Abstract
Introduction
Obese men with prostate cancer have an increased risk of biochemical recurrence, metastatic disease and mortality. For those undergoing androgen deprivation therapy (ADT), substantial increases in fat mass are observed in the first year of treatment. Recently, we showed that a targeted supervised clinic-based exercise and nutrition intervention can result in a substantial reduction in fat mass with muscle mass preserved in ADT-treated patients. However, the intervention needs to be accessible to all patients and not just those who can access a supervised clinic-based programme. The purpose of this study was to evaluate the efficacy of telehealth delivered compared with supervised clinic-based delivered exercise and nutrition intervention in overweight/obese patients with prostate cancer.
Methods and analysis
A single-blinded, two-arm parallel group, non-inferiority randomised trial will be undertaken with 104 overweight/obese men with prostate cancer (body fat percentage ≥25%) randomly allocated in a ratio of 1:1 to a telehealth-delivered, virtually supervised exercise and nutrition programme or a clinic-based, face-to-face supervised exercise and nutrition programme. Exercise will consist of supervised resistance and aerobic exercise performed three times a week plus additional self-directed aerobic exercise performed 4 days/week for the first 6 months. Thereafter, for months 7–12, the programmes will be self-managed. The primary endpoint will be fat mass. Secondary endpoints include lean mass and abdominal aortic calcification, anthropometric measures and blood pressure assessment, objective measures of physical function and physical activity levels, patient-reported outcomes and blood markers. Measurements will be undertaken at baseline, 6 months (post intervention), and at 12 months of follow-up. Data will be analysed using intention-to-treat and per protocol approaches.
Ethics and dissemination
Ethics approval has been obtained from the Edith Cowan University Human Research Ethics Committee (ID: 2021–02157-GALVAO). Outcomes from the study will be published in academic journals and presented in scientific and consumer meetings.
Trial registration number
ACTRN12621001312831.
Keywords: Prostate disease, Adult oncology, Nutritional support
Strengths and limitations of this study.
Direct comparison between telehealth and clinic-based supervised exercise and nutrition programme in obese/overweight men with prostate cancer.
Year-long trial comprising 6 months of direct intervention followed by 6 months of self-management.
Comprehensive assessment of objective and patient-reported outcomes including dual-energy X-ray absorptiometry for the primary outcome.
The study is limited to patients previously treated or currently undergoing androgen deprivation therapy.
Introduction
Prostate cancer is one of the most prevalent cancers worldwide, accounting for ~1.4 million new cancer cases and more than 300 000 deaths in 2020.1 Among the treatments available, androgen deprivation therapy (ADT) has been extensively used in the management of localised and advanced disease to delay cancer progression and improve survival.2 However, several adverse effects including increases in fat mass and reductions in muscle mass are common especially during the first year of ADT,3 increasing or aggravating obesity and metabolic syndrome.4 In turn, obesity increases the risk of complications during radical prostatectomy and radiation therapy,5 6 as well as the risk of biochemical recurrence, metastatic disease and mortality.7–9
We10–17 and others18–23 have shown that exercise can counteract several treatment-related toxicities such as reducing or mitigating fatigue, improving muscle mass and strength, bone mass and physical function during or following ADT. However, the effects of exercise alone on fat mass are modest with reductions of ~0.7 kg observed in a recent meta-analysis of overweight men with prostate cancer17 compared with the substantial gains of ~2.3 kg that can be experienced during the first year of treatment.3 As a result, exercise undertaken in trials to date has been largely insufficient to counteract the treatment-related gains in fat mass, which may be especially problematic for men already overweight or obese.
Recently, we presented new evidence that in obese ADT-treated patients with prostate cancer, a targeted and supervised clinic-based 12-week exercise programme allied with protein supplementation and energy restriction resulted in a substantial reduction of ~2.8 kg in fat mass while preserving muscle mass.24 However, it is necessary to expand such programmes to alternative exercise settings where overweight/obese patients living in underserved areas, such as those in regional and rural settings, or those without the financial capacity to access such programmes. Recently, telehealth has emerged as a viable method to deliver health-related services such as exercise and nutrition interventions25 and, if effective in this group of patients with prostate cancer, could be available at a lower cost and reach larger numbers of patients irrespective of their geographical location. Therefore, we propose to undertake a 6-month non-inferiority randomised trial to evaluate the efficacy of a telehealth delivered compared with a supervised clinic-based delivered exercise and nutrition intervention in overweight and obese patients with prostate cancer. We will compare our previously reported supervised clinic-based exercise and nutrition weight loss programme24 to a programme modified for delivery via telehealth in overweight/obese men with prostate cancer, with subsequent follow-up over 6 months to monitor sustainability. The primary outcome will be fat mass with secondary outcomes including lean mass and objective and patient-reported outcomes.
Methods and analysis
This is a single-blinded, two-arm parallel group, non-inferiority randomised trial designed to examine the efficacy of implementing a telehealth-delivered, virtual supervised, exercise and nutrition (TENUT) programme compared with a clinic-based, face-to-face supervised exercise and nutrition (CENUT) programme on fat mass in overweight/obese men with prostate cancer (figure 1). The protocol has been approved (ID: 2021–02157-GALVAO) by the Edith Cowan University Human Research Ethics Committee. This trial expects to enrol participants to baseline testing between December 2021 and December 2023.
Figure 1.
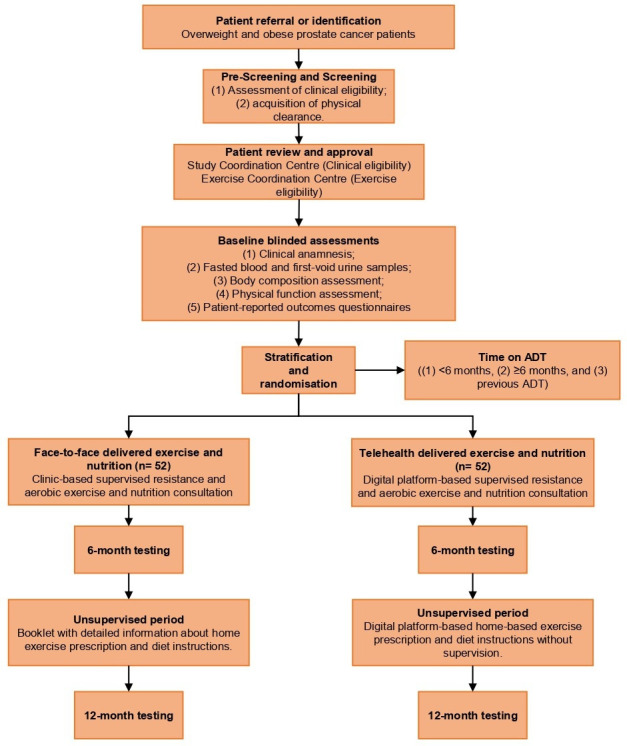
Consolidated Standards of Reporting Trials diagram depicting the participants throughout the trial. ADT, androgen deprivation therapy.
Patients and methods
One-hundred and four overweight/obese men (52 participants per arm) undergoing treatment or previously treated (ie, those who had completed treatment and are no longer on treatment) for prostate cancer involving ADT will be identified and recruited through attending physicians (general practitioner/radiation oncologist/urologist), specialist nurses, advertisements in local newspapers and presentations at cancer support groups and related events in Western Australia. Inclusion criteria are (1) body fat percentage of ≥25%26 and (2) ability to walk 400 m. Exclusion criteria are (1) acute illness or any musculoskeletal, cardiovascular or neurological disorder that could inhibit exercise performance or put participants at risk from exercising, and (2) inability to read and understand English. Eligible patients will undertake baseline measurements prior to randomisation. All patients must provide written informed consent prior to participation in addition to a physician clearance form. The study coordinator will obtain the consent and clearance forms from patients and physicians. Patients with metastases will be required to present their last bone imaging scan to establish location and extent of bone lesions with the exercise prescription modified according to Galvão et al12 and to the Exercise and Sports Science Australia exercise and cancer position statement.27 All data relevant to the study will be kept on password-encrypted computers accessible only by study investigators situated in the Exercise Medicine Research Institute (Perth, Western Australia, Australia).
Patient and public involvement
We conducted a health consumer workshop reaching out to 14 men with prostate cancer who had completed our most recent exercise and nutrition intervention study.24 The men were overweight or obese and completed the 3-month diet and exercise programme in our exercise clinics. We sought their input on the programme they had just completed and how they would view a telehealth intervention. This input was used to inform this project and ensure that it engages participants in a respectful, ethical and impactful way. We also worked closely with the Prostate Cancer Foundation of Australia (PCFA), their support groups and state offices. As the project evolves, PCFA will assist in the dissemination of findings to cancer support groups and the general public, while study participants will receive their individual results as well as overall study findings.
Randomisation
Patients will be randomly allocated by a computer random assignment programme to the two study arms: (1) TENUT and (2) CENUT in a ratio of 1:1, subject to maintaining approximate balance regarding stratification for time on ADT (<6 months, ≥6 months and previous ADT). An investigator with no patient contact will be responsible for randomisation. The allocation sequence will be concealed from exercise physiologists involved in assigning participants to groups or conducting the study measures. In addition, participants will be requested to not reveal their group allocation to any members of the research team. The exercise will be provided by exercise physiologists not in the research team or performing the tests.
Measurements
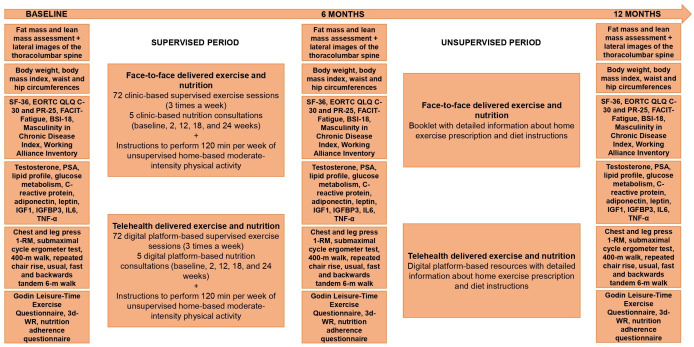
All measurement study endpoints will take place at baseline, 6 months (end of intervention) and 12 months (6 months post intervention) and will be undertaken in person at the Exercise Medicine Research Institute at Edith Cowan University in Perth, Australia (figure 2). All assessment tools/procedures have established validity and reliability and are used widely in clinical research including by our team.10–15
Figure 2.
Study design, exercise and nutrition interventions and timeline assessments. BSI-18, Brief Symptom Inventory-18; 3d-WR, 3-day weighed food record; EORTC QLQ-C30, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire 30; EORTC QLQ-PR25, European Organization for Research and Treatment of Cancer Prostate Cancer-Specific Module; FACIT-Fatigue, Functional assessment of Chronic Illness Therapy–Fatigue; IGF1, insulin-like-growth factor-1; IGFBP3, IGF-binding protein-3; IL, interleukin; PSA, prostate-specific antigen; TNF-α, tumour necrosis factor alpha.
Study endpoints
Primary study endpoint
Fat mass
Regional and whole-body fat mass will be derived from a whole-body dual-energy X-ray absorptiometry (DXA, Horizon A; Hologic, Waltham, Massachusetts, USA) scan. Trunk adiposity, visceral fat and adipose indices will be assessed using standard procedures.13 14 28
Secondary study endpoints
Lean mass and abdominal aortic calcification
Regional and whole-body lean mass will be assessed by DXA. In addition, lateral spine images will be collected for abdominal aortic calcification assessment as a surrogate of cardiovascular disease.29
Anthropometric measures and blood pressure assessment
Central adiposity will be assessed by waist circumference (WC) and hip circumference (HC).30 WC will be measured at the level of the narrowest point between the lower costal (rib) border and the iliac crest. HC will be measured at the level of the greatest posterior protuberance of the buttocks, which usually corresponds anteriorly to the level of the symphysis pubis. Body mass index (kg/m2) will also be used to assess weight (kg) relative to height (m) squared. A validated oscillometric device (HEM-705CP, Omron Corporation, Japan) will be used to record brachial systolic and diastolic blood pressures at the dominant arm in triplicate.
Objective measures of physical function and physical activity levels
A battery of standard tests will be used to assess physical function: (1) one-repetition maximum (1RM) test for chest press and leg press strength, (2) submaximal cycle ergometer test for estimation of maximal oxygen uptake (VO2max), (3) 400 m walk test for aerobic capacity and walking endurance, (4) repeated chair rise for lower body function, (5) 6 m usual and fast walk for gait speed and (6) 6 m backwards tandem walk for dynamic balance.11–15 31 Self-reported physical activity will be assessed by the leisure score index from the Godin Leisure-Time Exercise Questionnaire modified to include a question on resistance training.32
Intervention adherence and monitoring
Adherence to the direct supervised exercise component will be defined as the number of sessions attended divided by the total number of sessions scheduled in both TENUT (ie, telehealth programme) and CENUT (clinic-based programme) groups. For the self-managed phase of the study, patients in the TENUT will continue with the digital platform for recording, while the CENUT group will receive a self-managed exercise log with instructions to be completed.
Adherence to dietary recommendations will be assessed using an adapted customised adherence questionnaire24 33 34 designed to provide an estimated frequency of consumption and number of serves of food of interest based on the nutrition advice given. Food items of interest include fruit and vegetables, nuts, high protein foods, dairy, grains and cereals, beverages and alcoholic drinks, discretionary and take-away items. Patients will be asked 25 yes/no questions, where a score of 1 is given if the patient met a predetermined desired outcome, or a 0 if they did not. A higher total score indicates greater compliance with a maximum score of 25. For nutrition monitoring, patients will complete a 3-day weighed food record (3d-WR) over three consecutive days (one weekend day and two weekdays) at baseline and 6 and 12 months. This will provide an estimate of total energy intake (kJ/d) and macro- and micronutrients consumed. The 3d-WR data will be analysed using FoodWorks (FoodWorks 10 Professional, Xyris Software Pty, Queensland, Australia).
Patient-reported outcomes
Health-related quality of life will be assessed using the Medical Outcomes Short Form 36,35 while cancer-related quality of life will be measured using the EORTC QLQ-C30 (European Organization for Research and Treatment of Cancer Prostate Cancer-Specific Module)36 and the EORTC-PR25 (European Organization for Research and Treatment of Cancer Quality of Life Questionnaire 30) for disease-specific health-related quality of life.37 Fatigue will be assessed using the Functional assessment of Chronic Illness Therapy–Fatigue questionnaire. The Brief Symptom Inventory-18 will be used to assess psychological distress across the domains of anxiety, depression and somatisation, and global distress severity.38 These validated instruments are an integrated system to assess quality of life and psychological distress in patients with cancer and has been extensively employed in clinical trials of exercise medicine.10–13 39 In addition, the Masculinity in Chronic Disease Index will be used to assess the extent to which men identify with six masculine values: strength, sexual importance/priority, family responsibilities, emotional self-reliance, optimistic capacity and action approach,40 41 while an adapted Working Alliance Inventory for General Practice tool will be used to identify and explain the mechanism or process that underlies the delivery of exercise and nutrition as well as benefits derived from these programmes in men with prostate cancer.42 43
Blood markers
Testosterone, prostate-specific antigen (PSA), lipid profile, insulin, glucose, glycated haemoglobin, C reactive protein, adiponectin, leptin, insulin-like-growth factor-1 (IGF1), IGF-binding protein-3 (IGFBP3), interleukin 6 (IL-6) and tumour necrosis factor alpha (TNF-α) will be measured commercially by an accredited Australian National Association of Testing Authorities laboratory (Pathwest Diagnostics, Perth, Western Australia, Australia).11 13 24
Safety and monitoring
Patients will be monitored for any adverse events during training and testing by accredited exercise physiologists (AEPs) with study clinicians overseeing aspects of patient management where required. During the self-management phase of the study, participants will record any adverse events which will be monitored by AEPs via monthly phone calls. The National Cancer Institute’s Common Terminology Criteria for Adverse Events V.5.0 will be used for grading the severity of adverse events during the study.44
Exercise interventions
Telehealth and face-to-face delivered exercise and nutrition programmes
The interventions will consist of 300 min/week of moderate to vigorous exercise per week for 6 months comprising a supervised resistance and aerobic exercise programme performed three times per week for 60 min per session delivered face-to-face in an exercise clinic or via telehealth by AEP, plus 30 min of moderate/vigorous physical activity self-managed 4 days/week. For both TENUT and CENUT, resistance training will comprise two to four sets for six to eight exercises targeting the major upper and lower body muscle groups performed using equipment such as exercise machines, dumbbells and elastic bands at an intensity of 6–12 repetition maximum (ie, the maximal weight that can be lifted 6–12 times, which is equivalent to ~60% to 85% of 1RM). The supervised aerobic exercise component will involve 15 to 20 min of moderate to vigorous intensity cardiovascular exercise using a variety of modes such as walking, jogging or cycling. This approach has been extensively used by our team,11 13 14 24 providing optimal stimulus while maximising safety, compliance and retention in clinic-based exercise programmes. The self-directed aerobic component will comprise these modes for 30 min 4 days/week. For the telehealth intervention, we will implement the latest digital platforms that we developed during COVID-19 restrictions in 2020 and related technological advancements in wearable sensors (Fitbit Charge 5, Fitbit, USA), online monitoring and video chat (Microsoft Teams, Microsoft, Redmond, Washington, USA) and cloud-based platforms (MyWellness TechnoGym Cloud platform, TechnoGym Australia Pty, Australia). Participants will receive their exercise programme via their smart device or computer, communicate with the AEP and fellow participants by video chat, and be monitored in real-time through the internet.
For the nutrition intervention, all participants will receive a total of five face-to-face or online consultations over the first 6 months of intervention (baseline, 2, 12, 18 and 24 weeks) with an accredited practising dietitian (APD) aiming to (1) achieve an energy deficit of 2100–4200 kJ/day (500–1000 kcal/day); (2) reducing discretionary items including alcoholic drinks and foods containing refined sugars and (3) maintain protein intake. In addition, participants will consume 40 g of a whey protein supplement three times per week immediately after the resistance exercise sessions.
At the end of the first 6-month period, participants from CENUT will receive a booklet with detailed information about a home exercise prescription, while the telehealth programme will be maintained without supervision for participants from TENUT. Instructions on performing the home-based exercises and achieving dietary recommendations will be provided by the AEP and APD.
Calculation of sample size and statistical analysis
From our previous research in obese patients with prostate cancer, we reported that the SD for change in fat mass equates to 2.6 kg (mean change of −2.8 kg) following 3 months of combined resistance and aerobic exercise with protein supplementation and caloric restriction.24 A priori, 43 patients per group will be required to achieve 80% power at an α level of.025 (one-tailed) and to demonstrate a non-inferiority limit below 1.4 kg of fat mass between the TENUT and CENUT groups. Therefore, to adequately ensure that we have a sufficient number of participants at the end of the study (accounting for a drop-out rate of 20%), 52 participants will be randomised to each group.
Normality of the data will be assessed using the Kolmogorov-Smirnov test. Baseline characteristics will be analysed using Student’s t-tests or the Mann-Whitney U test for continuous measures, as appropriate, and χ2 for categorical variables. For the study outcomes, data will be analysed using intention-to-treat and per protocol approaches. Testing for longitudinal changes will be performed using linear mixed models. Non-inferiority of the intervention for the primary outcome will be implied if the lower limit of a one-sided 95% CI of the difference between groups between baseline and 6 months is within the prestated limit of 1.4 kg for fat mass change.
Ethics and dissemination
Outcomes from the study will be published in peer-reviewed academic journals and presented in scientific, consumer and clinical meetings. The study investigators and trial coordinator will have access to the data.
Discussion
Men with prostate cancer undergoing ADT experience increased fat and reduced muscle mass, placing them at increased risk of morbidity and mortality from cardiovascular and metabolic diseases.3 8 45 46 Targeted exercise medicine interventions for men with prostate cancer can improve quality of life, reduce treatment-related side effects and improve both physical and psychological health.10–17 More recently, we have shown that in obese men with prostate cancer, a targeted supervised clinic-based exercise and nutrition intervention resulted in a substantial reduction in fat mass (~3 kg), while muscle mass was preserved.24 This is a new finding; however, availability of these clinic-based services and patient support is limited and the vast majority of patients cannot access due to issues of distance, transport, inconvenience and financial capacity. The result is an unacceptable disparity between patients who have access to such supportive care and those who do not, resulting in suboptimal quality of life and, ultimately, survival for those men who cannot access current best practice care.
These issues are particularly pertinent to patients in Western Australia due to our geography (comprises about a third of the country with only one major metropolitan area), resulting in the majority of men with prostate cancer having limited or no capacity to access exercise and nutrition programmes face-to-face with health professionals. Access to the latest exercise medicine and nutrition services has been unfortunately further impacted by the COVID-19 pandemic due to personal isolation, physical distancing and changes to public transport and procedures within cancer care clinics.47 Moreover, changes in physical activity behaviour can be challenging in this group,48 49 with common barriers being treatment-related symptoms and lack of time.50 Therefore, telehealth exercise and nutrition interventions have the potential to overcome a number of these issues providing high-quality, effective and safe supportive care at a time and in a place of the patient’s choosing. Translation of the outcomes of this research can be immediate. The underlying knowledge required to take this programme out into the community will be a result of the research project.
Supplementary Material
Footnotes
Twitter: @ChambersInOz, @AdMandydevine, @profrobnewton
Contributors: DAG, DRT, DH, PL, PL-W, CIT, SKC, NS, CK and RUN collaboratively developed the concept and protocol, including intervention, outcomes of interests and planned data analysis procedures, and contributed to writing, reviewing, editing and the final approval of the manuscript. AD, EJ and DJ further contributed to the study protocol, editing and final approval of the manuscript.
Funding: This work was supported by Cancer Council Western Australia Prostate Cancer Research Initiative grant (2021–2023 Prostate Cancer Research Initiative). DAG and RUN are funded by the National Health and Medical Research Council (NHMRC) Centre of Research Excellence (CRE) in Prostate Cancer Survivorship. PL is supported by the NHMRC CRE in Prostate Cancer Survivorship Scholarship.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, conduct, reporting or dissemination plans of this research. Refer to the Methods and analysis section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–49. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.D'Amico AV, Chen M-H, Renshaw AA, et al. Androgen suppression and radiation vs radiation alone for prostate cancer: a randomized trial. JAMA 2008;299:289–95. 10.1001/jama.299.3.289 [DOI] [PubMed] [Google Scholar]
- 3.Galvão DA, Spry NA, Taaffe DR, et al. Changes in muscle, fat and bone mass after 36 weeks of maximal androgen blockade for prostate cancer. BJU Int 2008;102:44–7. 10.1111/j.1464-410X.2008.07539.x [DOI] [PubMed] [Google Scholar]
- 4.Bhindi B, Locke J, Alibhai SMH, et al. Dissecting the association between metabolic syndrome and prostate cancer risk: analysis of a large clinical cohort. Eur Urol 2015;67:64–70. 10.1016/j.eururo.2014.01.040 [DOI] [PubMed] [Google Scholar]
- 5.Di Bella CM, Howard LE, Oyekunle T, et al. Abdominal and pelvic adipose tissue distribution and risk of prostate cancer recurrence after radiation therapy. Prostate 2020;80:1244–52. 10.1002/pros.24054 [DOI] [PubMed] [Google Scholar]
- 6.Uchida T, Higure T, Kawakami M, et al. What factors affect the operative time of robot-assisted laparoscopic radical prostatectomy? Surg Endosc 2021;35:4436–43. 10.1007/s00464-020-07946-1 [DOI] [PubMed] [Google Scholar]
- 7.Bonn SE, Wiklund F, Sjölander A, et al. Body mass index and weight change in men with prostate cancer: progression and mortality. Cancer Causes Control 2014;25:933–43. 10.1007/s10552-014-0393-3 [DOI] [PubMed] [Google Scholar]
- 8.Cao Y, Ma J. Body mass index, prostate cancer-specific mortality, and biochemical recurrence: a systematic review and meta-analysis. Cancer Prev Res 2011;4:486–501. 10.1158/1940-6207.CAPR-10-0229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Troeschel AN, Hartman TJ, Jacobs EJ, et al. Postdiagnosis body mass index, weight change, and mortality from prostate cancer, cardiovascular disease, and all causes among survivors of nonmetastatic prostate cancer. J Clin Oncol 2020;38:2018–27. 10.1200/JCO.19.02185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galvão DA, Newton RU, Chambers SK, et al. Psychological distress in men with prostate cancer undertaking androgen deprivation therapy: modifying effects of exercise from a year-long randomized controlled trial. Prostate Cancer Prostatic Dis 2021;24:758–66. 10.1038/s41391-021-00327-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galvão DA, Spry N, Denham J, et al. A multicentre year-long randomised controlled trial of exercise training targeting physical functioning in men with prostate cancer previously treated with androgen suppression and radiation from TROG 03.04 radar. Eur Urol 2014;65:856–64. 10.1016/j.eururo.2013.09.041 [DOI] [PubMed] [Google Scholar]
- 12.Galvão DA, Taaffe DR, Spry N, et al. Exercise preserves physical function in prostate cancer patients with bone metastases. Med Sci Sports Exerc 2018;50:393–9. 10.1249/MSS.0000000000001454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galvão DA, Taaffe DR, Spry N, et al. Combined resistance and aerobic exercise program reverses muscle loss in men undergoing androgen suppression therapy for prostate cancer without bone metastases: a randomized controlled trial. J Clin Oncol 2010;28:340–7. 10.1200/JCO.2009.23.2488 [DOI] [PubMed] [Google Scholar]
- 14.Newton RU, Galvão DA, Spry N, et al. Exercise mode specificity for preserving spine and hip bone mineral density in prostate cancer patients. Med Sci Sports Exerc 2019;51:607–14. 10.1249/MSS.0000000000001831 [DOI] [PubMed] [Google Scholar]
- 15.Taaffe DR, Galvão DA, Spry N, et al. Immediate versus delayed exercise in men initiating androgen deprivation: effects on bone density and soft tissue composition. BJU Int 2019;123:261–9. 10.1111/bju.14505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lopez P, Taaffe DR, Newton RU, et al. What is the minimal dose for resistance exercise effectiveness in prostate cancer patients? systematic review and meta-analysis on patient-reported outcomes. Prostate Cancer Prostatic Dis 2021;24:465–81. 10.1038/s41391-020-00301-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lopez P, Taaffe DR, Newton RU, et al. Resistance exercise dosage in men with prostate cancer: systematic review, meta-analysis, and meta-regression. Med Sci Sports Exerc 2021;53:459–69. 10.1249/MSS.0000000000002503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bourke L, Gilbert S, Hooper R, et al. Lifestyle changes for improving disease-specific quality of life in sedentary men on long-term androgen-deprivation therapy for advanced prostate cancer: a randomised controlled trial. Eur Urol 2014;65:865–72. 10.1016/j.eururo.2013.09.040 [DOI] [PubMed] [Google Scholar]
- 19.Segal RJ, Reid RD, Courneya KS, et al. Resistance exercise in men receiving androgen deprivation therapy for prostate cancer. J Clin Oncol 2003;21:1653–9. 10.1200/JCO.2003.09.534 [DOI] [PubMed] [Google Scholar]
- 20.Segal RJ, Reid RD, Courneya KS, et al. Randomized controlled trial of resistance or aerobic exercise in men receiving radiation therapy for prostate cancer. J Clin Oncol 2009;27:344–51. 10.1200/JCO.2007.15.4963 [DOI] [PubMed] [Google Scholar]
- 21.Ndjavera W, Orange ST, O'Doherty AF, et al. Exercise-induced attenuation of treatment side-effects in patients with newly diagnosed prostate cancer beginning androgen-deprivation therapy: a randomised controlled trial. BJU Int 2020;125:28–37. 10.1111/bju.14922 [DOI] [PubMed] [Google Scholar]
- 22.Winters-Stone KM, Dobek JC, Bennett JA, et al. Skeletal response to resistance and impact training in prostate cancer survivors. Med Sci Sports Exerc 2014;46:1482–8. 10.1249/MSS.0000000000000265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bressi B, Cagliari M, Contesini M, et al. Physical exercise for bone health in men with prostate cancer receiving androgen deprivation therapy: a systematic review. Support Care Cancer 2021;29:1811–24. 10.1007/s00520-020-05830-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilson RL, Newton RU, Taaffe DR, et al. Weight loss for obese prostate cancer patients on androgen deprivation therapy. Med Sci Sports Exerc 2021;53:470–8. 10.1249/MSS.0000000000002509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collins IM, Burbury K, Underhill CR. Teletrials: implementation of a new paradigm for clinical trials. Med J Aust 2020;213:263–5. 10.5694/mja2.50741 [DOI] [PubMed] [Google Scholar]
- 26.Kennedy AP, Shea JL, Sun G. Comparison of the classification of obesity by BMI vs. dual-energy X-ray absorptiometry in the Newfoundland population. Obesity 2009;17:2094–9. 10.1038/oby.2009.101 [DOI] [PubMed] [Google Scholar]
- 27.Hayes SC, Newton RU, Spence RR, et al. The exercise and sports science Australia position statement: exercise medicine in cancer management. J Sci Med Sport 2019;22:1175–99. 10.1016/j.jsams.2019.05.003 [DOI] [PubMed] [Google Scholar]
- 28.Messina C, Albano D, Gitto S, et al. Body composition with dual energy X-ray absorptiometry: from basics to new tools. Quant Imaging Med Surg 2020;10:1687–98. 10.21037/qims.2020.03.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lewis JR, Schousboe JT, Lim WH, et al. Long-term atherosclerotic vascular disease risk and prognosis in elderly women with abdominal aortic calcification on lateral spine images captured during bone density testing: a prospective study. J Bone Miner Res 2018;33:1001–10. 10.1002/jbmr.3405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Evans DJ, Hoffmann RG, Kalkhoff RK, et al. Relationship of androgenic activity to body fat topography, fat cell morphology, and metabolic aberrations in premenopausal women. J Clin Endocrinol Metab 1983;57:304–10. 10.1210/jcem-57-2-304 [DOI] [PubMed] [Google Scholar]
- 31.Mijwel S, Cardinale D, Ekblom-Bak E, et al. Validation of 2 submaximal cardiorespiratory fitness tests in patients with breast cancer undergoing chemotherapy. Rehabil Oncol 2016;34:137–43. 10.1097/01.REO.0000000000000030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Can J Appl Sport Sci 1985;10:141–6. [PubMed] [Google Scholar]
- 33.Martínez-González MA, García-Arellano A, Toledo E, et al. A 14-item Mediterranean diet assessment tool and obesity indexes among high-risk subjects: the PREDIMED trial. PLoS One 2012;7:e43134. 10.1371/journal.pone.0043134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Erdrich S, Bishop KS, Karunasinghe N, et al. A pilot study to investigate if New Zealand men with prostate cancer benefit from a Mediterranean-style diet. PeerJ 2015;3:e1080. 10.7717/peerj.1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. conceptual framework and item selection. Med Care 1992;30:473–83. [PubMed] [Google Scholar]
- 36.Aaronson NK, Ahmedzai S, Bergman B, et al. The European organization for research and treatment of cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365–76. 10.1093/jnci/85.5.365 [DOI] [PubMed] [Google Scholar]
- 37.Chu D, Popovic M, Chow E, et al. Development, characteristics and validity of the EORTC QLQ-PR25 and the FACT-P for assessment of quality of life in prostate cancer patients. J Comp Eff Res 2014;3:523–31. 10.2217/cer.14.41 [DOI] [PubMed] [Google Scholar]
- 38.Derogatis LR. BSI 18, brief symptom inventory 18: administration, scoring and procedures manual: NCS Pearson, incorporated, 2001. [Google Scholar]
- 39.Galvão DA, Taaffe DR, Chambers SK, et al. Exercise intervention and sexual function in advanced prostate cancer: a randomised controlled trial. BMJ Support Palliat Care 2022;12:29–32. 10.1136/bmjspcare-2020-002706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chambers SK, Hyde MK, Oliffe JL, et al. Measuring masculinity in the context of chronic disease. Psychol Men Masc 2016;17:228–42. 10.1037/men0000018 [DOI] [Google Scholar]
- 41.Hyde MK, Zajdlewicz L, Wootten AC, et al. Medical help-seeking for sexual concerns in prostate cancer survivors. Sex Med 2016;4:e7–17. 10.1016/j.esxm.2015.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sturgiss EA, Rieger E, Haesler E, et al. Adaption and validation of the working alliance inventory for general practice: qualitative review and cross-sectional surveys. Fam Pract 2019;36:516–22. 10.1093/fampra/cmy113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tracey TJ, Kokotovic AM. Factor structure of the working alliance inventory. Psychol Assess 1989;1:207–10. 10.1037/1040-3590.1.3.207 [DOI] [Google Scholar]
- 44.National Cancer Institute . Common terminology criteria for adverse events (CTCAE), 2017. Available: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf
- 45.Keating NL, O'Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol 2006;24:4448–56. 10.1200/JCO.2006.06.2497 [DOI] [PubMed] [Google Scholar]
- 46.Smith MR, Lee H, Nathan DM. Insulin sensitivity during combined androgen blockade for prostate cancer. J Clin Endocrinol Metab 2006;91:1305–8. 10.1210/jc.2005-2507 [DOI] [PubMed] [Google Scholar]
- 47.Lopez P, Taaffe DR, Newton RU, et al. Can exercise adaptations be maintained in men with prostate cancer following supervised programmes? implications to the COVID-19 landscape of urology and clinical exercise. Eur Urol Open Sci 2020;21:47–50. 10.1016/j.euros.2020.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bressi B, Iotti C, Cagliari M, et al. Physical exercise habits, lifestyle behaviors, and motivation to change among men with prostate cancer: a cross-sectional study. Support Care Cancer 2022;30:5017-5026. 10.1007/s00520-022-06911-z [DOI] [PubMed] [Google Scholar]
- 49.Galvão DA, Newton RU, Gardiner RA, et al. Compliance to exercise-oncology guidelines in prostate cancer survivors and associations with psychological distress, unmet supportive care needs, and quality of life. Psychooncology 2015;24:1241–9. 10.1002/pon.3882 [DOI] [PubMed] [Google Scholar]
- 50.Galvão DA, Chambers SK. Exercise medicine in men with prostate cancer: breaking barriers to increase participation. Prostate Cancer Prostatic Dis 2021;24:942–3. 10.1038/s41391-021-00406-4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.