Abstract
Connexins are assembled into dodecamer intercellular channels, a collection of which is termed a gap junction, and their canonical function allowing direct exchange of ions and metabolites has been unequivocally established. When initially assembled into undocked cell surface connexin hemichannels, healthy cells may also engage in cell signaling via a regulated small-molecule release. Recent advances in the field have led to an expanded view of the functional roles of intercellular channels and hemichannels in both physiology and pathology. As more of the 21-member human connexin family is intensely interrogated, mounting evidence points to the biological uniqueness of each member, and no longer can we confidently refer to all connexins engaging in the same cellular processes. Innovations in high-resolution cryo-electron microscopy have revealed important insights into the structure of functionally important domains of both hemichannels and channels. These and other studies have established a foundation of knowledge that should allow inhibitory smart drug design for situations where enhanced intercellular or hemichannel activity is at the root of a connexin-linked disease. Assessment of the connexin interactome, which varies widely for each connexin subtype, continues to provide regulatory insights into the assembly and function of connexins that exhibit a short half-life. As the most intensely studied, Cx43 is found in about 50% of all human cell types and is extensively regulated by multiple inhibitory and enhancing phosphorylation events that have direct implications on tissue function and outcomes of disease, including cancer. Here, we briefly discuss these advances and give our thoughts on where the field is headed.
Keywords: connexin, gap junctions, channel, hemichannel
Introduction
The most well-known canonical functions of gap junctions are to permit and regulate the intercellular exchange of hundreds of small cellular metabolome constituents1,2. Gap junctions play both structural and intercellular communication roles to help regulate many cell processes, including cell migration, cell proliferation, embryonic development, differentiation, wound repair, and the coordinated contraction of heart and smooth muscle2. There is unequivocal evidence that, in humans, the channel-lining proteins of gap junctions are composed of proteins from the 21-member connexin gene family3 (Figure 1). Genetic linkage analyses have associated 11 of these connexins to at least 30 human diseases with broad phenotypes, including deafness, syndactyly, skin diseases, neuropathies, lymphedema, cataracts, and developmental defects, emphasizing the fact that connexins are expressed in a tissue-specific manner4. However, not only are connexins linked to disease through gene mutations, but their role in cancer continues to be a focal point of research as the community continues to assess the potential value of prioritizing at least some connexins as therapeutic targets for specific tumor types or in cancer stem cells5–8. Still, other studies have identified the value of transiently downregulating connexin expression or function in diseases of the eye and in diabetic wounds2,9,10. There remains no doubt that connexins play critical roles in healthy cells as well as in pathophysiology. New advances in the areas of gap junction structure and regulation continue to expand the breadth of functions linked to connexins in cell biology.
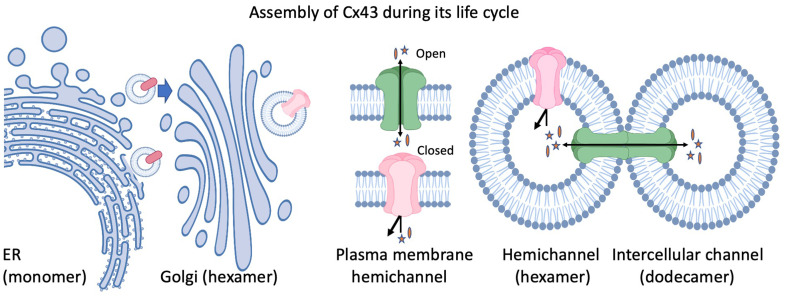
Figure 1. Assembly of Cx43 during its life cycle.
Cx43 is produced in the endoplasmic reticulum (ER) and is exported to the Golgi apparatus as a monomer. In the Golgi, Cx43 oligomerizes into hexamers and is exported to the plasma membrane, where it can exist as a closed (red channel) or open (green) hemichannel that can allow the regulated passage of specific small molecules (denoted by stars and ovals). Hemichannels from adjoining cells dock and form a head-to-head dodecameric structure (gap junction channel) to permit the regulated intercellular passage of small molecules and metabolites.
Revisiting the canonical function of connexins
Interrogation by X-ray diffraction, freeze-fracture electron microscopy, nuclear magnetic resonance, and atomic force microscopy, together with sophisticated image analysis, revealed that the dodecameric arrangement of connexins was needed to form a complete intercellular channel11–14. Direct confirmation of the connexin arrangement and molecular details of the pore-lining domains were lacking until 2009 when Maeda and colleagues solved the structure of the Cx26 gap junction channel at a resolution of 3.5 Å15. New details, such as the identification of positively charged residues at the cytoplasmic entrance to the channel, began to emerge from these high-resolution structures15. More recently, it was shown that, when calcium bound to Cx26, the electrostatic barrier changed to prevent the passage of channel permeants, a process previously proposed to be related to a wholesale movement of connexin subunits16. The emergence of single-particle image analysis and cryo-electron microscopy (cryo-EM) accelerated the structural revolution as high-resolution images of native lens gap junctions composed of Cx46 and Cx50 were obtained17. Here, the authors definitively proved the transmembrane domain arrangements and found open conformational states that were distinct from Cx2617, possibly revealing differences between alpha and beta connexin family members. This same team further resolved the structure of Cx46/50 channels in a dynamic aqueous-lipid context at near atomic resolution (1.9 Å), where the connexin proteins were found to stabilize the local lipid microenvironment18. This level of molecular detail is now sufficiently informative to serve as a model for rational drug design for the possible treatment of any number of inherited connexin-linked diseases4,8 and the countless pathologies linked to connexin regulation in diseased tissue and cancer19.
While connexins form intercellular channels that permit the direct passage of metabolomes, the 1991 discovery that macrophages could release ATP, via what the authors termed “half-gap junctions” or what today we would call hemichannels, expanded our view of connexin function20. In the years that followed, the community had to consider that connexins may, in fact, have a second canonical function in forming cell surface channels capable of releasing (or, on occasion, internalizing) metabolites or a variety of signaling molecules or both. While this core cellular function has molecular rivals, most notably pannexin channels21,22, there was no doubt that connexin hemichannel functions needed to be considered in both physiological and pathological contexts. The question continues to arise as to where and when connexin hemichannels play a role in normal healthy physiology and where and when aberrant hemichannel activity would lead to cell death or disease. No fewer than 1500 peer-reviewed papers have featured the existence and functional role of connexin hemichannels in virtually every organ of the human body. These studies range from Cx43 hemichannels engaging in a ventricular arrhythmogenic mechanism within microdomains of cardiac intercalated discs23 to Cx43 hemichannels in astrocytes serving as a therapeutic target in spinal cord injury24 and to Cx43 hemichannels in osteocytes playing a critical role in mechanical load-induced bone formation25. Intuitively, connexin intercellular channels tend to favor an open state, as supported by recent structural experimental evidence, but our view is that hemichannels must have a resting closed state under homeostatic conditions in order to protect the integrity of the cytosol17,18,26–30.
However, evidence continues to mount that hemichannels play a more universal and canonical functional role in non-diseased tissues. As an example, considerable evidence suggests that Cx31.3 may not even have the capacity to form traditional gap junction intercellular channels, raising the notion that their main role in cells may be to act as functional hemichannels31. Likewise, the evidence for functional Cx46 and Cx50 hemichannels in highly specialized lens tissue in the presence and absence of disease is compelling32,33. Still, other less well-studied connexins like Cx62 may have dual roles in platelets, functioning as both hemichannels and intercellular channels34. One must also consider that connexin hemichannels may only partially open, permitting the smallest of channel permeants to pass. Insight into this notion was provided when cryo-EM was used to solve the hemichannel structure of a connexin that appears unable to form functional gap junction channels, Cx31.327,31. Resolution analysis that exceeded 2.6 Å revealed that Cx31.3 hemichannels adapt to a partially closed state that would allow the passage of chloride ions through the 8 Å pore while preventing the passage of other cell metabolites27. Cryo-EM was also used to examine the open conformation of Cx26-N176Y mutant hemichannels within dynamic lipid bilayer nanodiscs28. Here, the alpha-helical structures found within the mutant Cx26 hemichannels were found to be the same as reported for Cx26 intercellular channels while the flexibility identified within the extracellular loops would probably serve to facilitate hemichannel docking in cases where complete gap junction channels are formed28. However, likely owing to their intrinsic disorder, we still know little about the structure of intracellular loop and C-terminal tail regions. Thus, there is no doubt that further high-resolution analysis of different connexin hemichannels and intercellular channels will continue to inform on their open and closed states.
To our minds, there remains little doubt that aberrant connexin hemichannel function is at the root of several inherited connexin-linked diseases and cases of tissue injury as well as in chronic and acute diseases. Many missense and truncating connexin-gene mutations lead to hyperactive or leaky hemichannels that appear especially prevalent in connexin-linked skin diseases4,35,36. Mutations in this class often lead to the loss of cell integrity and cell death, but other mechanisms of action need to be considered4,35,36. Blocking connexin hemichannels as a means of regulating the inflammatory response has given credence to the notion that this may be an effective strategy to treat chronic inflammatory eye diseases and eye injuries9. In fact, hyperactive or leaky hemichannels in disease or injury are somewhat ideal targets when considering commercialization and deployment of connexin hemichannel blockers2,8,37. Preferably, such hemichannel blockers should be specific to the aberrant hemichannel in question, a consideration that aligns with smart small-molecule design modeled from high-resolution connexin hemichannel structures, peptide mimetics that take into account both structure and domain flexibilities, and high-avidity antibodies. As an example, an antagonist antibody was shown to have efficacy in blocking leaky mutant Cx30 hemichannels in the treatment of Clouston syndrome38. Similarly, the hemichannel blocker flufenamic acid was found to inhibit aberrant Cx26-G45E hemichannel function, reducing the symptoms of keratitis ichthyosis deafness found in mutant mice39. As noted by others, several connexin-based therapeutics that have entered clinical trials need to consider the specificity of the connexin blocking agent and whether the pathological features of hemichannels can be selectively and effectively targeted40.
Finally, while connexins are foundational molecules needed to assemble both hemichannels and gap junction channels, we would be remiss if we did not draw attention to the connexin interactome. The connexin interactome is highly dependent on the connexin family member as some interactomes are small (e.g., Cx26), while others are in excess of 50 proteins (e.g., Cx43)41. In the case of Cx43, the interactome includes proteins involved in protein trafficking, connexin turnover, connexin assembly, connexin post-translational modification, scaffolding, and other functions that have been extensively reviewed elsewhere41,42. The gap junction scaffold is also functionally important for other connexins, as has been shown for Cx30 in the cochlea, where ephrin-B2 interacts at the periphery of the gap junction regulating gap junction turnover43 in a manner perhaps analogous to ZO-1 interaction with Cx4344. We believe the scaffold function of gap junctions will be found to play critical regulatory roles as more research is performed on other connexins, and we look forward to new revelations.
Regulation of gap junction channels by phosphorylation
Our current understanding of the regulation of gap junctions is heavily biased toward Cx43. Cx43 is expressed in most cell lines even if they originated from cells that do not typically express Cx43 (e.g., hepatocytes express Cx32 and Cx26, but cell lines derived from them typically express only Cx43). Furthermore, our analysis of the literature suggests that Cx43 is natively expressed in nearly half of the more than 200 cell types found in the human body. Many Cx43 post-translational modifications, including ubiquitination, acylation, hydroxylation, carboxylation, methylation, sumoylation, and nitrosylation, have been reported, but we know the most about the functional consequences of Cx43 phosphorylation45. It is now clear that more than eight protein kinases phosphorylate Cx43 at most of the 21 serine residues and at least three of the six tyrosine residues found within the cytoplasmic exposed carboxy terminus (Figure 2). We also know that phosphorylation regulates the transport of Cx43 to the plasma membrane and its assembly into gap junctions and channel gating46, but more recent studies have also linked Cx43 phosphorylation events to gap junction stability and turnover46–50. At the level of tissue and organ physiology, recent evidence indicates that the Cx43 phosphorylation status is intimately linked to cardiac disorders, cardioprotection, ameloblast differentiation, oocyte maturation, angiotensin II-induced renal damage, B-lymphocyte spreading, epidermal wound repair, and autophagy51–57. Evidence for the essential role of some of these Cx43 phosphorylation events has been obtained via the use of genetically modified mice where known phosphorylation sites were modified to unphosphorylatable residues (e.g., serine to alanine) or to mimic a constitutively phosphorylated residue (e.g., serine to aspartate). Via this approach, casein kinase 1 phosphorylation of Cx43 was found to regulate several critical physiological events, including (a) proper cardiac beat rhythm58 and response to ischemia55,56, (b) efficient epidermal wound healing57, and (c) the effects of stromal fibroblasts on promoting pancreas cancer progression59, while (d) MAPK phosphorylation was shown to regulate neuroprotection during stroke60.
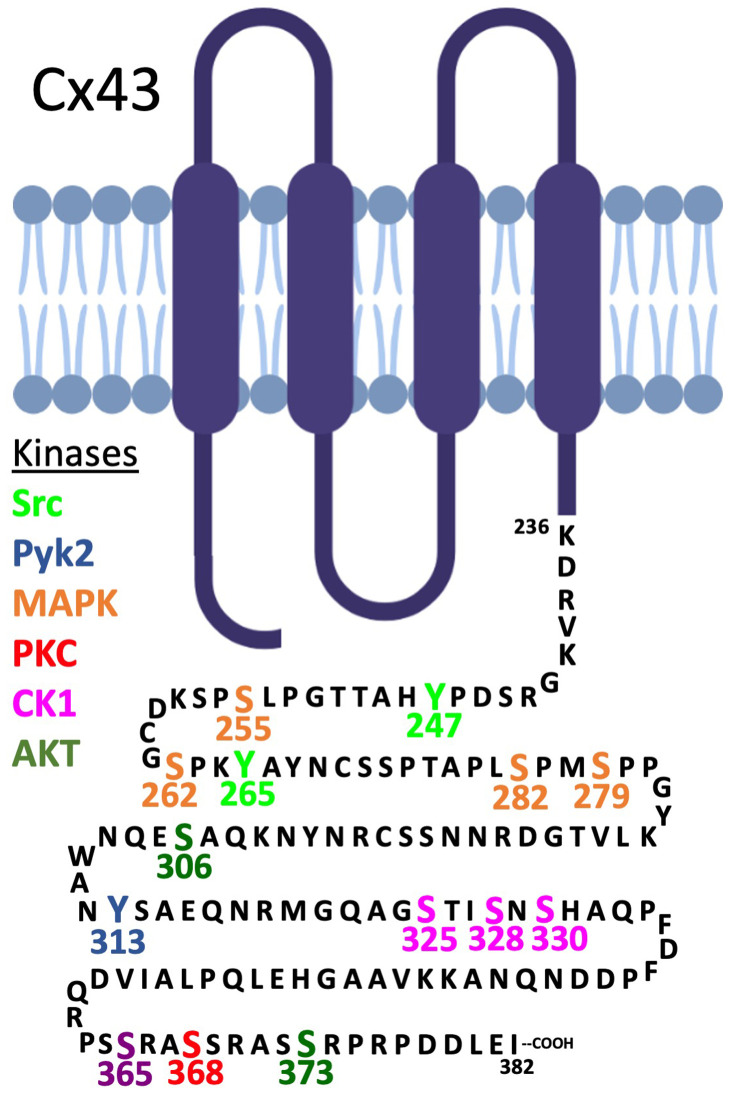
Figure 2. Map of the C-terminal tail of Cx43 with phosphorylation sites having known regulatory functions indicated.
The specified protein kinases and the Cx43 residues they are known to be phosphorylated by are denoted by distinct colors. The site at S365 (purple) is phosphorylated by an unknown kinase that is influenced by protein kinase A activity (i.e., the effect is likely indirect). CAMKII has also been reported to phosphorylate several additional residues in vitro.
Our knowledge of phosphorylation of connexins other than Cx43 and the resulting physiological effects is in general less well-defined. There are reports that Cx31, Cx32, Cx36, Cx37, Cx40, Cx43, Cx46, Cx47, and Cx50 are phosphorylated45, but in some cases the specific residue or direct linkage of the phosphorylation event to an effect on connexin-linked physiology is lacking. We conclude that many of the alpha subfamily of connexins that have been studied have shown at least some evidence of regulation by phosphorylation. However, some connexins, particularly those of the beta subfamily that have short C-terminal regions, have not been shown to be specifically regulated by phosphorylation (e.g., Cx26, likely Cx23), although caution should be exercised as the effects of phosphorylation might be apparent only under very specific conditions. In any case, we believe new interest in the less common connexins that are expressed in diverse tissues will more clearly delineate the roles connexin phosphorylation plays in gap junction and hemichannel regulation.
Looking toward the future of connexin and gap junction research
After an exhaustive review of over 180 peer-reviewed papers related to endogenous connexin expression, we conclude that the 21-member connexin family can be mapped to over 110 distinct cell types found within all 12 human body systems. On one end of this spectrum, it is unclear what human cells express Cx23, while at the other end, Cx43 has been convincingly shown to be expressed in 92 cell types, reflecting its dominance as the most widely distributed human connexin. After decades of intense investigation, the gap junction community not surprisingly is best equipped to describe where and when Cx43 functions regulate cell and tissue physiology. That said, the functional roles of lesser studied connexins (e.g., Cx25, Cx59, and Cx62) remain poorly understood, a situation made more difficult by the lack of reliable antibodies to specifically detect their expression in situ. While connexin family members share considerable sequence homology, it is now clear that connexin members are remarkably diverse with unique structures, regulatory motifs, post-translational modifications, interactomes, cell expression profiles, and subcellular distributions that all contribute to diverse intercellular channel and hemichannel functions in cells and tissues.
New advances in understanding hemichannel functions, intercellular channel and hemichannel structures, genetic linkages to disease, and the connexin interactome all point to critical and canonical roles for connexin hemichannel and channel functions in both disease and normal physiology. Some new areas of intensive study include the role of hemichannels in cardiac conduction23,61, connexin expression at non-canonical sites such as exosomes62 and mitochondrial outer membranes63, as well as the role that connexins play in cancer invasion, metastasis, and resistance to treatment64. There remains no doubt that connexins regulate a variety of cellular functions in almost every human organ. However, the challenge remains to sort out the tissue-context roles played by hemichannels/channels, a task made more complicated by our relatively rudimentary knowledge of what metabolites pass through each channel subtype in situ and the fact that connexin subtypes can intermix to form heteromeric and heterotypic channels. As more and more connexins get linked to diseases, a broad spectrum of organ-specific investigators from around the world are being attracted to the gap junction field in search of new therapeutic targets. We wholeheartedly welcome these new investigators to the field and believe they will pave the way to a better understanding of the functional importance of connexin-based cellular communication in all tissues.
Acknowledgments
The authors thank Ryan Yee (University of Western Ontario) for tabulating connexin expression in human tissues and Tiffany Nguyen (Fred Hutchinson Cancer Center) for their help with Figure 2. Both figures contain images created with Biorender.com.
The peer reviewers who approve this article are:
Steve L. Reichow, Department of Chemistry, Portland State University, Portland, OR, USA
Robert Gourdie, Virginia Tech Carilion School of Medicine, Roanoke, VA, USA
Jean Jiang, Department of Biochemistry and Structural Biology, University of Texas Health Science Center at San Antonio, TX, USA
Funding Statement
Research in PDL’s laboratory was supported by US National Institutes of Health grant GM556032. Research in DWL’s laboratory was supported by Canadian Institutes of Health Research grants 148630 and 148584.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Loewenstein WR: Junctional intercellular communication: The cell-to-cell membrane channel. Physiol Rev. 1981; 61(4): 829–913. 10.1152/physrev.1981.61.4.829 [DOI] [PubMed] [Google Scholar]
- 2. Leybaert L, Lampe PD, Dhein S, et al. : Connexins in Cardiovascular and Neurovascular Health and Disease: Pharmacological Implications. Pharmacol Rev. 2017; 69(4): 396–478. 10.1124/pr.115.012062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Willecke K, Eiberger J, Degen J, et al. : Structural and functional diversity of connexin genes in the mouse and human genome. Biol Chem. 2002; 383(5): 725–37. 10.1515/BC.2002.076 [DOI] [PubMed] [Google Scholar]
- 4. Laird DW, Lampe PD: Cellular mechanisms of connexin-based inherited diseases. Trends Cell Biol. 2022; 32(1): 58–69. 10.1016/j.tcb.2021.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nalewajska M, Marchelek-Myśliwiec M, Opara-Bajerowicz M, et al. : Connexins-Therapeutic Targets in Cancers. Int J Mol Sci. 2020; 21(23): 9119. 10.3390/ijms21239119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mulkearns-Hubert EE, Reizes O, Lathia JD: Connexins in Cancer: Jekyll or Hyde? Biomolecules. 2020; 10(12): 1654. 10.3390/biom10121654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aasen T, Sansano I, Montero MÁ, et al. : Insight into the Role and Regulation of Gap Junction Genes in Lung Cancer and Identification of Nuclear Cx43 as a Putative Biomarker of Poor Prognosis. Cancers (Basel). 2019; 11(3): 320. 10.3390/cancers11030320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Laird DW, Lampe PD: Therapeutic strategies targeting connexins. Nat Rev Drug Discov. 2018; 17(12): 905–21. 10.1038/nrd.2018.138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mugisho OO, Rupenthal ID, Paquet-Durand F, et al. : Targeting connexin hemichannels to control the inflammasome: The correlation between connexin43 and NLRP3 expression in chronic eye disease. Expert Opin Ther Targets. 2019; 23(10): 855–63. 10.1080/14728222.2019.1673368 [DOI] [PubMed] [Google Scholar]
- 10. Montgomery J, Ghatnekar GS, Grek CL, et al. : Connexin 43-Based Therapeutics for Dermal Wound Healing. Int J Mol Sci. 2018; 19(6): 1778. 10.3390/ijms19061778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Benedetti EL, Dunia I, Recouvreur M, et al. : Structural organization of gap junctions as revealed by freeze-fracture and SDS fracture-labeling. Eur J Cell Biol. 2000; 79(8): 575–82. 10.1078/0171-9335-00081 [DOI] [PubMed] [Google Scholar]
- 12. Unger VM, Kumar NM, Gilula NB, et al. : Expression, two-dimensional crystallization, and electron cryo-crystallography of recombinant gap junction membrane channels. J Struct Biol. 1999; 128(1): 98–105. 10.1006/jsbi.1999.4184 [DOI] [PubMed] [Google Scholar]
- 13. Yeager M, Unger VM, Falk MM: Synthesis, assembly and structure of gap junction intercellular channels. Curr Opin Struct Biol. 1998; 8: 517–24. 10.1016/s0959-440x(98)80131-0 [DOI] [PubMed] [Google Scholar]
- 14. Saez JC, Berthoud VM, Branes MC, et al. : Plasma membrane channels formed by connexins: Their regulation and functions. Physiol Rev. 2003; 83(4): 1359–400. 10.1152/physrev.00007.2003 [DOI] [PubMed] [Google Scholar]
- 15. Maeda S, Nakagawa S, Suga M, et al. : Structure of the connexin 26 gap junction channel at 3.5 A resolution. Nature. 2009; 458(7238): 597–602. 10.1038/nature07869 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 16. Bennett BC, Purdy MD, Baker KA, et al. : An electrostatic mechanism for Ca(2+)-mediated regulation of gap junction channels. Nat Commun. 2016; 7: 8770. 10.1038/ncomms9770 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 17. Myers JB, Haddad BG, O'Neill SE, et al. : Structure of native lens connexin 46/50 intercellular channels by cryo-EM. Nature. 2018; 564(7736): 372–7. 10.1038/s41586-018-0786-7 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 18. Flores JA, Haddad BG, Dolan KA, et al. : Connexin-46/50 in a dynamic lipid environment resolved by CryoEM at 1.9 Å. Nat Commun. 2020; 11: 4331. 10.1038/s41467-020-18120-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Aasen T, Mesnil M, Naus CC, et al. : Gap junctions and cancer: Communicating for 50 years. Nat Rev Cancer. 2017; 17: 74. 10.1038/nrc.2016.142 [DOI] [PubMed] [Google Scholar]
- 20. Beyer EC, Steinberg TH: Evidence that the gap junction protein connexin-43 is the ATP-induced pore of mouse macrophages. J Biol Chem. 1991; 266(13): 7971–4. [PubMed] [Google Scholar]
- 21. Laird DW, Penuela S: Pannexin biology and emerging linkages to cancer. Trends Cancer. 2021; 7(12): 1119–31. 10.1016/j.trecan.2021.07.002 [DOI] [PubMed] [Google Scholar]
- 22. Jin Q, Zhang B, Zheng X, et al. : Cryo-EM structures of human pannexin 1 channel. Cell Res. 2020; 30(5): 449–51. 10.1038/s41422-020-0310-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. de Smet MA, Lissoni A, Nezlobinsky T, et al. : Cx43 hemichannel microdomain signaling at the intercalated disc enhances cardiac excitability. J Clin Invest. 2021; 131(7): e137752. 10.1172/JCI137752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang C, Yan Z, Maknojia A, et al. : Inhibition of astrocyte hemichannel improves recovery from spinal cord injury. JCI Insight. 2021; 6(5): e134611. 10.1172/jci.insight.134611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhao D, Riquelme MA, Guda T, et al. : Connexin hemichannels with prostaglandin release in anabolic function of bone to mechanical loading. eLife. 2022; 11: e74365. 10.7554/eLife.74365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Oshima A, Tani K, Hiroaki Y, et al. : Projection structure of a N-terminal deletion mutant of connexin 26 channel with decreased central pore density. Cell Commun Adhes. 2008; 15(1): 85–93. 10.1080/15419060802013588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lee HJ, Jeong H, Hyun J, et al. : Cryo-EM structure of human Cx31.3/GJC3 connexin hemichannel. Sci Adv. 2020; 6(35): eaba4996. 10.1126/sciadv.aba4996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Khan AK, Jagielnicki M, Bennett BC, et al. : Cryo-EM structure of an open conformation of a gap junction hemichannel in lipid bilayer nanodiscs. Structure. 2021; 29(9): 1040–1047.e3. 10.1016/j.str.2021.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tong JJ, Khan U, Haddad BG, et al. : Molecular mechanisms underlying enhanced hemichannel function of a cataract-associated Cx50 mutant. Biophys J. 2021; 120(24): 5644–56. 10.1016/j.bpj.2021.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 30. Yue B, Haddad BG, Khan U, et al. : Connexin 46 and connexin 50 gap junction channel properties are shaped by structural and dynamic features of their N-terminal domains. J Physiol. 2021; 599(13): 3313–35. 10.1113/JP281339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liang WG, Su CC, Nian JH, et al. : Human connexin30.2/31.3 (GJC3) does not form functional gap junction channels but causes enhanced ATP release in HeLa cells. Cell Biochem Biophys. 2011; 61(1): 189–97. 10.1007/s12013-011-9188-2 [DOI] [PubMed] [Google Scholar]
- 32. Beyer EC, Berthoud VM: Connexin hemichannels in the lens. Front Physiol. 2014; 5: 20. 10.3389/fphys.2014.00020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Berthoud VM, Minogue PJ, Osmolak P, et al. : Roles and regulation of lens epithelial cell connexins. FEBS Lett. 2014; 588(8): 1297–303. 10.1016/j.febslet.2013.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sahli KA, Flora GD, Sasikumar P, et al. : Structural, functional, and mechanistic insights uncover the fundamental role of orphan connexin-62 in platelets. Blood. 2021; 137(6): 830–43. 10.1182/blood.2019004575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Srinivas M, Verselis VK, White TW: Human diseases associated with connexin mutations. Biochim Biophys Acta Biomembr. 2018; 1860(1): 192–201. 10.1016/j.bbamem.2017.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lilly E, Sellitto C, Milstone LM, et al. : Connexin channels in congenital skin disorders. Semin Cell Dev Biol. 2016; 50: 4–12. 10.1016/j.semcdb.2015.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Becker DL, Phillips AR, Duft BJ, et al. : Translating connexin biology into therapeutics. Semin Cell Dev Biol. 2016; 50: 49–58. 10.1016/j.semcdb.2015.12.009 [DOI] [PubMed] [Google Scholar]
- 38. Kuang Y, Zorzi V, Buratto D, et al. : A potent antagonist antibody targeting connexin hemichannels alleviates Clouston syndrome symptoms in mutant mice. EBioMedicine. 2020; 57: 102825. 10.1016/j.ebiom.2020.102825 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 39. Sellitto C, Li L, White TW: Connexin hemichannel inhibition ameliorates epidermal pathology in a mouse model of keratitis ichthyosis deafness syndrome. Sci Rep. 2021; 11(1): 24118. 10.1038/s41598-021-03627-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Acosta ML, Mat Nor MN, Guo CX, et al. : Connexin therapeutics: Blocking connexin hemichannel pores is distinct from blocking pannexin channels or gap junctions. Neural Regen Res. 2021; 16(3): 482–8. 10.4103/1673-5374.290097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Leithe E, Mesnil M, Aasen T: The connexin 43 C-terminus: A tail of many tales. Biochim Biophys Acta Biomembr. 2018; 1860(1): 48–64. 10.1016/j.bbamem.2017.05.008 [DOI] [PubMed] [Google Scholar]
- 42. Sorgen PL, Trease AJ, Spagnol G, et al. : Protein⁻Protein Interactions with Connexin 43: Regulation and Function. Int J Mol Sci. 2018; 19(5): 1428. 10.3390/ijms19051428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Defourny J, Audouard C, Davy A, et al. : Efnb2 haploinsufficiency induces early gap junction plaque disassembly and endocytosis in the cochlea. Brain Res Bull. 2021; 174: 153–60. 10.1016/j.brainresbull.2021.06.008 [DOI] [PubMed] [Google Scholar]
- 44. Montgomery J, Richardson WJ, Marsh S, et al. : The connexin 43 carboxyl terminal mimetic peptide αCT1 prompts differentiation of a collagen scar matrix in humans resembling unwounded skin. FASEB J. 2021; 35(8): e21762. 10.1096/fj.202001881R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Aasen T, Johnstone S, Vidal-Brime L, et al. : Connexins: Synthesis, Post-Translational Modifications, and Trafficking in Health and Disease. Int J Mol Sci. 2018; 19(5): 1296. 10.3390/ijms19051296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Solan JL, Lampe PD: Src Regulation of Cx43 Phosphorylation and Gap Junction Turnover. Biomolecules. 2020; 10(12): 1596. 10.3390/biom10121596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kells-Andrews RM, Margraf RA, Fisher CG, et al. : Connexin-43 K63-polyubiquitylation on lysines 264 and 303 regulates gap junction internalization. J Cell Sci. 2018; 131(15): jcs204321. 10.1242/jcs.204321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yang Y, Yan X, Xue J, et al. : Connexin43 dephosphorylation at serine 282 is associated with connexin43-mediated cardiomyocyte apoptosis. Cell Death Differ. 2019; 26(7): 1332–45. 10.1038/s41418-019-0277-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zheng L, Li H, Cannon A, et al. : Phosphorylation of Cx43 residue Y313 by Src contributes to blocking the interaction with Drebrin and disassembling gap junctions. J Mol Cell Cardiol. 2019; 126: 36–49. 10.1016/j.yjmcc.2018.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zheng L, Trease AJ, Katsurada K, et al. : Inhibition of Pyk2 and Src activity improves Cx43 gap junction intercellular communication. J Mol Cell Cardiol. 2020; 149: 27–40. 10.1016/j.yjmcc.2020.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Duan J, Chen H, Li Y, et al. : 17β-Estradiol Enhances Porcine Meiosis Resumption from Autophagy-Induced Gap Junction Intercellular Communications and Connexin 43 Phosphorylation via the MEK/ERK Signaling Pathway. J Agric Food Chem. 2021; 69(40): 11847–55. 10.1021/acs.jafc.1c04212 [DOI] [PubMed] [Google Scholar]
- 52. Gómez GI, Velarde V, Sáez JC: Role of a RhoA/ROCK-Dependent Pathway on Renal Connexin43 Regulation in the Angiotensin II-Induced Renal Damage. Int J Mol Sci. 2019; 20(18): 4408. 10.3390/ijms20184408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pournia F, Dang-Lawson M, Choi K, et al. : Identification of serine residues in the connexin43 carboxyl tail important for BCR-mediated spreading of B-lymphocytes. J Cell Sci. 2020; 133(5): jcs237925. 10.1242/jcs.237925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yamada A, Yoshizaki K, Ishikawa M, et al. : Connexin 43-Mediated Gap Junction Communication Regulates Ameloblast Differentiation via ERK1/2 Phosphorylation. Front Physiol. 2021; 12: 748574. 10.3389/fphys.2021.748574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hirschhäuser C, Lissoni A, Görge PM, et al. : Connexin 43 phosphorylation by casein kinase 1 is essential for the cardioprotection by ischemic preconditioning. Basic Res Cardiol. 2021; 116(1): 21. 10.1007/s00395-021-00861-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Solan JL, Márquez-Rosado L, Lampe PD: Cx43 phosphorylation-mediated effects on ERK and Akt protect against ischemia reperfusion injury and alter the stability of the stress-inducible protein NDRG1. J Biol Chem. 2019; 294(31): 11762–71. 10.1074/jbc.RA119.009162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lastwika KJ, Dunn CA, Solan JL, et al. : Phosphorylation of connexin 43 at MAPK, PKC or CK1 sites each distinctly alter the kinetics of epidermal wound repair. J Cell Sci. 2019; 132(18): jcs234633. 10.1242/jcs.234633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Remo BF, Qu J, Volpicelli FM, et al. : Phosphatase-resistant gap junctions inhibit pathological remodeling and prevent arrhythmias. Circ Res. 2011; 108(12): 1459–66. 10.1161/CIRCRESAHA.111.244046 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 59. Solan JL, Hingorani SR, Lampe PD: Cx43 phosphorylation sites regulate pancreatic cancer metastasis. Oncogene. 2021; 40(10): 1909–20. 10.1038/s41388-021-01668-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Freitas-Andrade M, Wang N, Bechberger JF, et al. : Targeting MAPK phosphorylation of Connexin43 provides neuroprotection in stroke. J Exp Med. 2019; 216(4): 916–35. 10.1084/jem.20171452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Veeraraghavan R, Hoeker GS, Alvarez-Laviada A, et al. : The adhesion function of the sodium channel beta subunit (β1) contributes to cardiac action potential propagation. eLife. 2018; 7: e37610. 10.7554/eLife.37610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Soares AR, Martins-Marques T, Ribeiro-Rodrigues T, et al. : Gap junctional protein Cx43 is involved in the communication between extracellular vesicles and mammalian cells. Sci Rep. 2015; 5: 13243. 10.1038/srep13243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Shimura D, Shaw RM: GJA1-20k and Mitochondrial Dynamics. Front Physiol. 2022; 13: 867358. 10.3389/fphys.2022.867358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Aasen T, Leithe E, Graham SV, et al. : Connexins in cancer: Bridging the gap to the clinic. Oncogene. 2019; 38(23): 4429–51. 10.1038/s41388-019-0741-6 [DOI] [PMC free article] [PubMed] [Google Scholar]