828-T + rs1050829-C), Mediterranean (rs5030868-A) or Sierra Leona (rs1050829-C) alleles (i.e., patients with partial or total enzyme deficiency). Controls were patients with G6PD normal phenotype matched with cases according to age, disease severity, sex and race (5 controls per case). Clinical data were retrieved from medical records of Hospital Universitario de La Princesa. AHA was considered if diagnosed or suspected in the clinical record as decrease > 1g/dl in hemoglobin during treatment with no other obvious causes.
Results: Five patients out of 817 (4.05 %) with partial G6DP deficiency were identified, four with the *1/*A- diplotype and one with the *1/*Sierra Leona. All of them were women and the average age was 60.4 years; four were original from República Dominicana and one from Argentina. Controls selected were all American women with similar age and same level of disease severity as the case matched. From a total population of 30 patients (5 cases and 25 controls), data from the 5 cases were analyzed so far, and we found 2 cases out of 5 with anemia during treatment. Further studies are being made, controls information has not been reviewed yet.
Conclusion: Subjects with G6PD deficiency may be at increased risk of hemolysis when receiving hydroxychloroquine.
236
Estimation of Impact of Implementing Pre-emptive Pharmacogenetic Testing in Hospital Treated Patients in Finland
Kulla N1,2, Cajanus K1,2, Kytö V3,4, Niemi M5,6,7, Tornio A1,2
1 Integrative Physiology and Pharmacology, Institute of Biomedicine, University Of Turku, Turku, Finland
2 Unit of Clinical Pharmacology, Turku University Hospital, Turku, Finland
3 Heart Center, Turku University Hospital and University of Turku, Turku, Finland
4 Research Center of Applied and Preventive Cardiovascular Medicine, University of Turku, Turku, Finland
5 Department of Clinical Pharmacology, Individualized Drug Therapy Research Program, University of Helsinki, Helsinki, Finland
6 HUS Diagnostic Center, Helsinki University Hospital, Helsinki, Finland
7 Individualized Drug Therapy Research Program, University of Helsinki, Helsinki, Finland
Introduction: Drugs associated with clinically actionable pharmacogenetic prescribing guidelines are frequently used. A potential target population to implement pre-emptive pharmacogenetic testing is hospitalized patients who are already in contact with healthcare system and use drugs more commonly than the general population. Real-world data, however, on drug utilisation in the intended target population is needed to evaluate the impact and cost-effects of pharmacogenetic testing.
Objectives: Aim of this study was to analyze the utilization of pharmacogenetically actionable drugs in hospital treated patients in a nationwide-level cohort in Finland.
Methods: Altogether 33 pharmacogenetically actionable drugs were identified from prescribing guidelines compiled by the Clinical Pharmacogenetics Implementation Consortium. Utilization of drugs of interest was investigated using register-based data in a nationwide cohort of surgical and internal medicine hospital patients. Prevalence of drug use was defined as a drug purchase during 6 months prior to hospitalisation and incidence was defined as a drug purchase during two years after hospitalization without previous purchases of the drug during the preceding year of hospitalisation. Similar analyses were performed for each 10 genes and 3 HLA-alleles of interest. Impact in Finnish population was studied by combining the drug incidence data with Finnish phenotype frequencies reported in the literature. Differences in post-discharge drug purchases between different variables such as surgical and internal medicine wards, patients’ age and hospital type were studied with Cox proportional-hazards model.
Results: During the two-year follow-up, 60% of patients discharged from surgical or internal medicine ward (n = 1.42 million) purchased at least one of the drugs of interest, and 25% of the patients purchased at least 2 drugs of interest. Most frequently initiated drugs during the two-year follow-up were ibuprofen (25.0%), codeine (19.4%), and pantoprazole (12.5%). Factors associated with a greater probability for overall drug purchases included treatment at a surgical ward (Hazard ratio [HR] 1.23, 95% confidence interval [CI] 1.22-1.23), an initial hospital period in a university hospital (HR 1.04, CI 1.03-1.04). Age between 65-80 years and >80 years was associated with lower probability of drug purchases when compared to age group 25-44 years with a HR of 0.86 (CI 0.85-0.86) and 0.58 (CI 0.57-0.59), respectively.
Conclusion: This study shows that pharmacogenetically actionable drugs are commonly used in patients discharged from hospital. Based on the phenotype frequencies from the literature, prescriptions requiring changes to drug or dose occur frequently in Finnish patients. As expected, frequent use of codeine and non-steroidal anti-inflammatory drugs explained the more frequent overall use of drugs in the surgical cohort. Analysing the factors predicting pharmacotherapy with the pharmacogenetically actionable drugs might advise in targeting pre-emptive pharmacogenetic testing.
240
Toxicokinetics and muscle related toxicity of atorvastatin in vitro
Hoste E1, Paquot A3, Nadtha P2, Muccioli G3, Haufroid V2,4, Elens L1
1 Integrated PharmacoMetrics, pharmacoGenomics and pharmacokinetics, LDRI, UCLouvain, Brussels, Belgium
2 Louvain Center for for Toxicology and Applied Pharmacology, IREC, UCLouvain, Brussels, Belgium
3 Bioanalysis and Pharmacology of Bioactive Lipids, LDRI, UCLouvain, Brussels, Belgium
4 Department of Clinical Chemistry, Saint-Luc university clinics, Brussels, Belgium
Introduction: Atorvastatin (ATV) is commonly used to fight hypercholesterolemia, a major predisposing factor for the development of cardiovascular diseases. Although generally well tolerated, patients can suffer from muscle complaints that can lead to poor compliance. However, the occurrence of this daily discomfort is difficult to predict due to its high inter-individual variability.
Objectives: Intuitively, muscle complaints were assumed to be influenced by intracellular ATV accumulation that might in turn be controlled by the balanced activities of main influx and efflux proteins expressed in the muscle tissue such as OATP2B1 and MRP1. Also, the impact of single nucleotide polymorphisms (SNPs) will be assessed in OATP2B1 and in MRP1 with the study of rs12422149 (Arg 312 Gln) and rs45511401 (Gly 671 Val), respectively. The goal is to assess the impact of both transporters, wild-type or variant, on the intracellular ATV accumulation in overexpression conditions.
Methods: HEK293 recombinant models stably overexpressing OATP2B1 (influx) and MRP1 (efflux) proteins were created either in single or double transfectant models. Initial lipofection was achieved using plasmids coding for each protein and for a respective fluorescence. Point mutations were introduced within the template plasmids c.DNA sequences through site-directed mutagenesis. ATV accumulation experiments were performed in cells that expressed the transporters previously selected in culture with an antibiotic and subsequently sorted by Fluorescence Activated Cell Sorting. Confirmation of the overexpression was obtained by fluorescence microscopy.
Results: Results confirmed the implication of both OATP2B1 and MRP1 in ATV transport in single models. Interestingly, ATV intracellular accumulation induced by OATP2B1 influx was being counteracted by MRP1 efflux in double transfectant models. Also, a decreased influx activity of OATP2B1 when affected by c.935G>A SNP was observed whereas c.2012G>T SNP affecting MRP1 appeared to increase its efflux activity.
Conclusion: Intracellular ATV accumulation is being ruled by influx and efflux proteins that can potentially be affected by SNPs. This mechanism could play a role in ATV muscle side effect development.
Tabel/Image
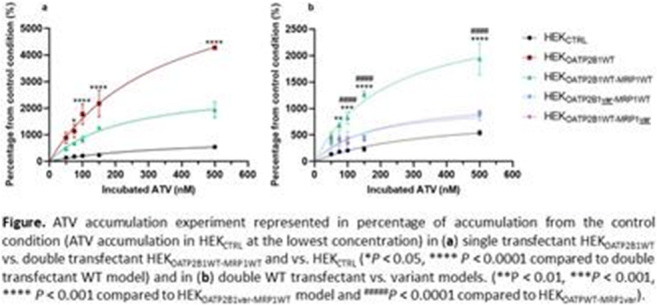
252
INFLUENCE OF CYP2D6 AND OTHER GENES’ PHENOTYPE ON THE SAFETY AND EFFICACY OF TRAMADOL IN PATIENTS WITH ACUTE POSTSURGICAL PAIN.
Casajus Rey A1, Zubiaur P1, Alday E1, Aragonés C1, Campodónico D1, Gómez A1, Navares Gomez M1, Villapalos G1, Soria Chacartegui P1, Novalbos J1, Mejía G1, Ochoa D1, Abad F1
1 Hospital de La Princesa, Madrid, Spain
Introduction: Pain is the most common physical symptom encountered in medicine. Both its perception and relief are subject to interindividual differences that affect analgesic dosing in the population. Tramadol is an analgesic drug used for the treatment of moderate to severe pain. It is metabolized in the liver primarily to M1 by cytochrome P450 (CYP) 2D6 (CYP2D6) and to M2 by CYP2B6 and CYP3A4. Poor metabolizers for CYP2D6 obtain less M1, so a lower analgesic effect is expected, while ultrarapid metabolizers have a higher efficacy and risk for adverse drug reactions (ADRs).
Objectives: This study aimed to evaluate both the analgesic effect of tramadol and the incidence of ADRs in patients with acute post-surgical pain depending on CYP2D6 phenotype. In addition, CYP3A5, CYP2C19, CYP2C9 and SLCO1B1 phenotypes were evaluated.
Methods: The present work was designed as an observational, prospective study that included 91 patients over 18 years old who presented acute post-surgical pain with a score greater than or equal to 4 cm on the visual analog scale (VAS) and who were treated with tramadol 100 mg IV. The primary outcomes were the reduction in VAS score between baseline and 30 minutes later (± 15 min) and between baseline and 2 h later or at the moment of discharge from the postanaesthesia care and recovery unit (URPA)). As secondary variables, the incidence of ADRs, need of rescue treatment with other analgesics, type of surgery, demographic data, CYPD6 phenotype and 4 other genes were analyzed.
Results: The patients were divided into 53 men and 38 women with a mean age (SD) of 59.48 (17.24) and a body mass index (BMI) of 26.15 (4.64) with no differences between sexes. The surgery in which tramadol was most commonly used was abdominal surgery. VAS reduction at 30 minutes was 53.53% (28.53) with a significant difference between CYP3A5 intermediate and poor metabolizers (29.65% vs. 56.45% p=0.10) and 74.22% (22.57) at discharge from the URPA with no differences according to any phenotype. The incidence of ADRs was 38.5%, with no differences according to sex or phenotypes. Rescue treatment was necessary in 25.3% of patients, especially in males (p=0.029).
Conclusiones: The variables studied had no apparent effect on the reduction of pain in patients with acute postoperative pain. However, this is a preliminary study and concomitant medications and other confounding factors will be considered in future analyses.
276
The role of pharmacogenetic testing in tamoxifen therapy: Drug licencing, clinical practice guidelines, and physician opinion.
Browne E1, Byrne P1, Gallagher H1
1 School of Medicine, Conway Institute, University College Dublin, Dublin 4, Ireland
Introduction: Tamoxifen is an important endocrine treatment in oestrogen receptor-positive breast cancer. The conversion of tamoxifen to its most active metabolite, endoxifen, is catalysed mainly by the cytochrome P450 2D6 enzyme (CYP2D6). Genetic polymorphisms in CYP2D6 and other medicines can alter endoxifen plasma concentration, which may potentially impact tamoxifen efficacy. The association between CYP2D6 genotype and clinical outcome of tamoxifen therapy is controversial, with a large body of conflicting evidence.
Objectives: The aim of this study was to examine current recommendations and practices relating to pharmacogenetic testing in tamoxifen therapy,
Methods: National summaries of product characteristics (SPCs), and clinical practice guidelines for tamoxifen prescribing, which were available in English, were reviewed. A survey of Irish and Canadian medical oncologists and general practitioners (GPs) was conducted to explore knowledge and attitudes to CYP2D6 testing. A literature search was carried out to further explore pharmacogenetic research undertaken in Canada and Japan of relevance to tamoxifen therapy.
Results: This review found that Japan and Canada are the sole drug licensing authorities that recommend CYP2D6 testing prior to tamoxifen prescription (Table 1). Additionally, this testing is not recommended by the vast majority of national breast cancer treatment guidelines. The results of the survey indicate that most Irish and Canadian oncologists currently do not consider patient genetics prior to prescribing tamoxifen. Awareness of the potential role of pharmacogenetics appeared higher among Canadian oncologists - with 40% of them considering CYP2D6 polymorphisms following tamoxifen unresponsiveness, compared to only 14% of Irish oncologists. The disparity in awareness around pharmacogenetics, and their application to tamoxifen treatment, was much greater amongst GPs; none of the Irish GPs who responded indicated an awareness of the clinical application of pharmacogenetics, compared to 50% of their Canadian counterparts. This indicates that despite the widespread use of tamoxifen, pharmacogenetic awareness is sub-optimal among primary care prescribers in Ireland
Conclusion: The majority of national drug licensing authorities do not recommend CYP2D6 testing prior to tamoxifen prescription, with the exception of the Pharmaceuticals and Medical Devices Agency (Japan) and Health Canada. It is also not recommended by most national breast cancer treatment guidelines, including those of the European Society for Clinical Oncology, American Society of Clinical Oncology and Cancer Australia. The few groups that do recommend CYP2D6 testing include the Canadian Pharmacogenomics Network for Drug Safety and the Clinical Pharmacogenetics Implementation Consortium. It appears that groups working more closely with pharmacogenomics will recommend genetic testing with less extensive evidence than that required by licensing authorities. Further research, such as large-scale randomised controlled trials, must be completed before the debate on pharmacogenetic testing in tamoxifen therapy can be resolved.
Tabel/Image
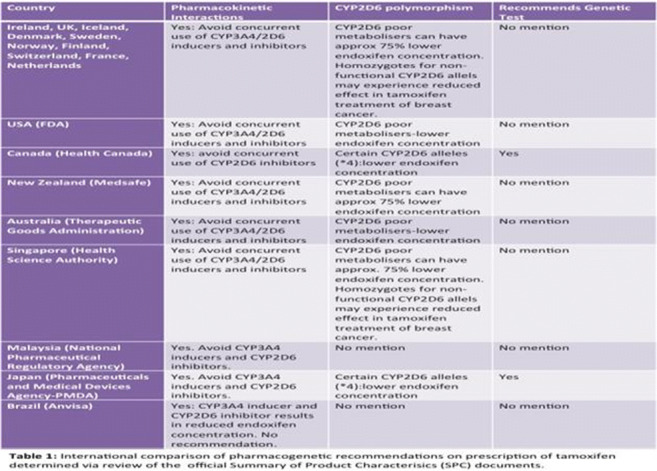
Keywords
CYP2D6; medical oncology; pharmacogenetic testing, tamoxifen
Pharmacokinetics / pharmacodynamics
32
Effect of Food and Meal Timing on the Pharmacokinetics of DWN12088 Enteric Coated Tablet in Healthy Subjects
Park S1, Kim B1, Park M2, Nam J2, Choi J2, Oh J1, Hwang S1, Jang I1, Lee S1, Oh J1
1 Department Of Clinical Pharmacology And Therapeutics, Seoul National University College Of Medicine And Hospital, Seoul, Republic of Korea
2 Clinical Development Center, Daewoong Pharmaceutical Co., Ltd., Seoul, Republic of Korea
Introduction: Idiopathic pulmonary fibrosis (IPF) is a type of lung disease that causes fibrosis in the lungs. Fibrosis is the result of excessive formation of connective tissue and causes organ sclerosis, which causes a gradual decrease in lung function and death. DWN12088 is a prolyl-tRNA synthetase inhibitor that is under clinical development for the treatment of IPF.
Objectives: The aim of this study was to evaluate the effect of food and meal timing on the pharmacokinetics (PKs) of DWN12088 enteric coated tablet.
Methods: A randomized, open-label, three-treatment, three-period, six-sequence, single-dose crossover study was conducted in healthy subjects. All subjects received a single dose of DWN12088 200 mg enteric coated tablet at fasted state, 0.5- or 2-hour after high-fat meal. The PK parameters were estimated by non-compartmental method. The geometric mean ratios (GMRs) and their 90% confidence interval (CIs) for maximum plasma concentration (Cmax) and area under the plasma concentration-time curve from the 0 to 48 hours (AUC0-48h) were estimated.
Results: A total of 36 subjects were randomized and included in the PK and safety analysis. The median time to reach Cmax was delayed for 3 hours in both fed states compared to fasted state. The GMRs (90% CIs) of 0.5-hour after high-fat meal to fasted state for Cmax and AUC0-48h were 0.5262 (0.4334 - 0.6388) and 0.9380 (0.8500 - 1.0351), respectively; and those values of 2-hour after high-fat meal to fasted state were 0.6744 (0.5540 - 0.8211) and 0.9944 (0.9000 - 1.0988), respectively. DWN12088 was well tolerated in fed and fasted state.
Conclusion: The food delayed the oral absorption rate of DWN12088 enteric coated tablet and lowered the maximum plasma concentration, but did not affect overall systemic exposure in healthy subjects.
41
Transport of statins by skeletal muscle efflux transporters
Tuomi S1,2, Deng F1,2, Niemi M1,2,3
1 Department of Clinical Pharmacology, Faculty of Medicine, University of Helsinki, Helsinki, Finland
2 Individualized Drug Therapy Research Program, Faculty of Medicine, University of Helsinki, Helsinki, Finland
3 Department of Clinical Pharmacology, HUS Diagnostic Center, Helsinki University Hospital, Helsinki, Finland
Introduction: ATP-binding cassette (ABC) transporters are among the key factors that regulate drug levels and subsequently determine drug efficacy and safety. Several transporters are known to be involved in the transport of various statins, which cause an array of adverse effects ranging from common and mild myalgia to rare and life-threatening rhabdomyolysis. Because efflux transporters may determine the tissue and plasma levels of statins, alteration of their function may affect statin toxicity and pharmacodynamics. Multidrug resistance-associated protein (MRP) 1 and 5 are ABC efflux transporters highly expressed in the skeletal muscle and therefore might regulate intramuscular levels of statins.
Objectives: The aim of this study was to characterize and compare the transport of atorvastatin, fluvastatin, pitavastatin, pravastatin, rosuvastatin, and simvastatin acid by MRP1 and MRP5, and thus improve the understanding of statin transport and role of efflux transporters in muscle toxicity.
Methods: Vesicular transport assay was used to study transport of statins. Commercially available membrane vesicles derived from human embryonic kidney cells and overexpressing human MRP1 and MRP5 were utilized. The ATP-dependent transport of the seven statins were investigated in MRP1 and MRP5 vesicles in order to determine substrate status and pharmacokinetic parameters of each statin.
Results: Initial screening results showed that both fluvastatin enantiomers and pitavastatin were MRP5 substrates. In addition, the data suggest that atorvastatin could also be an MRP5 substrate. Moreover, atorvastatin was found to inhibit MRP5 activity. Pravastatin, rosuvastatin and simvastatin were not transported by MRP5. ATP-dependent transport of all the seven statins will also be studied with MRP1. Furthermore, transport kinetic parameters will be evaluated for statins transported by MRP1 and MRP5. The final results will be presented in the conference.
Conclusion: These data indicate that MRP1 and MRP5 may play a role in transporting statins in the skeletal muscle. The findings shed light on the pharmacokinetic mechanisms affecting the local exposure and toxicity of statins.
46
Urate transporters may contribute to the difference in cardiovascular outcome between allopurinol and febuxostat
van der Pol K1, Koenderink J2, van den Heuvel J2, van den Broek P2, Pertijs J2, Russel F2, Koldenhof J3, Schirris T2, van der Meer A3, Rongen G1
1 Department of Pharmacology-Toxicology and Internal Medicine, Radboud Institute for Health Sciences, Radboudumc, Nijmegen, the Netherlands
2 Department of Pharmacology-Toxicology, Radboud Institute fro Molecular Life Sciences, Radboudumc, Nijmegen, the Netherlands
3 Applied Stem Cell Technologies, University of Twente,, Enschede, the Netherlands
Introduction: Xanthine oxidase inhibitors febuxostat and allopurinol may alter urate transporter function as an off-target effect. We hypothesize that this action contributes to their different impact on cardiovascular mortality (CARES trial, 2018).
Objectives: Consequently, we explored urate transporters gene expression in endothelial cells and tested the hypothesis that febuxostat and allopurinol differently affect endothelial urate uptake and transcription of urate transporter genes.
Methods: First, we explored gene expression of a panel of urate transporter genes (NPT1, OAT1, OAT3, NADC3, GLUT9, MRP4, BCRP and URAT1) in isolated human umbilical vein endothelial cells (HUVEC; 4 donors) and in endothelial cells isolated from human aorta (HAEC; 3 donors) using GAPDH mRNA as an internal reference. Next, cultured HUVEC (6 donors) were studied for the effect of allopurinol and febuxostat on urate transport (intracellular urate concentration as measured with LCMSMS, normalized to protein concentration and expressed as percentage difference with intracellular urate in absence of drug) and gene expression of transporters (mRNA, qPCR) after standardized exposure to urate in medium.
Results: HUVEC and HAEC significantly expressed GLUT9, MRP4 and BRCRP, and showed a similar profile of expression. Allopurinol (20 and 40 μM) did not significantly affect urate transport or gene expression. In contrast, 10 and 20 μM febuxostat significantly increased intracellular urate concentration by (median) 32% (range: 0-42%) and 75% (21-111%) during exposure to 100 μM urate, respectively (p=0.006, Friedman’s ANOVA), and by 62% (23-98%) and 88% (58-150%) during exposure to 350 μM urate, respectively (p=0.002). Allopurinol did not significantly change mRNA expression of the three transporters. In contrast, febuxostat (20 μM) significantly increased GLUT9 (p=0.038) and reduced MRP4 (p=0.013) but did not significantly affect BCRP mRNA expression.
Conclusion: Febuxostat increased endothelial uptake of urate, increased gene expression of influx transporter GLUT9 and reduced gene expression of efflux transporter MRP4. Allopurinol did not affect urate transport or gene expression under the experimental condition. This action of febuxostat could contribute to the higher cardiovascular mortality for febusostat as compared with allopurinol treatment of patients with gout.
123
Population pharmacokinetic models for direct oral anticoagulants: a systematic review and clinical appraisal
Terrier J1,3,6, Gaspar F2,5, Guidi M2,5, Fontana P4,6, Daali Y1,6, Csajka C2,5, Reny J3,6
1 Clinical Pharmacology and Toxicology Service, Anesthesiology, Pharmacology and Intensive Care Department, Geneva University Hospitals, Geneva, Switzerland
2 Institute of Pharmaceutical Sciences of Western Switzerland, University of Geneva, University of Lausanne, Geneva, Switzerland
3 Division of General Internal Medicine, Geneva University Hospitals, Geneva, Switzerland
4 Division of Angiology and Haemostasis, Geneva University Hospitals, Geneva, Switzerland
5 Service of Clinical Pharmacology, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland
6 Geneva Platelet Group, Faculty of Medicine, University of Geneva, Geneva, Switzerland
Available data has shown an association between direct oral anticoagulant (DOAC) plasma concentration and clinical, particularly bleeding events. Factors that may influence DOAC plasma concentration are therefore the focus of particular attention. Population pharmacokinetic (POPPK) analyses can help in identifying such factors while providing predictive models. The main aim of this systematic review was to identify all the POPPK models to date for the four most frequently used DOACs (dabigatran, apixaban, rivaroxaban and edoxaban). The secondary aim was to use these models to simulate different DOAC plasma concentration-time profiles in relevant clinical scenarios. The results of our model-based simulations confirm the clinical relevance of the known major factors influencing DOAC exposure and support the current approved dose adaptation, at least for atrial fibrillation. They also highlight how the accumulation of co-factors, not currently considered for dose adaptation due to their seemingly minor influence on DOAC exposure, lead to a dangerous increase in blood levels and thus enhance the risk of major bleeding. The present results therefore question DOAC dose adaptation in the presence of these co-factors such as drug-drug interaction (DDI) or genotypes alongside the known existing co-factors. As the overall effect of accumulation of several co-factors could be difficult to apprehend for the clinicians, POPPK modeling could represent an interesting approach for informed precision dosing and to improve personalized prescription of DOACs.
128
Inter-individual pharmacokinetic variability of torsemide in healthy Korean males considering OATP1B1 and CYP2C9 genetic polymorphisms
Lee Y1, Jeong S1, Jang J1, Cho H2
1 College of Pharmacy, Chonnam National University, Gwangju, Republic of Korea
2 College of Pharmacy, CHA University, Seongnam-si,, Republic of Korea
Introduction: Torsemide is a diuretic drug used for several cardiovascular and chronic diseases. With regard to clinical application of torsemide, studies on individualized pharmacotherapy and modeling that take variability in pharmacokinetics (PKs) within a population into account have been rarely reported.
Objectives: The objective of this study was to perform population pharmacokinetic (Pop-PK) modeling and to identify effective covariates that could explain the inter-individual PK variability of torsemide.
Methods: Pop-PK modeling for torsemide was performed based on serum concentration data obtained from 112 healthy Korean males and analysis of various genetic and physicochemical parameters. Modeling was performed with nonlinear mixed-effects (NLME) using Phoenix NLME. The finally developed model was fully verified. The model was also reconfirmed using NONMEM software.
Results: As a basic model, PKs of torsemide within the population were well described by a two-compartment model reflecting the lag-time on oral absorption. According to genetic polymorphisms of OATP1B1 and CYP2C9, significant associations were found in V/F, CL/F, and CL2/F of torsemide. These were reflected as effective covariates in the final Pop-PK model of torsemide. Considering that torsemide is a substrate for CYP2C9 and OATP1B1, it was very effective to search for genetic polymorphisms in CYP2C9 and OATP1B1 as covariates to explain the PK diversity of torsemide between individuals. Differences in CL/F and CL2/F between phenotypes of CYP2C9 were approximately 36.5-51%. The difference in V/F between phenotypes of OATP1B1 was approximately 41-64.6%.
Conclusion: These results suggested that phenotypes of CYP2C9 and OATP1B1 produced significant differences in torsemide PKs. There was no significant difference in the parameter estimates between modeling software (Phoenix NLME vs. NONMEM). In this study, the torsemide PK variability between individuals was largely explained. In the future, individualized effective drug therapy of torsemide taking individual patient's genotypes into account might become possible.
135
Pharmacokinetic variability of imatinib mesylate in Indian patients of chronic myeloid leukaemia
Mohan P2, Uppal R1, Chandgude S3
1 Armed Forces Medical College, Pune, India
2 Armed Forces Medical Services, Shillong, India
3 Armed Forces Medical College, Pune, India
Background: Imatinib is the current standard of care in the treatment of Chronic Myeloid Leukemia (CML). It produces significant inter-individual and intra-individual pharmacokinetic variability and hence, plasma exposure to the drug from a given dosing regimen can vary widely among patients. Factors underlying such variability include environmental factors, dietary, diseases (abnormal liver function, altered protein profile), drug interactions (Cytochrome P 450 inducers and inhibitors), genetic polymorphisms (mainly Cytochrome P450 3A5, influx or efflux transport proteins) and compliance. Despite such variability, trough plasma levels (C0) above 1000 ng/mL are associated with better clinical outcomes.
Objectives: To estimate the proportion of patients of CML on stable doses, having subtherapeutic C0 levels (< 1000 ng/ml) of imatinib
To estimate the mean C0 levels of imatinib in study population
To study the interindividual variability in C0 levels of imatinib in patient cohort.
Materials & Methods: 28 adult patients of CML, on stable treatment of imatinib for the past 30 days, were enrolled. Trough levels of imatinib were estimated by High performance Liquid Chromatography (HPCL) with Diode-Array Detection (DAD) detector. Mobile phase was 70:30 (0.05M KH2PO4: Acetonitrile); pH 3.0 and the column was C18 150 X 4.6 5μm. The eluent was detected at 254 nm after 3 minutes of retention time.
Results: The mean plasma imatinib level was 1083 +/- 570.1 ng/ml (single sample t-test p value was 0.44; considering 1000 ng/ml i.e. optimal plasma value as the hypothetical population mean) with coefficient of variation (CV) of 52.63 %. 17 patients (60.7%) had levels below 1000 ng/ml.
Conclusion: There is significant inter-patient variability in imatinib levels, although all included patients were on identical dose for the same clinical condition. Majority of patients were having suboptimal trough plasma imatinib levels, the pharmacodynamic impact of which, is being investigated on follow up. Nonetheless, findings from our study highlight the importance of Therapeutic Drug Monitoring (TDM) of imatinib. Though the usually employed method of estimating plasma levels of Imatinib is Liquid chromatography–mass spectrometry (LCMS), we have described a HPLC based method of doing the same which is economical and more readily available.
136
Individual maternal pharmacokinetics of antenatal corticosteroids in pregnancy: the influences of co-variates on drug exposure and peak concentration.
Schoenmakers S1, Li L, DeKoninck P, Ronde E, van Zelst B, van Schaik R, van den Berg S, Koch B, Reiss I
1 Erasmus University Medical Center, Rotterdam, Netherlands
Introduction: The dosing scheme of antenatal corticosteroids (ACS) in case of imminent preterm birth has been the same “one dose fits all” since its introduction fifty years ago. However, some co-variates such as multiple gestation seem to impact the individual drug’s pharmacokinetic properties, hereby influencing the available maternal concentrations of ACS. In addition, the safety of the universal dosage ACS regime was recently questioned as in utero overexposure resulted in a higher incidence of mental and behavioral disorders later in childhood.
Objectives: The aim of the study was to determine individual differences and co-variates affecting the maternal pharmacokinetics of the standard treatment regime of ACS (betamethasone) during pregnancy.
Methods: A prospective single center pilot pharmacokinetic study was designed. Twenty-eight pregnant women (23+5 – 33+4 weeks of gestation), who were admitted for imminent preterm birth and received two doses of 12 mg betamethasone intra-muscular given once daily. Maternal characteristics were collected as well as serial blood samples after each administered dose. Betamethasone plasma concentrations were determined by liquid chromatography mass spectrometry. Individual betamethasone pharmacokinetic parameters and crucial clinical covariates were estimated by Non-linear mixed effects models (NONMEM).
Results: One hundred and ninety four blood samples from twenty eight patients were collected and analysed (6.9 sample per patient)by a two compartment model. The crude data showed high variability in the measured maternal peak concentration of betamethasone as well as individual and serial betamethasone concentration at the same time points. The co-variates pre-eclampsia and lean body weight seem to highly influence individual maternal pharmacokinetics. In case of pre-eclampsia, betamethasone clearance was significantly reduce, resulting in higher betamethasone concentrations in maternal blood for a longer period of time, thus higher area under the curve (AUC). High lean bodyweight resulted in a significant lower Cmax, with peal concentrations being half as low, as compared to normal bodyweight, resulting in significantly lower concentrations of betamethason in the maternal circulation.
Conclusion: The results show that several individual maternal characteristics seem to influence pharmacokinetics and hereby the availability of ACS. ACS are primarily intended as fetal therapy, which reach the fetal end-organs via maternal administration. Fetal exposure to ACS is depended on a combination of maternal availability of betamethasone and transplacental maternal-to-fetal passage. Maternal pharmakinetics and betamethason concentration will mostly likely also be reflected in the fetal endresult and as such, will be influenzed by maternal co-variates. Based on our results, an individualized treatment of ACS based on a set of maternal co-variates to optimize fetal efficacy and safety needs to be explored.
167
Population pharmacokinetics and pharmacodynamics of ustekinumab in patients with psoriasis
Teeuwisse P1, Atalay S2, Berends S1, Reek J2, Njoo M3, de Vries A4, Mommers R5, Ossenkoppele P6, Koetsier M7, Berends M8, de Jong E2, Mathôt R1
1 Amsterdam UMC, Amsterdam, The Netherlands
2 Radboud University Medical Center, Nijmegen, The Netherlands
3 Ziekenhuis Groep Twente, Hengelo, The Netherlands
4 Sanquin, Amsterdam, The Netherlands
5 Anna Ziekenhuis, Geldrop, The Netherlands
6 Ziekenhuis Groep Twente, Hengelo, The Netherlands
7 Gelre ziekenhuizen, Apeldoorn, The Netherlands
8 Slingelandziekenhuis, Doetinchem, The Netherlands
Introduction: There is a large interpatient variability between patients in terms of response to treatment with ustekinumab. The possible cause is variability in the relationship between dosage, serum concentration (pharmacokinetics (PK)) and response (pharmacodynamics (PD)).
Objective: To quantify the interpatient variability in the relationship between dosage and serum concentration of ustekinumab and the Psoriasis Area and Severity Index (PASI score). Moreover, by determining which factors influence PK and PD, it should be possible to individualize therapy.
Methods: Forty psoriasis patients using ustekinumab with low disease activity (PASI ≤5) and low quality of life score ≤5 (DLQI) at baseline were included from the CONDOR study (NCT02602925). In the study patients were randomized to a dose interval extension group and a usual care group and, subsequently, followed for 12 to 24 months. Ustekinumab serum concentrations and PASI scores were modelled using published PKPD models. A new PKPD model was developed using nonlinear mixed effects modelling (NONMEM) Furthermore, it was assessed how a PKPD model may assist to control interval extension and individualize therapy.
Results: A total of 205 ustekinumab serum samples and 222 PASI scores were available for PKPD analysis. Because the previously published PK model underestimated the ustekinumab concentrations in the current population, we modified the model. In the novel population PK model, pharmacokinetics were described based on a 1-compartment model. Typical values for volume of distribution and clearance were 9.0 L and 0.27 L/h normalized to 86 kg body weight, with inter-individual variability (IIV) of 18% for the latter. The relationship between serum concentration and PASI scores was described by a turnover model, in which ustekinumab inhibited the formation rate of psoriatic skin lesions (Kin) according to an Emax function. The value of IC50 was 0.11 μg/ml and the remission of lesions (Kout) was 0.0093 h-1. IIV for IC50 was large with a value of 133% (RSE 13%), no examined covariates explained this IIV. Bayesian estimation of individual PD parameters helped identifying patients who were eligible to reduce dose. The possible added value of TDM is demonstrated in figure 1.
Conclusion: We developed a population PKPD model for ustekinumab in patients with psoriasis and low disease activity. In the future this model could be used for TDM of ustekinumab in patients with psoriasis.
Tabel/Image
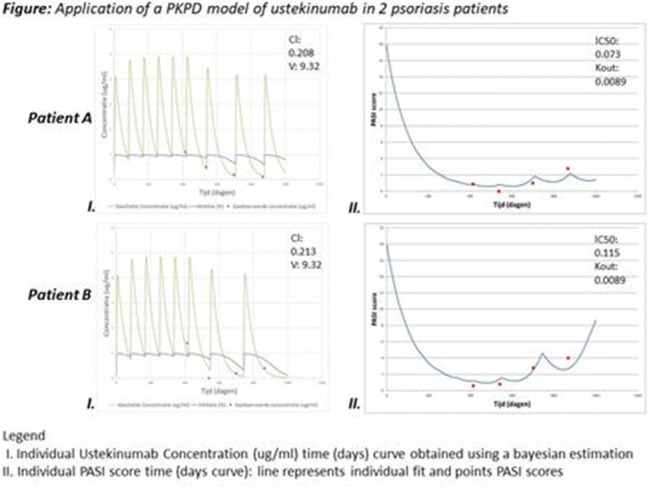
172
Overview of pharmacological and therapeutic properties of Euphorbia humifusa Willd
Gani G1, Zhakipbekov K1, Datkhayev U1
1 School of Pharmacy, Asfendiyarov Kazakh National Medical University, Almaty, the Republic of Kazakhstan
Gulfariza M. Gani, Kairat S. Zhakipbekov, Ubaidilla M. Datkhayev
School of Pharmacy, Asfendiyarov Kazakh National Medical University, Almaty, the Republic of Kazakhstan, e-mail: flower_fariza@inbox.ru
*Corresponding author: Gulfariza M. Gani, School of Pharmacy, Asfendiyarov Kazakh National Medical University, Almaty, the Republic of Kazakhstan,
e-mail: flower_fariza@inbox.ru
Overview of pharmacological and therapeutic properties of Euphorbia humifusa Willd
Topic: Pharmacological and therapeutic properties of E. humifusa Willd.
Keywords: Euphorbia humifusa Willd., biological activity, therapeutic pharmacological properties
Introduction: Euphorbia humifusa Willd. - is an annual herbaceous plant of the Euphorbiaceae family. The natural range of E. humifusa Willd. extends from the Caucasus to East Asia; as a drift species it is found in the Mediterranean, Asia Minor, Central and Eastern Europe. The components of E. humifusa Willd. have a multifaceted biological activity.
Objectives: Domestic and foreign journals.
Methods: In vitro, preclinical methods.
Results: Experimental studies of the biological activity of E. humifusa Willd. components revealed antitumor activity against breast cancer. The ethyl acetate fraction of E. humifusa Willd. extract at a concentration of 5 mcg / ml showed an antimetastatic effect on the human metastatic breast cancer line MDA-MB-231. Prenylated chalcone - paratocarpine E isolated from E. humifusa Willd. demonstrated significant cytotoxicity (IC50 at 19.6 μM) against MCF-7 cells.
Flavonoids isolated from E. humifusa Willd. have anti-inflammatory activity. In an experiment on macrophage cells of the RAW 264.7 line, the production of nitric oxide and tumor necrosis factor alpha (TNF-α) was inhibited with IC50 values ranging from 11.1 ± 0.9 to 45.3 ± 1.6 μM.
The chemical components of E. humifusa Willd. in experiments also showed a good vasorelaxant effect. The effect of the sum of flavonoids of this plant on the isolated rat aorta caused concentration-dependent vasorelaxation. Maximum relaxation was observed at a flavonoid concentration of 100 mcg / ml.
Experimental studies have shown that E. humifusa Willd. contains chemical components that have the potential as a new anti-influenza therapeutic agent with such a new mechanism of action. In particular, the substance 1,3,4,6-tetra-O-galloyl-β-D glucopyranoside isolated from this plant showed a good inhibitory effect against seasonal influenza strains A/California/07/2009 (H1N1), A/Perth/16/2009 (H3N2) and B/Florida/04/2006.
The chemical components of E. humifusa Willd. also showed an antifungal effect. In the experiment, they inhibited the development of fungi Trichophyton rubrum and T. mentagrophytes.
Conclusion: Experimental studies in recent years have shown that the chemical components contained in E. humifusa Willd. have antitumor and anti-inflammatory activity, vasorelaxant, anti-influenza and antifungal action.
191
Variability of voriconazole levels in renal replacement therapy patients.
Alonso Gomez B1, García Saiz M1, Sánchez Santiago M1, Vega Gil N1, Cuéllar Gómez D1, Bautista Blázquez A1
1 H. Universitario Marqués de Valdecilla, SANTANDER, España
Introduction: Voriconazole is an antifungal drug of the triazole derivative group, frequently used in immunocompromised patients both as prophylaxis and treatment of systemic fungal infections. Renal replacement therapy (RRT) results in an increase in the renal clearance rate of voriconazole, requiring patients on RRT to increase the daily dose of voriconazole to achieve adequate antimicrobial efficacy.
Objectives: To compare plasma levels of voriconazole in patients with known systemic fungal infection before and after initiation of renal replacement therapy to corroborate, after calculation of pharmacokinetic and pharmacodynamic parameters, the information obtained from our database with that present in the scientific literature.
Methodology: Observational descriptive study based on our database, taking information from 7 patients (6 men and 1 woman) with a mean age of 62.7 years, admitted to our hospital under treatment with voriconazole and requiring renal replacement support during their admission, either because they developed acute renal failure or because they were already on haemodialysis, most of them under continuous haemodiafiltration (CHDF) (57%).
The main variables to be studied were the plasma levels of voriconazole obtained before, during and after the start of renal replacement therapy. As secondary variables, the elimination half-life and the type of membrane used in renal replacement therapy were studied.
Results: In two of the patients, CHDF significantly decreased voriconazole blood levels (by 78- 87%), requiring a significant increase in subsequent doses to reach therapeutic plasma levels. In the patient on chronic pure intermittent haemodialysis, with regular doses of voriconazole, plasma levels remained stable, highlighting the importance of the type of membrane used in renal replacement therapy.
In another case, the patient suffered concomitant liver failure, so that plasma levels of voriconazole rose significantly into the toxicity range, requiring discontinuation of treatment.
In the remaining three patients, no plasma levels were obtained after initiation of RRT due to withdrawal of voriconazole.
The results confirm that the increase in renal clearance associated with the use of continuous RRT therapy secondarily implies an increase in the dose of voriconazole to be administered in these patients in order to achieve good therapeutic efficacy.
Conclusions: The results obtained can help to understand more accurately the pharmacokinetic and pharmacodynamic parameters that occur with the use of voriconazole in patients with RRT, depending on replacement modality. Because of individual variability, monitoring voriconazole levels allows us to adjust the doses of these patients earlier and appropriate to the type of fungal infection that they present.
203
Estimating transplant patient exposure to everolimus using Machine Learning and simulations, as compared with Maximum a Posteriori Bayesian estimation
Labriffe M1,2, Woillard J1,2, Debord J1,2, Marquet P1,2
1 Pharmacology & Transplantation, INSERM U1248, Université de Limoges, Limoges, France
2 Department of Pharmacology, Toxicology and Pharmacovigilance, CHU de Limoges, Limoges, France
Introduction: Everolimus is an immunosuppressant with a small therapeutic index and large between-patient variability. The best marker of everolimus exposure is the inter-dose area under the concentration vs. time curve (AUC) but measuring it requires collecting many blood samples. The objectives of this study were to: (i) train XGBoost machine learning (ML) algorithms using pharmacokinetic (PK) profiles from kidney transplant patients, simulated profiles, or both; and (ii) compare their performance for everolimus AUC0-12h estimation using a limited number of predictors as compared to maximum a posteriori Bayesian estimation (MAP-BE) in an independent set of full PK profiles from kidney transplant recipients.
Methods: XGBoost was first trained on 508 interdose AUC0-12h estimated using MAP-BE, and then on 250 up to 15,000 rich interdose PK profiles at steady-state, simulated for drug doses uniformly distributed from 0.5 to 4.5 mg using previously published population PK parameters. Random noise was independently added to simulated blood sampling times in order to introduce uncertainty on this feature. The predictors tested were: predose, ~1h and ~2h whole blood concentrations, differences between these concentrations, relative deviations from theoretical sampling times, morning dose, patient age, and time elapsed since transplantation.
Results: The best results were obtained with XGBoost trained on 5016 simulated profiles and informed with 3 concentrations (predose, 1h and 2h post-dose). AUC0-12h estimation achieved in an external dataset of 114 full-PK profiles was excellent (RMSE = 10.8μg*h/L) and slightly better than the 3-point MAP-BE (RMSE = 11.9μg*h/L). Using more profiles (n = 15,051) did not improve the Machine Learning algorithm performance. The 2-sample XGBoost algorithm trained on the same 5016 simulated data provided slightly less precise AUC estimations (RMSE = 12.9μg*h/L). As compared with patient data alone (RMSE = 18.0 μg*h/L), mixing patients’ and simulated profiles improved significantly the performance only when they were in balanced numbers, with approximatively 500 of each (RMSE = 12.5 μg*h/L), yet it did not reach the same performance as MAP-BE or XGBoost trained on a larger simulated dataset.
Conclusion: Everolimus AUC0-12h could be accurately estimated using an XGBoost algorithm by training it with ca. 5,000 full-PK profiles simulated using a population PK model, rather than with actual data from ca. 500 transplant patients. Even using one sampling time less, this algorithm yields performance similar to their reference MAP-BE, which should favor its clinical transfer.
211
Development and validation of a simple and reliable HPLC-UV method for determining gemcitabine levels: application in pharmacokinetic analysis.
Lafazanis K1, Begas E1, Sakellaridis N1, Iatrou H2, Vlassopoulos D3,4, Dimas K1
1 Department of Pharmacology, School of Medicine, University of Thessaly, Larissa, Greece
2 Industrial Chemistry Laboratory, Department of Chemistry, National and Kapodistrian University of Athens, Athens, Greece
3 FORTH, Institute for Electronic Structure and Laser, Heraklion, Greece
4 Department of Materials Science and Technology, University of Crete, Heraklion, Greece
Background: Gemcitabine (Gem) is an important anticancer chemotherapy drug, used for the treatment of pancreatic cancer, non-small cell lung cancer, metastatic breast cancer soft-tissue sarcoma and ovarian cancer as monotherapy or in combination with other medicines for example paclitaxel.
Objectives: The present study aimed to develop a simple, reliable and inexpensive HPLC-UV method for determining gemcitabine in serum and its application in pharmacokinetic studies.
Methods: Sample preparation consisted of a single protein precipitation step with perchloric acid using mouse and human serum. Analysis was accomplished by a reversed-phase column eluted isocratically by sodium phosphate buffer (pH 6.6) and methanol (97/3, v/v) at flow rate 1 mL/min, detection wavelength at 267 nm and column temperature at 40 ºC. 1,7-dimethyluric acid (1,7 U) was used as an internal standard. The pharmacokinetic analysis was conducted using male immunodeficient mice. The animals were weighed and divided into 8 groups (n= 5 / group). The average bodyweight of the mice was 27±1 gr. The first group was used as control and the other 7 groups received a single dose of gem (100mg/kg). Gem was administered either subcutaneous (sc) or intraperitoneal (ip). Blood samples were collected at 5, 15, 30 min and 1, 2, 4 and 6 h post-drug administration and analyzed as described previously.
Results: The retention times of gem and 1,7 U were determined at 9,51±0,36 and 12,16±0,44 min, respectively. Calibration curve was linear with r2= 0.999 over the range 1-400 μM, coefficient of variation was < 6.52% and bias < -7.77 %. The mean recovery of gem was 96.53 % and the limit of detection was 0.17 μM. In the case of spiked human samples, no interferences were observed at the elution time points of gem and the IS. The pharmacokinetic study was performed in serum collected from mice. The PK parameters for the sc administration T1/2, Tmax, Cmax and AUC0-t were calculated to be 1.03 h, 0.083 h, 272.14 μmol/L and 135.99 μmol/L*h respectively, while the corresponding values for the ip administration were 0.85 h, 0.083 h, 291.542 μmol/L and 121.227 μmol/L*h.
Conclusion: We developed a simple, valid and low-cost HPLC method coupled to UV detection of gemcitabine for the determination of the drug in serum which may be proved an important tool for monitoring gem in human serum samples aiming the personalized treatment of cancer patients.
Acknowledgment: This research has been co‐financed by the European Union and Greek national funds through the Operational Program Competitiveness, Entrepreneurship and Innovation, under the call RESEARCH – CREATE – INNOVATE (project code:T1EDK-01612)
216
Guanabenz Repurposing for Early-Childhood Onset Vanishing White Matter; translational PKPD Modeling to Define the Dose in Phase One
Bartelink I1, Bet P1, Verbeek R1, Abbink T1, Berg van den E1, Honeywell R1, Laan van der D1, Voermans M1, Knaap van der M1
1 Amsterdam University Medical Centers, Amsterdam, The Netherlands
Introduction: Vanishing White Matter (VWM) is a leukodystrophy caused by variants in the genes EIF2B1-5, encoding eIF2B. This complex is a key factor in the integrated stress response (ISR), a protective response activated by physical stresses. VWM leads to chronic neurological decline characterized by ataxia, spasticity and cognitive decline, and episodes of subacute major neurological decline, which can lead to coma and death. In VWM, the ISR is continuously activated in absence of stress. Guanabenz, an old alfa2-adrenergic antihypertensive drug, decreases ISR activation. In a mutant VWM mouse model, beneficial effects of Guanabenz were observed on motor function, brain white matter integrity and ISR activation. Furthermore, Literature, PK- and toxicity studies over a large range of doses were available to define the maximum tolerated dose and exposure in rodents.
Objectives: To assist in selection of the maximum safe dose in children with VWM and define the optimal PK-sampling protocol, we applied PKPD modeling. We currently conduct an open-label clinical trial with this dosing regimen for Guanabenz in children with early onset VWM, using a historical control group for comparison.
Methods: Rodent plasma and brain PK profiles showing benefit, without toxicity were integrated with adult PK literature to predict the human concentration time curves in plasma and brain tissue. To predict pediatric PK we applied allometric scaling (CL*W0.75 and volume of distribution *W1 and unlimited drug brain penetration), presuming linear pharmacokinetics to help set the maximum safe dose in children age 2-14 years. Based on the PKPD simulations, a target dose of 2 mg/kg/day, a minimum dose of 1 mg/kg/day and a maximum dose of 10 mg/kg/day was determined. In the clinical trial, children with genetically proven VWM, clinical onset <6 years of age and brain MRI compatible with VWM, who are still be able to walk 10 steps or more, are included. We evaluate (1) safety and tolerability of Guanabenz, (2) plasma pharmacokinetic profile, (3) efficacy (clinical outcome andquantitative brain MRI parameters), and (4) potential biomarkers for future studies. We expect that in 2 years, 30-40 patients can be included. The total trial duration will be 4 years.
Results: In 2021, 9 patients were treated with Guanabenz (dose range 1.05-1.78 mg/kg/day). Median age at time of inclusion was 7 (range 2-11) years, median body weight was 24.2 kg (range 12-49) and median disease duration at start of the trial was 2 (range 1-8) years. Plasma PK-samples have been collected up to 6 months of treatment. An LCMS method has been validated. Comparison of observed vs model-predicted PK is currently ongoing. Common side-effects were drowsiness, fatigue, nausea and nightmares. In all patients, tolerance was observed. Until now, one patient lost the ability to walk.
Conclusions: Preliminary results suggest the minimum dose was reached and tolerated by VWM patients. Longer follow-up is needed to evaluate efficacy. The presented model based translational PKPD-approach can be broadly applied to select doses drug repurposing for any disease and patient population.
217
Pharmacokinetic profile of Risperidone ISM® after switching from oral EU risperidone at steady-state: an open-label, one-sequence bioavailability trial (BORIS-2)
Walling D1, Anta L2, Hassman H3, Litman R4, Ochoa L2, Llaudó J2, Martínez J2, Gutierro I2
1 Collaborative Neuroscience Network, LLC, Garden Grove, USA
2 Laboratorios Farmacéuticos ROVI, S.A., Madrid, Spain
3Hassman Research Institute, Berlin, USA, 4CBH Health, LLC, Gaithersburg, USA
Introduction: isperidone ISM is a new intramuscular long-acting injectable formulation of risperidone providing plasma levels in the therapeutic range by two hours after administration which are maintained for up to four weeks without oral risperidone supplementation or loading doses.
Objectives: To evaluate the steady-state comparative bioavailability of Risperidone ISM and oral risperidone sourced from European Union (EU) in patients stabilized on oral risperidone treatment, as well as provide evidence that the direct switch from oral risperidone to Risperidone ISM is appropriate.
Methods: Multicenter, open-label, once-sequence, comparative bioavailability study (clinicaltrials.gov#NCT05179525). Once daily 4 mg oral EU risperidone for 7 days was followed by 4 intramuscular injections of Risperidone ISM 100 mg at 28-day intervals. Mean steady-state concentration versus time profiles for risperidone, 9-OH risperidone, and risperidone active moiety (RAM) were characterized.
Results: From 116 patients with schizophrenia assessed for eligibility, 77 received at least one dose of study drug (safety population) and 55 completed the study. After first injection, mean (SD) RAM plasma concentrations at 2 hours achieved similar levels [31.43(14.012) ng/mL] to mean average concentration observed on oral treatment at steady-state [28.37(14.490) ng/mL]. Steady-state concentrations were achieved following dose 1 of Risperidone ISM and were maintained to the end of the dosing period. Fluctuation in plasma concentrations (Fluc) of RAM after Risperidone ISM met bioequivalence criteria compared to oral risperidone. Steady-state minimum concentration (Cmin ss) approached bioequivalence criteria and steady-state maximum concentration (Cmax,ss), area under the curve during the dosing interval (AUCtau) and average concentration (Cave), were slightly higher for Risperidone ISM compared to oral risperidone (for RAM) (Table 1).
Conclusion: The release profile of Risperidone ISM allows for rapid achievement of plasma levels similar to those observed at steady-state after oral risperidone treatment. Therefore, direct switch after 24 hours from the last oral EU risperidone dose (after 7 days usage) to Risperidone ISM treatment can be done in stable patients with schizophrenia with no time lag, maintaining steady-state levels of the active moiety throughout treatment and without oral supplementation or loading doses.
Tabel/Image
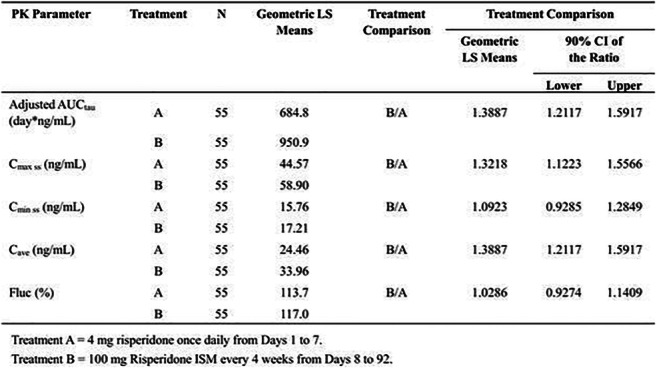
229
The probiotic mix of Saccharomyces boulardii, Bifidbacterium lactis, Lactobacillus acidophilus, Lactobacillus plantarum regulates chemokine responses in human intestinal subepithelial myofibroblasts
Tarapatzi G1,2, Filidou E1,2, Kandilogiannakis L1,2, Spathakis M1,2, Arvanitidis K1,2, Kotzampassi K3, Manolopoulos V1,2, Kolios G1,2, Vradelis S4
1 Laboratory of Pharmacology, Faculty of Medicine, Democritus University Of Thrace, Alexandroupolis, Greece
2 Individualised Medicine & Pharmacological Research Solutions Center (IMPReS), Alexandroupolis, Greece
3 Department of Surgery, Aristotle University of Thessaloniki, Thessaloniki, Greece
4 University Hospital of Alexandroupolis, Democritus University of Thrace, Alexandroupolis, Greece
Introduction: The intestinal mucosal immune system is responsible for guarding the gut surface against pathogenic entry and regulating reactions to the commensal flora. The symbiotic relationship between microbiota and mucosal cell populations is beneficial in gut homeostasis and intestinal inflammation. The strains Lactobacillus acidophilus, Lactobacillus plantarum, Bifidobacterium lactis and Saccharomyces boulardii seem to ameliorate the inflammatory responses and therefore are widely used among probiotic supplements. Intestinal Subepithelial Myofibroblasts (SEMFs) are key mediators in fibrosis, but they are also involved in mucosal inflammatory responses.
Objectives: The aim of this study was to investigate the inflammatory effects of the probiotic mix of L. acidophilus, L. plantarum, B. lactis and S. boulardii in the chemokine responses of SEMFs.
Methods: A probiotic mix of the aforementioned strains was reconstituted in SEMFs culture medium, identified by Gram staining and their viability was assessed by Trypan Blue staining. Primary SEMFs were isolated from colonic biopsies from healthy individuals and stimulated with 10² and 104 cfu/ml of the mix for 6h, when mRNA expression of the chemokines CCL2, CCL5, CCL20, CXCL1, CXCL2, CXCL3, CXCL4, CXCL5, CXCL6, CXCL7, CXCL8, CXCL9, CXCL10, CXCL11, CXCL12 and CXCL14 was assessed by quantitative PCR.
Results: Trypan Blue staining confirmed the viability of the studied probiotics, and the bacteria and the yeasts were stained Gram positive. SEMFs express basal levels of the studied chemokines. Stimulation of SEMFs with 10² cfu/ml mix resulted in the upregulated mRNAs of CXCL1 (2.7-fold, IQR: 1.9-6.2, p<0.01), CXCL2 (1.8-fold, IQR: 1.3-4.9, p<0.05), CXCL8 (2.3-fold, IQR: 1.9-3.3, p<0.001) and CCL2 (2.2-fold, IQR: 1.5-2.9, p<0.001), while stimulation with 104 cfu/ml also upregulated the mRNA expression of CXCL1 (2.4-fold, IQR: 1.6-4.1, p<0.05) and CCL2 (2.6-fold, IQR: 1.6-3.1, p<0.01), as well as CXCL4 (1.2-fold, IQR: 1.0-1.8, p<0.05), CXCL10 (1.8-fold, IQR: 1.4-2.6, p<0.001) and CCL5 (1.5-fold, IQR: 1.2-1.6, p<0.01). It is worth mentioning that the probiotic mix did not affect the mRNA expression of the chemokines CXCL3, CXCL5, CXCL6, CXCL7, CXCL9, CXCL11, CXCL12, CXCL14 and CCL20.
Conclusion: These results indicate a symbiosis of probiotics and SEMFs. In addition, they suggest that this probiotic mix could regulate the production of chemokines by myofibroblasts, contributing to the alertness of the normal mucosal immune system and possibly the regulation of mechanisms that govern intestinal inflammation.
Funding: This study was supported by «Strategic expansion of the Greek Biobanking Infrastructure» (BBMRI‐GR, NSRF 2014-2020), by “AMKE KLEON TSETIS” Foundation, Athens, Greece and by the project IMPReS (MIS 5047189), financially supported by the Program “Competitiveness, Entrepreneurship and Innovation” (NSRF 2014–2020), co-financed by Greece and the European Union (European Regional Development Fund).
242
Development of a population pharmacokinetic covariate model of rifampicin in healthy Caucasian volunteers
Bilal M1,2, Ullah S1, Trueck C1, Wachall B3, Wargenau M4, Scheidel B5, Zaremba D1, Gazzaz M1,7, Wiesen M6, Jaehde U2, Taubert M1, Dokos C1, Büsker S1, Fuhr U1
1 Department I of Pharmacology, Faculty of Medicine and University Hospital Cologne, Center for Pharmacology, University of Cologne, Cologne, Germany
2 Department of Clinical Pharmacy, Institute of Pharmacy, University of Bonn, Bonn, Germany
3 InfectoPharm Arzneimittel und Consilium GmbH, Heppenheim, Germany
4 M.A.R.C.O. GmbH & Co. KG, Düsseldorf, Germany
5 ACC GmbH Analytical Clinical Concepts, Leidersbach, Germany
6 Division of Therapeutic Drug Monitoring, Faculty of Medicine and University Hospital Cologne, Center for Pharmacology, University of Cologne, Cologne, Germany
7 Department of Clinical Pharmacy, College of Pharmacy, Umm Al-Qura University, Makkah, Saudi Arabia
Introduction: Rifampicin is one of the key drugs to treat tuberculosis (TB), a disease that is associated with high morbidity and mortality worldwide. Currently, weight-based dosing is recommended. Lately, fat-free mass (FFM) was reported to be superior to body weight.
Objectives: To develop a population pharmacokinetic (PK) model of rifampicin and to determine the influence of potential covariates on PK parameters in healthy Caucasian volunteers.
Methods: The data were obtained from a phase I/IV randomized, cross-over, open-label bioequivalence study (EUDRACT-No: 2017 004418-24). A total of 24 healthy individuals were enrolled, each receiving a test and a reference tablet of 600 mg of rifampicin, separated by a wash-out period of at least 9 days. Twenty samples up to 24 hours post-dose were taken per period. The median age and body weight of the subjects (11 male / 13 female) was 39.5 years and 68.0 kg, respectively.
Monolix version 2021R1 was used for non-linear mixed effect modeling. Absorption models with zero and first order, with and without lag time, and with transit compartments including linear and non-linear elimination were evaluated. Covariates assessed included sex, height, weight, body mass index (BMI), fat-free mass estimated from sex, body height and body weight (FFM), and identity of preparation.
Results: A one-compartment model with first-order absorption and transit compartments, and with nonlinear (Michaelis-Menten) elimination best described the data. A covariate model including both sex and weight on the volume of distribution (V), and maximum elimination rate (Vmax) distinctly improved the model (Akaike information criteria [AIC] decreased by 55). In an alternative model, FFM was a significant covariate on V and Vmax, decreasing AIC by 59. The point estimates of the (sex + body weight) covariate model for absorption rate constant (ka), transit time (Mtt), V, Vmax, Km (Michaelis-Menten constant), and transit rate constant were 8.95 h-1, 0.37 h, 14.5 L, 74.9 mg/h, 13.2 mg/L, and 54.6 h-1 respectively. The IIV (coefficient of variation [CV]) for Ka, Mtt, V and Vmax were 137%, 23.3%, 8.70% and 19.2% respectively. IIV CV% of both covariate models were highly similar. The identity of the preparation (test or reference) had no significant effect on any of the parameters.
Conclusion: Estimated FFM explained the inter-individual variability in PK of rifampicin in healthy volunteers to a similar extent as the combination of body weight and sex only.
258
Interaction between lamotrigine and other antiepileptic: pharmacokinetic study
Daldoul M1, Ben Sassi M1, Gaires E1, Charfi R1, El Jebari H1, Jebali N1, Salouage I1, Daghfous R1, Trabelsi S1
1 Service de Pharmacologie Clinique-National Centre Chalbi Belkahia of Pharmacovigilance (CNPV), Tunis, Tunisie
Introduction: Lamotrigine (LTG) is one of the newest anti-epileptic drugs used especially for absence seizures. It has a broader spectrum of action than the old antiepileptic drugs.
LTG can be used either as monotherapy or in combination with other antiepileptic drugs, which can induce variations in its plasma concentrations (C0), thus affecting its efficacy and tolerance.
Objectives: The main objectives of our study are to analyze C0 of LTG in combination with other antiepileptic drugs and in monotherapy and to study the importance of variation of C0 of LTG according to C0 of other antiepileptics in order to adapt the dosage.
Methods: We conducted a longitudinal retrospective descriptive study over a period of five years from September 2012 to December 2017. It involved 659 plasma lamotrigine (LTG) determinations performed in 393 patients. The LTG dose was performed by: High Performance Liquid Chromatography and an immunological technique. Therapeutic range for LTG was betaween 3 to 14 μg/mL.
Results: LTG was administered as monotherapy in 474 samples (72.9%). Correlation between dose and C0 of LTG was 0.459. Median C0 of LTG prescribed as monotherapy was 4 μg/mL (0.3 - 19.3 μg/mL).
LTG was prescribed in combination with other antiepileptic drugs in 106 patients (176 samples). LTG was associated with one antiepileptic in 86 patients (140 samples). With similar doses, C0 of LTG was 5.55 μg/mL with valproic acid, 2.2 μg/mL with carbamazepine and 2.05 μg/mL with phenobarbital.
Comparisons between C0 and C0/Dose of LTG monotherapy and polytherapy showed significant differences (p = 0.01 and <0.001, respectively).
We found a low correlation between ratio C0/Dose of LTG and carbamazepine C0 (r2=0.78) and between the same ratio and phenobarbital C0 (r2=0.6). We also found no correlation between this ratio and valproic acid (r2=0.399) and levetiracetam (r2=0.286).
Conclusion: The combination of other antiepileptic drugs, may influence the C0 and C0/D ratio of LTG.
Thus, Pharmacological therapeutic monitoring of LTG seems therefore necessary especially in polytherapy in order to adjust the posology and bring C0 into therapeutic range.
303
Multiplex phenotyping of human drug metabolizing enzymes in cell lines from different organs– assessing drug metabolism activity in vitro
Koczera P1, Yamoune S1,2, Tupiec J1, Stingl J1
1 Institute of Clinical Pharmacology, University Hospital of RWTH Aachen, Aachen, Germany
2 Federal Institute for Drugs and Medical Devices, Research Division, Bonn, Germany
Introduction: Drug metabolism is an interplay of many factors and influenced by various aspects. While its investigation is crucial for understanding specific mechanisms of actions for various drugs, identifying potential new pharmaceutical agents or drug targets, quantification of activity and modulation by interactions using in vitro methods are limited. The use of cultivated cell lines from specific organ sites involved in drug metabolism like the liver, colon or kidney enables the investigation of drug metabolism but quantification of the activity of single DME and measuring modulation or interactions are difficult due to the low activity of these enzymes in artificial culture conditions. The evaluation of substrate turnover mainly metabolized by cytochrome P450 enzymes, is done using different approaches from individual groups, but no standardized procedure has been published how to quantify CYP enzyme substrate metabolism for comparison studies in human organ cell lines.
Objectives: Quantify metabolite formation of specific substrates in whole cells across different cell lines to investigate drug metabolism using a standardized protocol and identifying potential problems and obstacles. Compare effects of green tea components epigallocatechin and epigallocatechin gallate known to modulate CYP3A4 among others (Satoh et al. 2016) in CYP enzymes derived from cell lines or commercially available and identify potential new interactions.
Methods: To compare turnover rates of various CYP substrates the liver cell line HepaRG, the small intestine cell line Caco-2 and the kidney cell line HEK-293 were seeded in 6-well plates at various cell numbers and kept in culture for a predetermined time. After 24 hours the medium was changed with medium containing single substrates for CYP3A4, CYP2B6, CYP2C9 or CYP2D6 of different concentrations. Following different incubation periods formation of potential metabolites in medium and cell lysates were analyzed via LC-MS/MS. Furthermore, mRNA expression of various CYPs and CYP activity of enzyme isolates from HepaRG and Caco-2 Cell in presence of epigallocatechin and epigallocatechin gallate were evaluated and compared with human liver microsomes and supersomes containing single CYPs.
Results: Highest activity could be observed in HepaRG cells with measurable activity of CYP3A4, CYP2B6 and CYP2C9, Caco-2 cells showed activity of CYP3A4 and HEK-293 cells showed no measurable activity. While overall activity was low, the used protocol provided most reliable and comparable data with cell numbers 800.00 or more and measurement of cell lysates rather than medium supernatant. An incubation time of 1 hour showed sufficient metabolite formation. Epigallocatechin and epigallocatechin gallate showed significant influence mRNA expression and activity of drug metabolizing enzymes from HepaRG and Caco-2 cells which could be confirmed with HLM and supersomes. CYP3A4 showed the highest activity and great modulatory properties.
Conclusion: While quantification is problematic due to the degradation of enzymes and low activity, a standardized procedure and comparison of different drug metabolizing enzymes in a panel of cell lines in a multiplex approach offers the possibility to detect substrate interactions on enzyme activity from cultivation dependent modulation of enzyme activity.
306
ESTABLISHMENT AND VALIDATION OF MARE’S MILK-DERIVED EXOSOMES EXTRACTION METHOD
Aljofan M1, Issayev A1, Sergazy S1, Zhetkenev S1
1 Nazarbayev University School Of Medicine, Nur-Sultan, Kazakhstan
Introduction: Exosomes are nanosized (30-150nm) extracellular vesicles with double layer lipid membrane, that present in milk of farm animals. These vesicles maintain endocrine, paracrine and autocrine signaling. They produced by parental cell as major substance of cell-cell communications. Their main physiological function is to transmit cellular signals and are capable of carrying big and charged molecules, which otherwise would not be able to penetrate the plasma membrane.
Objective: To establish a reliable extraction method of exosomes obtained from a mare’s milk, for future use as drug-delivery system.
Methods: Three different isolation methods were investigated including; isoelectric precipitation (IP), size-exclusion chromatography (SEC), and total exosome isolation (TEI). Each approach differs in price, equipment and materials, duration and complexity of implementation as well as mechanism of extraction.
Results: All methods resulted in the extraction of smooth membrane exosomes with no surface rupture and damage, which indicates their possible further loading of drugs and use for therapeutic purposes in experiments. However, TEI and SEC extraction methods excluded dead cells, cell debris, and fat globules, but IP system did not. Also, exosomes obtained using all three methods were of optimal size with TEI and SEC showed good clearance of casein, the main protein contaminant in milk.
Conclusion: TEI method appeared to be the most suitable extraction method for both laboratory research and large-scale production. In comparison to the other methods, TEI approach requires less time for extraction, more sensitive, and consistent with small volumes of biological samples.
309
Carvedilol pharmacokinetics and PBPK modeling in obesity and post-Roux-en-Y gastric bypass patients
Yamamoto P1, Salgado Jr. W2, dos Santos J2, Kemp R2, Sankarankutty A2, de Gaitani C1, da Silva R1, de Moraes N3
1 School of Pharmaceutical Sciences of Ribeirao Preto, University of Sao Paulo, Ribeirao Preto, Brazil
2 School of Medicine of Ribeirao Preto, University of Sao Paulo, Ribeirao Preto, Brazil
3 College of Pharmacy, University of Florida, Orlando, USA
Introduction: Roux-en-Y gastric bypass (RYGB) surgery is one of the most frequent bariatric surgery techniques applied for long-term weight loss and to improve obesity-related comorbidities. Anatomical and physiological changes in post-RYGB patients are likely to impact drug pharmacokinetics (PK). Physiologically-based pharmacokinetic (PBPK) models have been developed to predict the effect of these changes on PK, which can be useful for drugs with solubility-limited absorption, such as carvedilol.
Objectives: We conducted a clinical trial to assess the effect of obesity and RYGB on PK of a single oral dose of carvedilol. Our observed individual level PK data is being used to support PBPK modeling in obese and post-RYGB patients.
Methods: Obese (n=15, body mass index ≥ 30 kg/m², age: 24-56 years old) and post-RYGB patients (n=17, time post-RYGB: 356-2752 days, age: 31-57 years old) received a single oral dose of 25 mg of carvedilol. Blood was sampled up to 24 hours post-dose and carvedilol concentration was determined in plasma by LC-MS/MS. PK parameters of carvedilol were estimated by non-compartmental analysis (NCA) using Phoenix (version 8.3.4). PBPK model for carvedilol was elaborated on a previously developed model (1) using SimCyp® (v21). Healthy and obese models were available in the simulator and the post-RYGB population was adapted from the previous model (2). Simulated PK parameters and profiles were compared to observed individual data from obese and post-RYGB patients.
Results: The maximum plasma concentrations (Cmax) decreased 2-fold in post-RYGB patients (59.2±38.5 ng/mL and 25.8±14.5 ng/mL, p<0.001). In addition, a delay to reach the Cmax was observed post-RYGB (2.7±1.7 h) when compared to obese (1.2±0.9 h, p<0.05). No significant differences were observed in the area under the plasma concentration versus time curve (AUC) and apparent total clearance (CL/F) between obese (267.8±170.4 ng.h/mL and 144.6±1109.7 L/h, respectively) and post-RYGB (207.4±127.1 ng.h/mL and 173.5±117.7 L/h, respectively) groups. The apparent volume of distribution (Vd/F) was higher in post-RYGB (1405.2±692.6 L) than in obese patients (877.8±477.4 L, p<0.05). The current PBPK model for carvedilol accurately predicted the observed data in obese population with predicted/observed ratios of AUC, Cmax and Tmax within a 2-fold error. Carvedilol AUC was reasonably well predicted by the post-RYGB model (predicted/observed AUC ratio = 1.3), while Cmax was overestimated and Tmax underestimated. Additional research, including transcriptomic analysis of key enzymes and drug transporters on carvedilol’s PK, is being conducted to support PBPK modeling in post-RYGB patients.
Conclusion: A delayed oral absorption for carvedilol was observed in post-RYGB patients with a 2-fold reduction in Cmax and a 2-fold increase in Tmax. The current PBPK model for carvedilol accurately predicted the exposure in the obese population, while the post-RYGB model requires further development.
(1) Rasool M. F.; Khalil F.; Läer S. Clinical Pharmacokinetics 54, 943, 2015.
(2) Darwich A. S. et al., Journal of Pharmacy and Pharmacology 64, 1008, 2012.
Pharmacovigilance
12
Adverse drug reaction reporting: doctors' knowledge, attitudes and practice at the Clinical Center of Vojvodina
Rašković A1, Stilinović N1, Tomas A1, Panjković K1, Paut Kusturica M1
1 University Of Novi Sad, Faculty Of Medicine, Novi Sad, Serbia
Introduction: Adverse drug reactions are one of the main causes of morbidity and mortality around the world, increased hospital admissions, and financial burden for patients and health care system. Health care professionals play a vital role in the pharmacovigilance system, especially in detection, identification, managing and reporting of adverse drug reactions (ADR).
Objectives: The study aimed to examine the knowledge, attitudes and practices of doctors at Clinical centre of Vojvodina towards adverse drug reaction reporting.
Methods: The prospective research was conducted on doctors employed at the Clinical Center of Vojvodina, Novi Sad municipality. Respondents completed an anonymous questionnaire that was conducted in one month period. The questionnaire contained socio-demographic questions, knowledge test, given attitudes on ADR reporting and questions about everyday reporting practice. The collected data were processed in IBM SPSS version 23.
Results: A total of 150 respondents completed the survey. The survey tool showed acceptable validity and reliability. The median knowledge score was 5 out of 10 points. Doctors were of the opinion that they did not have adequate knowledge on ADR reporting and that prevention measures are needed. Underreporting was very high (68%). The results showed a clear link between higher reporting rates and parameters such as work years, postgraduate qualifications and professional membership.
Conclusion: Underreporting of ADRs by doctors is highly prevalent. Further studies are needed to find the main cause of this trend. Initiatives to educate and train doctors on ADR reporting and simplifying the reporting procedure may improve reporting practices.
47
A case report of posterior reversible encephalopathy syndrome associated with bortezomib for multiple myeloma during the COVID-19 pandemic
Anderssen-Nordahl E1, Bellas L1, Leguízamo-Martínez L1,2
1 Department of Clinical Pharmacology, Vall d'Hebron University Hospital, Barcelona, Spain
2 Department of Pharmacology, Therapeutics and Toxicology, Universitat Autònoma de Barcelona, Barcelona, Spain
Introduction: Posterior reversible encephalopathy syndrome (PRES) is a clinical and radiological condition, with a controversial pathophysiological mechanism. Multiple risk factors are associated, such as cytotoxic and immunosuppressive drugs, blood pressure fluctuation, infections, kidney diseases and others. COVID-19 has also been identified as a potential risk factor. Regarding cytotoxic drugs, bortezomib should be highlighted, with PRES as a known extremely rare side effect. If PRES is not appropriately recognised and treated, it may lead to life-threatening sequelae.
Objective: To describe a case report of PRES associated with bortezomib and research previous similar cases.
Methods: A 51-year-old female who was diagnosed with stage III multiple myeloma, and treatment with lenalidomide, bortezomib and dexamethasone was started. As additional problems, she presented obliterative bronchiolitis due to past infection of SARS-CoV2. After the third cycle of bortezomib, she developed neurological symptoms. The computed tomography scan showed hypodense lesions in both hemispheres which suggested leukoencephalopathy, therefore PRES was suspected. Bortezomib and lenalidomide were suspended and she was admitted to the intensive care unit. She presented a persistent non-convulsive epileptic status and continued deteriorating associating distributive shock and renal failure. The magnetic resonance imaging confirmed PRES. The patient died 12 days after the last administration of bortezomib.
Results: This case of PRES was reported as a possible adverse drug reaction (ADR) associated with bortezomib exposure; however, a multifactorial origin cannot be ruled out due to presence of exacerbated renal failure, administration of other drugs and SARS CoV-2 infection. In Spanish pharmacovigilance databases, 735 cases of ADR associated with bortezomib have been reported, with 199 neurological disorders, without further specifications. In the review of the published literature, 7 similar cases were found, presented in Table 1.
Conclusion: Our patient had several risk factors for developing PRES, but treatment with bortezomib was associated as the main trigger due to its temporal correlation. We aim to promote the use of pharmacovigilance sources and proper reporting of ADRs.
Tabel/Image
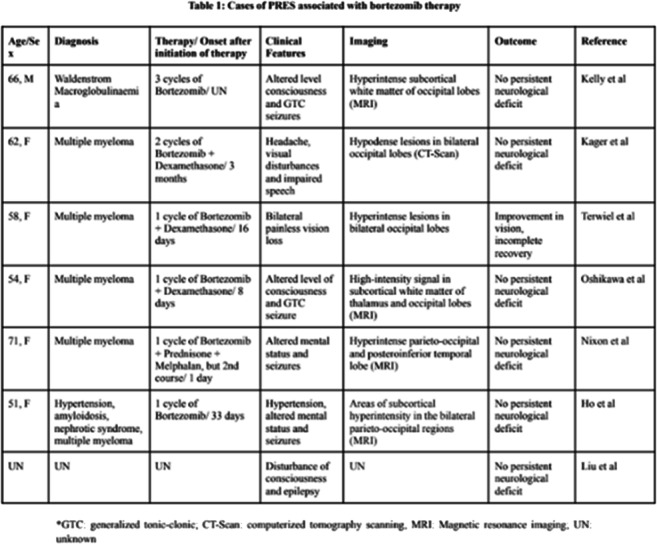
73
Efficacy of tacrolimus once-daily dose LCTP (Envarsus®) formulation to prevent post kidney transplant diabetes in at-risk patients. Preliminary safety results
Rodríguez-Fortúnez P1, Rodríguez-Jiménez C2, Pérez-Carreño E1, Masiero-Aparicio P1, Díaz-Martínez L3, Rodríguez-Adanero C3, González-Rinne A3, Marrero D3, Pérez-Tamajón L3, Rodríguez A3, Alvarez A3, de Bonis-Redondo E3, Fernández-Rivera C4, Porrini E5, Torres-Ramírez A5
1 Clinical Trials Unit. Pharmacology Service. University Hospital of the Canary Islands. Spanish Clinical Research Network (SCReN), Tenerife, Spain
2 Clinical Trials Unit. Pharmacology Service. University Hospital of the Canary Islands. Spanish Clinical Research Network (SCReN). La Laguna University, Tenerife, Spain
3 Nephrology Service. University Hospital of the Canary Islands, Tenerife, Spain
4 Nephrology Service. University Hospital Juan Canalejo, A Coruña, Spain
5 Nephrology Service. University Hospital of the Canary Islands. La Laguna University, Tenerife, Spain
Introduction: De novo post kidney transplant diabetes, now called post-transplantation diabetes mellitus (PTDM) (1), is a common complication that occurs in 20-30% of recipients (2). PTMD negatively influences the outcome of renal transplantation as it has been associated with increased cardiovascular and overall morbidity and mortality in this population (3,4). The importance of post-transplant immunosuppressive medication as a risk factor for PTDM should be highlighted. Of these drugs, it is worth highlighting the anti-calcineurin drugs, Tacrolimus and Cyclosporin A.
Objective: Since LCTP Envarsus tacrolimus formulation has pharmacokinetics advantages over immediate release tacrolimus (Prograf) that could spare beta cell toxicity the aim of this clinical trial is to investigate whether de novo immunosuppression with the extended-release Tacrolimus formulation Envarsus® reduces the incidence of post-transplant diabetes defined by baseline blood glucose, after glucose overload or by the use of anti-diabetic drugs compared to the standard Tacrolimus formulation Prograf® in patients at high risk of post-transplant diabetes, without increasing the risk of Acute Rejection.
Methods: Randomised, open-label, phase IV, parallel, controlled, two-arm (Envarsus® vs Prograf®) clinical trial. Preliminary results from 57 patients are presented. This CT has been approved by the Spanish Medicines Agency and the Ethics Committee of the University Hospital of the Canary Islands. EudraCT No: 2017-000718-52.
Results: 57 kidney post-transplant patients were recruited between Junio 2017 and January 2022. The mean age of the series was 60 years old, 68% were male. They were randomised in two parallel arms: Arm 1 (Prograf®): 26 patients vs Arm 2 (Envarsus®): 31 patients.
We collected 68 serious adverse events (SAEs) (33 patients, mean age 64 years old, 73% male). No deaths have been reported. The reported SAEs were summarized as follows: (see attached table)
28 SAEs were considered related to the investigational products and all of them were considered as expected adverse reactions (listed in the product data sheet). These data were consistent with the known safety profile of both drugs.
Regarding the outcome of the SAEs, 82% recovered (56 patients), 5% did not recover (3 patients) and 13% are recovering (9 patients).
Conclusion: To the date, no relevant differences in terms of pharmacological safety have been found in the use of Envarsus vs Prograf in patients after kidney transplantation.
Tabel/Image
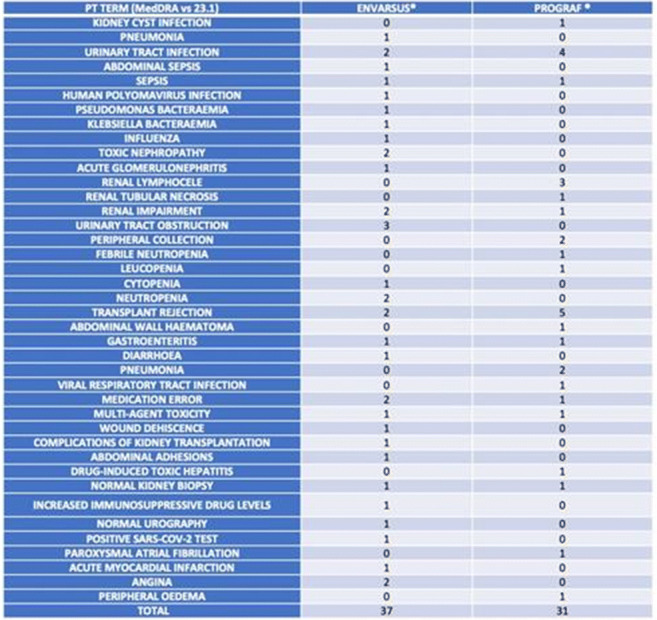
100
Acute adverse reactions to COVID-19 Comirnaty mRNA vaccine: a cross-sectional study in healthcare workers from a tertiary care hospital
Paiva P1, António N1, Dias P1, Carvalho C1, Jordão I1, Prata I1, Feio J1, Parente F1
1 Centro Hospitalar E Universitário De Coimbra, Coimbra, Portugal
Introduction: Until May 31, 2021, 177 million doses of the Comirnaty vaccine were administered in the European Union. This is a mRNA vaccine against COVID-19 and was the first vaccine that became available to employees of the Coimbra Hospital and University Center. Because it is a drug that has been on the market for less than 2 years, all suspected adverse reactions associated with the vaccine should be reported.
Objectives: Considering the problem of spontaneous underreporting in Portugal, it is important to conduct Pharmacovigilance studies that contribute to better continuous safety monitoring and consequent benefit/risk assessment of the COVID-19 vaccines.
Material and Methods: Cross-sectional study conducted in healthcare professionals of Centro Hospitalar e Universitário de Coimbra, through the application of a questionnaire to identify possible adverse reactions to the Comirnaty vaccine and to characterize its severity. The questionnaire was submitted by institutional email to all employees of the institution who had complied with the initial protocol, i.e., the inoculation of the first two doses of Comirnaty vaccine, between December 27, 2020 and February 15, 2021. In this email survey, sent during the month of March 2021, the questionnaire included the questions listed in the adverse reaction reporting portal of the National Authority of Medicines and Health Products, IP. (INFARMED).
Results: We obtained 2909 responses to the questionnaire, out of a universe of 4369 healthcare professionals. The mean age was 44.9±11.8 years and 75.2% of the employees were female. In 60.2% (N=1750) collaborators there were suspected adverse drug reactions (ADR) after the 1st inoculation and 70.0% (N=2037) reported suspected ADR after the 2nd inoculation. After the 1st inoculation, the side effects described in the literature as most common (1/10) and common (1/100) were also the most prevalent in this sample, occurring in 58.1% (N=1689) and 12.2% of the collaborators (N=354), respectively. After the 2nd inoculation, the most common, uncommon, and common side effects had the frequency of 68.7% (N=1999), 18.8% (N=548), and 15.1% (N=440), respectively. It should be noted that some employees had more than one adverse reaction. According to the severity criterion, most employees considered the side effects as "Non-Severe" or "Clinically Important", the latter including absenteeism/absence from work, the need to take medication or the need to go to the emergency room. No serious adverse reactions were identified. Only 6.9% of employees (N=201) after the first inoculation and 9.5% (N=277) after the second inoculation spontaneously reported the suspected adverse reaction to the vaccine. Regarding analgesic prophylaxis, 96.8% (N=2815) of the employees did not use any prophylaxis before the 1st inoculation, while in the second inoculation only 89.8% (N=2612) did not use any prophylaxis. SARS-CoV-2 coronavirus infection was reported in 4.6% of the staff (N=134), in 1.4% (N=40) before the first inoculation, in 2.4% (N=71) after the first dose, and in 0.8% (N=23) after the second dose.
Conclusions: ADRs to COVID-19 Comirnaty vaccine are very frequent, especially after the 2nd inoculation, but all not serious. Underreporting is a present and current reality.
106
QT prolonging drug use in children and adolescents in psychiatry outpatients in an Indian tertiary care medical institution
Das B1, Rawat V1, Sarkar C2
1 Professor, Department of Pharmacology, All India Institute Of Medical Sciences(AIIMS) Rishikesh, Rishikesh, India
2 Associate Professor, Department of Pharmacology, All India Institute Of Medical Sciences(AIIMS) Rishikesh, Rishikesh, India
3 Professor, Department of Pharmacology, NEIGRIHMS Shillong, Shillong, India
Introduction: Child and adolescent mental health problems are common in the community, but the scientific basis for the treatment of many of these conditions is still in its infancy. Prescriptions of psychotropic medications have become an important intervention for many children and adolescents with mental disorders, and the rise of these prescriptions is debated intensively both among experts and the public.
Objectives: The overall prescription rates of psychotropics in general and the major medication subgroups prescribed to children and adolescents vary substantially between countries. Therefore, they are also at escalated risk of QT interval protracting drug-drug interactions. Therefore, we have analysed the prescribing pattern of QT prolonging drugs and drug-drug interactions in children and adolescent psychiatric patients.
Methods: This was a cross-sectional prospective study conducted in Psychiatry OPD at All India Institute of Medical Sciences(AIIMS), Rishikesh, Uttarakhand from October 1, 2016 to December 31, 2018 employing the pertinent prescriptions.
Results: Prescriptions for 1682 patients(aged 0 to 18 years) during the aforementioned study period were investigated. 1146 patients were males and 536 were females in our study. 1317(78.3%) patients were using drugs with a capacity to induce TdP.2894 QT prolonging drug-drug interaction pairs have been identified. As per CredibleMeds(AzCERT) Classification, 2881(53.2%), 1982(36.6%) and 472(8.7%) interacting drugs were identified with known, possible and conditional risk of TdP, respectively. The common interacting medications belong to psychostimulant(968), antimicrobial (548), proton pump inhibitor(517), antinausea (467), antitubercular(462), antidepressant(436), and antipsychotic(421) therapeutic categories.
Conclusion: Many children and adolescent psychiatric patients in the OPD were prescribed drugs and drug combinations. Many of these have an elevated risk of prolonging QT interval and precipitating TdP. Therefore, it is imperative to adopt precautionary measures, to be watchful, and prevent QT prolongation in clinical settings. Various online evidence based drug-drug interaction resources can help physicians to select appropriate medications judiciously for children and adolescent psychiatric outpatients.
116
Hypophosphatemia Induced by Ferric Carboxymaltose Treatment before Surgery in Oncological Patients
Nuñez M1, Montané E1,2, Hladun O1, Jimenez Y3, Joaquin C4, Ramos A4, Morales A5, Sancho A6, Martínez S6, de La Rosa G1, Guardiola H7
1 Clinical Pharmacology Department - Germans Trias i Pujol Hospital, Badalona, Spain
2 Pharmacology, Therapeutics and Toxicology Department, Autonomous University of Barcelona, Cerdanyola del Vallès, Spain
3 Anesthesiology Department - Germans Trias i Pujol Hospital, Badalona, Spain
4 Endocrinology Department - Germans Trias i Pujol Hospital, Badalona, Spain
5 Pharmacy Department - Germans Trias i Pujol Hospital, Badalona, Spain
6 Biochemistry Department - Germans Trias i Pujol Hospital, Badalona, Spain
7 Internal Medicine Department - Germans Trias i Pujol Hospital, Badalona, Spain
Introduction: A recent meta-analysis showed that almost half of the patients treated with intravenous ferric carboxymaltose (FCM) had hypophosphatemia (HP). In these patients, however, periodic phosphatemia controls are not usually performed.
Objectives: Describe the characteristics and risk factors of a cohort of patients who developed HP after treatment with FCM for iron deficiency anemia (IDA) detected and treated before oncological surgery.
Methods: We conducted a retrospective analysis of patients with HP registered in the hospital pharmacovigilance database. We included patients with phosphate levels <2 mg/dL. We considered as risk factors long treatment duration, high dosages, advanced age, prior chronic kidney disease (CKD), low phosphate, ferritin, and transferrin saturation baseline levels.
Results: We evaluated eleven patients with a mean age of 67 years old (range 53–81). Because of the underlying neoplasia, all had IDA (hemoglobin range 6.5 – 11.4 g/dL). There was no history of CKD in any of them, and their phosphate baseline levels were normal. FCM total dosages varied from 1–3.5 g, distributed in 1-4 doses at 6–20 day intervals. Phosphate nadir levels ranged from 0.6–1.8 mg/dL. Five patients (45.4%) had moderate HP, four (36.4%) had severe HP, and two (18.2%) had life-threatening HP (<1 mg/dL), with one having to postpone surgery. All of them were asymptomatic. Nine patients (81.8%) needed intravenous phosphate to recover. Seven patients (63.6%) had one or no risk factors, and four (36.3%) had two or more. According to the Naranjo algorithm, nine patients (81.8%) scored 7 points (probable), and two (18.2%) scored 9 points (definite) for causality assessment.
Conclusion: Oncological patients are at risk for severe HP even if they have few risk factors or have received single doses of FCM before surgery for IDA. Therefore, in this population, phosphatemia should be closely monitored.
131
Adverse drug reactions with positive rechallenge: a retrospective analysis of cases through a hospital pharmacovigilance program
Hladun Alvaro O1, Farré Albaladejo M1,2, Montané Esteva E1,2
1 Clinical Pharmacology Department - Germans Trias i Pujol Hospital, Badalona, Spain
2 Pharmacology, Therapeutics and Toxicology Department, Autonomous University of Barcelona, Cerdanyola del Vallès, Spain
Introduction: Positive rechallenge was considered when a drug suspected of being a possible cause of an adverse drug reaction (ADR) was re-administered and the same reaction occurred again. ADRs with positive rechallenge can increase patient’s morbidity and mortality. Little is known about characteristics of patients with positive rechallenge.
Objectives: To describe the characteristics of ADRs with positive rechallenge that were reported to a tertiary hospital's pharmacovigilance program.
Methods: From January 2017 to December 2021, we did a retrospective analysis of all ADRs with positive rechallenge that were registered in the hospital pharmacovigilance program database. We collected demographic and drug-related data.
Results: There were 53 (5.7%) cases of positive rechallenge out of 922 ADRs reported during the study period. The median age of patients was 59 years old (range 5-85) and 54.7% were men. Immune system responses were the most common ADRs observed (39.6%, 21 cases), such as infusional reactions that were reported in 12 (22.6%). Sixteen cases (30.2%) were serious, 34 (64.2%) were hospital-acquired, and 34 (64.2%) were type B reactions. In 5 (9.4%) cases more than one drug was involved in the ADR. Antiinfectives for systemic use, and antineoplastic and immunomodulating drugs were the most commonly involved (33.3 % and 23.3% respectively), and specifically amoxicillin-clavulanate (4 cases). In 32 (60.4%) the cases, the latency period was <7 days. After rechallenge, the ADR became more serious in 16 (30.2%) cases and the latency period was shorter in 22 (41.5%). Rechallenge was conducted in 30 cases (56.6%) because the benefits outweigh the risks; and it was unintentional in 23 cases (43.4%), of which five (9.4%) were preventable because the first episode was properly recorded in the patient's medical history.
Conclusions: More than half of the rechallenge cases were done intentionally because the benefits outweigh the risks. ADR after rechallenge was more severe in only a quarter of patients and had a shorted latency period in nearly half. Cases of preventable rechallenge could be avoided by carefully reviewing the patient's medical history before prescribing drugs.
144
Portuguese single-centre series of suspected drug-inducedliver injury (DILI) cases included in the Pro-Euro-DILIRegistry
Mainoli B1,2,3, Avó-Baião R1,2,3, Craciun A3,4, Carvalhana S3,4, Moura M3,4, Freitas C3,4, Rosa M1,2,3, Cortez-Pinto H3,4,5
1 Laboratory of Clinical Pharmacology and Therapeutics, Faculdade de Medicina, Universidade de Lisboa, Lisboa, Portugal
2 IMM - Instituto de Medicina Molecular, Faculdade de Medicina, Universidade de Lisboa., Lisbon, Portugal
3 Centro Hospitalar Universitário Lisboa Norte, Lisboa, Portugal
4 Departamento de Gastrenterologia, Centro Hospital Universitário Lisboa Norte, Lisboa, Portugal
5 Clínica Universitária de Gastrenterologia, Laboratório de Nutrição, Faculdade de Medicina, Universidade de Lisboa, Lisbon, Portugal
Introduction: Drug-induced liver injury (DILI) is an adverse drug reaction that is often idiosyncratic and constitutes a challenge for clinicians due to a pathological mechanism not fully understood and its unpredictability. (1)
Objectives: The Pro-Euro-DILI Registry is a multicentre and multidisciplinary European registry of suspected DILI cases. Biological samples of participants are collected and stored in the Pro-Euro-DILI Biobank to establish a large international patient/control cohort and support assessment and validation of new biomarkers with potential application in DILI. (2)
Methods: We report here, cases of suspected DILI enrolled by a single centre participating in Pro-Euro-DILI Network during a 5-year period. Data were prospectively collected in Hepatology Department of a large tertiary hospital. Clinical data were recorded in detailed case report forms by delegated investigators, and participants’ blood samples were stored. All the patients gave informed consent to participate and donate biological samples. Declaration of Helsinki requirements were satisfied to ensure protection of patients' rights.
Results: In our centre, a total of 18 suspected cases were included and discussed by the international panel of experts of Pro-Euro-DILI Network. 10 cases were adjudicated as DILI, and 4 were included as acute liver damage control cases due to other cause, mainly autoimmune hepatitis. 4 cases were excluded, mostly for inconclusive diagnosis (2), for samples unavailability (1) or for not fulfilling DILI criteria (1). Concerning cases adjudicated as DILI, most patients were female, with a mean age of 48.9 years. The most common symptoms at onset were jaundice, choluria, pruritus, and dyspepsia; one patient was asymptomatic, and one had acute liver failure. At the time of admission, 80% of patients presented hepatocellular type of liver injury. No patients were transplanted or died due to DILI and 70% of patients had complete recovery. Causative agents were mainly antimicrobials (40%) and mean RUCAM (Roussel Uclaf Causality Assessment Method) score was 6.9.
Conclusions: DILI is a rare situation, often presenting with difficulties in differential diagnosis with autoimmune hepatitis. The main associated drugs are still antibiotics.
Tabel/Image
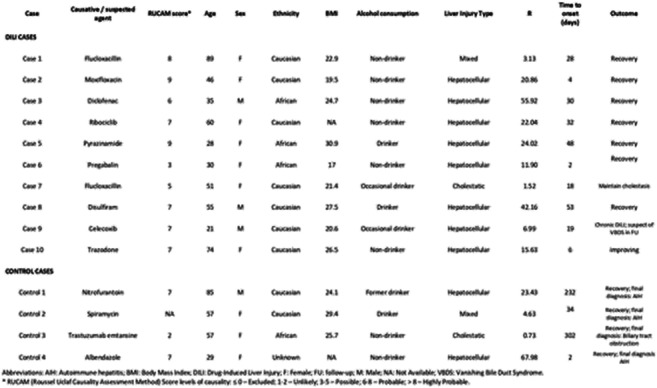
146
Safety of remdesivir in COVID-19 patients: post-marketing evaluation on spontaneous adverse event reporting system
Cesaro M1, Cesaro M1, Rafaniello C1, Di Mauro C1, Zinzi A1, Ferrajolo C1, Scavone C1, Sportiello L1, Sullo M1, Rossi F1, Capuano A1
1 Regional Center of Pharmacovigilance and Pharmacoepidemiology of Campania Region, Department of Experimental Medicine - University of Campania "Luigi Vanvitelli", Naples (Italy), Napoli, Italy
Introduction: Since of recent introduction of remdesivir on the market as antiviral treatment for coronavirus disease 2019 (COVID-19), several evidences from clinical experience provided effectiveness data. The only few evidences on safety profile focused on its hepatotoxicity and hypersensitivity reactions. Data on other potential risks of remdesivir are limited.
Aim: To evaluate the safety of remdesivir bv analyzing real world data on adverse drug reactions (ADR).
Methods: Individual case safety reports (ICSR) involving remdesivir were retrieved from Eudravigilance, centralized European database of reporting ADRs from marketing authorization date to December 2020. Information on patient characteristics, adverse events, seriousness, outcomes, suspected or concomitant medications, or dosage have been analyzed for all ICSRs and for ICSRs with fatal outcome (named fatal ICSRs) or ICSR with at least one adverse event belonging to cardiac system (named cardiac ICSRs). The Reporting Odds Ratio (ROR), with 95% of Confidence Interval (95% CI) were computed to assess the probability of reporting cardiac ICSRs for remdesivir compared to other two medicines used in the pandemic of COVID-19 and described as cardiotoxic drugs, such as azithromycin or hydroxychloroquine.
Results: Among 1,375 ICSRs with remdesivir, 62.8% were related to male and 43.3% to 18-64 years age old. Events fully resolved in one fourth of ICSRs, improved in 11%, and not resolved yet in 13.5% of cases. Almost one third of the total ICSRs reported fatal outcome. The 82.2% of all events was issued as serious. The mostly frequently reported events referred to hepatic/hepatobiliary disorders (19.4%), followed by renal/urinary disorders (11.1%), cardiac events (8.1%). The median duration of remdesivir treatment was 3 days (IQR: 2- 4). Among 221 ICSRs with at least one cardiovascular event, the gender difference still remained but was lower. Sixty-nine ICSRs (31.2%) resulted in death, while 60 ICSRs were fully resolved (27.1%). Other cardiac therapies were reported as suspected/concomitant together with remdesivir in 166 ICSRs (75.1%), 62 of which had fatal outcome. Remdesivir was associated with higher probability to be reported in cardiac ICSRs compared to azithromycin (ROR 2.1, 95% CI 1.8-2.5) and to hydroxychloroquine (2.3; 1.9-2.7).
Conclusion: Liver, kidney and heart have been mostly frequently reported as target of toxicity of remdesivir. However, due to pathogenic mechanism of underling COVID-19 disease involving mainly these organs, our results suggest to in-depth investigate risk-effectiveness of remdesivir in patients at higher risk.
148
From biological plausibility to the occurrence of neurological clinical manifestations due to immune checkpoint inhibitors: an example of translational research
Fraenza F1, Scavone C1, di Mauro G1, Ruggiero R1, Ferrajolo C1, Rafaniello C1, Sportiello L1, Sullo M1, Rossi F1, Capuano A1
1 Regional Center Of Pharmacovigilance And Pharmacoepidemiology Of Campania Region, Department Of Experimental Medicine – University of Campania “Luigi Vanvitelli”, Naples (Italy), Naples, Italy
Introduction: Neurological adverse events (nAEs) related to immune checkpoint inhibitors (ICIs) are potentially life-threatening consequences, infrequently reported in pre-marketing studies.
Objective: To assess the onset and characteristics of ICI-related nAEs in the real clinical practice.
Methods: We screened all cases of nervous disorders occurred in patients treated with ICIs, including ipilimumab, nivolumab, pembrolizumab, cemiplimab, atezolizumab, durvalumab, avelumab, or their combinations, collected in Eudravigilance until February 7th, 2020. We analyzed the characteristics of cases of nAEs involving ICI-treatments (either mono- or combined therapies) in terms of therapeutic indication, types of event and the MedDRA HLGT belonging, gender distribution, outcome or fatality. Finally, we examined the combination of more nAEs in the same patient-report, focusing on the events of greatest clinical interest.
Results: A total of 4875 cases describing 6429 ICI-related nAEs were collected in Eudravigilance. ICIs were mainly used as treatment of lung cancers (35%) and melanoma (26%). The reported nAEs were mainly related to nivolumab (39%) and pembrolizumab (32%) and occurred in males (59%). No substantial differences emerged in the type of neurological complication between male and female patients. The 23% of the events had unfavourable fallouts, including fatal outcome (7%), no resolution of the events (15%) or resolution with sequalae (1%). Overall, myasthenia gravis (N=107), neuropathy peripheral (N=82) and cerebral infarction (N=53) were the events mainly represented among the nAEs with unfavourable outcomes. Moreover, nivolumab and pembrolizumab were the treatments mostly involved in the fatal cases (168/429 and 160/429, respectively). Majority of nAEs were categorized as “Neurological disorders NEC” (32%), “Peripheral neuropathies” (12%), or “Central nervous system vascular disorders” (10%) HLGTs. Finally, 1094 cases (22%) described combination of nAEs. Seizures, encephalitis, and meningitis were the neurological complications of major interest more frequently to other nAEs.
Conclusions: The ICIs neurologic safety profiles need a continue monitoring of data coming from the clinical contexts and the identification of possible related biomarkers would be desirable to prevent these nAEs.
150
Correlation among the oral anticoagulants and haemorrhagic risk by applying Correspondence Analysis to the Italian Spontaneous Reporting System
Gaio M1, Mascolo A1, Ferrajolo C1, Rafaniello C1, Scavone C1, Sportiello L1, Sullo M1, Rossi F1, Capuano A1
1 Regional Center of Pharmacovigilance and Pharmacoepidemiology of Campania Region, Department of Experimental Medicine – University of Campania “Luigi Vanvitelli”, Naples, Italy
Introduction: Post-marketing data on risks associated with direct oral anticoagulants (DOACs) are conflicting and only few studies compared risks between different DOACs. Real-world data from pharmacovigilance databases can help to better define safety profile of each DOAC and warfarin. In this context, the “Correspondence Analysis (CA)“ could represent a useful tool of pharmacovigilance studies to explore the relationship among events and drugs.
Objective: We aimed to conduct a comparative analysis of the reports of suspected adverse drug reactions (ADRs) associated with the use of oral anticoagulants.
Methods: Study based on ADRs sent to the Italian National database for Pharmacovigilance by Campania Region from 2008 to 2021, in which warfarin, dabigatran, apixaban, edoxaban or rivaroxaban were reported as suspected drug. ADRs were clustered into three Standardized MedDRA Queries: cerebral haemorrhage (HCNS), gastrointestinal haemorrhage (HGI) and other haemorrhages (HH). Non-haemorrhagic ADRs were included in a fourth cluster (NOH). In order to illustrate the most important relationships among the frequencies of the different SMQs within each suspected drug (i.e. variables’ response categories), we applied the CA as statistical method designed for “inertia”, to measure variance/dispersion of the individual profiles around the average profile and represents a measure of deviation from independence.
Results: We retrieved 1,161 reports: 41.5% are associated to warfarin, 21.0% to dabigatran, 17.8% to rivaroxaban, 13.9% to apixaban and 5.8% to edoxaban. No significant differences in age distribution were observed. There were differences of distribution of ADRs among different drugs (P<0.05). Results of CA showed that dabigatran and warfarin have the highest contribution (44.910 and 47.656, respectively) to the Λ² of Dim. 1 as well as apixaban and dabigatran to the inertia of Dim. 2 (53.768 and 30.488, respectively). Edoxaban and rivaroxaban showed a negligible total contribution. CA biplot showed positive associations between warfarin and HH, apixaban and CNSH and dabigatran and NOH.
Conclusion: Results suggest that DOACs are not interchangeable. Apixaban was surprisingly associated with a higher risk of cerebral haemorrhage. As expected, our data support the better safety profile of DOACs than warfarin in terms of skin and respiratory tract hemorrhagic risks. Finally, we showed how CA could play a complementary role in analyzing data from pharmacovigilance databases.
Tabel/Image
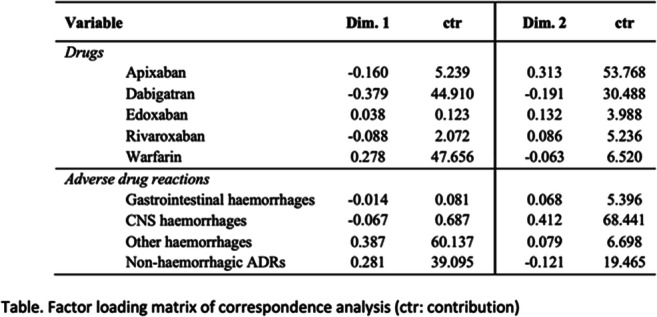
169
DRUG INDUCED RETINAL DISORDERS IDENTIFIED THROUGH SPONTANEOUS REPORTING SYSTEM
Guillen E1,4, Filippi-Arriaga F1, Cereza G2,3,4
1 Department of Clinical Pharmacology, Vall d’Hebron Hospital Universitari, Barcelona, Spain
2 Catalan Centre of Pharmacovigilance, Barcelona, Spain
3 Fundació Institut Català de Farmacologia, Barcelona, Spain
4 Department of Pharmacology, Therapeutics and Toxicology, Universidad Autonoma de Barcelona, Bellaterra, Spain
Introduction: The retina is a highly specialized sense organ subjected to constant exposure to systemic toxins and oxidative stress. The frequency and etiology of drug-induced retinopathy, as well as the number of new potential drugs involved, are largely unknown.
Objective: Describe the most frequent drug-induced retinal disorders and the drugs implicated gathered through the spontaneous report registry of the Spanish System of Pharmacovigilance (SSP).
Methods: All spontaneous reported cases describing “Retinal structural change, deposit and degeneration”, “Retinopathies not elsewhere classified”, and “Retinal bleeding and vascular disorders (excluded retinopathy)” (MedDRA-HLT) in the SSP database from 1983 to January 2022 were selected. Medical devices and marketing authorisation holder cases were excluded. The variables studied were age and sex of the patients, characteristics of adverse drug reactions (ADRs) (seriousness, outcome) and suspect drugs (active substance, anatomical therapeutic chemical code, previous knowledge of drug-reaction association, rechallenge and existence of alternative causes).
Results: Out of 175 spontaneous reports (0.1% of the spontaneous reports in the SSP database) that describe 210 ocular ADRs and/or adverse events, the most frequent (MedDRA-HLT) were retinal bleeding and vascular disorders (111, 52.9%), ocular structural change, deposit and degeneration (59, 28.1%) and vision disorders (12, 5.7%). For MedDRA-PT; retinal vein thrombosis (38, 18.1%), retinal detachment (22, 10.5%) and retinal hemorrhage (20, 9.5%). In only 8 cases (3.8%) drug administration was ophthalmic. Patient’s median age was 57.65 (IQR 48-67.5) years; 68.6% (120) were adults and 56.6% (99) were women. 153 reports (87.4%) were serious. 10.9% (19) cases resolved after withdrawal of the suspect drug and 12.6% (22) resolved with sequelae. A total of 220 drugs were suspected, of which 55 (25%) were COVID-19 vaccines -vector vaccine ChAdOx1 nCoV-19/AZD1222, Oxford-AstraZeneca (27) and mRNA vaccine BNT162b2, Pfizer-BioNTech (23)-, followed by sex hormones (21), immunostimulants (16) and antiprotozoals (14). Of the 175 reports, 56% (98) were poorly or unknown ADR associations. Alternative causes were excluded in 46 (26.3%) cases of which 12 (26%) were poorly or unknown ADR associations and no cases had a positive rechallenge.
Conclusion: Our study shows that drug-induced retinopathy is an infrequent but serious complication. In the SSP database more than half of ADRs were retinal bleeding and vascular disorders. A quarter of the suspected drugs were new COVID-19 vaccines, followed by other drugs for which retinal disorders are well known. Although striking, it is important to contextualize this data in the current situation, considering the particularities of pharmacovigilance in vaccines, the massive rollout campaign and the nascent and evolving data on COVID-19 vaccines. Thus, further studies are needed to confirm such associations. Moreover, clinicians should be aware of drug-induced retinal disorders, even when not listed in the product information leaflet.
176
Generalized Exanthematous Pustulosis-induced by antibiotics
Fathallah N1, Ouni B1, Mokni S2, Kenani Z2, Denguezli M2, Ben Salem C1, Slim R1
1 Department of Pharmacology, Laboratoire de Biophysique Métabolique et Toxicologie Professionnelle et Environnementale Appliquée LR12ES02. Université de Sousse, Faculté de Medecine, Sousse
2 Department of Dermatology, Farhat Hached University Hospital, Sousse
Introduction: Acute generalized exanthematous pustulosis (AGEP) is a rare and potentially lifethreatening acute cutaneous reaction. It is usually caused by a drug reaction and its onset is generally about 48 hours after medication exposure. Antibiotics are the most common trigger.
Objectives: Herein we report sociodemographic and drug characteristics of a serial cases of antibiotic-induced AGEP addressed to the pharmacovigilance of Sousse Tunisia.
Methods: We included in our study all cases of AGEP notified in the pharmacovigilance of Sousse in a period of 10 years (from January 2010 to January 2020).
Results: Antibiotics were reported in 8 patients of all cases of AGEP notified. The time of onset varied from 15 hours to 2.8 days. The most reported antibiotics were the betalactamins (amoxicillin, amoxicillin and clavulanate acid, oxacillin and cefazolin). Eruption was a widespread erythematous eruption with numerous non follicular pinpoint pustules, covering generally the trunk in 4 cases, and proximal limbs in 4 cases. In all cases, blood analysis showed raised white cell count with a predominant neutrophilia. Biospy was performed in all cases, showing features consistent with AGEP. Patch tests were performed in all patients, showing positive results in two cases (amoxicillin and oxacillin).
Conclusions: Drugs are incriminated in more than 90% of cases of AGEP. Medications from many different pharmacological classes, especially antibiotics such as sulfonamides, aminopenicillins, quinolones, lincosamides and pristinamycin, have been suspected in the development of AGEP. β-Lactams account for 80% of antibiotics implicated in AGEP.
Drug patch tests have been recently confirmed to be safe, with few reported relapses or severe reactions, and helpful to assess drug imputability in AGEP, with a high proportion of positive results as compared with patients with other drug-related eruptions.
178
A recurrent drug rash with eosinophilia and systemic symptoms: initially to valproate and recurring with phenobarbital
Fathallah N1, Slim R1, Mokni S2, Denguezli M2, Ben Salem C1, Ouni B1
1 Department of Pharmacology, Laboratoire de Biophysique Métabolique et Toxicologie Professionnelle et Environnementale Appliquée LR12ES02 Université de Sousse, Faculté de Medecine, Sousse
2 Department of Dermatology, Farhat Hached University Hospital of Sousse, Sousse
Introduction: Drug rash with eosinophilia and systemic symptoms (DRESS) is characterized by fever, rash and internal organ involvement after exposure to certain drugs. Most of the aromatic anticonvulsants, such as phenytoin, phenobarbital, and carbamazepine, can induce DRESS. Nonaromatic drugs such as lamotrigine and valproate are known to be more safe than aromatic anticonvulsants and are less respounsible of DRESS. We report here an occurrence of DRESS following exposure to valproate and recurring after phenobarbital intake.
Case Report: A 54-year-old man without previous significant medical history had been diagnosed with brain tumor. He was treated by surgery and received valproate (400 mg daily). No other medications had been taken. Three weeks later, the patient was admitted to the hospital with fever and general eruption. The patient’s face was edematous and erythematous papules were scattered over his entire body. Lymph nodes were palpable. Laboratory findings showed hypereosinophilia and elevated liver enzymes. Viral serology was negative for hepatitis A, B, and C, cytomegalovirus and Epstein–Barr virus. On suspicion of DRESS, valproate was stopped. While clinical symptoms and laboratory findings improved progressively, phenobarbital was started. The patient developed again a generalized rash and fever few days after phenobarbital administartion. The neurosurgeon decided to stop phenobarbital. Symptoms resolved few days later without complications.
Discussion: Drug rash with eosinophilia and systemic symptoms is a severe adverse reaction with high mortality rates. The aromatic anticonvulsants are the most frequently incriminated drugs. In DRESS, discontinuation of the offending aromatic anticonvulsant is essential for improving the prognosis. In this case, valproate is usually a safe alternative for aromatic anticonvulsants. In fact, Sodium valproate is very rarely responsible for DRESS. As far as we know, DRESS syndrome cases related solely to the use of valproate have not been previously reported. Herin, we report the first case of DRESS primarily induced by sodium valproate and secondarily to phenobarbital. This case illustrates a possible cross-reactivity between valproate and phenobarbital, which are non aromatic and aromatic anticonvulsants, respectively.
Conclusion: Switching anticonvulsants is not usually safe. Clinicians should be more vigilant when adverse events occur first with non aromatic anticonvulsants.
180
Clozapine-induced adverse reactions: about a case series of 62 patients
Fathallah N1, Ouni B1, Ladhari C1, Mtiraoui A2, Nakhli J2, Ben Salem C1
1 Department of Pharmacology, Laboratoire de Biophysique Métabolique et Toxicologie Professionnelle et Environnementale Appliquée LR12ES02. Université de Sousse, Faculté de Medecine, Sousse
2 Department of Psychiatry, Farhat Hached University Hospital, Sousse
Introduction: Clozapine is the only medication with evidence-based effectiveness in the treatment of resistant schizo phrenia, being effective in 50 – 60 % of cases. However, it is associated with a broad range of adverse effects (AEs).
Objectives: We conducted this study to identify Clozapine’s AEs reported at the regional centre of pharmacovigilance of Sousse (CRPV), to study their epidemiological and clinical characteristics and to identify their management.
Methods: We conducted a retrospective and descriptive study of the clozapine-induced AEs notified to the CRPV between January 2009 and July 2017. We have included the notifications where clozapine was the suspected drug. All the cases with AEs more likely induced by other drugs were excluded from our study. The causality assessment was evaluated by the updated French method of imputability and the Naranjo probability scale.
Results: This study included 62 patients presenting 97 AEs. The most common AE was hypersialorrhea, found among 53.2% of patients with a frequency of 34%, an average of delay-onset of 123 days and an average dose of 471.9 mg. Hematologic disorders included hypereosinophila and agranulocytosis. Hematologic disorders had a frequency of 16.5%. The mean delay of onset of eosinophilia was 42.3 days and 30 days for agranulocytosis.
In our case-series, we had noticed some transitory hypereosinophila especially at the beginning of the treatment by Clozapine. However we noted 2 severe cases oh hypereosinophilia associated to cytolysis and episcleritis for the 2nd case. Among the two cases of agranulocytosis, one rechallange was undertaken and was successful. Endocrine disorders accounted for 6.1% and were as the following: one case of metabolic syndrome and 5 cases of weight gain. Anti-cholinergic manifestations had a frequency of 10.3% and were as following: dry mouth and constipation. Other AEs accounted over 10% and were tremor, sexual impotence, parotitis, tachycardia, urinary incontinence, episcleritis, hair loss and headache.
Conclusion: Through this study, we showed the variability of clozapine AEs. For mild and transient reactions, Clozapine didn’t need to be withdrawn. In the case of serious AEs such as agranulocytosis, the rechallange could be undertaken after a multidisciplinary decision.
185
COVID-19 Vaccine safety update
Quijada Manuitt M1, Amaro Hosey K1, Antonijoan Arbos R1
1 Hospital De Santa Creu i Sant Pau, Barcelona, España
Introduction: Due to COVID-19 pandemics, vaccines were approved by regulatory authorities in an accelerated procedure, arising concerns about safety. This abstract refers to the experience of a Pharmacovigilance unit in a third level hospital in Barcelona, Spain.
Objectives: To assess the adverse drug reactions (ADR) reported to COVID-19 vaccines and their characteristics based on spontaneous notification.
Methods: An observational study of reported ADR to COVID-19 vaccines from January 2021 to February 2022 was carried out. A descriptive analysis of demographic characteristics, COVID-19 vaccines and ADR was performed. ADR incidence was estimated.
Results: The estimated ADR incidence was 8.8%. A total of 684 suspected ADR cases were reported, corresponding to 441 users. Mean age was 43.0 (SD 12.0) years and 385 (87.3%) were women. One hundred eleven users (25.2%) had previously documented COVID-19 infection. Two-hundred eighty (40.9%) ADR suspected cases were reported after the first dose (Comirnaty® = 225 [80.4%], Spikevax® = 54 [19.3%]), 369 (49.6%) occurred after the second dose (Comirnaty® = 305 [90.0%] and Spikevax® = 34 [10.0%]), and 65 (9.5%; Spikevax® = 100%) regarding third doses.
Regarding severity, 2.4% were serious: the most frequently were nervous system disorders (paraesthesia = 3, paresis = 2, neuralgia = 2, dysesthesia = 1, vertigo = 1 and allodynia = 1), respiratory disorders (dyspnoea = 8 and COVID-19 pneumonia = 1), Immune system disorders (Eyelid oedema = 4, Palatal oedema = 1 and Swelling face = 1), and reproductive (Intermenstrual bleeding= 3, abortion = 1, amenorrhoea =1 and Galactorrhoea= 1). Only two ADR caused hospital admission, and the rest were considered clinically relevant. Severe ADR were reported to the Pharmacovigilance System. General disorders, nervous system and musculoskeletal represented globally the most common ADR: injection site pain, headache and fatigue related to the first dose; and fever, myalgia and arthralgia and fatigue in relation to the second and third dose. Positive reexposition was observed in 117 cases, 6 of whom were considered severe.
Conclusion: The ADR incidence to covid vaccines was less than 10%. Most ADR cases reported were considered non-serious and less than 3% were considered serious. Vaccines safety profile observed is consistent as described in clinical trials.
Tabel/Image
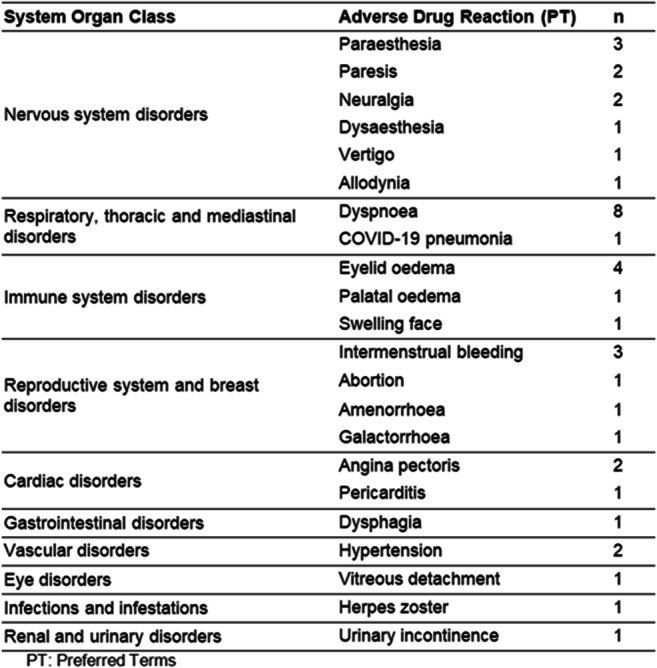
197
CHARACTERIZATION OF DRUG-INDUCED LIVER INJURY CASES WITH DRUG REACTION WITH EOSINOPHILIA AND SYSTEMIC SYMPTOMS: DEMOGRAPHICS, CLINICAL FEATURES AND CULPRIT DRUGS
Villanueva-Paz M1, Sanabria-Cabrera J1, Medina-Caliz I1, Niu H1, Alvarez-Alvarez I1, Aukstikalne L2, Robles-Diaz M1, Garcia-Cortes M1, Ortega-Alonso A1, Bessone F3, Forns X4, Romero-Gomez M5, Soriano G6, Hernandez N7, Lucena M1, Andrade R1
1 UGC Aparato Digestivo y Servicio de Farmacología Clínica. Instituto de Investigación Biomédica de Málaga (IBIMA). Hospital Universitario Virgen de la Victoria. Universidad de Málaga, CIBERehd, Málaga, Spain
2 Lithuanian University of Health Sciences, Institute of Physiology and Pharmacology, Kaunas, Lithuania
3 Hospital Provincial del Centenario, Rosario, Argentina
4 Hospital Clinic de Barcelona. CIBERehd, Barcelona, España
5 Hospitales Universitarios Virgen Macarena-Virgen del Rocío. CIBERehd, Sevilla, España
6 Hospital de la Santa Creu i Sant Pau. CIBERehd, Barcelona, España
7 Hospital de Clínicas, Montevideo, Uruguay
Introduction: Drug-induced liver injury (DILI) may develop in the context of an immune-mediated severe cutaneous adverse reaction, including drug reaction with eosinophilia and systemic symptoms (DRESS). However, the characterization of DILI in the setting of DRESS is a subject yet undefined.
Objectives: We aimed to comprehensively assess the clinical characteristics, outcomes, and causative agents in a cohort of well-defined DILI cases with DRESS from the Spanish DILI Registry and the LATINDILI Network.
Methods: Data from 55 well-characterized DILI cases with DRESS enrolled in the Spanish DILI Registry (N=31) and LATINDILI Network (N=24) between 1994 and 2020 were retrieved. DRESS was defined according to the European Registry of Severe Cutaneous Adverse Reactions (RegiSCAR) diagnostic criteria. Steven Johnson syndrome and toxic epidermal necrolysis cases, and cases with missing or incomplete information of any of the hypersensitivity features were excluded.
Results: DILI-DRESS patients were aged 48±20 years, and 51% were female. Hepatocellular damage was predominant (44%). Most patients (80%) had eosinophilia and were hospitalized. Over half of the patients showed a moderate liver injury (55%), while the 15% developed a severe damage. Only one patient progressed to acute liver failure and died. DILI-DRESS cases showed a distinctive causative agent pattern compared to DILI cases included in the registries. The most frequent drugs were antiepileptic drugs (carbamazepine, lamotrigine, and phenytoin, 24%), amoxicillin-clavulanate (13%), anti-tuberculosis drugs (9.1%) and allopurinol (7.3%). Antiepileptic drug-induced cases were younger and predominantly women. In allopurinol cases, mixed pattern of liver injury was distinctive. Anti-tuberculosis drugs were associated with a more severe liver injury and the fatal outcome, and a prolonged time to resolution.
Conclusion: Obtaining a complete clinical picture of DRESS represents a major challenge. In this well-characterized cohort, DILI-DRESS cases coursed with great severity, but low mortality. Antiepileptic drugs were the leading causative agent, causing predominantly a hepatocellular pattern of liver injury. These findings represent a major step to understand the distinctive clinical features and outcomes of DRESS in the context of DILI.
199
Improved, but still limited coding of medication-related problems – application of the new WHO ICD-11 code-set to clinical routine data
Jung-Poppe L1, Andrikyan W1, Altenbuchner A1, Pfistermeister B2,3, Dormann H2, Fromm M1, Maas R1
1 Friedrich-Alexander-Universität Erlangen-Nürnberg, Institute of Experimental and Clinical Pharmacology and Toxicology, Fahrstraße 17, 91054 Erlangen, Germany
2 Fürth Hospital, Study Center of the Emergency Department, Jakob-Henle-Str.1, 90766 Fürth, Germany
3 Fürth Hospital, Hospital Pharmacy, Jakob-Henle-Str.1, 90766 Fürth, Germany
Introduction: Medication-related problems (MRP) such as adverse drug reactions or events (ADR/ADE) and medication errors (ME) are a common and serious threat for patient wellbeing and safety. Documentation of MRP is a prerequisite for reporting (i.e., quality control and research) and prevention. In clinical routine, documentation of MRP is often insufficient due to limited codability using the ‘International Statistical Classification of Diseases and Related Health Problems’ (ICD) 10. In January 2022 the 11th revision of ICD (ICD-11) came into effect. This version contains a new code-set for reporting different aspects of quality of care and patient safety by allowing post-coordination (combination of codes), which could potentially help to improve documentation of MRP.
Objectives: The aim of this work was to assess the practical usability of ICD-11 to systematically code and document MRP in clinical routine.
Methods: In a cooperation of projects funded by the German Federal Ministry of Health (BMG grant number ZMVI1-2519ATS004Z) and the German Federal Ministry of Education and Research (BMBF grant number 01ZZ1910O) we assessed 100 different anonymized MRP patient cases (50 adverse drug reactions/events and 50 medication errors) verified by an interdisciplinary team of pharmacists and physicians from the ‘medication safety stewardship’ project (German Clinical Trial Register ID 00017534). The MRP cases were observed in the clinical routine of internal and surgical wards at a large tertiary care hospital and chosen to represent a broad clinical spectrum of MRP. Codability and usability of MRP were assessed by application of the WHO´s proposed three-part quality and safety model and a method for terminology mapping (ISO/TR 12300:2014).
Results: Of 50 ADR/ADE 13 (26%) were fully classifiable and codable by ICD-11 according to the three-part quality and safety model, whereas 37 (74%) could not be fully classified due to ‘sanctioning rules’ of the ICD-11 preventing the combination of specific codes. As for terminology matching, 34 (68%) ADR/ADE could be matched with an equivalent ICD-11 code. In 4 (8%) cases the ADR/ADE as detailed in the patient record was broader and in 12 (24%) cases it was narrower than the corresponding ICD-11 code. The three-part model is not specifically designed for reporting ME (without harm or injury). However, 41 (82%) of the 50 ME were codable with codes describing circumstances influencing the episode of care without injury or harm (ICD-11, Chapter 24). None of these codes allows post-coordination and therefore prevents the assignment of the event with its causing drug(s).
Conclusion: The ICD-11 code-set for quality and patient safety enables more detailed documentation of MRP than previous ICD versions. For the majority of ADR/ADE matching codes exist. Nevertheless, the three-part quality and safety model is only applicable for a minority of ICD-11 coded MRP. Codability, documentation, and reporting of MRP could be significantly improved by modifying current ICD-11 ‘sanctioning rules’ and adding new codes to Chapter 23 (Subchapter: ‘Causes of healthcare related harm or injury’).
Tabel/Image

201
COVID-19 vaccines or infection possibly increase risk of agranulocytosis in patients on potentially hematologically toxic treatment
Vukovic J1, Walker M1, Massy-Chalverat M1, Rochat C1, Panait C1, Blondel N1
1 Department of Internal Medicine, Cantonal Hospital Fribourg, Switzerland, Fribourg, Switzerland
Introduction: Department of Internal Medicine in Cantonal Hospital Fribourg, Switzerland, has a regular activity of monitoring and reporting of adverse drug effects to Swiss Agency for Therapeutic Products. During 2021 special attention was directed to the safety of COVID-19 mRNA vaccines. COVID-19 infection and vaccines trigger immune response, which may cause adverse reactions to vaccines or may modify the responses to drugs. The immune response may also modify enzymes’ activity (e.g. CYP P450).
Objectives: Our objective is to indicate a possible increased risk of hematological adverse drug effects due to disturbed immune response.
Methods: We present two case reports of patients treated with drugs with potential for hematological toxicity without previously detected neutropenia, who developed agranulocytosis after the COVID-19 vaccine or infection.
Results:
Case 1: Patient, male, 34 years old, obese, receives the 1st dose of COVID mRNA vaccine Spikevax®. Few days later he notices the painful induration around the anus. In order to threat the pain, he takes metamizole for 3 days, already used in the past, but he develops fever. His GP discontinues metamizole and starts paracetamol. Patient’s status deteriorates and he is hospitalized due to febrile agranulocytosis, 15 days after the vaccine and 7 days after the initiation of metamizole. He has a negative PCR COVID test on entry. His hospitalization is marked with many complications; however, he recovers from agranulocytosis after 10 days treatment. He doesn’t receive the 2nd dose of the vaccine.
Case 2: Patient, female, 55 years old, who suffers from multiple sclerosis, is treated by ocrelizumab (anti-CD20), with known risk of late onset neutropenia. She is regularly examined by neurologist and neutropenia was never detected. The last dose was received 2 months before hospitalization. She is hospitalized for febrile agranulocytosis with acute mild COVID infection. PCR is positive and patient history confirms start of symptoms one week before. All other infections known to may cause neutropenia are excluded. The patient is recovered from agranulocytosis 5 days later without receiving G-CSF.
Conclusion: These cases alert to possible link between increased risk of agranulocytosis in patients on potentially hematologically toxic treatment and COVID mRNA vaccines or infection.
204
Adherence to Clinical Guidelines and Patients' Compliance to First Year Essential Hypertension Medication Treatment in Adults Over 65 Years
Lavon O1,2, Omri S1
1 Clinical Pharmacology and Toxicology Unit, Carmel Medical Center, Haifa, Israel
2 Rappaport Faculty of Medicine, Technion-Israel Institute of Technology, Haifa, Isarel
Introduction: Hypertension (HTN) is a common disease with serious complications. Its prevalence is increasing with age. Treatment of HTN in the elderly population is challenging.
Objectives: To examine the scope of adherence to clinical guidelines for first-line medication treatment in HTN, patients' compliance to this treatment, and the impact on real-world clinical outcomes.
Methods: A retrospective cohort study was conducted based on electronic medical records of patients over the age of 65 years, who were diagnosed with primary HTN, with no previous history of heart events, strokes or advanced kidney disease, and without prior treatment with HTN drugs in the year before the diagnosis of HTN. Demographic and clinical data were collected and a comprehensive statistical analysis was performed.
Results: After screening and exclusion, the study sample included 2,934 patients. Most patients (81.2%) start HTN treatment with a single medication, not dual, contrary to curreent guidelines. About 10% were treated with drugs that are not recommended as first line, mainly B-blockers and alpha blockers. In 10.6% of the study population, the medication was replaced within the first year. About half of the medication changes were with ACE inhibitors (11% of patients that started treatment with ACE inhibitors). This is a similar rate to the prevalence of a common adverse reaction to ACE inhibitors, cough, which usually leads to discontinuation of the drug. A relatively low compliance, below 80%, according to PDC (Proportion of Days Covered) calculation, was found in about a third of the patients. A statistically significant low incidence of strokes was found among patients with higher compliance to treatment. A tendency for a lower all-cause mortality rate was found with higher treatment compliance. Adverse safety signals, secondary to higher treatment compliance, were not observed.
Conclusion: There are gaps in the adherence to guidelines and in patients' compliance regarding HTN medications in the first year of treatment among the elderly. Compliance to treatment was significantly linked to reduced incidence of stroke. It is essential to promote interventions aimed at improvement of HTN medication adherence to guidelines and patients' compliance.
213
Two profiles of drug-induced Selective Eating Disorder characterized by Disproportionality Analysis
MERINO D1,2, GERARD A2,3, PIERRE M4, BEN OTHMAN N2, CHARRIE C2, ETTORE E1, GIORDANA B1, VIARD D2, ROCHER F2, DESTERE A2, BENOIT M1, DRICI M2
1 Department of Psychiatry, University Hospital of Nice, Nice, France
2 Department of Pharmacology and Pharmacovigilance Center of Nice, University Hospital of Nice, Nice, France
3 Department of Nephrology-Dialysis-Transplantation, University Hospital of Nice, Nice, France
4 Department of Obstetrics and Gynaecology, Montpellier University Hospital, Montpellier, France
Introduction: Selective eating (SE) consists of the consumption of a limited variety of foods and a reluctance to try new ones. Its prevalence (higher in early childhood) and persistence may lead to nutritional deficiencies and psychosocial dysfunction. Several drug classes are associated with appetite disturbances, yet their association with SE remains unclear.
Objectives: We aimed to characterize which drugs are strongly and disproportionaly associated with SE.
Methods: The World Health Organization (WHO) pharmacovigilance database (VigiBase®) was queried for all reports of “SE disorder” ever registered, until February 24, 2022. All drugs deemed suspect or interacting in 10 or more cases were included. Disproportionality analysis relied on the Reporting Odds Ratio (ROR) with its 95% Confidence Interval (CI) and the lower end of the 95% CI of the Information Component (IC025). A positive IC025 is required to detect a signal.
Results: In VigiBase®, 1,711 cases of drug-associated SE reports were collected. SE was deemed serious in 1,167 reports, among which 48 cases of deaths. New-borns accounted for 427 cases, venlafaxine (8.0%), paroxetine (5.9%) and lamotrigine (5.6%) being the most suspected. Reports from infants (28 days to 23 months) mainly involved pneumococcal vaccine (39.8%), rotavirus vaccine (37.1%) and Haemophilus influenzae type b vaccine (24.0%). Significant disproportionate reporting was found for over 50 drugs, including rotavirus vaccine (58.0; 95%CI 50.2-67.1), palivizumab (56.0; 95%CI 41.4-75.9), DTP‐HBV vaccine (52.3; 95%CI 37.0-73.8),ondansetron (49.9; 95%CI 39.0-63.8), as well as various psychotropic drugs.
Conclusion: This study clearly yields two profiles of safety concerns: SE apparently results from maternal drug exposure in newborns (psychotropic drugs, ondansetron) whereas in older infants, SE could be associated with vaccination-induced fussiness. Even if causality remains unclear from this study, such potential drug triggers, especially when taken by the mother, may guide physicians dealing with a SE disorder in infants.
218
Is Drug-associated Merycism a Chinese peculiarity?
MERINO D1,2, GERARD A2,3, FARRUGIA M4, BEN OTHMAN N2, ETTORE E1, GIORDANA B1, VIARD D2, ROCHER F2, DESTERE A2, BENOIT M1, DRICI M2
1 Department of Psychiatry, University Hospital of Nice, Nice, France
2 Department of Pharmacology and Pharmacovigilance Center of Nice, University Hospital of Nice, Nice, France
3 Department of Nephrology-Dialysis-Transplantation, University Hospital of Nice, Nice, France
4 Department of Gastroenterology and Hepatology, University Hospital of Nice, Nice, France
Introduction: Merycism (or rumination syndrome) is characterized by the effortless and repetitive regurgitation of recently ingested food into the oral cavity aftermeals. The regurgitated material may be chewed, swallowed again or spat out. The origin of this functional disorder (usually affecting children but also found in adults) is unclear, but has been occasionally associated with drugs.
Objectives: We aimed to assess by disproportionality analysis which medications were linked to merycism in the World Health Organization (WHO) pharmacovigilance database (VigiBase®).
Methods: We queried VigiBase® for all reports of “Merycism”, up to February 24, 2022. All medications deemed suspect or interacting in 10 or more reports were included. Analysis was based on the Reporting Odds Ratio (ROR) with its 95% Confidence Interval (CI) and the lower end of the 95% CI of the Information Component (IC025). A positive IC025 determining a statistical signal.
Results: The query yielded 1,450 cases of drug-associated merycism, 1316 (90.8%) originating from China. Merycism was deemed serious in 185 reports, including 4 cases of deaths. “Nausea” (38.6%), “vomiting” (14.0%) and “dizziness” (10.3%) were frequently co-reported. The drugs mostly involved were indapamide (5.8%), levofloxacin (3.6%) and azithromycin (2.8%). Significant disproportionality was found for 23 medications including indapamide (145.7; 95%CI 116.8-181.8), tinidazole (41.6; 95%CI 25.4-68.1), ornidazole (29.3; 95%CI 17.9-48.0), roxithromycin (16.9; 95%CI 9.3-30.5) and serotonin reuptake inhibitor antidepressants.
Conclusion: Merycism was disproportionately reported with antiinfectives but also with antidepressants and antihypertensives. The geographical distribution of this peculiar disorder was strikingly unusual, overwhelmingly coming from China, reflecting perhaps prescribing behaviors. Our analysis underlines the necessity to address a drug-associated adverse event in the light of the sociocultural context. However, causality cannot be ascertained with this sole approach. Further studies are also necessary to investigate the origins of the geographical discrepancy such as possible underdiagnosis of merycism in western countries.
245
Carbamazepine-Induced DRESS Syndrome in a child Confirmed by Positive Patch Test
Slim R1, Fathallah N1, Ouni B1, Ben Salem C1
1 Department of Pharmacology, Laboratoire de Biophysique Métabolique et Toxicologie Professionnelle et Environnementale Appliquée LR12ES02. Université de Sousse, Faculté de Medecine, Sousse
Background: Drug reaction with eosinophilia and systemic symptoms (DRESS) is a severe and potentially life-threatening disease. Carbamazepine is among the most frequently reported anticonvulsant drug causes of this syndrome in adults, but it is rarely reported in children. Here, we present a case of carbamazepine induced DRESS in a 15-year-old girl confirmed by positive patch test.
Case Report: A 15-year-old girl was treated with carbamazepine and sertaline for depressive disorder. Six weeks later, she presented with generalized erythematous skin eruption and fever. Examination revelateddiffuse erythematous rash with evident facial edema. She had palpable bilateral cervical lymphadenopathy. Initial laboratory tests showed leukocytosis with eosinophilia and elevated liver enzymes. Viral serology and antinuclear antibodies were all negative. By fulfilling the proposed criteria of the scoring system for classifying DRESS, our patient was a definite case of carbamazepine-induced DRESS.
Her symptoms resolved after withdrawal of carbamazepine. Her pathological laboratory findings returned to normal ranges within 3 weeks. Patch tests for carbamazepine performed 7months after complete recovery induced a strongly positive skin reaction in 48 hours.
Discussion: DRESS-syndrome is a rare drug-induced hypersensitivity reaction.It is mainly associated with antibacterial sulphonamides and antconvulsivants. DRESS syndrome typically manifests 2–6 weeks after the beginning of the administration of the offending drug. It is characterized by the presence of fever, skin eruption and systemic symptoms including lymphadenopathy, abnormal liver functionthat may progress to liver failure, which is the primary cause of death in DRESS syndrome. In children, Diagnosisof DRESS syndrome may be difficult because it is rarely describedand can mimic many different conditions for example as infections, neoplastic and immunologic conditions. This may delay the diagnosis and the prompt management. Patch testing is a useful tool for identifying the inducing agent of DRESS syndrome and for determining a safe anticonvulsant drug.
Conclusion: The knowledge of DRESS syndrome clinical symptoms in children is essential for clinicians. Early diagnosis and immediate cessation of the suspected drug is primordial to prevent potentially fatal outcomes. Patch test is considered as a useful complementary investigation to confirm the immutability of carbamazepine in DRESS.
248
Does Spontaneous reporting of ADRs through portal funnels patients’ discontent? The illustrating case of Levothyrox®
DRICI M1, MARCHELLO G2, ROCHER F1, MERINO D1,3, GERARD A1,4, BEN OTHMAN N1, BERRIRI S1, VIARD D1, DESTERE A1
1 Department of Pharmacology and Pharmacovigilance Center of Nice, University Côte d’Azur Hospital, France, Nice, France
2 Maasai team, Laboratory J.A.Dieudonné, INRIA, National Center for Scientific Research (CNRS), Côte d’Azur University, Nice, France
3 Department of Psychiatry, University Côte d’Azur Hospital, Nice, France
4 Department of Nephrology-Dialysis-Transplantation, University Côte d’Azur Hospital, Nice, France
Introduction: Patients’ spontaneous reporting of adverse drug reactions (ADR) has proven reliable to help monitor drug safety. Yet, after minor changes of excipients, the launch of a new formula of levothyroxine (LNF: Levothyrox® new formula) led to an unexpected and atypical amount of notifications to our center. Thousands of such reportings ensued nationally, mainly through a dedicated portal. After noting striking differences from other drugs, we aimed to characterize patients’ LNF reports and to compare them with control notifications. We also used artifical intelligence (AI) to screen out LNF amidst different passed drug-related crises.
Methods: Reports involving LNF or control drugs were sampled (200 notifications each) randomly from the national pharmacovigilance database between March 2017 and March 2018. We evaluated the number of ADRs and incriminated drugs per notification, as well as their medical pertinence between groups. We also used the entire base of our Center to apply a generative co-clustering model, named dynamic latent block model (dLBM), which extends the classical binary latent block model to the case of dynamic count data in order to identify time-ADRs-drugs clusters.
Results: Age did not differ significantly and females predominated in LNF (94.5%) compared to controls (60.8%, p<0.001). LNF notifications reported more adverse reactions (8±4) than controls (2±2, p<0.01), mentioning mostly one drug (98.5%) versus 2 in controls (p<0.001). The quantitative distribution for ADRs was Gaussian for LNF compared with Poisson-like for controls (p<0.001). One third of LNF-ADRs only was deemed “expected” (two-third for controls, p<0.001). The application to our large-scale ADRs dataset pointed out that dLBM was not only able to identify clusters that are coherent with retrospective knowledge, in particular for major drug-related crises, but also to detect atypical behaviors, which the health professionals were unaware. AI clearly pointed out a time-ADRs-Levothyrox® signal.
Conclusion: We confirm the atypical profile of ADR reports from patients treated with Levothyrox®. We suggest a “typical” profile for such spontaneous notifications, from which an “atypical” signal may stray. This typical profile features more than one suspected or concomitant drug, less than 5 symptoms, at least half of them being “expected” (mentioned in the SmPC of the drug). Health authorities should address or even prevent atypical notifications, and could do so with AI and dLBM models, in order not to impair the acuity of the European pharmacovigilance system.*
* The views expressed in this article are the authors’ personal views and may not be understood or quoted as being made on behalf of, or reflect in any way the position of the ANSM, the EMA or one of their committees or working parties
284
Community pharmacists’ challenges regarding adverse drug reaction reporting: an example from Serbia
Paut Kusturica M1, Stilinović N1, Tomas A1, Panjković K1, Rašković A1
1 University Of Novi Sad, Faculty Of Medicine, Novi Sad, Serbia
Introduction: The effectiveness of the national drug safety monitoring program directly depends on the active participation of healthcare professionals in reporting suspected adverse drug reactions (ADRs).
Objectives: The aim of the study was to explore community pharmacists’ comprehension of pharmacovigilance, their perspectives toward reporting ADRs, and investigate the current practice of ADR reporting among pharmacists in Serbia.
Methods: This descriptive cross-sectional study was performed on a sample of pharmacists in Serbia between November 2019 and March 2020 using a pre-tested questionnaire distributed online. Eligible participants were community pharmacists in Serbia who were willing to participate in the study during the data collection period. Non-parametric statistical tests were used in analysis of knowledge, perspectives and ADR reporting. Exploratory factor analysis was used to measure the validity and reliability of the survey.
Results: The median knowledge score was 6 out of 10 (interquartile range 5-7, range 2-10). There were no significant differences in the knowledge scores of pharmacists based on hours worked per week (U=24805, p=0.374), working experience (χ2=4.011, DF=2, p=0.135), having a professional membership (U=24312, p=0.209), or highest level of pharmacy qualification obtained (χ2=3.233, DF=3, p=0.506). Only 28.8% of pharmacists reported ADR at least once a year, while the majority of them have never reported any ADRs.
Conclusions: Despite the community pharmacists’ positive attitude toward adverse drug reporting and their role in the process, they show limited knowledge regarding the issue and highly prevalent under-reporting of ADRs. Educational programs are necessary to increase ADRs reporting.
291
ACUTE DRUG-INDUCED PANCREATITIS
BOURAOUI H1, FATHALLAH N1, SLIM R1, BEN AZOUZ C1, OUNI B1, BEN SALEM C1
1 Department of Pharmacology, University of Sousse, Faculty of medecine, Sousse, TUNISIA
Introduction: Drug-Induced Pancreatitis is a difficult diagnosis to establish. Drugs are considered to be a possible cause of pancreatitis when no other cause can be found and the patient is on a medication that has been previously reported to cause pancreatitis. In general, it has been estimated that medications are etiologic factors in less than 2% of acute pancreatitis.
Objectives: We conducted this study to analyse the clinical, biological, chronological and evolutive features of acute drug-induced pancreatitis.
Methods: We conducted a descriptive, retrospective study of all cases of acute pancreatitis that are caused by drugs, notified to the regional centre of pharmacovigilance of Sousse (CRPV) between January 2009 and January 2022.
Results: This study included 6 patients with acute drug-induced pancreatitis assessed as probable by the imputability score. The mean age was 46 years with extremes ranging from 22 to 63 years. Our series included 4 women and 2 men (sex ratio 0.5). 5 of the patients had single drug-induced pancreatitis and 1 had pancreatitis as part of a drug hypersensitivity disorder. The drugs involved were furosemide, allopurinol, meprobamate, secnidazole, tamoxifen and the anti-hypertensive association (enalapril-lercanidipine). The time of onset was variable, ranging from two weeks to several months. The most cases had a favourable outcome. One death was notified in the case of acute pancreatitis associated with hypersensitivity syndrome.
Conclusion: Drug-induced pancreatitis is currently a well-recognised entity. The chronology of symptoms, the onset of an offending drug, the clinical evolution and laboratory test kinetics are key to the diagnosis. However, a delay of several months does not exclude a drug related aetiology. Detailed lists of the most frequently culprit drugs are available in the literature, but it is still the responsibility of each health care professional to notify a pharmacovigilance centre of any suspected drug-induced pancreatitis.
292
Intolerance to non steroidal anti-inflammatory drugs
BOURAOUI H1, SLIM R1, FATHALLAH N1, BEN AZOUZ C1, OUNI B1, BEN SALEM C1
1 Department of Pharmacology, Metabolic Biophysics and Applied Pharmacology Laboratory LR12ES02, Faculty of medecine,University of Sousse, Sousse, TUNISIA
Introduction: Nonsteroidal anti-inflammatory drugs (NSAIDs) are one of the most common groups of drugs implicated in hypersensitivity reactions (HS). These reactions are classified into allergic and pseudo-allergic (NSAIDs intolerance) reactions.
Objectives: We conducted this study to describe the epidemiological, clinical and evolutive characteristics of NSAIDs intolerance and to evaluate the tolerance to paracetamol of these patients.
Patients and methods: We conducted a descriptive study carried out in the regional pharmacovigilance centre of Sousse during 4 years (January 2016-December 2019). All patients with NSAIDs intolerance were included.
Results: This study included 14 patients. The mean age was 31.64 years. The sex ratio was 1. A history of atopy was present in 6 patients (42%). All patients had a history of previous hypersensitivity to one or more NSAIDs. The clinical manifestations of hypersensitivity were dominated by skin reactions in 85% of cases, followed by respiratory signs in 64% of cases. The average time to onset of symptoms was 146 minutes. According to Ring and Messmer classification, anaphylaxis was grade I in 4 of our patients, grade II and III in 5 patients each. Acetylsalicylic acid (Aspegic) was the most common drug responsible for the occurrence of clinical manifestations. Most of our patients (85.71%) tolerated paracetamol without any problems. The tolerated dose was between 500 and 2000 mg.
Conclusion: Through this study, we have noticed that acetylsalicylic acid (Aspegic) is the most responsible molecule for the occurrence of clinical manifestations of intolerance to NSAIDs. Because of the risk of cross-reactions, tolerance to paracetamol in patients with pharmacological HS to NSAIDs should be evaluated.
294
Terbinafine-induced acute generalized exanthematous pustulosis
BEN AZOUZ C1, BOURAOUI H1, FATHALLAH N1, SLIM R1, OUNI B1
1 Department of Pharmacology, Metabolic Biophysics and Applied Pharmacology Laboratory LR12ES02, Faculty of medecine, University of Sousse, Sousse, TUNISIA
Introduction: Acute generalized exanthematous pustulosis (AGEP) is a rare and severe adverse cutaneous reaction (SCAR) that is characterized by the widespread appearance of numerous subcorneal pustules affecting large areas of the body.It is typically caused by drugs such as ampicillin/amoxicillin, quinolones, sulfonamides, and diltiazem.
Objectives: We report a case of AGEP induced by terbinafine.
Methods: A 47-year-old male with medical history of diabetes treated with insulin presented a maculopapular eruption that started on the face then extended to the entire body. According to the patient, skin rash was developed 20 days after starting oral and topic terbinafine for resistant onychomycosis. He denied previous therapy with terbinafine and reported no adverse reaction to other drugs. The patient had no history of psoriasis. On admission, the patient’s vital signs were normal. He had disseminated nonfollicular small pustules on erythematous skin. His temperature was 37°C. Laboratory tests revealed an elevated white blood count of (29 × 103 / mm3) with a neutrophilic predominance (17× 103 / mm 3), normal count of eosinophils and an elevated C-reactive protein level (268 mg/L). Liver tests showed an elevated rate of γ-glutamyl transferase (143 UI/L). Terbinafine induced AGEP was suspected and drug was withdrawn. Skin biopsy confirmed the diagnosis of AGEP and showed spongiotic pustules in the epidermis; a perivascular mixed inflammatory infiltrate including eosinophils. After terbinafine withdrawal, the pustular lesions disappeared within a week, followed by desquamation (Fig. 3). Results of microbiologic tests ruled out other causes of AGEP. Drug-induced AGEP was retained and according the European Study of Severe Cutaneous Adverse Reactions criteria; causality relationship of terbinafine was certain (EuroSCAR= 8).
Results: Terbinafine, an allylamine, is an antifungal agent used for the management of onychomycosis that is well documented to induce skin adverse drug reaction 3–6. In our case, the responsibility of terbinafine was retained because of the temporal correlation between drug intake and onset of skin eruption, improvement of symptoms after suspected drug is withdrawn, skin biopsy findings, and exclusion of other AGEP causes.
Conclusion: Terbinafine-induced SCAR, and more specifically AGEP is rare, but probably more frequent than most believe. Patients and treating physicians must be informed and educated about this adverse reaction that can be life threatning.
295
Characteristics of fixed drug reaction and its management in clinical pharmacology
Jellazi M1, Ben Fadhel N1
1 Fattouma Bourguiba University Hospital, Department of Clinical Pharmacology, Monastir, Tunisia
Introduction: Fixed drug eruption (FDE) is a hypersensitivity reaction to drugs in the vast majority of cases. The identification of the culprit drugs is mandatory in order to prevent the extension of the lesions that in some cases may involve the aesthetic prognosis or be life-threatening.
Objectives: The main goals of this study were to describe the epidemiological characteristics and clinical features of the FDE, to determine the different responsible drugs and to evaluate the risk factors behind the development of the severe forms of FDE.
Methods: We have conducted a longitudinal descriptive study in the Clinical Pharmacology Department of Monastir over a period of 17 years. We have included all the patients with a confirmed FDE. Allergological workup studies included three parts:
Clinical history (semiology, chronology, and culprit drugs), skin patch testing (performed according to the European Network of Drug Allergy and International Contact Dermatitis Research Group recommendation) and oral challenge testing. An allergy ID card was issued to all the patients.
Results: We have included 70 patients. The majority of our patients were adults (mean age = 45,6 years) and a female predominance was noted (57.1%). The positive diagnosis of FDE was made clinically in 60% of the patients. The most common form of the FDE was multiple (74.3%). Bullous FDE presented 38.6% of the cases. Locations included above all the limbs (n=45) and the trunk (n=19). Lesions generally appear 5 minutes to 10 days after the exposure with a median of 48 hours. Allergological workup helped to identify the responsible drugs in 62 (88%) patients. NSAIDs were present in 33 cases (53%) and included mefenamic acid (n=15) and piroxicam (n=14). Antibiotics were present in 14 cases(22%) followed by paracetamol in 11 cases(17%). Patch test was positive in all patients with bullous FDE and the probability to have a positive patch test was higher in case of NSAIDs induced FDE. Multivariable regression showed that paracetamol consumption was a risk factor for the development of bullous FDE with an Odds Ratio of 4.5; (p=0.03; [1.06-19.02]).
Conclusion: At the end of this study, NSAIDs were the most common drugs involved in FDE. Paracetamol, which is widely used for self-medication, was associated with the severe form of the FDE. This should encourage doctors to educate their patients about the risks of self-medication with such drugs usually perceived as harmless.
297
The analysis of pharmacotherapy and kidney function of patients hospitalized at the department of internal medicine of the University Hospital
Poruba M1, Zvárová E1, Urbánek K1
1 Department of Pharmacology, Faculty of Medicine and Dentistry, Palacký University Olomouc, Olomouc,
Introduction: Chronic kidney disease (CKD) is at least 3-month long condition of gradual loss of kidney function or any other kidney abnormality. From stage G3, there is a risk for the cumulation of drugs, primary eliminated through the kidney.
Objective: The study aimed to evaluate the incidence of CKD and its impact on drug dosing in hospitalized patients.
Methods: This prospective observational study aimed to analyze the medical history, pharmacotherapy and kidney function of patients hospitalized in the department of internal medicine of the University Hospital in Olomouc. For each patient, the creatinine clearance and eGFR were individually calculated, and all patients were stratified according to risk factors that include kidney function, polypharmacy, treatment with drugs with low therapeutic index, or risk of drug-drug interactions.
Results: Among 695 patients hospitalized from September 2021 to March 2022, the were 218 (31.4 %) with chronic kidney disease of stage G3a or worsen. Of these, 186 (85.3 %) were treated with a drug where the dose adjustment according to the kidney function is of interest. In 96 patients (44 % of patients with altered kidney function), the dose did not correlate with the SmPC of the medicine. The used dose of drugs and SmPC did not correlate most commonly for ACE inhibitors, diuretics, antidiabetics, statins, DOAC, acetylsalicylic acid, and methotrexate. Subsequent analysis showed that patients with altered kidney function were at higher risk for polypharmacy (8.9±4.0 drugs) than patients at stage G3 or better (4.9±4.3; p < 0.001). Also, the drugs with low therapeutic index were more common in patients with CKD stage G3 or worsen.
Conclusion: CKD is a common condition in patients who are hospitalized in internal clinics. Many of these patients are treated with drugs that are eliminated through the kidney. In many inpatients, the drug dose does not correlate with the actual SmPC, leading potentially to patient overdose. On the other hand, not all drugs should be dosed only according the kidney function and other factors should be considered.
Funding: This study was supported by grant IGA_LF_2022_006 of the Internal Grant Agency of Palacký University in Olomouc.
300
STATIN-ASSOCIATED NECROTIZING AUTOIMMUNE MYOPATHY
Salgueiro E1, Vázquez L1, Revuelta M1, Valencia J1
1 Universidad De Oviedo, Oviedo, Spain
Introduction: Over the past few years, there has been an increase in cases published of necrotizing autoimmune myopathy (NAM) associated with statin use. How Hydroxymethylglutaryl-CoA reductase (HMGCR) inhibitors (statin) trigger an immunological response in some individuals remains uncertain so we need to get a better understanding of this rare muscle disorder.
Objectives: To analyse the main features of statin-associated NAM cases reports and to assess response to treatment in order to improve their medical control.
Methods: PubMed was searched for case reports and case series of statin-associated NAM from inception until December 31, 2019. We did a descriptive analysis of the main characteristics of the patients, drug exposure, clinical presentation and laboratory findings of NAM, and their response to treatment.
Results: A total of 66 NAM cases reports associated with atorvastatin (50), simvastatin (8), rosuvastatin (6) and pravastatin (2) were analysed. With 36 male patients, 25 females and 5 patients of unknown gender, the median age at onset was 65.5 (range of 47- 84) years, the mean comorbidity (n= 39) was 2.03 ± 0.15. 5 of the 35 patients screened for cancer had history or concomitant cancer. The mean latency period (time between the statin start date and NAM onset) was 3 ± 0.6 years. Althout not statistically significant, some delay in statin withdrawal was observed after NAM onset. All patients had proximal (n= 52) or generalized (n= 14) weakness. Laboratory studies showed significantly elevated creatine kinase (mean ± SE: 12113.2 ± 2044.6). Specific anti-HMGCR autoantibody was positive in almost all cases where it was checked (46/47). 28 patients had MHC-1 (major histocompatibility complex-1)/HLA-1 (human leucocyte antigen class 1) overexpression. Unlike toxic myopathy, which improves when the statin is discontinued, NAM progressed and often intense immunotherapy was required in most patients. Relapse after lowering treatment was informed in 14 patients. Treatment with “glucocorticosteroids (GC) + methotrexate (MTX)” or “GC + intravenous immunoglobulin + MTX” were the most used drug combinations.
Conclusions: Early diagnosis of NAM, statin withdrawal and early onset of immunosuppressive therapy are essential for a proper patient management. Rituximab was effective in some patients who had resistance to several immunosuppressive agents. Further controlled research is needed to define the optimal therapeutic strategy.
301
Corticosteroid-induced allergic skin reaction: cross reaction between methylprednisolone and hydrocortisone hemisuccinate
BEN AZOUZ C2, BOURAOUI H1, FATHALLAH N1, SLIM R1, OUNI B1, BEN SALEM C1
1 Department of Pharmacology, Metabolic Biophysics and Applied Pharmacology Laboratory LR12ES02, Faculty of medecine, Univ, Sousse, TUNISIA
2 Faculty of pharmacy of Monastir, Monastir, TUNISIA
Introduction: Corticosteroids (CS) are widely used drugs for their anti-inflammatory and immunomodulatory properties, often used to treat allergic reactions. However, allergic-type reactions to these agents can occur. The severity of those reactions can vary from a rash to anaphylaxis or death. Both immediate and delayed reactions are rarely reported.
Objectives: We report a case of hypersensitivity cutaneous reaction due to methylprednisolone, with cross reaction to hydrocortisone hemisuccinate.
Results: A 25 year-old-man with a history of multiple sclerosis (MS), developed few minutes after intravenous administration of methylprednisolone, an urticarial reaction predominant in the upper limbs. The rash cleared within two hours with oral antihistamine. The patient reported that he had the same cutaneous reaction after injection of intravenous hydrocortisone hemisuccinate as a premedication before natalizumab indicated for his disease, with positive rechallenge. The patient noted that the oral prednisolone was well tolerated. Skin tests were performed showing a positive result to methylprednisolone.
Discussion: In our case, clinical manifestations and positive skin Prick test were consistent with the diagnostic of allergy to methylprednisolone and hydrocortisone hemisuccinate.
For cross-reactions, prednisolone and hydrocortisone (belonging to group A) are most often concerned. There are not necessarily cross-reactions between the different CS in group A. Some patients allergic to hydrocortisone or methylprednisolone tolerate prednisone and/or prednisolone as in our patient. It would also seem that the succinate ester forms of these molecules used intravenously or intra-articularly are most often implicated. In patients allergic to hydrocortisone or methylprednisolone, good tolerance of halogenated CS such as betamethasone or dexamethasone has been demonstrated.
Conclusion: CS are used to treat allergic reactions and anaphylaxis but in patients who are often treated with systemic steroids, as it was the case in our patient with MS, allergic reaction to steroids can develop. Therefore, allergologic exploration is required for all suspected CS allergic reactions.
307
Recurrent DRESS syndrome to aromatic anti-epileptic drugs
BOURAOUI H1, BEN AZOUZ C2, FATHALLAH N1, LAHOUEL M3, SLIM R1, BOURAOUI O1
1 Department of Pharmacology, Metabolic Biophysics and Applied Pharmacology Laboratory LR12ES02, Faculty of medecine,Univ, Sousse, TUNISIA
2 Faculty of pharmacy of Monastir, Monastir, TUNISIA
3 Department of Dermatology, Farhat Hached Hospital, Sousse, Sousse, TUNISIA
Introduction: Aromatic antiepileptic drugs (AEDs) are common causes of severe cutaneous adverse drug reactions (SCAR), including drug reaction with eosinophilia and systemic symptoms (DRESS), Stevens–Johnson syndrome, and toxic epidermal necrolysis.
Objectives: Herein we describe a case of recurrent DRESS syndrome induced by different AEDs.
Methods: A 18-year-old man with a medical history of birth mental deficiency and seizure disorder since 2018 currently treated with levetiracetam admitted to the dermatology department for a recurrent DRESS syndrome. According to the patient’s father, in 2018 his son developed a maculopapular rash with fever and vomiting, seven days after the beginning of phenobarbital for epilepsy. He was admitted to the dermatology department for DRESS syndrome to phenobarbital(RegiSCAR=5, DRESS probable). The patient was switched to carbamazepine. Two days later, symptoms worsened and carbamazepine was withdrawn. In 2019, when patient’s neurologist added lamotrigine because of occurrence of seizure disorder under levetiracetam., the patient presented skin eruption few days after lamotrigine intake and symptoms resolved after lamotrigine withdrawal. Currently episode dates back to one month (March 2022), when patient’s neurologist intensified his treatment by adding phenytoin. Nearly twenty days later, the patient experienced a widespread pruritic maculo-papular confluent eruption over the face, the trunk and limbs with fever and lymphadenopathy. The physical examination revealed several lymphadenopathy in axillary, inguinal and cervical zones. Laboratory tests revealed leukocytosis (12600/mm3), and elevated liver enzyme levels (alanine aminotransferase >4×N (Normal 35 UI/L), aspartate amino-transferase > 2×N (Normal 30 UI/L)). Results of microbiologic and autoimmunity tests ruled out other causes. Skin biopsy showed parakeratosis and dyskeratotic keratinocytes in the epidermis and a perivascular lymphocytic infiltrate compatible with drug reaction. The diagnosis of DRESS was retained as probable based on RegiSCAR diagnostic criteria(=5). Phenytoin therapy was discontinued immediately. Skin eruptions resolved after corticosteroid therapy without 2 weeks later with desquamation.
Results: The aromatic anticonvulsants (Phenytoin, phenobarbital and carbamazepine) are the most frequently incriminated. Cross-reactivity has been widely described among the aromatic anticonvulsants and occurs at a rate as high as 80%. This finding has been explained by the fact that these drugs are metabolized into structurally similar reactive intermediates, such as arene oxides. These latters may bind covalently to cellular macromolecules, directly causing cell damage or initiating immune responses. Lamotrigine, which has a non-aromatic structure, is rarely associated with DRESS. It has been thought that the hypersensitivity reactions are an immunological response to metabolically generated drug-protein adducts, but many data demonstrate a lack of evidence of significant reactive metabolite formation in the skin, so the drug reactions caused by lamotrigine may be caused by the parent drug instead of a reactive metabolite. In fact, the positivity of patch test with lamotrigine in many cases of lamotrigine-induced DRESS in the literature confirm this hypothesis.
Conclusion: The hypersensitivity syndrome or DRESS is a potentially life-threatening adverse drug reaction. Patients and treating physicians must be aware about the causal drug and its potential immunologic or toxicologic cross-reactivity with other compounds particularly among aromatic AEDs.
Professional development
105
Regulatory submission process improvements in the clinical pharmacology area
Paczkowska M1, Andrzejewska-Górecka D1, Malewicka D1, Burden A2, Subramony P2
1 Clinical Pharmacology and Quantitative Pharmacology, Clinical Pharmacology and Safety Sciences, R&D, AstraZeneca, Warsaw, Poland
2 Clinical Pharmacology and Quantitative Pharmacology, Clinical Pharmacology and Safety Sciences, R&D, AstraZeneca, Gatihersburg, United States
Introduction: A regulatory submission is required to release new medicines to the market and is critical for patients waiting for new therapies. The pharmaceutical companies analyze data from clinical trials, prepare filing documents, and submit them to health authorities within required timelines. Project management tools and knowledge sharing within an organization could increase the efficiency of the regulatory submission process.
Objectives: We aimed to improve the process of regulatory submissions in a clinical pharmacology area by introducing selected project management tools, which are often used in other industries. The purpose of implemented solutions was to increase the quality of documents included in the submission package, decrease the time spent on regulatory submission preparation, optimize resources management, reduce the number of questions received from Health Authorities, and increase the overall efficiency of the process.
Methods: Lessons learned were introduced in two regulatory submission projects led by Clinical Pharmacology and Quantitative Pharmacology department at AstraZeneca. In order to identify process improvements, surveys were sent out to all team members one week after the regulatory submission. Collected answers were then analyzed and presented during a workshop organized for the regulatory submission team, where all issues were discussed in detail. Finally, lessons learned document for each project was created and made widely available to all teams involved in regulatory submissions within the department.
Results: Lessons Learned repository with documents regarding regulatory submission projects in a clinical pharmacology area was established. During lessons learned run in both projects, process bottlenecks and areas of improvement were identified. Interestingly, after sharing the information discussed during lessons learned workshop in the first project with the team in the second project, we observed improved ways of working on documents, increased quality of submission package, and an accelerated timeline, among other benefits.
Conclusions: Lessons learned can be successfully used to improve current and future regulatory submission projects in the clinical pharmacology area providing benefits for both: the organization and patients.
117
Relationship between hair cortisol levels, stress and burnout questionnaires amongst resident doctors: a protocol description
Bautista A1, Lavín L2, Cuellar D1, Alonso B1, Mazón I2, Valencia D1, Vega N1, García M1
1 Department of Clinical Pharmacology Marqués de Valdecilla University Hospital, Santander, Spain
2 IDIVAL, Santander, Spain
Introduction: The hormone released in stressful situations is cortisol, a steroid hormone stored in the adrenal cortex and released under the control of the hypothalamus through the circadian cycles of ACTH secreted by the pituitary gland. During the pandemic, health professionals have been exposed to multiple stressors from which it is not easy to recover. Chronic exposure to these stressors and the repeated failure of coping strategies, in addition to having a negative impact on the health of these workers, reduces the quality of care and favours the development of burnout syndrome.
Objectives: To compare the levels of stress between first and fourth year resident doctors during on-call shifts and to correlate the levels with the number of on-call duties performed by the residents in a period of one month. As secondary objectives, to compare the values obtained with the stress and burnout questionnaires carried out by the residents.
Methodology: Descriptive Observational study, which includes an analysis of quantitative data obtained from determinations of cortisol levels from biological hair samples of participants and quantitative data (scores) extracted from the questionnaires completed by participants. The main study variables will be hair cortisol levels, and the quantitative values obtained from the stress questionnaires carried out by the residents: Perceived Stress Scale and Maslach Burnout Inventory. Inclusion criteria for the study will be residents of medical specialties who perform (both non-paediatric emergency and internal medicine) in the first year and in the third, fourth or fifth year. The exclusion criteria for the study will be residents who do not perform on-call duties, who are unable to obtain a biological hair sample at the specific times of the study or who do not sign the informed consent form. The results of cortisol levels will be correlated with the answers obtained in the burnout and stress questionnaires to study a possible correlation.
Results: The results obtained for hair cortisol levels are expected to correlate with a higher number of on-call shift than those performing less on-call shifts and with a lower degree of responsibility. Such results are expected to be supported by higher questionnaire scores.
Conclusions: The results obtained will contribute to an understanding of stress levels derived from a pandemic situation and to establish coping strategies to reduce the development of burnout syndrome.
262
Quality Indicators for appropriate in-hospital pharmacotherapeutic stewardship: An international RAND-modified Delphi study
Mahomedradja R1,2, Tichelaar J1,2, Mokkink L3, Sigaloff K1,4, van Agtmael M1,2
1 Amsterdam UMC, Vrije Universiteit Amsterdam, Department of Internal Medicine, Section Pharmacotherapy, De Boelelaan 1117, Amsterdam, the Netherlands, Amsterdam, The Netherlands
2 Amsterdam UMC, Vrije Universiteit Amsterdam, Department of Internal Medicine, Research and Expertise Center in Pharmacotherapy Education (RECIPE), De Boelelaan 1117, Amsterdam, the Netherlands, Amsterdam, The Netherlands
3 Amsterdam UMC, Vrije Universiteit Amsterdam, Department of Epidemiology and Data Science, Amsterdam Public Health research institute, Amsterdam, the Netherlands, Amsterdam, The Netherlands
4 Amsterdam UMC, Vrije Universiteit Amsterdam, Department of Internal Medicine, Division of Infectious Diseases, Amsterdam Institute for Infection and Immunity, De Boelelaan 1117, Amsterdam, Netherlands, Amsterdam, The Netherlands
Introduction: Prescribing of medication is one of the corner stones of in-hospital medical treatment. Slips, mistakes or lapses in the decision making or writing process of medication may result in prescribing errors (PEs), causing distress to patients and their family; burden hospital capacity; and are accompanied with significant healthcare costs. Despite efforts, PE-related harm is not declining. In-depth analyses shows that there are diverse outcome measures being used in this matter; diverse professionals performed PE-reducing interventions; the generalizability of successful interventions from one in-hospital settings to another is challenging; and that the targeted patient population is diverse. Therefore, consensus on several topics is needed, before developing new or more effective strategies.
We used a modified Delphi study to obtain input from international experts associated with the European Association of Clinical Pharmacology and Therapeutics (EACPT), in order to establish a set of QIs forming a framework to ‘in-hospital pharmacotherapeutic stewardship’.
Objectives: To develop, in consensus with international experts associated with the EACPT, a set of QIs forming a framework to in-hospital pharmacotherapeutic stewardship.
Methods: A 3-rounds modified Delphi procedure was used and ran between June 7 and September 21 2021. The procedure included 3 phases: The preparation phase (phase 1) including preliminary research; systematic literature searches; and selection of a European expert panel ; the study phase (phase 2) including two web-based, written questionnaires rounds with an online face-to-face focus group meeting in between to identify the ‘why’ behind the QIs labeled ‘rejected’ and ‘up for discussion’ instead of reaching consensus; and the final phase (phase 3) including final decisions and translation to the final framework.
Results: A total of 183 experts were invited of which 61 experts (34%) from 23 countries completed the first written questionnaire. In the second written questionnaire round, a total of 194 experts were invited, and was completed by 56 experts (29%) from 24 countries. 67% of the experts in the first written questionnaire also participated in the second.
Overall, experts rated 63 proposed QIs and 4 general statements divided over two web-based questionnaires. In the first written questionnaire, 17 (50%; N = 34) of the proposed QIs were accepted; 41% was labelled ‘up for discussion’ and 9% was labelled ‘rejected’. All four general statements assessing the experts’ support of the framework and need for European consensus on this topic were accepted.
Based on the first written questionnaire and input from the focus group meeting, 2 QIs were rejected; 14 QIs were rephrased and 11 new QIs emerged. After the second written questionnaire, 84% (n = 25) was accepted and 4 QIs were left labelled ‘up for discussion’. These proposed QIs were excluded from the final set. The final set included 35 QIs.
Conclusion: This is the first study establishing in international consensus a framework for ‘in-hospital pharmacotherapeutic stewardship’. The framework should enable hospitals to set up this program locally, aiming to improvement in-hospital medication safety and reduce in-hospital PEs.
282
EFFECTS OF CYP3A4 GENETIC POLYMORPHISMS ON THE EFFICACY AND SAFETY PROFILES OF DIAZEPAM IN PATIENTS WITH ALCOHOL WITHDRAWAL SYNDROME
Skryabin V1,2, Zastrozhin M1,2
1 Moscow Research And Practical Centre For Narcology, Moscow, Russian Federation
2 Russian Medical Academy for Continuous Professional Education, Moscow, Russian Federation
Background: Diazepam therapy is often ineffective and some patients suffer from dose-dependent adverse drug reactions, reducing the efficacy of the therapy of alcohol withdrawal syndrome. The presence of some polymorphic markers of CYP3A4 decreases the amount of isoenzyme to be expressed or reduces its activity resulting in the changes biotransformation and elimination rates of the medication. Currently there is no data on correlation between the CYP3A4 genetic polymorphisms and efficacy and safety of diazepam among the Russian patients.
Objective: To investigate the effects of CYP3A4 genetic polymorphisms on the efficacy and safety of diazepam in patients with alcohol withdrawal syndrome in order to develop the algorithms of optimization of diazepam therapy for reducing the risk of dose-dependent undesirable side effects and pharmacoresistance.
Methods: The study involved 95 male patients (average age: 36.42±9.72 years) with alcohol withdrawal syndrome who were hospitalized in Moscow Research and Practical Centre of Addictions of the Moscow Department of Healthcare. Inclusion criteria were 5-day diazepam therapy in intramuscular (IM) injections and age of 18 to 75. Exclusion criteria were presence of any antipsychotics in the treatment regimen, creatinine clearance values <50 mL/min, creatinine concentration in plasma ≥1.5 mg/dL (133 mcmol/L); body weight less than 60 kg or greater than 100 kg; and presence of any contraindications for diazepam use. Venous blood samples collected in vacuum tubes VACUETTE® (Greiner Bio-One, Austria) on the sixth day of the diazepam therapy were used for genotyping. The real-time polymerase chain reaction was performed using DNA amplifiers “Dtlite” by DNA Technology (Moscow, Russia) and CFX96 Touch Real Time System with CFX Manager software of Bio-Rad Laboratories Inc. (USA) and sets “SNP-screen” by “Syntol” (Russia). A series of psychometric scales were used in the research. Genotyping of C>T intron 6 of CYP3A4*22 (rs35599367) was performed using the real-time polymerase chain reaction.
Results: According to results of U-test Mann-Whitney, statistically significant differences between the efficacy and safety of diazepam were obtained on the 1st and 6th days of therapy in patients with CC and CT+TT genotypes (Differences in mean CIWA-Ar scores: -9.0 [-12.0; -7.0] for CC genotype carriers vs -13.0 [-14.25; -12.75] (p < 0.001) for CT and TT genotype carriers; differences in mean Udvald for Kliniske Undersogelser Side Effect Rating Scale scores: 8.0 [6.0; 9.0] (p < 0.001) for CC genotype carriers vs 10.0 [9.5; 12.0] for CT and TT genotype carriers. The results of our study should be taken into consideration when prescribing diazepam to patients with alcohol withdrawal syndrome since it will allow increasing the efficacy of the therapy and decreasing the risk of undesirable side effects.
Conclusion: This study demonstrated the higher efficacy and lower safety of diazepam in patients with alcohol withdrawal syndrome carrying the CT and TT genotypes of CYP3A4*22 intron 6 C>T polymorphism (rs35599367). This should be considered when prescribing this medication to such patients to reduce the risk of undesirable side effects and pharmacoresistance.
Psychopharmacology
15
1MeTIQ reverses the disturbances of the serotonergic system caused by NMDA receptor antagonists
Wąsik A1, Białoń M1, Żarnowska M1
1 Maj Institute of Pharmacology PAS, Krakow, Poland
Introduction: Dysregulation of monoaminergic systems can cause both positive and negative symptoms of schizophrenia. Ketamine and MK-801 act as an antagonists of NMDA receptors, and may induce schizophrenia-like symptoms [Rung et al. 2005]. 1-Methyl-1,2,3,4-tetrahydroisoquinoline (1MeTIQ) is reversible MAO inhibitor and exhibits the neuroprotective, antidepressant- and anxiolytic-like effect [Wąsik et al. 2015; 2019].
Objectives and Aims: The aim of the present study was to investigate the impact of acute 1MeTIQ administration on the disturbances in the release of serotonin (5-HT) in the rat’s hippocampus (HIP) and striatum (STR) caused by MK-801 or ketamine; 1MeTIQ was administered 20 minutes before MK-801 or ketamine injection using in vivo microdialysis study.
Methods: The release of 5-HT was measured in the HIP and STR in freely moving rats. After the microdialysis experiment, the dialysate samples were analyzed on an HPLC apparatus with electrochemical detection.
Results: In vivo microdialysis study showed that in the STR combined treatment 1MeTIQ with ketamine increased the release of 5-HT (approx. 60%) and decreased the level of 5-HIAA in the rat striatum. In vivo microdialysis study showed that in the HIP MK-801 significantly decreased the release of 5-HT (up to 50%). In the combined group, 1MeTIQ completely reversed this effect and increased the release of 5-HT (approx. 180%) while 5-HIAA level was significantly reduced (approx. 20%) in this group.
Conclusion: Our study indicated the ability of 1MeTIQ to reverse NMDA receptors antagonists-induced disturbances in the activity of serotonergic system.
Acknowledgements: The project was financed by PROM
49
Prescriptions of zolpidem and zopiclone in Greece: an analysis of the Greek prescription database before and after mandatory electronic prescription
Siafis S1,2, Fragkidis V3, Papazisis G1,4
1 Department Of Clinical Pharmacology, School Of Medicine, Aristotle University Of Thessaloniki, Thessaloniki, Greece, THESSALONIKI, GREECE
2 Department of Psychiatry and Psychotherapy, School of Medicine, Technical University of Munich, Munich, Germany, MUNICH, GERMANY
3 Substitution Treatment Unit of Thessaloniki, Agios Dimitrios General Hospital, Organisation Against Drugs (O.KA.NA), Thessaloniki, Greece, THESSALONIKI, GREECE
4 Clinical Trials Unit, Special Unit for Biomedical Research and Education, School of Medicine, Aristotle University of Thessaloniki, Greece, THESSALONIKI, GREECE
Introduction: Zolpidem and zopiclone are widely used for sleep disorders, yet their abuse and dependence potential has been underestimated. The electronic prescription of zolpidem/zopiclone became mandatory on 17.07.2019 in Greece.
Objectives: To investigate descriptive characteristics of zolpidem/zopiclone prescriptions and the impact of the mandatory electronic prescription mandate.
Methods: Anonymized prescriptions of zopiclone (ATC: N05CF01) and/or zolpidem (ATC: NC05CF02) that were executed in pharmacies between 01.10.2018 and 01.10.2021 were obtained from the Greek nationwide prescription database. The database covers almost the entire Greek population and it is administrated by IDIKA of the Greek Ministry of Health. We investigated descriptive characteristics of prescriptions, and calculated the monthly number of prescriptions taking into consideration dates with potential impact, i.e., the date of the mandatory electronic prescription mandate (on 17.07.2019) and the date of the first case of COVID-19 in Greece (on 26.02.2020).
Results and Conclusion: During the investigated period of three years, there were 1229842 executed prescriptions of zolpidem (89.4%), zopiclone (10.4%) or both (0.3%), considering 156554 unique patients. The patients were mainly elderly (73.1% were ≥ 65 years old) and women (64.5%). The majority of the prescription physicians (69.9%) were general practitioners or internists, followed by 17% psychiatrists or neurologists, 5.3% cardiologists, 4.5% physicians in specialty training, 1% nephrologists and 2.4% of physicians with another specialty.
After the mandatory electronic prescription mandate and before COVID-19 in Greece, i.e., between 08.2019 to 03.2020, there was a notable increase of prescriptions in comparison to the previous period from 10.2018 to 07.2019 (median 37267 vs median 34106; Mann–Whitney U=9, p-value=0.009). After COVID-19, the median monthly number of prescriptions was 36363, yet there were variations ranging from 16963 to 39956.
In conclusion, the mandatory electronic prescription system could increase the surveillance of drugs with abuse potential such as zolpidem and zopiclone. Nevertheless, the large number of prescriptions in elderly patients and prescribed by primary care physicians is worrisome and warrants further investigation.
74
Immunomodulatory properties of GABAergic agents in an in vivo model of Multiple Sclerosis
Stamoula E1, Psathas T2, Dardalas I1, Vavilis T3, Ainatzoglou A2, Siafis S4, Stamatellos V5, Boskou I2, Ilia T2, Papazisis G1,6
1 Department of Clinical Pharmacology, School of Medicine, Thessaloniki, Greece
2 School of Medicine, Aristotle University of Thessaloniki, Thessaloniki, Thessaloniki, Greece
3 Department of Biology and Genetics, School of Medicine, Aristotle University of Thessaloniki, Thessaloniki, Greece
4 Department of Psychiatry and Psychotherapy, School of Medicine, Technical University of Munich, Munich, Germany, Munich, Germany
5 Department of Neurology, Klinik fur Neurologie, DRK Kliniken Berlin Kopenick, Berlin 12559, Germany, Berlin, Germany
6 Clinical Trials Unit, Special Unit for Biomedical Research and Education, School of Medicine, Aristotle University of Thessaloniki, Thessaloniki, Greece
Introduction: Multiple sclerosis (MS) is a lifelong autoimmune disease of the central nervous system, which is characterized by a wide variety of symptoms. Neurological symptoms are central to the disease, arising from the deterioration of the myelin seath of the nerves. Cells of the immune system are known to express GABA receptors and utilize GABAergic transmission in order to modulate a host of responses such as cell migration, proliferation and cytokine production. Consequently, agents acting upon the GABA system, have the potential to serve as an auxiliary therapy, contributing to better quality of life for MS patients by lessening the symptoms of the disease.
Objectives: This study aims to showcase the potential benefits of GABAergic agents as an adjunct treatment for MS patients, by providing relevant evidence supporting their use in disease management.
Methods: A literature review was conducted utilizing Scopus and Pubmed databases for GABA affecting agents such as antagonists, agonists and various modulators in accordance with their disease modifying use in the in vivo model of experimental autoimmune encephalomyelitis (EAE). The resulting material was filtered using specific inclusion and exclusion criteria.
Results: Both GABA-a and GABA-b agonists and modulators has shown in vivo immunomodulatory action in EAE mice. The immunomodulation manifested in the form of reduction in pro-inflammatory cytokines, decrease of inflammatory infiltration of mononuclear cells and a reduction in the formation of reactive oxygen species. (ROS). These findings were accompanied by a rescuing effect on myelinated sensitive fibers and amelioration of CNS axonal damage. As far as the clinical aspect is concerned, the treated animals exhibited decreased peak disease severity, duration, clinical scores and EAE incidence.
Conclusion: The results highlight the possible therapeutic potential of GABA agonists and modulators in MS related pathology, since such agents demonstrated efficiency in in vivo models of EAE. The significant neuroprotection offered by their immunomodulatory action, could constitute another therapeutic approach in the treatment of CNS conditions with autoimmune aspects. Hence, GABA agonists and modulators appear to be promising candidates for clinical use and as such, further clinical studies are required to establish their utilization in clinical setting.
88
Multiple Sclerosis and Glutamatergic modulation: A Review οf in vivo preclinical studies
Stamoula E1, Ainatzoglou A2, Vavilis T3, Dardalas I1, Psathas T2, Siafis S4, Matsas A5, Stamoulas K2, Ilia T2, Boskou I2, Papazisis G1,6
1 Department of Clinical Pharmacology, School of Medicine, Aristotle University of Thessaloniki, Thessaloniki, Thessaloniki, Greece
2 School of Medicine, Aristotle University of Thessaloniki, Thessaloniki, Greece
3 Department of Biology and Genetics, School of Medicine, Aristotle University of Thessaloniki, Thessaloniki, Greece
4 Department of Psychiatry and Psychotherapy, School of Medicine, Technical University of Munich, Munich, Germany
5 School of Medicine, National and Kapodistrian University of Athen, Athens, Greece
6 Clinical Trials Unit, Special Unit for Biomedical Research and Education, School of Medicine, Aristotle University of Thessaloniki, Thessaloniki, Greece
Introduction: Multiple sclerosis (MS) is a demyelinating disease of autoimmune etiology. Studies in MS patients as well as in in vivo model of experimental autoimmune encephalomyelitis (EAE), have shown that impaired glutamate metabolism and dysregulation at the level of its receptors and cellular signaling are implicated in the pathophysiology of the disease.
Objectives: The aim of this study was to investigate the effects of drugs known to affect glutamatergic activity in the EAE model of MS.
Methods: A systematic review of the literature was undertaken, utilizing Scopus and Pubmed databases, following by filtering the results according to specific inclusion/exclusion criteria. The search focused on agents that can have an effect on the biosynthesis, release and action of the excitatory neurotransmitter glutamate.
Results: In vivo studies in AEA MS model reveal an immunomodulatory role for drug molecules that regulate glutamate action. Molecules possessing such activity improve the clinical image of AEA laboratory animals by decreasing both the intensity and the severity of symptoms observed during the acute and the chronic phase of the disease. Moreover, a notable decrease of leucocyte’s pathological tissue infiltration and action is reported, coupled with amelioration of demyelination and neural cell death. On a cellular and molecular level, those agents significantly diminish intracellular oxidative stress, production of free radicals and release of pro-inflammatory factors (chemokines, cytokines, RANTES). The activation of intracellular signaling pathways promoting neuronal survival and growth, has also been credited for some of the neuroprotective qualities that glutamate modulating agents possess.
Conclusion: Utilization of drug molecules that exert their actions through regulation of glutamate action, constitutes a possible and attractive therapeutical approach targeted towards the symptoms of MS.
94
THERAPEUTIC OPTIONS IN CONCOMITANT INFECTION WITH BORRELIA AND SARS-COV2 – A CASE PRESENTATION
Schulz-Falten S1, Bán E1, Brassai A1, Schreiber H1, Buchta T1, Schäfer K1
1 University Of Medicine, Pharmacy, Sciences And Technology Targu-mures, Târgu Mureş, Romania
Introduction: Both Covid and Lyme disease individually are known to cause, besides physical illness, also psychosocial symptoms. We did not find reports so far about a concomitant occurrence of both infectious diseases necessitating psychiatric treatment.
Objectives: The aim of this case-based approach is to bring this case to public notice and evaluate pharmacological treatment options.
Methods: A 47-year-old female patient with no significant medical history presented in august 2020 with a mild case of Covid. In the first month after the first positive test she developed panic attacks followed by depression.
Results: The timeline of the case progression showed a fulminant appearance of symptomatology with a poor outcome in just one year. For SARS-COV-2 infection the patient received only symptomatic therapy with NSAIDs. The psychiatric therapy was initiated with Alprazolam plus Sertraline-Olanzapine combination, which after proven unsuccessful was completed with Mirtazapine beside dose increase. The high-dose quadruple association did not lead to control of the symptomatology, therefore it was changed to a Sertraline-Risperidone high-dose association with slight improvement after the appearance of suicidal ideation. After independent discontinuation-induced relapse Risperidone and Sertraline treatment was continued resulting in Parkinson-like side effects. Therefore, the treatment was changed consecutively to Quetiapine, Olanzapine, Sertraline-Lorazepam, Escitalopram-Venlafaxine-Clomipramine with little to no success.
Concurrently with the Sertraline-Risperidone therapy the patient underwent further clinical evaluation which only revealed positive Borrelia antibodies, confirmed by western blot, for which she received 28-day Ceftriaxone treatment.
Psychiatric symptoms did not cease; she was diagnosed with psychotic depression and therapy was continued.
Conclusion: We have performed a retrospective clinical pharmacological evaluation of the case. The step-by-step analysis of therapy vs. diagnosis revealed a frequent change of the medication with high incidence of the side effects. We also correlated the administered treatment with actual guidelines and the presence of concomitant infectious pathology. The psychological deterioration can, besides infectious etiology, be partially associated to the adverse event profile observed or to certain differential diagnostic possibilities not evaluated like encephalitis or neurodegenerative or demyelinating disorders.
125
A randomized controlled trial to evaluate the placebo effect on psychomotor functions in adult healthy volunteers
Reeta K1, Mishra A1, Sarangi S1
1 All India Institute Of Medical Sciences, New Delhi, India
Introduction: Any effect attributable to any pill, potion or procedure but not explained by its pharmacodynamic or specific properties is called placebo. These have been commonly used as control intervention in randomized controlled trials establishing efficacy and safety of new pharmaceuticals. The common practice in clinical trials is to subtract the effect of placebo to obtain pure intervention effect. But placebo has been used in clinical practice and has been shown to be effective in certain pathologies like pain, depression and parkinsonism etc
Objectives: The study evaluates the effect of placebo deceived as stimulant when compared with placebo given as an inert substance and with no treatment.
Methods: An open label, assessor-blinded, randomized 3-periods, 6-sequence, crossover trial was conducted in 54 healthy volunteers. After initial screening for eligibility, patients were randomized into sequences. The participants were given an initial training for three days and then psychomotor function tests for simple reaction time, choice discrimination test, digit symbol substitution test were carried out at baseline and after 30 minutes of administration of intervention.
Results: No carryover effect was expected as the intervention was placebo. Sequence effect was not statistically significant. A significant difference was obtained in change in mean reaction time between placebo as stimulant and placebo as inert substance in simple reaction time (47.15; 95% CI: 30.99 to 63.32; p < 0.001), choice discrimination test (16.62; 95% CI:5.76 to 27.48, p<0.001) and digit picture substitution test (182.12; 95% CI: 116.0 to 248.24; p<0.001). The difference in change from baseline in placebo as stimulant arm over no treatment arm was also statistically significant for simple reaction time (44.06; 95% CI: 25.54 to 62.58; p <0.001), choice discrimination test (18.22; 95%CI: 9.52 to 26.91; p<0.001) and digit picture substitution test (194.22; 95%CI: 117.74 to 270.70; p<0.001). There was no significant difference between placebo as inert substance and no treatment groups.
Conclusion: Placebo deceived as stimulant improved psychomotor function when compared with placebo administered as inert substance; similar findings were observed when placebo as stimulant was compared with no treatment. Placebo given as stimulant mimics situation in clinical practice and placebo as inert substance mimics trial situation. Placebo effect which may be explained by reward mechanism of our central nervous system is more prominent in clinical practice when compared to clinical trial settings.
244
Cannabidiol's effects on a pharmacological model of schizophrenia induced by subanesthetic ketamine
Brakatselos C1, Ntoulas G1, Asprogerakas M1, Tsarna O1, Polissidis A2, Antoniou K1
1 Department of Pharmacology, Faculty of Medicine, School of Health Sciences, University of Ioannina, 45110 Ioannina, Greece, Ioannina, Greece
2 Center of Clinical, Experimental Surgery and Translational Research, Biomedical Research Foundation of the Academy of Athens, 4 Soranou Efesiou Str, 11527 Athens, Greece, Athens, Greece
Background and Objectives: Repeated subanesthetic ketamine (KET) administration has been used to induce a schizophrenia -like behavioral profile in rodents, while the neurobiological underpinnings are yet to be elucidated. Cannabidiol (CBD), a psychoactive but non addictive cannabis compound is reported to present antipsychotic properties in humans and animal models of psychosis and schizophrenia, but the mechanisms involved are poorly understood. This study aims to evaluate the antipsychotic effect on CBD on indices of positive, negative, and cognitive symptomatology. Additionally, we aim to investigate specific neurochemical, neurobiological and neurostructural aspects of the schizophrenia-like profile induced by repeated subanesthetic KET administration and to explore the potential mitigating role of CBD.
Methods: Male adult Sprague-Dawley rats have been treated with 30 mg/kg/day KET or saline (SAL) for 10 days. Subsequently, rats received 10 mg/kg/day of CBD, or vehicle (VEH). Two days after the last injection, rats underwent a battery of behavioral tests consisting of open field activity, object recognition task (ORT), social interaction (SI), pre-pulse inhibition (PPI) and amphetamine challenge on the open field. Moreover, the dopaminergic, glutamatergic, and GABAergic activity in specific brain areas involved in the neurobiological substrate of schizophrenia (prefrontal cortex, dorsal and ventral hippocampus, medial and lateral striatum and the nucleus accumbens) was estimated with High Performance Liquid Chromatography (HPLC). Protein expression levels of neurobiological indices associated with neuroplasticity processes, glutamatergic function and downstream signaling were measured with western blot in crude tissue lysates and/or synaptosomes. Additionally, spine density and dendritic arborization analyses were performed in pyramidal cells from hippocampus and mPFC of Golgi-cox-stained brains.
Results: KET-treated rats displayed hyperlocomotion, impaired recognition memory in the ORT, social dysfunction in SI and sensorimotor gating deficits as deduced by the reduced PPI. The treatment with CBD ameliorated all these behavioral effects. Neurochemical analyses have shown region-specific KET effects in dopamine biosynthesis that are counteracted by CBD, while western blot analyses demonstrated an opposite modulation of NMDA receptor subunit composition in frontal and limbic areas, an effect that CBD partially mitigates. Synaptosomal expression of BDNF was affected in the hippocampus only by KET per se, while the ongoing neurostructural analyses reveal prominent KET effects.
Conclusion: Repeated KET administration induced a schizophrenia-related bio-phenotype in terms of behavior and the subsequent neurochemical and neurobiological analyses. CBD ameliorated or reversed the behavioral aspects of this schizophrenia-like model while affected KET-induced neurochemical alterations and modulated the neurobiological underpinnings of the bio-phenotype in a region-specific manner. The abovementioned findings characterize further the schizophrenia-like bio-phenotype induced by repeated KET, provide insights regarding schizophrenia pathophysiology, and enrich our understanding of the antipsychotic potential of CBD.
Funding: The research work was supported by the Hellenic Foundation for Research and Innovation (HFRI) under the HFRI PhD Fellowship grant (Fellowship Number: 1203).
264
Use of Novel Psychoactive Substances (NPSs) of Natural Origin in the United Kingdom Population
Deligianni E1,2,3, Lione L2, Kontogiorgis C4, Papazisis G1,5, Goulas A6, Lazari D3
1 Department of Clinical Pharmacology, School of Medicine, Aristotle University of Thessaloniki, Thessaloniki, Greece
2 School of Life and Medical Sciences, University of Hertfordshire, Hatfield, United Kingdom
3 Laboratory of Pharmacognosy, Department of Pharmacy, Aristotle University of Thessaloniki, Thessaloniki, Greece
4 Laboratory of Hygiene and Environmental Protection, School of Medicine, Democritus University of Thrace, Alexandroupolis, Greece
5 Clinical Research Unit, Special Unit for Biomedical Research and Education (SUBRE), School of Medicine, Aristotle University of Thessaloniki, Thessaloniki, Greece
6 1st Laboratory of Pharmacology, School of Medicine, Aristotle University of Thessaloniki, Thessaloniki, Greece
Introduction: In the last decade, there has been an emerging threat to public health due to the increase of recreational use of novel psychoactive substances (NPSs) that are mostly derived or modified from natural products. There is an urgent need for regulatory authorities, governments and scientific experts to tackle this issue.
Objectives: The aim of this pharmacoepidemiological study was to investigate in the UK population the use of natural NPSs and the perception of potential associated health risks.
Methods: The Bristol Online Survey was distributed on the Bluelight drug forum, social media pages and via University emails during 1 July and 17 November 2018. For the data analysis, the SPSS software was used (IBM SPSS version 26).
Results: 207 UK responses were received from which 76 (36.7%) were users. The main motivations for natural NPSs use were “to experience something new and different” (70.1%) and “to fight depression/anxiety symptoms” (53.2%); 90% of users reported satisfaction after the use of natural NPSs. The most preferred natural NPS was magic mushrooms (psilocybin, 92%) often combined with cannabis (69%); with the most favourable place for taking them being outdoors in nature (67%). More than half of the users (56.6%) have stopped using natural NPSs in the past; one out of two users stated that they could not function normally throughout the day whilst taking natural NPSs. The majority (67.5%) believed that there is no risk or just a low risk by consuming their favourite natural NPSs when only 19 % stated that there is medium or high risk. Gender, age, employment, smoking frequency and religion significantly affected (P<0.001) natural NPSs use. Male respondents, middle-aged, habitual smokers, atheists and agnostics but also frequent alcohol consumers represented the majority of natural NPSs users as well as the employed, the unable to work (due to disability/accident) and retired groups.
Conclusion: UK users’ low perception of natural NPSs safety profile and intoxication risks indicates a need for enhanced prevention interventions through education and drug policy updates.
Therapeutic drug monitoring
6
Impact of tacrolimus intra-patient variability on the development of drug-related toxicities in a group of lung transplant recipients
Nogueiras-Álvarez R1, Mora Cuesta V2, de Cos Cossío M3, Cifrián Martínez J2, Iturbe Fernández D2, Tello Mena S2, García Sáiz M3
1 Clinical Pharmacologist, Spain
2 Pneumology Service. Hospital Universitario Marqués de Valdecilla, Santander, Spain
3 Clinical Pharmacology Service. Hospital Universitario Marqués de Valdecilla, Santander, Spain
Introduction: In recent years, tacrolimus intra-patient variability (IPV) has shown to be an useful biomarker for the study of the evolution of different types of transplantation (Tx).
Objectives: To analyze whether a high tacrolimus IPV (Tac-IPV) is associated with an increased risk of developing cardiovascular risk factors and/or impaired renal function in lung transplant recipients (LTR).
Methods: A retrospective review (between 01/01/2015 and 09/12/2018) of 142 LTR on tacrolimus as maintenance immunosuppression treatment was performed. Those with no previous history of arterial hypertension (HTN), diabetes mellitus (DM), hypercholesterolemia, hyperuricemia in their pre-transplant medical history were included as cases.
Recording of tacrolimus blood levels started from the third month post-transplantation (to avoid variability due to clinical changes and medication modifications during early post-Tx) and only patients with at least 3 tacrolimus trough (C0) determinations were included. To allow comparisons between patients, the recorded levels were set to coincide with routine post-Tx follow-up.
As a Tac-IPV measure, the coefficient of variation of the level/dose ratios corresponding to each point was obtained.
Blood concentrations of tacrolimus were determined in the Clinical Pharmacology laboratory of the centre by chemiluminescent microparticle immunoassay on an Abbott Diagnostics® ARCHITEC i-1000 platform.
Results: Of the total number of patients reviewed: 39 developed HTN, 39 DM, 72 hypercholesterolaemia, 25 hyperuricemia and 109 impaired renal function.
After classifying recipients according to their Tac-IPV, no statistically significant differences were found for the development of HTN [OR=0.932; 95% CI: 0.446-1.947], DM [OR=0.701; 95% CI: 0.334-1.472], hypercholesterolaemia [OR=1.119; 95% CI: 0.580-2.162], hyperuricemia [OR=1.634; 95% CI: 0.679-3.933] or impaired renal function [OR=1.488; 95% CI: 0.678-3.265].
Time to onset of each of these events was calculated, with the shortest time being the development of hypercholesterolaemia, with a median of 49 days [interquartile range: 20.5-305.25].
Conclusion: In this review of 142 LTR, no statistically significant differences were observed in terms of high Tac-IPV and increased development of cardiovascular risk factors and renal impairment.
7
Variability of immunosuppression blood levels and its influence on allograft evolution in lung transplant recipients
Nogueiras-Álvarez R1, Mora Cuesta V2, de Cos Cossío M3, Cifrián Martínez J2, Iturbe Fernández D2, Tello Mena S2, García Sáiz M3
1 Clinical Pharmacologist, Spain
2 Pneumology Service. Hospital Universitario Marqués de Valdecilla, Santander, Spain
3 Clinical Pharmacology Service. Hospital Universitario Marqués de Valdecilla, Santander, Spain
Introduction: High tacrolimus intra-patient variability (IPV) has recently become recognized as a biomarker to identify transplant recipients at risk for poor outcomes such as acute rejection and graft loss.
Objective: To evaluate the potential association between development of chronic lung allograft dysfunction (CLAD) and high tacrolimus IPV (Tac-IPV) following lung transplantation (LTx).
Patients and Methods: We performed a retrospective review (between 01/01/2015 and 09/12/2018) of 142 lung transplant recipients (LTR) on tacrolimus treatment, including tacrolimus trough (C0) blood levels from the third month post-transplant (to avoid variability due to clinical changes and medication modifications during early post-Tx). To allow comparisons between patients, the recorded levels were set to coincide with routine post-LTx follow-up. Only patients with at least 3 tacrolimus trough (C0) determinations were included.
As measure of Tac-IPV, both the coefficient of variation (measured in %) and the standard deviation were calculated. Since not all patients receive a constant dose of tacrolimus, the C0 levels obtained were corrected for their daily dose (i.e., level/dose index).
Blood concentrations of tacrolimus were measured by chemiluminescent microparticle immunoassay on an Abbott Diagnostics® ARCHITEC i-1000 platform in the Clinical Pharmacology laboratory.
Results: We found 20 LTR (14.1%) who developed CLAD. Mean time from transplant to CLAD onset was 951.1 ± 467.56 days.
After classifying recipients according to their Tac-IPV, no statistically significant results were obtained both when using the coefficient of variation (p=0.790) and standard deviation (p=0.208) as a measure of Tac-IPV.
Conclusion: Different studies in solid organ transplantation have demonstrated that high Tac-IPV can predict poor outcomes in transplant recipients. In our LTR retrospective review, no statistically significant differences were observed in terms of high Tac-IPV and CLAD; nevertheless, this could be due to the quite short follow-up time available for each patient, which makes it difficult to assess the occurrence of chronic graft rejection in this type of solid organ transplantation.
17
Investigation of the Effect of Epalrestat and Sitagliptin on Alleviation of the Pain in Neuropathic Pain Model in Rats
Hasanvand A 1
1 Department of Pharmacology, Faculty of Medicine, Lorestan University of Medical Sciences, Khorramabad, Iran
Background and Objectives: Neuropathic pains are one of the most important consequences of the damage to the nervous system that plays an important role in the social and personal life. This type of pain can appear in different forms such as hyperlagesia and Allodynia. It is also shown that microglias play a significant role in increasing the neuropathic pain. In the present study, the alleviatory role of Epalrestat and Sitagliptin is investigated on the Chronic Constriction Injury (CCI) Model of Neuropathic Pain in rats by examining the level of inflammatory cytokines and anti-oxidative factors.
Methodology: To conduct this study, 50 male rates were used. The animals were randomly divided into five groups: the first group: control group, the second group: CCI, the third group: CCI + Epalrestat at the dose 100 mg/kg, the fourth group: CCI + Sitagliptin at the dose 10 mg/kg, and the fifth group: CCI + Epalrestat at the dose 100 mg/kg + Sitagliptin at the dose 10 mg/kg. The behavioral tests (e.g. Radiant Heat Planter Test, Acetone Test and Von ferry Test) in animals were applied 4, 7 and 14 days after the surgery and were compared to the CSF samples prepared on the 14th day and the inflammatory and oxidative factors.
Findings: CCI-induced pain increased the level of inflammatory cytokines and oxidative stress and ultimately severe pain by reducing the pain threshold. Nonetheless, it was shown that Epalrestat and Sitagliptin can be used separately and simultaneously as effective and safe drugs to treat neuropathic pain, which increase the pain threshold by inhibiting the power of inflammatory cytokines and oxidative stress.
Conclusion: Research results showed that using Epalrestat and Sitagliptin can alleviate and reduce neuropathic pain in this experimental model and play an important role in inhibiting the increase of pain by balancing the level of inflammatory and oxidative factors.
18
Progesterone Receptor plays a protective role in Diabetic neuropathy through anti-oxidative and anti-inflammatory effects
Hasanvand A1
1 Department of Pharmacology, Faculty of Medicine, Lorestan University of Medical Sciences, Khorramabad, Iran., Khorramabad, Iran
Introduction: Diabetic neuropathy (DN) is a debilitating outcome of diabetes, which affects many patients. Development of demyelinated neurons is the main cause of diabetic neuropathy. It has been demonstrated that progesterone can prevent neurons from damage. Therefore, the aim of this experiment was evaluate the neuroprotective effect of progesterone receptor against diabetic neuropathy.
Methods: Forty male Sprague-Dawley rats were divided into 4 groups, including control group, diabetic control group, diabetic control group + Progesterone (30 mg/kg, ), diabetic control group + combination of Progesterone (30 mg/kg, ) and RU486 (10 mg/kg). Diabetes was induced in rats using streptozotocin (55 mg/kg, ). After the induction of diabetes, blood glucose level, body weight, behavioral tests, electrophysiological tests, oxidative as well as inflammatory factors, and histological parameters were measured.
Results: Progesterone treatment did not significantly modify the amount of blood glucose level. The sensitivity level to hot plate remarkably was decreased on the 14th, 21th and 28th days. Significant changes also were observed in the results of tail flick tests. In addition, the results showed that the administration of progesterone could improve MNCV. Progesterone could also significantly attenuate the serum level of oxidative stress and inflammatory factors. Inflammation and edema diminished around sciatic nerve in progesterone treated groups. RU486 reversed the beneficial effect of Progesterone.
Conclusion: Progesterone can be considered as a protective agent in the reduction of DN due to its capacity to reduce inflammation and nerve damage. In addition, RU486, progesterone receptor blocker, inhibits the beneficial effects of progesterone in DN, resulting in progesterone receptors play a crucial role in the neuroprotective influence of progesterone.
35
Comparison of Mw\Pharm 3.30 (DOS) and Mw\Pharm++ (Windows) Versions of Pharmacokinetic Software for PK/PD Monitoring of Vancomycin in continuous Administration
Hricova M1, Koristkova B1,2, Vavreckova E1, Kacirova I1,2, Brozmanova H1,2, Grundmann M1,2
1 Dept. Clin. Pharmacol., Faculty Of Medicine, University of Ostrava, Ostrava, Czech Republic
2 Dept. Clin. Pharmacol., Inst. Lab. Medicine, Ostrava University Hospital, Ostrava, Czech Republic
Introduction: For a long time, the Mw\Pharm software suite (MEDI\WARE, Prague, Czech Republic / Groningen, Netherlands) has been used for PK/PD modelling in therapeutic drug monitoring (TDM).
Objective:The aim of this study was to find the best model in the newer Windows version of Mw\Pharm++ 1.3.5.558 (WIN) for continuous administration of vancomycin.
Methods: Twenty adult patients with mean age 66±12 years, body weight 85±16 kg, and median dose 1,625 g/24h were repeatedly examined for vancomycin. Concentrations predicted by “#vancomycin_adult_k_C2“ (WIN1), ”#vancomycin_adult_C2“ (WIN2), ”vancomycin_adult_C2“ (WIN3), “vancomycin_C1“ (WIN4) WIN models and ”vancomycine (cont.inf.) %ahz“ (DOS1) and ”vancomycin adult“ (DOS2) DOS models were compared with the measured value and with DOS1 model. Statistics: Percentage prediction error (%PE) calculated as (predicted–measured)/measured or WIN-DOS1/DOS1, RMSE, Blandt-Altman bias, Pearson’s coefficient of rank correlation (R), Student’s t-test. Statistical analysis was performed using GraphPad Prism version 5.00 for Windows.
Results: %PE value varied between -7.4% and -3.2%, with the exception of WIN4, the only one-compartment model, where it was -20.8%. Best outcomes were achieved with WIN3 model. WIN1 produced the lowest %PE, RMSE and Blandt-Altman bias among the WIN models, but its correlation (Pearson’s R) was less tight (Table 1). RMSE was the same in WIN3 while %PE and Bland-Altman bias were similar, with slightly better correlation when compared to WIN1. WIN1-3 models were more similar to DOS1 as %PE was -1,4 – 1,7%, whereas %PE value between the two DOS models was 4.1 ± 13.9% (NS). DOS2 produced slightly better outcomes than DOS1.
Conclusion: “vancomycin_adult_C2“ and “#vancomycin_adult_k_C2“ produced the best outcomes between WIN models. Both DOS models produced lower bias and their prediction was comparable.
Acklowledgement: Supported by grant University of Ostrava SGS08/LF2019-2020
Tabel/Image
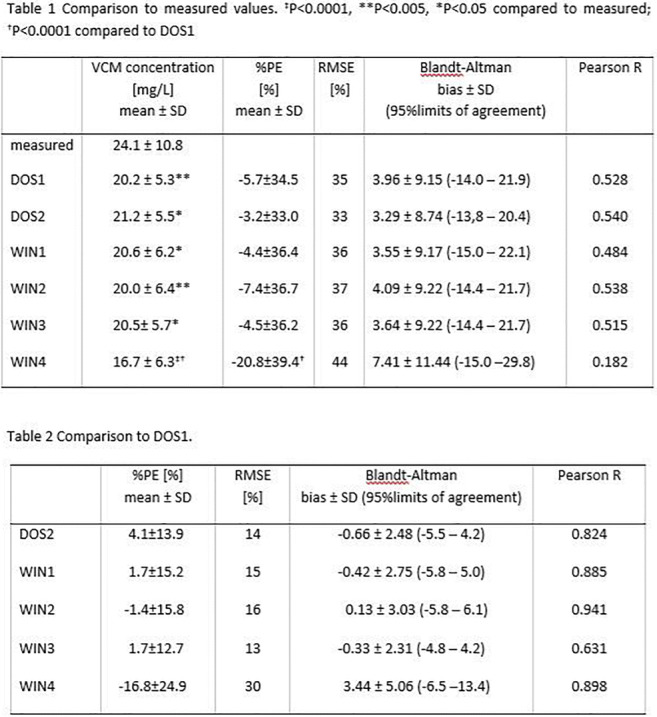
48
Therapeutic drug monitoring of vancomycin as one of the methods for detecting drug-induced nephropathy in patients with orthopedic infection
Kasimova A1,2, Tufanova O2
1 Pavlov First Saint Petersburg State Medical University, Saint-Petersburg, Russia
2 Russian Scientific Research Institute of Traumatology and Orthopedicsnamed after R.R. Vreden, Saint-Petersburg, Russia
Introduction: Infectious complications in modern orthopedics are a serious problem. The widespread use of methicillin-resistant strains of staphylococci in the etiology of infection causes the empirical administration of vancomycin to patients with orthopedic infection. Vancomycin is an antibiotic with a narrow therapeutic window. Its use requires mandatory monitoring of renal function in patients. According to studies, nephrotoxicity phenomena are diagnosed in 8% of patients treated with vancomycin. The probability of developing drug nephropathy in patients with periprosthetic infection is high due to the use of high doses and long courses of antibiotic therapy. For the timely detection of the development of nephrotoxicity, the determination of its serum concentration in the blood is used. It is known that its level above 20 mcg/ml significantly increases the risk of acute renal failure.
Objectives: To evaluate the role of drug monitoring of vancomycin in the detection of drug nephrotoxicity in patients with orthopedic infection for the period 2019-2021.
Methods: A retrospective analysis of 457 results of drug monitoring of vancomycin in patients with orthopedic infection for the period 2019-2021 was carried out. Serum creatinine levels were found in all patients with toxic vancomycin concentrations (above 20 mcg/ml). Patients are divided into groups depending on the number of studies conducted.
Results: It was revealed that in the first study (on the 3rd day of vancomycin therapy), the serum concentration of the drug exceeded 20 mcg/ml in 29 (6.3%) people and in the second (on the 6th day of therapy) in 22 (12.4%) people. At the same time, a simultaneous significant increase in serum creatinine levels in combination with the clinical picture of kidney damage developed in 8 (1.6%) patients, 6 of them in the first study and 2 in the second. All patients received timely care and were discharged in a stable condition.
Conclusion: Therapeutic drug monitoring of vancomycin is one of the auxiliary methods that allow timely detection of the development of drug nephropathy against the background of vancomycin therapy.
75
Main factors impacting therapeutic valproate trough levels in epileptic children
Ferchichi K1,2, Charfi R1,2, Affes R1,2, Ben Sassi M1,2, Gaies E1,2, El Jebari H1, Jebabli N1, Salouage I1,2, Daghfous R1,2, Trabelsi S1,2
1 National Centre Chalbi Belkahia Of Pharmacovigilance, Tunis, Tunisia
2 University of Tunis El Manar - Faculty of Medicine of Tunis, Tunis, Tunisia
Introduction: Valproic acid (VA) is established as a major antiepileptic drug with a broad spectrum of activity. In children, VA therapeutic drug monitoring (TDM) is recommended because of the large interindividual variability of its pharmacokinetics and the high non-adherence, making this molecule one of the first and most common drugs. TDM optimizes VA dose adjustment in order to maximize efficiency and minimize adverse reactions risks. Ideally, VA treatment entails achieving complete seizure freedom without significant adverse reactions, but for many patients, achieving optimum seizure control with minimal adverse reactions is the best compromise.
Objective: In this study, we aimed to assess the predictive factors in order to reach therapeutic VA trough levels (TL).
Methods: We conducted an observational study in the department of clinical pharmacology for 12 years (January 2009-December 2021). Patients aged between two and 18 years, regularly treated with VA, who had a TDM of VA with at least two VA TL measurements were included.
Patients’ VA levels measured before reaching the steady-state and those with missing data were excluded.
For each measurement, the following data were collected: age, sex, pathology, medical history, type and frequency of seizures, compliance, dose, rate of administration of VA, associated drugs, and adverse reactions observed.
The measurements were performed with the Architect® automated system from Abbott Laboratories using FPIA method. The TR for VA was between 50 and 100 μg/mL and the low limit of quantification was 0.7μg/mL.
Our population was divided into two groups: group A (GpA) with a VA TL outside the therapeutic range (TR) and group B (GpB) with a final VA TL outside the TR.
Results: We included 805 patients (2538 TL). The median age was 6.24 and the sex ratio (M/F) was 1.45. A median of two measurements per patient, varying between two and 15 measurements was reported. The median VA daily dose was 27.27 mg/kg/day varying between 8mg/kg/day and 100 mg/kg/day. The median VA TL was 57 μg/mL (18.8-177.5 μg/mL).
The population was divided into two groups of patients: GpA (540 children) represented 67.08% of the children and GpB (265 children) 32.92% (table 1).
Conclusions: This study suggests that the follow-up duration and the continuous and rigorous TDM of valproic acid is mandatory to reach and to maintain a VA through concentration in the TR. Because of missing data and epilepsy resistance, remission was probably underestimated in this study.
Tabel/Image
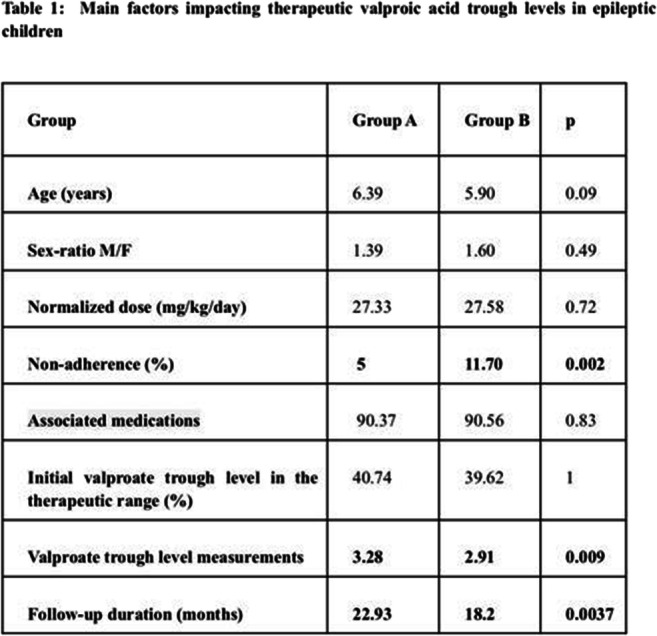
85
Implementation of a vancomycin dose-optimization protocol in neonates: impact on vancomycin exposure, biological parameters, and clinical outcomes
Tonini J1, Gomez L1, Boegler D1, Epiard C1, Alin L1, Arata-Bardet J1, Caspar Y1, Debillon T1, Stanke-Labesque F1, Gautier-Veyret E1
1 Grenoble Alpes University Hospital, Grenoble, France
Introduction: Vancomycine exhibits high pharmacokinetic variability, especially in neonates, and a narrow therapeutic range, justifying therapeutic drug monitoring. Moreover, vancomycin frequently exhibits insufficient steady-state concentrations (Css) in neonates.
Objectives: We aimed to compare vancomycin exposure and biological and clinical parameters before and after implementation of a vancomycin dose optimization protocol in the neonatal intensive care unit of the Grenoble Alpes University Hospital.
Methods: We performed a monocenter retrospective study. The primary endpoint was the proportion of initial vancomycin Css in the therapeutic range (15-25 mg/L).
Results: In total, 60 and 59 courses of vancomycin treatment in 45 and 49 patients were analyzed in groups before and after implementation, respectively. Initial vancomycin Css were more frequently in the therapeutic range in the group after implementation (74.6% versus 28.3%, p < 0.001), with 1.6-fold higher Css (20.3 [17.0-22.2] mg/L versus 12.9 [11.3-17.0] mg/L, p < 0.001). Considering all Css during longitudinal therapeutic drug monitoring, the frequency of therapeutic Css was higher in the group after implementation (74.8% [n = 103] versus 31% [n = 116], p < 0.001). The dose optimization protocol was also associated with a reduced time to obtain a negative blood culture (p < 0.001) and fewer antibiotic switches (p = 0.025), without increasing the frequency of nephrotoxicity. Clinical outcomes also appeared to be improved, with less periventricular leukomalacia (p = 0.021) and trended towards less respiratory instability (p = 0.15) and a shorter duration of vasoactive drug use (p = 0.18) for neonates receiving personalized doses of vancomycin.
Conclusion: This personalized vancomycin dose protocol improves vancomycin exposure in neonates, with good safety, and suggests an improvement in biological and clinical outcomes.
Tabel/Image
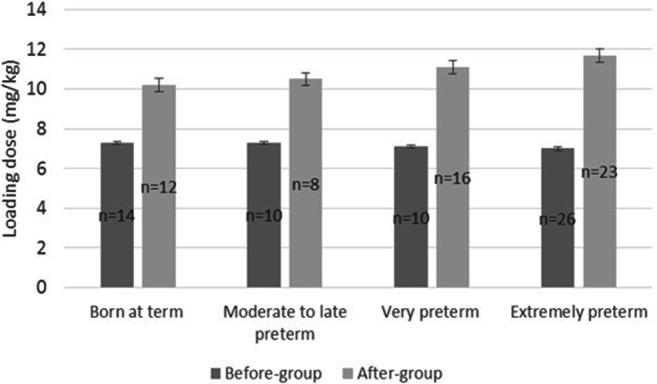
87
Variability of isavuconazole trough concentrations during longitudinal therapeutic drug monitoring
Tonini J1, Bolcato L1, Thiebaut-Bertrand A1, Stanke-Labesque F1, Gautier-Veyret E1
1 Grenoble Alpes University Hospital, Grenoble, France
Introduction: Isavuconazole (ISA), a broad-spectrum triazole antifungal agent, is licensed for the treatment of invasive aspergillosis and mucormycosis. Therapeutic drug monitoring is a cornerstone of treatment efficacy for triazole antifungals due to their pharmacokinetic variability, except for ISA, for which the utility of therapeutic drug monitoring is still uncertain.
Objectives: We therefore performed a retrospective study that aimed to assess the inter- and intra-individual variability of ISA trough concentrations (Cmin), to identify the determinants involved in such variability and explore the concentration-effect relationship.
Methods: We perform a retrospective study of ISA Cmin determined at pharmacokinetic steady state in hospitalized adult patients between January 2018 and August 2020.
Results: In total, 304 ISA Cmin were determined from 33 patients. The median ISA Cmin was 2.8 (range: 0.7-6.5) mg/L. The inter- and intra-individual variability was 41.5% and 30.7%, respectively. Multivariate analysis showed independent covariate effects of ISA dose (β=0.004 ± 3.56.10-4, p<0.001), ASAT (β=0.002 ± 5.41.10-4, p=0.002) and protein levels (β=0.022 ± 0.004, p<0.001) on ISA Cmin, whereas CRP levels did not show any association. Those data do not demonstrate any concentration-clinical efficacy relationship.
Conclusion: This study conducted on a large number of ISA Cmin shows that ISA exposure exhibits variability, explained, in part, by the ISA dose, ASAT and albumin levels.
Tabel/Image
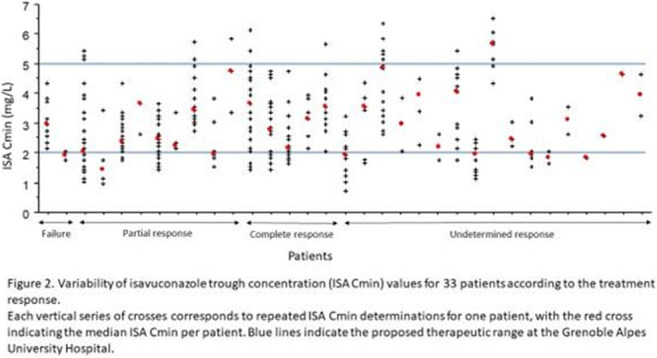
89
Development and validation of a new High Performance Liquid Chromatography technique for determination of paracetamol
Charfi R1, Staali H1, Daldoul M1,2, El Jebari H1, Ben Sassi M1,2, Gaïes E1,2, Jebabli N1, Daghfous R1,2, Trabelsi S1,2
1 National Centre Chalbi Belkahia of Pharmacovigilance, Department of clinical pharmacology, LR16SP02, Tunis, Tunisia
2 University of Tunis El Manar, Faculty of Medicine of Tunis, Tunis, Tunisia
Background: Paracetamol is a synthetic compound used as a medicine to relieve and reduce fever, usually taken as tablets. The pharmacological therapeutic monitoring of paracetamol is justified by the interindividual variability of its pharmacokinetics, the risk of drug interactions and therapeutic non-compliance.
Aim: In this context, we have developed and validated a plasma assay technique for paracetamol using High Performance Liquid Chromatography with UV detection (HPLC-UV).
Methods: Initially, we set the chromatographic conditions which were a mobile phase (95% distilled water + 5% acetonitrile), a C18 column (250 mm), a wavelength equal to 254nm, a flow rate of 1 ml/min, and a furnace temperature equal to 40°C.
Results: The liquid/liquid extraction of our analyte from human plasma was carried out by a perchloric acid solution 17.5% This technique is linear in a concentration range from 5 to 250 μg/ml (r² = 0.9997). The detection and quantification limits are in the range of 0.041 μg/ml and 0.01 μg/ml, respectively. Repeatability and reproducibility were checked for three concentration levels with coefficients of variation ranging from 0.76 to 4.28% for repeatability and from 1.57 to 4.34% for reproducibility. The retention time of paracetamol is 8.8 min.
Conclusion: We were able to develop and validate a HPLC paracetamol assay technique with UV detection that is sensitive, specific, reliable and easy to perform for routine use in pharmacological therapeutic monitoring.
102
Ischemic stroke in atrial fibrillation patients on new oral anticoagulants: assessment of therapeutic adherence and dose regimen
Paiva P1, Carvalho I1, Matos S1, Miller J1, Silva F1, Santo G1, Cunha L1, Gonçalves L1, Sargento-Freitas J1, António N1
1 Centro Hospitalar E Universitário De Coimbra, Coimbra, Portugal
Introduction: Currently, new oral anticoagulants (NOACs) are the therapeutic option of choice for the prevention of ischemic stroke in patients with non-valvular atrial fibrillation (AF). However, the risk of developing ischemic stroke even in patients prescribed NOACs is not negligible.
Objective: To evaluate whether therapeutic compliance and daily or bi-daily regimen of taking NOAC are associated with clinical outcome and occurrence of large and medium vessel occlusion (defined by occlusion of the internal carotid, basilar and posterior cerebral arteries, segments M1-M2 of the middle cerebral artery and A1 of the anterior cerebral artery) in cases of therapeutic failure.
Methodology: Prospective observational study: 171 patients admitted to the neurology service of a tertiary hospital over a two-year period with a diagnosis of ischemic stroke despite being prescribed oral anticoagulation for non-valvular atrial fibrillation were selected. Possible etiologies for stroke were assessed, including adequacy of NOAC dose and therapeutic adherence, as assessed by 2 scales: Brief Medication Questionnaire (BMQ) and Measure Treatment Adherence (MTA).
Results: 171 patients were included with a mean age of 79.65±8.53 [49-103] years, 57.89% women. 87 (50.88%) were taking daily NOAC and 84 (49.12%) were taking bi-daily NOAC. The population characteristics, pattern and duration of AF were similar between the groups.
Inadequate adherence to therapy (33.3% BMQ scale and 28.0% MAT scale) and underdosage (32.3%) were common in the overall population. No statistically significant differences in adherence were identified between groups with 70.00% of patients reporting adequate adherence in the daily-dose group and 62.90% in the bi-daily-dose group on the BMQ scale (p=0.39); 75.71% vs 67.74% on the MAT scale (p=0.31).
Large and medium vessel occlusion was verified in 122 patients. Univariable analysis showed that age (p=0.001), presence of non-paroxysmal AF (p=0.002), left ventricular ejection fraction (p=0.001), therapeutic compliance according to the ATM scale (p=0.015) and BMQ (p=0.02) were associated with large and medium vessel occlusion. Daily or bi-daily taking was not associated with increased risk (p=0.322). The association was maintained after adjustment for confounding variables for left ventricular ejection fraction and therapeutic adherence according to the MTA scale.
NIHSS scale at admission (p=0.09) and functional status at discharge (p=0.089) did not show a statistically significant difference between patients on daily or bi-daily NOAC, even after adjustment for possible confounders.
Discussion/Conclusions: In a significant percentage of patients, the occurrence of stroke can be explained by poor compliance, underdose or non-cardioembolic etiology. Therapeutic compliance is an independent predictor for the occurrence of large and medium vessel occlusion. Dose regimen does not seem to influence compliance, large and medium vessel occlusion, stroke severity at admission, and disability at discharge.
115
Comparison of Mw\Pharm 3.30 (DOS) and Mw\Pharm++ (Windows) Versions of Pharmacokinetic Software for PK/PD Monitoring of Vancomycin for dialyzed patients
Koristkova B1,2, Vavreckova E1, Hricova M1, Kacirova I1,2, Brozmanova H1,2, Grundmann M1,2
1 Dept. Clin. Pharmacol., Faculty of Medicine, University of Ostrava, Ostrava, Czech Republic
2 Dept. Clin. Pharmacol., Inst. Lab. Medicine, Ostrava University Hospital, Ostrava, Czech Republic
Introduction: For a long time, the Mw\Pharm software suite (MEDI\WARE, Prague, Czech Republic / Groningen, Netherlands) has been used for PK/PD modelling in therapeutic drug monitoring (TDM).
Objective: The aim of this study was to find the best model in the newer Windows version of Mw\Pharm++ 1.3.5.558 (WIN) for patient on intermittent hemodialysis.
Methods: Twenty-two adult patients with mean age 65±15 years, body weight 88±25 kg, were repeatedly examined for vancomycin. Cmin and concentration before hemodialysis (CBH) predicted by Windows models “vancomycin_dialysis_c2 “ (WINd), ”vancomycin_adult_C2“ (WINa), and DOS 3.30 models ” vancomycin (dialysis)“ (DOSd), ”vancomycin adult“ (DOSa) were compared with the with the measured value and with DOSd model. Statistics: Percentage prediction error (%PE) calculated as (predicted–measured)/measured or (predicted-DOSd)/DOSd, RMSE, Blandt-Altman bias, Pearson’s coefficient of rank correlation (R), Student’s t-test. Statistical analysis was performed using GraphPad Prism version 5.00 for Windows.
Results: Models WINd and DOSd produced better Cmin prediction then respective adult models. DOSd model produced lower %PE, Blandt-Altman bias and Pearson R, while slightly higher RMSE than WINd model. CBH prediction (available only in 7 cases) was better by WINa compared to WINd, while similar by DOSa and DOSd (Table 1). WINd and DOSd models use higher population V1 and Clm while lower fr than WINa and DOSa models (Table 2). V1, k12 were higher, while k21 was lower in all models than respective population data. Clm was higher in DOSa, while lower in WINa and fixed in WINd. Fr was lower in WINa and DOSa.
Conclusion: DOS and WIN models are not identical despite similar name and the same population data. Dialysed models should be use for TDM in dialysed patients. DOSd model produced the best prediction while WINd was poorer. Implementation of DOSd model into WIN version of MW\Pharm is recommended.
Abreviations: Clm – non-renal clearance, fr – renal fraction, k12, k21– distribution rate constants, RMSE – root mean square error, V1 – volume of distribution related to lean body mass
Acklowledgement: Supported by grant University of Ostrava SGS08/LF2019-2020
Tabel/Image
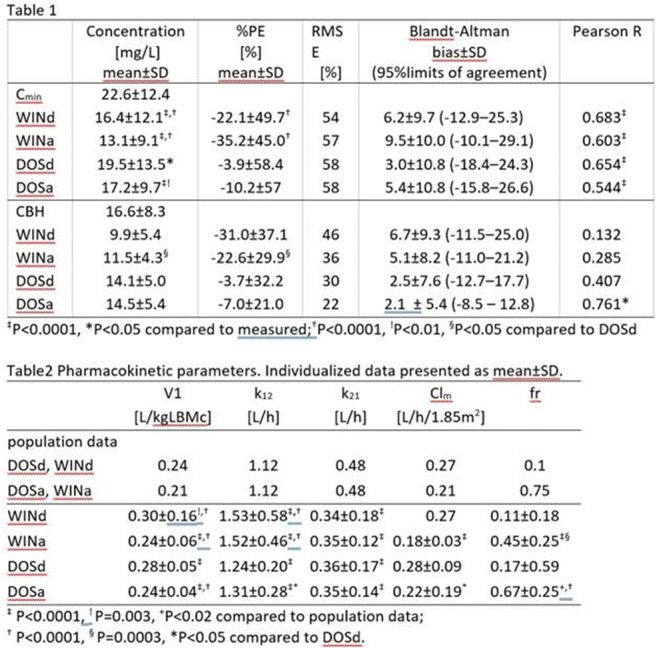
122
Tacrolimus and everolimus high blood levels after reversion of ileostomy: a case report and review of the literature
Nikolaou A1, Craig A2, Ing Lorenzini K1, Desmeules J1, Samer C1, Rodieux F1
1 Division of Clinical Pharmacology and Toxicology, Geneva University Hospitals, Geneva, Switzerland
2 CHUV, centre médical universitaire vaudois, Lausanne, Switzerland
Case Description: A 56-year-old woman known for Gardner's syndrome, multi-visceral transplantation (duodenum, pancreas, spleen, small intestine, right colon) and ileostomy treated, among others, with prolonged-release tacrolimus tablets 6mg qd and everolimus 5mg bid, was electively hospitalized for ileostomy closure and restoration of colonic continuity. 48h after surgery, the tacrolimus whole blood trough concentration increased significantly (28.7 μg/l; target concentration 3-5 μg/l). Everolimus trough concentrations increased 96h postoperatively as shown (Table 1). The patient’s symptoms were consistent with tacrolimus toxicity (headache, hypertension, nausea). Both treatment dosages were decreased.
A pre-analytical error was eliminated. Hematocrit values were stable. The patient’s medication compliance was adequate and no pharmaceutical change was reported. Despite the presence of comedication inhibiting CYP3A4/5 and Pgp activity, no recent drug treatment introduction or change could explain the sudden increase in tacrolimus concentrations. Transit was normal 3 days after intervention and no diet changes occurred.
Discussion: Our case is the first reported case of high tacrolimus and everolimus levels after reversion of ileostomy. We conducted a literature review about tacrolimus and everolimus absorption. Data are scarce, but, it appears that tacrolimus is absorbed from the duodenum up to the colon, in particular when administered as a prolonged-release form. Ileostomy reversal could thus increase the colonic absorption of tacrolimus and contribute to the rise of tacrolimus trough levels and risk toxicity. We found no data about everolimus, however, in this patient, the increase in everolimus concentrations is probably explained by the same mechanism.
Conclusion: Our case clearly illustrates the short transit time and the poor bioavailability, particularly of long-acting formulations, in case of short bowel syndrome and the risk of toxicity after ileostomy closure. This risk is particularly important for drugs with a narrow therapeutic margin such as tacrolimus and everolimus. Close monitoring of drug concentrations is extremely important in this situation.
Tabel/Image
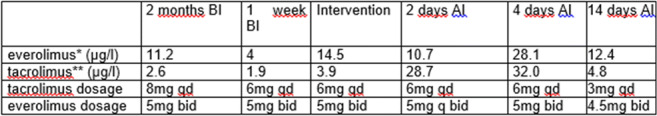
142
The impact of the pandemic of COVID-19 on therapeutic drug monitoring
Jerbi A1,2, Ben Sassi M1,2,3, Charfi R1,2,3, Gaies E1,2,3, El Jebari H1,2, Daghfous R1,2,3, Trabelsi S1,2,3
1 National Center Of Pharmacovigilance of Tunis, Tunisia
2 Clinical and Experimental Pharmacology Research Laboratory LR16SP02, Tunisia
3 Faculty of Medecine of Tunis, Tunisia
Introduction: The pandemic of COVID-19 impacted the health care system worldwide. It changed our health care priorities and drug prescription.
When it comes to therapeutic drug monitoring, we noticed changes in our daily activity in the department of Clinical Pharmacology in the National Center of Pharmacovigilance in Tunis.
Objectives: To describe variation in therapeutic drug monitoring and analyze reasons behind it in order to provide better health care for patients during the pandemic of COVID-19.
Methodology: We collected data from our database. We analyzed a number of dosages from 2016 to 2020. Data for each drug was analyzed separately. The average number of samples analyzed between 2016 and 2019 was calculated and then compared to the total number of samples analyzed in 2020.
Results: The total number of samples analyzed in 2020 dropped by 11% compared to the average of total samples between 2016 and 2019. For the number of samples analyzed for anti-infectious drugs, we noted a drop of 23 % only for vancomycin and an increase of 20% for amikacin, 16% for gentamicin, 117 % for rifampicin, 79.3% for teicoplanin, and 22% for voriconazole. For immunosuppressors, the number dropped by 14% for mycophenolic acid, 8% for ciclosporin, 14% for tacrolimus, and an increase of 2.6% for sirolimus, and 29.8 % for azathioprine (It should be noted that in 2016, there was a lack of reagents for dosage of azathioprine). For antiepileptics, the number dropped by 30% for valproic acid and carbamazepine, 28.2% for lamotrigine, 36.5% for phenobarbital, 95% for phenytoin, and an increase of 46.3% for levetiracetam, and 35.3 % for clonazepam (dosage of these 2 last drugs was introduced in 2017).
Conclusion: The pandemic of COVID-19 reduced the number of dosages of certain drugs despite the need for this dosage in the management of patients. This decrease can be explained by fear of patients to go to hospitals or lowering prescription of therapeutic drug monitoring.
158
A centralized automated controlling system of treatment compliance – “Medreminder”.
Shopabayeva A, Sydykov S, Zhakipbekov K
1 KaZNMU, Almaty, Kazakhstan
Introduction: “Medreminder” - is a mobile app (app) based on Android and iOS operating systems. The functions, inform the attending physician about the patient's health and adverse effects on the medicine, also allows keeping record and controlling patients receiving medications prescribed by the attending physician.
Objectives: Evaluate the contribution of the “Medreminder” on improving patient treatment compliance using app.
Matherials and Methods: The market of mobile operational systems, which are more frequently used in Kazakhstan, was carefully researched to choose the platform of programming language. The Android operational system takes 74,37%, iOS- 22,73% and others- 2,9%. So, the mobile application “MedReminder” was developed on Android and iOS operational systems.
Results: The selection criteria of the patients were a verified diagnosis, the availability of all laboratory and instrumental results, receiving medical therapy at least a month before the study and informed consent for the study.
The research included the observation of two groups of patients:
1) The first group of 25 patients who were using the mobile application.
2) The second control group of 20 patients who were not using the application.
All patients were specially surveyed and questioned; moreover, careful consideration was given to all the data from clinical-laboratory and instrumental research.
During the survey, the following data, not related to the disease, was collected: gender, age, marital status, residence, education, occupation and experience in long-term use of medicines in case of chronic diseases in anamnesis.
Every participant of the research was assigned to have a unique two-digit number, which matched the patient’s serial number in the centre. All the patients included in the research were registered in the research centre, the registered data included a serial number of a patient, the date of inclusion in the study and other information. The observation period lasted for 3 months.
Both groups of patients filled in the questionnaire during the appointments, including Morisky-Green test. It includes 4 questions:
1) Have you ever forgotten to take your medication?
2) Are you sometimes careless about the time when to take medicines?
3) Do you miss taking medicines if you are feeling well?
4) If you are feeling unwell or worse after you have taken medicines, do you miss the following medication?
Results of test in the research group and control group at first visit: 1,8 and 1,9 points. After 3 months of using an app, treatment compliance in the study group increased to 3.9 and in the control group to 2.9 points. It was noted that on the background of the treatment there was a significant increase in compliance in both of the groups. However, in the research group there was a more dramatic change in the answers.
Discussion: The analysis of the results and doctor’s interviews showed that during the study, patients who used the application became more careful about following the regime for medications intakes, doctor’s prescriptions and sending information about health, which allows making such a conclusion about formation of compliance to treatment and an increase in patient’s responsibility.
166
Safety Evaluation with Plasma Voriconazole Concentrations in Patients with Hematologic Malignancies
Ertem O1, Demirkan F2, Sarac A1, Arici M1, Gelal A1, Yavuz B2, Tuncok Y1, Alp Cavus S3, Gumustekin M
1 Dokuz Eylul University, School of Medicine, Department of Medical Pharmacology, Izmir, Turkey
2 Dokuz Eylul University, School of Medicine, Department of Internal Medicine, Division of Hematology, Izmir, Turkey
3 Dokuz Eylul University, School of Medicine, Department of Infectious Diseases and Clinical Microbiology, Izmir, Turkey
Introduction: Voriconazole is the guideline-recommended first-line treatment for invasive Aspergillus. It is also effective against Fusarium, Scedosporium, and Candida infections. However, it has a narrow therapeutic window; subtherapeutic concentrations cause decreased efficacy, and supratherapeutic concentrations predispose to voriconazole toxicity. Despite therapeutic drug monitoring (TDM) being recommended to ensure safe and effective treatment, it is not part of routine clinical care in Turkey. Interindividual variability up to 50% in voriconazole pharmacokinetics that increases the likelihood of voriconazole toxicity or treatment failure is also attributable to polymorphisms in CYP2C19.
Objectives: The study aims to evaluate trough concentrations of voriconazole in patients with hematological malignancies regarding the safety evaluation of the drug. As a part of the ongoing project entitled "Influence of CYP2C19 genotype on safety and efficacy of voriconazole in patients with hematologic malignancies", current data regarding the relationship between trough concentrations of voriconazole and adverse reactions are presented.
Methods: This is a single-center, observational cohort study. Patients aged 18 and older that have been hospitalized with the diagnosis of hematologic malignancies at hematology inpatient clinics of Dokuz Eylul University Hospital and that have been given voriconazole for the prophylaxis or the treatment of invasive fungal infections have enrolled in the study (n=15). Before the 9th dose of voriconazole (total daily (maintenance dose of 400 mg), whole blood samples of the patients were collected and analyzed by Liquid chromatography-sequential mass spectrometry.
Results: While two patients received voriconazole for the prophylaxis of invasive fungal infection, others (n=13) received voriconazole for the treatment. The median trough plasma voriconazole concentrations of the patients were 4.41 mcg/mL (IQR: 2.36). Target voriconazole concentration (1.0-5.5 mcg/mL) was achieved in 14 (93.3 %) patients, while one (6.7 %) patient had a supratherapeutic level of 6.6 mcg/mL). Adverse reactions were observed in six patients (40 %), between Day 1 and Day 18 of the voriconazole treatment. While in only one patient (6.7 %) with supratherapeutic voriconazole levels blurred vision, tremor, and skin rashes were observed, plasma voriconazole levels were normal range in two patients (13.3%) with hepatobiliary adverse reactions and in 3 patients (20%) with hallucinations. Voriconazole treatment was discontinued in all patients who had an adverse reaction.
Conclusion: The preliminary findings suggested a relatively high incidence of adverse reactions leading to treatment discontinuation in 40 % of the patients. To maintain an optimized and uninterrupted treatment in critically ill patients, the dose of voriconazole should be personalized. Once the ongoing project is completed after achieving the larger sample size, the causality assessment will be done by revealing the polymorphisms in the CYP2C19 gene that metabolizes the drug.
171
Determination of colistin A and B in human plasma: Application for verification of adsorption on the ECMO circuit and TDM
Kubickova V1, Soukop J1, Rychlickova J2,3, Urbanek K1
1 Department of Pharmacology, Faculty of Medicine and Dentistry, Palacký University Olomouc, Olomouc, Czech Republic
2 Department of Pharmacology, Faculty of Medicine, Masaryk University, Brno, Czech Republic
3 International Clinical Research Center, St. Anne´s University Hospital Brno, Brno, Czech Republic
Colistin also known as polymyxin E, is a narrow-spectrum lipopeptide antimicrobial agent used in the therapy of infections caused by multi-resistant bacteria. The resistance of gram-negative bacteria to most of the currently used antibiotics and the lack of new antimicrobial agents have prompted the re-emergence of colistin as potent treatment of infection caused by gram-negative microorganisms [1,2].
Colistin is administered in the form of a prodrug - colistin methanesulfonate (CMS) usually with a loading dose of 9 MIU and 4.5 MIU is continued 12 hours apart. The bactericidal effect of colistin is dependent on the concentration and AUC/MIC. A mean steady state plasma concentration of 2 mg/L is a suitable target concentration but difficult to achieve in critically ill patients. The conversion of CMS to colistin is highly variable. About 30-60 % of CMS is converted to colistin with target plasma concentrations of 2-5 mg/L, in fact 0,6-13 mg/L [3,4].
Therefore, we have introduced a method for the determination of colistin A a colistin B in human blood plasma by high performance liquid chromatography with MS detection. Solid phase extraction preceded chromatography separation on a Kinetex EVO C18 (100x2,1; 5 μm) column with a mobile phase consist of 0.1% formic acid in water and 0.1% formic acid in methanol (85/15) at 0.5 mL/min. Polymyxin B was used as an intermediate standard. Ions were generated using electrospray ionization and detected in the positive ion mode at the following transitions of mass to charge (m/z): colistin A 585.55 → 101.05; colistin B 578.5 → 101.15; and polymyxin B 602.4 → 101.1, 120.15, 86.15. The total analysis time was 5min.
This poster summarizes the pharmacological knowledge of colistin and presents the development of a rapid LC-MS method suitable for the determination of colistin A and colistin B and TDM purposes.
1. Gikas E, Bazoti FN, Katsimardou M, Anagnostopoulos D, Papanikolaou K, Inglezos I, Skoutelis A, Daikos GL, Tsarbopoulos A. Determination of colistin A and colistin B in human plasma by UPLC–ESI high resolution tandem MS: Application to a pharmacokinetic study. J Pharmaceut Biomed. 2013; 83, 228–236.
2. Ma Z, Wang J, Gerber JP, Milne RW. Determination of colistin in human plasma, urine and other biological samples using LC–MS/MS. J Chromatogr B. 2008; 862(1-2), 205–212.
3. Grayson ML, Cosgrove S, Crowe SM, et al. Kucers’ the use of antibiotics: A clinical review of antibacterial, antifungal, antiparasitic, and antiviral drugs, seventh edition. Boca Raton: CRC Press, 2018. ISBN 9781498747950.
4. Zabidi MS, Abu Bakar R, Musa N, Mustafa S, Wan Yusuf WN. Population Pharmacokinetics of Colistin Methanesulfonate Sodium and Colistin in Critically Ill Patients: A Systematic Review. Pharmaceuticals. 2021;14, 903.
173
Association of plasma tacrolimus but not whole-blood tacrolimus with c-reactive protein in transplant recipients
Zijp T1, Knobbe T2, Gan C3, Blokzijl H4, Bakker S2, Touw D1,5, TransplantLines investigators6
1 University of Groningen, Department of Clinical Pharmacy and Pharmacology, University Medical Center Groningen, Groningen, The Netherlands
2 University of Groningen, Department of Internal Medicine, Division of Nephrology, University Medical Center Groningen, Groningen, The Netherlands
3 University of Groningen, University Medical Center Groningen, Department of Pulmonary Diseases and Tuberculosis, Groningen, The Netherlands
4 University of Groningen, University Medical Center Groningen, Department of Gastroenterology and Hepatology, Groningen, The Netherlands
5 University of Groningen, Department of Pharmaceutical Analysis, Groningen Research Institute of Pharmacy, University of Groningen, Groningen, The Netherlands
6 University Medical Center Groningen Transplant Center, University Medical Center Groningen, Groningen, The Netherlands
Introduction: Tacrolimus is the cornerstone in prevention of graft dysfunction in transplant (Tx) recipients and requires therapeutic drug monitoring (TDM). Inflammation, as indicated by increased c-reactive protein concentrations (CRP), may cause down-regulation of liver enzymes that metabolize tacrolimus, therefore increasing trough tacrolimus concentrations (C0). The “free” unbound fraction is in rule pharmacologically active and related to effectivity and safety, and a previous study showed plasma C0 to be linearly related to unbound tacrolimus. Therefore, a method was developed to analyse plasma C0 in addition to routine whole blood C0.
Objectives: We aimed to compare the associations of plasma and whole blood C0 with CRP in stable lung, kidney and liver Tx recipients.
Methods: Plasma and whole blood C0, and CRP were measured in samples of the ongoing TransplantLines Biobank and Cohort study. Samples were collected from stable liver, kidney and lung Tx recipients at ≥ 12 months after transplantation. Linear relationships were assessed with Pearson’s correlation on log2-transformed data. Non-parametric differences for plasma and whole blood C0 were determined per CRP group (<1.0 mg/L, 1.0-3.0 mg/L and >3.0mg/L) and per Tx group with the Kruskal Wallis test.
Results: Complete data were available of 1016 Tx recipients at median 2.2 years after transplantation. Median CRP was 1.9 (interquartile range 0.8-4.9) mg/L. There was a linear relationship between plasma C0 and CRP (Pearson’s R = 0.11, P < 0.001), but not between whole blood C0 and CRP (R=-0.02, P=0.43). Median plasma C0 was 0.12 (0.08-0.17) μg/L for Tx recipients with CRP <1.0mg/L, and 0.11 (0.09-0.17) μg/L for CRP 1.0-3.0mg/L, and 0.14 (0.09-0.20) μg/L for CRP>3.0mg/L (P<0.01). Plasma C0 of liver Tx recipients did not differ between CRP groups, while there was a significant difference for kidney and lung Tx recipients (P<0.01). For whole blood C0, there were no significant differences between CRP groups.
Conclusion: Increased plasma C0 is associated with CRP in kidney and lung Tx recipients, who have higher therapeutic ranges than liver Tx recipients. This relation was not identified for whole-blood C0, routinely used for TDM.
Tabel/Image
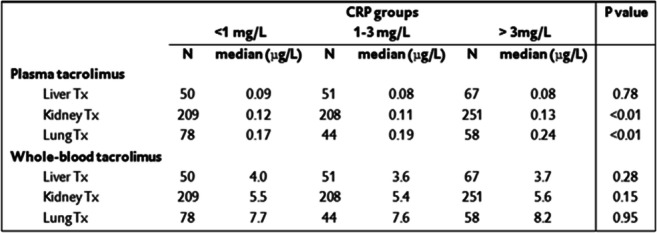
193
Cozapine, Norclozapine plasma concentrations and the Clozapine/Norclozapine ratio in resistant schizophrenia
Daly W1,3, Gaies E1,2,3, El Jebari H1, Ben Sassi M1,2,3, Rim C1,2,3, Trabelsi 1,2,3, Daghfous R1,2,3
1 Service de Pharmacologie Clinique-National Centre Chalbi Belkahia of Pharmacovigilance (CNPV), Tunis, Tunisia
2 Laboratoire de Recherche de Pharmacologie Clinique et expérimentale LR16SP02, Tunis, Tunisia
3 Faculté dde Médecine de Tunis-Université de Tunis El Manar., Tunis, Tunisia
Background: Clozapine (CLZ) is an effective antipsychotic drug for treating refractory schizophrenia. Therapeutic drug monitoring (TDM) is recommended in clozapine therapy. CLZ undergoes oxidative metabolism via cytochrome-P450 into two principal metabolites, principally norclozapine which is known to be pharmacologically active and has different pharmacokinetics proprieties. Therefore, TDM of CLZ and NCLZ is justified. A cut off of 2 for CLZ/NCLZ ratio seems to be associated with a better efficiency and tolerance and a ratio >2 suggests a defectuous sample, metabolism saturation or inhibition.
Objective: The aim of this study was to determine the plasma concentration of CLZ, NCLZ and to calculate CLZ/NCLZ ratio in schizophrenic patients.
Method: It is a retrospective study, established in the Clinical Pharmacology Department of CNPV-Tunisia over a 3-month period from February 18, to May 13, 2021.
TDM of CLZ and NCLZ was made using a validated chromatographic method.
Results: This study has included 40 patients, corresponding to 52 samples. Median age was 33 years with a sex-ratio M/F of 12.3. Median daily dose of CLZ was 450 mg/day and median weight-based dosing was 5.26 mg/kg/day. Median trough plasma concentration was 235 ng/mL (23-1211 ng/ml) for CLZ, 94 ng/ml (33-1004 ng/ml) for NCLZ and 2.18 (0.3-12.38) for CLZ/NCLZ ratio. CLZ/NCLZ ratio was < 2 in 20 samples with a median of 1.22 and > 2 in 32 samples with a median of 3.76. The ratio was < 0.5 in 3 patients who had low serum levels of CLZ and NCLZ. This study shows an increase of CLZ concentration and CLZ/NCLZ ratio according to CLZ dose. NCLZ concentration is relatively constant above 300 mg/day. This probably explain the saturation of CLZ metabolism.
Conclusion: TDM of CLZ, NCLZ and CLZ/NCLZ ratio allow us to have a better idea about therapeutic observance, metabolism saturation and possible drug interactions.
195
Therapeutic drug monitoring of clozapine in Tunisian psychiatric patients: What about the dose-related reference range?
Sahnoun D1, Ben-Hammamia S1,2, Ben Sassi M1,2, El Jebari H1,2, Charfi R1,2, Daghfous R1,2, Gaies E1,2, Trabelsi S1,2
1 Centre National Chalbi Belkahia De Pharmacovigilance, Tunis, Tunisia
2 Laboratoire de recherche en pharmacologie Clinique et expérimentale (LR16SP02), Tunis, Tunisia
Therapeutic drug monitoring of clozapine in Tunisian psychiatric patients: What about the dose-related reference range?
Introduction: Clozapine (Clz) is an atypical antipsychotic which therapeutic drug monitoring (TDM) is clinically important because of its narrow therapeutic range and its toxicity, especially hematological [1]. Interpretation of Clz plasma concentration is usually based on therapeutic reference range (TR). The dose-related reference range (DRR) is a new pharmacokinetic approach based on drug dosage and rhythm of administration [2]. It allows a comparison of a measured drug concentration with a theoretically expected concentration range and thus an individual dose adjustement according to the dosage received.
Objectives: To compare Clz plasma concentrations obtained by TDM to the DRR on the Tunisian psychiatric patients treated by Clz.
Methods: This is a retrospective study (2014 to 2021) conducted in the Clinical Pharmacology Department of the Centre National Chalbi belkahia de Pharmacovigilance, including Tunisian patients, treated by Clz, without pharmacokinetically relevant comorbidity or comedication. Clz monitoring was done using High Performance Liquid Chromatography (HPLC). The TR is: 350 to 600 ng/mL. DRR was determined, based on Drug Related Concentration (DRC) factor, using this formula [3]:
Lower limit = daily dosage (mg) x DRC factor low.
Upper limit = daily dosage (mg) x DRC factor high.
For Clz taken once daily, DRC factor low was 0.21 and DRC factor high was 0.79.
For Clz taken twice daily, DRC factor low was 0.43 and DRC factor high was 1.59.
When a patient’s drug concentration measured by TDM was found within the DRR, the concentration was defined as normal. Concentrations out of range may indicate a partial non-adherence, drug interactions, genetic polymorphisms or metabolic failure.
Results: Our study recorded 324 blood samples from 239 patients aged between 18 and 65 years who met the selected inclusion criteria. The average weight was 71.5 kg (60-80 kg). About a quarter of samples (27,8%) were within the TR. Applying DRR, the percentage rose to 85,2 %. The rate of infra and supratherapeutic concentrations were 53,1% and 19,1%, respectively. By applying the DRR approach, these percentages have dropped to 3,1% and 11,7%. All patients whose dosage does not exceed 200 mg daily, were infratherapeutic. Applying DRR, the infratherapeutic rate decreased to 33%. For dosage between 200 and 450 mg daily, we found 68.4% infratherapeutic. After applying DRR, 92% of these returned to normal. As for dosage above 450 mg daily, we only found 23% in the TR and 77% supratherapeutic. After applying DRR, normals amounted to 65% and supratherapeutic lowered to 35%.
Conclusion: It seems that DRR contribute to better correlated clozapine concentrations with dose changes compared to the traditional TR. Further studies are needed to assess clinical impact of the DRR application, in terms of efficacy and tolerability.
References
1. Miller DD. Review and Management of Clozapine Side Effects. J Clin Psychiatry. 2000;61:14-7.
2. Hiemke C, Bergemann N, Clement H, Conca A, Deckert J, Domschke K, et al. Consensus Guidelines for Therapeutic Drug Monitoring in Neuropsychopharmacology: Update 2017. Pharmacopsychiatry. 2018;51:9-62
205
Risk factors for delayed methotrexate elimination in patients with osteosarcoma
Ferchichi K1,2, Ben Sassi M1,2, Charfi R1,2, Affes R1, Gaies E1,2, El Jebari H1, Trabelsi S1,2, Daghfous R1,2
1 Centre Chalbi Belkahia of Pharmacovigilance, Department of clinical pharmacology, Tunis, Tunisia
2 Faculty of medecine of Tunis, University of Tunis El Manar, Tunis, Tunisia
Introduction: High-dose methotrexate (HD-MTX) is the main chemotherapy for patients with osteosarcoma. Yet, there is a significant individual variance in MTX elimination. Identifying factors related to DEM will enable the early onset of preventative measures to reduce serious adverse effects including hepatotoxicity, nephrotoxicity, and hepatotoxicity.
Objective: The aim of this study was to explore risk factors for delayed elimination of MTX in patients with osteosarcoma.
Methods: We conducted a retrospective study, over 6 years from January 2015 to December 2021, including all MTX plasma concentration (MPC) measurements for patients treated for osteosarcoma. Measures are collected on a database of the Clinical Pharmacology Department of the National Center of Pharmacovigilance of Tunisia. The standard of care was to measure MPC at 24, 48, and 72 hours after the starting of MTX administration which is considered as one cycle. We included only 24 hours measure of each cycle. Patients using concomitant drugs that could potentially affect MTX elimination are excluded.
Delayed methotrexate elimination (DME) was defined as MPC > 10 μmol/L at 24 hours.
Cycles were then divided into two groups:
Group 1: cycles with DME
Group2: cycles without delayed DME
The measurements were performed using the immuno-enzymatic technique with the Architect® automated system from Abbott Laboratories using the FPIA method.
Results: We extracted data from 136 patients for whom a total of 2383 measurements of MPC were performed. We included only 24 hours measurement from each cycle which corresponds to n= 108 patients who received a total of 678 cycles of HD-MTX therapy for osteosarcoma. The sex ratio (M/F) was 1,63 with a median age of 11,75 years varying between 4,85 and 31,96-year old. The median MTX dose administrated was 11,74 g/m2 with a body surface varying between 0,64 and 2,2 m2. For group 1 was included 45 patients with 153 cycles. The sex ratio was 3,44 with a median age of 11,75 years varying between 9 and 31,94-year old. The median normalized dose was 11,74 g/m2. The median of MPC was 57,3 μmol/l. Toxicity signs were detected during 10 cycles (22,22%). For group 2 was included 101 patients with 525 cycles. The sex ratio was 1,58 with a median age of 13,74 years varying between 4,85 and 16,49-year old. the median normalized dose was 9,94 g/m2. The median of MPC was 1,62 μmol/l. Toxicity signs were detected during 31 cycles (30,69%). No significant difference in toxicity signs was identified between the two groups p=0,77. The age was identified as an independent risk factor with p<0.0001. Table I shows the comparison of patients' characteristics of the two groups.
Conclusion: In conclusion, our results indicate that age can be a risk factor for DME in patients treated with HD MTX for osteosarcoma, lining with previous studies. Identifying the factors associated with delayed elimination of MTX could lead to safer and optimized chemotherapy for patients with osteosarcoma.
Tabel/Image
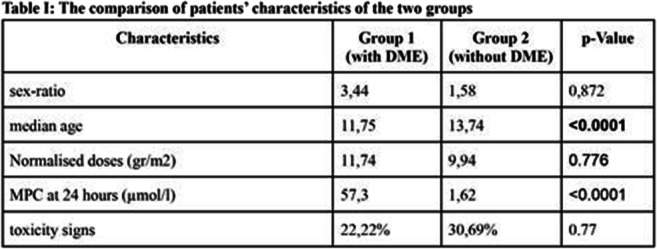
206
Does concomitant administration of proton pump inhibitors with methotrexate cure delay its elimination?
Ferchichi K1,2, Ben Sassi M1,2, Charfi R1,2, Affes R1, Gaies E1,2, El Jebari H1, Trabelsi S1,2, Daghfous R1,2
1 Centre Chalbi Belkahia of Pharmacovigilance, Department of clinical pharmacology, Tunis, Tunisia
2 Faculty of medecine of Tunis, University of Tunis El Manar, Tunis, Tunisie
Introduction: To detect delayed methotrexate (MTX) elimination, which could result in serious and potentially life‐threatening toxicity, continuous monitoring of methotrexate plasma concentration (MPC) is essential. MTX elimination delays can be induced by MTX's direct nephrotoxicity, insufficient hydration or urine alkalinization, or drug interaction. This study aimed to investigate the impact of proton-pump inhibitors (PPIs) on delayed MTX elimination in patients treated with high‐dose MTX.
Methodology: This is a non-interventional retrospective study including all MPC measures for patients treated with high‐dose MTX (≥1 g/m2 ) over 6 years from January 2015 to December 2021. Measures are collected on a database of the Clinical Pharmacology Department of the National Center of Pharmacovigilance of Tunisia. The standard of care was to measure MPC at 24, 48, and 72 hours after the starting of MTX administration which is considered as one cycle. We included all patients with concomitant use of IPPs and MTX for at least one cycle to avoid potential interindividual variability. Patients using another concomitant drug that could potentially affect MTX elimination are excluded.
Delayed methotrexate elimination (DME) was defined as MPC >0.1 μmol/L at 72 hours.
Cycles were then divided into two groups:
Group 1: cycles with DME
Group2: cycles without delayed DME
The measurements were performed using the immuno-enzymatic technique with the Architect® automated system from Abbott Laboratories using the FPIA method.
Results: From a total of 10710 MPC measures, 380 measures were selected corresponding to 153 cycles of MTX administration to 20 patients with concomitant use of IPPs and MTX for at least one cycle. The median age was 31,44 years old. The sex ratio (M/F) was 1,5. Patients were treated for either osteosarcoma in 55% of cases and hematological malignancy in 45%. The median MTX dose administrated was 4,97 g/m2. A total of n=102 cycles with MPC performed at 72 hours were identified: ie, MTX was correctly eliminated by 48 hours. DME was observed in 27,45% of cycles. The use of PPIs was identified in 15,69% of all cycles and 14,29% of cycles with DME. There was no significant difference between G1 and G2 in the elimination of MTX (p=0,81).
Conclusion: The concomitant use of PPIs did not increase the risk of methotrexate delayed elimination. This retrospective study suggests that there is no association between concomitant use of PPIs and delayed methotrexate elimination.
209
Impact of gender and age on levetiracetam pharmacokinetic profile
Guettari M2, Ben Sassi M1,2, Sahnoun D1,2, Gaies E1,2, El Jabari H2, Charfi R1,2, Trabelsi S1,2, Daghfous R1,2
1 Faculty Of Medecine Of Tunis, University Of El Manar, Tunis, Tunisia
2 Tunisian National Pharmacovigilance Center, Tunis, Tunisia
Introduction: Second generation antiepileptic drugs (AEDs), such as levetiracetam (LEV), are characterized by a more predictable and consistent pharmacokinetic (PK) parameters compared to first generation AEDs. Therapeutic Drug Monitoring (TDM) is, as a consequence, not fully required, especially for the proved linear relationship between the dose and the serum levels of LEV. However, LEV blood concentrations can be affected by several parameters such as age, gender, polymedication, physiological and pathophysiological states. Based on this understanding, this study will focus on the effect of gender and age on blood levels of LEV
Objectives: To evaluate the influence of gender and age on LEV PKs in clinical practice for the purpose on improving drug dosing.
Methods: This was a retrospective study of epileptics receiving LEV treatments. Patients were categorized into subgroups according to gender (Men/Women) and age (Infants 0-18years old (y.o.), adults 18-65 y.o. and elderly >65 y.o.). LEV trough levels (LEV C0) were achieved by HPLC with Ultraviolet (UV) detection. The concentration/dose ratio (C0/D) was calculated as LEV C0 divided by the weight-normalized dose, which is used as a measure of apparent oral clearance of the drug. Therapeutic range (TR) was between 10 and 40μg/mL. Mann Withney u test was used to compare different parameters.
Results: Overall, 922 LEV C0 corresponding to 554 patients were identified (281 men and 263 women).Table 1 shows several studied parameters among subgroups. Significant differences in C0/D were noted between adults and elderly (p<0.001) and infants and elderly (p<0.001). Whereas, no significant difference was observed between infants and adults (P=0.128). In another hand, no gender-related difference was observed (p>0.05).
Conclusion: The study findings emphasize the need to monitor relatively LEV, especially among infants and elderly.No conclusive relationship could be established for gender. TDM is as a consequence useful to personalize and optimize LEV therapy.
Tabel/Image
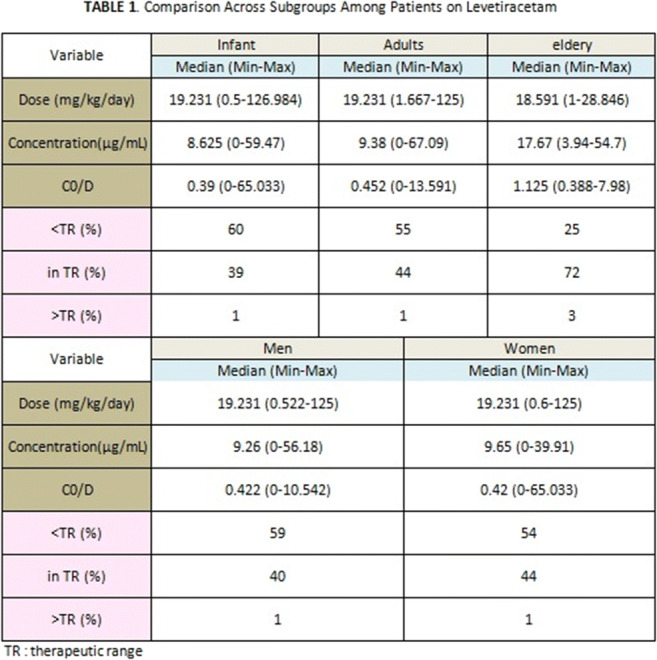
224
Evolution of Therapeutic Drug Monitoring requests in a tertiary University Hospital in the last 16 years
Kazaki E1,2, Tsikrikas P1,2, Paraskeva T1,2, Arvanitidis K1,2,3, Kolios G1,2,3, Manolopoulos V1,2,3
1 Laboratory of Pharmacology, Medical School, Democritus University of Thrace, Alexandroupolis, Greece
2 Individualised Medicine & Pharmacological Research Solutions Center (IMPReS), Alexandroupolis, Greece
3 Clinical Pharmacology Unit, Academic General Hospital of Alexandroupolis, Alexandroupolis, Greece
Introduction: TDM has been widely used in clinical practice for many years and has application in various groups of drugs, mainly in drugs with pharmacokinetic variability, concentration related therapeutic and adverse effects, narrow therapeutic index, defined therapeutic concentration range and desired therapeutic effect difficult to monitor.
Objectives: The aim of this study is to assess the evolution of TDM application throughout the years and its usefulness and necessity nowadays in a tertiary University Hospital.
Methods: Data of Therapeutic Drug Monitoring requests were collected for the years 2005, 2010, 2015, 2019, 2020 and 2021 from the records of the Laboratory of Pharmacology General University Hospital of Alexandroupolis. Drugs monitored included digoxin, valproic acid, carbamazepine, phenytoin, phenobarbital, cyclosporine, tacrolimus, amikacin, gentamycin, vancomycin, serum benzodiazepines, methotrexate, cortisol, acetaminophen, salicylate, theophylline, tobramycin and primidone. More specifically, the data collected were the drug’s level measurement and the clinic that requested the specific drug order.
Results: A total of 1357 drug level measurement records were found for 2005, 1442 for 2010, 766 for 2015, 520 for 2019, 442 for 2020 and 622 for 2021. During these years the most frequent drug requested for TDM was digoxin (2005), cyclosporine (2010, 2019), valproic acid (2015, 2020), and tacrolimus (2021). In regards to the drugs requested, digoxin was predominantly requested by Cardiology and Pathology, cyclosporine and tacrolimus by Nephrology and valproic acid by Psychiatry. Furthermore, the percentage of non optimal therapeutic levels that required dose adjustments were calculated and will be presented.
Conclusion: TDM retains a degree of its value as shown by the number of incidents requiring dose modifications. In addition, the presented results obtained from 2005 to 2021 show that there is a notable decrease in the number of requests for TDM per year. This can be imputed to several reasons such as the replacement of specific drugs with new therapeutic regimens and the evolution of therapeutic drug protocols in several diseases. Another major factor was the measures taken against the Covid-19 pandemic in the last two years which resulted to a substantial decrease of routine health examinations and scheduled appointments in the Hospital.
Funding: Financial support for project IMPReS (MIS 5047189) was provided by the Program “Competitiveness, Entrepreneurship and Innovation” (NSRF 2014-2020) co-financed by Greece and the European Union (European Regional Development Fund).
241
Does concomitant administration of proton pump inhibitors with methotrexate cure delay its elimination?
Ferchichi K1,2, Ben Sassi M1,2, Charfi R1,2, Affes R1, El Jebari H1, Gaies E1,2, Trabelsi S1,2, Daghfous R1,2
1 Centre Chalbi Belkahia of Pharmacovigilance, Department of clinical pharmacology, Tunis, Tunisia
2 Faculty of medecine of Tunis, University of Tunis El Manar, Tunis, Tunisia
Introduction: To detect delayed methotrexate (MTX) elimination, which could result in serious and potentially life‐threatening toxicity, continuous monitoring of methotrexate plasma concentration (MPC) is essential. MTX elimination delays can be induced by MTX's direct nephrotoxicity, insufficient hydration or urine alkalinization, or drug interaction. This study aimed to investigate the impact of proton-pump inhibitors (PPIs) on delayed MTX elimination in patients treated with high‐dose MTX.
Methodology: This is a non-interventional retrospective study including all MPC measures for patients treated with high‐dose MTX (≥1 g/m2 ) over 6 years from January 2015 to December 2021. Measures are collected on a database of the Clinical Pharmacology Department of the National Center of Pharmacovigilance of Tunisia. The standard of care was to measure MPC at 24, 48, and 72 hours after the starting of MTX administration which is considered as one cycle. We included all patients with concomitant use of IPPs and MTX for at least one cycle to avoid potential interindividual variability. Patients using another concomitant drug that could potentially affect MTX elimination are excluded.
Delayed methotrexate elimination (DME) was defined as MPC >0.1 μmol/L at 72 hours.
Cycles were then divided into two groups:
Group 1: cycles with DME
Group2: cycles without delayed DME
The measurements were performed using the immuno-enzymatic technique with the Architect® automated system from Abbott Laboratories using the FPIA method.
Results: From a total of 10710 MPC measures, 380 measures were selected corresponding to 153 cycles of MTX administration to 20 patients with concomitant use of IPPs and MTX for at least one cycle. The median age was 31,44 years old. The sex ratio (M/F) was 1,5. Patients were treated for either osteosarcoma in 55% of cases and hematological malignancy in 45%. The median MTX dose administrated was 4,97 g/m2.
A total of n=102 cycles with MPC performed at 72 hours were identified: ie, MTX was correctly eliminated by 48 hours. DME was observed in 27,45% of cycles. The use of PPIs was identified in 15,69% of all cycles and 14,29% of cycles with DME. There was no significant difference between G1 and G2 in the elimination of MTX (p=0,81).
Conclusion: The concomitant use of PPIs did not increase the risk of methotrexate delayed elimination. This retrospective study suggests that there is no association between concomitant use of PPIs and delayed methotrexate elimination.
261
QUININE TOXICITY WHEN ASSOCIATED WITH DOXYCYCINE AND THE ROLE OF THERAPEUTIC DRUG MONITORING: A CASE REPORT
Daldoul M1, Gaies E1, El Jebari H1, Ben Sassi M1, Charfi R1, Ammari L2, Daghfous R1, Trabelsi S1
1 Service de Pharmacologie Clinique-National Centre Chalbi Belkahia of Pharmacovigilance (CNPV)-Tunis-Tunisia, Tunis, Tunisie
2 Service d’infectiologie- Hôpital La Rabta-Tunis-Tunisia, Tunis, Tunisie
Introduction: Quinine is an antimalarian agent mainly used for the treatment of uncomplicated, drug-resistant P. falciparum and for severe falciparum malaria. It is characterized by a narrow therapeutic range and a risk of toxicity, as well as numerous and important drug interactions, justifying, consequently, the indication of a therapeutic drug monitoring.
Objectives: Herein we report a case of a patient who was treated with quinine and doxycycline for a relapse of an uncomplicated falciparum malaria attack and who develops quinine toxicity illustrated by a high plasma concentration of quinine.
Methods: Therapeutic drug monitoring was performed using a chromatograpic method
Results: A 39 year old male patient was admitted to the infectious diseases departement for a falciparum malaria. During the acute episode of infection he was treated by Quinine at 750 mg orally three times a day and doxycycline 100 mg per day. Forty –eight hours after, he developed dizziness and tinnitus. Therapeutic drug monitoring was performed and quinine concentration was 33.82 μg/ml (therapeutic range: 10-12 μg/mL). One day later the patient developed auditory and visual hallucinations, nightmares and increased QT interval. Quinine treatement was stopped with maintenance of the doxycycline dose. Twenty four hours later, the evolution was marqued by an improvement of dizziness and tinnitus and a decrease in the quinine concentration wihch was 19.82 μg/ml.
Conclusion: The concentrations of quinine obtained from this patient were higher suggesting a possible interaction between quinine and doxycycline wihch may be explained by inibition of the metabolism of quinine. So, a daily therapeutic drug monitoring of quinine during the first 3 days is therefore necessary to adjust the dosage and to avoid toxicity as it is recommended by the 2007 Consensus Conference of the French Language Infectious Diseases Society.
267
Direct oral anticoagulants (DOAC), therapeutic drug monitoring and creatinine clearance
Bula M1, Simms C1, Ablett C1, Ahmed M1, Tempany J1, Tsakiroglou M2, Pirmohamed M2
1 Liverpool University Hospitals NHS Foundation Trust, Liverpool, United Kingdom
2 University of Liverpool, Liverpool, United Kingdom
Introduction: Direct oral anticoagulants (DOAC) demonstrate pharmacokinetic and pharmacodynamic profiles which are more predictable compared to vitamin K antagonists (VKA). Routine monitoring is not recommended, but dose adjustments in accordance with peak levels are performed in high risk patients in clinical settings.
Objectives: We investigated the relationship between drug levels and Creatinine Clearance (CrCl) using the Cockcroft-Gault formula to identify high risk patients who might benefit from DOAC monitoring to minimise the risk of adverse events.
Method: In a retrospective study, the records of patients on a DOAC who were admitted at the Royal Liverpool University Hospital, UK, between June 2018 and January 2021 were reviewed. Patients who had levels performed at least once using anti-factor Xa assays (Hemosilliquid anti-Xa) 4-hours post dose (peak levels) were included in the analysis.
Results: Of the 400 records reviewed, 43 (11%) patients had peak DOAC levels measured and 8 out of 43 (19%) patients had multiple measurements. The reasons for repeating peak levels were previous high levels with a cut-off at 200 ng/mL (n=6, 14%) and recurrence of thrombosis (n=2, 5%). Of note, one patient with recurrent brain infracts on rivaroxaban had peak levels above 200 ng/mL and he was switched to apixaban. Patient characteristics are shown in the table 1.
Self-limiting minor bleeding including haematuria, epistaxis, spontaneous bruising and wound bleeding were recorded in 11 (25%) patients. Apixaban was the culprit in 64% of the bleeding adverse reactions. DOAC peak levels were above 198 ng/ml in 8 (72%) out of 11 patients with reported bleeds. In this group, we found 3 patients were also receiving concomitant therapy with clopidogrel ( n=2, 18%) and aspirin (n=1, 9%).
Four patients (9%) developed a thrombotic event. A patient with recurrent PE on apixaban was converted to LMWH, a patient with cephalic vein thrombus was continued edoxaban following a removal of midline catheter, a NSTEMI patient completed 6-month course of apixaban, while the last patient was converted from rivaroxaban to apixaban. All four patients’ DOAC levels were above 150 ng/mL and CrCl was between 25 ml/min and 116 ml/min.
In all 43 patients, there was a negative correlation between CrCL and DOAC levels (p=0.0255). We found a linear correlation between CrCl and estimated glomerular fitration rate (eGFR).
Conclusion: Our findings suggest that patients with a normal CrCl may still have high peak DOAC levels increasing the risk of bleeding. Moreover, in patients with increased cardiovascular risk, who have a lower CrCl, DOAC monitoring may be useful for improving the benefit-risk ration of DOACs. Further data is required to explore the indications for DOAC drug level monitoring in clinical practice.
Tabel/Image
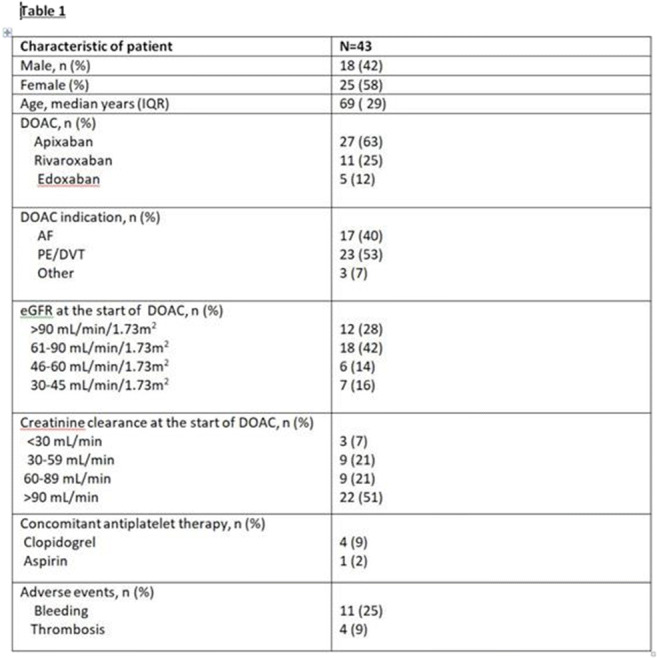
311
Therapeutic Drug Monitoring of Cyclosporine in Heart Transplant Patients
Sahnoun D1, Charfi R1, Guettari M1, Ziadi J2, Ouali S3, Taamallah K4, Ben Sassi M1, Gaies E1, Ben-Hammamia S1, El Jebari H1, Salouage I1, Daghfous R1, Trabelsi S1
1 University of Tunis El Manar, Faculty of Medicine of Tunis. National Centre Chalbi Belkahia of Pharmacovigilance, Department of clinical pharmacology, Research Laboratory of Clinical and Experimental Pharmacology (LR16SP02), 1006 Tunis, Tunisia, Tunis, Tunisia
2 University of Tunis El Manar, Faculty of Medicine of Tunis. Rabta Hospital, Department of Cardiac Surgery, Tunis, Tunisia
3 University of Tunis El Manar, Faculty of Medicine of Tunis. Rabta Hospital, Department of Cardiology, Tunis, Tunisia
4 Military Hospital of Tunis, Department of Cardiology, Tunis, Tunisia
Introduction: The low therapeutic index of cyclosporine (CsA) and its broad intraindividual and interindividual pharmacokinetic variability requires close therapeutic drug monitoring (TDM). Therefore, CsA dose individualisation by TDM is mandatory in order to optimize efficacy and safety of CsA therapy.
Objectives: We aimed to assess the TDM of CsA in heart transplant patients and their clinical evolution.
Methods: Our study is conducted a retrospective study from 2009 to 2021 in the department of Clinical Pharmacology. We included patients, undergoing their first orthotopic heart transplantation and who were adressed for TDM of CsA. CsA blood levels were measured by CMIA technique. Therapeutic levels were considered for pre-dose levels C0 (250–350 ng/mL during the first 6 months and reduced to 120–200 ng/mL from the beginning of the first year). And for 2-hour post-dose (C2) levels (800–1000 ng/mL during the first period and lowered to 600–800 ng/mL after six months).
Results: In this study we included 116 blood samples from 12 patients, we described two groups; the first for whom we measured C0 levels for 7 patients and the second including 5 other patients for whom C2 levels were measured.
In the first group they were all male aged between 8 and 64 years. In the second, they were 4 male and 1 female aged between 43 and 54 years. The average weight was 86.6 kg (22-110 kg). The mean of years post-transplantation was 13.2 for the first group and 6 for the second.
There is some variation in immunosuppressive combinations utilized for our study population: The drugs associated with CsA in the first group were mycophenolate mofetil (MMF) and prednisone (Pred) in 7 patients, 2 patients were taking azathioprine and Pred with CsA, for the second group all patients were taking MMF and Pred in association with CsA.
About a 38.8 % of samples including C0 and C2 monitoring were within the therapeutic range (TR). The rate of infra and supratherapeutic concentrations were 19.8 % and 41.4 %, respectively. The proportion of samples undergoing CsA dose decrease was 15.5 %, there is no dose optimization for 49.1 % of the total blood samples and we increased the CsA dose in 35.4 % of them. Table
In our study there is three patients who had an arterial hypertension and one person suffered from vomiting.
The evolution was favorable for three patients but it was fatal for five of them: One person presented with hyperacute rejection after four months after transplantation due to discontinuation of prednisone and an other patient presented with chronic rejection.
There was an adult who suffered from strock and two others were died by infection. The evolution was unfortunately unknown for four other patients.
Conclusion: C2 and C0 monitoring of CsA allowed individualization of immunosuppression with optimization of CsA efficacy and tolerability. We emphasize according to our study the interest of TDM for cardiac transplantation patients undergoing CsA therapy.
AUTHOR INDEX
A
| Altenbuchner, A | 199 | ||
| Abad, F | 252 | Alvarez, A | 73 |
| Abad Santos, F | 215 | Alvarez-Alvarez, I | 197 |
| Abadías, M | 230 | Aly, R | 44 |
| Abad-Santos, F | 168, 225 | Aman, J | 210 |
| Abad-Santos, F | 67 | Amaro Hosey, K | 185 |
| Abbink, T | 216 | Ammari, L | 261 |
| Abde-kader Martín, L | 138 | Anatolou, D | 237 |
| Abdel-kader Martín, L | 137 | Andersen, L | 293 |
| Ablett, C | 267 | Andersen, S | 8 |
| Abo-Loha, C | 97 | Anderssen-Nordahl, E | 47 |
| Abouir, K | 233 | Andrade, R | 197 |
| Achard, M | 174 | André, P | 97 |
| Achour, B | 54 | Andrikyan, W | 198, 199 |
| Affes, R | 75, 99 | Andrushchyshina, T | 4 |
| Affes, R | 205, 206, 241 | Andrzejewska-Górecka, D | 105 |
| Agustí, A | 119, 230 | Anta, L | 217 |
| Ahmed, M | 267 | Antonijoan Arbos, R | 185 |
| Ahmetova, Z | 299 | António, N | 100, 102 |
| Aidinis, V | 259 | Antoniou, K | 249, 244 |
| Ainabekova, B | 299 | Aounallah, A | 181 |
| Ainatzoglou, A | 74, 88 | Aparicio, L | 130 |
| Aissi, M | 86 | Aragonés, C | 252 |
| Akpan, A | 235 | Arapovic Dzakula, S | 20 |
| Akpunonu, P | 188 | Arata-Bardet, J | 85 |
| Al- Humadi, A | 263 | Arellano, L | 130, 202 |
| Albendin, H | 23 | Arévalo-Lorido, J | 53, 55 |
| Alcubilla, P | 130, 202 | Arici, M | 166 |
| Alday, E | 252 | Arvanitidis, K | 224, 232, 229 |
| Aldiyarova, N | 243, 253 | Antoniou, K | 249, 244 |
| Aldy, K | 188 | Ascencao, R | 177 |
| Aleksic, D | 143 | Ashirov, M | 160 |
| Alerany, C | 119, 230 | Asprogerakas, M | 244, 249 |
| Alexopoulos, N | 170 | Assan, A | 253 |
| Algren, D | 188 | Astasio, O | 29 |
| Alin, L | 85 | Atabey, N | 164 |
| Aljofan, M | 306, 310 | Atalay, S | 167 |
| Al-Majdoub, Z | 54 | Atig, A | 181 |
| Almurzayeva, A | 160 | Atzemian, N | 257, 208 |
| Alonso, B | 117 | Aukstikalne, L | 197 |
| Alonso Gomez, B | 191 | Aurinsalo, L | 219 |
| Alonso Gómez, B | 271 | Auriola, S | 78 |
| Alonso-Gomez, B | 155 | Avendaño-Solá, C | 59, 109, 108, 67, 168 |
| Alp Cavus, S | 166 | Avó-Baião, R | 144 |
| Al-Majdoub, Z | 54 | Azevedo, D | 103 |
| Almurzayeva, A | 160 |
Á
| Álvarez del Vayo Benito, C | 139 |
B
| Backman, J | 68, 219, 251, 80 | Blaise, S | 38, 22 |
| Backman, J | 78 | Blanquer-Blanquer, M | 23 |
| Backman, J | 192 | Blokzijl, H | 173 |
| Bae, S | 51 | Blondel, N | 201 |
| Báez Gutiérrez, N | 137 | Bocsan, I | 81, 290 |
| Bahar, J | 235 | Boegler, D | 85 |
| Bahmany, S | 304 | Bogaard, H | 210 |
| Bakker, S | 173 | Bolcato, L | 87 |
| Bakkum, M | 127 | Boldueva, S | 157 |
| Bakuridze, A | 45, 101 | Bondarenko, N | 156 |
| Bakuridze, K | 45, 101 | Bosch, J | 119 |
| Balan, M | 113, 114 | Bosch, T | 226 |
| Balderas Vazquez, C | 315 | Bosch-Llonch, F | 26 |
| Ballarín, E | 230 | Boskou, I | 74, 88 |
| Balzano, N | 283, 288, 289 | Bostanitis, C | 269 |
| Ban, E | 113, 114 | Bottiger, Y | 212 |
| Bán, E | 94 | Bouazzaoui, L | 96 |
| Banos, A | 153 | BOUAZZAOUI, L | 36 |
| Baos Muñoz, E | 317 | Bouloutsou, E | 231 |
| Barber, J | 54 | Boumpas, D | 153 |
| Bardinet, C | 97 | BOURAOUI, H | 291, 292, 294, 301, 307 |
| Bartelink, I | 210, 216 | BOURAOUI, O | 307 |
| Bartolomé Arcilla, J | 317 | Boureka, E | 50 |
| Bartra, J | 314 | Boussageon, R | 5 |
| Bascuas, J | 314 | Bouwhuis, J | 226 |
| Batenova, G | 124 | Boy, R | 285 |
| Bautista, A | 117 | Bozkurt, O | 164 |
| Bautista Blázquez, A | 191 | Bozovic, I | 143 |
| Bautista Blázquez, A | 271 | Brakatselos, C | 249 |
| Bautista-Blazquez, A | 155 | Brakatselos, C | 244 |
| Begas, E | 211 | Brassai, A | 94 |
| Bellas, L | 47 | Bratberg, A | 104 |
| BEN AZOUZ, C | 291, 292, 294, 301, 307 | Breining, P | 13, 14 |
| Ben Fadhel, N | 295 | Brent, J | 188 |
| Ben Hammamia, S | 313 | Breza jr., J | 141 |
| BEN OTHMAN, N | 213, 218, 248 | Brinkman, D | 147 |
| Ben Salem, C | 183, 181, 176, 178, 180, 245 | Brossier, C | 238 |
| BEN SALEM, C | 291, 292, 301 | Browne, E | 276 |
| Ben Sassi, M | 75, 89, 99, 142, 186, 193, 195, 205, 206, 209, 241, 258, 261, 311, 313 | Brozmanova, H | 35, 115 |
| Ben-Hammamia, S | 195, 311 | Brunak, S | 8 |
| Benitez Fuentes, J | 317 | Buchta, T | 94 |
| BENOIT, M | 213, 218 | ||
| BERARD, A | 72 | Buclin, T | 97 |
| Berends, M | 167 | Bukonjic, A | 143 |
| Berends, S | 167 | Bukumiric, Z | 308 |
| Berg van den, E | 216 | Bukumirić, D | 43 |
| Berkovitch, M | 93 | Bula, M | 267 |
| Berlin, M | 93 | Bulekbayeva, S | 243 |
| Bernal Morales, B | 315 | Burden, A | 105 |
| BERRIRI, S | 248 | Burhenne, J | 37 |
| BERTOLETTI, L | 302 | Burns, M | 188 |
| Bessone, F | 197 | Burunat, L | 130 |
| Bet, P | 216 | Buzoianu, A | 81, 290 |
| Bet, P | 210 | Büsker, S | 242 |
| Bethlehem, C | 21 | Byrne, P | 276 |
| Bezin, J | 22 | Böhringer, S | 196 |
| Białoń, M | 15 | Böttiger, Y | 147 |
| Bifano, M | 275 | Baalbaki, N | 210 |
| Bijl, G | 27 | Baars, A | 196 |
| Bilal, M | 242 | ||
| Bingöl, M | 34 | ||
| BINQUET, C | 72 | ||
| Bisseling, E | 129 |
C
| Caballero-Bermejo, A | 109, 108, 59, 67, 168 | Chenoweth, J | 188 |
| Cabrera, N | 5 | Cho, H | 128 |
| Cacowski, J | 28 | Cho, J | 30, 31, 63 |
| Cadenas Manceñido, Á | 271 | ||
| Cai, W | 68 | Cho, S | 51, 71 |
| Cajanus, K | 236 | Choi, J | 32 |
| Calmy, A | 233 | Chretien, B | 96 |
| Calvo, G | 314 | Christiaens, T | 127, 147 |
| Camacho, C | 314 | Chtioui, H | 97 |
| Campisi, V | 189 | Chung, W | 31, 71 |
| Campleman, S | 188 | Cifrián Martínez, J | 6, 7 |
| Campo Hoyos, C | 110 | Cizmaș, I | 290 |
| Campodónico, D | 215, 225, 252 | Cohen, E | 187 |
| Canning, J | 188 | Cohen, N | 188 |
| Capuano, A | 283, 288, 289, 146, 148, 150 | Cole, C | 107 |
| Caravati, M | 188 | Cole, S | 97 |
| Carballo, D | 174 | Coorevits, P | 27 |
| Carey, J | 188 | Cordeiro, G | 273 |
| CARLE, Q | 72 | Cornu, C | 5 |
| Carrillo-Alcaraz, A | 23 | CORNU, C | 72 |
| Carvalhana, S | 144 | Cortés, J | 119 |
| Carvalho, C | 100 | Cortés, S | 230 |
| Carvalho, I | 102 | Cortez-Pinto, H | 144 |
| Casajús, A | 225 | Cos Cossio, M | 271 |
| Casajus Rey, A | 252 | Costa, J | 127, 147, 177 |
| Casino, G | 26 | Cottin, J | 5 |
| Caspar, Y | 85 | Craciun, A | 144 |
| Castells, A | 314 | Cracowski, J | 38, 22 |
| Castro-Rebollo, P | 23 | Craig, A | 122 |
| Cats, A | 196 | Creemers, G | 196 |
| Cereza, G | 169 | Csajka, C | 123 |
| Cesaro, M | 146 | Cucherat, M | 5 |
| CHAILLOT, F | 36 | Cuellar, D | 117 |
| Chalikias, G | 208 | Cuéllar Gómez, D | 155, 191, 271 |
| Chandgude, S | 135 | Cui, Y | 132, 133 |
| Charfi, R | 90, 186, 86, 92, 75, 89, 99, 142, 195, 205, 206, 209, 241, 258, 261, 311, 313 | Cunha, L | 102 |
| CHARRIE, C | 213 | Curtin, F | 39 |
| Chatellier, A | 96 | Czarnetzki, C | 39 |
| Czock, D | 37 |
d
| da Silva, R | 309 | de Moraes, N | 309 |
| de Bonis-Redondo, E | 73 | de Ponti, F | 127 |
| de Cos Cossío, M | 6, 7 | de Vries, R | 70 |
| de Gaitani, C | 309 | de Vries, A | 167 |
| de Jong, E | 167 | de Winter, B | 304 |
| de La Rosa, G | 116 | di Mauro, G | 148 |
| de Luna Aguilar, A | 317 | dos Santos, J | 309 |
| de Man, F | 196 |
D
| Daelmans, H | 56, 57 | Desmeules, J | 11 |
| Daghfous, R | 90, 186, 86, 92, 75, 89, 99, 142, 209, 261 | Desmeules, J | 233 |
| Daghfous, R | 258, 193, 195, 311 | DESTERE, A | 213, 218, 248 |
| Daghfous, R | 313, 205, 206, 241 | Dezentjé, V | 196 |
| Dagklis, T | 50 | Dho-Nagy, E | 113, 114 |
| Daldoul, M | 92, 258, 89, 261 | Di Mauro, C | 146 |
| Dalla, C | 249 | Di Napoli, R | 283 |
| Dalm, V | 70 | Dias, P | 100 |
| Daly, W | 186, 193 | Díaz Del Gobbo, A | 285 |
| Danés, I | 119, 230 | Díaz-Martínez, L | 73 |
| Dardalas, I | 74, 88 | Dickman, A | 246 |
| Darnaude Ximénez, I | 59, 67 | Diezi, L | 97 |
| Darnaude-Ximénez, I | 109, 108, 168 | Dijkman, M | 69 |
| Das, B | 95, 106 | Dima, L | 127, 266 |
| Datkhayev, U | 172 | Dimakos, J | 132, 133 |
| Davis, A | 188 | Dimas, K | 118, 231, 211 |
| De Dios, V | 202 | Dimopoulos, A | 170 |
| De Groot, J | 83 | Diogène, E | 230 |
| De Haan, T | 93 | Djanic, M | 308 |
| De La Rosa Loppacher, G | 234 | Dobbins, B | 279 |
| De Ponti, F | 147 | Doffey Lazeyras, F | 11 |
| De Santis, A | 84 | Dokos, C | 242 |
| De Santis, A | 82 | Dolladille, C | 96 |
| Debillon, T | 85 | Donker, E | 147 |
| Debord, J | 203 | Doornebal, J | 129 |
| Decosterd, L | 97 | Dormann, H | 199 |
| Dedroogh, M | 65, 66 | Douros, A | 132, 133 |
| Dedroogh, S | 65, 66 | Dovrolis, N | 237 |
| Defer, G | 96 | Dovrolis, N | 214, 257, 250 |
| DeKoninck, P | 136 | DRICI, M | 213, 218, 248 |
| Del Bene Simonetti, C | 189 | Droogendijk, H | 196 |
| Deleanu, D | 81 | Dubravicky, J | 141 |
| Delgado-Iribarren García-Campero, A | 317 | Dueñas-Laita, A | 108 |
| Deligianni, E | 264 | Dueñas-Ruiz, A | 108 |
| Demirkan, F | 166 | Duijvelaar, E | 210 |
| Demirtsoglou, D | 255, 256 | Dunvald, A | 159 |
| Deng, F | 25, 41 | Dürr, P | 198 |
| Denguezli, M | 176, 178 | Dürr, P | 194 |
| Denisenko, N | 4 | Daabees, T | 44 |
| Desfontaine, V | 97 | Daali, Y | 11 |
| Desmeules, J | 39, 122 | Daali, Y | 233, 123 |
E
| El Jabari, H | 209 | ERPELDINGER, S | 72 |
| El Jebar, H | 186 | Ertem, O | 166 |
| El Jebari, H | 75, 89, 99, 142, 193, 195, 205, 206, 241, 258, 261, 311, 313 | Esen, A | 164 |
| Elens, L | 240 | ETTORE, E | 213, 218 |
| Elseviers, M | 27 | Evdoridis, C | 170 |
| Engels, F | 69 | Eyal, S | 93 |
| Epiard, C | 85 |
F
| Farker, K | 198 | Filppula, A | 192 |
| Farré, M | 234 | Finkelstein, Y | 187, 188, 175 |
| Farré Albaladejo, M | 131 | Flores Navarro, P | 317 |
| FARRUGIA, M | 218 | Folliot, A | 112 |
| Fathallah, N | 183, 181, 176, 178, 180, 245, 291, 292, 294, 301, 307 | Follonier, C | 174 |
| Fedrizzi, S | 96 | Fontana, P | 123 |
| Feio, J | 100 | Fontseca Martin, E | 275 |
| Ferchichi, K | 313, 75, 99, 205, 206, 241 | Forns, X | 197 |
| FERNANDEZ, J | 16 | Fraenza, F | 283, 148 |
| Fernández González, M | 140 | Fragkidis, V | 49 |
| Fernández-Rivera, C | 73 | Freedman, S | 187, 188 |
| Ferrajolo, C | 146, 148, 150 | Freitas, C | 144 |
| Figueiras, A | 76, 79, 103 | Frías Iniesta, J | 110 |
| Filia, A | 153 | Froberg, B | 188 |
| Filidou, E | 232, 229 | Fromm, M | 194, 198, 199 |
| Filion, K | 132, 133 | Fuhr, U | 242 |
| Filippi-Arriaga, F | 169, 285 | Förster, K | 37 |
G
| Gaies, E | 75, 89, 99, 142, 186, 193, 195, 205, 206, 209, 241 261, 311, 313 | Goel, L | 134 |
| GAILLARD, C | 36 | Goldstein, L | 93 |
| Gaio, M | 288, 289, 150 | Golik, A | 93 |
| Gaires, E | 258 | Gomez, L | 85 |
| Galarraga, F | 82, 84 | Gómez, A | 225, 252 |
| Gallagher, H | 278, 276 | Gonçalves, L | 102 |
| Gan, C | 173 | Gongadze, N | 45, 101 |
| Gani, G | 172 | Gonzalez, L | 53, 55 |
| Gara, S | 269 | González, E | 314 |
| Garantziotis, P | 153 | González Samperio, C | 155, 271 |
| Garcia, J | 165 | González-Rinne, A | 73 |
| GARCIA, E | 16 | González-Rodríguez, L | 55 |
| GARCIA, S | 16 | Goorhuis, A | 70 |
| García, J | 189 | Gorgas, M | 230 |
| García, M | 117 | Gosselin, P | 11, 233 |
| García Bravo, L | 317 | Goudon, H | 38 |
| García Montalvo, E | 315 | Goulas, A | 254, 264 |
| García Sáiz, M | 6, 7, 191, 271 | GOURIO, C | 36 |
| García-Arenillas, M | 29 | Goutsiou, R | 269 |
| García-Bea, A | 110 | Graf, K | 65, 66 |
| García-Clavel, J | 23 | Granholm, A | 293 |
| Garcia-Cortes, M | 197 | Grapsa, D | 270 |
| Garcia-Saiz, M | 155 | Grau, M | 24 |
| Gaspar, F | 123 | Greene, S | 188 |
| Gatti, M | 147 | Grenet, G | 5 |
| Gauna, F | 28 | Grigoriou, K | 170 |
| Gautier-Veyret, E | 85, 87 | Grijmans, E | 56, 57 |
| Gavrielatos, G | 170 | Grosgurin, O | 233 |
| Gaztelumendi Martín, G | 271 | Grundmann, M | 35, 115 |
| Gazzaz, M | 242 | Guardiola, H | 116 |
| Geib, A | 188 | Guchelaar, H | 196 |
| Gelal, A | 166 | Guerra López, P | 110 |
| Gelderblom, H | 196 | Guerrier, S | 233 |
| Gentile, S | 8 | Gueta, I | 93 |
| GERARD, A | 213, 218, 248 | Guettari, M | 92, 209, 311 |
| Gervasini, G | 53, 55 | Guevara-Hoyer, K | 317 |
| Gessner, A | 194 | Gueyffier, F | 5 |
| GeurtsvanKessel, C | 70 | Guidi, M | 97, 123 |
| Gholami, O | 3 | Guillen, E | 169 |
| Ghongadze, M | 45, 101 | Gulyayev, A | 310 |
| Gil Useros, S | 317 | Gumustekin, M | 164, 166 |
| Gilani, A | 91 | Gunst, J | 14 |
| Gill, D | 120 | Gupta, P | 134 |
| Gill, P | 175 | Gustavsen, I | 104 |
| Gil-Wey, B | 39 | Gutierro, I | 217 |
| GINHOUX, T | 72 | Guy-Alfandary, S | 93 |
| GIORDANA, B | 213, 218 | Gümüs, K | 37 |
| Girardin, F | 174 | ||
| Glamoclija, U | 298 | ||
| Glasa, J | 274, 239 | ||
| Glasova, H | 274, 239 | ||
| Gloor, Y | 11, 39 |
H
| Haefeli, W | 37 | HO, A | 16 |
| Hagovska, M | 141 | Hoek, K | 21 |
| Hamberg, P | 196 | Hoffmann, M | 112 |
| Hanchanale, S | 246 | Hogdman, M | 188 |
| Handgraaf, S | 174 | Holstege, C | 188 |
| Hartjes, M | 111 | Homar-Amengual, C | 108 |
| Hasanpour, K | 3 | Honeywell, R | 216 |
| Hasanvand, A | 17, 18 | Horbach, A | 286, 287 |
| Hassman, H | 217 | Horvat, O | 43, 162, 308 |
| Haufroid, V | 240 | Horváth-Puhó, E | 187 |
| Hautaniemi, S | 25 | Hoste, E | 240 |
| He, G | 228 | Hoxha, M | 10 |
| Hendrickson, R | 188 | Hoyte, C | 188 |
| Henricks, L | 196 | Hricova, M | 35, 115 |
| Herdeiro, M | 79, 103, 76 | Huckriede, A | 70 |
| Hernandez, N | 197 | Huduti, D | 298 |
| Heron, O | 235 | Huhtinen, K | 25 |
| Herrmann, F | 174 | Husic-Selimovic, A | 298 |
| Hesselink, D | 304, 305 | Hussain, M | 91 |
| Hirvensalo, P | 78 | Hwang, S | 32, 71 |
| Hladun, O | 116, 234 | Hynninen, J | 25 |
| Hladun Alvaro, O | 131 | Hämäläinen, K | 78 |
I
| I. Plácido, A | 103 | Ioannou, M | 118, 231 |
| Iatrou, H | 211 | Ioniță, I | 266 |
| Ikhambaeva, A | 243 | Issayev, A | 306 |
| Ilia, T | 74, 88 | Issilbayeva, A | 299 |
| Imholz, A | 196 | Iturbe Fernández, D | 6, 7 |
| Incir, C | 164 | Ivanyuk, A | 97 |
| Ing Lorenzini, K | 122 |
J
| Jaehde, U | 242 | Jerbi, A | 142 |
| Jambon-Barbara, C | 38 | Jeurissen, F | 196 |
| Jang, I | 30, 31, 32, 50, 63, 71 | Jimenez, Y | 116 |
| Jang, J | 128 | Jiménez Ortega, A | 317 |
| Jankovic, G | 228 | Jiménez-Mercado, M | 110 |
| Jankovic, S | 143 | Jin, S | 68 |
| Jansen, R | 196 | Jlassi, S | 186 |
| Jara-Rubio, R | 23 | Joaquin, C | 116 |
| Jardou, M | 238 | Jordão, I | 100 |
| Javid, F | 279 | Jovančević, B | 43 |
| Jebabli, N | 75, 89, 186, 258, 313 | Judge, B | 188 |
| Jang, J | 128 | Jullien, A | 38 |
| Jellazi, M | 295 | Jung-Poppe, L | 198, 199 |
| Jensen, A | 293 | Juricic Nahal, D | 20 |
| Jeong, S | 63 | Jürgens, G | 8 |
| Jeong, S | 30, 128 |
K
| Kachanova, A | 4 | Koch, B | 304, 305, 136 |
| Kacirova, I | 35, 115 | Koczera, P | 303 |
| Kahma, H | 68 | Koenderink, J | 46 |
| Kahma, H | 192 | Koetsier, M | 167 |
| Kalaitzidou, A | 255, 256 | Kokras, N | 249 |
| Kalfsvel, L | 21 | Kolbin, A | 260, 265, 153 |
| Kamperi, N | 270 | Koldenhof, J | 46 |
| Kandilogiannakis, L | 232, 229 | Kolios, G | 250, 232, 208, 214 229, 224, 237, 257 |
| Kanjuh, V | 228 | Konteles, V | 231 |
| Kappers, M | 305 | Kontogiorgis, C | 264 |
| Karailidou, P | 255, 256 | Koons, A | 188 |
| Karampelas, T | 270 | Koopman, M | 196 |
| Karandov, A | 156 | Koopmans, M | 70 |
| Karatzas, E | 232 | Kootstra, N | 70 |
| Karinauske, E | 222 | Koppen, A | 69 |
| Kasimova, A | 260, 265, 153, 48 | Korhan, P | 164 |
| Katavati, T | 259 | Koristkova, B | 35, 115 |
| Kazaki, E | 224 | Kornelsen, E | 175 |
| Kazantzidis, L | 255, 256 | Koß, R | 65, 66 |
| Kazzi, Z | 188 | Kotsovolis, G | 254 |
| Kebaili, R | 183 | Kotteas, E | 270 |
| Kehl, N | 194 | Kotzampassi, K | 229 |
| Kemp, R | 309 | Kourounakis, A | 259, 272 |
| Kenani, Z | 176 | Koutsougianni, F | 118, 231 |
| Kerckhoffs, A | 129 | Kovačević, Z | 43 |
| Kerimbayeva, Z | 253 | Kramers, C | 127, 147 |
| Khammassi, G | 86 | Krčméryová, T | 274 |
| Khammassi, N | 90, 86 | Kubickova, V | 171 |
| Khaziakhmetova, V | 121 | Kuijper, M | 226 |
| Khouri, C | 38, 28, 22 | Kulla, N | 236 |
| Khwarg, J | 31 | Kumar, L | 134 |
| KIEFFER, F | 72 | Kumar, V | 120 |
| Kiiski, J | 219 | Kupka, R | 129 |
| Kilo, R | 302 | Kuppermann, N | 175 |
| Kim, B | 51, 30, 32, 71 | Kurkela, M | 68 |
| Kim, H | 31 | Kurkela, M | 192 |
| Kim, H | 63 | Kushugulova, A | 299 |
| Kim, Y | 51 | Kytö, V | 236 |
| Kleftaras, A | 272 | Kaartinen, T | 251 |
| Klumpers, U | 129 | Kaas-Hansen, B | 293, 8 |
| Knobbe, T | 173 | ||
| Knaap van der, M | 216 |
L
| Labriffe, M | 203 | Lehtonen, R | 25 |
| Ladhari, C | 180 | Lenoir, C | 11 |
| Lafazanis, K | 211 | Lévyová, M | 239 |
| Lafazanis, K | 118 | Li, L | 136 |
| Lafeber, M | 304, 70 | Li, S | 188 |
| LAHOUEL, M | 307 | Li, Y | 25 |
| Lallas, K | 50 | Liapi, C | 263 |
| Lambrianidou, A | 231 | Likic, R | 127 |
| LAMORT-BOUCHE, M | 72 | Likic, R | 147 |
| LAMOTTE-FELIN, A | 72 | Lilius, T | 80 |
| Lamprinou, M | 40 | Limo, S | 298 |
| Landeta Manzano, A | 110 | Linardakis, S | 170 |
| Lange, T | 293 | Lione, L | 264 |
| Lapatto, L | 25 | Lipperheide, I | 109 |
| Lapatto-Reiniluoto, O | 219 | Litman, R | 217 |
| Larabi, A | 28 | Llaudó, J | 217 |
| Laroche, M | 24 | Llorente Cantalapiedra, A | 271 |
| Lasys, T | 222 | Longo, C | 210 |
| Lauria, B | 320 | Loobeek, B | 127 |
| Lavín, L | 117 | Lopes, D | 273 |
| Lavin-Alconero, L | 155, 271 | LOPEZ, C | 16 |
| Lavon, O | 200, 204 | López-Bernús, A | 23 |
| Lavrnic, D | 143 | Lopez-Gomez, J | 53, 55 |
| Lawson, R | 238 | López-Suñé, E | 314 |
| Lazari, D | 264 | Lorenzini, K | 233 |
| Lechsner, P | 113, 114 | Lourbopoulos, A | 161 |
| LECRUX, B | 36 | Lovrek Romcevic, M | 20 |
| Lee, A | 30 | Lucena, M | 197 |
| Lee, D | 31 | Lugovkina, T | 52 |
| Lee, M | 31 | Lukina, M | 4 |
| Lee, S | 31, 51, 30, 32, 63, 71 | Lunenburg, C | 196 |
| Lee, Y | 128 | Løhne, K | 104 |
| Lega, J | 5, 302 | Laan van der, D | 216 |
| Leguízamo-Martínez, L | 47 | Laasik, M | 25 |
| Lehtonen, M | 78 |
l
| le, h | 226 |
M
| Mach, F | 174 | ||
| Magalhães Silva, T | 79 | ||
| Magouliotis, D | 118 | ||
| Magzumova, R | 253 | ||
| Mahmoud, K | 210 | ||
| Mahomedradja, R | 262 | ||
| Mainoli, B | 144, 177 | Mijatovic-Jovin, V | 308 |
| Maitland-van der Zee, A | 210 | Mikros, E | 153 |
| Makalkina, L | 243 | Mikroulis, D | 232 |
| Makhmudova, O | 121 | Milijasevic, B | 308 |
| Malewicka, D | 105 | Miller, J | 102 |
| Malik, A | 91 | Milojevic, P | 228 |
| Malo de Molina, R | 109 | Milosavljevic, J | 143 |
| Mandigers, C | 196 | Milosavljevic, M | 143 |
| Manolopoulos, V | 208, 214,229, 232 257, 250, 237, 224 | Miron, N | 81 |
| MARCHELLO, G | 248 | Mishra, A | 125, 126 |
| Marilly, E | 5 | Mitrov-winkelmolen, L | 226 |
| Maring, J | 83 | Moga, M | 266 |
| Marquet, P | 203 | Mohamed Mohamed, K | 317 |
| Marrero, D | 73 | Mohan, P | 207, 135 |
| Martic, N | 308 | Mokkink, L | 262 |
| MARTIN, F | 16 | Mokni, S | 181, 178 |
| Martínez, J | 217 | Mokni, S | 176 |
| Martínez, S | 116 | Mommers, R | 167 |
| Martínez-Mellado, A | 23 | Montané, E | 116, 234 |
| Martinez-Muñoz, M | 109 | Montané Esteva, E | 131 |
| Mascolo, A | 150, 288, 289 | Mora Cuesta, V | 6, 7 |
| Mashida, K | 235 | Moraleda-Jiménez, J | 23 |
| Masiero-Aparicio, P | 73 | Morales, A | 116 |
| Maslarinou, A | 250 | MORDEL, P | 36 |
| Massy-Chalverat, M | 201 | MORELLO, R | 36 |
| Mateos-Campos, R | 103 | MORENO, F | 16 |
| Mateus Rodriguez, J | 275 | Morozova, T | 4 |
| Mathew, M | 188 | Morris, C | 278 |
| Mathijssen, R | 196 | Mota-Zamorano, S | 53, 55 |
| Mathôt, R | 167 | Mottes, Y | 200 |
| Matos, S | 102 | Moulfi, F | 97 |
| Matralis, A | 259 | Moulin, P | 5 |
| Matsas, A | 88 | Mouna, M | 86 |
| Mazón, I | 117 | Moura, M | 144 |
| Mazon-Maraña, I | 155, 271 | Mourouzis, I | 170, 161 |
| McFalls, J | 188 | Mousavi, S | 3 |
| Medina-Caliz, I | 197 | Mtiraoui, A | 180 |
| Mehic, M | 298 | Muccioli, G | 240 |
| Mehmood, M | 91 | Mukhitova, D | 190 |
| Meid, A | 37 | Muller, M | 56, 57 |
| Mejía-Abril, G | 168, 225 | Muntean, I | 81 |
| Mendez Placencia, C | 275 | Murzina, A | 157 |
| MERINO, D | 213, 218, 248 | Mykkänen, A | 80 |
| Merino Bohorquez, V | 139 | Müller, J | 112 |
| MEUNIER-BEILLARD, N | 72 | Myllymäki, L | 212 |
| Michailidou, M | 223, 277, 318 | Myrianthopoulos, V | 153 |
| Meiramova, A | 299 | Møller, M | 293 |
| Mejía, G | 252 | ||
| Mejía Abril, G | 67, 215 | Maas, R | 198, 194, 199 |
N
| Na, J | 71 | Niemi, M | 25, 41, 78, 80, 192, 219, 251, 236 |
| Nadtha, P | 240 | Nijenhuis, T | 129 |
| Nakas, G | 249 | Nikolakis, D | 153 |
| Nakhli, J | 180 | Nikolaou, A | 122 |
| Nam, J | 32 | Nishijima, D | 175 |
| Navares Gomez, M | 67, 168, 215, 225, 252 | Niu, H | 197 |
| Nazarzadeh, M | 3 | Njoo, M | 167 |
| Neculau, A | 266 | Nogueiras-Álvarez, R | 6, 7 |
| Nenezic, D | 228 | Nongpiur, A | 95 |
| Neuvonen, M | 78, 68, 219, 80 | Novakovic, A | 228 |
| Nguyen, S | 96 | Novalbos, J | 252 |
| Nicot, R | 96 | Ntenti, C | 254 |
| Nieboer, P | 196 | Ntoulas, G | 249, 244 |
| Niederer, A | 11 | Nuñez, M | 116 |
| Nielsen, A | 8 | Núñez, M | 234 |
| Nielsen, L | 13 | Nurgaziyev, M | 299 |
| Nygaard, I | 104 |
O
| Ochoa, D | 252 | Orfanou, I | 270 |
| Ochoa, L | 217 | Ortega-Alonso, A | 197 |
| Offer, S | 196 | Osanlou, R | 64 |
| Oh, J | 32, 51, 71 | Ossenkoppele, P | 167 |
| Olesti, E | 202 | Otero Candelera, R | 137, 138 |
| Olivé Torralba, A | 275 | Othong, R | 188 |
| Omri, S | 204 | Ouali, S | 311 |
| Onteniente, M | 23 | Ouni, B | 183, 181, 176, 178, 180, 245, 291, 292, 294, 301 |
| Opdal, M | 104 | Ozer, M | 164 |
P
| Paczkowska, M | 105 | Pérez-Carreño, E | 73 |
| Page, C | 120 | Pérez-Mañá, C | 234 |
| Paile-Hyvärinen, M | 80 | Pérez-Tamajón, L | 73 |
| Paiva, P | 100, 102 | Perner, A | 293 |
| Palčevski, D | 182 | Pertijs, J | 46 |
| Paloumpis, N | 270 | Peters, S | 54 |
| Paludetto, M | 68, 192 | Peyro-Saint-Paul, L | 36, 96 |
| Panait, C | 201 | Pfistermeister, B | 199 |
| Pancere, J | 222 | Phillips, R | 279 |
| Pandit, R | 147 | PIERRE, M | 213 |
| Panjković, K | 12, 284 | Pinault, E | 238 |
| Pantelidaki, A | 161 | Pinto, R | 273 |
| Pantos, C | 170, 161 | Pires, K | 226 |
| Papadopoulos, A | 255, 256 | Pirmohamed, M | 64, 107, 267 |
| Papaioannidou, P | 147, 247, 223, 269, 277, 312, 316, 318, 319, 127 | Pissimisis, E | 170 |
| Papakonstantinou, N | 170 | Pistis, M | 320 |
| Papapostolou, I | 231 | Pizon, A | 188 |
| Papaseit, E | 234 | Placido, D | 8 |
| Papavramidis, T | 254 | Plácido, A | 76 |
| Papazisis, G | 40, 50, 49, 74, 88, 264 | Platt, R | 132 |
| Papiashvili, N | 45, 101 | Platt, R | 133 |
| Paquot, A | 240 | POITE, M | 72 |
| Paraskeva, T | 224 | Polissidis, A | 249, 244 |
| Pareja, A | 23 | Pop, R | 290 |
| Parente, F | 100 | Porrini, E | 73 |
| PARIS, A | 72 | Portielje, J | 196 |
| Park, J | 30 | Portokallidou, K | 214 |
| Park, M | 32 | Portolés-Pérez, P | 29 |
| Park, S | 31, 51, 32 | Poruba, M | 297 |
| Park, Y | 63 | Postma, D | 70 |
| Parra, R | 225 | Postma, M | 83 |
| Patsourakos, N | 170 | Pottegård, A | 159 |
| Paut Kusturica, M | 43, 162, 12, 284 | Poultsidi, A | 231 |
| Payares-Herrera, C | 59, 67, 109, 168 | Pourzitaki, C | 254 |
| Payerl-Pal, M | 182 | Prabhakaran, K | 207 |
| Péchère-Bertschi, A | 174 | Prado Ochoa, M | 296 |
| Pedersen, L | 187 | Prados-Bo, A | 26 |
| Peeters, L | 304, 305 | Prata, I | 100 |
| Pena Pardo, M | 271 | Prod’Hom, S | 97 |
| Penengo, M | 165, 189 | Prodanović, D | 162 |
| Perez, J | 22 | PROVENCHER, S | 302 |
| Pérez Hurtado, J | 139 | Psathas, T | 74, 88 |
| Pérez Segura, P | 317 | Puiguriguer-Ferrando, J | 108 |
| Peric, S | 143 | ||
| Perin, F | 165 |
Q
| Quang, L | 188 | Quijada Manuitt, M | 185 |
| Quesada, S | 314 |
R
| Rad, A | 3 | Rodríguez Ramallo, H | 137, 138, 139, 140, 139 |
| Rafaniello, C | 146, 148, 150 | Rodríguez-Adanero, C | 73 |
| Rafaniello, C | 283 | Rodríguez-Aguilar, L | 108 |
| Ragia, G | 214, 257, 250, 237, 208 | Rodríguez-Fortúnez, P | 23, 73 |
| Rajasundaram, S | 120 | Rodríguez-Jiménez, C | 23, 73 |
| Rakhimova, Z | 121 | Rogozea, L | 266 |
| Ramakers, C | 304 | Rollason, V | 11 |
| Ramírez-García, A | 109, 108, 59, 67, 168 | Romero-Gomez, M | 197 |
| Ramos, A | 116 | Ronde, E | 136 |
| Rašković, A | 12, 43, 162, 284, 308 | Rongen, G | 46 |
| Rawat, V | 106 | Roque, F | 76, 79, 103 |
| Reek, J | 167 | Rosa, M | 144 |
| Reeta, K | 125, 126 | Rosellini, P | 27 |
| Reina-Couto, M | 273 | Rossi, F | 283, 146, 148, 150, 288, 289 |
| Reiss, I | 136 | Rostami-Hodjegan, A | 54 |
| Ren, L | 175 | Rothuizen, L | 97 |
| Renoux, C | 132, 133 | Roustit, M | 38, 22 |
| Reny, J | 11, 123, 233 | Roux, B | 24 |
| Reumerman, M | 56, 57, 58 | Rowland, T | 246 |
| Revuelta, M | 300 | Roy, N | 207 |
| Richir, M | 56, 57, 58, 111, 127, 147 | Roy Millan, P | 275 |
| Rietdijk, W | 70 | Rubinić, I | 182 |
| Rim, C | 193 | Ruggiero, R | 283, 288, 289, 148 |
| Robles, N | 53, 55 | Ruiz-Antorán, B | 109, 108, 59, 67, 168 |
| Robles-Diaz, M | 197 | Rumantir, M | 175 |
| Rocha, V | 76, 79 | Russel, F | 46 |
| Rochat, C | 201 | Rychlickova, J | 171 |
| ROCHER, F | 213, 218, 248 | Ryu, C | 31 |
| Rodieux, F | 122 | ||
| Rodrigues, D | 76, 103 | ||
| RODRIGUEZ, C | 16 | ||
| Rodríguez, A | 73 | ||
| Rodríguez, C | 8 | ||
| Rodríguez Gallego, A | 285 | ||
| Rodríguez Landa, J | 315 | ||
| RODRIGUEZ, F | 16 |
S
| Sabin, O | 81, 290 | Sfar, I | 92 |
| Sablerolles, R | 70 | Shakenov, M | 243 |
| Sabo, A | 162 | Shatskiy, D | 4 |
| Sachinidis, A | 40 | Shihmanter, R | 93 |
| Saedder, E | 13, 14 | Shikh, E | 4 |
| Sáez, J | 202 | Shikh, N | 4 |
| Sáez-Peñataro, J | 130, 314 | Shopabayeva, A | 158 |
| Saha, A | 279 | Shumkov, V | 157 |
| Sahnoun, D | 195, 209, 311 | Siafis, S | 49, 74, 88 |
| SAIDI, M | 72 | Sigaloff, K | 262 |
| Saied, S | 235 | Silva, F | 102 |
| Sakellaridis, N | 118, 211 | Simms, C | 267 |
| Saleh, A | 279 | Singh, A | 134 |
| Salgado Jr., W | 309 | Sinha, S | 207 |
| Salgado-García, E | 108 | Sivac, N | 298 |
| Salgueiro, E | 300 | Skoumpa, K | 269 |
| Salminen, L | 25 | Skryabin, V | 282 |
| Salouage, I | 186, 313, 258, 75, 311 | Slim, R | 176,183, 181, 178, 245, 291, 292, 294, 301, 307 |
| Salvo, F | 27 | Smith, D | 120 |
| Samer, C | 11, 122, 233, 269 | ||
| Sanabria-Cabrera, J | 197 | ||
| Sanaï, S | 90, 86 | ||
| Sánchez, C | 130 | ||
| Sánchez Martín, A | 139 | 239, 274 | |
| Sánchez Santiago, B | 110 | Sogaard, O | 14 |
| Sánchez Santiago, M | 271 | Solano, E | 23 |
| Sánchez Santiago, M | 191 | Sonneveld, M | 304 |
| Sánchez-Ramón, S | 317 | Sood, M | 126 |
| Sanchez-Santiago, M | 155 | Soria Chacartegui, P | 215, 252 |
| Sancho, A | 116 | Soria-Chacartegui, P | 225 |
| Sancho-López, A | 108, 59, 67, 168 | Soriano, G | 197 |
| Sankaranarayanan, R | 235 | Soukop, J | 171 |
| Sankarankutty, A | 309 | Soultati, I | 254 |
| Sarıkaya, A | 164 | Souto, M | 273 |
| Sans Pola, C | 119 | Spathakis, M | 232, 229 |
| Santo, G | 102 | Speranza, N | 82, 84 |
| Sanz, E | 127, 147 | Spitaleri Timpone, P | 147 |
| Sarac, A | 166 | ||
| Sarangi, S | 125, 126 | Stage, T | 159 |
| Sargento-Freitas, J | 102 | Stamatellos, V | 74 |
| Sarkar, C | 95, 106 | Stamoula, E | 40, 74, 88 |
| Sarmanaev, S | 156 | Stamoulas, K | 88 |
| Sauvage, F | 238 | Stanke-Labesque, F | 85, 87 |
| Scavone, C | 146, 148, 150 | Stankeviciute, S | 222 |
| Scheidel, B | 242 | Stefanovic, S | 143 |
| Schellens, J | 196 | Steiropoulos, P | 232 |
| Schirris, T | 46 | Stelitano, B | 288, 289 |
| Schjørring, O | 293 | Stergiopoulou, T | 247, 312, 316, 319 |
| Schlichtig, K | 194 | Stilinovic, N | 308 |
| Schmiedl, S | 65, 66 | Stilinović, N | 162, 12, 284 |
| Schoenmakers, S | 136 | Stingl, J | 98, 112, 303 |
| Schrama, Y | 34 | Stojanovic, I | 228 |
| Schreiber, H | 94 | Stougiannos, P | 170 |
| Schuh, S | 188 | Strilakou, A | 263 |
| Schulz-Falten, S | 94 | Staali, H | 89 |
| Schuster, A | 198 | Subramony, P | 105 |
| Schwarz, E | 188 | Sukalo, A | 298 |
| Schäfer, K | 94 | Sullo, M | 146, 148, 150 |
| Seifert, S | 188 | Sultan, R | 56, 57 |
| Seikenova, Z | 243 | Svihra, J | 141 |
| Senbel, A | 44 | Swart, E | 210 |
| Sereti, E | 231 | Swen, J | 196 |
| Sergazy, S | 306, 310 | Sychev, D | 253, 4 |
| Serrano, S | 314 | Sydykov, S | 158 |
| Severs, D | 304 | Syrigos, K | 270 |
| Sportiello, L | 146, 148, 150, 288, 289 | Szépligeti, S | 187 |
| Springer, H | 57 | Sørensen, H | 187 |
| Spyrou, G | 232 |
T
| Taha, S | 44 | Tomić, Z | 162 |
| Tamvakopoulos, C | 270 | Tonini, J | 85, 87 |
| Tanuarbek, U | 190 | Tornio, A | 78, 219, 251, 80, 236 |
| Tapaninen, T | 80 | Torres, F | 314 |
| Tarapatzi, G | 232, 229 | Torres-Ramírez, A | 73 |
| Tarkiainen, K | 80 | Tortajada, M | 314 |
| Taskinen, S | 80 | Tortora, L | 188 |
| Tataridis, G | 255, 256 | Tounta, D | 184 |
| Taubert, M | 242 | Touw, D | 83, 173 |
| Taudte, V | 194 | ||
| Tavares, A | 103, 76 | Trabelsi, S | 90, 186, 86, 92, 258, 75, 89, 99, 193, 195 142, 209, 311, 205, 206, 241, 261, 313 |
| Tebbenjohanns, J | 65, 66 | Tramèr, M | 39 |
| Teegelbekkers, A | 37 | ||
| Teeuwisse, P | 167 | Trikas, A | 170, 250, 161, 208 |
| Tej, A | 183 | Trilla, A | 314 |
| Tello Mena, S | 6, 7 | Trontzas, I | 270 |
| Temirgalieva, E | 190 | Trueck, C | 242 |
| Tempany, J | 267 | Tsakiridis, I | 50 |
| Terrier, J | 11, 123 | Tsakiroglou, M | 107, 267 |
| Tesseromatis, C | 184 | Tsalidou, M | 269 |
| Tessieras, J | 97 | Tsaliki, V | 208 |
| Tessitore, E | 174 | Tsaluchidu, S | 223, 312 |
| Thal, S | 65, 66 | Tsaousi, G | 254 |
| Theis, C | 65, 66 | Tsarna, O | 244 |
| Then, M | 198 | Tseti, I | 161 |
| Theodosis-Georgilas, A | 170 | Tsezou, A | 231 |
| Thiebaut-Bertrand, A | 87 | Tsikrikas, P | 224 |
| Thomopoulos, T | 250, 208 | Tsoy, L | 253 |
| Thorsen-Meyer, H | 8 | Tufanova, O | 48 |
| Thürmann, P | 65, 66 | Tuncok, Y | 164, 166 |
| Tichelaar, F | 83 | Tuomi, S | 41 |
| Tichelaar, J | 56, 57, 111, 127, 147, 58, 262 | Tupiec, J | 303 |
| Tobias, A | 177 | Tweet, M | 188 |
| Toledo, M | 165, 189 | Twisk, J | 210 |
| Tomas, A | 43, 162, 308, 12, 284 | Tzara, A | 272 |
| Tomek, D | 239 | Tziakas, D | 208 |
| Tomic, S | 20 | Taamallah, K | 311 |
| Tomic, Z | 308 |
U
| Ullah, S | 242 | Urbánek, K | 297 |
| Uppal, R | 135 | Ussai, S | 320 |
| Urbanek, K | 171 | Utepova, D | 253 |
V
| VALDES, S | 16 | Vega Gil, N | 191, 155, 110, 271 |
| Valdivielso, J | 55 | Veluwenkamp-Worawutputtapong, P | 83 |
| Valencia, D | 117 | Verbeek, R | 216 |
| Valencia, J | 300 | Verger, P | 28 |
| Valencia López, D | 155, 271 | Verginis, P | 153 |
| Valenzuela Limón, O | 315 | Versmissen, J | 304, 305, 21 |
| Valsivielso, J | 53 | ||
| Van Agtmael, M | 147 | VIARD, D | 213, 218, 248 |
| Van Den Oever, F | 34 | Vila, M | 230 |
| Vancells, G | 230 | Vilella, A | 314 |
| Vander Stichele, R | 27 | Vilium, I | 260, 265 |
| Varea, S | 202 | Villanueva-Paz, M | 197 |
| Vargas-Castrillón, P | 29 | Villapalos, G | 252 |
| Vargovcak, M | 141 | Villapalos-García, G | 225 |
| Varsou, E | 269 | Visser, L | 70 |
| Vasbinder, E | 34 | Vlahović-Palčevski, V | 182 |
| Vasilogianni, A | 54 | Vlassopoulos, D | 211 |
| Vavilis, T | 40, 74, 88 | Voermans, M | 216 |
| Vavreckova, E | 35, 115 | Vousnakis, D | 255, 256 |
| Vázquez, L | 300 | Vradelis, S | 229 |
| Vearrier, D | 188 | Vukmirovic, S | 308 |
| Vega, N | 117 | Vukovic, J | 201 |
v
| van Agtmael, M | 56, 57, 58, 111, 127, 262 | van der Pol, K | 46 |
| van Baarle, D | 70 | van der Sluis, I | 69 |
| van de Poel, M | 196 | van der Aa, M | 129 |
| van den Bemt, P | 34 | van Doorn, A | 27 |
| van den Berg, S | 136 | van Gelder, T | 304, 34 |
| van den Broek, P | 46 | van Gelder, T | 305 |
| van den Broek, W | 21 | van Gurp, E | 34 |
| van den Heuvel, J | 46 | van Rosse, F | 21 |
| van den Hoogen, M | 304 | van Schaik, R | 136 |
| van der Kuy, H | 70, 21 | van Schaik, R | 196 |
| van der Lee, M | 196 | van Smeden, J | 127 |
| van der Meer, A | 46 | van Zelst, B | 136 |
W
| Wachall, B | 242 | Wax, P | 188 |
| Wahlang, J | 95 | Weisbach, L | 198 |
| Walker, L | 235, 246, 64 | Weiß, J | 37 |
| Walker, M | 201 | Wiegand, T | 188 |
| Walling, D | 217 | Wiesen, M | 242 |
| WALLON, M | 72 | Wightman, F | 246 |
| Ward, J | 28 | Woillard, J | 203 |
| Wargenau, M | 242 | Wood, I | 82 |
| Wąsik, A | 15 |
Y
| Yamamoto, P | 309 | Yavuz, B | 166 |
| Yamoune, S | 98, 112, 303 | Ygiyeva, D | 124 |
| Yang, E | 31, 63 | Yurina, Y | 156 |
| Yang, Q | 228 |
Z
| Zacharouli, K | 118 | Zhetkenev, S | 306 |
| Zacharoulis, D | 118 | Zhumadilov, Z | 310 |
| Zagorodnikova, K | 157 | Zhurat, S | 93 |
| Zahiragic, N | 298 | Zia, Z | 66 |
| Zappacosta, B | 10 | Ziadi, J | 311 |
| Zaremba, D | 242 | Zijp, T | 173 |
| Zastrozhin, M | 282 | Zinzi, A | 146 |
| Zaychenko, G | 286, 287 | Zittema, D | 129 |
| Zhai, Q | 196 | Zorrilla Martínez, I | 110 |
| Zhakipbekov, K | 158, 160, 172 | Zubiaur, P | 67, 168, 225, 252 |
| Zhashkeyev, A | 310 | Zubiaur Precioso, P | 215 |
| Zhetkenev, S | 299 | Zvárová, E | 297 |
Ż
| Żarnowska, M | 15 |
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.


