Significance
APOBEC3A (A3A) is a DNA binding enzyme that introduces mutations through its cytidine deaminase activity. In addition, A3A can repress proviral HIV-1 and retroelements within the host genome by a deaminase-independent mechanism. Here, we demonstrate that A3A binds to the promoter sequence of interferon (IFN)-stimulated gene (ISG)15 and suppresses IFN-stimulated response element activity. In a deaminase-independent manner, A3A reduces ISG15 expression in response to IFN stimulation. A3A overexpression decreases and A3A knockout increases the expression of several ISGs in response to IFN-α treatment. Since A3A itself is an ISG, our data suggest that A3A plays a role in a negative feedback loop to control ISG expression.
Keywords: A3A, ISG, ISRE, LTR, HIV
Abstract
APOBEC3A (A3A) is a cytidine deaminase that inactivates a variety of viruses through introduction of lethal mutations to the viral genome. Additionally, A3A can suppress HIV-1 transcription in a deaminase-independent manner by binding to the long terminal repeat of proviral HIV-1. However, it is unknown whether A3A targets additional host genomic loci for repression. In this study, we found that A3A suppresses gene expression by binding TTTC doublets that are in close proximity to each other. However, one TTTC motif is sufficient for A3A binding. Because TTTC doublets are present in interferon (IFN)-stimulated response elements (ISRE), we hypothesized that A3A may impact IFN-stimulated gene (ISG) expression. After scanning the human genome for TTTC doublet occurrences, we discovered that these motifs are enriched in the proximal promoters of genes associated with antiviral responses and type I IFN (IFN-I) signaling. As a proof of principle, we examined whether A3A can impact ISG15 expression. We found that A3A binding to the ISRE inhibits phosphorylated STAT-1 binding and suppresses ISG15 induction in response to IFN-I treatment. Consistent with these data, our RNA-sequencing analyses indicate that A3A loss results in increased IFN-I–dependent induction of several ISGs. This study revealed that A3A plays an unexpected role in ISG regulation and suggests that A3A contributes to a negative feedback loop during IFN signaling.
The APOBEC3 (A3) family of cytidine deaminase induces lethal mutations in viral genomic DNA or RNA in infected cells and is an important cell-intrinsic immune defense mechanism against viral infection (1). There are seven different A3 family genes (A3A-H) in the human and primate genomes, whereas the mouse genome has one A3 gene member (2). The expansion of the A3 gene family likely reflects an evolutionary arms race between the host defense and retroelement genome insertions (3). Most A3 gene expression is induced by the type I interferon (IFN-I) response during viral infections, except for A3B and A3C (4). In particular, A3A expression is induced by HIV type 1 (HIV-1), human papillomavirus (HPV), hepatitis B virus (HBV), human cytomegalovirus (HCMV), and coronavirus infection, and A3A is the most strongly induced A3 gene following IFN-I stimulation (5–10).
A3A targets a broad spectrum of infectious viruses. A3A not only suppresses retroviral infections by HIV-1 and human T cell leukemia virus type 1 (HTLV-1) but also inhibits adeno-associated virus, herpes simplex virus 1 (HSV-1), HPV, HBV, and HCMV via cytidine deamination-dependent mutagenesis of the viral genome (5, 11–15). In addition to exogenous viruses, A3A inhibits endogenous retroelements, like LINE-1, Alu, and long terminal repeat (LTR) retrotransposons, by cytidine deamination-dependent or -independent mechanisms (8, 16–19). In several types of cancer, A3A consistently mutagenizes protein-coding genes by cytidine deamination of transiently exposed single-stranded DNA (ssDNA) (20, 21). Previously, we reported that A3A directly binds to ssDNA in the HIV-1 LTR and recruits KAP-1 silencing machinery to suppress HIV-1 LTR activity in latently infected CD4 T cells (22). A3A-dependent repression of HIV transcription was independent of its deamination activity (22). Previous biochemical binding, enzymatic activity, and co-crystal structure studies have utilized random sequence oligonucleotide (oligo) targets to elucidate the A3A target motif (23–26). However, the actual A3A target binding motifs in the human genome have not yet been identified.
Previous in vitro experiments indicate that A3A preferentially binds ssDNA substrates containing a minimal TTC motif (23, 26). Since our previous study determined that A3A binds exposed ssDNA in the proviral 5′ LTR of HIV-1 (22), we hypothesized that the paired TTTC motifs located within the U3/R region of this LTR are bound by A3A as ssDNA is exposed at the transcription bubble. Furthermore, activation-induced cytidine deaminase, another member of the APOBEC family of proteins, binds to greater than 5-nt-long ssDNA bubbles, which are formed by R-loops during transcription (27, 28). Activation-induced cytidine deaminase induces somatic hypermutation and class-switch recombination of the immunoglobulin heavy-chain locus (27–29). Based on these previous findings, we hypothesized that A3A could bind ssDNA in R-loops formed by the transcription bubble and block HIV-1 transcription. Here, we identified a paired TTTC sequence as a putative binding motif of A3A by focusing on the proviral HIV-1 LTR. We further extend this observation to IFN-stimulated response elements (ISREs) containing TTTC tandem motifs as a regulatory target of A3A.
Results
A3A Binding Motif within the HIV-1 LTR.
First, we analyzed recombinant A3A binding and deamination activity on synthetic ssDNA and multiple double-stranded DNA (dsDNA) oligos, which encode the U3/R sequence of the HIV-1 LTR (Fig. 1 A and B, Left). To form ssDNA bubbles of varying sizes, these dsDNA oligos encoded internal mismatches ranging from 0 to 15 nt (Fig. 1 B, Left). Our DNA pull-down assay indicated that A3A bound ssDNA (Fig. 1 B, Right Upper). Consistent with a previous report (29), dsDNA oligos containing ssDNA bubbles of at least 5 nt also pulled down A3A, but with weaker affinity (Fig. 1 B, Right Upper). To determine if A3A deamination activity varies across our oligos, we performed in vitro deamination assays with fluorescently labeled oligos (Materials and Methods). Briefly, substrates that are successfully deaminated by A3A in this assay will generate cleaved products that migrate based on the location of deaminated cytidines. Our deamination assay indicated that A3A only deaminates ssDNA at TTTC motifs (position 31 nt and 17 nt, resulting in 30 mer and 16 mer bands), but generally not complete or mismatch-containing dsDNA oligos (Fig. 1 B, Right Lower). Our quantification data indicated that A3A exhibits a slight deamination activity on dsDNA substrates with a 15-nt bubble (Fig. 1C). These results suggested that while A3A is capable of binding short, exposed ssDNA bubbles, it does not induce deamination.
Fig. 1.
A3A binds TTTC motifs located in the HIV-1 LTR. (A) The sequence encoding the U3/R region of the HXB2 HIV-1 LTR contains TTTC motifs (highlighted in blue) predicted to be bound by A3A. (B) 5′-biotinylated or Alexa 488-conjugated HIV-1 LTR oligos were used for DNA pull-down and deamination assays, respectively. These oligos encompassed WT sense-stranded ssDNA, WT dsDNA, and dsDNA containing 3- to 15-nt-long mismatches at the A3A-targeted TTTC motifs. Recombinant A3A protein was pulled down using biotinylated-oligo–linked dynabeads. Ten percent of input and bound A3A protein was analyzed by Western blot (B, Upper). Deamination activity of A3A-transfected 293T cell lysate was analyzed using Alexa 488-conjugated oligos (B, Lower). As shown in the Right, nondeaminated oligos migrate as a 50 mer-DNA band. Cytidine deamination within TTTC motifs at positions 31 and 17 results in the appearance of 30 mer- and 16 mer-DNA bands, respectively. (C) The relative fluorescent intensity of deamination products (shown in B) was compared to WT dsDNA. (D) 293T cells were transfected with WT, ΔκB, ΔSp1 HIV-1 LTR luciferase constructs and cotransfected with or without A3A. Twenty-four hours after transfection, cells were treated with 10 nM PMA for 48 h. Then, luciferase activity was measured for each LTR and normalized to spontaneous reporter signal in the absence of A3A. Data are means ± SE (n = 3). Sample means were compared to ΔκB signal and assessed by ANOVA with Tukey–Kramer’s test. ***P < 0.001, **P < 0.01, *P < 0.05; n.s., not significant. (E) Recombinant A3A protein was pulled down using ssDNA oligos with indicated TTTC motif mutations. Five percent of input and DNA-bound A3A protein was analyzed by Western blot.
Based on a previous study that showed preferential binding of A3A to T over A (23), we performed DNA pull-down assays on ssDNA oligos containing TTT → AAA mutations to examine whether A3A directly binds TTTC motifs within the HIV-1 LTR. Because the TTTC motif (underlined) is a core component of the NF-κB binding site (GGGACTTTCC) in the HIV-1 LTR (30) and A3A-dependent suppression of HIV-1 LTR activity was attenuated by mutation of this site (ΔκB) (Fig. 1D), we also used ΔκB mutant oligos in our DNA pull-down assays (Fig. 1E). Except for the double TTTC mutation, A3A could bind all ssDNA oligo substrates (Fig. 1E). These data indicate that one TTTC motif is sufficient for A3A to bind HIV-1 LTR encoding ssDNA oligos. Our deamination assays also indicated that one TTTC motif within the ssDNA LTR is needed for A3A-mediated deamination (SI Appendix, Fig. S1). These results indicate that while a single TTTC motif within a short exposed ssDNA is sufficient for A3A binding, a long stretch of at least 15 nt of ssDNA (Fig. 1C) surrounding a single TTTC motif is required for deamination.
A3A Suppression of HIV-1 Transcription Requires TTTC Motifs within the LTR.
Both WT and deaminase mutant A3A (C106S) suppress HIV-1 mRNA and protein expression (22). To examine the function of A3A in suppressing LTR activity in vivo, we introduced TTTC mutations into the NL4-3 plasmid, which encodes a full-length infectious HIV-1 genome, and measured HIV-1 Gag transcript and p24 protein expression. As expected, A3A suppressed HIV-1 transcription and production of WT NL4-3. However, A3A failed to suppress NL4-3 expression when either single or double TTTC mutations were introduced into the LTR (Fig. 2 A and B). While it is apparent that mutating TTTC motifs in the NF-κB binding site strongly reduces basal HIV-1 transcription, these results are consistent with our hypothesis that double TTTC motifs are necessary for A3A-dependent HIV-1 suppression.
Fig. 2.
A3A suppresses HIV-1 production via TTTC motifs. (A and B) 293T cells were transfected with WT, single-, or double-TTTC motif mutant NL4- (1 μg) and A3A-HA (0.5, 1 μg) plasmids. Total transfected plasmid quantity was normalized with pcDNA3.1 empty plasmid. (A) Total RNA was recovered 24 h after transfection and analyzed for HIV-1 Gag and 18s mRNA expression by qRT-PCR; 18s mRNA was used as an internal control. (B) The whole-cell lysate was recovered 48 h after transfection and HIV-1 p24 protein expression was measured by ELISA. For A and B, data are means ± SE (n = 3). Sample means were compared to control and assessed by ANOVA with Tukey–Kramer’s test. ***P < 0.001, **P < 0.01, *P < 0.05; n.s., not significant. (C and D) Recombinant A3A protein was pulled down using biotinylated HIV-1 LTR encoding ssDNA oligos with indicated spacer lengths between the two A3A-targeted TTTC motifs. The amount of bound A3A was measured by Western blot (C). A3A binding intensity for oligos with indicated spacer lengths was normalized to the WT LTR oligo (9 nt) signal (D).
Because a previous report suggested that dimerized A3A binds to ssDNA (25), we hypothesized that the distance between TTTC motifs might affect A3A binding to the HIV-1 LTR. To address this question in vitro, we examined A3A binding to HIV-1 LTR encoding ssDNA oligos containing different spacer lengths ranging from −1 to 20 nt (Fig. 2 D, Left). A3A binding required at least a 1-nt spacer between the TTTC motifs and diminished when the spacer was longer than 10 nt (Fig. 2 C and D). A3A binding to oligos with a 5-nt spacer was weaker than those with 4- or 6-nt spacers, which may be attributable to ssDNA secondary structure. These results indicated that A3A target binding minimally requires a TTTC motif within an extended stretch of ssDNA. A3A-mediated transcriptional suppression requires two TTTC motifs that are separated by a spacer of 1 to 10 nt.
A3A Overexpression Suppresses IFN-α–Induced IFN-Stimulated Gene Expression.
Since ISREs are defined by the presence of TTTC pairs with 2-nt spacers (31, 32), we hypothesized A3A may impact IFN-stimulated gene (ISG) expression. We scanned the human genome for ISREs (Fig. 3A) and found 412 unique gene promoters that contain at least one ISRE. Subsequent gene ontology (GO) enrichment analysis indicated that innate immune signaling and antiviral response terms are enriched in ISRE-containing genes (Fig. 3B). Notably, ISG15 and OAS1 promoters contain bona fide ISREs that are bound by phosphorylated STAT-1 (p-STAT-1) and expressed in response to IFN-α treatment (Fig. 4A) (31, 33). To examine whether A3A affects ISRE-driven transcription, we first confirmed that neither the WT nor deaminase mutant C106S A3A affect STAT-1 phosphorylation in response to IFN-α treatment (Fig. 4B). Next, we examined whether ISG expression was impacted by the presence or absence of WT or C106S A3A. When overexpressed, both WT and C106S A3A inhibited ISG15 and OAS1 induction by IFN-α but did not affect basal ISG15 expression (Fig. 4 C and D and SI Appendix, Fig. S2 A–C). Furthermore, qRT-PCR analysis demonstrated that WT A3A overexpression in 293T cells suppressed IFN-α–dependent induction of several other ISGs (Fig. 4E and SI Appendix, Fig. S3), indicating A3A-dependent repression extends to multiple ISGs.
Fig. 3.
ISREs are enriched in promoters of genes associated with antiviral responses. (A) Genome scanning strategy to identify genes with ISRE-containing proximal promoters. Genomic locations of ISREs (yellow box) were identified using HOMER2 (48) (Materials and Methods) and intersected with the genomic locations of GENCODEV36-annotated genes (blue box) (50). An ISRE motif was considered to be present in a proximal promoter if at least 1 nt overlapped with the promoter window defined as −200 bp to +50 bp relative to the gene start. (B) For genes identified in A, a GO enrichment analysis was performed using ClusterProfiler (51). Significantly enriched biological processes are shown in the dot plot. Dot size reflects number of genes that fall within indicated biological processes. Dot color denotes Benjamini–Hochberg-adjusted P value.
Fig. 4.
A3A overexpression suppresses ISRE activity and IFN-α-induced ISG15 expression. (A) The ISG15 proximal promoter sequence encodes TTTC-motif-containing ISREs in the region located −129 bp to −81 bp relative to the gene start. (B) 293T cells were transfected with Control (pcDNA3.1), WT A3A-HA, or C106S A3A-HA plasmid and treated with IFN-α (1,000 IU/mL) for 3 h. Nuclear protein was isolated by nuclear fractionation and STAT1, p-STAT1, A3A-HA, HDAC1 protein expression were analyzed by Western blot. HDAC1 expression served as an internal loading control. (C) 293T cells were cotransfected with the pGL4-ISRE reporter and pRL-TK plasmids, and one of Control (pcDNA3.1), WT A3A-HA or C106S A3A-HA plasmids. Twenty-four hours posttransfection, cells were treated with indicated IFN-α concentrations for 24 h, and relative luciferase activity was measured. (D) ISG15 and 18s mRNA expression was analyzed in pcDNA3.1, WT A3A-HA or C106S A3A-HA transfected 293T cells treated with indicated concentrations of IFN-α for 18 h. For C and D, data are means ± SE (n = 3). Sample means were compared to Control and assessed by ANOVA with Tukey–Kramer’s test. ***P < 0.001, *P < 0.05; n.s., not significant. (E) pcDNA3.1 or WT A3A-HA transfected 293T cells were treated with IFN-α (1,000 IU/mL) for 12 h (n = 3 per condition). ISGs, inflammatory cytokines, and A3A mRNA expression were analyzed by qRT-PCR. 18S-normalized expression levels of these genes are summarized in the heatmap. Log-transformed values were used to generate the heatmap. The results for individual genes can be found in SI Appendix, Fig. S3.
A3A Suppresses IFN-α Induced ISG15 Promoter Activity by Binding TTTC/GAAA Motifs Located in ISREs.
Since chromatin immunoprecipitation (ChIP)-qPCR experiments indicated that A3A bound ISG15 and OAS1 promoters and suppressed pSTAT1 binding (Fig. 5A and SI Appendix, Fig. S2D), we hypothesized that A3A may bind TTTC motifs located in the ISREs of the ISG15 promoter (Fig. 4A), which were previously identified as critical for ISG15 induction upon IFN-α treatment (34). To address whether A3A specifically binds TTTC motifs in the ISG15 promoter, we performed DNA pull-down assays using ISG15 promoter-encoding dsDNA, sense-ssDNA, and antisense-ssDNA oligos with intact TTTC motifs. We also tested ssDNA oligos encoding single- (mt1, mt2, mt3) and triple-TTTC (mt123) mutant sequences (Fig. 5B). A3A could only be pulled down with the unmutated and mt2 antisense-ssDNA oligos (Fig. 5C). These results indicated that A3A binds TTTC motifs No.1 and No.2 (Fig. 4A) and suggested that A3A binding depends on optimal TTTC motif pair spacing (here 8 nt) to inhibit IFN-α–induced ISG15 expression. These data suggest that A3A could simultaneously occupy both ISREs within the ISG15 promoter, which consists of three TTTC motifs spaced 2 or 3 nt apart. Because A3A inhibits IFN-α–induced ISG15 promoter activity but not a basal activity (Fig. 4C), we speculate that pSTAT-1–dependent transcription initiation at the ISG15 promoter drives the formation of an R-loop that exposes TTTC motif-containing ssDNA that are susceptible to A3A binding and result in repression of ISG15 transcription (35).
Fig. 5.
A3A binds to TTTC/GAAA motifs in the ISG15 promoter and suppresses IFN-α–induced pSTAT1 recruitment. (A) 293T cells were transfected with WT A3A-HA or pcDNA3.1 and treated with IFN-α (1,000 IU/mL) for 3 h. A3A-HA and p-STAT-1 binding to the ISRE region of the ISG15 promoter was analyzed using ChIP-qPCR. Data are means ± SE (n = 3). For ***P < 0.001, **P < 0.01, the sample means were compared to nontreated control. For †††P < 0.001, the indicated sample mean was compared to IFN-α–treated control. All comparisons were assessed by ANOVA with Tukey–Kramer’s test. n.s., not significant. (B and C) WT dsDNA, WT sense-, WT antisense-ssDNA, and indicated TTTC mutant (TTT to AAA) antisense-ssDNA oligos were used to pull down recombinant A3A protein (B). Five percent of input and DNA bound A3A protein was analyzed by Western blot (C).
A3A Deficiency Enhances IFN-α–Induced ISG Expression.
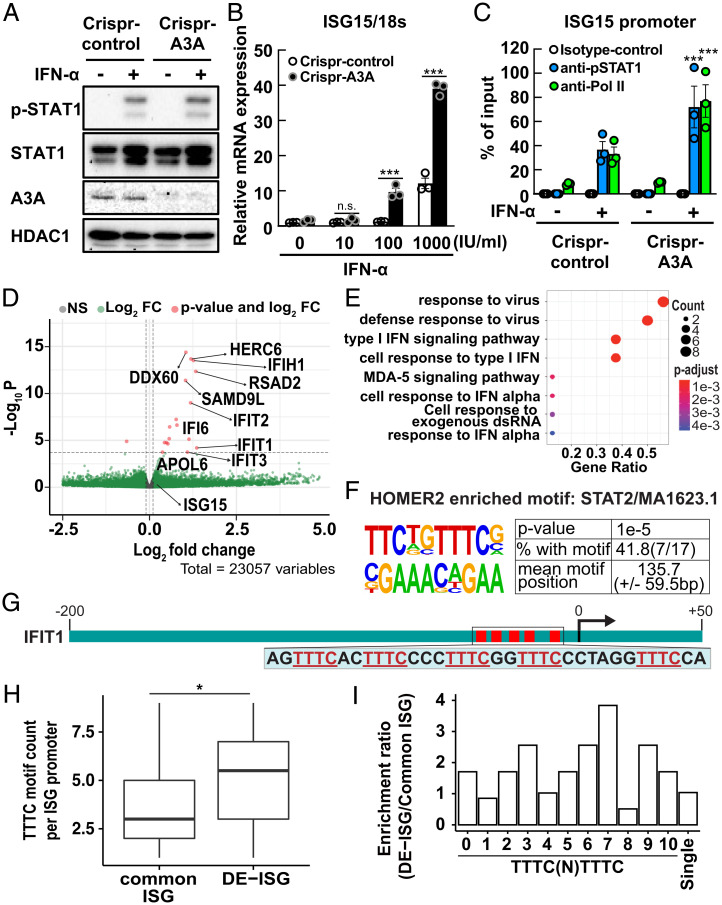
Finally, we examined the consequences of A3A loss on ISRE activity. A3A knockout by CRISPR in J-Lat10.6 cells (22) showed that A3A deficiency did not affect STAT-1 phosphorylation (Fig. 6A). However, A3A deficiency enhanced IFN-α–induced transcription as well as pSTAT-1, and RNA Pol II binding to the ISG15 and OAS1 promoters (Fig. 6 B and C and SI Appendix, Fig. S2 E and F).
Fig. 6.
A3A knockout enhanced IFN-α–induced ISG expression. (A) CRISPR-control or -A3A J-Lat10.6 cells were treated with IFN-α (1,000 IU/mL) for 3 h. Nuclear STAT1, p-STAT1, A3A, HDAC1 protein expression was measured by Western blot. HDAC1 expression served as an internal loading control. (B) ISG15 and 18s mRNA were measured in CRISPR-control or -A3A J-Lat10.6 cells treated with IFN-α (1,000 IU/mL) for 18 h. Data are means ± SE (n = 3). Sample means were compared to CRISPR-control and assessed by ANOVA with Tukey–Kramer’s test. ***P < 0.001; n.s., not significant. (C) CRISPR-control and -A3A J-Lat10.6 cells were treated with IFN-α (1,000 IU/mL) for 3 h and p-STAT-1 and RNA Pol II binding to the ISRE region in the ISG15 promoter was analyzed by ChIP-qPCR. Data are means ± SE (n = 3). Sample means were compared to IFN-α–treated CRISPR-control and assessed by ANOVA with Tukey–Kramer’s test. ***P < 0.001. (D) CRISPR-control or CRISPR-A3A J-Lat10.6 cells were treated with IFN-α (1,000 IU/mL) for 18 h and gene expression was measured by RNA-seq (n = 3). Volcano plot (59) depicts the difference in IFN-α–responsive gene induction in CRISPR-A3A vs. -control J-Lat10.6 cells. Genes with significant differential expression (Benjamini–Hochberg false-discovery rate < 0.1) (58) are highlighted in red. (E) ClusterProfiler (51) GO enrichment analysis of genes significantly up-regulated (log2 fold-change ≥ 0.1, Benjamini–Hochberg P-adjusted ≤ 0.1) in CRISPR-A3A cells. (F) ISRE-like motif identified by HOMER2 (48) enrichment analysis. (G) TTTC motif locations and sequences (red) within the IFIT1 promoter are shown. (H) Boxplots denote the distribution of TTTC motif counts per promoter sequence of common and DE-ISGs. *P ≤ 0.05, Welch’s two-sample t test. (I) HOMER2 enrichment analyses were performed using a custom library encompassing the indicated TTTC motifs. Enrichment ratios are reported from the HOMER2 ‘known’ output.
To examine the effect A3A has on overall ISG induction resulting from IFN-α treatment, we performed RNA-sequencing (RNA-seq) analyses of control and A3A-deficient J-Lat10.6 cells treated with and without IFN-α. Both control and A3A-deficient J-Lat10.6 cells showed robust up-regulation of ISGs and antiviral gene expression (SI Appendix, Fig. S4 B and C) in response to IFN-α treatment. Critically, we found that 17 ISGs are modestly but significantly differentially elevated in IFN-α–treated A3A knockout cells relative to identically treated control cells, which we define as DE-ISG (Fig. 6D). While ISG15 was not significantly elevated in A3A knockout cells over WT cells, its expression was subtly elevated by 1.19-fold (Fig. 6D). This discrepancy in ISG15 expression relative to experiments performed in 293T cells is likely attributable to ∼104-fold higher A3A expression in pkA3A-HA–transfected 293T cells relative to endogenous A3A expression in JLat cells (SI Appendix, Fig. S5). GO analysis of DE-ISGs revealed enrichment of terms associated with responses to IFN-I signaling and viral infection (Fig. 6E), confirming these are indeed ISGs.
To confirm the presence of A3A-binding TTTC motifs within DE-ISG promoters, we used HOMER2 to perform a motif enrichment analysis (Materials and Methods) and found that the majority of DE-ISGs contains a bona fide ISRE motif TTTCNNTTTC (Fig. 6F). For example, the IFIT1 promoter region encodes five TTTC motifs that makeup two ISREs upstream of the gene start (Fig. 6G). Additionally, the ISRE motif is highly similar to other motifs targeted by IFN signaling-associated transcription factors (SI Appendix, Fig. S6). To understand if TTTC motif content differs between the promoter sequences of DE-ISGs and ISGs induced equally in both cell lines (common ISG), we counted the number of TTTC motifs per promoter and found that DE-ISGs generally had significantly more motifs (navg = 5.13) compared to common ISGs (navg = 3.69) (Fig. 6H). To determine if optimally spaced TTTC pairs are enriched in DE-ISGs, we used the HOMER2 script ‘seq2profile.pl’ to generate a custom motif matrix file containing TTTC motif pairs with 0- to 10-nt-long spacers [TTTCN(0-10)TTTC]. We then performed a HOMER2 motif enrichment analysis of DE-ISGs against a background of common ISGs (Materials and Methods). This analysis indicated that A3A-accessible TTTC motif pairs tend to be enriched in DE-ISG promoters (Fig. 6I). Visual inspection of DE-ISG promoter sequences suggests that TTTC motif pairs tend to be clustered ∼200 bp up- and 50 bp downstream of the gene start (SI Appendix, Fig. S7). These results indicate that DE-ISG promoters are generally defined by a greater abundance of TTTC motifs that are more likely to be optimally spaced and near the gene start. In summary, transcriptome, ChIP-qPCR, and genome sequence analyses are consistent with our model that A3A suppresses IFN-I–induced ISG expression by inhibiting p-STAT-1 binding to ISREs.
Discussion
In this study, we showed that A3A binds to TTTC motifs within a stretch of ssDNA and found that an intact TTTC motif is necessary for A3A-induced deamination. We also showed that a double TTTC motif with a spacer length of 1 to 10 nt is needed for optimal A3A binding. These motifs, which are present in ISREs within the HIV-1 LTR of several HIV-1 strains (SI Appendix, Fig. S8) and ISREs upstream of ISGs, can be bound by A3A to suppress gene transcription. We found that ISRE loci contain optimal TTTC-TTTC doublet motifs, enabling high-affinity binding of A3A to these sites. We showed that A3A inhibits p-STAT-1 binding to ISG15 ISREs.
Type I IFNs (IFN-α, -β, -ω) inhibit the HIV-1 preintegration (36). However, during latency, IFN-α treatment reactivates proviral HIV-1 by inducing STAT-1, -3, and -5 phosphorylation, which in turn bind and drive transcription from the HIV-1 LTR (36, 37). Here, we showed that A3A inhibits HIV-1– and STAT-1–mediated ISG expression. As an ISG, A3A could suppress IFN-α–induced HIV-1 reactivation and potentially modulate ISG activation.
Synthetic linear and stem-loop structures containing ssRNA and ssDNA substrates have previously been used to study A3A binding and deamination (21, 38, 39). In this study, we used mismatch containing dsDNA oligos to model exposed ssDNA that may be found in R-loops during transcription. We found that while A3A could bind these substrates, deamination was extremely inefficient (Fig. 1C). Since genome integrity is essential to cell survival, our data are consistent with previous reports that A3A can restrict viral infection and retrotransposition by deaminase-dependent and -independent mechanisms (40). During retroviral infection, A3A may canonically mutate retroviral genomes during reverse transcription where ssRNA and ssDNA are readily accessible. However, since genomic ssDNA is generally only exposed when R-loops are formed in healthy cells during RNA Pol II transcription and pausing (41), A3A can mediate transcriptional repression of viral sequences and host genes without causing DNA deamination. While we found that A3A can suppress ISG expression, it is unclear whether this effect relies on KAP-1–dependent epigenetic silencing. Further genome-wide analyses of A3A, KAP1, p-STAT1, and several IRF family genome binding are required to examine the general function of A3A in regulating human ISGs and potentially retroelements.
By retrospectively searching for mutational footprints, previous reports indicated that A3A mediates deamination on viral genomes, retroelements, and protein-coding genes (3, 5, 8, 11, 14, 15, 20, 21). In prospective analyses, further viral and host genes could be examined for the presence of TTTC motifs that could be targeted by A3A. Because endogenous retroviruses harbor LTR sequences that drive their expression, A3A might bind ISREs present in endogenous retrovirus LTRs to suppress their aberrant expression (42, 43). Future studies are needed to probe the link between A3A and transposable element regulation.
A3A is conserved in primates but not mice (2). Therefore, the function of A3A cannot be analyzed by making a knockout mouse. It is also difficult to study the systemic function of A3A in vivo. A previous study made a human A3A whole-body transgenic mouse to analyze the antiviral effect of A3A, and a recent study generated a transient A3A gene-expression mouse model to examine how A3A contributes to deamination dependent mutagenesis and carcinogenesis (44, 45). Both models clearly showed A3A mutation signatures were observed in viral and tumor genomes. Our motif may be useful for further dissecting A3A deamination targets in these models.
In conclusion, our study demonstrated that A3A regulates ISG expression via directly binding to paired TTTC motifs located within ISREs. While this study only focused on the role of A3A in ISRE regulation, A3A might bind other sites in the human genome. Future studies are needed to fully characterize the genomic binding sites of A3A. Given that A3A is an ISG itself, our results suggest A3A may play a previously unappreciated role in regulating ISG expression during a viral infection. These data suggest that A3A binding to genomic TTTC motifs may play an important role in gene regulation, and contribute to negative feedback regulation of interferon stimulated genes.
Materials and Methods
Reagents and Antibodies.
UltraPure salmon sperm DNA (15632011), Dynabeads Protein G, Dynabeads M-280 Streptavidin were purchased from Thermo Fisher Scientific. Glycogen (AB00670-0020) was purchased from American Bio. Recombinant A3A (TP320995) was purchased from OriGene. ProLong Gold Antifade Reagent with DAPI (8961) was purchased from Cell Signaling Technology. Human IFN-α (IF007) was purchased from Millipore. Anti-A3A (ab38641 for Western blot), anti-KAP1 (ab22553), anti-Myc tag (ab9132), anti-HA (ab9110 for immunofluorescence), rabbit control IgG (ab172730) antibodies were purchased from Abcam. Anti-RNA Pol II (05-623) and mouse control IgG (12-371) antibodies were purchased from Millipore. Anti-HA (901503 for ChIP) and anti-HDAC1 (607401) antibodies were purchased from BioLegend. Anti-HA (H3663 for IP and Western blot) and anti–β-actin (A1978) antibodies were purchased from Sigma. Phosphorylated-STAT1 (9167), total STAT-1 (9172), HRP-conjugated anti-rabbit (7074), and anti-mouse (7076) antibodies were purchased from Cell Signaling Technology. HRP-conjugated anti-GAPDH antibody (GTX627408-01) was purchased from GeneTex. Anti-A3A (AP20219a for ChIP) was purchased from Abgent. Anti-rabbit Cy5 (111-176-144) and HRP-conjugated anti-goat (705-035-147) antibodies were purchased from Jackson ImmunoResearch. APOBEC3A Taqman gene-expression assay probes were purchased from ThermoFisher (4331182). All oligonucleotides used for qRT-PCR, plasmid construction, DNA pull-down assay, and deamination assay were purchased from Integrated DNA Technologies.
Cell Lines and Plasmids.
293T cells were purchased from ATCC and cultured in DMEM supplemented with 10% FBS, 1% antibiotic-antimycotic solution, and 1 mM sodium pyruvate. CRISPR-control and CRISPR-A3A J-Lat10.6 cells were established as described previously (46). J-Lat10.6 cells were cultured in RPMI supplemented with 10% heat inactivated FBS and 1% penicillin-streptomycin. pK-A3A WT and C106S plasmids were provided by J. Moran, University of Michigan Medical School, Ann Arbor, MI. A3A-Myc plasmid (RC220995) was purchased from OriGene. pGL4.11 (E6661), pGL4-ISRE (E414A), pRL-TK (E2241) plasmids were purchased from Promega. J-Lat10.6 cell and pNL4-3 plasmids were provided by E. Verdin and M. Martin through AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, NIH.
TTTC-mutated NL4-3 plasmids and methionine mutated A3A-HA plasmids were generated by using QuikChange II XL Site-Directed Mutagenesis Kit (Agilent). Primers used in mutagenesis are listed in SI Appendix, Table S1.
ISG15 promoter constructs were directly cloned into the pGL4.11 luciferase empty vector. Sense and antisense human ISG15 promoter sequence containing ISRE observed in the National Center for Biotechnology Information sequence database was modified by adding restriction enzymatic cut sites (KpnI and XhoI) and ordered as oligonucleotides. Sense and antisense sequences of ISRE mutants (mt1, mt2, mt3, mt23, mt13, mt12, mt123) were also ordered as oligonucleotides. Sense and antisense of WT and all mutant oligonucleotides were annealed at 95 °C for 10 min to make inserts. pGL4.11 empty plasmids and all annealed oligonucleotides were double digested with XhoI and KpnI for 2 h. Digested pGL4.11 vector and inserts were isolated with the QIAquick Gel Extraction Kit (Qiagen) and ligated with the Quick Ligation Kit (New England Biolabs). Ligated plasmids were transformed into TOP10 competent cell and selected on ampicillin plates. Positive clones were confirmed by DNA sequencing (Yale Keck DNA Sequencing Facility). All oligonucleotides used in ISG15 promoter cloning are listed in SI Appendix, Table S2.
qRT-PCR and Western Blot.
The qRT-PCR procedure was previously described (47). The oligonucleotide primers used for qPCR are shown in SI Appendix, Table S3. Data are representative of at least three independent experiments. Taqman Gene Expression assays were conducted following the manufacturer’s instructions. For Western blot, nuclear and cytosolic fractions were prepared from cells as previously described (22). Whole-cell lysate was prepared with RIPA buffer (47). Protein samples were fractionated by SDS/PAGE and transferred to a polyvinylidene difluoride membrane. Each protein was probed with the appropriate antibodies as listed in reagents and antibodies and the blot was visualized with SuperSignal West Pico or Femto Chemiluminescent Substrate (Thermo Fisher Scientific).
DNA Pull-Down Assay, Deamination Assay.
DNA pull-down assays were performed as previously described (46). Briefly, biotinylated dsDNA was prepared by annealing nonbiotinylated ssDNA and biotinylated ssDNA for 10 min at 95 °C and cooled slowly, as previously described (38). Streptavidin-Dynabeads (M280) and 300 pmol of Biotinylated ssDNA or dsDNA were mixed and incubated in 100 μL of DB buffer (20 mM Tris⋅HCl [pH 8.0], 2 M NaCl, 0.5 mM EDTA, 0.03% NP-40) for 30 min at room temperature. After DNA-linked to dynabeads, beads were washed once with 1 mL of DB buffer and two times with 1 mL of PB buffer (50 mM Tris⋅HCl [pH 8.0], 150 mM NaCl, 10 mM MgCl2, 0.5% Nonidet P-40, proteinase inhibitor). DNA-linked beads were incubated with 0.2 μg of recombinant A3A protein for 2 h at room temperature. Beads were washed three times with 1 mL of PB buffer, eluted by 2× Laemmli Sample Buffer (1610737, Bio-Rad), and subjected to immunoblot. Deamination activity of cell lysate was measured as in previous reports (46). Briefly, pK-A3A WT transfected 293T lysate was prepared using lysis buffer (20 mM Tris⋅HCl [pH 7.5], 1% Nonidet P-40, 150 mM NaCl, proteinase inhibitor mixture [Roche]). Lysates were mixed with Alexa 488-labeled oligonucleotides for 2 h at 37 °C in deamination buffer (100 mM Tris⋅HCl [pH 8.0], 500 mM NaCl, 100 mM DTT, 50 mM EDTA). Samples were incubated with UDG enzyme (M0280, New England Biolabs) in UDG buffer (100 mM Tris⋅HCl [pH 8.0], 500 mM NaCl, 50 mM EDTA) for 1 h at 37 °C and incubated with ProteinaseK (Qiagen) for 20 min at 65 °C. The reaction was stopped with 0.15 N NaOH for 15 min at 37 °C and neutralized with HCl. Samples were electrophoresed in 15% TBE-UREA PAGE gel, and visualized by ChemiDoc Touch Imaging System (Bio-Rad). Deamination activity was monitored through the appearance of a 16 mer and 30 mer bands. Biotinylated oligonucleotides and Alexa 488-labeled oligonucleotide sequences are listed in SI Appendix, Tables S5–S7.
ELISA.
The amount of p24 antigen in HIV-1-infected cells was determined with an HIV-1 p24 antigen ELISA kit 2.0 (ZeptoMetrix). Briefly, the lysate of plasmid transfected 293T cells was recovered using RIPA buffer and diluted using the kit’s lysing buffer. Diluted samples were subsequently incubated with the primary and secondary antibodies according to the manufacturer’s directions. Data are representative of at least three independent experiments.
Luciferase Assay.
293T cells were plated on 24-well plates and transfected with 0.1 μg of ISRE-luc or ISG15 promoter-luc, together with 20 ng of Renilla luciferase plasmid (pRL-TK), A3A WT, C106S expression plasmids and/or pcDNA3.1 empty vector (control) using Lipofectamine 2000 (Thermo Fisher Scientific). Luciferase activity was determined using a Dual-Luciferase Reporter assay system (Promega) as previously described (46). Data are representative of at least three independent experiments.
ChIP-qPCR.
ChIP-qPCR was performed as described previously (46). Briefly, protein–DNA complex was cross-linked with 1% formaldehyde for 5 min and the reaction was quenched with 0.125 M of glycine for 5 min. Cells were washed and lysed with cell lysis buffer (50 mM Hepes, pH 7.4, 1 mM EDTA, 85 mM KCl, 10% glycerol, 0.5% Nonidet P-40, supplemented with protease inhibitor mixture) and incubated for 20 min on ice. The cytosol fraction was removed by centrifugation (1,200 × g, 4 °C, 5 min) and the nuclear pellet was suspended using nuclear lysis buffer (50 mM Tris⋅HCl, pH 8.0, 2 mM EDTA, 150 mM NaCl, 5% glycerol, 1% Triton-X 100, 0.1% SDS, supplemented with protease inhibitor mixture) and incubated for 10 min on ice. The nuclear suspension was sonicated using a Bioruptor (30-s sonic and 30-s cooling cycle 35 times) to recover a fragmented chromatin. For IP of the chromatin, 2 to 5 μg of antibody was added and rotated overnight at 4 °C. After incubation with Dynabeads Protein G under rotation for 1 h at room temperature, beads were washed with ChIP wash buffer 1 (20 mM Tris⋅HCl, pH 8.0, 150 mM NaCl, 1% Triton-X 100, 0.1% SDS, 2 mM EDTA) twice, ChIP wash buffer 2 (20 mM Tris⋅HCl, pH 8.0, 500 mM NaCl, 1% Triton-X 100, 0.1% SDS, 2 mM EDTA) twice, ChIP wash buffer 3 (20 mM Tris⋅HCl, pH 8.0, 150 mM NaCl, 500 mM LiCl, 1% Nonidet P-40, 1% deoxycholate, 1 mM EDTA) once, and then with TE buffer (10 mM Tris⋅HCl, pH 8.0, 1 mM EDTA) once. The bead-bound DNA was eluted using 150 μL of elution buffer (1% SDS, 0.1 M NaHCO3) at 65 °C for 15 min twice. The eluted sample was decross-linked by adding 18 μL of 5 M NaCl and incubated at 65 °C for 5 h. The DNA in the sample was precipitated by adding 2 μL 5 mg/mL glycogen and 2.5 times the volume of ethanol and incubated at −80 °C overnight. DNA was recovered by centrifugation and treated with Proteinase K for 1 to 2 h at 45 °C. Proteinase K-treated DNA was isolated by using a QIAquick PCR Purification Kit (Qiagen). For qPCR of immunoprecipitated DNA, targeting primers were used to analyze each protein-binding at ISG15 and OAS1 promoters. The oligonucleotide primers used for ChIP-qPCR are listed in SI Appendix, Table S4. Data are representative of at least three independent experiments.
Genome-Wide TTTC Pair Enrichment Analysis.
To identify TTTC motif pairs located in gene promoters within the human genome, we generated a custom motif library, which is composed of TTTC motif pairs separated by spacers of 0 to 10 N. Using this library and the HOMER2 (48) ‘scanMotifGenomeWide.pl’ program, we identified all TTTC motif pairs within the hg38 genome assembly. We then used BEDTools (49) ‘closest’ to filter on genes with TTTC motif pairs located upstream of or within annotated human genes (GENCODEV36) (50). Finally, we used ClusterProfiler (51) to perform a Biological Process Gene Ontology enrichment analysis on genes with TTTC motif pairs located in their core promoters (defined as −200 to +50 relative to the annotated gene start). See Code Availability section for code details.
RNA-Seq, Differential Expression Analysis, and GO Analysis.
We performed RNA-seq on JLat 10.6 CRISPR-Control and CRISPR-A3A cells treated with IFN-α (1,000 U/mL, 18 h) or PBS (n = 3 per treatment). Total RNA was prepared from 1.5 × 106 cells per replicate treatment using the RNeasy Mini Kit (Qiagen 74104). Contaminating genomic DNA was removed by on-column DNase digestion as described in the RNeasy Mini Kit.
After validating RNA quality (Agilent 2200 Tapestation) and concentration (Thermo Nanodrop 8000), poly-A RNA from 1 μg of total RNA and cDNA libraries were prepared as described in the New England Biolabs polyA purification (E7490L) and library preparation kits (E7770L).
Our cDNA libraries were sequenced on an Illumina NextSeq 500 platform with 42 bp by 42-bp chemistry. Raw reads are accessible at BioProject ID: PRJNA742400. Read quality assessment and trimming was performed using fastp (52). Trimmed reads were mapped to the GRCh38/hg38 human genome assembly using STAR (53). Gene expression was quantified with featureCounts (54) according to the hg38 RefSeq gene annotations (55, 56) obtained from the University of California, Santa Cruz genome Browser (57).
We performed differential expression analysis of genes using DESeq2 (58). In these analyses we made three comparisons: IFN-α vs. PBS in CRISPR-Control, IFN-α vs. PBS in CRISPR-A3A, and IFN-α/PBS in CRISPR-A3A vs. IFN-α/PBS in CRISPR-Control samples. For IFN-α vs. PBS comparisons a gene was considered significantly induced by IFN-α if log2 fold-change ≥ 1 and adjusted P ≤ 0.1 (Wald Test with Benjamini–Hochberg correction). Expression of an IFN-α–inducible gene was considered significantly different between cell lines if log2 fold-change ≥ ±0.1 and adjusted P ≤ 0.1 (Wald Test with Benjamini–Hochberg correction). The results of our DESeq2 analysis were plotted using the EnhancedVolcano (59) package.
GO enrichment analyses for differentially expressed genes were performed using ClusterProfiler (51).
Motif Enrichment Analysis.
We extracted putative promoter and regulatory sequences of IFN-α–responsive ISGs using biomaRt (49, 60) and GenomicRanges (61) from the GRCh38/hg38 human genome assembly. Promoter and regulatory sequences were defined as a genomic window of 200 bp upstream and 50 bp downstream of the ensemble annotated gene start position. FASTA sequences corresponding to these regulatory regions were extracted from the GRCh38/hg38 assembly with BEDTools (62). To determine if ISREs are enriched in differentially elevated ISGs, we used the HOMER2 findMotifs.pl algorithm using scrambled regulatory sequences (HOMER2: scrambleFasta.pl) as the background.
To determine if there is a difference in the abundance of TTC motifs in the promoters of DE-ISGs and common ISGs, we first used HOMER2 ‘find’ to identify all motifs. We then used R (4.0.2) to sum the total number of solo TTTC motifs in DE-ISG and common ISG promoters and normalized this count to (i.e., divided by) the total number of input (DE-ISG, n = 17) and background (common ISG, n = 87) sequences, respectively.
To characterize whether TTTC motif spacing was correlated with ISG responsiveness to A3A loss, we first performed a motif enrichment analysis using the ‘homer2 known’ algorithm with the hypergeometric test statistic (-stat hypergeo). For this analysis, we first generated a custom motif library (-m) consisting of a solo TTTC and TTTCN(n)TTTC motifs with the HOMER2 script ‘seq2profile.pl’, where the number (n) of n ranged from 0 to 10. Differentially elevated ISGs were used as our input (-i) and shared IFN-α–responsive ISGs served as our background (-b).
Code Availability.
Code used to perform the described genome-wide motif scan and RNA-seq, differential expression, GO enrichment, and motif enrichment analyses are available at GitHub, https://github.com/jofrank1988/PNAS_A3A_2022 .
Statistical Analysis.
For statistical analysis, the data were analyzed by ANOVA with Tukey’s multiple comparison test (Prism v7.0 software, GraphPad), as indicated in each figure legend. For analysis in SI Appendix, Fig. S3, Bonferroni multiple comparison tests were used for comparison as indicated. Bioconductor R software (R v4.0.5) was used to generate heatmaps in Fig. 4E. P < 0.05 is considered statistically significant.
Supplementary Material
Acknowledgments
This work was supported by the Howard Hughes Medical Institute (A.I.). M. Taura was a Japan Society for the Promotion of Science (JSPS) fellow and in part supported by the Kanzawa Medical Research Foundation and Mochida Memorial Foundation for Medical and Pharmaceutical Research. J.A.F. is a Cancer Research Institute Irvington Fellow supported by the Cancer Research Institute (CRI4017). T.T. was a JSPS fellow and in part supported by Uehara Memorial Foundation. E.K. is a JSPS fellow and in part supported by The Naito Foundation. E.S. was supported in part by T32 MSTP Fellowship 2T32GM007205-41.
Footnotes
Reviewers: J.D., The University of Texas at Austin; and S.G., Columbia University Irving Medical Center.
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2011665119/-/DCSupplemental.
Data Availability
RNA-seq data have been deposited in the publicly accessible BioSample database (accession nos. SAMN19947880, SAMN19947881, SAMN19947882, SAMN19947883). Raw reads are available at BioProject (ID no. PRJNA742400) (63). All study data other than raw RNA-seq data are included in SI Appendix.
References
- 1.Salter J. D., Bennett R. P., Smith H. C., The APOBEC protein family: United by structure, divergent in function. Trends Biochem. Sci. 41, 578–594 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.LaRue R. S., et al. , Guidelines for naming nonprimate APOBEC3 genes and proteins. J. Virol. 83, 494–497 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ito J., Gifford R. J., Sato K., Retroviruses drive the rapid evolution of mammalian APOBEC3 genes. Proc. Natl. Acad. Sci. U.S.A. 117, 610–618 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peng G., Lei K. J., Jin W., Greenwell-Wild T., Wahl S. M., Induction of APOBEC3 family proteins, a defensive maneuver underlying interferon-induced anti-HIV-1 activity. J. Exp. Med. 203, 41–46 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berger G., et al. , APOBEC3A is a specific inhibitor of the early phases of HIV-1 infection in myeloid cells. PLoS Pathog. 7, e1002221 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kondo S., et al. , APOBEC3A associates with human papillomavirus genome integration in oropharyngeal cancers. Oncogene 36, 1687–1697 (2017). [DOI] [PubMed] [Google Scholar]
- 7.Vartanian J. P., et al. , Massive APOBEC3 editing of hepatitis B viral DNA in cirrhosis. PLoS Pathog. 6, e1000928 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weisblum Y., et al. , APOBEC3A is upregulated by human cytomegalovirus (HCMV) in the maternal-fetal interface, acting as an innate anti-HCMV effector. J. Virol. 91, e01296-17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Milewska A., et al. , APOBEC3-mediated restriction of RNA virus replication. Sci. Rep. 8, 5960 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koning F. A., et al. , Defining APOBEC3 expression patterns in human tissues and hematopoietic cell subsets. J. Virol. 83, 9474–9485 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ooms M., Krikoni A., Kress A. K., Simon V., Münk C., APOBEC3A, APOBEC3B, and APOBEC3H haplotype 2 restrict human T-lymphotropic virus type 1. J. Virol. 86, 6097–6108 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen H., et al. , APOBEC3A is a potent inhibitor of adeno-associated virus and retrotransposons. Curr. Biol. 16, 480–485 (2006). [DOI] [PubMed] [Google Scholar]
- 13.Suspène R., et al. , Genetic editing of herpes simplex virus 1 and Epstein-Barr herpesvirus genomes by human APOBEC3 cytidine deaminases in culture and in vivo. J. Virol. 85, 7594–7602 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vartanian J. P., Guétard D., Henry M., Wain-Hobson S., Evidence for editing of human papillomavirus DNA by APOBEC3 in benign and precancerous lesions. Science 320, 230–233 (2008). [DOI] [PubMed] [Google Scholar]
- 15.Lucifora J., et al. , Specific and nonhepatotoxic degradation of nuclear hepatitis B virus cccDNA. Science 343, 1221–1228 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bogerd H. P., et al. , Cellular inhibitors of long interspersed element 1 and Alu retrotransposition. Proc. Natl. Acad. Sci. U.S.A. 103, 8780–8785 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richardson S. R., Narvaiza I., Planegger R. A., Weitzman M. D., Moran J. V., APOBEC3A deaminates transiently exposed single-strand DNA during LINE-1 retrotransposition. eLife 3, e02008 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bogerd H. P., Wiegand H. L., Doehle B. P., Lueders K. K., Cullen B. R., APOBEC3A and APOBEC3B are potent inhibitors of LTR-retrotransposon function in human cells. Nucleic Acids Res. 34, 89–95 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee Y. N., Malim M. H., Bieniasz P. D., Hypermutation of an ancient human retrovirus by APOBEC3G. J. Virol. 82, 8762–8770 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nik-Zainal S., et al. , Association of a germline copy number polymorphism of APOBEC3A and APOBEC3B with burden of putative APOBEC-dependent mutations in breast cancer. Nat. Genet. 46, 487–491 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buisson R., et al. , Passenger hotspot mutations in cancer driven by APOBEC3A and mesoscale genomic features. Science 364, eaaw2872 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taura M., et al. , COMMD1/Murr1 reinforces HIV-1 latent infection through IκB-α stabilization. J. Virol. 89, 2643–2658 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silvas T. V., et al. , Substrate sequence selectivity of APOBEC3A implicates intra-DNA interactions. Sci. Rep. 8, 7511 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kouno T., et al. , Crystal structure of APOBEC3A bound to single-stranded DNA reveals structural basis for cytidine deamination and specificity. Nat. Commun. 8, 15024 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bohn M. F., et al. , The ssDNA mutator APOBEC3A is regulated by cooperative dimerization. Structure 23, 903–911 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Byeon I. J., et al. , NMR structure of human restriction factor APOBEC3A reveals substrate binding and enzyme specificity. Nat. Commun. 4, 1890 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larijani M., Martin A., Single-stranded DNA structure and positional context of the target cytidine determine the enzymatic efficiency of AID. Mol. Cell. Biol. 27, 8038–8048 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feng Y., Seija N., Di Noia J. M., Martin A., AID in antibody diversification: There and back again: (Trends in Immunology 41, 586–600; 2020). Trends Immunol. 42, 89 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu Z., Zan H., Pone E. J., Mai T., Casali P., Immunoglobulin class-switch DNA recombination: Induction, targeting and beyond. Nat. Rev. Immunol. 12, 517–531 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duh E. J., Maury W. J., Folks T. M., Fauci A. S., Rabson A. B., Tumor necrosis factor alpha activates human immunodeficiency virus type 1 through induction of nuclear factor binding to the NF-kappa B sites in the long terminal repeat. Proc. Natl. Acad. Sci. U.S.A. 86, 5974–5978 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Antonczyk A., et al. , Direct inhibition of IRF-dependent transcriptional regulatory mechanisms associated with disease. Front. Immunol. 10, 1176 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Michalska A., Blaszczyk K., Wesoly J., Bluyssen H. A. R., A positive feedback amplifier circuit that regulates interferon (IFN)-stimulated gene expression and controls type I and type II IFN responses. Front. Immunol. 9, 1135 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sadler A. J., Williams B. R., Interferon-inducible antiviral effectors. Nat. Rev. Immunol. 8, 559–568 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reich N., et al. , Interferon-induced transcription of a gene encoding a 15-kDa protein depends on an upstream enhancer element. Proc. Natl. Acad. Sci. U.S.A. 84, 6394–6398 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen X., et al. , Crystal structure of a tyrosine phosphorylated STAT-1 dimer bound to DNA. Cell 93, 827–839 (1998). [DOI] [PubMed] [Google Scholar]
- 36.Van der Sluis R. M., et al. , Diverse effects of interferon alpha on the establishment and reversal of HIV latency. PLoS Pathog. 16, e1008151 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Selliah N., et al. , The gammac-cytokine regulated transcription factor, STAT5, increases HIV-1 production in primary CD4 T cells. Virology 344, 283–291 (2006). [DOI] [PubMed] [Google Scholar]
- 38.Mitra M., et al. , Structural determinants of human APOBEC3A enzymatic and nucleic acid binding properties. Nucleic Acids Res. 42, 1095–1110 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sharma S., et al. , APOBEC3A cytidine deaminase induces RNA editing in monocytes and macrophages. Nat. Commun. 6, 6881 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sadeghpour S., Khodaee S., Rahnama M., Rahimi H., Ebrahimi D., Human APOBEC3 variations and viral infection. Viruses 13, 1366 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Santos-Pereira J. M., Aguilera A., R loops: New modulators of genome dynamics and function. Nat. Rev. Genet. 16, 583–597 (2015). [DOI] [PubMed] [Google Scholar]
- 42.Tokuyama M., et al. , ERVmap analysis reveals genome-wide transcription of human endogenous retroviruses. Proc. Natl. Acad. Sci. U.S.A. 115, 12565–12572 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chuong E. B., Elde N. C., Feschotte C., Regulatory evolution of innate immunity through co-option of endogenous retroviruses. Science 351, 1083–1087 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stavrou S., et al. , Different modes of retrovirus restriction by human APOBEC3A and APOBEC3G in vivo. PLoS Pathog. 10, e1004145 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Law E. K., et al. , APOBEC3A catalyzes mutation and drives carcinogenesis in vivo. J. Exp. Med. 217, e20200261 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taura M., Song E., Ho Y. C., Iwasaki A., Apobec3A maintains HIV-1 latency through recruitment of epigenetic silencing machinery to the long terminal repeat. Proc. Natl. Acad. Sci. U.S.A. 116, 2282–2289 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hayashi K., Taura M., Iwasaki A., The interaction between IKKα and LC3 promotes type I interferon production through the TLR9-containing LAPosome. Sci. Signal. 11, eaan4144 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heinz S., et al. , Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell 38, 576–589 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Durinck S., Spellman P. T., Birney E., Huber W., Mapping identifiers for the integration of genomic datasets with the R/Bioconductor package biomaRt. Nat. Protoc. 4, 1184–1191 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Frankish A., et al. , GENCODE reference annotation for the human and mouse genomes. Nucleic Acids Res. 47, D766–D773 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yu G., Wang L. G., Han Y., He Q. Y., clusterProfiler: An R package for comparing biological themes among gene clusters. OMICS 16, 284–287 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen S., Zhou Y., Chen Y., Gu J., fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34, i884–i890 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dobin A., et al. , STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liao Y., Smyth G. K., Shi W., featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930 (2014). [DOI] [PubMed] [Google Scholar]
- 55.Pruitt K. D., Tatusova T., Maglott D. R., NCBI Reference Sequence (RefSeq): A curated non-redundant sequence database of genomes, transcripts and proteins. Nucleic Acids Res. 33, D501–D504 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pruitt K. D., et al. , RefSeq: An update on mammalian reference sequences. Nucleic Acids Res. 42, D756–D763 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Haeussler M., et al. , The UCSC Genome Browser database: 2019 update. Nucleic Acids Res. 47, D853–D858 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Love M. I., Huber W., Anders S., Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Blighe K., Rayna S., Lewis M., EnhancedVolcano: Publication-ready volcano plots with enhanced colouring and labeling (2018). https://github.com/kevinblighe/EnhancedVolcano. Accessed 3 June 2021.
- 60.Durinck S., et al. , BioMart and Bioconductor: A powerful link between biological databases and microarray data analysis. Bioinformatics 21, 3439–3440 (2005). [DOI] [PubMed] [Google Scholar]
- 61.Lawrence M., et al. , Software for computing and annotating genomic ranges. PLOS Comput. Biol. 9, e1003118 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Quinlan A. R., Hall I. M., BEDTools: A flexible suite of utilities for comparing genomic features. Bioinformatics 26, 841–842 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.J. A. Frank et al., Examining impacts of APOBEC3A on Interferon alpha induced gene expression. NCBI BioProject. https://www.ncbi.nlm.nih.gov/Traces/study/?acc=PRJNA742400&o=acc_s%3Aa. Deposited 26 June 2021. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
RNA-seq data have been deposited in the publicly accessible BioSample database (accession nos. SAMN19947880, SAMN19947881, SAMN19947882, SAMN19947883). Raw reads are available at BioProject (ID no. PRJNA742400) (63). All study data other than raw RNA-seq data are included in SI Appendix.