Highlights
-
•
In recent decades, extensive studies have been performed on breast cancer prevalence, causes, risk factors, prevention, diagnosis, and treatment.
-
•
On the other hand, traditional treatment methods suffer from limitations that prevent optimized clinical outcomes.
-
•
Different natural ingredients from various sources have shown the anticancer effects via physiological pathways.
-
•
A promising solution could be identified, including the usage of new adjuvants along with existing drugs.
-
•
Nanotechnology due to the presentation of remarkable properties has overcome this limitation through enhanced the solubility and bioavailability of AP.
Keywords: Apigenin, Breast cancer, Bioavailability, Nanostructure systems
Abstract
This review highlights using nanotechnology in increasing the bioavailability of AP (Apigenin) to enhance its therapeutic efficacy in breast cancer treatment. Breast cancer is one of the most leading causes of cancer death in women both in developed and developing countries. According to several epidemiological and clinical trial studies that indicate progestin-stimulated breast cancer in post-menopausal women; it is necessary to determine compounds to suppress or attenuate the tumor-promoting effects of progestins in breast cells. For this purpose, using the natural anti-progestins, including AP compared with the chemical ones could be significantly effective due to the lack of toxicities and contradiction effects. However, AP is categorized as a Class II drug of Biopharmaceutical Classification System with low solubility in water which limited its therapeutic effects. Therefore, nanotechnology due to the presentation of remarkable properties has overcome this limitation through enhanced the solubility and bioavailability of AP. In this regard, various nanocarriers such as nanocrystals, micelles, liposomes, PLGA, etc., have highlighted the significantly increased bioavailability and therapeutic efficacy of AP. Therefore, we will focus on the anticancer effects of AP in breast cancers, including involved mechanisms, the chemistry of AP and its bioavailability, finally different nanostructure systems to enhance the bioavailability of AP.
Graphical abstract
Abbreviations
- AP
Apigenin
- BAX
Bcl-2-associated X protein
- Bcl-2
B-cell lymphoma 2
- BCRP
Breast cancer resistance protein
- CAFs
Cancer-associated fibroblasts
- COX2
Cyclooxygenase-2
- CSCs
Cancer stem cells
- CXCL12
Chemokine (C-X-C motif) ligand 12
- CXCR4
C-X-C chemokine receptor 4
- ER
Estrogen receptor
- ERK 1
Extracellular signal-regulated kinase 1
- FASN
Fatty acid synthase
- GMCSF
Macrophage colony-stimulating factor
- HIF-1
Hypoxia-inducible factor-1
- HRE
Hypoxia response element
- LSP
Leukocyte sub-populations
- MAMs
Metastasis-associated macrophages
- MDSCs
Myeloid-derived suppressor cells
- MPA
Medroxy progesterone acetate
- PARP
poly (ADP-ribose) polymerase
- PD-L1
Programmed death ligand 1
- PR
Progesterone receptor
- PUMA
p53 upregulated modulator of apoptosis
- ROS
Reactive oxygen species
- STAT3
Signal transducer and activator of transcription 3
- TAMs
Tumor-associated macrophages
- TANs
Tumor-associated neutrophils
- Th17s
T helper IL-17-producing cells
- TNFR
Tumor necrosis factor receptors
- TRAIL
TNF-related apoptosis-inducing ligand
- Tregs
T-regulatory cells
- VEGF
Vascular endothelial growth factor
1. Introduction
Breast cancer (BC) is one of the most common cancers diagnosed among women and ranked as the second cause of cancer-related death among women, after lung cancer [1]. WHO reported in 2021, that cancer is a leading cause of death worldwide, accounting for over 10 million deaths in 2020. The most common cancer in 2020 was breast cancer with over 2.26 million cases [2]. In recent decades, extensive studies have been performed on breast cancer prevalence, causes, risk factors, prevention, diagnosis, and treatment [3, 4]. However, there has been a significant increase in breast cancer prevalence and its risk factors [3]. Complicated pathogenesis and several subtypes including basal-like and HER2-positive, luminal A, and luminal B make breast cancer challenging cancer to track and treat [5]. Breast cancer risk factors, including exogenous factors like early menarche, hormone replacement therapy, obesity, diabetes, and endogenous genetic factors like breast cancer susceptibility gene 1 (BRCA1) and BRCA2 mutations are growing worldwide [5]. On the other hand, traditional treatment methods suffer from limitations that prevent optimized clinical outcomes. For example, surgical procedures, multidrug resistance (MDR), and side effects of chemotherapy and radiotherapy reduce the survival rate and efficient treatment of breast cancer [4, 5]. Major problems still seen in the clinical experiences of many patients with breast cancer include poor response to treatment and the recurrence of tumor [6]. drug-resistance among BC patients can be intrinsic or acquired which lead to the drug failure [7]. This phenomenon is caused by the survival of heterogeneous tumor cells having drug-resistance characteristics [8]. Actually, tumor recurrence is induced by these heterogeneous cells popular as the residual disease.
To overcome these drawbacks, various research fields have been focused on novel diagnosis, prevention, and treatment methods [9], [10], [11]. As a result, a range of natural and synthetic materials have been proposed and developed for therapeutic applications. Among them, natural materials especially plant-derived compounds have been used for the synthesis of more than 50% of anticancer drugs [3].
Different natural ingredients from various sources have shown the anticancer effects via physiological pathways. In recent years naturally derived compounds have been increasingly used in cancer prevention and treatment [2, 12]. It has been reported that dietary natural products derived from fruits, vegetables, soy, spices, and cereals are useful in breast cancer prevention and treatment [5]. Their cellular mechanisms include apoptosis induction, metastasis and angiogenesis inhibition, and downregulation of the expression and activity of ER-α. Among plant-derived materials, flavonoids with a various diversity of over 5000 ubiquitous compounds have been examined in cancer research showing potential impacts on prevention and treatment of different cancer models especially in breast cancer [13], [14], [15]. Flavonoids present properties like antioxidant, anti-inflammatory, phyto-estrogens, CYP1A1 inhibitor, ABC (ATP-binding cassette) transporter regulator, and apoptotic effects substances that make them promising in breast cancer studies [13].
Different human studies have confirmed the anticancer effects of flavonoids like luteolin, myricetin, kaempferol, quercetin, and AP (AP) against lung [16], colorectal [17], and ovarian [18] cancers. A study in Italy on 2569 women with breast cancer showed an inverse association between 6 classes of flavones and breast cancer risk [19]. AP, 4′, C15H10O5; 5,7-trihydroxyflavone, is one of the flavone subclasses with a molecular weight of 270.24 MW [20]. AP presents in plant-derived beverages, fruits, and vegetables like parsley and tea [20, 21]. AP has potent antioxidant and anti-inflammatory activities with low intrinsic toxicity, making it a cancer chemopreventive agent [20]. Various studies have applied AP against breast cancer. Li1 et al. [22] reported the AP potency in inhibiting the proliferation, migration, and stemness features of triple-negative breast cancer (TNBC), in vitro and in vivo. It has been found that AP attenuates YAP/TAZ activity, CTGF and CYR61 expression, and disrupts the YAP/TAZ-TEADs protein-protein interaction. Another study used AP for suppressing TNFα related metastasis in human breast cancer cells [23]. AP suppressed TNFα releasing of CCL2 chemotactic protein through blocking mRNA and protein synthesis of IKBKe and phosphorylated extracellular signal-regulated kinase 1 (ERK 1/ 2) suppression.
Furthermore, recent studies have expanded the AP applications in breast cancer-related research and revealed more involving parameters which are crucial in the promising treatments. Drug resistance is an important hindering factor in existing breast cancer treatments. This mechanism hinders the successful treatment of patients. A promising solution could be identified, including the usage of new adjuvants along with existing drugs. The advantages of dietary flavonoids like AP could be beneficial in this field and improve the treatment efficacy of drug-resistant breast cancers. In addition, most conventional studies on analyzing the effects of different drugs and natural ingredients have been conducted on traditional two-dimensional (2D) cell cultures. The 2D models show limitations in fully mimicking native human three-dimensional (3D) tissues. Similarly, these models are incapable of imitating the complex structure of tumors, resulting in limited in vivo treatment efficacy and translation into clinical applications. Several groups have been considering these issues and proposing more innovative treatment mechanisms. Sudhakaran et al. developed triple-negative breast cancer (TNBC) spheroids with high cellular uptake and showed that AP induced apoptosis and inhibited the growth of TNBC patient-derived organoids as a chemotherapeutic adjuvant [24]. They regarded AP as chemo-sensitizers and showed that AP sensitized spheroids allowed doxorubicin to trigger the caspase-9-mediated intrinsic apoptotic pathway and induce DNA damage. They also showed that AP regulates the expression of ABCC4 and ABCG2 drug efflux transporters and involves apoptosis by targeting hnRNPA2. Another similar study showed that AP and hesperidin intensified the cytotoxic effect of doxorubicin on MCF-7 breast cancer cells [25]. Another potential mechanism is resistance to endocrine therapies by hyperactivation of Akt in the estrogen receptor (ER) expressed breast cancer cells. Pham et al. showed that AP exerts an antiproliferative effect on the active form of the Akt protein in MCF-7 cells by inhibition of Akt/FOXM1 signaling pathway and inducing G2/M phase cell cycle arrest and apoptosis [26]. Shendge et al. isolated AP from Clerodendrum- viscosum leaves and evaluated its anticancer activity in MCF-7 cells. They reported that AP shows selective cytotoxicity, intracellular ROS, and nuclear fragmentation, and dose-dependent apoptosis. They also examined the role of p53 in AP-induced apoptosis in cells. They reported AP induced p53 expression, and as a result activation of the caspase-cascade pathway, and cleavage of PARP [27].
This research aimed to critically review, the different molecular mechanisms of AP against breast cancer, including induction of apoptosis and cell cycle arrest, inhibition of fatty acid synthase, tumor angiogenesis, anti-invasive and metastasis properties, inhibition of drug resistance and YAP/TAZ activity, and finally, improving the immune response. The chemistry of AP and enhancing the bioavailability of AP via nanoparticles was discussed in depth.
2. Molecular mechanism of AP in breast cancer
So far, extensive investigations either in vitro or in vivo have been demonstrated the AP mechanisms in breast cancers, including induction of apoptosis and cell cycle arrest, inhibition of fatty acid synthase (FASN), aromatase inhibition, inhibition of tumor angiogenesis, anti-invasive and metastasis, inhibition of drug-resistance, inhibition of YAP/TAZ activity, improved the immune response. AP function as a chemo-preventive or chemotherapeutic agent in breast cancer has been highlighted in the majority of reported documents. Interestingly, the AP efficiency in most types of breast cancer, such as HER2 positive, ERα positive, ERβ positive, triple-negative breast cancer, and drug-resistant species has been reported. It was shown that exposure to high doses of phytoestrogens like AP had the same efficiency in both ER+ and ER- breast cancer cells [28]. High doses of AP (50 µМ) lead to “switching-off” the hormonal receptor of breast cancer cells.
A summary of AP molecular targets is shown in Table 1 Induction of apoptosis and cell cycle arrest have been reported as the most important mechanism of AP in the suppression of breast cancer. Apoptosis is a programmed cell death causing the elimination of cells without releasing harmful substances into the surrounding area. Two core pathways are considered for apoptosis, including the intrinsic – mitochondrial pathway and extrinsic – death receptor pathway which are schematically shown in Fig. 1. Various studies on breast cancer in vitro or in vivo demonstrated the strong apoptotic effect of AP in a dose- and time-dependent approach through both apoptosis pathways, including extrinsic and intrinsic pathways. Moreover, according to several epidemiological and clinical trial studies that indicate progestin-stimulated breast cancer in post-menopausal women [29, 30]; in vivo studies show that AP as the natural anti-progestin has the inhibitory effects on the (MPA)-dependent 7, 12-dimethylbenz (a) anthracene (DMBA)-dependent tumors through apoptosis, and cell proliferation inhibition.
Table 1.
Molecular targets of AP in breast cancer.
| Mechanism | Cell Type | Animal model | Outcome/Molecular targets | Ref. |
|---|---|---|---|---|
| Induction of apoptosis and cell cycle arrest | BT-474 cell (MPA)-dependent 7, 12-dimethylbenz(a)anthracene (DMBA)-dependent tumors SKBR3 - MCF-7 - MDA-MB-231 - MBA-MB-468 - MCF-7 - MDA-MB-453 |
medroxy progesterone acetate (MPA)-dependent BT-474 xenograft tumors | - ↓ cell growth in a dose- and time-dependent - ↑ sub-G0/G1 apoptotic population - caspase-dependent extrinsic apoptosis - the same efficiency in both ER+ and ER- breast cancer cells - ↓ caspases-8, -3, and PARP - ↑ active p53 (p-p53) - ↓ p-STAT3, p-JAK1 and p-JAK2 (upstream kinase of STAT3), and VEGF (STAT3 target gene) - ↓ STAT3 signaling pathway - ↑ ROS - ↓ DNA synthesis in a subcytotoxic dose - ↓ cyclin-dependent kinase - ↓ cyclin B-associated cdc2 activity - ↓ phosphorylation of serine/threonine kinase Akt (protein kinase B) in subcytotoxic concentration - ↓ Akt in dose-dependent - ↓ cyclooxygenase-2 (COX2) - “switching-off” the hormonal receptor of breast cancer cells - HER2/neu degradation - ↓ release of cytochrome c - ↓ receptor tyrosine kinases - ↓ expression of growth factors - ↓ critical transcription factors - ↑ p53 activation |
(28, [44], [45], [46], [47], [48]) |
| Inhibition of fatty acid synthase (FASN) | HER2+ breast cancer cells such as SKBR3 and MCF-7 cells | - | - ↑ apoptosis through ↓ FASN enzyme | (31, 32) |
| Aromatase inhibition | MCF7 cells and the antiestrogen-resistant sublines | human placental microsomes | - ↓ aromatase enzyme, - ↓ aromatase mRNA |
([33]) |
| Inhibition of tumor angiogenesis | T47-D cells BT-474 |
nude mice | - ↓ HIF-1α and VEGF in both hypoxic and normoxic situations - ↓ HIF-1α binding to Hsp90 - ↓ phosphorylation of AKT - ↓ HIF-1α through AKT signaling - ↓ VEGF through ↓ STAT3 - ↓ progestin-dependent induction of VEGF mRNA and protein |
(37, 44, 49) |
| Anti-invasive and metastasis | MDA-MB-231 Efficient in both TNBC and receptor positive breast cancer cells |
MDA-MB-231-derived xenograft tumors | - ↓ TNFα - ↓ CCL2 via ↓ IKBKe and other LSP - ↓ phosphorylated ERK 1/ 2 - ↓ GMCSF - ↓ IL-1α - ↓ IL-6 - ↓ NF-kB which lead to ↓ TGF-b and MMP by ↓ TAM/ TANs and Treg - ↓ HGF - ↓ PI3K/Akt - ↓ CXCR4 - having potential to ↓ VEGF-C, MMP-2/ MMP-9, and Cox-2 |
(23, 50, 51) |
| Inhibition of drug-resistance | -BCRP positive breast cancer cells (MCF-7 MX100) -MCF‑7/ADR cells -antiestrogen‑resistant breast cancer cell |
- | - ↓ BCRP-mediated efflux of mitoxantrone - ↓ mRNA expression of MDR1, - ↓ MRPs, - ↓ P‑gp expression, STAT3, - ↓ p-STAT3, - ↓ nuclear translocation of STAT3, - ↓ STAT3 target genes: VEGF and MMP-9 - ↓ ERα, - ↓ AIB1, - ↓ p38, - ↓ protein kinase A, - ↓ mitogen-activated protein kinase, - ↓ AKT |
([52], [53], [54]) |
| Inhibition of YAP/TAZ activity | TNBC cells | in vivo limited dilution assay | - ↓ CTGF, - ↓ CYR61, - disrupt YAP/TAZ-TEAD interaction, - ↓ TAZ expression |
(55) |
| Improved the immune response | triple-negative MDA-MB-468, HER2+ SK-BR-3, and 4T1 mouse mammary carcinoma cells, as well as human mammary epithelial cells | - | - ↓ PD-L1 upregulation induced by interferon (IFN)-ϒ - ↓ STAT1 phosphorylation, - ↑ interleukin-2 |
(43) |
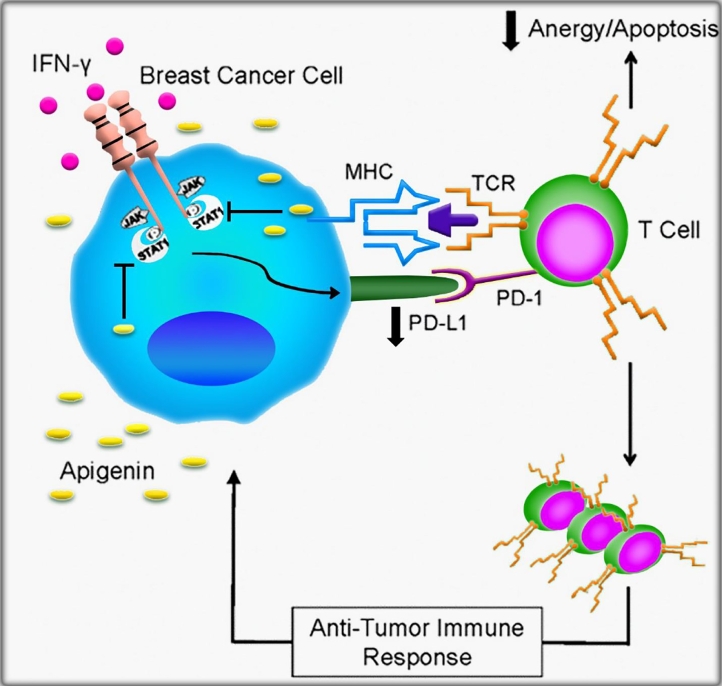
Fig. 1.
Schematic illustration of AP role in down-regulating of PD-L1 induced by IFN-γ by BC cells. AP suppresses IFN-γ-induced STAT1 phosphorylation, which down-regulates inducible PD-L1 by breast cancer cells. An anti-tumor immune response can be created by tumor-reactive T cells in the absence of PD-L1 interaction with PD-1. Reproduced with permission from the Elsevier [43].
Noticeably, it was also shown that a sub-cytotoxic dose of AP suppressed DNA synthesis in a panel of human breast cancer cell lines including MDA-MB-231, MBA-MB-468, MCF-7, and SK-BR-3. In other words, cell viability was not affected by AP at 30 µM concentrations, while the same ones resulted in a dramatic decrease in DNA synthesis at 24 and 72 h of all breast cancer cell lines treatment. This effect of AP which is not restricted to breast cancer cells indicates a common mechanism of action including suppression of cyclin-dependent kinase by p21Cip1 and p27Kip1 and blockade of cyclin B-associated cdc2 activity. Increased ROS generation and decreased phosphorylation of serine/threonine kinase Akt (protein kinase B) in the phosphatidylinositide 3-kinase pathway has been reported as the further AP mechanism at sub-cytotoxic concentrations.
AP as a multi-target compound suppressed the growth of cancer cells by blocking the receptor tyrosine kinases, reduced expression of growth factors, p53 activation, and inhibition of critical transcription factors [28]. AP can also activate apoptosis by preventing fatty acid synthase, a key lipogenic enzyme overexpressed in various human malignancies, including breast cancer [31, 32]. On the other hand, the aromatase enzyme, CYP19, plays a key role in the conversion of androgens to estrogens which express over 60% in breast cancers. Aromatase suppression by phytoestrogens, including AP not only performs at the aromatase enzyme level but also takes place at the gene expression level [33].
Moreover, according to recent clinical trials, treatment with the combination of estrogen and progestin hormones resulted in increased breast cancer among postmenopausal women than therapy with estrogen alone or placebo [29, 34]. In this regard, it has been demonstrated that progestins stimulate breast tumor progression through induction of VEGF which in turn triggers angiogenesis [35, 36]. Mafuvadze and et al., found that AP (50 or 100 µM) suppresses progestin-dependent induction of VEGF mRNA and protein, and inhibits the PR (progesterone receptor) protein expression in T47-D cells. Moreover, progestin-dependent VEGF secretion from BT-474 was blocked by AP. These results indicated that AP as a chemopreventive agent has significant potential in post-menopausal women treated with oral progestins [37].
Worth mentioning that mortality of breast cancer is induced by aggressive metastasis in which AP through blockade of TNFα, IL-6, and chemokines play an essential role in the suppression of breast cancer development, invasion, and metastasis. Moreover, administration of flavonoids accompanied with cytotoxic agents, apart from MDR reversal could provide extra anticancer mechanisms by inhibiting multiple pathways that the tumor cells can survive. Therefore, needs to be determined the potent and nontoxic BCRP inhibitors because of their potential clinical application to reverse MDR. In this regard, AP as a potential chemosensitizing agent showed strong BCRP-suppressing effects in various studies through several mechanisms (Table 1).
Furthermore, overexpression of YAP/TAZ resulted in various biological processes, including epithelial-mesenchymal transition (EMT), tumor metastasis, and tumorigenesis [38], [39], [40], [41], [42]. Li and et al., [22] indicated that proliferation and migration of TNBC cells were significantly prevented by AP. It has also been reported that AP suppressed stemness properties of TNBC cells in both in vitro and in vivo investigations. Interestingly, the function of AP as an immune system modulator specifically in breast cancer has also been investigated in recent years [43]. Coombs and et al., [43] found that AP suppressed the upregulation of PD-L1 induced by interferon (IFN)-ϒ in various breast cancer cell types. The mechanistic basis of downregulation IFN-ϒ-induced PD-L1 expression by AP in MDA-MB-468 and 4T1 cells has been illustrated in Fig. 1.
3. Chemistry of AP
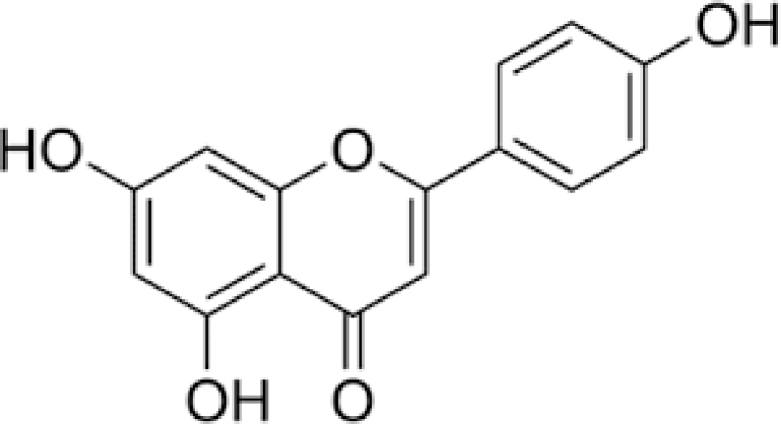
AP, 4,5,7-trihydroxyglavone, is a compound based on flavonoid subgroup that has a skeleton of 2-phenylchromen-4-one (2-phenyl-1-benzopyran-4-one) (Fig. 2). AP has a pale yellow color, and low solubility in alcohol, however, is fully soluble in DMSO. Working with AP is really hard and needed thermodynamically controlled conditions due to the instability at room temperature. AP occasionally happens in plants as aglycone; usually is established as a conformation of glycoside form. Some researchers even support the notion that free API is produced of postharvest degradation procedure [12, 56]. In nature, the public flavonoid feature is development of O-glycosides but other properties of flavones are the formation of C-glycosides recognized by a “carbon-carbon bond among the anomeric carbon of the sugar molecule and the C-6 or C-8 carbon of the flavone nucleus” [57]. In the various groups have recognized various acylated glycosides forms of API [58, 59]. In plants, API is in an extensive kind of forms of glycosides that existence and ratio are influenced through genetic background, environmental growth situation, development phase [60]. Furthermore in glycosylation, in several plants, API forms dimer molecules to form bioflavonoids and other diverse structural groups. The greatest studied API dimer of pharmacological importance is amentoflavone (3, 8-biAP) which is established in recognized medicinal herbs such as St John's wort [61], ginko (Ginkgo biloba L.) [62], and spike mosses [63]. AP is synthesized in the cytoplasmic surface of the endoplasmic reticulum and the reaction is catalyzed through a class of enzymes [64]. The significant stage in the synthesis of flavonoids is the production of naringenin chalcone by condensation and then intramolecular cyclization of three malonyl-CoAs and Coumaroyl-CoAs through chalcone synthesis (CHS). Additional action through the stereospecific catalysis of chalcone isomerase (CHI) outcomes in the synthesis of naringenin. Lastly, naringenin works as the substrate in order to the flavone synthase I (FSI) which catalyzes the construction of the compound [65]. API is irregularly established in its free form, so its creation is typically followed through additional action through formation of methyltransferases, glycosyltransferases, and hydroxyl transferases which catalyze methylation and hydroxylation of API to form varied derivatives. It has been described that one operative manner of AP glucosides synthesis is via the glycosylation reaction by uridine diphosphate-glucosyltransferase YjiC, from Bacillus licheniformis DSM 13. Numerous approaches are also accessible in order to the synthesis of AP such as microwaves irradiation of ketoester as the starting material or commercially phloroglucinol. Extensive kinds of various synthetic API derivatives are also synthesized as pharmacologically active compounds [66], [67], [68], [69].
Fig. 2.
Schematic diagram of the chemical structure of AP.
4. Increase bioavailability of AP
Based on the literature, there are several methods to increase the bioavailability of AP in different forms and concentrations, for different types of applications. Based on the biopharmaceutics classification systems, AP is a class II drug with considerable intestinal membrane permeability and also has very poor solubility in green media, especially water [70, 71]. There is wide interest in improving the bioavailability of AP via cross-linking them with biocompatible linkers, and also modifying the chemical structure with reactive functional groups; however, the most important factor in the improving of AP bioavailability, generally any compound, is considering the factors that may play a role in the mass production or entry of these compounds into the clinical phase [72, 73]. In this manner, Rabiee's Theory (Variable Laws) [74] would be considered as a promising point. Rabiee's theory is as follow:
| Vb + Vh ∝ Vtotal |
The number of biomaterials (Vb) and host variables (Vh) depends on a variety of factors, and by increasing the number of biomaterials and host variables, the amount of total variables also increases and as a result, performance and, consequently, biomaterial behavior in the host environment will have less control and predictive capabilities. For an external substance that is supposed to be in the human body, it must be predictable and controllable. In addition, according to the principle that the host in an individual does not have the ability to change, therefore, by using the simpler biomaterials (with fewer variables), the above goal is more accessible.
The biocompatibility examinations in order to a biomaterial based on obtainable protocols and standards, the appropriate compatibility (AC) parameter is also needed in agreement with Rabiee's theory. The summary of this theory and relationship is as follows:
| AC ∝ Vnb |
| AC ∝ Vnh |
| AC ∝ Vnb × Vnh |
| AC = α × Vnb × Vnh |
In the above equations, Vnb is the amount of variation numbers of biomaterials and Vnh is the amount of variation numbers of the host. In general, the host is not controllable, and it varies in individual numbers and any part, therefore, we assume the amount of variation numbers of the host (Vnh) is 100. α is the biomaterial constant that depends on the component's variations (Vc) and the morphology simplicity variation (Vms), therefore, the final equation is:
| AC = 100 × α × Vnb |
In the case of AP bioavailability, the correct logic is that based on the above mentioned, it should improve the bioavailability of the AP at the lowest cost as well as maximum efficiency by a simple, tunable and fully accessible protocol. There are limited articles that emphasize improving the bioavailability of AP by the mentioned protocol, but encapsulating the AP into biocompatible, biodegradable and low-cost polymers is the desired protocol [75], [76], [77], [78].
5. Enhanced bioavailability of AP via nanoparticles
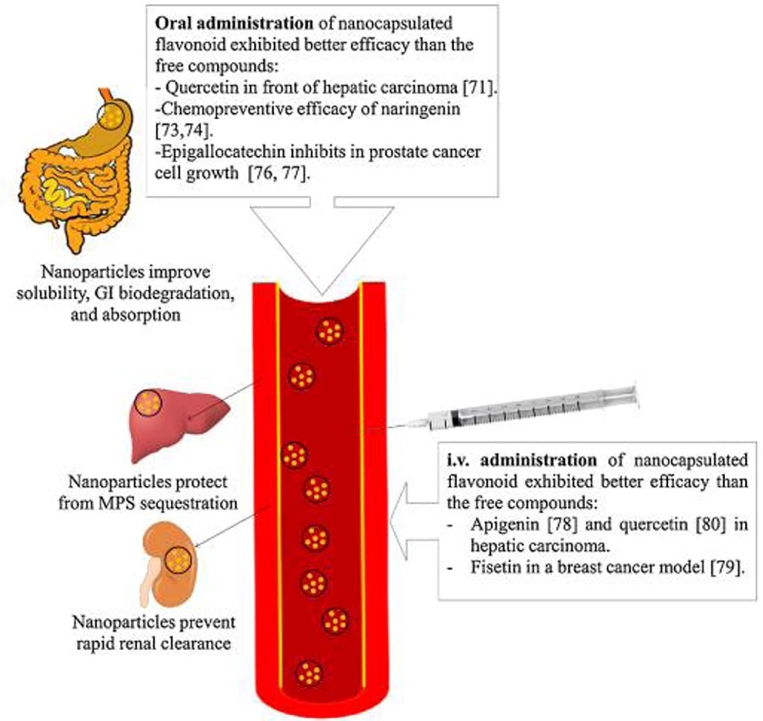
Nanomaterials/nanoparticles (NP) consider as emerging materials in medicine with application from drugs, genes and other medical fields [79]. Examples are recent COVID-19 vaccines from both Pfizer's and Modena's contain mRNA wrapped in lipid nanoparticles (LNPs) that support carrying it to human cells nevertheless also act as an adjuvant, a vaccine ingredient that bolsters the immune response [80]. The nanoparticles are <100 nm in size, and a wide range of drugs, including hydrophilic and hydrophobic small drugs, flavonoids, vaccines and biological molecules, can be delivered by these NPs [81], [82], [83]. Application of NPs in drug delivery can be mentioned in diseases such as cancer, cardiovascular and Alzheimer's disease [84]. The NPs help improve several features to attain improved bioavailability, as shown in Fig. 3 [85].
Fig. 3.
Effect of NPs formulation on bioavailability of drugs. Reprinted with permission from the Elsevier [85].
AP acts well in pharmacological aspect, but due to its poor solubility, its clinical application has been limited. Therefore, new formulations or methods should be developed to improve solubility of AP and its bioavailability [86]. Numerous kinds of nanomaterials such as metallic–based nanomaterials, lipid-based and polymeric nanoparticles were developed in order to the highly effective delivery of AP [86], [87], [88]. The oral bioavailability of AP nanoparticles was about 5 fold upper compared to the naked AP, and this formulation shows no toxic outcome on the organs of mice [89]. Various studies have revealed that solid dispersion (SD) can be effectively applied in order to the improving the dissolution level of poorly water-soluble drugs [90]. Metallic nanoparticles and carbon-based nanomaterials have been broadly applied as drug delivery systems, predominantly in targeted therapy [91]. Lipid-based nanocarriers are applied to progress the high-efficiency delivery and bioavailability of AP. Furthermore, the appliances of lipid-based nanoparticles to overcome the MDR have been proposed based on improved permeability of membrane [92, 93]. Examples of applications of NPs in the delivery of AP have been shown in Table 2. It demonstrated that NPs significantly improve solubility and bioavailability of AP in vitro and preclinical animal models.
Table 2.
Summary of report on the production of AP-based NPs, particle size and their importance in increasing bioavailability. Keys: Carbon nanopowders: CNPs; AP-phospholipid phytosome: APLC.
| Types of nanomaterials | Production techniques used | Particle size (nm) | Bioavailability improved | Drug delivery | Type of study | Year | Ref |
| AP nanocrystals | Supercritical antisolvent process | 400–800 | 3.4-fold | Oral | In vitro | 2013 | [94] |
| AP-loaded polymeric micelles | Spray drying technique | – | 2.5-fold | Oral | In vitro, in vivo (Male Wister Albino rats) | 2018 | [95] |
| AP-loaded mixed micelles | Ethanol thin-film hydration method | 178 | 4.03- fold | Oral | In vitro, in vivo (Male Sprague-Dawley rats) | 2017 | [78] |
| APLC | – | 107 | bioavailability of AP after APLC administration was 82% | Oral | In vitro, in vivo (Male and female albino rats, Wistar strain) | 2016 | [96] |
| AP NPs | Liquid antisolvent precipitation technique | 159 | 4.96-fold | Oral | In vitro | 2017 | [97] |
| AP liposomes | lipid film hydration | 103 | – | Vein | In vitro, in vivo (Athymic nude mice (nu/nu,4–6 week) | 2017 | [87] |
| Mesoporous silica | physical absorption | 49 | Enhanced bioavailability | Oral | In vitro, in vivo(female Sprague-Dawley rats) | 2019 | [86] |
| CNPs | Solvent evaporation | 40 | Increased the bioavailability of AP by approximately 183% | Oral | In vitro, in vivo (Male Sprague-Dawley rats) | 2014 | [98] |
| AP-PLGA | Multiple emulsion solvent evaporation | 226 | Enhanced bioavailability | Intraperitoneal | In vitro, in vivo (Swiss albino mice) | 2018 | [99] |
Solid dispersion (SD) is an extensively applied method to improve dissolution of poorly water-soluble drugs [100]. Carbon nano powders (CNPs) are carbon nanomaterials with less than 100 nm, can be used as nanocarriers for SD preparation and to increase drug solubility. These materials have properties such as large specific surface area and high dispersibility, which reduces drug particle size and improved SD. For preclinical research, oral administration of AP resulted in low blood levels, with a Cmax of 1.33 μg/mL and AUC 0–t° of 11.76 μg h/mL. With CNPs drug nanomaterial of SD, the oral bioavailability of AP was increased by approximately 183% [98].
Mesoporous silica NPs (MSNs) are insoluble nanomaterials, so these carriers can be classified into the last generation SD. These particles have many advantages such as high biocompatibility and biodegradability, pore size with narrow distribution, and stability [101]. As a result, MSNs are a good choice in order to drug delivery according to their exclusive properties and low toxicity. In a study, MSN showed increased solubility and oral bioavailability of AP. AP-MSN was prepared by physical absorption, and the AP-MSN SD was synthesized at the weight ratio of 1:1 to get the high solubility. As a result, this solubility of AP-MSN SD was larger than AP. The results of the study showed that the concentration of AP is usually very low, but oral bioavailability of AP-MSN SD enhanced by 8.32 times than AP in 2.5–8 h. So, AP-MSN SD has a good outlook to be applied as new oral formulation for clinical application [102].
Various methodologies have been used to enhance the AP dispersion (SD) and improve its bioavailability. Spray drying technique was established to increase the bioavailability of AP by formation of AP-loaded Pluronic F-127 (PL-F127) polymeric micelle (95). PLF-127 is a non-ionic amphiphilic copolymer comprised of ethylene oxide (PEO) and propylene oxide. At high copolymer concentrations, micelles are packed leading to gel-like performance. These polymers can be widely used in increasing solubility and bioavailability of different molecules [103, 104]. Mixed micelles system act as an effective drug delivery system, which has the capability to control the drug's release, due to its core-shell structure, and can also improve the solubility of hydrophobic drugs. An AP-loaded mixed micelles (AP-M) system comprising two copolymers of soluplus and PL F-127 polymers with size 178.5 nm, prepared by ethanol thin-film hydration method. This system has been helping to improve oral bioavailability (more than 4-fold), and increase water solubility, high Caco-2 cellular uptake and gastrointestinal absorption of AP than free AP in rats [78]. Poly lactic co-glycolic acid (PLGA), as polymeric NPs, have unique properties such as nontoxic and biodegradable, and are highly popular as nanocarrier. These NPs have been approved by the Food and Drug Administration (FDA) in US as an intravenous drug delivery system [105, 106].
Evaluation of human hepatocellular carcinoma cell line such as HepG2 and Huh-7, after administration of AP-loaded NPs (20 mg/kg body weight per week) in rats, showing reduced nodule size, number, and area liver lesions after apigenin treatment. These NPs were more effective for the inhibition of tumor growth, associated with a sustained release of AP-loaded NPs than the free form. This formulation of AP improved its bioavailability and made it more tumor site specific in nature [107]. PLGA NPs can reduce cancer cell survival without damaging healthy cells.
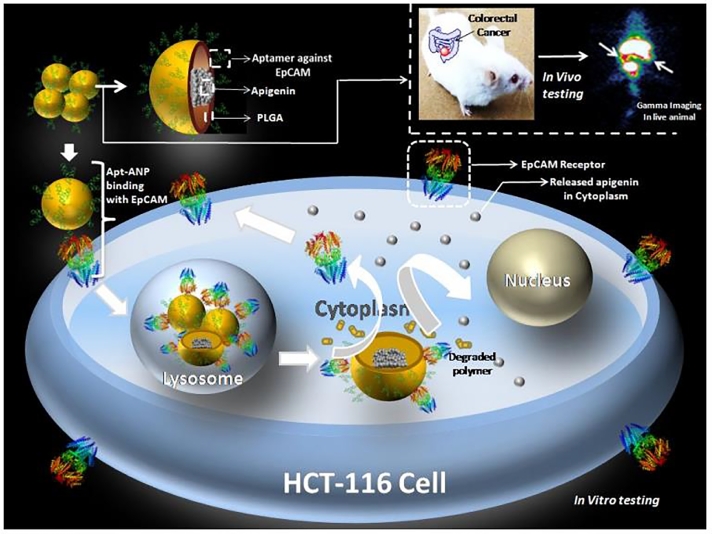
The PLGA-based nanoformulation containing aptamer-conjugated AP-loaded PLGA NPs with average size of 226 nm, was found to have anti proliferative action both in vitro and in vivo colorectal cancer model as shown in Fig. 4. These nanocarriers were effectively delivered AP to the targeted region. This nanoplatform accumulated in the colon, which increases therapeutic efficacy to colorectal cancer cells and decreases off-target cytotoxicity. The outcome was AP bioavailability in colorectal tissue and plasma increased significantly after AP-ANP treatment, with low levels of toxicities [99]. The recent development of graphene and its derivatives are emerging as effective gene/drug delivery system. Functionalized graphene oxides (FGO) are nontoxic, biocompatible and have properties including strong, light and conductive. A recent study of paclitaxel with FGO-AP produces synergistic effects in human ovarian cancer cells via the modulation of anti-apoptotic genes and reduced side effects of paclitaxel chemotherapy [108]
Fig. 4.
Schematic illustration of the efficacy of HCT-116 cell-targeted aptamer-conjugated AP-loaded PLGA NPs, followed by in vivo efficacy in a mouse model of colorectal cancer. Reprinted with permission from the American Chemical Society [99].
The oral administration of flavone AP conjugated with meso‑2,3-dimercaptosuccinic acid (DMSA) showed higher lung bioavailability, and improved plasma half-life than naked AP in C57BL/6 melanoma metastatic mouse models. This nano-formulation exhibited considerably probable to accumulate in the lungs than naked flavone AP, and can act as a therapeutic agent in treatment of lung melanoma metastasis. The results showed DMSA-conjugated flavone AP causes the COX-2 expression to be down regulated COX-2 expression in B16F10 cells. In fact, decrease in the COX-2 level can be an inhibitor of melanoma cell invasion [88]. Encapsulated AP with nanomaterials such as PLGA showed enhanced effectiveness in treatment of cancer. This is due to NPs helping take the AP to enter the nucleus of the cells and causing higher mitochondrial apoptosis in A375 cells. NAp also inhibited A375 cells proliferation in a dose dependent effect with the IC50 of 15 µM, therefore, there was no cytotoxic effect on the normal skin keratinocyte (HaCaT) cells [109].
The role of AP as a protective agent in IR-induced acute kidney injury (AKI) was developed. Intraperitoneal injection of AP at specific doses (5, 10 and 20 mg/kg) was performed on mice. Loading of AP in degradable polymer nanoparticles can increase its bioavailability. AP reduces inflammation by silencing the NF-B pathway through increasing miR-140–5p expression and decreasing expression in CXCL12 in vivo and in vitro. As a result, it can act as a therapeutic agent in patients with IR-related kidney injury [110].
There are lots of NPs and lipid-based carrier systems such as phospholipids that can increase the overall bioavailability and solubility of some compounds, like curcumin, chrysophanol, and naringenin with plenty in vitro and early phase of the preclinical trial on a rodent model. Examples of this work are carried out on AP-phospholipid phytosome (APLC) to increase the drug's solubility, antioxidant activity, and in vivo oral bioavailability. APLC enhances the aqueous solubility of AP in water by 37-fold (22 µg/mL), compared with AP (0.62 µg/mL). It establishes that phytosome is a capable formulation approach for increasing the delivery of poorly water-soluble drugs [96].
The liposome nanoparticles are most interesting and used clinically nanoparticles. The liposome is made of nanobubble (vesicle), it is the same material as a cell membrane. It can be filled with drugs and then deliver to target cells. It is an excellent drug delivery vehicle, transporting a cargo of interest within a protective, outer layer of lipids. In fact, they are an innovative drug delivery system; and comprising of bilayers that form extemporaneously when phospholipids are dispersed in water [111]. Liposomes are generally used in the form of several commercial products, including d-alpha-tocopheryl polyethylene glycol succinate (TPGS), which is a water-soluble solution derived from esterified polyethylene glycol, vitamin E succinate, and is used as a nanocarrier. Promoting the bioavailability of anticancer drugs and inhibiting the resistance of several drugs are the benefits of this nanocarrier [112]. The combination of AP -TPGS and Tyroservatide (YSV), which showed important anti-tumor effects against A549 cancer cells [113].
The application of liposome with capsulation AP, with nano size of 103 nm, tested in vitro cells work with CRC cell lines HT-29. The experimental results confirmed liposome-AP improved cytotoxicity and the bioavailability of AP. The results also showed that low hemolysis caused by liposomes made their hemo-compatibility and made them suitable for intravenous injection [87]. Encapsulated AP with SLNP exhibited improved effectiveness in the treatment of diabetes mellitus. This highly bioavailable AP-SLNP with the size of about 150 nm, cab be enhanced HO-1 and Nrf2 expression and decreased the NF-kB activity, leads a protective effect against diabetic properties by anti-inflammatory and anti-oxidant activity, as well as reduced level of glucose in the blood in rats [114]. However, Lipid-based carriers have been shown to be suitable as oral delivery vehicles, but, high lipolysis of lipid-based carriers in the gastrointestinal tract reduces their lifespan in the circulation [87, 115]. Moreover, studies have shown that lipid -reconstitution after lipolysis in vivo using non-water-quenching dye. The encapsulated in the lipid-based carrier can be monitored the reconstitution of lipolytic products offers record for the gastrointestinal tract condition of lipid-based nanocarriers [116, 117]. Table 3 shows a list of some clinical trials used for Apigenin in various interventions in the https://clinicaltrials.gov/ site.
Table 3.
A list of AP clinical trials studies.
| Studies | Trial ID | Mechanism of action | Clinical Phases/Study design |
| Chemotherapy-induced Oral Mucositis | NCT04317183 | Topical chamomile oral gel may affect the prevention of chemotherapy-induced oral mucositis | Recruiting/Phase 2 |
| Diabetes Mellitus Type 2 | NCT04233658 | Chlorogenic acid, luteolin and AP can be improved antidiabetic effects through downregulation of gluconeogenesis. | Phase 3 |
| Pancreatic Cancer | NCT00609310 | The effect of AP on GLUT-1, HIF and VEGF prevents the proliferation of PC cells | Suspended/Phase 2 |
| COVID | NCT04404218 | The use of natural extracts such as AP to diminution inflammation in patients with SARS-COV-2 | Recruiting/ Phase 2 |
| Cardiovascular Risk (NUT) | NCT04114916 | Changes in the dilatation of the Humeral artery | Completed |
| Allergic Rhino Conjunctivitis | NCT03365648 | The use of rosmarinic acid, AP, luteolin and chrysoeriol can be prevent the release of histamine and interleukins | Completed |
6. Conclusion and future prospective
Breast cancer is the second leading cause of death from cancer in women worldwide. Moreover, one of the breast cancer subtypes, TNBC has attracted a wide scientific and clinical attentions because of its heterogeneity, poor prognosis, and lack of the targeted therapy strategies. Based on several studies, the risk of this subtype as a heterogeneous disease varies in different western and Asian populations. The differences are related to its prevalence, while the risk factors and treatment options are in common [118]. Surgery, radiation and chemotherapy are some of the treatments that can be used for breast cancer. However, it is important to find and develop chemo-preventive factors to prevent and / or manage the breast cancer. A wide variety of epidemiological and experimental researches showed the efficacy of dietary phytochemicals in breast cancer prevention [119].
The above discussed literature emphasized the anticancer potential of apigenin. It was demonstrated that apigenin as a potent agent suppress almost all types of breast cancer. Nevertheless, the most findings are resulted from in vitro studies which cannot be extended the dosage and efficacy to living systems because of their various enzymes and immune system, leading apigenin degradation and regulation the pathways respectively. Albeit, some in vivo studies have also demonstrated cancer-preventing properties of apigenin, but unfortunately, the studied variable numbers are restricted [120].
Since ancient times, natural products (NPs) and their derivatives have played a major role in pharmacotherapy, including cancer diseases. However, these materials also suffer from challenges of drug discovery, like screening, isolation, characterization and optimization. Recently, various scientific and technological advances, containing genome mining and engineering approaches, and analytical tools improvement, and microbial culturing developments consider these challenges and create the novel opportunities. Therefore, using the natural products in pharmacotherapy, especially for circumvent antimicrobial resistance is revived [121].
The dietary-derived natural materials have been traditionally used for the prevention and treatment of cancers, and many new synthetic drugs are the extract of these materials. Hence further research should be carried out on the effectiveness of these materials for the treatment of cancer. In the last decades, various research studies have confirmed the multiple anticancer effects of flavonoids, especially their subclass, AP. Different molecular mechanisms against breast cancer make AP a promising potential for breast cancer prevention and treatment. AP has different physicochemical and biological properties that are essential in cancer treatment. The AP can be stable and reactive; however, it can interact in different stages of breast cancer development and active enzymes and molecules. In this regard, the chemistry of AP, the main molecular mechanisms of AP against breast cancer, and the different methods to improve its bioavailability were discussed. Among different approaches of bioavailability enhancing, NPs can enhance the delivery and interaction of AP into cancer cells. The mechanisms of enhancing the bioavailability of AP via NPs were discussed in detail. It has been demonstrated that conjugation or encapsulation of AP with NPs, can enhance the solubility, biodegradation, bioavailability, and absorption of AP, leading to better chemo-preventive and chemotherapeutic efficacy. In this regard, different NPs, their synthesis methods and the mechanism of enhancing bioavailability were reported.
AP has been introduced as an anticancer agent. However, further studies are required to fully understand its mechanism of action on cancer cells and tumors in a clinical setting. The exact mechanism should be investigated in therapeutic effects against every specific cancer cell, the interactions among the involved mechanisms, and modulating them. Any further study will complete the existing data and can optimize the therapeutic effects of AP. In addition, their synergistic anticancer effects with NPs and cell-specific delivery by nanomedicine through targeting approaches can be studied in future researches. More in vivo results and clinical data of these AP-nanomedicine systems can help to fight against breast cancer.
Author contributions
AM performed different analyses. All authors helped in performing and drafting the manuscript. The authors read and approved the final manuscript.
Funding
Not Applicable.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request
Ethics approval and consent to participate
Not Applicable
Consent for publication
Not Applicable
Competing Interests
The authors declare that they have no competing interests.
Acknowledgments
The authors would like to thank Department of Medical Nanotechnology, Faculty of Advanced Medical Science, Tabriz University of Medical Sciences.
Contributor Information
Masoumeh Zahmatkeshan, Email: zahmatkeshanm1@gmail.com.
Abolfazl Akbarzadeh, Email: akbarzadehab@tbzmed.ac.ir.
Alexander Marcus Seifalian, Email: a.seifalian@gmail.com.
References
- 1.Zheng Q., Zhang M., Zhou F., Zhang L., Meng X. The breast Cancer stem cells traits and drug resistance. Front. Pharmacol. 2021:2120. doi: 10.3389/fphar.2020.599965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mitra S., Dash R. Natural products for the management and prevention of breast cancer. Evidence-based complementary and alternative medicine. 2018;2018.
- 3.Mitra S., Dash R. Natural products for the management and prevention of breast cancer. Evidence-based complementary and alternative medicine. 2018;2018:8324696. [DOI] [PMC free article] [PubMed]
- 4.Group EBCTC. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. The Lancet. 2005;366(9503):2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 5.Zhou Y., Li Y., Zhou T., Zheng J., Li S., Li H.-.B. Dietary natural products for prevention and treatment of liver cancer. Nutrients. 2016;8(3):156. doi: 10.3390/nu8030156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harbeck N., Penault-Llorca F., Cortes J., Gnant M., Houssami N., Poortmans P., et al. Breast cancer. Nat. Rev. Dis. Primers. 2019;5(1):1–31. doi: 10.1038/s41572-019-0111-2. [DOI] [PubMed] [Google Scholar]
- 7.Abad E., Graifer D., Lyakhovich A. DNA damage response and resistance of cancer stem cells. Cancer Lett. 2020;474:106–117. doi: 10.1016/j.canlet.2020.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Eiro N., Gonzalez L.O., Fraile M., Cid S., Schneider J., Vizoso F.J. Breast cancer tumor stroma: cellular components, phenotypic heterogeneity, intercellular communication, prognostic implications and therapeutic opportunities. Cancers (Basel) 2019;11(5):664. doi: 10.3390/cancers11050664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zahmatkeshan M., Ilkhani H., Paknejad M., Adel M., Sarkar S., Saber R. Analytical characterization of label-free immunosensor subsystems based on multi-walled carbon nanotube array-modified gold interface. Comb. Chem. High Throughput Screen. 2015;18(1):83–88. doi: 10.2174/1386207318666141212165513. [DOI] [PubMed] [Google Scholar]
- 10.Zahmatkeshan M., Gheybi F., Rezayat S.M., Jaafari M.R. Improved drug delivery and therapeutic efficacy of PEgylated liposomal doxorubicin by targeting anti-HER2 peptide in murine breast tumor model. Eur. J. Pharmaceutical Sci. 2016;86:125–135. doi: 10.1016/j.ejps.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 11.Mirzaei-Parsa M.J., Najafabadi M.R.H., Haeri A., Zahmatkeshan M., Ebrahimi S.A., Pazoki-Toroudi H., et al. Preparation, characterization, and evaluation of the anticancer activity of artemether-loaded nano-niosomes against breast cancer. Breast Cancer. 2020;27(2):243–251. doi: 10.1007/s12282-019-01014-w. [DOI] [PubMed] [Google Scholar]
- 12.Nabavi S.F., Khan H., D'onofrio G., Šamec D., Shirooie S., Dehpour A.R., et al. Apigenin as neuroprotective agent: of mice and men. Pharmacol. Res. 2018;128:359–365. doi: 10.1016/j.phrs.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 13.Martinez-Perez C., Ward C., Cook G., Mullen P., McPhail D., Harrison D.J., et al. Novel flavonoids as anti-cancer agents: mechanisms of action and promise for their potential application in breast cancer. Biochem. Soc. Trans. 2014;42(4):1017–1023. doi: 10.1042/BST20140073. [DOI] [PubMed] [Google Scholar]
- 14.Romagnolo D.F., Selmin O.I. Flavonoids and cancer prevention: a review of the evidence. J. Nutr. Gerontol. Geriatr. 2012;31(3):206–238. doi: 10.1080/21551197.2012.702534. [DOI] [PubMed] [Google Scholar]
- 15.Pan H., Zhou W., He W., Liu X., Ding Q., Ling L., et al. Genistein inhibits MDA-MB-231 triple-negative breast cancer cell growth by inhibiting NF-κB activity via the Notch-1 pathway. Int. J. Mol. Med. 2012;30(2):337–343. doi: 10.3892/ijmm.2012.990. [DOI] [PubMed] [Google Scholar]
- 16.Knekt P., Järvinen R., Seppänen R., Hellövaara M., Teppo L., Pukkala E., et al. Dietary flavonoids and the risk of lung cancer and other malignant neoplasms. Am. J. Epidemiol. 1997;146(3):223–230. doi: 10.1093/oxfordjournals.aje.a009257. [DOI] [PubMed] [Google Scholar]
- 17.Hoensch H., Groh B., Edler L., Kirch W. Prospective cohort comparison of flavonoid treatment in patients with resected colorectal cancer to prevent recurrence. World J. Gastroenterol. 2008;14(14):2187–2193. doi: 10.3748/wjg.14.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rossi M., Negri E., Lagiou P., Talamini R., Dal Maso L., Montella M., et al. Flavonoids and ovarian cancer risk: a case-control study in Italy. Int. J. Cancer. 2008;123(4):895–898. doi: 10.1002/ijc.23549. [DOI] [PubMed] [Google Scholar]
- 19.Bosetti C., Spertini L., Parpinel M., Gnagnarella P., Lagiou P., Negri E., et al. Flavonoids and breast cancer risk in Italy. Cancer Epidemiol. Prevention Biomarkers. 2005;14(4):805–808. doi: 10.1158/1055-9965.EPI-04-0838. [DOI] [PubMed] [Google Scholar]
- 20.Shukla S., Gupta S. Apigenin: a promising molecule for cancer prevention. Pharm. Res. 2010;27(6):962–978. doi: 10.1007/s11095-010-0089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yan X., Qi M., Li P., Zhan Y., Shao H. Apigenin in cancer therapy: anti-cancer effects and mechanisms of action. Cell Biosci. 2017;7(1):1–16. doi: 10.1186/s13578-017-0179-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y.W., Xu J., Zhu G.Y., Huang Z.J., Lu Y., Li X.Q., et al. Apigenin suppresses the stem cell-like properties of triple-negative breast cancer cells by inhibiting YAP/TAZ activity. Cell Death Discov. 2018;4:105. doi: 10.1038/s41420-018-0124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bauer D., Redmon N., Mazzio E., Soliman K.F. Apigenin inhibits TNFα/IL-1α-induced CCL2 release through IKBK-epsilon signaling in MDA-MB-231 human breast cancer cells. PLoS ONE. 2017;12(4) doi: 10.1371/journal.pone.0175558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sudhakaran M., Parra M.R., Stoub H., Gallo K.A., Doseff A.I. Apigenin by targeting hnRNPA2 sensitizes triple-negative breast cancer spheroids to doxorubicin-induced apoptosis and regulates expression of ABCC4 and ABCG2 drug efflux transporters. Biochem. Pharmacol. 2020;182 doi: 10.1016/j.bcp.2020.114259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Korga-Plewko A., Michalczyk M., Adamczuk G., Humeniuk E., Ostrowska-Lesko M., Jozefczyk A., et al. Apigenin and hesperidin downregulate DNA repair genes in MCF-7 breast cancer cells and augment doxorubicin toxicity. Molecules. 2020;25(19):4421. doi: 10.3390/molecules25194421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pham T.H., Page Y.L., Percevault F., Ferriere F., Flouriot G., Pakdel F. Apigenin, a partial antagonist of the estrogen receptor (ER), inhibits ER-positive breast cancer cell proliferation through Akt/FOXM1 signaling. Int .J. Mol. Sci. 2021;22(1):470. doi: 10.3390/ijms22010470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shendge A., Chaudhuri D., Basu T., Mandal N. A natural flavonoid, apigenin isolated from Clerodendrum viscosum leaves, induces G2/M phase cell cycle arrest and apoptosis in MCF-7 cells through the regulation of p53 and caspase-cascade pathway. Clin. Translational Oncol. 2021;23(4):718–730. doi: 10.1007/s12094-020-02461-0. [DOI] [PubMed] [Google Scholar]
- 28.Scherbakov A., Andreeva O. Apigenin inhibits growth of breast cancer cells: the role of ERα and HER2/neu. Acta Naturae (англоязычная версия) 2015;7:133–139. (3 (26)) [PMC free article] [PubMed] [Google Scholar]
- 29.Ross R.K., Paganini-Hill A., Wan P.C., Pike M.C. Effect of hormone replacement therapy on breast cancer risk: estrogen versus estrogen plus progestin. J. Natl. Cancer Inst. 2000;92(4):328–332. doi: 10.1093/jnci/92.4.328. [DOI] [PubMed] [Google Scholar]
- 30.Chlebowski R.T., Anderson G.L., Gass M., Lane D.S., Aragaki A.K., Kuller L.H., et al. Estrogen plus progestin and breast cancer incidence and mortality in postmenopausal women. JAMA. 2010;304(15):1684–1692. doi: 10.1001/jama.2010.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brusselmans K., Vrolix R., Verhoeven G., Swinnen J.V. Induction of cancer cell apoptosis by flavonoids is associated with their ability to inhibit fatty acid synthase activity. J. Biol. Chem. 2005;280(7):5636–5645. doi: 10.1074/jbc.M408177200. [DOI] [PubMed] [Google Scholar]
- 32.Menendez J.A., Vazquez-Martin A., Oliveras-Ferraros C., Garcia-Villalba R., Carrasco-Pancorbo A., Fernandez-Gutierrez A., et al. Analyzing effects of extra-virgin olive oil polyphenols on breast cancer-associated fatty acid synthase protein expression using reverse-phase protein microarrays. Int. J. Mol. Med. 2008;22(4):433–439. [PubMed] [Google Scholar]
- 33.Lephart E.D. Modulation of aromatase by phytoestrogens. Enzyme Res. 2015 doi: 10.1155/2015/594656. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen W.Y., Hankinson S.E., Schnitt S.J., Rosner B.A., Holmes M.D., Colditz G.A. Association of hormone replacement therapy to estrogen and progesterone receptor status in invasive breast carcinoma. Cancer. 2004;101(7):1490–1500. doi: 10.1002/cncr.20499. [DOI] [PubMed] [Google Scholar]
- 35.Hyder S.M., Murthy L., Stancel G.M. Progestin regulation of vascular endothelial growth factor in human breast cancer cells. Cancer Res. 1998;58(3):392–395. [PubMed] [Google Scholar]
- 36.Hyder S.M., Chiappetta C., Stancel G.M. Pharmacological and endogenous progestins induce vascular endothelial growth factor expression in human breast cancer cells. International J. Cancer. 2001;92(4):469–473. doi: 10.1002/ijc.1236. [DOI] [PubMed] [Google Scholar]
- 37.Mafuvadze B., Benakanakere I., Hyder S.M. Apigenin blocks induction of vascular endothelial growth factor mRNA and protein in progestin-treated human breast cancer cells. Menopause. 2010;17(5):1055–1063. doi: 10.1097/gme.0b013e3181dd052f. [DOI] [PubMed] [Google Scholar]
- 38.Zanconato F., Cordenonsi M., Piccolo S. YAP/TAZ at the roots of cancer. Cancer Cell. 2016;29(6):783–803. doi: 10.1016/j.ccell.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao B., Li L., Lei Q., Guan K.-.L. The Hippo–YAP pathway in organ size control and tumorigenesis: an updated version. Genes Dev. 2010;24(9):862–874. doi: 10.1101/gad.1909210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lei Q.-.Y., Zhang H., Zhao B., Zha Z.-.Y., Bai F., Pei X.-.H., et al. TAZ promotes cell proliferation and epithelial-mesenchymal transition and is inhibited by the hippo pathway. Mol. Cell. Biol. 2008;28(7):2426–2436. doi: 10.1128/MCB.01874-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harvey K.F., Zhang X., Thomas D.M. The Hippo pathway and human cancer. Nat. Rev. Cancer. 2013;13(4):246–257. doi: 10.1038/nrc3458. [DOI] [PubMed] [Google Scholar]
- 42.Marti P., Stein C., Blumer T., Abraham Y., Dill M.T., Pikiolek M., et al. YAP promotes proliferation, chemoresistance, and angiogenesis in human cholangiocarcinoma through TEAD transcription factors. Hepatology. 2015;62(5):1497–1510. doi: 10.1002/hep.27992. [DOI] [PubMed] [Google Scholar]
- 43.Coombs M.R.P., Harrison M.E., Hoskin D.W. Apigenin inhibits the inducible expression of programmed death ligand 1 by human and mouse mammary carcinoma cells. Cancer Lett. 2016;380(2):424–433. doi: 10.1016/j.canlet.2016.06.023. [DOI] [PubMed] [Google Scholar]
- 44.Seo H.-.S., Jo J.K., Ku J.M., Choi H.-.S., Choi Y.K., Woo J.-.K., et al. Induction of caspase-dependent extrinsic apoptosis by apigenin through inhibition of signal transducer and activator of transcription 3 (STAT3) signalling in HER2-overexpressing BT-474 breast cancer cells. Biosci. Rep. 2015;35(6) doi: 10.1042/BSR20150165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mafuvadze B., Liang Y., Besch-Williford C., Zhang X., Hyder S.M. Apigenin induces apoptosis and blocks growth of medroxyprogesterone acetate-dependent BT-474 xenograft tumors. Hormones and Cancer. 2012;3(4):160–171. doi: 10.1007/s12672-012-0114-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seo H.S., Ku J.M., Choi H.S., Woo J.K., Jang B.H., Go H., et al. Apigenin induces caspase-dependent apoptosis by inhibiting signal transducer and activator of transcription 3 signaling in HER2-overexpressing SKBR3 breast cancer cells. Mol. Med. Rep. 2015;12(2):2977–2984. doi: 10.3892/mmr.2015.3698. [DOI] [PubMed] [Google Scholar]
- 47.Bai H., Jin H., Yang F., Zhu H., Cai J. Apigenin induced MCF-7 cell apoptosis-associated reactive oxygen species. Scanning: J. Scanning Microscopies. 2014;36(6):622–631. doi: 10.1002/sca.21170. [DOI] [PubMed] [Google Scholar]
- 48.Harrison M.E., Coombs M.R.P., Delaney L.M., Hoskin D.W. Exposure of breast cancer cells to a subcytotoxic dose of apigenin causes growth inhibition, oxidative stress, and hypophosphorylation of Akt. Exp. Mol. Pathol. 2014;97(2):211–217. doi: 10.1016/j.yexmp.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 49.Fang J., Zhou Q., Liu L.Z., Xia C., Hu X., Shi X., et al. Apigenin inhibits tumor angiogenesis through decreasing HIF-1α and VEGF expression. Carcinogenesis. 2007;28(4):858–864. doi: 10.1093/carcin/bgl205. [DOI] [PubMed] [Google Scholar]
- 50.Lee H.H., Jung J., Moon A., Kang H., Cho H. Antitumor and anti-invasive effect of apigenin on human breast carcinoma through suppression of IL-6 expression. Int. J. Mol. Sci. 2019;20(13):3143. doi: 10.3390/ijms20133143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang L., Kuang L., Hitron J.A., Son Y.-.O., Wang X., Budhraja A., et al. Apigenin suppresses migration and invasion of transformed cells through down-regulation of CXC chemokine receptor 4 expression. Toxicol. Appl. Pharmacol. 2013;272(1):108–116. doi: 10.1016/j.taap.2013.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Long X., Fan M., Bigsby R.M., Nephew K.P. Apigenin inhibits antiestrogen-resistant breast cancer cell growth through estrogen receptor-α-dependent and estrogen receptor-α-independent mechanisms. Mol. Cancer Ther. 2008;7(7):2096–2108. doi: 10.1158/1535-7163.MCT-07-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang S., Yang X., Morris M.E. Flavonoids are inhibitors of breast cancer resistance protein (ABCG2)-mediated transport. Mol. Pharmacol. 2004;65(5):1208–1216. doi: 10.1124/mol.65.5.1208. [DOI] [PubMed] [Google Scholar]
- 54.Seo H.S., Ku J.M., Choi H.S., Woo J.K., Lee B.H., Kim D.S., et al. Apigenin overcomes drug resistance by blocking the signal transducer and activator of transcription 3 signaling in breast cancer cells. Oncol. Rep. 2017;38(2):715–724. doi: 10.3892/or.2017.5752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li Y.-.W., Xu J., Zhu G.-.Y., Huang Z.-.J., Lu Y., Li X.-.Q., et al. Apigenin suppresses the stem cell-like properties of triple-negative breast cancer cells by inhibiting YAP/TAZ activity. Cell Death Discov. 2018;4(1):1–9. doi: 10.1038/s41420-018-0124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bagwe-Parab S., Kaur G., Buttar H.S., Singh Tuli H. Absorption, metabolism, and disposition of flavonoids and their role in the prevention of distinctive cancer types. Current Aspects of Flavonoids: Their Role in Cancer Treatment. 2019:125–137. [Google Scholar]
- 57.Kanha N., Surawang S., Pitchakarn P., Regenstein J.M., Laokuldilok T. Copigmentation of cyanidin 3-O-glucoside with phenolics: thermodynamic data and thermal stability. Food Biosci. 2019;30 [Google Scholar]
- 58.Pilon A.C., Gu H., Raftery D., VdS Bolzani, Lopes N.P., Castro-Gamboa I., et al. Mass spectral similarity networking and gas-phase fragmentation reactions in the structural analysis of flavonoid glycoconjugates. Anal. Chem. 2019;91(16):10413–10423. doi: 10.1021/acs.analchem.8b05479. [DOI] [PubMed] [Google Scholar]
- 59.Madala N.E., Piater L., Dubery I., Steenkamp P. Distribution patterns of flavonoids from three Momordica species by ultra-high performance liquid chromatography quadrupole time of flight mass spectrometry: a metabolomic profiling approach. Revista Brasileira de Farmacognosia. 2016;26:507–513. [Google Scholar]
- 60.Muñoz C., Sánchez-Sevilla J.F., Botella M.A., Hoffmann T., Schwab W., Valpuesta V. Polyphenol composition in the ripe fruits of Fragaria species and transcriptional analyses of key genes in the pathway. J. Agric. Food Chem. 2011;59(23):12598–12604. doi: 10.1021/jf203965j. [DOI] [PubMed] [Google Scholar]
- 61.Wong I.L., Chan K.-.F., Zhao Y., Chan T.H., Chow L.M. Quinacrine and a novel apigenin dimer can synergistically increase the pentamidine susceptibility of the protozoan parasite Leishmania. J. Antimicrobial Chemotherapy. 2009;63(6):1179–1190. doi: 10.1093/jac/dkp130. [DOI] [PubMed] [Google Scholar]
- 62.Mandery K., Bujok K., Schmidt I., Keiser M., Siegmund W., Balk B., et al. Influence of the flavonoids apigenin, kaempferol, and quercetin on the function of organic anion transporting polypeptides 1A2 and 2B1. Biochem. Pharmacol. 2010;80(11):1746–1753. doi: 10.1016/j.bcp.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 63.Handayani S., Hermawan A., Meiyanto E., Udin Z. Induction of Apoptosis on MCF-7 cells by Selaginella Fractions. J. Appl. Pharmaceutical Sci. 2013;3(4):31. [Google Scholar]
- 64.Seo Y.J., Kim B.S., Chun S.Y., Park Y.K., Kang K.S., Kwon T.G. Apoptotic effects of genistein, biochanin-A and apigenin on LNCaP and PC-3 cells by p21 through transcriptional inhibition of polo-like kinase-1. J. Korean Med. Sci. 2011;26(11):1489–1494. doi: 10.3346/jkms.2011.26.11.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Luo Y., Li B.-.Z., Liu D., Zhang L., Chen Y., Jia B., et al. Engineered biosynthesis of natural products in heterologous hosts. Chem. Soc. Rev. 2015;44(15):5265–5290. doi: 10.1039/c5cs00025d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gurung R.B., Kim E.-.H., Oh T.-.J., Sohng J.K. Enzymatic synthesis of apigenin glucosides by glucosyltransferase (YjiC) from Bacillus licheniformis DSM 13. Mol. Cells. 2013;36(4):355–361. doi: 10.1007/s10059-013-0164-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Seijas J.A., Vázquez-Tato M.P., Carballido-Reboredo R. Solvent-free synthesis of functionalized flavones under microwave irradiation. J. Org. Chem. 2005;70(7):2855–2858. doi: 10.1021/jo048685z. [DOI] [PubMed] [Google Scholar]
- 68.Verma A.K., Pratap R. Chemistry of biologically important flavones. Tetrahedron. 2012;68(41):8523–8538. [Google Scholar]
- 69.Chauthe S.K., Sharma R.J., Aqil F., Gupta R.C., Singh I.P. Quantitative NMR: an applicable method for quantitative analysis of medicinal plant extracts and herbal products. Phytochem. Anal. 2012;23(6):689–696. doi: 10.1002/pca.2375. [DOI] [PubMed] [Google Scholar]
- 70.S-m Ding, Z-h Zhang, Song J., X-d Cheng, Jiang J., X-b Jia. Enhanced bioavailability of apigenin via preparation of a carbon nanopowder solid dispersion. Int. J. Nanomed. 2014;9:2327. doi: 10.2147/IJN.S60938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hanske L., Loh G., Sczesny S., Blaut M., Braune A. The bioavailability of apigenin-7-glucoside is influenced by human intestinal microbiota in rats. J. Nutr. 2009;139(6):1095–1102. doi: 10.3945/jn.108.102814. [DOI] [PubMed] [Google Scholar]
- 72.Falé P.L., Ascensão L., Serralheiro M.L. Effect of luteolin and apigenin on rosmarinic acid bioavailability in Caco-2 cell monolayers. Food Funct. 2013;4(3):426–431. doi: 10.1039/c2fo30318c. [DOI] [PubMed] [Google Scholar]
- 73.Telange D.R., Patil A.T., Pethe A.M., Fegade H., Anand S., VS Dave. Formulation and characterization of an apigenin-phospholipid phytosome (APLC) for improved solubility, in vivo bioavailability, and antioxidant potential. Eur. J. Pharmaceutical Sci. 2017;108:36–49. doi: 10.1016/j.ejps.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 74.Rabiee M., Rabiee N., Salarian R., Rabiee G. Morgan & Claypool Publishers; 2019. Introduction to Nanomaterials in Medicine. [Google Scholar]
- 75.Kim B.-.K., Cho A.-.R., Park D-J. Enhancing oral bioavailability using preparations of apigenin-loaded W/O/W emulsions: in vitro and in vivo evaluations. Food Chem. 2016;206:85–91. doi: 10.1016/j.foodchem.2016.03.052. [DOI] [PubMed] [Google Scholar]
- 76.Huang Y., Zu Y., Zhao X., Wu M., Feng Z., Deng Y., et al. Preparation of inclusion complex of apigenin-hydroxypropyl-β-cyclodextrin by using supercritical antisolvent process for dissolution and bioavailability enhancement. Int. J. Pharm. 2016;511(2):921–930. doi: 10.1016/j.ijpharm.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 77.Jain A.K., Thanki K., Jain S. Co-encapsulation of tamoxifen and quercetin in polymeric nanoparticles: implications on oral bioavailability, antitumor efficacy, and drug-induced toxicity. Mol. Pharm. 2013;10(9):3459–3474. doi: 10.1021/mp400311j. [DOI] [PubMed] [Google Scholar]
- 78.Zhang Z., Cui C., Wei F., Lv H. Improved solubility and oral bioavailability of apigenin via Soluplus/Pluronic F127 binary mixed micelles system. Drug Dev. Ind. Pharm. 2017;43(8):1276–1282. doi: 10.1080/03639045.2017.1313857. [DOI] [PubMed] [Google Scholar]
- 79.Khan I., Saeed K., Khan I. Nanoparticles: properties, applications and toxicities. Arabian J. Chem. 2019;12(7):908–931. [Google Scholar]
- 80.Tavakol S., Zahmatkeshan M., Mohammadinejad M.R., Mehrzadi S., Joghataei M.T., Alavijeh M.S., et al. The role of nanotechnology in current COVID-19 outbreak. Heliyon. 2021:e06841. doi: 10.1016/j.heliyon.2021.e06841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.De Jong W.H., Borm P.J. Drug delivery and nanoparticles:applications and hazards. Int. J. Nanomed. 2008;3(2):133–149. doi: 10.2147/ijn.s596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Aiello P., Consalvi S., Poce G., Raguzzini A., Toti E., Palmery M., et al. Dietary flavonoids: nano delivery and nanoparticles for cancer therapy. Semin Cancer Biol. 2019;24(19) doi: 10.1016/j.semcancer.2019.08.029. 30217-2. [DOI] [PubMed] [Google Scholar]
- 83.Lambricht L., Peres C., Florindo H., Préat V., Vandermeulen G., Skwarczynski M., Toth I. William Andrew Publishing; 2017. Chapter Ten - Polymer-Based Nanoparticles as Modern Vaccine Delivery Systems; pp. 185–203. Micro and Nanotechnology in Vaccine Development. [Google Scholar]
- 84.Singh A.P., Biswas A., Shukla A., Maiti P. Targeted therapy in chronic diseases using nanomaterial-based drug delivery vehicles. Signal Transduction and Targeted Therapy. 2019;4(1):33. doi: 10.1038/s41392-019-0068-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Khan H., Ullah H., Martorell M., Valdes S.E., Belwal T., Tejada S., et al. Flavonoids nanoparticles in cancer: treatment, prevention and clinical prospects. Semin. Cancer Biol. 2019;30(19):30182–30188. doi: 10.1016/j.semcancer.2019.07.023. [DOI] [PubMed] [Google Scholar]
- 86.Huang Y., Zhao X., Zu Y., Wang L., Deng Y., Wu M., et al. Enhanced solubility and bioavailability of apigenin via preparation of solid dispersions of mesoporous silica nanoparticles. Iran J. Pharm. Res. 2019;18(1):168–182. [PMC free article] [PubMed] [Google Scholar]
- 87.Banerjee K., Banerjee S., Mandal M. Enhanced chemotherapeutic efficacy of apigenin liposomes in colorectal cancer based on flavone-membrane interactions. J. Colloid Interface Sci. 2017;491:98–110. doi: 10.1016/j.jcis.2016.12.025. [DOI] [PubMed] [Google Scholar]
- 88.Sen R., Ganguly S., Ganguly S., Debnath M.C., Chakraborty S., Mukherjee B., et al. Apigenin-loaded PLGA-DMSA nanoparticles: a novel strategy to treat melanoma lung metastasis. Mol. Pharm. 2021;18(5):1920–1938. doi: 10.1021/acs.molpharmaceut.0c00977. [DOI] [PubMed] [Google Scholar]
- 89.Guo D., Shen Y., Li W., Li Q., Zhao Y., Pan C., et al. 6-Bromoindirubin-3′-Oxime (6BIO) suppresses the mTOR pathway, promotes autophagy, and exerts anti-aging effects in rodent liver. Front. Pharmacol. 2019;10(320) doi: 10.3389/fphar.2019.00320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Leuner C., Dressman J. Improving drug solubility for oral delivery using solid dispersions. Eur. J. Pharmaceutics and Biopharmaceutics: Official J. Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik eV. 2000;50(1):47–60. doi: 10.1016/s0939-6411(00)00076-x. [DOI] [PubMed] [Google Scholar]
- 91.Xie J., Lee S., Chen X. Nanoparticle-based theranostic agents. Adv. Drug Deliv. Rev. 2010;62(11):1064–1079. doi: 10.1016/j.addr.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Karim R., Palazzo C., Laloy J., Delvigne A.-.S., Vanslambrouck S., Jerome C., et al. Development and evaluation of injectable nanosized drug delivery systems for apigenin. Int. J. Pharm. 2017;532(2):757–768. doi: 10.1016/j.ijpharm.2017.04.064. [DOI] [PubMed] [Google Scholar]
- 93.Fernandes F., Dias-Teixeira M., Delerue-Matos C., Grosso C. Critical review of lipid-based nanoparticles as carriers of neuroprotective drugs and extracts. Nanomaterials. 2021;11(3):563. doi: 10.3390/nano11030563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang J., Huang Y., Liu D., Gao Y., Qian S. Preparation of apigenin nanocrystals using supercritical antisolvent process for dissolution and bioavailability enhancement. Eur. J. Pharm. Sci. 2013;48(4–5):740–747. doi: 10.1016/j.ejps.2012.12.026. [DOI] [PubMed] [Google Scholar]
- 95.Altamimi M.A., Elzayat E.M., Alshehri S.M., Mohsin K., Ibrahim M.A., Al Meanazel O.T., et al. Utilizing spray drying technique to improve oral bioavailability of apigenin. Adv. Powder Technol. 2018;29(7):1676–1684. [Google Scholar]
- 96.Telange D., Patil A., Pethe A., Fegade H., Anand S., Dave V. Formulation and characterization of an apigenin-phospholipid phytosome (APLC) for improved solubility, in vivo bioavailability, and antioxidant potential. Eur. J. Pharmaceutical Sci. 2016;108 doi: 10.1016/j.ejps.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 97.Wu W., Zu Y., Wang L., Wang L., Wang H., Li Y., et al. Preparation, characterization and antitumor activity evaluation of apigenin nanoparticles by the liquid antisolvent precipitation technique. Drug Deliv. 2017;24:1713–1720. doi: 10.1080/10717544.2017.1399302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ding S.M., Zhang Z.H., Song J., Cheng X.D., Jiang J., Jia X.B. Enhanced bioavailability of apigenin via preparation of a carbon nanopowder solid dispersion. Int. J. Nanomed. 2014;9:2327–2333. doi: 10.2147/IJN.S60938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dutta D., Chakraborty A., Mukherjee B., Gupta S. Aptamer-conjugated apigenin nanoparticles to target colorectal carcinoma: a promising safe alternative of colorectal cancer chemotherapy. ACS Appl. Bio. Mater. 2018;1(5):1538–1556. doi: 10.1021/acsabm.8b00441. [DOI] [PubMed] [Google Scholar]
- 100.Kumar S., Gupta S.K. Pharmaceutical solid dispersion technology: a strategy to improve dissolution of poorly water-soluble drugs. Recent Pat. Drug Deliv. Formul. 2013;7(2):111–121. doi: 10.2174/18722113113079990009. [DOI] [PubMed] [Google Scholar]
- 101.Bharti C., Nagaich U., Pal A.K., Gulati N. Mesoporous silica nanoparticles in target drug delivery system: a review. Int. J. Pharm. Investig. 2015;5(3):124. doi: 10.4103/2230-973X.160844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Huang Y., Zhao X., Zu Y., Wang L., Deng Y., Wu M., et al. Enhanced solubility and bioavailability of apigenin via preparation of solid dispersions of mesoporous silica nanoparticles. Iranian J. Pharmaceutical Res. IJPR. 2019;18(1):168. [PMC free article] [PubMed] [Google Scholar]
- 103.Diniz I.M.A., Chen C., Xu X., Ansari S., Zadeh H.H., Marques M.M., et al. Pluronic F-127 hydrogel as a promising scaffold for encapsulation of dental-derived mesenchymal stem cells. J. Mater. Sci. Mater. Med. 2015;26(3):153. doi: 10.1007/s10856-015-5493-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Taha E.I., Badran M.M., El-Anazi M.H., Bayomi M.A., El-Bagory I.M. Role of Pluronic F127 micelles in enhancing ocular delivery of ciprofloxacin. J. Mol. Liq. 2014;199:251–256. [Google Scholar]
- 105.Chenthamara D., Subramaniam S., Ramakrishnan S.G., Krishnaswamy S., Essa M.M., Lin F.-.H., et al. Therapeutic efficacy of nanoparticles and routes of administration. Biomater. Res. 2019;23(1):20. doi: 10.1186/s40824-019-0166-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sharma S., Parmar A., Kori S., Sandhir R. PLGA-based nanoparticles: a new paradigm in biomedical applications. TrAC Trends in Analytical Chem. 2016;80:30–40. [Google Scholar]
- 107.Bhattacharya S., Mondal L., Mukherjee B., Dutta L., Ehsan I., Debnath M.C., et al. Apigenin loaded nanoparticle delayed development of hepatocellular carcinoma in rats. Nanomedicine. 2018;14(6):1905–1917. doi: 10.1016/j.nano.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 108.Pal M.K., Jaiswar S.P., Dwivedi A., Goyal S., Dwivedi V.N., Pathak A.K., et al. Synergistic effect of graphene oxide coated nanotised apigenin with paclitaxel (GO-NA/PTX): a ROS dependent mitochondrial mediated apoptosis in ovarian cancer. Anticancer Agents Med. Chem. 2017;17(12):1721–1732. doi: 10.2174/1871520617666170425094549. [DOI] [PubMed] [Google Scholar]
- 109.Das S., Das J., Samadder A., Paul A., Khuda-Bukhsh A.R. Strategic formulation of apigenin-loaded PLGA nanoparticles for intracellular trafficking, DNA targeting and improved therapeutic effects in skin melanoma in vitro. Toxicol. Lett. 2013;223(2):124–138. doi: 10.1016/j.toxlet.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 110.He X., Wen Y., Wang Q., Wang Y., Zhang G., Wu J., et al. Apigenin nanoparticle attenuates renal ischemia/reperfusion inflammatory injury by regulation of miR-140-5p/CXCL12/NF-κB signaling pathway. J. Biomed. Nanotechnol. 2021;17(1):64–77. doi: 10.1166/jbn.2021.3010. [DOI] [PubMed] [Google Scholar]
- 111.Drescher S., van Hoogevest P. The phospholipid research center: current research in phospholipids and their use in drug delivery. Pharmaceutics. 2020;12(12):1235. doi: 10.3390/pharmaceutics12121235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang Z., Tan S., Feng S.S. Vitamin E TPGS as a molecular biomaterial for drug delivery. Biomaterials. 2012;33(19):4889–4906. doi: 10.1016/j.biomaterials.2012.03.046. [DOI] [PubMed] [Google Scholar]
- 113.Jin X., Yang Q., Zhang Y. Synergistic apoptotic effects of apigenin TPGS liposomes and tyroservatide: implications for effective treatment of lung cancer. Int. J. Nanomed. 2017;12:5109–5118. doi: 10.2147/IJN.S140096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Li P., Bukhari S.N.A., Khan T., Chitti R., Bevoor D.B., Hiremath A.R., et al. Apigenin-loaded solid lipid nanoparticle attenuates diabetic nephropathy induced by Streptozotocin Nicotinamide through Nrf2/HO-1/NF-kB signalling pathway. Int. J. Nanomed. 2020;15:9115–9124. doi: 10.2147/IJN.S256494. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 115.Thomas N., Holm R., Rades T., Müllertz A. Characterising lipid lipolysis and its implication in lipid-based formulation development. AAPS J. 2012;14(4):860–871. doi: 10.1208/s12248-012-9398-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Xia F., Fan W., Jiang S., Ma Y., Lu Y., Qi J., et al. Size-dependent translocation of nanoemulsions via oral delivery. ACS Appl. Mater. Interfaces. 2017;9(26):21660–21672. doi: 10.1021/acsami.7b04916. [DOI] [PubMed] [Google Scholar]
- 117.Hu X., Zhang J., Yu Z., Xie Y., He H., Qi J., et al. Environment-responsive aza-BODIPY dyes quenching in water as potential probes to visualize the in vivo fate of lipid-based nanocarriers. Nanomed. Nanotechnol. Biol. Med. 2015;11(8):1939–1948. doi: 10.1016/j.nano.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 118.Wang C., Kar S., Lai X., Cai W., Arfuso F., Sethi G., et al. Triple negative breast cancer in Asia: an insider's view. Cancer Treat. Rev. 2018;62:29–38. doi: 10.1016/j.ctrv.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 119.Nabavi S.M., Habtemariam S., Daglia M., Nabavi S.F. Apigenin and breast cancers: from chemistry to medicine. Anticancer Agents Med. Chem. 2015;15(6):728–735. doi: 10.2174/1871520615666150304120643. [DOI] [PubMed] [Google Scholar]
- 120.Imran M., Aslam Gondal T., Atif M., Shahbaz M., Batool Qaisarani T., Hanif Mughal M., et al. Apigenin as an anticancer agent. Phytotherapy Res. 2020;34(8):1812–1828. doi: 10.1002/ptr.6647. [DOI] [PubMed] [Google Scholar]
- 121.Atanasov A.G., Zotchev S.B., Dirsch V.M., Supuran C.T. Natural products in drug discovery: advances and opportunities. Nat. Rev. Drug Discovery. 2021;20(3):200–216. doi: 10.1038/s41573-020-00114-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request