Version Changes
Revised. Amendments from Version 1
The main points suggested by reviewers were considered in the new version to improve the quality of the manuscript. As suggested by reviewers, some changes have been made to the introduction part, where the subsections "emotion model" and "electrocardiogram & emotion" have been removed and the explanation of what ECG images are added in the manuscript. We expanded the related works section with Table 1, which compares several previous studies that used 1-D and 2-D ECG. In this new version, we better describe the proposed method for both input formats, including the preprocessing of ECG signals and the transformation from 1-D to 2-D ECG. The results have been updated according to the results of the latest experiment. Additionally, the analysis of the computing complexity was added along with the text in Table 7. The discussion and conclusion parts also go through some modifications to address comments and suggestions from the reviewer.
Abstract
Background: The electrocardiogram (ECG) is a physiological signal used to diagnose and monitor cardiovascular disease, usually using 2- D ECG. Numerous studies have proven that ECG can be used to detect human emotions using 1-D ECG; however, ECG is typically captured as 2-D images rather than as 1-D data. There is still no consensus on the effect of the ECG input format on the accuracy of the emotion recognition system (ERS). The ERS using 2-D ECG is still inadequately studied. Therefore, this study compared ERS performance using 1-D and 2-D ECG data to investigate the effect of the ECG input format on the ERS.
Methods: This study employed the DREAMER dataset, which contains 23 ECG recordings obtained during audio-visual emotional elicitation. Numerical data was converted to ECG images for the comparison. Numerous approaches were used to obtain ECG features. The Augsburg BioSignal Toolbox (AUBT) and the Toolbox for Emotional feature extraction from Physiological signals (TEAP) extracted features from numerical data. Meanwhile, features were extracted from image data using Oriented FAST and rotated BRIEF (ORB), Scale Invariant Feature Transform (SIFT), KAZE, Accelerated-KAZE (AKAZE), Binary Robust Invariant Scalable Keypoints (BRISK), and Histogram of Oriented Gradients (HOG). Dimension reduction was accomplished using linear discriminant analysis (LDA), and valence and arousal were classified using the Support Vector Machine (SVM).
Results: The experimental results show 1-D ECG-based ERS achieved 65.06% of accuracy and 75.63% of F1 score for valence, and 57.83% of accuracy and 44.44% of F1-score for arousal. For 2-D ECG-based ERS, the highest accuracy and F1-score for valence were 62.35% and 49.57%; whereas, the arousal was 59.64% and 59.71%.
Conclusions: The results indicate that both inputs work comparably well in classifying emotions, which demonstrates the potential of 1-D and 2-D as input modalities for the ERS.
Keywords: Emotion recognition, electrocardiogram, numerical ECG, image ECG, DREAMER
Introduction
Medical professionals have been actively using electrocardiogram (ECG) wave images as a tool for monitoring 1 – 3 and diagnosing cardiovascular diseases, 4 – 6 such as heart attacks, dysrhythmia, and pericarditis, with some reported accuracy of more than 99% in the past decade. Fundamentally, ECG is used to measure electrical activity in the human heart by attaching electrodes to the human body. Due to the continual blood pumping action to the body, the electrical activity of the heart can be found in the sinoatrial node. The electrocardiogram signal is composed of three basic components: P, QRS, and T waves ( Figure 1). P waves are produced during atrium depolarization, QRS complexes are produced during ventricular depolarization, and T waves are produced during ventricle recovery.
Figure 1.
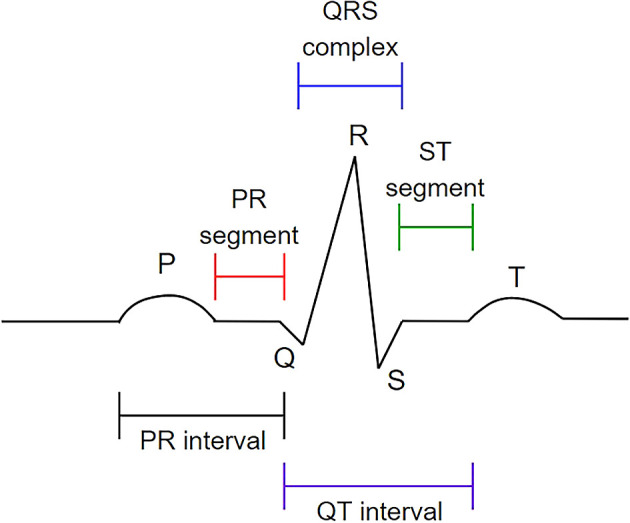
The P wave, QRS complex, and T wave in the standard electrocardiogram (ECG). This figure has been reproduced with permission from Ref. 7.
Today’s ECG devices have advanced from large and immobile to compact, wearable, and portable. Additionally, the signal accuracy of portable devices is comparable to that of traditional medical devices and can be used for the same purposes as traditional devices, including the study of human emotions. Many studies have proven that ECG which is associated with autonomic nervous system’s (ANS) physiological responses can be used to identify human emotions. 8 – 11 Different emotions influence human heart activities differently; these influences may be hidden in the ECG wave and can be detected through closer monitoring of the main features of ECG, namely, heart rate (HR) and heart rate variability (HRV).
Previous research on human emotions has primarily relied on either direct analysis of 1-D data 12 – 14 or the conversion of 1-D data to a 2-D spectral image 15 prior to identifying the emotions. Despite this, majority of the portable devices record the ECG signal as images (2-D images) in a PDF file rather than as raw numerical data (1-D data). 16 – 18 The example of a PDF-based 2-D ECG is depicted in Figure 2. Due to this problem, researchers were required to convert the PDF file of the ECG into 1-D data before performing further emotion analysis, adding complexity to the pre-processing process. On this account, given the positive results obtained in monitoring and diagnosing cardiovascular-related diseases, the efficacy of 2-D ECG in emotion studies also warrants further investigation.
Figure 2.
The example of 2-D ECG in a PDF file.
As far as our knowledge is concerned, despite numerous attempts to recognize emotions using ECG signals, the effects of employing various types of ECG inputs to recognise emotions in the emotion recognition system (ERS) have yet to be closely studied. In addition, there is no consensus on whether or not the type of ECG input format affects the emotion classification accuracy. Therefore, to address this gap, the contribution of this study is to compare emotion classification performance using 1-D and 2-D ECGs to investigate the effect of the ECG input format on the ERS.
This study analysed ECG data from the DREAMER dataset, a multimodal database. In DREAMER, ECG signals were recorded from 23 participants using 18 audio-visual stimuli for the elicitation of various emotions. The Augsburg BioSignal Toolbox (AUBT) 19 and the Toolbox for Emotional Feature Extraction from Physiological Signals (TEAP) 20 were used to help extract features from the 1-D ECG. Prior to emotion classification, the dimension of the extracted ECG features was reduced using linear discriminant analysis (LDA). On the other hand, the 2-D ECG was obtained by converting the 1-D ECG, and six different feature extractors were used to extract features from the 2-D ECG, namely Oriented FAST and Rotated BRIEF (ORB), Scale Invariant Feature Transform (SIFT), KAZE, Accelerated-KAZE (AKAZE), Binary Robust Invariant Scalable Keypoints (BRISK), and Histogram of Oriented Gradients (HOG). The Support Vector Machine (SVM) classifier is used, and the ERS results for both ECG inputs are compared to examine the effect of signal input on ERS performance. The finding indicates no substantial difference between the two ECG inputs since both produce a promising outcome within same range of accuracy for emotion recognition.
The next section discusses related works. The following section describes the dataset and the proposed methods in depth. The results are then provided. Finally, the study is concluded in the final section.
Related works
Researchers in the emotion recognition field have been proposing multiple approaches using electrocardiogram signals. For instance, Minhad, Ali, and Reaz 21 used 1-D ECG to classify emotions of happiness and anger. They achieved 83.33% accuracy using the SVM classification method. Besides, Tivatansakul and Ohkura 22 used 1-D ECG from the AUBT dataset to detect emotions for the emotional healthcare system. K-Nearest Neighbour (KNN) successfully classified three emotions (joy, anger, and sadness) with an accuracy 85.75%, 82.75%, and 95.25%, respectively. The MPED database for ERS was proposed by Song et al. 23 using ECG numerical data to recognise discrete emotions (joy, humour, disgust, anger, fear, sadness, and neutrality). Attention Long Short-Term Memory (A-LSTM) was used as a feature extractor to extract the frequency and time-domain features from the physiological signal. The A-LSTM was used as a classifier along with SVM, KNN, and Long Short-Term Memory (LSTM). Averagely, A-LSTM achieved better results of 40% to 55% compared to those of other classifiers.
Katsigiannis and Ramzan 13 suggested that ERS should use low-cost and off-the-shelf devices to collect ECG signals based on numerical format. Their dataset was called DREAMER, and the classification using SVM with a radial basis function kernel successfully achieved 62.37% for valence and arousal. This dataset is adopted here. Additionally, numerous other researchers also used the ECG signals from the DREAMER dataset to perform emotion recognition. For instance, 1-D ECG data from the DREAMER dataset is utilized by Wenwen He et al. 24 that suggested an approach for emotion recognition using ECG contaminated by motion artefacts. The proposed approach improved classification accuracy by 5% to 15%. Additionally, Pritam and Ali 25 also employed 1-D ECG from the DREAMER dataset to develop the self-supervised deep multi-task learning framework ERS, which consists of two stages of learning: ECG representation learning and emotion classification learning. The accuracy gained in this study was greater than 70%. Hasnul et al. 12 also used the 1-D ECG by DREAMER dataset to compare the performance of two feature extractor toolboxes. They noted that the dataset’s size and the type of emotion classified might affect the suitability of the extracted features.
As mentioned before, the 2-D ECG was widely used for a variety of other purposes, including human authentication, ECG classification, and cardiovascular-related diseases. For example, Ref. 26 and Ref. 27 developed authentication systems based on printout-based 2-D ECG and 2-D ECG spectral images that achieved greater than 99% accuracy using CNN. Additionally, Klosowski et al. 28 reached the highest accuracy rate of 100% by classifying ECG signals into several categories, including normal ECG, brachy cardia, and premature ventricular contraction (PVC). Meanwhile, Ref. 4 and Ref. 29 employ 2-D ECG to detect and diagnose cardiovascular disease, specifically myocardial infarction (MI) and arrhythmia. Additionally, Mandal et al. 5 published a study comparing 1-D and 2-D ECGs for the diagnosis of Ventricular Arrhythmia. They concluded that both ECG inputs are effective at detecting the disease.
Despite rising popularity among medical practitioners in assessing patients’ cardiac disease, 2-D ECG remains inadequate compared to 1-D ECG usage as a type of input in emotion recognition studies. As a result, the number of studies employing 1-D ECG in ERS is higher than that utilizing 2-D ECG in ERS. However, rather than employing a printout-based 2-D ECG, emotion researchers classified human emotions using 2-D ECG spectral images. For example, Ref. 15 determines the R-peaks of the electrocardiogram prior to generating the R-R interval (RRI) spectrogram. Following that, CNN was used to classify the emotions, with an accuracy rate greater than 90%. Elalamy et al. 30 used ResNet-50 to extract features from a 2-D ECG spectrogram. Then, Logistic Regression (LR) was employed as a classifier and achieved an accuracy of 78.30% in classifying emotions.
Table 1 summarises these works, including the reference to the work, the dataset details (number of participants, number of stimuli), the signal used, the ECG input, the purpose of the work, the features extracted, the classifiers, and their accuracy—the accuracy denoted by an asterisk (*) refers to the accuracy of works that do not mainly focus on ERS.
Table 1.
The summary of existing works using 1-D and 2-D ECG input.
| Ref | Dataset | Signal used | ECG input | Purposes | Feature extracted | Classifier | Result (%) |
|---|---|---|---|---|---|---|---|
| 21 | Own dataset
69 subjects, 20 stimuli |
ECG | 1-D | ERS | Statistical features from the time and frequency domains | SVM, NB, KNN, Gaussian | SVM – 69.23
NB – 53.83 KNN – 61.83 Gaussian – 70.00 |
| 22 | AUBT | ECG | 1-D | ERS | Local pattern description using Local Binary Pattern (LBP) and Local Ternary Pattern (LTP) | KNN | LBP – 84.17
LTP – 87.92 |
| 23 | (MPED)
23 subjects, 28 stimuli |
ECG | 1-D | ERS | Statistical features from the time and frequency domains | SVM, KNN, LSTM, A-LSTM | SVM – 42.66
KNN – 40.02 LSTM – 49.77 A-LSTM – 51.66 |
| 13 | (DREAMER)
23 subject, 18 stimuli |
ECG, EEG | 1-D | ERS | Statistical features from the time and frequency domains | SVM, KNN, LDA | Valence – 62.37
Arousal – 62.37 |
| 24 | DREAMER | ECG | 1-D | ERS | Statistical features from the time, frequency, time-frequency domains, and nonlinear analysis-related | SVM | Valence – 86.09
Arousal – 87.80 |
| 25 | DREAMER | ECG | 1-D | ERS | Deep-learning | Convolutional Neural Network (CNN) | Valence – 74.90
Arousal – 77.10 |
| 12 | DREAMER | ECG | 1-D | ERS | Statistical features from the time and frequency domains | SVM | Valence – 65.80
Arousal – 65.40 |
| 26 | (MWM-HIT)
100 subjects |
ECG | 2-D | Authentication System | PQRST peaks | CNN | 99.99* |
| 27 | PhysioNet dataset (Fantasia and ECG-ID) | ECG | 2-D spectral | Authentication system | Spectrogram | CNN | 99.42* |
| 28 | Own dataset generated by FLUKE “ProSim 4 Vital Sign
and ECG Simulator” |
ECG | 2-D spectral | ECG classification | Instantaneous frequency and spectral entropy | LSTM | 100* |
| 4 | Zhejiang dataset | ECG | 2-D | Myocardial infarction screening | Object detection | DenseNet, KNN, SVM | DenseNet – 94.73*
KNN – 89.84* SVM – 92.19* |
| 29 | MIT-BIH arrhythmia dataset | ECG | 2-D spectral | Arrhythmia classification | Local features from 2-D images using deep learning | CNN | 99.11* |
| 5 | Physiobank dataset | ECG | 1-D and 2-D | Ventricular Arrhythmia detection | ECG beat images | SVM, Probabilistic Neural Network (PNN), KNN, Random Forest (RF) | 99.99* (both are useful) |
| 15 | Own dataset
11 subjects, 6 stimuli |
ECG and EEG | 2-D spectral | ERS | Statistical features from the time and frequency domains (R-R interval spectrogram) | CNN | ECG – 91.67
EEG – 90.00 |
| 30 | AMIGOS, DEAP | ECG, PPG, EDA | 2-D spectral | ERS | Features extracted from spectrogram by ResNet-50 | Logistic Regression | AMIGOS – 78.30
DEAP – 69.45 |
Although considerable research has been conducted using ECG for ERS, the majority of research has focused on 1-D ECG analysis rather than 2-D ECG analysis, despite the fact that systems based on 2-D ECG have achieved excellent results in detecting cardiovascular-related diseases and human authentication. Additionally, no comparison of 1-D and 2-D ECG input was found in the emotion studies. As a result, it is unknown whether the ECG input format has an effect on the ERS’s emotional classification accuracy. The significance of this study is that it compares emotion classification performance between 1-D and 2-D ECGs to determine the effect of the ECG input format on the ERS.
Methods
In this section, the details of the dataset are described, and the experimental setup for 1-D and 2-D ECGs is explained. The current study began in September 2020. MATLAB version 9.7 was utilized for data conversion and feature extraction, whereas Python version 3.8.5 was used for feature dimension reduction (for 1-D ECG) and classification. The analysis code used in this study is available from GitHub and archived with Zenodo. 47
The dataset (DREAMER)
This study used ECG signals from Katsigiannis and Ramzan 13 called DREAMER. The DREAMER dataset is a freely accessible database of electroencephalogram (EEG) and electrocardiogram (ECG) signals used in emotion research. However, EEG signals were removed from this study because the primary focus is on ECG signals. The ECG was recorded using the SHIMMER ECG sensor at 256 Hz and stored in 1-D format. The DREAMER dataset contains 414 ECG recordings from 23 subjects who were exposed to 18 audio-visual stimuli designed to evoke emotion. Each participant assessed their emotions on a scale of 1 to 5 for arousal, valence, and dominance. However, because this study was primarily concerned with arousal and valence ratings, participants’ evaluations of dominance were discarded. The summary of the DREAMER dataset is tabulated in Table 2.
Table 2.
The summary of the DREAMER dataset.
| No of subject | 23 |
|---|---|
| No of videos | 18 audio-visual stimuli |
| Type of stimuli | Audio-video |
| Used Signal (Hz) | ECG (256) |
| Rating scales | Valence, Arousal |
| Rating values | 1–5 |
Experimental setup
1) 1-D ECG
The proposed ERS for 1-D ECG consists of three stages: feature extraction, feature dimension reduction, and emotion classification. The structure of the proposed 1-D ECG-based ERS is illustrated in Figure 3.
Figure 3.
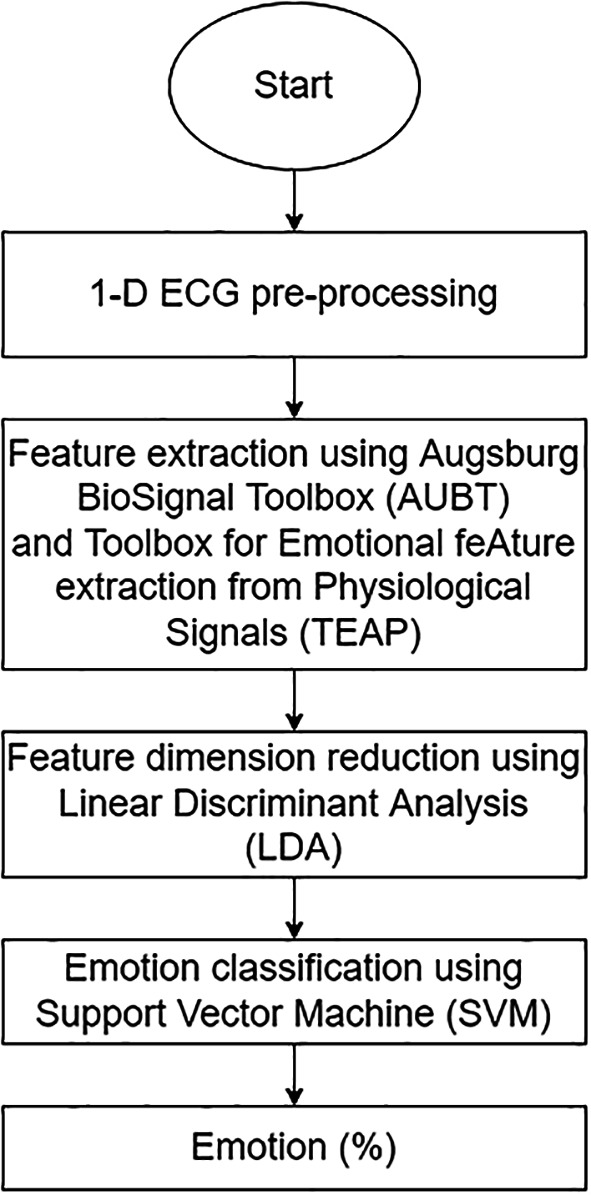
The overall structure of 1-D ECG-based ERS.
Two open-source toolboxes, namely, Augsburg BioSignal Toolbox (AUBT) 19 and Toolbox for Emotional feature extraction from Physiological signals (TEAP), 20 were employed to facilitate feature extraction from the ECG signals. AUBT provides tools for the analysis of physiological signals such as the ECG, RESP, EMG, and GSR. These tools are available for Windows with MATLAB 7.1. On the other hand, TEAP is compatible with the MATLAB and Octave software packages operating on Windows and can analyse and compute features from physiological data such as EEG, GSR, PPG, and EMG.
The AUBT and TEAP feature extractors were included with the Low Pass Filter (LPF), a filter meant to reject all undesirable frequencies in a signal. The LPF was one of the most widely used filters before the computation of statistical features for physiological signals. 31 , 32 As a result, automated 1-D ECG pre-processing utilizing LPF was performed in this study to reduce muscle and respiratory noise in ECG signals.
AUBT extracted 81 features in the time and frequency domains from each 1-D ECG signal, including the mean, median, and standard deviation for each PQRST wave, HRV, frequency spectrum range, and amplitude signal. Sixteen (16) statistical features are extracted, including mean, IBI, HRV, and multiscale entropy in the time and frequency domains. Table 3 and Table 4 provide abbreviations and descriptions of AUBT and TEAP features, respectively.
Table 3.
Features extracted from Augsburg Bio-signal Toolbox (AUBT).
| Features | Description |
|---|---|
| P, Q, R, S, T | P-, Q-, R-, S-, T-peaks (ECG) |
| HRV | Heart rate variability |
| Ampl | Amplitude signal |
| Mean | Mean value |
| Median | Median value |
| Std | Standard deviation |
| Min | Minimum value |
| Max | Maximum value |
| SpecRange | Mean of the frequency spectrum in a given range |
Table 4.
Features extracted from Toolbox for Emotional feature extraction from Physiological signals (TEAP).
| Features | Description |
|---|---|
| meanIBI | Mean inter-beat interval |
| HRV | Heart Rate Variability |
| MSE | Multiscale entropy at 5 levels |
| sp0001/0102/0203/0304 | Spectral power 0-0.1 Hz, 0.1-0.2 Hz, 0.2-0.3 Hz, 0.3-0.4 Hz |
| energyRatio | Spectral energy ratio between f<0.08 Hz/f>0.15 Hz and f<5.0 Hz |
| tachogram_LF/MF/HF | Spectral power in tachogram (HRV) for low, medium, and high frequencies. |
| tachogram_energy_ratio | Energy ratio for tachogram spectral content (MF/(LF+HF)) |
Additionally, to prevent the “Curse of Dimensionality,” dimensionality reduction is employed to further reduce the number of high-dimensional features to low-dimensional features. The dimensions of the features were decreased using linear discriminant analysis (LDA), a well-known approach for reducing the dimensions of features. 33 LDA is a supervised algorithm that can reduce dimensionality while retaining as much class-discrimination information as possible. Following that, the low-dimensional features were fed into a Support Vector Machine (SVM) classifier for emotion classification. The following section will outline the classifying process.
2) 2-D ECG
The duration of the ECG recording varies according to the duration of the video (average = 199 seconds). As Katsigiannis and Ramzan proposed, this study analysed the final 60 seconds of each recording to allow time for a dominant emotion to emerge. 13 Following that, 1-D ECG was pre-processed using a simple MATLAB function by Ref. 34 to eliminate baseline wander caused by breathing, electrically charged electrodes, or muscle noise. The signal was then divided into four segments corresponding to 15 seconds each. Then, using MATLAB version 9.7, the 1-D ECG was transformed into a 2-D ECG ( Figure 4). The image has a width of 1920 pixels and a height of 620 pixels.
Figure 4.
The 2-D ECG converted from 1-D ECG.
Due to the fact that the 2-D ECG was converted to a rectangle shape, it is not easy to resize the photos to the standard input image sizes of 224×224 and 299×299. As a result, the converted 2-D ECG was resized to 60% of its original size using Python version 3.8.5. This scale percentage was chosen after considering the quality of the image, the type of feature extractor used, and the computational cost the system can afford. The coloured images were converted into greyscale images. Then, binarization of the image using an Otsu’s automatic image thresholding method 35 was done. This method ascertains the optimal threshold values from pixel values of 0 to 255 by calculating and evaluating their within-class variance. 36
The area of interest for 2-D ECG is laying on the PQRST waves, making the peaks detector the best approach to be employed. Therefore, six different feature extractors that could extract peaks, edges, or corners were applied to extract features from 2-D ECGs using Python version 3.8.5:
-
1.
ORB 37 : ORB features are invariant to rotation and noise because they are a combination of Features from Accelerated Segment Test (FAST) detection and Binary Robust Independent Elementary Features (BRIEF) description methods.
-
2.
SIFT 38 : SIFT identifies feature points by searching for local maxima on the images using Difference-of-Gaussians (DoG) operators. The description approach generates a 16x16 neighbourhood around each identified feature and sub-blocks the region. SIFT is also rotation and scale invariant.
-
3.
KAZE 39 : KAZE is based on the scale of the normalised determinant of the Hessian Matrix, with the maxima of detector responses being captured as feature points using a moving window. Additionally, KAZE makes use of non-linear space via non-linear diffusion filtering to reduce noise while keeping the borders of regions in images.
-
4.
AKAZE 40 : AKAZE is a more sophisticated version of KAZE that is based on the Hessian Matrix determinant. Scharr filters were employed to enhance the quality of the invariance rotation, rendering AKAZE features rotation- and scale-invariant.
-
5.
BRISK 41 : While searching for maxima in the scale-space pyramid, BRISK detects corners using the AGAST algorithm and filters them using the FAST Corner Score. Additionally, the BRISK description is based on the recognised characteristic direction of each feature, which is necessary for rotation invariance.
-
6.
HOG 42 : HOG is a feature descriptor that is used to compute the gradient value for each pixel. The image shape denoted the edge or gradient structure derived using a high-quality local gradient intensity distribution.
All of the extractors successfully extracted the ECG features, including the peaks, edges, and corners from the PQRST waves. The extracted features were then given to the classifier (SVM) to classify the emotions. Figure 5 illustrates the structure of the proposed 2-D ECG-based.
Figure 5.
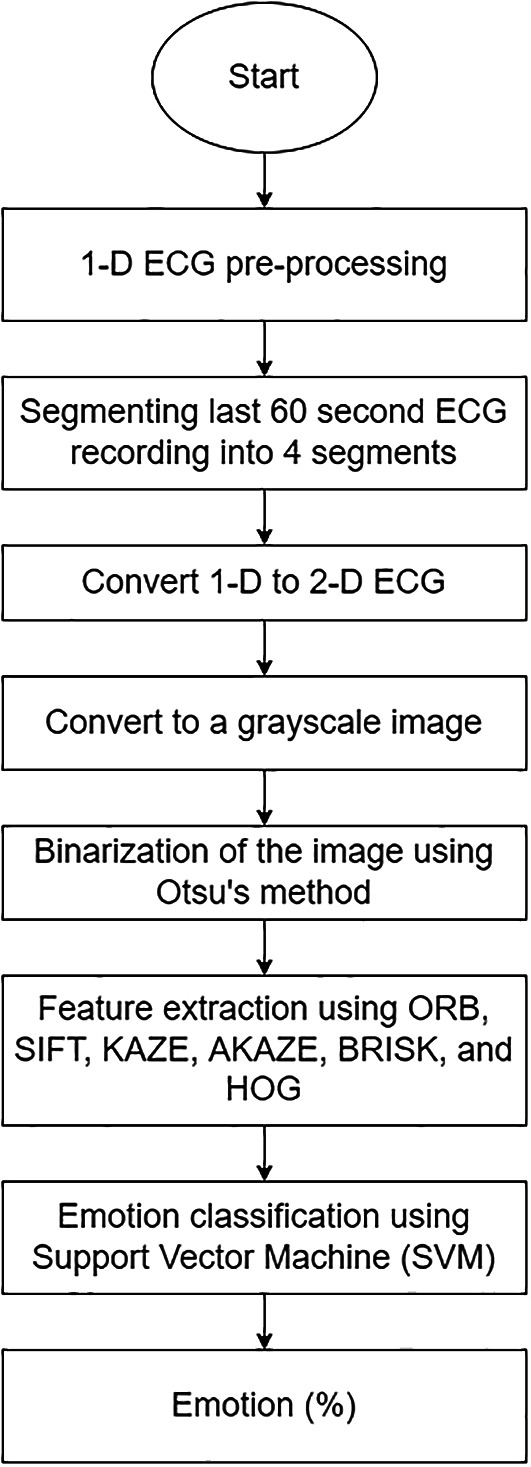
The overall structure of 1-D ECG-based ERS.
Support vector machine
Emotion classification was performed using SVM. The SVM works by separating the class data points and drawing a boundary called the hyperplane between them. Each hyperplane has what are known as “decision boundaries” to determine which side of each class resides. As reported in previous studies, SVM has a low computational cost and shows excellent performance in classifying emotions, as reported in previous studies. 13 , 21 , 24 , 43 , 44
Experimental setting
The scale of self-assessed emotions, which ranges from 1 (lowest) to 5 (highest), was classified using a five-point scale with middle-point thresholds (an average rate of 3.8). As a result, scales four and five were assigned to the high class, while the remaining scales were assigned to the low class. This results in an imbalanced distribution of DREAMER classes: valence has 39% of high valence and 61% of low valence; arousal has 44% of low arousal and 56% of high arousal.
The hyperparameters for SVM were tuned using an exhaustive parameter search tool, GridSearchCV, from Scikit-learn that automates the tuning procedure. 45 This study tuned only the parameters with a high and relative tuning risk and left the remainder at their default values because they are the least sensitive to the hyperparameter tuning process, as suggested by Weerts, Mueller, and Vanschoren. 46
The dataset was split into a reasonable proportion of training and testing sets to evaluate the model’s performance on new unseen data. This study used a stratified train-test split of 80:20 for the training and testing sets. This strategy guarantees the dataset’s exact proportions of samples in each class are preserved.
Additionally, as we had a small dataset size, this study applied KFold Cross-Validation, with the number of folds set to 10, the most commonly used number in prior research to improve ERS performance. The experimental setting is tabulated in Table 5.
Table 5.
The experimental setting values.
| Setting | Value | |
|---|---|---|
| SVM hyperparameter | Kernel | {linear, rbf} |
| C | [0.1,1,10] | |
| Gamma | [0.1,1,10] | |
| Train-test split | Stratified 70:30 | |
| Kfold cross-validation | 10 | |
Results
The testing performance of the ERS in classifying emotions using two different types of ECG data, 1-D and 2-D, is summarised in Table 6. The result denoted by an asterisk (*) corresponds to the original DREAMER publication 13, whereas the best accuracy and F1 score for classifying valence and arousal were bolded and shaded.
Table 6.
Testing emotion classification accuracy and F1-score for 1-D and 2-D electrocardiogram (ECG).
| Type of ECG input | Feature extractor | Valence | Arousal | ||
|---|---|---|---|---|---|
| Accuracy | F1-Score | Accuracy | F1-Score | ||
| 1-D | *AUBT and BioSig | 62.37 | 53.05 | 62.37 | 57.98 |
| AUBT | 63.86 | 72.73 | 57.83 | 44.44 | |
| TEAP | 65.06 | 75.63 | 54.22 | 42.42 | |
| 2-D | ORB | 61.75 | 47.76 | 56.33 | 40.59 |
| KAZE | 62.35 | 49.57 | 56.33 | 40.59 | |
| SIFT | 61.14 | 46.4 | 56.33 | 40.59 | |
| AKAZE | 61.75 | 47.76 | 59.64 | 59.71 | |
| BRISK | 61.75 | 47.76 | 56.02 | 40.23 | |
| HOG | 61.14 | 46.4 | 56.33 | 40.59 | |
For 1-D input, the features extracted using the TEAP feature extractor obtain the best valence performance with an accuracy of 65.06% and an F1 score of 75.63%. The best arousal performance is obtained using features extracted by the AUBT feature extractor, which has a 57.83% and a 44.44% F1 score.
On the other hand, the KAZE feature extractor achieves the best valence performance with 2-D input, achieving 62.35% accuracy and a 49.57% F1 score. Simultaneously, with 59.64% accuracy and a 59.71% F1 score, the AKAZE feature extractor achieves the best performance in arousal emotion.
For comparison purposes, the computation time for both ECG inputs was recorded and reported in Table 7. The average time required to compute 1-D is 1.58 ± 0.07 seconds. In comparison, the average computation time for 2-D is 3377.425 ± 3138.875 seconds. Therefore, according to the observation, 2-D took the longest computation time, whereas 1-D obtained the shortest.
Table 7.
Computation Time for Each Feature Extractor using Support Vector Machine (SVM).
| Type of ECG input | Feature extractor | Computational time (sec) | |
|---|---|---|---|
| Valence | Arousal | ||
| 1-D | AUBT | 1.65 | 1.63 |
| TEAP | 1.51 | 1.55 | |
| 2-D | ORB | 1473.07 | 1461.77 |
| KAZE | 4486.27 | 6034.26 | |
| SIFT | 239.31 | 238.55 | |
| AKAZE | 2950.23 | 3308.23 | |
| BRISK | 4926.46 | 3610.64 | |
| HOG | 6516.30 | 6431.28 | |
Discussion & conclusions
The results indicate that both inputs work comparably well in classifying emotions. This finding is demonstrated by the fact that the best valence performance was obtained using a 1-D ECG, and the best arousal performance was acquired using a 2-D ECG. Additionally, ERS with 1-D ECG was combined with dimensionality reduction, called LDA. The presence of LDA improved the ERS performance in valence emotion but not in arousal. In terms of computational cost, 1-D ECG is better to 2-D ECG since it requires less computation time.
However, it is worth mentioning that the results obtained using 2-D ECG demonstrated potential for use as an input modality for the ERS. Additionally, 2-D ECGs are appealing because the format enables the use of a variety of image-based methods such as image augmentation to increase the data size, convolution neural networks (CNN), and the application of transfer learning from models trained using large data. To summarise, the ERS performance of the two ECG inputs is comparable since both yield a promising outcome for emotion recognition.
Data availability
Source data
The DREAMER dataset was first presented here: https://doi.org/10.1109/JBHI.2017.2688239 and can be found on Zenodo. Access is restricted and users are required to apply. The decision whether to grant/deny access is solely under the responsibility of the record owner.
Extended data
Analysis code available from: https://github.com/nr-isml/ECG-Numerical-Vs.-Image-Data-for-Emotion-Recognition-System
Archived analysis code as at time of publication: https://doi.org/10.5281/zenodo.5542739. 47
License: Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
Acknowledgements
The authors would like to thank those who were involved in this experiment, either directly or indirectly.
Funding Statement
This project is funded by the TM Research & Development Grant (RDTC/190988), awarded to Multimedia University (MMU).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 2; peer review: 2 approved
References
- 1. Tayel MB, El-Bouridy ME: ECG images classification using artificial neural network based on several feature extraction methods. 2008 Int. Conf. Comput. Eng. Syst. ICCES 2008. 2008; pp.113–115.
- 2. Mohamed B, Issam A, Mohamed A, et al. : ECG Image Classification in Real time based on the Haar-like Features and Artificial Neural Networks. Procedia Comput. Sci. 2015;73(Awict):32–39. 10.1016/j.procs.2015.12.045 [DOI] [Google Scholar]
- 3. Yeh LR, et al. : Integrating ECG monitoring and classification via iot and deep neural networks. Biosensors. 2021;11(6):1–12. 10.3390/bios11060188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hao P, Gao X, Li Z, et al. : Multi-branch fusion network for Myocardial infarction screening from 12-lead ECG images. Comput. Methods Programs Biomed. 2020;184:105286. 10.1016/j.cmpb.2019.105286 [DOI] [PubMed] [Google Scholar]
- 5. Mandal S, Mondal P, Roy AH: Detection of Ventricular Arrhythmia by using Heart rate variability signal and ECG beat image. Biomed. Signal Process. Control. 2021;68(May):102692. 10.1016/j.bspc.2021.102692 [DOI] [Google Scholar]
- 6. Du N, et al. : FM-ECG: A fine-grained multi-label framework for ECG image classification. Inf. Sci. (Ny). 2021;549:164–177. 10.1016/j.ins.2020.10.014 [DOI] [Google Scholar]
- 7. C. U. First Faculty of Medicine: Electrocardiogram - WikiLectures. 2018.[Accessed: 04-Oct-2021]. Reference Source
- 8. Soleymani M, Lichtenauer J, Pun T, et al. : A multimodal database for affect recognition and implicit tagging. IEEE Trans. Affect. Comput. 2012;3(1):42–55. 10.1109/T-AFFC.2011.25 [DOI] [Google Scholar]
- 9. Abadi MK, Subramanian R, Kia SM, et al. : DECAF: MEG-Based Multimodal Database for Decoding Affective Physiological Responses. IEEE Trans. Affect. Comput. 2015;6(3):209–222. 10.1109/TAFFC.2015.2392932 [DOI] [Google Scholar]
- 10. Subramanian R, Wache J, Abadi MK, et al. : ASCERTAIN: Emotion and personality recognition using commercial sensors. IEEE Trans. Affect. Comput. 2018;9(2):147–160. 10.1109/TAFFC.2016.2625250 [DOI] [Google Scholar]
- 11. Siddharth S, Jung T-P, Sejnowski TJ: Utilizing Deep Learning Towards Multi-modal Bio-sensing and Vision-based Affective Computing. IEEE Trans. Affect. Comput. 2019; pp.1–1. 10.1109/TAFFC.2019.2916015 [DOI] [Google Scholar]
- 12. Hasnul MA, Ab Aziz NA, Aziz AA: Evaluation of TEAP and AuBT as ECG’s Feature Extraction Toolbox for Emotion Recognition System. IEEE 9th Conf. Syst. Process Control (ICSPC 2021), no. December,2022; pp.52–57. [Google Scholar]
- 13. Katsigiannis S, Ramzan N: DREAMER: A Database for Emotion Recognition Through EEG and ECG Signals from Wireless Low-cost Off-the-Shelf Devices. IEEE J. Biomed. Heal. Informatics. 2018;22(1):98–107. 10.1109/JBHI.2017.2688239 [DOI] [PubMed] [Google Scholar]
- 14. Koelstra S, et al. : DEAP: A database for emotion analysis; Using physiological signals. IEEE Trans. Affect. Comput. 2012;3(1):18–31. 10.1109/T-AFFC.2011.15 [DOI] [Google Scholar]
- 15. Fangmeng Z, Peijia L, Iwamoto M, et al. : Emotional changes detection for dementia people with spectrograms from physiological signals. Int. J. Adv. Comput. Sci. Appl. 2018;9(10):49–54. 10.14569/IJACSA.2018.091006 [DOI] [Google Scholar]
- 16. AliveCor :EKG Anywhere, Anytime|AliveCor.2022. [Online]. [Accessed: 07-Jan-2022]. https://www.kardia.com/
- 17. EMAY :Wireless EKG Monitor – EMAY.2022. [Online]. [Accessed: 25-Mar-2022]. https://www.emaycare.com/products/wireless-ekg-monitor-blue
- 18. Liu J, et al. : CRT-Net: A Generalized and Scalable Framework for the Computer-Aided Diagnosis of Electrocardiogram Signals. arXiv. 2021:1–25. [Google Scholar]
- 19. Wagner J: Augsburg biosignal toolbox (aubt). Univ. Augsbg;2014. [Google Scholar]
- 20. Soleymani M, Villaro-Dixon F, Pun T, et al. : Toolbox for Emotional feAture extraction from Physiological signals (TEAP). Front. ICT. 2017;4(FEB):1–7. 10.3389/fict.2017.00001 [DOI] [Google Scholar]
- 21. Minhad KN, Ali SHM, Reaz MBI: Happy-anger emotions classifications from electrocardiogram signal for automobile driving safety and awareness. J. Transp. Heal. 2017;7(November):75–89. 10.1016/j.jth.2017.11.001 [DOI] [Google Scholar]
- 22. Tivatansakul S, Ohkura M: Emotion Recognition using ECG Signals with Local Pattern Description Methods. Int. J. Affect. Eng. 2015;15(2):51–61. 10.5057/ijae.IJAE-D-15-00036 [DOI] [Google Scholar]
- 23. Song T, Zheng W, Lu C, et al. : MPED: A multi-modal physiological emotion database for discrete emotion recognition. IEEE Access. 2019;7(October):12177–12191. 10.1109/ACCESS.2019.2891579 [DOI] [Google Scholar]
- 24. He W, Ye Y, Pan T, et al. : Emotion Recognition from ECG Signals Contaminated by Motion Artifacts. 2021 Int. Conf. Intell. Technol. Embed. Syst. ICITES. 2021; pp.125–130. [Google Scholar]
- 25. Sarkar P, Etemad A: Self-supervised ECG Representation Learning for Emotion Recognition. IEEE Trans. Affect. Comput. 2020:1–1. 10.1109/TAFFC.2020.3014842 [DOI] [Google Scholar]
- 26. Hammad M, Zhang S, Wang K: A novel two-dimensional ECG feature extraction and classification algorithm based on convolution neural network for human authentication. Futur. Gener. Comput. Syst. 2019;101:180–196. 10.1016/j.future.2019.06.008 [DOI] [Google Scholar]
- 27. Bento N, Belo D, Gamboa H: ECG Biometrics Using Spectrograms and Deep Neural Networks. Int. J. Mach. Learn. Comput. 2020;10(2):259–264. 10.18178/ijmlc.2020.10.2.929 [DOI] [Google Scholar]
- 28. Kłosowski G, Rymarczyk T, Wójcik D, et al. : The use of time-frequency moments as inputs of lstm network for ECG signal classification. Electron. 2020;9(9):1–22. 10.3390/electronics9091452 [DOI] [Google Scholar]
- 29. Ullah A, Anwar SM, Bilal M, et al. : Classification of arrhythmia by using deep learning with 2-D ECG spectral image representation. Remote Sens. 2020;12(10):1–14. 10.3390/rs12101685 [DOI] [Google Scholar]
- 30. Elalamy R, Fanourakis M, Chanel G: Multi-modal emotion recognition using recurrence plots and transfer learning on physiological signals. 2021 9th Int. Conf. Affect. Comput. Intell. Interact. ACII 2021,2021. [Google Scholar]
- 31. Guo X: Study of emotion recognition based on electrocardiogram and RBF neural network. Procedia Eng. 2011;15:2408–2412. [Google Scholar]
- 32. Schmidt P, Reiss A, Duerichen R, et al. : Introducing WeSAD, a multimodal dataset for wearable stress and affect detection. ICMI 2018 - Proc. 2018 Int. Conf. Multimodal Interact. 2018; pp.400–408. [Google Scholar]
- 33. Velliangiri S, Alagumuthukrishnan S, Thankumar Joseph SI: A Review of Dimensionality Reduction Techniques for Efficient Computation. Procedia Comput. Sci. 2019;165:104–111. 10.1016/j.procs.2020.01.079 [DOI] [Google Scholar]
- 34. Rahman MA, et al. : A statistical designing approach to MATLAB based functions for the ECG signal preprocessing. Iran J. Comput. Sci. 2019;2(3):167–178. 10.1007/s42044-019-00035-0 [DOI] [Google Scholar]
- 35. Otsu N: A Threshold Selection Method from Gray-Level Histograms. IEEE Trans. Syst. Man Cybern. 1979;9(1):62–66. 10.1109/TSMC.1979.4310076 [DOI] [Google Scholar]
- 36. Trier OD, Taxt T: Evaluation of Binarization Methods for Document Images. IEEE Trans. Pattern Anal. Mach. Intell. 1995;17(3):312–315. 10.1109/34.368197 [DOI] [Google Scholar]
- 37. Rublee E, Rabaud V, Konolige K, et al. : ORB: An efficient alternative to SIFT or SURF. Proc. IEEE Int. Conf. Comput. Vis. 2011 May: pp.2564–2571. [Google Scholar]
- 38. Shi Y, Lv Z, Bi N, et al. : An improved SIFT algorithm for robust emotion recognition under various face poses and illuminations. Neural Comput. Appl. 2020;32(13):9267–9281. 10.1007/s00521-019-04437-w [DOI] [Google Scholar]
- 39. Alcantarilla PF, Bartoli A, Davison AJ: KAZE features. Lect. Notes Comput. Sci. (including Subser. Lect. Notes Artif. Intell. Lect. Notes Bioinformatics). 2012; vol.7577 LNCS(no.PART 6): pp.214–227. 10.1007/978-3-642-33783-3_16 [DOI] [Google Scholar]
- 40. Tareen SAK, Saleem Z: A comparative analysis of SIFT, SURF, KAZE, AKAZE, ORB, and BRISK. 2018 Int. Conf. Comput. Math. Eng. Technol. Inven. Innov. Integr. Socioecon. Dev. iCoMET 2018 - Proc. 2018; vol.2018-Janua: pp.1–10. [Google Scholar]
- 41. Liu Y, Zhang H, Guo H, et al. : A FAST-BRISK feature detector with depth information. Sensors (Switzerland). 2018;18(11). 10.3390/s18113908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rathikarani V, Dhanalakshmi P, Vijayakumar K: Automatic ECG Image Classification Using HOG and RPC Features by Template Matching. 2016; pp.117–125.
- 43. Bulagang AF, Weng NG, Mountstephens J, et al. : A review of recent approaches for emotion classification using electrocardiography and electrodermography signals. Informatics Med. Unlocked. 2020;20:100363. 10.1016/j.imu.2020.100363 [DOI] [Google Scholar]
- 44. Zhai J, Barreto A: Stress detection in computer users based on digital signal processing of noninvasive physiological variables. Annu. Int. Conf. IEEE Eng. Med. Biol. - Proc. 2006; (no.May): pp.1355–1358. [DOI] [PubMed]
- 45. Pedregosa F, Varoquaux G, Gramfort A, et al. : Scikit-learn: Machine Learning in Python. J. Machine Learn. Res. 2011;12:2825–2830. [Google Scholar]
- 46. Weerts HJP, Mueller AC, Vanschoren J: Importance of Tuning Hyperparameters of Machine Learning Algorithms. 2020.
- 47. nr-isml: nr-isml/ECG-Numerical-Vs.-Image-Data-for-Emotion-Recognition-System: First release (ECG). Zenodo. 2021. 10.5281/zenodo.5542739 [DOI]