Abstract
Since the anti-inflammatory effect of hydrogen has been widely known, it was supposed that hydrogen could suppress tissue damage by inhibiting virus-related inflammatory reactions. However, hydrogen is slightly soluble in water, which leads to poor effect of oral hydrogen-rich water therapy. In this study, the nano-bubble hydrogen water (nano-HW) (about 0.7 ppm) was prepared and its therapeutic effect against viral infection was investigated by utilizing spring viraemia of carp virus (SVCV)-infected zebrafish as model. Three-month-old zebrafish were divided into nano-HW treatment–treated group and aquaculture water treated group (control group). The results revealed that the cumulative mortality rate of SVCV-infected zebrafish was reduced by 40% after treatment with nano-bubble hydrogen water, and qRT-PCR results showed that SVCV replication was significantly inhibited. Histopathological examination staining showed that SVCV infection caused tissue damage was greatly alleviated after treatment with nano-bubble hydrogen water. Futhermore, SVCV infection caused reactive oxygen species (ROS) accumulation was significantly reduced upon nano-HW treatment. The level of proinflammatory cytokines IL-1β, IL-8, and TNF-α was remarkably reduced in the nano-HW-treated group in vivo and in vitro. Taken together, our data demonstrated for the first time that nano-HW could inhibit the inflammatory response caused by viral infection in zebrafish, which suggests that nano-HW can be applied to antiviral research,and provides a novel therapeutic strategy for virus-caused inflammation related disease.
Keywords: Nano-bubble hydrogen water (nano-HW), Spring viraemia of carp virus (SVCV), Zebrafish, Inflammation
Highlights
-
•
Nano-HW treatment reduces the mortality of SVCV-infected zebrafish.
-
•
Nano-HW alleviates the inflammatory response caused by SVCV infection.
-
•
Nano-HW inhibits SVCV replication.
1. Introduction
Since the first report about clinical application of high-pressure H2 on treating squamous cell carcinoma in hairless albino mice (Dole et al., 1975), the gas has been demonstrated to have significant anti-tumor, anti-inflammatory, and anti-oxidation effect (Iuchi et al., 2016; Watanabe et al., 2017; Sim et al., 2020). Recent studies of clinical study of H2 mainly focus on its anti-tumor effect which can effectively eliminate endometrial cancer growth in a xenograft mouse model by facilitating the death of cancerous cells and initiating pyroptosis mediated by the GSDMD pathway (Yang et al., 2020b). Cancer prognosis can be improved in guinea pigs by inhalation of H2 due to its effect on reducing the number of terminal PD-1+CD8+T cells by stimulating mitochondrial activity (Xu et al., 2018). In addition, H2 can disturb cancer growth by relieving oxidation stress (Wu et al., 2019), and it can also alleviate side effects caused by chemotherapy and radiotherapy (Ishibashi et al., 2015). Clinical research conducted by Chen et al. has shown that physical conditions of 41.5% of patients with stage III or stage IV cancer was ameliorated after four weeks of H2 inhalation (Chen et al., 2019). Chen et al. has also shown that combined treatment of H2 and chemotherapy alleviates lung cancer symptom more significantly than using chemotherapy individually (Chen et al., 2020).
Apart from cancer treatment, H2 has also been reported to have effects on regulating endoplasmic reticulum stress (Zhao et al., 2019), treating Parkinson's disease by restraining nigrostrital degeneration development (Fu et al., 2009), and maintaining cardiac health and for cardiovascular disease prophylaxis (Zhuang et al., 2019). Though there are many reports of H2 treatment from a great variety of diseases, research on H2 treatment of viral infected diseases is still limited. China National Health Commission stated in Chinese Clinical Guidance for COVID-19 Pneumonia Diagnosis and Treatment (7th edition) suggests that inhaling H2 can improve respiratory system condition, which especially results in dyspnea amelioration (Guan et al., 2020). When treating COVID-19 using ventilators, inhaling H2 contained gas can result in alleviation of lung injury due to its effect on promoting anti-apoptotic protein expression (Huang et al., 2010), which may also eliminate P53 signaling pathway and apoptosis activation therefore dwindle risk of developing lymphopenia (Xiong et al., 2020). However, the mechanism for its therapeutic effect is still unclear.
Although H2 has been widely accepted as antioxidant and antitumor reagent, the particle utilization of H2 treatment is still limited due to poor accessibility of hydrogen to body parts (Yang et al., 2020a). H2 has been reported to be slightly soluble in water, making effectiveness of oral hydrogen-rich water therapy lower than expected (Iida et al., 2016). Recently, nano-bubble technology was established that has been developed in medical, pharmaceutical, and dental applications (Kato et al., 2020; Xiao et al., 2021). Nano-bubbles in liquids exhibit excellent stability and permeability. Recent technology can increase H2 solubility by producing nano-bubbles hydrogen water, which may lead to stronger effectiveness of hydrogen treatments.
Spring viremia of carp virus (SVCV) is a pathogen causing spring viremia of carp (SVC), a highly contagious and haemorrhagic disease in cyprinids. As a member of the genus Spirivivirus belonging to Rhabdoviridae family, SVCV is an enveloped, bullet-shaped virus with a size of 80–180 nm in length and 60–90 nm in diameter. The genome of SVCV is composed of linear, negative-sense, single-stranded RNA (ssRNA). SVCV is a notifiable pathogen on the OIE-World Organization for Animal Health list. The fatality rate of SVC can reach up to 90% in spring when water temperature is between 10 and 17 °C (Ahne et al., 2002). The virus has been detected in spleen, liver, brain, kidney, and intestinal of infected fish. Multifocal necrosis and non-purulent inflammation often occur in the pancreas of affected fish, and the heart shows pericarditis and discontinuous myodegeneration (Misk et al., 2016). In the intestine, perivasculitis with subsequent atrophy of the villi is often observed (Ahne et al., 2002; Misk et al., 2016). However, currently, there is no effective therapeutic method or drug for SVCV infection.
In this study, we used SVCV infected zebrafish as a model and found that nano-HW can reduce the inflammatory response induced by SVCV infection and increase the host's resistance to viral infections. These findings may provide new understanding on functions of nano-HW about anti-inflammatory response in zebrafish model in addition to its effect on anti-tumor, anti-inflammatory, and anti-oxidation.
2. Methods and materials
2.1. Preparation and measurement of nano-bubble hydrogen water
An apparatus (a microporous hydrogen-gas bubbling emittance-terminal from Japan), which can produce nano-bubble hydrogen-dissolved water, produces nano-bubble hydrogen water (nano-HW) by shearing bubbles in hydrogen water. We prepared nano-bubble hydrogen-dissolved water from the purified water using the apparatus. Nano-sized hydrogen bubbles in nano-HW were analyzed using Nanoparticle Tracking Analysis (Zetasizer; Malvern, UK). The water samples were stored at 4 °C in a screwcapped aluminum container without air exchange. Each sample was tested 2 times.
2.2. Fish cell lines and viruses
The zebrafish embryo (ZF4) cells (ATCC® CRL-2050™) were maintained in DMEM/F-12 (Hyclone, USA) with 10% FBS (Gibco, Australia) at 28 °C. The epithelioma papulosum cyprini (EPC) cells (ATCC CRL-2872) were maintained at 28 °C in Medium 199 (Hyclone, USA) supplemented with 10% FBS (Gibco, Australia). SVCV (ATCC: VR-1390) was used for viral infection in this study and viral titers were determined by plaque assay.
2.3. Zebrafish
Zebrafish were purchased from China Zebrafish Resource Centre (CZRC) and the care, breeding, feeding and challenging were carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health.
2.4. Experimental infection
Adult zebrafish (0.6 ± 0.1 g, n = 120) were divided into four groups, with two groups maintained at ordinary aquaculture water and two groups in nano-bubble hydrogen water. In nano-HW treated group, zebrafish were raised in receptacles containing 2 L of nano-HW. The water was changed every 4 h by removing 1 L of water in the receptacle and replacing it with 1 L of nano-HW, to maintain the concentration of hydrogen in the water at a relatively high level. In order to be consistent, the other two groups were also replaced half of the previous water with fresh water. The fish were adapted to their respective feeding conditions for 7 days before infection. Two out of four groups were intraperitoneally (i.p.) injected with 10 μL (∼2 × 108 PFU/mL) SVCV suspension per fish. The i.p. Injection of Medium 199 was used as a control.
2.5. Virus infection of ZF4 cells
For virus infection assays, ZF4 cells were infected with SVCV at a multiplicity of infection (MOI) of 1 at 28 °C. After 1 h virus absorption, cells were washed with PBS for three times, and subsequently maintained in M199 supplemented with 5% FBS.
2.6. Quantitative real-time PCR (qRT-PCR)
Total SVCV RNA was extracted using TRIzol Reagent (TAKARA, Japan) according to the instruction of manufacturer. The reverse transcription was carried out using the ReverTra Ace qPCR RT kit (TAKARA). The relative expression of each cDNA was determined by qRT-PCR using TB Green Realtime PCR Master Mix (TAKARA). Amplification was performed for 5 min at 95 °C, followed by 40 cycles of 95 °C for 15 s, 60 °C for 20 s, and 72 °C for 20 s. Fluorescent signals were analyzed by a Light Cycler/Light Cycler 480 System (Roche, Switzerland). The relative mRNA levels were calculated using the 2−ΔΔCT (where CT is threshold cycle) method. All the primers employed in this study are listed in Supplementary Table S1.
2.7. Pathological section for Hematoxylin and eosin (H&E) staining
As for the pathological sections, the intestinal and brain were fixed in 4% (v/v) paraformaldehyde and were routinely processed, sectioned at 5 mm. The standard H&E staining protocol was followed for tissue staining. The H&E-stained sections were analyzed for virus-induced damages. Images were obtained using a Zeiss Axio Imager Z2 microscope and analyzed with the ZEN software (Carl Zeiss MicroImaging).
2.8. Viral plaque assay
Viruses were serially diluted and inoculated onto monolayers of EPC cells. After 1 h of absorption, cells were washed with serum-free DMEM and cultured in DMEM containing 3% fetal bovine serum and 1.5% sodium carboxymethyl cellulose (Sigma-Aldrich, USA). Visible plaques were counted, and viral titers were calculated after 3 days of incubation.
2.9. Reactive oxygen species (ROS) and O2− staining assay
Total intracellular ROS in the mixed tissues (kidney, intestine, brain, liver and spleen) of mock-infected and SVCV-infected zebrafish at 1, 3, 5 and 7 days post-infection based on 2,7-dichlorofuorescin diacetate (DCFH-DA) was measured with a Reactive Oxygen Species Assay Kit according to the manufacturer's instructions (E004, Jiancheng, Nanjing, China). O2− in the mixed tissues was also examined with Dihydroethidium (DHE) probes according to the manufacturer's instructions (S0063, Beytome, Hangzhou, China). The fluorescence intensities were measured on the Synergy HTX Multi-Mode Reader (Winooski, USA) with excitation at 300 nm and emission at 535 nm.
2.10. Statistics analysis
All statistical analyses and calculations were done using GraphPad Prism 7.0 (GraphPad Software Inc, USA). The significance of the variability between different treatment groups was determined by two-way analysis of variance. A P value of <0.05 was considered statistically significant. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, ∗∗∗∗P < 0.0001. ns means no significant difference. Data are expressed as means ± standard deviations of results from three independent experiments.
3. Results
3.1. Characteristics of hydrogen nano-bubble water (nano-HW)
Given that it is easy for the dissolved hydrogen to escapes from the water, the hydrogen molecules were wrapped in nano-sized fine bubbles, which increases the stability of hydrogen molecules in water. The Zetasizer analysis showed that the average size of the bubbles was about 200 nm in fresh nano-HW (Fig. 1A). Nano-HW had a dissolved hydrogen concentration of about 0.7 ppm, and the hydrogen nano-bubble can exist for about 12 h. However, in aquaculture water rearing zebrafish, hydrogen nano-bubbles reduced more quickly. In addition, in supernatant of ZF4 cell culture, hydrogen can be maintained for about 4 h above 0.09 ppm (Fig. 1B). These data suggest that hydrogen concentrations in nano-HW decrease slowly with time.
Fig. 1.

Parameters of hydrogen nano-bubbles in nano-bubble hydrogen water (nano-HW). A Sizes of hydrogen bubbles in nano-HW were measured with a Nano Sight LM10V-HS system and analyzed using Zetasizer software. B Concentration of hydrogen nano-bubbles in nano-HW samples at different time post-treatment. The experiment was repeated for two times.
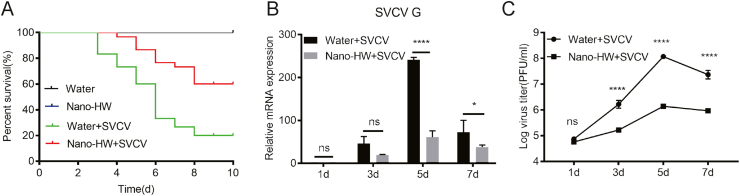
3.2. Nano-HW treatment reduces the mortality of SVCV-infected zebrafish
To evaluate the effect of nano-HW on virus-caused inflammation, zebrafish was infected with SVCV and then treated with nano-HW. The cumulative mortality rate of SVCV-infected zebrafish reached up to 80% at 10 dpi. Upon nano-HW treatment, the cumulative mortality rate of zebrafish was reduced to 40% (Fig. 2A). To further determine the anti-viral activity of nano-HW, the expressions of SVCV G protein mRNA and the virus titer in zebrafish were measured. The result showed that nano-HW significantly inhibits SVCV replication and viral particle production (Fig. 2B and C).
Fig. 2.
Antiviral activity of nano-HW against SVCV in zebrafish. A Survival of zebrafish in the presence and absence of HW treatment. Size- and age-matched zebrafish (0.6 ± 0.1 g) were injected with 10 μL of SVCV suspension (∼2 × 108 PFU/mL) per fish, and then treated with nano-HW. B Viral gene expression in nano-HW-treated and untreated zebrafish. The relative mRNA expression of SVCV G protein was examined by qRT-PCR in mixed tissues from 3 fish on 1, 3, 5 and 7 dpi. C The virus titer in nano-HW-treated and untreated zebrafish. The viral titer was measured by standard plaque assay with EPC cells. Viral particles in mixed tissues were titered on days 1, 3, 5, and 7 post-infection. Each value was represented as mean ± SEM of three fishes. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, ∗∗∗∗P < 0.0001. ns means no significant difference.
3.3. Nano-HW improves intestinal and brain damage in SVCV-infected zebrafish
Hydrogen can ameliorate tissue damage caused by inflammation. The role of nano-HW in tissue damage induced by SVCV was verified. Samples at 5 dpi were collected, since the most severe clinical symptoms appeared on day 5 post-infection, as shown in Fig. 3A and Fig. 3B, the midgut of zebrafish is selected for H&E staining. The intestinal villi and intestinal wall of zebrafish were severely damaged after the virus infection. Compared with the zebrafish in untreated group, the intestinal tissue damage of those in nano-HW treated group was obviously alleviated in general, although intestinal villi were still shorted, and lymphocyte infiltration was slightly increased, compared to the zebrafish in uninfected group. The brain tissues were also harvest for H&E staining (Fig. 3C and D). The histopathological pictures showed obvious necrotic changes in the brains of SVCV-infected zebrafish compared with those of uninfected zebrafish. As expected, the brain tissue damage was potently attenuated after the nano-HW treatment. The results further support the conclusion that nano-HW treatment alleviated the tissue damage of SVCV infected zebrafish.
Fig. 3.
Histopathology of tissues from nano-HW-treated and untreated zebrafish. Tissue section of intestines (A and B) and brain (C–D) on day 5 post-infection were subjected to H&E stain. Images represent the 20 × (Scale bar = 100 μm in A and C) or 40 × (Scale bar = 50 μm in B and D) of H&E staining.
3.4. Repressive effects of Nano-HW on SVCV-Induced ROS generation in zebrafish
Since the nano-HW is reported to affect oxidative stress (Ohsawa et al., 2007; Hirayama et al., 2018), total ROS in mock-infected and SVCV-infected zebrafish was monitored using a Reactive Oxygen Species Assay Kit based on 2, 7-dichlorofuorescin diacetate (DCFH-DA) probes. SVCV infection in zebrafish increased ROS accumulation at 3, 5 and 7 days post infection and peaked at 5 days post infection. After nano-HW treatment, the total ROS was significantly reduced (Fig. 4A). Further, dihydroethidium (DHE), a non-fluorescent cell-permeable indicator for O2−, was used to quantify the amount of O2− after SVCV infection in zebrafish. The amount of O2− at 1, 3, 5 and 7 days post infection was significantly increased comparing with that in mock-infected zebrafish. However, the amount of O2− was significantly reduced upon nano-HW treatment (Fig. 4B).
Fig. 4.
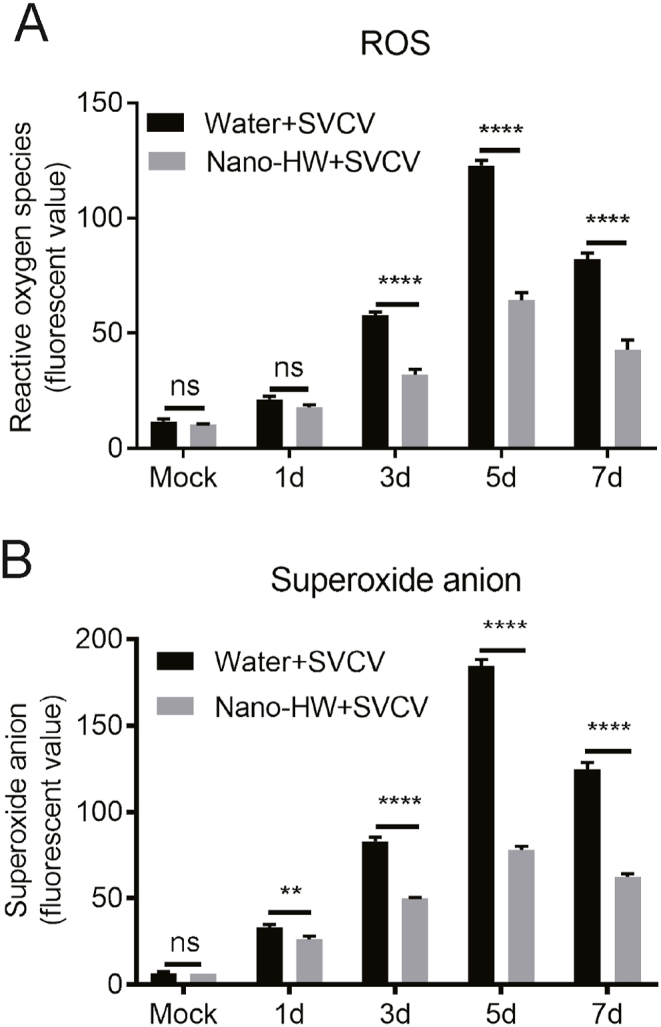
Effects of nano-HW on SVCV-Induced ROS production in zebrafish. ATotal reactive oxygen species were measured in mixed tissues (kidney, intestine, brain, liver and spleen) at 1, 3, 5, and 7 dpi with 2, 7-dichlorofuorescin diacetate (DCFH-DA) probes. B Superoxide anion was monitored in mixed tissues (kidney, intestine, brain, liver and spleen) using dihydroethidium (DHE) at 1, 3, 5, and 7 dpi. Each value was represented as mean ± SEM of three fish. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, ∗∗∗∗P < 0.0001. ns means no significant difference.
3.5. Nano-HW alleviates the inflammatory response caused by SVCV infection in zebrafish
Since it is well known that hydrogen can reduce inflammation (Liu et al., 2013; Kajisa et al., 2017), the physiological role of nano-HW on SVCV-caused inflammation was determined. To this end, the mRNA levels of pro-inflammatory cytokines IL-1β, IL-8, and TNF-α in response to viral infection was examined. As shown in Fig. 5A–C, nano-HW treatment resulted in reduced expression of IL-1β, IL-8, and TNF-α in SVCV infected Zebrafish.
Fig. 5.
Effect of nano-HW on the inflammatory response in SVCV- infected zebrafish. The IL-1β (A), IL-8 (B), and TNF-α (C) mRNA levels were examined in mixed tissues (kidney, intestine, brain, liver and spleen) of SVCV- infected zebrafish on 1, 3, 5 and 7 dpi. Each value represented the mean ± SEM of three fish. The experiment was repeated for three times. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, ∗∗∗∗P < 0.0001. ns means no significant difference.
3.6. Nano-HW reduces SVCV-induced inflammatory response in ZF4 cells
To further confirm the effect of nano-HW on SVCV caused inflammation in vitro, the impact of nano-HW on the inflammation was examined in ZF4 cells. As expected, treatment with nano-HW prepared Medium resulted in reduced expression of IL-1β, IL-8, and TNF-α in SVCV infected ZF4 cells compared with the untreated cells (Fig. 6A–C). In addition, we further evaluated the titers of SVCV in nano-HW treated ZF4 cells. The results show that there is a significant difference between the two groups at 12 h after SVCV infection, but there is no significant difference between 24 h and 36 h after SVCV infection (Fig. 6D).
Fig. 6.
Effect of nano-HW on the inflammatory response in SVCV- infected ZF4 cells. ZF4 cells were cultured in DMEM/F-12 medium or nano-HW-prepared DMEM-F12 medium to reach to a logarithmic growth. ZF4 cells were infected with SVCV (1 MOI), and the mRNA expression levels of IL-1β (A), IL-8 (B), and TNF-α (C) were quantified by qRT-PCR at 12 hpi. The SVCV titer (D) of infected ZF4 cells was determined at 6, 12, 24, and 36 hpi. All data are representatives of three independent experiments. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, ∗∗∗∗P < 0.0001. ns means no significant difference.
4. Discussion
Since hydrogen has great potential in the regulation of oxidative stress, inflammation, energy metabolism of organelles, and programmed cell death, many in vivo experiments and clinical trials have established the protective effects of hydrogen on different organs and systems. For example, hydrogen rich water can suppress oxidative stress and inflammatory response and thus provide protective effect on permanent focal cerebral ischemia (Kato et al., 2020). However, hydrogen molecules are very small and hardly soluble in water, which limits the clinical effectiveness of hydrogen. In order to increase the solubility of hydrogen in water, nano-bubbles wrapping the hydrogen like a capsule were prepared by a microporous hydrogen-gas bubbling emittance-terminal. Due to the high stability of nano-bubbles in water, hydrogen-rich water with higher concentration was made, which would be beneficial for clinical utilization. In the present study, we found that the number of hydrogen nano-bubbles remained at high level in HW for a long storage time, supporting that hydrogen nano-bubbles are relatively stable in water (Fig. 1B).
Most of previous studies about clinical use of hydrogen focused on its effect on cancer therapy. For instance, it was shown that nano-HW could suppress human esophagus cancer with the present of platinum–povidone (Jackson et al., 2018), and it can be utilize in high-fat-diet nonalcoholic fatty liver disease using mouse model (Li et al., 2012). The concentration of HW used in the above experiment is 0.2 mg/L (approximately 0.2 ppm). Our study for the first time utilized the nano-HW with concentration of 0.7 ppm for therapy of inflammation caused by viral infection. The high concentration of nano-HW may provide stronger therapeutic effect than that used in the previous studies.
To date, zebrafish have been widely employed as an animal model to investigate the pathogenesis of cancer and viral diseases (Novoa and Figueras, 2012; Brown et al., 2017; Zou and Nie, 2017; Yao et al., 2020). Infection with SVCV can result in body surface bleeding of zebrafish and eventual death (Wang et al., 2017). Zebrafish live in an aquatic environment, which benefits the absorption of hydrogen from nano-HW. Therefore, in this study, the SVCV-infected zebrafish were employed as an in vivo model to verify the function of nano-HW on viral-infection induced inflammation. We found that nano-HW can reduce the inflammatory response induced by SVCV infection and increase the host's resistance to viral infections. However, whether the similar effect of Nano-hydrogen would be shown in mammals is still needed for further investigation. It is possible to increase the absorption in mammals by making nano-HW using normal saline and inject in to the mammalians. Nevertheless, the safety of this method will need to be evaluated.
In our experiment, a decreased mortality rate of SVCV-infected zebrafish was observed upon nano-HW treatment (Fig. 2A), which is possibly related with the inhibitory effect of hydrogen molecules on inflammation response. Apart from the suppression of inflammatory factors, our experiment has also shown the effect of hydrogen on reducing viral load in infected organs (Fig. 2C). This is possibly due to the improvement of antiviral environment of host by alleviation of over-activated inflammation response.
We also examined the effects of nano-HW on tissue damage induced by SVCV. The H&E staining results suggest that treatment of nano-HW could alleviate the tissue damage of intestinal but not eliminate the damage. Compared with the zebrafish in uninfected group, intestinal villi were still shortened, and lymphocyte infiltration was slightly increased after nano-HW treatment. This may be caused by the oxidative damage. Molecular oxygen in water may be decreased on the process of creating nano-HW. Therefore, oxygen deprivation may also result in oxidative damage. However, further studies are needed to clarify this issue.
Molecular hydrogen is known to possess anti-oxidative properties (Ohsawa et al., 2007). Therefore, nano-HW may protect zebrafish against SVCV induced oxidative damage. In this study, the ROS level was lower in nano-HW-treated group than in the control group (Fig. 4). The results indicate that nano-HW could reduce oxidative damage in zebrafish tissues.
The anti-inflammatory effect of hydrogen has already been reported in many studies. Therefore, we expected that nano-HW would reduce expression of inflammatory factors in zebrafish. In this study, nano-HW treatment down-regulated the mRNA expression of inflammatory factors in SVCV-infected zebrafish (Fig. 5). In an in vitro study, we also found that nano-HW can reduce the expression of inflammatory factors and inhibit the production of virus particles. However, the plaque results showed that the virus titer can be significantly inhibited only at 12 h post infection. According to the results in Fig. 1B, we suspected that it was caused by the exhaustion of hydrogen in the medium. However, further research is required to clarify the underlying mechanisms.
In conclusion, our study demonstrated for the first time the anti-inflammation effect of Nano-hydrogen bubbles in virus infected zebrafish and cells. We found that nano-HW exhibits excellent stability, and fishes can fully absorb Nano-hydrogen molecules that dissolved in water as they live in aquatic environment. This study provides us new understanding of nano-HW function and novel therapeutic strategy for virus-caused inflammation.
Data availability
The datasets supporting the conclusions of this article are included within the article.
Ethics statement
All animal procedures were carried out strictly accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. All animal infections did not involve endangered or protected species. Experiments using zebrafish were performed under the approval of the Animal Ethics Committee of Huazhong Agriculture University (HZAU). The infection and dissection experiments were performed under 3-Aminobenzoic acid ethyl ester methanesulfonate (MS-222) (Sigma, USA) anesthesia to minimize fish suffering.
Authors contributions
Chen Li: writing – original draft, data curation, formal analysis, visualization. Yiran Cao: data curation, formal analysis, visualization. Fukuda Kohei: data curation, formal analysis. Haihong Hao: data curation, formal analysis. Guiqing Peng: resources, validation. Can Cheng: writing – review & editing. Jing Ye: conceptualization, writing – review & editing.
Conflict of interest
The authors have no financial conflicts of interest.
Acknowledgement
This work was supported by Natural Science Foundation of China (31972834,32022082, 31972721).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.virs.2022.01.023.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Ahne W., Bjorklund H.V., Essbauer S., Fijan N., Kurath G., Winton J.R. Spring viremia of carp (SVC) Dis. Aquat. Org. 2002;52:261–272. doi: 10.3354/dao052261. [DOI] [PubMed] [Google Scholar]
- Brown H.K., Schiavone K., Tazzyman S., Heymann D., Chico T.J. Zebrafish xenograft models of cancer and metastasis for drug discovery. Expet Opin. Drug Discov. 2017;12:379–389. doi: 10.1080/17460441.2017.1297416. [DOI] [PubMed] [Google Scholar]
- Chen J.B., Kong X.F., Lv Y.Y., Qin S.C., Sun X.J., Mu F., Lu T.Y., Xu K.C. Real world survey" of hydrogen-controlled cancer: a follow-up report of 82 advanced cancer patients. Med. Gas Res. 2019;9:115–121. doi: 10.4103/2045-9912.266985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.B., Kong X.F., Mu F., Lu T.Y., Lu Y.Y., Xu K.C. Hydrogen therapy can be used to control tumor progression and alleviate the adverse events of medications in patients with advanced non-small cell lung cancer. Med. Gas Res. 2020;10:75–80. doi: 10.4103/2045-9912.285560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dole M., Wilson F.R., Fife W.P. Hyperbaric hydrogen therapy: a possible treatment for cancer. Science. 1975;190:152–154. doi: 10.1126/science.1166304. [DOI] [PubMed] [Google Scholar]
- Fu Y., Ito M., Fujita Y., Ito M., Ichihara M., Masuda A., Suzuki Y., Maesawa S., Kajita Y., Hirayama M., Ohsawa I., Ohta S., Ohno K. Molecular hydrogen is protective against 6-hydroxydopamine-induced nigrostriatal degeneration in a rat model of Parkinson's disease. Neurosci. Lett. 2009;453:81–85. doi: 10.1016/j.neulet.2009.02.016. [DOI] [PubMed] [Google Scholar]
- Guan W.J., Wei C.H., Chen A.L., Sun X.C., Guo G.Y., Zou X., Shi J.D., Lai P.Z., Zheng Z.G., Zhong N.S. Hydrogen/oxygen mixed gas inhalation improves disease severity and dyspnea in patients with Coronavirus disease 2019 in a recent multicenter, open-label clinical trial. J. Thorac. Dis. 2020;12:3448–3452. doi: 10.21037/jtd-2020-057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirayama M., Ito M., Minato T., Yoritaka A., LeBaron T.W., Ohno K. Inhalation of hydrogen gas elevates urinary 8-hydroxy-2'-deoxyguanine in Parkinson's disease. Med. Gas Res. 2018;8:144–149. doi: 10.4103/2045-9912.248264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C.S., Kawamura T., Lee S., Tochigi N., Shigemura N., Buchholz B.M., Kloke J.D., Billiar T.R., Toyoda Y., Nakao A. Hydrogen inhalation ameliorates ventilator-induced lung injury. Crit. Care. 2010;14 doi: 10.1186/cc9389. R234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida A., Nosaka N., Yumoto T., Knaup E., Naito H., Nishiyama C., Yamakawa Y., Tsukahara K., Terado M., Sato K., Ugawa T., Nakao A. The clinical application of hydrogen as a medical treatment. Acta Med. Okayama. 2016;70:331–337. doi: 10.18926/AMO/54590. [DOI] [PubMed] [Google Scholar]
- Ishibashi T., Ichikawa M., Sato B., Shibata S., Hara Y., Naritomi Y., Okazaki K., Nakashima Y., Iwamoto Y., Koyanagi S., Hara H., Nagao T. Improvement of psoriasis-associated arthritis and skin lesions by treatment with molecular hydrogen: a report of three cases. Mol. Med. Rep. 2015;12:2757–2764. doi: 10.3892/mmr.2015.3707. [DOI] [PubMed] [Google Scholar]
- Iuchi K., Imoto A., Kamimura N., Nishimaki K., Ichimiya H., Yokota T., Ohta S. Molecular hydrogen regulates gene expression by modifying the free radical chain reaction-dependent generation of oxidized phospholipid mediators. Sci. Rep. 2016;6:18971. doi: 10.1038/srep18971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson K., Dressler N., Ben-Shushan R.S., Meerson A., LeBaron T.W., Tamir S. Effects of alkaline-electrolyzed and hydrogen-rich water, in a high-fat-diet nonalcoholic fatty liver disease mouse model. World J. Gastroenterol. 2018;24:5095–5108. doi: 10.3748/wjg.v24.i45.5095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajisa T., Yamaguchi T., Hu A., Suetake N., Kobayashi H. Hydrogen water ameliorates the severity of atopic dermatitis-like lesions and decreases interleukin-1beta, interleukin-33, and mast cell infiltration in NC/Nga mice. Saudi Med. J. 2017;38:928–933. doi: 10.15537/smj.2017.9.20807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato S., Saitoh Y., Miwa N. Hydrogen-bubbled platinum-colloid suppresses human esophagus- or tongue-carcinoma cells with intracellular platinum-uptake and the diminished normal-cell mortality. Hum. Cell. 2020;33:1294–1301. doi: 10.1007/s13577-020-00402-1. [DOI] [PubMed] [Google Scholar]
- Li J., Dong Y., Chen H., Han H., Yu Y., Wang G., Zeng Y., Xie K. Protective effects of hydrogen-rich saline in a rat model of permanent focal cerebral ischemia via reducing oxidative stress and inflammatory cytokines. Brain Res. 2012;1486:103–111. doi: 10.1016/j.brainres.2012.09.031. [DOI] [PubMed] [Google Scholar]
- Liu W., Shan L.P., Dong X.S., Liu X.W., Ma T., Liu Z. Combined early fluid resuscitation and hydrogen inhalation attenuates lung and intestine injury. World J. Gastroenterol. 2013;19:492–502. doi: 10.3748/wjg.v19.i4.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misk E., Garver K., Nagy E., Isaac S., Tubbs L., Huber P., Al-Hussinee L., Lumsden J.S. Pathogenesis of spring viremia of carp virus in emerald shiner Notropis atherinoides Rafinesque, fathead minnow Pimephales promelas Rafinesque and white sucker Catostomus commersonii (Lacepede) J. Fish. Dis. 2016;39:729–739. doi: 10.1111/jfd.12405. [DOI] [PubMed] [Google Scholar]
- Novoa B., Figueras A. Zebrafish: model for the study of inflammation and the innate immune response to infectious diseases. Adv. Exp. Med. Biol. 2012;946:253–275. doi: 10.1007/978-1-4614-0106-3_15. [DOI] [PubMed] [Google Scholar]
- Ohsawa I., Ishikawa M., Takahashi K., Watanabe M., Nishimaki K., Yamagata K., Katsura K., Katayama Y., Asoh S., Ohta S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007;13:688–694. doi: 10.1038/nm1577. [DOI] [PubMed] [Google Scholar]
- Sim M., Kim C.S., Shon W.J., Lee Y.K., Choi E.Y., Shin D.M. Hydrogen-rich water reduces inflammatory responses and prevents apoptosis of peripheral blood cells in healthy adults: a randomized, double-blind, controlled trial. Sci. Rep. 2020;10:12130. doi: 10.1038/s41598-020-68930-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Zhang H., Lu Y., Wang F., Liu L., Liu J., Liu X. Comparative transcriptome analysis of zebrafish (Danio rerio) brain and spleen infected with spring viremia of carp virus (SVCV) Fish Shellfish Immunol. 2017;69:35–45. doi: 10.1016/j.fsi.2017.07.055. [DOI] [PubMed] [Google Scholar]
- Watanabe M., Kamimura N., Iuchi K., Nishimaki K., Yokota T., Ogawa R., Ohta S. Protective effect of hydrogen gas inhalation on muscular damage using a mouse hindlimb ischemia-reperfusion injury model. Plast. Reconstr. Surg. 2017;140:1195–1206. doi: 10.1097/PRS.0000000000003878. [DOI] [PubMed] [Google Scholar]
- Wu Y., Yuan M., Song J., Chen X., Yang H. Hydrogen gas from inflammation treatment to cancer therapy. ACS Nano. 2019;13:8505–8511. doi: 10.1021/acsnano.9b05124. [DOI] [PubMed] [Google Scholar]
- Xiao L., Miwa N. Hydrogen nano-bubble water suppresses ROS generation, adipogenesis, and interleukin-6 secretion in hydrogen-peroxide- or PMA-stimulated adipocytes and three-dimensional subcutaneous adipose equivalents. Cells. 2021:10. doi: 10.3390/cells10030626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y., Liu Y., Cao L., Wang D., Guo M., Jiang A., Guo D., Hu W., Yang J., Tang Z., Wu H., Lin Y., Zhang M., Zhang Q., Shi M., Liu Y., Zhou Y., Lan K., Chen Y. Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID-19 patients. Emerg. Microb. Infect. 2020;9:761–770. doi: 10.1080/22221751.2020.1747363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F., Yu S., Qin M., Mao Y., Jin L., Che N., Liu S., Ge R. Hydrogen-rich saline ameliorates allergic rhinitis by reversing the imbalance of Th1/Th2 and up-regulation of CD4+CD25+Foxp3+Regulatory T cells, interleukin-10, and membrane-bound transforming growth factor-beta in Guinea pigs. Inflammation. 2018;41:81–92. doi: 10.1007/s10753-017-0666-6. [DOI] [PubMed] [Google Scholar]
- Yang M., Dong Y., He Q., Zhu P., Zhuang Q., Shen J., Zhang X., Zhao M. Hydrogen: a novel option in human disease treatment. Oxid. Med. Cell. Longev. 2020;2020:8384742. doi: 10.1155/2020/8384742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Liu P.Y., Bao W., Chen S.J., Wu F.S., Zhu P.Y. Hydrogen inhibits endometrial cancer growth via a ROS/NLRP3/caspase-1/GSDMD-mediated pyroptotic pathway. BMC Cancer. 2020;20:28. doi: 10.1186/s12885-019-6491-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y., Wang L., Wang X. Modeling of solid-tumor microenvironment in zebrafish (Danio rerio) larvae. Adv. Exp. Med. Biol. 2020;1219:413–428. doi: 10.1007/978-3-030-34025-4_22. [DOI] [PubMed] [Google Scholar]
- Zhao Y.S., An J.R., Yang S., Guan P., Yu F.Y., Li W., Li J.R., Guo Y., Sun Z.M., Ji E.S. Hydrogen and oxygen mixture to improve cardiac dysfunction and myocardial pathological changes induced by intermittent hypoxia in rats. Oxid. Med. Cell. Longev. 2019;2019:7415212. doi: 10.1155/2019/7415212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang K., Zuo Y.C., Sherchan P., Wang J.K., Yan X.X., Liu F. Hydrogen inhalation attenuates oxidative stress related endothelial cells injury after subarachnoid hemorrhage in rats. Front. Neurosci. 2019;13:1441. doi: 10.3389/fnins.2019.01441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou P.F., Nie P. Zebrafish as a model for the study of host-virus interactions. Methods Mol. Biol. 2017;1656:57–78. doi: 10.1007/978-1-4939-7237-1_2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting the conclusions of this article are included within the article.