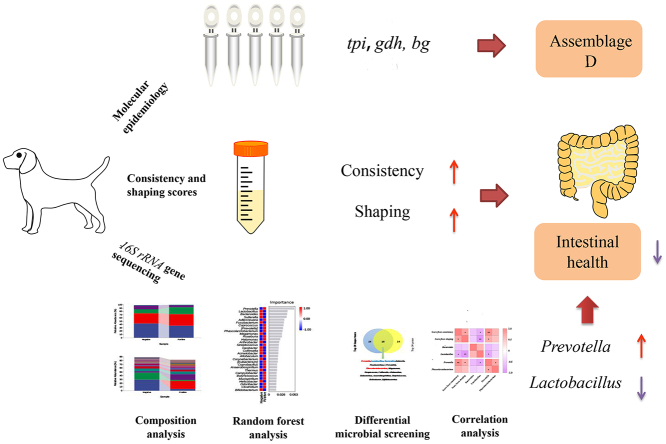
Abstract
As a common zoonotic intestinal parasite, Giardia duodenalis could infect humans and various mammals worldwide, including pet dogs, leading to giardiasis. This study detected the infection of G. duodenalis in asymptomatic pet dogs in Zhengzhou, and evaluated the possibility of zoonosis and the relationship between gut microbiota and fecal characteristics. We randomly collected 448 fresh fecal samples from Zhengzhou, and G. duodenalis was screened based on the beta-giardin (bg), glutamate dehydrogenase (gdh), and triose phosphate isomerase (tpi) genes. The difference of gut microbiota between five G. duodenalis-positive and five G. duodenalis-negative samples was investigated by 16S rRNA gene sequencing. The overall prevalence of G. duodenalis was 7.1% (32/448) based on bg, gdh, and tpi locus, two G. duodenalis assemblages (C = 13, D = 14) and five (15.6%) mixed infection (C + D) were identified. Moreover, compared with the G. duodenalis-negative group, the diversity of gut microbiota increased in G. duodenalis-positive group. The decrease of Lactobacillus spp. and considerable increase of Prevotella spp. were associated with the fecal characteristics. These results show that the transmission of zoonotic giardiasis between humans and pet dogs is rare in Zhengzhou, central China, and support the use of Lactobacillus spp. as a potential probiotic agent to improve intestinal health in dogs, or even humans, by treating G. duodenalis. Therefore, the public health significance of G. duodenalis to humans, companion animals, and the environment should be further evaluated from One Health perspective.
Keywords: Giardia duodenalis, Genetic diversity, Gut microbiota, Pet dogs, Fecal characteristics
Graphical abstract
Highlights
-
•
Prevalence of G. duodenalis was 7.1% in pet dogs in Zhengzhou, central China.
-
•
G. duodenalis assemblage D infection is associated to higher gut microbial diversity.
-
•
The abundance of Prevotella and Lactobacillus may affect fecal characteristics.
-
•
Lactobacillus may be used as a potential feed additive to treat giardiasis.
-
•
Effective strategies should be taken to minimize the threat posed by G. duodenalis.
1. Introduction
Giardia duodenalis is a foodborne and waterborne parasite, which predominantly parasitizes the duodenum of humans and various mammals with zoonosis potential [1]. The simple life cycle consists of trophozoites that cause symptoms and infective cysts that accompany host fecal shedding [2]. Susceptible hosts ingest cysts through contaminated food or water, or via the fecal-oral route, and then the fresh cysts are discharged from the host with the feces and can infect other susceptible hosts through contaminating food or water, or via the fecal-oral route [3].
Eight G. duodenalis assemblages have been named and classified to date. Infections in humans and other mammals are associated with assemblages A and B, and dogs and other canines often report being infected with assemblages C and D [3]. Sporadic reports of assemblages C, D, E and F in humans have also impacted the concept that assemblages C—H is widely considered to be host specific to a certain extent [[4], [5], [6], [7]]. G. duodenalis, a global parasite, is estimated to infect more than 280 million people worldwide [8]. At the same time, its clinical manifestations range from asymptomatic to acute or chronic diarrhea with abdominal pain and nausea [9]. However, there is still no effective and safe vaccine to prevent G. duodenalis infection, and synthetic drugs have some limitations [10].
The gut microbiota plays a key role in the homeostasis and overall health of the gut and is frequently found to be altered during parasitic infection [11]. For example, the destruction of the microbial biofilm structure, the change of virulence, abundance, and diversity of species frequently occur in the course of G. duodenalis infection [[12], [13], [14]]. Conversely, the disrupted microbiota can also affect the pathogenesis of G. duodenalis, including colonization resistance, immune response, and brush edge defects [14].
Approximately 17% of Chinese households own at least one companion animal, among which dogs are the most popular [15]. Owing to close contact with humans, dogs play an increasingly important role in the study of zoonotic pathogens transmission [16]. In addition, dogs are increasingly regarded as an ideal model system for human gut microbiota research since they often share the environment with humans, eat a similar omnivorous diet, and are often infected with intestinal parasites in early life [11].
G. duodenalis is widely detected in humans, non-human primates (NHPs), ruminants, companion animals, livestock, and wild animals and even in the environment in China [17]. The estimated prevalence of G. duodenalis among dogs in China was 9.3% and 14.3% by microscopy and serology, respectively [18]. Molecular analysis revealed that G. duodenalis was detected in approximately 12.3% of dogs in China, including aggregates A, B, C, D, E and F [18]. However, limited information about pet dogs infected with G. duodenalis is available in central China [19,20]. It has been reported that fecal characteristics are an important indicator of intestinal health [21]. The damage caused by G. duodenalis to the host intestine has also attracted substantial attention; however, the relationship between fecal characteristics and gut microbiota during infection remains unclear. Hence, this study focused on investigating the prevalence of G. duodenalis, and evaluating its genetic diversity and zoonosis potential of pet dogs in Zhengzhou, central China. The relationship between fecal characteristics and gut microbiota during infection was also investigated.
2. Materials and methods
2.1. Fecal collection and DNA extraction
From September 2017 to March 2019, 448 fresh fecal samples of asymptomatic pet dogs mainly living indoors were randomly collected in pet hospitals and pet shops in Zhengzhou, central China, and the owner's questionnaire was also collected during the collection of fecal samples. Each specimen (30–50 g) was collected immediately after being defecated and placed separately in sterile gloves marked with sample information, including ID number, date, origin, age, sex, scores of consistency and shape of feces (scored by an objective third party among six experimenters). Fecal consistency was scored on a 5-point scale proposed by Moxham [22]. Through the Zieger method [23], fecal shape was scored using a 4-point scale. After being stored in containers with ice, the specimens were immediately sent to laboratory. Fresh fecal samples were stored at −80°C after collection, and genomic DNA was extracted within one week with the E.Z.N.A. ® Stool DNA Kit (Omega Biotek Inc., Norcross, GA, USA).
2.2. PCR amplification
PCR amplification targeting the bg, gdh, and tpi genes was performed by the nested-PCR method. The resulting fragments were of 511 bp, 520 bp, and 530 bp in length, respectively (Table S1) [19,[24], [25], [26]]. The nested-PCR results of each target loci (bg, gdh, and tpi) were detected by electrophoresis with 1% agarose gel. Staining screening was performed by SYBR green (TIANDZ Inc., Beijing, China).
PCR amplification of the microbiota 16 S rRNA genes V3–V4 region of 10 fecal samples (Five were identified as G. duodenalis assemblage D only by the bg locus, while the other were negative) was performed by forward primer 338F and reverse primer 806R (Cryptosporidium and Enterocytozoon bieneusi were not detected in the 10 fecal samples) (Table S1). According to the owner questionnaire, all 10 dogs were asymptomatic males (≥12 months old), dewormed and vaccinated, lived in the house with their owners and fed commercial dog food.
2.3. Sequencing and the sequence analysis
The commercial sequencing company (SinoGenoMax, Beijing, China) conducted bidirectional sequencing for the positive PCR amplification products of the each target loci (bg, gdh, and tpi). Chromas Pro, version 2.182 was used for assemble the sequence, and resultant sequences was aligned in GenBank (http://blast.ncbi.nlm.nih.gov) by Clustal X 2.1 (http://www.clustal.org/). The nucleotide sequences of G. duodenalis have been submitted to the GenBank database (GenBank accession No. ON168743-ON168749 and ON243609).
Illumina sequencing was completed by Shanghai personal Biotechnology Co., Ltd. (Shanghai, China). QIIME2-DADA2 pipeline or Vsearch software was used to process and analyze the sequencing data. The primer fragments and mismatched primer sequences were removed using the cutadapt tool. Quality control, denoising, splicing, and chimera removal were performed by DADA2. Amplicon sequence variants (ASVs) clustering was performed with 100% sequence similarity [27]. Alpha and beta diversity indices were evaluated using the Kruskal–Wallis post-hoc test and the Bray–Curtis distance, respectively. The similarity of the bacterial community structure was evaluated by principal coordinates analysis (PCoA). In addition, 30 important ASVs were selected by the random forest algorithm. The entire 16 s rRNA gene sequence dataset in this paper has been submitted to the Sequence Read Archive (SRA)(https://www.ncbi.nlm.nih.gov/sra/)(Accession No. SUB10505899).
2.4. Statistical analysis
Data were represented by mean ± S·D, and homogeneity and normal distribution were obtained by Student's t-test in SPSS software (version 20.0, Chicago, Illinois). The correlations were determined by Spearman correlation analysis. Differences were considered statistically significant when P < 0.05.
3. Result
3.1. Prevalence of G. duodenalis in pet dogs
Simultaneous analysis of 448 fecal samples for amplification of the three loci (bg, gdh, and tpi) revealed that 32 were positive, with a positive rate of 7.1% (Table 1). It was also found that G. duodenalis existed in pet dogs of all age groups, among which the highest detection rate (9.6%, 10/104) was 6–12 months old dogs and the lowest detection rate (5.6%, 8/144) in dogs less than 6 months old (Table 1). In different genders, the infection rate of female dogs (7.2%, 13/180) was higher than that of male dogs (7.1%, 19/268). There was no significant difference between gender and age (P > 0.05) (Table 1).
Table 1.
Prevalence and genotype distribution of G. duodenalis among pet dogs of different ages and genders in Zhengzhou, central China.
| Factor |
Variable |
No. positive/no. tested (%) |
G. duodenalis Assemblages (n) |
P-value |
95% CI |
OR (95% CI) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| tpi | gdh | bg | tpi/gdh | tpi/bg | gdh/bg | tpi/gdh/bg | ||||||
| Age (month) | ≤6 | 8/144 (5.6%) | D (3) C (1) | C (1) | C/D (1) | D/D (1) | C/C/C (1) | 0.47 | 1.8–9.3 | Reference | ||
| 6–12 | 10/104 (9.6%) | D (1) | C (2) D (1) |
C/D (2) C/C (1) |
D/D (1) C/D (1) |
C/C/C (1) | 3.9–15.4 | 1.8 (0.7–4.8) |
||||
| ≥12 | 14/200 (7.0%) | C (2) | C (1) D (2) |
D (5) C (1) |
C/C (1) | C/C (1) | C/D/D (1) | 3.4–10.6 | 1.3 (0.5–3.1) |
|||
| Gender | Male | 19/268 (7.1%) | C (1) | C (1) D (4) |
D (2) C (4) |
C/D (2) | C/D (1) D/D (1) |
C/D/D (1) C/C/C (2) |
0.957 | 2.8–8.4 | Reference | |
| Female | 13/180 (7.2%) | C (1) | C (1) D (2) |
D (4) | C/D (1) | C/C (2) | C/C (1) D/D (1) |
5.1–13.8 | 1.0 (0.5–2.1) |
|||
| Total | 32/448 (7.1%) | C (2) | C (2) D (6) |
C (4) D (6) |
C/D (1) | C/C (2) C/D (2) |
C/C (1) C/D (1) D/D (2) |
C/C/C (2) C/D/D (1) |
4.7–9.5 | |||
3.2. Genotypes of G. duodenalis in pet dogs
32 samples were genotyped at one or more loci, and 21 bg, 16 gdh and 10 tpi loci sequences were successfully obtained (Table 1). Sequence analysis identified two known G. duodenalis assemblages, namely, C (40.6%, 13/32) and D (43.8%, 14/32), and mixed infections of assemblages C and D were found in five samples (15.6%, 5/32) (Table 1). These assemblages were detected in all age and gender groups (Table 1).
For the bg locus, the 21 sequences obtained were identified as one assemblage C sequence (ON168745) and two assemblage D sequences (ON168746 and ON168747). The assemblage C isolate (n = 9) shared 100% similarity with isolates collected from dogs in Croatia (JN416534), China (MK968845).The sequence ON168746 (n = 10) was identical to a Canis lupus familiaris isolate in Spain (MF285585), while the other sequence ON168747 (n = 2) was 100% similarity with isolates collected from dog in China (MN044604).
For the gdh locus, of the 16 G. duodenalis isolates successfully sequenced, 6 were identified as assemblage C sequence (ON243609), while 10 were identified as two assemblage D sequences (ON168748 and ON168749). The assemblage C sequence was identical to the reference sequence from C. lupus familiaris isolate in Japan (LC437367). The two assemblage D sequences (ON168748 and ON168749) were identical to the reference sequences from a dog isolate in China (MK968852) (n = 4), and a dog isolate in Japan (KY608978) (n = 6), respectively.
For the G. duodenalis tpi loci, 2 assemblage C sequences were identified in 10 isolates, and the sequence (ON168743) (n = 6) shared 100% homology with isolates from C. lupus familiaris in Japan (LC437552). Another assemblage C isolate (ON168744) (n = 4) shared 100% similarity with isolates collected from dogs in the Germany (KY608998), and China (KY979493).
3.3. Effects of G. duodenalis on the consistency and shape of feces in pet dogs
According to the collected questionnaire from owners and the fecal score provided by the experimenters, most of the dogs in the G. duodenalis-positive group demonstrated diarrhea, depression, and loss of appetite. The scores of consistency and shape of feces in the G. duodenalis-positive group were higher than those in the G. duodenalis-negative group (P < 0.05) (Table 2).
Table 2.
Effects of G. duodenalis (assemblage D) on fecal consistency and shape scores in pet dogs.
| Item | Score feces consistency | Score feces shape |
|---|---|---|
| Negative group | 2.83 ± 0.12 | 2.47 ± 0.41 |
| Positive group | 3.70 ± 0.07 | 3.57 ± 0.19 |
| P-value | 0.000001 | 0.002 |
3.4. Effects of G. duodenalis on the diversity and composition
1,138,613 paired-end raw reads were obtained by the Illumina platform, and 843,058 Non-Chimeric reads were generated. Finally, a total of 477,099 high-quality reads were screened (mean: 47,709.9; range: 54,554–123,303).
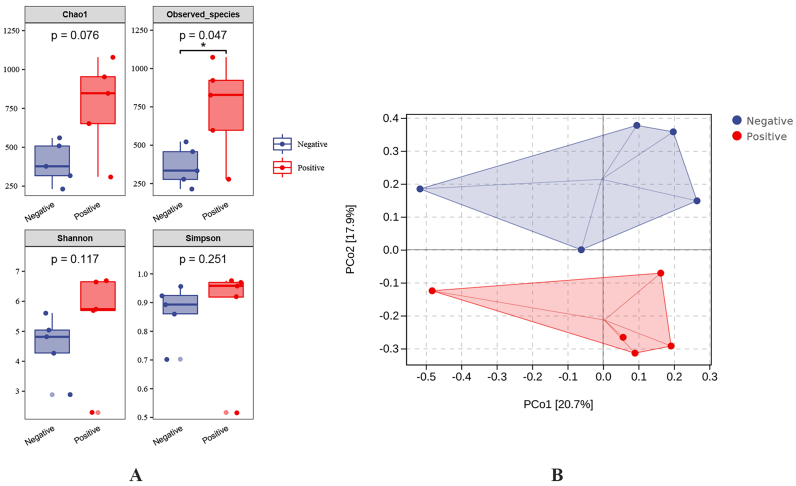
As shown in Fig. 1A, Chao1, Observed species, Shannon and Simpson indices with a higher ratio than those in the G. duodenalis-negative group all occurred in the G. duodenalis-positive group (P = 0.076, P = 0.047, P = 0.117 and P = 0.251, respectively), while the differences in Chao1, Shannon and Simpson indices were not statistically significant. PCoA showed a significant separation of gut microbiota between the two groups (PCo1: 20.7%, PCo2: 17.9%, P = 0.044) (Fig. 1B).
Fig. 1.
Alpha diversity (A) and principal coordinate analysis (PCoA) by Bray-Curtis distance (B).
3.5. Changes in the gut microbiota caused by G. duodenalis infection
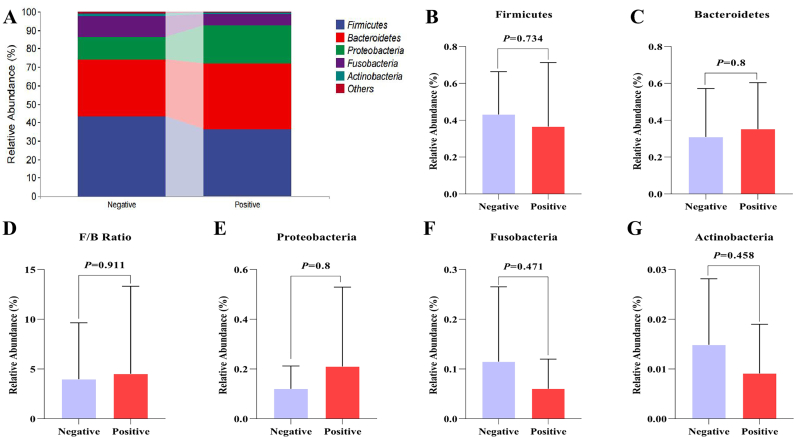
As depicted in Fig. 2A–G, the top five phyla in the two groups are Firmicutes, Bacteroides, Proteobacteria, Fusobacteria, and Actinobacteria, and the proportion of Bacteroidetes and Proteobacteria in the G. duodenalis-positive group (35.22% and 21.03%, respectively) was relatively higher than G. duodenalis-negative group (30.97% and 12.02%, respectively), whereas the proportion of Firmicutes, Fusobacteria and Actinobacteria in the G. duodenalis-positive group (36.55%, 6.04%, and 0.91%, respectively) was lower than the pet dogs that G. duodenalis-negative group (43.18%, 11.50%, and 1.49%, respectively). Furthermore, a slightly higher ratio of Firmicutes to Bacteroides (F/B) occurred in the G. duodenalis-positive group (P > 0.05).
Fig. 2.
Changes of gut microbiota at the phylum level during G. duodenalis infection. (A) The top 5 abundances of gut microbiota at the phylum level. (B–G) Differences in the abundance of Firmicutes, Bacteroidetes, Firmicutes/Bacteroidetes (F/B) ratio, Proteobacteria, Fusobacteria, and Actinobacteria, respectively.
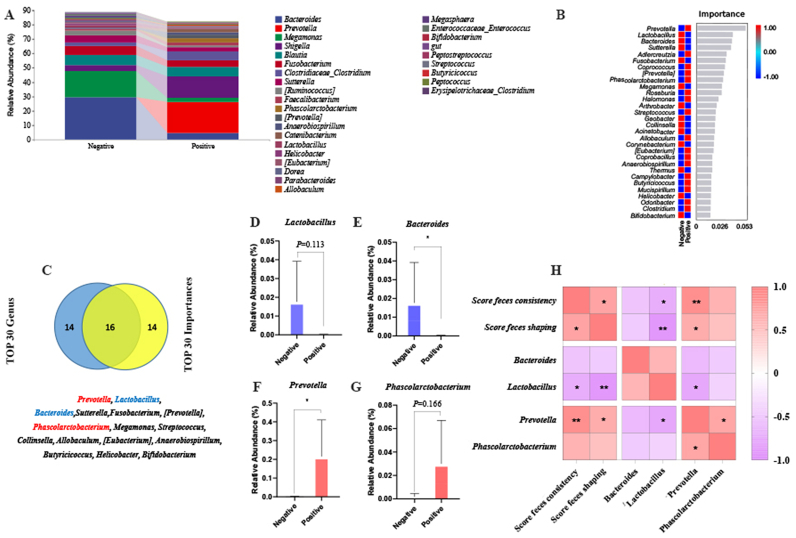
Among the Top 30 genera, Bacteroides spp., Megamonas spp., and Blautia spp. were dominant in the G. duodenalis-negative group, whereas Prevotella spp., Shigella spp., and Blautia spp. were dominant in the G. duodenalis-positive group (Fig. 3A). Furthermore, the Top 30 important genera was screen out by random forest analysis, and the highest importance included Prevotella spp., Lactobacillus spp., Bacteroides spp., and so forth (Fig. 3B). 16 genera were screened when analyzed the important Top 30 and the abundant Top 30 by Venn diagram, namely, Prevotella spp., Lactobacillus spp., Bacteroides spp., Sutterella spp., Fusobacterium spp., [Prevotella] spp., Phascolarctobacterium spp., Megamonas spp., Streptococcus spp., Collinsella spp., Allobaculum spp., Eubacterium spp., Anaerobiospirillum spp., Butyricicoccus spp., Helicobacter spp., and Bifidobacterium spp. (Fig. 3C). Next, we noticed that the abundance of Lactobacillus spp. and Bacteroides spp. was lower in the G. duodenalis-positive group, while Prevotella spp. and Phascolarctobacterium spp. were higher than G. duodenalis-negative group (P = 0.113, P = 0.048, P = 0.04 and P = 0.166, respectively; Fig. 3D–G). By Spearman correlation analysis, the consistency was significantly negatively correlated with Lactobacillus spp. (r = −0.710, P = 0.022; Fig. 3h), while it was significantly positively correlated with Prevotella spp. (r = 0.881 and P = 0.001). For shaping, Lactobacillus spp. (r = −0.892 and P = 0.001) showed a significant negative correlation, while it had a significant positive correlation with Prevotella spp. (r = 0.646 and P = 0.044).
Fig. 3.
Effects of G. duodenalis on the gut microbiota at the genus level in pet dogs. (A) The top 30 abundances of gut microbiota at the genera level. (B) Analysis of gut microbiota by the random forest algorithm. The value of the importance of genus is in the abscissa and the taxon name is in the ordinate. (C) Venn diagrams of the 30 genera with the highest abundance and the highest importance. (D–G) Differences of gut microbiota abundance at the genus level (Lactobacillus spp., Bacteroides spp., Prevotella spp., and Phascolarctobacterium spp., respectively). (H) Heat map of the correlation between gut microbiota and the consistency and shape of feces.
4. Discussion
In our study, the detection rate of G. duodenalis in pet dogs mainly living indoors from Zhengzhou, central China, was similar to that reported in Brazil (6.9%)[28], and Poland (6.0%)[29]. However, it is lower than the prevalence of rural dogs in Argentina (44.4%)[30], and Italy (15%)[31]. In this study, pet dogs living indoors rarely had the chance to contact other animals and contaminated environments [32]. Moreover, pet dogs living with their owners tended to live in more hygienic living conditions, which may also be an important factor leading to the relatively low prevalence rate of G. duodenalis [33].
Sequence analysis identified only the host-specific assemblages C and D were detected in G. duodenalis isolates from dogs in the present study [19,34,35]. Although in Egypt [36], China [37], and Colombia [38], assemblages C and D were occasionally found in human, the potential for zoonotic transmission of G. duodenalis from dogs to humans is still largely an unresolved issue [36]. Compared with the zoonotic assemblages A and B that usually exist in humans, zoonotic transmission of giardiasis has rarely occurred between humans and dogs in Zhengzhou, central China in our study. Previous studies mostly reported that mixed infection of G. duodenalis in dogs is usually composed by C and D assemblages [32,35]. Similarly, mixed combination of C and D assemblages was found in dogs in this study, revealing the diversity of G. duodenalis in our investigation area.
Fecal characteristics represent important intestinal health indicators for dogs, especially fecal consistency and shape [39]. In this study, G. duodenalis-negative dogs had significantly lower fecal consistency and shape scores than G. duodenalis-positive dogs and were closer to the ideal optimal score (score 2) [40]. Thus, this study shows that G. duodenalis will have an adverse impact on the intestinal health of pet dogs.
In the intestines of humans and other mammals, G. duodenalis infection is often associated with host flora dysbiosis [41]. Using 16S sequencing analysis, we found higher diversity and species richness in the G. duodenalis-positive group. Similar to the gut protozoan G. duodenalis, higher gut microbial diversity was also reported in E. bieneusi-positive foxes and Blastocystis-positive children [42,43]. Beta-diversity showed significant changes between the two groups, and a similar phenomenon of significant dispersion during G. duodenal infection was also reported in domestic dogs in the United States [11].
In this study, the most abundant phyla in the two groups were Firmicutes and Bacteroidetes, together accounting for more than 70% of the total. The high abundance of Firmicutes and Bacteroidetes is consistent with previous studies on canines [44,45]. Both Firmicutes and Bacteroidetes participate in the metabolic processes of the host, and Firmicutes can produce volatile fatty acids and other by-products from the metabolic process [46]. Bacteroidetes can degrade proteins, carbohydrates, and compounds in plant cell walls [47]. The Firmicutes/Bacteroidetes ratio was slightly increased in the G. duodenalis-positive group, which may be related to the pathological changes of intestinal metabolic homeostasis and inflammatory markers [48]. There was no significant difference in the ratio of Firmicutes/Bacteroides between the two groups, which may be due to the small sample size of 16S amplicon sequencing.
Moreover, through genus level screening, we found four key genera, namely, Prevotella spp., Lactobacillus spp., Bacteroides spp., and Phascolarctobacterium spp., among which, the relative abundance of Prevotella spp. and Lactobacillus spp. was significantly correlated with the consistency score and shape score of pet dog feces. Prevotella spp. plays a key role in host microbial interactions, especially in nutrition and metabolism [49]. Some studies have shown that Prevotella spp. may disrupt the intestinal microbiota and act as a proinflammatory mediator in the intestine by reducing the level of short-chain fatty acids and reducing the protective mucus layer in experimental mouse models [50,51]. Lactobacillus spp. can effectively balance the intestinal microbial environment, improve the digestibility of nutrients, promote growth and immune status, and exert a beneficial impact on pet dogs [52].Our results showed that G. duodenalis may damage the fecal characteristics by increasing and decreasing the abundance of Prevotella spp. and Lactobacillus spp., respectively.
Furthermore, the direct cytotoxicity against G. duodenalis trophozoites and the same ecological niches highlight Lactobacillus spp. as a good probiotic candidate strain for the prevention and treatment of giardiasis [[53], [54], [55]]. These studies show that Lactobacillus spp. can be given priority in the future research of probiotic preparations, which may improve the intestinal health of pet dogs by treating giardiasis. However, the mechanism by which Lactobacillus spp. improves host fecal characteristics after G. duodenalis infection is not clear, which is worthy of further study.
5. Conclusion
The total prevalence of G. duodenalis in pet dogs in Zhengzhou, central China, was 7.1% based on bg, gdh, and tpi locus, where aggregates C and D were detected in all age and gender groups. Although no zoonotic genotypes were found in this study, the possibility of potential transmission of G. duodenalis should not be ignored to reduce the possibility of intraspecific transmission. Moreover, G. duodenalis may damage the fecal characteristics by increasing and decreasing the abundance of Prevotella spp. and Lactobacillus spp., respectively. Some measures that effectively minimize the threat posed to animals and public health by G. duodenalis should be taken in Zhengzhou, central China.
Ethics statement
The review and approval of this project was conducted by the Research Ethics Committee of Henan Agricultural University (approval No. IRB-HENAU-20180914-01) and was implemented in accordance with the Guide for the Care and Use of Laboratory Animals. Prior to collecting fecal samples, the owner's consent has been obtained.
Funding
This research was supported by the National Natural Science Foundation of China (grant number 31402187); the Young Backbone Teachers Project of Colleges and Universities in Henan Province (grant number 2018GGJS031); and Project of Tackling Key Problems in Science and Technology of Henan Province (grant number 92102110079).
The following are the supplementary data related to this article.
Primer sequences for PCR amplification.
CRediT authorship contribution statement
Yuzhen Sui: Writing – original draft, Software, Formal analysis, Visualization. Xiangqian Zhang: Investigation, Formal analysis. Haidong Wang: Methodology. Liping Zheng: Methodology. Yunan Guo: Investigation. Ying Lu: Investigation. Minghui Chen: Methodology. Bukang Wang: Methodology. Hongyu Dai: Methodology. Fang Liu: Methodology. Haiju Dong: Resources, Supervision, Project administration. Chao Tong: Supervision, Resources. Longxian Zhang: Supervision, Resources.
Declaration of Competing Interest
None to report.
Acknowledgments
Thanks to Editage(www.editage.com)for the language help provided in this manuscript.
Contributor Information
Haiju Dong, Email: dongju0528@163.com.
Chao Tong, Email: chaotong@henau.edu.cn.
Longxian Zhang, Email: zhanglx8999@henau.edu.cn.
References
- 1.Barbosa J., Costa-de-Oliveira S., Rodrigues A.G., Pina-Vaz C. Optimization of a flow cytometry protocol for detection and viability assessment of Giardia lamblia. Travel Med. Infect. Dis. 2008;6(4):234–239. doi: 10.1016/j.tmaid.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Resi D., Varani S., Sannella A.R., De Pascali A.M., Ortalli M., Liguori G., Benvenuti M., Re M.C., Pirani R., Prete L., Mazzetti C., Musti M., Pizzi L., Sanna T., Cacciò S.M. November 2018 to April 2019, Euro surveillance : bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin. Vol. 26. 2021. A large outbreak of giardiasis in a municipality of the Bologna province, north-eastern Italy. (35) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang Y., Mi R., Yang L., Gong H., Xu C., Feng Y., Chen X., Huang Y., Han X., Chen Z. Wildlife is a potential source of human infections of Enterocytozoon bieneusi and Giardia duodenalis in southeastern China. Front. Microbiol. 2021;12:692837. doi: 10.3389/fmicb.2021.692837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Broglia A., Weitzel T., Harms G., Cacció S.M., Nöckler K. Molecular typing of Giardia duodenalis isolates from German travellers. Parasitol. Res. 2013;112(10):3449–3456. doi: 10.1007/s00436-013-3524-y. [DOI] [PubMed] [Google Scholar]
- 5.Gelanew T., Lalle M., Hailu A., Pozio E., Cacciò S.M. Molecular characterization of human isolates of Giardia duodenalis from Ethiopia. Acta Trop. 2007;102(2):92–99. doi: 10.1016/j.actatropica.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 6.Scalia L.A., Fava N.M., Soares R.M., Limongi J.E., da Cunha M.J., Pena I.F., Kalapothakis E., Cury M.C. Multilocus genotyping of Giardia duodenalis in Brazilian children. Trans. R. Soc. Trop. Med. Hyg. 2016;110(6):343–349. doi: 10.1093/trstmh/trw036. [DOI] [PubMed] [Google Scholar]
- 7.Štrkolcová G., Maďar M., Hinney B., Goldová M., Mojžišová J., Halánová M. Dog’s genotype of Giardia duodenalis in human: first evidence in Europe. Acta Parasitol. 2015;60(4):796–799. doi: 10.1515/ap-2015-0113. [DOI] [PubMed] [Google Scholar]
- 8.Einarsson E., Ma’ayeh S., Svärd S.G. An up-date on Giardia and giardiasis. Curr. Opin. Microbiol. 2016;34:47–52. doi: 10.1016/j.mib.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 9.Halliez M.C., Buret A.G. Extra-intestinal and long term consequences of Giardia duodenalis infections. World J. Gastroenterol. 2013;19(47):8974–8985. doi: 10.3748/wjg.v19.i47.8974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alnomasy S., Al-Awsi G.R.L., Raziani Y., Albalawi A.E., Alanazi A.D., Niazi M., Mahmoudvand H. Systematic review on medicinal plants used for the treatment of Giardia infection. Saudi J. Biol. Sci. 2021;28(9):5391–5402. doi: 10.1016/j.sjbs.2021.05.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berry A.S.F., Johnson K., Martins R., Sullivan M.C., Farias Amorim C., Putre A., Scott A., Wang S., Lindsay B., Baldassano R.N., Nolan T.J., Beiting D.P. Natural infection with Giardia is associated with altered community structure of the human and canine gut microbiome. mSphere. 2020;5(4) doi: 10.1128/mSphere.00670-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toro-Londono M.A., Bedoya-Urrego K., Garcia-Montoya G.M., Galvan-Diaz A.L., Alzate J.F. Intestinal parasitic infection alters bacterial gut microbiota in children. PeerJ. 2019;7 doi: 10.7717/peerj.6200. e6200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dann S.M., Le C.H.Y., Hanson E.M., Ross M.C., Eckmann L. Giardia infection of the small intestine induces chronic colitis in genetically susceptible hosts. J. Immunol. (Baltimore, Md.: 1950) 2018;201(2):548–559. doi: 10.4049/jimmunol.1700824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fekete E., Allain T., Siddiq A., Sosnowski O., Buret A.G. Giardia spp. and the gut microbiota: dangerous liaisons. Front. Microbiol. 2020;11:618106. doi: 10.3389/fmicb.2020.618106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.https://www.chyxx.com/industry/202006/874331.html
- 16.Esch K.J., Petersen C.A. Transmission and epidemiology of zoonotic protozoal diseases of companion animals. Clin. Microbiol. Rev. 2013;26(1):58–85. doi: 10.1128/CMR.00067-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li J., Wang H., Wang R., Zhang L. Giardia duodenalis infections in humans and other animals in China. Front. Microbiol. 2017;8:2004. doi: 10.3389/fmicb.2017.02004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao Z.Y., Li M.H., Lyu C., Meng X.Z., Qin Y.F., Yang X.B., Ma N., Zhao Q., Zhang Y., Jiang J. Prevalence of Giardia duodenalis among dogs in China from 2001 to 2021: a systematic review and Meta-analysis. Foodborne Pathog. Dis. 2022;19(3):179–191. doi: 10.1089/fpd.2021.0073. [DOI] [PubMed] [Google Scholar]
- 19.Qi M., Dong H., Wang R., Li J., Zhao J., Zhang L., Luo J. Infection rate and genetic diversity of Giardia duodenalis in pet and stray dogs in Henan Province, China. Parasitol. Int. 2016;65(2):159–162. doi: 10.1016/j.parint.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 20.Yang D., Zhang Q., Zhang L., Dong H., Jing Z., Li Z., Liu J. Prevalence and risk factors of Giardia doudenalis in dogs from China. Int. J. Environ. Health Res. 2015;25(2):207–213. doi: 10.1080/09603123.2014.915021. [DOI] [PubMed] [Google Scholar]
- 21.Bednar G.E., Murray S.M., Patil A.R., Flickinger E.A., Merchen N.R., Fahey G.C., Jr. Selected animal and plant protein sources affect nutrient digestibility and fecal characteristics of ileally cannulated dogs. Arch. Tierernahr. 2000;53(2):127–140. doi: 10.1080/17450390009381942. [DOI] [PubMed] [Google Scholar]
- 22.Clapper G., Grieshop C., Merchen N., Russett J., Brent J., Fahey G. Ileal and total tract nutrient digestibilities and fecal characteristics of dogs as affected by soybean protein inclusion in dry, extruded diets. J. Anim. Sci. 2001;79(6):1523–1532. doi: 10.2527/2001.7961523x. [DOI] [PubMed] [Google Scholar]
- 23.Zieger A.L. Tierärztliche Hochschule; Hannover: 2015. Untersuchungen zum Einsatz und Futterwert asche- und protein- bzw. keratinreicher Nebenprodukte der Geflügelschlachtung in der Fütterung von Hunden. [Google Scholar]
- 24.Cacciò S.M., Beck R., Lalle M., Marinculic A., Pozio E. Multilocus genotyping of Giardia duodenalis reveals striking differences between assemblages A and B. Int. J. Parasitol. 2008;38(13):1523–1531. doi: 10.1016/j.ijpara.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 25.Lalle M., Pozio E., Capelli G., Bruschi F., Crotti D., Cacciò S.M. Genetic heterogeneity at the beta-giardin locus among human and animal isolates of Giardia duodenalis and identification of potentially zoonotic subgenotypes. Int. J. Parasitol. 2005;35(2):207–213. doi: 10.1016/j.ijpara.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 26.Sulaiman I.M., Fayer R., Bern C., Gilman R.H., Trout J.M., Schantz P.M., Das P., Lal A.A., Xiao L. Triosephosphate isomerase gene characterization and potential zoonotic transmission of Giardia duodenalis. Emerg. Infect. Dis. 2003;9(11):1444–1452. doi: 10.3201/eid0911.030084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Edgar R.C., Haas B.J., Clemente J.C., Quince C., Knight R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics (Oxford, England) 2011;27(16):2194–2200. doi: 10.1093/bioinformatics/btr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chiebao D., Martins C., Pena H., Gabriel F., Turazza J., Soares H., Merlo A.J.Z. Epidemiological study of Giardia duodenalis infection in companion dogs from the metropolitan area of São Paulo Brazil. Zoonoses Public Health. 2020;67(7):765–773. doi: 10.1111/zph.12710. [DOI] [PubMed] [Google Scholar]
- 29.Piekara-Stępińska A., Piekarska J., Gorczykowski M., Bania J.J.A.P. Genotypes of Giardia duodenalis in household dogs and cats from Poland. 2021;66(2):428–435. doi: 10.1007/s11686-020-00292-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuthyar S., Kowalewski M., Seabolt M., Roellig D., Gillespie T.J.T., e. 2021. Diseases, Molecular Characterization of Giardia duodenalis and Evidence for Cross-Species Transmission in Northern Argentina. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berrilli F., Di Cave D., De Liberato C., Franco A., Scaramozzino P., Orecchia P. Genotype characterisation of Giardia duodenalis isolates from domestic and farm animals by SSU-rRNA gene sequencing. Vet. Parasitol. 2004;122(3):193–199. doi: 10.1016/j.vetpar.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 32.Andrea W., Rebecca R.-G., Valeria S., Philip L., Michael R.L. Prevalence of Giardia and Cryptosporidium species in dog park attending dogs compared to non-dog park attending dogs in one region of Colorado. Vet. Parasitol. 2012;184(2):335–340. doi: 10.1016/j.vetpar.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 33.Zottler E.M., Bieri M., Basso W., Schnyder M. Intestinal parasites and lungworms in stray, shelter and privately owned cats of Switzerland. Parasitol. Int. 2019;69:75–81. doi: 10.1016/j.parint.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y., Zhong Z., Deng L., Wang M., Li W., Gong C., Fu H., Cao S., Shi X., Wu K., Peng G. Detection and multilocus genotyping of Giardia duodenalis in dogs in Sichuan province, China. Parasite (Paris, France) 2017;24:31. doi: 10.1051/parasite/2017032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Y.G., Zou Y., Yu Z.Z., Chen D., Gui B.Z., Yang J.F., Zhu X.Q., Liu G.H., Zou F.C. Molecular Investigation of Zoonotic Intestinal Protozoa in Pet Dogs and Cats in Yunnan Province, Southwestern China. Pathogens (Basel, Switzerland) 2021;10(9) doi: 10.3390/pathogens10091107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soliman R.H., Fuentes I., Rubio J.M. Identification of a novel assemblage B subgenotype and a zoonotic assemblage C in human isolates of Giardia intestinalis in Egypt. Parasitol. Int. 2011;60(4):507–511. doi: 10.1016/j.parint.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 37.Liu H., Shen Y., Yin J., Yuan Z., Jiang Y., Xu Y., Pan W., Hu Y., Cao J. Prevalence and genetic characterization of Cryptosporidium, Enterocytozoon, Giardia and Cyclospora in diarrheal outpatients in China. BMC Infect. Dis. 2014;14:25. doi: 10.1186/1471-2334-14-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Villamizar X., Higuera A., Herrera G., Vasquez A.L., Buitron L., Muñoz L.M., Gonzalez C.F., Lopez M.C., Giraldo J.C., Ramírez J.D. Molecular and descriptive epidemiology of intestinal protozoan parasites of children and their pets in Cauca, Colombia: a cross-sectional study. BMC Infect. Dis. 2019;19(1):190. doi: 10.1186/s12879-019-3810-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Acuff H.L., Aldrich C.G. Evaluation of graded levels of Bacillus coagulans GBI-30, 6086 on apparent nutrient digestibility, stool quality, and intestinal health indicators in healthy adult dogs. J. Anim. Sci. 2021;99(5) doi: 10.1093/jas/skab137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ingenpaß L., Abd El-Wahab A., Ullrich C., Kölln M., Ahmed M.F.E., Visscher C., Kamphues J. Nitrogen output in the urban environment using a vegetarian canine diet. PLoS One. 2021;16(9) doi: 10.1371/journal.pone.0257364. e0257364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burgess S.L., Gilchrist C.A., Lynn T.C., Petri W.A., Jr. Parasitic protozoa and interactions with the host intestinal microbiota. Infect. Immun. 2017;85(8) doi: 10.1128/iai.00101-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kodio A., Coulibaly D., Koné A.K., Konaté S., Doumbo S., Guindo A., Bittar F., Gouriet F., Raoult D., Thera M.A., Ranque S. Blastocystis colonization is associated with increased diversity and altered gut bacterial communities in healthy Malian children. Microorganisms. 2019;7(12) doi: 10.3390/microorganisms7120649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sui Y., Tong C., Li X., Zheng L., Guo Y., Lu Y., Huang S., Wang H., Chen M., Xu C., Dai H., Liu F., Dong H., Zhang L. Molecular detection and genotyping of Enterocytozoon bieneusi in captive foxes in Xinxiang, Central China and its impact on gut bacterial communities. Res. Vet. Sci. 2021;141:138–144. doi: 10.1016/j.rvsc.2021.10.023. [DOI] [PubMed] [Google Scholar]
- 44.Wu X., Zhang H., Chen J., Shang S., Wei Q., Yan J., Tu X. Comparison of the fecal microbiota of dholes high-throughput Illumina sequencing of the V3-V4 region of the 16S rRNA gene. Appl. Microbiol. Biotechnol. 2016;100(8):3577–3586. doi: 10.1007/s00253-015-7257-y. [DOI] [PubMed] [Google Scholar]
- 45.Handl S., Dowd S.E., Garcia-Mazcorro J.F., Steiner J.M., Suchodolski J.S. Massive parallel 16S rRNA gene pyrosequencing reveals highly diverse fecal bacterial and fungal communities in healthy dogs and cats. FEMS Microbiol. Ecol. 2011;76(2):301–310. doi: 10.1111/j.1574-6941.2011.01058.x. [DOI] [PubMed] [Google Scholar]
- 46.Huang S., Zhang H. The impact of environmental heterogeneity and life stage on the hindgut microbiota of Holotrichia parallela larvae (Coleoptera: Scarabaeidae) PLoS One. 2013;8(2):e57169. doi: 10.1371/journal.pone.0057169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu H., Li Z., Si H., Zhong W., Fan Z., Li G. Comparative analysis of the gut microbiota of the blue fox (Alopex lagopus) and raccoon dog (Nyctereutes procyonoides) Arch. Microbiol. 2020;202(1):135–142. doi: 10.1007/s00203-019-01721-0. [DOI] [PubMed] [Google Scholar]
- 48.Magne F., Gotteland M., Gauthier L., Zazueta A., Pesoa S., Navarrete P., Balamurugan R. The firmicutes/bacteroidetes ratio: a relevant marker of gut dysbiosis in obese patients? Nutrients. 2020;12(5) doi: 10.3390/nu12051474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tett A., Pasolli E., Masetti G., Ercolini D., Segata N.J.N.R.M. 2021. Prevotella Diversity, Niches and Interactions with the Human Host. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Macia L., Tan J., Vieira A., Leach K., Stanley D., Luong S., Maruya M., Ian McKenzie C., Hijikata A., Wong C., Binge L., Thorburn A., Chevalier N., Ang C., Marino E., Robert R., Offermanns S., Teixeira M., Moore R., Flavell R., Fagarasan S., Mackay C.J.N.C. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. 2015;6:6734. doi: 10.1038/ncomms7734. [DOI] [PubMed] [Google Scholar]
- 51.Rolhion N., Chassaing B., Nahori M., de Bodt J., Moura A., Lecuit M., Dussurget O., Bérard M., Marzorati M., Fehlner-Peach H., Littman D., Gewirtz A., Van de Wiele T., Cossart P.J.C.H. A Listeria monocytogenes bacteriocin can target the commensal Prevotella copri and modulate intestinal infection. Microbe. 2019;26(5) doi: 10.1016/j.chom.2019.10.016. 691–701.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jang H.J., Son S., Kim J.A., Jung M.Y., Choi Y.J., Kim D.H., Lee H.K., Shin D., Kim Y. Characterization and functional test of canine probiotics. Front. Microbiol. 2021;12:625562. doi: 10.3389/fmicb.2021.625562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Allain T., Chaouch S., Thomas M., Travers M.A., Valle I., Langella P., Grellier P., Polack B., Florent I., Bermúdez-Humarán L.G. Bile salt hydrolase activities: a novel target to screen anti-Giardia lactobacilli? Front. Microbiol. 2018;9:89. doi: 10.3389/fmicb.2018.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Amer E.I., Mossallam S.F., Mahrous H. Therapeutic enhancement of newly derived bacteriocins against Giardia lamblia. Exp. Parasitol. 2014;146:52–63. doi: 10.1016/j.exppara.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 55.Travers M.A., Sow C., Zirah S., Deregnaucourt C., Chaouch S., Queiroz R.M., Charneau S., Allain T., Florent I., Grellier P. Deconjugated bile salts produced by extracellular bile-salt hydrolase-like activities from the probiotic Lactobacillus johnsonii La1 inhibit Giardia duodenalis in vitro growth. Front. Microbiol. 2016;7:1453. doi: 10.3389/fmicb.2016.01453. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primer sequences for PCR amplification.