Abstract
Radiomics is a promising mathematical tool for characterizing disease and predicting clinical outcomes from radiological images such as CT. Photon-counting-detector (PCD) CT provides improved spatial resolution and dose efficiency relative to conventional energy-integrating-detector CT systems. Since improved spatial resolution enables visualization of smaller structures and more details that are not typically visible at routine resolution, it has a direct impact on textural features in CT images. Therefore, it is of clinical interest to quantify the impact of the improved spatial resolution on calculated radiomic features and, consequently, on sample classification. In this work, organic samples (zucchini, onions, and oranges) were scanned on both clinical PCD-CT and EID-CT systems at two dose levels. High-resolution PCD-CT and routine-resolution EID-CT images were reconstructed using a dedicated sharp kernel and a routine kernel, respectively. The noise in each image was quantified. Fourteen radiomic features of relevance were calculated in each image for each sample and compared between the two scanners. Radiomic features were plotted pairwise to evaluate the resulting cluster separation of the samples by their type between PCD-CT and EID-CT. Thirteen out of 14 studied radiomic features were notably changed by the improved resolution of the PCD-CT system, and the cluster separation was better when assessing features derived from PCD-CT. These results show that features derived from high-resolution PCD-CT, which are subject to higher noise compared to EID-CT, may impact radiomics-based clinical decision making.
Keywords: radiomics, x-ray computed tomography, spatial resolution, image segmentation, noise measurement
1. INTRODUCTION
Radiomics is a process in which numerical values are computed from an image, such as a CT image, to characterize features in the image that may or may not be recognizable to a human observer (1). Differences in these features between normal and diseased anatomy can then be used to detect or characterize the disease or to predict clinical outcomes, such as predicting major adverse cardiac events from coronary artery plaque composition (2). The pathological volume in some diseases can be difficult to visualize in CT due to the small size of the impacted volume and/or the lack of ability to sufficiently resolve fine structures. Examples where radiomics is of clinical interest include soft plaque characterization in coronary artery disease (3) or quantitation of potential biomarkers for osteoarthritis in condylar bone (4). In such cases, the spatial resolution of the CT image is an important factor to consider in preventing loss of texture information.
Conventional CT scanners use energy-integrating detectors (EID) that are based on indirect conversion technology. The conversion of x-rays to visible light requires that opaque septa be present between detector elements to reduce optical crosstalk. To achieve improved spatial resolution imaging, energy-integrated detector elements – and the required septa - need to be smaller, which could decrease geometric efficiency (fill factor) if the septa are not small enough. Currently one commercial EID-CT system is available that achieves 150 micron limiting spatial resolution using typical CT doses (5). In another approach to improving spatial resolution, attenuating “comb” filters are used to reduce the pixel size by blocking incoming photons. The use of post-patient collimation substantially decreases overall dose efficiency (6). A third approach to improving spatial resolution in CT imaging uses direct conversion photon-counting detectors (PCD), which eliminate the need for septa between detector pixels or comb filters (6, 7). PCD-CT systems that are capable of whole-body full field-of-view imaging and provide multi-energy and improved-resolution capabilities recently became commercially available for routine clinical use (8, 9).
Noise variations in conventional CT images have been shown to affect radiomic features (10), and differences in radiomic texture and morphology features have been demonstrated between conventional EID-CT and PCD-CT imaging when varying parameters such as dose and slice thickness (11). Radiomic features extracted from a public dataset of EID-CT images from two scanner models has been shown to change by varying the in-plane resolution by means of resampling methods (12). However, to the best of our knowledge, a systematic investigation of the impact of improved spatial resolution on radiomic features has yet to be investigated in the context of PCD-CT technology. In this work, we quantify the effects of varying spatial resolution and noise levels on radiomic features derived from organic samples imaged with a PCD-CT system capable of a limiting resolution of 125 microns and compare the results to a conventional EID-CT system.
2. METHODS
2.1. CT systems
A clinical dual-source PCD-CT system (NAEOTOM Alpha, Siemens Healthineers GmbH, Malvern, PA) was used (9). The scanner is equipped with two cadmium telluride PCD arrays and Vectron x-ray tubes (Siemens Healthineers GmbH) with the smallest focal spot size of 0.4 mm x 0.5 mm for high-resolution imaging. The non-comb-based ultra-high-resolution (UHR) acquisition mode (120 x 0.2 mm collimation) in single-source geometry was used in this study. A conventional EID-CT system (SOMATOM Force, Siemens Healthineers, GmbH, Forchheim, Germany) with a beam collimation of 192 x 0.6 mm was used for comparison to PCD-CT.
2.2. Image Acquisition and Reconstruction
An organic phantom consisting of twelve samples was assembled: four zucchinis, four small red Cipollini onions, and four mandarin oranges. Samples were affixed to three platforms each made of acrylic (Figure 1) and scanned using both the PCD-CT system with a helical thorax protocol using the HR mode and the EID-CT system with a routine helical thorax protocol. The CT acquisition and reconstruction parameters are shown in Table 1. The CTDIvol of 10 mGy was chosen to represent routine dose and a CTDIvol of 60 mGy was chosen to minimize noise influence (for comparison, routine chest CT exams in normal weight adults typically use 10 mGy).
Figure 1:
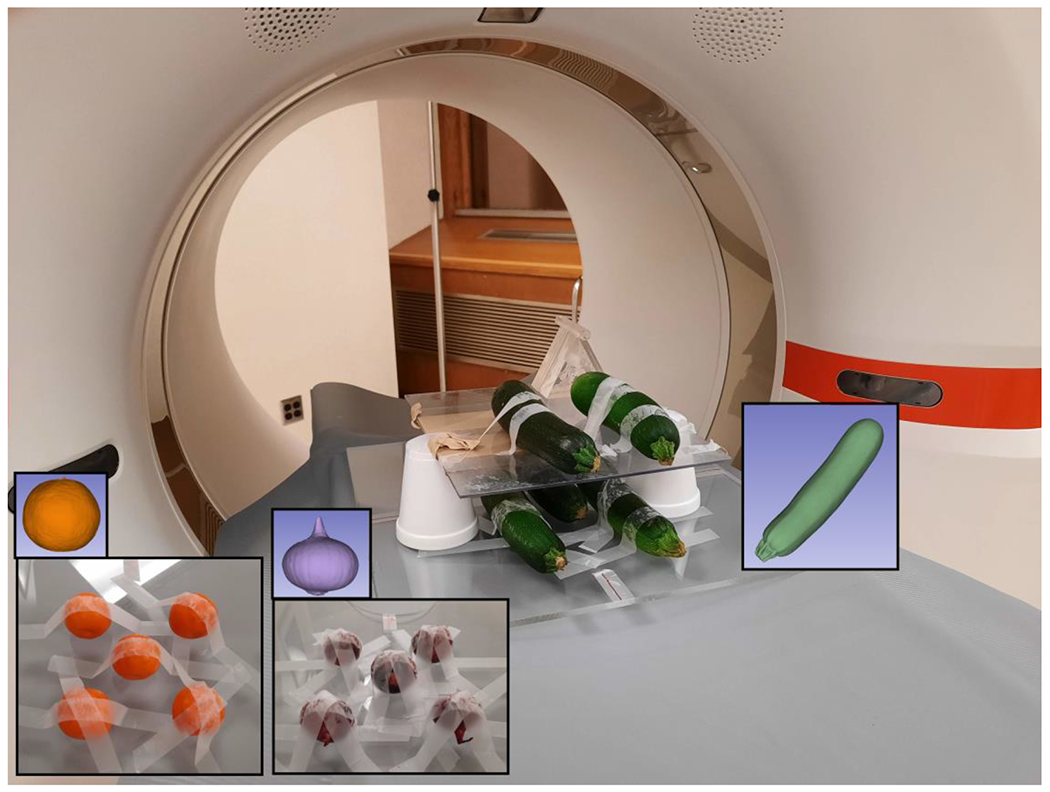
Photos of organic samples on the PCD-CT scanner bed, with examples of the segmented volume (insets).
Table 1:
Parameters for CT acquisition and reconstruction.
| Modality | PCD-CT | EID-CT |
|---|---|---|
| Tube potential (kV) | 120 | 120 |
| Effective mAs | 125, 748 | 149, 895 |
| CTDIvol (mGy) | 10, 60 | 10, 60 |
| Rotation time (s) | 0.5 | 0.5 |
| Helical pitch | 0.2 | 0.35 |
| Reconstruction technique | Weighted filtered back-projection | Weighted filtered back-projection |
| Reconstruction kernel | Qr80 | Qr40 |
| Slice thickness/increment (mm) | 0.6 / 0.6 | 0.6 / 0.6 |
| Field-of-view (mm) | 200 | 200 |
| Matrix size | 1024 x 1024 | 1024 x 1024 |
Images were reconstructed using the parameters in Table 1. Qr40 is a quantitative kernel with typical sharpness used in routine clinical practice, while Qr80 is a very sharp quantitative kernel that is only available on the PCD-CT system.
The noise in each CT image was measured as the standard deviation of CT numbers in a region of interest (ROI) shown in Figure 2. The ROI was chosen as an oval shape coinciding with a slice in one of the orange samples due to the large area of uniform contrast in the object.
Figure 2.
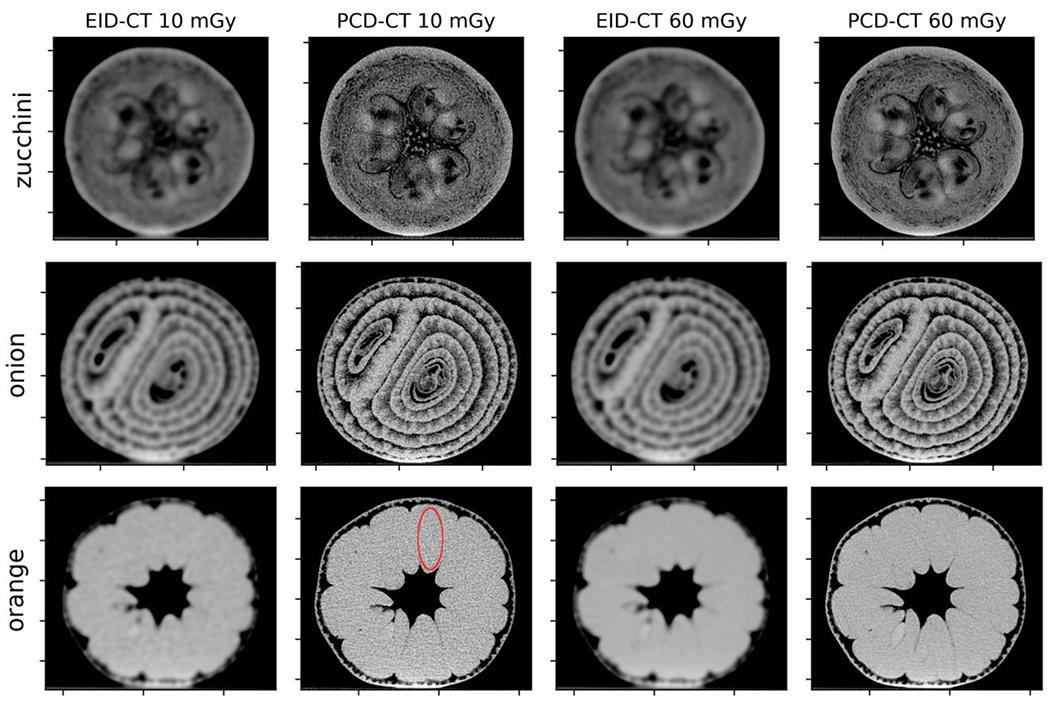
Axial EID-CT and high-resolution PCD-CT slices (window/level = 600/0) of representative zucchini, onion, and orange samples. The red elliptical ROI used for noise measurement is shown in the 10 mGy PCD-CT image for clarity.
2.3. Segmentation
The open-source software Slicer (slicer.org, version 4.11.20210226) (13) was used to semi-automatically segment each sample in its entirety in the 3D image volume using a built-in grow-from-seeds algorithm (Figure 1). The segmentation volume was manually adjusted to ensure satisfactory overlap with the sample surface, then convolved using a 1 mm kernel size to smooth out the surface boundary. The result was saved as a binary mask. This segmentation process was repeated for each individual sample in both PCD-CT and EID-CT images at both dose levels.
2.4. Radiomic Calculations
Radiomic feature extraction was performed using PyRadiomics version 2.2.0b1 (14), an open source radiomics package based in Python 3.7.9. Fourteen radiomic features (Table 2) were extracted from each image series for each segmented object. Radiomic features belonging to matrix-based classes were calculated using a bin width setting of 25 for gray value discretization. Other settings included a resampled pixel spacing of 1 mm3 and a voxel array shift (CT number offset) of 1024 HU. These radiomic features were chosen based on importance as biomarkers in other literature and are robust to quantum noise, segmentation variability, and image acquisition (10, 15). Feature definitions can be found at https://pyradiomics.readthedocs.io/ and are mostly consistent with the Imaging Biomarker Standardization Initiative definitions (16). The ratios of radiomic feature values derived from 10 mGy and 60 mGy PCD-CT and EID-CT images were calculated.
Table 2:
Radiomic features examined in this study.
| Feature # | Radiomic Feature Name | Feature Class |
|---|---|---|
| 1 | Entropy | First-order statistics |
| 2 | Uniformity | First-order statistics |
| 3 | Cluster Shade | Gray-level co-occurrence matrix (GLCM) |
| 4 | Maximum Probability | GLCM |
| 5 | Low Gray Level Run Emphasis | Gray-level run length matrix (GLRLM) |
| 6 | Short Run Low Gray Level Emphasis | GLRLM |
| 7 | Short Run High Gray Level Emphasis | GLRLM |
| 8 | Long Run Low Gray Level Emphasis | GLRLM |
| 9 | Long Run High Gray Level Emphasis | GLRLM |
| 10 | Large Dependence High Gray Level Emphasis | Gray-level dependence matrix (GLDM) |
| 11 | Contrast | Neighboring gray tone difference matrix (NGTDM) |
| 12 | Busyness | NGTDM |
| 13 | Coarseness | NGTDM |
| 14 | Strength | NGTDM |
To compare the classification of the fruits and vegetables using the radiomic features derived from PCD-CT and EID-CT images, each radiomic feature was normalized to the maximum absolute value among the twelve samples then compared pairwise in a series of two-dimensional planes. For a given pairing, the data points were assigned to a cluster based on the fruit or vegetable and the centroids of each cluster were calculated. The overall cluster separation was evaluated using both the Dunn index (17) and Davies-Bouldin index (18). The Dunn index (DI) for a given pairing was calculated by the following equation:
where N = 3 is the number of clusters, Ci,j is the distance between the centroids of clusters i and j, and Sk is the average distance between data points to the centroid within cluster k. The Davies-Bouldin index (DBI) was calculated by the following equation using the same definitions above:
The mean Dunn index and Davies-Bouldin index was evaluated across all 91 possible unique pairings of radiomic features derived from 10 mGy and 60 mGy PCD-CT and EID-CT images. A high Dunn index and a low Davies-Bouldin index indicate better cluster separation.
RESULTS AND DISCUSSION
3.1. CT images and noise
The EID-CT and PCD-CT images of each organic sample are shown in Figure 2. Improved spatial resolution is evident for high-resolution PCD-CT at both dose levels, with smaller structures and finer details visible. The tradeoff is that compared to the EID-CT images, the increased kernel strength of the PCD-CT image increases the noise. The image noise of EID-CT and PCD-CT images were 5.72 HU and 46.04 HU at 10 mGy dose, and 2.61 HU and 20.35 HU at 60 mGy dose, respectively.
3.2. Radiomic Features
Figure 3 shows both the ratio of the values between high-resolution PCD-CT and EID-CT and the ratio between 10 mGy and 60 mGy doses for each radiomic feature for the various samples. Thirteen out of 14 radiomic features changed by at least 50% between PCD-CT and EID-CT at both dose levels. Up to 4 features had greater than an order of magnitude difference in value between PCD-CT and EID-CT at 10 mGy dose in zucchinis and oranges. While the focus of this study was the impact of better spatial resolution on radiomic features using PCD-CT, the increased image noise also impacted the radiomic features. From Figure 3 c) and d), the dose level did not impact the features as much as the spatial resolution. At least 10 features in EID-CT and 8 features in PCD-CT were within a factor of 2 difference in value between 10 mGy and 60 mGy dose levels, with oranges predominantly affected. Potential solutions to decouple noise and spatial resolution include varying the effective mAs during acquisition and the use of texture-preserving denoising techniques.
Figure 3.
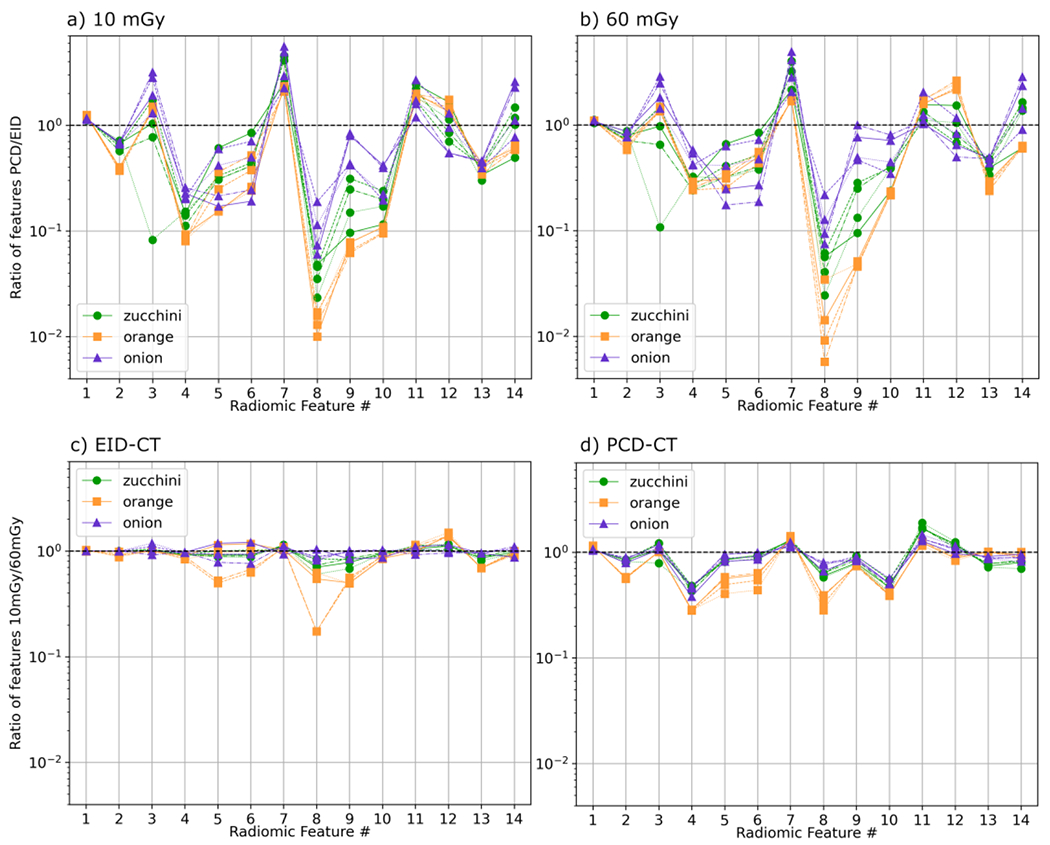
Ratio of PCD-CT-derived over EID-CT-derived radiomic features at a) 10 mGy and b) 60 mGy doses. Ratio of radiomic features from 10 mGy over 60 mGy doses for c) EID-CT and d) PCD-CT images. Unity is shown as a dashed black line.
Pair plots for the radiomic features Uniformity, Short Run High Gray Level Emphasis, and Busyness are shown in Figure 4; these features were selected for brevity. The zucchini, orange, and onion clusters appear to be tighter and more distinguishable in PCD-CT compared to EID-CT. The mean Dunn index for all pair plots was 1.99 and 1.80 for PCD-CT and EID-CT at 10 mGy and 2.44 and 1.65 for PCD-CT and EID-CT at 60 mGy, respectively. The Dunn index was overall higher for PCD-CT images at both dose levels, indicating better cluster separation for classification using these 14 radiomic features compared to EID-CT. The mean Davies-Bouldin index for all pair plots was 0.74 and 0.86 for PCD-CT and EID-CT at 10 mGy and 0.60 and 0.85 for PCD-CT and EID-CT at 60 mGy, respectively. The Davies-Bouldin index was overall lower for PCD-CT images at both dose levels, which is also consistent with PCD-CT images having better cluster separation for fruit and vegetable classification. The differences in the Dunn index and Davis-Bouldin index between PCD-CT and EID-CT at 10 mGy was smaller than at 60 mGy, this indicates that noise does have an impact on cluster separation at routine dose levels.
Figure 4.
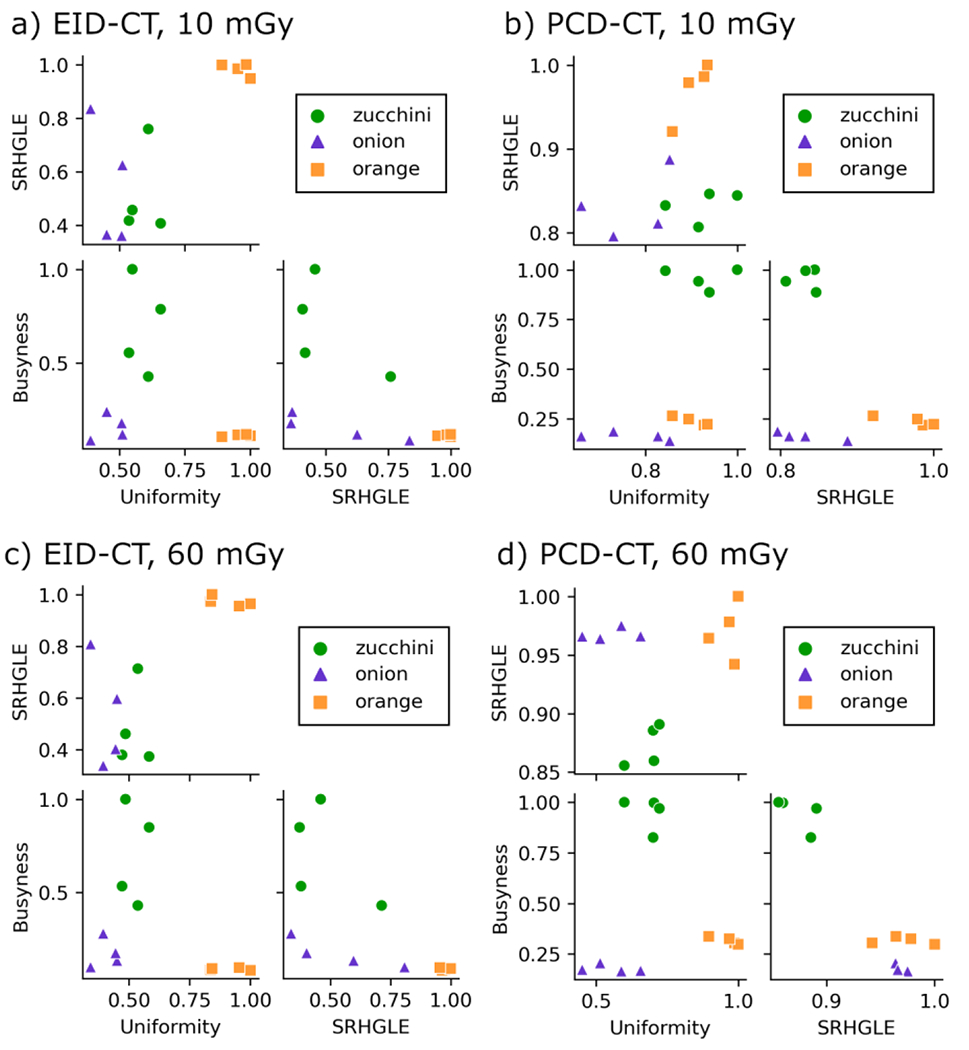
Pair plots of three radiomic features (Uniformity, Busyness, and Short Run High Gray Level Emphasis (SRHGLE)) whose data points form clusters representing zucchini, onion, and oranges. Many clusters appear smaller and more separated in features derived from PCD-CT images compared to EID-CT images.
4. CONCLUSIONS
Several radiomic features were identified that differ strongly in images of twelve organic samples obtained with high-resolution-mode PCD-CT and EID-CT scanners. Improved resolution PCD-CT showed a strong change in radiomic feature values compared to routine resolution EID-CT and better differentiation of radiomic features clustered by organic sample types. High-resolution PCD-CT has the potential to change radiomics-based clinical interpretation or classification for applications involving pathology with rich textures or small volumes, such as bone condyle or coronary plaque analysis. Further studies with patient images on specific clinical applications are warranted.
REFERENCES
- 1.Gillies RJ, Kinahan PE, and Hricak H, “Radiomics: Images Are More than Pictures, They Are Data,” Radiology 278(2), 563–577 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kolossvary M et al. , “Radiomic Features Are Superior to Conventional Quantitative Computed Tomographic Metrics to Identify Coronary Plaques With Napkin-Ring Sign,” Circ Cardiovasc Imaging 10(12), (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kolossvary M et al. , “Cardiac Computed Tomography Radiomics: A Comprehensive Review on Radiomic Techniques,” J Thorac Imaging 33(1), 26–34 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Paniagua B et al. , “Validation of CBCT for the computation of textural biomarkers,” Proc SPIE Int Soc Opt Eng 9417((2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hernandez AM et al. , “Validation of synthesized normal-resolution image data generated from high-resolution acquisitions on a commercial CT scanner,” Med Phys 47(10), 4775–4785 (2020). [DOI] [PubMed] [Google Scholar]
- 6.Leng S et al. , “Dose-efficient ultrahigh-resolution scan mode using a photon counting detector computed tomography system,” J Med Imaging (Bellingham) 3(4), 043504 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leng S et al. , “Photon-counting Detector CT: System Design and Clinical Applications of an Emerging Technology,” Radiographics 39(3), 729–743 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rajendran K et al. , “Full field-of-view, high-resolution, photon-counting detector CT: technical assessment and initial patient experience,” Physics in Medicine & Biology (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rajendran K et al. , “First Clinical Photon-counting Detector CT System: Technical Evaluation,” Radiology 212579 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tu SJ, Chen WY, and Wu CT, “Uncertainty measurement of radiomics features against inherent quantum noise in computed tomography imaging,” Eur Radiol (2021). [DOI] [PubMed] [Google Scholar]
- 11.Rajagopal J et al. , “Accuracy and variability of radiomics in photon-counting CT: texture features and lung lesion morphology,” in Medical Imaging 2019: Physics of Medical Imaging (2019). [Google Scholar]
- 12.Ibrahim A et al. , “The Effects of In-Plane Spatial Resolution on CT-Based Radiomic Features’ Stability with and without ComBat Harmonization,” Cancers (Basel) 13(8), (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fedorov A et al. , “3D Slicer as an image computing platform for the Quantitative Imaging Network,” Magn Reson Imaging 30(9), 1323–1341 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Griethuysen JJM et al. , “Computational Radiomics System to Decode the Radiographic Phenotype,” Cancer Research 77(21), e104–e107 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Le EPV et al. , “Assessing robustness of carotid artery CT angiography radiomics in the identification of culprit lesions in cerebrovascular events,” Sci Rep 11(1), 3499 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zwanenburg A et al. , “The Image Biomarker Standardization Initiative: Standardized Quantitative Radiomics for High-Throughput Image-based Phenotyping,” Radiology 295(2), 328–338 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dunn JC, “A Fuzzy Relative of the ISODATA Process and Its Use in Detecting Compact Well-Separated Clusters,” Journal of Cybernetics 3(3), 32–57 (1973). [Google Scholar]
- 18.Davies DL, and Bouldin DW, “A Cluster Separation Measure,” IEEE Transactions on Pattern Analysis and Machine Intelligence PAMI-1(2), 224–227 (1979). [PubMed] [Google Scholar]


