Recent approvals of antisense, exon skipping, and small interfering RNA (siRNA) therapeutics, along with the highly successful mRNA-based COVID-19 vaccines, have invigorated the field of nucleic acid therapeutics and stimulated interest in other mechanisms by which oligonucleotides might elicit therapeutic effects.1 Oligonucleotides can be used to guide Adenosine Deaminases Acting on RNA (ADARs) to correct disease-causing mutations, suggesting the possibility of therapeutic RNA editing.2, 3, 4, 5 The manuscript by Monian and Shivalila et al. recently published in Nature Biotechnology describes the recruitment of endogenously expressed ADARs with short, chemically modified oligonucleotides (AIMers) for efficient directed RNA editing in non-human primates (NHPs) for the first time. Importantly, the authors show that a fully modified 30-nucleotide (nt) AIMer bearing GalNAc to facilitate liver uptake yielded 50% target transcript editing without measurable off-target editing.6 This advance sets the stage for translation of oligonucleotide-directed RNA editing technologies to the clinic.
The ADAR family of enzymes deaminates adenosine (A) to inosine (I) in double-stranded RNA. Inosine is recognized during translation as guanosine (G), thereby affording an A-to-G transition. This is significant because most human genetic variants associated with disease are point mutations, a majority of which could be reversed by A-to-G transitions.7 In addition, recent reports detailing genomic editing of the pathogenic sickle cell hemoglobin allele to another naturally occurring variant has demonstrated the potential to reverse disease phenotypes without restoration of the original codon.8 In fact, single transversion mutations can have implications beyond codon substitutions; to alter translation, skip or induce exons, alter epitranscriptomic modifications, and activate or inactivate enzymes.
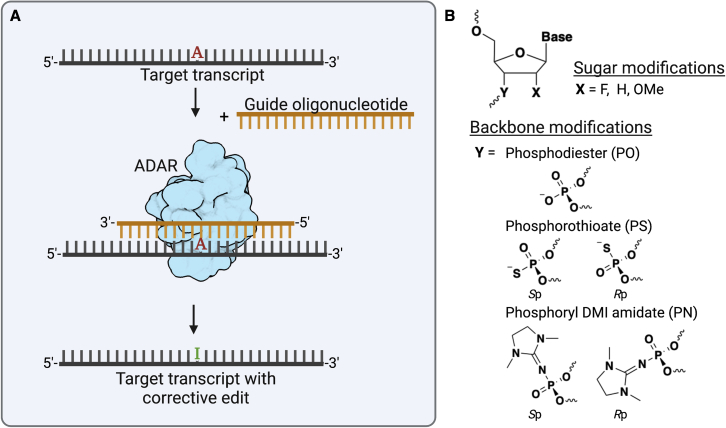
ADARs are uniquely suited for directed editing applications, as they only act on double-stranded substrates. This provides the opportunity to design oligonucleotides that hybridize to a target sequence and thereby guide enzyme activity to a desired adenosine (Figure 1A). In addition, the use of endogenous human enzymes circumvents potential issues that occur with nucleic acid editing technologies requiring the use of exogenous or bacterial-derived enzymes, such as delivery barriers or immune stimulation. Directing edits at the transcript level has some advantages for clinical applications in comparison with genome editing. Of particular importance, any potential off-target transcript editing does not result in a permanent change to the genome.
Figure 1.
Oligonucleotide-directed RNA editing via endogenous ADARs
(A) Scheme depicting the use of guide oligonucleotides to direct endogenous ADAR editing at a target adenosine. (B) Chemical modifications used in guide oligonucleotides (AIMers). Figure created with BioRender.com.
Initial strategies for site-directed RNA editing via ADAR enzymes relied on enzyme overexpression to achieve significant editing levels. However, these approaches resulted in prominent off-target editing and still required exogenous enzyme delivery. More recent reports have shown success in employing endogenous ADAR enzymes for directed editing, primarily by optimizing long guide oligonucleotides (>100 nt) to induce efficient editing in cells.3, 4, 5 Complications observed in cellular studies have included limited editing efficacy and specificity, delivery efficiency, and siRNA-like effects due to the requirement for longer RNAs to achieve desired editing levels. Thus far, in vivo applications have been limited to mouse models, depend on ancillary delivery vehicles, and typically demonstrate relatively low levels of editing (<20%).
Therefore, challenges remain for the development of guide oligonucleotides that can induce efficient directed editing via endogenous ADARs, and effectively translate activity into animal models. It is known that chemical modifications of the guide RNA can impact ADAR activity.9 In their recent study, Monian et al. provided a structure activity relationship analysis of backbone and 2′- modifications in vitro to identify AIMers that would induce efficient editing in cells and in animals. Their primary findings revealed that a phosphorothioate (PS) backbone increases editing, as well as selectively placed 2′-deoxy, 2′-fluoro, and 2′-O-methyl modifications (Figure 1B). In addition, they found that ADAR1 p110 and p150 isoforms are impacted by PS backbone chirality: Sp PS modifications increased activity, while Rp PS had a detrimental effect. The inclusion of neutral phosphoryl DMI amidate (PN) backbone linkages of either stereoisomer (Rp or Sp) also showed an enhancement in editing as compared with AIMers lacking PN linkages. Furthermore, backbone features were found to differentially influence editing between ADAR1 and ADAR2. Modifications that preferentially enhanced ADAR1 activity were carried forward since cellular studies suggested that editing via endogenous ADARs is primarily supported by ADAR1. In addition to optimizing chemical modifications to impact editing activity, they included a GalNAc modification that is known to improve oligonucleotide delivery to hepatocytes via uptake by the asialoglycoprotein receptor.10 In primary hepatocytes, these GalNAc-conjugated AIMers outperformed AIMers without a GalNAc conjugate and were shown to restore protein function of targets implicated in alpha-1 antitrypsin deficiency.
While translation to animal models has been a challenge for directed editing via ADARs, optimized GalNAc-AIMers were found to support efficient editing in vivo. Proof-of-concept studies in NHPs were conducted after finding rapid accumulation of AIMers in mouse liver without signs of hepatotoxicity. In cynomolgus monkeys, AIMers induced up to 50% editing for a target site in the 3′ UTR of the ACTB transcript. While the change in editing over time was distinct for each AIMer, editing persisted at least 45 days post dose. Importantly, there were no signs of hepatotoxicity in NHPs and editing was found to be highly specific. No additional editing was observed within the region of the transcript complementary to the AIMer (bystander region), and RNA sequencing from treated animals revealed that all potential off-target sites identified through in vitro studies had <5% editing in vivo. This serves as the first example of specific and robust editing via endogenous ADARs in NHPs.
This study furthered our understanding of ADAR’s chemical modification tolerance and preferences to enable endogenous editing with guide RNAs shorter than previously reported. This is an important advance given the benefits of shorter oligonucleotides in manufacture and in cell uptake. Chemical modifications used in this study also allowed for the elimination of mismatched and wobble base pairs that were vestiges of the original guide design based on the structure of the well-studied GRIA2 R/G editing site. In addition, the authors assessed the pharmacology of oligonucleotides containing modifications that are common in the field of site-directed RNA editing. Their results suggest that designs containing similar chemical modification patterns may also be amenable in vivo. Serving as an example of a guide RNA design that was successfully translated from in vitro to animal studies, this can act as a model workflow for the rational design of oligonucleotide platforms aiming to achieve robust editing via endogenous ADARs.
The demonstration of relatively high levels of editing in vivo with short oligonucleotides and without ADAR overexpression is likely to encourage the field’s current momentum toward further optimization of chemically modified guide strands. The limited off-target profile also illustrates the motivation for exploring nucleic acid editing technologies capable of employing endogenous enzymes as opposed to ectopic expression, which is required for some Cas-based technologies already in the clinic. While this proof-of-concept study demonstrated effective editing in vivo, one caveat is that it did not show restoration of functional protein in NHPs. Also, the current method demonstrated editing in the NHP liver at a 5′-UAG-3′ sequence in the 3′-UTR of the ACTB mRNA. This model substrate is not therapeutically relevant and the editing site’s location on the transcript (3′-UTR) and its flanking sequence make it relatively low-hanging fruit for targeting by ADARs. Moving forward, more ADAR guide strands need to be tested that target pathogenic mutations for reversing disease phenotypes in animals. These targets must include editing sites within coding sequences, outside the liver, and with non-ideal nearest neighbor nucleotides for ADARs before the full scope and limitations of this approach can be known. Nevertheless, the results reported in Monian et al. represent a highly significant advance on the way to therapeutic oligonucleotides that recruit ADARs to correct disease-causing mutations.
References
- 1.Damase T.R., Sukhovershin R., Boada C., Taraballi F., Pettigrew R.I., Cooke J.P. The limitless future of RNA therapeutics. Front. Bioeng. Biotechnol. 2021;9:628137. doi: 10.3389/fbioe.2021.628137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woolf T.M., Chase J.M., Stinchcomb D.T. Toward the therapeutic editing of mutated RNA sequences. Proc. Natl. Acad. Sci. U S A. 1995;92:8298–8302. doi: 10.1073/pnas.92.18.8298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qu L., Yi Z., Zhu S., Wang C., Cao Z., Zhou Z., Yuan P., Yu Y., Tian F., Liu Z., et al. Programmable RNA editing by recruiting endogenous ADAR using engineered RNAs. Nat. Biotechnol. 2019;37:1059–1069. doi: 10.1038/s41587-019-0178-z. [DOI] [PubMed] [Google Scholar]
- 4.Merkle T., Merz S., Reautschnig P., Blaha A., Li Q., Vogel P., Wettengel J., Li J.B., Stafforst T. Precise RNA editing by recruiting endogenous ADARs with antisense oligonucleotides. Nat. Biotechnol. 2019;37:133–138. doi: 10.1038/s41587-019-0013-6. [DOI] [PubMed] [Google Scholar]
- 5.Katrekar D., Chen G., Meluzzi D., Ganesh A., Worlikar A., Shih Y.-R., Varghese S., Mali P. In Vivo RNA editing of point mutations via RNA-guided adenosine deaminases. Nat. Methods. 2019;16:239–242. doi: 10.1038/s41592-019-0323-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monian P., Shivalila C., Lu G., Shimizu M., Boulay D., Bussow K., Byrne M., Bezigian A., Chatterjee A., Chew D., et al. Endogenous ADAR-mediated RNA editing in non-human primates using stereopure chemically modified oligonucleotides. Nat. Biotechnol. 2022 doi: 10.1038/s41587-022-01225-1. [DOI] [PubMed] [Google Scholar]
- 7.Rees H.A., Liu D.R. Base editing: precision chemistry on the genome and transcriptome of living cells. Nat. Rev. Genet. 2018;19:770–788. doi: 10.1038/s41576-018-0059-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Newby G.A., Yen J.S., Woodard K.J., Mayuranathan T., Lazzarotto C.R., Li Y., Sheppard-Tillman H., Porter S.N., Yao Y., Mayberry K., et al. Base editing of haematopoietic stem cells rescues sickle cell disease in mice. Nature. 2021;595:295–302. doi: 10.1038/s41586-021-03609-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doherty E.E., Wilcox X.E., van Sint Fiet L., Kemmel C., Turunen J.J., Klein B., Tantillo D.J., Fisher A.J., Beal P.A. Rational design of RNA editing guide strands: cytidine analogs at the orphan position. J. Am. Chem. Soc. 2021;143:6865–6876. doi: 10.1021/jacs.0c13319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nair J.K., Willoughby J.L.S., Chan A., Charisse K., Alam M.R., Wang Q., Hoekstra M., Kandasamy P., Kel’in A.V., Milstein S., et al. Multivalent N-acetylgalactosamine-conjugated SiRNA localizes in hepatocytes and elicits robust RNAi-mediated gene silencing. J. Am. Chem. Soc. 2014;136:16958–16961. doi: 10.1021/ja505986a. [DOI] [PubMed] [Google Scholar]