Abstract
Youth living with HIV (YLWH) in the US have low rates of viral suppression (VS). In a prospective randomized clinical trial (ATN152) that enrolled 89 YLWH on antiretroviral therapy (ART) with detectable viral load, we evaluated a 12 week triggered escalating real-time adherence (TERA) intervention with remote coaching, electronic dose monitoring (EDM), and outreach for missed/delayed doses compared to standard of care (SOC). Median [Q1, Q3] percent days with EDM opening was higher in TERA (72% (47%, 89%)) versus SOC (41% (21%, 59%); p < 0.001) and incidence of numbers of 7 day gaps between openings were lower (TERA to SOC ratio: 0.40; 95% CI 0.30, 0.53; p < 0.001). There were no differences in VS at week 12 (TERA 35%; 95% CI 21%, 51% versus SOC 36%; 95% CI 22%, 51%; p > 0.99) or later time-points. The intervention improved adherence but not VS in heavily ART-experienced YLWH. Remote coaching more closely tailored to the unique dosing patterns and duration of need for youth struggling to reach VS warrants further investigation.
Keywords: YLWH, Viral suppression, Intervention, mHealth, Coaching, EDM, Youth
Introduction
Youth (13–24 years) living with HIV (YLWH) have the lowest rates of viral suppression (VS) among people living with HIV in the United States (US) [1, 2]. Among YLWH in the US, low rates of viral suppression, from 12% [3] to 30% [4] are attributed to loss across the HIV cascade of care (knowing one’s status, linking to care, receiving antiretroviral therapy (ART), adhering to ART and sustaining engagement in care and ART). Among YLWH prescribed ART, about 60% are virally suppressed [5], far below the national target of 90% [6]. For youth with detectable viral load, negative consequences include worse clinical outcomes such as AIDS defining illness, hospitalization, death, issues related to chronic inflammation, and increased odds of repeat virologic failure, as well as greater odds for onward transmission [7–10]. Achieving and sustaining viral suppression in youth on ART depends primarily on consistent adherence [11]. ART has dramatically improved over the past decade in terms of tolerability, potency and pill-burden [9], and the US Food and Drug Administration (FDA) recently approved the first complete injectable long acting ART regimen for adults with suppressed viral load [12]. However, for youth with known difficulties achieving viral suppression, daily oral ART remains the main treatment strategy, suggesting that adherence interventions tailored to YLWH, and specifically to those with detectable viral load, remain critically needed [13, 14].
The Adolescent Medicine Trials Network for HIV/AIDS Interventions (ATN) 152 study (NCT03292432) was a Phase II, randomized, open-label trial that evaluated the efficacy of a 12 week triggered escalating real-time adherence (TERA) intervention in YLWH with detectable viral load on ART in the US. Enrollees were not virally suppressed at time of enrollment despite being prescribed ART for at least 24 weeks. The 12 week TERA intervention, described in greater detail previously [15], provided remote coaching sessions at baseline, Weeks 4 and 12, and had several mHealth components, including actively monitored dosing using an electronic dose monitoring (EDM) device, with coach outreach by text and phone for delayed and missed doses. EDM devices were issued to all participants to use throughout study participation to measure adherence. We hypothesized that participants randomized to the 12 week TERA intervention would achieve viral suppression after 12 weeks at higher rates than those continuing with standard of care (SOC).
We present the study’s primary and secondary outcomes using data collected before study visits were paused on March 20, 2020 due to the COVID-19 pandemic [16, 17]. All participants had reached or passed their Week 12 study visit before the pause. Given the potential impact of COVID-19 on experiences and outcomes [18–21], we revised the protocol and approach to focus outcomes and related interpretations on pre-COVID-19 pandemic data.
Methods
Study Design
Participants were recruited at ATN sites across the US, with a targeted enrollment of 120 youth. Inclusion criteria in addition to living with HIV (vertically or perinatally acquired) included: (i) 13–24 years of age, (ii) prescribed ART for at least 24 weeks, (iii) documented HIV-1 RNA plasma ≥ 200 copies/ml within 45 days of study entry, (iv) on an ART regimen taken once daily, (v) having a cellphone able to send and receive text messages, (vi) able to communicate in spoken English, and (vii) willing to provide contact information for at least one person who could assist in locating youth if the study team could not reach them directly. Use of EDM devices outside the study, enrollment in another active adherence intervention study, and issues that would impair or interfere with ability to provide informed consent or assent at enrollment were exclusion criteria. All participants completed consent procedures per site specific consent and assent requirements. Enrollees were stratified by age (< 18 years vs. ≥ 18 years) and randomized with equal probability to TERA or continuing SOC. Stratification was to ensure balance in treatment assignment within age stratum. Half the enrollees were randomly selected to engage in additional in-depth interviews about their experiences at Weeks 12 and 48. The qualitative data is presently undergoing analysis for presentation elsewhere.
Participants were followed for 48 weeks with study visits at screening, entry (which could be the same day as screening), and Weeks 4, 12 (each ± 2 weeks), 24, 36, and 48 (each ± 4 weeks). In addition to clinical data collection, participants completed an audio computer-assisted self interview (ACASI) at each study visit except Week 4. Participants in both arms were given an AdhereTech™ EDM pill bottle (https://adheretech.com/how-it-works) to store ART medication and record dosing data (date and time for each bottle opening). Participants with multiple pills in their daily dose of ART were asked to store only one of their medications in the bottle, and use that prompt to remind themselves of all accompanying pills. For participants in the TERA arm, only the first 12 weeks (active intervention phase) involved active monitoring of the EDM data wirelessly transmitted to a central website and used by the intervention coaches and monitors to identify and respond to late or missed doses. For the SOC participants, EDM dosing data were collected passively (opening event date and time were captured and battery strength monitored, but no outreach for non-dosing) for the full 48 week study period; for the TERA participants, passive collection started post intervention (Week 12) and continued through to the end of their study participation.
The Triggered Escalating Remote Coaching Adherence (TERA) Intervention Condition
Described in detail elsewhere [15], the TERA intervention paired participants with a remote coach for 12 weeks. The coaches were study team members with diverse educational backgrounds (undergraduate degree to Masters in Social Work) and unrelated to the clinical care sites where youth received treatment. Over the course of the study, four coaches of diverse ethnic and racial background and sexual and gender identity were trained and delivered the intervention. Although not a requirement, all coaches participating in the study were at or below 30 years of age. Coaches completed basic Motivational Interviewing and TERA intervention training as part of study specific preparation. Coaches engaged with participants remotely through planned sessions on VSee, a secure video conferencing platform, at clinic visits at baseline, week 4 and week 12, and as needed through SMS and phone calls throughout the 12 week period when the EDM signaled late or missed dosing. Planned sessions included exploration of values and needs through a visual activity mapping out one’s “Lifespace”, identification of facilitators and challenges to adherence through an adapted Next Step Counseling approach, and development of an adherence plan. At week 4 and 12 sessions, coaches additionally used a readiness ruler around adherence and reviewed participant dosing data presented in a calendar graphic display. For as needed outreach, when doses appeared late per EDM data, an automated text asking about dosing plans was sent to the participant’s cell phone with options of (a) taking now, (b) taking later, and (c) skipping. Follow-up from coaches was based on the specific answer provided or if there was no reply, and included two-way texting and phone-call outreach. Exploring the specific situation causing delays in dosing or missed doses and brainstorming potential solutions was generally the focus of follow-up outreach. Thus, the intervention approach used remote coaches and EDM data collection to provide support triggered by late dosing and escalating in terms of initiation and progression of outreach efforts.
Analysis Population and Follow-up
Analyses included all enrolled participants with any data collected. Follow-up included data collected through March 20, 2020, the start of the study’s COVID-19 pandemic related pause, by which time all participants had reached Week 12 and all but one had reached Week 24. The study pause followed FDA and regulatory guidelines applicable to our behavioral study [16, 17]. Because participant behavior after the study pause might differ [22], the protocol team revised the statistical analysis plan and study protocol to base primary results on data collected before the pause. A Letter of Amendment was released to the sites in June 2020 modifying the post Week 12 secondary outcome measures, and institutional review board (IRB) approval obtained for the revised approach. Post COVID-19 pause data collection was completed in October 2020. While primary results focus on pre COVID-19 pandemic related pause data, we repeated analyses with all collected data as a supplement to the primary analyses [23, 24].
Virologic Suppression
The primary objective of the study was to estimate and compare HIV VS rates at the completion of the intervention phase of the study at Week 12. The primary definition of VS was a composite outcome where successful VS was defined as a plasma HIV-1 RNA < 200 copies/ml at Week 12 (± 2 weeks) while failure was defined as either having a Week 12 HIV-1 RNA of ≥ 200 copies/ml or missing the viral load test. Thus, participants with no viral load collected within the 2 week window of the study visit for any reason except a non-HIV-related death before Week 12 were classified as failures for the primary analysis. Two supplementary analyses were conducted: HIV-1 RNA < 200 copies/ml at Week 12 (± 2 weeks) with missing HIV-1 RNA values excluded (complete case) and (ii) HIV-1 RNA < 200 copies/ml with missing HIV-1 RNA values classified as failures, but allowing a wider window (8–18 weeks) for capturing HIV-1 RNA (wide window). This set of analyses was repeated for HIV-1 RNA < 50 copies/ml.
Additional objectives included VS over longer time periods using both cross-sectional (HIV-1 RNA < 200 (or < 50 copies/ml) at Weeks 24, 36, and 48 (± 4 weeks)) and longitudinal outcome measures (achieved HIV-1 RNA < 200 copies/ml by Week 12 and maintain VS through Week 48). Participants with no HIV-1 RNA measurement within the ± 4 week visit window for any reason besides non HIV-related death were classified as failures. Because of the administrative censoring due to the COVID-19 pause, only participants with a study visit or with the opportunity to have had a study visit (i.e., time since randomization was longer than the acceptable upper window for the study visit to have taken place) before the COVID-19 pandemic related study pause were included in the primary post intervention analyses. For comparison, Week 48 results were also summarized using all data collected through the end of the study for all participants.
Adherence Outcomes
Adherence was measured through the collection of bottle-opening events from the EDM Smart Bottle, which registered the date and time of device opening, and transmitted this information wirelessly to a data collection dashboard through a cellular network. When out of network, the device stored the data, which were transmitted once the device was again in range. The scheduled daily dose time was stored on the dashboard and the Smart Bottle emitted a blue pulsing light around that dose time when the bottle remained unopened. This feature was active for all participants throughout the study to promote engagement with the EDM.
Following recommendations and relevant methodological guidelines for summarizing adherence [25, 26], a dose was considered as taken if the device was opened at least once during the 24 h period starting at midnight. Two adherence outcome measures were used to compare arms: (i) percent of days device was opened over a full 12 week period (PCT12), and (ii) an incidence rate (IR) representing the number of 7 day gaps (defined as 7 consecutive days without opening the EDM) over 12 weeks (GAP IR) calculated as:
When summarizing adherence with EDM data it was assumed that opening of the EDM device reflected actual dosing. To check this assumption, we evaluated the association between EDM openings and viral load (see second paragraph of statistical methods) and only used EDM data for 12 week periods having statistically significant (p ≤ 0.05) correlations with viral load of at least moderate size (described below).
Statistical Methods
Categorical outcomes were summarized using proportions with exact (Clopper-Pearson) 95% confidence intervals (CIs) and arms compared using Fisher’s exact tests. Continuous outcomes were summarized using medians [Q1, Q3] and distributions compared by arm using Wilcoxon rank sum tests.
For each 12 week interval, percentages of participants with VS and the difference in percentages between arms were summarized. Three covariates were identified a priori by the Study Team to be included in adjusted analyses: age (years), mode of transmission, and sex at birth. Any baseline characteristics with differences by arm at baseline (p < 0.05) and associated with the primary virologic outcome measure (p < 0.10) were also included in adjusted analyses. Log binomial regression models were fit on the VS outcomes with main effects for the intervention and adjusted for each covariate. Adjusted analyses were also fit using repeated measures log binomial models including VS at Weeks 12, 24, 36 and 48. Models were fit using generalized estimating equations with an exchangeable correlation structure, robust variances, and included study visit week in the model. Median [Q1, Q3] HIV-1 RNA data (log10-transformed) were presented graphically and numbers of participants with at least a one log10 decline in HIV-1 RNA levels from baseline summarized.
We explored associations of EDM openings (adherence) with viral load using Locally Estimated Scatterplot Smoothing (LOESS) curves and Spearman correlations (ρ) between each 12 week adherence outcome (PCT12 and GAP IR) and HIV-1 RNA levels (log10-transformed) at the end of the 12 week period. If dose monitoring was capturing actual dose taking, the summary measures should be associated with the viral outcome. For each 12 week period where the adherence summary measures were associated with HIV-1 RNA levels (a moderate correlation of |ρ|> 0.35 and p < 0.05) [27], distributions of PCT12 and GAP IR were compared by VS using Wilcoxon rank sum tests. By arm comparisons of PCT12 used Wilcoxon rank sum tests and IR ratios of GAP IR used generalized linear models with a Poisson link and Wald confidence limits.
Differences significant at the p < 0.05 level were highlighted in the text. No adjustments were made for multiple testing, and since there were many comparisons, results should be interpreted with care. Analyses were done in SAS 9.4 (SAS Institute, Cary, NC).
Community Engagement
Components of the intervention and study procedures were reviewed by a youth advisory board who met virtually each month, facilitated by one of the intervention coaches. Each participating clinical research site was asked to identify one to two youth who could serve in the virtual youth advisory board (vYAB). vYAB members could remain engaged throughout the entire study, and were compensated for their participation. Recommendations from the vYAB were incorporated throughout the study, including their feedback on interpretation of results and findings.
Ethics
Study procedures were reviewed and approved by a central IRB (the ATN Coordinating Center at University of North Carolina, Chapel Hill). In addition, participating research sites engaged their local IRB as per site policy. Sites varied in their institutional approaches for obtaining informed consent versus assent with parental/guardian consent per their local guidelines. The study was reviewed by the ATN’s Safety Monitoring Committee (SMC) twice a year. The SMC reviewed any adverse events and made recommendations for the continuation of the study, given both safety and progress of the overall study in terms of recruitment and enrollment targets.
Results
Sample
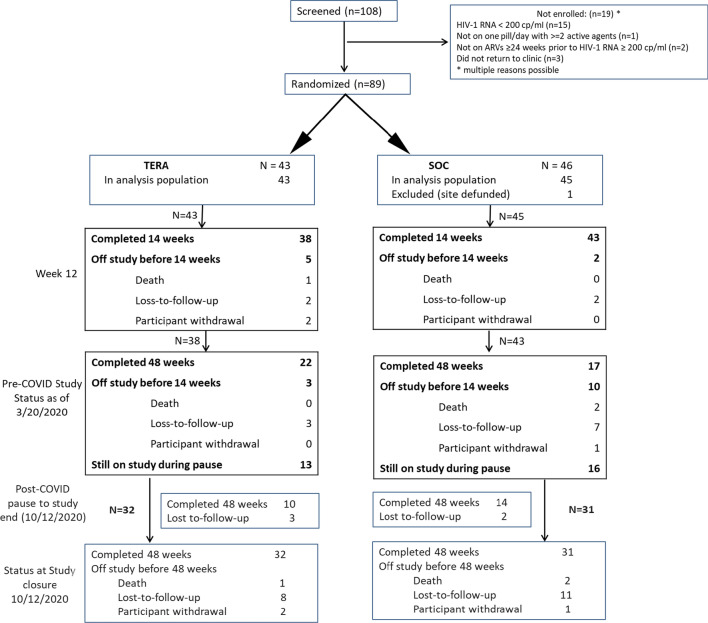
Eighty-nine youth (74% of the targeted 120) were recruited at ten participating ATN sites located in the southern US (five sites), east coast (two sites), mid-west (one site) and western US (two sites) between April 2018 and September 2019. Accrual was closed before reaching enrollment targets per the recommendation of the SMC, which also recommended study follow-up continue as planned. Of the 269 participants approached for enrollment, 110 (41%) consented to being screened for eligibility. As indicated in Fig. 1, of the 108 screened for enrollment, three potential participants did not return to the clinic for further evaluation. Of the 16 eligibility failures, the most common reason for ineligibility (15) was having an HIV-1 RNA less than the 200 copies/ml minimum set by the inclusion criteria. One participant enrolled at a site that was immediately defunded, leaving 88 participants in the analysis population followed until March 20, 2020.
Fig. 1.
Consort diagram. Grayed text represents post COVID-19 pandemic related pause in data collection- not used in primary analyses
Sample Characteristics
Participants were 55% male, 85% Black, with a median age of 22 years (range 13–24) (Table 1). Forty-four percent had acquired HIV at birth. When reporting sexual identity, 45% selected ‘gay’, 41% ‘heterosexual’, and 11% ‘bisexual’. Twenty-percent (20%) were classified as having moderate to high alcohol use, 54% moderate to high cannabis use, just under half had used tobacco products (45%), but use of other substances was minimal (injection drugs (0%), amphetamine (5%), cocaine (6%), hallucinogens (1%), inhalants (2%), opioids (7%), sedatives (8%), other (2%)). Median HIV-1 RNA at screening was 3571 copies/ml (range 210–1,440,000), with median values slightly higher in the TERA arm. About one third (32%) of participants had an ongoing psychiatric disorder and 18% (30% in the TERA arm and 7% in the SOC arm) were on at least one psychiatric medication at study entry. Most participants (49%) were on their second ART regimen, 30% were on at least their third regimen, and 22% were continuing on their first line regimen. Most participants (78%) were on an integrase strand transfer inhibitor based combination antiretroviral therapy. Nineteen participants (22%) had switched to a new regimen in the 30 days prior to entering the study. A total of eight participants (9%) switched regimens while on study and all were after Week 12 (3 in the TERA arm and 5 in the SOC arm).
Table 1.
Baseline characteristics of analysis population (Triggered escalating real-time adherence intervention [TERA] versus standard of care [SOC])
| Characteristic | Level | SOC (N = 45) | TERA (N = 43) | Total (N = 88) |
|---|---|---|---|---|
| Site N (%) | Bronx-Lebanon | 7 (16%) | 8 (19%) | 15 (17%) |
| Emory | 4 (9%) | 3 (7%) | 7 (8%) | |
| Ft. Lauderdale | 6 (13%) | 4 (9%) | 10 (11%) | |
| Jacksonville | 3 (7%) | 3 (7%) | 6 (7%) | |
| Johns Hopkins | 4 (9%) | 3 (7%) | 7 (8%) | |
| St. Jude | 5 (11%) | 6 (14%) | 11 (13%) | |
| Univ. of Alabama | 0 (0%) | 1 (2%) | 1 (1%) | |
| Univ. of Colorado | 5 (11%) | 6 (14%) | 11 (13%) | |
| Wayne State | 11 (24%) | 9 (21%) | 20 (23%) | |
| Sex at birth N (%) | Female | 19 (42%) | 21 (49%) | 40 (45%) |
| Male | 26 (58%) | 22 (51%) | 48 (55%) | |
| Race N (%) | Black or African American | 40 (89%) | 34 (81%) | 74 (85%) |
| Other | 4 (9%) | 6 (14%) | 10 (11%) | |
| Unknown | 1 (2%) | 2 (5%) | 3 (3%) | |
| Missing | 0 | 1 | 1 | |
| Ethnicity N (%) | Not Hispanic or Latino | 41 (91%) | 34 (81%) | 75 (86%) |
| Hispanic or Latino | 4 (9%) | 5 (12%) | 9 (10%) | |
| Unknown | 0 (0%) | 3 (7%) | 3 (3%) | |
| Missing | 0 | 1 | 1 | |
| Age (years) | Median (Min, Max) | 22.1 (16.5, 24.8) | 22.3 (13.7, 24.8) | 22.2 (13.7, 24.8) |
| Age category N (%) | 13–17 yrs | 5 (11%) | 4 (9%) | 9 (10%) |
| 18–21 yrs | 16 (36%) | 13 (30%) | 29 (33%) | |
| 22- < 25 yrs | 24 (53%) | 26 (60%) | 50 (57%) | |
| Mode of HIV transmission N (%) | Horizontal | 24 (53%) | 25 (58%) | 49 (56%) |
| Vertical | 21 (47%) | 18 (42%) | 39 (44%) | |
| Sexual identity N (%) | Gay | 21 (47%) | 18 (43%) | 39 (45%) |
| Heterosexual | 20 (44%) | 16 (38%) | 36 (41%) | |
| Bisexual | 3 (7%) | 7 (17%) | 10 (11%) | |
| Other/Prefer not to answer | 1 (2%) | 1 (2%) | 2 (2%) | |
| Missing | 0 | 1 | 1 | |
| ASSIST [28] Any substance use N (%) | Yes | 35 (78%) | 33 (79%) | 68 (78%) |
| No | 8 (18%) | 9 (21%) | 17 (20%) | |
| Preferred not to answer > 1 question | 2 (4%) | 0 (0%) | 2 (2%) | |
| Missing | 0 | 1 | 1 | |
| Alcohol risk score [28] category N (%) | Never used/Low | 34 (76%) | 32 (76%) | 66 (76%) |
| Moderate/High | 10 (22%) | 7 (17%) | 17 (20%) | |
| Used substance but invalid risk score | 0 (0%) | 3 (7%) | 3 (3%) | |
| Prefer not to answer | 1 (2%) | 0 (0%) | 1 (1%) | |
| Missing | 0 | 1 | 1 | |
| Cannabis risk score [28] category N (%) | Never used/Low | 13 (29%) | 19 (45%) | 32 (37%) |
| Moderate/High | 27 (60%) | 20 (48%) | 47 (54%) | |
| Used substance but invalid risk score | 2 (4%) | 2 (5%) | 4 (5%) | |
| Prefer not to answer | 3 (7%) | 1 (2%) | 4 (5%) | |
| Missing | 0 | 1 | 1 |
| Characteristic | Level | SOC (N = 45) | TERA (N = 43) | Total (N = 88) | |
|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | |||
| Ever used tobacco N (%) | Yes | 22 (49%) | 17 (40%) | 39 (45%) | |
| No | 22 (49%) | 25 (60%) | 47 (54%) | ||
| Prefer not to answer | 1 (2%) | 0 (0%) | 1 (1%) | ||
| Missing | 0 | 1 | 1 | ||
| Screening HIV-1 RNA (copies/ml) | Median (Min, Max) | 2630 (210, 209,000) | 5010 (240, 1,440,000) | 3571 (210, 1,440,000) | |
| Screening log10 HIV-1 RNA (copies/ml) | Median (Min, Max) | 3.42 (2.32, 5.32) | 3.70 (2.38, 6.16) | 3.55 (2.32, 6.16) | |
| Psychiatric disorder present N (%) | Yes | 14 (31%) | 14 (33%) | 28 (32%) | |
| No | 31 (69%) | 29 (67%) | 60 (68%) | ||
| On psychiatric medications N (%) | Yes | 3 (7%) | 13 (30%) | 16 (18%) | |
| No | 42 (93%) | 30 (70%) | 72 (82%) | ||
| Number prior ARV regimens N (%) | 1 | 10 (22%) | 9 (21%) | 19 (22%) | |
| 2 | 21 (47%) | 22 (51%) | 43 (49%) | ||
| ≥ 3 | 14 (31%) | 12 (28%) | 26 (30%) | ||
| Center for epidemiological studies depression scale (CESD) [29–31] category N (%) | Not depressed (< 10) | 22 (50%) | 13 (33%) | 35 (42%) | |
| Depressed (≥ 10) | 22 (50%) | 27 (68%) | 49 (58%) | ||
| Missing | 1 | 3 | 4 | ||
| Medical outcomes study social support survey [32] overall index score | N | 43 | 39 | 82 | |
| Median (Q1, Q3) | 72.4 (43.4, 94.7) | 71.1 (52.6, 97.4) | 71.7 (50.0, 96.1) | ||
| EQ-5D-Y [33, 34] VAS score | N | 45 | 42 | 87 | |
| Median (Q1, Q3) | 80.0 (70.0, 95.0) | 80.0 (65.0, 90.0) | 80.0 (70.0, 95.0) | ||
| Emotional regulation questionnaire (ERQ) [35, 36]: Cognitive reappraisal scale | N | 43 | 41 | 84 | |
| Median (Q1, Q3) | 31.0 (26.0, 38.0) | 35.0 (27.0, 39.0) | 32.0 (27.0, 38.5) | ||
| ERQ Expressive suppression scale | N | 44 | 41 | 85 | |
| Median (Q1, Q3) | 18.0 (13.7, 22.0) | 19.0 (13.0, 22.0) | 18.0 (13.0, 22.0) | ||
| Adolescent decision making questionnaire (ADMQ) [37, 38]: Self-confidence score | N | 43 | 40 | 83 | |
| Median (Q1, Q3) | 3.2 (2.8, 3.5) | 3.0 (2.6, 3.5) | 3.2 (2.7, 3.5) | ||
| ADMQ vigilance score | N | 42 | 40 | 82 | |
| Median (Q1, Q3) | 3.0 (2.5, 3.8) | 3.0 (2.4, 3.6) | 3.0 (2.5, 3.7) | ||
| ADMQ panic score | N | 42 | 39 | 81 | |
| Median (Q1, Q3) | 2.3 (1.8, 2.8) | 2.0 (1.5, 2.8) | 2.0 (1.5, 2.8) | ||
| ADMQ evasiveness score | N | 42 | 40 | 82 | |
| Median (Q1, Q3) | 1.5 (1.3, 1.8) | 1.4 (1.2, 2.2) | 1.5 (1.2, 2.0) | ||
| ADMQ complacency score | N | 42 | 38 | 80 | |
| Median (Q1, Q3) | 1.8 (1.4, 2.1) | 1.7 (1.4, 2.0) | 1.8 (1.4, 2.1) | ||
| HIV stigma framework (HIV stigma) [39–41]: anticipated | N | 43 | 40 | 83 | |
| Median (Q1, Q3) | 2.0 (1.0, 2.4) | 1.9 (1.0, 2.3) | 1.9 (1.0, 2.3) | ||
| HIV stigma: Enacted | N | 42 | 38 | 80 | |
| Median (Q1, Q3) | 1.2 (1.0, 2.0) | 1.0 (1.0, 1.7) | 1.1 (1.0, 1.8) | ||
| HIV stigma: Internalized | N | 43 | 39 | 82 | |
| Median (Q1, Q3) | 2.7 (1.0, 4.0) | 2.8 (1.0, 4.0) | 2.8 (1.0, 4.0) | ||
| Information motivation behavioral ART adherence questionnaire (IMB-AAQ) [42, 43]: Information score | N | 43 | 39 | 82 | |
| Median (Q1, Q3) | 3.6 (3.1, 4.1) | 3.8 (3.3, 4.1) | 3.7 (3.2, 4.1) | ||
| IMB-AAQ: Motivation scale | N | 43 | 39 | 82 | |
| Median (Q1, Q3) | 3.3 (2.7, 4.1) | 3.0 (2.6, 3.9) | 3.2 (2.7, 4.0) | ||
| IMB-AAQ: Personal motivation scale | N | 43 | 39 | 82 | |
| Median (Q1, Q3) | 2.8 (2.3, 4.0) | 2.5 (2.0, 3.8) | 2.8 (2.0, 3.8) | ||
| HIV adherence self-efficacy scale (HIV-ases) [44, 45]: Overall self-efficacy | N | 43 | 39 | 82 | |
| Median (Q1, Q3) | 5.3 (5.0, 6.0) | 5.3 (5.0, 6.3) | 5.3 (5.0, 6.0) |
Study Status as of COVID-19 Pause
By March 20, 2020, all 88 participants in the analysis population were evaluable for Week 12 VS analyses (i.e., had been enrolled at least 14 weeks before the study pause), 87 for Week 24, 68 for Week 36, and 54 for Week 48. Thirty-nine (44%) participants (22 in the TERA arm and 17 in the SOC arm) had completed the Week 48 clinic visit and 29 (33%) remained on study (Fig. 1). Seven (8%) participants went off study before 14 weeks of follow-up and an additional 13 went off study after Week 14 and before completing the Week 48 visit (Fig. 1). Reasons for early study discontinuation included three deaths, 14 losses-to-follow-up, and three withdrawals. Time to off study did not differ by arm (Logrank p = 0.31).
Attendance at each study visit among participants on study was above 85%, although visits were not always within the required visit windows. Among participants who attended study visits, only three ACASIs (one at study entry), two qualitative interviews, and two coaching sessions were not completed. Problems with the EDM device were reported for eight SOC and nine TERA arm participants over the course of the study. Two participants in the SOC arm and five in the TERA arm required new devices due to devices being lost, stolen or otherwise no longer available.
Adverse Events
Up to the COVID-19 study pause, ten serious adverse events (SAEs) in seven participants had been reported and none were related to the study. In the SOC arm, three participants (7%) experienced one SAE each: cardiac event resulting in death at 35 weeks follow-up (not HIV-related), gunshot resulting in death at 51 weeks (not HIV-related), and hospitalization due to esophageal candidiasis and pelvic inflammatory disease. In the TERA arm, four participants (9%) experienced seven SAEs: abscess, death due to AIDS complications at 13 weeks (HIV-related), pneumothorax (twice), and one participant with herpes simplex virus proctitis, pneumonia and cellulitis. No additional SAEs were reported between March 2020 and when follow-up was completed in October 2020.
Intervention Effect on Virologic Success at Week 12
All 88 participants were included in the Week 12 analysis, including the one HIV-related death which occurred within the Week 12 study visit window. Virologic success (primary: HIV-1 RNA < 200 copies/ml measured between weeks 10–14) was achieved in 16 of 45 (36%; 95% CI 22%, 51%) SOC arm participants and 15 of 43 (35%; 95% CI 21%, 51%) TERA arm participants (Table 2). The difference between arms (TERA–SOC) was − 1% (95% CI − 21%, 20%; p > 0.99). About half (56%) the failures were HIV-1 RNA measurements ≥ 200 copies/ml (62% in the SOC arm and 50% in the TERA arm). One third (32%) of participants had no HIV-1 RNA measurements available within a wider window of 8–18 weeks (31% in the SOC arm and 32% in the TERA arm). The rest (12%) had an HIV-1 RNA < 200 copies/ml measurement within the wider window (7% in the SOC arm and 18% in the TERA arm). The potential impact of these small imbalances in reasons for failure were explored through the two supplementary analyses described in the next paragraph. Numbers of participants within sites were small (Table 1) and differences between arms within sites highly variable, ranging from – 32–50% (data not shown).
Table 2.
Percent (95% confidence interval (CI)) of participants achieving primary and secondary VS outcome measures by study arm (Triggered escalating real-time adherence intervention [TERA] versus standard of care [SOC])
| Outcome | Week | Arm | VS (N) | Total (N) | VS (%) | VS (%) (95% CI) | TERA–SOC | pa | |
|---|---|---|---|---|---|---|---|---|---|
| % | (95% CI) | ||||||||
| HIV-1 RNA < 200 copies/ml at 12 weeks | |||||||||
| ± 2 weeks (Primary MEF) | 12 | SOC | 16 | 45 | 35.6 | (21.9, 51.2) | |||
| TERA | 15 | 43 | 34.9 | (21.0, 50.9) | − 0.7 | (− 20.9, 19.6) | > 0.99 | ||
| ± 2 weeks (Complete case) | 12 | SOC | 16 | 36 | 44.4 | (27.9, 61.9) | |||
| TERA | 15 | 34 | 44.1 | (27.2, 62.1) | − 0.3 | (− 24.6, 23.1) | > 0.99 | ||
| 8—18 weeks (Wide window, MEF) | 12 | SOC | 18 | 45 | 40.0 | (25.7, 55.7) | |||
| TERA | 20 | 43 | 46.5 | (31.2, 62.3) | 6.5 | (− 14.9, 27.1) | 0.67 | ||
| HIV-1 RNA < 50 copies/ml at 12 weeks | |||||||||
| ± 2 weeks (Primary MEF) | 12 | SOC | 11 | 45 | 24.4 | (12.9, 39.5) | |||
| TERA | 9 | 43 | 20.9 | (10.0, 36.0) | − 3.5 | (− 21.8, 15.2) | 0.80 | ||
| ± 2 weeks (Complete case) | 12 | SOC | 11 | 36 | 30.6 | (16.3, 48.1) | |||
| TERA | 9 | 34 | 26.5 | (12.9, 44.4) | − 4.1 | (− 25.4, 17.7) | 0.79 | ||
| 8—18 weeks (Wide window, MEF) | 12 | SOC | 13 | 45 | 28.9 | (16.4, 44.3) | |||
| TERA | 12 | 43 | 27.9 | (15.3, 43.7) | − 1.0 | (− 20.1, 18.4) | > 0.99 | ||
| HIV-1 RNA < 200 copies/ml at 24, 36 and 48 weeks | |||||||||
| ± 4 weeks (MEF) | 24 | SOC | 18 | 44 | 40.9 | (26.3, 56.8) | |||
| TERA | 12 | 43 | 27.9 | (15.3, 43.7) | − 13.0 | (− 32.7, 7.3) | 0.26 | ||
| 36 | SOC | 7 | 32 | 21.9 | (9.3, 40.0) | ||||
| TERA | 12 | 36 | 33.3 | (18.6, 51.0) | 11.5 | (− 11.2, 32.8) | 0.42 | ||
| 48 | SOC | 8 | 25 | 32.0 | (14.9, 53.5) | ||||
| TERA | 8 | 29 | 27.6 | (12.7, 47.2) | − 4.4 | (− 29.5, 20.7) | 0.77 | ||
| ± 4 weeks (Complete case) | 24 | SOC | 18 | 34 | 52.9 | (35.1, 70.2) | |||
| TERA | 12 | 31 | 38.7 | (21.8, 57.8) | − 14.2 | (− 37.9, 10.5) | 0.32 | ||
| 36 | SOC | 7 | 22 | 31.8 | (13.9, 54.9) | ||||
| TERA | 12 | 29 | 41.4 | (23.5, 61.1) | 9.6 | (− 18.7, 35.5) | 0.57 | ||
| 48 | SOC | 8 | 16 | 50.0 | (24.7, 75.3) | ||||
| TERA | 8 | 20 | 40.0 | (19.1, 63.9) | − 10.0 | (− 41.9, 23.3) | 0.74 | ||
| ± 6 weeks (Wide windows, MEF) | 24 | SOC | 19 | 44 | 43.2 | (28.3, 59.0) | |||
| TERA | 13 | 43 | 30.2 | (17.2, 46.1) | − 12.9 | (− 32.9, 7.7) | 0.27 | ||
| 36 | SOC | 10 | 32 | 31.3 | (16.1, 50.0) | ||||
| TERA | 13 | 36 | 36.1 | (20.8, 53.8) | 4.9 | (− 18.3, 27.7) | 0.80 | ||
| 48 | SOC | 9 | 25 | 36.0 | (18.0, 57.5) | ||||
| TERA | 8 | 29 | 27.6 | (12.7, 47.2) | − 8.4 | (− 33.6, 17.3) | 0.57 | ||
| HIV-1 RNA < 50 copies/ml at 24, 36 and 48 weeks | |||||||||
| ± 4 weeks (MEF) | 24 | SOC | 16 | 44 | 36.4 | (22.4, 52.2) | |||
| TERA | 10 | 43 | 23.3 | (11.8, 38.6) | − 13.1 | (− 32.1, 7.2) | 0.24 | ||
| 36 | SOC | 5 | 32 | 15.6 | (5.3, 32.8) | ||||
| TERA | 7 | 36 | 19.4 | (8.2, 36.0) | 3.8 | (− 16.0, 22.6) | 0.76 | ||
| 48 | SOC | 6 | 25 | 24.0 | (9.4, 45.1) | ||||
| TERA | 8 | 29 | 27.6 | (12.7, 47.2) | 3.6 | (− 21.8, 27.4) | > 0.99 | ||
| ± 4 weeks (Complete case) | 24 | SOC | 16 | 34 | 47.1 | (29.8, 64.9) | |||
| TERA | 10 | 31 | 32.3 | (16.7, 51.4) | − 14.8 | (− 38.7, 9.5) | 0.31 | ||
| 36 | SOC | 5 | 22 | 22.7 | (7.8, 45.4) | ||||
| TERA | 7 | 29 | 24.1 | (10.3, 43.5) | 1.4 | (− 24.2, 26.2) | > 0.99 | ||
| 48 | SOC | 6 | 16 | 37.5 | (15.2, 64.6) | ||||
| TERA | 8 | 20 | 40.0 | (19.1, 63.9) | 2.5 | (− 30.8, 34.5) | > 0.99 | ||
| HIV-1 RNA < 50 copies/ml | |||||||||
| ± 6 weeks (Wide windows, MEF) | 24 | SOC | 17 | 44 | 38.6 | (24.4, 54.5) | |||
| TERA | 11 | 43 | 25.6 | (13.5, 41.2) | − 13.1 | (− 32.4, 7.2) | 0.25 | ||
| 36 | SOC | 8 | 32 | 25.0 | (11.5, 43.4) | ||||
| TERA | 8 | 36 | 22.2 | (10.1, 39.2) | − 2.8 | (− 24.1, 17.9) | > 0.99 | ||
| 48 | SOC | 7 | 25 | 28.0 | (12.1, 49.4) | ||||
| TERA | 8 | 29 | 27.6 | (12.7, 47.2) | − 0.4 | (− 25.4, 24.1) | > 0.99 | ||
| Sustained HIV-1 RNA < 200 copies/ml Weeks 12–48 | |||||||||
| SOC | 2 | 25 | 8.0 | (1.0, 26.0) | |||||
| TERA | 4 | 29 | 13.8 | (3.9, 31.7) | 5.8 | (− 14.6, 25.3) | 0.67 | ||
Complete case—based on available viral load data (missing excluded); Wide windows—extended windows to include any available viral load data within ± 6 weeks from study visit (− 4– + 6 weeks for Week 12 timepoint)
VS viral suppression per definition of outcome measure, MEF missing equals failure
aFisher’s exact p-value
Using only complete case HIV-1 RNA measurements within the Week 12 window, VS rates were 44% in both arms. Expanding the allowable window for viral load data while retaining the missing equals failure approach (wide window) resulted in a slight but non statistically significant advantage for those in the TERA arm (47%; 95% CI 31%, 62%) versus (40%; 95% CI 26%, 56%) in the SOC arm (p = 0.67). Also presented in Table 2 are results of analyses using the more stringent VS definition of HIV-1 RNA < 50 copies/ml. VS rates were comparable across approaches, with small, non-statistically significant advantages in the SOC arm.
No covariates (those summarized in Table 1) met criteria to be included in adjusted analyses. In an unadjusted log binomial model, the relative risk of achieving HIV-1 RNA < 200 copies/ml (± 2 weeks, primary) for TERA to SOC was 0.98 (95% CI 0.56, 1.73). In all log binomial regression models with study arm and each covariate identified a prior for inclusion in adjusted analyses (age (years), mode of transmission, and sex at birth), the magnitude of the risk ratios (RR) for TERA to SOC changed very little (a maximum of 0.03). Older YLWH were less likely to achieve VS using the wide window outcome (age (years): RR = 0.91; 95% CI 0.85, 0.98; p = 0.014 and age ≥ 21 years vs. < 21 years: RR = 0.67; 95% CI 0.42, 1.07; p = 0.09). Neither sex at birth nor mode of infection were associated with levels of viral suppression at Week 12.
Intervention Effect on Virologic Suppression at Weeks 24, 36 and 48
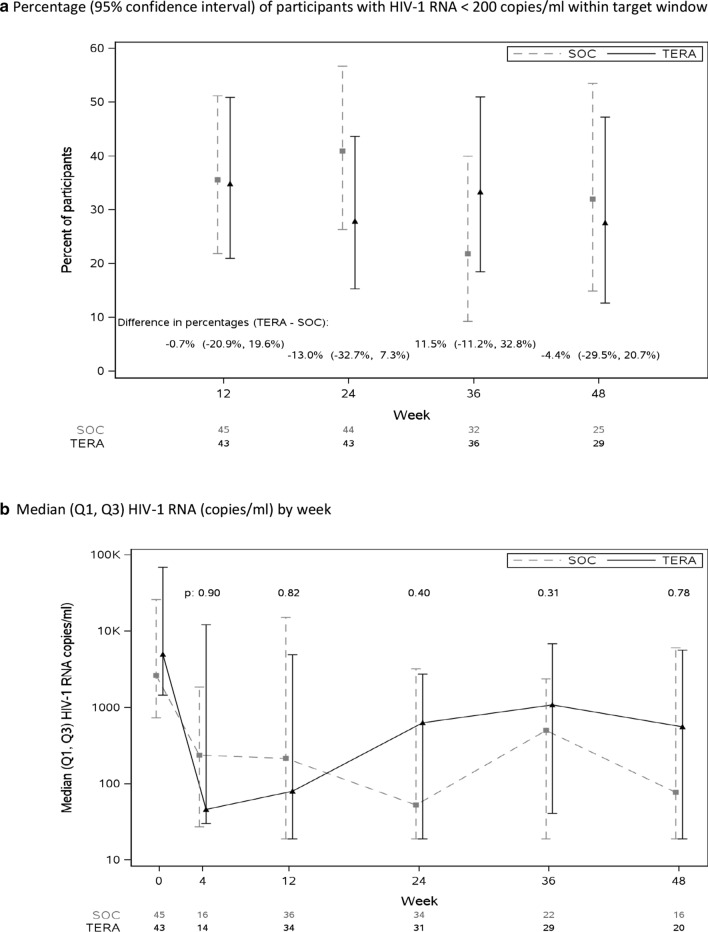
Figure 2 (primary) and Table 2 show cross-sectional percentages of participants with HIV-1 RNA < 200 copies/ml from Weeks 12–48. There were no consistent differences between arms across study visits (Fig. 2a). At least half the failures at each timepoint were HIV-1 RNA measurements ≥ 200 copies/ml within ± 4 weeks (58% at Week 24, 57% at Week 36 and 50% at Week 48), with higher percentages in the TERA arm, i.e., more failures due to missing HIV-1 measurements in the SOC arm.
Fig. 2.
Viral Suppression and Viral Load Outcomes
In repeated measures log binomial regressions, the RR of viral suppression across Weeks 12 to 48 for the primary VS outcome (HIV-1 RNA < 200 copies/ml) for TERA to SOC was 0.90 (95% CI 0.58, 1.41). This RR varied little in models adjusted for age, mode of transmission, or sex at birth. As with the cross-sectional analysis at Week 12, older age was associated with a lower risk of achieving HIV-1 RNA < 200 copies/ml (wide window) (age (years): RR = 0.94; 95% CI 0.88, 0.99; p = 0.032) and age ≥ 21 years vs. < 21 years: RR = 0.72; 95% CI 0.48, 1.06; p = 0.09). There were marginally significant associations of sex at birth with HIV-1 RNA < 200 (and < 50) copies/ml (complete case), with females less likely to achieve VS than males (HIV-1 RNA < 200 copies/ml RR for females vs. males = 0.69; 95% CI 0.45, 1.05; p = 0.09 and for HIV-1 RNA < 50 copies/ml = 0.63; 95% CI 0.38, 1.03; p = 0.07).
Intervention Effect on Sustained Virologic Suppression
Of the 54 participants with the opportunity for follow-up to Week 48, 14% (4 of 29) in the TERA arm and 8% (2 of 25) in the SOC arm achieved consistent HIV-1 RNA < 200 copies/ml (TERA–SOC: 6%; 95% CI − 15%, 25%; p = 0.67) (Table 2). Among the 31 participants with HIV-1 RNA < 200 copies/ml at Week 12, 15 had the opportunity for 48 weeks of follow-up. Of the 15, two of five in the SOC arm (40%; 95% CI 5%, 85%) and four of 10 in the TERA arm (40%; 95% CI 12%, 74%) had sustained virologic control through Week 48.
Intervention Effect on HIV-1 RNA (Continuous)
Median [Q1, Q3] log10 HIV-1 RNA (copies/ml) by week is graphed in Fig. 2b. There were no statistically significant differences between the arms in HIV-1 RNA levels at any timepoint (p ≥ 0.31). However, over half the participants in each arm experienced at least a 1 log10 HIV-1 RNA decline at Week 4 (TERA 64%, SOC 50%), 12 (TERA 59%, SOC 53%), and 24 (TERA 52%, SOC 59%).
Intervention Effect on Post COVID-19 Pandemic Related Pause Week 48 Viral Suppression
By the time study visits resumed after the COVID-19 pandemic related pause, all participants were approaching or past their Week 48 visit. Using all HIV-1 RNA data, the proportion of participants with VS at Week 48 (using the primary (MEF) definition including all enrolled participants not dying from non HIV-related causes (N = 86)) in the TERA arm was 26% (95% CI 14%, 41%) versus 23% (12%, 39%) in the SOC arm (p > 0.99). Proportions able to achieve (by Week 12) and maintain HIV-1 RNA through Week 48 were 12% (95% CI 4%, 25%) in the TERA arm and 7% (95%CI 2%, 19%) in the SOC arm (p = 0.71). These results were consistent with results in the pre-COVID-19 pandemic related timepoint analysis showing no differences between arms in long term VS.
EDM Openings and Association with VS
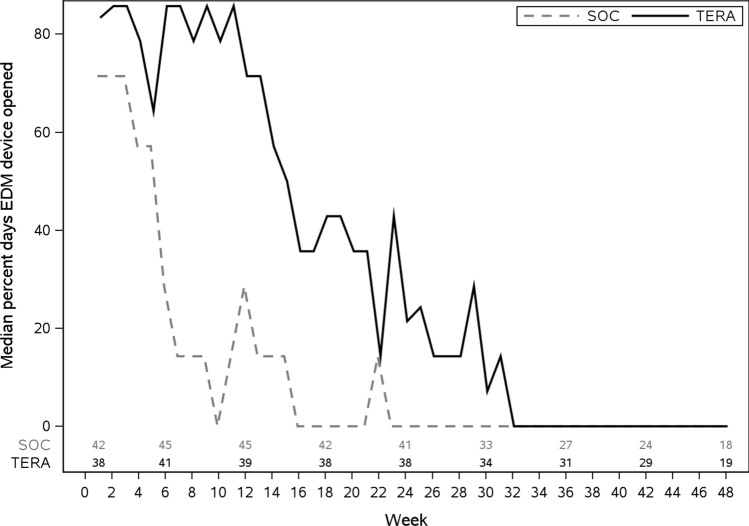
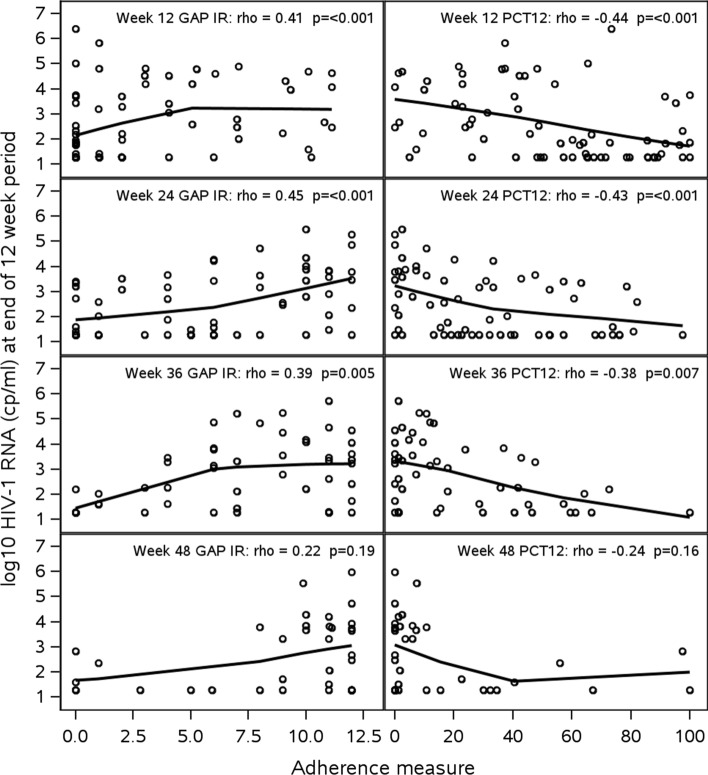
There were statistically significant associations between VS (HIV-1 RNA < 200 copies/ml) and adherence per EDM dosing (PCT12) over the initial 12 weeks (52% virally suppressed among those with 80% or higher adherence (11/21) versus 30% among those with lower than 80% adherence (20/66); Wilcoxon p-value < 0.001), and with occurrence of at least one gap of at least 7 days between openings (51% of participants with no gaps (20/39) were suppressed versus 23% among those with at least one gap (11/48), Wilcoxon p-value 0.001). As illustrated in Fig. 3, the median percentage of days opening the EDM device by week declined in both arms over time. To determine the appropriateness of using EDM data to characterize adherence at more distal timepoints, we evaluated associations of the 12 week summary adherence measures (PCT12 and GAP IR) with viral load over the course of the study. Figure 4 shows scatter plots of HIV-1 RNA levels against each adherence summary measure with a LOESS curve superimposed (smoothing parameter of 95%). HIV-1 RNA levels were consistently negatively correlated with PCT12 and positively correlated with GAP IR. Associations were statistically significant (p < 0.05) up to Week 36 for both measures (ρ ≥ 0.39 for GAP IR and ρ ≤ − 0.38 for PCT12). Accordingly, arm comparisons of EDM data were conducted up to Week 36.
Fig. 3.
Median percent days EDM device opened by week
Fig. 4.
Scatterplot (LOESS line) of log10 HIV-1 RNA vs. GAP IR and percent days opened (PCT12) by 12-week period
Intervention Effects on Adherence and Gaps in Adherence
As presented in Table 2, the distribution of PCT12 during the intervention period was higher for participants in the TERA arm (median [Q1, Q3] of 72% [47%, 89%]) versus 41% [21%, 59%] in the SOC arm (p < 0.001). Adherence in the TERA arm remained significantly higher than the SOC arm during the 12 weeks following the intervention. GAP IRs were significantly lower in the TERA arm (TERA to SOC IR ratio 0.40; 95% CI 0.30, 0.53) during the intervention (Table 2) and remained significantly lower for the 12 weeks following the intervention.
Discussion
In our sample of YLWH in the US with detectable viral load despite being prescribed ART at least 24 weeks, while a 12 week Triggered Escalating Real-Time Adherence intervention compared to standard of care was associated with improvement in indicators of EDM assessed adherence to medications, there was no association with improved viral load suppression. It is sobering to note that achieving virologic success at 12 weeks (HIV-1 RNA < 200 copies/mL within a 4 week window) was realized by only about 35% of study participants (Table 3).
Table 3.
Adherence outcomes by study arm (Triggered escalating real-time adherence intervention (TERA) versus standard of care (SOC))
| Adherence summary | Weeks | SOC median (Q1, Q3) | TERA median (Q1, Q3) | pa | |
|---|---|---|---|---|---|
| % doses taken (PCT12) | 0–12 | 41 (21, 59) | 72 (47, 89) | < 0.001 | |
| 13–24 | 14 (1, 32) | 41 (11, 70) | < 0.001 | ||
| 25–36 | 2 (0, 14) | 17 (2, 41) | 0.06 |
| Adherence summary | Weeks | IR (95% CI) | IR (95% CI) | TERA to SOC IR Ratiob | pb |
|---|---|---|---|---|---|
| GAP IR | 0–12 | 4.2 (3.6, 4.8) | 1.7 (1.3, 2.1) | 0.40 (0.30, 0.53) | < 0.001 |
| 13–24 | 6.9 (6.2, 7.8) | 4.4 (3.8, 5.2) | 0.64 (0.53, 0.77) | < 0.001 | |
| 25–36 | 8.2 (7.3, 9.2) | 6.7 (5.8, 7.6) | 0.81 (0.68, 0.97) | 0.02 |
PCT12 percent doses taken over the 12-week period, IR incidence rate
aWilcoxon p-value
bWald confidence intervals and p-values from generalized linear model with Poisson link
ART attributes and participant characteristics may provide some insight into why the TERA intervention was not associated with improved virologic success (primary outcome measure) despite an association between EDM-estimated adherence and virologic success, and significantly higher adherence (secondary outcome measure) in the TERA arm. As an overarching consideration, contemporary combination ART regimens are highly efficacious when taken and relatively forgiving to non-adherence in the short term. In hindsight, the 12 week intervention period may have been too short to assess the impact of the TERA intervention. The study participants were treatment experienced, some to multiple regimens. Nearly half (44%) had perinatally acquired HIV, 30% were on or beyond their third ART regimen, and three in four participants continued on the same ART regimen they were on prior to study entry. These ART history attributes may have blunted virologic response in the short run and with it the ability to ascertain a clinically meaningful difference in viral suppression between the two study arms within the 12 week intervention period. At the same time, the greater than 1 log10 drop in HIV-1 RNA relative to baseline through 12 weeks in more than half the participants in both study arms demonstrates the effect of participating in the study and with it for many study participants, potentially re-engaging in care. This may have further blunted the ability to ascertain differences in HIV-1 viral load in the short-term between the two study arms. We examined the possibility of whether youth in the TERA arm may have made an effort to open and close the device to “quiet” coach outreach or just because they were aware of the study’s “ask” to do so. While the possibility of such cannot be ruled out, the significant association of the EDM data with virologic suppression at week 12, would indicate that if such “gaming” of the system was being done by the participants, it was an exception rather than the norm and unlikely to explain the results.
Because using the EDM device waned over time, with very few devices in use after 36 weeks, robust longitudinal modeling of EDM adherence and viral load outcomes was not possible. Other intervention research has found similar effects on adherence in the absence of viral load effects [14, 46–50], although these were typically with self-reported adherence measures. Reasons for affecting EDM measured adherence but not viral load are not clear. In addition to adherence, factors influencing viral suppression include viral load at diagnosis, history with viral suppression, suboptimal pharmacokinetics, lower genetic barriers to resistance, drug resistance and drug-drug interactions [51]. Resistance testing, pharmacokinetics and other relevant moderators or mediators of an adherence-to-viral load relationship were not assessed in this study.
Study limitations include the small sample size, which limited ability to evaluate the intervention in relation to geographic or clinic-level differences. The study did not reach enrollment targets (n = 120) due to slow accrual. Participating network sites were all well-established clinical care centers and had variable success reaching and enrolling viremic youth who are often not engaged in clinical HIV-care. We used a composite outcome measure for viral success, which required attendance at clinics for viral load testing within relatively narrow permissible study windows, and which proved difficult. For 44% of participants, failure was because we had no viral load result within the 2 week window surrounding the Week 12 study visit. A planned sensitivity analysis using a wider window from 8 to 18 weeks but retaining the missing equals failure criterion, resulted in 32% of participants with missing values set to failure. Using wider windows and using only complete cases yielded results similar to the primary analysis, so there were no indications the primary results were biased by differential missingness by arm. Our intervention focused on engaging youth both at clinic visits, and extensively outside of clinic visits, with monitoring and outreach. The focus on adherence did not expressly include retention in care. Future interventions that either facilitate viral load monitoring outside of a clinical care setting (e.g., home testing) or that incorporate strategies to assist in showing up for clinical care visits should be considered.
EDM data, while high resolution compared to snapshot assessments based on participant recall, also had some limitations when estimating adherence. Although EDM devices similar to the one used in this study have been used in numerous studies and amassed considerable validation data, they have limitations [51]. Such devices can create a ‘bump’ in adherence (increased adherence through novelty of and reactivity to the device [52]) that dissipates around 40 days into use [53], they measure adherence related behaviors rather than ingestion of a dose, and require adherence to the monitoring device itself [25]. In our study, we saw an initial drop in viral load in both arms, which may have been facilitated by study participation, and specifically use of the EDM bottle. Over time, however, participants did appear to disengage with the EDM. Limited research is available for validity of EDM data over extended periods of time [54]. We observed periods of association between EDM dosing data and viral outcomes, and later periods when the EDM was only passively monitored and youth appeared to have largely abandoned its use. In these later periods there were no associations between EDM and viral load.
Overall, our results show improvement in ART adherence during the active TERA intervention phase that was not sustained post intervention. A similar tapering of benefits post intervention has been reported with directly observed therapy interventions [55], which share some similarity with TERA in daily monitoring of dosing. While the results did not indicate an effect on viral suppression, the impact on improved adherence is clinically meaningful and warrants future studies to examine alternate approaches to deploy the TERA intervention including longer or tailored/titrated or pulsed delivery. Investigations into the potential impact of the intervention on psychosocial factors (e.g., adherence related information, motivation or skills, decision making, emotional regulation, mental health or stigma) are underway. Qualitative data are being examined for perceived impact of the intervention and related recommendations. With the recent proliferation of mHealth remote service delivery strategies due to the COVID pandemic, components of TERA, such as use of a remotely located coach, as-needed SMS-based outreach and video-conference support sessions, are well-matched to services offered outside of brick-and-mortar clinical care sites. Based on our experiences with implementation of TERA and data gathered to date, adaptations to the intervention that could potentially improve adherence further and assist youth in reaching viral suppression include: (1) untethering the coaching session from attendance in clinic, allowing for more and as-needed full coaching sessions in places of highest convenience for youth, (2) opening communication channels between remote coaches and the on-site clinical care team to coordinate efforts or alert local outreach teams of potential gaps in adherence, and (3) allowing greater flexibility in implementation which would allow for shortening the intervention experience for those who do not “need” the support and elongating it for those who do. There are a multitude of barriers to optimal adherence to medications in youth living with HIV and tailoring of interventions to improve adherence to each individual remains important. We have shown the feasibility of the TERA intervention and its short-term impact on adherence. Pending the noted additional studies the TERA intervention holds promise both as an adherence improvement intervention in itself as well as providing the platform to incorporate and deliver other intervention strategies.
Acknowledgements
This manuscript is presented on behalf of and thanks to the entire TERA Study Team, with staff and collaborators from all research sites (Bronx-Lebanon Hospital Center, Emory, Johns Hopkins University, South Florida CDTC, St. Jude Children’s Hospital, University of Colorado Denver Children’s Hospital, University of Florida Jackson, Wayne State University, University of Alabama, and University of California Los Angeles), youth advisory board members, program management staff at the University of North Carolina’s Collaborative Studies Coordinating Center, NICHD program officer, data management and analysis collaborators at Frontier Science Foundation and the Harvard T.H. Chan School of Public Health, and the intervention and project implementation and qualitative data analysis teams at the University of Michigan.
Author Contributions
All authors made contributions to the implementation of the study and interpretation of study findings. Conceptualization and Methods [KRA, JCL, MH, RD, KJH, AD, RG, MMJ, BH, JC, AG]; Site-level Implementation [ES, MP, DR, MR, LGR, AG]; Implementation Support [AD, RG, BH]; Primary Analyses [JCL]; Results Interpretation [KRA, JCL, MH, AG]; Original Draft [KRA, JCL]; Review, Editing and Finalization of manuscript [KRA, JCL, MH, RD, KJH, AD, RG, MMJ, BH, JC, ES, MP, DR, MR, LGR, AG].
Funding
This study is supported by the Adolescent Medicine Trials Network for HIV/AIDS Interventions (ATN) from the National Institutes of Health (U24HD089880) through the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), National Institute on Minority Health and Health Disparities (NIMHD), National Institute of Mental Health (NIMH), and National Institute on Drug Abuse (NIDA).
Declarations
Conflict of interest
The authors report no financial interests and no conflicts of interest relevant to the subject matter discussed in this manuscript.
Ethical Approval
Ethical approval was obtained by the Institutional Review Board at the University of North Carolina (location of the coordinating center) as the single IRB of reference, and clinic and other participating sites and institutions reviewed all approved materials and protocols. This research was performed in line with the principles of the Declaration of Helsinki.
Informed Consent
Written informed consent was obtained from all participants included in the study. The content in this manuscript is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Harris NS, Johnson AS, Huang YA, Kern D, Fulton P, Smith DK, et al. Vital signs: status of human immunodeficiency virus testing, viral suppression, and HIV preexposure prophylaxis - United States, 2013–2018. MMWR Morb Mortal Wkly Rep. 2019;68(48):1117–1123. doi: 10.15585/mmwr.mm6848e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. HIV surveillance report, 2018 (Updated); vol. 31. Published May 2020.
- 3.Kapogiannis BG, Koenig LJ, Xu J, Mayer KH, Loeb J, Greenberg L, et al. The HIV continuum of care for adolescents and young adults attending 13 urban US HIV care centers of the NICHD-ATN-CDC-HRSA SMILE collaborative. J Acquir Immune Defic Syndr. 2020;84(1):92–100. doi: 10.1097/QAI.0000000000002308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. HIV and Youth. 2020. https://www.cdc.gov/hiv/group/age/youth/incidence.html Accessed March 4 2022.
- 5.Centers for Disease Control and Prevention. Monitoring selected national HIV prevention and care objectives by using HIV surveillance data: United States and 6 dependent areas, 2018. HIV Surveillance Report 2020; 25(No. 2). https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-supplemental-report-vol-25-2.pdf Accessed March 4 2022.
- 6.Fauci AS, Redfield RR, Sigounas G, Weahkee MD, Giroir BP. Ending the HIV epidemic: a plan for the United States. JAMA, J Am Med Assoc. 2019;321(9):844–845. doi: 10.1001/jama.2019.1343. [DOI] [PubMed] [Google Scholar]
- 7.Neilan AM, Karalius B, Patel K, Van Dyke RB, Abzug MJ, Agwu AL, et al. Association of risk of viremia, immunosuppression, serious clinical events, and mortality with increasing age in perinatally human immunodeficiency virus-infected youth. JAMA Pediatr. 2017;171(5):450–460. doi: 10.1001/jamapediatrics.2017.0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kacanek D, Huo Y, Malee K, Mellins CA, Smith R, Garvie PA, et al. Nonadherence and unsuppressed viral load across adolescence among US youth with perinatally acquired HIV. AIDS. 2019;33(12):1923–1934. doi: 10.1097/QAD.0000000000002301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foster C, Ayers S, Fidler S. Antiretroviral adherence for adolescents growing up with HIV: understanding real life, drug delivery and forgiveness. Ther Adv Infect Dis. 2020;7:2049936120920177. doi: 10.1177/2049936120920177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shoko C, Chikobvu D. A superiority of viral load over CD4 cell count when predicting mortality in HIV patients on therapy. BMC Infect Dis. 2019;19(1):169. doi: 10.1186/s12879-019-3781-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kahana SY, Rohan J, Allison S, Frazier TW, Drotar D. A meta-analysis of adherence to antiretroviral therapy and virologic responses in HIV-infected children, adolescents, and young adults. AIDS Behav. 2013;17(1):41–60. doi: 10.1007/s10461-012-0159-4. [DOI] [PubMed] [Google Scholar]
- 12.US Food and Drug Administration. FDA Approves First Extended-Release, Injectable Drug Regimen for Adults Living with HIV. US Food and Drug Administration press release December 20 2021. https://www.fda.gov/news-events/press-announcements/fda-approves-first-injectable-treatment-hiv-pre-exposure-prevention#:~:text=Today%2C%20the%20U.S.%20Food%20and,risk%20of%20sexually%20acquired%20HIV. Accessed March 3 2022.
- 13.Lee S, Kapogiannis BG, Allison S. Improving the youth HIV prevention and care continuums: the adolescent medicine trials network for HIV/AIDS interventions. JMIR Res Protoc. 2019;8(3):e12050. doi: 10.2196/12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaw S, Amico KR. Antiretroviral therapy adherence enhancing interventions for adolescents and young adults 13–24 years of age: a review of the evidence base. JAIDS J Acquir Immune Defic Syndr. 2016;72(4):387–399. doi: 10.1097/QAI.0000000000000977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amico KR, Dunlap A, Dallas R, Lindsey J, Heckman B, Flynn P, et al. Triggered escalating real-time adherence intervention to promote rapid HIV viral suppression among youth living with HIV failing antiretroviral therapy: protocol for a triggered escalating real-time adherence intervention. JMIR Res Protoc. 2019;8(3):e11416–e11416. doi: 10.2196/11416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turner JR. New FDA guidance on general clinical trial conduct in the era of COVID-19. Ther Innov Regul Sci. 2020;54:723–724. doi: 10.1007/s43441-020-00160-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pollard V, Ryan M, Geetter F. FDA offers guidance on clinical trials during COVID-19 Pandemic. The Natl Law Rev. 2020;10(84):1205–1214. [Google Scholar]
- 18.Sathian B, Asim M, Banerjee I, Pizarro AB, Roy B, van Teijlingen ER, et al. Impact of COVID-19 on clinical trials and clinical research: a systematic review. Nepal J Epidemiol. 2020;10(3):878–887. doi: 10.3126/nje.v10i3.31622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Asaad M, Habibullah NK, Butler CE. The Impact of COVID-19 on Clinical Trials. Ann Surg. 2020;272(3):e222–e223. doi: 10.1097/SLA.0000000000004113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mara CA, Peugh JL. Validity of data collected from randomized behavioral clinical trials during the COVID-19 pandemic. J Pediatr Psychol. 2020;45(9):971–976. doi: 10.1093/jpepsy/jsaa078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fleming TR, Labriola D, Wittes J. Conducting clinical research during the COVID-19 pandemic: protecting scientific integrity. JAMA J Am Med Assoc. 2020;324(1):33–34. doi: 10.1001/jama.2020.9286. [DOI] [PubMed] [Google Scholar]
- 22.Krukowski B, Arigo D, Graetz I. New Approaches for Conducting Behavioral Research. In; 2020
- 23.US Food and Drug Administration. Statistical Considerations for Clinical Trials During the COVID-19 Public Health Emergency: Guidance for Industry. June 2020. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/statistical-considerations-clinical-trials-during-covid-19-public-health-emergency-guidance-industry Accessed March 3 2022.
- 24.Chow S-C, Zhang W. Statistical evaluation of clinical trials under COVID-19 pandemic. Ther Innov Regul Sci. 2020;54(6):1551–1556. doi: 10.1007/s43441-020-00182-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Denhaerynck K, Schäfer-Keller P, Young J, Steiger J, Bock A, De Geest S. Examining assumptions regarding valid electronic monitoring of medication therapy: development of a validation framework and its application on a European sample of kidney transplant patients. BMC Med Res Methodol. 2008;8:5. doi: 10.1186/1471-2288-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGrady ME, Ramsey RR. Using electronic monitoring devices to assess medication adherence: a research methods framework. J Gen Intern Med. 2020;35(9):2707–2714. doi: 10.1007/s11606-020-05905-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taylor R. Interpretation of the correlation coefficient: a basic review. J Diagn Med Sonogr. 1990;6(1):35–39. doi: 10.1177/875647939000600106. [DOI] [Google Scholar]
- 28.Humeniuk R, Henry-Edwards S, Ali R, Poznyak V, Monteiro MG. The alcohol, smoking and substance involvement screening test (ASSIST): manual for use in primary care/prepared by R HumeniukƯ et al. Geneva: World Health Organization; 2010. [Google Scholar]
- 29.Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CES-D (center for epidemiologic studies depression scale) Am J Prev Med. 1994;10:77–84. doi: 10.1016/S0749-3797(18)30622-6. [DOI] [PubMed] [Google Scholar]
- 30.Bradley KL, Bagnell AL, Brannen CL. Factorial validity of the center for epidemiological studies depression 10 in adolescents. Issues Ment Health Nurs. 2010;31(6):408–412. doi: 10.3109/01612840903484105. [DOI] [PubMed] [Google Scholar]
- 31.Shrout PE, Yager TJ. Reliability and validity of screening scales: effect of reducing scale length. J Clin Epidemiol. 1989;42(1):69–78. doi: 10.1016/0895-4356(89)90027-9. [DOI] [PubMed] [Google Scholar]
- 32.Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med. 1991;32(6):705–714. doi: 10.1016/0277-9536(91)90150-B. [DOI] [PubMed] [Google Scholar]
- 33.Group TE, van Reenen M, Janssen B, Oppe M, Kreimeier S, Greiner W. EQ-5D-Y User Guide: Basic information on how to use the EQ-5D-Y instrument 2014. https://euroqol.org/wp-content/uploads/2019/10/EQ-5D-Y-User-Guide.pdf Accessed March 3 2022.
- 34.Wille N, Badia X, Bonsel G, Burström K, Cavrini G, Devlin N, et al. Development of the EQ-5D-Y: a child-friendly version of the EQ-5D. Qual Life Res. 2010;19(6):875–886. doi: 10.1007/s11136-010-9648-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gross JJ, John OP. Individual differences in two emotion regulation processes: implications for affect, relationships, and well-being. J Pers Soc Psychol. 2003;85(2):348–362. doi: 10.1037/0022-3514.85.2.348. [DOI] [PubMed] [Google Scholar]
- 36.Gullone E, Taffe J. The emotion regulation questionnaire for children and adolescents (ERQ–CA): a psychometric evaluation. Psychol Assess. 2012;24(2):409. doi: 10.1037/a0025777. [DOI] [PubMed] [Google Scholar]
- 37.Tuinstra J, van Sonderen FLP, Groothoff JW, van den Heuvel WJA, Post D. Reliability, validity and structure of the adolescent decision making questionnaire among adolescents in The Netherlands. Pers Indiv Differ. 2000;28(2):273–285. doi: 10.1016/S0191-8869(99)00096-3. [DOI] [Google Scholar]
- 38.Black DS, Sun P, Rohrbach LA, Sussman S. Decision-making style and gender moderation of the self-efficacy-condom use link among adolescents and young adults: informing targeted STI/HIV prevention programs. Arch Pediatr Adolesc Med. 2011;165(4):320–325. doi: 10.1001/archpediatrics.2011.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Earnshaw VA, Chaudoir SR. From conceptualizing to measuring HIV stigma: a review of HIV stigma mechanism measures. AIDS Behav. 2009;13(6):1160–1177. doi: 10.1007/s10461-009-9593-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Earnshaw VA, Bogart LM, Dovidio JF, Williams DR. Stigma and racial/ethnic HIV disparities: moving toward resilience. Am Psychol. 2013;68(4):225–236. doi: 10.1037/a0032705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Earnshaw VA, Smith LR, Chaudoir SR, Amico KR, Copenhaver MM. HIV stigma mechanisms and well-being among PLWH: a test of the HIV stigma framework. AIDS Behav. 2013;17(5):1785–1795. doi: 10.1007/s10461-013-0437-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fisher JD, Amico KR, Fisher WA, Cornman DH, Shuper PA, Trayling C, et al. Computer-based intervention in HIV clinical care setting improves antiretroviral adherence: the LifeWindows project. AIDS Behav. 2011;15(8):1635–1646. doi: 10.1007/s10461-011-9926-x. [DOI] [PubMed] [Google Scholar]
- 43.Amico KR, Fisher WA, Cornman DH, Shuper PA, Redding CG, Konkle-Parker DJ, et al. Vis ual analog scale of ART adherence: association with 3-day self-report and adherence barriers. J Acquired Immune Deficiency Syndromes. 2006;42(4):455–459. doi: 10.1097/01.qai.0000225020.73760.c2. [DOI] [PubMed] [Google Scholar]
- 44.Johnson MO, Neilands TB, Dilworth SE, Morin SF, Remien RH, Chesney MA. The role of self-efficacy in HIV treatment adherence: validation of the HIV Treatment Adherence Self-Efficacy Scale (HIV-ASES) J Behav Med. 2007;30(5):359–370. doi: 10.1007/s10865-007-9118-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Erlen JA, Cha ES, Kim KH, Caruthers D, Sereika SM. The HIV Medication Taking Self-efficacy Scale: psychometric evaluation. J Adv Nurs. 2010;66(11):2560–2572. doi: 10.1111/j.1365-2648.2010.05400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shah R, Watson J, Free C. A systematic review and meta-analysis in the effectiveness of mobile phone interventions used to improve adherence to antiretroviral therapy in HIV infection. BMC Public Health. 2019;19(1):915. doi: 10.1186/s12889-019-6899-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Amico KR. Evidence for Technology Interventions to Promote ART Adherence in Adult Populations: a Review of the Literature 2012–2015. Curr HIV/AIDS Rep. 2015;12(4):441–450. doi: 10.1007/s11904-015-0286-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Amico KR, Harman JJ, Johnson BT. Efficacy of antiretroviral therapy adherence interventions: a research synthesis of trials, 1996 to 2004. J Acquir Immune Defic Syndr. 2006;41(3):285–297. doi: 10.1097/01.qai.0000197870.99196.ea. [DOI] [PubMed] [Google Scholar]
- 49.Simoni JM, Pearson CR, Pantalone DW, Marks G, Crepaz N. Efficacy of interventions in improving highly active antiretroviral therapy adherence and HIV-1 RNA viral load. A meta-analytic review of randomized controlled trials. J Acquir Immune Defic Syndr. 2006;43(1):S23–35. doi: 10.1097/01.qai.0000248342.05438.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hine P, Smith R, Eshun-Wilson I, Orrell C, Cohen K, Leeflang MMG, et al. (2018) Measures of antiretroviral adherence for detecting viral non-suppression in people living with HIV. Cochrane Database Syst Rev. 2018;7:CD013080. doi: 10.1002/14651858.CD013080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Garrison LE, Haberer JE. Technological methods to measure adherence to antiretroviral therapy and preexposure prophylaxis. Curr Opin HIV AIDS. 2017;12(5):467–474. doi: 10.1097/COH.0000000000000393. [DOI] [PubMed] [Google Scholar]
- 52.Sutton S, Kinmonth AL, Hardeman W, Hughes D, Boase S, Prevost AT, et al. Does electronic monitoring influence adherence to medication? Randomized controlled trial of measurement reactivity. Ann Behav Med. 2014;48(3):293–299. doi: 10.1007/s12160-014-9595-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Deschamps AE, De Geest S, Vandamme A-M, Bobbaers H, Peetermans WE, Van Wijngaerden E. Diagnostic value of different adherence measures using electronic monitoring and virologic failure as reference standards. AIDS Patient Care STDS. 2008;22(9):735–743. doi: 10.1089/apc.2007.0229. [DOI] [PubMed] [Google Scholar]
- 54.van Heuckelum M, van den Ende CHM, Houterman AEJ, Heemskerk CPM, van Dulmen S, van den Bemt BJF. The effect of electronic monitoring feedback on medication adherence and clinical outcomes: a systematic review. PLoS ONE. 2017;12(10):e0185453. doi: 10.1371/journal.pone.0185453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gaur AH, Belzer M, Britto P, Garvie PA, Hu C, Graham B, et al. Directly observed therapy (DOT) for nonadherent HIV-infected youth: lessons learned, challenges ahead. AIDS Res Hum Retrovir. 2010;26(9):947–953. doi: 10.1089/aid.2010.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]