Abstract
Purpose
Hospitalization affords an opportunity to reduce smoking, but fewer than half of patients who smoke receive evidence-based cessation treatment during inpatient stays. This study evaluated a pharmacist-led, electronic health record (EHR)–facilitated opt-out smoking cessation intervention designed to address this need.
Methods
Analyses of EHR records for adult patients who smoked in the past 30 days admitted to an academic medical center in the upper Midwest were conducted using the Reach Effectiveness Adoption Implementation Maintenance (RE-AIM) framework. The reach of a pharmacist-led, EHR-facilitated protocol for smoking cessation treatment was assessed by comparing patients’ receipt of nicotine replacement therapy (NRT) and tobacco quitline referral before and after implementation. χ2 tests, t tests, and multiple logistic regression models were used to compare reach across patient demographic groups to assess treatment disparities and the representativeness of reach. Adoption of the program by hospital services was also assessed.
Results
Of the 70 hospital services invited to implement the program, 88.6% adopted it and 78.6% had eligible admissions. Treatment reach increased as rates of delivering NRT rose from 43.6% of eligible patients before implementation to 50.4% after implementation (P < 0.0001) and quitline referral rates rose from 0.9% to 11.9% (P < 0.0001). Representativeness of reach by sex and ethnicity improved after implementation, although disparities by race and age persisted after adjustment for demographics, insurance, and primary diagnosis. Pharmacists addressed tobacco use for eligible patients in 62.5% of cases after protocol implementation.
Conclusion
Smoking cessation treatment reach and representativeness of reach improved after implementation of a proactive, pharmacist-led, EHR-facilitated opt-out smoking cessation treatment protocol in adult inpatient services.
Keywords: electronic health records, healthcare disparities, implementation science, medication reconciliation, pharmacists, tobacco use cessation
Nearly 1 in 7 US adults (representing over 34 million Americans) smoke cigarettes, and many of these individuals are hospitalized each year, providing an underutilized opportunity for smoking cessation.1-4 Although the majority of adults who smoke want to quit and more than half try to quit annually, few use broadly available evidence-based smoking cessation treatments.5 Evidence suggests that system changes in healthcare delivery (eg, systematic assessment and documentation of smoking status, training staff to refer patients to smoking cessation treatment, and hospital policies to support smoking cessation) can help to address this gap in smoking cessation treatment reach, but effect sizes are modest and there are many implementation challenges.6,7
Low reach of smoking cessation treatments is a problem across all levels of care, including in inpatient hospitalization. Although hospitalizations afford opportunities to enhance motivation to quit for health reasons and to leverage temporary abstinence to kickstart attempts at permanent abstinence, few patients who smoke at admission receive evidence-based smoking cessation treatment during or after their hospitalizations.3,4,8-12 Promising models of inpatient smoking cessation treatments are effective in helping patients quit smoking and refrain from smoking following discharge.4,6,13 Implementation of such programs is not simple, and achieving broad and equitable reach of inpatient smoking cessation treatments can be challenging.14-18
Making cessation treatment the default for all patients who smoke is an approach that shows promise to enhance the reach and equitable delivery of tobacco cessation treatments. Thus, opt-out programs make treating tobacco use the default for patients, unless they actively opt out of cessation treatment.19-23 System changes that prompt healthcare personnel to proactively provide smoking cessation treatment to all patients who smoke also seem to be particularly effective in reaching typically underserved populations that are disproportionately affected by tobacco use and historically less likely to receive cessation treatment (ie, socioeconomically disadvantaged people and members of racial/ethnic minority groups).24-26 For example, a recent cluster-randomized controlled trial showed that implementing electronic health record (EHR)–facilitated workflows that cue up smoking cessation treatment referrals in routine care at least tripled the reach of state quitline services in primary care settings and had especially high reach among African American patients and Medicaid-eligible patients.24
The first aim of the present study was to assess the reach of the new pharmacist-led inpatient smoking cessation treatment intervention by comparing rates of nicotine replacement therapy (NRT) prescribing and Wisconsin Tobacco Quit Line (WTQL) referral before and after implementation. Pharmacotherapy reach analyses also assessed shifts in NRT prescribing from physicians to pharmacists with implementation of the new workflow to identify who addressed tobacco use during the inpatient stay. The second aim was to assess the representativeness of reach of the program across patient subgroups defined by age, sex, race, ethnicity, insurance status, and primary diagnoses at discharge.
Methods
The current study evaluated a proactive, opt-out approach to treating combustible tobacco use among adult inpatients in a tertiary care university hospital in the upper Midwest. A workflow was developed to enhance implementation using the Reach Effectiveness Adoption Implementation Maintenance (RE-AIM) framework for implementation planning and evaluation.27,28 Specifically, a multidisciplinary team worked together to design implementation strategies that would (1) have broad and equitable reach by taking a population-based approach that targeted all inpatients who smoked, (2) be readily adopted by hospital services, (3) connect patients with effective treatments, and (4) be implemented in a sustainable manner.
Clinical pharmacists were targeted as the ideal group to implement the opt-out intervention. Studies have shown pharmacist-led programs addressing tobacco use to be both cost effective and efficacious.29,30 Pharmacists are well suited to offer evidence-based smoking cessation treatment, as they have the skills and training needed to recommend smoking cessation pharmacotherapies (eg, NRT). As best practices include providing both NRT and cessation counseling, pharmacists can also facilitate connections with cessation counseling through referrals to telephone tobacco quitlines, available throughout the United States.31,32 Workflows, EHR tools, and training materials needed to support implementation of the program by pharmacists and nurses were developed by the research team. Details regarding the development, formative testing, and refinement of the implementation strategy are described elsewhere.33
The intervention and implementation strategies were designed with health equity in mind. To minimize cost and access barriers to treatment, the counseling portion of the intervention included referral to free quitline telephone and/or web coaching services available 7 days a week and optional mailed 2-week NRT kits. In Wisconsin, quitline services (ie, the WTQL) are available at no cost to all state residents. Quitlines offer services in many languages and have the capacity to deliver targeted interventions to groups disproportionately affected by tobacco. For example, the WTQL offers an intensive treatment program to quit commercial tobacco use for callers who identify as American Indian or Native American.34 As such, quitlines offer promise as a way to reduce disparities in treatment access and success.
Study design.
A pre-post design was used to evaluate changes in rates of delivering smoking cessation treatment among all smokers admitted to a large Midwestern hospital from a 10-month baseline (preimplementation) period to a 10-month postimplementation period.
Intervention.
The intervention involved 2 components for eligible patients who smoked: (1) pharmacists providing NRT (eg, nicotine patches and/or lozenges or gum) during the inpatient stay and upon discharge (absent contraindication) and (2) pharmacists providing referral to the WTQL at discharge.10,31 Pharmacists ordered NRT under a standing delegation protocol, such that individual orders did not need approval from a physician. The delegation protocol did not extend to varenicline or bupropion.
At admission, nurses documented tobacco use in the 30 days before hospitalization. Then, as part of the medication history and via an EHR adaptation, pharmacists informed eligible patients who smoked that they would receive NRT during hospitalization and upon discharge and that they would be referred to the WTQL for counseling, unless they actively opted out of these treatments.
Referral to the WTQL was ordered via an EHR-based electronic referral, or “eReferral,” that prompted the WTQL to proactively call referred patients within 3 days of discharge. WTQL services include, at minimum, one 20-minute counseling call with a quit-coach and unlimited patient-initiated calls for support available 24 hours a day, 7 days a week. WTQL services also include a free mailed 2-week supply of nicotine patches, gum, or lozenges once per year (unless the patient is medically ineligible for NRT). Details regarding the EHR tools that prompted and supported the intervention are presented elsewhere.33
The hospital pharmacy and therapeutics committee and medical board approved the smoking cessation protocol. The Health Sciences institutional review board at the University of Wisconsin–Madison deemed this project exempt from review as a quality improvement study.
Inclusion/exclusion criteria.
The smoking cessation protocol specified inclusion and exclusion criteria designed with end-user input. Eligible patients were adults (≥18 years old) identified upon hospital admission as smoking cigarettes (with or without use of other tobacco products) in the past 30 days. Patients were excluded if they exclusively used tobacco products other than combustible cigarettes; were pregnant; had a cerebrovascular bypass in the past 12 weeks; had a cerebrovascular aneurysm or vasospasm in the past 4 weeks; were admitted for flap or free-flap breast reconstruction or vascular, orthopedic, or spinal surgery; were receiving intensive care unit (ICU) care; were on the burn service; or had open wounds (ie, surgical wounds not sutured/stapled, pressure ulcers of stage 2 or higher, wounds with vacuum-assisted closure). Exclusion criteria were determined by the multidisciplinary implementation team (eg, agitation due to the excitatory effects of nicotine in the ICU, elective surgeries that require smoking cessation, etc). eFigure 1 details exclusions among admissions by 10-month time period (preimplementation vs postimplementation). For patients with multiple admissions within each period, only the first eligible admission was analyzed to avoid inflation of reach estimates by inclusion of patients with multiple treatment opportunities due to rehospitalization.
Measures.
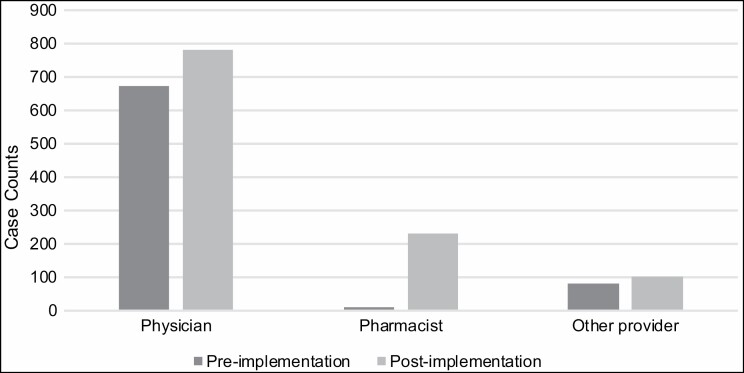
The current analyses used data collected for clinical care during hospitalizations between July 2018 and April 2019 (preimplementation) and between May 2019 and February 2020 (postimplementation). Adoption was defined as the proportion of invited hospital services that agreed to implement the intervention. Hospital services are administrative designations (eg, oncology, psychiatry, urology, etc) that can overlap hospital units. Reach was defined as the proportion of eligible inpatients who were offered and agreed to medication and/or quitline referral at their first hospitalization during the relevant period (pre- or postimplementation). All patients who met the eligibility criteria could receive some form of smoking cessation treatment from hospital pharmacists. Each treatment component was coded as binary at the patient level: pharmacist-recorded offer of treatment vs none; NRT ordered vs none; WTQL referral placed vs none; any treatment ordered (NRT or WTQL referral) vs none. For NRT ordering, we additionally assessed who ordered the medication (physician, pharmacist, or other provider) to assess prescribing task-sharing among hospital teams (Figure 1). Pharmacists could also recommend other pharmacotherapies (eg, varenicline, bupropion) not included in the delegation protocol, but a provider needed to place these medication orders.
Figure 1.
Number of patients for whom nicotine replacement therapy was ordered by physicians, pharmacists, and other hospital providers, by implementation period (n = 768 before implementation and 1,114 after implementation; n = 1 medication order was missing information on who placed the order and was omitted from this analysis).
Patient demographics, insurance status, and diagnoses were extracted from the EHR for analyses of the representativeness of reach of the intervention. Patient variables included age in years (capped at 85 years for deidentification purposes), sex (male or female), race (Black, White, or other/unknown), ethnicity (Hispanic, non-Hispanic, or unknown), primary insurance source (commercial, Medicare, Medicaid, or other/unknown), and primary diagnosis at discharge. Primary diagnosis was created using International Classification of Diseases, 10th Revision (ICD-10) codes and the Centers for Disease Control and Prevention schema for smoking-attributable mortality.35 Under this schema, 3 categories of smoking-attributable disease were identified: (1) cancers (ICD groups C18-C20 and C22), (2) cardiovascular and metabolic diseases (CVMD; ICD groups I00-I09, I20-I28, I30-I52, I60-I78, and E10-E14), and (3) pulmonary diseases (ICD groups A16-A19, J10-J18, and J40-J44). A fourth category included all other primary diagnoses.
Data analyses.
Reach was computed as the proportion of eligible patients who received an intervention at their first intervention-eligible hospitalization within the data collection periods. Postimplementation rates at which patients opted out and rates at which patients who initially opted out subsequently received treatment were also computed. Rates of reach were compared across periods (pre- vs postimplementation) and across categorical patient groups (eg, men vs women) using χ2 tests. We analyzed differences in age in those who received vs did not receive an intervention via t tests. In addition to these bivariate analyses, we conducted multivariate logistic regression models on pre- and postimplementation data to identify factors associated with intervention engagement and delivery. We also performed sensitivity analyses to assess whether our findings were robust when analyzing cumulative exposure to treatment across multiple hospitalizations rather than just first eligible hospitalizations.
Results
Adoption.
A total of 70 nonpediatric hospital services were eligible for the program. Of these services, 62 (88.6%) adopted the program. The services that declined to participate included burn, critical care, orthopedic surgery, emergency general surgery, emergency medicine, neurocritical care, surgical critical care, and vascular surgery.
Descriptive statistics.
eFigure 1 depicts hospital admission data inclusion and exclusion criteria used to select EHR records for analyses in the preimplementation and postimplementation periods. All adult hospital admissions were considered for inclusion in each time period. Following exclusions (ie, unit nonparticipation, no history of tobacco use, no current tobacco use, tobacco use without cigarette use, clinician exclusion, or diagnosis-based exclusion), the first eligible admission for each patient was selected for inclusion in analyses. These decision rules left a final sample of 1,761 eligible admissions in the preimplementation period and 2,214 eligible admissions in the postimplementation period.
Characteristics of patients who smoked before implementation and those eligible for the intervention after implementation are summarized in Table 1. No patient characteristics were significantly different between the pre- and postimplementation periods.
Table 1.
Demographics, Insurance, and Diagnoses of Eligible Patients; Rates of Treatment Receipt by Demographic, Insurance, and Diagnostic Group; and Association of Age With Treatment Receipt, by Implementation Period
| Variablea | Preimplementation Period | Postimplementation Period | |||||||
|---|---|---|---|---|---|---|---|---|---|
| All (n = 1,761)b | Received NRT (n=768) | WTQL Referral (n=16) | Any Treatment (n=772) | All (n=2,214) | Pharmacist Addressed Tobacco Use (n=1,383) | Received NRT (n=1,115) | WTQL Referral (n=264) | Any Treatment (n=1,164) | |
| Sex | |||||||||
| Female | 792 (45.0) | 321 (40.5)c | 5 (0.6) | 324 (40.9)c | 1,004 (45.4) | 616 (61.4) | 498 (49.6) | 117 (11.7) | 523 (52.1) |
| Male | 969 (55.0) | 447 (46.1) | 11 (1.1) | 448 (46.2)c | 1,210 (54.7) | 767 (63.4) | 617 (51) | 147 (12.2) | 641 (53) |
| Race | |||||||||
| Black | 175 (9.9) | 64 (36.6) | 0 (0) | 64 (36.6) | 237 (10.7) | 138 (58.2) | 108 (45.6) | 30 (12.7) | 114 (48.1) |
| Other/unknown | 61 (3.5) | 25 (41) | 0 (0) | 25 (41) | 89 (4) | 55 (61.8) | 44 (49.4) | 6 (6.7) | 44 (49.4) |
| White | 1,525 (86.6) | 670 (43.9) | 16 (1.1) | 683 (44.8) | 1,888 (85.3) | 1,190 (63) | 963 (51) | 228 (12.1) | 1,006 (53.3) |
| Ethnicity | |||||||||
| Hispanic | 47 (2.7) | 12 (25.5)c | 1 (2.1) | 12 (25.5)c | 77 (3.5) | 48 (62.3) | 32 (41.6) | 7 (9.1) | 34 (44.2) |
| Other/unknown | 27 (1.5) | 15 (55.6) | 0 (0) | 15 (55.6) | 34 (1.5) | 25 (73.5) | 20 (58.8) | 3 (8.8) | 20 (58.8) |
| Non-Hispanic | 1,687 (95.8) | 741 (43.9) | 15 (0.9) | 745 (44.2) | 2,103 (95) | 1,310 (62.3) | 1,063 (50.5) | 254 (12.1) | 1,110 (52.8) |
| Insurance | |||||||||
| Commercial | 695 (39.5) | 286 (41.2) | 7 (1) | 287 (41.3) | 795 (35.9) | 491 (61.8) | 370 (46.5)c | 103 (13) | 392 (49.3)c |
| Medicaid | 493 (28) | 251 (50.9)c | 3 (0.6) | 253 (51.3)c | 675 (30.5) | 413 (61.2) | 376 (55.7)c | 75 (11.1) | 388 (57.5)c |
| Medicare | 546 (31) | 221 (40.5) | 6 (1.1) | 222 (40.7) | 719 (32.5) | 467 (65) | 355 (49.4) | 85 (11.8) | 370 (51.5) |
| Other/unknown | 27 (1.5) | 10 (37) | 0 (0) | 10 (37) | 25 (1.1) | 12 (48) | 14 (56) | 1 (4) | 14 (56) |
| Primary diagnosis | |||||||||
| Cancer | 39 (2.2) | 12 (30.8) | 0 (0) | 12 (30.8) | 66 (3) | 47 (71.2) | 34 (51.5) | 12 (18.2) | 35 (53) |
| CVMD | 265 (15.1) | 116 (43.8) | 4 (1.5) | 117 (44.2) | 333 (15) | 206 (61.9) | 180 (54.1) | 54 (16.2)c | 194 (58.3) |
| Pulmonary | 41 (2.3) | 22 (53.7) | 2 (4.9) | 22 (53.7) | 62 (2.8) | 44 (71) | 35 (56.5) | 7 (11.3) | 36 (58.1) |
| Other | 1,416 (80.4) | 618 (43.6) | 10 (0.7) | 621 (43.9) | 1,753 (79.2) | 1,086 (62) | 866 (49.4) | 191 (10.9)c | 899 (51.3) |
| Age, mean (SD), years | 51.2 (15.3) | 50.2 (14.7) | 59.1 (6.8)c | 50.2 (14.7) | 51.5 (15.1) | 52.4 (15) | 50.9 (14.7) | 52.8 (13)c | 50.9 (14.7)c |
| Mean difference (standard error), yearsd | 1.9 (0.7) | –7.9 (3.8) | 1.8 (0.7) | –0.3 (0.5) | –2.4 (0.7) | 1.2 (0.6) | –1.5 (1) | 1.2 (0.6) | |
| Total | 1,761 (100) | 768 (43.6) | 16 (0.9) | 772 (43.8) | 2,214 (100) | 1,383 (62.5) | 1,115 (50.4) | 264 (11.9) | 1,164 (52.6) |
Abbreviations: CVMD, cardiovascular or metabolic disease; NRT, nicotine replacement therapy; WTQL, Wisconsin Tobacco Quit Line.
aData are shown as No. (%) unless indicated otherwise.
bExcludes 4 pilot services.
cStatistically significant difference (P < 0.05). Comparison group is all other groups.
dMean difference between those who received treatment and those who did not: negative values indicate that those who received treatment were older than those who did not, while positive values indicate that those who received treatment were younger than those who did not, on average.
Reach.
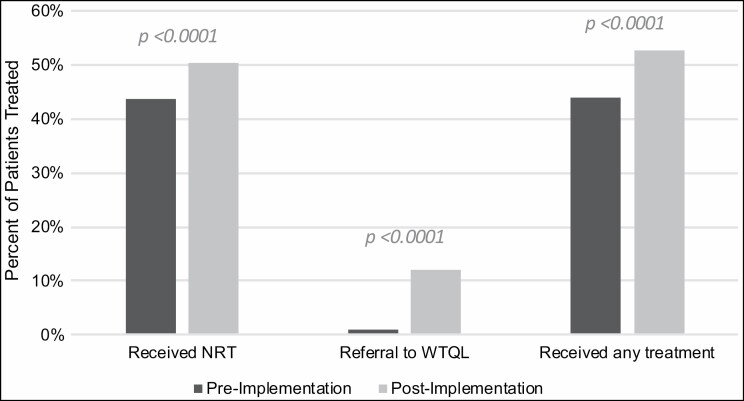
Overall, pharmacists recorded addressing tobacco use in 62.5% of eligible admissions in the postimplementation period (Table 1). Rates of providing treatment increased significantly from before to after implementation (Figure 2). Rates of ordering NRT increased from 43.6% to 50.4% (χ 2(n = 3,975) = 17.92, P < 0.0001), WTQL referral rates increased 10-fold from 0.9% to 11.9% (χ2(n = 3,975) = 181.77, P < 0.0001), and rates of any treatment (either NRT or WTQL referral, or both) increased such that a majority of eligible patients (52.6%; χ2(n = 3,975) = 29.96, P < 0.0001) received at least one treatment after implementation.
Figure 2.
The percentage of patients who received nicotine replacement therapy, Wisconsin Tobacco Quit Line referral, or any treatment (either nicotine replacement therapy or Wisconsin Tobacco Quit Line referral, or both) in their first eligible hospital admission during the preimplementation period (n = 1,761) or the postimplementation period (n = 2,214). NRT indicates nicotine replacement therapy; WTQL, Wisconsin Tobacco Quit Line.
NRT ordering increased from before to after implementation for physicians (MD, DO, or MBBS) and other hospital providers (DNP, APNP, NP, or PA), as well as pharmacists (RPh), as shown in Figure 1, but the greatest increase in ordering was observed for pharmacists (from 11 patients in the preimplementation period to 229 patients in the postimplementation period). Pharmacists placed a significantly higher proportion of all NRT orders after protocol implementation (20.6%) than before implementation (1.4%) (χ2(n = 1,882) = 149.44, P < 0.0001), while the proportion of NRT orders from physicians declined (from 87.8% to 70.2%; χ2(n = 1,882) = 80.1, P < 0.0001) and the proportion of NRT orders from other providers decreased slightly from 10.8% to 9.3% (χ2(n = 1,882) = 1.24, P = 0.26). Prescriptions for varenicline were ordered by clinicians for only 14 patients before implementation and for 15 patients after implementation, corresponding to only 1.8% and 1.4% of cessation medication orders, respectively. Bupropion was not prescribed during the study periods.
Among smoking patients for whom tobacco use was addressed, 52.8% (n = 730) and 72.9% (n = 1,008) opted out of NRT and quitline referral, respectively. However, over the course of their hospital stay, some patients who initially opted out later accepted treatment. Specifically, 25.3% (n = 185) of these “opt-out” patients received NRT during their hospital stay or at discharge, and 2.2% (n = 22) received quitline referral at discharge.
Representativeness of reach.
Table 1 displays rates of treatment reach by patient demographic, insurance, and diagnostic group. Before implementation, rates of NRT treatment and receipt of any cessation treatment were significantly lower for female than for male patients. This gender difference was not significant after implementation when rates of NRT receipt among female patients increased. Similarly, statistically significant preimplementation disparities in NRT provision by patient ethnicity (Hispanic vs non-Hispanic/unknown) were no longer significant after implementation of the protocol. No significant differences by race were observed in bivariate analyses before or after implementation.
Both before and after implementation, patients referred to the WTQL were older than those not referred, although the mean age difference was reduced after implementation as overall rates of WTQL referral increased. There was a significant age difference in receipt of any treatment after implementation, such that those treated were slightly younger than those who were not treated. Rates of NRT receipt and any treatment were significantly higher for those with Medicaid insurance as compared to other insurance types, both before and after protocol implementation. After implementation, those with commercial insurance had significantly lower rates of receiving NRT and any treatment than those with public or no insurance.
Rates of intervention did not differ significantly across primary diagnostic groups before implementation, but rates of WTQL referral were significantly higher for those with CVMD diagnoses than for other diagnoses after implementation. Those with diagnoses not closely tied to smoking were referred to the WTQL at significantly lower rates than those with cancer, CVMD, or pulmonary diagnoses after implementation.
In multivariate logistic regression analyses (Table 2), several factors were significantly related to preimplementation treatment reach, including sex, ethnicity, race, and insurance status. As in bivariate analyses, female and Hispanic patients had lower log odds of receiving NRT or any treatment when compared to male and non-Hispanic patients, respectively. Although race was unrelated to treatment in bivariate analyses, in multiple logistic regression models, Black patients had significantly lower log odds of receiving NRT or any treatment when compared to White patients. Having Medicaid as a primary insurance source was associated with a higher log odds of receiving NRT or any treatment.
Table 2.
Multiple Logistic Regression Model Adjusted Odds of Protocol Action and Treatment Delivery, by Implementation Period
| Variablea | Preimplementation Period (n = 1,761)b | Postimplementation Period (n = 2,214) | |||||
|---|---|---|---|---|---|---|---|
| Received NRT | Referred to WTQL | Any Treatment | Pharmacist Addressed Tobacco Use | Received NRT | Referred to WTQL | Any Treatment | |
| Age | 0.99 (0.99, 1.00) | 1.04 (0.99, 1.09) | 0.99 (0.99, 1.00) | 1.01 (1.00, 1.02)c | 0.99 (0.99, 1.00)c | 1.01 (1.00, 1.02) | 0.99 (0.99, 1.00)c |
| Sex (male reference group) | |||||||
| Female | 0.75 (0.62, 0.92)c | 0.55 (0.19, 1.63) | 0.76 (0.63, 0.93)c | 0.89 (0.75, 1.06) | 0.93 (0.79, 1.11) | 0.96 (0.74, 1.25) | 0.96 (0.81, 1.14) |
| Race (White reference group) | |||||||
| Black | 0.62 (0.45, 0.87)c | NAd | 0.62 (0.44, 0.86)c | 0.84 (0.64, 1.11) | 0.72 (0.54, 0.95)c | 1.10 (0.73, 1.66) | 0.73 (0.55, 0.97)c |
| Other/unknown | 0.81 (0.45, 1.46) | NAd | 0.80 (0.45, 1.44) | 0.78 (0.47, 1.31) | 0.77 (0.46, 1.29) | 0.50 (0.19, 1.35) | 0.70 (0.42, 1.17) |
| Ethnicity (Non-Hispanic reference group) | |||||||
| Hispanic | 0.38 (0.19, 0.75)c | 3.90 (0.46, 33.05) | 0.38 (0.19, 0.75)c | 1.14 (0.70, 1.84) | 0.64 (0.40, 1.03) | 0.83 (0.37, 1.86) | 0.66 (0.41, 1.06) |
| Other/unknown | 1.60 (0.68, 3.74) | NAd | 1.59 (0.68, 3.73) | 2.14 (0.89, 5.18) | 1.67 (0.74, 3.76) | 1.22 (0.30, 4.96) | 1.64 (0.73, 3.71) |
| Insurance (commercial reference group) | |||||||
| Medicaid | 1.53 (1.21, 1.95)c | 0.98(0.24, 3.98) | 1.56 (1.22, 1.98)c | 1.05(0.85, 1.31) | 1.50 (1.21, 1.86)c | 0.90(0.65, 1.25) | 1.45 (1.17, 1.79)c |
| Medicare | 1.08(0.84, 1.39) | 0.67(0.20, 2.27) | 1.07(0.83, 1.38) | 0.99(0.78, 1.25) | 1.24(0.99, 1.56) | 0.82(0.58, 1.15) | 1.21(0.96, 1.51) |
| Other/unknown | 0.84(0.37, 1.9) | NAd | 0.83(0.37, 1.88) | 0.47(0.21, 1.05) | 1.49(0.66, 3.37) | 0.24(0.03, 1.80) | 1.33(0.59, 3.00) |
| Primary diagnosis (diagnoses other than those listed below reference group) | |||||||
| Cancer | 0.61(0.3, 1.22) | NAd | 0.60(0.30, 1.21) | 1.33(0.77, 2.31) | 1.21(0.73, 1.99) | 1.69(0.88, 3.24) | 1.19(0.72, 1.96) |
| CVMD | 1.06(0.8, 1.39) | 1.74(0.52, 5.77) | 1.06(0.81, 1.40) | 0.91(0.71, 1.17) | 1.32 (1.03, 1.68)c | 1.54 (1.10, 2.16)c | 1.46 (1.14, 1.86)c |
| Pulmonary | 1.8(0.96, 3.41) | 5.69 (1.12, 29.02)c | 1.78(0.94, 3.35) | 1.34(0.76, 2.37) | 1.55(0.92, 2.61) | 0.95(0.42, 2.14) | 1.52(0.90, 2.57) |
Abbreviations: CVMD, cardiovascular or metabolic disease; NA, not available; NRT, nicotine replacement therapy; WTQL, Wisconsin Tobacco Quit Line.
aData are shown as odds ratio (95% confidence interval).
bExcludes 4 pilot services.
cStatistically significant difference (P < 0.05).
dModel estimates did not converge due to low numbers of observations.
After implementation, the only factor significantly related to whether pharmacists addressed tobacco use was age; older patients had higher log odds of receiving pharmacist intervention. Black patients had significantly lower log odds of receiving NRT or any treatment than White patients after implementation. However, in contrast to the preimplementation period, differences by sex and ethnicity were not significant for any measures of reach. Medicaid insurance was associated with increased log odds of receiving NRT or any treatment (relative to commercial insurance). In addition, rates of receiving NRT, referral to the WTQL, and any treatment were higher among those with CVMD primary diagnoses than among those with diagnoses not attributable to smoking.
Analyses of NRT orders indicated that there was greater engagement in NRT delivery by multiple clinician groups (physicians, pharmacists, and other providers) after launch of the program and that pharmacists had a greater role in providing NRT to patients who smoke.
Sensitivity analyses.
Because the primary analysis included only the first eligible hospitalization for patients, sensitivity analyses were conducted to assess the robustness of findings when all possible admissions for patients were included. Because most patients had only one admission, this sensitivity analysis used a cumulative indicator for each treatment across all admissions (eg, WTQL referral was coded as 1 if the patient was referred to the quitline at any admission and 0 otherwise), rather than a multilevel approach. Cumulative rates of treatment were only slightly higher than rates based on the first eligible admission (eTable 1). Multiple logistic regression models of cumulative treatment reach, controlling for number of admissions, yielded results similar to those of the primary analyses and are provided in eTable 2.
Discussion
The novel pharmacist-led opt-out smoking cessation treatment program appears to increase rates of receipt of smoking cessation pharmacotherapy and counseling referral. The new program connected a majority (52.6%) of eligible adult inpatients to one or more evidence-based treatments. Rates of pharmacotherapy (50.4%) were higher than rates of quitline referral (11.9%), but both treatments reached more patients after protocol implementation than before. Also, although many patients initially declined treatment, at least 1 in 4 changed their mind during hospitalization and later accepted treatment, as often happens when treatment is offered repeatedly.36 The program appears to be implemented by pharmacists in an equitable manner across gender, race, ethnic, and insurance groups and to improve treatment equity by reaching more female and Hispanic patients than usual care and by reaching many patients eligible for Medicaid. These findings add to data suggesting that programs and policies that prompt healthcare providers to proactively offer treatment to all patients who smoke may attenuate some disparities in treatment access.16,24-26 Taken together, these findings highlight the potential impact of engaging pharmacists to offer treatment to all patients who smoke at multiple points during hospital stays (eg, during medication reconciliation at admission and at discharge).
Some patient groups may benefit from targeted approaches to boost treatment reach, as racial disparities emerged when other demographic and insurance factors were controlled for, despite similar rates of pharmacist intervention with patients. Those with commercial (ie, employer-sponsored or marketplace) insurance accepted medication and referral at lower rates and may also benefit from targeted outreach regarding smoking cessation treatment coverage available under Affordable Care Act provisions.37 Future research could illuminate barriers to treatment engagement that may depress treatment reach in Black and commercially insured patients.
The program evaluated in this study has broad potential for dissemination to other hospitals. With modifications to existing EHR tools and modest training, pharmacists offered treatment to nearly two-thirds of eligible patients and ordered treatment for more than half of their patients. Importantly, the development team identified pharmacists as team members with consistent and nearly universal access to patients and then integrated smoking treatment into existing pharmacist workflows. The team also selected pharmacists as interventionists because they routinely engage in medication review at admission and discharge and are well prepared to recommend NRT to patients. Quitline referral may be less familiar and readily integrated into workflows, and this may have contributed to the lower reach of quitline services in comparison to NRT. Markedly higher rates of patients opting out of quitline referral (72.9%) in comparison to NRT (52.8%) suggest that lower patient interest in quitline services may contribute as well. Additional details regarding the development of this pharmacist-centered approach are available in Trapskin et al.33
Limitations.
The cross-sectional pre-post design of this study cannot rule out secular trends that may have contributed to increases in treatment reach and equity following implementation. Additionally, this evaluation was limited to one hospital, and results may not generalize to other settings. Also, not all hospital services participated in the initiative, although 88.6% did. In addition, treatment reach is a function of both clinician behavior (offering treatment) and patient interest in quitting and treatment. Our secondary data do not afford opportunities to model patient interest and decision-making. Survey results provide some insight into barriers perceived by participating pharmacists and patients.33 Additional barriers, facilitators, costs, and the cost-effectiveness of pharmacist engagement in the protocol merit investigation in future studies. While we were able to assess the percentage of patients for whom pharmacists provided treatment, we were not able to determine whether patients accepted quitline services or filled their prescriptions, limiting our understanding of patients’ postvisit behavior. Finally, we had some missing data. In analyses, we did not correct for false discovery rate or experiment-wise error in analyses of representativeness of reach, as we wanted to explore reach equity with maximal power.
Conclusion
Engaging clinical pharmacists in EHR-facilitated efforts with a delegation protocol to treat cigarette smoking has the potential to reach the majority of eligible patients with pharmacotherapy and/or tobacco cessation quitline services. Pharmacists may also be able to share the task of providing smoking cessation pharmacotherapy, which often falls on physicians. Proactively providing cessation treatment during bedside practice via an opt-out approach may also extend reach in an equitable manner and reduce disparities in treatment access.
Supplementary Material
Acknowledgments
We acknowledge support for this research by UW Health and the University of Wisconsin Hospitals and Clinics. We acknowledge the cooperation of Epic Systems Corporation. We thank the treating pharmacists who participated in this quality improvement project. We also acknowledge the contribution of Bill Marten at UW Health Enterprise Analytics, who provided invaluable data assistance with this manuscript.
Contributor Information
Paul D Creswell, UW Center for Tobacco Research and Intervention (UW-CTRI), University of Wisconsin School of Medicine and Public Health, Madison, WI, USA.
Danielle E McCarthy, UW Center for Tobacco Research and Intervention (UW-CTRI), University of Wisconsin School of Medicine and Public Health, Madison, WI, USA.
Philip Trapskin, UW Health, University of Wisconsin School of Pharmacy, Madison, WI, USA.
Ann Sheehy, Department of Medicine, University of Wisconsin School of Medicine and Public Health, Madison, WI, USA.
Amy Skora, UW Center for Tobacco Research and Intervention (UW-CTRI), University of Wisconsin School of Medicine and Public Health, Madison, WI, USA.
Robert T Adsit, UW Center for Tobacco Research and Intervention (UW-CTRI), University of Wisconsin School of Medicine and Public Health, Madison, WI, USA.
Mark E Zehner, UW Center for Tobacco Research and Intervention (UW-CTRI), University of Wisconsin School of Medicine and Public Health, Madison, WI, USA.
Timothy B Baker, UW Center for Tobacco Research and Intervention (UW-CTRI), University of Wisconsin School of Medicine and Public Health, Madison, WI, USA.
Michael C Fiore, UW Center for Tobacco Research and Intervention (UW-CTRI), University of Wisconsin School of Medicine and Public Health, Madison, WI, USA.
Disclosures
The authors disclose receipt of the following financial support for the research, authorship, and/or publication of this article. This work was supported by the National Cancer Institute (grant number R35CA197573). The authors have declared no potential conflicts of interest.
References
- 1. Creamer MR, Wang TW, Babb S, et al. Tobacco product use and cessation indicators among adults-United States, 2018. MMWR Morb Mortal Wkly Rep. 2019;68(45):1013-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Office on Smoking and Health, National Center for Chronic Disease Prevention and Health Promotion. Smoking Cessation: A Report of the Surgeon General. Department of Health and Human Services; 2020. https://www.hhs.gov/sites/default/files/2020-cessation-sgr-full-report.pdf [PubMed] [Google Scholar]
- 3. McBride CM, Emmons KM, Lipkus IM. Understanding the potential of teachable moments: the case of smoking cessation. Health Educ Res. 2003;18(2):156-170. [DOI] [PubMed] [Google Scholar]
- 4. Rigotti NA, Clair C, Munafò MR, Stead LF. Interventions for smoking cessation in hospitalised patients. Cochrane Database Syst Rev. 2012;5(5):CD001837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Babb S, Malarcher A, Schauer G, Asman K, Jamal A. Quitting smoking among adults-United States, 2000-2015. MMWR Morb Mortal Wkly Rep. 2017;65(52):1457-1464. [DOI] [PubMed] [Google Scholar]
- 6. Thomas D, Abramson MJ, Bonevski B, George J. System change interventions for smoking cessation. Cochrane Database Syst Rev. 2017;2(2):CD010742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Papadakis S, Cole AG, Reid RD, et al. Increasing rates of tobacco treatment delivery in primary care practice: evaluation of the Ottawa model for smoking cessation. Ann Fam Med. 2016;14(3):235-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Adams J, Cymbala AA, Delate T, et al. Cluster-randomized trial of clinical pharmacist tobacco cessation counseling among patients with cardiovascular disease. Popul Health Manag. 2015;18(4):300-306. [DOI] [PubMed] [Google Scholar]
- 9. Chui CY, Thomas D, Taylor S, et al. Factors associated with nicotine replacement therapy use among hospitalised smokers. Drug Alcohol Rev. 2018;37(4):514-519. [DOI] [PubMed] [Google Scholar]
- 10. Bjornson WG, Gonzales DH, Markin CJ, et al. Two years in the life of a university hospital tobacco cessation service: recommendations for improving the quality of referrals. Jt Comm J Qual Patient Saf. 2016;42(5):209-218. [DOI] [PubMed] [Google Scholar]
- 11. Braun BL, Fowles JB, Solberg LI, Kind EA, Lando H, Pine D. Smoking-related attitudes and clinical practices of medical personnel in Minnesota. Am J Prev Med. 2004;27(4):316-322. [DOI] [PubMed] [Google Scholar]
- 12. Thorndike AN, Rigotti NA, Stafford RS, Singer DE. National patterns in the treatment of smokers by physicians. JAMA. 1998;279(8):604-608. [DOI] [PubMed] [Google Scholar]
- 13. Rigotti NA, Stoney CM. CHARTing the future course of tobacco-cessation interventions for hospitalized smokers. Am J Prev Med. 2016;51(4):549-550. [DOI] [PubMed] [Google Scholar]
- 14. Bailey SR, Heintzman J, Jacob RL, Puro J, Marino M. Disparities in smoking cessation assistance in US primary care clinics. Am J Public Health. 2018;108(8):1082-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mullen KA, Manuel DG, Hawken SJ, et al. Effectiveness of a hospital-initiated smoking cessation programme: 2-year health and healthcare outcomes. Tob Control. 2017;26(3):293-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tan ASL, Young-Wolff KC, Carter-Harris L, Salloum RG, Banerjee SC. Disparities in the receipt of tobacco treatment counseling within the US context of the Affordable Care Act and meaningful use implementation. Nicotine Tob Res. 2018;20(12):1474-1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen J, Grossman E, Link A, Wang B. Disparities in hospital smoking cessation treatment by immigrant status. J Ethn Subst Abuse. 2020;19(1):44-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Faseru B, Yeh HW, Ellerbeck EE, Befort C, Richter KP. Prevalence and predictors of tobacco treatment in an academic medical center. Jt Comm J Qual Patient Saf. 2009;35(11):551-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kale D, Gilbert H, Sutton S. An exploration of the barriers to attendance at the English Stop Smoking Services. Addict Behav Rep. 2019;9:005-5. doi: 10.1016/j.abrep.2018.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wu Q, Gilbert H, Nazareth I, et al. Cost-effectiveness of personal tailored risk information and taster sessions to increase the uptake of the NHS stop smoking services: the Start2quit randomized controlled trial. Addiction. 2018;113(4):708-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Faseru B, Ellerbeck EF, Catley D, et al. Changing the default for tobacco-cessation treatment in an inpatient setting: study protocol of a randomized controlled trial. Trials. 2017;18(1):379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Buchanan C, Nahhas GJ, Guille C, Cummings KM, Wheeler C, McClure EA. Tobacco use prevalence and outcomes among perinatal patients assessed through an “opt-out” cessation and follow-up clinical program. Matern Child Health J. 2017;21(9):1790-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nahhas GJ, Wilson D, Talbot V, et al. Feasibility of implementing a hospital-based “opt-out” tobacco-cessation service. Nicotine Tob Res. 2017;19(8):937-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Baker TB, Berg KM, Adsit RT, et al. Closed-loop electronic referral from primary care clinics to a state tobacco cessation quitline: effects using real-world implementation training. Am J Prev Med. 2021;60(3 suppl 2):S113-S122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Adsit RT, Fox BM, Tsiolis T, et al. Using the electronic health record to connect primary care patients to evidence-based telephonic tobacco quitline services: a closed-loop demonstration project. Transl Behav Med. 2014;4(3):324-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fiore M, Adsit R, Zehner M, et al. An electronic health record-based interoperable eReferral system to enhance smoking quitline treatment in primary care. J Am Med Inform Assoc. 2019;26(8-9):778-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Glasgow RE, Estabrooks PE. Pragmatic applications of RE-AIM for health care initiatives in community and clinical settings. Prev Chronic Dis. 2018;15:E02. doi: 10.5888/pcd15.170271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Glasgow RE, Klesges LM, Dzewaltowski DA, Estabrooks PA, Vogt TM. Evaluating the impact of health promotion programs: using the RE-AIM framework to form summary measures for decision making involving complex issues. Health Educ Res. 2006;21(5):688-694. [DOI] [PubMed] [Google Scholar]
- 29. Dent LA, Harris KJ, Noonan CW. Randomized trial assessing the effectiveness of a pharmacist-delivered program for smoking cessation. Ann Pharmacother. 2009;43(2):194-201. [DOI] [PubMed] [Google Scholar]
- 30. O’Reilly E, Frederick E, Palmer E. Models for pharmacist-delivered tobacco cessation services: a systematic review. J Am Pharm Assoc. 2019;59(5):742-752. [DOI] [PubMed] [Google Scholar]
- 31. Fiore MC, Jaén CR, Baker TB, et al. Treating Tobacco Use and Dependence: 2008 Update. Department of Health and Human Services; 2008. https://www.ahrq.gov/sites/default/files/wysiwyg/professionals/clinicians-providers/guidelines-recommendations/tobacco/clinicians/update/treating_tobacco_use08.pdf [Google Scholar]
- 32. Centers for Disease Control and Prevention. 1-800-QUIT-NOW: 15 years of helping people quit smoking. Accessed November 16, 2020. https://www.cdc.gov/tobacco/features/quitlines/15th-anniversary/index.html
- 33. Trapskin PJ, Sheehy A, Creswell PD, et al. Development of a pharmacist-led opt-out cessation treatment protocol for combustible tobacco smoking within inpatient settings. Hosp Pharm. Published online March 5, 2021. doi: 10.1177/0018578721999809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wisconsin Tobacco Quit Line. American Indian Program. Accessed April 16, 2021. https://quitline.wisc.edu/american-indian-program
- 35. Centers for Disease Control and Prevention. Smoking-attributable mortality, morbidity, and economic costs (SAMMEC)—smoking-attributable expenditures (SAE). Accessed December 16, 2020. https://chronicdata.cdc.gov/Health-Consequences-and-Costs/Smoking-Attributable-Mortality-Morbidity-and-Econo/ezab-8sq5
- 36. Peters EN, Hughes JR. The day-to-day process of stopping or reducing smoking: a prospective study of self-changers. Nicotine Tob Res. 2009;11(9):1083-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Office on Smoking and Health, National Center for Chronic Disease Prevention and Health Promotion. The Health Consequences of Smoking—50 Years of Progress. Centers for Disease Control and Prevention; 2014. https://www.ncbi.nlm.nih.gov/books/NBK179276/pdf/Bookshelf_NBK179276.pdf [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.