Summary
Within the central nervous system, Wnt7a/b are unambiguously discriminated from other Wnt ligands by an endothelial receptor complex made of the glycosylphosphatidylinositol (GPI)-anchored Reck and the adhesion G protein-coupled receptor (GPCR) Gpr124. Reck is a Wnt7a/b-specific receptor, while Gpr124 facilitates the delivery of Reck-bound Wnt7a/b ligands to Frizzled, through partially characterized mechanisms. We report that, in zebrafish, the Gpr124-Frizzled interactions are dominated by intracellular scaffolds that exploit the striking molecular mimicry between Gpr124 and Frizzled intracellular domains (ICDs): an internal Dvl-binding motif and a C-terminal ETTV motif that recruits Dlg4 and Magi3. By contrast, mammalian Gpr124 receptors exhibit an ICD-independent interaction mechanism governed by species-specific attributes of their transmembrane and extracellular domains. This mechanism seemingly evolved to replace the Dvl-mediated mechanism. By contrasting zebrafish, mouse, and human Gpr124, this study provides insights into the evolution of Gpr124/Reck function across the vertebrate clade, a receptor complex uniquely implicated in Wnt ligand-specific cellular responses.
Keywords: Gpr124, Reck, Wnt7a, Wnt7b, Dlg4, Magi3, brain angiogenesis, blood-brain barrier, BBB, Wnt/β-catenin signaling
Graphical abstract

Highlights
-
•
Gpr124 displays mechanistic diversification in vertebrates
-
•
At least two partially redundant mechanisms operate in each Gpr124 ortholog
-
•
The distinct mechanisms converge on mediating Gpr124/Frizzled interactions
Brain vessel physiology is regulated by an endothelial receptor complex containing Gpr124 and Reck. Here, America et al. study zebrafish, mouse, and human Gpr124 to uncover the diversity of its mechanistic underpinnings in Wnt7a/b-specific signaling across the vertebrate clade. They reveal common mechanisms, as well as important species-specific functional diversifications.
Introduction
Within the central nervous system (CNS), endothelial cells (ECs) display neuroprotective adaptations, collectively called the blood-brain barrier (BBB). These adaptations, instructed by neighboring cells, are essential in governing CNS homeostasis and protection against blood-borne harmful components (Obermeier et al., 2013; Blanchette and Daneman, 2015; Chow and Gu, 2015; Zhao et al., 2015; Profaci et al., 2020). Wnt7a/b ligands are arguably the most investigated BBB-inducing signals (Liebner et al., 2008; Stenman et al., 2008; (Daneman et al., 2009)). They control BBB formation and brain angiogenesis by selectively regulating the invasive properties of the tip cells of the nascent CNS sprouts (Vanhollebeke et al., 2015).
Wnt7a and Wnt7b belong to an evolutionarily conserved family of 19 closely related ligands that control cell differentiation and migration in various biological contexts, by activating Frizzled (Fz) receptors and Lrp5/6 co-receptors (Clevers et al., 2014; Nusse and Clevers, 2017; Steinhart and Angers, 2018). Wnt7a/b discrimination by brain ECs was found to rely on an atypical receptor complex composed of the glycosylphosphatidylinositol (GPI)-anchored glycoprotein Reck and the orphan adhesion G protein-coupled receptor Gpr124 (also referred to as adhesion G protein-coupled receptor A2 or Adgra2) (Zhou and Nathans, 2014; Posokhova et al., 2015; Vanhollebeke et al., 2015; Ulrich et al., 2016). At present, the Gpr124/Reck complex, besides being an important therapeutic target in neurological disorders (Chang et al., 2017; Martin et al., 2022), represents the only known Wnt ligand-specific receptor complex. Its mechanism is, therefore, explored as a paradigm for how Wnt ligand-specific signaling is achieved in vertebrates.
Reck functions as a Wnt7a/b-specific receptor and stabilizer (Eubelen et al., 2018; Vallon et al., 2018; Cho et al., 2019a). In the absence of Gpr124, Reck is a sink for Wnt7a/b, as Reck-bound ligands remain unavailable for Fz (Eubelen et al., 2018). Gpr124, however, switches the Wnt signaling output from severe inhibition to potent activation. Gpr124 was proposed to act as a signaling-deficient transmembrane tethering molecule, contacting Reck extracellularly and Fz intracellularly, thereby bringing the Reck-associated Wnt7a/b ligands near to Fz receptors, within higher order Gpr124/Reck/Fz/Lrp5/6 complexes (Eubelen et al., 2018; Vallon et al., 2018; Cho et al., 2019a).
Studies in zebrafish revealed that the interaction between Gpr124 and Fz is indirect, occurring via a common adaptor, the scaffolding protein Dishevelled (Dvl). Dvl, a well-known binding partner of Fz, was indeed shown to bind the intracellular domain (ICD) of Gpr124, as well as the closely related Gpr125 (Li et al., 2013; Eubelen et al., 2018). However, in cell cultures, Gpr124 variants lacking the ICD retain some signaling activity, implying that Gpr124 operates, at least in part, in an ICD- and therefore possibly Dvl-independent manner (Vallon et al., 2018). In agreement with this hypothesis, the mere addition of the recombinant human GPR124 ectodomain in the culture medium of Reck-expressing cells could trigger Wnt7a/b signaling (Vallon et al., 2018). More generally, as no mouse or zebrafish germline mutant for Gpr124 ICD has been analyzed thus far, our understanding of this domain, and hence of Gpr124 itself, remains fragmentary.
Here, we undertook to decipher the mode of action of Gpr124 by comparing human, mouse, and zebrafish orthologs in signaling systems in vitro and during the process of brain angiogenesis. Combining gain-of-function settings with newly generated mutant zebrafish lines lacking different segments of the ICD, we reveal common mechanisms as well as significant structure-function divergences in the evolution of Gpr124 function during Wnt7a/b-dependent brain angiogenesis.
Results
The C-terminal intracellular domain of zebrafish Gpr124 contains two functional motifs
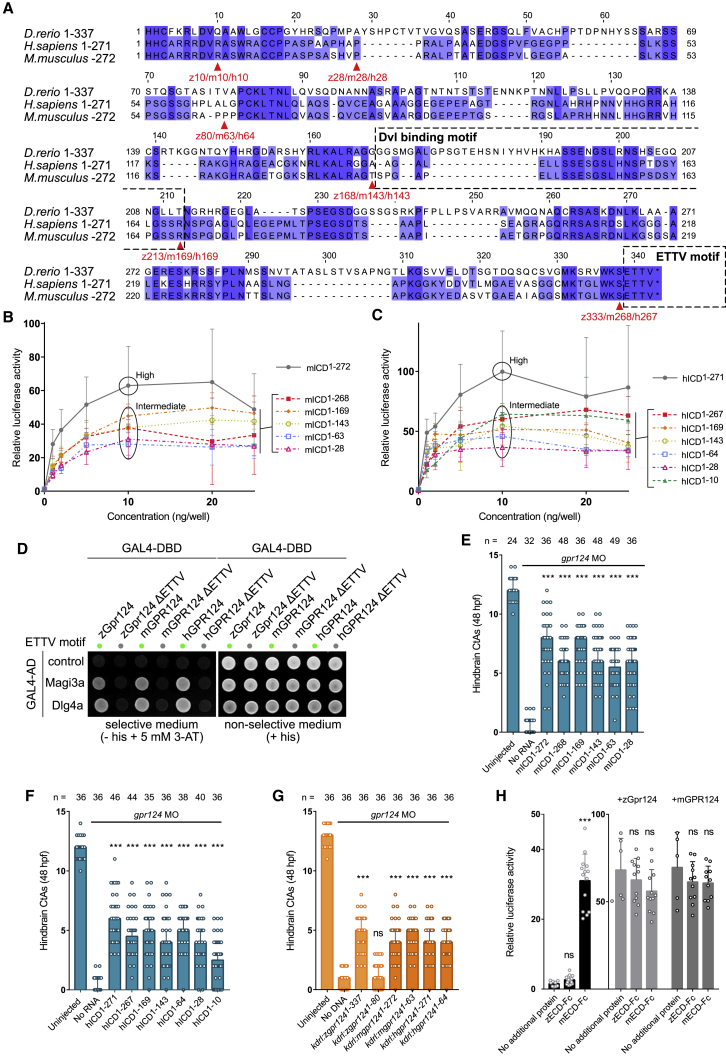
We started exploring the Gpr124 mechanism by analyzing a collection of FLAG-tagged zebrafish Gpr124 ICD truncations (Figure 1A; ICD1–337 corresponds to the full-length zebrafish Gpr124). We first analyzed their subcellular localization using immunofluorescence staining (Figure 1B) and cell surface ELISA (Figure 1C) in HEK293T cells. The variants reached the plasma membrane at levels similar to full-length Gpr124 except those with an ICD shorter than 28 residues (ICD0, ICD1–3, and ICD1–10), disqualifying these variants from further investigation. The defective variants accumulated in the endoplasmic reticulum similar to a previously characterized Gpr124 variant lacking the extracellular leucine-rich repeat (LRR) motifs (ΔLRR) (Bostaille et al., 2016).
Figure 1.
The intracellular domain of zebrafish Gpr124 contains two important motifs
(A) Schematic representation of zebrafish Gpr124 and its variants. Protein domains from N to C terminus are as follows: the extracellular domain (ECD), leucine-rich repeats (LRR), immunoglobulin-like domain (Ig-like), hormone receptor motif (HRM), GPCR autoproteolysis-inducing (GAIN) domain with GPCR proteolysis site (GPS), 7 transmembrane domain (7TM), and intracellular domain (ICD), containing a Dvl-binding motif and a C-terminal PDZ-binding motif or ETTV motif.
(B and C) Anti-FLAG immunostaining (B) and cell-surface ELISA (C) of transiently expressed FLAG-tagged zebrafish Gpr124 or its ICD truncation variants in HEK293T cells; n = 3.
(D) Luciferase activities of HEK293 STF cells co-transfected with Wnt7a, Fz1, Reck, and increasing amounts of Gpr124 or its ICD truncation variants. Data are normalized to ELISA data; n = 3.
(E) Setup for mRNA-injection-based brain angiogenesis assays in zebrafish embryos and representative 3D rendering of the cerebrovasculatures.
(F) Anti-FLAG immunostaining of zebrafish blastulas injected with mRNA encoding FLAG-tagged zebrafish Gpr124 together with membrane-localized tdTomato; n = 3.
(G and H) Hindbrain CtAs of gpr124 morphant embryos at 48 hpf injected at the one-cell stage with 100 pg of the indicated mRNAs (G) or with 7 pg of the indicated DNA construct and 25 pg Tol2 transposase mRNA (H).
Scale bars, 10 μm. Related to Figure S1. In this and all other figures, ns, not significant; ∗p < 0.05; ∗∗p < 0.01; and ∗∗∗p < 0.001, dual luciferase assay data and ELISA data are represented as mean ± SD, and in vivo data are represented as median ± 95 confidence interval (CI).
The Wnt/β-catenin signaling potential of the retained ICD variants was analyzed using dual luciferase assays in HEK293 STF cells (Figure 1D). As it is difficult to predict the physiologically relevant concentration of Gpr124 to be expressed in such in vitro systems, we transfected Gpr124-expressing constructs at doses ranging from 1 ng to 25 ng. Cells were co-transfected with 5 ng of mouse Reck, 5 ng of Fz1, and 20 ng of Wnt7a-encoding plasmids to reconstitute the signaling module (LRP5/6 are endogenously expressed). All variants displayed well-behaved dose-response curves with plateau values reached at the 5- or 10-ng doses. Three distinct activity classes emerged, with the highest Wnt/β-catenin signaling values resulting from the full-length ICD1–337 construct. Constructs with an ICD even modestly shorter than full-length, including ICD1–333, but longer than ICD1–213 showed a partial decrease in signaling capacity (average ratio to full-length Gpr124 of ∼65%). In sharp contrast, constructs with an ICD equal to or shorter than ICD1–168 displayed a marginal, yet not null, signaling capacity (∼10% activity). These results suggest that the most important functional motifs are located after residue 168. Accordingly, an internal deletion variant, lacking ICD residues 1–168 (ICD169–337), retained Wnt signaling activity.
Zebrafish gpr124 knockdown embryos were subsequently used to establish Gpr124 structure-function relationships during brain angiogenesis by mRNA injections of Gpr124 variants (Figure 1E). The proper trafficking and expression of the different variants were confirmed in blastulas, using anti-FLAG immunofluorescence (Figure 1F), and semi-quantitative dot blot analyses (Figure S1A). Mirroring the in vitro situation, injection of 100 pg of mRNA encoding Gpr124 with an ICD composed of at least the first 213 residues was sufficient to partially restore central arteries (CtAs) formation in 48 hours postfertilization (hpf) embryos. Shorter variants, starting with ICD1–168, were almost entirely inactive. The complementary variant ICD169–337 was active in vivo, as it was in vitro (Figure 1G). The 100-pg mRNA dose was chosen, as it yields a robust and easily scorable rescue phenotype (Vanhollebeke et al., 2015). As assessed by qRT-PCR, the total Gpr124 mRNA levels in injected embryos (endogenous and ectopic) were ∼5.5× and ∼1.6× higher than in uninjected controls at 24 and 48 hpf, respectively (Figures S1B and S1C). As Gpr124 is required selectively within a limited number of endothelial tip cells (<20 cells) derived from the primordial hindbrain channels (PHBCs) within this time interval (Vanhollebeke et al., 2015), the local endothelial overexpression is likely very modest. As a further control, restoring ICD1–337 and ICD1–80 by transgenic expression under the control of the endothelial kdrl promoter resulted in similar differential rescues (Figure 1H). Consistent with previous reports, these experiments reveal two functionally important discontinuous motifs in the intracellular domain of zebrafish Gpr124. The first one corresponds to a 45-amino acid (aa)-long internal region spanning amino acids 169–213. This motif is coined the “Dvl-binding motif” as it lies within the previously identified Dvl-binding region, spanning amino acids 80–213 (Eubelen et al., 2018). The other one, the so-called “ETTV motif” is made up of the last four Glu-Thr-Thr-Val amino acids, and constitutes a PDZ-binding motif (PBM) (Figure 1A; Posokhova et al., 2015; Eubelen et al., 2018; Liu and Fuentes, 2019).
The intracellular domain of Gpr124 is essential for proper brain angiogenesis in zebrafish
To validate the role of Gpr124 ICD in vivo, we used CRISPR-Cas9 to introduce a premature stop codon upstream of the two identified functional motifs of the zebrafish Gpr124 ICD. This mutant allele is designated gpr124ulb13 (Figures 2A and S1D–S1F). gpr124ulb13 was compared with two previously generated alleles: (1) gpr124s984, a presumptive null, and (2) gpr124s985, a more downstream frameshift mutation allele (Vanhollebeke et al., 2015; Figure 2A). All three homozygous mutant embryos remain morphologically indistinguishable from wild-type (WT) embryos at 60 hpf (Figure 2B). gpr124ulb13 adults exhibit disrupted skin pigmentation patterns of the most dorsal stripes, as previously reported for gpr124s984 and gpr124s985 (Figure 2C). qRT-PCR analysis of gpr124ulb13 at 30 hpf revealed no significant mutant mRNA degradation (Figure S1G). The CNS vasculature of the three mutants was quantified at 48 hpf, 60 hpf, and 5 dpf by counting the number of hindbrain CtAs. As shown previously for gpr124s984 and gpr124s985 mutants, the gpr124ulb13 mutants display a severe reduction in CNS vessels at all stages (Figures 2D–2H). Dorsal root ganglia (DRG) neurogenesis, another Gpr124-controlled Wnt signaling process, was also strongly impaired by the absence of the Gpr124 ICD (Figures 2I and 2J). However, consistent with the 10% residual in vitro activity of severely truncated ICD variants, gpr124ulb13 mutants exhibited CtAs counts marginally superior to those observed in gpr124s985 and gpr124s984 mutants at 48 or 60 hpf. At 5 dpf, gpr124s984, gpr124s985, and gpr124ulb13 displayed a stepwise increase in CtAs. Together with previous somatic gene-disruption experiments (Eubelen et al., 2018), these findings establish that the Gpr124 ICD is essential at the onset of brain angiogenesis in zebrafish but that a weak ICD-independent signaling activity is sufficient to facilitate recovery of blood vessels at later stages.
Figure 2.
Germline mutants of the zebrafish Gpr124 ICD exhibit brain vascular defects
(A) Scheme of zebrafish Gpr124 highlighting the TALEN or CRISPR-Cas9 target sites corresponding to the gpr124s984, gpr124s985, and gpr124ulb13 alleles.
(B) Lateral views of WT, gpr124s984/s984(s984−/−), gpr124s985/s985(s985−/−), and gpr124ulb13/ulb13(ulb13−/−) embryos at 60 hpf.
(C) Lateral views of the adult WT and gpr124 mutant skin pigmentation patterns. Arrowheads point at stripe discontinuities.
(D) Dorsal views of hindbrain vasculatures of WT and gpr124 mutants.
(E) Quantification of hindbrain CtAs of WT and gpr124 mutant embryos at 48 hpf.
(F) Same as (E) at 60 hpf.
(G) 3D representation of the cerebrovasculature of 60 hpf WT and gpr124 mutant embryos. Red vessels are Wnt7a/Gpr124/Reck-dependent CtAs that sprout from white perineural vessels.
(H) Same as (E) in 5 dpf larvae.
(I and J) Dorsal views (I) and quantification (J) of dorsal root ganglia in the trunk region of WT and gpr124 mutant larvae at 72 hpf.
Scale bars, 0.5 mm for (B) and 100 μm for (D), (G), and (I). Related to Figure S1.
The Dvl and ETTV motifs are partially redundant for brain angiogenesis
The position of the gpr124ulb13 genetic lesion does not permit defining the respective contribution of the Dvl-binding and ETTV motifs. We, therefore, generated the gpr124ulb14 allele (ΔETTV), where a premature stop codon was introduced downstream of the Dvl-binding motif but upstream of the ETTV motif, thereby selectively impairing the latter (Figures 3A and S1D–S1F).
Figure 3.
The Dvl and ETTV motifs act in a partially redundant manner during zebrafish brain angiogenesis
(A) Scheme of zebrafish Gpr124 highlighting the CRISPR-Cas9 target site corresponding to the gpr124ulb14(ΔETTV) mutant allele.
(B) Lateral views of WT and ΔETTV−/− mutant embryos at 60 hpf.
(C) Lateral views of the skin-pigmentation patterns of WT and ΔETTV−/− mutant adults.
(D) Dorsal views of hindbrain vasculature of WT and ΔETTV−/− mutants.
(E) Quantification of CtAs of WT and ΔETTV−/− mutant embryos at 48 hpf.
(F) Same as (E) at 60 hpf.
(G) 3D representation of cerebrovasculature of 60 hpf WT and ΔETTV mutant embryos.
(H) Same as (E) in 5 dpf larvae.
(I) Quantification of the number of lumenized CtAs in 48 hpf WT and ΔETTV mutant embryos.
(J and K) Dorsal views (J) and quantification (K) of dorsal root ganglia in the trunk region of WT and ΔETTV mutant larvae at 72 hpf.
(L) Luciferase activities of HEK293 STF cells co-transfected with Wnt7a, Fz1, Reck, and increasing amounts of the indicated Gpr124 ICD variants; n = 3.
(M) Quantification of hindbrain CtAs of gpr124 morphant embryos at 48 hpf injected at the one-cell stage with 100 pg of the indicated mRNAs.
Scale bars, 0.5 mm for (B) and 100 μm for (D), (G), and (J). Related to Figure S1.
ΔETTV mutants do not display detectable morphological phenotypes from embryogenesis to adulthood (Figures 3B and 3C). Quantification of CtAs at three different stages (48 hpf, 60 hpf, and 5 dpf) revealed a partial impairment of angiogenesis that resolved over time (Figures 3D–3H). The phenotype was not explained by RNA degradation (Figure S1G). At 48 hpf, half of the CtAs failed to form a lumen (Figure 3I). ΔETTV mutants did, however, not exhibit detectable DRG defects (Figures 3J and 3K). By comparing gpr124ulb13 and gpr124ΔETTV mutants, we conclude that, while the ETTV motif is required for full activity, it is not strictly necessary for brain angiogenesis or DRG neurogenesis when Gpr124 harbors a functional Dvl-binding motif.
We were unsuccessful in generating the symmetric tool, i.e., an in-frame gpr124 deletion allele lacking the Dvl-binding motif but maintaining the C-terminal ETTV motif. We, therefore, resorted to a gain-of-function strategy and measured the impact of fusing a C-terminal ETTV motif to three inactive Gpr124 variants (ICD1–28, ICD1–80, and ICD1–168) in vitro (Figure 3L) and in vivo (Figure 3M). In both settings, the mere addition of the ETTV motif increased their activity significantly, with ICD1–168+ETTV and ICD1–80+ETTV being the most active variants.
Dlg4 and Magi3 as Gpr124-ETTV-dependent brain angiogenic regulators
The ICD was documented to operate by recruiting the adaptor protein Dvl, whose simultaneous binding to Fz allows for the organization of higher order Gpr124/Reck/Fz/Lrp5/6 complexes (Eubelen et al., 2018). As expected, Dvl-GFP recruitment to membrane-localized Gpr124 variants in Fz1–10−/− cells perfectly correlated with an intact Dvl-binding motif, irrespective of the presence or absence of the C-terminal ETTV motif (Figures 4A and 4B). These interactions were confirmed in pull-down assays in WT cells (Figure 4C). As a comparison, Dvl-GFP bound to Fz with variable strength in a similar setting, with Fz4 displaying the strongest interaction and Fz5 and Fz6 the weakest (Figure 4D).
Figure 4.
Dlg4 and Magi3 interact with the ETTV motif of Gpr124 during brain angiogenesis
(A and E) Distribution of Dvl-GFP co-expressed with zebrafish Gpr124 or its variants in FZ1–10−/− HEK293T cells. Arrowheads point to Dvl-GFP signal at the plasma membrane.
(B and F) Quantification of the fraction of FZ1–10−/− HEK293T cells showing membrane localization of Dvl-GFP when co-expressed with zebrafish Gpr124 or its variants.
(C) GFP-Trap co-immunoprecipitation assays in total lysates of HEK293T cells co-expressing Dvl-GFP and N-terminally FLAG-tagged Gpr124 or its ICD variants.
(D) GFP-Trap co-immunoprecipitation between Dvl-GFP and Gpr124 or different Fz receptors. Arrowheads and asterisks label Fz monomers and likely multimers, respectively.
(G) Agar plate growth of spotted yeast cultures co-transformed with different combinations of GAL4-DBD and GAL4-AD fusion constructs onto selective or non-selective conditions. Green and gray dots illustrate, respectively, the presence or absence of the Dvl binding or ETTV motif in the GAL4-DBD fusions.
(H and I) Dorsal views (H) and quantification (I) of 48 hpf hindbrain CtAs of WT embryos injected at the one-cell stage with 150 pg of zCas9 mRNA and 30 pg of the illustrated sgRNAs.
(J) Same as (I) after injection with a combination of different sgRNAs, each 30 pg.
(K) Same as (I) in ΔETTV−/− mutant embryos.
(L) Quantification of 48 hpf hindbrain CtAs of gpr124 morphant embryos injected at the one-cell stage with 100 pg of Gpr124-ΔICD-GFP mRNA together with the indicated nanobody fusion mRNAs.
(M) Heatmap representation of average luciferase activities 48 h after transfection of the indicated constructs in FZ1–10−/− HEK293T cells. Boxed area highlights the conditions most affected by ETTV and Dlg4; n = 3.
Scale bars, 10 μm for cultured cells and 100 μm for zebrafish images. Related to Figure S2.
The binding of Dvl-GFP to ICD1–333 indicates that interaction between Gpr124 and Dvl can occur in the absence of the C-terminal ETTV but does not exclude that ETTV might constitute a second Dvl-binding mechanism. We therefore evaluated the artificial ETTV motif fusions to ICD variants lacking the Dvl-binding motif (ICD1–28, ICD1–80, and ICD1–168). Dvl-GFP membrane recruitment ratios reached ∼50% of ICD1–337 with ICD1–168+ETTV, while ICD1–80+ETTV and ICD1–28+ETTV only marginally recruited Dvl-GFP (Figures 4E and 4F). Although Dvl binding to ETTV cannot be excluded, these data suggest that binding partners other than Dvl are implicated in ETTV-dependent Gpr124 function.
To identify the Gpr124 ETTV effectors, we used a two-step yeast two-hybrid screening strategy. First, the ICD fragment of Gpr124 was screened against an embryonic (18–20 hpf) zebrafish cDNA library. This primary screen returned 84 positive clones from a total of 84.6 million analyzed interactions. Out of these, six corresponded to unique potential intracellular protein-protein interactions. In a second step, all six potential partners were re-cloned as GAL4-activation domain (AD) fusions and tested against Gpr124 GAL4-DNA-binding domain (DBD) fusions harboring or not the Dvl-binding and ETTV motifs (Figure 4G). Three interactors (Prickle1b, Magi3a, and Dlg4a) could be confirmed in this secondary mapping screen. While the Prickle1b binding site did not match the Gpr124 functional motifs, the PDZ proteins membrane-associated guanylate kinase, WW, and PDZ domain containing 3 (Magi3a) and discs large MAGUK scaffold protein 4 (Dlg4a) bound to Gpr124 via its C-terminal ETTV motif.
As the diffuse whole-mount in situ hybridization expression patterns of dlg4a, dlg4b, magi3a, and magi3b did not permit to establish their vascular expression unambiguously (Figure S2A), we isolated 48 hpf kdrl:GFP+ ECs by fluorescence-activated cell sorting (FACS) and determined their expression levels by qRT-PCR analysis. dlg4b, magi3a, and magi3b transcripts could readily be amplified, while dlg4a transcripts were not (Figure S2B).
To assess the function of Dlg4 and Magi3 in Gpr124-dependent brain angiogenesis, we performed somatic gene-disruption experiments. Targeting magi3a, magi3b, or dlg4b, but not dlg4a, partially impaired brain vascularization (Figures 4H and 4I), in agreement with the expression data. No other vascular phenotypes were detected, including in the trunk intersegmental vessels (ISVs). As a control for each single-guide RNA (sgRNA), a scrambled version with a protospacer adjacent motif (PAM)-proximal 6-bp mismatch was injected, which did not result in a detectable phenotype. Efficient targeting of all four genes was confirmed by high-resolution melt analysis, genome-editing test (get)PCR on cDNA, and next-generation sequencing (Figures S2C–S2F). Combined injections of dlg4a and dlg4b, magi3a and magi3b, or all four sgRNAs did not worsen the phenotype (Figure 4J). This lack of additive effect suggests that Dlg4b, Magi3a, and Magi3b act within the same pathway, in which they are individually required.
Strikingly, magi3a, magi3b, or dlg4b crispant phenotypes were indistinguishable from the ΔETTV phenotypes (Figures 4H and 4I), with an identical reduction in number of cerebral vessels and a similar defect in vessel lumenization. This observation is compatible with a role for Dlg4 and Magi3 as Gpr124 ETTV adaptor proteins, acting in parallel to Dvl. Accordingly, injections of magi3a, magi3b, dlg4a, or dlg4b sgRNAs did not affect the gpr124ΔETTV mutant vasculature (Figure 4K), suggestive of an interaction between the ETTV motif of Gpr124 and these PDZ proteins. To further evaluate this interaction, we resorted to a previously developed bimolecular complementation assay in zebrafish gpr124 morphants (Eubelen et al., 2018). In this assay, an inactive form of Gpr124 in which most of the ICD is replaced by GFP (Gpr124ICD1–28-GFP or ICD1–28-GFP) is co-expressed with an anti-GFP VhhGFP4 nanobody fusion construct. The GFP-nanobody interaction will force the artificial recruitment of candidate proteins to Gpr124ICD1–28-GFP, thereby establishing their capacity to complement the lack of the ICD. As a proof of concept, the recruitment of Gpr124 ICD itself (nano-ICD), but not the control TagRFP (nano-tagRFP), partially restored CNS angiogenesis in this assay (Figure 4L). Similarly, the recruitment of the ETTV motif, DLG4, and less potently MAGI3 could restore brain angiogenesis. These data thus identify Dlg4 and Magi3 as potential regulators of brain angiogenesis acting via the Gpr124 ETTV motif. Intriguingly, Fz1, Fz2, Fz4, and Fz7 contain a similar E-T-X-V sequence, through which they were shown to bind Dlg4 and Magi3 (Hering and Sheng, 2002; Yao et al., 2004; Wawrzak et al., 2009; Schulte, 2010).
We therefore performed Wnt signaling synergy studies between Gpr124 and Fz1, in the absence or presence of Dlg4, in Fz1–10−/− cells (Figure 4M). In the absence of exogenous Dlg4 expression, Fz1 and Gpr124 synergized across a range of concentrations. When Dlg4 was co-expressed, the synergy was increased, in particular at low Fz1 doses (0.6 ng). This effect was strongly reversed when Fz1 was substituted with a variant lacking its ETTV motif (Fz1 ΔETTV), confirming an ETTV-dependent cooperation mechanism between Gpr124 and Fz that is enhanced by Dlg4.
Contrasting zebrafish, mouse, and human GPR124-signaling modalities
We contrasted zebrafish Gpr124 with mammalian GPR124, starting with sequence alignments. The zebrafish Gpr124 ICD exhibits 37% to 38% amino acid identity with the mouse and human ICDs (Figure 5A). As a comparison, the identity reaches 55% for the extracellular domain and 58% to 59% for the transmembrane domain (Figure S3). With the exception of the C-terminal ETTV motif, which is conserved from zebrafish to humans, the overall level of conservation of the ICD is thus comparatively modest, including within the region corresponding to the zebrafish Dvl-binding motif.
Figure 5.
ICD-deleted mouse and human GPR124 are competent for brain angiogenesis
(A) Sequence alignment of zebrafish, mouse, and human Gpr124 intracellular domains. Dvl-binding and ETTV motifs are boxed, and truncations sites are indicated by red arrowheads.
(B and C) Luciferase activities of HEK293 STF cells co-transfected with Wnt7a, Fz1, Reck, and increasing amounts of mouse (B) or human (C) GPR124 or their ICD truncation variants. Data are normalized to ELISA data; n = 3.
(D) Agar plate growth of spotted yeast cultures co-transformed with different combinations of GAL4-DBD and GAL4-AD fusion constructs onto selective or non-selective conditions. Green and gray dots illustrate, respectively, the presence or absence of the ETTV motif in the GAL4-DBD fusions.
(E and F) Quantification of hindbrain CtAs in 48 hpf gpr124 morphant embryos injected at the one-cell stage with 100 pg of the indicated mRNAs of mouse (E) and human (F) Gpr124 and their ICD variants.
(G) Quantification of hindbrain CtAs of gpr124 morphant embryos at 48 hpf injected at the one-cell stage with 7 pg of the indicated DNA constructs and 25 pg Tol2 transposase mRNA. Zebrafish data are duplicated from Figure 1H.
(H) Luciferase values of HEK293 STF cells co-transfected with Wnt7a, Fz1, and Reck, with or without zebrafish Gpr124 or mouse Gpr124, and supplemented or not with 10 mM of the indicated recombinant protein, n = 3.
Related to Figures S3 and S4.
We next evaluated a collection of ICD truncation variants of mouse and human GPR124, guided by the zebrafish collection (Figure 5A). As before, trafficking and expression were tested by immunostaining (Figure S4A) and cell-surface ELISA (Figures S4B and S4C). All constructs, except mouse ICD1–10, reached the plasma membrane at levels similar to the full-length constructs. Much like the situation in zebrafish, removal of the C-terminal ETTV motif in human or mouse GPR124 lowered the in vitro signaling capacity of the receptors by ∼35%–40% (Figures 5B and 5C). Not unexpectedly, the ETTV motif of mouse and human GPR124 also interacted with Dlg4a and Magi3a in a yeast two-hybrid assay (Figure 5D). In addition, mouse GPR124 similarly synergized with Dlg4 in cell culture signaling assays, in an ETTV-dependent manner, especially at low doses of mouse GPR124 (Figure S4D).
In sharp contrast with their zebrafish ortholog, however, longer truncations of mouse or human GPR124 did not further reduce their Wnt/β-catenin signaling activity in cell cultures (Figures 5B and 5C). Mouse and human GPR124 variants thus exhibit only two activity classes (high and moderate) and retain ∼60%–65% of their signaling potential when the ICD is deleted. As predicted from their higher Wnt signaling activities in vitro, human and mouse GPR124 variants lacking most of the ICD exhibited robust brain angiogenic potential in vivo (Figures 5E and 5F), in contrast with equivalent zebrafish Gpr124 truncations (Figures 1G and 2D–2H). Mouse, human, and zebrafish Gpr124 were expressed at comparable levels in zebrafish (Figure S1A) and similarly reached the plasma membrane (Figure S4E). Transgenic endothelial expression strategies confirmed the differential species-specific requirement of the Gpr124 ICD (Figure 5G).
To test whether these specificities could be explained, at least in part, by evolutionary differences in the extracellular domain of Gpr124, recombinant Fc fusions of zebrafish or mouse ectodomains were added to the supernatant of Reck-expressing STF cells, as previously done with the human GPR124 extracellular domain (ECD) (Vallon et al., 2018). Recombinant mouse ECD, but not zebrafish ECD, induced Wnt signaling in such assay. When the cells additionally expressed full-length Gpr124, the recombinant proteins had little effect, suggesting that the sole ectodomain is unable to outcompete the full-length receptor (Figure 5H).
These data suggest that, during the evolution of mammalian vertebrates, the function of the Gpr124 Dvl-binding motif became dispensable, possibly at the benefit of other Gpr124/Reck/Fz/Lrp5/6-clustering mechanisms, which could at least in part implicate the ECD.
Mouse and human GPR124 interact with Frizzled in the absence of Dishevelled
Reinforcing this model, neither mouse nor human GPR124 recruited Dvl-GFP, irrespective of the presence of their ICD in Fz1–10−/− cells, suggesting that mammalian GPR124 function does not rely on Dvl binding (Figures 6A and 6B). These data are consistent with the low level of conservation of the Dvl-binding motif (Figure 5A) and reduce the likelyhood of a convergent evolution mechanism involving Dvl recruitment to other parts of mammalian GPR124. Of note, from Figure 6A onwards, for the sake of concision, we will refer to zGpr1241−337, mGpr1241−272, and hGpr1241−271 as full-length (FL) and to zGpr1241−80, mGpr1241−63, and hGpr1241−64 as ΔICD.
Figure 6.
A Dvl-independent interaction mechanism between mammalian GPR124 receptors and Frizzled
(A) Distribution of Dvl-GFP co-expressed with full-length (FL) zebrafish (z), mouse (m), or human (h) Gpr124 or their ΔICD (ICD1–80 and corresponding) variants in FZ1–10−/− HEK293T cells. The arrowhead points to Dvl-GFP signal at the plasma membrane.
(B) GFP-Trap co-immunoprecipitation assays in total lysates of FZ1–10−/− HEK293T cells co-expressing Dvl-GFP and N-terminally FLAG-tagged zebrafish, mouse, or human FL Gpr124 and their ΔICD variants; n = 3.
(C) Distribution of individually expressed TagRFP-fused zebrafish or mouse FL Gpr124, or their ΔICD variants, as well as Fz4-GFP in zebrafish blastula deep-layer cells.
(D) Representative images of zebrafish blastula deep-layer cells co-expressing Fz4-GFP and zebrafish FL or ΔICD Gpr124-TagRFP, with or without Dvl; n = 3.
(E) Quantification of the enrichment level of Fz4-GFP in the Gpr124-TagRFP+ membranes in the presence or absence of Dvl.
(F) Same as (D) with corresponding mouse GPR124 constructs. For (C), (D), and (F), the cells annotated with an asterisk are magnified, and the pixel intensity of the green and magenta channels along a virtual clockwise path following the cell cortex from a to b is plotted.
(G) Same as (E) for mouse GPR124 constructs.
(H) Distribution of mouse Fz4-GFP in zebrafish blastula deep-layer cells in the presence or absence of Dvl.
(I) Bimolecular complementation assay strategy. Illustration created with BioRender.com.
(J) Representative images of zebrafish blastula deep-layer cells co-expressing the indicated bimolecular complementation constructs, with or without Dvl. Venus junctional signal is indicated with arrowheads; n = 3.
Data are represented as mean ± SD. Scale bars, 10 μm. Related to Figure S4.
The binding of Dvl to zebrafish Gpr124 has been shown to promote the association of Frizzled and Gpr124 in membrane subdomains of the intercellular junctions (Eubelen et al., 2018). This association is easily tractable in zebrafish blastula deep-layer cells. When expressed individually, full-length zebrafish or mouse Gpr124-TagRFP reside in the junctions, while Fz4-GFP is distributed all around the cell membrane (Figure 6C). When co-expressed in the absence of exogenous Dvl, the localization of zebrafish Gpr124-TagRFP and Fz4-GFP is unchanged, and the receptors thus only partially co-localize at the cellular cortex (Figures 6D and 6E). Co-expression of Dvl does not alter Gpr124-TagRFP localization but quantitatively relocalizes Fz4-GFP to the Gpr124+ membrane pool (Figures 6Dʹ and 6E). As anticipated, this relocalization of Fz4-GFP does not occur when the ICD of Gpr124 is deleted (Figures 6Dʺ, 6D‴, and 6E). Of note, the C-terminal fluorescent fusions used in these assays intentionally mask the C-terminal ETTV motif of Gpr124 and Fz4, thereby prohibiting potential interactions between Gpr124 and Frizzled via adaptor proteins binding the PDZ-binding motif, including Dlg4 and Magi3.
By contrast, mouse GPR124-TagRFP and GPR124ΔICD-TagRFP constitutively recruited Fz4-GFP to the intercellular junctions, even in the absence of Dvl (Figures 6F and 6G). These data imply that an alternative interaction modality exists between Fz4 and mouse GPR124, which occurs independently of the recruitment of intracellular proteins to the GPR124 ICD. This possibly more direct interaction mechanism explains the higher Wnt signaling activity and angiogenic potential of the mammalian GPR124ΔICD variants. As an additional control, co-expression of Fz4-GFP and Dvl, in the absence of Gpr124, did not result in the junctional relocalization of Fz (Figure 6H).
Bimolecular Venus fluorescence complementation assays in zebrafish blastula deep-layer cells were used to further evaluate the requirement of Dvl in mediating mouse or zebrafish Gpr124 interactions with Fz (Figure 6I). As previously reported (Eubelen et al., 2018), co-injection of zebrafish Gpr124-VN155 (I152L) and mouse Fz1-VC155 or Fz4-VC155 generated fluorescent signals at intercellular junctions, in a Dvl-dependent manner. By contrast, upon injection of mouse GPR124-VN155, junctional signals were seen in both the absence and presence of Dvl (Figure 6J), irrespective of the Fz isoform. As controls, no signal was observed after injection of the constructs individually (Figure S4F) or upon co-injection of GPR124-VN155 with other unrelated plasma-membrane proteins (mouse GPR116-VC155 or zebrafish Tnfrsf9a-VC155; Figure S4G).
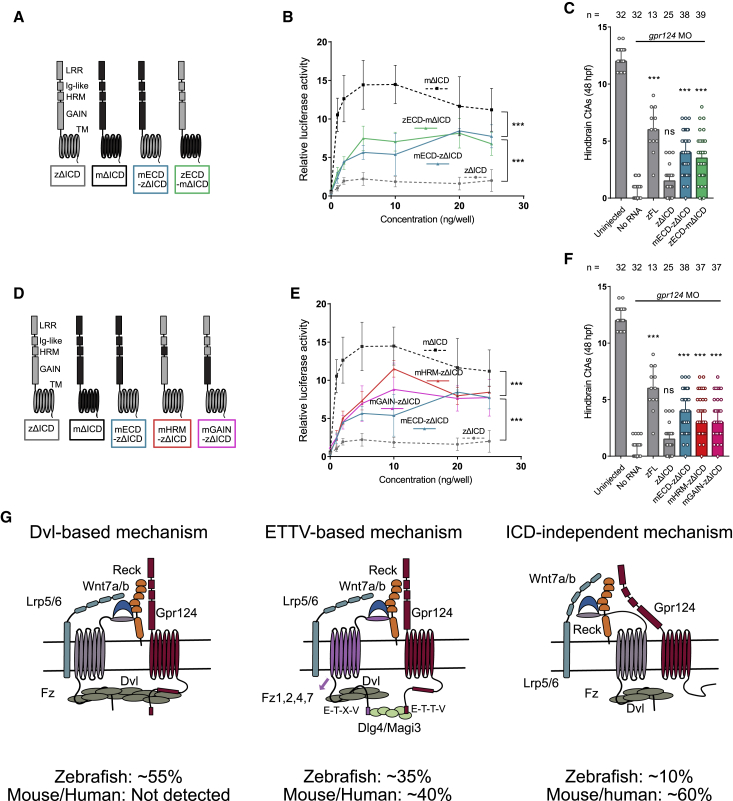
Mapping the differential activities between mouse and zebrafish Gpr124 through chimeragenesis
To map the differential capacity of mammalian and zebrafish Gpr124 to signal and recruit Fz in an ICD-independent manner, we investigated the role of the 7 transmembrane domain (7TM) and ECD by analyzing ICD-truncated chimeric receptors whose proper trafficking was evaluated as before (Figure S5). Substituting zebrafish 7TM or ECD with their mouse counterpart increased the signaling levels but failed to reach the activity levels of mouse GPR124ΔICD (Figures 7A and 7B). We did not dissect the 7TM further but pursued the analysis of the constitutive domains of the ECD (Figures 7D and 7E). Within the ECD, the hormone receptor motif (HRM) and the G protein-coupled receptor (GPCR) autoproteolysis-inducing (GAIN) domain seemed accountable for the increased signaling capacity of the mouse ECD, as substituting each of these domains was equivalent to the substitution of the full ECD. On a cautionary note, the contribution of the immunoglobulin (Ig)-like and the LRR domains were possibly underestimated by virtue of suboptimal trafficking (Figures S5D–S5F). The activities of the chimeras were confirmed in zebrafish (Figures 7C and 7F). As before, proper trafficking and expression levels were verified by immunostaining (Figure S6A) and dot blot analysis (Figure S1A).
Figure 7.
Functional comparison of mouse and zebrafish Gpr124 extracellular and transmembrane domains
(A and D) Schematics of the investigated mouse and zebrafish Gpr124 chimeras.
(B and E) Luciferase dose-response assays in HEK293 STF cells co-transfected with Wnt7a, Fz1, Reck, and the indicated Gpr124 chimeras. Data are normalized to ELISA data, n = 3.
(C and F) Quantification of hindbrain CtAs in 48 hpf gpr124 morphant embryos injected at the one-cell stage with 100 pg of the indicated mRNAs.
(G) Model for the various modalities of Gpr124/Reck-dependent Wnt7a/b signaling and the importance of their relative contribution in different species.
Related to Figures S5 and S6.
Using the reciprocal strategy of swapping zebrafish protein domains into mouse GPR124ΔICD, only the LRR chimera reached the signaling level of mouse GPR124ΔICD (Figures S6B and S6C). This unambiguously disqualifies the LRR domain as driver of the species-specific functional differences. Swapping the entire ECD, HRM, GAIN, or 7TM systematically reduced signaling. Proper trafficking of the constructs was assessed by immunostaining (Figures S5G–S5I).
Combined, these results assign the differential activity of mouse versus zebrafish Gpr124 to the transmembrane segment, the GAIN domain, and the HRM, which each individually can raise the signaling capacity of zebrafish Gpr124ΔICD when permuted with the mouse versions. However, none of these single-domain substitutions is sufficient to reach the high signaling levels of mouse GPR124ΔICD. We therefore performed combined substitutions of the 7TM and each of the ECD constitutive domains. Substituting both the 7TM and the HRM or the GAIN domain achieved signaling activity levels similar to mouse GPR124ΔICD (Figures S6D and S6E). This suggests that the higher ICD-independent activity of mouse GPR124 has been gained within different parts of the transmembrane and extracellular domains of the receptor (Figure 7G), possibly reflecting the evolutionary pressures to make the formation of the Gpr124/Reck/Fz/Lrp5/6 assembly mechanism more robust in higher vertebrates.
Discussion
The experiments reported here reveal multiple modalities governing Gpr124 function in Wnt7a/b-specific signaling throughout the vertebrate clade. In all examined orthologs, full activity requires an intact ICD, including the terminal ETTV PDZ-binding motif, which we show, in a genome-wide yeast two-hybrid screen, binds Dlg4 and Magi3. Genetic analyses in zebrafish reveal that both adaptor proteins contribute to brain angiogenesis, in a Gpr124 ETTV-dependent manner. Previous evidence supports a role of Dlg proteins in Fz or Gpr124 functions. Dlg1 was reported to interact genetically with Fz4 in the process of retinal angiogenesis and blood-retina barrier formation by activating β-catenin signaling (Cho et al., 2019b), and Dlg1 has been shown to be able to bind the ETTV sequence of Gpr124 (Yamamoto et al., 2004; Posokhova et al., 2015). Besides this common theme across the vertebrate clade, we reveal divergent modalities of Gpr124 signaling between mammalian and non-mammalian vertebrates. In zebrafish, Gpr124 strongly relies on its ICD, which contains a second functional motif that binds Dvl. The ETTV and Dvl motifs are each sufficient, but not individually necessary, for zebrafish Gpr124 function in Wnt signaling. Their combined deletion, however, reduces Gpr124 activity below the Wnt activity thresholds required for zebrafish brain angiogenesis. Remarkably, the proteins known to be recruited to these motifs also bind Fz receptors, highlighting a form of molecular mimicry between Gpr124 and Fz and reinforcing the concept that oligomerizing intracellular scaffolds are implicated in assembling the Gpr124/Reck/Fz/Lrp5/6 signalosome (Eubelen et al., 2018).
Phylogenetic alignments and functional analyses reveal no zebrafish-like Dvl-binding sequence or activity in mouse and human GPR124. Although alternative Dvl-binding mechanisms cannot be excluded, the ETTV motif accounts for all ICD-related activity in mammlian GPR124. Instead, the extracellular domain, more specifically the hormone-binding motif, the GAIN domain, and the transmembrane domain appear to have gained a function in receptor complex assembly. Consequently, ICD-deleted mouse and human GPR124 can trigger brain angiogenesis in zebrafish.
In sum, this work reveals that, in order to deliver the Reck-bound Wnt7a/b ligands to the signal-transducing Fz/Lrp5/6 receptors, the adhesion GPCR Gpr124 operates through at least two partially redundant mechanisms in every examined species (Figure 7G). In zebrafish, this function critically relies on the ICD that recruits Dvl to an internal motif and Dlg4/Magi3 to the C-terminal ETTV PDZ-binding motif. Mouse and human GPR124 also rely on the C-terminal ETTV for full activity, but the Dvl-binding internal motif is lost, at the benefit of an alternative extracellular and transmembrane interaction mechanism, relieving the stringent requirement of the ICD typical of the zebrafish.
What are the biological implications of having distinct Gpr124-Fz bridging mechanism acting in a partially redundant manner?
The teleost Dvl-based mechanism could contribute a generic and non-discriminative bridging mechanism to most, if not all, Frizzled family members. Indeed, all 10 Frizzled receptors bind Dvl through two conserved motifs of the third intracellular loop and a membrane-distal K-T/S-X-X-X-W motif on the ICD (Umbhauer et al., 2000; Wong et al., 2003; Punchihewa et al., 2009; Tauriello et al., 2012; Sharma et al., 2018). The Dvl-based bridging mechanism is thus presumably non-discriminatory for the different Fz receptors. Similarly, the mammalian ICD-independent mechanism might be non-discriminative, as mouse GPR124 stimulates Wnt7 signaling via most Fz receptors (Zhou and Nathans, 2014; Cho et al., 2017). These two mechanisms could act as a default basal mechanism, possibly sufficient in most cellular settings.
By contrast, the evolutionarily conserved ETTV-dependent mechanism, via Dlg4 and Magi3, might result in a more selective recruitment of specific Fz receptors. Only a subset of them, i.e., Fz1, Fz2, Fz4, and Fz7, indeed contain a similar E-T-X-V sequence, through which they were shown to bind Dlg4 and Magi3 (Hering and Sheng, 2002; Yao et al., 2004; Wawrzak et al., 2009; Schulte, 2010). Interestingly, Wnt7a/b signaling by Fz1 and Fz4 is more potently stimulated by Gpr124/Reck than by other Fz receptors (Zhou and Nathans, 2014; Vanhollebeke et al., 2015; Cho et al., 2019a), which is consistent with this hypothesis. As different Fz receptors can initiate distinct signal transduction cascades, this preferential Fz recruitment could influence the cellular response (van Amerongen and Nusse, 2009; van Amerongen, 2012; Schulte, 2015; Wang et al., 2016).
More so, besides affecting the preferential recruitment of the Fz receptors themselves, we propose that distinct Gpr124-associated scaffolding structures could result in a differential recruitment of other essential or accessory Wnt signalosome components (Tan et al., 2001; Yao et al., 2001, 2004; Wawrzak et al., 2009; Wheeler et al., 2011). Thereby, the different modalities of Gpr124-Fz interactions revealed in these experiments could fine-tune the intensity, dynamics, or nature of the endothelial Wnt signaling cascades at play during CNS angiogenesis and BBB formation and maintenance. This mechanistic diversity could contribute to the increasingly recognized molecular and functional heterogeneity of CNS endothelial cells (Sabbagh et al., 2018; Vanlandewijck et al., 2018).
Limitations of the study
The ICD-independent modalities of mammalian Gpr124 and Fz interaction remain to be molecularly characterized. Our results are compatible with both a direct and indirect interaction mechanism, the latter involving yet-to-be-identified partners. In the search for such partners, we propose using zebrafish Gpr124 as a contrasting reagent. The proper trafficking and expression levels of the different constructs used in this study were not assessed directly in CNS ECs but instead inferred from in vitro systems and zebrafish blastulas. Finally, as no ICD-deleted mouse GPR124 allele was analyzed, the functionality of such variant in mice, although likely, awaits functional confirmation.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Mouse monoclonal ANTI-FLAG M2 antibody | Sigma Aldrich | Cat# F1804; RRID:AB_262044 |
| Alexa Fluor 488 rabbit anti-mouse IgG | Invitrogen | Cat# A11059; RRID:AB_2534106 |
| Donkey anti-mouse IgG-HRP | Santa Cruz | Cat# sc-2096; RRID:AB_641168 |
| GFP-Trap Agarose | Chromotek | Cat# gta-100 |
| Anti-FLAG DYKDDDDK Tag Monoclonal Antibody (L5), Alexa Fluor 488 | Invitrogen | Cat# MA1-142-A488; RRID:AB_2610653 |
| Chicken anti-GFP | Aves | Cat# GFP-1020; RRID:AB_10000240 |
| Goat anti-chicken IgY-HRP | Santa Cruz | Cat# sc-2428; RRID:AB_650514 |
| Anti-Digoxigenin-AP, Fab fragments | Roche | Cat# 11093274910; RRID:AB_2313640 |
| Bacterial and virus strains | ||
| DH5α E.coli MAX Efficiency | Thermo Fisher Scientific | Cat# 18258012 |
| MaV203 Competent Yeast Cells | Thermo Fisher Scientific | Cat# 11445012 |
| Chemicals, peptides, and recombinant proteins | ||
| UltraPure Low Melting Point Agarose | Invitrogen | Cat# 15517–022 |
| Trizol TRI Reagent | Sigma Aldrich | Cat# T9424 |
| PBS 1X | Lonza | Cat# 17–516F |
| 2-Propanol. Puriss. P.a., ACS reagent, ≥99.8% (GC) | Sigma Aldrich | Cat# 59300 |
| Ethanol Absolute | VWR | Cat# 20821.321 |
| Methanol | VWR | Cat# 20903.368 |
| TB Green Premix Ex Taq II (Tli Rnase H Plus) | Takara | Cat# RR820L |
| Poly-L-lysine | Sigma Aldrich | Cat# P1399 |
| Hoechst 33342 (10 mg/mL) | Thermo Fisher Scientific | Cat# H3570 |
| Prolong Gold antifade Reagent with DAPI | Cell Signaling | Cat# 8961S |
| Triton X-100 | MP Biomedicals | Cat# MP B07426 |
| Tween 20 | Sigma Aldrich | Cat# 07949 |
| DMSO | Sigma Aldrich | Cat# D2438 |
| 16% Formaldehyde (w/v), Methanol-free | Thermo Fisher Scientific | Cat# 28908 |
| Paraformaldehyde | Sigma Aldrich | Cat# P6148 |
| Albumin Bovine, Fraction V | MP Biomedicals | Cat# MP 160069 |
| Normal Goat Serum | Invitrogen | Cat# 10000c |
| Lipofectamine 2000 Transfection Reagent | Invitrogen | Cat# 11668019 |
| PEI Max | Polysciences Inc. | Cat# 24765–1 |
| TMB stabilized chromogen substrate | Thermo Fisher Scientific | Cat# SB02 |
| Sulfuric acid (H2SO4) | Merck | Cat# 160313 |
| Glycine | Sigma Aldrich | G8898 |
| Sodium Chloride | Sigma Aldrich | S7653 |
| Tris Base | MP Biomedicals | Cat# MP TRIS01KG |
| Hydrochloric acid 37% | Carl Roth | Cat# 9277.1 |
| Benzyl Alcohol | Riedel-de Haën/Honeywell | Cat# 24122 |
| Benzyl Benzoate | Sigma Aldrich | Cat# B6630 |
| Glycerol | Invitrogen | Cat# 15514011 |
| Sodium dodecyl sulfate GPR Rectapur | VWR | Cat# 27926.238 |
| 4X Bolt LDS Sample Buffer | Thermo Fisher Scientific | Cat# B0007 |
| 10X Bolt Sample Reducing Agent | Thermo Fisher Scientific | Cat# B0009 |
| NP-40/IGEPAL | Sigma Aldrich | Cat# CA-630 |
| cOmplete, EDTA-free Protease Inhibitor Cocktail | Roche | Cat# 05056489001 |
| Western Lightning Plus, Chemiluminescent Substrate | Perkin Elmer | Cat# NEL03001-EA |
| T7 RNA polymerase | Roche | Cat# RPOLT7-RO 10881767001 |
| DIG RNA labeling mix | Roche | Cat# 11277073910 |
| NBT/BCIP | Roche | Cat# 11681451001 |
| Critical commercial assays | ||
| Dual luciferase reporter assay system | Promega | Cat# E1980 |
| MEGAshortscript T7 transcription kit | Thermo Fisher Scientific | Cat# AM1354 |
| mMESSAGE mMACHINE T3 transcription Kit | Thermo Fisher Scientific | Cat# AM1348 |
| mMESSAGE mMACHINE SP6 transcription Kit | Thermo Fisher Scientific | Cat# AM1340 |
| HiScribe T7 High Yield RNA Synthesis Kit | NEB | Cat# E2040S |
| SuperScript III First-Strand Synthesis System | Thermo Fisher Scientific | Cat# 18080–051 |
| In-Fusion HD Cloning Kit | Takara | Cat# 639650 |
| Phusion High-Fidelity PCR Kit | NEB | Cat# E0553L |
| Experimental models: Cell lines | ||
| HEK293T | ATCC | CRL-3216; RRID:CVCL_0063 |
| HEK293 STF | ATCC | CRL-3249; RRID:CVCL_AQ26 |
| GPR124−/−;RECK−/− HEK293 STF | (Eubelen et al., 2018) | N/A |
| FZ1-10−/− HEK293T | (Eubelen et al., 2018) | N/A |
| Experimental models: Organisms/strains | ||
| Danio rerio (zebrafish): gpr124s984 | (Vanhollebeke et al., 2015) | N/A |
| Danio rerio (zebrafish): gpr124s985 | (Vanhollebeke et al., 2015) | N/A |
| Danio rerio (zebrafish): gpr124ulb13 | This paper | N/A |
| Danio rerio (zebrafish): gpr124ulb14 | This paper | N/A |
| Danio rerio (zebrafish): Tg(kdrl:eGFP)s843 | (Jin et al., 2005) | N/A |
| Danio rerio (zebrafish): Tg(-17.0neurog1:eGFP)w61 | (McGraw et al., 2008) | N/A |
| Oligonucleotides | ||
| gpr124ulb13 sgRNA: ulb13-fwd 5′-TAGGGTAATGGTTTGGGTCCGT-3′ | This paper | N/A |
| gpr124ulb13 sgRNA: ulb13-rev 5′-AAACACGGACCCAAACCATTAC-3′ | This paper | N/A |
| gpr124ulb14 sgRNA: ulb14-fwd 5′-TAGGGGTATGAAGAGCAGGGTA-3′ | This paper | N/A |
| gpr124ulb14 sgRNA: ulb14-rev 5′-AAACTACCCTGCTCTTCATACC-3′ | This paper | N/A |
|
mag3a sgRNA#1: 5′-GACGG AGGGGTTCGCGAAAG-3′ |
This paper | N/A |
|
magi3a sgRNA#2: 5′-GGAGGG GTTCGCGAAAGTGG-3′ |
This paper | N/A |
|
magi3b sgRNA#1: 5′-GGGGGA TTTTCGCATCCTGG-3′ |
This paper | N/A |
|
magi3b sgRNA#2: 5′-GACAGT AATCCACTCTCCTCC-3′ |
This paper | N/A |
|
dlg4a sgRNA#1: 5′-GCACCAG CGATAAGAACCTGG-3′ |
This paper | N/A |
|
dlg4a sgRNA#2: 5′-CATGGAGT CCATGGCCGCCC-3′ |
This paper | N/A |
|
dlg4b sgRNA#1: 5′-GGGATGG GGGAGTAACGGCG-3′ |
This paper | N/A |
|
dlg4b sgRNA#2: 5′-GCCTTAG GGATGGGGGAGTAA-3′ |
This paper | N/A |
|
magi3a scrambled sgRNA#1: 5′-GAC GGAGGGGTTCGGCTTTC-3′ |
This paper | N/A |
|
magi3a scrambled sgRNA#2: 5′-GGA GGGGTTCGCGATTCACC-3′ |
This paper | N/A |
|
magi3b scrambled sgRNA#1: 5′-GG GGGATTTTCGCAAGGACC-3′ |
This paper | N/A |
|
magi3b scrambled sgRNA#2: 5′-GAC AGTAATCCACTCAGGAGG-3′ |
This paper | N/A |
|
dlg4a scrambled sgRNA#1: 5′-GCAC CAGCGATAAGATGGACC-3′ |
This paper | N/A |
|
dlg4a scrambled sgRNA#2: 5′-GCA TGGAGTCCATGGGGCGGC-3′ |
This paper | N/A |
|
dlg4b scrambled sgRNA#1: 5′-GGG ATGGGGGAGTATGCCGC-3′ |
This paper | N/A |
|
dlg4b scrambled sgRNA#2: 5′-CCTT AGGGATGGGGCTCATT-3′ |
This paper | N/A |
| Universal Bottom Strand Ultramer: 5′-AAA AGCACCGACTCGGTGCCACTTTTTCAAG TTGATAACGGACTAGCCTTATTTTAACTT GCTATTTCTAGCTCTAAAAC -3′ |
(Varshney et al., 2016) | N/A |
| gpr124-MO: ACTGATATTGATTTAACTCACCACA | (Vanhollebeke et al., 2015) | GeneTools |
|
gpr124 qPCR: fwd 5′-ATCTGAG CAACAACCGTATTCG-3′ |
This paper | N/A |
|
gpr124 qPCR: rev 5′-AATGGAAGT TCACTAGCTTGAGAG-3′ |
This paper | N/A |
|
rps16 qPCR: fwd 5′-CTCGGTATCT GAAAGAATCATGCC-3′ |
This paper | N/A |
| rps16 qPCR: rev 5′-GTGACGG GCTCAATCATCTC-3′ |
This paper | N/A |
|
tie1 qPCR: fwd 5′-TGAGCGA TGATGGAACCTGG-3′ |
(Gjini et al., 2011) | N/A |
|
tie1 qPCR: rev 5′-GCTGAAGA GGCCTGACTACC-3′ |
(Gjini et al., 2011) | N/A |
|
actb1 qPCR: fwd 5′-TGAATCC CAAAGCCAACAGAAG-3′ |
(Zada et al., 2016) | N/A |
|
actb1 qPCR: rev 5′-CCAGAGT CCATCACAATACCAG-3′ |
(Zada et al., 2016) | N/A |
|
gpr124s984 HRM: fwd 5′-CAA TGGAAAGGCAGCCTG-3′ |
(Vanhollebeke et al., 2015) | N/A |
|
gpr124s984 HRM: rev 5′-AGG GTCCACTGGAACTGC-3′ |
(Vanhollebeke et al., 2015) | N/A |
|
gpr124s985 HRM: fwd 5′-GCACA CTCTCCTGAACACTAG-3′ |
(Vanhollebeke et al., 2015) | N/A |
|
gpr124s985 HRM: rev 5′-TGCC TGGCATACAATTGGG-3′ |
(Vanhollebeke et al., 2015) | N/A |
|
gpr124ulb13 HRM: fwd 5′-GTGTT GTCCAGGCTATCATCGC-3′ |
This paper | N/A |
|
gpr124ulb13 HRM: rev 5′-GGACACTT GGAGGAGGTTAGTAAGC-3′ |
This paper | N/A |
|
gpr124ulb14 HRM: fwd 5′-CTGGAC ACTAGTGGAACTGACC-3′ |
This paper | N/A |
|
gpr124ulb14 HRM: rev 5′-AACTTT CAAAGTCTGTTAACTCTACCTG-3′ |
This paper | N/A |
| dlg4a HRM: fwd 5′-CCCCTGGAGCACAGCC-3′ | This paper | N/A |
| dlg4a HRM: rev 5′-ATGTGCGTGTGAGGAGCG-3′ | This paper | N/A |
|
dlg4b HRM: fwd 5′-CATGTC TGATTACCCGCAAGC-3′ |
This paper | N/A |
|
dlg4b HRM: rev 5′-ATAATCAT GTGTGTGTGTGACCTGG-3′ |
This paper | N/A |
|
magi3a HRM: fwd 5′-GGAGA TGTGCTTCTGGAGGTG-3′ |
This paper | N/A |
|
magi3a HRM: rev 5′-AGCGTCACA AACAGATAACTCAGG-3′ |
This paper | N/A |
|
magi3b HRM: fwd 5′-AGGTAC CACTCGGCTGCCC-3′ |
This paper | N/A |
|
magi3b HRM: rev 5′-ATCATAT GTCCCACTCTCCAACAG-3′ |
This paper | N/A |
|
dlg4a getPCR: fwd 5′-ACACCAG CGATAAGAACCTGGC-3′ |
This paper | N/A |
|
dlg4a getPCR: rev 5′-GGATCG TCTCCTATATGAGGGT-3′ |
This paper | N/A |
|
dlg4b getPCR: fwd 5′-AGTCCCT CGTCTCCGCGCC-3′ |
This paper | N/A |
|
dlg4b getPCR: rev 5′-ATCTACAC CGTTCACACTCAGTATC-3′ |
This paper | N/A |
|
magi3a getPCR: fwd 5′-GAAACAC TGGTCCAGCAAAGTGC-3′ |
This paper | N/A |
|
magi3a getPCR: rev 5′-CGAAAGT GGCGGATGACGGC-3′ |
This paper | N/A |
|
magi3b getPCR: fwd 5′-TCCGAGA CAACCTGTACCTGC-3′ |
This paper | N/A |
|
magi3b getPCR: rev 5′-TCCAGG ATGCGAAAATCCCCC-3′ |
This paper | N/A |
|
dlg4a NGS: fwd 5′-GCAGTCAT GAATAATTCAGGGGG-3′ |
This paper | N/A |
|
dlg4a NGS: rev 5′-TCAGGGTC TTTTCTCTGAGAAACGG-3′ |
This paper | N/A |
|
dlg4b NGS: fwd 5′-GTGTTTTCA GCATATTCTCCTCA-3′ |
This paper | N/A |
|
dlg4b NGS: rev 5′-TAGTTAGACT GAGCTGAATTAGGCC-3′ |
This paper | N/A |
|
magi3a NGS: fwd 5′-GGGACA GGTGTTGTCGGACG-3′ |
This paper | N/A |
|
magi3a NGS: rev 5′-TCTGCTAA AAGCGATGCTAACCC-3′ |
This paper | N/A |
|
magi3b NGS: fwd 5′-GGATATTC TCACCAGAAGTACATTGC-3′ |
This paper | N/A |
|
magi3b NGS: rev 5′-CCATCCA CAATAATTTTGATCTCC-3′ |
This paper | N/A |
|
kdrl ISH probe: fwd 5′-TGGCAGG ATTCACTTTGAGTGG-3′ |
This paper | N/A |
|
kdrl ISH probe: rev 5′-TAATACG ACTCACTATAGGGTA GTGTAGGGCTCAATCCGCAG-3′ |
This paper | N/A |
|
dlg4a ISH probe: fwd 5′-CGGCTA CACATGAACAAGCAGC-3′ |
This paper | N/A |
|
dlg4a ISH probe: rev 5′-TAATAC GACTCACTATAGGGGACG CTCCCTGGTGGGAATCC-3′ |
This paper | N/A |
|
dlg4b ISH probe: fwd 5′-CATATGG ACATGTCTGATTACCCG-3′ |
This paper | N/A |
|
dlg4b ISH probe: rev 5′-TAATAC GACTCACTATAGGGACGG CCTGCGACAGAAAACC-3′ |
This paper | N/A |
|
magi3a ISH probe: fwd 5′-CCTGC CAGCCGAGAAGACAGG-3′ |
This paper | N/A |
|
magi3a ISH probe: rev 5′-TAAT ACGACTCACTATAGGGCTTTC CAGGGACCTGGAGTTATGG-3′ |
This paper | N/A |
|
magi3b ISH probe: fwd 5′-CGCTCA AGAGGAAGAAACACTGG-3′ |
This paper | N/A |
|
magi3b ISH probe: rev 5′-TAATA CGACTCACTATAGGGTCCAA CAGTAATCCACTCTCCTCC–¬¬3′ |
This paper | N/A |
| Recombinant DNA | ||
| pT7- gRNA | (Jao et al., 2013) | Addgene #46759 |
| pT3TS- nCas9n | (Jao et al., 2013) | Addgene #46757 |
| pCS2- mWNT7a | (Vanhollebeke et al., 2015) | N/A |
| pCS2- mRECK | (Vanhollebeke et al., 2015) | N/A |
| pRK5- mFZ1 | (Yu et al., 2012) | Addgene #42253 |
| pRL- SV40 | (Chen and Prywes, 1999) | Addgene #27163 |
| pCS2- FLAG-zGpr124 | (Eubelen et al., 2018) | N/A |
| pCS2- FLAG-zGpr124 ICD0 | This paper | N/A |
| pCS2- FLAG-zGpr124 ICD1-3 | This paper | N/A |
| pCS2- FLAG-zGpr124 ICD1-10 | This paper | N/A |
| pCS2- FLAG-zGpr124 ICD1-28 | (Eubelen et al., 2018) | N/A |
| pCS2- FLAG-zGpr124 ICD1-80 | (Eubelen et al., 2018) | N/A |
| pCS2- FLAG-zGpr124 ICD1-168 | This paper | N/A |
| pCS2- FLAG-zGpr124 ICD1-213 | (Eubelen et al., 2018) | N/A |
| pCS2- FLAG-zGpr124 ICD1-251 | (Eubelen et al., 2018) | N/A |
| pCS2- FLAG-zGpr124 ICD1-296 | (Eubelen et al., 2018) | N/A |
| pCS2- FLAG-zGpr124 ICD1-333 | (Eubelen et al., 2018) | N/A |
| pCS2- FLAG-zGpr124 ICD-169-337 | This paper | N/A |
| pCS2- FLAG-zGpr124 ΔLRR | (Eubelen et al., 2018) | N/A |
| pCS2- zGpr124 ICD-1-28 | (Eubelen et al., 2018) | N/A |
| pCS2- zGpr124 ICD-1-80 | (Eubelen et al., 2018) | N/A |
| pCS2- zGpr124 ICD-1-168 | This paper | N/A |
| pCS2- zGpr124 ICD1-28 + ETTV | This paper | N/A |
| pCS2- zGpr124 ICD1-80 + ETTV | This paper | N/A |
| pCS2- zGpr124 ICD1-168 + ETVV | This paper | N/A |
| pCS2- FLAG-mGPR124 | This paper | N/A |
| pCS2- FLAG-mGPR124 ICD1-10 | This paper | N/A |
| pCS2- FLAG-mGPR124 ICD1-28 | This paper | N/A |
| pCS2- FLAG-mGPR124 ICD1-80 | This paper | N/A |
| pCS2- FLAG-mGPR124 ICD1-168 | This paper | N/A |
| pCS2- FLAG-mGPR124 ICD1-213 | This paper | N/A |
| pCS2- FLAG-mGPR124 ICD1-333 | This paper | N/A |
| Human GPR124 cDNA clone | K.K. DNAFORM | H05D147H24 |
| pCS2- FLAG-hGPR124 | This paper | N/A |
| pCS2- FLAG-hGPR124 ICD1-10 | This paper | N/A |
| pCS2- FLAG-hGPR124 ICD1-28 | This paper | N/A |
| pCS2- FLAG-hGPR124 ICD1-80 | This paper | N/A |
| pCS2- FLAG-hGPR124 ICD1-168 | This paper | N/A |
| pCS2- FLAG-hGPR124 ICD1-213 | This paper | N/A |
| pCS2- FLAG-hGPR124 ICD1-333 | This paper | N/A |
| pPC97- Gal4 (DBD) | (Chevray and Nathans, 1992) | N/A |
| pPC86- Gal4 (AD) | (Chevray and Nathans, 1992) | N/A |
| pPC97- Gal4 (DBD)-zGpr1241−337 | This paper | N/A |
| pPC97- Gal4 (DBD)-zGpr124169−337 | This paper | N/A |
| pPC97- Gal4 (DBD)-zGpr1241−333 | This paper | N/A |
| pPC97- Gal4 (DBD)-zGpr1241−296 | This paper | N/A |
| pPC97- Gal4 (DBD)-zGpr1241−251 | This paper | N/A |
| pPC97- Gal4 (DBD)-zGpr1241−213 | This paper | N/A |
| pPC97- Gal4 (DBD)-zGpr1241−80 | This paper | N/A |
| pPC86- Gal4 (AD)-Prickle1b | This paper | N/A |
| pPC86- Gal4 (AD)-Atf4a | This paper | N/A |
| pPC86- Gal4 (AD)-Magi3a | This paper | N/A |
| pPC86- Gal4 (AD)-Snx27a | This paper | N/A |
| pPC86- Gal4 (AD)-Zbtb16a | This paper | N/A |
| pPC86- Gal4 (AD)-Dlg4a | This paper | N/A |
| pPC97- Gal4 (DBD)-mGPR1241−272 | This paper | N/A |
| pPC97- Gal4 (DBD)-mGPR1241−268 | This paper | N/A |
| pPC97- Gal4 (DBD)-hGPR1241−271 | This paper | N/A |
| pPC97- Gal4 (DBD)-hGPR1241−267 | This paper | N/A |
| pCS2- Dvl-GFP | Randall Moon Laboratory | Addgene #16788 |
| pCS2- Dvl | (Eubelen et al., 2018) | N/A |
| pCS2- mFZ4-GFP | (Eubelen et al., 2018) | N/A |
| pCS2- zGpr124-TagRFP | (Eubelen et al., 2018) | N/A |
| pCS2- zGpr124-TagRFP ΔICD | This paper | N/A |
| pCS2- mGPR124-TagRFP | This paper | N/A |
| pCS2- mGPR124-TagRFP ΔICD | This paper | N/A |
| pCS2- FLAG-zGpr124 zECD-m7TM | This paper | N/A |
| pCS2- FLAG-zGpr124 ΔICD-mLRR | This paper | N/A |
| pCS2- FLAG-zGpr124 ΔICD-mIg-like | This paper | N/A |
| pCS2- FLAG-zGpr124 ΔICD-mHRM | This paper | N/A |
| pCS2- FLAG-zGpr124 ΔICD-mGAIN | This paper | N/A |
| pCS2- FLAG-mGPR124 mECD-z7TM | This paper | N/A |
| pCS2- FLAG-mGPR124 ΔICD-zLRR | This paper | N/A |
| pCS2- FLAG-mGPR124 ΔICD-zIg-like | This paper | N/A |
| pCS2- FLAG-mGPR124 ΔICD-zHRM | This paper | N/A |
| pCS2- FLAG-mGPR124 ΔICD-zGAIN | This paper | N/A |
| pCS2- FLAG-zGpr124 zECD-mLRR-m7TM | This paper | N/A |
| pCS2- FLAG-zGpr124 zECD-mIg-like-m7TM | This paper | N/A |
| pCS2- FLAG-zGpr124 zECD-mHRM-m7TM | This paper | N/A |
| pCS2- FLAG-zGpr124 zECD-mGAIN-m7TM | This paper | N/A |
| pCS2- membrane tdTomato | This paper | N/A |
| pCS2- Transposase | (Kwan et al., 2007) | N/A |
| pmT2- kdrl:zGpr124 1–337 | This paper | N/A |
| pmT2- kdrl:zGpr124 1–80 | This paper | N/A |
| pmT2- kdrl:mGPR124 1–268 | This paper | N/A |
| pmT2- kdrl:mGPR124 1–63 | This paper | N/A |
| pmT2- kdrl:hGPR124 1–269 | This paper | N/A |
| pmT2- kdrl:hGPR124 1–64 | This paper | N/A |
| pCS2- FLAG-FZ1 | This paper | N/A |
| pCS2- FLAG-FZ4 | This paper | N/A |
| pCS2- FLAG-FZ5 | This paper | N/A |
| pCS2- FLAG-FZ6 | This paper | N/A |
| PSD95-FLAG | (Zhang et al., 2007) | Addgene #15463 |
| pCS2- zGpr124-ICD1-28-GFP | (Eubelen et al., 2018) | N/A |
| pCS2- RnDLG4-VhhGFP4 | This paper | N/A |
| pCS2- VhhGFP4-HsMAGI3 | This paper | N/A |
| pCS2- VhhGFP4-ICD | (Eubelen et al., 2018) | N/A |
| pCS2- VhhGFP4-ETTV | This paper | N/A |
| pCS2- VhhGFP4-TagRFP | (Eubelen et al., 2018) | N/A |
| pS521- zECD(Gpr124)-Fc | This paper | N/A |
| pS521- mECD(GPR124)-Fc | This paper | N/A |
| pCS2- zGpr124-VN155 | (Eubelen et al., 2018) | N/A |
| pCS2- mGPR124-VN155 | This paper | N/A |
| pCS2- mFZ1-VC155 | (Eubelen et al., 2018) | N/A |
| pCS2- mFZ4-VC155 | This paper | N/A |
| pCS2- mGPR116-VC155 | This paper | N/A |
| pCS2- zTnfrsf9a-VC155 | This paper | N/A |
| Software and algorithms | ||
| Prism7 | GraphPad Software www.graphpad.com |
N/A |
| ImageJ | Open source imagej.nih.gov/ij/ |
N/A |
| Clustal Omega | (Sievers et al., 2011) | N/A |
| Jalview | (Waterhouse et al., 2009) | N/A |
| CRISPOR | crispor.tefor.net | N/A |
| CRISPR design tool | crispr.mit.edu | N/A |
| EcoStudy software V5.0 | Illumina.com | N/A |
| PerlPrimer | perlprimer.sourceforge.net/index (Marshall, 2004) | N/A |
| Other | ||
| Fetal Bovine Serum (FBS) | Biowest | Cat# S1810 |
| Milk powder | Nestlé | N/A |
| Penicillin-Streptomycin | Sigma Aldrich | Cat# P4333 |
| DMEM/F12 1:1 medium | Lonza | Cat# BE04-687F/U1 |
| FreeStyle 293 expression medium | Gibco | Cat# 12338–018 |
| HBSS 1x Ca2+/Mg2+-free | Gibco | Cat# 14175–095 |
| HBSS 1x (with Ca2+/Mg2+) | Gibco | Cat# 14025–092 |
| Opti-MEM Reduced Serum Medium | Gibco | Cat# 31985062 |
| TrypLE select enzyme 1x | Gibco | Cat# 12563–011 |
| 7.5% Mini-PROTEAN TGX Precast Protein Gels | Biorad | Cat# 4561025 |
| Vivaspin 20 10kDa column | Cytiva | Cat# 28-9323-60 |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Benoit Vanhollebeke (Benoit.Vanhollebeke@ulb.be).
Materials availability
Zebrafish lines and constructs generated in this study are available upon request.
Experimental model and subject details
Zebrafish husbandry and imaging
Zebrafish (Danio rerio) were maintained at 28°C on a 14 h light/10 h dark cycle. Embryos were obtained and raised under standard conditions in accordance with European and national ethical and animal welfare guidelines (Protocol approval number: CEBEA-IBMM-2017-22:65). Mutant and transgenic lines used in this paper are referenced in the Key resources table. The two to five days post fertilization embryos and larvae used in this study were staged according to Kimmel et al. (Kimmel et al., 1995). Embryos were imaged using a Zeiss LSM900 confocal microscope using the 20x objective after being anesthetized with tricaine, dechorionated and immobilized in 1% low melting point agarose in a glass-bottom Petri dish (MatTek Corporation, Ashland, MA). After imaging, the embryos were genotyped using high resolution melt analysis (Eco Illumina real-time PCR system).
Cell culture
HEK293T and HEK293 STF cells, kindly provided by Jeremy Nathans (John Hopkins), were grown in a 37°C incubator equilibrated at 5% CO2 in DMEM/F12 1:1 medium, supplemented with 10% Fetal bovine serum and Penicillin/Streptomycin. CRISPR/Cas9-engineerd mutant cell lines used are (1) GPR124−/−;RECK−/− HEK293 STF; and (2) FZ1-10−/− HEK293T, as described before (Eubelen et al., 2018).
Method details
CRISPR/Cas9-mediated gene disruption in zebrafish
Germline zebrafish gpr124ulb13 and gpr124ulb14 alleles were engineered using CRISPR/Cas9 according to the protocol described by Jao et al. (Jao et al., 2013). Target sites were designed using the crispr.mit.edu website. The sgRNA constructs were cloned into pT7-gRNA using the following primer pair for gpr124ulb13: 5′-TAGGGTAATGGTTTGGGTCCGT-3′ and 5′-AAACACGGACCCAAACCATTAC-3′, and the following pair for gpr124ulb14: 5′-TAGGGGTATGAAGAGCAGGGTA-3′ and 5′-AAACTACCCTGCTCTTCATACC-3′. The sgRNAs were transcribed from the BamHI linearized pT7-gRNA vector using the MEGAshortscript T7 kit (Thermo Fisher Scientific). The synthetic Cas9 mRNA was transcribed from the XbaI linearized pT3TS-nls-zCas9-nls vector (Addgene #46757) using the mMESSAGE mMACHINE T3 Kit (Ambion). sgRNAs (30 pg) and nls-zCas9-nls mRNA (150 pg) were injected into one-cell stage zebrafish embryos. For somatic gene disruption each gene was targeted by a pair of partially overlapping sgRNAs as described by Wu et al. (Wu et al., 2018). Control sgRNAs were obtained by scrambling the last 6 bp before the PAM sequence. Target sites were identified using the CRISPOR tool (crispor.tefor.net). The sgRNAs were generated according to the protocol developed by Varshney et al. (Varshney et al., 2016). sgRNA-corresponding oligonucleotides were annealed in vitro before transcription using the HiScribe T7 High Yield RNA Synthesis Kit. 15 pg of each sgRNA of a pair was injected at the one-cell stage together with 150 pg of Cas9 mRNA.
To evaluate the efficiency of the somatic gene disruption, the targeted genomic loci were investigated by high resolution melt analysis using an Illumina Eco Real-Time PCR System. The mRNA from target genes was subjected to the genome editing test (get) PCR (Li et al., 2019) using an Applied Biosystems StepOnePlus Real-Time PCR System in order to evaluate the combined effects of non-sense mediated decay and sequence editing. Illumina pair-end sequencing was performed by Genewiz on PCR amplicons flanking the target sites from 12 embryos derived from 4 different injected clutches.
Zebrafish RNA and DNA rescue experiments, morpholino injections, and blastula deep layer cells imaging
In brain angiogenesis assays, one-cell stage embryos were injected with a combination of 2 ng of a splice-blocking morpholino targeting gpr124 (ACTGATATTGATTTAACTCACCACA, Gene Tools, Eugene, OR), and 100 pg of synthetic mRNAs transcribed from NotI linearized pCS2 using the mMESSAGE mMACHINE SP6 Kit (Thermo Fisher Scientific). For the GFP and nano-GFP bimolecular complementation assay, 100 pg of each construct was injected at the one-cell stage. For the DNA rescues, 2 ng of gpr124 morpholino together with 7 pg of pTol2-vector DNA, and 25 pg Tol2 transposase mRNA, were injected at the one-cell stage. For the blastula imaging, one-cell stage embryos were injected with a combination of 300 pg of Gpr124-TagRFP and 300 pg of Fz4-GFP +/− 100 pg of Dvl. 30–50% epiboly blastula deep layer cells were imaged using a Zeiss LSM900 confocal microscope and a 40x long distance objective. The enrichment score represents the ratio of the mean intensity of Fz4-GFP signal within TagRFP + junctional areas, over the mean intensity outside of the junctions. Junctions were defined by the expression of Gpr124-TagRFP. Similarly, for the bimolecular complementation assay, 200 pg of zebrafish or mouse Gpr124-VN155 and 200 pg of Fz1-or Fz4- VC155 were injected with or without 100 pg of Dvl.
In vivo membrane localization and protein expression level assessments
Embryos were dechorionated at the blastula stage and fixed overnight at 4°C with 4% PFA. The blastulas were carefully washed 3x with PBS and 2x with PBS/Tween 20 0.5%, before being blocked for 1 h at RT with in PBS/Tween 20 0.5% supplemented with 10% normal goat serum and 1% DMSO. The blastulas were stained for 48 h at 4°C with Alexa Fluor 488-conjugated anti-FLAG antibody diluted 1:300 in blocking solution and were then washed 3x for 3 h with PBS/Tween 20 0.5% and 2x with PBS before being imaged using a Zeiss LSM900 confocal microscope and a 40x long distance objective.
To measure total protein expression in zebrafish, blastula stage embryos were collected and snap frozen in liquid nitrogen. The embryos were then homogenized using pestles and resuspended for 30 min in lysis buffer at 4°C (150 mM NaCl, 25 mM Tris-HCl, 1% NP-40, and EDTA-free proteinase inhibitors). The samples and dilutions thereof were deposited onto a nitrocellulose membrane using a dot blot apparatus. The membrane was blocked for 1 h at RT with 5% milk in TBS/Tween 20 0.05%. The membrane was stained with a 1:1,000 dilution of an anti-FLAG M2 antibodies in 1% milk in TBS/Tween 20 0.05% overnight at 4°C. The membranes were washed 3x with TBS/Tween 20 0.05%, before adding a 1:10,000 dilution of an anti-mouse-HRP antibody in 1% BSA in TBS/Tween 20 0.05% for 1 h at RT. The membrane was then washed 3x with TBS/Tween 20 0.05% and 2x with TBS. The blot was revealed with the ECL substrate (PerkinElmer).
RT-qPCR analysis
After a tail biopsy was collected for genotyping, total RNA of deeply anesthetized individual 30 hpf embryos was extracted with Trizol and precipitated with isopropanol. cDNA synthesis was performed using the Superscript III First-Strand Synthesis System. TB Green RT-qPCR analysis was performed with the following primers: gpr124-fwd 5′-ATCTGAGCAACAACCGTATTCG-3′, gpr124-rev 5′-AATGGAAGTTCACTAGCTTGAGAG-3′, rps16-fwd 5′-CTCGGTATCTGAAAGAATCATGCC-3′, and rps16-rev 5′-GTGACGGGCTCAATCATCTC-3′ using an Applied Biosystems StepOnePlus Real-Time PCR System. Primers were designed using PerlPrimer software, set at default settings (Real-Time PCR). Embryos were collected from four independent crosses.
FACS sorting
Three independent pools of 300 48 hpf dechorionated Tg(kdrl:eGFP)s843 embryos were washed in Ca2+/Mg2+-free HBSS (Gibco) before being dissociated in 2 mL TrypLE Select (Gibco) at 28.5°C for 40 min under gentle agitation. The dissociation was stopped by adding 100 μL FBS (Westburg) on ice. The cells were collected by centrifugation and resuspended in 1 mL HBSS (with Ca2+/Mg2+) and 5% FBS. The cells were then filtered and GFP+ cells were isolated on a BD Biosciences FACSAria III. The sorted cells were collected by centrifugation and total RNA was extracted using Trizol and precipitated with isopropanol. cDNA was synthesized using the Superscript III First-Strand Synthesis System for qPCR analysis.
Whole-mount in situ hybridization
Whole mount in situ hybridization was performed using standard procedures (Thisse and Thisse, 2008). The embryos were fixed in 4% PFA (Sigma Aldrich) and dehydrated in methanol before being hybridized with riboprobes that were generated from PCR products with the following primers: kdrl-fwd 5′-TGGCAGGATTCACTTTGAGTGG-3′ and kdrl-rev 5′-TAATACGACTCACTATAGGGTAGTGTAGGGCTCAATCCGCAG-3′; dlg4a-fwd: 5′-CGGCTACACATGAACAAGCAGC-3′ and dlg4a-rev: 5′-TAATACGACTCACTATAGGGGACGCTCCCTGGTGGGAATCC-3′; dlg4b-fwd: 5′-CATATGGACATGTCTGATTACCCG-3′ and dlg4b-rev: 5′-TAATACGACTCACTATAGGGACGGCCTGCGACAGAAAACC-3′; magi3a-fwd: 5′-CCTGCCAGCCGAGAAGACAGG-3′ and magi3a-rev: 5′-TAATACGACTCACTATAGGGCTTTCCAGGGACCTGGAGTTATGG-3′; magi3b-fwd: 5′-CGCTCAAGAGGAAGAAACACTGG-3′ and magi3b-rev: 5′-TAATACGACTCACTATAGGGTCCAACAGTAATCCACTCTCCTCC-3′
The probes were then transcribed with a T7 RNA polymerase (Roche) and digoxigenin-RNA labeling mix (Roche) and an anti-DIG AP (1:5000) and NBT/BCIP substrate (Roche) was used to detect them. The embryos were cleared with Benzyl Alcohol/Benzyl Benzoate (BA/BB) before being transferred into glycerol for storage and imaging using a Leica M165 stereoscope.
HEK293(T) cell imaging
HEK293(T) cells were transfected at 70% confluency in 24-well plates using lipofectamine 2000 and 400 ng of total plasmid DNA. 24 h after transfection, cells were re-seeded for 24 h on poly-L-lysine coated (0.1 mg.mL-1) μ-slide 8 well IBIDI plates (IBIDI 80824), or 12 mm glass coverslips (VWR 13mm CB00130RAC33BDH0) in 24-well plates. When imaging endogenous fluorescence from Dvl-GFP expressing cells, cells were stained with 10 μg.mL-1 Hoechst before imaging. For immunostainings, cells were rinsed once with PBS, fixed for 12 min in 4% PFA, washed 3x with PBS, permeabilized for 3 min with PBS-Triton X-100 0.15%, washed 3x with PBS for 1 min, before being blocked for 1 h at room temperature with 4% BSA in PBS/Tween 20 0.05% (Further referred to as ‘blocking solution’). After blocking, the cells were incubated at room temperature for 2 h with a 1:300 dilution of anti-FLAG M2 antibodies in the blocking solution, washed 3 × 5 min with PBS/Tween 20 0.05%, incubated with a 1:500 dilution of Alexa Fluor 488 rabbit anti-mouse IgGs in the blocking solution, washed 3 × 10 min with PBS/Tween 20 0.05%, 1x with PBS, and with water before being mounted on a glass slide with Prolong antifade + DAPI and sealed with clear nail polish. The images were taken using a LSM710 Zeiss confocal microscope, using a 63x objective.
Cell surface ELISA
HEK293(T) cells were transfected at 70% confluency in 24-well plates using lipofectamine 2000 and 400 ng of total plasmid DNA. 24 h after transfection, cells were split into four poly-L-lysine coated wells of a 96-well plate. After 24 h, the cells were rinsed once with PBS, fixed for 12 min with 4% PFA, and washed 3x with PBS before half of the wells were permeabilized for 3 min with PBS-Triton X-100 0.15%. Two experimental replicates were permeabilized (P) (overall expression of a construct, used for normalization) and two experimental replicates were not permeabilized (NP) (cell surface expression of a construct). All wells were subsequently rinsed 3 × 1 min with PBS and blocked for 1h at room temperature with 4% BSA in PBS (NP-blocking solution) or PBS/Tween 20 0.05% (P-blocking solution). After blocking, the cells were incubated at room temperature for 1 h with a 1:500 dilution of anti-FLAG M2 antibodies in the blocking solution, washed 3 × 5 min with PBS (NP) or PBS/Tween 20 0.05% (P), incubated for 1 h with a 1:1000 dilution of donkey anti-mouse IgG-HRP in blocking solution, before being washed 3 × 10 min with PBS (NP) or PBS/Tween 20 0.05% (P) and 1x with PBS. After washing, cells were exposed to 50 μL of TMB stabilized chromogen substrate for 1–5 min. The reaction was stopped using 25 μL 2N H2SO4 and the absorbance was read using a Multiskan FC Microplate Photometer (Thermo Fisher Scientific). Background absorbance levels were determined in cells transfected with an empty pCS2 plasmid. Cell surface expression is determined by the ratio between NP and p values.
Co-immunoprecipitation
HEK293T Fz1-10−/− were seeded at 70% confluency in 6-well plates and were transfected 24 h later with 1 μg of the Dvl-GFP construct and 1 μg of the FLAG construct using polyethylenimine “Max” (PEI, Polysciences). 48 h after transfection, the cells were washed 2x with PBS before being collected at 4°C by centrifugation. Cell pellets were resuspended for 30 min at 4°C in lysis buffer under agitation (150 mM NaCl, 25 mM Tris-HCl, 1% NP-40 and EDTA-free proteinase inhibitors). Cell debris were precipitated and part of the lysate was saved as “input” material in Bolt LDS sample buffer supplemented with the Bolt sample reducing agent. The remaining cell lysate was added to 25 μL of pre-washed GFP-Trap beads and the suspension was rotated for 1 h at 4°C. The beads were then washed 3x with the lysis buffer before being resuspended in reducing Bolt LDS sample buffer and heated at 70°C for 5 min. After SDS-PAGE, membranes were blocked for 1 h with 5% milk in TBS/Tween 20 0.05%, and incubated with a 1:1,000 dilution of mouse anti-FLAG M2 and chicken anti-GFP antibodies diluted in 1% milk in TBS/Tween 20 0.05% overnight at 4°C. Before adding the secondary antibodies, the membranes were washed 3x with TBS/Tween 20 0.05%. Anti-mouse-HRP and anti-Chicken-HRP were diluted 1:10 000 in 1% BSA in TBS/Tween 20 0.05% and membranes were stained for 1 h at RT before being washed 3x with TBS/Tween 20 0.05% and 2x with TBS. The blot was revealed with the ECL substrate (PerkinElmer).
Recombinant proteins
HEK293T cells were seeded into 10 cm dishes at 80% confluency and after 24 h were transfected with 15 μg of a construct encoding mouse or zebrafish Gpr124 extracellular domain fused to the Fc region using PEI. 24 h after transfection the serum-containing medium was replaced by FreeStyle 293 (Gibco) medium. After 48 h of secretion, the medium was collected and concentrated on a Vivaspin 20 column. Protein concentration were determined using BCA and the purity evaluated >95% by Coomasie-blue staining. 10 mM of recombinant protein was added to the cells in the dual luciferase assays 24 h after transfection of the plasmid constructs.
Yeast two-hybrid screen
The primary screen was performed by Hybrigenics (Paris, France) using the coding sequence of zebrafish Gpr124 ICD as bait and a cDNA library of 18–20 hpf zebrafish embryos as prey. This primary screen returned 84 positive clones from a total of 84.6 million analyzed interactions, six of which corresponded to unique potential intracellular interactions. Each potential partner was subcloned, and retested against a collection ICD variants of different lengths, under permissive (+histidine) and selective medium (- histidine +5 mM 3-amino-1,2,4-triazole), using pPC86 and pPC97 plasmids (Chevray and Nathans, 1992), in MaV203 competent yeast cells (Invitrogen).
Cloning strategy
All plasmids used in STF assays and cell imaging were expressed from the CMV promoter of the pCS2 vector, with the exception of Fz1, which is expressed from the pRK5 vector. Gpr124 ICD truncations and chimeras were cloned using the In-Fusion cloning strategy, using overlapping PCR products generated with Phusion DNA polymerase. All constructs were confirmed by Sanger sequencing. Zebrafish and mouse Gpr124 cDNAs were obtained previously (Zhou and Nathans, 2014; Vanhollebeke et al., 2015), human Gpr124 was cloned from a cDNA vector sourced at DNAFORM. The FLAG tag was inserted after residue 47, 56 and 55 in zebrafish, mouse, and human Gpr124, respectively. The mouse and zebrafish extracellular domain fused to the Fc region were cloned into the PS521 vector (provided by P. Schneider, University of Lausanne).
STF dual luciferase assays
HEK293 STF cells were transfected at 70% confluency in 96-well plates using lipofectamine 2000 and 5 ng of Reck, 5 ng of Fz1, 20 ng of Wnt7a, and 0.5 ng of Renilla luciferase constructs. Gpr124 constructs were transfected at the indicated doses, and the total amount of DNA was adjusted to 55.5 ng with the empty pCS2 vector. 48 h after transfection, firefly and Renilla luciferase were measured sequentially according to the manufacturer recommendation (Dual luciferase reporter assay system, Promega). The data is normalized to an empty pCS2 transfection control.
Quantification and statistical analysis
Statistical analysis was performed using the GraphPad Prism 7 software. For the cell surface ELISA data, statistical tests were performed by one-way ANOVA analysis. All experiments were done 3–4 times independently. For the dual luciferase assay data, a two-way ANOVA with Tukey’s post-hoc analysis was performed. All experiments were done 3–4 times independently. For the zebrafish CtAs and DRG quantification, Kruskal-Wallis tests with a Dunn’s multiple comparison post-hoc analysis were used for multiple comparisons. When comparing two independent conditions, a Mann-Whitney test was used. All data points are indicated in the graphs and were collected from 3 to 4 independent experiments. Finally, for the blastula enrichment score, Mann-Whitney tests were done. All data points are indicated in the graphs and were collected from 3 to 4 different experiments. ns = not significant; ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001.
Acknowledgments
We thank S. Rosar, P. Poelvoorde, E. Dupont, P. Tebabi, M. Adam, and S. Reischauer for their technical support and help. We thank P. Schneider and J. Nathans for the PS521 vector and the HEK293 STF cells, respectively. The graphical abstract was created with BioRender.com. N.B. and M.E. are FRIA fellows from the FRS - FNRS. Work in the B.V. laboratory is supported by the FNRS, the Fondation ULB, the Queen Elisabeth Medical Foundation (QEMF), the FRFS-WELBIO (CR-2017S-05R), and the ERC (CoG Ctrl-BBB 865176).
Author contributions
All authors performed research and/or analyzed data. All authors discussed results and edited the manuscript. M.A. and B.V. wrote the manuscript. B.V. supervised the study.
Declaration of interests
The ULB (B.V.) has filed for patent protection for Gpr124/Reck-specific agonism. B.V. is a founder, shareholder, and consultant of NeuVasQ Biotechnologies.
Published: May 31, 2022
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.celrep.2022.110902.
Supplemental information
Data and code availability
The data reported in this paper will be shared by the lead contact upon request.
No code was generated in this study.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- Blanchette M., Daneman R. Formation and maintenance of the BBB. Mech. Dev. 2015;138:8–16. doi: 10.1016/j.mod.2015.07.007. [DOI] [PubMed] [Google Scholar]
- Bostaille N., Gauquier A., Twyffels L., Vanhollebeke B. Molecular insights into Adgra2/Gpr124 and Reck intracellular trafficking. Biol. Open. 2016;5:1874–1881. doi: 10.1242/bio.021287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J., Mancuso M.R., Maier C., Liang X., Yuki K., Yang L., Kwong J.W., Wang J., Rao V., Vallon M., et al. Gpr124 is essential for blood-brain barrier integrity in central nervous system disease. Nat. Med. 2017;23:450–460. doi: 10.1038/nm.4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Prywes R. Serum-induced expression of the cdc25A gene by relief of E2F-mediated repression. Mol. Cell Biol. 1999;19:4695–4702. doi: 10.1128/MCB.19.7.4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevray P.M., Nathans D. Protein interaction cloning in yeast: identification of mammalian proteins that react with the leucine zipper of Jun. Proc. Natl. Acad. Sci. U S A. 1992;89:5789–5793. doi: 10.1073/pnas.89.13.5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho C., Smallwood P.M., Nathans J. Reck and Gpr124 are essential receptor cofactors for Wnt7a/Wnt7b-specific signaling in mammalian CNS angiogenesis and blood-brain barrier regulation. Neuron. 2017;95:1056–1073.e5. doi: 10.1016/j.neuron.2017.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho C., Wang Y., Smallwood P.M., Williams J., Nathans J. Molecular determinants in Frizzled, Reck, and Wnt7a for ligand-specific signaling in neurovascular development. Elife. 2019;8 doi: 10.7554/eLife.47300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho C., Wang Y., Smallwood P.M., Williams J., Nathans J. Dlg1 activates beta-catenin signaling to regulate retinal angiogenesis and the blood-retina and blood-brain barriers. Elife. 2019;8:e45542. doi: 10.7554/elife.45542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow B.W., Gu C. The molecular constituents of the blood-brain barrier. Trends Neurosci. 2015;38:598–608. doi: 10.1016/j.tins.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H., Loh K.M., Nusse R. Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science. 2014;346:1248012. doi: 10.1126/science.1248012. [DOI] [PubMed] [Google Scholar]
- Daneman R., Agalliu D., Zhou L., Kuhnert F., Kuo C.J., Barres B.A. Wnt/β-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc. Natl. Acad. Sci. U S A. 2009;106:641–646. doi: 10.1073/pnas.0805165106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eubelen M., Bostaille N., Cabochette P., Gauquier A., Tebabi P., Dumitru A.C., Koehler M., Gut P., Alsteens D., Stainier D.Y.R., et al. A molecular mechanism for Wnt ligand-specific signaling. Science. 2018;361 doi: 10.1126/science.aat1178. [DOI] [PubMed] [Google Scholar]
- Gjini E., Hekking L.H., Küchler A., Saharinen P., Wienholds E., Post J.-A., Alitalo K., Schulte-Merker S. Zebrafish Tie-2 shares a redundant role with Tie-1 in heart development and regulates vessel integrity. Dis. Model. Mech. 2011;4:57–66. doi: 10.1242/dmm.005033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hering H., Sheng M. Direct interaction of Frizzled-1, -2, -4, and -7 with PDZ domains of PSD-95. FEBS Lett. 2002;521:185–189. doi: 10.1016/S0014-5793(02)02831-4. [DOI] [PubMed] [Google Scholar]
- Jao L.-E., Wente S.R., Chen W. Efficient multiplex biallelic zebrafish genome editing using a CRISPR nuclease system. Proc. Natl. Acad. Sci. U S A. 2013;110:13904–13909. doi: 10.1073/pnas.1308335110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin S.-W., Beis D., Mitchell T., Chen J.-N., Stainier D.Y.R. Cellular and molecular analyses of vascular tube and lumen formation in zebrafish. Development. 2005;132:5199–5209. doi: 10.1242/dev.02087. [DOI] [PubMed] [Google Scholar]
- Kimmel C.B., Ballard W.W., Kimmel S.R., Ullmann B., Schilling T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Kwan K.M., Fujimoto E., Grabher C., Mangum B.D., Hardy M.E., Campbell D.S., Parant J.M., Yost H.J., Kanki J.P., Chien C.-B. The Tol2kit: a multisite gateway-based construction kit for Tol2 transposon transgenesis constructs. Dev. Dyn. 2007;236:3088–3099. doi: 10.1002/dvdy.21343. [DOI] [PubMed] [Google Scholar]
- Li X., Roszko I., Sepich D.S., Ni M., Hamm H.E., Marlow F.L., Solnica-Krezel L. Gpr125 modulates Dishevelled distribution and planar cell polarity signaling. Development. 2013;140:3028–3039. doi: 10.1242/dev.094839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B., Ren N., Yang L., Liu J., Huang Q. A qPCR method for genome editing efficiency determination and single-cell clone screening in human cells. Sci. Rep. 2019;9:18877. doi: 10.1038/s41598-019-55463-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebner S., Corada M., Bangsow T., Babbage J., Taddei A., Czupalla C.J., Reis M., Felici A., Wolburg H., Fruttiger M., et al. Wnt/β-catenin signaling controls development of the blood–brain barrier. J. Cell Biol. 2008;183:409–417. doi: 10.1083/jcb.200806024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Fuentes E.J. Emerging themes in PDZ domain signaling: structure, function, and inhibition. Int. Rev. Cell Mol. Biol. 2019;343:129–218. doi: 10.1016/bs.ircmb.2018.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall O.J. PerlPrimer: cross-platform, graphical primer design for standard, bisulphite and real-time PCR. Bioinformatics. 2004;20:2471–2472. doi: 10.1093/bioinformatics/bth254. [DOI] [PubMed] [Google Scholar]
- Martin M., Vermeiren S., Bostaille N., Eubelen M., Spitzer D., Vermeersch M., Profaci C.P., Pozuelo E., Toussay X., Raman-Nair J., et al. Engineered Wnt ligands enable blood-brain barrier repair in neurological disorders. Science. 2022;375:eabm4459. doi: 10.1126/science.abm4459. [DOI] [PubMed] [Google Scholar]
- McGraw H.F., Nechiporuk A., Raible D.W. Zebrafish dorsal root ganglia neural precursor cells adopt a glial fate in the absence of neurogenin1. J. Neurosci. 2008;28:12558–12569. doi: 10.1523/JNEUROSCI.2079-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusse R., Clevers H. Wnt/β-Catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169:985–999. doi: 10.1016/j.cell.2017.05.016. [DOI] [PubMed] [Google Scholar]
- Obermeier B., Daneman R., Ransohoff R.M. Development, maintenance and disruption of the blood-brain barrier. Nat. Med. 2013;19:1584–1596. doi: 10.1038/nm.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posokhova E., Shukla A., Seaman S., Volate S., Hilton M.B., Wu B., Morris H., Swing D.A., Zhou M., Zudaire E., et al. GPR124 functions as a WNT7-specific coactivator of canonical β-catenin signaling. Cell Rep. 2015;10:123–130. doi: 10.1016/j.celrep.2014.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Profaci C.P., Munji R.N., Pulido R.S., Daneman R. The blood-brain barrier in health and disease: important unanswered questions. J. Exp. Med. 2020;217 doi: 10.1084/jem.20190062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punchihewa C., Ferreira A.M., Cassell R., Rodrigues P., Fujii N. Sequence requirement and subtype specificity in the high-affinity interaction between human frizzled and dishevelled proteins. Protein Sci. 2009;18:994–1002. doi: 10.1002/pro.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbagh M.F., Heng J.S., Luo C., Castanon R.G., Nery J.R., Rattner A., Goff L.A., Ecker J.R., Nathans J. Transcriptional and epigenomic landscapes of CNS and non-CNS vascular endothelial cells. Elife. 2018;7 doi: 10.7554/eLife.36187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte G. International union of basic and clinical pharmacology. LXXX. The class frizzled receptors. Pharmacol. Rev. 2010;62:632–667. doi: 10.1124/pr.110.002931. [DOI] [PubMed] [Google Scholar]
- Schulte G. Frizzleds and WNT/β-catenin signaling--The black box of ligand-receptor selectivity, complex stoichiometry and activation kinetics. Eur. J. Pharmacol. 2015;763:191–195. doi: 10.1016/j.ejphar.2015.05.031. [DOI] [PubMed] [Google Scholar]
- Sharma M., Castro-Piedras I., Simmons G.E., Pruitt K. Dishevelled: a masterful conductor of complex Wnt signals. Cell. Signal. 2018;47:52–64. doi: 10.1016/j.cellsig.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sievers F., Wilm A., Dineen D., Gibson T.J., Karplus K., Li W., Lopez R., McWilliam H., Remmert M., Söding J., et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011;7:539. doi: 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhart Z., Angers S. Wnt signaling in development and tissue homeostasis. Development. 2018;145:dev146589. doi: 10.1242/dev.146589. [DOI] [PubMed] [Google Scholar]
- Stenman J.M., Rajagopal J., Carroll T.J., Ishibashi M., McMahon J., McMahon A.P. Canonical Wnt signaling regulates organ-specific assembly and differentiation of CNS vasculature. Science. 2008;322:1247–1250. doi: 10.1126/science.1164594. [DOI] [PubMed] [Google Scholar]
- Tan C., Deardorff M.A., Saint-Jeannet J.-P., Yang J., Arzoumanian A., Klein P.S. Kermit, a frizzled interacting protein, regulates frizzled 3 signaling in neural crest development. Development. 2001;128:3665–3674. doi: 10.1242/dev.128.19.3665. [DOI] [PubMed] [Google Scholar]
- Tauriello D.V.F., Jordens I., Kirchner K., Slootstra J.W., Kruitwagen T., Bouwman B.A.M., Noutsou M., Rüdiger S.G.D., Schwamborn K., Schambony A., Maurice M.M. Wnt/β-catenin signaling requires interaction of the Dishevelled DEP domain and C terminus with a discontinuous motif in Frizzled. Proc. Natl. Acad. Sci. U S A. 2012;109:E812–E820. doi: 10.1073/pnas.1114802109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thisse C., Thisse B. High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat. Protoc. 2008;3:59–69. doi: 10.1038/nprot.2007.514. [DOI] [PubMed] [Google Scholar]
- Ulrich F., Carretero-Ortega J., Menéndez J., Narvaez C., Sun B., Lancaster E., Pershad V., Trzaska S., Véliz E., Kamei M., et al. Reck enables cerebrovascular development by promoting canonical Wnt signaling. Development. 2016;143:1055. doi: 10.1242/dev.136507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbhauer M., Djiane A., Goisset Â., Penzo-me A. The C-terminal cytoplasmic Lys-Thr-X-X-X-Trp motif in frizzled receptors mediates Wnt/b -catenin signalling. EMBO J. 2000;19:4944–4954. doi: 10.1093/emboj/19.18.4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallon M., Yuki K., Nguyen T.D., Chang J., Yuan J., Siepe D., Miao Y., Essler M., Noda M., Garcia K.C., Kuo C.J. A RECK-WNT7 receptor-ligand interaction enables isoform-specific regulation of Wnt bioavailability. Cell Rep. 2018;25:339–349.e9. doi: 10.1016/j.celrep.2018.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Amerongen R. Alternative Wnt pathways and receptors. Cold Spring Harb. Perspect. Biol. 2012;4:a007914. doi: 10.1101/cshperspect.a007914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Amerongen R., Nusse R. Towards an integrated view of Wnt signaling in development. Development. 2009;136:3205–3214. doi: 10.1242/dev.033910. [DOI] [PubMed] [Google Scholar]
- Vanhollebeke B., Stone O.A., Bostaille N., Cho C., Zhou Y., Maquet E., Gauquier A., Cabochette P., Fukuhara S., Mochizuki N., et al. Tip cell-specific requirement for an atypical Gpr124- and Reck-dependent Wnt/β-catenin pathway during brain angiogenesis. Elife. 2015;4:1–25. doi: 10.7554/eLife.06489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanlandewijck M., He L., Mäe M.A., Andrae J., Ando K., Del Gaudio F., Nahar K., Lebouvier T., Laviña B., Gouveia L., et al. A molecular atlas of cell types and zonation in the brain vasculature. Nature. 2018;554:475–480. doi: 10.1038/nature25739. [DOI] [PubMed] [Google Scholar]
- Varshney G.K., Carrington B., Pei W., Bishop K., Chen Z., Fan C., Xu L., Jones M., LaFave M.C., Ledin J., et al. A high-throughput functional genomics workflow based on CRISPR/Cas9-mediated targeted mutagenesis in zebrafish. Nat. Protoc. 2016;11:2357–2375. doi: 10.1038/nprot.2016.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Chang H., Rattner A., Nathans J. In: Current Topics in Developmental Biology. Wassarman P.M., editor. Academic Press; 2016. Chapter seven - frizzled receptors in development and disease; pp. 113–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse A.M., Procter J.B., Martin D.M.A., Clamp M., Barton G.J. Jalview Version 2--a multiple sequence alignment editor and analysis workbench. Bioinformatics. 2009;25:1189–1191. doi: 10.1093/bioinformatics/btp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wawrzak D., Luyten A., Lambaerts K., Zimmermann P. Frizzled–PDZ scaffold interactions in the control of Wnt signaling. Adv. Enzyme Regul. 2009;49:98–106. doi: 10.1016/j.advenzreg.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Wheeler D.S., Barrick S.R., Grubisha M.J., Brufsky A.M., Friedman P.A., Romero G. Direct interaction between NHERF1 and Frizzled regulates β-catenin signaling. Oncogene. 2011;30:32–42. doi: 10.1038/onc.2010.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong H.-C., Bourdelas A., Krauss A., Lee H.-J., Shao Y., Wu D., Mlodzik M., Shi D.-L., Zheng J. Direct binding of the PDZ domain of dishevelled to a conserved internal sequence in the C-terminal region of frizzled. Mol. Cell. 2003;12:1251–1260. doi: 10.1016/S1097-2765(03)00427-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu R.S., Lam I.I., Clay H., Duong D.N., Deo R.C., Coughlin S.R. A rapid method for directed gene knockout for screening in G0 zebrafish. Dev. Cell. 2018;46:112–125.e4. doi: 10.1016/j.devcel.2018.06.003. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y., Irie K., Asada M., Mino A., Mandai K., Takai Y. Direct binding of the human homologue of the Drosophila disc large tumor suppressor gene to seven-pass transmembrane proteins, tumor endothelial marker 5 (TEM5), and a novel TEM5-like protein. Oncogene. 2004;23:3889–3897. doi: 10.1038/sj.onc.1207495. [DOI] [PubMed] [Google Scholar]
- Yao R., Maeda T., Takada S., Noda T. Identification of a PDZ domain containing golgi protein, GOPC, as an interaction partner of frizzled. Biochem. Biophys. Res. Commun. 2001;286:771–778. doi: 10.1006/bbrc.2001.5430. [DOI] [PubMed] [Google Scholar]
- Yao R., Natsume Y., Noda T. MAGI-3 is involved in the regulation of the JNK signaling pathway as a scaffold protein for frizzled and Ltap. Oncogene. 2004;23:6023–6030. doi: 10.1038/sj.onc.1207817. [DOI] [PubMed] [Google Scholar]
- Yu H., Ye X., Guo N., Nathans J. Frizzled 2 and frizzled 7 function redundantly in convergent extension and closure of the ventricular septum and palate: evidence for a network of interacting genes. Development. 2012;139:4383–4394. doi: 10.1242/dev.083352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zada D., Tovin A., Lerer-Goldshtein T., Appelbaum L. Pharmacological treatment and BBB-targeted genetic therapy for MCT8-dependent hypomyelination in zebrafish. Dis. Model. Mech. 2016;9:1339–1348. doi: 10.1242/dmm.027227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Vinuela A., Neely M.H., Hallett P.J., Grant S.G.N., Miller G.M., Isacson O., Caron M.G., Yao W.-D. Inhibition of the dopamine D1 receptor signaling by PSD-95. J. Biol. Chem. 2007;282:15778–15789. doi: 10.1074/jbc.M611485200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z., Nelson A.R., Betsholtz C., Zlokovic B.V. Establishment and dysfunction of the blood-brain barrier. Cell. 2015;163:1064–1078. doi: 10.1016/j.cell.2015.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Nathans J. Gpr124 controls CNS angiogenesis and blood-brain barrier integrity by promoting ligand-specific canonical Wnt signaling. Dev. Cell. 2014;31:248–256. doi: 10.1016/j.devcel.2014.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data reported in this paper will be shared by the lead contact upon request.
No code was generated in this study.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.