Abstract
Objectives
The overuse and misuse of antibiotics has accelerated the rapid emergence of antibiotic resistance. The aim of the study was to review interventions conducted in China to optimize use of antibiotics in humans, animals, and the environment from a One Health perspective.
Methods
The literature review for this study was limited to English and Chinese articles published from January 1985 to May 2021. Literature review searches were conducted using Web of Science, Scopus, PubMed and three biomedical databases from China (the Chinese Scientific Journals database, the Wanfang Database, and China National Knowledge Infrastructure). We used Arksey and O’Malley's step-wise methodological framework as the basis for our scoping review.
Results
A total of 53 studies met our inclusion criteria, of which 51 (96%) were from human healthcare settings, one from environment health that pertained to rural ponds, and no studies were found that met our criteria on interventions used to improve antibiotic use in animals. For human health, the majority of the research was related to antibiotic intervention programs performed in public institutions, and only one policy assessment study included private institutions. Interventions were classified into four broad categories: 1) Knowledge interventions; 2) decision support; 3) financial incentives; and 4) organizational/management systems. Our findings indicated that combinations of multiple interventions were more effective in promoting the rational use of antibiotics in China.
Conclusions
China has made major efforts on improving rational use of antibiotics in the past decades. Most policies or interventions, however, focused mainly on the human health aspect, less effort targeted toward the environment and animal health sectors. For further optimizing use of antibiotics, the cross-disciplinary and coordinated multi-faceted interventions guided by the One Health perspective should be developed and implemented. Meanwhile, the cross-departmental collaborative mechanism leading by the Chinese central government should be further strengthened to play a greater and more active role in fighting against antibiotic resistance wholly.
Keywords: One Health, Antibiotic use, Intervention, Antibiotic resistance, China
Highlights
-
•
Antibiotic resistance is a complex global health challenge.
-
•
China is one of the largest producers and consumers of antibiotics in the world.
-
•
Based on One Health perspective, we provide a compendium of evaluations of different sectoral intervention.
-
•
For improving antibiotic use, more cross-disciplinary coordinated efforts are suggested.
1. Introduction
Antibiotic overuse and misuse has led to the emergence of antibiotic resistance [1]. Nowadays, antibiotic resistance has become a significant problem in public health, resulting in the delayed application of effective interventions, and increased mortality, morbidity, and costs [2,3]. If no urgent action is taken, is estimated that by 2050, antibiotic resistance will cause an estimated loss of 10 million lives and $US 100 trillion [4]. Antibiotic resistance affects all countries; however, the burden is disproportionately higher in low- and middle-income countries (LMICs) [4,5].
Worldwide, China is among largest manufacturers and consumers of antibiotics. Antibiotics are used widely to treat human and livestock diseases in China. Moreover, China uses antibiotics as prophylactics and growth promoters in livestock [6]. One study indicated that 162,000 tons of antibiotics (inclusive of 36 groups of antibiotics) were consumed in China; 48% of which were consumed by humans and the remainder by animals [7]. Approximately 46% of antibiotics were ultimately released into rivers through sewage effluent. The remaining percentage ended up in the environment, either unmetabolized or metabolized and in an active form [6,8].
Currently, to restrain antibiotic resistance, the United Nations is promoting a “One Health” approach. “One Health” deals with questions at the intersection of human, animal, and environmental health, and emphasizes that efforts must be made across all these sectors [9,10]. Based on the “One health” approach, bacterial resistance was included as a focus of the “Global Action Plan on Antimicrobial Resistance”. This plan was passed at the 68th World Health Assembly (WHA) in 2015 and aimed to optimize antimicrobial medicine application in animal and human health [10]. In 2016, the National Action Plan to Contain Antimicrobial Resistance (2016–2020) was published by the Chinese government, reinforcing its determination to control bacterial resistance. Since then, China's healthcare and agriculture departments implemented actions designed to reduce inappropriate and unnecessary antibiotic use in animal feed and to treat patients, while making sure that, when required, antibiotic therapy is available and effective.
Previously published systematic reviews revealed evidence that supported certain safe and effective interventions to optimize the use of antibiotics in humans within health settings [11,12]. More effective results were obtained by combining interventions comprising both enabling and restrictive policies [9]. Furthermore, multi-faceted and interactive interventions, such as education with feedback and monitoring mechanisms were found to be most effective at encouraging the appropriate use of antibiotics. However, in the present review, most of the included studies were performed in higher-income countries. Surprisingly, limited evidence exists for the utilization of effective and safe interventions that optimize the use of antibiotics in LMICs [12]. China, an LMIC, is the most populous country in the world and therefore has a significant influence on global health. The international community has high expectations of China with respect to global health management, especially antibiotic resistance. Therefore, this study aims to review interventions conducted in China to optimize the use of antibiotics in humans, animals, and the environment under the “One Health” approach. Our research provides important insights and implications for effective approaches to reduce antibiotic resistance.
2. Methods
2.1. Study design
The scoping study was used to overview the empirical evidence of effective antibiotic resistance strategies. Arksey and O’Malley's step-wise methodological framework was used as the basis of this scoping review [13]. The step-wise framework approach included: (1) Composing the research question; (2) identifying related studies; (3) selecting the studies; (4) data analysis; and (5) collating, summarizing, and reporting the results.
2.2. Stage 1: Composing the research question
The study aimed to find answers to the questions (for environmental, animal and human health systems):
-
1.
Where in China have the interventions been enacted? e.g., regions and type of healthcare setting.
-
2.
What types of interventions have been conducted to promote the rational use of antibiotics in China?
-
3.
Which interventions have the potential to decrease the inappropriate use of antibiotics in China?
-
4.
What lessons have we learned from these interventions?
2.3. Stage 2: Identifying related studies
A PICO (Population, Intervention, Comparison, and Outcome) combination was used to guide the systematic search. We were concerned with all interventions conducted in China to improve antibiotic use in humans, animals. and the environment (see Table 1). Intervention targets include two populations: antibiotic providers and antibiotic consumers. We looked for all interventions aiming to improve antibiotic use, for example communication and education, stewardship programs, incentives and policies and regulations, such as National Essential Medicines Policy (NEMP) and the New Cooperative Medical Scheme (NCMS) (details see Appendix 1). The outcomes we were interested in were quantifiable improvement in antibiotic supply (e.g., a decrease in inappropriate sales and prescribing of antibiotics, adherence to the outline by health workers), reported alterations in understanding regarding use of antibiotics, and health outcomes (adverse, unaffected, or improved).
Table 1.
Search parameters.
| Parameter | Definition |
|---|---|
| Population | 1) Antibiotic providers (referred to as P1), e.g., physicians, other healthcare providers, drug vendors, informal physicians, pharmacists, and vets. 2) Antibiotic consumers (referred to as P2), e.g., patients, caregivers, and farmers. |
| Intervention | Any intervention aiming to promote the rational use of antibiotics, e.g., communication and education, stewardship programs, incentives, peer or community oversight (details see the Appendix 1). |
| Comparison | Not applicable. The scoping review is not limited the comparative or controlled research designs. We included studies that reported interventions with/without comparison groups. |
| Outcome | Primary outcome: Quantifiable improvement in the use of antibiotics (e.g., decreased prescribing of unnecessary antibiotics, improved conformation to guidelines). Secondary outcomes: Reported alterations in understanding around use of antibiotics, e.g., unintended consequences, health outcomes (adverse, unaffected or improved), and levels of antibiotic resistance. |
The Web of Science, Scopus, PubMed and three Chinese biomedical databases (China National Knowledge Infrastructure (CNKI), Wanfang Database, and Chinese Scientific Journals database) were searched to identify studies. The search was performed using Boolean operators for antibiotics, antimicrobial, antibacterial, or anti-bacterial agents of various types. This was combined with terms for a variety of types of interventions and study settings in China. Details of the search terms are shown in Appendix 1.
Studies published between 1985 and May 2021 were searched. In 1985, China issued the Pharmaceutical Administration Law of the People's Republic of China. This was the first drug administration regulation to strengthen drug supervision and management, after which management of the problem of antibiotic resistance was initiated. Table 2 contains search results for each database used in our study.
Table 2.
Search results.
| Database | Result |
|---|---|
| Web of Science | 7470 |
| Scopus | 6328 |
| PubMed | 5808 |
| CNKI | 68 |
| Wanfang Database | 50 |
| Chinese Scientific Journals database | 43 |
| Total | 19,767 |
2.4. Stage 3: Selecting the studies
Based on the predefined inclusion and exclusion criteria, abstracts were reviewed by two research assistants. Any controversies were resolved via discussion among the team.
Inclusion criteria:
-
1.
Reports on the intervention aiming to improve use of antibiotics, such as influencing the prescribing and sale of antibiotics (formal and informal) and raising awareness of antimicrobial resistance for residents.
-
2.
Any healthcare setting in China.
-
3.
Outcome
Independently measured alteration in the prescription, sale, or use of antibiotics.
Independently measured alteration in the knowledge surrounding the use of antibiotics.
Self-reported alterations in the prescription, sale, or use of antibiotics.
Self-reported alteration in the knowledge surrounding the use of antibiotics.
Observed alteration in the quality of the prescription of antibiotics.
Exclusion criteria:
-
1.
Studies dealing with alternative methods of dealing with antibiotic resistance, e.g., hygiene, control measures, e.g., vaccines.
-
2.
Reports examining the effects of new tools in the clinic to promote the use of antibiotics, e.g., C-reactive protein and Procalcitonin (PCT) guidance, etc.
-
3.
Reports examining adherence to guidelines for the use antibiotics or other medicines.
-
4.
Reports dealing with environmental antibiotic transmission and resistance.
-
5.
Reports dealing with other antimicrobials, excluding antibiotics.
-
6.
Reports dealing with patient demand for antibiotics and self-use.
-
7.
Reports assessing antibiotic treatment effectiveness in clinical care, e.g., comparing different treatments or administering routes of antibiotics.
-
8.
Context studies that only describe the background but not implemented intervention.
2.5. Stage 4: Data analysis
We extracted the information from the included studies including author, year of publication, country, target population, study design, study goal, study discipline, main findings in process of improving antibiotic use.
2.6. Stage 5: Results collation, analysis, and reporting
We summarized the studies by disease focus, country, and publication. The extracted data related to the evidence and findings of all the studies were analyzed thematically using inductive and deductive coding according to Braun and Clarke [14]. Subgroup analyses were performed with respect to study dimension, target population, and geographical regions. The human, animal, and environment dimensions were included in this study, and the target populations were divided into the antibiotic provider group and the consumer group. Rural and urban areas were also considered in the study.
For interventional studies focusing on human health, subgroup analyses were conducted by hospital level and institutional characteristics. Chinese health-care facilities are divided into three levels (tertiary hospitals, secondary hospitals, and primary healthcare facilities). In China, tertiary hospitals comprise large medical centers located in big cities, secondary hospitals comprise county hospitals, and primary healthcare facilities comprise community hospitals or village clinics, which are often small and deal with a limited range of basic medical services. The institutional characteristics were divided into public and private medical institutions.
In the intervention impact analysis, studies were classified according to whether they reported either positive, negative, mixed, or no effects based on their report of increased or decreased rates of antibiotic prescription, a mixture, or no change, respectively. Reports describing appropriate reduces in antibiotic prescription or improved adherence to current guidelines were identified as positive. Reports describing inappropriately increased rates of antibiotic prescription or lack of adherence to the current guidelines were identified as negative. Studies were classified as mixed if they reported both positive and negative effects on various prescribing indicators.
2.7. Stage 6: Consulting stakeholders
We invited two experts who were familiar with the “One Health” approach to gain their feedback regarding the original findings and suggestions for additional studies that could meet the eligibility criteria. The expert's feedback stated our findings were plausible in a regional context; however, neither expert suggested the inclusion of further papers.
3. Results
The database search yielded 19,767 results and an additional 123 studies were identified through other sources. The exclusion of irrelevant studies according to their titles and abstracts left 137 articles, which were downloaded and detailed full-text evaluation was performed. According to the inclusion criteria, we included 53 studies in English but none in Chinese for this review. Intervention studies are currently published primarily in international journals; therefore, there was a paucity of studies meeting our criteria in Chinese databases. In fact, no study met our inclusion criteria in the Chinese databases because of limited manuscript descriptions (details see Fig. 1).
Fig. 1.
Flow diagram of study identification.
3.1. Intervention settings
We could not find a single published intervention designed to improve antibiotic use in animal health. For environmental health, we found only one intervention study that pertained to a rural pond. Fifty-one (96%) studies were from human healthcare settings. In total 53 studies, 94% (50/53) of the interventions targeted antibiotic providers, such as doctors and pharmacist. In total, 66% (35/53) of the reports examined interventions caried out in urban areas, and three reports addressed both rural and urban areas (see Fig. 2). Four studies did not include information about the facilities; therefore, it was difficult to assess where the studies were conducted.
Fig. 2.
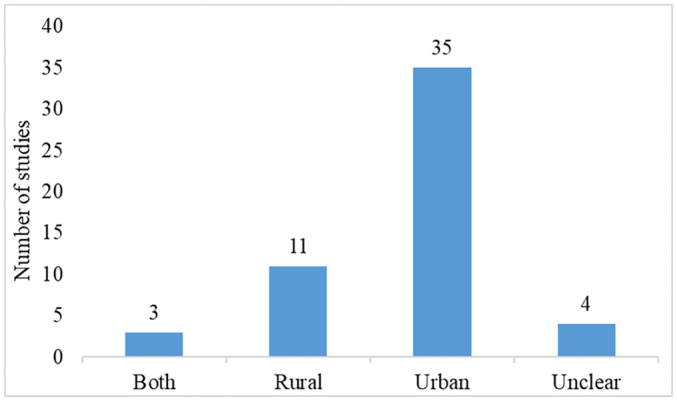
Interventions attempted in different locations (urban or rural).
For interventions in human healthcare settings, 29 studies were from tertiary or secondary hospitals, 21 were from primary healthcare facilities, and only 1 study included both hospital and primary care facilities (Fig. 3). Most of these studies were from public medical institutions, and only one policy assessment study included both public and private medical institutions (Fig. 3). This was especially obvious for the studies carried out in primary care facilities, with approximately 90% (19/21) focusing on the public sector. Similarly, 86% (25/29) of the studies were focused on hospitals in public settings. Five studies were unclear because they did not mention the names of the institutions and other related information, meaning that we could not determine if the facilities described were private or public.
Fig. 3.
Sectors undertaking intervention testing, separated according to the kind of health setting.
3.2. Intervention types
Table 3 shows the kinds of intervention undertaken in various settings. Blank cells reveal that no evidence exists showing that interventions were conducted in that setting. Most interventions have only been assessed once or twice, frequently in only a single setting. Only educational, audit/feedback interventions, and evaluation implementation effects of National Essential Medicines Policy and Antimicrobial Stewardship Program (AMS) have been carried out in all settings. The evidence base is uneven, for example, only one educational intervention was addressed in animal health, one regarding the environment, and only one study targeted ecopharmacovigilance (EPV).
Table 3.
The interventions of various types.
| Types of intervention | Human |
Animal |
Environment |
Total | |||
|---|---|---|---|---|---|---|---|
| P1⁎ |
P2⁎⁎ |
P2 |
P2 |
||||
| Hospital | Primary care facility | Mixed Setting | Village | Village | Village | ||
| Knowledge intervention | |||||||
| Education | 2 | 2 | 1⁎⁎⁎ | 1⁎⁎⁎ | 5 | ||
| Education/Feedback | 1⁎⁎⁎⁎ | 1⁎⁎⁎⁎ | 1 | ||||
| Education/Guideline/Audit/Feedback | 1 | 1 | |||||
| Audit/Feedback | 3 | 1 | 4 | ||||
| Guidelines | 1 | 1 | |||||
| Decision support | |||||||
| Electronic medical records (EMR) | 1 | 1 | |||||
| Pharmacist intervention | 8 | 8 | |||||
| Financial incentives | |||||||
| Funding | 1 | 1 | |||||
| Policy: Capitation with pay-for-performance | 1 | 1 | |||||
| Policy: New Cooperative Medical Scheme | 3 | 3 | |||||
| Organizational/management systems | |||||||
| Policy: National Essential Medicines Policy | 2 | 8 | 1 | 11 | |||
| Public reporting | 1 | 1 | |||||
| AMS | 12 | 2 | 14 | ||||
| Targeted ecopharmacovigilance (EPV) | 1 | 1 | |||||
| Total | 29 | 21 | 1 | 2 | 1 | 1 | 53 |
P1: Antibiotic provider group.
P2: Antibiotic consumer group.
These belong to the same article.
These belong to the same article.
Table 3 reveals certain gaps in the evidence base. At present, the intervention for primary care mainly comes from the implementation of relevant policies, such as the National Essential Medicines Policy, the New Cooperative Medical Scheme, Pharmacist Intervention, and AMS. These types of interventions are often implemented in hospitals in comparison to primary care. AMS studies are those that combine change and policy review (e.g., incentives/disincentives, new guideline development, and new targets), the creation of committees/working groups dealing with antimicrobial resistance, monitoring, auditing, and education. Pharmacist interventions are those in which pharmacists compose drug treatment plans, participate in ward rounds, communicate promptly with physicians if they suspect inappropriate prescription of antibiotics; provide medical teams with educational handouts and sessions concerning antibiotic prophylaxis; report the data on the inappropriate prophylactic use of antibiotics on a regular basis (e.g., every week/month). This might reflect the completeness of the hospital system, which is mainly reflected by the high knowledge level of medical staff and good hospital management systems.
3.3. Intervention impact
For the interventions, 35 studies showed positive effects, 9 documented mixed effects, 4 showed no effect, and 6 demonstrated negative effects. Most of the reports (86%, 25/29) from hospitals showed positive effects; however, only 38% (8/21) of the studies from primary health care facilities showed positive effects. For each category of intervention, Table 4 details the number of reports disclosing each type of impact. Notably, one study that included both positive and no effects was conducted with different intervention subjects (village household/farmer) [15].
Table 4.
The intervention impacts of studies.
| Types of intervention | Positive |
Mixed |
Negative |
No effect |
Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Human |
Environment |
Human |
Human |
Human |
Animal |
||||||||
| P1⁎ |
P2⁎⁎ |
P2 |
P1 |
P1 |
P1 |
P2 |
|||||||
| Hospital | Primary care facility | Village | Village | Hospital | Primary care facility | Mixed | Hospital | Primary care facility | Hospital | Primary care facility | Village | ||
| Knowledge intervention | |||||||||||||
| Education | 1 | 1 | 1⁎⁎⁎ | 1 | 1 | 1⁎⁎⁎ | 5 | ||||||
| Education/Feedback | 1 | 2 | |||||||||||
| Education/Guideline/Audit/Feedback | 1⁎⁎⁎⁎ | 1⁎⁎⁎⁎ | 1 | ||||||||||
| Audit/feedback | 3 | 1 | 4 | ||||||||||
| Guidelines | 1 | 1 | |||||||||||
| Decision support | |||||||||||||
| Electronic medical records (EMR) | 1 | 1 | |||||||||||
| Pharmacist intervention | 8 | 8 | |||||||||||
| Financial incentives | |||||||||||||
| Funding | 1 | 1 | |||||||||||
| Policy: Capitation with pay-for-performance | 1 | 1 | |||||||||||
| Policy: New Cooperative Medical Scheme | 1 | 2 | 3 | ||||||||||
| Organizational/management systems | |||||||||||||
| Policy: National Essential Medicines Policy | 1 | 2 | 2 | 1 | 3 | 2 | 11 | ||||||
| Public reporting | 1 | 1 | |||||||||||
| AMS | 11 | 1 | 1 | 1 | 14 | ||||||||
| Targeted ecopharmacovigilance (EPV) | 1 | 1 | |||||||||||
| Total | 25 | 8 | 2 | 1 | 3 | 5 | 1 | 1 | 5 | 3 | 1 | 53 | |
P1: Antibiotic provider group.
P2: Antibiotic consumer group.
These belong to the same article.
These belong to the same article.
Among the reports detailing positive results, organizational/management systems had the strongest evidence, with 14 studies for AMS. There were eight positive evaluations of pharmacist interventions and four positive evaluations of audit/feedback. Audit/feedback studies comprise those that introduced audit measures (such as a review of prescriptions carried out following the guidelines) combined with feedback mechanisms (such as reports and meetings).
Twenty-nine studies were classified as utilizing multiple intervention strategies, whereas 24 studies used a single intervention pathway (i.e., implementation of one policy, e.g., the NEMP). According to Table 5, despite both single and multifaceted interventions resulting in positive and mixed results, most of the positive studies were reported by multifaceted studies (24 vs. 11) and no or negative effects were reported more often by studies evaluating single interventions (no effect/negative combined = 1 vs. 9).
Table 5.
Results by intervention approach.
| Intervention Pathway |
|||
|---|---|---|---|
| Reported Results | Multifaceted | Single | Total |
| Positive | 24 | 11⁎ | 35 |
| Mixed | 4 | 5 | 9 |
| Negative | 1 | 5 | 6 |
| No effect | 0 | 4⁎ | 4 |
| Total | 29 | 24 | 53 |
There was one study that included both positive and no effect in a single intervention, and it was conducted among different intervention subjects (village household/farmer).
3.4. Intervention content and results
This review identified 53 studies, from 16 different provinces in China (for details, see Appendix 2). Thirteen studies were multi-province, while the remainder described specific area contexts (details see Appendix 3). Interventions were grouped into four broad categories: 1) Knowledge interventions; 2) decision support; 3) financial incentives; and 4) organizational/management systems.
3.4.1. Knowledge based strategies
Several interventions commenced with training, supplemented by further components. A study of 12 villages in Shandong, China, found that in terms of the human use of antibiotics, health education-based interventions were effective to improve knowledge, attitudes, and reported practices; however, they barely affected antibiotic use in pigs [15]. In addition, Chen et al. [16] found that a single education training program for doctors did not effectively change the doctors' antibiotic prescribing behavior. Wei et al. [17] found that the combined education support and monitoring multi-faceted interventions was effective. This included: using up-to-date International and Chinese guidelines for the use of antibiotics focusing on infections of the upper respiratory tract with/without fever to develop guidelines, and then providing a 2-h interactive training session for doctors. Doctors were also asked to participate in monthly peer-review meetings. Finally, a video and leaflets designed to educate caregivers about antibiotics were developed by the researchers. Such pragmatic interventions reduced the prescription of antibiotics for infections of the upper respiratory tract in children [17]. A similar phenomenon was also reported in Chen et al.'s study [18], which indicated that raising awareness and an education program combined with surveillance could reduce unnecessary antibiotic use in general hospitals. However, at 18-months of follow-up, the improvements in antibiotic prescribing had declined without long-term effects for primary care facilities, and the authors indicated that a combination of enabling and restrictive policies might have produced better results [19].
3.4.2. Decision support strategies
Some studies promote the rational use of antibiotics through pharmacist interventions, which mainly include: patient education (as required), more frequent training of physicians, feedback at monthly intervals, structured review of medical orders, and accompanying daily physician ward rounds. Three studies found that interventions with the active involvement of professional pharmacists could positively influence physicians' prescribing behavior and knowledge regarding antibiotic use [[20], [21], [22]]. This significantly reduced decisions regarding the inappropriate use of antibiotics, irrational choices, and unnecessary prolongation of prophylactic treatment, resulting in reduced antibiotic costs and good economic outcomes [23,24].
With the development of internet technology, the working mode of pharmacist's interventions have gradually become combined with information technology. The form of intervention was changed from the regular review of medical orders to monthly indicator feedback (after the prescription) to guide the prescribing process [25]. The integrated electronic medical records (EMR) system adheres strictly to the guidelines. When prescribing antibiotics, the system can provide doctors with reminders and recommendations for the appropriate use of antibiotics. Li et al. [25] found that comprehensive EMR systems were successful in curbing antibiotic misuse and contributed to a significant reduction in antibiotic consumption.
3.4.3. Financial strategies
Economic stimulus is a key factor affecting doctors' prescribing behavior [26]. In 2003, the Chinese government launched the NCMS to help finance rural healthcare. Only one study compared antibiotic prescribing in township hospitals in areas with and without the NCMS, and found that over-prescribing is common in villages and is more severe in areas with NCMS health insurance [27]. However, evidence shows that significant alterations to the structures of financial incentives could positively affect antibiotic use within public primary care settings. In 2014, a policy intervention was conducted in 28 primary healthcare settings (township hospitals and village clinics) in China, which replaced fee-for-service NCMS payments with pay-for-performance with a capitated budget [28]. Capitation with pay-for-performance could reduce over prescribing and inappropriate prescribing. In addition, the results indicated the feasibility of diligent assessments of health system interventions when carried out in close cooperation with government agencies. Moreover, Sun et al. found that too little Government health funding (GHF) is one of the reasons for antibiotics abuse [27]. Importantly, systemic arrangements of health funding by government policymakers are required.
3.4.4. Organizational/management systems strategies
Healthcare system reform is under way, and the Chinese government is attempting to restrain antibiotic abuse once and for all. It is making every possible effort to pursue appropriate prescription policies and to reduce antibiotic overuse and misuse across the country. Since 2009, in China, the prescription of antibiotics has been regulated by enacted national health policy reforms [29]. First, the NEMP, together with a matching policy comprising Zero Mark-up, was introduced. The NEMP used a multifaceted approach to establish a list of essential medicines and introduced price control and supply chain measures. Although the effects were mixed, the NEMP effectively removed economic incentives for hospitals to over-use medicines. However, it had little demonstrable impact on antibiotic prescription. Two studies investigated the impact of NEMP policy on the use of antibiotics in hospitals [30,31], whereas other studies investigated its impact in primary care settings [29,[32], [33],34]. Depending on implementation, mixed results were achieved in different regions (rural and urban areas) and different institutions. Its inclusion on the list of lists of essential medicines determined whether an antibiotic's use increased or decreased. Some interventions were run concurrently with other mechanisms, making it a challenge to separate their effects, such as broader healthcare system reforms (e.g., the zero mark-up policy) and delivery (e.g., healthcare medical insurance programs), which might have affected the changes in antibiotic use [34]. The effects of these organizational/management systems strategies in encouraging rational medicine use were greater in public institutions than in private institutions [35]. This finding is significant, because improvement in prescription patterns is evident in township hospitals in China. However, prominent regional disparities remain significant. Studies found that irrational antibiotic use and unnecessary parenteral administration are still highly prevalent. Antibiotic overuse remained an extensive problem in the treatment of many diseases, and the NEMP plays a limited role [29,31,36,37].
In 2001, the WHO initiated measures to curb the spread of bacterial resistance and strongly recommended that governments implement AMS. In response to bacterial resistance, the National Health and Family Planning Commission of the People's Republic of China (NHFPC) proposed National Special Stewardship in the Clinical Use of Antibiotics, the strictest regulation of antibiotics in history so far. Interventions based on AMS comprised: the constitution of interdisciplinary team of experts to encourage data verification and management, feedback, academic engagement (such attendance at seminars and conference), monitoring, clinical training, dissemination of knowledge, and oversight and guidance; the re-development of targets and guidelines; and financial penalties. Two studies investigated the impact of the stewardship program in primary health care [38,39], while other studies reviewed the effects at secondary or tertiary hospitals [[40], [41], [42], [43], [44]]. Eleven studies had positive intervention results, which were effective in reducing antibiotic consumption. Although the results supported the implementation of antimicrobial stewardship strategies nationally; continuous assessment, monitoring, and tracking are required to determine the long-term effects of these strategies [45].
Only one study covered the control of residual water pollution of antibiotic drugs [46]. The EPV was used as the intervention tool. In the Chinese rural aquatic environment, residual ofloxacin levels decreased significantly as a result of targeted intervention using EPV. Interestingly, EPV measures that targeted and reduced ofloxacin levels also effectively decreased environmental pollution by other, non-targeted, antibiotics.
4. Discussion
As far as we know, this is the largest review of interventions for the rational use of antibiotics in China. The results found that China has made major efforts on improving rational use of antibiotics in the past decades, and indicated that interventions with multiple strategies are more effective than single pathways, which was in line with previous findings [12,47,48]. In addition, interventions that target both consumers and providers could be more effective compared with targeting either group singly in reducing the rate of antibiotic prescription [17,18,49].
This review found that most policies or interventions only focused on the human health aspect, with less targeting of the environment and animal health sectors. However, one of the important drivers of bacterial resistance is antibiotic misuse in the environment, humans, and animals, and the spread of resistant bacteria between these sectors [50]. The “One Health” approach, advocated by The United Nations, is designed to contain bacterial resistance [51], and recognizes the importance of interlinks among the environment, animal health, and human health, emphasizing that efforts must be made to involve interdisciplinary and inter-departmental collaboration. China responded positively, being committed to the “One Health” approach to fight bacterial resistance, with 14 ministerial departments meeting regularly to discuss progress, resulting in the issue of the National Action Plan for Containing Bacterial Resistance (2016–2020). The effects of the program were obvious, and antibiotic prescription rates were reduced dramatically below a predetermined level during the program, but only in human health [52]. For animal health, the actions focused on a surveillance plan for animal-derived bacterial resistance, rather than on optimizing the use of antibiotics [53]. In addition, in the environmental setting, there has been a lack of proposed actions or policies. Previous studies found that antibiotics are used in animals for a wide variety of nontherapeutic purposes, including growth promotion [54]. Farmers tend to be more dependent on antibiotics to control animal diseases, because they affect the income of millions of households in rural areas [55,56]. To date, the toughest challenge is still to reduce the use of antibiotics as growth promoters in animal husbandry; therefore, inter-departmental collaboration guided by the One Health perspective needs to be further strengthened.
The most interventions are targeted at public hospitals, while private medical institutions, such as retail pharmacies, lack effective regulation. However, in China, one of the main sources of antibiotic misuse is retail pharmacies. Previous research showed that about 70–84% of pharmacies in China dispensed antibiotics without a prescription for adults with acute upper respiratory infections [57]. Moreover, a multi-country public awareness survey also found that an average of 93% of people reported to have obtained their most recently used antibiotics from pharmacies as non-prescribed antibiotics [58]. This suggests lax enforcement of the regulations for antibiotic prescription and dispensing. In China, public medical institutions are managed by the NHCPRC, and retail pharmacies are supervised by National Medical Products Administration (NMPA). The NHCPRC usually adopts a range of policies to improve the rational use of antibiotics from a health perspective. However, the NMPA's main responsibilities for drugs are regulating their registration and undertaking quality management. Thus, function barriers between agencies make it impossible to form synergistic strategies to promote rational use of antibiotics. In addition, poor practices are also attributed to knowledge gaps and a lack professionalism among pharmacists [59], consumer demand [60], and a focus on profits [59]. Therefore, we suggest that not only is there a need to strengthen the enforcement of regulations for the dispensation of antibiotics, improve the public's perception of antibiotic use, and provide more training for pharmacy staff, but also encouragement of cross-departmental collaboration between government departments is needed.
We found that the primary care facilities lack long-term effective interventions to improve antibiotic use, as reported previously [61]. A range of policies have been introduced to improve the use of antibiotics; however, studies have shown that they were ineffective at reducing the prescription of antibiotics [29,34,35]. Wei et al. [17] found that a comprehensive health education intervention for providers and caregivers achieved impressive outcomes: for children with upper respiratory tract infections, prescription of antibiotics was reduced by 29%. Unfortunately, at 18 months of follow-up, the improvements in antibiotic prescribing had declined [19]. These interventions were shown to be important, but they may not be sufficient to change physicians' behaviors. Interventions the effectively combine restrictive and enabling policies might have long-term effects [12,19]. Therefore, in primary care facilities, there is an urgent need to develop multifaceted and context-adapted interventions to sustainably improve the use of antibiotics.
4.1. Strengths and weaknesses
Our review has several strengths. First, a scoping review was carried out to obtain an overview of the evidence base, which contrast with systematic reviews that narrow the focus to evidential sub-sets. We looked for large numbers of different types of intervention approaches aiming to improve the use of antibiotics. Second, the studies used in our scoping review followed specific criteria for inclusion to ensure the quality of the included research and we did not use studies that failed meet the strict inclusion criteria. For example, three Chinese databases were excluded from our research because of inadequate descriptions and detail. This study does have limitations. The inappropriate of antibiotic use accelerating the spread of antibiotic resistance directly, and this review just focusses on efforts of optimizing use of antibiotic in China. Further research is need to focus on more efforts on combating antibiotic resistance.
5. Conclusion
China has made major efforts on improving rational use of antibiotics in the past decades. Most policies or interventions, however, focused mainly on the human health aspect, with less targeted at the environmental and animal health sectors. In human health, most interventions targeted hospitals, while retail pharmacies and primary healthcare institutions are lacking effective measures. In the animal and environmental settings, few policies or actions were proposed on optimizing use of antibiotics.
For further improving the rational use of antibiotics, the cross-disciplinary and coordinated multi-faceted interventions guided by the One Health perspective should be developed and implemented. Meanwhile, the cross-departmental collaborative mechanism leading by the Chinese central government should be further strengthened to play a greater and more active role in fighting against antibiotic resistance wholly.
Author contributions
The study was conceived and designed by all the authors. Conceptualization, LYS; Data curation, LYS and QS; Formal analysis, LYS; Writing - original draft, LYS and QS; Writing - review & editing, XLW, JY, DRH and CSL. All the authors read and approved the final the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (grant number 71774103). The funders have had no role in the study design, data collection, data analysis, interpretation, or manuscript production.
Declaration of Competing Interest
The authors declare that they have no competing interests.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.onehlt.2022.100388.
Appendix A. Supplementary data
Supplementary material 1: The terms for types of interventions and study settings
Supplementary material 2
Supplementary material 3
References
- 1.Wise R., Hart T., Cars O., et al. Antimicrobial resistance. Is a major threat to public health. Bmj. 1998;317:609–610. doi: 10.1136/bmj.317.7159.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morehead M.S., Scarbrough C. Emergence of global antibiotic resistance. Primary Care. 2018;45:467–484. doi: 10.1016/j.pop.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 3.Ferri M., Ranucci E., Romagnoli P., et al. Antimicrobial resistance: a global emerging threat to public health systems. Crit. Rev. Food Sci. Nutr. 2017;57:2857–2876. doi: 10.1080/10408398.2015.1077192. [DOI] [PubMed] [Google Scholar]
- 4.O’Neill J. The Review on Antimicrobial Resistance. May 2016. In. 2017. Tackling drug-resistant infections globally: Final report and recommendations. [Google Scholar]
- 5.Klein E.Y., Tseng K.K., Pant S., et al. Tracking global trends in the effectiveness of antibiotic therapy using the drug resistance index. BMJ Glob. Health. 2019;4 doi: 10.1136/bmjgh-2018-001315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Q.Q., Ying G.G., Pan C.G., et al. Comprehensive evaluation of antibiotics emission and fate in the river basins of China: source analysis, multimedia modeling, and linkage to bacterial resistance. Environ. Sci. Technol. 2015;49:6772–6782. doi: 10.1021/acs.est.5b00729. [DOI] [PubMed] [Google Scholar]
- 7.Qiao M., Ying G.G., Singer A.C., et al. Review of antibiotic resistance in China and its environment. Environ. Int. 2018;110:160–172. doi: 10.1016/j.envint.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 8.Marshall B.M., Levy S.B. Food animals and antimicrobials: impacts on human health. Clin. Microbiol. Rev. 2011;24:718–733. doi: 10.1128/cmr.00002-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lebov J., Grieger K., Womack D., et al. A framework for one health research. One Health (Amsterdam, Netherlands) 2017;3:44–50. doi: 10.1016/j.onehlt.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO member states adopt global action plan on antimicrobial resistance . Vol. 20. 2015. Euro surveillance: bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin. [PubMed] [Google Scholar]
- 11.Van Dijck C., Vlieghe E., Cox J.A. Antibiotic stewardship interventions in hospitals in low-and middle-income countries: a systematic review. Bull. World Health Organ. 2018;96:266–280. doi: 10.2471/blt.17.203448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cross E.L., Tolfree R., Kipping R. Systematic review of public-targeted communication interventions to improve antibiotic use. J. Antimicrob. Chemother. 2017;72:975–987. doi: 10.1093/jac/dkw520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arksey H., O'Malley L. 2005. Scoping Studies: Framework, Towards a Methodological. [Google Scholar]
- 14.Braun V., Clarke V. Using thematic analysis in psychology. Qual. Res. Psychol. 2006;3:77–101. doi: 10.1191/1478088706qp063oa. [DOI] [Google Scholar]
- 15.Shen L., Dyar O.J., Sun Q., et al. 2021. The Effectiveness of an Educational Intervention on Knowledge, Attitudes and Reported Practices on Antibiotic Use in Humans and Pigs: A Quasi-Experimental Study in Twelve Villages in Shandong Province, China. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Y., Yang K., Jing T., et al. Use of text messages to communicate clinical recommendations to health workers in rural China: a cluster-randomized trial. Bull. World Health Organ. 2014;92:474–481. doi: 10.2471/blt.13.127076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wei X., Zhang Z., Walley J.D., et al. Effect of a training and educational intervention for physicians and caregivers on antibiotic prescribing for upper respiratory tract infections in children at primary care facilities in rural China: a cluster-randomised controlled trial. Lancet Glob. Health. 2017;5:e1258–e1267. doi: 10.1016/s2214-109x(17)30383-2. [DOI] [PubMed] [Google Scholar]
- 18.Chen J., Xu M., Zhu R., et al. Development of a model to reduce antibiotic consumption: a quasi-experimental study in all 202 general hospitals in Zhejiang province, China. Lancet (London, England). 2019;394:42. [Google Scholar]
- 19.Wei X., Zhang Z., Hicks J.P., et al. Long-term outcomes of an educational intervention to reduce antibiotic prescribing for childhood upper respiratory tract infections in rural China: follow-up of a cluster-randomised controlled trial. PLoS Med. 2019;16 doi: 10.1371/journal.pmed.1002733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen J., Sun Q., Zhou X., et al. Pharmacist interventions on antibiotic use in inpatients with respiratory tract infections in a Chinese hospital. Int. J. Clin. Pharm. 2011;33:929–933. doi: 10.1007/s11096-011-9577-z. [DOI] [PubMed] [Google Scholar]
- 21.Zhang H.X., Li X., Huo H.Q., et al. Pharmacist interventions for prophylactic antibiotic use in urological inpatients undergoing clean or clean-contaminated operations in a Chinese hospital. PLoS One. 2014;9 doi: 10.1371/journal.pone.0088971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou Y., Ma L.Y., Zhao X., et al. Impact of pharmacist intervention on antibiotic use and prophylactic antibiotic use in urology clean operations. J. Clin. Pharm. Ther. 2015;40:404–408. doi: 10.1111/jcpt.12275. [DOI] [PubMed] [Google Scholar]
- 23.Zhou L., Ma J., Gao J., et al. Optimizing prophylactic antibiotic practice for cardiothoracic surgery by pharmacists’ effects. Medicine. 2016;95 doi: 10.1097/md.0000000000002753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang P., Jiang S.-P., Lu X.-Y. Effectiveness of continuous improvement by a clinical pharmacist-led guidance team on the prophylactic antibiotics usage rationality in intervention procedure at a Chinese tertiary teaching hospital. Ther. Clin. Risk Manag. 2017;13 doi: 10.2147/tcrm.S131937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li J.-S., Zhang X.-G., Wang H.-Q., et al. The meaningful use of EMR in Chinese hospitals: a case study on curbing antibiotic abuse. J. Med. Syst. 2013;37 doi: 10.1007/s10916-013-9937-4. [DOI] [PubMed] [Google Scholar]
- 26.Melku L., Wubetu M., Dessie B. Irrational drug use and its associated factors at Debre Markos referral Hospital’s outpatient pharmacy in East Gojjam, Northwest Ethiopia. SAGE Open Med. 2021;9 doi: 10.1177/20503121211025146. 20503121211025146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun X., Jackson S., Carmichael G.A., et al. Prescribing behaviour of village doctors under China’s new cooperative medical scheme. Soc. Sci. Med. 1982;68(2009):1775–1779. doi: 10.1016/j.socscimed.2009.02.043. [DOI] [PubMed] [Google Scholar]
- 28.Yip W., Powell-Jackson T., Chen W., et al. Capitation combined with pay-for-performance improves antibiotic prescribing practices in rural China. Health Affairs (Project Hope). 2014;33:502–510. doi: 10.1377/hlthaff.2013.0702. [DOI] [PubMed] [Google Scholar]
- 29.Ding D., Pan Q., Shan L., et al. Prescribing patterns in outpatient clinics of township hospitals in China: a comparative study before and after the 2009 health system reform. Int. J. Environ. Res. Public Health. 2016;13 doi: 10.3390/ijerph13070679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tao J., Zhang T., Xu J., et al. Analysis of the current situation of antibiotics use in China: a hospital-based perspective. Therap. Innovat. Regulat. Sci. 2013;47:23–31. doi: 10.1177/0092861512466397. [DOI] [PubMed] [Google Scholar]
- 31.Wei X., Yin J., Walley J.D., et al. Impact of China's essential medicines scheme and zero-mark-up policy on antibiotic prescriptions in county hospitals: a mixed methods study. Trop. Med. Int. Health: TM & IH. 2017;22:1166–1174. doi: 10.1111/tmi.12922. [DOI] [PubMed] [Google Scholar]
- 32.Song Y., Bian Y., Petzold M., et al. The impact of China’s national essential medicine system on improving rational drug use in primary health care facilities: an empirical study in four provinces. BMC Health Serv. Res. 2014;14:507. doi: 10.1186/s12913-014-0507-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yao Q., Liu C., Ferrier J.A., et al. Urban-rural inequality regarding drug prescriptions in primary care facilities - a pre-post comparison of the National Essential Medicines Scheme of China. Int. J. Equity Health. 2015;14:58. doi: 10.1186/s12939-015-0186-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiao Y., Wang J., Shen P., et al. Retrospective survey of the efficacy of mandatory implementation of the essential medicine policy in the primary healthcare setting in China: failure to promote the rational use of antibiotics in clinics. Int. J. Antimicrob. Agents. 2016;48:409–414. doi: 10.1016/j.ijantimicag.2016.06.017. [DOI] [PubMed] [Google Scholar]
- 35.Gong Y., Yang C., Yin X., et al. The effect of essential medicines programme on rational use of medicines in China. Health Policy Plan. 2016;31:21–27. doi: 10.1093/heapol/czv008. [DOI] [PubMed] [Google Scholar]
- 36.Yang L., Liu C., Ferrier J.A., et al. The impact of the National Essential Medicines Policy on prescribing behaviours in primary care facilities in Hubei province of China. Health Policy Plan. 2013;28:750–760. doi: 10.1093/heapol/czs116. [DOI] [PubMed] [Google Scholar]
- 37.Chen M., Wang L., Chen W., et al. Does economic incentive matter for rational use of medicine? China's experience from the essential medicines program. PharmacoEconomics. 2014;32:245–255. doi: 10.1007/s40273-013-0068-z. [DOI] [PubMed] [Google Scholar]
- 38.Tang Y., Liu C., Zhang Z., et al. Effects of prescription restrictive interventions on antibiotic procurement in primary care settings: a controlled interrupted time series study in China. Cost Effectiv. Res. Allocat. C/E. 2018;16:1. doi: 10.1186/s12962-018-0086-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao H., Bian J., Wei L., et al. Validation of an algorithm to evaluate the appropriateness of outpatient antibiotic prescribing using big data of Chinese diagnosis text. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2019-031191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bao L., Peng R., Wang Y., et al. Significant reduction of antibiotic consumption and patients’ costs after an action plan in China, 2010-2014. PLoS One. 2015;10 doi: 10.1371/journal.pone.0118868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma X., Xie J., Yang Y., et al. Antimicrobial stewardship of Chinese ministry of health reduces multidrug-resistant organism isolates in critically ill patients: a pre-post study from a single center. BMC Infect. Dis. 2016;16:704. doi: 10.1186/s12879-016-2051-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou W.-J., Luo Z.-N., Tang C.-M., et al. Is there an improvement of antibiotic use in China? Evidence from the usage analysis of combination antibiotic therapy for type I incisions in 244 hospitals. J. Huazhong Univ. Sci. Technol. Med. Sci. 2016;36:772–779. doi: 10.1007/s11596-016-1660-1. [DOI] [PubMed] [Google Scholar]
- 43.Chang Y.-Y., Chen H.-P., Lin C.-W., et al. Implementation and outcomes of an antimicrobial stewardship program: effectiveness of education. J. Chinese Med. Assoc. 2017;80:353–359. doi: 10.1016/j.jcma.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 44.Liu J., Li N., Hao J., et al. Impact of the antibiotic stewardship program on prevention and control of surgical site infection during peri-operative clean surgery. Surg. Infect. 2018;19:326–333. doi: 10.1089/sur.2017.201. [DOI] [PubMed] [Google Scholar]
- 45.Hou D., Wang Q., Jiang C., et al. Evaluation of the short-term effects of antimicrobial stewardship in the intensive care unit at a tertiary hospital in China. PLoS One. 2014;9 doi: 10.1371/journal.pone.0101447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang J., Zhang M.-Y., Liu J., et al. Using a targeted ecopharmacovigilance intervention to control antibiotic pollution in a rural aquatic environment. Sci. Total Environ. 2019;696 doi: 10.1016/j.scitotenv.2019.134007. [DOI] [PubMed] [Google Scholar]
- 47.Arnold S.R., Straus S.E. Interventions to improve antibiotic prescribing practices in ambulatory care. Cochrane Database Syst. Rev. 2005;2005 doi: 10.1002/14651858.CD003539.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Davey P., Marwick C.A., Scott C.L., et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst. Rev. 2017;2:Cd003543. doi: 10.1002/14651858.CD003543.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gong S., Qiu X., Song Y., et al. Effect of financially punished audit and feedback in a pediatric setting in China, within an antimicrobial stewardship program, and as part of an international accreditation process. Front. Public Health. 2016;4:99. doi: 10.3389/fpubh.2016.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McEwen S.A., Collignon P.J. Antimicrobial resistance: a one health perspective. Microbiol. Spectrum. 2018;6 doi: 10.1128/microbiolspec.ARBA-0009-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.United Nations meeting on antimicrobial resistance. Bull. World Health Organ. 2016;94:638–639. doi: 10.2471/blt.16.020916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao H., Wei L., Li H., et al. Appropriateness of antibiotic prescriptions in ambulatory care in China: a nationwide descriptive database study. Lancet Infect. Dis. 2021;21:847–857. doi: 10.1016/s1473-3099(20)30596-x. [DOI] [PubMed] [Google Scholar]
- 53.Yin J., Wang Y., Xu X., et al. The progress of global antimicrobial resistance governance and its implication to China: A review. Antibiotics (Basel, Switzerland). 2021;10 doi: 10.3390/antibiotics10111356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Coyne L.A., Latham S.M., Dawson S., et al. Antimicrobial use practices, attitudes and responsibilities in UK farm animal veterinary surgeons. Prev. Vet. Med. 2018;161:115–126. doi: 10.1016/j.prevetmed.2018.10.021. [DOI] [PubMed] [Google Scholar]
- 55.Visschers V.H.M., Iten D.M., Riklin A., et al. Swiss pig farmers’ perception and usage of antibiotics during the fattening period. Livest. Sci. 2014;162:223–232. doi: 10.1016/j.livsci.2014.02.002. [DOI] [Google Scholar]
- 56.Chen X., Wu L., Xie X. Assessing the linkages between knowledge and use of veterinary antibiotics by pig farmers in rural China. Int. J. Environ. Res. Public Health. 2018;15 doi: 10.3390/ijerph15061126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shi L., Chang J., Liu X., et al. Dispensing antibiotics without a prescription for acute cough associated with common cold at community pharmacies in Shenyang, Northeastern China: a cross-sectional study. Antibiotics (Basel, Switzerland). 2020;9 doi: 10.3390/antibiotics9040163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gong Y., Jiang N., Chen Z., et al. Over-the-counter antibiotic sales in community and online pharmacies, China. Bull. World Health Organ. 2020;98:449–457. doi: 10.2471/blt.19.242370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zawahir S., Lekamwasam S., Aslani P. A cross-sectional national survey of community pharmacy staff: knowledge and antibiotic provision. PLoS One. 2019;14 doi: 10.1371/journal.pone.0215484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Barker A.K., Brown K., Ahsan M., et al. What drives inappropriate antibiotic dispensing? A mixed-methods study of pharmacy employee perspectives in Haryana, India. BMJ Open. 2017;7 doi: 10.1136/bmjopen-2016-013190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.He P., Sun Q., Shi L., et al. Rational use of antibiotics in the context of China's health system reform. Bmj. 2019;365 doi: 10.1136/bmj.l4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1: The terms for types of interventions and study settings
Supplementary material 2
Supplementary material 3




