Abstract
Aureobasidium pullulans ATCC 42023 was cultured under aerobic conditions with glucose, mannose, and glucose analogs as energy sources. The exopolymer extracts produced under these conditions were composed of glucose and mannose. The molar ratio of glucose to mannose in the exopolymer extract and the molecular weight of the exopolymer varied depending on the energy source and culture time. The glucose content of exopolymer extracts formed with glucose and mannose as the carbon sources was between 91 and 87%. The molecular weight decreased from 3.5 × 106 to 2.12 × 106 to 0.85 × 106 to 0.77 × 106 with culture time. As the culture time increased, the glucose content of the exopolymer extract formed with glucosamine decreased from 55 ± 3 to 29 ± 2 mol%, and the molecular weight increased from 2.73 × 106 to 4.86 × 106. There was no evidence that glucosamine was directly incorporated into exopolymers. The molar ratios of glucose to mannose in exopolymer extracts ranged from 87 ± 3:13 ± 3 to 28 ± 2:72 ± 2 and were affected by the energy source added. On the basis of the results of an enzyme hydrolysis analysis of the exopolymer extracts and the compositional changes observed, mannose (a repeating unit) was substituted for glucose, which gave rise to a new family of exopolymer analogs.
Pullulan is one of the few neutral water-soluble microbial polysaccharides that can be produced in large quantities by fermentation (31, 38). Pullulan is an extracellular, unbranched homopolysaccharide which consists of maltotriose and maltotetraose units with both α-(1→6) and α-(1→4) linkages (3, 6, 9, 38). The regular alternation of α-1,4 and α-1,6 bonds results in two distinctive properties, structural flexibility and enhanced solubility (22). These properties suggest that pullulan may be used for both medical and industrial purposes (23). Pullulan produces high-viscosity solutions at relatively low concentrations and can be utilized to form oxygen-impermeable films, thickening or extending agents, or adhesives (28). Films formed from pullulan are suitable for coating foods and pharmaceuticals, especially when exclusion of oxygen is desirable (46).
Pullulan biosynthesis is accomplished through mediation of sugar nucleotide-lipid carrier intermediates associated with the cell membrane fraction (8, 38). Some important parameters that control the production of pullulan are temperature (28), the initial pH of the medium (15, 18, 30), the oxygen supply (32, 41), the nitrogen concentration (1, 35), and the carbon source (2). The molecular weight of pullulan varies depending on the culture conditions and strain (31, 36, 44).
In our attempts to modify the native structure of polysaccharides, we recently examined the effects of glucose analogs when they were used as energy sources for the production of exopolymers (24–26). In some cases, novel exopolymers were formed, or the glucose analogs affected the molecular weight or composition of the native exopolymers synthesized. In this paper we describe the influence of glucose analogs, such as 3-O-methyl-d-glucose (3-O-methylglucose), 2-amino-2-deoxy-d-glucose (glucosamine), and 2-acetamino-2-deoxy-d-glucose (N-acetylglucosamine) on the yield and composition of exopolymers synthesized by Aureobasidium pullulans. We also explored the possibility that these glucose analogs might be directly polymerized by A. pullulans. Our goals were to devise biosynthesis strategies which modulate the composition and structural features of exopolymers and to gain insight into the metabolic flexibility of the biosynthesis and secretion pathways.
MATERIALS AND METHODS
Bacterial strain.
A. pullulans ATCC 42023 (47) was obtained from the American Type Culture Collection and was transferred monthly to fresh nutrient agar medium. The medium used for cell growth and exopolymer production contained (per liter) 5.0 g of K2HPO4, 1.0 g of NaCl, 0.2 g of MgSO4 · 7H2O, 0.6 g of (NH4)2SO4, 2.5 g of yeast extract, and 20 g of glucose (39). The pH of medium was adjusted to 6.5 to 6.7 before sterilization. Each carbohydrate source was autoclaved separately for 20 min at 121°C and was added to the medium under aseptic conditions.
Production of exopolymer.
Starter cultures were prepared by transferring cells from agar slants to 50-ml portions of medium containing 2% (wt/vol) glucose in 250-ml Erlenmeyer flasks. The resulting cultures were incubated for 2 days at 30°C and 180 rpm. Each starter culture was used as an inoculum (3%, vol/vol) for 150 ml of medium supplemented with an energy source (2%, wt/vol) in a 500-ml Erlenmeyer flask. The cultures were incubated for 5 days under the same conditions used to prepare the starter cultures. The energy sources, including glucose (>99.5% pure) and glucose-related sugars (>99.0% pure), such as 3-O-methylglucose, glucosamine, and N-acetylglucosamine, were purchased from Sigma Chemical Co. (St. Louis, Mo.). Samples were periodically withdrawn from the cultures to examine cell growth and exopolymer production.
Purification of exopolymer.
Cell broth was centrifuged at 15,000 × g for 20 min at 4°C to remove the cells. To determine biomass, the cells were washed with distilled water and dried at 100 to 105°C until the weight was constant. Supernatant fluids were mixed with 2 volumes of 95% ethanol and incubated at 4°C for 24 h to precipitate the crude products, which were separated by centrifugation at 15,000 × g for 30 min. The precipitated material was repeatedly washed with acetone and ether, dissolved in deionized water, and dialyzed against deionized water by using dialysis tubing with a molecular weight cutoff of 12,000 to 14,000. After dialysis for 2 to 3 days with four or five changes of deionized water, the solution was lyophilized, and the exopolymer yield was determined by weighing.
Treatment with pullulanase and chemical analysis.
Exopolymers were assayed for sensitivity to pullulanase (21, 43). Exopolymers were suspended at a concentration of 1 mg/ml (0.1%, wt/vol) in 50 mM sodium acetate buffer (pH 5.0). Pullulanase from Klebsiella pneumoniae (Sigma Chemical Co.) was added to a concentration of 0.1 U/ml. After mixing, the treated samples were incubated for 42 h at 25°C. Authentic pullulan (Sigma Chemical Co.) was digested as a control, and data are reported below as percentages of reducing sugars relative to complete hydrolysis to maltotriose units.
Total-sugar and reducing-sugar contents were determined by the phenol sulfuric acid method (12) and the dinitrosalicylic acid (DNS) method, respectively (29, 33). DNS reagent was prepared by first dissolving 7.46 g of 3,5-DNS and 13.98 g of NaOH pellets in 1 liter of deionized water. Then 216.1 g of Rochelle Salt (potassium sodium tartarate tetrahydrate), 5.38 ml of saturated phenol, and 5.85 g of sodium metabisulfite were added, and the reagent was aged for 2 weeks. DNS reagent was added to the same volume of an enzyme-substrate solution, and the preparation was placed in a boiling water bath for 15 min. After the preparation cooled to room temperature, the concentration of reducing sugars was determined spectrophotometrically at 550 nm with a spectrophotometer (Beckman Instrument Co.). The calibration curve used for reducing-sugar determinations was generated by using maltotriose (Sigma Chemical Co.).
Composition analysis by GC.
Gas chromatography (GC) and GC-mass spectrometry (MS) were used to determine the carbohydrate composition after methanolysis and trimethylsilyation of the product (10). Samples were prepared for GC-MS analyses as described elsewhere (10, 24). GC-MS analyses were performed with a Hewlett-Packard gas chromatograph (model 5890 series II) equipped with an Hewlett-Packard model 7673 injector and coupled to a mass selective detector (Hewlett-Packard model 5971 series). The capillary column used was a cross-linked 5% phenylmethyl silicone fused-silica column (HP Ultra MS 5; 30 m by 0.25 mm; film thickness, 0.33 μm). Dry oxygen-free helium (flow rate, 0.8 ml/min) was used as the carrier gas, and the column temperature was programmed so that it was 140°C for 2 min and then increased at a rate of 8°C per min to 260°C. One-microliter samples were injected, and the injector was purged for 0.6 min after injection. m-Inositol was used as the internal standard.
Determination of molecular weight by GPC.
The number average molecular weight (Mn) (average molecular weight divided by the number of molecules) and the weight average molecular weight (Mw) (average molecular weight divided by the weight of each polymer chain), as well as the polydispersity (Mw/Mn) (the breadth of the molecular weight distribution) of pullulan samples, were determined by gel permeation chromatography (GPC) by using a Waters model 600E system controller equipped with Shodex KB800 series columns (two KB80M columns and one KB805 column) and a model 410 refractive index detector. All data processing was carried out by using Millennium version 2.15 software. Pullulan standards (Polysciences) with narrow polydispersity and with molecular weights ranging from 5.80 × 103 to 8.53 × 105 were used to construct a calibration curve. Deionized water containing 0.05% (wt/vol) sodium azide was used as the mobile phase at a flow rate of 1.0 ml/min. The sample concentration and injection volume were 5.0 mg/ml and 100 μl, respectively. All of the sample solutions were filtered through 0.45-μm-pore-size filters before injection. For the samples which produced multimodal distribution GPC chromatograms, a curve fit software program (Peakfit, version 4.0, for Win32; Jandel Scientific Software Inc.) was used to analyze the GPC chromatograms (r2 > 0.950). The GPC chromatograms were estimated, relative to the pullulan standards, by assuming Bernoullian shape curves and using three molecular weight range components for each chromatogram.
RESULTS
Monomeric composition of exopolymers.
The GC chromatograms of the exopolymers purified from the culture grown with 2% (wt/vol) glucose showed that the major component was glucose, as identified by the peak retention times for the α and β anomers (9.34 and 9.82 min, respectively) and the area ratios of these anomers to a pure standard (10.5 to 1.0) (10). The exopolymer synthesized with 2% (wt/vol) glucosamine consisted of glucose and mannose, as determined by GC. The identities of the glucose and mannose in the GC-MS chromatograms of exopolymers were confirmed by using reference spectra in the GC-MS data bank (data not shown).
Production of exopolymer with glucose analogs.
Cell growth and exopolymer production were greater with glucose than with any of the other energy sources used under the conditions used (Table 1). 3-O-Methylglucose and 2-deoxyglucose were not utilized effectively by A. pullulans ATCC 42023. Cell growth with glucosamine was similar to cell growth with N-acetylglucosamine. The exopolymers synthesized with glucose, 3-O-methylglucose, glucosamine, N-acetylglucosamine, 2-deoxyglucose, and mannose were analyzed by GC. All of the exopolymers produced similar GC peaks which corresponded to only glucose and mannose. Based on the relative peak areas and the response factors for corresponding standard sugars, the relative glucose and mannose contents of the exopolymers were calculated (Table 1). The glucose and mannose contents of the exopolymer synthesized with 2% (wt/vol) glucose were 87 ± 3 and 13 ± 3 mol%, respectively, whereas the glucose and mannose contents of the exopolymer synthesized with 2% (wt/vol) glucosamine were 30 ± 7 and 70 ± 7 mol%, respectively. The ratio of glucose to mannose in the exopolymer synthesized with 2% (wt/vol) mannose was similar to the ratio of glucose to mannose in the exopolymer synthesized with 2% (wt/vol) glucose (Table 1).
TABLE 1.
Production of exopolymers with glucose and its analogs by A. pullulansa
| Carbon source | Final pHb | Cell dry wt (mg/ml) | Yield (mg/ml)c | Specific yield (mg/mg [dry wt] of cells)d | Mol% of:
|
|
|---|---|---|---|---|---|---|
| Glucosee | Mannosef | |||||
| Glucose (2%) | 6.19 | 9.79 | 3.21 ± 0.35 | 0.34 | 87 ± 3 | 13 ± 3 |
| 3-O-Methylglucose (2%) | 7.47 | 1.74 | 0.47 ± 0.10 | 0.36 | 57 ± 6 | 43 ± 6 |
| Glucosamine (2%) | 5.75 | 3.94 | 0.70 ± 0.11 | 0.21 | 30 ± 7 | 70 ± 7 |
| N-Acetylglucosamine (2%) | 7.62 | 3.15 | 0.51 ± 0.12 | 0.22 | 43 ± 4 | 57 ± 4 |
| 2-Deoxyglucose (2%) | 6.82 | 0.43 | 0.35 ± 0.08 | 1.09 | 54 ± 5 | 46 ± 5 |
| Mannose (2%) | 5.40 | 7.56 | 2.63 ± 0.07 | 0.35 | 89 ± 4 | 11 ± 4 |
The data are data for 5-day cultures grown at 30°C, and the values are means based on triplicate experiments. The values for controls included to account for medium or exopolymer carryover were as follows: for cell dry weight, 0.23 ± 0.00 mg/ml; and for yield, 0.17 ± 0.01 mg/ml. The control values should be subtracted from the gross values shown.
The initial medium pH was adjusted to 6.5 to 6.7 prior to sterilization.
Total amount of exopolymers, including pullulan.
Specific yield of exopolymers, including pullulan.
Moles percent of glucose = [glucose/(glucose + mannose)] × 100.
Moles percent of mannose = [mannose/(glucose + mannose)] × 100.
Production of exopolymers as a function of cultivation time.
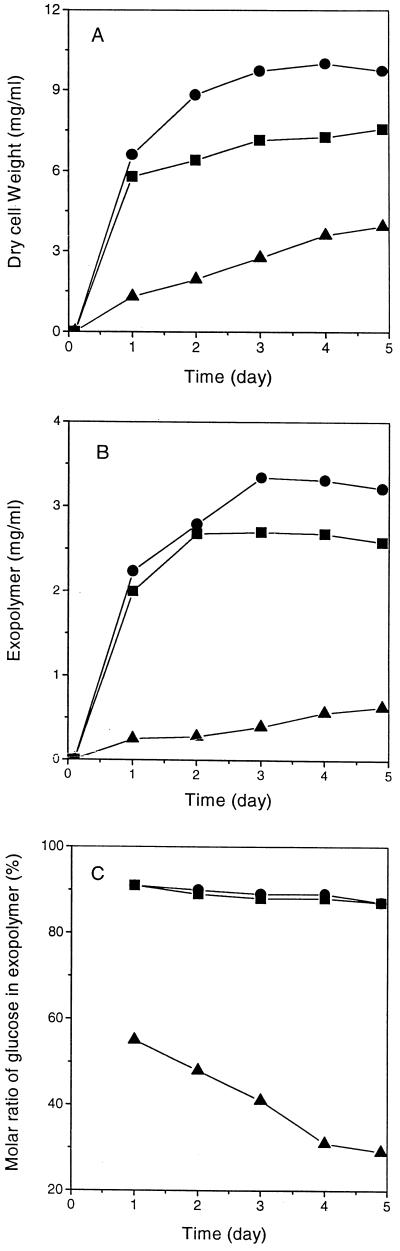
Cell growth and production of exopolymers when glucose, mannose, and glucosamine were used as energy sources were compared as a function of time. On the basis of cell dry weight, the growth of A. pullulans cells depended on the energy source (Fig. 1A). The highest exopolymer yields with glucose and mannose were obtained after 3 and 2 days of growth, respectively (Fig. 1B). The production of exopolymers in the presence of glucose, mannose, and glucosamine paralleled cell growth. The molar ratio of glucose to mannose in exopolymers synthesized with glucose and mannose remained almost constant as a function of culture time (about 90:10) (Fig. 1C). The molar ratio of glucose to mannose in the exopolymer synthesized with glucosamine decreased with culture time. The initial ratio of glucose to mannose was 55 ± 3:45 ± 3; after 5 days the ratio was 29 ± 2:71 ± 2.
FIG. 1.
Production of exopolymers with glucose (●), mannose (■), and glucosamine (⧫) as a function of time of cultivation of A. pullulans ATCC 42023. (a) Cell growth. (b) Exopolymer production. (C) Molar ratio of glucose to mannose in exopolymers.
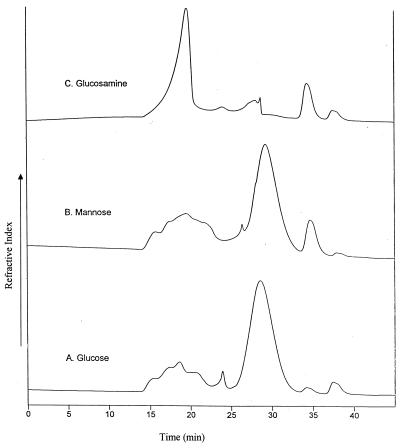
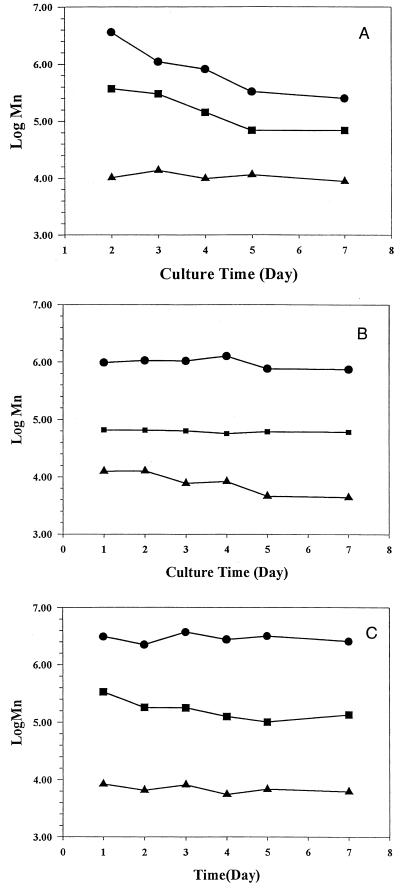
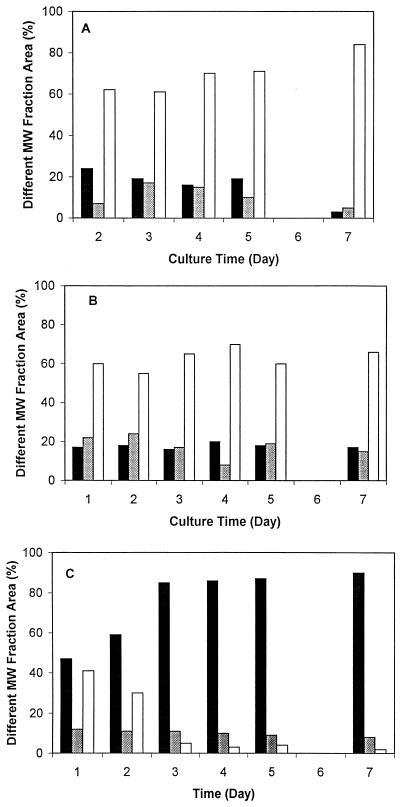
GPC chromatograms of exopolymers purified from 5-day cultures in which glucose, mannose, and glucosamine were the energy sources showed that the molecular weight of each exopolymer was heterogeneous (Fig. 2). On the basis of molecular weight, the exopolymers could be divided into high-molecular-weight (molecular weight, more than 1.0 × 106), medium-molecular-weight (1.0 × 106 to 5.0 × 104), and low-molecular-weight (less than 5.0 × 104) fractions. The relative amounts of the high- and medium-molecular-weight fractions of exopolymer synthesized with glucose decreased as the amount of the low-molecular-weight fraction of exopolymer increased (Fig. 3). As a function of culture time, the low-molecular-weight fractions of the exopolymers synthesized with glucose and mannose dominated, whereas the high-molecular-weight fractions of the exopolymer synthesized with glucosamine accounted for 90% of total exopolymer (Fig. 4). Thus, the major fraction of the exopolymers synthesized with glucose and mannose was the low-molecular-weight fraction, but the major fraction of the exopolymer synthesized with glucosamine was the high-molecular-weight fraction.
FIG. 2.
GPC chromatograms of exopolymers purified from 5-day cultures grown on glucose (A), mannose (B), and glucosamine (C).
FIG. 3.
Molecular weight fractional patterns as a function of culture time. (A) Glucose. (B) Mannose. (C) Glucosamine. Symbols: ●, high-molecular-weight fraction; ■, medium-molecular-weight fraction; ▴, low-molecular-weight fraction.
FIG. 4.
Patterns of molecular fraction areas as a function of culture time when glucose (A), mannose (B), and glucosamine (C) were the carbon sources. Black bars, high-molecular-weight fraction; gray bars, medium-molecular-weight fraction; white bars, low-molecular-weight fraction. MW, molecular weight.
Production of exopolymers with mixed carbon sources.
The molar ratios of glucose to mannose in the exopolymers synthesized after incubation for 5 days with 2% (wt/vol) glucose and 2% (wt/vol) glucosamine as the sole energy sources were 87 ± 3:13 ± 3 and 28 ± 2:72 ± 2, respectively (Table 2). The molar ratios of glucose to mannose in exopolymers synthesized with glucose and glucosamine together were between the molar ratios in the exopolymers synthesized with the individual energy sources. The cell growth and exopolymer yield obtained with glucose plus glucosamine as the carbon source exhibited responses which reflected the relative ratios of glucose and mannose in the polymer. The exopolymer synthesized with a higher relative concentration of glucosamine in the mixed energy source had a higher mannose content.
TABLE 2.
Production of exopolymers with mixed carbon sources by A. pullulansa
| Concn (%) of Carbon sources
|
Final pHb | Cell dry wt (mg/ml) | Yield (mg/ml)c | Specific yield (mg/mg [dry wt] of cells)d | Mol% of
|
||
|---|---|---|---|---|---|---|---|
| Glucose | Glucosamine | Glucosee | Mannosef | ||||
| 2.0 | 0.0 | 5.80 | 9.61 | 3.10 ± 0.30 | 0.31 | 87 ± 3 | 13 ± 3 |
| 1.5 | 0.5 | 4.86 | 8.07 | 2.29 ± 0.03 | 0.28 | 80 ± 2 | 20 ± 2 |
| 1.0 | 1.0 | 5.80 | 8.33 | 1.60 ± 0.05 | 0.19 | 75 ± 3 | 25 ± 3 |
| 0.5 | 1.5 | 5.80 | 8.30 | 1.14 ± 0.05 | 0.14 | 61 ± 3 | 39 ± 3 |
| 0.0 | 2.0 | 5.80 | 3.57 | 0.74 ± 0.02 | 0.21 | 28 ± 2 | 72 ± 2 |
The data are data for 5-day cultures grown at 30°C, and the values are means ± standard deviations based on results of triplicate experiments.
The initial medium pH was adjusted to 6.5 to 6.7 prior to sterilization.
Total amount of exopolymers, including pullulan.
Specific yield of exopolymers, including pullulan.
Moles percent of glucose = [glucose/(glucose + mannose)] × 100.
Moles percent of mannose = [mannose/(glucose + mannose)] × 100.
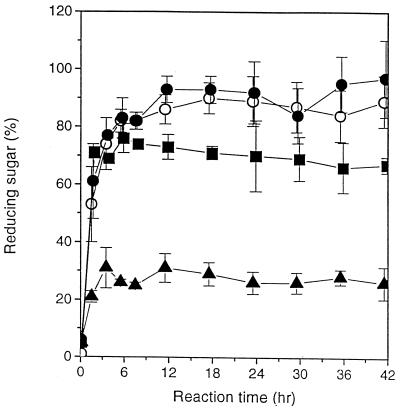
Pullulan and exopolymers synthesized with glucose and mixtures of glucose and glucosamine contained increased concentrations of reducing sugars after treatment with pullulanase (Fig. 5). After a 12-h treatment, the reducing sugars from pullulan were 86 ± 5.1% maltotriose equivalents. The reducing-sugar contents of exopolymers with molar ratios of glucose to mannose of 89:11 (exopolymer 1), 79:21 (exopolymer 2), and 55:45 (exopolymer 3) were 93 ± 9.2, 73 ± 4.3, and 31 ± 5.0%, respectively.
FIG. 5.
Treatment of pullulan (○), exopolymers synthesized with glucose (molar ratio of glucose to mannose, 89:11) (●), exopolymers synthesized with glucose and glucosamine (molar ratio of glucose to mannose, 79:21) (■), and exopolymers synthesized with glucosamine (molar ratio of glucose to mannose, 55:45) (▴) with pullulanase.
DISCUSSION
Based on the data reported here, exopolymers synthesized by A. pullulans may consist of a family of glucose-mannose copolymers or may involve modulation of the composition of pullulan itself. It is important to note that in previous studies of pullulan the workers usually did not clarify the level of mannose in the polymer(s) and there was no attempt to separate different polymers, if they were present. The yield of exopolymers produced by A. pullulans and the relative amount of pullulan (usually defined as the ethanol precipitate) have been reported to vary with the culture conditions and strain (4, 21, 30, 42, 43). Depending on the strain and carbon source, the pullulan content of the exopolymer produced by A. pullulans has varied from 95 to 73% (30), from 76 to 51% (43), or from 100 to 45% (43). Exopolymers purified from the fermentation broth media of A. pullulans cultures grown with various agroindustrial wastes were heterogeneous with respect to monomeric composition and molecular weight (16). For example, the glucose contents of the exopolymers synthesized with olive oil waste effluents and molasses as the carbon sources were about 33 and 25%, respectively, while all of the monomeric components of exopolymers synthesized with starch waste and grape skin pulp extract were glucose. The exopolymer synthesized with carob pod extract as the energy source contained only glucose, and the glucosidic linkages were primarily α-(1→4) (68%) and α-(1→6) (31%) linkages (34).
The pullulan content of exopolymers synthesized with glucose as the energy source decreased with culture time (4). The major component of the extracellular polysaccharides produced by A. pullulan with glucose as the energy source was glucose, and the minor components were mannose and galactose (47). In the present study, the exopolymers produced by A. pullulan ATCC 42023 in the presence of glucose and its analogs consisted of glucose and mannose. The molar ratio of glucose to mannose in the exopolymers varied from 90:10 to 30:70 depending on the energy source and culture time. The energy source was presumably responsible for determining the repeat unit composition of the exopolymer produced by A. pullulan.
Beijerinckia indica utilized glucose, as well as glucose analogs, such as 3-O-methylglucose, glucosamine, N-acetylglucosamine, and 2-deoxyglucose, for growth and produced polysaccharide 7. However, there was no evidence that these sugars were directly incorporated into exopolymers, and there was no change in the repeat unit composition of polysaccharide 7 (25). Two of the glucose analogs, 3-O-methylglucose and N-acetylglucosamine, were directly incorporated into exopolysaccharides produced by Agrobacterium sp. (26). When 3-O-methylglucose was used, 8 to 12 mol% of the curdlan repeats were 3-O-methylglucose based on GC and 1H nuclear magnetic resonance spectrometry data. When glucose analogs were used as energy sources, they did not support Zoogloea ramigera cell growth. However, when mixtures of glucose and these sugars were used, as cosubstrates, they supported cell growth and resulted in a significant change in the internal ratio of components (26). The use of glucose analogs as cosubstrates during Z. ramigera cultivation resulted in dramatic changes in sugar metabolism due to the competition of these compounds with glucose (11, 40) and in alterations in the gene expression involved in the biosynthetic pathway of zooglan (13). When glucose analogs are used as energy sources with A. pullulan, they appear to modulate sugar metabolism, which results in changes in the composition of exopolysaccharides.
The average molecular weight of pullulan ranges from 1.5 × 104 to 1.0 × 107 depending on the culture conditions and strain used (31, 36, 44). The molecular weight of pullulan decreased late in the stationary growth phase due to the presence of the less frequent amylase-sensitive maltotetraose sites among the predominantly maltotriose units in pullulan and due to α-amylase secreted into the medium (5, 20, 31). New strains were isolated, and the pH used for cultivation was optimized to produce higher-molecular-weight pullulan (27, 31).
Exopolymers produced by A. pullulans with different substrates had different molecular weights and repeat unit compositions (16). The molecular weights of exopolymers synthesized with agricultural wastes were higher than the molecular weights of exopolymers synthesized with glucose as the energy source. In this study, exopolymers synthesized with glucose as well as mannose had a monomeric composition similar to that of pullulan. The molecular weights of these exopolymers decreased with culture time, while the molecular weights of exopolymers synthesized with glucose analogs, which had high mannose contents, did not decrease with culture time. Apparently, the α-amylase or the pullulan-degrading enzymes produced by the cells (20) cannot hydrolyze the exopolymers synthesized with glucose analogs due to the change in mannose content.
Pullulanase is one of the starch-debranching enzymes that specifically attacks the branch points of amylopectin, hydrolyzing α-(1,6)glucosidic linkages to produce maltotriose (17, 37, 45). The increase in reducing sugar content after pullulanase treatment of pullulan and the exopolymers synthesized with glucose and glucose analogs indicated that the exopolymers have α-(1,6)glucosidic linkages like those in pullulan. These data suggest that the exopolymers have triose units, like pullulan. We confirmed that the exopolymers purified from the cultures grown on glucose had a structure identical to that of pullulan based on 13C nuclear magnetic resonance chromatograms (data not shown). The exopolymers with increased mannose contents exhibited resistance to hydrolysis by α-amylase.
The biosynthetic pathway for the exopolymers produced by A. pullulan has not been well established (8, 38). There are two possibilities for incorporation of mannose into the exopolymers synthesized by A. pullulans. One option involves an epimerase that converts glucose to mannose after polymerization. Epimerization by C-5-mannuronan epimerase has been found in Pseudomonas aeruginosa (14) but has not been reported in A. pullulans. The monomeric ratio of mannuronate to guluronate in exopolymer synthesized by P. aeruginosa changed depending on the activity of C-5 epimerase, which was affected by the concentration of salts in culture medium (19). The other possibility for incorporation of mannose into the exopolymer is direct incorporation. The only monomeric components of exopolymers synthesized with energy sources including glucose analogs in this experiment were glucose and mannose, but the ratios were different depending on the growth conditions. Direct incorporation of glucose and mannose into exopolymers may be affected by the physiological conditions, including energy source and culture time, that were changed in this study.
In this study, exopolymers with various molar ratios of glucose to mannose, which ranged from 87:13 to 28:72, were synthesized during growth on glucose and glucose analogs. The ratio of glucose to mannose in exopolymers can be controlled by the ratio of mixed carbon sources. Therefore, it is possible to produce exopolymers with a more defined ratio of glucose to mannose. These exopolymers have higher molecular weights and narrower polydispersity than pullulan. Further work to characterize the structural and functional properties of these exopolymers with various molar ratios of glucose to mannose produced by A. pullulans is needed.
REFERENCES
- 1.Auer D P F, Seviour R J. Influence of varying nitrogen sources on polysaccharide production by Aureobasidium pullulans in batch culture. Appl Microbiol Biotechnol. 1990;32:637–644. [Google Scholar]
- 2.Badr-Eldin S M, El-Tayeb O M, El-Masry E G, Mohamad O A, El-Rahman O A A. Polysaccharide production by Aureobasidium pullulans: factors affecting polysaccharide formation. World J Microbiol Biotechnol. 1994;10:423–426. doi: 10.1007/BF00144465. [DOI] [PubMed] [Google Scholar]
- 3.Bouveng H O, Kiessling H, Lindberg B, McKay J. Polysaccharides elaborated by Pullularia pullulans. Acta Chem Scand. 1963;17:797–800. [Google Scholar]
- 4.Catley B. Utilization of carbon sources by Pullularia pullulans for the elaboration of extracellular polysaccharides. Appl Microbiol. 1971;22:641–649. doi: 10.1128/am.22.4.641-649.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Catley B J. Pullulan, a relationship between molecular weight and fine structure. FEBS Lett. 1970;10:190–193. doi: 10.1016/0014-5793(70)80450-1. [DOI] [PubMed] [Google Scholar]
- 6.Catley B J, Whelan W J. Observations on the structure of pullulan. Arch Biochem Biophys. 1971;143:138–142. doi: 10.1016/0003-9861(71)90193-7. [DOI] [PubMed] [Google Scholar]
- 7.Catley B J. Pullulan synthesis by Aureobasidium pullulans. In: Berkeley R C W, Gooday G W, Ellwood D C, editors. Microbial polysaccharides and polysaccharases. London, United Kingdom: Academic Press Ltd.; 1979. pp. 69–84. [Google Scholar]
- 8.Cately B J, McDowell W. Lipid-linked saccharides formed during pullulan biosynthesis in Aureobasidium pullulans. Carbohydr Res. 1982;103:65–75. [Google Scholar]
- 9.Catley B J, Ramsay A, Servis C. Observations on the structure of fungal extracellular-polysaccharide, pullulan. Carbohydr Res. 1986;153:79–86. [Google Scholar]
- 10.Chaplin M. A rapid and sensitive method for the analysis of carbohydrate components in glycoproteins using gas-liquid chromatography. Anal Biochem. 1982;123:336–341. doi: 10.1016/0003-2697(82)90455-9. [DOI] [PubMed] [Google Scholar]
- 11.Cloherty E K, Diamond D L, Heard K S, Carruthers A. Regulation of GLUT1-mediated sugar transport by an antiport/uniport switch mechanism. Biochemistry. 1996;35:13231–13239. doi: 10.1021/bi961208t. [DOI] [PubMed] [Google Scholar]
- 12.Dubois M, Gilles K A, Hamilton J K, Rebers P A, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. [Google Scholar]
- 13.Easson D D, Jr, Sinskey A J, Peoples O P. Isolation of Zoogloea ramigera I-16-M exopolysaccharide biosynthetic genes and evidence for instability within this region. J Bacteriol. 1987;169:4518–4524. doi: 10.1128/jb.169.10.4518-4524.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franklin M J, Chitnis C E, Gacesa P, Sonesson A, White D C, Ohman D. Pseudomonas aeruginosa AlgG is a polymer level alginate C5-mannuronan epimerase. J Bacteriol. 1994;176:1821–1830. doi: 10.1128/jb.176.7.1821-1830.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Imshenetskii A A, Kondrat’eva T F, Smut’ko A N. Influence of the acidity of the medium, conditions of aeration, and temperature on pullulan biosynthesis by polyploid strains of Pullularia (Aureobasidium) pullulans. Microbiology (Engl Transl Mikrobiologiya) 1981;50:330–333. [PubMed] [Google Scholar]
- 16.Israilides C, Scanlon B, Smith A, Harding S E, Jumel K. Characterization of pullulans from agro-industrial wastes. Carbohydr Polym. 1994;25:203–209. [Google Scholar]
- 17.Koch R, Canganella F, Hippe H, Jahnke K D, Antranikian G. Purification and properties of a thermostable pullulanase from a newly isolated thermophilic anaerobic bacterium, Fervidobacterium pennavrans Ven5. Appl Environ Microbiol. 1997;63:1088–1094. doi: 10.1128/aem.63.3.1088-1094.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lacroix C, LeDuy A, Noel G, Choplin L. Effect of pH on the batch fermentation of pullulan from sucrose medium. Biotechnol Bioeng. 1985;27:202–207. doi: 10.1002/bit.260270216. [DOI] [PubMed] [Google Scholar]
- 19.Larsen B, Haug A. Biosynthesis of alginate. I. Composition and structure of alginate synthesis and enzymes in Pseudomonas aeruginosa variants. J Gen Microbiol. 1971;138:605–610. doi: 10.1099/00221287-138-3-605. [DOI] [PubMed] [Google Scholar]
- 20.Leathers T D. Host amylase and pullulan production. In: Kaplan D L, editor. Materials Biotechnology Symposium Proceedings. Natick, Mass: U. S. Army Natick Research, Development and Engineering Center; 1987. pp. 175–185. [Google Scholar]
- 21.Leathers T D, Nofsinger G W, Kurtzman C P, Bothast R J. Pullulan production by color variant strains of Aureobasidium pullulans. J Ind Microbiol. 1988;3:231–239. [Google Scholar]
- 22.Leathers T D. Substrate regulation and specificity of amylases from Aureobasidium strain NRRL Y-12,974. FEMS Microbiol Lett. 1993;110:217–222. [Google Scholar]
- 23.LeDuy A, Zajic J E, Luong J H T, Choplin L. Pullulan. In: Mark H F, Bikales N M, Overberger C G, Menges G, Kroschwitz J I, editors. Encyclopedia of polymer science and engineering. 2nd ed. Vol. 13. Toronto, Ontario, Canada: Wiley; 1988. pp. 650–660. [Google Scholar]
- 24.Lee J W, Yeomans W G, Allen A L, Kaplan D L, Deng F, Gross R A. Exopolymers from curdlan production—incorporation of glucose-related sugars by Agrobacterium sp. ATCC 31749. Can J Microbiol. 1997;43:149–156. [Google Scholar]
- 25.Lee J W, Yeomans W G, Allen A L, Gross R A, Kapan D L. Production of zoogloea gum by Zoogloea ramigera with glucose analogs. Biotechnol Lett. 1997;19:799–802. [Google Scholar]
- 26.Lee J W, Yeomans W G, Allen A L, Gross R A, Kaplan D L. Compositional consistency of hetropolysaccharide-7 by Beijerinckia indica. Biotechnol Lett. 1997;19:803–807. [Google Scholar]
- 27.Lee K Y, Yoo Y J. Optimization of pH for high molecular weight pullulan. Biotechnol Lett. 1993;15:1021–1024. [Google Scholar]
- 28.McNeil B, Kristiansen B. Temperature effects on polysaccharide formation by Aureobasidium pullulans in stirred tanks. Enzyme Microb Technol. 1990;12:521–526. [Google Scholar]
- 29.Miller G L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 1959;31:426–428. [Google Scholar]
- 30.Ono K, Yasuda N, Ueda S. Effect of pH on pullulan elaboration by Aureobasidium pullulans S-1. Agric Biol Chem. 1977;41:2113–2118. [Google Scholar]
- 31.Pollock T J, Thorne L, Armentrout R W. Isolation of new Aureobasidium strains that produce high-molecular-weight pullulan with reduced pigmentation. Appl Environ Microbiol. 1992;58:877–883. doi: 10.1128/aem.58.3.877-883.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rho D, Mulchandsani A, Luong J H T, LeDuy A. Oxygen requirement in pullulan fermentation. Appl Microbiol Biotechnol. 1988;28:361–366. [Google Scholar]
- 33.Roesser D S, McCarthy S P, Gross R A, Kaplan D L. Effects of substitution site on acetyl amylose biodegradability by amylase enzymes. Macromolecules. 1996;29:1–9. [Google Scholar]
- 34.Roukas T, Biliaderis C G. Evaluation of carob pod as a substrate for pullulan production by Aureobasidium pullulans. Appl Biochem Biotechnol. 1995;55:27–44. [Google Scholar]
- 35.Seviour R J, Kristiansen B. Effect of ammonium ion concentration on polysaccharide production by Aureobasidium pullulans in batch culture. Eur J Appl Microbiol Biotechnol. 1983;17:178–181. [Google Scholar]
- 36.Slodki M E, Cadmus M C. Production of microbial polysaccharides. Adv Appl Microbiol. 1978;23:19–54. doi: 10.1016/s0065-2164(08)70064-9. [DOI] [PubMed] [Google Scholar]
- 37.Smith K A, Salyers A A. Cell-associated pullulanase from Bacteroides thetaiotaomicron: cloning, characterization, and insertional mutagenesis to determine role in pullulan utilization. J Bacteriol. 1989;171:2116–2123. doi: 10.1128/jb.171.4.2116-2123.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taguchi R, Kikuchi Y, Sakano Y, Kobayashi T. Structural uniformity of pullulan produced by several strains of Pullularia pullulans. Agric Biol Chem. 1973;37:1583–1588. [Google Scholar]
- 39.Ueda S, Fujita K, Komatsu K, Nakashima Z. Polysaccharide produced by the genus Pulularia. Appl Microbiol. 1963;11:211–215. doi: 10.1128/am.11.3.211-215.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vera J C, Reyes A M, Crácamo J G, Velásquez F V, Rivas C I, Zhang R H, Strobel P, Iribarren R, Scher H I, Slebe J C, Golde D W. Genistein is a natural inhibitor of hexose and dehydroascorbic acid transport through the glucose transporter, GLUT1. J Biol Chem. 1996;271:8719–8724. doi: 10.1074/jbc.271.15.8719. [DOI] [PubMed] [Google Scholar]
- 41.Wecker A, Onken U. Influence of dissolved oxygen concentration and shear rate on the production of pullulan by Aureobasidium pullulans. Biotechnol Lett. 1991;13:155–160. [Google Scholar]
- 42.West P T, Reed-Hamer B. Polysaccharide production by a reduced pigmentation mutant of the fungus Aureobasidium pullulans. FEMS Microbiol Lett. 1993;113:345–350. [Google Scholar]
- 43.West P T, Reed-Hamer B. Elevated polysaccharide production by mutants of the fungus Aureobasidium pullulans. FEMS Microbiol Lett. 1994;124:167–171. [Google Scholar]
- 44.Wiley B J, Ball D H, Arcidiacono S M, Mayer J M, Kaplan D L. Control of molecular weight distribution of the biopolymer pullulan produced by Aureobasidium pullulans. J Environ Polym Degrad. 1993;1:3–9. [Google Scholar]
- 45.Yamashita M, Kinoshita T, Ihara M, Mikawa T, Murooka Y. Random mutagenesis of pullulanase from Klebsiella aerogenes for studies of the structure and function of the enzyme. J Biochem. 1994;116:1223–1240. doi: 10.1093/oxfordjournals.jbchem.a124669. [DOI] [PubMed] [Google Scholar]
- 46.Yuen S. Pullulan and its applications. Process Biochem. 1974;9:7–9. [Google Scholar]
- 47.Zajic J E, LeDuy A. Flocculant and chemical properties of a polysaccharide from Pullularia pullulans. Appl Microbiol. 1973;25:628–635. doi: 10.1128/am.25.4.628-635.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]