Abstract
The effect of meat peptone type I (Sigma) on the growth of Escherichia coli cells under hyperosmotic stress has been investigated. Peptone is a complex mixture of peptides with a small content of free amino acids, which resembles nutrients found in natural environments. Our data showed that peptone enhances the growth of E. coli cells in high-osmolarity medium to levels higher than those achieved with the main compatible solute in bacteria, glycine betaine. The mechanism of osmoprotection by peptone comprises the uptake and accumulation of the compatible solute, proline. The main role of the peptides contained in peptone is the provision of nutrients rather than the intracellular accumulation of osmolytes. In contrast to Listeria monocytogenes (M. R. Amezaga, I. Davidson, D. McLaggan, A. Verheul, T. Abee, and I. R. Booth, Microbiology 141:41–49, 1995), E. coli does not accumulate exogenous peptides for osmoprotection and peptides containing proline do not lead to the accumulation of proline as a compatible solute. In late-logarithmic-phase cultures of E. coli growing at high osmolarity plus peptone, proline becomes the limiting factor for growth, and the intracellular pools of proline are not maintained. This is a consequence of the low concentration of free proline in peptone, the catabolism of proline by E. coli, and the inability of E. coli to utilize proline-containing peptides as a source of compatible solutes. Our data highlight the role that natural components in food such as peptides play in undermining food preservation regimes, such as high osmolarity, and also that the specific mechanisms of osmoprotection by these compounds differ according to the organism.
Under favorable conditions for growth, nutrient availability is the main factor that dictates the rate of growth of a microorganism. However, when cells encounter conditions of stress, such as an increase in external osmolarity, media components surpass their nutritional role and can confer protection. The main strategy of nonhalophilic bacteria to adapt to high osmolarity is the accumulation of organic solutes, known as compatible solutes or osmoprotectants, and this mechanism is associated with enhanced osmotolerance (5, 12, 13). It is well established that the intracellular accumulation of compatible solutes prevents the loss of water caused by high external osmolarity and allows the maintenance of the outwardly directed turgor pressure required for growth. Concurrently, compatible solutes do not interfere with macromolecules and allow the maintenance of high levels of cellular function (3, 5, 13, 15). The main compatible solutes in bacteria are glycine betaine (N,N,N-trimethylglycine), proline, ectoine (1,4,5,6-tetrahydro-2-methyl-4-pyrimidine carboxylic acid), and trehalose (5, 6, 12). In enterobacteria, two osmotically regulated permeases, ProP and ProU, transport glycine betaine and proline into the cell, whereas ectoine is taken up via ProP (6, 12). In addition, the permease PutP transports proline independently of medium osmolarity (27). There is a hierarchy of compatible solutes and glycine betaine is the primary physiological substrate for ProP and ProU, hence it is accumulated preferentially over proline (6, 24). In the absence of osmoprotectants in the medium, enterobacteria can also synthesize de novo the compatible solute trehalose via the otsBA operon (9, 17).
Lowering the water activity in food by adding salt or sugar is a traditional method commonly used to preserve food from spoilage and pathogenic bacteria. However, the inhibition of bacterial growth caused by high external osmolarity in food can be undermined by the presence of compatible solutes (2). For example, glycine betaine is found in higher plants, such as spinach and sugar beets, and, therefore, it is expected to be a constituent of foods containing plant tissues (29). An alternative source of glycine betaine for many bacteria is its precursor choline which is a product of the degradation of phosphatidylcholine (14, 16). This compound is an integral component of biological membranes and, therefore, is potentially abundant in many foods. Another important source of compatible solutes in food is peptides. Proteins can be broken down into peptides via the proteolytic activity associated with the normal processing of food or via the release of extracellular proteases by contaminating microorganisms (2, 26). Bacteria can transport peptides of up to eight amino acid residues via specific transport systems, and peptides are subsequently hydrolyzed to free amino acids by intracellular peptidases (2, 26).
The role of exogenous peptides in the osmoprotection of bacteria was first reported in Listeria monocytogenes (1). Although the accumulation of peptides at high osmolarity had been reported previously, γ-glutamyl-glutamine and glutathione in Escherichia coli and N-acetylglutaminyl-glutamine amide in Rhizobium meliloti and Pseudomonas aeruginosa, these peptides were synthesized de novo rather than taken up from the media (10, 21, 31). In L. monocytogenes, the uptake of peptides containing glycine, hydroxyproline, and proline leads to the intracellular accumulation of peptides and free amino acids. Both pools increase with the external osmolarity in a manner consistent with a role in osmoprotection (1). L. monocytogenes possesses at least two peptide transport systems, a ditripeptide system and an oligopeptide uptake system (32, 33). Compatible solutes such as glycine betaine are generally not catabolized in bacteria (5). An exception to this occurs in R. meliloti, which can utilize glycine betaine as a carbon and/or nitrogen source under conditions of low osmolarity (23). However, at high osmolarity, the demethylation of glycine betaine is inhibited so that it can be accumulated by R. meliloti as a compatible solute (23). In contrast, peptides can play an important role in cell metabolism by supplying essential amino acids and metabolic energy under conditions of both low and high osmolarity (26, 32). Consequently, in the mechanism of osmoprotection by exogenous peptides there is a potential nutritional component in addition to the osmotic effect. In L. monocytogenes, the identification of single peptides, such as prolyl-hydroxyproline, prolyl-glycyl-glycine, and prolyl-glycine, that behaved as compatible solutes only stimulating growth at high osmolarity, allowed the separation of the osmoprotective and the nutritional effects of peptides (1).
In this study, the effect of meat peptone type I (Sigma) on the growth of E. coli under conditions of hyperosmotic stress has been investigated. Peptone type I is a complex mixture of peptides, with a small content of free amino acids, obtained from enzymatic hydrolysis of animal proteins, that mimics the peptide-rich environment that bacteria encounter in many foods. We have established that the presence of peptone in the growth medium enhances the growth of E. coli cells under conditions of hyperosmotic stress. The osmotic component of this growth stimulation is the uptake and accumulation of free proline, whereas the main role of the peptides in peptone is nutritional supplementation. Our data emphasize the osmoprotective effect that natural components of food such as peptides and proline confer on E. coli cells, leading to a potential threat to human health by permitting their replication to high numbers before consumption, even under conditions of low water activity.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The bacterial strains used in this study are described in Table 1. Cells were grown in McIlvaine’s buffer, pH 7.0 (20), modified to include a source of potassium and adjusted to a final osmolarity of 220 mosM. The final buffer contained per liter: 60.4 mM Na2HPO4, 5 mM K2HPO4 · 3 H2O, and 7 mM anhydrous citric acid. To this buffer standard supplements were added to the following final concentrations: 400 μM MgSO4 · 7 H2O, 6 μM (NH4)2SO4FeSO4 · 6 H2O (in 0.1 mM HCl), and 1 μg · ml−1 thiamine HCl. Glucose was used as carbon source at 0.2% (wt/vol) in growth experiments and at 0.04% (wt/vol) for overnight growth under limiting glucose conditions. High osmolarity medium was prepared by adding 0.5 M NaCl to McIlvaine’s medium. We selected for our studies peptone type I (P7750; Sigma), which is an enzymatic hydrolysate of meat and is therefore free of plant produce that may contain glycine betaine (29). The glycine betaine content of our stocks of peptone was measured by 1H-nuclear magnetic resonance on a Varian 400 MHz nuclear magnetic resonance spectrometer, and it was found to be below the limit of resolution (20 μM) for such a complex mixture. The effect of peptone was assessed at a concentration of 0.5% (wt/vol). Single peptides, leucyl-proline (LP), prolyl-glycyl-glycine (PGG), and prolyl-hydroxyproline (PHP) (Sigma) were used at concentrations of 2 mM.
TABLE 1.
Bacterial strains used in this study
| Strain | Genotype | Source |
|---|---|---|
| NCIMB 10214 | Wild type | NCIMBa |
| MC4100 | F−araD139 Δ(argF lac)U169 rpsL150 (Strr) relA1 flbB5301 deoC1 ptsF25 rbsR | |
| EF063 | MC4100, ΔputPA 101 ΔproU 600 Φ(proP-lacZ+)1 [λplac-Mu55] (Kanr) | E. Bremer |
| MK11 | MC4100, ΔproU 608 (Spcr) | E. Bremer |
| Frag1 | F−thi rha gal lacZ | |
| MJF383 | Frag1, ΔlacU169 proC::Tn5 | I. R. Booth |
| J1 | Commensal | I. R. Booth |
The National Collections of Industrial and Marine Bacteria.
Exponential-phase cultures were prepared by growing overnight cultures in McIlvaine’s medium under limiting glucose conditions (0.04%, wt/vol) at 37°C with agitation (300 rpm) in a shaker-incubator (New Brunswick Scientific Co., Edison, N.J.) for approximately 16 h. Cells were subsequently supplemented with glucose (0.2%, wt/vol) and were allowed to double once. Cultures were then diluted in fresh, prewarmed medium to give a starting optical density at 650 nm (OD650) of 0.05 to 0.1 and were grown to an OD650 of 0.4. Exponential-phase cultures were then diluted in the appropriate prewarmed medium (37°C) to give a starting OD650 of 0.05 to 0.1.
Intracellular pools of amino acids and peptides.
Cells were harvested by filtration (0.45-μm-pore-size filter, Whatman) in the mid-exponential phase of growth (OD650 = 0.2), unless otherwise stated. Filters were washed immediately with prewarmed (37°C) McIlvaine’s buffer made slightly hypertonic (0.6 M NaCl) with respect to the growth medium (0.5 M NaCl) to ensure that no loss of solutes occurred. Intracellular solutes were extracted in 1 ml of ice-cold trifluoroacetic acid (0.1%, vol/vol) containing norleucine as an internal standard and were kept on ice for at least 30 min prior to the removal of filters. Lysates were stored at −20°C prior to the analysis for free amino acids exactly as described previously (1). To determine the presence of peptides, lysates were hydrolyzed prior to amino acid analysis to obtain the total concentration of amino acids. The difference between total and free amino acid pools reflected the concentration of that amino acid present as part of a peptide (1).
Extracellular proline.
One milliliter of each culture was filtered (0.45-μm-pore-size filter, Whatman), and the supernatant was recovered in an Eppendorf tube. The supernatant was stored at −20°C until subsequent analysis for free amino acids and peptides as described previously (1).
RESULTS
Peptone stimulates the growth of E. coli NCIMB 10214 under conditions of hyperosmotic stress to higher levels than glycine betaine.
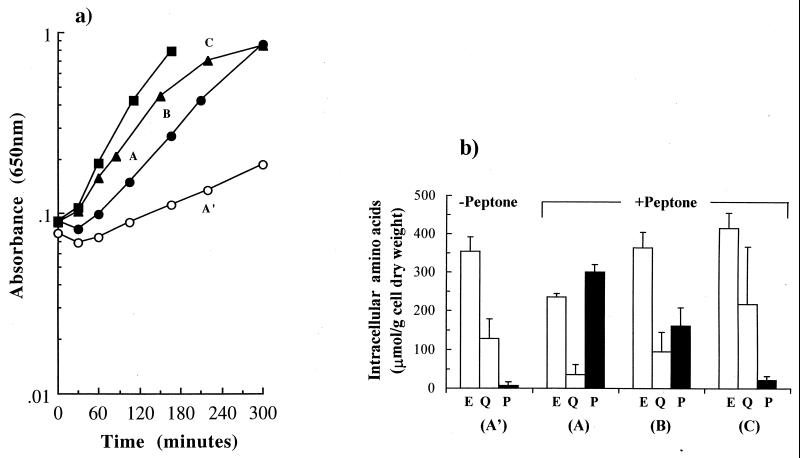
It has been shown previously that peptone protects the food-borne pathogen L. monocytogenes from hyperosmotic stress via the accumulation of peptides (1). We investigated the effect of peptone on the growth of E. coli under similar conditions. The effect of peptone (0.5%, wt/vol) on exponential-phase E. coli NCIMB 10214 cells was determined in modified McIlvaine’s medium in the presence and absence of 0.5 M NaCl (Table 2 and Fig. 1a). The growth inhibition caused by 0.5 M NaCl was reversed by the addition of peptone; the specific growth rate was stimulated (3.4 ± 0.3)-fold (Table 2 and Fig. 1a). In contrast, the growth stimulation achieved with the major compatible solute in bacteria, glycine betaine (1 mM), was lower ([2.6 ± 0.3]-fold) (Table 2 and Fig. 1a). Peptone and glycine betaine together stimulated growth to a greater extent than either alone ([4.3 ± 0.0]-fold) (Table 2 and Fig. 1a), indicating that no significant glycine betaine was present in peptone. As predicted for nutritional supplementation, peptone also stimulated the growth of E. coli at low osmolarity ([2.3 ± 0.1]-fold) but to a lesser extent than observed at high osmolarity (Table 2) so that the contribution of an osmoprotective component in addition to the nutritional effect of peptone was expected at high osmolarity.
TABLE 2.
Specific growth rates of E. coli NCIMB 10214 in the presence of peptone and glycine betaine, at low and high osmolarities
| Growth medium osmolarity | Medium content of:
|
Specific growth rate (n)a | |
|---|---|---|---|
| Peptone (0.5%, wt/vol) | Glycine betaine (1 mM) | ||
| Low | − | − | 0.67 ± 0.01 (5) |
| + | − | 1.55 ± 0.11 (4) | |
| High (+ 0.5 M NaCl) | − | − | 0.22 ± 0.02 (6) |
| + | − | 0.74 ± 0.03 (4) | |
| − | + | 0.59 ± 0.04 (2) | |
| + | + | 0.98 ± 0.07 (2) | |
Specific growth rates (shown per hour ± standard deviations) were calculated during exponential phase of growth.
FIG. 1.
Effect of peptone on the growth and the intracellular amino acid pools of E. coli NCIMB 10214 at high osmolarity. (a) Comparison of the growth stimulation by peptone and glycine betaine in the following: 0.5 M NaCl (○); 0.5 M NaCl plus 0.5% (wt/vol) peptone (▴); 0.5 M NaCl plus 1 mM glycine betaine (●); and 0.5 M NaCl plus 0.5% (wt/vol) peptone plus 1 mM glycine betaine (■). Cells were grown to exponential phase in McIlvaine’s medium exactly as described in Materials and Methods. Samples for amino acid analysis of high osmolarity cultures in the presence (A) and absence (A′) of peptone were taken in mid-log phase of growth. In addition, samples for amino acid analysis in the presence of peptone were taken at points B and C. (b) Main intracellular pools of amino acids at high osmolarity in the absence and presence of peptone (0.5%, wt/vol). Samples were taken at points indicated in panel a, and the intracellular pools of amino acids were determined exactly as described in Materials and Methods. Levels for pools of free glutamate (E), glutamine (Q), and proline (P) are indicated.
To ascertain the mechanism by which peptone stimulated the growth of E. coli at high osmolarity, the intracellular pools of free amino acids and peptides were measured in exponential-phase cells (Fig. 1a and b). The analysis of the intracellular pools of free amino acids showed that, without peptone, E. coli cells predominantly accumulated glutamate and glutamine (Fig. 1b). When peptone was added, proline was the only amino acid that E. coli cells accumulated to high concentrations, and this was accompanied by a decrease in the concentration of both glutamate and glutamine (Fig. 1b). The intracellular pools of the other amino acids were very small and did not show any significant changes in the presence of peptone (data not shown). The accumulation of a large pool of proline, which would not have occurred in the presence of the preferred compatible solute glycine betaine (6, 24), was indicative of the absence of glycine betaine in peptone-containing medium, and, therefore, glycine betaine did not contribute to the osmoprotection by peptone. In E. coli, we did not find evidence for the accumulation of peptides from peptone, since the amino acid levels in pre- and posthydrolysis samples were within experimental error of each other (data not shown). These data suggest that the accumulation of the free amino acid proline is the major osmoprotective component in the stimulatory effect of peptone at high osmolarity.
The proline pool is not maintained at high cell densities.
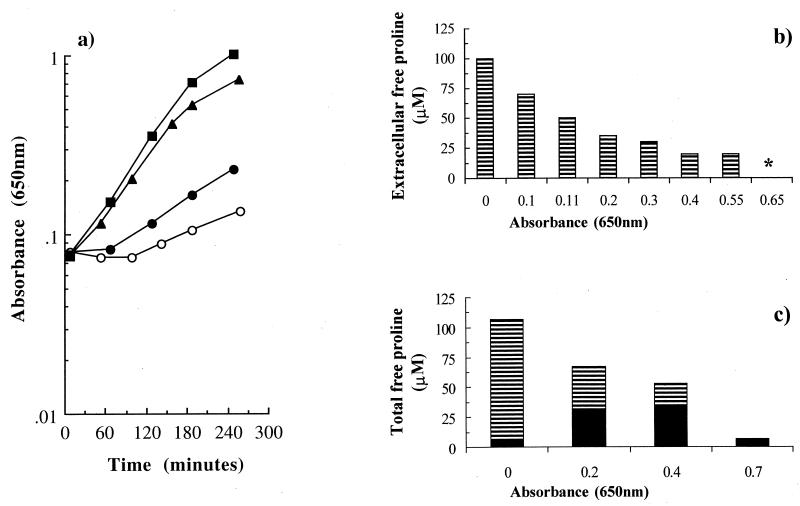
We consistently found that the stimulation of growth by peptone addition to high osmolarity medium was not sustained (Fig. 1a). As the OD650 increased, the growth rate declined (Fig. 1a). In contrast, when the effect of peptone on growth was monitored by viable counts using lower cell numbers than in our growth experiments (inoculum size, 103 cells · ml−1 instead of 107 cells · ml−1), exponential growth was sustained throughout the incubation (data not shown). These data suggest that a component in peptone became limiting for the growth of E. coli in high osmolarity medium when cultures reached high cell densities. The growth rate could be sustained at its initial value if the medium was further supplemented with either glycine betaine (Fig. 1a) or proline (Fig. 2a). A similar effect was observed in other strains of E. coli, such as the laboratory strain Frag1 and a commensal strain, J1 (data not shown). This suggested that the reduced growth rate at higher cell densities was a general phenomenon in E. coli and that it was due to the loss of the contribution to osmoregulation. Therefore, the proline pool in the cells was investigated at different cell densities (Fig. 1b). As the growth progressed, the pool of proline gradually decreased to very low concentrations, equivalent to the levels found in the absence of proline in the medium, and this was accompanied by the concomitant increase in the pools of glutamate and glutamine. Measurements of the extracellular proline confirmed that approximately 100 μM of free proline was available at the start of the experiment, but that as growth progressed, the extracellular concentration decreased to undetectable levels (Fig. 2b). The comparison of the extracellular and intracellular proline pools at set time points indicated that the total proline in the culture declined to undetectable levels over the course of the experiment (Fig. 2c). In contrast, extracellular proline was present as a component of the peptides in the medium (equivalent to 4 mM proline) and did not change significantly throughout growth (data not shown). From these data, we conclude that catabolism of the free proline leads to growth limitation of E. coli at high osmolarity and that proline as peptides is not readily metabolized by E. coli cells.
FIG. 2.
Proline becomes limiting to the growth of E. coli NCIMB 10214 at high osmolarity in the presence of peptone. (a) Growth stimulation by peptone and proline in the following: 0.5 M NaCl (○); 0.5 M NaCl plus 0.5% (wt/vol) peptone (▴); 0.5 M NaCl plus 1 mM proline (●); and 0.5 M NaCl plus 0.5% peptone plus 1 mM proline (■). (b) Extracellular free proline measured in cultures growing in the presence of peptone. Samples were taken when the culture reached the OD650s indicated, and amino acid analyses were performed exactly as described in Materials and Methods. The asterisk represents undetectable levels. (c) Composite figure of the intracellular (filled bars) and extracellular (hatched bars) concentrations of free proline in cultures growing in the presence of peptone, at the OD650s indicated.
The role of peptides in the growth of E. coli at high osmolarity is nutritional supplementation.
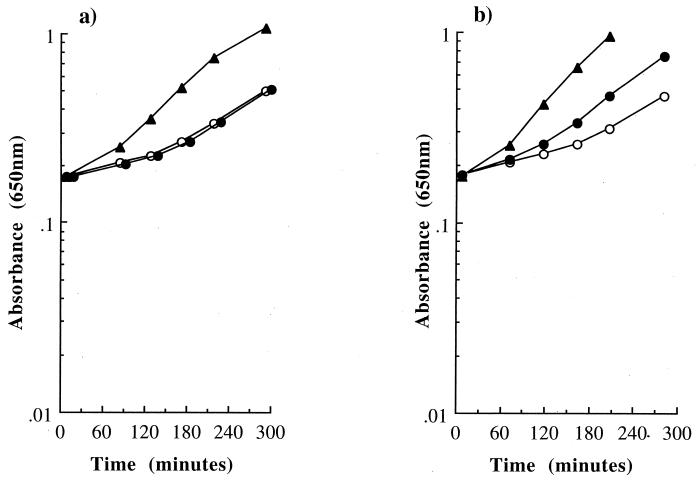
We have established that the accumulation of a large intracellular pool of free proline is an important component in the mechanism by which peptone stimulates the growth of E. coli at high osmolarity. However, supplementation of medium solely with proline (1 mM) only caused a modest stimulation of growth (Fig. 2a) despite a large intracellular proline pool (912 ± 48 μmol · g of cell [dry weight]−1). This is a much smaller growth stimulation than with peptone (μ = 0.35 ± 0.01 and 0.74 ± 0.03 h−1, for proline and peptone supplementation, respectively) (Fig. 2a). This observation suggests that the other constituents of peptone, which are mainly peptides, are largely responsible for the greater stimulation of the specific growth rate at high osmolarity. To separate the mechanisms by which the proline and peptides in peptone stimulated the growth of E. coli at high osmolarity, a mutant deficient in the transport systems for proline (and hence glycine betaine), EF063 (PutP−, ProP−, ProU−), was investigated. Strain EF063 could not take up proline, and, therefore, any stimulation of growth in the presence of peptone at high osmolarity would be due solely to the peptide fraction (Fig. 3a). The growth stimulation by peptone in EF063 was slightly lower ([1.5 ± 0.0]-fold) (Fig. 3a) than observed in an isogenic strain that can take up proline (MK11) ([2.1 ± 0.2]-fold) (Fig. 3b), and the difference could be accounted for by the effect of proline. Moreover, the growth stimulation by peptone in EF063 was not accompanied by the cytoplasmic accumulation of either free proline (8 μmol · g of cell [dry weight]−1) or proline-containing peptides (data not shown). This supports a model in which the peptide component of peptone stimulated the growth of E. coli at high osmolarity through the provision of nutrients rather than the intracellular accumulation of osmolytes, such as proline. The important nutritional role of peptides on the growth of E. coli at high osmolarity is consistent with the high level of nutritional stimulation provided by peptone at low osmolarity (Table 2). Peptone was also a more efficient source of nutrients than the free amino acids in casein hydrolysate at both low and high osmolarities (data not shown). Thus, in contrast to L. monocytogenes, we have not found evidence for an osmotic role of peptides at high osmolarity in E. coli.
FIG. 3.
Effect of addition of peptone (0.5%, wt/vol) at high osmolarity on the growth of an E. coli strain deficient in the three proline permeases (EF063). Cells were grown to exponential phase in McIlvaine’s medium exactly as described in Materials and Methods. (a) EF063, 0.5 M NaCl (○); 0.5 M NaCl plus 0.5% (wt/vol) peptone (▴); and 0.5 M NaCl plus 1 mM proline (●). (b) MK11 (isogenic strain with ProP activity). Symbols are the same as in panel a.
E. coli does not accumulate proline at high osmolarity via peptides.
Although proline-containing peptides are abundant in peptone, we have shown that in the presence of peptone, the growth of E. coli at high osmolarity becomes limited by the low concentration of free proline. By inference, proline-containing peptides do not provide E. coli with sufficient proline for osmoregulation. To investigate this, we analyzed the effect on growth of single proline-containing peptides, such as PGG, PHP, and LP. These peptides were unable to stimulate the growth of E. coli NCIMB 10214 at high osmolarity (data not shown). The analysis was extended by utilizing the peptides as a source of proline for an E. coli proline auxotroph, MJF383. Poor stimulation of growth was seen with two peptides, PPG and PHP, at either low or high osmolarity (data not shown). In contrast, the addition of LP (2 mM) was found to significantly enhance the specific growth rate of the mutant at low osmolarity (Table 3). At high osmolarity, LP also enhanced the specific growth rate of the mutant but less effectively than free proline and only to the extent of the parent strain in the absence of proline (Table 3). Similar data were obtained with the parent strain growing at high osmolarity, in that, unlike proline, LP had no osmoprotective effect (Table 3). This suggests that E. coli can utilize LP as a source of proline as a nutrient but cannot generate a large pool of proline as a compatible solute at high osmolarity.
TABLE 3.
Effect of proline and LP on the growth of a proline auxotroph of Frag1, MJF383, at low and high osmolarities
| Strain | Growth medium
|
Specific growth rate (n)a | ||
|---|---|---|---|---|
| NaCl (0.5 M) | Proline (1 mM) | LP (2 mM) | ||
| MJF383 | − | − | − | —b |
| − | + | − | 0.87 ± 0.10 (2) | |
| − | − | + | 0.81 ± 0.05 (2) | |
| + | − | − | —c | |
| + | + | − | 0.34 ± 0.01 (3) | |
| + | − | + | 0.29 ± 0.01 (3) | |
| Frag1 | − | − | − | 0.87 ± 0.07 (4) |
| + | − | − | 0.31 ± 0.02 (6) | |
| + | + | − | 0.42 ± 0.05 (4) | |
| + | − | + | 0.29 ± 0.00 (2) | |
Specific growth rates are shown per hour ± standard deviations.
Doubling time > 10 h.
Doubling time > 16 h.
Our data lead to the conclusion that in peptide-rich environments, osmotically stressed E. coli cells rely on the uptake and accumulation of free proline for osmoregulation and that the uptake of peptides provides a significant source of nutrients, but it does not provide proline as a compatible solute.
DISCUSSION
In this study, we set out to investigate the effect that natural components of food, such as peptides, have on the growth of E. coli cells under conditions of hyperosmotic stress. Peptone of animal origin was used to mimic peptide-rich foods, and the effect of single di- and tripeptides was also assessed. We have shown that in E. coli, peptone counteracts the growth inhibition caused by high osmolarity, and this occurs via the accumulation of the compatible solute proline and the nutritional stimulation by peptides. Peptone has proved to be more effective at enhancing the growth of E. coli at high osmolarity than the most powerful compatible solute in bacteria, glycine betaine. Similar effect by peptone was found in the gram-positive food-borne pathogen L. monocytogenes (1), but the detailed mechanisms of osmoprotection differ in these two organisms. The only amino acid that E. coli accumulates to a large intracellular pool when grown with peptone at high osmolarity is proline, whereas L. monocytogenes also accumulates hydroxyproline and glycine. The accumulation of proline as a compatible solute in E. coli is mediated exclusively by the uptake of free proline, whereas the transport of proline-containing peptides does not contribute to this process. The oligopeptide and dipeptide permeases of E. coli efficiently mediate the provision of proline as a nutrient, but these permeases do not seem to have evolved to meet the demand for proline as a compatible solute. Peptidase activity is not expected to be the limiting step in the ability to accumulate proline, as it is generally accepted that the intracellular peptidase activity in E. coli is high and, consequently, peptides that enter the cell will be fully hydrolyzed. In contrast, it can be hypothesized that because L. monocytogenes lacks efficient transport systems for proline, this organism has evolved an alternative route for the accumulation of proline as a compatible solute via the uptake of proline-containing peptides and their subsequent intracellular hydrolysis (1). Another main difference between these two organisms is that L. monocytogenes accumulates exogenous peptides in a manner consistent with a role in osmoprotection (1), whereas no significant accumulation of exogenous peptides was found in E. coli.
We have shown that the uptake of peptides contained in peptone plays an important role in stimulating the growth rate of E. coli at high osmolarity through the provision of nutrients rather than the intracellular accumulation of compatible solutes. Therefore, sensu stricto, the peptides in peptone are not osmoprotectants for E. coli in that, unlike L. monocytogenes, their uptake does not lead to the intracellular accumulation of free amino acids or peptides. However, sensu lato, these peptides share with other osmoprotectants the ability to counteract the inhibitory effect caused by high osmolarities in E. coli. It is becoming evident that food preservation regimes such as the addition of weak acids can affect the metabolic activity of the cell. The inhibitory effect of acetate on E. coli cells can be greatly relieved by the presence of methionine in the growth medium (30). Similarly, it is possible that high osmolarity affects the biosynthesis of a certain amino acid(s) and that the addition of peptides could compensate for such a deficiency.
We have presented data showing the disappearance of the intracellular pool of proline accumulated by osmotically stressed E. coli cells growing with glucose as a carbon source, suggesting that proline was catabolized under these conditions. In enterobacteria, proline can be utilized as the sole carbon, nitrogen, or energy source via the putPA operon (18). PutA is a bifunctional membrane-associated enzyme with both oxidase and dehydrogenase activities required to convert proline to Δ-1-pyrroline-5-carboxylate and subsequently to glutamate (22). The expression of the putPA operon is regulated at several levels; induction occurs in the presence of high intracellular proline, and it is subjected to catabolite repression via cyclic adenosine monophosphate receptor protein-cyclic AMP (4, 7, 19, 25, 27, 28). This would suggest that under our growth conditions, which include proline but also glucose as a carbon source, the catabolism of proline would be repressed. However, in E. coli, unlike in Salmonella typhimurium, if proline is present as an inducer, the repression by glucose is relieved during nitrogen starvation (25). Furthermore, in E. coli, there is a basal activity of proline oxidase, independently of nitrogen metabolism or cyclic adenosine monophosphate receptor protein-cyclic AMP (25), which is in agreement with our data. The effect of high osmolarity on the catabolism of proline has been investigated in enterobacteria in the presence of carbon sources other than glucose. In E. coli, induction of putA was shown in cells growing in 0.3 M NaCl with fructose (24). In S. typhimurium growing with succinate, 0.65 M NaCl caused 50% inhibition of the activity of proline oxidase (11). However, in E. coli cells growing with glycerol as carbon source, 0.5 M NaCl only caused a slight inhibition of the catabolism of proline, and this occurred by a general metabolic response rather than by a direct effect of high osmolarity (8). We can infer from these observations that, in E. coli, the basal activity of proline oxidase is maintained at high osmolarity (0.5 M NaCl) and that it can account for the decrease in the intracellular pool of proline that we observed under our growth conditions. The impact of proline catabolism on the effectiveness of proline as a compatible solute has been questioned before in cells growing with carbon sources other than glucose (8, 11, 24). However, to our knowledge, this is the first report in which the catabolism of proline in the presence of glucose, coupled with the inability to accumulate proline as a compatible solute from peptides, has been shown to be detrimental to the growth of osmotically stressed E. coli cultures. This would occur when high cell densities are reached in natural environments where peptides are the main source of proline and low levels of the free amino acid are available. It is therefore seemingly paradoxical that, under these growth conditions, the growth of the cell can be compromised by its own catabolic activity.
Our results lead to the conclusion that the presence of natural components of food such as peptides protects E. coli cells from hyperosmotic stress. If proline is also present, the growth will be further enhanced. This study highlights the importance that availability of osmoprotectants and nutrients in the medium has on the growth of potential pathogenic bacteria in low-water-activity environments. The ability of these compounds to protect cells against osmotic stress will depend primarily on the presence of efficient transport systems, and this will vary according to the organism. In the case of peptides and proline, other physiological mechanisms, such as intracellular peptidases and catabolic activity, will also be important considerations.
ACKNOWLEDGMENTS
M.-R.A. was funded by MAFF (Project FS 1527 to I.R.B. and J.I.P.), and I.R.B. is a Wellcome Trust Research leave Fellow.
We acknowledge the support of Debbie McLaggan. We thank Erhard Bremer for the provision of strains. We thank Marcel Jaspars (Department of Chemistry, University of Aberdeen) for the NMR measurements of glycine betaine in peptone.
REFERENCES
- 1.Amezaga M R, Davidson I, McLaggan D, Verheul A, Abee T, Booth I R. The role of peptide metabolism in the growth of Listeria monocytogenes ATCC 23074 at high osmolarity. Microbiology. 1995;141:41–49. doi: 10.1099/00221287-141-1-41. [DOI] [PubMed] [Google Scholar]
- 2.Amezaga-Johnstone M R, McLaggan D, Booth I R. Surviving osmotic stress: the role of natural food components in limiting preservative action. In: Roos Y H, Leslie R B, Lillford P J, editors. Water management in the design and distribution of quality foods. Lancaster, Pa: Technomic Publishing Company Inc.; 1999. pp. 325–351. [Google Scholar]
- 3.Booth I R, Cairney J, Sutherland L, Higgins C F. Enteric bacteria and osmotic stress: an integrated homeostatic system. J Appl Bacteriol. 1988;65(SS):35S–49S. [PubMed] [Google Scholar]
- 4.Chen L-M, Maloy S. Regulation of proline utilization in enteric bacteria: cloning and characterization of the Klebsiella put control region. J Bacteriol. 1991;173:783–790. doi: 10.1128/jb.173.2.783-790.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Csonka L N. Physiological and genetic responses of bacteria to osmotic stress. Microbiol Rev. 1989;53:121–147. doi: 10.1128/mr.53.1.121-147.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Csonka L N, Epstein W. Osmoregulation. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. Washington, D.C.: American Society for Microbiology; 1996. pp. 1210–1223. [Google Scholar]
- 7.Dendinger S, Brill W J. Regulation of proline degradation in Salmonella typhimurium. J Bacteriol. 1970;103:144–152. doi: 10.1128/jb.103.1.144-152.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deutch C E, Hasler J M, Houston R M, Sharma M, Stone V J. Nonspecific inhibition of proline dehydrogenase synthesis in Escherichia coli during osmotic stress. Can J Microbiol. 1989;35:779–785. doi: 10.1139/m89-130. [DOI] [PubMed] [Google Scholar]
- 9.Dinnbier U, Limpinsel E, Schmid R, Bakker E P. Transient accumulation of potassium glutamate and its replacement by trehalose during adaptation of growing cells of Escherichia coli K-12 to elevated sodium chloride concentrations. Arch Microbiol. 1988;150:348–357. doi: 10.1007/BF00408306. [DOI] [PubMed] [Google Scholar]
- 10.D’Souza-Ault M R, Smith L T, Smith G M. Roles of N-acetylglutaminylglutamine amide and glycine betaine in adaptation of Pseudomonas aeruginosa to osmotic stress. Appl Environ Microbiol. 1993;59:473–478. doi: 10.1128/aem.59.2.473-478.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ekena K, Maloy S. Regulation of proline utilization in Salmonella typhimurium: how do cells avoid a futile cycle? Mol Gen Genet. 1990;220:492–494. doi: 10.1007/BF00391761. [DOI] [PubMed] [Google Scholar]
- 12.Galinski E A. Osmoadaptation in bacteria. In: Poole R K, editor. Advances in microbial physiology. London, England: Academic Press; 1995. pp. 273–328. [PubMed] [Google Scholar]
- 13.Imhoff J F. Osmoregulation and compatible solutes in Eubacteria. FEMS Microbiol Rev. 1986;39:57–66. [Google Scholar]
- 14.Kaenjak A, Graham J E, Wilkinson B J. Choline transport activity in Staphylococcus aureus induced by osmotic stress and low phosphate concentrations. J Bacteriol. 1993;175:2400–2406. doi: 10.1128/jb.175.8.2400-2406.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koch A. The surface stress theory of microbial morphogenesis. Adv Microb Physiol. 1983;24:301–366. doi: 10.1016/s0065-2911(08)60388-4. [DOI] [PubMed] [Google Scholar]
- 16.Landfald B, Strøm A R. Choline-glycine betaine pathway confers a high level of osmotic tolerance in Escherichia coli. J Bacteriol. 1986;165:849–855. doi: 10.1128/jb.165.3.849-855.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Larsen P I, Sydness L K, Landfald B, Strøm A R. Osmoregulation of Escherichia coli by accumulation of organic osmolytes: betaines, glutamic acid, and trehalose. Arch Microbiol. 1987;147:1–7. doi: 10.1007/BF00492896. [DOI] [PubMed] [Google Scholar]
- 18.Maloy S. The proline utilization operon. In: Neidhart F C, Ingram J L, Low K B, Magasanik B, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. Washington, D.C.: American Society for Microbiology; 1987. pp. 1513–1519. [Google Scholar]
- 19.Maloy S R, Roth J R. Regulation of proline utilization in Salmonella typhimurium: characterization of put::Mu d(Ap lac) operon fusions. J Bacteriol. 1983;154:561–568. doi: 10.1128/jb.154.2.561-568.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McIlvaine T C. A buffer solution for colorimetric comparison. J Biol Chem. 1921;49:183–186. [Google Scholar]
- 21.McLaggan D, Logan T M, Lynn D G, Epstein W. Involvement of γ-glutamyl peptides in osmoadaptation of Escherichia coli. J Bacteriol. 1990;172:3631–3636. doi: 10.1128/jb.172.7.3631-3636.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Menzel R, Roth J. Purification of the putA gene product: a bifunctional membrane-bound protein from Salmonella typhimurium responsible for the two-step oxidation of proline to glutamate. J Biol Chem. 1981;256:9755–9761. [PubMed] [Google Scholar]
- 23.Miller K J, Wood J W. Osmoadaptation by rhizosphere bacteria. Annu Rev Microbiol. 1996;50:101–136. doi: 10.1146/annurev.micro.50.1.101. [DOI] [PubMed] [Google Scholar]
- 24.Milner J L, McClellan D J, Wood J M. Factors reducing and promoting the effectiveness of proline as an osmoprotectant in Escherichia coli K-12. J Gen Microbiol. 1987;133:1851–1860. doi: 10.1099/00221287-133-7-1851. [DOI] [PubMed] [Google Scholar]
- 25.Pahel G, Zelenetz A D, Tyler B M. gltB gene and regulation of nitrogen metabolism by glutamine synthetase in Escherichia coli. J Bacteriol. 1978;133:139–148. doi: 10.1128/jb.133.1.139-148.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Payne J W. Transport and utilization of peptides by bacteria. In: Payne J W, editor. Microorganisms and nitrogen sources. New York, N.Y: John Wiley & Sons, Inc.; 1980. pp. 211–256. [Google Scholar]
- 27.Ratzkin R, Grabnar M, Roth J. Regulation of the major proline permease gene of Salmonella typhimurium. J Bacteriol. 1978;133:737–743. doi: 10.1128/jb.133.2.737-743.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ratzkin R, Roth J. Cluster of genes controlling proline degradation in Salmonella typhimurium. J Bacteriol. 1978;133:744–754. doi: 10.1128/jb.133.2.744-754.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rhodes D, Hanson A D. Quaternary ammonium and tertiary sulfonium compounds in higher plants. Annu Rev Plant Physiol. 1993;44:357–384. [Google Scholar]
- 30.Roe, A. J., D. McLaggan, C. O’Byrne, and I. R. Booth. Unpublished data.
- 31.Smith L T, Smith G M. An osmoregulated dipeptide in stressed Rhizobium meliloti. J Bacteriol. 1989;171:4714–4717. doi: 10.1128/jb.171.9.4714-4717.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Verheul A, Hagting A, Amezaga M R, Booth I R, Rombouts F M, Abee T. A di- and tripeptide transport system can supply Listeria monocytogenes Scott A with amino acids essential for growth. Appl Environ Microbiol. 1995;61:226–233. doi: 10.1128/aem.61.1.226-233.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verheul A, Rombouts F M, Abee T. Utilization of oligopeptides by Listeria monocytogenes Scott A. Appl Environ Microbiol. 1998;64:1059–1065. doi: 10.1128/aem.64.3.1059-1065.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]