Abstract
Purpose
In acute respiratory distress syndrome (ARDS), physiological parameters associated with outcome may help defining targets for mechanical ventilation. This study aimed to address whether transpulmonary pressures (PL), including transpulmonary driving pressure (DPL), elastance-derived plateau PL, and directly-measured end-expiratory PL, are better associated with 60-day outcome than airway driving pressure (DPaw). We also tested the combination of oxygenation and stretch index [PaO2/(FiO2*DPaw)].
Methods
Prospective, observational, multicentre registry of ARDS patients. Respiratory mechanics were measured early after intubation at 6 kg/ml tidal volume. We compared the predictive power of the parameters for mortality at day-60 through receiver operating characteristic (ROC) and assessed their association with 60-day mortality through unadjusted and adjusted Cox regressions. Finally, each parameter was dichotomized, and Kaplan–Meier survival curves were compared.
Results
385 patients were enrolled 2 [1–4] days from intubation (esophageal pressure and arterial blood gases in 302 and 318 patients). As continuous variables, DPaw, DPL, and oxygenation stretch index were associated with 60-day mortality after adjustment for age and Sequential Organ Failure Assessment, whereas elastance-derived plateau PL was not. DPaw and DPL performed equally in ROC analysis (P = 0.0835). DPaw had the best-fit Cox regression model. When dichotomizing the variables, DPaw ≥ 15, DPL ≥ 12, plateau PL ≥ 24, and oxygenation stretch index < 10 exhibited lower 60-day survival probability. Directly measured end-expiratory PL ≥ 0 was associated with better outcome in obese patients.
Conclusion
DPL was equivalent predictor of outcome than DPaw. Our study supports the soundness of limiting lung and airway driving pressure and maintaining positive end-expiratory PL in obese patients.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00134-022-06724-y.
Keywords: Acute respiratory distress syndrome, Artificial respiration, Mechanical ventilation, Positive-pressure respiration, Respiratory mechanics
Introduction
Mechanical ventilation is lifesaving but can generate ventilator-induced lung injury (VILI) [1]. A protective ventilatory strategy which combined lower tidal volume (VT) normalized to predicted body weight (PBW) and airway plateau pressure (Pplat) in patients with acute respiratory distress syndrome (ARDS) reduced mortality [2].
Several parameters have been suggested to monitor ventilatory strategy. Amato et al. [3] proposed that airway driving pressure (DPaw) is physiologically sounder than using VT/PBW for limiting VILI. Their retrospective analysis demonstrated that DPaw was better associated with outcome than VT/PBW or Pplat [3]. Further prospective, large observational studies also confirmed this strong association [4, 5]. DPaw is thus an essential monitoring parameter and is a potential target for lung protective ventilation strategies [6].
VILI is related to the degree of lung stretch, and thus theoretically transpulmonary driving pressure (DPL) or tidal lung stress may be a better determinant of VILI than DPaw by removing the chest wall contribution to DPaw [7]. Other calculated indices related to transpulmonary pressures (PL) represent possible physiological meaning for limiting VILI. Particularly, because of the existence of pleural pressure gradient [8], PL at dorsal lung differs from PL at ventral lung, which may have different implications for monitoring VILI. For example, because the position of the esophagus is close to dorsal lung, the directly measured end-expiratory PL through esophageal pressure (Pes) provides PL at the dorsal lung, representing the degree of collapse [9–11]. On the other hand, the tidal change of pleural pressure is relatively uniform. In other words, delta Pes (i.e., delta pleural pressure at dorsal lung) is similar with the delta pleural pressure at ventral lung [10]. Thanks to this feature, the elastance-derived plateau transpulmonary pressure [12] calculated through delta Pes (see Appendix) thus represent total lung stress of ventilated “baby lung”, particularly at the ventral lung, which is more susceptible to overdistension [10, 13]. All these values of transpulmonary pressure require measurement of Pes [14], which is rarely monitored in routine clinical practice. A previous study from our centre demonstrated the feasibility of embedding this technique into clinical practice [15], providing a foundation for the present study.
Our ultimate goal was to find out the most important variables that can be used as ventilatory targets for limiting the risk of VILI. However, since there is no direct, reliable approach (including biomarkers) to assess VILI at bedside, we decided to look at the associations between mechanics and mortality as an indirect approach. Certainly, mortality was not determined only by VILI but we think that, if one variable in mechanics can well represent the risk of VILI, it should be associated with mortality.
The present study describes a multicentre study to examine the relative importance on outcome of the global and partitioned respiratory mechanics in ARDS patients. Our main hypothesis was that transpulmonary driving pressure (DPL) is a better predictor for outcome than DPaw. Secondary hypothesis was that oxygenation stretch index, a composite variable using PaO2/FiO2 divided by DPaw [15], can enhance the predictive power of DPaw.
Methods
Design and settings
This was a prospective, observational, multicentre study (ClinicalTrials.gov NCT02623192). Participating centres are listed in the Supplementary file. The study was approved by research ethics boards from each centre. Informed consent was waived at the centre from Canada since mechanics measurement is a part of the standard of care in patients with moderate and severe ARDS [15]. In all remaining centres, informed consent was obtained from patient’s substitute decision maker.
Patients
Inclusion criteria were the following: (1) age > 18 years; (2) ARDS per the Berlin definition [16]; (3) receiving assist/control ventilation with sedation; (4) within the first week of intubation. Exclusion criteria were the following: (1) known esophageal pathology, active upper gastrointestinal bleeding or other contraindications to gastric tube insertion; (2) severe hemodynamic instability (> 30% increase in vasopressors in the prior 6 h, or norepinephrine > 0.5 mcg/kg/min).
Measurements
Respiratory mechanics were measured in a standardized fashion as previously described [15]. Briefly, we studied patients at semi-recumbent position [17] who were not generating spontaneous breathing (no triggering and no effort during an end-expiratory occlusion) during the measurement. To achieve this, patients were receiving deep sedation with or without neuromuscular blockade (sometimes as boluses). Patients were not on extracorporeal life support and ventilated with the following settings: volume control with VT 6 ml/kg of predicted body weight (PBW), constant inspiratory flow 50–60 L/min, 0.3-s end-inspiratory pause, and positive end-expiratory pressure (PEEP) set by the attending clinician. Pes measurements followed a standardized protocol [15], which was adapted from the procedures of EPVent study [9], in all centres. Briefly, Pes catheter was firstly passed into stomach (a depth of 60 cm and confirmed by gentle, swift compression of abdomen). The catheter was then withdrawn to a depth of 40 cm. The esophageal placement was confirmed by the presence of cardiac artifact and the apparent difference between tidal change in Pes and airway driving pressure (to exclude misplacement in trachea). For each patient, the position of the catheter was also validated through Lanteri’s modification of Baydur’s occlusion test at the bedside [18, 19]. Central inspection of Pes waveforms (e.g., the presence of cardiac artifact, the absence of negative Pes swing, the validity of occlusion test) was also performed by the author L.C. Pes was only measured in patients with moderate or severe ARDS and in the absence of spontaneous breathing effort. Mechanics and arterial blood gases were measured at the clinical PEEP level and were repeated 10 min after PEEP was modified by 5 cmH2O (increased in most cases without changing any other settings). Whether PEEP was increased (preferable option) or decreased from clinical PEEP level was decided by investigators and clinicians. The principle was to maintain Pplat ≤ 35 cmH2O and keep the patient hemodynamically stable. Notice that the Pplat limit is only for the measurement safety, not for clinical practice. The measurements were repeated at a different PEEP level to observe the responses to PEEP.
Definitions in respiratory mechanics
Detailed calculations in mechanics are provided in Appendix (see supplement). We refer to global mechanics for calculations based on airway pressure, such as DPaw. We calculated DPaw as the difference between Pplat and total PEEP, not set PEEP. We refer to lung and chest wall mechanics when Pes and PL were used. We partitioned DPaw into DPL, and the chest wall driving pressure (i.e., ∆Pes during a tidal breath). Since the position of the esophagus is close to dorsal lung, we further partitioned PL specific to the ventral or dorsal lungs. Concretely, the directly measured PL represents end-expiratory PL across the dorsal lung, whereas the elastance-derived PL represents end-inspiratory PL across the ventral lung [10, 11]. We also calculated the oxygenation stretch index [(PaO2/(FiO2 × DPaw)] a previously described composite index of oxygenation and mechanics [15]. Mechanical power was calculated using the formula: 0.098 × RR × [VT2 × [0.5 × Ers + RR × (1 + I:E)/(60 × I:E) × Rrs] + VT × PEEP] [20].
Sample size estimation
The study was aimed to show that the discriminant power for predicting mortality, measured with the area under curve (AUC) of receiver operating characteristic (ROC) curve, of DPaw and DPL differed. Using Hanley and McNeils’ method [21], a sample of 64 non-survivors and 160 survivors at 60 days achieve 95% power to detect a difference of 0.092 between DPaw with an AUC of 0.590 and DPL with an AUC of 0.498, using a two-sided z-test at a significance level of 0.05. The correlation between the two diagnostic tests was assumed 0.834 for the survivor group and 0.870 for the non-survivor group. Assuming a dropout rate of 20%, the dropout-inflated enrollment sample size was 280 patients (200 survivors and 80 non-survivors) with Pes measured. Assumptions and data for sample size calculation was based on EPVent study [9].
Statistical analysis
All analyses were prospective unless explicitly indicated as post-hoc. For descriptive analysis, data are presented as mean ± standard derivation or median [25th–75th interquartile range (IQR)] according to skewness and normality of data checked through Shapiro–Wilk test. Characteristics and mechanics between survivors and non-survivors were compared using t-test or Mann–Whitney U-test, depending on normality, without P-value correction for multiple comparisons.
To compare the predictive power of mechanics, we used mortality at day-60 as a binary outcome and estimated ROC curves for mechanics as continuous variables. The AUCs were then compared by using bootstrap test for two correlated ROC curves. To test the association between outcome and mechanics, Cox proportional-hazards regression models were carried out using mechanics as continuous predictors. Association between each covariate and outcome was quantified by hazard ratio (HR) and 95% confidence interval (CI). Adjusted Cox regression models were performed to adjust the HR estimate for baseline characteristics—age and Sequential Organ Failure Assessment (SOFA) score. Proportional hazard assumption was tested for all Cox models (based on the scaled Schoenfeld residuals). Adjusted models were ranked by their Akaike information criterion (AIC). AIC addresses both goodness-of-fit and simplicity of a model. Since we compared models with the same number of independent variables for the same set of patients, the lowest AIC represented the best fit model.
Furthermore, for each mechanics parameter, we grouped patients into the lower or the higher (or equal) dichotomized group according to thresholds proposed in previous studies: 12 cmH2O for DPL, 15 cmH2O for DPaw, 24 cmH2O for elastance-derived plateau PL, 0 cmH2O for directly measured end-expiratory PL, and 10 mmHg/cmH2O for oxygenation stretch index [3, 5, 14, 15]. The Kaplan–Meier approach was used to estimate survival functions in each group and unadjusted log-rank test was used to compare them.
We did not impute missing data, assuming missingness was at random; when addressing the different research questions, we used subsets with complete information.
All p-values were two-sided, with p-values < 0.05 considered statistically significant. Statistical analyses were performed using R version 3.5.2 [22] with package survminer version 0.4.9.
Results
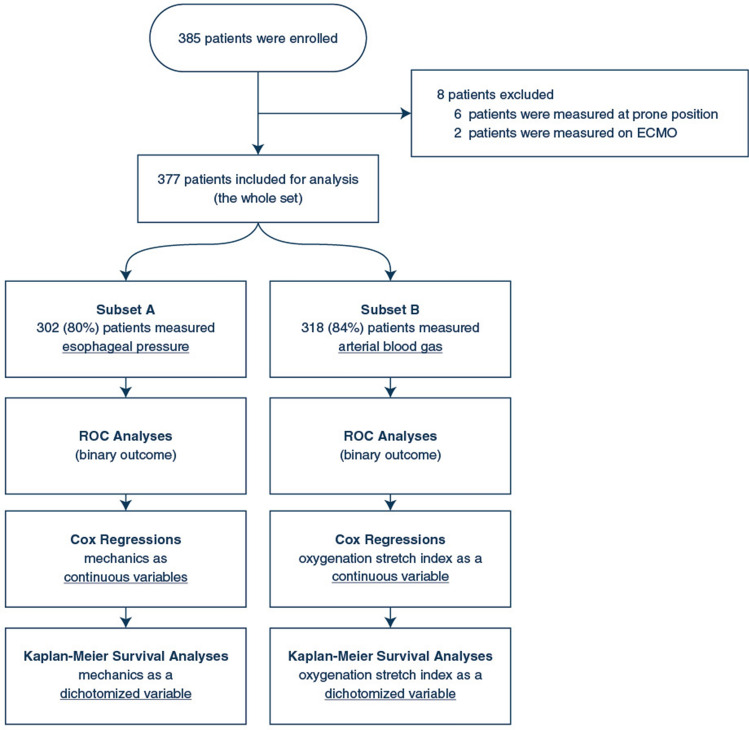
As illustrated in the flowchart (Fig. 1), 385 patients were enrolled; 8 patients were excluded from analysis since measurements were obtained in the prone position, or under extracorporeal membrane oxygenation (ECMO). The remaining 377 patients formed the study cohort.
Fig. 1.
Flowchart of the study
Characteristics of the cohort are provided in Table 1. Mortality at day 60 was 37.7%. Measurements were made within 2 [IQR 1–4] days from intubation at a median clinical PEEP of 12 [IQR 10–14] cmH2O. In univariate analysis non-survivors had higher age, APACHE II at ICU admission, higher SOFA and lower PaO2/FiO2 at enrollment. Respiratory mechanics are reported in Table 1. Univariate analysis suggested that non-survivors had higher Pplat, DPaw, DPL, elastance-derived plateau PL, respiratory rate, and lower oxygenation stretch index. We did not find any statistical differences in oxygenation, blood pressure, and DPaw responses to higher PEEP among survivors and non-survivors.
Table 1.
General characteristics and respiratory mechanics of patients
| General characteristics | Overall | Survivors | Non-survivors | P value* |
|---|---|---|---|---|
| (n = 377) | (n = 235) | (n = 142) | ||
| Males—no. (%) | 266 (70.6) | 169 (71.9) | 97 (68.3) | 0.530 |
| Age—years | 60 [46, 69] | 56 [43, 66] | 65 [53, 76] | < 0.001 |
| Height—cm | 171 [164, 178] | 171 [164, 178] | 170 [163, 178] | 0.980 |
| Body Mass Index—kg/m2 | 28 [24, 34] | 28 [24, 33] | 28 [24, 35] | 0.674 |
| APACHE II at ICU admission | 24 [16, 30] | 23 [16, 28] | 27 [17, 32] | 0.016 |
| ICU stay prior to enrollment—days | 2 [1, 5] | 2 [1, 6] | 2 [1, 4] | 0.963 |
| IMV days prior to enrollment—days | 2 [1, 4] | 2 [1, 5] | 2 [1, 4] | 0.350 |
| SOFA at enrollment | 11 [8, 14] | 10 [7, 13] | 12 [9, 16] | < 0.001 |
| Clinical PEEP at enrollment—cmH2O | 12 [10, 14] | 12 [10, 14] | 12 [10, 14] | 0.615 |
| PaO2/FiO2 at enrollment—mmHg | 128 [100, 162] | 138 [103, 168] | 115 [88, 150] | 0.001 |
| VE,corr—L/min | 12.3 [10.4, 14.5] | 12.3 [10.3, 14.2] | 12.3 [10.5, 14.8] | 0.630 |
| Severity of ARDS—no. (%) | 0.024 | |||
| Mild | 38 (11.1) | 28 (13.2) | 10 (7.6) | |
| Moderate | 232 (67.6) | 148 (69.8) | 84 (64.1) | |
| Severe | 73 (21.3) | 36 (17) | 37 (28.2) | |
| Pulmonary ARDS—no. (%) | 262 (69.5) | 169 (72) | 93 (65.2) | 0.197 |
| 60-day mortality—no. (%) | 142 (37.7) | |||
| Global mechanics | (n = 377) | (n = 235) | (n = 142) | |
| PEEP—cmH2O | 12 [10, 15] | 12 [10, 15] | 12 [10, 15] | 0.153 |
| VT/PBW—ml/kg | 6.2 [5.9, 6.5] | 6.1 [5.9, 6.5] | 6.3 [5.9, 6.5] | 0.542 |
| Auto-PEEP—cmH2O | 1 [0, 1] | 1 [0, 1] | 1 [0, 1] | 0.522 |
| Pplat—cmH2O | 25 [23, 28] | 25 [22, 28] | 26 [24, 30] | < 0.001 |
| DPaw—cmH2O | 12 [10, 14] | 11 [9, 13] | 13 [10, 15] | 0.001 |
| RR—cmH2O | 28 [24, 30] | 27 [24, 30] | 28 [25, 30] | 0.061 |
| Crs—ml/cmH2O | 35 [27, 43] | 36 [29, 44] | 32 [25, 41] | 0.003 |
| Rrs—cmH2O/L/s | 12 [9, 14] | 12 [9, 14] | 12 [9, 14] | 0.337 |
| Oxygenation stretch index†—mmHg/cmH2O | 11.7 [8.1, 16.2] | 12.3 [9.1, 16.9] | 10.8 [6.8, 14.9] | 0.005 |
| Partitioned mechanics | (n = 302) | (n = 195) | (n = 107) | |
| DPL—cmH2O | 8.3 [6.3, 11] | 8 [6.1, 10.6] | 9.3 [6.9, 12.1] | 0.010 |
| DPcw—cmH2O | 3 [2.7 4.1] | 2.7 [2.7, 4.1] | 3 [2.7, 4.1] | 0.111 |
| CL– ml/cmH2O | 48 [35, 62] | 50 [38, 62] | 44 [30, 61] | 0.009 |
| Ccw—ml/cmH2O | 127 [92, 163] | 130 [98, 165] | 120 [85, 155] | 0.180 |
| EL/Ers ratio | 0.73 [0.63, 0.8] | 0.73 [0.64, 0.79] | 0.73 [0.63, 0.81] | 0.638 |
| Elastance-derived plateau PL—cmH2O | 18.1 [14.5, 21.6] | 17.5 [14.2, 20.8] | 19.1 [15.4, 22.9] | 0.012 |
| Directly-measured end-expiratory PL—cmH2O | − 2.3 ± 5 | − 1.9 ± 4.7 | − 3 ± 5.5 | 0.077 |
| Response to higher PEEP†† | (n = 311) | (n = 194) | (n = 117) | |
| Higher PEEP—cmH2O | 15 [14, 17] | 15 [14, 17] | 15 [14, 17] | 0.660 |
| Lower PEEP—cmH2O | 10 [9, 12] | 10 [9, 12] | 10 [9, 12] | 0.307 |
| ΔPaO2/FiO2—mmHg | 14 [− 3, 36] | 16 [− 4, 36] | 14 [− 2, 34] | 0.696 |
| ΔMAP—mmHg | − 2 [− 5, 2] | − 1 [− 5, 2] | − 2 [− 5, 1] | 0.735 |
| ΔDPaw—cmH2O | 1 [0, 2] | 1 [0, 2] | 1 [0, 2] | 0.503 |
| ΔDPL—cmH2O | 1 [0, 2] | 1 [0, 2] | 1.4 [0, 3] | 0.110 |
| ΔEnd-expiratory PL—cmH2O | 3.6 [2.6, 5] | 3.6 [2.6, 5] | 3.6 [2.3, 4.4] | 0.276 |
Categorical variables are described in numbers (percentage); continuous variables are described as mean ± standard derivation or median [interquartile ranges], as appropriate
*Comparing survivors with non-survivors using t-test or Mann–Whitney U-test or chi-square, as appropriate. P values were not corrected for multiple comparisons
†Oxygenation stretch index was measured in total 318 patients, of which 194 survivors and 124 non-survivors
††Measured variables at higher PEEP minus those at lower PEEP. For example, PaO2/FiO2 at high PEEP minus PaO2/FiO2 at lower PEEP
APACHE II Acute Physiology and Chronic Health Evaluation II score, IMV invasive mechanical ventilation, SOFA Sequential Organ Failure Assessment score, PEEP positive end-expiratory pressure, PaO2/FiO2 the ratio of partial pressure of arterial oxygen and fraction of inspired oxygen, VE,corr expired volume per minute corrected by arterial carbon dioxide, PEEP positive end-expiratory pressure, VT/PBW tidal volume per predicted body weight, Pplat airway plateau pressure, DPaw airway driving pressure which is the difference between Pplat and total PEEP, RR respiratory rate, Crs respiratory system compliance, Rrs respiratory system resistance, oxygenation-stretch index PaO2/FiO2 divided by DPaw, DPL transpulmonary driving pressure which is the tidal change in transpulmonary pressure, DPcw chest wall driving pressure which is the tidal change in esophageal pressure, CL lung compliance, Ccw chest wall compliance, EL/Ers ratio lung elastance to respiratory system elastance ratio, PL transpulmonary pressure, PaO2/FiO2 the ratio of partial pressure of arterial oxygen and fraction of inspired oxygen, MAP mean arterial pressure
Mechanics and outcome
Pes was measured in 302 patients (subset A: 80% of the cohort). The median occlusion test ratio (∆Paw/∆Pes) for validating the measured Pes was 0.91 [IQR 0.84–1.00].
AUCs of the ROC curves of DPaw, DPL, elastance-derived plateau PL, and directly measured end-expiratory PL for predicting mortality at day 60 were 0.62, 0.59, 0.58, and 0.53, respectively. There was no difference in AUCs between DPaw and DPL (P = 0.0835, Figure S1).
Unadjusted Cox regressions showed that higher DPaw, higher DPL, and higher elastance-derived plateau PL were associated with higher HR of death (Table 2). Directly measured end-expiratory PL was not statistically associated with 60-day mortality in this subset. DPaw and DPL remained associated with 60-day mortality after adjustment for age and SOFA (Table 2), with an attenuation of HR at low values of DPaw and DPL (Figure S2 and S3).
Table 2.
Cox proportional-hazards regression models (subset A*)
| Covariate | Unadjusted model | Adjusted model with DPaw | Adjusted model with DPL | Adjusted model with plateau PL | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) |
p value | HR (95% CI) |
p value | HR (95% CI) |
p value | HR (95% CI) |
p value | |
| Age |
1.026 (1.013–1.039) |
< 0.0001 |
1.025 (1.013–1.038) |
< 0.0001 |
1.026 (1.013–1.039) |
< 0.0001 |
1.026 (1.013–1.039) |
< 0.0001 |
| SOFA |
1.157 (1.103–1.214) |
< 0.0001 |
1.147 (1.091–1.206) |
< 0.0001 |
1.154 (1.098–1.213) |
< 0.0001 |
1.153 (1.097–1.212) |
< 0.0001 |
| DPaw |
1.116 (1.062–1.172) |
< 0.0001 |
1.093 (1.040–1.148) |
0.0004 | ||||
| DPL |
1.089 (1.036–1.144) |
0.0007 |
1.080 (1.028–1.134) |
0.0023 | ||||
| Plateau PL |
1.045 (1.009–1.082) |
0.0140 |
1.034 (0.999–1.070) |
0.0533 | ||||
| End-expiratory PL |
0.982 (0.942–1.023) |
0.3900 | ||||||
| AIC† of adjusted model | 1119 | 1123 | 1128 | |||||
*Subset A included 302 patients with measured esophageal pressure. Number of deaths was 107
†The model with lowest AIC is the one that explains the greatest amount of variation using the fewest possible independent variables. It addresses goodness of fit and simplicity of the model. Since the models presented in the Table have the same number of independent variables and the same sample, the model with lowest AIC is the best-fit model
AIC Akaike information criterion, SOFA Sequential Organ Failure Assessment score, DPaw airway driving pressure which is the difference between Pplat and total PEEP, DPL transpulmonary driving pressure which is the tidal change in transpulmonary pressure
Comparing adjusted Cox models, DPaw had slightly better goodness-of-fit (lower AIC when the number of covariates were the same) than DPL (Table 2). We performed post-hoc explanatory analyses to understand this result (See “Discussion”) and found that chest wall driving pressure was correlated with non-pulmonary SOFA (Pearson’s correlation coefficient = 0.150, P = 0.0090) and associated with 60-day mortality in unadjusted Cox regression (HR: 1.136, 95% CI 1.018–1.268, P = 0.023).
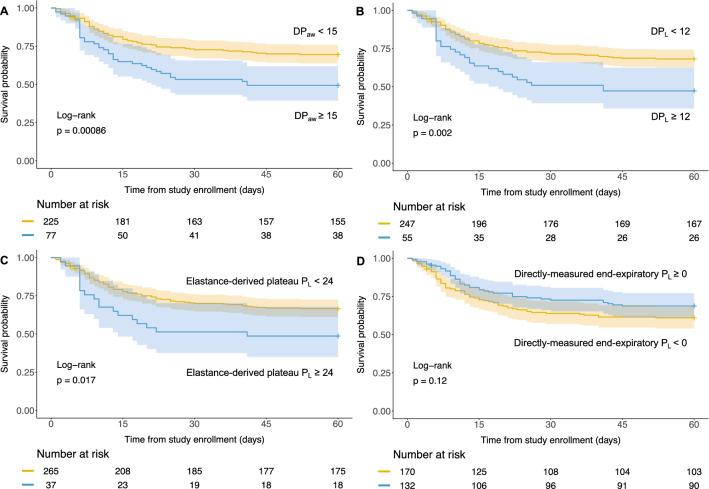
By grouping patients using thresholds used in previous studies, patients with high DPaw (≥ 15 cmH2O), high DPL (≥ 12 cmH2O) and high elastance-derived plateau PL (≥ 24 cmH2O) had lower 60-day survival probability (log-rank test, P < 0.05) as shown with Kaplan–Meier survival curves (Fig. 2). There was no difference in survival probability comparing positive (≥ 0 cmH2O) with negative end-expiratory PL (Fig. 2).
Fig. 2.
Kaplan–Meier plots for patients who were measured esophageal pressure (n = 302). Patients were grouped by the dichotomized airway driving pressure (DPaw), transpulmonary driving pressure (DPL), elastance-derived plateau PL, and directly-measured end-expiratory PL, respectively
Post-hoc analyses for the obese patients
Previous study [23] showed that higher Body Mass Index correlated with higher directly measured end-expiratory PL, supporting the hypothesis that PEEP might be particularly helpful for maintaining functional residual capacity in obese patients with more load (weight) due to fat. A recent study [24] also well demonstrated the usefulness of high airway pressure in this population. We thus conduct post-hoc analyses in patients with Pes measurement (n = 302) and found an interaction between directly measured end-expiratory PL and obesity (Body Mass Index ≥ 30 kg/m2) with regard to 60-day mortality in the adjusted Cox regression model (HR for the interaction term: 0.904, 95% CI 0.832–0.982, P = 0.0175). No significant interaction with obesity was found for DPaw, DPL, elastance-derived plateau PL. There were 123 obese patients according to the abovementioned classification. Among them, obese patients with positive end-expiratory PL had higher survival probability than those with negative end-expiratory PL (Fig. 3, Log-rank test: 0.0042).
Fig. 3.
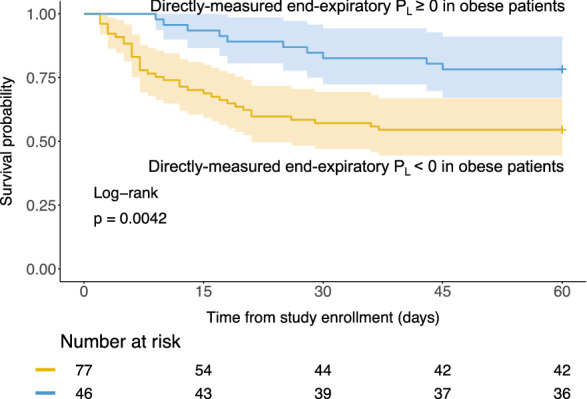
Kaplan–Meier plot for obese patients who were measured esophageal pressure (n = 123). Obesity was defined by body mass index ≥ 30 kg/m2, as per World Health Organization classification. Patients were grouped by the dichotomized end-expiratory PL. Notice that this is a post-hoc analysis
Oxygenation stretch index
We obtained arterial blood gases in 318 patients (subset B: 84% the cohort). Oxygenation stretch index did not present significant higher AUC for predicting mortality at day 60 (binary outcome) than DPaw (0.59 vs. 0.58, P = 0.6483, see Figure S4).
Unadjusted Cox regression (Table S1) showed that higher oxygenation stretch index was associated with markedly lower 60-day mortality (HR: 0.959, 95% CI 0.930–0.989, P = 0.0082). Since both SOFA and oxygenation stretch index include PaO2/FiO2 as a component, multicollinearity would exist if they were added together in the multivariable Cox regression model. We thus adjusted the Cox model by age and non-pulmonary SOFA rather than SOFA. After adjustment, oxygenation stretch index remained associated with outcome (HR: 0.969, 95% CI 0.939–0.999, P = 0.0490).
We repeated the modeling for DPaw on the same subset of patients to compare AIC. Oxygenation stretch index did not improve AIC comparing with DPaw (Table S1). To explain this result, we performed a post-hoc analysis to examine the association of oxygenation with outcome. Oxygenation (PaO2/FiO2) was not significantly associated with outcome in a Cox regression model after adjustment by DPaw (Table S2. HR: 0.998, 95% CI 0.994–1.001, P = 0.2161).
By dichotomizing oxygenation stretch index, patients with high oxygenation stretch index (≥ 10 mmHg/cmH2O) had higher survival probability than patients with low oxygenation stretch index (< 10 mmHg/cmH2O) (Fig. 4, unadjusted log-rank test: P = 0.0033).
Fig. 4.
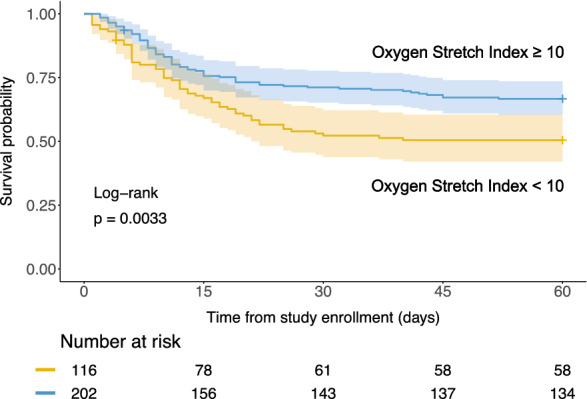
Kaplan–Meier plot for patients with arterial blood gas measurement (n = 318), who were grouped by the dichotomized oxygenation stretch index
Post-hoc analyses on mechanical power and DPaw × 4 + RR
Although respiratory rate did not reach to statistically significant in univariate comparison (P = 0.061) and unadjusted Cox regression (P = 0.084), recent studies have shown the importance of respiratory rate for VILI [25]. We thus performed post-hoc analyses on mechanical power [20] and a recently proposed composite index – DPaw × 4 + RR [26]. The ROC analyses showed that DPaw × 4 + RR and power did not present significantly higher AUC than DPaw alone for predicting the binary outcome—mortality at day 60 (Figure S5). After the adjustment for age and SOFA, the Cox regression models showed that DPaw × 4 + RR had lowest AIC (see Table S3). Mechanical power was associated with 60-day mortality (time-dependent outcome) only when it was normalized by respiratory system compliance (Table S3).
Post-hoc sensitivity analysis for the time of measurement
To assess the possible effect of measurement time on outcome, we tested in multivariable Cox regression models the interaction term between mechanical variable of interest and a dichotomous covariate represented the time point of measurement [early (≤ 2 days); late (> 2 days)]. We chose 2 days as threshold to define early and late measurement because it represents the median value of the measurement time. There was no significant interaction between early measurement and mechanics with respect to 60-day mortality (Table S5).
Post-hoc sensitivity analysis for excluding patients who were still ventilated at day 60
We excluded 37 patients who were possibly still ventilated after 60 days (of which 17 patients missing data on ventilator weaning date) from 302 patients with Pes measured. Eventually, 265 patients were involved in this sensitivity analysis. DPaw, DPL, elastance-derived plateau PL, remained associated with 60-day mortality after adjusted by age and SOFA. And again, the Cox regression model DPaw showed lowest AIC (Table S6).
Discussion
To our knowledge, this is the largest clinical study linking data of partitioned respiratory mechanics with outcome in patients with ARDS who are already receiving 6 ml/kg PBW of VT. Our data showed that DPaw and DPL have similar predictive power for mortality at day 60 (binary outcome) in ROC analysis. Cox regression confirmed that DPaw had the highest ranked association with 60-day mortality (time-dependent outcome) after adjustment for age and SOFA (or non-pulmonary SOFA), among DPL, elastance-derived plateau PL, directly-measured end-expiratory PL, and oxygenation stretch index. Our post-hoc explanatory analyses showed the following: (1) chest wall driving pressure (a component of DPaw) was also associated with disease severity and survival rate; (2) positive end-expiratory PL was associated with better outcome only in obese patients; (3) oxygenation was not significantly associated with outcome after adjustment for DPaw.
Prediction versus association
It is worthy to emphasize that our ultimate goal was not the mortality prediction but to look for the most important variable that can be targeted for limiting the risk of VILI. Thus, we tested the associations between mechanics and outcomes as an indirect approach. Our main method to test the associations between the outcome and mechanics is Cox regression by treating the physiologic variables as continuous variables. We then dichotomized these variables in Kaplan–Meier survival analysis as a secondary method to test associations. Any kind of dichotomization does have limitation, but we want to see whether the previously proposed thresholds could well differentiate outcomes, and if they could potentially be used in clinical practice. Regarding the low predictive power in the ROC analyses (see the supplement), we want to stress that the predictive validity for mortality of the old American-European Consensus Conference (AECC) definition of ARDS was 0.536, whereas the Berlin definition was slightly improved but only to 0.577, in terms of AUC [16]. These definitions have several criteria, which can be considered as composite variables. Hence, the AUC of 0.62 for DPaw, as a single variable, is probably a solid signal.
Lung versus respiratory system
The observation that DPL was not better associated with outcome than DPaw was unexpected [7]. We think that one explanation is the association between chest wall driving pressure, disease severity [27] and outcome. It indicates that DPaw may contain information about both the risk of VILI through the best indicator of lung distension, i.e., DPL, and also about general severity of the patient resulting in stiffer chest wall. As such, it is possible that DPaw is essentially a composite index, and DPL may remain more specific to VILI. Alternatively, the measurement of Pes changes over the low VT used for assessing the chest wall elastance may suffer from imprecision due to the magnitude of cardiac artifact. We implemented standardized training and a specific protocol to help with Pes measurements [15]. The occlusion test ratio, an index addressing the validity of Pes, was in a valid range (0.8 to 1.2) [14, 18, 28]. Moreover, we performed the same analysis by using Pes adjusted by the occlusion test ratio, assuming a linear relation between ∆Paw and ∆Pes. It slightly strengthened the adjusted Cox regression model (AIC = 1122) but did not change the conclusions.
Setting PEEP to reverse a negative end-expiratory PL, an indicator of collapse of dorsal lung, makes physiological sense [29] and has been used in randomized clinical trials but with no impact on outcome [30]. This is in keeping with our data which demonstrated that end-expiratory PL was not associated with outcome. We should, however, also keep in mind that the average clinical PEEP level was 12 [IQR 10–14] cmH2O. The risk of “atelectrauma” [31] in the dorsal lung might have been already been reduced by clinical PEEP. In other words, a relatively narrow variance of PEEP levels might be insufficient to detect any significance of end-expiratory PL.
Interestingly, the end-expiratory PL seemed to be highly relevant in obese patients, who have a higher fat “load” than non-obese patients. This load is counter-balanced by the outward recoil force of chest wall. But when respiratory muscles are relaxed due to sedation or paralysis, a high external PEEP might be necessary to counterbalance this load and maintain end-expiratory lung volume. Hence, keeping a positive end-expiratory PL in obese was associated with a more favorable outcome, as suggested by our data.
Oxygenation stretch index versus DP aw
Our finding that oxygenation stretch index, a composite index of oxygenation and DPaw, failed to strengthen the predictive power was unexpected. Interestingly, the association between oxygenation and outcome “disappeared” once it was adjusted by DPaw. This was even clearer when it was adjusted by age, non-pulmonary SOFA, and DPaw (Table S2. HR for oxygenation: 0.998, 95% CI 0.994–1.001, P = 0.2161). These results highlight the predominance of DPaw over oxygenation, which is currently used to classify the severity of ARDS [16].
Limitations
Our study has several limitations. One limitation is that the measurement was performed at a single time point at the early onset of mechanical ventilation like previous studies aiming to link mechanics with outcomes [3]. Since mechanics might alter during the process of ARDS, the measurements, ideally, should be repeated daily. The repeat measurements however can be practically challenging as patients often generate spontaneous breathing effort after a few days of intubation which would greatly complicate the interpretation of measurements. Second, we used a fixed balloon volume (e.g., 1 ml for Cooper catheter), similar to other trials for simplicity [9, 30]. Our conclusions remain unchanged after adjusting Pes by the occlusion test ratio. Third, complete airway closure occurs in almost one-third of ARDS patients which can impact the accuracy of the measurement of elastance, and hence on elastance-derived plateau PL. The present registry was done by measuring mechanics at clinical PEEP level. In our previous study [32], 15 out of 45 (33.3%) patients presented complete airway closure, which means, only 4 (8.8%) patients received a clinical PEEP below AOP. We reasoned that most of patients with airway closure should be receiving clinical PEEP higher than their airway opening pressure (AOP) in the present study, and thus, this would not substantially alter the interpretation of our results. Moreover, we also repeated the survival analysis by using mechanics measured at higher PEEP level (with the lowest likelihood of having the clinical PEEP below the AOP), and the conclusions remain unchanged except the elastance-derived plateau PL became non-significant (see Figure S6 in the Supplement). Fourth, the time of measurement was not homogeneous among patients. Our sensitivity analysis, however, showed that the measurement time did not change the effect of the mechanics variables on the outcome. Fifth, we have missing data as indicated in Fig. 1. Handling these missing data can be difficult, and the best approach is debatable. While multivariate imputation is an alternative option with strengths and weaknesses [33], we decided to use the conventional approach—complete case analysis. Sixth, there were 54 patients affected by coronavirus disease 2019 (COVID-19) enrolled into the registry but none of these COVID-19 patients measured esophageal pressure due to infection control precautions. In other words, our results showing that transpulmonary pressures did not have stronger association with outcome than DPaw, was found in non-COVID-19 patients. Last but not least, we did not collect data on key co-interventions for ARDS during the entire hospital stay, such as prone positioning, dose and duration of neuromuscular blockade, steroids, and ECMO. Particularly, prone positioning prior to the measurements might have been influenced respiratory mechanics.
Conclusion
Transpulmonary driving pressure, surprisingly, did not present a stronger association with outcome than airway driving pressure, which might partly be explained by the fact that chest wall driving pressure was associated with disease severity and outcome. Oxygenation stretch index, a composite index of oxygenation and airway driving pressure, did not outperform airway driving pressure alone. Indeed, oxygenation was no longer associated with outcome after adjustment for airway driving pressure. Our study enhances the rationale of limiting airway but also lung driving pressure during mechanical ventilation. In addition, targeting a positive end-expiratory transpulmonary pressure seems to be relevant in obese patients. These data may help in designing new ventilatory strategies based on respiratory mechanics.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (DOCX 868 KB) Supplemental data and figures
Supplementary file2 (DOCX 33 KB) STROBE statement
Acknowledgements
We are grateful for Stephen Loring and Daniel Talmor’s support, who kindly provide us EPVent study data for sample size estimation. We greatly appreciate the respiratory therapists in the Department of Respiratory Therapy, St Michael’s Hospital (Toronto, Canada) for their persistent support and active involvement in this project, with special thanks to Orest Shklar, Pamela Greco, Hilary Every, and Thomas Piraino. We are full of gratitude to Jianing Gu and Audery Kim for their essential work on data collection and organization. This study is a part of Lu Chen’s PhD program, supervised by Laurent Brochard and the Program Advisory Committee – Brian Kavanagh, John Laffey, Haibo Zhang, Arthur Slutsky, and Eddy Fan. We deeply thank their invaluable advice for improving the study.
Authors’ contributions
All authors made substantial contributions to the conception or design of the study, or the acquisition or interpretation of data for the study. LC, CL, and FM conducted the statistical analysis. LC and LB drafted the manuscript. All authors revised the draft of the manuscript. All authors read and approved the final manuscript.
Funding
Dr. François Beloncle received a grant from IRSR Pays de la Loire for conducting the study in his institute. The sponsor had no role in study design, data collection, data analysis, data interpretation, or writing of the report. Dr Laurent Brochard holds the Keenan Chair in Critical Care and Acute Respiratory Failure.
Data availability
The data used in the present study are available from the corresponding author upon reasonable request.
Declarations
Conflicts of interest
All authors declared having no conflict of interest related to this work. Unrelated potential conflict are as follows: FB received research support from GE Healthcare, Medtronic and Getinge Group, consulting fees from Löwenstein Medical and travel fees from Air Liquide Medical System, which are not related to the present study. LB’s laboratory received research grants from Medtronic and Draeger, equipment from Sentec, Fisher Paykel, Philips and Air Liquide, and lecture fees from Fisher Paykel.
Ethics approval
The study has been independently reviewed and approved by the local Institutional Review Board of each participated centre.
Footnotes
The original online version of this article was revised: The Supplementary file 1 contained mistakes and has been replaced with a corrected file.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
2/2/2023
A Correction to this paper has been published: 10.1007/s00134-023-06985-1
References
- 1.Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med. 2013;369:2126–2136. doi: 10.1056/NEJMra1208707. [DOI] [PubMed] [Google Scholar]
- 2.Acute Respiratory Distress Syndrome N. Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 3.Amato MB, Meade MO, Slutsky AS, Brochard L, Costa EL, Schoenfeld DA, Stewart TE, Briel M, Talmor D, Mercat A, Richard JC, Carvalho CR, Brower RG. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med. 2015;372:747–755. doi: 10.1056/NEJMsa1410639. [DOI] [PubMed] [Google Scholar]
- 4.Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, Gattinoni L, van Haren F, Larsson A, McAuley DF, Ranieri M, Rubenfeld G, Thompson BT, Wrigge H, Slutsky AS, Pesenti A, Investigators LS, Group ET. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315:788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 5.Urner M, Juni P, Hansen B, Wettstein MS, Ferguson ND, Fan E. Time-varying intensity of mechanical ventilation and mortality in patients with acute respiratory failure: a registry-based, prospective cohort study. Lancet Respir Med. 2020;8:905–913. doi: 10.1016/S2213-2600(20)30325-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pereira Romano ML, Maia IS, Laranjeira LN, Damiani LP, Paisani DM, Borges MC, Dantas BG, Caser EB, Victorino JA, Filho WO, Amato MBP, Cavalcanti AB. Driving pressure-limited strategy for patients with acute respiratory distress syndrome. a pilot randomized clinical trial. Ann Am Thorac Soc. 2020;17:596–604. doi: 10.1513/AnnalsATS.201907-506OC. [DOI] [PubMed] [Google Scholar]
- 7.Baedorf Kassis E, Loring SH, Talmor D. Mortality and pulmonary mechanics in relation to respiratory system and transpulmonary driving pressures in ARDS. Intensive Care Med. 2016;42:1206–1213. doi: 10.1007/s00134-016-4403-7. [DOI] [PubMed] [Google Scholar]
- 8.Loring SH, O'Donnell CR, Behazin N, Malhotra A, Sarge T, Ritz R, Novack V, Talmor D. (2010) Esophageal pressures in acute lung injury: do they represent artifact or useful information about transpulmonary pressure, chest wall mechanics, and lung stress? J Appl Physiol. 1985;108:515–522. doi: 10.1152/japplphysiol.00835.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Talmor D, Sarge T, Malhotra A, O'Donnell CR, Ritz R, Lisbon A, Novack V, Loring SH. Mechanical ventilation guided by esophageal pressure in acute lung injury. N Engl J Med. 2008;359:2095–2104. doi: 10.1056/NEJMoa0708638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoshida T, Amato MBP, Grieco DL, Chen L, Lima CAS, Roldan R, Morais CCA, Gomes S, Costa ELV, Cardoso PFG, Charbonney E, Richard JM, Brochard L, Kavanagh BP. Esophageal manometry and regional transpulmonary pressure in lung injury. Am J Respir Crit Care Med. 2018;197:1018–1026. doi: 10.1164/rccm.201709-1806OC. [DOI] [PubMed] [Google Scholar]
- 11.Tilmont A, Coiffard B, Yoshida T, Daviet F, Baumstarck K, Brioude G, Hraiech S, Forel JM, Roch A, Brochard L, Papazian L, Guervilly C. Oesophageal pressure as a surrogate of pleural pressure in mechanically ventilated patients. ERJ Open Res. 2021;7:2. doi: 10.1183/23120541.00646-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gattinoni L, Carlesso E, Cadringher P, Valenza F, Vagginelli F, Chiumello D. Physical and biological triggers of ventilator-induced lung injury and its prevention. Eur Respir J Suppl. 2003;47:15s–25s. doi: 10.1183/09031936.03.00021303. [DOI] [PubMed] [Google Scholar]
- 13.Grasso S, Terragni P, Birocco A, Urbino R, Del Sorbo L, Filippini C, Mascia L, Pesenti A, Zangrillo A, Gattinoni L, Ranieri VM. ECMO criteria for influenza A (H1N1)-associated ARDS: role of transpulmonary pressure. Intensive Care Med. 2012;38:395–403. doi: 10.1007/s00134-012-2490-7. [DOI] [PubMed] [Google Scholar]
- 14.Mauri T, Yoshida T, Bellani G, Goligher EC, Carteaux G, Rittayamai N, Mojoli F, Chiumello D, Piquilloud L, Grasso S, Jubran A, Laghi F, Magder S, Pesenti A, Loring S, Gattinoni L, Talmor D, Blanch L, Amato M, Chen L, Brochard L, Mancebo J, Group PL. Esophageal and transpulmonary pressure in the clinical setting: meaning, usefulness and perspectives. Intensive Care Med. 2016;42:1360–1373. doi: 10.1007/s00134-016-4400-x. [DOI] [PubMed] [Google Scholar]
- 15.Chen L, Chen GQ, Shore K, Shklar O, Martins C, Devenyi B, Lindsay P, McPhail H, Lanys A, Soliman I, Tuma M, Kim M, Porretta K, Greco P, Every H, Hayes C, Baker A, Friedrich JO, Brochard L. Implementing a bedside assessment of respiratory mechanics in patients with acute respiratory distress syndrome. Crit Care. 2017;21:84. doi: 10.1186/s13054-017-1671-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the berlin definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 17.Washko GR, O'Donnell CR, Loring SH. (2006) Volume-related and volume-independent effects of posture on esophageal and transpulmonary pressures in healthy subjects. J Appl Physiol. 1985;100:753–758. doi: 10.1152/japplphysiol.00697.2005. [DOI] [PubMed] [Google Scholar]
- 18.Lanteri CJ, Kano S, Sly PD. Validation of esophageal pressure occlusion test after paralysis. Pediatr Pulmonol. 1994;17:56–62. doi: 10.1002/ppul.1950170110. [DOI] [PubMed] [Google Scholar]
- 19.Baydur A, Behrakis PK, Zin WA, Jaeger M, Milic-Emili J. A simple method for assessing the validity of the esophageal balloon technique. Am Rev Respir Dis. 1982;126:788–791. doi: 10.1164/arrd.1982.126.5.788. [DOI] [PubMed] [Google Scholar]
- 20.Gattinoni L, Tonetti T, Cressoni M, Cadringher P, Herrmann P, Moerer O, Protti A, Gotti M, Chiurazzi C, Carlesso E, Chiumello D, Quintel M. Ventilator-related causes of lung injury: the mechanical power. Intensive Care Med. 2016;42:1567–1575. doi: 10.1007/s00134-016-4505-2. [DOI] [PubMed] [Google Scholar]
- 21.Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983;148:839–843. doi: 10.1148/radiology.148.3.6878708. [DOI] [PubMed] [Google Scholar]
- 22.R Core Team (2018) R: A Language and Environment for Statistical Computing. In: Book R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, City
- 23.Coudroy R, Vimpere D, Aissaoui N, Younan R, Bailleul C, Couteau-Chardon A, Lancelot A, Guerot E, Chen L, Brochard L, Diehl JL. Prevalence of complete airway closure according to body mass index in acute respiratory distress syndrome: pooled cohort analysis. Anesthesiology. 2020;2:2. doi: 10.1097/ALN.0000000000003444. [DOI] [PubMed] [Google Scholar]
- 24.De Santis SR, TeggiaDroghi M, Fumagalli J, Marrazzo F, Florio G, Grassi LG, Gomes S, Morais CCA, Ramos OPS, Bottiroli M, Pinciroli R, Imber DA, Bagchi A, Shelton K, Sonny A, Bittner EA, Amato MBP, Kacmarek RM, Berra L, Lung Rescue I. High pleural pressure prevents alveolar overdistension and hemodynamic collapse in ARDS with class III obesity. Am J Respir Crit Care Med. 2020;2:2. doi: 10.1164/rccm.201909-1687OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marini JJ, Rocco PRM, Gattinoni L. Static and dynamic contributors to ventilator-induced lung injury in clinical practice. pressure, energy, and power. Am J Respir Crit Care Med. 2020;201:767–774. doi: 10.1164/rccm.201908-1545CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Costa ELV, Slutsky AS, Brochard LJ, Brower R, Serpa-Neto A, Cavalcanti AB, Mercat A, Meade M, Morais CCA, Goligher E, Carvalho CRR, Amato MBP. Ventilatory variables and mechanical power in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2021;204:303–311. doi: 10.1164/rccm.202009-3467OC. [DOI] [PubMed] [Google Scholar]
- 27.Jardin F, Genevray B, Brun-Ney D, Bourdarias JP. Influence of lung and chest wall compliances on transmission of airway pressure to the pleural space in critically ill patients. Chest. 1985;88:653–658. doi: 10.1378/chest.88.5.653. [DOI] [PubMed] [Google Scholar]
- 28.Akoumianaki E, Maggiore SM, Valenza F, Bellani G, Jubran A, Loring SH, Pelosi P, Talmor D, Grasso S, Chiumello D, Guerin C, Patroniti N, Ranieri VM, Gattinoni L, Nava S, Terragni PP, Pesenti A, Tobin M, Mancebo J, Brochard L, Group PW. The application of esophageal pressure measurement in patients with respiratory failure. Am J Respir Crit Care Med. 2014;189:520–531. doi: 10.1164/rccm.201312-2193CI. [DOI] [PubMed] [Google Scholar]
- 29.Cavalcanti AB, Amato MBP, Serpa-Neto A. The elusive search for "Best PEEP" and whether esophageal pressure monitoring helps. JAMA. 2019;321:839–841. doi: 10.1001/jama.2019.0267. [DOI] [PubMed] [Google Scholar]
- 30.Beitler JR, Sarge T, Banner-Goodspeed VM, Gong MN, Cook D, Novack V, Loring SH, Talmor D, Group EPS. Effect of titrating positive end-expiratory pressure (PEEP) with an esophageal pressure-guided strategy vs an empirical high PEEP-Fio2 strategy on death and days free from mechanical ventilation among patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA. 2019;321:846–857. doi: 10.1001/jama.2019.0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muscedere JG, Mullen JB, Gan K, Slutsky AS. Tidal ventilation at low airway pressures can augment lung injury. Am J Respir Crit Care Med. 1994;149:1327–1334. doi: 10.1164/ajrccm.149.5.8173774. [DOI] [PubMed] [Google Scholar]
- 32.Chen L, Del Sorbo L, Grieco DL, Junhasavasdikul D, Rittayamai N, Soliman I, Sklar MC, Rauseo M, Ferguson ND, Fan E, Richard JM, Brochard L. Potential for lung recruitment estimated by the recruitment-to-inflation ratio in acute respiratory distress syndrome. a clinical trial. Am J Respir Crit Care Med. 2020;201:178–187. doi: 10.1164/rccm.201902-0334OC. [DOI] [PubMed] [Google Scholar]
- 33.Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, Wood AM, Carpenter JR. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. doi: 10.1136/bmj.b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file1 (DOCX 868 KB) Supplemental data and figures
Supplementary file2 (DOCX 33 KB) STROBE statement
Data Availability Statement
The data used in the present study are available from the corresponding author upon reasonable request.