Paired scRNA-seq and scTCR-seq reveals that diabetogenic CD8 T cells in the islets and spleens of NOD mice exhibit phenotypic and clonal heterogeneity despite restricted TCR gene usage. Expression of certain TCR genes correlates with clonal proliferation and effector phenotype.
Abstract
Type 1 diabetes (T1D) is an autoimmune disorder defined by CD8 T cell–mediated destruction of pancreatic β cells. We have previously shown that diabetogenic CD8 T cells in the islets of non-obese diabetic mice are phenotypically heterogeneous, but clonal heterogeneity remains relatively unexplored. Here, we use paired single-cell RNA and T-cell receptor sequencing (scRNA-seq and scTCR-seq) to characterize autoreactive CD8 T cells from the islets and spleens of non-obese diabetic mice. scTCR-seq demonstrates that CD8 T cells targeting the immunodominant β-cell epitope IGRP206-214 exhibit restricted TCR gene usage. scRNA-seq identifies six clusters of autoreactive CD8 T cells in the islets and six in the spleen, including memory and exhausted cells. Clonal overlap between IGRP206-214–reactive CD8 T cells in the islets and spleen suggests these cells may circulate between the islets and periphery. Finally, we identify correlations between TCR genes and T-cell clonal expansion and effector fate. Collectively, our work demonstrates that IGRP206-214–specific CD8 T cells are phenotypically heterogeneous but clonally restricted, raising the possibility of selectively targeting these TCR structures for therapeutic benefit.
Introduction
Type 1 diabetes (T1D) is an autoimmune condition that results in destruction of insulin-producing β cells in the pancreatic islets, thereby leaving affected patients dependent on exogenous insulin therapy (Bluestone et al, 2010). T1D is thought to be primarily mediated by T cells, with both CD4 and CD8 T cells involved in human disease and the non-obese diabetic (NOD) mouse model (Makino et al, 1980; Mullen, 2017). Over the last three decades, CD8 T cells have been studied more extensively in the context of chronic or relapsing inflammatory conditions such as chronic viral infections (Moskophidis et al, 1993; Zajac et al, 1998; Wherry et al, 2003), cancers (Ahmadzadeh et al, 2009; Siddiqui et al, 2019), and autoimmune conditions including T1D (McKinney et al, 2015; Long et al, 2016; Hu et al, 2020). Application of high-throughput sequencing, including single-cell RNA sequencing (scRNA-seq), to models of these various inflammatory states by our laboratory and others has shown previously unappreciated heterogeneity in the CD8 T-cell compartment, demonstrating the coexistence of progenitor-, effector-, and exhausted-like CD8 T cells in these conditions (He et al, 2016; Im et al, 2016; Chen et al, 2019, 2021; Hudson et al, 2019; Zander et al, 2019; Abdelsamed et al, 2020; Beltra et al, 2020; Sandu et al, 2020; Wiedeman et al, 2020; Zakharov et al, 2020; Ciecko et al, 2021; Connolly et al, 2021; Gearty et al, 2022).
Some works have suggested that an increased exhausted state, rather than a cytolytic effector state, in the CD8 T-cell compartment may be protective in autoimmune diseases (McKinney et al, 2015). In line with this, a recent study of T1D patients demonstrated a correlation between increased frequencies of TIGIT+ EOMES+ islet–specific CD8 T cells and slower disease progression (Wiedeman et al, 2020). These cells were found to exhibit an exhausted-like phenotype, similar to the increased expression of EOMES observed in exhausted CD8 T cells in murine models of chronic viral infection (Paley et al, 2012; Zander et al, 2019). Despite the important clinical impact and novel findings of this work, the data are limited as CD8 T cells were collected from peripheral blood, raising the question of whether CD8 T cells detected peripherally provide an accurate sampling of the autoreactive lymphocyte pool in the pancreatic islets.
Further investigation into the clonal landscape of CD8 T cells, in which a T-cell clone is defined as a group of T cells arising from the same naïve T cell and sharing the same unique T-cell receptor generated by V(D)J recombination (Oettinger et al, 1990), may provide further insight into the pathogenesis of insulitis. Mathematical estimates of TCR diversity have suggested that 1015–1020 unique TCRs are capable of being generated by random rearrangement (Davis & Bjorkman, 1988; Lieber, 1991), although individual organisms have far fewer naïve T cells in reality. Recent work from our laboratory using single-cell TCR sequencing (scTCR-seq) has demonstrated that virus-specific CD4 T cells during acute infection are highly clonally heterogeneous, with variable V(D)J gene usage detected and the vast majority of clones not shared among any of five mice studied (Khatun et al, 2021). However, CD8 T-cell clonal heterogeneity in the context of T1D remains understudied at the single-cell level. Given recent advancements in the use of high-throughput sequencing to better define T-cell phenotypes and clonotypes, and to build upon recent work from our laboratory and others demonstrating that islet-specific CD8 T cells are composed of self-renewing progenitor cells that give rise to effector cells (Ciecko et al, 2021; Gearty et al, 2022), we decided to investigate the phenotypic and clonal landscapes of CD8 T cells from the islets and spleens of prediabetic NOD mice using paired scRNA-seq and scTCR-seq with a particular focus on CD8 T cells targeting the immunodominant autoantigen IGRP206-214.
Results
Single-cell T-cell receptor sequencing reveals vast clonal heterogeneity among IGRP206-214–reactive CD8 T cells despite restricted TCR gene usage
To investigate the phenotypic and clonal landscapes of CD8 T cells in detail, we subjected CD8 T cells harvested from NOD mice to paired single-cell RNA sequencing (scRNA-seq) and single-cell T-cell receptor sequencing (scTCR-seq). We performed two independent experiments in which we harvested and pooled spleens and islets from 10 individual 10- to 15-wk-old prediabetic NOD females and sorted IGRP206-214 tetramer–positive CD8 T cells from both tissues as well as tetramer-negative CD8 T cells from islets (Figs 1A and S1A). Performing TCR sequencing at the single-cell level allowed us to define T-cell clones based on paired TCR α and β chain complementarity determining region 3 (CDR3) sequences at the nucleotide level. This approach provides a strong definition of T-cell clonality as the CDR3 region, the most variable portion of the TCR and the region that directly contacts peptides presented on MHC (Cole et al, 2014), includes germline information from V(D)J genes as well as N nucleotides randomly inserted into the junctions between these genes. Of 25,209 total CD8 T cells sequenced, we recovered paired TCR α and β chain sequences from 15,226 cells (60.4%).
Figure 1. Single-cell T-cell receptor sequencing reveals vast clonal heterogeneity among IGRP206-214–reactive CD8 T cells despite restricted TCR gene usage.
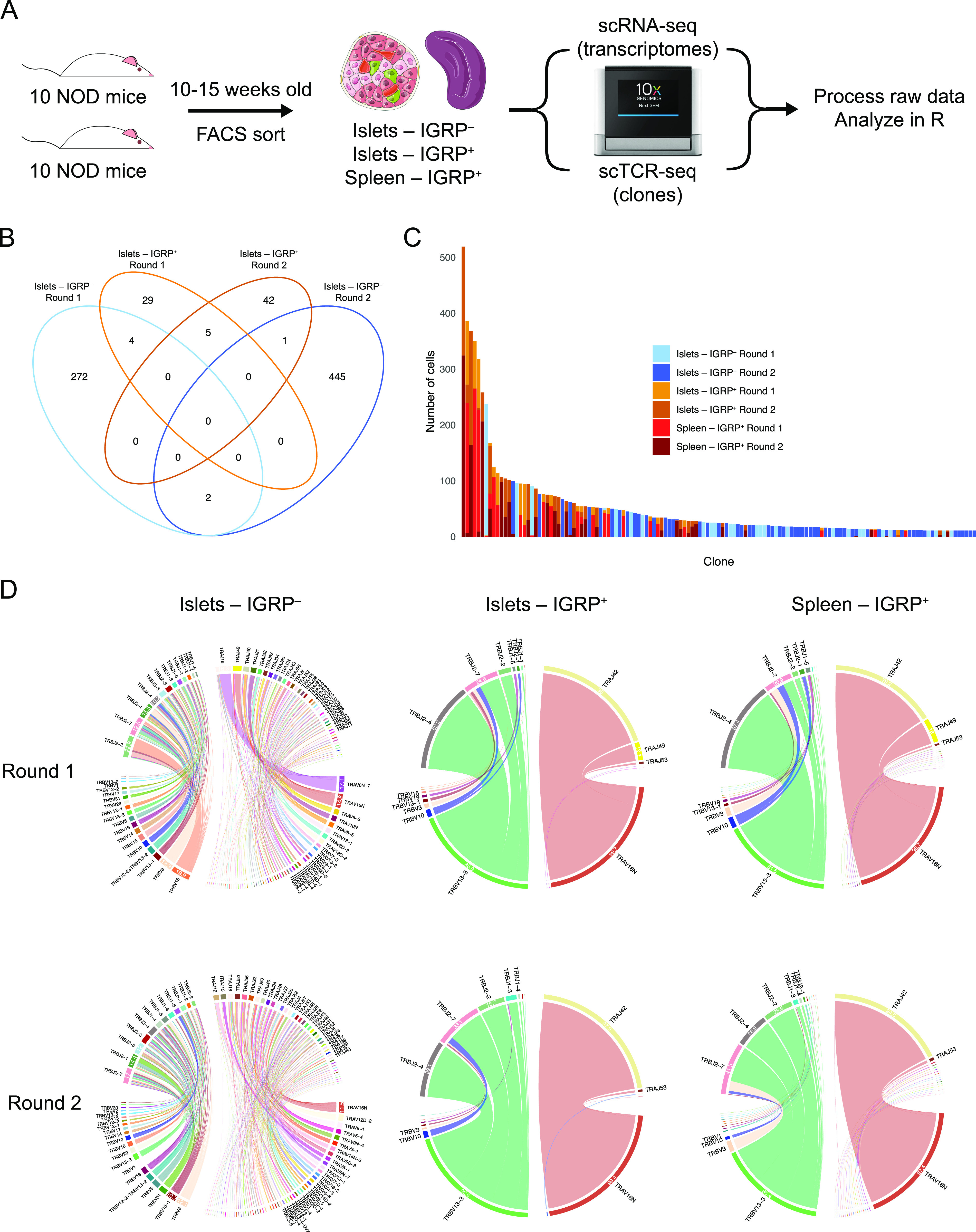
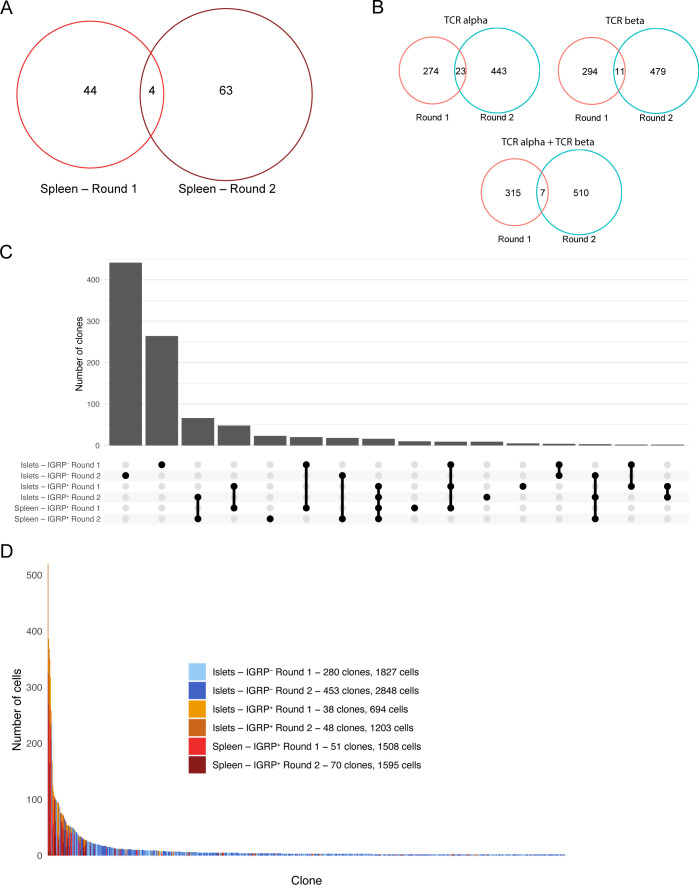
(A) Experimental design of paired scRNA-seq and scTCR-seq experiments performed on CD8 T cells isolated from the islets and spleens of non-obese diabetic mice. (B) Venn diagram showing clonal overlap among CD8 T cell clones isolated from islets. Clones are defined as groups of CD8 T cells sharing the same paired TCR α and β chain CDR3 nucleotide sequences. Singlet clones were excluded from this analysis as, by definition, they do not overlap between samples. (C) Bar plot showing size and sample origin of clones with at least 10 constituent cells. Each bar represents one clone, color denotes sample identity. (D) Chord diagrams showing V and J gene pairings within TCR α and β chains of IGRP206-214–reactive cells from islets and spleens and IGRP206-214–nonreactive cells from islets. Colors denote individual TCR genes, chord width correlates with frequency of cells using a gene. Gene usage frequencies are shown on the edge of each chord, with frequencies less than 10% not shown for visual clarity. TCR gene names are shown outside each chord, with genes used by less than 2% of cells not shown for visual clarity.
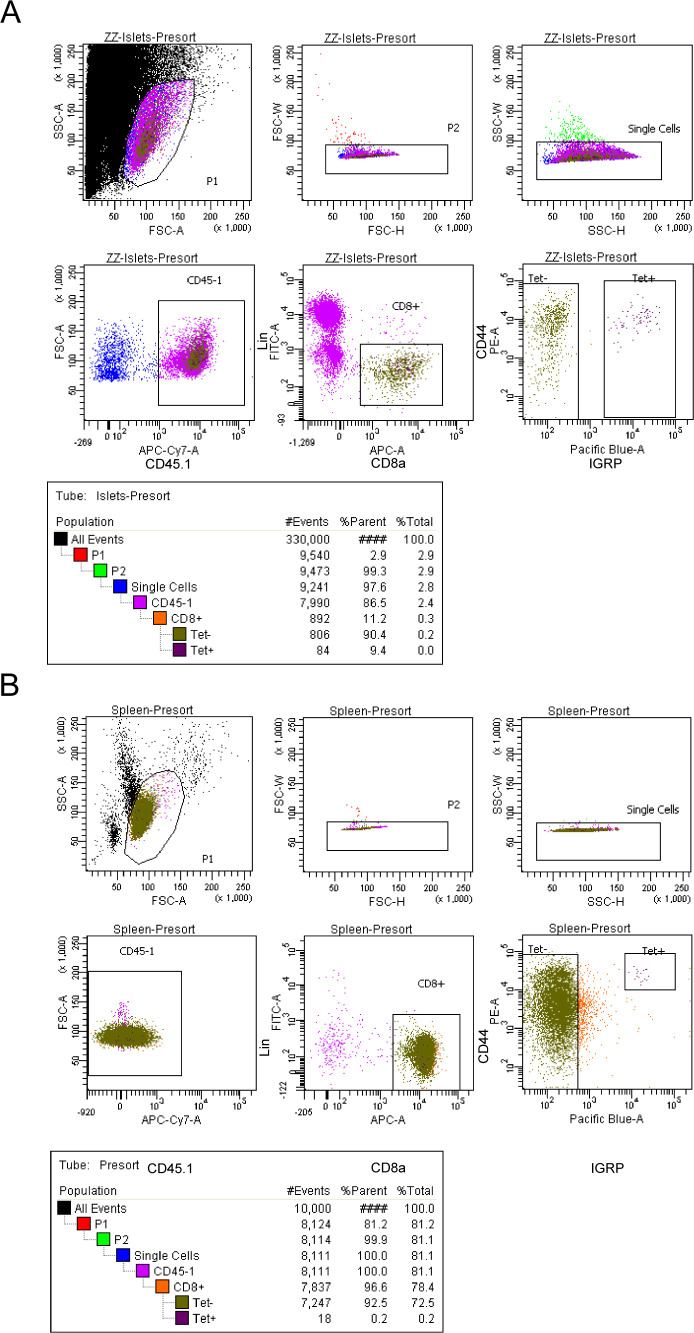
Figure S1. Sorting strategy for scRNA-seq experiments.
(A, B) Representative flow plot from FACS sorting of islets (A) and spleen (B). Sorted samples were at least 90% pure. For islets, the “Tet−” and “Tet+” gates were collected, whereas for spleens, only the “Tet+” gate was collected. Splenocytes were enriched for CD8 T cells via negative selection before sorting. Lin: CD4, B220, and Cd11b.
We used a Venn diagram to compare overlap of clones with at least two constituent CD8 T cells recovered from the islets in each of our two experiments (Fig 1B). We defined a clone as a group of T cells sharing the same paired TCR α and β chain CDR3 nucleotide sequence. Five of 81 IGRP206-214 tetramer–positive islet clones were shared between the two groups of mice (6.2% overlap), as were two out of 724 IGRP206-214 tetramer–negative clones (0.3% overlap). A total of five clones were also shared between IGRP206-214 tetramer–positive and –negative cells within individual experiments, likely the result of inherent limitations to the accuracy of FACS sorting. When examining clones isolated from the spleen, we found four of 111 clones shared between experiments (3.6% overlap) (Fig S2A). However, when clones from islets and spleens were combined, we found that only seven of 840 clones were shared between the two groups of 10 pooled mice (0.8% overlap) (Fig S2B). Moreover, if the same group of cells was analyzed with clones being defined using only TCR α or TCR β chain CDR3 nucleotide sequences, rather than paired CDR3 sequences, higher frequencies of overlapping clones (23/740 = 3.1% overlap and 11/784 = 1.4% overlap, respectively) were found (Fig S2B), thus emphasizing the increased resolution afforded by scTCR-seq compared to bulk TCR sequencing. These data suggest that clonal overlap of autoreactive CD8 T cells between any two genetically identical NOD mice is extremely low; this validates mathematical predictions of V(D)J recombination diversity (Davis & Bjorkman, 1988; Lieber, 1991), which suggest that 1015 to 1020 TCRs may be randomly generated by an organism, and our laboratory’s prior work on CD4 T-cell clonal diversity in acute viral infection (Khatun et al, 2021). Despite a relatively high level of diversity, there was higher clonal overlap between IGRP206-214–reactive clones compared with other CD8 T-cell clones, as visualized by an UpSet diagram of clonal overlap (Fig S2C). Of note, there is a large number of the same IGRP206-214–reactive clones present in both islets and spleens in each of the two experiments, suggesting continuous trafficking between islets and peripheral lymphoid organs.
Figure S2. Clonal distribution and overlap of diabetogenic CD8 T cells.
(A) Venn diagram showing clonal overlap of spleen clones from two experiments. Singlet clones were excluded from this analysis. (A, B) As in (A), but showing all non-singlet clones from each experiment. Clones are defined using CDR3 nucleotide sequences from the TCR α chain only (top left), TCR β chain only (top right), or both chains paired at the single-cell level (bottom). (C) UpSet diagram showing clonal overlap among all combinations of samples. (D) Bar plot showing size and sample origin of clones with at least two constituent cells. Each bar represents one clone, color denotes sample identity. Clone and cell counts shown in the legend are restricted to non-singlet clones.
IGRP206-214 tetramer–positive CD8 T cells, both in the islets and in the spleen, also exhibited high levels of clonal expansion compared with tetramer-negative cells (Fig 1C). When examining clones with at least two constituent cells, an average tetramer-positive clone consisted of ∼24 cells, whereas an average tetramer-negative clone consisted of approximately six cells (Fig S2D). Despite this, we still detected several large IGRP206-214 tetramer–negative clones, including one clone with more than 200 cells and nine clones with more than 50 cells. These data reinforce the fact that although IGRP is the major autoantigen in the islets in NOD mice during the progression of T1D (Lieberman et al, 2003; Trudeau et al, 2003), multiple epitopes such as insulin and glutamate decarboxylase are targeted by CD8 T cells in both NOD mice and human T1D patients (Amdare et al, 2021).
Intrigued by our findings that autoreactive CD8 T-cell clones exhibit low level of clonal overlap between organisms, we used chord diagrams to visualize V-J gene pairings of CD8 T cells recovered from the islets. D genes were excluded from this analysis as these are only found in the TCR β chain and the mouse genome only contains two D genes (TRBD1 and TRBD2). Although we expected to see highly variable V-J gene pairings similar to what we previously observed in CD4 T cells in the setting of acute viral infection (Khatun et al, 2021), we found different trends between IGRP206-214–reactive and –nonreactive CD8 T cells (Fig 1C). IGRP206-214–nonreactive (tetramer-negative) CD8 T cells from the islets exhibited highly diverse V-J gene pairings in the TCR α and β chain in both experiments, as expected (Fig 1D). However, IGRP206-214–reactive (tetramer-positive) cells had highly restricted V-J pairings in both the TCR α and β chains. Specifically, over 99% of these cells used TRAV16N and 86–92% of total cells paired this V gene with TRAJ42 to form a TCR α chain. Approximately 90% of tetramer-positive cells expressed TRBV13-3 and more than 90% used either TRBJ2-4, TRBJ2-7, or TRBJ2-2 for the β chain J gene. Similar trends were found in tetramer-positive CD8 T cells recovered from the spleen, with more than 97% of cells expressing TRAV16N; 79–94% TRAJ42; 81–85% TRBV13-3; and 90–92% TRBJ2-4, TRBJ2-7, or TRBJ2-2.
Single-cell RNA sequencing elucidates the phenotypic heterogeneity of diabetogenic CD8 T cells in the islets and spleen
To investigate the phenotypes of CD8 T cells in T1D we performed scRNA-seq of the IGRP206-214 tetramer–positive and tetramer-negative CD8 T cells isolated from the islets and spleens of NOD mice. To prevent overfitting phenotypes, sctransform integration and dimensionality reduction using Seurat’s UMAP algorithm was performed separately on samples from islets and spleens (see the Materials and Methods section). Analysis of integrated tetramer-positive and tetramer-negative cells from the islets revealed six clusters (Fig 2A). Identities of these CD8 T-cell clusters were determined using differentially expressed genes (Fig 2B). Bystander cells expressed Sell (encodes L-selectin) and had the lowest expression of Cd44; progenitor cells expressed Tcf7 and Slamf6 (encodes Ly108). Effector cells expressed the cytotoxic molecule Gzmb, the chemokine receptor Cxcr6, and coinhibitory receptors associated with T-cell exhaustion such as Pdcd1 (encodes PD-1) and Lag3. Recently activated CD8 T cells were identified by high expression of Cd69, an early marker of T-cell activation (Yokoyama et al, 1988), and Nr4a1 (encodes Nur77, a transcription factor [TF] downstream of TCR signaling) (Moran et al, 2011). Mitotic cells highly expressed Top2a (encodes DNA topoisomerase II alpha). Finally, anergic cells up-regulated Cblb (encodes a ubiquitin kinase that inhibits TCR signaling) (Bachmaier et al, 2000; Chiang et al, 2000; Jeon et al, 2004); Itpkb (encodes inositol triphosphate 3 kinase beta), which restrains T-cell cytokine production (Pouillon et al, 2013) and inhibits thymic TCR signaling (Westernberg et al, 2016); and Dgka (encodes diacylglycerol kinase alpha), a kinase that inhibits TCR signaling to promote anergy (Olenchock et al, 2006).
Figure 2. Single-cell RNA sequencing elucidates the phenotypic heterogeneity of diabetogenic CD8 T cells in the islets and spleen.
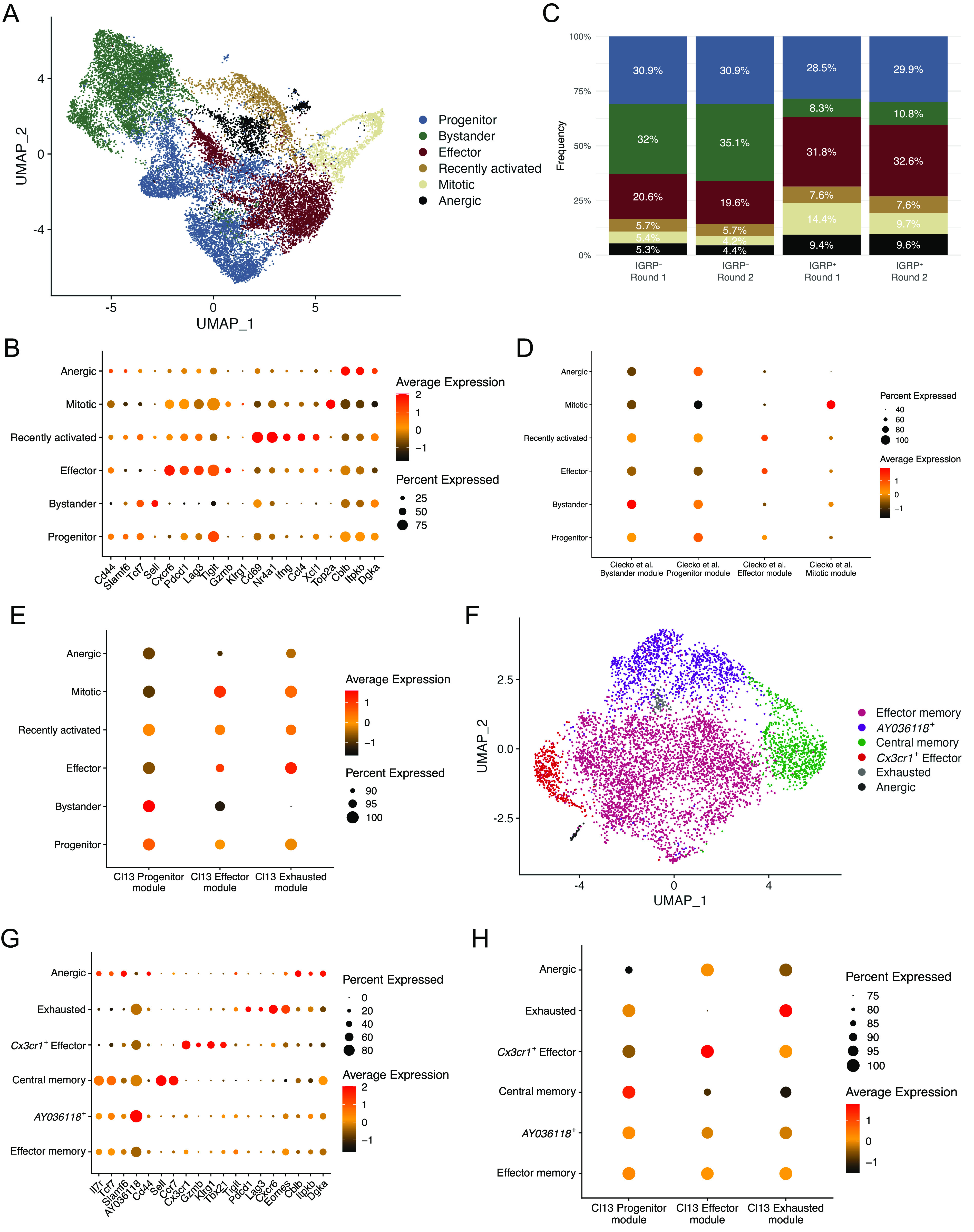
(A) UMAP plot showing cluster identities of individual CD8 T cells isolated from islets. Each dot represents one cell, colors denote cluster identity as determined by unsupervised clustering. (B) Dot plot showing expression of key marker genes among different CD8 T-cell clusters from islets. Dot size indicates the frequency of cells in a cluster expressing a particular gene, dot colors denotes average expression of that gene. (C) Bar graph showing islet cluster distribution frequencies of each sample. (A) Colors correspond to the clusters shown in (A). (A, D) Dot plot showing module scores within each cluster from (A) of the top 100 differentially expressed genes in the four CD8 T cell clusters previously identified in non-obese diabetic islets (Ciecko et al, 2021). (D, E) As in (D), but using the top 100 differentially expressed genes among previously identified splenic CD8 T cell clusters from LCMV Clone 13 infection (Zander et al, 2019). (A, F) As in (A), but showing IGRP206-214–reactive CD8 T cells isolated from spleens of non-obese diabetic mice. (B, G) As in (B), but showing gene expression among CD8 T cell clusters from spleen. (E, H) As in (E), but showing CD8 T cell clusters from spleens.
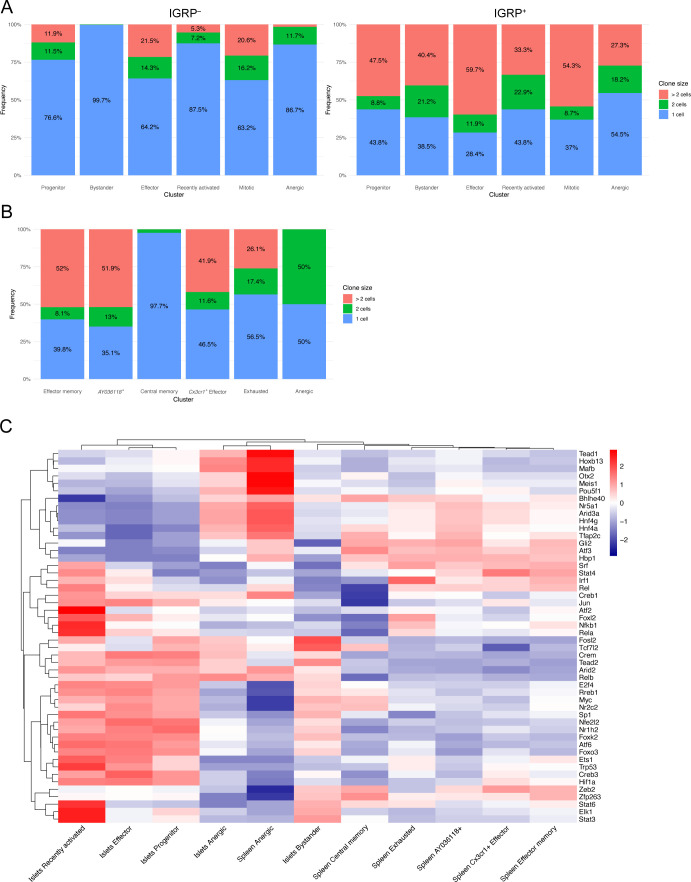
We also examined the relative frequencies of these clusters in islet tetramer-positive and -negative samples and found that bystander cells were enriched in tetramer-negative samples in both independent experiments (Figs S3A and 2C). Conversely, effector, mitotic, and anergic cells were enriched in tetramer-positive samples in both experiments. These data suggest that IGRP206-214–reactive effector CD8 T cells dominate the islet autoimmune response at 10–15 wk of age in NOD mice as they are more proliferative and able to adopt a cytotoxic phenotype. These cells may also be more prone to developing an anergic phenotype as a result of overstimulation. Development of anergy would also be expected to limit CD8 T cell clonal proliferative capacity. To test this, we used our scTCR-seq data to break down each islet cluster by frequency of clone size (Fig S3A) Indeed, IGRP206-214–nonreactive clones in the anergic cluster, as well as those in the progenitor and recently activated clusters, had relatively low expansion, with ∼80% being singlet clones. IGRP206-214–nonreactive clones with bystander constituent cells were even more extreme in their lack of expansion, with almost 99% being singlet clones. In contrast, IGRP206-214–nonreactive effector and mitotic clones proliferated much more, with ∼60% of clones being singlets and 25% having at least three cells. IGRP206-214–reactive clones, on the other hand, were all more expanded than their IGRP206-214–nonreactive counterparts. Despite this, similar trends among clusters could be seen, with effector and mitotic cells being more expanded than other clusters. IGRP206-214–reactive bystander cells exhibited the greatest difference in clonal expansion compared with IGRP206-214–nonreactive bystander cells, suggesting that these cells may have already undergone some degree of activation due to the immunodominance of IGRP206-214 as an autoantigen in the islets.
Figure S3. Phenotypic and clonal breakdown of diabetogenic CD8 T cell clusters.
(A) Bar plots showing frequency of clones with one cell, two cells, or more than two cells among islet clusters from IGRP206-214–nonreactive (left) and IGRP206-214–reactive (right) cells. (A, B) As in (A), but showing spleen clusters. (C) Gene regulatory network (regulon) heat map of predicted transcription factor activity within islet and spleen CD8 T-cell clusters constructed using DoRothEA.
We have previously used scRNA-seq to identify four major subsets of CD8 T cells (bystander, progenitor, effector, and mitotic) in the islets of NOD mice at 7 and 14 wk of age (Ciecko et al, 2021); our previous work, as well as that from other groups (Hu et al, 2020; Gearty et al, 2022), has shown that progenitor cells give rise to effector cells that then mediate insulitis. Our current study has much higher resolution as we were able to collect and pool a much greater number of cells for scRNA-seq, allowing us to identify greater heterogeneity in the diabetogenic CD8 T-cell pool compared with our previous work (Ciecko et al, 2021). To compare the cells in our current data with our previously identified subsets, we generated module scores from differentially expressed genes of the four subsets in our previous publication (Fig 2D). This analysis showed that the current bystander, progenitor, effector, and mitotic clusters scored highly for their respective module scores derived from our previous data. In addition, our newly identified recently activated and anergic clusters both scored highly for the progenitor module score, suggesting that these two clusters may have been part of our older progenitor cluster but were not identified because of limited scRNA-seq resolution at the time. Given that lymphocytic choriomeningitis virus Clone 13 (LCMV Cl13) chronic infection system is a common model system of persistent antigen stimulation and CD8 T-cell exhaustion, we also used module scores derived from one of our laboratory’s prior publications (Zander et al, 2019) to score our islet CD8 T-cell clusters (Fig 2E). This analysis confirmed that progenitor, bystander, and recently activated cells are more quiescent because they had the highest Cl13 Progenitor module scores. Autoreactive effector cells scored highly for Cl13 Effector modules as expected but also had high Cl13 Exhausted module scores in line with their expression of inhibitory coreceptors (Fig 2B). Mitotic cells had a reasonably high Cl13 Effector module score and an intermediate Exhausted module score. Anergic cells scored relatively low in all categories but had an especially low Effector module score. Collectively, these analyses suggest that CD8 T cells in the islets of NOD mice are phenotypically heterogeneous. Although we did not detect a discrete exhausted CD8 T-cell population in the islets, we did find that autoreactive effector cells express coinhibitory receptors and have a similar gene expression profile to both Effector and Exhausted cells from LCMV Cl13 chronic infection. Overall, our results concerning islet CD8 T-cell differentiation states is consistent with a recent report (Zakharov et al, 2020) but in addition reveals an anergic population, as supported by its gene expression profile and reduced clonal expansion.
We then turned our attention to IGRP206-214–reactive CD8 T cells isolated from the spleens of NOD mice. UMAP projection with unbiased clustering revealed 6 clusters (Fig 2F): effector memory, AY036118+, central memory, Cx3cr1+ effector, exhausted, and anergic CD8 T cells. As before, clusters were defined based on expression of key marker genes (Fig 2G). Effector memory and AY036118+ cells had a very similar gene expression profile and up-regulated memory genes such as Il7r (encodes IL-7R), Tcf7 (encodes TCF-1), and Slamf6 (encodes Ly108) as well as effector genes such as Tbx21 (encodes T-bet) and Klrg1; however, only the latter cluster up-regulated AY036118, which encodes the transcriptional repressor ERF1 (Verykokakis et al, 2007) and was recently reported to be up-regulated in CD8 T cells with repressed activation in a lung cancer model (Burger et al, 2021). Central memory cells highly expressed the aforementioned memory markers as well as Sell (encodes L-selectin) and Ccr7. Splenic Cx3cr1+ effector cells expressed Gzmb and Klrg1, similar to effector cells from the islets, but critically had minimal expression of coinhibitory receptor genes such as Pdcd1 and Lag3. Instead, these molecules were up-regulated by Exhausted cells, which also expressed the chemokine receptor Cxcr6 and the exhaustion-associated TF Eomes (Paley et al, 2012; Long et al, 2016; Siddiqui et al, 2019). Of note, the effector memory and AY036118+ clusters also expressed Eomes, albeit at a lower level, likely due to the fact that Eomes is involved in CD8 T cell memory formation (Banerjee et al, 2010; Kaech & Cui, 2012). Finally, splenic anergic cells were similar to their counterparts in the islets as they also up-regulated Cblb, Itpkb, and Dgka, genes encoding TCR inhibitory molecules. Scoring of spleen clusters using LCMV Cl13 module scores demonstrated expected trends (Fig 2H). The effector memory and AY036118+ clusters exhibited intermediate scores of all three Cl13 modules with a slightly higher Progenitor module score, as we have shown that Cl13 Progenitor cells are similar to memory precursor cells from acute viral infection (Chen et al, 2021). Central memory cells had a high Progenitor module score, Cx3cr1+ effector cells had a high Effector module score, and exhausted cells had a high Cl13 Exhausted module score. Anergic cells in the spleen had relatively low scores but scored slightly best for the Effector module, unlike islet anergic cells. Clonal expansion in splenic CD8 T cell clusters was more restrained compared with IGRP206-214–reactive islet clusters (Fig S3B): Effector memory, AY036118+, Cx3cr1+ effector, and exhausted clones had up to 50% of non-singlet clones. This was in line with IGRP206-214–reactive progenitor cells, which also have a memory-like phenotype. Only two clones were covered from the very small splenic anergic cluster, one singlet and one doublet clone. The major outlier was the central memory cluster, which was almost entirely (>97%) composed of singlet clones. Although it may be possible that some of the cells in this cluster were actually naïve cells, given that naïve and memory T cells share many transcriptomic and epigenetic similarities (Schauder et al, 2021), the splenic CD8 T cells we sequenced were sorted to be CD44+ (Fig S1B); these data therefore suggest that central memory CD8 T cells may undergo clonal contraction. In general, splenic CD8 T cell clusters were less clonally expanded than those in the islets, as would be expected given that the islets are the major source of autoantigens in T1D.
Finally, we leveraged the DoRothEA curated gene regulatory network (regulon) resource (Garcia-Alonso et al, 2019; Holland et al, 2020a, 2020b) to predict activity of the top 50 most globally variable TFs among clusters of CD8 T cells in our scRNA-seq data (Fig S3C). Regulon analysis circumvents difficulties detecting TFs by scRNA-seq because of low RNA copy numbers by also analyzing coexpression of TF target genes. These regulons, consisting of TFs and their targets, are then scored for putative activity. Islet mitotic cells were excluded from this analysis as their extremely high expression of cell cycle-related TFs skews scaled heat maps. Regulon analysis predicted enriched activity of TCR signaling-induced TFs such as Nfkb1 and Jun (Courtney et al, 2018) in recently activated islet CD8 T cells as would be expected. In addition, recently activated, bystander, and progenitor cells in the islets all had high predicted activity of Stat3, a TF downstream of IL-27 signaling; this is supported by our recent work showing that IL-27 signaling promotes progenitor to effector CD8 T-cell differentiation in the islets of NOD mice (Ciecko et al, 2021). Conversely, islet effector cells exhibited increased activity of TFs associated with aerobic glycolysis, such as Hif1a (Shi et al, 2011) and Foxk2 (Sukonina et al, 2019); aerobic glycolysis is crucial for the effector function of activated T cells (Gupta et al, 2020), and we have found that Hif1a is also up-regulated in effector-like tumor-infiltrating lymphocytes (Topchyan et al, 2021).
Interestingly, DoRothEA analysis predicted that the TF Zeb2, known to promote the function of long-lived effector cells (LLECs) and effector memory CD8 T cells in acute viral infection (Renkema et al, 2020), is active in splenic effector memory, AY036118+, and Cx3cr1+ effector cells. Moreover, although Zeb2 is also critical for short-lived effector cell (SLEC) function in acute viral infection (Dominguez et al, 2015; Omilusik et al, 2015; Guan et al, 2018), Zeb2 activity was predicted to occur at intermediate levels in recently activated, progenitor, and effector CD8 T cells in the islets. This analysis suggests that Zeb2 may differentially control the effector and memory potential of diabetogenic CD8 T cells in the islets and spleen. Regulon analysis also predicted that interferon response factor 1 (IRF1), a TF activated downstream of type I IFN signaling (Li et al, 1996), is the most active regulon in exhausted CD8 T cells in the spleen. In models of chronic viral infection, blocking type I IFN signaling promotes viral control, likely due to reduced CD8 T cell exhaustion (Teijaro et al, 2013; Wilson et al, 2013; Wu et al, 2016); similar mechanisms may therefore regulate the development of exhausted CD8 T cells in T1D. Overall, this analysis showed similarity in TF activity among the different populations within the islets compared with the spleen, except for anergic cells which display similar TF activity regardless of tissue localization. We have previously demonstrated the power of regulon analysis as a tool to investigate the gene regulatory networks that govern CD8 T cell differentiation and function in chronic viral infection (Chen et al, 2021); application of such analytical pipelines to T1D may provide further insight into how diabetogenic CD8 T cells are regulated at the transcriptomic and epigenetic levels.
IGRP206-214–reactive CD8 T cells exhibit characteristics of increased antigen exposure compared with IGRP206-214–nonreactive CD8 T cells
Given that IGRP206-214 is the predominant islet autoantigen in NOD mice (Lieberman et al, 2003; Trudeau et al, 2003), we decided to examine phenotypic differences between IGRP206-214 tetramer–positive (red) and -negative (blue) CD8 T cells in the islets. To do so, we examined differential expression of genes related to antigen stimulation within each islet cluster (Fig 3A). We found that the coinhibitory receptors Pdcd1, Tigit, and Lag3 were all up-regulated in IGRP206-214–reactive CD8 T cells, as were the exhaustion-related TFs Tox (Alfei et al, 2019; Khan et al, 2019; Scott et al, 2019; Seo et al, 2019; Yao et al, 2019) and Nr4a2 (Zander et al, 2019). Interestingly, up-regulation of these genes was not exclusive to less quiescent clusters such as effector cells and mitotic cells; IGRP206-214–reactive progenitor and bystander cells, as well as recently activated and anergic cells, up-regulated these genes compared with their IGRP206-214–nonreactive counterparts; this supports our data showing that IGRP206-214–reactive bystander cells are clonally expanded (Fig S3B) and further suggests that they may be antigen-experienced to some degree. Given their expression of genes encoding inhibitory coreceptors and Tox, IGRP206-214–reactive bystander cells are likely distinct from IGRP206-214–nonreactive bystander cells, which do not demonstrate expression of these activation markers and are clonally non-expanded (Fig S3B). Moreover, Tox expression was also observed in IGRP206-214–reactive CD8 T cells from the spleen, including in the memory-like Effector memory and AY036118+ clusters, albeit at lower levels than in exhausted or anergic splenocytes (Fig 3B). This observation is in line with several recent studies that have shown that a “molecular scar” of TOX expression and epigenetic accessibility is present in memory CD8 T cells generated in settings of persistent antigen exposure, such as chronic viral infection or T1D (Wieland et al, 2017; Abdelsamed et al, 2020; Charmoy et al, 2021; Heim et al, 2021; Hensel et al, 2021; Tonnerre et al, 2021; Yates et al, 2021). Curiously, the central memory and Cx3cr1+ effector cells in the spleen had minimal Tox expression despite being activated and therefore presumably antigen-experienced. We then quantified exhaustion more broadly by plotting module scores of our LCMV Cl13 Exhausted gene list (Fig 2E) among IGRP206-214–reactive and –nonreactive islet CD8 T cells (Fig 3C). This analysis demonstrated a statistically significant increase in Cl13 Exhausted module scores in IGRP206-214–reactive compared with –nonreactive CD8 T cells in all clusters except the mitotic cluster. Conversely, IGRP206-214–nonreactive CD8 T cells tended to up-regulate memory- and stem-like genes such as Slamf6, Ccr7, Il7r, Tcf7, and Klf2 compared with their IGRP206-214–reactive counterparts (Fig 3D). Expression of the first four genes was low in the effector and mitotic clusters, but even these clusters demonstrated differential expression of Klf2. Accordingly, a module of differentially expressed genes from LCMV Cl13 Progenitor cells was significantly enriched in IGRP206-214–nonreactive cells in every cluster (Fig 3E). These analyses suggest that autoreactive CD8 T cells specific for epitopes other than IGRP206-214 adopt a more quiescent transcriptional profile during the later stages of insulitis (10–15 wk of age) in NOD mice despite the fact that they do not form distinct phenotypes compared with IGRP206-214–reactive diabetogenic CD8 T cells.
Figure 3. IGRP206-214–reactive CD8 T cells exhibit characteristics of increased antigen exposure compared with IGRP206-214–nonreactive CD8 T cells.
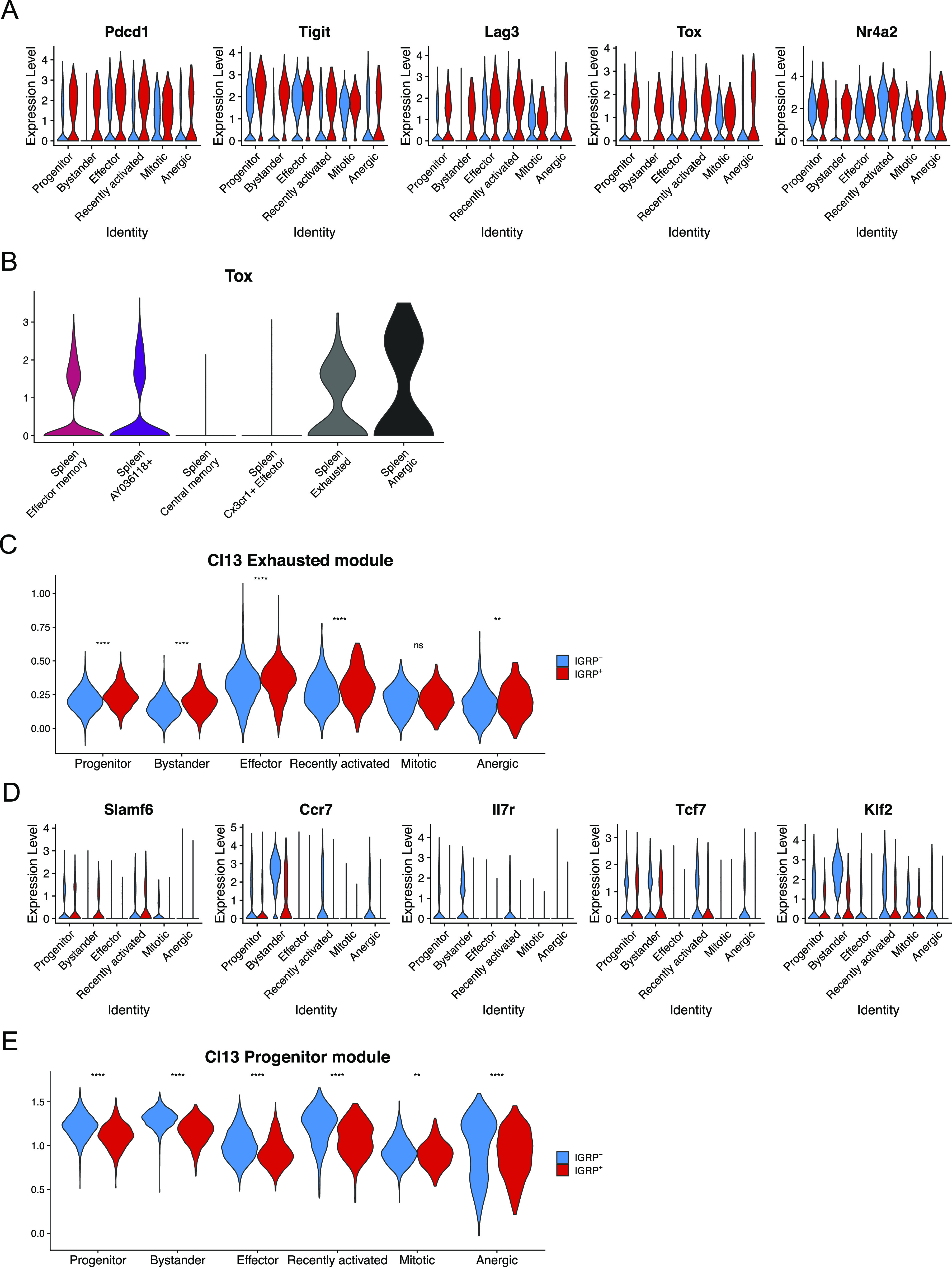
(A) Violin plots showing expression of key coinhibitory receptors, memory CD8 T cell-associated cell surface markers, and transcription factors regulating CD8 T-cell exhaustion. (B) Violin plot showing expression of Tox in spleen clusters. (C) Violin plot showing module scores of the top 100 differentially expressed genes from LCMV Clone 13 Exhausted CD8 T cells in islet CD8 T cells. Blue, IGRP206-214–nonreactive CD8 T cells. Red, IGRP206-214–reactive CD8 T cells. (A, D) As in (A) but showing markers of CD8 T-cell stemness. (C, E) As in (C), but showing module scores derived from the top 100 differentially expressed genes from LCMV Clone 13 Progenitor CD8 T cells. **P < 0.01, ****P < 0.0001 by the Wilcoxon test.
IGRP206-214–reactive CD8 T-cell phenotype correlates with T-cell receptor gene usage
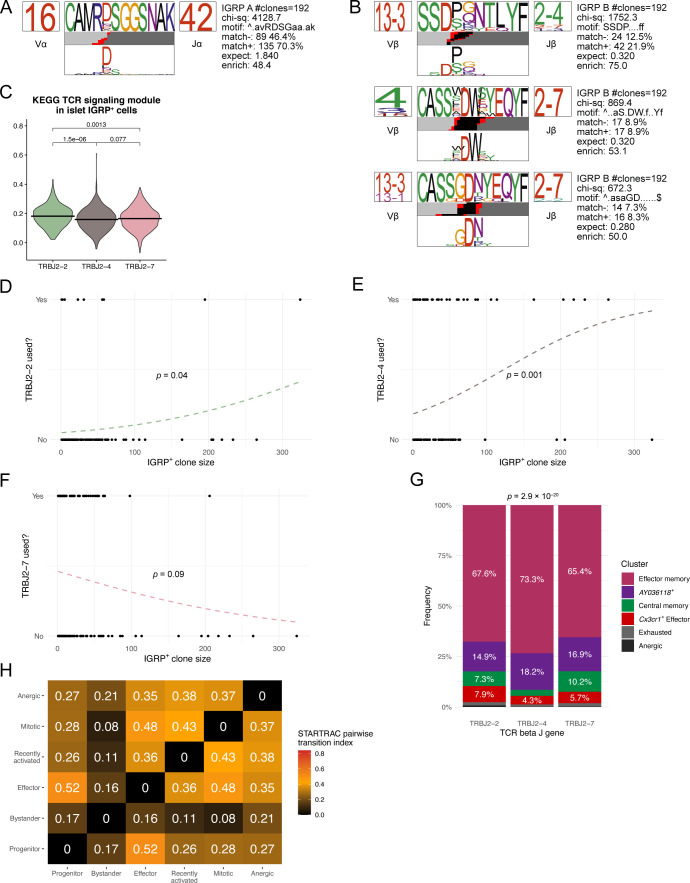
Given that IGRP206-214–reactive CD8 T cells exhibit striking clonal diversity (Fig 1B) despite restricted TCR gene usage (Fig 1D) and that diabetogenic CD8 T cells from transgenic mice or culture systems are known to have limited TCR diversity (Verdaguer et al, 1996; DiLorenzo et al, 1998), we hypothesized that most TCR sequences from IGRP206-214–reactive CD8 T cells would bear similar sequences. To test this hypothesis, we performed CDR3 amino acid motif analysis using TCRdist (Dash et al, 2017). Paired TCR α and β chain motif analysis of all IGRP206-214–reactive clones with at least two constituent cells revealed strong motif consensus between large numbers of IGRP206-214–reactive CD8 T-cell clones (Fig 4A). Of the 192 clones analyzed, 135 clones (70.3%) exhibited an “RDSG” amino acid motif in the α chain CDR3 (Fig S4A). 42 clones (21.9%) also demonstrated an “SSDP” motif at the beginning of the β chain CDR3 (Fig S4B). Other major β chain motifs included an “S-DW” motif found in 17 clones (8.9%) and a “GDN” motif found in 16 clones (8.3%) (Fig S4B). Conversely, IGRP206-214–nonreactive clones had a broad range of CDR3 motifs, with consensus motifs occurring among a far lower frequency of clones compared with IGRP206-214–reactive cones (Fig 4B). Interestingly, 10 of 724 clones analyzed (1.4%) had a fairly conserved “E-RGS” motif in the α chain CDR3 paired with an “RGQSN” motif in the β chain CDR3. These 10 clones also exhibited restricted TCR gene usage (TRAV4D-3, TRAJ18, TRVB13-1, and TRJB1-4/1-1); by analogy to similar characteristics found in IGRP206-214–reactive clones, these 10 IGRP206-214–nonreactive clones may all be specific for the same autoantigen. Returning to our IGRP206-214–reactive motif analysis (Fig 4A), we noticed that the three TCR β chain motifs primarily used the 2-2, 2–4, or 2–7 variant of the TCR β chain J gene, in line with these three genes collectively being used by ∼90% of IGRP206-214–reactive CD8 T cells (Fig 1D). We began to investigate differences imparted by differential TCR β J gene usage by quantifying TCR signaling within islet CD8 T cells. We used the “KEGG TCR Signaling Pathway” geneset (GSEA systematic name M9904) to score islet IGRP206-214–reactive CD8 T cells using Seurat’s Module Score function. Doing so showed that IGRP206-214–reactive cells using TRBJ2-2 express significantly higher levels of genes involved in TCR signaling than those cells using TRBJ2-4 or TRBJ2-7 (Fig S4C), suggesting that the TCR structure encoded by TRBJ2-2 may promote TCR signaling upon binding to H-2Kd:IGRP206-214. Given that TCR motifs correlate with cell fate (Khatun et al, 2021) and that TCR signaling has an impact on T-cell expansion (Ozga et al, 2016), we wondered if use of these TCR β chain J genes might correlate with clonal expansion. Using logistic regression analysis, we found that IGRP206-214–reactive clones that used TRBJ2-2 or TRBJ2-4 were significantly more likely to have a larger clone size than those that did not (Fig S4D and E). Conversely, a negative correlation approaching statistical significance was found between TRBJ2-7 gene usage and clone size (Fig S4F). Increased TCR signaling has also been shown to impact cell fate by promoting effector function in acute LCMV infection and a RIP-OVA model of T1D (King et al, 2012), so we examined the phenotypic distributions of islet IGRP206-214–reactive CD8 T cells expressing each major TCR β J gene. In parallel with their increased TCR signaling module scores, islet IGRP206-214–reactive CD8 T cells expressing TRBJ2-2 also had a significantly increased frequency of effector cells compared with TRBJ2-4– and TRBJ2-7–expressing cells (Fig 4C). Accordingly, islet IGRP206-214–reactive CD8 T cells expressing TRBJ2-2 had significantly higher LCMV Cl13 Effector and Exhausted module scores, and significantly lower Cl13 Progenitor Module scores, than those expressing either TRBJ2-4 or TRBJ2-7 (Fig 4D). Moreover, phenotypic differences among islet IGRP206-214–reactive CD8 T cells were also mirrored in the spleen, with TRBJ2-2 usage correlating with an increased frequency of Cx3cr1+ effector cells compared with TRBJ2-4 or TRBJ2-7 gene usage (Fig S4G). These findings demonstrate that although IGRP206-214–reactive CD8 T cell clones exhibit highly conserved CDR3 amino acid motifs, particularly in the TCR α chain, variable use of TCR β J genes may impact clonal expansion and effector potential of these cells.
Figure 4. Clonal analysis of autoreactive CD8 T cells in islets and spleen.
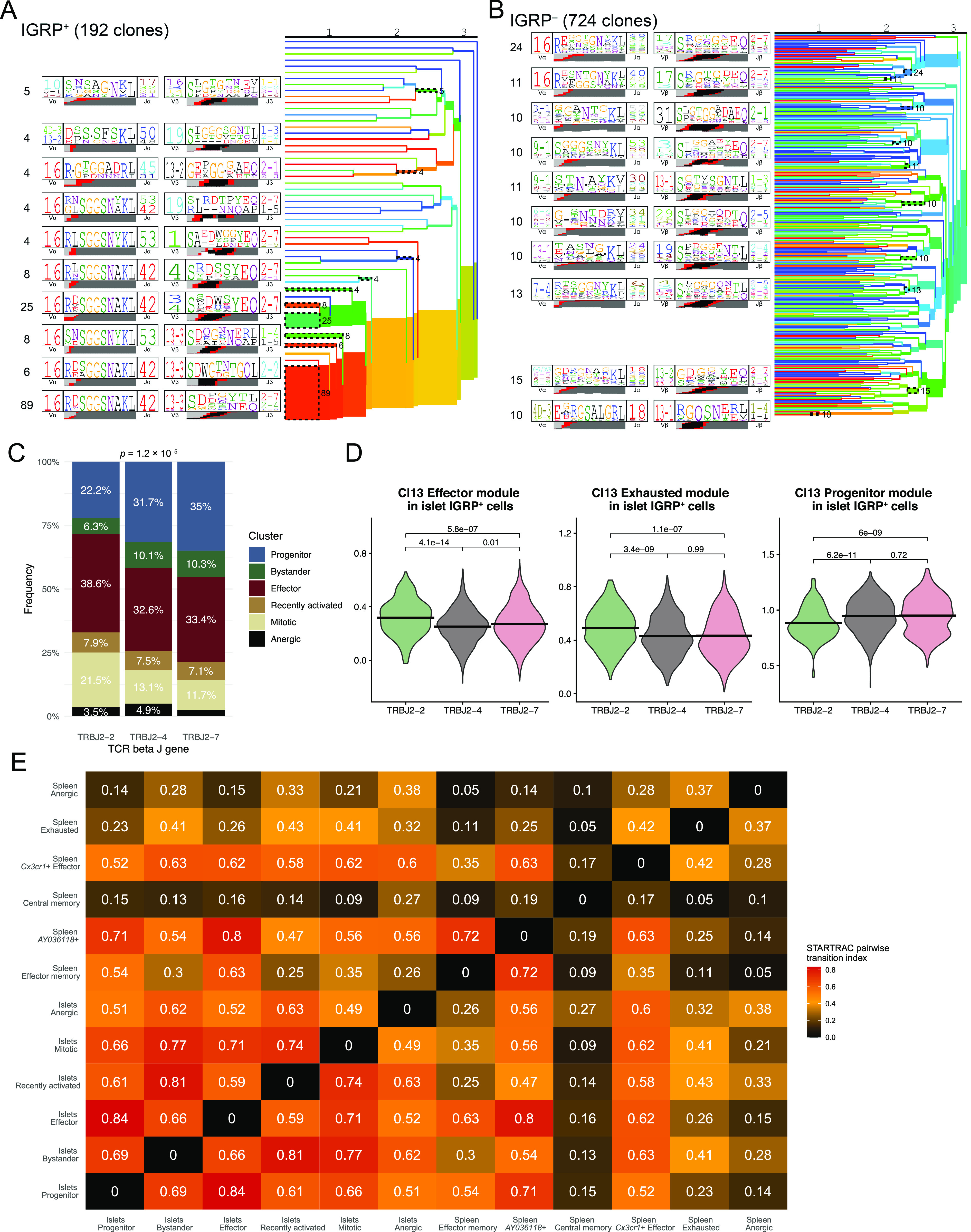
(A) Clonal motif clustering generated by TCRdist of paired α and β chain CDR3 sequences from IGRP206-214–reactive CD8 T cells with at least two cells per clone. Colors underneath each motif denote origin of each amino acid: light gray, V gene; black, D gene; red, N nucleotide; dark gray, J gene. (A, B) As in (A), but from IGRP206-214–nonreactive CD8 T cells. (C) Bar plot showing cluster distribution of islet IGRP206-214–reactive CD8 T cells using the three dominant TCR β J genes. (D) Violin plots showing LCMV Cl13 module scores among islet IGRP206-214–reactive cells grouped by TCR β J gene usage. (E) Heat map showing STARTRAC pairwise transition index of IGRP206-214–reactive CD8 T cells from islets and spleens. Higher values indicate greater predicted differentiation potential between pairs of clusters.
Figure S4. Correlations between TCR gene usage and phenotypes of autoreactive CD8 T cells.
(A) Position weight matrix of one conserved TCR α chain motif identified in IGRP206-214–reactive CD8 T cell clones with at least two cells. (A, B) As in (A), but showing motifs from the TCR β chain. (C) Violin plot showing KEGG TCR signaling module scores (GSEA ID: M9904) among islet IGRP206-214–reactive cells grouped by TCR β J gene usage. (D, E, F) Logistic regression of IGRP206-214–reactive CD8 T-cell clone size and use of TRBJ2-2 (D), TRBJ2-4 (E), or TRBJ2-7 (F). Analyses are restricted to non-singlet clones. (G) Bar plot showing cluster distribution of splenic IGRP206-214–reactive CD8 T cells using the three dominant TCR β J genes. (H) Heat map showing STARTRAC pairwise transition index of IGRP206-214–nonreactive CD8 T cells from islets. Higher values indicate greater predicted differentiation potential between pairs of clusters.
Finally, we applied our scTCR-seq data to the clusters identified by scRNA-seq to predict phenotypic transitions among autoreactive CD8 T cells in the islets and spleen. We used the R package STARTRAC (Zhang et al, 2018) to identify clonal overlap among phenotypic clusters, quantifying overlap using the STARTRAC pairwise transition index (Fig 4C). Given that we did not sequence IGRP206-214–negative cells from the spleen as these would not necessarily have diabetogenic potential, we performed this analysis only on IGRP206-214–reactive cells from the islets and spleen. In general, we found that islet clusters exhibited high intercluster transition, likely the result of an immunostimulatory environment leading to active CD8 T-cell differentiation. The highest pairwise transition index was between the islet progenitor and islet effector clusters, in line with our previous work showing that progenitor cells give rise to effector cells under the influence of IL-27 signaling (Ciecko et al, 2021). Mitotic cells exhibited strong clonal overlap with all islet clusters except the anergic cluster, which in turn had the most clonal overlap with the bystander and recently activated clusters. This may imply that CD8 T-cell anergy in the islets tends to occur early, following priming and before clonal proliferation. Similar trends were found when we performed STARTRAC pairwise transition index analysis on IGRP206-214–negative cells from the islets (Fig S4H): the progenitor and effector clusters exhibited strong pairwise transition scores, as did the anergic and recently activated clusters; on the other hand, bystander cells had low predicted transition with all clusters except the anergic cluster.
Effector memory, central memory, exhausted, and anergic IGRP206-214–positive cells in the spleen had lower transition indices, indicative of more quiescent phenotypes with lower differentiation potential (Fig 4C). Central memory cells, in particular, had an extremely low transition index, in line with their predominantly non-expanded clonal composition (Fig S3C). Of note, although effector memory cells had relatively low transition indices on average, there were noticeable higher transition indexes between these cells and spleen AY036118+, islet progenitor, and islet effector cells. In contrast, the AY036118+ and Cx3cr1+ effector clusters had high transition indices in general, suggesting that these cells are more phenotypically malleable. The spleen AY036118+ cluster and the islet effector cluster had a particularly high pairwise transition index, suggesting that the former may serve as an intermediate stage in memory formation from islet effector cells. Collectively, these data show that IGRP206-214–reactive CD8 T cell clones have constituent cells in both the islets and spleen and raise the possibility that effector cells from the islets may recirculate and generate memory cells in the spleen, as has been previously suggested (Chee et al, 2014).
Discussion
In this study we used paired scRNA-seq and scTCR-seq to investigate the phenotypic and clonal heterogeneity of IGRP206-214–reactive and –nonreactive diabetogenic CD8 T cells in the islets and spleens of the NOD murine model of T1DM. Our work demonstrates that IGRP206-214–reactive CD8 T cells are highly restricted in terms of TCR gene usage, with the vast majority of these cells exhibiting TRAV16N–TRAJ42–TRBV13-3 TCR gene pairings at the single-cell level. These findings suggest that autoreactive T cells may inherently have restricted TCR repertoires as only specific TCR gene pairings are able to successfully slip through the net of thymic selection. This confirms and expands upon previous findings from CD8 T cell lines generated from islet-infiltrating CD8 T cells, which used anchor PCR to identify unpaired TCR α and β chain sequences (Santamaria et al, 1995). Of note, another study found that IGRP206-214–reactive CD8 T cells from the islets of NOD mice exhibit avidity maturation at the population level, with Vα17.6 (TRAV16*04), then Vα17.4 (TRAV16*01), and finally Vα17.5 (TRAV16*02) TCR α chain V genes being selected for in most of the IGRP206-214–reactive CD8 T cells (Han et al, 2005b). Our data instead found a highly selective use of the TRAV16N gene, although some of this homogeneity may be due to the fact that the 10x Genomics Cell Ranger pipeline is limited in its ability to distinguish between all TRAV16 gene variants. It is therefore possible that the TCR α V gene variants previously reported by Han et al are present in our data. Alternatively, it may be possible that differences in the TCR repertoire of IGRP206-214–reactive CD8 T cells may vary in NOD mice based upon their housing and diet; it has been shown that the gut microbiome impacts both the onset of T1DM in NOD mice (Tai et al, 2016) as well as T-cell maturation during thymic selection (Ennamorati et al, 2020; Cheng et al, 2021; Zegarra-Ruiz et al, 2021). It is plausible that some peptides from commensal microbiota may directly impact the development of IGRP206-214–reactive CD8 T cells in the thymus by being presented on dendritic cells, as Tai et al demonstrated that the Mgt protein from Fusobacteria acts as an IGRP mimic and is capable of activating peripheral IGRP206-214–reactive NY8.3 TCR transgenic CD8 T cells in vivo by oral gavage.
Our data expand upon previous evidence by Han et al due to technological advancements in sequencing technology. Using scTCR-seq, our study was able to identify TCR gene restriction not only in the TCR α V gene but also in the α J, β V, and β J genes at the single-cell level; moreover, we demonstrate that this holds true across several thousand diabetogenic CD8 T cells, all of which come from endogenous T cell repertoires rather than transgenic T cells or cultured cell lines. These novel findings therefore demonstrate that TCR gene usage in IGRP206-214–reactive CD8 T cells is even more restricted than previously thought. Evidence of restricted TCR gene usage in IGRP206-214–reactive CD8 T cells may also warrant the investigation of TCR gene usage in T cells reactive to other commonly targeted β cell epitopes, such as insulin (Amdare et al, 2021), at the single-cell level; these data parallel findings of restricted TCR repertoires among autoreactive T cells found in other autoimmune disorders such as experimental autoimmune encephalomyelitis (Acha-Orbea et al, 1988; Urban et al, 1988; Sakai et al, 1989), polymyositis (Mantegazza et al, 1993), rheumatoid arthritis (Williams et al, 1992; Sharma et al, 2021), thyroiditis (Martin et al, 1999), and primary biliary cirrhosis (Moebius et al, 1990). It would also be valuable to apply scTCR-seq to CD8 T cells targeting other autoantigens in the islets of NOD mice to see if this trend holds for CD8 T cells targeting other antigens; although we studied IGRP206-214–nonreactive CD8 T cells in bulk in this study, we did not separately sort and sequence CD8 T cells targeting specific autoantigens other than IGRP206-214 because of technical limitations of cell recovery. Restricted TCR gene usage may therefore be a fundamental property conserved among autoimmune disorders, as only certain genes may encode TCR structures that bind a given self-antigen strongly enough to overcome positive selection but weakly enough to evade negative selection in the thymus. These findings provide a window of opportunity to therapeutically target autoreactive T cells via selective depletion, although previous work performed using epitope-specific TCR altered peptide ligands has shown there are critical nuances to these approaches (Han et al, 2005a).
In addition to restricted TCR gene usage, IGRP206-214–reactive CD8 T clones showed strong TCR motif overlap among many clones. The majority (70.3%) of IGRP206-214–reactive clones exhibited a highly conserved “RDSG” TCR α chain CDR3 motif, a result of both restricted TRAV16-N/TRAJ42 gene usage as well as N nucleotide additions within the CDR3. We also identified three consensus β chain CDR3 motifs arising from the three most dominant TCR β J genes: TRBJ2-2, TRBJ2-4, and TRBJ2-7. Expression of TRBJ2-2 significantly correlated with increased clone size, increased TCR signaling module scores, increased LCMV Cl13 Effector and Exhausted module scores among islet CD8 T cells, and increased frequencies of effector cells in the islets and spleen. Collectively, these analyses reveal striking TCR α chain CDR3 motif similarity among IGRP206-214–reactive CD8 T cells clones in the islets and spleen while also suggesting that the TCR β chain CDR3 may be slightly less restricted because of TCR β J gene heterogeneity; expression of TCR β J genes may also impact clonal expansion and effector function of IGRP206-214–reactive clones during the autoimmune response in NOD mice. Of note, our observations mirror TCR-seq data from human T1D patients, whose IGRP265-273–specific CD8 T cells also exhibited highly restricted TCR α gene usage but less restricted TCR β gene usage (Fuchs et al, 2017).
Despite the remarkable TCR similarly present among IGRP206-214–reactive clones, we found that IGRP206-214–reactive CD8 T-cell clones are extremely heterogeneous and rarely overlap (∼5% overlap for two groups of 10 mice) between biological organisms. This is similar to our previous findings that there is minimal clonal overlap in the virus-specific CD4 T-cell pool among several genetically identical organisms (Khatun et al, 2021). As these clones are highly restricted in terms of germline TCR gene usage, the clonal diversity in the IGRP-specific CD8 T-cell repertoire arises both from combinatorial diversity of V and J gene pairings, junctional diversity of N nucleotides added between TCR genes during V(D)J recombination, and paired diversity of α chain and β chain sequences for each cell. These details may have hitherto been obscured but are now coming to light given the resolution afforded by scTCR-seq. Application of this technique to other autoimmune disorders may unveil similar trends in both clonal restriction and heterogeneity to those we have identified among diabetogenic T cells.
Using scRNA-seq, we found several distinct phenotypes of diabetogenic CD8 T cells in the islets. Four of these clusters (bystander, progenitor, mitotic, and effector) were recently identified by our laboratory (Ciecko et al, 2021), whereas the recently activated cluster and anergic cluster were identified by our current work. We also identified six clusters of IGRP206-214–reactive CD8 T cells in the spleen. Effector memory and AY036118+ cells had a similar memory-like phenotype, suggesting that autoreactive memory CD8 T cells can be found in lymphoid tissues. Regulon analysis suggested that these memory T cell subsets may depend on the TF Zeb2. Generation of diabetogenic memory T cells is a critical barrier to the use of islet transplants (Ehlers & Rigby, 2015), so identification and inhibition of pathways that promote memory formation may ultimately be of clinical benefit. Splenic Cx3cr1+ effector cells were similar to islet effector CD8 T cells as both populations expressed the cytotoxic molecule Gzmb; however, the former expressed lower levels of coinhibitory receptors such as Pdcd1, Lag3, and Tigit and higher levels of the effector marker Klrg1 (Joshi et al, 2007; Zander et al, 2019). This gene expression profile suggests that Cx3cr1+ effector cells in the spleen have a more cytotoxic phenotype whereas effector cells in the islets exhibit characteristics of exhausted T cells. Although we did not find a discrete exhausted subset of CD8 T cells in the islets, we did identify a small number of exhausted cells in the spleen. These cells were characterized by expression of Cxcr6, Pdcd1, Tigit, and Eomes and were reminiscent of both CXCR6+ PD-1hi exhausted CD8 T cells from LCMV Cl13 infection (Zander et al, 2019) as well as TIGIT+ Eomeshi CD8 T cells found in the peripheral blood of T1D patients (Wiedeman et al, 2020). Finally, we identified central memory and anergic cells in the spleen, both of which had limited clonal expansion and predicted differentiation potential. Although the splenocytes we sequenced were sorted on CD44+ cells, it may be possible that the splenic central memory cluster may also contain some naïve CD8 T cells given that naïve and memory CD8 T cells share similar phenotypic and epigenetic profiles (Schauder et al, 2021). Collectively, our data therefore identify previously underappreciated phenotypic heterogeneity among diabetogenic CD8 T cells and show that several different CD8 T-cell populations exist in the islets and spleens of NOD mice during the onset of T1D.
Although our analyses identified variable clonal overlap between islet effector clusters and splenic effector and memory-like populations, there are still unresolved questions regarding the differentiation process of these cells. The relationship between islet effector cells and splenic Cx3cr1+ effector cells is of particular interest. Although it is possible that the former population could up-regulate the chemokine receptor CX3CR1 to exit the islets and enter the circulation to eventually travel to the spleen and become Cx3cr1+ effector cells, islet effector cells express the inhibitory coreceptors Pdcd1 and Tigit, typically expressed by exhausted cells, whereas these genes are not highly expressed in Cx3cr1+ effector cells. Moreover, islet effector cells express Tox, a master regulator of T-cell exhaustion (Alfei et al, 2019; Khan et al, 2019; Scott et al, 2019; Seo et al, 2019; Yao et al, 2019), whereas this key marker is not expressed by splenic Cx3cr1+ effector cells. Alternatively, splenic Cx3cr1+ effector cells may travel from pancreatic lymph nodes, where they are activated but have not yet migrated to pancreatic islets. Single-cell assay for transposase-accessible chromatin (scATAC-seq) may therefore be useful to map the epigenetic landscape of these two populations and splenic exhausted cells to determine whether transdifferentiation is possible. scATAC-seq was recently used to study autoreactive CD8 T cells found in the peripheral blood of T1D patients (Abdelsamed et al, 2020) and would provide a valuable comparison with future data from murine diabetogenic CD8 T cells. High resolution sequencing techniques such as combined scRNA-seq and scTCR-seq, as used in this work, may therefore shed new light on the phenotypic and clonotypic nuances of T cells in T1D and other autoimmune disorders.
Materials and Methods
Mice
NOD/ShiLtJ (NOD) mice were procured from The Jackson Laboratory and maintained at the Medical College of Wisconsin (MCW). All mice were bred and maintained under the guidelines of the MCW Institutional Animal Care and Use Committee (IACUC).
Isolation of cells from pancreatic islets and spleen
Pancreatic islets were isolated as previously described (Foda et al, 2020). Briefly, pancreatic islets were isolated by perfusing the pancreas via the common bile duct with collagenase P solution (0.5 units/ml). The inflated pancreata were incubated for 16 min at 37°C, agitated, and washed with Hank’s balanced salt solution (Sigma-Aldrich) plus 2% fetal bovine serum. Islets were hand-picked from the pancreas digestion slurry and dissociated in non-enzymatic cell dissociation buffer. Islet cell suspensions were resuspended in RPMI and incubated at 37°C for 1 h. Final islet single cell suspensions were washed and resuspended in RPMI. Spleens were harvested and mechanically processed with a 70 μm filter to obtain a single-cell suspension. Red blood cell lysis was performed using ACK lysing buffer (Lonza). Splenocytes were enriched for CD8 T cells using magnetic bead-based negative selection (CD8 mouse T-cell isolation kit; Miltenyi Biotec). Final spleen single-cell suspensions were washed and resuspended in RPMI. Islets and spleens were each pooled from 10 mice for both scRNA-seq experiments.
Cell sorting
Spleen and islet single cell suspensions were incubated with 5 μg/ml of FC block (anti-mouse CD16/CD32 clone 2.4G2; Bio X Cell). Cells were simultaneously incubated for 15 min at room temperature with MHC class I (Kd) tetramers loaded with mimotope peptide NRP-V7 (Trudeau et al, 2003). Cells were then stained with antibodies against CD45.1 (A20), CD8 (53-6.7), CD44 (IM7), and lineage markers (Lin) CD4 (RM4-5), B220 (RA3-6B2), and CD11b (M1/70) for 30 min at 4°C and were then washed and passed through a 30 μm filter. Single CD45.1+ Lin−CD8+ NRP-V7 Tet+ cells were sorted from spleen and islet samples and single CD45.1+ Lin−CD8+ NRP-V7 Tet− cells were sorted from islet samples using a FACSAria II cell sorter (BD Biosciences).
Single-cell RNA sequencing
Cells were loaded into the 10x Chromium Controller (10x Genomics) for barcoding. scRNA-seq and scTCR-seq libraries were prepared using the Chromium Single Cell 5′ v3 Reagent Kit according to the manufacturer’s protocol. Libraries were quantified using a KAPA library quantification kit (Roche Sequencing) and loaded onto an Illumina NextSeq 500 sequencer. A NextSeq 500/550 High Output Kit v2.5 (150 cycles) (20024907; Illumina) with 26 cycles for read 1, 91 cycles for read 2, and 8 cycles for the index read was used for scRNA-seq libraries from the first experiment (Round 1). A NextSeq 500/550 High Output Kit v2.5 (300 cycles) (20024908; Illumina) with 151 cycles for read 1, 151 cycles for read 2, and 8 cycles for the index read was used for scTCR-seq libraries from Round 1. A NextSeq 500/550 High Output Kit v2 (300 cycles) (20024908; Illumina) with 149 cycles for read 1, 149 cycles for read 2, and 10 cycles for index reads was used for pooled scRNA-seq and scTCR-seq libraries from the second experiment (Round 2). Raw sequencing data were downloaded from Illumina BaseSpace and demultiplexed using the “mkfastq” and “count” functions in Cell Ranger (v 6.0) (10x Genomics). scRNA-seq FASTQ files were aligned to the mm10 reference transcriptome (10x Genomics) using the “count” function, whereas scTCR-seq FASTQ files were aligned to the GRCm38 V(D)J reference transcriptome (10x Genomics). 7,302, 1,373, and 3,708 cells were recovered from islet IGRP−, islet IGRP+, and spleen IGRP+ Round 1 scRNA-seq samples, respectively. 5,550, 1,011, and 2,959 cells were recovered from islet IGRP−, islet IGRP+, and spleen IGRP+ Round 1 scTCR-seq samples, respectively. 9,877, 1,889, and 2,669 cells were recovered from islet IGRP−, islet IGRP+, and spleen IGRP+ Round 2 scRNA-seq samples, respectively. 8,495, 1,585, and 2,361 cells were recovered from islet IGRP−, islet IGRP+, and spleen IGRP+ Round 2 scTCR-seq samples, respectively.
Single-cell RNA sequencing analysis
Data analysis was performed in R (v 4.0.2) using the package Seurat (v 4.0.3) (Stuart et al, 2019), with the package tidyverse (v 1.2.1) (Wickham et al, 2019) used to organize data and the package ggplot2 (v 3.2.1) used to generate figures. scRNA-seq data were filtered to keep cells with a low percentage of mitochondrial genes in the transcriptome (<5%) and between 200 and 2000 (spleen) or 200 and 3,500 (islets) unique genes to exclude apoptotic cells, low quality cells, and doublets. Cell cycle scores were regressed when scaling gene expression values and T cell receptor genes were regressed during scTransform integration (Hafemeister & Satija, 2019) and clustering, which was performed using 50 principal components with the Louvain algorithm and visualized with UMAP plots. Islet and spleen samples were integrated and clustered separately to avoid overfitting phenotypes. From the islet data, one cluster of 237 pancreatic exocrine cells was excluded before analysis. A clone was defined as a group of cells sharing the same TCR α and β chain CDR3 nucleotide sequences. STARTRAC (v 0.1.0) (Zhang et al, 2018) was used to calculate clonal metrics of differentiation potential. TCRdist (Dash et al, 2017) was used to perform CDR3 motif analysis. Chord diagrams were generated with circlize (v 0.4.8) (Gu et al, 2014), Venn diagrams with VennDiagram (v 1.6.20) (Chen & Boutros, 2011), and UpSet diagrams with ggupset (v 0.3.0). Module scores were calculated with stated gene lists using Seurat’s AddModuleScore function with default parameters. Regulon analysis was performed using DoRothEA (v 1.0.1) (Garcia-Alonso et al, 2019; Holland et al, 2020a; 2020b) with default settings; only TFs with confidence ratings of A, B, or C (validated ChIP-seq binding and present in DoRothEA curated resources) were used for analysis.
Statistical analysis
Statistical tests were performed in R (v 4.0.2). Violin plots were compared pairwise using the Wilcoxon test with Holm–Sidak correction using the ggpubr package (v 0.4.0). Bar graphs and logistic regression plots were analyzed using the chi-squared test.
Data and code availability
Single-cell RNA sequencing and single-cell TCR sequencing data from this article are accessible in the GEO database (accession number GSE200608). All other raw data and scripts are available from the corresponding author upon request.
Supplementary Material
Acknowledgements
This work is supported by National Institutes of Health (NIH) grants DK107541 (Yi-Guang Chen), DK121747 (Yi-Guang Chen), AI125741 (W Cui), AI148403 (W Cui), DK127526 (MY Kasmani), and DK118786 (AE Ciecko); by an American Cancer Society Research Scholar Grant (W Cui); and by an Advancing a Healthier Wisconsin Endowment Grant (W Cui). MY Kasmani and AK Brown are members of the Medical Scientist Training Program at the Medical College of Wisconsin (MCW), which is partially supported by a training grant from the National Institute of General Medical Sciences (NIGMS) (T32-GM080202). This research was completed in part with computational resources and technical support provided by the Research Computing Center at MCW. NRP-V7 tetramer was obtained through the NIH Tetramer Core Facility.
Author Contributions
MY Kasmani: conceptualization, data curation, software, formal analysis, validation, investigation, visualization, and writing—original draft, review, and editing.
AE Ciecko: conceptualization, data curation, software, formal analysis, validation, investigation, visualization, and writing—original draft, review, and editing.
AK Brown: software, formal analysis, visualization, and writing—review and editing.
G Petrova: investigation.
J Gorski: formal analysis and writing—review and editing.
Y-G Chen: conceptualization, resources, supervision, funding acquisition, project administration, and writing—review and editing.
W Cui: conceptualization, resources, supervision, funding acquisition, project administration, and writing—review and editing.
Conflict of Interest Statement
The authors declare that they have no conflict of interest.
Footnotes
Moujtaba Y Kasmani and Ashley E Ciecko are co-first authors.
Yi-Guang Chen and Weiguo Cui are co-senior authors.
References
- Abdelsamed HA, Zebley CC, Nguyen H, Rutishauser RL, Fan Y, Ghoneim HE, Crawford JC, Alfei F, Alli S, Ribeiro SP, et al. (2020) Beta cell-specific CD8+ T cells maintain stem cell memory-associated epigenetic programs during type 1 diabetes. Nat Immunol 21: 578–587. 10.1038/s41590-020-0633-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acha-Orbea H, Mitchell DJ, Timmermann L, Wraith DC, Tausch GS, Waldor MK, Zamvil SS, McDevitt HO, Steinman L (1988) Limited heterogeneity of T cell receptors from lymphocytes mediating autoimmune encephalomyelitis allows specific immune intervention. Cell 54: 263–273. 10.1016/0092-8674(88)90558-2 [DOI] [PubMed] [Google Scholar]
- Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, Rosenberg SA (2009) Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood 114: 1537–1544. 10.1182/blood-2008-12-195792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfei F, Kanev K, Hofmann M, Wu M, Ghoneim HE, Roelli P, Utzschneider DT, von Hoesslin M, Cullen JG, Fan Y, et al. (2019) TOX reinforces the phenotype and longevity of exhausted T cells in chronic viral infection. Nature 571: 265–269. 10.1038/s41586-019-1326-9 [DOI] [PubMed] [Google Scholar]
- Amdare N, Purcell AW, DiLorenzo TP (2021) Noncontiguous T cell epitopes in autoimmune diabetes: From mice to men and back again. J Biol Chem 297: 100827. 10.1016/j.jbc.2021.100827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmaier K, Krawczyk C, Kozieradzki I, Kong YY, Sasaki T, Oliveira-dos-Santos A, Mariathasan S, Bouchard D, Wakeham A, Itie A, et al. (2000) Negative regulation of lymphocyte activation and autoimmunity by the molecular adaptor Cbl-b. Nature 403: 211–216. 10.1038/35003228 [DOI] [PubMed] [Google Scholar]
- Banerjee A, Gordon SM, Intlekofer AM, Paley MA, Mooney EC, Lindsten T, Wherry EJ, Reiner SL (2010) Cutting edge: The transcription factor eomesodermin enables CD8 + T cells to compete for the memory cell niche. J Immunol 185: 4988–4992. 10.4049/jimmunol.1002042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltra JC, Manne S, Abdel-Hakeem MS, Kurachi M, Giles JR, Chen Z, Casella V, Ngiow SF, Khan O, Huang YJ, et al. (2020) Developmental relationships of four exhausted CD8+ T cell subsets reveals underlying transcriptional and epigenetic landscape control mechanisms. Immunity 52: 825–841.e8. 10.1016/j.immuni.2020.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluestone JA, Herold K, Eisenbarth G (2010) Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature 464: 1293–1300. 10.1038/nature08933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger ML, Cruz AM, Crossland GE, Gaglia G, Ritch CC, Blatt SE, Bhutkar A, Canner D, Kienka T, Tavana SZ, et al. (2021) Antigen dominance hierarchies shape TCF1+ progenitor CD8 T cell phenotypes in tumors. Cell 184: 4996–5014.e26. 10.1016/j.cell.2021.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charmoy M, Wyss T, Delorenzi M, Held W (2021) PD-1+ Tcf1+ CD8+ T cells from established chronic infection can form memory while retaining a stableimprint of persistent antigen exposure. Cell Rep 36: 109672. 10.1016/j.celrep.2021.109672 [DOI] [PubMed] [Google Scholar]
- Chee J, Ko H-J, Skowera A, Jhala G, Catterall T, Graham KL, Sutherland RM, Thomas HE, Lew AM, Peakman M, et al. (2014) Effector-memory T cells develop in islets and report islet pathology in type 1 diabetes. J Immunol 192: 572–580. 10.4049/jimmunol.1302100 [DOI] [PubMed] [Google Scholar]
- Chen H, Boutros PC (2011) VennDiagram: A package for the generation of highly-customizable Venn and euler diagrams in R. BMC Bioinformatics 12: 35. 10.1186/1471-2105-12-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Zander RA, Wu X, Schauder DM, Kasmani MY, Shen J, Zheng S, Burns R, Taparowsky EJ, Cui W (2021) BATF regulates progenitor to cytolytic effector CD8+ T cell transition during chronic viral infection. Nat Immunol 22: 996–1007. 10.1038/s41590-021-00965-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Ji Z, Ngiow SF, Manne S, Cai Z, Huang AC, Johnson J, Staupe RP, Bengsch B, Xu C, et al. (2019) TCF-1-Centered transcriptional network drives an effector versus exhausted CD8 T cell-fate decision. Immunity 51: 840–855.e5. 10.1016/j.immuni.2019.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Yuan Y, Zhao F, Chen J, Chen P, Li Y, Yan X, Luo C, Shu D, Qu H, et al. (2021) Thymic T-cell production is associated with changes in the gut microbiota in young chicks. Front Immunol 12: 700603. 10.3389/fimmu.2021.700603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang YJ, Kole HK, Brown K, Naramura M, Fukuhara S, Hu RJ, Jang IK, Gutkind JS, Shevach E, Gu H (2000) Cbl-b regulates the CD28 dependence of T-cell activation. Nature 403: 216–220. 10.1038/35003235 [DOI] [PubMed] [Google Scholar]
- Ciecko AE, Schauder DM, Foda B, Petrova G, Kasmani MY, Burns R, Lin CW, Drobyski WR, Cui W, Chen YG (2021) Self-renewing islet TCF1+ CD8 T cells undergo IL-27–controlled differentiation to become TCF1− terminal effectors during the progression of type 1 diabetes. J Immunol 207: 1990–2004. 10.4049/jimmunol.2100362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole DK, Miles KM, Madura F, Holland CJ, Schauenburg AJA, Godkin AJ, Bulek AM, Fuller A, Akpovwa HJE, Pymm PG, et al. (2014) T-cell Receptor (TCR)-peptide specificity overrides affinity-enhancing TCR-major histocompatibility complex interactions. J Biol Chem 289: 628–638. 10.1074/jbc.M113.522110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly KA, Kuchroo M, Venkat A, Khatun A, Wang J, William I, Hornick NI, Fitzgerald BL, Damo M, Kasmani MY, et al. (2021) A reservoir of stem-like CD8 + T cells in the tumor-draining lymph node preserves the ongoing anti-tumor immune response. Sci Immunol 6: eabg7836. 10.1126/sciimmunol.abg7836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney AH, Lo WL, Weiss A (2018) TCR signaling: Mechanisms of initiation and propagation. Trends Biochem Sci 43: 108–123. 10.1016/j.tibs.2017.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash P, Fiore-Gartland AJ, Hertz T, Wang GC, Sharma S, Souquette A, Crawford JC, Clemens EB, Nguyen THO, Kedzierska K, et al. (2017) Quantifiable predictive features define epitope-specific T cell receptor repertoires. Nature 547: 89–93. 10.1038/nature22383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MM, Bjorkman PJ (1988) T-cell antigen receptor genes and T-cell recognition. Nature 334: 395–402. 10.1038/334395a0 [DOI] [PubMed] [Google Scholar]
- DiLorenzo TP, Graser RT, Ono T, Christianson GJ, Chapman HD, Roopenian DC, Nathenson SG, Serreze DV (1998) Major histocompatibility complex class I-restricted T cells are required for all but the end stages of diabetes development in nonobese diabetic mice and use a prevalent T cell receptor α chain gene rearrangement. Proc Natl Acad Sci U S A 95: 12538–12543. 10.1073/pnas.95.21.12538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez CX, Amezquita RA, Guan T, Marshall HD, Joshi NS, Kleinstein SH, Kaech SM (2015) The transcription factors ZEB2 and T-bet cooperate to program cytotoxic T cell terminal differentiation in response to LCMV viral infection. J Exp Med 212: 2041–2056. 10.1084/jem.20150186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers MR, Rigby MR (2015) Targeting memory T cells in type 1 diabetes. Curr Diab Rep 15: 84. 10.1007/s11892-015-0659-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennamorati M, Vasudevan C, Clerkin K, Halvorsen S, Verma S, Ibrahim S, Prosper S, Porter C, Yeliseyev V, Kim M, et al. (2020) Intestinal microbes influence development of thymic lymphocytes in early life. Proc Natl Acad Sci U S A 117: 2570–2578. 10.1073/pnas.1915047117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foda BM, Ciecko AE, Serreze DV, Ridgway WM, Geurts AM, Chen Y-G (2020) The CD137 ligand is important for type 1 diabetes development but dispensable for the homeostasis of disease-suppressive CD137 + FOXP3 + regulatory CD4 T cells. J Immunol 204: 2887–2899. 10.4049/jimmunol.1900485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs YF, Eugster A, Dietz S, Sebelefsky C, Kühn D, Wilhelm C, Lindner A, Gavrisan A, Knoop J, Dahl A, et al. (2017) CD8+ T cells specific for the islet autoantigen IGRP are restricted in their T cell receptor chain usage. Sci Rep 7: 44661. 10.1038/srep44661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Alonso L, Holland CH, Ibrahim MM, Turei D, Saez-Rodriguez J (2019) Benchmark and integration of resources for the estimation of human transcription factor activities. Genome Res 29: 1363–1375. 10.1101/gr.240663.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gearty SV, Dündar F, Zumbo P, Espinosa-Carrasco G, Shakiba M, Sanchez-Rivera FJ, Socci ND, Trivedi P, Lowe SW, Lauer P, et al. (2022) An autoimmune stem-like CD8 T cell population drives type 1 diabetes. Nature 602: 156–161. 10.1038/s41586-021-04248-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Z, Gu L, Eils R, Schlesner M, Brors B (2014) Circlize implements and enhances circular visualization in R. Bioinformatics 30: 2811–2812. 10.1093/bioinformatics/btu393 [DOI] [PubMed] [Google Scholar]
- Guan T, Dominguez CX, Amezquita RA, Laidlaw BJ, Cheng J, Henao-Mejia J, Williams A, Flavell RA, Lu J, Kaech SM (2018) ZEB1, ZEB2, and the miR-200 family form a counterregulatory network to regulate CD8+ T cell fates. J Exp Med 215: 1153–1168. 10.1084/jem.20171352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta SS, Wang J, Chen M (2020) Metabolic reprogramming in CD8+ T cells during acute viral infections. Front Immunol 11: 1013. 10.3389/fimmu.2020.01013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafemeister C, Satija R (2019) Normalization and variance stabilization of single-cell RNA-seq data using regularized negative binomial regression. Genome Biol 20: 296. 10.1186/s13059-019-1874-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han B, Serra P, Amrani A, Yamanouchi J, Marée AFM, Edelstein-Keshet L, Santamaria P (2005. a) Prevention of diabetes by manipulation of anti-IGRP autoimmunity: High efficiency of a low-affinity peptide. Nat Med 11: 645–652. 10.1038/nm1250 [DOI] [PubMed] [Google Scholar]
- Han B, Serra P, Yamanouchi J, Amrani A, Elliott JF, Dickie P, DiLorenzo TP, Santamaria P (2005. b) Developmental control of CD8+ T cell–avidity maturation in autoimmune diabetes. J Clin Invest 115: 1879–1887. 10.1172/JCI24219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He R, Hou S, Liu C, Zhang A, Bai Q, Han M, Yang Y, Wei G, Shen T, Yang X, et al. (2016) Follicular CXCR5-expressing CD8+ T cells curtail chronic viral infection. Nature 537: 412–416. 10.1038/nature19317 [DOI] [PubMed] [Google Scholar]
- Heim K, Binder B, Sagar AG, Wieland D, Hensel N, Llewellyn-Lacey S, Gostick E, Price DA, Emmerich F, Vingerhoet H, et al. (2021) TOX defines the degree of CD8+ T cell dysfunction in distinct phases of chronic HBV infection. Gut 70: 1550–1560. 10.1136/gutjnl-2020-322404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensel N, Gu Z, Sagar, Wieland D, Jechow K, Kemming J, Llewellyn-Lacey S, Gostick E, Sogukpinar O, Emmerich F, et al. (2021) Memory-like HCV-specific CD8+ T cells retain a molecular scar after cure of chronic HCV infection. Nat Immunol 22: 229–239. 10.1038/s41590-020-00817-w [DOI] [PubMed] [Google Scholar]
- Holland CH, Szalai B, Saez-Rodriguez J (2020. a) Transfer of regulatory knowledge from human to mouse for functional genomics analysis. Biochim Biophys Acta Gene Regul Mech 1863: 194431. 10.1016/j.bbagrm.2019.194431 [DOI] [PubMed] [Google Scholar]
- Holland CH, Tanevski J, Perales-Patón J, Gleixner J, Kumar MP, Mereu E, Joughin BA, Stegle O, Lauffenburger DA, Heyn H, et al. (2020. b) Robustness and applicability of transcription factor and pathway analysis tools on single-cell RNA-seq data. Genome Biol 21: 36. 10.1186/s13059-020-1949-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Zakharov PN, Peterson OJ, Unanue ER (2020) Cytocidal macrophages in symbiosis with CD4 and CD8 T cells cause acute diabetes following checkpoint blockade of PD-1 in NOD mice. Proc Natl Acad Sci U S A 117: 31319–31330. 10.1073/pnas.2019743117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson WH, Gensheimer J, Hashimoto M, Wieland A, Valanparambil RM, Li P, Lin JX, Konieczny BT, Im SJ, Freeman GJ, et al. (2019) Proliferating transitory T cells with an effector-like transcriptional signature emerge from PD-1+ stem-like CD8+ T cells during chronic infection. Immunity 51: 1043–1058.e4. 10.1016/j.immuni.2019.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im SJ, Hashimoto M, Gerner MY, Lee J, Kissick HT, Burger MC, Shan Q, Hale JS, Lee J, Nasti TH, et al. (2016) Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature 537: 417–421. 10.1038/nature19330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon MS, Atfield A, Venuprasad K, Krawczyk C, Sarao R, Elly C, Yang C, Arya S, Bachmaier K, Su L, et al. (2004) Essential role of the E3 ubiquitin ligase Cbl-b in T cell anergy induction. Immunity 21: 167–177. 10.1016/j.immuni.2004.07.013 [DOI] [PubMed] [Google Scholar]
- Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM (2007) Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity 27: 281–295. 10.1016/j.immuni.2007.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech SM, Cui W (2012) Transcriptional control of effector and memory CD8+ T cell differentiation. Nat Rev Immunol 12: 749–761. 10.1038/nri3307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan O, Giles JR, McDonald S, Manne S, Ngiow SF, Patel KP, Werner MT, Huang AC, Alexander KA, Wu JE, et al. (2019) TOX transcriptionally and epigenetically programs CD8+ T cell exhaustion. Nature 571: 211–218. 10.1038/s41586-019-1325-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatun A, Kasmani MY, Zander R, Schauder DM, Snook JP, Shen J, Wu X, Burns R, Chen Y-G, Lin C-W, et al. (2021) Single-cell lineage mapping of a diverse virus-specific naive CD4 T cell repertoire. J Exp Med 218: e20200650. 10.1084/jem.20200650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King CG, Koehli S, Hausmann B, Schmaler M, Zehn D, Palmer E (2012) T cell affinity regulates asymmetric division, effector cell differentiation, and tissue pathology. Immunity 37: 709–720. 10.1016/j.immuni.2012.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Leung S, Qureshi S, Darnell JE, Stark GR (1996) Formation of STAT1-STAT2 heterodimers and their role in the activation of IRF-1 gene transcription by interferon-α. J Biol Chem 271: 5790–5794. 10.1074/jbc.271.10.5790 [DOI] [PubMed] [Google Scholar]
- Lieber MR (1991) Site-specific recombination in the immune system. FASEB J 5: 2934–2944. 10.1096/fasebj.5.14.1752360 [DOI] [PubMed] [Google Scholar]
- Lieberman SM, Evans AM, Han B, Takaki T, Vinnitskaya Y, Caldwell JA, Serreze DV, Shabanowitz J, Hunt DF, Nathenson SG, et al. (2003) Identification of the β cell antigen targeted by a prevalent population of pathogenic CD8 + T cells in autoimmune diabetes. Proc Natl Acad Sci U S A 100: 8384–8388. 10.1073/pnas.0932778100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long SA, Thorpe J, DeBerg HA, Gersuk V, Eddy JA, Harris KM, Ehlers M, Herold KC, Nepom GT, Linsley PS (2016) Partial exhaustion of CD8 T cells and clinical response to teplizumab in new-onset type 1 diabetes. Sci Immunol 1: eaai7793. 10.1126/sciimmunol.aai7793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino S, Kunimoto K, Muraoka Y, Mizushima Y, Katagiri K, Tochino Y (1980) Breeding of a non-obese, diabetic strain of mice. Jikken Dobutsu 29: 1–13. 10.1538/expanim1978.29.1_1 [DOI] [PubMed] [Google Scholar]
- Mantegazza R, Andreetta F, Bernasconi P, Baggi F, Oksenberg JR, Simoncini O, Mora M, Cornelio F, Steinman L (1993) Analysis of T cell receptor repertoire of muscle-infiltrating T lymophocytes in polymyositis. J Clin Invest 91: 2880–2886. 10.1172/jci116533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A, Barbesino G, Davies TF (1999) T-cell receptors and autoimmune thyroid disease: Signposts for T-cell-antigen driven diseases. Int Rev Immunol 18: 111–140. 10.3109/08830189909043021 [DOI] [PubMed] [Google Scholar]
- McKinney EF, Lee JC, Jayne DRW, Lyons PA, Smith KGC (2015) T-cell exhaustion, co-stimulation and clinical outcome in autoimmunity and infection. Nature 523: 612–616. 10.1038/nature14468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moebius U, Manns M, Hess G, Kober G, zum Büschenfelde KHM, Meuer SC (1990) T cell receptor gene rearrangements of T lymphocytes infiltrating the liver in chronic active hepatitis B and primary biliary cirrhosis (PBC): Oligoclonality of PBC‐derived T cell clones. Eur J Immunol 20: 889–896. 10.1002/eji.1830200426 [DOI] [PubMed] [Google Scholar]
- Moran AE, Holzapfel KL, Xing Y, Cunningham NR, Maltzman JS, Punt J, Hogquist KA (2011) T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J Exp Med 208: 1279–1289. 10.1084/jem.20110308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskophidis D, Lechner F, Pircher H, Zinkernagel RM (1993) Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature 362: 758–761. 10.1038/362758a0 [DOI] [PubMed] [Google Scholar]
- Mullen Y (2017) Development of the nonobese diabetic mouse and contribution of animal models for understanding type 1 diabetes. Pancreas 46: 455–466. 10.1097/MPA.0000000000000828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oettinger MA, Schatz DG, Gorka C, Baltimore D (1990) RAG-1 and RAG-2, adjacent genes that synergistically activate V(D)J recombination. Science 248: 1517–1523. 10.1126/science.2360047 [DOI] [PubMed] [Google Scholar]
- Olenchock BA, Guo R, Carpenter JH, Jordan M, Topham MK, Koretzky GA, Zhong XP (2006) Disruption of diacylglycerol metabolism impairs the induction of T cell anergy. Nat Immunol 7: 1174–1181. 10.1038/ni1400 [DOI] [PubMed] [Google Scholar]
- Omilusik KD, Best JA, Yu B, Goossens S, Weidemann A, Nguyen JV, Seuntjens E, Stryjewska A, Zweier C, Roychoudhuri R, et al. (2015) Transcriptional repressor ZEB2 promotes terminal differentiation of CD8+ effector and memory T cell populations during infection. J Exp Med 212: 2027–2039. 10.1084/jem.20150194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozga AJ, Moalli F, Abe J, Swoger J, Sharpe J, Zehn D, Kreutzfeldt M, Merkler D, Ripoll J, Stein JV (2016) pMHC affinity controls duration of CD8+ T cell–DC interactions and imprints timing of effector differentiation versus expansion. J Exp Med 213: 2811–2829. 10.1084/jem.20160206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paley MA, Kroy DC, Odorizzi PM, Johnnidis JB, Dolfi DV, Barnett BE, Bikoff EK, Robertson EJ, Lauer GM, Reiner SL, et al. (2012) Progenitor and terminal subsets of CD8+ T cells cooperate to contain chronic viral infection. Science 338: 1220–1225. 10.1126/science.1229620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouillon V, Maréchal Y, Frippiat C, Erneux C, Schurmans S (2013) Inositol 1, 4, 5-trisphosphate 3-kinase B (Itpkb) controls survival, proliferation and cytokine production in mouse peripheral T cells. Adv Biol Regul 53: 39–50. 10.1016/j.jbior.2012.08.001 [DOI] [PubMed] [Google Scholar]
- Renkema KR, Huggins MA, Borges da Silva H, Knutson TP, Henzler CM, Hamilton SE (2020) KLRG1 + memory CD8 T cells combine properties of short-lived effectors and long-lived memory. J Immunol 205: 1059–1069. 10.4049/jimmunol.1901512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K, Zamvil SS, Mitchell DJ, Hodgkinson S, Rothbard JB, Steinman L (1989) Prevention of experimental encephalomyelitis with peptides that block interaction of T cells with major histocompatibility complex proteins. Proc Natl Acad Sci U S A 86: 9470–9474. 10.1073/pnas.86.23.9470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandu I, Cerletti D, Oetiker N, Borsa M, Wagen F, Spadafora I, Welten SPM, Stolz U, Oxenius A, Claassen M (2020) Landscape of exhausted virus-specific CD8 T cells in chronic LCMV infection. Cell Rep 32: 108078. 10.1016/j.celrep.2020.108078 [DOI] [PubMed] [Google Scholar]
- Santamaria P, Utsugi T, Park BJ, Averill N, Kawazu S, Yoon JW (1995) Beta-cell-cytotoxic CD8+ T cells from nonobese diabetic mice use highly homologous T cell receptor alpha-chain CDR3 sequences. J Immunol 154: 2494–2503. [PubMed] [Google Scholar]
- Schauder DM, Shen J, Chen Y, Kasmani MY, Kudek MR, Burns R, Cui W (2021) E2A-regulated epigenetic landscape promotes memory CD8 T cell differentiation. Proc Natl Acad Sci U S A 118: e2013452118. 10.1073/pnas.2013452118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott AC, Dündar F, Zumbo P, Chandran SS, Klebanoff CA, Shakiba M, Trivedi P, Menocal L, Appleby H, Camara S, et al. (2019) TOX is a critical regulator of tumour-specific T cell differentiation. Nature 571: 270–274. 10.1038/s41586-019-1324-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo H, Chen J, González-Avalos E, Samaniego-Castruita D, Das A, Wang YH, López-Moyado IF, Georges RO, Zhang W, Onodera A, et al. (2019) TOX and TOX2 transcription factors cooperate with NR4A transcription factors to impose CD8 + T cell exhaustion. Proc Natl Acad Sci U S A 116: 12410–12415. 10.1073/pnas.1905675116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma RK, Boddul SV, Yoosuf N, Turcinov S, Dubnovitsky A, Kozhukh G, Wermeling F, Kwok WW, Klareskog L, Malmström V (2021) Biased TCR gene usage in citrullinated Tenascin C specific T-cells in rheumatoid arthritis. Sci Rep 11: 24512. 10.1038/s41598-021-04291-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi LZ, Wang R, Huang G, Vogel P, Neale G, Green DR, Chi H (2011) HIF1α-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J Exp Med 208: 1367–1376. 10.1084/jem.20110278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui I, Schaeuble K, Chennupati V, Fuertes Marraco SA, Calderon-Copete S, Pais Ferreira D, Carmona SJ, Scarpellino L, Gfeller D, Pradervand S, et al. (2019) Intratumoral Tcf1+ PD-1+ CD8+ T cells with stem-like properties promote tumor control in response to vaccination and checkpoint blockade immunotherapy. Immunity 50: 195–211.e10. 10.1016/j.immuni.2018.12.021 [DOI] [PubMed] [Google Scholar]
- Stuart T, Butler A, Hoffman P, Hafemeister C, Papalexi E, Mauck WM, Hao Y, Stoeckius M, Smibert P, Satija R (2019) Comprehensive integration of single-cell data. Cell 177: 1888–1902.e21. 10.1016/j.cell.2019.05.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukonina V, Ma H, Zhang W, Bartesaghi S, Subhash S, Heglind M, Foyn H, Betz MJ, Nilsson D, Lidell ME, et al. (2019) FOXK1 and FOXK2 regulate aerobic glycolysis. Nature 566: 279–283. 10.1038/s41586-019-0900-5 [DOI] [PubMed] [Google Scholar]
- Tai N, Peng J, Liu F, Gulden E, Hu Y, Zhang X, Chen L, Wong FS, Wen L (2016) Microbial antigen mimics activate diabetogenic CD8 T cells in NOD mice. J Exp Med 213: 2129–2146. 10.1084/jem.20160526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teijaro JR, Ng C, Lee AM, Sullivan BM, Sheehan KCF, Welch M, Schreiber RD, Carlos de la Torre J, Oldstone MBA (2013) Persistent LCMV infection is controlled by blockade of type I interferon signaling. Science 340: 207–211. 10.1126/science.1235214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonnerre P, Wolski D, Subudhi S, Aljabban J, Hoogeveen RC, Damasio M, Drescher HK, Bartsch LM, Tully DC, Sen DR, et al. (2021) Differentiation of exhausted CD8+ T cells after termination of chronic antigen stimulation stops short of achieving functional T cell memory. Nat Immunol 22: 1030–1041. 10.1038/s41590-021-00982-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topchyan P, Xin G, Chen Y, Zheng S, Burns R, Shen J, Kasmani MY, Kudek M, Yang N, Cui W (2021) Harnessing the IL-21-BATF pathway in the CD8+ T cell anti-tumor response. Cancers (Basel) 13: 1263. 10.3390/cancers13061263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudeau JD, Kelly-Smith C, Verchere CB, Elliott JF, Dutz JP, Finegood DT, Santamaria P, Tan R (2003) Prediction of spontaneous autoimmune diabetes in NOD mice by quantification of autoreactive T cells in peripheral blood. J Clin Invest 111: 217–223. 10.1172/JCI200316409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban JL, Kumar V, Kono DH, Gomez C, Horvath SJ, Clayton J, Ando DG, Sercarz EE, Hood L (1988) Restricted use of T cell receptor V genes in murine autoimmune encephalomyelitis raises possibilities for antibody therapy. Cell 54: 577–592. 10.1016/0092-8674(88)90079-7 [DOI] [PubMed] [Google Scholar]
- Verdaguer J, Yoon JW, Anderson B, Averill N, Utsugi T, Park BJ, Santamaria P (1996) Acceleration of spontaneous diabetes in TCR-beta-transgenic nonobese diabetic mice by beta-cell cytotoxic CD8+ T cells expressing identical endogenous TCR-alpha chains. J Immunol 157: 4726–4735. [PubMed] [Google Scholar]
- Verykokakis M, Papadaki C, Vorgia E, Le Gallic L, Mavrothalassitis G (2007) The RAS-dependent ERF control of cell proliferation and differentiation is mediated by c-Myc repression. J Biol Chem 282: 30285–30294. 10.1074/jbc.M704428200 [DOI] [PubMed] [Google Scholar]
- Westernberg L, Conche C, Huang YH, Rigaud S, Deng Y, Siegemund S, Mukherjee S, Nosaka L, Das J, Sauer K (2016) Non-canonical antagonism of PI3K by the kinase Itpkb delays thymocyte β-selection and renders it Notch-dependent. Elife 5: e10786. 10.7554/eLife.10786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R (2003) Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol 77: 4911–4927. 10.1128/jvi.77.8.4911-4927.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham H, Averick M, Bryan J, Chang W, McGowan L, François R, Grolemund G, Hayes A, Henry L, Hester J, et al. (2019) Welcome to the tidyverse. J Open Source Softw 4: 1686. 10.21105/joss.01686 [DOI] [Google Scholar]
- Wiedeman AE, Muir VS, Rosasco MG, DeBerg HA, Presnell S, Haas B, Dufort MJ, Speake C, Greenbaum CJ, Serti E, et al. (2019) Autoreactive CD8+ T cell exhaustion distinguishes subjects with slow type 1 diabetes progression. J Clin Invest 130: 480–490. 10.1172/JCI126595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland D, Kemming J, Schuch A, Emmerich F, Knolle P, Neumann-Haefelin C, Held W, Zehn D, Hofmann M, Thimme R (2017) TCF1+ hepatitis C virus-specific CD8+ T cells are maintained after cessation of chronic antigen stimulation. Nat Commun 8: 15050. 10.1038/ncomms15050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams WV, Fang Q, Demarco D, VonFeldt J, Zurier RB, Weiner DB (1992) Restricted heterogeneity of T cell receptor transcripts in rheumatoid synovium. J Clin Invest 90: 326–333. 10.1172/JCI115866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson EB, Yamada DH, Elsaesser H, Herskovitz J, Deng J, Cheng G, Aronow BJ, Karp CL, Brooks DG (2013) Blockade of chronic type I interferon signaling to control persistent LCMV infection. Science 340: 202–207. 10.1126/science.1235208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Ji Y, Moseman EA, Xu HC, Manglani M, Kirby M, Anderson SM, Handon R, Kenyon E, Elkahloun A, et al. (2016) The TCF1-Bcl6 axis counteracts type I interferon to repress exhaustion and maintain T cell stemness. Sci Immunol 1: eaai8593. 10.1126/sciimmunol.aai8593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao C, Sun H-W, Lacey NE, Ji Y, Moseman EA, Shih H-Y, Heuston EF, Kirby M, Anderson S, Cheng J, et al. (2019) Single-cell RNA-seq reveals TOX as a key regulator of CD8+ T cell persistence in chronic infection. Nat Immunol 20: 890–901. 10.1038/s41590-019-0403-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates KB, Tonnerre P, Martin GE, Gerdemann U, Al Abosy R, Comstock DE, Weiss SA, Wolski D, Tully DC, Chung RT, et al. (2021) Epigenetic scars of CD8+ T cell exhaustion persist after cure of chronic infection in humans. Nat Immunol 22: 1020–1029. 10.1038/s41590-021-00979-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama WM, Koning F, Kehn PJ, Pereira GM, Stingl G, Coligan JE, Shevach EM (1988) Characterization of a cell surface-expressed disulfide-linked dimer involved in murine T cell activation. J Immunol 141: 369–376. [PubMed] [Google Scholar]
- Zajac AJ, Blattman JN, Murali-Krishna K, Sourdive DJD, Suresh M, Altman JD, Ahmed R (1998) Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med 188: 2205–2213. 10.1084/jem.188.12.2205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharov PN, Hu H, Wan X, Unanue ER (2020) Single-cell RNA sequencing of murine islets shows high cellular complexity at all stages of autoimmune diabetes. J Exp Med 217: e20192362. 10.1084/jem.20192362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zander R, Schauder D, Xin G, Nguyen C, Wu X, Zajac A, Cui W (2019) CD4+ T cell help is required for the Formation of a cytolytic CD8+ T cell subset that protects against chronic infection and cancer. Immunity 51: 1028–1042.e4. 10.1016/j.immuni.2019.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zegarra-Ruiz DF, Kim DV, Norwood K, Kim M, Wu WJH, Saldana-Morales FB, Hill AA, Majumdar S, Orozco S, Bell R, et al. (2021) Thymic development of gut-microbiota-specific T cells. Nature 594: 413–417. 10.1038/s41586-021-03531-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Yu X, Zheng L, Zhang Y, Li Y, Fang Q, Gao R, Kang B, Zhang Q, Huang JY, et al. (2018) Lineage tracking reveals dynamic relationships of T cells in colorectal cancer. Nature 564: 268–272. 10.1038/s41586-018-0694-x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Single-cell RNA sequencing and single-cell TCR sequencing data from this article are accessible in the GEO database (accession number GSE200608). All other raw data and scripts are available from the corresponding author upon request.