Abstract
Background
Hospital visitation has become challenging during the coronavirus disease 2019 pandemic because of quarantine measures and fear of infection. Consequently, newly diagnosed patients may present with more severe diseases during the pandemic. The present study analyzed the differences in the initial clinical presentations of newly diagnosed patients with type 1 diabetes (T1D) and type 2 diabetes (T2D), comparing pre-pandemic and pandemic periods.
Methods
Newly diagnosed patients with T1D or T2D and aged < 18 years during 2018–2020 were included in the study. Data were collected retrospectively from four academic centers in Gyeonggi-do, South Korea. Initial clinical data were compared between the pre-pandemic (2018–2019) and pandemic (2020) periods.
Results
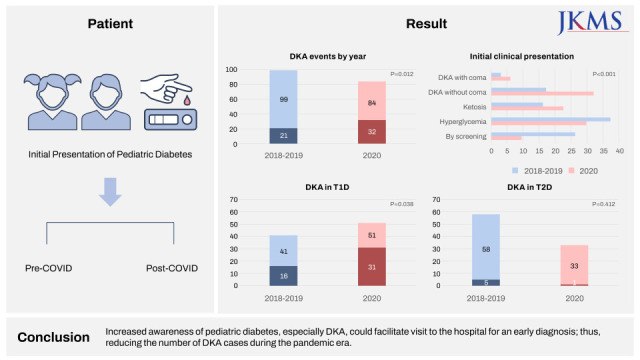
In the pre-pandemic and pandemic periods, 99 patients (41 T1D and 58 T2D patients) and 84 patients (51 T1D and 33 T2D patients) were identified, respectively. During the pandemic, the proportion of diabetic ketoacidosis (DKA) cases increased compared to the pre-pandemic period (21.2% during 2018–2019 vs. 38.1% in 2020; P = 0.012). In the pre-pandemic and pandemic periods, initial pH was 7.32 ± 0.14 and 7.27 ± 0.15, respectively (P = 0.040), and HbA1c values were 11.18 ± 2.46% and 12.42 ± 2.87%, respectively (P = 0.002). During the pandemic, there was an increased risk of DKA in patients with T1D (odds ratio, 2.42; 95% confidence interval, 1.04–5.62; P = 0.040).
Conclusion
During the pandemic, the proportion of DKA in newly diagnosed patients with T1D increased and clinical parameters showed a deteriorating pattern. Increased awareness of pediatric diabetes, especially DKA, could facilitate visit to the hospital for an early diagnosis; thus, reducing the number of DKA cases during the pandemic era.
Keywords: Diabetic Ketoacidosis, COVID-19, Diabetes Mellitus, Child, Adolescents
Graphical Abstract
INTRODUCTION
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2, first occurred in December 2019 in Wuhan, China, and has rapidly spread worldwide from the start of 2020. On March 11, 2020, the World Health Organization declared COVID-19 a pandemic and it was the most important health issue worldwide in 2020.1 In Korea, the first case of COVID-19 occurred on January 20, 2020, and since February 2020, it spread nationwide.2
The social distancing policy to prevent gatherings was implemented in Korea since March 21, 2020. Several quarantine measures to prevent the spread of COVID-19, such as social distancing and isolation, have made it challenging for patients to visit the hospitals. In addition, psychological factors such as fear of infection have decreased visits to the pediatric department.3,4 The tendency to avoid hospital visitation could lead to more severe diagnoses during the pandemic than in the pre-pandemic period.5
Diabetic ketoacidosis (DKA) is one of the most serious acute complications of diabetes mellitus (DM), a metabolic derangement of absolute or relative insulin deficiency and excess counter-regulatory hormones. DKA can manifest at diagnosis or result from poor glycemic control in patients with confirmed DM. Several studies reported that the incidence of DKA and severe DKA increased in children and adolescents with type 1 diabetes (T1D) during the COVID-19 pandemic period,6,7,8,9,10,11 along with increased incidence of DKA in patients with T2D.12 In contrast, some studies reported decreased number of admissions in pediatric patients with DKA during the pandemic period13 or no changes at all.14,15
During the study duration, the results of these changes were underappreciated throughout Korea. Furthermore, two studies conducted on pediatric patients with DM in Korea showed controversial results and had limitations, such as study population confined to patients with T1D and small sample size.16,17 Therefore, in the current study, we aimed to analyze differences in the initial clinical presentation of T1D and T2D over three years, between the pre-pandemic (2018–2019) and pandemic (2020) periods, in four medical centers in Korea.
METHODS
Study population
Newly diagnosed patients with T1D or T2D, aged < 18 years, from January 2018 to December 2020 were included in the study. Data were collected from four academic medical centers in Gyeonggi-do: Seoul National University Bundang Hospital, Korea University Ansan Hospital, Soonchunhyang University Bucheon Hospital, and Inje University Ilsan Paik Hospital.
DM diagnosis was based on the criteria of the American Diabetes Association: random plasma glucose ≥ 200 mg/dL with typical symptoms, fasting plasma glucose ≥ 126 mg/dL, or 2-hour plasma glucose value ≥ 200 mg/dL during a 75 g oral glucose tolerance test or glycosylated hemoglobin (HbA1c) level ≥ 6.5%.18 The diagnostic criteria of T1D included insulin treatment with a decreased C-peptide or positive autoantibodies or the presence of both criteria. The diagnostic criteria of T2D included symptoms and signs of insulin resistance such as acanthosis nigricans, presence of obesity, use of oral hypoglycemic agents, normal or increased level of C-peptide, and a family history of T2D. DKA diagnosis was based on the International Society for Pediatric and Adolescent Diabetes 2018 guidelines19; according to which, the following should be present: pH (< 7.3), bicarbonate (HCO3 −) level (< 15 mmol/L), and ketonemia (> 3 mmol/L) or ketonuria (> 2+). DKA severity was categorized as mild (pH < 7.3 or bicarbonate < 15 mmol/L), moderate (pH < 7.2 or bicarbonate < 10 mmol/L), or severe (pH < 7.1 or bicarbonate < 5 mmol/L). Patients diagnosed with other types of diabetes, including monogenic DM, were excluded.
Measures and outcomes
Data were obtained from a retrospective review of patient medical records. Data on clinical characteristics included sex, age, height, weight, body mass index (BMI), and sexual maturity rating (SMR) at diagnosis. In addition, personal information data included the interval from symptom onset to diagnosis, degree of body weight loss, birth weight, distance between home and hospital at first diagnosis, family history of DM, information regarding the patient’s home district, and insurance level. Height, weight, and BMI data were converted into a standard deviation score (SDS) using the 2017 Korean National Growth Charts.20 The distance between home and hospital was calculated via the shortest route possible as determined by a Korean Web map (https://map.naver.com). The insurance level pertained to medical expense coverage (National Health Insurance or Medical Aid). The patient’s information regarding home district was reported as either urban (address written with a city name [-si, -gu, or -dong]) or rural (with a village name [-eup, -myeon, or -ri]).
Laboratory data were collected as initial blood gas analysis results, including pH and bicarbonate. In addition, random glucose, serum electrolyte level, blood urea nitrogen (BUN), creatinine, anion gap, and level of total CO2, levels of HbA1c, insulin, C-peptide level, C-peptide in 24-hour urine, urine ketone, serum ketone, and diabetes-associated autoimmune antibodies were included. The estimated glomerular filtration rate was calculated using the Chronic Kidney Disease Epidemiology Collaboration creatinine equation.21 The initial presentation was classified into five classes: DKA with coma (less than 8 points on the Glasgow Coma Scale), DKA without coma, ketosis, hyperglycemia, and screening. Diabetes-associated antibodies included glutamic acid decarboxylase antibody, islet antigen-2 antibody, insulin antibody, and islet cell antibody.
The years 2018 to 2019 were defined as pre-pandemic period, while 2020 was defined as the pandemic period. The first COVID-19 case in Korea was reported on January 20, 2020 and since February 2020, this disease spread nationwide. In 2020, the first recorded patient with diabetes visited the hospital on January 23. Therefore, although the WHO declared the pandemic on March 11, 2020, all patients diagnosed in 2020 were classified into the pandemic group.
The primary outcome was comparing the DKA rate in newly diagnosed patients with DM between 2018–2019 and 2020. The secondary outcome was identifying risk factors correlating with DKA using patients’ clinical, social, and laboratory data.
Statistical analysis
All statistical analyses were performed using IBM SPSS software for Windows (version 25; IBM Corp., Armonk, NY, USA). We used an independent samples t-test to compare continuous variables between pre-pandemic and pandemic periods. Data were presented as mean and standard deviation for continuous variables. Pearson’s χ2 test, Fisher’s exact test, and linear-by-linear association were used to compare the proportions of events and categorical variables between the two groups.
The secondary outcomes for identifying clinical information associated with DKA were analyzed using binary logistic regression analysis. Differences were presented as odds ratios with 95% confidence intervals (CIs). Statistical significance was indicated by a 2-sided P value of < 0.05.
Ethics statement
The Institutional Review Boards (IRBs) of the four participating medical centers approved the study protocol with waived consent (IRB No.: B-2108/701-103, ISPAIK 2021-07-002, SCHBC 2021-09-009, and 2021AS0296).
RESULTS
The study enrolled 183 patients with newly diagnosed T1D or T2D between 2018 and 2020 from four medical centers. The pre-pandemic (2018–2019) and pandemic (2020) periods included, 99 patients (41 T1D and 58 T2D patients) and 84 patients (51 T1D and 33 T2D patients), respectively.
Clinical characteristics of patients during diagnosis by year
Table 1 presents the baseline characteristics of patients at diagnosis. In the pandemic period, mean weight SDS and BMI SDS decreased significantly and proportion of patients with T1D increased significantly. There was no difference between the pre-pandemic and pandemic periods in regards to age, sex, height SDS, SMR, duration from symptom onset to diagnosis, and degree of body weight loss. The severity of DKA, anion gap, and levels of random glucose, corrected sodium, BUN, creatinine, total CO2, C-peptide (serum and 24-hour urine), and ketone (urine and serum) showed no difference between the two groups. Additionally, personal information such as birth weight (whether small for gestational age or not), information concerning the patient’s home district (rural or urban), distance between home and hospital at first diagnosis, family history of diabetes mellitus, and insurance level did not show any differences.
Table 1. Clinical characteristics of patients at diagnosis by year.
| Clinical characteristics | 2018–2019 (n = 99) | 2020 (n = 84) | P value | ||
|---|---|---|---|---|---|
| Sex, male (%) | 51 (51.5) | 46 (54.8) | 0.611 | ||
| Age, yr | 12.17 ± 3.76 | 12.17 ± 3.51 | 0.998 | ||
| Height | 0.79 ± 1.13 | 0.55 ± 1.14 | 0.146 | ||
| Weight | 1.40 ± 1.94 | 0.58 ± 1.69 | 0.003 | ||
| BMIa | 1.29 ± 2.29 | 0.36 ± 1.93 | 0.004 | ||
| Sexual maturity rateb | 0.162 | ||||
| 1 | 14 (19.7) | 18 (29.0) | |||
| 2–3 | 16 (22.5) | 7 (11.3) | |||
| 4–5 | 41 (57.7) | 37 (59.7) | |||
| Diagnosis | 0.009 | ||||
| Type 1 diabetes | 41 (41.4) | 51 (60.7) | |||
| Type 2 diabetes | 58 (58.6) | 33 (39.3) | |||
| Duration since onset, daysc | 63.00 ± 113.76 | 88.86 ± 137.52 | 0.191 | ||
| Weight loss, kgd | 4.82 ± 6.56 | 5.25 ± 5.05 | 0.649 | ||
| Initial clinical presentation | |||||
| Initial presentation | < 0.001 | ||||
| By screening | 26 (26.3) | 8 (9.5) | |||
| Hyperglycemia | 37 (37.4) | 25 (29.8) | |||
| Ketosis | 16 (16.2) | 19 (22.6) | |||
| DKA without coma | 17 (17.2) | 27 (32.1) | |||
| DKA with coma | 3 (3.0) | 5 (6.0) | |||
| Ketonemia or ketonuriae | 34 (35.8) | 50 (59.5) | 0.001 | ||
| DKA | 21 (21.2) | 32 (38.1) | 0.012 | ||
| DKA by sex | 0.285 | ||||
| Male | 11 (52.4) | 12 (37.5) | |||
| Female | 10 (47.6) | 20 (62.5) | |||
| DKA by age group | 0.521 | ||||
| < 6 yr | 5 (23.8) | 4 (12.5) | |||
| 6–11 yr | 7 (33.3) | 14 (43.8) | |||
| 12–18 yr | 9 (42.9) | 14 (43.8) | |||
| Coma | 3 (14.3) | 6 (18.8) | 0.728 | ||
| Severity of DKA | 0.349 | ||||
| Mild | 5 (23.8) | 10 (31.3) | |||
| Moderate | 6 (28.6) | 13 (40.6) | |||
| Severe | 10 (47.6) | 9 (28.1) | |||
| Initial laboratory data | |||||
| pHf | 7.32 ± 0.14 | 7.27 ± 0.15 | 0.040 | ||
| Bicarbonate, mmol/Lf | 21.18 ± 8.31 | 18.22 ± 8.40 | 0.024 | ||
| Random glucose, mg/dLg | 366.07 ± 242.56 | 404.73 ± 249.80 | 0.292 | ||
| HbA1c, % | 11.18 ± 2.46 | 12.42 ± 2.87 | 0.002 | ||
| Corrected Na, mmol/Lh | 141.22 ± 4.15 | 140.38 ± 5.27 | 0.241 | ||
| Anion gap, mmol/Li | 21.31 ± 9.05 | 23.62 ± 9.97 | 0.111 | ||
| BUN, mg/dLj | 13.92 ± 7.18 | 13.55 ± 6.97 | 0.730 | ||
| Creatinine, mg/dLj | 0.70 ± 0.33 | 0.75 ± 0.40 | 0.305 | ||
| eGFR, mL/min/1.73 mj | 140.00 ± 28.89 | 134.90 ± 31.75 | 0.262 | ||
| Total CO2, mmol/Lk | 18.79 ± 7.57 | 16.02 ± 12.84 | 0.158 | ||
| Insulin, mIU/Ll | 15.85 ± 20.13 | 18.19 ± 30.47 | 0.562 | ||
| C-peptide (serum), ng/mLm | 2.60 ± 2.76 | 1.94 ± 2.25 | 0.087 | ||
| C-peptide (24-hr urine), μg/dayn | 27.89 ± 44.80 | 25.90 ± 45.69 | 0.821 | ||
| Ketone (urine)o | 1.18 ± 1.39 | 1.98 ± 1.47 | < 0.001 | ||
| Ketone (serum), mmol/Lp | 1.33 ± 1.78 | 2.33 ± 2.44 | 0.007 | ||
| Diabetes-associated antibodyq | 36 (37.5) | 43 (52.4) | 0.046 | ||
| Personal information | |||||
| SGAr | 15 (16.7) | 5 (7.2) | 0.076 | ||
| Home | 0.252 | ||||
| Urban | 84 (84.8) | 76 (90.5) | |||
| Rural | 15 (15.2) | 8 (9.5) | |||
| Distance from home, km | 13.95 (22.66) | 10.43 (10.37) | 0.170 | ||
| FHxs | 0.324 | ||||
| No | 25 (26.0) | 26 (33.3) | |||
| 1st degree | 22 (22.9) | 21 (26.9) | |||
| 2nd–3rd degree | 49 (51.0) | 31 (39.7) | |||
| Insurance | 0.144 | ||||
| National Health Insurance | 94 (94.9) | 83 (98.8) | |||
| Medical Aid | 5 (5.1) | 1 (1.2) | |||
Height, weight, and BMI data are converted into a mean with standard deviation score, other data are presented as mean with standard deviation or number of patients (%).
BMI = body mass index, DKA = diabetic ketoacidosis, HbA1c = glycosylated hemoglobin, BUN = blood urea nitrogen, eGFR = estimated glomerular filtration rate, SGA = small for gestational age, FHx = family history.
The items marked with small alphabet letters represent the total number of items, which are as follows:
aBMI (n = 182), bSexual maturity rate (n = 133), cDuration since onset (n = 163), dWeight loss (n = 154), eKetonuria or ketonemia (n = 179), fpH/Bicarbonate (n = 168), gRandom glucose (n=182), hCorrected Na (n = 175), iAnion gap (n = 175), jBUN/Creatinine/eGFR (n = 179), kTotal CO2 (n = 112), lInsulin (n = 160), mC-peptide (serum) (n = 180), nC-peptide (urine) (n = 109), oKetone (urine) (n = 176), pKetone (serum) (n = 131), qDiabetes-associated antibody (n = 178), rSGA (n = 159), sFHx (n = 174).
During the pandemic period, the proportion of DKA cases increased compared to previous years (21.2% during 2018–2019 vs. 38.1% in 2020; P = 0.012). The initial clinical presentation during diagnosis, which was classified into five groups based on severity, had a greater number of severe status than mild status in 2020 (P < 0.001). Although ketonemia or ketonuria cases increased in 2020 compared to those in 2018–2019 (35.8% vs. 59.5%; P = 0.012), DKA severity did not differ between the pre-pandemic and pandemic periods. Laboratory tests during diagnosis showed worse results in 2020. Decreased levels of initial pH and bicarbonate (pH 7.32 ± 0.14 during 2018–2019 vs. 7.27±0.15 in 2020, P = 0.040; bicarbonate 21.18 ± 8.31 mmol/L vs. 18.22 ± 8.40 mmol/L, P = 0.024) were noted. Although the mean random glucose level showed no significant difference, the HbA1c was higher in 2020 than in 2018–2019 (11.18 ± 2.46% vs. 12.42 ± 2.87%, P = 0.002). Levels of ketones in the urine and serum increased in 2020 compared to those during 2018–2019, indicating worse condition outcomes during the initial visit (urine ketone 1.18 ± 1.39 vs. 1.98 ± 1.47, P < 0.001; serum ketone 1.33 ± 1.78 mmol/L vs. 2.33 ± 2.44 mmol/L, P = 0.007).
A total of 39 newly diagnosed patients with DM underwent COVID-19 evaluation in 2020, and none of them were diagnosed with COVID-19.
Clinical characteristics of patients during diagnosis and type of diabetes by year
The baseline characteristics of the patients according to the type of diabetes are shown in Table 2. There were significant changes in patients with T1D during the pandemic era. The proportion of DKA in patients with T1D was higher in 2020 than in 2018–2019 (39.0% vs. 60.8%; P = 0.038). In addition, HbA1c and ketone levels in both the serum and urine were higher among patients with T1D during the pandemic period (HbA1c: 12.13 ± 2.08% vs. 13.44 ± 2.36%, P = 0.007; urine ketone: 1.93 ± 1.33 vs. 2.53 ± 1.17, P = 0.026; and serum ketone: 1.89 ± 1.62 mmol/L vs. 3.35 ± 2.46 mmol/L, P = 0.003). Patients with T1D showed a more severe initial presentation in 2020 than in 2018–2019 (P = 0.040).
Table 2. Clinical characteristics of patients at diagnosis by type of diabetes.
| Clinical characteristics | Type 1 DM (n = 92) | Type 2 DM (n = 91) | ||||||
|---|---|---|---|---|---|---|---|---|
| 2018–2019 (n = 41) | 2020 (n = 51) | P value | 2018–2019 (n = 58) | 2020 (n = 33) | P value | |||
| Sex, male | 15 (36.6) | 22 (43.1) | 0.524 | 36 (62.1) | 24 (72.7) | 0.302 | ||
| Age, yr | 10.00 ± 4.21 | 10.92 ± 3.76 | 0.272 | 13.71 ± 2.46 | 14.11 ± 1.89 | 0.421 | ||
| Height | 0.71 ± 0.97 | 0.37 ± 1.13 | 0.137 | 0.86 ± 1.24 | 0.82 ± 1.11 | 0.891 | ||
| Weight | 0.10 ± 1.49 | −0.38 ± 1.31 | 0.104 | 2.31 ± 24.39 | 2.05 ± 1.00 | 0.430 | ||
| BMIa | −0.32 ± 1.86 | −0.75 ± 1.41 | 0.217 | 2.39 ± 1.86 | 2.07 ± 1.23 | 0.381 | ||
| Sexual maturity rateb | 0.812 | 0.235 | ||||||
| 1 | 13 (38.2) | 17 (42.5) | 1 (2.7) | 1 (4.5) | ||||
| 2–3 | 7 (20.6) | 6 (15.0) | 9 (24.3) | 1 (4.5) | ||||
| 4–5 | 14 (41.2) | 17 (42.5) | 27 (73.0) | 20 (90.9) | ||||
| Duration since onset, daysc | 65.41 ± 129.76 | 47.62 ± 49.45 | 0.373 | 60.98 ± 99.78 | 178.52 ± 210.18 | 0.017 | ||
| Weight loss, kgd | 3.99 ± 4.30 | 4.71 ± 4.45 | 0.438 | 5.57 ± 8.07 | 6.61 ± 6.24 | 0.613 | ||
| Initial clinical presentation | ||||||||
| Initial presentation | 0.040 | 0.106 | ||||||
| By screening | 3 (7.3) | 1 (2.0) | 23 (39.7) | 7 (21.2) | ||||
| Hyperglycemia | 12 (29.3) | 10 (19.6) | 25 (43.1) | 15 (45.5) | ||||
| Ketosis | 10 (24.4) | 10 (19.6) | 6 (10.3) | 9 (27.3) | ||||
| DKA without coma | 14 (34.1) | 25 (49.0) | 3 (5.2) | 2 (6.1) | ||||
| DKA with coma | 2 (4.9) | 5 (9.8) | 1 (1.7) | 0 (0.0) | ||||
| Ketonemia or ketonuriae | 25 (62.5) | 40 (78.4) | 0.095 | 9 (16.4) | 10 (30.3) | 0.124 | ||
| DKA | 16 (39.0) | 31 (60.8) | 0.038 | 5 (8.6) | 1 (3.0) | 0.411 | ||
| DKA by sex | 0.581 | |||||||
| Male | 7 (43.8) | 11 (35.5) | 4 (80.0) | 1 (100.0) | ||||
| Female | 9 (56.3) | 20 (64.5) | 1 (20.0) | 0 (0.0) | ||||
| DKA by age group | 0.314 | |||||||
| < 6 yr | 5 (31.3) | 4 (12.9) | 0 (0.0) | 0 (0.0) | ||||
| 6–11 yr | 6 (37.5) | 14 (45.2) | 1 (20.0) | 0 (0.0) | ||||
| 12–18 yr | 5 (31.3) | 13 (41.9) | 4 (80.0) | 1 (100.0) | ||||
| Coma | 3 (18.8) | 6 (19.4) | 0.726 | 0 (0.0) | 0 (0.0) | |||
| Severity of DKA | 0.148 | 0.655 | ||||||
| Mild | 2 (12.5) | 10 (25.5) | 3 (60.0) | 0 (0.0) | ||||
| Moderate | 5 (31.3) | 12 (36.2) | 1 (20.0) | 1 (100.0) | ||||
| Severe | 9 (56.3) | 9 (38.3) | 1 (20.0) | 0 (0.0) | ||||
| Initial laboratory data | ||||||||
| pHf | 7.26 ± 0.18 | 7.21 ± 0.15 | 0.221 | 7.36 ± 0.07 | 7.37 ± 0.05 | 0.527 | ||
| Bicarbonate, mmol/Lf | 16.90 ± 8.85 | 15.51 ± 8.83 | 0.459 | 24.62 ± 5.99 | 23.44 ± 3.98 | 0.369 | ||
| Random glucose, mg/dLg | 477.02 ± 248.01 | 479.61 ± 265.82 | 0.962 | 286.26 ± 206.10 | 289.00 ± 169.39 | 0.949 | ||
| HbA1c, % | 12.13 ± 2.08 | 13.44 ± 2.36 | 0.007 | 10.51 ± 2.50 | 10.84 ± 2.90 | 0.565 | ||
| Corrected Na, mmol/Lh | 140.67 ± 2.93 | 140.76 ± 6.37 | 0.927 | 141.63 ± 4.85 | 139.73 ± 2.37 | 0.050 | ||
| Anion gap, mmol/Li | 23.71 ± 8.41 | 24.89 ± 9.39 | 0.537 | 19.52 ± 9.17 | 21.43 ± 10.70 | 0.395 | ||
| BUN, mg/dLh | 14.98 ± 6.05 | 14.37 ± 8.44 | 0.701 | 13.17 ± 7.87 | 12.16 ± 2.78 | 0.502 | ||
| Creatinine, mg/dLi | 0.71 ± 0.27 | 0.81 ± 0.46 | 0.220 | 0.69 ± 0.38 | 0.66 ± 0.28 | 0.718 | ||
| eGFR, mL/min per 1.73 m2j | 136.86 ± 30.75 | 129.41 ± 35.03 | 0.288 | 142.27 ± 27.53 | 144.21 ± 22.88 | 0.741 | ||
| Total CO2, mmol/Lk | 15.33 ± 8.10 | 14.78 ± 14.55 | 0.847 | 22.73 ± 4.45 | 18.92 ± 7.09 | 0.072 | ||
| Insulin mIU/Ll | 8.74 ± 16.61 | 12.55 ± 27.83 | 0.476 | 20.73 ± 21.01 | 26.95 ± 32.76 | 0.305 | ||
| C-peptide (serum), ng/mLm | 0.82 ± 0.78 | 0.67 ± 0.71 | 0.337 | 3.88 ± 2.96 | 3.84 ± 2.42 | 0.947 | ||
| C-peptide (24-hr urine), μg/dayn | 18.45 ± 46.85 | 9.78 ± 13.17 | 0.310 | 37.95 ± 40.92 | 63.93 ± 68.85 | 0.125 | ||
| Ketone (urine)o | 1.93 ± 1.33 | 2.53 ± 1.17 | 0.026 | 0.63 ± 1.18 | 1.07 ± 1.48 | 0.166 | ||
| Ketone (serum), mmol/Lp | 1.89 ± 1.62 | 3.35 ± 2.46 | 0.003 | 0.72 ± 1.76 | 0.65 ± 1.11 | 0.861 | ||
| Diabetes-associated antibodyq | 30 (73.2) | 41 (80.4) | 0.412 | 6 (10.9) | 2 (6.5) | 0.705 | ||
| Personal Information | ||||||||
| SGAr | 8 (20.0) | 1 (2.3) | 0.008 | 7 (14.0) | 4 (16.0) | > 0.999 | ||
| Home | 0.480 | 0.384 | ||||||
| Urban | 35 (85.4) | 46 (90.2) | 49 (84.5) | 30 (90.9) | ||||
| Rural | 6 (14.6) | 5 (9.8) | 9 (15.5) | 2 (9.1) | ||||
| Distance from home, km | 12.88 ± 20.31 | 11.56 ± 11.99 | 0.702 | 14.71 ± 24.32 | 8.73 ± 7.10 | 0.085 | ||
| FHxs | 0.898 | 0.103 | ||||||
| No | 13 (33.3) | 17 (36.2) | 12 (21.1) | 9 (29.0) | ||||
| 1st degree | 5 (12.8) | 7 (14.9) | 17 (29.8) | 14 (45.2) | ||||
| 2nd–3rd degree | 21 (53.8) | 23 (48.9) | 28 (49.1) | 8 (25.8) | ||||
| Insurance | 0.113 | > 0.999 | ||||||
| National health insurance | 39 (95.1) | 51 (100) | 55 (94.8) | 32 (97.0) | ||||
| Medical aid | 2 (4.9) | 0 (0) | 3 (5.2) | 1 (3.0) | ||||
Height, weight, and BMI data are converted into a mean with standard deviation score, other data are presented as mean with standard deviation or number of patients (%).
DM = diabetes mellitus, BMI = body mass index, DKA = diabetic ketoacidosis, HbA1c = glycosylated hemoglobin, BUN = blood urea nitrogen, eGFR = estimated glomerular filtration rate, SGA = small for gestational age, FHx = family history.
The items marked with small alphabet letters represent the total number of items, which are as follows:
Type 1 DM: aBMI (n =91), bSexual maturity rate (n = 74), cDuration since onset (n = 91), dWeight loss (n = 90), eKetonemia or ketonuria (n = 92), fpH/Bicarbonate (n = 91), hCorrected Na (n = 91), iAnion gap (n = 91), kTotal CO2 (n = 68), lInsulin (n = 80), mC-peptide (serum) (n = 90), nC-peptide (24-hr urine) (n = 65), oKetone (urine) (n = 91), pKetone (serum) (n = 75), rSGA (n = 84), sFHx (n = 86).
Type 2 DM: bSexual maturity rate (n = 59), cDuration since onset (n = 72), dWeight loss (n = 64), eKetonemia or ketonuria (n = 91), fpH/Bicarbonate (n = 77), gRandom glucose (n = 90), hCorrected Na (n = 84), iAnion gap (n = 84), jBUN/Creatinine/eGFR (n = 87), kTotal CO2 (n = 44), lInsulin (n = 80), mC-peptide (n = 90), nC-peptide (urine) (n = 44), oKetone (urine) (n = 85), pKetone (serum) (n = 56), qDiabetes-associated antibody (n = 86), rSGA (n = 75), sFHx (n = 88).
In patients with T2D in 2020, a higher number of patients had longer duration of symptoms than patients with T2D in 2018–2019 (60.98 ± 99.78 days vs. 178.52 ± 210.18 days, P = 0.017). No other differences were observed among patients with T2D.
Clinical characteristics affecting DKA during diagnosis
Factors affecting DKA during diagnosis are presented in Table 3. Newly diagnosed patients with DM in 2020 had 2.286-fold higher odds (95% CI, 1.190–4.390; P = 0.013) of DKA compared to those in 2018–2019. Lower weight (SDS), BMI (SDS), and higher HbA1c were independent risk factors for DKA. In addition, lower age and family history of a first-degree diabetic relative are risk factors for DKA.
Table 3. Factors affecting diabetic ketoacidosis at diagnosis by type of diabetes.
| Factors | Type 1 diabetes | Type 2 diabetes | Total | ||||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | ||
| Year | |||||||
| 2018–2019 | (ref.) | 0.331 (0.037–2.964) | (ref.) | ||||
| 2020 | 2.422 (1.043–5.624) | 0.040 | 0.331 (0.037–2.964) | 0.323 | 2.286 (1.190–4.390) | 0.013 | |
| Sex | |||||||
| Male | (ref.) | (ref.) | |||||
| Female | 1.177 (0.511–2.711) | 0.701 | 0.367 (0.041–3.285) | 0.370 | 1.724 (0.905–3.24) | 0.098 | |
| Age, yr | 0.955 (0.860–1.060) | 0.384 | 1.117 (0.753–1.657) | 0.582 | 0.852 (0.778–0.932) | < 0.001 | |
| Distance | 0.995 (0.970–1.022) | 0.730 | 0.990 (0.934–1.049) | 0.734 | 0.996 (0.977–1.015) | 0.649 | |
| Home | |||||||
| Urban | (ref) | (ref) | |||||
| Rural | 0.774 (0.219–2.740) | 0.691 | 1.345 (0.143–2.619) | 0.795 | 0.849 (0.315–2.286) | 0.745 | |
| Height SDS | 0.818 (0.554–1.209) | 0.314 | 0.886 (0.442–1.778) | 0.734 | 0.767 (0.575–1.023) | 0.071 | |
| Weight SDS | 0.700 (0.506–0.968) | 0.031 | 0.955 (0.551–1.656) | 0.869 | 0.559 (0.447–0.698) | < 0.001 | |
| BMI SDS | 0.756 (0.571–1.002) | 0.052 | 0.998 (0.605–1.647) | 0.994 | 0.604 (0.496–0.735) | < 0.001 | |
| SMR | |||||||
| 1 | (ref) | (ref) | |||||
| 2–3 | 0.655 (0.177–2.424) | 0.527 | 0.00 | 0.999 | 0.275 (0.086–0.879) | 0.029 | |
| 4–5 | 0.552 (0.200–1.524) | 0.252 | 0.068 (0.003–1.381) | 0.080 | 0.201 (0.083–0.488) | < 0.001 | |
| HbA1c, % | 1.333 (1.089–1.631) | 0.005 | 1.257 (0.893–1.769) | 0.189 | 1.461 (1.251–1.706) | < 0.001 | |
| Duration since onset, day | 0.996 (0.990–1.002) | 0.188 | 0.994 (0.983–1.006) | 0.356 | 0.995 (0.990–1.000) | 0.031 | |
| Weight loss, kg | 1.060 (0.960–1.170) | 0.252 | 1.011 (0.900–1.137) | 0.849 | 1.002 (0.947–1.061) | 0.937 | |
| Birth weight, kg | 1.048 (0.451–2.437) | 0.913 | 0.755 (0.179–3.189) | 0.702 | 1.016 (0.553–1.867) | 0.960 | |
| FHx | |||||||
| No | (ref) | (ref) | |||||
| 1st degree | 0.609 (0.238–1.558) | 0.301 | 3.226 (0.351–9.683) | 0.301 | 0.173 (0.053–0.599) | 0.003 | |
| 2nd–3rd degree | 0.333 (0.082–1.359) | 0.125 | 0.00 | 0.998 | 0.811 (0.389–1.692) | 0.577 | |
OR = odds ratio, CI = confidence interval, SDS = standard deviation score, BMI = body mass index, SMR = sexual maturity rating, HbA1c = glycosylated hemoglobin, FHx = family history.
During the pandemic period, there was an increased risk of DKA among patients with T1D (odds ratio, 2.42; 95% confidence interval, 1.04–5.62; P = 0.040). Moreover, lower weight (SDS) and higher HbA1c were proven independent risk factors for DKA. Conversely, no substantial differences were observed between the factors for patients with T2D and DKA.
DISCUSSION
Our study showed higher rates of DKA incidence during the pandemic period. Moreover, the overall poor metabolic status of DM during diagnosis was accompanied by higher HbA1c and serum and urine ketone levels; lower pH and bicarbonate levels; and more severe state of initial clinical presentation in the pandemic era. Our study revealed that COVID-19 was a risk factor for DKA, and younger age, low weight, low BMI, and high HbA1c were independently associated with DKA. Interestingly, these findings were predominant in patients with T1D.
The findings of this study are similar to those of previous studies, revealing increased number of children with T1D presenting with DKA during the COVID-19 pandemic.6,7,8,9,10,11 Kamrath et al.6 and Ho et al.11 reported increased incidences of both the DKA and severe DKA. In addition, a study in Romania showed an increased number of new patients with T1D in 2020 and increased incidences of DKA and severe DKA.22
In contrast, the current study showed increased incidences of DKA but not severe DKA. As mentioned above, studies in Germany and Canada have shown increases in both DKA and severe DKA.6,9,11 A study in Italy found little difference as, despite more severe DKA events, there was a similar incidence in the number of DKA events in 2020 compared with previous years.7 Alaqeel et al.8 reported similar results, which showed increase in DKA cases but not in its severity among patients with T1D. Because there was no strict lockdown policy in Korea, access to the hospitals was possible whenever necessary; this explains the lack of difference in the severity of DKA between pre-pandemic and pandemic periods in the current study.
The increase in the total number of T1D cases may have contributed to the rise in number of DKA because DKA at diagnosis is more prevalent among those with T1D than T2D. In our study, the number of patients with new-onset T1D tended to increase, with 22 patients in 2018, 20 patients in 2019, and 51 patients in 2020. This trend differs from previous studies in Canada, Germany, Saudi Arabia, and the United States, which found no significant difference in the number of patients newly diagnosed with diabetes.8,9,10,11 However, a recent study in the United States showed a trend similar to this study; the total number of newly diagnosed patients with T1D increased significantly during COVID-19 pandemic compared to that in the previous 5 years.23 Conflicting results from previous literatures which showed no difference of T1D prevalence in COVID-19 era, it is unclear whether that COVID-19 is the reason for the increase in the incidence of T1D. Moreover, the increased number of newly diagnosed patients with T1D may indicate a trend for the rise in the number of patients with diabetes, as shown in previous records in Korea.24,25
On the other hand, the incidence of DKA in patients with T2D did not significantly differ annually and total number of newly diagnosed patients with T2D was similar annually (24 patients in 2018, 34 patients in 2019, and 33 patients in 2020). A study in the United States conducted on patients with T2D showed heterogeneous result compared with our study result. In that study, although the DKA cases increased in 2020 compared to the previous years12; the total number of newly diagnosed patients with T2D doubled from 44 in 2018 to 82 in 2020, and the total number of DKA cases increased from < 10% in 2018–2019 to 20% in 2020. Another study in the UK reported similar severity or duration of DKA in patients with T2D between the pre-pandemic and pandemic groups.15 However, our study reported no changes in BMI (SDS) and weight (SDS) between the pre-pandemic and pandemic periods. This might be due to the lack of physical activity during the pandemic and more severe conditions during diagnosis.
The proportion of DKA cases observed in our study was higher than that in previous Korean studies. Kim et al.25 reported that DKA occurred in 39.7% of newly diagnosed patients with T1D between 2012 and 2014, and another study by Lee et al.26 revealed that 49.0% of new patients with T1D were diagnosed with DKA during diagnosis between 2000 and 2015. The higher DKA rate in patients with T1D in our study, especially 60.8% in 2020, might be the consequences of COVID-19. In our study, the proportion DKA in patients with T2D was 6.59% for three years (8.6% in 2018–2019, 3.0% in 2020). A previous study reported that the proportion of DKA in patients with T2D in a single-center study was 0% from 1980 to 2000.27 Another single-center study by Yun et al.28 reported that 4.35% (3/69) of newly diagnosed patients with T2D had DKA between 1991 and 2007. This reflects the overall increasing trend of T2D and DKA incidences in Korea.
There have been two studies in South Korea comparing the rate of DKA in patients with T1D between the pre-pandemic and pandemic periods. A study showed increased number of DKA cases in the emergency room during 2020 compared to the previous three years. However, the total number of subjects were 19 for four years, resulting in limitations due to the insufficient sample size.17 Another study in Daegu-Gyeongbuk revealed a similar incidence of DKA between the pre-pandemic and pandemic periods among newly diagnosed patients with T1D, although HbA1c significantly deteriorated at diagnosis in 2020.16 To the best of our knowledge, current study is the first multicenter study conducted in Korea that compared DKA incidence in newly diagnosed pediatric patients with both T1D and T2D in the COVID-19 pandemic period to that of the prior two years.
In the present study, social data was analyzed between 2018–2019 and 2020, such as distance between home and hospital, insurance level, and home district. Although we assumed that the distance between home and hospital would affect initial clinical manifestation in newly diagnosed DM patients, it did not have a significant effect, whether in the pre-pandemic or the pandemic period. The distance between home and hospital was insignificant because patients living in both the rural and urban areas might be similar in regards having access to hospital facilities. In addition, patients whose insurance was covered by medical aid and those who were covered by national health insurance showed similar clinical severity at diagnosis, suggesting similar accessibility to medical services. Most patients had good access to medical services; only a few lived in rural districts (12.6% Rural area vs. 87.43% Urban area) and received medical aid (3.28% Medical aid vs. 96.72% National health insurance).
The pandemic era has been a concern for medical personnel for severe illnesses. According to many reports, during the pandemic period, the tendency of delayed diagnosis with severe clinical outcomes is evident in chronic diseases, such as cancer. For example, although the number of new cancer diagnoses declined in 2020, the mortality increased because of decreased routine screening and non-urgent surgeries.29,30 This trend is the same for DM; Elbarbary et al.31 published a large global survey of 215 clinicians from diabetes centers during the COVID-19 pandemic, which showed that 22% of participants reported delayed diagnosis in children with new-onset DM. In the study, a 15% increase in DKA was observed and 68% of clinicians responded that caregivers or families had difficulty in contacting the diabetic team.31
The underlying causes for the increased number of DKA have not been elucidated. The governmental policies to minimize transmission and fear of infection contribute to limited number of hospital visits and subsequent significant deterioration in the patients’ first clinical presentations. This has greater influence on children and adolescents than on older people. Overall, the number of pediatric emergency department visits significantly declined after the COVID-19 pandemic in Korea, especially in Seoul and Gyeonggi-do,32 similar to the trends observed in the United States.3 However, the admission rate was higher in the pandemic period than in the pre-pandemic period, suggesting worsening disease severity.32
In addition, a decrease in diagnosis by screening examination may affect the severity of the initial clinical presentation. In Korea, all students undergo annual medical examinations in school and urinalysis for diabetic screening; however, these screenings were canceled in 2020 because of the prohibition of gatherings. Therefore, diagnosis through screening decreased from 26 patients (26.3%) in pre-pandemic period (2018–2019) to 8 patients (9.5%) in pandemic period (2020).
This study has several limitations. Since the data were confined to four medical centers in Gyeonggi-do, it is difficult to generalize nationwide trends. Therefore, further studies with larger cohorts are necessary to improve our knowledge. Due to the retrospective study design, lifestyle factors associated with the development of DM could not be investigated. However, a particular strength of our study is the individual analysis of the initial clinical presentation of the new-onset T1D and T2D subtypes. Our study analyzed the relationship between clinical and social data such as social-economic state, parents’ education level, the distance between home and hospital at first diagnosis, patients’ home districts, and insurance level to determine risk factors for DKA.
During the pandemic, the patients’ are afraid to access medical services due to fear of infection, resulting in delayed diagnosis and subsequent treatment of diseases. Accordingly, our study comprising newly diagnosed pediatric patients with DM observed increased DKA incidences and worse trend in the initial laboratory and clinical evaluations during the COVID-19 pandemic. In this context, it is necessary for healthcare providers to increase the awareness of pediatric diabetes, especially DKA, in the caregivers or families to facilitate hospital visits for early diagnosis and reduce the prevalence of DKA.
Footnotes
Disclosure: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Kim JH.
- Data curation: Lee YH, Kim MS, Oh KE, Kang E, Rhie YJ, Lee J, Hong YH, Shin YJ, Kim JH.
- Writing - original draft: Lee YH, Kim JH.
- Writing - review & editing: Kim MS, Oh KE, Kang E, Rhie YJ, Lee J, Hong YH, Shin YJ, Kim JH.
References
- 1.Jin Y, Yang H, Ji W, Wu W, Chen S, Zhang W, et al. Virology, epidemiology, pathogenesis, and control of COVID-19. Viruses. 2020;12(4):E372. doi: 10.3390/v12040372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim JY, Choe PG, Oh Y, Oh KJ, Kim J, Park SJ, et al. The first case of 2019 novel coronavirus pneumonia imported into Korea from Wuhan, China: implication for infection prevention and control measures. J Korean Med Sci. 2020;35(5):e61. doi: 10.3346/jkms.2020.35.e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boserup B, McKenney M, Elkbuli A. The impact of the COVID-19 pandemic on emergency department visits and patient safety in the United States. Am J Emerg Med. 2020;38(9):1732–1736. doi: 10.1016/j.ajem.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mantica G, Riccardi N, Terrone C, Gratarola A. Non-COVID-19 visits to emergency departments during the pandemic: the impact of fear. Public Health. 2020;183:40–41. doi: 10.1016/j.puhe.2020.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cherubini V, Gohil A, Addala A, Zanfardino A, Iafusco D, Hannon T, et al. Unintended consequences of coronavirus disease-2019: remember general pediatrics. J Pediatr. 2020;223:197–198. doi: 10.1016/j.jpeds.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kamrath C, Mönkemöller K, Biester T, Rohrer TR, Warncke K, Hammersen J, et al. Ketoacidosis in children and adolescents with newly diagnosed type 1 diabetes during the COVID-19 pandemic in Germany. JAMA. 2020;324(8):801–804. doi: 10.1001/jama.2020.13445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rabbone I, Schiaffini R, Cherubini V, Maffeis C, Scaramuzza A Diabetes Study Group of the Italian Society for Pediatric Endocrinology and Diabetes. Has COVID-19 delayed the diagnosis and worsened the presentation of type 1 diabetes in children? Diabetes Care. 2020;43(11):2870–2872. doi: 10.2337/dc20-1321. [DOI] [PubMed] [Google Scholar]
- 8.Alaqeel A, Aljuraibah F, Alsuhaibani M, Huneif M, Alsaheel A, Dubayee MA, et al. The impact of COVID-19 pandemic lockdown on the incidence of new-onset type 1 diabetes and ketoacidosis among Saudi children. Front Endocrinol (Lausanne) 2021;12:669302. doi: 10.3389/fendo.2021.669302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sellers EAC, Pacaud D. Diabetic ketoacidosis at presentation of type 1 diabetes in children in Canada during the COVID-19 pandemic. Paediatr Child Health. 2021;26(4):208–209. doi: 10.1093/pch/pxab017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loh C, Weihe P, Kuplin N, Placzek K, Weihrauch-Blüher S. Diabetic ketoacidosis in pediatric patients with type 1- and type 2 diabetes during the COVID-19 pandemic. Metabolism. 2021;122:154842. doi: 10.1016/j.metabol.2021.154842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ho J, Rosolowsky E, Pacaud D, Huang C, Lemay JA, Brockman N, et al. Diabetic ketoacidosis at type 1 diabetes diagnosis in children during the COVID-19 pandemic. Pediatr Diabetes. 2021;22(4):552–557. doi: 10.1111/pedi.13205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chao LC, Vidmar AP, Georgia S. Spike in diabetic ketoacidosis rates in pediatric type 2 diabetes during the COVID-19 pandemic. Diabetes Care. 2021;44(6):1451–1453. doi: 10.2337/dc20-2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Misra S, Barron E, Vamos E, Thomas S, Dhatariya K, Kar P, et al. Temporal trends in emergency admissions for diabetic ketoacidosis in people with diabetes in England before and during the COVID-19 pandemic: a population-based study. Lancet Diabetes Endocrinol. 2021;9(10):671–680. doi: 10.1016/S2213-8587(21)00208-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atlas G, Rodrigues F, Moshage Y, Welch J, White M, O’Connell MA. Presentation of paediatric type 1 diabetes in Melbourne, Australia during the initial stages of the COVID-19 pandemic. J Paediatr Child Health. 2020;56(10):1654–1655. doi: 10.1111/jpc.15081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kempegowda P, Melson E, Johnson A, Wallett L, Thomas L, Zhou D, et al. Effect of COVID-19 on the clinical course of diabetic ketoacidosis (DKA) in people with type 1 and type 2 diabetes. Endocr Connect. 2021;10(4):371–377. doi: 10.1530/EC-20-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee MS, Lee R, Ko CW, Moon JE. Increase in blood glucose level and incidence of diabetic ketoacidosis in children with type 1 diabetes mellitus in the Daegu-Gyeongbuk area during the coronavirus disease 2019 (COVID-19) pandemic: a retrospective cross-sectional study. J Yeungnam Med Sci. 2022;39(1):46–52. doi: 10.12701/yujm.2021.01221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han MJ, Heo JH. Increased Incidence of pediatric diabetic ketoacidosis after COVID-19: a two-center retrospective study in Korea. Diabetes Metab Syndr Obes. 2021;14:783–790. doi: 10.2147/DMSO.S294458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes-2019 . Diabetes Care. 2019;42(Suppl 1):S13–S28. doi: 10.2337/dc19-S002. [DOI] [PubMed] [Google Scholar]
- 19.Wolfsdorf JI, Glaser N, Agus M, Fritsch M, Hanas R, Rewers A, et al. ISPAD clinical practice consensus guidelines 2018: diabetic ketoacidosis and the hyperglycemic hyperosmolar state. Pediatr Diabetes. 2018;19(Suppl 27):155–177. doi: 10.1111/pedi.12701. [DOI] [PubMed] [Google Scholar]
- 20.Kim JH, Yun S, Hwang SS, Shim JO, Chae HW, Lee YJ, et al. The 2017 Korean National Growth Charts for children and adolescents: development, improvement, and prospects. Korean J Pediatr. 2018;61(5):135–149. doi: 10.3345/kjp.2018.61.5.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boboc AA, Novac CN, Ilie MT, Ieșanu MI, Galoș F, Bălgrădean M, et al. The impact of SARS-CoV-2 pandemic on the new cases of T1DM in children. A single-centre cohort study. J Pers Med. 2021;11(6):551. doi: 10.3390/jpm11060551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gottesman BL, Yu J, Tanaka C, Longhurst CA, Kim JJ. Incidence of new-onset type 1 diabetes among US children during the COVID-19 global pandemic. JAMA Pediatr. 2022;176(4):414–415. doi: 10.1001/jamapediatrics.2021.5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chae HW, Seo GH, Song K, Choi HS, Suh J, Kwon A, et al. Incidence and prevalence of type 1 diabetes mellitus among Korean children and adolescents between 2007 and 2017: an epidemiologic study based on a national database. Diabetes Metab J. 2020;44(6):866–874. doi: 10.4093/dmj.2020.0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim JH, Lee CG, Lee YA, Yang SW, Shin CH. Increasing incidence of type 1 diabetes among Korean children and adolescents: analysis of data from a nationwide registry in Korea. Pediatr Diabetes. 2016;17(7):519–524. doi: 10.1111/pedi.12324. [DOI] [PubMed] [Google Scholar]
- 26.Lee HJ, Yu HW, Jung HW, Lee YA, Kim JH, Chung HR, et al. Factors associated with the presence and severity of diabetic ketoacidosis at diagnosis of type 1 diabetes in Korean children and adolescents. J Korean Med Sci. 2017;32(2):303–309. doi: 10.3346/jkms.2017.32.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park JM, Yoo EG, Kim DH. Type 2 diabetes mellitus in children. J Korean Pediatr Soc. 2002;45(5):646–653. [Google Scholar]
- 28.Yun KA, Lee YA, Shin CH, Yang SW. Clinical course of childhood and adolescence onset type 2 diabetes mellitus. J Korean Soc Pediatr Endocrinol. 2009;14(1):19–24. [Google Scholar]
- 29.Patt D, Gordan L, Diaz M, Okon T, Grady L, Harmison M, et al. Impact of COVID-19 on cancer care: how the pandemic is delaying cancer diagnosis and treatment for American seniors. JCO Clin Cancer Inform. 2020;4(4):1059–1071. doi: 10.1200/CCI.20.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vose JM. Delay in cancer screening and diagnosis during the COVID-19 pandemic: what is the cost? Oncology (Williston Park) 2020;34(9):343. doi: 10.46883/ONC.2020.3409.0343. [DOI] [PubMed] [Google Scholar]
- 31.Elbarbary NS, Dos Santos TJ, de Beaufort C, Agwu JC, Calliari LE, Scaramuzza AE. COVID-19 outbreak and pediatric diabetes: perceptions of health care professionals worldwide. Pediatr Diabetes. 2020;21(7):1083–1092. doi: 10.1111/pedi.13084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi DH, Jung JY, Suh D, Choi JY, Lee SU, Choi YJ, et al. Impact of the COVID-19 outbreak on trends in emergency department utilization in children: a multicenter retrospective observational study in Seoul metropolitan area, Korea. J Korean Med Sci. 2021;36(5):e44. doi: 10.3346/jkms.2021.36.e44. [DOI] [PMC free article] [PubMed] [Google Scholar]


