Highlights
-
•
Clove oil has potent antimicrobial activity.
-
•
Nanoemulsion fabricated with clove oil enhances its solubility in aqueous system.
-
•
Clove oil nanoemulsion was stable against various processing parameters.
-
•
Study revealed enhanced antimicrobial activity with nanoemulsion than free oil.
Keywords: Nanoemulsion, Clove oil, Whey protein, Escherichia coli, Bacillus subtilis
Abstract
Clove oil has a high eugenol content, making it an effective antimicrobial essential oil; nevertheless, its low water solubility, high volatility, and organoleptic qualities limit its use in food systems. As a result, we created an antibacterial system using clove oil-in-water nanoemulsion. Clove oil nanoemulsions were produced using whey protein concentrate (0.1–1%) as an emulsifier by ultrasonication and various physico-chemical characteristics (stability, particle size, zeta-potential, and poly dispersity index) were investigated. Mean particle size, zeta potential and polydispersity index of the most stable nanoemulsion were 279.0 ± 8.43 nm, -34.5 ± 0.12 mV, and 0.179 ± 0.012, respectively. Most stable nanoemulsion was fairly stable at different processing parameters such as various pH (3.0 – 7.0), temperature ranges (63 – 121 °C), and ionic strengths (0.1 – 1.0 M NaCl). Finally, antimicrobial activities, such as minimum inhibitory concentration was found with 50 µL, whereas minimum bactericidal concentration was observed to be 90 µL after 8 h contact time, against E. coli and B. subtilis strains.
1. Introduction
Due to great escalation of illness, outbreak triggered by pathogenic and spoilage microorganisms, there is an emergent interest for the use of natural ingredients or additives as food preservatives. Hence, food manufacturers, food safety researchers and regulatory agencies are more concerned about natural preservatives for their effective antimicrobial activities as well as minimal changes in the organoleptic attributes of the food system. Due to an increase in the microorganisms’ resistance to antibiotics, consumers are interested in employing natural preservatives (essential oils) in food systems that have been designated as Generally Recognized as Safe (GRAS) by the Food and Drug Administration (FDA) [1]. Studies over the centuries concluded that essential oils (EOs) and their active components (phytochemicals) are powerful antimicrobials against a variety of foodborne pathogens [2], [3], [4].
Due to hydrophobic, highly reactive, and volatile in nature, EOs are very difficult to incorporate directly into the system. One approach that pursuits actively is to develop a novel delivery or encapsulation system for the EOs would enable more widespread use in various food systems with improved efficacies [5], [6]. Recent study has demonstrated nanoemulsion based delivery systems for d-limonene and terpenes mixture, and they were found an increase in the antimicrobial activity with negligible organoleptic properties [7]. Antimicrobial activity of EOs were found to be increased when encapsulated into the surfactant micelles, which increase the solubility in aqueous system [8]. Furthermore, multiple studies have shown that using pure essential oils as the dispersed phase is extremely challenging, resulting in a number of physical stability issues [9], [10], [11]. Clove essential oil derived from the stem, leaves and buds of Eugenia caryophyllata having a major constituent eugenol attributed to its potential antimicrobial activity. However, its use as oil in water nanoemulsion is under scanty. In addition, protein stabilized nanoemulsions showed small particle droplets with better stability and biocompatibility as compared to traditional emulsions. For nanoemulsions stabilization, a large amount of surfactant/emulsifier is required which hinders its therapeutic applications due to toxicological concerns for prolonged treatments [12], [13], [14]. Therefore, it is necessary to develop a stable nanoemulsion by using effective food grade emulsifiers. A working knowledge on nanoemulsions and the extensive use of synthetic antioxidants in the food processing industry motivated us to investigate on the antimicrobial studies of clove oil nanoemulsions.
In the present study, clove oil nanoemulsion by using whey protein concentrate (WPC) has been developed. The hydrophobic core (β-lactoglobulin) of whey proteins helps to bind many hydrophobic molecules of essential oil (clove oil). Being an efficient carrier for hydrophobic moieties, whey proteins can be a potential delivery vehicle for clove oil. Therefore, we investigated the synthesis of a stable clove oil nanoemulsion coated with WPC and their different physico-chemical and antimicrobial properties.
2. Materials and methods
2.1. Materials
The clove oil and whey protein concentrate were procured from Saachi Fragrance Ltd. (Uttar Pradesh, India) and Modern Dairy Pvt. Ltd. (Haryana, India), respectively. Microbial strains of Bacillus subtilis (NCDC – 70) and E. coli (NCDC – 249) were procured from National Collection of Dairy Cultures of our institute (NCDC, NDRI, India). To analyze the antimicrobial assays, the initial counts of microbial strains were kept about ∼106 cfu ml−1 (colony forming units per ml). All the chemicals and reagents used in the study were of analytical grade.
2.2. Preparation of clove oil nanoemulsion
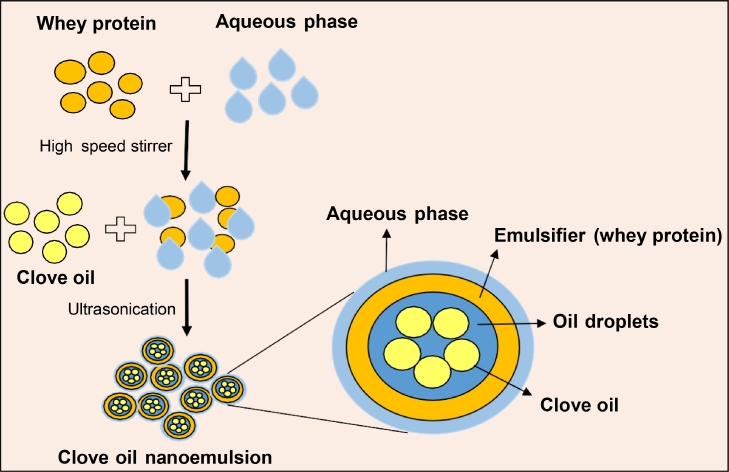
Oil-in-water nanoemulsions were developed in two stages as per the method described by Jafari et al [15], after slight modifications. As shown in Fig. 1, two stages involved: a) pre-emulsification step to produce coarse emulsions using high-speed magnetic stirrer, and b) ultrasonication was used to obtain final stable emulsion with nanosized particles. The inner oil phase (O) as core material was composed of clove oil (1 - 10 % w/v), while the outer aqueous phase (W) was prepared by mixing WPC-70 (0.1–1 percent w/v) as coating material with de-ionized water. Different ratio of core and coating materials were tried to get stable emulsions as shown in Table 1. The coarse emulsions were prepared by mixing above two phases using magnetic stirrer. Stable O/W clove oil nanoemulsions were formed by sonication (keeping on ice) for 10 min with an ultrasonicator (5 s off/on).
Fig. 1.
Schematic method for the preparation of clove oil nanoemulsions.
Table 1.
Formulations tried with different concentrations of clove oil and whey protein concentrate.
| Emulsion composition | Stability | Antimicrobial activity using 50 µl of emulsions | ||
| Clove oil (%) | WPC (%) | Before heating | After heating | |
| 10% oil | 0.1 | Unstable | Unstable | NA* |
| 0.4 | Unstable | Unstable | NA | |
| 0.5 | Stable | Stable | +** | |
| 0.6 | Unstable | Unstable | NA | |
| 1 | Unstable | Unstable | NA | |
| 4% oil | 0.1 | Unstable | Unstable | NA |
| 0.5 | Unstable | Unstable | NA | |
| 1 | Unstable | Unstable | NA | |
| 2% oil | 0.1 | Unstable | Unstable | NA |
| 0.2 | Stable | Unstable | NS*** | |
| 0.5 | Unstable | Unstable | NA | |
| 1 | Unstable | Unstable | NA | |
| 1% oil | 0.1 | Stable | Stable | NS |
| 0.5 | Stable | Stable | NS | |
| 1 | Stable | Unstable | NS | |
NA* Not analyzed.
+** showed antimicrobial activity, hence selected for further study.
NS*** Not showed the antimicrobial activity.
2.3. Stability of clove oil nanoemulsion
The emulsion stability towards phase separation of freshly prepared clove oil nanoemulsion was assessed by centrifugation at 1300 × g (Kubota, Tokyo, Japan) at 4 °C for 30 min and thermal treatment at 80 °C for 30 min as suggested by Sharma et al [16]. Among the different concentrations as shown in Table 1, highest clove oil containing formulation (0.5% WPC with 10% clove oil) was selected for different physico-chemical characteristics on the basis of stability on centrifugation (before and after heating).
2.4. Physico-chemical characterization of clove oil nanoemulsion
Physico-chemical parameters of the fresh and stored (30 days) clove oil nanoemulsions were examined using the dynamic light scattering method, including mean particle diameter, zeta-potential, and particle size distribution (PDI) (Malvern Instruments Ltd., Worcestershire, U.K). The net electrical charge of the nanoemulsion droplets was calculated using the same equipment at a holder temperature of 25 °C and an electric voltage of 3.9 V. To avoid multiple scattering during measurement, nanoemulsions were diluted with deionized water (1:50) before testing. All measurements were taken in triplicates.
2.5. Effect of processing conditions on physico-chemical characteristics
The clove oil (10%) nanoemulsion with WPC (0.5%) was observed with high stability against different processing conditions such as pH, thermal treatment and ionic strength, was chosen for further study. Briefly, the selected nanoemulsion was adjusted for different pH ranges (3.0 – 7.0) using 0.1 N NaOH or HCl. To analyze the thermal stability, the nanoemulsion was treated at different temperature conditions such as sterilization (121 °C/15 min), pasteurization (63 °C/30 min), forwarming (80 °C/10 min) and boiling (95 °C/10 min). To analyze the effect of ionic strength, the emulsion was subjected to different concentration of NaCl (0.1 – 1 M). The particle size, zeta potential and poly dispersity index were assessed after above treatments to observe the effect of processing conditions on stability.
2.6. Antimicrobial assay
The antimicrobial activity of nanoemulsion was tested against B. subtilis (NCDC - 70) and E. coli (NCDC - 249). The minimum inhibitory concentration (MIC) was assessed by using well assay method and minimum bactericidal concentration (MBC) was analyzed by time kill assay method.
2.6.1. Minimum inhibitory concentration
The MIC was determined using a modified agar well diffusion experiment [17]. To determine antibacterial activity, nutrient agar was placed into sterile petri plates and allowed to cool in an incubator at 37 °C for 24 h. The plates were then injected with 100 µL of bacterial cultures (∼ 106 cfu ml−1) in 7 ml of nutrient soft agar on the surface. Wells were punched out of the agar with a sterile well borer (9 mm diameter). Then, the different concentrations (10–200 µl) of nanoemulsion and free clove oil were added in the wells. To compare the antimicrobial activity of nanoemulsion, the free clove oil was considered as positive control while the sterile saline was taken as negative control. The well plate were incubated for 24 h at 37 °C. Zones of inhibition extending laterally across the wells were measured, and clear zones of 3 mm or larger (excluding well diameters) were termed as positive zones of inhibition.
2.6.2. Minimum bactericidal concentration
MBC was estimated by using time kill assay with some modifications [18] by employing nutrient agar. The lowest dose at which no observable growth on agar could be observed (99.9 percent killed) is termed as MBC. Screened and revived cultures in BHI broth having ∼106 cfu ml−1 (initial count) were considered to analyze the antimicrobial activity. Culture samples (1%) were added in 5 mL sterile BHI broth containing test tubes. Different concentrations (10–100 µl) of nanoemulsion and free clove oil were added in BHI broth tubes and incubated at 37 °C. To determine the inactivation kinetics, 1 ml of above samples were taken after 0, 2, 4, 8, 16 and 24 h of incubation and serially diluted with saline solution. Then serially diluted samples (1 ml) were placed on the petri plates and poured nutrient agar. Sterile saline treated as negative control. All plates were incubated for 24 h at 37 °C, and counts were represented as log cfu ml−1. The lowest concentration which causes complete inhibition of bacteria or no visible growth for more than 24 h was considered as MBC.
2.7. Statistical analysis
The MS-EXCEL-2010 software and GraphPad Prism 5.0 were used for statistical analysis.
The outcomes were presented as the mean ± standard error of means (SEM) of triplicates, with ANOVA used to test significance (P < 0.05).
3. Results
3.1. Selection of stable clove oil nanoemulsion
As shown in Table 1, we investigated different combinations of clove oil along with emulsifier (WPC). Based on the stability and preliminary antimicrobial studies, the most stable formulation was obtained by using 10% clove oil with 0.5% WPC and was selected in this study. Other combinations having more concentration of WPC (1%) that promoted free availability of protein in the emulsion system, which sediments after heating at 80 °C for 30 min and resulted in the instability of nanoemulsion. Therefore, on the basis of stability on centrifugation (after and before heating), we selected the nanoemulsion (0.5% WPC with 10% clove oil) for further physico-chemical characterization and antimicrobial studies.
3.2. Physico-chemical characterization of nanoemulsion
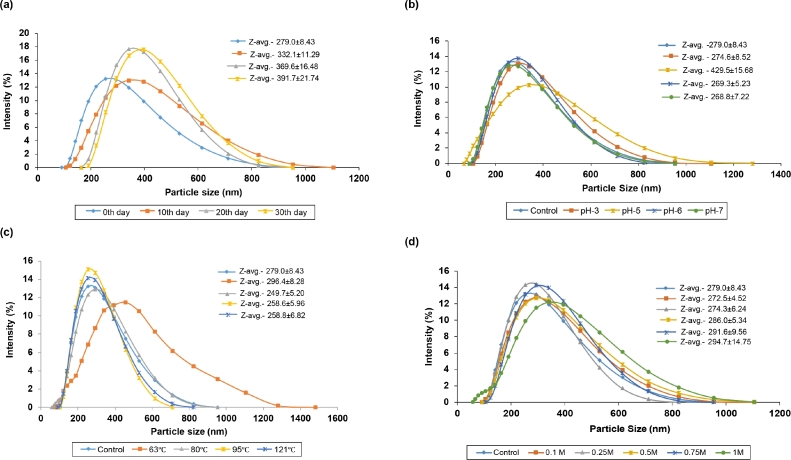
The mean particle size, zeta potential and polydispersity index of the stable nanoemulsion (10% clove oil + 0.5% WPC) were found to be 279.0 ± 8.43 nm, −34.5 ± 0.12 mV, and, 0.179 ± 0.02 respectively, as shown in Table 2. The results revealed that the mean particle size of developed nanoemulsion was increased during storage from 0th to 30th day as reported in Fig. 2a. The mean particle size of the nanoemulsion was gradually increased with the increase in storage time from 0 (279.0 ± 8.43 nm) to 30 days (391.7 ± 21.74 nm), but there was no visible phase separation. On the other hand, the nanoemulsion on storage was stable for 30 days without any creaming and aggregation.
Table 2.
Physico-chemical characteristics of clove oil (10%) + WPC (0.5%) nanoemulsion.
| Characteristics | Values |
| Size (nm) | 279.0 ± 8.43 |
| Zeta potential (mV) | -34.5 ± 0.12 |
| PDI | 0.179±0.02 |
| pH | 5.67±0.04 |
| Stability (d)* | 30±2.00 |
Values mentioned above is mean ± SEM (n = 3); *d= days.
Fig. 2.
Effect of the parameters (a) storage time; (b) pH; (c) heat treatments; and (d) ionic strength on the particle size distribution of stable clove oil (10%) nanoemulsion prepared using 0.5% whey protein concentrate.
3.3. Effect of different processing conditions on physico-chemical characteristics
The stability is a vital aspect of nanoemulsion. Fig. 2 represents the effect of different processing conditions such as pH (3.0 – 7.0), thermal treatments (sterilization, forwarming, boiling and pasteurization) and ionic strength (0.1 – 1.0 M NaCl) on particle size of the developed nanoemulsion. The mean particle size was decreased below and above the isoelectric point (pI) and the values were found to be at pH 3.0 (274.6 ± 8.52 nm) and 7.0 (268.8 ± 7.22 nm) (Fig. 2b).
Nanoemulsion was observed with deviations in the particle size after subjecting to the different thermal treatments as shown in Fig. 2c. With different thermal treatments, the particle size was varied from 249.7 ± 5.20 to 296.4 ± 8.28 nm. The particle size distribution appears to be similar in all heat-treated samples except for pasteurization, which observed with increased particle size distribution (296.4 ± 8.28 nm). This may be due to slight protein denaturation during the thermal treatments. The impact of salt ions on the particle size distribution of nanoemulsion was studied and the results are documented in Fig. 2d. There was no significant difference in the particle size was observed in between control sample and the sample treated up to 0.2 M salt concentration, but there was increased in the particle size was observed from 0.5 M to 1 M NaCl concentration (286.0 ± 5.34 to 294.7 ± 14.75 nm). The increase in the salt concentration (0.1 to 1 M NaCl) showed insignificant change in the particle size distribution but a slight linear increase was reported from 272.5 ± 4.52 to 294.7 ± 14.75 nm.
3.4. Antimicrobial assay
The antimicrobial activity of prepared nanoemulsion was assessed by using MIC and MBC by agar well and time kill assay methods, respectively. Antimicrobial activity was evaluated against all the stable formulations and results are shown in Table 1 and Table 3. No antimicrobial activity was observed in clove oil (1–4%) containing emulsions which could relate to its lower concentration. Hence, in the present investigation, O/W nanoemulsion containing 10% clove oil with 0.5% WPC was selected for better antimicrobial study against E. coli (NCDC-249) and B. subtilis (NCDC-70).
Table 3.
Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of clove oil nanoemulsion and unencapsulated clove oil against Escherichia coli and Bacillus subtilis.
| Antimicrobial formulations | E. coli | Bacillus subtilis |
|---|---|---|
| Minimum inhibitory concentrations (MICs) | ||
| Nanoemulsion | 50 µl | 50 µl |
| Unencapsulated oil (free oil) | 100 µl | 100 µl |
| Minimum bactericidal concentrations (MBCs) | ||
| Nanoemulsion | 90 µl/8h | 90 µl/8h |
| Unencapsulated oil (free oil) | 90 µl/16h | 90 µl/16h |
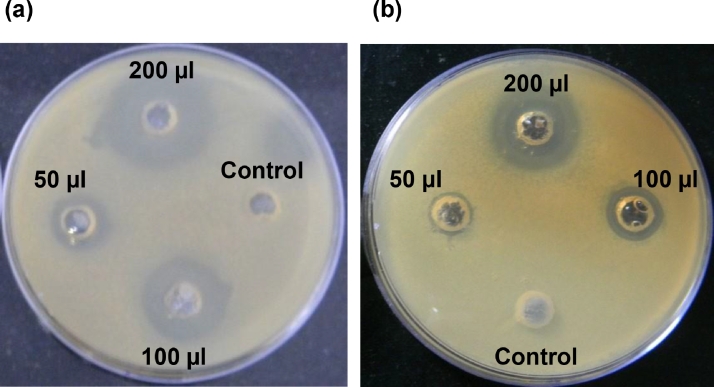
As shown in Table 3 and Fig. 3, the most stable clove oil nanoemulsion showed the MIC as 50 µl concentration which was quite half of the MIC of free clove oil (100 µl) against E. coli and B. subtilis. The diameter of zone of inhibition for B. subtilis and E. coli was found to be 21.15 ± 0.3 and 19.5 ± 0.3 mm, respectively using 100 µl concentration of nanoemulsion, whereas the zone of inhibition for free clove oil was observed to be 20.3 ± 0.25 and 18.3 ± 0.2 mm with 200 µl concentration as shown in Fig. 3. The significant difference was observed in MIC values of encapsulated and free clove oil as shown in Fig. 3, which illustrated larger diameter of zone of inhibition than the free oil against both microorganisms with respect to their concentrations.
Fig. 3.
Minimum inhibitory concentration as zone of inhibitions of (a) clove oil (10%) encapsulated in nanoemulsion prepared using 0.5% whey protein concentrate; and, (b) unencapsulated free clove oil (10%) in dimethyl sulphoxide (DMSO), against Escherichia coli by Agar-Well assay method using different concentrations.
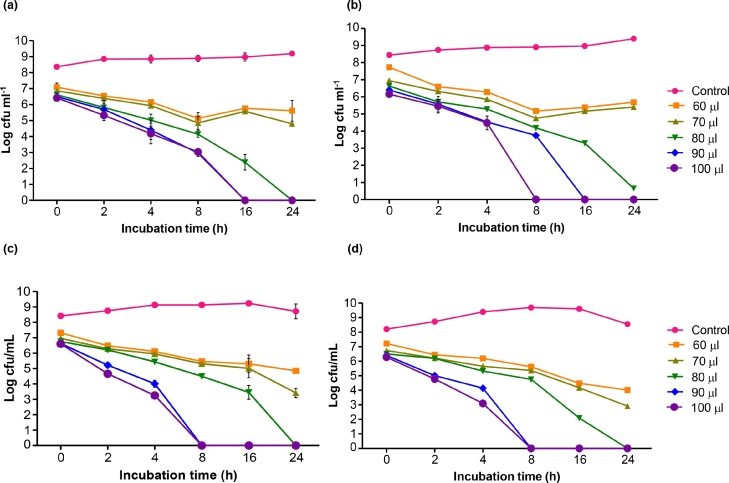
The MBC of clove oil nanoemulsion was analyzed through time kill assay against E. coli and B. subtilis and represented in Table 3 and Fig. 4. Time taken by clove oil for complete killing of bacteria when treated with it, was assessed by using time kill assay method. Different concentrations (10–100 µl) of free clove oil (in DMSO) were used and complete killing (8.97 log cfu ml−1 reduction) of E. coli and B. subtilis population was observed after 16 h of incubation using 90 µl concentration (Fig. 4a, b).
Fig. 4.
Minimum bactericidal concentration of free clove oil (10%) dissolved in dimethyl sulphoxide (DMSO) solvent (a & b); nanoemulsion prepared using clove oil (10%) and whey protein concentrate (0.5%) (c & d) against E. coli and Bacillus subtilis, respectively, by time kill assay method at different concentrations (60 – 100 µl) of free clove oil and nanoemulsion.
However, the treatment using different concentrations (10–100 µl) of nanoemulsion were found highly effective against the inhibition of E. coli and B. subtilis population. In case of nanoemulsion, the MBC of about 90 µl (concentration used) was found more effective as it inhibit the microbial population within 8 h of incubation with complete killing of tested microbes (8.97 log cfu ml−1 reduction) (Fig. 4b, c).
4. Discussion
More concentration of WPC (1%) promotes free availability of protein in the system which sediments during stability assessment of nanoemulsions and resulted unstable emulsions. Similarly, Lizarraga et al. concluded that the excess concentration of WPC affects the stability of an emulsion system by raising its viscosity [19]. Stability of lycopene microemulsion found to be decreased with increase in oil phase fractions, may be due to limited whey protein content results Ostwald ripening [5].
Moreover, the particle size was found decreased with increase in the concentration of emulsifier. At low concentration of emulsifier, there was an insufficient amount of emulsifier (WPC) to cover surface area of oil and water resulted in coalescence leads to larger droplets. At higher concentration, there is an appropriate amount of emulsifier present to encapsulate all the nanodroplets in emulsion system [20]. Our results are also corroborated that higher WPC concentration responsible for larger interfacial area, which significantly reduce the droplet diameter and improve emulsion stability [21].
The results of particle size distribution was corroborated with a study of He and his co-workers who reported that the proteins as a coating material are responsible to stabilized the nanoemulsions in narrow peak with a relatively lower particle size (about 200 - 250 nm) [22]. The zeta potential of the developed clove oil nanoemulsion was comparatively high because of the presence of highly negatively charged whey proteins which further supported by high electrostatic and steric repulsive forces [23].
Storage study have great influence on the mean particle size of the nanoemulsion. With progression in storage time, the mean particle size was increased from 0th to 30th day. Our another study also supported these findings, that there was increase in the particle size (172 nm to 415 nm) of nanoemulsion prepared by using sodium caseinate and pectin observed with the progression in storage time (0 to 20 days) [16]. The reason might be due to disbursement of smaller nano-particles into larger droplets which further leads to aggregation and gradually shift the monomodal to multimodal size distribution during storage [24].
Various pH ranges have a great influence on the particle size distribution within emulsion system. The emulsion system can be bimodal or multimodal, on the basis of pH of the emulsion system. The emulsion in the present study was in monomodal distribution except pH 4.0. Similarly, Onsaard and co-workers reported that the particle size of sesame oil nanoemulsion developed by using WPC was relatively low at various pH, except pH 4.0 – 5.0 [25]. It has been demonstrated that there is an electrostatic forces repulsion between protein-coated particles above and below the isoelectric point (pI), which prevent their aggregation [26]. However, at pI, electrostatic interaction is insufficient to overcome weak van der Waal and hydrophobic attraction, resulting in particle agglomeration [27]. In our previous study, curcumin nanoemulsion was prepared with WPC by using ultrasonication method demonstrated higher mean particle size with lower pH value was may be due to the aggregation of particles [28]. Whey proteins-stabilized emulsions with electrostatic repulsive forces are mainly sensitive to pH of the system [29]. The emulsions are stable to flocculation at above and below pI due to strong electrostatic repulsive forces within the system. At or near pI, the electrostatic repulsive forces are reduced, and attractive weak van der Wall forces leads to flocculation [30].
The particle size distribution appears to be similar in all heat-treated samples (forewarming, boiling and sterilization) except pasteurization (slightly increased). Similarly, Keowmaneechai and Mcclements reported that there was a little increased in the mean particle size with increase in temperature from 50 – 90 °C, which could be due to droplet aggregation [31]. Aggregation could be owing to increased intermolecular interactions between protein particles adsorbed on distinct droplets [32]. The association of denatured protein molecules is facilitated by thermal processing, hydrophilic attractions, and thioldisulphide interchange processes. Sometimes, heating causes a little reduction in particle size, which is likely related to the distribution of inter-particle hydrogen bonding [33]. Whey proteins are partially denatured to create soluble and aggregated forms with higher surface hydrophobicity, which favors adsorption at the oil-water interface and improves emulsifying properties [34]. The presence of denatured whey proteins in the emulsions increases viscosity and stability.
In our previous study, curcumin nanoemulsion was prepared using sodium caseinate that showed an increased in particle size (340 to 351 nm) with increase in temperature (63 – 95 °C) [35]. It seems possible that these results are due to hydrophobic residues, which are present in the protein interior, are responsible for the reduction in surface tension at oil/water interface. Thermal denaturation, leads to the opening of nonpolar disulfide links, which improves protein adsorption at the emulsion interface.
The impact of change in ionic strength, the particle size distribution did not alter significantly, although there was a minor linear rise in particle size when the salt content was increased. The average size of the nanoparticles was initially reduced slightly from 0 to 0.2 M NaCl concentration. Furthermore, the average size of the nanoparticles was also increased when the salt content was increased from 0.5 to 1 M.
Ye et al. demonstrated that average nanodroplet size was decreased from 0.73 – 0.60 µm when ionic strength increased (0 – 20 mM NaCl). They hypothesized that at relatively low concentration of salt ions, the electrostatic repulsion forces are still strong enough to withstand weak van der Walls and hydrophobic interactions in the system [36]. However, salt concentration exceeds or reached at a critical level the droplets fails to facilitate electrostatic repulsion strong enough and consequently the attractive forces dominate that leads to droplet aggregation [27]. Our findings suggest that the presence of salt in emulsion decreased electrostatic repulsion between the molecules which results in the reduction of surface charge and leads to aggregation of the particles. The destabilization of nanoemulsion on addition of salt can be explained by reduction in electrostatic repulsive forces between emulsion droplets [26].
In our study, no antimicrobial activity was observed by nanoemulsions containing lower concentration (1–4%) of clove oil which further supported by the study of Suriyarak and Weiss [37]. These researchers found that by increasing the eugenol level (up to 1%), there was no reduction in bacterial counts. They reported that the emulsion prepared using 50% eugenol reduced the bacterial load of S. carnosus by 3 logs/1 hour, however, 3 logs reduction was observed in 3 h using 20% eugenol emulsion [37].
In our study, there was significant reductions observed in the bacterial counts in case of nanoemulsion as compared to free clove oil. The MIC values of nanoemulsion was quite half (50 µl) as compared to free clove oil (100 µl) against both of the tested bacteria. The possible reason might be attributed to the enhancement of solubility of clove oil within the nanoemulsion results uniformly distribution of oil and release sufficiently to inhibit the bacterial growth due to the nanosized particles of nanoemulsion [38]. However, Wu et al. observed no difference between thyme oil emulsion and free thyme oil in the inhibition of L. monocytogenes, while the emulsion was significantly efficient than the free oil against E. coli O157:H7 and S. enteritidis [39]. Confounding results for the preparation of nanoemulsions against their antimicrobial activities was due to the oxidation of lipids in the droplets generated at high temperature during ultrasonification [40], [41].
Minimum bactericidal concentrations (MBC) were slightly higher than the MICs of the nanoemulsion and free clove oil. In similar fashion as in MICs results, the nanoemulsion showed better killing effects or reduction in bacterial counts as compared to the free clove oil against E. coli and B. subtilis. This might be due to the increased hydrophilicity of clove oil in aqueous system, as well as its smaller size as nano-fabricated particle within the nanoemulsion, which promote its functionality as potential antimicrobial agent. However, Shah et al. compared the antimicrobial potential of nano-dispersed and free eugenol against E. coli O157:H7 (ATCC43889) and Lm strains, and observe the MBC of both about 1.75 gl−1 without any significant difference [38].
The clove oil nanoemulsion against E. coli found to be more resistance than B. subtilis. A possible explanation for this might be due to the different composition of their cell walls with respect to their Gram-positive and Gram-negative nature. Due to this property, E. coli owns a unique cell wall with a periplasmic space which makes it more resistance to the antimicrobial agents [42]. The Gram-negative bacterial cell membrane enriched in lipopolysaccharides acts as a direct barrier to numerous antimicrobial compounds which leads to resistance towards antibiotic molecules [43].
5. Conclusions
The development of antimicrobial nanoemulsion with suitable ingredients provides several ways to modify the microbial cells of various food spoilage and pathogenic microbes, which are directly related to food spoilage and infectious diseases. Antimicrobial nanoemulsions have unique physicochemical and functional properties, such as increased bioavailability, regulated drug release, solubilization of non-polar molecules, and the capacity to protect the active potential of bioactive molecules [44], [45]. In the present study, the nanoemulsion was developed successfully using clove oil and whey protein by using ultra-sonification. The droplet sizes of the most stable nanoemulsion was evaluated through DLS technique with mean particle size, zeta potential and polydispersity index of 279.0 ± 8.43 nm, −34.5 ± 0.12 mV and 0.179 ± 0.012, respectively. For the screening of antimicrobial properties, food spoilage and pathogenic bacteria were treated with developed nanoemulsions. MBC (90 µL) of nanoemulsion was found effective to 8.69 log reductions when compared to control. Our results supported the potential role of nanoemulsion as antimicrobial agent for food spoilage and pathogenic microbes. Conclusively, the nanoemulsions could be promising approach against various food spoilage and pathogenic microorganism in food preservation. Beside this, they provide a inimitable mode to advance the antimicrobial efficacy of non-polar drugs by intensifying their effects at targeted locations.
Author contributions
MS: Formal analysis, investigation, data interpretation, writing—original draft preparation. BM: supervision, reviewing, editing and visualization. RP: data validation, reviewing, editing. RS and RK: supervision, reviewing, editing and visualization.
Declaration of Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgments
We thank to Director, ICAR-National Dairy Research Institute, Karnal, Haryana (India) for providing necessary facilities.
Contributor Information
Minaxi Sharma, Email: minaxi86sharma@gmail.com.
Bimlesh Mann, Email: bimleshmann@gmail.com.
References
- 1.Arfa A.B., Chrakabandhu Y., Preziosi-Belloy L., Chalier P., Gontard N. Coating papers with soy protein isolates as inclusion matrix of carvacrol. Food Res. Int. 2007;40:22–32. [Google Scholar]
- 2.Gill A., Holley R.A. Disruption of Escherichia coli, Listeria monocytogenes and Lactobacillus sakei cellular membranes by plant oil aromatics. Int. J. Food Microbiol. 2006;108:1–9. doi: 10.1016/j.ijfoodmicro.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 3.Gill A., Holley R.A. Inhibition of membrane bound ATPases of Escherichia coli and Listeria monocytogenes by plant oil aromatics. Int. J. Food Microbiol. 2006;111:170–174. doi: 10.1016/j.ijfoodmicro.2006.04.046. [DOI] [PubMed] [Google Scholar]
- 4.Di Pasqua R., Betts G., Hoskins N., Edwards M., Ercolini D., Mauriello G. Membrane toxicity of antimicrobial compounds from essential oils. J. Agric. Food Chem. 2007;55:4863–4870. doi: 10.1021/jf0636465. [DOI] [PubMed] [Google Scholar]
- 5.Mcclements D., Decker E., Weiss J. Emulsion-based delivery systems for lipophilic bioactive components. J. Food Sci. 2007;72:R109–R124. doi: 10.1111/j.1750-3841.2007.00507.x. [DOI] [PubMed] [Google Scholar]
- 6.De Vos P., Faas M.M., Spasojevic M., Sikkema J.J. Encapsulation for preservation of functionality and targeted delivery of bioactive food components. Int. Dairy J. 2010;20:292–302. [Google Scholar]
- 7.Donsì F., Annunziata M., Vincensi M., Ferrari G. Design of nanoemulsion-based delivery systems of natural antimicrobials: effect of the emulsifier. J. Biotechnol. 2012;159:342–350. doi: 10.1016/j.jbiotec.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Gaysinsky S., Davidson P.M., Bruce B.D., Weiss J. Growth inhibition of Escherichia coli O157: H7 and Listeria monocytogenes by carvacrol and eugenol encapsulated in surfactant micelles. J. Food Prot. 2005;68:2559–2566. doi: 10.4315/0362-028x-68.12.2559. [DOI] [PubMed] [Google Scholar]
- 9.Lim S.S., Baik M.Y., Decker E.A., Henson L., Popplewell L.M., Mcclements D.J., Choi S. Stabilization of orange oil-in-water emulsions: a new role for ester gum as an Ostwald ripening inhibitor. Food Chem. 2011;128:1023–1028. [Google Scholar]
- 10.Chang Y., Mclandsborough L., Mcclements D.J. Physical properties and antimicrobial efficacy of thyme oil nanoemulsions: influence of ripening inhibitors. J. Agric. Food Chem. 2012;60:12056–12063. doi: 10.1021/jf304045a. [DOI] [PubMed] [Google Scholar]
- 11.Terjung N., Löffler M., Gibis M., Hinrichs J., Weiss J.J. Influence of droplet size on the efficacy of oil-in-water emulsions loaded with phenolic antimicrobials. Food Funct. 2012;3:290–301. doi: 10.1039/c2fo10198j. [DOI] [PubMed] [Google Scholar]
- 12.Yan A., Von Dem Bussche A., Kane A.B., Hurt R. Tocopheryl polyethylene glycol succinate as a safe, antioxidant surfactant for processing carbon nanotubes and fullerenes. Carbon N Y. 2007;45:2463–2470. doi: 10.1016/j.carbon.2007.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiao J. Polyoxyethylated nonionic surfactants and their applications in topical ocular drug delivery. Adv. Drug Deliv. Rev. 2008;60:1663–1673. doi: 10.1016/j.addr.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Sivakumar S., Bansal V., Cortez C., Chong S.F., Zelikin A.N., Caruso F. Degradable, surfactant-free, monodisperse polymer-encapsulated emulsions as anticancer drug carriers. Adv. Mater. 2009;21:1820–1824. [Google Scholar]
- 15.Jafari S.M., He Y., Bhandari B. Production of sub-micron emulsions by ultrasound and microfluidization techniques. J. Food Eng. 2007;82:478–488. [Google Scholar]
- 16.Sharma M., Mann B., Sharma R., Bajaj R., Athira S., Sarkar P., Pothuraju R. Sodium caseinate stabilized clove oil nanoemulsion: physicochemical properties. J. Food Eng. 2017;212:38–46. [Google Scholar]
- 17.Bertrand-Harb C., Ivanova I., Dalgalarrondo M., Haertllé T. Evolution of β-lactoglobulin and α-lactalbumin content during yoghurt fermentation. Int. Dairy J. 2003;13:39–45. [Google Scholar]
- 18.Teixeira P.C., Leite G.M., Domingues R.J., Silva J., Gibbs P.A., Ferreira J.P. Antimicrobial effects of a microemulsion and a nanoemulsion on enteric and other pathogens and biofilms. Int. J. Food Microbiol. 2007;118:15–19. doi: 10.1016/j.ijfoodmicro.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 19.Lizarraga M., Pan L., Anon M., Santiago L. Stability of concentrated emulsions measured by optical and rheological methods. Effect of processing conditions—I. Whey protein concentrate. Food Hydrocoll. 2008;22:868–878. [Google Scholar]
- 20.Walstra P. Principles of emulsion formation. Chem. Eng. Sci. 1993;48:333–349. [Google Scholar]
- 21.Xue J., Zhong Q. Thyme oil nanoemulsions coemulsified by sodium caseinate and lecithin. J. Agric. Food Chem. 2014;62:9900–9907. doi: 10.1021/jf5034366. [DOI] [PubMed] [Google Scholar]
- 22.He W., Tan Y., Tian Z., Chen L., Hu F., Wu W. Food protein-stabilized nanoemulsions as potential delivery systems for poorly water-soluble drugs: preparation, in vitro characterization, and pharmacokinetics in rats. Int. J. Nanomedicine. 2011;6:521. doi: 10.2147/IJN.S17282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klang V., Valenta C. Lecithin-based nanoemulsions. J. Drug Deliv. Sci. Technol. 2011;21:55–76. [Google Scholar]
- 24.Zeeb B., Gibis M., Fischer L., Weiss J. Influence of interfacial properties on Ostwald ripening in crosslinked multilayered oil-in-water emulsions. J. Colloid Interface Sci. 2012;387:65–73. doi: 10.1016/j.jcis.2012.07.054. [DOI] [PubMed] [Google Scholar]
- 25.Onsaard E., Putthanimon J., Singthong J., Thammarutwasik P.T. Influence of maltodextrin and environmental stresses on stability of whey protein concentrate/κ-carrageenan stabilized sesame oil-in-water emulsions. Food Sci. Tech. Int. 2014;20:617–628. doi: 10.1177/1082013213498247. [DOI] [PubMed] [Google Scholar]
- 26.Mcclements D.J. CRC press; 2015. Food emulsions: principles, practices, and Techniques. [Google Scholar]
- 27.Qian C., Decker E.A., Xiao H., Mcclements D.J. Physical and chemical stability of β-carotene-enriched nanoemulsions: influence of pH, ionic strength, temperature, and emulsifier type. Food Chem. 2012;132:1221–1229. doi: 10.1016/j.foodchem.2011.11.091. [DOI] [PubMed] [Google Scholar]
- 28.Sari T., Mann B., Kumar R., Singh R., Sharma R., Bhardwaj M., Athira S. Preparation and characterization of nanoemulsion encapsulating curcumin. Food Hydrocoll. 2015;43:540–546. [Google Scholar]
- 29.Demetriades K., Coupland J.N., Mcclements D.J. Physical properties of whey protein stabilized emulsions as related to pH and NaCl. J. Food Sci. 1997;62:342–347. [Google Scholar]
- 30.Demetriades K., Mcclements D.J. Influence of pH and heating on physicochemical properties of whey protein-stabilized emulsions containing a nonionic surfactant. J. Food Sci. 1998;46:3936–3942. [Google Scholar]
- 31.Keowmaneechai E., Mcclements D.J. Influence of EDTA and citrate on thermal stability of whey protein stabilized oil-in-water emulsions containing calcium chloride. Food Res. Int. 2006;39:230–239. doi: 10.1021/jf020489a. [DOI] [PubMed] [Google Scholar]
- 32.Surh J., Decker E.A., Mcclements D.J. Influence of pH and pectin type on properties and stability of sodium-caseinate stabilized oil-in-water emulsions. Food Hydrocoll. 2006;20:607–618. [Google Scholar]
- 33.Shah B., Ikeda S., Davidson P.M., Zhong Q... Nanodispersing thymol in whey protein isolate-maltodextrin conjugate capsules produced using the emulsion–evaporation technique. J. Food Eng. 2012;113:79–86. [Google Scholar]
- 34.Mutilangi W., Panyam D., Kilara A. Functional properties of hydrolysates from proteolysis of heat-denatured whey protein isolate. J. Food Sci. 1996;61:270–275. [Google Scholar]
- 35.Kumar D.D., Mann B., Pothuraju R., Sharma R., Bajaj R. Formulation and characterization of nanoencapsulated curcumin using sodium caseinate and its incorporation in ice cream. Food Funct. 2016;7:417–424. doi: 10.1039/c5fo00924c. [DOI] [PubMed] [Google Scholar]
- 36.Ye A., Srinivasan M., Singh H. Influence of NaCl addition on the properties of emulsions formed with commercial calcium caseinate. Food Chem. 2000;69:237–244. [Google Scholar]
- 37.Suriyarak S., Weiss J.J.C. Cutoff Ostwald ripening stability of alkane-in-water emulsion loaded with eugenol. Colloids and Surfaces A: Physicochem. Eng. Aspects. 2014;446:71–79. [Google Scholar]
- 38.Shah B., Davidson P.M., Zhong Q. Nanodispersed eugenol has improved antimicrobial activity against Escherichia coli O157: H7 and Listeria monocytogenes in bovine milk. Int. J. Food Microbiol. 2013;161:53–59. doi: 10.1016/j.ijfoodmicro.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 39.Wu J.-.E., Lin J., Zhong Q. Physical and antimicrobial characteristics of thyme oil emulsified with soluble soybean polysaccharide. Food Hydrocoll. 2014;39:144–150. [Google Scholar]
- 40.Wu Z., Ondruschka B. Roles of hydrophobicity and volatility of organic substrates on sonolytic kinetics in aqueous solutions. J. Phys. Chem. A. 2005;109:6521–6526. doi: 10.1021/jp051768e. [DOI] [PubMed] [Google Scholar]
- 41.Salvia-Trujillo L., Rojas-Graü A., Soliva-Fortuny R., Martín-Belloso O. Physicochemical characterization of lemongrass essential oil–alginate nanoemulsions: effect of ultrasound processing parameters. Food Control. 2013;6:2439–2446. [Google Scholar]
- 42.Duffy C.F., Power R.F. Antioxidant and antimicrobial properties of some Chinese plant extracts. Int. J. of Antimicrob. Agents. 2001;6:527–529. doi: 10.1016/s0924-8579(01)00326-0. [DOI] [PubMed] [Google Scholar]
- 43.Gao Y., Van Belkum M.J., Stiles M.E. The outer membrane of Gram-negative bacteria inhibits antibacterial activity of brochocin-C. Appl. Environ. Microbiol. 1999;65:4329–4333. doi: 10.1128/aem.65.10.4329-4333.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jaiswal M., Dudhe R., Sharma P.K. Nanoemulsion: an advanced mode of drug delivery system. 3 Biotech. 2015;5:123–127. doi: 10.1007/s13205-014-0214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li M., Cui J., Ngadi M.O., Ma Y. Absorption mechanism of whey-protein-delivered curcumin using Caco-2 cell monolayers. Food Chem. 2015;180:48–54. doi: 10.1016/j.foodchem.2015.01.132. [DOI] [PubMed] [Google Scholar]