Abstract
In this study, the effect of the γ-valerolactone (GVL)/H2O pretreatment system on bamboo (Neosinocalamus affinis) for enzymatic hydrolysis and ethanol fermentation was investigated. The performance characterization of the pretreated bamboo substrates, including the chemical composition, the structural characteristics, and the ability to produce bioethanol, were evaluated. The recovered substrates were enzymatically hydrolyzed for 48 h and then fermented to bioethanol. For the cellulose in the raw bamboo material, the highest cellulose-to-glucose conversion yield (CGCY) was achieved at 140 °C for 2 h with GVL : H2O = 8 : 2, which was 73.39%, and the cellulose-to-ethanol conversion yield (CECY) was 67.00%. This indicated that 183.5 kg of bioethanol could be produced per ton of bamboo, which was 9.71-folds higher than that directly converted from the untreated raw bamboo powder. Under these conditions, 50.60% of the active lignin can be recovered and be used as a wood-derived feedstock for further high-valued utilization. Meanwhile, the maximum concentration of fermentation inhibitors formed after pretreatment was about 140.9 mmol L−1, and had weak inhibition to the subsequent reaction. It has been shown that the cellulose could be effectively separated from bamboo and converted into bioethanol through the GVL/H2O pretreatment system.
In this study, the potential production of bamboo by the GVL/H2O system for ethanol production was investigated.
1. Introduction
With the gradual consumption of unrenewable fossil resources and the rapidly growing demand for energy resources, the conversion, and utilization of renewable resources have attracted great interest in recent years.1,2 Biomass resources can not only replace fossil energy, but also meet the requirement of sustainable development by sequester carbon through photosynthesis, and therefore have promising development prospects.
Among the biomass resources, as one of the most promising feedstocks for producing biofuels and biochemicals, lignocellulosic biomass is abundant, inexpensive, renewable, and does not compete with grain crops.3 Wooden resources in China are scarce, while the bamboo resource is relatively abundant. As the fastest growing plant in the world, bamboo can grow as rapidly as 91 cm per day and reach maturity in 3–5 years.4 It was reported that the annual output value of the bamboo industry in China reach 300 billion RMB. However, owing to the hollow structure, large sharpness, and poor timber properties of bamboo green and yellow, the processing residues were increased with the rapid development of the bamboo processing industry and amount to about 2817 million tons currently.5 While the current disposal methods for bamboo processing residues still involve burning or landfilling directly, which causes serious environmental pollution.6 Therefore, if the bamboo processing residues can be fully employed for their high value, they will be equivalent to an inexhaustible renewable resource.
At present, the moso bamboo is used as the feedstock for more than 90% of the bamboo panel industry. While the clumping bamboo, which accounts for more than 20% of bamboo forest in China, is not effectively utilized.7,8Neosinocalamus affinis is a kind of clumping bamboo in the Sichuan province of China, which is widely grown, valuable and growing extremely fast. It can grow from bamboo shoots to 10 m in only 100 days under suitable conditions.9 In previous studies, the higher cellulose content of Neosinocalamus affinis makes it a preferable biomass raw material, and the thinner cell walls also make it easier to be transformed and utilized.10 However, there are still quite a few studies on the performance of Neosinocalamus affinis for bioethanol conversion and utilization.
The lignocellulosic cell wall of mature bamboo wood consists of 40–60% cellulose, 20–32% hemicellulose, and 20–30% lignin.7 The components are firmly bound by the combination of covalent bonds, hydrogen bonds, and van der Waals forces.11 Cellulose, as the skeleton part, is encapsulated by lignin and hemicellulose. Both conventional methods for producing bioethanol (i.e., biochemical and thermochemical processes) require pretreatment to destroy the crystalline structure of cellulose by removing a maximum amount of lignin and hemicellulose.12–14 Pretreatments can expose cellulose by separating hemicellulose and lignin and reduce the crystallinity and degree of polymerization of cellulose to improve the accessibility and enzymatic efficiency of cellulose. The pretreatment methods currently used are the physical pretreatments, such as crushing, chemical pretreatments, such as acid and alkali pretreatment,15 and physicochemical pretreatments including ionic liquids16,17 and steam explosion18,19 among these, organic solvents (and their mixtures) are effective methods to facilitate cell wall component removal, such as methanol, ethanol, and acetone are commonly used.
Recently, as a renewable and environmentally-friendly organic solvent, γ-valerolactone (GVL) has been widely applied in food additives, lubricants, and plasticizers. Since the solubility parameter is close to that of lignin, the GVL/H2O system can effectively extract lignin from lignocellulose-based biomass,20 and the soluble sugars in the spent liquor can be separated and purified by supercritical CO2 extraction.21 Recently, this system has been applied in the pretreatment of biomass such as cotton, straw, and eucalyptus.22,23 The cellulose content of the substrates was increased from 39.9% to 89.3% by pretreating sawdust at 156 °C for 0.5 h with a GVL/H2O (1 : 1) system. After enzymatic hydrolysis and fermentation of the cellulose-rich substrates, the bioethanol yield was equivalent to 94% of the theoretical yield, which also indicates that the GVL/H2O system is an effective method to convert biomass to bioethanol.23 When the bamboo powder was pretreated by the GVL/H2O system, the glucose yield from the enzymatic hydrolysis was also enhanced by 6.7 times over the untreated substrate.24 But there is still no detailed work on the performance of preparing bioethanol from bamboo substrates pretreated by the GVL/H2O pretreatment.
In this work, GVL/H2O system was employed for the pretreatment of bamboo (Neosinocalamus affinis). The modifications performed in the pretreated bamboo substrates, including chemical composition analysis, cellulase accessibility, and cellulose digestibility, were investigated comprehensively. To further investigate the bioethanol potential of the pretreated substrates, the enzymatic hydrolysate was fermented. In addition, the composition and content of carbohydrates and fermentation inhibitors in the pretreated spent liquor were analyzed. Furthermore, the structural changes in bamboo samples before and after pretreatment were characterized by the degree of polymerization (DP), Fourier transform infrared spectroscopy (FT-IR), X-ray diffraction (XRD), and scanning electron microscopy (SEM). The aim of this study was to provide methods to reduce carbon consumption and slow down the carbon emission of bamboo processing residues and offer the possibility of energizing the utilization of bamboo.
2. Materials and methods
2.1. Materials
The two-year-old bamboo (Neosinocalamus affinis) was harvested from the south Guangxi Region in China. The air-dried bamboo culm was smashed and then sieved. The powder with the size less than 40 mesh (0.425 mm) was extracted with ethanol and water using the Soxhlet apparatus for 10 h to remove the waxes from the surface and the extract was dried at 103 ± 2 °C to constant weight before use.
GVL (98% purity) used in the pretreatment and formic acid, acetic acid, furfural, glucose, and xylose (all analytical purity) used in the assay process were provided by Aladdin Biochemical Technology Co. (Shanghai). Cellulase (C1794-5KU, from Trichoderma sp.) and β-glucosidase (49290-1G, from almonds) were obtained from Sigma-Aldrich (Shanghai) and the enzyme activity was determined by filter paper enzyme activity (FPA).25Saccharomyces cerevisiae yeast used in the fermentation was purchased from Hubei Anqi Yeast Co. Ltd. The bicinchoninic acid (BCA) Protein Assay Kit used in the enzymatic adsorption experiment was provided by Biotech Bioengineering Co. Ltd (Shanghai). All chemicals were used directly without further purification.
2.2. Pretreatment
2.5 g of the dewaxed bamboo powder was loaded into a 50 mL vessel with different ratios of GVL/H2O solution and sulfuric acid to make a solid-to-liquid ratio of 1 : 10 and a sulfuric acid concentration of 75 mmol L−1. The vessel was placed into the hydrothermal synthesis reactor, and the programmed controllers were set at 20 rpm and the rate of 1.5 (°C minute−1) to heat the reactor from room temperature to the target temperature. After pretreatment, the reactor was cooled to room temperature by following a procedure.
After cooling, the mixture was separated by vacuum filtration and washed three times within a total of 25 mL of GVL/H2O solution, which was the same as used in pretreatment. Water was added to the recovered filtrate for GVL : H2O = 1 : 3 and kept the mixture at 4 °C for 48 h to precipitate lignin sufficiently. The recovered solid substrates were washed thoroughly with 100 mL of deionized water and then stored at 4 °C for the subsequent enzymatic hydrolysis and ethanol fermentation. Each pretreatment was performed in triplicate, with the data reported as the average ± standard deviation (SD).
2.3. Enzymatic hydrolysis
Enzymatic hydrolysis experiments were carried out in 50 mL plastic flasks with 1.5% substrate content (w/v) and 10 mL of sodium acetate–acetic acid buffer solution (pH = 4.8). The system was loaded with cellulose (15 FPU per g substrates) and β-glucosidase (30 IU per g substrates). The flasks were placed on a shaking incubator (KYC-100C, Shanghai Fuma Laboratory Instrument Co., China) and enzymatic hydrolysis was performed at 50 °C for 48 h with 200 rpm. During the hydrolysis, 0.1 mL of hydrolysate was sampled periodically and deactivated at 100 °C for the sugar concentration assay. All enzymatic hydrolysis experiments were performed in triplicate.
2.4. Ethanol fermentation
The yeast was placed in a 50 mL plastic flask containing 40 mL of a 2% glucose solution. The flask was held in the shaking incubator at 38 °C for 15 minutes, and then the temperature was adjusted to 34 °C and held for 1.5 h to activate the yeast.
The enzymatic hydrolysate obtained in 2.3 was submerged in water at 100 °C for 30 min to stop the enzymatic hydrolysis. After cooling, nutrients (peptone 5 g L−1, KH2PO4 2 g L−1, MgSO4 1 g L−1, and CaCl2 0.25 g L−1) were dissolved in deionized water and then added to the enzymatic hydrolysate. After adjusting the pH to 5.5 ± 0.1 using 0.6 mol L−1 NaOH, the former precultured yeast solution was inoculated into the prepared hydrolysate (2 w% of the substrate). The flasks were placed at 37 °C for 48 h at 150 rpm for ethanol fermentation. To avoid the activity of the yeast inhibited by the CO2 produced during the fermentation, the flasks were sealed with a film that could retain moisture while passing through the air. Fermentation was conducted in triplicate for each substrate.
2.5. Analytical methods
2.5.1. Determination of the contents of carbohydrates and inhibitors
The content of carbohydrates (cellulose and hemicellulose) and lignin in the bamboo samples before and after pretreatment were determined by the standard analytical procedures established by the National Energy Laboratory (NREL) TP-510-42618.26 The bamboo simple was prehydrolyzed with 72% sulfuric acid for 2 h at room temperature, and then, the mixture was diluted to 3% sulfuric acid and hydrolyzed at 121 °C for 1 h. The residual solids were separated by vacuum filtration and dried at 103 °C to measure the acid-insoluble lignin (AIL), and the acid-soluble lignin (ASL) in the filtrate was determined using a UV-Vis spectrophotometer at 205 nm. The lignin content was calculated as the sum of the above. The monosaccharide concentrations in the acid hydrolysates, enzymatic hydrolysates, and fermenting solution were measured by ion chromatography using an amperometric detector (Metrohm Corporation, Switzerland)27 equipped with a Hamilton RCX-30 column and Metrosep RP2 guard column. The eluent was 4 mM NaOH and 0.5 mM NaAc at a flow rate of 0.7 mL min−1. Oligosaccharides in the spent liquor were also hydrolyzed with 4% H2SO4 at 121 °C for 60 min and the carbohydrate composition was measured by the same method.
The concentrations of the fermentation inhibitors such as formic acid, acetic acid, levulinic acid, furfural, and 5-hydroxymethylfurfural (HMF) produced during the pretreatment were determined by high-performance liquid chromatography (HPLC; UV230II, Elite, Dalian, China)28 with an Aminex HPX-87H (300 mm × 7.8 mm) column. The eluent was 0.1% phosphoric acid at a flow rate of 0.7 mL min−1. Ethanol formed by fermentation was analyzed using a commercial biosensor analyzer (SBA-40E, Shandong Academy of Science, China).29 The detection limit of ethanol for the instrument was 2 g L−1 and the error between two measurements was less than 1%.
2.5.2. Characterization of the untreated raw bamboo and pretreated bamboo substrates
The delignification of bamboo samples before and after the pretreatment was performed using sodium chlorite and acetic acid at 70 °C. The filtered residues were washed, dried, and dissolved in cupriethylenediamine solvent at a concentration of 2 w%. The DP of cellulose was analyzed at 20 °C using the Ubbelohde viscometer and estimated as the following formula:DP0.95 = 0.75 × η
In which η is the intrinsic viscosity of the solution.
The crystallinity index (CrI)30 of cellulose in various bamboo substrates was evaluated using XRD (Philips, X PERTPRO-30X, The Netherlands) meter with Cu Kα radiation at 40 kV and 40 mA. The intensity was recorded in the range of 5–40° at a speed of 2.5° min−1 and the CrI was calculated using the following formula:
In which I002 is the diffracted intensity of 2θ = 22.5°, and Iam is the diffracted intensity of 2θ = 18.0°.
The infrared spectra of various bamboo simples were recorded using an FT-IR spectrophotometer (Nicolet 380, Thermo Electron Corporation, USA).31 All samples were oven-dried and mixed with KBr and scanned between 500–4000 cm−1.
The surface morphology and structure of various bamboo substrates were observed by SEM (SU8010, Hitachi, Japan).32 The samples were coated with gold to make them conductive and then enlarged 500 times.
The various substrates and untreated bamboo powder were added to sodium acetate–acetic acid buffer (pH = 4.8) with 1.5% substrate content (w/v) and were equilibrated at 4 °C for 12 h before the reaction. Cellulase was added at 100 (mg g−1 substrates) and adsorbed at 4 °C for 2 h to determine the amount of cellulase adsorbed on the substrate. The protein content in the liquid supernatant was determined by the BCA method.33,34 All experiments were performed in triplicate.
2.5.3. Calculation
In which CGCY is the cellulose-to-glucose conversion yield, CECY is the cellulose-to-ethanol conversion yield, and GECY is the glucose-to-ethanol conversion yield.


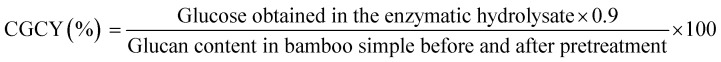


3. Results and discussion
3.1. Chemical composition of bamboo samples before and after pretreatment
During pretreatment, the reaction conditions have a significant effect on the structure and color of bamboo substrates. Therefore, in this work, we investigated the effect of temperature, reaction time, and GVL/H2O ratio on the chemical composition of bamboo substrates, and the removal (or retention) rates of the three components are shown in Fig. 1.
Fig. 1. The chemical composition of pretreated bamboo substrates and untreated raw bamboo powder.
When the pretreatment was maintained at 120 °C for 2 h, the solid retention rate dropped first and then increased as the ratio of GVL increased, and the color of substrates was gradually deepening (Fig. S1†). Specifically, when the ratio of GVL increased from 30% to 80%, the solid retention rate decreased from 62.52% to 47.44%. Although the feedstock was lost severely after the reaction, the removal rate of lignin increased from 37.48% to 87.12%, and the glucan retention rate varied between 82.53% and 87.51%. This indicated that the glucan in the bamboo powder remained intact after the pretreatment at 120 °C, while most of the xylan and arabin, which were part of hemicellulose and lignin were removed by the GVL dissolution. In contrast, the lignin removal rate decreased when the ratio of GVL was up to 90%, which was attributed to water and GVL, which could form hydrogen bonds together with the –OCH3 in the lignin structure. This effect could break the lignin–carbohydrate complex bonds and the hydrogen bonds in the lignin structure. Therefore, the optimal ratio of GVL/H2O was 8 : 2, and the glucan content of the substrate under this condition was 83.88%.
By increasing the pretreatment temperature with GVL/H2O = 8 : 2, the solid retention rate decreased from 47.44% (120 °C) to 40.73% (140 °C), while the glucan retention rate was up to 81.47% and the glucan content in the substrates increased significantly from 83.88% to 94.94%. By increasing the temperature to 160 °C, the glucan content in the substrates increased slightly to 96.55%, but the solid retention rate decreased to 9.01% sharply and the color was deepened. This may be attributed to the redeposition of dissolved lignin on the cellulose surface by the increase in temperature. Only 18.08% of the glucan was retained in the substrate under this condition and severe hydrolysis of glucan could lead to a decrease in the glucose produced in enzymatic hydrolysis. Therefore, the optimal temperature of pretreatment was 140 °C.
The solid retention rate of the substrates gradually decreased with the time extended to 4 h at 140 °C. The glucan content in substrates increased slightly from 94.94% to 97.87% while the retention rate of glucan gradually decreased from 80.20% to 56.39%, and the effect of lignin and hemicellulose removal from the substrate was not significant. This may indicate that the decrease in retention after extending the reaction time was attributed to the degradation of cellulose, and the temperature was a more significant influencing factor for cellulose hydrolysis.
The results showed that the GVL/H2O system could excellently remove a large amount of hemicellulose and lignin while retaining most of the cellulose with the maximum glucan content remaining at 97.86% in the substrate. These pretreatment effects can work together to promote the enzymatic hydrolysis efficiency and obtain more fermentable sugars. This method offers excellent potential for the conversion of bamboo to bioethanol.
3.2. Chemical composition of spent liquor
The comparative analysis of the sugar and the fermentation inhibitors in the spent liquor is shown in Table 1. The glucose content was only 0–0.07 g L−1 at the reaction temperature of 120 °C, while contents of xylose and arabinose were 5.32–7.62 g L−1 and 0.13–0.31 g L−1, respectively. This indicates that the glucan (cellulose) was preserved in the pretreated substrate and was almost intact at this temperature, while the xylan and arabin (hemicellulose) were significantly hydrolyzed. This is accountable to the results in Fig. 1, which may explain that cellulose is more difficult to hydrolyze than hemicellulose, thanks to the crystalline structure. The glucose content increased and the xylose content gradually decreased as the reaction time increased at 140 °C. This indicated that the hydrolysis of glucan was more serious at 140 °C, while xylose was converted to the fermentation inhibitor more easily. When the temperature further increased to 160 °C, the glucan was severely hydrolyzed, and the glucose content in the spent liquid was 7.53 g L−1.
Compositions of the spent liquors from GVL/H2O pretreatment at different conditions (based on 100 g oven-dry bamboo powder)a.
| Pretreatment conditions | Lignin | Sugars (g L−1) | Inhibitors (g L−1) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GVL : H2O | Temperature (°C) | Time (h) | Recovered lignin (g) | ASL (g L−1) | Araban | Xylan | Glucan | Formic acid | Acetic acid | Levulinic acid | Furfural | HMF |
| 3 : 7 | 120 | 2 | 2.50 ± 0.41 | 0.62 ± 0.06 | 0.26 ± 0.02 | 7.25 ± 0.58 | ND | 0.08 ± 0.01 | 2.15 ± 0.30 | ND | ND | ND |
| 5 : 5 | 6.98 ± 0.28 | 0.83 ± 0.02 | 0.23 ± 0.06 | 6.60 ± 0.13 | ND | 0.15 ± 0.02 | 2.52 ± 0.27 | ND | ND | ND | ||
| 7 : 3 | 7.38 ± 0.14 | 1.23 ± 0.12 | 0.12 ± 0.09 | 7.61 ± 0.85 | ND | 0.26 ± 0.06 | 3.04 ± 0.03 | ND | ND | ND | ||
| 8 : 2 | 7.68 ± 0.20 | 1.19 ± 0.31 | 0.31 ± 0.05 | 7.62 ± 0.62 | 0.06 ± 0.04 | 0.46 ± 0.03 | 3.32 ± 0.14 | ND | ND | 0.04 ± 0.01 | ||
| 9 : 1 | 7.27 ± 0.18 | 1.03 ± 0.13 | 0.13 ± 0.05 | 5.32 ± 0.15 | 0.07 ± 0.09 | 0.27 ± 0.05 | 3.93 ± 0.64 | ND | ND | 0.02 ± 0.00 | ||
| 8 : 2 | 140 | 2 | 12.24 ± 0.9 | 1.87 ± 0.08 | 0.62 ± 0.01 | 5.77 ± 0.36 | 2.87 ± 0.28 | 4.41 ± 0.45 | 2.70 ± 0.77 | ND | ND | 0.13 ± 0.06 |
| 3 | 12.12 ± 0.67 | 1.88 ± 0.19 | 0.61 ± 0.07 | 3.33 ± 0.21 | 5.14 ± 0.22 | 2.92 ± 0.06 | 2.55 ± 0.34 | 0.08 ± 0.04 | ND | 0.69 ± 0.27 | ||
| 4 | 15.34 ± 0.77 | 1.82 ± 0.01 | 0.43 ± 0.03 | 2.15 ± 0.16 | 5.93 ± 0.12 | 1.98 ± 0.15 | 2.38 ± 0.29 | 0.18 ± 0.04 | ND | 1.04 ± 0.08 | ||
| 8 : 2 | 160 | 2 | 11.23 ± 0.91 | 1.93 ± 0.15 | 0.23 ± 0.01 | 0.97 ± 0.07 | 7.53 ± 0.31 | 2.89 ± 0.71 | 4.51 ± 0.41 | 0.32 ± 0.04 | ND | 3.00 ± 0.36 |
ND: not detectable.
Fermentation inhibitors are formed during pretreatment (Table 1), with the major fermentation inhibitor, acetic acid, was measurable in the spent liquor up to 4.51 g L−1. This is likely attributed to the higher degree of acetylation of hemicellulose in neosinocalamus affins.9 The ionization of water under high-temperature pretreatment can produce a number of hydronium ions, which will catalyze the breakage of the acetyl group on hemicellulose to form acetic acid, and the acetic acid as a catalyst will further catalyze the breakage of acetylation, making the reaction go forward (Fig. 2). Other fermentation inhibitors in the spent liquor were lower. Furfural generated from the xylose degradation was not detected after the reaction, while the content of formic acid was significant. It might be argued that furfural was hydrolyzed to formyl acrylic acid and formic acid during pretreatment, which is extremely unstable and will further decompose into “WR” (water-soluble resin) acids and “SI” (solid indicator) acids.35 Low concentrations of acid (<100 mmol L−1) can promote the fermentation of ethanol, while high concentrations (>200 mmol L−1) will inhibit the fermentation.36,37 In this study, the content of levulinic acid was a trace, with a maximum of 0.32 g L−1, and the maximum content of total carboxylic acid was 140.87 mmol L−1, which implied that the inhibition of subsequent fermentation was weak. The content of HMF generated from glucose dehydration gradually increased due to significant hydrolysis of cellulose when the temperature was > 140 °C, and reaching the content of 3 g L−1 at 160 °C, which was also less than the 5 g L−1 that inhibited yeast significantly.38 Moreover, the increase of HMF content in the spent liquor did not lead to a decrease in glucose, which is likely explained by the fact that the hydrolysis of dextran into glucose was faster than the dehydration of glucose into HMF during the pretreatment.
Fig. 2. The inhibitors formed reactions during the pretreatment.
Besides sugars and fermentation inhibitors, the content of recovered lignin from spent liquors is also summarized in Table 1. Part of the lignin in the spent liquor could be recovered by watering down, and the recovery was higher with the enhancement delignification. When the reaction was reversed in the GVL : H2O = 8 : 2 system at 140 °C for 4 h, 15.34 g of lignin could be recovered from the spent liquor, accounting for 64.00% of the lignin content in the bamboo raw material.
3.3. Characterization of bamboo substrates
3.3.1. Analysis of the DP of cellulose
DP of cellulose is a notable factor in characterizing cellulose supramolecular structure, which can reflect the length of cellulose chains and has an important influence on enzymatic hydrolysis. The DP of the various substrates before and after pretreatment was measured using the Ubbelohde viscometer (Table 2). The decrease in the DP of the substrate after pretreatment indicates that the cellulose chains were disconnected and more reductive end groups were exposed to the higher temperature and the acid concentration in the pretreatment system,39,40 which could increase the accessibility of the substrate to cellulase. At 120 °C, the DP declined with a not significant increase in the GVL ratio (from 1562 to 967–1282). This may be explained by the fact that only low-energy bonds between cellulose and hemicellulose, lignin, or molecular chains in the non-crystalline regions of cellulose were broken at 120 °C, while the parts connected by high-energy bonds were not likely to be broken. The DP of the substrates dropped sharply from 1039 to 464 on raising the temperature from 120 °C to 140 °C. Under this condition, some of the molecular chains in the crystallization region break extensively, and cellulose was hydrolyzed severely. Therefore, it can be inferred that the GVL/H2O pretreatment system can break and de-polymerize the cellulose in the substrate.
DP and cellulase adsorption of bamboo powder before and after pretreatment.
| Samples | DP | Cellulase adsorption (mg g−1) | ||
|---|---|---|---|---|
| GVL : H2O | Temperature (°C) | Time (h) | ||
| Bamboo powder | 1562 ± 10 | 65.09 ± 0.75 | ||
| 3 : 7 | 120 | 2 | 1282 ± 14 | 63.86 ± 0.80 |
| 5 : 5 | 1299 ± 3 | 61.15 ± 0.89 | ||
| 7 : 3 | 1074 ± 12 | 62.84 ± 0.05 | ||
| 8 : 2 | 1039 ± 9 | 68.94 ± 1.63 | ||
| 9 : 1 | 967 ± 18 | 65.96 ± 1.11 | ||
| 8 : 2 | 140 | 2 | 464 ± 4 | 71.79 ± 0.56 |
| 3 | 360 ± 5 | 73.60 ± 1.38 | ||
| 4 | 312 ± 7 | 70.41 ± 1.02 | ||
| 8 : 2 | 160 | 2 | 293 ± 3 | 70.06 ± 0.46 |
3.3.2. XRD analysis of the CrI of cellulose
CrI is also an important factor that reflects the efficiency of enzymatic hydrolysis. In this work, the change in the crystal structure of the substrates could be measured by XRD. As seen in Fig. 3, all the bamboo samples displayed similar peaks at 16.5 °, 22.5 °, and 34.9 °, which matched the crystal surfaces (101), (002), and (034), respectively, of the crystalline cellulose I41 implying that the crystalline structure of bamboo substrates after pretreatment remained cellulose I. The diffraction peaks at 22.6 ° in the substrates after the pretreatment was narrower and higher, and the CrI values increased from 44.20% to 56.10–71.56%. This is due to the considerable increase in the crystalline region of cellulose in the substrate. This indicates that the cellulose structure was gradually exposed after pretreatment, which could increase the accessibility of enzymes in the subsequent process and promote enzymatic hydrolysis.42
Fig. 3. XRD patterns of pretreated bamboo substrates and untreated raw bamboo powder.
Moreover, the CrI/glucan ratio is probably to be a more reasonable indicator of the CrI of cellulose.43 As the pretreatment time at 140 °C was extended, the CrI of pretreated bamboo substrates to its glucan content (CrI/glucan) increased slightly, implying that the crystallinity of glucan itself increased slightly upon extending the pretreatment time. This may be attributed to the decrease in polymerization degree that could expose more hydroxyl groups and hydrogen bonding sites. However, the CrI/glucan of pretreated substrates (0.62–0.83) was much lower than that of the untreated bamboo powder (0.92) by the removal of lignin and hemicellulose, which could promote the subsequent enzymatic hydrolysis.
3.3.3. FT-IR spectroscopy analysis of the substrates
The changes in the absorption spectral bands of various functional groups in the pretreated substrates are shown in Fig. 4. The IR peaks before and after pretreatment were approximately the same, indicating that the cellulose structure was still characteristic of the bamboo powder after the pretreatment. While the intensity of some absorption peaks changed significantly, indicating that the structure and chemical composition of the pretreated bamboo substrate changed. The peaks around 1511 cm−1 and 1604 cm−1 correspond to the aromatic skeletal vibration, the peak at 1255 cm−1 is attributed to the C–O plane stretching vibration of the aryl group, and the peak at 833 cm−1 is assigned to the C–H single bond bending of stringy units. The intensity of these peaks gradually decreased corresponding to a sharp decrease in the lignin content of bamboo substrates from 24.19% to 1.56%, as shown in Fig. 1. The increase in the strength of characteristic bands at approximately 1034 cm−1 and 1057 cm−1, is designated to the stretching vibration peak of cellulosic alcohol –OH. The enhancement of these peaks also confirmed an increase in the cellulose content of the substrate as the pretreatment temperature increased. The C O stretching vibration, which matched the characteristic peak of the acetyl in hemicellulose, was observed around 1640 cm−1 and 1750 cm−1. These peaks were weaker after pretreatment at 140 °C for 4 h, indicating the significant removal of hemicellulose during the pretreatment, and leading to an increase in the acetic acid concentration in the spent liquor to 2.38 g L−1. The strong band between 3000 and 3600 cm−1 corresponds to the O–H stretching vibration and the peak near 2900 cm−1 corresponds to the C–H stretching vibration. These peak intensities demonstrate that the β-1,4 glycosidic bond is broken and the DP decreased upon pretreatment, exposing more of the hydroxyl groups. In addition, the weakening of the characteristic peaks of the crystalline region in cellulose at 1450 cm−1, with the CrI/glucan results, indicates that the crystallinity of the cellulose fraction was reduced after the reaction.
Fig. 4. FT-IR spectra of pretreated bamboo substrates and untreated raw bamboo powder.
3.3.4. Micromorphology of the pretreated bamboo substrates
The surface morphology of the substrates before and after pretreatment was studied by SEM analysis. As shown in Fig. 5, the surface of the untreated bamboo fibers was well arranged, uninterrupted, and smooth. After pretreatment at 120 °C for 2 h with GVL/H2O = 8 : 2, the surface of the bamboo fibers was slightly curling, and the internal structure was exposed. These effects promoted the access of cellulase to the inner fibril bundles44 and eventually led to a notable increase in CGCY (67.12%) compared to the raw material (22.59%) after enzymatic hydrolysis. After extending the pretreatment time to 4 h at 140 °C, the morphology of bamboo fibers was obviously disordered and numerous cracks appeared on the surface. The destruction of the bamboo fiber structure exposed more enzymatic reaction sites, which could result in a significant increase in the glucose yield to 93.18% after hydrolysis. In summary, the SEM revealed that the GVL/H2O pretreatment system could effectively break the physical barrier on the surface of bamboo powder and improve the CGCY of enzymatic hydrolysis.
Fig. 5. SEM images for pretreated bamboo substrates and untreated raw bamboo powder (×500 magnification).
3.3.5. The cellulase adsorption on bamboo substrates
As seen in Fig. S1,† the color of the substrates was gradually deepened as the ratio of GVL increased at 120 °C, indicating that the recombination of the removed lignin on the surface of substrates was violent after pretreatment. Therefore the cellulase adsorption on the bamboo substrates in the GVL/H2O pretreatment system can be influenced by both the content of lignin and the degree of recombination of dissolved lignin in the cellulose surface.45 The cellulase adsorption of substrates under different conditions is shown in Table 2. When the ratio of GVL of the pretreatment system was less than 80% at 120 °C, the cellulase adsorption of the pretreated substrates was less than that of the untreated bamboo powder. This may be explained by the fact that there is still a large amount of lignin in the substrate at this temperature, which can form a physical barrier to cellulose. In addition, dissolved lignin was re-depolymerized on the cellulose surface, which further hinders the cellulase adsorption efficiency. By increasing the GVL content of the pretreatment system, it was seen that as the cellulose content of the substrates increased, the higher was the adsorption of the pretreated substrate to the enzymes, indicating that despite the dissolved lignin deposited on the surface, the effect of significant lignin removal was more pronounced under these conditions. This may be attributed to the decrease in DP and CRI increasing the porosity of the biomass.46 After increasing the temperature to 140 °C, the color of the recovered substrates was lighter, meaning that the degree of the recombination of dissolved lignin at that temperature diminished as a large amount of lignin was removed. Therefore, the adsorption of enzymes under these conditions is mainly attributed to the lignin content in the substrates and the degree of crystallinity together. Therefore, the maximum adsorption of the pretreated substrates was achieved after 3 h of the reaction.
3.4. Enzymatic hydrolysis of the bamboo substrates
To evaluate the effect of the substrates, all bamboo samples before and after pretreatment were enzymatically hydrolyzed for 48 h, and CGCY is shown in Fig. 6. The results showed that the CCGY of the pretreated substrate ranged from 56.55% to 93.18%, all of which were significantly better than that of the untreated bamboo powder (22.59%). This is due to the obvious removal of lignin and hemicellulose from the bamboo powder by pretreatment, which can damage the cellular structure of the bamboo powder, and effectively improve the accessibility of cellulase and the hydrophilicity of the substrate.
Fig. 6. Effect of pretreatment conditions (A) GVL dosage, (B) temperature, and (C) time on the CGCY from enzymatic hydrolysis of various bamboo samples. (D) The CGCY calculated based on bamboo samples before and after pretreatment.
The rate of the enzymatic reaction was influenced by a combination of factors. As shown in Fig. 6A, when the GVL content of the system was increased to 50% at 120 °C for 2 h, the reaction rate gradually increased in the first 24 h of the enzymatic reaction. This was due to the removal of hemicellulose and lignin from the substrate, and the elimination of the physical barrier of lignin to the enzyme. When the ratio of GVL to the system continued to increase, the removed lignin was redeposited on the surface of the substrate, which will deepen the color of the substrate. This will not cause any productive adsorption of cellulase, and decrease the reaction rate gradually instead. Therefore, the reaction proceeded most rapidly when GVL : H2O = 5 : 5, which was almost completed within 12 h. After extending the time of enzymatic hydrolysis, the CGCY increased firstly and then decreased with the increase in the GVL rate, which was attributed to the fact that the amount of glucose produced after 48 h was mainly determined by the cellulose content of the substrate.
The effect of pretreatment temperature and time on the enzymatic digestion of the substrate was also investigated in this work. As shown in Fig. 6B, the CGCY increased from 67.12% to 92.18% after 48 h of hydrolysis when the pretreatment temperature was increased to 140 °C. However, when the pretreatment temperature was further increased to 160 °C, the CCGY did not increase, instead, it decreased to 68.84% caused by the decrease of cellulase adsorption. Therefore, 140 °C was selected as the optimal pretreatment temperature.
The substrates pretreated for 3 h had a greater rate of enzymatic digestion in the first 6 hours of enzymatic hydrolysis than substrates pretreated for 2 h and 4 h. The longer reaction time on the one hand can reduce the lignin content of the substrate and improve the accessibility to the enzyme. On the other hand, the slight elevation of the crystallinity of the cellulose fraction makes the enzymatic process take a longer time to break the barrier. This is in accord with the enzyme adsorption effect and the CRI/cellulose illustrated in the previous section. After the enzymatic hydrolysis for 48 h, the enzymatic yield finally increased slightly from 91.52% to 93.18% with the elevated cellulose content in the pretreated substrate.
As shown in Fig. 6D, the CGCY calculated based on pretreated substrates was optimal after 4 h of pretreatment. However, as the substrate recovery decreases substantially after extended pretreatment time, a great quantity of cellulose entered the spent liquor during the pretreatment. They are either hydrolyzed to glucan or further converted to fermentation inhibitors. Thus, for cellulose in the raw bamboo material, the highest CGCY was achieved at a pretreatment time of 2 h, which was 73.39%. The results show that the effect of the enzymatic hydrolysis was enhanced by the significant removal of lignin and hemicellulose from the substrate during the pretreatment, which reduced the DP and crystallinity of the CrI of the substrates and increased the adsorption of cellulase.
3.5. Ethanol fermentation of enzymatic hydrolysates
The fermentability of the enzymatic hydrolysate was evaluated using saccharomyces cerevisiae yeast, and the results are presented in Fig. 7. As can be seen, glucose was not detected in the liquid supernatant after 48 h of fermentation. Since the glucose generated by the enzymatic hydrolysis of the unpretreated bamboo powder was low, most of the glucose in the solution was consumed as the nutrient for yeast, and the GECY was only 34.34%. In contrast, the GECY of the pretreated substrates was between 83.96% and 104.81%. This means that only a few glucose molecules were consumed by the yeast as a nutrient for growth, while most of them were fermented into ethanol and a few of the xylose remaining in the enzymatic hydrolysate or glucose in the process of activating yeast was fermented at the same time.
Fig. 7. The CECY and The GECY after a 48 h fermentation.
The CECY calculated based on pretreated substrates was significantly increased after pretreatment. The CECY was 86.92% when GVL : H2O = 8 : 2 at 140 °C for 4 h, which was 12.45 times higher than that of the untreated bamboo powder. For cellulose in the bamboo powder, the highest CECY was achieved by pretreatment for 2 h under the same conditions, and 67.00% of the cellulose of bamboo material could be converted to bioethanol.
Table 3 summarizes the CGCY and CECY obtained in this study and previous studies by the different pretreatment systems. The CECY in this study was lower compared to that obtained using Eucalyptus obliqua sawdust under the same pretreatment system. This may be attributed to the acid generated during the pretreatment of bamboo causing a slight decrease in the retention of glucan, or to the greater influence on enzymes and yeast by microorganisms in the enzymatic hydrolysate and fermentation systems of different biomasses. The CECY of this study was comparable with other bamboo species, and in the case of Neosinocalamus affinis, the CECY obtained in the GVL/H2O pretreatment system was also better than that obtained using other methods. Together, the results showed that the GVL/H2O pretreatment system is an effective pretreatment system for converting bamboo powder into fermentable sugars for bioethanol production.
Comparison of the CGCY and CECY in this study and the previous studiesab.
| Biomass | Condition | CGCY (%) | CECY (%) | Ref. |
|---|---|---|---|---|
| Bamboo (Neoinocalamus affinis) | 80% GVL, 140 °C, 120 min, 75 mmol L−1 H2SO4 | 73.39 | 67.00 | This study |
| Bamboo | 60% GVL, 160 °C, 20 min, 50 mmol L−1 H2SO4 | 89.48 | —c | 24 |
| Eucalyptus obliqua sawdust | 42.5% GVL, 150 °C, 75 min, 50 mmol L−1 H2SO4 | —c | 80.47 | 23 |
| Bamboo (Yushania alpina) | Pressurized hot water pretreatment at 128 °C for 10 min | —c | 23.81 | 47 |
| Bamboo (Phyllostachys edulis) | Steam explosion (212.3 °C, 5 min), green liquor (total titratable alkali 31.01%, 28.01 min, 166.41 °C) | 100 | 67.29 | 48 |
| Bamboo (Phyllostachys edulis) | Alkaline pre-extraction (5% NaOH, 70 °C, 6 h), acid catalyzed steam pretreatment (3% SO2, 190 °C, 10 min) | 85.66 | 68.90 | 49 |
| Bamboo (Phyllostachys pubescens) | Hydrogen peroxide–acetic acid 85 °C, 120 min | 84.22 | 66.37 | 50 |
| Bamboo (Neosinocalamus affinis) | 3% H2O2 with 1% ethanol, 100 °C, 60 min | 79.88 | 63.39 | 51 |
| Bamboo (Neoinocalamus affinis) | 0.5% NaOH, 170 °C, for 2 h | 53.30 | 36.49 | 52 |
CGCY: the cellulose-to-glucose conversion yield.
CECY: the cellulose-to-ethanol conversion yield.
—: there are no relevant data can be found in the literature.
3.6. Mass balance of the whole process from raw bamboo to bioethanol
The mass balance of the substrates after pretreatment at 140 °C for 2 h with GVL/H2O was 8 : 2, as shown in Fig. 8. As was shown, about 40.73 g of the dried substrates could be recovered after pretreatment, including 38.67 g of glucan, 1.26 g of xylan and arabin, and 1.00 g of lignin. This indicates that over half of the bamboo powder was degraded by GVL/H2O pretreatment. Meanwhile, 12.24 g of lignin could be recovered by precipitation, which accounted for 50.60% of the lignin in the raw material. Meanwhile, the carbohydrates such as xylose 6.38 g, glucose 2.87 g, lignin 2.87 g, and the fermentation inhibitor, including HMF 0.19 g and 4.4 g formic acid, were retained in the spent liquor. HMF was generated from the degradation of glucose (Table 1). The levulinic acid produced in the process of formic acid generation from HMF and the furfural generated from xylose degradation were not detected, indicating that most of the formic acid in the hydrolysate under this condition was generated from the degradation of furfural. This means that 57.12% of the hemicellulose was converted into formic acid in the spent liquor by pretreatment. 35.38 g of glucose could be produced by enzymatic hydrolysis and the subsequent bioethanol yield was 18.35 g. The CGCY of the substrate was 91.49% and the GECY after fermentation was 101.48%, which means obtaining the highest ethanol yield of 18.35 (kg ton−1 of bamboo powder), and the bioethanol yield improved by about 9.17-fold compared to the direct conversion of untreated bamboo powder. The results showed that this pretreatment system is an effective way to co-produce fermentable glucose, lignin, and bioethanol from bamboo.
Fig. 8. Mass balances of bamboo substrates of ethanol production by GVL/H2O pretreatment (at 140 °C for 120 min with GVL : H2O = 8 : 2 and 75 mmol L−1 H2SO4).
4. Conclusion
In this work, the GVL pretreatment system could remove hemicellulose and lignin from bamboo powder effectively, and increase the cellulose content in the substrate. These effects combine to reduce the DP and CrI of the cellulose in the substrate and therefore enhance the enzymatic adsorption, thereby, enhancing the enzymatic hydrolysis of the bamboo powder. The maximum concentration of organic acids produced by pretreatment that would affect the fermentation was 140.87 mmol L−1, which was less inhibitory to the subsequent fermentation process. The optimal CECY of the substrate after pretreatment was 86.92% (GVL : H2O = 8 : 2, 140 °C, 4 h), and the highest CEGY of bamboo powder was 67.00% (GVL : H2O = 8 : 2, 140 °C, 2 h). The highest yield of bioethanol was 183.5 kg ton−1 of bamboo powder, which was 9.17 folds of direct conversion from the raw bamboo powder. Under the above conditions, 54.71% of lignin in bamboo powder can be recovered from spent liquor. This indicated that the GVL/H2O pretreatment system plays an important role in improving the effect of bamboo hydrolysis and fermentation, to be considered an environmentally friendly and effective method for improving the commercialization of biomass in the bioethanol industry.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
This research was supported by the Fundamental Research Fund of the International Centre for Bamboo and Rattan (Grant No. 1632019017).
Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2ra02421g
References
- Yu Q. Bai R. Wang F. Zhang Q. Sun Y. Zhang Y. Qin L. Wang Z. Yuan Z. J. Chem. Technol. Biotechnol. 2020;95:751–757. [Google Scholar]
- Liu S. He H. Fu X. Yuan T. Wang Q. Yang G. Zhang H. Ding M. Liao C. Bioresources. 2019;14:21–30. [Google Scholar]
- Jiang Z. Wang G. Fei B. Yu W. For. Res. 2002;15:712–718. [Google Scholar]
- Yang J. Xu H. Jiang J. Zhang N. Xie J. Zhao J. Wei M. Appl. Biochem. Biotechnol. 2021;193:1–12. doi: 10.1007/s12010-020-03472-x. [DOI] [PubMed] [Google Scholar]
- Wang H. Zuo X. Wang D. Bi Y. J. Cent. South Univ. For. Technol. 2017;37:29–38. [Google Scholar]
- Zhang H. Zhao M. Zhao T. Li L. Zhu Z. Green Chem. 2016;18:2296–2301. [Google Scholar]
- Li Z. Jiang Z. Fei B. Chem. Ind. Eng. 2012;31:533–540. [Google Scholar]
- Li Y. Xu B. Zhang Q. Jiang S. Journal of Forestry Engineering. 2016;1:2–7. [Google Scholar]
- Zhang B. Guan Y. Bian J. Peng F. Ren J. Yao C. Sun R. Cellul. Chem. Technol. 2016;50:189–198. [Google Scholar]
- Zhan Y. Cheng J. Liu X. Huang C. Wang J. Han S. Fang G. Meng X. Ragauskas A. J. Bioresour. Technol. 2022;349:126854. doi: 10.1016/j.biortech.2022.126854. [DOI] [PubMed] [Google Scholar]
- Zugenmaier P. Prog. Polym. Sci. 2001;26:1341–1417. [Google Scholar]
- Yin X. Wei L. Pan X. Liu C. Jiang J. Wang K. Front. Plant Sci. 2021;12:670061. doi: 10.3389/fpls.2021.670061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbe M. Wallberg O. Biotechnol. Biofuels. 2020;12:294. doi: 10.1186/s13068-019-1634-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey P. Pal P. Kevin J. D. Das D. B. Rev. Chem. Eng. 2020;36:333–367. [Google Scholar]
- Li K. Wan J. Wang X. Wang J. Zhang J. Ind. Crops Prod. 2016;83:414–422. [Google Scholar]
- Mohan M. Deshavath N. N. Banerjee T. Goud V. V. Dasu V. V. Ind. Eng. Chem. Res. 2018;57:10105–10117. [Google Scholar]
- Ninomiya K. Soda H. Ogino C. Takahashi K. Shimizu N. Bioresour. Technol. 2013;128:188–192. doi: 10.1016/j.biortech.2012.10.097. [DOI] [PubMed] [Google Scholar]
- Dai N. H. Huynh K. T. T. Nguyen T. A. D. Do V. V. T. Tran M. V. Waste Biomass Valorization. 2021;12:4103–4112. [Google Scholar]
- Li Z. Fei B. Jiang Z. Bioresources. 2015;10:1037–1047. [Google Scholar]
- Huy Q. Zaitseva A. Pokk J. P. Stahl M. Alopaeu V. Sixta H. ChemSusChem. 2016;9:2939–2947. doi: 10.1002/cssc.201600655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le H. Q. Pokki J. P. Borrega M. Uusi-Kyyny P. Alopaeus V. Sixta H. Ind. Eng. Chem. Res. 2018;57:15147–15158. doi: 10.1021/acs.iecr.8b03723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M. Yan Z. Zhang X. Xu F. Sun R. Bioresour. Technol. 2016;200:23–28. doi: 10.1016/j.biortech.2015.09.111. [DOI] [PubMed] [Google Scholar]
- Trevorah R. M. Huynh T. Vancov T. Othman M. Z. Bioresour. Technol. 2018;250:673–682. doi: 10.1016/j.biortech.2017.11.084. [DOI] [PubMed] [Google Scholar]
- Li S. Li M. Yu P. Fan Y. Shou J. Sun R. Bioresour. Technol. 2017;230:90–96. doi: 10.1016/j.biortech.2017.01.041. [DOI] [PubMed] [Google Scholar]
- Adney B. and Baker J., Measurement of cellulase activities, National Renewable Energy Laboratory, Golden, CO, 1996 [Google Scholar]
- Sluiter A., Hames B., Ruiz R., Scarlata C. and Crocker D., National Renewable Energy Laboratory (NREL) Analytical Procedures, 2004 [Google Scholar]
- Li Z. Fei B. Jiang Z. Chem. Technol. Fuels Oils. 2014;50:189–196. [Google Scholar]
- Zhang L. Liu Y. Li Z. RSC Adv. 2019;9:30489–30495. doi: 10.1039/c9ra06475c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z. Fei B. Li Z. Bioresour. Technol. 2016;214:876–880. doi: 10.1016/j.biortech.2016.05.025. [DOI] [PubMed] [Google Scholar]
- Segal L. Creely J. J. Martin A. E. Conrad C. M. Text. Res. J. 1959;29:786–794. [Google Scholar]
- Pandey K. K. J. Appl. Polym. Sci. 1999;71:1969–1975. [Google Scholar]
- Ma Y. Xia Q. Liu Y. Chen W. Liu S. Wang Q. Liu Y. Li J. Yu H. ACS Omega. 2019;4:8539–8547. doi: 10.1021/acsomega.9b00519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg R. C. Dong Y. Schwamborn K. Knuechel R. J. Biochem. Biophys. Methods. 2005;65:13–19. doi: 10.1016/j.jbbm.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Kumar R. Wyman C. E. Enzyme Microb. Technol. 2008;42:426–433. [Google Scholar]
- He J. Liu M. Huang K. Walker T. W. Maravelias C. T. Dumesic J. A. Huber G. Green Chem. 2017;19:3642–3653. [Google Scholar]
- Palmqvist E. Hahn-Hagerdal B. Bioresour. Technol. 2000;74:25–33. [Google Scholar]
- Dunlop P. A. Ind. Eng. Chem. Res. 1948;40:204–209. [Google Scholar]
- Nilvebrant N. O. Reimann A. deSousa F. Kleen M. Palmqvist E. Can. Pulp Pap. Assoc. 1997:791–794. [Google Scholar]
- Lin W. Xing S. Jin Y. Lu X. Huang C. Yong Q. Bioresour. Technol. 2020;306:123163. doi: 10.1016/j.biortech.2020.123163. [DOI] [PubMed] [Google Scholar]
- Meng X. Bhagia S. Wang Y. Zhou Y. Pu Y. Dunlap J. R. Shuai L. Ragauskas A. J. Yoo C. G. Ind. Crops Prod. 2020;146:112144. [Google Scholar]
- Wei W. Chen Z. Wang H. Ling R. Jin Y. Ind. Crops Prod. 2021;165:113440. [Google Scholar]
- Gong C. Cao N. Du J. Tsao G. T. Adv. Biochem. Eng./Biotechnol. 1999;65:207–241. doi: 10.1007/3-540-49194-5_9. [DOI] [PubMed] [Google Scholar]
- Alam A. Wang Y. Liu F. Kang H. Tang S. Wang Y. Cai Q. Wang H. Peng H. Li Q. Zeng Y. Tu Y. Xia T. Peng L. Renewable Energy. 2020;159:1128–1138. [Google Scholar]
- Zhang L. Li Z. J. Biobased Mater. Bioenergy. 2020;14:265–272. [Google Scholar]
- Huang C. Lin W. Lai C. Li X. Jin Y. Yong Q. Bioresour. Technol. 2019;285:121355. doi: 10.1016/j.biortech.2019.121355. [DOI] [PubMed] [Google Scholar]
- Alam A. Zhang R. Liu P. Huang J. Wang Y. Hu Z. Madadi M. Sun D. Hu R. Ragauskas A. Tu Y. Biotechnol. Biofuels. 2019;12:99. doi: 10.1186/s13068-019-1437-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsegaye M. Chandravanshi B. S. Feleke S. Redi-Abshiro M. Biofuels. 2021;12:1041–1050. [Google Scholar]
- Gao H. Wang Y. Yang Q. Peng H. Li Y. Zhan D. Wei H. Lu H. Bakr M. EI-Sheekh M. M. Qi Z. Peng L. Lin X. Renewable Energy. 2021;175:1069. [Google Scholar]
- Yuan Z. Li G. Wei W. Wang J. Fang Z. Energy. 2020;196:117156. [Google Scholar]
- Song Y. Lee Y. Lee D. Nguyen D. Bae H. Fuel. 2021;307:121892. [Google Scholar]
- Huang C. Zhan Y. Du X. Zhou Y. Yu L. Meng X. Jiao J. Fang G. Ragauskas A. Energy Convers. Manage. 2020;224:113365. [Google Scholar]
- Yang H. Shi Z. Xu G. Qin Y. Deng J. Yang J. Bioresour. Technol. 2019;274:261–266. doi: 10.1016/j.biortech.2018.11.088. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.










