Abstract
BACKGROUND
Frailty is associated with adverse events in elderly patients with acute coronary syndrome (ACS). Our aim was to compare the prognostic value of four frailty scales in patients aged ≥ 65 years hospitalized with ACS in a cardiac care unit (CCU).
METHODS
Patients aged ≥ 65 years with ACS were included. Frailty was assessed using the Fried frailty scale (reference standard), the Edmonton Frail Scale (EFS), the FRAIL scale, and the Clinical frailty scale (CFS). The primary end point was all-cause mortality and the secondary end point was unscheduled rehospitalization.
RESULTS
One hundred and seventy four patients aged ≥ 65 years with ACS were recruited. The median follow-up was 637.5 days. Frailty was identified in 41.4%, 40.2%, 39.1% and 36.3% patients by the Fried frailty scale, EFS, FRAIL scale and CFS, respectively. The agreement coefficients were 0.88, 0.86, and 0.79 for the FRAIL scale, EFS and CFS, respectively. In the Cox regression model, frailty was associated with all-cause mortality regardless of the scale used (univariate: hazard ratio [HR] 95% CI = 10.5, 2.4–46.8 Fried frailty scale; 12.0, 2.7–53.4 FRAIL scale; 7.1, 2.0–25.2 EFS; 8.3, 2.4–29.6 CFS. Multivariate: HR = 5.1, 1.1–23.8 Fried frailty scale; 5.7, 1.2–26.8 FRAIL scale; 3.7, 1.0–14.0 EFS; 4.2, 1.1–15.9 CFS). The FRAIL scale had the highest HR. In the univariate analysis, frailty was associated with unscheduled rehospitalization (HR = 3.2, 1.7–6.0 Fried frailty scale; 3.4, 1.8–6.3 FRAIL scale; 3.5, 1.8–6.6 EFS; 3.1, 1.7–5.8 CFS). In the multivariate analysis, only the EFS independently predicted unscheduled rehospitalization (HR = 2.2, 1.1–4.63).
CONCLUSIONS
Frailty assessed by the Fried frailty scale, FRAIL scale, EFS and CFS is associated with all-cause mortality and unscheduled rehospitalization in elderly patients hospitalized in a CCU with ACS. The adjusted HR of the FRAIL scale for all-cause mortality was the highest among the scales compared, whereas the EFS was an independent predictor of unscheduled rehospitalization. These data should be taken into consideration when choosing a frailty assessment tool.
Cardiovascular diseases, including ischemic heart disease, are the leading cause of death globally.[1] Age is one of the strongest predictors of adverse events in patients with coronary artery disease (CAD) and acute coronary syndrome (ACS). Over 60% of all people admitted to hospital with ACS are elderly and this population is projected to rise.[2-5] Older patients with comorbidities, and physical and cognitive disabilities are often excluded from clinical trials; therefore, current evidence-based recommendations are not applicable for these patients.[6] For ACS risk stratification in this population, we should also take into consideration geriatric syndromes, most importantly frailty.[7] Frailty is a loss of physiological reserves, which leads to a failure of homeostasis following stressful events and causes vulnerability to adverse outcomes, such as falls, hospitalizations or mortality. An example of a stressful event is ACS.[8,9] Current studies and structured literature reviews have shown that frailty is common in older people with ACS. The frailty prevalence is estimated at 5%–48%, with a median prevalence of 31.5%.[10,11] Moreover, frailty syndrome is an independent predictor of mortality and adverse events in ACS patients, and the relationship has been demonstrated by many studies using different frailty assessment tools.[12,13] There are many valid and reliable tools to identify frailty and there is evidence of usefulness for some of them in patients with ACS.[9,14] However, it remains unclear which tool is the best for detecting frailty in ACS patients that are hospitalized in a cardiac care unit (CCU).
The aim of this study is to compare the prognostic value of four different frailty scales in ACS patients aged ≥ 65 years who are hospitalized in a CCU, and to identify the most valuable frailty scale in these patients.
METHODS
Participants
This is a single-center prospective cohort study. Patients aged ≥ 65 years with diagnosed ACS were included. ACS was defined as one of three conditions: unstable angina (UA), non-ST-elevation myocardial infarction (NSTEMI) and ST-elevation myocardial infarction (STEMI), according to the European Society of Cardiology guidelines.[15] Exclusion criteria included a refusal to participate, inability to give consent, and any substantial visual, hearing or speech impairments that precluded a frailty assessment. Patients were recruited from the Department of Intensive Cardiac Therapy at the National Institute of Cardiology in Warsaw. The study protocol was approved by the Bioethics Committee and all participants signed informed consent forms.
Data Collection
The demographic and clinical characteristics of the participants were recorded. Details on medical history, previous medications and comorbidities were collected through interviews and patient medical files. Comorbidities included hypertension, diabetes mellitus, atrial fibrillation (AF), hyperlipidemia/hypercholesterolemia, congestive heart failure (CHF), valvular heart disease, previous coronary heart disease (including previous myocardial infarction (MI), percutaneous coronary intervention (PCI), or coronary artery bypass grafting), cerebrovascular disease, peripheral vascular disease, chronic pulmonary disease, chronic kidney disease (CKD), and a history of cancer. Frailty was assessed as soon as possible after admission with the exception of the physical tests, which were performed immediately after patients’ bed restrictions were removed. Physical tests were performed by a team of physical therapists from the National Institute of Cardiology. Four validated frailty scales were used: (1) the Fried frailty scale (as a reference scale); (2) the Edmonton Frail Scale (EFS); (3) the FRAIL scale; and (4) the Clinical Frailty Scale (CFS).
The Fried frailty scale consists of five criteria: (1) weight loss: self-reported unintentional weight loss (≥ 4.5 kg in the last year). (2) Exhaustion: identified by using two questions from the Center of Epidemiologic Studies-Depression (CES-D): “How often in the last week did you feel that everything you did was an effort?” and “How often in the last week did you feel that you could not get going?”. Participants answering “a moderate amount of the time (3–4 days)” or “most of the time” to either of these questions were categorized as frail by the exhaustion criterion. (3) Low physical activity: Based on the short version of the Minnesota Leisure Time Activity questionnaire. Physical activity was considered low if total physical activity was < 383 kcal/week (men) and < 270 kcal/week (women). (4) Walk time, stratified by gender and height: Time required to walk 4 .57 m in a straight line. (5) Grip strength, stratified by gender and body mass index (BMI) quartiles: assessed using a handgrip strength dynamometer.
Frailty was considered present when 3 or more of the 5 criteria were fulfilled. Pre-frailty was considered present when 1 or 2 of the 5 criteria were fulfilled. Participants with 0 criterion were considered not frail.[8]
The EFS is composed of series of simple questions and tasks that assess several conditions, such as cognitive function (by using the “clock test”), functional independence, previous hospitalization, social support, medications, nutrition, mood, incontinence and functional status (by using the “get up and go” test). Frailty was considered present when the total score was 8–17 points. Pre-frailty was considered present when the total score was 6–7 points. Participants with a total score between 0–5 points were considered not frail.[16,17]
The FRAIL scale is a simple questionnaire that includes 5 components: fatigue, resistance, ambulation, illness, and loss of weight. The maximum score is 5 and the minimum score is 0. Frailty was considered present when the total score was 3–5 points. Pre-frailty was considered present when the total score was 1–2 points. Participants with a total score of 0 points were considered not frail.[18,19]
The CFS is a 7-point scale that evaluates specific conditions such as comorbidity, functional status and cognition. Frailty was considered present when the patient was assigned to categories 5–7. Pre-frailty was present when the patient was assigned to category 4. Patients assigned to categories 1–3 were considered not frail.[20] However, the CFS is now more commonly used as a 9-point scale with the additional subgroups “very severe frailty” and “terminally ill”.
All scales are attached in the supplementary material (Supplementary Tables 1–4).
Clinical Outcomes
The primary end point was all-cause mortality and the secondary end point was unscheduled rehospitalization. The information was obtained by contacting the patient or patient’s family.
Statistical Analysis
Depending on the distribution of continuous variables as assessed by the Shapiro-Wilk test, the results are shown as either arithmetic means with standard deviations or as median values with inter-quartile ranges. The significance of differences between the mean values was evaluated using the Student’s t-test. Categorical variables are shown as frequencies and percentages. Comparisons of the proportional categorical data from two different groups were performed using the chi-squared test with the Yate’s correction for continuity, or the Fisher’s exact test if the minimum expected count in the cell was less than 5. Agreement between the binary variables of the paired samples was analyzed using the kappa coefficient and McNemar’s test. The relationships between frailty and the other examined variables are listed in Table 1. Outcome (all-cause death and unscheduled rehospitalization) was assessed with the Cox’s proportional hazard model using univariate and backward multivariate procedures. The multivariate analysis included variables for which the level of statistical significance in univariate analysis was P < 0.1 ( Table 2). Variables included in the multivariate analysis of all-cause death were gender, age, BMI, previous CKD, malignant disease, hypercholesterolemia or hyperlipidemia, hemoglobin (Hgb) on admission to hospital, and left ventricular ejection fraction (LVEF) assessed during hospitalization. The variables included in the multivariate analysis of unscheduled rehospitalization were age, previous MI, CHF with EF ≥ 50%, CHF with EF < 50%, previous AF, previous CKD, previous stroke/TIA, Hgb on admission to hospital, LVEF assessed during hospitalization, and coronary angiography results. Each of the analyzed frailty scores were assessed in separate multivariate models. The goodness of fit models were evaluated with Harrell’s C-index. A significance level of P< 0.05 was required for the variable to remain in the multivariate model. A test for non-proportionality of hazards based on Schoenfeld residuals did not reveal significant violations of the proportionality assumptions. The probabilities of survival and survival free of unscheduled rehospitalization were estimated using the Kaplan–Meier method. The homogeneities of the curves with a different status of frailty syndrome were assessed with the log-rank test. All tests were two-sided and aP-value of < 0.05 was considered statistically significant. All statistical analyses were performed using SAS 9.4 software (SAS Institute, Cary, NC, USA).
Table 1. Baseline patient characteristics.
| Variables | Fried frailty scale | FRAIL scale | Edmonton Frail Scale | Clinical Frailty Scale | ||||||||
| Data are presented as mean ± SD or n (%). ACS: acute coronary syndrome; AF: atrial fibrillation; BMI: body mass index; CABG: coronary artery bypass grafting; CHF: congestive heart failure; CKD: chronic kidney disease; COPD: chronic obstructive pulmonary disease; EF: ejection fraction; Hgb: hemoglobin; MI: myocardial infarction; NSTEMI: non-ST-segment elevation myocardial infarction; PCI: percutaneous coronary intervention; STEMI: ST-segment elevation myocardial infarction; TIA: transient ischemic attack; UA: unstable angina. | ||||||||||||
| Non-frail
(n = 102) |
Frail
(n = 72) |
P-value | Non-frail
(n = 106) |
Frail
(n = 68) |
P-value | Non-frail
(n = 104) |
Frail
(n = 70) |
P-value | Non-frail
(n = 111) |
Frail
(n = 68) |
P value | |
| Female | 34 (33.3%) | 44 (61.1%) | < 0.001 | 39 (36.8%) | 39 (57.4%) | 0.012 | 36 (34.6%) | 42 (60.0%) | 0.001 | 40 (36.0%) | 38 (60.3%) | < 0.001 |
| Age, yrs | 72.1 ± 5.6 | 78.3 ± 8.0 | < 0.001 | 71.8 ± 5.1 | 79.2 ± 8.0 | < 0.001 | 72.3 ± 5.7 | 78.2 ± 8.1 | < 0.001 | 72.1 ± 5.6 | 79.1 ± 8.0 | < 0.001 |
| BMI, kg/m2 | 27.7 ± 4.4 | 25.3 ± 4.2 | < 0.001 | 27.7 ± 4.4 | 25.2 ± 4.2 | < 0.001 | 27.6 ± 4.4 | 25.3 ± 4.2 | < 0.001 | 27.4 ± 4.4 | 25.5 ± 4.3 | 0.009 |
| Type of ACS | 0.095 | 0.141 | 0.238 | 0.322 | ||||||||
| STEMI | 58 (56.9%) | 30 (41.67%) | 59 (55.7%) | 29 (42.6%) | 58 (55.8%) | 30 (42.9%) | 58 (52.2%) | 30 (47.6%) | ||||
| NSTEMI | 42 (41.2%) | 38 (52.78%) | 45 (42.4%) | 35 (51.5%) | 43 (41.3%) | 37 (52.9%) | 51 (45.9%) | 29 (46.0%) | ||||
| UA | 2 (1.9%) | 4 (5.56%) | 2 (1.9%) | 4 (5.9%) | 3 (2.9%) | 3 (4.3%) | 2 (1.8%) | 4 (6.3%) | ||||
| Coronary angiography | 0.002 | 0.008 | 0.018 | 0.026 | ||||||||
| No significant lesions | 2 (2.0%) | 7 (10%) | 3 (2.8%) | 6 (9.1%) | 2 (1.9%) | 7 (10.3%) | 3 (2.7%) | 6 (9.7%) | ||||
| Single-vessel disease | 30 (29.4%) | 7 (10%) | 30 (28.3%) | 7 (10.6%) | 28 (26.9%) | 9 (13.2%) | 29 (26.4%) | 8 (12.9%) | ||||
| Two-vessel disease | 32 (31.4%) | 20 (28,6%) | 34 (32.1%) | 18 (27.3%) | 33 (31.7%) | 19 (27.9%) | 36 (32.7%) | 16 (25.8%) | ||||
| Three-vessel disease | 38 (37.2%) | 36 (51.4%) | 39 (36.8%) | 35 (53.0%) | 41 (39.4%) | 33 (48.5%) | 42 (38.2%) | 32 (51.6%) | ||||
| Treatment | 0.020 | 0.013 | 0.007 | 0.035 | ||||||||
| Conservative therapy | 15 (14.7%) | 20 (27.8%) | 15 (14.1%) | 20 (29.4%) | 14 (13.5%) | 21 (30.0%) | 17 (15.3%) | 18 (28.6%) | ||||
| PCI | 78 (76.5%) | 51 (70.8%) | 82 (77.4%) | 47 (69.1%) | 81 (77.9%) | 48 (68.6%) | 85 (76.6%) | 44 (69.8%) | ||||
| CABG | 9 (8.8%) | 1 (1.4%) | 9 (8.5%) | 1 (1,5%) | 9 (8.6%) | 1 (1.4%) | 9 (8.1%) | 1 (1.6%) | ||||
| Treatment | 0.034 | 0.014 | 0.008 | 0.036 | ||||||||
| Conservative therapy | 15 (14.7%) | 20 (27.8%) | 15 (14.1%) | 20 (29.4%) | 14 (13.5%) | 21 (30.0%) | 17 (15.3%) | 18 (28.6%) | ||||
| PCI or CABG | 87 (85.3%) | 52 (72.2%) | 91 (85.8%) | 48 (70.6%) | 90 (86.5%) | 49 (70.0%) | 94 (84.7%) | 45 (71.4%) | ||||
| Hypertension | 81 (79.4%) | 65 (90.3%) | 0.055 | 85 (80.2%) | 61 (89.7%) | 0.145 | 83 (79.8%) | 63 (90.0%) | 0.073 | 89 (80.2%) | 57 (90.5%) | 0.076 |
| Diabetes mellitus | 32 (31.4%) | 30 (41.7%) | 0.163 | 33 (31.1%) | 29 (42.6%) | 0.166 | 33 (31.7%) | 29 (41.4%) | 0.190 | 35 (31.5%) | 27 (42.9%) | 0.134 |
| Previous MI | 30 (29.4%) | 15 (20.8%) | 0.203 | 30 (28.3%) | 15 (22.1%) | 0.459 | 30 (28.8%) | 15 (21.4%) | 0.273 | 31 (27.9%) | 14 (22.2%) | 0.409 |
| Previous PCI | 29 (28.4%) | 16 (22.2%) | 0.366 | 28 (26.4%) | 17 (25.0%) | 0.976 | 29(27.9%) | 16 (22.9%) | 0.458 | 31(27.9%) | 14 (22.2%) | 0.409 |
| Previous CABG | 12 (11.8%) | 7 (9.7%) | 0.671 | 12 (11.3%) | 7 (10.3%) | 1.00 | 12 (11.5%) | 7 (10.0%) | 0.750 | 14 (12.6%) | 5 (7.9%) | 0.342 |
| CHF with EF ≥ 50% | 3 (2.9%) | 10 (13.9%) | 0.007 | 4 (3.8%) | 9 (13.2%) | 0.043 | 3 (2.9%) | 10 (14.3%) | 0.005 | 4 (3.6%) | 9 (14.3%) | 0.015 |
| CHF with EF < 50% | 5 (4.9%) | 8 (11.1%) | 0.125 | 6 (5.7%) | 7 (10.3%) | 0.402 | 6 (5.8%) | 7 (10.0%) | 0.298 | 7 (6.3%) | 6 (9.5%) | 0.550 |
| Previous AF | 13 (12.7%) | 22 (30.6%) | 0.004 | 13 (12.3%) | 22 (32.3%) | 0.002 | 13 (12.5%) | 22 (31.4%) | 0.002 | 14 (12.6%) | 21 (33.3%) | 0.001 |
| Previous CKD | 20 (19.6%) | 28 (38.9%) | 0.005 | 20 (18.9%) | 28 (41.2%) | 0.002 | 22 (21.1%) | 26 (37.1%) | 0.021 | 24 (21.6%) | 24 (38.1%) | 0.019 |
| COPD | 10 (9.8%) | 9 (12.5%) | 0.574 | 9 (8.5%) | 10 (14.7%) | 0.200 | 10 (9.6%) | 9 (12.9%) | 0.501 | 8 (7.2%) | 11 (17.5%) | 0.037 |
| Peripheral artery disease | 12 (11.8%) | 10 (13.9%) | 0.678 | 12 (11.3%) | 10 (14.7%) | 0.673 | 13 (12.5%) | 9 (12.9%) | 0.945 | 14 (12.6%) | 8 (12.7%) | 0.987 |
| Previous stroke/TIA | 8 (7.8%) | 13 (18.1%) | 0.042 | 7 (6.6%) | 14 (20.6%) | 0.012 | 9 (8.7%) | 12 (17.1%) | 0.092 | 8 (7.2%) | 13 (20.6%) | 0.009 |
| Malignant disease | 13 (12.7%) | 11 (15.3%) | 0.633 | 13 (12.3%) | 11 (16.2%) | 0.614 | 12 (11.5%) | 12 (17.1%) | 0.293 | 13 (11.7%) | 11 (17.5%) | 0.291 |
| Hypercholesterolemia or
hyperlipidemia |
81 (79.4%) | 48 (66.7%) | 0.059 | 85 (80.2%) | 44 (64.7%) | 0.023 | 83 (79.8%) | 46 (65.7%) | 0.037 | 92 (82.9%) | 37 (58.7%) | < 0.001 |
| Hgb on admission to
hospital, g/dL |
13.7 ± 1.7 | 12.5 ± 1.9 | < 0.001 | 13.7 ± 1.7 | 12.5 ± 2.0 | < 0.001 | 13.7 ± 1.6 | 12.5 ± 2.0 | < 0.001 | 13.7 ± 1.6 | 12.3 ± 2.0 | < 0.001 |
| EF assessed during hospitalization,% | 49.2 ± 11.8 | 44.3 ± 13.7 | 0.011 | 49.4 ± 11.9 | 43.7 ± 13.5 | 0.004 | 48.8 ± 11.9 | 44.8 ± 13.7 | 0.043 | 48.6 ± 12.2 | 44.7 ± 13.6 | 0.053 |
Table 2. Predictors of unscheduled rehospitalization and all-cause death (univariate analysis).
| Variables | Death
(n = 15) |
Survivals (n = 153) | P-value | HR [95% CI] | P-value | Rehosp. (+)
(n = 41) |
Rehosp. (-)
(n = 123) |
P-value | HR [95% CI] | P-value |
| Data are presented as mean ± SD or n (%). *NA: due to the small number of observations. ACS: acute coronary syndrome; AF: atrial fibrillation; BMI: body mass index; CABG: coronary artery bypass grafting; CHF: congestive heart failure; CKD: chronic kidney disease; COPD: chronic obstructive pulmonary disease; EF: ejection fraction; Hgb: hemoglobin; MI: myocardial infarction; NSTEMI: non-ST-segment elevation myocardial infarction; PCI: percutaneous coronary intervention; STEMI: ST-segment elevation myocardial infarction; TIA: transient ischemic attack; UA: unstable angina. | ||||||||||
| Female | 10 (66.7%) | 66 (43.1%) | 0.081 | 2.40 [0.82; 7.03] | 0.109 | 22 (53.7%) | 50 (40.6%) | 0.146 | 1.48 [0.80; 2.73] | 0.213 |
| Age, yrs | 78.7 ± 7.7 | 74.2 ± 7.3 | 0.022 | 1.07 [1.01; 1.14] | 0.025 | 78.4 ± 8.2 | 73.4 ± 6.7 | < 0.001 | 1.08 [1.04; 1.12] | < 0.001 |
| BMI, kg/m2 | 25.0 ± 3.8 | 27.0 ± 4.5 | 0.098 | 0.90 [0.79; 1.02] | 0.097 | 26.0 ± 3.3 | 27.1 ± 4.8 | 0.105 | 0.96 [0.89; 1.03] | 0.218 |
| Type of ACS | ||||||||||
| STEMI | 10 (66.7%) | 75 (49.2%) | 0.372 | Reference | 17 (41.5%) | 63 (51.2%) | 0.537 | Reference | ||
| NSTEMI | 5 (33.3%) | 72 (47.1%) | 0.54 [0.18; 1.58] | 0.260 | 22 (53.7%) | 56 (45.5%) | 1.31 [0.70; 2.46] | 0.405 | ||
| UA | 0 (0.0%) | 6 (3.9%) | NA* | 0.992 | 2 (4.9%) | 4 (3.3%) | 1.28 [0.30; 5.54] | 0.741 | ||
| Coronary angiography | ||||||||||
| I. no significant lesions | 2 (13.3%) | 6 (4.0%) | 0.224 | Reference | 0 (0.0%) | 7 (5.7%) | 0.061 | Reference
(I and II) |
||
| II. single-vessel disease | 2 (13.3%) | 34 (22.5%) | 0.18 [0.02; 1.28] | 0.087 | 5 (12.5%) | 30 (24.6%) | Reference
(I and II) |
|||
| III. multi-vessel disease | 11 (73.3%) | 111 (73.5%) | 0.29 [0.06; 1.32] | 0.110 | 35 (87.5%) | 85 (69.7%) | 2.62 [1.02; 6.68] | 0.044 | ||
| Treatment | ||||||||||
| Conservative therapy | 5 (33.3%) | 27 (17.6%) | 0.246 | Reference | 8 (19.5%) | 22 (17.9%) | 0.610 | Reference | ||
| PCI | 10 (66.7%) | 117 (76.5%) | 0.49 [0.17; 1.43] | 0.193 | 32 (78.1%) | 93 (75.6%) | 1.16 [0.53; 2.52] | 0.707 | ||
| CABG | 0 (0%) | 9 (5.9%) | NA* | 0.991 | 1 (2.4%) | 8 (6.5%) | 0.43 [0.05; 3.44] | 0.427 | ||
| Treatment | ||||||||||
| Conservative therapy | 5 (33.3%) | 27 (17.7%) | 0.166 | Reference | 8 (19.5%) | 22 (17.9%) | 0.816 | Reference | ||
| PCI or CABG | 10 (66.7%) | 126 (82.3%) | 0.46 [0.16; 1.33] | 0.152 | 33 (80.5%) | 101 (82.1%) | 1.10 [0.51; 2.39] | 0.804 | ||
| Hypertension | 11 (73.3%) | 129 (84.3%) | 0.281 | 0.55 [0.17; 1.72] | 0.304 | 37 (90.2%) | 101 (82.1%) | 0.217 | 1.84 [0.66; 5.18] | 0.245 |
| Diabetes mellitus | 5 (33.3%) | 54 (35.3%) | 0.879 | 0.92 [0.31; 2.69] | 0.877 | 19 (46.3%) | 40 (32.5%) | 0.110 | 1.64 [0.89; 3.02] | 0.116 |
| Previous MI | 2 (13.3%) | 41 (26.8%) | 0.359 | 0.43 [0.10; 1.89] | 0.263 | 16 (39.0%) | 29 (23.6%) | 0.055 | 1.78 [0.95; 3.33] | 0.073 |
| Previous PCI | 2 (13.3%) | 41 (26.8%) | 0.359 | 0.44 [0.10; 1.93] | 0.275 | 15 (36.6%) | 29 (23.6%) | 0.103 | 1.68 [0.89; 3.17] | 0.111 |
| Previous CABG | 1 (6.7%) | 17 (11.1%) | 1.000 | 0.65 [0.09; 4.96] | 0.680 | 3 (7.3%) | 15 (12.2%) | 0.566 | 0.72 [0.22; 2.34] | 0.587 |
| CHF with EF ≥ 50% | 2 (13.3%) | 10 (6.5%) | 0.291 | 2.12 [0.48; 9.40] | 0.322 | 9 (21.9%) | 3 (2.4%) | < 0.001 | 4.59 [2.17; 9.74] | < 0.001 |
| CHF with EF < 50% | 1 (6.7%) | 11 (7.2%) | 1.000 | 0.94 [0.12; 7.15] | 0.953 | 6 (14.6%) | 6 (4.9%) | 0.075 | 2.36 [0.99; 5.62] | 0.052 |
| Previous AF | 5 (33.3%) | 26 (17.0%) | 0.157 | 2.23 [0.76; 6.55] | 0.142 | 14 (34.1%) | 17 (13.8%) | 0.004 | 2.30 [1.21; 4.40] | 0.011 |
| Previous CKD | 7 (46.7%) | 39 (25.5%) | 0.125 | 2.42 [0.88; 6.66] | 0.088 | 18 (43.9%) | 27 (21.9%) | 0.006 | 2.31 [1.25; 4.30] | 0.008 |
| COPD | 2 (13.3%) | 15 (9.8%) | 0.651 | 1.41 [0.32; 6.23] | 0.654 | 6 (14.6%) | 12 (9.8%) | 0.395 | 1.33 [0.56; 3.16] | 0.524 |
| Peripheral artery
disease |
2 (13.3%) | 18 (11.8%) | 0.694 | 1.18 [0.27; 5.23] | 0.828 | 7 (17.1%) | 14 (11.4%) | 0.345 | 1.59 [0.70; 3.58] | 0.267 |
| Previous stroke/TIA | 3 (20.0%) | 18 (11.8%) | 0.406 | 1.81 [0.51; 6.41] | 0.395 | 8 (19.5%) | 11 (8.9%) | 0.089 | 2.06 [0.95; 4.46] | 0.067 |
| Malignant disease | 5 (33.3%) | 18 (11.8%) | 0.036 | 3.38 [1.15; 9.89] | 0.026 | 6 (14.6%) | 15 (12.2%) | 0.686 | 1.37 [0.58; 3.27] | 0.475 |
| Hypercholesterolemia or hyperlipidemia | 6 (40.0%) | 119 (77.8%) | 0.003 | 0.21 [0.08; 0.60] | 0.003 | 33 (80.5%) | 92 (74.8%) | 0.458 | 1.52 [0.70; 3.30] | 0.292 |
| Hgb on admission to hospital, g/dL | 11.6 ± 1.4 | 13.3 ± 1.8 | < 0.001 | 0.69 [0.56; 0.85] | < 0.001 | 12.8 ± 2.1 | 13.5 ± 1.7 | 0.020 | 0.84 [0.73; 0.97] | 0.021 |
| EF assessed during hospitalization, % | 39.1 ± 13.2 | 48.5 ± 12.3 | 0.006 | 0.95 [0.91; 0.99] | 0.008 | 42.3 ± 13.8 | 49.6 ± 11.4 | < 0.001 | 0.96 [0.94; 0.99] | 0.002 |
RESULTS
Baseline Patient Characteristics
A total of 174 patients aged ≥ 65 years with diagnosed ACS were recruited. 78 (44.8%) patients were female and 96 (55.2%) were male. The mean patient age was 74.8 years and the oldest was 96 years. 96 (55.2%) patients were 65–74 years of age, 64 (36.8%) were 75–84 years of age, and 14 (8%) were ≥ 85 years of age. STEMI was diagnosed in 88 (50.6%) patients, NSTEMI in 80 (46%) patients, and UA in 6 (3.4%) patients. The baseline patient characteristics were similar irrespective of the scale used, with a few exceptions (Table 1). Frail patients were more likely to be female, older than non-frail patients, with a mean age of 78 vs. 72 years according to the reference scale (Fried frailty scale, P < 0.001), have a lower BMI, a lower level of Hgb on admission to hospital, and a lower LVEF evaluated during hospitalization (on the CFS the difference was on the verge of statistical significance, P = 0.053). Frail patients had more comorbidities, including higher rates of AF, CHF with preserved EF ≥ 50% and CKD, whereas the incidence of hypercholesterolemia/hyperlipidemia was substantially lower (on the Fried frailty scale the difference was on the verge of statistical significance, P = 0.059). According to most of the scales (Fried frailty scale, FRAIL scale, CFS), frail patients had higher rates of previous stroke and TIA, except on the EFS (P = 0.092). On one scale (CFS), frail patients had slightly higher rates of chronic obstructive pulmonary disease, but the difference was marginal (P = 0.037). Frail patients were more likely to have multi-vessel disease and were more often qualified for conservative therapy.
Frailty Assessment
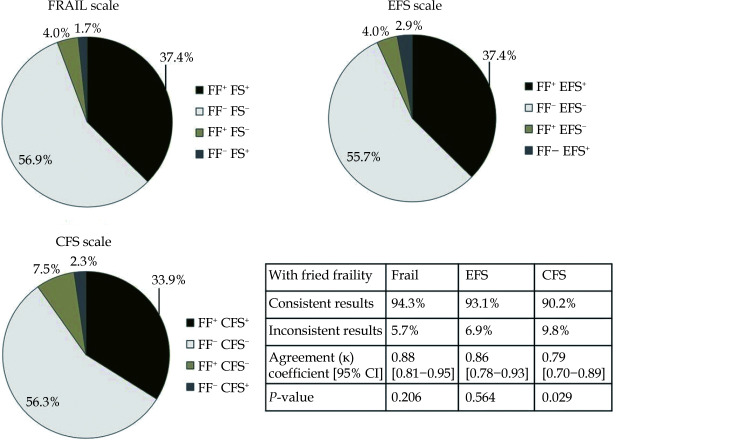
Figure 1 shows the differences in frailty detection between the FRAIL scale, EFS and CFS compared with the Fried frailty scale as a reference standard. Of the 174 patients, frailty was identified in 72 (41.4%) by the Fried frailty scale, 70 (40.2%) by the EFS, 68 (39.1%) by the FRAIL scale, and 63 (36.3%) by the CFS. The agreement coefficients were high for each scale and were 0.88, 0.86, 0.79 for the FRAIL scale, EFS and CFS, respectively. McNemar’s test showed that frailty was statistically significantly more often identified using the Fried frailty scale compared to the CFS (P = 0.029). In contrast, there were no statistically significant differences between the Fried frailty scale compared and the FRAIL scale (P = 0.206) or EFS (P = 0.564).
Figure 1.
Comparison of the scales abilities to detect frailty.
The Fried frailty scale is used as a reference standard (total number of frail patients: 72 [41.4%]). CFS: Clinical Frailty Scale; EFS: Edmonton Frail Scale; FF: Fried frailty scale; FS: FRAIL scale. "+" frailty occurred, “−” frailty not occurred.
Association of Frailty with All-cause Mortality and Unscheduled Rehospitalization
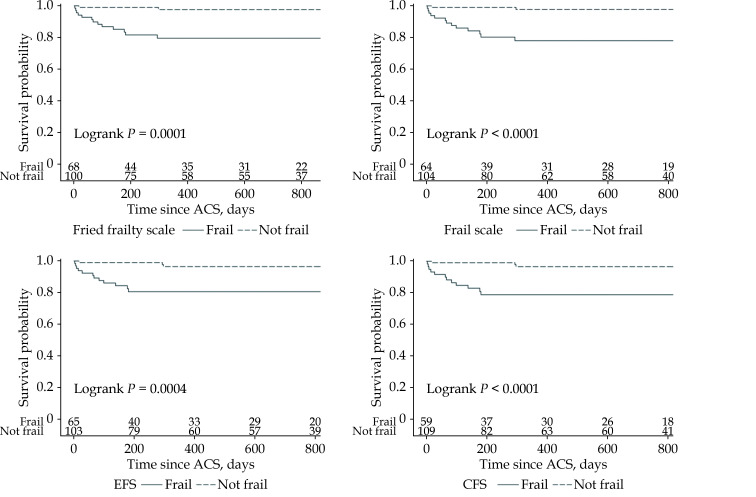
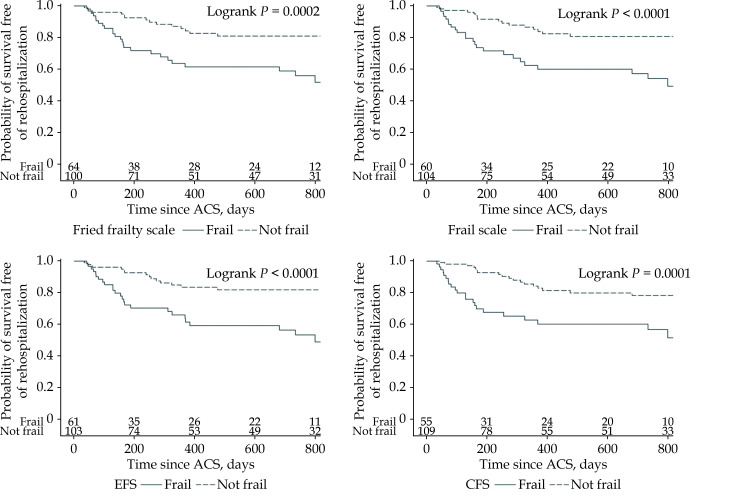
Patients were followed up by telephone consultation. The follow-up period ranged from 80–945 days and the median follow-up time was 637.5 days. Six patients were lost prior to follow-up. During the follow up period, 15 patients died (8 patients in hospital) and 41 patients had an unscheduled hospitalization. Frailty was associated with all-cause mortality regardless of the scale used and the association was demonstrated in both the univariate and multivariate Cox regression models. In both analyses, the FRAIL scale had the highest HR (univariate analysis: HR = 10.5, 95% CI: 2.4–46.8, P < 0.001 for the Fried frailty scale; 12.0, 2.7–53.4, P < 0.001 for the FRAIL scale; 7.1, 2.0–25.2, P < 0.001 for the EFS; 8.3, 2.4–29.6, P < 0.001 for the CFS; multivariate analysis: HR = 5.1, 95% CI: 1.1–23.8, P = 0.037 for the Fried frailty scale; 5.7, 1.2–26.8, P = 0.027 for the FRAIL scale; 3.7, 1.0–14.0, P = 0.05 for the EFS; 4.2, 1.1–15.9, P = 0.033 for the CFS). Other independent predictors were a low LVEF assessed during hospitalization and a low Hgb level on admission to hospital (Table 3, Figure 2). In the univariate Cox regression model, frailty was associated with an increased risk of unscheduled rehospitalization regardless of the scale used (HR = 3.2, 95% CI: 1.7–6.0, P < 0.001 for the Fried frailty scale; 3.4, 1.8–6.3, P < 0.001 for the FRAIL scale; 3.5, 1.8–6.6, P < 0.001 for the EFS; 3.1, 1.7–5.8, P < 0.001 for the CFS). Multivariate analysis showed that frailty assessed only with the EFS was an independent predictor of unscheduled rehospitalization (HR = 2.2, 95% CI: 1.1–4.5, P = 0.031). Other independent predictors were age and CHF with mildly reduced and reduced EF (< 50%;Table 3, Figure 3).
Table 3. Impact of frailty on unscheduled rehospitalization and all-cause death as assessed by the Cox regression model.
| Frail
group |
Non-frail
group |
Univariate | Multivariate | |||||
| Frailty: HR (95% CI)
|
P value | Frailty: HR (95% CI)
|
P value | Other significant predictors, HR [95% CI], P-value, Harrell’s C-index [95% CI] | ||||
| CHF with EF < 50%: congestive heart failure with ejection fraction < 50%; CHF: congestive heart failure; EF: ejection fraction; HR: hazard ratio; Hgb: hemoglobin. | ||||||||
| All-cause Death | ||||||||
| Fried frailty scale
N = 68, n = 100 |
13 (19.1%) | 2 (2.0%) | 10.5 [2.4−46.8] | < 0.001 | 5.1 [1.1−23.8] | 0.037 | EF: 0.94 [0.90−0.98]; P = 0.009HgB: 0.67 [0.51−0.88]; P = 0.005Harrell’s C-index: 0.86 [0.80−0.92] | |
| FRAIL scale
N = 64, n = 104 |
13 (20.3%) | 2 (1.9%) | 12.0 [2.7−53.4] | < 0.001 | 5.7 [1.2−26.8] | 0.027 | EF: 0.94 [0.90; 0.99]; P = 0.013HgB: 0.68 [0.51−0.90]; P = 0.007Harrell’s C-index: 0.87 [0.81−0.92] | |
| Edmonton frail scale
N = 65, n = 103 |
12 (18.5%) | 3 (2.9%) | 7.1 [2.0−25.2] | < 0.001 | 3.7 [1.0−14.0] | 0.050 | EF: 0.94 [0.90−0.98], P = 0.004HgB: 0.67 [0.51−0.88], P = 0.005Harrell’s C-index: 0.87 [0.82−0.93] | |
| Clinical frailty scale
N = 59, n = 109 |
12 (20.3%) | 3 (2.75%) | 8.3 [2.4−29.6] | < 0.001 | 4.2 [1.1−15.9] | 0.033 | EF: 0.94 [0.90−0.98], P = 0.005HgB: 0.68 [0.51−0.90], P = 0.007Harrell’s C-index: 0.87 [0.82−0.93] | |
| Unscheduled rehospitalization | ||||||||
| Fried frailty scale
N = 64, n = 100 |
26 (40.6%) | 15 (15.0%) | 3.2 [1.7−6.0] | < 0.001 | - | - | Age: 1.07 [1.03−1.12], P < 0.001CHF with EF < 50%: 3.68 [1.73−7.82], P < 0.001Harrell’s C-index: 0.70 [0.62−0.79] | |
| FRAIL scale
N = 60, n = 104 |
25 (41.7%) | 16 (15.4%) | 3.4 [1.8−6.3] | < 0.001 | - | - | Age: 1.07 [1.03−1.12], P < 0.001CHF with EF < 50%: 3.7 [1.8−7.9], P < 0.001Harrell’s C-index: 0.70 [0.62−0.80] | |
| Edmonton Frail Scale
N = 61, n = 103 |
26 (41.6%) | 15 (14.6%) | 3.5 [1.8−6.6] | < 0.001 | 2.2 [1.10−4.5] | 0.031 | Age: 1.05 [1.01−1.09], P = 0.026CHF with EF < 50%: 3.3 [1.5−7.0], P = 0.002Harrell’s C-index: 0.74 [0.67−0.81] | |
| Clinical Frailty Scale
N = 55, n = 109 |
23 (41.8%) | 18 (16.5%) | 3.1 [1.7−5.8] | < 0.001 | - | - | Age: 1.07 [1.03−1.12], P < 0.001CHF with EF < 50%: 3.7 [1.7−7.9], P < 0.001Harrell’s C-index: 0.70 [0.62−0.79] | |
Figure 2.
Kaplan–Meier curves.
All-cause mortality according to frailty status. ACS: acute coronary syndrome; CFS: Clinical Frailty Scale; EFS: Edmonton Frail Scale.
Figure 3.
Kaplan–Meier curves. Survival free of unscheduled rehospitalization according to frailty status.
ACS: acute coronary syndrome; CFS: Clinical Frailty Scale; EFS: Edmonton Frail Scale.
DISCUSSION
The present study confirmed a high prevalence of frailty in elderly patients with ACS, which varied from 36.3–41.4% depending on the scale used. High agreement coefficients denoted the high concordance in frailty identification between the Fried frailty scale (our reference scale) and the FRAIL scale, the EFS, and the CFS. At the same time, McNemar’s test showed that frailty was significantly more often identified using the Fried frailty scale compared to the CFS (but not compared to the FRAIL scale or the EFS), indicating that the CFS may underestimate frailty in elderly patients hospitalized in a CCU with ACS. Our study also showed that frailty, regardless of the scale used, was associated with an increased risk of rehospitalization or mortality during the follow-up period. Frailty was associated with all-cause mortality and the association was demonstrated in the univariate and multivariate Cox regression models. In both analyses, the FRAIL scale had the highest HR and it turned out to be the scale that best correlated with mortality in elderly patients hospitalized in a CCU with ACS. Unscheduled rehospitalization occurred 3.1–3.5 times (depending on the scale used) more often in patients with frailty. However, multivariate analysis showed that frailty assessed only with the EFS was an independent predictor of unscheduled rehospitalization.
There are two principal models of frailty: the phenotype model and the cumulative deficit model. The phenotype model was established by Fried and colleagues and considers frailty as poor physical functioning. Frailty criteria are included in the Fried frailty scale and it’s the most common frailty screening tool used worldwide, with multidomain evaluation and strong evidence of frailty identification. For these reasons, we chose this scale as a reference standard.[8,9] The Fried frailty scale requires an interview and physical testing; therefore, it can only be conducted in stable, mobile patients. This scale has strong evidence for usefulness in patients with ACS. Frailty assessed by this scale is strongly associated with long-term mortality and an increased risk of MI in elderly patients with CAD, ACS, or in those who underwent PCI.[11,21-25] The cumulative deficit model describes frailty as an accumulation of deficits, including symptoms, signs, abnormal laboratory values, comorbidities and disabilities. On the basis of this conception of frailty, the EFS,[16] the FRAIL scale,[18] and the CFS[20] were established. The CFS and the FRAIL scale are simple tools, based only on interviews without physical testing, and both are easy and fast to perform. The FRAIL scale is comprised of five items and contains multidomain evaluation, including for concomitant illnesses. The FRAIL scale independently predicts in-hospital, short-term and long-term outcomes (mortality and re-admission) in older patients with ACS.[19,26,27] The CFS is a 7-point scale (however, at present, a 9-point scale is more commonly used) and the level of frailty can be assessed by a few, simple questions, according to the description provided for every level. The identification of the category is based on the judgment of the physician; thus, there is a component of subjective consideration. Frailty assessed by the CFS is strongly and independently associated with in-hospital, 1-month and 1-year mortality, increased length of stay in hospital and unscheduled rehospitalization in elderly patients with ACS.[28-33] The EFS is a moderately complex multidimensional scale, which is based on a series of basic questions and tasks. Similar to the Fried frailty scale, the EFS requires an interview and physical testing, and can only be conducted in stable, mobile patients. Frailty assessed by the EFS is a strong and independent prognostic factor for mid-term and long-term mortality in elderly patients presenting with ACS.[23,34,35] A comparison of the frailty assessment tools is presented in Table 4.
Table 4. Comparison of the frailty assessment tools.[14].
| Evidence for
usefulness in ACS |
Physical test | Easy and fast to perform | Objective assessment | Practical in the
cardiac care unit |
|
| ACS: acute coronary syndrome. | |||||
| Fried frailty scale | + | + | - | + | - |
| Edmonton frail scale | + | + | - | + | - |
| Frail scale | + | - | + | + | + |
| Clinical frailty scale | + | - | + | - | + |
Frail patients represent a group at high risk of mortality, morbidity and medical complications. Thus, recent studies and reviews have highlighted the importance of frailty assessment for better risk stratification in elderly patients with ACS. Knowledge of a patient’s frailty status gives valuable information that may be useful in decision making regarding treatment strategy. At the same time, current studies are limited. Most clinical trials exclude this population; therefore, there are no guidelines from randomized controlled trials (RCTs) as to how and when frailty should be assessed, what scales are preferable, and how a frailty diagnosis should impact treatment (revascularization strategy, pharmacological treatment, rehabilitation).[11,13,14,36] Recommendations from the Geriatric Cardiology Section of the Spanish Society of Cardiology suggest the FRAIL scale or CFS to assess frailty in the acute phase of ACS, as they are simple and rapid to complete tools. By contrast, after the acute phase of ACS (24–48 hours after ACS onset), they suggest a more complete frailty assessment, with more extensive tools (e.g., the Fried frailty scale or the EFS).[14] Furthermore, Walker, et al.[36], in a paper from the Acute Cardiovascular Care Association, recommend the CFS as a generally useful tool to assess frailty at the time of an acute cardiovascular admission because of its practical use in the acute settings; however, the authors do not specify which tool is most useful in ACS patients.
In the current study, the adjusted HR of the FRAIL scale for all-cause mortality was the highest among the scales used, whereas the EFS turned out to be an independent predictor of unscheduled rehospitalization. These data should be taken into consideration when choosing a frailty assessment tool in elderly patients hospitalized in a CCU with ACS. Considering mortality as the most important factor, the FRAIL scale seems to be the most prognostically valuable scale, and we suggest this scale should be favored in elderly (≥ 65 years) patients hospitalized in a CCU with ACS. Moreover, our study showed no differences in frailty identification between the FRAIL scale and more comprehensive scales like the Fried frailty scale and the EFS. Therefore, we suggest the FRAIL scale as a useful tool both during and after the acute phase of ACS. The FRAIL scale is simple to use and takes less than 2 min to complete. Thus, it might be an easy and practical tool for everyday use in a CCU.
LIMITATIONS
The current study has several limitations. First, it was a single-center, non-randomized study with a relatively small number of patients. In addition, there is no consensus on the optimal frailty assessment tool in patients with ACS. We chose to compare 4 frailty scales. The Fried frailty scale is one of the most common frailty screening tools used worldwide, with strong evidence of frailty identification. The EFS, the CFS and the FRAIL scale were chosen for their feasibility and practicality. However, there are several different, valid scales with evidence for usefulness in ACS. Further research, especially a RCT, is needed to indicate the optimal frailty scale for elderly patients with ACS.
CONCLUSIONS
The current study demonstrates that frailty assessed by the Fried frailty scale, the FRAIL scale, the EFS and the CFS is strongly associated with an increased risk of all-cause mortality and unscheduled rehospitalization in an elderly population with ACS. Compared to the other scales, the FRAIL scale, turned out to have the best prognostic value and it may be a favored tool for elderly ACS patients in a CCU. However, the EFS better predicts unscheduled rehospitalization.
SUPPLEMENTARY DATA
Supplementary data to this article can be found online.
Acknowledgments
This study was found by statutory work number 3.19/III/18, National Institute of Cardiology, Warsaw, Poland. All authors had no conflicts of interest to disclose.
References
- 1.World Health Organization. The top 10 causes of death. Published 9 December 2020;https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on October 10, 2021).
- 2.Avezum A, Makdisse M, Spencer F, et al Impact of age on management and outcome of acute coronary syndrome: observations from the Global Registry of Acute Coronary Events (GRACE) Am Heart J. 2005;149:67–73. doi: 10.1016/j.ahj.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 3.Dai XM, Busby-Whitehead J, Alexander KP Acute coronary syndrome in the older adults. J Geriatr Cardiol. 2016;13:101–108. doi: 10.11909/j.issn.1671-5411.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Engberding N, Wenger NK Acute coronary syndromes in the elderly. F1000Res. 2017;6:1791. doi: 10.12688/f1000research.11064.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eurostat (2020). Older people-population overview, July 2020;https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Ageing_Europe_-_statistics_on_population_developments#Older_people_.E2.80.94_population_overview (accessed on October 10, 2021).
- 6.Rich MW, Chyun DA, Skolnick AH, et al Knowledge gaps in cardiovascular care of the older adult population: A Scientific Statement From the American Heart Association, American College of Cardiology, and American Geriatrics Society. J Am Coll Cardiol. 2016;67:2419–2440. doi: 10.1016/j.jacc.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Montilla Padilla I, Martín-Asenjo R, Bueno H Management of acute coronary syndromes in geriatric patients. Heart Lung Circ. 2017;26:107–113. doi: 10.1016/j.hlc.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 8.Fried LP, Tangen CM, Walston J, et al Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 9.Clegg A, Young J, Iliffe S, et al. Frailty in elderly people. Lancet 2013; 381: 752-762. Erratum in: Lancet. 2013 Oct 19; 382(9901): 1328.
- 10.Bebb O, Gd Smith F, Clegg A, et al Frailty and acute coronary syndrome: A structured literature review. Eur Heart J Acute Cardiovasc Care. 2018;7:166–175. doi: 10.1177/2048872617700873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tonet E, Pavasini R, Biscaglia S, Campo G Frailty in patients admitted to hospital for acute coronary syndrome: when, how and why? J Geriatr Cardiol. 2019;16:129–137. doi: 10.11909/j.issn.1671-5411.2019.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dou Q, Wang W, Wang H, et al Prognostic value of frailty in elderly patients with acute coronary syndrome: a systematic review and meta-analysis. BMC Geriatr. 2019;19:222. doi: 10.1186/s12877-019-1242-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chung KJNC, Wilkinson C, Veerasamy M, Kunadian V Frailty scores and their utility in older patients with cardiovascular disease. Interv Cardiol. 2021;16:e05. doi: 10.15420/icr.2020.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Díez-Villanueva P, Arizá-Solé A, Vidán MT, et al Recommendations of the geriatric cardiology section of the spanish society of cardiology for the assessment of frailty in elderly patients with heart disease. Rev Esp Cardiol (Engl Ed) 2019;72:63–71. doi: 10.1016/j.recesp.2018.06.015. [DOI] [PubMed] [Google Scholar]
- 15.Collet JP, Thiele H, Barbato E, et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J 2021; 42: 1289-1367. Erratum in: Eur Heart J 2021; 42: 1908. Erratum in: Eur Heart J 2021; 42: 1925.
- 16.Rolfson DB, Majumdar SR, Tsuyuki RT, et al Validity and reliability of the Edmonton Frail Scale. Age Ageing. 2006;35:526–529. doi: 10.1093/ageing/afl041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hilmer SN, Perera V, Mitchell S, et al The assessment of frailty in older people in acute care. Australas J Ageing. 2009;28:182–188. doi: 10.1111/j.1741-6612.2009.00367.x. [DOI] [PubMed] [Google Scholar]
- 18.Morley JE, Malmstrom TK, Miller DK A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African Americans. J Nutr Health Aging. 2012;16:601–608. doi: 10.1007/s12603-012-0084-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alegre O, Formiga F, López-Palop R, et al An easy assessment of frailty at baseline independently predicts prognosis in very elderly patients with acute coronary syndromes. J Am Med Dir Assoc. 2018;19:296–303. doi: 10.1016/j.jamda.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 20.Rockwood K, Song X, MacKnight C, et al A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh M, Rihal CS, Lennon RJ, et al Influence of frailty and health status on outcomes in patients with coronary disease undergoing percutaneous revascularization. Circ Cardiovasc Qual Outcomes. 2011;4:496–502. doi: 10.1161/CIRCOUTCOMES.111.961375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanchis J, Bonanad C, Ruiz V, et al Frailty and other geriatric conditions for risk stratification of older patients with acute coronary syndrome. Am Heart J. 2014;168:784–791. doi: 10.1016/j.ahj.2014.07.022. [DOI] [PubMed] [Google Scholar]
- 23.Qayyum S, Rossington JA, Chelliah R, et al Prospective cohort study of elderly patients with coronary artery disease: impact of frailty on quality of life and outcome. Open Heart. 2020;7:e001314. doi: 10.1136/openhrt-2020-001314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.White HD, Westerhout CM, Alexander KP, et al Frailty is associated with worse outcomes in non-ST-segment elevation acute coronary syndromes: Insights from the TaRgeted platelet Inhibition to cLarify the Optimal strateGy to medicallY manage Acute Coronary Syndromes (TRILOGY ACS) trial. Eur Heart J Acute Cardiovasc Care. 2016;5:231–242. doi: 10.1177/2048872615581502. [DOI] [PubMed] [Google Scholar]
- 25.Batty J, Qiu W, Gu S, et al One-year clinical outcomes in older patients with non-ST elevation acute coronary syndrome undergoing coronary angiography: An analysis of the ICON1 study. Int J Cardiol. 2019;274:45–51. doi: 10.1016/j.ijcard.2018.09.086. [DOI] [PubMed] [Google Scholar]
- 26.Rodríguez-Queraltó O, Formiga F, López-Palop R, et al Frail scale also predicts long-term outcomes in older patients with acute coronary syndromes. J Am Med Dir Assoc. 2020;21:683–687.e1. doi: 10.1016/j.jamda.2019.10.007. [DOI] [PubMed] [Google Scholar]
- 27.Calvo E, Teruel L, Rosenfeld L, et al Frailty in elderly patients undergoing primary percutaneous coronary intervention. Eur J Cardiovasc Nurs. 2019;18:132–139. doi: 10.1177/1474515118796836. [DOI] [PubMed] [Google Scholar]
- 28.Ekerstad N, Swahn E, Janzon M, et al Frailty is independently associated with short-term outcomes for elderly patients with non-ST-segment elevation myocardial infarction. Circulation. 2011;124:2397–2404. doi: 10.1161/CIRCULATIONAHA.111.025452. [DOI] [PubMed] [Google Scholar]
- 29.Ekerstad N, Swahn E, Janzon M, et al Frailty is independently associated with 1-year mortality for elderly patients with non-ST-segment elevation myocardial infarction. Eur J Prev Cardiol. 2014;21:1216–1224. doi: 10.1177/2047487313490257. [DOI] [PubMed] [Google Scholar]
- 30.Kang L, Zhang SY, Zhu WL, et al. Is frailty associated with short-term outcomes for elderly patients with acute coronary syndrome? J Geriatr Cardiol 2015; 12: 662-667.
- 31.Sujino Y, Tanno J, Nakano S, et al Impact of hypoalbuminemia, frailty, and body mass index on early prognosis in older patients (≥ 85 years) with ST-elevation myocardial infarction. J Cardiol. 2015;66:263–268. doi: 10.1016/j.jjcc.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 32.Murali-Krishnan R, Iqbal J, Rowe R, et al Impact of frailty on outcomes after percutaneous coronary intervention: a prospective cohort study. Open Heart. 2015;2:e000294. doi: 10.1136/openhrt-2015-000294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoshioka N, Takagi K, Morita Y, et al Impact of the clinical frailty scale on mid-term mortality in patients with ST-elevated myocardial infarction. Int J Cardiol Heart Vasc. 2019;22:192–198. doi: 10.1016/j.ijcha.2019.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Graham MM, Galbraith PD, O'Neill D, et al Frailty and outcome in elderly patients with acute coronary syndrome. Can J Cardiol. 2013;29:1610–1615. doi: 10.1016/j.cjca.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 35.Blanco S, Ferrières J, Bongard V, et al Prognosis impact of frailty assessed by the edmonton frail scale in the setting of acute coronary syndrome in the elderly. Can J Cardiol. 2017;33:933–939. doi: 10.1016/j.cjca.2017.03.026. [DOI] [PubMed] [Google Scholar]
- 36.Walker DM, Gale CP, Lip G, et al Editor’s Choice-Frailty and the management of patients with acute cardiovascular disease: A position paper from the Acute Cardiovascular Care Association. Eur Heart J Acute Cardiovasc Care. 2018;7:176–193. doi: 10.1177/2048872618758931. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data to this article can be found online.