Abstract
Camellia oleifera is a woody oil tree species unique to China that has been cultivated and used in China for more than 2,300 years. Most biological research on C. oleifera in recent years has focused on the development of new varieties and breeding. Novel genomic information has been generated for C. oleifera, including a high-quality reference genome at the chromosome level. Camellia seeds are used to process high-quality edible oil; they are also often used in medicine, health foods, and daily chemical products and have shown promise for the treatment and prevention of diseases. C. oleifera by-products, such as camellia seed cake, saponin, and fruit shell are widely used in the daily chemical, dyeing, papermaking, chemical fibre, textile, and pesticide industries. C. oleifera shell can also be used to prepare activated carbon electrodes, which have high electrochemical performance when used as the negative electrode of lithium-ion batteries. C. oleifera is an economically valuable plant with diverse uses, and accelerating the utilization of its by-products will greatly enhance its industrial value.
Keywords: camellia oil, by-products, medical value, activated carbon, applications
Introduction
Edible oil is an important food for humans that provides essential fatty acids and promotes the absorption of fat-soluble vitamins (Soussanaet 2014; Khatri and Jain, 2017). China is the world’s largest consumer and second-largest producer of edible oil (Cassiday 2019; Bai et al., 2021), and the demand for edible oil in China continues to increase with the continued growth of the economy and improvement in living standards.
Edible vegetable oils in China include rapeseed oil, soybean oil, peanut oil, cottonseed oil, sunflower oil, sesame oil, camellia oil, and linseed oil (Bai et al., 2021). Vegetable oils are rich in nutrients and provide various health benefits: camellia oil in particular shows antibacterial activity against Escherichia coli (Yang et al., 2018).
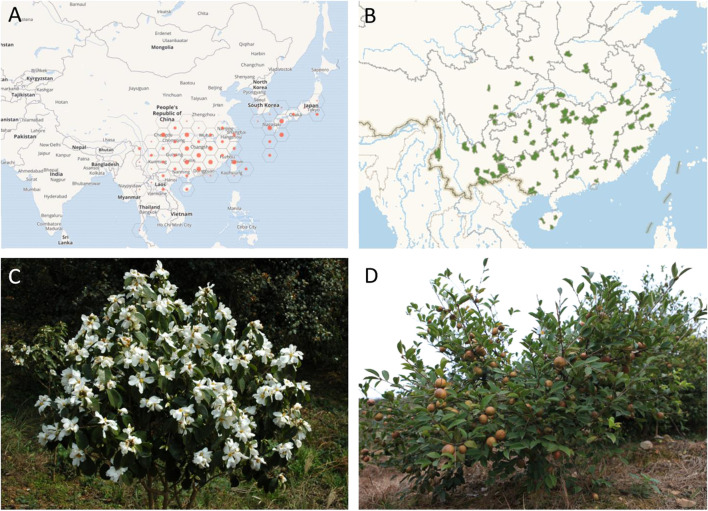
Camellia oleifera Abel. is one of the four major sources of the world’s edible oil, along with Olea europaea L., Elaeis guineensis Jacq., and Cocos nucifera L. (Ma et al., 2011). It is a perennial shrub or small arbour that grows in warm and humid hills and mountains and is mainly distributed in the southern provinces (regions) of China (Figure 1), including Zhejiang, Jiangxi, Henan, Hunan, and Guangxi Provinces; it also occurs in Thailand (Suealek et al., 2019). The varieties mainly include ordinary Camellia oleifera, Camellia yuhsiensis Hu, Camellia chekangoleosa Hu, Camellia meiocarpa Hu, Camellia vietnamensis T. C. Huang ex Hu, and Camellia reticulata Lindl (Liu et al., 2018a). Zhou et al. indicated that the germplasms of C oleifera possess high genetic diversity, as geographic isolation has affected the degree of genetic differentiation among populations (Zhou et al., 2015).
FIGURE 1.
Distribution (A,B) of C. oleifera and images of the flowers and fruits (C,D) of C. oleifera plants.
C. oleifera trees are evergreen and highly adaptable. The benefits of planting C. oleifera can be reaped for as long as a century. The total output value of the Chinese camellia industry was 116 billion yuan in 2019, and the plantation area of C. oleifera was 4.5 million hm2; the camellia industry is a source of income for a total of 1.73 million people.
Figure 1A,B were obtained from the Global Biodiversity Information Facility (https://www.gbif.org/) and Map Bio of China (http://map.especies.cn/), respectively.
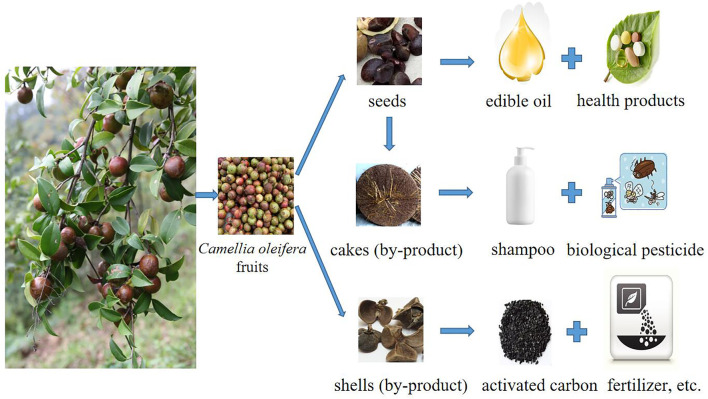
C. oleifera is an economically important tree species with high utilization value. The main product derived from C. oleifera is camellia oil, and other by-products include tea shell and tea meal. Tea meal can be further processed into tea saponin, and tea shell has been used to make furfural, xylitol, tannin extract, activated carbon, and culture medium (Robards et al., 2009).
Research on the Biology of C. oleifera
C. oleifera is a woody oil plant that is highly resistant to various types of stress. However, genetic and genomic information for this species is lacking (Yang et al., 2017). The large polyploid genome of C. oleifera makes genomic analyses rather challenging and hinders further molecular genetic improvement. Recently, an abundance of genomic information has been generated for C. oleifera. The published genome of Camellia lanceoleosa provides an important reference for analyzing the formation and regulation of important traits such as self-incompatibility and lipid synthesis (Gong et al., 2022). Construction of a high-quality reference genome at the chromosome level of C. oleifera has demonstrated that the alleles regulating the synthesis of C. oleifera have been under artificial selection, and this genome resource could provide new insights with implications for the genetic improvement of C. oleifera varieties (Lin et al., 2022). In addition, a high-quality, chromosome-level genome of Camellia chekiangoleosa has been published, and this has provided new insights into the adaptive evolution and oil metabolism of Camellia (Shen et al., 2022).
Wang et al. (2018) found that the total nitrogen content and dry weight accumulation of the seedlings are highest when NO3 − and NH4 + (ratio 1:1) are applied. Liu et al. (2019b) found that a total of 797 miRNAs are significantly differentially expressed in the flowers and fruits of C. oleifera. miR156, miR390, and miR395 regulate the expression of carbohydrate accumulation genes, and miR477 plays a key role in fatty acid synthesis. miR156 contributes to the expression of genes regulating glycolysis and nutrient transformation.
The high rate of flower and fruit drop in C. oleifera, especially under extreme climate conditions, affects C. oleifera yields. Hu et al. (2021) studied the relationship between ethylene and fruit abscission and found that the CoACO genes (CoACO1 and CoACO2) regulate fruit abscission.
C. oleifera is highly tolerant of drought. An understanding of the molecular mechanism of drought tolerance is important. Dong et al. (2017) identified several 76,585 unigenes under drought stress using transcriptome technology and obtained functional annotations for 52,531 of the unigenes.
Other studies have examined the high-affinity Pi transporter gene and have characterized rbcL and rbcS genes from C. oleifera (Chen et al., 2015; Zhou et al., 2020). These findings are useful for identifying promising cultivars (Figure 2)
FIGURE 2.
Diagram illustrating the various industrial uses of C. oleifera.
Camellia Oil and the Composition of its Main Fatty Acids
The oil content of the dry seeds of new cultivars and wild C. oleifera is approximately 47%; the dry seeds also possess volatile aroma components (Jia et al., 2021). The content of unsaturated fatty acids of C. oleifera oil is as high as 90%; oleic acid makes up more than 80% of these unsaturated fatty acids, and linoleic acid comprises 7–13% (Ma et al., 2011; Yang et al., 2016). Variation in the composition of unsaturated fatty acids mainly stems from differences in genotype and extraction method (Zeng et al., 2019a) (Table 1). Fatty acids can be extracted using the petroleum ether, hydrolytic, or potassium hydroxide/methanol extraction methods. The fatty acid composition of camellia oil is mainly determined using gas chromatography or gas chromatography–mass spectrometry.
TABLE 1.
Fatty acid composition of camellia oil according to studies using different extraction methods.
| No. | Analytical Method | Main Fatty Acids (% of the Total Fatty Acids) | References |
|---|---|---|---|
| 1 | Petroleum ether extraction and gas chromatography (GC) | 82–84% unsaturated fatty acids (UFA),68–77% monounsaturated fatty acids (MUFA), 7–14% polyunsaturated fatty acids (PUFA). | Ma et al. (2011) |
| 2 | gas chromatography–mass spectrometry (GC–MS) | 90% UFA, 66.54–83.24% oleic acid, 8.15–9.70% palmitic acid, 5.64–7.96% linoleic acid. | Yuan et al. (2012) |
| 3 | GC–MS | 87.45–90.17% UFA, 77.08–82.78% MUFA, 5.17–11.27% PUFA. | Yang et al. (2016) |
| 4 | Hydrolytic extraction and GC | 10–10.4% SFA, 89.55–90.00% UFA, 79.35–81.60% MUFA, 8.40–10.20% PUFA. | Cao et al. (2017) |
| 5 | GC | 12.65–12.40% SFA, 79.16–81.05% MUFA, 8.19–8.04% PUFA. | Zhang et al. (2019) |
| 6 | Methanol extraction and GC–MS | 87.85–91.44% UFA, 80.53– 86.18% oleic acid, 6.72–9.26% palmitic acid, 4.19–8.95% linoleic acid, 0.84–1.65% stearic acid, 0.09–0.26% eicosenoic acid | Liu et al. (2021) |
Oleic acid provides various health benefits (Farooqui 2013); olive oil is approximately 59–75% oleic acid (Newmark 1997), and palm oil contains 43% oleic acid (Waterman and Lockwood 2007). The main characteristic feature of camellia oil is its high oleic acid content compared with other woody edible oils.
C. oleifera Products
Medicinal Research on Camellia Oil
Camellia oil contains tocopherol, sterol, squalene, vitamin E, and flavonoids (Lee and Yen 2006; Robards 2009; Cao et al., 2017; Wang et al., 2017; Zeng and Endo 2019b), and these compounds are thought to aid weight loss and reduce the risks of cardiovascular and cerebrovascular diseases.
Camellia oil also contains large amounts of functional nutrients, such as squalene, plant sterols (e.g., β-sitosterol and campesterol), polyphenols (e.g., phenolic acid), tocopherols (α-, γ-, and δ-tocopherols), carotenoids (e.g., lycopene), β-carotene, and lutein (Wang et al., 2017; Wang et al., 2018; Yang et al., 2018). These active substances can delay the degradation of unsaturated fatty acids in camellia oil (Zhou et al., 2019), provide various health benefits (Luan et al., 2020), and show antioxidant, anti-inflammatory, and antibacterial activity (Zhu et al., 2019). These compounds can also lower cholesterol, blood sugar, and blood lipids, relieve constipation, and reduce liver and gastrointestinal damage (Table 2).
TABLE 2.
Specific medicinal uses of camellia oil.
| No. | Materials | Experimental Model | Specific Medicinal Use | References |
|---|---|---|---|---|
| 1 | Camellia seed | Male Wistar rats | Repair nonalcoholic fatty liver disease | Yeh et al. (2019) |
| 2 | Camellia oil | Male Sprague-Dawley rats | Repair oxidative damage in the stomach and intestine | Cheng et al. (2014) |
| 3 | Camellia oil | Male BALB/c mice | Ameliorate ethanol-induced acute gastric mucosal injury | Tu et al. (2017) |
| 4 | Camellia oil | Four-week-old male BALB/c mice | Repair gastrointestinal mucosal damage | Wang et al. (2019a) |
| 5 | Camellia oil | Human Int-407 cells; Female Sprague-Dawley rats | Mitigate Alzheimer’s disease (AD) | Weng et al. (2020) |
| 6 | Camellia oil | Hamsters | Reduce fat | Suealeket al. (2019) |
| 7 | Camellia oil | Female ovariectomized mice | Reduce fat | Tung et al. (2019) |
| 8 | Camellia seed | Five human cancer cell lines | Anticancer: saponin OSC6 is a potential therapeutic agent for the treatment of cancer | Zong et al. (2016) |
| 9 | Camellia seed | Male ICR mice | Anticancer: a new glycoprotein (COG2a) has anticancer action. | Li et al. (2019) |
| 10 | Camellia seed | Wistar rats | Hepatoprotective effects | Ko et al. (2019) |
| 11 | Camellia oil | Male Sprague-Dawley rats | Alleviates colitis | Lee et al. (2018) |
| 12 | Camellia oil | 2,2-diphenyl-1-picrylhydrazyl (DPPH) scavenging activity and Trolox equivalent antioxidant capacity | Free radical scavenging: two compounds isolated exhibit antioxidant activity. | Lee and Yen, (2006) |
Camellia oil can be used as a nutritional supplement and be further refined and processed into an advanced skin care product. Recently, the seed extract of camellia oil has been shown to reduce liver fat in rats (Yang et al., 2019).
The Main Nutrient Components of Camellia By-Products
Residues such as camellia seed cake, saponin, and fruit shells are widely used in the daily chemical, dyeing, papermaking, chemical fibre, textile, and pesticide industries (Liu et al., 2018b). Previous studies have shown that polysaccharides extracted from fruit shells have hypoglycemic effects (Zhang and Li, 2015; Gao et al., 2020).
The seeds remaining after oil extraction are by-products referred to as camellia seed cake (Xiao et al., 2017). Oil makes up approximately 5%–6% of the seed cake, and the remaining seed cake after extraction can be used to extract 4% of high-quality saponin (Zhu et al., 2018). Recent studies have shown that seed cakes contain large amounts of polyphenols and new saponins with antimelanogenic and hypoglycemic activity (Zhang et al., 2012; Zhang and Li 2018; Hong et al., 2019). For example, kaempferol extracted from seed cakes shows excellent scavenging activity of 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical (Zheng et al., 2019), saponins show anticancer activity (Di et al., 2017; Wang et al., 2019a), and polysaccharides show hypoglycemic activity (Zhang and Li 2018; Zhu et al., 2018; Jin et al., 2019).
Saponin from C. oleifera is a natural plant pesticide that shows potential to be used for the control of insect and fungal pests (Zhang et al., 2014). Contact toxicity tests and gastric toxicity tests have shown that saponin is an effective insecticide against Ectropis obliqua (Cui et al., 2019). Saponin mixtures can also be used as a potential plant insecticide to control Rhizoctonia damping-off in vegetable seedlings (Kuo et al., 2010).
Utilization of C. oleifera Shell
Morphological Changes of Camellia Shell
C. oleifera shell is mainly composed of cellulose, hemicellulose, and lignin; it is generally used as waste given its low utilization efficiency (Hu et al., 2015). Approximately 54% of C. oleifera fruits are shells (Zhu et al., 2013; Zhao et al., 2017). Camellia shell is thus a rich biomass resource; the shells contain rich quantities of lignin and are an ideal raw material for preparing activated carbon (Hu et al., 2018). Hu et al. (2018) and Wang et al. (2019b, 2021) showed that mature camellia shell is composed of stone cells, spiral vessels, and parenchyma, and the latter two are the main cell types (Figure 3).
FIGURE 3.
Structural characteristics (A) and morphological changes at different stages (B) of C. oleifera fruit.
Functions and Applications of Camellia Shell
The high-quality by-products of C. oleifera shell have been used in many industries. Camellia shell contains tannins, furfural, bioactive phenolic compounds (Zhang et al., 2013), and saponins (Chen et al., 2013; Xiong et al., 2018; Yu and Yong 2018), which are used to make tannins, furfural, activated carbon, and other chemical raw materials. Zhu et al. (2013) used camellia shell to produce ethanol, vanillin, and xylooligosaccharides; camellia shell can also be used as a natural colourant for pigment printing on cotton fabrics (Nakpathom et al., 2017).
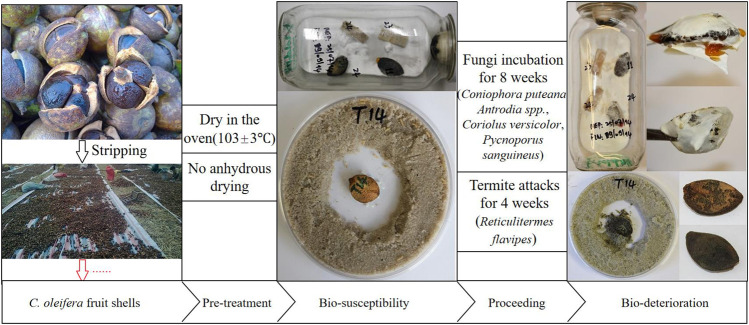
Previous studies have examined the ability of camellia shell extract to inhibit tyrosinase activity in vitro as well as the melanin inhibition of a cosmetic formula containing the extract in 30 female subjects. Camellia shell extract has been used as a skin whitening agent in cosmetic products (Liu et al., 2019a). Hu et al. (2015) investigated the resistance of camellia shell to fungi and termites; the shells appeared to be toxic to fungi and termites but did not completely eradicate them (Figure 4). This indicates that Camellia shell has the potential to be used as a green pesticide.
FIGURE 4.
Resistance of camellia shell powder to fungi and termites. Copyright 2015 Elsevier.
Application of Camellia Shell as High-Quality Activated Carbon
Activated carbon is widely used, and its most notable features are its large surface area, porosity, highly adsorptive internal porous structure, and low cost (Sakaray and Ramirez, 2014). C. oleifera shell is composed of cellulose, hemicellulose, and lignin, and highly developed mesoporous activated carbon can be formed through various technologies. The advantages of Camellia shell activated carbon mainly include its high yield and low cost, as well as the fact that it provides a guaranteed source of raw materials; Camellia shell activated carbon also shows high electrical conductivity and can be used as electrodes. Activated carbon has been obtained from many other plant materials, and these activated carbons, such as macadamia nut shell (Wang et al., 2002), Terminalia catappa shell (Inbaraj and Sulochana, 2006), peanut shell (Wu et al., 2013), durian fruit shell (Tey et al., 2016), baobab fruit shell (Vunain et al., 2017), Aegle marmelos Correa fruit shell (Sivarajasekar et al., 2018), and Swietenia macrophylla fruit shell (Hossain et al., 2021), show high application prospects.
Camellia shell activated carbon is a porous carbon material generated through the carbonization and activation process and an economically important chemical product. It shows high selective adsorption and is widely used in decolorization and water purification. Camellia shell activated carbon has more functions compared with the conventional activated carbon, and Camellia shell activated carbon products with different adsorption characteristics can be prepared using various methods (Sun et al., 2011; Guo et al., 2016, 2018; Fan et al., 2017; Nie et al., 2019). C. oleifera shell can be used to synthesize zirconium dioxide biochar and improve the removal of fluorine in water (Lei et al., 2019).
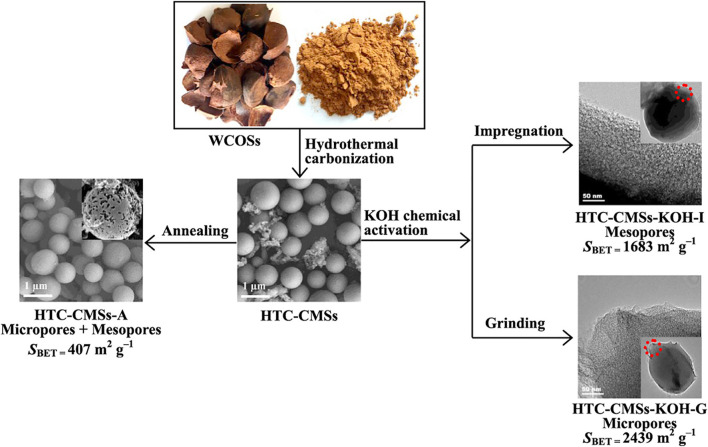
Activated carbon produced by the steam method has many micropores, which is suitable for adsorbing small molecular impurities. The activated carbon produced by the phosphoric acid method has many mesopores and is suitable for adsorbing macromolecular impurities. The phosphoric acid method has become the main method used in the industrial production of activated carbon from husks because it generates fewer pollutants compared with other methods. C. oleifera shell carbon can remove hexavalent chromium and methylene blue from water by adsorption (Ma et al., 2019); the shell has similar burning properties to ordinary wood (Tan et al., 2020). It can also rapidly remove phenolic pollutants in water (Figure 5) (Li et al., 2016).
FIGURE 5.
Porous carbon microspheres made from camellia shells by KOH chemical activation. Copyright 2016 Elsevier.
Activated carbon electrodes can be prepared from C. oleifera shell, and this is a particularly efficient method for using camellia resources. Zhang et al. (2012) used C. oleifera shell to prepare activated carbon electrodes using the ZnCl2 activation method. Ma et al. (2019) made a porous carbon material synthesized from C. oleifera shells via K2CO3 impregnation and pyrolysis, and this material has excellent electrochemical properties when it is used as the anode of Li-ion batteries. Porous carbon with a three-dimensional porous architecture, large surface area, and electrochemical-active oxygen functionalities has been prepared using the microwave-assisted carbonization/activation method (Liang et al., 2018).
Conclusion and Prospects
In this review, recent research on C. oleifera was summarized, including physiological and ecological research on C. oleifera trees, as well as research on the quality and function of camellia oil and the various uses of camellia by-products. The camellia industry has a long industrial chain, and the results of recent research have promoted the development of the entire industrial chain.
C. oleifera oil is a high-end edible oil with high medicinal value. The unsaturated fatty acid content of C. oleifera oil is greater than 80%. The oil can be used as a nutritional supplement and can also be further refined into an advanced skin care product. Camellia oil can aid weight loss and reduce the risk of cardiovascular and cerebrovascular diseases.
Camellia seed cake has high medicinal value and can be used to develop several products. The seed cake contains a large number of tea polyphenols and saponins, which show anti-melanin, hypoglycemic, antibacterial, and insecticidal activity.
Camellia shells can be used to prepare high-quality activated carbon and electrodes. The shell activated carbon is a porous carbon material generated through the carbonization and activation process that is widely used in decolourization and water purification. The shell is a residue produced during the production process and is not effectively utilized; making full use of this waste to prepare high value-added products should thus be a major focus of future research.
Author Contributions
CL conceived the structure of the manuscript. WQ, CG, and AW collected materials and data. WQ wrote the manuscript. CL revised and approved the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (32060331), the Science and Technology Project of Guizhou Province (QKHZC 2022 ZD017), and the Guizhou Provincial Characteristic Key Laboratory (QJHKY 2021002).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Bai Y., Zhai Y., Ji C., Zhang T., Chen W., Shen X., et al. (2021). Environmental Sustainability Challenges of China's Edible Vegetable Oil Industry: from Farm to Factory. Resour. Conservation Recycl. 170, 105606. 10.1016/j.resconrec.2021.105606 [DOI] [Google Scholar]
- Cao Y., Yao X., Ren H., Wang K. (2017). Determination of Fatty Acid Composition and Metallic Element Content of Four Camellia Species Used for Edible Oil Extraction in China. J. Consum. Prot. Food Saf. 12, 165–169. 10.1007/s00003-017-1104-2 [DOI] [Google Scholar]
- Cassiday L. (2017). China's Evolving Edible Oils Industry. Inform 28, 6–9. 10.21748/inform.04.2017.06 [DOI] [Google Scholar]
- Chen L., Chen J., Xu H. (2013). Sasanquasaponin from Camellia Oleifera Abel. Induces Cell Cycle Arrest and Apoptosis in Human Breast Cancer MCF-7 Cells. Fitoterapia 84, 123–129. 10.1016/j.fitote.2012.11.009 [DOI] [PubMed] [Google Scholar]
- Chen Y., Wang B., Chen J., Wang X., Wang R., Peng S., et al. (2015). Identification of Rubisco rbcL and rbcS in Camellia Oleifera and Their Potential as Molecular Markers for Selection of High Tea Oil Cultivars. Front. Plant Sci. 06, 189. 10.3389/fpls.2015.00189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y.-T., Wu S.-L., Ho C.-Y., Huang S.-M., Cheng C.-L., Yen G.-C. (2014). Beneficial Effects of Camellia Oil (Camellia Oleifera Abel.) on Ketoprofen-Induced Gastrointestinal Mucosal Damage through Upregulation of HO-1 and VEGF. J. Agric. Food Chem. 62, 642–650. 10.1021/jf404614k [DOI] [PubMed] [Google Scholar]
- Cui C., Yang Y., Zhao T., Zou K., Peng C., Cai H., et al. (2019). Insecticidal Activity and Insecticidal Mechanism of Total Saponins from Camellia Oleifera . Molecules 24, 4518. 10.3390/molecules24244518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di T.-M., Yang S. L., Yang S.-L., Du F.-Y., Zhao L., Xia T., et al. (2017). Cytotoxic and Hypoglycemic Activity of Triterpenoid Saponins from Camellia Oleifera Abel. Seed Pomace. Molecules 22, 1562. 10.3390/molecules22101562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong B., Wu B., Hong W., Li X., Li Z., Xue L., et al. (2017). Transcriptome Analysis of the Tea Oil Camellia (Camellia Oleifera) Reveals Candidate Drought Stress Genes. PLoS ONE 12, e0181835. 10.1371/journal.pone.0181835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan F., Zheng Y., Huang Y., Lu Y., Wang Z., Chen B., et al. (2017). Preparation and Characterization of Biochars from Waste Camellia Oleifera Shells by Different Thermochemical Processes. Energy Fuels 31, 8146–8151. 10.1021/acs.energyfuels.7b00269 [DOI] [Google Scholar]
- Farooqui A. A. (2013). “Beneficial Effects of Extra Virgin Olive Oil (N-9 Fatty Acids) on Neurological Disorders,” in Phytochemicals, Signal Transduction, and Neurological Disorders (New York, NY: Springer; ). 10.1007/978-1-4614-3804-5_2 [DOI] [Google Scholar]
- Gao C., Cai C., Liu J., Wang Y., Chen Y., Wang L., et al. (2020). Extraction and Preliminary Purification of Polysaccharides from Camellia Oleifera Abel. Seed Cake Using a Thermoseparating Aqueous Two-phase System Based on EOPO Copolymer and Deep Eutectic Solvents. Food Chem. 313, 126164. 10.1016/j.foodchem.2020.126164 [DOI] [PubMed] [Google Scholar]
- Gong W., Xiao S., Wang L., Liao Z., Chang Y., Mo W., et al. (2022). Chromosome‐level Genome of Camellia Lanceoleosa Provides a Valuable Resource for Understanding Genome Evolution and Self‐incompatibility. Plant J. 110, 881–898. 10.1111/tpj.15739 [DOI] [PubMed] [Google Scholar]
- Guo H., Bi C., Zeng C., Ma W., Yan L., Li K., et al. (2018). Camellia Oleifera Seed Shell Carbon as an Efficient Renewable Bio-Adsorbent for the Adsorption Removal of Hexavalent Chromium and Methylene Blue from Aqueous Solution. J. Mol. Liq. 249, 629–636. 10.1016/j.molliq.2017.11.096 [DOI] [Google Scholar]
- Guo H., Yan L., Song D., Li K. (2016). Citric Acid modifiedCamellia Oleiferashell for Removal of Crystal Violet and Pb(II): Parameters Study and Kinetic and Thermodynamic Profile. Desalination Water Treat. 57, 15373–15383. 10.1080/19443994.2015.1072057 [DOI] [Google Scholar]
- Hong C., Chang C., Zhang H., Jin Q., Wu G., Wang X. (2019). Identification and Characterization of Polyphenols in Different Varieties of Camellia Oleifera Seed Cakes by UPLC-QTOF-MS. Food Res. Int. 126, 108614. 10.1016/j.foodres.2019.108614 [DOI] [PubMed] [Google Scholar]
- Hossain M., Goni L. K. M. O., Muntaha N., Jamal M. S., Jamal M. S., Sujan S. M. A., et al. (2021). Box-Behnken Design-Based Optimization for Biodiesel Production from Waste Cooking Oil Using Mahogany (Swietenia Macrophylla) Fruit Shell Derived Activated Carbon as a Heterogeneous Base Catalyst. Reac. Kinet. Mech. Cat. 133, 117–138. 10.1007/s11144-021-01995-w [DOI] [Google Scholar]
- Hu J., Chang S., Peng K., Hu K., Thévenon M.-F. (2015). Bio-susceptibility of Shells of Camellia Oleifera Abel. Fruits to Fungi and Termites. Int. Biodeterior. Biodegrad. 104, 219–223. 10.1016/j.ibiod.2015.06.011 [DOI] [Google Scholar]
- Hu J., Shi Y., Liu Y., Chang S. (2018). Anatomical Structure of Camellia Oleifera Shell. Protoplasma 255, 1777–1784. 10.1007/s00709-018-1271-8 [DOI] [PubMed] [Google Scholar]
- Hu X., Yang M., Gong S., Li H., Zhang J., Sajjad M., et al. (2021). Ethylene-regulated Immature Fruit Abscission Is Associated with Higher Expression of CoACO Genes in Camellia Oleifera . R. Soc. Open Sci. 8, 202340. 10.1098/rsos.202340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inbaraj B., Sulochana N. (2006). Mercury Adsorption on a Carbon Sorbent Derived from Fruit Shell of Terminalia catappa . J. Hazard. Mater. 133, 283–290. 10.1016/j.jhazmat.2005.10.025 [DOI] [PubMed] [Google Scholar]
- Jia X., Deng Q., Yang Y., Xiang X., Zhou X., Tan C., et al. (2021). Unraveling of the Aroma-Active Compounds in Virgin Camellia Oil (Camellia Oleifera Abel) Using Gas Chromatography-Mass Spectrometry-Olfactometry, Aroma Recombination, and Omission Studies. J. Agric. Food Chem. 69, 9043–9055. 10.1021/acs.jafc.0c07321 [DOI] [PubMed] [Google Scholar]
- Jin R., Guo Y., Xu B., Wang H., Yuan C. (2019). Physicochemical Properties of Polysaccharides Separated from Camellia Oleifera Abel Seed Cake and its Hypoglycemic Activity on Streptozotocin-Induced Diabetic Mice. Int. J. Biol. Macromol. 125, 1075–1083. 10.1016/j.ijbiomac.2018.12.059 [DOI] [PubMed] [Google Scholar]
- Kang S., Jian-chun J., Dan-dan C. (2011). Preparation of Activated Carbon with Highly Developed Mesoporous Structure from Camellia Oleifera Shell through Water Vapor Gasification and Phosphoric Acid Modification. Biomass Bioenergy 35, 3643–3647. 10.1016/j.biombioe.2011.05.007 [DOI] [Google Scholar]
- Khatri P., Jain S. (2017). Environmental Life Cycle Assessment of Edible Oils: A Review of Current Knowledge and Future Research Challenges. J. Clean. Prod. 152, 63–76. 10.1016/j.jclepro.2017.03.096 [DOI] [Google Scholar]
- Ko J., Yeh W. J., Huang W. C., Yang H. Y. (2019). Camellia Oleifera Seed Extract Mildly Ameliorates Carbon Tetrachloride‐Induced Hepatotoxicity in Rats by Suppressing Inflammation. J. Food Sci. 84, 1586–1591. 10.1111/1750-3841.14645 [DOI] [PubMed] [Google Scholar]
- Kuo P.-C., Lin T.-C., Yang C.-W., Lin C.-L., Chen G.-F., Huang J.-W. (2010). Bioactive Saponin from Tea Seed Pomace with Inhibitory Effects against Rhizoctonia Solani. J. Agric. Food Chem. 58, 8618–8622. 10.1021/jf1017115 [DOI] [PubMed] [Google Scholar]
- Lee C.-P., Yen G.-C. (2006). Antioxidant Activity and Bioactive Compounds of Tea Seed (Camellia Oleifera Abel.) Oil. J. Agric. Food Chem. 54, 779–784. 10.1021/jf052325a [DOI] [PubMed] [Google Scholar]
- Lee W.-T., Tung Y.-T., Wu C.-C., Tu P.-S., Yen G.-C. (2018). Camellia Oil (Camellia Oleifera Abel.) Modifies the Composition of Gut Microbiota and Alleviates Acetic Acid-Induced Colitis in Rats. J. Agric. Food Chem. 66, 7384–7392. 10.1021/acs.jafc.8b02166 [DOI] [PubMed] [Google Scholar]
- Lei Z., Wang S., Fu H., Gao W., Wang B., Zeng J., et al. (2019). Thermal Pyrolysis Characteristics and Kinetics of Hemicellulose Isolated from Camellia Oleifera Shell. Bioresour. Technol. 282, 228–235. 10.1016/j.biortech.2019.02.131 [DOI] [PubMed] [Google Scholar]
- Li K., Liu S., Shu T., Yan L., Guo H., Dai Y., et al. (2016). Fabrication of Carbon Microspheres with Controllable Porous Structure by Using Waste Camellia Oleifera Shells. Mater. Chem. Phys. 181, 518–528. 10.1016/j.matchemphys.2016.06.089 [DOI] [Google Scholar]
- Li T., Meng X., Wu C., Fan G., Yang J., Pan W. (2019). Anticancer Activity of a Novel Glycoprotein from Camellia Oleifera Abel Seeds against Hepatic Carcinoma in vitro and in vivo . Int. J. Biol. Macromol. 136, 284–295. 10.1016/j.ijbiomac.2019.06.054 [DOI] [PubMed] [Google Scholar]
- Liang J., Qu T., Kun X., Zhang Y., Chen S., Cao Y.-C., et al. (2018). Microwave Assisted Synthesis of Camellia Oleifera Shell-Derived Porous Carbon with Rich Oxygen Functionalities and Superior Supercapacitor Performance. Appl. Surf. Sci. 436, 934–940. 10.1016/j.apsusc.2017.12.142 [DOI] [Google Scholar]
- Lin P., Wang K., Wang Y., Hu Z., Yan C., Huang H., et al. (2022). The Genome of Oil-Camellia and Population Genomics Analysis Provide Insights into Seed Oil Domestication. Genome Biol. 23, 14. 10.1186/s13059-021-02599-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Chen L., Tang W., Peng S., Li M., Deng N., et al. (2018a). Predicting Potential Distribution and Evaluating Suitable Soil Condition of Oil Tea Camellia in China. Forests 9, 487. 10.3390/f9080487 [DOI] [Google Scholar]
- Liu L., Cheng X. X., Cheng X., Zhao W., Wang Y., Dong X., et al. (2018b). Systematic Characterization of Volatile Organic Components and Pyrolyzates from Camellia Oleifera Seed Cake for Developing High Value-Added Products. Arabian J. Chem. 11, 802–814. 10.1016/j.arabjc.2017.12.031 [DOI] [Google Scholar]
- Liu L., Feng S., Chen T., Zhou L., Yuan M., Liao J., et al. (2021). Quality Assessment of Camellia Oleifera Oil Cultivated in Southwest China. Separations 8, 144. 10.3390/separations8090144 [DOI] [Google Scholar]
- Liu W., Wang M., Xu S., Gao C., Liu J. (2019a). Inhibitory Effects of Shell of Camellia Oleifera Abel Extract on Mushroom Tyrosinase and Human Skin Melanin. J. Cosmet. Dermatol. 18, 1955–1960. 10.1111/jocd.12921 [DOI] [PubMed] [Google Scholar]
- Liu X.-X., Luo X.-F., Luo K.-X., Liu Y.-L., Pan T., Li Z.-Z., et al. (2019b). Small RNA Sequencing Reveals Dynamic microRNA Expression of Important Nutrient Metabolism during Development of Camellia Oleifera Fruit. Int. J. Biol. Sci. 15, 416–429. 10.7150/ijbs.26884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan F., Zeng J., Yang Y., He X., Wang B., Gao Y., et al. (2020). Recent Advances in Camellia Oleifera Abel: A Review of Nutritional Constituents, Biofunctional Properties, and Potential Industrial Applications. J. Funct. Foods 75, 104242. 10.1016/j.jff.2020.104242 [DOI] [Google Scholar]
- Ma B., Huang Y., Nie Z., Qiu X., Su D., Wang G., et al. (2019). Facile Synthesis of Camellia Oleifera Shell-Derived Hard Carbon as an Anode Material for Lithium-Ion Batteries. RSC Adv. 9, 20424–20431. 10.1039/C9RA03345A [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J., Ye H., Rui Y., Chen G., Zhang N. (2011). Fatty Acid Composition of Camellia Oleifera Oil. J. Verbr. Leb. 6, 9–12. 10.1007/s00003-010-0581-3 [DOI] [Google Scholar]
- Nakpathom M., Somboon B., Narumol N., Mongkholrattanasit R. (2017). Fruit Shells of Camellia Oleifera Abel as Natural Colourants for Pigment Printing of Cotton Fabric. Prt 46, 56–63. 10.1108/PRT-01-2016-0010 [DOI] [Google Scholar]
- Newmark H. L. (1999). Squalene, Olive Oil, and Cancer Risk: Review and Hypothesis. Ann. N. Y. Acad. Sci. 889, 193–203. 10.1111/j.1749-6632.1999.tb08735.x [DOI] [PubMed] [Google Scholar]
- Nie Z., Huang Y., Ma B., Qiu X., Zhang N., Xie X., et al. (2019). Nitrogen-doped Carbon with Modulated Surface Chemistry and Porous Structure by a Stepwise Biomass Activation Process towards Enhanced Electrochemical Lithium-Ion Storage. Sci. Rep. 9, 15032. 10.1038/s41598-019-50330-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robards K., Prenzler P., Ryan D., Zhong H. (2009). “Camellia Oil and Tea Oil,” in Gourmet and Health-Promoting Specialty Oils. Editors Moreau Robert A., Kamal-Eldin Afaf. (AOCS Press; ), 313–343. 10.1016/B978-1-893997-97-4.50017-6 [DOI] [Google Scholar]
- Sakaray A., Ramirez D. (2014). “Manufacture and Physical Characterization of Wood-Derived Activated Carbon from South texas Mesquite for Environmental Applications,” in Environmental Sustainability Issues in the South Texas–Mexico Border Region. Editors Ramirez D., Ren J., Jones K., Lamm H. (Dordrecht: Springer; ). 10.1007/978-94-007-7122-2_6 [DOI] [Google Scholar]
- Shen T. F., Huang B., Xu M., Zhou P. Y., Ni Z. X., Gong C., et al. (2022). The Reference Genome of Camellia Chekiangoleosa Provides Insights into Camellia Evolution and Tea Oil Biosynthesis. Hortic. Res. 9, uhab083. 10.1038/hortres.2022.uhab08310.1093/hr/uhab083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivarajasekar N., Mohanraj N., Baskar R., Sivamani S. ((2018)). Fixed-bed Adsorption of Ranitidine Hydrochloride onto Microwave Assisted-Activated Aegle Marmelos Correa Fruit Shell: Statistical Optimization and Breakthrough Modelling. Arab. J. Sci. Eng. 43, 2205–2215. 10.1007/s13369-017-2565-4 [DOI] [Google Scholar]
- Soussana J.-F. (2014). Research Priorities for Sustainable Agri-Food Systems and Life Cycle Assessment. J. Clean. Prod. 73, 19–23. 10.1016/j.jclepro.2014.02.061 [DOI] [Google Scholar]
- Suealek N., Yokoyama W. H., Rojpibulstit P., Holt R. R., Hackman R. M. (2019). Thai Tea Seed ( Camellia Oleifera ) Oil Favorably Affects Plasma Lipid Responses in Hamsters Fed High‐Fat Diets. Eur. J. Lipid Sci. Technol. 121, 1800024. 10.1002/ejlt.201800024 [DOI] [Google Scholar]
- Tan M., Luo L., Wu Z., Huang Z., Zhang J., Huang J., et al. (2020). Pelletization of Camellia Oleifera Abel. Shell after Storage: Energy Consumption and Pellet Properties. Fuel Process. Technol. 201, 106337. 10.1016/j.fuproc.2020.106337 [DOI] [Google Scholar]
- Tey J. P., Careem M. A., Yarmo M. A., Arof A. K., Arof A. K. (2016). Durian Shell-Based Activated Carbon Electrode for EDLCs. Ionics 22, 1209–1216. 10.1007/s11581-016-1640-2 [DOI] [Google Scholar]
- Tu P.-S., Tung Y.-T., Lee W.-T., Yen G.-C. (2017). Protective Effect of Camellia Oil (Camellia Oleifera Abel.) against Ethanol-Induced Acute Oxidative Injury of the Gastric Mucosa in Mice. J. Agric. Food Chem. 65, 4932–4941. 10.1021/acs.jafc.7b01135 [DOI] [PubMed] [Google Scholar]
- Tung Y.-T., Hsu Y.-J., Chien Y.-W., Huang C.-C., Huang W.-C., Chiu W.-C. (2019). Tea Seed Oil Prevents Obesity, Reduces Physical Fatigue, and Improves Exercise Performance in High-Fat-Diet-Induced Obese Ovariectomized Mice. Molecules 24, 980. 10.3390/molecules24050980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vunain E., Kenneth D., Biswick T. (2017). Synthesis and Characterization of Low-Cost Activated Carbon Prepared from Malawian Baobab Fruit Shells by H3PO4 Activation for Removal of Cu(II) Ions: Equilibrium and Kinetics Studies. Appl. Water Sci. 7, 4301–4319. 10.1007/s13201-017-0573-x [DOI] [Google Scholar]
- Wang D., Huo R., Cui C., Gao Q., Zong J., Wang Y., et al. (2019a). Anticancer Activity and Mechanism of Total Saponins from the Residual Seed Cake of Camellia Oleifera Abel. In Hepatoma-22 Tumor-Bearing Mice. Food Funct. 10, 2480–2490. 10.1039/C9FO00069K [DOI] [PubMed] [Google Scholar]
- Wang Q., Chang S., Tan Y., Hu J. (2019b). Mesopore Structure in Camellia Oleifera Shell. Protoplasma 256, 1145–1151. 10.1007/s00709-019-01371-5 [DOI] [PubMed] [Google Scholar]
- Wang Q., Hu J., Yang T., Chang S. (2021). Anatomy and Lignin Deposition of Stone Cell in Camellia Oleifera Shell during the Young Stage. Protoplasma 258, 361–370. 10.1007/s00709-020-01568-z [DOI] [PubMed] [Google Scholar]
- Wang R., Chen L., Chen J., Chen Y., Zhang Z., Wang X., et al. (2018). Different Nitrate and Ammonium Ratios Affect Growth and Physiological Characteristics of Camellia Oleifera Abel. Seedlings. Forests 9, 784. 10.3390/f9120784 [DOI] [Google Scholar]
- Wang X., Zeng Q., del Mar Contreras M., Wang L. (2017). Profiling and Quantification of Phenolic Compounds in Camellia Seed Oils: Natural Tea Polyphenols in Vegetable Oil. Food Res. Int. 102, 184–194. 10.1016/j.foodres.2017.09.089 [DOI] [PubMed] [Google Scholar]
- Wang Z.-M., Kanoh H., Kaneko K., Lu G. Q., Do D. (2002). Structural and Surface Property Changes of Macadamia Nut-Shell Char upon Activation and High Temperature Treatment. Carbon 40, 1231–1239. 10.1016/S0008-6223(01)00286-X [DOI] [Google Scholar]
- Waterman E., Lockwood B. (2007). Active Components and Clinical Applications of Olive Oil. Altern. Med. Rev. 12, 331–342. [PubMed] [Google Scholar]
- Weng M.-H., Chen S.-Y., Li Z.-Y., Yen G.-C. (2020). Camellia Oil Alleviates the Progression of Alzheimer's Disease in Aluminum Chloride-Treated Rats. Free Radic. Biol. Med. 152, 411–421. 10.1016/j.freeradbiomed.2020.04.004 [DOI] [PubMed] [Google Scholar]
- Wu M., Guo Q., Fu G. (2013). Preparation and Characteristics of Medicinal Activated Carbon Powders by CO2 Activation of Peanut Shells. Powder Technol. 247, 188–196. 10.1016/j.powtec.2013.07.013 [DOI] [Google Scholar]
- Xiao X., He L., Chen Y., Wu L., Wang L., Liu Z. (2017). Anti-inflammatory and Antioxidative Effects of Camellia Oleifera Abel Components. Future Med. Chem. 9, 2069–2079. 10.4155/fmc-2017-0109 [DOI] [PubMed] [Google Scholar]
- Xiong W., Fu J.-P., Hu J.-W., Wang H.-B., Han X.-D., Wu L. (2018). Secondary Metabolites from the Fruit Shells of Camellia Oleifera . Chem. Nat. Compd. 54, 1189–1191. 10.1007/s10600-018-2592-8 [DOI] [Google Scholar]
- Yang C., Liu X., Chen Z., Lin Y., Wang S. (2016). Comparison of Oil Content and Fatty Acid Profile of Ten New Camellia Oleifera Cultivars. J. Lipids 2016, 3982486. 10.1155/2016/3982486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H.-Y., Yeh W.-J., Ko J., Chen J.-R. (2019). Camellia Oleifera Seed Extract Attenuated Abdominal and Hepatic Fat Accumulation in Rats Fed a High-Fat Diet. Appl. Physiol. Nutr. Metab. 44, 320–325. 10.1139/apnm-2018-0392 [DOI] [PubMed] [Google Scholar]
- Yang H., Zhou H. Y., Yang X. N., Zhan J. J., Zhou H., Wang C., et al. (2017). Transcriptomic Analysis of Camellia Oleifera in Response to Drought Stress Using High Throughput RNA-Seq. Russ. J. Plant Physiol. 64, 728–737. 10.1134/S1021443717050168 [DOI] [Google Scholar]
- Yang R., Zhang L., Li P., Yu L., Mao J., Wang X., et al. (2018). A Review of Chemical Composition and Nutritional Properties of Minor Vegetable Oils in China. Trends Food Sci. Technol. 74, 26–32. 10.1016/j.tifs.2018.01.013 [DOI] [Google Scholar]
- Yeh W.-J., Ko J., Huang W.-C., Cheng W.-Y., Yang H.-Y. (2019). Crude Extract of Camellia Oleifera Pomace Ameliorates the Progression of Non-alcoholic Fatty Liver Disease via Decreasing Fat Accumulation, Insulin Resistance and Inflammation. Br. J. Nutr. 123, 508–515. 10.1017/S0007114519003027 [DOI] [PubMed] [Google Scholar]
- Yu X.-L., He Y. (2018). Tea Saponins: Effective Natural Surfactants Beneficial for Soil Remediation, from Preparation to Application. RSC Adv. 8, 24312–24321. 10.1039/c8ra02859a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J. J., Wang C. Z., Chen H. X., Ye J. Z., Zhou H. (2012). Oil Content and Fatty Acid Composition Analysis of Different Varieties of Camellia Oleifera Seeds. China Oils Fats 37, 75–79. 10.3969/j.issn.1003-7969.2012.01.019 [DOI] [Google Scholar]
- Zeng W., Endo Y. (2019a). Effects of Cultivars and Geography in China on the Lipid Characteristics of Camellia Oleifera Seeds. J. Oleo Sci. 68, 1051–1061. 10.5650/jos.ess19154 [DOI] [PubMed] [Google Scholar]
- Zeng W., Endo Y. ((2019b)). Lipid Characteristics of Camellia Seed Oil. J. Oleo Sci. 68, 649–658. 10.5650/jos.ess18234 [DOI] [PubMed] [Google Scholar]
- Zhang J., Gong L., Sun K., Jiang J., Zhang X. (2012). Preparation of Activated Carbon from Waste Camellia Oleifera Shell for Supercapacitor Application. J. Solid State Electrochem. 16, 2179–2186. 10.1007/s10008-012-1639-1 [DOI] [Google Scholar]
- Zhang L. L., Wang Y. M., Wu D. M., Xu M., Chen J. H. (2013). Comparisons of Antioxidant Activity and Total Phenolics of Camellia Oleifera Abel Fruit Hull from Different Regions of China. J. Med. Plants Res. 4, 1407e1413. 10.5897/JMPR10.270 [DOI] [Google Scholar]
- Zhang S., Li X.-Z. (2015). Inhibition of α-glucosidase by Polysaccharides from the Fruit Hull of Camellia Oleifera Abel. Carbohydr. Polym. 115, 38–43. 10.1016/j.carbpol.2014.08.059 [DOI] [PubMed] [Google Scholar]
- Zhang S., Li X. (2018). Hypoglycemic Activity in vitro of Polysaccharides from Camellia Oleifera Abel. Seed Cake. Int. J. Biol. Macromol. 115, 811–819. 10.1016/j.ijbiomac.2018.04.054 [DOI] [PubMed] [Google Scholar]
- Zhang S., Pan Y. G., Zheng L., Yang Y., Zheng X., Ai B., et al. (2019). Application of Steam Explosion in Oil Extraction of Camellia Seed ( Camellia Oleifera Abel.) and Evaluation of its Physicochemical Properties, Fatty Acid, and Antioxidant Activities. Food Sci. Nutr. 7, 1004–1016. 10.1002/fsn3.924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.-F., Han Y.-Y., Bao G.-H., Ling T.-J., Zhang L., Gao L.-P., et al. (2012). A New Saponin from Tea Seed Pomace (Camellia Oleifera Abel) and its Protective Effect on PC12 Cells. Molecules 17, 11721–11728. 10.3390/molecules171011721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.-F., Yang S.-L., Han Y.-Y., Zhao L., Lu G.-L., Xia T., et al. (2014). Qualitative and Quantitative Analysis of Triterpene Saponins from Tea Seed Pomace (Camellia Oleifera Abel) and Their Activities against Bacteria and Fungi. Molecules 19, 7568–7580. 10.3390/molecules19067568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao K., Liu S., Li K., Hu Z., Yuan Y., Yan L., et al. (2017). Fabrication of −SO3H Functionalized Aromatic Carbon Microspheres Directly from Waste Camellia Oleifera Shells and Their Application on Heterogeneous Acid Catalysis. Mol. Catal. 433, 193–201. 10.1016/j.mcat.2017.02.032 [DOI] [Google Scholar]
- Zheng L., Chen L., Li J., Liang L., Fan Y., Qiu L., et al. (2019). Two Kaempferol Glycosides Separated from Camellia Oleifera Meal by High‐Speed Countercurrent Chromatography and Their Possible Application for Antioxidation. J. Food Sci. 84, 2805–2811. 10.1111/1750-3841.14765 [DOI] [PubMed] [Google Scholar]
- Zhou D., Shi Q., Pan J., Liu M., Long Y., Ge F. (2019). Effectively Improve the Quality of Camellia Oil by the Combination of Supercritical Fluid Extraction and Molecular Distillation (SFE-MD). LWT 110, 175–181. 10.1016/j.lwt.2019.04.075 [DOI] [Google Scholar]
- Zhou J., Lu M., Zhang C., Qu X., Liu Y., Yang J., et al. (2020). Isolation and Functional Characterisation of the PHT1 Gene Encoding a High-Affinity Phosphate Transporter in Camellia Oleifera . J. Hortic. Sci. Biotechnol. 95, 553–564. 10.1080/14620316.2019.1703562 [DOI] [Google Scholar]
- Zhou L.-Y., Wang X.-N., Wang L.-P., Chen Y.-Z., Jiang X.-C. (2015). Genetic Diversity of Oil-Tea Camellia Germplasms Revealed by ISSR Analysis. Int. J. Biomath. 08, 1550070. 10.1142/S1793524515500709 [DOI] [Google Scholar]
- Zhu C., Zhang M., Tang Q., Yang Q., Li J., He X., et al. (2019). Structure and Activity of the Camellia Oleifera Sapogenin Derivatives on Growth and Biofilm Inhibition of Staphylococcus aureus and Escherichia coli . J. Agric. Food Chem. 67, 14143–14151. 10.1021/acs.jafc.9b03577 [DOI] [PubMed] [Google Scholar]
- Zhu J., Zhu Y., Jiang F., Xu Y., Ouyang J., Yu S. (2013). An Integrated Process to Produce Ethanol, Vanillin, and Xylooligosaccharides from Camellia Oleifera Shell. Carbohydr. Res. 382, 52–57. 10.1016/j.carres.2013.10.007 [DOI] [PubMed] [Google Scholar]
- Zhu W.-F., Wang C.-L., Ye F., Sun H.-P., Ma C.-Y., Liu W.-Y., et al. (2018). Chemical Constituents of the Seed Cake ofCamellia Oleiferaand Their Antioxidant and Antimelanogenic Activities. Chem. Biodivers. 15, e1800137. 10.1002/cbdv.201800137 [DOI] [PubMed] [Google Scholar]
- Zong J., Wang D., Jiao W., Zhang L., Bao G., Ho C.-T., et al. (2016). Oleiferasaponin C6 from the Seeds of Camellia Oleifera Abel.: A Novel Compound Inhibits Proliferation through Inducing Cell-Cycle Arrest and Apoptosis on Human Cancer Cell Lines in vitro . RSC Adv. 6, 91386–91393. 10.1039/C6RA14467E [DOI] [Google Scholar]