Abstract
Background
Fibroblasts produce collagen molecules that support the structure of the skin. The decrease and hypersynthesis of collagen causes skin problems such as skin atrophy, wrinkles and scars.
Objective
The purpose of this study is to investigate the mechanism of mitoxantrone on collagen synthesis in fibroblasts.
Methods
Cultured fibroblasts were treated with mitoxantrone, and then collagen synthesis was confirmed by reverse transcription-polymerase chain reaction and Western blot.
Results
Mitoxantrone inhibited the expression of type I collagen in fibroblasts at both the mRNA and protein levels. In the collagen gel contraction assay, mitoxantrone significantly inhibited gel contraction compared to the control group. Mitoxantrone inhibited transforming growth factor (TGF)-β-induced phosphorylation of SMAD3. Finally, mitoxantrone inhibited the expression of LARP6, an RNA-binding protein that regulates collagen mRNA stability.
Conclusion
These results suggest that mitoxantrone reduces collagen synthesis by inhibiting TGF-β/SMAD signaling and LARP6 expression in fibroblasts, which can be developed as a therapeutic agent for diseases caused by collagen hypersynthesis.
Keywords: Collagen, Fibroblasts, LARP6, Mitoxantrone, SMAD
INTRODUCTION
Fibroblasts are the main cells that make up the dermis and are responsible for producing and secreting extracellular matrix molecules such as collagen, elastin and proteoglycans1. Among them, type 1 collagen is the most abundant protein that directly affects skin texture. As aging progresses, fibroblasts are less proliferative and collagen synthesis decreases, resulting in skin atrophy and wrinkles2. Conversely, in pathological situations such as keloids, transforming growth factor-β (TGF-β) signal is activated and collagen synthesis increases, so that the scar grows without spontaneous regression3. Because collagen synthesis of fibroblasts is associated with healthy skin texture, cosmetic problems, and pathological conditions, many researchers have attempted to develop substances that can regulate fibroblast activity and collagen synthesis.
Recently, drug repositioning technique has been spotlighted as an alternative method for developing new drugs4,5. This is because it can significantly save time and money spent on drug development. We purchased Screen-Well® FDA approved drug library V2 (Cat #BML-2843-0100; Enzo Life Sciences Inc., Farmingdale, NY, USA). This drug library contains more than 800 compounds that are FDA-approved and are in clinical use, all of which are well-known and well-characterized for their biological activity, safety and bioavailability. We conducted screening test and found that mitoxantrone has the effect of inhibiting collagen synthesis in fibroblasts.
Mitoxantrone is an anthraquinone used to treat several cancers including breast and prostate cancer, lymphoma and leukemia6. Mitoxantrone has been shown to inhibit TGF-β-induced transcription of collagen type I α1 chain (COL1A1) by suppressing the binding of the SP1 transcription factor to the COL1A1 gene promoter in cultured human dermal fibroblasts7. However, the exact action mechanism of mitoxantrone on collagen synthesis is not well understood. In this study, we show that mitoxantrone inhibits SMAD signaling and also inhibits the expression of RNA-binding protein LARP6 (La ribonucleoprotein 6, translational regulator), which is involved in the post-transcriptional regulation of collagen mRNA.
MATERIALS AND METHODS
The study was approved by the Institutional Review Board of the Chungnam National University Hospital (IRB no. 2016-07-009). Written informed consent was obtained from all donors.
Cell culture
Normal human skin fibroblasts were cultured as previously described8. Cells were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) (Welgene, Gyeongsan, Korea) supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin (Thermo Fisher Scientific, Waltham, MA, USA). Mitoxantrone was purchased from Enzo Life Sciences and dissolved as 1,000× concentrate in dimetylsulfoxide (DMSO) and diluted with culture medium. The control group was treated with the same amount of DMSO. For mitoxantrone treatment, cells were seeded in 60-mm culture dish and incubated overnight. The next day, an appropriate amount of mitoxantrone was added directly to the culture medium and the cells were incubated for an additional 24 hours. For treatment with TGF-β, cells were seeded in 60-mm culture dish and incubated overnight. Then, the cells were washed twice with phosphate-buffered saline, replaced with serum-free DMEM, and incubated overnight. The next day, TGF-β (R&D systems, Minneapolis, MN, USA) was added directly to the culture medium. Cells were cultured for an additional 1 hour (for detection of phosphor-SMAD) or 24 hours (for detection of collagens, LARP6, and HNRNPK [heterogeneous nuclear ribonucleoprotein K]).
Cell viability test
To examine the effect on cell viability, MTT assay was performed. Normal human skin fibroblasts were seeded in 12-well culture plate at a density of 1×105/well and treated with mitoxantrone for 24 hours. Then medium was replaced with fresh medium containing 0.5 mg/ml 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT). Cells were incubated for an additional 4 hours, and then formazan crystal was dissolved in DMSO. The optical density was measured at 570 nm using a microplate reader.
Reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted from fibroblasts using Easy-blue RNA extraction kit (Intron Biotechnology, Seongnam, Korea). Two µg of total RNAs were reversed transcribed with moloney-murine leukaemia virus (M-MLV) reverse transcriptase (RTase) (Elpis Biotech, Daejeon, Korea). A portion of cDNA was taken and used for the PCR reaction. The sequences for primers were as follows: COL1A1, 5′-ccaaatctgtctccccagaa and 5′-tcaaaaacgaaggggagatg; COL1A2, 5′-ctgcaagaacagcattgcat and 5′-ggcgtgatggcttatttgtt; LARP6, 5′-ttacacgggactggagaacc and 5′-cagctctctcccaggtttga; HNRNPK, 5′-atgtccctggcatctgttca and 5′-actgctccccaccttagttc; GAPDH, 5′-GTCAGTGGTGGACCTGACCT and 5′-AGGGGTCTACATGGCAACTG.
Collagen gel contraction assay
Gel contractions assay was performed using a Cell contraction assay kit (Cell Biolabs Inc., San Diego, CA, USA), according to the manufacturer’s protocol. Briefly, 5×105 cells were mixed with collagen gel and polymerized in Transwell permeable supports (Merck KGaA, Darmstadt, Germany). After collagen polymerizaiton, 5 ml of medium was added atop each collagen gel lattice.
Western blot
Cells were harvested by centrifugation and then lysed in Pro-Prep protein extraction solution (Intron Biotechnology). After vigorous pipetting, the extract was centrifuged for 15 minutes at 15,000 rpm. Total protein was measured using a BCA protein assay kit (Thermo Fisher Scientific). Samples (20~30 µg protein per lane) were run on SDS-polyacrylamide gel, transferred to nitrocellulose membrane, and incubated with appropriate antibody at 4℃ overnight with gentle agitation. The blot was then incubated with peroxidase-conjugated secondary antibody for 30 minutes at room temperature and visualized by enhanced chemiluminescence (Intron Biotechnology). The following primary antibodies were used in this study: COL1A1 (sc-8783), COL1A2 (sc-8786), HNRNPK (sc-28380), actin beta (ACTB) (sc-47778) (Santa Cruz Biotechnologies, Santa Cruz, CA, USA); LARP6 (H00055323-B01P; Abnova, Taipei, Taiwan); phosphorylated-SMAD family member 2 (p-SMAD2) (3108S), phosphorylated-SMAD family member 3 (p-SMAD2) (9520S) (Cell Signaling Technology, Danvers, MA, USA).
Statistical analysis
Data were evaluated statistically by one-way ANOVA or Student’s t-test using IBM SPSS software v 22.0 (IBM Corp., Armonk, NY, USA). Statistical significance was set at p<0.05.
RESULTS
Our pilot screening study showed that mitoxantrone inhibits collagen synthesis. To investigate the effect of mitoxantrone on cell viability, normal human skin fibroblasts were treated with various concentrations of mitoxantrone and MTT assay was performed. As a result, mitoxantrone induced cell death at doses of 1 µM or higher (Fig. 1A). To examine the effect of mitoxantrone, on collagen synthesis, we performed RT-PCR and Western blot analysis. As a result of mitoxantrone treatment, the mRNA expression of COL1A1 and COL1A2 decreased in a dose-dependent manner (Fig. 1A). Consistent with these results, the protein levels of COL1A1 and COL1A2 were also decreased by mitoxantrone (Fig. 1B).
Fig. 1. Effect of mitoxantrone on collagen synthesis. (A) Normal human skin fibroblasts were treated with mitoxantrone at the indicated concentrations for 24 hours. MTT assay was performed to determine cell viability. Mitoxantrone induced cell death at doses of 1 µM or higher. (B) Skin fibroblasts were treated with mitoxantrone at the indicated concentration for 24 hours. The mRNA levels of collagen type I α1 chain (COL1A1) and collagen type I α2 chain (COL1A2) were determined by reverse transcription-polymerase chain reaction (RT-PCR). Mitoxantrone decreased the mRNA expression of COL1A1 and COL1A2 in a dose-dependent manner. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a loading control. (C) The protein levels of COL1A1 and COL1A2 were determined by Western blot. Mitoxantrone decreased the protein level of COL1A1 and COL1A2 in a dose-dependent manner. Actin beta (ACTB) was used as a loading control. *p<0.05.
To further verify the effect of mitoxantrone, we performed the collagen gel contraction assay that well reflects collagen production by fibroblasts9. Gel contraction occurred well over time in the control groups (non-treated and DMSO-treated groups). However, gel contraction was remarkably suppressed in the mitoxantrone-treated group (Fig. 2). Together, these data clearly showed that mitoxantrone has the inhibitory effect on collagen production of fibroblasts.
Fig. 2. Collagen gel contraction assay. Collagen gels containing fibroblasts were prepared then treated with 0.5 µM of mitoxantrone for 7 days. Gel contraction was significantly inhibited in mitoxantrone treated group compared to control group. Dimethyl sulfoxide (DMSO) was used as a vehicle control. Right graph shows the results measured from three independent experiments. *p<0.05.
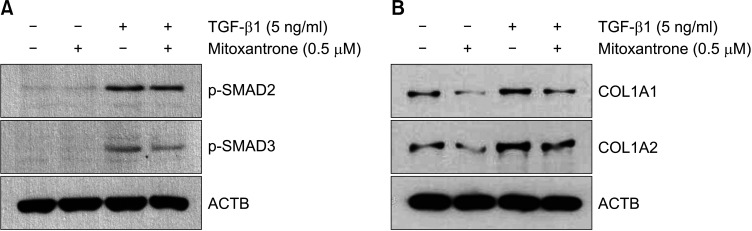
It is well known that TGF-β/SMAD signaling plays a central role in collagen synthesis10,11. To investigate the mechanism of action, we first identified the effect of mitoxantrone on TGF-β/SMAD signaling. When fibroblasts were treated with TGF-β, phosphorylation of SMAD2 and SMAD3 was induced. Pre-treatment of mitoxantrone did not affect TGF-β-induced phosphorylation of SMAD2, whereas phosphorylation of SMAD3 was slightly but significantly inhibited by mitoxantrone (Fig. 3A). As expected, mitoxantrone weakly but significantly inhibited TGF-β-induced collagen synthesis (Fig. 3B).
Fig. 3. Effect of mitoxantrone on transforming growth factor-β (TGF-β)/SMAD signaling. (A) Fibroblasts were treated with mitoxantrone for 1 hour then stimulated with TGF-β for 1 hour. Phosphorylations of SMAD2 and SMAD3 were determined by Western blot. Mitoxantrone slightly inhibited TGF-β-induced phosphorylation of SMAD3. (B) The protein levels of collagen type I α1 chain (COL1A1) and collagen type I α2 chain (COL1A2) were determined by Western blot. Mitoxantrone weakly but significantly inhibited TGF-β-induced collagen synthesis. Actin beta (ACTB) was used as a loading control.
Although mitoxantrone inhibited TGF-β-induced collagen synthesis, we found that mitoxantrone could still inhibit collagen synthesis even in the absence of TGF-β. Therefore, we tried to find other targets that mitoxantrone could have an effect on. Among the factors that regulate collagen synthesis, RNA-binding proteins including LARP6 and HNRNPK are known to play important roles by stabilizing collagen mRNA12,13. Therefore, we examined how mitoxantrone affects the expression of LARP6 and HNRNPK. As a result, mitoxantrone inhibited the expression of LARP6 at both the mRNA and protein levels. However, the expression of HNRNPK was not significantly affected by mitoxantrone (Fig. 4). These results suggest that mitoxantrone influences collagen synthesis by regulating the expression of the RNA-binding protein LARP6 in addition to inhibiting TGF-β/SMAD signaling.
Fig. 4. Effect of mitoxantrone on the expression of RNA-binding proteins. (A) Fibroblasts were treated with mitoxantrone for 1 hour, then stimulated with transforming growth factor-β (TGF-β) for 24 hours. The mRNA levels of LARP6 and HNRNPK were determined by reverse transcription-polymerase chain reaction (RT-PCR). Mitoxantrone inhibited the expression of LARP6 mRNA in both the absence and/or presence of TGF-β. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a loading control. (B) The protein levels of LARP6 and HNRNPK were determined by Western blot. Mitoxantrone weakly but significantly decreased LARP6 level in both the absence and/or presence of TGF-β. Actin beta (ACTB) was used as a loading control.
DISCUSSION
Collagen is an important factor that plays a decisive role in maintaining the shape of the skin. Too little production can cause wrinkles, but too much production can lead to wounds or keloids. From this point of view, there has been a continuous demand for the development of substances capable of regulating collagen synthesis. In this study, we showed that mitoxantrone has the potential to inhibit collagen synthesis in skin fibroblasts. We also demonstrated that mitoxantrone not only inhibits TGF-β/SMAD signaling but also affects the expression of LARP6, which regulates collagen mRNA stability.
The most central intracellular signaling in collagen synthesis is the TGF-β/SMAD pathway, but it has been found that several other important regulators are involved. For example, it has been reported that Wnt/β-catenin signaling contributes to the development of keloids by inducing fibroblast hyperproliferation14,15. Other factors involved in collagen synthesis include RNA-binding proteins such as LARP6 and HNRNPK. It is known that the mRNA of type I collagen has an evolutionarily conserved stem-loop structure in the 5′ untranslated region. LARP6 binds to this 5′ stem-loop in a sequence-specific manner to regulate the stability of collagen mRNA and its translatability. When LARP6 binds to the 5′ stem-loop of type I collagen mRNA, LARP6 acts as an adapter recruiting accessory translational factors, increasing the translational competency of type I collagen mRNA16. In contrast, HNRNPK increases mRNA stability by binding to the CU-rich element of the 3′untranslated region of type I collagen mRNA13. In this study, mitoxantrone inhibits the expression of LARP6, suggesting that mitoxantrone treatment might result in decrease of type I collagen mRNA stability thereby reducing the production of collagen. It is not known how mitoxantrone inhibits LARP6 expression. Interestingly, it has been reported that insulin-like growth factor-1 (IGF-1) promotes the expression of LARP6 via the PI3K/AKT/p70S6K pathway in human aortic smooth muscle cells17. Based on these results, it is possible that mitoxantrone suppresses the expression of LARP6 by inhibiting not only the TGF-β/SMAD signal but also the PI3K/AKT signal. The precise mechanism of action underlying the mitoxantrone-suppressed collagen expression should be investigated further.
In summary, we demonstrated that mitoxantrone inhibits the collagen synthesis in dermal fibroblasts. Our data suggest that mitoxantrone can be developed as therapeutics for diseases caused by hypersynthesis of collagen.
Footnotes
CONFLICTS OF INTEREST: The authors have nothing to disclose.
FUNDING SOURCE: This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2017R1A2B2005612).
DATA SHARING STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J Cell Sci. 2010;123(Pt 24):4195–4200. doi: 10.1242/jcs.023820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shin JW, Kwon SH, Choi JY, Na JI, Huh CH, Choi HR, et al. Molecular mechanisms of dermal aging and antiaging approaches. Int J Mol Sci. 2019;20:2126. doi: 10.3390/ijms20092126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang T, Wang XF, Wang ZC, Lou D, Fang QQ, Hu YY, et al. Current potential therapeutic strategies targeting the TGF-β/Smad signaling pathway to attenuate keloid and hypertrophic scar formation. Biomed Pharmacother. 2020;129:110287. doi: 10.1016/j.biopha.2020.110287. [DOI] [PubMed] [Google Scholar]
- 4.Pushpakom S, Iorio F, Eyers PA, Escott KJ, Hopper S, Wells A, et al. Drug repurposing: progress, challenges and recommendations. Nat Rev Drug Discov. 2019;18:41–58. doi: 10.1038/nrd.2018.168. [DOI] [PubMed] [Google Scholar]
- 5.Zhang QL, Lian DD, Zhu MJ, Li XM, Lee JK, Yoon TJ, et al. Antitumor effect of albendazole on cutaneous squamous cell carcinoma (SCC) cells. Biomed Res Int. 2019;2019:3689517. doi: 10.1155/2019/3689517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evison BJ, Sleebs BE, Watson KG, Phillips DR, Cutts SM. Mitoxantrone, more than just another topoisomerase II poison. Med Res Rev. 2016;36:248–299. doi: 10.1002/med.21364. [DOI] [PubMed] [Google Scholar]
- 7.Gaidarova S, Jiménez SA. Inhibition of basal and transforming growth factor-beta-induced stimulation of COL1A1 transcription by the DNA intercalators, mitoxantrone and WP631, in cultured human dermal fibroblasts. J Biol Chem. 2002;277:38737–38745. doi: 10.1074/jbc.M201742200. [DOI] [PubMed] [Google Scholar]
- 8.Zhou MW, Yin WT, Jiang RH, Lee JH, Kim CD, Lee JH, et al. Inhibition of collagen synthesis by IWR-1 in normal and keloid-derived skin fibroblasts. Life Sci. 2017;173:86–93. doi: 10.1016/j.lfs.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Ngo P, Ramalingam P, Phillips JA, Furuta GT. Collagen gel contraction assay. Methods Mol Biol. 2006;341:103–109. doi: 10.1385/1-59745-113-4:103. [DOI] [PubMed] [Google Scholar]
- 10.Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. FASEB J. 2004;18:816–827. doi: 10.1096/fj.03-1273rev. [DOI] [PubMed] [Google Scholar]
- 11.Bettinger DA, Yager DR, Diegelmann RF, Cohen IK. The effect of TGF-beta on keloid fibroblast proliferation and collagen synthesis. Plast Reconstr Surg. 1996;98:827–833. doi: 10.1097/00006534-199610000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, Stefanovic B. LARP6 meets collagen mRNA: specific regulation of type I collagen expression. Int J Mol Sci. 2016;17:419. doi: 10.3390/ijms17030419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thiele BJ, Doller A, Kähne T, Pregla R, Hetzer R, Regitz-Zagrosek V. RNA-binding proteins heterogeneous nuclear ribonucleoprotein A1, E1, and K are involved in post-transcriptional control of collagen I and III synthesis. Circ Res. 2004;95:1058–1066. doi: 10.1161/01.RES.0000149166.33833.08. [DOI] [PubMed] [Google Scholar]
- 14.Akhmetshina A, Palumbo K, Dees C, Bergmann C, Venalis P, Zerr P, et al. Activation of canonical Wnt signalling is required for TGF-β-mediated fibrosis. Nat Commun. 2012;3:735. doi: 10.1038/ncomms1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sato M. Upregulation of the Wnt/beta-catenin pathway induced by transforming growth factor-beta in hypertrophic scars and keloids. Acta Derm Venereol. 2006;86:300–307. doi: 10.2340/00015555-0101. [DOI] [PubMed] [Google Scholar]
- 16.Stefanovic L, Longo L, Zhang Y, Stefanovic B. Characterization of binding of LARP6 to the 5′ stem-loop of collagen mRNAs: implications for synthesis of type I collagen. RNA Biol. 2014;11:1386–1401. doi: 10.1080/15476286.2014.996467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blackstock CD, Higashi Y, Sukhanov S, Shai SY, Stefanovic B, Tabony AM, et al. Insulin-like growth factor-1 increases synthesis of collagen type I via induction of the mRNA-binding protein LARP6 expression and binding to the 5′ stem-loop of COL1a1 and COL1a2 mRNA. J Biol Chem. 2014;289:7264–7274. doi: 10.1074/jbc.M113.518951. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.