Abstract
The development of complement inhibitors represented one of the major breakthroughs in clinical nephrology in the last decade. Complement inhibition has dramatically transformed the outcome of one of the most severe kidney diseases, the atypical hemolytic uremic syndrome (aHUS), a prototypic complement-mediated disorder. The availability of complement inhibitors has also opened new promising perspectives for the management of several other kidney diseases in which complement activation is involved to a variable extent. With the rapidly growing number of complement inhibitors tested in a rapidly increasing number of indications, a rational use of this innovative and expensive new therapeutic class has become crucial. The present review aims to summarize what we know, and what we still ignore, regarding complement activation and therapeutic inhibition in kidney diseases. It also provides some clues and elements of thoughts for a rational approach of complement modulation in kidney diseases.
Keywords: complement, glomerular diseases, complement inhibitors
The introduction in clinical practice of complement inhibitors was one of the most significant therapeutic achievements during the last decade. The taming of the old and powerful complement system has opened new clinical perspectives that materialized with the dramatic impact of the first C5 blocker, eculizumab, on the prognosis of 2 severe diseases—aHUS1,2 and paroxysmal nocturnal hemoglobinuria (PNH).3
This breakthrough has fueled among clinicians and patients a renewed interest in complement and high expectations regarding the potential benefit of complement modulation in various kidney diseases, but also in the setting of kidney transplantation and of disorders affecting other organs. More than 10 years after the introduction of eculizumab, and with the growing number of complement inhibitors in development, it is time to reflect on the rational use of this innovative therapeutic class.
Where It All Started
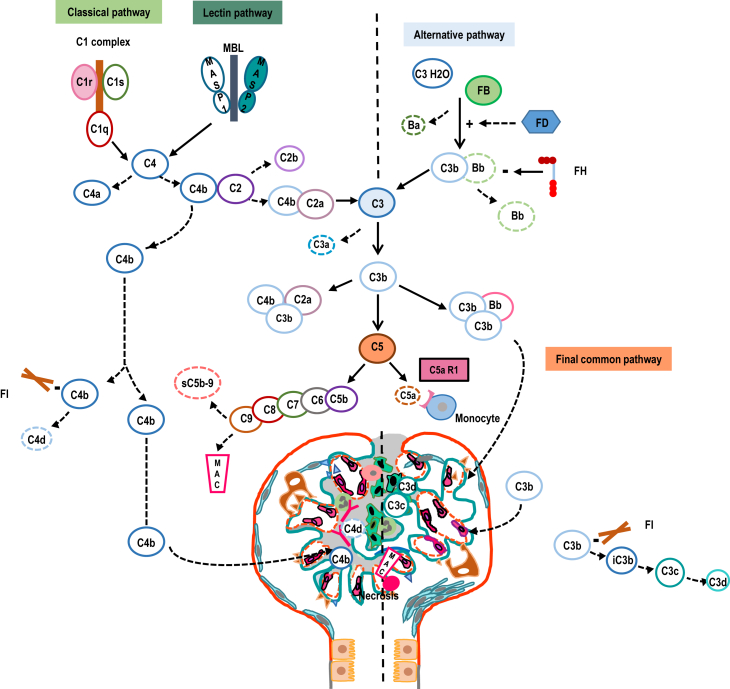
C5 blockade using eculizumab opened a new era for the therapeutic inhibition of the complex complement cascade (Figure 1). It was a turning point in the management of 2 rare but severe complement-mediated diseases, aHUS and PNH. C5 blockade provided a targeted, efficacious treatment, with a reasonable safety profile, for patients affected with these disorders.
Figure 1.
Representation of the 3 activation pathways of the complement cascade and complement-associated kidney involvement. Main targets of complement inhibitors are shown in full symbols. Complement biomarkers are shown in dashed symbols. C5aR1, C5a receptor 1; FB, factor B; FD, factor D; FH, factor H; FI, factor I; MAC, membrane attack complex; MBL, mannose-binding lectin.
In patients with PNH, eculizumab significantly reduced, if not halted, complement-induced hemolysis, alleviated or decreased the need for repeated transfusions, reduced thrombosis-related morbidity, but proved inefficacious for treating bone marrow failure associated to PNH.4,5 In aHUS, C5 blockade not only induced rapid and sustained hematological remission but also led to a significant improvement in kidney function of affected patients, and the risk of end-stage kidney disease decreased from ∼50% to 60% to ∼10% to 15%2,6 with the use of eculizumab.
The effect of C5 blockade on the natural history of PNH and aHUS was undoubtedly spectacular. However, this led to a misunderstanding and some level of confusion about what one may expect from complement blockade in other diseases.
Even though PNH and aHUS are 2 prototypic complement-mediated diseases, they diverge in various aspects related to complement involvement, a first illustration of the complex approach to therapeutic complement inhibition (Supplementary Figure S1). C5-induced cellular damage, on red blood cells in one case, and on endothelial cells in the other, is a hallmark of PNH and aHUS. However, in PNH, all affected patients carry an acquired, clonal dysregulation of the complement system, owing to the loss of anchoring at the surface of red blood cells of 2 complement inhibitors, CD55 and CD59. Furthermore, PNH can be diagnosed using rather simple tests.7
In contrast, aHUS is associated to a constitutional complement alternative pathway dysregulation in a subset of patients (30%–60%),8,9 and this dysregulation is only a risk factor for the disease. More importantly, there is to date no definite, fully reliable diagnostic test for aHUS in particular, and for complement-mediated thrombotic microangiopathies (TMAs) in general. The rational use of complement inhibitors should take into account the variable aspects of complement involvement in distinct diseases.
Furthermore, one should keep in mind that, in both PNH and aHUS, C5 blockade does not reverse the initial driver of the disease (mainly loss of the control of the alternative C3 convertase), but targets its downstream consequences (C5 activation).
Complement Inhibition in Kidney Diseases Beyond aHUS
The list of potential indications for complement inhibitors in kidney diseases is rapidly growing (Tables 1 and 2). It includes mostly glomerular diseases, in which one cannot easily transpose the approach of complement inhibition used in aHUS. For instance, aHUS is a rather homogeneous disorder (at least in its primary forms), usually acute and in which most, if not all, complement toxicity is mediated by C5 activation,10,11 particularly C5b.12 The effect of complement blockade on hematological features of aHUS can be expected within days and on the improvement of kidney function within days to weeks6,13 (Supplementary Figure S1).
Table 1.
The rationale for the use of therapeutic complement inhibition in various glomerular diseases is summarized
| Type of glomerular diseases | Rationale for therapeutic complement blockade | Registered or completed trials with complement inhibitors. Retrospective series and case reports |
|---|---|---|
| Nephropathies with exclusive or predominant C3 deposits | ||
| Acute postinfectious glomerulonephritis | Low C3 plasma levels and dominant C3 staining in kidney biopsy (with the absence of C1q and C4 deposits) are cardinal features of acute postinfectious glomerulonephritis. Acquired (C3 nephritic factor, anti-factor B antibodies) and more rarely constitutional dysregulation of the CAP has been reported in patients with acute postinfectious glomerulonephritis.46,72,73 Clinical and pathologic features of acute postinfectious glomerulonephritis may overlap with those of C3 glomerulopathy. |
No trials registered C5 inhibition used in case reports74,75 |
| C3 glomerulopathy | Predominant or exclusive C3 deposits are pathologic hallmarks of C3 glomerulopathy. Acquired (autoantibodies) or constitutional (genetic variants) dysregulation of the CAP has been reported in patients with C3 glomerulopathy.76 Animal models have linked C3 glomerulopathy to alternative C3 convertase dysregulation.77 |
Clinical trials registered for: anti-C5 (eculizumab), anti-C3 (AMY-101, ARO-C3, pegcetacopan), anti-C5a receptor (avacopan), anti-factor D (danicopan, BCX9930), anti-factor B (iptacopan), MAPS2 inhibitor (narsolimab) Use of eculizumab reported in retrospective series42 and a small prospective open-label trial.78 |
| Nephropathies with Igs, immune complexes, and complement deposits | ||
| Immunoglobulin A nephropathy | Co-dominant IgA and C3 glomerular deposits (90%), along with properdin, C4d, MBL, and C5b-9 deposits are characteristic pathologic features of IgA nephropathy. Markers of glomerular activation of the lectin pathway (MBL, L-ficolin, MASP2, MASP1/3, and C4d) have been associated with a worse outcome of IgA nephropathy.79,80 Variations in complement genes have been associated with better (CFHR3,1 deletion)81 or worse outcome (CFH, CFHR5)82, 83, 84 of IgA nephropathy. Plasma levels of FHR-1 and FHR-1/FH ratio are associated with a progressive course of IgA nephropathy.85 C3a receptor/C5a receptor deficiency in mice alleviates IgA nephropathy in mice.86 |
Clinical trials registered: anti-C5 (eculizumab, ravulizumab, cemdisiran), anti-C5a receptor (avacopan), anti-C3 (ARO-C3, pegcetacopan), anti-factor B (iptacopan, IONIS-FB-LRx), anti-factor D (vemircopan, BCX9930, ALXN2050), MAPS-2 inhibitor (narsoplimab). Salvage treatment with eculizumab in case reports.87 |
| Immunoglobulin-mediated membranoproliferative glomerulonephritis | Co-deposition in glomeruli of Igs and complement components characterize the disease. Features of CAP (and to much lesser extent of the CP) activation reported in up to 39%–59% of patients.88 Acquired CAP dysregulation detected in 45%–86% of patients89 | Clinical trials registered: anti-C3 (pegcetacopan), anti-factor D (danicopan) |
| Lupus nephritis | Glomerular co-localization of IgG, IgA, and IgM with C1q, C4 and C3, and C5b-9 (“full house” pattern) is characteristic of proliferative lupus nephritis. Decreased systemic levels of C3 and C4 reflect disease activity. The role of complement in lupus is dual: 1. Altered clearance of immune complexes by complement. Deficiency of C1q, C2, and C4 is associated with a high risk of systemic lupus erythematous. 2. Complement activation by immune complexes deposition contributes to glomerular inflammation through C3a and C5-dependent inflammatory cell recruitment and C5b-9-induced tissular damage. Earlier onset of lupus nephritis has been observed in patients with variants in CFH or CD46 (MCP) genes89 Animal models have underlined the role of CAP in the pathogenesis of lupus nephritis90,91 |
Clinical trials registered: anti-C5 (ravulizumab), anti-C3 (pegcetacopan), anti-factor D (vemircopan, ALXN2050), MAPS-2 inhibitor (narsoplimab). |
| Membranous nephropathy | C3 fragments and C5b-9 colocalize with IgG in subepithelial glomerular deposits (even though IgG4, the predominant IgG subclass in membranous nephropathy, do not activate complement). In experimental models of membranous nephropathy, C3 depletion prevents the development of proteinuria92 and C5b-9 is a key mediator of podocyte injury.93 CP,94 lectin95 and alternative96,97 pathways are likely to play a role in podocyte injury in the setting of membranous nephropathy. |
Registered trials: anti-C3 (pegcetacopan), anti-factor B (iptacopan), anti-factor D (BCX9930), MAPS2 inhibitor (narsoplimab). |
| Cryoglobulinemic glomerulopathy | Glomerular Igs and C3 deposits are characteristic of the cryoglobulinemic glomerulopathy. Severely decreased serum levels of C4 and low levels of C3 correlate with disease activity. In animal models, C5, C1q, C3, and factor B deficiency protect against or attenuate the severity of cryoglobulinemic glomerulopathy (reviewed in Trendelenburg et al.98). |
Eculizumab use reported in a single case.99 |
| Antiglomerular basement membrane antibody disease | Co-deposition of C1q and C3 and linear IgG along the glomerular basement membrane are characteristic of the disease. Presence of MBL, C4d, factor B, properdin, and C5b-9 deposits along the glomerular basement membrane suggest lectin and alternative pathway involvement.21 Murine models demonstrated that C3 and C4 deficiency prevented full manifestation of renal disease.100 |
No trials registered. Salvage treatment with eculizumab in case reports.101,102 |
| Nephropathies with nonspecific complement-induced inflammation | ||
| ANCA-renal vasculitis | Despite the absence of significant complement deposits in the kidney, high levels of circulating C3a, C5a, sC5b-9, and Bb have been reported in patients with active ANCA vasculitis.103 Low C3 levels are associated with worse prognosis.104 ANCA can induce NETosis by neutrophils that activate CAP. C5a activates neutrophils through C5a receptor (positive feedback loop)105 Murine models of antimyeloperoxidase antibody-associated vasculitis confirm the crucial role of C5a, C3 and factor B in the pathogenesis of the disease.106 |
Published clinical phase II and III trials: anti-C5a receptor (avacopan)40,107 Ongoing trials: anti-C5a receptor (vilobelimab) |
| Complement-mediated TMA | ||
| Atypical hemolytic uremic syndrome | Alternative pathway dysregulation (inherited or acquired) reported in up to 60% of pateints.2,44 Animal models confirm the central role of alternative C3 convertase dysregulation in the disease.108 |
Ongoing clinical trials: anti-C5 (eculizumab, ravulizumab, crovalimab), anti-C5a receptor (avacopan), anti-factor B (iptacopan), MAPS-2 inhibitor (narsolimab) Efficacy of eculizumab reported in prospective noncontrolled trials1,6,109 and in retrospective series.13 Data on ravulizumab use have been recently reported.110 |
ANCA, anti-neutrophil cytoplasmic autoantibody; CAP, complement alternative pathway; CP, complement classical pathway; FH, factor H; FHR-1, factor H-related protein 1; MBL, mannose-binding lectin; TMA, thrombotic microangiopathy.
A list of published, ongoing trials, retrospective series, and case reports regarding the use of complement inhibitors in glomerular diseases is provided.
Table 2.
Main complement inhibitors undergoing development in kidney diseases
| Target in the complement cascade | Mechanism of action | Drug | Pharmaceutical company | Type of inhibitor | Mode of administration | Phases of drug development | Potential indications in kidney diseases |
|---|---|---|---|---|---|---|---|
| C5 | Inhibition of the release of C5a and C5b, and ultimately of the formation of C5b9 | Eculizumab | Alexion Pharma/AstraZeneca | mAb | i.v. | Commercialized | aHUS |
| Ravulizumab | Alexion Pharma/AstraZeneca | mAb | i.v. | Commercialized, phase III | aHUS | ||
| Crovalimab | Roche | mAb | s.c. | Phases II–III | aHUS | ||
| C3 | Inhibition of the binding of C3 to the C3bBb and thus of the cleavage of C3 | Pegcetacoplan | Apellis Pharma/SOBI | Pegylated peptide | s.c. | Phase III | C3G, IgAN, MN |
| Factor B | Inhibition of the serine protease FB and thus of the cleavage of C3 and C5 | Iptacopan | Novartis | Small molecule | Oral | Phases II–III | aHUS, C3G, MN, IgAN |
| Factor D | Inhibition of the cleavage of FB | Danicopan | A Alexion Pharma/AstraZeneca | Small molecule | Oral | Phases II–III | C3G |
| MASP2 | Inhibition of the serine protease MASP2 | Narsoplimab | Omeros | mAb | i.v. | Phase II | IgAN |
| C5a receptor | Inhibition of the binding of C5a to its receptor | Avacopan | Chemocentrix | Small molecule | Oral | Phase III | ANCA-associated vasculitis aHUS |
aHUS, atypical hemolytic uremic syndrome; ANCA, anti-neutrophil cytoplasmic autoantibody; C3G, C3 glomerulopathy; IgAN, IgA nephropathy; mAb, monoclonal antibody; MN, membranous nephropathy; s.c., subcutaneously.
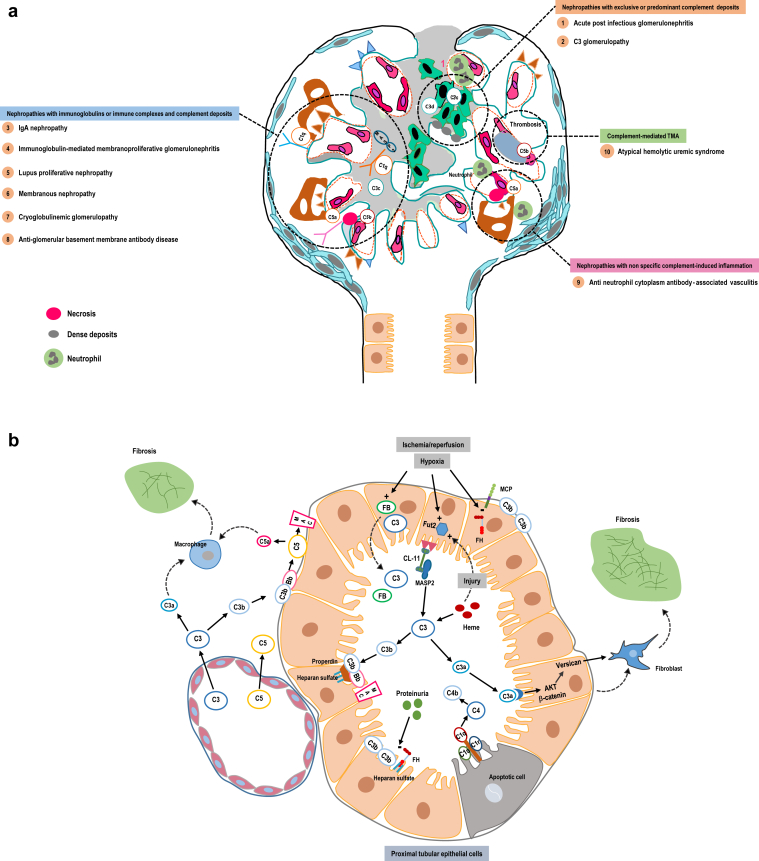
In contrast, glomerular diseases have, most frequently, subacute/chronic presentation, carry a significant clinical and pathologic heterogeneity, and the role of complement in these nephropathies is polymorph (Figure 2a and Table 1). In this context, one may expect that the effect of the complement blockade on urinary parameters, notably proteinuria, occurs within months and its impact on kidney function preservation within years.
Figure 2.
(a) Complement involvement in glomerular diseases is multiform: (i) deposition of complement (mainly C3) degradation products in glomeruli; (ii) activation by Igs and immune complexes deposited in glomeruli of the classical and/or the lectin pathway; (iii) C5-driven glomerular inflammatory changes; and (iv) complement-induced noninflammatory endothelial cell damage in complement-mediated renal thrombotic microangiopathy. The extent and weight of complement involvement are variable among distinct glomerular diseases (inset). (b) Complement activation also potentially contributes to kidney tubular and interstitial damage. This activation involves: (i) filtered (increased permeability of the glomerular basement membrane)29 or leaked (increased permeability of the peritubular capillaries) circulating complement components or (ii) locally synthetized ones. Hypoxia and ischemia/reperfusion in the kidney triggers complement activation through the induction of Fut2, the ensuing abnormal fucosylation of (mainly proximal) tubular cells, which activates MASP2 (lectin pathway) via CL-11.25,34,68 Hypoxia and ischemia/reperfusion also induces the synthesis of factor B (FB) and C3 by tubular cells, while potentially decreasing the expression of the inhibitory FH and MCP.69,70 After C3 activation, properdin provides a docking platform for tC3b and the C3 convertase assembly at the surface of the tubular cells,71 promoting the formation of the MAC. C3a binds to its receptor at the surface of the tubular cells, activates AKT and β-catenin pathway, and increases the secretion of versican that promotes the epithelial-mesenchymal transition and interstitial fibrosis.32 Similarly, C3a and C5a recruit inflammatory cells in the interstitium, which contributes to the development of interstitial fibrosis. Heme, which is released in the circulation during hemolysis and rhabdomyolysis, is filtered by the glomeruli and can directly activate C3 in the tubular lumen. Besides, proteinuria, a hallmark of glomerular diseases, inhibits the fixation of FH at the surface of the tubular cells, hence amplifying local complement activation.27 Finally, tubular apoptotic cells (notably after injury) are cleared via the activation of the classical complement pathway. CL-11, collectin-11; FB, factor B; FH, factor H; Fut2, fucosyl-transferase 2; MAC, membrane attack complex; TMA, thrombotic microangiopathy.
Furthermore, complement activation accounts for variables degrees and aspects in the pathogenesis of kidney diseases in general and of glomerular diseases in particular. In respect of the role of the complement, glomerular diseases can be broadly classified into 4, potentially intersecting, categories (Figure 2a and Table 1).
In the first category of kidney diseases, the complement is assumed to be a major, if not the sole, driver of the glomerular disease, acute postinfectious glomerulonephritis, and C3 glomerulopathy being the most illustrative examples of this category.14 In this setting, the simple deposition of complement degradation products (mainly C3c and C3d) is supposed to initiate glomerular injury. However, one cannot exclude that the deposition of complement degradation products simply reflects other yet not characterized drivers of the disease. Nevertheless, in this category of kidney diseases, the restoration of a normal control of the complement key enzymes, notably the alternative C3 convertase, is a potential curative option.
The second category encompasses autoantibody and immune complex-mediated glomerular diseases. In this setting, it is assumed that complement activation, notably of the classical pathway and to some extent of the lectin pathway, amplifies the initial insult due to autoantibodies or immune complexes. For these kidney diseases, the curative approach should aim, when feasible, to halt the deposition of autoantibodies and immune complexes within the glomeruli. Complement inhibition may only help attenuate kidney damage, while awaiting for the effect of immunosuppressive drugs on the production of pathogenic autoantibodies and immune complexes to occur. Complement inhibition should be considered as a complementary and transient therapeutic approach, and maintained only when the production of pathogenic autoantibodies is not or cannot be efficiently halted. This category includes among others, lupus nephritis, IgA nephropathy, membranous nephropathy, antiglomerular basement membrane antibody disease and potentially primary Ig-mediated membranoproliferative glomerulonephritis, and humoral rejection of the kidney graft.15, 16, 17 The respective role of Ig and complement deposition in Ig-mediated membranoproliferative glomerulonephritis is however still a matter of debate (reviewed in Fakhouri et al.14).
The third category represents inflammatory forms of any acute or subacute/chronic glomerular disease with or without immune deposits. Inflammatory changes may refer to mesangial, endo or extracapillary proliferation, glomerular capillary wall necrosis, arteritis, and/or peritubular capillaritis. These inflammatory changes are predominantly dependent on C5 activation and the subsequent release of C5a (recruitment of inflammatory cells) and C5b (membrane attack complex-induced tissular necrosis).18, 19, 20, 21 In this setting, C5 activation is a nonspecific inflammatory insult and complement blockade a nonspecific anti-inflammatory treatment. Nevertheless, C5 blockade may provide an alternative to corticosteroids for the management of acute inflammatory changes in most of the glomerular diseases, with 2 main potential advantages: a more acceptable safety profile and potentially an improved rate of complete short- and long-term renal remission, as compared with corticosteroids. This category includes antineutrophil cytoplasm antibody-associated vasculitis and exacerbation of several glomerular diseases (e.g., crescentic IgA nephropathy or C3 glomerulopathy).
The fourth category refers to complement-mediated kidney TMA. Complement-induced damage on the endothelial cells during kidney TMA may seem nonspecific (as in the previous category). However, the marked predominance, if not the exclusiveness, of kidney involvement during this disorder, combined with the absence of pathologic kidney inflammatory changes, is unique to complement-mediated TMA. This category includes obviously aHUS, but the exact spectrum of complement-mediated TMA is still a matter of debate, particularly in regard to TMA associated to coexistent disorders (malignancy, autoimmune diseases, drugs, and infections).22
These categories may overlap, and the role of the complement may vary in a single patient with a well-characterized type of a kidney disorder, over the course of the disease. For example, the glomerular deposition of C3 degradation products plays the predominant role during the chronic/subacute phase of a C3 glomerulopathy, whereas superimposed C5 activation may contribute to disease exacerbation, following infection for example.23,24 This also applies for chronic/subacute (amplifying role of the complement classical and lectin pathways) or rapidly progressive (C5-triggered inflammation) forms of IgA nephropathy.
Integration of the variable patterns of complement activation between kidney diseases, and between distinct phases of a kidney disease, will help select one or several concomitant therapeutic targets within the complement system.
Complement Activation in Kidney Diseases Beyond the Glomerulus
Complement activation is a ubiquitous phenomenon and is, obviously, not restricted to the glomeruli within the kidney. A body of experimental and clinical data indicates that complement activation may contribute to tubular cell injury in various situations (Figure 2b). For instance, kidney hypoxia and ischemia/reperfusion trigger complement activation at the surface of the (proximal) tubular cells via the induction of an abnormal fucosylation of these cells, with ensuing activation of the lectin pathway.25,26 Similarly, albuminuria impairs factor H binding to the proximal tubular cells and thus facilitates C3 convertase assembly and docking to properdin at the surface of these cells.27,28 In both situations, complement activation depends on locally synthetized and on filtered (increased glomerular permeability)29 complement components.30,31 It leads to the release of anaphylatoxins, C3a and C5a, that contribute directly32 or indirectly (recruitment of inflammatory cells) to interstitial fibrosis.33 Furthermore, complement activation is a main contributor to myoglobinuria- and hemoglobinuria-induced tubular damage, as heme can directly activate C3.34
In animal models, complement depletion or inhibition attenuates myoglobin,34 proteinuria,29 and ischemia/reperfusion35, 36, 37, 38-induced tubular damage and interstitial fibrosis. However, clinical data are particularly scarce and limited to trials assessing C5 blockade in renal transplantation associated-ischemia/reperfusion, and that has yielded disappointing results.39
The Rational Selection of a Therapeutic Target Within the Complement System
Several drugs targeting distinct components of the 3 complement pathways are under development (Figure 1 and Table 2). Inhibitors of C5 activation or of C5a receptor are already in clinical use for diseases driven mainly by C5-induced cellular injury (PNH, aHUS), or as nonspecific anti-inflammatory drugs in others (anti-neutrophil cytoplasmic autoantibody kidney vasculitis40). The alternative pathway, notably the C3 alternative convertase, is currently attracting most of the efforts for the design and development of specific modulators. The majority of these drugs inhibit central components or activators of the C3 alternative convertase. Recombinant or modified inhibitors (factor H41 or factor I) with enhanced potency are also potential therapeutic tools. Alternative pathway modulators are optimal tools for the treatment of glomerular diseases driven mainly or partially by the deposition of C3 activation products. Inhibitors of the initiation phases of the classical and lectin pathways are also available or ongoing development. They are optimal tools for the treatment of antibody and immune complex-mediated kidney diseases.
Ultimately, these drugs could be used as monotherapies but also in combination strategies aiming to the simultaneous inhibition of >1 level of the complement cascade. One such association may combine an inhibitor of the alternative C3 convertase and short-term C5 blockade in the management of an exacerbation of C3 glomerulopathy for example or an inhibitor of the classical pathway and a C5 blocker for antiglomerular basement membrane antibody disease or acute humoral rejection of a kidney allograft.
The selection of a target within the complement system is based on the assumption, corroborated by experimental and clinical data, of a direct or indirect pathogenic role of one or several complement components in a kidney disease.
Is It Complement Mediated? The New Conundrum in Kidney Diseases
The implication of complement activation in a kidney disease, and hence the potential benefit of complement blockade, can be inferred using a combination of clinical, pathologic, genetic, and experimental arguments.
The first set of argument is based on the detection of markers of complement activation in the circulation (in case of systemic diseases affecting the kidneys) and/or in the urine, or within the kidney tissue (isolated or predominant complement product deposits or in combination with antibody or immune complexes). These biomarkers include serum levels of C3, C4, sC5b-9, Bb, and Ba, urinary levels of Bb, and sC5b-9, and staining for C3c, C3d, C4d, C5b-9, and C1q in kidney biopsies. Nevertheless, a clear demonstration of the clinical impact of these complement biomarkers on the severity and outcome of the kidney disease is required; it is still lacking for a significant number of kidney diseases, including sC5b-9 in C3 glomerulopathy42 or in lupus nephritis.43 The selection of a complement inhibitor based on yet not validated biomarkers is one of the most significant potential biases in the clinical development of complement inhibitors (Table 3).
Table 3.
Some elements for the rational use of complement inhibitors in kidney diseases
| Inhibition of the complement alternative pathway is a potential treatment for kidney diseases primarily driven by C3 degradation production deposition. |
| Complement alternative pathway inhibitors (factor H, factor I) with enhanced potency may represent potential therapeutic agents in complement-driven diseases. |
| Inhibition of the initial phases of the classical and lectin pathways is a potential treatment in Ig and immune complex-mediated kidney diseases. |
| C5 and C5a blockers may represent an alternative to corticosteroids as more optimal anti-inflammatory drugs (improved quality of renal remission/decreased side effects). |
| Constitutional complement alternative pathway dysregulation is only a risk factor for complement-mediated kidney diseases, and not synonymous of continuous complement activation in all carriers. |
| In Ig- and immune complex-driven kidney diseases, the primary therapeutic target is Ig or immune complexes production and not complement inhibition. |
| For complement-mediated kidney diseases, distinct complement modulators may be required during the acute and chronic phases. |
| The potential clinical benefit should clearly outweigh the infectious risk resulting from the inhibition of complement cascade components. |
| The clinical relevance of anticomplement autoantibodies for the use of complement blockers is not established, except for high-titer anti-factor H antibodies in patients with aHUS. |
| No currently available biomarker can predict response to complement blockade in kidney diseases. The design of clinical trials based, even partially, on not yet validated biomarkers may prove, at least, misleading. |
| Complement genetics may help individualize the optimal duration of anticomplement therapy in a given patient with a kidney disease (e.g., aHUS). |
| The selection of the optimal target for complement inhibition should be individualized and integrates the patient’s specific clinical characteristics, complement biological and genetic profile and kidney pathologic features. |
aHUS, atypical hemolytic uremic syndrome.
The second set of arguments relates to the demonstration of a constitutional or acquired complement dysregulation in patients with a kidney disease. Constitutional complement dysregulation arises from loss-of-function variants in inhibitors mostly of the complement alternative pathway (complement factor H, complement factor I, MCP), or gain-of-function variants in the 2 genes encoding for the main components of the alternative C3 convertase, C3 and factor B. The weight of genetic complement dysregulation in the pathogenesis of a kidney disease depends first on the extent of the enrichment (increase in frequency) of the complement gene rare variants in the studied population, as compared with healthy individuals (3%–5%). To date, aHUS is the only kidney disease in which an enrichment of complement gene variants is clearly significant (30%–60%).44 Second, the assessment of the pathogenicity of a complement variant is paramount for the interpretation of complement genetics, and only variants with demonstrated or likely pathogenic effect on the function of the encoded proteins are to be taken into account for clinical decision, including the use of complement inhibitors.44 Finally, constitutional complement dysregulation is only a risk factor for some kidney diseases.
Acquired complement dysregulation refers to the occurrence of several autoantibodies directed against several components of the complement cascade: anti-factor H antibodies,45 C3 and C5 nephritic factors, anti-factor B,46 and anti-C3b and anti-C1q antibodies (reviewed in Fakhouri et al.14). However, except for high-titer anti-factor H antibodies in the context of aHUS, it remains, to date, unclear whether these autoantibodies are markers (bystanders) or significantly contribute to the pathogenesis of kidney diseases.
The third set of arguments derives from animal experiments in which the inhibition or the modulation of the complement system prevents, cure, or improves a model of kidney disease. This set of arguments carries all the caveats of the relevance of animal models for human clinical practice. They however provide a perfect illustration of the complexity of complement inhibition. In an animal model of C3 glomerulopathy (factor H-deficient mice), the inhibition of properdin, the unique positive regulator of the alternative C3 convertase, proved unexpectedly to be detrimental.47 In the same model, the deletion of complement factor I, a main inhibitor of the C3 alternative convertase, surprisingly prevented the development of C3 glomerulopathy.48
The last set of arguments is the reversibility or the marked clinical improvement of a kidney disease with the use of complement inhibitors. The analysis of these data, the most relevant clinically, is hindered by several factors, such as the following: the potential spontaneous improvement of a kidney disease, the concomitant use of other drugs, the absence of controlled studies in the context of (ultra)rare diseases, and the bulk of evidence, if any, being derived from retrospective small series or case reports. However, the beneficial effect of the inhibition of a complement cascade component provides hard evidence for the implication of a given complement pathway in the pathogenesis of a kidney disease.
All these sets of arguments may help clinicians not only to assess whether complement activation is involved in the pathogenesis of a kidney disease but also to select the optimal therapeutic target within the complement cascade.
Nevertheless, the untangling of complement involvement in a kidney disease is not straightforward. Indeed, aHUS, the prototypic complement-mediated kidney disease, is not characterized by complement deposition in the kidney, or at least its diagnosis does not rely on the detection of such deposits. Markers of complement activation in the circulation are detected in only a subset of patients (30%–50%).8,9 Currently, no fully reliable biomarker in the circulation or in the urine can help predict whether a TMA is predominantly complement mediated8,9,49—this applies to the recently developed in vitro tests (Ham modified test50 or complement deposition on endothelial cells in vitro51,52). Finally, the impact of eculizumab on the natural course of aHUS has been assessed only in prospective nonrandomized trials and in retrospective studies. However, the magnitude of the clinical improvement was highly significant as compared with historical controls and thus proved sufficient for the approval of this innovative drug for an ultrarare disease. The expected clinical benefit of complement blockade in other kidney diseases will not be as impressive and its demonstration will require randomized, controlled trials.
Finally, complement activation is only 1 feature of the presentation of a patient with a kidney disease. A more holistic approach combining clinical, biological, genetic, and pathologic features (a “cluster approach” previously used in C3 glomerulopathy and Ig-mediated membranoproliferative glomerulonephritis53) is a more appropriate guide for the decision to use or not complement inhibition and for the selection of the most relevant inhibitor.
Different Complement Therapeutic Targets for One Kidney Disease?
This question extends beyond the already discussed potential combination of multiple complement inhibitors for the treatment of acute inflammatory changes superimposed on a chronic/subacute kidney disease. It relates to distinct pathologic approaches of a single complement-mediated kidney disease, including diseases in which C5 blockade had already proven efficacious. Among patients with PNH treated with eculizumab, the quality of hemolysis remission is heterogeneous, and only one-third of the patients experience a complete normalization of hemoglobin and hemolysis parameters.54 This suboptimal control of complement-induced hemolysis results in part from C3d-mediated extravascular hemolysis and erythrophagocytosis by mononuclear cells.55 Inhibition of red blood cell opsonization with anticomplement therapies targeting C356 or factor B,57 alone or in combination with C5 blockade, has proved more efficacious in the control of hemolysis than sole anti-C5 therapies.
Would such approach be applicable to aHUS? Response to C5 blockade in aHUS is already very significant; the residual risk of end-stage disease being estimated around 10% to 15%. An additional effect of another complement inhibitor will be hard to detect. Nevertheless, the initial driver of aHUS is not C5 activation but the dysregulation of the alternative C3 convertase. Restoring a normal control of this key enzyme of the complement alternative pathway may represent a potential therapeutic strategy in patients with aHUS, most particularly as a maintenance therapy in the remission phase of the disease.
Different Inhibitors for One Complement Target
Several types of drugs targeting 1 component of the complement cascade are already available or under development, C5 being the complement protein that has attracted the highest level of interest. These drugs differ in terms of type (antibody, peptide, small interfering RNA), half-life (short- or long-acting drugs), and way of administration (i.v. or subcutaneous). The choice of a drug depends on the patient’s preference and on the setting or phase of the disease (short-acting drugs in the acute phase vs. long-acting drugs in the maintenance phase). Small interfering RNAs are not typically used in acute, rapidly progressive kidney disease, owing to their delayed therapeutic effect, but may prove cost-effective in long-term strategies. The treatment cost is also to be taken into consideration. The availability of cheaper anti-C5 biosimilars may increase the use of these therapies in low-income but also in some high-income countries.
The Delicate Equilibrium Between Complement Inhibition and Infectious Risk
As for any treatment, the benefit-to-risk ratio is central for the development and clinical use of anticomplement therapies. Complement inhibition increases the risk of infections, notably invasive meningococcal and, to a lesser extent, gonococcal infections.58 Anti-infectious complement properties derive from a direct membrane attack complex-mediated bactericidal activity, from C3-dependent germ opsonization and the activation of the C5a-C5a receptor axis.59 Thus, the inhibition of the complement final common pathway recapitulates the clinical pattern of patients with genetic deficiency in this pathway, mostly C5, and carries a 2000-fold increased risk of invasive meningococcal infections.60 The inhibition of the alternative C3 convertase also carries an infectious risk through a decrease in the opsonization of encapsulated and unencapsulated germs.61 It remains unknown whether a partial blockade of the alternative C3 convertase mitigates this infectious risk.
The decision to start an anticomplement therapy should be based on weighting on one hand the prognosis of a disease left untreated and on the other hand the potential infectious risk. The balance tips toward benefit when dealing with a severe, life-threatening condition, such as PNH and aHUS. The benefit/risk ratio may be more mitigated for a kidney disease that progresses toward end-stage kidney disease over several years, if not decades.
What Is the Optimal Duration of Anticomplement Therapies?
The duration of anticomplement therapies should be individualized based on the type of the disease requiring treatment and patient’s specificities and preferences. The risk of disease relapse or worsening after treatment cessation on one hand and treatment-related infectious risk and costs on the other hand provide the main arguments for or against the discontinuation of anticomplement treatments. The answer to the question of treatment duration is rather simple in some situations. In patients with PNH, discontinuation of anti-C5 treatment is invariably followed by a—potentially dramatic—relapse of hemolysis and an increase in the risk of thrombosis, and thus lifelong treatment is warranted. However, in the vast majority of diseases, notably kidney diseases, the optimal duration of complement inhibition is not clearly settled.
In patients with aHUS, lifelong anti-C5 treatment has been initially advocated with the assumption that patients with aHUS have a continuous systemic complement activation and hence a high risk of relapse in case of treatment discontinuation. However, there is no definite evidence sustaining this assumption. Not all carriers of complement gene variants have features of systemic complement activation. Besides, 40% to 60% of patients with aHUS do not carry a constitutional complement dysregulation. This has led some clinicians to consider the discontinuation of anti-C5 therapy in patients with aHUS.
Several retrospective series62, 63, 64, 65 and one more recent prospective trial66 indicate that the presence or absence of complement gene pathogenic variants is the main factor associated with aHUS relapse after C5 blockade cessation. One study66 suggested that increased serum levels of sC5b-9 may help refine the risk of aHUS relapse in carriers of complement gene variants. Overall, available data indicate that the risk of aHUS relapse after anti-C5 treatment cessation is <5%, in patients with no documented complement gene rare pathogenic variants. Hence, treatment cessation in these patients, who account for 40% to 60% of all aHUS patients, is safe. A strategy of anti-C5 treatment discontinuation in patients with aHUS based on complement genetics is thus rational and cost effective.
The case of aHUS illustrates the fact that complement genetics is a potential tool for an individualized use of complement inhibitors in some settings. However, the clinical relevance of complement genetics findings should be carefully assessed. For instance, not all detected variants in complement genes are pathogenic and consequently clinical decision based on complement gene variants of unknown significance is at least hazardous. The classification of complement gene variants as pathogenic, likely pathogenic, or of unknown significance has become central for the management of patients with aHUS in the maintenance phase.44 This classification requires an intimate knowledge of complement genetics and biology, mastered in few specialized centers. Only a rigorous characterization of complement variants allows a rational use of complement inhibitors.
The Revolution of Therapeutic Complement Inhibition: Time to Pause and Reflect
The therapeutic revolution because of the introduction of complement inhibitors in clinical practice has, rightly, ignited a great enthusiasm in the community of nephrologists and patients. This enthusiasm has been reinforced by the successful translational research in aHUS that materialized with the design of an efficacious treatment for a devastating disease. Enthusiasm should not however preclude caution and reflection. The activation of the complement system is not necessarily harmful in all settings, and hence complement inhibition is not necessarily beneficial in all conditions. Beyond its obvious anti-infectious properties, the complement cascade is central for the clearance of necrotic, apoptotic, and malignant cells. Inhibition of this beneficial effect of the complement may lead to the persistence of necrotic cells and inflammation, as recently illustrated by the deleterious effect of properdin deficiency in an experimental model of kidney ischemia/reperfusion.67 Furthermore, complement activation is a ubiquitous phenomenon that is usually self-limited and spontaneously resolving. Finally, the old and preserved complement cascade is complex and intuitive approaches to its inhibition may yield surprising and disappointing results. The rational use of the new complement inhibitors should also acknowledge our uncertainties.
Disclosure
FF has received consultancy and/or speaker honoraria from Roche, Alexion, Apellis, Achillion, Novartis, and Alnylam. VFB has received fees from Alexion Pharmaceuticals, Roche, BioCryps, and Apellis for invited lectures and/or board membership and is the recipient of a research grant from Alexion Pharmaceuticals. All the other authors declared no competing interests.
Footnotes
Figure S1. Various aspects of complement involvement and complement inhibitors use across a range of diseases. Abbreviations: PNH, paroxysmal nocturnal hemoglobinuria. aHUS, atypical haemolityc uremic syndrome. C3G, C3 glomerulopathy.
Supplementary Material
Figure S1. Various aspects of complement involvement and complement inhibitors use across a range of diseases. Abbreviations: PNH, paroxysmal nocturnal hemoglobinuria. aHUS, atypical haemolityc uremic syndrome. C3G, C3 glomerulopathy.
References
- 1.Legendre C.M., Licht C., Muus P., et al. Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N Engl J Med. 2013;368:2169–2181. doi: 10.1056/NEJMoa1208981. [DOI] [PubMed] [Google Scholar]
- 2.Fakhouri F., Zuber J., Fremeaux-Bacchi V., Loirat C. Haemolytic uraemic syndrome [published correction appears in Lancet. 2017;390:648] Lancet. 2017;390:681–696. doi: 10.1016/S0140-6736(17)30062-4. [DOI] [PubMed] [Google Scholar]
- 3.Hillmen P., Young N.S., Schubert J., et al. The complement inhibitor eculizumab in paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2006;355:1233–1243. doi: 10.1056/NEJMoa061648. [DOI] [PubMed] [Google Scholar]
- 4.Brodsky R.A. How I treat paroxysmal nocturnal hemoglobinuria. Blood. 2021;137:1304–1309. doi: 10.1182/blood.2019003812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sicre de Fontbrune F., Peffault de Latour R. Ten years of clinical experience with eculizumab in patients with paroxysmal nocturnal hemoglobinuria. Semin Hematol. 2018;55:124–129. doi: 10.1053/j.seminhematol.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Fakhouri F., Hourmant M., Campistol J.M., et al. Terminal complement inhibitor eculizumab in adult patients with atypical hemolytic uremic syndrome: a single-arm, open-label trial. Am J Kidney Dis. 2016;68:84–93. doi: 10.1053/j.ajkd.2015.12.034. [DOI] [PubMed] [Google Scholar]
- 7.Brodsky R.A., Mukhina G.L., Li S., et al. Improved detection and characterization of paroxysmal nocturnal hemoglobinuria using fluorescent aerolysin. Am J Clin Pathol. 2000;114:459–466. doi: 10.1093/ajcp/114.3.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fremeaux-Bacchi V., Fakhouri F., Garnier A., et al. Genetics and outcome of atypical hemolytic uremic syndrome: a nationwide French series comparing children and adults. Clin J Am Soc Nephrol. 2013;8:554–562. doi: 10.2215/CJN.04760512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noris M., Caprioli J., Bresin E., et al. Relative role of genetic complement abnormalities in sporadic and familial aHUS and their impact on clinical phenotype. Clin J Am Soc Nephrol. 2010;5:1844–1859. doi: 10.2215/CJN.02210310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith-Jackson K., Yang Y., Denton H., et al. Hyperfunctional complement C3 promotes C5-dependent atypical hemolytic uremic syndrome in mice. J Clin Invest. 2019;129:1061–1075. doi: 10.1172/JCI99296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Jorge E.G., Macor P., Paixao-Cavalcante D., et al. The development of atypical hemolytic uremic syndrome depends on complement C5. J Am Soc Nephrol. 2011;22:137–145. doi: 10.1681/ASN.2010050451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ueda Y., Miwa T., Ito D., et al. Differential contribution of C5aR and C5b-9 pathways to renal thrombic microangiopathy and macrovascular thrombosis in mice carrying an atypical hemolytic syndrome-related factor H mutation. Kidney Int. 2019;96:67–79. doi: 10.1016/j.kint.2019.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fakhouri F., Delmas Y., Provot F., et al. Insights from the use in clinical practice of eculizumab in adult patients with atypical hemolytic uremic syndrome affecting the native kidneys: an analysis of 19 cases. Am J Kidney Dis. 2014;63:40–48. doi: 10.1053/j.ajkd.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 14.Fakhouri F., Le Quintrec M., Fremeaux-Bacchi V. Practical management of C3 glomerulopathy and Ig-mediated MPGN: facts and uncertainties. Kidney Int. 2020;98:1135–1148. doi: 10.1016/j.kint.2020.05.053. [DOI] [PubMed] [Google Scholar]
- 15.Haas M., Rahman M.H., Racusen L.C., et al. C4d and C3d staining in biopsies of ABO- and HLA-incompatible renal allografts: correlation with histologic findings. Am J Transplant. 2006;6:1829–1840. doi: 10.1111/j.1600-6143.2006.01356.x. [DOI] [PubMed] [Google Scholar]
- 16.Locke J.E., Magro C.M., Singer A.L., et al. The use of antibody to complement protein C5 for salvage treatment of severe antibody-mediated rejection. Am J Transplant. 2009;9:231–235. doi: 10.1111/j.1600-6143.2008.02451.x. [DOI] [PubMed] [Google Scholar]
- 17.Stegall M.D., Diwan T., Raghavaiah S., et al. Terminal complement inhibition decreases antibody-mediated rejection in sensitized renal transplant recipients [published correction appears in Am J Transplant. 2013;13:241] Am J Transplant. 2011;11:2405–2413. doi: 10.1111/j.1600-6143.2011.03757.x. [DOI] [PubMed] [Google Scholar]
- 18.Itami H., Hara S., Samejima K., et al. Complement activation is associated with crescent formation in IgA nephropathy. Virchows Arch. 2020;477:565–572. doi: 10.1007/s00428-020-02800-0. [DOI] [PubMed] [Google Scholar]
- 19.Brilland B., Garnier A.S., Chevailler A., Jeannin P., Subra J.F., Augusto J.F. Complement alternative pathway in ANCA-associated vasculitis: two decades from bench to bedside. Autoimmun Rev. 2020;19:102424. doi: 10.1016/j.autrev.2019.102424. [DOI] [PubMed] [Google Scholar]
- 20.Chen M., Jayne D.R.W., Zhao M.H. Complement in ANCA-associated vasculitis: mechanisms and implications for management. Nat Rev Nephrol. 2017;13:359–367. doi: 10.1038/nrneph.2017.37. [DOI] [PubMed] [Google Scholar]
- 21.Ma R., Cui Z., Hu S.Y., et al. The alternative pathway of complement activation may be involved in the renal damage of human anti-glomerular basement membrane disease. PLoS One. 2014;9:e91250. doi: 10.1371/journal.pone.0091250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Le Clech A., Simon-Tillaux N., Provot F., et al. Atypical and secondary hemolytic uremic syndromes have a distinct presentation and no common genetic risk factors. Kidney Int. 2019;95:1443–1452. doi: 10.1016/j.kint.2019.01.023. [DOI] [PubMed] [Google Scholar]
- 23.Oosterveld M.J., Garrelfs M.R., Hoppe B., et al. Eculizumab in pediatric dense deposit disease. Clin J Am Soc Nephrol. 2015;10:1773–1782. doi: 10.2215/CJN.01360215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vernon K.A., Goicoechea de Jorge E., Hall A.E., et al. Acute presentation and persistent glomerulonephritis following streptococcal infection in a patient with heterozygous complement factor H-related protein 5 deficiency. Am J Kidney Dis. 2012;60:121–125. doi: 10.1053/j.ajkd.2012.02.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nauser C.L., Howard M.C., Fanelli G., et al. Collectin-11 (CL-11) is a major sentinel at epithelial surfaces and key pattern recognition molecule in complement-mediated ischaemic injury. Front Immunol. 2018;9:2023. doi: 10.3389/fimmu.2018.02023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farrar C.A., Tran D., Li K., et al. Collectin-11 detects stress-induced L-fucose pattern to trigger renal epithelial injury. J Clin Invest. 2016;126:1911–1925. doi: 10.1172/JCI83000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buelli S., Abbate M., Morigi M., et al. Protein load impairs factor H binding promoting complement-dependent dysfunction of proximal tubular cells. Kidney Int. 2009;75:1050–1059. doi: 10.1038/ki.2009.8. [DOI] [PubMed] [Google Scholar]
- 28.Zaferani A., Vives R.R., van der Pol P., et al. Factor H and properdin recognize different epitopes on renal tubular epithelial heparan sulfate. J Biol Chem. 2012;287:31471–31481. doi: 10.1074/jbc.M112.380386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abbate M., Zoja C., Corna D., et al. Complement-mediated dysfunction of glomerular filtration barrier accelerates progressive renal injury. J Am Soc Nephrol. 2008;19:1158–1167. doi: 10.1681/ASN.2007060686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Damman J., Nijboer W.N., Schuurs T.A., et al. Local renal complement C3 induction by donor brain death is associated with reduced renal allograft function after transplantation. Nephrol Dial Transplant. 2011;26:2345–2354. doi: 10.1093/ndt/gfq717. [DOI] [PubMed] [Google Scholar]
- 31.Laskowski J., Philbrook H.T., Parikh C.R., Thurman J.M. Urine complement activation fragments are increased in patients with kidney injury after cardiac surgery. Am J Physiol Ren Physiol. 2019;317:F650–F657. doi: 10.1152/ajprenal.00130.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Han R., Hu S., Qin W., et al. C3a and suPAR drive versican V1 expression in tubular cells of focal segmental glomerulosclerosis [published correction appears in JCI Insight. 2019;4:e130986] JCI Insight. 2019;4 doi: 10.1172/jci.insight.122912. [DOI] [Google Scholar]
- 33.Strainic M.G., Liu J., Huang D., et al. Locally produced complement fragments C5a and C3a provide both costimulatory and survival signals to naive CD4+ T cells. Immunity. 2008;28:425–435. doi: 10.1016/j.immuni.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boudhabhay I., Poillerat V., Grunenwald A., et al. Complement activation is a crucial driver of acute kidney injury in rhabdomyolysis. Kidney Int. 2021;99:581–597. doi: 10.1016/j.kint.2020.09.033. [DOI] [PubMed] [Google Scholar]
- 35.Peng Q., Li K., Smyth L.A., et al. C3a and C5a promote renal ischemia-reperfusion injury. J Am Soc Nephrol. 2012;23:1474–1485. doi: 10.1681/ASN.2011111072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eerhart M.J., Reyes J.A., Blanton C.L., et al. Complement blockade in recipients prevents delayed graft function and delays antibody-mediated rejection in a nonhuman primate model of kidney transplantation. Transplantation. 2022;106:60–71. doi: 10.1097/TP.0000000000003754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Vries B., Matthijsen R.A., Wolfs T.G., et al. Inhibition of complement factor C5 protects against renal ischemia-reperfusion injury: inhibition of late apoptosis and inflammation. Transplantation. 2003;75:375–382. doi: 10.1097/01.TP.0000044455.05584.2A. [DOI] [PubMed] [Google Scholar]
- 38.Pratt J.R., Basheer S.A., Sacks S.H. Local synthesis of complement component C3 regulates acute renal transplant rejection. Nat Med. 2002;8:582–587. doi: 10.1038/nm0602-582. [DOI] [PubMed] [Google Scholar]
- 39.Schroppel B., Akalin E., Baweja M., et al. Peritransplant eculizumab does not prevent delayed graft function in deceased donor kidney transplant recipients: results of two randomized controlled pilot trials. Am J Transplant. 2020;20:564–572. doi: 10.1111/ajt.15580. [DOI] [PubMed] [Google Scholar]
- 40.Jayne D.R.W., Merkel P.A., Schall T.J., et al. Avacopan for the treatment of ANCA-associated vasculitis. N Engl J Med. 2021;384:599–609. doi: 10.1056/NEJMoa2023386. [DOI] [PubMed] [Google Scholar]
- 41.Fakhouri F., de Jorge E.G., Brune F., et al. Treatment with human complement factor H rapidly reverses renal complement deposition in factor H-deficient mice. Kidney Int. 2010;78:279–286. doi: 10.1038/ki.2010.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Le Quintrec M., Lapeyraque A.L., Lionet A., et al. Patterns of clinical response to eculizumab in patients with C3 glomerulopathy. Am J Kidney Dis. 2018;72:84–92. doi: 10.1053/j.ajkd.2017.11.019. [DOI] [PubMed] [Google Scholar]
- 43.Wilson H.R., Medjeral-Thomas N.R., Gilmore A.C., et al. Glomerular membrane attack complex is not a reliable marker of ongoing C5 activation in lupus nephritis. Kidney Int. 2019;95:655–665. doi: 10.1016/j.kint.2018.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fakhouri F., Fremeaux-Bacchi V. Thrombotic microangiopathy in aHUS and beyond: clinical clues from complement genetics. Nat Rev Nephrol. 2021;17:543–553. doi: 10.1038/s41581-021-00424-4. [DOI] [PubMed] [Google Scholar]
- 45.Dragon-Durey M.A., Sethi S.K., Bagga A., et al. Clinical features of anti-factor H autoantibody-associated hemolytic uremic syndrome. J Am Soc Nephrol. 2010;21:2180–2187. doi: 10.1681/ASN.2010030315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chauvet S., Berthaud R., Devriese M., et al. Anti-factor B antibodies and acute postinfectious GN in children. J Am Soc Nephrol. 2020;31:829–840. doi: 10.1681/ASN.2019080851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ruseva M.M., Vernon K.A., Lesher A.M., et al. Loss of properdin exacerbates C3 glomerulopathy resulting from factor H deficiency. J Am Soc Nephrol. 2013;24:43–52. doi: 10.1681/ASN.2012060571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rose K.L., Paixao-Cavalcante D., Fish J., et al. Factor I is required for the development of membranoproliferative glomerulonephritis in factor H-deficient mice. J Clin Invest. 2008;118:608–618. doi: 10.1172/JCI32525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bu F., Meyer N.C., Zhang Y., et al. Soluble c5b-9 as a biomarker for complement activation in atypical hemolytic uremic syndrome. Am J Kidney Dis. 2015;65:968–969. doi: 10.1053/j.ajkd.2015.02.326. [DOI] [PubMed] [Google Scholar]
- 50.Gavriilaki E., Yuan X., Ye Z., et al. Modified Ham test for atypical hemolytic uremic syndrome. Blood. 2015;125:3637–3646. doi: 10.1182/blood-2015-02-629683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Noris M., Galbusera M., Gastoldi S., et al. Dynamics of complement activation in aHUS and how to monitor eculizumab therapy. Blood. 2014;124:1715–1726. doi: 10.1182/blood-2014-02-558296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Palomo M., Blasco M., Molina P., et al. Complement activation and thrombotic microangiopathies. Clin J Am Soc Nephrol. 2019;14:1719–1732. doi: 10.2215/CJN.05830519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Iatropoulos P., Daina E., Curreri M., et al. Cluster analysis identifies distinct pathogenetic patterns in C3 glomerulopathies/immune complex-mediated membranoproliferative GN. J Am Soc Nephrol. 2018;29:283–294. doi: 10.1681/ASN.2017030258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Risitano A.M., Marotta S., Ricci P., et al. Anti-complement treatment for paroxysmal nocturnal hemoglobinuria: time for proximal complement inhibition? A position paper from the SAAWP of the EBMT. Front Immunol. 2019;10:1157. doi: 10.3389/fimmu.2019.01157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lin Z., Schmidt C.Q., Koutsogiannaki S., et al. Complement C3dg-mediated erythrophagocytosis: implications for paroxysmal nocturnal hemoglobinuria. Blood. 2015;126:891–894. doi: 10.1182/blood-2015-02-625871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hillmen P., Szer J., Weitz I., et al. Pegcetacoplan versus eculizumab in paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2021;384:1028–1037. doi: 10.1056/NEJMoa2029073. [DOI] [PubMed] [Google Scholar]
- 57.Risitano A.M., Roth A., Soret J., et al. Addition of iptacopan, an oral factor B inhibitor, to eculizumab in patients with paroxysmal nocturnal haemoglobinuria and active haemolysis: an open-label, single-arm, phase 2, proof-of-concept trial. Lancet Haematol. 2021;8:e344–e354. doi: 10.1016/S2352-3026(21)00028-4. [DOI] [PubMed] [Google Scholar]
- 58.Crew P.E., Abara W.E., McCulley L., et al. Disseminated gonococcal infections in patients receiving eculizumab: a case series. Clin Infect Dis. 2019;69:596–600. doi: 10.1093/cid/ciy958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Konar M., Granoff D.M. Eculizumab treatment and impaired opsonophagocytic killing of meningococci by whole blood from immunized adults. Blood. 2017;130:891–899. doi: 10.1182/blood-2017-05-781450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Figueroa J.E., Densen P. Infectious diseases associated with complement deficiencies. Clin Microbiol Rev. 1991;4:359–395. doi: 10.1128/CMR.4.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McQuillen D.P., Ram S. No good deed goes unpunished: eculizumab and invasive neisserial infections. Clin Infect Dis. 2019;69:601–603. doi: 10.1093/cid/ciy959. [DOI] [PubMed] [Google Scholar]
- 62.Ardissino G., Possenti I., Tel F., Testa S., Salardi S., Ladisa V. Discontinuation of eculizumab treatment in atypical hemolytic uremic syndrome: an update. Am J Kidney Dis. 2015;66:172–173. doi: 10.1053/j.ajkd.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 63.Wetzels J.F., van de Kar N.C. Discontinuation of eculizumab maintenance treatment for atypical hemolytic uremic syndrome. Am J Kidney Dis. 2015;65:342. doi: 10.1053/j.ajkd.2014.04.039. [DOI] [PubMed] [Google Scholar]
- 64.Fakhouri F., Fila M., Provot F., et al. Pathogenic variants in complement genes and risk of atypical hemolytic uremic syndrome relapse after eculizumab discontinuation. Clin J Am Soc Nephrol. 2017;12:50–59. doi: 10.2215/CJN.06440616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Merrill S.A., Brittingham Z.D., Yuan X., et al. Eculizumab cessation in atypical hemolytic uremic syndrome. Blood. 2017;130:368–372. doi: 10.1182/blood-2017-02-770214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fakhouri F., Fila M., Hummel A., et al. Eculizumab discontinuation in children and adults with atypical haemolytic uremic syndrome: a prospective multicentric study. Blood. 2021;137:2438–2449. doi: 10.1182/blood.2020009280. [DOI] [PubMed] [Google Scholar]
- 67.Wu Y., Zwaini Z.D., Brunskill N.J., et al. Properdin deficiency impairs phagocytosis and enhances injury at kidney repair phase post ischemia-reperfusion. Front Immunol. 2021;12:697760. doi: 10.3389/fimmu.2021.697760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu W., Liu C., Farrar C.A., et al. Collectin-11 promotes the development of renal tubulointerstitial fibrosis. J Am Soc Nephrol. 2018;29:168–181. doi: 10.1681/ASN.2017050544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thurman J.M. Rhabdomyolysis and complement-once again, epithelial cells take center stage. Kidney Int. 2021;99:537–539. doi: 10.1016/j.kint.2020.10.045. [DOI] [PubMed] [Google Scholar]
- 70.Thurman J.M., Ljubanovic D., Royer P.A., et al. Altered renal tubular expression of the complement inhibitor Crry permits complement activation after ischemia/reperfusion. J Clin Invest. 2006;116:357–368. doi: 10.1172/JCI24521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zaferani A., Vives R.R., van der Pol P., et al. Identification of tubular heparan sulfate as a docking platform for the alternative complement component properdin in proteinuric renal disease. J Biol Chem. 2011;286:5359–5367. doi: 10.1074/jbc.M110.167825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fremeaux-Bacchi V., Weiss L., Demouchy C., et al. Hypocomplementaemia of poststreptococcal acute glomerulonephritis is associated with C3 nephritic factor (C3NeF) IgG autoantibody activity. Nephrol Dial Transplant. 1994;9:1747–1750. [PubMed] [Google Scholar]
- 73.Sethi S., Fervenza F.C., Zhang Y., et al. Atypical postinfectious glomerulonephritis is associated with abnormalities in the alternative pathway of complement. Kidney Int. 2013;83:293–299. doi: 10.1038/ki.2012.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chehade H., Guzzo G., Cachat F., et al. Eculizumab as a new treatment for severe acute post-infectious glomerulonephritis: two case reports. Front Med (Lausanne) 2021;8:663258. doi: 10.3389/fmed.2021.663258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chehade H., Rotman S., Fremeaux-Bacchi V., et al. Blockade of C5 in severe acute postinfectious glomerulonephritis associated with anti-factor H autoantibody. Am J Kidney Dis. 2016;68:944–948. doi: 10.1053/j.ajkd.2016.06.026. [DOI] [PubMed] [Google Scholar]
- 76.Fakhouri F., Fremeaux-Bacchi V., Noel L.H., et al. C3 glomerulopathy: a new classification. Nat Rev Nephrol. 2010;6:494–499. doi: 10.1038/nrneph.2010.85. [DOI] [PubMed] [Google Scholar]
- 77.Pickering M.C., Cook H.T., Warren J., et al. Uncontrolled C3 activation causes membranoproliferative glomerulonephritis in mice deficient in complement factor H. Nat Genet. 2002;31:424–428. doi: 10.1038/ng912. [DOI] [PubMed] [Google Scholar]
- 78.Ruggenenti P., Daina E., Gennarini A., et al. C5 convertase blockade in membranoproliferative glomerulonephritis: a single-arm clinical trial. Am J Kidney Dis. 2019;74:224–238. doi: 10.1053/j.ajkd.2018.12.046. [DOI] [PubMed] [Google Scholar]
- 79.Espinosa M., Ortega R., Sanchez M., et al. Association of C4d deposition with clinical outcomes in IgA nephropathy. Clin J Am Soc Nephrol. 2014;9:897–904. doi: 10.2215/CJN.09710913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Roos A., Rastaldi M.P., Calvaresi N., et al. Glomerular activation of the lectin pathway of complement in IgA nephropathy is associated with more severe renal disease. J Am Soc Nephrol. 2006;17:1724–1734. doi: 10.1681/ASN.2005090923. [DOI] [PubMed] [Google Scholar]
- 81.Kiryluk K., Li Y., Scolari F., et al. Discovery of new risk loci for IgA nephropathy implicates genes involved in immunity against intestinal pathogens. Nat Genet. 2014;46:1187–1196. doi: 10.1038/ng.3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhu L., Guo W.Y., Shi S.F., et al. Circulating complement factor H-related protein 5 levels contribute to development and progression of IgA nephropathy. Kidney Int. 2018;94:150–158. doi: 10.1016/j.kint.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 83.Guzzo G., Sadallah S., Fodstad H., et al. Case report: a rare truncating variant of the CFHR5 gene in IgA nephropathy. Front Genet. 2021;12:529236. doi: 10.3389/fgene.2021.529236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tortajada A., Gutierrez E., Goicoechea de Jorge E., et al. Elevated factor H-related protein 1 and factor H pathogenic variants decrease complement regulation in IgA nephropathy. Kidney Int. 2017;92:953–963. doi: 10.1016/j.kint.2017.03.041. [DOI] [PubMed] [Google Scholar]
- 85.Medjeral-Thomas N.R., Troldborg A., Constantinou N., et al. Progressive IgA nephropathy is associated with low circulating mannan-binding lectin-associated serine protease-3 (MASP-3) and increased glomerular factor H-related protein-5 (FHR5) deposition. Kidney Int Rep. 2018;3:426–438. doi: 10.1016/j.ekir.2017.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang Y., Yan X., Zhao T., et al. Targeting C3a/C5a receptors inhibits human mesangial cell proliferation and alleviates immunoglobulin A nephropathy in mice. Clin Exp Immunol. 2017;189:60–70. doi: 10.1111/cei.12961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ring T., Pedersen B.B., Salkus G., Goodship T.H. Use of eculizumab in crescentic IgA nephropathy: proof of principle and conundrum? Clin Kidney J. 2015;8:489–491. doi: 10.1093/ckj/sfv076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Servais A., Noel L.H., Roumenina L.T., et al. Acquired and genetic complement abnormalities play a critical role in dense deposit disease and other C3 glomerulopathies. Kidney Int. 2012;82:454–464. doi: 10.1038/ki.2012.63. [DOI] [PubMed] [Google Scholar]
- 89.Jonsen A., Nilsson S.C., Ahlqvist E., et al. Mutations in genes encoding complement inhibitors CD46 and CFH affect the age at nephritis onset in patients with systemic lupus erythematosus. Arthritis Res Ther. 2011;13:R206. doi: 10.1186/ar3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bao L., Haas M., Quigg R.J. Complement factor H deficiency accelerates development of lupus nephritis. J Am Soc Nephrol. 2011;22:285–295. doi: 10.1681/ASN.2010060647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mehta G., Ferreira V.P., Skerka C., et al. New insights into disease-specific absence of complement factor H related protein C in mouse models of spontaneous autoimmune diseases. Mol Immunol. 2014;62:235–248. doi: 10.1016/j.molimm.2014.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Salant D.J., Belok S., Madaio M.P., Couser W.G. A new role for complement in experimental membranous nephropathy in rats. J Clin Invest. 1980;66:1339–1350. doi: 10.1172/JCI109987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cybulsky A.V., Quigg R.J., Salant D.J. The membrane attack complex in complement-mediated glomerular epithelial cell injury: formation and stability of C5b-9 and C5b-7 in rat membranous nephropathy. J Immunol. 1986;137:1511–1516. [PubMed] [Google Scholar]
- 94.Lateb M., Ouahmi H., Payre C., et al. Anti-PLA2R1 antibodies containing sera induce in vitro cytotoxicity mediated by complement activation. J Immunol Res. 2019;2019:1324804. doi: 10.1155/2019/1324804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hayashi N., Okada K., Matsui Y., et al. Glomerular mannose-binding lectin deposition in intrinsic antigen-related membranous nephropathy. Nephrol Dial Transplant. 2018;33:832–840. doi: 10.1093/ndt/gfx235. [DOI] [PubMed] [Google Scholar]
- 96.Bally S., Debiec H., Ponard D., et al. Phospholipase A2 receptor-related membranous nephropathy and mannan-binding lectin deficiency. J Am Soc Nephrol. 2016;27:3539–3544. doi: 10.1681/ASN.2015101155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Luo W., Olaru F., Miner J.H., et al. Alternative pathway is essential for glomerular complement activation and proteinuria in a mouse model of membranous nephropathy. Front Immunol. 2018;9:1433. doi: 10.3389/fimmu.2018.01433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Trendelenburg M., Fossati-Jimack L., Cortes-Hernandez J., et al. The role of complement in cryoglobulin-induced immune complex glomerulonephritis. J Immunol. 2005;175:6909–6914. doi: 10.4049/jimmunol.175.10.6909. [DOI] [PubMed] [Google Scholar]
- 99.Hirt-Minkowski P., Trendelenburg M., Groschl I., Fischer A., Heijnen I., Schifferli J. A trial of complement inhibition in a patient with cryoglobulin-induced glomerulonephritis. Case Rep Nephrol Urol. 2012;2:38–45. doi: 10.1159/000339403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sheerin N.S., Springall T., Carroll M.C., Hartley B., Sacks S.H. Protection against anti-glomerular basement membrane (GBM)-mediated nephritis in C3- and C4-deficient mice. Clin Exp Immunol. 1997;110:403–409. doi: 10.1046/j.1365-2249.1997.4261438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nithagon P., Cortazar F., Shah S.I., et al. Eculizumab and complement activation in anti-glomerular basement membrane disease. Kidney Int Rep. 2021;6:2713–2717. doi: 10.1016/j.ekir.2021.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sobotta M., Moerer O., Gross O. Case report: eculizumab and ECMO rescue therapy of severe ARDS in Goodpasture syndrome. Front Med (Lausanne) 2021;8:720949. doi: 10.3389/fmed.2021.720949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gou S.J., Yuan J., Chen M., et al. Circulating complement activation in patients with anti-neutrophil cytoplasmic antibody-associated vasculitis. Kidney Int. 2013;83:129–137. doi: 10.1038/ki.2012.313. [DOI] [PubMed] [Google Scholar]
- 104.Augusto J.F., Langs V., Demiselle J., et al. Low serum complement C3 levels at diagnosis of renal ANCA-associated vasculitis is associated with poor prognosis. PLoS One. 2016;11 doi: 10.1371/journal.pone.0158871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Camous L., Roumenina L., Bigot S., et al. Complement alternative pathway acts as a positive feedback amplification of neutrophil activation. Blood. 2011;117:1340–1349. doi: 10.1182/blood-2010-05-283564. [DOI] [PubMed] [Google Scholar]
- 106.Xiao H., Schreiber A., Heeringa P., et al. Alternative complement pathway in the pathogenesis of disease mediated by anti-neutrophil cytoplasmic autoantibodies. Am J Pathol. 2007;170:52–64. doi: 10.2353/ajpath.2007.060573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jayne D.R.W., Bruchfeld A.N., Harper L., et al. Randomized trial of C5a receptor inhibitor avacopan in ANCA-associated vasculitis. J Am Soc Nephrol. 2017;28:2756–2767. doi: 10.1681/ASN.2016111179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pickering M.C., de Jorge E.G., Martinez-Barricarte R., et al. Spontaneous hemolytic uremic syndrome triggered by complement factor H lacking surface recognition domains. J Exp Med. 2007;204:1249–1256. doi: 10.1084/jem.20070301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Licht C., Greenbaum L.A., Muus P., et al. Efficacy and safety of eculizumab in atypical hemolytic uremic syndrome from 2-year extensions of phase 2 studies. Kidney Int. 2015;87:1061–1073. doi: 10.1038/ki.2014.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rondeau E., Scully M., Ariceta G., et al. The long-acting C5 inhibitor, ravulizumab, is effective and safe in adult patients with atypical hemolytic uremic syndrome naive to complement inhibitor treatment. Kidney Int. 2020;97:1287–1296. doi: 10.1681/ASN.2017050544. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.