Abstract
Background: Blockade of tumour necrosis factor (anti-TNF) is effective in patients with Crohn’s Disease but has been associated with infection risk and neurological complications such as demyelination. Niemann-Pick disease Type C1 (NPC1) is a lysosomal storage disorder presenting in childhood with neurological deterioration, liver damage and respiratory infections. Some NPC1 patients develop severe Crohn’s disease. Our objective was to investigate the safety and effectiveness of anti-TNF in NPC1 patients with Crohn’s disease.
Methods: Retrospective data on phenotype and therapy response were collected in 2019-2020 for the time period 2014 to 2020 from patients in the UK, France, Germany and Canada with genetically confirmed NPC1 defects and intestinal inflammation. We investigated TNF secretion in peripheral blood mononuclear cells treated with NPC1 inhibitor in response to bacterial stimuli .
Results: NPC1 inhibitor treated peripheral blood mononuclear cells (PBMCs) show significantly increased TNF production after lipopolysaccharide or bacterial challenge providing a rationale for anti-TNF therapy. We identified 4 NPC1 patients with Crohn’s disease (CD)-like intestinal inflammation treated using anti-TNF therapy (mean age of onset 8.1 years, mean treatment length 27.75 months, overall treatment period 9.25 patient years). Anti-TNF therapy was associated with reduced gastrointestinal symptoms with no apparent adverse neurological events. Therapy improved intestinal inflammation in 4 patients.
Conclusions: Anti-TNF therapy appears safe in patients with NPC1 and is an effective treatment strategy for the management of intestinal inflammation in these patients.
Keywords: Niemann-Pick disease Type C1
Introduction
Niemann-Pick disease Type C (NPC) is an autosomal recessive lysosomal storage disorder presenting most typically throughout childhood or adolescence 1 . Variants in the NPC1 gene account for 95% of the genetic defects in patients with NPC 1 . Patients experience progressive neurological impairment with reduced muscle tone, seizures, speech impairment and early onset dementia as well as hepatosplenomegaly, interstitial lung disease with recurrent pneumonia 1 .
Around 7% of patients with NPC1 develop inflammatory bowel disease (IBD), in particular Crohn’s disease with weight loss, diarrhoea, perianal fissures and fistula formation 2 . The NPC1 defect disrupts auto-phagosome maturation which impairs antibacterial responses towards Salmonella typhimurium and Adherent-invasive E coli (AIEC) 2 . Mice with NPC1 deficiency can develop mild intestinal inflammation 3 .
In paediatric and adult patients with Crohn’s disease anti-TNF agents (such as infliximab and adalimumab) are standard of care to induce and maintain remission 4, 5 . However, anti-TNF treatment can cause neurological side effects 6 such as demyelination 7, 8 and non-demyelinating central nervous system (CNS) inflammation including vasculitis 9, 10 . Since NPC1 patients could be particularly vulnerable to adverse neurological events due to their underlying progressive neurological disease we investigated if anti-TNF is a safe and effective treatment strategy for intestinal inflammation in NPC patients.
Methods
Study design
In this retrospective cohort study, we identified patients with NPC1 and intestinal inflammation by contacting specialist centres involved in their care. We recruited patients with NPC1 and intestinal inflammation who had been on anti-TNF agents as part of their management. Data collection took place in 2019 –2020 with data collection from 2014 until 2020. Selection bias was reduced by the use of pre-determined inclusion criteria, and as a consequence, patients with incomplete datasets were excluded. Data was collected from historical medical records to calculate wPCDAI scores to reduce memory bias.
Patient cohort
Patients with genetically confirmed NPC1 and confirmed intestinal inflammation (endoscopically and histologically) treated with anti-TNF agents were identified by contacting specialist facilities responsible for their care in Europe and Canada. We identified 5 patients and retrospective data was collected. Data was obtained from local hospital notes by the patients’ lead clinician. Out of five patients, one was excluded due to incomplete data being available.
De-identified patient information was provided by the clinicians responsible for their care.
Information on previous treatment and responses (dates of treatment, reason for stopping treatment and pre and post treatment severity of abdominal pain, stools per day, perianal disease and presence extraintestinal manifestations) were obtained in addition to response to anti-TNF therapy. The mathematically weighted paediatric Crohn’s disease activity index (wPCDAI) 11 was calculated at baseline, 4 weeks, 10 weeks, 6 months and a year after starting anti-TNF agents. A change in wPCDAI of >17.5 was used to indicate a response to treatment with a wPCDAI <12.5 suggesting remission, wPCDAI > 40 suggesting moderate disease and wPCDAI > 57.5 suggesting severe disease 11 . Potential side effects including unexpected changes in neurological function and infectious complications (e.g. increased frequency of respiratory infections) since starting anti-TNF therapy were recorded.
In vitro Infection model
Peripheral blood mononuclear cells (PBMCs) from healthy volunteers supplied from the NHS blood bank as leukocyte cones were obtained via the Oxford GI biobank.
PBMCs were separated from whole blood by using a Ficoll gradient. PBMCs (400,000 cells per condition) were either treated with 2ug/ml U18666A (Sigma Aldrich) drug for 24 hours or were left untreated. After U18666A treatment, PBMCs (400,000/well) were either stimulated with lipopolysaccharide (LPS) (200 ng/ml) orinfected with Salmonella typhimurium (Multiplicity of infection (MOI): 10) or Adherent invasic E. Coli ( AIEC) (MOI: 10). Supernatant was collected and frozen at 0, 2, 4 and 6 hours after stimulation. TNF quantification was performed using an eBioscience ELISA kit (catalogue number BMS223–4) as per manufacturer’s protocol.
Statistical analysis
Data analysis was performed using GraphPad Prism 9 software (GraphPad software, Inc., San Diego, CA). R is an open access alternative. Statistical significance was calculated using a Mann-Whitney U test or ANOVA for multiple test comparison. P < 0.05 were considered significant (* p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001).
Ethics
Written informed consent was obtained locally by the patients’ lead clinician from the patients or their guardians for deidentified data to be used for research purposes. Patient data were collected as part of the Oxford IBD cohort study and a sub-project to investigate rare diseases (The Oxford Gastrointestinal Illness Biobank IRAS ID 210441). Anonymous patient data were analyzed. Healthy donor samples were obtained via Oxford GI illnesses biobank.
Results
Small molecule inhibition of NPC1 increased TNF levels
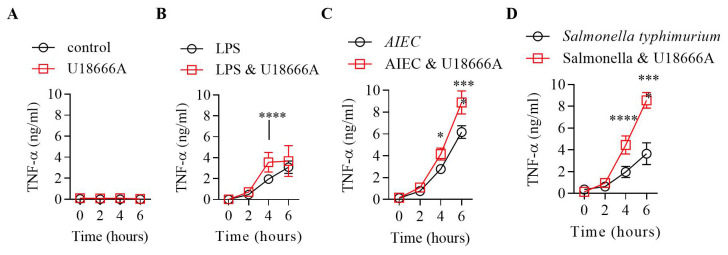
We investigated whether pharmacological inhibition of NPC1 with U18666A 2 caused a TNF associated inflammatory cytokine response. Healthy volunteer PBMCs were incubated with U18666A 2 before being stimulated with LPS, AIEC or S almonella. At baseline (0 hour) we did not observe any significant differences in TNF levels between U18666A treated and control cells ( Figure 1A) 12 . After stimulation with LPS we found significantly higher TNF levels at the 4-hour time point only ( Figure 1B). In contrast, stimulation with AIEC and S almonella resulted in significantly higher level of TNF production at the 4- and 6-hour time points in U18666A treated cells compared to controls (* p<0.01, **** p<0.0001) ( Figure 1C&D). This suggests increased TNF production in response to LPS and gut bacteria in patients with NPC1 may contribute to mucosal inflammation.
Figure 1. Tumour necrosis factor (TNF) quantification after bacterial exposure in U-drug treated or control PBMCs.
Peripheral blood mononuclear cells (PBMCs) (400,000) were either treated with U-drug for 24 hrs or left untreated. A-D: Post U-drug treatment PBMCs were stimulated with LPS, Salmonella typhimurium and AIEC and supernatants were collected at 0-, 2-, 4- and 6-hour time points for TNF quantification by ELISA. Statistical significance was determined using two-way ANOVA, multiple comparisons; Bonferroni’s multiple comparisons test (*p<0.01 and **** p<0.0001).
Anti-TNF treatment of NPC1 patients with Crohn’s disease (CD) like intestinal inflammation
We identified 4 patients with NPC1 and Crohn’s disease-like intestinal inflammation who received anti-TNF therapy. Two patients presented with complex perianal disease, fistulation, pain and weight loss ( Table 1). Patient numbers 1 and 4 have been described previously and we provide updated results with treatment response here 2 . The mean age of Crohn’s disease onset was 8.1 years with a mean treatment period of 27.75 months. Therapy used for control of intestinal inflammation was exclusive enteral nutrition (EEN) (n=1), local steroids (n=1), systemic steroids (n=4), 5-ASA (n=2), adalimumab (n=2), infliximab (n=4), methotrexate (n=1), anti-IL12/23p40 targeting ustekinumab (n=1) and the integrin antagonist vedolizumab (n=1). Two patients received both infliximab and adalimumab (patients 1 and 4) and patient 3 stopped infliximab and was switched to ustekinumab due to loss of effect before restarting both agents on family request. Complete remission was temporarily achieved in one patient (patient 1) using infliximab induction therapy. All other patients achieved a partial response (patients 2–4) ( Table 2). Two patients (patients 1 and 4) received adalimumab after loss of response (patient 1). Patient 4 was commenced on adalimumab first before switching to infliximab after loss of response to therapy. Infliximab trough-levels or anti-drug antibodies were not available. The duration of anti-TNF treatment ranged from 1.2 year to 5.4 years. The overall treatment period with anti-TNF was 9.25 patient years ( Figure 2).
Figure 2. Anti-TNF (tumour necrosis factor) therapy in patients with NPC1 genetic defect and Crohn’s disease (CD).
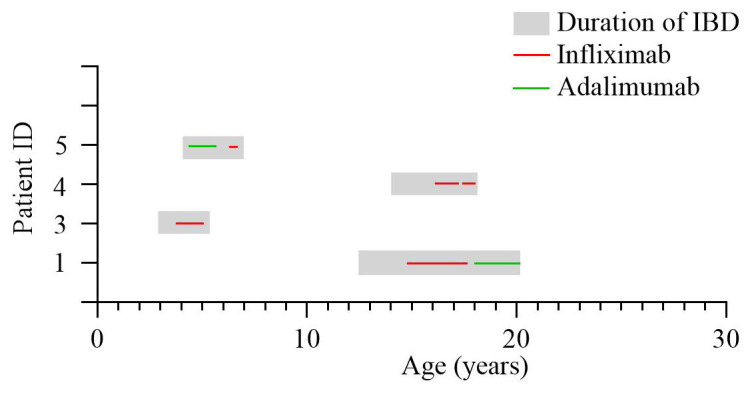
The grey shaded area represents time from age of IBD diagnosis to age at most recent follow up data. Red line represents duration of infliximab therapy with green line representing adalimumab therapy. IBD=inflammatory bowel disease.
Table 1. Baseline characteristics of patients.
| Patient
ID |
Sex | Age at IBD
diagnosis (years) |
Mutation | Intestinal
symptoms |
Endoscopic and
histological findings |
Paris
Classification |
Extra-intestinal
manifestations |
|---|---|---|---|---|---|---|---|
| 1 | F | 12.4 | c.3019C>G
(p.P1007A) and c.3731T>C (p.L1244P) |
Diarrhoea, rectal
bleeding, pain on defecation, perianal fissures, anorectal and vaginal fistula, arthritis |
Aphthous lesions in
the colon + fistulas Histology - inflammatory infiltrates, no granulomas. |
A1bL2B1p | |
| 2 | F | 2.9 | compound
heterozygous for c.2678dupT and c.3107C>T also heterozygous for c.29T>G |
Rectal bleeding
and diarrhoea |
Severe left sided
disease, inflammatory stricture of distal rectum, severe perianal skin tagging. |
A1aB2B3pL2G1 # | Respiratory
infections, growth delay, neurological deterioration with swallowing dysfunction |
| 3 | F | 13.6 | c.2848G>A and
c.423_424dupGA. |
Diarrhoea with
blood, weight loss |
Mild colitis
endoscopically with granulomas on biopsy |
A1bL2B1G0 | Progressive motor,
speech and bulbar dysfunction with swallowing dysfunction and moderate OSA (uses CPAP). Wilms tumour age 4 (surgery and chemotherapy) |
| 4 | M | 4.2 | p.Ile1061Thr and
p.Ser847Pro |
Perianal pain,
weight loss |
Perianal skin
tags and fissures, macroscopically normal colon and terminal ileum. Histology – IEL and granulomas |
A1aB1pL1G0 | Splenomegaly,
no neurological symptoms at present |
# : unable to have complete assessment at colonoscopy due to disease severity. 1* represents patient 8 in Schwerd et al., 4 + patient case report previously published 13
IEL: intraepithelial lymphocytes
Table 2. Response to treatment of 5 patients with NP-C and Crohn’s-like disease.
| Patient ID | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EEN | Steroids,
systemic |
Steroids,
local |
5-ASA | IFX | ADA | Vedo | MTX | AZA / 6-MP | AB | Other | Side effects | |
| 1 | - | I, partial | - | I, poor | I, complete, M, partial,
loss of response after 2.5 years |
M, partial | - | - | - | - | - | |
| 2 | I, poor | I, partial | - | - | M, partial | - | - | - | M, partial | M, partial | - | - |
| 3 | - | I, partial | - | I, poor | M, partial | - | - | - | M partial | M, partial | Ustekinumab (M, partial). | - |
| 4 | - | I, partial | I, poor | - | M, partial | M, partial | M partial | M, partial | - | - | 2- hydroxypropyl-β-cyclodextrin | - |
EEN = exclusive enteral nutrition, IFX = infliximab, ADA = adalimumab MTX = methotrexate, AZA = azathioprine, 6-MP = 6 mercaptopurine, AB = antibiotics. I= induction, M = maintenance therapy. Response classified as complete, partial or poor. Complete induction was defined as successful induction of remission, partial as improvement of disease activity and poor as little or no response. Maintenance treatment was classified complete if there was control of intestinal inflammation with no flares or complications, partial if there was good control of IBD activity and poor if there was little or no response.
Anti-TNF therapy appears safe in patients with NPC1 and Crohn’s disease like intestinal inflammation
In all 4 patients who received anti-TNF therapy, over the 1 year of follow up, we did not observe any adverse neurological events or worsening of neurological function suggesting anti-TNF therapy does not accelerate neurological decline in this patient group. Furthermore, there was no increase in respiratory complications or other serious infection in patients reported in this cohort at 1-year of follow up. Neurological decline was seen in some patients after the first year follow up period however, clinicians felt this was consistent with NPC1 progression. Patient 2 died whilst on anti-TNF therapy due to neurological and respiratory complications including inability to clear secretions. The clinicians felt this was due to NPC progression rather than anti-TNF treatment. However, this patient experienced an initial honeymoon period of improving motor skills, better respiratory function, nutrition, pain control (pain due to perianal disease and abscesses) during the first year of treatment with a significant improvement in quality of life. Patient 1 had an improvement in both a large perianal wound pocket and a vaginal fistula and became able to swallow small amounts again. Unfortunately, the patient did not have an endoscopy prior to starting treatment so it is unclear if this was due to improvement in neurological function or upper gastrointestinal manifestations of Crohn’s disease.
Efficacy of anti-TNF treatment in NPC1 patients with Crohn’s disease like intestinal inflammation
Four patients had a partial response to infliximab therapy. One patient had poor disease control on infliximab. Despite this, infliximab appeared to achieve better reported disease control on clinician global assessment than prior immunosuppression, steroids and exclusive enteral nutrition.
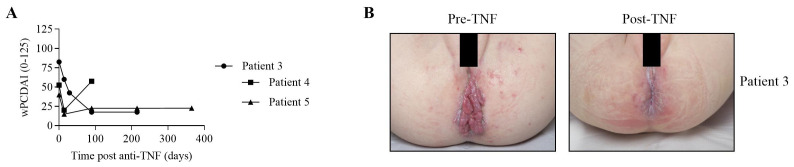
We assessed patient response to treatment using the wPCDAI scoring system to indicate severity of intestinal symptoms at baseline, 4 weeks, 10 weeks, 6 months and a year. Patient 2 initially had severe Crohn’s disease with a wPCDAI of 82.5 and showed a significant improvement in score to 17.5 over the first 90 days of treatment with anti-TNF therapy ( Figure 3A). This improvement was maintained for a further 125 days whilst continuing anti-TNF therapy. There was also a marked improvement in perianal disease ( Figure 3B). Patient 3 initially improved wPCDAI scores from 52.5 to 20. However, this improvement was not maintained and the wPCDAI increased to 57.5 due to worsening abdominal pain, increased ESR and weight loss. Patient 4 had an initial score of 40 improving to 15 after 2 weeks before increasing to 22.5 from week 14 to 52 (due to increase in stool frequency).
Figure 3. Clinical experience with anti-TNF (tumour necrosis factor) in Niemann-Pick Type C patients with inflammatory bowel disease (IBD).
A: Disease activity over time in patients 2, 3 and 4. Time points “0” indicated baseline disease activity before start of infliximab. Disease activity was determined by mathematically weighted Pediatric Crohn’s Disease Activity Index (wPCDAI, range 0-125 points). B: Anti-TNF therapy results in marked improvement of severe perianal disease in patient 2.
Discussion
NPC1 patients can present with severe Crohn’s-like disease in particular debilitating perianal disease. Anti-TNF therapy in NPC1-IBD patients results in symptom improvement without neurological deterioration at 1 year of follow up. We show in an in-vitro cell model increased levels of TNF upon LPS and bacterial stimulation suggesting that TNF is a differentially expressed cytokine in NPC1 and providing a molecular rationale of the treatment.
Patients with NPC1 suffer from a severe life-limiting disease, the potential risks of TNF therapy (demyelination and increased infection susceptibility) must be balanced against the improvement in quality of life due to effective IBD therapy and symptom control. Our data has shown improved management of Crohn’s-like disease in patients with NPC following anti-TNF therapy without worsening neurological function or infectious complications. Our data suggest that anti-TNF is safe to use in NPC from a neurological perspective. The improvement in IBD activity in NPC1 patients ( Figure 3) was associated with reduced pain, perianal disease and improved nutritional status. This suggests an improved quality of life for these patients. The response rate of anti-TNF in NPC1 patients is not complete and not sustainable (secondary loss of response) but very similar to patients with classical severe therapy-refractory Crohn’s disease 14 .
TNF can promote neuro-inflammation and neuronal damage as well as having a protective role in some diseases (such as ADA2 deficiency 15 ) 16 . Elevated levels of TNF within demyelinating plaques has been associated with the development of multiple sclerosis (MS) 17 . Polymorphism in both TNFR1 and TNFR2 have been linked to an increased risk of MS 18, 19 . Similarly, anti-TNF therapies have been linked to CNS demyelination and increased disease burden in patients with multiple sclerosis with a dose-dependent increase in relapse rate found in a trial of the TNF antagonist lenecept 17 . Despite this, anti-TNF associated demyelination occurs independently to the classical risk factors for MS 9 . Cases of anti-TNF associated demyelination have been described in patients with Crohn’s disease, psoriasis, rheumatoid arthritis and ankylosing spondylitis potentially hinting to a rare but relevant safety signal 20– 22 . For example, in 75 patients starting anti-TNF therapy for rheumatoid arthritis or spondyloarthropathies, three patients who developed neurological symptoms demonstrated new cortical lesions consistent with demyelination on MRI 6 . Another case series of four patients with neurological symptoms on anti-TNF therapy demonstrated MRI changes and CSF oligoclonal bands with three patients meeting the diagnostic criteria for multiple sclerosis 23 . However, pathophysiological mechanisms behind these effects are not completely understood 24 . Several mechanisms have been proposed, including poor cerebral penetration of anti-TNF agents due to large molecular size 25 which could prevent immunosuppressive actions in the brain. Another theory is based on the multiple forms and receptors for TNF. Soluble TNF signals through TNFR1 which is a ubiquitous receptor whose signalling can result in both pro and anti-apoptotic effects 26 but more commonly results in inflammation and increased apoptosis through caspase 8 activation 27 . In contrast, signalling from transmembrane TNF is largely through TNFR2. TNFR2 is found on immune cells, endothelial cells and some cells of the CNS and is thought to be neuroprotective and stimulate cell survival through phosphatidylinositol 3-kinase (PI3K) activity 26 . These opposing responses combined with variable receptor expression results in complex consequences of TNF manipulation and could explain why TNF may have both beneficial and deleterious role in multiple sclerosis 24 .
NPC1 is a disorder of hypomyelination 28 and is characterised by progressive neurological decline. It is therefore important to ensure no worsening of neurological function in patients treated with anti-TNF. Unfortunately, it is difficult to formally assess cognitive function in this patient group in retrospect and therefore clinician reported outcome was used. In this cohort none of the patients experienced symptomatic episodes suggestive of demyelination or unexpected worsening of neurological function within a year of follow up. Anti-TNF therapy can also be associated with non-demyelinating neuroinflammation such as meningoencephalitis and vasculitis 10 . Whilst these are rare events, we did not see any evidence of this in our cohort. The increased infection risk associated with anti-TNF therapy is also relevant in NPC1 patients, especially regarding respiratory infections, but there was no reported increase in infection rates ( Table 2). The improvement in intestinal disease activity in NPC1 patients ( Figure 3) was associated with reduced pain, perianal disease and improved nutritional status. This suggests an improved quality of life for these patients.
Limitations of this study include the small patient cohort and retrospective data collection from medical notes. Whilst a randomised, prospective study would be informative this would be very difficult to achieve given that NPC1 is a rare disease. As data was obtained from medical notes written before study recruitment, bias in data collection was reduced. However, as a result of this we had fewer variables that we could study, and this led to incomplete data collection for one patient. As a result of this missing data this patient was not included in data analysis.
Selection criteria included diagnosis of NPC1 with intestinal inflammation and treatment with anti-TNF agents. As a result, we did not expect or see changing eligibility over time.
In summary, our data advocates the use of anti-TNF therapy in the clinic to improve quality of life in patients with severe CD like intestinal inflammation in the context of NPC1. Our results in this high-risk group of patients complement previous findings that the incidence of adverse neurological effects associated with anti-TNF use is low in patients with paediatric IBD 8 .
Data availability
Zenodo: Anti-TNF therapy for inflammatory bowel disease in patients with neurodegenerative Niemann-Pick disease Type C. https://doi.org/10.5281/zenodo.5668321 12 .
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Acknowledgements
We would like to thank the patients and their families as well as healthy volunteers for consenting to this research.
We would like to thank Athena Cavounidis, Subra Kugathasan, Forbes D. Porter, Beth Jameson and Marianne Rohrbach for helpful and instructive discussions.
Funding Statement
We acknowledge the support of the BRC for the Oxford GI biobank (11/YH/0020, 16/YH/0247). The research was also supported by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre (BRC), University of Oxford. HHU and SP are supported by the Health Research (NIHR) Oxford Biomedical Research Centre and the The Leona M. and Harry B. Helmsley Charitable Trust. TS is supported by Reinhard-Frank Stiftung, German Research Foundation (Deutsche Forschungsgemeinschaft - DFG, grant number 395357507 – SFB 1371/P01) and The Leona M. and Harry B. Helmsley Charitable Trust. F.M.P. is a Royal Society Wolfson Research Merit Award holder and a Wellcome Trust Investigator in Science Wellcome grant number 202834.) NP and DS are supported by the Wellcome Trust. NAE by King Saud bin Abdulaziz University for Health Sciences, King Abdulaziz Medical City, National Guard Health Affairs and the Saudi Ministry of Higher Education.
[version 1; peer review: 2 approved]
References
- 1. Vanier MT: Niemann-Pick disease type C. Orphanet J Rare Dis. 2010;5:16. 10.1186/1750-1172-5-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schwerd T, Pandey S, Yang HT, et al. : Impaired antibacterial autophagy links granulomatous intestinal inflammation in Niemann-Pick disease type C1 and XIAP deficiency with NOD2 variants in Crohn's disease. Gut. 2017;66(6):1060–1073. 10.1136/gutjnl-2015-310382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cougnoux A, Movassaghi M, Picache JA, et al. : Gastrointestinal Tract Pathology in a BALB/c Niemann-Pick Disease Type C1 Null Mouse Model. Dig Dis Sci. 2018;63(4):870–880. 10.1007/s10620-018-4914-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lamb CA, Kennedy NA, Raine T, et al. : British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68(Suppl 3): s1–s106. 10.1136/gutjnl-2019-318484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ruemmele FM, Veres G, Kolho KL, et al. : Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn's disease. J Crohns Colitis. 2014;8(10):1179–207. 10.1016/j.crohns.2014.04.005 [DOI] [PubMed] [Google Scholar]
- 6. Kaltsonoudis E, Zikou AK, Voulgari PV, et al. : Neurological adverse events in patients receiving anti-TNF therapy: a prospective imaging and electrophysiological study. Arthritis Res Ther. 2014;16(3):R125. 10.1186/ar4582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shivaji UN, Sharratt CL, Thomas T, et al. : Review article: managing the adverse events caused by anti-TNF therapy in inflammatory bowel disease. Aliment Pharmacol Ther. 2019;49(6):664–680. 10.1111/apt.15097 [DOI] [PubMed] [Google Scholar]
- 8. Bertrand V, Massy N, Pigneur B, et al. : Neurological Adverse Effects Associated With Anti-tumor Necrosis Factor Alpha Antibodies in Pediatric Inflammatory Bowel Diseases. J Pediatr Gastroenterol Nutr. 2020;70(6):841–848. 10.1097/MPG.0000000000002654 [DOI] [PubMed] [Google Scholar]
- 9. Lin S, Green HD, Hendy P, et al. : Clinical features and genetic risk of demyelination following anti-TNF treatment. J Crohns Colitis. 2020;jjaa104. 10.1093/ecco-jcc/jjaa104 [DOI] [PubMed] [Google Scholar]
- 10. Kunchok A, Aksamit AJ Jr, Davis JM 3rd, et al. : Association Between Tumor Necrosis Factor Inhibitor Exposure and Inflammatory Central Nervous System Events. JAMA Neurol. 2020;77(8):937–946. 10.1001/jamaneurol.2020.1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Turner D, Griffiths AM, Walters TD, et al. : Mathematical weighting of the pediatric Crohn's disease activity index (PCDAI) and comparison with its other short versions. Inflamm Bowel Dis. 2012;18(1): 55–62. 10.1002/ibd.21649 [DOI] [PubMed] [Google Scholar]
- 12. Williams I, Panday S, Haller W, et al. : Anti-TNF therapy for inflammatory bowel disease in patients with neurodegenerative Niemann-Pick disease Type C [Data set]. Zenodo. 2021. 10.5281/zenodo.5668321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dike CR, Bernat J, Bishop W, et al. : Niemann-Pick disease type C presenting as very early onset inflammatory bowel disease. BMJ Case Rep. 2019;12(7):e229780. 10.1136/bcr-2019-229780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van Rheenen H, van Rheenen PF: Long-Term Efficacy of Anti-Tumor Necrosis Factor Agents in Pediatric Luminal Crohn's Disease: A Systematic Review of Real-World Evidence Studies. Pediatr Gastroenterol Hepatol Nutr. 2020;23(2):121–131. 10.5223/pghn.2020.23.2.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ombrello AK, Qin J, Hoffmann PM, et al. : Treatment Strategies for Deficiency of Adenosine Deaminase 2. N Engl J Med. 2019;380(16):1582–1584. 10.1056/NEJMc1801927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kemanetzoglou E, Andreadou E: CNS Demyelination with TNF-α Blockers. Curr Neurol Neurosci Rep. 2017;17(4):36. 10.1007/s11910-017-0742-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. TNF neutralization in MS: results of a randomized, placebo-controlled multicenter study. The Lenercept Multiple Sclerosis Study Group and The University of British Columbia MS/MRI Analysis Group. Neurology. 1999;53(3):457–65. [PubMed] [Google Scholar]
- 18. Gregory AP, Dendrou CA, Attfield KE, et al. : TNF receptor 1 genetic risk mirrors outcome of anti-TNF therapy in multiple sclerosis. Nature. 2012;488(7412):508–511. 10.1038/nature11307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ehling R, Gassner C, Lutterotti A, et al. : Genetic variants in the tumor necrosis factor receptor II gene in patients with multiple sclerosis. Tissue Antigens. 2004;63(1): 28–33. 10.1111/j.1399-0039.2004.00166.x [DOI] [PubMed] [Google Scholar]
- 20. Zhu TH, Nakamura M, Abrouk M, et al. : Demyelinating disorders secondary to TNF-inhibitor therapy for the treatment of psoriasis: A review. J Dermatolog Treat. 2016;27(5):406–13. 10.3109/09546634.2015.1136385 [DOI] [PubMed] [Google Scholar]
- 21. Andersen NN, Caspersen S, Jess T, et al. : Occurrence of demyelinating diseases after anti-TNFα treatment of inflammatory bowel disease: A Danish Crohn Colitis Database study. J Crohns Colitis. 2008;2(4):304–9. 10.1016/j.crohns.2008.04.001 [DOI] [PubMed] [Google Scholar]
- 22. Dreyer L, Magyari M, Laursen B, et al. : Risk of multiple sclerosis during tumour necrosis factor inhibitor treatment for arthritis: a population-based study from DANBIO and the Danish Multiple Sclerosis Registry. Ann Rheum Dis. 2016;75(4):785–6. 10.1136/annrheumdis-2015-208490 [DOI] [PubMed] [Google Scholar]
- 23. Andreadou E, Kemanetzoglou E, Brokalaki C, et al. : Demyelinating Disease following Anti-TNFa Treatment: A Causal or Coincidental Association? Report of Four Cases and Review of the Literature. Case Rep Neurol Med. 2013;2013:671935. 10.1155/2013/671935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pegoretti V, Baron W, Laman JD, et al. : Selective Modulation of TNF-TNFRs Signaling: Insights for Multiple Sclerosis Treatment. Front Immunol. 2018;9:925. 10.3389/fimmu.2018.00925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Clark IA, Vissel B: A Neurologist's Guide to TNF Biology and to the Principles behind the Therapeutic Removal of Excess TNF in Disease. Neural Plast. 2015;2015:358263. 10.1155/2015/358263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Marchetti L, Klein M, Schlett K, et al. : Tumor necrosis factor (TNF)-mediated neuroprotection against glutamate-induced excitotoxicity is enhanced by N-methyl-D-aspartate receptor activation. Essential role of a TNF receptor 2-mediated phosphatidylinositol 3-kinase-dependent NF-kappa B pathway. J Biol Chem. 2004;279(31):32869–81. 10.1074/jbc.M311766200 [DOI] [PubMed] [Google Scholar]
- 27. Micheau O, Tschopp J: Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114(2):181–90. 10.1016/s0092-8674(03)00521-x [DOI] [PubMed] [Google Scholar]
- 28. Walterfang M, Fahey M, Desmond P, et al. : White and gray matter alterations in adults with Niemann-Pick disease type C: a cross-sectional study. Neurology. 2010;75(1):49–56. 10.1212/WNL.0b013e3181e6210e [DOI] [PubMed] [Google Scholar]