Abstract
Background and aim:
Recurrent wheezing is often triggered by viral respiratory infections. The aims of our study were: i) to evaluate whether the addition of a nutraceutical (Leucodif®), could improve the efficacy of montelukast or inhaled steroids (ICS) compared to the single treatment; ii) to verify whether a treatment is more effective than another. Our study was biased by the COVID-19 pandemic, which resulted in a lockdown of almost two months in Italy.
Methods:
The multicenter, open-label study enrolled 84 children aged 2–6 years diagnosed with recurrent wheezing and randomized them into four treatment arms for three months: ICS treatment; ii) montelukast; iii) montelukast + Leucodif; iv) ICS + Leucodif. Children were assessed at baseline and after one, two, and three months of treatment using the TRACK score for both the caregiver and the physician.
Results:
Out of the 84 patients, 18 patients received ICS therapy, 22 patients ICS + Leucodif, 24 patients montelukast, and 20 patients montelukast + Leucodif. All four treatments resulted in a significant reduction in symptoms with no differences among the various groups.
Conclusions:
Our study demonstrates that montelukast therapy appears to be equally effective as ICS therapy and that the addition of the nutraceutical Leucodif does not appear to improve the treatment outcome. However, in our opinion our study was strongly influenced and biased by the lockdown due to the COVID-19 pandemic, which inherently resulted in reduced exposure to the viruses that commonly cause respiratory infections in children. (www.actabiomedica.it)
Keywords: wheezing, children, montelukast, inhaled steroid, leucodif, covid-19, SARS-CoV2, clinical trial
Introduction
The presence of wheezing in preschool children is a very frequent phenomenon with which a pediatrician is very often confronted in clinical practice. Epidemiological studies show that about 30% of children experience at least one episode of wheezing within the first three years of life and by the age of six, the cumulative prevalence of wheezing reaches almost 50% [1, 2]. Most episodes are triggered by viral infections as confirmed by studies on nasal washing, which have identified a viral agent in 80% to 90% of exacerbations in preschool age [3, 4]. Despite the high prevalence of wheezing, a minimal amount of evidence about the natural history and pharmacological treatment of wheezing in preschool children exists. Clinical heterogeneity, which reflects a multifactorial etiopathogenesis, is the main characteristic of this condition, thus requiring the use of two classifications based on different phenotypes:
A symptom-based classification, which distinguishes between episodic viral wheeze (EVW), which includes all forms of wheezing secondary to viral infections of the upper airways and is characterized by a state of well-being between each infectious episode and multiple-trigger wheeze (MTW) characterized by more frequent episodes of wheezing that is triggered by numerous factors (viral infections, exercise, exposure to environmental pollutants) [5].
A classification based on the evolution of the symptoms through time, which distinguishes between transient (symptoms that end before three years of age), persistent (symptoms that begin before three years of age and continue beyond six years), and late-onset (symptoms that begin after three years of age) wheezing [1].
Based on the present evidence, the European Respiratory Society (ERS) Task Force recommends the use of the inhaled steroid (ICS) in patients with MTW and montelukast in those with EVW [5]. However, it has been shown that wheezing phenotypes can vary over time and therefore the same child in 50% of cases can experience a change in wheezing characteristics [6,7]. Accordingly, although these classifications have been created for practical purposes, they are in reality impractical, and the latest Global Initiative for Asthma (GINA) guidelines (2020) do not recognize their use in patient management [8].
In light of these considerations, the same guidelines recommend use of low doses of ICS as the first choice in the treatment of the child with frequent episodes of wheezing (three or more episodes in a season) to be continued for at least three months regardless of the phenotype [8,9]. Montelukast is less effective than ICS but does represent a valid alternative in patients in whom compliance with inhalation therapy is not adequate [10–13]
Respiratory infections are very common in children and can be the cause of wheezing episodes in predisposed individuals. In this sense, some nutraceuticals are attributed a preventive role against respiratory infections [14–19]. Among these, Leucodif® (Konpharma, Rome, Italy) is a nutraceutical based on vitamin C, vitamin E, zinc, vitamin D3, Echinacea, beta-glucan, and blood orange extract and originated as a product for stimulating the immune system against viruses and bacteria. This product can be prescribed from two years of age onward according to a scheme that provides for the administration of one capsule a day for 14 days a month for three months. Considering that the event that triggers the wheezing episode in most cases is an infectious event, the aim of this study was to evaluate whether the addition of the Leucodif® nutraceutical to therapy with montelukast or ICS in patients with recurrent wheezing may result in an outcome gain over montelukast or ICS alone. Moreover, we further compared the various groups to establish whether a treatment is more effective than another.
Our clinical trial has had to address with the difficulties related to the COVID-19 pandemic and with the lockdown that ensued in Italy for almost two months [20–22]. This lockdown has led to an obligatory closure of the children at home with consequent repercussions on their health and a reduction in the incidence of respiratory infections [23]. This study will therefore be an opportunity to discuss the criticalities of clinical trials during the COVID-19 pandemic [24,25].
Materials and Methods
Study design
This trial was a multicenter, open-label, randomized controlled study involving children who met two inclusion criteria: (1) age between 2 and 6 years old and (2) diagnosis of recurrent wheezing (> 3 episodes in one season) [5]. To make the population homogeneous, the following exclusion criteria were applied: (1) concomitant chronic disease (syndromes or immunodeficiencies), (2) preterm births (< 37 weeks), and/or (3) use of steroids, immunostimulants, or antileukotrienes during the previous two weeks prior to the study.
Enrolled patients were randomized into four arms of the study based on a computerized procedure (REDcap web application): (1) ICS treatment (beclomethasone diproprionate 100 mcg x 2 given via a spacer device), (2) montelukast (4 or 5 mg/day according to child’s age),(3) montelukast + Leucodif® (one capsule per day for 14 days per month for three months), and (4) ICS + Leucodif.
At time 0 (T0), a clinical check of the child was expected with the administration of the test for respiratory and asthma control in kids (TRACK). The first part was completed by the caregiver and included a 33-item questionnaire divided into five sections, including questions about the frequency of respiratory symptoms (wheeze, cough, shortness of breath), activity limitation, and nighttime awakenings over the past four weeks, rescue medication use in the past three months, and oral corticosteroid use during the previous year. The second part of the questionnaire was completed by the physician and included five questions about respiratory symptoms and need for supplementary drugs [26].
This questionnaire was repeated by telephone interview at T1 and T2 after the start of treatment (only questions from 1–26 for parents) and therefore during the outpatient clinical check at the end of T3, questions from 1 to 30 were asked. The outcomes of the study were represented by the score of respiratory symptoms from the four analyzed arms. Physical examination of each child was performed by a pediatrician at the beginning and end of the study.
The study was conducted in Italy at the Pediatric Pulmonology Unit of Catania Policlinic, Pediatric Unit of University of Campania “Luigi Vanvitelli” in Naples, Pediatric Unit of Messina Policlinic, Pediatric Clinic of Pavia Policlinic, from January 2020 to May 2020.
Demographic data and other clinical characteristics of the study subjects were collected through questionnaires and extrapolated from medical records.
Ethical considerations
The study was explained in detail to the parents/guardians of the children who participated in the study and informed consent was acquired from each parent/guardian. The ethics committee of University Catania (Italy) approved the study.
Data analysis
Collected data were statistically analyzed by the GraphPad software (version 8.1.2). A p value <0.05 was considered to be statistically significant. Data were studied for normality and according to the results, statistical analysis was performed using analysis of variance (ANOVA). Application of Tukey’s multiple comparisons test for normally distributed data and Friedman’s test followed by the application of Dunn’s multiple comparisons test for not normally distributed data were applied.
Results
Eight-four patients (males/females = 42/42) with a mean age of 3.48 ± 1.8 years were enrolled. Eighty-two out of 84 completed the study protocol. Two patients did not complete the protocol study due to adverse effects (n = 1 patient in the montelukast arm for hyperactivity) and allergic reaction (n = 1 patient in the arm ICS+Leucodif with generalized pruritus and urticaria). Demographic and clinical characteristics of the enrolled population are shown in Table 1.
Table 1.
Overview of patients.
| n. | |
|---|---|
| Enrolled children | 84 |
| Males/Females | 42/42 |
| Mean age (y/o) ± standard deviation | 3.48 ± 1.8 |
| Children on ICS treatment | 18 |
| Children on ICS + Leucodif | 24 |
| Children on montelukast | 24 |
| Children on montelukast + Leucodif | 20 |
Out of the 84 patients, 18 patients received ICS therapy, 22 patients ICS + Leucodif, 24 patients montelukast, and 20 patients montelukast + Leucodif. Therapeutic regimens of all four groups lasted for three months.
Effectiveness of treatments
The temporal evolution of the scores is summarized in Table 2.
Table 2.
Test for respiratory and asthma control in kids (TRACK) score in the various arms, at baseline (T0) and after 1 (T1), 2 (T2) and 3 (T3) months of treatment.
| T0 | T1 | T2 | T3 | p-value | |
|---|---|---|---|---|---|
| ICS | |||||
| Caregiver Score | 114.22 ± 23.04 | 52.33 ± 14.26 | 48.33 ± 22.25 | 42.55 ± 24.51 | < 0.01 |
| Physician Score | 11.22 ± 3.34 | 6.55 ± 1.13 | 6.77 ± 2.77 | 4.88 ± 2.1 | < 0.01 |
| ICS + Leucodif | |||||
| Caregiver Score | 110.63 ± 17.68 | 42.18 ± 27.58 | 47 ± 26.72 | 53.36 ± 29.04 | < 0.01 |
| Physician Score | 11 ± 2.66 | 5.81 ± 4.26 | 6.81 ± 3.51 | 6.18 ± 3.42 | < 0.01 |
| Montelukast | |||||
| Caregiver Score | 108.58 ± 26 | 59.58 ± 20.47 | 65.66 ± 24.66 | 53.16 ± 26.68 | < 0.01 |
| Physician Score | 11.08 ± 2.06 | 7.25 ± 3.00 | 8.66 ± 3.25 | 6.83 ± 2.85 | < 0.05 |
| Montelukast + Leucodif | |||||
| Caregiver Score | 102.2 ± 16.38 | 54.6 ± 27.06 | 50.4 ± 34.78 | 48.2 ± 30.87 | < 0.01 |
| Physician Score | 11.2 ± 1.61 | 6.6 ± 3.16 | 5.6 ± 3.65 | 5.7 ± 3.83 | < 0.01 |
It can be observed that for all four treatments, a statistically significant reduction in the scores of both the caregiver and the physician occurred.
As for the ICS alone, the post hoc analysis with Dunn’s multiple comparison test showed that statistical significance was reached already after the first month with regard to caregiver score, while it was reached after three months of therapy for the physician score. With regard to the ICS + Leucodif association, statistical significance was reached for both scores already after the first month of treatment. With regard to montelukast alone, statistical significance was reached already in the first month according to the caregiver score and after three months according to the physician score.
Finally, as regards the montelukast + Leucodif association, statistical significance was reached already in the first month for the caregiver score and after the second month based on the physician score.
Comparison among groups
Comparing the percentage reduction of the score (from T0 to T3), no statistically significant differences in caregiver and the physician scores were noted. Figures 1 and 2.
Figure 1.
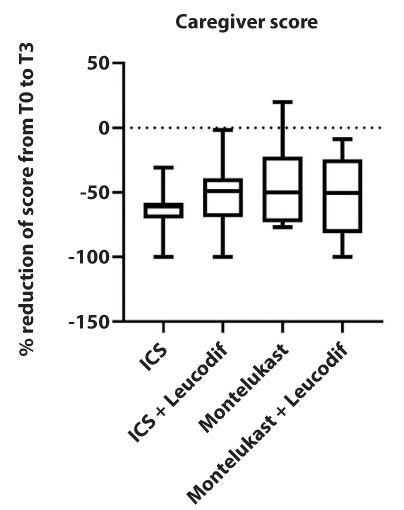
Caregiver test results for respiratory and asthma control in kids (TRACK) score in the four treatment arms expressed as a percentage difference between the initial and final values.
Figure 2.
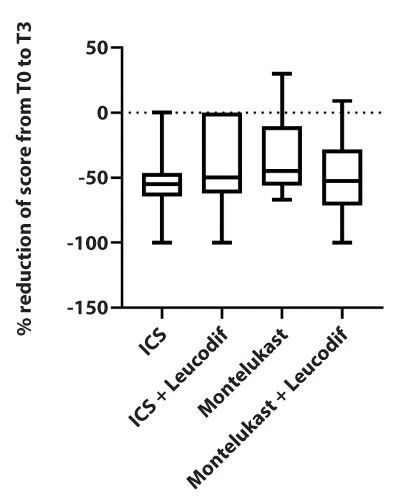
Physician test results for TRACK scores in the four treatment arms expressed as a percentage difference between the initial and final values.
In other words, no treatment seems to be more effective than any other, and they are all equally effective in reducing symptoms.
Safety data
A total of two patients dropped out of the study due to the appearance of adverse effects. One patient in the montelukast arm, suffering from autism spectrum disorder, had an accentuation of the state of hyperactivity, and the other patient, in the ICS + Leucodif arm, had an allergic reaction characterized by generalized itching and urticaria, which spontaneously regressed.
Discussion
Our study showed that all the children undergoing the various treatments improved from a clinical point of view as demonstrated by the reduction of caregiver and physician scores. Our goal was to demonstrate that the addition of a nutraceutical (based on vitamin C, vitamin E, zinc, vitamin D3, Echinacea, beta-glucan, and blood orange extract) would allow reduction of the incidence of respiratory infections, resulting in a therapeutic gain in children in treatment with montelukast or ICS. However, this randomized study demonstrated that in all four treatment arms, the children’s symptoms improved and no advantage of adding nutraceutical over conventional therapy was found.
The study lends itself to some considerations. The first is that this study was strongly influenced by the lockdown due to the COVID-19 pandemic. In fact, on March 11, 2020, the Italian government issued a decree that provided for the suspension of common retail commercial and educational activities and catering services and prohibited gatherings of people in public places or places open to the public [27]. That decree resulted in a reduced exposure to viral agents due to school closures, which certainly represent the area in which young patients, living in the community, would have been more exposed to respiratory diseases. This finding is confirmed by several studies, the last of which was conducted on 12 typical infections in children, some of which have been virtually eliminated. The study was conducted with over 375,000 children who arrived in one of the 80 clinics connected with Boston Children’s Hospital. Cases of 12 infections, otitis media, bronchiolitis, colds, laryngotracheobronchitis, gastroenteritis, influenza, streptococcal and non-streptococcal pharyngitis, pneumonia, sinusitis, and skin and urinary tract infections were evaluated in early 2019 and 2020. As a result of social distancing measures, all pathologies showed a decline. The greatest decrease was seen in the respiratory diseases: (1) influenza (99.5%), (2) laryngotracheobronchitis (96.5%), and (3) bronchiolitis (92.9%), all of which essentially disappeared. Nevertheless, the study was continued and the clinical follow-up was maintained through a very complex and well-articulated telephone questionnaire [23].
It is obvious that to confirm these findings, a placebo group would have been needed to understand whether isolation and social distancing would be sufficient measures to reduce the incidence of respiratory symptoms in children with recurrent wheezing. However, when we designed the study, we never thought we would face this type of pandemic and therefore it seemed unethical to withhold treatment from any patient. However, it could be speculated that the preventive effects of the nutraceutical were nullified by the lockdown that mitigated exposure to the viruses.
The second important point from this study is the revival of montelukast that regardless of the addition of the nutraceutical Leucodif seems to have an efficacy comparable to that of ICS. Montelukast is a leukotriene receptor antagonist drug (LTRA). It is known that the production of cysteinyl-leukotrienes increases during asthma, and a further increase is observed during exacerbations, whether they are secondary to allergens or induced by exercise [28]. Leukotrienes have a strong pro-inflammatory activity and are capable of inducing recruitment, activation, and migration of neutrophils, eosinophils, and monocytes. Furthermore, leukotrienes stimulate the production of interleukins by T lymphocytes and monocytes, leading to an increase in microvascular permeability and hyperproduction of mucous secretions [29]. Cysteinyl-leukotrienes, on the other hand, act on the smooth muscle cells of the airways and exhibit far more constrictive activity than that of methacholine and histamine. These agents are also responsible for the hypersecretion of mucus and the increase in vascular permeability in the airways in addition to the reduction of ciliary mucus clearance, all phenomena typically present in the airways during asthma [30]. LTRAs perform their action by binding to the leukotriene D4 receptor. The binding of the antagonists does not evoke any response but mechanically prevents physiological ligands from interacting with them [31, 32].
For years, montelukast has been used in the treatment of episodic viral wheezing, but the most recent literature has reported a reduction in its use both because administration would seem associated with a higher incidence of side effects [33,34] and because their efficacy, regardless of the wheezing phenotype, is considered lower than ICS [35]. This reduction may also be due to the fact that there are polymorphisms in the montelukast response pathway that would affect its effectiveness in some patients [36].
For all of these reasons, montelukast use has been downgraded to second or third choice in case of ICS therapy failure or non-compliance with inhalation therapy [8]. However, our study would appear to revive montelukast as its effectiveness appears to be comparable to that of ICS.
The third point to develop concerns the use of nutraceuticals for the prevention of infections. In the pediatric population one of the most frequent diseases is constituted by recurrent respiratory tract infections, which can often be resolved only with the administration of repeated courses of antibiotic therapy [37]. In light of these considerations in recent years, various supportive treatments have been proposed, including nutraceuticals, which can somehow provide an improvement both in clinical terms and in terms of quality of life [38]. Nieman and Sener report good results relating to the use of beta-glucan in upper airway infections [39,40]. Beta-glucan, an element found on the walls of yeast cells and in fungi, seems to be characterized by an immunomodulatory process resulting from an intrinsic and marked antioxidant action and a close link with receptors that are found on the surfaces of macrophages, granulocytes, natural killer (NK) cells, and leukocytes [39,40]. In the specific case of the nutraceutical Leucodif, in addition to beta-glucan, other substances, which have been attributed immunomodulating properties, such as vitamins C, D, E and zinc, have also been added [18, 41–43]. We have not been able to demonstrate that Leucodif facilitates an improvement in episodic viral wheezing, but its use in prevention of recurrent respiratory infections in the child remains rational.
Conclusions
In conclusion, the reading of our study can be two-fold. In the first instance, it could be argued that our study demonstrates how the lockdown due to the coronavirus pandemic may have influenced not only our clinical study but many other major clinical trials, often invalidating the results. Alternatively, taking the results for granted, it could be argued that the addition of the nutraceutical Leucodif does not seem to modify the natural history of recurrent wheezing and that the effectiveness of montelukast would seem comparable to that of ICS. However, further trial is needed to confirm these result and retest the role of the neutraceutical.
Conflict of Interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article
References
- Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N Engl J Med. 1995 Jan 19;332(3):133–38. doi: 10.1056/NEJM199501193320301. doi: 10.1056/NEJM199501193320301. PMID: 7800004. [DOI] [PubMed] [Google Scholar]
- Bisgaard H, Szefler S. Prevalence of asthma-like symptoms in young children. Pediatr Pulmonol. 2007 Aug;42(8):723–8. doi: 10.1002/ppul.20644. doi: 10.1002/ppul.20644. PMID: 17598172. [DOI] [PubMed] [Google Scholar]
- Rakes GP, Arruda E, Ingram JM, Hoover GE, Zambrano JC, Hayden FG, Platts-Mills TA, Heymann PW. Rhinovirus and respiratory syncytial virus in wheezing children requiring emergency care. IgE and eosinophil analyses. Am J Respir Crit Care Med. 1999 Mar;159(3):785–90. doi: 10.1164/ajrccm.159.3.9801052. doi: 10.1164/ajrccm.159.3.9801052. PMID: 10051251. [DOI] [PubMed] [Google Scholar]
- Jackson DJ, Gangnon RE, Evans MD, et al. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med. 2008 Oct 1;178(7):667–72. doi: 10.1164/rccm.200802-309OC. doi: 10.1164/rccm.200802-309OC. Epub 2008 Jun 19. PMID: 18565953; PMCID: PMC2556448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand PL, Baraldi E, Bisgaard H, et al. Definition, assessment and treatment of wheezing disorders in preschool children: an evidence-based approach. Eur Respir J. 2008 Oct;32(4):1096–110. doi: 10.1183/09031936.00002108. doi: 10.1183/09031936.00002108. PMID: 18827155. [DOI] [PubMed] [Google Scholar]
- Schultz A, Devadason SG, Savenije OE, Sly PD, Le Souëf PN, Brand PL. The transient value of classifying preschool wheeze into episodic viral wheeze and multiple trigger wheeze. Acta Paediatr. 2010 Jan;99(1):56–60. doi: 10.1111/j.1651-2227.2009.01508.x. doi: 10.1111/j.1651-2227.2009.01508.x. PMID: 19764920. [DOI] [PubMed] [Google Scholar]
- Doull IJ, Lampe FC, Smith S, Schreiber J, Freezer NJ, Holgate ST. Effect of inhaled corticosteroids on episodes of wheezing associated with viral infection in school age children: randomised double blind placebo controlled trial. BMJ. 1997 Oct 4;315(7112):858–62. doi: 10.1136/bmj.315.7112.858. doi: 10.1136/bmj.315.7112.858. PMID: 9353508; PMCID: PMC2127557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://ginasthma.org/ Accessed on 11th November 2020. [Google Scholar]
- Guilbert TW, Morgan WJ, Zeiger RS, et al. Long-term inhaled corticosteroids in preschool children at high risk for asthma. N Engl J Med. 2006 May 11;354(19):1985–97. doi: 10.1056/NEJMoa051378. doi: 10.1056/NEJMoa051378. PMID: 16687711. [DOI] [PubMed] [Google Scholar]
- Robertson CF, Price D, Henry R, et al. Short-course montelukast for intermittent asthma in children: a randomized controlled trial. Am J Respir Crit Care Med. 2007 Feb 15;175(4):323–9. doi: 10.1164/rccm.200510-1546OC. doi: 10.1164/rccm.200510-1546OC. Epub 2006 Nov 16. PMID: 17110643. [DOI] [PubMed] [Google Scholar]
- Bacharier LB, Phillips BR, Zeiger RS, et al. CARE Network Episodic use of an inhaled corticosteroid or leukotriene receptor antagonist in preschool children with moderate-to-severe intermittent wheezing. J Allergy Clin Immunol. 2008 Dec;122(6):1127–1135.e8. doi: 10.1016/j.jaci.2008.09.029. doi: 10.1016/j.jaci.2008.09.029. Epub 2008 Oct 30. PMID: 18973936; PMCID: PMC2753208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducharme FM, Lemire C, Noya FJ, et al. Preemptive use of high-dose fluticasone for virus-induced wheezing in young children. N Engl J Med. 2009 Jan 22;360(4):339–53. doi: 10.1056/NEJMoa0808907. doi: 10.1056/NEJMoa0808907. PMID: 19164187. [DOI] [PubMed] [Google Scholar]
- Papi A, Nicolini G, Baraldi E, Boner AL, Cutrera R, Rossi GA, Fabbri LM. BEclomethasone and Salbutamol Treatment (BEST) for Children Study Group. Regular vs prn nebulized treatment in wheeze preschool children. Allergy. 2009 Oct;64(10):1463–71. doi: 10.1111/j.1398-9995.2009.02134.x. doi: 10.1111/j.1398-9995.2009.02134.x. PMID: 19772514. [DOI] [PubMed] [Google Scholar]
- Souyoul SA, Saussy KP, Lupo MP. Nutraceuticals: A Review. Dermatol Ther (Heidelb) 2018;8(1):5–16. doi: 10.1007/s13555-018-0221-x. doi:10.1007/s13555-018-0221-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisi GF, Carota G, Castruccio Castracani C, et al. Nutraceuticals in the Prevention of Viral Infections, including COVID-19, among the Pediatric Population: A Review of the Literature. Int J Mol Sci. 2021 Feb 28;22(5):2465. doi: 10.3390/ijms22052465. doi: 10.3390/ijms22052465. PMID: 33671104; PMCID: PMC7957644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manti S, Parisi GF, Papale M, Licari A, et al. Bacteriotherapy with Streptococcus salivarius 24SMB and Streptococcus oralis 89a nasal spray for treatment of upper respiratory tract infections in children: a pilot study on short-term efficacy. Ital J Pediatr. 2020 Apr 3;46(1):42. doi: 10.1186/s13052-020-0798-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardi S, Parisi G, Papale M, et al. Small airways in children with allergic rhinoconjunctivitis: the potential role of a multicomponent nutraceutical. Acta Biomed. 2020;91(2):350–55. doi: 10.23750/abm.v91i2.9641. Published 2020 May 11. doi:10.23750/abm.v91i2.9641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulvirenti G, Parisi GF, Manti S, et al. The Immunomodulatory Role of Vitamin D in Respiratory Diseases. Current Respir Med Rev. 2019;15(3):238–45. DOI: 10.2174/1573398X15666191114144230. [Google Scholar]
- Cohen HA, Varsano I, Kahan E, Sarrell EM, Uziel Y. Effectiveness of an herbal preparation containing echinacea, propolis, and vitamin C in preventing respiratory tract infections in children: a randomized, double-blind, placebo-controlled, multicenter study. Arch Pediatr Adolesc Med. 2004 Mar;158(3):217–21. doi: 10.1001/archpedi.158.3.217. doi: 10.1001/archpedi.158.3.217. PMID: 14993078. [DOI] [PubMed] [Google Scholar]
- Manti S, Licari A, Montagna L, et al. SARS-CoV-2 infection in pediatric population. Acta Biomed. 2020 Sep 15;91(11-S):e2020003. doi: 10.23750/abm.v91i11-S.10298. doi: 10.23750/abm.v91i11-S.10298. PMID: 33004773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaferio L, Parisi GF, Brindisi G, et al. Cross-sectional survey on impact of paediatric COVID-19 among Italian paediatricians: report from the SIAIP rhino-sinusitis and conjunctivitis committee. Ital J Pediatr. 2020 Oct 6;46(1):146. doi: 10.1186/s13052-020-00906-4. doi: 10.1186/s13052-020-00906-4. PMID: 33023616; PMCID: PMC7538039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinale F, Ciprandi G, Barberi S, et al. and the SIAIP Task Force. Consensus statement of the Italian society of pediatric allergy and immunology for the pragmatic management of children and adolescents with allergic or immunological diseases during the COVID-19 pandemic. Ital J Pediatr. 2020 Jun 16;46(1):84. doi: 10.1186/s13052-020-00843-2. doi: 10.1186/s13052-020-00843-2. PMID: 32546234; PMCID: PMC7296524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatoun J, Correa ET, Donahue SMA, Vernacchio L. Social Distancing for COVID-19 and Diagnoses of Other Infectious Diseases in Children. Pediatrics. 2020 Oct;146(4):e2020006460. doi: 10.1542/peds.2020-006460. doi: 10.1542/peds.2020-006460. Epub 2020 Sep 2. PMID: 32879032. [DOI] [PubMed] [Google Scholar]
- Yang Y. Impact of the COVID-19 Pandemic on Biomedical and Clinical Research. Matter. 2020 Oct 7;3(4):970–973. doi: 10.1016/j.matt.2020.08.026. doi: 10.1016/j.matt.2020.08.026. Epub 2020 Aug 29. PMID: 32904589; PMCID: PMC7456300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuttle KR. Impact of the COVID-19 pandemic on clinical research. Nat Rev Nephrol. 2020 Oct;16(10):562–564. doi: 10.1038/s41581-020-00336-9. doi: 10.1038/s41581-020-00336-9. PMID: 32760016; PMCID: PMC7404075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy KR, Zeiger RS, Kosinski M, et al. Test for respiratory and asthma control in kids (TRACK): a caregiver-completed questionnaire for preschool-aged children. J Allergy Clin Immunol. 2009 Apr;123(4):833–9.e9. doi: 10.1016/j.jaci.2009.01.058. doi: 10.1016/j.jaci.2009.01.058. PMID: 19348922. [DOI] [PubMed] [Google Scholar]
- http://www.governo.it/it/articolo/coronavirus-conte-firma-il-dpcm-11-marzo-2020/14299. Accessed on 11th November 2020. [Google Scholar]
- Bisgaard H. Role of leukotrienes in asthma pathophysiology. Pediatr Pulmonol. 2000 Aug;30(2):166–76. doi: 10.1002/1099-0496(200008)30:2<166::aid-ppul15>3.0.co;2-l. doi: 10.1002/1099-0496(200008)30:2<166::aid-ppul15>3.0.co;2-l. PMID: 10922142. [DOI] [PubMed] [Google Scholar]
- Lewis RA, Austen KF, Soberman RJ. Leukotrienes and other products of the 5-lipoxygenase pathway. Biochemistry and relation to pathobiology in human diseases. N Engl J Med. 1990 Sep 6;323(10):645–55. doi: 10.1056/NEJM199009063231006. doi: 10.1056/NEJM199009063231006. PMID: 2166915. [DOI] [PubMed] [Google Scholar]
- Arm JP, Lee TH. Sulphidopeptide leukotrienes in asthma. Clin Sci (Lond) 1993 May;84(5):501–10. doi: 10.1042/cs0840501. doi: 10.1042/cs0840501. PMID: 8389264. [DOI] [PubMed] [Google Scholar]
- Bernstein PR. Chemistry and structure-activity relationships of leukotriene receptor antagonists. Am J Respir Crit Care Med. 1998 Jun;157(6 Pt 2):S220–5. discussion S225-6, S247-8. PMID: 9647603. [PubMed] [Google Scholar]
- Pecoraro R, Parisi GF, Lanzafame A, Trovato A, Barone P, Leonardi S. Leukotriene antagonist drugs: a tool to face Chronic Urticaria in Children? Journal of Pediatric Biochemistry. 2013;3:203–211. [Google Scholar]
- Haarman MG, van Hunsel F, de Vries TW. Adverse drug reactions of montelukast in children and adults. Pharmacol Res Perspect. 2017 Oct;5(5):e00341. doi: 10.1002/prp2.341. doi: 10.1002/prp2.341. PMID: 28971612; PMCID: PMC5625152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aagaard L, Hansen EH. Adverse drug reactions associated with asthma medications in children: systematic review of clinical trials. Int J Clin Pharm. 2014 Apr;36(2):243–52. doi: 10.1007/s11096-014-9924-y. doi: 10.1007/s11096-014-9924-y. Epub 2014 Feb 23. PMID: 24562976. [DOI] [PubMed] [Google Scholar]
- Castro-Rodriguez JA, Rodriguez-Martinez CE, Ducharme FM. Daily inhaled corticosteroids or montelukast for preschoolers with asthma or recurrent wheezing: A systematic review. Pediatr Pulmonol. 2018 Dec;53(12):1670–1677. doi: 10.1002/ppul.24176. doi: 10.1002/ppul.24176. Epub 2018 Nov 5. PMID: 30394700. [DOI] [PubMed] [Google Scholar]
- Lima JJ, Zhang S, Grant A, et al. Influence of leukotriene pathway polymorphisms on response to montelukast in asthma. Am J Respir Crit Care Med. 2006 Feb 15;173(4):379–85. doi: 10.1164/rccm.200509-1412OC. doi: 10.1164/rccm.200509-1412OC. Epub 2005 Nov 17. PMID: 16293801; PMCID: PMC2662939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patria MF, Esposito S. Recurrent lower respiratory tract infections in children: a practical approach to diagnosis. Paediatr Respir Rev. 2013 Mar;14(1):53–60. doi: 10.1016/j.prrv.2011.11.001. doi: 10.1016/j.prrv.2011.11.001. Epub 2011 Dec 8. PMID: 23347661. [DOI] [PubMed] [Google Scholar]
- Carr RR, Nahata MC. Complementary and alternative medicine for upper-respiratory-tract infection in children. Am J Health Syst Pharm. 2006 Jan 1;63(1):33–9. doi: 10.2146/ajhp040613. doi: 10.2146/ajhp040613. PMID: 16373463. [DOI] [PubMed] [Google Scholar]
- Nieman DC, Henson DA, et al. Beta-glucan, immune function, and upper respiratory tract infections in athletes. Med Sci Sports Exerc. 2008 Aug;40(8):1463–71. doi: 10.1249/MSS.0b013e31817057c2. doi: 10.1249/MSS.0b013e31817057c2. PMID: 18614945. [DOI] [PubMed] [Google Scholar]
- Sener G, Toklu H, Ercan F, Erkanli G. Protective effect of beta-glucan against oxidative organ injury in a rat model of sepsis. Int Immunopharmacol. 2005 Aug;5(9):1387–96. doi: 10.1016/j.intimp.2005.03.007. doi: 10.1016/j.intimp.2005.03.007. Epub 2005 Apr 9. PMID: 15953565. [DOI] [PubMed] [Google Scholar]
- Padhani ZA, Moazzam Z, Ashraf A, Bilal H, Salam RA, Das JK, Bhutta ZA. Vitamin C supplementation for prevention and treatment of pneumonia. Cochrane Database Syst Rev. 2020 Apr 27;4(4):CD013134. doi: 10.1002/14651858.CD013134.pub2. doi: 10.1002/14651858.CD013134.pub2. PMID: 32337708; PMCID: PMC7192369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dror DK, Allen LH. Vitamin E deficiency in developing countries. Food Nutr Bull. 2011 Jun;32(2):124–43. doi: 10.1177/156482651103200206. doi: 10.1177/156482651103200206. PMID: 22164974. [DOI] [PubMed] [Google Scholar]
- Lamberti LM, Fischer-Walker CL, Black RE. Prophylactic zinc supplementation for prevention of acute respiratory infections in infants and young children. Indian Pediatr. 2014 Oct;51(10):775–76. doi: 10.1007/s13312-014-0502-0. doi: 10.1007/s13312-014-0502-0. PMID: 25362005. [DOI] [PubMed] [Google Scholar]


