Significance
Tactile sensations on a moving hand are perceived weaker than when presented on the same but stationary hand. There is an ongoing debate about whether this weaker perception is based on sensorimotor predictions or is due to a blanket reduction in sensitivity. Here, we show greater suppression of sensations matching predicted sensory feedback. This reinforces the idea of precise estimations of future body sensory states suppressing the predicted sensory feedback. Our results shine light on the mechanisms of human sensorimotor control and are relevant for understanding clinical phenomena related to predictive processes.
Keywords: sensorimotor prediction, efference copy, tactile suppression, sensory gating, tactile attenuation
Abstract
The ability to sample sensory information with our hands is crucial for smooth and efficient interactions with the world. Despite this important role of touch, tactile sensations on a moving hand are perceived weaker than when presented on the same but stationary hand. This phenomenon of tactile suppression has been explained by predictive mechanisms, such as internal forward models, that estimate future sensory states of the body on the basis of the motor command and suppress the associated predicted sensory feedback. The origins of tactile suppression have sparked a lot of debate, with contemporary accounts claiming that suppression is independent of sensorimotor predictions and is instead due to an unspecific mechanism. Here, we target this debate and provide evidence for specific tactile suppression due to precise sensorimotor predictions. Participants stroked with their finger over textured objects that caused predictable vibrotactile feedback signals on that finger. Shortly before touching the texture, we probed tactile suppression by applying external vibrotactile probes on the moving finger that either matched or mismatched the frequency generated by the stroking movement along the texture. We found stronger suppression of the probes that matched the predicted sensory feedback. These results show that tactile suppression is specifically tuned to the predicted sensory states of a movement.
Tactile sensations on a moving limb are suppressed compared to rest (1, 2). Movement-induced suppression is a fundamental phenomenon of sensory processing found at both the neural and the perceptual level (3) and across various mammalian species, such as human and nonhuman primates (1, 4), cats (5), and rats (6–8). Tactile suppression, sometimes also called tactile gating (9), is commonly tested by applying externally generated tactile probes on a limb that is moving; a well-established paradigm that allows quantifying tactile suppression at various body parts and times (10–12). Tactile suppression is modulated by different spatial and temporal factors. It is most pronounced when the tactile probe is in close proximity to the muscles involved in the movement and gradually decreases when the probe is at more distant locations (13). It can precede the onset of muscle activity and persist throughout the movement (10, 13). Moreover, it can be modulated by task-relevancy, such that suppression is reduced or even eliminated entirely as the importance of sensory feedback increases (11, 14–16).
There is strong evidence that tactile suppression on a limb depends on a forward model. This model uses online feedback and an efference copy of the motor command to estimate the limb’s future sensory states and suppress the predicted sensory feedback (17). Indeed, suppression occurs during the planning phase of a movement (i.e., in the absence of movement) (12) and is stronger with more reliable predictions about the prevailing dynamics (16). Neural effects in central somatosensory and motor areas before movement (18, 19) further corroborate the involvement of central, predictive mechanisms. Yet, reports of tactile suppression shortly before and during passive movements indicate that peripheral, reafferent signals may also mask the detection of tactile probes (20), although suppression before active movements may precede the effects observed in passive movements (4, 21). The possibility of backward masking mechanisms as well as the fact that the external tactile probes cannot be predicted by an efference copy of the motor command have led to recent claims that the reduced sensitivity on a moving limb is rather caused by general cancellation policies, and tactile suppression of externally generated stimuli is independent of sensorimotor predictions (22, 23). Here, we aim to resolve this debate by investigating whether tactile suppression stems from such precise sensorimotor predictions or whether it originates from an unspecific mechanism that leads to a blanket reduction in tactile sensitivity and thus does not distinguish between predicted and unpredicted sensory feedback.
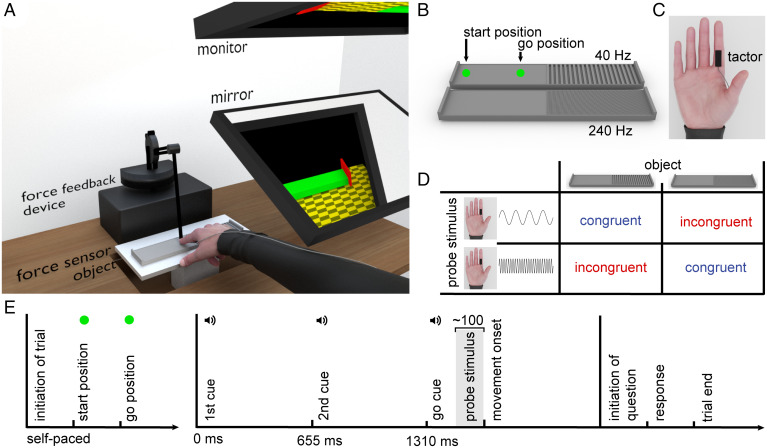
To determine highly specific suppression effects, we manipulated the congruency between the predicted sensory feedback and the external vibrotactile probes (see Fig. 1 for an overview of the methods). Participants performed single stroking movements along two textured objects at a designated speed. Depending on the spatial frequency of the object’s texture, they experienced either a low or a high frequency vibration on their fingertip. These two fundamental frequencies were chosen in a way that different mechanoreceptors were stimulated (24, 25). The objects were presented in a blocked manner so that the tactile feedback from the movement was fully predictable. Vibrotactile probe stimuli were applied to the stroking finger around movement onset. The frequency of these probes either matched (congruent condition) or mismatched (incongruent condition) the frequency elicited on the finger during movement along the textured object. This resulted in four movement conditions: low frequency congruent, low frequency incongruent, high frequency congruent, and high frequency congruent.
Fig. 1.
Illustration of the experimental setup and design. (A) Participants were seated in front of a desk with the force sensor, the object and the force feedback device placed in front of them. A mirror blocked the view of the hand and the object. Participants viewed the scene presented to them on the monitor through the mirror. (B) The objects with two different textured surfaces that would cause the participant to experience a low fundamental frequency (40 Hz) and a high fundamental frequency (240 Hz), respectively. The start position at which participants initially made contact with the object and the go position from which participants began the stroking movements along the texture are marked with arrows. These two positions were represented to participants visually by green spheres on the monitor and were not discernible by touching the object. (C) The tactile stimulation device (tactor) was placed on the ventral part of the proximal phalanx of the right index finger. (D) The combination of the low frequency (40 Hz) and high frequency (240 Hz) objects with the two probe stimulation frequencies that either matched or mismatched the fundamental frequencies experienced by moving along the textured objects resulted in four movement conditions: low frequency congruent, low frequency incongruent, high frequency congruent, low frequency incongruent. (E) Participants initiated each trial via a central, virtual button. A green circle (start position) appeared at the very left of the object in the virtual workspace. Once participants moved their finger to the start position, thus touching the real object, the circle vanished and reappeared at the go position, 7 cm further to the right (∼3 cm before the textured area). Participants moved their finger to this go position at their own pace. Once there, three auditory cues spaced 655 ms apart sounded, prompting participants to start a smooth continuous movement at the designated speed of 203 mm/s after the third cue (go cue). The probe stimulus was presented 100 ms before the anticipated movement onset. After reaching the end of the textured area, participants initiated the question about the tactile stimulus via a central, virtual button and responded with either “yes” or “no” by selecting the respective button.
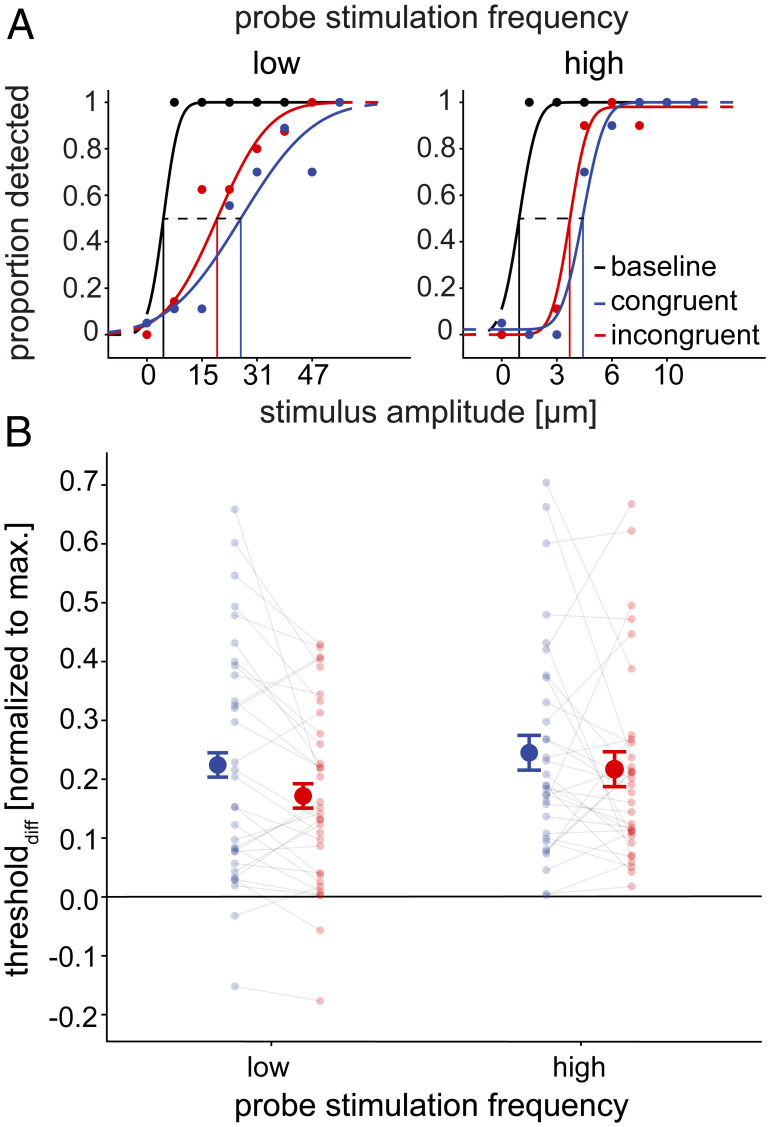
Participants were asked to respond whether they detected the probe stimulus in each trial. We fitted psychometric functions to the individual responses and first calculated the difference in tactile detection thresholds (thresholddiff) for each participant between each movement condition and a respective baseline condition (detection of the same probes at rest, see Fig. 2A). Positive values of thresholddiff indicate stronger tactile suppression during movement. If tactile suppression stems from sensorimotor predictions, we expect stronger suppression (increased thresholddiff) in congruent than in incongruent conditions, as the probe frequencies would match the movement-related predictions. If, on the other hand, tactile suppression stems from an unspecific mechanism, then we should observe similar suppression across all movement conditions (comparable thresholddiff) (22).
Fig. 2.
The results of the tactile detection task. (A) Example psychometric functions of a single participant for low and high frequency probes. Each panel shows psychometric functions for the baseline and the congruent and incongruent movement conditions for the respective probe frequency. Some datapoints are not visible due to overlap. The difference between the baseline and the movement conditions is exemplified by the dotted line representing thresholddiff for both movement conditions. (B) Individual (transparent data points; n = 32) and mean differences in detection of the probe stimulation between the baseline and the movement conditions as measured by the change in detection thresholds, averaged across all participants. Thresholddiff represents the difference between the movement condition and the respective baseline condition (i.e., the suppression effect). Thresholddiff values are normalized to the maximum possible suppression at the respective probe frequency. Greater values indicate impaired sensitivity to probes (i.e., tactile suppression) in the movement conditions, while zero indicates no difference from the baseline. The error bars display 95% Cousineau-Morey confidence intervals for the difference between congruent and incongruent conditions within each probe stimulation frequency (27, 28). Tactile suppression took place in all movement conditions and was generally greater in congruent compared to incongruent conditions.
Results
First, to determine whether tactile perception was affected during movement compared to rest, we performed two-tailed t tests against zero on thresholddiff. Data from a representative participant (Fig. 2A) show increased detection thresholds during movement compared to rest. In line with previous findings (16, 26), tactile probe stimuli were indeed suppressed in all movement conditions. Specifically, detection thresholds during movement were systematically larger than those at rest, indicated by all thresholdsdiff being significantly greater than zero (low frequency congruent: t [31] = 6.25, P < 0.001, d = 1.11; low frequency incongruent: t [31] = 6.24, P < 0.001, d = 1.10; high frequency congruent: t [31] = 7.61, P < 0.001, d = 1.35; and high frequency incongruent: t [31] = 7.39, P < 0.001, d = 1.31) (Fig. 2B).
Second, we examined whether this suppression was stronger in congruent than incongruent conditions, which would provide strong evidence for the involvement of sensorimotor predictions. To this end, we compared thresholddiff using a 2 (probe frequency: low vs. high) by 2 (congruency: congruent vs. incongruent) univariate, repeated-measures ANOVA. Importantly, tactile suppression, as reflected in thresholddiff, was stronger for congruent compared to incongruent conditions (main effect of congruency, F [1,31] = 5.35, P = 0.028, ηG2 = 0.013), whereas there was no effect of probe frequency (F [1,31] = 0.91, P = 0.347, ηG2 = 0.009) nor an interaction (F [1,31] = 0.47, P = 0.497, ηG2 = 0.001) (see Fig. 2B).
The congruency effect on suppression provides evidence for a strong sensorimotor prediction component. However, it may be contaminated by different response criteria across movement conditions. To control for this, we calculated for each movement condition performed by each participant the response criterion (according to signal detection theory, see Materials and Methods for details) (29), which we then submitted to a 2 (probe frequency: low vs. high) by 2 (congruency: congruent vs. incongruent) univariate, repeated-measures ANOVA. The results show that the response criterion did not significantly differ between conditions (mean ± SD: low frequency congruent = −1.89 ± 0.19, low frequency incongruent = −1.88 ± 0.21, high frequency congruent = −1.90 ± 0.15 and high frequency incongruent movement conditions = −1.90 ± 0.15), with neither significant main effects of congruency (F [1,31] < 0.03, P = 0.855, ηG2 < 0.001) or probe frequency (F [1,31] = 0.16, P = 0.690, ηG2 = 0.002), nor a significant interaction (F [1,31] = 0.05, P = 0.831, ηG2 < 0.001). Thus, the increased thresholdsdiff in congruent compared to incongruent conditions are unlikely to be explained by differences in the response criterion. The evidence for sensorimotor predictions is further corroborated by an additional analysis of the sensitivity measure d′ (SI Appendix, Fig. S2) (29), which also demonstrates poorer sensitivity in congruent than incongruent conditions (SI Appendix, Signal Detection Analysis for further details).
Last, to test if the differences in thresholddiff between the movement conditions may be due to differences in the movement itself, rather than to a prediction of the sensory states, we compared several kinematic parameters using 2 (probe frequency: low vs. high) by 2 (congruency: congruent vs. incongruent) univariate, repeated-measures ANOVAs. No statistically significant differences were found for the stimulation time (all F [1,31] ≤ 3.12, P ≥ 0.087, ηG2 ≤ 0.013), the reaction time (all F [1,31] ≤ 3.06, P ≥ 0.090, ηG2 ≤ 0.002), the movement duration (all F [1,31] ≤ 1.08, P ≥ 0.307, ηG2 ≤ 0.005), the average force exerted during the movement (all F [1,31] ≤ 3.10, P ≥ 0.088, ηG2 ≤ 0.012), the average movement velocity (all F [1,31] ≤ 1.56, P ≥ 0.221, ηG2 ≤ 0.007), the average acceleration (all F [1,31] ≤ 2.07, P ≥ 0.161, ηG2 ≤ 0.008), and the average deceleration (all F [1,31] ≤ 1.17, P ≥ 0.287, ηG2 ≤ 0.003) (SI Appendix, Fig. S5).
In summary, congruent vibrotactile probes matching the predicted sensations caused by the movement along textured objects were subject to stronger suppression than incongruent probes. No statistically significant differences in the response criteria or the movement kinematics were found across conditions, making the possibility of systematic biases or movement effects on the detection performance unlikely.
Discussion
Suppression of externally generated sensations shortly before and during voluntary movements is a well-established phenomenon (1, 14, 30). On one hand, it seems paradoxical to suppress sensations not caused by one’s own actions since survival and adaptation require to enhance, and not to suppress, novel and potentially harmful sensations. On the other hand, a surge of somatosensory afferents from the moving limb could overwhelm the system, so it may be beneficial to suppress somatosensory feedback from that limb altogether, including external stimuli. Considering this, the functional mechanisms governing sensory suppression of external stimulations are still a matter of debate. Although this phenomenon has been attributed to predictive mechanisms, such as internal forward models (31), other accounts claim that it stems from an unspecific mechanism, because the probe stimuli used to measure suppression cannot be predicted by an efference copy of the motor command itself (22, 23), or because backward masking may generally obscure any sensations on the moving limb (1). Here, we set out to address the debate on the origin of tactile suppression and show that tactile suppression of externally generated sensations originates from specific sensorimotor predictions.
We demonstrate that the well-established suppression of tactile sensations is stronger when the frequency of the probe stimulus’s vibration matches the predicted vibration caused by the finger movement along the object’s texture. In other words, suppression was stronger in the congruent than incongruent conditions as reflected in increased detection thresholds. This congruency effect cannot be attributed to a general cancellation policy that blankets any stimulus on the moving limb. It can neither be attributed to different response biases, nor to different kinematic performance across movement conditions, as our results show. Rather, a predictive mechanism is regulating the strength of suppression on the basis of specific sensorimotor predictions about the future sensory states generated by the movement.
We found elevated detection thresholds in all movement conditions compared to rest. This suppression effect is present in both congruent and incongruent conditions (i.e., unrelated to the congruency effect). Instead, it can be explained by sensorimotor predictions about the future state of the moving arm, which in turn leads to downweighed sensory processing around the movement (16, 30). As sensory feedback is subject to sensory noise and processing delays, predicting and, as a result discounting, such sensations can be advantageous and possibly desired (31). We suggest that the observed suppression effect comprises two components: one that originates from sensorimotor predictions about the sensory states of the moving arm, and a second one caused by the congruency between the probe stimulation and the predicted sensory consequences of the stroking movement.
Motor control theories involving feedforward control can account for tactile suppression (31, 32). Sensorimotor control is thought to depend on the optimal integration of a sensorimotor estimate about the future sensory states of the system and of peripheral sensory input (33). When the state of one’s own sensory system is uncertain, sensory feedback is downweighed and reliance on sensorimotor estimates is greater (34). Accordingly, in the presence of sensorimotor predictions, sensory feedback, including any external probes, is downweighed to different extents, depending on the reliability of the prediction. This is reflected in attenuated activity in central brain regions, such as sensory and motor cortices, to tactile probes on a limb shortly before its movement (18, 19). Tactile suppression of external probe stimuli is also modulated by feedback demands, such that it is weaker when task-relevant feedback gains importance (14–16) and stronger when movement feedback becomes more predictable (35). This suggests that suppression depends on a dynamic interplay between predictive and feedback signals (11). However, to the best of our knowledge, no study hitherto has distinguished whether this suppression is based on predictive mechanisms, leaving the phenomenon open to different interpretations. One such interpretation is that tactile suppression of external probes is caused by unpredictive mechanisms, such as backward masking of the probe stimuli by peripheral reafferences from the moving limb. Although backward masking may influence sensory processing briefly before and during the movement (1, 21), it does not always occur (11). Masking might have contributed to the generally elevated detection thresholds we observed during movement, however, we presented the probe stimuli well before movement onset (on average 119 ms before), which is substantially earlier than the time window of backward masking (∼50 ms before movement onset) (20, 35). Probes were also presented well before touching the object’s texture (at least 250 ms), which far exceeded the threshold of temporal tactile acuity (50–70 ms) (36), allowing for the perception of the probe stimulation and the texture as two distinct events. Importantly, the differences between the congruent and incongruent conditions can neither be explained by backward masking, nor by any other movement-related mechanism (35, 37, 38), as kinematic behavior was also similar between conditions.
Tactile suppression has also been discussed in the context of the opposing process theory (22), according to which predicted sensations may first be enhanced and later cancelled should unexpected sensations arise. This theory is based on the premise that tactile suppression does not differ between expected and unexpected sensations and is hence not predictive. Here, we show that exafferent tactile probes matching predicted sensations are suppressed more strongly than mismatching probes. Importantly these probes themselves are not the subject of precise sensorimotor predictions; instead, sensorimotor predictions are made about the sensory states associated with the movement (i.e., the vibrations caused by moving the finger across the textured objects). Critically, the exafferent stimulations can be used to probe the sensory suppression effect of those predictions. While our experiment was not designed to evaluate the opposing process theory directly, it is inconsistent with both our results and previous work from the tactile domain showing predictive suppression of self-generated (39) and externally generated tactile sensations (10, 12, 14) occurring up to 300 ms before movement onset (i.e., lacking preceding enhancement).
In sum, we conclude that tactile suppression of externally generated stimuli on a moving limb is modulated in a highly specific manner, as it is stronger when the generated stimuli match the predicted sensory feedback. This speaks in favor of sensorimotor predictions involved in tactile suppression. Our findings provide evidence for the origins and underlying mechanisms of tactile suppression and form the ground to unify the processes that govern sensory tuning, also in other modalities, such as vision (40–42 but see also 43) and audition (see 44 for a review). This contributes to a better understanding of the computational principles and neurobiological substrates of human sensorimotor control as well as of clinical phenomena related to predictive mechanisms, such as Parkinson’s disease (45), obsessive-compulsive disorder (46), schizophrenia (47, 48), and depression (49).
Materials and Methods
Participants.
A total of 48 participants completed the experiment and were compensated with either course credit or a payment of 8€/h. Due to exclusion criteria listed below, 32 participants (23 women, 9 men, range 19–30 y, 22.56 ± 3.03) were included in the final sample (see Quantification and Statistical Analysis for the exclusion criteria; SI Appendix, Fig. S4 for psychometric functions of all 48 participants; SI Appendix for the sample size considerations). The experiment was approved by the local ethics committee at the Justus Liebig University Giessen and was in accordance with the Declaration of Helsinki (2013). All participants provided their signed informed consent. Participants were right-handed according to the German translation of the Edinburgh Handedness Inventory (50) (77.94 ± 21.48). Furthermore, participants reported no current neurological symptoms, no issues with stereopsis or color vision, and had normal or corrected to normal eyesight. Participants also did not report any injuries to the right index finger and had a two-point-discrimination threshold of at least 3 mm on the right index finger (2.34 mm ± 0.48 mm; Two-Point Discriminator, North Coast Medical).
Apparatus.
To assess tactile suppression, a vibrotactile stimulation device (PiezoTac tactor; skin contactor diameter 6.4 mm; Engineering Acoustics) was attached to the ventral part of the proximal phalanx of the participants’ right index finger and was secured with medical tape. The tactor encompassed a skin contactor mounted on a shielded cantilever. Vibrations of the cantilever produced sinusoidal contactor oscillations perpendicular to the skin. We presented brief (100 ms) vibrotactile probes of eight varying oscillation amplitudes for each of the two probe frequencies that we used (40 Hz; 240 Hz). The peak-to-peak displacements of the oscillations ranged from 0 (no stimulation) to 54.45 µm and to 11.31 µm (SI Appendix) for probe frequencies of 40 Hz and 240 Hz, respectively. These displacements were identical in the baseline and movement conditions. White noise was played through over-ear-headphones to prevent participants from hearing the sound caused by the vibrotactile stimulation.
The textured objects (209 × 40 mm) were printed using a three-dimensional (3D) PolyJet printer (Stratasys; Objet30 Pro; printing resolution: 600–1600 dpi; material VeroClear). The right half of the object had a texture with an even cube-wave pattern. The left half of the object was smooth to mitigate the risk of participants adapting to the texture over time. To create two distinct objects, a texture with a high spatial period of 5.08 mm and another one with a smaller spatial period of 0.85 mm were chosen. When moving along these textures at a constant velocity of 203 mm/s (average recorded speed 203 ± 35 mm/s, range: 110–329 mm/s; SI Appendix, Fig. S6 for further details), these spatial periods elicited vibrations with frequencies of 40 Hz and 240 Hz, respectively, on the participants’ fingertip (see Fig. 1B). Two types of mechanoreceptors were targeted with these vibration frequencies to ensure the prediction of discrete sensory states. With frequencies of 40 Hz we mainly stimulated the Meissner corpuscles that have a receptive range of 10–100 Hz and are most sensitive to vibrations of 40–60 Hz (24, 25). The probes of 240 Hz lie in the response range of the Pacinian corpuscles reaching from 40 to 800 Hz (24), with their maximal sensitivity being between 200–300 Hz (25). While moving the finger along the textures may result in a complex set of vibrotactile sensations that go beyond a single frequency, we expect that the experienced vibrations are dominated by a fundamental frequency that follows the spatial frequency of the textured surface, along with some weaker noise and harmonic components (51) (fundamental temporal frequency = spatial frequency * velocity).
The experiment was conducted using a 3D virtual environment, wherein the position of the participants’ index finger as well as the exerted forces were collected through a force feedback device (PHANToM 1.5A, 3D Systems) and a force sensor (bending beam load cell LCB 130 and a measuring amplifier GSV-2AS, resolution 0.05 N, ME-Messsysteme GmbH). Custom-made software (C++) was used to run the experiment, collect responses, and record the force and finger position every 3 ms. Participants sat in front of a table and connected their hand to the force feedback device using a custom-made plastic fingernail which was attached to the nail of their index finger with moldable adhesive pads. This setup allowed keeping the finger pad free for haptic exploration. Participants had no view of their finger connected to the force feedback device or the objects and instead viewed a spatially aligned 3D representation of the scene on a 22” monitor (120 Hz, 786 × 1024 pixel) by looking through a mirror using a pair of stereo glasses (3D Vision 2, NVidia). Visual information associated with experimental control, such as questions and answers for the detection task, were presented on the monitor and corresponded to specific virtual positions in the workspace. Participants could navigate through the experiment and indicate answers by moving their finger and thus the force feedback device to those virtual buttons.
Procedure.
The experiment consisted of a sequence of five blocks, one for each movement condition (2 probe frequencies * 2 object frequencies), and one for the baseline condition. To familiarize participants with the use of the force feedback device and the nature of the tactile stimulations, they first performed 20 mock trials of the baseline condition. These data were not included in the analysis. Next, half of the participants continued with the baseline condition while the other half completed the baseline at the very end of the experimental session. Before the start of the first block of the movement condition, participants performed two practice blocks to train their movement along a smooth object at the designated speed. Before the second, third, and fourth block of the movement condition, they performed one practice block of eight trials (SI Appendix for details on the practice trials). The order of the four movement conditions was pseudorandomized with a Latin square design resulting in four possible sequences, with each condition being equally likely to occur at each position.
Participants were seated in front of a desk with the force sensor, the object and the force feedback device placed in front of them. A mirror blocked the view of the hand and the object. Participants viewed the scene presented to them on the monitor through the mirror. To probe tactile perception, a brief vibrotactile probe was delivered to the participants' index finger in both movement and baseline conditions. At the end of each trial, participants were to respond whether they detected the probe or not. To limit the possibility of a response criterion shift between the conditions, the experimenter instructed the participants to adopt a conservative response criterion before each block of the baseline and movement conditions. For each probing frequency, we presented 70 trials with stimulation (each of the seven stimulation amplitudes repeated 10 times) and 20 trials with no stimulation in a randomized order. This resulted in a total of 90 trials for each block of the movement condition and 180 trials for the block of the baseline condition that involved both probe frequencies.
In each of the four movement conditions, the participants’ right index finger was attached to the force feedback device. They were to move their finger along the textured objects at a constant speed of 203 mm/s while detecting the vibrotactile probes (see Fig. 1 for further details). If participants deviated considerably from the prescribed movement, the software repeated the respective trials and the experimenter gave feedback (SI Appendix for further details). To avoid any influence of the textured object and/or the movement itself on tactile suppression, we presented the probe stimuli shortly before the anticipated movement onset, specifically 100 ms before the median reaction time of the preceding five trials (19, 37). For the first five trials, the stimulation was presented 150 ms after the go cue. Upon initiating the trial by moving to a virtual button, a schematic representation of the object’s left and right outer borders was presented, along with a green circle at the very left of the object (start position). As soon as participants moved their right finger to that circle, thus touching the real object in the workspace, the circle vanished and reappeared at the go position, 7 cm further to the right (∼3 cm before the textured area). Participants moved their finger at their own pace to that new position while keeping contact with the object. To prevent participants from drifting back again after having reached the go position, a force “wall” was generated by the force feedback device to the left of this point. Once participants reached the go position, an untextured green block representing the remaining right part of the object appeared on the monitor followed by three auditory cues spaced 655 ms apart. This time interval of 655 ms corresponded to the time participants needed to travel the complete remaining distance of the object at an average speed of 203 mm/s and thus aided the participants to adhere to this designated speed. Accordingly, participants started a smooth, continuous movement with the last of the three consecutive auditory cues (go cue) and had to finish their movement at the time of an imagined fourth cue. Once they reached the end of the textured area, the object’s visual representation disappeared, and participants could initiate the detection question about the tactile stimulus via a central virtual button and responded with “yes” or “no” by selecting the respective virtual button with the right index finger.
The baseline condition served to determine each individual’s tactile detection performance for each probe frequency at rest. Participants placed their right hand comfortably on the table in front of them while the finger with the tactor rested on a pad to avoid the vibrations from reverberating through the table and distorting the measurement. In the baseline condition only, the participants’ left index finger was attached to the force feedback device to control the experiment in order to limit possible effects on tactile perception from subsequent movements of the stimulated hand. Participants started each trial by bringing their left finger to a virtual button. After 100 ms, a probing tactile stimulus was delivered to their right index finger followed by a delay of 500 ms. Then, a question asking whether the participants had felt a vibration was presented on the virtual monitor and could be answered as during the movement condition. Throughout the baseline condition, the right hand remained completely stationary.
Quantification and Statistical Analysis.
The kinematic data were processed using MATLAB R2021a (The MathWorks) (SI Appendix for further details). To avoid any possible effects of backward masking by the texture, only trials in which the stimulation ended at least 150 ms before participants reached the texture were included in the analysis of the tactile detection task (20, 21, 35, 52). Likewise, only trials with stimulations starting no earlier than 300 ms before movement onset were included, to avoid probing suppression at a moment that is too early to occur (10, 14). Based on these criteria, 88 ± 8% of trials were included on average per participant and movement condition (SI Appendix, Fig. S3 for detection thresholds derived without trial exclusion). One participant was excluded because less than 60% of the trials remained based on the two above-mentioned criteria. The average timespan between stimulation onset and the moment when the finger reached the texture was 360 ± 25 ms, while the probing stimulus was presented on average 119 ± 14 ms before movement onset. Thus, our probing stimuli were presented during the time window when tactile suppression already occurs (10, 14) (SI Appendix, Fig. S1).
Cumulative Gaussian functions were fitted to the responses of the tactile detection task, using the maximum likelihood procedure implemented in the psignifit 4 toolbox (53) for MATLAB. Separate functions were fitted for each of the four movement conditions and each participant. Two additional psychometric functions were fitted for responses to the low and the high frequency probes of the baseline condition. A technical issue erroneously changed the first period of the sinusoidal oscillation for trial n to the probe frequency of the trial n-1. This obviously does not affect the probes presented during the movement conditions, as each block of the movement condition only included a single probe frequency. However, since the two probe frequencies were intermixed randomly in the baseline condition, the frequency was mingled in some trials. To account for this, we fit baseline psychometric functions by using only those trials that had the same probe frequency as the trial before (50 ± 3% and 50 ± 4% for low and high probe stimulation frequency). Importantly, the exact strength of suppression in all movement conditions was affected equally. For each of the psychometric functions, we calculated the detection threshold as the stimulus amplitude that had a 50% probability of being detected. To account for differences across individual baseline detection sensitivities, we subtracted each participant’s baseline detection threshold from the respective value of each of their movement conditions, separately for low and high frequencies. This resulted in four thresholddiff values per participant, with higher values representing stronger suppression during movement compared to rest. To rule out any poorly fitted data, participants with detection thresholds exceeding the range of stimulation (n = 9) or with false alarm rates of 20% or more (n = 6) were excluded. The response criterion was calculated separately for each condition and each participant as the z-transformed false alarm rate (29). Rates of 0 and 1 were corrected to and , respectively, with n being the number of no stimulation trials (54). Averages are reported as mean ± SD throughout the manuscript. The statistical analyses were carried out with a significance level of α = 0.05 using R (R Foundation for Statistical Computing). Effect sizes are reported as Cohen’s d for t tests and as generalized Eta squared for ANOVAs. Cohen’s d was calculated as the difference between the means divided by the SD of the difference.
Supplementary Material
Acknowledgments
We thank Dr. Aaron Zöller for his advice regarding the software, as well as Daniel Famularo and Leah Trawnitschek for their valuable assistance in data collection. This work was supported by the German Research Foundation (DFG)-Collaborative Research Centre SFB/TRR 135, Project A4 and A5, Grant 222641018, and by “The Adaptive Mind,” funded by the Excellence Program of the Hessian Ministry for Science and the Arts.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2118445119/-/DCSupplemental.
Data Availability
Anonymized data have been deposited in Open Science Framework (DOI: 10.17605/OSF.IO/G5ZBJ).
References
- 1.Chapman C. E., Bushnell M. C., Miron D., Duncan G. H., Lund J. P., Sensory perception during movement in man. Exp. Brain Res. 68, 516–524 (1987). [DOI] [PubMed] [Google Scholar]
- 2.Cheron G., Borenstein S., Specific gating of the early somatosensory evoked potentials during active movement. Electroencephalogr. Clin. Neurophysiol. 67, 537–548 (1987). [DOI] [PubMed] [Google Scholar]
- 3.Lei Y., Ozdemir R. A., Perez M. A., Gating of sensory input at subcortical and cortical levels during grasping in humans. J. Neurosci. 38, 7237–7247 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chapman C. E., Jiang W., Lamarre Y., Modulation of lemniscal input during conditioned arm movements in the monkey. Exp. Brain Res. 72, 316–334 (1988). [DOI] [PubMed] [Google Scholar]
- 5.Coquery J.-M., Vitton N., Altérations des potentiels évoqués sur le cortex somesthésique du chat durant un mouvement conditionné. Physiol. Behav. 8, 963–967 (1972). [DOI] [PubMed] [Google Scholar]
- 6.Chapin J. K., Woodward D. J., Somatic sensory transmission to the cortex during movement: Phasic modulation over the locomotor step cycle. Exp. Neurol. 78, 670–684 (1982). [DOI] [PubMed] [Google Scholar]
- 7.Chapin J. K., Woodward D. J., Somatic sensory transmission to the cortex during movement: Gating of single cell responses to touch. Exp. Neurol. 78, 654–669 (1982). [DOI] [PubMed] [Google Scholar]
- 8.Shin H. C., Park H. J., Chapin J. K., Differential phasic modulation of short and long latency afferent sensory transmission to single neurons in the primary somatosensory cortex in behaving rats. Neurosci. Res. 19, 419–425 (1994). [DOI] [PubMed] [Google Scholar]
- 9.Chapman C. E., Tremblay F., “Tactile suppression” in Scholarpedia of Touch, Prescott T. J., Ahissar E., Izhikevich E., Eds. (Atlantis Press, 2016), pp. 293–300. [Google Scholar]
- 10.Colino F. L., Buckingham G., Cheng D. T., van Donkelaar P., Binsted G., Tactile gating in a reaching and grasping task. Physiol. Rep. 2, e00267 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Voudouris D., Fiehler K., Dynamic temporal modulation of somatosensory processing during reaching. Sci. Rep. 11, 1928 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Voss M., Ingram J. N., Haggard P., Wolpert D. M., Sensorimotor attenuation by central motor command signals in the absence of movement. Nat. Neurosci. 9, 26–27 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams S. R., Shenasa J., Chapman C. E., Time course and magnitude of movement-related gating of tactile detection in humans. I. Importance of stimulus location. J. Neurophysiol. 79, 947–963 (1998). [DOI] [PubMed] [Google Scholar]
- 14.Colino F. L., Binsted G., Time course of tactile gating in a reach-to-grasp and lift task. J. Mot. Behav. 48, 390–400 (2016). [DOI] [PubMed] [Google Scholar]
- 15.Manzone D. M., Inglis J. T., Franks I. M., Chua R., Relevance-dependent modulation of tactile suppression during active, passive and pantomime reach-to-grasp movements. Behav. Brain Res. 339, 93–105 (2018). [DOI] [PubMed] [Google Scholar]
- 16.Voudouris D., Broda M. D., Fiehler K., Anticipatory grasping control modulates somatosensory perception. J. Vis. 19, 4 (2019). [DOI] [PubMed] [Google Scholar]
- 17.Fiehler K., Brenner E., Spering M., Prediction in goal-directed action. J. Vis. 19, 10 (2019). [DOI] [PubMed] [Google Scholar]
- 18.Seki K., Fetz E. E., Gating of sensory input at spinal and cortical levels during preparation and execution of voluntary movement. J. Neurosci. 32, 890–902 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arikan B. E., Voudouris D., Voudouri-Gertz H., Sommer J., Fiehler K., Reach-relevant somatosensory signals modulate activity in the tactile suppression network. Neuroimage 236, 118000 (2021). [DOI] [PubMed] [Google Scholar]
- 20.Williams S. R., Chapman C. E., Time course and magnitude of movement-related gating of tactile detection in humans. III. Effect of motor tasks. J. Neurophysiol. 88, 1968–1979 (2002). [DOI] [PubMed] [Google Scholar]
- 21.Chapman C. E., Beauchamp E., Differential controls over tactile detection in humans by motor commands and peripheral reafference. J. Neurophysiol. 96, 1664–1675 (2006). [DOI] [PubMed] [Google Scholar]
- 22.Press C., Kok P., Yon D., The perceptual prediction paradox. Trends Cogn. Sci. 24, 13–24 (2020). [DOI] [PubMed] [Google Scholar]
- 23.Kilteni K., Ehrsson H. H., Predictive attenuation of touch and tactile gating are distinct perceptual phenomena. iScience 25, 104077 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bolanowski S. J. Jr., Gescheider G. A., Verrillo R. T., Checkosky C. M., Four channels mediate the mechanical aspects of touch. J. Acoust. Soc. Am. 84, 1680–1694 (1988). [DOI] [PubMed] [Google Scholar]
- 25.Johnson K. O., Yoshioka T., Vega-Bermudez F., Tactile functions of mechanoreceptive afferents innervating the hand. J. Clin. Neurophysiol. 17, 539–558 (2000). [DOI] [PubMed] [Google Scholar]
- 26.Voudouris D., Fiehler K., Enhancement and suppression of tactile signals during reaching. J. Exp. Psychol. Hum. Percept. Perform. 43, 1238–1248 (2017). [DOI] [PubMed] [Google Scholar]
- 27.Baguley T., Calculating and graphing within-subject confidence intervals for ANOVA. Behav. Res. Methods 44, 158–175 (2012). [DOI] [PubMed] [Google Scholar]
- 28.Morey R. D., Confidence intervals from normalized data: A correction to Cousineau (2005). Tutor. Quant. Methods Psychol. 4, 61–64 (2008). [Google Scholar]
- 29.Green D. M., Swets J. A., Signal Detection Theory and Psychophysics (Peninsula Publishing, 1988). [Google Scholar]
- 30.Voss M., Ingram J. N., Wolpert D. M., Haggard P., Mere expectation to move causes attenuation of sensory signals. PLoS One 3, e2866 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wolpert D. M., Flanagan J. R., Motor prediction. Curr. Biol. 11, R729–R732 (2001). [DOI] [PubMed] [Google Scholar]
- 32.Wolpert D. M., Miall R. C., Forward models for physiological motor control. Neural Netw. 9, 1265–1279 (1996). [DOI] [PubMed] [Google Scholar]
- 33.Franklin D. W., Wolpert D. M., Computational mechanisms of sensorimotor control. Neuron 72, 425–442 (2011). [DOI] [PubMed] [Google Scholar]
- 34.Körding K. P., Wolpert D. M., Bayesian integration in sensorimotor learning. Nature 427, 244–247 (2004). [DOI] [PubMed] [Google Scholar]
- 35.Fraser L. E., Fiehler K., Predicted reach consequences drive time course of tactile suppression. Behav. Brain Res. 350, 54–64 (2018). [DOI] [PubMed] [Google Scholar]
- 36.Humes L. E., Busey T. A., Craig J. C., Kewley-Port D., The effects of age on sensory thresholds and temporal gap detection in hearing, vision, and touch. Atten. Percept. Psychophys. 71, 860–871 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gertz H., Voudouris D., Fiehler K., Reach-relevant somatosensory signals modulate tactile suppression. J. Neurophysiol. 117, 2262–2268 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cybulska-Klosowicz A., Meftah M., Raby M., Lemieux M. L., Chapman C. E., A critical speed for gating of tactile detection during voluntary movement. Exp. Brain Res. 210, 291–301 (2011). [DOI] [PubMed] [Google Scholar]
- 39.Bays P. M., Flanagan J. R., Wolpert D. M., Attenuation of self-generated tactile sensations is predictive, not postdictive. PLoS Biol. 4, e28 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cardoso-Leite P., Mamassian P., Schütz-Bosbach S., Waszak F., A new look at sensory attenuation. Action-effect anticipation affects sensitivity, not response bias. Psychol. Sci. 21, 1740–1745 (2010). [DOI] [PubMed] [Google Scholar]
- 41.Gremmler S., Lappe M., Saccadic suppression during voluntary versus reactive saccades. J. Vis. 17, 8 (2017). [DOI] [PubMed] [Google Scholar]
- 42.Diamond M. R., Ross J., Morrone M. C., Extraretinal control of saccadic suppression. J. Neurosci. 20, 3449–3455 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schwarz K. A., Pfister R., Kluge M., Weller L., Kunde W., Do we see it or not? Sensory attenuation in the visual domain. J. Exp. Psychol. Gen. 147, 418–430 (2018). [DOI] [PubMed] [Google Scholar]
- 44.Schröger E., Marzecová A., SanMiguel I., Attention and prediction in human audition: A lesson from cognitive psychophysiology. Eur. J. Neurosci. 41, 641–664 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Railo H., et al. , Dopamine and eye movement control in Parkinson’s disease: Deficits in corollary discharge signals? PeerJ 6, e6038 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Belayachi S., Van der Linden M., Feeling of doing in obsessive-compulsive checking. Conscious. Cogn. 19, 534–546 (2010). [DOI] [PubMed] [Google Scholar]
- 47.Lindner A., Thier P., Kircher T. T. J., Haarmeier T., Leube D. T., Disorders of agency in schizophrenia correlate with an inability to compensate for the sensory consequences of actions. Curr. Biol. 15, 1119–1124 (2005). [DOI] [PubMed] [Google Scholar]
- 48.Ford J. M., Palzes V. A., Roach B. J., Mathalon D. H., Did I do that? Abnormal predictive processes in schizophrenia when button pressing to deliver a tone. Schizophr. Bull. 40, 804–812 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yaple Z. A., Tolomeo S., Yu R., Abnormal prediction error processing in schizophrenia and depression. Hum. Brain Mapp. 42, 3547–3560 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oldfield R. C., The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9, 97–113 (1971). [DOI] [PubMed] [Google Scholar]
- 51.Wiertlewski M., Hudin C., Hayward V., “On the 1/f noise and non-integer harmonic decay of the interaction of a finger sliding on flat and sinusoidal surfaces” in 2011 IEEE World Haptics Conference, L. A. Jones, M. Harders, Y. Yokokohji, Eds. (IEEE, 2011), pp. 25–30. [Google Scholar]
- 52.Laskin S. E., Spencer W. A., Cutaneous masking. II. Geometry of excitatory andinhibitory receptive fields of single units in somatosensory cortex of the cat. J. Neurophysiol. 42, 1061–1082 (1979). [DOI] [PubMed] [Google Scholar]
- 53.Schütt H. H., Harmeling S., Macke J. H., Wichmann F. A., Painfree and accurate Bayesian estimation of psychometric functions for (potentially) overdispersed data. Vision Res. 122, 105–123 (2016). [DOI] [PubMed] [Google Scholar]
- 54.Macmillan N. A., Kaplan H. L., Detection theory analysis of group data: Estimating sensitivity from average hit and false-alarm rates. Psychol. Bull. 98, 185–199 (1985). [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymized data have been deposited in Open Science Framework (DOI: 10.17605/OSF.IO/G5ZBJ).