Abstract
We found 73.1 to 96.9% similarity by aligning the cytolytic enterotoxin gene of Aeromonas hydrophila SSU (AHCYTOEN; GenBank accession no. M84709) against aerolysin genes of Aeromonas spp., suggesting the possibility of selecting common primers. Identities of 90 to 100% were found among the eight selected primers from those genes. Amplicons obtained from Aeromonas sp. reference strains by using specific primers for each gene or a cocktail of primers were 232 bp long. Of hybridization group 4/5A/5B (HG4/5A/5B), HG9, and HG12 or non-Aeromonas reference strains, none were positive. PCR-restriction fragment length polymorphism (PCR-RFLP) with HpaII yielded three types of patterns. PCR-RFLP 1 contained two fragments (66 and 166 bp) found in HG6, HG7, HG8, HG10, and HG11. PCR-RFLP 2 contained three fragments (18, 66, and 148 bp) found in HG1, HG2, HG3, and HG11. PCR-RFLP 3, with four fragments (7, 20, 66, and 139 bp), was observed only in HG13. PCR-amplicon sequence analysis (PCR-ASA) revealed three main types. PCR-ASA 1 had 76 to 78% homology with AHCYTOEN and included strains in HG6, HG7, HG8, HG10, and HG11. PCR-ASA 2, with 82% homology, was found only in HG13. PCR-ASA 3, with 91 to 99% homology, contained the strains in HG1, HG2, HG3, and HG11. This method indicated that 37 (61%) of the 61 reference strains were positive with the primer cocktail master mixture, and 34 (58%) of 59 environmental isolates, 93 (66%) of 141 food isolates, and 100 (67%) of 150 clinical isolates from around the world carried a virulence factor when primers AHCF1 and AHCR1 were used. In conclusion, this PCR-based method is rapid, sensitive, and specific for the detection of virulence factors of Aeromonas spp. It overcomes the handicap of time-consuming biochemical and other DNA-based methods.
Aeromonas spp. comprise mesophilic motile and psychrophilic nonmotile gram-negative ubiquitous bacteria. Worldwide studies have demonstrated that Aeromonas spp. are universally distributed and widely isolated from clinical (38), environmental (29, 38, 44), and food samples (2, 5), where they may grow even at low temperatures (46). They are an example of emerging bacterial pathogens. Even though they have been recognized as primary fish pathogens for a long time, their status as primary human pathogens was not clear until recently (18, 42). It is estimated that aeromonads may cause up to 13% of the reported gastroenteritis cases in the United States (10). Aeromonas spp. are opportunistic pathogens that are at the same time infectious (4, 33) and enterotoxigenic (12, 16).
Routine detection of pathogenic Aeromonas spp. was not efficient until now due to the diversity of the hybridization groups (HGs), and also, 17% of isolates could not be grouped into any of the known HGs with biochemical tests (19).
By definition, the detection of a pathogen requires rapid and specific methods for isolation, identification, and enumeration. Such procedures may assist in the control of potentially pathogenic microorganisms from environmental and food samples, which are mostly regarded as the main transport vectors to human populations.
According to the International Commission on Microbiological Specifications for Food (30), many classical microbial procedures for the detection of Aeromonas spp. are laborious and time consuming or do not allow quantitative assessment of these organisms. A complete review of methodologies for the isolation, identification, and enumeration of Aeromonas spp. from clinical, environmental, and food samples has been done by Joseph and coworkers (34). Since then, other isolation methods have been evaluated and compared for the ability to isolate or detect Aeromonas spp. in food and environmental items (20, 48, 51) and this indicates the need for a reliable, universal, and standard method for the detection of these pathogens in clinical, environmental, and food samples.
The identification of mesophilic Aeromonas spp. remains difficult in routine surveys due to the complex classification of Aeromonas bacteria into phenotypically defined phenospecies and into genomospecies delineated on the basis of DNA-DNA hybridization studies. At least 15 genospecies or HGs related to 14 phenospecies have been validated or proposed (13). Biochemical allocation of unknown aeromonads into HGs usually involves a large series of tests, ranging from 9 (1) to 136 (35) in number. Well-equipped laboratories can perform cellular fatty acid methyl ester (FAME) composition analysis (27), DNA fingerprinting by amplified fragment length polymorphism analysis (28), multilocus enzyme electrophoresis (MEE), or ribotyping (50) to identify Aeromonas spp. to the genomospecies level. Nevertheless, the above-mentioned methods cannot reveal the pathogenic or nonpathogenic character of unknown Aeromonas sp. isolates (39).
An interesting approach for the direct detection of potentially pathogenic Aeromonas sp. isolates is the use of virulence determinants as genetic markers. A significant number of Aeromonas sp. virulence genes have been described, including the aerolysin genes (15, 24, 25, 26); hemolysin genes from A. hydrophila and A. sobria (22), A. salmonicida (23), and A. caviae (52); an extracellular lipase gene from A. hydrophila (7); a cytolytic enterotoxin gene of A. hydrophila (16); and a hemolytic toxin gene of A. trota (36). The first study using the PCR technique for specific detection of an A. hydrophila virulence gene, the hole-forming toxin gene (25), was accomplished in Canada by Pollard and coworkers (47). Using a pair of primers designed on the basis of this known gene sequence, the authors were able to detect beta-hemolysin-positive A. hydrophila strains from patients with diarrhea, whereas control strains of hemolytic A. sobria, nonhemolytic Aeromonas spp., and A. caviae strains, even those producing cytotoxin or enterotoxin, were negative with these primers. A Swedish study (8) using the same primers confirmed the Canadian findings (47) when screening for the presence of the aerolysin gene in water, fish, and foods isolates. Another PCR technique for the detection of two hemolysin genes of A. sobria was conducted in Japan (49), where none of the virulence factors found with other Aeromonas spp. were detected.
The present report describes the development of a new PCR method that detects cytolytic enterotoxin and aerolysin genes in Aeromonas spp. by using one pair of primers for a specific gene or a cocktail of primers for the most known virulence genes of Aeromonas spp. In combination with PCR-restriction fragment length polymorphism (PCR-RFLP) and/or PCR-amplicon sequence analysis (PCR-ASA), the described PCR assay also allows partial determination of the HG of a potentially virulent isolate from a clinical, environmental, or food sample.
MATERIALS AND METHODS
Bacterial strains and cultural media. (i) Bacterial strains.
For the reference strains and the clinical, environmental, and food isolates used in this investigation, see Tables 2 and 3. All strains were identified by either FAME, MEE, or ribotyping as described previously (6, 27, 39, 50) or by serotyping with a unpublished serology test developed at the National Veterinary Laboratory, Aarhus, Denmark.
TABLE 2.
Results of PCR and characteristics of the 232-bp amplicons of reference strains
| Organism | Reference no. | Source | Country of origin | PCRa | No. of PCR-RFLPb fragments | % Similarity by PCR-ASAc | Gene similarity |
|---|---|---|---|---|---|---|---|
| A. hydrophila (HG1) | |||||||
| A17d | LMG 13439 | Unknown | Germany | + | 3 | 96 | Hemolysin-enterotoxin |
| A19 | LMG 13440 | Unknown | Germany | − | |||
| A22 | Not available | Feces | Switzerland | − | |||
| A34 | LMG 13441 | Feces | Switzerland | − | |||
| A52 | LMG 13442 | Feces | Switzerland | − | |||
| A82 | LMG 13656 | Feces | Switzerland | − | |||
| A145 | LMG 13657 | Feces | Switzerland | + | 3 | 99 | Hemolysin-enterotoxin |
| A162 | LMG 13658 | Feces | Switzerland | − | |||
| A167 | LMG 13659 | Feces | Switzerland | + | 3 | 98 | Hemolysin-enterotoxin |
| A306 | ATCC 7966Te | Milk | United States | + | 3 | 97 | Hemolysin-enterotoxin |
| A. bestiarum (HG2) | |||||||
| A2 | LMG 13445 | Human | Germany | + | 3 | 92 | Hemolysin-enterotoxin |
| A13 | LMG 13446 | Environmental | Germany | − | |||
| A39 | LMG 13447 | Water | Switzerland | + | 3 | 91 | Hemolysin-enterotoxin |
| A169 | LMG 13448 | Feces | Switzerland | + | 3 | 94 | Hemolysin-enterotoxin |
| A307 | CDC 9533-76T | Fish | France | + | 3 | 94 | Hemolysin-enterotoxin |
| A908 | ATCC 14715 | Fish | France | + | 3 | 94 | Hemolysin-enterotoxin |
| A936 | LMG 13664 | Unknown | United States | + | 3 | 94 | Hemolysin-enterotoxin |
| A937 | LMG 13665 | Unknown | United States | + | 3 | 94 | Hemolysin-enterotoxin |
| A976 | LMG 13666 | Water | United States | + | 3 | 94 | Hemolysin-enterotoxin |
| A1613 | LMG 13667 | Water | United States | + | 3 | 95 | Hemolysin-enterotoxin |
| A. hydrophila (HG3) | |||||||
| A7 | LMG 13452 | Environmental | Germany | + | 3 | 91 | Hemolysin-enterotoxin |
| A8 | LMG 13453 | Environmental | Germany | + | 3 | 91 | Hemolysin-enterotoxin |
| A14 | LMG 13674 | Environmental | Germany | + | 3 | 91 | Hemolysin-enterotoxin |
| A15 | LMG 13675 | Environmental | Germany | + | 3 | 91 | Hemolysin-enterotoxin |
| A63 | LMG 13450 | Feces | Switzerland | + | 3 | 91 | Hemolysin-enterotoxin |
| A99 | LMG 13449 | Feces | Switzerland | + | 3 | 91 | Hemolysin-enterotoxin |
| A220 | LMG 13661 | Feces | Switzerland | + | 3 | 91 | Hemolysin-enterotoxin |
| A308 | CDC 043-84 | Water | France | + | 3 | 91 | Hemolysin-enterotoxin |
| A938 | Not available | Unknown | United States | + | 3 | 91 | Hemolysin-enterotoxin |
| A1422 | Not available | Unknown | United States | + | 3 | 91 | Hemolysin-enterotoxin |
| A. salmonicida (HG3) | |||||||
| Not available | ATCC 33658T | Fish | United Kingdom | + | 3 | 91 | Hemolysin-enterotoxin |
| Not available | ATCC 33659 | Fish | United Kingdom | − | |||
| A. caviae (HG4) | |||||||
| A1 | LMG 13454 | Human | Germany | − | |||
| A3 | Not available | Unknown | Germany | − | |||
| A309 | ATCC 15468T | Guinea pig | France | − | |||
| A. caviae (HG5A) | |||||||
| A6 | LMG 13460 | Environmental | Germany | − | |||
| A75 | LMG 13461 | Feces | Switzerland | − | |||
| A310 | ATCC 51107 | Fish | France | − | |||
| A. caviae (HG5B) | |||||||
| A117 | LMG 13465 | Feces | Switzerland | − | |||
| A137 | Not available | Feces | Switzerland | − | |||
| A213 | LMG 13466 | Feces | Switzerland | − | |||
| A. eucrenophila (HG6) | |||||||
| A311 | ATCC 23309 | Fish | Unknown | + | 2 | 76 | Hemolysin |
| A914 | CDC 9179 | Unknown | Unknown | + | 2 | 76 | Hemolysin |
| A. sobria (HG7) | |||||||
| A312 | ATCC 43979T | Fish | France | + | 2 | 76 | Hemolysin |
| A915 | LMG 13469 | Fish | United States | − | |||
| A. veronii biovar sobria (HG8) | |||||||
| A10 | LMG 13067 | Environmental | Germany | + | 2 | 76 | Hemolysin |
| A11 | LMG 13693 | Environmental | Germany | + | 2 | 78 | Hemolysin |
| A27 | LMG 13694 | Unknown | Switzerland | + | 2 | 78 | Hemolysin |
| A132 | Not available | Feces | Switzerland | + | 2 | 76 | Hemolysin |
| A. veronii biovar veronii (HG10) | |||||||
| A901 | ATCC 35624T | Human | United States | + | 2 | 77 | Hemolysin |
| A. jandaei (HG9) | |||||||
| A179 | LMG 13064 | Feces | Switzerland | − | |||
| A919 | LMG 13065 | Feces | United States | − | |||
| A1642 | ATCC 49568T | Feces | United States | − | |||
| A. encheleia (HG11) | |||||||
| A902 | ATCC 35941 | Human | New Zealand | − | |||
| A926 | LMG 13076 | Water | United States | + | 2 | 76 | Hemolysin |
| A1653 | LMG 13061 | Water | Germany | + | 3 | 99 | Hemolysin-enterotoxin |
| A. schubertii (HG12) | |||||||
| A903 | ATCC 43700T | Human | United States | − | |||
| A922 | LMG 13473 | Human | United States | − | |||
| A. trota (HG14) | |||||||
| A1645 | ATCC 49659 | Human | United States | + | 4 | 82 | Hemolysin |
| A1646 | ATCC 49657T | Feces | India | + | 4 | 82 | Hemolysin |
| A1647 | ATCC 49660 | Feces | Thailand | + | 4 | 82 | Hemolysin |
| Non-Aeromonas spp. | |||||||
| Bacillus cereus | ATCC 11778 | Unknown | United States | − | |||
| Bacteroides coagulans | ATCC 7050 | Milk | United Kingdom | − | |||
| Clostridium perfringens | ATCC 13124 | Cow | United Kingdom | − | |||
| C. perfringens | CIP 60-19 | Sheep | France | − | |||
| C. perfringens | CIP 60-60 | Dog | France | − | |||
| Candida albicans | ATCC 10231 | Unknown | United States | − | |||
| Escherichia coli | ATCC 25922 | Clinical | United States | − | |||
| Listeria monocytogenes | ATCC 7644 | Human | Unknown | − | |||
| Micrococcus luteus | ATCC 9341 | Soil | United States | − | |||
| Proteus vulgaris | ATCC 13315 | Unknown | United Kingdom | − | |||
| Pseudomonas aeruginosa | ATCC 27853 | Blood | United States | − | |||
| Salmonella typhimurium | ATCC 14028 | Bovine | United States | − | |||
| Staphylococcus aureus | ATCC 25923 | Clinical | United States | − | |||
| S. aureus | ATCC 33862 | Unknown | Unknown | − | |||
| S. epidermidis | ATCC 12228 | Unknown | United States | − | |||
| Streptococcus agalactiae | ATCC 12386 | Unknown | Unknown | − | |||
| Vibrio parahemolyticus | ATCC 17802 | Food | Japan | − | |||
| V. vulnificus | CIP 75.4 | Blood | United States | − | |||
| V. vulnificus | CIP 81.90 | Blood | France | − | |||
| Yersinia enterocolitica | ATCC 23715 | Blood | Unknown | − |
PCR positivity.
PCR-RFLP fragments obtained when using HpaII endonuclease. PCR-RFLP 1 has two fragments, PCR-RFLP 2 has three fragments, and PCR-RFLP has four fragments.
PCR-ASA indicating similarity to the cytolytic enterotoxin gene (AHCYTOEN, GenBank accession no. M84709). PCR-ASA 1 has 76 to 78% similarity, PCR-RFLP 2 has 82% similarity, and PCR-ASA 3 has 91 to 99% similarity to the AHCYTOEN gene.
Altwegg reference number.
T, type strain in HG.
TABLE 3.
PCR results and characteristics of virulence gene amplicons in Aeromonas sp. isolates
| Sample | Origin | Organism | HG | No. of isolates | No. (%) PCR positive | HpaIIa | % Similarity by PCR-ASAb | No. of PCR-RFLP fragmentsc |
|---|---|---|---|---|---|---|---|---|
| Food | ||||||||
| Beef | Switzerland | A. bestiarum | 2 | 2 | 1 | 1 | 93 | 3 |
| A. caviae | 5A | 1 | 0 | |||||
| A. hydrophila | 3 | 12 | 10 | 9 | 90, 91, 92 | 3 | ||
| A. veronii | 8/10 | 2 | 1 | 1 | 77 | 2 | ||
| Aeromonas sp. | NDd | 4 | 3 | ND | ND | |||
| Total | 21 | 15 (71) | 11 | |||||
| Minced meat | Switzerland | A. bestiarum | 2 | 1 | 1 | ND | ND | |
| A. caviae | 5A | 2 | 0 | ND | ND | |||
| A. hydrophila | 3 | 9 | 9 | ND | ND | |||
| Aeromonas sp. | ND | 2 | 2 | ND | ND | |||
| Total | 14 | 12 (86) | ||||||
| Pork | Switzerland | A. hydrophila | 3 | 6 | 6 | 6 | 90, 91, 92 | 3 |
| Aeromonas sp. | ND | 4 | 3 | ND | ND | |||
| Total | 10 | 9 (90) | 6 | |||||
| Poultrye | Switzerland | A. hydrophila | 3 | 6 | 6 | 6 | 91, 92 | 3 |
| European Union | A. veronii | 8/10 | 1 | 1 | 1 | 77 | 2 | |
| Aeromonas sp. | ND | 10 | 3 | ND | ||||
| Total | 17 | 10 (59) | 7 | |||||
| Seafoodf | European Union | A. caviae | 5A | 1 | 0 | ND | ND | |
| A. hydrophila | 3 | 5 | 5 | 1 | 78 | 2 | ||
| Aeromonas sp. | ND | 6 | 4 | ND | ND | |||
| Total | 12 | 9 (75) | 1 | |||||
| Perch | European Union | A. bestiarum | 2 | 1 | 1 | ND | ND | |
| A. encheleia | 6B | 1 | 0 | ND | ND | |||
| A. hydrophila | 3 | 4 | 4 | 1 | 91 | 3 | ||
| Aeromonas sp. | ND | 5 | 4 | ND | ND | |||
| Total | 11 | 9 (82) | 1 | |||||
| Salmon | European Union | A. bestiarum | 2 | 1 | 1 | 1 | 94 | 3 |
| A. hydrophila | 1 | 1 | 1 | 1 | 95 | 3 | ||
| A. hydrophila | 3 | 15 | 15 | 5 | 91, 93 | 3 | ||
| Aeromonas sp. | ND | 5 | 3 | ND | ND | |||
| Total | 22 | 20 (91) | 7 | |||||
| Vegetables | Belgium | A. bestiarum | 2 | 2 | 0 | ND | ND | |
| A. caviae | 4/5A/5B | 15 | 2 | ND | ND | |||
| A. caviae | Complex | 6 | 4 | ND | ND | |||
| A. hydrophila | 3 | 1 | 1 | ND | ND | |||
| Aeromonas sp. | ND | 6 | 1 | ND | ND | |||
| Switzerland | Aeromonas sp. | ND | 4 | 1 | ND | ND | ||
| Total | 34 | 9 (27) | ||||||
| Environmental | ||||||||
| Water | Bangladesh | A. veronii | 8/10 | 5 | 5 | 5 | 77, 78 | 2 |
| Belgium | A. bestiarum | 2 | 4 | 4 | 4 | 94, 95 | 3 | |
| A. caviae | 4/5A | 5 | 0 | |||||
| A. hydrophila | 1 | 2 | 2 | 2 | 77, 94 | 2, 3 | ||
| A. hydrophila | 3 | 9 | 8 | 5 | 83, 91, 92 | 3 | ||
| Switzerland | A. bestiarum | 2 | 6 | 6 | 6 | 90, 93, 94 | 3 | |
| A. caviae | 4/5A/5B | 9 | 0 | |||||
| A. eucrenophila | 6 | 2 | 0 | |||||
| A. hydrophila | 1 | 1 | 1 | 1 | 98 | 3 | ||
| A. hydrophila | 3 | 4 | 3 | 2 | 91, 94 | 3 | ||
| A. veronii | 8/10 | 12 | 5 | 3 | 76, 78 | 2 | ||
| Total | 59 | 34 (58) | 28 | |||||
| Clinical | ||||||||
| Human | Bangladesh | A. caviae | 4 | 10 | 4 | 4 | 78, 93, 96, 100 | 2, 3 |
| A. hydrophila | 1 | 13 | 2 | ND | ND | |||
| Ivory Coast | A. hydrophila | 1 | 5 | 2 | 2 | 100 | 3 | |
| A. veronii | 8 | 6 | 6 | 6 | 76, 78 | 2 | ||
| Switzerland | A. bestiarum | 2 | 1 | 1 | 1 | 94 | 3 | |
| A. caviae | 4/5A/5B | 11 | 0 | ND | ND | |||
| A. eucrenophila | 6 | 1 | 1 | ND | ND | |||
| A. hydrophila | 1 | 7 | 3 | 3 | 97, 98 | 3 | ||
| A. veronii | 8 | 17 | 17 | 6 | 76, 77, 78 | 2 | ||
| Total | 71 | 36 (50) | 22 | |||||
| Fish | Switzerland | A. bestiarum | 2 | 1 | 1 | 1 | 94 | 3 |
| A. caviae | 4 | 3 | 0 | ND | ND | |||
| A. eucrenophila | 6 | 2 | 0 | ND | ND | |||
| A. hydrophila | ND | 5 | 3 | 2 | 77, 94 | 2, 3 | ||
| A. hydrophila | Ser.g | 7 | 6 | 5 | 77, 78, 91, 94 | 2, 3 | ||
| A. veronii | 8/10 | 1 | 1 | 1 | 77 | 2 | ||
| A. veronii | ND | 4 | 4 | 4 | 77, 78, 91 | 2, 3 | ||
| A. veronii | Ser. | 3 | 3 | 3 | 76, 78 | 2 | ||
| A. salmonicida | Ser. | 52 | 46 | 6 | 82, 91 | 3 | ||
| A. sobria | 7 | 1 | 0 | ND | ND | |||
| Total | 79 | 64 (81) | 23 | |||||
| Gross total | 350 | 227 (65) | 106 |
Number of amplicons used for restriction enzyme analysis with endonuclease HpaII.
PCR-ASA indicating similarity to cytolytic enterotoxin gene AHCYTOEN (GenBank accession no. M84709). PCR-ASA 1 has 76 to 78% similarity, PCR-ASA 2 has 82% similarity, and PCR-ASA 3 has 91 to 99% similarity to the AHCYTOEN gene.
Number of PCR-RFLP fragments obtained when using HpaII endonuclease. PCR-RFLP 1 has two fragments, PCR-RFLP has three fragments, and PCR-RFLP 3 has four fragments.
ND, not done.
Poultry including chicken, turkey, and duck.
Seafood including mussels, oysters, and scallops.
Ser., serotyped.
(ii) Isolate preservation.
All strains were purified on Columbia sheep blood agar (bio-Merieux, Geneva, Switzerland) upon receipt in our laboratory. Subsequently, a typical colony was grown in Tryptone soya broth (Oxoid CM 129; Fakola AG, Basel, Switzerland) at 28°C for 16 h. For preservation purposes, stock cultures were prepared in 30% sterilized glycerol (Glycerol G-5150; Sigma, Buchs, Switzerland) and maintained at −70°C.
Bacterial DNA extraction for PCR.
Approximately 100 μl of a Tryptone soya broth (Oxoid) culture grown for 16 h at 28°C was used for DNA extraction using the InstaGene matrix (Bio-Rad Laboratories AG, Glattbrugg, Switzerland) in accordance with the manufacturer’s instructions. Subsequently, 5 μl of the DNA solution was used as a template for PCR amplification.
Strategies for primer design.
An A. hydrophila cytolytic enterotoxin gene (AHCYTOEN) was described (16) as a multivirulence gene including lethality in mice, hemolysis, cytotoxicity, and enterotoxigenicity. Some of those activities are part of the virulence factors of other Aeromonas spp. For this reason, the AHCYTOEN gene was chosen as the reference gene in this investigation.
First, we aligned the AHCYTOEN gene with other aerolysin genes, i.e., GenBank accession no. M16495 (24), U40711 (52), and AF064068 (36) and EMBL accession no. X65043, X65044, X65045, X65046 (21), X65048 (22), and Y00559 (26), to find the related regions. This alignment was performed with the Bestfit program of the GCG software (Genetics Computer Group, Inc., Madison, Wisc.). Finally, unique and specific primers were selected from open reading frame 2 (ORF2) of the AHCYTOEN gene by using Primer Designer 3 software (Scientific & Educational Software, Durham, N.C.). The selected primers were then simultaneously compared to other sequences in the GenBank database to verify their identity and similarity to Aeromonas sp. genes and those of other species.
Master mixture preparation and PCR condition optimization.
As previewed by the Primer Designer 3 software, the eight selected primers (AHCF1, -2, and -3 and AHCR1, -2, -3, -4, and -5 [see Table 1]) constitute five combinations of primers or a cocktail of all of the primers that produce an amplicon of 232 bp under identical amplification conditions. The annealing temperature indicated by the software was increased by 10°C in order to increase the stringency of primer annealing and to avoid nonspecific binding. Of the five possible primer combinations, only the combination of AHCF1 and AHCR1 was used for clinical, environmental, and food isolates because they displayed 100% homology with the counterpart AHCYTOEN gene and an extracellular hemolysin gene (EMBL accession no. X65045) (21), which represented the two main groups of virulence factors in the genus Aeromonas, i.e., enterotoxins and hemolysins. The cocktail master mixture containing all eight primers was used only for the reference strains, which were found to be negative for the AHCF1-AHCR1 primer combination master mixture. All primers were purchased from MWG-Biotech GmbH (Ebersberg, Germany).
TABLE 1.
Sequence alignment and primer design for the detection of Aeromonas sp. virulence genes
| Organism | Nucleotide sequence accession no. | % Similaritya | Primer combinationb | Site locations | % Similarity by PCR-ASAc | No. of PCR-RFLP fragmentsd |
|---|---|---|---|---|---|---|
| A. hydrophila | GBeM84709 | 100.0 | C1, AHCF1-AHCR1 | 1661–1682, 1871–1892 | 100.0g | 3 |
| A. hydrophila | EMhX65045 | 96.9 | C1, AHCF1-AHCR1 | 919–940, 1871–1892 | 100.0 | 3 |
| A. hydrophila | GB M16495 | 86.9 | C2, AHCF1-AHCR2 | 1309–1330, 1519–1540 | 92.2 | 3 |
| A. hydrophila | EM X65043 | 84.7 | C2, AHCF1-AHCR2 | 1203–1224, 1413–1433 | 90.5 | 3 |
| A. hydrophila | EM X65044 | 84.7 | C2, AHCF1-AHCR2 | 1202–1223, 1413–1433 | 90.1 | 3 |
| A. sobria | EM X65046 | 74.4 | C3, AHCF1-AHCR3 | 1117–1138, 1327–1348 | 76.7 | 2 |
| A. salmonicida | EM X65048 | 74.7 | C3, AHCF1-AHCR3 | 1119–1140, 1329–1350 | 76.7 | 2 |
| A. caviae | GB U40711 | 77.5 | C4, AHCF2-AHCR4 | 985–1006, 1195–1216 | 84.1 | 2 |
| A. sobria | EM Y00559 | 73.6 | C5, AHCF3-AHCR5 | 1639–1660, 1849–1870 | 81.5 | 4 |
| A. trota | GB AF064068 | 73.1 | C5, AHCF3-AHCR5 | 1174–1195, 1383–1405 | 81.9 | 4 |
Compared to the 2,528 bp of AHCYTOEN (GenBank nucleotide sequence accession no. M84709).
Primer sequences with mismatched bases underlined: AHCF1, 5′-GAGAAGGTGACCACCAAGAACA-3′; AHCR1, 5′-AACTGACATCGGCCTTGAACTC-3′; AHCF2, 5′-GAGAAGGTCTCCACCAAGAACA-3′; AHCR2, 5′ AGCTGACATCGGCCTTGAACTC-3′; AHCF3, 5′-GAGAAGGTCTCGACCAAGAACA-3′; AHCR3, 5′-AATTGACCTCGGCCTTGAACTC-3′; AHCR4, 5′ AGCTGACATCCGCCTTGAACTC-3′; AHCR5, 5′ AGCTCATATCCGCTTTGAACTC-3′.
Compared to the 232-bp amplicon sequence of the AHCYTOEN gene.
PCR-RFLP of 232 bp using HpaII.
GB, GenBank.
Primer combination number.
Value of 100.0% = 232-bp amplicon size.
EM, European Molecular Biology Laboratory.
Optimization of the PCR protocol was performed by using 50 μl of a PCR mixture containing 5 μl of template DNA, 8 μl of a mixture containing each deoxynucleoside triphosphate at 0.2 mM (Roche Diagnostics AG, Rotkreuz, Switzerland), 5 μl of GeneAmp 10× PCR buffer II (Perkin-Elmer, Rotkreuz, Switzerland), 5 μl of a 25 mM MgCl2 solution (3.0 mM final concentration; Perkin-Elmer), 0.25 μl of a 200 μM solution of each primer (1 μM final concentration), 0.10 μl of Tween 20 (2% final concentration) (Sigma), 0.25 μl of AmpliTaq Gold at 5 U/μl (1.25-U final concentration) (Perkin-Elmer), and 26.15 μl of sterile double-distilled water, to make the final volume 50 μl. The PCR mixture was made in a sterile, laminar-airflow environment in a large volume to produce approximately 100 tubes of 45 μl each, combining all of the ingredients except the template DNA, and stored at −20°C. PCR amplification was performed with a GeneAmp 9600 PCR system (Perkin-Elmer) by using the following temperature program: 1 cycle of denaturation for 10 min at 95°C; 25 cycles of melting at 95°C for 15 s, annealing at 66°C for 30 s, and elongation at 72°C for 30 s; and a final extension round at 72°C for 10 min. The PCR amplicons were separated electrophoretically by loading a total amplicon volume of 20 μl including 6 μl of stop solution buffer onto a 2.5% agarose gel (pulsed-field-certified agarose; Bio-Rad Laboratories AG). Electrophoresis was performed in 0.5× Tris-borate-EDTA buffer–double-distilled water (Roche Diagnostics AG) for 1 h at 100 V. Amplicons were visualized with UV light after the agarose gel had been soaked for 15 min in an ethidium bromide solution (0.5 g/ml; Bio-Rad Laboratories AG).
Characterization of amplicons.
The PCR products were purified by using the QIAquick PCR Purification Kit (QIAGEN AG, Basel, Switzerland) and quantified by using the GeneQuant instrument (Pharmacia-Biotech Europe GmbH, Dübendorf, Switzerland) in accordance with the instructions provided by the manufacturers. PCR-RFLP analysis using the endonuclease HpaII as indicated by the Clone Manager software (Scientific & Educational Software) and PCR-ASA were used to characterize the 135 amplicons available for this purpose.
The following procedure was used for PCR-RFLP analysis. A 10-μg sample of the purified PCR product was digested for 16 h at 37°C with endonuclease HpaII (Roche Diagnostics AG) in accordance with the manufacturer’s instructions. The fragments were separated by electrophoresis of 20 μl of the digested product in 3.5% agarose gel (pulsed-field-certified agarose; Bio-Rad) and subsequently visualized with ethidium bromide fluorescence.
For PCR-ASA, the cycle sequencing reaction mixture was prepared with the Big Dye Terminator Cycle Sequencing Ready Reaction Kit (PE Applied Biosystems, Rotkreuz, Switzerland). Excess terminators were removed by the Centri-Sep Spin Column Purification System (PE Applied Biosystems) before preparation and loading of the samples onto the ABI PRISM 310 Genetic Analyzer System (PE Applied Biosystems) as specified by the manufacturer’s protocol. The obtained sequences were aligned with the amplicon of the AHCYTOEN gene predicted by the Primer Designer 3 software by using the Bestfit program of the GCG software.
RESULTS
Sequence alignment and primer design strategies.
Results of sequence alignment and the primer design strategies are detailed in Table 1. Alignment of the AHCYTOEN gene with the aerolysin genes of Aeromonas spp. revealed high sequence similarities ranging from 73.1 to 96.9% (Table 1). The most commonly shared region of the AHCYTOEN gene was ORF2, with its 1,515-bp length. The search for primers within this ORF provided two 22-bp primers unique to the AHCYTOEN gene, i.e., AHCF1 (5′-GAG AAG GTG ACC ACC AAG AAC A-3′) and AHCR1 (5′-AAC TGA CAT CGG CCT TGA ACT C-3′). Comparison of these primers to sequences in the GenBank database generated six more primers, including forward primers AHCF2 and AHCF3 and reverse primers AHCR2, AHCR3, AHCR4, and AHCR5 (Table 1), from the other aerolysin genes with high sequence identities to AHCF1 and AHCR1 ranging from 90 to 100%. More base mismatches in the generated primers were found with AHCR1 than with AHCF1.
PCR amplification.
Results of PCR amplification using well-characterized reference strains and wild-type isolates are reported in Tables 2 and 3. For the reference strains, PCR amplification using a specific combination of primers for a specific gene or using the cocktail master mixture produced an amplicon of 232 bp, as predicted by the Primer Designer 3 software. Of the 61 Aeromonas reference strains tested, the 30 (49.2%) that were positive for the primer combination AHCF1-AHCR1 master mixture were found in HG1 (A. hydrophila), HG2 (A. bestiarum), HG3 (A. hydrophila and A. salmonicida), HG6 (A. eucrenophila), HG7 (A. sobria), and HG8 (A. veronii biogroup sobria). When the cocktail master mixture was used, 37 (61%) of the 61 reference strains were positive. Some of the reference strains negative with the AHCF1-AHCR1 mixture, e.g., in HG6 (A. eucrenophila), HG10 (A. veronii biogroup veronii), HG11 (A. encheleia), and HG13 (A. trota), turned out to be positive with the primer cocktail master mixture. No representatives of HG4/5A/5B (A. caviae-A. media complex), HG9 (A. jandaei), or HG12 (A. schubertii) were found to be positive for the targeted genes. Two positive representative reference strains of each HG were tested with all five combinations, including the cocktail master mixture. Some of them produced more than one amplicon as evidence that they possess more than one virulence factor, e.g., strain ATCC 43979T (HG7) (results not shown). The application of this PCR procedure to isolates from clinical, environmental, and food isolates when using the primer combination AHCF1-AHCR1 showed that 227 (65%) of 351 strains were positive for the targeted virulence markers. Use of the primer combination AHCF1-AHCR1 for the clinical, environmental, and food isolates was justified by the fact that almost all of the isolates received in our laboratory for this investigation were in HG1, HG2, HG3, HG4, HG5A, HG5B, or HG8. The reference strains in these HGs always reacted positively or negatively with both primers AHCF1 and AHCR1 and the cocktail master mixture. Of the 55 isolates which were not characterized to the genotype (HG) level because they could not grow on the culture medium required for identification by the FAME method or which were only serotyped (fish isolates), 31 (56%) were positive for one of the virulence factors.
Specificity of primers.
The specificity of the primer combination AHCF1-AHCR1 and the cocktail master mixture for the amplification of the 232-bp amplicon in Aeromonas spp. was demonstrated by the negative PCR results obtained with all of the non-Aeromonas reference strains (Table 2).
PCR-RFLP characterization.
Results of the characterization of the reference strains and isolates by PCR-RFLP are presented in Tables 2 and 3, respectively. The characterization of the 232-bp PCR product from reference strains by restriction enzyme digestion (PCR-RFLP) using endonuclease HpaII revealed three types of amplicons, as predicted by the Clone Manager software (Fig. 1 and 2). PCR-RFLP type 1 (PCR-RFLP 1), exhibiting two restriction fragments of 66 and 166 bp, was found in HG6, HG7, HG8, HG10, and HG11 (e.g., strain A926; Table 2). PCR-RFLP 2, displaying three restriction fragments of 18, 66, and 148 bp, was found in HG1, HG2, HG3, and HG11 (e.g., strain A1653; Table 2). PCR-RFLP 3, with four fragments of 7, 20, 66, and 139 bp, was found only in HG13. PCR-RFLP characterization of amplicons of Aeromonas isolates from clinical, environmental, and food samples by either FAME, MEE, or ribotyping generally produced the same values as the reference strains. All 18 amplicons of the A. veronii complex isolates tested were found to be of PCR-RFLP 1. Of the 57 amplicons of HG1, HG2, HG3, and the HG4/5A/5B complex tested, only 2 (1 of HG1, isolated from water in Belgium, and 1 from a clinical isolate of HG4 from Bangladesh) were of PCR-RFLP 1 instead of PCR-RFLP 2. Divergent results were found when the HGs of the isolates were not identified or when they were serotyped by the Danish National Veterinary Laboratory serotyping system, except for A. salmonicida, for which all six of the amplicons tested were of PCR-RFLP 2.
FIG. 1.
PCR-RFLP maps of the 232-bp amplicon showing the three types of HpaII endonuclease restriction fragment patterns. The upper map represents PCR-RFLP 1 with two fragments of 66 and 166 bp. The second map represents PCR-RFLP 2 with three fragments of 18, 66, and 148 bp. The third map represent PCR-RFLP 3 with fragments of 7, 20, 66, and 139 bp. The lower map represents the scaled 232-bp amplicon. The arrows flanking the PCR-RFLP 1 map represent primers which may be AHCF1, -2, or -3 and AHCR1, -2, -3, -4, or -5.
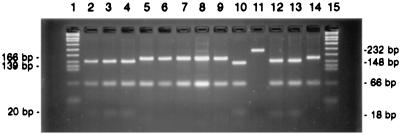
FIG. 2.
PCR amplicon of 232 bp and PCR-RFLP types of 232-bp amplicons of different HGs of reference strains of Aeromonas spp. PCR amplicon and PCR-RFLP types of reference and wild-type strains of Aeromonas spp. on 4% Metaphor agarose gel (Bioconcept, Allschwil, Switzerland) stained with ethidium bromide at 0.5 μg/ml (Bio-Rad). Lanes: 1 and 15, DNA size marker VIII (Roche Diagnostics AG; catalog no. 1336 045); 2, a PCR-RFLP 2 of A17 (ATCC 7966), a reference strain of HG1 isolated from canned milk from the United States; 3, PCR-RFLP 2 of A307 (ATCC 51108), a reference strain of HG2 isolated from a fish in France; 4, PCR-RFLP 2 of A14 (LMG 13674), a reference strain of HG3 that is an environmental isolate from Germany; 5, PCR-RFLP type 1 of A914 (CDC 9179), a reference strain of HG6 (source unknown); 6, PCR-RFLP 1 of A312 (ATCC 43979), a reference strain of HG7 that is a fish isolate from France; 7, PCR-RFLP 1 of A10 (CCUG 30356), a reference strain of HG8 that is an environmental isolate from Germany; 8, PCR-RFLP 1 of A901 (ATCC 35624), a reference strain of HG10 that is a clinical isolate (sputum) from the United States; 9, PCR-RFLP 1 of A926 (CDC 3136), a reference strain of HG11 that is a water isolate from the United States; 10, PCR-RFLP 3 of A1645 (ATCC 49659), a reference strain of HG13 that is a clinical isolate from the United States; 11, uncut 232-bp amplicon of strain A1645; 12, PCR-RFLP 2 of a wild-type strain (BP 3) of HG1 that is a clinical isolate of HG1 from Bangladesh; 13, PCR-RFLP 2 of a wild-type strain (CBK 45) of HG3 that is a pork meat isolate from Switzerland; 14, PCR-RFLP 1 of a wild-type strain (BE 6) of HG8 that is an environmental isolate from Bangladesh.
PCR-ASA characterization.
The results of PCR-ASA for the reference strains demonstrated the existence of three PCR-ASA groups in Aeromonas (Table 2). Similarly to PCR-RFLP 1, PCR-ASA group 1 (PCR-ASA 1) includes strains of the A. veronii complex in HG6, HG7, HG8, HG10, and HG11. All six of the positive strains of this complex tested for this analysis showed sequence similarities ranging from 76 to 78% compared to the reference amplicon of the AHCYTOEN gene. PCR-ASA 2, with 82% sequence similarity, was found exclusively in HG13. PCR-ASA 3 contained HG1, HG2, HG3, and HG11, with sequence similarities to the reference amplicon of the AHCYTOEN gene varying from 91 to 99%. The results of PCR-ASA of amplicons from clinical, environmental, and food isolates characterized by either FAME, MEE, or ribotyping as for PCR-RFLP characterization were similar to those obtained with the reference strains. Again, as for the PCR-RFLP test, the isolates which were not characterized with respect to HG and serotype gave divergent results (Table 3), except for isolates identified as A. salmonicida by serotyping. All six of these isolates of A. salmonicida had the PCR-ASA 3 profile.
DISCUSSION
The results of alignment analysis have shown that high sequence homologies exists between the AHCYTOEN gene and other aerolysin genes, ranging from 73.1 to 96.7%, thus confirming clearly the high level of DNA relatedness of Aeromonas sp. virulence factors. This enabled us to retrieve specific primers from their common regions (Table 1). We found that the ORF2 region within the AHCYTOEN gene was the most common region of the aligned genes. The systematic search for specific primers from ORF2 within the AHCYTOEN gene led to the selection of primers AHCF1 (forward) and AHCR1 (reverse). These primers were selected for their uniqueness to this gene, but when these primers were compared to sequences in the GenBank database, high sequence similarities to eight other described virulence genes of the Aeromonas complex were revealed by gene alignment analysis (Table 1). From the primer comparison results, six more primers were generated, including AHCF2, AHCF3, AHCR2, AHCR3, AHCR4, and AHCR5, each with the ability to amplify the same amplicon from its specific gene. The master mixture using all eight primers (cocktail master mixture) also produced the 232 bp in reference strains.
Among the described virulence factors of Aeromonas spp., two major groups are frequently associated with its pathogenicity, namely, the aerolysins and the enterotoxins (11). The specific and rapid detection of the virulence genes (Table 1) by means of molecular techniques has been the subject of many studies during the last decade (8, 14, 35, 41, 47). However, many of these investigations were limited to the detection of one specific virulence gene. Theoretically, primers AHCF1 and AHCR1 used in this investigation were designed to target both the AHCYTOEN gene and the extracellular hemolysin gene with EMBL database accession no. X65045 (22) of A. hydrophila on the basis of 100% sequence homology. In practice, however, these primers produced amplicons in almost all of the HGs of the reference strains except HG9, HG10, HG11, HG12, and HG13. Also, we observed that these particular HGs were practically absent in the wild-type strains we received from different laboratories for this investigation. This observation demonstrates the multigene detection characteristics of the two-primer set AHCF1-AHCR1 used in this work for wild-type isolates and its greater usefulness than previously described primers (14, 46, 49).
The results of PCR-RFLP analysis of Aeromonas reference strains with endonuclease HpaII have revealed that three main types or clusters of virulence genes coexist in the 15 HGs of Aeromonas spp. (Fig. 1 and 2).
As for the PCR-RFLP, the PCR-ASA of the 232-bp amplicons showed that representatives of all known Aeromonas HGs can also be divided into three types (Tables 2 and 3). In Aeromonas sp. taxa, amplicons from A. hydrophila HG1 and A. encheleia HG11 (e.g., strain A1653; this strain was recently moved from HG6 [A. eucrenophila] to HG11 [A. encheleia]) reference strains were most closely related to the AHCYTOEN gene sequence, with homologies between 97 and 99%. The strains of A. bestiarum HG2 were more versatile in their homology to the AHCYTOEN gene, with 91 to 95% sequence homology, whereas all of the reference strains of HG3, including A. hydrophila and A. salmonicida, exhibited 91% of homology to the AHCYTOEN gene. These data clearly illustrated the stability of this gene in these HGs (Table 2).
The reference strains of A. caviae (HG4/5A/5B) could not be classified in any of the defined PCR-RFLP or PCR-ASA types because all representatives were negative for the 232-bp amplicon with the primer set AHCF1-AHCR1 and the cocktail master mixtures. The lack of these genes in A. caviae (HG4/5A/5B) is in agreement with previous reports (3, 21, 31, 32, 53) which suggested that this group of aeromonads are less virulent and less cytotoxigenic than other Aeromonas taxa. Nevertheless, recent studies have demonstrated the production of cytotoxin by A. caviae strains under specific culture conditions and its implications for the generation of diarrhea in very young children and old people (40, 45). Negative 232-bp amplicon detection results were also obtained with the A. jandaei (HG9) and A. schubertii (HG12) reference strains. Since only a limited number of strains were included, it is not possible to report on the distribution of hemolytic or enterotoxin genes in these Aeromonas HGs.
The PCR-RFLP and PCR-ASA assays performed on Aeromonas reference strains have proven to be helpful tools for the detection and characterization of potential enterotoxigenic and hemolytic aeromonads. This characterization can be performed in any molecular laboratory, depending on the availability of equipment, principally a thermocycler and the restriction endonuclease HpaII. The application of these procedures to clinical, environmental, and food isolates will permit not only the detection of the virulence genes but also their characterization and the distribution of these genes in wild isolates from different sources or in their HGs.
Of the total of 350 clinical, environmental, and food isolates of Aeromonas spp. included in this study, 65% harbored virulence genes. Fifty percent of the human clinical isolates originating from Bangladesh, Ivory Coast, and Switzerland were positive by PCR for virulence factors and belonged mainly to the A. caviae complex (HG4/5A/5B), A. hydrophila (HG1), and A. veronii (HG8/10) (Table 3). Although it is very likely that clinical isolates possess at least one virulence gene, it should be kept in mind that Aeromonas spp. are well recognized as opportunistic organisms that may be present in diarrheal stool as commensals rather than as primary pathogens (3). In addition, the primers used for PCR identification in this study may not be specific for other virulence genes of Aeromonas spp. (Table 1). Interestingly, all of the Swiss human and environmental isolates of A. caviae were found to be negative for the targeted genes whereas 25% of the A. caviae isolates originating from Bangladesh harbored virulence genes. This may confirm the previously published observations that geographical variation in virulence factors for this Aeromonas species may exist (3).
A total of 22 amplicons from human clinical isolates belonging to A. bestiarum (n = 1), A. caviae (n = 4), A. hydrophila (n = 5), and A. veronii (n = 12) were included for PCR-RFLP and PCR-ASA assays. All positive A. caviae isolates from Bangladesh were classified as members of HG4 and belonged to PCR-RFLP 2 and PCR-ASA 3 with amplicon sequence homologies ranging from 93 to 100%. Clearly, these amplicons show high relatedness to the reference sequence of the multivirulence AHCYTOEN gene found mostly in A. hydrophila HG1. In this study, amplicons of reference strains and clinical and environmental isolates of HG1 exhibited 97 to 100% homology with the AHCYTOEN gene (Table 3). In addition, it was found that the PCR-RFLP and PCR-ASA profiles of HG1 isolates were similar to those of HG1 reference strains, suggesting that these isolates possess a multiple-virulence gene comparable to the AHCYTOEN gene. Previous clinical studies (37, 43) have suggested that the expression of multiple biological activities, as in the case of the AHCYTOEN gene, is necessary for the expression of microbial pathogenicity.
All of the 23 clinical isolates of A. veronii included in this investigation contained the 232-bp amplicon. Of the 12 amplicons characterized by PCR-RFLP and PCR-ASA assays, all were of PCR-ASA 1 and PCR-RFLP 1, regardless their origin. From the literature, it is known that A. veronii represents one of the more virulent species in the genus Aeromonas, as was proven by its pronounced invasiveness and lower 50% lethal doses (17). Similar to A. hydrophila HG1, A. veronii isolates originated mainly from clinical and environmental samples, suggesting that the aquatic environment acts as a reservoir of potentially virulent Aeromonas spp.
Fresh water is known to be a source and reservoir of Aeromonas spp. Among the 60 water isolates, 58% were found to be potentially pathogenic. Virulence genes were found in a high proportion in all species but in none of 15 A. caviae (HG4/5A/5B) isolates. All A. bestiarum and A. hydrophila isolates were found to be of PCR-ASA 3 and PCR-RFLP 2. A strain of A. hydrophila of HG1 and one of A. caviae of HG4 were found to be of PCR-ASA 1 and PCR-RFLP 1; these were the only cases of FAME-characterized isolates to be found so among all of the reference strains and wild isolates.
The Aeromonas sp. fish isolates under study were dominated by A. salmonicida (n = 52), followed by A. hydrophila (n = 12) and A. veronii (n = 8), as determined by serotyping. In contrast to the reference strains, the distribution of virulence genes determined by the PCR-RFLP and PCR-ASA tests among the PCR-RFLP 1 or 2 and PCR-ASA 1 or 2 amplicons in isolates classified as A. veronii or A. hydrophila demonstrated the lack of specificity of the serotyping technique in the classification of Aeromonas spp. Furthermore, it was interesting to note such a proportion (85%) of PCR-positive A. salmonicida isolates, among which almost all of the amplicons belonged to PCR-RFLP 2 and PCR-ASA 3. The fact that all of the fish isolates were isolated from moribund fish may confirm the high pathogenicity of A. salmonicida for fish.
A total of 67% of the food isolates originating from beef, minced meat, pork, poultry, seafoods, perch, salmon, and vegetables possessed one of the targeted virulence genes. The majority of these food isolates were identified as A. bestiarum HG2 and A. hydrophila HG3. The virulence genes of these isolates, as shown by molecular analysis, belonged to PCR-RFLP 2 and PCR-ASA 2. Representatives of the A. caviae complex were rarely isolated from foods of animal origin, and none of them was positive for the virulence genes. These strains mostly originated from vegetables sampled in Belgium, and 29% were found to be positive for the targeted virulence genes. Also, A. veronii HG8/10 was not frequently found among the food isolates; the few positive amplicons all belonged to PCR-RFLP 1 and PCR-ASA 1.
In conclusion, we found that the alignment and comparison analysis of the described aerolysins, hemolysins, and AHCYTOEN genes of Aeromonas spp. confirm the high homologies reported in the literature. The primers designed from the 10 Aeromonas sp. virulence factor genes permit the detection of any of the above virulence markers according to a specific need. The results of the application of the PCR-RFLP and PCR-ASA assays to Aeromonas reference strains and clinical, environmental, and food isolates demonstrated that the detection and identification of potentially pathogenic Aeromonas spp. have become more specific, faster, and easier to perform than conventional phenotyping methods. The PCR procedure did not detect the targeted virulence genes in reference strains of A. caviae; however, there were 10 (15%) of 68 well FAME-characterized positive A. caviae isolates, including 4 clinical isolates of HG4 from Bangladesh and 6 vegetable isolates from Belgium, of which 4 were identified as belonging to the A. caviae complex. In this context, the geographic variation in virulence and/or the failure of actual characterization systems for A. caviae isolates should be considered. Virulence genes were detected and characterized in well-typed reference strains. They were also found and characterized in 65% of wild isolates of Aeromonas spp. from around the world which originated from different samples, proving the universality of the procedures described here. The characterization of the PCR products by PCR-RFLP using endonuclease HpaII and PCR-ASA revealed three major types or clusters of amplicons. It may suggest the classification of pathogenic Aeromonas sp. virulence genes into three main groups (aerolysins-hemolysins, cytolytic enterotoxins, and cytotonic enterotoxins) with PCR-RFLP 1, 2, and 3 and PCR-ASA 1, 2, and 3. The PCR, PCR-RFLP, and PCR-ASA systems may prove to be important tools for the detection, identification, differentiation, and distribution of virulence markers in HGs. These tools will give microbiologists an alternative way to understand pathogenicity in Aeromonas spp. and their distribution in isolates from different sources and HGs.
ACKNOWLEDGMENTS
We thank M. Altwegg and J. Lüthy-Hottenstein, University of Zürich, Zürich, Switzerland; T. Wahli and J. Graf, University of Bern, Bern, Switzerland; M. Jermini of the Cantonal Health Laboratory of Ticino, Lugano, Switzerland; M. Uyttendaele and K. Neyts of the University of Ghent for providing us with Aeromonas sp. isolates; A. Caminada of the Cantonal Institute of Bacteriology, Lugano, Switzerland; P. Meyer of Perkin-Elmer Switzerland; and E. Lüthi and D. Howald of the Swiss Federal Veterinary Office for their exceptional technical support.
REFERENCES
- 1.Abbott S L, Cheung W K W, Kroske-Bystrom S, Malekzadeh T, Janda J M. Identification of Aeromonas strains to the genospecies level in the clinical laboratory. J Clin Microbiol. 1992;30:1262–1266. doi: 10.1128/jcm.30.5.1262-1266.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abeyta C, Jr, Kaysner C A, Wekell M M, Sullivan J J, Stelma G N. Recovery of Aeromonas hydrophila from oysters implicated in an outbreak of foodborne illness. J Food Prot. 1986;49:643–646. doi: 10.4315/0362-028X-49.8.643. [DOI] [PubMed] [Google Scholar]
- 3.Altwegg M. Aeromonas caviae: an enteric pathogen? Infection. 1985;13:228–230. doi: 10.1007/BF01667217. [DOI] [PubMed] [Google Scholar]
- 4.Altwegg M, Geiss H K. Aeromonas as human pathogen. Crit Rev Microbiol. 1989;16:253–286. doi: 10.3109/10408418909105478. [DOI] [PubMed] [Google Scholar]
- 5.Altwegg M, Martinetti Lucchini G, Lüthy-Hottenstein J, Rohrbach M. Aeromonas-associated gastroenteritis after consumption of contaminated shrimp. Eur J Clin Microbiol Infect Dis. 1990;10:44–45. doi: 10.1007/BF01967100. [DOI] [PubMed] [Google Scholar]
- 6.Altwegg M, Lüthi-Hottenstein J. Methods for the identification of DNA hybridization groups in the genus Aeromonas. Experientia. 1991;47:403–406. [PubMed] [Google Scholar]
- 7.Anguita J, Aparicio L B R, Naharro G. Purification, gene cloning, amino acid sequence analysis, and expression of an extracellular lipase from an Aeromonas hydrophila human isolate. Appl Environ Microbiol. 1993;59:2411–2417. doi: 10.1128/aem.59.8.2411-2417.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baloda S B, Krovacek K, Eriksson L, Linne T, Mansson I. Detection of aerolysin gene Aeromonas strain isolates from drinking water, fish, and foods by polymerase chain reaction. Comp Immunol Microbiol Infect Dis. 1995;18:17–26. doi: 10.1016/0147-9571(94)e0001-a. [DOI] [PubMed] [Google Scholar]
- 9.Balows A, Hausler W J Jr, Herrmann K L, Isenberg H D, Shadomy H J, editors. Manual of clinical microbiology. 5th ed. Washington, D.C.: American Society for Microbiology; 1991. [Google Scholar]
- 10.Buchanan R L. The new pathogens. An update of selected examples. Assoc Food Drug Off Q Bull. 1984;48:142–155. [Google Scholar]
- 11.Burke V, Robinson J, Beaman J, Gracey M, Lesmana M, Rockhill R, Echeverria P, Janda J M. Correlation of endotoxicity with biotype in Aeromonas spp. J Clin Microbiol. 1983;18:1196–1200. doi: 10.1128/jcm.18.5.1196-1200.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cahill M M. Virulence factors in motile Aeromonas species: a review. J Appl Bacteriol. 1990;69:1–16. doi: 10.1111/j.1365-2672.1990.tb02905.x. [DOI] [PubMed] [Google Scholar]
- 13.Carnahan A M, Altwegg M. Taxonomy. In: Austin B, Altwegg M, Gosling P J, Joseph S, editors. The genus Aeromonas. Chichester, United Kingdom: John Wiley & Sons; 1996. pp. 1–38. [Google Scholar]
- 14.Cascón A, Anguita J, Hernanz C, Sánchez M, Fernández M, Naharro G. Identification of Aeromonas hydrophila hybridization group I by PCR assays. Appl Environ Microbiol. 1996;62:1167–1170. doi: 10.1128/aem.62.4.1167-1170.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chakraborty T, Huhle B, Bergbauer H, Goebel W. Cloning, expression, and mapping of the Aeromonas hydrophila aerolysin gene determinant in Escherichia coli. J Bacteriol. 1986;167:368–374. doi: 10.1128/jb.167.1.368-374.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chopra A K, Houston C W, Peterson J W, Jin G-F. Cloning, expression, and sequence analysis of a cytolytic enterotoxin gene from Aeromonas hydrophila. Can J Microbiol. 1993;39:513–523. doi: 10.1139/m93-073. [DOI] [PubMed] [Google Scholar]
- 17.Daily O P, Joseph S W, Coolbaulgh J C, Walker R I, Merrell B R, Rollins D M, Seidler R J, Colwell R R, Lissner C R. Association of Aeromonas sobria with human infection. J Clin Microbiol. 1981;13:769–777. doi: 10.1128/jcm.13.4.769-777.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foster E M. Historical overview of key issues in food safety. Emerg Infect Dis. 1997;3:481–482. doi: 10.3201/eid0304.970410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.George W L, Jones J M, Nakata M M. Phenotypic characteristics of Aeromonas species isolated from adult humans. J Clin Microbiol. 1986;23:1026–1029. doi: 10.1128/jcm.23.6.1026-1029.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gobat P-F, Jemmi T. Comparison of seven selective media for the isolation of mesophilic Aeromonas species in fish and meat. Int J Food Microbiol. 1994;24:375–384. doi: 10.1016/0168-1605(94)00043-6. [DOI] [PubMed] [Google Scholar]
- 21.Gracey M, Burke V, Robinson J. Aeromonas-associated gastroenteritis. Lancet. 1982;ii:1304–1306. doi: 10.1016/s0140-6736(82)91510-0. [DOI] [PubMed] [Google Scholar]
- 22.Hirono I, Aoki T, Asao T, Kozaki S. Nucleotide sequences and characterization of hemolysin genes from Aeromonas hydrophila and Aeromonas sobria. Microb Pathog. 1992;13:433–446. doi: 10.1016/0882-4010(92)90011-c. [DOI] [PubMed] [Google Scholar]
- 23.Hirono I, Aoki T. Cloning and characterization of hemolysin genes from Aeromonas salmonicida. Microb Pathog. 1993;15:269–282. doi: 10.1006/mpat.1993.1077. [DOI] [PubMed] [Google Scholar]
- 24.Howard S P, Buckley J T. Molecular cloning and expression in Escherichia coli of the structural gene for the hemolytic toxin aerolysin from Aeromonas hydrophila. Mol Gen Genet. 1986;204:289–295. doi: 10.1007/BF00425512. [DOI] [PubMed] [Google Scholar]
- 25.Howard S P, Garland W J, Green M J, Buckley J T. Nucleotide sequence of the gene for the hole-forming toxin aerolysin of Aeromonas hydrophila. J Bacteriol. 1987;169:2869–2871. doi: 10.1128/jb.169.6.2869-2871.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25a.Hui Y H, et al., editors. Foodborne disease handbook, diseases caused by bacteria. Vol. 1. New York, N.Y: Marcel Dekker Inc.; 1994. pp. 1–27. [Google Scholar]
- 26.Husslein V, Huhle B, Jarchau T, Lurz R, Goebel W, Chakraborty T. Nucleotide sequence and transcriptional analysis of the AerCaerA region of Aeromonas sobria encoding aerolysin and its regulatory region. Mol Microbiol. 1988;2:507–517. doi: 10.1111/j.1365-2958.1988.tb00057.x. [DOI] [PubMed] [Google Scholar]
- 27.Huys G, Vancanneyt M, Coopman R, Janssen P, Falsen E, Altwegg M, Kersters K. Cellular fatty acid composition as a chemotaxonomic marker for the differentiation of phenospecies and hybridization groups in the genus Aeromonas. Int J Syst Bacteriol. 1994;44:651–658. [Google Scholar]
- 28.Huys G, Coopman R, Janssen P, Kersters K. High-resolution genotypic analysis of genus Aeromonas by AFLP fingerprinting. Int J Syst Bacteriol. 1996;46:572–580. doi: 10.1099/00207713-46-2-572. [DOI] [PubMed] [Google Scholar]
- 29.Huys G, Kesters I, Coopman R, Janssen P, Kersters K. Genotypic diversity among Aeromonas isolates recovered from drinking water production plants as revealed by AFLP™ analysis. Syst Appl Microbiol. 1996;19:428–435. [Google Scholar]
- 30.International Commission on Microbiological Specifications for Food. Microorganisms in foods. 5. Microbiological specifications of food pathogens. London, England: Blackie Academic & Professional; 1996. Aeromonas; pp. 5–19. [Google Scholar]
- 31.Janda J M, Reitano M, Bottone E J. Biotyping of Aeromonas isolates as correlate to delineating a species-associated disease spectrum. J Clin Microbiol. 1984;19:44–47. doi: 10.1128/jcm.19.1.44-47.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Janda J M, Clark R B, Brenden R. Virulence of Aeromonas species as assessed though mouse lethality studies. Curr Microbiol. 1985;12:163–168. [Google Scholar]
- 33.Janda J M. Recent advances in study of the taxonomy, pathogenicity, and infectious syndromes associated with the genus Aeromonas. Clin Microbiol Rev. 1991;4:397–410. doi: 10.1128/cmr.4.4.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joseph S W, Janda M, Carnahan A. Isolation, enumeration and identification of Aeromonas spp. J Food Safety. 1988;9:23–35. [Google Scholar]
- 35.Kaznowski A. Identification of Aeromonas strains of different origin to the genomic species level. J Appl Microbiol. 1998;84:423–430. doi: 10.1046/j.1365-2672.1998.00364.x. [DOI] [PubMed] [Google Scholar]
- 36.Khan A A, Kim E, Cerniglia C E. Molecular cloning, nucleotide sequence, and expression in Escherichia coli of a hemolytic toxin (aerolysin) gene from Aeromonas trota. Appl Environ Microbiol. 1998;64:2473–2478. doi: 10.1128/aem.64.7.2473-2478.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kirov S M, Rees B, Wellock R C, Goldsmid J M, Van Galen A D. Virulence characteristics of Aeromonas spp. in relation to source and biotype. J Clin Microbiol. 1986;24:827–834. doi: 10.1128/jcm.24.5.827-834.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kühn I, Allestam G, Huys G, Janssen P, Kersters K, Krovacek K, Stenström T-A. Diversity, persistence, and virulence of Aeromonas strains isolated from drinking water distribution systems in Sweden. Appl Environ Microbiol. 1997;63:2708–2715. doi: 10.1128/aem.63.7.2708-2715.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kühn I, Albert M J, Ansaruzzaman M, Bhuiyan N A, Alabi S A, Sirajul Islam M, Neogi P K B, Huys G, Janssen P, Kersters K, Möllby R. Characterization of Aeromonas spp. isolated from humans with diarrhea, from healthy controls, and from surface water in Bangladesh. J Clin Microbiol. 1997;35:369–373. doi: 10.1128/jcm.35.2.369-373.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuijper E J, Zanen H C, Peeters M F. Aeromonas-associated diarrhea in The Netherlands. Ann Intern Med. 1987;106:640–641. doi: 10.7326/0003-4819-106-4-640_2. [DOI] [PubMed] [Google Scholar]
- 41.Lior H, Johnson W M. Application of polymerase chain reaction (PCR) to detection of the aerolysin gene in whole cell cultures of B-hemolysin Aeromonas hydrophila. Experientia. 1991;47:421–4249. [PubMed] [Google Scholar]
- 42.Molenda J R, Cottingham J M. Emerging infectious diseases of particular relevance to environmental health professionals. Dairy Food Environ Sanit. 1998;18:427–435. [Google Scholar]
- 43.Morgan D R, Johnson P C, Dupont H L, Satterwhite T K, Wood L V. Lack of correlation between known virulence properties of Aeromonas hydrophila and enteropathogenicity for humans. Infect Immun. 1985;50:62–65. doi: 10.1128/iai.50.1.62-65.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakano H, Kameyama T, Venkateswaran K, Kawamaki H, Hashimoto H. Distribution and characterization of hemolytic and enteropathogenic motile Aeromonas in aquatic environment. Microbiol Immunol. 1990;34:447–458. doi: 10.1111/j.1348-0421.1990.tb01027.x. [DOI] [PubMed] [Google Scholar]
- 45.Namdari H, Bottone E J. Microbiological and clinical evidence supporting the role of Aeromonas caviae as a pediatric enteric pathogen. J Clin Microbiol. 1990;28:837–840. doi: 10.1128/jcm.28.5.837-840.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pin C, Benito Y, Carcia M L, Selgas D, Tormos J, Casas C. Influence of temperature, pH, sodium chloride, and sodium nitrite on the growth of clinical and food motile Aeromonas sp. strains. Arch Lebensmittelhyg. 1996;47:35–56. [Google Scholar]
- 47.Pollard D R, Johnson W M, Lior H, Tyler S D, Rozee K R. Detection of the aerolysin gene in Aeromonas hydrophila by the polymerase chain reaction. J Clin Microbiol. 1999;28:2477–2481. doi: 10.1128/jcm.28.11.2477-2481.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schweizer R, Kaderli M, Spahr U. Aeromonas hydrophila in Rohmilch in der Schweiz. Schweiz Milchwirtsch Forsch. 1995;24:9–11. [Google Scholar]
- 49.Shibata M, Morita K, Wanatabe N, Wada H, Okitsu T, Yamai S, Itoh K, Shimada T, Wanatabe H, Kanamori M. Rapid detection of the hemolysin genes in Aeromonas sobria by polymerase chain reaction. Kansenshogaku Zasshi. 1996;70:1266–1270. doi: 10.11150/kansenshogakuzasshi1970.70.1266. [DOI] [PubMed] [Google Scholar]
- 50.Tonolla M, Demarta A, Peduzzi R. Multilocus genetic relationships between clinical and environmental Aeromonas strains. FEMS Microbiol Lett. 1991;81:193–200. doi: 10.1016/0378-1097(91)90302-q. [DOI] [PubMed] [Google Scholar]
- 51.Umadatt S. Isolation and identification of Aeromonas spp. from ground meats in eastern Canada. J Food Prot. 1997;60:125–130. doi: 10.4315/0362-028X-60.2.125. [DOI] [PubMed] [Google Scholar]
- 52.Wang G, Tyler K D, Munro C K, Johnson W M. Characterization of cytotoxic, hemolytic Aeromonas caviae clinical isolates and their identification by determining presence of a unique hemolysin gene. J Clin Microbiol. 1996;34:3203–3205. doi: 10.1128/jcm.34.12.3203-3205.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Watson I M, Robinson J O, Burke V, Gracey M. Invasiveness of Aeromonas spp. in relation to biotype virulence factors and clinical features. J Clin Microbiol. 1985;22:48–51. doi: 10.1128/jcm.22.1.48-51.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]