Significance
Recovering relevant information, while ignoring the irrelevant, is crucial for episodic memory (remembering a particular event at a specific temporal and spatial context). Information presented at any time could drive the retrieval of more than one memory trace; thus, there should be a mechanism to select the retrieval of the most relevant trace. However, how the brain controls memory interference is not well understood. Here, we analyzed the communication between ventral hippocampus (vHPC) and medial prefrontal cortex (mPFC) during the resolution of an episodic memory task in rats. We found an increased synchronization between the vHPC and mPFC and identified specific mPFC neural subpopulations that selectively respond to object–context associations, and their firing preference correlates with the animals’ behavioral responses.
Keywords: mPFC, hippocampus, theta oscillations, behavior, memory retrieval
Abstract
Remembering life episodes is a complex process that requires interaction among multiple brain areas. It is thought that contextual information provided by the hippocampus (HPC) can trigger the recall of a past event through the activation of medial prefrontal cortex (mPFC) neuronal ensembles, but the underlying mechanisms remain poorly understood. However, little is known about the coordinated activity between these structures during recall. We performed electrophysiological recordings in behaving rats during the retrieval phase of the object-in-context (OIC) memory task. Context-guided recognition of objects in this task requires the activity of both the mPFC and the ventral HPC (vHPC). Coherence, phase locking, and theta amplitude correlation analysis showed an increase in vHPC-mPFC LFP synchronization in the theta range when animals explore contextually mismatched objects. Moreover, we identified ensembles of putative pyramidal cells in the mPFC that encode specific object–context associations. Interestingly, the increase of vHPC-mPFC synchronization during exploration of the contextually mismatched object and the preference of mPFC incongruent object neurons predicts the animals’ performance during the resolution of the OIC task. Altogether, these results identify changes in vHPC-mPFC synchronization and mPFC ensembles encoding specific object–context associations likely involved in the recall of past events.
Episodic memory deficits are hallmark deficits in aging as well as numerous neurodegenerative and psychiatric disorders, yet the mechanisms underlying memory retrieval are not fully understood. The hippocampus (HPC) has been postulated as a key structure in encoding and retrieval of episodic memories due to its ability to encode contextual information (1) and its importance for spatial navigation (2, 3). During recall, contextual information impacts the activity of neocortical areas, also identified as nodes, involved in episodic memory retrieval (4, 5). The prefrontal cortex (PFC), especially, is postulated to exert a controlling role over HPC-dependent memories by establishing relationships among memories and monitoring retrieval (4, 6). Imaging studies as well as medial PFC (mPFC) lesions in humans strongly suggest that this structure is recruited specifically under conditions of memory interference or distractions (7, 8). Moreover, tracing studies have shown a direct, monosynaptic, ipsilateral (IPSI) projection from the ventral HPC (vHPC) to the mPFC (9) as well as indirect connections through the reuniens nucleus (RE) of the thalamus (10, 11) and other polysynaptic pathways. It is hypothesized that during retrieval, the vHPC sends contextual information that engages previously formed contextual rules in the mPFC to support memory recall and to inhibit interference from less-relevant memory traces (8). However, the mechanisms underlying this vHPC-mPFC interaction remain unknown.
Slow rhythms allow information sharing by synchronizing distant brain areas. Particularly, theta rhythms in the HPC have been linked with exploratory behavior (12, 13), memory formation, and recall (14–16). In the case of the mPFC, theta rhythms had been associated with attention processes (17) and memory recall (18, 19). Furthermore, it has been shown that coordination of theta oscillations between the HPC and the mPFC increases during free exploration (20), anxiety-related behaviors (21, 22), decision making (23), and mnemonic processes that are spatial/contextually guided and rewarded (24, 25). Also, single-cell activity in the mPFC is modulated by theta rhythms in a task-specific manner (21). However, little is known about the interplay between the vHPC and the mPFC during retrieval of episodic-like memories and how the flow of information between these structures depends on contextual cues.
Novel object recognition (NOR) tasks are nonrewarded paradigms used to investigate episodic-like memory in rodents (26, 27) including versions that require contextually guided recall to recognize object–context matches. This is the case of the object-in-context (OIC) task, where animals need to recognize which of two familiar objects is contextually mismatched (28). Both the mPFC and the vHPC, as well as their functional interaction, are required for the resolution of this task (29). In this work, we analyzed the functional connectivity between the mPFC and the vHPC in rats by simultaneously recording neural activity from the vHPC and mPFC in freely moving animals during the resolution of the OIC task. We found an increase in theta oscillation power in the mPFC and vHPC as well as an increased synchronization between both structures during the retrieval phase, as assessed by coherence analysis and correlation analysis. The increase in coherence is maximal when the animals are exploring the contextually incongruent object, and it correlates with recall performance during the task. We also identified cells in the mPFC that respond differently to the OIC combinations and found a significant correlation between the cell preference and the animals’ recall performance.
Materials and Methods
Ethical Statement.
All experimental procedures were in accordance with the institutional Animal Care and Use Committee (School of Medicine, University of Buenos Aires, ASP # 49527/15, and University of Favaloro, #DCT0205-18) and government regulations (SENASAARS617.2002). All efforts were made to minimize the number of animals used and their suffering.
Subjects.
A total of 22 Wistar male rats were used: 9 for electrophysiological recordings and 13 for pharmacological experiments (7 for the OIC and 6 for the NOR). Three animals were excluded due to poor electrophysiological signal-to-noise ratio or electrode misplacement, and another could not be used for the local field potential (LFP) analysis due to problems in the vHPC electrode.
At the time of the surgery, animals were >P60, weighted 250 to 300 g, and housed in groups. Rats for chronic electrophysiological recordings were single housed after surgery. Rats were kept with water and food ad libitum under a 12-h light/dark cycle (lights on at 0700) at a constant temperature range of 21 to 23 °C. Experiments took place during the light phase of the cycle (between 1000 and 1700) in quiet rooms with dim light.
Surgical Procedures.
For electrophysiological recordings.
Animals were anesthetized with isoflurane, treated with local anesthetic (bupivacaine hydrochlorate solution, 5% wt/vol 0.2 to 0.3 mL s.c.) in the scalp and pressure points, and secured within a stereotaxic frame. The temperature was maintained using a heating pad. Small metal screws were placed as anchors for the implant. Electrodes were placed IPSI above the mPFC (+3.2 mm anterior, ± 1.2 mm lateral from bregma, and −2 mm from brain surface) and in the vHPC (−5.75 mm anterior, ± 4.6 mm lateral from bregma, and −6.3 mm from brain surface), with angles of 10° and 350°, respectively (Fig. 2A). The mPFC electrode array consisted of four tetrodes (20 μm HML-heavy polyimide insulated tungsten wire, final impedance adjusted by gold plating to 150 to 250 kOhms) separated by 250 μm in a square shape (Fig. 2A). For the vHPC, two bipolar electrodes, separated by 250 μm, were used (50 μm Formvar-insulated nickel chrome wire) (Fig. 2A). A screw located on the occipital bone was used for grounding. Dental cement was used to anchor the electrodes. Animals were then treated with antibiotics (gentamicin, 1 mg/mL) and a nonsteroidal anti-inflammatory agent (flunixin, 4 mg/mL) and allowed to recover for a week before conducting the experiments.
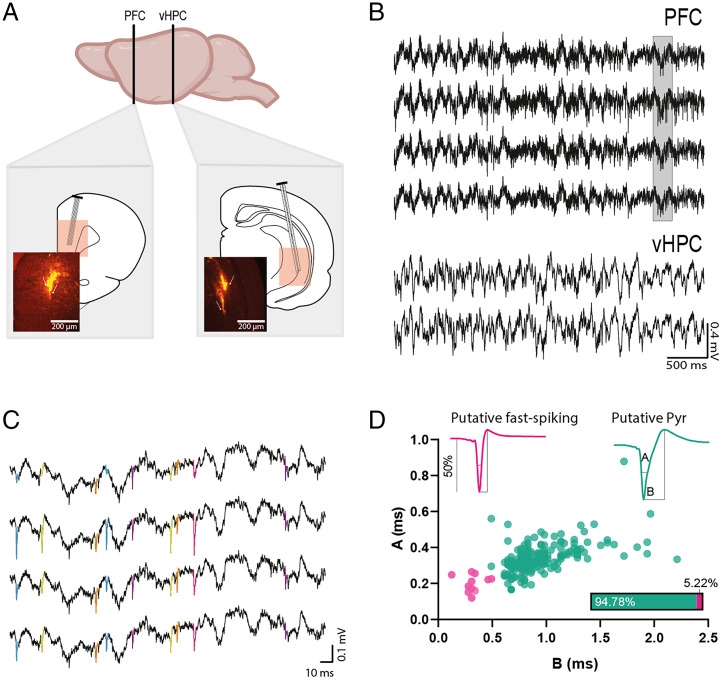
Fig. 2.
Electrophysiological recordings obtained during the resolution of the OIC task. (A) Electrode location. Dye-staining of the trace of mPFC tetrodes and vHPC stereodes. (B) Raw LFP signal from one mPFC tetrode (Top) and a vHPC stereode (Bottom) recorded during the test phase of the task. (C) Example of mPFC raw signal showing spikes colored according to their clustering into separate SUs (signal corresponds to the area painted in B). (D) mPFC pyramidal cell and interneuron classification based on their electrophysiological properties. Examples of isolated unit waveforms classified as interneurons (pink) and pyramidal (green) cells. The parameters used for the classification were A, half-amplitude duration; and B, trough to peak time. The parameters A and B were plotted for each cell, and two clear clusters were formed and used to sort neurons into putative pyramidal and interneurons.
Pharmacological disconnection experiments.
Animals were deeply anesthetized with ketamine (Inducmina, 80 mg/kg i.p.) and xylazine (Richmond, 8 mg/kg i.p) and placed in a stereotaxic frame. Two pairs of guide cannulae (22G) were implanted bilaterally into the mPFC (anteroposterior [AP]: +3.20 mm; L: ±0.75 mm; dorsoventral [DV]: −3.50 mm) and vHPC (AP: −6.30 mm; L: ±5.50 mm; DV: −5.50 mm). Dummy injectors (30G) were used to preclude their obstruction until infusions were made. After the behavioral experiments, animals were infused with 1 µl methylene blue, deeply anesthetized, and transcardially perfused to assess histological localization of the infusion sites (see SI Appendix, Fig. S2B and S3C).
Pharmacological Disconnection Intervention.
All animals were injected in four sites (mPFC and vHPC on both hemispheres) 15 min before the test session using a 10 μL Hamilton syringe. Both the mPFC and vHPC were treated with muscimol (MUS, 0.1 mg/mL, 1 µl per injection site, Alamone Lab, Cat #: M-240) in the same (IPSI) or contralateral (CONT) hemisphere in a counterbalanced manner. Vehicle (saline 1 µl/side) was injected in the remaining sites. For example, animals in the CONT group received either MUS in the right mPFC and in the left vHPC, or MUS in the left mPFC and in the right vHPC (see Fig. 1E). We used a within-subject design. Each animal was tested in both conditions (IPSI and CONT) in a pseudorandom manner following the same protocol used for the electrophysiological experiment.
Fig. 1.
vHPC and PFC are functionally connected during the resolution of the OIC task. (A) Each OIC block is a 5-d procedure. Each column represents a day. Animals were first habituated to two different contexts and then trained to two object–context associations. Then, 24 h after the training session animals are re-exposed to one of the contexts and copies of both previously presented objects. (B) Mean exploration time and (C) number of exploration events for the congruent (blue bar) and incongruent objects (purple bar) obtained during 25 test sessions from six rats. Paired t test, n = 25, **** P < 0.0001, tB = 40.82, tC = 4.87. Graphs represent mean ± SEM. (D) DI calculated for all sessions. One-sample t test against zero, ****P < 0.0001, t = 7.27, n = 25. The graph represents mean ± max and min. (E) Example of a possible combination for the infusions procedure. MUS (red) was infused 15 min before each test session IPSI or CONT, choosing the injection configuration sites in a counterbalanced manner. Saline was infused in the non-MUS-treated sites. All animals received IPSI and CONT treatment in a counterbalanced manner across trials. (F) Example of a heatmap representing the centroid of the same animal in IPSI and CONT conditions. (G) DI calculated in IPSI and CONT conditions. Paired t test, n = 7 rats per group, ***P = 0.003, t = 7.62. (Inset) Total object exploration time. Paired t test, P = 0.59, t = 0.56. The red line indicates the example shown in F. Graphs represent median, 25th to 75th quartiles, and range.
Behavioral Paradigm.
Apparatus.
We used two differently shaped acrylic mazes (50-cm width × 50-cm length × 39-cm height and 60-cm width × 40-cm length × 50-cm height arenas) as previously described (29). To change the context, we designed removable walls with different visual cues. Duplicate copies of objects made from plastic, glass, and aluminum with no natural relevance for the animals were used. Objects were randomly assigned to the different phases of the experiments. The objects were always located along the central line of the maze, away from the walls and equidistant from each other. The objects, floor, and walls were cleaned with ethanol 50% between sessions. Animals were actively exploring an object when their nose was at a distance of <2 cm or less. Turning around or sitting on the object was not considered exploratory behavior.
OIC task.
This task is a three-trial procedure that allows evaluation of the congruency between the context and the object (30, 31). First, animals are habituated to each context for 10 min. During the training phase, rats were sequentially exposed, 1 h apart, for 30 min to the contexts, each containing a different pair of identical objects. In the test phase, carried out 24 h later, a new copy of each object is presented in one of the contexts (counterbalanced) for 10 min. Thus, one of the objects is presented in an “incongruent” context (incongruent object), while the other is presented in a “congruent” one (congruent object) (30). To increase the number of recording sessions, we modified the task to increase the number of exposures. We divided the task into blocks. Each block involved a pair of distinct and different contexts, four pairs of objects, and lasted 5 d. The objects were used in two pair sets (e.g., A & B) and were randomly chosen and assigned to each context in a balanced way across subjects. The basic procedure was: 1) day 1, habituation to contexts 1 and 2; 2) day 2, training phase 1 (novel pairs of objects A or B were presented in context 1 or 2); 3) day 3, test session 1 (copies of objects A and B were presented in either context 1 or 2); 4) day 4, training phase 2 (novel objects C or D were presented in context 1 or 2); and 5) day 5, test session 2 (copies of objects C and D objects were presented in either context 1 or 2). At the end of test session 2, animals were allowed to rest for 2 d before a different block was started (Fig. 1A). Animals were then habituated to a novel set of contexts and presented with novel pairs of objects as previously described. All behavioral sessions were video recorded (Genius, FaceCam 1000×). Object exploratory events were online recorded and synchronized with the electrophysiological recordings. Based on the exploratory time, a discrimination index (DI) was calculated as tIncongruent − tcongruent/total exploratory time. We referred to the objects in the text as congruent and incongruent for descriptive purposes.
NOR task.
We also adapted the spontaneous NOR task (32). Animals were exposed to one block that started with a habituation session of 10 min to the context. On day 2, rats were exposed to a pair of novel identical objects for 30 min. On day 3 (test session 1), animals were reintroduced to the context and were allowed to explore the familiar object together with a novel one for 10 min. On day 4, animals were reintroduced to the familiar context with a novel pair of objects, and 24 h later, the second test session was conducted. All behavioral sessions were video recorded. DI was calculated as tnovel − tfamiliar/total exploratory time.
Electrophysiology.
Neural activity was recorded using a 32-channel Cheetah setup (Neuralynx). Neural signals were digitized, bandpass filtered (0.1 to 6000 Hz), and continuously sampled at 32 kHz. mPFC tetrodes were attached to a micromanipulator that allowed us to move them daily.
LFP spectral analysis.
Since all wires within each brain structure showed similar LFP signals, only one wire from the mPFC and vHPC was used hereon for the spectral analysis. LFP segments recorded during events of either congruent or incongruent object exploration were detrended, digitally filtered (2 to 625 Hz, plus notch filter), and concatenated using a Hamming window. Only exploration events longer than the median exploration time were considered for the analysis. The rate and duration of object exploration were higher for the incongruent object than for the congruent one. Thus, we randomly selected incongruent object exploration events to match the length of the congruent exploration time. For the nonobject exploration events, we randomly selected periods of time when animals were in the task but not exploring the objects. The segments were concatenated until reaching the duration of the less-explored object time. The same procedure was applied for the baseline condition using the signal acquired while animals were in their homecage. The power spectral density (PSD), cross-PSD, and coherence analyses were performed using the coherencyc function in the Chronux toolbox (https://chronux.org/). For PSD, cross-PSD, and coherence comparisons between conditions, we extracted each value at 7.5 Hz.
For the coherence peri-event spectrograms, we used the wcoherence function from the Matlab signal processing toolbox. The coherence between the mPFC and the vHPC was calculated using signal segments of 4 s centered on the beginning and the end of the object exploration events. The signal was analyzed using 500-ms windows with 70% overlap, and a mean matrix (frequency × time) was calculated for each session. All sessions were averaged for the congruent and incongruent events (Fig. 4E). The coherence maximum values from these spectrograms were extracted at 7 to 8 Hz for the first and last 500 ms of the object exploration events (ON and OFF peri-event coherence spectrograms, respectively). The same procedure was used to evaluate changes in the vHPC-mPFC coherence across the duration of the test session. To do this, the total number of exploration events for each object was equally divided into three segments, and the mean time × frequency matrices were constructed. Only sessions with at least five exploration events were used, and only the beginning of the exploration events was considered (Fig. 4G).
Fig. 4.
vHPC-mPFC coherence is enhanced during incongruent object exploration. (A) vHPC-mPFC cross-spectrum analyzed during different behavioral events. Cross-PSD analysis was performed using LFP segments recorded during baseline condition (gray) and during the test session separating non-object (orange), congruent object (blue), and incongruent object (purple) events. (B) Quantification of theta cross-PSD for all conditions. One-way repeated measure ANOVA, n = 21 sessions, ***P = 0.0009, F = 6.31. Bonferroni’s post hoc test, ***pI-B = 0.0005, *pC-B = 0.035. (C) vHPC-mPFC coherence spectrum for all conditions. (D) Quantification of coherence analysis. One-way repeated measure ANOVA, n = 21 sessions, P < 0.0001, F = 11.46. Bonferroni’s post hoc test, ***pI-B = 0.0005, ***pI-N = 0.0007, **pI-C = 0.001. Bars represent mean ± SEM. (E) Peri-event coherence spectrograms centered at the beginning (ON, Left) and at the end (OFF, Right) of the congruent (Top) and incongruent (Bottom) object exploration events. (F) Comparisons between coherence max value in the theta range during the beginning and the end of the congruent (blue) and incongruent (purple) events. Two-way ANOVA, *pInteraction = 0.03, F(1,20) = 5.28. Bonferroni’s post hoc test, **P = 0.008, ***P = 0.0003, ****P < 0.0001. (G) Mean peri-event coherence spectrograms centered at the beginning of the congruent (Top) and incongruent (Bottom) exploration events across the duration of the test session of the OIC task. (H) Object exploration events were split into three segments, and the maximum coherence value was compared for both objects and across time. Two-way ANOVA, *pinteraction = 0.04, F(1.98,29,7) = 3.53. Bonferroni’s post hoc test, *P = 0.023 (cong1 vs. incong1), **P = 0.008 (Incong1 vs. Incong3).
LFP amplitude cross-correlation analysis.
We performed a vHPC-mPFC amplitude correlation as it was described elsewhere (21). First, we extracted mPFC and vHPC LFP signals during congruent and incongruent object exploration events. LFP signals were filtered between 6 and 10 Hz, and a Hilbert transform was applied. The absolute value of the instantaneous Hilbert amplitude was used to compute a cross-correlation between the vHPC and mPFC theta oscillations amplitude using a lag of ± 200 ms for each object condition (Fig. 5H). We also compared the area under the curve of the theta oscillation envelope of the object exploration events to the average z-score throughout the session (SI Appendix, Fig. S4A).
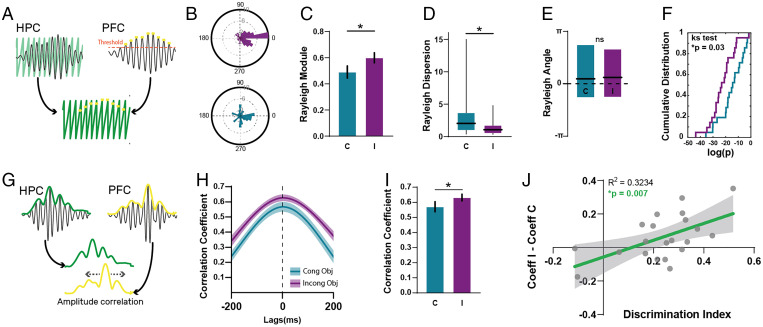
Fig. 5.
Synchronization of vHPC-mPFC theta oscillations is enhanced during exploration of the incongruent object, and it correlates with the animals’ performance in the task. (A) Phase-locking analysis of vHPC-mPFC oscillations was performed by determining the vHPC phase at which mPFC theta peaks occur. (B) Example session showing the circular phase distributions during the exploration of the congruent (blue, module: 0.1, dispersion: 14.77, P value: 0.18) and incongruent objects (purple, module: 0.56, dispersion: 1.06, P value: 4.34 × 10−9). (C) Mean vector module obtained from the phase circular distribution of each test session during the exploration of the congruent (blue) and incongruent (purple) objects. Paired t test, n = 21 sessions per group, *P = 0.017, t = 2.59. Bars represent mean ± SEM. (D) Median Rayleigh’s vector dispersion for both conditions. Paired Wilcoxon test, n = 21, *P = 0.035. Graph represents median, 25th to 75th quartiles, and range. (E) Angle of the mean vector obtained from circular phase distributions for congruent and incongruent conditions. Graph represents mean and 25th to 75th quartiles. Wilcoxon paired test P = 0.95. (F) Cumulative frequency function of Rayleigh’s test P values for the congruent (blue) and incongruent (purple) objects. Two-sample Kolmogorov-Smirnov test, *P = 0.03. (G) Scheme showing the method for calculating the cross-correlation of the instantaneous theta oscillation amplitude of the vHPC and the mPFC. (H) Mean correlation coefficient for congruent (blue line) and incongruent (purple line) conditions. Shaded areas represent ± SEM. (I) Correlation coefficient for both conditions at zero lag. Paired t test, *P = 0.04, t = 2.09. Bars represent mean ± SEM. (J) Linear regression analysis between the behavioral DI and the difference of the correlation coefficient determined when animals explore the incongruent (coeff I) and congruent objects (coeff C).
Phase-locking analysis of LFP signals.
mPFC and vHPC theta oscillations were filtered (6 to 10 Hz), and the phase angle at every timestamp of the vHPC signal (“instantaneous phase”) was obtained from the Hilbert transform. mPFC theta oscillations peaks were detected, and the instantaneous phase at which they occurred in the vHPC LFP recording was determined (Fig. 5A) and plotted in a circular histogram for each object condition (Fig. 5B). Phase locking of mPFC theta peaks to HPC theta oscillation was determined by assessing deviation from uniformity in these circular plots using the Rayleigh test.
Tetrodes SU detection and clustering.
Tetrode recordings from the mPFC were analyzed to extract single units (SUs) as previously described (33). Spikes were detected after high-pass filtering of the tetrodes signal (median filter, half-window length: 2.4 ms) using NDManager (34). Spikes were then assigned to SUs by a semiautomatic spike-sorting method based on the spike’s relative amplitudes across the tetrode and PCA (Klusters software). Semiautomatic verification of cluster’s quality was performed using autocorrelograms, average spikes’ shape, and correlation matrices (Klusters) (Fig. 2C and SI Appendix, Table S1). Recording sessions where cluster stability could not be confirmed were excluded. SUs were divided into two subclasses (putative pyramidal cells and putative interneurons, Fig. 2D) based on waveform shape as described previously (35).
mPFC SU population analysis locked to object exploration.
mPFC neurons’ firing rates were normalized (z-score), and the activity of all cells was locked to the object exploration onset event. The z-score was calculated using ± 20-s histograms centered on this event using a 0.2-s bin. Neurons were then sorted according to their mean maximum activity in a +/− 0.2-s window around time 0 at the peri-event histogram (Fig. 6A). mPFC neurons were considered to respond to the congruent or incongruent objects if they showed a change in activity in this time window greater than ± 1 SD.
Fig. 6.
The incongruent object–context preference of mPFC neurons correlates with the animals’ performance during the resolution of the OIC task. (A) PETHs locked to the beginning of the congruent (Left) and incongruent (Right) object exploration events for all SUs recorded during the OIC recall. SUs were sorted in descending order according to their mean z-score in a 400 ms time window (Bottom, indicated with a line) centered on the beginning of the congruent object exploration event. Then, the same sorting order was used to plot the activity of the neurons locked to the incongruent object event (Right). Time = 0 represents the onset of object exploration events. (B) Percentage of mPFC neurons responding to the congruent (blue), incongruent (purple), and both objects (intersection, nonselective). (C) Percentage of neuronal responses classified as excitations (dark gray) and inhibitions (light gray) according to their object preference (C, congruent; I, incongruent; C + I, nonselective; χ2= 8.66, *P = 0.0131). (D) Average response of cells classified as congruent selective when animals explore the congruent (blue) and incongruent objects (purple). (Left) Mean response of excitatory congruent cells (paired t test, n = 14, ****P < 0.0001, t = 9.36). (Right) Mean response of inhibitory responses (paired t test, n = 10, **P = 0.0016, t = 4.43). (E) As in D, but for neurons classified as selective for the incongruent object (Left, paired t test, n = 9, ***P = 0.0005, t = 5.70; Right, paired t test, n = 16, ****P < 0.0001, t = 6.56). (F) Average z-score for nonselective objects cells. Only cells responding with excitations were used (paired t test, n = 9, *P = 0.039, t = 2.46). (G) Cumulative frequency distribution of absolute z-score values for congruent (blue line) and incongruent (purple line) selective population responses. Kolmogorov-Smirnov test, P = 0.21. (Inset) Median, 25th to 75th quartiles, and range of the absolute z-score responses for both cell populations. Mann-Whitney test, *P = 0.12, U = 213. (H) Linear correlation between mPFC neuronal object preference index (|z-score in preferred condition − z-score in non-preferred condition|) and the behavioral DI. Linear regressions were calculated for the congruent (blue) and incongruent (purple) selective cell populations. (I) Frequency of behavioral DIs obtained for all test sessions. The distribution was split into two areas: left tail including sessions with the 40% smaller DI (bad performance) and right tail including sessions with the 40% higher DI values (good performance). The envelope represents the probability density. (J) Neuronal preference index for congruent and incongruent selective cells in sessions where rats showed bad or good performance. Two-way ANOVA, *pcell = 0.04, F(1,31) = 4.61. Bonferroni's post hoc test, *P = 0.04. Bars represent mean ± SEM.
SU-LFP phase-locking analysis.
The vHPC LFP signal of object exploration events was filtered in the theta range. A Hilbert transform was used to obtain the phase angle at every point of vHPC theta oscillations. The number of mPFC SU spikes occurring at different phase angles of the vHPC theta rhythm was depicted in circular plots (SI Appendix, Fig. 5A). Phase locking of mPFC SU discharges to vHPC theta oscillations was determined by assessing deviation from uniformity in these circular plots with the Rayleigh test. To guarantee sufficient statistical power, only neurons that fired at least 25 spikes during the events of interest were considered.
Postmortem histology.
At the end of the experiments, rats were anesthetized and transcardially perfused (4% paraformaldehyde [PFA] in phosphate-buffer saline [PBS]). Brains were removed, immersed overnight in PFA at 4 °C, and cryopreserved in 30% sucrose. Coronal brain sections were serially cut (40 µm) using a microtome for histologic reconstructions. Electrode localization was assessed by mechanical tissue damage and visualization of a red fluorescent dye (1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate, 100 mg/mL in acetone; DiI, Invitrogen) (Fig. 2A).
Statistical analysis.
Statistical analyses were performed with GraphPad 8. Data were analyzed using two tailed unpaired Student’s t tests when two groups were compared. For comparisons between two repeated-measured groups, a two-tail paired Student’s t test was used. Paired one-way ANOVA followed by Bonferroni post hoc test was performed for comparisons among more than two groups. If normality test failed (using Shapiro-Wilk test), nonparametric test versions were performed (Mann-Whitney for two groups or Kurskal-Wallis test for more than two groups). For correlations between continuous variables, we performed linear regressions. For distribution comparisons, a two-sample Kolmogorov-Smirnov test was applied. In all cases, P values were considered to be statistically significant when P < 0.05.
Results
In rodents, episodic-like memories can be evaluated using object recognition paradigms based on the natural tendency of these animals to explore novelty. Particularly, the OIC task is characterized by a strong contextual component (29) in which animals are trained to learn two different object–context associations. In this setting, both objects as well as the contexts are familiar to the animals. However, during the test phase, one of the objects is presented in a novel context, generating a contextual incongruency (from now on, “incongruent object”; Fig. 1A). Then, to solve the task, animals have to recognize a novel combination of previously known contexts and objects. Here, we used a slightly modified version of the OIC already published (see Materials and Methods) to maximize the object exploration events for electrophysiological analysis of brain activity. Similar to what we have previously reported (29, 31), animals showed increased exploratory time and a higher number of exploratory events for the incongruent object compared to the congruent one during the test phase of the task (Fig. 1 B and C).
Thus, the DI was significantly different from zero (Fig. 1D). This preference for the incongruent object was observed and maintained across the entire test session (SI Appendix, Fig. S1B). We also found a decrease in object exploration time across training sessions, suggesting equal habituation to the contexts and objects during the training phase (SI Appendix, Fig. S1A). These results suggest that animals present a robust preference for the contextually mismatched object and that they can solve the task in this modified version (see Materials and Methods).
vHPC-mPFC Functional Connectivity Is Required for the Resolution of the OIC Task.
The vHPC and the mPFC appear as fundamental components of the circuit required to solve the OIC task (29). To establish if the interaction between vHPC and mPFC is required during the resolution of the OIC protocol we are using here, we performed a pharmacological disconnection experiment under these conditions. We trained cannulated rats in the OIC task (SI Appendix, Fig. S2) and infused MUS affecting one vHPC and one mPFC unilaterally (both in the same hemisphere: IPSI) or asymmetrically (one structure of each hemisphere: CONT) (Fig. 1E and SI Appendix, Fig. S2) before the test session. The IPSI-treated animals, who conserved one functional vHPC-mPFC circuit, could solve the OIC task showing high DI. However, the CONT treatment group lost the preference for exploring the incongruent object (Fig. 1F) and, thus, presented a significant decrease in the DI (Fig. 1G). This result cannot be explained by differences in exploratory behavior during the training phase of the task since all animals explored the objects similarly (SI Appendix, Fig. S2A). Also, we did not find differences in the total time animals spent exploring both objects for any of the conditions tested (Fig. 1G, Inset) suggesting that MUS infusions did not affect motor activity. Interestingly, we did not observe any differences in the DI when the same disconnection manipulation was performed during a NOR task, which does not depend on contextual information (SI Appendix, Fig. S3). This suggests that the interaction between vHPC and mPFC is necessary for the resolution of the task and that this circuit is recruited during recognition memory recall when the task relies on contextual information. Thus, the protocol implemented here was effective to generate long-lasting OIC memories that require a functional vHPC-mPFC communication during the retrieval phase.
mPFC and vHPC Theta Oscillations Increase during Object Exploration.
We studied the oscillatory profile of the vHPC-mPFC circuit and mPFC SU activity during the recall of episodic-like memories guided by contextual information. Rats were ipsilaterally implanted with four tetrodes in the mPFC and two stereodes in the vHPC (Fig. 2A). The local field potential activity and 211 mPFC SUs were recorded during the test sessions (Fig. 2 B and C). Isolated units were classified offline into two subclasses (putative pyramidal cells and interneurons) based on waveform and firing characteristics (Fig. 2D).
Putative pyramidal neurons were defined as cells with relatively broad spike waveforms and with negative curvilinear shapes in the after-hyperpolarization phase. Based on their relatively narrow waveforms, 5% of all recorded cells were classified as putative interneurons, most likely fast spiking. Since putative interneurons were very few, all mPFC cells were analyzed together.
To evaluate if theta oscillations were modulated during object exploration, a PSD analysis of the LFP signal from the mPFC and the vHPC was performed on different behavioral periods. LFP activity was recorded prior to the beginning of the session, while the animals were still in their homecage, and it was used as a baseline to assess changes in LFP power during the task. The test session was divided into periods of object exploration (incongruent and congruent) and time intervals where animals were not actively exploring the objects (nonobject). We found that theta oscillations (6 to 10 Hz) were significantly enhanced in the mPFC and vHPC during object exploration periods (Fig. 3 A and C). In the vHPC, we found that theta power was significantly increased during exploration of both objects compared to baseline and nonobject periods (Fig. 3B). In the mPFC, we observed an increase in theta oscillations during the exploration of the incongruent object compared with the baseline and the nonobject conditions (Fig. 3D). In this structure, we did not observe statistical differences in theta power oscillation between the incongruent and congruent conditions (Bonferroni’s post hoc test, P = 0.415). These results indicate that theta oscillations are enhanced during active episodes of object exploration, suggesting that they may be involved in the behaviors and cognitive processes associated with object recognition and retrieval.
Fig. 3.
Theta oscillations are increased in the vHP and mPFC during object exploration. Power auto-spectrum of vHPC (A) and PFC (C) LFP signals. PSD analysis was performed using LFP segments recorded during baseline condition (gray) and during the test session separating non-object (orange), congruent object (blue), and incongruent object (purple) events. (B) Quantification of theta power in the vHPC for all conditions. One-way repeated measure ANOVA, n = 21 sessions, **P = 0.002, F = 7.44. Bonferroni's post hoc, *pC-B = 0.042, *pI-B = 0.049, **pI-N = 0.002, ****pC-N < 0.0001. (D) Quantification of theta power in the mPFC. One-way repeated measure ANOVA, n = 21 sessions, P = 0.01, F = 4.02. Bonferroni's post hoc test, *pI-B = 0.01, *pI-N = 0.03. Bars represent mean ± SEM.
vHPC-mPFC LFP Coordination Shows High Coherence in the Theta Range during the Exploration of a Contextually Mismatched Object.
Next, we addressed whether the functional connectivity between these structures changed dynamically during the test phase of the OIC task. We found a significant increase in the cross-PSD in the theta range when animals were actively exploring an object compared to the baseline condition (Fig. 4 A and B). Interestingly, vHPC-mPFC theta oscillation coherence only increased when animals explored the contextually mismatched object, while in the other conditions, it did not significantly differ from baseline (Fig. 4 C and D).
Thus, while the mPFC and vHPC share an increase in theta activity irrespective of the explored object, they show a significant oscillatory synchronization only when the incongruent object is explored. To further address the dynamic modulation of this synchronization during object exploration, we built coherograms where coherence at different frequencies is plotted across time and centered at the beginning of the object exploration events (Fig. 4 E and G). Interestingly, we found that the vHPC-mPFC coherence is higher predominantly at the beginning of the exploratory bout of the incongruent object (Fig. 4 E and F). To further analyze the temporal dynamics of this process, we divided the test sessions into three segments and found that theta synchronization decreased throughout the session, specifically for the incongruent object (Fig. 4 G and H). This suggests that an increase in vHPC-mPFC theta synchronization is required at the beginning of the session to recognize the object in context association as novel and to control the correct resolution of the task.
We performed two additional analyses assessing the phase synchronization between these structures. First, we performed a phase-locking analysis to determine how consistent the vHPC-mPFC theta oscillation phase was between structures. We looked at the vHPC phase at which mPFC theta oscillations peaks occurred (Fig. 5A) and compared the phase circular distributions obtained when animals explored either object using the Rayleigh test (Fig. 5B). We found that vHPC-mPFC theta oscillations showed increased phase locking in the incongruent condition compared with the congruent one.
This was evidenced by a larger vector module (Fig. 5C), and smaller dispersion (Fig. 5D) and P value (obtained when evaluating if the phases follow a uniform distribution; Fig. 5F) of the circular phase distributions when animals explore the contextually mismatched object. We did not find significant differences between the angles of the phase distributions when comparing the congruent and incongruent conditions (Fig. 5E). In addition, we performed a cross-correlation of instantaneous amplitudes of LFP theta oscillations (21). We found that the amplitude cross-correlation between vHPC and mPFC theta oscillations (Fig. 5G) was significantly higher during the exploration of the incongruent object compared with the congruent one (Fig. 5 H and I). This increase was not explained by an increase in theta amplitude, per se, since we did not find a significant difference in theta amplitudes when animals explore the objects (SI Appendix, Fig. S4). Interestingly, the difference between the cross-correlation value at zero lag between the incongruent and congruent conditions positively correlated with the animals’ behavioral DI (Fig. 5J). This suggests that an increase in vHPC-mPFC synchronization during the exploration of the incongruent object is instrumental for the correct solving of the OIC task. Overall, the analysis of coherence, phase locking, and instantaneous amplitude cross-correlation show a higher and tighter vHPC-mPFC synchronization during the exploration of the incongruent object than during the recognition of a known object–context match.
mPFC SUs Differentially Encoding OIC Stimuli Correlate with Animals’ Recall Performance.
Since vHPC-mPFC theta oscillations and functional connectivity are increased during object exploration, we addressed whether vHPC theta rhythms can modulate mPFC SU activity in the present task. We recorded mPFC SU activity during the test phase of the OIC task and found that 22.5% of mPFC recorded neurons (32/142) were significantly phase locked to HPC theta rhythms (SI Appendix, Fig. S5A). Among this neuronal subpopulation, some units showed significant phase locking when animals explored a particular object, and some were nonselectively phase locked to object exploration (SI Appendix, Fig. S5B). These results suggest that vHPC theta oscillations impact the mPFC activity, modulating the firing pattern.
Previous reports have shown that mPFC neuronal ensembles are modulated during learning and memory (36) and working-memory tasks (37). But it is still unclear if mPFC SUs can encode OIC information. To address this, we normalized the firing rate of individual mPFC neurons (z-score) and built peri-event time histograms (PETHs) for every recorded cell centered on the beginning of each object exploration period (for congruent and incongruent objects). Neurons were then sorted according to their maximal responses in a small time window around the moment animals begin exploring the objects (Fig. 6A). Responses to object exploration were considered significant for cells showing changes in firing rate greater or smaller than mean ±1 SD within this time window (SI Appendix, Fig. S6 A and B). Overall, 59 of 211 (29%) neurons responded to object exploration (58 putative pyramidal cells and 1 putative fast-spiking interneuron; Fig. 6B). Once cells were sorted by their maximal response to both objects, two extra PETHs were built to analyze how neurons showing significant responses to one object responded to the other. For example, Fig. 6A shows the PETHs of all neurons sorted by their maximal responses to the congruent object, and the same sorting order was used to evaluate how these cells respond to the incongruent object. We found that 24 and 25 out of 211 mPFC neurons (Fig. 6B) showed a significant change in firing rate during the congruent and incongruent object exploration periods, respectively, while 10 neurons responded to both objects (SI Appendix, Fig. S6 and Fig. 6C). While the object-selective neurons showed a very strong preference for either the congruent or incongruent object (Fig. 6 D and E), the nonselective object–responding cells showed, on average, a slight preference for the congruent one (Fig. 6F). Further analysis of the type of object responses showed that the proportion of excitations and inhibitions was different among groups (Fig. 6C). To assess if the object-selective responses were of similar amplitude irrespective of the congruent or incongruent nature of the context, we compared both cumulative frequency distributions of response amplitudes and found no significant differences between them (Fig. 6G). Altogether, these results suggest that distinct mPFC SU subpopulations could differentially encode the representations of the congruent and incongruent objects.
Finally, since we clustered distinct mPFC neuronal populations responding differently to the congruent and incongruent objects, we asked if this neuronal OIC discrimination correlated with recall performance assessed with the DI. We evaluated how congruent and incongruent object-selective cells responded to their preferred and nonpreferred objects by calculating a neuronal preference index. We found that the congruent-selective cells showed high object discrimination neural activity irrespective of the animals’ behavior. On the other hand, the neuronal object discrimination activity observed in the incongruent-selective cells significantly and negatively correlated with the behavioral DI (Fig. 6H). To further analyze this phenomenon, we compared the difference in the neuronal response to both objects for a subgroup of OIC tests where animals showed either good or bad performance in the task (Fig. 6I). We found that when animals show a poor behavioral performance, both selective neuronal populations clearly discriminate between the contextually congruent and incongruent objects with changes in firing rate (selectively changing their firing for their preferred object). However, in those sessions where animals had a good behavioral performance and explored the incongruent object more, only the congruent cell subpopulation showed high neuronal preference index, while the incongruent cells fired similarly during exploration of both objects (Fig. 6J). Overall, strong congruent object-selective responses do not inform about performance in the task. By contrast, a poor neuronal discrimination associated with a particular object correlates with a failure to recognize its association with the context. Together with the above findings, these data suggest that lack of object discrimination at the neuronal level in the mPFC during enhanced theta synchronization with the vHPC might reflect the identification of a mismatch between the explored object–context pair and previously stored experiences.
Discussion
This study examined the vHPC-mPFC coordination and mPFC activity during OIC retrieval. Our main findings are as follows: 1) vHPC-mPFC functional connectivity is required for the resolution of the OIC task and not for the NOR task; 2) theta oscillation power increases during object exploration in the mPFC and vHPC; 3) the coherence, amplitude cross-correlation, and phase locking of theta oscillations between these structures increase significantly during the exploration of the contextually mismatched object; 4) this increase in vHPC-mPFC synchronization is time restricted to the beginning of the test session and object exploratory bouts; 5) a greater vHPC-mPFC synchronization during the exploration of the contextually mismatched object correlates with the behavioral performance in the task; 6) there are subpopulations of putative pyramidal cells in the mPFC that selectively respond to object–context associations; and 7) their neuronal preference for one object correlates with the animals’ behavioral response during the resolution of the OIC task.
Episodic memory is the memory of events in their original spatiotemporal context: what happened and when and where it happened. Though the combination of these characteristics generates unique events, many times, specific features may be part of different memories. The mPFC is involved in memory processes mainly through strategic or cognitive control over retrieval processes. Eichenbaum and Preston proposed that the mPFC stores information during acquisition that is then used to flexibly select the memory trace to be retrieved, particularly when the animal is presented with conflictive information (38). The resolution of the OIC requires the recognition of the correct object–context association when the animal is simultaneously presented with two object–context pairs. Thus, two possible traces can be retrieved: the congruent memory appears as the most relevant to solve the task, while the incongruent object memory could interfere by activating a different episode (29, 30). Then, selecting the most relevant one is indispensable for the correct resolution of the task. The mPFC is suggested as a structure that could receive contextual information through the direct vHPC connection to solve possible conflicting data arising from related memories (9, 38). Consistent with this theory, we found that vHPC-mPFC functional communication was: 1) necessary to solve an object recognition task that depends on contextual information (OIC); and 2) not required in the NOR task, where contextual information is not needed to identify an object as familiar or novel. The vHPC and mPFC are connected through monosynaptic (9, 39, 40) and polysynaptic pathways (41, 42). Our results do not allow to dissect the type of connectivity between both structures, but they demonstrate that their functional IPSI communication is instrumental in the resolution of the OIC task.
Low-frequency brain rhythms, like theta oscillations, are usually coherent over long distances and thought to link distributed cell assemblies. Thus, they have been proposed as attractors to control local and distant structure activity (43, 44). HPC theta oscillations are closely related to locomotion and animals’ speed, and they carry spatial information (43, 45). We found that the power of vHPC theta oscillations increases during object exploration, suggesting that vHPC theta oscillations in this task might be associated with the cognitive processes underlying the behavioral response. In addition, several studies reported an increase of mPFC theta power during decision making, attentional processes, and, recently, in a NOR task (23, 46, 47). We observed an increase in mPFC theta power during the exploration of the contextually incongruent object.
Since the vHPC and the mPFC showed increased theta power during object exploration, we analyzed the synchronization between both structures in the theta range during these events. Based on the anatomical connectivity and pharmacological experiments, we predicted that the resolution of the task would involve tight communication between both structures. Increased theta vHPC-mPFC connectivity has been observed in spatial navigation, working memory (17, 48), a NOR task, and rewarded object–context associations (25, 47, 49). Consistently, we found that the vHPC and mPFC share an increase in theta activity irrespective of the explored object. Interestingly, further analysis demonstrated that the vHPC-mPFC theta synchronization increases significantly only when animals explore the incongruent object, suggesting that increase in theta synchronization might be the mechanism by which the novelty of the object–context association is established. We also observed that vHPC-mPFC communication in the theta range modulates the firing pattern of a proportion of mPFC neurons. This is evidenced by an increase in neuronal phase locking to this rhythm during object exploratory bouts. Altogether, this provides strong evidence for a differential requirement of vHPC-mPFC communication during object exploration in the resolution of the task. Interestingly, it was shown that long-range inputs from vHPC impact mPFC pyramidal and local interneurons (46, 50, 51). Thus, theta oscillation entrainment could be critical to determine the coordination of local faster mPFC oscillations by acting in a coordinated manner on both types of cells (52).
As we mentioned, the resolution of the OIC task requires the selection of the most relevant memory trace. This selection, as it happens in other tasks involving competing information, appears to involve the mPFC. Previous studies in humans (53, 54) and in rodents (55) have demonstrated that mPFC activity decreases as the cognitive control demands decline, suggesting that prefrontal interaction with downstream structures is a dynamic and active process. Here, we observed that the vHPC-mPFC synchronization increases during the initial incongruent object exploration and then declines as object novelty decreases, supporting the view that the involvement of the mPFC during memory retrieval is an adaptive process that diminishes over time.
Moreover, higher vHPC-mPFC synchronization during the exploration of the incongruent object, relative to the congruent one, correlates with the nature of the behavioral response (Fig. 5J). The exploration of the incongruent object involves the recognition of the object itself (familiar to the animal) as well as the contextual mismatch. We hypothesize that the relative increase in vHPC-mPFC theta synchronization observed for the incongruent object may help identify the object–context pair as novel and, thus, facilitate the correct selection of the relevant memory trace. This hypothesis is supported by the fact that the coherence between mPFC and vHPC is higher at the beginning of the test session and declines as the object exploration progresses, and a new context–object linkage is probably created.
Due to the vast projections that arrive at the mPFC from associative regions (56), the mPFC has been postulated as the orchestrator of memory reactivation in downstream structures for different tasks (4, 23, 46, 47). We have previously shown that activation of the vHPC, as well as its interaction with the mPFC, affects the labilization of OIC memory traces in the perirhinal cortex (29). Interestingly, we found that it is the memory trace of the congruent object the one that normally was reactivated, supporting that it is the most relevant trace to solve the task. Additionally, when both memory traces were reactivated, animals showed a poor behavioral performance in the test. Then, it is relevant to analyze the cellular signature within the mPFC during the resolution of the task. We identified two neuronal subpopulations that responded selectively to the congruent and incongruent objects. In addition, the preference of the incongruent cell subpopulation correlated with the animals' performance in the task. We hypothesize that the activation of specific neuronal ensembles with high preference for a particular object–context pair could drive the retrieval of the original memory trace for that match. In the context of the present task, we find the following: 1) when both neuronal subpopulations show high preference, the incongruent object cells would be controlling the retrieval of an incorrect trace, related to a past experience of this object in another context and leading to a poor OIC recognition; and 2) when the incongruent object subpopulation shows low preference index, only the most relevant memory trace will be retrieved. We interpret that this low preference index of the incongruent object ensemble generates a noisy signaling within the mPFC that helps inhibit the retrieval of the less-relevant memory trace in downstream structures.
Human studies have shown that prefrontal regions are engaged during retrieval inhibition and that they modulate the activity of downstream areas (57). Interestingly, functional homologous regions in rodents, like the mPFC, have also been associated with retrieval inhibition (55). What are the local processes that enable the cortex to control retrieval interference? Though our work does not answer this question, it does provide some interesting observations. mPFC ensembles were suggested to select the most appropriate behavioral response by acting as comparators (58) between the present information and that acquired during training. When animals show good performance, the lack of contextual coincidence could be reflected in the incongruent neuronal subpopulation as a decrease in the signal-to-noise ratio (i.e., a small difference between the signals evoked during exploration of the object–context pairs) that drives the inhibition of the memory trace evoked by the incongruent object. However, the type of information that drives the change of ratio observed is still unknown. It could arise from a local circuit controlling the firing pattern of mPFC cells, or, as mentioned above, it could be driven by other structures like the vHPC that send contextual information to the mPFC relevant to solve the task. For instance, the contextual inconsistency might be coded as an increase in vHPC-mPFC synchronization during the incongruent object exploration and might be read by mPFC neurons to compare recent and old information. But which structure integrates the vHPC-mPFC synchronization is still unclear. There are two other areas that are known to be important in the OIC task: the PRH, which integrates different features of the objects and generates complex object representations (59); and the dorsal HPC, which receives and integrates information regarding the context and the objects (38, 59–61). We speculate that these structures could integrate the vHPC-mPFC coordinated activity during retrieval in the context of previously generated ensembles associated with the task. How might the vHPC-mPFC interact with these structures? The RE of the thalamus appears as a strong candidate to mediate the interaction between these structures and the dorsal HPC. It has been postulated that the RE promotes mPFC-HPC interactions possibly through synchronization at different oscillation frequencies facilitating communication (41, 62, 63). However, the ability of the RE to direct the synchronization of these structures in the theta range is still unclear and requires further analysis (41).
Overall, we identified and analyzed the interaction between key structures involved in the retrieval and resolution of an episodic-like memory task in rats. We found that the correct resolution of the OIC task requires an increased synchronization between the vHP and the mPFC. Also, during the resolution of the OIC task, mPFC neuronal ensembles might drive the retrieval by inhibiting the less-relevant trace through a decrease in the signal-to-noise ratio in the ensemble responding to the incongruent object. As episodic memories are one of the hallmark processes affected in aging and in neurological and psychiatric disorders, the identification of the circuits underlying the retrieval of a specific memory is essential to better understand the mechanisms involved and to identify new therapeutics targets.
Supplementary Material
Acknowledgments
We thank Dr. Gustavo Murer, Dr. Juan Belforte, and Dr. Pedro Bekinschtein for their critical reading and comments on the manuscript. We would also like to thank Graciela Ortega, Veronica Risso, and Jessica Unger for technical support.
This work was supported by research grants from the National Agency of Scientific and Technological Promotion of Argentina to N.V.W. (PICT 2015–2344, PICT 2018–1063, Ben Barres Spotlife Award from eLife) and C.L.Z. (PICT 2014-2828, PICT 2017-2254) and from the University of Buenos Aires to C.L.Z. (Ubacyt 2018-2020).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2203024119/-/DCSupplemental.
Data Availability
All study data is available at the Open Science Framework (https://doi.org/10.17605/OSF.IO/M59NJ) or are included in the article and/or SI Appendix.
References
- 1.Zemla R., Basu J., Hippocampal function in rodents. Curr. Opin. Neurobiol. 43, 187–197 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eichenbaum H., The role of the hippocampus in navigation is memory. J. Neurophysiol. 117, 1785–1796 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moser E. I., Moser M. B., McNaughton B. L., Spatial representation in the hippocampal formation: A history. Nat. Neurosci. 20, 1448–1464 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Eichenbaum H., Memory: Organization and control. Annu. Rev. Psychol. 68, 19–45 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wirt R. A., Hyman J. M., ACC theta improves hippocampal contextual processing during remote recall. Cell Rep. 27, 2313–2327.e4 (2019). [DOI] [PubMed] [Google Scholar]
- 6.Zhang W., van Ast V. A., Klumpers F., Roelofs K., Hermans E. J., Memory contextualization: The role of prefrontal cortex in functional integration across item and context representational regions. J. Cogn. Neurosci. 30, 579–593 (2018). [DOI] [PubMed] [Google Scholar]
- 7.Tomita H., Ohbayashi M., Nakahara K., Hasegawa I., Miyashita Y., Top-down signal from prefrontal cortex in executive control of memory retrieval. Nature 401, 699–703 (1999). [DOI] [PubMed] [Google Scholar]
- 8.Eichenbaum H., Prefrontal-hippocampal interactions in episodic memory. Nat. Rev. Neurosci. 18, 547–558 (2017). [DOI] [PubMed] [Google Scholar]
- 9.Hoover W. B., Vertes R. P., Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct. Funct. 212, 149–179 (2007). [DOI] [PubMed] [Google Scholar]
- 10.Varela C., Kumar S., Yang J. Y., Wilson M. A., Anatomical substrates for direct interactions between hippocampus, medial prefrontal cortex, and the thalamic nucleus reuniens. Brain Struct. Funct. 219, 911–929 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoover W. B., Vertes R. P., Collateral projections from nucleus reuniens of thalamus to hippocampus and medial prefrontal cortex in the rat: A single and double retrograde fluorescent labeling study. Brain Struct. Funct. 217, 191–209 (2012). [DOI] [PubMed] [Google Scholar]
- 12.Buzsáki G., Moser E. I., Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nat. Neurosci. 16, 130–138 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buzsáki G., Tingley D., Space and time: The hippocampus as a sequence generator. Trends Cogn. Sci. 22, 853–869 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hasselmo M. E., Eichenbaum H., Hippocampal mechanisms for the context-dependent retrieval of episodes. Neural Netw. 18, 1172–1190 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lopes-Dos-Santos V., et al. , Parsing hippocampal theta oscillations by nested spectral components during spatial exploration and memory-guided behavior. Neuron 100, 940–952.e7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boyce R., Glasgow S. D., Williams S., Adamantidis A., Causal evidence for the role of REM sleep theta rhythm in contextual memory consolidation. Science 352, 812–816 (2016). [DOI] [PubMed] [Google Scholar]
- 17.O’Neill P. K., Gordon J. A., Sigurdsson T., Theta oscillations in the medial prefrontal cortex are modulated by spatial working memory and synchronize with the hippocampus through its ventral subregion. J. Neurosci. 33, 14211–14224 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marko M., Cimrová B., Riečanský I., Neural theta oscillations support semantic memory retrieval. Sci. Rep. 9, 17667 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riddle J., Scimeca J. M., Cellier D., Dhanani S., D'Esposito M., Causal evidence for a role of theta and alpha oscillations in the control of working memory. Curr. Biol. 30, 1748–1754.e1744 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siapas A. G., Lubenov E. V., Wilson M. A., Prefrontal phase locking to hippocampal theta oscillations. Neuron 46, 141–151 (2005). [DOI] [PubMed] [Google Scholar]
- 21.Adhikari A., Topiwala M. A., Gordon J. A., Synchronized activity between the ventral hippocampus and the medial prefrontal cortex during anxiety. Neuron 65, 257–269 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adhikari A., Topiwala M. A., Gordon J. A., Single units in the medial prefrontal cortex with anxiety-related firing patterns are preferentially influenced by ventral hippocampal activity. Neuron 71, 898–910 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang W., Shin J. D., Jadhav S. P., Multiple time-scales of decision-making in the hippocampus and prefrontal cortex. eLife 10, e66227 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benchenane K., et al. , Coherent theta oscillations and reorganization of spike timing in the hippocampal- prefrontal network upon learning. Neuron 66, 921–936 (2010). [DOI] [PubMed] [Google Scholar]
- 25.Place R., Farovik A., Brockmann M., Eichenbaum H., Bidirectional prefrontal-hippocampal interactions support context-guided memory. Nat. Neurosci. 19, 992–994 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morici J. F., Bekinschtein P., Weisstaub N. V., Medial prefrontal cortex role in recognition memory in rodents. Behav. Brain Res. 292, 241–251 (2015). [DOI] [PubMed] [Google Scholar]
- 27.Barker G. R., Warburton E. C., Multi-level analyses of associative recognition memory: The whole is greater than the sum of its parts. Curr. Opin. Behav. Sci. 32, 80–87 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barker G. R. I., Warburton E. C., Putting objects in context: A prefrontal-hippocampal-perirhinal cortex network. Brain Neurosci. Adv. 4, 2398212820937621 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morici J. F., et al. , 5-HT2a receptor in mPFC influences context-guided reconsolidation of object memory in perirhinal cortex. eLife 7, e33746 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eacott M. J., Norman G., Integrated memory for object, place, and context in rats: A possible model of episodic-like memory? J. Neurosci. 24, 1948–1953 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bekinschtein P., Renner M. C., Gonzalez M. C., Weisstaub N., Role of medial prefrontal cortex serotonin 2A receptors in the control of retrieval of recognition memory in rats. J. Neurosci. 33, 15716–15725 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barker G. R., Bird F., Alexander V., Warburton E. C., Recognition memory for objects, place, and temporal order: A disconnection analysis of the role of the medial prefrontal cortex and perirhinal cortex. J. Neurosci. 27, 2948–2957 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alvarez R. J., Pafundo D. E., Zold C. L., Belforte J. E., Interneuron NMDA receptor ablation induces hippocampus-prefrontal cortex functional hypoconnectivity after adolescence in a mouse model of schizophrenia. J. Neurosci. 40, 3304–3317 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hazan L., Zugaro M., Buzsáki G., Klusters, NeuroScope, NDManager: A free software suite for neurophysiological data processing and visualization. J. Neurosci. Methods 155, 207–216 (2006). [DOI] [PubMed] [Google Scholar]
- 35.Barthó P., et al. , Characterization of neocortical principal cells and interneurons by network interactions and extracellular features. J. Neurophysiol. 92, 600–608 (2004). [DOI] [PubMed] [Google Scholar]
- 36.Park A. J., et al. , Reset of hippocampal-prefrontal circuitry facilitates learning. Nature 591, 615–619 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spellman T., et al. , Hippocampal-prefrontal input supports spatial encoding in working memory. Nature 522, 309–314 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Preston A. R., Eichenbaum H., Interplay of hippocampus and prefrontal cortex in memory. Curr. Biol. 23, R764–R773 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jay T. M., Glowinski J., Thierry A. M., Selectivity of the hippocampal projection to the prelimbic area of the prefrontal cortex in the rat. Brain Res. 505, 337–340 (1989). [DOI] [PubMed] [Google Scholar]
- 40.Jay T. M., Witter M. P., Distribution of hippocampal CA1 and subicular efferents in the prefrontal cortex of the rat studied by means of anterograde transport of Phaseolus vulgaris-leucoagglutinin. J. Comp. Neurol. 313, 574–586 (1991). [DOI] [PubMed] [Google Scholar]
- 41.Dolleman-van der Weel M. J., et al. , The nucleus reuniens of the thalamus sits at the nexus of a hippocampus and medial prefrontal cortex circuit enabling memory and behavior. Learn. Mem. 26, 191–205 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vertes R. P., Interactions among the medial prefrontal cortex, hippocampus and midline thalamus in emotional and cognitive processing in the rat. Neuroscience 142, 1–20 (2006). [DOI] [PubMed] [Google Scholar]
- 43.Buzsáki G., Theta oscillations in the hippocampus. Neuron 33, 325–340 (2002). [DOI] [PubMed] [Google Scholar]
- 44.Stella F., Treves A., Associative memory storage and retrieval: Involvement of theta oscillations in hippocampal information processing. Neural Plast. 2011, 683961 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O’Keefe J., Recce M. L., Phase relationship between hippocampal place units and the EEG theta rhythm. Hippocampus 3, 317–330 (1993). [DOI] [PubMed] [Google Scholar]
- 46.Liu T., Bai W., Xia M., Tian X., Directional hippocampal-prefrontal interactions during working memory. Behav. Brain Res. 338, 1–8 (2018). [DOI] [PubMed] [Google Scholar]
- 47.Wang C., et al. , Hippocampus-prefrontal coupling regulates recognition memory for novelty discrimination. J. Neurosci. 41, 9617–9632 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sigurdsson T., Stark K. L., Karayiorgou M., Gogos J. A., Gordon J. A., Impaired hippocampal-prefrontal synchrony in a genetic mouse model of schizophrenia. Nature 464, 763–767 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alemany-González M., et al. , Prefrontal-hippocampal functional connectivity encodes recognition memory and is impaired in intellectual disability. Proc. Natl. Acad. Sci. U.S.A. 117, 11788–11798 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dégenètais E., Thierry A. M., Glowinski J., Gioanni Y., Synaptic influence of hippocampus on pyramidal cells of the rat prefrontal cortex: An in vivo intracellular recording study. Cereb. Cortex 13, 782–792 (2003). [DOI] [PubMed] [Google Scholar]
- 51.Canetta S., et al. , Differential synaptic dynamics and circuit connectivity of hippocampal and thalamic inputs to the prefrontal cortex. Cereb. Cortex. Commun. 1, tgaa084 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sirota A., et al. , Entrainment of neocortical neurons and gamma oscillations by the hippocampal theta rhythm. Neuron 60, 683–697 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kuhl B. A., Dudukovic N. M., Kahn I., Wagner A. D., Decreased demands on cognitive control reveal the neural processing benefits of forgetting. Nat. Neurosci. 10, 908–914 (2007). [DOI] [PubMed] [Google Scholar]
- 54.Wimber M., Alink A., Charest I., Kriegeskorte N., Anderson M. C., Retrieval induces adaptive forgetting of competing memories via cortical pattern suppression. Nat. Neurosci. 18, 582–589 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bekinschtein P., Weisstaub N. V., Gallo F., Renner M., Anderson M. C., A retrieval-specific mechanism of adaptive forgetting in the mammalian brain. Nat. Commun. 9, 4660 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Klune C. B., Jin B., DeNardo L. A., Linking mPFC circuit maturation to the developmental regulation of emotional memory and cognitive flexibility. eLife 10, e64567 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Anderson M. C., Floresco S. B., Prefrontal-hippocampal interactions supporting the extinction of emotional memories: The retrieval stopping model. Neuropsychopharmacology 47, 180–195 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McKenzie S., et al. , Representation of memories in the cortical-hippocampal system: Results from the application of population similarity analyses. Neurobiol. Learn. Mem. 134 Pt A, 178–191 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bussey T. J., Saksida L. M., Murray E. A., Perirhinal cortex resolves feature ambiguity in complex visual discriminations. Eur. J. Neurosci. 15, 365–374 (2002). [DOI] [PubMed] [Google Scholar]
- 60.Ahn J. R., Lee I., Neural correlates of both perception and memory for objects in the rodent perirhinal cortex. Cereb. Cortex 27, 3856–3868 (2017). [DOI] [PubMed] [Google Scholar]
- 61.Squire L. R., Wixted J. T., Clark R. E., Recognition memory and the medial temporal lobe: A new perspective. Nat. Rev. Neurosci. 8, 872–883 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hartung H., Brockmann M. D., Pöschel B., De Feo V., Hanganu-Opatz I. L., Thalamic and entorhinal network activity differently modulates the functional development of prefrontal-hippocampal interactions. J. Neurosci. 36, 3676–3690 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hauer B. E., Pagliardini S., Dickson C. T., The reuniens nucleus of the thalamus has an essential role in coordinating slow-wave activity between neocortex and hippocampus. eNeuro 6, ENEURO.0365-19.2019 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data is available at the Open Science Framework (https://doi.org/10.17605/OSF.IO/M59NJ) or are included in the article and/or SI Appendix.