Abstract
This paper reports the results of leaching experiments conducted with and without Thiobacillus ferrooxidans at the same conditions in solution. The extent of leaching of ZnS with bacteria is significantly higher than that without bacteria at high concentrations of ferrous ions. A porous layer of elemental sulfur is present on the surfaces of the chemically leached particles, while no sulfur is present on the surfaces of the bacterially leached particles. The analysis of the data using the shrinking-core model shows that the chemical leaching of ZnS is limited by the diffusion of ferrous ions through the sulfur product layer at high concentrations of ferrous ions. The analysis of the data shows that diffusion through the product layer does not limit the rate of dissolution when bacteria are present. This suggests that the action of T. ferrooxidans in oxidizing the sulfur formed on the particle surface is to remove the barrier to diffusion by ferrous ions.
Thiobacillus ferrooxidans is the microorganism that is primarily associated with the oxidation of sulfide minerals. The bacterial interaction with sulfide minerals is a significant factor in the formation of acid mine drainage, and large amounts of effort are invested in the remediation of sites of acid mine drainage (9). On the other hand, this process has been exploited in the extraction of gold, nickel, copper, and cobalt from sulfide ores (8).
The dissolution of zinc sulfide (sphalerite) by ferric sulfate has been well studied. Ferric ions oxidize the sphalerite to form zinc and ferrous ions in solution and elemental sulfur (5–7, 25, 26). This reaction is illustrated as follows:
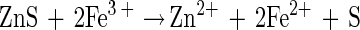 |
1 |
The ferrous ions formed in the leaching reaction can be oxidized to ferric ions by T. ferrooxidans in the following reaction:
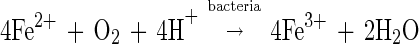 |
2 |
Because T. ferrooxidans accelerates the rate of the oxidation of ferrous ions, given by equation 2, by a factor of 106 (19), the presence of bacteria can significantly increase the rate of the overall leaching process. In addition to the above reactions, T. ferrooxidans is capable of oxidizing the sulfur formed in the dissolution reaction by the following reaction:
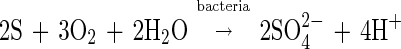 |
3 |
In the set of reactions represented by equations 1 to 3, the role of the bacteria is to oxidize the products of the dissolution reaction, that is, the ferrous ions and the sulfur. However, Silverman and Ehrlich (17) proposed in 1964 that T. ferrooxidans enhances the rate of oxidation of sulfide minerals above that achieved by a chemical reaction with ferric ions at the same conditions. They proposed a mechanism of “direct oxidative attack on metal sulfide minerals independent of the action of the ferric sulfate” (17). This mechanism was envisaged as contributing to the overall leaching process by the following reaction:
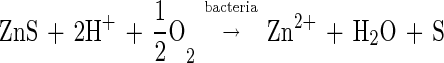 |
4 |
The distinguishing feature between the two mechanisms is the substitution of ferric ions as the oxidant at the surface of the zinc sulfide by an unidentified biological process. Thus, Silverman and Ehrlich (17) proposed that the rate of dissolution of the sulfide mineral with bacteria is greater than that without bacteria at the same conditions in solution. However, there has been no research work that has directly confirmed that bacteria increase the rate of leaching above that achieved by chemical leaching at the same solution conditions. As a result, the debate concerning these mechanisms has not been settled (2).
In addition, the interpretation of the data from previously reported experiments on bacterial leaching has proved difficult. This is because in all previous work the concentrations of ferric and ferrous ions varied considerably during the experiment (1, 3, 4, 27). This has made it difficult to compare data from experiments with and without bacteria at the same solution conditions.
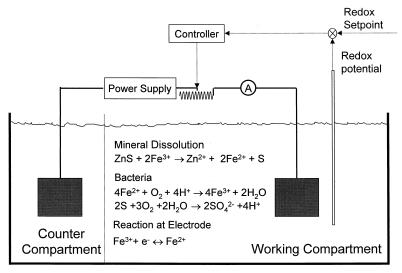
However, by performing experiments in which the solution concentrations are maintained at a set value, we are able to compare directly the extents of leaching with and without bacteria. We previously described an experimental apparatus in which the concentrations of ferrous and ferric ions are controlled at the initial value for the duration of the experiment (10, 11). This apparatus is a two-compartment electrolytic cell, and the redox potential in the compartment in which leaching occurs is controlled by manipulating the electrolytic current. This apparatus is shown in Fig. 1. Previous experiments using this apparatus showed that the rate of growth of T. ferrooxidans is unaffected by the small electrolytic current (<2 A) (11).
FIG. 1.
Schematic diagram of the experimental apparatus. The working compartment is that in which leaching experiments are conducted. The flow of current is regulated by adjusting the variable resistor so that the redox potential in the working compartment remains at the setpoint value.
In this way, we can directly test the proposal of Silverman and Ehrlich (17). In this paper, we report results on the leaching of zinc sulfide with and without T. ferrooxidans at high concentrations of ferrous ions.
MATERIALS AND METHODS
Apparatus.
The electrolysis cell was made of Plexiglas and was divided into two sections by an anion-exchange membrane (Sybron Chemicals Inc., Birmingham, N.J.). The electrolysis cell was fitted with a Plexiglas lid to minimize evaporation of the solution. The working volume of the cell was 2 liters. The contents of the working compartment were stirred by a three-bladed impeller driven by an overhead motor, and the compartment was sparged with air. Electrodes to measure the redox potential and the concentration of oxygen were suspended in the solution of the working compartment.
Redox potential measurements were made with a platinum electrode and an Ag-AgCl reference electrode by using a high-impedance galvanically isolated differential amplifier and an analog-to-digital control card (type PC30; Eagle Technology, Cape Town, South Africa) and were recorded by computer. A computer program determined the values of the output signals from the PC30 card to the relay switch and to the variable resistor that varied the direction and magnitude of the current. The current was measured by determining the potential difference across a precision resistor.
The redox potential was controlled within 0.1% of the setpoint for the period of the experiment. All the solution samples were analyzed for ferrous ions in order to confirm that the control of the redox potential maintained the concentration of ferrous ions at a constant value. Typical results showed that the concentration of the ferrous ions differed from the initial value by less than 0.8%.
The pH of the solution in the working compartment was 1.6. The pH was measured throughout each experiment and remained within 0.05 pH unit of the initial value. The concentration of dissolved oxygen was measured (Hanna Instruments) and was maintained manually at a value of 5.9 mg/liter. The electrolysis cell was placed in a water bath, and the temperature was maintained at 35 ± 0.1°C.
Bacterial culture.
A pure strain of T. ferrooxidans (strain FC1) was used. This organism, which was supplied by D. Rawlings, University of Cape Town, Cape Town, South Africa, has been thoroughly characterized (16). The bacteria were cultured on a medium which contained (per liter) 1.5 g of (NH4)2SO4, 0.5 g of K2HPO4, 0.5 g of MgSO4 · 7H2O, and 45 g of FeSO4 · 7H2O (18). The pH of the medium was adjusted to 1.6 by adding H2SO4. The bacteria were maintained in the exponential growth phase by subculturing one-third of the culture volume on a daily basis.
Preparation and characterization of the ore.
The sphalerite concentrate, which was from the Gamsberg deposit, was supplied by Gold Fields Research Laboratories. This sample was milled and wet screened to a size fraction of between 53 and 45 μm. The ore contained 53.3% Zn, 7.84% Fe, 32.5% S, 1.19% Mn, and 0.24% Pb. The metallic element contents were determined by analyzing the solution by atomic adsorption spectrophotometery with a Varian Spectra AA30 spectrophotometer after acid digestion. The sulfur content was determined with a LECO model SC32 DB64 sulfur determinator. X-ray diffraction at a scan rate of 0.05°/s indicated that the sample contained 98% sphalerite, which supported the conclusion that the iron was present in solid solution in ZnS rather than as a separate mineral (6). The ore sample was washed with a 0.5 M solution of sodium sulfide to remove flotation agents and to sulfidize the mineral surface (5, 12, 20, 22).
Analytical techniques.
The number of bacterial cells in a solution was determined by counting with a hemacytometer (depth, 0.1 mm; area, 0.0025 mm2). The cells were stained by using crystal violet in a citric acid solution as the stain. The standard deviation for the cell number determinations was 1.2% of the mean (10 replicates).
The concentration of ferrous ion in solution was determined by titration with potassium dichromate, with sodium diphenylamine sulfonate as the indicator (28). The standard deviation for the determinations of ferrous ion concentrations of was 1.1% of the mean (10 replicates). The total concentration of iron in solution was determined by using the titration for ferrous ions once the iron had been reduced to the ferrous state with stannous chloride. The concentration of ferric ions was calculated by determining the difference. It must be emphasized that the measurement of the redox potential was used for control purposes only and not for chemical analysis.
The concentration of zinc in solution was determined by atomic adsorption spectrophotometery with the Varian Spectra AA30 spectrophotometer. The standard deviation for the determinations of the concentration of zinc in solution was 0.3% of the mean (10 replicates).
Procedure.
All experiments were conducted in the same medium but with different ferrous ion concentrations. The concentration of ferric ions was 1 g/liter for all experiments. The loading concentration of solids was 5 g of sphalerite per liter. The bacterial leaching experiment preparations were inoculated with a number of T. ferrooxidans cells equivalent to 10% (by volume) of the reactor size. Samples were withdrawn from the working compartment at the same time intervals for every leaching experiment performed.
Part of the sample was used to determine the bacterial cell population in suspension. The remaining part of each sample was immediately filtered by using a Millipore filter (Sterifil aseptic system with a sterile, individually sealed, 0.45-μm-pore-size filter membrane). The filtrate was used to determine the concentrations of ferrous and ferric ions and the concentration of zinc ions in solution. The solid residues on the filter membrane were immersed in a 95% solution of ethanol to fix the bacteria (in bacterial leaching experiments) on the surfaces of the mineral particles. These preparations were dried at the critical point and coated for investigation with a scanning electron microscope (SEM). Samples were withdrawn at regular intervals from the counter compartment. These samples were also analyzed to determine the concentration of zinc ions in solution. Analyses revealed that no zinc was transferred from the working compartment to the counter compartment through the anion-exchange membrane.
The solution samples obtained from the sterile (chemical leaching) experiments were checked for contamination by T. ferrooxidans. No bacteria were detected by a microscopic investigation. Between successive experiments, the electrolysis cell was soaked in hydrochloric acid, rinsed with water, cleaned with an ammonia-based cleaning solution (pH 10), and finally rinsed with distilled water.
RESULTS
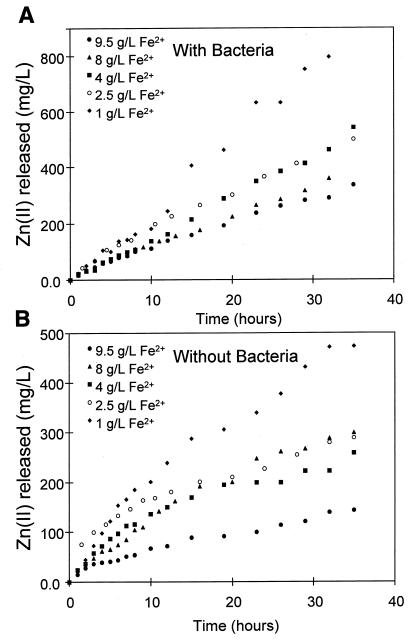
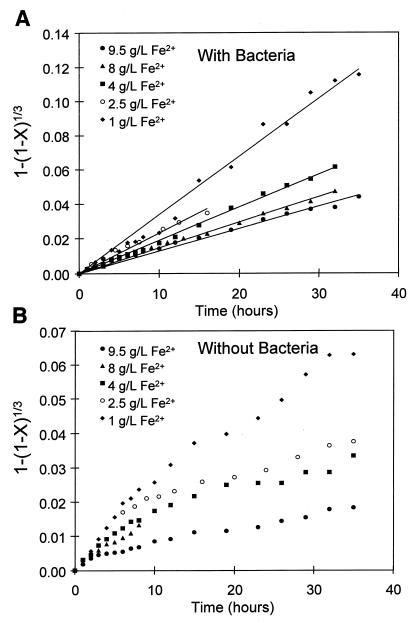
The results for the leaching of sphalerite with and without bacteria in the controlled redox potential apparatus are shown in Fig. 2. These experiments were conducted at various concentrations of ferrous ions. The concentration of ferric ions was the same for each experiment. The concentrations of ferrous and ferric ions in solution were constant to within 1% of their initial concentrations for the duration of the experiment. Because the solution conditions were controlled in the leaching experiments, the only difference between these two sets of experiments is the presence or absence of bacteria.
FIG. 2.
Effect of the concentration of ferrous ions in solution on the bacterial and chemical leaching of sphalerite. (A) Results in the presence of 10% (vol/vol) T. ferrooxidans inoculum; (B) results in absence of bacteria (i.e., chemical leaching). The solution conditions were as follows: Fe3+ concentration, 1.0 g/liter; density of solids, 5 g/liter; temperature, 35°C; pH, 1.6; O2 concentration, 5.9 mg/liter.
It is clear from the experiments that an increase in the concentration of ferrous ions resulted in a decrease in the rate of leaching. It is also apparent that the extent of leaching is higher in the presence of bacteria.
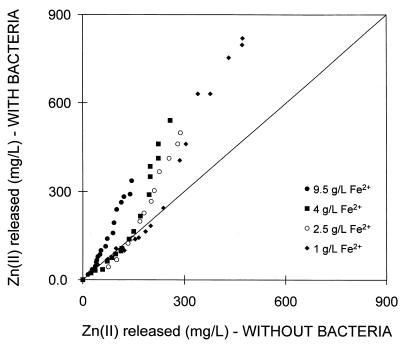
A comparison of the amounts of zinc dissolved with and without bacteria is shown in Fig. 3. This figure shows that the extent of leaching with bacteria was the same as that without bacteria during the early stages of the reaction, for the first 8 to 12 h. At longer reaction times, the rate of dissolution of sphalerite with bacteria was higher than that without bacteria. It is clear from Fig. 3 that the extent to which the bacteria enhance the extent of dissolution is dependent on the concentration of ferrous ions. The bacteria have a greater effect on the extent of dissolution at higher concentrations of ferrous ions. Because all other conditions were controlled, an increase in the extent of dissolution in the presence of bacteria indicates a contribution to the dissolution of the mineral by the bacteria beyond their ability to oxidize ferrous ions.
FIG. 3.
Comparison of the amounts of zinc dissolved from sphalerite with and without bacteria at various concentrations of ferrous ions. The data is that presented in Fig. 2. The experiments with and without bacteria were conducted at constant solution conditions throughout each experiment. The solution conditions are given in the legend for Fig. 2.
The concentration of bacterial cells in solution during the experiment increased typically from 3 × 108 to 11 × 108 cells/ml. These measurements underestimate the total number of cells in the leaching reactor because of cells attached to the zinc sulfide particles.
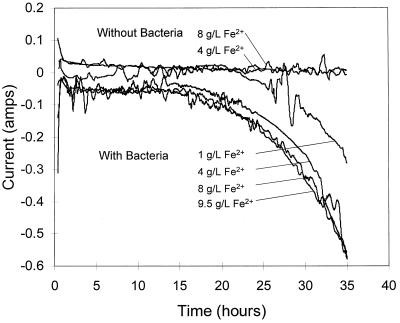
The currents that are applied to the electrolysis cell in order to maintain conditions are a direct measure of the rates of the processes occurring in the reactor. These currents can be used to estimate the bacterial growth rates in the reactor. Figure 4 shows the currents that were passed through the electrolytic cell in these experiments. Positive currents represent the electrolytic oxidation of ferrous ions, and negative currents represent the electrolytic reduction of ferric ions. Thus, positive currents indicate that the leaching reaction is dominant, since ferrous ions, which are the product of the leaching reaction, must be oxidized to maintain the solution conditions. Negative currents, on the other hand, mean that the bacterial oxidation of ferrous ions is dominant, since ferric ions, which are the product of the bacterial reaction, must be reduced to maintain the solution conditions. In the chemical leaching experiment, the current is a measure of the rate of leaching by ferric ions. In the bacterial leaching experiment, the current is the difference between the rates of leaching by ferric ions and the rate of oxidation of ferrous ions by bacteria. Thus, subtracting the current for the chemical leaching experiment from that for the bacterial experiment gives the rate of bacterial oxidation of ferrous ions.
FIG. 4.
Current data required to maintain the redox potential at the initial value. Solution conditions are given in the legend for Fig. 2. A 10% (vol/vol) T. ferrooxidans inoculum was used.
Since the logarithm of the current passed through the electrolysis cell during the experiments with bacteria is a straight line with time, the rate of bacterial oxidation increases exponentially with time during these experiments. Estimates of the bacterial growth rate from this data and the yield coefficient give doubling times of between 6.1 and 9.6 h. These values compare favorably with the doubling times of between 8.8 and 10.3 h obtained from batch growth studies of the same sample of T. ferrooxidans with ferrous ions as the energy source. Therefore, estimates of the bacterial growth from the cell numbers in solution are moderate in comparison with those obtained by calculation from the electrolysis current. This indicates that substantial growth of cells attached to the mineral surface occurs.
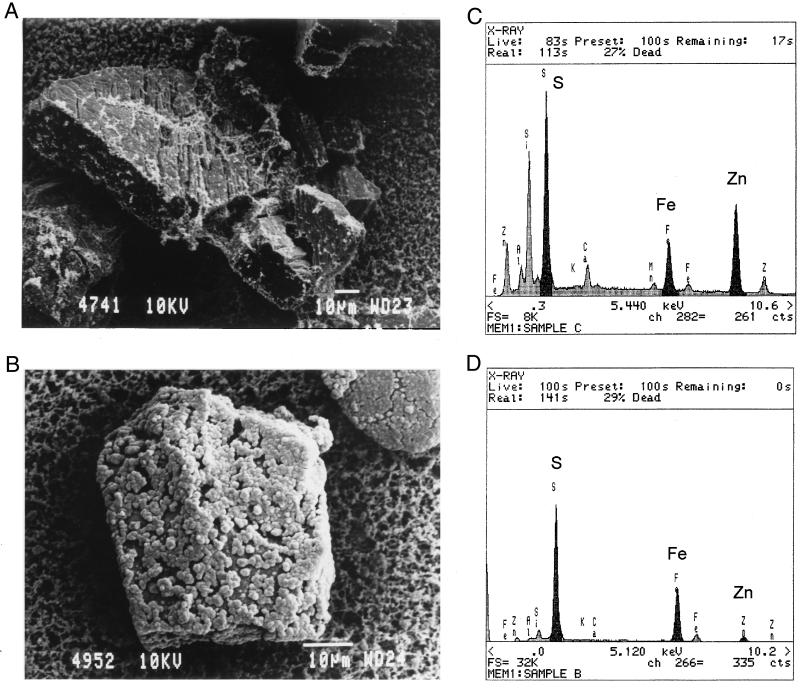
The bacteria attached to the mineral particles were examined by SEM. The SEM analysis indicated that the bacteria attached rapidly to the mineral particles, forming large amounts of exopolymer. Typical results are shown in Fig. 5 and 6. SEM photographs of bacterially treated and chemically treated particles obtained without critical-point drying are shown in Fig. 5A and B. The attached bacteria and exopolymer are dehydrated in Fig. 5A due to the sample preparation. The dried remains of the exopolymer material present on the surfaces of the bacterially leached particles are apparent in Fig. 5A.
FIG. 5.
SEM photographs of bacterially and chemically leached zinc sulfide after 100 h. (A) Surface of bacterially leached zinc sulfide. Note that the biofilm has been dehydrated due to the SEM preparation technique. (B) Surface of chemically leached zinc sulfide. Note the presence of porous sulfur on the surface. (C) EDAX scan of the surface in panel A showing that the surface is fresh zinc sulfide. (D) EDAX scan of the surface in panel B showing that the surface is coated with sulfur. Conditions: Fe3+ concentration, 1.0 g/liter; Fe2+ concentration, 4.0 g/L; density of solids, 5 g/liter; temperature, 35°C; pH, 1.6; O2 concentration, 5.9 mg/liter.
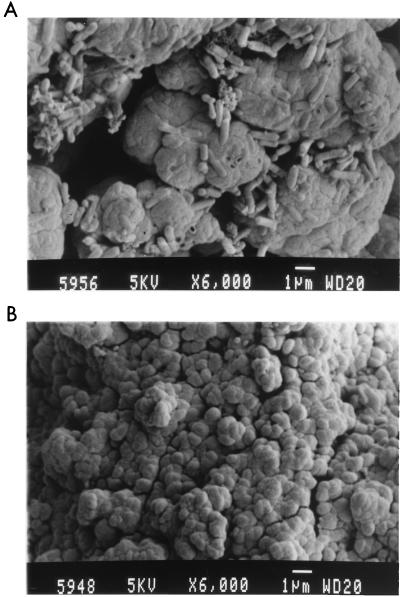
FIG. 6.
Comparison of bacterial and chemical surfaces after 69 h of leaching. A SEM preparation technique was used to minimize sample dehydration. (A) Surface of bacterially leached sample, indicating large amounts of attachment of rod-shaped T. ferrooxidans. (B) Surface of chemically leached sample, indicating the presence of sulfur, as seen in Fig. 5B. Conditions: Fe3+ concentration, 1.0 g/liter; Fe2+ concentration, 8.0 g/liter; density of solids, 5 g/liter; temperature, 35°C; pH, 1.6; O2 concentration, 5.9 mg/liter.
A comparison of Fig. 5A and B indicates that the mineral surface of the bacterially leached sample is clear, while that of the chemically leached sample is covered with a porous layer of sulfur. The EDAX analysis of the surfaces is given in Fig. 5C and D. The EDAX analysis confirmed that sulfur is present as a reaction product on the surface of the chemically leached sample and that the surface of the bacterially leached sample is clear of sulfur coating. This suggests that T. ferrooxidans oxidized the sulfur formed on the surface of the sphalerite to sulfate (15, 21, 24).
Figure 6 and shows typical SEM results for bacterially and chemically leached surfaces obtained with critical-point drying. The surface of the bacterially leached sample, shown in Fig. 6A, indicates the presence of large quantities of attached bacteria. The bacteria appear to be embedded in large quantities of exopolymer material. (This material is not sulfur, since it is dehydrated in the SEM preparation process without critical-point drying. In addition, the bacteria cannot be embedded in the mineral surface, because no indentations caused by the bacteria were detected in the SEM study in which the samples were dehydrated, shown in Fig. 5A.) The surface of the chemically leached sample is similar to that of the sample shown in Fig. 5B, showing the presence of a thick sulfur layer.
DISCUSSION
The results shown in Fig. 3 differ from our previous finding that the bacteria do not enhance the rate of dissolution of sphalerite (10). The results given in Fig. 3 were obtained at high concentrations of ferrous ions, while those reported previously were obtained at low concentrations of ferrous ions. However, it is clear from Fig. 5 that the chemically leached particles are coated with the porous sulfur that is formed during dissolution. This sulfur is not present on the particle surface in the leaching experiments with bacteria. Thus, in interpreting the data, we need to account for the following observations: (i) T. ferrooxidans does not enhance the rate of dissolution at low concentrations of ferrous ions (10), (ii) T. ferrooxidans does enhance the rate of dissolution at high concentrations of ferrous ions, (iii) the extent to which T. ferrooxidans enhances the rate of dissolution at high concentrations of ferrous ions is dependent on the concentration of ferrous ions, (iv) at low reaction times (less than 12 h), the rates of chemical and bacterial leaching are similar, and (v) T. ferrooxidans removes the sulfur product from the particle surface.
In order to build an understanding of these observations, the chemical dissolution of zinc sulfide, given by equation 1, was examined first, and then the leaching of zinc sulfide in the presence of bacteria was examined.
Analysis of the chemical leaching of zinc sulfide.
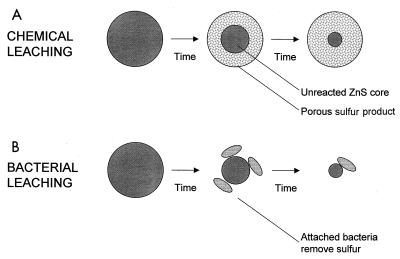
The chemical leaching of sphalerite by ferric ions has been studied in detail (5–7, 12). This is a heterogeneous reaction in which the size of the unreacted sphalerite diminishes with reaction time. As the sphalerite dissolves, a porous product layer of sulfur forms on the core of unreacted sphalerite. This is shown schematically in Fig. 7. It is well known that the formation of product layers on the surfaces of particles may limit the rate of reaction by hindering the diffusion of soluble reactants or products through this layer (13, 23), and it is known that this is true for the chemical leaching of sphalerite by ferric ions (5–7, 12). The processes that govern the rate of dissolution are the transport of ferric, ferrous, and zinc ions through the porous sulfur layer and the intrinsic reaction, equation 1, at the surface of unreacted sphalerite.
FIG. 7.
Schematic diagram of a reacting sphalerite particle. (A) Chemical leaching mechanism, showing the formation of a porous layer of sulfur on the core of unreacted sphalerite. The reaction given by equation 1 occurs at the surface of the unreacted core of zinc sulfide. Diffusion of soluble reactant and products through the sulfur may control the rate of dissolution under some conditions. (B) Leaching mechanism in the presence of bacteria. T. ferrooxidans oxidizes sulfur, removing any barrier to diffusion.
The theory of heterogeneous reactions describes the decrease in particle size based on the geometry of the particles and the rate processes contributing to the overall reaction (13, 23). Two limiting forms of this unreacted shrinking-core theory are used here to analyze the results given in Fig. 2. These are the case in which the reaction at the sphalerite surface controls the overall rate of dissolution and the case in which diffusion through the porous product layer of sulfur controls the overall rate of dissolution.
If the reaction at the surface of the unreacted core of sphalerite controls the overall rate of dissolution, then the extent of reaction, X, is given by (13, 23):
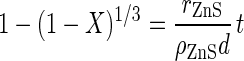 |
5 |
where rZnS is the intrinsic rate of dissolution of sphalerite (in moles per meter squared per hour), d is the initial particle size (48.8 × 10−6 m), ρZnS is the molar density (42,098 mol/m3), and t is the reaction time (in hours). The extent of reaction, or conversion, is the amount of sphalerite dissolved divided by the total amount of sphalerite initially in the reactor. This equation is valid only if the concentrations of reactants in solution are constant and the particles are of uniform initial size. The controlled leaching experiments reported here meet these criteria.
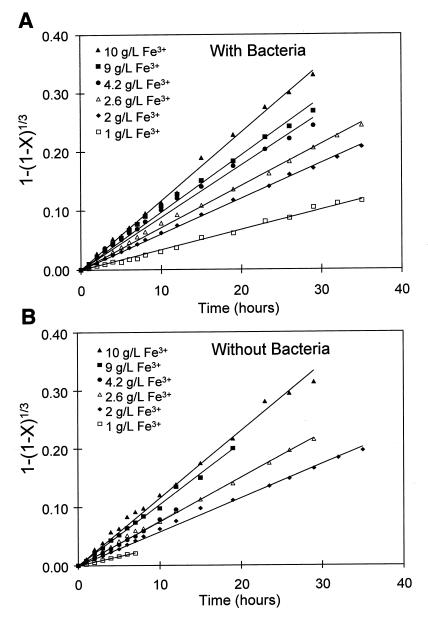
Equation 5 indicates that if the surface reaction controls the overall rate of dissolution, then a plot of 1 − (1 − X)1/3 against t should be a straight line through the origin with slope proportional to rZnS. Plots of 1 − (1 − X)1/3 versus time are shown in Fig. 8B for the experimental results reported here for the chemical leaching of zinc sulfide at various concentrations of ferrous ions. This figure shows that the plot is linear only for the first 8 to 12 h of the reaction time. After this time, the data deviates from the linear relationship expected from equation 5. This result suggests that the reaction at the surface controls the overall rate of dissolution only in the early stages of the reaction. As the thickness of the sulfur product layer increases, the diffusion resistance caused by the sulfur product layer plays an increasingly important role. This deviation from equation 5 because of product layer diffusion is a well-known phenomenon in the literature on the chemical leaching of zinc sulfide (5, 6, 12).
FIG. 8.
Plot of 1 − (1 − X)1/3 versus time for the bacterial and chemical leaching data. (A) Plot of the bacterial leaching data from Fig. 2A, confirming that the shrinking-particle model with surface reaction control describes the leaching of sphalerite in the presence of bacteria at high concentrations of ferrous ions. (B) Plot of chemical leaching data from Fig. 2B showing that the shrinking-particle model with surface reaction control does not describe the leaching of sphalerite in the absence of bacteria at high concentrations of ferrous ions, except for the first 8 to 10 h.
If the diffusion of ferric and ferrous ions through the porous sulfur layer controls the overall rate of chemical leaching, then X is described by the following equation (13, 23):
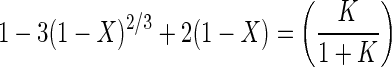 |
6 |
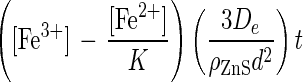 |
where K is the equilibrium constant, De is the effective diffusion coefficient in the porous product layer (in meters squared per hour), and [Fe3+] and [Fe2+] are the concentrations of ferric and ferrous ions (in moles per cubic meter), respectively. Equation 6 is valid only if the concentrations of reactants in solution are constant and the particles are of uniform initial size. The controlled leaching experiments described here meet these criteria.
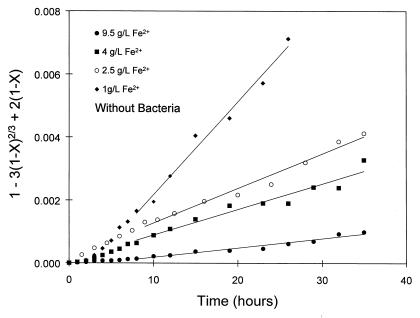
Equation 6 indicates that if diffusion through the sulfur layer controls the overall rate of dissolution, then a plot of 1 − 3(1 − X)2/3 + 2(1 − X) versus time should be a straight line. The plots of 1 − 3(1 − X)2/3 + 2(1 − X) versus time for the chemical leaching results, shown in Fig. 9, are linear after about 10 h. This result indicates that the controlling mechanism in the chemical leaching of sphalerite changes from surface reaction control to product layer diffusion as the thickness of the sulfur product layer increases. The slopes of the lines in Fig. 9 are linearly proportional to the concentration of ferrous ions with a slope of −1, in accordance with equation 6. This indicates that ferrous ions provide the resistance to diffusion.
FIG. 9.
Plot of 1 − 3(1 − X)2/3 + 2(1 − X) versus time showing that the shrinking-core model with diffusion through the product layer as the rate-controlling step describes the leaching of sphalerite at high concentrations of ferrous ions in the absence of bacteria after about 10 h of reaction time. X is calculated from the data presented in Fig. 2B.
If this mechanism is correct, then the chemical dissolution of zinc sulfide should not be affected by the diffusion of ferrous ions through the product layer at low concentrations of ferrous ions. In this case, the chemical leaching results should be described by equation 5 over the entire leaching period. Plots of 1 − (1 − X)1/3 versus time are shown in Fig. 10B for the experimental results reported previously at a low concentration of ferrous ions (10). These plots are straight lines through the origin, with no deviation, even at high conversions. This indicates that the chemical dissolution of sphalerite at low concentrations of ferrous ions is controlled by the intrinsic reaction at the sphalerite surface. Under these conditions, diffusion through the product layer does not play a role in controlling the overall rate of reaction in the chemical leaching of sphalerite.
FIG. 10.
Plot of 1 − (1 − X)1/3 versus time showing that the shrinking-particle model with surface reaction control describes the leaching of sphalerite at high concentrations of ferric ions both in the presence and absence of bacteria. The original data was presented in Fig. 4 of our previous study (10). (A) Bacterial leaching of zinc sulfide. (B) Chemical leaching of zinc sulfide. The solution conditions were as follows: Fe2+ concentration, 1.0 g/liter; density of solids, 5 g/liter; temperature, 35°C; pH, 1.6; O2 concentration, 5.9 mg/liter.
From this analysis, it is clear that the chemical dissolution of zinc sulfide is controlled by two linked processes: reaction at the surface of the zinc sulfide and diffusion of ferrous ions through a product layer of sulfur present on the surface of the zinc sulfide. It must be emphasized that the species whose rate of transport is limited by the formation of the sulfur layer is ferrous ions. At low concentrations of ferrous ions in the bulk solution, the product layer diffusion does not influence the rate of the overall process. At high concentrations of ferrous ions, there is a change in the controlling mechanism. In the initial stages of the reaction, the intrinsic chemical reaction at the surface controls the rate of dissolution. However, with time a porous sulfur product accumulates on the particle surface, and this hinders the rate of diffusion of ferrous ions from the reaction surface. Eventually, this becomes the controlling mechanism.
Analysis of the bacterial leaching of zinc sulfide.
The leaching of zinc sulfide in the presence of T. ferrooxidans was examined in the light of the understanding of the chemical dissolution of zinc sulfide. SEM photographs and the EDAX analysis indicate that there is no sulfur present on the surface of the zinc sulfide leached in the presence of T. ferrooxidans. This is because T. ferrooxidans oxidizes the sulfur formed at the surface. From this observation, it is expected that T. ferrooxidans will enhance the rate of leaching above that of chemical leaching only when diffusion through the sulfur layer controls the rate of dissolution. It was shown above that product layer diffusion controls the rate of chemical leaching when the ferrous ion concentration is higher than 1 g/liter, and only once the sulfur layer is of sufficient thickness to provide a barrier to diffusion. Therefore, if the contribution of T. ferrooxidans is to oxidize sulfur, the rate of dissolution of zinc sulfide will be enhanced only when the ferrous ion concentration is above 1 g/liter and once the sulfur product layer is sufficiently thick for it to control the rate of chemical leaching. These are exactly the observations made in points (i) to (v) listed at the beginning of Discussion.
During the first 8 to 12 h of chemical leaching, the sulfur product is not sufficiently thick to hinder the rate of reaction and the reaction at the surface controls the overall rate of dissolution. Removal of the sulfur product would have no effect on the rate of chemical leaching during this initial period. This accounts for the observation that T. ferrooxidans does not enhance the rate during the first 12 h. After this period, the sulfur layer hinders the rate of dissolution of the chemical leaching reaction, and since the bacteria remove the sulfur, the bacterial leaching reaction occurs at a greater rate than the chemical reaction. This explains the results shown in Fig. 3.
The conclusions that the sulfur hinders the rate of chemical leaching after 12 h and that it is removed by T. ferrooxidans in the experiments with bacteria indicate that the ferrous ion-grown T. ferrooxidans shows very little lag phase for growth on sulfur.
If the only contribution of T. ferrooxidans in these experiments is to oxidize the sulfur formed in accordance with equation 1, then the rate of dissolution of sphalerite in the presence of T. ferrooxidans should be described by equation 5 for all sets of experiments. Plots of 1 − (1 − X)1/3 versus time are shown in Fig. 8A and 10A for the experimental results at various concentrations with T. ferrooxidans reported here. These plots are straight lines through the origin, indicating that the dissolution of sphalerite in the presence of T. ferrooxidans at high concentrations of ferrous ions is controlled by the reaction at the sphalerite surface. This further confirms that the reason for the difference between the rates of chemical and bacterial leaching shown in Fig. 3 is the removal of the sulfur product from the surface of the zinc sulfide.
The rates of reaction, rZnS, may be calculated from the slopes of the lines in Fig. 10. The effect of the concentration of ferric ions on the rate of reaction is expressed in terms of the order of reaction. The orders of reaction for ferric ions with and without bacteria are 0.47 and 0.50, respectively. This result is consistent with results previously reported (5–7, 12, 25, 26). In addition, these orders of reaction are consistent with the electrochemical theory of dissolution, which predicts values between 0.4 and 0.6 (14). The orders of reaction with respect to the ferrous ions are obtained from the data presented in Fig. 8. The orders of reaction with and without bacteria are −0.41 and −0.40, respectively. Again, this result is consistent with the electrochemical theory, which predicts values of between −0.4 and −0.6 (14).
Thus, it is clear from this analysis of the orders of reaction that T. ferrooxidans does not affect the mechanism of the intrinsic reaction at the zinc sulfide surface. This provides additional evidence for the view that the role of T. ferrooxidans in enhancing the rate of dissolution of zinc sulfide is the removal of sulfur from the particle surface.
Thus, all of the observations are accounted for by the model of dissolution suggested by the above analysis. This model may be summarized as follows: (i) at low concentrations of ferrous ions, the rate of dissolution of sphalerite is controlled by the reaction at the mineral surface, (ii) at high concentrations of ferrous ions in the bulk solution, the rate of chemical leaching of sphalerite is limited by the diffusion of ferrous ions through a porous sulfur layer, and (iii) T. ferrooxidans enhances the rate of leaching above that achieved without bacteria when diffusion through the sulfur layer is limiting by removing the sulfur layer.
In conclusion, we have shown that at high concentrations of ferrous ions, the rate of dissolution of zinc sulfide is higher in the presence of T. ferrooxidans than it is in the absence of T. ferrooxidans. We showed that the particle surface of chemically leached zinc sulfide is covered in sulfur, while sulfur is not present on the bacterially leached zinc sulfide. We have argued that the chemical leaching of zinc sulfide is controlled by two mechanisms, reaction at the surface and diffusion of ferrous ions through a growing layer of sulfur on the surface of the zinc sulfide. We have shown that T. ferrooxidans enhances the rate of leaching of sphalerite at conditions under which chemical leaching is controlled by diffusion through the sulfur layer. Thus, the observations and the analysis of the data lead to the conclusion that the bacteria enhance the rate of dissolution of sphalerite only under conditions in which diffusion through the sulfur layer controls the overall rate of chemical leaching. This is achieved by the oxidation of the sulfur by the bacteria.
ACKNOWLEDGMENTS
We thank Billiton Process Research and the Foundation for Research Development for funding this project.
We also thank D. Rawlings (University of Cape Town) and E. Lawson (University of the Witwatersrand, Johannesburg) for supplying the bacterial culture and their valuable assistance.
REFERENCES
- 1.Boon M, Heijnen J J. Chemical oxidation kinetics of pyrite in bioleaching processes. Hydrometallurgy. 1998;48:27–41. [Google Scholar]
- 2.Boon M, Luyben K C A M, Heijnen J J. The use of on-line off-gas analyses and stoichiometry in the bio-oxidation kinetics of sulfide minerals. Hydrometallurgy. 1998;48:1–26. [Google Scholar]
- 3.Boon M, Snijder M, Hansford G S, Heijnen J J. The oxidation kinetics of zinc sulfide with Thiobacillus ferrooxidans. Hydrometallurgy. 1998;48:171–186. [Google Scholar]
- 4.Choi W K, Torma A E, Ohline R W, Ghali E. Electrochemical aspects of zinc sulfide leaching by Thiobacillus ferrooxidans. Hydrometallurgy. 1993;33:137–152. [Google Scholar]
- 5.Crundwell F K. Kinetics and mechanism of the oxidative dissolution of a zinc sulfide concentrate in ferric sulfate solution. Hydrometallurgy. 1987;19:227–242. [Google Scholar]
- 6.Crundwell F K. Effect of iron impurity in zinc sulfide concentrates on the rate of dissolution. Am Inst Chem Eng J. 1988;34:1128–1134. [Google Scholar]
- 7.Crundwell F K, Verbaan B. Kinetics and mechanisms of the non-oxidative dissolution of sphalerite (zinc sulfide) Hydrometallurgy. 1987;17:369–384. [Google Scholar]
- 8.Dew D W, Lawson E N, Broadhurst J L. The BIOX® process for biooxidation of gold-bearing ore or concentrates. In: Rawlings D E, editor. Biomining: theory, microbes and industrial processes. Berlin, Germany: Springer-Verlag; 1998. pp. 45–80. [Google Scholar]
- 9.Evangelou V P. Pyrite oxidation and its control: solution chemistry, surface chemistry, acid mine drainage (AMD), molecular oxidation mechanisms. Boca Raton, Fla: CRC Press; 1995. p. 1. [Google Scholar]
- 10.Fowler T A, Crundwell F K. Leaching of zinc sulfide by Thiobacillus ferrooxidans: experiments with a controlled redox potential indicate no direct bacterial mechanism. Appl Environ Microbiol. 1998;64:3570–3575. doi: 10.1128/aem.64.10.3570-3575.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harvey P I, Crundwell F K. Growth of Thiobacillus ferrooxidans: a novel experimental design for batch growth and bacterial leaching studies. Appl Environ Microbiol. 1997;63:2586–2592. doi: 10.1128/aem.63.7.2586-2592.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jin Z-M, Warren G W, Henein H. Reaction kinetics of the ferric chloride leaching of sphalerite—an experimental study. Metall Trans B. 1984;15B:5–12. [Google Scholar]
- 13.Levenspiel O. Chemical reaction engineering. 2nd ed. New York, N.Y: John Wiley & Sons; 1972. p. 368. [Google Scholar]
- 14.Nicol M J, Needes C S R, Finkelstein N P. Electrochemical model for the leaching of uranium dioxide. In: Burkin A R, editor. Leaching and reduction in hydrometallurgy. London, United Kingdom: Institute of Mining and Metallurgy; 1975. pp. 1–11. [Google Scholar]
- 15.Porro S, Ramirez S, Reche C, Curutchet G, Alonso-Romanowski S, Donati E. Bacterial attachment—its role in bioleaching processes. Process Biochem. 1997;32:573–578. [Google Scholar]
- 16.Rawlings D E. Restriction enzyme analysis of 16s rRNA genes for the rapid identification of Thiobacillus ferrooxidans, Thiobacillus thiooxidans and Leptospirillum ferrooxidans strains in leaching environments. In: Jerez C A, Vargas T, Toledo H, Wiertz J V, editors. Biohydrometallurgical processing. II. Santiago, Chile: University of Chile Press; 1995. pp. 9–17. [Google Scholar]
- 17.Silverman M P, Ehrlich H L. Microbial formation and degradation of minerals. Adv Appl Microbiol. 1964;6:181–183. [Google Scholar]
- 18.Silverman M P, Lundgren D G. Studies on the chemoautotrophic iron bacterium Ferrobacillus ferrooxidans. I. An improved medium and a harvesting procedure for securing high cell yields. J Bacteriol. 1959;77:642–647. doi: 10.1128/jb.77.5.642-647.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singer P C, Stumm W. Acid mine drainage: the rate limiting step. Science. 1970;167:1121–1123. doi: 10.1126/science.167.3921.1121. [DOI] [PubMed] [Google Scholar]
- 20.Somasundaran P, Moudgil B M. Reagents in mineral technology. New York, N.Y: Marcel Dekker Inc.; 1988. [Google Scholar]
- 21.Sugio T, Katagiri T, Moriyama M, Zhen Y L, Inagaki K, Tano T. Existence of a new type of sulfite oxidase which utilizes ferric ions as an electron acceptor in Thiobacillus ferrooxidans. Appl Environ Microbiol. 1988;54:153–157. doi: 10.1128/aem.54.1.153-157.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suni J, Henein H, Warren G W, Reddy D. Modelling the leaching kinetics of a sphalerite concentrate size distribution in ferric chloride solution. Hydrometallurgy. 1989;22:22–38. [Google Scholar]
- 23.Szekely J, Evans J W, Sohn H Y. Gas-solid reactions. New York, N.Y: Academic Press; 1976. p. 76. [Google Scholar]
- 24.Torma A E. The role of Thiobacillus ferrooxidans in hydrometallurgical processes. Adv Biochem Eng. 1977;6:1–37. [Google Scholar]
- 25.Verbaan B. The leaching of sphalerite in acidic ferric sulfate media in the absence of elemental oxygen, report 2038. Randburg, South Africa: National Institute for Metallurgy; 1980. [Google Scholar]
- 26.Verbaan B, Crundwell F K. An electrochemical model for the leaching of a sphalerite concentrate. Hydrometallurgy. 1986;16:345–359. [Google Scholar]
- 27.Verbaan B, Huberts R. An electrochemical study of the bacterial leaching of synthetic Ni3S2. Int J Mineral Processing. 1988;24:185–202. [Google Scholar]
- 28.Vogel A I. A textbook of quantitative inorganic analysis. London, United Kingdom: Longman; 1962. pp. 309–319. [Google Scholar]