Abstract
The coronavirus disease‐2019 (COVID‐19) has spread worldwide. Previous studies indicated a low prevalence of COVID‐19 induced acute exacerbations of asthma. We present a 39‐year‐old‐male obese asthmatic case who experienced acute asthma exacerbation during COVID‐19. On day 8 of infection, his cough and dyspnea worsened with hypoxia and wheezing. Laboratory test results revealed elevated interleukin‐6 (IL‐6) and total immunoglobulin E (IgE) levels without eosinophilia. Two months since the onset, hypoxia disappeared with decreased IL‐6 and IgE levels. Our case suggested that obesity and high serum IL‐6 and IgE levels may have contributed to atypical asthma exacerbation in COVID‐19.
Keywords: asthma, COVID‐19, immunoglobulin E, interleukin‐6, obesity
The coronavirus disease‐2019 (COVID‐19), an emerging infectious disease caused by Severe Acute Respiratory Syndrome‐Coronavirus 2 (SARS‐CoV2), emerged in December 2019 and has spread worldwide. Old age, hypertension, diabetes, and smoking were identified risk factors for aggravation. Obesity also increases the severity and mortality of COVID‐19. However, the relationship between asthma and COVID‐19 infection remains unclear. We present a rare case of an obese asthmatic patient who experienced asthma exacerbation during COVID‐19 infection.
![]()
INTRODUCTION
Asthma pertains to chronic airway inflammation, resulting in paroxysmal cough, wheezing, and dyspnea. About 5%–10% of asthma patients have severe asthma, insensitive to standard treatments, including inhaled corticosteroids (ICS). Obesity is an intractable comorbidity for asthma that predisposes patients to steroid resistance and disease severity. Viral infections, especially those induced by rhinovirus, respiratory syncytial virus, and influenza virus, most frequently cause acute asthma exacerbation and worsen asthma control.
The coronavirus disease‐2019 (COVID‐19), an emerging infectious disease caused by Severe Acute Respiratory Syndrome‐Coronavirus 2 (SARS‐CoV2), emerged in December 2019 and has spread worldwide. Old age, hypertension, diabetes, and smoking were identified risk factors for aggravation. Obesity also increases the severity and mortality of COVID‐19. However, the relationship between asthma and COVID‐19 infection remains unclear. The risk of intubation significantly increased in COVID‐19 asthmatic patients aged 50 years or younger. 1 We present a rare case of an obese asthmatic patient who experienced asthma exacerbation during COVID‐19 infection.
CASE REPORT
A 39‐year‐old man with asthma had specific IgE antibodies against cedar, house dust, and mites. He was obese with a body mass index (BMI) of 34 kg/m2. He has never smoked and had a medical history of hypertension. His asthma was well‐controlled with an ICS, long‐acting β‐agonist (LABA) (budezonide formoterol; 320 μg/9 μg/day), and leukotriene receptor antagonist (montelukast, 10 mg/day). He had a fever of 39.2°C, headache, sore throat, and general fatigue. The diagnosis of COVID‐19 was confirmed by a positive reaction for SARs‐CoV2 of the nasopharyngeal swabs on the reverse transcription‐polymerase chain reaction test. He was hospitalized 3 days after the onset of symptoms. On admission, he had a body temperature of 38.4°C and SpO2 of 95% at room air. Wheezes during exhalation were not heard. Laboratory tests showed that the peripheral white blood cell and eosinophil counts were 4940/μl and 10/μl, respectively. He had a C‐reactive protein (CRP) of 1.45 mg/dl and IL‐6 of 17 pg/ml. The serum IL‐5 level was below the detection limit. The chest X‐ray findings were unremarkable (Figure 1A). However, chest computed tomography (CT) showed bilateral non‐segmented ground glass opacities in the peripheral areas of the lower lobes without bronchial wall thickening (Figure 1B). The patient was treated with acetaminophen. From the date of admission to day 7, he remained febrile with no wheezes during exhalation. On day 8, his cough and dyspnea worsened with hypoxia (SpO2 88% at room air). Wheezes during exhalation were heard on physical examination, suggesting acute asthma exacerbation. Chest X‐ray (anterior–posterior view) showed bilateral infiltrative shadows in all lung fields (Figure 1C). Laboratory test revealed elevated levels of CRP (2.43 mg/dl), IL‐6 (22 pg/ml), and total IgE (1,718 IU/ml). His weight did not change from admission to exacerbation. These findings indicated COVID‐19 and asthma aggravation. Thus, an antivirus drug, remdesivir (200 mg/day), was administered. Moreover, the ICS/LABA dose was increased (budesonide formoterol: 1,280 μg/36 μg/day), and inhaled procaterol was added. However, these therapeutic changes were not effective to control respiratory symptoms. On day 11, oral dexamethasone (6 mg/day) was added for worsening hypoxia with persisting wheezes. After treatment, wheezes gradually improved and completely disappeared on day 16. However, mild hypoxia persisted (SpO2 93% at room air) on day 20. Chest CT demonstrated bilateral ground glass shadows with bronchial wall thickening with no pleural effusions and cardiac hypertrophy (Figure 1D). After 2 months from the onset, hypoxia disappeared (SpO2 97% at room air). The serum IL‐6 and IL‐5 levels were below the detection limit, while the CRP level was 0.02 mg/dl. Blood eosinophil count and total serum IgE level were 101/μl and 805 IU/ml, respectively. The key findings of the laboratory tests are summarized in Table 1.
FIGURE 1.
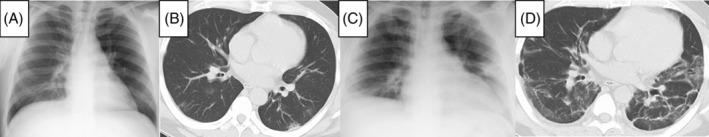
Radiological findings. (A) Chest X‐ray (posterior–anterior view) on admission showed no obvious abnormal findings. (B) Chest computed tomography (CT) on admission showed non‐segmented ground glass opacities only in the peripheral areas of both lower lobes with no bronchial wall thickening. (C) Chest X‐ray (Anterior–Posterior view) on day 8 from the onset of coronavirus disease‐2019 (COVID‐19) showed bilateral infiltrative shadows mainly in peripheral lung fields. (D) Chest CT on day 20 from the onset of COVID‐19 showed bilateral ground‐glass shadows with bronchial wall thickening. No pleural effusions and cardiac hypertrophy were observed.
TABLE 1.
Laboratory findings
| BEC (/μl) | IgE (IU/ml) | IL‐5 (pg/ml) | IL‐6 (pg/ml) | CRP (mg/ml) | |
|---|---|---|---|---|---|
| On admission (day 3) | 11 | 1,787 | ND | 17 | 1.45 |
| Acute exacerbation (day 8) | 8 | 1,718 | ND | 22 | 2.43 |
| After discharge (day 105) | 101 | 805 | ND | ND | 0.02 |
Abbreviations: BEC, blood eosinophil count; CRP, C‐reactive protein; IL, interleukin; ND, not detected.
DISCUSSION
SARS‐CoV2‐induced asthma exacerbations rarely affected hospitalized asthmatic patients. 2 The low prevalence of coronavirus‐induced asthma exacerbation was likely related to an immunological response, distinct from those against other pathogens (rhinoviruses, respiratory syncytial viruses, etc.) causing respiratory infections. In this case, however, asthma exacerbation was observed in a patient with asthma and worsening COVID‐19.
Specific host receptors have been identified as virus entry for infection. SARS‐CoV2 utilizes angiotensin‐converting enzyme 2 (ACE2), which is expressed in the alveolar epithelial cells of the lungs. IL‐4 and IL‐13 establish allergic airway inflammation in asthma and reduce ACE2 expression of epithelial cells. 3 Lowered ACE2 expression possibly induces a protective effect against COVID‐19 infection in asthmatic patients.
Obesity is a possible cause of asthma exacerbation in this case. A high BMI is closely associated with the frequency of asthma exacerbations and obese patients experiencing frequent exacerbations had higher serum IL‐6 concentrations. High IL‐6 trans‐signalling activates inflammatory genes in epithelial cells associated with airway remodelling, frequent exacerbation, and eosinophilia. 4 In this case, obesity and high serum IL‐6 level contributed to the atypical asthma exacerbation during COVID‐19 infection.
The total serum IgE increased during COVID‐19 aggravation, indicating an association with asthma exacerbation. Increased expression of high‐affinity FcεRI in the plasmacytoid dendritic cell surface decreases interferon‐α and λ1 production in human rhinovirus infection, which is inversely correlated with serum IgE level. 5 Thus, the increase in IgE during a viral infection possibly suppressed interferon production and induced asthma exacerbation in this case.
The levels of IL‐6 and IgE changed in parallel during disease course (Table 1), indicative of its close relationship. Previous studies showed that IL‐6 potentiated IgE synthesis and IL‐4 and anti‐CD40 signalling induced both IgE and IL‐6 productions in B cells. These findings suggest that both molecules synergistically enhance inflammatory responses during asthma exacerbation in this patient.
We encountered a rare case of asthma exacerbation during the disease course of COVID‐19. Future investigations are warranted to improve the therapeutic strategy for asthma exacerbation during COVID‐19 infection, especially in obese patient.
AUTHOR CONTRIBUTIONS
Hisashi Sasaki and Jun Miyata conceived the idea. Akihiko Kawana overviewed the research project. Hisashi Sasaki and Jun Miyata wrote the paper. Ai Kobayashi, Shuichi Kawano critically contributed to the accomplishment of this research.
CONFLICT OF INTEREST
None declared.
ETHICS STATEMENT
The authors declare that appropriate written informed consent was obtained for the publication of this manuscript and accompanying images.
Sasaki H, Miyata J, Kobayashi A, Kawano S, Kawana A. A case report of coronavirus disease‐2019‐induced acute asthma exacerbation in an obese patient. Respirology Case Reports. 2022;10:e0979. 10.1002/rcr2.979
Associate Editor: Arata Azuma
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Hussein MH, Elshazli RM, Attia AS, Nguyen TP, Aboueisha M, Munshi R, et al. Asthma and COVID‐19; different entities, same outcome: a meta‐analysis of 107,983 patients. J Asthma. 2021;59:1–8. [DOI] [PubMed] [Google Scholar]
- 2. Grandbastien M, Piotin A, Godet J, Amougou IA, Ederlé C, Enache I, et al. SARS‐CoV‐2 pneumonia in hospitalized asthmatic patients did not induce severe exacerbation. J Allergy Clin Immunol Pract. 2020;8:2600–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kimura H, Francisco D, Conway M, Martinez FD, Vercelli D, Polverino F, et al. Type 2 inflammation modulates ACE2 and TMPRSS2 in airway epithelial cells. J Allergy Clin Immunol. 2020;146:80–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Peters MC, McGrath KW, Hawkins GA, Hastie AT, Levy BD, Israel E, et al. Plasma interleukin‐6 concentrations, metabolic dysfunction, and asthma severity: a cross‐sectional analysis of two cohorts. Lancet Respir Med. 2016;4:574–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Durrani SR, Montville DJ, Pratt AS, Sahu S, DeVries MK, Rajamanickam V, et al. Innate immune responses to rhinovirus are reduced by the high‐affinity IgE receptor in allergic asthmatic children. J Allergy Clin Immunol. 2012;130:489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


