The coronavirus disease-19 (COVID-19) pandemic has dramatically burdened healthcare systems worldwide by creating new public health priorities. In addition to the most prevalent presentation of COVID-19 with mild to moderate respiratory symptoms, which occasionally progresses to acute respiratory distress syndrome, cardiovascular complications of COVID-19 include arterial and venous thromboembolic events (pulmonary embolism, acute myocardial infarction (MI) and ischemic stroke), myocarditis/myocardial injury, type 2 MI, takotsubo cardiomyopathy, heart failure, arrhythmias and sudden cardiac death.[1,2] By producing such effects, COVID-19 may exacerbate pre-existing chronic cardiovascular diseases or precipitate the onset of acute cardiac complications in previously apparently healthy individuals.[1]
Respiratory and other viral infections increase the risk of MI in the short, intermediate, and long term. Enhanced inflammatory activity in atheromatous coronary plaques coupled with the infection-associated prothrombotic state increase the risk of coronary atherothrombosis three to six times during the week after laboratory-confirmed infection with influenza and other respiratory viruses.[3] Paradoxically, a significant reduction in hospital admissions for all types of acute coronary syndrome (ACS) during the first wave of COVID-19 pandemic has been reported by multiple single- and multi-center studies, and analysis of national data registries.[4-12]
The processes of aging and senescence are accompanied with decreased protective mechanisms and increased frailty of body functions, including the cardiovascular system and reduced efficiency of thrombolysis.[13-15] Aging probably represents the most detrimental risk factor for cardiovascular system dysfunction.[16] At the same time, age is associated with a greater prevalence of cardiovascular risk factors, and individuals with hypertension, diabetes, and obesity have proved more susceptible to severe COVID-19 disease.[1,2,17-20] Therefore, it is not surprising that clinical experience during the pandemic clearly showed that elderly patients with underlying structural heart disease represent the highest risk subgroup for severe COVID-19 disease, its complications and death.[19-22] However, the impact of COVID-19 on the elderly population in terms of detection and treatment of ACS is more complex than in other population subgroups because of age-related psychosocial factors, more frequent atypical clinical presentation, and more frequent coexisting comorbidities.
After the early spread in China and East Asian countries, the pandemic in other world regions began by late January 2020, and the number of confirmed cases and deaths in Europe and the USA started to increase since the end of February 2020 rapidly. A lower incidence of ACS, mainly during March and April, compared to 2019 numbers, was observed around the globe: in Europe, India, Latin America and the USA (Figure 1).[4-12] Considering MI subtypes, with rare exceptions,[6] the majority of studies have shown a significant reduction in hospital admissions for ST-segment elevation MI (STEMI)[4,5,7-12] (Figure 1). Several additional studies have confirmed the decline in STEMI cases during the pandemic: 51% in China,[23] 40% in Spain,[24] and 27% in Pakistan.[25] Given the between-study heterogeneity, a more recent meta-analysis revealed an average 25.5% reduction of STEMI admissions.[26] Except in India, with an extreme 83% decrease in STEMIs,[11] the decline in admissions, ranging between 37% and 65%, generally was even more impressive for non-STEMI (NSTEMI) (Figure 1).[4-10,12]
Figure 1.
Change in hospital admissions for subtypes of ACS during the first wave of the COVID-19 pandemic.
The total number of admissions in studies from Austria, England, Germany and Latin America represents ACS and in other studies represents acute MI. Among the European countries, the reduction of all ACS types was somewhat less pronounced in Sweden where the lockdown was not introduced. In Germany, a nonsignificant increase was observed for STEMI. The reduction was generally more pronounced for NSTEMI than STEMI. Adapted from references 4 to 12. ACS: acute coronary syndrome; MI: myocardial infarction; NSTEMI: non-ST-segment elevation myocardial infarction; STEMI: ST-segment elevation myocardial infarction.
The number of admissions commonly began to decline during February 2020, with progressive reduction during March and April, reaching the nadir by late April and early May, usually within a couple of weeks after the introduction of lockdown restrictions (Figure 2). The studies that include data from May 2020 in the analysis revealed a reversed trend with increasing ACS numbers.[5,10,27-30] The UK and the US reports described almost fully restored number of admissions occurring in late June, reaching the pre–pandemic incidence.[29,30] Importantly, the UK study also documented a new fall in admissions, resembling the first one, which accompanied the second pandemic wave in October and the subsequent lockdown in November 2020.[29] In contrast, there were no significant declines in MI in the US during the second (summer 2020) or the third (winter 2020) pandemic wave.[30]
Figure 2.
Factors influencing the number of hospital admissions for ACS during the first pandemic wave.
A decline in admissions during the early phase of COVID-19 pandemic was mainly driven by fear of the infection followed by disturbed function of healthcare system, redistribution of cardiology resources, and several factors associated with challenging diagnostics and unrecognized or untimely treated ACS. Most of these factors more intensively have affected elderly population. While the impact of daily life triggers is questionable in the early pandemic stage, a lower exposure to the triggers in countries where the lockdown was introduced probably contributed to the nadir of admissions. Gradual weakening of public restrictions with restored trigger exposure, newly established triggers and attenuation of the early phase factors probably contributed to a reversed trend of reported coronary events in late April and May. Adapted according to references 5, 10, 12, 28 and 29. ACS: acute coronary syndrome.
The fluctuation in hospital admissions for ACS associated with the first wave of COVID-19 is most likely multifactorial and caused by several pandemic-related factors, primarily including fear of the infection, difficulties in reaching a timely diagnosis, and reduced exposure to ACS triggers caused by the anti-pandemic public health measures and lifestyle changes (Figure 2). Many of those factors more intensively affect the elderly, and a significant portion of the “missing” ACSs could have been “lost” in this population subgroup.
As the reduction in ACS admissions began during the early pandemic when infection and fatality rates were low, fear of acquiring the infection has been proposed as a major contributor to the phenomenon.[5-7,10-12,23] Fear, anxiety and distress accompany natural psychological responses to the randomly changing condition. The fear of getting COVID-19 was occasionally enhanced by media-released misinformation during the initial pandemic period.[31] The fear of not receiving proper treatment or the desire to not overload the emergency service were also present. This perception combined with guidance on social distancing and sometimes inaccurate or misleading recommendations to avoid hospitals unless suffering from severe symptoms[31] could have led patients not only to avoid regular check-ups, but also to delay or completely avoid seeking care.
The consequences of fear may well be reflected in the study involving 51 New York State hospitals and reporting a 43% decrease in PCI procedures/week for STEMI in counties with high-density COVID-19 spreading and only a 4% decrease in counties with low-density spreading.[32] The second UK fall in MI hospitalizations, resembling the first wave, started before the new October/November lockdown, and has also been linked chiefly to public fear of hospitals.[29]
Several studies reported that patients with MI hospitalized during the COVID-19 period, compared to the pre-COVID-19 period, were one to three years younger supporting the notion that older patients are more reluctant to seek medical attention.[5,6,28] The reduction of hospital admission for MI was particularly conspicuous among those over 80 years of age.[10] A plausible explanation is that older individuals with multiple serious preexisting comorbidities are aware of their vulnerability to severe disease and worse outcomes in case of contracting COVID-19.[31,33] In addition to this understandable reasoning, the process of aging also may hamper cognitive skills and flexibility, and the ability to process a number of frightening and less understood information. Many elderly patients do not have biological, social, material and informatic resources to deal with the stress associated with COVID-19. Finally, frail and cognitively impaired individuals are probably the most affected by such tendencies.
The adverse effect of social isolation on well-being in individuals of all ages is especially pronounced in older adults.[34,35] Social isolation often results in loneliness, a factor significantly associated with depression in elderly adults.[36] Loneliness, isolation, and depression have been linked to worse disease outcomes and mortality in older populations, particularly in those over 65 years of age regardless of disease progression or severity.[37,38]
The first pandemic wave has been characterized by a more than two-fold increase in incidence of out-of-hospital cardiac arrest (OHCA) and a significantly prolonged time from call to ambulance arrival.[39] Although most OHCA events have occurred at home, there has been a significantly higher frequency of unwitnessed OHCA.[39] Strict self-quarantine measures and isolation could have forced the vulnerable elderly population to live in different areas at home away from family members and isolated from their visits.[40] Even when the onset of ACS or an OHCA event has occurred at home in the presence of family members, we may assume that, at least in some cases, they could have been reluctant to call the ambulance or to perform CPR assigning the event to COVID-19 and fearing of further spread of the infection. While direct COVID-19 deaths account for a proportion of increased deaths at home, a portion was undoubtedly due to ACS cases in which delayed seeking of help, avoiding to do so, or avoiding to perform CPR was caused by fear of the infection.[39]
Age seems to be one of the most important predictors of atypical presentation of ACS.[41-44] Accordingly, a relatively high incidence of unrecognized MIs in patients aged 55 and older[45] could be explained by a tendency toward atypical MI presentation with increasing age.[46] Most importantly, even before the COVID-19 pandemic, it has been well-documented that patients with atypical MI presentation are at increased risk for delays in seeking medical attention, less aggressive treatments, and in-hospital mortality.[42,44]
A body of evidence strongly suggests that patients with NSTEMI, compared to those with STEMI, are older, more often have comorbidities and almost 2-times more often to have an atypical presentation at the onset.[42,47,48] This fact has led to the hypothesis that they could have been more reluctant to seek help and could more easily be misdiagnosed as COVID-19 leading to a greater number of “missing” NSTEMIs.[5,49] In contrast, STEMIs are more typical in symptom presentation and electrocardiographic changes, so we may assume that STEMI patients have been more accurately recognized and treated. Even among STEMI patients, hospital mortality is still significantly higher in those without than in those with typical chest pain.[42] However, in a group of older patients aged 69 years on average, Baldi, et al.[50] have reported that they voluntarily delay the call to emergency service despite typical MI symptoms, i.e., the time from symptoms to call nearly tripled during the pandemic compared to non-pandemic periods.
A linkage among the older age, NSTEMI, and atypical symptom presentation, could have contributed to the reduced hospital admissions. This linkage could have underlaid a portion of unrecognized NSTEMIs where a delay in seeking help and untimely or inappropriate treatment has caused the development of OHCA. This possibility has been further corroborated by the observation that the incidence of OHCA among those presenting with MI markedly increased following implementation of the lockdown and social distancing measures.[51] The incidence of such cases during the first pandemic wave almost doubled the pre-pandemic incidence and patients presenting with OHCA were more likely to be older, women or of Asian origin.[51]
Tropism and interaction of COVID-19 virus with ACE2 receptors and the renin–angiotensin–aldosterone system can enhance systemic inflammatory response involving the heart.[1,2] Early at the beginning of the pandemic it was established that COVID-19 patients often have myocardial injury characterized by increased troponin levels.[1,2,52,53] Patients with myocardial injury also have a high incidence of concomitant elevation of D-dimer levels, markedly elevated inflammatory biomarkers,[1,2] more common complications including acute respiratory distress syndrome, coagulation disorders, acute kidney injury, electrolyte disturbances and a twofold to fourfold higher mortality.[54,55] Furthermore, patients with myocardial injury are on average 10 to 15 years older than those without cardiac injury[54,55] and patients over 60 years of age with COVID-19 are more likely to suffer from an acute myocardial injury in case of a pre-existing cardiovascular disease.[56] However, elderly patients are significantly more susceptible to myocardial injury, regardless of the presence of previous cardiovascular disease, particularly those over 75 years of age.[56,57]
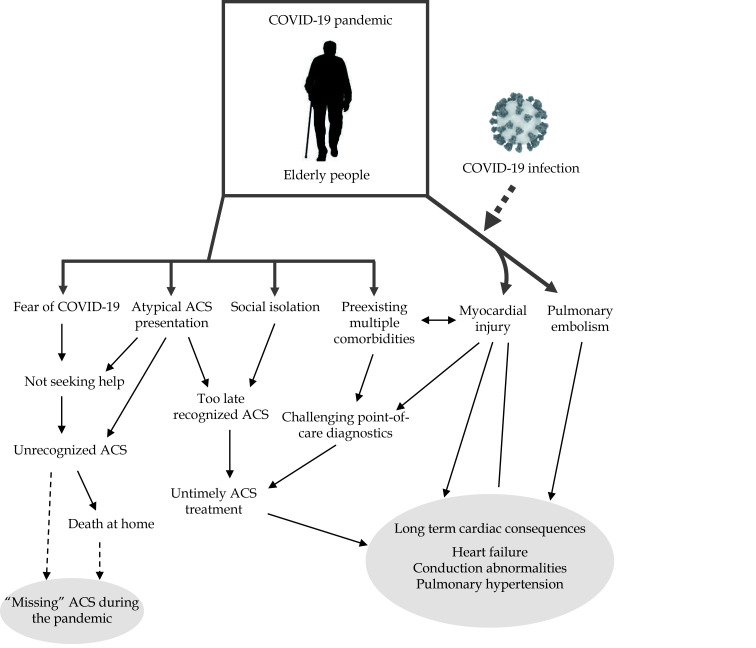
Elderly patients with coexisting COVID-19 disease and NSTEMI represent a subgroup with particularly challenging point-of-care diagnostics (Figure 3). Due to a greater likelihood for less typical symptoms and NSTEMI, more frequent pre-existing cardiac disease, and expected troponin rise with the COVID-19 infection, it is likely that this situation in the elderly could be more often misinterpreted as being exclusively related to COVID-19 disease. Evolving and sometimes conflicting guidance about the treatment of patients having an ACS with or without OHCA during the first pandemic wave could have also burdened point-of-care diagnostics and prompt treatment. In this line, those hospitalized with MI after OHCA had longer delays to emergency reperfusion, were less likely to receive invasive coronary angiography and specialist care, and had higher in-hospital mortality.[51]
Figure 3.
Factors associated with a specific impact of the COVID-19 pandemic in the elderly population.
Multiple factors could have been responsible for more frequent “missing” ACS during the first pandemic wave among the elderly. In addition, long-term consequences and sequelae of the COVID-19 disease will also be more severe with increasing age. ACS: acute coronary syndromes.
During the pandemic, several factors associated with the functioning and organization of healthcare systems additionally hampered the timely recognition and treatment of ACS patients. Frequent emergency calls overwhelmed and disturbed the regular running of emergency services whose personnel were busy transporting COVID-19 patients which often resulted in delayed transport and treatment of MI patients.[7,49] In addition to fear of the infection, the stay-at-home order has strongly influenced decision to call for help for medical emergencies.[8,58,59] The prolongation of time from call to ambulance arrival in OHCA cases supports the possibility that a number of “missing” ACSs were unrecognized at the time and ended up as OHCA.[26]
A diversion of resources caused by altered urgent pathology has reduced non-urgent cardiologic procedures, including elective coronarography, valvuloplasty, percutaneous aortic valve implantation, atrial septal defect closure and other interventions has dropped between 50% and 90% worldwide.[9,24] In some elderly patients, postponing the procedure could have resulted in the progression of cardiac disease and could have produced a greater vulnerability to more severe COVID-19 disease. A weakening of the first pandemic wave in June 2020 and smaller number of COVID-19 patients allowed healthcare systems to consolidate gradually. The return to a full scale of the primary function of cardiology personnel and facilities undoubtedly has contributed to better detection and treatment of ACS patients.
Although the lockdown has varied between countries in duration and measures imposed, a substantial reduction of individual, public and economic activities during the lockdown, some of which may act as triggers of ACS,[60,61] is the only factor that could have actually reduced the occurrence of ACS events and enhanced the nadir of ACS admissions (Figure 2). About 6% of all MIs may be assigned to physical exertion[60] and an additional 4% to moderate physical activity.[62] During the lockdown, fitness centers and other locations for physical activities in many countries were temporarily closed and people were requested to stay at home in self-quarantine. Regular physical activity among older adults was also hampered by the cancellation of group-based exercise classes and because participants were reluctant to participate in group sessions for fear of contracting the infection.[63] A body of evidence suggests a significant decrease in physical activities of all types among middle-aged and older adults during the pandemic.[64-68] During the lockdown, public and international transport was suspended or extremely reduced, and the non-commuting work from home probably has also reduced work-related stress, an additional well-recognized MI trigger.[60]
Gradual alleviation of the restrictions by the end of the pandemic waves and after the lockdowns restored the population exposure to the triggers, probably contributing to the post nadir rise in ACSs during the first wave (Figure 2). However, a reduced triggering of ACS by physical activities could have been counterbalanced by adverse effects of social distancing, uncertainty regarding the future, loneliness, loss of job, financial stress, and fear of contacting COVID-19, which have already become additional important MI triggers.[69]
Healthcare providers should further raise awareness of ACS symptoms and encourage healthcare-seeking behavior. This should reduce the number of unrecognized cases, delays in action, and consequent unnecessary OHCA events in all population subgroups, especially in the elderly. For example, the UK national campaign to encourage patients experiencing symptoms of ACS to call an ambulance promptly[70] might have contributed to the recovery in hospital admissions by the end of May 2020.[5] Healthy behavior, particularly diet and recreational habits at home, should be promoted during the current unusual pandemic circumstances to reduce the overall risk of ACS. This is of utmost importance because the decrease in physical activity during the pandemic was associated with changes toward a sedentary lifestyle, and accompanied by an increase in food and alcohol consumption, body weight, anxiety, depression, general stress levels, and poor quality of sleep.[64,65,68,71]
There is a consensus that people aged 65 years or older and upper-risk people below age 65 represent high-risk groups that should be offered vaccination with priority. Based on a review of the literature, Ioannidis provided estimates of the COVID-19 infection case fatality rate by risk group, ranging from 1% in people over 65 years of age, 2% in those over 75 years of age to 25% in institutionalized frail elderly.[72] However, fully vaccinated adults over 65 years of age are 94%, and those partially vaccinated 64% less likely to be hospitalized with COVID-19 than unvaccinated people of the same age.[73] Accordingly, there are big hopes that the adverse effects of the pandemic in the high-risk older populations can be largely overcome by achieving timely completion of vaccination. Vaccinated older people could be less reluctant to seek help in the presence of ACS symptoms, and if infected, they should have less severe COVID-19 disease with a lesser potential to activate preexisting coronary disease.
The use of statin therapy is a conditio sine qua non for the treatment of patients with known coronary artery disease.[74,75] Studies on statin use and outcomes in patients with COVID-19 have produced conflicting results.[76-80] The overall benefit of statins in older patients should be carefully weighted, and possible options include continuation with lower doses or discontinuation by switching into other lipid-lowering management.[81]
The question of long-term cardiovascular consequences is particularly intriguing (Figure 3). COVID-19 is a predominantly respiratory virus with a tendency for myocardial injury and thromboembolic incidents, particularly pulmonary embolism, through which the infection may compromise both left and right heart function. In addition to recognized and unrecognized ACSs, the sequelae of COVID-19 disease probably will substantially increase the incidence of heart failure, conduction disturbances, and pulmonary hypertension, particularly in the elderly population (Figure 3).
Fear of acquiring the infection and lockdown measures favor physical inactivity, sedentary behavior, weight gain and adverse effects on the psycho-emotional well-being in the elderly.[64-68,71] Eventually, these trajectories may be expected to independently increase the number of vulnerable coronary patients in the population.[49] Moreover, given the observed decline in physical activity levels with increasing age,[82] now coupled with a further decline during the pandemic, the possibility that older adults will never reach the pre-pandemic activity levels is particularly worrisome.[66]
The future pandemic trends and transmission dynamics process will be determined by multiple regional socioeconomic, lifestyle and cultural factors, and counter-pandemic public health measures. In any scenario, the elderly will be the most severely affected. Every possible measure should be undertaken to provide better protection for the highly vulnerable elderly population during the pandemic, particularly the timely diagnostics and treatment of ACS.
Disclosures
The authors have no conflicts of interest to disclose.
References
- 1.Azevedo RB, Botelho BG, Hollanda JVG, et al COVID-19 and the cardiovascular system: a comprehensive review. J Hum Hypertens. 2021;35:4–11. doi: 10.1038/s41371-020-0387-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonow RO, O’Gara PT, Yancy CW Cardiology and COVID-19. JAMA. 2020;324:1131–1132. doi: 10.1001/jama.2020.15088. [DOI] [PubMed] [Google Scholar]
- 3.Musher DM, Abers MS, Corrales-Medina VF Acute infection and myocardial infarction. N Engl J Med. 2019;380:171–176. doi: 10.1056/NEJMra1808137. [DOI] [PubMed] [Google Scholar]
- 4.Metzler B, Siostrzonek P, Binder RK, et al Decline of acute coronary syndrome admissions in Austria since the outbreak of COVID-19: the pandemic response causes cardiac collateral damage. Eur Heart J. 2020;41:1852–1853. doi: 10.1093/eurheartj/ehaa314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mafham MM, Spata E, Goldacre R, et al COVID-19 pandemic and admission rates for and management of acute coronary syndromes in England. Lancet. 2020;396:381–389. doi: 10.1016/S0140-6736(20)31356-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rattka M, Baumhardt M, Dreyhaupt J, et al 31 days of COVID-19—cardiac events during restriction of public life—a comparative study. Clin Res Cardiol. 2020;109:1476–1482. doi: 10.1007/s00392-020-01681-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Rosa S, Spaccarotella C, Basso C, et al; Società Italiana di Cardiologia and the CCU Academy investigators group Reduction of hospitalizations for myocardial infarction in Italy in the COVID-19 era. Eur Heart J. 2020;41:2083–2088. doi: 10.1093/eurheartj/ehaa409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Solomon MD, McNulty EJ, Rana JS, et al The Covid-19 pandemic and the incidence of acute myocardial infarction. N Engl J Med. 2020;383:691–693. doi: 10.1056/NEJMc2015630. [DOI] [PubMed] [Google Scholar]
- 9.Mayol J, Artucio C, Batista I, et al; STEMI Working Group of Stent-Save a Life! LATAM and SOLACI (Latin American Society of Interventional Cardiology) An international survey in Latin America on the practice of interventional cardiology during the COVID-19 pandemic, with a particular focus on myocardial infarction. Neth Heart J. 2020;28:424–430. doi: 10.1007/s12471-020-01440-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mesnier J, Cottin Y, Coste P, et al Hospital admissions for acute myocardial infarction before and after lockdown according to regional prevalence of COVID-19 and patient profile in France: a registry study. Lancet Public Health. 2020;5:e536–e542. doi: 10.1016/S2468-2667(20)30188-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meenakshisundaram R, Senthilkumaran S, Thirumalaikolundusubramanian P, et al Status of acute myocardial infarction in Southern India during COVID-19 lockdown: a multicentric study. Mayo Clin Proc Innov Qual Outcomes. 2020;4:506–510. doi: 10.1016/j.mayocpiqo.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mohammad MA, Koul S, Olivecrona GK, et al Incidence and outcome of myocardial infarction treated with percutaneous coronary intervention during COVID-19 pandemic. Heart. 2020;106:1812–1818. doi: 10.1136/heartjnl-2020-317685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soysal P, Arik F, Smith L, Jackson SE, Isik AT Inflammation, frailty and cardiovascular disease. Adv Exp Med Biol. 2020;1216:55–64. doi: 10.1007/978-3-030-33330-0_7. [DOI] [PubMed] [Google Scholar]
- 14.Napoli C, Cacciatore F, Bonaduce D, et al Efficacy of thrombolysis in younger and older adult patients suffering their first acute Q-wave myocardial infarction. J Am Geriatr Soc. 2002;50:343–348. doi: 10.1046/j.1532-5415.2002.50068.x. [DOI] [PubMed] [Google Scholar]
- 15.Abete P, Ferrara N, Cacciatore F, et al. Angina-induced protection against myocardial infarction in adult and elderly patients: a loss of preconditioning mechanism in the aging heart? J Am Coll Cardiol 1997; 30: 947–954.
- 16.Obas V, Vasan RS The aging heart. Clin Sci. 2018;132:1367–1382. doi: 10.1042/CS20171156. [DOI] [PubMed] [Google Scholar]
- 17.Hendren NS, de Lemos JA, Ayers C, et al Association of body mass index and age with morbidity and mortality in patients hospitalized with COVID-19: results from the American Heart Association COVID-19 Cardiovascular Disease Registry. Circulation. 2021;143:135–144. doi: 10.1161/CIRCULATIONAHA.120.051936. [DOI] [PubMed] [Google Scholar]
- 18.Apicella M, Campopiano MC, Mantuano M, et al COVID-19 in people with diabetes: understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol. 2020;8:782–92. doi: 10.1016/S2213-8587(20)30238-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xie J, Tong Z, Guan X, et al Clinical characteristics of patients who died of coronavirus disease 2019 in China. JAMA Netw Open. 2020;3:e205619. doi: 10.1001/jamanetworkopen.2020.5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu Z, McGoogan JM Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 21.Zhou F, Yu T, Fan G, et al Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tomasoni D, Inciardi RM, Lombardi CM, et al Impact of heart failure on the clinical course and outcomes of patients hospitalized for COVID-19. Results of the Cardio-COVID-Italy multicentre study. Eur J Heart Fail. 2020;22:2238–2247. doi: 10.1002/ejhf.2052. [DOI] [PubMed] [Google Scholar]
- 23.Zhang F, Song X, Dang Y Experience of ST segment elevation myocardial infarction management during COVID-19 pandemic from the mainland of China. Cardiovasc Revasc Med. 2021;28:92–94. doi: 10.1016/j.carrev.2020.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodríguez-Leor O, Cid-Álvarez B, Ojeda S, et al Impact of the COVID-19 pandemic on interventional cardiology activity in Spain. REC Interv Cardiol. 2020;2:82–89. [Google Scholar]
- 25.Mengal N, Saghir T, Hassan Rizvi SN, et al. Acute ST-elevation myocardial infarction before and during the COVID-19 pandemic: what is the clinically significant difference? Cureus 2020; 12: e10523.
- 26.Rattka M, Dreyhaupt J, Winsauer C, et al. Effect of the COVID-19 pandemic on mortality of patients with STEMI: a systematic review and meta-analysis. Heart. Published Online First: 17 Dec 2020; doi: 10.1136/heartjnl-2020-318360.
- 27.Fardman A, Oren D, Berkovitch A, et al Post COVID-19 acute myocardial infarction rebound. Can J Cardiol. 2020;36:1832.e15–1832.e16. doi: 10.1016/j.cjca.2020.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gluckman TJ, Wilson MA, Chiu ST, et al Case rates, treatment approaches, and outcomes in acute myocardial infarction during the coronavirus disease 2019 pandemic. JAMA Cardiol. 2020;5:1–6. doi: 10.1001/jamacardio.2020.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu J, Mamas MA, de Belder MA, et al Second decline in admissions with heart failure and myocardial infarction during the COVID-19 pandemic. J Am Coll Cardiol. 2021;77:1141–1143. doi: 10.1016/j.jacc.2020.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Solomon MD, Nguyen-Huynh M, Leong TK, et al Changes in patterns of hospital visits for acute myocardial infarction or ischemic stroke during COVID-19 surges. JAMA. 2021;77:1141–1143. doi: 10.1001/jama.2021.8414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dubey S, Biswas P, Ghosh R, et al Psychosocial impact of COVID-19. Diabetes Metab Syndr. 2020;14:779–788. doi: 10.1016/j.dsx.2020.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hannan EL, Wu Y, Cozzens K, et al Percutaneous coronary intervention for ST-elevation myocardial infarction before and during COVID in New York. Am J Cardiol. 2021;142:25–34. doi: 10.1016/j.amjcard.2020.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Q, Guan X, Wu P, et al Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shankar A, Rafnsson SB, Steptoe A Longitudinal associations between social connections and subjective wellbeing in the English longitudinal study of ageing. Psychol Health. 2015;30:686–698. doi: 10.1080/08870446.2014.979823. [DOI] [PubMed] [Google Scholar]
- 35.Windle G, Woods RT Variations in subjective wellbeing: the mediating role of a psychological resource. Ageing Soc. 2004;24:583–602. doi: 10.1017/S0144686X04002107. [DOI] [Google Scholar]
- 36.Adams KB, Sanders S, Auth EA Loneliness and depression in independent living retirement communities: risk and resilience factors. Aging Ment Health. 2004;8:475–485. doi: 10.1080/13607860410001725054. [DOI] [PubMed] [Google Scholar]
- 37.Tomaka J, Thompson S, Palacios R The relation of social isolation, loneliness, and social support to disease outcomes among the elderly. J Aging Health. 2006;18:359–384. doi: 10.1177/0898264305280993. [DOI] [PubMed] [Google Scholar]
- 38.Blazer DG, Hybels CF, Pieper CF The association of depression and mortality in elderly persons: a case for multiple, independent pathways. J Gerontol A Biol Sci Med Sci. 2001;56:M505–M509. doi: 10.1093/gerona/56.8.M505. [DOI] [PubMed] [Google Scholar]
- 39.Lim ZJ, Ponnapa Reddy M, Afroz A, et al Incidence and outcome of out-of-hospital cardiac arrests in the COVID-19 era: a systematic review and meta-analysis. Resuscitation. 2020;157:248–258. doi: 10.1016/j.resuscitation.2020.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baldi E, Sechi GM, Mare C, et al COVID-19 kills at home: the close relationship between the epidemic and the increase of out-of-hospital cardiac arrests. Eur Heart J. 2020;41:3045–3054. doi: 10.1093/eurheartj/ehaa508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Čulić V, Eterović D, Mirić D, Silić N Symptom presentation of acute myocardial infarction: influence of gender, age and risk factors. Am Heart J. 2002;144:1012–1017. doi: 10.1067/mhj.2002.125625. [DOI] [PubMed] [Google Scholar]
- 42.Canto AJ, Kiefe CI, Goldberg RJ, et al Differences in symptom presentation and hospital mortality according to type of acute myocardial infarction. Am Heart J. 2012;163:572–579. doi: 10.1016/j.ahj.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 43.Čulić V, Mirić D, Eterović D Correlation between symptomatology and site of acute myocardial infarction. Int J Cardiol. 2001;77:163–168. doi: 10.1016/S0167-5273(00)00414-9. [DOI] [PubMed] [Google Scholar]
- 44.Canto JG, Shlipak MG, Rogers WJ, et al Prevalence, clinical characteristics, and mortality among patients with myocardial infarction presenting without chest pain. JAMA. 2000;283:3223–3329. doi: 10.1001/jama.283.24.3223. [DOI] [PubMed] [Google Scholar]
- 45.de Torbal A, Boersma E, Kors JA, et al Incidence of recognized and unrecognized myocardial infarction in men and women aged 55 and older: the Rotterdam Study. Eur Heart J. 2006;27:729–736. doi: 10.1093/eurheartj/ehi707. [DOI] [PubMed] [Google Scholar]
- 46.Čulić V Atypical presentation and unrecognized myocardial infarction. Eur Heart J. 2006;27:2607. doi: 10.1093/eurheartj/ehl286. [DOI] [PubMed] [Google Scholar]
- 47.Fu R, Song CX, Dou KF, et al Differences in symptoms and pre-hospital delay among acute myocardial infarction patients according to ST-segment elevation on electrocardiogram: an analysis of China Acute Myocardial Infarction (CAMI) registry. Chin Med J (Engl) 2019;132:519–524. doi: 10.1097/CM9.0000000000000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Canto JG, Rogers WJ, Goldberg RJ; NRMI Investigators Association of age and sex with myocardial infarction symptom presentation and in-hospital mortality. JAMA. 2012;307:813–822. doi: 10.1001/jama.2012.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Čulić V, AlTurki A, Proietti R Covid-19 pandemic and possible rebound phenomenon in incidence of acute myocardial infarction. Can J Cardiol. 2021;37:1294. doi: 10.1016/j.cjca.2020.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baldi E, Auricchio A, Cresta R, et al Patient voluntarily delays call to emergency medical system for ST-elevation myocardial infarction during COVID-19 pandemic. Int J Cardiol Heart Vasc. 2021;35:100824. doi: 10.1016/j.ijcha.2021.100824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rashid M, Gale CP, Curzen N, et al Impact of coronavirus disease 2019 pandemic on the incidence and management of out-of-hospital cardiac arrest in patients presenting with acute myocardial infarction in England. J Am Heart Assoc. 2020;9:e018379. doi: 10.1161/JAHA.120.018379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alzahrani SH, Al-Rabia MW Cardiac injury biomarkers and the risk of death in patients with COVID-19: a systematic review and meta-analysis. Cardiol Res Pract. 2021;2021:9363569. doi: 10.1155/2021/9363569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.García de Guadiana-Romualdo L, Morell-García D, Rodríguez-Fraga O, et al Cardiac troponin and COVID-19 severity: results from BIOCOVID study. Eur J Clin Invest. 2021;51:e13532. doi: 10.1111/eci.13532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smilowitz NR, Jethani N, Chen J, et al Myocardial injury in adults hospitalized with COVID-19. Circulation. 2020;142:2393–2395. doi: 10.1161/CIRCULATIONAHA.120.050434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shi S, Qin M, Shen B, et al Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wei C, Liu Y, Liu Y, et al Clinical characteristics and manifestations in older patients with COVID-19. BMC Geriatrics. 2020;20:395. doi: 10.1186/s12877-020-01811-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ruan Q, Yang K, Wang W, et al Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goldberg SA, Cash RE, Peters G, et al The impact of COVID-19 on statewide EMS use for cardiac emergencies and stroke in Massachusetts. J Am Coll Emerg Physicians Open. 2021;2:e12351. doi: 10.1002/emp2.12351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wong LE, Hawkins JE, Langness S, et al. Where are all the patients? Addressing Covid-19 fear to encourage sick patients to seek emergency care. https://catal yst.nejm.org/doi/full/10.1056/CAT.20.0193 (accessed on March 7, 2022).
- 60.Nawrot TS, Perez L, Künzli N, et al Public health importance of triggers of myocardial infarction: a comparative risk assessment. Lancet. 2011;377:732–740. doi: 10.1016/S0140-6736(10)62296-9. [DOI] [PubMed] [Google Scholar]
- 61.Čulić V Acute risk factors for myocardial infarction. Int J Cardiol. 2007;117:260–269. doi: 10.1016/j.ijcard.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 62.Čulić V Triggers of myocardial infarction. Lancet. 2011;377:2175. doi: 10.1016/S0140-6736(11)60953-7. [DOI] [PubMed] [Google Scholar]
- 63.Goethals L, Barth N, Guyot J, et al Impact of home quarantine on physical activity among older adults living at home during the COVID-19 pandemic: qualitative interview study. JMIR Aging. 2020;3:e19007. doi: 10.2196/19007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lipert A, Kozłowski R, Timler D, et al Physical activity as a predictor of the level of stress and quality of sleep during COVID-19 lockdown. Int J Environ Res Public Health. 2021;18:5811. doi: 10.3390/ijerph18115811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dobrowolski H, Włodarek D Body mass, physical activity and eating habits changes during the first COVID-19 pandemic lockdown in Poland. Int J Environ Res Public Health. 2021;18:5682. doi: 10.3390/ijerph18115682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Joseph RP, Pituch KA, Guest MA, et al Physical activity among predominantly white middle-aged and older US adults during the SARS-CoV-2 pandemic: results from a national longitudinal survey. Front Public Health. 2021;9:652197. doi: 10.3389/fpubh.2021.652197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schuch FB, Bulzing RA, Meyer J, et al. Moderate to vigorous physical activity and sedentary behavior changes in self-isolating adults during the COVID-19 pandemic in Brazil: a cross-sectional survey exploring correlates. Sport Sci Health 2021: 1–9.
- 68.Di Santo SG, Franchini F, Filiputti B, et al The effects of COVID-19 and quarantine measures on the lifestyles and mental health of people over 60 at increased risk of dementia. Front Psychiatry. 2020;11:578628. doi: 10.3389/fpsyt.2020.578628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hammoudeh A, Abu-Hantash H, Tabbalat R, et al The COVID-19 pandemic and triggered acute myocardial infarction among non-infected individuals. Int J Clin Cardiol. 2020;7:185. [Google Scholar]
- 70.Herz N. Why are thousands fewer people being treated for a heart attack? April 23, 2020. https://www.bhf.org.uk/informationsupport/heart-matters-magazine/news/behind-the-headlines/coronavirus/coronavirus-and-reduction-in-heart-attack-treatment (accessed on June 24, 2021).
- 71.Stanton R, To QG, Khalesi S, et al Depression, anxiety and stress during COVID-19: associations with changes in physical activity, sleep, tobacco and alcohol use in Australian adults. Int J Environ Res Public Health. 2020;17:4065. doi: 10.3390/ijerph17114065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ioannidis JPA Global perspective of COVID-19 epidemiology for a full-cycle pandemic. Eur J Clin Invest. 2020;50:e13423. doi: 10.1111/eci.13423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tenforde MW, Olson SM, Self WH, et al; IVY Network; HAIVEN Investigators Effectiveness of Pfizer-BioNTech and Moderna vaccines against COVID-19 among hospitalized adults aged ≥ 65 years - United States, January-March 2021. MMWR Morb Mortal Wkly Rep. 2021;70:674–679. doi: 10.15585/mmwr.mm7018e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Libby P The changing landscape of atherosclerosis. Nature. 2021;592:524–533. doi: 10.1038/s41586-021-03392-8. [DOI] [PubMed] [Google Scholar]
- 75.Crea F, Libby P Acute coronary syndromes: the way forward from mechanisms to precision treatment. Circulation. 2017;136:1155–1166. doi: 10.1161/CIRCULATIONAHA.117.029870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hippisley-Cox J, Young D, Coupland C, et al Risk of severe COVID-19 disease with ACE inhibitors and angiotensin receptor blockers: cohort study including 8.3 million people. Heart. 2020;106:1503–1511. doi: 10.1136/heartjnl-2020-317393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang XJ, Qin JJ, Cheng X, et al In-hospital use of statins is associated with a reduced risk of mortality among individuals with COVID-19. Cell Metab. 2020;32:176–187. doi: 10.1016/j.cmet.2020.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.An C, Lim H, Kim DW, et al Machine learning prediction for mortality of patients diagnosed with COVID-19: a nationwide Korean cohort study. Sci Rep. 2020;10:18716. doi: 10.1038/s41598-020-75767-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Inciardi RM, Adamo M, Lupi L, et al Characteristics and outcomes of patients hospitalized for COVID-19 and cardiac disease in Northern Italy. Eur Heart J. 2020;41:1821–1829. doi: 10.1093/eurheartj/ehaa388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lohia P, Kapur S, Benjaram S, Mir T Association between antecedent statin use and severe disease outcomes in COVID-19: a retrospective study with propensity score matching. J Clin Lipidol. 2021;15:451–459. doi: 10.1016/j.jacl.2021.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hariyanto TI, Kurniawan A Statin and outcomes of coronavirus disease 2019 (COVID-19): a systematic review, meta-analysis, and meta-regression. Nutr Metab Cardiovasc Dis. 2021;31:1662–1670. doi: 10.1016/j.numecd.2021.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lounassalo I, Salin K, Kankaanpää A, et al Distinct trajectories of physical activity and related factors during the life course in the general population: a systematic review. BMC Public Health. 2019;19:271. doi: 10.1186/s12889-019-6513-y. [DOI] [PMC free article] [PubMed] [Google Scholar]