Significance
Arginine–vasopressin (AVP) acting on V1a receptors (Avpr1as) represents a key signaling mechanism in a brain circuit that increases the expression of social communication and aggression. We produced Syrian hamsters that completely lack Avpr1as (Avpr1a knockout [KO] hamsters) using the CRISPR-Cas9 system to more fully examine the role of Avpr1a in the expression of social behaviors. We confirmed the absence of Avpr1as in these hamsters by demonstrating 1) a complete lack of Avpr1a-specific receptor binding throughout the brain, 2) a behavioral insensitivity to centrally administered AVP, and 3) an absence of the well-known blood-pressure response produced by activating Avpr1as. Unexpectedly, however, Avpr1a KO hamsters displayed more social communication behavior and aggression toward same-sex conspecifics than did their wild-type (WT) littermates.
Keywords: aggression, social communication, flank marking, oxytocin, social behavior neural network
Abstract
Studies from a variety of species indicate that arginine–vasopressin (AVP) and its V1a receptor (Avpr1a) play a critical role in the regulation of a range of social behaviors by their actions in the social behavior neural network. To further investigate the role of AVPRs in social behavior, we performed CRISPR-Cas9–mediated editing at the Avpr1a gene via pronuclear microinjections in Syrian hamsters (Mesocricetus auratus), a species used extensively in behavioral neuroendocrinology because they produce a rich suite of social behaviors. Using this germ-line gene-editing approach, we generated a stable line of hamsters with a frame-shift mutation in the Avpr1a gene resulting in the null expression of functional Avpr1as. Avpr1a knockout (KO) hamsters exhibited a complete lack of Avpr1a-specific autoradiographic binding throughout the brain, behavioral insensitivity to centrally administered AVP, and no pressor response to a peripherally injected Avpr1a-specific agonist, thus confirming the absence of functional Avpr1as in the brain and periphery. Contradictory to expectations, Avpr1a KO hamsters exhibited substantially higher levels of conspecific social communication (i.e., odor-stimulated flank marking) than their wild-type (WT) littermates. Furthermore, sex differences in aggression were absent, as both male and female KOs exhibited more aggression toward same-sex conspecifics than did their WT littermates. Taken together, these data emphasize the importance of comparative studies employing gene-editing approaches and suggest the startling possibility that Avpr1a-specific modulation of the social behavior neural network may be more inhibitory than permissive.
A key challenge in social neuroscience is to understand how genes act within defined neural circuits to regulate social and affective behaviors. The social behavior neural network (SBNN) is a complex circuit composed of an interconnected series of brain regions, or “nodes,” that govern many social behaviors, ranging from fear and aggression to affiliation (1–4). Much of the diversity and complexity of social behavior across species and among individuals is hypothesized to emerge from the functional interactions of elements of the entire SBNN circuit, and not from the activity of its individual components (3, 5). To address how social behavior emerges from such a complex neural network, it is essential to have advanced genetic tools capable of manipulating key neurochemical pathways across the entire circuit and to be able to compare the effects of these manipulations in a variety of species that exhibit rich and varied patterns of social behavior.
In the present study, we employed a CRISPR-Cas9 pronuclear germ-line gene-editing approach in Syrian hamsters (Mesocricetus auratus) to eliminate arginine–vasopressin (AVP) V1a receptors (Avpr1as) throughout the nodes of the SBNN (6–8). Syrian hamsters have been used extensively in studies of social neuroscience because of their well-described patterns of social behavior and are the species in which AVP was first demonstrated to regulate social behavior (9). Subsequent pharmacological studies using highly selective agonists and antagonists for Avpr1as in hamsters, as well as in other species, such as voles and mice, have reinforced the critical role of AVP and Avpr1as in regulating discrete social behaviors, including aggression and social communication (10–13). Despite the substantial body of evidence that Avpr1as regulate aggression in several rodent species, including mice, aggression exhibited by Avpr1a knockout (KO) male (M) mice was found to be no different from that produced by wild-type (WT) controls (14). In order to more fully investigate the functional role of Avpr1as in governing social behaviors in hamsters, we capitalized on the recently sequenced hamster transcriptome to design a Cas9/single-guide RNA (sgRNA) construct targeting the start codon of the hamster Avpr1a gene (15, 16). Surprisingly, we report here that the global deletion of the hamster Avpr1a gene paradoxically increases aggression and social communication.
Results
CRISPR-Cas9 Indels.
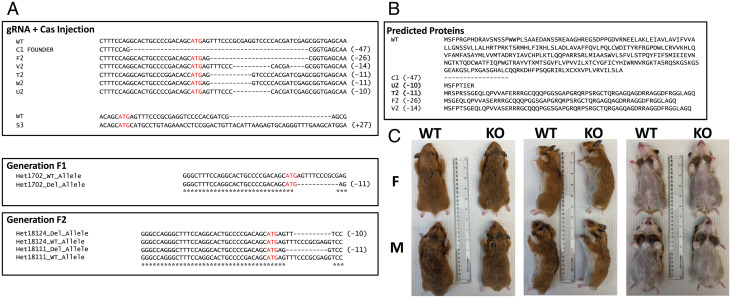
Injections of sgRNA/Cas9 plasmid targeting the Avpr1a gene into hamster embryos produced a variety of indels at and around the target site in hamsters that survived to weaning (Fig. 1). Of these hamsters, a single female (F) (C1) had a WT allele and a 47-base-pair deletion beginning 16 bases upstream from the start codon and ending 28 bases downstream from the start codon, and she produced two litters with WT sires. Her descendants, however, were all found to have either an 11-base-pair or 10-base-pair deletion, which was subsequently passed on to their offspring (generations F2, F3, and F4). These results suggest that the founder (C1) likely exhibited somatic mosaicism. The start codon is present in the 11-base-pair and 10-base-pair deletions, but both produced a frame-shift mutation resulting in a premature stop codon. An off-target analysis was conducted with F3 and F4 hamsters after two heterozygote × WT outcrossings. Six potential off-target genes were identified (Table 1). Of these, we were able to amplify, subclone, and sequence PROB1 (n = 3) and SCARF2 (n = 2) from KO hamsters with sequencing revealing a precise match to the corresponding WT sequence from the reference genome.
Fig. 1.
Injections of sgRNA/Cas9 plasmid targeting the Avpr1a gene into hamster embryos. (A) Alignments of various indels produced by CRISPR-Cas9 editing. Only C1 FOUNDER was able to produce offspring resulting in generations F1 and F2. (B) Predicted proteins produced by indels. (C) F (Top of each photo) and M (Bottom of each photo) Avpr1a KOs (Right side of each photo) exhibit no obvious physical differences compared to WT (Left side of each photo).
Table 1.
Potential off-target genes
| gRNA | GACAGCATGAGTTTCCCGCGAGG |
| Scarf2 | –––––––––––––GTTTCCCGCGCGG |
| Paics | ––––––––––––––TTTCCCGCGCGG |
| PROB1 | –––––––––––––GTTTCCCGCGAGG |
| Pyurf | ––––––––––GAGTTTCCCGCGGGG |
| Hoxa10 | ––––––––––––––TTTCCCGCGGGG |
| Dus3l | ––––––––––––––TTTCCCGCGTGG |
| ********* ** |
Avpr1a Binding Is Absent in KO Hamsters and Reduced in Heterozygotes.
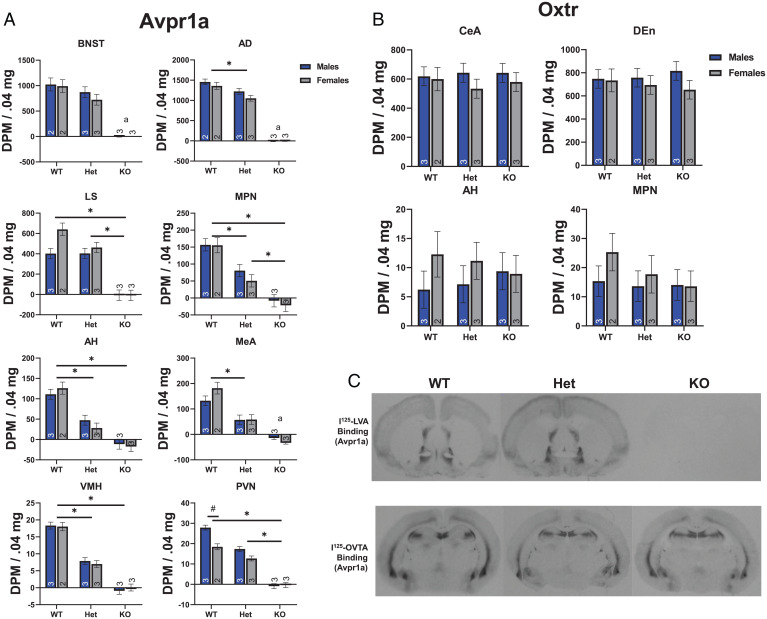
To confirm the absence of functional Avpr1as, we first used receptor autoradiography to determine whether disruption of the Avpr1a gene resulted in the loss of specific Avpr1a binding in the brain. Autoradiograms revealed that specific Avpr1a binding was nonexistent in the brains of M and F homozygous KOs (Fig. 2 A and C). Statistical analysis of specific Avpr1a binding revealed significantly lower binding in KOs than in heterozygous or WT hamsters in selected brain regions rich in Avpr1a and/or known to be involved in social behavior (6, 7), including the lateral septum [LS; F(2,11) = 57.79, P < 0.001, eta2 = 0.913), the medial preoptic nucleus [MPN; F(2,11) = 39.10, P < 0.001, eta2 = 0.88], the anterior hypothalamus [AH; F(2,11) = 52.48, P < 0.001, eta2 = 0.91], the ventromedial hypothalamus [VMH; F(2,11) = 148.7, P < 0.001, eta2 = 0.96], and the paraventricular nucleus [PVN; F(2,11) = 184.09, P < 0.001, eta2 = 0.97] (Fig. 2A). Analysis of the bed nucleus of the stria terminalis [BNST; F(1,6) = 3.218, P = 0.123], the anterodorsal thalamus [AD; F(1,7) = 12.66, P = 0.009, eta2 = 0.64], and the medial amygdala [F(1,7) = 24.52, P = 0.002, eta2 = 0.78] violated the assumption of heteroscedasticity due to low variability among KOs compared to WTs and heterozygotes, and so the statistics presented here represent comparisons between WTs and heterozygotes only. However, these regions followed a pattern of Avpr1a binding similar to other regions in which binding in KOs was nonexistent. In all regions investigated, except the BNST and LS, heterozygotes had binding densities that were intermediate between KOs and WTs. There was also a genotype × sex interaction in the PVN, in which M had higher Avpr1a binding density than F among WTs, but this difference was not apparent among heterozygotes or KOs [F(2,11) = 7.58, P = 0.009, eta2 = 0.58].
Fig. 2.
Localization of Avpr1a and Oxtr with receptor autoradiography. (A) Quantification (disintegrations per minute per .04 mg standard) of Avpr1a binding in regions important for social behavior or in regions with a large number of Avpr1as. Bars connected by lines and * indicate significant post hoc differences. Bars connected by lines and # indicate significant sex differences. An “a” notation indicates that KOs were excluded from ANOVA. (B) Quantification of Oxtr binding in regions important for social behavior or in regions with a large number of Oxtrs. (C) Representative Avpr1a (Upper) and Oxtr (Lower) photomicrographs from WT, heterozygote (Het), and KO hamsters. Numbers inside or above bars indicate n. OVTA, ornithine vasotocin analog; LVA, linear vasopressin antagonist.
To investigate the specificity of the disruption of Avpr1as, we also examined the density and distribution of receptor binding of the closely related oxytocin receptor (Oxtr) in brain sections adjacent to those where Avpr1a binding was analyzed (Fig. 2 B and C). In contrast to Avpr1a, there were no differences among KOs, heterozygotes, and WTs with respect to specific Oxtr binding in various brain regions rich in Oxtrs [central amygdala (CeA), F(2,11) = 0.072, P = 0.931, η2 = 0.013; dorsal endopiriform cortex (DEn), F(2, 11) = 0.015, P = 0.985, η2 = 0.003] or in regions associated with social communication [AH, F(2,11) = 0.001, P = 0.999, η2 = 0.000; MPN, F(2,10) = 0.705, P = 0.517, η2 = 0.124] (6, 7). There was no main effect of sex or genotype × sex interaction for any brain region or receptor type (all F’s < 1.40; all P’s > 0.261).
Avpr1a-Mediated Blood-Pressure Regulation Is Lost in KO Hamsters.
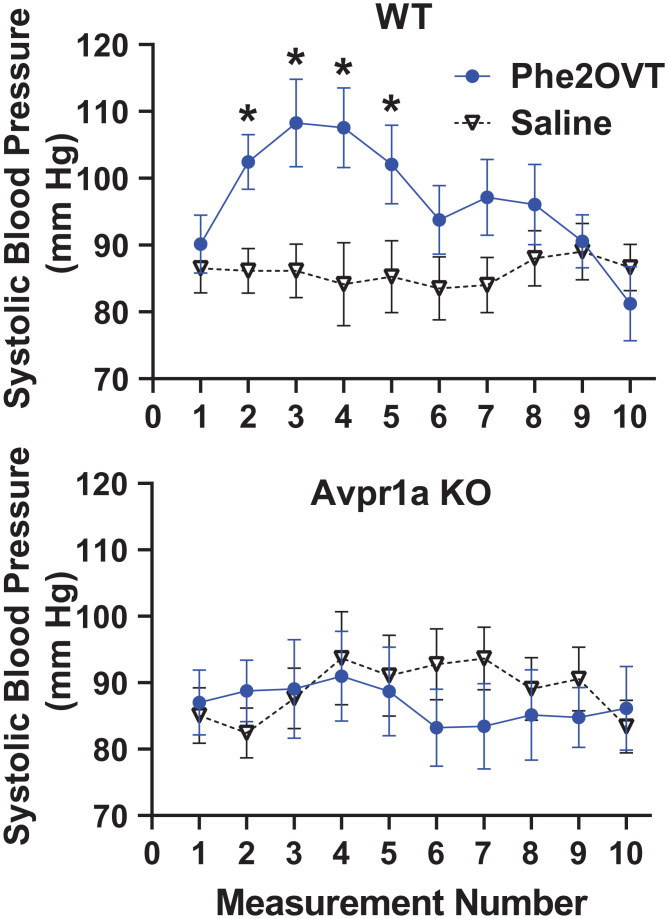
To further confirm the absence of functional Avpr1a in Avpr1a KO hamsters, we examined the well-known effect of Avpr1a activation on blood pressure (17, 18). Increasing blood pressure is a critical function of peripheral Avpr1as; thus, we tested the hypothesis that the “pressor” effect of Avpr1as is impaired in Avpr1a KO hamsters. Treatment with a highly selective Avpr1a agonist (Phe2OVT) briefly, but robustly, increased systolic blood pressure in WTs [F(9,189) = 2.18, P = 0.025, η2 = 0.094; Fig. 3]. Systolic blood pressure was significantly higher during measurements following systemic injection of Phe2OVT compared with saline, after which blood pressure returned to levels similar to those following saline injection. In contrast, injection of Phe2OVT in Avpr1a KO hamsters did not affect systolic blood pressure in M or F. There was no genotype × sex × treatment × measurement interaction [F(9,189) = 0.96, P = 0.471, η2 = 0.044].
Fig. 3.
Systolic blood pressure from anesthetized WT (7 F, 7 M) and Avpr1a KO (5 F, 6 M) hamsters injected IP with saline or the selective Avpr1a agonist (Phe2OVT). Injections occurred at measurement 0, and each measurement lasted approximately 1 min. * Indicates a significant post hoc difference from saline.
AVP-Stimulated Flank Marking Is Abolished in KO Hamsters.
We next confirmed that the absence of Avpr1as in Avpr1a KO hamsters eliminated the ability of Phe2OVT to induce social communication, namely, flank marking. Flank marking communicates a variety of socially important information, such as dominance status and mate choice (19, 20). Hamsters flank mark during social interactions and in response to odors of conspecifics. There is considerable evidence from structure–activity studies and the administration of highly selective Avpr1a agonists and antagonists that centrally administered AVP stimulates flank marking by acting through Avpr1as (21, 22). To test whether this Avpr1a-mediated behavior remains in the absence of Avpr1a, we injected AVP, Phe2OVT, and saline in counterbalanced order into the ventricles of M and F KO and WT hamsters. AVP and Phe2OVT each induced robust flank marking in WT M and F compared with saline. In contrast, neither AVP nor Phe2OVT stimulated flank marking in KO M or F when compared with saline injection [F(2, 14) = 10.598, P < 0.001, η2 = 0.43; Fig. 4]. There was no genotype × sex interaction [F(2,28) = 1.371, P = 0.242, η2 = 0.89].
Fig. 4.
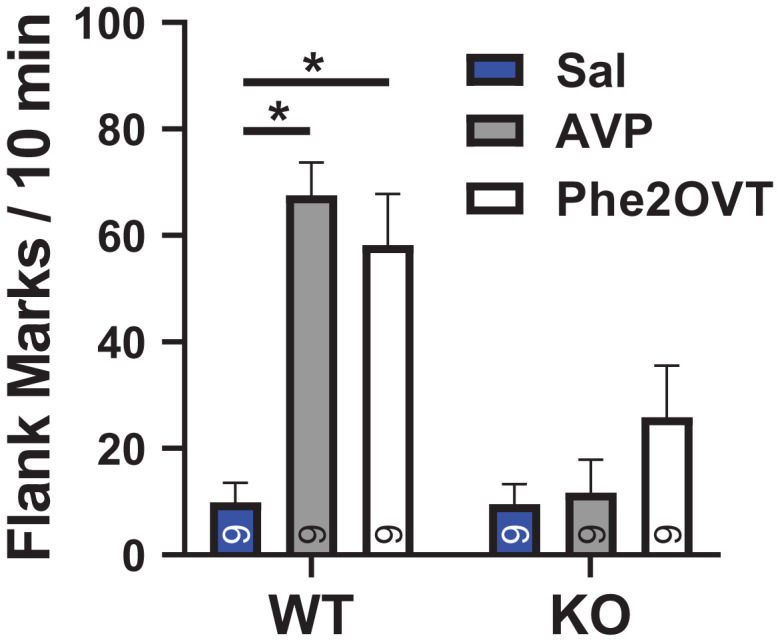
Drug-induced flank marking in WT and Avpr1a KO hamsters. Hamsters were injected just prior to being placed in a clean cage. Bars connected by lines indicate significant post hoc differences. Numbers inside or above bars indicate n. Sal, saline.
Odor-Stimulated Flank Marking Occurs at Higher Levels in KO than in WT Hamsters.
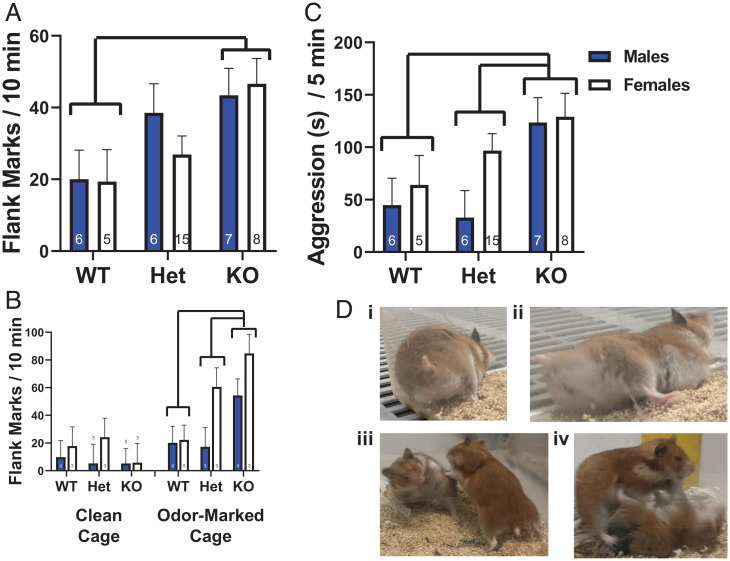
Given the pharmacological evidence that centrally administered AVP acts via Avpr1as to induce flank marking, we tested the hypothesis that a complete absence of Avpr1as would also eliminate the expression of odor-stimulated flank marking. Surprisingly, all hamsters, regardless of genotype, exhibited robust flank marking when exposed to the odors of a same-sex conspecific, with KOs marking approximately twice as much as WTs. These differences translated into a significant main effect of genotype [F(2,41) = 5.156, P = 0.01, eta2 = 0.201; Fig. 5A]. There was no genotype × sex interaction [F(2,41) = 0.594, P = 0.557, eta2 = 0.028]. Lastly, we examined whether the expression of flank marking in KO hamsters required the presence of social stimulation (i.e., conspecific odor). While KO hamsters flank marked more than WTs in odor-marked cages, neither KOs nor WTs flank marked in the absence of conspecific odors [genotype × condition effect; F(2,31) = 5.443, P = 0.009, eta2 = 0.260; Fig. 5B].
Fig. 5.
Flank marking and aggression in WT, heterozygote (Het), and KO hamsters. (A) Flank marking exhibited by WT, heterozygote, and KO hamsters when exposed to a cage previously marked by a same-sex conspecific. (B) Flank marking exhibited by WT, heterozygote, and KO hamsters exposed to clean and odor-marked cages. (C) Aggression exhibited by WT, heterozygote, and KO hamsters when exposed to a same-sex conspecific. Bars connected by brackets and lines indicate significant post hoc differences. Numbers inside bars or above indicate n. (D) Representative behavioral images. In i and ii, a M hamster flank marks the corner of a Plexiglas cage. In iii and iv, a larger F attacks and pins a smaller F.
KO Hamsters Exhibit Increased Aggression.
Aggressive behavior in hamsters is highly stereotyped and is exhibited at high levels in both M and F (23). Avpr1a activation has sex-specific effects on aggressiveness in M and F, with Avpr1a activation increasing aggression in M and reducing aggression in F (24–26). All hamsters, regardless of genotype or sex, exhibited aggression (total duration: chasing, biting, and pinning) when exposed to a nonaggressive, same-sex conspecific in a neutral arena. Moreover, there was a significant main effect of genotype [F(2,41) = 5.406, P = 0.008, eta2 = 0.209; Fig. 5C] with M and F KO hamsters displaying more aggression than either WT or heterozygote hamsters. M and F heterozygotes did not differ from WTs in their expression of aggression. There was no genotype × sex interaction [F(2,41) = 0.938, P = 0.400, eta2 = 0.044]. There was no effect of genotype on the latency to initiate aggression [F(2,41) = 2.415, P = 0.102, eta2 = 0.11]. There was, however, a significant main effect of sex, with F (mean = 79.11, SEM ± 19.3 s) initiating aggression earlier than M [mean = 145.2, SEM ±21.3 s; F(1,41) = 5.28, P = 0.027, eta2 = 0.114].
Discussion
Here, we report the successful use of CRISPR-Cas9 for the generation of Avpr1a KO Syrian hamsters. Through the breeding of a CRISPR-Cas9–edited founder and heterozygote progeny, we were able to successfully produce M and F Syrian hamsters completely lacking a functional Avpr1a gene. Avpr1a KO hamsters exhibited 1) a complete lack of Avpr1a binding throughout the brain, 2) insensitivity to the behavioral effects of centrally administered AVP, and 3) no changes in blood pressure in response to a peripherally injected Avpr1a agonist. Despite these expected changes, it is remarkable that Avpr1a KO hamsters expressed double the levels of odor-stimulated flank marking and aggression toward same-sex conspecifics than WTs.
Importantly, we showed haploinsufficiency in flank marking and aggressive behaviors and in Avpr1a binding in the brain, indicating that heterozygotes will be useful for investigating the effects of reduced, but not absent, Avpr1a expression. The translational relevance of behavioral genetic approaches should improve by increasing the variety of animal models and approaches, and these results demonstrate the utility of CRISPR-Cas9 gene editing in Syrian hamsters to interrogate gene function. Comparison of behavioral genetic data obtained from nontraditional model species with data obtained in KO mice (e.g., effects on aggression) provides important context that may help generalize findings to other rodents or to humans. During the generation and phenotyping of the hamsters described here, a KO was generated via CRISPR-Cas9 in prairie voles (27). Notably, these voles lack a functional Oxtr gene, and due to the relationship between Oxtrs and Avpr1a, they provide a useful comparison to our results in hamsters. In both species, CRISPR-Cas9 induced mosaics in edited animals. Mosaicism after Cas9-mediated gene editing is not uncommon (28–30), and, when generating a new KO model, it underscores the value of careful selective breeding of edited founders and descendants. Comparisons across species will likely provide new insight into the function and evolution of the various molecular substrates of behavior.
Flank marking plays a critical role in social communication in hamsters. M and F hamsters flank mark in response to odors of same-sex conspecifics or to hypothalamic injection of AVP without conspecific odors (9, 22, 31). In the present study, we again showed that intracerebroventricular (ICV) injection of AVP or a selective Avpr1a agonist in WT hamsters produced robust flank marking in odor-free environments; however, ICV injection of AVP or a selective Avpr1a agonist in Avpr1a KO hamsters had no effect on the expression of flank marking, demonstrating insensitivity of KOs to exogenous AVP. Surprisingly, we found that odor-stimulated flank marking was twofold higher in Avpr1a KO hamsters than in WT hamsters. This increase was specific to the presence of conspecific odor, as Avpr1a KO hamsters marked at the same low levels as WT hamsters in a clean cage. These findings indicate that Avpr1a activation is not necessary for the expression of odor-stimulated flank marking. Indeed, the present data are not alone in indicating that a disassociation between the number of Avpr1as and the expression of flank marking can occur. Housing in “summer-like” photoperiods (i.e., >12 h of light per day) dramatically increases the expression of hypothalamic Avpr1as compared with “winter-like” photoperiods (32). Interestingly, however, odor-stimulated and AVP-induced flank marking are expressed at the same levels, regardless of photoperiod (11, 33). The mechanisms responsible for the uncoupling of flank marking from the number of hypothalamic Avpr1as in short-photoperiod-exposed hamsters are not known, although photoperiod-induced compensatory changes in the response to neurochemical signals, such as serotonin or galanin, that can influence flank marking do not appear to be involved (34). Certainly, the investigation of the compensatory mechanisms that mediate the robust, odor-stimulated flank marking in Avpr1a KO hamsters is a necessary next step. Taken together, these data indicate that, although the presence of Avpr1as are necessary for exogenous AVP to induce flank marking, Avpr1as are not necessary for the expression of odor-stimulated flank marking; thus, it is clear that the neurochemical mechanisms regulating flank marking are more complex than previously thought.
Another intriguing and surprising finding emerged when we examined the result of eliminating functional Avpr1as on aggressive behavior. Previous studies have found that injection of AVP into the AH stimulates aggression in M hamsters and inhibits aggression in F hamsters and that injection of a selective Avpr1a antagonist into the AH inhibits aggression in M and stimulates aggression in F hamsters (13, 24, 25). Therefore, we predicted that the absence of Avpr1as would reduce aggression in M and increase aggression in F hamsters. As predicted, aggression was higher in Avpr1a KO F than in WTs, and aggression in heterozygote F was intermediate to that seen in KO and WT F. In Avpr1a KO M, however, aggression was unexpectedly higher than in heterozygotes or WTs. Indeed, aggression was twofold higher in M Avpr1a KO hamsters than it was in WT M. Thus, the elimination of functional Avpr1as eliminated the previously established sex differences in AVP-mediated aggression. These data are consistent with the hypothesis that Avpr1as have inhibitory effects on aggression in F and raise the possibility that the global loss of functional Avpr1as can increase aggression in M. Interestingly, aggression significantly increases in M and F hamsters exposed to short photoperiods compared with hamsters housed in long photoperiods (35, 36), under which there is a reduction of Avpr1as within key sites of the SBNN (32, 33). Similar to the increase in aggression seen in Avpr1a KO M hamsters, increased aggressiveness in short-photoperiod-exposed M hamsters does not depend on activation of Avpr1as (37). The short-photoperiod-induced increase in aggression may be the result of changes in the secretion of pineal melatonin that serve to increase aggression (38–42), and it will be interesting to investigate if M Avpr1a KOs have similar compensatory increases in melatonin secretion.
As discussed above, the diversity and complexity of social behaviors across species and among individuals is hypothesized to emerge from the functional interactions among the multiple nodes of SBNN circuitry, and not from the activity of its individual components (3, 5). Investigation of SBNN neurocircuitry has been restricted almost exclusively to studies of how individual nodes influence social behavior because of the inability to manipulate the entire circuit concurrently. Global KOs like those employed in the present study provide one approach, albeit an imperfect one, to manipulate key neurochemical signals across the entire circuit. The dramatic differences in aggression and social communication between the KO and WT hamsters seen in the present study were not predicted by studies employing pharmacological inhibition of Avpr1a activity within specific SBNN nodes. Therefore, elimination of Avpr1a activity throughout the entire SBNN circuit can impact social behavior very differently than does inhibiting Avpr1a activity in individual nodes of the circuit. As such, these data support the hypothesis that social behavior can be an emergent property coming from the interactions across nodes of the entire circuit. It is, however, important to emphasize the possibility that these differences could also be due to elimination of Avpr1as in regions outside the SBNN or as the result of developmental compensation. Nevertheless, the data obtained by using the KO approach suggest some fascinating questions about the putative functions of Avpr1a at a circuit level that are deserving of future study. For example, do Avpr1as operating across the circuit serve to inhibit the expression of at least some social behaviors, even though their activation in specific individual nodes can induce those behaviors? Are sex differences in aggression the result of sex differences in the effects of Avpr1a across the SBNN with activation of Avp1ar reducing aggression in M hamsters and enhancing aggression in F hamsters?
The phenotype of Avpr1a KO hamsters also differs greatly from the phenotype of Avpr1a KO mice, indicating that there are important species differences in Avpr1a function and/or compensatory mechanisms. M and F Avpr1a KO hamsters were more aggressive than were WT hamsters, whereas Avpr1a KO M mice display no differences in aggression from WTs (14). The increase in aggression in Avpr1a KO hamsters relative to WTs is informed by the concomitant increase in odor-stimulated flank marking, suggesting the possible existence of a hypersensitivity to and/or hyperresponsiveness to social olfactory stimuli in the main olfactory system (43). In contrast, Avpr1a KO mice exhibit reduced social investigation and impaired olfactory processing compared with WTs (14, 44). Taken together, these comparisons suggest that as CRISPR-Cas9 and related gene-editing technologies continue to be applied to new species and gene targets, more species-specific differences in gene function and developmental compensation will continue to be discovered. These data certainly emphasize the necessity of using species in addition to mice to interrogate the role of specific neurochemical signaling pathways in generating social behavior.
One critical aspect of genetic models is determining the influence of a gene (or its lack) throughout development (reviewed in ref. 45). It is difficult or impossible to separate the acute effects of eliminating a gene from its developmental effects in mammalian KO models, perhaps most notably in potential developmental compensation. For instance, it is possible that odor-stimulated flank marking in Avpr1a KO hamsters is “rescued” by other receptors. Two obvious candidates for compensation are Oxtr or Avpr1b. However, given that AVP, which binds and activates Oxtr and Avpr1b (46, 47), did not stimulate flank marking in KOs, this explanation is unlikely. Though we found no differences in Oxtr binding density in the brain nuclei examined, considering the strong link between the OT and AVP systems, it is possible that some developmental disruptions occurred in the Oxtr system in hamster Avpr1a KOs. Viral rescue strategies offer the potential to parse developmental and activational influences of Avpr1a KO (see ref. 48 for an example). The potential developmental consequences from the lack of Avpr1a will be an important target and consideration in future research using Avpr1a KO hamsters.
In conclusion, the unexpected behavioral phenotypes of Avpr1a KO Syrian hamsters reveal insight into the function of Avpr1as in facilitating social behavior. It was long thought that activation of Avpr1a was both necessary and sufficient for the expression of flank marking, but it now appears that flank marking can occur in the absence of Avpr1a activation in certain situations. This raises new questions regarding non-Avpr1a-mediated mechanisms of social communication. Gene-edited hamsters will provide an important tool in these future studies.
Materials and Methods
All procedures were approved by Georgia State University’s Institutional Animal Care and Use Committee and conformed to the NIH Guide for the Care and Use of Laboratory Animals (49), as well as the Animal Welfare Act Code of Federal Regulations. The Georgia State University animal care and use program is fully accredited by AAALAC International. Syrian hamsters (M. auratus) were purchased from Charles River Laboratories, housed in a 14-h light/10-h dark cycle, and provided food and water ad libitum.
CRISPR-Cas9 Constructs, Embryo Collection, Pronuclear Microinjections, and Embryo Transfer.
In order to generate CRISPR-Cas9–mediated nonhomologous end joining mutations, an sgRNA was designed that targeted the start codon of the hamster Avpr1a gene. The web tool CHOPCHOP (50) was used to query the G(N)20GG search motif against the published hamster genome (https://www.ncbi.nlm.nih.gov/genome/11998). The selected sgRNA (Table 1) was then cloned into a pX330-U6-Chimeric_BB_CBh_hSpCas9 bicistronic plasmid (Addgene ID 42230) as described (51, 52) using a NEBuilder HiFi DNA Assembly kit. This AVPR1A sgRNA/Cas9 plasmid was then quantified and prepared for injection.
F hamsters, 3 mo old, were induced to superovulate by intraperitoneal (IP) injection of 20 IU of pregnant mare serum gonadotropin (PMSG; EMD Millipore) in the morning of the day of postestrus discharge. They were mated with sexually mature M hamsters 82 h after PMSG injection. Zygotes were collected from oviducts ∼20 h after hand mating and cultured in M199TE medium at 37.5 °C, 10% CO2, 5% O2, and 85% N2. The Avpr1a sgRNA/Cas9 plasmid (2.5 ng/µL) was injected into the pronucleus of zygotes. The injected embryos were incubated in M199TE medium to recover for 1 h and then washed through M2 medium and transferred into the oviducts of pseudopregnant hamsters (53, 54).
Genotyping.
The genomic region containing the deletion site was amplified from ear punches (Qiagen, catalog no. 69504) using the following PCR conditions: 0.5 to 2 μL of genomic DNA, 1 μL of forward primer (10 mM; 5′-TTTAGGGCACGGGTCTTTG-3′), 1 μL of reverse primer (10 mM; 5′-GGCTCGGGAAATGGTCTTTA-3′), and 21 to 22.5 μL of PCR Supermix (catalog no. 12532024). The thermocycler conditions were as follows: 1) 95 °C for 2 min, 2) 95 °C for 30 s, 3) 59 °C for 30 s, 4) 68 °C for 1 min, 5) repeat steps 2 through 4 40×, and 5) 68 °C for 10 min. Amplicons of the expected length were then either subcloned and sequenced or sequenced as purified PCR products. The potential for CRISPR-Cas9 editing at off-target gene sites was evaluated after two outcrossings in F3 and F4 offspring. The genomic RNA (gRNA) plus each potential protospacer-adjacent motif (PAM) site (AGG, GGG, CGG, and TGG) were entered into National Center for Biotechnology Information BLAST against all predicted M. auratus genes. Potential off-target sites were evaluated by PCR amplification and sequencing if the results were within the coding region of a gene and matched the three-nucleotide PAM site and the last nine nucleotides of the gRNA query.
Subjects, Breeding, and Housing.
A single founder F was bred to produce two litters by WT sires. Offspring were outbred to WTs for two more generations. M and F heterozygotes from F3 were crossed to produce WTs, heterozygotes, and KOs. All subjects used for data collection were from F3 or their descendants. WTs generated from heterozygote × heterozygote or heterozygote × WT crossings were used whenever possible, but some WTs were from WT litters or purchased from Charles River Laboratories. All subjects were housed singly for at least 14 d prior to behavioral testing. Estrus cycles were monitored daily via vaginal discharge, and M hamsters were also handled daily. Weanlings were kept in same-sex groups of three to six until adulthood.
Receptor Autoradiography and Quantification.
Brains from Avpr1a KO, heterozygote, and WT animals were processed as described (6). The nuclei measured for Avpr1a were the BNST, the LS, the MPN, the AH, the VMH, the PVN. and the AD; and the nuclei for the Oxtr were the DEn, the AH, the MPN, and the CeA. Tissue in the same sections of those quantified for receptor binding that were not dense in Avpr1a or Oxtr binding in WT tissues (i.e., caudate, putamen, or ventromedial thalamus) was subtracted to provide an estimate of specific binding. Binding densities were averaged across both hemispheres, and at least two sections per brain, per nucleus were sampled.
Blood Pressure.
Systolic blood pressure was measured by using a Muromachi 50370 Non-Invasive Blood Pressure Monitor with a medium-sized hamster arm cuff. M and F WT (7 F, 7 M) and KO (5 F, 6 M) hamsters were anesthetized with 2% isoflurane until immobile. Hamsters were placed in a supine position and thermally supported. Isoflurane depresses blood pressure, so measurements were continually taken until systolic blood pressure stabilized. Then, saline was injected IP at a volume of 1 mL per kg of body weight. Ten measurements were taken (∼10-min duration), and then the selective Avpr1a agonist Phe2OVT (Bachem; Chemical Abstracts Service no. 2480-41-3) was injected IP on the contralateral side at a volume of 1 mL per kg of body weight and a dose of 3 mg per kg of body weight, followed by 10 blood-pressure measurements.
Brain Cannulation Surgeries.
Subjects were anesthetized with isoflurane and mounted in a stereotaxic apparatus. After leveling the skull, a single hole was drilled, and a 4-mm guide cannula aimed at the lateral ventricle was implanted (ICV: 0° angle, +0.8 mm anterior from bregma, ±1.4 mm from midline, −2.8 mm ventral from top of skull, cannula length 1.2 mm beyond guide). Stainless steel mounting screws were implanted, and hardware was affixed with dental acrylic. Ketoprofen (5 mg/kg) was administered before the first incision and again 24 h later. Subjects were allowed at least 3 d of recovery before behavioral testing began. After testing, hamsters were euthanized with an overdose of sodium pentobarbital, and ink was injected to verify correct cannula placement.
Behavioral Testing.
Drug-induced flank marking.
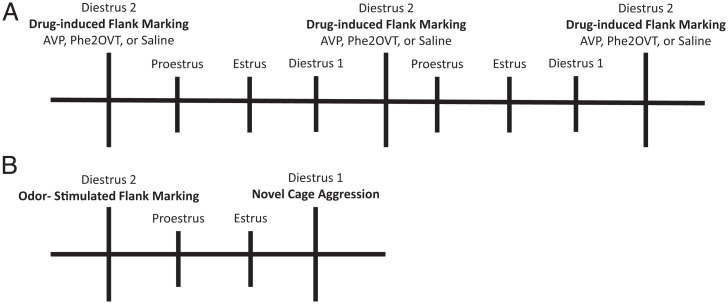
Immediately prior to testing, subjects were infused with either AVP (Sigma; 0.9 μM), the selective Avpr1a agonist Phe2OVT (a gift from Maurice Manning, The University of Toledo College of Medicine and Life Sciences, Toledo, OH; 0.27 μM), or saline, each at a volume of 1,000 nL. They were then placed in a clean cage and observed for 10 min. Four days (one estrus cycle) elapsed between drug treatments. The estrus cycle was monitored daily in F hamsters by the presence and quality of vaginal discharge. M hamsters were yoked to F hamsters and were handled daily and tested on the same days as F hamsters. Behavioral observations in all experiments occurred in the first 3 h of the dark phase.
Odor-stimulated flank marking.
Subjects were exposed for 10 min to a cage that had been flank marked by a novel, same-sex conspecific 40 to 50 times (Fig. 6B). Flank marks were scored when the subject pressed its flank glands against the walls of the cage and moved forward. In another experiment, using a between-subject design, M and F KO and WT hamsters were exposed to a clean cage or an odor-marked cage. Testing occurred on the second day of diestrus in F hamsters.
Fig. 6.
Schematic of experimental timelines for drug-induced flank marking (A) and aggression and odor-stimulated flank marking (B). M hamsters were yoked to F hamsters and tested on the same days.
Neutral arena aggression.
A neutral arena was used for testing aggression so that the data would be comparable to a previous study in Avpr1a KO M mice (14). Three days after testing for odor-stimulated flank marking (Fig. 6B), subjects were placed in a clean cage with a smaller, group-housed, same-sex conspecific, observed for 5 min, and scored for aggressive behavior (total duration: chasing, biting, and pinning).
Data Analysis.
Avpr1a and Oxtr binding.
We tested the hypothesis that genomic disruption of the Avpr1a gene results in a lack of specific Avpr1a binding in the brain in M and F hamsters (Avpr1a KO: 3 M, 3 F; heterozygote: 3 M, 3 F; and WT: 3 M, 2 F). Binding densities for Avpr1a and Oxtr were analyzed by using 2 (sex) × 3 (genotype) ANOVAs. Appropriate Fisher’s least significant difference (LSD) post hoc tests were done to probe significant main effects and interactions.
Systolic blood pressure.
We examined whether Avpr1as in the periphery are responsible for pressor responses to vasopressin in M and F hamsters (WT: n = 7 M, 7 F; and KO: n = 6 M, 5 F). Systolic blood pressure following saline and Phe2OVT treatment was analyzed by using a 2 (sex) × 2 (genotype) × 2 (treatment) × 10 (measurement) mixed ANOVA. Appropriate Fisher’s LSD post hoc tests were done to probe significant main effects and interactions.
AVP-stimulated flank marking.
We tested the hypothesis that, in the absence of Avpr1a, AVP does not induce flank marking in hamsters. KO (n = 5 M, 4 F) and WT (n = 5 M, 4 F) hamsters were exposed to the drug-induced flank-marking procedures described above after ICV treatment with AVP, Phe2OVT, or saline. Flank marking was analyzed using 2 (sex) × 2 (genotype) × 3 (treatment) mixed ANOVAs. Appropriate Fisher’s LSD post hoc tests were done to probe significant main effects and interactions.
Odor-stimulated flank marking and same-sex aggression.
We tested the hypothesis that Avpr1a is required for the expression of flank marking in KO M and F hamsters and that activation of Avpr1a enhances the expression of aggression in M hamsters. WT (n = 6 M, 5 F), heterozygote (n = 6 M, 15 F), and KO (n = 8 M, 7 F) M and F hamsters were exposed to the odor-stimulated flank marking (WT = 6 M, 5 F; heterozygote = 6 M, 15 F; KO = 8 M, 7 F) and neutral arena aggression (WT = 5 M, 6 F; heterozygote = 6 M, 15 F; KO = 8 M, 7 F) procedures described above. Flank marking and aggression were analyzed by using 2 (sex) × 3 (genotype) mixed ANOVAs. Appropriate Fisher’s LSD post hoc tests were done to probe significant main effects and interactions.
Acknowledgments
This work was supported by NSF Grant IOS-1035960 (to H.E.A.) and NIH Grants MH109302 and MH122622 (to K.L.H. and H.E.A.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2121037119/-/DCSupplemental.
Data Availability
All study data and Statistical Package for the Social Sciences analysis code are included in the article and/or supporting information.
References
- 1.Albers H. E., The regulation of social recognition, social communication and aggression: Vasopressin in the social behavior neural network. Horm. Behav. 61, 283–292 (2012). [DOI] [PubMed] [Google Scholar]
- 2.Goodson J. L., The vertebrate social behavior network: Evolutionary themes and variations. Horm. Behav. 48, 11–22 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newman S. W., The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavior network. Ann. N. Y. Acad. Sci. 877, 242–257 (1999). [DOI] [PubMed] [Google Scholar]
- 4.O’Connell L. A., Hofmann H. A., Genes, hormones, and circuits: An integrative approach to study the evolution of social behavior. Front. Neuroendocrinol. 32, 320–335 (2011). [DOI] [PubMed] [Google Scholar]
- 5.Albers H. E., Species, sex and individual differences in the vasotocin/vasopressin system: Relationship to neurochemical signaling in the social behavior neural network. Front. Neuroendocrinol. 36, 49–71 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grieb Z. A., et al. , Sex-dependent effects of social status on the regulation of arginine-vasopressin (AVP) V1a, oxytocin (OT), and serotonin (5-HT) 1A receptor binding and aggression in Syrian hamsters (Mesocricetus auratus). Horm. Behav. 127, 104878 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ross A. P., et al. , Sex-dependent effects of social isolation on the regulation of arginine-vasopressin (AVP) V1a, oxytocin (OT) and serotonin (5HT) 1a receptor binding and aggression. Horm. Behav. 116, 104578 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DiBenedictis B. T., Nussbaum E. R., Cheung H. K., Veenema A. H., Quantitative mapping reveals age and sex differences in vasopressin, but not oxytocin, immunoreactivity in the rat social behavior neural network. J. Comp. Neurol. 525, 2549–2570 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferris C. F., Albers H. E., Wesolowski S. M., Goldman B. D., Luman S. E., Vasopressin injected into the hypothalamus triggers a stereotypic behavior in golden hamsters. Science 224, 521–523 (1984). [DOI] [PubMed] [Google Scholar]
- 10.Bester-Meredith J. K., Martin P. A., Marler C. A., Manipulations of vasopressin alter aggression differently across testing conditions in monogamous and non-monogamous Peromyscus mice. Aggress. Behav. 31, 189–199 (2005). [Google Scholar]
- 11.Caldwell H. K., Lee H.-J., Macbeth A. H., Young W. S. III, Vasopressin: Behavioral roles of an “original” neuropeptide. Prog. Neurobiol. 84, 1–24 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gobrogge K. L., Liu Y., Jia X., Wang Z., Anterior hypothalamic neural activation and neurochemical associations with aggression in pair-bonded male prairie voles. J. Comp. Neurol. 502, 1109–1122 (2007). [DOI] [PubMed] [Google Scholar]
- 13.Terranova J. I., Ferris C. F., Albers H. E., Sex differences in the regulation of offensive aggression and dominance by arginine-vasopressin. Front. Endocrinol. (Lausanne) 8, 308 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wersinger S. R., et al. , Vasopressin 1a receptor knockout mice have a subtle olfactory deficit but normal aggression. Genes Brain Behav. 6, 540–551 (2007). [DOI] [PubMed] [Google Scholar]
- 15.McCann K. E., Sinkiewicz D. M., Rosenhauer A. M., Beach L. Q., Huhman K. L., Transcriptomic analysis reveals sex-dependent expression patterns in the basolateral amygdala of dominant and subordinate animals after acute social conflict. Mol. Neurobiol. 56, 3768–3779 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCann K. E., Sinkiewicz D. M., Norvelle A., Huhman K. L., De novo assembly, annotation, and characterization of the whole brain transcriptome of male and female Syrian hamsters. Sci. Rep. 7, 40472 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aoyagi T., Koshimizu T. A., Tanoue A., Vasopressin regulation of blood pressure and volume: Findings from V1a receptor-deficient mice. Kidney Int. 76, 1035–1039 (2009). [DOI] [PubMed] [Google Scholar]
- 18.Koshimizu T. A., et al. , V1a vasopressin receptors maintain normal blood pressure by regulating circulating blood volume and baroreflex sensitivity. Proc. Natl. Acad. Sci. U.S.A. 103, 7807–7812 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferris C. F., Axelson J. F., Shinto L. H., Albers H. E., Scent marking and the maintenance of dominant/subordinate status in male golden hamsters. Physiol. Behav. 40, 661–664 (1987). [DOI] [PubMed] [Google Scholar]
- 20.Huck U. W., Lisk R. D., Gore A. C., Scent marking and mate choice in the golden hamster. Physiol. Behav. 35, 389–393 (1985). [DOI] [PubMed] [Google Scholar]
- 21.Albers H. E., Pollock J., Simmons W. H., Ferris C. F., A V1-like receptor mediates vasopressin-induced flank marking behavior in hamster hypothalamus. J. Neurosci. 6, 2085–2089 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferris C. F., Singer E. A., Meenan D. M., Albers H. E., Inhibition of vasopressin-stimulated flank marking behavior by V1-receptor antagonists. Eur. J. Pharmacol. 154, 153–159 (1988). [DOI] [PubMed] [Google Scholar]
- 23.Albers H. E., Huhman K. L., Meisel R. L., “Hormonal basis of social conflict and communication” in Hormones, Brain and Behavior, Pfaff D. W., Arnold A. P., Fahrbach S. E., Etgen A. M., Rubin R. T., Eds. (Academic Press, New York, 2002), pp. 393–433. [Google Scholar]
- 24.Ferris C. F., et al. , Vasopressin/serotonin interactions in the anterior hypothalamus control aggressive behavior in golden hamsters. J. Neurosci. 17, 4331–4340 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gutzler S. J., Karom M., Erwin W. D., Albers H. E., Arginine-vasopressin and the regulation of aggression in female Syrian hamsters (Mesocricetus auratus). Eur. J. Neurosci. 31, 1655–1663 (2010). [DOI] [PubMed] [Google Scholar]
- 26.Terranova J. I., et al. , Serotonin and arginine-vasopressin mediate sex differences in the regulation of dominance and aggression by the social brain. Proc. Natl. Acad. Sci. U.S.A. 113, 13233–13238 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horie K., et al. , Oxytocin receptor knockout prairie voles generated by CRISPR/Cas9 editing show reduced preference for social novelty and exaggerated repetitive behaviors. Horm. Behav. 111, 60–69 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fan Z., et al. , Efficient gene targeting in golden Syrian hamsters by the CRISPR/Cas9 system. PLoS One 9, e109755 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mehravar M., Shirazi A., Nazari M., Banan M., Mosaicism in CRISPR/Cas9-mediated genome editing. Dev. Biol. 445, 156–162 (2019). [DOI] [PubMed] [Google Scholar]
- 30.Yen S.-T., et al. , Somatic mosaicism and allele complexity induced by CRISPR/Cas9 RNA injections in mouse zygotes. Dev. Biol. 393, 3–9 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnston R. E., Scent marking by male golden hamsters (Mesocricetus auratus). II. The role of the flank gland scent in the causation of marking. Z. Tierpsychol. 37, 138–144 (1975). [PubMed] [Google Scholar]
- 32.Caldwell H. K., Albers H. E., Photoperiodic regulation of vasopressin receptor binding in female Syrian hamsters. Brain Res. 1002, 136–141 (2004). [DOI] [PubMed] [Google Scholar]
- 33.Caldwell H. K., Albers H. E., Short-photoperiod exposure reduces vasopressin (V1a) receptor binding but not arginine-vasopressin-induced flank marking in male Syrian hamsters. J. Neuroendocrinol. 15, 971–977 (2003). [DOI] [PubMed] [Google Scholar]
- 34.Gutzler S. J., Karom M., Erwin W. D., Albers H. E., Seasonal regulation of social communication by photoperiod and testosterone: Effects of arginine-vasopressin, serotonin and galanin in the medial preoptic area-anterior hypothalamus. Behav. Brain Res. 216, 214–219 (2011). [DOI] [PubMed] [Google Scholar]
- 35.Badura L. L., Nunez A. A., Photoperiodic modulation of sexual and aggressive behavior in female golden hamsters (Mesocricetus auratus): Role of the pineal gland. Horm. Behav. 23, 27–42 (1989). [DOI] [PubMed] [Google Scholar]
- 36.Garrett J. W., Campbell C. S., Changes in social behavior of the male golden hamster accompanying photoperiodic changes in reproduction. Horm. Behav. 14, 303–318 (1980). [DOI] [PubMed] [Google Scholar]
- 37.Caldwell H. K., Albers H. E., Effect of photoperiod on vasopressin-induced aggression in Syrian hamsters. Horm. Behav. 46, 444–449 (2004). [DOI] [PubMed] [Google Scholar]
- 38.Bartness T. J., Goldman B. D., Mammalian pineal melatonin: A clock for all seasons. Experientia 45, 939–945 (1989). [DOI] [PubMed] [Google Scholar]
- 39.Demas G. E., Polacek K. M., Durazzo A., Jasnow A. M., Adrenal hormones mediate melatonin-induced increases in aggression in male Siberian hamsters (Phodopus sungorus). Horm. Behav. 46, 582–591 (2004). [DOI] [PubMed] [Google Scholar]
- 40.Jasnow A. M., Huhman K. L., Bartness T. J., Demas G. E., Short days and exogenous melatonin increase aggression of male Syrian hamsters (Mesocricetus auratus). Horm. Behav. 42, 13–20 (2002). [DOI] [PubMed] [Google Scholar]
- 41.Munley K. M., Deyoe J. E., Ren C. C., Demas G. E., Melatonin mediates seasonal transitions in aggressive behavior and circulating androgen profiles in male Siberian hamsters. Horm. Behav. 117, 104608 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rendon N. M., Rudolph L. M., Sengelaub D. R., Demas G. E., The agonistic adrenal: Melatonin elicits female aggression via regulation of adrenal androgens. Proc. Biol. Sci. 282, 20152080 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnston R. E., Vomeronasal and/or olfactory mediation of ultrasonic calling and scent marking by female golden hamsters. Physiol. Behav. 51, 437–448 (1992). [DOI] [PubMed] [Google Scholar]
- 44.Egashira N., et al. , Impaired social interaction and reduced anxiety-related behavior in vasopressin V1a receptor knockout mice. Behav. Brain Res. 178, 123–127 (2007). [DOI] [PubMed] [Google Scholar]
- 45.Gingrich J. A., Hen R., The broken mouse: The role of development, plasticity and environment in the interpretation of phenotypic changes in knockout mice. Curr. Opin. Neurobiol. 10, 146–152 (2000). [DOI] [PubMed] [Google Scholar]
- 46.Manning M., et al. , Oxytocin and vasopressin agonists and antagonists as research tools and potential therapeutics. J. Neuroendocrinol. 24, 609–628 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Song Z., Albers H. E., Cross-talk among oxytocin and arginine-vasopressin receptors: Relevance for basic and clinical studies of the brain and periphery. Front. Neuroendocrinol. 51, 14–24 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kasahara Y., et al. , Oxytocin receptor in the hypothalamus is sufficient to rescue normal thermoregulatory function in male oxytocin receptor knockout mice. Endocrinology 154, 4305–4315 (2013). [DOI] [PubMed] [Google Scholar]
- 49.National Research Council, Guide for the Care and Use of Laboratory Animals (National Academies Press, Washington, DC, ed. 8, 2011). [Google Scholar]
- 50.Montague T. G., Cruz J. M., Gagnon J. A., Church G. M., Valen E., CHOPCHOP: A CRISPR/Cas9 and TALEN web tool for genome editing. Nucleic Acids Res. 42, W401–W407 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cong L., et al. , Multiplex genome engineering using CRISPR/Cas systems. Science 339, 819–823 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cong L., Zhang F., Genome engineering using CRISPR-Cas9 system. Methods Mol. Biol. 1239, 197–217 (2015). [DOI] [PubMed] [Google Scholar]
- 53.Fan Z., Meng Q., Bunch T. D., White K. L., Wang Z., Effective cryopreservation of golden Syrian hamster embryos by open pulled straw vitrification. Lab. Anim. 50, 45–53 (2016). [DOI] [PubMed] [Google Scholar]
- 54.Haigo K., Yamauchi Y., Yazama F., Yanagimachi R., Horiuchi T., Full-term development of hamster embryos produced by injection of round spermatids into oocytes. Biol. Reprod. 71, 194–198 (2004). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All study data and Statistical Package for the Social Sciences analysis code are included in the article and/or supporting information.