Abstract
High pathogenicity avian influenza virus (HPAIV) clade 2.3.4.4b has re-emerged in the United Kingdom in 2021–2022 winter season, with over 90 cases of HPAIV detected among poultry and captive birds in England, Scotland, Wales, and Northern Ireland. Globally, HPAIV H5N1 has also had a wide geographical dispersion, causing outbreaks in Europe, North America, Asia, and Africa, impacting on socioeconomic and wildlife conservation. It is important to raise awareness of the gross pathological features of HPAIV and subsequently aid disease investigation through definition of pathological indicators following natural infection. In this study, we report on the gross pathology of HPAI H5N1 in poultry species (chicken, turkey, pheasant, guineafowl, duck, goose), and captive or wild birds (mute swan, tufted duck, jackdaw, peahen, white-tailed eagle) that tested positive between October 2021 and February 2022. Pancreatic and splenic necrosis were the common pathological findings in both Galliformes and Anseriformes. In addition to the more severe lesions documented in Galliformes, we also noted increased detection of pathological changes in a broader range of Anseriformes particularly in domestic ducks, in contrast to those reported in previous seasons with other H5Nx HPAIV subtypes. A continual effort to characterise the pathological impact of the disease is necessary to update on the presentation of HPAIV for both domestic/captive and wild birds whilst guiding early presumptive diagnosis.
Keywords: HPAIV, Gross pathology, Poultry, Wild bird, Galliformes, Anseriformes
1. Introduction
High pathogenicity avian influenza viruses (HPAIV) of clade 2.3.4.4b have re-emerged in Europe during the winter of 2021–2022. The dominant virus responsible for the epizootic is HPAIV subtype H5N1 [1,2]. In the UK, more than 90 HPAIV epizootic outbreaks have been detected in England, Scotland, Wales and Northern Ireland (up until 24/02/2021) [2]. This represents a greater detection of HPAIV in poultry and captive birds than previous seasons in 2020–2021 (28 cases) and 2016–2017 (13 cases) [3,4]. To control the impact of HPAIV epizootic in poultry, up to 2.4 million birds have been culled in the UK during the current season and the strict indoor housing measures applied have also impacted on the marketing of free-range poultry products. Further, the global dissemination of HPAIV clade 2.3.4.4 has also led to outbreaks and mortalities in poultry, captive and wild birds across Europe, North America, Middle East, Africa, and the Far East [1,5,6], causing significant economic impact on producers as well as negative impact on wildlife conservation [7]. To understand and define the pathogenicity of changing HPAIV's in avian species, we describe the gross pathology findings of infection in poultry species, captive, and wild birds.
2. Materials and methods
The same inclusion criteria for data analysis and case confirmatory testing were used as per previous report [3]. Briefly, this included confirmation of HPAIV status utilising a HP subtype-specific real-time polymerase chain reaction (RT-PCR) [8], and exclusion of avian paramyxovirus type-1 by RT-PCR. Carcasses were necropsied by veterinary pathologists within the BSL3+ high containment facility at APHA Weybridge. In addition to poultry and captive birds, wild birds present in infected premises were also submitted for necropsy. The macroscopic changes included cyanosis, facial oedema, hydropericardium, epicardial petechiae, pneumonia, splenic necrosis, pancreatic necrosis, renal petechiae, ascites (defined as accumulation of clear fluid), serosal haemorrhage (excluding cases of yolk peritonitis where exudate and yolk materials are evident), hepatic necrosis, proventricular haemorrhage, coelomic surface (including rib spaces, sternum, coelomic fat pad) and skeletal muscle petechiae. GraphPad Prism 8 and Adobe Photoshop CS6 were used to create graphs and figures.
3. Results
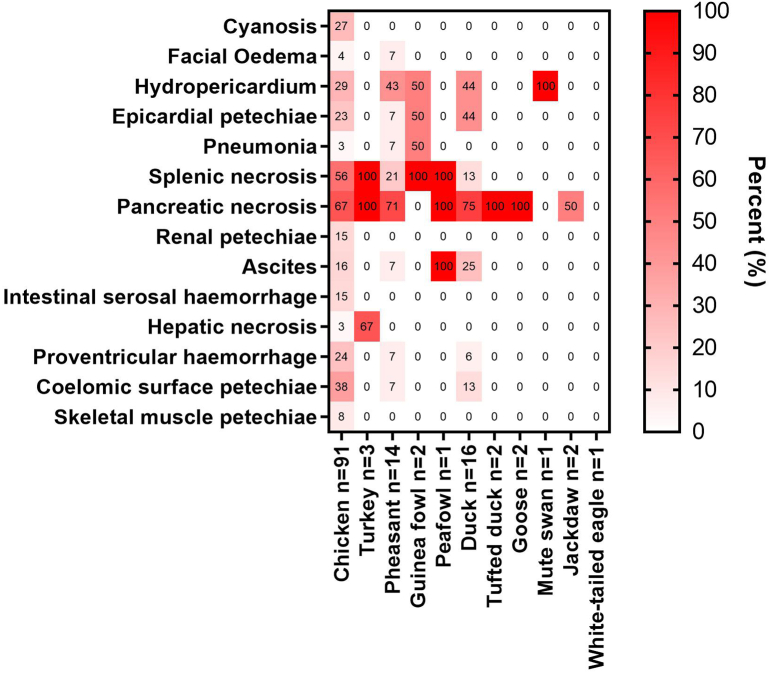
The most common lesions associated with HPAIV H5N1 infection were pancreatic and splenic necrosis (Fig. 1). Pancreatic necrosis was frequently observed in poultry Galliformes, including chickens, turkeys, and pheasants. However, pancreatic necrosis was also notable in Anseriformes examined including domestic ducks (Fig. 2a), tufted ducks, and domestic geese. Furthermore, pancreatic necrosis was also seen in a peafowl (Galliformes) and jackdaw (Passeriformes). Splenic necrosis was also commonly observed in Galliformes including chickens, turkeys, pheasants, guineafowl, and peafowl. This was infrequently observed in the domestic ducks. The incidence of pancreatic and splenic necrosis was similar in both layer and broiler chickens. Cardiac lesions such as epicardial petechiae and hydropericardium were also commonly observed in chickens, pheasants, guinea fowls and ducks.
Fig. 1.
Gross lesions observed from a subset of birds submitted for necropsy and tested positive for high pathogenicity avian influenza virus H5N1 examined over the period covering 12 October 2021 to 24 February 2022.
Species examined included chicken (Gallus gallus domesticus, n = 91), turkey (Meleagris gallopavo, n = 3), pheasant (Phasianus colchicus, n = 14), guineafowl (Numida meleagris, n = 2), peafowl (Pavo sp., n = 1), domestic duck (Anas platyrhynchos domesticus, n = 16), tufted duck (Aythya fuligula, n = 2), domestic goose (Anser anser, n = 2), mute swan (Cygnus olor, n = 1), jackdaw (Corvus monedula, n = 2), and white-tailed eagle (Haliaeetus albicilla, n = 1). The numbers in the heat map indicate the percentage of birds observed with stated pathological changes.
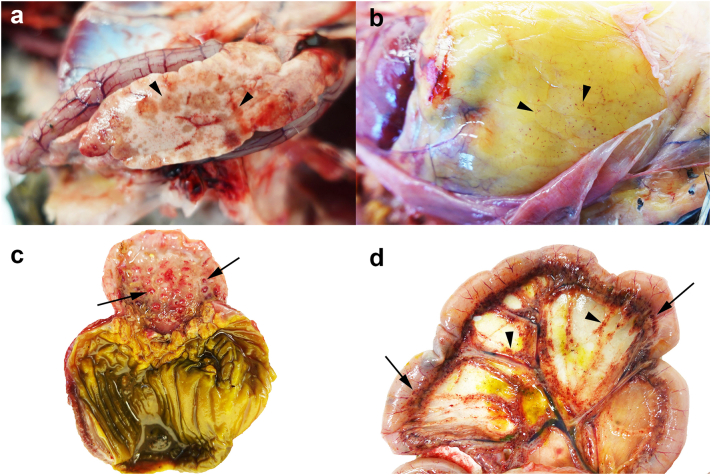
Fig. 2.
Gross lesions of high pathogenicity avian influenza virus H5N1-infected birds during the 2021–2022 season.
Lesions observed including pancreatic necrosis, commonly multifocal to coalescing tan discoloration and occasionally haemorrhagic (arrowheads; duck, a), petechiae on the coelomic fat (arrowheads; chicken, b), haemorrhage on the proventricular mucosa centred on the proventricular ducts (arrow; chicken, c), and haemorrhages on the intestinal serosa (arrow, mesenteric border) and on the mesentery (arrowheads; chicken, d).
During this 2021–2022 season, we also noted occasional haemorrhage and petechiae in coelomic structures including the coelomic fat (Fig. 2b), proventricular mucosa (Fig. 2c), intestinal serosa (Fig. 2d), renal/peri-renal fat, typically among the chickens and rarely in the ducks. Further, skeletal muscle petechiae were also observed in chickens, often around the abductor, abdominal and tibial muscle groups. Other rare lesions observed included ascites in chickens, pheasants, peafowl and ducks, and cyanosis in the chickens usually presenting in the combs or feet. Pneumonia was uncommon and was only present in small numbers of chickens, pheasants, and guinea fowl.
Overall, pancreatic and splenic necrosis were the most common features presented across various avian species infected with HPAIV. Furthermore, Galliformes particularly chicken, pheasant and guinea fowl presented a range of vascular lesions.
4. Discussion
This report provides an overview to the pathological presentation of HPAIV H5N1 infection from the 2021–2022 epizootic outbreak in the UK which is now the largest recorded UK outbreak of HPAIV [2]. Consequently, there was an increased number of carcasses and wider avian taxa under examination. The most common pathological findings associated with HPAIV H5N1 infection in poultry species include pancreatic and splenic necrosis. However, there were many instances where gross pathological changes compatible for HPAIV were lacking during necropsy.
Chickens infected with HPAIV H5N1 exhibited diverse lesions, showing some similarities to that reported in HPAIV H5N1 cases in 2020–2021 [3]. In addition, vascular lesions were more commonly observed during the 2021–2022 season, including petechiae on the epicardium and coelomic surfaces. In contrast to the previous season (H5N8 HPAIV clade 2.3.4.4b), proventricular haemorrhage was also observed, although infrequently, in chickens. Petechiae and haemorrhages in these coelomic structures, likely a reflection of acute systemic infection and endotheliotropism of HPAIV, can be very subtle and careful examination is needed to identify these changes. In addition, gross pathology changes were also frequently encountered in domestic ducks sampled during 2021–2022, in direct contrast to the unremarkable gross pathological findings from ducks either naturally or experimentally infected with other H5 clade 2.3.4.4 viruses [3,[9], [10], [11], [12]]. Pancreatic necrosis was also noted in the tufted duck, similar to other reports [13,14]. Nevertheless, the lesions observed in HPAIV H5N1 cases from 2021 to 2022 and 2020–2021 were generally more prominent than those of HPAIV H5N8 2020–2021. Moreover, the severity and diversity of pathology observed could be reflective of the increased case submissions, greater awareness of HPAIV by the field services, or could potentially relate to the pathogenicity, kinetics, and clinical progression of HPAIV H5N1. In-depth mechanistic modelling is warranted to further understand the relationship of the current circulating virus and disease outcome.
The detection of disease in minor gallinaceous species such as pheasant and guinea fowl, which are known to be highly susceptible to HPAIV, as well as apex predatory or scavenging birds infected with HPAIV, underlines the porosity of the poultry-wildlife-ecosystem interface. Minor gallinaceous species have been suggested as bridging between susceptible wild birds and poultry given the free-ranging nature attributed to farming and recreational purposes [15,16]. The incursions of HPAIV in apex predator species including the great skuas (Stercorarius skua) or common buzzards (Buteo buteo) have led to mortality events associated with virus infection in these species [7,17]. Although rare, there have been several detections of HPAIV infection of marine and terrestrial mammals where infection has occurred following likely contact with HPAIV H5N8-infected birds [18,19]. In addition, the close linkages between the Animal and Plant Health Agency (APHA) and Public Health Agency (UK Health Security Agency) have facilitated active surveillance among people exposed to the HPAIV, which enabled to the detection of HPAIV H5N1 in an asymptomatic human case during the current avian influenza season [20] These cases highlighted the importance of HPAIV disease investigations and surveillance in poultry and wild birds to recognise the risk in order to safeguard animal, environmental and public health.
The evolution of HPAIV H5 clade 2.3.4.4 presents multi-faceted challenges for poultry health and wild bird conservation [21] where reported mass mortality events present real threats to internationally threated populations and underlines the important role pathology has to play in providing insights to diseases outcomes and causal effects. Continual virological surveillance of HPAIV in wild birds and pathological investigation of HPAIV-associated mortality events helps furthering our understanding of the properties of emerging viruses to enable better risk mitigation and countermeasures. Further, this type of analysis serves as a useful resource for pathological investigation of suspected cases of HPAIV globally.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Author contributions
Conceptualisation - F.Z.X.L, A.N., R.D.E.H.; Investigation - F.Z.X.L., A.G.V., S.M.R, A.N.; Formal analysis - F.Z.X.L.; Formal analysis - F.Z.X.L.; Resources – A.C.B., I.H.B., R.D.E.H., Writing - Original Draft – F.Z.X.L.; Writing - Review & editing – All authors.
Disclosure statement
Authors declare no conflict of interest.
Acknowledgements
The authors would like to thank the laboratory members in supporting notifiable avian disease investigation. This work was funded by the Department for Environment, Food and Rural Affairs (DEFRA) and the devolved governments (Wales and Scotland) via surveillance and diagnostic contract SV3400-Monitoring for statutory and exotic virus diseases of avian species and research project, and SE2213 FluFutures 2.0 - Understanding of the diverse spectrum influenza virus-based threats to the UK.
References
- 1.Adlhoch C., Fusaro A., Gonzales J.L., Kuiken T., Marangon S., Niqueux É., et al. Avian influenza overview September – December 2021. EFSA J. 2021;(19) doi: 10.2903/j.efsa.2021.7108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Defra. 2022. Updated Outbreak Assessment #11 Highly Pathogenic Avian Influenza (HPAI) in the UK and Europe. [Google Scholar]
- 3.Lean F.Z.X., Núñez A., Banyard A.C., Reid S.M., Brown I.H., Hansen R.D.E. Gross pathology associated with highly pathogenic avian influenza H5N8 and H5N1 in naturally infected birds in the UK (2020−2021) Vet. Rec. 2021;190(1):e731. doi: 10.1002/vetr.731. [DOI] [PubMed] [Google Scholar]
- 4.Alarcon P., Brouwer A., Venkatesh D., Duncan D., Dovas C.I., Georgiades G., et al. Comparison of 2016-17 and previous epizootics of highly pathogenic avian influenza H5 Guangdong lineage in Europe. Emerg. Infect. Dis. 2018;24:2270–2283. doi: 10.3201/eid2412.171860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.OFFLU . Offlu; Canada: 2021. OFFLU Statement on Outbreak of H5N1 High Pathogenicity Avian Influenza in Newfoundland. [Google Scholar]
- 6.Caliendo V., Lewis N.S., Pohlmann A., Waldenstrom J., van Toor M., Lameris T., et al. Transatlantic spread of highly pathogenic avian influenza H5N1 by wild birds from Europe to North America in 2021. bioRxiv. 2022 doi: 10.1101/2022.01.13.476155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banyard A.C., Lean F.Z.X., Robinson C., Howie F., Tyler G., Nisbet C., et al. Detection of highly pathogenic avian influenza virus H5N1 clade 2.3.4.4b in great Skuas: a species of conservation concern in Great Britain. Viruses. 2022;14 doi: 10.3390/v14020212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.James J., Seekings A.H., Skinner P., Purchase K., Mahmood S., Brown I.H., et al. Rapid and sensitive detection of high pathogenicity Eurasian clade 2.3.4.4b avian influenza viruses in wild birds and poultry. J. Virol. Methods. 2022;114454 doi: 10.1016/j.jviromet.2022.114454. [DOI] [PubMed] [Google Scholar]
- 9.Nunez A., Brookes S.M., Reid S.M., Garcia-Rueda C., Hicks D.J., Seekings J.M., et al. Highly pathogenic avian influenza H5N8 clade 2.3.4.4 virus: equivocal pathogenicity and implications for surveillance following natural infection in breeder ducks in the United Kingdom. Transbound. Emerg. Dis. 2016;63:5–9. doi: 10.1111/tbed.12442. [DOI] [PubMed] [Google Scholar]
- 10.Pantin-Jackwood M.J., Costa-Hurtado M., Bertran K., DeJesus E., Smith D., Swayne D.E. Infectivity, transmission and pathogenicity of H5 highly pathogenic avian influenza clade 2.3.4.4 (H5N8 and H5N2) United States index viruses in Pekin ducks and Chinese geese. Vet. Res. 2017;48:33. doi: 10.1186/s13567-017-0435-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Slomka M.J., Puranik A., Mahmood S., Thomas S.S., Seekings A.H., Byrne A.M.P., et al. Ducks are susceptible to infection with a range of doses of H5N8 highly pathogenic avian influenza virus (2016, clade 2.3.4.4b) and are largely resistant to virus-specific mortality, but efficiently transmit infection to contact turkeys. Avian Dis. 2019;63:172–180. doi: 10.1637/11905-052518-Reg.1. [DOI] [PubMed] [Google Scholar]
- 12.van den Brand J.M.A., Verhagen J.H., Veldhuis Kroeze E.J.B., van de Bildt M.W.G., Bodewes R., Herfst S., et al. Wild ducks excrete highly pathogenic avian influenza virus H5N8 (2014-2015) without clinical or pathological evidence of disease. Emerg. Microb. Infect. 2018;7:67. doi: 10.1038/s41426-018-0070-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bröjer C., Ågren E.O., Uhlhorn H., Bernodt K., Mörner T., Désirée, et al. Pathology of natural highly pathogenic avian influenza H5N1 infection in wild tufted ducks (Aythya Fuligula) J. Vet. Diagn. Investig. 2009;21:579–587. doi: 10.1177/104063870902100501. [DOI] [PubMed] [Google Scholar]
- 14.Keawcharoen J., van Riel D., van Amerongen G., Bestebroer T., Beyer W.E., van Lavieren R., et al. Wild ducks as long-distance vectors of highly pathogenic avian influenza virus (H5N1) Emerg. Infect. Dis. 2008;14:600–607. doi: 10.3201/eid1404.071016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bertran K., Dolz R., Majo N. Pathobiology of avian influenza virus infection in minor gallinaceous species: a review. Avian Pathol. 2014;43:9–25. doi: 10.1080/03079457.2013.876529. [DOI] [PubMed] [Google Scholar]
- 16.Liang Y., Krog J.S., Ryt-Hansen P., Pedersen A.G., Kvisgaard L.K., Holm E., et al. Molecular characterization of highly pathogenic avian influenza viruses H5N6 detected in Denmark in 2018–2019. Viruses. 2021;13:1052. doi: 10.3390/v13061052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valentina C., Lonneke L., VDB Marco W.G., Ron A.M.F., Jolianne M.R., Thijs K. Pathology and virology of natural highly pathogenic avian influenza H5N8 infection in wild common buzzards (Buteo buteo) Sci. Rep. 2022;12:920. doi: 10.1038/s41598-022-04896-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rijks J.M., Hesselink H., Lollinga P., Wesselman R., Prins P., Weesendorp E., et al. Highly pathogenic avian influenza A(H5N1) virus in wild red foxes, the Netherlands, 2021. Emerg. Infect. Dis. 2021;27:2960–2962. doi: 10.3201/eid2711.211281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Floyd T., Banyard A.C., Lean F.Z.X., Byrne A.M.P., Fullick E., Whittard E., et al. Encephalitis and death in wild mammals at a rehabilitation center after infection with highly pathogenic avian influenza A(H5N8) virus, United Kingdom. Emerg. Infect. Dis. 2021;27:2856–2863. doi: 10.3201/eid2711.211225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oliver I., Roberts J., Brown C.S., Byrne A.M., Mellon D., Hansen R., et al. A case of avian influenza A(H5N1) in England, January 2022. Euro surveillance : bulletin Europeen sur les maladies transmissibles = Eur. Commun. Dis. Bull. 2022;27 doi: 10.2807/1560-7917.ES.2022.27.5.2200061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lewis N.S., Banyard A.C., Whittard E., Karibayev T., Al Kafagi T., Chvala I., et al. Emergence and spread of novel H5N8, H5N5 and H5N1 clade 2.3.4.4 highly pathogenic avian influenza in 2020. Emerg. Microb. Infect. 2021;10:148–151. doi: 10.1080/22221751.2021.1872355. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.