Abstract
Tertiary outpatient ophthalmology clinics are high-risk environments for COVID-19 transmission, especially retina clinics, where regular follow-up is needed for elderly patients with multiple comorbidities. Intravitreal injection therapy (IVT) for chronic macular diseases, is one of the most common procedures performed, associated with a significant burden of care because of the vigorous treatment regimen associated with multiple investigations. While minimizing the risk of COVID-19 infection transmission is a priority, this must be balanced against the continued provision of sight-saving ophthalmic care to patients at risk of permanent vision loss. This review aims to give evidence-based guidelines on managing IVT during the COVID-19 pandemic in common macular diseases such as age-related macular degeneration, diabetic macula edema and retinal vascular disease and to report on how the COVID-19 pandemic has affected IVT practices worldwide.
To illustrate some real-world examples, 18 participants in the International Retina Collaborative, from 15 countries and across four continents, were surveyed regarding pre- and during- COVID-19 pandemic IVT practices in tertiary ophthalmic centers. The majority of centers reported a reduction in the number of appointments to reduce the risk of the spread of COVID-19 with varying changes to their IVT regimen to treat various macula diseases. Due to the constantly evolving nature of the COVID-19 pandemic, and the uncertainty about the normal resumption of health services, we suggest that new solutions for eye healthcare provision, like telemedicine, may be adopted in the future when we consider new long-term adaptations required to cope with the COVID-19 pandemic.
Keywords: COVID-19, Intravitreal injections, Age-related macular degeneration, Diabetic macula edema, Practice patterns, Recommendations
Background
As the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-induced COVID-19 [1] emerged as a global pandemic with significant morbidity and mortality, massive disruptions in healthcare, financial, and social sectors have occurred [1]. To allow healthcare systems to adequately cope with COVID-19, governments around the world have placed strict measures in place to curb the spread of the disease.
Ophthalmologists are at particularly high risk due to their close proximity to patients during slit-lamp and indirect ophthalmoscope evaluations [2]. There is also a risk of virus transmission through aerosol contact with the conjunctiva and exposed mucous membranes [2]. Retinal providers and clinics face additional challenges in crowded clinics with predominantly elderly patients, who have multiple comorbidities, requiring multiple investigations and long-waiting times [3]. Furthermore, most of these patients have sight-blinding chronic diseases such as neovascular age-related macular degeneration (nAMD), diabetic macula edema (DME), and macular edema associated with retinal vascular occlusion (ME-RVO), necessitating frequent intravitreal injection therapy (IVT), imposing a substantial burden on physicians, staff, patients, and caregivers, even in routine care.
The COVID-19 pandemic imposes additional barriers to the management of retinal diseases, in terms of non-adherence to long-term treatment and follow-up regimens [4]. In addition, many health authorities and hospital management teams have mandated that, during this high-risk COVID-19 period only urgent and emergent care should be provided and that all routine clinical activity be deferred, to allow redirection of available resources to those at high risk for permanent visual loss [5, 6].
This study aims to summarize the literature, current guidelines, and evidence-based recommendations with regards to managing IVT during the COVID-19 pandemic and report on the effect of the COVID-19 pandemic on the visual outcomes, number of injections and adherence to follow up in IVT patients. In addition, we illustrate variability in changes to IVT practices in response to the COVID-19 pandemic in the early days from examples of tertiary ophthalmic centers worldwide and provide updated evidence on recommended best practices for IVT regimens and administration.
Methods
A comprehensive literature review was performed based on a search of previous published papers (including original articles, reviews, editorials) in English, relevant to medical retina management or IVT treatment during the COVID-19 pandemic (keywords: guidelines, COVID-19, SARS-CoV-2, intravitreal injections, medical retina, age-related macular degeneration, diabetic macula edema, retinal vein occlusion) up to 22nd April 2022, available on the PubMed database and included published guidelines from various professional ophthalmology societies (e.g. American Academy of Ophthalmology, Royal College of Ophthalmologists, United Kingdom, Canadian Retinal Society). Data were stored using Microsoft Excel (Microsoft, Redmond, WA), and absolute and relative (%) numbers are presented.
To illustrate real-world examples of the varied effect of the COVID-19 pandemic on routine IVT practice, 18 participants in the International Retina Collaborative, from 18 different cities in 15 countries and across four continents, were surveyed regarding pre- and during- COVID-19 pandemic IVT practices in tertiary ophthalmic centers (Tables 1, 2, 3). The responses were collected from 24th March to 22nd April 2020 (last response update). Participant agreement/consent was implied by completion or return of the questionnaire.
Table 1.
Summary of the global routine intravitreal injection therapy (IVT) practices during the pre-COVID-19 pandemic time
| Country (city/region) | Type of institution | Prior to intravitreal injections being administered | Intravitreal Injection procedure | |||||
|---|---|---|---|---|---|---|---|---|
| Imaging with OCT done at every visit | Ophthalmology consult performed at every visit | Setting where the majority of IVTs are performed | Skilled manpower used to administer IVT | Routine equipment used to administer IVT (Surgical mask, drape, gown, sterile gloves, speculum, iodine) |
Bilateral injections allowed on the same day | |||
| Asia and Pacific Region | ||||||||
| China (Guangzhou) | Tertiary stand-alone ophthalmology centre | Yes | Yes | Operating theatre | Senior/Junior Ophthalmologists | All | No | |
| Israel (Tel Aviv) | Ophthalmology department within General Hospital | Noa | Noa | Separate treatment room | Senior/Junior Ophthalmologists | All except gowns | Yes | |
| Malaysia (Kuala Lumpur) | Ophthalmology department within General Hospital | Yes | Yes | Separate treatment room | Junior ophthalmologists | All | Yes | |
| Singapore | Tertiary stand-alone ophthalmology centre | Noa | Noa | Separate treatment room | Senior/Junior Ophthalmologist, Specialised nurses | All except gowns | Yes | |
| Thailand (Bangkok) | Ophthalmology department within General Hospital | Noa | Noa | Separate treatment room or stand-alone IVT clinics | Senior/Junior Ophthalmologists/Residents | All except gowns | Yes | |
| Europe | ||||||||
| France (Paris) | Ophthalmology department within General Hospital | Yes | Yes | Separate treatment room | Junior ophthalmologist | All | Yes | |
| Germany (Berlin) | Tertiary stand-alone ophthalmology centre | Noa | Noa | Operating theatre | Senior ophthalmologist | All except gowns | No | |
| Germany (Munich) | Tertiary stand-alone ophthalmology centre | Yes | Yes | Separate treatment room/ Operating theatre | Junior/Senior ophthalmologist | All except gowns | Yes | |
| Greece (Athens) | Ophthalmology department part of General Hospital | Yes | Yes | Separate treatment room | Senior/Junior Ophthalmologists | All except gowns | Yes | |
| Italy (Rome) | Ophthalmology department part of General Hospital | No (only after 3 loading doses) | No (only after 3 loading doses) | Separate treatment room / Operating theatre | Senior ophthalmologist | All except gown (drape recommended) | No | |
| Italy(Milan) | Ophthalmology department part of General Hospital | Noa | Noa | Separate treatment room/Operating theatre | Senior/Junior Ophthalmologists | All | No | |
| Switzerland (Lucerne) | Ophthalmology department part of General Hospital | No | No (only at fixed time points) | Operating theatre | Junior Ophthalmologist, Specialised nurses | All | Yes | |
| United Kingdom(London) | Tertiary stand-alone ophthalmology centre | Yes | Yes | Separate treatment room | Senior/Junior Ophthalmologist, Specialised nurses | All | Yes | |
| United Kingdom (Wales) | Ophthalmology department part of General Hospital | Yes | Yes (most done virtually) | Within the outpatient clinic | Senior/Junior Ophthalmologist, Specialised nurses | All (drape and masks only recommended) | Yes | |
| North and South America | ||||||||
| Brazil (Recife) | Tertiary stand-alone ophthalmology centre | Yes | Yes | Operating theatre | Senior/Junior Ophthalmologist | All | Yes | |
| Canada (Vancouver) | Tertiary stand-alone ophthalmology centre | Yes | Yes | Within the outpatient clinic | Senior Ophthalmologist | Iodine only (some substitute chlorhexidine for iodine), speculum optional | No | |
| Colombia (Cali) | Tertiary stand-alone ophthalmology centre | Yes | Yes | Within the outpatient clinic | Senior/Junior Ophthalmologist | All | Yes | |
| United States of America (Chicago) | Ophthalmology department part of General Hospital | Yes | Yes | Within the outpatient clinic | Senior ophthalmologist | All except gown | Yes (rarely) | |
aIVT also administered in injection only clinics/appointments with no imaging or ophthalmologist consult
Table 2.
The global timeline of when changes to intravitreal injection therapy (IVT) practices were instituted during the COVID-19 pandemic, in the context of the magnitude of the COVID-19 problem in various countries
| Country (city) | Estimated date the changes started | Number of cases of COVID-19 in the country on that datea | Other restrictions within the country at that date | Main reasons for the change in practice | Changes in practice patterns during the COVID-19 pandemic with regards to various chronic macula diseases receiving IVT | ||
|---|---|---|---|---|---|---|---|
| nAMD | DME | ME-RVO | |||||
| Asia and Pacific Region | |||||||
| China (Guangzhou) | 1/2/2020 | 14,380 | Travel ban, lockdown | High risk of hospital transmitted infections | All IVT postponed in February, given in March | All IVT postponed in February, given in March | All IVT postponed in February, given in March |
| Israel (Tel Aviv) | 17/3/2020 | 337 | Travel ban, close borders, lockdown | High risk of hospital transmitted infections | No IVT injections postponed, some loading doses could be extended | No IVT injections postponed, some loading doses could be extended | No IVT injections postponed, some loading doses could be extended |
| Malaysia (Kuala Lumpur) | 18/3/2020 | 790 | Travel ban, lockdown | High risk of hospital transmitted infections | All IVT postponed with exceptions | All IVT postponed with exceptions | All IVT postponed with exceptions |
| Singapore | 7/4/2020 | 1418 | Travel ban, close borders, partial lockdown | High risk of hospital transmitted infections | All IVT postponed for 4 weeks except patients with only 1 seeing eye can receive IVT | All IVT postponed for 4 weeks exceptions based on clinician discretion | All IVT postponed for 4 weeks exceptions based on clinician discretion |
| Thailand (Bangkok) | 23/3/2020 | 721 | Travel ban, partial lockdown | High risk of hospital transmitted infection, Lack of resources | Some IVT postponed except those based on individual clinician’s discretion | All IVT postponed for 2–3 months | Some IVT injections postponed based on individual clinician’s discretion |
| Europe | |||||||
| France (Paris) | 16/3/2020 | 6663 | Complete lockdown, travel ban | High risk of hospital transmitted infections | No IVT injections postponed | All postponed for 2/3 months except in single eye patients or threatening situations | All postponed for 2/3 months except in single eye patients or threatening situations |
| Germany (Berlin) | 23/3/2020 | 29056 | Travel ban Reduce close contacts, schools closed | High risk of hospital transmitted infection | No IVT injections postponed | No IVT injections postponed | No IVT injections postponed |
| Germany (Munich) | 16/3/2020 | 7272 | Travel ban, Reduce close contacts, schools closed | High risk of hospital transmitted infection | No IVT injections postponed | No IVT injections postponed | No IVT injections postponed |
| Greece (Athens) | 17/3/2020 | 387 | Travel ban, Lockdown, schools closed | High risk of hospital transmitted infection | All IVT injections postponed, exceptions allowed based on clinician’s discretion | All IVT injections postponed | All IVT injections postponed, exceptions allowed based on clinician’s discretion |
| Italy (Rome) | 16/3/2020 | 27980 | Travel ban, Lockdown, Close borders | Hospital policy, High risk of hospital transmitted infection | All IVT postponed except patients with only 1 seeing eye can receive IVT | All IVT postponed | All IVT postponed |
| Italy (Milan) | 9/3/2020 | 9172 | Travel ban, Lockdown, Close borders | High risk of hospital transmitted infection | All IVT postponed except patients with only 1 seeing eye can receive IVT | All IVT postponed | All IVT postponed except patients with neovascular glaucoma |
| Switzerland (Lucerne) | 16/3/2020 | 2353 | Travel ban, Lockdown, Close borders | High risk of hospital transmitted infection | No IVT injections postponed | No IVT injections postponed | No IVT injections postponed |
| United Kingdom (London) | 19/3/2020 | 3269 | Travel ban, Lockdown, Close borders | High risk of hospital transmitted infection | No IVT injections postponed but to continue on a fixed treatment regiment | All IVT postponed for 6 months | All IVT postponed for 6 months for BRVO, IVT given to CRVO based on clinician discretion |
| United Kingdom (Wales) | 30/3/2020 | 22,141 | Travel ban, Lockdown, Close borders | High risk of hospital transmitted infection | No injections postponed – Extended by 4 weeks rather than 2 where needed | No injections postponed – Extended by 4 weeks rather than 2 where needed | No injections postponed – Extended by 4 weeks rather than 2 where needed |
| North and South America | |||||||
| Brazil (Recife) | 20/3/2020 | 640 | Travel ban, quarantine | High risk of hospital transmitted infectionFlatten the curve | IVT injections postponed in elderly and high-risk patients if vision and OCT were stable on last visit | Some IVT injections postponed in elderly and high-risk patients if vision and OCT were stable on last visit | Some IVT injections postponed in elderly and high-risk patients if vision and OCT were stable on last visit |
| Canada (Vancouver) | 20/3/2020 | 1067 | Travel Ban, State of Emergency, Close borders | High risk of hospital transmitted infection | Some IVT injections postponed for 3-month stable patients | All IVT injections postponed | Some IVT injections postponed for 3-month stable patients |
| Colombia (Cali) | 24/3/2020 | 378 | Travel Ban, State of Emergency, Lockdown, Close borders | Hospital policy, High risk of hospital transmitted infection | All IVT injections postponed for at least 1 month, exceptions allowed based on clinician’s discretion | All IVT injections postponed for at least 1 month, exceptions allowed based on clinician’s discretion | All IVT injections postponed for at least 1 month, exceptions allowed based on clinician’s discretion |
| United States of America (Chicago) | 16/3/2020 | 4596 | Travel restriction, lockdown |
High risk of hospital transmitted infection Limited manpower |
Some IVT postponed for patient with long IVT intervals, patients with shorter IVT maintained according to clinician’s discretion | Some IVT postponed for patient with long IVT intervals, patients with shorter IVT maintained according to clinician’s discretion | Some IVT postponed for patient with long IVT intervals, patients with shorter IVT maintained according to clinician’s discretion |
nAMD: neovascular AMD, DME: Diabetic macula edema, ME-RVO: macula edema related to retinal vein occlusion, BRVO: branch retinal vein occlusion, CRVO: central retinal vein occlusion
aData obtained from ref 73: https://www.worldometers.info/coronavirus/ unless specified otherwise
Table 3.
Summary of changes to logistics and procedural practices of intravitreal injection therapy (IVT) during the COVID-19 pandemic time
| Country (city/region) | Prior to intravitreal injections being administered | Intravitreal Injection procedure | |||||
|---|---|---|---|---|---|---|---|
| Screening for high risk COVID patients performed (temperature screen, symptoms, travel history) | High-risk COVID patients allowed to the specialist outpatient clinic for IVT | Changes to performing OCT imaging | Changes to performing ophthalmology consult | Changes to the setting where the majority of IVTs was performed | Changes to the skilled manpower used to administer IVT | Changes to the IVT administration procedure or equipment | |
| Asia- Pacific | |||||||
| China(Guangzhou) | Yes | No | No | No | Yes, reduced numbers, social distancing, reduced follow up appointments | No | No |
| Israel(Tel Aviv) | Yes | No, deferred for 2 weeks | Yes, reduced OCT performed | Yes, reduced VA, slit-lamp exam | Yes, Reduced numbers and social distancingAn additional injection clinic opened outside the hospitalHome injections in selected cases | No | Yes, face shield and gown worn. N95 mask was available at physician’s discretion |
| Malaysia (Kuala Lumpur) | Yes | No, deferred | No | No | Yes, reduced numbers, social distancing, reduced follow up appointments | No | Yes, face shield was worn |
| Singapore | Yes | No, deferred | Yes, reduced OCT performed | Yes, reduced VA, slit-lamp exam | Yes, reduced numbers, reduced time in clinic, social distancing | No | Yes, goggles of face-shield recommended |
| Thailand (Bangkok) | Yes | Yes, if no fever detected | No | No | Yes, IVT clinic/OT stopped only IVT in the treatment room | No | No |
| Europe | |||||||
| France(Paris) | Yes | Yes | Yes, reduced OCT performed (no OCT in patients with known interval) | Yes, no slit lamp exam in patients with known interval | Yes, reduced numbers, reduced time in clinic, social distancing | No | No |
| Germany (Berlin) | Not formally | Yes, obviously sick patients asked to return later | No | Yes, telephone consults for patients instead of routine follow up examination | Yes, reduced numbers | No | No |
| Germany(Munich) | Yes | Yes, high risk cases screened in isolation | No | Yes, only VA, IOP, OCT taken no slit lamp exam | Yes, reduced numbers | No | No |
| Greece(Athens) | Yes | No | Yes, reduced OCT performed | Yes, reduced VA and slit-lamp exam | Yes, reduced numbers, social distancing | No | No |
| Italy(Rome) | Yes | No, deferred for 2 weeks | No | No | Yes, reduced numbers, social distancing | No | Yes, face shields worn by all staff |
| Italy(Milan) | Yes | Yes, high risk cases screened in isolation | No | Yes, telephone consults for symptoms screening | Yes, no injections in OT all IVT done in small procedures room | Yes, more senior ophthalmologists performing IVT as junior staff are deployed elsewhere | Yes, face shields worn by all staff |
| Switzerland (Lucerne) | No | Yes | Yes, reduced OCT, done only in treatment naïve patients and those patients with significant vision loss | Yes, no routine VA, IOP and slit lamp examination telephone consults done | Yes, reduced numbers, waiting time, social distancing | No | No |
| United Kingdom(London) | Yes | No, deferred for 2 weeks | Yes, reduced OCT performed | Yes, no routine VA, IOP or slit lamp exam performed | Yes, reduced numbers, waiting time, social distancing | No | No |
| United Kingdom (Wales) | Yes (department dependent) | No, deferred for 2 weeks | Yes, reduced OCT performed | Yes, no routine VA, IOP or slit lamp exam performed, virtual consults continue | Yes, reduced numbers, waiting time, social distancing | No | Yes, surgical mask strongly recommended |
| North and South America | |||||||
| Brazil(Recife) | Yes | No | No | Yes, included virtual consultations | Yes, reduced numbers, waiting time, social distancing | No | No |
| Canada(Vancouver) | No | Yes | No | Yes, DME and RVO patients contacted by telephone | No | No | Yes, gloves, goggles and masks for all staff, masks for any sick patients |
| Colombia(Cali) | Yes | No | Yes, reduced OCT performed | Yes, no pinhole or IOP, virtual consults where possible | No | No | Yes, face shield and gown worn. N95 mask was available at physician’s discretion |
| United States of America (Chicago) | Yes | Yes, high risk cases screened in isolation | Yes, only basic OCT allowed | No | Yes, reduced numbers, waiting time, social distancing | No | Yes |
VA: visual acuity, IOP: intraocular pressure, OCT: optical coherence tomography, DME: diabetic macula edema, RVO: retinal vein occlusions
Results
Global pre-pandemic routine IVT practice and the effects of the COVID-19 pandemic on the IVT practices surveyed.
Routine clinical examination, investigations and IVT procedures performed pre-pandemic were summarized in Table 1. The approximate dates in which changes to the IVT practices occurred in respective countries are summarized in Table 2 and Fig. 1. The changes to the appointments with regards to diagnosis (nAMD, DME and ME-RVO), changes to clinical assessments and PPE use in various centers is summarized in Table 3. Factors that were likely to influence the decision to implement changes in IVT practice included: the date when the first case of COVID-19 was detected, the rate of COVID-19 infection in the community, the speed of response of the respective governments in implementing lockdown policies, and the availability of the healthcare resources (Table 2). The most common reasons cited for implementing these changes included the high risk of COVID-19 transmission and the need to comply with hospital policies. Less common reasons included a lack of manpower and resources.
Fig. 1.
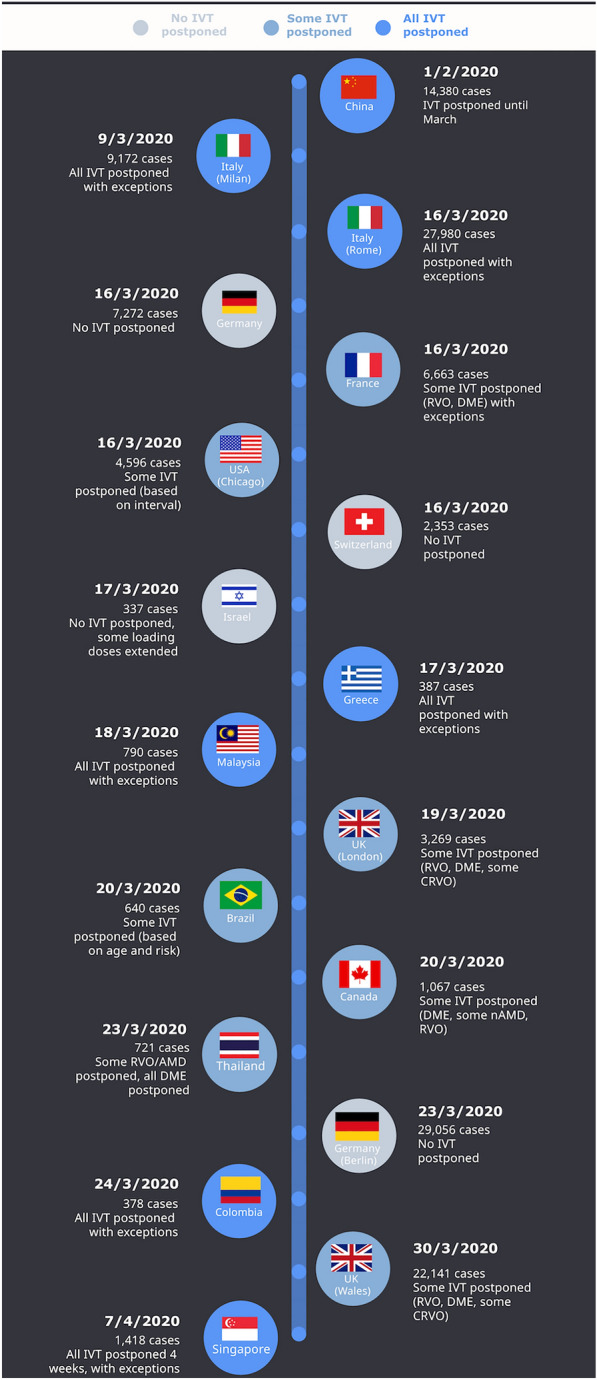
Timeline of the dates when changes to IVT practice occurred in the different centers surveyed and the number of COVID-19 cases on that date
Discussion
The multiple tertiary ophthalmic centers around the world included in our study reported varying responses to changes in their IVT practice in response to the COVID-19 pandemic, with the majority of centers reducing the number of appointments to reduce the risk of the spread of COVID-19 among staff and patients. Most centers reported having routine screening for high risk COVID-19 patients and about half of the tertiary centers reported the additional use of PPE for IVT procedures (in most of the other centers full PPE was already worn pre-COVID-19 pandemic).
The recommended best practice guidelines for IVT during the COVID-19 pandemic.
The main guiding principles of planning IVT treatment in times of the COVID-19 pandemic include (1) minimizing the risk of COVID-19 infection between healthcare workers and patients; (2) continuing to provide IVT to patients to prevent permanent vision loss from the progression of their chronic macular disease [3, 7]. In addition, these decisions should be made in the context of other factors such as the number of COVID-19 cases within the country, the risk of COVID-19 transmission, the availability of healthcare resources, and government policies.
A recent paper by the Vision Academy Steering Committee outlined various guidelines on the treatment regimens for various common macular diseases requiring IVT, during the time when the COVID-19 pandemic began [5, 7]. External factors, such as the strain on the healthcare system caused by the pandemic, government-imposed restrictions, and the need to reduce the risk of virus transmission all led to global recommendations in IVT delivery: the number of visits should be kept to a minimum, the time within visits shortened, exposure should be minimized to the lowest number of the staff, and priority should be given to patients at greatest risk of vision loss [2, 7]. One strategy proposed in some of our surveyed centers and in previous studies is having two types of appointments: (1) an assessment appointment performed at baseline, after the 3rd injection of the anti-VEGF loading-dose, at regular intervals after, and at physician discretion in case of reported vision loss, consisting in a VA assessment, slit-lamp examination, and OCT and (2) an injection-only appointment, where IVT only is performed without any eye assessments [3, 7].
A proactive T&E regimen is ideal during the COVID-19 pandemic, as it reduces the number of visits and injections while maintaining visual outcomes [8]. However, a disadvantage of T&E is that the decision about the next treatment interval is made based on VA measurement and OCT findings, which need to be done at every visit, adding to the time spent in the clinic and increased close contact with patients [9]. Table 4 summarizes the benefits, risks, and recommendations for each routine assessment procedures done prior to IVT. Newer intravitreal drugs or drug delivery systems such as faricimab and port-delivery systems that are being currently developed aims to increased injection intervals with the potential to further reduce the number of clinic visits [10–12].
Table 4.
Benefits, risks and recommendations for assessment procedures done prior to administering intravitreal injection treatment
| Procedure | Benefits | Risks during COVID-19 pandemic | Risk of deferring procedure | Situations where the procedure is indicated | Situations where the procedure can be deferred | Modifications to the procedure during COVID-19 pandemic |
|---|---|---|---|---|---|---|
| VA testing |
Widely accepted functional visual assessment Can be used to determine T&E decisions |
Increasing contact time with patient and staff |
Patients may not report vision loss Visual outcomes less closely monitored |
Treatment naïve patients Patients who complain of visual loss |
Patients receiving loading doses Long-term patients with stable disease |
Take VA starting from smallest letter and work upwards to save time Pinhole vision may not be necessary |
| IOP measurement | Monitor glaucoma risk in IVT patients |
Increased contact time with patient and staff Aerosolized droplets from non-contact/pneumatic tonometry |
Undetected IOP rise |
High risk glaucoma patients Cupped disc Post intravitreal steroid injection for the first time |
Routine follow up No history of glaucoma or disc cupping Already has separate glaucoma follow-up appointment |
Suspend the use of non-contact tonometry, use Goldmann applanation or I-care tonometry |
| Pupil dilation | Allows the examination of the peripheral retina | Increased contact time with patient and staff; spread of COVID-19 from contaminated eye drops | Risk of missing retinal pathology |
Treatment naive High myopia Extra-foveal disease Visual field loss |
Long-term patients with stable disease | Dilation eye drops should be administered only once on arrival, if needed patient can be given disposable minims of eye drops for repeated administration |
| OCT |
Objective structural assessment of active disease Can be used to determine T&E decisions |
Increased contact time with staff |
Undetected Worsening disease activity Early recurrence with no VA loss not detected Missed screening of fellow eye |
Treatment naïve 4 weeks after 3rd loading dose |
Patients receiving loading doses Long-term patients with stable disease Known maximum treatment interval |
Plastic shield in machines where patient faces the technician Keep scanning protocol to a minimum Decentralise imaging service |
| Slit-lamp examination |
Detection on non-retinal pathology Assessment of the retinal periphery Detection of new areas of bleeding |
Increased close contact with staff |
Undetected Non-retinal pathology and peripheral retinal pathology Undetected new retinal hemorrhages or rubeosis |
Treatment naïve cases Patients with worsening visual acuity |
Patients receiving loading doses Long-term patients with stable disease |
Plastic shield in machines where patient faces the doctor N95 masks and goggles for high risk patients |
| Ophthalmology consultation |
Direct reporting of symptoms Patient doctor rapport |
Increased prolonged close contact with doctor | Undetected pathology not picked up by imaging | Treatment naïve cases |
Patients receiving loading doses Long-term patients with stable disease |
To be replaced by telephone or video consultation |
VA: visual acuity, IOP: intra-ocular pressure, OCT: optical coherence tomography, loading doses refer to intravitreal anti-VEGF therapy
Neovascular age-related macular degeneration
Multiple lines of evidence recommend that patients with nAMD in their first two years of treatment should be prioritised [5, 7]. Previous studies on the natural history of nAMD show that delaying IVT treatment results in vision loss(control arm of MARINA and ANCHOR) [13] and quarterly IVT after the 3 monthly loading doses anti-VEGF loading doses has inferior visual outcomes compared to monthly treatment (PIER and EXCITE study). Hence, an intensive treatment regimen for nAMD should be recommended from a vision standpoint in the treatment and consent discussions during the COVID-19 pandemic, despite the risk of being infected. For treatment naïve patients, OCT and/or OCT angiography (OCTA) should be preferred for confirming the diagnosis in place of dye angiography, which is time-consuming and requires increased person-to-person contact [7]. In nAMD patients, a modified T&E approach mixed with fixed dosing interval has been proposed to minimize the need for VA, OCT, and slit-lamp examination at every visit, while avoiding the risk of under-treatment [3]. An example of this was the TriPla regimen was proposed, which was a hybrid of fixed dosing and T&E, with an aim to still provide an individualized approach but minimizing the number of examinations and risk of COVID-19 exposure [15]. The ALTAIR study showed that increasing intervals by 4 weeks in a T&E regimen with aflibercept carried no differences in the visual outcomes or the number of injections compared to the traditionally adopted 2-week extension. Results from the FLUID study also showed that visual outcomes are comparable using a relaxed approach in patients versus a strict no tolerance to subretinal fluid approach. This meant that IVT interval for patients with stable VA and minimal stable subretinal fluid could continue to be extended as long as they did not deteriorate [14]. These added treatment strategies may also help in reducing the number of follow-up visits.
Diabetic macular edema and diabetic retinopathy
Diabetic patients are at higher risk of COVID-19 complications; therefore, extra care should be provided to these patients to minimize the risk of infection. General recommendations for DME management are to defer all IVT treatments and follow-up unless the patient is monocular, has significant vision loss from DME, or has severe non-proliferative or proliferative diabetic retinopathy (in this case, pan-retinal photocoagulation should be considered) [5, 7]. Previous studies have shown that the long-term risk of vision loss in DME patients is lower than in nAMD (control/laser arms of RISE, RIDE, RESTORE, VIVID, VISTA) [16]. In treatment naïve patients, a delay in anti-VEGF IVT treatment may result in a higher risk of suboptimal long-term visual outcomes (crossover arms RISE, RIDE,VIVID, and VISTA, RESTORE extension study). Hence, for both treatment naïve and DME patient on treatment, guidelines state that follow-up appointments should be deferred, but should not be postponed for more than 4–6 months as this could lead to irreversible vision loss [7, 17]. When treatment is initiated, 6 monthly loading anti-VEGF injection doses (as recommended by the DRCRnet: Protocol T) can be performed as an injection-only appointment to reduce time spent in the clinic. Sustained-release intravitreal corticosteroid implants can also be considered as an alternative therapy in suitable patients to adequately treat DME and reduce the number of injections and follow-up visits, however additional visits for intra-ocular pressure checks may be required in higher risk cases.
Macular edema related to retinal vein occlusion
Similar to DME, natural history studies show the risk of long-term vision loss from ME-RVO is low (control arms-VIBRANT, CRUISE, CORPENICUS, and GALILEO). Nevertheless, macular edema associated with central retinal vein occlusion (ME-CRVO) can be associated with a higher risk of suboptimal long-term visual outcomes in case of significant delay in anti-VEGF IVT treatment (crossover arms, CRUISE, COPERNICUS, and GALILEO). Recommendations for macular edema associated with branch retinal vein occlusion (ME-BRVO) is to defer all IVT treatments. Intensive monthly IVT anti-VEGF loading doses (done as injection only appointments) are recommended for the treatment naïve ME-CRVO [7]. In patients with ME-CRVO treated with monthly bevacizumab or ranibizumab, that have persistent activity or have recurrences, when monthly intervals are extended past 4 weeks, a switch to aflibercept or the dexamethasone implant may allow increased treatment intervals (NEWTON, SCORE 2).
Reducing the risk of COVID-19 transmission within the IVT clinic
Recommendations to reduce the risk of COVID-19 transmission within the clinic include well-organized efforts to reschedule appointments for non-urgent patients, by giving them clear advice to postpone their visits and to contact the hospital only if their condition deteriorates or they require a prescription for drug-refill [2, 7]. Increased manpower should be provided for walk-in or emergency services, to address a potential rise in patients whose appointments have been rescheduled. Clear communications on public health recommendations should be given to patients before they attend the clinic, which include limiting accompanying persons, social distancing, hand hygiene, and wearing masks at all times (Fig. 2) [2, 7]. As countries start to relax confinement measures, patients will need to be continually reminded of the importance of maintaining a high degree of vigilance and compliance to all the public health recommendations specified above while within hospitals and clinics.
Fig. 2.

An example of a pre-screening counter for COVID-19 located at the entrance of the tertiary center (top left image), a government supported digital application (top right image) is used to record the patients entry details, symptoms, previous exposure to Covid-19 and travel history (also used for contact tracing if needed), an automatic thermal scanner (top right image) to detect patients with a fever as they enter the center. Examples of signs on clinic waiting room seats used to encourage social distancing (left image) and an example of patients in the waiting room of the clinic (right image) and staff wearing surgical masks and practicing social distancing
PPE is extremely important to prevent COVID-19 transmission, and it is recommended that at the very least surgical masks are worn by staff, patients, and caregivers [2, 7] (Fig. 3). Routine screening for respiratory symptoms, travels, or previous COVID-19 contact history, 2–14 days prior to the clinic visit, and temperature checks on arrival, have been recommended for all patients and caregivers before entering the eye clinic (Fig. 2) [2]. Some studies have suggested an increased risk of endophthalmitis associated with surgical masks worn by patients [18, 19], however a large multi-center study, showed no difference in the culture positive endophthalmitis rates between cohorts with no masks and those where both patients and physicians wore masks [20]. Prolonged mask wear of more than 4 h was also suggested as having a higher bacterial load that can be reduced with povidone iodine administration [21]. Taping of the top of masks or using a sealed drape before IVT administration has also been suggested as another alternative to decrease aerosolized particles from the patient’s mouth that may carry oral pathogens [22].
Fig. 3.
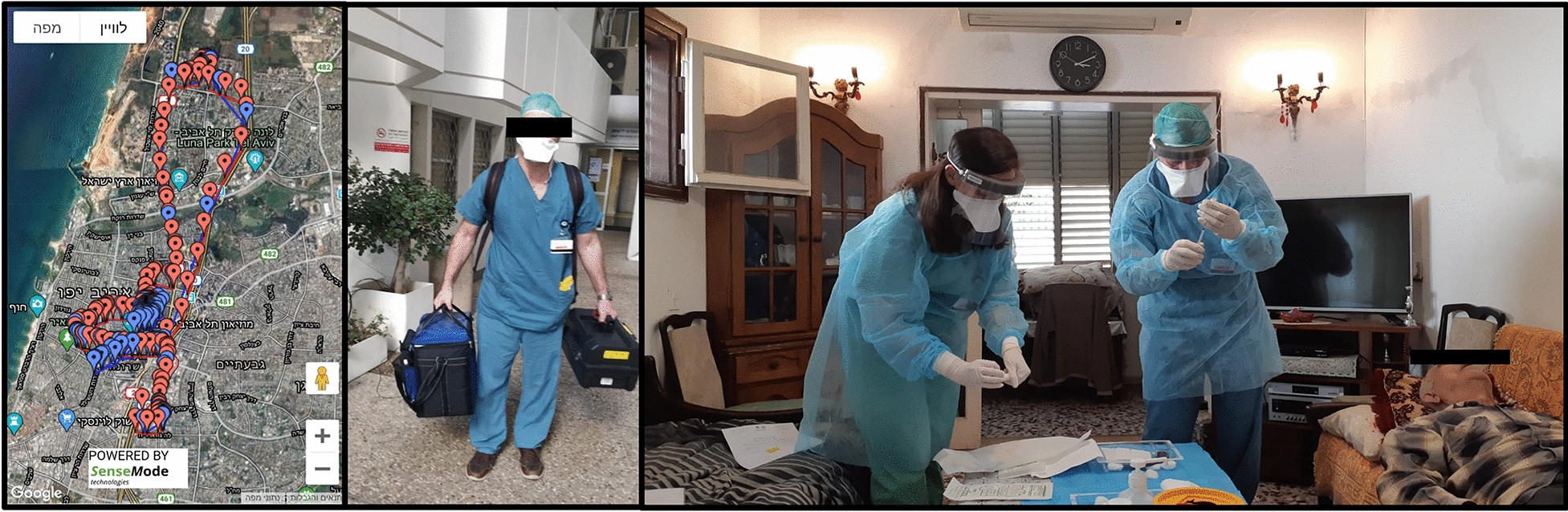
Decentralized home intravitreal therapy (IVT) service shown by the map illustrating the planned route of the home IVT service (left image), medical staff carrying the portable home IVT equipment (middle image) and medical team administering IVT to a patient at his home (left image
In general, all assessment procedures should be kept to a minimum and deferred where possible (Table 4). Suggested modifications to respective procedures are outlined to minimize the total time taken and contact with staff which include adequate social distance, with a clear outlined delineation between surfaces in contact with the patient and staff. A recent study described the development of a new intravitreal injection center based on “LEAN” principles (creating more value for customers with fewer resources, minimizing waste), resulting in better quality and efficiency, speed of the overall procedures and clinical capacity of the IVT service, with an aim to reduce the risk of COVID-19 transmission [23]. Recently revised recommendations released by the Vision Academy Steering Committee, included guidelines on IVT treatment based on the local epidemic pressure, to ensure the safety of patients and staff and the sustainability of healthcare resources, in era of easing COVID-19 measures leading to a resurgence of disease in many areas [17]. Apart from local epidemic pressure, the vaccination rates should also be considered when assessing the risk of COVID-19 transmission during IVT [17]. In particular, due to the long lasting effect of endemic COVID-19, there was an emphasis on maintaining treatment intervals wherever possible to avoid risk of permanent visual changes especially in patients with DME and BRVO who have had their treatment postponed for more than 6 months during the initial wave of the COVID-19 pandemic [17].
There also should be a shift towards telemedicine, with models of care such as virtual clinics, where clinical decisions are based on imaging such as color fundus photography and OCT. Patients are then contacted remotely and their management plan conveyed through phone, messaging service, or video consultation [17, 24–26]. In further efforts to reduce crowding in the tertiary centers, decentralization of services into the community, such as primary eye care centers, imaging centers, satellite clinics, and even home intravitreal services can be considered (Fig. 3) [24, 27]. The COVID-19 pandemic presents a unique opportunity to incentivize governments and insurance companies to provide healthcare remuneration for new services and initiatives [24].
The effect of COVID-19 on IVT adherence rates and visual acuity outcomes of patients receiving IVT
The added challenges during the COVID-19 pandemic, such as the fear of visiting hospitals for appointments, difficulties with accessing healthcare, rescheduling missed appointments, and the reduced patient capacity of hospitals and eye clinics to maintain adequate social distancing may increase the risk of non-adherence to IVT. Overall, numerous studies worldwide report the adherence rates for IVT being reduced significantly during the COVID-19 pandemic [28–31]. One Italian study reported better adherence rates associated with younger patients, worser vision in fellow eye and during period of no lockdown [28]. A German study reported that during the first wave [32]of the pandemic, risk factors for poor adherence included low VA of the treated eye, high VA of the untreated eye, COVID-19 in the family and DME [31].
A French study also reported, that during lockdown, there was a relatively marked decrease in IVT procedures that did not return to pre-lockdown levels despite subsequent opening up [30]. Even though overall IVT numbers have decreased during the pandemic and immediately post-pandemic, it will be inevitable in the endemic COVID-19 era, that there will be a “rebound” number of patients who will need IVT treatment, that may have a delayed presentation with more advanced disease [33].
Fight Retinal Blindness Registry is a large international data base that published data from 8 countries showed that 6 month drop-out rates were higher for ME-RVO (28%), DME (27%) and lower for AMD (20%) [34]. Eyes with AMD loss more vision in proportion with the number of injections than eyes with DME or ME-RVO [34]. Other studies have also reported significant short term and long term vision loss in all patients receiving IVT [35], especially AMD patients who have had lapses in treatment due to COVID-19 [29, 32, 36]. Interestingly, one study reported that AMD eyes with active disease, with a high injection demand (intervals less than 6 weeks) were able to be extended to 10–12 weeks with stable VA, however when intervals were extended to more than 12 weeks there was a risk short term vision loss [37]. A Chinese study, reported that patients on the T&E regiment versus those on pro nata (prn) regimen showed better visual outcomes when their therapy was halted during COVID-19 especially in eyes with Type 1 neovascularisation [38]. Devastating VA outcomes due to submacular hemorrhages in AMD eyes have also been reported when IVT treatment was delayed due to COVID-19 [39, 40].
In contrast to the current UK guidelines, recommending delaying all ME-RVO injections [5], one UK-based study showed that when IVT was delayed and then restarted, more DME eyes were able to regain vision, however VA in nAMD and ME-RVO eyes were less likely to return to baseline [41]. Another study examining the short- and long-term effects of delayed IVT of more than 8 weeks, showed that in the short-term vision loss was more marked in the DR and CRVO eyes compared to nAMD, while long-term vision loss was more commonly observed in CRVO and nAMD eyes, with BRVO patients least effected by the IVT delay [42].
Patient adherence in this setting may be improved through other solutions that include digital interactive education programs, digital home monitoring programs [43], a hotline that gives direct access to doctors or nurses counselors, an online appointment scheduling service and private video consultation services [7].
Conclusion
In this review, we summarize the current IVT recommendations during the COVID-19 pandemic and justify these recommendations based on previous published pivotal trials and current published studies, outlining the effects of the COVID pandemic on various retinal diseases treated with IVT. We describe the effect COVID-19 with both published reports and real-world examples from various tertiary centers around the world and suggest recommendations that may improve future resilience in providing continued IVT for patients with chronic retinal diseases despite challenges from the pandemic.
Acknowledgements
Allergan for organising the International Retinal Collaborative
Abbreviations
- IVT
Intravitreal injection therapy
- WHO
World Health Organization
- NAMD
Neovascular age-related macular degeneration
- DME
Diabetic macula edema
- ME-RVO
Macular edema associated with retinal vascular occlusion
- VA
Visual acuity
- OCT
Optical coherence tomography
- OT
Operating theatre
- Anti-VEGF
Anti-vascular endothelial growth factor
- PPE
Personal protective equipment
- T&E
Treat-and-extend
Author contributions
TACS, SR, AD, CI, YM, CMV, FL, MM, PN, PD, RR, RA, SA, TS, VK, VCV, VD made substantial contributions to conception and design of the work; or the acquisition, analysis. TACS drafted the work. All authors revised it critically for important intellectual content; and gave final approval of the version to be published with an agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
Open Access funding enabled and organized by Projekt DEAL. All authors above have received travel expenses from Allergan (Abbvie) for participation in the IRC. In addition: Anna Tan has received travel expenses from Bayer, Novartis, Allergan, Zeiss and Nidek, a consultant for Bayer, Novartis and Zeiss and receives grant funding from Novartis and Nidek. Amir Rosenblatt receives lecture fees from Allergan Bayer and Novartis in additional to travel fees from Allergan. Nopasak Phasukkijwatana received travel expenses from Allergan and honorarium from Novartis and Bayer. Dominika Pohlmann received travel expense from Allergan (now Abbvie) and research grant from Bayer. Rhianon Reynolds has received travel expenses from Allergan and honorarium from Novartis. Camila Ventura is on the adviser board for Novartis. Jayakrishna Ambati is a co-founder of iVeena Holdings, iVeena Delivery Systems and Inflammasome Therapeutics; he has received consultancy fees from Allergan, Biogen, Boehringer Ingelheim, Immunovant, Janssen, Olix Pharmaceuticals, Retinal Solutions, and Saksin LifeSciences,
Availability of data and materials
Not Applicable.
Declarations
Ethics approval and consent to participate
No individual patient data was used and questionnaires were completed by the retinal specialists where agreement/consent was implied by completion or return of the questionnaire.
Consent for publication
All the authors have consented for publication.
Competing interests
The authors report no competing interest with the contents of this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
A. C. S. Tan, Email: annacstan@gmail.com
D. Pohlmann, Email: dominika.pohlmann@charite.de
References
- 1.Sohrabi C, Alsafi Z, O'Neill N, et al. World Health Organization declares global emergency: a review of the 2019 novel coronavirus (COVID-19) Int J Surg. 2020;76:71–76. doi: 10.1016/j.ijsu.2020.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lai THT, Tang EWH, Chau SKY, Fung KSC, Li KKW. Stepping up infection control measures in ophthalmology during the novel coronavirus outbreak: an experience from Hong Kong. Graefes Arch Clin Exp Ophthalmol. 2020;258(5):1049–1055. doi: 10.1007/s00417-020-04641-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antaki F, Dirani A. Treating neovascular age-related macular degeneration in the era of COVID-19. Graefes Arch Clin Exp Ophthalmol. 2020 doi: 10.1007/s00417-020-04693-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ehlken C, Helms M, Böhringer D, Agostini HT, Stahl A. Association of treatment adherence with real-life VA outcomes in AMD, DME, and BRVO patients. Clin Ophthalmol. 2018;12:13–20. doi: 10.2147/OPTH.S151611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ophthalmologists RCo. COVID-19 clinical guidance for ophthalmologists. 2020. https://www.rcophth.ac.uk/2020/03/covid-19-update-and-resources-for-ophthalmologists/.
- 6.Ophthalmology AAo. New recommendations for urgent and non-urgent patient care. United States of America. 2020. https://www.aao.org/headline/new-recommendations-urgent-nonurgent-patient-care.
- 7.Korobelnik JF, Loewenstein A, Eldem B, et al. Guidance for anti-VEGF intravitreal injections during the COVID-19 pandemic. Graefes Arch Clin Exp Ophthalmol. 2020 doi: 10.1007/s00417-020-04703-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freund KB, Korobelnik JF, Devenyi R, et al. Treat-and-extend regimens with anti-vegf agents in retinal diseases: a literature review and consensus recommendations. Retina. 2015;35(8):1489–1506. doi: 10.1097/IAE.0000000000000627. [DOI] [PubMed] [Google Scholar]
- 9.Lanzetta P, Loewenstein A, Committee VAS. Fundamental principles of an anti-VEGF treatment regimen: optimal application of intravitreal anti-vascular endothelial growth factor therapy of macular diseases. Graefes Arch Clin Exp Ophthalmol. 2017;255(7):1259–1273. doi: 10.1007/s00417-017-3647-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heier JS, Khanani AM, Quezada Ruiz C, et al. Efficacy, durability, and safety of intravitreal faricimab up to every 16 weeks for neovascular age-related macular degeneration (TENAYA and LUCERNE): two randomised, double-masked, phase 3, non-inferiority trials. Lancet. 2022;399(10326):729–740. doi: 10.1016/S0140-6736(22)00010-1. [DOI] [PubMed] [Google Scholar]
- 11.Wykoff CC, Abreu F, Adamis AP, et al. Efficacy, durability, and safety of intravitreal faricimab with extended dosing up to every 16 weeks in patients with diabetic macular oedema (YOSEMITE and RHINE): two randomised, double-masked, phase 3 trials. Lancet. 2022;399(10326):741–755. doi: 10.1016/S0140-6736(22)00018-6. [DOI] [PubMed] [Google Scholar]
- 12.Khanani AM, Callanan D, Dreyer R, et al. End-of-study results for the ladder phase 2 trial of the port delivery system with ranibizumab for neovascular age-related macular degeneration. Ophthalmol Retina. 2021;5(8):775–787. doi: 10.1016/j.oret.2020.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 14.Arnold JJ, Markey CM, Kurstjens NP, Guymer RH. The role of sub-retinal fluid in determining treatment outcomes in patients with neovascular age-related macular degeneration–a phase IV randomised clinical trial with ranibizumab: the FLUID study. BMC Ophthalmol. 2016;16:31. doi: 10.1186/s12886-016-0207-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sacconi R, Borrelli E, Vella G, et al. TriPla Regimen: a new treatment approach for patients with neovascular age-related macular degeneration in the COVID-19 "era". Eur J Ophthalmol. 2021;31(3):849–852. doi: 10.1177/1120672120963448. [DOI] [PubMed] [Google Scholar]
- 16.Brown DM, Nguyen QD, Marcus DM, et al. Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials: RISE and RIDE. Ophthalmology. 2013;120(10):2013–2022. doi: 10.1016/j.ophtha.2013.02.034. [DOI] [PubMed] [Google Scholar]
- 17.Korobelnik JF, Loewenstein A, Eldem B, et al. Anti-VEGF intravitreal injections in the era of COVID-19: responding to different levels of epidemic pressure. Graefes Arch Clin Exp Ophthalmol. 2021;259(3):567–574. doi: 10.1007/s00417-021-05097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hadayer A, Zahavi A, Livny E, et al. Patients wearing face masks during intravitreal injections may be at a higher risk of endophthalmitis. Retina. 2020;40(9):1651–1656. doi: 10.1097/IAE.0000000000002919. [DOI] [PubMed] [Google Scholar]
- 19.Blom K, Bragadóttir R, Sivertsen MS, Moe MC, Jørstad Ø. Mask use by patients in the context of COVID-19 can increase the risk of postinjection endophthalmitis. Acta Ophthalmol. 2022;100(3):e859–e860. doi: 10.1111/aos.14945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel SN, Tang PH, Storey PP, et al. The influence of Universal face mask use on endophthalmitis risk after intravitreal anti-vascular endothelial growth factor injections. Ophthalmology. 2021;128(11):1620–1626. doi: 10.1016/j.ophtha.2021.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marín-Nieto J, Reino-Perez C, Santillana-Cernuda G, Díaz-Bernal JM, Luque-Aranda R, García-Basterra I. Face mask contamination during COVID-19 pandemia a study on patients receiving intravitreal injections. Retina. 2021;41(11):2215–2220. doi: 10.1097/IAE.0000000000003202. [DOI] [PubMed] [Google Scholar]
- 22.Schultheis WG, Sharpe JE, Zhang Q, et al. Effect of taping face masks on quantitative particle counts near the eye: implications for intravitreal injections in the COVID-19 Era. Am J Ophthalmol. 2021;05(225):166–171. doi: 10.1016/j.ajo.2021.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verolino M, Grassi P, Sosto G, D'Onofrio G, De Simone S, Costagliola C. Lean approach to the management of patients undergoing intravitreal injections during COVID-19 pandemic. Ther Adv Ophthalmol. 2021;13:25158414211018893. doi: 10.1177/25158414211018893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohannessian R, Duong TA, Odone A. Global telemedicine implementation and integration within health systems to fight the COVID-19 pandemic: a call to action. JMIR Public Health Surveill. 2020;6(2):e18810. doi: 10.2196/18810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith WR, Atala AJ, Terlecki RP, Kelly EE, Matthews CA. Implementation guide for rapid integration of an outpatient telemedicine program during the COVID-19 pandemic. J Am Coll Surg. 2020 doi: 10.1016/j.jamcollsurg.2020.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mintz J, Labiste C, DiCaro MV, McElroy E, Alizadeh R, Xu K. Teleophthalmology for age-related macular degeneration during the COVID-19 pandemic and beyond. J Telemed Telecare. 2020 doi: 10.1177/1357633X20960636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kern C, Fu DJ, Kortuem K, et al. Implementation of a cloud-based referral platform in ophthalmology: making telemedicine services a reality in eye care. Br J Ophthalmol. 2020;104(3):312–317. doi: 10.1136/bjophthalmol-2019-314161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Viola F, Milella P, Pozzo Giuffrida F, Ganci S, Invernizzi A. Impact of coronavirus disease pandemic on intravitreal injections treatment for macular diseases: report from a referral hospital in Milan. Retina. 2021;41(4):701–705. doi: 10.1097/IAE.0000000000002941. [DOI] [PubMed] [Google Scholar]
- 29.Arruabarrena C, Toro MD, Onen M, et al. Impact on visual acuity in neovascular age related macular degeneration (nAMD) in Europe Due to COVID-19 pandemic lockdown. J Clin Med. 2021 doi: 10.3390/jcm10153281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Billioti Gage S, Drouin J, Desplas D, et al. Intravitreal anti-vascular endothelial growth factor use in france during the coronavirus disease 2019 pandemic. JAMA Ophthalmol. 2021;139(2):240–242. doi: 10.1001/jamaophthalmol.2020.5594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stemplewitz B, Luethy J, Eddy MT, et al. Impact of the COVID-19 pandemic's first wave on the care and treatment situation of intravitreal injections in a German metropolitan region. Graefes Arch Clin Exp Ophthalmol. 2022 doi: 10.1007/s00417-021-05521-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Borrelli E, Grosso D, Vella G, et al. Short-term outcomes of patients with neovascular exudative AMD: the effect of COVID-19 pandemic. Graefes Arch Clin Exp Ophthalmol. 2020;258(12):2621–2628. doi: 10.1007/s00417-020-04955-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Borrelli E, Grosso D, Vella G, et al. Impact of COVID-19 on outpatient visits and intravitreal treatments in a referral retina unit: let's be ready for a plausible "rebound effect". Graefes Arch Clin Exp Ophthalmol. 2020;258(12):2655–2660. doi: 10.1007/s00417-020-04858-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zarranz-Ventura J, Nguyen V, Creuzot-Garcher C, et al. International impact of the COVID-19 pandemic lockdown on intravitreal therapy outcomes: fight retinal blindness registry. Retina. 2022;42(4):616–627. doi: 10.1097/IAE.0000000000003368. [DOI] [PubMed] [Google Scholar]
- 35.Rush RB, Rush SW. Outcomes in patients resuming intravitreal anti-vascular endothelial growth factor therapy following treatment delay during the coronavirus-19 pandemic. Retina. 2021;41(12):2456–2461. doi: 10.1097/IAE.0000000000003276. [DOI] [PubMed] [Google Scholar]
- 36.Sekeroglu MA, Kilinc Hekimsoy H, Horozoglu Ceran T, Doguizi S. Treatment of neovascular age related macular degeneration during COVID-19 pandemic: The short term consequences of unintended lapses. Eur J Ophthalmol. 2021 doi: 10.1177/11206721211010613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Teo KYC, Nguyen V, Barthelmes D, Arnold JJ, Gillies MC, Cheung CMG. Extended intervals for wet AMD patients with high retreatment needs: informing the risk during COVID-19, data from real-world evidence. Eye. 2021;35(10):2793–2801. doi: 10.1038/s41433-020-01315-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiang B, Gao L, Dong S, et al. Correction to: the influence of COVID-19 on the stability of patients with neovascular age-related macular degeneration with different treatment regimens. Adv Ther. 2022;39(4):1611. doi: 10.1007/s12325-022-02045-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Romano F, Monteduro D, Airaldi M, et al. Increased number of submacular hemorrhages as a consequence of coronavirus disease 2019 lockdown. Ophthalmol Retina. 2020;4(12):1209–1210. doi: 10.1016/j.oret.2020.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hanhart J, Wiener R, Totah H, et al. Effects of delay in anti-vascular endothelial growth factor intravitreal injections for neovascular age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2022 doi: 10.1007/s00417-021-05505-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stone LG, Grinton ME, Talks JS. Delayed follow-up of medical retina patients due to COVID-19: impact on disease activity and visual acuity. Graefes Arch Clin Exp Ophthalmol. 2021;259(7):1773–1780. doi: 10.1007/s00417-021-05174-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Douglas VP, Vavvas DG, Miller JW, Miller JB. Short- and Long-term visual outcomes in patients receiving intravitreal injections: the impact of the coronavirus 2019 disease (COVID-19)-related lockdown. J Clin Med. 2022 doi: 10.3390/jcm11082097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chew EY, Clemons TE, Harrington M, et al. Effectiveness of different monitoring modalities in the detection of neovascular age-related macular degeneration: the Home Study, Report Number 3. Retina. 2016;36(8):1542–1547. doi: 10.1097/IAE.0000000000000940. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not Applicable.


