Abstract
In recent decades, life expectancy has been increasing significantly. In this scenario, health interventions are necessary to improve prognosis and quality of life of elderly with cardiovascular risk factors and cardiovascular disease. However, the number of elderly patients included in clinical trials is low, thus current clinical practice guidelines do not include specific recommendations. This document aims to review prevention recommendations focused in patients ≥ 75 years with high or very high cardiovascular risk, regarding objectives, medical treatment options and also including physical exercise and their inclusion in cardiac rehabilitation programs. Also, we will show why geriatric syndromes such as frailty, dependence, cognitive impairment, and nutritional status, as well as comorbidities, ought to be considered in this population regarding their important prognostic impact.
In recent decades, life expectancy has been increasing significantly.[1] Age is a cardiovascular (CV) risk factor (CVRF) and, therefore, we are facing a growing population of elderlies with cardiovascular events. In addition, most of them have a low short-term mortality due to advances in treatment. This makes essential to develop health interventions to improve their long-term prognosis and quality of life.
Nevertheless, clinical practice guidelines do not include elderly-specific recommendations, since the representation of this age group in clinical trials is meaningly low. This document aims to address scientific evidence for CV prevention in patients ≥ 75 years with high or very high CV risk focusing in therapeutic goals, medical treatment, physical exercise and their inclusion in cardiac rehabilitation (CR) programs, as well as the adaptation of these interventions based on comorbidities and geriatric syndromes (frailty, comorbidities, dependence, cognitive impairment and nutritional status).
Elderly patients with CV disease present some characteristics that may differ from the general population, with relevant influence in diagnosis, treatment, and prognosis.[2] This population is a heterogeneous group, ranging from robust, independent individuals with no comorbidities to severely dependent individuals. Therefore, an individualized approach to variables beyond age is essential to guide CV prevention goals and strategies.
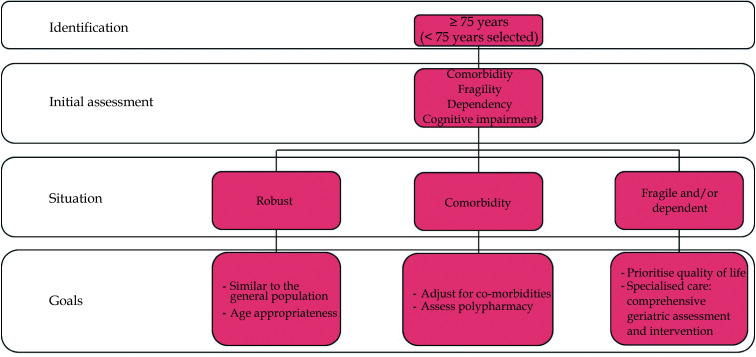
In this scenario, a lax threshold to identify possible risk situations is recommended. Age is the most objective and immediate criterion. Every patient aged 75 years or older (or even younger in selected patients) should have an initial brief and systematic approach, including comorbidity, functional, cognitive, social, nutritional assessment, as well as polypharmacy.[3] This multidimensional assessment strategy allows CV prevention strategies to be optimized and individualized, trying to establish a realistic adaptation of objectives without falling into either therapeutic obstinacy or nihilism. Finally, decision-making must consider the expectations and possibilities of the patient and his or her environment (Figure 1).
Figure 1.
Identifying and assessing elderly patients in cardiovascular prevention.
Robust patient: no relevant comorbidities, independent and not presenting frailty criteria. In this patient, the general CV prevention strategy will be similar to that of the general population, although CVRF targets might need to be modified according to age-specific evidence when available.
Patient with comorbidities: individual severe comorbidities or multiple comorbidities. In this case, rethink the therapeutic goals is essential (especially if they pose a relevant impact on life expectancy) and take into account polypharmacy in relation to possible interactions and problems with therapeutic compliance.
Frail and/or dependent patient: frailty is a situation of vulnerability that goes beyond one’s biological age and dependency is the need for help with daily life activities. Physical frailty is a stage prior to dependency that can be attenuated or even reversed with appropriate interventions. It is important to differentiate between those with mild, moderate, or severe dependency to determine therapeutic goals. What is clear is that as the degree of dependency progresses, quality of life aspects should be prioritized over survival or major CV events, ensuring that strict control does not result in a negative impact. In these patients, referral to specialists should be considered for a comprehensive geriatric assessment and an appropriate approach to geriatric syndromes.
The latest cardiovascular prevention clinical practice guidelines[4] propose the SCORE2-OP for classification of CV risk in apparently healthy people over 70 years of age, estimating the risk of death due to CV events and non-fatal events at 5-10 years adjusted for competitive risk (age increases the risk of death without CV disease as well as an attenuation of risk by classical CV risk factors). Reclassifying older patients in very high CV risk those with a risk ≥ 15%, high risk 7.5%-15% and low or moderate risk < 7.5%, the treatment objectives being risk factors CV based on risk category.
CONTROL OF CVRF
Dyslipidaemia
In the last year, several studies have been published that have demonstrated the prognostic benefit associated with lowering low density lipoprotein cholesterol (LDL-C) levels in this population. Mortensen, et al.[5] observed that those aged ≥ 70 years with elevated plasma LDL-C levels are the most likely to develop atherosclerotic disease and acute myocardial infarction. On the other hand, a recent meta-analysis, including data on lipid-lowering treatments from different randomized clinical trials with more than 21,000 patients aged ≥ 75 years, mainly in secondary prevention, observed that after a mean follow-up of 2.2 to 6 years, the reduction of LDL-C levels was associated with a significant reduction in the combined outcome of major CV events (26% for every 1 mmol/L reduction), with no statistical difference compared to the benefit obtained in younger patients.[6] The authors also observed that each of the components of the combined outcome (cardiovascular death, acute myocardial infarction, stroke, and coronary revascularization) was significantly reduced in elderly patients.
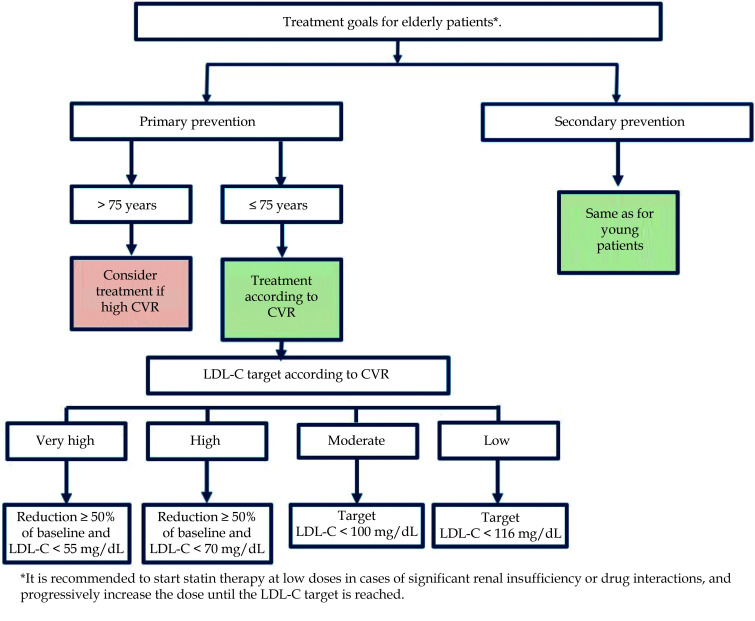
Current guidelines establish that the approach to dyslipidemia in older patients at high or very high CV risk should follow the same recommendations as in their younger counterparts [7] with the same objectives (reduction ≥ 50% of LDL-C levels and reaching a target of LDL-C < 55 mg/dL in very high CV risk and < 70 mg/ dL in high CV risk) ( Figure 2). The greatest prognostic benefit from lowering LDL-C levels is obtained in patients with both higher levels and higher CV risk.[8] Regarding pharmacological treatment, statins are also the drug of choice and ezetimibe and proprotein convertase subtilisin/kexin type 9 inhibitors as second and third level drugs.[7] In this respect, sub-analyses of the Odyssey Outcomes study with alirocumab and the FOURIER study with evolocumab did also demonstrate CV benefits in patients over 65 and over 69 years of age, respectively.[9,10]
Figure 2.
LDL-C targets for elderly patients.
CVR: cardiovascular risk; LDL-C: low density lipoprotein cholesterol.
Other new treatments for LDL-C reduction are bempedoic acid and inclisiran. Bempedoic acid is a small molecule that reduces cholesterol synthesis by inhibiting ATP-citrate lyase.[11] Added to lipid-lowering treatment, it effectively and safely reduces LDL cholesterol, also in patients over 65 and 75 years of age, and independently of the baseline treatment.[11,12] Inclisiran, a small interfering RNA, is another recent addition to the therapeutic arsenal. It has been shown in several trials to reduce LDL-C levels by half in patients with established CV disease (ORION-10), or established CV disease and equivalent risk (ORION-11) and in patients with familial hypercholesterolemia (ORION-9) despite lipid-lowering therapy at maximally tolerated doses. This benefit also extended to patients over 65 and 75 years of age included in the trials.[13-15]
Finally, and especially in elderly patients, combinations of different drugs in monotherapy improve adherence and compliance with treatment, thus enabling the targets to be achieved.
Hypertension
The approach to hypertension in the elderly population is more complex because it is more severe,[16] there is more resistance to treatment and a higher risk of hypotension in patients with more comorbidities and/or frailty.[17,18] The most common form is isolated systolic hypertension. Before starting or increasing the dose of a pharmacological treatment, it is also very important to evaluate the presence of postural hypotension. In the Systolic Blood Pressure Intervention Trial (SPRINT), which included a cohort of patients aged 75 years and older, more intensive blood pressure treatment was associated with a reduction in CV complications and mortality.[19] Any reduction in blood pressure is beneficial, although in frail patients target values should be reconsidered as they may increase the risk of adverse events.
It is recommended to initiate treatment in patients over 75 years of age with systolic blood pressure ≥ 140 mmHg and ≥ 160 mmHg in those over 80 years of age, aiming for 130-139 mmHg.[20,21] It is not recommended to achieve blood pressure < 130/70 mmHg in these patients, because of possible deleterious effects and a possible J-curve. [22]
The recommended measures in older patients, as in younger patients, include dietary and behaviour measures, such as losing weight, reducing salt in the diet and physical activity. Recommendations regarding pharmacological treatment are summarized in Table 1.[20]
Table 1. Pharmacological treatment recommendations for hypertension in older patients.
| Initiate monotherapy in patients with grade I hypertension, age > 80 years or frail. In frail patients requiring dual therapy, start at low doses. |
| Angiotensin converting enzyme inhibitors are first-line drugs. |
| Look for or rule out possible postural hypotension. |
| Avoid diuretics and alpha-blockers because of possible adverse effects (falls). |
| Monitor renal function. |
| Close monitoring, to minimise adverse effects, tolerance problems and increase adherence to treatment.[23] |
Type 2 diabetes
When managing diabetes in this population, we must take into account the CV benefit of the pharmacological treatment used, the metabolic control and the risk of hypoglycaemia. Again, a comprehensive geriatric assessment together with management of comorbidities are of great importance.[24]
Data from several clinical trials demonstrate the CV benefits of the use of glucagon-like peptide-1 receptor agonists and inhibitors of sodium-glucose linked transporter-2 in patients with cardiovascular disease.[25-30] Although there are few specific trials of these drugs in the elderly, sub-analyses by age confirm that the cardio-renal benefits are maintained in these patients without compromising their safety (Tables 2 and 3).[31-36]
Table 2. Sub-analysis of pivotal trials with iSGLT2 in older population.
| N | Older patients | HR (95% CI) | |
| CI: confidence interval; CV: cardiovascular; HF: heart failure; HR: hazard ratio; iSGLT2: inhibitors of sodium-glucose linked transporter-2; MACE: major adverse cardiac events; MI: myocardial infarction. | |||
| Empagliflozin[34] | 7,020 | ≥ 75 years: 652 | 3P-MACEAll: 0.86 (0.74-0.99)≥ 75 years: 0.68 (0.46-1.00)Cardiovascular deathAll: 0.62 (0.49-0.77)≥ 75 years: 0.55 (0.32-0.94)HF hospitalisation All: 0.65 (0.50-0.85)≥ 75 years: 0.45 (0.22-0.89) |
| Dapagliflozin[54] | 4,744 | ≥ 75 years: 1,149 |
Combined HF hospitalisation/CV death
All: 0.68 (0.53-0.88) |
| Dapagliflozin[36] | 17,160 | ≥ 75 years: 1,096 |
Combined HF hospitalisation/CV death
All: 0.94 (0.65-1.36) |
| Canagliflozin[26] | 10,142 | ≥ 75 years: 4,564 | CV death, non-fatal MI or non-fatal strokeAll: 0.86 (0.75-0.97)≥ 75 years: 0.80 (0.67-0.95)Hospitalisation for HFAll: 0.67 (0.52-0.87)≥ 75 years: 0.65 (0.47-0.90) |
| Ertugliflozin[37] | 8,238 | ≥ 75 years: 903 | CV death, non-fatal MI or non-fatal strokeAll: 0.97 (0.85-1.11)≥ 65 years: 1.03 (0.86-1.22) |
| Sotagliflozin[38] | 1,222 | ≥ 65 years: 858 | CV death, hospitalisation and urgent care for HFAll: 0.67 (0.52-0-85)≥ 65 years: 0.62 (0.47-0.82) |
Table 3. Sub-analyses of the pivotal trials glucagon-like peptide-1 receptor agonist.
| N | Older patients | HR (95% CI) | |
| AMI: acute myocardial infarction; CI: confidence interval; CV: cardiovascular; HR: hazard ratio. | |||
| Dulaglutide[98] | 9901 | ≥ 65 years: 5,256 | CV mortality or death of unknown cause, non-fatal AMI, non-fatal strokeAll: 0.88 (0.79-0.99)≥ 65 years: 0.89 (0.78-1.03) |
| Liraglutide[40] | 9340 | ≥ 75 years: 83660-74 years: 6,183
< 60 years: 2,321 |
CV mortality, non-fatal AMI and non-fatal strokeAll: 0.77 (0.62-0.97)< 60 years: 0.87 (0.78- 0.97)60-74 years: 0.95 (0.83-1.09)≥ 75 years: 0.66 (0.49-0.89) |
| Semaglutide[29] | 3297 | ≥ 65 years: 1,598 | CV death, non-fatal AMI or non-fatal strokeAll: 0.58-0.95)≥ 65 years: 0.72 (0.51-1.02) |
Metabolic control targets as well as the integrated approach to diabetes treatment are summarised in Tables 4 and 5.
Table 4. General goals and recommendations in diabetic patients.[41,42].
| Control target: HbA1c < 7% (provided it is achieved with drugs that do not cause hypoglycaemia), although less stringent targets such as HbA1c < 8% may be considered in the elderly with long-standing diabetes mellitus and frailty. |
| Plan treatment according to the comprehensive geriatric assessment (frailty, comorbidities, renal function, cardiovascular risk). |
| Prioritise the use of drugs with proven cardiovascular benefit. |
| Avoid acute hyperglycaemia which can lead to complications. |
| Simplify complex insulin regimens for the elderly to reduce the risk of hypoglycaemia. |
| Important: in this population, a high HbA1c does not exclude the risk of hypoglycaemia. |
| Screening for complications should be aimed at reducing cognitive impairment. |
Table 5. Targets and specific recommendations in diabetic patients.[41,43].
| CV: cardiovascular: DPP4i: dipeptidyl peptidase-4 inhibitors; GFR: glomerular filtration rate; GLP-1: glucagon-like peptide-1; iSGLT-2: sodium-glucose cotransporter type 2 inhibitors | |
| Non-pharmacological treatment | Nutritional advice avoiding very low-calorie diets (sarcopenia, increased risk of malnutrition). |
| Prescription of adapted physical activity: aerobic, endurance, coordination and balance. | |
| Pharmacological treatment | 1stchoice• Metformin It does not usually cause hypoglycaemia. Monitoring of renal function (decrease dose if GFR < 45 mL/min and discontinue if GFR < 30 mL/min) and liver function (risk of lactic acidosis). Vitamin B12 monitoring (especially if anaemia or peripheral neuropathy). Gastrointestinal intolerance may be greater in the elderly.• iSGLT2 Do not induce hypoglycaemia. Blood pressure control in concomitant use with diuretics. Educate on genital hygiene to avoid genital and urinary tract infections. CV and renal benefit.• GLP-1 receptor agonists They do not induce hypoglycaemia. Drug of choice in parenteral treatment* (ADA Guidelines). [41] Decreased appetite, weight loss and gastrointestinal discomfort, therefore use with caution in frail elderly people with hyporexia and malnutrition. Subcutaneous administration. CV and renal benefit. |
| In the event of not controlling with the Metformin + iSGLT2 + GLP1 receptor agonist combination or contraindication/intolerance to any of them. Insulin High hypoglycaemic power. Important to monitor the possibility of hypoglycaemia. Weight gain. Insulin Glargine and Degludec, lower rate of hypoglycaemia. Close monitoring in frail patients. | |
| Other | |
| • DDP4i Few side effects. Low risk of hypoglycaemia. No CV benefit. Saxagliptin risk of heart failure. Linagliptin and Sitagliptin are neutral in cardiovascular risk.[44,45]• Sulphonylureas, glinides: Not recommended due to risk of hypoglycaemia.• Thiazolidinediones: Not recommended due to side effects and risk of heart failure. | |
Other CVRF
Tobacco
Elderly smokers tend to have greater nicotine dependence and a long history of smoking.[46] Quitting smoking should be strongly advised regardless of age, offering help with quitting smoking and pharmacological treatment if necessary.[18] E-cigarettes are not harmless either, as they emit other fine and ultrafine particles, and the general recommendation is to stop using them.[47]
A correct assessment should include the presence or absence of smoking but also, in smokers, the severity and the motivation to quit. For this purpose, the Fagerström and Richmond scales have been validated. Anti-smoking advice in the consultation room and pharmacological support if necessary are recommended just as for the rest of the population.
The use of drugs to quit smoking might be indicated, the treatment of choice being varenicline, with an efficacy of 30% abstinence without an increase in side effects in the elderly population, with the dose having to be adjusted for patients with glomerular filtration rate < 30 mL/min. [48-50] Bupropion has demonstrated CV safety[51] but is contraindicated in this age group due to the risk of seizures.[46] Nicotine replacement therapy has certain limitations in the population at very high CV risk, as well as lower efficacy.[50]
Quitting smoking is probably the single most effective measure of lifestyle changes for the prevention of cardiovascular disease, regardless of age.[20]
Obesity
More than 20% of the population over 65 years of age is obese, the presence of which increases CV risk and mortality.[52] The advantages of weight loss in obese elderly people not only include improved long-term prognosis, but also improved functional capacity and quality of life.
A personalised diet tailored to the patient is recommended, matching caloric intake to the patient’s energy expenditure, considering the risk of malnutrition and/or sarcopenia in frail patients. In patients over 60 years of age, the target body mass index may be higher than 25.[7]
ISCHAEMIC HEART DISEASE: ANTIPLATELET THERAPY
In older patients with ischemic heart disease, the recommendations in clinical practice guidelines are followed regarding drugs for secondary prevention, as well as antiplatelet therapy depending on the scenario.[18,53,54] In patients with acute coronary syndromes or undergoing percutaneous coronary intervention, double antiplatelet therapy should be prescribed for the duration established in the most recent European guidelines.[18,53,54] The duration will depend on the ischemic and bleeding risk and will be determined on an individual basis according to the score on recommended bleeding risk scales such as the ARC Bleeding risk and the PRECISE-DAPT scale.[53] The PRECISE-DAPT scale is assessed in peri-coronary intervention and we must bear in mind that it tends to overestimate bleeding risk as age plays a significant factor.[55] Guerrero, et al.[56] demonstrated in a cohort of 208 octogenarians with ST-segment elevation myocardial infarction that 92% of patients had a score greater than 25, yet only 12% had hemorrhagic events, suggesting better risk stratification with other cut-off points adapted to the elderly population.
The antiplatelet agents of choice in this context would be ticagrelor and clopidogrel, both in terms of invasive and conservative management, and prasugrel would be relegated due to the results of TRITON-TIMI 38, to younger populations, who are not underweight and with no previous stroke.[57,58] In any case, the dose to be used would be 5 mg, which has not been shown to be superior to clopidogrel in several clinical trials.[59,60] Ticagrelor has been shown to be safe and effective in patients over 75 years of age, so in those patients with high ischemic risk and low bleeding risk it should be considered.[61,62]
Regarding the prolongation of dual antiplatelet therapy, the patient’s ischemic risk would be assessed one year after tolerating dual antiplatelet therapy, so that a score above two on this scale with a low hemorrhagic risk would lead us to consider prolonging double antiplatelet therapy for 30 months.[53,55] There are 2 strategies: PEGASUS with ticagrelor and COMPASS with rivaroxaban with different populations (see table/figure).[63,64] It should be noted that the DAPT, PEGASUS and COMPASS trials included a small percentage of patients over 75 years of age: 11%, 15% and 20%, respectively.[63-65]
FRAILTY AND COMORBIDITY
Elderly patients often have comorbidities that may hinder the diagnosis of CV disease, limit the use of drugs with no proven prognostic benefit and affect prognosis (poorer quality of life and mortality).[66,67] For this reason, information from clinical guidelines is insufficient for decision-making, requiring a multidisciplinary approach and adapted recommendations, and it is essential to perform a comprehensive geriatric assessment that includes a series of aspects reflected in Table 6.
Table 6. Comprehensive geriatric assessment and situations to be assessed for decision-making.
| Medical aspects | Polypharmacy (≥ 5 drugs per day).Risk of side effects attributed to the drugs (anticholinergic effects, drug interactions, bleeding, renal failure, hypotension, etc).Recurrence of hospital admissions.Degree of stability and baseline functional class of the patient's diseases (chronic obstructive pulmonary disease, heart failure, etc).Definition of short- and medium-term objectives (life expectancy vs. quality of life).Risk of falling. |
| Comorbidity | Charlson Index. |
| Social situation | The Gijón scale. |
| Physical functionality | Physical frailty (Short Physical Performance Battery).Basic (Barthel index) and instrumental activities of daily living (Lawton-Brody index). |
| Mental situation | 4AT, Pfeiffer Index, Mini-Mental-Status-Test. |
| Prognostic indices integrating comprehensive geriatric assessment and co-morbidity | Simple Comorbidity Index validated in acute coronary syndrome.[68]Cumulative illness rating scale for geriatrics.[69]MPI-Age.[70] |
Frailty
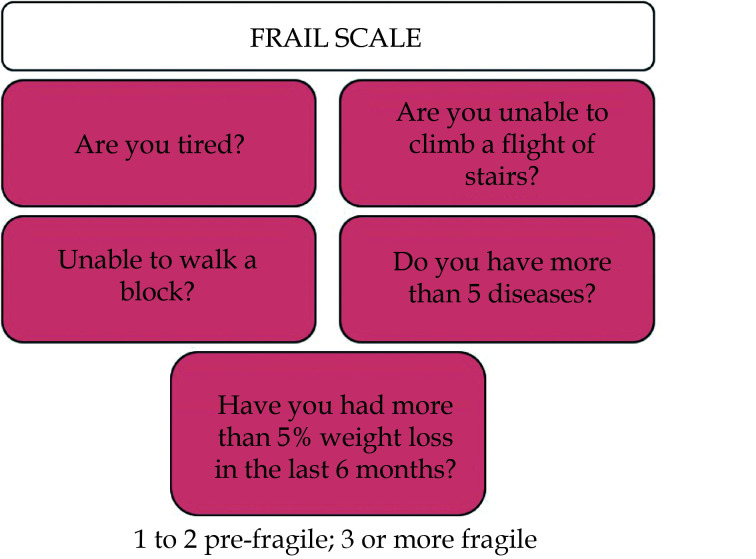
A distinction should be made between physical and multidimensional frailty. To assess physical frailty, the Short Physical Performance Battery (SPPB) is recommended for independent or mildly dependent patients. For multidimensional frailty, the Clinical Frailty Scale is recommended.[71] The FRAIL scale is a multidimensional scale that encompasses questions on physical frailty and the presence of comorbidity (Figure 3).[72] This scale can be useful to individualize prevention targets, being more stringent in robust patients and less stringent (even avoiding certain futile drugs) in very frail patients and/or those with advanced disability.[3]
Figure 3.
FRAIL scale (Fatigue, Resistance, Aerobic, Illnesses, Weight loss) (adapted from Morley, et al.).[72]
Assessment of Co-morbidity
It is essential to assess the mental status of patients, as depression is prevalent in this population and may be confused or overlap with cognitive impairment, increasing complications and CV morbidity and mortality. The heart-brain interaction is important, as both diseases share pathophysiological aspects as well as CVRF.[73] The prevalence of some degree of cognitive impairment in patients with previous acute coronary syndrome, associated comorbidities and multiple admissions are often associated with a progressive deterioration of mental function.[74] Assessing them should also guide us when planning goals.
In patients who have had an acute coronary syndrome, it is recommended that both the doses of antithrombotics and those of secondary prevention drugs be adapted to renal function and that specific contraindications for these drugs be assessed (Table 6) (class I recommendation, level B).[53] In older patients, we must be especially careful with the choice and dose of antithrombotics, as well as their duration, individualizing double and triple therapy.[53] Given that there is a greater vulnerability to bleeding due to antithrombotics in this age group, and in the event of detecting anemia or iron deficiency correct management and diagnosis should be carried out, with referral to specialists if necessary. Similarly, active questioning for the presence of macroscopic bleeding is necessary. In patients with chronic obstructive pulmonary disease, caution should be exercised in the use of beta-blockers, choosing those with higher B1 specificity and carefully titrating them (Table 7). In the next years, sodium-glucose cotransporter-2 inhibitors are likely to emerge as potential therapies for renal and CV protection in our patients due the promising results in chronic kidney disease.[78,79,80]
Table 7. Recommendations for the use of drugs in cardiovascular prevention in elderly people with renal failure.
| ACE: angiotensin converting enzyme; ASA: acetylsalicylic acid; GFR: glomerular filtration rate; iSGLT2: sodium-glucose cotransporter-2 inhibitors; RAA: renin-angiotensin-aldosterone; SC: subcutaneous. | |
| ACE/RAA-II inhibitors | Adjust based on renal function.Minimise the risk of hypotension.[18,20]Monitorize hyperkalemia. |
| Mineralocorticoid receptor antagonists | Patients with left ventricular ejection fraction < 35% and ischemic heart disease when Cr > 2.5 or K>5.Monitorize hyperkalemia. |
| Beta-blockers | Dose need not be modified due to renal insufficiency.Careful titration due to risk of side effects (asthenia, hypotension, sinus dysfunction, conduction disturbances).Evidence of good tolerance of nebivolol in elderly patients. |
| Statins | Creatin kinase titration (discontinue if elevated X10).Monitor symptoms (myalgias).Use of moderate-intensity doses if targets are achieved 6.52. |
| Antithrombotics | Monitor risk of bleeding and/or anaemia. ASA does not require adjustment.Clopidogrel: no dose modification required. P2Y12 receptor inhibitor of choice over ticagrelor or prasugrel in patients with a higher risk of bleeding, always assessing risk/benefit. Ticagrelor: no dosage modification required.Prasugrel: dose of 5 mg/day in patients aged 75 years or older.Enoxaparin: dose of 0.75 mg/kg/12 h SC if age ≥ 75 years; if glomerular filtration rate < 30 mL/min, dose is 1 mg/kg/24 h SC; contraindicated if GFR < 15 ml/min.Fondaparinux: contraindicated if GFR < 30 mL/min. |
| iSGLT2 (Dapaglizofin or Empaglizofin) | Patients with heart failure and left ventricular ejection fraction > 40%. [75,76,77] |
| Sacubitril/Valsartan | Patients with heart failure and left ventricular ejection fraction > 40%. |
Treatment Adherence
Adherence to medication is key to the management and control of cardiovascular diseases, and in older patients there are factors that make this difficult (Table 8). In addition, when prescribing drugs, it is essential to consider polypharmacy (≥ 5 drugs per day), as inappropriate prescribing is associated with worse prognosis. The STOPP/START criteria help to tailor treatment regimens and avoid potentially inappropriate prescribing.[81] The STOPP criteria refer to those drugs that should be avoided in older people, the START criteria to those drugs that should be started. In terms of treatment in CV prevention, the START criteria include antihypertensive treatment when systolic blood pressure is typically > 160 mmHg and/or diastolic blood pressure is typically > 90 mmHg (>140 mmHg and 90 mmHg if diabetes mellitus is present), statins if there is coronary, cerebral or peripheral arterial atherosclerotic disease (unless the patient is at the end of life or aged > 85 years), angiotensin converting enzyme inhibitors in systolic heart failure and/or ischemic heart disease, and beta-blockers in ischemic heart disease and systolic heart failure. [81]
Table 8. Complexity and factors influencing adherence to treatment.
| Complexity of adherence | Social, economic, cognitive and demographic factors. Several predictors of poor adherence often coexist in older patients.There are no adherence studies in elderly patients. |
| Factors influencing improved adherence | A multidimensional assessment of adherence is highly recommended.The Morisky scale (8 items) is a sensitive tool for the detection of poor adherence.Nurses play an important role in providing health education, training and tools to the patient.Promotion of continuity of care with primary care, single e-prescription and shared medical records. Use of new technologies with applications and technological supports to trigger reminders. |
To facilitate adherence, drug combinations with polypills and avoidance of split doses should be a priority option in these patients.[82,83] On the other hand, nursing intervention in the follow-up of the elderly after acute coronary syndrome can make a marked contribution to improving adherence at 12 months.[84]
CR in Older Patients
Physical exercise reduces mortality and CV disease, and also improves quality of life.[85] For this reason, structured physical exercise within a CR program has a level IA recommendation.[4] In the elderly general population, the Leisure World Cohort study (> 13,000 people, mean age 74 years) reported a 35% decrease in total mortality with physical activity. Less data is available in the over-85 s, although heart-healthy habits are recommended.[86] In the general elderly population, the European Society of Cardiology and American Heart Association guidelines[86,87] concur in recommending exercise adapted to comorbidity and baseline functional capacity, avoiding exercise with abrupt postural changes.[88]
Moreover, a recent article regarding CR in elderly patients with acute heart failure has been published. Its conclusion was that in older patients who were hospitalized for acute decompensated heart failure, an early, transitional, tailored, progressive rehabilitation intervention that included multiple physical-function domains resulted in greater improvement in physical function than usual care.[89]
The characteristics of physical exercise are summarized in Table 9. The intensity of physical exercise can also be measured with subjective exertion scales, such as the Börg scale or adapted scales, which are easier for the elderly, and as a last resort the talk test.[91]
Table 9. Characteristics of physical exercise in the older population.
| Frequency | Intensity | Duration | |
| *40%-69% of VO2 max or 55-74% of heart rate reserve.[90] **40% of 1 RM (maximum load you are able to lift in a single repetition); ***20%-40% of heart rate reserve; #10-15 reps / 8-10 muscle groups; ##20 seconds per static stretch with 3-4 repetitions. | |||
| Aerobic | > 5 days/week | Moderate* | > 30 min/session |
| Force | 2 days/week | Moderate** | 1-2 sets# |
| Flexibility/balance | > 5 days/week | Moderate*** | > 10 min/session## |
Physical exercise in the elderly population reduces CV events, improves CVRF control and reduces the prevalence of obesity, osteoporosis, cancer, anxiety, and depression. It counteracts both central and peripheral functional limitation, improves pain control, muscle control and reduces sarcopenia, reducing the risk of falls and allowing functional independence to be maintained for longer, even in frail populations of nonagenarians, and is one of the pillars for promoting the reversibility of frailty in its initial stages.[92] Regarding structured physical exercise within a CR program, both the recommendations and the benefits for older patients with a previous CV event are the same, with the following considerations: the benefit is greater the higher the number of sessions,[93] the worse the baseline functional capacity (mean peak VO2 16 mL/kg per minute in the EU-caRE study with a mean age of 73 years, post-coronary event or valve replacement).[94] In the Dutch record,[95] the 3286 patients aged > 70 years who underwent a CR program compared to the non-CR group had a decrease in mortality of up to 50% at 12 months and 32% at 48 months. It was observed in a cohort of patients over 70 years of age who underwent CR, greater comorbidity, atrial fibrillation and greater residual ischemia than in younger patients, with exercise being safe on a cardiovascular level and with little interruption of the program due to osteoarticular issues, achieving a clear improvement in functional capacity of 27% and in quality of life (EuroQol5D). Kitzman, et al.[89] have shown that mobility-, strength-, endurance- and power-based rehabilitation in older patients with heart failure improve functionality.
CR and Physical Exercise According to Co-morbidities
Frailty and comorbidity in the elderly population may be a cause of non-referral to CR programmes.[96] However, patients > 75 years account for a third of the total in some of these programmes, [97] therefore, the assessment of frailty should be a quality objective in CR.[98] The ideal tools for such assessment in this area are not yet well determined, although scales validated in other settings show potential usefulness,[99] especially those that are easier to implement with associated physical assessment,[3] such as the modified Fried scale or the SPPB.
Although there are no large studies assessing the effect of CR on physical capacity in frail elderly patients,[100] studies based on community exercise programs or institutionalized patients do point to an improvement in quality of life and physical capacity. It may be a priority to focus on strength and balance exercises that help them to carry out daily life activities.[101]
But beyond underlying heart disease or simple physical capacity, counselling within CR programs needs to cover specific aspects of the older population as addressed in Table 10.
Table 10. Specific issues to be addressed in cardiac rehabilitation programmes in the older population.
| Nutritional status | Addressing nutritional deficits would complement the exercise programme to improve the patient's physical performance, both in pre-frailty and established frailty. |
| Psychological state | Depression and anxiety mask incipient cognitive impairment, physical exercise can improve cognitive abilities in older patients.[102] |
| Cognitive status | Cognitive impairment may make it difficult to learn heart-healthy habits and may limit the beneficial effect of programmes in this population. |
| Social situation | Lack of social support may complicate the continuation of measures implemented during the programme,[103] it is important to involve family and carers, and in some cases an assessment by a social worker may be necessary. |
TELEMEDICINE IN SECONDARY PREVENTION IN THE ELDERLY POPULATION
Remote consultation in the field of cardiology is already a reality,[104] especially in the current pandemic.[105] In older patients, specific and prevalent considerations and problems must be taken and addressed. There are guidelines and recommendations that allow us to guide and improve teleconsultation and thus the care provided, which can be summarized in the 5M framework.[106] Using this framework, the patient is asked simple structured questions about morbidity, mobility, mental state, medication and, most importantly, the patient’s preferences and priorities.
Physical training and the inclusion of older patients in secondary prevention and CR units is highly recommended and independent of age. The implementation of these programs remotely represents a special challenge in older patients.[107] There is scientific evidence of good acceptance of the technology in a high percentage of older patients.[108] There are even randomized clinical trials showing significant benefit of digital intervention in addition to standard CR in this population.[109]
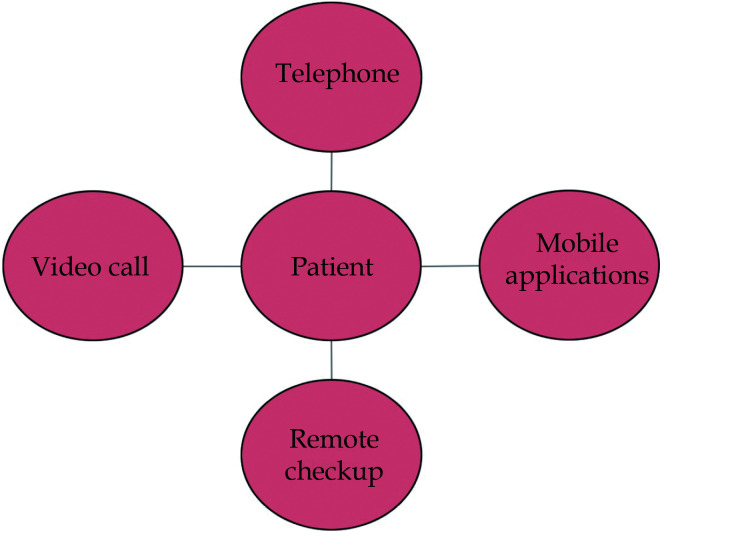
Physical exercise could be prescribed in an unsupervised way or in a supervised way online, within a prevention and CR program. Hybrid programs are one of the most recommended options, initiating face-to-face training for correct learning with direct supervision and continuing with remote online supervision. There are different options for cardiac telerehabilitation as shown in Figure 4, which can be used individually or integrated into a single CR program.
Figure 4.
Different possibilities for telemedicine for secondary prevention among the elderly population.
Among these options, videoconferencing could be the best choice for these patients, as it allows direct supervision at home and real-time correction of aerobic training, balance and strength exercises, and inspiratory muscle training. However, to carry out this telemedicine system, equipment is required for resistance exercise (such as an exercise bike), to work on strength, technological means (a mobile phone or tablet with a camera and video, and internet connection), and family support, especially in patients with sensory deficits.
We can also use simple online prevention and CR tools such as the aularc.es platform, which provides patients and their families access to many resources of a CR programme.
PSYCHOSOCIAL ASPECTS
For the management of CV disease in the elderly, mental health and social support issues must be addressed. An individual’s social network is defined by the size, structure and frequency of contact of the group of people in his or her usual environment. Poor social support is associated with poorer prognosis in patients with ischemic heart disease and heart failure. Adequate social support may reduce stress secondary to the disease itself and help with self-care and adherence to treatment. The DUKE-UNK-11 or Gijón scales may be useful to measure the degree of social support. On the other hand, the impact could be modulated by the presence of depression or cognitive impairment.
Depression is common in patients with cardiovascular disease and is likely to be related to sustained symptomatology, also influencing the worsening of the heart disease itself, and may contribute to a greater tendency to social withdrawal and isolation.
CONCLUSIONS
There is a lack of representation of the elderly population in clinical practice guidelines, which is not always accompanied by a lack of scientific evidence. In this document, we have sought to compile the specific evidence on cardiovascular prevention in the population over 75 years of age, focusing on the classic CVRF and on the use of the latest treatments with clear prognostic benefit in these patients. On the other hand, we address how to approach CR in these patients and how to make a comprehensive assessment, so that, in cases where it is necessary, they are less strict in the control of some of CVRF.
In general, chronological age would not be a reason per se for inequity and for not offering treatments based on scientific evidence and pursuing lipid, diabetes, and hypertension targets (Table 11).
Table 11. Summary table.
| Valuation | Comorbidity, frailty, dependency, cognitive impairment.Robust, co-morbid, frail patient. |
| Frailty | Frail Scale. |
| Comorbidity | Assessment: cognitive impairment, depressive syndromes, chronic kidney disease, chronic obstructive pulmonary disease, neoplastic diseases. Adjustment of medication based on renal function.STOPP/START pharmacological treatment criteria.Treatment adherence. |
| Dyslipidaemia | Control targets at very high risk > 55 mg/dL and at high risk < 70 mg/dL (and 50 % reduction).Statins treatment of choice. Monitor side effects.Safety ezetimibe and proprotein convertase subtilisin/kexin type 9 inhibitors. |
| Hypertension | In frail patients or those over 80 years of age, start treatment when blood pressure > 160/90 mmHg. Preferably initiate treatment in monotherapy or combination at low doses.Monitor postural hypotension and renal function.Angiotensin converting enzyme inhibitors as treatment of choice. |
| Diabetes | Hb1a control targets ≤ 7% or < 8% in patients with long-standing diabetes.Monitor for hypoglycaemia or hyperglycaemia.Metformin, sodium-glucose cotransporter-2 inhibitors and agonists of glucagon-like peptide-1 as treatment of choice.Avoid complex insulin regimens, especially in frail patients. |
| Other | Address nutrition: obesity, malnutrition, sarcopenia.Tobacco: quit smoking with the possibility of using varenicline.Total drug withdrawal (non-alcohol). Influenza and pneumococcal vaccination. |
| Antithrombotic therapy | Dual antiplatelet therapy up to 12 months after acute coronary syndromes.Haemorrhagic and ischaemic risk assessment for dual antiplatelet therapy prolongation.Treatment of choice acetylsalicylic acid + clopidogrel/ticagrelor. |
| Pharmacological adherence | Out of choice, drug combinations and polypills. |
| Cardiac Rehabilitation | Demonstrated benefit in this population.Individually adapted physical exercise to improve physical condition and prevent frailty. Avoid sudden changes in posture.Assessment: nutritional, social and psychological status. |
| Telemedicine | Benefits in adherence and secondary prevention programmes.Hybrid programmes (supervised and remote).Video calls, phones, app.Web platforms (aularc.es). |
Acknowledgements
We would like to thank the Geriatric Cardiology Section and Cardiovascular Risk Section of the Spanish Society of Cardiology. This work was funded by Instituto de Salud Carlos III and FEDER founds (Exp. JR/21/00041)
References
- 1.INE. National Statistics Institute. INE. https://www.ine.es/ (accessed on March 10, 2021).
- 2.Madhavan V, Gersh J, Alexander P, et al Coronary artery disease in patients ≥ 80 years of age. J Am Coll Cardiol. 2018;71:2015–2040. doi: 10.1016/j.jacc.2017.12.068. [DOI] [PubMed] [Google Scholar]
- 3.Díez-Villanueva P, Arizá-Solé A, Vidán MT, et al Recommendations of the Geriatric Cardiology Section of the Spanish Society of Cardiology for the assessment of frailty in the elderly with heart disease. Rev Esp Cardiol. 2019;72:63–71. doi: 10.1016/j.recesp.2018.06.015. [DOI] [PubMed] [Google Scholar]
- 4.Visseren FLJ, Mach F, Smulders YM, et al 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2021;42:3227–3337. doi: 10.1093/eurheartj/ehab484. [DOI] [PubMed] [Google Scholar]
- 5.Mortensen MB, Nordestgaard BG Elevated LDL cholesterol and increased risk of myocardial infarction and atherosclerotic cardiovascular disease in individuals aged 70-100 years: a contemporary primary prevention cohort. Lancet. 2020;396:1644–1652. doi: 10.1016/S0140-6736(20)32233-9. [DOI] [PubMed] [Google Scholar]
- 6.Gencer B, Marston NA, Im K, et al Efficacy and safety of lowering LDL cholesterol in older patients: a systematic review and meta-analysis of randomised controlled trials. Lancet. 2020;396:1637–1643. doi: 10.1016/S0140-6736(20)32332-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mach F, Baigent C, Catapano AL, et al 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) Eur Heart J. 2020;41:111–188. doi: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- 8.Robinson JG, Jayanna MB, Bairey Merz CN, et al Clinical implications of the log linear association between LDL-C lowering and cardiovascular risk reduction: Greatest benefits when LDL-C > 100 mg/dL. PLoS One. 2020;15:e0240166. doi: 10.1371/journal.pone.0240166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sinnaeve PR, Schwartz GG, Wojdyla DM, et al Effect of alirocumab on cardiovascular outcomes after acute coronary syndromes according to age: an ODYSSEY OUTCOMES trial analysis. Eur Heart J. 2020;41:2248–2258. doi: 10.1093/eurheartj/ehz809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sever P, Gouni-Berthold I, Keech A, et al. LDL-cholesterol lowering with evolocumab, and outcomes according to age and sex in patients in the FOURIER Trial. Eur J Prev Cardiol 2020; 2047487320902750.
- 11.Ballantyne CM, Laufs U, Ray KK, et al Bempedoic acid plus ezetimibe fixed-dose combination in patients with hypercholesterolemia and high CVD risk treated with maximally tolerated statin therapy. Eur J Prev Cardiol. 2020;27:593–603. doi: 10.1177/2047487319864671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ray KK, Bays HE, Catapano AL, et al Safety and efficacy of bempedoic acid to reduce LDL cholesterol. N Engl J Med. 2019;380:1022–1032. doi: 10.1056/NEJMoa1803917. [DOI] [PubMed] [Google Scholar]
- 13.Ray KK, Wright RS, Kallend D, et al Two Phase 3 trials of inclisiran in patients with elevated LDL cholesterol. N Engl J Med. 2020;382:1507–1519. doi: 10.1056/NEJMoa1912387. [DOI] [PubMed] [Google Scholar]
- 14.Wong ND, Toth PP, Amsterdam EA, et al Most important advances in preventive cardiology during this past decade: Viewpoint from the American Society for Preventive Cardiology. Trends Cardiovasc Med. 2021;31:49–56. doi: 10.1016/j.tcm.2019.11.013. [DOI] [PubMed] [Google Scholar]
- 15.Ruscica M, Ferri N, Santos RD, et al Lipid lowering drugs: present status and future developments. Curr Atheroscler Rep. 2021;23:17. doi: 10.1007/s11883-021-00918-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lionakis N, Mendrinos D, Sanidas E, et al Hypertension in the elderly. World J Cardiol. 2012;4:135–147. doi: 10.4330/wjc.v4.i5.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yusuf S, Hawken S, Ounpuu S, et al Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 18.Knuuti J, Wijns W, Saraste A, et al 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. The Task Force for the diagnosis and management of chronic coronary syndromes of the European Society of Cardiology (ESC) Eur Heart J. 2020;41:407–477. doi: 10.1093/eurheartj/ehz425. [DOI] [PubMed] [Google Scholar]
- 19.SPRINT Research Group, Wright JT, Williamson JD, et al A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103–2116. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams B, Mancia G, Spiering W, et al 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–3104. doi: 10.1093/eurheartj/ehy339. [DOI] [PubMed] [Google Scholar]
- 21.Beckett NS, Peters R, Fletcher AE, et al Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358:1887–1898. doi: 10.1056/NEJMoa0801369. [DOI] [PubMed] [Google Scholar]
- 22.Denardo SJ, Gong Y, Nichols WW, et al Blood pressure and outcomes in very old hypertensive coronary artery disease patients: an INVEST substudy. Am J Med. 2010;123:719–726. doi: 10.1016/j.amjmed.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sierra C Arterial hypertension in the elderly. Hipertens Riesgo Vasc. 2017;34:26–29. doi: 10.1016/S1889-1837(18)30072-2. [DOI] [PubMed] [Google Scholar]
- 24.Gómez-Huelgas R, Gómez Peralta F, Rodríguez Mañas L, et al Treatment of type 2 diabetes mellitus in the elderly patient. Rev Clin Esp. 2018;218:74–88. doi: 10.1016/j.rce.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 25.Zinman B, Wanner C, Lachin JM, et al Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 26.Neal B, Perkovic V, Mahaffey KW, et al Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 27.Wiviott SD, Raz I, Bonaca MP, et al Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347–357. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 28.Marso SP, Daniels GH, Brown-Frandsen K, et al Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marso SP, Bain SC, Consoli A, et al Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834–1844. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 30.Gerstein HC, Colhoun HM, Dagenais GR, et al Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394:121–130. doi: 10.1016/S0140-6736(19)31149-3. [DOI] [PubMed] [Google Scholar]
- 31.Hanefeld M, Berria R, Lin J, et al Lixisenatide treatment for older patients with type 2 diabetes mellitus uncontrolled on oral antidiabetics: meta-analysis of five randomized controlled trials. Adv Ther. 2014;31:861–872. doi: 10.1007/s12325-014-0146-4. [DOI] [PubMed] [Google Scholar]
- 32.Sinclair AJ, Bode B, Harris S, et al Efficacy and safety of canagliflozin in individuals aged 75 and older with type 2 diabetes mellitus: a pooled analysis. J Am Geriatr Soc. 2016;64:543–552. doi: 10.1111/jgs.14028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bode BW, Brett J, Falahati A, et al Comparison of the efficacy and tolerability profile of liraglutide, a once-daily human GLP-1 analog, in patients with type 2 diabetes ≥ 65 and < 65 years of age: a pooled analysis from phase III studies. Am J Geriatr Pharmacother. 2011;9:423–433. doi: 10.1016/j.amjopharm.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 34.Monteiro P, Bergenstal RM, Toural E, et al Efficacy and safety of empagliflozin in older patients in the EMPA-REG OUTCOME® trial . Age Ageing. 2019;48:859–866. doi: 10.1093/ageing/afz096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martinez FA, Serenelli M, Nicolau JC, et al Efficacy and safety of dapagliflozin in heart failure with reduced ejection fraction according to age: insights from DAPA-HF. Circulation. 2020;141:100–111. doi: 10.1161/CIRCULATIONAHA.119.044133. [DOI] [PubMed] [Google Scholar]
- 36.Cahn A, Mosenzon O, Wiviott SD, et al Efficacy and safety of dapagliflozin in the elderly: analysis from the DECLARE-TIMI 58 study. Diabetes Care. 2020;43:468–475. doi: 10.2337/dc19-1476. [DOI] [PubMed] [Google Scholar]
- 37.Cannon CP, Pratley R, Dagogo-Jack S, et al Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med. 2020;383:1425–1435. doi: 10.1056/NEJMoa2004967. [DOI] [PubMed] [Google Scholar]
- 38.Bhatt DL, Szarek M, Steg PG, et al Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med. 2021;384:117–128. doi: 10.1056/NEJMoa2030183. [DOI] [PubMed] [Google Scholar]
- 39.Riddle MC, Gerstein HC, Xavier D, et al Efficacy and safety of dulaglutide in older patients: a post hoc analysis of the REWIND trial. J Clin Endocrinol Metab. 2021;106:1345–1351. doi: 10.1210/clinem/dgab065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gilbert MP, Bain SC, Franek E, et al Effect of liraglutide on cardiovascular outcomes in elderly patients: a post hoc analysis of a randomized controlled trial. Ann Intern Med. 2019;170:423–426. doi: 10.7326/M18-1569. [DOI] [PubMed] [Google Scholar]
- 41.Association AD Older adults: standards of medical care in diabetes-2021. Diabetes Care. 2021;44:S168–S179. doi: 10.2337/dc21-S012. [DOI] [PubMed] [Google Scholar]
- 42.Cosentino F, Grant PJ, Aboyans V, et al 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41:255–323. doi: 10.1093/eurheartj/ehz486. [DOI] [PubMed] [Google Scholar]
- 43.Castro-Conde A, Marzal-Martín D, Arrarte V, et al Comprehensive approach to the patient with type 2 diabetes mellitus and cardiovascular disease or very high cardiovascular risk. REC: Cardio Clinics. 2019;54:183–192. doi: 10.1016/j.rccl.2019.04.005. [DOI] [Google Scholar]
- 44.Green JB, Bethel MA, Armstrong PW, et al Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373:232–242. doi: 10.1056/NEJMoa1501352. [DOI] [PubMed] [Google Scholar]
- 45.Rosenstock J, Perkovic V, Johansen OE, et al Effect of linagliptin vs. placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk:the CARMELINA randomized clinical trial . JAMA. 2019;321:69–79. doi: 10.1001/jama.2018.18269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carreras-Castellet J, Quesada-Laborda M, Sánchez-Agudo L Treatment of smoking in the elderly. Rev Esp Geriatr Gerontol. 2001;36:36–44. [Google Scholar]
- 47.Estruch R, Ruilope LM, Cosentino F The year in cardiovascular medicine 2020: epidemiology and prevention. Eur Heart J. 2021;42:813–821. doi: 10.1093/eurheartj/ehaa1062. [DOI] [PubMed] [Google Scholar]
- 48.Windle SB, Bata I, Madan M, et al A randomized controlled trial of the efficacy and safety of varenicline for smoking cessation after acute coronary syndrome: design and methods of the Evaluation of Varenicline in Smoking Cessation for Patients Post-Acute Coronary Syndrome trial. Am Heart J. 2015;170:635–640. doi: 10.1016/j.ahj.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 49.Eisenberg MJ, Windle SB, Roy N, et al Varenicline for smoking cessation in hospitalized patients with acute coronary syndrome. Circulation. 2016;133:21–30. doi: 10.1161/CIRCULATIONAHA.115.019634. [DOI] [PubMed] [Google Scholar]
- 50.Anthenelli RM, Benowitz NL, West R, et al Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet. 2016;387:2507–2520. doi: 10.1016/S0140-6736(16)30272-0. [DOI] [PubMed] [Google Scholar]
- 51.Benowitz NL, Pipe A, West R, et al Cardiovascular safety of varenicline, bupropion, and nicotine patch in smokers: a randomized clinical trial. JAMA Intern Med. 2018;178:622–631. doi: 10.1001/jamainternmed.2018.0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Allo G, Diez-Villanueva P Type 2 diabetes, obesity and nutrition. In: Manual de Cardiopatía En El Paciente Anciano. Editorial IMC. 2018:71–78. [Google Scholar]
- 53.Collet J-P, Thiele H, Barbato E, et al 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: the task force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC) Eur Heart J. 2021;42:1289–1367. doi: 10.1093/eurheartj/ehaa575. [DOI] [PubMed] [Google Scholar]
- 54.Ibanez B, James S, Agewall S, et al 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC) Eur Heart J. 2018;39:119–177. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 55.Valgimigli M, Bueno H, Byrne RA, et al 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS) Eur Heart J. 2018;39:213–260. doi: 10.1093/eurheartj/ehx419. [DOI] [PubMed] [Google Scholar]
- 56.Guerrero C, Ariza-Solé A, Formiga F, et al Applicability of the PRECISE-DAPT score in elderly patients with myocardial infarction. J Geriatr Cardiol. 2018;15:713–717. doi: 10.11909/j.issn.1671-5411.2018.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wiviott SD, Braunwald E, McCabe CH, et al Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–2015. doi: 10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
- 58.Cannon CP, Harrington RA, James S, et al Comparison of ticagrelor with clopidogrel in patients with a planned invasive strategy for acute coronary syndromes (PLATO): a randomised double-blind study. Lancet. 2010;375:283–293. doi: 10.1016/S0140-6736(09)62191-7. [DOI] [PubMed] [Google Scholar]
- 59.Cayla G, Cuisset T, Silvain J, et al Platelet function monitoring to adjust antiplatelet therapy in elderly patients stented for an acute coronary syndrome (ANTARCTIC): an open-label, blinded-endpoint, randomised controlled superiority trial. Lancet. 2016;388:2015–2022. doi: 10.1016/S0140-6736(16)31323-X. [DOI] [PubMed] [Google Scholar]
- 60.Savonitto S, Ferri LA, Piatti L, et al Comparison of reduced-dose prasugrel and standard-dose clopidogrel in elderly patients with acute coronary syndromes undergoing early percutaneous revascularization. Circulation. 2018;137:2435–2445. doi: 10.1161/CIRCULATIONAHA.117.032180. [DOI] [PubMed] [Google Scholar]
- 61.Husted S, James S, Becker RC, et al Ticagrelor versus clopidogrel in elderly patients with acute coronary syndromes: a substudy from the prospective randomized PLATelet inhibition and patient Outcomes (PLATO) trial. Circ Cardiovasc Qual Outcomes. 2012;5:680–688. doi: 10.1161/CIRCOUTCOMES.111.964395. [DOI] [PubMed] [Google Scholar]
- 62.Schmucker J, Fach A, Mata Marin LA, et al Efficacy and safety of ticagrelor in comparison to clopidogrel in elderly patients with ST-segment-elevation myocardial infarctions. J Am Heart Assoc. 2019;8:e012530. doi: 10.1161/JAHA.119.012530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bonaca MP, Bhatt DL, Cohen M, et al Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. 2015;372:1791–1800. doi: 10.1056/NEJMoa1500857. [DOI] [PubMed] [Google Scholar]
- 64.Eikelboom JW, Connolly SJ, Bosch J, et al Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med. 2017;377:1319–1330. doi: 10.1056/NEJMoa1709118. [DOI] [PubMed] [Google Scholar]
- 65.Mauri L, Kereiakes DJ, Yeh RW, et al Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med. 2014;371:2155–2166. doi: 10.1056/NEJMoa1409312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tinetti ME, Fried TR, Boyd CM Designing health care for the most common chronic condition-multimorbidity. JAMA. 2012;307:2493–2494. doi: 10.1001/jama.2012.5265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Marengoni A, Angleman S, Melis R, et al Aging with multimorbidity: a systematic review of the literature. Ageing Res Rev. 2011;10:430–439. doi: 10.1016/j.arr.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 68.Sanchis J, Soler M, Núñez J, et al Comorbidity assessment for mortality risk stratification in elderly patients with acute coronary syndrome. Eur J Intern Med. 2019;62:48–53. doi: 10.1016/j.ejim.2019.01.018. [DOI] [PubMed] [Google Scholar]
- 69.Linn BS, Linn MW, Gurel L Cumulative illness rating scale. J Am Geriatr Soc. 1968;16:622–626. doi: 10.1111/j.1532-5415.1968.tb02103.x. [DOI] [PubMed] [Google Scholar]
- 70.Bureau ML, Liu E, Christiaens L, et al Using a multidimensional prognostic index (MPI) based on comprehensive geriatric assessment (CGA) to predict mortality in elderly undergoing transcatheter aortic valve implantation. Int J Cardiol. 2017;236:381–386. doi: 10.1016/j.ijcard.2017.02.048. [DOI] [PubMed] [Google Scholar]
- 71.Rockwood K, Song X, MacKnight C, et al A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Morley JE, Vellas B, van Kan GA, et al Frailty consensus: a call to action. J Am Med Dir Assoc. 2013;14:392–397. doi: 10.1016/j.jamda.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Doehner W, Ural D, Haeusler KG, et al Heart and brain interaction in patients with heart failure: overview and proposal for a taxonomy. A position paper from the Study Group on Heart and Brain Interaction of the Heart Failure Association. Eur J Heart Fail. 2018;20:199–215. doi: 10.1002/ejhf.1100. [DOI] [PubMed] [Google Scholar]
- 74.Deckers K, Schievink SHJ, Rodriquez MMF, et al Coronary heart disease and risk for cognitive impairment or dementia: Systematic review and meta-analysis. PLoS One. 2017;12:e0184244. doi: 10.1371/journal.pone.0184244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McMurray JJV, Solomon SD, Inzucchi SE, et al Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. doi: 10.1056/NEJMoa1911303. [DOI] [PubMed] [Google Scholar]
- 76.Packer M, Butler J, Filippatos GS, et al Evaluation of the effect of sodium-glucose co-transporter 2 inhibition with empagliflozin on morbidity and mortality of patients with chronic heart failure and a reduced ejection fraction: rationale for and design of the EMPEROR-Reduced trial. Eur J Heart Fail. 2019;21:1270–1278. doi: 10.1002/ejhf.1536. [DOI] [PubMed] [Google Scholar]
- 77.Packer M, Anker SD, Butler J, et al Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413–1424. doi: 10.1056/NEJMoa2022190. [DOI] [PubMed] [Google Scholar]
- 78.Wanner C, Inzucchi SE, Lachin JM, et al Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323–334. doi: 10.1056/NEJMoa1515920. [DOI] [PubMed] [Google Scholar]
- 79.Perkovic V, Jardine MJ, Neal B, et al Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295–2306. doi: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 80.Heerspink HJL, Stefánsson BV, Correa-Rotter R, et al Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383:1436–1446. doi: 10.1056/NEJMoa2024816. [DOI] [PubMed] [Google Scholar]
- 81.O’Mahony D, O’Sullivan D, Byrne S, et al STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing. 2015;44:213–218. doi: 10.1093/ageing/afv114.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Castellano JM, Sanz G, Peñalvo JL, et al A polypill strategy to improve adherence: results from the FOCUS project. J Am Coll Cardiol. 2014;64:2071–2082. doi: 10.1016/j.jacc.2014.08.021. [DOI] [PubMed] [Google Scholar]
- 83.Masjuan J, Gállego J, Aguilera JM, et al Use of the cardiovascular polypill in the secondary prevention of cerebrovascular disease. Neurología. 2021;36:1–8. doi: 10.1016/j.nrl.2017.10.013. [DOI] [PubMed] [Google Scholar]
- 84.Calvo E, Izquierdo S, Castillo R, et al Can an individualized adherence education program delivered by nurses improve therapeutic adherence in elderly people with acute myocardial infarction: A randomized controlled study. Int J Nurs Stud. 2021;120:103975. doi: 10.1016/j.ijnurstu.2021.103975. [DOI] [PubMed] [Google Scholar]
- 85.Anderson L, Oldridge N, Thompson DR, et al Exercise-based cardiac rehabilitation for coronary heart disease: Cochrane systematic review and meta-analysis. J Am Coll Cardiol. 2016;67:1–12. doi: 10.1016/j.jacc.2015.10.044. [DOI] [PubMed] [Google Scholar]
- 86.Vanhees L, De Sutter J, Gelada SN, et al Importance of characteristics and modalities of physical activity and exercise in defining the benefits to cardiovascular health within the general population: recommendations from the EACPR (Part I) Eur J Prev Cardiol. 2012;19:670–686. doi: 10.1177/2047487312437059. [DOI] [PubMed] [Google Scholar]
- 87.Nelson ME, Rejeski WJ, Blair SN, et al Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116:1094–1105. doi: 10.1161/CIRCULATIONAHA.107.185650. [DOI] [PubMed] [Google Scholar]
- 88.Piepoli MF, Corrà U, Benzer W, et al Secondary prevention through cardiac rehabilitation: from knowledge to implementation. A position paper from the Cardiac Rehabilitation Section of the European Association of Cardiovascular Prevention and Rehabilitation. Eur J Cardiovasc Prev Rehabil. 2010;17:1–17. doi: 10.1097/HJR.0b013e3283313592. [DOI] [PubMed] [Google Scholar]
- 89.Kitzman DW, Whellan DJ, Duncan P, et al Physical rehabilitation for older patients hospitalized for heart failure. N Engl J Med. 2021;385:203–216. doi: 10.1056/NEJMoa2026141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pelliccia A, Sharma S, Gati S, et al 2020 ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease: The Task Force on sports cardiology and exercise in patients with cardiovascular disease of the European Society of Cardiology (ESC) Eur Heart J. 2021;42:17–96. doi: 10.1093/eurheartj/ehaa605. [DOI] [PubMed] [Google Scholar]
- 91.Zanettini R, Centeleghe P, Franzelli C, et al Validity of the Talk Test for exercise prescription after myocardial revascularization. Eur J Prev Cardiol. 2013;20:376–382. doi: 10.1177/2047487312438982. [DOI] [PubMed] [Google Scholar]
- 92.Cadore EL, Casas-Herrero A, Zambom-Ferraresi F, et al Multicomponent exercises including muscle power training enhance muscle mass, power output, and functional outcomes in institutionalized frail nonagenarians. Age. 2014;36:773–785. doi: 10.1007/s11357-013-9586-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vanhees L, Rauch B, Piepoli M, et al Importance of characteristics and modalities of physical activity and exercise in the management of cardiovascular health in individuals with cardiovascular disease (Part III) Eur J Prev Cardiol. 2012;19:1333–1356. doi: 10.1177/2047487312437063. [DOI] [PubMed] [Google Scholar]
- 94.Prescott E, Mikkelsen N, Holdgaard A, et al Cardiac rehabilitation in the elderly patient in eight rehabilitation units in Western Europe: baseline data from the EU-CaRE multicentre observational study. Eur J Prev Cardiol. 2019;26:1052–1063. doi: 10.1177/2047487319839819. [DOI] [PubMed] [Google Scholar]
- 95.de Vries H, Kemps HMC, van Engen-Verheul MM, et al Cardiac rehabilitation and survival in a large representative community cohort of Dutch patients. Eur Heart J. 2015;36:1519–1528. doi: 10.1093/eurheartj/ehv111. [DOI] [PubMed] [Google Scholar]
- 96.Brown TM, Hernandez AF, Bittner V, et al Predictors of cardiac rehabilitation referral in coronary artery disease patients: findings from the American Heart Association's get with the guidelines program. J Am Coll Cardiol. 2009;54:515–521. doi: 10.1016/j.jacc.2009.02.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Giallauria F, Vigorito C, Tramarin R, et al Cardiac rehabilitation in very old patients: data from the Italian Survey on Cardiac Rehabilitation-2008 (ISYDE-2008) - official report of the Italian Association for Cardiovascular Prevention, Rehabilitation, and Epidemiology. J Gerontol A Biol Sci Med Sci. 2010;65:1353–1361. doi: 10.1093/gerona/glq138. [DOI] [PubMed] [Google Scholar]
- 98.Vigorito C, Abreu A, Ambrosetti M, et al Frailty and cardiac rehabilitation: A call to action from the EAPC Cardiac Rehabilitation Section. Eur J Prev Cardiol. 2017;24:577–590. doi: 10.1177/2047487316682579. [DOI] [PubMed] [Google Scholar]
- 99.Graham MM, Galbraith PD, O'Neill D, et al Frailty and outcome in elderly patients with acute coronary syndrome. Can J Cardiol. 2013;29:1610–1615. doi: 10.1016/j.cjca.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 100.Ushijima A, Morita N, Hama T, et al Effects of cardiac rehabilitation on physical function and exercise capacity in elderly cardiovascular patients with frailty. J Cardiol. 2021;77:424–431. doi: 10.1016/j.jjcc.2020.11.012. [DOI] [PubMed] [Google Scholar]
- 101.Faber MJ, Bosscher RJ, Chin A, et al Effects of exercise programs on falls and mobility in frail and pre-frail older adults: a multicenter randomized controlled trial. Arch Phys Med Rehabil. 2006;87:885–896. doi: 10.1016/j.apmr.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 102.Blondell SJ, Hammersley-Mather R, Veerman JL Does physical activity prevent cognitive decline and dementia: a systematic review and meta-analysis of longitudinal studies. BMC Public Health. 2014;14:510. doi: 10.1186/1471-2458-14-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kjesbu IE, Mikkelsen N, Sibilitz KL, et al. Greater burden of risk factors and less effect of cardiac rehabilitation in elderly with low educational attainment: The Eu-CaRE study. Eur J Prev Cardiol 2020; 2047487320921485.
- 104.Barrios V, Cosín-Sales J, Bravo M, et al Telematic consultation for the clinical cardiologist in times of COVID-19: present and future. Consensus document of the Spanish Society of Cardiology. Rev Esp Cardiol. 2020;73:910–918. doi: 10.1016/j.rec.2020.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bonanad C, García-Blas S, Tarazona-Santabalbina FJ, et al Coronavirus: the geriatric emergency of 2020. Joint document of the Geriatric Cardiology Section of the Spanish Society of Cardiology and the Spanish Society of Geriatrics and Gerontology. Rev Esp Cardiol. 2020;73:569–576. doi: 10.1016/j.rec.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Díez-Villanueva P, Bonanad C, Ariza-Solé A, et al Telematic cardiology consultation in the elderly. The 5M framework can help. Rev Esp Cardiol. 2021;74:116–117. doi: 10.1016/j.rec.2020.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kumar KR, Pina IL Cardiac rehabilitation in older adults: New options. Clin Cardiol. 2019;43:163–170. doi: 10.1002/clc.23296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Widmer RJ, Allison TG, Lennon R, et al Digital health intervention during cardiac rehabilitation: A randomized controlled trial. Am Heart J. 2017;188:65–72. doi: 10.1016/j.ahj.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 109.Varnfield M, Karunanithi M, Lee CK, et al Smartphone-based home care model improved use of cardiac rehabilitation in postmyocardial infarction patients: results from a randomised controlled trial. Heart. 2014;100:1770–1779. doi: 10.1136/heartjnl-2014-305783. [DOI] [PubMed] [Google Scholar]