Introduction
Progress in short-term kidney graft survival has decreased since 2000 which suggests an unmet need for innovation regarding early kidney graft function optimization.1 Previous studies have revealed that optimal perioperative fluid therapy was associated with a lower incidence of delayed graft function after kidney transplantation.2, 3, 4 Contrary to earlier studies, large volume administration is no longer recommended, and an individualized approach is now encouraged to balance the graft perfusion and the possible complications of fluid overload.5
We recently reported that intra-abdominal hypertension, a surrogate of fluid overload, was frequent and associated with impaired graft function in a cohort of kidney transplant recipients in whom fluid administration aimed to reach a hyperhydration state.6 This suggests that intra-abdominal pressure (IAP) might be useful to guide fluid management in these patients, though several questions remained: (i) What IAP level should be targeted in clinical practice? (ii) Are other fluid status indicators relevant in this setting?
Here, we investigated the association between the levels of IAP, weight gain, central venous pressure (CVP), mean arterial pressure (MAP), mean perfusion pressure (MPP), and abdominal perfusion pressure (APP) within the first 72 hours after kidney transplantation and day 30 estimated glomerular filtration rate (eGFR).
Results
We performed a single-center study at the University Hospital of Reims (Reims, France) using the data set of a previous prospective study of our group in which patients were well-characterized for both donor, recipient, and perioperative characteristics and for all fluid status indicators of interest. The Methods section can be found in the Supplementary Material. A total of 55 kidney transplant recipients, mostly from cadaveric donors (89%), were included in the analysis with a mean age of 49 ± 12 years. The characteristics of all recipients and donors are found in Supplementary Table S1. Mean day 3 creatinine level was 307 ± 227 μmol/l. There were 10 patients (18%) who presented delayed graft function, and none of them were diagnosed with having acute rejection within the first month after kidney transplantation. On day 30, mean eGFR was 47 ± 22 ml/min per 1.73 m2.
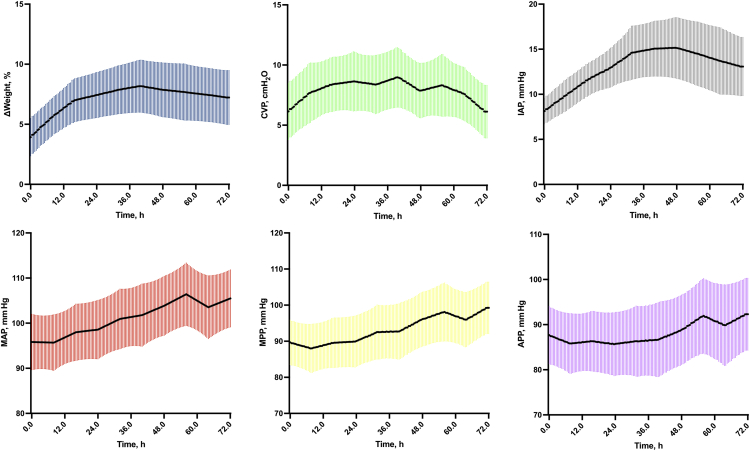
The first 48 hours cumulative fluid balance was +2311 ± 2403 ml (Supplementary Figure S1). Consistently, weight gain, CVP, and IAP levels increased by 8 ± 4%, 3 ± 0 cm H2O and +7 ± 4 mm Hg (Figure 1). MAP level continuously increased within the first 72 hours (+10 ± 0 mm Hg), whereas both MPP and APP levels increased mostly on day 3 after kidney transplantation (+10 ± 2 mm Hg and +5 ± 3 mm Hg).
Figure 1.
Time series of fluid status indicators within the first 72 hours after kidney transplantation. Evolution of each fluid status indicator (ΔWeight, MAP, CVP, IAP, MPP, and APP) within the first 72 hours after kidney transplantation. Results are illustrated as mean and SD. ΔWeight, weight gain; APP, abdominal perfusion pressure; CVP, central venous pressure; h, hour; IAP, intra-abdominal pressure; MAP, mean arterial pressure; MPP, mean perfusion pressure.
In univariate analyses, area above threshold (AAT) for MAP levels of 85 and 95 mm Hg within the first 72 hours was positively associated with day 30 eGFR, including AAT for MPP and APP, regardless of the threshold considered. AAT for IAP was negatively associated with day 30 eGFR, regardless of the threshold considered. All AAT for both weight gain and CVP were not associated with day 30 eGFR (Supplementary Table S2).
After adjustment for covariates, all AAT for IAP and APP were independently associated with day 30 eGFR, regardless of the threshold considered (Table 1 and Supplementary Figure S2). The strongest associations with day 30 eGFR were found while considering the lowest IAP and the highest APP levels. For each mm Hg/h increase in IAP >12 mm Hg within the first 72 hours, the day 30 eGFR decreased by 0.5 ± 0.1 ml/min per 1.73 m2 (r2 = 0.62, P < 0.01). For each mm Hg/h increase in APP >85 mm Hg within the first 72 hours, the day 30 eGFR increased by 0.3 ± 0.1 ml/min per 1.73 m2 (r2 = 0.60, P = 0.02). We did not find any independent association between day 30 eGFR and AAT for MAP or MPP and in multivariate analysis, regardless of the threshold considered. All AAT for IAP and APP remained independently associated with day 30 eGFR, regardless of the threshold considered, after exclusion of recipients from living kidney donors (Supplementary Table S3).
Table 1.
Association between fluid status indicator levels within the first 72 hours after kidney transplantation and day 30 eGFR: summary of the different regression models
| Variables | Multivariate analysis |
|
|---|---|---|
| β Coefficient ± SE | P value | |
| Weight gain | ||
| AAT ΔWeight >0, %/h | – | – |
| AAT ΔWeight >5, %/h | – | – |
| AAT ΔWeight >10, %/h | – | – |
| AAT ΔWeight >15, %/h | – | – |
| MAP | ||
| AAT MAP >65 mm Hg, mm Hg/h | 0.03 ± 0.14 | 0.80 |
| AAT MAP >75 mm Hg, mm Hg/h | 0.04 ± 0.14 | 0.81 |
| AAT MAP >85 mm Hg, mm Hg/h | 0.04 ± 0.14 | 0.75 |
| AAT MAP >95 mm Hg, mm Hg/h | 0.05 ± 0.14 | 0.71 |
| CVP | ||
| AAT CVP >5 cm H2O, mm H2O/h | – | – |
| AAT CVP >10 cm H2O, cm H2O/h | – | – |
| AAT CVP >15 cm H2O, cm H2O/h | – | – |
| AAT CVP >20 cm H2O, cm H2O/h | – | – |
| IAP | ||
| AAT IAP >12 mm Hg, mm Hg/h | −0.45 ± 0.11 | <0.01 |
| AAT IAP >15 mm Hg, mm Hg/h | −0.41 ± 0.11 | <0.01 |
| AAT IAP >20 mm Hg, mm Hg/h | −0.39 ± 0.11 | <0.01 |
| AAT IAP >25 mm Hg, mm Hg/h | −0.32 ± 0.11 | <0.01 |
| MPP (MPP = MAP − CVP) | ||
| AAT MPP >55 mm Hg, mm Hg/h | 0.09 ± 0.13 | 0.50 |
| AAT MPP >65 mm Hg, mm Hg/h | 0.10 ± 0.13 | 0.44 |
| AAT MPP >75 mm Hg, mm Hg/h | 0.11 ± 0.14 | 0.43 |
| AAT MPP >85 mm Hg, mm Hg/h | 0.11 ± 0.14 | 0.43 |
| APP (APP = MAP − IAP) | ||
| AAT APP >55 mm Hg, mm Hg/h | 0.29 ± 0.12 | 0.02 |
| AAT APP >65 mm Hg, mm Hg/h | 0.30 ± 0.12 | 0.01 |
| AAT APP >75 mm Hg, mm Hg/h | 0.31 ± 0.12 | 0.01 |
| AAT APP >85 mm Hg, mm Hg/h | 0.32 ± 0.12 | 0.01 |
ΔWeight, weight gain; AAT, area above threshold; APP, abdominal perfusion pressure; CVP, central venous pressure; eGFR, estimated glomerular filtration rate; IAP, intra-abdominal pressure; MAP, mean arterial pressure; MPP, mean perfusion pressure.
Each multivariate analysis model included the AAT corresponding to 1 of the fluid status indicators (weight gain, MAP, CVP, IAP, MPP, or APP) and 1 predefined threshold within the first 72 hours after kidney transplantation, including all the following confounding variables: recipient age, donor creatininemia, anti-human leukocyte antigen immunization status (presence or absence of anti-human leukocyte antigen antibodies), donor type (cadaveric or living kidney donor), calcineurin inhibitor (ciclosporin or tacrolimus), and cold ischemia time.
Discussion
Current practice consisting in administering large fluid volumes to increase graft perfusion may promote hyperhydration in most patients, though fluid overload could be harmful for both the graft function and the recipient. However, the best modalities to evaluate fluid status in this particular setting remain controversial. After adjustment for all the confounding characteristics of donors and recipients, our results suggest both IAP and APP as relevant fluid status indicators in terms of graft function recovery after kidney transplantation.
To our knowledge, our study is the first to investigate the association of early fluid status indicator levels with graft function recovery after kidney transplantation by calculating the AAT, which reflect both the magnitude and duration of deviation above predefined thresholds. This new data analysis methodology allows to identify (i) the most relevant variables of interest in this particular setting and (ii) the more clinically relevant thresholds for each variable of interest.
In kidney transplant recipients, fluid administration aims to improve cardiac output, MAP, and ultimately renal blood flow to optimize the graft perfusion.2,3 We did find a positive association between AAT for MAP levels of 85 and 95 mm Hg and day 30 eGFR, meaning that higher MAP levels could be beneficial in early postkidney transplantation period. Nevertheless, we failed to demonstrate that this association remained after adjustment for confounders, possibly because of the small sample size of our study. Excessive fluid administration however can promote harmful fluid overload complications, as we and others have previously reported.6, 7, 8
Because MAP and IAP play opposite roles for renal hemodynamics, APP (= MAP−IAP) may be of interest for clinicians to approach kidney perfusion as this indicator includes both upper (IAP) and lower (MAP) bounds to guide fluid administration.9 Our study is the first to highlight its positive correlation with graft function recovery in a dose-dependent manner (i.e., the higher the APP, the higher the day 30 eGFR).
CVP is widely used to approximate patients’ hydration status in kidney transplant recipients. High CVP levels have been found to correlate with worsening of acute kidney failure, possibly through a backpressure effect as GFR is determined by the arteriovenous pressure difference among the glomerulus.4 This driving pressure can be approached by MPP (= MAP−CVP). Interestingly, although no relevant association was found with AAT for CVP, AAT for MPP was associated with day 30 eGFR in univariate analysis, regardless of the threshold considered.
We acknowledge some limitations. First, our sample size is relatively small though we used a prospective cohort of our group in which patients were well-characterized for each variable of interest and were treated accordingly with current standards for kidney transplant recipients. Second, though the temporal association between high fluid load administration and the rise in IAP suggests that the former is primary, we cannot ignore that the secondary rise in IAP could have in return facilitated fluid overload itself by impairing the graft function, leading to a vicious cycle. Third, day 30 cystatin C measurements were not available in our cohort. Finally, our data set did not include any data regarding fluid status biomarkers or echocardiographic measurements though it would be of interest to better understand the repartition of fluid load in this particular setting.
Overall, this pilot study suggests APP and IAP, 2 routinely measurable variables, as relevant early fluid status indicators that may be used after kidney transplantation to guide fluid management. These findings need to be confirmed by larger studies. A strategy consisting in administering fluids to increase MAP but stopping fluid administration when IAP reaches a 12 mm Hg threshold (resulting in an increased APP) could represent a feasible and promising intervention to optimize allograft perfusion while avoiding renal congestive injury. Our work serves as a foundation for future interventional studies that are warranted to test the impact of an early goal-directed fluid therapy on graft function recovery after kidney transplantation.
Disclosure
All the authors declared no competing interests.
Footnotes
Table S1. Characteristics of patients included in the study.
Table S2. Univariate analysis of factors associated with day 30 estimated glomerular filtration rate.
Table S3. Association between fluid status indicators levels within the first 72h after kidney transplantation from cadaveric donors and day-30 eGFR: summarize summary of the different regression models after inclusion of KDPI.
Figure S1. Daily fluid load and fluid balance within the first 72 h after kidney transplantation.
Figure S2. Predicted day 30 eGFR versus fluid status indicators levels within the first 72h after kidney transplantation (PDF).
Supplementary Material
Table S1. Characteristics of patients included in the study.
Table S2. Univariate analysis of factors associated with day 30 estimated glomerular filtration rate.
Table S3. Association between fluid status indicators levels within the first 72h after kidney transplantation from cadaveric donors and day-30 eGFR: summarize summary of the different regression models after inclusion of KDPI.
Figure S1. Daily fluid load and fluid balance within the first 72 h after kidney transplantation.
Figure S2. Predicted day 30 eGFR versus fluid status indicators levels within the first 72h after kidney transplantation (PDF).
References
- 1.Coemans M., Süsal C., Döhler B., et al. Analyses of the short- and long-term graft survival after kidney transplantation in Europe between 1986 and 2015. Kidney Int. 2018;94:964–973. doi: 10.1016/j.kint.2018.05.018. [DOI] [PubMed] [Google Scholar]
- 2.Campos L., Parada B., Furriel F., Castelo D., Moreira P., Mota A. Do intraoperative hemodynamic factors of the recipient influence renal graft function? Transplant Proc. 2012;44:1800–1803. doi: 10.1016/j.transproceed.2012.05.042. [DOI] [PubMed] [Google Scholar]
- 3.Snoeijs M.G.J., Wiermans B., Christiaans M.H., et al. Recipient hemodynamics during non-heart-beating donor kidney transplantation are major predictors of primary nonfunction. Am J Transplant. 2007;7:1158–1166. doi: 10.1111/j.1600-6143.2007.01744.x. [DOI] [PubMed] [Google Scholar]
- 4.Calixto Fernandes M.H., Schricker T., Magder S., Hatzakorzian R. Perioperative fluid management in kidney transplantation: a black box. Crit Care. 2018;22:14. doi: 10.1186/s13054-017-1928-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wagener G., Bezinover D., Wang C., et al. Fluid management during kidney transplantation: a consensus statement of the Committee on Transplant Anesthesia of the American Society of Anesthesiologists. Transplantation. 2021;105:1677–1684. doi: 10.1097/TP.0000000000003581. [DOI] [PubMed] [Google Scholar]
- 6.Dupont V., Debrumetz A., Leguillou A., et al. Intra-abdominal hypertension in early post-kidney transplantation period is associated with impaired graft function. Nephrol Dial Transplant. 2020;35:1619–1628. doi: 10.1093/ndt/gfaa104. [DOI] [PubMed] [Google Scholar]
- 7.Gueutin V., Ficheux M., Châtelet V., et al. Hydration status of patients with end-stage renal disease after kidney transplantation. Clin Transpl. 2011;25:E656–E663. doi: 10.1111/j.1399-0012.2011.01496.x. [DOI] [PubMed] [Google Scholar]
- 8.Mottola C., Girerd N., Coiro S., et al. Evaluation of subclinical fluid overload using lung ultrasound and estimated plasma volume in the postoperative period following kidney transplantation. Transplant Proc. 2018;50:1336–1341. doi: 10.1016/j.transproceed.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 9.Cheatham M.L., White M.W., Sagraves S.G., Johnson J.L., Block E.F. Abdominal perfusion pressure: a superior parameter in the assessment of intra-abdominal hypertension. J Trauma. 2000;49:621–627. doi: 10.1097/00005373-200010000-00008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.