Abstract
Introduction
Whether in advanced countries lead exposure still contributes to renal impairment is debated, because blood lead (BL) level is declining toward preindustrial levels and because longitudinal studies correlating renal function and BL changes over time are scarce.
Methods
The Study for Promotion of Health in Recycling Lead (SPHERL) evaluated the 2-year renal function responses in 251 workers (mean age, 29.7 years) transiting from environmental to occupational exposure. Main study end point was the estimated glomerular filtration rate (eGFR) derived from serum creatinine (eGFRcrt), cystatin C (eGFRcys), or both (eGFRcc). BL level was measured by inductively coupled plasma mass spectrometry (detection limit 0.5 μg/dl).
Results
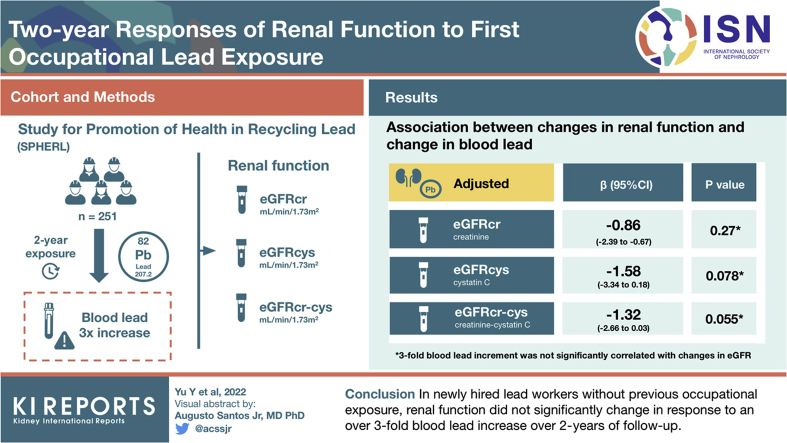
In the follow-up, mean baseline BL level of 4.13 μg/dl increased 3.30-fold. In fully adjusted mixed models, additionally accounting for the within-participant clustering of the 1- and 2-year follow-up data, a 3-fold BL level increment was not significantly correlated with changes in eGFR with estimates amounting to −0.86 (95% CI: −2.39 to 0.67), −1.58 (−3.34 to 0.18), and −1.32 (−2.66 to 0.03) ml/min per 1.73 m2 for eGFRcrt, eGFRcys, or eGFRcc, respectively. Baseline BL level and the cumulative lead burden did not materially modify these estimates, but baseline eGFR was a major determinant of eGFR changes showing regression to the mean during follow-up. Responses of serum osmolarity, urinary gravity, or the urinary albumin-to-creatinine ratio (ACR) were also unrelated to the BL level increment. The age-related decreases in eGFRcrt, eGFRcys, and eGFRcc were −1.41, −0.96, and −1.10 ml/min per 1.73 m2, respectively.
Conclusion
In the current study, the 2-year changes in renal function were unrelated to the increase in BL level. However, given the CIs around the point estimates of the changes in eGFRcc and eGFRcys, a larger study with longer follow-up is being planned.
Keywords: cystatin C, glomerular filtration rate, lead, occupational medicine, renal function
Graphical abstract
According to the 2017 Global Burden of Disease report,1 lead exposure causes or aggravates chronic kidney disease (CKD). From 2007 to 2017, global mortality and disability adjusted life-years related to lead exposure amounted to 43,000 and 56,000 deaths and 1,094,000 and 1,297,000 disability adjusted life-years, respectively. However, in the same time interval, lead exposure (−27.7%) and the age-standardized mortality and disability-adjusted life year rates shrank by 10%. Limitations of the Global Burden of Disease results included the following: residual confounding, for instance by co-exposure to other pollutants; uncertainty about the generalizability of the association sizes; and the impossibility to account for secular trends in exposure. The latter issue is not trivial, because among American adults enrolled in consecutive cycles of the National Health and Nutrition Examination Survey, mean BL level dropped from 13.1 μg/dl in 1976 to 19802 to 1.2 to 2.76 μg/dl in 1988 to 1994,2 and further to 1.64 μg/dl in 1999 to 2002,3,4 which is close to the estimated BL level in preindustrial humans (2 μg/dl), only exposed by natural sources.1
Several studies of populations5, 6, 7, 8, 9, 10 or workers11 reported an inverse association between eGFR and BL level, but given that 70% of lead is renally excreted,12,13 it cannot be distinguished whether higher BL level induces renal impairment or vice versa. Experts argue as to whether current-day lead exposure still contributes to CKD rates.14,15 Furthermore, the high prevalence of comorbid conditions and the lack of longitudinal data associating temporal trends in renal function and BL level hamper interpretation of the literature.15 SPHERL (NCT02243904) is addressing these knowledge gaps by evaluating the 2-year responses of the eGFR16,17 as primary renal end point in newly hired workers not occupationally exposed before. eGFR was determined not only from serum creatinine but also from serum cystatin C, which is less vulnerable to confounding by ethnicity, sex, age, muscle mass, and protein intake.18
Methods
SPHERL is a longitudinal study of newly hired lead workers at battery manufacturing and lead recycling plants in the United States.16,17 SPHERL complies with the Helsinki declaration for investigations in humans. The Ethics Committee of the University Hospitals Leuven (Belgium) approved the study protocol (document number, B322201421631). The health of the labor force was protected in compliance with the US Occupational Safety and Health Administration Standard (www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.1025), which includes regular health check-ups, proper workplace ventilation, and the obligatory use of personal protective equipment. The predefined primary renal end point was the eGFR response to lead exposure in newly hired workers not occupationally exposed before.16 With 2-tailed significance and power set at 0.05 and 0.90, respectively, SPHERL was powered to detect a doubling of the age-related eGFR decline, if 250 workers would be followed up for 2 years.
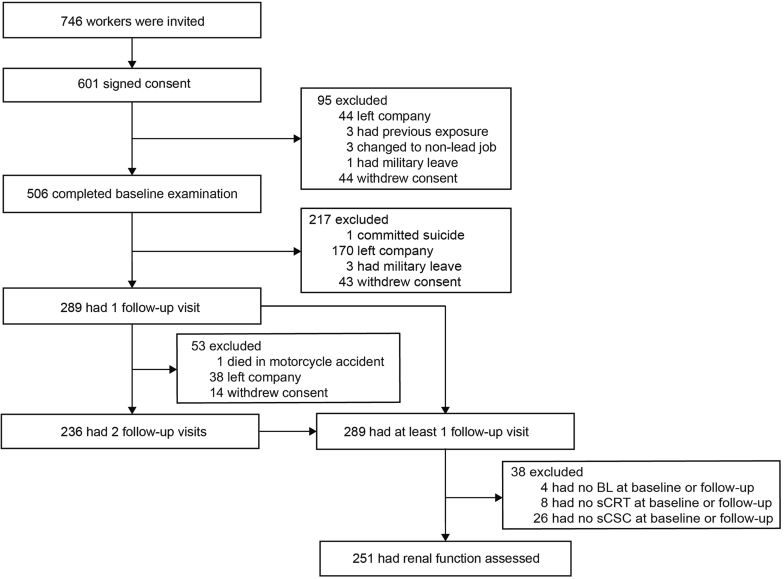
Of 746 newly hired workers invited to participate, 601 (80.6%) consented. However, in the interval between consent and the planned baseline examination (median: 19 days; fifth to 95th percentile interval: 9–59 days), 95 laborers left the workplace or withdrew. From January 25, 2015, to September 19, 2017, a total of 506 workers underwent the baseline examination, of whom 289 (57.1%) had at least 1 follow-up visit and 236 (46.6%) had 2 follow-up visits (Figure 1). Of 289 participants with at least 1 follow-up visit, 61 were excluded because baseline/follow-up levels of BL (n = 1/3), serum creatinine (n = 7/1), or serum cystatin C (n = 3/23) had not been measured. This left 251 workers, including 21 women, for statistical analysis. The clinical variables and the analytical methods of laboratory tests, including the measurements of renal function, hematological parameters, and BL level, the quality control of these measurements, and the supporting references are given in the Supplementary Data. In short, BL level was determined on whole blood by inductively coupled plasma mass spectrometry at an analytical laboratory certified for BL level analysis in compliance with the provisions of the Occupational Safety and Health Administration Lead Standard, 29CFR 1910.1025 (Occupational Safety and Health Administration [www.osha.gov]). The BL level detection limit was 0.5 μg/dl. Supplementary Table S1 in the Supplementary Data lists the formulas to estimate the glomerular filtration rate from serum creatinine, serum cystatin C, or both (Supplementary Table S1).18 Serum osmolality (mOsm/kg) was computed as 2 × (serum sodium ion [mmol/l]) + (blood glucose [mg/dl] / 18) + (blood urea nitrogen [mg/dl] / 2.8).19 Hemoglobin level and the red blood cell count were measured using a fully automated analyzer. From these 2 measurements, hematocrit, mean corpuscular volume, mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration, and the red cell distribution width were calculated by the device software (Supplementary Data, pages 5 to 6).
Figure 1.
Chart showing the flow of study participants. BL, blood lead; sCRT, serum creatinine; sCSC, serum cystatin C.
For database management and statistical analysis, we used the SAS software, version 9.4, maintenance level 5 (SAS Institute Inc., Cary, NC). Departure from normality was evaluated by the Shapiro-Wilk statistic. We applied a logarithmic transformation (base 10) to normalize the distributions of serum cystatin C, ACR, γ-glutamyltransferase, and BL. We reported the central tendency and spread of continuously distributed variables as mean and SD or as geometric mean and the interquartile range (IQR) or the fifth to 95th percentile data range (PDR) for logarithmically transformed variables. To compare means and proportions, we applied the t statistic or analysis of variance for continuous variables and the Fisher exact test for categorical variables, respectively.
In exploratory analyses, renal function changes were first assessed across quartiles of the follow-up-to-baseline BL ratio without any adjustment. In the next step of the analyses, point estimates with 95% CIs of the association sizes between the changes in the outcome variables and in BL levels were derived from mixed models with random intercept and random slope. Unadjusted, adjusted, and fully adjusted models were constructed with the follow-up-to-baseline BL ratio as the independent variable and the changes in renal function and hematological variables as the dependent variables. Irrespective of the adjustment, all models included a random effect term to account for clustering of observations within study participants, of whom 236 (94.0%) had 2 follow-up visits (Figure 1). Adjusted models accounted for age, the change of age from baseline to follow-up (equivalent to the between-visit interval), the time of day of the blood sampling (nighttime vs. daytime), and the baseline renal function measurement being analyzed. Fully adjusted models additionally accounted for baseline body mass index, change in body weight, and the baseline values of and changes during follow-up in smoking status, mean arterial pressure, antihypertensive medication (yes vs. no), the total-to-high–density lipoprotein cholesterol ratio, and γ-glutamyltransferase (index of alcohol intake). These covariables were considered based on previous publications.17,18,20 In the next analysis step, adjusted mixed models were constructed to account for the combined effects of the BL change and the baseline BL on eGFR. Association sizes were expressed for a 3-fold BL level increment from baseline over follow-up and for a 3-fold higher baseline BL level. Finally, sensitivity analyses were conducted, excluding workers on antihypertensive drug treatment at baseline, follow-up, or both. Moreover, the influence of the lead body burden at enrolment was assessed by stratifying study participants according to the median age, the median baseline BL level, and the median cumulative BL index (CBLI).21 To compute CBLI,21 age for workers leaving school at less than, at, and above the 12th grade was assumed to be 14, 18, and 23 years, respectively; based on the National Health and Nutrition Examination Survey data (1988–1994), the corresponding BL levels were set at 2.2, 1.4, and 1.5 μg/dl, respectively.22
Results
Baseline Characteristics
Of 251 workers, 230 were men (91.6%), 122 were White (48.6%), 107 were Hispanic (42.6%), 12 were Black (4.8%), and 10 had other self-reported ethnicities (4.0%). At baseline, age averaged 29.7 years, body mass index 28.9 kg/m2, mean arterial pressure 93.1 mm Hg, total and high-density lipoprotein serum cholesterol 171.8 mg/dl and 46.3 mg/dl, respectively, the total-to-high-density lipoprotein cholesterol ratio 3.91, blood glucose 94.3 mg/dl, and γ-glutamyltransferase 22.6 U/l (Table 1). The characteristics of the workers analyzed and not analyzed were broadly similar (Supplementary Table S2).
Table 1.
Baseline characteristics of 251 workers
| Characteristic | n (%) | Characteristic | Mean (SD/IQR) |
|---|---|---|---|
| Male | 230 (91.6) | Age, yr | 29.7 (9.8) |
| White ethnicity | 122 (48.6) | Body mass index, kg/m2 | 28.9 (6.1) |
| Hispanic ethnicity | 107 (42.6) | Systolic blood pressure, mm Hg | 120.0 (10.2) |
| Black ethnicity | 12 (4.8) | Diastolic blood pressure, mm Hg | 79.7 (8.8) |
| Other ethnicities | 10 (4.0) | Mean arterial pressure, mm Hg | 93.1 (8.7) |
| Current smokers | 67 (27.0) | Total cholesterol, mg/dl | 171.8 (37.8) |
| Alcohol intake | 110 (44.4) | HDL cholesterol, mg/dl | 46.3 (12.0) |
| Hypertension stage ≥1 | 125 (49.8) | Total-to-HDL cholesterol ratio | 3.91 (1.3) |
| Hypertension stage ≥2 | 46 (18.3) | Blood glucose, mg/dl | 94.3 (15.8) |
| Treated hypertension | 17 (6.8) | γ-glutamyltransferase, U/l | 22.6 (16.0, 33.0) |
| Diabetes mellitus | 12 (4.8) | Blood lead, μg/dl | 4.13 (2.40, 7.80) |
ACC, American College of Cardiology; AHA, American Heart Association; HDL, high-density lipoprotein; IQR, interquartile range.
Values are number of participants (%), arithmetic mean (SD), or geometric mean (IQR). Blood pressure was the average of 5 readings. Hypertension was categorized according to the 2017 ACC/AHA guideline, irrespective of treatment status.36 Mean arterial pressure was diastolic blood pressure plus one-third of pulse pressure. Diabetes mellitus was a self-reported diagnosis, a fasting blood glucose of ≥126 mg/dl, or use of antidiabetic drugs. To convert total or HDL serum cholesterol to mmol/l, multiply by 0.0259; to convert blood glucose to mmol/l, multiply by 0.0559.
Blood Lead
Median follow-up was 2.0 years (PDR: 1.0–2.3 years). At baseline, the geometric mean BL level was 4.13 μg/dl (IQR: 0.40–7.80 μg/dl; PDR: 1.00–14.8 μg/dl; Supplemetary Figure S1A) and at the last follow-up 13.6 μg/dl (IQR, 10.2–22.5 μg/dl; PDR, 3.30–30.5 μg/dl; Supplementary Figure S1B). At the 1- and 2-year follow-up visits, these BL values were 14.0 μg/dl (IQR: 10.5–21.8 μg/dl; PDR: 3.70–30.4 μg/dl) and 13.5 μg/dl (IQR: 10.4–22.0 μg/dl; PDR: 3.20–30.4 μg/dl), respectively. The last-follow-up-to-baseline BL ratio averaged 3.30 (IQR: 1.91–5.72; PDR: 0.73–14.3 μg/dl; Supplementary Figure S1C). In the follow-up, participants with lower baseline BL level experienced larger increases in the biomarker of exposure than those with higher baseline levels and vice versa (Supplementary Figure S2A).
Renal Function at Baseline and Follow-Up
Levels of serum creatinine and cystatin C averaged 0.958 mg/dl and 0.676 mg/l at baseline and increased (P < 0.001) in the follow-up by 0.0609 mg/dl and 0.0339 mg/l, respectively (Table 2). Consequently, eGFRcrt, eGFRcys, and eGFRcc decreased (P < 0.001) by 7.33, 4.52, and 6.44 ml/min per 1.73 m2 from their baseline level of 105.4, 125.1, and 114.7 ml/min per 1.73 m2. In the follow-up (Table 2), serum osmolality level increased by 2.32 mOsm/kg from 286.8 mOsm/kg at baseline and urinary gravity by 0.0027 from a starting value of 1.020 (P < 0.001). ACR did not significantly change from baseline to follow-up (P = 0.52). To estimate the aging effect on eGFR, age at the last follow-up was introduced in the formula to compute eGFRcrt, eGFRcys, and eGFRcc from the baseline values of serum creatinine, serum cystatin C, or both; the resulting estimates were −1.41, −0.96, and −1.10 ml/min per 1.73 m2, respectively.
Table 2.
Renal function at baseline and follow-up
| Characteristic | Baseline | Year 1 | Year 2 | Δ (95% CI) | P value |
|---|---|---|---|---|---|
| SCRT, mg/dl | 0.958 (0.168) | 1.002 (0.175) | 1.015 (0.174) | 0.0609 (0.0437–0.0780) | <0.001 |
| Serum cystatin C, mg/l | 0.676 (0.106) | 0.696 (0.132) | 0.714 (0.161) | 0.0339 (0.0187–0.0491) | <0.001 |
| eGFRcrt, ml/min per 1.73 m2 | 105.4 (17.0) | 100.4 (17.3) | 97.8 (16.4) | −7.33 (−8.94 to −5.71) | <0.001 |
| eGFRcys, ml/min per 1.73 m2 | 125.1 (13.3) | 122.6 (16.6) | 119.8 (19.9) | −4.52 (−6.27 to −2.77) | <0.001 |
| eGFRcc, ml/min per 1.73 m2 | 114.7 (14.4) | 110.5 (15.2) | 107.8 (16.0) | −6.44 (−7.85 to −5.04) | <0.001 |
| Serum osmolality, mOsm/kg | 286.8 (3.8) | 288.5 (3.9) | 289.1 (4.6) | 2.32 (1.67–2.97) | <0.001 |
| Serum sodium, mmol/l | 138.3 (1.8) | 139.1 (1.8) | 139.3 (2.2) | 1.05 (0.73–1.37) | <0.001 |
| Blood glucose, mg/dl | 94.3 (15.8) | 85.5 (16.2) | 89.2 (19.0) | −5.91 (−8.32 to −3.51) | <0.001 |
| Serum insulin, U/l | 7.21 (3.70–13.2) | 8.24 (4.30–15.2) | 9.24 (4.70–18.6) | 22.8 (8.77–38.7) | 0.001 |
| BUN, mg/dl | 14.0 (3.5) | 15.3 (4.0) | 15.5 (3.7) | 1.55 (1.09–2.02) | <0.001 |
| BUN-to-SCRT ratio | 14.9 (4.1) | 15.5 (3.8) | 15.5 (3.7) | 0.59 (0.08–1.09) | 0.023 |
| Urinary gravity | 1.020 (0.0077) | 1.022 (0.0072) | 1.022 (0.0069) | 0.0027 (0.0016–0.0038) | <0.001 |
| ACR, mg/g | 4.49 (2.86–6.33) | 4.29 (2.73–6.01) | 4.61 (2.86–6.63) | 2.90 (−5.80 to 12.4) | 0.52 |
ACR, urinary albumin-to-creatinine ratio; BUN, blood urea nitrogen; eGFR, estimated glomerular filtration rate; eGFRcc, eGFR derived from SCRT and cystatin C; eGFRcrt, eGFR derived from SCRT; eGFRcys, eGFR derived from cystatin C; IQR, interquartile range; SCRT, serum creatinine.
eGFRcrt, eGFRcys, and eGFRcc refer to the glomerular filtration rate estimated from SCRT, serum cystatin C, or both, respectively.18 Values are arithmetic mean (SD) or geometric mean (IQR). Changes from baseline to last follow-up are given with 95% CI; for logarithmically transformed serum insulin and ACR, changes are expressed as percentage. P values denote the significance of the changes from baseline to last follow-up. To convert SCRT to μmol/l, multiply by 88.42; to convert cystatin C from mg/l to nmol/l, multiply by 74.9; to convert blood glucose to mmol/l, multiply by 0.0559; to convert BUN to mmol/l, multiply by 0.3571.
At baseline and last follow-up, eGFRcrt, eGFRcys, and eGFRcc were 8.96 ml/min per 1.73 m2 (P < 0.001), 4.68 ml/min per 1.73 m2 (P = 0.004), and 7.19 ml/min per 1.73 m2 (P < 0.001) lower, respectively, if determined from night shift blood samples compared with morning and evening blood samples (Supplementary Figure S3). At baseline and follow-up, 2 workers had an eGFRcrt of <60 ml/min per 1.73 m2, whereas their eGFRcys and eGFRcc were above this limit. In addition, 13 workers (5.2%) reported a history of nephrolithiasis at enrolment, but no new or recurrent cases occurred during follow-up.
Categorical Analysis
In unadjusted categorical analyses, the changes in renal function, also including serum osmolality, urinary gravity, and urinary ACR (Table 3), and the hematological measurements (Supplementary Table S3) were analyzed by quartiles of the distribution of the follow-up-to-baseline BL ratio. None of these measurements showed a significant trend across increasing categories of the follow-up-to-baseline BL ratio. Supplementary Tables S4 and S5 exclude that the renal results given in Table 3 might be explained by opposing trends in the baseline or follow-up data.
Table 3.
Renal function changes from baseline to last follow-up by quartiles of the distribution of the follow-up-to-baseline blood lead concentration ratio
| Characteristic | <1.91 | 1.91–3.45 | 3.45–5.66 | ≥5.66 | P value |
|---|---|---|---|---|---|
| Number in group | 62 | 63 | 63 | 63 | … |
| SCRT, mg/dl | 0.0624 (0.0275–0.0973) | 0.0833 (0.0505–0.1160) | 0.0335 (0.0072–0.0742) | 0.0643 (0.0382–0.0903) | 0.57 |
| Serum cystatin C, mg/l | 0.0221 (−0.0054 to 0.0496) | 0.0249 (−0.0041 to 0.0539) | 0.0432 (0.0075–0.0789) | 0.0451 (0.0168–0.0734) | 0.21 |
| eGFRcrt, ml/min per 1.73 m2 | −7.38 (−10.4 to −4.32) | −9.33 (−12.6 to −6.04) | −4.91 (−8.68 to −1.14) | −7.68 (−10.3 to −5.10) | 0.63 |
| eGFRcys, ml/min per 1.73 m2 | −3.12 (−6.08 to −0.17) | −3.70 (−7.00 to −0.40) | −5.19 (−9.38 to −1.00) | −6.04 (−9.45 to −2.63) | 0.20 |
| eGFRcc, ml/min per 1.73 m2 | −5.82 (−8.56 to −3.08) | −6.91 (−9.42 to −4.39) | −5.57 (−8.85 to −2.28) | −7.47 (−10.1 to −4.88) | 0.57 |
| Serum osmolality, mOsm/kg | 2.39 (0.84–3.95) | 2.42 (1.09–3.74) | 1.99 (0.81–3.17) | 2.49 (1.38–3.59) | 0.96 |
| Serum sodium, mmol/l | 1.13 (0.33–1.93) | 0.92 (0.28–1.56) | 0.97 (0.39–1.54) | 1.17 (0.64–1.71) | 0.90 |
| Blood glucose, mg/dl | −4.74 (−9.55 to 0.06) | −5.01 (−9.20 to −0.82) | −8.41 (−13.7 to −3.12) | −5.47 (−10.3 to −0.62) | 0.61 |
| Insulin, % | 37.0 (7.84–74.0) | 8.91 (−15.6 to 40.5) | 19.3 (−5.67 to 50.9) | 28.0 (0.84–62.6) | 0.85 |
| BUN, mg/dl | 1.11 (0.27–1.96) | 2.40 (1.36–3.43) | 1.46 (0.70–2.22) | 1.24 (0.24–2.23) | 0.78 |
| BUN-to-SCRT ratio | 0.31 (−0.67 to 1.29) | 0.95 (−0.12 to 2.01) | 0.99 (0.07–1.90) | 0.11 (−0.94 to 1.15) | 0.80 |
| Urinary gravity, | 0.0044 (0.0023–0.0064) | 0.0028 (0.0005–0.0052) | 0.0009 (−0.0014 to 0.0032) | 0.0027 (0.0007–0.0047) | 0.17 |
| ACR, % | −1.54 (−18.0 to 18.3) | 2.00 (−15.6 to 23.2) | 10.6 (−7.03 to 31.7) | 0.85 (−13.9 to 18.1) | 0.71 |
ACR, albumin-to-creatinine ratio; BUN, blood urea nitrogen; eGFR, estimated glomerular filtration rate; eGFRcc, eGFR derived from SCRT and cystatin C; eGFRcrt, eGFR derived from SCRT; eGFRcys, eGFR derived from cystatin C; IQR, interquartile range; SCRT, serum creatinine.
eGFRcrt, eGFRcys, and eGFRcc refer to the glomerular filtration rate estimated from SCRT, serum cystatin C, or both, respectively.18 Within-group changes are arithmetic mean (SD) or geometric mean (IQR). For logarithmically transformed serum insulin and urinary ACR, changes are expressed as percentage. P values denote the significance of the between-group differences. To convert SCRT to μmol/l, multiply by 88.42; to convert cystatin C from mg/l to nmol/l, multiply by 74.9; to convert blood glucose to mmol/l, multiply by 0.0559; to convert blood urea nitrogen to mmol/l, multiply by 0.3571.
Mixed Model Regression Analysis
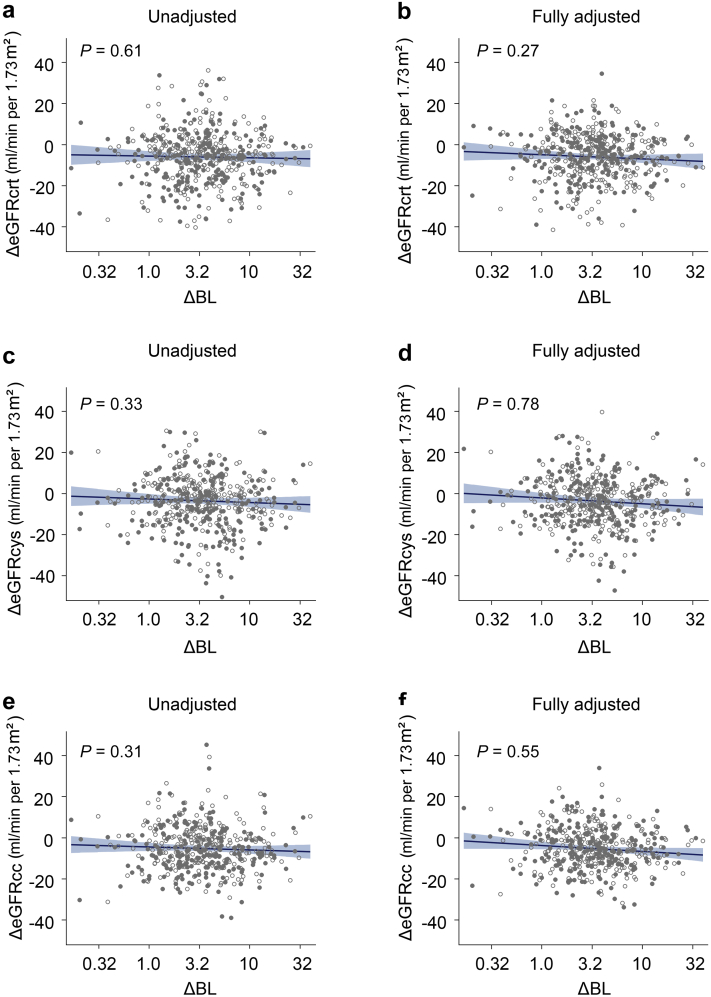
All analyses of the relation between the changes in the renal and other outcome measurements accounted for clustering of the data within individuals (Table 4). Results are presented without any further adjustment, with partial adjustment for sex, enrolment age, follow-up duration, the time of day of the blood sampling (nighttime vs. daytime), and the baseline renal function measure being analyzed. Fully adjusted models additionally accounted for baseline body mass index, change in body weight, and the baseline values of and changes during follow-up in smoking status, mean arterial pressure, antihypertensive medication (yes vs. no), total -to-high-density lipoprotein cholesterol ratio, and γ-glutamyltransferase. In unadjusted (Table 4 and Figure 2a, c and e) and in partially and fully adjusted models (Table 4), the changes in levels of serum creatinine, serum cystatin C, eGFRcrt, eGFRcys, and eGFRcc were not significantly correlated with the BL changes. In fully adjusted models (Table 4), the association sizes for a 3-fold BL increment amounted to 0.0072 mg/dl (95% CI: −0.0081 to 0.0226 mg/dl) for serum creatinine, 0.0118 mg/l (95% CI: −0.0033 to 0.0268 mg/l) for serum cystatin C, −0.86 ml/min per 1.73 m2 for eGFRcrt (95% CI: −2.39 to 0.67 ml/min per 1.73 m2), −1.58 ml/min per 1.73 m2 (95% CI: −3.34 to 0.18 ml/min per 1.73 m2) for eGFRcys, and −1.32 ml/min per 1.73 m2 (95% CI: −2.66 to 0.03 ml/min per 1.73 m2) for eGFRcc. In fully adjusted models, a 3-fold BL level increase was not significantly associated with the decreases in eGFRcrt, eGFRcys and eGFRcc, amounting to 0.86, 1.58, and 1.32 ml/min per 1.73 m2, respectively (Table 4 and Figure 2b, d and f). Given the whole range of the follow-up-to-baseline BL ratio (range: 0.16–34.0; Supplementary Figure S1C), the predicted mean changes in eGFRcrt, eGFRcys, and eGFRcc amounted to −5.91 ml/min per 1.73 m2 (P = 0.30), −3.46 ml/min per 1.73 m2 (P = 0.082), and −5.11 ml/min per 1.73 m2 (P = 0.045), respectively. Finally, in unadjusted and adjusted analyses, changes in serum osmolarity, its constituents, urinary gravity and ACR (Table 4), and in the hematological measurements (Supplementary Table S6) were not significantly correlated with the change in BL level during follow-up.
Table 4.
Association between changes in renal function and change in blood lead
| Variables | Unadjusted |
Adjusted |
Fully adjusted |
|||
|---|---|---|---|---|---|---|
| β (95% CI) | P value | β (95% CI) | P value | β (95% CI) | P value | |
| SCRT, ×10−2 mg/dl | 0.28 (−1.37 to 1.92) | 0.74 | 0.74 (−0.74 to 2.23) | 0.32 | 0.72 (−0.81 to 2.26) | 0.35 |
| Serum cystatin C, ×10−2 mg/l | 0.65 (−0.89 to 2.19) | 0.41 | 1.05 (−0.42 to 2.52) | 0.16 | 1.18 (−0.33 to 2.68) | 0.12 |
| eGFRcrt, ml/min per 1.73 m2 | −0.43 (−2.08 to 1.23) | 0.61 | −0.86 (−2.34 to 0.61) | 0.25 | −0.86 (−2.39 to 0.67) | 0.27 |
| eGFRcys, ml/min per 1.73 m2 | −0.88 (−2.67 to 0.91) | 0.33 | −1.42 (−3.13 to 0.30) | 0.11 | −1.58 (−3.34 to 0.18) | 0.078 |
| eGFRcc, ml/min per 1.73 m2 | −0.75 (−2.21 to 0.71) | 0.31 | −1.21 (−2.51 to 0.09) | 0.069 | −1.32 (−2.66 to 0.03) | 0.055 |
| Serum osmolality, mOsm/kg | 0.08 (−0.57 to 0.74) | 0.80 | 0.02 (−0.47 to 0.50) | 0.95 | −0.05 (−0.55 to 0.46) | 0.85 |
| Serum sodium, mmol/l | 0.13 (−0.19 to 0.45) | 0.42 | 0.09 (−0.16 to 0.34) | 0.48 | 0.06 (−0.20 to 0.31) | 0.66 |
| Blood glucose, mg/dl | −2.19 (−4.67 to 0.29) | 0.084 | −0.78 (−2.91 to 1.34) | 0.47 | −0.93 (−3.07 to 1.22) | 0.40 |
| Insulin, % | −4.44 (−15.4 to 7.94) | 0.46 | −4.30 (−14.6 to 7.26) | 0.45 | −3.75 (−13.6 to 7.19) | 0.48 |
| BUN, mg/dl | −0.13 (−0.63 to 0.38) | 0.62 | −0.18 (−0.61 to 0.25) | 0.40 | −0.13 (−0.57 to 0.32) | 0.57 |
| BUN-to-SCRT ratio | −0.23 (−0.76 to 0.29) | 0.38 | −0.40 (−0.83 to 0.03) | 0.067 | −0.35 (−0.80 to 0.09) | 0.12 |
| Urine specific gravity, ×10−2 | −0.03 (−0.14 to 0.09) | 0.65 | −0.03 (−0.11 to 0.06) | 0.54 | −0.00 (−0.09 to 0.08) | 0.94 |
| ACR, % | 1.31 (−8.03 to 11.6) | 0.79 | −0.37 (−8.34 to 8.29) | 0.93 | 0.23 (−7.94 to 9.12) | 0.96 |
ACR, albumin-to-creatinine ratio; BUN, blood urea nitrogen; eGFR, estimated glomerular filtration rate; eGFRcc, eGFR derived from SCRT and cystatin C; eGFRcrt, eGFR derived from SCRT; eGFRcys, eGFR derived from cystatin C; HDL, high-density lipoprotein; SCRT, serum creatinine.
eGFRcrt, eGFRcys, and eGFRcc refer to the glomerular filtration rate estimated from SCRT, serum cystatin C, or both, respectively.18 Changes in serum insulin and the urinary ACR are expressed as percentage differences from baseline to follow-up. Association sizes (β), given with 95% CI, express the change in the dependent variable for a 3-fold increase in the blood lead concentration. Adjusted models accounted for sex, age, follow-up duration, the time of day of blood sampling (nighttime vs. daytime), and the baseline renal function measure being analyzed. Fully adjusted models additionally accounted for baseline body mass index, change in body weight, and the baseline values of and changes during follow-up in smoking status, mean arterial pressure, antihypertensive medication (yes vs. no), the total-to-HDL cholesterol ratio, and γ-glutamyltransferase.
Figure 2.
Association of the change in eGFRcrt, eGFRcys, eGFRcc with the ΔBL. All plotted associations account for clustering of data within participants. Open symbols represent the first-year data and closed symbols second-year data. The regression line and 95% CI were derived from a mixed model, unadjusted (a, c, e) or adjusted (b, d, f) for sex, age, follow-up duration, the time of day of blood sampling, the baseline eGFR being analyzed, body mass index, change in body weight, and the baseline values of and changes during follow-up in smoking status, mean arterial pressure, antihypertensive medication (yes vs. no), the total-to-HDL cholesterol ratio, and γ-glutamyltransferase. ΔBL, follow-up-to-baseline blood lead concentration ratio; eGFR, estimated glomerular filtration rate; eGFRcc, eGFR derived from serum creatinine and cystatin C; eGFRcrt, eGFR derived from serum creatinine; eGFRcys, eGFR derived from cystatin C; HDL, high-density lipoprotein.
Baseline Effect Modifiers
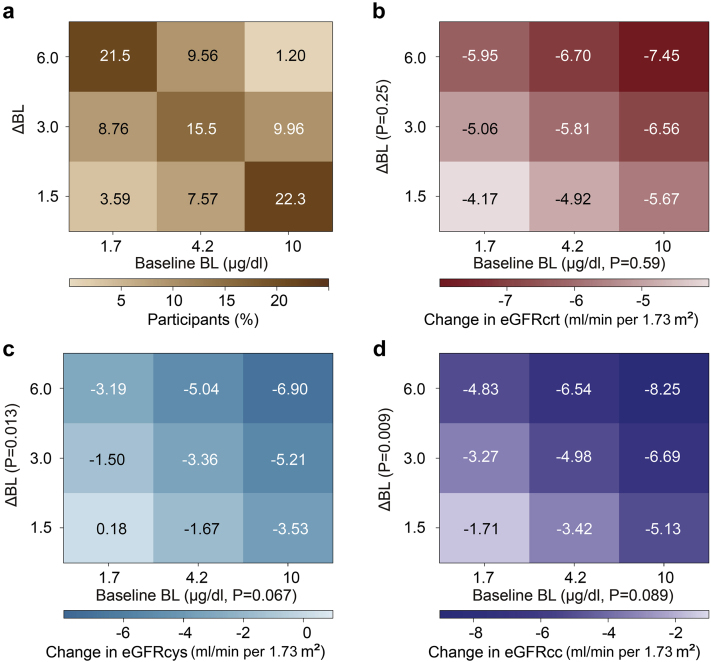
In the next analysis step, with full adjustment for covariables applied, heat maps based on the fully adjusted mixed model were constructed to account for the combined effects of baseline BL level and the BL level change on eGFR (Figure 3). Baseline BL level was not significantly associated with the changes during follow-up in eGFRcrt (β:−0.65 μg/dl; 95% CI: −3.03 to 1.74 μg/dl; P = 0.59), eGFRcys (β:−2.43 μg/dl; 95% CI: −5.02 to 0.17 μg/dl; P = 0.067), or eGFRcc (β:−1.81 μg/dl; 95% CI: −3.90 to 0.28 μg/dl; P = 0.089). However, accounting for baseline BL level made the eGFR estimates associated with the BL ratio borderline significant for eGFRcys (P = 0.013) and eGFRcc (P = 0.009). Figure 3a shows the percentages of the participants contributing to each box in the heat maps. The predicted values in the central boxes of heat maps (Figure 3) were consistent with the predicted eGFR values derived from the fully adjusted model not including baseline BL level: −5.81 versus −5.91 ml/min per 1.73 m2 for eGFRcrt (Figure 3b), −3.36 versus −3.46 ml/min per 1.73 m2 for eGFRcys (Figure 3c), and −4.98 versus −5.11 ml/min per 1.73 m2 in eGFRcc (Figure 3d). The absence of a significant interaction between baseline BL level and the BL level changes indicated that the effect modification of baseline BL level was steady over its range (P value for interaction, ≥0.38).
Figure 3.
Heat maps relating the changes in renal function to baseline BL level and the follow-up-to-baseline BL concentration ratio. Participants were cross-classified by thirds of the distributions of baseline BL and the follow-up-to-baseline BL ratio. The percentage of participants contributing to each cell of the heat maps is given in a. Results for the eGFRcrt, eGFRcys, or eGFRcc are presented in b, c, and d, respectively. The color codes and numbers in the grids are the longitudinal changes in glomerular filtration as estimated from the corresponding thick marks at mean values of the covariables, including sex, age, follow-up duration, the time of day of blood sampling (daytime vs. nighttime), the baseline eGFR being analyzed, body mass index, change in body weight, and the baseline values of and changes during follow-up in smoking status, mean arterial pressure, antihypertensive medication (yes vs. no), the total-to-HDL cholesterol ratio, and γ-glutamyltransferase. The P values for the interaction terms between baseline BL and the BL change were 0.56, 0.44, and 0.38 for eGFRcrt (b), eGFRcys (c), and eGFRcc (d), respectively. ΔBL, follow-up-to-baseline blood lead concentration ratio; BL, blood lead; eGFR, estimated glomerular filtration rate; eGFRcc, eGFR derived from serum creatinine and cystatin C; eGFRcrt, eGFR derived from serum creatinine; eGFRcys, eGFR derived from cystatin C; HDL, high-density lipoprotein.
Furthermore, heat maps were constructed to assess the influence of the baseline eGFR in combination with baseline BL level (Supplementary Figure S4) or the BL level change (Supplementary Figure S5) on eGFR. Baseline eGFR was a major determinant of the eGFR changes (P ≤ 0.001), higher baseline values being associated with more eGFR decline during follow-up, and vice versa, however, without significant interaction (P ≥ 0.11) between baseline eGFR and the baseline BL level or BL level change (Supplementary Figures S4 and S5).
Sensitivity Analyses
Sensitivity analyses from which 28 workers on antihypertensive drug treatment at baseline (n = 17), follow-up (n = 28), or both (n = 17) were excluded confirmed the main findings (Supplementary Table S7 vs. Table 4). Furthermore, the cross-sectional relations of the renal and associated measurements with BL level at baseline and at last follow-up were similar; none of the differences in the slopes reached significance (P ≥ 0.074; Supplementary Table S8). The β-coefficients for a 3-fold increment of BL at baseline versus a 3-fold increment of BL level at follow-up in adjusted models were 0.26 versus 2.96 mg/dl for cystatin C (P = 0.13), 1.31 versus −0.80 ml/min per 1.73 m2 (P = 0.29) for eGFRcrt, −0.19 versus −3.36 ml/min per 1.73 m2 (P = 0.099) for eGFRcys, and 0.63 versus −2.33 ml/min per 1.73 m2 for eGFRcc (P = 0.082).
To assess the influence of the BL burden, the 251 workers were categorized by median age (Supplementary Table S9), median BL at enrolment (Supplementary Table S10), or the median CBLI (Supplementary Table S11). The multivariable-adjusted slopes of any eGFR measure did not differ, if dichotomized by baseline BL level (P value for interaction ≥ 0.45, Supplementary Table S10), but were slightly but significantly different between older and younger workers for serum sodium (0.39 vs. −0.32 mmol/l), blood glucose (−1.91 vs. 1.75 mg/dl), and urine specific gravity −0.0011 vs. 0.0011), if categorized by baseline age (P ≥ 0.002; Supplementary Table S9). Moreover, if categorized by median CBLI (P ≥ 0.054; Supplementary Table S11), the slope of eGFRcc (−0.93 vs. −3.46 ml/min per 1.73 m2) slightly but not differed between the low versus high CBLI group.
Discussion
In a real-world experiment, conducted in an occupational setting, 2 years of lead exposure was not significantly associated with eGFR decline, the co-principal renal SPHERL end point.16,17 In fully adjusted mixed models, accounting for covariables and the within-participant clustering of the 1- and 2-year follow-up data, a 3-fold BL level increment generated estimates of the lead-related eGFR changes (Table 4) amounting to −0.86, −1.58, and −1.32 ml/min per 1.73 m2 for eGFRcrt, eGFRcys, or eGFRcc, respectively. These eGFR changes must be gauged against the expected age-related decline in eGFR, estimated in the current study to be −1.41, −0.96, and −1.10 ml/min per 1.73 m2, for eGFRcrt, eGFRcys, and eGFRcc, respectively. The responses of serum osmolality, urine specific gravity, ACR (Table 4), and the hematological measurements (Supplementary Table S6) were not significantly associated to the increment in lead exposure.
In addition to regression to the mean, the present study highlights the importance to account in longitudinal studies for concealed albeit not unexpected confounders and to distinguish crudely observed eGFR changes in the context of the biomarker of exposure of interest, BL level in the current study. Supplementary Figure S3 shows that eGFRcrt, eGFRcys, and eGFRcc were 8.96, 4.68, and 7.18 ml/min per 1.73 m2, respectively, lower when determined from night shift blood samples compared with morning or evening blood samples. Both serum creatinine and serum cystatin C, from which eGFR is derived, show a diurnal rhythm with little influence of meals or meat ingestion on serum cystatin C level, whereas these confounders increase serum creatinine level.23 Along similar lines, during sleep, urine flow decreases and the tubular reabsorption of water increases.24,25 The newly hired workers recruited into SPHERL transited not only from environmental to occupational exposure but also from a sedentary to a physically demanding lifestyle. On the basis of the published tables,26 the jobs offered to the workers required an energy expenditure of 6 to >8 metabolic equivalents defined as the amount of oxygen consumed while resting in the sitting position. Physical labor induces acute renal changes in healthy adults.27 In young adults, exercise reduces renal plasma flow and eGFR with smaller effects on eGFRcys than on eGFRcrt.28 Strenuous physical work is also associated with sodium and water loss through sweating and an increased respiration rate and with higher insulin sensitivity, hence increases in serum sodium and urine specific gravity, lower blood glucose, and higher serum insulin during follow-up compared with baseline (Table 2).
Several studies of populations5, 6, 7, 8,10 or workers11 in Europe,5,8,10 the United States,6,7,11 and Taiwan9 reported an inverse association between eGFR and BL level or an increased risk of CKD in relation to BL. In a study of randomly recruited Belgians published in 1992,5 mean BL level was 7.5 μg/dl in 1016 women and 11.4 μg/dl in 965 men; in sex-stratified analyses, a 10-fold higher BL level was associated with a 13 and 10 ml/min lower creatinine clearance in women and men, respectively. However, given that approximately 70% of lead excretion occurs via the urine,12,13 the observational studies referenced previously5, 6, 7, 8, 9, 10, 11 could not ascertain the directionality of the association between eGFR and BL level, reduced eGFR being associated with lesser urinary lead excretion and higher BL level, or higher lead exposure as reflected by BL level reducing eGFR. Lead is a cumulative contaminant, which is for 90% to 95% stored in the bone.29,30 Bone lead level, for 99% carried by the red blood cells, correlates with BL level30,31 and explains around 20% of the variance in BL level, depending on seasonality30 and hormonal and other endogenous and environmental stimuli influencing the balance between bone formation and resorption.31 Both bone lead and BL levels increase with age. However, the sensitivity analyses dichotomized by median age at baseline (Supplementary Table S9), median baseline BL level (Supplementary Table S10), or the median CBLI (Supplementary Table S11) addressed modification of the association between eGFR and BL level by the BL burden before starting a job in the lead industry. The multivariable-adjusted association sizes between changes in eGFR and in BL did not differ, if dichotomized by age or baseline BL level, but were slightly but significantly higher for eGFRcys and eGFRcc, if categorized by median CBLI. However, the categorization by median CBLI21 relied on extrapolation of BL level at 14, 18, and 23 years of age, so that the latter findings must be carefully interpreted.22
Among the strong points of our study are its longitudinal design with annual follow-up visits, the stringent quality control of the BL level measurement maintained during the 2-year course of the study, and the use of serum cystatin C in addition to serum creatinine to derive eGFR. Notwithstanding these strong points, our study also has limitations. First, the attrition rate among the 506 workers who participated in the baseline examination, but defaulted from follow-up, amounted to 217 (42.9%), mainly because they left employment (Figure 1). The small sample size and the 2-year follow-up of the current SPHERL cohort warrant a cautious interpretation of the findings. According to the published SPHERL protocol,16 the anticipated attrition rate was 50%. To meet the sample size required to address renal function as primary end point, 500 workers had to be enrolled, a target that was met. According to recently updated plans, consenting workers remaining employed at the study sites are now being followed up for an additional 2 years, using a simplified protocol focusing on the key traits potentially associated with chronic lead exposure, also including early markers of tubular dysfunction. In addition, to enlarge the sample size, 200 new participants are being recruited, of whom presumably 100 will have at least one follow-up examination. Third, the healthy worker effect32 might partially account for the nonsignificant results in relation to lead exposure in this occupational cohort with mean age of 29.7 years. The current observations should not be unthoughtfully generalized and are therefore not applicable to older individuals or patients with comorbidities, such as diabetes,33 which increases the vulnerability of renal function. Finally, co-exposure to cadmium is common in lead recycling plants. This metal accumulates in the kidneys, where its half-life exceeds 30 years.34 Cadmium adversely affects renal tubular and glomerular function.35 In keeping with the Occupational Safety and Health Administration standard, blood cadmium levels were monitored in workers operating the blast furnaces, but not in other study participants, so that adjustment for co-exposure to cadmium was not feasible. Although residual confounding by unmeasured risk factors can never be excluded in observational studies, SPHERL did address a wide array of potential confounders.
In conclusion, in a real-world experiment, conducted in an occupational setting, there was no association between the 2-year changes in renal function,16 the co-principal SPHERL end point, and the increment of BL level. However, given the CIs around the point estimates of the changes in eGFRcc and eGFRcys (Table 4), a larger study with longer follow-up, as planned, should consolidate the current findings. However, the current findings cast doubt on the premise that at the current environmental exposure levels, which in advanced countries are much lower than in an occupational setting,15 lead exposure is still a major contributor to CKD.15 This might not apply to patients with comorbidities, such as hypertension or diabetes, which predispose to CKD. An important concept highlighted by the SPHERL experience is that in longitudinal studies in an occupational setting not accounting for confounders, such as aging, the diurnal variation in body functions, shift work, or the physiological changes normally associated with physically strenuous work might lead to falsely attributing noxious effects to the pollutant under study.
Disclosure
All the authors declared no competing interests.
Acknowledgments
The authors gratefully acknowledge the nursing staff employed at the study sites in the United States and the expert clerical assistance of Renilde Wolfs at the Studies Coordinating Centre in Leuven, Belgium. The International Lead Association (www.ila-lead.org) provided an unrestricted grant to the Research Unit Hypertension and Cardiovascular Epidemiology partially supporting the data management and statistical analysis. The Non-Profit Research Association Alliance for the Promotion of Preventive Medicine, Mechelen, Belgium (URL, http://www.appremed.org) received a nonbinding grant from OMRON Healthcare Co., Ltd., Kyoto, Japan.
Data Sharing Statement
In consultation with the Ethics Committee that approved the study protocol, SPHERL data cannot be made publicly available. Reasons are that the informed consent signed by the workers did not cover data sharing and that an anonymized and deidentified data set still contains elements, which could potentially lead to the identification of the study participants. Only JAS (the principal investigator and corresponding author), YLY, and LT had full access to the SPHERL database. They will provide additional results according to a scientifically justified statistical analysis plan proposed by other investigators in a request addressed to JAS.
Footnotes
Supplementary Expanded Methods.
Table S1. Chronic Kidney Disease Epidemiology Collaboration equations for estimating glomerular filtration rates derived from serum creatinine, serum cystatin C, and both.
Table S2. Baseline characteristics of workers followed up and not followed up.
Table S3. Red blood cell biomarker changes from baseline to last follow-up by quartiles of the distribution of the follow-up-to-baseline blood lead concentration ratio.
Table S4. Renal function at baseline by quartiles of the distribution of the follow-up-to-baseline blood lead ratio.
Table S5. Renal function at follow-up by quartiles of the distribution of the follow-up-to-baseline blood lead ratio.
Table S6. Association between changes in red blood cell biomarkers and in blood lead.
Table S7. Association between changes in renal function and in blood lead in 223 workers not on antihypertensive drug treatment.
Table S8. Association between renal function and blood lead at baseline and last follow-up.
Table S9. Association between changes in renal function and in blood lead stratified by the median baseline age.
Table S10. Association between changes in renal function and in blood lead stratified by the median baseline blood lead.
Table S11. Association between changes in renal function and in blood lead stratified by the median cumulative blood lead index.
Figure S1. Distributions of blood lead at baseline (A) and last follow-up (B) and of the last-follow-up-to-baseline blood lead ratio (C).
Figure S2. Linear associations between baseline blood lead (bBL) and the last-follow-up-to-baseline BL ratio (rBL; A), between bBL and the residual of rBL regressed on bBL (B), and between rBL and the residual of bBL regressed on rBL (C).
Figure S3. Boxplots showing the distributions of the glomerular filtration rate derived from serum creatinine (A), serum cystatin C (B), or both serum creatinine and cystatin C (C) by study phase and work shift.
Figure S4. Heat maps relating the changes in glomerular filtration to their baseline values and the baseline blood lead concentration.
Figure S5. Heat maps relating the changes in glomerular filtration to their baseline values and the follow-up-to-baseline blood lead concentration ratio.
Supplementary Material
Supplementary Expanded Methods
Table S1. Chronic Kidney Disease Epidemiology Collaboration equations for estimating glomerular filtration rates derived from serum creatinine, serum cystatin C, and both.
Table S2. Baseline characteristics of workers followed up and not followed up.
Table S3. Red blood cell biomarker changes from baseline to last follow-up by quartiles of the distribution of the follow-up-to-baseline blood lead concentration ratio.
Table S4. Renal function at baseline by quartiles of the distribution of the follow-up-to-baseline blood lead ratio.
Table S5. Renal function at follow-up by quartiles of the distribution of the follow-up-to-baseline blood lead ratio.
Table S6. Association between changes in red blood cell biomarkers and in blood lead.
Table S7. Association between changes in renal function and in blood lead in 223 workers not on antihypertensive drug treatment.
Table S8. Association between renal function and blood lead at baseline and last follow-up.
Table S9. Association between changes in renal function and in blood lead stratified by the median baseline age.
Table S10. Association between changes in renal function and in blood lead stratified by the median baseline blood lead.
Table S11. Association between changes in renal function and in blood lead stratified by the median cumulative blood lead index.
Figure S1. Distributions of blood lead at baseline (A) and last follow-up (B) and of the last-follow-up-to-baseline blood lead ratio (C).
Figure S2. Linear associations between baseline blood lead (bBL) and the last-follow-up-to-baseline BL ratio (rBL; A), between bBL and the residual of rBL regressed on bBL (B), and rBL and the residual of bBL regressed on rBL (C).
Figure S3. Boxplots showing the distributions of the glomerular filtration rate derived from serum creatinine (A), serum cystatin C (B), or both serum creatinine and cystatin C (C) by study phase and work shift.
Figure S4. Heat maps relating the changes in glomerular filtration to their baseline values and the baseline blood lead concentration.
Figure S5. Heat maps relating the changes in glomerular filtration to their baseline values and the follow-up-to-baseline blood lead concentration ratio.
References
- 1.GBD 2017 Risk Factor Collaborators Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risk factors or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017 [published correction appears in Lancet. 2019;393:132] [published correction appears in Lancet. 2019;393:e44] Lancet. 2018;392:1923–1994. doi: 10.1016/S0140-6736(18)32225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pirkle J.L., Brody D.J., Gunter E.W., et al. The decline in blood lead levels in the United States. The National Health and Nutrition Examination Surveys (NHANES) JAMA. 1994;272:284–291. [PubMed] [Google Scholar]
- 3.Muntner P., Menke A., DeSalvo K.B., Rabito F.A., Batuman V. Continued decline in blood lead levels among adults in the United States : the National Health and Nutrition Examination Surveys. Arch Intern Med. 2005;165:2155–2161. doi: 10.1001/archinte.165.18.2155. [DOI] [PubMed] [Google Scholar]
- 4.Hara A., Thijs L., Asayama K., et al. Blood pressure in relation to environmental lead exposure in the National Health and Nutrition Examination Survey 2003 to 2010. Hypertension. 2015;65:62–69. doi: 10.1161/HYPERTENSIONAHA.114.04023. [DOI] [PubMed] [Google Scholar]
- 5.Staessen J.A., Lauwerys R.R., Buchet J.P., et al. Impairment of renal function with increasing blood lead concentrations in the general population. N Engl J Med. 1992;327:151–156. doi: 10.1056/NEJM199207163270303. [DOI] [PubMed] [Google Scholar]
- 6.Kim R., Rotnitzky A., Sparrow D., et al. A longitudinal study of low-level lead exposure and impairment of renal function. The Normative Aging Study. JAMA. 1996;275:1177–1181. [PubMed] [Google Scholar]
- 7.Muntner P., He J., Vupputuri S., Coresh J., Batuman V. Blood lead and chronic kidney disease in the general United States population: results from NHANES III. Kidney Int. 2003;63:1044–1050. doi: 10.1046/j.1523-1755.2003.00812.x. [DOI] [PubMed] [Google Scholar]
- 8.Åkesson A., Lundh T., Vahter M., et al. Tubular and glomerular kidney effects in Swedish women with low environmental cadmium exposure. Environ Health Perspect. 2005;113:1627–1631. doi: 10.1289/ehp.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lai L.H., Chou S.Y., Wu F.Y., Chen J.J.H., Kuo H.W. Renal dysfunction and hyperuricemia with low blood lead levels and ethnicity in community-based study. Sci Tot Environ. 2008;401:39–43. doi: 10.1016/j.scitotenv.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 10.Harari F., Sallsten G., Christensson A., et al. Blood lead levels and decreased kidney function in a population-based cohort. Am J Kidney Dis. 2018;72:381–389. doi: 10.1053/j.ajkd.2018.02.358. [DOI] [PubMed] [Google Scholar]
- 11.Chowdhury R., Darrow L., McClellan W., Sarnat S., Steenland K. Incident ESRD among participants in a lead surveillance program. Am J Kidney Dis. 2014;64:25–31. doi: 10.1053/j.ajkd.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 12.O’Flaherty E.J. Physiologically based methods for bone-seeking elements. IV. Kinetics of lead disposition in humans. Toxicol Appl Pharmacol. 1993;118:16–29. doi: 10.1006/taap.1993.1004. [DOI] [PubMed] [Google Scholar]
- 13.Leggett R.W. An age-specific kinetic model of lead metabolism in humans. Environ Health Perspect. 1993;101:598–616. doi: 10.1289/ehp.93101598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ekong E.B., Jaar B.G., Weaver V.M. Lead-related nephrotoxicity : a review of the epidemiologic evidence. Kidney Int. 2006;70:2074–2084. doi: 10.1038/sj.ki.5001809. [DOI] [PubMed] [Google Scholar]
- 15.Evans M., Elinder C.G. Chronic renal failure from lead: myth or evidence-based fact? Kidney Int. 2011;79:272–279. doi: 10.1038/ki.2010.394. [DOI] [PubMed] [Google Scholar]
- 16.Hara A., Gu Y.M., Petit T., et al. Study for promotion of health in recycling lead - rationale and design. Blood Press. 2015;24:147–157. doi: 10.3109/08037051.2014.996409. [DOI] [PubMed] [Google Scholar]
- 17.Mujaj B., Yang W.Y., Zhang Z.Y., et al. Renal function in relation to low-level environmental lead exposure. Nephrol Dial Transplant. 2018;34:941–946. doi: 10.1093/ndt/gfy279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inker L.A., Schmid C.H., Tighiouart H., et al. Estimating glomerular filtration rate from serum creatinine and cystatin C [published correction appears in N Engl J Med. 2012;367:681] [published correction appears in N Engl J Med. 201;367:2060] N Engl J Med. 2012;367:20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rasouli M. Basic concepts and practical equations on osmolality: biochemical approach. Clin Biochem. 2016;49:936–941. doi: 10.1016/j.clinbiochem.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 20.Levey A.S., Stevens L.A., Schmid C.H., et al. A new equation to estimate glomerular filtration rate [published correction appears in Ann Intern Med. 2011;155:408] Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roels H., Konings J., Green S., Bradley D., Chettle D., Lauwerys R. Time-integrated blood lead concentration is a valid surrugate for estimating the cumulative lead dose assessed by tibial lead measurement. Environ Res. 1995;69:75–82. doi: 10.1006/enrs.1995.1027. [DOI] [PubMed] [Google Scholar]
- 22.Fadrowski J.J., Navas-Acien A., Tellez-Plaza M., Guallar E., Weaver V.M., Furth S.L. Blood lead level and kidney function in US adolescents. The Third National Health and Nutrition Examination Survey. Arch Intern Med. 2010;170:75–82. doi: 10.1001/archinternmed.2009.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hilderink J.M., van der Linden N., Kimenai D.M., et al. Biological variation of creatinine, cystatin C, and eGFR over 24 hours. Clin Chem. 2018;64:851–860. doi: 10.1373/clinchem.2017.282517. [DOI] [PubMed] [Google Scholar]
- 24.Sirota J.H., Baldwin D.S., Villareal H. Diurnal variation of renal function in man. J Clin Invest. 1950;29:187–192. doi: 10.1172/JCI102245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dyer A.R., Martin G.J., Burton W.N., Levin M., Stamler J. Blood pressure and diurnal variation in sodium, potassium, and water excretion. J Hum Hypertens. 1998;12:363–371. doi: 10.1038/sj.jhh.1000601. [DOI] [PubMed] [Google Scholar]
- 26.Ainsworth B.E., Haskel W.L., Hermann S.D., et al. Compendium of physical activities: a second update of codes and MET values. Med Sci Sports Exerc. 2011;43:1575–1581. doi: 10.1249/MSS.0b013e31821ece12. [DOI] [PubMed] [Google Scholar]
- 27.Poortmans J.R., Ouchinsky M. Glomerular filtration rate and albumin excretion after maximal exercise in aging sendentary and active men. J Gerontol A Biol Sci Med Sci. 2006;61:1181–1185. doi: 10.1093/gerona/61.11.1181. [DOI] [PubMed] [Google Scholar]
- 28.Mingels A., Jacobs L., Kleijnen V., et al. Cystatin C a marker for renal function after exercise. Int J Sports Med. 2009;30:668–671. doi: 10.1055/s-0029-1220733. [DOI] [PubMed] [Google Scholar]
- 29.Rabinowitz M.B. Toxicokinetics of bone lead. Environ Health Perspect. 1991;91:33–37. doi: 10.1289/ehp.919133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oliveira S., Aro A., Sparrow D., Hu H. Season modifies the relationship between bone and blood lead levels : the Normative Aging Study. Arch Environ Health. 2002;57:466–472. doi: 10.1080/00039890209601439. [DOI] [PubMed] [Google Scholar]
- 31.Korrick S.A., Schwartz J., Tsaih S.W., et al. Correlates of bone and blood lead levels among middle-aged and elderly women. Am J Epidemiol. 2002;156:335–343. doi: 10.1093/aje/kwf042. [DOI] [PubMed] [Google Scholar]
- 32.Nuyts G.D., Elseviers M.M., De Broe M.E. Healthy worker effect in a cross-sectional study of lead workers. J Occup Med. 1993;35:387–391. [PubMed] [Google Scholar]
- 33.Tsaih S.W., Korrick S., Schwartz J., et al. Lead, diabetes, hypertension, and renal function: the Normative aging study. Environ Health Perspect. 2004;112:1178–1182. doi: 10.1289/ehp.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nordberg GF, Kjellström T, Nordberg M. Kinetics and metabolism. In: Friberg L, Elinder CG, Kjellström T, Nordberg GF, eds. Cadmium and Health : A Toxicological and Epidemiological Appraisal. Volume I. Exposure, Dose, and Metabolism. CRC Press Inc; 1985:103-178.
- 35.Staessen J.A., Lauwerys R.R., Ide G., Roels H.A., Vyncke G., Amery A. Renal function and historical environmental cadmium pollution from zinc smelters. Lancet. 1994;343:1523–1527. doi: 10.1016/s0140-6736(94)92936-x. [DOI] [PubMed] [Google Scholar]
- 36.Whelton P.K., Carey R.M., Aronow W.S., et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127–e248. doi: 10.1016/j.jacc.2017.11.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.