Significance
The increase in multidrug-resistant bacteria highlights the urgent need for compounds with novel target sites that can be developed as antibiotics. The argyrins represent a family of naturally produced octapeptides that display promising activity against Pseudomonas aeruginosa by inhibiting protein synthesis. Our structural and kinetic analyses reveal that argyrins inhibit protein synthesis by interacting with, and trapping, the translation elongation factor G (EF-G) on the ribosome, analogous to that reported previously for the unrelated antibiotic fusidic acid. However, the binding site of argyrin on EF-G is distinct from that of fusidic acid, indicating that intramolecular movements at the domain III/V interface of EF-G are also essential for facilitating late events in the translocation mechanism.
Keywords: antibiotic, argyrin, fusidic acid, ribosome, translocation
Abstract
Argyrins are a family of naturally produced octapeptides that display promising antimicrobial activity against Pseudomonas aeruginosa. Argyrin B (ArgB) has been shown to interact with an elongated form of the translation elongation factor G (EF-G), leading to the suggestion that argyrins inhibit protein synthesis by interfering with EF-G binding to the ribosome. Here, using a combination of cryo-electron microscopy (cryo-EM) and single-molecule fluorescence resonance energy transfer (smFRET), we demonstrate that rather than interfering with ribosome binding, ArgB rapidly and specifically binds EF-G on the ribosome to inhibit intermediate steps of the translocation mechanism. Our data support that ArgB inhibits conformational changes within EF-G after GTP hydrolysis required for translocation and factor dissociation, analogous to the mechanism of fusidic acid, a chemically distinct antibiotic that binds a different region of EF-G. These findings shed light on the mechanism of action of the argyrin-class antibiotics on protein synthesis as well as the nature and importance of rate-limiting, intramolecular conformational events within the EF-G-bound ribosome during late-steps of translocation.
The steady increase of antimicrobial resistance (AMR) represents a global threat (1). The identification of new compounds with novel binding sites to circumvent cross-resistance is a central aspect in combating AMR. To address AMR, previously discovered compounds are being “dusted-off” and revisited (2). Such an example are argyrins, a family of cyclic octapeptides that were first isolated some 25 y ago from Actinoplanes sp. (3). Subsequently, argyrins were shown to be produced by myxobacterial species, including Archangium gephyra (4) and more recently Cystobacter sp. SBCb004 (5). Argyrin A (ArgA) and argyrin B (ArgB) consist of glycine (Gly), sarcosine (Sarc), dehydroalanine (Dha), both an unmodified tryptophan (Trp1) and a 4-methoxy-tryptophan (Trp2), a thiazole ring (Thiaz), and either D-alanine (Ala) or D-aminobutyrate (Abu), respectively (Fig. 1A) (6, 7). Argyrins C to H (ArgC-H) have additional alterations at three further positions (Fig. 1A) (7).
Fig. 1.
Argyrins inhibit translation by trapping EF-G on the ribosome. (A) Chemical structure of argyrins A-H. (B) Relative luminescence from in vitro translation of a firefly luciferase (Fluc) reporter in absence and presence of increasing concentrations (0–50 µM) of FA and ArgA-D. Reactions performed in the absence of antibiotic were defined as 100% luminescence and all error bars represent the SD from the mean for three independent experiments. (C) SDS-PAGE analysis of cosedimentation assays monitoring EF-G binding to E. coli 70S ribosomes, in the presence of GTP (lanes 1–3), GTP + FA (lanes 4–6), and GTP + ArgB (lanes 7–9). Reactions (R) were centrifuged through a sucrose cushion and then separated into supernatant (SN) and pellet (P) fractions. The position of EF-G and molecular weight markers is indicated.
Total chemical synthesis has been accomplished for ArgA, ArgB, ArgE, and ArgF (8–10), allowing research into modifications to improve tolerability, increase specificity, and monitor pharmacokinetics (11–13), thereby making the argyrins an attractive family of compounds for further antibiotic development. Additionally, argyrins display promising activity against Pseudomonas aeruginosa (3, 14–17), with ArgB at the forefront (4). This is of particular interest since carbapenem-resistant P. aeruginosa is considered a pathogen with critical priority by the World Health Organization. Moreover, the cytotoxicity of argyrins is relatively low, as less than 5% of tested cell lines showed sensitivity at concentrations lower than 10 µM (16). The mechanism of antimicrobial action of argyrins was first assessed using a hyperpermeable Salmonella strain revealing that protein synthesis was rapidly inhibited upon drug addition, while RNA and DNA synthesis remained unaffected (3). In vitro, argyrins were shown to inhibit Escherichia coli poly(U)-dependent poly(Phe) synthesis (3), an observation later substantiated by the selection of resistance mutations in P. aeruginosa identifying translation elongation factor G (EF-G) as the physiological target (16–18).
EF-G is a ribosome-associated GTPase that catalyzes forward progression, or translocation, of the messenger RNA (mRNA) and transfer RNA (tRNA) through the intersubunit space of the ribosome during the elongation phase of protein synthesis. During translocation, the mRNA, which is situated between the small subunit body and head domains, slides forward by one codon while the peptidyl- and deacyl-tRNAs shift from the aminoacyl (A) and peptidyl (P) sites to the P and the exit (E) sites, respectively (19). Translocation begins spontaneously after transfer of the nascent peptide chain from the P- to the A-site tRNA and its consequent extension by one amino acid. In this pretranslocation ribosome complex (PRE), the tRNA CCA′ ends oscillate between classical A/A and P/P sites and so-called hybrid A/P (A site on the small subunit and P site on the large subunit) and P/E (P site on the small subunit and E site on the large subunit) states. The adoption of hybrid-state tRNA positions is loosely coupled to rotation of the ribosomal subunits (20) and such rotated, hybrid-state ribosome conformations are preferentially recognized by EF-G(GTP) (20, 21). Directional tRNA and mRNA translocation is dramatically accelerated by EF-G-catalyzed GTP hydrolysis, coupled with large-scale conformational changes in the ribosome (21–29). Such changes occur both within and between the large and small subunits, most notably an intramolecular process that entails reversal of small subunit body domain rotation and swivel of the small subunit head domain in the direction of translocation (21–29).
ArgB was reported to bind directly to EF-G from P. aeruginosa (EF-G1) with the binding site located between domains III and V (16). The current model for ArgB action on EF-G proposes that argyrins interact tightly with free EF-G and by stabilizing an elongated conformation, prevent EF-G from binding to the ribosome (16). The only other antibiotic known to bind directly to EF-G is fusidic acid (FA), which occupies a distinct site between domains I and III that is vacated by the released inorganic phosphate (Pi) and the switch-I element after GTP hydrolysis (21, 25, 29–32). FA binding at this site traps EF-G(GDP) on the ribosome in a late, intermediate state (INT2), which is rate-determining to complete translocation (21, 25, 29, 31, 32). In this trapped state, the small subunit body has begun the process of reverse rotation, while the small subunit head domain remains highly swiveled and tethered to the tRNAs and mRNA, carrying them forward toward their posttranslocation (POST) positions (23). These unexpected findings collectively support that posthydrolysis conformational changes within EF-G are required to convert the EF-G-bound ribosome into the POST translocation state (20–22, 24).
In this study, we present two cryo-electron microscopy (cryo-EM) structures of E. coli EF-G-ArgB-ribosome complexes and complementary single-molecule fluorescence resonance energy transfer (smFRET) studies to demonstrate that ArgB does not prevent EF-G from binding to the ribosome, as suggested previously (16). Rather, ArgB binds specifically to EF-G on the ribosome to trap a late, intermediate state of the translocation process via a binding pocket that is functionally linked but physically distinct from FA. Analysis of the EF-G-ArgB-ribosome complexes suggests that, like FA, ArgB also allows EF-G to hydrolyze GTP and release Pi, trapping the ribosome within the conformationally dynamic, INT2 translocation intermediate whose passage is rate limiting to the final steps of translocation that form the POST state. These data highlight the nature and critical importance of conformational changes within EF-G while bound to the ribosome to facilitate mRNA and tRNA movement on the ribosome.
Results
Translation Inhibition by Argyrins.
Argyrins have been observed previously to inhibit translation using an E. coli in vitro poly(U)-dependent poly(Phe) assay, however, these assays were performed with an impure ArgA-B mixture (3). To more accurately assess the inhibitory activity of individual argyrin family members, we used an E. coli in vitro translation system to monitor expression of a firefly luciferase (Fluc) reporter in the presence of increasing concentrations of ArgA, ArgB, ArgC, ArgD, or the control antibiotic FA (Fig. 1 A and B). Similar to FA, all four argyrins displayed dose-dependent translation inhibition, with IC50 values in the range of 1.2–2.4 µM. However, despite similar IC50 values, higher concentrations (50 µM) of FA were required to completely inhibit translation, whereas complete or near-complete inhibition was observed for argyrins at lower concentrations (5–7.5 µM) (Fig. 1B). Since ArgB and FA interact with EF-G at distinct sites, we also assessed whether there was any synergy in their inhibition using in vitro translation assays (SI Appendix, Fig. S1). From these assays, we conclude that there is no obvious synergy between ArgB and FA, whereas clear synergy was observed for the positive control using the streptogramin A and B combination of virginiamycin M1 and S1 (SI Appendix, Fig. S1).
Next, we directly assessed whether ArgB interferes with the binding of EF-G to the ribosome, as originally proposed (16). To do this, we employed cosedimentation assays to monitor the association of EF-G with the E. coli 70S ribosome in the presence of GTP, and absence or presence of 50 µM ArgB (Fig. 1C). As a positive control, we also performed a binding reaction in the presence of GTP and 50 µM FA. For reactions conducted in the presence of GTP, but absence of antibiotic (R, lane 1), the majority of EF-G was detected in the supernatant (S, lane 2), with only a minor fraction pelleting (P, lane 3) with the ribosomes. By contrast, in the presence of GTP and FA (lanes 4–6), a strong band for EF-G was observed in the pellet fraction that was near stoichiometric with the ribosomal proteins (lane 6), consistent with FA trapping EF-G on the ribosome (21, 29–34). Unexpectedly, we also observed that for reactions performed in the presence of GTP and ArgB (lanes 7–9), EF-G remained strongly associated with the ribosome (lane 8), suggesting that ArgB, like FA, traps EF-G on the ribosome rather than interfering with its binding as previously proposed (16).
Cryo-EM Structure of an E. coli EF-G-ArgB-70S Complex.
To directly visualize EF-G trapped by ArgB on the ribosome, we incubated E. coli EF-G with E. coli 70S ribosomes in the presence of GTP and 50 µM ArgB, analogous to the reactions used for the binding assays (Fig. 1C). The resulting complexes were then applied to cryo-grids and analyzed using single-particle cryo-EM methods. Focused sorting identified a class with well-resolved EF-G and an average resolution 2.9 Å (Fig. 2A and SI Appendix, Fig. S2). While multibody refinement using three separate masks encompassing (1) the small (30S) subunit body/platform domains, (2) the small subunit head domain, and (3) the large (50S) subunit and EF-G led to only slight numerical improvements in the average resolution, improvements were evident in the local resolution, with the large subunit core reaching ∼2.6 Å (SI Appendix, Fig. S2). EF-G exhibited some flexibility with respect to the ribosomal subunits, with local resolution ranging between 3.3–4.5 Å for the majority of the factor, allowing for unambiguous domain assignment (Fig. 2B and SI Appendix, Fig. S2). Additional density was evident between domain III and V of EF-G, which was assigned to ArgB (Fig. 2B), analogous to the binding site observed in the crystal-structure of P. aeruginosa EF-G1-ArgB complex (Fig. 2C) (16), providing direct evidence that EF-G-ArgB is able to engage the ribosome.
Fig. 2.
Cryo-EM structure reveals the conformation of EF-G-ArgB on the ribosome. (A) Cryo-EM map of the 70S ribosome with isolated densities for small (30S) subunit body (light blue), 30S head (light purple), large (50S) subunit (gray), EF-G (red), argyrin B (ArgB; purple), and E-site tRNA (orange), threshold σ = 2. (B) Cryo-EM density with molecular model of EF-G (colored by domain) and ArgB (purple), threshold σ = 2. (C) Alignment of EF-G from the EF-G-ArgB-70S complex (colored as in B) to P. aeruginosa free EF-G bound to ArgB (PDB ID 4FN5, gray) on the basis of either the ArgB binding site (Top) or the G domain (Bottom) (16). (D–F) Alignment of the G domain of EF-G from the EF-G-ArgB-70S complex (colored as in B–D) T. thermophilus EF-G(GDP) in solution (PDB ID: 2BM0, gray) (38), (E) ribosome-bound E. coli EF-G(GDPCP) (PDB ID: 4V9O, gray) (39), and (F) translocation intermediate (INT2) E. coli EF-G(GDP)-fusidic acid (FA, PDB ID: 7N2C, gray) (29).
EF-G-GDP-ArgB Adopts a GTP-Like Conformation on the Ribosome.
The original basis for proposing that ArgB blocks binding of EF-G to the ribosome was because the conformation of EF-G in the crystal structure of P. aeruginosa EF-G1-ArgB complex was elongated and incompatible with ribosome binding (16). By contrast, we observe an EF-G conformation in the E. coli EF-G-ArgB-70S complex, in which domains III–V are shifted by 9–31 Å (G-domain alignment) relative to the P. aeruginosa EF-G1-ArgB complex (Fig. 2C). The conformation of EF-G in the E. coli EF-G-ArgB-70S complex is also differentiated from that observed for GDP-bound T. thermophilus EF-G in isolation (35–38) (Fig. 2D). We note in this context that aside from the switch-I region, the conformation of EF-G in the presence of ArgB is indistinguishable (all-atom root-mean-square deviation of 0.8 Å–1.6 Å, respectively) from the ribosome-bound conformations of EF-G observed in the presence of the nonhydrolysable GTP analog GDPCP (39, 40) (Fig. 2E) or in the presence of FA (29–31) (Fig. 2F). This observation suggests that ArgB either prevents EF-G from hydrolyzing GTP to GDP or, like FA, it allows GTP hydrolysis and switch-I remodeling but prevents downstream conformational changes required for completion of the translocation mechanism and factor dissociation. To distinguish between these two modes of action, we carefully analyzed the nucleotide binding site of domain I (G domain) of EF-G within the E. coli EF-G-ArgB-70S complex structure. The observed nucleotide density was compatible with GDP (SI Appendix, Fig. S3A), as observed in the nucleotide-binding site of EF-G stalled on the ribosome by FA (29–31) as well as to unbound EF-G-GDP (35–38). The switch-I region (aa38–aa64) was disordered, indicative of a posthydrolysis GDP state (SI Appendix, Fig. S3B) (35–38). Alignment of EF-G complexed with the nonhydrolysable GTP analog GDPCP (39, 40) further suggested that density for the nucleotide γ-phosphate was absent (SI Appendix, Fig. S3C). We therefore conclude that ArgB traps EF-G on the ribosome after GTP hydrolysis and Pi release, analogous to the mechanism described for FA (21, 25, 29, 30, 33).
Argyrin B Stalls the Elongating Ribosome in an Intermediate State of Translocation.
The shared mechanism of stabilizing EF-G on the ribosome, and the similar EF-G conformations stalled by ArgB and FA, led us to ask whether ArgB, like FA, is able to trap intermediate states along the translocation reaction pathway (21, 25, 26, 31, 32). We therefore performed presteady-state smFRET imaging of translocation in the presence of either ArgB or FA, wherein a donor fluorophore (LD550) was placed on the N terminus of ribosomal protein uS13 and an acceptor fluorophore (LD650) was placed on ribosomal protein uL1 (21). This FRET perspective reports on the PRE state, the rate-limiting, late intermediate state (INT2) of translocation trapped by FA (21, 29) and the POST state (Fig. 3A). When EF-G(GTP) was delivered to pretranslocation ribosomes in the absence of drug, we observed rapid progression from the PRE state, through the FA-sensitive INT2 intermediate, to the POST state, as expected (Fig. 3B and SI Appendix, Fig. S4A). By contrast, when EF-G(GTP) was delivered together with either 10 µM ArgB or 10 µM FA (Fig. 3 C and D and SI Appendix, Fig. S4A), we observed prolonged stalls during late steps of translocation. The FRET efficiency of ArgB- and FA-stalled states were indistinguishable (apo 0.47 ± 0.11, ArgB 0.47 ± 0.09, FA 0.49 ± 0.08) (Fig. 3 C and D), suggesting that the two drugs block translocating ribosomes within the intrinsically dynamic INT2 intermediate state (21). By reducing the imaging time resolution and extending the imaging duration, we found that both ArgB- and FA-treated ribosomes eventually reached an EF-G-bound drug-stalled POST state after prolonged stalling (SI Appendix, Fig. S4A). It has been suggested that ArgB, unlike FA, forms a tight complex with EF-G in solution (16). We therefore carried out analogous experiments where EF-G was supplied to PRE ribosomes in fivefold excess over ArgB (SI Appendix, Fig. S4B). Under such conditions, we observed that, similar to when supplying ArgB in excess over EF-G, all ribosomes were inhibited. This finding indicates that rather than forming a tight complex with EF-G in solution, ArgB—like FA—preferentially binds with high affinity to EF-G when it is bound to the ribosome.
Fig. 3.
Argyrin B stalls translating ribosomes in a state highly similar to that stalled by the unrelated antibiotic fusidic acid. (A) Schematic of the long-lived states observed during the smFRET experiments between donor-labeled uS13 (green circle) and acceptor-labeled uL1 (red circle). Before delivery of EF-G, the pretranslocation ribosome occupies an ensemble of interconverting hybrid states with FRET efficiencies around 0.65 (Left). Delivery of EF-G into the flow cell is rapidly followed by factor binding (no observable change in FRET efficiency), followed by conversion of the ribosome to an intermediate state through back-rotation of the small subunit (30S) body (white arrow) and swivel of the small subunit head (purple arrow), lowering the FRET efficiency to around 0.46 (Middle). In the absence of drugs, this state is rapidly resolved to the POST state through back-swivel of the head domain and dissociation of EF-G-GDP, leading to a further reduction in the FRET efficiency to 0.25 (Right). (B–D) Population FRET histogram showing the time-evolution of the FRET signal at 40 ms time-resolution after delivery of EF-G to the microscope flow cell and FRET histogram with fits of Gaussian functions to estimate FRET efficiencies of the prevalent states, performed in (B) the absence of drugs, or the presence of (C) 10 µM argyrin B (ArgB) or (D) 10 µM fusidic acid (FA). N represents the number of observed molecules. ArgB and FA display similar behavior, both causing a prolonged stall with a FRET efficiency of 0.46, similar to the uninhibited intermediate state. The solid red lines are fits of Gaussian functions to the data, the solid black line is the sum of the three fitted functions. Error bars represent SEM.
Capture of an ArgB-EF-G-Ribosome Translocation Intermediate Structure.
To investigate the physiological mechanism of action of ArgB, we sought to visualize the translocation intermediate stalled in our smFRET experiments by performing presteady-state cryo-EM measurements, as recently described (29). Employing the same conditions and reagents used for smFRET, we reconstituted elongating ribosome complexes containing tRNAfMet (deacyl-tRNA) in the P site and fMet-Phe-tRNAPhe (peptidyl-tRNA) in the A site. In the presence of 10 µM ArgB, translocation reactions were initiated by addition of EF-G(GTP) before rapid transfer (within 20 s) to cryo-EM grids. Following focused classification for the presence of EF-G, we identified a class containing EF-G and two tRNAs in intermediate states of translocation that were refined to an average resolution of 2.6 Å (Fig. 4A and SI Appendix, Fig. S5).
Fig. 4.
Argyrin B interacts with EF-G similarly during translocation as in solution. (A) Cryo-EM density map of the EF-G-ArgB-translocation intermediate (INT2) structure colored by small (30S) subunit body (light blue), 30S head (light purple), large (50S) subunit (gray), peptidyl-tRNA (green), deacyl-tRNA (orange), EF-G (red), and argyrin B (ArgB; purple). Threshold σ = 4. (B) Global alignment of EF-G from the EF-G-ArgB-INT2 structure (colored by domain) from the EF-G in the EF-G-ArgB-70S complex (gray). Cryo-EM density and molecular model of ArgB binding pocket from the EF-G-ArgB-INT2 structure (Inlay). Threshold σ = 3.5.
All five domains of EF-G were well resolved, with clear cryo-EM density for ArgB in the junction between EF-G domains III and V (Fig. 4B), as observed previously in a crystal structure of EF-G with ArgB (16) and in the ArgB-EF-G-70S structure (Fig. 2B and SI Appendix, Fig. S6 A and B). The unmodified Trp1 moiety of ArgB formed van der Waals interactions with Met617, Leu660, and Met682 within domain V of EF-G and the backbone amide of Ala489 of EF-G was positioned within hydrogen bonding distance to the Sarcosine moiety of ArgB (Fig. 4B). In P. aeruginosa EF-G1, mutation of Leu663 (E. coli Leu660) or Met685 (E. coli Met682) to Met/Gln or Arg, respectively, confers resistance to argyrin (16, 17) (SI Appendix, Fig. S7), presumably by steric occlusion. Pro414 of EF-G domain III, which is also commonly mutated in ArgB-resistant strains (17), stacks on the indole of Trp2 of ArgB (Fig. 4B and SI Appendix, Fig. S7). Similarly, Ser417Leu mutations that confer argyrin resistance (16, 17) suggest that hydrogen bonding between Ser417 and the backbone carboxyl linking Trp1 and the thiazole ring of ArgB may be important for binding (Fig. 4B and SI Appendix, Fig. S7). By contrast, Ser459 in P. aeruginosa EF-G1 is unlikely to be critical for drug binding as E. coli EF-G contains Ala at the equivalent position (Fig. 4B and SI Appendix, Fig. S7). Instead, the Ser459Phe mutation likely gives rise to argyrin resistance because the bulky Phe sidechain prevents ArgB binding to EF-G, as reported previously (17). Consistent with the high conservation in the ArgB binding site between P. aeruginosa and E. coli, the interactions of ArgB with E. coli EF-G on the ribosome appear to be similar to those described between ArgB and P. aeruginosa EF-G1 in the absence of the ribosome (16) (SI Appendix, Fig. S6A). Modeling of ArgA, ArgC, and ArgD based on the binding position of ArgB suggests that these argyrins can also interact with EF-G analogously to ArgB (SI Appendix, Fig. S8), consistent with their similar inhibitory activities in vitro (Fig. 1B).
ArgB Stabilizes a Late Translocation Intermediate State.
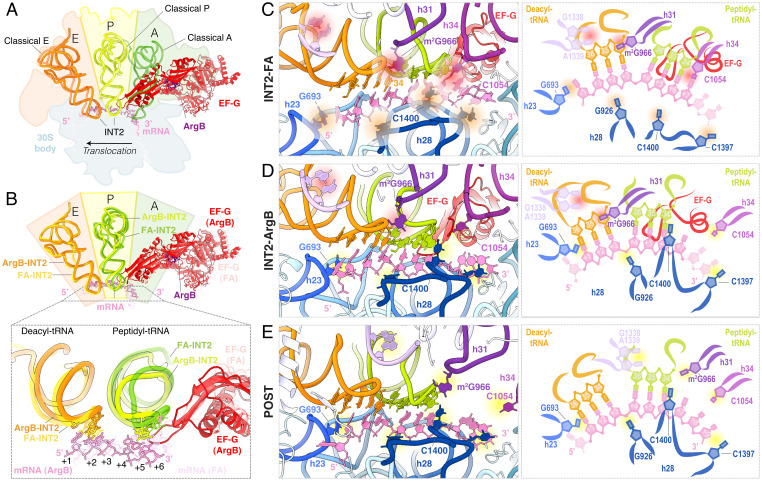
Consistent with our smFRET experiments and structures of the FA-stalled ribosome complex (25, 29), the translocation intermediate structure captured by ArgB contains tRNAs in partially translocated positions between PRE and POST states (Fig. 5 A and B and SI Appendix, Fig. S9) (25, 29). However, in the structure stalled by ArgB, we found that the tRNAs traveled slightly further toward the POST state as compared to the FA-stalled structure (Fig. 5B). This ∼5 Å-progression along the reaction coordinate allowed both the deacyl- and peptidyl-tRNAs to adopt a chimeric intersubunit hybrid position (aP/P, pE/E) (23, 25, 31), as defined by simultaneous contact between PRE-like positions on the small subunit head domain and POST-like contacts between the tRNA anticodons and the small subunit body as well as the 3′-CCA termini and the large subunit (Fig. 5 C–E). These POST-like small subunit body contacts include capture of the deacyl-tRNA anticodon-codon pair by helix 23 in the E site (Fig. 5 C–E). Forward tRNA-mRNA progression beyond that observed in the FA-stalled complex at comparable resolution (29) is also evidenced. This shift includes disengagement of small subunit head domain residue C1054 from peptidyl-tRNA and replacement of this stacking interaction by the C1400 residue of helix 28 in the small subunit body domain. Nonetheless, peptidyl-tRNA remained incompletely translocated as the G966 residue of the small subunit head domain remained associated with deacyl-tRNAfMet. Both shifts in tRNA positions seem to be enabled, at least in part, by a continuation of small subunit body back rotation on the trajectory from rotated PRE to POST (3.9° compared to POST) and an increase in the extent of head domain swivel (18.6° compared to POST; SI Appendix, Fig. S9 A and B). These domain conformations appear further along the translocation trajectory observed in the FA-stalled complex, which displayed 8.6° and 16.1° small subunit body rotation and head domain swivel, respectively (29) (Fig. 5 C–E).
Fig. 5.
Argyrin B inhibits a late intermediate state of tRNA translocation. (A) Overview of the EF-G (red) and tRNA positioning in the EF-G-ArgB-INT2 structure (solid, outlined) with classical tRNA positions from PRE and POST complexes (transparent, PDB ID: 7N1P and 7N31, respectively) (29). tRNAs are colored according to position from the A (green, Right) to P (yellow, Middle) to E (orange, Left) sites. (B) tRNA positioning in the EF-G-ArgB-INT2 structure compared to the late translocation intermediate stalled by fusidic acid (FA, PDB ID: 7N2C, transparent) (29) as viewed by the small subunit (30S) head domain (Top) and from beneath the small subunit (Bottom), colored as in (A). (C–E) Overview of the 30S helical architecture involved in translocation depicted with molecular models (Left) and schematic representation (Right), for (C) an FA-stalled intermediate structure, as in (B), (D) the EF-G-ArgB-INT2 structure, and (E) a POST structure, as in (A). Distances in the schematic are not to scale. Dotted residues display weak cryo-EM map density. Red circles depict contacts from the PRE state, orange circles depict contacts in an intermediate state, and yellow circles depict contacts from the POST state. Green, peptidyl-tRNA; orange, deacyl-tRNA. Camera perspective is identical for all images. Alignment on the large subunit core.
Despite the modest differences in tRNA positioning of the ArgB- compared to the FA-stalled intermediate, the distance between uS13 and uL1 was highly similar (∼70 Å), explaining the similarities observed in our smFRET experiments (Fig. 3 and SI Appendix, Fig. S9C). These findings are consistent with ArgB trapping EF-G(GDP) on the ribosome within a slightly more progressed region of the dynamic INT2 conformational basin, which displays a range of small subunit body rotation and head swivel angles (21, 25, 27–29, 41, 42). Completion of translocation from the EF-G-ArgB-INT2 state would therefore require further large-scale intramolecular conformational changes within both EF-G and the ribosome (21, 29), which both FA and ArgB similarly prevent via their distinct binding sites.
ArgB Stabilizes a GTPase State Similar to FA But Using a Different Mechanism.
Our structural findings raise the question of how ArgB prevents the conformational changes in EF-G that normally allow for completion of translocation. In the GTP-bound conformation, switch I is structured, contacting the rotated small subunit body, the catalytic sarcin–ricin loop (SRL) and the γ-phosphate moiety of GTP, but becomes disordered upon GTP hydrolysis (27–29, 39, 43). EF-G stalled during translocation by ArgB adopted a remarkably similar overall conformation as on the vacant 70S ribosome, as well as in the translocation intermediate stalled by FA (29) (Fig. 6A and SI Appendix, Fig. S10A), with a loss of cryo-EM density for both the nucleotide γ-phosphate and the switch-I loop (Fig. 6B and SI Appendix, Fig. S10 B and C). After GTP hydrolysis, FA directly interacts with EF-G switch II and domain III to stabilize a GTP-like conformation of the switch-II element (29–31). By contrast, in the EF-G-ArgB-ribosome complexes, switch II is stabilized in a GTP-like conformation without a direct interaction with ArgB (Fig. 6B). As switch-II conformational change is canonically observed in the GTP- to GDP-bound transition of GTPases (44), this configuration suggests that ArgB indirectly stabilizes the switch-II region by preventing movement of domain III relative to domain V after GTP hydrolysis. We infer from these findings that the relative movements of domains III and V are critical for destabilizing switch II and that this step is associated with late steps of the translocation mechanism.
Fig. 6.
Argyrin B inhibits conformational changes in EF-G required to complete translocation. (A) Global alignment of EF-G from the EF-G-ArgB-INT2 complex (solid) to EF-G from a late translocation intermediate stalled by fusidic acid (FA, PDB ID: 7N2C, transparent) (29) as viewed from the large (50S) subunit GTPase activating center and sarcin–ricin loop (SRL, dark gray). Cryo-EM density for GDP (gray blue), ArgB (purple), and FA (dark yellow) are shown in mesh representation. EF-G domain III (DIII, pink), DV (orange), switch I (light yellow), and switch II (light green). Incomplete modeling is designated by a dashed line. (B) EF-G overlay from (A), highlighting the intersection between EF-G switch I, switch II, and domains III and V. Colored as in (A). Threshold σ = 3.5. (C) Model for the mechanism of action of argyrin during translocation. Following peptide bond formation, the ribosome is in a PRE state; the tRNAs adopt a P/E and A/P hybrid state, whereas the small subunit (30S) fluctuates between rotated and unrotated states (Left). Binding of EF-G to the PRE ribosome leads to stabilization of the rotated state and facilitates translocation through an intermediate (INT2) state, which is characterized by strong head swivel (purple arrow) and back rotation of the body (white arrow, Middle). After translocation, the tRNAs have moved to the E- and P-sites and the 30S head has reverse-swiveled (Right). EF-G dissociates through conformational changes and is subsequently recycled for a new round of translocation. Argyrin B displays low affinity toward EF-G off the ribosome, but high affinity to EF-G bound to the ribosome. Here, it locks the ribosome in an intermediate translocation state by inhibiting conformational changes in EF-G.
In this context, we note that resistance to ArgB in P. aeruginosa is conferred by a Thr671Ala mutation in domain V of EF-G1 (16, 17) (SI Appendix, Fig. S7A), distal to the ArgB binding site. In the ArgB translocation intermediate complex, the equivalent residue, Thr668, appears to form a hydrogen bond with Glu463 in domain III (SI Appendix, Fig. S7C). We infer that the Thr671Ala mutation may confer resistance to ArgB by weakening the interaction between domains III and V and thereby permitting relative movements of these domains, even in the presence of ArgB. Analogously, mutations in EF-G have been reported to confer FA resistance by allowing conformation changes in EF-G even in the presence of the drug (37).
Discussion
Collectively, the findings presented here enable us to propose a revised model for the mechanism of action of ArgB and to elaborate on the nature of conformational changes within EF-G required for the completion of translocation (Fig. 6C). In contrast to previous suggestions, ArgB does not prevent EF-G binding to the ribosome, but rather traps EF-G in a GTP-like conformation on the ribosome after Pi release (Fig. 6C). Moreover, our findings suggest that ArgB has a lower affinity for EF-G in solution than on the ribosome, and therefore specifically targets the ribosome-bound form of EF-G (Fig. 6C). We speculate that ArgB specificity for EF-G after ribosome binding may arise from a defined conformation in which domains III and V of EF-G(GDP) are optimally arranged for high-affinity ArgB binding.
In the cryo-EM structures presented here, EF-G had hydrolyzed GTP to GDP, yet switch II remained in a GTP-like conformation. Consistent with our presteady-state smFRET studies of translocation, this implies that ArgB stabilizes EF-G on the ribosome within the highly dynamic INT2 conformational basin, in which small subunit head domain swivel and simultaneous body back rotation carry the mRNA and tRNA anticodon stem loops forward along the translocation reaction coordinate. Within the INT2 basin, conformational changes of the EF-G-bound ribosome complex after GTP hydrolysis have been shown to be required to complete translocation (21) (Fig. 6C). This mechanism of action parallels that defined for FA (21, 25, 29, 31, 32).
In the absence of antibiotic, hydrolysis of GTP to GDP likely gives rise to increased flexibility within EF-G that contributes to increased conformational degrees of freedom within the INT2 basin (21). Consequent destabilization of both the switch-I and -II regions of EF-G allows conformational rearrangements required for completing translocation and subsequent EF-G(GDP) dissociation (Fig. 6C). FA inhibits translocation and prevents EF-G(GDP) dissociation by directly interacting with switch II and domain III (30, 31). ArgB appears to trap a similar intermediate state as FA, where the switch-II region of EF-G also remains in a GTP-bound conformation despite complete disordering of the switch-I element. Rather than directly interacting with the switch-II region, as FA does, ArgB provides additional contacts between domain III and V, allosterically blocking switch-II remodeling. The ArgB-bound structures suggest that GTP hydrolysis and Pi release per se are necessary, but not sufficient, to destabilize the switch-II region, and relative movements between domains III and V are required both for switch-II remodeling and completion of translocation. These observations imply that the conformational change within switch-II from its active GTP to GDP conformation contributes to the rate-limiting, intramolecular conformational changes in the EF-G-bound ribosome complex that facilitate the final steps of mRNA and tRNA translocation and EF-G(GDP) dissociation after translocation is complete. Changes in intermolecular interactions between the G domain and domains III and V of EF-G may be analogous to release of aminoacyl-tRNA by EF-Tu during tRNA selection following GTP hydrolysis, supporting a conserved mechanism of translational GTPases on the ribosome (45). Interestingly, the binding site of ArgB on bacterial EF-G is similar to that of the unrelated antifungal Sordarin on the yeast homolog, eEF2 (SI Appendix, Fig. S11) (46–48). Like ArgB, Sordarin binds between domains III and V of eEF2 and has been used to trap eEF2 on the yeast 80S ribosome for structural analysis (49–52). It will be interesting to ascertain whether Sordarin also traps eEF2 on the fungal 80S ribosome through mechanisms similar to FA and ArgB.
Materials and Methods
Translation inhibition efficiency was measured by employing firefly luciferase in an in vitro transcription-translation system, as previously described (53, 54). Complex-formation for the EF-G-ArgB-70S complex was achieved by mixing 0.4 μM 70S, 1 μM EF-G, 500 µM GTP, and 50 μM ArgB followed by data collection using a Titan Krios 300 kV TEM equipped with a Falcon II DED (FEI). Particle images for the EF-G-ArgB-70S complex were aligned with MotionCor2 (55), picked using GAUTOMATCH (https://www.mrc-lmb.cam.ac.uk/kzhang) and processed (including final sharpening and automated b-factor application) using RELION 3.0 (56). Resolution calculations and locally filtered maps were computed using SPHIRE 1.3 (57) for the EF-G-ArgB-70S complex. Complex-formation for the EF-G-ArgB-INT2 complex was achieved by mixing 1 μM 70S initiation complexes bearing fMet-tRNAfMet in the P site, 1 μM ternary complex containing EF-Tu(GTP) and Phe-tRNAPhe, 5 μM EF-G, 1 mM GTP, and 10 μM ArgB followed by data collection using a Titan Krios 300 kV TEM equipped with a K3 direct electron detector and post column GIF (energy filter). Particle images for the EF-G-ArgB-INT2 complex were aligned with MotionCor2 (55), picked and processed using Relion 4.0 (58). Resolution was estimated based on gold-standard criterion (FSC0.143) for all structures. Molecular models were fitted and adjusted by using Coot (59) and refined in Phenix 1.14 (60). Model validation was carried out by using the MolProbity server (61) and the final model statistics are presented in SI Appendix, Table S1. Figures showing atomic models were made using UCSF Chimera X (62) or PyMOL Molecular Graphic Systems (Schrödinger). smFRET data collection was carried out as previously described (21). Further details can be found in the SI Appendix, Material and Methods.
Supplementary Material
Acknowledgments
We acknowledge support from the Cryo-Electron Microscopy and Tomography Center, the High-Performance Computing Center, and the Single-Molecule Center at St. Jude Children’s Research Hospital. We specifically acknowledge guidance from I. Fernandez from the St. Jude Cryo-Electron Microscopy and Tomography Center for his for his guidance, training and technical expertise on cryo-EM methodologies, including grid preparations, data collections to final map generation. We thank S.K. Natchiar, D. Terry, R. Kiselev, and other members of the Blanchard laboratory for their expertise and efforts to enable the molecular model building and single-molecule investigations to be performed. This work was supported by grant funding from the Deutsche Forschungsgemeinschaft (WI3285/6-1 to D.N.W.), Russian Science Foundation (20-74-10031 and 18-44-04005 to I.A.O.), National Institutes of Health (GM079238 to S.C.B. and NIH T32 [GM115327-Tan] to E.J.R.), and the Swedish Research Council (2017-06313 to M.H.).
Footnotes
Competing interest statement: The authors declare a competing interest. S.C.B. holds an equity interest in Lumidyne Technologies.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2114214119/-/DCSupplemental.
Data Availability
Cryo-EM map and models were deposited in the EMDatabank (EF-G-ArgB-70S, EMD-13058; EF-G-ArgB-INT2, EMD-26486) and RCSB Protein Data Bank (EF-G-ArgB-70S, PDB ID: 7OTC; EF-G-ArgB-INT2, PDB ID: 7UG7).
References
- 1.Gandra S., et al. , Antimicrobial resistance surveillance in low- and middle-income countries: Progress and challenges in eight South Asian and Southeast Asian countries. Clin. Microbiol. Rev. 33, e00048-19 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arenz S., Wilson D. N., Blast from the past: Reassessing forgotten translation inhibitors, antibiotic selectivity, and resistance mechanisms to aid drug development. Mol. Cell 61, 3–14 (2016). [DOI] [PubMed] [Google Scholar]
- 3.Selva E., et al. , Antibiotics A21459 A and B, new inhibitors of bacterial protein synthesis. I. Taxonomy, isolation and characterization. J. Antibiot. (Tokyo) 49, 145–149 (1996). [DOI] [PubMed] [Google Scholar]
- 4.Sasse F., et al. , Argyrins, immunosuppressive cyclic peptides from myxobacteria. I. Production, isolation, physico-chemical and biological properties. J. Antibiot. (Tokyo) 55, 543–551 (2002). [DOI] [PubMed] [Google Scholar]
- 5.Pogorevc D., et al. , Biosynthesis and heterologous production of argyrins. ACS Synth. Biol. 8, 1121–1133 (2019). [DOI] [PubMed] [Google Scholar]
- 6.Ferrari P., et al. , Antibiotics A21459 A and B, new inhibitors of bacterial protein synthesis. II. Structure elucidation. J. Antibiot. (Tokyo) 49, 150–154 (1996). [DOI] [PubMed] [Google Scholar]
- 7.Vollbrecht L., et al. , Argyrins, immunosuppressive cyclic peptides from myxobacteria. II. Structure elucidation and stereochemistry. J. Antibiot. (Tokyo) 55, 715–721 (2002). [DOI] [PubMed] [Google Scholar]
- 8.Ley S. V., Priour A., Heusser C., Total synthesis of the cyclic heptapeptide Argyrin B: A new potent inhibitor of T-cell independent antibody formation. Org. Lett. 4, 711–714 (2002). [DOI] [PubMed] [Google Scholar]
- 9.Wu W., Li Z., Zhou G., Jiang S., Total synthesis of argyrins A and E. Tetrahedron Lett. 52, 2488–2491 (2011). [Google Scholar]
- 10.Bülow L., et al. , Synthesis and biological characterization of argyrin F. ChemMedChem 5, 832–836 (2010). [DOI] [PubMed] [Google Scholar]
- 11.Siebert D. C. B., et al. , Chemical synthesis of tripeptide thioesters for the biotechnological incorporation into the myxobacterial secondary metabolite argyrin via mutasynthesis. Beilstein J. Org. Chem. 15, 2922–2929 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stempel E., Kaml R. F., Budisa N., Kalesse M., Painting argyrins blue: Negishi cross-coupling for synthesis of deep-blue tryptophan analogue β-(1-azulenyl)-l alanine and its incorporation into argyrin C. Bioorg. Med. Chem. 26, 5259–5269 (2018). [DOI] [PubMed] [Google Scholar]
- 13.Chen C. H., Genapathy S., Fischer P. M., Chan W. C., A facile approach to tryptophan derivatives for the total synthesis of argyrin analogues. Org. Biomol. Chem. 12, 9764–9768 (2014). [DOI] [PubMed] [Google Scholar]
- 14.Nickeleit I., et al. , Argyrin a reveals a critical role for the tumor suppressor protein p27(kip1) in mediating antitumor activities in response to proteasome inhibition. Cancer Cell 14, 23–35 (2008). [DOI] [PubMed] [Google Scholar]
- 15.Stauch B., et al. , Elucidation of the structure and intermolecular interactions of a reversible cyclic-peptide inhibitor of the proteasome by NMR spectroscopy and molecular modeling. Angew. Chem. Int. Ed. Engl. 49, 3934–3938 (2010). [DOI] [PubMed] [Google Scholar]
- 16.Nyfeler B., et al. , Identification of elongation factor G as the conserved cellular target of argyrin B. PLoS One 7, e42657 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bielecki P., et al. , Mutation in elongation factor G confers resistance to the antibiotic argyrin in the opportunistic pathogen Pseudomonas aeruginosa. ChemBioChem 13, 2339–2345 (2012). [DOI] [PubMed] [Google Scholar]
- 18.Jones A. K., et al. , Determinants of antibacterial spectrum and resistance potential of the elongation factor G inhibitor argyrin B in key gram-negative pathogens. Antimicrob. Agents Chemother. 61, e02400-16 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noller H. F., Lancaster L., Zhou J., Mohan S., The ribosome moves: RNA mechanics and translocation. Nat. Struct. Mol. Biol. 24, 1021–1027 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Munro J. B., Altman R. B., Tung C. S., Sanbonmatsu K. Y., Blanchard S. C., A fast dynamic mode of the EF-G-bound ribosome. EMBO J. 29, 770–781 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wasserman M. R., Alejo J. L., Altman R. B., Blanchard S. C., Multiperspective smFRET reveals rate-determining late intermediates of ribosomal translocation. Nat. Struct. Mol. Biol. 23, 333–341 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pan D., Kirillov S. V., Cooperman B. S., Kinetically competent intermediates in the translocation step of protein synthesis. Mol. Cell 25, 519–529 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ratje A. H., et al. , Head swivel on the ribosome facilitates translocation by means of intra-subunit tRNA hybrid sites. Nature 468, 713–716 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen J., Petrov A., Tsai A., O’Leary S. E., Puglisi J. D., Coordinated conformational and compositional dynamics drive ribosome translocation. Nat. Struct. Mol. Biol. 20, 718–727 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramrath D. J., et al. , Visualization of two transfer RNAs trapped in transit during elongation factor G-mediated translocation. Proc. Natl. Acad. Sci. U.S.A. 110, 20964–20969 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Belardinelli R., et al. , Choreography of molecular movements during ribosome progression along mRNA. Nat. Struct. Mol. Biol. 23, 342–348 (2016). [DOI] [PubMed] [Google Scholar]
- 27.Petrychenko V., et al. , Structural mechanism of GTPase-powered ribosome-tRNA movement. Nat. Commun. 12, 5933 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carbone C. E., et al. , Time-resolved cryo-EM visualizes ribosomal translocation with EF-G and GTP. Nat. Commun. 12, 7236 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rundlet E. J., et al. , Structural basis of early translocation events on the ribosome. Nature 595, 741–745 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao Y. G., et al. , The structure of the ribosome with elongation factor G trapped in the posttranslocational state. Science 326, 694–699 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou J., Lancaster L., Donohue J. P., Noller H. F., Crystal structures of EF-G-ribosome complexes trapped in intermediate states of translocation. Science 340, 1236086 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Borg A., et al. , Fusidic acid targets elongation factor G in several stages of translocation on the bacterial ribosome. J. Biol. Chem. 290, 3440–3454 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bodley J. W., Zieve F. J., Lin L., Zieve S. T., Formation of the ribosome-G factor-GDP complex in the presence of fusidic acid. Biochem. Biophys. Res. Commun. 37, 437–443 (1969). [DOI] [PubMed] [Google Scholar]
- 34.Belardinelli R., Rodnina M. V., Effect of fusidic acid on the kinetics of molecular motions during EF-G-induced translocation on the ribosome. Sci. Rep. 7, 10536 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.al-Karadaghi S., Aevarsson A., Garber M., Zheltonosova J., Liljas A., The structure of elongation factor G in complex with GDP: Conformational flexibility and nucleotide exchange. Structure 4, 555–565 (1996). [DOI] [PubMed] [Google Scholar]
- 36.Czworkowski J., Wang J., Steitz T. A., Moore P. B., The crystal structure of elongation factor G complexed with GDP, at 2.7 A resolution. EMBO J. 13, 3661–3668 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laurberg M., et al. , Structure of a mutant EF-G reveals domain III and possibly the fusidic acid binding site. J. Mol. Biol. 303, 593–603 (2000). [DOI] [PubMed] [Google Scholar]
- 38.Hansson S., Singh R., Gudkov A. T., Liljas A., Logan D. T., Structural insights into fusidic acid resistance and sensitivity in EF-G. J. Mol. Biol. 348, 939–949 (2005). [DOI] [PubMed] [Google Scholar]
- 39.Pulk A., Cate J. H., Control of ribosomal subunit rotation by elongation factor G. Science 340, 1235970 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tourigny D. S., Fernández I. S., Kelley A. C., Ramakrishnan V., Elongation factor G bound to the ribosome in an intermediate state of translocation. Science 340, 1235490 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Whitford P. C., Blanchard S. C., Cate J. H., Sanbonmatsu K. Y., Connecting the kinetics and energy landscape of tRNA translocation on the ribosome. PLOS Comput. Biol. 9, e1003003 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou J., Lancaster L., Donohue J. P., Noller H. F., How the ribosome hands the A-site tRNA to the P site during EF-G-catalyzed translocation. Science 345, 1188–1191 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li W., et al. , Activation of GTP hydrolysis in mRNA-tRNA translocation by elongation factor G. Sci. Adv. 1, e1500169 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vetter I. R., Wittinghofer A., The guanine nucleotide-binding switch in three dimensions. Science 294, 1299–1304 (2001). [DOI] [PubMed] [Google Scholar]
- 45.Schmeing T. M., et al. , The crystal structure of the ribosome bound to EF-Tu and aminoacyl-tRNA. Science 326, 688–694 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jørgensen R., et al. , Two crystal structures demonstrate large conformational changes in the eukaryotic ribosomal translocase. Nat. Struct. Biol. 10, 379–385 (2003). [DOI] [PubMed] [Google Scholar]
- 47.Jørgensen R., et al. , Crystal structure of ADP-ribosylated ribosomal translocase from Saccharomyces cerevisiae. J. Biol. Chem. 279, 45919–45925 (2004). [DOI] [PubMed] [Google Scholar]
- 48.Søe R., et al. , Sordarin derivatives induce a novel conformation of the yeast ribosome translocation factor eEF2. J. Biol. Chem. 282, 657–666 (2007). [DOI] [PubMed] [Google Scholar]
- 49.Spahn C. M., et al. , Domain movements of elongation factor eEF2 and the eukaryotic 80S ribosome facilitate tRNA translocation. EMBO J. 23, 1008–1019 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taylor D. J., et al. , Structures of modified eEF2 80S ribosome complexes reveal the role of GTP hydrolysis in translocation. EMBO J. 26, 2421–2431 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abeyrathne P. D., Koh C. S., Grant T., Grigorieff N., Korostelev A. A., Ensemble cryo-EM uncovers inchworm-like translocation of a viral IRES through the ribosome. eLife 5, e14874 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pellegrino S., et al. , Structural insights into the role of diphthamide on elongation factor 2 in mRNA reading-frame maintenance. J. Mol. Biol. 430, 2677–2687 (2018). [DOI] [PubMed] [Google Scholar]
- 53.Mardirossian M., et al. , Proline-rich peptides with improved antimicrobial activity against E. coli, K. pneumoniae, and A. baumannii. ChemMedChem 14, 2025–2033 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Seefeldt A. C., et al. , The proline-rich antimicrobial peptide Onc112 inhibits translation by blocking and destabilizing the initiation complex. Nat. Struct. Mol. Biol. 22, 470–475 (2015). [DOI] [PubMed] [Google Scholar]
- 55.Zheng S. Q., et al. , MotionCor2: Anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zivanov J., et al. , New tools for automated high-resolution cryo-EM structure determination in RELION-3. eLife 7, e42166 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moriya T., et al. , High-resolution single particle analysis from electron cryo-microscopy images using SPHIRE. J. Vis. Exp. (123), 55448 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kimanius D., Dong L., Sharov G., Nakane T., Scheres S. H. W., New tools for automated cryo-EM single-particle analysis in RELION-4.0. Biochem. J. 478, 4169–4185 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Emsley P., Cowtan K., Coot: Model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr 60, 2126–2132 (2004). [DOI] [PubMed] [Google Scholar]
- 60.Adams P. D., et al. , PHENIX: A comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen V. B., et al. , MolProbity: All-atom structure validation for macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr. 66, 12–21 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pettersen E. F., et al. , UCSF Chimera--A visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Cryo-EM map and models were deposited in the EMDatabank (EF-G-ArgB-70S, EMD-13058; EF-G-ArgB-INT2, EMD-26486) and RCSB Protein Data Bank (EF-G-ArgB-70S, PDB ID: 7OTC; EF-G-ArgB-INT2, PDB ID: 7UG7).