Abstract
Psoriasis is a chronic immune-mediated inflammatory skin disease, characterized by well-demarcated scaly, erythematous, infiltrated plaques. The cutaneous-to-systemic expansion of the inflammation in psoriasis leads to the concept of “psoriatic march” or “inflammatory skin march”. Accordingly, psoriasis is thought to be a systemic inflammatory disease associated with numerous comorbidities. Indeed, it’s currently considered an independent risk factor for cardiovascular diseases. Here, we discuss the current knowledge on TNF-α and IL-23/IL-17 mediated pathways linking the psoriatic plaque to the cardiovascular compartment. We further argue the possible involvement of the endothelial compartment in the psoriatic plaque- cardiovascular system crosstalk.
Keywords: psoriasis, cytokines, endothelial dysfunction (ED), cardiovascular diseases, inflammation
The Psoriatic March (From the Cutaneous to Systemic Spreading of the Inflammation)
Psoriasis is a chronic cutaneous disease resulting from a complex interplay between immunological, environmental and genetic factors, with a global prevalence ranging from 0.09 to 5.1% of adult population (1). It predominantly affects the skin with a broad spectrum of cutaneous manifestations that comprise either solitary lesions, varying from pinpoint to large plaques, or generalized erythroderma. 85-90% of all patients present a plaque-type psoriasis that appears with demarcated, scaly, erythematous and infiltrated lesions. These manifestations are usually located in specific body sites, as for instance the extensor surfaces of the limbs (knee and elbows) and other mechanically stressed sites (lumbar region) (2, 3). At the bottom of psoriasis clinical signs there is a complex multistep process involving a dysregulated activation of both the innate and adaptive immunity. Indeed, nowadays the prevailing model for psoriatic pathophysiology implies crucial abnormalities in the antigen presentation by dendritic cells (DCs), and the concomitant altered differentiation of T helper (TH) cell populations. Here, the enhanced (IL)-17-producing T cell response promotes the recruitment of immune cells, thus driving the psoriatic plaque formation (4–6).
Beyond the skin, systemic inflammation has been reported in psoriatic patients leading to the concept of “psoriatic march”, or “inflammatory skin march” (7). In accordance with this hypothesis, the inflammatory cutaneous-to-systemic expansion in psoriatic patients might cause the insurgence of systemic immune-mediated alterations, subsequently leading to important comorbidities such as diabetes mellitus, metabolic disorders, including obesity, hypertension, dyslipidemia, and cardio-vascular diseases (CVDs) (8, 9). Consistently, Sokolova et al. reported an increase of inflammatory serum markers lipocalin 2, beta-defensin 2, IL-22, IL-8 and calprotectin in psoriatic patients (9, 10). Moreover, several circulating biomarkers of inflammation, as C-reactive protein (CRP), erythrocyte sedimentation rate and the platelet activation marker P-selectin, positively correlate with the disease severity (11–13). In this line, elevated levels of pro-inflammatory S100A12, CCL22, IL1RN, CCL2, VEGF, ICAM1, IL-15, TNF, CCL5 have been identified both in the skin and in the blood of psoriatic patients, thus highlighting that psoriatic plaque pathological products might circulate toward the vascular network (14). Indeed, numerous foci of inflammation within the skin, liver, joints, tendons, and aorta have been observed in psoriasis patients by (18)F-fluorodeoxyglucose positron emission tomography computed tomography (FDG-PET/CT) (15). These results not only point out the systemic burden of psoriasis, but also suggest that psoriatic inflammation might act at distant sites.
In this review, we will overview current knowledge on the association between psoriasis and cardiovascular (CV) risk with a special focus on immune players contributing to the pathogenic link between psoriasis and CVDs.
Psoriasis as an Independent Cardiovascular Risk Factor
A high prevalence of underdiagnosed and undertreated CV risk factors, including hypertension, diabetes, hyperlipidemia, obesity, and metabolic syndrome, is commonly reported in psoriatic patients (Table 1) (16–31). However, the undoubted causality of this correlation is still questioned.
Table 1.
Summary of systemic reviews and meta-analysis analyzing the association between psoriasis and traditional cardiovascular risk factors.
| References | Study population | Traditional cardiovascular risk factors and relative risk of measures |
|---|---|---|
| Armsrong et al., (17) | Psoriasis: 20831 Control: 1898169 |
Obesity OR 1.66 (1.46-1.89); mild psoriasis OR 1.46 (1.17–1.82); severe psoriasis OR 2.23 (1.63–3.05) |
| Armstrong et al., (16) | Psoriasis: 309469 Controls: 2088197 |
Hypertension: all psoriasis, OR = 1.58 (1.42–1.76); mild psoriasis, OR = 1.30 (1.15–1.47); severe psoriasis, OR = 1.49 (1.20–1.86) |
| Ma et al., (18) | Psoriasis: 238385 Controls: 2102220 |
Dyslipidemia OR (1.04-5.05) |
| Armstrong et al., (19) | Psoriasis: 314036 Control: 3717217 |
Diabetes OR 1.59 (1.38–1.83); mild psoriasis pooled OR 1.53 (1.16–2.04); severe psoriasis pooled OR 1.97 (1.48–2.62) |
| Armstrong et al., (20) | Psoriasis: 41853 Control: 135814 |
Metabolic syndrome OR 2.26 (1.70–3.01) |
| Coto-Segura et al., (21) | Psoriasis: 557697 Control: 5186485 |
Type 2 diabetes pooled OR 1.76 (1.59–1.96) |
| Miller at al., (22) | Psoriasis: 503686 Control: 2686694 |
Diabetes OR 1.9 (1.5–2.5); hypertension OR 1.8 (1.6–2.0), dyslipidemia OR 1.5 (1.4–1.7); obesity OR 1.8 (1.4–2.2); metabolic syndrome OR 1.8 (1.2–2.8) |
| Rodriguez-Zuniga et al., (23) | Psoriasis: 25042 Control: 131609 |
Metabolic syndrome pooled OR 1.42 (1.28–1.65) |
| Singh et al., (24) | Psoriasis: 46714 Control: 1403474 |
Metabolic syndrome pooled OR 2.14 (1.84–2.48) |
| Mamizadeh et al., (25) | Psoriasis: 922870 Control: 12808071 |
Diabetes OR 1.69 (1.51–1.89) |
| Choudhary et al., (26) | Psoriasis: 15939 Control: 103984 |
Metabolic syndrome OR 2.077 (1.84–2.34) |
| Choudhary et al., (27) | Psoriasis: 17672 Control: 66407 |
Increased systolic blood pressure OR 2.31 (1.12–4.74); diastolic blood pressure OR 2.31 (1.58–3.38); abdominal obesity OR 1.90 (1.45–2.50); Triglycerides OR 1.80 (1.29–2.51) |
| Phan et al., (28) | Pediatric psoriasis: 43808 Control: 5384057 |
Obesity OR 2.45 (1.73–3.48); diabetes OR 2.32 (1.34–4.03); hypertension OR 2.19 (1.62–2.95); hyperlipidemia OR 2.01 (1.66–2.42); metabolic syndrome OR 1.75 (1.75–7.14) |
| Duan et al., (29) | Psoriasis: 255132 Control: 814631 |
Hypertension OR 1.43 (1.25–1.64) |
| Cho et al., (30) | Pediatric psoriasis: 20676 Controls: 5239197 |
Obesity pooled OR 2.4 (1.6-3.59); diabetes OR 2.01 (1.09-3.73); hypertension OR 2.73 (1.79-4.17); dyslipidemia OR 1.67 (1.42-1.97); metabolic syndrome OR 7.49 (1.86-30.07) |
OR, odds ratio. Values in brackets indicate 95% confidence intervals.
Obesity itself is an independent risk factor for the psoriatic development due to the massive induction of systemic inflammatory responses (17). Nevertheless, multiple studies have demonstrated an increased prevalence of obesity among patients with psoriasis. Although the molecular mechanisms have still to be elucidated, a severity-dependent relationship between the two diseases has been demonstrated (32, 33). Similar findings were found for hypertension, having psoriatic patients higher rate incidence of hypertension in comparison to the global population (34). To note, severe psoriasis patients showed a higher prevalence of hypertension compared with mild psoriasis patients (29). Several pathways have been proposed to unfold this association, as the higher serum levels of the renin and angiotensin-converting enzyme (ACE) in psoriatic patients (34). Furthermore, reduced blood levels of high-density lipoprotein (HDL) were described in psoriatic patients compared to age-matched controls (18, 35, 36). Intriguingly, moderate-to-severe psoriasis has been often associated to metabolic syndrome (MetS), which represents a well-known set of risk factors, including obesity and hypertension, but also dyslipidemia, insulin resistance related with an elevated risk of type 2 diabetes and CVD (19). Recent meta-analyses and epidemiological studies further highlighted the prevalence of MetS in 20-50% of psoriatic patients in comparison to healthy subjects (20, 24, 37, 38). In this line, Langan et al. demonstrated that psoriasis was independently associated with MetS even after adjustments for multiple parameters including age, sex, follow-up, smoking, and social class and that importantly, MetS prevalence directly correlates with the cutaneous disease extent (37). Together, these reports indicated that conventional CV risk factors are prevalent among psoriatic patients. Beside this notion, psoriasis per se is increasingly thought to be an independent risk factor for CVD and a “dose effect” of psoriasis’ activity on the patients’ cardiovascular risk has been demonstrated (39). Systemic reviews and meta-analysis evaluating the association between psoriasis and CVDs are summarized in Table 2 (22, 28, 40–49).
Table 2.
Summary of systemic reviews and meta-analysis assessing the risk of cardiovascular diseases in psoriasis.
| References | Study population | CV outcomes and relative risk of measures |
|---|---|---|
| Xu et al., (40) | Psoriasis: 326598 Controls: 5230048 |
MI and stroke RR 1.2 (1.1-1.31); MI RR 1.22 (1.05-1.42; Stroke RR 1.21 (1.04-1.40) |
| Armstrong et al., (41) | Psoriasis: 218654 (mild 201239; severe 17415) Controls: 9914799 |
Mild Psoriasis: MI RR 1.29 (1.02–1.63), stroke RR 1.12 (1.08–1.16) Severe Psoriasis: MI RR 1.70 (1.32–2.18), stroke RR 1.56 (1.32–1.84), CV mortality RR 1.39 (1.11–1.74) |
| Gaeta et al., (42) | Psoriasis: 1862297 Controls: 43407300 |
Overall CV RR 1.24 (1.18–1.31), MI RR 1.24 (1.11–1.39) Vascular disease RR 1.27 (1.12–1.43), CV Mortality RR 1.41 (0.97–2.04) MI and stroke RR 1.20 (1.10–1.31) |
| Gu et al., (43) | Psoriasis and controls: 6230774 | Stroke RR 1.26 (1.12–1.41) MI RR 1.32 (1.13–1.55) CVD RR 1.47 (1.30–1.60) CV mortality RR 1.33 (1.00–1.77) |
| Horreau et al., 2013 (44) | Psoriasis: 324650 Controls: 5309087 |
Cohort studies: MI OR 1.25 (1.03–1.52), CAD OR 1.20 (1.13–1.27), stroke OR 1.02 (0.92–1.14) Cross-sectional studies: MI OR 1.57 (1.08–2.27), CAD OR 1.84 (1.09-3.09), stroke OR 1.14 (1.08–1.19) |
| Miller et al., (22) | Psoriasis: 503686 Controls: 29686694 |
Overall CVD OR 1.4 (1.2–1.7) IHD OR 1.5 (1.2–1.9) Cerebrovascular disease OR 1.1 (0.9–1.3) CV mortality OR 0.9 (0.4–2.2) |
| Pietrzak et al., (45) | Psoriasis: 367358 Controls: 9199656 |
CV events OR 1.28 (1.18–1.38) |
| Samarasasekera et al., (46) | Psoriasis: 488315 (mild: 327418; severe: 12854) Controls: 10024815 |
All psoriasis: MI HR 1.40 (1.03–1.89), stroke HR 1.13 (1.01–1.26) Mild psoriasis: CVD mortality SMR 1.03 (0.86–1.25), MI HR 1.34 (1.07– 1.68), stroke HR 1.15 (0.98–1.35) Severe psoriasis: CVD mortality SMR 1.37 (1.17–1.60), CVD mortality HR 1.57 (1.26–1.96), MI HR 3.04 (0.65–14.35), stroke HR 1.59 (1.34–1.89) |
| Richard et al., (47) | NA | Cohort studies: MI OR = 1.25 (1.03-1.52); CAD1.20 (1.13-1.27); Cross-sectional studies: MI OR = 1.57 (1.08-2.27)], CAD OR = 1.19 (1.14-1.24); Case-control studies: CAD OR = 1.84 (1.09-3.09) |
| Raaby et al., (48) | NA | Mild psoriasis: MI HR 1.2 (1.06-1.35); stroke HR 1.10 (1.0-1.19); CV death HR 1.06 (0.90-1.24) Severe psoriasis: MI HR 1.70 (1.18-2.43); stroke HR 1.38 (1.20-1.60); CV death HR 1.37 (1.13-1.67) |
| Dhana et al., (49) | Psoriasis: 285675 Controls: NA |
All psoriasis: CV mortality pooler RR 1.15 (1.09-1.21); mild psoriasis: CV mortality pooled RR 1.05 (0.92-1.20); severe psoriasis: CV mortality pooled RR 1.38 (1.09-1.74) |
| Phan et al., (28) | Pediatric psoriasis: 43808 Controls: 5384057 |
IHD or HF OR 3.15 (1.06-9.42) |
CAD, coronary artery disease; CV, cardiovascular; HF, heart failure; HR, hazard ratio; IHD, ischemic heart disease; MACE, major adverse cardiovascular events; MI, myocardial infarction; OR, odds ratio; RR, relative risk; SMR, standardized mortality ratio. Values in brackets indicate 95% confidence intervals. NA, not applicable.
In 2006, Gelfand et al. conducted the seminal study identifying psoriasis as an independent risk factor for myocardial infarction (MI) taking advantage of prospective data from the United Kingdom General Practice Research Database (50). Here, the incidence of MI per 1000 person-years was 3.58 for control patients (95% CI 3.52-3.65), 4.04 for patients with mild psoriasis (95% CI 3.88-4.21) and 5.13 for patients with severe psoriasis (95% CI 4.22-6.17). Furthermore, after adjusting for hypertension, diabetes mellitus, and hyperlipidemia younger patients showed a greater relative risk (RR) for MI. In line with this, Samarasekera et al. and Armstrong et al. assessed the risk of CVDs considering the severity of psoriasis and noticed an increased risk of major cardiovascular events (MACE), namely myocardial infarction and stroke, and CV mortality in severe psoriasis patients (41, 46). Moreover, Egeberg et al. found that longer disease duration had a stronger association with the risk of MACE (51).
Accordingly, psoriasis affects the Framingham Risk Score for over 60% of patients being thus identified as an independent CV risk factor (52). Strengthened by this convincing evidence, psoriasis has been recently included in the European and American Guidelines on Cardiovascular disease prevention as a (1.5) risk factor multiplier for CV risk (53) and, in adults 40 to 75 years of age without diabetes mellitus and a CV intermediate risk, as a risk-enhancing factor favoring initiation of statin therapy (54). Moreover, the Joint American Academy of Dermatology- National Psoriasis Foundation Guidelines recommend that patients with psoriasis should be advised of their increased cardiovascular risk and referred to the primary care physician or cardiologist (Table 3) (55).
Table 3.
Psoriasis and cardiovascular diseases comorbidity strength of recommendation and level of evidence.
| Recommendation | Strength of recommendation | Level of evidence |
|---|---|---|
| Risk assessment Recommended for all patients with psoriasis |
B | II-III |
| Screening Early and more frequent screening for hypertension, diabetes, and hyperlipidemia in patients who are candidates for systemic or phototherapy or who have psoriasis involving >10% body surface area |
B | II-III |
| Risk score models Should be adapted by introducing a 1.5 multiplication factor for patients with either >10% body surface area involvement or those who are candidates for systemic or phototherapy |
C | II-III |
| Risk management Should be carried out according to national guidelines and performed by either primary care physician or dermatologist. Target blood pressure and lipid levels are based on risk as previously calculated Antihypertensives and statins may be used as in general population |
C | III |
Psoriasis and Microvascular Dysfunction
Beyond the well-documented association between psoriasis and CVD, current hypothesis placed the vascular network at the center of this circuit, describing a crucial role of the endothelium in the insurgence of psoriatic-dependent CVD. Indeed, it has been proposed that the cutaneous psoriatic lesion might induce a systemic inflammation through the immune-mediated activation of the endothelial compartment (56). In turn, this endothelial dysfunction, insurged at distant sites, could participate and concur to the development of cardiovascular pathological events (57).
Interestingly, psoriasis has been showed to impact early on the vascular compartment, even before the development of CV diseases, causing subclinical alterations typical of CVDs. Ludwig et al. used computed tomography to quantify the coronary artery calcification (CAC) as a biomarker of coronary atherosclerosis and a predictor of future cardiovascular events in patients with psoriasis and controls (58). An increased prevalence (59.4% vs 28.1%, p=0.015) and severity (3.7 vs 0.0, p=0.019) of CAC, assessed with Agatston score, has been demonstrated in psoriatic patients with a negative history of current or previous heart problems. In the same study, psoriasis has been pointed as an independent risk factor for CAC after multiple linear regression calculations. Multiple studies highlighted the presence of microvascular dysfunction, one of the earliest signs of CVDs, in psoriatic patients. Coronary microvascular dysfunction indeed indicates an abnormal regulation of the coronary microcirculation resulting in reduced myocardial blood flow in the absence of epicardial coronary arteries stenosis. The coronary flow reserve (CFR) analysis detects the damage to the microvascular heart circulation. CFR is the capacity of the coronary circulation to dilate and therefore to enhance its flow following an increased metabolic demand and it is related to the microvascular function as the 90% of the resistances of the coronary circulation are localized in the endocardial small vessels. The ratio between the maximal possible and the resting blood flow in normal subjects is >2.5. Intriguingly, we recently evaluated the CFR by transthoracic echocardiography inducing the hyperaemic stimulus with adenosine, showing a reduction of CFR in patients with psoriasis compared with healthy controls (p=0.02) (59). The risk of an abnormal CFR was higher in patients with a greater degree of psoriasis severity and this association was independent of traditional cardiovascular risk factors (60). Arterial stiffness, a surrogate of endothelial dysfunction and predictor of CV risk, was measured by Gisondi et al. in patients with moderate-severe psoriasis, by determining carotid-femoral and carotid-radial pulse wave velocity (PWVcf, PWVcr) (61). These patients, as compared to controls, showed a significantly increased PWVcf, even after adjustment for age, gender, smoking, hypertension and body mass index (8.78 ± 1.98 vs 7.78 ± 2.0 m/s; p=0.03). Moreover, a direct correlation between PWVcf and psoriasis duration (years) was evidenced (r=0.58; p=0.0001).
Taken together, different methodical approaches suggest an association between psoriasis and the microvascular dysfunction. Intriguingly, the immune-mediated dysfunction of endothelial cells in the vasculature is profoundly associated to the pathogenesis of several cardiovascular disorders. Indeed, the affected coronary arteries’ ability to increase coronary blood flow (vasodilatory abnormality) and/or the impaired coronary blood flow (coronary microvascular spasm) could insurge in severe psoriatic patients (62). Thus, data provide proof that psoriasis could be considered as an independent CV risk factor.
Putative Inflammatory Circulating Factors Inducing Microvascular Dysfunction in Psoriasis
The immune-mediated endothelial activation is a process in which pro-inflammatory cytokines, integrins and multiple analytes contribute to trigger and propagate inflammation (63). A prolonged immune stimulation and an excessive or unbalanced production of inflammatory mediators might dysregulate this finely-tuned process.
Notably, the pathogenesis of psoriasis is considerably mediated by T helper (Th) type 1 activation and Th17 immune responses and the consequent overproduction of cytokines (e.g., Tumor necrosis factor-α TNF-α, IL-23, and IL-17) that ultimately generate a systemic proinflammatory environment. In this context, the chronic exposure of multiple circulating factors might play a key role in the endothelial priming and microvascular dysfunction at distant sites.
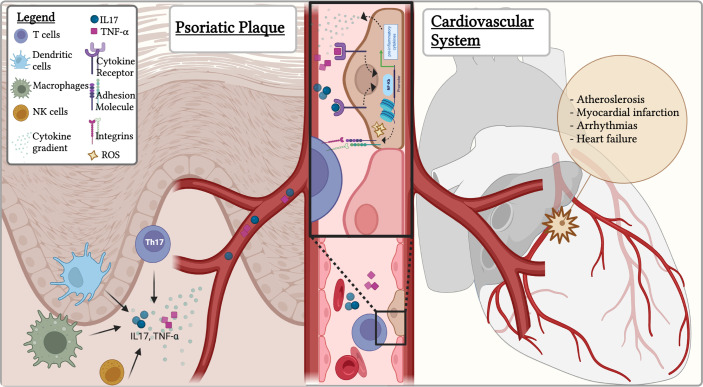
Endothelial dysfunction is referred to the imbalance between the release of vasoprotective/vasorelaxant mediators and pathological vasoconstricting substances. Despite the precise mechanisms mediating these vascular impairments are unclear, putative circulating analytes mediating the psoriatic immune-mediated endothelial dysfunction have been proposed. Among them, IL-17, TNF-α, reactive oxygen species (ROS), IFN-γ and Angiotensin-converting Enzyme, Renin and Endothelin-1(ET1) seem to play a crucial role (64–67) (Figure 1).
Figure 1.
The immune-mediated pathogenetic link between psoriasis and cardiovascular disorders: triggering the endothelial dysfunction. In the psoriatic plaque, immune cells, including Th17 promote the generation and release of circulating inflammatory mediators as IL-17 and TNF-α. TNF-α caused NF-κB activation, subsequently leading to increased expression of multiple pro-inflammatory cytokines in the psoriatic skin. In endothelial cells, IL-17A receptor drives the production of TNF-α, IL-1β, CCL2, and the expression of ICAM- 1 leading to the endothelial dysfunction involved in the pathophysiology of multiple immune-mediated CVDs. The IL-17 also triggers the accumulation of ROS, in particular within the endothelium further contributing to endothelial dysfunction and CVD progression.
IL-17 is a pro-inflammatory cytokine formerly thought to be generated in the psoriatic plaque only by a subset of CD4+ T cells (Th17), concomitantly with IL-6, IL-21, IL-22 and TNF-α. Nowadays, also dendritic cells, natural killer cells, macrophages, and γδ-T cells have been recognized as important players in the IL-17 generation (68). A variety of mechanisms, including exogenous and inflammatory stimuli, might trigger the initial activation of Th17 cells, leading to the strong IL-17 release within the skin. Notably, the IL-17 receptor is ubiquitously expressed in endothelial cells of the vascular network. Here, once activated, IL-17 receptor drives the production of TNF-α, IL-1β, CCL2, and the expression of adhesion molecules as intercellular adhesion molecule 1 (ICAM-1). The upregulation of chemokines and adhesion molecules is associated to the endothelial activation, a process that has been recently linked to the endothelial dysfunction featuring the pathophysiology of multiple immune-mediated CVDs (67). To note, IL-17 might be responsible for an additional determinant of the endothelial dysfunction in the setting of vascular disease: the massive accumulation of reactive oxygen species, in particular of the vascular superoxide ( ) within the endothelium. In pre-clinical atherosclerosis model induced by a high-fat-diet-feeding, it has been reported that IL-17 caused the increased production of superoxide in the aortic vessel (69). Nonetheless, precise mechanisms by which IL-17, induces vascular production remain unclear. Mounting evidence reveals that IL-17 accumulation might underpin i) NADPH oxidase activation in vascular smooth muscle cells, and ii) superoxide production via recruiting inflammatory cells (70) (Figure 1). Overall, the oxidative stress has a key role in the development of CVD in patients with psoriasis. Indeed, CVDs are generally characterized by an imbalance between ROS generation and degradation made by antioxidants or ROS-catalysing enzymes, finally leading to a significant deviation from the homeostatic state (71).
Together with IL-17, TNF-α contributes to the pathogenesis of psoriasis. TNF-α is a multifunctional cytokine that play a fundamental role in mediating inflammation, immune responses, and apoptosis. Notably, higher levels of TNF-α were found in skin lesions of psoriatic patients (72, 73). More, locally produced TNF-α caused NF-κB activation in psoriatic lesions, subsequently leading to increased expression of multiple pro-inflammatory cytokines; possibly concurring to the endothelial activation, and eventually dysfunction, at distant sites (Figure 1). Moreover, TNF supports the proinflammatory effects of IL-17 (74). The two cytokines indeed synergistically promote the release of key molecules for psoriasis pathogenesis, such as β-defensin 4 and S100A7 (75).
Accordingly, these results support the assumption that a local induction of multiple inflammatory mediators in psoriasis plaques determines an effect in cells outside the skin, such as endothelial cells, altering the vascular function and eventually leading to CVDs. Unquestionably, a deep investigation of the cellular and molecular interplay involved in the skin/endothelium/cardiovascular system crosstalk will be crucial for further characterize the pathophysiological link between psoriasis and CVDs. For instance, extracellular vesicles-dependent mechanisms could be an interesting target that still remains to be addressed. Accordingly, future studies aimed at investigating this possibility might also pave the way for innovative therapeutic strategies.
Targeting Key Inflammatory Pathways to Reduce CV Risk in Psoriatic Patients
Beside the evidence of the psoriasis-CVD crosstalk, several efforts have been made to understand whether a systemic treatment of psoriasis may lead to the control of the systemic inflammation associated with the skin manifestations, and to limit the risk of CV events (55, 76–78). The 2021 Group for Research and Assessment of Psoriasis and Psoriatic arthritis (GRAPPA) treatment recommendations for psoriasis are summarized in (79). Interestingly, EULAR guidelines report that disease activity should be controlled to lower CVD risk in all patients with rheumatoid arthritis, ankylosing spondylitis or psoriatic arthritis (80).
Classical disease-modifying antirheumatic drugs (DMARDs) routinely used in psoriasis include methotrexate, cyclosporine and acitretin. Among them, methotrexate (MTX) has been shown to be superior not only to topical treatments and phototherapy, but also to cyclosporine and acitretin in reducing the risk of MACE in patients with psoriasis (77, 81). In 2005, Prodanowich et al. firstly demonstrated the protective role of MTX on vascular disease in patients with psoriasis (82). In line with this, MTX has been associated with 21% lower risk for CVD and a 18% lower risk for MI in a systematic review and meta-analysis of 10 observational studies in which MTX was administered in patients with psoriasis, rheumatoid arthritis or polyarthritis (83). On the other hand, the role of cyclosporin and acitretin is still considered controversial. Indeed, they are associated with side effects such as renal failure, hypertension and dyslipidemia that can negatively impact on CV risk (84).
In the recent years, the attention has been focused on biological drugs as they target key inflammatory molecules involved in the pathogenesis of the disease, allowing a complete clearance of the skin lesion in almost all patients and a long-term control of the condition (6).
TNFα inhibitors commonly used in psoriatic disease include infliximab, etanercept, adalimumab and certolizumab. Patients treated with anti TNF-α for over 24 weeks demonstrated a reduction in inflammation markers related with endothelial dysfunction, such as CRP e vascular endothelial growth factor (VEGF) (85). These data have been confirmed in a randomized controlled trial reporting a reduction in inflammatory CVD markers such as GlycA, IL6, CRP, and TNF-α (86). Consistently, it has been noted a reduction in vascular inflammation assessed by the decreased signal intensity on FDG PET/CT in 115 patients after one year of treatment. Interestingly, a reduction of 75% in skin diseases severity was associated with a greater improvement in aortic vascular inflammation (87). The relevant role of TNF-α inhibitors in modulating the CV risk in psoriasis was also demonstrated by their ability to reverse the microvascular dysfunction measured by CFR. Indeed, a prospective study evaluating CFR in 37 consecutive patients with moderate to severe psoriasis before and after 6 months of treatment with TNF-α inhibitors reported a CFR increase from 2.2 ± 0.7 to 3.02 ± 0.8 (p<0.0001) (59). Other studies evaluated the impact of TNF-α inhibitors in psoriasis on several different CV imaging biomarkers. In particular, TNF-α inhibitors demonstrated a favourable impact on the arterial stiffness, assessed by the gold standard aortic pulse wave velocity (aPWV) (88), and decreased burden of noncalcified coronary plaques (89). Moreover, subclinical left and right ventricular myocardial dysfunctions have demonstrated to be ameliorated following anti TNF-α therapy in patients with severe psoriasis (90, 91). Although, to date, randomized placebo-controlled trials (RCT) evaluating the impact of biologics on CV risk in psoriasis are lacking, a systemic meta-analysis revealed that TNF-α inhibitors were related with fewer cardiovascular events compared to topical treatment/phototherapy (RR 0.58) or methotrexate (RR 0.67) (92). A retrospective cohort study demonstrated the beneficial effect of TNF-α inhibitors on myocardial protection by reducing the risk of MI compared with topical agents (adjusted hazard ratio, 0.50; 95% CI, 0.32-0.79) (93). The same result was reached by a large-scale observational study that confirmed a reduction by 11.2% of CV event risk in psoriasis patients exposed to TNF-α inhibitors for 6 months compared to those who received phototherapy (94). Similarly, in another study, TNF-α inhibitors have proved to be superior to methotrexate in reducing the risk of MACE (95).
Recently developed IL-17 inhibitors include the anti-IL-17 antibodies secukinumab and ixekizumab and the anti-IL-17 receptor antibody brodalumab. Preclinical studies have shown that IL-17 inhibitors diminished peripheral oxidative stress levels, proinflammatory cytokines, and vascular inflammation in psoriasis (96). In a prospective, observational study including 215 patients treated with different biologic therapies and completing one year of follow up, patients undergoing IL-17 inhibitors, along with the reduction of CRP and HDL serum levels, showed the greatest reduction in coronary plaque indices assessed by coronary computed tomography angiography as compared to groups treated with other biologics (89). The CARIMA study found an improvement of the endothelial function assessed by a reversal of baseline flow-mediated dilation, in psoriasis patients treated with secukinumab, revealing its beneficial effect on CV risk (97). On the contrary, another paper showed no effects on aortic vascular inflammation and cardiometabolic biomarkers in moderate-severe psoriasis patients treated with secukinumab compared to placebo (98).
The anti- IL-12/IL-23 antibody ustekinumab and the anti-IL-23/IL-39 antibodies guselkumab, risankizumab and tildrakizumab are currently approved for the treatment of psoriasis. In several studies, ustekinumab has demonstrated to have a neutral impact on MACE (99, 100). A recent meta-analysis of RCT, attempting to explore the impact of biologics on serologic and imaging biomarkers of CV risk, pointed out that adalimumab and secukinumab did not cause a significant improvement in imaging markers. Conversely, ustekinumab treatment promoted the reduction of aortic vascular inflammation at week 12, albeit this was not confirmed at week 52. Among all biologics, adalimumab was the most efficient in inducing a reduction of the serum markers CRP, TNF-α, IL-6, and GlycA (101).
Overall, these data point toward the improvement of imaging and serum biomarkers for CVD risk in patients with psoriasis treated with biologics targeting TNF-α, IL-23 and IL-17; moreover, therapies with biologics, by controlling remote skin inflammation, may have the potential to prevent the development of CVDs.
Conclusions
The association between psoriasis and CVD is promoted by the coexistence of traditional modifiable CV risk factors (hypertension, diabetes, hyperlipidemia, obesity, and metabolic syndrome) and chronic systemic inflammation. The latter might directly impinge on the vascular compartment leading to a pan-arterial inflammation and, in particular, to a microvascular dysfunction. In this context, biological drugs, targeting psoriasis key inflammatory molecules, demonstrated a positive outcome on both skin manifestations and cardiovascular involvement. However, further clinical studies are needed to investigate the potential beneficial effects of biologic agents in the reduction of CV risk in psoriatic patients.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
Funding
BM and RA received individual research grants from Istituto di Ricerca Pediatrica Fondazione Città della Speranza.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Michalek IM, Loring B, John SM. A Systematic Review of Worldwide Epidemiology of Psoriasis. J Eur Acad Dermatol Venereol (2017) 31(2):205–12. doi: 10.1111/jdv.13854 [DOI] [PubMed] [Google Scholar]
- 2. Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med (2009) 361(5):496–509. doi: 10.1056/NEJMra0804595 [DOI] [PubMed] [Google Scholar]
- 3. di Meglio P, Villanova F, Nestle FO. Psoriasis. Cold Spring Harbor Perspect Med (2014) 4(8):a015354–a015354. doi: 10.1101/cshperspect.a015354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lowes MA, Suárez-Fariñas M, Krueger JG. Immunology of Psoriasis. Annu Rev Immunol (2014) 32(1):227–55. doi: 10.1146/annurev-immunol-032713-120225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mahil SK, Capon F, Barker JN. Update on Psoriasis Immunopathogenesis and Targeted Immunotherapy. Semin Immunopathol (2016) 38(1):11–27. doi: 10.1007/s00281-015-0539-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Benezeder T, Wolf P. Resolution of Plaque-Type Psoriasis: What Is Left Behind (and Reinitiates the Disease) . Semin Immunopathol (2019) 41(6):633–44. doi: 10.1007/s00281-019-00766-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Boehncke W-H, Boehncke S, Tobin A-M, Kirby B. The ‘Psoriatic March’: A Concept of How Severe Psoriasis May Drive Cardiovascular Comorbidity. Exp Dermatol (2011) 20(4):303–7. doi: 10.1111/j.1600-0625.2011.01261.x [DOI] [PubMed] [Google Scholar]
- 8. Gisondi P, Bellinato F, Girolomoni G, Albanesi C. Pathogenesis of Chronic Plaque Psoriasis and Its Intersection With Cardio-Metabolic Comorbidities. Front Pharmacol (2020) 11. doi: 10.3389/fphar.2020.00117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Takeshita J, Grewal S, Langan SM, Mehta NN, Ogdie A, van Voorhees AS, et al. Psoriasis and Comorbid Diseases. J Am Acad Dermatol (2017) 76(3):377–90. doi: 10.1016/j.jaad.2016.07.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sokolova MV, Simon D, Nas K, Zaiss MM, Luo Y, Zhao Y, et al. A Set of Serum Markers Detecting Systemic Inflammation in Psoriatic Skin, Entheseal, and Joint Disease in the Absence of C-Reactive Protein and Its Link to Clinical Disease Manifestations. Arthritis Res Ther (2020) 22(1):26. doi: 10.1186/s13075-020-2111-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Montaudié H, Albert-Sabonnadière C, Acquacalda E, Fontas E, Danré A, Roux C, et al. Impact of Systemic Treatment of Psoriasis on Inflammatory Parameters and Markers of Comorbidities and Cardiovascular Risk: Results of a Prospective Longitudinal Observational Study. J Eur Acad Dermatol Venereol (2014) 28(9):1186–91. doi: 10.1111/jdv.12255 [DOI] [PubMed] [Google Scholar]
- 12. Beygi S, Lajevardi V, Abedini R. C-Reactive Protein in Psoriasis: A Review of the Literature. J Eur Acad Dermatol Venereol (2014) 28(6):700–11. doi: 10.1111/jdv.12257 [DOI] [PubMed] [Google Scholar]
- 13. Garbaraviciene J, Diehl S, Varwig D, Bylaite M, Ackermann H, Ludwig RJ, et al. Platelet P-Selectin Reflects a State of Cutaneous Inflammation: Possible Application to Monitor Treatment Efficacy in Psoriasis. Exp Dermatol (2009) 19(8):736–41. doi: 10.1111/j.1600-0625.2010.01095.x [DOI] [PubMed] [Google Scholar]
- 14. Suárez-Fariñas M, Li K, Fuentes-Duculan J, Hayden K, Brodmerkel C, Krueger JG. Expanding the Psoriasis Disease Profile: Interrogation of the Skin and Serum of Patients With Moderate-To-Severe Psoriasis. J Invest Dermatol (2012) 132(11):2552–64. doi: 10.1038/jid.2012.184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mehta NN. Systemic and Vascular Inflammation in Patients With Moderate to Severe Psoriasis as Measured by [18F]-Fluorodeoxyglucose Positron Emission Tomography –Computed Tomography (Fdg-Pet/Ct). Arch Dermatol (2011) 147(9):1031. doi: 10.1001/archdermatol.2011.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Armstrong AW, Harskamp CT, Armstrong EJ. The Association Between Psoriasis and Hypertension. J Hypertens (2013) 31(3):433–43. doi: 10.1097/HJH.0b013e32835bcce1 [DOI] [PubMed] [Google Scholar]
- 17. Armstrong AW, Harskamp CT, Armstrong EJ. The Association Between Psoriasis and Obesity: A Systematic Review and Meta-Analysis of Observational Studies. Nutr Diabetes (2012) 2(12):e54–4. doi: 10.1038/nutd.2012.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ma C, Harskamp CT, Armstrong EJ, Armstrong AW. The Association Between Psoriasis and Dyslipidaemia: A Systematic Review. Br J Dermatol (2013) 168(3):486–95. doi: 10.1111/bjd.12101 [DOI] [PubMed] [Google Scholar]
- 19. Armstrong AW, Harskamp CT, Armstrong EJ. Psoriasis and the Risk of Diabetes Mellitus. JAMA Dermatol (2013) 149(1):84. doi: 10.1001/2013.jamadermatol.406 [DOI] [PubMed] [Google Scholar]
- 20. Armstrong AW, Harskamp CT, Armstrong EJ. Psoriasis and Metabolic Syndrome: A Systematic Review and Meta-Analysis of Observational Studies. J Am Acad Dermatol (2013) 68(4):654–62. doi: 10.1016/j.jaad.2012.08.015 [DOI] [PubMed] [Google Scholar]
- 21. Coto-Segura P, Eiris-Salvado N, González-Lara L, Queiro-Silva R, Martinez-Camblor P, Maldonado-Seral C, et al. Psoriasis, Psoriatic Arthritis and Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Br J Dermatol (2013) 169(4):783–93. doi: 10.1111/bjd.12473 [DOI] [PubMed] [Google Scholar]
- 22. Miller IM, Ellervik C, Yazdanyar S, Jemec GBE. Meta-Analysis of Psoriasis, Cardiovascular Disease, and Associated Risk Factors. J Am Acad Dermatol (2013) 69(6):1014–24. doi: 10.1016/j.jaad.2013.06.053 [DOI] [PubMed] [Google Scholar]
- 23. Rodríguez-Zúñiga MJM, García-Perdomo HA. Systematic Review and Meta-Analysis of the Association Between Psoriasis and Metabolic Syndrome. J Am Acad Dermatol (2017) 77(4):657–666.e8. doi: 10.1016/j.jaad.2017.04.1133 [DOI] [PubMed] [Google Scholar]
- 24. Singh S, Young P, Armstrong AW. An Update on Psoriasis and Metabolic Syndrome: A Meta-Analysis of Observational Studies. PloS One (2017) 12(7):e0181039. doi: 10.1371/journal.pone.0181039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mamizadeh M, Tardeh Z, Azami M. The Association Between Psoriasis and Diabetes Mellitus: A Systematic Review and Meta-Analysis. Diabetes Metab Syndr: Clin Res Rev (2019) 13(2):1405–12. doi: 10.1016/j.dsx.2019.01.009 [DOI] [PubMed] [Google Scholar]
- 26. Choudhary S, Pradhan D, Pandey A, MohdK K, Lall R, Ramesh V, et al. The Association of Metabolic Syndrome and Psoriasis: A Systematic Review and Meta-Analysis of Observational Study. Endocr Metab Immune Disord - Drug Targets (2020) 20(5):703–17. doi: 10.2174/1871530319666191008170409 [DOI] [PubMed] [Google Scholar]
- 27. Choudhary S, Patel R, Pradhan D, Deval R, Singh H, Thomas G, et al. Psoriasis and Cardiovascular Disorders: Association or Epiphenomenon? Meta-Analysis of Observational Studies. 3 Biotech (2020) 10(3):104. doi: 10.1007/s13205-020-2089-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Phan K, Lee G, Fischer G. Pediatric Psoriasis and Association With Cardiovascular and Metabolic Comorbidities: Systematic Review and Meta-Analysis. Pediatr Dermatol (2020) 37(4):661–9. doi: 10.1111/pde.14208 [DOI] [PubMed] [Google Scholar]
- 29. Duan X, Liu J, Mu Y, Liu T, Chen Y, Yu R, et al. A Systematic Review and Meta-Analysis of the Association Between Psoriasis and Hypertension With Adjustment for Covariates. Medicine (2020) 99(9):e19303. doi: 10.1097/MD.0000000000019303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cho SI, Kim YE, Jo SJ. Association of Metabolic Comorbidities With Pediatric Psoriasis: A Systematic Review and Meta-Analysis. Ann Dermatol (2021) 33(3):203. doi: 10.5021/ad.2021.33.3.203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Garshick MS, Ward NL, Krueger JG, Berger JS. Cardiovascular Risk in Patients With Psoriasis. J Am Coll Cardiol (2021) 77(13):1670–80. doi: 10.1016/j.jacc.2021.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Duarte GV, Oliveira M de FSP, Cardoso TM, Follador I, Silva TS, Cavalheiro CMA, et al. Association Between Obesity Measured by Different Parameters and Severity of Psoriasis. Int J Dermatol (2013) 52(2):177–81. doi: 10.1111/j.1365-4632.2011.05270.x [DOI] [PubMed] [Google Scholar]
- 33. Jensen P, Skov L. Psoriasis and Obesity. Dermatology (2016) 232(6):633–9. doi: 10.1159/000455840 [DOI] [PubMed] [Google Scholar]
- 34. deShazo RA, Secrest AM, Armstrong AW, Duffin KC. Addressing Hypertension in Patients With Psoriasis: Review and Recommendations. J Psoriasis Psoriatic Arthritis (2020) 5(4):129–38. doi: 10.1177/2475530320936373 [DOI] [Google Scholar]
- 35. Pietrzak A, Michalak-Stoma A, Chodorowska G, Szepietowski JC. Lipid Disturbances in Psoriasis: An Update. Mediat Inflamm (2010) 2010:1–13. doi: 10.1155/2010/535612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wu S, Li W-Q, Han J, Sun Q, Qureshi AA. Hypercholesterolemia and Risk of Incident Psoriasis and Psoriatic Arthritis in US Women. Arthritis Rheumatol (2014) 66(2):304–10. doi: 10.1002/art.38227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Langan SM, Seminara NM, Shin DB, Troxel AB, Kimmel SE, Mehta NN, et al. Prevalence of Metabolic Syndrome in Patients With Psoriasis: A Population-Based Study in the United Kingdom. J Invest Dermatol (2012) 132(3):556–62. doi: 10.1038/jid.2011.365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gisondi P, Fostini AC, Fossà I, Girolomoni G, Targher G. Psoriasis and the Metabolic Syndrome. Clinics Dermatol (2018) 36(1):21–8. doi: 10.1016/j.clindermatol.2017.09.005 [DOI] [PubMed] [Google Scholar]
- 39. Boehncke W-H. Systemic Inflammation and Cardiovascular Comorbidity in Psoriasis Patients: Causes and Consequences. Front Immunol (2018) 9. doi: 10.3389/fimmu.2018.00579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Xu T, Zhang Y -H. Association of Psoriasis With Stroke and Myocardial Infarction: Meta-Analysis of Cohort Studies. Br J Dermatol (2012) 167(6):1345–50. doi: 10.1111/bjd.12002 [DOI] [PubMed] [Google Scholar]
- 41. Armstrong EJ, Harskamp CT, Armstrong AW. Psoriasis and Major Adverse Cardiovascular Events: A Systematic Review and Meta-Analysis of Observational Studies. J Am Heart Assoc (2013) 2(2). doi: 10.1161/JAHA.113.000062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gaeta M, Castelvecchio S, Ricci C, Pigatto P, Pellissero G, Cappato R. Role of Psoriasis as Independent Predictor of Cardiovascular Disease: A Meta-Regression Analysis. Int J Cardiol (2013) 168(3):2282–8. doi: 10.1016/j.ijcard.2013.01.197 [DOI] [PubMed] [Google Scholar]
- 43. Gu W-J, Weng C-L, Zhao Y-T, Liu Q-H, Yin R-X. Psoriasis and Risk of Cardiovascular Disease: A Meta-Analysis of Cohort Studies. Int J Cardiol (2013) 168(5):4992–6. doi: 10.1016/j.ijcard.2013.07.127 [DOI] [PubMed] [Google Scholar]
- 44. Horreau C, Pouplard C, Brenaut E, Barnetche T, Misery L, Cribier B, et al. Cardiovascular Morbidity and Mortality in Psoriasis and Psoriatic Arthritis: A Systematic Literature Review. J Eur Acad Dermatol Venereol (2013) 27:12–29. doi: 10.1111/jdv.12163 [DOI] [PubMed] [Google Scholar]
- 45. Pietrzak A, Bartosińska J, Chodorowska G, Szepietowski JC, Paluszkiewicz P, Schwartz RA. Cardiovascular Aspects of Psoriasis: An Updated Review. Int J Dermatol (2013) 52(2):153–62. doi: 10.1111/j.1365-4632.2012.05584.x [DOI] [PubMed] [Google Scholar]
- 46. Samarasekera EJ, Neilson JM, Warren RB, Parnham J, Smith CH. Incidence of Cardiovascular Disease in Individuals With Psoriasis: A Systematic Review and Meta-Analysis. J Invest Dermatol (2013) 133(10):2340–6. doi: 10.1038/jid.2013.149 [DOI] [PubMed] [Google Scholar]
- 47. Richard M-A, Barnetche T, Horreau C, Brenaut E, Pouplard C, Aractingi S, et al. Psoriasis, Cardiovascular Events, Cancer Risk and Alcohol Use: Evidence-Based Recommendations Based on Systematic Review and Expert Opinion. J Eur Acad Dermatol Venereol (2013) 27:2–11. doi: 10.1111/jdv.12162 [DOI] [PubMed] [Google Scholar]
- 48. Raaby L, Ahlehoff O, de Thurah A. Psoriasis and Cardiovascular Events: Updating the Evidence. Arch Dermatol Res (2017) 309(3):225–8. doi: 10.1007/s00403-016-1712-1 [DOI] [PubMed] [Google Scholar]
- 49. Dhana A, Yen H, Yen H, Cho E. All-Cause and Cause-Specific Mortality in Psoriasis: A Systematic Review and Meta-Analysis. J Am Acad Dermatol (2019) 80(5):1332–43. doi: 10.1016/j.jaad.2018.12.037 [DOI] [PubMed] [Google Scholar]
- 50. Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of Myocardial Infarction in Patients With Psoriasis. JAMA (2006) 296(14):1735. doi: 10.1001/jama.296.14.1735 [DOI] [PubMed] [Google Scholar]
- 51. Egeberg A, Skov L, Joshi AA, Mallbris L, Gislason GH, Wu JJ, et al. The Relationship Between Duration of Psoriasis, Vascular Inflammation, and Cardiovascular Events. J Am Acad Dermatol (2017) 77(4):650–656.e3. doi: 10.1016/j.jaad.2017.06.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mehta NN, Krishnamoorthy P, Yu Y, Khan O, Raper A, van Voorhees A, et al. The Impact of Psoriasis on 10-Year Framingham Risk. J Am Acad Dermatol (2012) 67(4):796–8. doi: 10.1016/j.jaad.2012.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, et al. European Guidelines on Cardiovascular Disease Prevention in Clinical Practice. Eur Heart J (2016) 37(29):2315–81. doi: 10.1093/eurheartj/ehw106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/Apha/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: Executive Summary. J Am Coll Cardiol (2018) 73(24):3168–209. doi: 10.1016/j.jacc.2018.11.002 [DOI] [PubMed] [Google Scholar]
- 55. Elmets CA, Leonardi CL, Davis DMR, Gelfand JM, Lichten J, Mehta NN, et al. Joint AAD-NPF Guidelines of Care for the Management and Treatment of Psoriasis With Awareness and Attention to Comorbidities. J Am Acad Dermatol (2019) 80(4):1073–113. doi: 10.1016/j.jaad.2018.11.058 [DOI] [PubMed] [Google Scholar]
- 56. Woo YR, Park CJ, Kang H, Kim JE. The Risk of Systemic Diseases in Those With Psoriasis and Psoriatic Arthritis: From Mechanisms to Clinic. Int J Mol Sci (2020) 21(19):7041. doi: 10.3390/ijms21197041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sajja AP, Joshi AA, Teague HL, Dey AK, Mehta NN. Potential Immunological Links Between Psoriasis and Cardiovascular Disease. Front Immunol (2018) 9. doi: 10.3389/fimmu.2018.01234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ludwig RJ, Herzog C, Rostock A, Ochsendorf FR, Zollner TM, Thaci D, et al. Psoriasis: A Possible Risk Factor for Development of Coronary Artery Calcification. Br J Dermatol (2007) 156(2):271–6. doi: 10.1111/j.1365-2133.2006.07562.x [DOI] [PubMed] [Google Scholar]
- 59. Piaserico S, Osto E, Famoso G, Zanetti I, Gregori D, Poretto A, et al. Treatment With Tumor Necrosis Factor Inhibitors Restores Coronary Microvascular Function in Young Patients With Severe Psoriasis. Atherosclerosis (2016) 251:25–30. doi: 10.1016/j.atherosclerosis.2016.05.036 [DOI] [PubMed] [Google Scholar]
- 60. Osto E, Piaserico S, Maddalozzo A, Forchetti G, Montisci R, Famoso G, et al. Impaired Coronary Flow Reserve in Young Patients Affected by Severe Psoriasis. Atherosclerosis (2012) 221(1):113–7. doi: 10.1016/j.atherosclerosis.2011.12.015 [DOI] [PubMed] [Google Scholar]
- 61. Gisondi P, Fantin F, del Giglio M, Valbusa F, Marino F, Zamboni M, et al. Chronic Plaque Psoriasis Is Associated With Increased Arterial Stiffness. Dermatology (2009) 218(2):110–3. doi: 10.1159/000182256 [DOI] [PubMed] [Google Scholar]
- 62. Alba BK, Greaney JL, Ferguson SB, Alexander LM. Endothelial Function is Impaired in the Cutaneous Microcirculation of Adults With Psoriasis Through Reductions in Nitric Oxide-Dependent Vasodilation. Am J Physiology-Heart Circulatory Physiol (2018) 314(2):H343–9. doi: 10.1152/ajpheart.00446.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mizuguchi S, Gotoh K, Nakashima Y, Setoyama D, Takata Y, Ohga S, et al. Mitochondrial Reactive Oxygen Species Are Essential for the Development of Psoriatic Inflammation. Front Immunol (2021) 12. doi: 10.3389/fimmu.2021.714897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bohm F, Pernow J. The Importance of Endothelin-1 for Vascular Dysfunction in Cardiovascular Disease. Cardiovasc Res (2007) 76(1):8–18. doi: 10.1016/j.cardiores.2007.06.004 [DOI] [PubMed] [Google Scholar]
- 65. Mehta NN, Teague HL, Swindell WR, Baumer Y, Ward NL, Xing X, et al. Ifn-γ and TNF-α Synergism May Provide a Link Between Psoriasis and Inflammatory Atherogenesis. Sci Rep (2017) 7(1):13831. doi: 10.1038/s41598-017-14365-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Armstrong AW, Voyles SV, Armstrong EJ, Fuller EN, Rutledge JC. A Tale of Two Plaques: Convergent Mechanisms of T-Cell-Mediated Inflammation in Psoriasis and Atherosclerosis. Exp Dermatol (2011) 20(7):544–9. doi: 10.1111/j.1600-0625.2011.01308.x [DOI] [PubMed] [Google Scholar]
- 67. von Stebut E, Boehncke W-H, Ghoreschi K, Gori T, Kaya Z, Thaci D, et al. Il-17A in Psoriasis and Beyond: Cardiovascular and Metabolic Implications. Front Immunol (2020) 10. doi: 10.3389/fimmu.2019.03096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Onishi RM, Gaffen SL. Interleukin-17 and its Target Genes: Mechanisms of Interleukin-17 Function in Disease. Immunology (2010) 129(3):311–21. doi: 10.1111/j.1365-2567.2009.03240.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Madhur MS, Funt SA, Li L, Vinh A, Chen W, Lob HE, et al. Role of Interleukin 17 in Inflammation, Atherosclerosis, and Vascular Function in Apolipoprotein E–Deficient Mice. Arterioscler Thromb Vasc Biol (2011) 31(7):1565–72. doi: 10.1161/ATVBAHA.111.227629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Pietrowski E, Bender B, Huppert J, White R, Luhmann HJ, Kuhlmann CRW. Pro-Inflammatory Effects of Interleukin-17A on Vascular Smooth Muscle Cells Involve Nad(P)H- Oxidase Derived Reactive Oxygen Species. J Vasc Res (2011) 48(1):52–8. doi: 10.1159/000317400 [DOI] [PubMed] [Google Scholar]
- 71. Daiber A, Chlopicki S. Revisiting Pharmacology of Oxidative Stress and Endothelial Dysfunction in Cardiovascular Disease: Evidence for Redox-Based Therapies. Free Radical Biol Med (2020), 157:15–37. doi: 10.1016/j.freeradbiomed.2020.02.026 [DOI] [PubMed] [Google Scholar]
- 72. Mease P. TNF Therapy in Psoriatic Arthritis and Psoriasis. Ann Rheumatic Dis (2004) 63(7):755–8. doi: 10.1136/ard.2004.020719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Veale DJ. Immunopathology of Psoriasis and Psoriatic Arthritis. Ann Rheumatic Dis (2005) 64(suppl_2):ii26–9. doi: 10.1136/ard.2004.031740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Liang SC, Tan X-Y, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, et al. Interleukin (IL)-22 and IL-17 Are Coexpressed by Th17 Cells and Cooperatively Enhance Expression of Antimicrobial Peptides. J Exp Med (2006) 203(10):2271–9. doi: 10.1084/jem.20061308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Guilloteau K, Paris I, Pedretti N, Boniface K, Juchaux F, Huguier V, et al. Skin Inflammation Induced by the Synergistic Action of IL-17a, Il-22, Oncostatin M, Il-1α, and TNF-α Recapitulates Some Features of Psoriasis. J Immunol (2010) 184(9):5263–70. doi: 10.4049/jimmunol.0902464 [DOI] [PubMed] [Google Scholar]
- 76. Cai J, Cui L, Wang Y, Li Y, Zhang X, Shi Y. Cardiometabolic Comorbidities in Patients With Psoriasis: Focusing on Risk, Biological Therapy, and Pathogenesis. Front Pharmacol (2021) 12. doi: 10.3389/fphar.2021.774808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Caiazzo G, Fabbrocini G, di Caprio R, Raimondo A, Scala E, Balato N, et al. Psoriasis, Cardiovascular Events, and Biologics: Lights and Shadows. Front Immunol (2018) 9. doi: 10.3389/fimmu.2018.01668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Weber B, Merola JF, Husni ME, di Carli M, Berger JS, Garshick MS. Psoriasis and Cardiovascular Disease: Novel Mechanisms and Evolving Therapeutics. Curr Atheroscl Rep (2021) 23(11):67. doi: 10.1007/s11883-021-00963-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Coates LC, Soriano E, Corp N, Bertheussen H, Callis-Duffin K, Barbosa Campanholo C, et al. Op0229 the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (Grappa) Treatment Recommendations 2021. Ann Rheumatic Dis (2021) 80(Suppl 1):139–40. doi: 10.1136/annrheumdis-2021-eular.4091 [DOI] [Google Scholar]
- 80. Agca R, Heslinga SC, Rollefstad S, Heslinga M, McInnes IB, Peters MJL, et al. EULAR Recommendations for Cardiovascular Disease Risk Management in Patients With Rheumatoid Arthritis and Other Forms of Inflammatory Joint Disorders: 2015/2016 Update. Ann Rheumatic Dis (2017) 76(1):17–28. doi: 10.1136/annrheumdis-2016-209775 [DOI] [PubMed] [Google Scholar]
- 81. Ahlehoff O, Skov L, Gislason G, Lindhardsen J, Kristensen SL, Iversen L, et al. Cardiovascular Disease Event Rates in Patients With Severe Psoriasis Treated With Systemic Anti-Inflammatory Drugs: A Danish Real-World Cohort Study. J Internal Med (2013) 273(2):197–204. doi: 10.1111/j.1365-2796.2012.02593.x [DOI] [PubMed] [Google Scholar]
- 82. Prodanowich S, Ma F, Taylor J, Pezon C, Fasihi T, Kirsner R. Methotrexate Reduces Incidence of Vascular Diseases in Veterans With Psoriasis or Rheumatoid Arthritis. J Am Acad Dermatol (2005) 52(2):262–7. doi: 10.1016/j.jaad.2004.06.017 [DOI] [PubMed] [Google Scholar]
- 83. Micha R, Imamura F, Wyler von Ballmoos M, Solomon DH, Hernán MA, Ridker PM, et al. Systematic Review and Meta-Analysis of Methotrexate Use and Risk of Cardiovascular Disease. Am J Cardiol (2011) 108(9):1362–70. doi: 10.1016/j.amjcard.2011.06.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Hu S, Lan C-CE. Psoriasis and Cardiovascular Comorbidities: Focusing on Severe Vascular Events, Cardiovascular Risk Factors and Implications for Treatment. Int J Mol Sci (2017) 18(10):2211. doi: 10.3390/ijms18102211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Boehncke S, Salgo R, Garbaraviciene J, Beschmann H, Hardt K, Diehl S, et al. Effective Continuous Systemic Therapy of Severe Plaque-Type Psoriasis is Accompanied by Amelioration of Biomarkers of Cardiovascular Risk: Results of a Prospective Longitudinal Observational Study. J Eur Acad Dermatol Venereol (2011) 25(10):1187–93. doi: 10.1111/j.1468-3083.2010.03947.x [DOI] [PubMed] [Google Scholar]
- 86. Mehta NN, Shin DB, Joshi AA, Dey AK, Armstrong AW, Duffin KC, et al. Effect of 2 Psoriasis Treatments on Vascular Inflammation and Novel Inflammatory Cardiovascular Biomarkers. Circulation: Cardiovasc Imaging (2018) 11(6). doi: 10.1161/CIRCIMAGING.117.007394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Dey AK, Joshi AA, Chaturvedi A, Lerman JB, Aberra TM, Rodante JA, et al. Association Between Skin and Aortic Vascular Inflammation in Patients With Psoriasis. JAMA Cardiol (2017) 2(9):1013. doi: 10.1001/jamacardio.2017.1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Pina T, Corrales A, Lopez-Mejias R, Armesto S, Gonzalez-Lopez MA, Gómez-Acebo I, et al. Anti-Tumor Necrosis Factor-Alpha Therapy Improves Endothelial Function and Arterial Stiffness in Patients With Moderate to Severe Psoriasis: A 6-Month Prospective Study. J Dermatol (2016) 43(11):1267–72. doi: 10.1111/1346-8138.13398 [DOI] [PubMed] [Google Scholar]
- 89. Elnabawi YA, Dey AK, Goyal A, Groenendyk JW, Chung JH, Belur AD, et al. Coronary Artery Plaque Characteristics and Treatment With Biologic Therapy in Severe Psoriasis: Results From a Prospective Observational Study. Cardiovasc Res (2019) 115(4):721–8. doi: 10.1093/cvr/cvz009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ahlehoff O, Hansen PR, Gislason GH, Frydland M, Bryld LE, Elming H, et al. Myocardial Function and Effects of Biologic Therapy in Patients With Severe Psoriasis: A Prospective Echocardiographic Study. J Eur Acad Dermatol Venereol (2016) 30(5):819–23. doi: 10.1111/jdv.13152 [DOI] [PubMed] [Google Scholar]
- 91. Herédi E, Végh J, Pogácsás L, Gáspár K, Varga J, Kincse G, et al. Subclinical Cardiovascular Disease and It’s Improvement After Long-Term TNF-α Inhibitor Therapy in Severe Psoriatic Patients. J Eur Acad Dermatol Venereol (2016) 30(9):1531–6. doi: 10.1111/jdv.13649 [DOI] [PubMed] [Google Scholar]
- 92. Yang Z, Lin N, Li L, Li Y. The Effect of TNF Inhibitors on Cardiovascular Events in Psoriasis and Psoriatic Arthritis: An Updated Meta-Analysis. Clin Rev Allergy Immunol (2016) 51(2):240–7. doi: 10.1007/s12016-016-8560-9 [DOI] [PubMed] [Google Scholar]
- 93. Wu JJ, Poon K-YT, Channual JC, Shen AY-J. Association Between Tumor Necrosis Factor Inhibitor Therapy and Myocardial Infarction Risk in Patients With Psoriasis. Arch Dermatol (2012) 148(11):1244. doi: 10.1001/archdermatol.2012.2502 [DOI] [PubMed] [Google Scholar]
- 94. Wu JJ, Sundaram M, Cloutier M, Gauthier-Loiselle M, Guérin A, Singh R, et al. The Risk of Cardiovascular Events in Psoriasis Patients Treated With Tumor Necrosis Factor–α Inhibitors Versus Phototherapy: An Observational Cohort Study. J Am Acad Dermatol (2018) 79(1):60–8. doi: 10.1016/j.jaad.2018.02.050 [DOI] [PubMed] [Google Scholar]
- 95. Wu JJ, Guérin A, Sundaram M, Dea K, Cloutier M, Mulani P. Cardiovascular Event Risk Assessment in Psoriasis Patients Treated With Tumor Necrosis Factor-α Inhibitors Versus Methotrexate. J Am Acad Dermatol (2017) 76(1):81–90. doi: 10.1016/j.jaad.2016.07.042 [DOI] [PubMed] [Google Scholar]
- 96. Schüler R, Brand A, Klebow S, Wild J, Veras FP, Ullmann E, et al. Antagonization of IL-17a Attenuates Skin Inflammation and Vascular Dysfunction in Mouse Models of Psoriasis. J Invest Dermatol (2019) 139(3):638–47. doi: 10.1016/j.jid.2018.09.021 [DOI] [PubMed] [Google Scholar]
- 97. von Stebut E, Reich K, Thaçi D, Koenig W, Pinter A, Körber A, et al. Impact of Secukinumab on Endothelial Dysfunction and Other Cardiovascular Disease Parameters in Psoriasis Patients Over 52 Weeks. J Invest Dermatol (2019) 139(5):1054–62. doi: 10.1016/j.jid.2018.10.042 [DOI] [PubMed] [Google Scholar]
- 98. Gelfand JM, Shin DB, Duffin KC, Armstrong AW, Blauvelt A, Tyring SK, et al. A Randomized Placebo-Controlled Trial of Secukinumab on Aortic Vascular Inflammation in Moderate-To-Severe Plaque Psoriasis (Vip-S). J Invest Dermatol (2020) 140(9):1784–93.e2. doi: 10.1016/j.jid.2020.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Reich K, Langley RG, Lebwohl M, Szapary P, Guzzo C, Yeilding N, et al. Cardiovascular Safety of Ustekinumab in Patients With Moderate to Severe Psoriasis: Results of Integrated Analyses of Data From Phase II and III Clinical Studies. Br J Dermatol (2011) 164(4):862–72. doi: 10.1111/j.1365-2133.2011.10257.x [DOI] [PubMed] [Google Scholar]
- 100. Ryan C, Leonardi CL, Krueger JG, Kimball AB, Strober BE, Gordon KB, et al. Association Between Biologic Therapies for Chronic Plaque Psoriasis and Cardiovascular Events. JAMA (2011) 306(8). doi: 10.1001/jama.2011.1211 [DOI] [PubMed] [Google Scholar]
- 101. González-Cantero A, Ortega-Quijano D, Álvarez-Díaz N, Ballester MA, Jimenez-Gomez N, Jaen P, et al. Impact of Biological Agents on Imaging and Biomarkers of Cardiovascular Disease in Patients With Psoriasis: A Systematic Review and Meta-Analysis of Randomized Placebo-Controlled Trials. J Invest Dermatol (2021) 141(10):2402–11.s. doi: 10.1016/j.jid.2021.03.024 [DOI] [PubMed] [Google Scholar]