Significance
Here we show that most chemokine receptors (CRs) form heteromeric complexes with α1-adrenergic receptors (ARs) in recombinant systems and that such heteromers are detectable in human monocytes and in the human monocytic leukemia cell line THP-1. Furthermore, we provide evidence that α1B/D-ARs control the function of their CR heteromerization partners. Our findings suggest that heteromeric complexes between α1B/D-ARs and CRs are necessary for normal function of CR heteromerization partners, indicate receptor heteromerization as a molecular mechanism by which stress hormones regulate leukocyte trafficking in health and disease, and offer opportunities to modulate leukocyte and/or cancer cell trafficking in disease processes.
Keywords: chemokine receptors, G protein–coupled receptor hetero-oligomers, α1-adrenergic receptors, CRISPR-Cas9 gene editing, chemotaxis
Abstract
It is known that catecholamines regulate innate immune functions. The underlying mechanisms, however, are not well understood. Here we show that at least 20 members of the human chemokine receptor (CR) family heteromerize with one or more members of the α1-adrenergic receptor (AR) family in recombinant systems and that such heteromeric complexes are detectable in human monocytes and the monocytic leukemia cell line THP-1. Ligand binding to α1-ARs inhibited migration toward agonists of the CR heteromerization partners of α1B/D-ARs with high potency and 50 to 77% efficacy but did not affect migration induced by a noninteracting CR. Incomplete siRNA knockdown of α1B/D-ARs in THP-1 cells partially inhibited migration toward agonists of their CR heteromerization partners. Complete α1B-AR knockout via CRISPR-Cas9 gene editing in THP-1 cells (THP-1_ADRA1BKO) resulted in 82% reduction of α1D-AR expression and did not affect CR expression. Migration of THP-1_ADRA1BKO cells toward agonists of CR heteromerization partners of α1B/D-ARs was reduced by 82 to 95%. Our findings indicate that CR:α1B/D-AR heteromers are essential for normal function of CR heteromerization partners, provide a mechanism underlying neuroendocrine control of leukocyte trafficking, and offer opportunities to modulate leukocyte and/or cancer cell trafficking in disease processes.
It is well established that the autonomic nervous system regulates innate immune functions through the release of endogenous catecholamines, which activate the G protein–coupled receptor (GPCR) family of adrenergic receptors (ARs) expressed in the immune system (1–5). Increased autonomic nervous system activity occurs in numerous physiological and pathological conditions, such as exercise, anxiety, trauma, or infection, and has been correlated with disease activity in various autoimmune diseases (6–8). Furthermore, evidence has been provided that catecholamines can be synthesized and released from lymphocytes, macrophages, and neutrophils, which are thought to mediate para- or autocrine signaling to control leukocyte function (9–12). While previous studies focused on the contribution of β- and α2-ARs, the roles of α1-ARs in the regulation of immune functions are poorly understood (13). Furthermore, the detailed molecular mechanisms underlying cross-talk between the neurohormonal and the innate immune system remain to be determined.
The 7-transmembrane domain protein family of chemokine receptors (CRs) is composed of 18 GPCRs, four atypical chemokine receptors (ACKR1 to ACKR4) and chemokine (C-C motif) receptor-like (CCRL) 2, designated ACKR5 pending confirmation (14, 15). CRs are essential for the regulation of leukocyte positioning, trafficking, and recruitment and play important roles in all aspects of inflammation, including numerous disease processes, as diverse as infections, autoimmune diseases, cancer, and tissue injury and repair (14, 16–18). All CRs except chemokine (C-X-C motif) receptor (CXCR) 5, ACKR1, and ACKR3 have been described to be expressed by human monocytes (19–22).
Recently, we showed that α1-ARs form hetero-oligomeric complexes with CXCR4 and ACKR3 in recombinant systems and in vascular smooth muscle cells, through which the receptors cross-talk (23–26). Similarly, recombinant CXCR2 has been reported to heteromerize with α1a-AR, and the endogenously expressed receptors were found to colocalize in prostate smooth muscle (27). The existence and possible function of such heteromeric receptor complexes in human leukocytes, however, is unknown. Furthermore, it is unknown whether other members of the human CR family may also heteromerize with α1-ARs.
In the present study, we sought to evaluate the interactome between the family of human CRs and α1-ARs and to assess the functional roles of such heteromers in the regulation of CR-mediated chemotaxis utilizing freshly isolated human monocytes and the human monocytic leukemia cell line THP-1 as model systems. Our findings suggest that most CRs heteromerize with α1-ARs. Moreover, we provide evidence that α1B/D-AR:CR heteromers are required for CR-mediated chemotaxis of α1B/D-AR heteromerization partners and that α1-ARs within these heteromers mediate the inhibitory effects of α1-AR ligands on directed cell migration toward cognate agonists of their CR heteromerization partners.
Results and Discussion
Bioluminescence Resonance Energy Transfer Identifies Multiple CR:α1a/b/d-AR Heteromers.
To evaluate the interactome between CRs and α1-ARs, we employed bioluminescence resonance energy transfer (BRET) to screen for interactions between α1a/b/d-ARs and all 23 members of the human CR family in human embryonic kidney 293T (HEK293T) cells. We transfected cells with one α1-AR subtype C-terminally ligated to the luminescence donor Renilla Luciferase (α1a/b/d-AR-RLuc) plus each of the CRs C-terminally ligated to enhanced yellow fluorescent protein (CR-YFP) in parallel and measured BRET. As a control for nonspecific bystander BRET signals, cells were transfected with α1a/b/d-AR-RLuc and yellow fluorescent protein (YFP) alone or with metabotropic glutamate receptor 1 (mGlu1R)-YFP at various energy acceptor:donor ratios (28). Fig. 1 A–E shows the results from representative BRET screening experiments for interactions between all CRs and α1a-AR (Fig. 1 A and B), α1b-AR (Fig. 1C), and α1d-AR (Fig. 1 D and E). To minimize type I error (false-positive identification), we considered mean BRET signals corresponding to heteromeric complexes between α1a/b/d-AR-RLuc and CR-YFP above the 99% prediction band for nonspecific interactions (gray areas in Fig. 1 A–E) as positive signals. While we recognize that this threshold may underestimate the number of true-positive receptor-receptor interactions, we believe that this approach permits identification of CR heteromerization partners with high confidence. SI Appendix, Fig. S1 summarizes the results from three independent screening experiments for each α1-AR subtype. We observed positive BRET signals for interactions between 20 of the 23 members of the CR family with at least one of the α1-AR subtypes in three of three screening experiments (19, 7, and 10 positive signals in all screening experiments for interactions between CRs and α1a-AR, α1b-AR, and α1d-AR, respectively). Chemokine (X-C motif) receptor 1 (XCR1) was the only CR for which BRET signals for interactions with α1a/b/d-ARs were below the 99% prediction band for nonspecific interactions in all screening experiments. The findings that BRET signals for interactions between ACKR3 and α1a/b-ARs were positive in only one of the three screening experiments and that BRET signals for interactions between ACKR3 and α1d-AR were negative could be explained by our previous observations, which suggested that α1-ARs preferentially hetero-oligomerize with ACKR3 within the CXCR4:ACKR3 heterodimer (24).
Fig. 1.
BRET screening to identify CR heteromerization partners of α1a/b/d-ARs. HEK293T cells were transfected with α1a/b/d-AR-RLuc plus each CR-YFP in triplicate. YFP fluorescence and luminescence were read as described in Methods. Net BRET (528/460 nm) was plotted against YFP fluorescence/luminescence (YFP/Lum). Net BRET signals are mean ± SD. Cells transfected with α1a/b/d-AR-RLuc and YFP or mGlu1R-YFP at various acceptor:donor ratios served as nonspecific controls; nonspecific BRET signals were analyzed by linear regression analysis. The black line shows the regression line, and dashed lines indicate 99% prediction bands. The gray area indicates the expected distribution of nonspecific BRET signals. BRET signals above the 99% prediction band for nonspecific interactions were considered positive signals for interactions between CRs (colored symbols) and α1a/b/d-AR. Graphs represent one of three screening experiments for interactions between CRs and α1a-AR (A and B), α1b-AR (C), or α1d-AR (D and E). ctrl., control.
To confirm the results from the BRET screening experiments, we then performed saturation BRET experiments. For each α1-AR subtype, we randomly selected several CRs that showed positive BRET signals and at least one CR with negative BRET signals in the screening experiments (Fig. 2). Consistent with the results from BRET screening, we observed hyperbolic progressions of the BRET signals for interactions between α1a-AR and chemokine (C-C motif) receptor (CCR) 1 (Fig. 2A), CXCR4 (Fig. 2B), and CXCR5 (Fig. 2C) with increasing energy acceptor:donor ratios, whereas BRET signals for interactions between α1a-AR and XCR1 (Fig. 2D) or ACKR4 (Fig. 2E) were indistinguishable from nonspecific bystander signals (Fig. 2B). Similarly, saturation BRET confirmed constitutive heteromerization between α1b-AR and CCR1, CCR2, CCR4, CCR10, CXCR4, ACKR1, or ACKR2 (Fig. 2 F–L) and between α1d-AR and CCR6 (Fig. 2N) and CXCR2 (Fig. 2O) and nonspecific interactions between α1b-AR and CCR8 (Fig. 2M) and between α1d-AR and CCR9 (Fig. 2P). These findings validate positive and negative BRET signals for interactions between α1-ARs and CRs from our screening approach. Collectively, BRET indicates that at least 20 recombinant members of the human CR family constitutively heteromerize with at least one recombinant member of the α1-AR family.
Fig. 2.
Saturation BRET confirms CR heteromerization partners of α1a/b/d-ARs. HEK293T cells were transfected with a fixed amount of α1a/b/d-RLuc and with increasing amounts of CR-YFP or YFP. Figures show saturation BRET signals representative of n = 3 independent experiments per receptor-receptor combination. YFP fluorescence and luminescence were read as described in Methods. Net BRET (528/460 nm) was plotted against YFP/Lum. (A–E) Saturation BRET between α1a-AR and CCR1 (A), CXCR4 and YFP (B), CXCR5 (C), XCR1 (D), or ACKR4 (E). (F–M). Saturation BRET between α1b-AR and CCR1 and YFP (F), CCR2 (G), CCR4 (H), CCR10 (I), CXCR4 (J), ACKR1 (K), ACKR2 (L), or CCR8 (M). (N–P). Saturation BRET between α1d-AR and CCR6 and YFP (N), CXCR2 (O), or CCR9 (P).
Chemokine Receptor:α1-AR Heteromers Are Detectable in THP-1 Cells and in Human Monocytes.
To assess whether these findings on recombinant receptors translate to endogenously expressed receptors in leukocytes, we performed proximity ligation assays (PLAs) in the human monocytic leukemia cell line THP-1 and in freshly isolated human monocytes to visualize α1-ARs and selected CRs individually and to assess α1-AR:CR interactions (29). Representative images for the detection of individual receptors and receptor-receptor interactions in THP-1 cells are shown in Fig. 3 A and B, respectively. While PLA signals for α1A-AR were negative in THP-1 cells, we observed positive PLA signals for α1B/D-ARs (Fig. 3A, Top). qPCR confirmed that α1A-AR mRNA was not detectable in THP-1 cells under our cell culture conditions, whereas mRNA for all α1-AR subtypes was detectable in human primary aortic smooth muscle cells, which are known to express all α1-AR subtypes on the cell surface (difference in cycle threshold values [ΔCt] normalized to β-actin: THP-1-α1A-AR, not detectable; α1B-AR, 17.4; α1D-AR, 16.8; human primary aortic smooth muscle cells: α1A-AR, 16.5; α1B-AR, 10.3; α1D-AR, 7.1) (23, 24, 30). Furthermore, we observed positive PLA signals for CCR1, CCR2, CCR8, and CXCR4 in THP-1 cells. When PLA was performed to visualize receptor-receptor interactions, we observed positive PLA signals corresponding to interactions between α1B/D-ARs and CCR1, CCR2, and CXCR4 (Fig. 3B). PLA signals for interactions between α1B/D-ARs and CCR8 and for interactions between α1A-AR and CRs were not detectable (Fig. 3B). These data agree with the expression profile of α1-AR subtypes that we determined in THP-1 cells and with the interactions between α1-ARs and CRs that we observed in BRET experiments.
Fig. 3.
CR:α1A/B/D-AR heteromers are detectable in THP-1 cells and in human monocytes. Representative PLA images for the detection of individual receptors (A and C) and receptor-receptor interactions (B and D) in THP-1 cells (A and B) and freshly isolated monocytes (C and D). Images show merged DAPI (nuclear counterstain) and PLA signals (red, λexcitation/emission 598/634 nm) acquired from z stack images (n = 10; thickness = 0.5 µm, Bottom to Top) and are representative of n = 3 independent experiments. As controls, cells were incubated with IgG (A and C) or with a combination of IgG and anti-CR (B and D). Scale bars, (A and B) 10 µm and (C and D) 5 µm.
In contrast to THP-1 cells, all α1-AR subtypes, as well as CCR1, CCR2, CCR8, and CXCR4, were detectable in freshly isolated human monocytes by PLA (Fig. 3C). Consistent with our BRET findings, we observed positive PLA signals for interactions between α1A-AR and the selected CRs and for interactions between α1B/D-ARs and CCR1, CCR2, and CXCR4 but not for interactions between α1B/D-ARs and CCR8 (Fig. 3D). Thus, our findings suggest that positive BRET signals for recombinant receptor-receptor interactions are applicable to endogenously expressed receptors and that such heteromeric complexes are constitutively expressed in human monocytes and THP-1 cells.
Ligand Binding to α1B/D-ARs Inhibits Chemotaxis Mediated by CR Heteromerization Partners.
To evaluate the functional roles of α1-ARs in the regulation of CR-mediated chemotaxis, we tested the effects of the pan–α1-AR agonist phenylephrine on directed migration of THP-1 cells and human monocytes toward cognate agonists of the selected CRs in transwell migration assays. We first determined the chemotactic dose-response for each chemokine in THP-1 cells and utilized the concentrations that resulted in maximal chemotaxis for subsequent experiments (SI Appendix, Fig. S2 A–D). CCL23 (chemokine [C-C motif] ligand [CCL] 23), CCL1, and CXCL12 are selective agonists for CCR1, CCR8, and CXCR4, respectively, in THP-1 cells. CCL2, however, is a cognate agonist of CCR2, CCR3, and CCR5, all of which are known to be expressed in THP-1 cells (31, 32). To evaluate which of these receptors mediates CCL2-induced chemotaxis, THP-1 cells were exposed to the CCR2 antagonist INCB3284, the CCR3 antagonist SB328437, the CCR5 antagonist Maraviroc, or combinations of the antagonists, and migration toward CCL2 tested. As shown in SI Appendix, Fig. S2E, INCB3284 dose-dependently inhibited CCL2-induced chemotaxis by more than 95%. SB328437, Maraviroc, or combinations of both inhibited CCL2-induced chemotaxis at the highest concentration of 10 μM by less than 30%, indicating that CCL2-induced chemotaxis in THP-1 cells is primarily mediated via CCR2.
Fig. 4 A and B show the effects of phenylephrine on chemotaxis of THP-1 cells and human monocytes, respectively. Cells were exposed to various concentrations of phenylephrine, and their migration toward the various chemokines was tested. Phenylephrine dose-dependently inhibited chemotaxis mediated via CR heteromerization partners of α1B/D-ARs in THP-1 cells by 50 to 55% and in human monocytes by 60 to 77% with high potency (IC50 [half maximal inhibitory concentration] [in nM; mean ± SE] in THP-1 cells: CCR1, 5.0 ± 6.7; CCR2, 7.5 ± 7.7; CXCR4, 17 ± 29; IC50 in human monocytes: CCR1, 12 ± 8; CCR2, 37 ± 26; CXCR4, 6 ± 3). Phenylephrine, however, did not affect chemotaxis induced by the cognate agonist of CCR8, a CR that does not heteromerize with α1B/D-ARs. Because human monocytes constitutively express α1A-AR and CCR8:α1A-AR heteromers (Fig. 3 C and D), the finding that CCL1-induced chemotaxis was not affected by phenylephrine indicates distinct functional roles of α1-AR subtypes in the regulation of CR-mediated chemotaxis. While our findings imply that the inhibitory effects of phenylephrine on CR-induced chemotaxis are primarily mediated via α1B/D-AR, the functional role of α1A-AR and the CCR8:α1A-AR heteromer in human monocytes remains to be determined.
Fig. 4.
Ligands of α1-ARs inhibit chemotaxis mediated via CR heteromerization partners of α1B/D-ARs. CI (mean ± SE). (A and B). THP-1 cells (A) or freshly isolated human monocytes (B) were exposed to various concentrations of phenylephrine (PE), and chemotaxis toward CCL23 (10 nM), CCL2 (10 nM), CCL1 (1 μM), or CXCL12 (100 nM) was tested. CI(%), chemotactic index in the percentage of cells not exposed to PE. n = 3–4 independent experiments. (C) THP-1 cells were exposed to various concentrations of phentolamine, and chemotaxis toward CCL23 (10 nM), CCL2 (10 nM), CCL1 (1 μM), or CXCL12 (100 nM) was tested. CI(%), chemotactic index in the percentage of cells not exposed to phentolamine. n = 3 independent experiments. (D–F). THP-1 cells were exposed to various concentrations of 5-methylurapidil, L-786314, or BMY7378, and chemotaxis toward CCL23 (10 nM, D), CCL2 (10 nM, E), or CXCL12 (100 nM, F) was tested. CI(%), chemotactic index in the percentage of cells not exposed to inhibitors. n = 3 independent experiments. (G–I) Chemotactic dose-responses for CCL23 (G, n = 3), CCL2 (H, n = 6), and CXCL12 (I, n = 3) in THP-1 cells exposed to 10 μM PE, 10 μM phentolamine, or vehicle. *P < 0.05 for PE vs. vehicle, #P < 0.05 for phentolamine vs. vehicle (two-way ANOVA with Dunnett’s multiple comparisons test). (J) Radioligand competition binding assays with crude membrane preparations from THP-1 cells exposed to vehicle, 10 μM PE, or 10 μM phentolamine. Specific [125I]-CCL2 binding (as a percentage): (counts per minute [cpm] − nonspecific cpm at bottom plateau) / (cpm in absence of CCL2 − nonspecific cpm) × 100. Data are mean ± SE from n = 3 independent experiments performed in duplicate.
Because the observed phenylephrine-mediated inhibition of chemotaxis could be a result of downstream signaling events upon activation of α1-ARs, independent of α1-AR:CR heteromerization, we tested whether exposure of THP-1 cells to α1-AR antagonists would also affect CR-mediated chemotaxis. As shown in Fig. 4C, the pan–α1-AR antagonist phentolamine dose-dependently inhibited chemotaxis mediated via CCR1, CCR2, and CXCR4 by 42, 66, and 58%, respectively. The IC50 of phentolamine for inhibition of CR-mediated chemotaxis was 0.03 ± 0.08, 2.7 ± 3.5, and 0.2 ± 0.2 nM for CCR1, CCR2, and CXCR4, respectively. In contrast, phentolamine did not affect CCR8-mediated chemotaxis.
To confirm these findings, we then utilized α1-AR subtype-selective antagonists and tested their effects on CCR1-, CCR2-, and CXCR4-mediated chemotaxis (Fig. 4 D–F). In agreement with α1-AR subtype expression in THP-1 cells, the α1A-AR–selective antagonist 5-methylurapidil did not affect CR-mediated chemotaxis. In contrast, CCR1-, CCR2-, and CXCR4-mediated chemotaxis could be inhibited with the α1B-AR–selective antagonist L-765314 by 64, 55, and 58% and with the α1D-AR–selective antagonist BMY7378 by 57, 47, and 54%, respectively. The IC50 of L-765314 for inhibition of CCR1-, CCR2-, and CXCR4-induced chemotaxis was 0.34 ± 0.29, 0.51 ± 0.35, and 2.65 ± 0.41 nM and that of BMY7378 was 10.5 ± 0.91, 0.93 ± 0.61, and 2.87 ± 0.6 nM, respectively.
To exclude that the observed inhibitory effects of α1-AR ligands on migration of cells toward single optimized concentrations of chemokines are caused by a shift of their bell-shaped chemotactic dose-response curves, we compared the chemotactic dose-responses for CCL23, CCL2, and CXCL12 in the presence and absence of 10 μM phenylephrine or phentolamine in THP-1 cells. As shown in Fig. 4 G–I, phenylephrine and phentolamine inhibited chemotaxis over the complete range of chemokine concentrations that induced chemotaxis in vehicle-treated cells. Moreover, we observed in radioligand competition binding experiments (Fig. 4J) that the IC50 of CCL2 to displace [125I]-CCL2 from crude membrane preparations of THP-1 cells that were pretreated with vehicle, phenylephrine, and phentolamine were indistinguishable (IC50 [mean ± SE, n = 3]: vehicle, 167 ± 23 pM; phenylephrine, 150 ± 23 pM; phentolamine, 169 ± 30 pM) and consistent with the reported affinity of CCL2 for CCR2 (33–35). Thus, these findings suggest that the inhibitory effects of α1B/D-AR ligands are primarily mediated by reducing efficacy of the CR heteromerization partners of α1B/D-AR to induce chemotaxis, rather than modulating affinity for their agonists.
Our observations on the chemotactic behavior of normal human monocytes and the leukemia cell line THP-1 are consistent with our previous observations that phenylephrine, phentolamine, prazosin (inverse α1A/B/D-AR agonist), and cyclazosin (inverse α1B/D-AR agonist and α1A-AR antagonist) inhibit CXCR4-mediated chemotaxis of primary human vascular smooth muscle cells (25, 36). This indicates that the inhibitory effects of α1-AR ligands are generalizable to CR heteromerization partners of α1B/D-ARs and to other normal and malignant cell types and tissues.
The finding that both agonist and antagonist binding to α1-ARs inhibited chemotaxis toward agonists of CR-heteromerization partners of α1B/D-AR implies that the observed inhibitory effects are not related to downstream signaling of α1-ARs. Because none of the α1-AR ligands demonstrated chemotactic activity in THP-1 cells (SI Appendix, Fig. S2F), their inhibitory effects on CR-mediated chemotaxis can also not be attributed to a reverse chemoattractant gradient in our experiments. However, phentolamine and all subtype-selective α1-AR inhibitors that we employed in the present study are known to function as inverse agonists at α1-ARs (37, 38). This suggests that conformational changes of α1B/D-AR upon ligand binding regulate the CR heteromerization partners of α1B/D-AR within α1B/D-AR:CR heteromeric complexes, leading to inhibition of chemokine-induced chemotaxis.
Furthermore, the binding affinity of phenylephrine for α1-ARs is comparable or slightly lower than the binding affinity of endogenous catecholamines for α1-ARs (15). Because the determined IC50 of phenylephrine for inhibition of CR-mediated chemotaxis is in the range of physiologically and pharmacologically relevant catecholamine concentrations in humans (39, 40), our findings suggest that circulating catecholamines in humans inhibit chemotactic activity of the cognate agonists of the CR heteromerization partners of α1B/D-ARs in health and disease processes. This assumption is consistent with the significant negative correlation between endogenous norepinephrine levels and CXCL12-induced chemotaxis of peripheral blood mononuclear cells that has been observed at baseline and during acute psychological stress in healthy humans (41).
Depletion of α1B/D-ARs Inhibits Chemotaxis Mediated by CR Heteromerization Partners.
To assess the roles of heteromeric complexes between α1B/D-ARs and CRs in the regulation of CR-mediated chemotaxis per se, we utilized small interfering RNA (siRNA) gene silencing in THP-1 cells to deplete α1B/D-AR from the cell surface. Fig. 5 shows representative images for the visualization of individual receptors after incubation of cells with nontargeting (NT) and α1B/D-AR siRNA and the quantification of PLA signals from three independent experiments. Incubation of THP-1 cells with α1B/D-AR siRNA selectively reduced PLA signals for the target receptor without affecting PLA signals for other receptors that were studied. Compared with cells incubated with NT siRNA, PLA signals for α1B-AR were reduced by 57 ± 7% and PLA signals for α1D-AR were undetectable after incubation with the corresponding siRNA. Fig. 6 shows representative images for the visualization of receptor-receptor interactions after incubation of cells with NT and α1B/D-AR siRNA and the quantification of PLA signals from three independent experiments. PLA signals corresponding to CCR1:α1B/D-AR heteromers were reduced consistent with the degree of α1B/D-AR depletion after siRNA treatment. In contrast, incubation of cells with α1B-AR siRNA reduced PLA signals for CCR2:α1B-AR and CXCR4:α1B-AR interactions, as well as PLA signals for CCR2:α1D-AR and CXCR4:α1D-AR interactions, compared with cells incubated with NT siRNA. Because α1B-AR is known to form heteromeric complexes with α1A-AR and α1D-AR in recombinant systems and in human vascular smooth muscle (42–44), our findings suggest that CCR1 primarily interacts with α1B/D-AR protomers or homodimers, whereas CCR2 and CXCR4 form higher-order hetero-oligomeric complexes with the α1B-AR:α1D-AR heteromer. Although the existence of higher-order hetero-oligomeric GPCR complexes composed of more than two GPCR protomers in native cells or tissues has not yet been unequivocally confirmed due to methodological limitations, we recently provided evidence that a class A hetero-oligomeric GPCR complex composed of four distinct protomers can be formed and exhibits pharmacological properties distinct from the individual protomers in a recombinant system (28).
Fig. 5.
Depletion of α1B/D-ARs by siRNA gene silencing does not affect CR expression. THP-1 cells were incubated with NT, α1B-AR, or α1D-AR siRNA, and receptor expression was measured by PLA. Images show merged DAPI/PLA signals for the detection of individual receptors, as indicated, and are representative of n = 3 independent experiments. Scale bars, 10 μm. Bottom row: Quantification of the number of PLA signals per cell for individual receptors in THP-1 cells after NT siRNA (ctrl., black bars), α1B-AR siRNA (light gray bars), or α1D-AR siRNA (dark gray bars) treatment (n = 3). Data (mean ± SE) are expressed as the percentage of cells treated with NT siRNA. *P < 0.05 vs. cells treated with NT siRNA (one-way ANOVA with Dunnett’s multiple comparisons test).
Fig. 6.
CR:α1B/D-AR heteromers after α1B/D-AR siRNA knockdown. THP-1 cells were incubated with NT, α1B-AR, or α1D-AR siRNA (same cells as in Fig. 5), and receptor-receptor interactions were measured by PLA. Images show merged DAPI/PLA signals for the detection of receptor-receptor interactions, as indicated, and are representative of n = 3 independent experiments. Scale bars, 10 μm. Bottom row: Quantification of the number of PLA signals per cell for receptor-receptor interactions in THP-1 cells after NT siRNA (ctrl., black bars), α1B-AR siRNA (light gray bars), or α1D-AR siRNA (dark gray bars) treatment (n = 3). Data (mean ± SE) are expressed as the percentage of cells treated with NT siRNA. *P < 0.05 vs. cells treated with NT siRNA (one-way ANOVA with Dunnett’s multiple comparisons test).
The chemotactic dose-response curves for each chemokine in THP-1 cells after incubation with NT and α1B-AR siRNA are shown in Fig. 7 A–D and after incubation with NT and α1D-AR siRNA are shown in Fig. 7 E–H. Compared with cells incubated with NT siRNA, incubation of cells with α1B-AR siRNA partially reduced chemotactic activity of the CCR1, CCR2, and CXCR4 agonists (Fig. 7 A, B, and D). Incubation of cells with α1D-AR siRNA also inhibited the chemotactic activity of the CCR2 and CXCR4 agonists (Fig. 7 F and H) but did not affect CCR1 (Fig. 7E) when compared with cells incubated with NT siRNA. Consistent with the lack of effect of α1-AR ligands on CCR8 function, CCR8-mediated chemotaxis was not affected by α1B/D-AR knockdown.
Fig. 7.
α1B/D-AR siRNA knockdown partially inhibits chemotaxis mediated via CR heteromerization partners of α1b/d-ARs. THP-1 cells were treated as in Figs. 5 and 6, and chemotaxis toward CCL23 (A and E), CCL2 (B and F), CCL1 (C and G), and CXCL12 (D and H) was tested. CI (mean ± SE, n = 3–4/condition). *P < 0.05 vs. cells incubated with NT siRNA (two-way ANOVA with Dunnett’s multiple comparisons test).
The finding that α1B-AR, as well as α1D-AR, knockdown partially inhibited CCR2- and CXCR4-mediated chemotaxis is consistent with our observations indicating that these CRs hetero-oligomerize with the α1B-AR:α1D-AR heteromer. Similarly, the finding that α1B-AR knockdown inhibited CCR1-mediated chemotaxis, whereas α1D-AR knockdown was ineffective, further supports the assumption that CCR1:α1B-AR and CCR1:α1D-AR heteromers exist as separate and independent entities. Although the function of CCR1:α1D-AR heteromers remains to be determined, our findings are consistent with the notion that the presence of α1B-AR or α1B/D-AR heteromers facilitates normal function of their CR heteromerization partners.
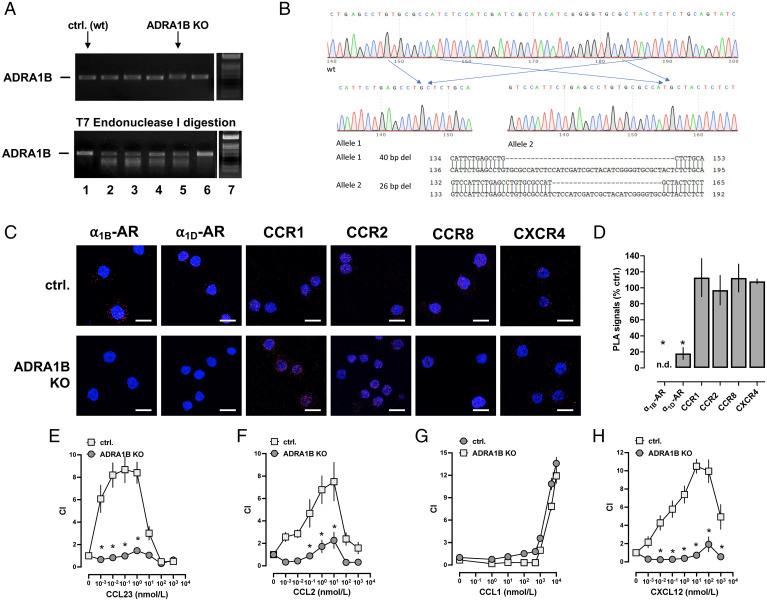
To consolidate these findings, we utilized CRISPR-Cas9 gene editing to generate a THP-1 cell line that lacks α1B-AR. Fig. 8A shows the PCR-amplified ADRA1B genomic DNA before and after T7 endonuclease I (T7EI) digestion from a wild-type THP-1 cell clone (lane 1) and from puromycin-selected THP-1 cell clones that were transduced with lentivirus encoding single guide RNA (sgRNA) targeting α1B-AR and Cas9 (lanes 2 to 6). Among the clones that showed cleaved mismatch products after T7EI digestion (lanes 2 to 5), lane/clone 5 was selected, and then DNA was PCR amplified with the ADRA1B primers and subcloned to the thymine adenine (TA) cloning vector. Sequencing of the plasmids showed a 40-bp deletion in allele 1 and a 26-bp deletion in allele 2 (Fig. 8B). Clone 5, designated THP-1_ADRA1BKO, was then expanded for further experiments.
Fig. 8.
CR heteromerization partners of α1B/D-ARs require α1B/D-ARs to mediate chemotactic responses. (A and B) CRISPR-Cas9 gene editing to generate a THP-1 cell line that lacks α1B-AR, designated THP-1_ADRA1BKO. (A) T7 surveyor assay to confirm gene modification in the targeted region of ADRA1B. Images from agarose gel electrophoresis for the detection of PCR-amplified ADRA1B genomic DNA before (Top) and after (Bottom) T7EI digestion from a wild-type (WT) THP-1 cell clone (lane 1, ctrl. [WT]) and from puromycin-selected THP-1 cell clones that were transduced with lentivirus encoding sgRNA targeting α1B-AR and Cas9 (lanes 2 to 6). Lane 7: DNA ladder. (B) Scheme depicting the modified genomic region of ADRA1B in THP-1_ADRA1BKO cells. (C) Detection of individual receptors in a WT THP-1 clone (ctrl., Top) and in THP-1_ADRA1BKO cells (Bottom) by PLA. Images show merged DAPI/PLA signals and are representative of n = 3 independent experiments. Scale bars, 10 μm. (D) Quantification of PLA signals per cell for the detection of individual receptors in THP-1_ADRA1BKO cells. Data (mean ± SE) are expressed as the percentage of a WT THP-1 cell clone (% ctrl.). *P < 0.05 vs. ctrl. (unpaired Student’s t test). (E–H) Chemotaxis of THP-1_ADRA1BKO cells and WT THP-1 cells toward CCL23 (E), CCL2 (F), CCL1 (G), and CXCL12 (H). CI (mean ± SE, n = 3–4 independent experiments). *P < 0.05 for THP-1_ADRA1BKO cells vs. WT THP-1 cells (two-way ANOVA with Dunnett’s multiple comparisons test).
Fig. 8C shows representative PLA images for the detection of individual receptors, and Fig. 8D shows quantification of PLA signals from three independent experiments in the THP-1 wild-type clone (control) and in THP-1_ADRA1BKO cells. As expected, PLA signals for α1B-AR were absent from THP-1_ADRA1BKO cells. When compared with control THP-1 cells, PLA signals for α1D-AR were reduced by 82 ± 7% in THP-1_ADRA1BKO cells. This observation can be explained by previous findings demonstrating that quantitative translocation of α1D-AR to the cell surface depends on the coexpression of α1B-AR (45).
PLA signals for CCR1, CCR2, CCR8, and CXCR4 in THP-1_ADRA1BKO cells were indistinguishable from corresponding PLA signals in control THP-1 cells, which is consistent with our findings after siRNA knockdown of α1B/D-ARs (Fig. 5). Fig. 8 E–H shows the migration of control and THP-1_ADRA1BKO cells toward agonists of the selected CRs. The chemotactic responses of THP-1_ADRA1BKO cells mediated by CCR1 (Fig. 8E), CCR2 (Fig. 8F), and CXCR4 (Fig. 8H) were reduced 95, 82, and 91%, respectively, compared with control cells. Migration of THP-1_ADRA1BKO and wild-type control cells toward the CCR8 agonist was indistinguishable (Fig. 8G). These findings demonstrate that CR heteromerization partners of α1B/D-ARs depend on the presence of α1B/D-ARs to induce migration toward their chemokine agonists.
In conclusion, the present study provides a systematic investigation of the heteromerization propensity between the GPCR families of CRs and α1-ARs. Our findings suggest that at least 20 members of the human CR family can physically interact with one or more members of the α1-AR family and that such heteromers are expressed in native human monocytes and in the monocytic leukemia cell line THP-1. While the functional roles of α1A-AR:CR heteromers remain to be determined, CR heteromerization partners of α1B/D-AR depend on the formation of heteromeric complexes with α1B/D-AR to mediate chemotactic responses toward their cognate agonists, and ligand binding to α1B/D-AR within the CR:α1B/D-AR heteromeric complex inhibits CR-mediated migration toward their cognate agonists with high potency.
We believe that our findings provide insights into the mechanisms by which the neuroendocrine system regulates leukocyte and/or cell migration in health and disease. The proposed mechanisms offer opportunities to modulate leukocyte or cancer cell trafficking, for example, by the development of drugs that interfere with CR:α1B/D-AR heteromerization or by repurposing of US Federal Drug Administration–approved α1-AR ligands for the treatment of disease processes in which chemokines play essential roles.
Materials and Methods
Proteins, Antibodies, and Reagents.
CCL1, CCL2, N-terminally truncated CCL23 (amino acids 25 to 99), and CXCL12 were purchased from Protein Foundry (Milwaukee, WI). CCL23 exists in multiple forms within the human body due to alternative splicing and postprocessing; CCL23 (amino acids 25 to 99) was selected because it has been reported to bind to and activate CCR1 with higher affinity and potency than the longer CCL23 variants (46, 47). [125I]-CCL2 was purchased from Perkin-Elmer (Shelton, CT).
Antibodies were obtained from Abcam (Cambridge, United Kingdom): anti-α1A-AR (host: rabbit; catalog: ab137123), anti-α1B-AR (host: rabbit; catalog: ab169523), anti-α1D-AR (host: rabbit; catalog: ab84402), and anti-CXCR4 (host: goat, ab1670); LifeSpan Biosciences (Seattle, WA): anti-CCR8 (host: goat; catalog: LS-C187704); and R&D Systems (Minneapolis, MN): anti-CCR1 (host: mouse; catalog: MAB145), anti-CCR2 (host: mouse; catalog: MAB48607), Immunoglobulin G (IgG) isotype control (host: rabbit; catalog: MAB150), IgG isotype control (host: mouse; catalog: MAB004), and IgG isotype control (host: goat; catalog: AB-108-C).
Phenylephrine, phentolamine, 5-methylurapidil, L-765314, BMY7378, and poly-l-lysine were purchased from Sigma-Aldrich (St. Louis, MO). α1B/D-AR siRNA, NT siRNA, and Accell transfection media were purchased from GE Dharmacon (Lafayette, CO). INCB3284 (CCR2 antagonist), SB328437 (CCR3 antagonist), and Maraviroc (CCR5 antagonist) were obtained from R&D Systems. All reagents for PLAs were from Sigma-Aldrich.
Plasmids.
cDNA for CCR1, CCR9, XCR1, ACKR1, ACKR2, and ACKR5 were obtained from Arizona State University. cDNA for all other CRs, α1a/b/d-AR, and mGlu1R was from Addgene. Upper and lowercase subscripts for α1-ARs are used to denote endogenous and recombinant α1-ARs, respectively (48). α1a/b/d-AR subtypes and mGlu1R were ligated at the C termini between Age I and Xba I sites with RlucII, which was PCR amplified from CXCR4-RlucII that was provided by Dr. Michel Bouvier. All CRs were ligated at the C termini between Age I and Xba I sites with enhanced YFP. All plasmids were sequenced and verified.
Cells and Cell Lines.
The human monocytic leukemia cell line THP-1 was from American Type Culture Collection (ATCC, Manassas, VA) and cultured as previously described (49). Briefly, cells were cultured and maintained in Roswell Park Memorial Institute 1640 medium (RPMI 1640) (Sigma) supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin and 100 μg/mL streptomycin (Invitrogen) in 100-mm Nunc tissue culture dishes from Thermo Fisher Scientific (Waltham, MA). THP-1 cells were used at a passage number of less than 10. HEK293T cells were obtained from ATCC and maintained in Dulbecco’s modified Eagle’s medium (Sigma) supplemented with HyClone FBS from Cytiva, 100 μg/mL penicillin (Invitrogen), and 100 μg/mL streptomycin (Invitrogen) in 100-mm Nunc tissue culture dishes (Thermo Fisher Scientific). Human monocytes were isolated from whole blood from healthy volunteers, according to our institutional review board (IRB) protocol approved by the University of South Florida. Whole blood was drawn by venipuncture into sodium citrate CPT mononuclear cell preparation tubes from Becton Dickinson (Franklin Lakes, NJ), and peripheral blood mononuclear cells were isolated by density gradient centrifugation at 1,800 × g for 20 min. CD14+/CD16− monocytes were then isolated via negative selection using magnetic-activated cell sorting LS columns (MACS LS), an indirect magnetic labeling system from Miltenyi Biotech (Bergisch Gladbach, Germany). MACS LS utilizes a mixture of biotin-conjugated monoclonal anti-human antibodies against CD3, CD7, CD16, CD19, CD56, CD123, and CD235a for negative selection (catalog: 130-117-337). Purity, and composition of the monocyte preparations were assessed by morphology and by measuring cell size, granularity, and expression of CD14/CD16 by flow cytometry.
Gene Silencing by RNA Interference.
Gene silencing with siRNA was performed as described (23, 30, 49). In brief, to deplete α1B/D-ARs from the cell surface, cells were incubated in Nunc six-well plates (Thermo Fisher Scientific) for 3 d in Accell transfection media (GE Dharmacon) with α1B-AR, α1D-AR, or NT (negative control) siRNA (GE Dharmacon) at a concentration of 1 µM. On day 3, cells were centrifuged at 300 × g for 5 min and resuspended in RPMI 1640 (Sigma) supplemented with 10% HyClone FBS from Cytiva (Marlborough, MA), 100 μg/mL penicillin from Invitrogen (Waltham, MA), and 100 μg/mL streptomycin (Invitrogen). Cells were utilized on day 4 for experimentation.
CRISPR-Cas9 Gene Editing.
CRISPR-Cas9 gene editing was performed according to previously established criteria (50, 51). To generate the lentivirus encoding both sgRNA targeting α1B-AR (target sequence TCCATCGATCGCTACATCG) and Cas9, 293T cells were cotransfected with the LV01 lentivirus plasmid (synthesized by Sigma) and lentiviral packing mix (Sigma) using Lipofectamine 2000 (Invitrogen). After overnight incubation, the culture medium was replaced with THP-1 medium RPMI 1640. Two days posttransfection, the supernatants containing the lentiviral particles were collected and spun at 500 × g for 5 min. The resultant supernatant was filtered and used to transduce THP-1 cells. Two days after transduction, cells were selected by addition of puromycin (1 µg/mL). To check the efficiency of CRISPR, the genomic DNA from transduced cells was extracted with DNAzol (Invitrogen). The DNA sequence flanking the targeting region was amplified by PCR with primers α1B-F (CGCCCACCAACTACTTCATT) and α1B-R (ACTCCTGCCTCTAGGTTCTT) using Platinum Blue PCR supermix (Invitrogen) according to the manufacturer’s instructions. The PCR product was examined for mutations with the T7EI assay kit (Integrated DNA Technologies [IDT]) following the manufacturer’s instructions. After confirming efficient editing of α1B-AR genomic DNA, the transduced cells were replated in 96-well plates at 1 cell/well in the conditioned medium containing 20% FBS. Three weeks later, clones were replated in 24-well plates and subjected to screening with the T7EI assay kit. To detect the sequences of both alleles, DNAs from the edited clones were PCR amplified with the above primers and subcloned to the TA cloning vector. The resulting plasmids were sequenced. The clones containing out-ot-frame inserts or deletions in both alleles (designated THP-1_ADRA1BKO) were expanded for experiments.
BRET.
BRET assays were performed in HEK293T cells as described previously (28, 52–54). HEK293T cells were seeded in 12-well plates and transfected with the indicated plasmids using Lipofectamine 3000 as a transfection reagent (Thermo Fisher Scientific). For BRET screening assays, α1a/b/d-RLuc was transfected at a fixed amount of 5 ng alone, with increasing amounts of YFP or mGlu1R-YFP or a fixed amount of 25 ng of CR-YFP; for saturation BRET experiments, α1a/b/d-RLuc was transfected at a fixed amount of 5 ng with increasing amounts of CR-YFP. In all assays, empty vector pcDNA3.1 was added to keep the total amount DNA for each transfection constant. Cells were incubated overnight, subsequently replated to 96-well white plates (Greiner Bio-One, Frickenhausen, Germany) coated with poly-l-lysine (Sigma), and incubated again overnight. Cells were then washed with phosphate-buffered saline (PBS), and fluorescence was measured in a plate reader (Cytation 1 cell imaging multi-mode reader, BioTek, Winooski, VT; λexcitation 485 nm, λemission 528 nm). For BRET measurements, coelenterazine H was added at a final concentration of 5 μM. After 10 min of incubation at room temperature, luminescence was measured at 460 and 528 nm. The BRET signal was calculated as the ratio of the relative luminescence units (RLUs) measured at 528 nm over RLUs measured at 460 nm. The net BRET was calculated by subtracting the BRET signal detected when α1a/b/d-RLuc was transfected alone. For screening and saturation experiments, net BRET ratios are expressed as a function of fluorescence/total luminescence.
PLAs.
PLAs were performed as previously described (23, 24, 26, 54). In brief, THP-1 cells were deposited in a monolayer on glass slides (Thermo Fisher Scientific) by centrifugation at 800 × g using a Cytospin 4 centrifuge (Thermo Fisher Scientific). Cell monolayers were isolated into individual wells using a water-repellent solution (super PAP pen, Thermo Fisher Scientific). Subsequently, cells were fixed with 4% (wt/vol) paraformaldehyde for 15 min at room temperature and then blocked overnight at 4 °C with the Sigma-Aldrich Duolink PLA blocking reagent. Blocked slides were incubated with the indicated primary antibody or antibodies in dilutions of 1 µg/mL corresponding to the receptor or receptors of interest. IgG isotype antibodies were utilized as controls. Slides were subsequently washed with PBS and incubated (60 min at 37 °C in a humidifying chamber) with secondary species‐specific antibodies conjugated to plus and minus PLA probes (1:5). Probed slides were then washed with Sigma-Aldrich Duolink wash buffer A and incubated with ligation reagent (30 min at 37 °C in a humidifying chamber). After ligation, slides were washed with wash buffer A again and then incubated with amplification reagent (105 min at 37 °C in a humidifying chamber). Slides were then washed twice with wash buffer B and then once with 0.01 × wash buffer B in ddH2O and allowed to dry. Treated slides were mounted with 50 µL per well of Duolink in situ mounting medium with DAPI overnight at −20 °C. PLA signals (Duolink in situ detection reagents red; λexcitation/emission 598/634 nm) were identified as red fluorescent spots under a Keyence (Osaka, Japan) BZ-X710 fluorescence microscope (60×/1.50 oil) at room temperature. PLA signals were quantified using ImageJ (NIH). Images were imported in merged TIFFs containing both signal and nuclei channels. Merged images were visually verified for analytical quality. Comparisons and statistical analyses were performed only when PLA assays were performed on the same day in parallel experiments. Fluorescence microscopy was performed with the identical settings. For each experiment and condition, 10 randomly selected nonoverlapping vision fields were analyzed.
Chemotaxis Assays.
Cell migration was measured employing the Neuroprobe (Gaithersburg, MD) ChemoTx disposable chemotaxis system. A 96-well Boyden chamber (30 µL/well, 8-µm pores, 1 × 105 pores/cm2 pore density) was selected for all chemotaxis experiments. Bottom wells of the Boyden chamber were loaded with 30 µL of test substances at various concentrations. Top wells of the membrane were loaded with 25 µL of THP-1 cells, THP-1_ADRA1BKO, wild-type THP-1 cells after clonal expansion (=control for THP-1_ADRA1BKO), or freshly isolated monocytes (200 cells/μL). THP-1 cells were suspended in depleted RPMI 1640 (0.5% HyClone FBS, Sigma); Freshly isolated monocytes were suspended in RPMI 1640 (0.5% human platelet poor plasma, Sigma). After 3 h, transmigrated cells were counted utilizing the Cytation 1 plate reader (BioTek) by direct imaging in high-contrast bright field (4x) and postimaging particle analyses with Gen5 (v3.05) imaging and microscopy software (BioTek). The chemotactic index (CI) was calculated as the ratio of cells that transmigrated in the presence vs. the absence of the test solutions.
qPCR.
Total RNA was extracted from cells using TRIzol from Invitrogen. RNA was reverse transcribed to cDNAs with the Applied Biosystems High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific) following the manufacturer manual. Premixed primers and probes for α1A-AR, α1B-AR, α1D-AR, and β-actin were synthesized by IDT. qPCR was performed with the Bio-Rad CFX Connect real-time PCR system.
Radioligand Competition Binding Assays.
THP-1 cells were incubated with vehicle, 10 μM phenylephrine, or 10 μM phentolamine (108 cells, 30 min at 37 °C) in cell culture medium, cooled on ice for 5 min, centrifuged (300 × g, 4 °C), washed with 20 mL of cold PBS, and resuspended in 10 mL of hypotonic buffer (10 mM HEPES, 0.2 mM CaCl2, 1 mM MgCl2, 0.02% bovine serum albumin [BSA], pH 7.2). Cells were then centrifuged at 30,000 × g (20 min, 4 °C), the supernatant was discarded, and pelleted cell fragments were snap-frozen in liquid nitrogen. The cell pellets were resuspended in 50 mM HEPES, 5 mM MgCl2, 1 mM CaCl2, and 0.1% (wt/vol) BSA (pH 7.2) and then incubated with 50 pM [125I]-CCL2, 1 × Xpert Protease Inhibitor Mixture (GenDEPOT), and varying concentrations of CCL2 for 1.5 h at 25 °C. Bound [125I]-CCL2 was collected via vacuum filtration using buffer containing 10 mM HEPES (pH 7.4) and 0.5 M NaCl at 4 °C and a cell harvester (Brandel) equipped with glass fiber filters. Radioactivity was measured in a gamma counter at 70% efficiency.
Data Analyses.
Data are expressed as mean ± SE from n independent experiments that were performed on different days or as mean ± SD from triplicate measurements for representative BRET screening experiments. Data were analyzed using GraphPad Prism v. 9.02 software. BRET and dose-response curves were analyzed using nonlinear regression analyses. Unpaired Student’s t test, one-way analysis of variance (ANOVA) with Dunnett’s multiple comparisons test, and two-way ANOVA with Dunnett’s multiple comparisons test were used as appropriate. A two-tailed P < 0.05 was considered significant.
Supplementary Material
Acknowledgments
Research reported in this publication was supported by the NIH under Awards R01GM139811 and R21AI139827. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2123511119/-/DCSupplemental.
Data Availability
All study data are included in the article and/or SI Appendix. All unique materials and plasmids are available on request by qualified researchers for their own use.
References
- 1.Scanzano A., Cosentino M., Adrenergic regulation of innate immunity: A review. Front. Pharmacol. 6, 171 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pongratz G., Straub R. H., The sympathetic nervous response in inflammation. Arthritis Res. Ther. 16, 504 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tracey K. J., Reflex control of immunity. Nat. Rev. Immunol. 9, 418–428 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dhabhar F. S., Effects of stress on immune function: The good, the bad, and the beautiful. Immunol. Res. 58, 193–210 (2014). [DOI] [PubMed] [Google Scholar]
- 5.Barnes M. A., Carson M. J., Nair M. G., Non-traditional cytokines: How catecholamines and adipokines influence macrophages in immunity, metabolism and the central nervous system. Cytokine 72, 210–219 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dimitrijevic M., Stanojevic S., Kustrimovic N., Leposavic G., End-point effector stress mediators in neuroimmune interactions: Their role in immune system homeostasis and autoimmune pathology. Immunol. Res. 52, 64–80 (2012). [DOI] [PubMed] [Google Scholar]
- 7.Grisanti L. A., et al. , α1-adrenergic receptors positively regulate Toll-like receptor cytokine production from human monocytes and macrophages. J. Pharmacol. Exp. Ther. 338, 648–657 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brosnan C. F., et al. , Prazosin, an alpha 1-adrenergic receptor antagonist, suppresses experimental autoimmune encephalomyelitis in the Lewis rat. Proc. Natl. Acad. Sci. U.S.A. 82, 5915–5919 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bergquist J., Tarkowski A., Ekman R., Ewing A., Discovery of endogenous catecholamines in lymphocytes and evidence for catecholamine regulation of lymphocyte function via an autocrine loop. Proc. Natl. Acad. Sci. U.S.A. 91, 12912–12916 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flierl M. A., et al. , Phagocyte-derived catecholamines enhance acute inflammatory injury. Nature 449, 721–725 (2007). [DOI] [PubMed] [Google Scholar]
- 11.Flierl M. A., et al. , Upregulation of phagocyte-derived catecholamines augments the acute inflammatory response. PLoS One 4, e4414 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Musso N. R., Brenci S., Setti M., Indiveri F., Lotti G., Catecholamine content and in vitro catecholamine synthesis in peripheral human lymphocytes. J. Clin. Endocrinol. Metab. 81, 3553–3557 (1996). [DOI] [PubMed] [Google Scholar]
- 13.Grisanti L. A., Perez D. M., Porter J. E., Modulation of immune cell function by α(1)-adrenergic receptor activation. Curr. Top. Membr. 67, 113–138 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bachelerie F., et al. , International Union of Pharmacology. LXXXIX. Update on the extended family of chemokine receptors and introducing a new nomenclature for atypical chemokine receptors. Pharmacol. Rev. 66, 1–79 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alexander S. P., et al. ; CGTP Collaborators, THE CONCISE GUIDE TO PHARMACOLOGY 2017/18: G protein-coupled receptors. Br. J. Pharmacol. 174 (suppl. 1), S17–S129 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olson T. S., Ley K., Chemokines and chemokine receptors in leukocyte trafficking. Am. J. Physiol. Regul. Integr. Comp. Physiol. 283, R7–R28 (2002). [DOI] [PubMed] [Google Scholar]
- 17.Busillo J. M., Benovic J. L., Regulation of CXCR4 signaling. Biochim. Biophys. Acta 1768, 952–963 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karin N., The multiple faces of CXCL12 (SDF-1alpha) in the regulation of immunity during health and disease. J. Leukoc. Biol. 88, 463–473 (2010). [DOI] [PubMed] [Google Scholar]
- 19.Hohenhaus D. M., et al. , An mRNA atlas of G protein-coupled receptor expression during primary human monocyte/macrophage differentiation and lipopolysaccharide-mediated activation identifies targetable candidate regulators of inflammation. Immunobiology 218, 1345–1353 (2013). [DOI] [PubMed] [Google Scholar]
- 20.Tripathi A., Davis J. D., Staren D. M., Volkman B. F., Majetschak M., CXC chemokine receptor 4 signaling upon co-activation with stromal cell-derived factor-1α and ubiquitin. Cytokine 65, 121–125 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma W., Liu Y., Ellison N., Shen J., Induction of C-X-C chemokine receptor type 7 (CXCR7) switches stromal cell-derived factor-1 (SDF-1) signaling and phagocytic activity in macrophages linked to atherosclerosis. J. Biol. Chem. 288, 15481–15494 10.1074/jbc.M112.445510. (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoshimura T., Oppenheim J. J., Chemokine-like receptor 1 (CMKLR1) and chemokine (C-C motif) receptor-like 2 (CCRL2); two multifunctional receptors with unusual properties. Exp. Cell Res. 317, 674–684 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tripathi A., et al. , Heteromerization of chemokine (C-X-C motif) receptor 4 with α1A/B-adrenergic receptors controls α1-adrenergic receptor function. Proc. Natl. Acad. Sci. U.S.A. 112, E1659–E1668 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Albee L. J., et al. , α1-Adrenergic Receptors Function Within Hetero-Oligomeric Complexes With Atypical Chemokine Receptor 3 and Chemokine (C-X-C motif) Receptor 4 in Vascular Smooth Muscle Cells. J. Am. Heart Assoc. 6, e006575 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao X., Albee L. J., Volkman B. F., Gaponenko V., Majetschak M., Asymmetrical ligand-induced cross-regulation of chemokine (C-X-C motif) receptor 4 by α1-adrenergic receptors at the heteromeric receptor complex. Sci. Rep. 8, 2730 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Evans A. E., et al. , New Insights into Mechanisms and Functions of Chemokine (C-X-C Motif) Receptor 4 Heteromerization in Vascular Smooth Muscle. Int. J. Mol. Sci. 17, 971 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mustafa S., et al. , Identification and profiling of novel α1A-adrenoceptor-CXC chemokine receptor 2 heteromer. J. Biol. Chem. 287, 12952–12965 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao X., Enten G. A., DeSantis A. J., Majetschak M., Class A G protein-coupled receptors assemble into functional higher-order hetero-oligomers. FEBS Lett. 595, 1863–1875 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Söderberg O., et al. , Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nat. Methods 3, 995–1000 (2006). [DOI] [PubMed] [Google Scholar]
- 30.Tripathi A., Gaponenko V., Majetschak M., Commercially available antibodies directed against α-adrenergic receptor subtypes and other G protein-coupled receptors with acceptable selectivity in flow cytometry experiments. Naunyn Schmiedebergs Arch. Pharmacol. 389, 243–248 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martinelli R., Sabroe I., LaRosa G., Williams T. J., Pease J. E., The CC chemokine eotaxin (CCL11) is a partial agonist of CC chemokine receptor 2b. J. Biol. Chem. 276, 42957–42964 (2001). [DOI] [PubMed] [Google Scholar]
- 32.Giri R. K., Rajagopal V., Shahi S., Zlokovic B. V., Kalra V. K., Mechanism of amyloid peptide induced CCR5 expression in monocytes and its inhibition by siRNA for Egr-1. Am. J. Physiol. Cell Physiol. 289, C264–C276 (2005). [DOI] [PubMed] [Google Scholar]
- 33.Coulin F., et al. , Characterisation of macrophage inflammatory protein-5/human CC cytokine-2, a member of the macrophage-inflammatory-protein family of chemokines. Eur. J. Biochem. 248, 507–515 (1997). [DOI] [PubMed] [Google Scholar]
- 34.Mirzadegan T., et al. , Identification of the binding site for a novel class of CCR2b chemokine receptor antagonists: Binding to a common chemokine receptor motif within the helical bundle. J. Biol. Chem. 275, 25562–25571 (2000). [DOI] [PubMed] [Google Scholar]
- 35.Uguccioni M., et al. , High expression of the chemokine receptor CCR3 in human blood basophils. Role in activation by eotaxin, MCP-4, and other chemokines. J. Clin. Invest. 100, 1137–1143 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gao X., et al. , Partial agonist activity of α1-adrenergic receptor antagonists for chemokine (C-X-C motif) receptor 4 and atypical chemokine receptor 3. PLoS One 13, e0204041 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kolarovszki-Sipiczki Z., et al. , Effect of alpha-adrenoceptor subtype-selective inverse agonists on non-pregnant and late-pregnant cervical resistance in vitro in the rat. Clin. Exp. Pharmacol. Physiol. 34, 42–47 (2007). [DOI] [PubMed] [Google Scholar]
- 38.García-Sáinz J. A., Torres-Padilla M. E., Modulation of basal intracellular calcium by inverse agonists and phorbol myristate acetate in rat-1 fibroblasts stably expressing alpha1d-adrenoceptors. FEBS Lett. 443, 277–281 (1999). [DOI] [PubMed] [Google Scholar]
- 39.Dodt C., Breckling U., Derad I., Fehm H. L., Born J., Plasma epinephrine and norepinephrine concentrations of healthy humans associated with nighttime sleep and morning arousal. Hypertension 30, 71–76 (1997). [DOI] [PubMed] [Google Scholar]
- 40.Ensinger H., Stein B., Jäger O., Grünert A., Ahnefeld F. W., Relationship between infusion rates, plasma concentrations, and cardiovascular and metabolic effects during the infusion of norepinephrine in healthy volunteers. Crit. Care Med. 20, 1250–1256 (1992). [DOI] [PubMed] [Google Scholar]
- 41.Redwine L., et al. , Differential immune cell chemotaxis responses to acute psychological stress in Alzheimer caregivers compared to non-caregiver controls. Psychosom. Med. 66, 770–775 (2004). [DOI] [PubMed] [Google Scholar]
- 42.Uberti M. A., Hall R. A., Minneman K. P., Subtype-specific dimerization of alpha 1-adrenoceptors: Effects on receptor expression and pharmacological properties. Mol. Pharmacol. 64, 1379–1390 (2003). [DOI] [PubMed] [Google Scholar]
- 43.Albee L. J., Gao X., Majetschak M., Plasticity of seven-transmembrane-helix receptor heteromers in human vascular smooth muscle cells. PLoS One 16, e0253821 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hague C., et al. , Heterodimers of alpha1B- and alpha1D-adrenergic receptors form a single functional entity. Mol. Pharmacol. 69, 45–55 (2006). [DOI] [PubMed] [Google Scholar]
- 45.Hague C., Uberti M. A., Chen Z., Hall R. A., Minneman K. P., Cell surface expression of alpha1D-adrenergic receptors is controlled by heterodimerization with alpha1B-adrenergic receptors. J. Biol. Chem. 279, 15541–15549 (2004). [DOI] [PubMed] [Google Scholar]
- 46.Nardelli B., et al. , Characterization of the signal transduction pathway activated in human monocytes and dendritic cells by MPIF-1, a specific ligand for CC chemokine receptor 1. J. Immunol. 162, 435–444 (1999). [PubMed] [Google Scholar]
- 47.Berkhout T. A., et al. , Selective binding of the truncated form of the chemokine CKbeta8 (25-99) to CC chemokine receptor 1(CCR1). Biochem. Pharmacol. 59, 591–596 (2000). [DOI] [PubMed] [Google Scholar]
- 48.Bylund D. B., et al. , International Union of Pharmacology nomenclature of adrenoceptors. Pharmacol. Rev. 46, 121–136 (1994). [PubMed] [Google Scholar]
- 49.Saini V., Marchese A., Majetschak M., CXC chemokine receptor 4 is a cell surface receptor for extracellular ubiquitin. J. Biol. Chem. 285, 15566–15576 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Benyoucef A., Marchitto L., Touzot F., CRISPR gene-engineered CYBB(ko) THP-1 cell lines highlight the crucial role of NADPH-induced reactive oxygen species for regulating inflammasome activation. J. Allergy Clin. Immunol. 145, 1690–1693 (2020). [DOI] [PubMed] [Google Scholar]
- 51.Baker P. J., Masters S. L., Generation of genetic knockouts in myeloid cell lines using a lentiviral CRISPR/Cas9 system. Methods Mol. Biol. 1714, 41–55 (2018). [DOI] [PubMed] [Google Scholar]
- 52.Gao X., et al. , Characterization of heteromeric complexes between chemokine (C-X-C motif) receptor 4 and α1-adrenergic receptors utilizing intermolecular bioluminescence resonance energy transfer assays. Biochem. Biophys. Res. Commun. 528, 368–375 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gao X., et al. , Regulation of the thrombin/protease-activated receptor 1 axis by chemokine (CXC motif) receptor 4. J. Biol. Chem. 295, 14893–14905 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Albee L. J., et al. , Identification and functional characterization of arginine vasopressin receptor 1A : Atypical chemokine receptor 3 heteromers in vascular smooth muscle. Open Biol. 8, 170207 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or SI Appendix. All unique materials and plasmids are available on request by qualified researchers for their own use.