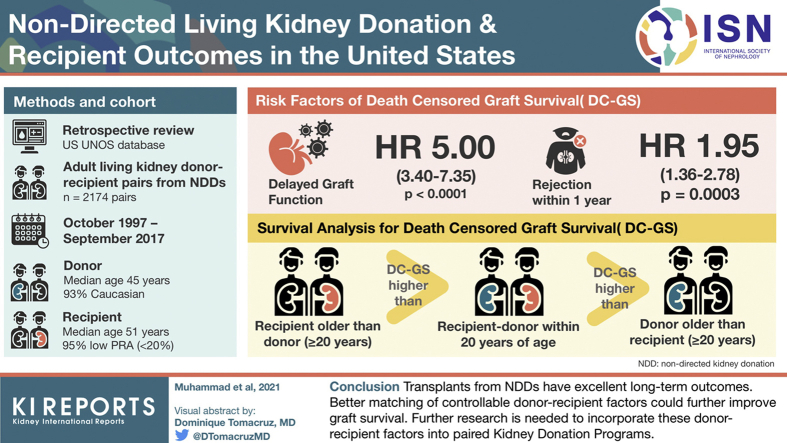
Abstract
Introduction
Nondirected donation (NDD) of the kidneys is a growing practice where donors who do not have any genetic or emotional relationship are selected to donate to a wide variety of recipients with a range of selection criteria and decisions which are left up to individual transplant centers.
Methods
We review all adult living kidney donor-recipient (DR) pairs and outcomes from NDDs who were recorded in United Network for Organ Sharing (UNOS) database as code 10 (anonymous) from October 1997 to September 2017 for demographics and outcomes.
Results
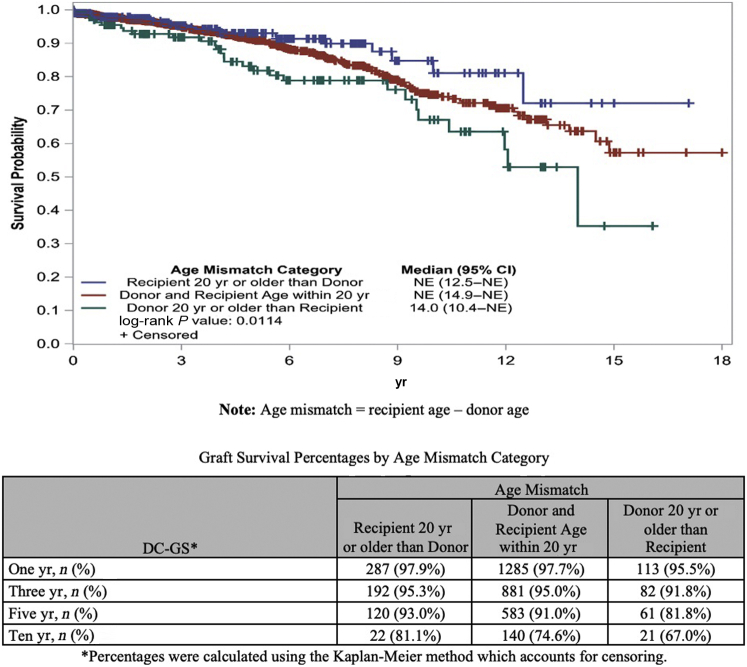
A total of 2174 DR pairs were identified. The number of NDDs increased from 18 in 2000 to 256 in 2016. Survival analysis showed higher death-censored–graft survival (DC-GS) when recipient was 20 years or more older than donor followed by recipient–donor within 20 years of age and lowest when donor was 20 years or more older than recipient (P = 0.0114).
Conclusion
Overall, the number of NDDs has increased significantly in the 20-year review period. Transplants from NDDs have excellent long-term outcomes. Better matching of controllable DR factors, such as age and body mass index (BMI), could further improve GS. Further research is needed to incorporate these DR factors into paired kidney donation programs potentially enhancing the utility and beneficence of this invaluable donation.
Keywords: donor-recipient matching, living kidney donation, nondirected kidney donation, paired exchange
Graphical abstract
Kidney transplant is the treatment of choice for end-stage kidney disease (ESKD) and has been found to have the best outcomes at 1 year and 5 years compared with dialysis.1 Unfortunately, there is a large gap between the demand for kidneys and kidney donations with >91,000 people awaiting kidney transplantation in the United States.2 Living kidney donations (LKDs) contribute significantly to the kidney donor pool comprising approximately 22% to 31% of all kidney transplants performed in the United States between 2015 and 2020.3
Overall, in the last 10 years, LKDs have not increased in the same proportion compared with deceased donations. Currently, one of the barriers in expanding LKDs is the lack of enough suitable living donors. NDDs are an invaluable resource in this regard for kidney donations contributing 5.6% (387 of 6863) of all LKDs done in the United States in 2019.4 NDD is also referred to as “anonymous” or “altruistic” donation.5 In these instances, the recipients do not know the donors and vice versa and have no genetic or emotional relationship. NDDs can significantly add to the expansion of the LKD pool and help bridge the gap between the need and availability of the kidneys. Its utility can further be optimized with its potential to start paired exchanges and benefit at least 2 or more recipients.
NDDs have increased in the United States in the past 20 years, a trend seen in other countries where legislations were passed for NDDs during this time. For comparison, in the United Kingdom, NDDs were legalized in 2006 and expanded significantly in the after 10 years.6 A study from the United Kingdom in 2018 reviewed 358 NDDs and compared them with outcomes in directed donors and reported no difference in graft outcomes.7 Similarly, single-center experiences with NDDs have been described8, 9, 10 previously; however, to best of our knowledge, our study is the first from the United States that reports the demographics and outcomes of NDDs nationally in a 20-year period.
To highlight this important group of donors who contribute the gift of a kidney, we reviewed NDDs in the UNOS database. Our study aims to (i) review the characteristics of nondirected living donors, (ii) review the characteristics of recipients who received living donor kidneys from these NDDs, and (iii) to look for any associations between donor or recipient characteristics with living donor kidney transplant (LDKT) survival where a better optimal match (age/BMI) would have been more ideal if offered through a kidney-paired exchange program. We hypothesize that kidney transplants from NDDs have excellent outcomes and that information from this study will be beneficial for transplant centers and NDDs alike in encouraging and guiding them to make informed decisions for kidney donation.
Methods
We performed a retrospective review and analysis of NDDs in the UNOS database. The Organ Procurement and Transplantation Network via UNOS mandates documentation of relationships between donor and recipient for every transplanted organ. Donors tagged with “code 4,” that is, nonbiological, unrelated (paired donation, anonymous donation, other unrelated directed donors), were identified and further classified based on subcategories. “Code 10” refers to nondirected donors who were labeled as “nonbiological, unrelated: anonymous donation.” All living donors in the UNOS database recorded as code 10 who donated between 1997 and September 2017 were reviewed for demographics. Similarly, recipients of LKDs who were older than 18 years at the time of transplant who were donated to by such donors were reviewed for demographics and physical characteristics. This information was analyzed with Statistical Package for Social Sciences version 27 (IBM Corporation, Armonk, NY) and SAS version 9.4 (SAS Institute, Cary, NC). The study involved national registry data from UNOS ensuring compliance with the Declaration of Helsinki regarding ethical standards as set forth for all transplants reviewed in the study.
Statistical Methods and Analysis
Continuous variables were summarized using mean, SD, median, minimum, and maximum. Categorical variables were summarized using frequency and percentages. Overall GS and DC-GS were calculated. Overall survival was calculated from date of transplant to date of death from any cause. Patients remaining alive were censored at their last known alive date. Median GS, DC-GS, and overall GS were estimated using the Kaplan–Meier method. Cox proportional hazards models were used to evaluate the association between risk factors and GS, first among all patients and then conditional on 1-year survival. Risk factors included recipient characteristics (age, sex, race, diabetes, BMI, whether the transplant was pre-emptive, and dialysis duration), transplant characteristics (DR age mismatch, BMI mismatch, and sex mismatch), and transplant outcomes (delayed graft function [DGF] and treatment for rejection within 1 year). Variables with a P < 0.10 in the univariable analyses were included in a multivariable Cox regression model. P < 0.05 was considered statistically significant.
Results
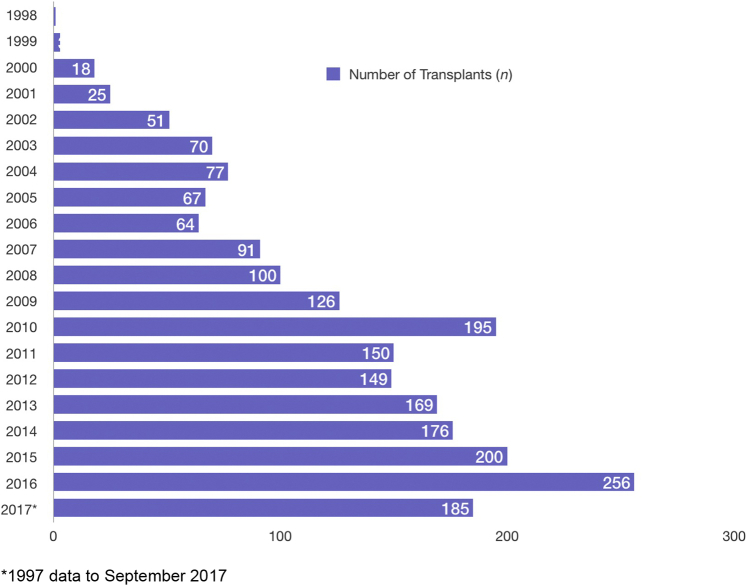
A total of 2174 nondirected DR pairs were identified during the review time period between October 1997 and September 2017. The trend for NDDs of the kidneys increased throughout this time frame with the highest seen in 2016 as illustrated in Figure 1. Of the transplants performed, 22.4% were pre-emptive (i.e., recipient not yet on dialysis) and 14.7% of the recipients received a retransplant.
Figure 1.
Number of nondirected kidney donations in the United States from 1997 to 2017. Graph of yearly nondirected donor transplant activity.
Donor Characteristics
Over the 20 years, the number of NDDs increased from <10 in 1998 to >250 in 2016, being the highest (full calendar year). Median age was 45 years with the oldest donor being 76 years old. An overwhelming majority (92.7%) of the donors were White, and females comprised 56.3% of all the donors. Median BMI was 25.4 kg/m2. A very small minority of the donors had a history of hypertension (2.1%).
The New York State had the highest number of donors (12.3%), followed by California (7.1%). In terms of education level, the largest group consisted of donors who have had an associate degree or higher (51.2%). The greatest number of donations from NDDs was performed in UNOS region 9 (17.5%), which comprises the New York State and western Vermont (Table 1).
Table 1.
Donor characteristics
| Characteristics | Value |
|---|---|
| Age, mean ± SD; median (min–max) | 44.0 ± 12.2; 45 (18–76) |
| Race, n (%) | |
| White | 2015 (92.7) |
| African American | 51 (2.4) |
| Other | 108 (5.0) |
| Ethnicity, n (%) | |
| Hispanic | 57 (2.6) |
| Non-Hispanic | 2117 (97.4) |
| Sex, n (%) | |
| Female | 1224 (56.3) |
| Male | 950 (43.7) |
| BMI, mean ± SD; median (min–max) | 25.7 ± 3.9; 25.4 (15.7–42.2) |
| History of hypertension, n (%) | 42 (2.1) |
| Blood type, n (%) | |
| O | 1003 (46.1) |
| A | 824 (37.9) |
| B | 261 (12.0) |
| AB | 86 (4.0) |
| Top 3 donor home states, n (%) | |
| New York | 266 (12.3) |
| California | 152 (7.1) |
| Minnesota | 105 (4.9) |
| Highest education level, n (%) | |
| Less than high school | 15 (0.7) |
| High school | 368 (16.9) |
| Some college/technical | 445 (20.5) |
| Associate/bachelor’s degree | 663 (30.5) |
| Postcollege/grad | 450 (20.7) |
| Unknown | 233 (10.7) |
| Top 3 transplant UNOS region, n (%) | |
| 9 | 381 (17.5) |
| 5 | 353 (16.2) |
| 7 | 277 (12.7) |
BMI, body mass index; max, maximum; min, minimum; UNOS, United Network for Organ Sharing.
Recipient Characteristics
Median age for recipients of NDD kidneys was 51 years with the oldest recipient being 79 years old. Of the recipients, 67.7% were White and 59.4% were males. Median wait time for the recipients was 485 days, and most of the recipients (94.8%) had low panel reactive antibody level (≤20%). In addition, 27.5% of the recipients had diabetes mellitus, and 21% of ESKD was caused by diabetes mellitus (Table 2).
Table 2.
Recipient characteristics
| Characteristics | Value |
|---|---|
| Age, mean ± SD; median (min–max) | 49.4 ± 13.2; 51 (18–79) |
| Race, n (%) | |
| White | 1472 (67.7) |
| African American | 337 (15.5) |
| Other | 365 (16.8) |
| Ethnicity, n (%) | |
| Hispanic | 194 (8.9) |
| Non-Hispanic | 1980 (91.1) |
| Sex, n (%) | |
| Female | 883 (40.6) |
| Male | 1291 (59.4) |
| BMI, mean ± SD; median (min–max) | 27.9 ± 5.6; 27.5 (11.3–52.1) |
| Retransplants, n (%) | 319 (14.7) |
| Pre-emptive, n (%) | 486 (22.4) |
| Waiting time (on list), mean ± SD; median (min–max) | 656.8 ± 597.5; 485 (0–4941) |
| Most recent peak PRA, n (%) | |
| ≤20 | 2060 (94.8) |
| 21–80 | 80 (3.7) |
| >80 | 34 (1.6) |
| DM, n (%) | 597 (27.5) |
| PVD, n (%) | 110 (5.1) |
| Cause of ESKD, n (%) | |
| DM | 457 (21.0) |
| HTN | 342 (15.7) |
| PKD | 298 (13.7) |
| FSGS | 151 (6.9) |
| IgA | 122 (5.6) |
| Others | 804 (37.0) |
BMI, body mass index; DM, diabetes mellitus; ESKD, end-stage kidney disease; FSGS, focal segmental glomerular sclerosis; HTN, hypertension; max, maximum; min, minimum; PKD, polycystic kidney disease; PRA, panel reactive antibody.
Transplant and Matching Characteristics
Left donor nephrectomy was done in most of the donors (87.4%). ABO blood group was identical in 89.2% of the DR pairs, whereas only 9.2% donor and recipients had identical human leukocyte antigen—donor–recipient DR isotype (HLA-DR) match status. Zero HLA mismatch overall was only 1%. BMI was fairly well matched overall with a mean mismatch of 2.2 kg/m2 with SD of 6.6 kg/m2 and median of 2.1 kg/m2. However, 11.9% of the recipients had a BMI of 10 kg/m2 or greater than the donor. For age mismatch, mean age difference between recipient and donor was 5.4 years with SD of 16 years and median difference of 4 years. A total of 25.5% DR pairs showed 20 years or more age differences, where 19% pairs had younger donors than recipients and only 6.5% younger recipients than donors (Table 3).
Table 3.
Transplant characteristics
| Characteristics | Value |
|---|---|
| Donor nephrectomy side, n (%) | |
| Left | 1899 (87.4) |
| Right | 275 (12.7) |
| ABO match, n (%) | |
| Identical | 1940 (89.2) |
| Compatible | 217 (10.0) |
| Incompatible | 17 (0.8) |
| DR mismatch level, n (%) | |
| 0 MM | 197 (9.2) |
| 1 MM | 991 (46.1) |
| 2 MM | 960 (44.7) |
| HLA mismatch level, n (%) | |
| 0 MM | 21 (1.0) |
| 1 MM | 30 (1.4) |
| 2 MM | 112 (5.2) |
| 3 MM | 308 (14.3) |
| 4 MM | 608 (28.3) |
| 5 MM | 702 (32.7) |
| 6 MM | 367 (17.1) |
| BMI mismatch (recipient–donor), mean ± SD; median (min–max) | 2.2 ± 6.6; 2.1 (−19.2 to 31) |
| BMI mismatch category, n (%) | |
| Donor BMI 10 kg/m2 or greater than recipient | 137 (6.3) |
| Donor and recipient BMI within 10 kg/m2 | 1778 (81.8) |
| Recipient BMI 10 kg/m2 or greater than donor | 259 (11.9) |
| Age mismatch (recipient–donor), mean ± SD; median (min–max) | 5.4 ± 16.0; 4 (−40 to 51) |
| Age mismatch category, n (%) | |
| Donor 20 yr or older than recipient | 141 (6.5) |
| Donor and recipient BMI within 20 yr | 1620 (74.5) |
| Recipient 20 yr or older than donor | 413 (19.0) |
| Sex mismatch | |
| Female-to-female | 517 (23.8) |
| Male-to-male | 584 (26.9) |
| Female-to-male | 707 (32.5) |
| Male-to-female | 366 (16.8) |
BMI, body mass index; DR, donor–recipient; HLA, human leukocyte antigen; max, maximum; min, minimum.
Transplant Outcomes, Overall Survival, and DC-GS
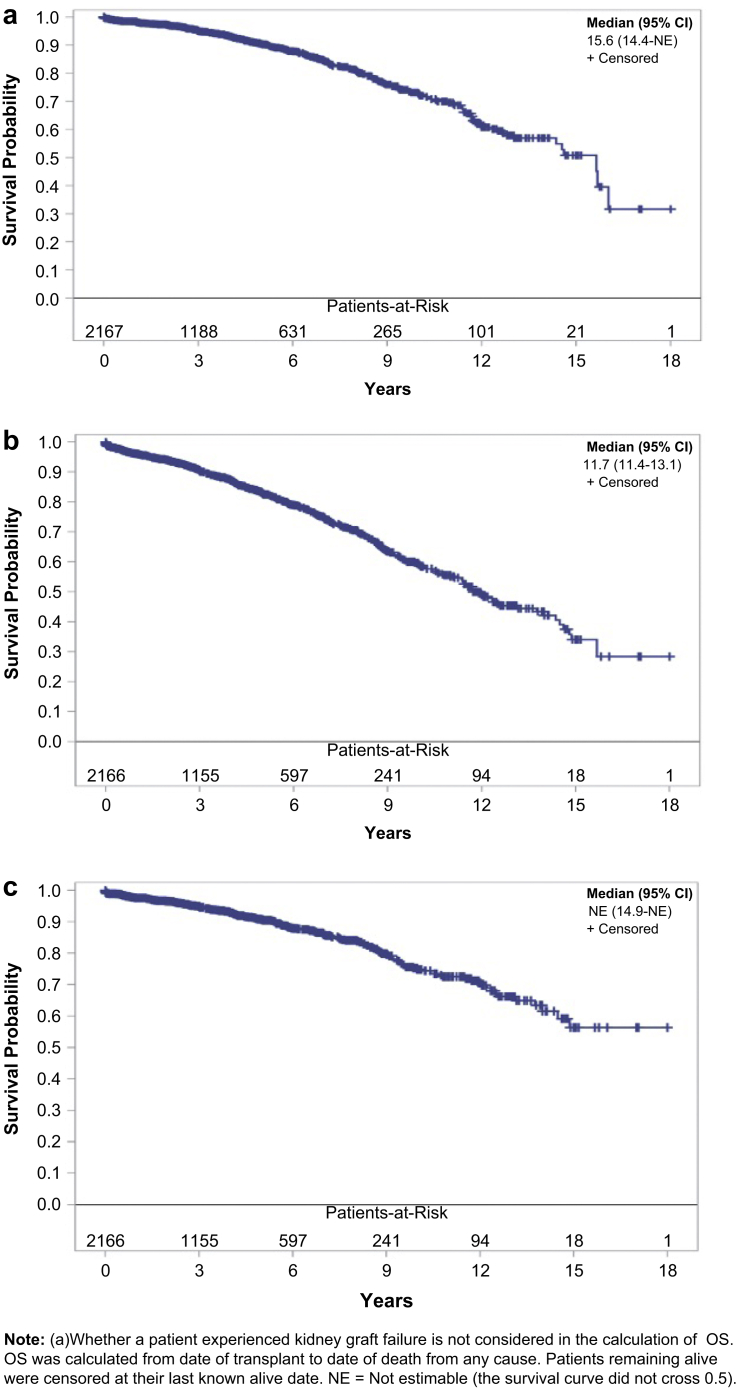
The overall unadjusted 1-year and 5-year GS rates were 96.1% and 82.9%, respectively, when both graft failure and death were counted for graft failures (GS). DC-GS at 1 year and 5 years was 97.6% and 90.6%, respectively. DGF and 1-year acute rejection rates were 4.1% and 7.6%, respectively (Table 4 and Figure 2).
Table 4.
Transplant outcomes
| Characteristics | Value |
|---|---|
| 1-yr acute rejection rate, n (%) | 166 (7.6) |
| Delayed graft function, n (%) | 90 (4.1) |
| GS, n (%)a | |
| 1 yr | 1685 (96.1) |
| 3 yr | 1155 (90.4) |
| 5 yr | 764 (82.9) |
| 10 yr | 183 (59.1) |
| DC-GS, n (%)a | |
| 1 yr | 1685 (97.6) |
| 3 yr | 1155 (94.8) |
| 5 yr | 764 (90.6) |
| 10 yr | 183 (74.8) |
DC-GS, death-censored GS; GS, graft survival.
Percentages were calculated using the Kaplan–Meier method that accounts for censoring.
Figure 2.
(a) Overall patient survival, (b) graft survival, and (c) death-censored graft survival. NE, not estimable; OS, overall survival.
Independent Risk Factors of GS and DC-GS
Analytical results from univariable Cox regression are presented in Tables 5 and 6. Recipient diabetes mellitus (hazard ratio [HR] = 1.62, P < 0.0001) and DGF (HR = 3.40, P < 0.0001) were significantly associated with increased hazards of graft failure. Besides, older recipient age (HR = 1.02, P < 0.0001), higher recipient BMI (HR = 1.03, P = 0.0011), and receiving dialysis before transplant (HR = 1.32, P = 0.0383) are significant risk factors associated with graft failure. Race of recipients and BMI mismatch category presented significant differences on GS as well (P = 0.0018 and P = 0.0494, respectively). For DC-GS, DGF (HR = 5.59, P < 0.0001) was significantly associated with an increased hazard of graft failure. Older recipient age (HR = 0.98, P = 0.0017) was significantly associated with a decreased hazard of graft failure. Race of recipients and age mismatch category presented significant differences on DC-GS as well (P = 0.0346 and P = 0.0127, respectively).
Table 5.
Analytical results of graft survival from univariable Cox regression
| Variables | HR | 95% CI | P value |
|---|---|---|---|
| Recipient diabetes | <0.0001a | ||
| Yes | 1.62 | 1.33–1.99 | |
| No (reference) | |||
| Recipient BMI, 1-unit increase | 1.03 | 1.01–1.05 | 0.0011a |
| Recipient age, 1-yr increase | 1.02 | 1.01–1.03 | <0.0001a |
| Sex | |||
| Male | 1.00 | 0.82–1.21 | 0.9889 |
| Female (reference) | |||
| Race | 0.0018a | ||
| Black | 1.10 | 0.86–1.42 | |
| Other | 0.60 | 0.45–0.82 | |
| White (reference) | |||
| Delayed graft function | <0.0001a | ||
| Yes | 3.40 | 2.44–4.73 | |
| No (reference) | |||
| Pre-emptive transplant | 0.0383a | ||
| No | 1.32 | 1.02–1.72 | |
| Yes (reference) | |||
| Dialysis duration, 1-yr increase | 1.02 | 0.99–1.05 | 0.1858 |
| Age mismatch category | 0.8061 | ||
| Donor 20+ than recipient | 1.04 | 0.73–1.48 | |
| Recipient 20+ than donor | 1.09 | 0.84–1.41 | |
| Within 20 yr (reference) | |||
| BMI mismatch category | 0.0494a | ||
| Donor 10+ kg/m2 than recipient | 1.21 | 0.88–1.66 | |
| Recipient 10+ kg/m2 than donor | 1.39 | 1.05–1.85 | |
| Within 10 kg/m2 (reference) | |||
| Sex mismatch category | 0.7482 | ||
| Male-to-male | 1.11 | 0.85–1.45 | |
| Female-to-male | 0.99 | 0.76–1.29 | |
| Male-to-female | 1.11 | 0.82–1.49 | |
| Female-to-female (reference) |
BMI, body mass index; HR, hazard ratio.
P < 0.10.
Table 6.
Analytical results of DC-GS from univariable Cox regression
| Variables | HR | 95% CI | P value |
|---|---|---|---|
| Recipient diabetes | |||
| Yes | 1.00 | 0.74–1.36 | 0.9862 |
| No (reference) | |||
| Recipient BMI, 1-unit increase | 1.02 | 0.99–1.04 | 0.1648 |
| Recipient age, 1-yr increase | 0.98 | 0.97–0.99 | 0.0017a |
| Sex | |||
| Male | 0.89 | 0.68–1.16 | 0.3768 |
| Female (reference) | |||
| Race | |||
| Black | 1.42 | 1.02–1.97 | 0.0346a |
| Other | 0.82 | 0.56–1.20 | |
| White (reference) | |||
| Delayed graft function | |||
| Yes | 5.59 | 3.84–8.14 | <0.0001a |
| No (reference) | |||
| Pre-emptive transplant | |||
| No | 1.38 | 0.95–2.00 | 0.0895a |
| Yes (reference) | |||
| Dialysis duration, 1-yr increase | 1.01 | 0.97–1.05 | 0.6361 |
| Age mismatch category | |||
| Donor 20+ than recipient | 1.62 | 1.08–2.41 | 0.0127a |
| Recipient 20+ than donor | 0.73 | 0.48–1.11 | |
| Within 20 yr (reference) | |||
| BMI mismatch category | |||
| Donor 10+ kg/m2 than recipient | 1.06 | 0.67–1.69 | 0.3996 |
| Recipient 10+ kg/m2 than donor | 1.31 | 0.89–1.95 | |
| Within 10 kg/m2 (reference) | |||
| Sex mismatch category | |||
| Male-to-male | 0.81 | 0.55–1.20 | 0.6301 |
| Female-to-male | 0.98 | 0.69–1.38 | |
| Male-to-female | 1.05 | 0.70–1.56 | |
| Female-to-female (reference) |
BMI, body mass index; DC-GS, death-censored graft survival; HR, hazard ratio.
P < 0.10.
Risk Factors of GS and DC-GS in Multivariable Cox Regression Models
For GS, recipient with diabetes mellitus (HR = 1.39, P = 0.0031), older recipient age (HR = 1.01, P = 0.0022), DGF (HR = 2.96, P < 0.0001), not having a pre-emptive transplant (HR = 1.32, P = 0.0422), and race (P = 0.0042) were all significantly associated with higher graft failure hazards. For DC-GS, DGF (HR = 5.26, P < 0.0001) was significantly associated with higher graft failure hazard (Tables 7 and 8).
Table 7.
Analytical results of graft survival from multivariable Cox regression
| Variables | HR | 95% CI | P value |
|---|---|---|---|
| Recipient diabetes | |||
| Yes | 1.39 | 1.12–1.73 | 0.0031 |
| No (reference) | |||
| Recipient BMI, 1-unit increase | 1.01 | 0.99–1.03 | 0.3559 |
| Recipient age, 1-yr increase | 1.01 | 1.01–1.02 | 0.0022 |
| Race | 0.0042 | ||
| Black | 1.05 | 0.81–1.37 | |
| Other | 0.61 | 0.44–0.83 | |
| White (reference) | |||
| Delayed graft function | |||
| Yes | 2.96 | 2.10–4.19 | <0.0001 |
| No (reference) | |||
| Pre-emptive transplant | |||
| No | 1.32 | 1.01–1.73 | 0.0422 |
| Yes (reference) | |||
| BMI mismatch category | |||
| Donor 10+ kg/m2 than recipient | 1.44 | 1.03–2.03 | 0.0724 |
| Recipient 10+ kg/m2 than donor | 1.18 | 0.83–1.66 | |
| Within 10 kg/m2 (reference) |
BMI, body mass index; HR, hazard ratio.
Table 8.
Analytical results of DC-GS from multivariable Cox regression
| Variables | HR | 95% CI | P value |
|---|---|---|---|
| Recipient age, 1-yr increase | 0.99 | 0.97–1.00 | 0.0325 |
| Race | |||
| Black | 1.08 | 0.77–1.51 | 0.1935 |
| Other | 0.73 | 0.49–1.07 | |
| White (reference) | |||
| Delayed graft function | |||
| Yes | 5.26 | 3.58–7.74 | <0.0001 |
| No (reference) | |||
| Pre-emptive transplant | |||
| No | 1.33 | 0.92–1.94 | 0.1325 |
| Yes (reference) | |||
| Age mismatch category | |||
| Donor 20+ than recipient | 1.16 | 0.74–1.81 | 0.7434 |
| Recipient 20+ than donor | 0.89 | 0.57–1.41 | |
| Within 20 yr (reference) |
DC-GS, death-censored graft survival; HR, hazard ratio.
Risk Factors of GS and DC-GS Conditional on 1-Year Survival
By excluding patients who were lost to follow-up, graft loss, or patient death during the first year, analyses based on univariable and multivariable Cox regression models were performed to identify risk factors of GS (Tables 9 and 10) and DC-GS (Tables 11 and 12) conditional on 1-year survival. Compared with analyses represented by Table 5, Table 6, Table 7, Table 8, both the risk factors selected for the multivariable models and their estimated HRs remain close. For DC-GS conditional on 1-year survival (Table 12), not having a pre-emptive transplant was identified as an additional significant risk factor (HR = 1.54, P = 0.0391), as compared with the complete analysis on all patients (Table 8). Conditional on 1-year survival, rejection within 1 year was significantly associated with decreased GS (HR = 1.57, P = 0.0039) and DC-GS (HR = 2.15, P < 0.0001) (Table 9, Table 10, Table 11, Table 12).
Table 9.
Analytical results of 1-year conditional unadjusted graft survival from univariable Cox regression (n = 1714)
| Variables | HR | 95% CI | P value |
|---|---|---|---|
| Recipient diabetes | <0.0001a | ||
| Yes | 1.63 | 1.31–2.01 | |
| No (reference) | |||
| Recipient BMI, 1-unit increase | 1.03 | 1.01–1.05 | 0.0021a |
| Recipient age, 1-yr increase | 1.02 | 1.01–1.03 | 0.0001a |
| Sex | |||
| Male | 1.00 | 0.81–1.23 | 0.9960 |
| Female (reference) | |||
| Race | 0.0181a | ||
| Black | 1.09 | 0.83–1.42 | |
| Other | 0.66 | 0.49–0.90 | |
| White (reference) | |||
| Delayed graft function | <0.0001a | ||
| Yes | 3.22 | 2.24–4.64 | |
| No (reference) | |||
| Treated for rejection within 1 yr | 0.0019a | ||
| Yes | 1.61 | 1.19–2.16 | |
| No (reference) | |||
| Pre-emptive transplant | 0.0071a | ||
| No | 1.50 | 1.12–2.02 | |
| Yes (reference) | |||
| Dialysis duration, 1-yr increase | 1.01 | 0.98–1.04 | 0.6265 |
| Age mismatch category | 0.8130 | ||
| Donor 20+ than recipient | 0.98 | 0.67–1.42 | |
| Recipient 20+ than donor | 0.91 | 0.68–1.22 | |
| Within 20 yr (reference) | |||
| BMI mismatch category | 0.0492a | ||
| Donor 10+ kg/m2 than recipient | 1.19 | 0.85–1.67 | |
| Recipient 10+ kg/m2 than donor | 1.43 | 1.06–1.93 | |
| Within 10 kg/m2 (reference) | |||
| Sex mismatch category | 0.5826 | ||
| Male-to-male | 1.14 | 0.86–1.52 | |
| Female-to-male | 0.96 | 0.72–1.27 | |
| Male-to-female | 1.09 | 0.80–1.50 | |
| Female-to-female (reference) |
BMI, body mass index; HR, hazard ratio.
P < 0.10.
Table 10.
Analytical results of 1-yr conditional graft survival from multivariable Cox regression (n = 1714)
| Variables | HR | 95% CI | P value |
|---|---|---|---|
| Recipient diabetes | |||
| Yes | 1.42 | 1.13–1.79 | 0.0030 |
| No (reference) | |||
| Recipient BMI, 1-unit increase | 1.01 | 0.99–1.03 | 0.4128 |
| Recipient age, 1-yr increase | 1.01 | 1.00–1.02 | 0.0100 |
| Race | |||
| Black | 1.03 | 0.78–1.37 | 0.0256 |
| Other | 0.66 | 0.48–0.90 | |
| White (reference) | |||
| Delayed graft function | |||
| Yes | 2.66 | 1.81–3.90 | <0.0001 |
| No (reference) | |||
| Treated for rejection within 1 yr | |||
| Yes | 1.57 | 1.16–2.14 | 0.0039 |
| No (reference) | |||
| Pre-emptive transplant | |||
| No | 1.53 | 1.13–2.06 | 0.0058 |
| Yes (reference) | |||
| BMI mismatch category | |||
| Donor 10+ kg/m2 than recipient | 1.49 | 1.05–2.11 | 0.0501 |
| Recipient 10+ kg/m2 than donor | 1.22 | 0.85–1.77 | |
| Within 10 kg/m2 (reference) |
BMI, body mass index; HR, hazard ratio.
Table 11.
Analytical results of 1-yr conditional DC-GS from univariable Cox regression (n = 1714)
| Variables | HR | 95% CI | P value |
|---|---|---|---|
| Recipient diabetes | |||
| Yes | 0.97 | 0.70–1.33 | 0.8335 |
| No (reference) | |||
| Recipient BMI, 1-unit increase | 1.02 | 0.99–1.04 | 0.2169 |
| Recipient age, 1-yr increase | 0.98 | 0.97–0.99 | 0.0006a |
| Sex | |||
| Male | 0.90 | 0.68–1.18 | 0.4345 |
| Female (reference) | |||
| Race | |||
| Black | 1.55 | 1.10–2.18 | 0.0243a |
| Other | 0.92 | 0.63–1.37 | |
| White (reference) | |||
| Delayed graft function | |||
| Yes | 4.82 | 3.16–7.33 | <0.0001a |
| No (reference) | |||
| Treated for rejection within 1 yr | |||
| Yes | 2.28 | 1.59–3.27 | <0.0001a |
| No (reference) | |||
| Pre-emptive transplant | |||
| No | 1.53 | 1.02–2.30 | 0.0400a |
| Yes (reference) | |||
| Dialysis duration, 1-yr increase | 1.01 | 0.97–1.06 | 0.5701 |
| Age mismatch category | |||
| Donor 20+ than recipient | 1.57 | 1.04–2.38 | 0.0032a |
| Recipient 20+ than donor | 0.55 | 0.33–0.90 | |
| Within 20 yr (reference) | |||
| BMI mismatch category | |||
| Donor 10+ kg/m2 than recipient | 1.14 | 0.71–1.82 | 0.3950 |
| Recipient 10+ kg/m2 than donor | 1.32 | 0.87–2.00 | |
| Within 10 kg/m2 (reference) | |||
| Sex mismatch category | |||
| Male-to-male | 0.84 | 0.56–1.26 | 0.6954 |
| Female-to-male | 0.99 | 0.69–1.43 | |
| Male-to-female | 1.09 | 0.72–1.65 | |
| Female-to-female (reference) |
BMI, body mass index; DC-GS, death-censored graft survival; HR, hazard ratio.
P < 0.10.
Table 12.
Analytical results of 1-yr conditional DC-GS from multivariable Cox regression (n = 1714)
| Variables | HR | 95% CI | P value |
|---|---|---|---|
| Recipient age, 1-yr increase | 0.99 | 0.97–1.00 | 0.0288 |
| Race | |||
| Black | 1.19 | 0.84–1.69 | 0.2911 |
| Other | 0.83 | 0.56–1.23 | |
| White (reference) | |||
| Delayed graft function | |||
| Yes | 4.15 | 2.69–6.40 | <0.0001 |
| No (reference) | |||
| Treated for rejection within 1 yr | |||
| Yes | 2.15 | 1.49–3.09 | <0.0001 |
| No (reference) | |||
| Pre-emptive transplant | |||
| No | 1.54 | 1.02–2.32 | 0.0391 |
| Yes (reference) | |||
| Age mismatch category | |||
| Donor 20+ than recipient | 1.10 | 0.69–1.75 | 0.3932 |
| Recipient 20+ than donor | 0.70 | 0.41–1.19 | |
| Within 20 yr (reference) |
DC-GS, death-censored graft survival; HR, hazard ratio.
GS and DC-GS by Age Mismatch
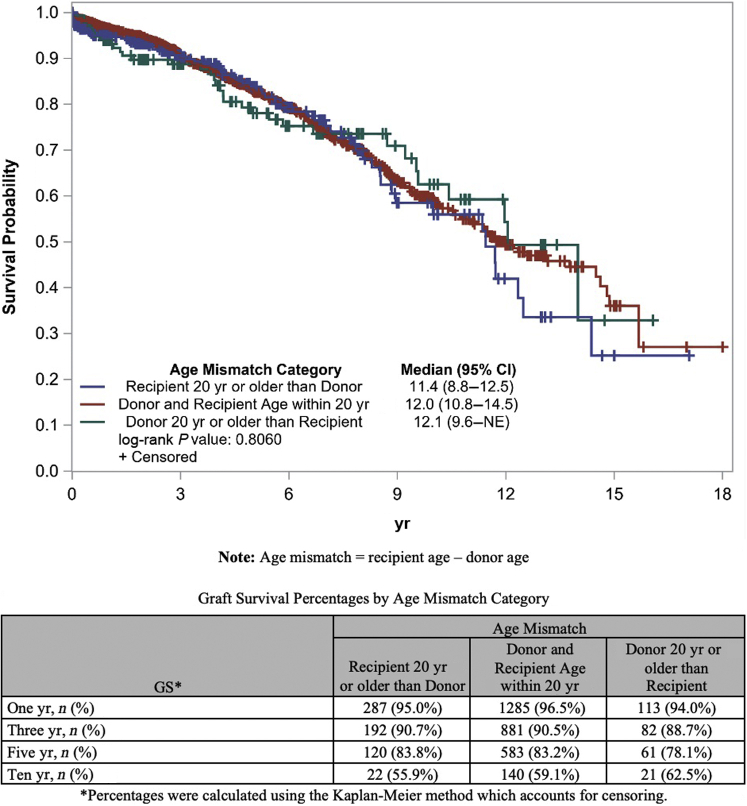
Analysis based on log-rank tests for GS among age mismatch groups showed no significant differences between groups. However, survival analysis showed significant differences in DC-GS among different age mismatch groups. Specifically, we observed a higher DC-GS when recipient was 20 years or more older than donor followed by recipient–donor within 20 years of age and lowest when donor was 20 years or more older than recipient (P = 0.0114) (Figures 3 and 4).
Figure 3.
Graft survival using Kaplan–Meier curve by age mismatch category. GS, graft survival; NE, not estimable.
Figure 4.
Death-censored graft survival Kaplan–Meier curve by age mismatch category. DC-GS, death-censored graft survival; NE, not estimable.
GS and DC-GS by BMI Mismatch
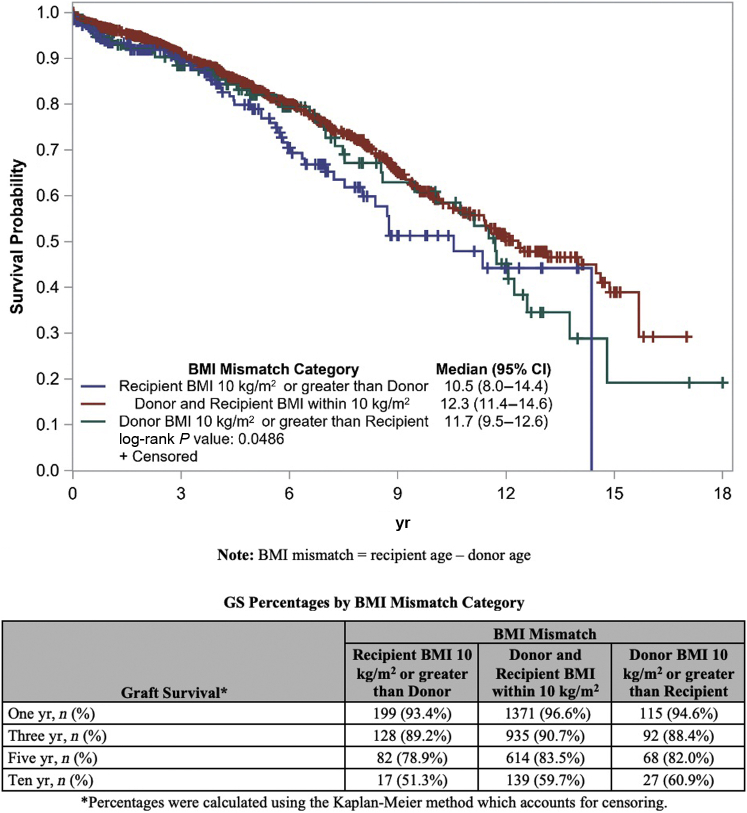
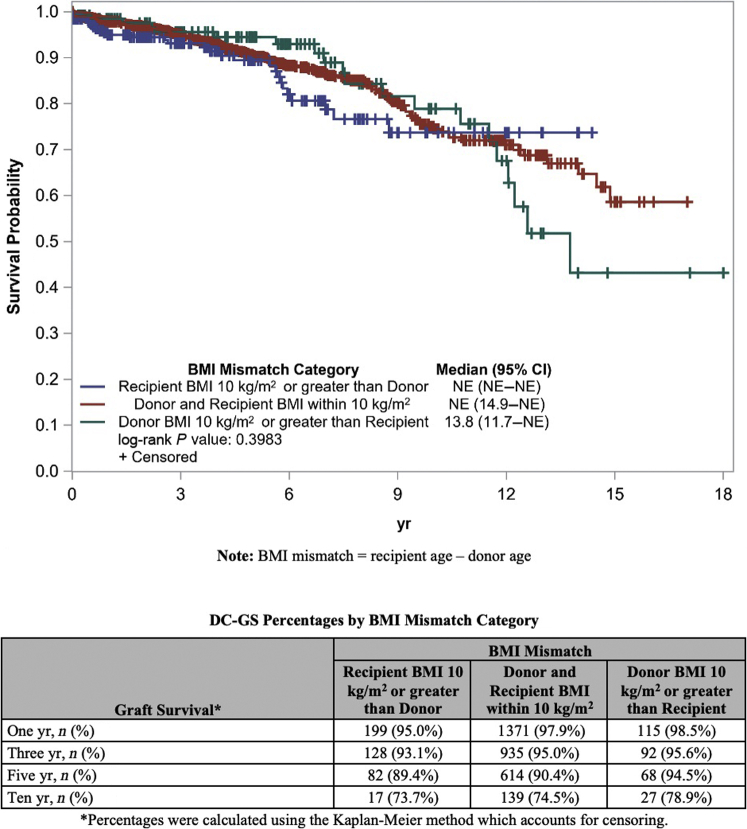
Survival analysis based on the log-rank test showed significant differences in GS among different BMI mismatch groups. Specifically, we observed reduced GS when the recipient had a BMI 10 kg/m2 or greater than the donor (P = 0.0486). However, a similar analysis for DC-GS among BMI mismatch groups showed no significant differences between groups (Figures 5 and 6).
Figure 5.
Graft survival Kaplan–Meier curve by BMI mismatch category. BMI, body mass index.
Figure 6.
Death-censored graft survival Kaplan–Meier curve by BMI mismatch category. BMI, body mass index; NE, not estimable.
Discussion
NDD of Kidneys and Donor Profile
To our knowledge, this is the first national study of its kind reviewing NDDs in the United States over a 20-year period with a large sample size. Our study highlights the increased NDDs over this time period, which is a trend seen in other countries where NDD is allowed.7 Overall, this trend is attributed to a number of factors, including legislations passed over the years to facilitate NDDs, increased public awareness and education, and improvement and innovation in donor surgical techniques which have advanced LKD in general improving safety and reducing donor recovery times.
We further highlight the unique characteristics of this subset of population. Most of the donors in our study were young (median age 45 years), and many (51.2%) had associate degrees or higher education. This may reflect the higher likelihood of more educated people to access resources in the media to explore the possibility of donation. Previous studies have also shown that NDDs belong to higher socioeconomic strata of the society.11
Other studies reporting characteristics of nondirected donors have shown different profiles. A study from the United Kingdom7 showed that nondirected donors were on average 10 years older compared with directed donors. In their study, blood group A was found to be more common in NDDs, as blood group O was more common in directed donors.7 In the United States, blood group O is the most common blood group among living kidney donors,4 and this trend was seen in our study as well. A study done among the National Kidney Registry participating centers (National Kidney Registry is a coalition of transplant programs in the United States National Kidney Registry) maintaining a database of nondirected donors used for kidney transplant exchange showed an average age of 45.6 years for donors and that most were White and female.11 Our study shows that the average age of nondirected donors was 44 years with 56.3% females and 92.7% White, which is comparable with the abovementioned study.
Most centers in the United States accept NDDs, as shown by a study in 2016. This study reported that most of the transplant centers sampled, accepted such donors (67 of 73 centers interviewed)12; however, the number of such donations varied by center and region. This may represent a variation in practice and attitude toward such type of donation and has been reported to be a significant barrier in the past for acceptance of nondirected donors, although this has significantly improved in the past 3 decades from 8% in 1989 to 61% in 2000.13,14 Transplant center–specific factors may include their level of experience, comfort levels, risk tolerance for outcomes, and legal, medical risk, and any social repercussions.12 There also quite often are different medical and/or psychological criteria for NDDs as compared with directed or related donors in accepting such donors.15
Recipient Profile of Nondirected Donors
The mean recipient age of 49.4 years for such NDDs was similar to what has been described in the study from the United Kingdom,7 although there was variation that led to age mismatch in several cases. Greater number of recipients was males, which is likely a reflection of higher incidence and prevalence of ESKD in males.
Most common reason for ESKD among recipients was diabetes and hypertension accounting for 36.7% of the cases. This is not surprising given that diabetes and hypertension are the topmost identifiable causes of ESKD among people on dialysis. Most of the recipients were not highly sensitized (panel reactive antibody <20%), likely reflecting practice where centers choose not to have a high immunologic risk in selecting the appropriate recipients for NDDs. Interestingly enough, there was only 1% of DR pairs with 0 HLA mismatch. A large registry review of LDKTs has shown lower GS with higher HLA mismatch in DR pairs.16 The percentage of recipients who got transplanted pre-emptively was 22.4%, which is lower than the 31% overall rate of pre-emptive transplants in LDKT.17 This again largely reflects center practice and participation in kidney-paired donation programs. Many centers choose to enter a pair into kidney-paired donation based on ABO incompatibility, positive crossmatches, surgical incompatibility, and size and age mismatch.
Outcomes
Overall post-transplant rate of DGF was 4.1% of the transplants. In our study, DGF is a significant risk factor (HR = 2.96 for GS and 5.26 for DC-GS) when controlling for other factors. Effect of DGF on GS among living kidney recipients has been well described, and its incidence ranges between 1.6% and 3.6% among LDKT recipients.18,19 DGF has been associated with higher incidence of acute episodes of rejection and reduced GS.20 Although the fact that DGF was associated with poor long-term outcomes is not a novel finding, it should be kept in mind that despite typical DGF rates being lower in LDKT transplants as compared with deceased donor kidney transplantation, this could represent a recipient selection criteria scenario, where it led to inferior outcomes.
Effect of Age, Sex, and BMI Mismatch Between Donors and Recipients on GS and DC-GS
Age mismatch had a profound and statistically significant effect on DC-GS in our study. For DC-GS, in the DR pairs with recipients older than donor, longer survival was noted when the age difference was >20 years compared with an age difference of 20 years or less. A recipient and donor age difference within 20 years of each other showed intermediate survival. Among the DR pairs with a younger recipient compared with the donor, an age difference of 20 years or less had superior outcomes than an age difference of >20 years. For GS, no such differences among DR pairs were seen. Although we could not discern the exact granular reasons for why certain age mismatch transplants were done, this reflection of practice sheds light on how centers are attempting not to transplant elderly NDD’s into younger recipients.
Studies of living kidney donors and recipients have showed mixed findings with respect to age mismatch. Findings of Ferrari et al.21 from the Australia and New Zealand Transplant Registry review showed a higher risk of graft failure when the DR age difference was >30 years when compared with between (−10 to +20 years) group during the first-year post-transplant. Similarly, a study22 from Japan reviewed 24 years of living DR pairs and found that recipients of kidneys from people older than 50 years affected their long-term GS when transplanted into individuals younger than 30 years of age compared with older recipients. Other studies23, 24, 25 have shown no difference in GS when analyzed for age difference between donors and recipients although some of these studies were among living related pairs where survival can potentially be influenced by better immunologic matching. These studies do show that a younger donor to an older recipient has better overall survival compared with a younger recipient of an older kidney most likely related to the quality of the kidneys transplanted.
Sex mismatch in DR pairs has been shown to affect GS with studies revealing different outcomes among sex-mismatched pairs. A study by Naderi et al.26 showed that better outcomes were observed when donors were male and that longest GS was seen in male-to-male followed by male-to-female kidney donation. Similarly, Shahani et al.23 reviewed 500 LDKTs and found that male-to-male kidney donation had better GS compared with female-to-male kidney donation. Nevertheless, some studies have shown that male donor to female recipient is a combination with the poorer GS.27,28 This has been postulated to be due to a number of factors including minor non-HLA incompatibility,29 greater body surface area correlating with larger kidney size in males compared with females,30 and hence higher volume of nephron mass transplanted in male kidneys.31 Our study did not show any statistically significant difference in sex-mismatched DR pairs.
Similarly, a mismatch in DR size where the recipient has higher BMI than donor (with BMI taken as a proxy for kidney size) has also been shown to negatively impact graft function and survival. Miller et al.32 showed that if recipients were >30 kg above donors irrespective of DR sex mismatch, there was a HR of 1.5 and 1.35 in male-to-female and female-to-male donations, respectively. Hwang et al.33 reviewed 123 adult LDKTs and found that a low donor kidney to recipient weight ratio (smaller kidney into a recipient with higher BMI) had the lowest 5- and 10-year GS. Several studies31,34 from deceased donor kidney transplant pairs have shown that smaller kidney transplanted into larger recipients resulted in compensatory hyperfiltration, increased creatinine clearance, and higher risks of proteinuria compared with a better-sized match or larger kidneys into smaller recipients. Our study showed that donor and recipients were well matched in most of the cases with a mean (DR) BMI difference of 2.2 kg/m2 and only 11.9% (n = 259) of recipients had a BMI greater than the donor by 10 kg/m2 or more and 6.3% (n = 141) of donors had a BMI greater than the recipient by 10 kg/m2 or more. No statistically significant difference in DC-GS was seen in our study among DR pairs. Unadjusted differences in GS, however, were observed, with recipients who had a BMI 10 kg/m2 or greater than the donor having reduced GS (P = 0.0494). Overall, it appears that there is high variability in BMI mismatch acceptance criteria and centers may not typically base their preference of a particular NDD BMI solely when selecting a recipient who may be extremely mismatched from a weight perspective.
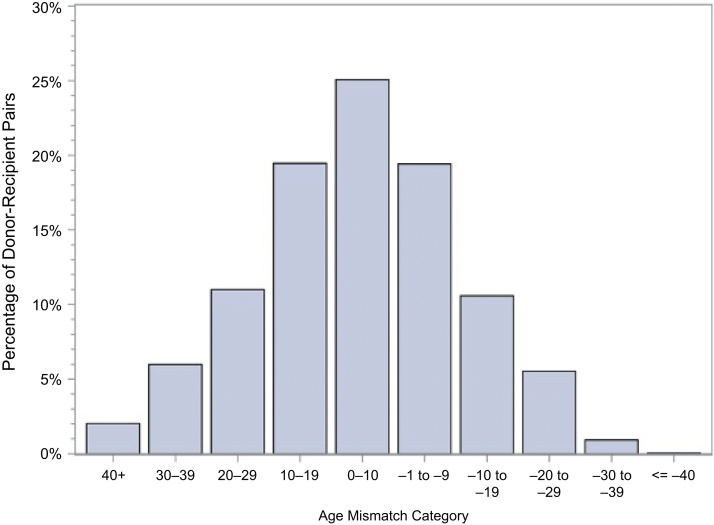
Overall, our study shows the complex interplay among several of these factors that affect GS and that most of nondirected donated kidneys are well matched for age and BMI (see Figures 7 and 8). It reflects a system that is functioning well. It shows the evaluation of all. Currently matching and allocation guidelines are followed as per UNOS guidelines; however, specific guidelines for allocation of nondirected donated kidneys are generally left up to the center to ensure better matching and avoid bias toward recipients. Efforts that can potentially improve this process and long-term survival include better immunologic matching and avoiding significant age and BMI mismatch.
Figure 7.
Distribution of age mismatch, where age mismatch = recipient age – donor age.
Figure 8.
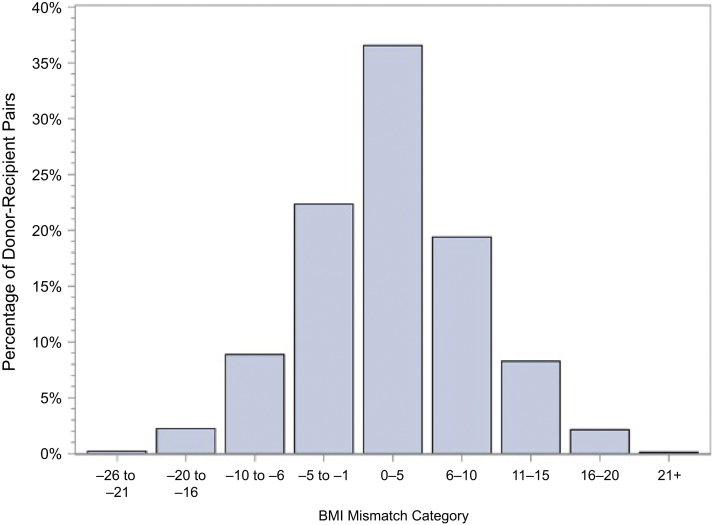
Distribution of BMI mismatch, where BMI mismatch = recipient BMI – donor BMI. BMI, body mass index.
Another important aspect of allocation could be the use of such nondirected donors to start and/or extend paired donor or domino chains to maximize the benefits; however, currently, there is no systematic process in place to integrate these donors with paired donation chains unless centers participate through kidney-paired donation programs or have large internal chains.
Our review shows excellent outcomes overall at par with living kidney donor transplants, which is reflective of the current system in place and serves to reinforce the confidence in the system of utilizing and allocating kidneys from NDDs. Areas where this can be even further improved include situations with age and BMI mismatches by including them into the matching algorithm to further enhance the utility of these donated kidneys.
Limitations
Limitations of our study include retrospective nature of study from a registry with potential for errors in coding for NDDs. It was also not possible to discern reasons for allocation of kidneys to extreme age mismatches or BMI, such as vascular anatomy and crossmatches. Similarly, we did not assess recipient outcomes that could have been related to adherence, different immunosuppression protocols, or other complications, such as BK viremia, cytomegalovirus disease, or malignancies post-transplantation. We were also not able to assess the impact of such donations on paired exchange chains owing to lack of specific coding data for these over this time frame. In evaluation of GS, recipient independent characteristics and adherence to and regimen of immunosuppression were not assessed owing to nonavailability of these data.
Conclusions
We present the largest and first study of its kind describing long-term outcomes and factors associated with NDDs of kidney in the United States. Overall, the number of NDDs significantly increased over the review time period between 1997 and 2017. We describe the characteristics and general outcomes of these donations. Further research and collaboration are required to better match NDDs with recipients and avoid extreme age and BMI mismatches, which are known to have inferior outcomes. We suggest developing an algorithm and integrating that with the kidney-paired exchange donation programs to aid in optimizing and maximizing the utility and beneficence of such donors who exhibit this most selfless act of generosity.
Disclosure
All the authors declared no competing interests.
Acknowledgments
Organ Procurement and Transplantation Network: This work was supported in part by the Health Resources and Services Administration contract HHSH250-2019-00001C. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
References
- 1.United States Renal Data System USRDS Previous ADRs. United States Renal Data System USRDS. Published 2019. https://usrds.org/annual-data-report/previous-adrs/
- 2.OPTN 2018 Annual report of the U.S. Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients. OPTN. https://optn.transplant.hrsa.gov/data/view-data-reports/annual-report/ Published 2018.
- 3.National Institutes of Health, US Renal Data System 2019 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. National Institutes of Health, US Renal Data System. https://usrds.org/media/2371/2019-executive-summary.pdf Published 2019.
- 4.HRSA Organ Procurement and Transplantation Network: National Data. HRSA. https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/ Published 2020.
- 5.Dor F.J.M.F., Massey E.K., Frunza M., et al. New classification of ELPAT for living organ donation. Transplantation. 2011;91:935–938. doi: 10.1097/TP.0b013e3182129236. [DOI] [PubMed] [Google Scholar]
- 6.Maple H., Chilcot J., Burnapp L., et al. Motivations, outcomes, and characteristics of unspecified (nondirected altruistic) kidney donors in the United Kingdom. Transplantation. 2014;98:1182–1189. doi: 10.1097/TP.0000000000000340. [DOI] [PubMed] [Google Scholar]
- 7.Nath J., Patel K., Field M., et al. Recipient outcomes from nondirected live kidney donors: a UK-based cohort study. Transplant Direct. 2018;4:e406. doi: 10.1097/TXD.0000000000000847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodrigue J.R., Schutzer M.E., Paek M., Morrissey P. Altruistic kidney donation to a stranger: psychosocial and functional outcomes at two US transplant centers. Transplantation. 2011;91:772–778. doi: 10.1097/TP.0b013e31820dd2bd. [DOI] [PubMed] [Google Scholar]
- 9.Jacobs C.L., Roman D., Garvey C., Kahn J., Matas A.J. Twenty-two nondirected kidney donors: an update on a single center’s experience. Am J Transplant. 2004;4:1110–1116. doi: 10.1111/j.1600-6143.2004.00478.x. [DOI] [PubMed] [Google Scholar]
- 10.Lennerling A., Fehrman-Ekholm I., Nordén G. Nondirected living kidney donation: experiences in a Swedish transplant centre. Clin Transplant. 2008;22:304–308. doi: 10.1111/j.1399-0012.2007.00785.x. [DOI] [PubMed] [Google Scholar]
- 11.Nassiri N., Baskin A.S., Herbert L.K., et al. Socioeconomic status in non-directed and voucher-based living kidney donation. Eur Urol Focus. 2018;4:185–189. doi: 10.1016/j.euf.2018.07.020. [DOI] [PubMed] [Google Scholar]
- 12.Kazley A.S., Amarnath R., Palanisamy A. Anonymous altruistic living kidney donation in the US: reality and practice. Int J Transplant Res Med. 2016;2:021. [Google Scholar]
- 13.Rodrigue J.R., Pavlakis M., Danovitch G.M., et al. Evaluating living kidney donors: relationship types, psychosocial criteria, and consent processes at US transplant programs. Am J Transplant. 2007;7:2326–2332. doi: 10.1111/j.1600-6143.2007.01921.x. [DOI] [PubMed] [Google Scholar]
- 14.Spital A. Unconventional living kidney donors--attitudes and use among transplant centers. Transplantation. 1989;48:243–248. doi: 10.1097/00007890-198908000-00012. [DOI] [PubMed] [Google Scholar]
- 15.Jendrisak M.D., Hong B., Shenoy S., et al. Altruistic living donors: evaluation for nondirected kidney or liver donation. Am J Transplant. 2006;6:115–120. doi: 10.1111/j.1600-6143.2005.01148.x. [DOI] [PubMed] [Google Scholar]
- 16.Williams R.C., Opelz G., Weil E.J., McGarvey C.J., Chakkera H.A. The risk of transplant failure with HLA mismatch in first adult kidney allografts 2: living donors, summary, guide. Transplant Direct. 2017;3:e152. doi: 10.1097/TXD.0000000000000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jay C.L., Dean P.G., Helmick R.A., Stegall M.D. Reassessing preemptive kidney transplantation in the United States: are we making progress? Transplantation. 2016;100:1120–1127. doi: 10.1097/TP.0000000000000944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park H.S., Hong Y.A., Kim H.G., et al. Delayed graft function in living-donor renal transplantation: 10-year experience. Transplant Proc. 2012;44:43–46. doi: 10.1016/j.transproceed.2011.11.057. [DOI] [PubMed] [Google Scholar]
- 19.Redfield R.R., Scalea J.R., Zens T.J., et al. Predictors and outcomes of delayed graft function after living-donor kidney transplantation. Transpl Int. 2016;29:81–87. doi: 10.1111/tri.12696. [DOI] [PubMed] [Google Scholar]
- 20.Damodaran S., Bullock B., Ekwenna O., et al. Risk factors for delayed graft function and their impact on graft outcomes in live donor kidney transplantation. Int Urol Nephrol. 2021;53:439–446. doi: 10.1007/s11255-020-02687-5. [DOI] [PubMed] [Google Scholar]
- 21.Ferrari P., Lim W., Dent H., McDonald S.P. Effect of donor–recipient age difference on graft function and survival in live-donor kidney transplantation. Nephrol Dial Transplant. 2010;26:702–708. doi: 10.1093/ndt/gfq383. [DOI] [PubMed] [Google Scholar]
- 22.Tasaki M., Saito K., Nakagawa Y., et al. Effect of donor-recipient age difference on long-term graft survival in living kidney transplantation. Int Urol Nephrol. 2014;46:1441–1446. doi: 10.1007/s11255-014-0655-8. [DOI] [PubMed] [Google Scholar]
- 23.Shahani M., Iqbal T., Idrees M. Impact of age and gender matching on long-term graft function and actual graft survival in live-related renal transplantation: retrospective study from Sindh Institute of Urology and Transplantation, Pakistan. Saudi J Kidney Dis Transplant. 2019;30:365–375. doi: 10.4103/1319-2442.256844. [DOI] [PubMed] [Google Scholar]
- 24.Lee Y.J., Chang J.H., Choi H.N., et al. Donor-recipient age difference and graft survival in living donor kidney transplantation. Transplant Proc. 2012;44:270–272. doi: 10.1016/j.transproceed.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 25.Trnka P., McTaggart S., Francis A. The impact of donor/recipient age difference and HLA matching on graft outcome in pediatric kidney transplantation. Transplantation. 2018;102:S455. doi: 10.1111/petr.13265. [DOI] [PubMed] [Google Scholar]
- 26.Naderi G., Azadfar A., Yahyazadeh S.R., Khatami F., Aghamir S.M.K. Impact of the donor-recipient gender matching on the graft survival from live donors [published correction appears in BMC Nephrol. 2020;21:487] BMC Nephrol. 2020;21:5. doi: 10.1186/s12882-019-1670-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McGee J., Magnus J.H., Islam T.M., et al. Donor-recipient gender and size mismatch affects graft success after kidney transplantation. J Am Coll Surg. 2010;210:718–725.e1. doi: 10.1016/j.jamcollsurg.2009.12.032. 725-716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tan J.C., Kim J.P., Chertow G.M., Grumet F.C., Desai M. Donor-recipient sex mismatch in kidney transplantation. Gend Med. 2012;9:335–347.e2. doi: 10.1016/j.genm.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tan J.C., Wadia P.P., Coram M., et al. H-Y antibody development associates with acute rejection in female patients with male kidney transplants. Transplantation. 2008;86:75–81. doi: 10.1097/TP.0b013e31817352b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neugarten J., Kasiske B., Silbiger S.R., Nyengaard J.R. Effects of sex on renal structure. Nephron. 2002;90:139–144. doi: 10.1159/000049033. [DOI] [PubMed] [Google Scholar]
- 31.Giral M., Nguyen J.M., Karam G., et al. Impact of graft mass on the clinical outcome of kidney transplants. J Am Soc Nephrol. 2005;16:261–268. doi: 10.1681/ASN.2004030209. [DOI] [PubMed] [Google Scholar]
- 32.Miller A.J., Kiberd B.A., Alwayn I.P., Odutayo A., Tennankore K.K. Donor-recipient weight and sex mismatch and the risk of graft loss in renal transplantation. Clin J Am Soc Nephrol. 2017;12:669–676. doi: 10.2215/CJN.07660716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hwang J.K., Kim Y.K., Kim S.D., et al. Does donor kidney to recipient body weight ratio influence long-term outcomes of living-donor kidney transplantation? Transplant Proc. 2012;44:276–280. doi: 10.1016/j.transproceed.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 34.Chertow G.M., Milford E.L., Mackenzie H.S., Brenner B.M. Antigen-independent determinants of cadaveric kidney transplant failure. JAMA. 1996;276:1732–1736. [PubMed] [Google Scholar]